The Placenta
Anatomy, Physiology, and Transfer of Drugs
Mark I. Zakowski MD, Andrew Geller MD
Chapter Outline
The placenta is a critical organ of great importance to obstetric anesthesia. Revered by ancient cultures as “the seat of the external soul” or “the bundle of life,” the placenta is involved in many cultural rituals.1 However, understanding of the indispensable role of the placenta in the development of the fetus did not start to evolve until the 17th century and continues today via microanatomic, biochemical, and molecular biologic techniques. The concept of the placenta as a passive sieve (acting only as a conduit for oxygen, nutrients, and waste) has been dispelled with the realization that the placenta is a complex and dynamic organ. Indeed, new studies show the critical importance of placental function in the metabolism, nutrition, and hormonal maintenance of pregnancy. Maternal-placental conditions can affect the fetus not only during pregnancy but also in adulthood and beyond into the next generation via epigenetic mechanisms.2
The placenta brings the maternal and fetal circulations into close apposition without substantial interchange of maternal and fetal blood for the physiologic transfer of gases, nutrients, and wastes. This important exchange is accomplished within a complex structure that is almost entirely of fetal origin.
Anatomy
Embryology
The blastocyst initially attaches to endometrial pinopodes (uterodomes), which express markers of endometrial receptivity (e.g., galectin-9).3 The remodeling of uterine extracellular matrix starts with serine proteases and metalloproteinases (e.g., MMP-2 and MMP-9). The developing blastocyst erodes the surrounding decidua, leaving the cellular debris on which it survives. The syncytiotrophoblasts (invasive cells located at the margin of the growing conceptus) continue to erode the surrounding decidua and its associated capillaries and arterioles until the blastocyst is surrounded by a sea of circulating maternal blood (trophoblastic lacunae). The vitelline vein system develops in the yolk sac of the embryo to enhance the transport of nutrients, which diffuse from the maternal blood through the trophoblast layer and chorionic plate into the chorionic cavity. The embryo undergoes a dramatic acceleration in growth as its dependence on simple diffusion diminishes.4
At 2 weeks of development, the primitive extraembryonic mesoderm (cytotrophoblast layer) begins to proliferate as cellular columns into the syncytiotrophoblast. These columns with their syncytiotrophoblast covering extend into the maternal blood lacunae and represent primary villi. Further mesodermal invasion into the core of these primary villi marks the metamorphosis into secondary villi. Cellular differentiation of the villi mesoderm results in the formation of a network of blood cells and vessels; this transition allows their classification as tertiary villi. The vascular components of each villus develop connections within the chorionic plate and into the stalk that connects the developing embryo and primitive placenta. Penetration of the cytotrophoblast continues through the syncytiotrophoblastic layer until many of the villi reach the decidua and form anchoring villi (Figure 4-1).4,5
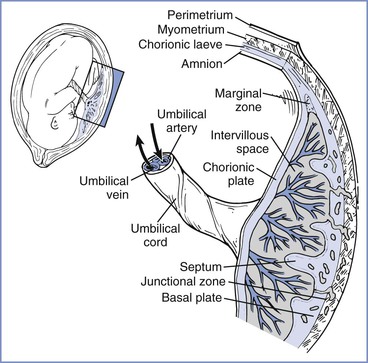
FIGURE 4-1 The placenta is a complex structure that brings the maternal and fetal circulations into close apposition for exchange of substances. (Redrawn from Kaufmann P, Hans-Georg F. Placental development. In Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3rd edition. Philadelphia, Saunders, 2004:85-96.)
Villi continue to develop and undergo extensive branching into treelike structures; the branches, which extend into the lacunar (or intervillous) spaces, enlarge the surface area available for exchange. Further villous maturation results in a marked reduction in the cytotrophoblastic component and a shortening of the distance between the fetal villi and maternal intervillous blood.4
The growing embryo within the blastocyst attaches to the chorion through a connecting or body stalk. Mesodermal components of this stalk coalesce to form the allantoic (or rudimentary umbilical) vessels. As the embryo continues its exponential growth phase, the connecting stalk shifts ventrally from its initial posterior attachment. The expansive open region at the ventral surface of the embryo constricts as the body wall grows and closes. By so doing, the body wall surrounds the yolk stalk, allantois, and developing vessels within the connecting stalk to form the primitive umbilicus. As the expanding amnion surrounds and applies itself over the connecting stalk and yolk sac, the cylindrical umbilical cord takes on its mature form.4
Placental development is a dynamic process influenced by many factors. Nitric oxide plays an important role in embryo development, implantation, and trophoblast invasion in diverse species.6 Human endothelial nitric oxide synthase (eNOS) expression in the syncytiotrophoblast and early endothelium occurs in the first trimester. Later in pregnancy, eNOS increases and becomes more prominent in the syncytiotrophoblast and endothelial cells. Vasculogenesis and angiogenesis depend on vascular endothelial growth factor (VEGF) and its receptors VEGFR-1 (Flt-1) and VEGFR-2 (Flk-2), transforming growth factor-β1 (TGF-β1), and angiopoietin 1 and 2, which exert their effects in part through nitric oxide. Hypoxia also plays an important role in placental development and angiogenesis by stimulating trophoblast invasion and differentiation via hypoxia-inducible factor-alpha, which activates VEGF and eNOS. Relative hypoxia must be maintained in early placental development because the placental-fetal unit cannot tolerate the oxidative stress of reactive oxygen species during organogenesis.7 Oxygen levels influence the placental vascular sensitivity to vasodilators and constrictors. In vitro studies have shown that NOS inhibition and hypoxia independently increase placental perfusion pressure. Both of these effects are prevented by nitric oxide donors, suggesting a common pathway with the effect of hypoxia mediated partly by low NOS activity.6
The development of preeclampsia is related, at least in part, to abnormal placental growth and implantation at this early stage of development (see Chapter 36). In patients with preeclampsia, the villous tree has longer capillaries with fewer branches.6 Vascular dysfunction occurs mainly from changes in vascular structure and activation of nitric oxide synthesis rather than from altered responses to nitric oxide and vasoconstrictors.
DNA, gene expression, and manipulation of gene expression control placental development, fetal development, adult phenotype expression, and clinical diseases, even into subsequent generations.2,8 The evolving field of epigenetics explores the prolonged effect of maternal and paternal environmental influences; gene expression becomes altered by DNA methylation, histone modification, and noncoding RNA. At fertilization, global DNA methylation is erased so at the blastocyst stage (implantation) the genome is hypomethylated.8 DNA methylation occurs in a specific manner so the trophectoderm (which becomes the placenta) remains relatively hypomethylated (50% to 70%) compared with the inner cell mass tissue (which becomes somatic human tissue). Genomic imprinting causes the silencing of one allele-specific copy of a gene. DNA methylation of imprinted genes occurs at the germ cell stage but is not involved in the methylation remodeling. Indeed, the human placenta exhibits extensive intraplacental mosaicism in an X-chromosome inactivation pattern. Individual placental cotyledons are derived from only a few cells, leading to cotyledon mosaicism. Even the process of in vitro fertilization produces an altered imprinted gene methylation pattern in the placenta.
Altered gene methylation has been linked to clinical disease states.8 Increased long interspersed nuclear element-1 (LINE1) gene methylation is associated with early-onset preeclampsia. Compared with disease-free matched tissue, early-onset preeclampsia is associated with hypomethylation of 34 specific genes, whereas only 4 hypomethylated genes were associated with late-onset preeclampsia.9 Thus, DNA and DNA regulatory changes influence not only early placental development but also the occurrence of pregnancy-associated disease.
Human studies have demonstrated fetal programming of childhood and adult disease. For example, a study showed that adults who were exposed in utero to episodes of malnutrition developed reduced glucose tolerance, atherogenic lipid profiles, and a doubled rate of cardiovascular diseases; these disease states were associated with hypomethylation of regulatory areas for insulin-like growth factor-2 and other genes.2 In utero exposure to a high-fat diet can lead to an increased incidence of diabetes in offspring. Maternal stress during pregnancy can lead to infant neurodevelopmental disorders.2
The placenta grows dramatically from the third month of gestation until term, with a direct correlation between placental growth and fetal growth. By term, the mature placenta is oval and flat, with an average diameter of 18.5 cm, weight of 500 g, and thickness of 23 mm. At term, the human fetal-placental weight constitutes 6% of maternal weight. Placental weight increases 0.7% per day, with active fetal growth contributing up to 1.5% of fetal body mass per day.10 The allocation of nutrient and metabolic resources for fetal growth potentially come at the expense of the mother. The growth of the placenta and fetus is influenced by maternal anabolic status, placental growth hormone, insulin-like growth factor-1, leptin, and glucocorticoids.11 Whether maternal or fetal in origin, increased glucocorticoids signal adverse environmental conditions and result in reduced glucose and amino acid transfer to the fetus. Indeed, competition between mother and fetus for resource allocation has been termed the kinship theory, in which imprinted genes influence the balance of nutrient allocation in a context-specific manner.11
Comparative Anatomy
The placentas of different species differ greatly, beginning with their method of uterine attachment, which can include adhesion, interdigitation, and fusion. In addition, the number of tissue layers between the maternal and fetal circulations differ. The most commonly used placental categorization system, the Grossner classification, uses the number of tissue layers in the placental barrier to help differentiate species (Figure 4-2).12
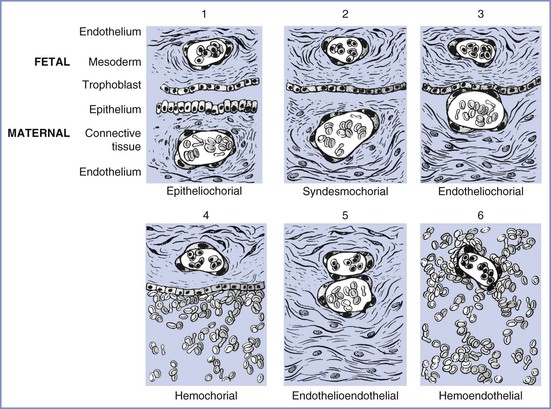
FIGURE 4-2 Modification of Grossner’s original classification scheme, showing the number and types of tissue layers between the fetal and maternal circulations. Examples of each are as follows: (1) epitheliochorial, sheep; (2) syndesmochorial, no known examples; (3) endotheliochorial, dogs and cats; (4) hemochorial, human and hamster; (5) endothelioendothelial, bandicoot (Australian opossum); and (6) hemoendothelial, Rocky Mountain pika. (Modified from Ramsey EM. The Placenta: Human and Animal. New York, Praeger Publishers, 1982.)
The ability of the placenta to transfer various substances differs among species. The markedly thicker epitheliochorial placenta found in sheep, a species commonly used for placental transfer studies, has three maternal layers (epithelium, connective tissue, and endothelium) that separate maternal from fetal blood. By contrast, the human hemochorial placenta lacks these maternal layers, which allows maternal blood to bathe fetal tissues directly (see Figure 4-2). As a result, species differ in the transfer of substances through the placenta; for example, fatty acids cannot cross through the placenta in sheep as they do in humans.13 This wide diversity in placental structure and function among species makes extrapolation from animal investigations to clinical medicine tenuous.
Vascular Architecture
Maternal
Under the initial hormonal influences of the corpus luteum, the spiral arteries of the uterus become elongated and more extensively coiled. In the area beneath the developing conceptus, the compression and erosion of the decidua induces lateral looping of the already convoluted spiral arteries,14 accessing the intervillous spaces. In late pregnancy, the growing demands of the developing fetus use approximately 200 spiral arteries that directly feed the placenta to handle a blood flow of approximately 600 mL/min.14 The vasodilation required to accommodate this flow is the result of the replacement of the elastic and muscle components of the artery, initially by cytotrophoblast cells and later by fibroid cells. This replacement reduces the vasoconstrictor activity of these arteries and exposes the vessels to the dilating forces of the greater blood volume of pregnancy, especially at the terminal segments, where they form funnel-shaped sacs that enter the intervillous space.14 The increased diameter of the vessels decreases blood velocity and reduces blood pressure.
The intervillous space is a large cavernous expanse that develops from the fusion of the trophoblastic lacunae and the erosion of the decidua by the expanding blastocyst, forming a huge blood sinus bounded by the chorionic plate and the decidua basalis (i.e., the maternal or basal plate). Folds in the basal plate form septa that separate the space into 13 to 30 anatomic compartments known as lobules. Each lobule contains numerous villous trees that are also known as cotyledons or placentomes. Although tightly packed with highly branched villous trees, the intervillous space of the mature placenta can accommodate approximately 350 mL of maternal blood.
Maternal arterial blood leaves the funnel-shaped spiral arteries and enters the intervillous space. The blood moves into the nearly hollow, low-resistance area, where villi are very loosely packed (the intercotyledonary space), before entering another region of densely packed intermittent and terminal villi (Figure 4-3).15 The terminal villi represent the areas where placental exchange predominates. After passing through this dense region, maternal venous blood collects between neighboring villous trees in an area called the perilobular zone.16 Collecting veins penetrate the maternal plate at the periphery of the villous trees to drain perilobular blood from the intervillous space.
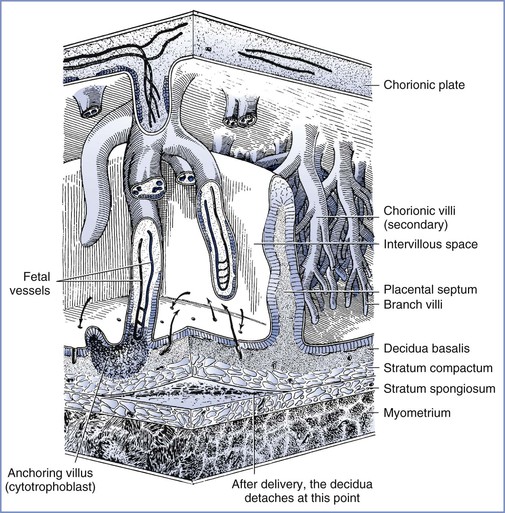
FIGURE 4-3 The relationship between the villous tree and maternal blood flow. Arrows indicate the maternal blood flow from the spiral arteries into the intervillous space and out through the spiral veins. (Modified from Tuchmann-Duplessis H, David G, Haegel P. Illustrated Human Embryology. Volume 1. Embryogenesis. New York, Springer Verlag, 1972:73.)
Fetal
Two coiled arteries bring fetal blood within the umbilical cord toward the placenta. On the placental surface, these arteries divide into chorionic arteries that supply the 50 villous trees located in the placental lobules. At the base of each villous tree, the chorionic arteries are considered the main villous stem or truncal arteries (first-order vessels), which in turn branch into four to eight ramal or cotyledonary arteries (second-order vessels); as they pass toward the maternal plate, they further subdivide into ramulus chorii (third-order vessels) and, finally, terminal arterioles. The terminal arterioles lead through a neck region into a bulbous enlargement where they form two to four narrow capillary loops. Here the large endothelial surface area and the near-absence of connective tissue allow optimal maternal-fetal exchange (Figure 4-4).16,17
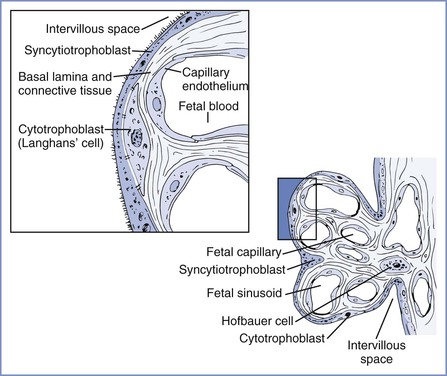
FIGURE 4-4 Left, Cellular morphology of two terminal villi. Right, Higher magnification of the boxed region exhibiting the placental barrier between fetal and maternal blood. (Redrawn from Kaufmann P. Basic morphology of the fetal and maternal circuits in the human placenta. Contrib Gynecol Obstet 1985; 13:5-17.)
The venous end of the capillaries loops, narrows, and returns through the neck region to the collecting venules, which coalesce to form the larger veins in the stem of the villous trees. Each villous tree drains into a large vein, which, as it perforates the chorionic plate, becomes a chorionic vein. All of the venous tributaries course toward the umbilical cord attachment site, where they empty into the one umbilical vein that delivers blood back to the fetus.
Physiology
Barrier Function
The placenta is an imperfect barrier that allows many substances to cross from the maternal to the fetal circulation and from the fetal to the maternal circulation. The rate and amount of placental transfer depend on the permeability and the ability of various mechanisms to restrict movement. A vast array of cytochrome P450 isoenzymes and transporters are found within the placenta; some of these are inducible, whereas others are constitutive. In addition, a number of substances undergo specific or nonspecific binding within the placental tissues, thereby minimizing fetal exposure to and accumulation of the substances. Finally, the thickness of the placental membranes, which diminishes as gestation progresses, may influence the rate of diffusion.18 Of interest, the rate of transfer of certain substances (e.g., glucose, water) differs very little among species, even though the placental thickness varies greatly.19
Fetal cells have been detected in maternal circulation, before organogenesis and full maternal arterial perfusion of the placenta, and maternal cells have also been shown to enter the fetal circulation.20 Maternal-fetal cell transfer may occur by disruption of the trophoblastic layer or by active adhesion and transmigration (similar mechanism to blood-brain barrier migration). Fetal cells may be pluripotent, and the DNA may be found in maternal organs for decades. Murine fetal progenitor cells have been found to migrate and assist with maternal wound healing.21 These microchimeric fetal cells may contribute to maternal immunomodulation, development or worsening of autoimmune diseases (e.g., thyroiditis, lupus, and asthma), and healing of wounds, including neuronal tissue.22 Indeed, placental exosomes, nanovesicles 30 to 100 nm in size found in maternal circulation that contain proteins and transcription-related materials, exert a maternal immunosuppressive effect. Placental microparticles, vesicular products of syncytiotrophoblast greater than 100 nm, also contain RNA and DNA fragments and affect fetal and maternal apoptosis, angiogenesis, and inflammation. An excess of microparticles has been observed in early-onset preeclampsia. The placenta and fetal-maternal interactions are certainly complex and worthy of further study.
Cell-free fetal DNA has been shown to be present in the plasma of pregnant women.23 This discovery has facilitated the development of a range of noninvasive diagnostic investigations, including tests for fetal sex assessment, fetal rhesus D blood group genotyping, fetal chromosomal aneuploidy detection, and other genetic abnormalities.24
Hormonal Function
A sophisticated transfer of precursor and intermediate compounds in the maternal-fetal-placental unit allows placental enzymes to convert steroid precursors into estrogen and progesterone. This steroidogenic function of the placenta begins very early in pregnancy; by 35 to 47 days after ovulation, the placental production of estrogen and progesterone exceeds that of the corpus luteum (i.e., the ovarian-placental shift).25
The placenta also produces a wide array of enzymes, binding proteins, and polypeptide hormones. For example, the placenta produces human chorionic gonadotropin, human placental lactogen (a growth hormone also known as human chorionic somatomammotropin), and factors that control hypothalamic function.25 This ability to produce proteins and steroid hormones allows the placenta to influence and control the fetal environment.26
Regulation of Placental Blood Flow
Maternal Blood Flow
The trophoblastic invasion and functional denervation of the musculoelastic lining of the spiral arteries may represent adaptive mechanisms to decrease vascular reactivity and promote vasodilation. These alterations allow the spiral arteries to vasodilate as much as 10 times their normal diameter, thereby lowering resistance for the passage of blood through the intervillous spaces.27
Maternal blood enters the intervillous cotyledon space at a pressure of 70 to 80 mm Hg in an area that has relatively few villi.14 The pressure and velocity of blood flow rapidly diminishes to approximately 10 mm Hg as the blood passes into an area of higher resistance created by the densely packed villi of the placentome.18
Fetal Blood Flow
In contrast to maternoplacental blood flow, the gestational increases in fetoplacental blood flow primarily results from vascular growth rather than vasodilation of the villous beds. Fetal perfusion of the placenta is not classically autoregulated; the placental vasculature has no innervation by the sympathetic nervous system. However, the fetus can modulate fetoplacental perfusion in a number of ways: (1) via endocrine effects of adrenomedullin, (2) via net efflux/influx of water regulated by fetal blood pressure, and (3) via local autoregulatory effects mediated by the paracrine vasodilators nitric oxide and acetylcholine.28,29 Adrenomedullin release by the fetal adrenal glands assists in maintenance of tone in placental vessels. Fetal blood pressure changes cause net influx/efflux of water across the placenta that affects fetal intravascular volume and perfusion. Maternal hyperglycemia30 and hypoxemia31 are examples of derangements that can alter regional fetal blood flow, probably through vascular mediators. Endothelium-derived relaxing factors, especially prostacyclin32 and nitric oxide,33 are important in the control of fetoplacental circulation. Hypoxia-induced fetoplacental vasoconstriction is mediated by a reduction in the basal release of nitric oxide.34 This vasoconstrictor activity is functionally similar to that found in the lung (i.e., hypoxic pulmonary vasoconstriction) and allows optimal fetal oxygenation through redistribution of fetal blood flow to better-perfused lobules.31 The placental vasculature constricts in response to graded hypoxia.35
Transport Mechanisms
Substances are transferred across the placenta by one of several mechanisms.
Passive Transport
The passive transfer of molecules across a membrane depends on (1) concentration and electrochemical differences across the membrane, (2) molecular weight, (3) lipid solubility, (4) degree of ionization, and (5) membrane surface area and thickness. This process requires no expenditure of cellular energy, with transfer driven principally by the concentration gradient across a membrane. Simple transmembrane diffusion can occur either through the lipid membrane (e.g., lipophilic molecules and water) or within protein channels that traverse the lipid bilayer (e.g., charged substances such as ions) (Figure 4-5).36,37 Drugs with a molecular weight less than 600 daltons cross the placenta by passive diffusion.38
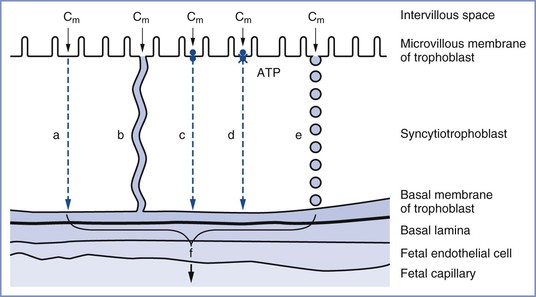
FIGURE 4-5 The transfer mechanisms used for the transfer of substances across the placental barrier: a, simple diffusion; b, simple diffusion through channels; c, facilitated diffusion; d, active transport; e, endocytosis; f, substance available for transfer into fetal circulation; Cm, intervillous concentration of substance at the trophoblastic membrane. (Modified from Atkinson DE, Boyd RDH, Sibley CP. Placental transfer. In Neill JD, Plant TM, Pfaff DW, et al., editors. Knobil and Neill’s Physiology of Reproduction. 3rd edition. St. Louis, Academic Press, 2006:2787-846.)
Facilitated Transport
Carrier-mediated adenosine triphosphate (ATP)–independent transport of relatively lipid-insoluble molecules down their concentration gradient is called facilitated diffusion.36 Facilitated diffusion differs from simple diffusion in several ways. Specifically, this mode of transfer exhibits (1) saturation kinetics, (2) competitive and noncompetitive inhibition, (3) stereospecificity, and (4) temperature influences (e.g., a higher temperature results in greater transfer). With simple diffusion, the net rate of diffusion is proportional to the difference in concentration between the two sides of the membrane. This rate limitation is valid for facilitated diffusion only when transmembrane concentration differences are small. At higher concentration gradients, a maximum rate of transfer (Vmax) is reached; thereafter, further increases in the concentration gradient do not affect the rate of transfer. The rate of transfer is determined by the number of membranous carrier protein complexes and the extent of interaction between the carrier and the substance undergoing transport.37 An example of facilitated diffusion is the transplacental transfer of glucose.
A special type of facilitated diffusion involves the “uphill” transport of a molecule linked to another substance traveling down its own concentration gradient. As such, the transfer is not directly driven by cellular energy expenditure. In most cases, sodium is the molecule that facilitates transport. For the membrane-bound carrier to transfer these molecules, both molecules must be bound to the carrier. This hybrid system is called secondary active transport or co-transport.37 The transplacental transport of amino acids appears to occur principally through secondary active transport. Transporters may be affected by disease states (e.g., preeclampsia) or signaling molecules (e.g., elevated steroid levels).39
Active Transport
Active transport involves the movement of any substance across a cell membrane; the process requires cellular energy. In general, active transport occurs against a concentration, electrical, or pressure gradient.
Like facilitated diffusion, active transport requires a protein membrane carrier that exhibits saturation kinetics and competitive inhibition.36 However, unlike secondary active transport, the movement of a substance against its concentration gradient is directly linked to the hydrolysis of high-energy phosphate bonds of ATP. The best known example of primary active transport is the translocation of sodium and potassium through the sodium-potassium adenosine triphosphatase (Na+/K+ ATPase) pump.
Active transport proteins include P-glycoprotein, breast cancer resistance protein, multidrug resistance protein, and the sodium/multivitamin transporter, as well as the many proteins involved in monoamine transport and xenobiotics.39 These transport proteins play an important role in protecting the fetus from foreign and potentially teratogenic compounds. Drugs may compete with endogenous compounds of similar shape and charge for active transport.39 P-glycoprotein transports many lipophilic drugs and antibiotics and removes cytotoxic compounds from the fetus; it exists on the maternal side of the trophoblastic cell membrane of the placenta and prevents compounds such as methadone and saquinavir (a protease inhibitor) from leaving the maternal blood, thus limiting fetal exposure.40 Inhibition of these transporter proteins (e.g., inhibition of P-glycoprotein by verapamil) can significantly increase the fetal transfer of certain drugs, including midazolam, which is a substrate for P-glycoprotein. DNA transcription of transporters may be induced by drugs or disease states. Expression of transporters may change with gestational age.41
Pinocytosis
Large macromolecules (e.g., proteins that exhibit negligible diffusion properties) can cross cell membranes via the process of pinocytosis (a type of endocytosis). Pinocytosis is an energy-requiring process in which the cell membrane invaginates around the macromolecule. Although the contents of pinocytotic vesicles are subject to intracellular digestion, electron microscopic studies have demonstrated that vesicles can move across the cytoplasm and fuse with the membrane at the opposite pole. This appears to be the mechanism by which immunoglobulin G is transferred from the maternal to the fetal circulation.36
Other Factors That Influence Placental Transport
Other factors that affect maternal-fetal exchange include (1) maternal and fetal blood flow, (2) placental binding, (3) placental metabolism, (4) diffusion capacity, (5) maternal and fetal plasma protein binding, and (6) gestational age (the placenta is more permeable in early pregnancy).42 Lipid solubility, pH gradients between the maternal and fetal environments for certain basic drugs (“ion trapping”), and alterations in maternal or fetal plasma protein concentrations found in normal pregnancy43 and other disease states (e.g., preeclampsia) may also alter placental transport.
Transfer of Respiratory Gases and Nutrients
Oxygen
Our knowledge of oxygen transfer physiology in the placenta is largely derived from the lung. The placenta must provide approximately 8 mL O2/min/kg fetal body weight for fetal growth and development, while adults only require 3 to 4 mL O2/min/kg at rest.44 As the “lung” for the fetus, the placenta has only one fifth the oxygen transfer efficiency of the adult lung.45 The human lung, with a surface area of 50 to 60 m2 and a thickness of only 0.5 µm, has a very large oxygen diffusion capacity; in comparison, the placenta has a lower diffusion capacity because of its smaller surface area of 16 m2 and a thicker membrane of 3.5 µm. Furthermore, 16% of uterine blood flow and 6% of umbilical blood flow are shunted through the placenta.18
Oxygen transfer across the placenta depends on the membrane surface area, membrane thickness, oxygen partial pressure gradient between maternal blood and fetal blood, affinity of maternal and fetal hemoglobin, and relative maternal and fetal blood flows. As physically dissolved oxygen diffuses across the villous membranes, bound oxygen is released by maternal hemoglobin in the intervillous space and diffuses across the placenta. Several factors affect the fetal blood PO2 once it reaches equilibration in the villi end-capillaries. First, the concurrent and countercurrent arrangements of maternal and fetal blood flow play a key role for placental oxygen transfer in various species. The almost complete equilibration of maternal and fetal PO2 values suggests that a concurrent (or parallel) relationship between maternal blood and fetal blood exists within the human placenta (Figure 4-6),18,46 although others have described a multivillous, cross-current flow pattern.
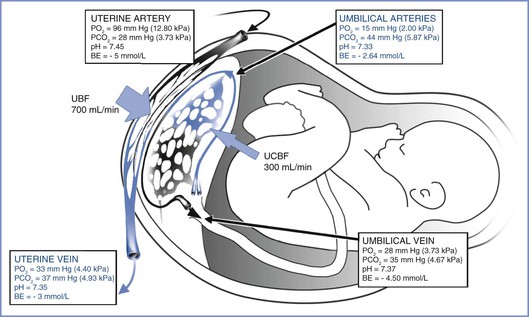
FIGURE 4-6 The concurrent relationship between the maternal and fetal circulations within the placenta and the way this arrangement affects gas transfer. These values were obtained from patients’ breathing room air during elective cesarean delivery. BE, base excess; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; UBF, uterine blood flow; UCBF, umbilical cord blood flow. (Blood gas data from Ramanathan S. Obstetric Anesthesia. Philadelphia, Lea & Febiger, 1988:27. Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Much of the literature in this area is based on animal studies. Because the functional anatomy of the placenta in many mammals involves more layers than the human placenta (e.g., the epitheliochorial placenta of the sheep has three layers), results of animal models can only provide evidence for trends, not values, in humans.
In humans, oxygen solubility is 10−4 M in plasma and 10−2 M in hemoglobin; thus, 99% of the oxygen content in blood is bound to hemoglobin. With an inspired oxygen fraction of 1.0, the maximum maternal arterial PO2 was 425 mm Hg, but the fetal umbilical venous PO2 was only 47 mm Hg, indicating a low diffusion capacity of oxygen across the placenta.47 In addition, the placenta receives less than 50% of the fetal cardiac output, and blood returning from the placenta admixes with the nonoxygenated blood in the fetal inferior vena cava, thus limiting fetal arterial PO2.
Although some have called the human placenta “diffusion limited” because of the decreased ability of oxygen to cross the intervillous membrane, the delivery of oxygen to the fetus is predominantly flow limited. Maternal delivery of blood (i.e., oxygen) to the uterus is the predominant factor controlling fetal oxygen transfer. The high fetal hemoglobin concentration (17 mg/dL) accounts for the large oxygen content and the net delivery of large quantities of oxygen to the fetus. Fetal hemoglobin has a higher oxygen affinity and therefore a lower partial pressure at which it is 50% saturated (P50: 18 mm Hg) than maternal hemoglobin (P50: 27 mm Hg). This gradient produces a “sink” effect that enhances oxygen uptake by fetal red blood cells, keeping fetal PO2 lower and promoting the transfer of additional oxygen across the placenta (see Figure 5-7). The Bohr effect also augments the transfer of oxygen across the placenta. Specifically, fetal-maternal transfer of carbon dioxide makes maternal blood more acidic and fetal blood more alkalotic. These alterations of pH cause shifts in the maternal and fetal oxyhemoglobin dissociation curves, further enhancing the maternal oxygen transfer to the fetus in what is termed the “double” Bohr effect. This accounts for 2% to 8% of the transplacental transfer of oxygen.48
The placenta normally has a 50% reserve for changes in maternal or fetal blood flow by increasing venous extraction, a mechanism similar to that in adults. Based on umbilical venoarterial difference, human fetal oxygen uptake at term is 0.25 mmol/kg/min.49 The metabolic activity of the placenta itself consumes up to 40% of the oxygen uptake. Placental oxygen consumption is stable even with changes in maternal blood pressure and PO2; 30% of placental oxygen is used for protein synthesis and almost 30% for Na+/K+ ATPase. The human placenta has a villous structure, which may be an adaptation for greater maternal flow and thus oxygen delivery, but at the expense of a smaller surface area and cross-current exchange mechanism.50 However, the placenta does change in response to chronic hypoxia found at high altitudes, with an increased capillary volume and decreased capillary thickness providing a near-doubling of the oxygen diffusion capacity.51
Carbon Dioxide
The transfer of CO2 occurs through a number of different forms, including dissolved CO2, carbonic acid (H2CO3), bicarbonate ion (HCO3−), carbonate ion (CO32−), and carbaminohemoglobin. Equilibrium between CO2 and HCO3− is maintained by a reaction catalyzed by carbonic anhydrase in red blood cells. The PCO2 gradient between fetal and maternal blood (i.e., 40 versus 34 mm Hg, respectively) drives fetal-maternal transfer. Carbon dioxide is 20 times more diffusible than oxygen and readily crosses the placenta,52 although dissolved CO2 is the form that actually crosses. The rapid movement of CO2 from fetal capillary to maternal blood invokes a shift in the equilibrium of the carbonic anhydrase reaction (La Chatelier’s principle) that produces more CO2 for diffusion. The transfer of CO2 is augmented further by the production of deoxyhemoglobin in the maternal blood, which has a higher affinity for CO2 than oxyhemoglobin (the Haldane effect). The resulting affinity may account for as much as 46% of the transplacental transfer of carbon dioxide.46 Although a significant fetal-maternal concentration gradient exists for HCO3−, its charged nature impedes its transfer and contribution to CO2 transport except as a source for CO2 production through the carbonic anhydrase reaction.53
Glucose
Simple diffusion alone cannot account for the amount of glucose required to meet the demands of the placenta and fetus. To assist the movement of glucose down its concentration gradient, stereospecific-facilitated diffusion systems have been described with glucose transporters such as GLUT1 and GLUT3; the system is independent of insulin, a sodium gradient, or cellular energy.54 Insulin does not cross the placenta; however, insulin receptors in the maternal side of the syncytiotrophoblast regulate nutrient transport through a signaling cascade involving the mammalian target of rapamycin complex (mTORC). Nutrient sensors for glucose, amino acids, oxygen, cytokines, growth factors, and energy levels stimulate mTORC1, a key sensing and signaling protein in the syncytiotrophoblast that regulates nutrient transport and growth.55
Amino Acids
Concentrations of amino acids are highest in the placenta, followed by umbilical venous blood and then maternal blood. The maternal-fetal transplacental transfer of amino acids is an active process that occurs principally through a linked translocation with sodium. The energy required for this transfer comes from the large sodium gradient established by the Na+/K+ ATPase pump, resulting in increased intracellular concentrations of amino acids, which then “leak” down their gradients into the fetal circulation. This transport mechanism may not apply to all amino acids and may be susceptible to inhibitors. Transport also occurs via transport exchangers of amino acids on both maternal and fetal sides of the placenta as well as facilitated diffusion. Pregnancies with fetal growth restriction (also known as intrauterine growth restriction) have reduced amino acid transport with an inability to increase transport in spite of higher maternal levels of essential amino acids than occur in healthy pregnancies.56
Fatty Acids
Free fatty acids readily cross the human, but not ovine, placenta. The essential fatty acids, linoleic and alpha-linolenic acid, must be transferred across the placenta. Lipid transfer to the fetus reaches a peak of 7 g/day at term. The placental basal membrane has specific binding sites for very low-density, low-density, and high-density lipoproteins. Lipase activity in the placenta is responsible for converting triglycerides to nonessential fatty acids. The placenta does not elongate fatty acid chains, whereas the fetus does. Fatty acid transport occurs primarily by simple diffusion; however, fatty acid–binding proteins (FABPpm, FAT/CD36, and FATP), which facilitate transport, have been discovered. Nonessential fatty acids are albumin bound and may displace other protein-bound substances.57
Drug Transfer
Placental permeability and pharmacokinetics determine the fetal exposure to maternal drugs. Animal models (e.g., pregnant ewes, guinea pigs) have been used to assess the placental transport of drugs; however, interspecies differences in placental anatomy and physiology limit the application of these data to humans.58 Human placental transport mechanisms have been studied in placental slices, isolated villi, membrane vesicles, homogenates, and tissue culture cells. The direct application of these data, however, is in question because these methods do not account for the dual (i.e., maternal and fetal) perfusion of the intact placenta in situ.58
The inaccessibility of the placenta in situ and concerns for maternal and fetal safety have limited direct studies of the placenta in humans. Data regarding the transplacental transfer of anesthetic agents have been extrapolated primarily from single measurements of drug concentrations in maternal and umbilical cord blood samples obtained at delivery. Most studies have reported fetal-to-maternal (F/M) ratios of drug concentration. In these studies, the umbilical vein blood concentration represents the fetal blood concentration of the drug. Maternal and fetal concentrations of a drug are influenced by drug metabolism in the mother, the placenta, and the fetus and also by changes during delivery (e.g., altered uteroplacental blood flow).58
A dual-perfused, in vitro human placental model has been developed to allow for the independent perfusion of the maternal and fetal sides of the placenta and thereby investigate maternal-fetal (or fetal-maternal) transport.58 Equilibration studies (i.e., recirculating maternal and fetal perfusates) using this model are not directly applicable to the placenta in vivo. However, when a non-recirculating design is used, steady-state drug clearance can be determined for either direction (maternal to fetal or fetal to maternal) and may have direct clinical application. This method has been used to assess the placental transfer of anesthetic agents (e.g., thiopental,59 methohexital,60 propofol,61 bupivacaine,62 ropivacaine,63 alfentanil,64 and sufentanil65,66). Transfer across the placenta may be reported as drug clearance or as a ratio referred to as the transfer index (i.e., drug clearance/reference compound clearance). The use of a transfer index allows for interplacental comparisons by accounting for differences between placentas (e.g., lobule sizes). Commonly used reference compounds are either flow limited (e.g., antipyrine, tritiated water) or membrane limited (e.g., creatinine). These studies have enhanced our understanding of the placental transfer of anesthetic drugs (Box 4-1).
Pharmacokinetic Principles
Factors affecting drug transfer across the human placenta include lipid solubility, protein binding, tissue binding, pKa, pH, and blood flow (Table 4-1). High lipid solubility may readily enable cell membrane (lipid bilayer) penetration but may also cause the drug (e.g., sufentanil) to be trapped within the placental tissue.66 Highly protein-bound drugs are affected by the concentration of maternal and fetal plasma proteins, which varies with gestational age and disease. Some drugs (e.g., diazepam) bind to albumin, whereas others (e.g., sufentanil, cocaine) bind predominantly to α1-acid glycoprotein (AAG) (Table 4-2). Although the free, unbound fraction of drug equilibrates across the placenta, the total drug concentration is greatly affected by both the extent of protein binding and the quantity of maternal and fetal proteins; fetal blood typically contains less than half the concentration of AAG than maternal blood.67 One study of the placental transfer of sufentanil in vitro noted different results when fresh frozen plasma, rather than albumin, was used as a perfusate. Albumin binds primarily acidic and lipophilic compounds, whereas AAG binds more basic compounds. Indeed, the fetal levels of both albumin and AAG increase from first trimester to term.68
TABLE 4-1
Factors Affecting Placental Transfer of Drug (Maternal to Fetal)
| Increased Transfer | Decreased Transfer | |
| Size: molecular weight (Da) | <1000 | >1000 |
| Charge of molecule | Uncharged | Charged |
| Lipid solubility | Lipophilic | Hydrophilic |
| pH versus drug pKa* | Higher proportion of un-ionized drug in maternal plasma | Higher proportion of ionized drug in maternal plasma |
| Placental efflux transporter† proteins (e.g., P-glycoprotein) | Absent | Present |
| Binding protein type | Albumin (lower binding affinity)‡ | α1-Acid glycoprotein (AAG) (higher binding affinity) |
| Free (unbound) drug fraction | High | Low |
* The pH relative to the pKa determines the amount of drug that is ionized and un-ionized in both maternal and fetal plasma. Fetal acidemia enhances the maternal-to-fetal transfer (i.e., “ion trapping”) of basic drugs such as local anesthetics and opioids.
† The efflux transporter pumps substances in a fetal-to-maternal direction.
‡ Note: albumin concentration is higher in the fetus, and AAG concentration is higher in the maternal circulation.
Da, dalton.
TABLE 4-2
Concentrations of Proteins That Bind Drugs
| Maternal | Umbilical Cord | |
| Albumin | 33.1 g/L | 37.1 g/L* |
| Alpha1-acid glycoprotein (AAG) | 0.77 g/L | 0.26 g/L* |
* P < .05.
Data from Sudhakaran S, Rayner CR, Li J, et al. Differential protein binding of indinavir and saquinavir in matched maternal and umbilical cord plasma. Br J Clin Pharmacol 2006; 63:315-21.
The pKa of a drug determines the fraction of drug that is non-ionized at physiologic pH. Thus, fetal acidemia greatly enhances the maternal-fetal transfer (i.e., “ion trapping”) of many basic drugs, such as local anesthetics and opioids (Figure 4-7) (see Chapter 13).69 Most anesthetic drugs are passively transferred, with the rate of blood flow (hence drug delivery) affecting the amount of drug that crosses the placenta.70 One of the authors (M.I.Z.) has used the in vitro perfused human placenta model to perform a number of studies of the placental transfer of opioids (Table 4-3).
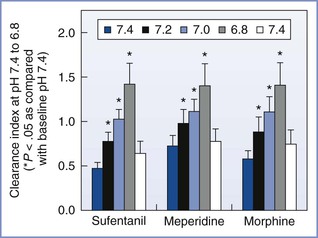
FIGURE 4-7 The effects of changes in fetal pH on the transfer of opioids during in vitro perfusion of the human placenta. This figure demonstrates the “ion trapping” of opioids, which is similar to that of local anesthetics. Clearance index = clearance drug/clearance creatinine (a reference compound). (Modified from Zakowski MI, Krishna R, Grant GJ, Turndorf H. Effect of pH on transfer of narcotics in human placenta during in vitro perfusion. Anesthesiology 1995; 85:A890.)
TABLE 4-3
Opioid Transfer during In Vitro Perfusion of the Human Placenta
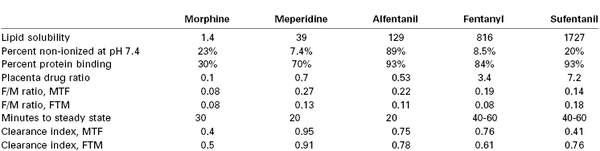
Clearance index, clearance drug/clearance antipyrine (a flow-limited reference compound); FTM, fetal-to-maternal (direction); MTF, maternal-to-fetal (direction); placenta drug ratio, placenta drug concentration/g placental tissue/maternal drug concentration.
Data from non-recirculated experiments, using perfusate Media 199 without protein, with maternal flow 12 mL/min and fetal flow 6 mL/min.64,66,102,105,110
Inhalation Anesthetic Agents
The lipid solubility and low molecular weight of inhalation anesthetic agents facilitate rapid transfer across the placenta. A prolonged induction-to-delivery interval results in lower Apgar scores.71
When administered during cesarean delivery, halothane is detectable in both umbilical venous blood and arterial blood within 1 minute. Even with a relatively short induction-to-delivery time, an F/M ratio of 0.71 to 0.87 is established.72,73 Isoflurane distributes rapidly across the placenta during cesarean delivery, resulting in an F/M ratio of approximately 0.71.73 Sevoflurane has an F/M ratio of 0.38, similar to that of other inhaled agents.74 Sevoflurane causes a dose-dependent vasodilation of the placental vessels that is not mediated by nitric oxide.75 To our knowledge, there are no published data regarding the placental transfer of desflurane.
Nitrous oxide also rapidly crosses the placenta, with an F/M ratio of 0.83 within 3 minutes.76 Maternal administration of nitrous oxide decreases fetal central vascular resistance by 30%,77 and a prolonged induction to delivery interval may cause neonatal depression. Diffusion hypoxia may occur during the rapid elimination of nitrous oxide from the neonate; supplemental oxygen for any neonate exposed to nitrous oxide immediately before delivery appears prudent.
Induction Agents
The lipophilic characteristics that make anesthetic agents ideal for the induction of anesthesia also enhance their transfer across the placenta. The understanding of the transplacental transfer of these drugs is better than for any other group of anesthetic agents.
Propofol
A 2- to 2.5-mg/kg bolus dose of propofol, the most widely used induction agent for general anesthesia, results in a mean F/M ratio between 0.65 and 0.85.78–80 A bolus dose of 2 mg/kg followed by a continuous infusion of 6 mg/kg/h or 9 mg/kg/h of propofol resulted in mean F/M ratios of 0.50 and 0.54, respectively.81 These F/M ratios are similar to those found when propofol is given in early gestation (at 12 to 18 weeks).82 Propofol may have sedative effects on the neonate; in a randomized trial of propofol compared with thiopental for the induction of anesthesia for elective cesarean delivery, the maternal administration of propofol (2.8 mg/kg) resulted in lower 1- and 5-minute Apgar scores than thiopental (5 mg/kg).83 Plasma levels of propofol in the neonate depend on the maternal dose and the time elapsed between drug administration and delivery of the neonate. In one study, when delivered within 10 minutes of induction, neonates whose mothers were given propofol (2 mg/kg) had an average umbilical vein propofol concentration of 0.32 µg/mL.84
Several factors that affect propofol transfer have been investigated with in vitro human placental perfusion models.85–87 Increased maternal blood flow and reduced protein binding increase both placental tissue uptake and transplacental transfer of propofol.81 Propofol is highly protein bound to albumin. Thus, altered albumin concentrations in mother or fetus will affect transplacental transfer and the total, but not free, concentration in umbilical vein.87 Propofol causes calcium channel-dependent vasodilation of human placental vessels in vitro.88
Ketamine
Ketamine, a phencyclidine derivative, rapidly crosses the placenta. Ketamine 2 mg/kg reached a mean F/M ratio of 1.26 in as little as 97 seconds when administered to the mother for vaginal delivery.89 In a sheep study, fetal concentration was 25% less than maternal concentration at 10 minutes.90
Etomidate
Etomidate, a carboxylated imidazole, has long been used for the induction of general anesthesia in obstetric patients. A dose of 0.3 to 0.4 mg/kg administered for cesarean delivery resulted in an F/M ratio of approximately 0.5,91 which is similar to the ratio found in sheep.92
Barbiturates
Previously a popular agent for the induction of general anesthesia in parturients, thiopental is the most extensively studied barbiturate. An extremely short-acting agent, it quickly appears in umbilical venous blood after maternal injection, with a mean F/M ratio between 0.4 and 1.1.93,94 The high F/M ratios suggest that thiopental is freely diffusible; however, there is wide intersubject variability in umbilical cord blood concentration at delivery. Both maternal-fetal and fetal-maternal transfer of thiopental are strongly influenced by maternal and fetal protein concentrations.59
The rapid transfer of the oxybarbiturate methohexital into the fetal circulation, with simultaneous peak concentrations in maternal blood and fetal blood, has been demonstrated by in vivo studies.95 Human in vitro placental perfusion studies in which the concentration of albumin was equal in the maternal and fetal perfusates confirm that methohexital rapidly crosses the placenta in both maternal-to-fetal and fetal-to-maternal directions, with transfer indices of less than 0.5 at 30 minutes.60
Dexmedetomidine
In humans, dexmedetomidine, an α2-adrenergic agonist, has an F/M ratio of 0.12, with evidence of significant placental tissue binding due to high lipophilicity.96 At 10 minutes, fetal concentration of medetomidine was about 28% less than maternal concentration in the sheep model.90
Benzodiazepines
Highly un-ionized, lipophilic, and 95% protein-bound diazepam is associated with an F/M ratio of 1 within minutes of maternal administration and a ratio of 2 at 60 minutes after maternal administration.97 Less lipophilic, lorazepam requires almost 3 hours after administration for the F/M ratio to reach unity.98 Midazolam is more polar, with an F/M ratio of 0.76 at 20 minutes after administration. The F/M ratio of midazolam, unlike that of other benzodiazepines, decreases rapidly; by 200 minutes it is only 0.3.99
Opioids
Meperidine has been associated with neonatal central nervous system and respiratory depression. Intravenous administration results in rapid transfer across the human placenta within 90 seconds after maternal administration.100 F/M ratios for meperidine may exceed 1.0 after 2 to 3 hours; maternal levels fall more rapidly than fetal levels because of the mother’s greater capacity for metabolism of the drug.101 This same time interval is associated with the greatest likelihood of neonatal depression, in part because of the active drug metabolite normeperidine. Human placental perfusion studies in vitro have demonstrated rapid placental transfer in both maternal-to-fetal and fetal-to-maternal directions with equal clearance profiles, minimal placental tissue binding, and no placental drug metabolism.102 As maternal levels fall, the meperidine and normeperidine will transfer from the fetus back to the mother, correlating with the clinically observed decrease in neonatal sedation 4 hours after maternal administration.
Morphine also rapidly crosses the placenta. One study demonstrated a mean F/M ratio of 0.61, a mean umbilical venous blood concentration of 25 ng/mL, and a significant reduction in the biophysical profile score (primarily as a result of decreased fetal breathing movements and fewer fetal heart rate accelerations) within 20 to 30 minutes of maternal administration.103 Intrathecal administration of morphine results in a high F/M ratio (0.92), although the absolute fetal concentrations are less than those associated with fetal and neonatal side effects.104 Human placental perfusion studies in vitro have demonstrated that morphine, which is a hydrophilic compound, exhibits membrane-limited transfer with a low placental tissue content and a fast washout.105 Concurrent naloxone administration does not affect the placental transfer of morphine.106
Fentanyl and its analogues are administered via the epidural, intrathecal, and intravenous routes. Fentanyl has a high lipophilicity and albumin binding (74%).107 Maternal epidural administration results in an F/M ratio between 0.37 and 0.57.108 During early pregnancy, fentanyl is rapidly transferred and may be detected not only in the placenta but also in the fetal brain.109 Perfusion of the human placenta in vitro results in rapid transfer in both maternal-to-fetal and fetal-to-maternal directions, with the placenta acting as a moderate drug depot.110,111
Despite a relatively low F/M ratio (0.30),112 maternal administration of alfentanil has been associated with a reduction of 1-minute Apgar scores when administered to the mother immediately before the induction of anesthesia.113 Perfusion of the human placenta in vitro shows rapid and symmetric maternal-fetal and fetal-maternal transfers of alfentanil, with low placental drug uptake and rapid washout.64
Maternal administration of sufentanil results in a high F/M ratio, 0.81. Compared with fentanyl, sufentanil has higher lipid solubility and more rapid uptake by the central nervous system, resulting in less systemic absorption from the epidural space; lower maternal and umbilical vein concentrations reduce fetal exposure and the associated potential risk for neonatal respiratory depression.108 Human placental perfusion studies in vitro have confirmed the rapid transplacental transfer of sufentanil, which is influenced by differences in maternal and fetal plasma protein binding and fetal pH. High placental tissue uptake suggests that the placenta serves as a drug depot.65,66
Remifentanil undergoes rapid placental transfer. During cesarean delivery, average F/M ratios were 0.88 when remifentanil was administered by intravenous infusion (0.1 µg/kg/min) during epidural anesthesia114 and 0.73 when it was given as a single bolus (1 µg/kg) at induction of general anesthesia.115 Excessive maternal sedation without adverse neonatal effects has been reported with the use of remifentanil during labor; presumably, the rapid metabolism of remifentanil by nonspecific esterases (context-sensitive half-time of 3 minutes) results in minimal fetal exposure.116 When remifentanil was used for patient-controlled analgesia during labor, bolus doses of 0.5 µg/kg resulted in an F/M ratio of approximately 0.5 and a 20% incidence of fetal heart rate changes.117 With continuous infusion of 0.33 µg/kg/min, the F/M ratio in plasma rapidly reached 0.1 to 0.3 in sheep.116
The systemic administration of an opioid agonist/antagonist for labor analgesia has been associated with few maternal, fetal, and neonatal side effects. Both butorphanol and nalbuphine rapidly cross the placenta, with mean F/M ratios of 0.84 and 0.74 to 0.97, respectively.118,119 In one study, maternal administration of nalbuphine resulted in “flattening” of the fetal heart rate tracing in 54% of cases.119
Local Anesthetics
Local anesthetic agents readily cross the placenta (see Chapter 13). The enantiomers of bupivacaine cross the placenta at the same rate as racemic bupivacaine.120
Muscle Relaxants
As fully ionized, quaternary ammonium salts, muscle relaxants do not readily cross the placenta; however, single doses of muscle relaxants can result in detectable fetal blood concentrations. Maternal administration of muscle relaxants for the induction of general anesthesia for cesarean delivery rarely affects neonatal muscle tone at delivery.
After a standard induction dose, succinylcholine is not detectable in umbilical venous blood at delivery; maternal doses larger than 300 mg are required before the drug can be detected.121 Neonatal neuromuscular blockade can occur when high doses are given repeatedly or when both the parturient and fetus are homozygous for atypical pseudocholinesterase deficiency.122
The administration of nondepolarizing muscle relaxants results in low F/M ratios: 0.19 to 0.26 for pancuronium,123–125 0.06 to 0.11 for vecuronium,125,126 0.16 for rocuronium,127 and 0.07 for atracurium.128 The F/M ratio may be the result of expedient fetal/neonatal blood sampling; in a study in rats, the F/M ratio of vecuronium nearly doubled as the induction-to-delivery interval increased from 180 to 420 seconds.126 No published study has investigated the placental transfer of the atracurium isomer cisatracurium. However, laudanosine, a metabolite of atracurium and cisatracurium, has an F/M ratio of 0.14.129
Although nondepolarizing muscle relaxant transfer rates are low, the fetal blood concentrations increase over time.126 Fetal blood concentrations of nondepolarizing muscle relaxants can be minimized by giving succinylcholine to facilitate intubation, followed by a nondepolarizing muscle relaxant to maintain paralysis.124
Anticholinergic Agents
The placental transfer rate of anticholinergic agents directly correlates with their ability to cross the blood-brain barrier. Atropine is detected in the umbilical circulation within 1 to 2 minutes of maternal administration, and an F/M ratio of 0.93 is attained at 5 minutes.130 Scopolamine also crosses the placenta easily; intramuscular administration results in an F/M ratio of 1.0 within 55 minutes.131 By contrast, glycopyrrolate is poorly transferred across the placenta, with maternal intramuscular administration resulting in a mean F/M ratio of only 0.22.132 Maternal intravenous administration of glycopyrrolate does not result in a detectable fetal hemodynamic response, whereas atropine and scopolamine may directly increase fetal heart rate.
Anticholinesterase Agents
Neostigmine, pyridostigmine, and edrophonium are quaternary ammonium compounds that are ionized at physiologic pH and consequently undergo limited transplacental transfer.133 For example, maternal administration of neostigmine does not reverse atropine-induced fetal tachycardia. However, small amounts of these agents do cross the placenta, and fetal bradycardia after maternal administration of neostigmine and glycopyrrolate has been reported.134 Because neostigmine may cross the placenta to a greater extent than glycopyrrolate, the combination of neostigmine and atropine should be considered for the reversal of nondepolarizing muscle relaxants in pregnant patients.134 Physostigmine crossed the placenta in 9 minutes and reversed the fetal heart rate effect of scopolamine.135
Antihypertensive Agents
Beta-adrenergic receptor antagonists have been commonly used as antihypertensive agents in pregnancy, despite early investigations noting an association with fetal growth restriction and neonatal bradycardia, hypoglycemia, and respiratory depression.136 Although a single dose of propranolol administered 3 hours before cesarean delivery has been shown to lead to an F/M ratio of 0.26,137 long-term administration during pregnancy results in F/M ratios greater than 1.0.138 Maternal administration of atenolol and metoprolol leads to mean F/M ratios of 0.94 and 1.0, respectively.139,140
Labetalol, the most commonly used antihypertensive during pregnancy, has a low F/M ratio of 0.38 with long-term oral administration, despite reports of mild neonatal bradycardia.141,142 National data from Denmark showed that use of beta-adrenergic receptor antagonists during pregnancy, including labetalol, approximately doubles the risk for small-for-gestational-age preterm births and for perinatal mortality, even after adjusting for preeclampsia.143 Preterm hypertensive women receiving labetalol had no acute change in umbilical artery or fetal middle cerebral resistance indices of flow.144
The ultra-short-acting beta-adrenergic receptor antagonist esmolol has been used to attenuate the hypertensive response to laryngoscopy and intubation. A mean F/M ratio of 0.2 after maternal administration of esmolol was observed in gravid ewes.145 However, a few cases of significant and prolonged fetal bradycardia requiring the performance of emergency cesarean delivery have been reported.146
Clonidine and methyldopa act through the central stimulation of α2-adrenergic receptors; studies have reported mean F/M ratios of 0.89147 and 1.17,148 respectively, for these agents. In concentrations likely to be present in maternal blood during clinical use, magnesium and nifedipine, but not clonidine, produce fetal vasodilation in human placental perfusion studies in vitro.149 Phenoxybenzamine, an alpha-adrenergic receptor antagonist, is commonly used to treat hypertension in patients with pheochromocytoma and has an F/M ratio of 1.6 with long-term maternal administration.150
Direct-acting vasodilators are used for short-term management of severe hypertension in pregnant women. Administration of hydralazine, which is often given to lower blood pressure in preeclampsia, results in an F/M ratio of 1.0151 and causes fetal vasodilation in in vitro studies.152 Hydralazine increased the umbilical artery resistance index, indicating vasodilation, in hypertensive women.144
Sodium nitroprusside is lipid soluble, rapidly crosses the placenta, and can produce cyanide as a byproduct.153 Sodium thiosulfate, the agent used to treat cyanide toxicity, does not cross the placenta in gravid ewes; it can be used to treat fetal cyanide toxicity by lowering maternal cyanide levels, thereby enhancing fetal-maternal transfer of cyanide.154
Glyceryl trinitrate (nitroglycerin) crosses the placenta to a limited extent, with an F/M ratio of 0.18, and results in minimal changes in fetal umbilical blood flow, blood pressure, heart rate, and blood gas measurements in gravid ewes.155 However, dinitrate metabolites found in both maternal and fetal venous blood indicate the capacity for placental biotransformation.156 Indeed, placental tissue production of nitric oxide enhances the uterine relaxation caused by nitroglycerin in vivo.157 In one in vitro study, in which prostaglandin F2α was used to create fetal vasoconstriction, the following order of nitrovasodilator compound potency was observed: glyceryl trinitrate ≥ sodium nitroprusside ≥ sodium nitrite (NaNO2) ≥ S-nitroso-N-acetylpenicillamine (SNAP) = S-nitroso-N-glutathione (SNG).158 SNG and NaNO2 were significantly more potent under conditions of low oxygen tension. The antioxidants cysteine, glutathione, and superoxide dismutase significantly enhanced the vasodilatory effects of NaNO2 only.158
Placental transfer of angiotensin-converting enzyme inhibitors may adversely affect fetal renal function. Enalaprilat rapidly crosses the placenta, and its maternal administration in high doses resulted in a 20% reduction in fetal arterial pressure in rhesus monkeys.159
Vasopressor Agents
Vasopressor agents are often administered to prevent or treat hypotension during the administration of neuraxial anesthesia in obstetric patients. Ephedrine readily crosses the placenta and results in an F/M ratio of approximately 0.7.160 In an in vitro human perfusion model that required supraphysiologic doses to obtain any effect, phenylephrine increased placental arterial pressure, but less so than ephedrine, whereas epinephrine, norepinephrine, and methoxamine had no effect.161
Ephedrine possesses 10 times greater lipid solubility than phenylephrine, with F/M ratios of 1.1 versus 0.17, respectively, in humans.162 Indeed, when either ephedrine or phenylephrine was given during spinal anesthesia for cesarean delivery, the ephedrine group had lower pH and base excess, higher PCO2, and higher glucose, lactate, epinephrine, and norepinephrine concentrations in umbilical arterial blood than the phenylephrine group.162 These differences may be due to the beta-adrenergic agonist effects of ephedrine in the fetus.162,163
Cocaine, a common drug of abuse during pregnancy (see Chapter 54), has potent vasoconstrictor activity. Human placenta perfusion studies in vitro have demonstrated the rapid transfer of cocaine in both maternal-to-fetal and fetal-to-maternal directions; transfer is constant over a wide range of concentrations.164 The active cocaine metabolites norcocaine and cocaethylene, but not the inactive metabolite benzoylecgonine, are also rapidly transferred across the placenta.165 Chronic maternal exposure to cocaine increases fetal concentrations; however, they remain lower than maternal peak levels.166
In a study using the in vitro dually perfused human placental lobule, fetal-side administration of vasoconstrictors was found to raise fetal placental perfusion pressure, thus causing a shift of fluid from the fetus to the maternal circulation.167
Anticoagulants
Anticoagulation therapy is often necessary during pregnancy. Maternal administration of warfarin in the first trimester results in placental transfer to the fetus, causing a higher rate of fetal loss and congenital anomalies.168 After the first trimester, warfarin may be used in the setting of stroke or mechanical heart valves.169 In contrast, heparin does not appear to cross the placenta, as measured by neonatal coagulation studies and the measurement of radiolabeled heparin in fetal lambs.170 Low-molecular-weight heparin appears to have limited placental transfer; maternal administration of enoxaparin does not alter fetal anti-IIa or anti-Xa activity.171 Even when enoxaparin or fondaparinux (a pentasaccharide that selectively inhibits factor Xa) was given at doses used for acute thromboembolic therapy, human placental perfusion studies in vitro demonstrated no placental transfer.172,173 Several case reports discussed use of direct thrombin inhibitors as early as 9 weeks’ gestation with successful delivery of normal neonates.174,175 Antiplatelet therapy (e.g., aspirin, clopidogrel) has been used successfully in the first trimester in dual therapy for coronary artery disease in the setting of drug-eluting stents.176 Abciximab, a glycoprotein IIb/IIIa platelet inhibitor, did not transfer across the in vitro perfused human placenta but did bind to the trophoblastic layer of the placenta.177
Drug Delivery Systems
New drug delivery systems may influence drug transfer and distribution across the human placenta. Liposome encapsulation, depending on the type and ionic charge, can affect placental transfer; anionic and neutral liposomes increase placental transfer, whereas cationic liposomes decrease placental transfer and placental tissue uptake.178 Liposome encapsulation of valproic acid significantly reduces drug transfer and placental uptake.179
Disease States
Disease states, such as diabetes, may affect the placental transfer of drugs. Glyburide, a second-generation sulfonylurea, is partially dependent on a P-glycoprotein active transport mechanism and demonstrates a lower F/M ratio (0.3) than the first-generation agents, even in the presence of a P-glycoprotein inhibitor.180 A high level of protein binding (99.8%) may also contribute to the low transplacental transfer of glyburide; when protein levels are reduced in vitro, higher transfer rates are observed.181,182 Some investigators have speculated that the thickened placenta found in diabetic patients is a cause of low transfer rates; however, no difference in maternal-fetal transfer of metformin has been observed between placentas from parturients with gestational diabetes and those from healthy parturients.183
Gestational age may alter placental transfer, although the direction of the alteration requires further evaluation. Although traditional belief holds that placentas from younger fetuses are more likely to transfer substances, one study has demonstrated that methadone transfer is 30% lower in human preterm placentas than in term placentas.184 Dexamethasone and betamethasone, corticosteroids that are often given to accelerate fetal lung maturity, increase ABCB1 gene expression fourfold. ABCB1 is an efflux transporter protein; hence increased gene expression may increase fetal-maternal transfer of substrate molecules.185
Oxidative stress increases in preeclampsia, fetal growth restriction, and diabetes. New studies have shown that nitrative stress, the covalent modification of proteins and DNA by peroxynitrite (formed by nitric oxide reacting with superoxide), also occurs.186 Peroxynitrite reacts with tyrosine to form nitro-tyrosine, a negatively charged group, which may mimic phosphorylation. Nitration may result in loss or gain of protein function.
Vitamin D helps modulate cytokines, inflammation, and insulin sensitivity, and a deficiency leads to increased risk for gestational diabetes and preeclampsia.187
Placental Pathology
There has been a growing interest in the clinicopathologic correlation between placental abnormalities and adverse obstetric outcomes. In some cases, a skilled and systematic examination of the umbilical cord, fetal membranes, and placenta may provide insight into antepartum pathophysiology; in most of these cases, examination of the placenta confirms the clinical diagnosis (e.g., chorioamnionitis). When adverse outcomes occur, often the “disorder that was not suspected clinically may be revealed by placental pathology.”188 Drugs may produce placental abnormalities (e.g., cocaine causes chorionic villus hemorrhage and villous edema).189 The significance of many findings (e.g., villous edema, hemorrhagic endovasculitis, chronic villitis), however, is unclear.
The following factors limit the assessment of placental pathology: (1) “the paucity of properly designed studies of adequate size with appropriate outcome parameters,”188 which impairs the correlation of placental abnormalities with adverse clinical outcomes; (2) the limited number of pathologists with expertise in the recognition and interpretation of subtle abnormalities of the placenta; and (3) the cost associated with a routine assessment of placental pathology.
References
1. Haynes DM. The human placenta: historical considerations. Lavery JP. The Human Placenta: Clinical Perspectives. Aspen Publishers: Rockville, MD; 1987:1–10.
2. Roseboom TJ, Watson ED. The next generation of disease risk: are the effects of prenatal nutrition transmitted across generations? Evidence from animal and human studies. Placenta. 2012;33(Suppl 2):e40–e44.
3. James JL, Carter AM, Chamley LW. Human placentation from nidation to 5 weeks of gestation. I. What do we know about formative placental development following implantation? Placenta. 2012;33:327–334.
4. Sadler TW, Langman J. Langman’s Medical Embryology. 12th edition. Lippincott Williams & Wilkins: Philadelphia; 2012.
5. Kaufmann P, Hans-Georg F. Placental development. Polin RA, Fox WW, Abman SH. Fetal and Neonatal Physiology. 3rd edition. Saunders: Philadelphia; 2004:85–96.
6. Krause BJ, Hanson MA, Casanello P. Role of nitric oxide in placental vascular development and function. Placenta. 2011;32:797–805.
7. Tuuli MG, Longtine MS, Nelson DM. Review: Oxygen and trophoblast biology—a source of controversy. Placenta. 2011;32(Suppl 2):S109–S118.
8. Novakovic B, Saffery R. The ever growing complexity of placental epigenetics—role in adverse pregnancy outcomes and fetal programming. Placenta. 2012;33:959–970.
9. Yuen RK, Penaherrera MS, von Dadelszen P, et al. DNA methylation profiling of human placentas reveals promoter hypomethylation of multiple genes in early-onset preeclampsia. Eur J Hum Genet. 2010;18:1006–1012.
10. Salafia CM, Popek PJ. Placental development and early pregnancy pathology. Sciarra JJ. Gynecology and Obstetrics. Lippincott Williams & Wilkins: Philadelphia; 2004.
11. Fowden AL, Moore T. Maternal-fetal resource allocation: co-operation and conflict. Placenta. 2012;33(Suppl 2):e11–e15.
12. Flexner LB, Gellhorn A. The comparative physiology of placental transfer. Am J Obstet Gynecol. 1942;43:965–974.
13. James E, Meschia G, Battaglia FC. A-V differences of free fatty acids and glycerol in the ovine umbilical circulation. Proc Soc Exp Biol Med. 1971;138:823–826.
14. Ramsey EM, Donner MW. Placental Vasculature and Circulation: Anatomy, Physiology, Radiology, Clinical Aspects: Atlas and Textbook. Saunders: Philadelphia; 1980.
15. Freese UE. The fetal-maternal circulation of the placenta. I. Histomorphologic, plastoid injection, and x-ray cinematographic studies on human placentas. Am J Obstet Gynecol. 1966;94:354–360.
17. Kaufmann P. Basic morphology of the fetal and maternal circuits in the human placenta. Contrib Gynecol Obstet. 1985;13:5–17.
18. Faber JJ, Thornburg KL. Placental Physiology: Structure and Function of Fetomaternal Exchange. Raven Press: New York; 1983.
19. Russo P. Maternal-fetal exchange of nutrients. Nutrition and Metabolism in Pregnancy: Mother and Fetus. Oxford University Press: New York; 1990:133–167.
20. Dawe GS, Tan XW, Xiao ZC. Cell migration from baby to mother. Cell Adh Migr. 2007;1:19–27.
21. Nassar D, Droitcourt C, Mathieu-d’Argent E, et al. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26:149–157.
22. Clifton VL, Stark MJ, Osei-Kumah A, Hodyl NA. Review: The feto-placental unit, pregnancy pathology and impact on long term maternal health. Placenta. 2012;33(Suppl):S37–S41.
23. Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487.
24. Chiu RW, Lo YM. Clinical applications of maternal plasma fetal DNA analysis: translating the fruits of 15 years of research. Clin Chem Lab Med. 2013;51:197–204.
25. Siler-Khodr TM. Endocrine and paracrine function of the human placenta. Polin RA, Fox WW, Abman SH. Fetal and Neonatal Physiology. 3rd edition. Saunders: Philadelphia; 2004:122–130.
26. Hill M, Parizek A, Cibula D, et al. Steroid metabolome in fetal and maternal body fluids in human late pregnancy. J Steroid Biochem Mol Biol. 2010;122:114–132.
27. Greiss FC. Uterine blood flow in pregnancy: an overview. Moawad AH, Lindheimer MD. Uterine and Placental Blood Flow. Masson Publishing: New York; 1982:19–26.
28. Sastry BV. Human placental cholinergic system. Biochem Pharmacol. 1997;53:1577–1586.
29. Jerat S, DiMarzo L, Morrish DW, Kaufman S. Adrenomedullin-induced dilation of human placental arteries is modulated by an endothelium-derived constricting factor. Regul Pept. 2008;146:183–188.
30. Roth JB, Thorp JA, Palmer SM, et al. Response of placental vasculature to high glucose levels in the isolated human placental cotyledon. Am J Obstet Gynecol. 1990;163:1828–1830.
31. Howard RB, Hosokawa T, Maguire MH. Hypoxia-induced fetoplacental vasoconstriction in perfused human placental cotyledons. Am J Obstet Gynecol. 1987;157:1261–1266.
32. Kuhn DC, Stuart MJ. Cyclooxygenase inhibition reduces placental transfer: reversal by carbacyclin. Am J Obstet Gynecol. 1987;157:194–198.
33. Myatt L, Brewer A, Brockman DE. The action of nitric oxide in the perfused human fetal-placental circulation. Am J Obstet Gynecol. 1991;164:687–692.
34. Coumans AB, Garnier Y, Supcun S, et al. The role of nitric oxide on fetal cardiovascular control during normoxia and acute hypoxia in 0.75 gestation sheep. J Soc Gynecol Investig. 2003;10:275–282.
35. Ramasubramanian R, Johnson RF, Downing JW, et al. Hypoxemic fetoplacental vasoconstriction: a graduated response to reduced oxygen conditions in the human placenta. Anesth Analg. 2006;103:439–442.
36. Atkinson DE, Boyd RDH, Sibley CP. Placental transfer. Neill JD, Plant TM, Pfaff DW, et al. Knobil and Neill’s Physiology of Reproduction. 3rd edition. Academic Press: St. Louis; 2006:2787–2846.
37. Hall JE, Guyton AC. Transport of substances through a cell membrane. Guyton and Hall Textbook of Medical Physiology. 12th edition. Saunders/Elsevier: Philadelphia; 2011.
38. Pollex EK, Feig DS, Koren G. Oral hypoglycemic therapy: understanding the mechanisms of transplacental transfer. J Matern Fetal Neonatal Med. 2010;23:224–228.
39. Ghosh C, Marchi N. Drug permeation across the fetal maternal barrier. Janigro D. Mammalian Brain Development. Humana Press; 2009:153–170.
40. Wang JS, Newport DJ, Stowe ZN, et al. The emerging importance of transporter proteins in the psychopharmacological treatment of the pregnant patient. Drug Metab Rev. 2007;39:723–746.
41. Prouillac C, Lecoeur S. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos. 2010;38:1623–1635.
42. Jauniaux E, Gulbis B. In vivo investigation of placental transfer early in human pregnancy. Eur J Obstet Gynecol Reprod Biol. 2000;92:45–49.
43. Tsen LC, Tarshis J, Denson DD, et al. Measurements of maternal protein binding of bupivacaine throughout pregnancy. Anesth Analg. 1999;89:965–968.
44. Longo LD. Respiration in the fetal-placental unit. Cowett RM. Principles of Perinatal-Neonatal Metabolism. Springer-Verlag: New York; 1991:304–315.
45. Dancis J, Schneider H. Physiology: transfer and barrier function. Gruenwald P. The Placenta and its Maternal Supply Line: Effects of Insufficiency on the Foetus. University Park Press: Baltimore; 1975:98–124.
46. Wilkening RB, Meschia G. Current topic: comparative physiology of placental oxygen transport. Placenta. 1992;13:1–15.
47. Ramanathan S, Gandhi S, Arismendy J, et al. Oxygen transfer from mother to fetus during cesarean section under epidural anesthesia. Anesth Analg. 1982;61:576–581.
48. Hill EP, Power GG, Longo LD. A mathematical model of carbon dioxide transfer in the placenta and its interaction with oxygen. Am J Physiol. 1973;224:283–299.
49. Radaelli T, Boito S, Taricco E, et al. Estimation of fetal oxygen uptake in human term pregnancies. J Matern Fetal Neonatal Med. 2012;25:174–179.
50. Carter AM. Evolution of factors affecting placental oxygen transfer. Placenta. 2009;30(Suppl A):S19–S25.
51. Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am J Obstet Gynecol. 1994;171:1560–1565.
52. Battaglia FC, Meschia G. Foetal and placental metabolisms: their interrelationship and impact upon maternal metabolism. Proc Nutr Soc. 1981;40:99–113.
53. Longo LD, Delivoria-Papadopoulos M, Forster RE 2nd. Placental CO2 transfer after fetal carbonic anhydrase inhibition. Am J Physiol. 1974;226:703–710.
54. Novakovic B, Gordon L, Robinson WP, et al. Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation. J Nutr Biochem. 2013;24:282–288.
55. Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012;33(Suppl 2):e23–e29.
56. Avagliano L, Garo C, Marconi AM. Placental amino acids transport in intrauterine growth restriction. J Pregnancy. 2012;2012:972562.
57. Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–255.
58. Dancis J. Why perfuse the human placenta. Contrib Gynecol Obstet. 1985;13:1–4.
59. Herman NL, Li AT, Bjoraker RW, et al. The effects of maternal-fetal perfusate protein differences on the bidirectional transfer of thiopental across the human placenta (abstract). Anesthesiology. 1998;89:A1046.
60. Herman NL, Li AT, Van Decar TK, et al. Transfer of methohexital across the perfused human placenta. J Clin Anesth. 2000;12:25–30.
61. Herman N, Van Decar TK, Lanza M, et al. Distribution of propofol across the perfused human placenta (abstract). Anesthesiology. 1994;81:A1140.
62. Johnson RF, Herman N, Arney TL, et al. Bupivacaine transfer across the human term placenta: a study using the dual perfused human placental model. Anesthesiology. 1995;82:459–468.
63. Johnson RF, Cahana A, Olenick M, et al. A comparison of the placental transfer of ropivacaine versus bupivacaine. Anesth Analg. 1999;89:703–708.
64. Zakowski MI, Ham AA, Grant GJ. Transfer and uptake of alfentanil in the human placenta during in vitro perfusion. Anesth Analg. 1994;79:1089–1093.
66. Krishna BR, Zakowski MI, Grant GJ. Sufentanil transfer in the human placenta during in vitro perfusion. Can J Anaesth. 1997;44:996–1001.
67. Yang YQ, Lee MP, Schenken RS, et al. Effects of binding on human transplacental transfer of cocaine. Am J Obstet Gynecol. 1995;172:720–722.
68. Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514.
69. Zakowski MI, Krishna R, Grant GJ, Turndorf H. Effect of pH on transfer of narcotics in human placenta during in vitro perfusion (abstract). Anesthesiology. 1995;85:A890.
70. Giroux M, Teixera MG, Dumas JC, et al. Influence of maternal blood flow on the placental transfer of three opioids—fentanyl, alfentanil, sufentanil. Biol Neonate. 1997;72:133–141.
71. Lumley J, Walker A, Marum J, Wood C. Time: an important variable at Caesarean section. J Obstet Gynaecol Br Commonw. 1970;77:10–23.
72. Kangas L, Erkkola R, Kanto J, Mansikka M. Halothane anaesthesia in caesarean section. Acta Anaesthesiol Scand. 1976;20:189–194.
73. Dwyer R, Fee JP, Moore J. Uptake of halothane and isoflurane by mother and baby during caesarean section. Br J Anaesth. 1995;74:379–383.
74. Satoh D, Iwatsuki N, Naito M, et al. Comparison of the placental transfer of halothane, enflurane, sevoflurane, and isoflurane during cesarean section. J Anesth. 1995;9:220–223.
75. Farragher R, Maharaj CH, Higgins BD, et al. Sevoflurane and the feto-placental vasculature: the role of nitric oxide and vasoactive eicosanoids. Anesth Analg. 2008;107:171–177.
76. Marx GF, Joshi CW, Orkin LR. Placental transmission of nitrous oxide. Anesthesiology. 1970;32:429–432.
77. Polvi HJ, Pirhonen JP, Erkkola RU. Nitrous oxide inhalation: effects on maternal and fetal circulations at term. Obstet Gynecol. 1996;87:1045–1048.
78. Dailland P, Cockshott ID, Lirzin JD, et al. Intravenous propofol during cesarean section: placental transfer, concentrations in breast milk, and neonatal effects: a preliminary study. Anesthesiology. 1989;71:827–834.
79. Valtonen M, Kanto J, Rosenberg P. Comparison of propofol and thiopentone for induction of anaesthesia for elective caesarean section. Anaesthesia. 1989;44:758–762.
80. Gin T, Gregory MA, Chan K, Oh TE. Maternal and fetal levels of propofol at caesarean section. Anaesth Intensive Care. 1990;18:180–184.
81. Gin T, Yau G, Chan K, et al. Disposition of propofol infusions for caesarean section. Can J Anaesth. 1991;38:31–36.
82. Jauniaux E, Gulbis B, Shannon C, et al. Placental propofol transfer and fetal sedation during maternal general anaesthesia in early pregnancy. Lancet. 1998;352:290–291.
83. Celleno D, Capogna G, Tomassetti M, et al. Neurobehavioural effects of propofol on the neonate following elective caesarean section. Br J Anaesth. 1989;62:649–654.
84. Sanchez-Alcaraz A, Quintana MB, Laguarda M. Placental transfer and neonatal effects of propofol in caesarean section. J Clin Pharm Ther. 1998;23:19–23.
85. He YL, Tsujimoto S, Tanimoto M, et al. Effects of protein binding on the placental transfer of propofol in the human dually perfused cotyledon in vitro. Br J Anaesth. 2000;85:281–286.
86. He YL, Seno H, Tsujimoto S, Tashiro C. The effects of uterine and umbilical blood flows on the transfer of propofol across the human placenta during in vitro perfusion. Anesth Analg. 2001;93:151–156.
87. He YL, Seno H, Sasaki K, Tashiro C. The influences of maternal albumin concentrations on the placental transfer of propofol in human dually perfused cotyledon in vitro. Anesth Analg. 2002;94:1312–1314.
88. Soares de Moura R, Silva GA, Tano T, Resende AC. Effect of propofol on human fetal placental circulation. Int J Obstet Anesth. 2010;19:71–76.
89. Ellingson A, Haram K, Sagen N, Solheim E. Transplacental passage of ketamine after intravenous administration. Acta Anaesthesiol Scand. 1977;21:41–44.
90. Netto JD, Musk GC, Maker GL, Trengove RD. Liquid chromatography tandem mass spectrometry for the simultaneous quantitative analysis of ketamine and medetomidine in ovine plasma. Biomed Chromatogr. 2011;25:1374–1380.
91. Gregory MA, Davidson DG. Plasma etomidate levels in mother and fetus. Anaesthesia. 1991;46:716–718.
92. Fresno L, Andaluz A, Moll X, et al. Placental transfer of etomidate in pregnant ewes after an intravenous bolus dose and continuous infusion. Vet J. 2008;175:395–402.
93. Levy CJ, Owen G. Thiopentone transmission through the placenta. Anaesthesia. 1964;19:511–513.
94. Morgan DJ, Blackman GL, Paull JD, Wolf LJ. Pharmacokinetics and plasma binding of thiopental. II. Studies at cesarean section. Anesthesiology. 1981;54:474–480.
95. Marshall JR. Human antepartum placental passage of methohexital sodium. Obstet Gynecol. 1964;23:589–592.
96. Ala-Kokko TI, Pienimaki P, Lampela E, et al. Transfer of clonidine and dexmedetomidine across the isolated perfused human placenta. Acta Anaesthesiol Scand. 1997;41:313–319.
97. Myllynen P, Vahakangas K. An examination of whether human placental perfusion allows accurate prediction of placental drug transport: studies with diazepam. J Pharmacol Toxicol Methods. 2002;48:131–138.
98. McBride RJ, Dundee JW, Moore J, et al. A study of the plasma concentrations of lorazepam in mother and neonate. Br J Anaesth. 1979;51:971–978.
99. Wilson CM, Dundee JW, Moore J, et al. A comparison of the early pharmacokinetics of midazolam in pregnant and nonpregnant women. Anaesthesia. 1987;42:1057–1062.
100. Shnider SM, Way EL, Lord MJ. Rate of appearance and disappearance of meperidine in fetal blood after administration of narcotics to the mother (abstract). Anesthesiology. 1966;27:227–228.
101. Caldwell J, Wakile LA, Notarianni LJ, et al. Transplacental passage and neonatal elimination of pethidine given to mothers in childbirth (abstract). Br J Clin Pharmacol. 1977;4:715P–716P.
102. Zakowski MI, Krishna BR, Wang SM, et al. Uptake and transfer of meperidine in human placenta during in vitro perfusion (abstract). Presented before the annual meeting of the Society for Obstetric Anesthesia and Perinatology, 1997:A104.
103. Kopecky EA, Ryan ML, Barrett JF, et al. Fetal response to maternally administered morphine. Am J Obstet Gynecol. 2000;183:424–430.
104. Hee P, Stampe Sorensen S, Bock JE, et al. Intrathecal administration of morphine for the relief of pains in labour and estimation of maternal and fetal plasma concentration of morphine. Eur J Obstet Gynecol Reprod Biol. 1987;25:195–201.
105. Bui T, Zakowski MI, Grant GJ, Turndorf H. Uptake and transfer of morphine in human placenta during in vitro perfusion (abstract). Anesthesiology. 1995;83:A932.
106. Kopecky EA, Simone C, Knie B, Koren G. Transfer of morphine across the human placenta and its interaction with naloxone. Life Sci. 1999;65:2359–2371.
107. Bower S. Plasma protein binding of fentanyl. J Pharm Pharmacol. 1981;33:507–514.
108. Loftus JR, Hill H, Cohen SE. Placental transfer and neonatal effects of epidural sufentanil and fentanyl administered with bupivacaine during labor. Anesthesiology. 1995;83:300–308.
109. Cooper J, Jauniaux E, Gulbis B, et al. Placental transfer of fentanyl in early human pregnancy and its detection in fetal brain. Br J Anaesth. 1999;82:929–931.
110. Zakowski MI, Schlesinger J, Dumbroff S, et al. In vitro human placental uptake and transfer of fentanyl (abstract). Anesthesiology. 1993;79:A1006.
111. de Barros Duarte L, Moises EC, Carvalho Cavalli R, et al. Distribution of fentanyl in the placental intervillous space and in the different maternal and fetal compartments in term pregnant women. Eur J Clin Pharmacol. 2009;65:803–808.
112. Gepts E, Heytens L, Camu F. Pharmacokinetics and placental transfer of intravenous and epidural alfentanil in parturient women. Anesth Analg. 1986;65:1155–1160.
113. Gin T, Ngan-Kee WD, Siu YK, et al. Alfentanil given immediately before the induction of anesthesia for elective cesarean delivery. Anesth Analg. 2000;90:1167–1172.
114. Kan RE, Hughes SC, Rosen MA, et al. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467–1474.
116. Coonen JB, Marcus MA, Joosten EA, et al. Transplacental transfer of remifentanil in the pregnant ewe. Br J Pharmacol. 2010;161:1472–1476.
117. Volikas I, Butwick A, Wilkinson C, et al. Maternal and neonatal side-effects of remifentanil patient-controlled analgesia in labour. Br J Anaesth. 2005;95:504–509.
118. Pittman KA, Smyth RD, Losada M, et al. Human perinatal distribution of butorphanol. Am J Obstet Gynecol. 1980;138:797–800.
119. Nicolle E, Devillier P, Delanoy B, et al. Therapeutic monitoring of nalbuphine: transplacental transfer and estimated pharmacokinetics in the neonate. Eur J Clin Pharmacol. 1996;49:485–489.
120. de Barros Duarte L, Moises EC, Carvalho Cavalli R, et al. Placental transfer of bupivacaine enantiomers in normal pregnant women receiving epidural anesthesia for cesarean section. Eur J Clin Pharmacol. 2007;63:523–526.
121. Kvisselgaard N, Moya F. Investigation of placental thresholds to succinylcholine. Anesthesiology. 1961;22:7–10.
122. Baraka A, Haroun S, Bassili M, Abu-Haider G. Response of the newborn to succinylcholine injection in homozygotic atypical mothers. Anesthesiology. 1975;43:115–116.
123. Duvaldestin P, Demetriou M, Henzel D, Desmonts JM. The placental transfer of pancuronium and its pharmacokinetics during caesarian section. Acta Anaesthesiol Scand. 1978;22:327–333.
124. Abouleish E, Wingard LB Jr, de la Vega S, Uy N. Pancuronium in caesarean section and its placental transfer. Br J Anaesth. 1980;52:531–536.
125. Dailey PA, Fisher DM, Shnider SM, et al. Pharmacokinetics, placental transfer, and neonatal effects of vecuronium and pancuronium administered during cesarean section. Anesthesiology. 1984;60:569–574.
126. Iwama H, Kaneko T, Tobishima S, et al. Time dependency of the ratio of umbilical vein/maternal artery concentrations of vecuronium in caesarean section. Acta Anaesthesiol Scand. 1999;43:9–12.
127. Abouleish E, Abboud T, Lechevalier T, et al. Rocuronium (Org 9426) for caesarean section. Br J Anaesth. 1994;73:336–341.
128. Shearer ES, Fahy LT, O’Sullivan EP, Hunter JM. Transplacental distribution of atracurium, laudanosine and monoquaternary alcohol during elective caesarean section. Br J Anaesth. 1991;66:551–556.
129. Fodale V, Santamaria LB. Laudanosine, an atracurium and cisatracurium metabolite. Eur J Anaesthesiol. 2002;19:466–473.
130. Kivalo I, Saarikoski S. Placental transmission of atropine at full-term pregnancy. Br J Anaesth. 1977;49:1017–1021.
131. Kanto J, Kentala E, Kaila T, Pihlajamaki K. Pharmacokinetics of scopolamine during caesarean section: relationship between serum concentration and effect. Acta Anaesthesiol Scand. 1989;33:482–486.
132. Ali-Melkkila T, Kaila T, Kanto J, Iisalo E. Pharmacokinetics of glycopyrronium in parturients. Anaesthesia. 1990;45:634–637.
133. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 9th edition. Lippincott Williams & Wilkins: Philadelphia; 2011.
134. Clark RB, Brown MA, Lattin DL. Neostigmine, atropine, and glycopyrrolate: does neostigmine cross the placenta? Anesthesiology. 1996;84:450–452.
135. Boehm FH, Egilmez A, Smith BE. Physostigmine’s effect on diminished fetal heart rate variability caused by scopolamine, meperidine and propiomazine. J Perinat Med. 1977;5:214–222.
136. Witter FR, King TM, Blake DA. Adverse effects of cardiovascular drug therapy on the fetus and neonate. Obstet Gynecol. 1981;58(Suppl):100S–105S.
137. Erkkola R, Lammintausta R, Liukko P, Anttila M. Transfer of propranolol and sotalol across the human placenta: their effect on maternal and fetal plasma renin activity. Acta Obstet Gynecol Scand. 1982;61:31–34.
138. Cottrill CM, McAllister RG Jr, Gettes L, Noonan JA. Propranolol therapy during pregnancy, labor, and delivery: evidence for transplacental drug transfer and impaired neonatal drug disposition. J Pediatr. 1977;91:812–814.
139. Melander A, Niklasson B, Ingemarsson I, et al. Transplacental passage of atenolol in man. Eur J Clin Pharmacol. 1978;14:93–94.
140. Lindeberg S, Sandstrom B, Lundborg P, Regardh CG. Disposition of the adrenergic blocker metoprolol in the late-pregnant woman, the amniotic fluid, the cord blood and the neonate. Acta Obstet Gynecol Scand. 1984;118(Suppl):61–64.
141. Michael CA. Use of labetalol in the treatment of severe hypertension during pregnancy. Br J Clin Pharmacol. 1979;8(Suppl 2):211S–215S.
142. Macpherson M, Broughton Pipkin F, Rutter N. The effect of maternal labetalol on the newborn infant. Br J Obstet Gynaecol. 1986;93:539–542.
143. Meidahl Petersen K, Jimenez-Solem E, Andersen JT, et al. β-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open. 2012;2:e001185 http://bmjopen.bmj.com/content/2/4/e001185.full.pdf+html.
144. Baggio MR, Martins WP, Calderon AC, et al. Changes in fetal and maternal Doppler parameters observed during acute severe hypertension treatment with hydralazine or labetalol: a randomized controlled trial. Ultrasound Med Biol. 2011;37:53–58.
145. Ostman PL, Chestnut DH, Robillard JE, et al. Transplacental passage and hemodynamic effects of esmolol in the gravid ewe. Anesthesiology. 1988;69:738–741.
146. Ducey JP, Knape KG. Maternal esmolol administration resulting in fetal distress and cesarean section in a term pregnancy. Anesthesiology. 1992;77:829–832.
147. Hartikainen-Sorri AL, Heikkinen JE, Koivisto M. Pharmacokinetics of clonidine during pregnancy and nursing. Obstet Gynecol. 1987;69:598–600.
148. Jones HM, Cummings AJ, Setchell KD, Lawson AM. A study of the disposition of α-methyldopa in newborn infants following its administration to the mother for the treatment of hypertension during pregnancy. Br J Clin Pharmacol. 1979;8:433–440.
149. David R, Leitch IM, Read MA, et al. Actions of magnesium, nifedipine and clonidine on the fetal vasculature of the human placenta. Aust N Z J Obstet Gynaecol. 1996;36:267–271.
150. Santeiro ML, Stromquist C, Wyble L. Phenoxybenzamine placental transfer during the third trimester. Ann Pharmacother. 1996;30:1249–1251.
151. Liedholm H, Wahlin-Boll E, Hanson A, et al. Transplacental passage and breast milk concentrations of hydralazine. Eur J Clin Pharmacol. 1982;21:417–419.
152. Magee KP, Bawdon RE. Ex vivo human placental transfer and the vasoactive properties of hydralazine. Am J Obstet Gynecol. 2000;182:167–169.
153. Naulty J, Cefalo RC, Lewis PE. Fetal toxicity of nitroprusside in the pregnant ewe. Am J Obstet Gynecol. 1981;139:708–711.
154. Graeme KA, Curry SC, Bikin DS, et al. The lack of transplacental movement of the cyanide antidote thiosulfate in gravid ewes. Anesth Analg. 1999;89:1448–1452.
155. Bootstaylor BS, Roman C, Parer JT, Heymann MA. Fetal and maternal hemodynamic and metabolic effects of maternal nitroglycerin infusions in sheep. Am J Obstet Gynecol. 1997;176:644–650.
156. Bustard MA, Farley AE, Smith GN. The pharmacokinetics of glyceryl trinitrate with the use of the in vitro term human placental perfusion setup. Am J Obstet Gynecol. 2002;187:187–190.
157. Segal S, Csavoy AN, Datta S. Placental tissue enhances uterine relaxation by nitroglycerin. Anesth Analg. 1998;86:304–309.
158. Zhang XQ, Kwek K, Read MA, et al. Effects of nitrovasodilators on the human fetal-placental circulation in vitro. Placenta. 2001;22:337–346.
159. Ducsay CA, Umezaki H, Kaushal KM, et al. Pharmacokinetic and fetal cardiovascular effects of enalaprilat administration to maternal rhesus macaques. Am J Obstet Gynecol. 1996;175:50–55.
160. Hughes SC, Ward MG, Levinson G, et al. Placental transfer of ephedrine does not affect neonatal outcome. Anesthesiology. 1985;63:217–219.
161. Minzter BH, Johnson RF, Paschall RL, et al. The diverse effects of vasopressors on the fetoplacental circulation of the dual perfused human placenta. Anesth Analg. 2010;110:857–862.
162. Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
164. Krishna RB, Levitz M, Dancis J. Lack of effect of cocaine on lysine and alanine uptake in human placental villi or transfer in perfused human placenta. Reprod Fertil Dev. 1995;7:1495–1497.
165. Simone C, Derewlany LO, Oskamp M, et al. Transfer of cocaine and benzoylecgonine across the perfused human placental cotyledon. Am J Obstet Gynecol. 1994;170:1404–1410.
166. Zhou M, Song ZM, Lidow MS. Pharmacokinetics of cocaine in maternal and fetal rhesus monkeys at mid-gestation. J Pharmacol Exp Ther. 2001;297:556–562.
167. Brownbill P, Sibley CP. Regulation of transplacental water transfer: the role of fetoplacental venous tone. Placenta. 2006;27:560–567.
168. Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med. 1980;68:122–140.
169. Tettenborn B. Stroke and pregnancy. Neurol Clin. 2012;30:913–924.
170. Andrew M, Boneu B, Cade J, et al. Placental transport of low molecular weight heparin in the pregnant sheep. Br J Haematol. 1985;59:103–108.
171. Dimitrakakis C, Papageorgiou P, Papageorgiou I, et al. Absence of transplacental passage of the low molecular weight heparin enoxaparin. Haemostasis. 2000;30:243–248.
172. Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90:67–76.
173. Lagrange F, Vergnes C, Brun JL, et al. Absence of placental transfer of pentasaccharide (Fondaparinux, Arixtra) in the dually perfused human cotyledon in vitro. Thromb Haemost. 2002;87:831–835.
174. Chapman ML, Martinez-Borges AR, Mertz HL. Lepirudin for treatment of acute thrombosis during pregnancy. Obstet Gynecol. 2008;112:432–433.
175. Ekbatani A, Asaro LR, Malinow AM. Anticoagulation with argatroban in a parturient with heparin-induced thrombocytopenia. Int J Obstet Anesth. 2010;19:82–87.
176. De Santis M, De Luca C, Mappa I, et al. Clopidogrel treatment during pregnancy: a case report and a review of literature. Intern Med. 2011;50:1769–1773.
177. Miller RK, Mace K, Polliotti B, et al. Marginal transfer of ReoPro (Abciximab) compared with immunoglobulin G (F105), inulin and water in the perfused human placenta in vitro. Placenta. 2003;24:727–738.
178. Bajoria R, Contractor SF. Effect of surface charge of small unilamellar liposomes on uptake and transfer of carboxyfluorescein across the perfused human term placenta. Pediatr Res. 1997;42:520–527.
179. Barzago MM, Bortolotti A, Stellari FF, et al. Placental transfer of valproic acid after liposome encapsulation during in vitro human placenta perfusion. J Pharmacol Exp Ther. 1996;277:79–86.
180. Kraemer J, Klein J, Lubetsky A, Koren G. Perfusion studies of glyburide transfer across the human placenta: implications for fetal safety. Am J Obstet Gynecol. 2006;195:270–274.
181. Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006;27:1096–1102.
182. Nanovskaya TN, Nekhayeva I, Hankins GD, Ahmed MS. Effect of human serum albumin on transplacental transfer of glyburide. Biochem Pharmacol. 2006;72:632–639.
183. Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, et al. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006;195:1081–1085.
184. Nanovskaya TN, Nekhayeva IA, Hankins GD, Ahmed MS. Transfer of methadone across the dually perfused preterm human placental lobule. Am J Obstet Gynecol. 2008;198:126.
185. Manceau S, Giraud C, Decleves X, et al. ABC drug transporter and nuclear receptor expression in human cytotrophoblasts: influence of spontaneous syncytialization and induction by glucocorticoids. Placenta. 2012;33:927–932.
186. Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–S69.
187. Shin JS, Choi MY, Longtine MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta. 2010;31:1027–1034.
188. American College of Obstetricians and Gynecologists. Committee on Obstetrics: Maternal and Fetal Medicine. Placental pathology. ACOG Committee Opinion No. 125, July 1993. Int J Gynaecol Obstet. 1993;42:318–319.
189. Mooney EE, Boggess KA, Herbert WN, Layfield LJ. Placental pathology in patients using cocaine: an observational study. Obstet Gynecol. 1998;91:925–929.