Carcinoma of the Pancreas
Lauren A. Mauro, Joseph M. Herman, Elizabeth M. Jaffee and Daniel A. Laheru
• Pancreatic adenocarcinoma is the tenth leading cause of cancer in the United States, but the fourth leading cause of cancer-related deaths.
• The overall 5-year survival for all patients is <5%: 15% to 20% of patients present with resectable/ borderline resectable disease with median survival of 20 to 24 months; 25% to 30% present with locally advanced/unresectable with median survival of 8 to 14 months; 50% to 60% present with metastatic disease with median survival of 4 to 6 months.
• Familial atypical multiple-mole melanoma syndrome, hereditary pancreatitis, Peutz-Jeghers syndrome, and a strong family history of pancreatic cancer are the strongest risk factors and these patients should undergo screening. Risk factors also include smoking, heavy alcohol consumption, chronic pancreatitis, obesity and diabetes.
• Screening of the general population is not indicated at this time.
• Mutations in the KRAS oncogene and the CDKN2A tumor suppressor gene are found in more than 80% of pancreatic tumors and are thought to be early mutations. Inactivation of tumor suppressor genes TP53 and SMAD4 are thought to occur later in the tumorigenesis process.
• Pancreatic intraepithelial neoplasms (PanINs) are microscopic precursor lesions. PanIN-3 lesions should be fully resected when found. Intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) are noninflammatory cysts that can lead to pancreatic cancer. All MCNs, main duct IPMNs, and symptomatic branch duct IPMNs should be resected.
• Jaundice, abdominal pain, malabsorption, weight loss, back pain, and nausea/vomiting are common manifesting symptoms.
• Pancreatic protocol computed tomography (or magnetic resonance imaging) should be used for diagnosing and staging. Endoscopic ultrasound may offer additional staging information. It is critical to determine resectability on imaging as that will determine the course of treatment.
• All patients should be evaluated in a multidisciplinary setting when possible.
• Surgery should be considered upfront in patients with resectable disease unless treated on a neoadjuvant clinical trial.
• Patients should receive adjuvant 5-FU– or gemcitabine-based chemotherapy with 5-FU– or gemcitabine-based chemoradiation when appropriate as adjuvant therapy leads to longer survivals.
• Neoadjuvant chemotherapy followed by chemoradiation can be given in patients with borderline resectable disease to increase the likelihood of R0 resections, treat micrometastatic disease earlier, and avoid surgery in patients who demonstrate early progression.
• Locally advanced and metastatic disease patients can be treated with the same systemic chemotherapy regimens. Approved regimens include gemcitabine single-agent therapy, gemcitabine combination therapy, and 5-FU combination therapy.
• Chemoradiation or stereotactic body radiotherapy can be considered for locally advanced patients with good performance status if there is no disease progression after initial systemic chemotherapy.
• Palliative measures should be introduced early for advanced patients and focus on relieving pain, biliary obstruction, gastric outlet obstruction, and malnutrition.
• Additional studies are needed to evaluate the ability of novel biomarkers to guide pancreatic cancer management.
• Research focused on developing new therapies should take its cues from preclinical studies that are dissecting the stromal/tumor/inflammatory cascade of signals unique to pancreatic cancers.
Introduction
Pancreatic adenocarcinoma (PC) has one of the highest incidence-to-mortality ratios of any disease. Although it represents the tenth leading cause of cancer in the United States, it is the fourth leading cause of cancer-related deaths1 because the vast majority of patients will die from their disease. The overall 5-year survival for all patients with PC is less than 5%.4–4 This is in part because there is no appropriate screening test for the general population and manifesting symptoms are vague. Once patients are diagnosed, only 15% to 20% of patients present with resectable, and thus potentially curable, disease.2,5–8 However, even in the patients with early stage disease, median survival is 20 to 24 months with a 5-year survival of only 15% to 20%,2,7,9–20 as the majority will eventually recur despite surgery and adjuvant or neoadjuvant therapy. PC demonstrates complex biological features, presents with aggressive growth and is poorly responsive to chemotherapy. Patients with locally advanced (25% to 30% at presentation) and metastatic disease (50% to 60% at presentation) have median survivals of only 8 to 14 months and 4 to 6 months,2,5,7,21–28 respectively. However, some of the more recent trials with combination chemotherapy regimens provide some promise with improved survival in selected patients. As many patients suffer from biliary obstruction, diarrhea, pain, and malnutrition, palliative care can provide great benefit. While there has been improved understanding in the biology of PC translating in some modest improvements using contemporary chemotherapy and/or chemoradiotherapy, there are still significant gaps of understanding that must be identified in order to advance the treatment of this disease. This chapter reviews carcinoma of the exocrine pancreas, the most common form of cancers of the pancreas.
Epidemiology
In 2013, there will be an estimated 45,220 new cases and 38,460 deaths from PC in the United States alone,1 accounting for 12.1 new cases/year per 100,000 people. Worldwide, there are more than 278,600 new cases and more than 266,600 deaths per year, with an average of 4.1 new cases/year per 100,000 people. This rate does vary according to location, with more developed regions having a higher rate of 13.5 on average (Japan, Czech Republic, Hungary, and Finland have the highest rate of 18) as compared with an average rate of 2 for less developed regions (Africa and South-Central Asia have the lowest with <1 new case).29 These numbers are expected to increase as the world’s population grows and ages. The male-to-female ratio is virtually identical, with only a slight male predominance.29 This is a cancer of older adults, with the median age at diagnosis of 72 years in the United States, and with more than 66% of cases being diagnosed at an age older than 65 years. Only 2.6% of cases are diagnosed in persons younger than the age of 45 years and it is virtually never found in children. There are some racial disparities in the United States, as there is a slightly higher incidence in African Americans (17.5 new cases/year per 100,000 people) as compared with whites (13.5 new cases), Hispanics (11.4 new cases), and Asians (10.6 new cases; all incidences reported for men),30,31 and the age of diagnosis is younger in the African American and Hispanic groups.32
Risk Factors
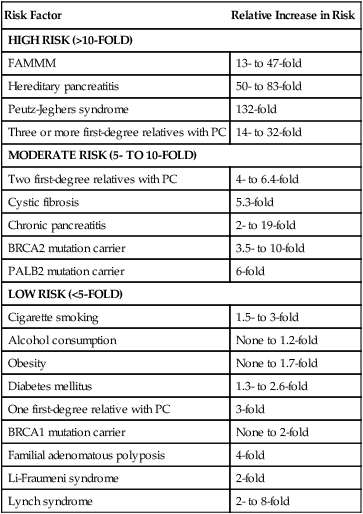
As PC is relatively uncommon yet incredibly aggressive, numerous risk factors have been identified that aid in preventative measures (Table 81-1). Cigarette smoking is one of the most clearly proven and most preventable risk factors. The risk increases with longer duration and higher number of cigarettes smoked, but can return to normal after 10 years of cessation.33,34 Cigarette smoke includes more than 50 carcinogens, the most important being nitrosamines, which promote tumor growth via various pathways, including activating the KRAS oncogene, and cause inflammation in the pancreas.35–35 It is believed to cause 15% to 25% of PC, with a relative risk of 1.5- to threefold in smokers as compared to nonsmokers.2,33–37 Chronic pancreatitis, often the result of chronic and heavy alcohol consumption or biliary duct blockages, leads to an increased risk of two- to 16-fold, although less than 5% of patients with chronic pancreatitis will develop PC. The chronic inflammation is thought to activate macrophages that produce cytokines that induce cell proliferation, angiogenesis, and inhibit apoptosis.34–36,38–42 Chronic pancreatitis may also be a manifesting symptom of PC from biliary obstruction caused by the tumor. Consequently, PC should be in the differential for a person who develops pancreatitis without any known risk factor.32,42,43 Patients with hereditary pancreatitis, caused by a mutation in PRSS1 in the autosomal dominant form leading to premature activation of trypsin while still in the pancreas as opposed to in the duodenum, or by a mutation in SPINK1 or CFTR in the autosomal-recessive form, have a 40- to 85-fold increased risk and a cumulative risk of 30% to 55% of developing PC in their lifetime.35,36,44,45 Alcohol is clearly a causative factor for cancers of the upper aerodigestive tract; however, it appears that only heavy alcohol use, defined as 3 or more alcoholic drinks per day, has a slightly increased relative risk of 1.2, whereas mild or moderate intake poses no increased risk.33,35–37 Obesity, defined as body mass index equal to or greater than 30 and accounting for 33% of adults in the United States, may lead to an increased risk of 1.2- to 1.7-fold and possibly higher in the severely obese.35,36,46 This link may result from increased circulating insulin and insulin growth factors, lower amounts of antiinflammatory cytokines, and a chronic low-grade inflammatory state throughout the bodies of obese people.46
The relationship between diabetes mellitus and PC is difficult to ascertain as type 2 diabetes can be both an early sign of PC and a risk factor. PC is thought to lead to diabetes through destruction of glands causing decreased production of insulin; however, the presence of diabetes does not appear to correlate with the size or stage of the tumor as this theory would suggest,47 so other mechanisms must be at play. Long-term diabetes is thought to increase the risk for PC because the increased secretion of insulin and insulin-like growth factors promote cell growth and the heightened state of inflammation leads to proinflammatory cytokines that contribute to angiogenesis and tumorigenesis.48 Studies have demonstrated that 55% to 85% of PC patients were diagnosed with glucose intolerance or diabetes either concurrently or within the 2 years preceding their cancer diagnosis.35,47,48 But, given the very high incidence of diabetes in the population, the relative risk of developing PC is still only 1.3- to 2.6-fold normal, when considered independently of obesity,35,37,47,48 and does not imply that all newly diagnosed diabetic patients warrant screening. However it should be considered in the atypical patient with newly diagnosed diabetes who has a steady or decreased weight, no change in activity and no family history of diabetes.36 Diets high in animal protein, history of gallstones, Helicobacter pylori infection, and some occupational exposures may lead to an increased risk.2,41,43,49,50 Allergies such as hay fever, increased vitamin D intake, blood type O, and diets high in fruits, vegetables, and citrus fruits may have a protective effect.2,35,36,44,51–54
Although the majority of PC are sporadic, 5% to 10% of patients have a family history.2,34,44 Familial PCc are defined as two or more first-degree relatives with the disease.35 Having one first-degree relative with PC gives a person a threefold increased risk, two first-degree relatives has a 4- to 6.4-fold increase risk, and three or more first-degree relatives poses a 14- to 32-fold increased risk.34,44,45 Although some patients with familial pancreatic cancer do not have identifiable genes, many syndromes have been described. Carriers of the BRCA2 gene (associated with breast, ovarian, and pancreatic cancers) have a 3.5- to 10-fold increased risk and account for 5% to 17% of families with familial PC. Mutations in PALB2 (binds to BRCA2) carry a sixfold increased risk,2,44 whereas BRCA1 carriers may have a twofold increased risk.2,35,36,44,55,56 Germline mutations in STK11 cause the autosomal dominant Peutz-Jeghers syndrome, characterized by hamartomatous polyps, pigmented macules, and a more than 90% chance of developing some form of cancer including a 132-fold increased risk for PC.2,35,36,44,50 Familial atypical mole-multiple melanoma patients typically have a mutation in CDKN2A and carry a 13- to 47-fold increased risk of PC.2,35,36,44,45 Germline mutations in DNA mismatch repair genes causing Lynch syndrome carry a two- to eightfold increased risk.35,36,44,57 Cystic fibrosis, characterized by a mutation in CFTR that produces viscous mucus that obstructs pancreatic ducts, carries an increased risk of 5.3-fold,35,44 whereas familial adenomatous polyposis (germline mutation in APC gene) has a four fold increased risk.44,45 Mutations in the tumor suppressor gene TP53 causing Li-Fraumeni syndrome results in a doubling of the risk.44
Etiological and Biological Characteristics
Molecular Biology
Understanding the molecular characteristics of PC is critical in order to aid in the development of targeted therapies. Several oncogenes and tumor suppressor genes are involved in the development of PC both by contributing to the growth of the tumor itself as well as to the support cells. Oncogenes, typically inactive in the normal cell, cause uncontrolled cell proliferation by inhibiting apoptosis and activating the cell cycle when mutations make them constitutively active. The KRAS oncogene, located on chromosome 12 and the most frequently mutated oncogene in PC (mutated in 80% to 90% or more of tumors), encodes a membrane-bound protein that has guanosine triphosphatase activity and is involved in signal transduction. When a mutation, typically a point mutation in codon 12, makes it constitutively active, its functions are independent of growth factors, which leads to chronic activation of its downstream pathways PI3K, MAPK, and RAF causing inhibition of apoptosis, activation of the cell cycle, migration, angiogenesis, cytoskeleton remodeling, and unchecked proliferation.5,35,45,54,58–62 When the AKT1 oncogene is activated through phosphorylation in the PTEN/PI3K/AKT pathway, it helps the cancer cells overcome cell-cycle arrest, blocks apoptosis, and promotes angiogenesis.35 Other oncogenes that are mutated in some pancreatic adenocarcinomas include BRAF (in KRAS wild-type tumors), MYC, MYB, and AKT2.45,54,59,60,62 Tumor suppressor genes, normally active, act in the opposite manner by activating apoptosis and inhibiting cell proliferation. However, tumor suppressor genes can be silenced when mutations make them inactive. CDKN2A, a cell-cycle control gene, is the most commonly inactivated tumor suppressor gene in PC, (lost in 80% to 95% of cases), leading to increased cell-cycle progression.5,35,45,54,60–62 TP53 is activated when there is DNA damage to either stop the cell cycle and repair the DNA or initiate apoptosis. Mutations in this tumor suppressor gene are found in 50% to 75% of tumors.5,35,45,54,58,61,62 Inactivation of SMAD4 (DPC4), involved in regulating cell cycle progression through the TGFB pathway, is seen in more than 50% of PC and is associated with worse prognosis and the development of metastases.5,35,45,54,58,62–64 Inactivation of RB1, in less than 10% of PC, and STK11, responsible for Peutz-Jeghers syndrome, is also seen.45,54 Other genes that have been demonstrated to be abnormal include the overexpression of IGF1, which leads to the abnormal activation of many downstream pathways, CDKN1A and CDKN1B mutations that cause loss of regulation at G1 checkpoint, EGFR TGFB mutations causing activation of MAPK pathway, and overexpression of ERBB2 (HER2/neu).58,62,63
There are several main signaling pathways involved in pancreatic tumorigenesis. The hedgehog signaling pathway, critical in embryogenesis, regulates the cell cycle and apoptosis, aids with the formation of the tumor stroma, and is often upregulated and abnormal in pancreatic cancers.54,61,63 The NOTCH pathway, also important in normal embryogenesis to prevent terminal differentiation of cells until appropriate, can be abnormally activated in pancreatic cancer, which allows cells to stay in an undifferentiated state contributing to tumor growth.54,63 When the WNT pathway, involved in maintaining the endocrine pathway, is activated, β-catenin is stabilized and migrates into the nucleus where it activates its target genes.35,54 The EGFR, TGFB, and JAK/STAT pathways have been found to be abnormal in PC cells leading to the promotion of cell growth, proliferation, differentiation and cell survival.35,54,63 The MET/HGF pathway is upregulated in most PC, and activates the downstream pathways including Ras, PI3K, JAK/STAT, and WNT, promotes angiogenesis and encourages cancer cell dissemination.35 NFKB1 proteins are a family of transcription factors that control numerous genes involved in inflammatory response, immune response, cell proliferation, apoptosis, differentiation and tumorigenesis. When its regulation is impaired, NFKB1 translocates into the nucleus and activates its target genes.54,63
Epigenetic modification, the process by which gene expression is altered by mechanisms other than changes in the actual DNA sequence, also has a role in PC tumorigenesis. DNA methylation and histone modification are two examples of this.2,45,65–67 Telomere shortening and overexpression of microRNAs lead to chromosomal instability and dysregulation of gene expression, respectively.2,45,58 In addition to the cancer cells themselves, the tumor microenvironment is comprised of stromal cells, inflammatory cells, and endothelial cells. The cancer cells secrete growth factors IGF1, FGF, TGFB, VEGF, and PDGFR that stimulate the pancreatic stellate cell (also called myofibroblasts) to secrete excess amounts of extracellular matrix. The matrix and its stromal cells in turn secrete cytokines and growth factors that promote cancer cell growth, invasion, and dissemination and protect the cancer cells from apoptosis. TGFB also leads to a decrease in activity of helper T cells, which suppresses the body’s immune system reaction against the abnormal cancer cells.5,54,58,63,68
Precursor Lesions
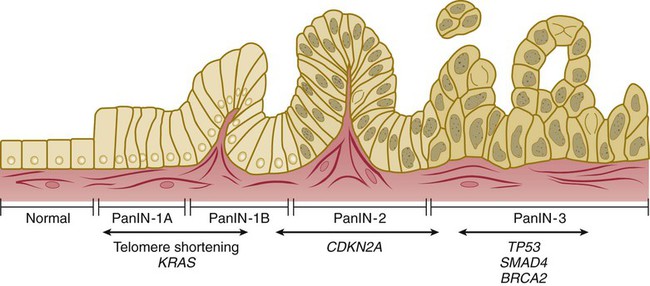
Approximately 85% of PC develops from the ductal epithelial, termed pancreatic ductal adenocarcinoma (PDAC). PDACs are thought to arise from precursor lesions: pancreatic intraepithelial neoplasms (PanINs), intraductal papillary mucinous neoplasms (IPMNs), and mucinous cystic neoplasms (MCNs). PanINs, the most common precursor lesions, are defined as “microscopic papillary or flat noninvasive epithelial neoplasms [of <5 mm diameter, not visible by imaging] arising from the pancreatic ducts…characterized by mucin-containing cuboidal to columnar cells [with varying degrees of nuclear and architectural atypia].”45,62 PanINs, are classified from low-grade atypical lesions (PanIN-1A or -1B) to high-grade atypical lesions (PanIN-2 or -3) as molecular alterations accumulate and more dysplasia appears (Figs. 81-1 and 81-2).2,35,54,58,63,69,70,356 One reason for their identification as precursor lesions is the presence of similar mutations as compared with invasive carcinoma. Activating mutations of the KRAS oncogene are one of the earliest abnormalities in the development of PC, as they appear in 30% to 45% of PanIN-1 lesions and more than 90% of PanIN-3 lesions. BRAF mutations are also found in the early precursor lesions in the 5% of patients that are KRAS wild-type. Telomere shortening also occurs early and is present in 90% of PanIN-1 lesions and leads to chromosomal instability making further mutations more likely. Inactivating mutations of CDKN2A begin to occur in PanIN-2 lesions and inactivation of TP53, BRCA2, and SMAD4 are seen in PanIN-3 lesions.2,45,54,58–61,70 Low-grade PanIN lesions are common in older individuals; however, higher grade PanINs are typically seen in association with invasive cancer, especially in those with familial PC.2,71,72 Although all precursor lesions have a risk of progressing to invasive carcinoma, the risk is very low for PanIN-1 lesions and very high for PanIN-3 lesions, as patients with PanIN-3 lesions at the margins of a resection virtually all recur with invasive carcinoma. Consequently, it is recommended that all margins should be clear of PanIN-3, even if this requires re-resection.62,70
IPMNs and MCNs are two types of noninflammatory cysts in the pancreas that are diagnosed secondary to symptoms or from incidental findings on imaging. Cysts are found incidentally on 1.2% to 2.6% of the over 50 million computed tomography (CT) scans done annually in the United States. Given their malignant potential, recommendations have been developed for their management.73 IPMNs are grossly visible, mucin-producing, epithelial neoplasms, typically with papillary architecture, that arise from either the main pancreatic duct or branch ducts and are graded according to degree of dysplasia. They range from 5 mm to 150 mm, are more commonly located in the pancreatic head, and can manifest with abdominal or back pain, weight loss, or anorexia from ductal obstruction from the mucin or the tumor itself. The average age at onset is 65 years and they have a 2 : 1 male-to-female predominance. Endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) can visualize the degree of dilation of the main pancreatic duct to help determine the place of origin. They are less frequent precursors to PC than PanINs, but they do share some of the same molecular aberrations. KRAS mutations are seen in 50% of low-grade IPMNs, whereas inactivating mutations of CDKN2A and TP53 are seen in high-grade lesions. Loss of SMAD4 is only rarely seen in even high-grade lesions, while mutations of GNAS are commonly seen. IPMNs arising from the branch ducts are less aggressive than those arising from the main duct with rates of malignancy and invasive malignancy (either mucinous noncystic carcinoma/colloid carcinoma or PDAC) of 25% and 15% for branch duct origin and 70% and 43% for main duct origin. Patients with main duct IPMNs should undergo resection. Their 5-year survival rate is 77% to 96% for the noninvasive neoplasms and 40% to 70% in the invasive lesions. Symptomatic patients with branch duct IPMNs have a higher risk of malignancy and should undergo resection. Asymptomatic branch duct IPMNs can be monitored with imaging (every 3 to 12 months depending on size) as they have a 0% to 5% chance of malignancy, especially if there is no main duct dilation, no mural nodules and the neoplasm is smaller than 30 mm. They should be removed if they become symptomatic or increase in size. Patients should be followed even after resection as well, yearly for benign IPMNs and twice yearly for invasive IPMNs, to catch any recurrence.2,45,70,74,75
MCNs also produce mucin; however, they are characterized by ovarian-type stroma. They are found almost exclusively in women, typically in the fourth and fifth decades, and 95% are in the pancreatic body and tail. They tend to be slow growing, but the average size is 5 to 12.5 cm. They have an orangelike gross appearance and only rarely communicate with the pancreatic duct. All MCNs should be resected with a distal pancreatectomy and proper lymph node dissection as they may progress to mucinous cystadenocarcinomas which have a very poor prognosis. Patients with benign MCNs do not need to be followed after resection because they do not recur; however, patients with malignant MCNs (6% to 36%) should be followed by imaging every 6 months.2,45,70,74,75
Prevention and Early Detection
Given the poor outcomes for patients diagnosed with PC, risk factor reduction and early detection are critical. Smoking cessation is the most important preventive measure and is strongly encouraged.2,33–37,76 Avoidance of heavy alcohol consumption can both reduce the risk of chronic pancreatitis, which is itself a risk factor, as well as reduce the carcinogens delivered to the pancreas from the alcohol.2,33–37 Decreasing the incidence of diabetes by maintain a healthy weight, a diet high in fruits and vegetables, and regular exercise will lower the risk.35–37,46–48,77,78 Unfortunately, the more severe risk factors are related to family history and congenital syndromes, which cannot be modified.
Screening the general population is not feasible because of the relatively low incidence of PC and the lack of an inexpensive, accurate, and noninvasive test. A biomarker would be ideal for this situation, however, none have shown to be appropriate. CA19-9, a sialylated Lewis A blood group antigen, is the most studied biomarker that is expressed and shed in pancreatic and hepatobiliary diseases. It is often used after the diagnosis has been made in order to predict prognosis and chance of recurrence. However it is not a reliable screening tool for the general public as it can be elevated from other causes, such as cholestasis, cholangitis, pancreatitis, neuroendocrine tumors, and other gastrointestinal cancers, and 5% to 10% of people are nonsecretors as they do not express Lewis A antigen. As obstructive jaundice can falsely raise the CA19-9 value, it should be checked after biliary drainage if possible. Its sensitivity ranges from 80% to 100% and specificity from 68% to 98.5%.5,35,76,79–92 Although the positive predictive value is higher in symptomatic individuals, especially when combined with imaging, it cannot be used alone for screening.
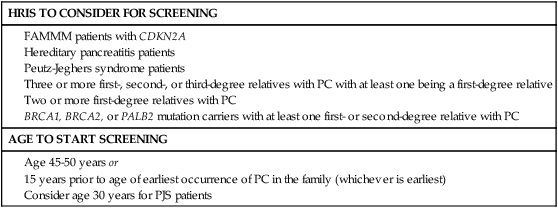
Individuals who are at high risk for developing PC, on the other hand, are recommended to undergo screening in order to catch premalignant lesions. The American Cancer of the Pancreas Screening (CAPS) Consortium did a prospective trial screening of high-risk individuals, defined as Peutz-Jeghers syndrome patients, familial breast-ovarian cancer patients with at least one affected first- or second-degree relative with PC, and relatives of patients with familial PC with at least one affected first-degree relative. They screened 225 high-risk individuals using CT, magnetic resonance imaging (MRI), and EUS, of which 82 had proven or suspected IPMNs and three had pancreatic endocrine tumors. Surgery was offered for suspected pancreatic neoplasms if there were solid masses, main duct or mixed duct IPMNs, branch duct IPMNs 2 cm or larger, or if there were concerning features. MRI and EUS was repeated in 6 to 12 months if lesions were found but no surgery was done, and repeated in 1 to 3 years if no mass was found. In the five patients who underwent pancreatectomy, all had high-grade dysplastic IPMN and/or PanIN but no invasive PC. The CAPS group considered this rate of identifying premalignant conditions adequate and recommended screening for this population starting at age 50 years, or 10 years before the earliest incidence in the family, or age 30 years for Peutz-Jeghers syndrome patients.93 The Fourth International Symposium of Inherited Diseases of the Pancreas also recommends screening high-risk individuals, defined as patients with greater than a 10-fold risk as per Table 81-1, as well as patients with three or more PC cases among first-, second-, and third-degree relatives, with at least one being a first-degree relative, and known BRCA1, BRCA2, and PALB2 mutation carriers who have at least one first- or second-degree relative with PC. Screening should start at age 45 years or 15 years before the earliest relative’s cancer onset, and should be done with EUS given its ability to detect pancreatic lesions smaller than 1 cm. Although MRI may be better, it has not been directly compared to EUS, and CT is less reliable for detecting small lesions.76,94 Several smaller studies have also demonstrated benefit of screening by finding precursor lesions in 8 of 72 high-risk individuals and 12 of 46 high-risk individuals,87,95 whereas others have shown only minimal yield.96 Therefore it is first recommended to test affected individuals in kindreds with familial PC for a causative mutation in order to properly test the unaffected individuals. Second, it is reasonable to screen high-risk individuals as defined per the studies above; however, it should be done in a clinical trial until randomized data proving its efficacy is available. Table 81-2 summarizes screening recommendations.2,35,44,58,76,93
Table 81-1
Risk Factors for Pancreatic Cancer
| Risk Factor | Relative Increase in Risk |
| HIGH RISK (>10-FOLD) | |
| FAMMM | 13- to 47-fold |
| Hereditary pancreatitis | 50- to 83-fold |
| Peutz-Jeghers syndrome | 132-fold |
| Three or more first-degree relatives with PC | 14- to 32-fold |
| MODERATE RISK (5- TO 10-FOLD) | |
| Two first-degree relatives with PC | 4- to 6.4-fold |
| Cystic fibrosis | 5.3-fold |
| Chronic pancreatitis | 2- to 19-fold |
| BRCA2 mutation carrier | 3.5- to 10-fold |
| PALB2 mutation carrier | 6-fold |
| LOW RISK (<5-FOLD) | |
| Cigarette smoking | 1.5- to 3-fold |
| Alcohol consumption | None to 1.2-fold |
| Obesity | None to 1.7-fold |
| Diabetes mellitus | 1.3- to 2.6-fold |
| One first-degree relative with PC | 3-fold |
| BRCA1 mutation carrier | None to 2-fold |
| Familial adenomatous polyposis | 4-fold |
| Li-Fraumeni syndrome | 2-fold |
| Lynch syndrome | 2- to 8-fold |
FAMMM, Familial atypical multiple-mole melanoma; PC, pancreatic cancer.
Table 81-2
Pathology and Pathways of Spread
Pathology
The pancreas is composed of both exocrine and endocrine cells. The endocrine cells account for 1% to 2% of the pancreas and form the islets of Langerhans where they secrete insulin, glucagon, and somatostatin. The exocrine pancreas includes the acinar cells that produce and secrete digestive enzymes in their inactive forms, the cuboidal cells that line the smaller ducts, and the mucin-producing columnar cells that line the larger ducts which carry these enzymes to the duodenum. Fibroblasts, pancreatic stellate cells, endothelial cells, nerves, and inflammatory cells create the stroma surrounding the endocrine and exocrine pancreas.54,75 Of pancreatic epithelial neoplasms, 85% develop from the ductal cells and are identified by their glandular or ductal differentiation. These PDACs arise from the pancreatic head in 60% of cases, typically presenting earlier because they obstruct the distal common bile duct, the pancreatic body in 15% of cases, tail in 5%, and diffusely in 20%. Tubular adenocarcinoma is the most common histology; adenosquamous carcinoma, colloid carcinoma, hepatoid carcinoma, medullary carcinoma, signet ring cell carcinoma, undifferentiated carcinoma, and undifferentiated carcinoma with osteoclastlike giant cells are less common variants. Medullary and colloid variants have a better prognosis than the typical tubular adenocarcinoma, whereas adenosquamous and undifferentiated have an even worse prognosis. The remaining 15% of pancreatic epithelial neoplasms are composed of 1% to 2% serous cystadenomas, 1% to 2% MCNs, 3% to 5% IPMNs, 1% to 2% acinar cell carcinomas, less than 1% pancreatoblastomas, 3% to 4% pancreatic endocrine neoplasms, and 1% to 2% solid-pseudopapillary neoplasms.45,62,75,97,98
Pathways of Spread
Invasive PC can spread through local invasion or through perineural, lymphatic, or vascular invasion, producing sites of distant metastasis. Given its location in the retroperitoneum, the most common sites of direct local invasion are to the stomach, duodenum, liver, common bile duct, gallbladder, spleen, superior mesenteric vein (SMV), superior mesenteric artery (SMA), and other surrounding arteries, veins, and lymph nodes. Perineural invasion is defined as the presence of cancer cells along nerves and/or within the neuronal sheath. Neurotrophins that are secreted by both the cancer cells and the nerve cells help support the survival and growth of these cells and promote neurogenesis, whereas chemokines like CX3CR1 appear to increase the cancer cells’ migration toward the nerve.99,100 The head of the pancreas is innervated by the superior mesenteric ganglion, the celiac plexus, and it branches which are the main routes for perineural invasion by the cancer cells. The main route of spread for cancers of the body and tail is through the celiac plexus and the splenic plexus. Intrapancreatic and/or extrapancreatic neural invasion is seen in virtually all advanced stage tumors, and even in more than 70% to 75% of stage I tumors, implying that this is an early event in the tumor’s progression. Given that some studies have reported worse prognosis for patients with perineural invasion, pathologists are now recommended to report the tumor’s perineural invasion status.99–105 In a retrospective analysis of 193 patients with resected PDACs, those without nodal metastases or perineural invasion had a 5-year survival of 75% as compared with 29% of those with perineural invasion. In patients with nodal metastases, 5-year survival was 17% when there was no perineural invasion and only 10% when there was invasion.103 Perineural invasion is one reason that many PC patients experience difficult to control abdominal and back pain.100
Vascular invasion is critical for both resectability and prognosis. The head and neck of the pancreas are supplied from superior pancreaticoduodenal arteries arising from the celiac trunk, and the inferior pancreaticoduodenal arteries arising from the SMA. These areas drain into the SMV, inferior vena cava, portal vein, and inferior mesenteric vein. The body and tail are supplied by the splenic artery arising from the celiac trunk and from pancreatic branches off the SMA and mostly drain into the splenic vein. Given their proximity to the pancreas, the SMV and SMA are the most commonly involved artery and vein by the tumor. The celiac artery, splenic artery, portal vein, hepatic artery, and, more rarely, the inferior vena cava and aorta can also be involved.6,106,107 On pathological review of more than 1100 resections, 53% had evidence of vascular invasion.108
Lymphatic invasion, and specifically nodal involvement, is the strongest predictor for survival. Lymphangiogenesis occurs during embryonic development and again during regeneration after a trauma and is thought to also occur during tumor growth. Tumor cells enter the lymphatic system by either migration through the interendothelial valves or by destroying the vessel walls. They will enter the smaller intralobular lymphatic vessels located within the pancreas and subsequently drain into the four main lymphatic branches of the pancreas: anterior, posterior, inferior, and superior pancreaticoduodenal lymphatic vessels. These then drain into regional lymph nodes that are typically located larger blood vessels: the celiac artery, common hepatic artery, SMA, and aorta for head/neck tumors and the splenic artery for body/tail tumors. At least 75% of all resections have positive lymph nodes.108–113
Tumor cells travel through the nerves, lymphatics, or vasculature to form distant metastases. The liver is the most common site of metastasis, possibly because tumor cells travel through the portal vein, followed by the peritoneal cavity. Lung metastases are the next most common, especially in long-term survivors.20,26,111,114–116 In a phase III trial for first-line metastatic PC, 88% of the patients had liver metastases, 24% had lung, 19% had peritoneal, and 14% had other sites.26 In a second-line therapy trial, 70% had liver disease, 30% had peritoneal, and 17% had lung involvement.116 Brain119–119 and skeletal metastases120 are quite uncommon and there have only been case reports of metastases to the appendix,121 kidneys,122 skin,123–126 prostate,127 stomach,128 heart,129 thyroid,130 and muscles.131
Clinical Manifestations, Patient Evaluation, and Staging
Signs and Symptoms
Although PC can cause numerous signs and symptoms, they often occur after the tumor is already at an advanced stage, making cure impossible and treatment challenging. When patients are diagnosed at early stages from incidental findings on scans, they can be asymptomatic.132 When the tumor has grown enough to cause biliary obstruction, symptoms include dark urine, light/clay-colored stool, pruritus, jaundice, scleral icterus, pancreatitis, and infectious complications such as cholangitis. As biliary obstruction typically occurs sooner for tumors of the pancreatic head, patients present with these tumors at an earlier stage than patients with body/tail tumors. Pancreatic enzyme insufficiency may cause malabsorption, increased flatulence, fatty stools, weight loss, and ascites. The pancreatic mass itself can cause compression or obstruction of the digestive tract, leading to nausea, anorexia, dyspepsia, pain, gastric or duodenal obstruction, and gastrointestinal bleeding. Pain may also be caused from perineural invasion or compression of the nerves surrounding the pancreas.2,5,88,98 It is usually dull, deep abdominal or middle back pain and is a poor prognostic sign, likely because of its association with larger tumors and micrometastatic disease.133 Approximately 85% of patients will have diabetes mellitus or glucose intolerance at diagnosis, with 55% to 85% of them being diagnosed within the preceding 2 years.35,47,48,134 Ten percent of patients may present with migratory thrombophlebitis with either deep or superficial venous thrombosis from the increase in procoagulants and platelet aggregation factors from the tumor.75 Many patients will report vague constitutional symptoms, such as generalized malaise, weakness, and depression, and most patients will report multiple symptoms at presentation.2,5,88,98 In several reviews describing the presenting symptoms of patients, 4.7% to 7% were asymptomatic, 34% to 51% had weight loss, 64% to 75% had jaundice, 39% to 55% had abdominal pain, 20% had back pain, and 13% had nausea/vomiting.98,108,135–137
Diagnosis
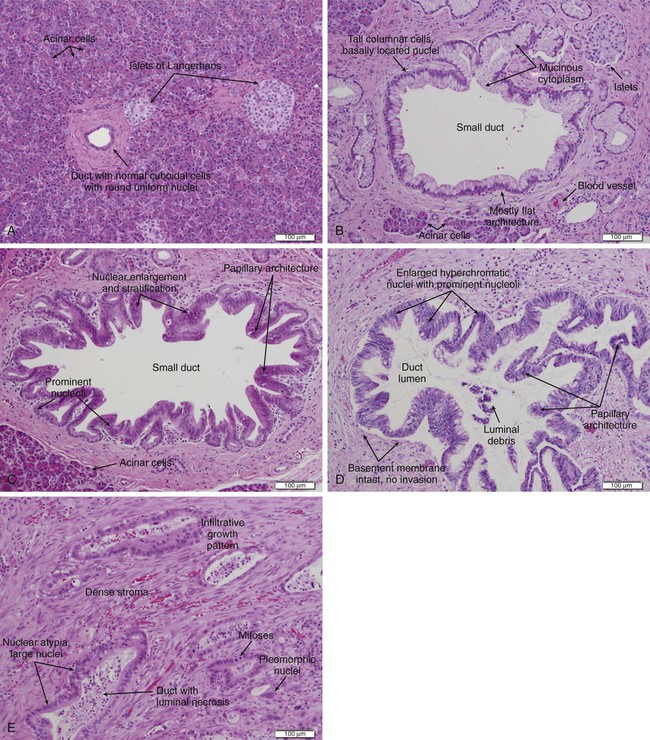
When evaluating a patient for pancreatic cancer, it is recommended to involve a multidisciplinary team that includes medical oncology, surgical oncology, radiation oncology, pathology, and radiology.88,138 Figure 81-3 depicts a diagnostic algorithm. The goals of the imaging, labs, and biopsies are to help clarify the diagnosis, stage, resectability status, prognosis, and possible palliative options. As no patient should be denied a potentially curative surgery if the patient qualifies, the imaging of choice should emphasize specificity over sensitivity. Multiphase pancreatic protocol CT with IV contrast is typically the first study ordered (Fig. 81-4A-E). This should include a noncontrast phase as well as contrast-enhanced arterial (when tumor has poor enhancement as compared to pancreas parenchyma given its hypovascularity), pancreatic parenchymal, and portal venous phases, and include thin cuts of 3 mm or less to help detail the location of the tumor in relationship to the main vessels, find signs of mass effect or ductal obstruction, and detect small tumors and sites of metastasis.2,5,88,139–143 3D reconstruction can add additional information for surgeons planning the operation.88,142,144 CT has a 74% to 98% accuracy in detecting pancreatic cancer with sensitivities of 77% to 97% and specificities of 85% to 100%, with the lower numbers accounting for the smaller tumors that CT may miss because they have not caused any biliary or vasculature obstruction or impingement. Additionally, CTs have sensitivities of 55% to 97% and specificities of 91% to 100% for predicting vascular invasion, and sensitivities of 76% to 92% and specificities of 82% to 100% for predicting resectability. Typically, 10% to 30% of patients taken to surgery will have their surgery aborted or altered to a palliative only procedure because of more serious vascular involvement or metastatic disease that was not detected on CT scan.2,5,6,88,140–142,145–152 The technology has greatly improved and CTs have a higher accuracy now that the multiphase pancreatic protocols are being used. Chest CTs are also recommended to assess for pulmonary metastases prior to resection.2 Multiphase pancreatic protocol MRI can be used when CT is contraindicated and may be able to detect smaller lesions and better differentiate between cancer and pancreatitis. However, it hasn’t been shown to be superior to multiphase CT. MRI can also detail the biliary and pancreatic ductal systems via magnetic resonance cholangiography. Its sensitivities and specificities for diagnosis are 84% to 98% and 82% to 88%, and for predicting resectability are 82% to 88% and 78%, respectively.2,88,139,142,143,150,153–155
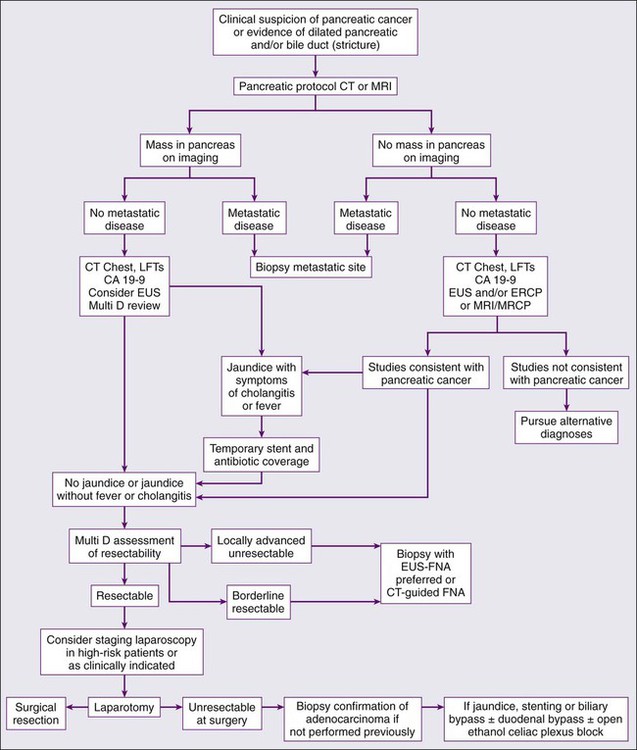
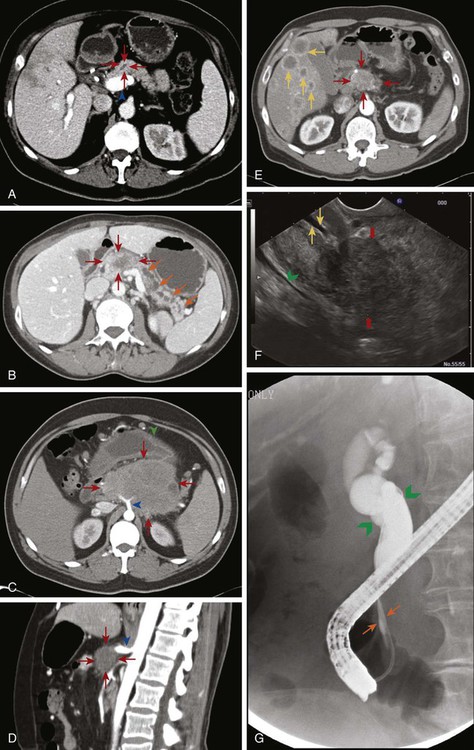
EUS is used in addition to either CT or MRI to obtain further information about nodal or vasculature involvement, to better identify suspected small lesions that were not seen on the prior imaging, and to help with staging (Fig. 81-4F). PDAC appears as a solid mass with irregular borders, is not homogeneous, and appears hypoechoic compared with the surrounding normal pancreatic parenchyma. Vascular invasion is determined when there is loss of the hyperechoic vessel wall and hypoechoic tumor interface, evidence of tumor within the vessel, irregularity of the vessel wall with tumor within 3 mm, inability to visualize a major vessel where collaterals are present, and vascular encasement. EUS may be superior to CT or MRI in differentiating cystic versus solid lesion, finding lesions smaller than 2 cm, noting pathological lymph nodes, and more accurately predicting the size of the tumor, but it is not very good at assessing SMA involvement. It does have the additional benefit of allowing a biopsy with fine-needle aspiration (FNA) or celiac plexus block to be performed at the same time. EUS has an accuracy of diagnosing PC of 86% to 96% with sensitivity of 85% to 100% and specificity of 98%.2,5,6,88,139,142,143,145,151,152,156–158 Although it is invasive, the complication rate is less than 2% and risk of major complications is 1 : 2500.159 Therefore EUS can be used as an adjunct to CT or MRI. ERCP combines endoscopy and fluoroscopy to map out the biliary system and may be beneficial if there is a duct stricture with no obvious mass as it does allow for ductal brushings. It also enables the placement of a biliary stent to decompress an obstructed system for patients who are receiving chemotherapy or for surgical patients who have a delay before surgery or have cholangitis (Fig. 81-4G).2,88,139,142,160
Positron emission tomography (PET)-CT may also be used in addition to pancreatic protocol CT or MRI to help clarify the presence of metastatic disease if this distinction would change management. With sensitivities of 85% to 100% and specificities of 67% to 99%, there can still be false negatives when tumors are smaller than 1 cm.2,5,6,88,142,161–163 Abdominal ultrasound, on the other hand, may be one of the first tests that is ordered to evaluate jaundice or abdominal pain and can demonstrate a hypoechoic mass or biliary dilatation, but it cannot firmly differentiate between PC, neuroendocrine tumor, and chronic pancreatitis. The addition of color Doppler does aid in detecting vascular invasion, but its accuracy of diagnosis is around 75%. Consequently, this will always need to be followed by further imaging.6,164–166 The use of staging laparoscopy has changed over time. It has been used in patients in whom unresectable disease is considered likely in order to prevent a larger laparotomy procedure that would need to be aborted. These higher-risk patients are those with CA19-9 higher than 100, head tumors larger than 3 cm, questionable findings on CT, or a body/tail lesion. Past studies did demonstrate that laparoscopy can prevent unnecessary laparotomies in 20% of patients, however these numbers are dropping to 5% to 8% as imaging studies have gotten better, making laparoscopy less likely to be helpful.6,88,139,167–169
Although a biopsy is not required when a patient with a high suspicion for PC goes directly to surgery, it is required prior to any neoadjuvant treatment or treatment of locally advanced or metastatic disease. FNA via EUS or CT-guidance are the two most common approaches to obtain a tissue sample. EUS-FNA is preferred because of the higher diagnostic yield, lower chance of seeding the peritoneum causing carcinomatosis, and the benefit of additional staging information from EUS. EUS-FNA has a 92% accuracy of correctly diagnosing a pancreatic mass and a 84% accuracy when performed in patients with negative CT-guided biopsies. It also carries a low complication rate of 0.5% to 2%.2,88,139,145,148,160,170–173 CT-guided FNA may also be performed to biopsy a metastatic site. ERCP brushings may also be done and should be considered when there is a biliary stricture but no mass, but cytology from brushings has a low yield of positive results.88,160 Although tumor-associated markers like CA19-9 can also assist in the diagnosis, as discussed previously, the sensitivity and specificity lead to a fair number of false positives and false negatives. The absolute value is often higher in more advanced stages, especially when there are liver metastases,86 and the value can provide useful prognostic information.
Staging
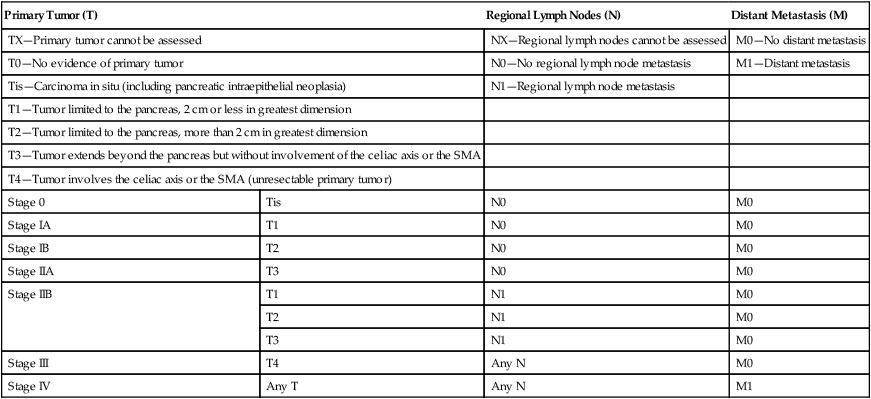
Classically, PC has been staged according to the TNM (tumor–node–metastasis) staging system per the American Joint Committee on Cancer, as shown in Table 81-3.174 The distinction of T1 and T2 tumors at 2 cm is secondary to the association of tumor size with survival in resected patients. Numerous reviews have demonstrated improved survival in patients with smaller tumors with 5-year survival improvements by 10% to 20% and increase in median survival by 5 to 20 months and hazard ratios around 1.6. Unfortunately, only a minority of patients (6% to 25%) present with tumors smaller than 2 cm.8,98,101,108,175–181 The classification of patients with nodal disease separately as stage IIB is because of the clear associated of nodal disease with worse survival, as described further below.175
Table 81-3
TNM Staging for Pancreatic Carcinoma per Seventh Edition of AJCC174
| Primary Tumor (T) | Regional Lymph Nodes (N) | Distant Metastasis (M) | |
| TX—Primary tumor cannot be assessed | NX—Regional lymph nodes cannot be assessed | M0—No distant metastasis | |
| T0—No evidence of primary tumor | N0—No regional lymph node metastasis | M1—Distant metastasis | |
| Tis—Carcinoma in situ (including pancreatic intraepithelial neoplasia) | N1—Regional lymph node metastasis | ||
| T1—Tumor limited to the pancreas, 2 cm or less in greatest dimension | |||
| T2—Tumor limited to the pancreas, more than 2 cm in greatest dimension | |||
| T3—Tumor extends beyond the pancreas but without involvement of the celiac axis or the SMA | |||
| T4—Tumor involves the celiac axis or the SMA (unresectable primary tumor) | |||
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2 | N0 | M0 |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| Stage III | T4 | Any N | M0 |
| Stage IV | Any T | Any N | M1 |
More recently, most centers and current clinical trials are classifying patients based on their resectability status (Table 81-4) because surgery is critical to achieve a cure in this disease. This characterizes stage I and II patients as resectable and divides stage III patients into those who are borderline resectable and may benefit from neoadjuvant therapy prior to surgery from those who are unresectable and are not surgical candidates. Tumors that encase the SMA or celiac artery would require technically difficult resections and reconstructions that carry a high perioperative mortality risk and are likely to leave some tumor behind and are therefore categorized as unresectable. In addition, there is a greater chance of perineural infiltration with SMA or celiac artery involvement because those arteries have a perineural sheath unlike the SMV or portal vein.88,139,175 Tumors involving the SMV and portal vein are considered borderline resectable, on the other hand, because SMV and portal vein resection does not affect surgical outcome or overall survival when done at experienced centers and therefore is not an absolute contraindication to surgery.19,175,182 Unfortunately, only 15% to 20% of patients present with resectable or borderline resectable disease.
Table 81-4
Classification of Resectability of Pancreatic Cancer per the National Comprehensive Cancer Network and Surgical Oncology Expert Consensus Statement2,5,7,21–28,88,139,175
| Resectability Status | Criteria | Median Survival |
| Resectable | No distant metastases; no radiographic evidence of SMV and portal vein abutment, distortion, tumor thrombus, or venous encasement; clear fat planes around the celiac axis, hepatic artery and SMA (Fig. 81-4A) | 20–24 months |
| Borderline resectable | No distant metastases; venous involvement of the SMV/portal vein demonstrating tumor abutment with or without impingement and narrowing of the lumen, encasement of the SMV/portal vein but without encasement of the nearby arteries, or short segment venous occlusion resulting from either tumor thrombus or encasement but with suitable vessel proximal and distal to the area of vessel involvement, allowing for safe resection and reconstruction; gastroduodenal artery encasement up to the hepatic artery with either short segment encasement or direct abutment of the hepatic artery without extension to the celiac axis; tumor abutment of the SMA not to exceed >180 degrees of the circumference of the vessel wall (Fig. 81-4B) | 20 months |
| Unresectable/locally advanced | HEAD: No distant metastases; >180 degrees SMA encasement or any celiac abutment; unreconstructable SMA/portal occlusion; aortic invasion or encasement BODY: No distant metastases; SMA or celiac encasement >180 degrees; unreconstructable SMV/portal occlusion; aortic invasion TAIL: No distant metastases; SMA or celiac encasement >180 degrees ALL: Metastases to lymph node beyond the field of resection (Fig. 81-4C and D) |
8–14 months |
| Metastatic | Any presence of distant metastases (Fig. 81-4E) | 4–6 months |
Primary Therapy
Surgery
Surgery is required to achieve a cure in patients with PC with resectable disease and improves overall survival. Patients with resectable tumors that undergo resection have a significantly longer survival of 20 to 24 months as compared with 10 to 12 months for patients with resectable tumors who do not have a resection.175 It is recommended that they be performed at a high-volume institution given the lower rate of operative mortality and morbidity.2,183 Resections are classified as R0 resections when the margins are clear of tumor, R1 resections where there is microscopic evidence of tumor at the margin, and R2 resections if there is grossly visible tumor at the margin. All attempts should be made to achieve an R0 resection as positive margins are a poor prognostic sign. There are three main surgeries for PC. The most common is a pancreaticoduodenectomy (Whipple procedure), indicated for pancreatic head tumors (Fig. 81-5). Prior to any resection, patients are first explored for resectability by evaluating for metastatic disease to the liver, peritoneum, or other viscera, examining the celiac and small bowel lymph nodes, and palpating the SMA, SMV, and portal hepatis.184 The surgeon will then resect the pancreatic head, distal bile duct, most of the duodenum, and proximal jejunum en bloc, described as a pylorus-preserving technique. At times, the remainder of the duodenum and gastric antrum must be resected to achieve a clear margin or when the duodenum is ischemic.108 Anastomoses will be created between the pancreas, bile duct, and stomach or duodenum to the remaining jejunum. Utilizing a pylorus-preserving technique is preferred because it maintains the complete gastric reservoir, preserves the pyloric sphincter, and shortens the operative time. Although it can be done in 70% to 85% of patients, it has not been shown to produce a better quality of life as compared with the classic procedure.88,108,184 Patients with resectable and borderline resectable tumors are candidates for this procedure, however the borderline patients will require scrupulous dissection around the vessels, vascular resection, and reconstruction to achieve an R0 resection.2,6,19,88,175,182
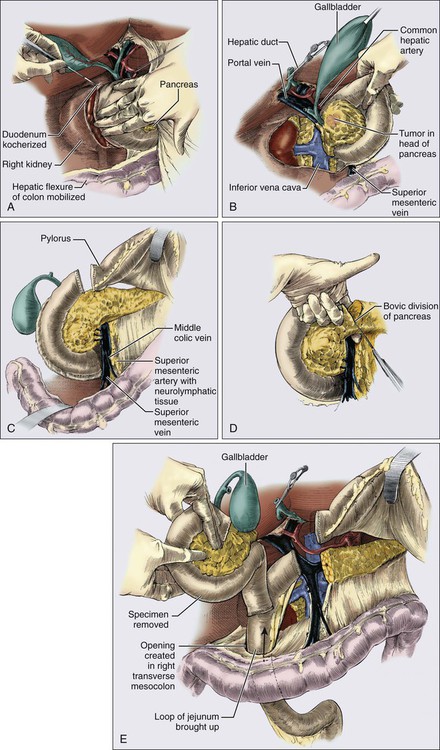
A minimum of 12 to 15 lymph nodes should be resected.5 Analyses have demonstrated that 90% of patients with node-positive disease will be identified when 15 nodes are removed.185 In addition, when patients were randomized in several trials to pancreaticoduodenectomy with standard node resection (designated N1 nodes) versus extended node resection (N1 plus N2 nodes), there was no difference in survival between the two groups despite removing on average 13 to 15 nodes versus 36 to 40 nodes. The extended group did have more diarrhea and worse bowel control.188–188 Identifying nodal involvement is also helpful in terms of prognosis as patients with node positive disease fare worse than those with node negative disease. Patients with N0 disease have a longer overall survival of 25.3 months and a hazard ratio of 0.63 as compared to patients with N1 disease with overall survival around 16.5 months. The survival decreases as the ratio of positive lymph nodes to total lymph nodes removed increases. In addition, patients who are node negative but had fewer than 12 to 15 nodes removed had similar survivals to those that had a few positive lymph nodes.191–191
The perioperative mortality rate is less than 3% to 5% at high-volume centers and as low as 1% in the recent decade.108,192,193 Perioperative morbidity is around 40% to 50%. The most common complications include pancreatic anastomotic leaks, pancreatic fistula development, intraabdominal abscess, hemorrhage, wound infection, metabolic abnormalities, diabetes, pancreatic enzyme insufficiency, cardiac events, and delayed gastric emptying. Less common complications include pneumonia, urinary tract infection, sepsis, respiratory failure, cholangitis, pulmonary embolism, and acute pancreatitis.2,88,108,184,194–196 Preoperative biliary drainage is not indicated unless patients have evidence of cholangitis, have a long delay to surgery, or are receiving neoadjuvant therapy as it leads to a significant increase in complications, as demonstrated in a randomized trial.2,5,88,193
Pancreatic tail lesions require a distal pancreatectomy to remove the body and tail, splenectomy, and regional lymph node resection. This can sometimes be done via a laparoscopic approach but should start with an assessment of metastatic disease.2,88,184 Distal pancreatectomies account for 6% to 9% of all resections performed, because the body/tail lesions typically manifest at a later stage because of a long delay to produce symptoms.20 Total pancreatectomy may be required when tumors are multifocal or when negative margins cannot be obtained with a pancreas preserving approach. In a review of 3280 patients with pancreatic head tumors and 383 patients with body/tail tumors, approximately 9% of patients in both groups required a total pancreatectomy. The 1-month mortality and overall survival was not different between the patients who underwent total pancreatectomy versus partial pancreatectomy and did improve survival as compared to patients who had margin-positive disease with the partial resection. Patients that have lost their entire pancreas do require long-term insulin and pancreatic enzyme replacement.88,197,198
It is recommended to check a CA19-9 level both preoperatively and postoperatively to assist with prognosis. Lower CA19-9 levels preoperatively were more likely in patients with T1/2 tumors and N0 resections and correlated with improved survival, especially when it was associated with a drop in level after surgery.86,199–201 Postoperative CA19-9 levels equal to or greater than 180 U/mL were significantly associated with worse median survival of 9 months as compared with 21 months for those with values less than 180 U/mL in the Radiation Therapy Oncology Group (RTOG) 9704 trial with a hazard ratio of 3.58. Lewis antigen-negative patients do slightly worse than patients with low CA19-9 levels, but not as bad as the high group.90 Similar results have been shown in other trials202 and a decrease with adjuvant treatment may imply improved response to chemotherapy and improved survival, and act as a surrogate marker for outcome.83,203–205 Pretreatment CA19-9 and levels checked during chemotherapy can also be used as a prognostic tool and surrogate marker of response when treating advanced PC as well.206–206
From large reviews of patients undergoing resection for PC, 1-year survival was 65%, 2-year survival was 37%, 5-year survival was 10% to 27%, and 10-year survival was 5% to 14%.20,108,207,208 Good prognostic factors include tumors smaller than 2 to 3 cm, negative lymph nodes, negative margins, well or moderately differentiated tumors, lack of perineural/perivascular invasion, CA19-9 less than 90 to 180, absence of chronic obstructive pulmonary disease, absence of bile leak, administration of adjuvant therapy, absence of diabetes mellitus, age younger than 65 years, no intraoperative blood transfusions, and absence of postoperative pneumonia or sepsis. Of those, the first four (specifically well differentiated) are the most significant factors in predicting long-term survival after resection and have been proven repeatedly in multiple trials.2,5,17,20,90,101,105,108,180,189,191,199,200,207–210 This may suggest that patients without these good prognostic factors should especially be encouraged to receive adjuvant therapy, although it is indicated in all patients who can tolerate it as described below.
Adjuvant Therapy
Given that the majority of patients who undergo resection for tumors that will recur, adjuvant therapy is indicated to decrease the risk of locoregional and metastatic recurrence. It is typically started 1 to 2 months after surgery to allow the patient to recover from the operation and complications associated with the underlying cancer. Although no regimen has been proven substantially more effective than others, 6 months of adjuvant therapy with 5-fluorouracil (5-FU)- or gemcitabine-based chemotherapy is standard with gemcitabine preferred because of a better toxicity profile. The integration of approximately 6 weeks of 5-FU–based chemoradiation therapy (45 to 46 Gy directed to the tumor bed, surgical anastomoses, and peripancreatic nodes with an additional 5 to 15 Gy boost to the tumor bed (Fig. 81-6A) using 3D planning or intensity-modulated radiotherapy (IMRT) during this 6-month period is an option and may be more favored for R1 resections and cases when the risk of locoregional recurrence is high. Radiation is a local therapy, similar to surgery, with chemotherapy being utilized as a radiosensitizer. The appropriate sequencing of adjuvant chemoradiation therapy is unclear. As more than 70% of patients will recur with distant disease, systemic chemotherapy is usually given first to be followed by chemoradiation if the disease is still controlled. As capecitabine has been shown in other studies to be equally effective as continuous infusion (CI) 5-FU, it can be substituted in adjuvant therapy as well. All patients should be restaged with CT or MRI prior to beginning adjuvant therapy and again before starting chemoradiation to rule out metastatic disease.2,5,88,211
The GI Tumor Study Group (GITSG) trial was the first to show a survival benefit for adjuvant chemoradiation in the 1980s. It randomized patients with resected PC to either observation or chemoradiation with bolus 5-FU for 2 weeks followed by bolus 5-FU weekly for up to 2 years. The medial overall survival (OS) was 20 months in the treatment arm compared to 11 months in the observation arm.212 This was followed by the European Organization for Research and Treatment of Cancer (EORTC) trial that randomized patients to observation or 40 Gy of radiation over 2 weeks with CI 5-FU on the days of radiation. Although the OS was 12.6 months in the observation arm compared to 17.1 months in the treatment arm, this was not statistically significant. Therefore this study could not conclude that chemoradiation was beneficial, but it was shown to be well tolerated.213 The ESPAC-1 trial also put the use of chemoradiation into question when it compared adjuvant chemoradiation, chemotherapy, both, or neither. Patients were randomized to either 20 Gy total of radiation with bolus 5-FU over 2 weeks repeated again after a 2-week break, bolus 5-FU daily for 5 days that was repeated every 4 weeks for a total of 6 cycles, chemoradiation as described followed by chemotherapy as described, or observation. With approximately 71 patients in each arm, median OS were 13.9 months, 21.6 months, 19.9 months and 16.9 months, respectively. Patients who received radiation had a median OS of 15.9 months compared with 17.9 months for those who didn’t receive radiation, suggesting that radiation was detrimental. Patients who received systemic chemotherapy had a median OS of 20.1 months compared to 15.5 months for those who didn’t, demonstrating that systemic chemotherapy is beneficial. Quality of life was similar in all groups and patients who received radiation did not have lower rates of local recurrence than those who didn’t. This trial was continued and demonstrated similar results after an additional 256 patients were added; however, these patients were not randomized in the original 2×2 fashion. Therefore systemic chemotherapy was recommended but chemoradiation was not, and should not be used alone.16,214 This trial has been criticized, however, for its nonstandard chemoradiation regimen and its unusual enrollment.
The CONKO-001 trial also demonstrated the benefit of adjuvant systemic chemotherapy by randomizing 354 patients to either observation or adjuvant gemcitabine given at 1000 mg/m2 IV weekly for 3 weeks on, 1 week off for a total of 6 cycles. Disease-free survival and OS were 6.9 months and 20.5 months for the observation arm and 13.4 months and 24.2 months for the treatment arm, respectively. The lack of a greater difference in OS was attributed to the ability for crossover from the observation group at time of recurrence. There were rare grade 3 or 4 toxicities in the treatment group but quality of life improved in both groups. Therefore it was concluded that gemcitabine improves disease-free survival and likely OS without compromising quality of life.14 Similarly, the ESPAC-3 phase III trial randomized 1088 patients to bolus 5-FU daily with folinic acid for 5 days every 4 weeks or gemcitabine weekly for 3 weeks every 4 weeks for 6 cycles total. OS was 23.0 months in the 5-FU group and 23.6 months in the gemcitabine group, with higher rates of stomatitis and diarrhea in the 5-FU group and higher rates of hematologic toxicity in the gemcitabine group but without any difference in quality of life.17 The ESPAC-1 trial, CONKO-001, and ESPAC-3 trial made both 5-FU and gemcitabine possibilities for adjuvant chemotherapy.
The RTOG 9704 trial compared these agents when used with chemoradiation. The trial randomized 451 patients to either CI 5-FU at 250 mg/m2 per day for 3 weeks followed by chemoradiation to 50.4 Gy with CI 5-FU followed by 2 cycles of 4 weeks on/2 weeks off CI 5-FU or to gemcitabine 1000 mg/m2 once weekly for 3 weeks followed by the same chemoradiation with CI 5-FU followed by 3 more cycles of gemcitabine. Radiation treatment planning and delivery were standardized. Radiation was delivered to the tumor bed and peripancreatic lymph nodes with a boost to the tumor bed plus a margin. There were more hematologic and overall toxicities in the gemcitabine arm but the same percentage of patients completed therapy in each arm. There was no statistically significant difference in survival, rate of local relapse (approximately 25%), and rate of distant relapse (approximately 70%) between the groups. Patients with pancreatic head tumors had a median OS of 17.1 months in the 5-FU group and 20.5 months in the gemcitabine group. This trial concluded that gemcitabine may be slightly favored; however, either option is appropriate, and chemoradiation can be given safely with similar outcomes to other trials when done in a standard fashion.15,209 Several large, single-institution, retrospective reviews have also demonstrated a benefit of combining 5-FU chemoradiation with systemic chemotherapy in the adjuvant setting.18,210,215 Gemcitabine-based chemoradiation has also been demonstrated to be effective in a phase II study when combined with systemic gemcitabine with a similar median OS of 24.4 months.216 There have been advancements in the delivery and management of radiation since these trials. IMRT is also becoming more commonly used as compared with the standard 3D dosing that was used in the RTOG 9704 trial. IMRT allows the radiation oncologist to improve targeting to the desired area while decreasing radiation dose to organs at risk of toxicity, such as the kidneys, liver, small bowel, and spinal cord.219–219 The use of antiemetics during radiation therapy has improved tolerability of radiation therapy and the use of proton pump inhibitors has decreased the rate of radiation duodenitis and ulcer formation.
Smaller trials have also looked at other regimens for adjuvant therapy. The combination of interferon-α, cisplatin, and 5-FU with chemoradiation reported a favorable OS but was significantly too toxic.220 Biweekly gemcitabine with daily erlotinib for 4 months followed by maintenance erlotinib for 8 months also is effective.221 Erlotinib has also been tested with Xeloda and IMRT and can be safely administered.222 The ongoing RTOG 0848 trial is randomizing patients to gemcitabine with or without erlotinib for 5 cycles and then randomizing patients again to a final cycle of chemotherapy versus 5-FU–based chemoradiation therapy. The results will help clarify the role of erlotinib and chemoradiation in the adjuvant setting. A phase II trial evaluated the efficacy of 5-FU chemotherapy, 5-FU chemoradiation therapy, and a vaccine of irradiated granulocyte-macrophage colony-stimulating factor transfected allogenic whole-cell tumor lines. Median OS was 24.8 months and those who demonstrated a CD8+ T-cell response had higher likelihoods of remaining disease free.223 KRAS–mutant vaccines224 and MUC1 peptide-loaded dendritic cell vaccines225 also have shown early promising results. Unfortunately, despite the above trials, median OS remains 20 to 24 months for patients with resected PC, demonstrating the need for more research in adjuvant regimens.
Neoadjuvant Therapy
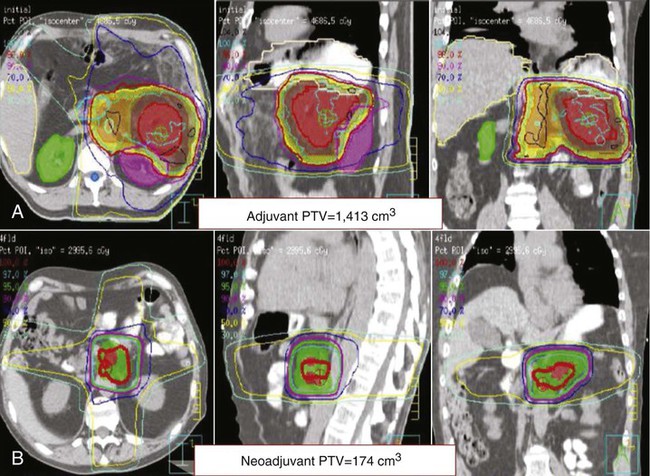
Neoadjuvant therapy remains controversial in the treatment of PC. It does have the advantage of downstaging borderline resectable patients who achieve a partial response with therapy allowing them to undergo surgery with a higher likelihood of an R0 resection.10,11,13,226–228 In fact, 15% to 40% of patients with initially borderline or unresectable tumors can eventually undergo surgery.10,12,13,228–233 More importantly, it spares patients who will quickly develop metastatic disease the risks and stress of a large surgery because their restaging scan after neoadjuvant therapy would show the progression and surgery would be avoided. This can range from 15% to 35% of patients initially considered for surgery9–11,226,234,235 and as high as 48% in some smaller studies.229,236,237 As 15% to 20% of resected patients fail to receive adjuvant therapy, neoadjuvant therapy guarantees that a majority of patients will receive some form of chemotherapy ± chemoradiation. Studies show that 73% to 100% of patients are able to complete the majority of their neoadjuvant regimens.12,226,230,234,235,237,238 Any micrometastatic disease will be treated earlier with the systemic chemotherapy and it allows for monitoring of response to specific chemotherapy or targeted agents. In regards to radiation therapy, neoadjuvant therapy allows for a smaller irradiated volume (Fig. 81-6B), limits exposure to other organs, and potentially allows for a higher dose of radiation to the pancreatic tumor. The chemoradiation component of neoadjuvant therapy also decreases local recurrence rates in patients who undergo surgery.11,227,230
However, one potential limitation with neoadjuvant therapy is that patients may progress locally or distantly and may miss their “window” for potentially curative surgery. Although the same percentage of patients ultimately undergo surgery, there are no randomized trials favoring neoadjuvant over adjuvant therapy. In addition, patients who undergo neoadjuvant therapy do not have the same opportunity for their pathology to be reviewed and to be considered for potential studies that require operative tissue prior to starting their therapy. Although it varies by institution, at this time neoadjuvant therapy is usually reserved for borderline resectable patients, whereas resectable patients are taken to surgery immediately and then given adjuvant therapy.2,5,88,138,211,239 Both groups have similar survivals when they are resected.9–13,226,228,230,233–235 These decisions should be made in a multidisciplinary manner to achieve the timeliest and most coordinated treatments.2,5,88,138,211,239
Although there are no clear standards established for neoadjuvant therapy, most centers use similar regimens as for locally advanced/unresectable disease.11,229,234,238 FOLFIRINOX (5-FU, irinotecan, oxaliplatin),26 GTX (gemcitabine, docetaxel, capecitabine),240 and gemcitabine are common chemotherapy regimens that are typically followed by CI 5-FU, capecitabine, or gemcitabine-based chemoradiation to 45 to 54 Gy in 1.8 to 2.5 Gy fractions or 36 Gy in 2.4 Gy fractions. Trials have been done using other regimens, such as gemcitabine and cisplatin,226,231,235,236,238 gemcitabine and oxaliplatin,241,242 and CI 5-FU,232 but the former regimens are more tested and preferred at this time. An upcoming intergroup study will evaluate FOLFIRINOX followed by capecitabine-based chemoradiation followed by surgical resection in patients who are stable or improved after therapy. Generally, surgery should be performed within 6 to 8 weeks following completion of neoadjuvant therapy. Further delays may lead to increased radiation-induced fibrosis, more challenging operations, longer operating times, and increased length of stay. Surgery is often followed with an additional 2 to 3 cycles of adjuvant chemotherapy, but may vary based on the final pathological features and regimens received previously.2,5,88,243
Locally Advanced and Metastatic Disease
Locally advanced and metastatic PC is treated with similar chemotherapy regimens outlined in the neoadjuvant and adjuvant sections of this chapter and summarized in Table 81-5. Chemotherapy is reserved for patients with adequate performance status, with Eastern Cooperative Oncology Group (ECOG) 0 to 2, while best supportive care and palliative therapy is recommended for patients with ECOG 3 to 4. The addition of chemoradiation can be considered for locally advanced disease only and is discussed later in this section.2,5,88,244
Table 81-5
Acceptable First- and Second-Line Chemotherapy Regimens for Locally Advanced and Metastatic Diseasea
| First-Line Therapy (Level of Recommendation) | Second-Line Therapy After Gemcitabine-Based First Line |
| Gemcitabine single agent, standard rate (1, 2B)b | Capecitabine single agent (2B)c Capecitabine + oxaliplatin (2B)c |
| Fixed-dose rate gemcitabine (2B) | Modified FOLFOX (2B)c |
| Gemcitabine + erlotinib (1)c Gemcitabine + capecitabine (2B)c |
Second-Line Therapy After 5-FU–Based First Line |
| Capecitabine single agent (2B)c | Gemcitabine single agent (2B)c |
| Gemcitabine + platinum in patients with BRCA1/2 mutation and/or family history (2B)c GTX (2B)c |
Gemcitabine-based combination therapy (2B)c |
| Clinical trial always recommended | |
| FOLFIRINOX (1, 2B)d | |
| Modified FOLFOX (2B)c | |
| Gemcitabine + nab-paclitaxel (2B)c | |
| Clinical trial always recommended |
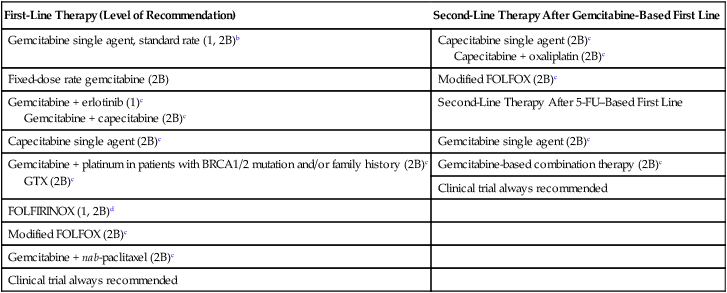
aLevel of Recommendation is based on NCCN Guidelines. Category 1 is based on high-level evidence with uniform NCCN consensus that the intervention is appropriate. Category 2B is based on lower-level evidence with NCCN consensus that the intervention is appropriate.
bGemcitabine single-agent is category 1 for locally advanced with poor performance status and metastatic disease for any performance status and is category 2B for locally advanced with good performance status.
cRecommended for locally advanced or metastatic disease with good performance status only.
dFOLFIRINOX is category 1 for metastatic disease with good performance status and category 2B for locally advanced with good performance status.
Chemotherapy for Locally Advanced and Metastatic Disease
For the last several decades of the 20th century, 5-FU was the standard of care to treat advanced PDAC.2,5,88,244 A metaanalysis of trials done from 1970 to 2003 demonstrated that 5-FU was superior to best supportive care.245 In 1997, a randomized phase III trial of patients with metastatic disease demonstrated a survival benefit for gemcitabine (dose at 1000 mg/m2 weekly for 7 weeks with a 1-week break followed by cycles of 3 weeks on/1 week off) over bolus 5-FU with a median survival of 5.65 months as compared to 4.41 months and 1-year survivals of 18% versus 2%. Gemcitabine also had a superior clinical benefit response described as improvement in pain, performance status or weight in 24% of patients versus 5% in the 5-FU group. Therefore it can be given to patients with moderate performance status of ECOG 2. Both were well tolerated with similar toxicities.24 Based on this trial, gemcitabine was FDA approved for PC and became the standard of care.2,5,88,244 Fixed-dose gemcitabine, given at a fixed rate of 10 mg/m2 per minute to lengthen the infusion time, is an alternative to the standard regimen above as the prolonged exposure leads to an increased intracellular gemcitabine triphosphate concentration.2,5,88,244 A phase II trial demonstrated improved survival for fixed-dose gemcitabine compared with standard gemcitabine, but was associated with increased hematologic toxicity.246 A phase III trial, however, did not show any difference in survival, therefore either regimen is considered viable options.247
Numerous gemcitabine-based combinations have been tested with mixed results.2,5,88,244 Metaanalyses have demonstrated that combination regimens should be considered in patients with good performance status (ECOG 0 to 1).248,249 Gemcitabine combined with bolus 5-FU in a phase III trial did not result in a statistically significant improvement in OS compared with gemcitabine alone. Standard dose gemcitabine has also been compared with gemcitabine plus twice-a-day capecitabine at various doses. Capecitabine is an oral medication that is converted to 5-FU in the tumor and inhibits thymidylate synthase. One phase III study had no statistically significant benefit in OS with the combination when all 319 patients were analyzed, but did have a benefit in good performance status patients without any difference in toxicity.250,251 A second phase III study did demonstrate an improvement in OS from 6.2 months for the gemcitabine-only arm compared to 7.1 months for the combination arm, with no difference in quality of life.25 Therefore gemcitabine plus capecitabine can be considered as a first-line option in patients with good performance status, although it is not standard of care.2,5,88,244 S-1 is a new oral drug that contains tegafur (prodrug of 5-FU), gimeracil (inhibits degradation of 5-FU), and oteracil (reduces gastrointestinal toxicity of 5-FU). When patients were randomized to S-1 alone, gemcitabine alone, or the combination in a phase III trial, there was no difference in OS, but there was a quality of life benefit in the combination arm as compared to the single-agent arms.252,253 Capecitabine has also been studied alone and does provide clinical benefit response and could be considered as an alternative to single-agent gemcitabine in patients with moderate performance status.254,255
The combination of gemcitabine and platinums (DNA-binding alkylating agents) have not shown improved efficacy in general.2,5,88,244 Gemcitabine plus oxaliplatin showed no statistically significant improvement in survival in phase III trials, although there may have been a trend in that direction. There was more neuropathy, thrombocytopenia, and vomiting with the combination.247,256 Randomized phase III trials with gemcitabine plus cisplatin had more toxicity in the combination arm with no statistical significant OS difference.257,258 Retrospective analyses have demonstrated, however, that patients with BRCA1 or -2 mutations and/or a family history of pancreatic, breast, or ovarian cancer responded much better to gemcitabine-platinum combinations, with longer median survivals compared with those who were not given a platinum. This is thought to be because the BRCA1/2 complex is needed to repair DNA double-stranded breaks and tumors are very sensitive to DNA-damaging agents like platinums if both alleles are inactive.261–261 Gemcitabine combinations with irinotecan262 and pemetrexed263 did not show any benefit in phase III trials. The addition of docetaxel and capecitabine to gemcitabine (in a regimen referred to a GTX) has demonstrated good activity. Two phase II trials and a retrospective review have demonstrated disease control rates of 63% to 80%, with median survival for locally advanced patients of 25 months and metastatic patients of 11 months. The most common toxicities were neutropenia, diarrhea, and hand-foot syndrome.240,264,265 Although these are smaller trials, this is an approved first-line therapy for patients with good performance status. Phase III trials are planned.2,5,88,244 A phase I/II trial published in 2011 demonstrated exciting results with combining gemcitabine with nab-paclitaxel (albumin-bound paclitaxel) as first-line therapy for metastatic PDAC patients. Gemcitabine was given at the standard dose of 1000 mg/m2 and nab-paclitaxel (maximum tolerated dose was 125 mg/ m2) was given weekly for 3 weeks, and repeated every 4 weeks. Median OS was 12.2 months with tolerable toxicity, and a negative PET-CT at 3 months was a predictor for good response. A current phase III trial is further testing this regimen.28
Gemcitabine has also been combined with targeted therapies; however, erlotinib is the only agent shown to improve OS, albeit minimally.2,5,88,244 A phase III randomized trial demonstrated a statistically significant improvement in OS from 5.91 months with single-agent gemcitabine to 6.24 months with gemcitabine plus erlotinib (an EGFR inhibitor, recommended dose of 100 mg by mouth daily).27 Erlotinib was FDA approved after this trial and can be considered as first-line therapy in patients with good performance status, although one needs to weigh the small benefit versus the toxicity and cost.2,5,88,244 The addition of cetuximab (monoclonal antibody to EGFR) to gemcitabine,266 sorafenib (VEGF inhibitor) to gemcitabine,267,268 bevacizumab (VEGF inhibitor) to gemcitabine ± erlotinib,269,270 or axitinib (VEGF inhibitor) to gemcitabine271 all demonstrated no survival benefit and an increase in toxicities in phase II and phase III trials. Trastuzumab (HER2 monoclonal antibody) and capecitabine also did not show any benefit.272
The newest combination regimen that has been demonstrated to be effective in a phase III trial for metastatic PDAC patients is FOLFIRINOX. It was initially described in France as oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 180 mg/m2, 5-FU bolus 400 mg/m2 on day 1 followed by a 46-hour CI-FU 2400 mg/m2 repeated every 2 weeks. When 342 patients were randomized, disease control rate and median OS were 70% and 11.1 months in the FOLFIRINOX arm, compared to 51% and 6.8 months in the gemcitabine arm. Although there were more grades 3 and 4 toxicities in the FOLFIRINOX arm, quality of life was not different.26 This, therefore, has been recommended as an option for first-line therapy in patients with ECOG 0 to 1 status; however, the doses have been reduced and adjusted when given in the United States because of the greater toxicity seen here.2,5,88,244
Second-line treatment is not very well established, partially because many patients are not strong enough to undergo further treatment. When patients do have adequate performance status, ones who progressed on a gemcitabine-based regimen should be treated with a 5-FU–based regimen and vice versa. Single-agent capecitabine is well tolerated and can be considered in patients with moderate performance status.255 Capecitabine and oxaliplatin (referred to as CAPOX or XELOX), provided 28% of patients with disease control with median OS of 23 weeks in a second-line phase II trial.273 Capecitabine plus erlotinib was reasonably tolerated and partial responses were seen in 10%.274 Modified 5-FU and oxaliplatin (FOLFOX)275,276 and 5-FU and irinotecan (FOLFIRI)275 regimens can also be considered as second-line options, with the former demonstrating a 2-month survival advantage over best supportive care and typically being the preferred regimen. Gemcitabine is also well tolerated and provides a clinical benefit response to patients who are 5-FU refractory.277
If patients recur after resection and adjuvant therapy, biopsy can be considered. If the disease relapse is locoregional only, chemoradiation can be performed as detailed below if no prior radiation was given. In regards to chemotherapy, if relapse occurs more than 6 months after completion of adjuvant therapy, the same regimen can be considered. If relapse occurs earlier, a different regimen should be chosen.88 Metastasectomy can be considered in very fit patients who have isolated metastases for a reasonable duration. Although resection of liver metastases have shown mixed results in terms of improving survival as compared with a palliative procedure only,278,279 pulmonary and brain metastasectomy have improved survival in a carefully selected group of patients.114,280
Radiation for Locally Advanced Disease
Chemoradiation can be considered for patients with locally advanced disease with adequate performance status to provide consolidative local therapy and potentially downstage approximately 10% to 15% of patients to allow them to undergo surgical resection.2,5,10,88,281–283 Although it can be given before or after systemic chemotherapy, 2 to 6 cycles of chemotherapy are usually given first.23 This allows for the treatment of micrometastatic disease upfront and avoids local therapy in patients who will develop metastatic disease early. However, chemoradiation may be preferred to be given first if patients are having pain or obstructive symptoms from the tumor. Regardless of the order, a total of at least 6 cycles of systemic chemotherapy are typically given either before or after chemoradiation. In some cases, patients will continue chemotherapy until progression or treatment related toxicity becomes prohibitive. Gemcitabine, CI 5-FU, and capecitabine can all be used as radiosensitizers. Radiation is usually dosed at 45 to 56 Gy in 1.8 to 2.4 Gy fractions for 5-FU–based therapy and 36 Gy in 2.4 Gy fractions for full-dose gemcitabine-based therapy. The fields will be the same as used in neoadjuvant therapy and IMRT can be used to spare radiation to healthy tissue and minimize treatment-related toxicity.2,5,10,88 Some phase III trials have favored the addition of chemoradiation, whereas others have not. ECOG4201 randomized locally advanced/unresectable patients to either gemcitabine alone or gemcitabine-based chemoradiation followed by gemcitabine. Although the grade 4 toxicities were significantly higher in the chemoradiation arm, the OS was 11.1 months compared to 9.2 months in the chemotherapy-alone arm.21 On the other hand, the FFCD-SFRO trial randomized locally advanced patients to gemcitabine alone or CI 5-FU and cisplatin chemoradiation followed by gemcitabine. The chemoradiation arm also had more increased toxicity and OS was shorter as compared with chemotherapy alone.22 A retrospective analysis was done of the GERCOR (Groupe Cooperateur Multidisciplinaire en Oncologie) phases II and III trials was conducted where locally advanced patients were given systemic chemotherapy upfront with the option of getting CI 5-FU–based chemoradiation if there was no progression. The OS of the group that did receive chemoradiation was superior at 15.0 months compared to 11.7 months for the patients who did not receive chemoradiation.23 When gemcitabine-based chemoradiation was compared with CI 5-FU–based chemoradiation in a prospective trial, there was a trend toward improved survival in the gemcitabine group that was not statistically significant but there was higher toxicity.284 In a retrospective analysis285 and a metaanalysis,286 the gemcitabine groups did have a longer survival compared with CI 5-FU or capecitabine. At this time, all three are considered options for radiosensitizers. Gemcitabine can be given at full dose (1000 mg/m2 weekly) safely during the radiation.287,288 Radiation with gemcitabine and oxaliplatin has also been studied in phase II trials and has good efficacy and toxicity profiles, but has not been compared to standard radiosensitizers in phase III trials.283,289 A recent phase III trial randomized patients to CI 5-FU standard radiation with or without an intratumorally injected TNFerade biological, which delivers TNFA to tumor cells by gene transfer. Patients were then given gemcitabine with/without erlotinib after completing chemoradiation. Patients with T1 to T3 disease did have a slight 1.9-month survival advantage in the TNF group, compared with the standard therapy group, with a minimal increase in toxicity. Otherwise, the addition of TNF failed to improve survival when compared to the standard arm.290
Stereotactic body radiation therapy (SBRT) is similar to IMRT in that it uses multiple fields of radiation therapy to conformally treat the planning treatment volume. However, it is delivered over one to five treatments as opposed to 25 to 30 treatments, and uses a smaller margin around the tumor in order to limit dose to normal tissues. The dose of radiation per day is also higher (5 to 25 Gy) as opposed to standard therapy (1.8 to 2.5 Gy). Chemotherapy is often given before and after SBRT as opposed to concurrently. Early data with SBRT (25 Gy × 1 dose) demonstrated disease stability with high rates of duodenal toxicity.291 Use of fractionated SBRT delivered in 3 to 5 fractions with specific constraints for duodenal radiation appears to result in improved tumor responses (approximately 40%) and less toxicity.292–295 Additionally, a prospective multicenter clinical trial evaluating SBRT in patients with locally advanced pancreatic cancer results in less-acute toxicity and improved quality of life when compared to standard chemoradiation.296 Additional studies are needed to evaluate the optimal dose of fractionated SBRT and determine how to best integrate SBRT into the neoadjuvant and adjuvant settings. Given the low toxicity and short course, SBRT may be beneficial in the palliative setting in patients with a poor performance status.
Palliative Therapy
PC patients develop many symptoms and problems that can burden their quality of life. Multidisciplinary management along with a palliative care medicine team is recommended. Approximately 65% to 80% of patients with pancreatic head tumors develop jaundice requiring biliary decompression.2,88,108,137,297–300 For patients with unresectable disease or receiving neoadjuvant therapy, endoscopically placed self-expanding metal stents are the preferred therapy to relieve this obstruction. Metal stents are recommended as they have a larger diameter, lower occlusion rates, and lower complication rates than plastic stents but they can become embedded in the bile duct and be more difficult to replace.300–305 The use of surgical biliary bypass can be done in patients who are found to be unresectable at the time of surgery. This is more uncommon today as higher-quality imaging can better predict resectability. Both surgery and endoscopy have similar success rates for palliating jaundice; however, surgical intervention has a longer hospital stay and a higher complication rate and is typically reserved for patients who are already in surgery or in whom endoscopic maneuvers fail.299,300,306–308 Percutaneous biliary drainage is another option when internal drainage cannot be done.2,88,297
Gastric outlet obstruction is seen in 10% to 25% of patients, and duodenal obstruction in less than 5%.300,309 Endoscopically placed enteral stents are often placed to relieve the obstruction and have good short-term outcomes with 15% stent dysfunction; however, they do not last longer than 1 year.88,300,310–313 Gastroduodenal surgical decompression and bypass is another option. Similar to above, surgical decompression can be done when patients are found to be unresectable during surgery and can prevent future gastric outlet obstructions. Surgical bypass may also be considered in patients with longer life expectancies as it has a lower failure rate than stents and better long-term outcomes, but it is more expensive, prolongs the hospital stay, has a higher complication rate, and has a longer time to resumption or oral intake.2,88,306,308,314–320 Percutaneous endoscopic gastrostomy tube and jejunostomy tubes may also be placed in patients with shorter life expectancies for decompression and nutrition.5 It is clear that planned palliative resections lead to increased morbidity and mortality and worse quality of life as compared with surgical bypass alone, therefore resections should not be attempted when gross tumor will be left behind.323–323
Pain is a very common symptom because of the perineural invasion and celiac plexus infiltration of the tumor and leads to decreased quality of life. Management with oral opioids is the first step; however, one third of patients cannot obtain reasonable pain control with oral medications alone. Neurolytic celiac plexus block with an injection of ethyl alcohol has a 70% to 95% success rate in controlling pain with a low complication rate and can last from 1 to 12 months. This can be done percutaneously, endoscopically, or through an open procedure if surgery was being done for another reason. It can also be considered as a prophylactic measure during surgery or EUS to prevent future pain.2,88,297,300,314,324–330 Palliative radiation with or without chemotherapy can also be given to control pain from local symptoms with a typical course of 30 Gy divided into 10 fractions.88 Epidural anesthesia and high-intensity, focused ultrasound therapy are other options.88,297,331
Venous thromboembolism (VTE) is seen in 8% to 28% of patients, as PC is one of most hypercoagulable malignancies. VTE commonly manifests as a pulmonary embolism, deep venous thrombosis, or thrombophlebitis migrans. However, other less-typical presentations can occur, which include splenic vein thrombosis causing portal hypertension, portal or SMV thrombosis causing pain, jaundice or ascites, or arterial thromboembolism causing ischemia. Low-molecular-weight heparin has been shown to be more effective at preventing recurrent VTE in malignancy and is recommended over oral warfarin therapy. VTE prophylaxis should also be used after pancreatic cancer surgery.2,88,301,332–335
Pancreatic insufficiency from destruction of pancreatic parenchyma or obstruction of bile ducts can lead to malabsorption, steatorrhea, diarrhea, weight loss, and abdominal cramps. Pancreatic enzyme replacement should be initiated after pancreatic resections and in symptomatic patients with unresectable disease.2,88,327,336,337
Controversies, Problems, Challenges, and Future Possibilities and Clinical Trials
The future for the development of new treatments is promising as there has been significant progress in the understanding of the genetics and molecular biology of PC.338–341 It has been long appreciated that KRAS is often mutated in pancreatic cancer tumors. However, many inhibitors targeting either KRAS or upstream/downstream of KRAS to date have been largely unsuccessful. More recent strategies have included integrating downstream pathways such as MEK, ERK, and AKT.342,343 The development of a PC mouse model that reliably recapitulates human PC has allowed for opportunities to not only learn about how PC grows, develops, and metastasizes, but also provides a unique platform with which to test new treatments in a methodical and systematic manner.59,344,345 In addition, much has been learned about the supportive tumor-stromal cell environment that is thought to not only influence the development of PC progression but also support mechanisms for resistance to effective treatment given its inherent protective properties.346,347 SMAD4 protein expression has been found to correlate local versus distant progression in an autopsy series and in patients with locally advanced pancreatic cancer289 and is being evaluated as a potential biomarker in an upcoming intergroup study for patients with locally advanced PC.
There is also a significant interest in understanding the complex relationship between inflammation and the immune suppressive tumor microenvironment in PC. Already there are new treatment opportunities targeting some of these pathways that can reverse immune suppression348 or immunotherapy approaches that lead to immune activation.223 Finally, there is significant research addressing the mechanisms associated with cancer cell metabolism, including pathways associated with glucose and glutamine metabolism as well as mechanisms associated with altered metabolic pathways and amino acid supply.349–353 Improved radiation techniques such as IMRT and SBRT have resulted in less treatment related toxicity, and when used with aggressive chemotherapeutic regimens can improve downstaging, tumor response, and survival in patients with borderline and unresectable PC. In addition, studies are underway to evaluate the role of limited radiation given to tumors to alter the tumor microenvironment in favor of improved access for other therapies (chemotherapy and immunotherapy).






