Postpartum Tubal Sterilization
Joy L. Hawkins MD
Chapter Outline
Many parous women choose tubal ligation for permanent contraception. Half are performed postpartum (over 350,000 annually in the United States) and half as ambulatory interval procedures.1 Although the interval sterilization rate has declined by 12% in the United States, the postpartum sterilization rate remains stable, and postpartum sterilization is performed after 8% to 9% of all live births.1 The considerations and controversies regarding the administration of anesthesia for postpartum tubal sterilization are discussed in this chapter.
American Society of Anesthesiologists Guidelines
The American Society of Anesthesiologists (ASA) has published “Practice Guidelines for Obstetric Anesthesia,”2 which includes a discussion of postpartum tubal ligation. (See Appendix B.) The Task Force recommendations can be summarized as follows:
Surgical Considerations
Tubal sterilization can be performed satisfactorily at any time, but the early postpartum period has several advantages for women who have had an uncomplicated vaginal delivery.3 The patient avoids the cost and inconvenience of a second hospital visit. The uterine fundus remains near the umbilicus for several days postpartum, which allows easy access to the fallopian tubes. Minilaparotomy and laparoscopy have similar rates of serious complications (e.g., bowel laceration, vascular injury).4
There are at least two potential disadvantages to immediate postpartum sterilization. First, parous women are at increased risk for uterine atony and postpartum hemorrhage. This risk decreases substantially 12 hours after delivery. Second, immediate surgery results in sterilization before assessment of the newborn is complete. Postpartum tubal ligation is not wise if the patient is ambivalent regarding permanent sterilization. However, women who undergo postpartum sterilization have a similar probability of regret within 1 year of delivery (23.7%) as women who undergo interval sterilization (22.3%), although the risk is markedly increased when the woman is younger than 25 years of age.5
Several techniques are used for postpartum tubal sterilization (Figure 25-1).6 Puerperal sterilization has a failure rate that is lower than most interval procedures, and the failure rate is lowest (approximately 0.75%) if some form of tubal resection occurs.7 With the Irving procedure, the obstetrician buries the cut ends of the tubes in the myometrium and mesosalpinx. This technique is least likely to fail, but it requires more extensive exposure and increases the risk for hemorrhage. The Pomeroy procedure is simplest. The surgeon ligates a loop of oviduct and excises the loop above the suture. With the Parkland procedure, the obstetrician ligates the tube proximally and distally and then excises the midsegment. The last two methods are most commonly performed during postpartum tubal ligations. Regardless of the technique, the obstetrician should document that fimbriae are present to preclude ligation of another structure such as the round ligament. The excised portions typically are sent to a pathologist for verification.
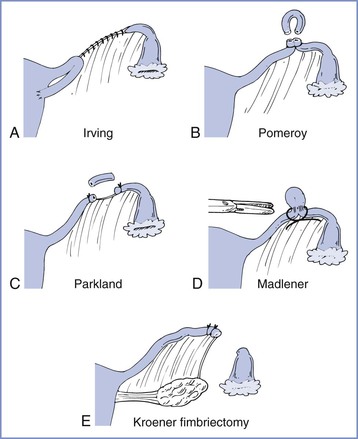
FIGURE 25-1 Techniques for tubal sterilization. A, Irving procedure. The medial cut end of the oviduct is buried in the myometrium posteriorly, and the distal cut end is buried in the mesosalpinx. B, Pomeroy procedure. A loop of oviduct is ligated, and the knuckle of tube above the ligature is excised. C, Parkland procedure. A midsegment of tube is separated from the mesosalpinx at an avascular site, and the separated tubal segment is ligated proximally and distally and then excised. D, Madlener procedure. A knuckle of oviduct is crushed and then ligated without resection; this technique has an unacceptably high failure rate of approximately 7%. E, Kroener procedure. The tube is ligated across the ampulla, and the distal portion of the ampulla, including all of the fimbria, is resected; some studies have reported an unacceptably high failure rate with this technique. (From Cunningham FG, MacDonald PC, Gant NF, et al. Williams Obstetrics, 20th edition. Stamford, CT, Appleton & Lange, 1997:1376.)
A randomized clinical trial found a significantly increased risk for pregnancy at 24 months after use of the titanium clip for postpartum tubal occlusion (i.e., a cumulative pregnancy rate of 1.7 pregnancies per 1000 women who had titanium tubal occlusion versus 0.04 pregnancies per 1000 women in whom the Pomeroy technique was used).8 Therefore, evidence does not support routine use of the titanium clip for postpartum sterilization.8
Nonmedical Issues
Nonmedical issues affect decisions regarding the timing of tubal sterilization. The obstetrician must obtain and document informed consent for surgery.5 Tubal ligation should be considered an irreversible procedure. Therefore, most obstetricians require a discussion with the patient before labor and delivery. Postpartum partial salpingectomy has 5-year and 10-year failure rates of 6.3 and 7.5 per 1000 patients, respectively, the lowest of all sterilization procedures.5 Physicians should be aware of state laws or insurance regulations that may require a specific interval between obtaining consent and performance of sterilization procedures. Regulations often do not allow the woman to give consent while in labor or immediately after delivery. For example, the Medicaid reimbursement program includes the following requirements for sterilization8:
• The patient must be at least 21 years of age and mentally competent when consent is obtained.
• Informed consent may not be obtained while the patient is in labor or during childbirth.
In some cases the obstetrician may schedule a patient for a postpartum tubal ligation because of a fear that the patient will not return for interval tubal sterilization 6 weeks after delivery. Concerns regarding patient compliance should not prompt the performance of postpartum tubal ligation in patients with significant medical or obstetric complications. However, women who request postpartum tubal sterilization but do not receive it are more likely to become pregnant within 1 year of delivery (46.7%) than are women who did not request the procedure (22.3%).9
Preoperative Evaluation
The patient scheduled for postpartum tubal ligation requires a thorough preoperative evaluation, and a reevaluation should be performed even if the patient is known to the anesthesia provider as a result of the provision of labor analgesia. A cursory evaluation should not be performed simply because the patient is young and healthy. Patients with pregnancy-induced hypertension may safely receive neuraxial or general anesthesia for postpartum tubal ligation provided that there is no evidence of pulmonary edema, oliguria, or thrombocytopenia.10
Physicians and nurses often underestimate blood loss during delivery.11 Excessive blood loss from uterine atony is not uncommon in parous women. Orthostatic changes in blood pressure and heart rate should be excluded, especially if an immediate postpartum procedure is to be performed. At the University of Colorado, for surgery performed the day after delivery, the patient’s hematocrit is determined several hours after delivery (to allow for equilibration) and compared with the antepartum measurement. A hematocrit is not obtained before an immediate postpartum tubal sterilization (performed < 8 hours after delivery), provided that the antepartum hematocrit was acceptable, there are no orthostatic vital sign changes, and there was no evidence of excessive blood loss during delivery.
No absolute value of hematocrit requires a delay of surgery, but physical signs of hemodynamic instability or laboratory evidence of excessive blood loss should prompt postponement of the procedure until 6 to 8 weeks postpartum. Fever may signal the presence of endometritis or urinary tract infection and also may require postponement of surgery until a later date. Finally, the condition of the neonate should be confirmed before surgery to exclude any unexpected problems.
Mothers may be concerned that medications administered during surgery might affect their ability to breast-feed or that these medications might harm the newborn. Any drug present in the mother’s blood will be present in breast milk, with the concentration dependent on factors such as protein binding, lipid solubility, and degree of ionization.12 Typically, the amount of drug present in breast milk is small. Opioids, barbiturates, and propofol administered during anesthesia are excreted in insignificant amounts. (See Chapter 14 for a detailed discussion of interactions between drugs and breast-feeding.)
Risk for Aspiration
Historically, anesthesiologists have considered maternal aspiration the major risk associated with anesthesia for postpartum tubal ligation, although the evidence for this is scant and conflicting. A review of anesthesia-related maternal mortality found no maternal deaths associated with aspiration during postpartum tubal ligation, despite tracking deaths for 1 year after delivery.13 However, several factors may place the pregnant woman at increased risk for aspiration. Some but not all of these factors are resolved at delivery. The placenta is the primary site of progesterone production, and progesterone concentrations fall rapidly after delivery of the placenta (Figure 25-2).14,15 Typically, progesterone concentrations decline within 2 hours of delivery; and by 24 hours postpartum, progesterone concentrations are similar to those found during the luteal phase of the menstrual cycle.
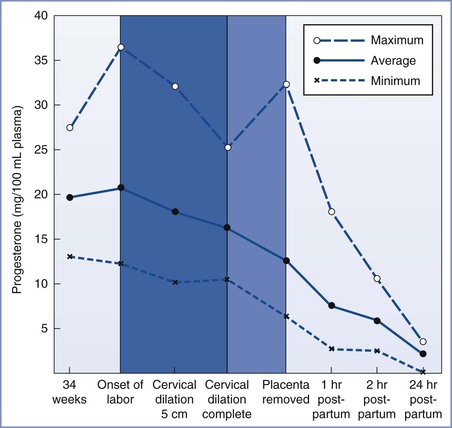
FIGURE 25-2 Average progesterone concentrations with the highest and lowest measurements of 13 pregnant women at given time intervals. (From Llauro JL, Runnebaum B, Zander J. Progesterone in human peripheral blood before, during and after labor. Am J Obstet Gynecol 1968; 101:871.)
Two important questions to address during the preanesthetic evaluation are (1) What is the duration of the fast for solids? (2) Were parenteral opioids administered during labor?
Gastric Emptying
Several studies have assessed gastric emptying in pregnant and postpartum women. In summary, the preponderance of evidence suggests that (1) administration of an opioid during labor increases the likelihood of delayed gastric emptying during the early postpartum period, (2) gastric emptying of solids is delayed during labor in all parturients, and (3) gastric emptying of clear liquids is probably not delayed unless parenteral opioids were administered. However, there are few data on gastric emptying during the first 8 hours postpartum.
O’Sullivan et al.16 used an epigastric impedance technique to compare gastric emptying times for solids and liquids in women during the third trimester of pregnancy, in women during the first hour postpartum, and in nonpregnant controls. The investigators observed that the overall rate of gastric emptying was lower in the postpartum patients than in pregnant or nonpregnant patients. When patients who had received parenteral opioids in labor were separated from those who had not, rates of gastric emptying for women who had not received opioids were similar to those for nonpregnant controls. The investigators concluded that the rate of gastric emptying in postpartum women is delayed only if opioids have been administered during labor.
Other studies have used the acetaminophen (paracetamol) absorption technique to assess gastric emptying. Gin et al.17 studied women on the first and third days after delivery and at 6 weeks postpartum. They found comparable times to peak concentration of acetaminophen in all three groups. They concluded that gastric emptying was no different in the immediate postpartum period than 6 weeks later, and they recommended that “the approach to prophylaxis against acid aspiration should be more consistent between nonpregnant and postpartum patients.” Whitehead et al.18 observed no significant delay in gastric emptying during the first, second, or third trimesters of pregnancy or between 18 and 48 hours postpartum when compared with gastric emptying in nonpregnant controls. They observed that gastric emptying was significantly delayed during the first 2 hours after vaginal delivery, but at least 4 of the 17 women studied received intramuscular meperidine during labor. The researchers did not measure gastric emptying between 2 and 18 hours postpartum. Despite the confounding use of opioids, they concluded, “The presence of delayed gastric emptying in the immediate (within 2 hours) postpartum period confirms that strict precautions against acid aspiration should be provided to mothers who are newly delivered and requiring anaesthesia.”19
Sandhar et al.19 used applied potential tomography to measure gastric emptying in 10 patients at term gestation, 2 to 3 days postpartum, and 6 weeks postpartum. The 6-week measurement served as each woman’s control value. All measurements were made after administration of an H2-receptor antagonist. The times to 50% emptying after ingestion of 400 mL of water were not different among the three periods of testing (Figure 25-3).
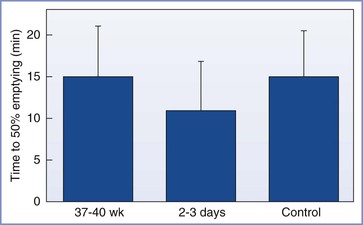
FIGURE 25-3 Mean (SEM) times to 50% gastric emptying (min). No significant differences were noted between term pregnant, postpartum, and nonpregnant control women. (From Sandhar BK, Elliott RH, Windram I, Rowbotham DJ. Peripartum changes in gastric emptying. Anaesthesia 1992; 47:197.)
Wong et al.20 assessed gastric emptying in nonlaboring pregnant women at term gestation, after ingestion of either 50 or 300 mL of water, by using two techniques: (1) serial assessment of acetaminophen absorption and (2) use of ultrasonography to determine gastric antrum cross-sectional areas. Gastric emptying was significantly faster after ingestion of 300 mL of water, consistent with the observation that a liquid meal may actually accelerate gastric emptying. Repeating the study in obese women showed similar results.21 Kubli et al.22 compared the effects of isotonic “sport drinks” versus water on residual gastric volume in women in early labor. Women who received isotonic “sports drinks” had similar gastric volumes and a similar incidence of vomiting as compared with those who received water, but the ingestion of “sport drinks” prevented the increase in ketone production that occurred in the control (water) group. Altogether, these studies suggest that gastric emptying of clear liquids is not delayed during pregnancy, early labor, or the postpartum period unless an opioid has been administered.
In contrast, Jayaram et al.23 found that 39% of postpartum patients, but not nonpregnant patients presenting for gynecologic surgery, had solid food particles in the stomach, as demonstrated by ultrasonography. Four hours after a standardized meal, 95% of postpartum women—compared with only 19% of nonpregnant subjects—still had solid food particles in the stomach. Prior administration of an opioid did not seem to be a risk factor in this study. Scrutton et al.24 randomized 94 women presenting in early labor to receive either a light diet or water only during labor. The mothers who ate a light diet had significantly larger gastric antrum cross-sectional areas (determined by ultrasonography) and were twice as likely to vomit at or around delivery as those who had water only. Also, the volumes vomited were significantly larger in the women who ate a light diet.
During the preoperative assessment of any woman scheduled for postpartum tubal ligation, the anesthesia provider should determine when the patient last consumed solids and whether opioids were administered by any route. Systemic absorption of an opioid occurs after epidural administration. However, published studies have provided conflicting results regarding the effect of epidural opioid administration on gastric emptying. Wright et al.25 observed that epidural administration of 10 mL of 0.375% bupivacaine with fentanyl 100 µg caused a modest prolongation of gastric emptying during labor when compared with epidural administration of bupivacaine alone. However, Kelly et al.26 found that intrathecal, but not epidural, fentanyl delayed gastric emptying. Metoclopramide may not accelerate gastric emptying in patients who have received an opioid.
Gastric Volume and pH
There is little evidence that postpartum women are at greater risk for sequelae of aspiration than patients undergoing elective surgery based solely on pregnancy-induced changes in gastric pH and volume. The conventional wisdom is that a gastric volume of more than 25 mL and a gastric pH of less than 2.5 are risk factors for aspiration pneumonitis. Coté27 noted that this dogma was derived from unpublished animal studies and that it assumes that every milliliter of gastric fluid is directed into the trachea. A marked disparity exists between the incidence of patients labeled “at risk” and the incidence of patients with clinically significant aspiration pneumonitis.
Blouw et al.28 measured gastric volume and pH in nonpregnant women undergoing gynecologic surgery and postpartum women 9 to 42 hours after delivery. They found no significant difference between the groups. Approximately 75% of women in both groups had a gastric pH of less than 2.5. When the combination of volume and pH was used to determine the risk for aspiration, 64% of the control patients but only 33% of postpartum patients were at risk. The researchers concluded that 8 hours after delivery, postpartum patients are not at greater risk than nonpregnant patients undergoing elective surgery. They did not examine patients earlier than 8 hours after delivery. In addition, they acknowledged that a large number of patients in both groups are at risk.
James et al.29 attempted to determine the “safe” interval after delivery. They compared gastric pH and gastric volume in postpartum women 1 to 8 hours, 9 to 23 hours, and 24 to 45 hours after delivery with a control group of nonpregnant women undergoing elective surgery. There were no significant differences in either parameter between the group of patients undergoing elective surgery and any of the postpartum groups (Table 25-1). Approximately 60% of all patients were considered “at risk” for aspiration pneumonitis. The investigators concluded that there was no difference in the risk for sequelae if aspiration should occur, but they speculated that hormonal changes or mechanical factors might make aspiration more likely during the postpartum period.
TABLE 25-1
Gastric Volume and pH at Intervals after Delivery
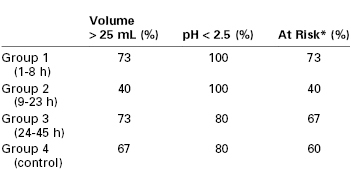
* Gastric contents with pH < 2.5 and volume > 25 mL.
From James CF, Gibbs CP, Banner T. Postpartum perioperative risk of aspiration pneumonia. Anesthesiology 1984; 61:756-9.
Finally, Lam et al.30 administered 150 mL of water to 50 women 2 to 3 hours before tubal ligation that was performed 1 to 5 days postpartum. Another 50 postpartum and 50 nonpregnant women fasted after midnight. The authors found no differences in gastric pH or volume among the postpartum-water group, the postpartum-fasted group, and the group of nonpregnant controls undergoing elective surgery.
Gastroesophageal Reflux
Women in the third trimester of pregnancy have decreased lower esophageal barrier pressures as compared with nonpregnant controls.31 Those with symptoms of heartburn have even lower pressures and a higher incidence of gastric reflux. Vanner and Goodman32 asked parturients to swallow a pH electrode to measure lower esophageal pH at term and on the second postpartum day. Patients were placed in four positions: supine with tilt, left lateral, right lateral, and lithotomy, and were then asked to perform a Valsalva and other maneuvers to promote reflux. A total of 17 of 25 patients had reflux at term, whereas only 5 of 25 had reflux after delivery. The investigators concluded that the incidence of reflux returns toward normal by the second day after delivery. However, this conclusion is arguable given the fact that they did not determine normal by defining the incidence of reflux before or 6 to 8 weeks after pregnancy.
Summary
No data indicate that the postpartum patient’s safety is enhanced by delaying surgery or is compromised by proceeding with surgery immediately after delivery. This situation has led to confusion and inconsistency in the development of policies for the performance of postpartum tubal ligation.33 No waiting interval guarantees that the postpartum patient is free of risk for aspiration. It is probably prudent to use some form of aspiration prophylaxis in all patients undergoing postpartum tubal ligation. However, significant aspiration pneumonitis is so rare that it will be difficult to document cost-effectiveness and decreased rates of morbidity and mortality from the use of these measures. H2-receptor antagonists and antacids do not reduce the possibility of regurgitation and aspiration, but they may make the consequences less severe. Metoclopramide (a prokinetic agent) may decrease the incidence of reflux by increasing lower esophageal sphincter tone and hastening gastric emptying.32 None of these medications can guarantee that gastric contents will not enter the lungs. Aspiration is best prevented by an experienced anesthesia provider using careful airway management and/or by use of a neuraxial anesthetic technique.
In summary, at the University of Colorado, immediate postpartum tubal ligation is performed in patients who have a functioning epidural catheter in place. These patients are given an H2-receptor antagonist and metoclopramide intravenously during labor, and a clear (nonparticulate) antacid is administered just before taking the patient to the operating room. In other patients who do not want (or are unable to receive) epidural analgesia for labor, an H2-receptor antagonist and metoclopramide are given intravenously after delivery followed by a wait of at least 1 hour for a therapeutic effect. This is an elective procedure, and patients should not consume solid food for 6 to 8 hours preoperatively. (NPO policies for postpartum tubal sterilization should not differ from those used for elective surgery in the main operating rooms.) Before surgery, estimated blood loss is reviewed and orthostatic vital signs are assessed. A clear antacid is given just before the patient goes to the operating room. Most of these patients (without preexisting epidural analgesia) receive spinal anesthesia for postpartum tubal ligation. However, general anesthesia can be provided using rapid-sequence induction with cricoid pressure.
Anesthetic Management
Local, general, or neuraxial anesthesia may be used successfully for postpartum tubal sterilization. Physiology remains altered in the postpartum patient and requires some modification in anesthetic technique. It seems reasonable to give all postpartum patients some form of aspiration prophylaxis. This may include a clear (nonparticulate) antacid, an H2-receptor antagonist, and/or metoclopramide to increase lower esophageal sphincter tone and hasten gastric emptying. Metoclopramide also may prevent emesis during and after surgery. Patients with additional risk factors for aspiration (e.g., morbid obesity, diabetes mellitus) warrant prophylaxis with all three classes of drugs. Tubal sterilization does not require administration of preoperative antibiotics.34
Local Anesthesia
Local anesthesia is used for more than 75% of tubal sterilizations worldwide, although neuraxial anesthesia is most often administered for postpartum tubal sterilization in the United States.3 Several reports have documented the efficacy and safety of local anesthesia for postpartum or laparoscopic tubal ligation in the hospital operating room or a free-standing outpatient facility. Cruikshank et al.35 described the use of intraperitoneal lidocaine for postpartum tubal ligation. After intravenous administration of diazepam, lidocaine 100 mg was used to infiltrate the skin and subcutaneous tissue. The peritoneum was entered, and 400 mg of lidocaine (80 mL of 0.5% solution) was instilled into the peritoneal cavity. A Pomeroy tubal ligation was performed 5 minutes later. All patients had complete peritoneal anesthesia, and all patients stated they would have the same procedure again. None recalled any pain or discomfort 24 hours later. There were no signs of lidocaine toxicity in any patient, and the maximum lidocaine blood level obtained was 5.3 µg/mL. Surgeons rated the conditions excellent. This study was published in 1973 when anesthesiologists may or may not have been involved and before ASA monitoring standards such as pulse oximetry existed.
Poindexter et al.36 described almost 3000 laparoscopic tubal sterilization procedures performed with local anesthesia in an ambulatory surgical facility. After intravenous sedation with midazolam (5 to 10 mg) and fentanyl (50 to 100 µg), the skin was infiltrated with 10 mL of 0.5% bupivacaine. After insertion of the trocar, the abdomen was insufflated with nitrous oxide. Each tube was sprayed with 5 mL of 0.5% bupivacaine, and a Silastic ring was applied. Patients were discharged home after approximately 1 hour in the postanesthesia care unit. The authors reported a technical failure rate of 0.14% and no unintended laparotomies or intraoperative complications. They reported that this technique reduced surgical time by 33% and cost by 68% to 85% when compared with general anesthesia. The investigators presented no data regarding patient satisfaction, and they made no comment on the use of pulse oximetry or blood pressure monitors. Four percent of patients, however, required oxygen therapy for “adequate tissue perfusion.” This study was done in the 1980s, before many ambulatory surgery facilities had institutional guidelines for sedation.
General Anesthesia
Much of the impetus for performing sterilization procedures under local anesthesia came from two reports in 1983 indicating that morbidity and mortality were much higher when general anesthesia was used. The first report involved 3500 interval (not postpartum) laparoscopic tubal sterilizations at nine university medical centers.37 Among all patients, the risk for intraoperative or postoperative complications was 1.75%, but the risk was five times higher with general anesthesia than with local anesthesia. (In this report, local anesthesia included local, epidural, and spinal anesthesia.) The reason(s) for the difference was unclear. In the second report, the U.S. Centers for Disease Control and Prevention examined deaths attributed to tubal sterilization procedures from 1977 to 1981.38 Both immediate postpartum laparotomies and interval laparoscopic procedures were included. Of the 29 deaths, 11 followed complications of general anesthesia and were caused by hypoventilation or cardiorespiratory arrest. Aspiration was not reported as a cause of death. Of the six patients whose deaths were definitely attributed to hypoventilation, none had undergone tracheal intubation. Five of the 11 deaths attributed to general anesthesia occurred during postpartum laparotomy. Of these, only one woman had undergone tracheal intubation; all others underwent mask ventilation. The investigators concluded, “It appears that for tubal sterilization, like abortion, the greatest risk for death is that associated with the anesthesia used during the procedure.”39
These reports preceded the mandatory use of pulse oximetry and capnography and do not reflect modern anesthesia care. In the 30 years since those reports, appropriate airway management with tracheal intubation has become standard practice. Thorough adherence to ASA standards for basic anesthesia monitoring (including use of pulse oximetry and capnography to monitor oxygenation and ventilation) should help prevent morbidity and mortality associated with general anesthesia. At the University of Colorado, rapid-sequence induction (with cricoid pressure) is performed and all patients undergo tracheal intubation during administration of general anesthesia for postpartum tubal ligation.
Volatile anesthetic agents cause uterine relaxation and could potentially increase the risk for postpartum hemorrhage if administered to women in the immediate postpartum period. Therefore, the question arises as to whether the anesthesia provider should use an inhalation or an intravenous technique to maintain general anesthesia for postpartum tubal ligation. Marx et al.39 measured postpartum uterine activity and the response to oxytocin with different concentrations of halothane or enflurane (Figure 25-4). Impairment of spontaneous uterine activity occurred at 0.5 minimum alveolar concentration (MAC) of both agents, and loss of the response to oxytocin occurred near 1.0 MAC. Spontaneous contractions reappeared when anesthetic concentrations were reduced below these levels. Parous women are at risk for postpartum uterine atony, and administration of a high concentration of a volatile halogenated agent may precipitate postpartum hemorrhage.
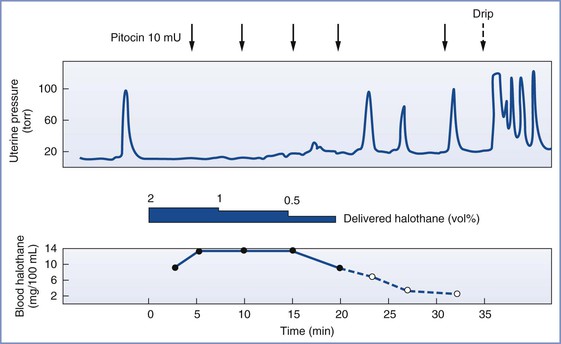
FIGURE 25-4 Halothane anesthesia blocked the normal response to oxytocin when arterial blood levels exceeded 10.5 mg/100 mL or approximately 0.8 MAC. (From Marx GF, Kim YI, Lin CC, et al. Postpartum uterine pressures under halothane or enflurane anesthesia. Obstet Gynecol 1978; 51:697.)
Two studies have determined the MAC of isoflurane during the postpartum period. Chan et al.40 found a positive correlation between MAC and the length of time after delivery, with nonpregnant values achieved by 72 hours postpartum. Zhou et al.41 determined that MAC of isoflurane was approximately 0.75% in the first 12 hours postpartum and 1.04% in patients who were 12 to 24 hours postpartum. No significant difference in MAC existed between the latter group and a control group of nonpregnant gynecologic patients. Together these results demonstrate that the reduced MAC observed during pregnancy persists for a variable period between 12 and 36 hours postpartum.
Propofol has some advantages (e.g., rapid awakening, decreased incidence of emesis) that make it attractive as an induction agent for short sterilization procedures. When propofol was used for induction and maintenance of anesthesia for cesarean delivery, breast milk samples obtained at 4 and 8 hours postpartum had a low concentration of the drug, which suggested a negligible newborn exposure to propofol.42 Use of sodium thiopental for induction of anesthesia also results in negligible newborn exposure during subsequent breast-feeding.
Alterations occur in the activity of both depolarizing and nondepolarizing muscle relaxants during the postpartum period. Evans and Wroe43 described the changes in plasma cholinesterase activity during pregnancy. A rapid decline in activity occurred during the first trimester. This low level of activity was maintained until delivery and was followed by an even lower level of activity during the first week postpartum. Ganga et al.44 found that a lower dose of succinylcholine was required to achieve 80% twitch suppression in postpartum women than in nonpregnant women. Time to recovery also was prolonged and correlated with lower cholinesterase activity in the postpartum patients. Leighton et al.45 studied four groups of patients: nonpregnant, nonpregnant using oral contraceptives, term pregnant, and postpartum women. Cholinesterase activity was significantly lower in both term pregnant and postpartum women. Recovery time was 25% longer in the postpartum patients than in other groups (685 seconds versus approximately 500 seconds). Although a 3-minute prolongation of paralysis may not seem clinically significant, it could be important if airway difficulties occur.46 Metoclopramide prolongs neuromuscular block with succinylcholine by 135% to 228% because of its inhibition of plasma cholinesterase.46 Ranitidine does not affect either plasma cholinesterase activity or the duration of action of succinylcholine.47
Several studies have evaluated the use of the nondepolarizing muscle relaxants rocuronium, mivacurium, vecuronium, atracurium, and cisatracurium in postpartum patients. Rocuronium’s duration of action is prolonged by approximately 25% in postpartum patients,48 and mivacurium’s duration of action is prolonged by approximately 20%.49 In postpartum patients, the duration of action of vecuronium is prolonged by more than 50%.50 In contrast, the duration of action for atracurium is unchanged51 (Figure 25-5) and that of cisatracurium is significantly shorter in the postpartum period.52 Prolongation of neuromuscular block could be clinically significant during a short procedure. Khuenl-Brady et al.52 suggested that a relative decrease in hepatic blood flow and/or competition between vecuronium and steroid hormones for hepatic uptake may interfere with the hepatic clearance of vecuronium in postpartum women. Alternatively, Gin et al.53 concluded that the duration of action for rocuronium is not prolonged in postpartum women if lean body mass—rather than total body weight—is used to calculate dose. These researchers speculated that the prolonged duration noted earlier49 might be explained by relative drug overdose if the dose of rocuronium is based on the patient’s temporarily increased body weight.54
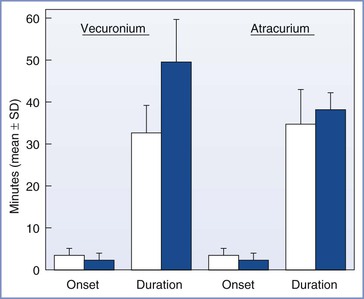
FIGURE 25-5 Onset and duration of action of vecuronium (0.1 mg/kg) and atracurium (0.5 mg/kg) in postpartum (solid bar) and nonpregnant control (open bar) patients. P < .001 for duration of vecuronium in postpartum compared with nonpregnant patients. (From Khuenl-Brady KS, Koller J, Mair P, et al. Comparison of vecuronium- and atracurium-induced neuromuscular blockade in postpartum and nonpregnant patients. Anesth Analg 1991; 72:112.)
Neuraxial Anesthesia
Spinal and epidural anesthesia both provide excellent operating conditions for postpartum tubal ligation. Airway obstruction, hypoventilation, and aspiration are much less likely during and after neuraxial anesthesia. A sensory level of T4 is needed to block visceral pain during exposure and manipulation of the fallopian tubes. The choice between spinal and epidural anesthesia is a matter of personal preference for the patient and the anesthesia provider.
Epidural Anesthesia
When the performance of postpartum tubal ligation is anticipated in a parous patient, I encourage administration of epidural analgesia for labor and delivery. The epidural anesthetic can be extended for immediate postpartum tubal ligation if appropriate. I avoid administration of parenteral opioids during labor if immediate postpartum tubal ligation is planned. Immediate postpartum tubal ligation may save the patient the cost and inconvenience of an extra day in the hospital, allow her to eat shortly after delivery (and surgery), and enable her to avoid the apprehension of undergoing a surgical procedure the following day. The avoidance of opioids helps maintain normal gastric emptying, which should decrease any risk for aspiration during postpartum surgery. If the patient is stable and personnel are available, the procedure may be performed immediately after delivery, once the patient is moved to the operating room. The obstetrician must exclude excessive intrapartum blood loss and document that the patient has given informed consent.5 The patient should also be asked whether the epidural catheter provided adequate analgesia for her delivery. A catheter that was inadequate for labor analgesia is unlikely to provide adequate surgical anesthesia.
Additional intravenous crystalloid may be administered, an epidural test dose is given to rule out intrathecal or intravascular migration of the epidural catheter, and the sensory level is extended with a concentration of local anesthetic suitable for surgical anesthesia. A short-acting local anesthetic (e.g., 3% 2-chloroprocaine) is usually appropriate because the procedure is quite short. An alternative choice is 2% lidocaine with epinephrine 1 : 200,000. Appropriate sedative drugs also may be given, if the patient requests. The anesthesia provider should be cautious about giving sedative drugs that may cause prolonged postpartum amnesia. Women want to remember the first several hours of contact with their newborn. In some cases, peripartum administration of a benzodiazepine may cause retrograde amnesia, and the patient may not recall childbirth.54
If surgery is not performed immediately, the catheter may be left in place for later postpartum tubal ligation. Several studies have evaluated the efficacy of using a previously placed epidural catheter for a tubal ligation performed several hours after delivery. Vincent and Reid55 found that the mean delivery-to-surgery interval was shorter in those patients who had adequate epidural anesthesia than in those without adequate anesthesia (10.6 versus 14.8 hours). The chance of successful epidural anesthesia was greatest if the catheter was used within 4 hours of delivery. Lawlor et al.56 reported an 87% success rate using an indwelling epidural catheter for postpartum tubal ligation, and they observed no difference in the catheter placement-to-surgery interval between the successful epidural and failed epidural groups (21.4 versus 20.5 hours). In this study, each epidural catheter was threaded 4 to 7.5 cm into the epidural space. Similarly, Goodman and Dumas57 reported an overall success rate of 92% with the use of an indwelling epidural catheter for postpartum tubal ligation. The success rate was 93% among patients who underwent surgery less than 24 hours after delivery and 80% among the 10 patients who underwent surgery more than 24 hours after delivery (Figure 25-6). This difference was not significant; however, this study lacked sufficient power to identify a difference of this magnitude. Clinical experience suggests that if the anesthesia provider uses an epidural catheter placed for labor, the risk for anesthesia failure may be greater if surgery is delayed more than 10 hours after delivery. To ensure maximal success when using a multi-orifice catheter, the anesthesia provider should thread the catheter 4 to 6 cm into the epidural space and have the patient assume a deflexed position before taping the catheter to the skin.58,59
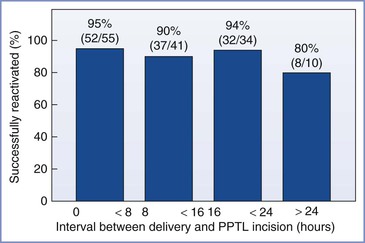
FIGURE 25-6 Rate of successful reactivation of epidural catheters for various intervals between delivery and the incision for postpartum tubal ligation (PPTL). There was no difference among groups in the success rate. (From Goodman EJ, Dumas SD. The rate of successful reactivation of labor epidural catheters for postpartum tubal ligation surgery. Reg Anesth Pain Med 1998; 23:260.)
Spinal Anesthesia
Spinal anesthesia for postpartum tubal ligation has several advantages over epidural anesthesia. Epidural anesthesia requires the use of a large volume of concentrated local anesthetic and thereby introduces the risk for intravascular injection and cardiotoxicity.60 Epidural anesthesia also is time consuming; the induction of epidural anesthesia may require more time than the tubal ligation itself. Spinal anesthesia is simple to perform, is rapid in onset, and provides dense sensory and motor block. In one study, spinal anesthesia for postpartum tubal ligation was associated with lower professional fees and operating room charges than attempted reactivation of an epidural catheter placed during labor.61 The ability to reinject a catheter intraoperatively is not necessary for a short procedure such as postpartum tubal ligation, and there is no need for prolonged postoperative analgesia. The risk for post–dural puncture headache is low if a small-gauge (25- or 27-gauge) pencil-point or non-cutting spinal needle is used. Indeed, some anesthesiologists have suggested that the incidence of post–dural puncture headache in obstetric patients is no different after spinal anesthesia with a 25-gauge Whitacre needle from that after planned epidural anesthesia (Table 25-2).
TABLE 25-2
Risk of Post–Dural Puncture Headache (PDPH) after Spinal and Epidural Anesthesia in Obstetric Patients
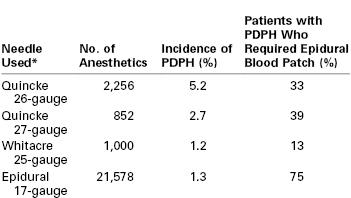
* There was a significant difference in the incidence of post–dural puncture headache between the 26-gauge Quincke needle and the epidural needle (P < .05), but not between the Whitacre needle or the 27-gauge Quincke needle and the epidural needle.
Quincke needles have a cutting bevel. Whitacre needles have a pencil-point tip.
Data from Lambert DH, Hurley RJ, Hertwig L, Datta S. Role of needle gauge and tip configuration in the production of lumbar puncture headache. Reg Anesth 1997; 22:66-72.
Local anesthetic requirements for spinal and epidural anesthesia are decreased during pregnancy, but studies have demonstrated a return to nonpregnant requirements by 36 hours postpartum. Assali and Prystowsky62 demonstrated a return to nonpregnant requirements by 36 to 48 hours postpartum. Abouleish63 prospectively compared the dose of spinal bupivacaine required for cesarean delivery with that required for postpartum tubal ligation. He noted that 30% more bupivacaine was required to achieve a T4 dermatomal level in women who were 8 to 24 hours postpartum. The reason for the rapid decrease in sensitivity to local anesthetics is unclear but may be related to the rapid fall in progesterone levels after delivery of the placenta.
Datta et al.64 examined plasma and cerebrospinal fluid (CSF) progesterone concentrations and spinal lidocaine requirements in nonpregnant, term pregnant, and postpartum women 12 to 18 hours after delivery. Plasma progesterone levels in pregnant women were 60 times higher than in nonpregnant women but only seven times higher than those in postpartum women. CSF progesterone concentrations were eight times higher in term pregnant women and three times higher in postpartum women than in nonpregnant women. Intrathecal lidocaine requirements were similar in pregnant and postpartum patients, even though plasma and CSF progesterone concentrations were lower in the postpartum women. The authors suggested “that a minimum level of progesterone in the CSF and/or plasma is necessary for this heightened local anesthetic activity” associated with progesterone. Together these studies suggest that local anesthetic requirements return to nonpregnant requirements 12 to 36 hours after delivery.63–65
Huffnagle et al.65 gave hyperbaric intrathecal lidocaine 75 mg to postpartum women to determine whether age, weight, height, body mass index, vertebral column length, or time from delivery to placement of the block correlated with the spread of sensory block. Only patient height had a weak positive correlation, and it accounted for less than 15% of the variance in height of the block. Because of the large variation in the spread of sensory block among patients of the same height, the investigators concluded that there was little use in adjusting the dose of local anesthetic on the basis of height.
Many anesthesia providers have discontinued the use of hyperbaric lidocaine for spinal anesthesia because of concern about transient neurologic symptoms or transient radicular irritation, but obstetric patients may be at lower risk for this complication. A prospective nonrandomized study of 303 obstetric patients who received spinal anesthesia during a 9-month period observed a 0% incidence of transient radicular irritation (95% confidence interval 0% to 4.5%).66 Patients underwent a variety of procedures, including cesarean delivery, postpartum tubal ligation, cerclage, and other cases. The number of patients was too small to determine the true incidence of transient radicular irritation in obstetric patients, but the investigators concluded that the true incidence is likely less than 5%. In a randomized controlled trial, Philip et al.67 compared spinal administration of hyperbaric 5% lidocaine with that of hyperbaric 0.75% bupivacaine for postpartum tubal ligation. They observed a 3% incidence of transient neurologic symptoms with the use of lidocaine, compared with a 7% incidence with bupivacaine, a nonsignificant difference. In an editorial accompanying their report, Schneider and Birnbach68 acknowledged that “there are no very short-acting hyperbaric spinal local anesthetics that have taken the place of lidocaine for these short procedures and many believe that spinal bupivacaine lasts too long to be a reasonable choice of anesthetic for a procedure that will last less than 20 minutes.” However, they concluded, “Because pregnant patients represent a population that lies to the extreme in terms of the criteria for safety and lack of morbidity, we believe that for the present, there is still insufficient safety evidence to suggest that spinal hyperbaric 5% lidocaine be routinely used in obstetrics.” At the University of Colorado, some of the anesthesia providers use 5% lidocaine because of its short duration of action, whereas others prefer to avoid spinal lidocaine despite the low risk for transient neurologic symptoms in obstetric patients.
Postpartum women seem to be at lower risk for hypotension during spinal anesthesia than pregnant women, and maintenance of uteroplacental perfusion is not a concern after delivery. Abouleish64 gave ephedrine to correct maternal hypotension in 83% of pregnant women who received spinal bupivacaine anesthesia for cesarean delivery. In contrast, only 7% of postpartum women who received spinal anesthesia for tubal ligation required ephedrine. An autotransfusion of blood occurs immediately after delivery. The greater intravascular volume and the lack of aortocaval compression may help protect postpartum patients from hypotension during spinal anesthesia. Sharma et al.69 compared the use of crystalloid with the use of 6% hetastarch for the prevention of hypotension during spinal anesthesia for postpartum tubal ligation. They observed a 52% incidence of hypotension in the crystalloid group and a 16% incidence in the hetastarch group. However, they acknowledged that the greater expense of colloid, as well as the risk for an allergic reaction, might not be justifiable. Suelto et al.70 compared normotensive and hypertensive patients receiving hyperbaric lidocaine for postpartum tubal ligation and found no difference in the use or dose of ephedrine for treatment of hypotension.
Preservative-free intrathecal meperidine can be used as an alternative to local anesthetic for postpartum tubal ligation. The typical dose is 1 mg per kilogram prepregnant weight (50 to 80 mg) for cesarean delivery or tubal ligation. With an onset time of 3 to 5 minutes and a duration of 30 to 60 minutes, intrathecal meperidine compares favorably with 5% lidocaine. In a study that compared intrathecal lidocaine 70 mg with intrathecal meperidine 60 mg for postpartum tubal ligation, patients who received meperidine had more pruritus but longer postoperative analgesia (448 versus 83 minutes, respectively).71 There was no difference between groups in rates of nausea, hemoglobin desaturation, or patient satisfaction. Intrathecal meperidine may be an alternative to lidocaine for postpartum tubal ligation.
Box 25-1 summarizes a personal approach to anesthetic management for postpartum tubal ligation.
Postoperative Analgesia
Postpartum tubal ligation produces modest postoperative pain of short duration. Patients may receive one dose of parenteral opioid postoperatively, followed by oral analgesics. Optimal analgesia encourages early ambulation, interaction with the newborn, and early discharge from the hospital. An oral nonsteroidal anti-inflammatory drug (NSAID) such as ibuprofen may be given to supplement other analgesics. When epinephrine 0.2 mg was added to lidocaine with fentanyl 10 µg for spinal anesthesia, the duration of complete and effective analgesia was prolonged and the incidence of pruritus was decreased, but the time to complete motor recovery was also prolonged.72 Habib et al.73 reported that adding intrathecal morphine 0.05 mg to intrathecal bupivacaine and fentanyl for postpartum tubal ligation resulted in less intense pain at rest and with movement at 4 hours after surgery than with saline control. However, patients who received morphine had more vomiting and pruritus. Despite side effects, patients who received morphine were significantly more satisfied. Similarly, Marcus et al.74 found that epidural morphine 2 mg provided better analgesia without increasing the need to treat side effects, in comparison with a regimen of oral opioids and NSAIDs without epidural morphine. Although effective, spinal and epidural morphine analgesia should be used with caution because these patients could be discharged soon after postpartum tubal ligation, before the risk for delayed respiratory depression has lapsed. The method of postoperative analgesia chosen should not delay patient discharge because of side effects or the need for postoperative respiratory monitoring.
Local anesthetic infiltration of the mesosalpinx with bupivacaine or topical application of a local anesthetic to the fallopian tubes significantly decreases opioid requirements postoperatively.75 These are simple, rapid techniques that can be used by the obstetrician. Wittels et al.76 reported that use of multimodal therapy with spinal or epidural anesthesia consisting of intravenous ketorolac 60 mg, intravenous metoclopramide 10 mg, and infiltration of the incised skin, fallopian tubes, and mesosalpinx with 0.5% bupivacaine prevented pain, nausea, and painful uterine cramping in both the immediate postoperative period and for 7 days after postpartum tubal ligation in 9 of 10 patients. The manufacturer of ketorolac has stated that ketorolac is contraindicated in nursing mothers because of the possible adverse effects of prostaglandin synthetase inhibitors on neonates. In contrast, the American Academy of Pediatrics considers ketorolac to be compatible with breast-feeding.77
References
1. Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1–6.
2. Practice Guidelines for Obstetric Anesthesia. An updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–863.
3. Peterson HB. Sterilization. Obstet Gynecol. 2008;111:189–203.
4. Kulier R, Boulvain M, Walker DM, et al. Minilaparotomy and endoscopic techniques for tubal sterilization. Cochrane Database Syst Rev. 2004;(3).
5. American College of Obstetricians and Gynecologists. Benefits and risks of sterilization. ACOG Practice Bulletin No. 133. Washington, D.C., February 2013. Obstet Gynecol. 2013;121:392–404.
6. Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 23rd edition. McGraw-Hill: New York; 2009.
7. Peterson HB, Zhisen X, Hughes JM, et al. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol. 1996;174:1161–1170.
8. Medicaid. Sterilizations, hysterectomies and abortions. Consent form, Form 499-A, MED-178 (rev 11/85). [Available at] http://hlunix.hl.state.ut.us/medicaid/pdfs/forms/sterilizationconsent.pdf.
9. Thurman AR, Janecek T. One-year follow-up of women with unfulfilled postpartum sterilization requests. Obstet Gynecol. 2010;116:1071–1077.
10. Vincent RD, Martin RQ. Postpartum tubal ligation after pregnancy complicated by preeclampsia or gestational hypertension. Obstet Gynecol. 1996;88:119–122.
11. Toledo P, McCarthy RJ, Hewlett BJ, et al. The accuracy of blood loss estimation after simulated vaginal delivery. Anesth Analg. 2007;105:1736–1740.
12. Ito S. Drug therapy: drug therapy for breast-feeding women. N Engl J Med. 2000;343:118–125.
13. Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States. Obstet Gynecol. 2011;117:69–74.
14. Löfgren M, Bäckström T. Serum concentrations of progesterone and 5 alpha-pregnane-3,20-dione during labor and early post partum. Acta Obstet Gynecol Scand. 1990;69:123–126.
15. Llauro JL, Runnebaum B, Zander J. Progesterone in human peripheral blood before, during and after labor. Am J Obstet Gynecol. 1968;101:867–873.
16. O’Sullivan GM, Sutton AJ, Thompson SA, et al. Noninvasive measurement of gastric emptying in obstetric patients. Anesth Analg. 1987;66:505–511.
17. Gin T, Cho AMW, Lew JKL, et al. Gastric emptying in the postpartum period. Anaesth Intens Care. 1991;19:521–524.
18. Whitehead EM, Smith M, Dean Y, O’Sullivan G. An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48:53–57.
19. Sandhar BK, Elliott RH, Windram I, Rowbotham DJ. Peripartum changes in gastric emptying. Anaesthesia. 1992;47:196–198.
20. Wong CA, Loffredi M, Ganchiff JN, et al. Gastric emptying of water in term pregnancy. Anesthesiology. 2002;96:1395–1400.
21. Wong CA, McCarthy RJ, Fitzgerald PC, et al. Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105:751–755.
22. Kubli M, Scrutton MJ, Seed PT, et al. An evaluation of isotonic “sport drinks” during labor. Anesth Analg. 2002;94:404–408.
23. Jayaram A, Bowen MP, Deshpande S, Carp HM. Ultrasound examination of the stomach contents of women in the postpartum period. Anesth Analg. 1997;84:522–526.
24. Scrutton MJ, Metcalfe GA, Lowy C, et al. Eating in labour: a randomized controlled trial assessing the risks and benefits. Anaesthesia. 1999;54:329–334.
25. Wright PMC, Allen RW, Moore J, Donnelly JP. Gastric emptying during lumbar extradural analgesia in labor: effect of fentanyl supplementation. Br J Anaesth. 1992;68:248–251.
26. Kelly MC, Carabine UA, Hill DA, Mirakhur RK. A comparison of the effect of intrathecal and extradural fentanyl on gastric emptying in laboring women. Anesth Analg. 1997;85:834–836.
27. Coté CJ. Aspiration: An overrated risk in elective patients. Stoelting RK, Barash PG, Gallagher TJ. Advances in Anesthesia. Mosby: St. Louis; 1992:5–6.
28. Blouw R, Scatliff J, Craig DB, Palahniuk RJ. Gastric volume and pH in postpartum patients. Anesthesiology. 1976;45:456–457.
29. James CF, Gibbs CP, Banner T. Postpartum perioperative risk of aspiration pneumonia. Anesthesiology. 1984;61:756–759.
30. Lam KK, So HY, Gin T. Gastric pH and volume after oral fluids in the postpartum patient. Can J Anaesth. 1993;40:218–221.
31. Brock-Utne JG, Dow TGB, Welman S, et al. The effect of metoclopramide on the lower oesophageal sphincter in late pregnancy. Anaesth Intens Care. 1978;6:26–29.
32. Vanner RG, Goodman NW. Gastro-oesophageal reflux in pregnancy at term and after delivery. Anaesthesia. 1989;44:808–811.
33. Bogod DG. The postpartum stomach: when is it safe? (editorial). Anaesthesia. 1994;49:1–2.
34. American College of Obstetricians and Gynecologists. Antibiotic prophylaxis for gynecologic procedures. ACOG Practice Bulletin No. 104. Washington, D.C., May 2009. Obstet Gynecol. 2009;113:1180–1189.
35. Cruikshank DP, Laube DW, DeBacker LJ. Intraperitoneal lidocaine anesthesia for postpartum tubal ligation. Obstet Gynecol. 1973;42:127–130.
36. Poindexter AN, Abdul-Malak M, Fast JE. Laparoscopic tubal sterilization under local anesthesia. Obstet Gynecol. 1990;75:5–8.
37. DeStefano F, Greenspan JR, Dicker RC, et al. Complications of interval laparoscopic tubal sterilization. Obstet Gynecol. 1983;61:153–158.
39. Marx GF, Kim YI, Lin CC, et al. Postpartum uterine pressures under halothane or enflurane anesthesia. Obstet Gynecol. 1978;51:695–698.
40. Chan MTV, Gin T. Postpartum changes in the minimum alveolar concentration of isoflurane. Anesthesiology. 1995;82:1360–1363.
41. Zhou HH, Norman P, DeLima LGR, et al. The minimum alveolar concentration of isoflurane in patients undergoing bilateral tubal ligation in the postpartum period. Anesthesiology. 1995;82:1364–1368.
42. Dailland P, Cockshott ID, Lorzin JD, et al. Intravenous propofol during cesarean section: placental transfer, concentrations in breast milk, and neonatal effects. A preliminary study. Anesthesiology. 1989;71:827–834.
43. Evans RT, Wroe JM. Plasma cholinesterase changes during pregnancy. Anaesthesia. 1980;35:651–654.
44. Ganga CC, Heyduk JV, Marx GF, Sklar GS. A comparison of the response to suxamethonium in postpartum and gynaecological patients. Anaesthesia. 1982;37:903–906.
45. Leighton BL, Cheek TG, Gross JB, et al. Succinylcholine pharmacodynamics in peripartum patients. Anesthesiology. 1986;64:202–205.
46. Kao YJ, Turner DR. Prolongation of succinylcholine block by metoclopramide. Anesthesiology. 1989;70:905–908.
47. Woodworth GE, Sears DH, Grove TM, et al. The effect of cimetidine and ranitidine on the duration of action of succinylcholine. Anesth Analg. 1989;68:295–297.
48. Puhringer FK, Spart HJ, Mitterschiffthaler G, et al. Extended duration of action of rocuronium in postpartum patients. Anesth Analg. 1997;84:352–354.
49. Gin T, Derrick JL, Chan MTV, et al. Postpartum patients have slightly prolonged neuromuscular block after mivacurium. Anesth Analg. 1998;86:82–85.
50. Hawakins JL, Adenwala J, Camp C, Joyce TH. The effect of H2-receptor antagonist premedication on the duration of vecuronium-induced neuromuscular blockade in postpartum patients. Anesthesiology. 1989;71:175–177.
51. Khuenl-Brady KS, Koller J, Mair P, et al. Comparison of vecuronium and atracurium-induced neuromuscular blockade in postpartum and nonpregnant patients. Anesth Analg. 1991;72:110–113.
52. Pan PH, Moore C. Comparison of cisatracurium-induced neuromuscular blockade between immediate postpartum and nonpregnant patients. J Clin Anesth. 2001;13:112–117.
53. Gin T, Chan MTV, Chan KL, et al. Prolonged neuromuscular block after rocuronium in postpartum patients. Anesth Analg. 2002;94:686–689.
54. Camann W, Cohen MB, Ostheimer GW. Is midazolam desirable for sedation in parturients (letter)? Anesthesiology. 1986;65:441.
55. Vincent RD, Reid RW. Epidural anesthesia for postpartum tubal ligation using epidural catheters placed during labor. J Clin Anesth. 1993;5:289–291.
56. Lawlor M, Weiner M, Fantauzzi M, Johnson C. Efficacy of epidural anesthesia for post-partum tubal ligation utilizing indwelling labor epidural catheters. Reg Anesth. 1994;19:54.
57. Goodman EJ, Dumas SD. The rate of successful reactivation of labor epidural catheters for postpartum tubal ligation surgery. Reg Anesth Pain Med. 1998;23:258–261.
58. Beilin Y, Bernstein HH, Zucker-Pinchoff B. The optimal distance that a multiorifice epidural catheter should be threaded into the epidural space. Anesth Analg. 1995;81:301–304.
59. Hamilton CL, Riley ET, Cohen SE. Changes in the position of epidural catheters associated with patient movement. Anesthesiology. 1997;86:778–784.
60. Abouleish EI, Elias M, Nelson C. Ropivacaine-induced seizure after extradural anaesthesia. Br J Anaesth. 1998;80:843–844.
61. Vixcomi CM, Rathmell JP. Labor epidural catheter reactivation or spinal anesthesia for delayed postpartum tubal ligation: a cost comparison. J Clin Anesth. 1995;7:380–383.
62. Assali NS, Prystowsky H. Studies on autonomic blockade. I. Comparison between the effects of tetraethylammonium chloride (TEAC) and high selective spinal anesthesia on blood pressure of normal and toxemic pregnancy. J Clin Invest. 1950;29:1354–1366.
63. Abouleish EI. Postpartum tubal ligation requires more bupivacaine for spinal anesthesia than does cesarean section. Anesth Analg. 1986;65:897–900.
64. Datta S, Hurley RJ, Naulty JS, et al. Plasma and cerebrospinal fluid progesterone concentrations in pregnant and nonpregnant women. Anesth Analg. 1986;65:950–954.
65. Huffnagle S, Norris MC, Leighton BL, et al. Do patient variables influence the subarachnoid spread of hyperbaric lidocaine in the postpartum patient? Reg Anesth. 1994;19:330–334.
66. Wong CA, Slavenas P. The incidence of transient radicular irritation after spinal anesthesia in obstetric patients. Reg Anesth Pain Med. 1999;24:55–58.
67. Philip J, Sharma SK, Gottumukkala VNR, et al. Transient neurologic symptoms after spinal anesthesia with lidocaine in obstetric patients. Anesth Analg. 2001;92:405–409.
68. Schneider MC, Birnbach DJ. Lidocaine neurotoxicity in the obstetric patient: is the water safe? Anesth Analg. 2001;92:287–290.
69. Sharma SK, Gajraj NM, Sidawi JE. Prevention of hypotension during spinal anesthesia: a comparison of intravascular administration of hetastarch versus lactated Ringer’s solution. Anesth Analg. 1997;84:111–114.
70. Suelto MD, Vincent RD, Larmon JE, et al. Spinal anesthesia for postpartum tubal ligation after pregnancy complicated by preeclampsia or gestational hypertension. Reg Anesth Pain Med. 2000;25:170–173.
71. Norris MC, Honet JE, Leighton BL, et al. A comparison of meperidine and lidocaine for spinal anesthesia for postpartum tubal ligation. Reg Anesth. 1996;21:84–88.
72. Malinow AM, Mokriski BLK, Nomura MK, et al. Effect of epinephrine on intrathecal fentanyl analgesia in patients undergoing postpartum tubal ligation. Anesthesiology. 1990;73:381–385.
73. Habib AS, Muir HA, White WD, et al. Intrathecal morphine for analgesia after postpartum bilateral tubal ligation. Anesth Analg. 2005;100:239–243.
74. Marcus RL, Wong CA, Lehor A, et al. Postoperative epidural morphine for postpartum tubal ligation analgesia. Anesth Analg. 2005;101:876–881.
75. Alexander CD, Wetchler BV, Thompson RE. Bupivacaine infiltration of the mesosalpinx in ambulatory surgical laparoscopic tubal sterilization. Can J Anaesth. 1987;34:362–365.
76. Wittels B, Faure EAM, Chavez R, et al. Effective analgesia after bilateral tubal ligation. Anesth Analg. 1998;87:619–623.
77. American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137–150.