Trauma and Critical Care
Luis Pacheco MD, Paul Howell MBChB, FRCA, Edward R. Sherwood MD, PhD
Chapter Outline
The management of critically ill obstetric patients most commonly involves treatment of disease processes that occur as a direct consequence of pregnancy. Although sometimes life threatening, these conditions are usually reversible. Delivery of the infant often attenuates or ablates the disease process, and the mother typically recovers with supportive and resuscitative measures. Primary obstetric disorders account for 50% to 80% of intensive care unit (ICU) admissions for pregnant patients; approximately 80% of these admissions result from preeclampsia, sepsis, and/or hemorrhage (Box 55-1).1,2 The estimated ICU admission rate for obstetric patients is 0.5% to 1% in the United States; the mortality rate in this population is 12% to 20%.3
Trauma accounts for 45% to 50% of all maternal deaths in the United States, and it is the most common nonobstetric cause of maternal death.4 Other common nonobstetric causes of ICU admission are respiratory failure, endocrine disorders, preexisting autoimmune disease, and thromboembolic disorders.5 Ethnic minorities and women with low socioeconomic status have the highest rates of morbidity and mortality. Modern medicine has allowed women with complex medical problems such as congenital heart disease and cystic fibrosis to survive into childbearing age, and these patients are at increased risk for complications during pregnancy and have a higher incidence of ICU admission. Among critically ill obstetric patients admitted to an ICU, the most common cause of death is acute respiratory distress syndrome (ARDS), which can complicate both obstetric and nonobstetric disease processes.6
Critical maternal illness places the fetus at significant risk for morbidity and mortality. Important fetal risk factors include maternal shock, transfusion of blood products, and early gestational age at the time of critical maternal illness.7
Trauma during Pregnancy
Epidemiology
Trauma affects 5% to 7% of pregnancies in the United States and is the leading nonobstetric cause of maternal death; as many as 20% of affected women require emergency surgery.8,9 Motor vehicle accidents are the most common cause of injury-related maternal death (49% to 70%), followed by domestic violence (11% to 25%) and falls (9% to 23%).10–12 Not using a seat belt is a major risk factor for maternal and fetal injury in motor vehicle trauma.13 Penetrating trauma and burns are far less common than blunt mechanisms of injury. The rate of maternal trauma admission to an ICU increases with each trimester: 8% occur in the first trimester, 40% in the second trimester, and 52% in the third trimester.14 Most women are able to continue their pregnancy at home, but up to 38% are hospitalized until delivery.
Risk factors for maternal trauma include age younger than 25 years, low socioeconomic status, minority race, use of illicit drugs or alcohol, and domestic violence.14,15 It is important to remember that any female patient of reproductive age who is a victim of trauma could be pregnant at the time of injury.
Complications and Outcomes
As in the general population, hemorrhagic shock and brain injury are the most common mechanisms of death in pregnant trauma victims.16 Pelvic and acetabular fractures also pose a significant risk. Injuries and complications that are unique to pregnant trauma victims include uterine rupture, placental abruption, preterm labor, and direct fetal injury. Although rare (0.6% of injuries), uterine rupture is a major threat to the life of both the mother (10% mortality) and the fetus (near 100% mortality).17 Placental abruption complicates 1% to 5% of minor injuries and 20% to 60% of major trauma and usually occurs from 16 weeks’ gestation onward.18 Placental abruption can cause major overt and occult hemorrhage and coagulopathy and should be considered as a possible source of bleeding in the unstable pregnant trauma patient. Preterm labor is a common (25%) complication of trauma and can be precipitated even in cases of apparently minor injury.19
Premature rupture of membranes (PROM) increases the risk for both preterm labor and infection. Amniotic fluid embolism is a rare complication of maternal trauma, but it should be considered as part of the differential diagnosis in patients who are refractory to resuscitation.
Fetal-maternal (transplacental) hemorrhage can occur after trauma and result in maternal isoimmunization with the D antigen of the fetal red blood cell Rhesus protein complex (Rh0[D]) in the Rh-negative mother (see Chapter 6). The Kleihauer-Betke test is used to identify fetal blood in the maternal circulation after maternal injury. When fetal-maternal hemorrhage is present, treatment with Rh0(D) immune globulin (RhoGAM) is generally indicated. In a study performed at the R. Adams Cowley Shock Trauma Center of the University of Maryland, more than 50% of evaluated pregnant trauma victims were positive for fetal-maternal hemorrhage as determined by a positive Kleihauer-Betke test.20 Essentially all patients with a positive test had uterine contractions, whereas patients with a negative Kleihauer-Betke test did not have contractions. The investigators concluded that the Kleihauer-Betke test was a sensitive and specific predictor of preterm labor in pregnant trauma patients and should be performed in all victims regardless of blood Rh phenotype.
Fetal Trauma and Outcome
The fetal mortality rate after maternal blunt trauma has been reported to range from 3.4% to 38.0%; placental abruption, uterine rupture, maternal shock, and maternal death are the most frequent factors associated with fetal demise (Box 55-2).15,21 The risk for direct fetal trauma increases with gestational age because the bony pelvis protects the uterus and fetus prior to 13 weeks’ gestation. Pregnant women who sustain blunt trauma have a lower risk for bowel injury than nonpregnant patients because the uterus acts as a shield and pushes the abdominal contents into the upper abdomen.22,23 Maternal pelvic fractures are associated with uteroplacental injury and fetal skull fractures. Skull fracture is the most common direct fetal injury and has a reported fetal mortality rate of 42%.24
The relationship between the Injury Severity Score (ISS) (see later discussion) and fetal outcome is controversial. Some studies have shown a direct relationship between the ISS and the incidence of fetal demise, whereas others have not. Analysis of outcomes from 1195 pregnant trauma victims showed that an ISS of greater than 15 was associated with increased risk for fetal demise.25,26 Evidence suggests that decreased serum bicarbonate, an indicator of systemic hypoperfusion, is associated with fetal demise after maternal trauma. Altered maternal mental status and the presence of head trauma have also been linked to adverse fetal outcomes.
It is crucial to preserve maternal cardiac output, blood pressure, and oxygen delivery to optimize maternal recovery and protect fetal well-being. However, fetal loss can occur even if the mother has not incurred serious injuries. Thus, all pregnant women should be evaluated in a medical setting after trauma, regardless of the apparent severity of injury. The fetus remains at risk for delayed complications after maternal discharge from the hospital. Delayed complications include a twofold increase in the risk for preterm delivery and a ninefold increase in the risk for fetal death.27 Late complications of trauma, such as cerebral palsy, have also been reported in children born to mothers who experienced trauma during pregnancy.28,29
Initial Assessment and Resuscitation
The initial assessment and resuscitation should focus on the mother; it is axiomatic that maternal resuscitation typically facilitates fetal resuscitation. A systematic approach to initial resuscitation and stabilization should be used (Figure 55-1).4 Immediate interventions are initiated to identify and treat life-threatening conditions based on the principles of Advanced Trauma Life Support (ATLS).30 Initial focus should be placed on ensuring adequate airway protection, ventilation (breathing), and circulation (the “ABCs” of resuscitation). Pregnant women experience significant changes in cardiopulmonary and metabolic function that must be considered during resuscitation (Table 55-1).
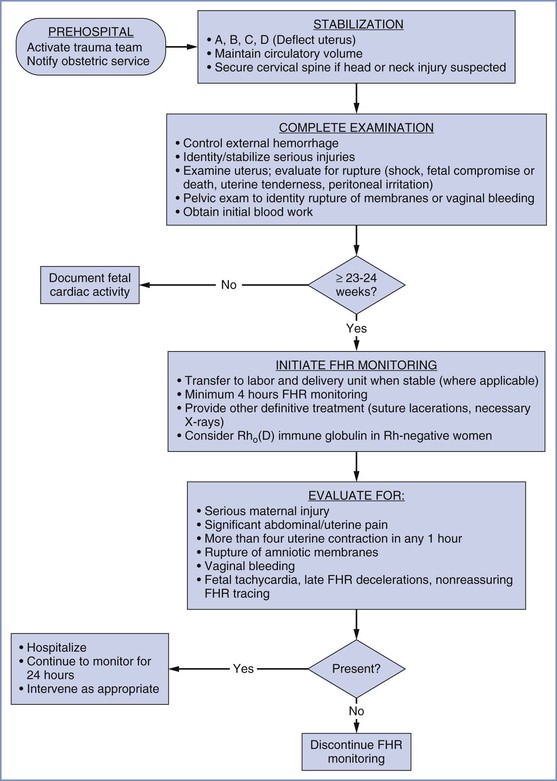
FIGURE 55-1 Algorithm for management of the pregnant woman after trauma. FHR, fetal heart rate. (Modified from Chames MC, Pearlman MD. Trauma during pregnancy: outcomes and clinical management. Clin Obstet Gynecol 2008; 51:398-408.)
TABLE 55-1
Physiologic Changes of Pregnancy That May Affect Trauma Management
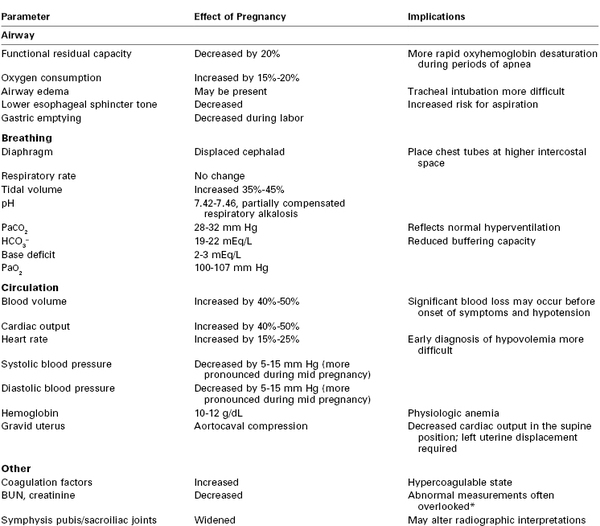
* The reported measurements may fall within the normal range for the hospital laboratory, but the measurements may actually be abnormally high for a pregnant patient.
BUN, blood urea nitrogen.
Airway
Airway patency, stabilization, and protection should be ensured as quickly as possible in all critically injured patients, including those who are pregnant. The status of the patient’s airway can be quickly assessed by eliciting a verbal response. The inability to speak is an indication of severely impaired mental status or the inability to move adequate air to mediate phonation, either of which should prompt interventions to secure and protect the airway. Additional means of assessing airway patency include auscultation of the chest, assessment of chest movement, and assessment of air movement at the oral/nasal openings. Immediate interventions to establish airway patency include head tilt and jaw thrust maneuvers, as well as placement of an oral or nasopharyngeal airway to facilitate bag-mask ventilation and oxygenation.
It is essential to consider the possibility of cervical spine injury, facial fractures, and skull base injuries. Excessive head tilt maneuvers can worsen injury if a cervical spine fracture is present.31 Patients who have sustained severe trauma should be suspected of having a cervical spine injury until proven otherwise. Cervical spine injury must be ruled out by radiographic and physical examination criteria.32,33 The cervical spine should be stabilized with a hard collar and in-line stabilization until the severity of injury has been established. In cases of documented cervical spine injury, great care must be taken not to worsen spinal cord injury. A nasopharyngeal airway should not be placed in patients with suspected facial or skull base fractures to avoid further trauma and worsening of preexisting conditions.
Most patients who require interventions to open or support the airway, as just described, will ultimately require tracheal intubation. Indications for tracheal intubation include impaired mental status, airway obstruction, inability to clear secretions or blood from the airway, inadequate spontaneous ventilation, and hypoxemia that is refractory to supplemental oxygen administration.34 Tracheal intubation of pregnant patients is complicated by changes in respiratory system structure and function (see Table 55-1) (see Chapter 30).18 Among the most prominent alterations are airway (including vocal cord) edema, decreased functional residual capacity, and increased oxygen consumption. Airway edema impairs vocal cord visualization, thus complicating laryngoscopy and tracheal intubation. Decreased functional residual capacity and increased oxygen consumption result in more rapid oxyhemoglobin desaturation during periods of apnea. These factors increase the risk for failed tracheal intubation and hypoxemia.
Gastric emptying is normal in pregnant women before the onset of labor. However, lower esophageal sphincter tone is commonly decreased in pregnant women (see Table 55-1). Thus, pregnant women are at increased risk for regurgitation and pulmonary aspiration of gastric contents, although all trauma victims are considered to have a full stomach on arrival in the emergency department or operating room. Therefore, in most cases, rapid-sequence induction of general anesthesia is performed to facilitate tracheal intubation. However, the specific tracheal intubation technique will depend on the practitioner’s skills and resources, as well as on the location of the patient’s injuries. Alternative approaches to rapid-sequence induction include awake tracheal intubation and tracheostomy.
Several factors can complicate tracheal intubation in the trauma patient. The patient may be combative, which complicates awake tracheal intubation strategies. Blood in the airway can also limit the use of a fiberoptic bronchoscope and impair visualization of the glottis when using a standard or video laryngoscope. The presence of facial fractures, direct airway injuries, trauma-induced airway edema, and tracheal deviation can limit access to the airway.
Finally, airway management, including tracheal intubation, is more challenging in the presence of cervical spine injury. If cervical spine injury is present or suspected, it is crucial to avoid flexion, extension, or lateral movement of the neck. The spine is protected using in-line stabilization and/or a hard cervical collar. Airway management devices such as a gum elastic bougie, a video laryngoscope, a lighted intubating stylette, and/or an intubating laryngeal mask airway (LMA), among others, should be available for use if standard laryngoscopy is difficult or impossible. A supraglottic airway device such as an LMA can be used to temporarily provide ventilation in cases in which mask ventilation and tracheal intubation have failed, but an LMA will not provide protection from aspiration and should be replaced by a secure airway device as soon as possible. In some cases, cricothyroidotomy or tracheostomy may be necessary to provide a secure airway.
Breathing
Adequate ventilation and oxygenation should be ensured for the benefit of both the mother and the fetus. Supplemental oxygen should be administered immediately, even if the patient is breathing spontaneously. Mechanical ventilation is often necessary after tracheal intubation in patients with respiratory failure and/or hypoxemia. Ventilation can be compromised by trauma-associated factors such as pneumothorax, hemothorax, lung contusion, mediastinal compression, and chest wall injuries. These problems must be identified during the primary survey and treated to optimize ventilation and oxygenation. In women with advanced pregnancy, it may be necessary to place chest tubes more cephalad than normal owing to the cephalad displacement of the diaphragm and intra-abdominal structures by the gravid uterus. Pregnant trauma patients should be ventilated to maintain PaCO2 at a level that is normal for pregnancy (28 to 32 mm Hg) (see Table 55-1). Positive end-expiratory pressure (PEEP) may be added to improve oxygenation, if indicated; however, PEEP should be titrated carefully in the hypovolemic patient because it may impair venous return and worsen cardiac output and organ perfusion.
Circulation
Once respiratory stabilization has been achieved, it is essential to assess cardiovascular function and to determine whether the patient is in shock. Two large-bore peripheral intravenous catheters should be placed in the upper extremities to facilitate resuscitation. Central venous access facilitates rapid resuscitation but may be difficult to obtain. Intraosseous cannulation should be considered if it is difficult or impossible to obtain peripheral or central venous access.
Fluid resuscitation should be initiated using crystalloid solution, but blood transfusion should be considered if significant blood loss is apparent or suspected. Left uterine displacement should be initiated immediately to prevent or minimize aortocaval compression by the gravid uterus. The adverse effects of aortocaval compression may be exacerbated during periods of trauma-associated hypovolemia. The use of the pneumatic antishock garment to stabilize fractures or control hemorrhage is relatively contraindicated in pregnant women owing to its adverse effects on venous return.
The hallmark clinical signs of shock are listed in Box 55-3. The presence of these signs indicates a need for timely and appropriate fluid resuscitation. A rapid assessment of sources of blood loss should be performed. In trauma victims, the most common locations of exsanguinating blood loss are the chest, abdomen, retroperitoneum, long bones, and external sites. In the pregnant trauma patient, placental abruption and uterine rupture are also potential sources of hemorrhage. A brief physical examination will identify fractures of the long bones and external sites of bleeding. Thoracic blood loss and pelvic fractures can be identified by chest and pelvic radiographs, respectively. Focused abdominal sonography in trauma (FAST) or diagnostic peritoneal lavage can be used to identify intra-abdominal bleeding. However, diagnostic peritoneal lavage may be difficult to perform safely in advanced pregnancy. FAST can be rapidly performed to assess the hepatorenal, splenorenal, and pelvic spaces, which are the most common sites of major hemorrhage in trauma patients. FAST can also be used to assess uteroplacental integrity and the presence of intrauterine bleeding. Finally, ultrasonography facilitates assessment of cardiac filling and recognition of cardiac tamponade in patients with thoracic trauma.
It is important to recognize that pregnant trauma patients may lose a significant amount of blood before the development of hypotension. Pregnant patients have a 40% to 50% increase in blood volume by the third trimester. Classic signs of hypovolemia such as tachycardia, hypotension, and poor capillary refill may not be evident until blood loss approaches 1.5 to 2 liters. Therefore, it is likely that a pregnant trauma victim will have lost significantly more blood volume and oxygen-carrying capacity than a comparable nonpregnant patient when signs of cardiovascular deterioration become evident. Resuscitation should be guided by apparent blood loss to maintain adequate maternal cardiac output and uteroplacental perfusion. Because of the physiologic anemia of pregnancy, oxygen-carrying capacity may be significantly impaired at the time that hypovolemia becomes evident. In addition, maternal perfusion of vital organs is often sustained at the expense of uteroplacental perfusion. Uterine blood flow may decrease by as much as 30% before the mother shows signs of hypovolemia. Therefore, a nonreassuring fetal heart rate (FHR) pattern may be the first sign of significant intravascular volume loss. Fluids should be warmed to minimize the risk for hypothermia, which can contribute to coagulopathy, arrhythmias, and altered drug responses.
Fluid Resuscitation.
Current practice supports the use of crystalloid solutions to resuscitate the hypovolemic trauma victim. However, the crystalloid versus colloid debate remains to be fully resolved. The Saline versus Albumin Fluid Evaluation (SAFE)35 did not show any difference in survival in nonpregnant trauma patients randomized to receive resuscitation with either colloid or crystalloid, with the exception of patients with head trauma, who had worse outcomes when resuscitated with albumin. However, colloid solutions are anecdotally preferred in some trauma centers. The current ATLS guidelines advocate the use of lactated Ringer’s solution for initial fluid resuscitation.30 Lactated Ringer’s solution has significant buffering properties and is less likely to cause hyperchloremic metabolic acidosis during high-volume resuscitation than normal saline solution. Other buffered salt solutions such as Plasma-Lyte, Ringer’s ethyl pyruvate, and Ringer’s hydroxybutyrate also may have value. Currently, no evidence supports the use of one buffered isotonic crystalloid solution over another.
The use of hypertonic crystalloid solutions such as 3% sodium chloride is controversial; currently no evidence supports their use in pregnant trauma victims. Hypernatremia is a risk in patients resuscitated with hypertonic saline, and some studies have shown increased mortality in patients resuscitated with hypertonic crystalloid solutions.36
Some practitioners have advocated hypovolemic resuscitation in patients with major hemorrhage after trauma.37 This technique employs permissive hypotension (systolic blood pressure of 80 to 90 mm Hg) until hemorrhage can be controlled in the operative setting. The underlying premise of hypovolemic (hypotensive) resuscitation is that over-resuscitation worsens ongoing blood loss as a result of higher perfusion pressure and dilution of clotting factors. Small boluses of fluids are administered to maintain perfusion in patients without evidence of closed head injury. The use of hypotensive resuscitation is likely to be detrimental in patients with closed head injury because it is crucial to maintain adequate cerebral perfusion pressure (CPP) in patients with elevated intracranial pressure (ICP).38 (CPP is the difference between mean arterial pressure [MAP] and ICP.) No definitive published data support the use of hypotensive resuscitation in pregnant trauma patients. Current guidelines do not support this approach because it may compromise uteroplacental perfusion.
Damage Control Principles and Resuscitation.
The traditional approach to treatment of traumatic life-threatening injuries has been definitive operative repair. However, some patients experience progressive physiologic decline during long surgical procedures and develop severe derangements such as hypothermia, metabolic acidosis, and coagulopathy, a combination that has become known as the deadly triad.39 These pathologic alterations require rapid and effective treatment to prevent severe morbidity and death. More recently, some practitioners have advocated the use of a more targeted approach, termed damage control, which is initiated to control hemorrhage without providing early definitive repair of injuries.40 Major surgical bleeding is controlled, and the thoracic and abdominal cavities are packed to provide hemostasis. Gastrointestinal diversion is performed, and body cavities are temporarily closed, often using vacuum-type closure systems. Active volume resuscitation is performed to achieve metabolic homeostasis. On achievement of stable hemodynamic and acid-base status, coagulation function, and temperature, the patient is taken back to the operating room for definitive repair of injuries.
Blood Products.
All trauma centers should have rapid access to type O, Rh-negative blood for emergency use before type-specific or crossmatched blood is available. Recently, some trauma specialists have advocated damage-control resuscitation using packed red blood cells and fresh frozen plasma mixed in equal proportions (1 : 1) (see Chapter 38). Several investigators have reported the value of this approach in military practice, and they have specifically observed that this approach results in more effective resuscitation, less coagulopathy, and improved survival than more traditional approaches.41 It remains unclear whether this approach will be advantageous in civilian practice, but many centers are investigating its use.
In the setting of uncontrolled hemorrhage, recombinant activated factor VII (rFVIIa) has been shown to be effective in treating severe coagulopathy in a small number of observational studies. Case reports and case series have described the efficacy of rFVIIa during massive hemorrhage in trauma and obstetric patients (see Chapter 38).37 A multidisciplinary group in Israel reported that rFVIIa was effective in decreasing severe bleeding in 36 trauma patients.42 European consensus guidelines also advocate the use of rFVIIa in trauma patients with massive uncontrolled hemorrhage that is refractory to conventional transfusion of blood products.43 However, further research is needed before rFVIIa can be endorsed for treatment of massive hemorrhage, owing to its high cost and an incomplete risk-benefit analysis. A major concern is the potential increased risk for thromboembolism in patients treated with rFVIIa.
Secondary Survey
As in all trauma cases, it is crucial to evaluate the mother for significant abdominal, thoracic, orthopedic, and neurologic injuries. A head-to-toe examination should be performed to determine the presence of injuries and the need for intervention. A more detailed evaluation of neurologic function, as well as examination of the head and neck, should be performed. This survey includes examination of posterior structures that may be obscured by the supine position and the presence of a cervical collar. The torso should be examined to identify thoracic and abdominal injuries. The thoracic examination should include chest auscultation, inspection, and palpation. Palpation of the abdomen should be performed to evaluate abdominal tenderness, and a rectal examination should be performed to identify evidence of intraluminal bleeding. The extremities must be examined to identify deformities, and each joint should be manipulated. Distal perfusion of the extremities must be continuously monitored, especially in limbs that show signs of significant injury. This is accomplished by evaluation of distal pulses and capillary refill. In cases of penetrating injury, the sites of entry and exit should be identified. It is especially important to examine carefully the areas that are difficult to access such as the oral cavity, perineum, axilla, scalp, and back. Once the secondary survey has been performed, more targeted assessments of suspected injuries can be performed using radiologic imaging.
Fetal Survey.
After initial stabilization of the mother, information about the pregnancy should be gathered through a focused history and physical examination. The history should include the date of the last menstrual period, expected date of delivery, and status of the pregnancy. In cases in which there is uncertainty regarding fetal age, an approximate determination can be made by measuring fundal height. The fetal age is estimated to be 1 week for each centimeter of fundal height above the symphysis pubis. In addition to the assessment of fundal height, the abdominal examination should include an assessment of uterine tenderness and consistency, the presence or absence of uterine contractions, and fetal position and movement.
A pelvic examination should be performed to evaluate cervical dilation and effacement, fetal station, and the presence of amniotic fluid and blood. The FHR is assessed by Doppler auscultation or ultrasonography. If maternal stability permits, ultrasonography facilitates estimation of fetal age and assessment of uteroplacental injury.
If no fetal cardiac activity is identified, fetal resuscitation should not be attempted (see Figure 55-1). In a series of 441 pregnant trauma patients, the absence of a detectable FHR was associated with fetal death in all cases.9 When a FHR is detected, an assessment of fetal viability should be performed. An estimated gestational age of 24 to 25 weeks and an estimated fetal weight of 500 g are common thresholds for extrauterine fetal viability. The FHR should be monitored in cases in which the fetus is determined to be viable. In cases in which a nonviable fetus is present, the importance of FHR monitoring is unclear. However, alterations in FHR and FHR variability may signal maternal deterioration and serve as a good monitor of the effectiveness of maternal resuscitation.
FHR monitoring is generally performed with external Doppler auscultation, and a tocodynamometer is used to assess uterine contractions. Adverse fetal outcomes are unlikely in cases with a reassuring FHR tracing and no early warning signs of uteroplacental injury (bleeding, abdominal pain).44 In contrast, an abnormal FHR tracing or evidence of uteroplacental injury (vaginal bleeding, uterine contractions, uterine tenderness, presence of amniotic fluid on vaginal examination) predicts poor fetal outcome in approximately 50% of cases.45
Fetal Monitoring
Continuous electronic FHR monitoring is the current standard of care for pregnant trauma victims with a viable fetus.9,14 FHR monitoring should be initiated as soon as maternal stabilization is complete, because placental abruption can occur early during the course of resuscitation. Continuous electronic FHR monitoring is more sensitive for placental abruption than ultrasonography, physical examination, or Kleihauer-Betke testing. Occasional uterine contractions are common after trauma and are usually not associated with poor fetal outcome.11,45,46 Random uterine contractions usually resolve within a few hours of the accident. However, regular and prolonged uterine contractions (eight contractions per hour for more than 4 hours) are associated with placental abruption, which has a high fetal mortality rate.46 The diagnosis of placental abruption should trigger immediate cesarean delivery; a large percentage of viable fetuses can be rescued if expedited delivery is performed. The presence of fetal bradycardia and frequent late FHR decelerations should also prompt delivery if the mother is stable and adequately resuscitated.
The ideal duration of FHR monitoring has not been determined. However, a common practice, based on a prospective study of 60 pregnant trauma patients at more than 20 weeks’ gestation, is to monitor the FHR for 4 hours.47 If maternal-fetal abnormalities are not detected within 4 hours, it is generally considered safe to discontinue FHR monitoring because a normal FHR tracing has a negative predictive value of 100% when combined with a normal maternal physical examination. However, the presence of abnormalities such as vaginal bleeding, spontaneous rupture of membranes, category II and III FHR patterns, persistent uterine contractions, uterine tenderness, abdominal pain, and/or need for maternal analgesia should prompt further FHR monitoring. The sensitivity of ultrasonography for placental abruption ranges from 50% to 80%, but ultrasonography is a safe and effective means of assessing fetal viability, FHR, placental location, gestational age, and amniotic fluid volume. It is particularly valuable in the presence of maternal tachycardia, when it can be difficult to differentiate maternal and fetal heart rates using Doppler auscultation.
Laboratory Studies
Laboratory evaluation in pregnant trauma patients does not differ from the evaluation for nonpregnant patients, with a few exceptions. As for all trauma patients, the laboratory evaluation will be driven by the type and severity of injury. For most patients with significant injury, standard analysis includes a complete blood cell count with a platelet count, coagulation studies, serum electrolyte measurements, blood glucose and lactate levels, liver function tests, arterial blood gas analysis, urinalysis, and toxicology screening, as well as sending a blood sample for typing and crossmatching for compatible blood products (Box 55-4).
The presence of disseminated intravascular coagulation (DIC) and low blood bicarbonate levels is associated with poor fetal outcome.10,25 Both abnormalities reflect severe maternal injury and should prompt aggressive maternal resuscitation. Of special consideration in pregnant trauma patients is maternal-fetal hemorrhage. The Kleihauer-Betke acid elution assay is used to detect the entry of fetal blood into the maternal circulation.20 It is typically performed in Rh0(D) antigen–negative mothers to detect transplacental hemorrhage and the potential for developing Rh0(D) sensitization, which can be prevented in 99% of cases by early treatment with Rh0(D) immune globulin (RhoGAM). However, this test may help predict adverse fetal outcomes in all pregnant trauma patients (see earlier discussion).
Imaging
The pregnant trauma patient often requires imaging to evaluate orthopedic, head, thoracic, and abdominal injuries. Although many patients and physicians are concerned about the fetal effects of ionizing radiation, the risks for teratogenesis, malignancy, and gene mutation are small with a radiation exposure less than 5 to 10 rads (see Chapter 17).48 Less than 1% of trauma patients receive more than 3 rads of radiation exposure. When possible, the fetus should be shielded with lead. Intravenous pyelography subjects the fetus to as much as 1.4 rads of exposure, but the test can be invaluable in diagnosing injuries to the kidneys, ureters, and bladder. Computed tomography (CT) is associated with greater radiation exposure than plain radiography, but exposure levels are generally below that considered to be dangerous to the fetus. Modern multidetector (multislice) CT results in higher fetal radiation exposure, but it has significant advantages in speed and image resolution. Overall, the small risk for fetal radiation exposure is outweighed by the benefits to the injured mother and, by extension, the fetus.
Injury Scoring
Several injury scoring scales have been developed over the past 40 years. The scoring systems provide a framework for standardizing clinical management, benchmarking outcomes, and planning research. Presently, no reliable scoring tool exists for predicting maternal or fetal outcome after trauma. Currently used scoring systems include (1) anatomic injury scales that rely on physical examination and diagnostic procedures, (2) physiologic injury scales that rely on assessment of physiologic responses and function, and (3) combination injury scales.
One of the first anatomy-based injury scales was the Abbreviated Injury Scale (AIS) developed by the Association for the Advancement of Automotive Medicine.49 Each of nine body regions is given an injury severity score that ranges from 1 (minor) to 6 (maximal [currently untreatable]). Although the AIS effectively describes the severity of injuries at specific locations, it provides a limited assessment of the overall pathophysiologic impact of all injuries. The Injury Severity Score (ISS), which was developed to address this issue, is obtained by summing the square of the three highest severity scores from the AIS. The ISS ranges from 1 to 75. Minor injuries are classified as an ISS of less than 9; moderate, from 9 to 15; serious, from 16 to 25; and severe, greater than 25. The ISS correlates with the risk for preterm delivery after trauma, but its value in predicting fetal death, placental abruption, and other adverse outcomes is controversial.15 The American Association for the Surgery of Trauma developed the Organ Injury Scale (OIS)50; this is an organ-based severity scale designed to facilitate clinical investigation and outcomes research. The value of the OIS in predicting adverse maternal and fetal outcomes remains to be established.
Anatomic scoring systems have value in describing the extent and severity of injuries to specific organ systems. However, physiologic scoring systems may add prognostic value. The Glasgow Coma Scale, which is among the most widely used physiologic scoring systems, evaluates the neurologic status of the trauma patient. The Glasgow Coma Scale evaluates eye opening, verbal response, and motor activity; scores range from 3 to 15, with 3 indicating the absence of neurologic activity and 15 representing intact neurologic function. A Glasgow Coma Scale score of less than 9 reflects severe impairment, whereas as a score of 9 to 12 reflects moderate disability. However, concerns have been raised about inter-rater reliability and lack of prognostic utility for the Glasgow Coma Scale. Some researchers have proposed that a simplified motor score would be more reliable. Evidence suggests that the Glasgow Coma Scale has a poor correlation with fetal outcome.15
Traumatic Brain Injury
Brain injury is the most common severe injury in patients who suffer from motor vehicle accident, and it is a major cause of mortality among pregnant trauma victims.51 It is important to perform a thorough neurologic examination with particular attention to level of consciousness. Altered mental status may be an indicator of evolving intracranial pathology, intoxication, hypoperfusion, and/or metabolic disturbances. Mental status should be reevaluated frequently because intracranial pathologic processes may not be apparent on initial evaluation and may evolve during the course of resuscitation.
Elevated ICP is a common finding in patients with traumatic brain injury and may be a significant threat to life. Head CT is the imaging study of choice for identifying the site and severity of intracranial pathologic processes in trauma patients, and it should be performed within 1 hour of arrival in the emergency department.
Crystalloid fluid resuscitation should be used for resuscitation in patients with traumatic brain injury; resuscitation with albumin is deleterious in patients with traumatic brain injury (see earlier discussion).35 Hypotensive resuscitation is contraindicated in patients with traumatic brain injury and elevated ICP. It is crucial to maintain CPP to minimize the risk for brain ischemia and permanent brain injury. The extent of brain injury will worsen if CPP is not maintained. It is also important to maintain cerebral oxygen delivery by optimizing maternal cardiac output and blood oxygen-carrying capacity.
It may be necessary to intubate the trachea of patients with deteriorating mental status for airway protection and provision of ventilatory support. Hypoventilation should be avoided because it increases ICP. Hyperventilation to a PaCO2 between 25 and 30 mm Hg will provide a transient decrease in ICP and may be useful until definitive treatment can be initiated. However, hyperventilation can be disadvantageous for the fetus because it can decrease uteroplacental blood flow by decreasing maternal cardiac output and blood pressure, and perhaps by causing uteroplacental vasoconstriction. Therefore, it is prudent to maintain PaCO2 at levels that are normal in pregnant females (28 to 32 mm Hg). Additional maneuvers to decrease ICP include treatment with a diuretic such as mannitol or furosemide and elevation of the head 30 to 45 degrees.
Corticosteroids are no longer recommended for patients with traumatic brain injury because their administration in this setting is associated with increased mortality. Barbiturates decrease cerebral oxygen use and blood flow and may provide cerebral protection in patients with severe impairment. Both mannitol and furosemide cross the placenta and could cause alterations in fetal plasma osmolality and decrease fetal intravascular volume. However, concern regarding adverse fetal effects should be overridden by the needs of the mother in cases of traumatic brain injury.
Cardiopulmonary Resuscitation
The incidence of cardiac arrest in pregnancy is reported to be 1 : 20,000 to 1 : 30,000 patients in industrialized countries.52 The most frequent causes are trauma, peripartum hemorrhage, embolic phenomena, stroke, preeclampsia/eclampsia, sepsis, status asthmaticus, and anesthesia-related complications.53 Cardiopulmonary resuscitation should be initiated immediately (see Chapters 17 and 42). The 2010 American Heart Association (AHA) guidelines highlight the importance of initiating high-quality chest compressions to facilitate circulation.52,54 The hands should be placed slightly above the center of the sternum because the diaphragm and abdominal contents are displaced cephalad during the third trimester of pregnancy (Box 55-5). In the hospital setting, the airway should be secured and the mother ventilated with 100% oxygen. Intravenous or intraosseous access should be secured to facilitate resuscitation. Advanced Cardiac Life Support (ACLS) guidelines should be followed to identify and treat causes of cardiopulmonary arrest.52
Cardiac arrest in the pregnant patient is complicated by the physiologic changes of pregnancy, particularly the effect of the gravid uterus on aortocaval blood flow. Well-performed chest compressions in the nonpregnant patient typically result in cardiac output that is approximately 30% of normal. In pregnant patients, aortocaval compression reduces the cardiac output that results from chest compressions. Although it is typically advised to tilt the patient 15 to 30 degrees to facilitate left uterine displacement and optimize venous return and cardiac output, such a maneuver may impede the effectiveness of chest compressions. Therefore, the AHA guidelines advocate manual left uterine displacement rather than the usual whole-body tilt (Figure 55-2).52 If this technique is not successful, a firm wedge may be placed under a resuscitation board to tilt the patient approximately 30 degrees. In the field, the responder may use his or her knees to tilt the patient.
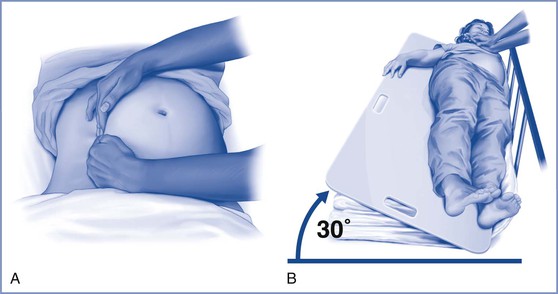
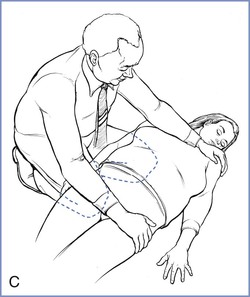
FIGURE 55-2 Methods to relieve aortocaval compression during cardiopulmonary resuscitation in maternal cardiac arrest. A, Manual left lateral displacement with two-handed technique. B, Patient in a 30-degree left-lateral tilt using a firm wedge to support the pelvic and thorax. C, The human wedge position. (A and B from Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: Cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:S829-61; C, modified from Harnett M, Tsen LC. Cardiovascular disease. In Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut’s Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009.)
Chest impedance is unchanged during pregnancy. Therefore, the usual voltage levels for defibrillation should be used in pregnant patients.52 Electric cardioversion during pregnancy appears to be safe for the fetus (see Chapter 42). If spontaneous circulation does not return within 4 minutes of cardiac arrest, immediate hysterotomy or cesarean delivery should be performed if gestational age is 20 weeks or greater, aiming for delivery within 5 minutes of cardiac arrest. Timely delivery facilitates successful resuscitation of both the mother and the infant.52,54 Furthermore, early hysterotomy and uterine evacuation optimizes maternal resuscitation, even if the fetus has already died. Extrauterine fetal survival is unlikely before 24 to 25 weeks’ gestation.
Management of the Brain-Dead Patient
Brain death in the pregnant patient is a rare occurrence. Esmaeilzadeh et al.55 summarized 30 cases published between 1982 and 2010. Nontraumatic brain injury, primarily intracranial hemorrhage, was the cause of death in 26 of 30 cases. The mean gestational ages at times of injury and delivery were 22 and 29.5 weeks, respectively. Twelve viable infants survived beyond the neonatal period.
In cases of maternal brain death, care providers should focus on saving the life of the fetus; maternal organ preservation for harvest and donation is a secondary consideration. Maintenance of vital functions in mothers with catastrophic brain injury is justified to meet these two goals, but in many cases ethical and legal concerns must be addressed. Consideration must be given to gestational age and the chance for fetal survival. Before 24 weeks’ gestation, the chance for extrauterine fetal survival is small. In general, management should follow current guidelines for organ preservation therapy.
The question of whether to preserve maternal circulation and organ function to facilitate fetal development is an ethical dilemma. A fundamental issue relates to the support of the brain-dead mother as an incubator for the unborn fetus. Some professionals argue such an approach is unethical, whereas others view prolonged somatic support as a case of organ donation with the fetus as the recipient. In many cases, the mother’s wishes are not known. If the mother indicated a wish to donate organs, prolonged somatic maternal support may be appropriate. Currently, there is no generally accepted lower limit of gestational age for maintenance of maternal support. Each case must be addressed on an individual basis, with close communication among the family, a cohort of care providers, and the hospital ethics committee.
Critical Care during Pregnancy
Stroke
Ischemic Stroke
Pregnant women are at increased risk for ischemic stroke compared with their nonpregnant counterparts.56 Pregnancy is a hypercoagulable state characterized by decreased fibrinolysis, increased levels of clotting factors, and decreased levels of certain natural anticoagulants (e.g., protein S). Clinical manifestations of ischemic stroke are similar to those seen in the nonpregnant population and include focal neurologic symptoms, seizures, decreased level of consciousness, and abnormal cranial nerve function. Once the diagnosis is suspected, and initial evaluation and therapy—including airway protection—have been addressed, non–contrast-enhanced CT should follow immediately. The fetal radiation exposure from this test is less than 1 rad.57 If CT shows no evidence of hemorrhagic stroke, the patient is assumed to have an ischemic stroke. Consideration of thrombolytic therapy should follow, including evaluation of contraindications for thrombolytic therapy (Box 55-6). Thrombolytic therapy with recombinant tissue plasminogen activator (r-tPA) has been reported during pregnancy and appears to be safe for the fetus; transplacental passage of r-tPA is minimal. However, retroplacental bleeding with pregnancy loss has been reported.58–60 Consultation with an experienced neurologist is recommended.
The r-tPA is administered intravenously at a dose of 0.9 mg/kg (maximum, 90 mg) over 1 hour (10% of the dose is commonly administered as an initial bolus over the first minute). The therapeutic window (i.e., time from onset of symptoms to administration of the agent) is 4.5 hours.61 Patients who are candidates for this therapy should be watched closely in an ICU environment that includes fetal surveillance by a maternal-fetal medicine specialist. Blood pressure should be maintained below 180/105 mm Hg during r-tPA therapy. Neurologic deterioration during thrombolytic infusion should raise suspicion of hemorrhagic transformation; the infusion should be stopped immediately, and the head CT should be repeated. Transfusion of platelets (e.g., 10 units of pooled random donor platelet concentrate or 1 to 2 units of apheresis platelet concentrate) and 10 units of cryoprecipitate is recommended if hemorrhagic transformation of the stroke is documented.62 Other potential therapies include fresh frozen plasma, activated rFVIIa, and antifibrinolytic therapy.62
Patients with ischemic stroke who are not candidates for r-tPA therapy should receive aspirin (162 to 325 mg/day) for 2 weeks; subsequently, the dose may be decreased to 100 mg/day.61 Deep vein thrombosis prophylaxis with unfractionated heparin should also be started on admission unless contraindicated. Outcome data related to blood pressure management in patients with acute ischemic stroke are inconsistent; however, in 2007, the AHA and the American Stroke Association recommended that, in patients receiving thrombolytic therapy, emergency antihypertensive medication should be withheld unless the blood pressure is above 220/120 mm Hg.63 If the patient has received thrombolytic therapy, aspirin and prophylactic doses of heparin should be withheld for 24 hours after completion of the infusion. Fever should be aggressively treated to achieve normothermia. Blood glucose measurements higher than 140 to 180 mg/dL should be treated with insulin. Seizure prophylaxis in ischemic stroke patients is not recommended.63
Cerebral sinus and vein thrombosis may occur during pregnancy and the puerperium, but most cases occur postpartum. The clinician should suspect cerebral sinus thrombosis in the presence of severe headache, focal neurologic symptoms, and/or papilledema. The diagnostic test of choice is magnetic resonance venography (MRV). Most cases involve the transverse sinuses. Initial imaging reveals concomitant areas of hemorrhage in as many as 40% of these cases. However, treatment involves immediate therapeutic anticoagulation with unfractionated heparin or low-molecular-weight heparin unless massive hemorrhage is present.61,64
Ischemic strokes that involve more than 50% of the territory of the middle cerebral artery are known as “malignant strokes.” They occur more commonly in young people. In the vast majority of cases, these patients require early decompression hemicraniectomy because the stroke is usually associated with massive cerebral edema that frequently is not responsive to medical therapy.65
Hemorrhagic Stroke
Pregnancy typically is accompanied by a 40% to 50% increase in both cardiac output and effective blood volume. These changes, coupled with hormone-induced changes in the vessel wall structure, may render pregnant patients more susceptible to hemorrhagic strokes resulting from intracerebral and subarachnoid hemorrhage.66
Intracerebral Hemorrhage.
Intracerebral hemorrhage is usually a secondary complication of hypertensive emergencies (e.g., preeclampsia, hypertensive encephalopathy). Clinical presentation is similar to that in nonpregnant individuals. The diagnosis is confirmed with non–contrast-enhanced CT.
As in any other stroke victim, initial management involves securing the airway and facilitating oxygenation and ventilation. Intracranial pressure monitoring should be considered in patients with a Glasgow Coma Scale score less than 8, clinical evidence of transtentorial herniation, and evidence of significant intraventricular hemorrhage or hydrocephalus.67
Blood pressure control in this setting is controversial. Current guidelines recommend antihypertensive therapy when the blood pressure exceeds 180/110 mm Hg or when MAP exceeds 130 mm Hg.67,68 Some experts argue that hypertension leads to hematoma expansion and may worsen cerebral edema. Recent evidence suggests that it is safe to use antihypertensive agents to achieve a systolic blood pressure less than 140 mm Hg. Maintenance of systolic blood pressure below 140 mm Hg leads to decreased hematoma expansion, but the effect on outcomes is unknown.68 In cases of intracerebral hemorrhage associated with preeclampsia, some practitioners recommend maintenance of systolic and diastolic blood pressures between 140 to 160 mm Hg and 90 to 110 mm Hg, respectively, to maintain uteroplacental perfusion pressure. Blood pressure should be monitored invasively and treated with a titratable intravenous agent such as labetalol or nicardipine.
In the setting of intracerebral hemorrhage secondary to the use of warfarin, rapid reversal of the anticoagulation effect is of paramount importance. Vitamin K (10 mg intravenously over a minimum of 20 minutes) should be administered.69 Recent guidelines from the American College of Chest Physicians suggest that prothrombin complex concentrate, which contains the vitamin K–dependent clotting factors II, VII, IX, and X, should be first-line therapy.69 Advantages of prothrombin complex concentrate over fresh frozen plasma include no requirement for blood typing and crossmatching, a lower risk for bloodborne infection, and little risk for volume overload. Prothrombin complex concentrate can be infused over 15 to 30 minutes.69 The role of rFVIIa in this setting is limited, and its use cannot be recommended at this time.
The risk for seizures after intracerebral hemorrhage is higher in cases of lobar hemorrhage; the risk is small if hemorrhage is localized to the basal ganglia, and even less if limited to the posterior fossa. Unless clinical seizures are observed or nonconvulsive activity is noted on the electroencephalogram (EEG), routine seizure prophylaxis in patients with intracerebral hemorrhage is not recommended. Therapy may be associated with worse long-term functional outcome.67 As with most brain injuries, glucose control is paramount in the management of intracerebral hemorrhage. Blood glucose should be maintained between 140 and 180 mg/dL in critically ill patients who require insulin therapy.67,70 Once bleeding cessation is documented by repeat imaging, deep vein thrombosis prophylaxis with unfractionated heparin or low-molecular-weight heparin should be initiated (usually 1 to 4 days after the intracerebral hemorrhage).
Subarachnoid Hemorrhage.
A subarachnoid hemorrhage may be traumatic or nontraumatic. This discussion is limited to nontraumatic forms of subarachnoid hemorrhage. The most common cause of nontraumatic subarachnoid hemorrhage is rupture of a berry aneurysm. The clinical presentation varies from the complaint of the “worst headache in my life” to profound coma. The diagnosis is made by CT followed by cerebral angiography to locate the source of the bleeding. Abdominal shielding is essential during all radiographic procedures to limit fetal radiation exposure. Once the aneurysm is located, two management options exist. Craniotomy with clipping of the aneurysm has been the traditional treatment. More recently, coiling of the aneurysm has emerged as a less invasive option. Controversy exists regarding which option results in the best outcome (see Chapter 49). The largest randomized study comparing both treatment modalities found that endovascular coiling resulted in a lower risk for death at 5 years71; however, the risk for rebleeding was higher in the endovascular coiling group. The 2009 AHA guidelines suggested that “endovascular coiling can be beneficial.” 72 Endovascular coiling has not been specifically studied in the pregnant population, although several cases of successful endovascular treatment of ruptured intracranial aneurysms in pregnant women have been reported.
Regardless of the treatment modality, it is crucial to secure the aneurysm as early as possible. Before the aneurysm is secured, blood pressure control should target a systolic blood pressure below 160 mm Hg.73
Delayed vasospasm is one of the most serious complications of subarachnoid hemorrhage. The onset is usually 4 to 10 days after the hemorrhage and manifests as worsening of the neurologic examination (either new focal symptoms or decreased level of consciousness). If a change in the neurologic status is noted, immediate CT should be performed to rule out rebleeding or hydrocephalus; if absent, vasospasm is likely. Vasospasm is confirmed with cerebral angiography. All patients should be treated with nimodipine (60 mg orally every 4 hours for 21 days) as prophylaxis against delayed-onset ischemia secondary to cerebral vasospasm. Other potential treatments to prevent delayed vasospasm include magnesium sulfate and statins.74 However, a 2012 meta-analysis does not lend support to the efficacy of magnesium sulfate,74 and statins should be avoided during pregnancy because of the potential risk for teratogenicity.
Once surgical or endovascular treatment is completed, blood pressure control may be less rigorous because hypertension may be a compensatory mechanism to maintain cerebral perfusion pressure in the setting of vasospasm. During pregnancy, extremely high blood pressure may lead to placental abruption and should be avoided.
Historically, patients presenting with symptomatic delayed cerebral vasospasm have been treated with “triple H therapy.” Triple H therapy consists of inducing hypervolemia (through administration of crystalloids or colloids), leading to hemodilution, accompanied by induced hypertension (by using vasopressors) in an attempt to increase cerebral perfusion. Evidence of the efficacy of triple H therapy is extremely limited.74 The use of hypervolemia and subsequent hemodilution may even be detrimental as a result of a decrease in arterial oxygen content.75 Instead, many practitioners recommend induced hypertension with intravenous infusion of norepinephrine, phenylephrine, or dopamine and titration of systolic blood pressure to measurements higher than 180 mm Hg (MAP > 120 mm Hg). However, during pregnancy, the use of high-dose vasopressors to induce hypertension may lead to uteroplacental vasoconstriction and hypoperfusion and subsequent fetal demise. Additionally, the hypertension may increase the risk for placental abruption. Pregnant patients requiring induced hypertension for delayed vasospasm present a significant clinical dilemma. If the fetus is sufficiently mature (e.g., more than 32 weeks’ gestation), it may be advisable to deliver the fetus before initiation of induced hypertension. If delivery is not an option, balloon angioplasty of the constricted vessels by an interventional radiologist may be considered.
Subarachnoid hemorrhage may also lead to extracerebral manifestations, the most common of which are hyponatremia, cardiac dysfunction, and neurogenic pulmonary edema. Hyponatremia occurs secondary to increased secretion of atrial and ventricular natriuretic peptides (cerebral salt wasting syndrome).73 Treatment should focus on isotonic sodium replacement (0.9% saline) and diuresis. The massive liberation of catecholamines that accompanies subarachnoid hemorrhage is believed to cause subendocardial ischemia, leading to “cardiac stunning” with concomitant arrhythmias and decreased cardiac output. Patients may require inotropic support and antiarrhythmic therapy. Neurogenic pulmonary edema has both a hydrostatic component (from cardiac stunning and pulmonary venous constriction as a result of increased catecholamine secretion) and a noncardiogenic component (from endothelial injury owing to activation of the inflammatory cascade). Treatment of neurogenic pulmonary edema is supportive and requires low tidal volume ventilation strategies and the use of PEEP to improve oxygenation.
Subarachnoid hemorrhage may also occur secondary to rupture of brain arteriovenous malformations.76 General intensive care provided to these patients is similar to that used in aneurysmal subarachnoid hemorrhage, with a few exceptions. The risk for vasospasm after subarachnoid hemorrhage from arteriovenous malformations is lower and rarely warrants therapy. Once removed (surgically), adjacent brain tissue will be exposed to increased cerebral blood flow leading to cerebral edema. Localized cerebral edema may be prevented by avoidance of severe hypertension. The decision to surgically treat a ruptured arteriovenous malformation is controversial and depends on the location and type of venous drainage (superficial or deep).76 The risk for rebleeding during pregnancy is increased, and treatment (e.g., surgical resection, embolization) is commonly recommended.77
As with intracerebral hemorrhage in general, pregnant patients with subarachnoid hemorrhage should be normothermic and the maximum blood glucose level should be maintained between 140 and 180 mg/dL. Seizure prophylaxis in the setting of subarachnoid hemorrhage is controversial. Prolonged use of phenytoin has been associated with poor neurologic outcomes and should be avoided.78 A systematic review suggested that 3 days of seizure prophylaxis therapy provides similar seizure prevention with better outcomes compared with longer-term treatment.78
Status Epilepticus
Status epilepticus is defined as a continuous, generalized convulsive seizure lasting more than 5 minutes or two or more seizures with no return to baseline consciousness between the seizures.79 Status epilepticus may be caused by noncompliance with antiepileptic medications, stroke, brain tumor, central nervous system infection, head trauma, metabolic derangements (e.g., uremia, hepatic encephalopathy, electrolyte abnormalities), cerebral hypoxia, and hypoglycemia/hyperglycemia. Rarely, eclamptic seizures may progress to status epilepticus.
Initial management should include protecting the airway and arresting the epileptic convulsions. Intravenous access should be obtained and hypoglycemia ruled out promptly. If in doubt, a 50-mL bolus of intravenous 50% dextrose with 100 mg of thiamine should be administered. Adequate hydration is of paramount importance because seizure-induced muscle breakdown may lead to myoglobin-induced kidney injury.79 Initial pharmacologic therapy includes an intravenous benzodiazepine (e.g., lorazepam 0.1 mg/kg) followed by a standard intravenous antiepileptic agent (e.g., phenytoin). If intravenous access is difficult, intramuscular drug administration is an option. A recent study showed that intramuscular midazolam (10 mg) was at least as effective as intravenous lorazepam in the prehospital management of status epilepticus.80 If seizure control is not achieved, a continuous infusion of propofol, midazolam, or a barbiturate may be required.81 There is no agreement on the recommended EEG titration goal (burst suppression versus seizure control).81 Many practitioners recommend 24 to 48 hours of seizure control documented by EEG before slowly weaning the infusion.
Acute Respiratory Distress Syndrome and Acute Lung Injury
Acute respiratory distress syndrome (ARDS) is a severe form of noncardiogenic pulmonary edema; it is a common cause of mortality in the critically ill obstetric patient. Traditionally, it has been defined as (1) pulmonary edema manifested on chest radiograph as bilateral acute infiltrates, (2) normal left ventricular function (pulmonary artery occlusion pressure [PAOP] below 18 mm Hg or normal heart function as determined by transthoracic echocardiography), and (3) a PaO2/FIO2 ratio below 200.82 If the same conditions are present but the PaO2/FIO2 ratio is between 200 and 300, then the term acute lung injury (ALI) is used. The mortality rate from ARDS is approximately 40%.83
ALI/ARDS may occur secondary to a pulmonary or extrapulmonary pathologic process. The common denominator is activation of the inflammatory cascade, leading to inflammation-induced endothelial/epithelial injury in the lung with subsequent leaking of protein-rich fluid into the alveoli. Direct (pulmonary) insults that may lead to ALI/ARDS include pneumonia, aspiration pneumonitis, pulmonary contusion, and smoke inhalation during burns. Indirect (extrapulmonary) causes include sepsis, pancreatitis, trauma, and massive transfusion. Similar to the nonobstetric population, the most common cause of ARDS/ALI in pregnant women is sepsis.84 Certain obstetric conditions (e.g., placental abruption, amniotic fluid embolism, preeclampsia) may also cause ALI/ARDS because these conditions are associated with inflammation and subsequent diffuse endothelial injury. Also, patients with severe placental abruption or amniotic fluid embolism complicated by DIC are at increased risk for massive transfusion of blood products.
Initial treatment for the patient with acute hypoxemic respiratory failure of noncardiac origin falls into ventilatory and nonventilatory strategies (Box 55-7). Of vital importance, the underlying cause of the ARDS must be addressed simultaneously with institution of supportive measures (e.g., broad-spectrum antibiotics and surgical drainage if indicated in cases of sepsis-related ARDS; immediate delivery in patients with chorioamnionitis).
Ventilatory Strategies
The only intervention that has convincingly decreased mortality in ARDS is lung-protective mechanical ventilation.85 ALI/ARDS is a heterogeneous disease in which some areas of the lung are affected (consolidated with edema) and others are not. During tidal volume delivery, gas flow is predominantly distributed to unaffected portions of the lung. If large tidal volumes are used, these “normal” areas of the lung are exposed to excessive volumes and pressures that lead to volutrauma and barotrauma, respectively. Overdistention of the lung provokes a local inflammatory response that further injures the lung parenchyma; locally produced inflammatory mediators (i.e., cytokines) may translocate to the systemic circulation and provoke worsening multiorgan failure (biotrauma).86 If large tidal volumes are used with low PEEP, the constant opening and closing of the recruited alveoli (atelectrauma) will also worsen lung inflammation. These four insults (volutrauma, barotrauma, biotrauma, and atelectrauma) constitute what is known as ventilator-induced lung injury.87
In a randomized clinical trial involving 861 patients with ALI/ARDS, those randomized to receive mechanical ventilation with a small tidal volume (6 mL/kg lean body weight) and a limitation of plateau pressure to less than 30 cm H2O had a mortality of 31% compared with 40% in the group randomized to receive a larger tidal volume (12 mL/kg lean body weight).85 Since the publication of this trial in 2000, most intensivists have adopted strategies that limit tidal volumes to decrease ventilator-induced lung injury and improve outcomes in patients with ARDS. By limiting tidal volumes, minute ventilation is invariably decreased, leading to hypercarbia and respiratory acidemia. To improve minute ventilation, the operator may increase the respiratory rate up to 35 breaths per minute to maintain, ideally, an arterial pH above 7.3.87
Significant maternal hypercarbia may result in decreased removal of carbon dioxide from the fetus, leading to fetal acidemia. Tidal volumes from 6 to 8 mL/kg (lean body weight) can be used to ventilate pregnant women with ARDS; attempts should be made to maintain maternal PaCO2 below 60 mm Hg. Because of decreased compliance of the chest wall during pregnancy, plateau pressures of up to 35 cm H2O may be tolerated. Continuous electronic FHR monitoring is recommended because abnormal FHR patterns may occur during periods of fetal acidemia.
The use of low tidal volumes to limit ventilator-induced lung injury must be accompanied by the use of adequate levels of PEEP to recruit alveolar units. A discussion of optimization of PEEP is beyond the scope of this chapter, but the reader may access FIO2-PEEP tables that titrate PEEP according to oxygen requirements.88 The oxygenation goal is to achieve a PaO2 of at least 55 mm Hg or higher with an SaO2 (as measured by pulse oximetry) of at least 88% or higher.88 The use of high PEEP (levels up to 15 cm H2O or higher) has been associated with decreased mortality in the subset of patients with the most severe forms of ARDS.89
Other ventilatory strategies that may be used in ARDS, but have not been associated with reduced mortality, include the use of recruitment maneuvers, airway pressure release ventilation, high-frequency oscillatory ventilation, extracorporeal membrane oxygenation (ECMO), and prone ventilation.88,90,91 When patients with ARDS are turned from the supine to the prone position, oxygenation often greatly improves, likely secondarily to anterior displacement of the heart with resultant recruitment of the posterior lung segments and improved ventilation-perfusion matching.92 However, improved survival has not been documented.93 A survival benefit may be gained in the subgroup of patients with the most severe forms of ARDS and if the periods of prone ventilation are extended to 12 to 20 hours per day.94,95 Use of prone ventilation during the second half of pregnancy is obviously technically demanding (and may not be possible) because of the enlarged gravid uterus.
Nonventilatory Strategies
Nonventilatory strategies play an important role in the management of patients who suffer ALI/ARDS (see Box 55-7). The early use of neuromuscular blockade has recently been shown to improve survival in patients with ARDS.96 A possible advantage of cisatracurium is its significant anti-inflammatory activity; the infusion should be limited to the first 48 hours of mechanical ventilation.96 Pregnancy is not a contraindication to application of this treatment, if required.
Because ALI/ARDS occurs secondary to inflammation-mediated lung injury, interest has risen regarding the possible benefits of corticosteroid therapy. Theoretically, low-dose corticosteroids could be immunomodulatory (not immunosuppressive), leading to downregulation of excessive inflammation, and thus limiting acute lung injury.97 Overall, published evidence indicates that low-dose corticosteroids reduce systemic inflammation, improve oxygenation, reduce multiorgan dysfunction, and decrease the duration of mechanical ventilation and ICU stay in patients with ALI/ARDS.97 If used, low-dose corticosteroids (see Box 55-7) should be initiated before day 14 of the disease; after day 14, corticosteroid therapy is not recommended. The effect on survival is controversial, although some evidence suggests that corticosteroids decrease mortality in patients with ALI/ARDS.98 Interestingly, the use of these low doses has not been associated with an increased risk for gastrointestinal hemorrhage, hyperglycemia, nosocomial infection, or myopathy.97 If corticosteroids are used, the use of muscle relaxants should be avoided to prevent critical illness polyneuromyopathy. During pregnancy, the physician should consider the possible risk for fetal cleft lip (with and without cleft palate) associated with corticosteroid administration during the first trimester of pregnancy.99
Fluid Management.
Management of fluid balance is fundamental in the care of patients with ALI/ARDS. A randomized trial found that conservative fluid management (mean fluid balance in the first 7 days, −136 mL) was associated with a shorter duration of mechanical ventilation and a shorter length of stay in the ICU than liberal fluid management (mean fluid balance, +6992 mL).100 At the time of enrollment most patients had been fluid resuscitated and were hemodynamically stable. The incidence of adverse effects (e.g., shock, need for renal dialysis) did not differ between groups.
Fluid restriction in patients with ALI/ARDS is usually initiated on day 2 to 3 of the disease process; the first several days are commonly associated with hypotension and shock that invariably requires fluid resuscitation. Provided shock has resolved and vasopressors are not required to support blood pressure, fluids should be restricted to the amount required to maintain hemodynamic stability. Enteral feeding should be the main source of fluids in lieu of “maintenance fluids.” If a diuretic is used to achieve a negative fluid balance, judicious use is recommended. Continuous electronic FHR monitoring is recommended to assess the adequacy of uteroplacental perfusion.
Right ventricular failure is a common occurrence in patients with ALI/ARDS. Alveolar “flooding” limits oxygenation with resultant hypoxic pulmonary vasoconstriction. The increase in pulmonary vascular resistances may lead to acute cor pulmonale and right ventricular failure, accompanied by a severe decrease in cardiac output (from both right-sided failure and left ventricular diastolic dysfunction). The administration of an inhaled pulmonary vasodilator such as nitric oxide or prostacyclin often leads to improved oxygenation and right ventricular function. However, there is no evidence that these interventions improve survival.101 Pregnancy is not a contraindication for pulmonary vasodilator therapy. Pharmaconutritional therapy for ALI/ARDS (e.g., omega-3 fatty acids, linolenic acid, antioxidants) has not been shown to be beneficial and is not recommended.102
Nutrition and Glucose Control
Critically ill patients require nutrition to heal. Early aggressive enteral nutrition is of paramount importance and should be implemented within 24 to 48 hours of admission to the ICU.103 Enteral nutrition (either through a nasogastric tube or a Dobhoff tube with the tip placed in the duodenum) helps maintain gut barrier integrity, thus preventing bacterial and cytokine translocation from the intestine. Enteral nutrition is generally preferred over parenteral nutrition because it is associated with less infectious morbidity and mortality. Enteral nutrition should be discontinued in patients requiring high doses of vasopressors because of a possible increase in the risk for bowel ischemia.103
The total amount of calories needed during critical illness is unknown.104 More than 200 formulas exist to calculate daily energy requirements while in the ICU; many of these formulas coincide with a simple calculation of 25 Kcal/kg/day (ideal body weight).103 An extra 300 Kcal/day should be added during pregnancy (500 Kcal/day in patients with multiple gestation). Overfeeding should be avoided because it may lead to fatty liver, volume overload, excessive carbon dioxide production, hyperglycemia, infection, and immunosuppression. Protein delivery should not be restricted, and critically ill patients should receive 1.2 to 2.0 g/kg/day of protein.103 Most available enteral feeding formulas do not contain sufficient protein; it may be necessary to add additional protein to standard formulas.
Stress hyperglycemia refers to the elevation in blood glucose concentration associated with critical illness. Stress hyperglycemia is caused by multiple factors, including massive catecholamine release and systemic inflammation. Hyperglycemia is commonly worsened with initiation of nutritional support. Hyperglycemia worsens oxidative injury and potentiates inflammation and clotting. In 2001, a landmark paper suggested that tight glycemic control during critical illness (intravenous use of an insulin infusion to maintain blood glucose level between 80 and 110 mg/dL) was associated with (1) decreased mortality; (2) a lower incidence of sepsis, critical illness polyneuropathy, and liver injury; (3) reduced transfusion requirements; (4) fewer days of mechanical ventilation; and (5) a decreased need for renal replacement therapy.105 These findings were not replicated in later studies; the largest available randomized controlled trial actually found that tight glucose control led to a 2.6% increase in mortality compared with less stringent glucose control. Maintaining blood glucose between 80 and 110 mg/dL commonly leads to episodes of iatrogenic hypoglycemia, which may worsen outcome. Current guidelines recommend a target blood glucose level between 140 and 180 mg/dL in ICU patients who are receiving insulin therapy.70
Transfusion Triggers
Most packed red blood cell (PRBC) transfusions in the ICU are used to treat anemia in hemodynamically stable patients who are not actively bleeding.106 The efficacy of such an approach has not been demonstrated. Anemia in the critically ill patient commonly results from inflammation-induced inhibition of erythropoiesis and excessive phlebotomy.106 Administration of PRBCs theoretically leads to an increase in arterial blood oxygen content, oxygen delivery, and, ultimately, oxygen consumption. Unfortunately, no clear evidence indicates that PRBC transfusions improve oxygen consumption.107,108 During storage in the blood bank, red blood cells undergo a series of changes that include anomalies in the cytoskeleton, which hinders the red blood cells’ capacity for deformability. As a result, transfusion of these rigid red blood cells may cause occlusion of the microvasculature and distal ischemia. These cells do not effectively release oxygen in the tissues because of a deficit in 2,3-diphosphoglycerate (2,3-DPG). Thus, oxygen-carrying capacity often is not improved by PRBC transfusion. In reality, significant risks are associated with transfusion of blood products in the ICU (Table 55-2).109
TABLE 55-2
Potential Complications Associated with Transfusion of Blood Products

DIC, disseminated intravascular coagulopathy; HIV, human immunodeficiency virus.
Modified from Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology 2011; 115:635-49.
The largest published trial to date evaluating the role of PRBC transfusion in hemodynamically stable ICU patients found no difference in outcome between patients randomized to a liberal transfusion strategy (transfuse to maintain hemoglobin above 10 g/dL) or a restrictive strategy (transfuse to maintain a hemoglobin > 7 g/dL).110 A subgroup analysis revealed that mortality was increased with the liberal transfusion strategy in the subgroup of patients younger than 50 years old and with less severe disease (Acute Physiology and Chronic Health Evaluation II [APACHE II] score < 20). Based on these findings, most intensivists do not transfuse hemodynamically stable patients in the ICU until the hemoglobin level is less than 7 g/dL. (In patients with acute coronary syndrome, the threshold may be higher at 8 g/dL.106) Although each case should be individualized, it seems reasonable to apply these guidelines to critically ill pregnant patients. Fresh frozen plasma, cryoprecipitate, and platelets should not be transfused for the sole purpose of correcting laboratory measurements in patients who are not actively bleeding and are not undergoing an invasive procedure.
Sepsis
Sepsis occurs as the result of a maladaptive systemic inflammatory response to an infectious insult. It is the leading cause of mortality in ICUs in developed countries, and the incidence is increasing worldwide.111 Sepsis is also one of the leading causes of maternal mortality.112 The incidence of death from severe sepsis in obstetric patients is lower than that of nonobstetric patients. This likely reflects the fact that pregnant women are younger and have fewer coexisting medical pathologic processes.
Pregnancy affects both humoral and cell-mediated immunologic functions. The white blood cell count rises as pregnancy progresses, and some authors have described neutrophils in pregnant patients as “activated,” thus favoring severe inflammatory reactions to infectious stimuli.113 Cellular immunity is altered as a consequence of a decline in T-helper type 1 cell and natural killer cell function. The impaired cellular immunity may predispose pregnant women to infections from viruses and parasites. In contrast, antibody-mediated immunity is enhanced in pregnancy despite depressed levels of immunoglobulins (likely from hemodilution). Pregnancy is not a state of generalized immunosuppression; rather, it is a state of immunomodulation with compromised cellular and enhanced humoral immunity. Unfortunately, published information regarding the management of sepsis in pregnant women is limited; pregnant women typically have been excluded from large trials that have guided the evolution of the management of sepsis over the past several decades.
Definitions
Traditionally, sepsis has been defined as the presence of a systemic inflammatory response syndrome (SIRS) in a patient with a defined or suspected infection. The concept of SIRS was introduced by the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) in 1992.114 A patient is considered to have SIRS if two or more of the following criteria are present:
• Temperature greater than 38° C (100.4° F) or less than 36° C (96.8° F)
• Heart rate greater than 90 beats per minute
• Respiratory rate greater than 20 breaths per minute or a PaCO2 less than 32 mm Hg
• White blood cell count greater than 12,000/mm3 or less than 4000/mm3 or bandemia greater than 10%
Severe sepsis is defined as sepsis with signs of dysfunction of at least one organ (e.g., confusion, respiratory failure, acute kidney injury, thrombocytopenia, prolonged clotting times, elevated liver enzymes, hypotension). Septic shock is defined as severe sepsis with hypotension despite adequate fluid resuscitation, accompanied by a requirement for vasopressor therapy.
This definition of sepsis has been criticized for being too sensitive and nonspecific because most ICU patients will meet SIRS criteria.115 Defining sepsis in pregnant patients is complicated by the fact that normal physiologic changes of pregnancy may include a heart rate above 90 beats per minute, a PaCO2 below 32 mm Hg, and a white blood cell count above 12,000/mm3.
In 2001, extended criteria were developed to improve the diagnosis of sepsis by expanding the list of signs and symptoms of sepsis to reflect bedside clinical experience (Box 55-8).115 These signs and symptoms serve as clinical guidelines; not every patient with sepsis will manifest these alterations, and some alterations may be present in nonseptic patients.
Pathophysiology
The pathophysiology of sepsis is complicated and not fully understood. After exposure to a microorganism (bacteria, virus, parasite, fungus), the inflammatory cascade is activated. Massive production and release of inflammatory (interleukin-1 beta [IL-1β], tumor necrosis factor-alpha [TNF-α], IL-6, IL-8) and anti-inflammatory (IL-4, IL-10) cytokines, endothelial factors (nitric oxide), and other mediators (prostaglandins, leukotrienes, complement) results in loss of vasomotor tone, profound vasodilation, and increased vascular permeability (secondary to cytokine-induced endothelial injury). Fluids subsequently move out of the vascular space, resulting in so-called third spacing.111
The profound decrease in systemic vascular resistance and accompanying tachycardia results in the so-called hyperdynamic state seen in septic patients. However, myocardial function is profoundly altered by the actions of nitric oxide, IL-1, oxygen-derived free radicals, and TNF-α. Up to 60% of patients with sepsis have an ejection fraction less than 45%. Both systolic and diastolic dysfunction may occur. Not infrequently, myocyte injury from proinflammatory cytokines may lead to leakage of troponins. Typically, patients with systolic dysfunction tend to present with biventricular dilation. This dilation appears to be an adaptive response that allows for increased intracavitary filling, leading to an increased stroke volume despite a decrease in ejection fraction (preload recruitment). These cardiac changes tend to resolve spontaneously among survivors of sepsis.
Almost all patients with severe sepsis have clotting abnormalities, ranging from asymptomatic changes to severe DIC.116 Activation of the clotting cascade results from tissue factor production by macrocytes, neutrophils, and the endothelium as part of the inflammatory response. Tissue factor expressed in the surface of these cells binds factor VII, thus activating the clotting cascade. Excessive activation of the clotting cascade may lead to consumptive coagulopathy and the development of DIC. Development of DIC contributes to organ hypoperfusion (secondary to microvascular occlusion) and multiorgan system failure.
Natural anticoagulant proteins are often consumed in patients with severe sepsis111; alterations in protein C, antithrombin III, and plasminogen activator inhibitor have been described. Consumption of activated protein C has been associated with poor outcome in septic patients. Activated protein C is generated by thrombin-thrombomodulin–mediated protein C cleavage. Activated protein C, which inhibits clotting factors V and VIII, promotes fibrinolysis and has anti-inflammatory properties. Cytokines decrease the activity of thrombomodulin, leading to a lack of protein C activity during sepsis. Thus, the decline in activated protein C observed in severe sepsis results in amplified inflammation and a disruption in the normal balance between procoagulant and anticoagulant activity.
Mitochondrial dysfunction is also common in severe sepsis.116 Even in the presence of adequate oxygen delivery, oxygen consumption cannot be guaranteed if the mitochondria are dysfunctional and cannot extract oxygen and use it in oxidative respiration. This pathologic process explains why patients with sepsis may have normal or above normal oxyhemoglobin saturation in the central or pulmonary circulation despite poor tissue oxygen utilization.
Management
Of pivotal importance in the management of sepsis is achieving early infection source control and instituting adequate antibiotic therapy and resuscitation. Infected fluid collections or tissues should be drained/excised if clinically indicated.117 Broad-spectrum antibiotics should be initiated quickly (ideally after cultures have been obtained); narrow-spectrum antibiotic administration should follow once culture results are available.
Fluid Management.
The cornerstone of resuscitation in sepsis is early goal-directed fluid administration. Classically, hemodynamic resuscitation in severe sepsis has been directed to achieve a MAP of 65 mm Hg. The placenta should be regarded as the maternal end organ that is most sensitive to hypoperfusion. For this reason, FHR decelerations are often the first sign of maternal hypoperfusion.
Early goal-directed fluid resuscitation improves tissue perfusion by increasing driving pressure and modulating early inflammation by decreasing concentrations of proinflammatory cytokines. The Surviving Sepsis Campaign guidelines recommend the use of either crystalloid or colloid for the early resuscitation of sepsis.35,117 Crystalloids (normal saline, Ringer’s lactate, Plasma-Lyte) have an intravascular half-life of 30 to 60 minutes compared with 16 hours for colloids such as albumin. Theoretically, the use of colloid leads to a more efficient resuscitation. The largest published trial comparing the use of crystalloids and colloids in critically ill patients found no difference in outcomes between groups.35 However, a subgroup analysis suggested that patients with sepsis might benefit from albumin administration.35 A 2011 systematic review and meta-analysis118 found a small reduction in mortality in septic patients randomized to receive albumin rather than crystalloid for resuscitation (pooled estimate of the odds ratio, 0.82; 95% confidence interval, 0.62 to 1.0; P = .047). The authors suggested that additional study is required before a definitive conclusion can be made.118
Randomized trials (and a meta-analysis) that compared hydroxyethyl starch (e.g., 6% hetastarch solution) with crystalloid therapy in patients with severe sepsis found an increased incidence of 90-day mortality and an increased need for renal replacement therapy in the hydroxyethyl starch group.119,120 Therefore, carbohydrate-based colloid solutions may not be the optimal choice for resuscitation of the septic patient.
One of the most challenging clinical decisions in daily critical care practice is the precise identification of adequate fluid resuscitation. Goal-directed fluid therapy (defined as “adjustments in cardiac preload, afterload, and contractility to balance oxygen delivery with oxygen demand”121) is fundamental in the initial management of sepsis, but one must consider the endpoints of resuscitation. Premature initiation of vasopressors may be harmful because it will worsen tissue ischemia. On the other hand, excessive fluid resuscitation and a positive fluid balance have consistently been associated with increased mortality in critically ill patients. It appears that early in sepsis (the first 6 hours), patients benefit from goal-directed fluid therapy.121 Later, a conservative fluid strategy may be beneficial. In a randomized trial, Rivers et al.121 compared early goal-directed fluid therapy with standard care in patients with severe sepsis or septic shock. Goal-directed therapy consisted of early aggressive fluid resuscitation (central venous pressure [CVP] goal of 8 to 12 mm Hg), coupled with the use of vasopressors, inotropes, and blood transfusions to achieve a central venous oxygen saturation of greater than 70%. The in-hospital mortality was 30.5% in the goal-directed therapy group compared with 46.5% in the standard therapy group.121
A subsequent trial in patients with ALI or ARDS showed that after the initial phase of resuscitation, patients who were randomized to receive a conservative fluid regimen had improved lung function and a shorter duration of mechanical ventilation and ICU stay compared with patients who received a liberal fluid regimen.100 The study did not have sufficient power to identify a difference in 60-day mortality.
Traditionally, clinicians have titrated fluid therapy to static measurements such as CVP or the PAOP. The Surviving Sepsis Campaign still recommends fluid therapy that targets a CVP between 8 to 12 mm Hg (12 to 15 mm Hg in the setting of mechanical ventilation).117 This recommendation also likely applies to pregnant patients because neither CVP nor PAOP change during gestation. Unfortunately, static measurements of preload are less than ideal for predicting response to fluid administration. Echocardiographic-derived measurements provide further data on the adequacy of fluid resuscitation but suffer from the same limitations.
Current evidence supports the titration of fluid resuscitation to dynamic rather that static measurements of preload.122 For example, an increase in stroke volume as assessed by transthoracic echocardiography during passive leg raising is an accurate predictor of response to fluid administration.122 The validity of dynamic measures of preload during pregnancy requires investigation.
Vasopressors and Inotropes.
When fluid therapy alone is unable to achieve a MAP above 60 to 65 mm Hg, vasopressors are commonly used. The vasopressors of choice in septic shock are dopamine and norepinephrine.123 Norepinephrine increases blood pressure primarily by increasing systemic vascular resistance; dopamine’s main effect is to increase stroke volume. Although most studies have not found one vasopressor to be clearly superior to others, a 2008 meta-analysis of randomized controlled and observational trials found that dopamine was associated with greater mortality and a higher incidence of arrhythmic events than norepinephrine.124 Currently, it appears appropriate for the clinician to use the agent with which he or she is most familiar.
Obstetricians have traditionally expressed concern regarding the potential adverse effects of vasopressors on uteroplacental perfusion. However, in the setting of septic shock, restoration of maternal organ perfusion pressure is essential for fetal survival. Multiple case reports have described improved fetal status with the use of vasopressors to increase MAP.
Vasopressin is a peptide hormone synthesized in the hypothalamus and stored in the pituitary gland. A relative deficiency of vasopressin has been described during septic shock. Vasopressin causes direct vascular smooth muscle constriction via stimulation of V1 receptors. It also increases the response to catecholamines, likely by increasing cortisol secretion through its action on V3 receptors in the pituitary gland. Additionally, vasopressin achieves vasoconstriction by closing ATP-dependent potassium channels. Observational studies have shown that the addition of low-dose vasopressin (0.04 U/min) to traditional vasopressor therapy can raise blood pressure in vasopressor-refractory septic shock. A large randomized clinical trial reported no difference in mortality in patients with vasopressor-dependent septic shock who were randomized to receive either vasopressin or norepinephrine in addition to open-label vasopressor therapy.124 However, the trial did not address the use of vasopressin as rescue therapy for septic shock that is resistant to conventional vasopressors.
No good data exist regarding the use of vasopressin during septic shock in pregnant women. Theoretically, it may activate uterine V1a receptors, leading to uterine contractions. Extreme caution is recommended if this agent is used during pregnancy.
Contrary to popular belief, myocardial contractility is compromised in septic shock because TNF-α, IL-1, and nitric oxide lead to myocardial depression. Patients may develop both systolic and diastolic dysfunction, commonly involving both ventricles. In the subgroup of patients with mainly systolic dysfunction, the ventricles dilate to accommodate increased preload. This adaptive mechanism allows a transient increase in stroke volume and has been associated with improved outcomes. Patients with predominantly diastolic dysfunction do not usually develop this compensatory chamber dilation.
Septic cardiomyopathy may be “unmasked” when vasopressors are used to increase systemic vascular resistance. Assessment of cardiac output (e.g., bedside transthoracic echocardiography) is beneficial when vasopressors (primarily norepinephrine and vasopressin) are used without concomitant inotropic support. If worsening cardiac output is noted after initiating vasoconstrictor therapy, inotropic support (dobutamine or dopamine) may be beneficial.
Anecdotal data suggest that catecholamines may not be effective in patients with severe acidemia. However, data do not support the use of sodium bicarbonate therapy during resuscitation. In septic patients, bicarbonate therapy is not indicated if the pH is above 7.15.117 If the pH is less than 7.15, the use of sodium bicarbonate should be individualized. Particular care should be taken if the clinician chooses to use bicarbonate during pregnancy because bicarbonate does not cross the placenta, but the carbon dioxide generated from its administration crosses the placenta to the fetal compartment, leading to potential fetal acidemia.
Corticosteroids.
Approximately 50% to 75% of patients with severe sepsis/septic shock have critical illness–related corticosteroid insufficiency. Cytokines lead to a dysfunctional hypothalamic-pituitary-adrenal axis with a consequent decrease in cortisol secretion. Cortisol plays a pivotal role in the up-regulation of catecholamine receptors at the vascular level (leading to an increased response to endogenous and exogenous catecholamines).
The use of corticosteroid therapy in patients with septic shock has long been controversial. In the past, large doses of corticosteroids were used; however, evidence suggested that the use of high-dose corticosteroids in patients with sepsis/septic shock may be harmful. Studies performed in the past decade suggest that low-dose corticosteroids (hydrocortisone ≤ 300 mg/day) may be beneficial.125 Currently, physiologic doses of corticosteroids are recommended in patients who fail to respond to catecholamine therapy.125 Patients who receive vasopressors and are unable to maintain a systolic blood pressure above 90 mm Hg are candidates for corticosteroid therapy.125 The agent of choice is hydrocortisone (50 mg bolus intravenously every 6 hours or a continuous intravenous infusion at 10 mg/h). This low dose of glucocorticoid is believed to be immunomodulatory; the excessive immune response that leads to shock is down-regulated without causing immunosuppression. Once begun, treatment should be maintained for at least 4 to 7 days, and the dose should be tapered over 1 week to avoid rebound inflammation and shock. The adrenocorticotropic hormone (ACTH) stimulation test should not be used to identify patients with septic shock who should receive glucocorticoids. The same guidelines are applied during pregnancy to decide who might benefit from corticosteroid therapy. The clinician should be cognizant of the possible association between corticosteroid use during the first trimester of pregnancy and the development of fetal cleft lip and palate.99
Monitoring Resuscitation.
Traditionally, the main goal of resuscitation efforts in sepsis has been to achieve “normal” vital signs (MAP greater than 65 mm Hg, urine output greater than 0.5 mL/kg/h, normal heart rate).117 Unfortunately, clinical signs and symptoms lack sensitivity for detection of tissue hypoperfusion. Patients may have normal vital signs and still have organ hypoperfusion and anaerobic metabolism. Different strategies have been proposed to detect these patients with “occult shock.” Resuscitation may be guided by blood lactate levels. Patients with persistent lactic acidosis, despite apparently normal vital signs, may require further resuscitation to increase tissue oxygen delivery.
Another option is to optimize hemodynamic support using central ( ) and mixed venous (
) and mixed venous ( ) oxyhemoglobin saturation monitoring.
) oxyhemoglobin saturation monitoring. 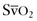 is the saturation of hemoglobin obtained from blood sampled from the pulmonary artery. It requires placement of a pulmonary artery catheter; a normal value is greater than 65%. Pregnancy per se does not alter the
is the saturation of hemoglobin obtained from blood sampled from the pulmonary artery. It requires placement of a pulmonary artery catheter; a normal value is greater than 65%. Pregnancy per se does not alter the 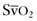 .126
.126
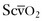 is the oxyhemoglobin saturation of a blood sample obtained from the junction of the superior vena cava and the right atrium. This measurement is readily achieved by obtaining a blood sample from a central venous catheter. The normal value is greater than 70%. To our knowledge, normal values for
is the oxyhemoglobin saturation of a blood sample obtained from the junction of the superior vena cava and the right atrium. This measurement is readily achieved by obtaining a blood sample from a central venous catheter. The normal value is greater than 70%. To our knowledge, normal values for 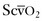 during pregnancy have not been described. Patients with tissue hypoperfusion will extract more oxygen in an attempt to increase aerobic metabolism. The increased extraction will lead to a decrease in the oxygen saturation of hemoglobin returning to the central circulation. Low values of either
during pregnancy have not been described. Patients with tissue hypoperfusion will extract more oxygen in an attempt to increase aerobic metabolism. The increased extraction will lead to a decrease in the oxygen saturation of hemoglobin returning to the central circulation. Low values of either 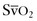 or
or 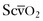 may warrant an attempt to increase oxygen delivery by volume expansion, blood transfusion, or the use of an inotrope.
may warrant an attempt to increase oxygen delivery by volume expansion, blood transfusion, or the use of an inotrope.
The Fetus during Critical Maternal Illness
In the vast majority of cases, therapeutic interventions in the critically ill pregnant patient should not be withheld because of fetal concerns. When the alternative is death or severe injury, very few drugs or diagnostic or therapeutic maneuvers are contraindicated. Most medications (sedatives, neuromuscular blocking agents, corticosteroids, vasopressors, inotropes, antibiotics) may be used safely (or with minimal risks that are commonly outweighed by the benefits) during pregnancy. Some of the common agents used in the ICU setting, and the associated fetal risks, are listed in Table 55-3.
TABLE 55-3
Common Medications Used during Critical Illness
| Medication | FDA Pregnancy Category* | Comments |
| Norepinephrine | C | Uterine vessels are very sensitive to vasoconstriction because they are rich in alpha-adrenergic receptors. As with any vasopressor, this agent may be used after adequate volume resuscitation and with continuous electronic FHR monitoring. Recent evidence suggests that norepinephrine may be the vasopressor of choice during sepsis. |
| Dopamine | C | Many clinicians are more familiar with the use of dopamine as a vasopressor in sepsis during pregnancy. Dopamine is associated with more tachyarrhythmias than norepinephrine. As with norepinephrine, it should be used after adequate fluid resuscitation and with continuous electronic FHR monitoring. |
| Vasopressin | C | Low doses used for the treatment of diabetes insipidus have not been associated with fetal harm. No data exist on its use as a continuous infusion for septic shock in pregnancy. Potentially, vasopressin may lead to activation of V1a receptors and uterine contractions; thus, extreme caution is recommended if used during pregnancy. Continuous electronic FHR monitoring is highly desirable. |
| Dobutamine | B | |
| Milrinone | C | |
| Phenylephrine | C | |
| Epinephrine | C | |
| Hydrocortisone | C | Some data suggest an association between corticosteroid use during the first trimester and fetal cleft lip/palate. However, if required to improve maternal outcomes, use should not be deferred. |
| Midazolam | D | No clear correlation between the use of midazolam and fetal anomalies has been described. Use of any sedative close to the time of delivery is associated with neonatal depression. |
| Lorazepam | D | Limited data have associated prolonged use with fetal anomalies. It is unlikely that its use during a few days of critical illness outside the first trimester is teratogenic. |
| Propofol | B | |
| Dexmedetomidine | C | This agent crosses the placenta, but no published studies link dexmedetomidine to fetal malformations. |
| Cisatracurium | B | May have anti-inflammatory properties |
| Vecuronium | C | |
| Morphine | C | |
| Fentanyl | C | |
| Hydromorphone | C | |
| Haloperidol | C |
FHR, fetal heart rate.
Imaging studies should be performed as needed with the use of abdominal shielding to limit fetal radiation exposure. When possible, ultrasonography and magnetic resonance imaging (MRI) are preferred because no ionizing radiation is used. Radiation exposure from common studies, such as a chest or abdominal radiography or CT of the head or chest, are all below the upper limit of safety (5 rads) recommended by the American College of Obstetricians and Gynecologists (ACOG).57 Similarly, the use of radiopaque (iodide containing) or paramagnetic contrast media during pregnancy is acceptable, provided the study is considered fundamental to the care of the patient.57
Critically ill pregnant patients should receive continuous electronic FHR monitoring after 24 weeks’ gestation (lower age limit of viability) to continuously evaluate fetal well-being. The presence of fetal bradycardia, tachycardia, or persistent FHR decelerations may signal uterine hypoperfusion that requires improved resuscitation efforts. Each case should be evaluated individually with the aid of a maternal-fetal medicine specialist. Pregnant women beyond 20 weeks’ gestation should be positioned either in the left or right decubitus position to avoid uterine compression of the inferior vena cava, which results in a decrease in cardiac preload.
References
1. Karnad DR, Guntupalli KK. Critical illness and pregnancy: review of a global problem. Crit Care Clin. 2004;20:555–576.
2. Soubra SH, Guntupalli KK. Critical illness in pregnancy: an overview. Crit Care Med. 2005;33(Suppl 10):S248–S255.
3. Naylor DF Jr, Olson MM. Critical care obstetrics and gynecology. Crit Care Clin. 2003;19:127–149.
4. Chames MC, Pearlman MD. Trauma during pregnancy: outcomes and clinical management. Clin Obstet Gynecol. 2008;51:398–408.
5. Munnur U, Bandi V, Guntupalli KK. Management principles of the critically ill obstetric patient. Clin Chest Med. 2011;32:53–60.
6. Price LC, Slack A, Nelson-Piercy C. Aims of obstetric critical care management. Best Pract Res Clin Obstet Gynaecol. 2008;22:775–799.
7. Cartin-Ceba R, Gajic O, Iyer VN, Vlahakis NE. Fetal outcomes of critically ill pregnant women admitted to the intensive care unit for nonobstetric causes. Crit Care Med. 2008;36:2746–2751.
8. Jacob S, Bloebaum L, Shah G, Varner MW. Maternal mortality in Utah. Obstet Gynecol. 1998;91:187–191.
9. Rogers FB, Rozycki GS, Osler TM, et al. A multi-institutional study of factors associated with fetal death in injured pregnant patients. Arch Surg. 1999;134:1274–1277.
10. Baerga-Varela Y, Zietlow SP, Bannon MP, et al. Trauma in pregnancy. Mayo Clin Proc. 2000;75:1243–1248.
11. Drost TF, Rosemurgy AS, Sherman HF, et al. Major trauma in pregnant women: maternal/fetal outcome. J Trauma. 1990;30:574–578.
12. Pearlman MD, Tintinalli JE, Lorenz RP. Blunt trauma during pregnancy. N Engl J Med. 1990;323:1609–1613.
13. Metz TD, Abbott JT. Uterine trauma in pregnancy after motor vehicle crashes with airbag deployment: a 30-case series. J Trauma. 2006;61:658–661.
14. Curet MJ, Schermer CR, Demarest GB, et al. Predictors of outcome in trauma during pregnancy: identification of patients who can be monitored for less than 6 hours. J Trauma. 2000;49:18–24.
15. Aboutanos SZ, Aboutanos MB, Dompkowski D, et al. Predictors of fetal outcome in pregnant trauma patients: a five-year institutional review. Am Surg. 2007;73:824–827.
16. Chesnut RM. Care of central nervous system injuries. Surg Clin North Am. 2007;87:119–156.
17. El Kady D, Gilbert WM, Anderson J, et al. Trauma during pregnancy: an analysis of maternal and fetal outcomes in a large population. Am J Obstet Gynecol. 2004;190:1661–1668.
18. Grossman NB. Blunt trauma in pregnancy. Am Fam Physician. 2004;70:1303–1310.
19. Melamed N, Aviram A, Silver M, et al. Pregnancy course and outcome following blunt trauma. J Matern Fetal Neonatal Med. 2012;25:1612–1617.
20. Muench MV, Baschat AA, Reddy UM, et al. Kleihauer-Betke testing is important in all cases of maternal trauma. J Trauma. 2004;57:1094–1098.
21. Brown HL. Trauma in pregnancy. Obstet Gynecol. 2009;114:147–160.
22. Lavin JP Jr, Polsky SS. Abdominal trauma during pregnancy. Clin Perinatol. 1983;10:423–438.
23. Richards JR, Ormsby EL, Romo MV, et al. Blunt abdominal injury in the pregnant patient: detection with US. Radiology. 2004;233:463–470.
24. Morgan JA, Marcus PS. Prenatal diagnosis and management of intrauterine fracture. Obstet Gynecol Surv. 2010;65:249–259.
25. Scorpio RJ, Esposito TJ, Smith LG, Gens DR. Blunt trauma during pregnancy: factors affecting fetal outcome. J Trauma. 1992;32:213–216.
26. Patteson SK, Snider CC, Meyer DS, et al. The consequences of high-risk behaviors: trauma during pregnancy. J Trauma. 2007;62:1015–1020.
27. Schiff MA, Holt VL. The injury severity score in pregnant trauma patients: predicting placental abruption and fetal death. J Trauma. 2002;53:946–949.
28. Baethmann M, Kahn T, Lenard HG, Voit T. Fetal CNS damage after exposure to maternal trauma during pregnancy. Acta Paediatr. 1996;85:1331–1338.
30. Driscoll P, Wardrope J. ATLS: past, present, and future. Emerg Med J. 2005;22:2–3.
31. Hastings RH, Marks JD. Airway management for trauma patients with potential cervical spine injuries. Anesth Analg. 1991;73:471–482.
32. Clayton JL, Harris MB, Weintraub SL, et al. Risk factors for cervical spine injury. Injury. 2012;43:431–435.
33. Markandaya M, Stein DM, Menaker J. Acute treatment options for spinal cord injury. Curr Treat Options Neurol. 2012;14:175–187.
34. Oxford CM, Ludmir J. Trauma in pregnancy. Clin Obstet Gynecol. 2009;52:611–629.
35. SAFE Study Investigators. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884.
36. Brasel KJ, Bulger E, Cook AJ, et al. Hypertonic resuscitation: design and implementation of a prehospital intervention trial. J Am Coll Surg. 2008;206:220–232.
37. Lippi G, Favaloro EJ, Cervellin G. Massive posttraumatic bleeding: epidemiology, causes, clinical features, and therapeutic management. Semin Thromb Hemost. 2013;39:83–93.
38. Fowler R, Pepe PE. Fluid resuscitation of the patient with major trauma. Curr Opin Anaesthesiol. 2002;15:173–178.
39. Arul GS, Pugh HE, Mercer SJ, Midwinter MJ. Optimising communication in the damage control resuscitation—Damage Control Surgery sequence in major trauma management. J R Army Med Corps. 2012;158:82–84.
40. Curry N, Davis PW. What’s new in resuscitation strategies for the patient with multiple trauma? Injury. 2012;43:1021–1028.
41. Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am. 2012;92:877–891.
42. Martinowitz U, Michaelson M. Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli Multidisciplinary rFVIIa Task Force. J Thromb Haemost. 2005;3:640–648.
43. Lau P, Ong V, Tan WT, et al. Use of activated recombinant factor VII in severe bleeding—evidence for efficacy and safety in trauma, postpartum hemorrhage, cardiac surgery, and gastrointestinal bleeding. Transfus Med Hemother. 2012;39:139–150.
44. Weinberg L, Steele RG, Pugh R, et al. The pregnant trauma patient. Anaesth Intensive Care. 2005;33:167–180.
45. Connolly AM, Katz VL, Bash KL, et al. Trauma and pregnancy. Am J Perinatol. 1997;14:331–336.
46. Shah S, Miller PR, Meredith JW, Chang MC. Elevated admission white blood cell count in pregnant trauma patients: an indicator of ongoing placental abruption. Am Surg. 2002;68:644–647.
47. Pearlman MD, Tintinallli JE, Lorenz RP. A prospective controlled study of outcome after trauma during pregnancy. Am J Obstet Gynecol. 1990;162:1502–1507.
48. McCollough CH, Schueler BA, Atwell TD, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27:909–917.
49. Association for the Advancement of Automotive Medicine. Abbreviated Injury Scale. [Available at] http://www.aaam1.org/ais/ [Accessed April 2013] .
50. Esposito TJ, Tinkoff G, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale (OIS): past, present, and future. J Trauma Acute Care Surg. 2013;74:1163–1174.
51. Harris T, Davenport R, Hurst T, et al. Improving outcome in severe trauma: what’s new in ABC? Imaging, bleeding and brain injury. Postgrad Med J. 2012;88:595–603.
52. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: Cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–S861.
53. Jeejeebhoy FM, Zelop CM, Windrim R, et al. Management of cardiac arrest in pregnancy: a systematic review. Resuscitation. 2011;82:801–809.
54. Hui D, Morrison LJ, Windrim R, et al. The American Heart Association 2010 guidelines for the management of cardiac arrest in pregnancy: consensus recommendations on implementation strategies. J Obstet Gynaecol Can. 2011;33:858–863.
55. Esmaeilzadeh M, Dictus C, Kayvanpour E, et al. One life ends, another begins: management of a brain-dead pregnant mother—a systematic review. BMC Med. 2010;8:74.
56. Wiebers DO. Ischemic cerebrovascular complications of pregnancy. Arch Neurol. 1985;42:1106–1113.
57. American College of Obstetricians and Gynecologists. Guidelines for diagnostic imaging during pregnancy. [ACOG Committee Opinion No. 299. Washington, DC] September 2004.
58. Wiese KM, Talkad A, Mathews M, Wang D. Intravenous recombinant tissue plasminogen activator in a pregnant woman with cardioembolic stroke. Stroke. 2006;37:2168–2169.
59. Li Y, Margraf J, Kluck B, et al. Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: a case report and literature review. Neurologist. 2012;18:44–48.
60. Vaid U, Baram M, Marik PE. Thrombolytic therapy in a patient with suspected pulmonary embolism despite a negative computed tomography pulmonary angiogram. Respir Care. 2011;56:336–338.
61. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke. [In Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines] Chest. 2012;141(Suppl 2):e601S–e636S.
62. French KF, White J, Hoesch RE. Treatment of intracerebral hemorrhage with tranexamic acid after thrombolysis with tissue plasminogen activator. Neurocrit Care. 2012;17:107–111.
63. Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Circulation. 2007;115:e478–e534.
64. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–1798.
65. Wartenberg KE. Malignant middle cerebral artery infarction. Curr Opin Crit Care. 2012;18:152–163.
66. Karnad DR, Guntupalli KK. Neurologic disorders in pregnancy. Crit Care Med. 2005;33(Suppl 10):S362–S371.
67. Flower O, Smith M. The acute management of intracerebral hemorrhage. Curr Opin Crit Care. 2011;17:106–114.
68. Grise EM, Adeoye O. Blood pressure control for acute ischemic and hemorrhagic stroke. Curr Opin Crit Care. 2012;18:132–138.
69. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy. [In Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines] Chest. 2012;141(Suppl 2):e44S–88S.
70. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocrine Practice. 2009;15:353–369.
71. Raper DM, Allan R. International subarachnoid trial in the long run: critical evaluation of the long-term follow-up data from the ISAT trial of clipping vs coiling for ruptured intracranial aneurysms. Neurosurgery. 2010;66:1166–1169.
72. Bederson JB, Connolly ES Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
73. Wartenberg KE. Critical care of poor-grade subarachnoid hemorrhage. Curr Opin Crit Care. 2011;17:85–93.
74. Muroi C, Seule M, Mishima K, Keller E. Novel treatments for vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care. 2012;18:119–126.
75. Rabinstein AA. Vasospasm and statin therapy: yet another cautionary tale. Neurocrit Care. 2010;12:310–312.
76. van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA. 2011;306:2011–2019.
77. Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356:2704–2712.
79. Marik PE, Varon J. The management of status epilepticus. Chest. 2004;126:582–591.
80. Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600.
81. Claassen J, Silbergleit R, Weingart SD, Smith WS. Emergency neurological life support: status epilepticus. Neurocrit Care. 2012;17(Suppl 1):S73–S78.
82. Raghavendran K, Napolitano LM. Definition of ALI/ARDS. Crit Care Clin. 2011;27:429–437.
83. Blank R, Napolitano LM. Epidemiology of ARDS and ALI. Crit Care Clin. 2011;27:439–458.
84. Cole DE, Taylor TL, McCullough DM, et al. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005;33(Suppl 10):S269–S278.
85. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
86. Kuiper JW, Groeneveld AB, Slutsky AS, Plotz FB. Mechanical ventilation and acute renal failure. Crit Care Med. 2005;33:1408–1415.
87. Kolobow T. Acute respiratory failure: on how to injure healthy lungs (and prevent sick lungs from recovering). ASAIO Trans. 1988;34:31–34.
88. Haas CF. Mechanical ventilation with lung protective strategies: what works? Crit Care Clin. 2011;27:469–486.
89. Phoenix SI, Paravastu S, Columb M, et al. Does a higher positive end expiratory pressure decrease mortality in acute respiratory distress syndrome? A systematic review and meta-analysis. Anesthesiology. 2009;110:1098–1105.
90. Park PK, Napolitano LM, Bartlett RH. Extracorporeal membrane oxygenation in adult acute respiratory distress syndrome. Crit Care Clin. 2011;27:627–646.
91. Maung AA, Kaplan LJ. Airway pressure release ventilation in acute respiratory distress syndrome. Crit Care Clin. 2011;27:501–509.
92. Douglas WW, Rehder K, Beynen FM, et al. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis. 1977;115:559–566.
93. Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573.
94. Anzueto A, Guntapalli K. Adjunctive therapy to mechanical ventilation: surfactant therapy, liquid ventilation, and prone position. Clin Chest Med. 2006;27:637–654.
95. Dickinson S, Park PK, Napolitano LM. Prone-positioning therapy in ARDS. Crit Care Clin. 2011;27:511–523.
96. Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116.
97. Marik PE, Meduri GU, Rocco PR, Annane D. Glucocorticoid treatment in acute lung injury and acute respiratory distress syndrome. Crit Care Clin. 2011;27:589–607.
98. Tang BM, Craig JC, Eslick GD, et al. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–1603.
99. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal And Neonatal risk. 9th edition. Lippincott Williams & Wilkins: Philadelphia; 2011.
100. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575.
101. Puri N, Dellinger RP. Inhaled nitric oxide and inhaled prostacyclin in acute respiratory distress syndrome: what is the evidence? Crit Care Clin. 2011;27:561–587.
102. Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581.
103. Martindale RG, McClave SA, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757–1761.
104. Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake!. Crit Care Med. 2011;39:2619–2626.
105. van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367.
106. Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67:1439–1442.
107. Vincent JL, Sakr Y, De Backer D, Van der Linden P. Efficacy of allogeneic red blood cell transfusions. Best Pract Res Clin Anaesthesiol. 2007;21:209–219.
108. Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024–3029.
109. Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115:635–649.
110. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417.
111. Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677–702.
112. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
113. Naccasha N, Gervasi MT, Chaiworapongsa T, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–1123.
114. Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–1483.
115. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256.
116. Marshall JC, al Naqbi A. Principles of source control in the management of sepsis. Crit Care Clin. 2009;25:753–768.
117. Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
118. Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39:386–391.
119. Estrada CA, Murugan R. Hydroxyethyl starch in severe sepsis: end of starch era? Crit Care. 2013;17:310.
120. Patel A, Waheed U, Brett SJ. Randomised trials of 6 % tetrastarch (hydroxyethyl starch 130/0.4 or 0.42) for severe sepsis reporting mortality: systematic review and meta-analysis. Intensive Care Med. 2013;39:811–822.
121. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
122. Préau S, Saulnier F, Dewavrin F, et al. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–825.
123. De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med. 2012;40:725–730.
124. Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887.
125. Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–2375.
126. Hankins GD, Clark SL, Uckan E, Van Hook JW. Maternal oxygen transport variables during the third trimester of normal pregnancy. Am J Obstet Gynecol. 1999;180:406–409.