Uterine Cancer
John F. Boggess and Joshua E. Kilgore
• 46,000 estimated new cases per year
• Approximately 8,100 deaths per year from the disease
• Endometrioid adenocarcinoma arises in the endometrium and accounts for approximately 90% of cases
• Most common presenting symptom is irregular vaginal bleeding
• Risk factors include unopposed estrogen therapy, obesity, chronic anovulation, tamoxifen use, diabetes, and nulliparity
• Staging is surgical according to International Federation of Gynecology and Obstetrics (FIGO) guidelines
• Uterine sarcomas and carcinomas are staged differently
• Primary surgery with hysterectomy, bilateral salpingo-oophorectomy and lymphadenectomy is curative in majority of cases
• Radiation alone may be used for inoperable patients and/or palliation
• Risk factors for recurrence or extrauterine spread include deep myometrial invasion, moderate- to high-grade histology, and lymphovascular space invasion (LVSI)
• Adjuvant radiation may be used in patients with high-intermediate and high-risk disease
• Adjuvant chemotherapy with or without radiation therapy may be administered in cases with aggressive histology, such as serous, clear cell, carcinosarcoma, or leiomyosarcoma and undifferentiated endometrial sarcoma
Locally Advanced, Metastatic, or Recurrent Disease
• Chemotherapy, radiation, or combination therapy is used in locally advanced, metastatic, or recurrent endometrial cancer
• Cytoreductive surgery is associated with improved overall survival
• Salvage radiotherapy is utilized for recurrences after surgery alone
• Endocrine therapy with progestins and/or tamoxifen may be used when avoidance of cytotoxic chemotherapy is desired
• Tumor-directed radiation therapy may be employed in cases of locally advanced, metastatic, or recurrent disease
• Survival is strongly correlated with surgical stage, tumor grade, and histology
• Uterine clear cell and serous carcinomas are associated with a worse prognosis than endometrioid adenocarcinomas
Introduction
Endometrial cancer, or uterine cancer, is a malignancy arising from the endometrium. Women have a 1-in-40 lifetime risk of being diagnosed with endometrial cancer, the fourth most common malignancy among women. Uterine cancer is the most common gynecologic malignancy in the United States.1 Stage I malignancies comprise the majority of endometrial cancers. Postmenopausal bleeding is the most common presentation. Cancer arising from endometrial glands is referred to as carcinoma, compared to the less common uterine sarcoma that arises in mesenchymal elements such as smooth muscle or connective tissue.
Epidemiology
An estimated 46,000 new diagnoses of uterine cancer and 8,100 deaths from the disease occurred in 2011.2 Median age at diagnosis is 63 years. The Surveillance, Epidemiology, and End Results program (SEER) reported that 70% of patients are diagnosed with localized disease, 17% with regional spread, 9% with distant disease, and 4% were unstaged.1 Five-year survival rates are approximately 80% to 90% for disease confined to the uterus (Table 88-1).3
Table 88-1
| Stage | 5-year Survival (%) |
| IA | 90 |
| IB | 78 |
| II | 74 |
| IIIA | 56 |
| IIIB | 36 |
| IIIC1 | 57 |
| IIIC2 | 49 |
| IVA | 22 |
| IVB | 21 |
Etiology and Biological Characteristics
Genetics
Endometrial cancer is uncommon in premenopausal women and genetic factors account for only 1% of newly diagnosed cases.4,5 Lynch syndrome or hereditary nonpolyposis colorectal cancer syndrome (HNPCC) is associated with a relative risk of 1.5 for the development of endometrial cancer before menopause.5 Women with Lynch syndrome have a 27% to 71% risk of endometrial cancer.6,7 Mutations in the DNA mismatch repair genes impair the ability to maintain genomic integrity. Patients with Lynch syndrome have a germline mutation in one mismatch repair allele and the second allele is inactivated through mutation, loss of heterozygosity, or epigenetic silencing by promoter hypermethylation. Inactivation of both genes leads to increased DNA mutations and alteration of microsatellite regions in the tumor compared to normal tissue. Microsatellite instability refers to the contraction or expansion of short repetitive DNA sequences and is due to loss of DNA mismatch repair. Microsatellite instability is found in 90% of tumor tissue from patients with Lynch syndrome. Mutations in MSH2 or MLH1 account for approximately 90% of the identified mutations associated with Lynch syndrome. Other mutations in PMS1, MSH6, and MLH3 have also been described. The age of diagnosis in women with Lynch syndrome is 46 to 54 years.8–11 Endometrioid histology is the most common Lynch syndrome–associated endometrial cancer; however, nonendometrioid tumors have been reported.12,13 The majority of tumors are diagnosed with early-stage disease, similar to women with sporadic endometrial cancer. Women with Lynch syndrome are at risk for synchronous or metachronous cancers.14,15 The Amsterdam Criteria have been developed to identify families at risk for Lynch syndrome (Table 88-2). The Society of Gynecologic Oncologists published committee guidelines for genetic testing of individuals at risk for Lynch syndrome (Table 88-3).16
Table 88-2

Table 88-3
Society of Gynecologic Oncologists Statement Guidelines on Risk Assessment for Lynch Syndrome
| GENETIC EVALUATION STRONGLY RECOMMENDED IF RISK OF LYNCH SYNDROME IS 20%–25% |
| GENETIC ASSESSMENT MAY BE “HELPFUL” IF RISK OF LYNCH SYNDROME IS 5%–10% |
|
1. Patients with endometrial or colorectal cancer before age 50 years 2. Patients with endometrial and/or ovarian cancer and another Lynch-associated malignancy before age 50 years 3. Patients with endometrial or colorectal cancer and a first-degree relative with a Lynch syndrome–associated malignancy before age 50 year 4. Patients with endometrial or colorectal cancer at any age with 2 or more first- or second-degree relatives of any age with a Lynch syndrome–associated malignancy 5. Patients with first- or second-degree relative who meets above criteria |
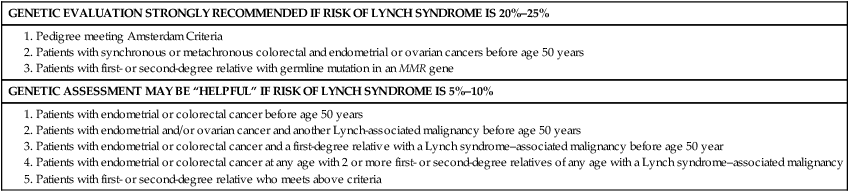
From Lancaster JM, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 2007;107(2):159–62.
Risk Factors
Table 88-4 lists many of the known risk factors for endometrial adenocarcinomas. Endogenous estrogen exposure associated with nulliparity, early menarche, late menopause, obesity, and estrogen-producing tumors are associated with an increased risk of endometrial cancer.17,18 Risk factors are associated with the unopposed estrogen effect, or lifetime estrogen exposure. Exposure to unopposed estrogen leads to increased endometrial cell proliferation, resulting in increased DNA replication errors and somatic mutations. Exogenous estrogen sources, such as hormone replacement therapy without progestins, increases the risk for endometrial cancer fivefold.19 The use of progestins with hormone replacement therapy may decrease the risk of uterine cancer through downregulation of hormone receptors. Cancers that occur in women on combined hormone replacement therapy tend to be of low stage and grade. Endogenous hormones, such as androstenedione, estrone, and estradiol, are associated with a threefold to fourfold increased risk.20
Table 88-4
Risk Factors for Endometrial Cancer
| Risk Factor | Relative Risk |
| Unopposed estrogen therapy | 10-20 |
| Tamoxifen | 2.5 |
| Polycystic ovarian syndrome | 3-5 |
| Obesity | 2-5 |
| Diabetes | 2-3 |
| Nulliparity | 2-3 |
| Estrogen-producing ovarian tumors | 5 |
The National Surgical Adjuvant Breast Project (NSABP) and the Netherlands Cancer Institute clarified a causal link between the treatment of breast cancer with tamoxifen and endometrial cancer.21,22 Tamoxifen, a selective estrogen receptor modulator, was first described as a risk factor for uterine cancer in 1985. The NSABP evaluated 2834 women treated for breast cancer treated with tamoxifen. Twenty-four cases of endometrial cancer were diagnosed among the study patients. Nearly 96% (N = 23) of endometrial cancer cases occurred in women taking tamoxifen. The annual hazard rate of endometrial cancer development was 1.6 per 1000 patient-years in the tamoxifen group and 0.2 per 1000 patient-years in the placebo group. In addition, 88% of endometrial cancers diagnosed were stage I, and 78% were of low or intermediate grade. The use of tamoxifen results in a 38% improvement in disease-free survival for breast cancer, which far outweighs the risk of endometrial cancer, from which there were only four deaths. A subsequent analysis by the NSABP showed the risk was predominately in women 50 years of age or older, with an incidence of approximately 2 per 1000.23 Regular gynecologic follow-up is recommended for patients on tamoxifen.
The Netherlands Cancer Institute performed a case–control study by identifying 98 patients in whom endometrial cancer developed after treatment for breast cancer compared with controls in whom endometrial cancer did not develop.24 Twenty-four percent of the patients with endometrial cancer had taken tamoxifen, compared with 20% of the controls. Treatment with tamoxifen for 5 years or more was associated with a 3.0 risk of endometrial cancer compared with controls.
Previous Irradiation
The etiology of uterine sarcomas is not well understood, but may be related to prior exposure to pelvic irradiation. Uterine sarcomas and carcinomas have been reported after irradiation for cervix and rectal cancer.25,26 Radiation-associated uterine cancers are usually of higher grade and stage, with a more unfavorable histology.27
Other Comorbidities
Systemic disease and lifestyle factors influence the risk of endometrial cancer. Hypertension, diabetes, and obesity all increase the risk. Nearly 40% of uterine cancers can be attributed to obesity.28 Increasing body mass indices (BMIs) are associated with higher relative risks of developing a uterine malignancy. Overweight women (BMI 28 to 29.9) have a relative risk of 1.5 compared with women with a normal BMI. Obese women (BMI 30 to 33.9) have a relative risk of 2.9 and markedly obese women (BMI >34) have a relative risk of 6.3.29 The increased risk of endometrial carcinoma associated with increasing BMI may be explained by higher levels of endogenous estrogen. The conversion of androstenedione to estrone and the aromatization of androgens to estradiol occurs in peripheral adipose tissue. Severely obese women are more likely than nonobese women to have a less-aggressive histology and present with stage I disease.30
Protective Factors
Known protective factors against endometrial cancer include full-term pregnancy, multiparity, older age of menarche, and oral contraceptive use. Use of oral contraceptives for up to 5 years is associated with a relative risk of 0.2, and its use for at least 1 year reduces endometrial cancer risk by approximately 45%.31 Interestingly, cigarette smoking decreases the risk of endometrial cancer; however, the clear adverse risks of smoking greatly outweigh any potential benefit with respect to endometrial cancer.
Prevention and Early Detection
Abnormal uterine bleeding is the most common presentation in women diagnosed with endometrial carcinoma. Approximately 80% of women with endometrial carcinoma present with abnormal uterine bleeding; however, the amount of bleeding does not correlate with the risk of cancer.32,33 Less commonly, abnormal cervical cytology may be the first indication of uterine cancer. The risk of endometrial carcinoma and the need for endometrial evaluation depends on age, symptoms, and the presence of risk factors.32,34–36 Postmenopausal bleeding, including spotting, carries a 3% to 20% risk of endometrial carcinoma. Diagnosis of endometrial carcinoma before age 45 years is uncommon; however, intermenstrual bleeding or prolonged periods of amenorrhea (6 or more months) after age 45 years should be evaluated. Nineteen percent of endometrial carcinomas occur between ages 45 and 64 years.37 The risk of uterine cancer before age 45 years is low and increases with advancing age. The majority of abnormal bleeding is due to benign uterine pathology, but further evaluation is warranted and recommended by the American College of Obstetricians and Gynecologists.36
Women presenting with clinical signs suspicious for endometrial carcinoma should undergo a physical examination, including pelvic examination. Urine or serum human chorionic gonadotropin testing to exclude pregnancy should be performed on all reproductive-age women prior to any endometrial sampling. Pelvic ultrasound is often ordered to assess the endometrial thickness. In postmenopausal women, transvaginal ultrasound to evaluate the endometrial thickness may be used for endometrial neoplasia in selected women. In women with postmenopausal bleeding, an endometrial thickness less than 4 mm is associated with a low risk of endometrial carcinoma40–40; however, any focal endometrial lesion requires a biopsy. In contrast to postmenopausal women, the utility of transvaginal ultrasound is not well established in premenopausal women.41,42 There is no standard threshold for endometrial thickness in premenopausal women. Transvaginal ultrasound also does not appear to be an effective screening tool for women on hormone replacement therapy.43,44 Endometrial sampling is the gold standard for premenopausal women and women taking hormone replacement therapy.
Pathology and Pathways of Spread
Pathogenesis Overview
Endometrioid adenocarcinoma arises from the endometrium and is the most common pathological subtype (95% of cases) (Table 88-5). Uterine serous and clear cell carcinomas are less common adenocarcinomas but more aggressive and associated with a worse prognosis. Uterine sarcomas comprise 2% to 5% of uterine malignancies and arise from the myometrium or other mesenchymal elements (Table 88-6).45 Current classification guidelines for sarcomas include leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated endometrial sarcoma.
Table 88-5
Classifications of Endometrial Carcinomas
Table 88-6
Gynecologic Oncology Group Classifications of Uterine Sarcomas
| NONEPITHELIAL TUMORS |
| MIXED EPITHELIAL–NONEPITHELIAL TUMORS |
Endometrial adenocarcinomas appear to have two distinct mechanisms of pathogenesis.46 The most common adenocarcinoma, type I, arises in normal-functioning endometrium. Type I tumors tend to be associated with hyperplasia, be well differentiated, and express steroid hormone receptors. Type II adenocarcinomas arise in atrophic endometrium, are associated with endometrial intraepithelial carcinomas, and have a worse prognosis than type I tumors.
Endometrial Hyperplasia
Simple hyperplasia is the most common type and is a benign, diffuse thickening of the endometrium. Histologically, simple hyperplasia is characterized by dilated and increased numbers of endometrial glands, but minimal crowding or glandular complexity. Malignant transformation from simple hyperplasia is rare (Table 88-7). Complex hyperplasia is characterized by increased endometrial thickness because of increased numbers and crowding of the endometrial glands. The glands have irregular contours and markedly diminished stromal spaces. Complex hyperplasia without atypia is associated with a 3% rate of malignant transformation.47 Both simple and complex hyperplasia without atypia tend to regress with treatment.
Table 88-7
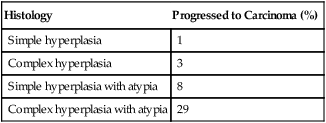
Progression of Endometrial Hyperplasia
| Histology | Progressed to Carcinoma (%) |
| Simple hyperplasia | 1 |
| Complex hyperplasia | 3 |
| Simple hyperplasia with atypia | 8 |
| Complex hyperplasia with atypia | 29 |
From Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56(2):403–12.
Atypical hyperplasia is characterized by an increased number of cellular atypia. Glandular morphology appears simple or complex and includes nuclear enlargement, increased nucleus-to-cytoplasmic ratio, nuclear hyperchromatism, prominent nucleoli, and loss of normal orientation of the epithelial cells. If atypia is present, the reported risk of finding an adenocarcinoma after hysterectomy ranges from 14% to 57%.48 Among such cancers, myometrial invasion is common, and up to 20% of cases will be grade II or III.
Endometrioid Adenocarcinoma
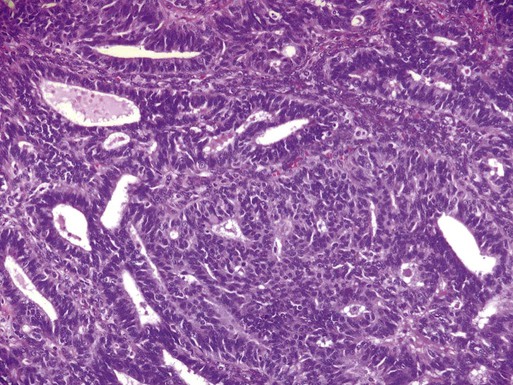
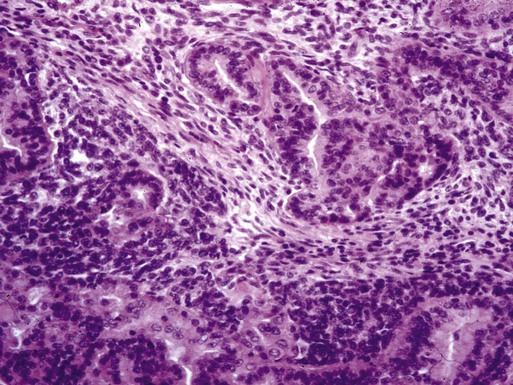
Adenocarcinomas arising from hypertrophic endometrium or associated with atypical hyperplasia are typically endometrioid type. For this histologic subtype, the median age of diagnosis is 65 years. Seventy-five percent of uterine cancers are endometrioid adenocarcinoma in histology. Variations include villoglandular, secretory, ciliated cell, adenocarcinoma with squamous differentiation, and not otherwise specified (NOS). Adenocarcinomas are further categorized by depth of invasion and grade. International Federation of Gynecology and Obstetrics (FIGO) uses a combination of architectural patterns and nuclear features for histologic grading of tumors. Low-grade, or grade I, lesions are well-differentiated tumors composed of cells and glands that closely resemble those of the normal endometrium (Figure 88-1). Glandular growth patterns show 5% or less solid growth in grade I tumors. Grade II lesions contain 6% to 50% solid growth pattern, and grade III tumors are composed of more than 50% nonsquamous solid growth pattern.49 Although this system relies predominantly on the glandular architecture, FIGO recommends the overall grade of the tumor should be raised by one grade in cases with severe nuclear atypia. Higher-grade tumors are associated with increased incidence of lymph node metastasis, myometrial invasion, and a poorer overall prognosis.50 Depth of invasion is determined by measuring the percentage of cancer extension into the uterine wall, or myometrium. For staging purposes, FIGO classifies depth of invasion as less than 50% of the myometrium or greater than 50% of the myometrium.
Uterine Serous Carcinoma
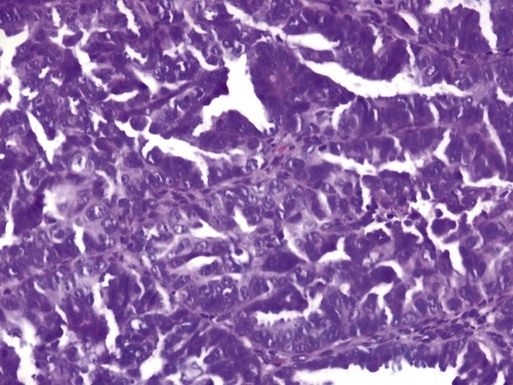
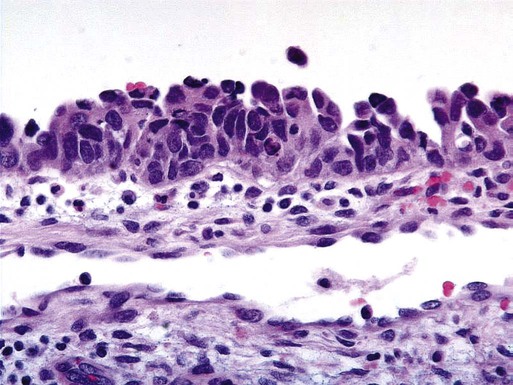
Uterine serous carcinomas represent 5% to 10% of endometrial malignancies. The median age at diagnosis is 63 years. Serous uterine carcinomas are not hormonally mediated and arise from endometrial intraepithelial carcinoma, a recognized precursor lesion (Figure 88-2). These cancers are considered high-grade with marked nuclear pleomorphism, multinucleated cells, psammoma bodies, hobnail cells, uneven gland borders, and papillary or solid growth patterns with slitlike spaces (Figure 88-3).51 Lymphovascular invasion is common and lymph node metastases are present in 36% of women with no myometrial invasion, 50% with less than one-half invasion, and 40% with greater than one-half invasion.52 Serous uterine cancers have a high rate of metastasis to the omentum and peritoneal surfaces.53 Survival rates are only 30% to 50% even when disease is confined to the uterus, and median survival is significantly shorter than that for endometrioid adenocarcinoma.
Clear Cell Carcinoma
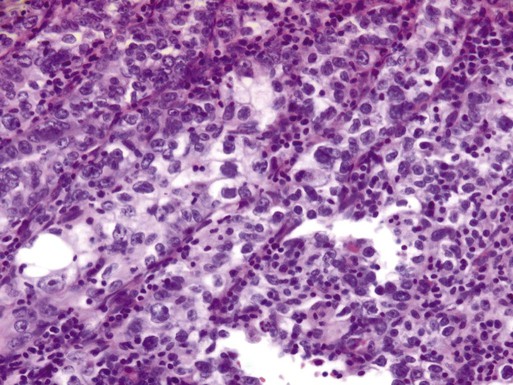
In utero exposure to the synthetic estrogen diethylstilbestrol (DES) is associated with clear cell carcinomas of the uterus, vagina, ovary, and cervix. DES was prescribed to pregnant women between 1940 and 1971 for various indications, including prevention of miscarriage. The risk of developing clear cell carcinoma of the genital tract is 0.1% in a woman exposed to DES in utero.54 The median age of diagnosis is 66 years and clear cell carcinomas comprise 5% of adenocarcinomas.55 Cells form cystic solid or tubular cellular patterns whose cytoplasm appears clear or light pink on hematoxylin-eosin staining. Papillary formations, when present, often have a characteristic hyalinized core, and hobnail cells are frequently seen (Figure 88-4). Nuclear size varies, but high mitotic count is often present and lesions are considered high grade. Although pelvic failures occur in about one-third of patients postoperatively without adjuvant radiation, unlike serous carcinoma, this subtype has only a 15% rate of intraabdominal failure. Relapse in the lung, liver, and bone occurs in 25% of patients.56 The 5-year disease-free survival rate is approximately 40%.
Carcinosarcoma
Uterine carcinosarcomas are highly aggressive tumors with a poor prognosis. These tumors were previously classified as uterine sarcomas and termed malignant mixed müllerian tumors; however, they are now classified as carcinomas arising from a monoclonal cancer cell that exhibits sarcomatous metaplasia. Uterine carcinosarcoma is rare, with an incidence of 1 to 4 per 100,000 in the United States in women older than age 35 years.57 African Americans have a twofold increase in incidence compared with non-Hispanic whites.58,59 A history of pelvic radiation exposure is associated with an increased risk of developing uterine carcinosarcoma.27,60 Uterine carcinosarcomas contain both sarcomatous and carcinomatous elements (Figure 88-5). The most common combination is a mixed homologous tumor consisting of an endometrial stromal sarcoma and a high-grade serous carcinoma. The carcinomatous or epithelial elements predominate metastatic sites and determine the overall course in advanced cases.61,62
Leiomyosarcoma
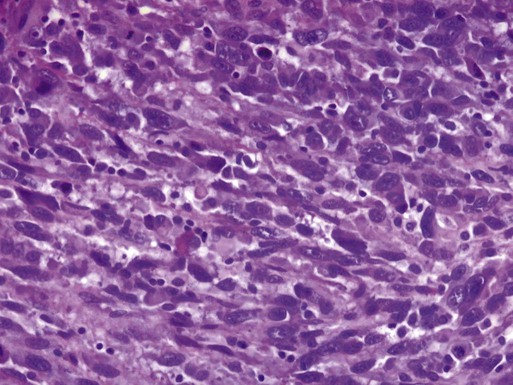
Leiomyosarcoma makes up about 1% of uterine malignancies. Median age of diagnosis is 52 years. Leiomyosarcoma are thought to arise independently from leiomyomas (uterine fibroids). These tumors are characterized by abundant mitoses (greater than 10 per 10 high-power fields), prominent cellular atypia, and necrosis (Figure 88-6). The presence of two of the three features indicates a risk of metastasis greater than 10%.63 There are two variations of leiomyosarcoma: epithelioid leiomyosarcoma and myxoid leiomyosarcoma. Epithelioid leiomyosarcoma tumors are characterized by abundant eosinophilic cytoplasm, atypia, and >5 mitoses per 10 high-power fields. Benign pathology is not confirmed by the absence of coagulative necrosis. Myxoid leiomyosarcomas are highly malignant tumors characterized by a dense myxoid appearance.64
Endometrial Stromal Sarcoma
Stromal sarcomas have been divided into two categories: benign stromal nodule if the margins are smooth, and endometrial stromal sarcoma (ESS) if the margins are infiltrating or lymphovascular invasion is present (Figure 88-7). Previous classifications included low-grade sarcomas with fewer than 10 mitoses per 10 high-power fields, and high grade sarcomas with 10 or more mitoses per 10 high-power field.65
ESSs are malignant tumors composed of cells similar to those of the endometrial stroma. Histologically, the cells have scant cytoplasm and serpentine processes that infiltrate between muscle fibers and into lymphatic spaces. Mild nuclear atypia is present and mitoses are usually less than 10 per 10 high-power fields. Estrogen and progesterone receptors are generally expressed and are responsive to progesterone therapy.66 The tumors are characterized by long disease-free intervals; however, up to 36% of patients with stage I disease will recur.
Mixed Epithelial–Nonepithelial Tumors
Adenosarcoma is a rare mixed tumor with a benign epithelial component and a malignant stromal element.68 These tumors generally have a low malignant potential and good prognosis. The majority of women present with stage I disease.69 Adenosarcoma with sarcomatous overgrowth is a variation of adenosarcoma and tends to have a worse prognosis.70
Molecular Pathways
Type 1 and type 2 endometrial cancers demonstrate unique molecular pathways. Type 1 lesions are commonly associated with unopposed estrogen, endometrial hyperplasia and younger age. Type 1 tumors are thought to be a result of genetic alteration and hormones. Gene expression within the endometrium is closely regulated by systemic hormonal changes. As such, genes expressed in type 1 cancers resemble those of proliferative endometrium consistent with unopposed estrogen and endometrial proliferation. Loss of pentaerythritol tetranitrate (PTEN) function is thought to occur early during carcinogenesis. Up to 83% of endometrioid carcinomas demonstrate loss of PTEN.71 Mutations in microsatellite instability, K-ras, and β-catenin are also associated with type 1 endometrial cancers.72–76 Approximately 20% of sporadic endometrioid-type endometrial cancers demonstrate microsatellite instability. Nonendometrioid endometrial cancers are rarely associated with microsatellite instability. Both PTEN mutations and microsatellite instability have been demonstrated in precursor lesions such as complex atypical hyperplasia.71,77
In contrast, type 2 endometrial cancers are most commonly associated with mutation in p53, HER-2/neu amplification, and p16 inactivation. Up to 90% of serous carcinomas are associated with mutations in the tumor suppressor gene p53.74 Identification of genetic mutations has led to the development and study of targeted therapy for endometrial cancer. Prognosis has been correlated with several biological markers. Overexpression of p53, abnormal DNA ploidy, increased DNA methylation and expression of p21 has been associated with a worse prognosis; however, preserved expression of progesterone and estrogen receptors may indicate a more favorable prognosis.
Endometrial adenocarcinomas express estrogen receptors (ERs) and progesterone receptors (PRs) in approximately 57% of cases, whereas 24% are negative for either receptor.78 Expression correlates with stage and tumor differentiation. Eighty percent of grade I tumors and 60% to 70% of grade II tumors express both ER and PR compared with 46% ER and 59% PR expression in grade III tumors.79 Higher-grade histologies, such as uterine serous carcinoma, also express lower levels of receptors compared with endometrioid carcinomas. Survival is better in ER-positive/PR-positive and ER-negative/PR-positive tumors compared with ER-negative/PR-negative or ER-positive/PR-negative tumors.80 ER status has been shown to be prognostic in some studies. Loss of PR expression is more common in metastatic tumors than loss of ER expression.81
Clinical Features
Approximately 80% to 90% of women with endometrial cancer present with postmenopausal vaginal bleeding. In premenopausal women, endometrial cancer may present with abnormal bleeding patterns or menorrhagia. In either case, other malignancies such as cervical or vaginal cancer must be excluded. Bladder and gastrointestinal cancers can also present as irregular vaginal bleeding and should be evaluated accordingly.82 Less commonly, patients present with metastatic disease in the absence of vaginal bleeding. The severity of vaginal bleeding does not correlate with the risk of malignancy. Increasing age and vaginal bleeding is strongly associated with risk of malignancy. Postmenopausal bleeding, including spotting, is attributed to endometrial carcinoma in up to 20% of cases.35,36 Premenopausal women are less likely to be diagnosed with endometrial malignancy, but menorrhagia and menometrorrhagia should be evaluated as recommended by the American College of Obstetricians and Gynecologists.83 Endometrial cancer may present as abnormal cytology during routine pelvic examination with a pap smear. Atypical glandular or endometrial cells diagnosed on cervical cytology must be evaluated keeping in mind that malignant cells may arise from either the cervix or uterus. Advanced disease may present as pelvic pain, hematuria, hematochezia, renal obstruction, abdominal distention, or pulmonary symptoms.
Staging
Endometrial carcinoma is surgically staged according to FIGO guidelines.84,85 In 1988, FIGO instituted surgical staging for endometrial cancer. Prior to this system, physical examination was used to clinically stage patients. In addition to hysterectomy, FIGO guidelines recommend the assessment of adnexa, pelvic, and paraaortic lymph nodes. In addition to revised endometrial carcinoma staging in 2009, FIGO implemented separate staging systems for uterine sarcomas (Tables 88-8 through 88-10).
Table 88-8
FIGO 2009 Staging for Endometrial Carcinoma (Including Carcinosarcoma)
| Stage | |
| IA | Tumor confined to uterine corpus, <50% myometrial invasion |
| IB | Tumor confined to uterine corpus, ≥50% myometrial invasion |
| II | Tumor invades cervical stroma but confined to uterus |
| IIIA | Tumor invades uterine serosa or adnexa |
| IIIB | Involvement of vagina or parametrium |
| IIIC1 | Metastasis to pelvic lymph nodes |
| IIIC2 | Metastasis to paraaortic lymph nodes |
| IVA | Invasion of bladder or bowel mucosa |
| IVB | Distant metastases including intraabdominal metastasis and/or inguinal lymph nodes |
Table 88-9
FIGO 2009 Staging for Leiomyosarcoma and Endometrial Stromal Sarcomas
| Stage | |
| IA | Tumor limited to uterus, size <5 cm |
| IB | Tumor limited to uterus, size >5 cm |
| IIA | Adnexal involvement |
| IIB | Extrauterine pelvic tissue involvement |
| III | Involvement of abdominal tissues |
| IIIA | 1 site |
| IIIB | >1 site |
| IIIC | Metastases to pelvic and/or paraaortic lymph nodes |
| IVA | Tumor invades bladder or rectum |
| IVB | Distant metastases |
Table 88-10
FIGO 2009 Staging for Uterine Adenosarcoma
| Stage | |
| IA | Tumor limited to endometrium |
| IB | Tumor limited to uterus, <50% myometrial invasion |
| IC | Tumor limited to uterus, ≥50% myometrial invasion |
| IIA | Adnexal involvement |
| IIB | Extrauterine pelvic tissue involvement |
| III | Involvement of abdominal tissues |
| IIIA | 1 site |
| IIIB | >1 site |
| IIIC | Metastases to pelvic and/or paraaortic lymph nodes |
| IVA | Tumor invades bladder or rectum |
| IVB | Distant metastases |
The most recent classification system for endometrial carcinoma was changed to include only two substages of stage I. Stage IA tumors are limited to the inner one-half of the myometrium. Stage IB tumors invade one-half or more of the myometrium. Total hysterectomy with bilateral salpingo-oophorectomy with pelvic and paraaortic lymphadenectomy is the standard procedure for endometrial carcinoma.86 In contrast to prior endometrial carcinoma staging guidelines, intraperitoneal cytology is not part of FIGO 2009 staging. More than 50% of patients with positive peritoneal washings will have extrauterine disease involving the pelvis or abdomen; however, approximately 10% of FIGO stage I tumors will have positive peritoneal washings.
One of the most important prognostic factors for uterine cancer is the presence of extrauterine disease. The approach to lymph node assessment in patients with early-stage disease is controversial. The rate of nodal involvement varies with stage and histology (Tables 88-11 and 88-12). Well-differentiated tumors with superficial invasion have a 3% to 5% risk of nodal metastasis, whereas deeply invasive poorly differentiated tumors have up to a 20% risk of nodal involvement.50,87 High-grade histology, such as serous or clear cell tumors, is also associated with increased risk of nodal metastasis. Myometrial invasion greater than one-half and tumors larger than 2 cm are also associated with an increased risk of nodal disease. There is no evidence that lymphadenectomy is beneficial in cases of uterine sarcoma.
Table 88-11
Frequency of Positive Pelvic Lymph Nodes
| Depth of Myometrial Invasion | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) |
| Endometrium only | 0 | 3 | 0 |
| Inner one-third | 3 | 5 | 9 |
| Middle one-third | 0 | 8 | 4 |
| Outer one-third | 11 | 19 | 34 |
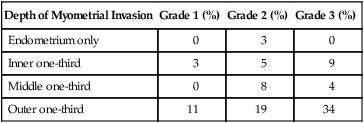
From Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105(2):109.
Table 88-12
Frequency of Positive Paraaortic Lymph Nodes
| Depth of Myometrial Invasion | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) |
| Endometrium only | 0 | 3 | 0 |
| Inner one-third | 1 | 4 | 4 |
| Middle one-third | 5 | 0 | 0 |
| Outer one-third | 6 | 14 | 23 |
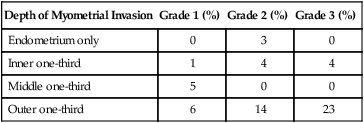
From Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105(2):109.
Stage
Patients with disease confined to the uterus have an excellent prognosis compared with patients with extrauterine or metastatic disease. Current FIGO surgical staging guidelines more accurately predict survival than clinical staging.88 Stage I and II survival rates are approximately 80% to 90%, compared to 50% to 60% and 20% for stage III and IV disease, respectively.
Depth of Invasion
Myometrial invasion is the most important pathological feature influencing risk of lymph node metastasis. Twenty-five percent of deeply invasive (outer third of myometrium) tumors have pelvic lymph node involvement, compared with 1% of superficially invasive tumors, 5% of tumors involving the inner third, and 6% of tumors involving the middle third of the myometrium.50 Paraaortic lymph node metastasis are seen less often with 1% of superficial, 3% of inner third, 1% of middle third, and 17% of outer third tumors.
Grade
Histologic grade correlates with depth of invasion, and more than half of grade 3 cancers are deeply invasive. Higher grade is also associated with lymph node metastasis. Grade 1 cancers carry a 3% risk of pelvic lymph node involvement, compared with 9% for grade 2 and 18% for grade 3 tumors.50
Histologic Subtype
Endometrioid adenocarcinoma is the most common and most favorable histology. Uterine serous carcinoma and clear cell carcinoma are adenocarcinomas with a much higher propensity for nodal involvement and extrauterine spread, leading to a poorer overall prognosis. Uterine carcinosarcoma (previously called malignant mixed müllerian tumors) is also considered a high-risk variant of adenocarcinoma. Overall survival for endometrioid adenocarcinoma approaches 90%, compared with only 33% for other combined subtypes of adenocarcinoma.89
Therapy
Surgery as a Single Modality
Traditionally, surgical staging was performed via laparotomy; however, minimally invasive surgery has gained wide acceptance as an alternative to laparotomy in the evaluation of gynecologic malignancies, in particular endometrial cancer. Minimally invasive surgery is associated with less postoperative pain, quicker postoperative recovery, and lower blood loss compared with traditional laparotomy.92–92 In addition, the use of laparoscopy for staging and treatment of endometrial carcinoma does not adversely affect recurrence or survival outcomes.93,94 A Gynecologic Oncology Group study randomly assigned more than 2600 patients with endometrial cancer to surgical staging via laparotomy or laparoscopy.93,94 The study concluded that laparoscopic surgical staging is a reasonable option with no differences in recurrence or survival. Patients who underwent laparoscopic surgical staging had fewer moderate to severe postoperative complications, equivalent detection rates of advanced-stage disease, and an improved quality of life through 6 weeks after surgery.
Advances in surgical technology have introduced robotic-assisted surgical staging for endometrial cancer. The da Vinci surgical system was approved by the FDA for gynecologic use in 2005. The advantages of robotic-assisted surgery include 3D operative visualization, improved ergonomics during surgery, and seven degrees of freedom of articulation offering improved dexterity compared with laparoscopy. As in traditional laparoscopy, robotic-assisted surgical procedures are associated with lower blood loss, shorter hospital stays, and fewer perioperative complications compared to laparotomy.90,91 Long-term oncologic outcomes appear to be equivalent to traditional laparoscopy, but further follow-up is necessary.
For patients with metastatic disease at the time of staging, more aggressive surgical options exist. Radical hysterectomy that involves removal of the uterus, cervix, parametria, and upper vagina may be an option for selected patients.95 Patients infrequently present with stage IV disease, but there may be a role for aggressive cytoreduction to improve survival.96,97 Randomized trials are lacking, but a meta-analysis of 14 retrospective case series demonstrated a survival benefit associated with complete cytoreduction in women with primary or recurrent endometrial cancer.98
Intermediate-risk patients have tumors confined to the uterus (stage IA or IB) or occult cervical involvement (stage II). Other prognostic factors used to further stratify intermediate-risk tumors include outer-third myometrial invasion, grade 2 or 3 differentiation, or presence of lymphovascular invasion. High-intermediate risk is based on a combination of age and number of prognostic factors present. Patients of any age with all three risk factors, patients 50 to 69 years of age with 2 risk factors, and patients over age 70 years with one risk factor are considered high-intermediate risk (Table 88-13). All others are considered low-intermediate risk. High-intermediate risk patients are at increased risk for locoregional relapse and may benefit from postoperative radiation therapy.
Table 88-13
Gynecologic Oncology Group Criteria for High Intermediate-Risk
| Risk Factors | Group |
| Grade 2 or 3 tumor | <50 years with all 3 risk factors |
| Lymphovascular invasion (LVSI) present | 50-69 years with 2 risk factors |
| Deep myometrial invasion (outer third) | ≥70 years with 1 risk factor |
Race, age, and positive peritoneal cytology also affect the risk of recurrence after surgical staging. African American women consistently have a poorer outcome than Caucasians, a disparity that is not completely explained by imbalances in psychosocial, clinicopathological, and treatment factors.99–102 A higher incidence of high-risk (nonendometrioid or grade 3) tumors has been attributed to some of the racial disparity. Increasing age has been associated with a worse prognosis in several, but not all, trials.105–105 Women over the age of 65 have, on average, higher stage and grade tumors compared with younger patients. In addition, deeper myometrial invasion is more commonly present in older patients. Age is used to stratify patients after surgery to determine the role of adjuvant therapy, and less aggressive therapy could account for poorer outcomes among the elderly; however, older women have higher rates of recurrence and inferior survival compared with younger patients even when all were treated uniformly.106,107
Most patients with positive peritoneal cytology have advanced or extrauterine disease at the time of surgery. A systematic review of 50 studies demonstrated the prognostic significance of isolated positive peritoneal washings in the absence of extrauterine disease. Women with positive peritoneal cytology in the absence of other risk factors (lymphovascular invasion, >50% myometrial invasion, cervical involvement, grade 3 tumors) had a significantly lower rate of recurrence compared with women with positive peritoneal washings and risk factors (4.1% vs. 32%).108 However, a review of SEER data reported positive peritoneal cytology to be an independent predictor of mortality, regardless of histology or tumor grade.109
The Role of Lymphadenectomy
The most commonly involved lymph nodes are the external iliac and obturator lymph nodes.110 Common iliac lymph node involvement is more frequent in cancers involving the uterine cervix. Spread to the paraaortic lymph nodes usually occurs in the presence of involved pelvic lymph nodes, but isolated paraaortic lymph node metastasis can occur. The presence of lymphatic spread significantly changes treatment planning; however, routine lymphadenectomy has not been shown to improve survival in several large trials, including two randomized controlled trials.113–113
Risk factors for metastases to lymph nodes include myometrial invasion, cervical involvement, high-grade histology, and lymphovascular involvement. Lymphadenectomy may be omitted in stage I tumors without any risk factors given the low risk of nodal involvement.114,115 A frozen section of the uterine specimen at the time of surgery can help identify patients with risk factors who need lymphadenectomy performed; however, to date no surgical algorithm has been proved to definitely predict which patients are at low risk for nodal spread of tumor.116,117 Significant differences in practice can occur within institutions and between practitioners. The necessity of lymphadenectomy and the extent of dissection has not been standardized. In a review of 328 patients by Mariani and colleagues, no patients with grade 1 or 2 disease, tumors ≤2 cm and myometrial invasion less than 50% had positive pelvic lymph nodes or died of endometrial cancer.118 They concluded that patients with grade 1 or 2 disease, tumors ≤2 cm, myometrial invasion less than 50%, and no evidence of macroscopic metastasis should be treated with hysterectomy alone.
A large, prospective European study examined the role of lymphadenectomy in the ASTEC trial (Adjuvant External Beam Radiotherapy in the Treatment of Endometrial Cancer).111 The ASTEC trial was a multisite prospective study involving more than 1400 patients with clinical stage I endometrial carcinoma. Patients were randomly assigned to standard surgery with hysterectomy, bilateral salpingo-oophorectomy, peritoneal washings, and palpation of paraaortic lymph nodes or standard surgery with bilateral pelvic lymphadenectomy. Paraaortic lymphadenectomy was performed at the discretion of the individual surgeons. A second group of patients were randomly assigned of intermediate-risk and high-risk early-stage patients to pelvic radiotherapy versus no treatment. Patients were assigned independent of lymphadenectomy. There was no difference in overall survival or disease-related deaths. After adjustment for differences in baseline characteristics (aggressive histology and depth of myometrial invasion), no difference in recurrence-free survival was noted. A major limitation of the study was the large number of patients with low-risk disease who were included in the lymphadenectomy group and would not normally have undergone lymphadenectomy or benefited from such a procedure. Other limitations included the low number of resected lymph nodes per patient (median number 12) and nonstandardization of paraaortic lymphadenectomy leading to an unknown number of isolated paraaortic metastases.
No survival benefit or improvement in disease-free survival was found in a second prospective, randomized trial of more than 500 patients (CONSORT Trial).113 Compared with ASTEC, a higher number of lymph nodes were removed per patient, but only 26% of patients in the lymphadenectomy group underwent paraaortic lymphadenectomy. Similar to the ASTEC trial, the CONSORT trial is limited by nonstandardization of the indications for and the extent of lymphadenectomy.
With the previously mentioned studies and ongoing debate regarding the role of lymphadenectomy, sentinel lymph node (SLN) mapping has been proposed as a possible alternative. SLN mapping has been well established in other solid tumors, such as breast cancer and melanoma. The sentinel lymph node hypothesis states that tumor cells from a primary tumor metastasize first to one or a few lymph nodes before spreading to other lymph nodes. Blue dye (such as isosulfan blue) and/or radiolabeled colloid can be used via injection in the vicinity of the tumor to map lymphatic drainage and identify sentinel lymph nodes. Additional studies using indocyanine green fluorescent dye and a special laparoscopic camera with fluorescent capabilities during robotic surgery have been performed with promising results.119 A recent meta-analysis of 26 studies evaluated the role of SLN mapping in endometrial cancer and found a detection rate of 78% and sensitivity of 93%.120 The reported detection rate is lower than that for breast cancer or melanoma, but may not represent the full potential of SLN mapping in endometrial cancer. The authors concluded that further studies are needed to evaluate the true performance of SLN biopsy in endometrial cancer.
Adjuvant Therapy
Adjuvant Treatment of Low- and Intermediate-Risk Endometrial Cancer
The majority of women with endometrial cancer have stage I disease. As such, surgical resection may be curative in a majority of these patients. Women with grade 1 or 2 disease confined to the endometrium are considered low-risk. Vaginal vault recurrence is the primary risk following surgery in low-risk patients; however, adjuvant radiation therapy is not recommended because of the low recurrence rate of less than 5%.114,121 Radiation therapy can reduce the risk of local recurrence, but does not improve overall survival.122 In a randomized controlled study of 645 women with grade 1 or 2 surgical stage IA tumors, patients were assigned to vaginal brachytherapy or observation following surgery. There were no differences in survival or recurrence rates; however, an increase in dysuria, incontinence, and urinary frequency was seen in the vaginal brachytherapy group. Neither chemotherapy nor progestin therapy is used in the adjuvant setting for patients with low-risk disease.
Endometrial tumors that invade the myometrium (stage IA or IB) or have occult cervical stromal invasion are classified as intermediate-risk. A subset of these women are considered high intermediate-risk depending upon age and presence of risk factors (Tables 88-13 and 88-14). The treatment of high intermediate-risk patients will be discussed later. Patients who do not meet criteria for high intermediate risk are classified as low intermediate risk. Patients with low intermediate-risk disease have low rates of recurrence and a good prognosis. A Gynecologic Oncology Group (GOG) study evaluated the role of radiation therapy in 448 patients with intermediate-risk endometrial cancer.123 Patients with stage IB-II disease were randomly assigned to either observation or pelvic radiation therapy after complete surgical staging with both pelvic and paraaortic lymphadenectomy. The addition of radiation therapy resulted in lower local recurrences but no difference in distant recurrences or overall survival (Table 88-15). The term high intermediate-risk was also provided by the results of GOG-99. High intermediate-risk patients are defined as (1) 70 years of age or greater with either lymphovascular space involvement (LVSI), outer-third myometrial invasion, or grade 2 or 3 tumors; (2) 50 to 69 years of age and two of the three mentioned risk factors; (3) less than 50 years of age and all three risk factors (Table 88-13). A subgroup analysis of these high intermediate-risk patients showed a lower risk of local recurrence, but no improvement in survival with pelvic radiotherapy (2% vs. 9%, HR = 0.42). The authors concluded that as in the low-risk patients, the risks associated with radiation therapy outweigh the benefits and it is generally not recommended for patients with low intermediate-risk disease.
Table 88-14
PORTEC Criteria for High Intermediate-Risk
| Risk Factor (2 of 3 required to meet criteria) |

Table 88-15
Results of Adjuvant Radiation Therapy for Early-Stage Endometrial Cancer
| Trial | No. of Patients | Stages | Radiation Technique | Local Control Rate | Overall Survival Rate |
| Keys et al. (GOG-99) | 190 | IB, IC, II | EBRT | 97 | 92 |
| Creutzberg et al. (PORTEC-1) | 354 | I | EBRT | 96 | 81 |
| Nout et al. (PORTEC-2)* | 427 | I or IIA | EBRT or VBT | EBRT 78.1 (DFS) VBT 82.7 (DFS) |
EBRT 79.6 VBT 84.8 |
| Aalders et al. | 263 | I | VBT ± EBRT | 98 | 89 |
| Irwin et al. | 322 | I | EBRT, VBT, or EBRT +VBT | 77 (DFS) | 81 |
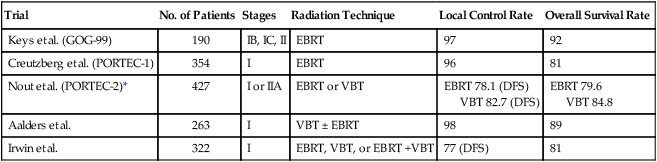
*No significant differences in local control or overall survival rates between EBRT and VBT
Similar results were found in the Post Operative Radiation Therapy in Endometrial Carcinoma (PORTEC-1) trial.124 More than 700 patients with stage I endometrial carcinoma underwent randomization after total abdominal hysterectomy, bilateral salpingo-oophorectomy without lymphadenectomy. The following tumor characteristics were required for enrollment: grade 1 tumors with at least 50% invasion, grade 2 with any degree of invasion, and grade 3 with less than 50% invasion. Patients were randomly assigned to pelvic radiotherapy or observation. Locoregional recurrence was 14% in the observation group and 4% in the radiotherapy group (P < 0.001). However, there was no difference in the overall survival (85% in observation vs. 81% in radiotherapy, P = 0.31). A subsequent analysis after central pathology review that excluded more than 100 patients with grade 1 tumors and superficial myometrial invasion found no difference in overall survival at 10 years (70% in observation vs. 65% in radiotherapy, P = 0.23). As in GOG-99, the authors concluded that adjuvant treatment of intermediate-risk patients with pelvic radiation is not justified in the majority of cases.
With the results of PORTEC-1 and GOG-99 in mind, Nout and colleagues (PORTEC-2) performed a noninferiority trial of vaginal brachytherapy versus external pelvic radiotherapy. PORTEC-2 included more than 400 patients with stage I or IIA endometrial carcinoma. Patients underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy with lymphadenectomy for suspicious nodes followed by random assignment to vaginal brachytherapy or external pelvic radiotherapy. The rates of recurrence at 5 years were not significantly different between the two groups (5.1 for vaginal brachytherapy vs. 2.1% for external pelvic radiotherapy, P = 0.17). There were no differences in disease-free survival or overall survival (Table 88-14). However, external pelvic radiotherapy was associated with significantly more cases of acute grade 1 or 2 gastrointestinal toxicities (54% vs. 13%). A subsequent quality of life analysis showed better social functioning and lower symptom scores for diarrhea and fecal incontinence, with less limitations in daily activities for the vaginal brachytherapy group. Based on PORTEC-2 and the follow-up quality of life study, vaginal brachytherapy is the preferred choice for treatment of patients with intermediate-risk disease that need adjuvant therapy to decrease local recurrences; however, the role of radiation therapy to improve survival remains less clear.
Adjuvant Treatment of High-Risk Endometrial Cancer
Any stage uterine serous carcinoma, clear cell adenocarcinoma, and uterine carcinosarcoma, and FIGO stage III carcinomas of any histology are considered high-risk endometrial cancers. Patients with stage IV disease will be discussed separately in a section on treating metastatic and recurrent disease. Five-year survival rates for women with uterine serous or clear cell carcinoma from the SEER study are significantly less than women with endometrioid adenocarcinoma (45%, 65%, and 91%, respectively). Compared with women with surgical stage I or II disease, women with stage III disease have a much lower 5-year survival rate (97% and 80% vs. 60%, respectively).102
Uterine Serous Carcinoma
Uterine serous carcinoma, a type II endometrial cancer, is staged according to FIGO guidelines with hysterectomy and bilateral salpingo-oophorectomy. Pelvic and paraaortic lymphadenectomy is also performed even in the absence of myometrial invasion due to higher rates of metastasis to lymph nodes when compared with similar-stage endometrioid adenocarcinoma. Several studies, including one by Gehrig and colleagues, have reported that extrauterine metastases are encountered in more than one-third of women with no myometrial invasion (endometrium only).125,126 Recurrence rates for uterine serous carcinoma without myometrial invasion treated with observation alone varies from 0% to 30%.127,128 Patients with any degree of myometrial invasion treated with surgery alone have a recurrence rate ranging from 29% to 80%. For patients with uterine serous carcinoma confined to a polyp or those without residual carcinoma at the time of hysterectomy, few recurrences have been demonstrated.127 Recurrence patterns for uterine serous carcinoma are similar to patterns seen in ovarian cancer and guides treatment decisions. With these recurrence patterns and rates in mind, there is much debate over the type and extent of adjuvant treatment (Table 88-16).
Table 88-16
NCCN Guidelines for Treatment of Uterine Serous, Clear Cell Endometrial Carcinoma and Carcinosarcoma
| Stage | Adjuvant Treatment |
| IA without myometrial invasion | Observe, or chemotherapy, or VBT, or combination therapy |
| IA with myometrial invasion, IB, II | Chemotherapy ± tumor-directed RT, or Whole abdominopelvic RT ± VBT |
| III, IV (adequately debulked) | Chemotherapy ± tumor-directed RT, or Whole abdominopelvic RT ± VBT |
| III, IV (inadequately debulked) | Chemotherapy |
The Uterine Papillary Serous Carcinoma Consortium study, which included 142 patients with stage I serous carcinoma, evaluated the role of adjuvant radiation therapy and chemotherapy.129 Compared with radiation therapy or surgery alone, chemotherapy (carboplatin and paclitaxel) was associated with a significant reduction in recurrence rates (30% and 25% vs. 11%, respectively). A statistically significant improvement in 5-year progression-free survival was also noted, but no improvement in 5-year cancer-specific survival.
A retrospective analysis by Kelly and colleagues analyzed 74 patients with stage I uterine serous carcinoma treated with chemotherapy and/or radiation therapy.130 Platinum-based chemotherapy was associated with improved disease-free and overall survival (P < 0.01, and P < 0.05, respectively). No recurrences occurred in patients with no residual tumor in the hysterectomy specimen (tumor diagnosed in preoperative biopsy, but no cancer discovered at time of surgery), regardless of treatment regimen. In addition, no vaginal vault recurrences were documented in patients who received adjuvant vaginal brachytherapy; however, 6 of the 31 (19%) patients not treated with radiation therapy were found to have vaginal recurrences. The authors concluded that patients with stage IA disease and no residual tumor in the hysterectomy specimen may be observed, but combination vaginal brachytherapy and platinum-based chemotherapy should be offered to all other stage I patients.
Based on the results from PORTEC-2, vaginal brachytherapy rather than whole pelvic radiation therapy is preferred if adjuvant radiation is recommended for early-stage uterine serous carcinoma.131 In the PORTEC-2 trial, 427 women with high intermediate-risk endometrial cancer were randomly assigned to whole pelvic radiotherapy (external beam radiotherapy) or vaginal brachytherapy. There were no differences in locoregional control rates, distant metastasis, or overall survival; however, external beam radiotherapy was associated with higher rates of treatment-related diarrhea and bowel symptoms.
There is growing evidence for the use of adjuvant platinum-based chemotherapy for early-stage uterine serous carcinoma. Carboplatin and paclitaxel chemotherapy is an established, active treatment regimen for recurrent and metastatic endometrial cancer. Many of the trials evaluating carboplatin and paclitaxel for recurrent or advanced endometrial cancer have disproportionate numbers of patients with uterine serous carcinoma, leading to extrapolation of results and regimens to treat early-stage uterine serous cancer. Several retrospective studies have evaluated adjuvant chemotherapy for early-stage disease. The UPSC consortium reported a recurrence rate of 9.2% after carboplatin and paclitaxel chemotherapy with or without radiation therapy; however, recurrence rates of 24% and 30% were seen after radiation therapy and observation, respectively. Another retrospective multiinstitutional study by Dietrich and colleagues found that adjuvant therapy with carboplatin and paclitaxel was associated with improved overall survival and decreased recurrences.132
Patients with stage II uterine serous carcinoma have an even higher risk of recurrence. In a retrospective study by Fader and colleagues, primary recurrence was documented in 36% of patients with stage IIA or IIB disease.133 The majority of recurrences were extrapelvic (70%) and not salvageable (84%). As in other retrospective studies of early uterine serous cancer, chemotherapy (carboplatin/paclitaxel) with or without radiation was associated with significantly improved survival rates compared with observation or radiation therapy alone. Although the numbers were small, another point of interest was that no recurrences were documented in patients receiving both radiation therapy and chemotherapy.
Clear Cell Carcinoma
The optimal management of women with clear cell carcinoma is unclear. Similar to serous uterine cancers, clear cell carcinomas are associated with a worse prognosis than endometrioid carcinomas.37 A retrospective study of 80 patients with clear cell cancer of the uterine corpus showed that vaginal brachytherapy was associated with a significant improvement in overall survival (median, 140 vs. 50 months) compared with no adjuvant treatment.134 In this small, retrospective study, adjuvant chemotherapy was not associated with an improvement in progression-free or overall survival. However, given the high rates of recurrence and paucity of high-quality data, it is reasonable to consider chemotherapy combined with radiation therapy for these patients.
Uterine Carcinosarcomas
Uterine carcinosarcomas are rare, aggressive tumors that present with extrauterine disease in 60% of cases. Surgical staging follows FIGO recommendations for endometrial cancer that includes hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy. As in endometrial adenocarcinoma tumors, cytoreductive surgery is associated with a significant survival advantage and should be considered in cases of extrauterine spread.135
Given the poor prognosis associated with carcinosarcoma, adjuvant treatment is often recommended, even for early-stage disease. However, few high-quality studies exist to validate a survival benefit from adjuvant treatment for stage IA disease. In a multiinstitutional retrospective study of 111 women with early-stage carcinosarcoma, administration of chemotherapy was associated with a significant improvement in progression-free survival, but not overall survival.136 A study by the GOG evaluated the role of adjuvant doxorubicin and found no improvement in progression-free or overall survival.137 Postoperative radiation therapy has not demonstrated an improvement in progression-free or overall survival, but may decrease locoregional recurrences.138 Nonetheless, given the aggressive nature of carcinosarcoma, many experts recommend adjuvant chemotherapy for stage IA disease.
Adjuvant chemotherapy was evaluated in 206 women with stage I-IV uterine carcinosarcoma.139 Patients were randomly assigned to whole abdominal radiation therapy or chemotherapy with cisplatin, ifosfamide, and mesna. Chemotherapy was associated with a lower risk of recurrence (HR = 0.79, 95% CI = 0.53 to 0.79), and a lower rate of abdominal relapse (29% vs. 19%). Chemotherapy was, however, associated with higher rates of vaginal recurrence and neuropathy compared with the radiation group. Choice of chemotherapy is based on a GOG study that enrolled 55 patients with stage III or IV, persistent, or recurrent carcinosarcoma.140 Treatment with carboplatin plus paclitaxel was administered every 3 weeks until disease progression or side effects occurred. Median progression-free and overall survivals were 8 and 15 months, respectively. Compared with ifosfamide-based regimens, carboplatin and paclitaxel is associated with less toxicity. Ifosfamide plus paclitaxel was evaluated in 179 women with previously untreated stage III-IV, recurrent, or advanced carcinosarcoma. In contrast to single-agent ifosfamide, combination ifosfamide and paclitaxel resulted in a higher response rate (45% vs. 29%) and an improved overall survival (14 vs. 6 months). However, sensory neuropathy was significantly worse in the combination treatment group. Ifosfamide plus cisplatin was evaluated in another GOG trial that found a significant improvement in progression-free survival (6 vs. 4 months), but no improvement in overall survival (9 vs. 8 months).141 With the previously mentioned study results and toxicity profiles in mind, carboplatin and paclitaxel is often suggested as first-line therapy for carcinosarcoma. As in uterine serous carcinoma, some experts recommend combined vaginal brachytherapy and chemotherapy to reduce both local and distant recurrences, but high-quality data from randomized trials is lacking. Enrollment in GOG-261, a comparison of carboplatin plus paclitaxel to ifosfamide plus paclitaxel, should be encouraged when appropriate.
Advanced-Stage Endometrial Cancer
The initial treatment of advanced stage endometrial cancer is surgery with cytoreductive surgery, when feasible, followed by platinum-based combination chemotherapy. Active chemotherapeutic agents have been evaluated in several GOG studies. The most commonly used regimens are carboplatin and paclitaxel or the triple-drug combination cisplatin, doxorubicin, and paclitaxel (TAP). Doxorubicin and cisplatin were some of the first drugs evaluated by the GOG.142 A trial evaluating combination cisplatin and doxorubicin compared with doxorubicin alone demonstrated an overall response rate in favor of combined therapy (42% vs. 25%, P = 0.004). Improved progression-free survival was also noted in the combination group (5.7 vs. 3.8 months, P = 0.014); however, there was no significant difference in overall survival. A subsequent study by the GOG assessed the role of triple-drug therapy, namely TAP.143 GOG-177 enrolled 273 previously untreated women with stage III or IV disease to receive cisplatin and doxorubicin (AP) or TAP. A significant improvement in overall response rate, progression-free survival, and overall survival was seen in the TAP arm. However, TAP was also associated with increased grade 3 neurotoxicity (12% vs. 1%).
The ability to exclude doxorubicin, and hopefully improve toxicity profiles, was demonstrated in a phase II trial of cisplatin with paclitaxel.144 A subsequent retrospective review of carboplatin with paclitaxel provided further data to support a randomized trial of TAP versus carboplatin and paclitaxel.145 The GOG recently completed enrollment in a phase III trial to compare TAP with carboplatin and paclitaxel. The results have yet to be published, but preliminary data show similar response rates, progression-free survival, and overall survival in the two treatment arms.
The role of radiation therapy for advanced endometrial cancer changed after the results of a randomized trial comparing whole-abdomen radiotherapy (WART) with combination cisplatin and doxorubicin. The propensity of endometrial cancer to recur within the abdomen led investigators to evaluate the role of WART in women with maximally debulked stage III and IV disease.146,147 The trial demonstrated improved progression-free and overall survival in the AP arm compared with WART.148
A phase II study by Greven and colleagues evaluated the recurrence rates and survival in women with high-risk endometrial cancer treated with both chemotherapy and radiation therapy.149 Patients with grade 2 or 3 disease, greater than 50% myometrial invasion, cervical stromal invasion, or extrauterine disease confined to the pelvis were eligible for enrollment. After completion of postoperative whole-pelvic radiotherapy and vaginal brachytherapy, four cycles of cisplatin and paclitaxel were administered every 4 weeks. At 4 years, there were no recurrences documented in patients with stage IC, IIA, or IIB disease. Progression-free and survival rates at 4 years were 72% and 77%, respectively.
Similar to the previously mentioned study by Secord et al., Geller and colleagues performed a phase II study evaluating the role of sequencing treatment in a “sandwich” fashion.150 Forty-two patients received three cycles of carboplatin and docetaxel (every 21 days) followed by pelvic radiotherapy with or without vaginal brachytherapy and an additional three cycles of carboplatin and docetaxel. Estimated 5-year progression-free and overall survival rates were 64% and 71%, respectively. Grade 3 or 4 hematologic toxicities were common, occurring 30 times during the study.
As mentioned in an earlier section, positive peritoneal cytology is no longer part of FIGO staging; however, positive peritoneal cytology may be an independent predictor of mortality. A review of more than 14,000 patients with stage I–II disease from the SEER database reported higher rates of positive peritoneal cytology with grade 3 tumors or high-risk histology. The study also reported a decrease in overall survival associated with positive peritoneal cytology, regardless of tumor grade or histology.109 On the other hand, a systematic review by Wethington and colleagues found that positive peritoneal cytology was significant only in the presence of other risk factors (stage II disease, deep myometrial invasion, LVSI, grade 3 disease).108 With these studies in mind, peritoneal cytology can assist treatment planning, but decisions should be based primarily on pathological findings at the time of comprehensive surgical staging.
Treatment of Uterine Sarcomas
Primary surgery with hysterectomy, with or without oophorectomy, and consideration of cytoreduction is recommended for uterine sarcoma. Surgery on advanced-stage uterine sarcoma can be complicated by a high risk of morbidity. Preoperative imaging with computed tomography (CT) or magnetic resonance imaging (MRI) can help with surgical plans and decisions regarding possible tumor debulking. However, preoperative biopsy reveals malignancy in less than 50% of patients with leiomyosarcoma, meaning that the diagnosis is usually made after hysterectomy. Metastases to lymph nodes occur in 3% to 11% of cases, usually in the context of other metastatic spread.151,152 As such, lymphadenectomy is not part of FIGO comprehensive surgical staging, but may provide prognostic information.
Limited studies exist to assess the role of chemotherapy in treating uterine sarcomas. Radiation therapy decreases local recurrences, but most studies did not show a survival benefit.138,153–156 Leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated endometrial sarcoma differ clinically. As such, treatment regimens should be tailored to each type of uterine sarcoma.
Leiomyosarcomas are aggressive tumors with high rates of distant failure. Systemic therapy with gemcitabine and docetaxel has shown improvements in progression-free survival.157 Single-agent therapy with doxorubicin, ifosfamide, paclitaxel, and gemcitabine have also shown complete or partial response rates.158–162 Adjuvant therapy for early-stage disease has not been fully evaluated, but treatment with gemcitabine and docetaxel may be of benefit. Radiation therapy may reduce local recurrence rates, but an improvement in survival has not been demonstrated.151,154,155,163–165
Treatment of endometrial stromal sarcoma starts with hysterectomy with or without bilateral salpingo-oophorectomy. As in leiomyosarcoma, lymphadenectomy does not appear to improve survival.166 Endometrial stromal sarcomas often express estrogen and progesterone receptors and may be sensitive to hormonal agents; however, bilateral oophorectomy has not been shown to be of benefit, and ovarian preservation may be considered in young, premenopausal women.166–169 When recurrence does occur, multiple cytoreductive surgeries may be recommended because of the indolent nature of the disease. Adjuvant treatment of endometrial stromal sarcoma is based on expression of estrogen and progesterone receptors. Medroxyprogesterone acetate and megestrol acetate have shown benefit in the adjuvant setting.167,170–172 Amant and colleagues reported a response rate of 76% associated with treatment of progestins. Gonadotropin-releasing hormone (GnRH) agonists may also be used for adjuvant treatment, especially in premenopausal women with retained ovaries.173,174 Letrozole, an aromatase inhibitor, demonstrated a response rate of 88% in one study.175 Determining which patients receive hormonal treatment and for how long remains controversial. Some recommend treating all patients with positive hormone receptors, whereas others recommend treating only locally advanced disease.176 Given the indolent nature of ESS, radiation therapy is generally not recommended but may be considered in select cases. If chemotherapy is necessary after exhausting hormonal options, ifosfamide and doxorubicin have shown antitumor activity.177
Undifferentiated endometrial sarcomas are aggressive tumors with a poor prognosis, but data regarding adjuvant treatment is sparse. A case series by Tanner and colleagues reported on 21 patients treated after primary surgery.178 Median survival was 11.8 months, with an overall response rate of 62% for those treated with gemcitabine plus docetaxel or doxorubicin-based regimens.
Radiation for Inoperable Patients
Comprehensive surgical staging is the recommended treatment for the majority of endometrial cancer patients; however, some patients have significant medical comorbidities that add an unacceptable level of risk to surgery. In these patients, primary treatment with radiation therapy may be an option. Primary radiation therapy for medically inoperable patients has been shown to be effective in several studies, but increased failure rates are seen in tumors of higher grade and stage (Table 88-17).181–181
Table 88-17
Results of Radiation Only for Inoperable Patients
| Study | No of Patients | Stages | Local Control Rates (%) | Overall Survival (%) |
| Patanaphan et al. | 54 | I | NR | 75 (OS) |
| Langren et al. | 124 | I, II | I: 78 II: 82 |
I: 77 (OS) II: 65 (OS) |
| Kupelian et al. | 137 | I, II | 86 | I: 87 (DSS) II: 88 (DSS) |
| Chao | 101 | I | IA: 100 IB: 88 |
IA: 80 (DFS) IB: 84 (DFS) |
| Fishman et al. | 54 | I, II | NR | I: 80 (CSS) II: 85 (CSS) |
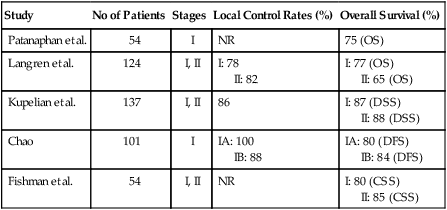
Patients with clinical stage I disease with a low risk for extrauterine spread may be treated with high-dose-rate (HDR) intracavitary brachytherapy. Uterine control rates of 88% can be achieved using HDR brachytherapy.182 Combination HDR brachytherapy and external beam radiation therapy can be used for those patients at risk for extrauterine disease. Long-term results after HDR brachytherapy for medically inoperable patients with endometrial cancer approach results achieved with surgery. Higher tumor grade is associated with increasing rates of failure. Disease-specific survival rates for clinical stage I–II disease may be as high as 91% for grade 1 tumors, but decreases to 67% for grade 2 and 3 tumors.179
Treatment of Advanced Endometrial Cancer
Endometrial cancer involves the adnexa, lymph nodes, and extrapelvic organs in 10% to 15% of cases. These patients are categorized as having stage III or IV disease. Treatment of advanced endometrial cancer typically involves surgical resection and adjuvant chemotherapy with or without radiation therapy (Tables 88-18 and 88-19). The goal of surgical resection is optimal cytoreduction when appropriate.
Table 88-18
National Comprehensive Cancer Network Treatment Guidelines for Endometrial Carcinoma After Comprehensive Surgical Staging
| STAGE IA | |
| Grade 1 without ARF | Observe |
| Grade 1 with ARF | Observe or VBT |
| Grade 2 or 3 without ARF | Observe or VBT |
| Grade 2 or 3 with ARF | Observe or VBT and/or pelvic RT |
| STAGE IB | |
| Grade 1 without ARF | Observe |
| Grade 1 with ARF | Observe or VBT |
| Grade 2 without ARF | Observe or VBT |
| Grade 2 with ARF | Observe or VBT and/or pelvic RT |
| Grade 3 without ARF | Observe or VBT and/or pelvic RT |
| Grade 3 with ARF | Observe or VBT and/or pelvic RT ±chemotherapy |
| STAGE II | |
| Grade 1 | VBT and/or pelvic RT |
| Grade 2 | Pelvic RT and VBT |
| Grade 3 | Pelvic RT and VBT ±chemotherapy |
| STAGE IIIA | Chemotherapy ±pelvic RT or tumor-directed RT ±chemotherapy or pelvic RT ±VBT |
| STAGE IIIB-IIIC | Chemotherapy and/or tumor directed RT |
| STAGE IVA-IVB | Chemotherapy ±RT |
Table 88-19
National Comprehensive Cancer Network Treatment Guidelines for Uterine Sarcoma After Comprehensive Surgical Staging (Leiomyosarcoma and Undifferentiated Endometrial Sarcoma)
| Stage I | Observe, or chemotherapy, or pelvic RT ±VBT |
| Stage II, III | Tumor-directed RT or chemotherapy |
| Stage IVA | Chemotherapy ± tumor directed RT |
| Stage IVB | Chemotherapy ± palliative RT |
The most effective chemotherapy regimens have been established by several GOG studies. The combination of doxorubicin and cisplatin compared with doxorubicin alone was evaluated in 281 patients. Response rates in the combination group were significantly higher than single-agent doxorubicin (42% vs. 25%, P = 0.004).142 Cisplatin plus doxorubicin also resulted in improved progression-free survival (5.7 vs. 3.8 months, P = 0.014), but no difference in overall survival. However, greater toxicities, including grade 3 and 4 hematologic toxicities, and increased nausea and vomiting were found in the combination chemotherapy group. Adjuvant therapy with cisplatin and doxorubicin became the standard regimen for advanced or recurrent endometrial cancer.
The addition of paclitaxel to cisplatin and doxorubicin (TAP) for the treatment of recurrent stage III and IV endometrial cancer resulted in improved outcomes. GOG 177, which evaluated cisplatin and doxorubicin plus paclitaxel versus cisplatin and doxorubicin in recurrent stage III and IV endometrial cancer, showed improved response rates (57% vs. 34%, P < 0.01), progression-free survival (8.3 vs. 5.3 months, P < 0.01) and overall survival (15.3 vs. 12.3 months) in the three-drug cohort.143 However, increased grade 3 neurotoxicity was noted in the TAP cohort (12% vs. 1%).
Given the toxicity of TAP chemotherapy, regimens without doxorubicin have been investigated in several studies. Cisplatin plus doxorubicin was evaluated in a phase II trial of advanced or recurrent endometrial carcinoma and showed a response rate of 67%.144 A phase II study of carboplatin for the treatment of recurrent or advanced endometrial cancer demonstrated an overall response rate of 24%.183
Preliminary results from GOG 209, a comparison of TAP and carboplatin/paclitaxel in 1300 patients with stage III, IV, and recurrent endometrial cancer, showed no significant differences in response rates, progression-free survival or overall survival.184 Statistically significant reductions in neuropathy, thrombocytopenia, emesis, and diarrhea were seen in the carboplatin/paclitaxel group. Given these early results combined with retrospective studies, carboplatin and paclitaxel is often prescribed for high-risk and advanced or recurrent endometrial cancer.
The role of radiation therapy for advanced or recurrent disease has been investigated. The sensitivity of endometrial cancer to whole-abdomen radiation therapy (WART) was evaluated in patients with advanced-stage disease in a phase III trial conducted by the GOG. After optimal cytoreductive surgery (residual tumor less than 2 cm), patients were enrolled and randomized to WART or cisplatin with doxorubicin (AP).148 Twenty-one percent of patients had uterine serous carcinoma whereas only 50% had endometrioid tumors. Improved progression-free and overall survival was seen in the AP arm compared with WART. However, higher toxicity rates were seen in the chemotherapy cohort compared to the WART group, including grade 3 and 4 cardiac toxicities.
Treatment regimens for stage III and IV are similar; however, 5-year survival rates for stage IV disease are approximately 20% compared to 40% to 50% for women with stage III disease. When disease is intraabdominal, surgical cytoreduction should be considered. A meta-analysis of 14 studies demonstrated a significant improvement in overall survival associated with surgical cytoreduction.98 Following surgery, carboplatin and paclitaxel is considered first-line treatment for the reasons mentioned earlier. Hormonal agents, including progestins and/or tamoxifen, may be considered in lieu of cytotoxic therapy and will be discussed in the subsequent section.
Treatment of Recurrent Disease and Palliation
Treatment of recurrent endometrial cancer depends on disease location and prior therapy. For women with isolated vaginal recurrences and no prior radiation therapy, treatment with radiation therapy alone is associated with excellent results.187–187 Five-year local control rates range from 42% to 65%, whereas overall survival rates range from 31% to 53%.190–190 The Postoperative Radiation Therapy after Endometrial Cancer (PORTEC) trial reported on 46 recurrences from the group initially randomly assigned to no therapy following surgery. In this study, the 5-year overall survival after vaginal vault recurrence was 65%; however, this is much higher than other studies and may be attributed to early detection. As expected, the extent and size of the recurrence is related to outcome. It should also be mentioned that the high survival rate after relapse only pertains to isolated vaginal vault recurrence, not pelvic or other sites of recurrence. A study by Jhingran and colleagues reported an overall survival of 43% and a 5-year local control rate of 75% for isolated vaginal recurrences.191 In addition, salvage radiation therapy is associated with a risk of significant complications as it involves whole-pelvic radiation therapy with or without vaginal brachytherapy. The rate of grade 4 complications approaches 10% and needs to be considered when counseling patients for adjuvant radiation.
Patients with vaginal recurrences and prior radiation therapy have a worse prognosis than radiation-naïve patients.185 Adjuvant radiation therapy reduces isolated vaginal recurrence rates but has no impact on overall survival.124 Treatment options for recurrent endometrial cancer after radiation therapy generally includes chemotherapy, but may include additional radiation therapy or surgical resection. Additional radiation therapy requires tailored treatment approaches and may not be available in many institutions. Surgical resection may be considered for isolated recurrences in previous irradiated fields. Five-year survival after pelvic exenteration for recurrent endometrial cancer ranges from 20% to 40%.192 Postoperative complications after pelvic exenteration are as high as 50%, and therefore such procedures should be reserved for highly motivated patients with surgically resectable disease.
In women with pelvic or intraabdominal disease, surgical cytoreduction may be associated with an improved overall survival.98 A significant increase in survival of 9 to 35 months may be achieved with cytoreductive surgery, but high-quality data are lacking and patient selection is paramount.
Platinum-based combination chemotherapy is the recommended first-line treatment for metastatic endometrial cancer. First-line treatment with multiagent chemotherapy is associated with an improvement in progression-free survival and overall survival.193 Choice of treatment regimen after adjuvant chemotherapy depends on the treatment-free interval. Repeat platinum-based chemotherapy is often used if 6 months or more have passed since the completion of chemotherapy. In a study by the GOG, a significant reduction in the risk of death was seen if more than 6 months passed since completion of adjuvant chemotherapy and the initiation of second-line treatment.194
Single-agent therapy is often recommended for treatment-free intervals less than 6 months and disease progression during first-line treatment. Doxorubicin, paclitaxel, liposomal doxorubicin, ifosfamide, and docetaxel are commonly used second-line agents.195 Other choices include bevacizumab, oxaliplatin, and topotecan.
Endocrine Therapy
Advanced or recurrent endometrial cancer may be treated with hormonal agents. Therapy with progestins is well tolerated and is a reasonable alternative to cytotoxic chemotherapy. Response rates range from 15% to 30%.196 Tumors of lower grade with positive progesterone and estrogen receptors are more likely to respond to hormonal treatment.196,197 Treatment regimens include progestins, tamoxifen, or progestins alternating with tamoxifen.
A GOG study by Thigpen and colleagues randomly assigned 299 patients with advanced or recurrent endometrial cancer to 200 mg/d or 1000 mg/d of oral medroxyprogesterone acetate (MPA).198 Surprisingly, among women receiving the high-dose MPA, the overall response rate was 15% compared with 25% for the low-dose MPA. In addition, the median progression-free survival for the high-dose and low-dose MPA was 2.5 and 3.2 months, respectively. The low-dose MPA was also associated with an overall survival of 11 months compared with 7 months for high-dose MPA. Patients with well-differentiated tumors and positive progesterone status were associated with improved response rates. The authors concluded that daily oral medroxyprogesterone acetate was a reasonable treatment for well-differentiated and/or progesterone receptor–positive advanced or recurrent endometrial cancer.
A similar study by Lentz and colleagues evaluated the role of megestrol acetate to treat recurrent or advanced endometrial carcinoma. This study enrolled cytotoxic and hormonal therapy-naïve patients to receive divided doses of megestrol acetate 800 mg/d until disease progression or intolerable toxicity. The progression-free and overall survival were 2.5 and 7.6 months, respectively.199 As in the MPA study, grade 1 and 2 tumors were associated with significantly improved response rates when compared to grade 3 tumors (30% vs. 8%, P = 0.02).
Given the improved response rates associated with progesterone receptor positive status, the GOG investigated progestin therapy coupled with tamoxifen, a selective estrogen receptor modulator known to increase the number of progesterone receptors in endometrial carcinoma.200 Women with measurable advanced or recurrent disease were treated with daily tamoxifen (40 mg) and alternating weekly MPA (200 mg/d).201 The overall response rate was 33%, whereas the partial and complete response rates were 23% and 10%, respectively. This phase II trial also showed a progression-free and overall survival of 3 months and 13 months, respectively. Another study of tamoxifen plus MPA demonstrated similar results with progression-free and overall survival rates of 2.7 and 14 months, respectively.202 The authors from both studies concluded that progestins combined with tamoxifen warrants further investigation in randomized trials.
Despite multiple studies, including a Cochrane review of six randomized trials evaluating the effectiveness of hormonal therapy in advanced or recurrent endometrial cancer, progestin therapy has not demonstrated a benefit in the adjuvant setting.203 As such, progestin therapy should be individualized and reserved for palliation.
Novel Targeted Therapies
PTEN loss of function and PI3K mutations are the most common genetic aberrations encountered in endometrial cancer. As such, inhibition of the PI3K/AKT/mTOR pathway may be of benefit in treating endometrial cancer. Treatment with mTOR inhibitors, such as everolimus, has resulted in prolonged stable disease in small studies (43% at 8 weeks, 21% at 20 weeks).204 Treatment with temsirolimus, another mTOR inhibitor, resulted in both prolonged stable disease and partial response in 44% and 7.4% of patients, respectively.205 A phase II study of ridaforolimus in 45 women showed complete (n = 2) or partial responses (n = 7) in 28%, but 18% of the women discontinued treatment due to disease progression.
Treatment with ixabepilone, a microtubule-stabilizing agent similar to taxanes, resulted in stable disease for 8 weeks in 60% of patients previously treated with taxane chemotherapy.206 Median progression-free survival was 2.9 months.
A study of arzoxifene, a third-generation selective estrogen receptor modulator, showed an overall response rate of 31% in patients who previously responded to progestin therapy. Side effects were minimal, with hot flashes as the most common toxicity (grade 1/2).207
The anti-VEGF monoclonal antibody bevacizumab is being studied in the treatment of endometrial cancer. Overall response rate, median progression-free survival, and overall survival were 15%, 4 months, and 11 months, respectively.208
Epidermal growth factor receptor inhibitors have also been evaluated in patients with recurrent or metastatic endometrial cancer. Erlotinib was associated with an overall response rate of 13%.209 A study of cetuximab, a human epidermal growth factor receptor monoclonal antibody, showed a partial response in 5% and stable disease in 10% of patients.
Fertility-Sparing Treatment
Young, nulliparous women account for approximately 5% of endometrial carcinoma.210,211 These cases present the clinician with questions about fertility preservation. Important considerations include tumor histology and grade and hormone receptor status. In addition, depth of myometrial invasion and risk of metastatic disease should be considered. Conservative, fertility-sparing treatments are not recommended to patients with grade 3 tumors or high-risk histologies because of the increased risk of extrauterine spread. Fortunately, younger women are more likely to have endometrioid adenocarcinomas of lower grade with a more favorable prognosis. In addition, low-grade endometrioid adenocarcinoma tumors are more likely to express progesterone receptors compared with high-grade tumors. A previous study has shown that tumors expressing progesterone receptors are more likely to respond to oral medroxyprogesterone than tumors not expressing the receptor.198
Another important consideration is the risk of metastasis to the ovary or a synchronous primary ovarian cancer. Up to 5% of grade 1 tumors will have endometrial cancer metastatic to an ovary.212 Synchronous primary ovarian cancers are found in 11% to 29% of young, premenopausal endometrial cancer patients.211,213 Thus, appropriate counseling and evaluation of ovaries should take place before fertility-sparing measures are undertaken.
In a meta-analysis of 280 patients with grade 1 endometrial cancer treated with progestins, an initial response was noted in 75% of patients with a median time to response of 6 months; however, disease recurrence was documented in 35% of cases despite continued treatment.214 In this study, the majority of patients were treated with medroxyprogesterone or megestrol acetate. Less data exists regarding the use of levonorgestrel-releasing intrauterine devices (IUDs), but data are forthcoming. Initial response rates of 75% have been reported with levonorgestrel containing IUDs, but additional studies are necessary.215 Due to a paucity of data and lower rates of progesterone receptor expression in grade 2 tumors, caution should be exercised when fertility-sparing treatments are desired in women with moderately differentiated tumors.
Surveillance with repeat endometrial sampling to assess response every 3 months is recommended. Ramirez and colleagues reported a median time to response of 12 weeks following treatment with oral progestins in women with grade 1 tumors.216 However, 24% of women in the study experienced a recurrence with a median time of 19 months. Surgery may be warranted if disease progression or persistence is demonstrated despite medical therapy. Given the recurrence rate and likelihood that risk factors for endometrial cancer remain, hysterectomy should be considered after completion of childbearing.