Cardiovascular Disease
Mladen I. Vidovich MD, FACC, FSCAI
Chapter Outline
CARDIOVASCULAR PHYSIOLOGIC CHANGES OF PREGNANCY
CARDIAC EXAMINATION DURING PREGNANCY
CARDIOPULMONARY RESUSCITATION DURING PREGNANCY
Evidence suggests that gender has a significant impact on cardiovascular disease. The etiology of coronary thrombosis causing acute myocardial infarction is different in women than in men. Plaque erosion, rather than plaque rupture, occurs at a higher rate in women than in men. Coronary artery diameter is smaller in women,1 and women frequently develop more diffuse atherosclerosis than men. Female aortas appear to be stiffer due to underlying fibrosis or remodeling. Women have more microvascular coronary artery dysfunction than men and frequently demonstrate impaired coronary artery vasodilator response.
Autoimmune rheumatic diseases are associated with premature atherosclerosis; vasculitides such as Takayasu’s arteritis, temporal arteritis, rheumatoid vasculitis, lupus vasculitis, and polymyalgia rheumatica are all more common in women than in men. During exercise stress testing, women more often have atypical and nonanginal pain than men, who more often have typical angina.2 Women with acute myocardial infarction more frequently do not have chest pain, especially women who have the infarction at a younger age. In-hospital mortality for acute myocardial infarction is higher for women than for men.3 Although women who have non–ST-segment elevation myocardial infarction have a worse risk profile than men, the infarction is frequently treated less aggressively.4 Yet women are more likely than men to summon emergency medical services during a myocardial infarction.
Whether women have worse outcome after percutaneous coronary intervention has been a matter of debate.5 At the time of presentation for both percutaneous and surgical coronary revascularization procedures, women are older than men and have more cardiovascular risk factors and comorbid conditions.5 Older studies demonstrated worse overall outcome in women than in men; however, more recent studies have demonstrated a narrowing or disappearance of the gender outcome gap. Similarly, several studies have demonstrated that female gender was an independent predictor of coronary artery bypass (CABG) operative mortality. However, after extensive baseline risk adjustment, outcomes after CABG or aortic valve replacement have been reported to be similar for men and women.
The number of pregnancies has been associated with the future risk for coronary artery disease6 and progression of atherosclerosis.7 Hypertensive disorders of pregnancy, preeclampsia, and gestational diabetes mellitus are risk factors for future development of cardiovascular disease.8,9 Earlier identification of these women with an increased lifetime risk for developing cardiovascular disease may present a unique opportunity for prevention of subsequent cardiovascular events.
Historically, rheumatic mitral stenosis represented the most common cardiac condition encountered in pregnant women. This disease continues to be a major problem in the developing world and in certain immigrant populations in the United States. In the industrialized countries, congenital heart disease has become the most common cardiac condition complicating pregnancy. This demographic change is a result of significant advances in the treatment of complex congenital heart conditions and survival of these patients into childbearing age. In the United States, maternal mortality due to hemorrhage and hypertensive disorders of pregnancy has declined, whereas mortality due to cardiovascular conditions has steadily increased.10
The optimal management of women with cardiovascular disease begins before conception. Normal physiologic changes of pregnancy may exacerbate preexisting cardiovascular disease. For most women with heart disease, pregnancy is associated with favorable outcome; however, even with modern advances in treatment and monitoring, there remains a high incidence of morbidity and mortality for some conditions. Thus, for some women, it may be advisable to avoid pregnancy.
There is significant individual variability in the severity of specific cardiovascular disease entities. Additionally, several cardiovascular conditions may be simultaneously present in one individual. Management may be further complicated by the presence of noncardiovascular pathologic processes. The anesthetic management of the parturient with cardiovascular disease should be individualized, and a multidisciplinary team should plan peripartum care. Some case reports and small series have described the anesthetic management of these patients, but, in general, few data justify choosing one anesthetic technique over another. Therefore, the anesthesiologist must have a thorough understanding of the normal physiologic changes of pregnancy as well as the individual parturient’s pathophysiology, and then plan anesthetic management that best achieves the desired hemodynamic goals. Optimal analgesia is often an important part of safe childbirth in these patients.
The anesthetic care of women with cardiovascular disease does not end with labor and delivery; rather, it continues postpartum when the physiologic changes of pregnancy may be at their greatest. Inadequate postpartum analgesia may be associated with hypertension and tachycardia. Postoperative shivering increases oxygen consumption and may cause myocardial ischemia in patients with limited cardiac reserve.
Cardiovascular Physiologic Changes of Pregnancy
The electrocardiogram (ECG) typically changes during pregnancy. During the third trimester, the enlarging gravid uterus causes upward and lateral rotation of the heart, which may result in left-axis deviation of 15 to 20 degrees. Overall, however, the QRS axis is quite variable during pregnancy. At rest, nonspecific ST-segment and T-wave changes are very common during normal pregnancy.11 Exercise in healthy pregnant women does not cause distinctive ECG changes when compared with nonpregnant subjects.
No repolarization abnormalities are observed with uncomplicated vaginal delivery. ST-segment elevation is never seen in normal pregnancy and should always be considered pathologic. ST-segment depression is seen in 25% to 81% of parturients undergoing cesarean delivery, regardless of the type of anesthesia. Oxytocin administration during the third stage of labor has been associated with ST-segment depression.12,13 However, these oxytocin-associated ECG changes are not associated with myocardial damage. Whether these ECG changes are caused by underlying ischemia or some other mechanism remains unclear.14
Left ventricular mass increases during normal pregnancy.15,16 The increase in left ventricular mass is greater in multiple gestation than in singleton gestation.17 Preeclampsia also results in a greater increase in left ventricular mass.18
Plasma lipid concentrations, including total serum cholesterol, triglycerides, and low-density lipoprotein cholesterol concentrations, increase during pregnancy.19 Obese pregnant women have an even greater increase in plasma lipids. This increase in plasma lipids results in part from insulin resistance and an increase in estrogen levels during pregnancy. The effects of these physiologic changes in plasma lipid concentrations on long-term cardiovascular outcomes are unclear.
Brain natriuretic peptide (BNP) is a natriuretic hormone synthesized primarily in the heart ventricles. BNP levels increase as a response to increased filling pressures in patients with heart failure. Physiologically, BNP has hypotensive, diuretic, and natriuretic effects. During uncomplicated normal pregnancy, BNP levels are unchanged (< 20 pg/mL).20 The lack of change in BNP levels during normal pregnancy suggests that the heart adapts to the increased volume load associated with pregnancy. By contrast, BNP levels are increased in preeclamptic women20 and in pregnant women with heart disease.21 A correlation exists between BNP and the increases in left ventricular mass and end-diastolic and end-systolic volumes observed in preeclampsia.18 The increase in BNP with fluid administration in preeclamptic women further confirms that this hormone is secreted in response to increased intracavitary pressures. Intravenous fluid administration does not increase BNP levels in healthy women.22,23 BNP is elevated in women with complex congenital heart disease, but it varies considerably among anomalies. Therefore, its use for individual patient management remains unclear.24
Cardiac enzyme levels may be altered by pregnancy or pregnancy-associated disease. Myocardial cell death is associated with elevation of sensitive and specific cardiac biomarkers—creatine kinase MB fraction (CK-MB) and cardiac troponins.25 Cardiac troponin levels are not elevated above the upper limits of normal during uncomplicated pregnancy. Troponin levels are elevated in women with gestational hypertension or preeclampsia.26,27 By contrast, CK-MB levels may be elevated up to two to four times the upper limit of normal owing to the presence of these enzymes in the uterus and placenta (see Figure 47-1). Thus, elevated CK-MB levels are not specific for the diagnosis of myocardial infarction during pregnancy.27,28 In patients with preeclampsia who have concurrent myocardial infarction, the observed troponin levels are higher than expected for the underlying preeclampsia.29 Heterophil antibody interference with the troponin assay may cause a false-positive increase in troponin levels during pregnancy. However, both CK-MB and troponin are sensitive markers for the diagnosis of myocardial infarction during pregnancy.
Cardiac output increases as early as 5 weeks’ gestation and continues to increase throughout the second trimester until it is approximately 50% greater than nonpregnant values (see Figure 2-1). Cardiac output does not change from this level during the third trimester; it may actually be reported as decreased in the third trimester if measurements are made in the supine position, which causes aortocaval compression. Both an increase in heart rate and stroke volume contribute to the increase in cardiac output. Distribution of cardiac output to the uterine circulation increases from 1% in the nonpregnant state to 12% during the second half of pregnancy (see Chapter 2).
Cardiac Examination during Pregnancy
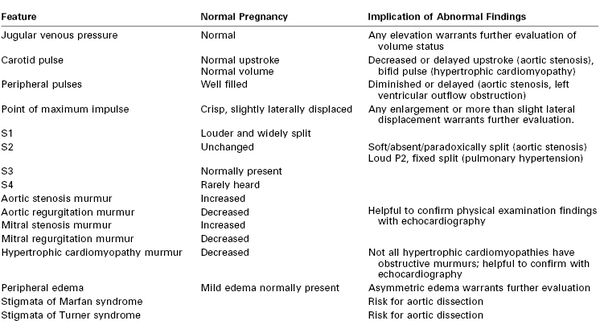
Pregnant women frequently complain of mild dyspnea at rest and exertion; on occasion, exercise tolerance is decreased. Therefore, it is important to recognize normal changes in the physical examination associated with pregnancy (Table 42-1).
Resting heart rate is higher in pregnancy, and peripheral pulses are “well filled” with rapid upstroke and collapse, primarily owing to lower systemic vascular resistance (SVR). The central venous pressure remains unchanged during pregnancy, and any elevation of jugular venous pressure is an abnormal finding. Basilar rales may be heard on lung auscultation; however, these are no longer heard after deep inspiration, a brief breath-hold, or a cough. These evanescent rales likely result from basilar atelectasis.
The heart examination during pregnancy is altered as a result of uterine enlargement. Consequently, the point of maximum impulse (left ventricular apex) is displaced superiorly and laterally during advanced pregnancy. It remains crisp, well defined, and hyperdynamic. In thin women, the right ventricular impulse may become visible owing to an increase in circulating blood volume and the proximity of this chamber to the anterior chest wall.
Recognition of normal auscultatory changes helps distinguish pathologic from physiologic changes. New murmurs are heard in more than 90% of pregnant women. The loudest murmurs are heard between 15 and 25 weeks’ gestation; murmur intensity decreases toward term and increases again during labor and the early postpartum period. This peripartum increase in murmurs is followed by a gradual decrease; most of these pregnancy-associated murmurs are no longer appreciated by 6 weeks postpartum.30 Importantly, there is no correlation between the disappearance of physiologic murmurs of pregnancy and the return of cardiac output and blood volume to prepregnancy levels.31
The first heart sound (S1) becomes louder and is widely split owing to early closure of the mitral valve during pregnancy. The second heart sound (S2) is unchanged. It is quite common to appreciate the third heart sound (S3), although considerable expertise and a quiet environment are necessary because of the presence of underlying tachycardia and an increased basal respiratory rate. The fourth heart sound (S4) is rarely appreciated. Owing to increased cardiac output and increased flow through cardiac valves, a systolic ejection murmur, usually soft (grade 2 to 3/6), is appreciated over the upper sternal border and the right side of the heart.
The murmurs of aortic and mitral regurgitation generally decrease and may become inaudible during pregnancy owing to the decrease in SVR. However, administration of phenylephrine or development of hypertension during pregnancy, both of which increase the SVR, increases the intensity of these murmurs.32
The murmur associated with aortic stenosis increases in intensity during pregnancy from increased flow through the stenotic valve. A diminished carotid upstroke, soft or inaudible S2, and a grade 4/6 murmur are almost always indicative of severe aortic stenosis. An audible, physiologic split S2 almost invariably rules out severe aortic stenosis. Diastolic murmurs during pregnancy are almost always associated with an underlying pathologic process.
The murmur of hypertrophic cardiomyopathy may have decreased intensity because the pregnancy-associated increase in intravascular volume may result in decreased outflow tract obstruction. The murmur of an atrial septal defect may become more audible during pregnancy.
Mammary souffle (“soo-fuhl”) is a noncardiac sound; it describes the continuous hum heard over the breasts. It becomes audible during late pregnancy and lactation, and it disappears at the end of lactation.
Most pregnant women display some degree of peripheral edema, in part owing to uterine compression of the inferior vena cava, which impedes venous return. This physiologic edema is symmetric and decreases with leg elevation and the left lateral decubitus position. The pathologic edema of preeclampsia should be differentiated from the physiologic edema of pregnancy. Asymmetric lower extremity edema is almost invariably pathologic. A tender and warm lower extremity may suggest deep vein thrombosis or cellulitis.
Funduscopic examination in pregnancy may help differentiate chronic hypertension from hypertensive disease of pregnancy (preeclampsia/eclampsia) and may identify changes due to long-standing diabetes.
It is important to look for stigmata of Marfan syndrome. Tall stature, large arm span, or other stigmata may alert the practitioner to the presence of a previously undiagnosed condition. Patients with Marfan syndrome frequently demonstrate scoliosis and may have dural ectasia. Turner syndrome is characterized by short stature and webbed neck. Both conditions predispose pregnant women to aortic dissection (see later discussion).
Cardiac Risk Prediction
The New York Heart Association (NYHA)33 and Heart Failure Stage34 classifications describe symptoms and predict risk in the nonpregnant population (Box 42-1). Several classifications have been proposed to specifically predict cardiac risk during pregnancy (Boxes 42-2, 42-3, and 42-4).35–38 These classifications may help predict the individual pregnant woman’s cardiac risk and, combined with the clinical constellation and results of cardiac imaging, may help guide clinical management.35,37–39 Implementation of a standardized and guideline-based approach to care, based on risk assessment, provides consistency in treating pregnant women with cardiac disease.
Maternal cardiac disease is associated with an increased incidence of neonatal complications. The most widely accepted associations are cyanosis, NYHA functional class greater than II, presence of a mechanical valve prosthesis, heparin or warfarin use during pregnancy, multiple gestation, smoking during pregnancy, left-sided heart obstruction, and use of cardiac medications before pregnancy.35,39
Cardiovascular Imaging during Pregnancy
Echocardiography
Echocardiography allows for safe and noninvasive assessment of heart structure and function. Both transthoracic and transesophageal echocardiography can be performed at any stage of pregnancy. Echocardiography helps predict overall cardiac risk and guides anesthetic management in pregnant women with cardiac disease. Echocardiography also allows assessment of intravascular volume and may obviate the need for right-sided heart catheterization to determine ventricular filling pressures.
One of the most commonly used echocardiographic assessments of left ventricular function—left ventricular ejection fraction—remains unchanged during pregnancy. Echocardiographic measurements of cardiac output increase during pregnancy owing to increases in stroke volume and heart rate. Importantly, stroke work is increased during pregnancy, which is consistent with augmented myocardial fiber function. Both the right and left ventricular chamber size are increased in end diastole and end systole, and the heart becomes more globular. This increase in chamber size results in increased left ventricular end-systolic and end-diastolic volumes and is accompanied by an increase in left ventricular wall thickness (eccentric left ventricular hypertrophy). These parameters return to baseline within 3 to 6 months postpartum.40 Left ventricular end-diastolic and end-systolic volumes are further increased with multiple gestation; the stroke volume is increased an additional 15%. Combined with a small additional increase in heart rate, this change results in a 20% greater increase in maternal cardiac output with multiple gestation compared with singleton gestation.17
Left atrial size is increased during pregnancy16; the increase is even greater with multiple gestation than with singleton gestation.17 Preeclampsia results in an adaptive concentric hypertrophy and an increase in left ventricular mass due to increased afterload.
In normal pregnancy, left ventricular diastolic function is increased in the first two trimesters and declines in the third trimester.15 Twenty percent of women with preeclampsia have evidence of global diastolic dysfunction.41 Diastolic function is most commonly assessed echocardiographically by evaluating mitral valve inflow, pulmonary venous flow, and myocardial tissue motion (tissue Doppler imaging).
Cardiac Magnetic Resonance Imaging
The risks of magnetic resonance imaging (MRI) in pregnant women are similar to those in nonpregnant patients. The potential risks to the fetus are teratogenicity and acoustic damage. There are no published reports of untoward fetal effects resulting from MRI in pregnant women. Owing to limited evidence on safety during organogenesis, it may be prudent to limit use of MRI during the first trimester. Cardiac magnetic resonance (CMR) imaging should be performed during pregnancy only after consideration of both the maternal and fetal benefits and risks. However, given the quality of images and diagnostic yield, CMR is preferable to any other modality that uses ionizing radiation. Gadolinium use during pregnancy should be avoided; it should be used only if absolutely required. Gadolinium is a pregnancy category C drug; it crosses the placenta and has been shown to be teratogenic in animal studies.
Left- and Right-Sided Heart Catheterization
Left-sided heart catheterization remains the gold standard for diagnosis of coronary artery disease, and it can be performed at any time during pregnancy. Radial arterial access is preferable to femoral access because it is associated with earlier ambulation, increased patient comfort, and a significant reduction in access-related bleeding complications. Additionally, during the procedure, the left lateral decubitus position can be more easily maintained with radial access. Cardiologists should strictly adhere to the ALARA principle (as low as reasonably achievable) to limit both maternal and fetal ionizing radiation exposure.
The use of pulmonary artery catheterization has significantly declined in the United States in recent years. Similar information can be obtained noninvasively by transthoracic or transesophageal echocardiography. However, thermodilution and Fick cardiac output measurements can be obtained only with the use of a pulmonary artery catheter. Similarly, right-sided heart catheterization is required for vasoreactivity testing in patients with pulmonary hypertension. Historically, pulmonary artery catheterization has helped guide fluid management in women with severe preeclampsia and eclampsia, particularly those who develop renal failure and pulmonary edema (see Chapter 36). In the future, ventricular filling in these patients may be assessed noninvasively with the bedside use of portable, hand-held transthoracic echocardiography devices.
Iodinated Contrast Use during Pregnancy
The use of iodinated contrast media in pregnant women appears safe. To date, there have been no reports of fetal teratogenic or mutagenic effects after maternal administration of iodinated contrast media. Free iodide in the contrast medium administered to the mother may depress fetal, and subsequently neonatal, thyroid function. Therefore, it has been suggested that neonatal thyroid function be checked during the first week postpartum. Minimal amounts of iodinated contrast media are excreted in breast milk; even smaller amounts are absorbed by the neonate’s gastrointestinal tract. The slight potential risk associated with absorption of contrast medium is thought to be insufficient to recommend interruption of breast-feeding after maternal administration of iodinated contrast media.
Computed Tomographic Angiography
Multidetector computed tomography (CT) allows noninvasive imaging of the coronary arteries along with cardiac structure and function. Use of contemporary CT with aggressive dose-reduction techniques can significantly limit the radiation dose. The overall radiation dose of coronary CT angiography may be equivalent to, or even lower than, radiation doses delivered with conventional invasive coronary angiography. Although soft cardiac structures not seen by conventional coronary angiography are well visualized by CT angiography, the intravenous contrast medium load is greater with CT angiography, and coronary intervention cannot be performed at the time of imaging. Thus, in pregnancy, invasive coronary angiography appears preferable under most circumstances.
Ionizing Radiation Risks to the Fetus
The ionizing radiation dose to the fetus can be limited by the use of echocardiography, intracardiac echocardiography, and markedly reduced fluoroscopy frame rates. Because the fetus is not directly within the radiation beam for cardiac procedures, the fetal exposure occurs through indirect scatter radiation. Therefore, external shielding of the fetus is ineffective. The fetal radiation dose cannot be measured and is therefore estimated. It is reassuring that the estimated fetal doses are low. Nonetheless, the safest approach is to avoid ionizing radiation during pregnancy if possible, and to assess both the maternal and fetal risks and benefits before deciding on the most appropriate imaging modality.
Cardiac Drugs and Pregnancy
Angiotensin-Converting Enzyme Inhibitors
Angiotensin-converting enzyme (ACE) inhibitors are the mainstay of coronary artery disease treatment, left ventricular dysfunction, and hypertension in nonpregnant patients. These drugs are particularly useful in nonpregnant patients with diabetes mellitus.
In the first trimester, the use of ACE inhibitors has been associated with an increased risk for fetal cardiovascular and central nervous system malformations. Use of ACE inhibitors during the second and third trimesters of pregnancy has also been associated with adverse fetal and neonatal outcomes as a result of the ACE inhibitors’ effect on fetal renal vascular tone. These drugs cause fetal kidney malfunction and decreased fetal urine output, which results in oligohydramnios. Positional limb deformities, skull ossification retardation, and lung hypoplasia are seen with ACE inhibitor−associated oligohydramnios. These effects are not thought to be caused by fetal exposure to ACE inhibitors during the first trimester. Thus, despite the exceptionally useful profile of ACE inhibitors in nonpregnant patients with cardiovascular disease, their use in pregnancy is contraindicated (pregnancy category D) (see Chapter 14).42
Angiotensin Receptor–Blocking Agents
Fewer data are available on the use of angiotensin receptor–blocking agents in pregnancy. Nonetheless, given the adverse outcomes associated with use of ACE inhibitors in pregnancy and the mechanism of action of angiotensin receptor–blocking agents, the use of these drugs is not recommended in pregnancy.42
Beta-adrenergic Receptor Antagonists
Beta-adrenergic receptor antagonists are often used for treatment of coronary artery disease, myocardial infarction, hypertension, many arrhythmias, and a wide spectrum of cardiomyopathies. There is no evidence that beta-adrenergic receptor antagonists are teratogenic. Prolonged and high-dose use of these drugs has been associated with fetal growth restriction (also known as intrauterine growth restriction); however, the overall risk is likely small.42 Neonatal bradycardia, hypotension, and hyperglycemia are rarely encountered.
Calcium Entry−Blocking Agents
First-trimester maternal use of verapamil and diltiazem is likely not teratogenic. Both drugs appear to be safe and effective treatments for cardiac arrhythmias in the second and third trimesters.42 The use of amlodipine, a dihydropyridine calcium entry−blocking agent, appears safe during pregnancy.42
Other Drugs
Hydralazine is used in the treatment of cardiomyopathy and is also an excellent antihypertensive agent. It has a long track record of safe use during pregnancy.42
The use of nitrates for treatment of angina during pregnancy appears safe. Nitrates likely can be safely used long term in pregnant women with cardiomyopathy.42
Digoxin is quite useful for the treatment of various cardiomyopathies and some arrhythmias. Digoxin freely crosses the placenta, but its use has not been associated with congenital abnormalities or untoward fetal effects.42 Digoxin’s pharmacokinetics are altered during pregnancy, and attention to blood levels is recommended.
Eptifibatide, tirofiban, and abciximab are potent intravenous platelet aggregation inhibitors (IIB/IIIA receptor inhibitors) used to inhibit platelet aggregation during percutaneous coronary intervention. Both the American Society of Regional Anesthesia and Pain Medicine (ASRA) and the European Society of Anaesthesiology (ESA) guidelines strongly advise avoidance of neuraxial anesthesia until platelet function has recovered after administration of these agents.43,44
Statins interrupt cholesterol synthesis and result in a lowering of plasma cholesterol levels. The widespread use of statins (which are associated with exceptionally robust cardiovascular outcome data) has transformed the treatment of coronary artery disease. Nonetheless, given the critical importance of cholesterol synthesis in the normal development of the embryo and placenta, and thus their potential for teratogenicity, statins are contraindicated in pregnancy (pregnancy category X).42
Thiazide diuretics do not appear to be teratogenic. Long-term use may result in a reduction in uteroplacental perfusion, which may be associated with fetal growth restriction and oligohydramnios.42 Neonatal hypoglycemia and thrombocytopenia have been reported.
Loop diuretics are likely not teratogenic.42 Similar to thiazide diuretics, fetal growth restriction may be associated with long-term use during pregnancy. Spironolactone is an aldosterone antagonist frequently used in the treatment of patients with congestive heart failure and cardiomyopathy, but because of its antiandrogenic potential its use during pregnancy is not recommended.42
Aortic Diseases and Aortic Dissection
The cardiovascular changes of pregnancy may to lead to increased arterial wall tension and intimal shear forces. However, the full impact of pregnancy on changes in aortic wall structure is not fully understood. Estrogen-induced changes in collagen deposition, as well as circulating elastases and relaxin, may weaken the aortic media and thus predispose the aorta to dissection during pregnancy. Approximately half of aortic dissections and ruptures in women younger than 40 years of age are associated with pregnancy.45 Dissection of the ascending aorta (Stanford type A or DeBakey type I or II) is a surgical emergency, whereas dissection of the descending aorta (Stanford type B or DeBakey type III) is predominantly treated medically.
Conditions that predispose women to aortic dissection during pregnancy include Marfan syndrome, Ehlers-Danlos syndrome, bicuspid aortic valve,46 Turner syndrome, and non–Marfan syndrome−associated familial thoracic aneurysms.47 Aortic dissection has been associated with preeclampsia and chronic hypertension in pregnancy.48 Most aortic dissections that occur during pregnancy are type A (ascending aorta); the average aortic diameter at the time of dissection is 4.8 cm.49 The majority of dissections occur in the third trimester of pregnancy, but aortic dissections may also occur at the time of delivery or in the early postpartum period. It has been hypothesized that contraction of the uterus causes outflow resistance, thus predisposing to aortic dissection after delivery.
Marfan Syndrome
Marfan syndrome is an autosomal-dominant connective tissue disorder. The penetrance is high, but the expression is variable. Marfan syndrome is caused by a mutation in the FBN1 gene encoding fibrillin-1, a glycoprotein. Sporadic mutations are seen in approximately 25% of patients without a family history of this syndrome. Aortic dilation and aortic dissection contribute significantly to cardiovascular complications in these patients. In addition to aortic disease, affected patients often have valvular disease (e.g., aortic regurgitation, mitral regurgitation, mitral valve prolapse). Aortic dissection has been observed during pregnancy and in the peripartum period.50,51 Most patients have type A aortic dissection; type B aortic dissection and abdominal aortic aneurysm are less commonly seen.
Based on observational studies, current guidelines recommend that women with Marfan syndrome who are planning pregnancy undergo replacement of the ascending aorta and the aortic root if the diameter is greater than 4.0 cm (class IIa, level of evidence C).47,52 The aortic root dilation rate increases during pregnancy and does not return to baseline after delivery. Subsequent pregnancies further increase the aortic dilation rate.53
Aortic Disease Associated with a Bicuspid Aortic Valve
Bicuspid aortic valve has been associated with dissection during pregnancy.46 Affected women are slightly younger, and the dissection occurs earlier in pregnancy than in patients with Marfan syndrome.49 Because the disease is familial, first-degree relatives of patients with a bicuspid aortic valve should be screened for this disease.54
Ehlers-Danlos Syndrome
Ehlers-Danlos syndrome is an inherited connective tissue disorder. Ehlers-Danlos syndrome type IV has been associated with severe complications and maternal mortality. Rupture of the bowel, aorta, vena cava, and uterus may occur.47 Pregnancy outcomes in patients with Ehlers-Danlos syndrome types I, II, and III are generally favorable, although these women have a higher incidence of pelvic instability, preterm delivery, perineal lacerations, and postpartum hemorrhage than the general population.55
Turner Syndrome
Turner syndrome is caused by complete or partial absence of an X chromosome. In addition to short stature, webbed neck, and characteristic facial features, Turner syndrome is associated with aortic coarctation and hypertension. During pregnancy, the syndrome is associated with aortic dissection.56,57 Moreover, approximately 30% of patients with Turner syndrome have a bicuspid aortic valve, which is also a risk factor for aortic dissection. Preconception echocardiographic evaluation of patients with Turner syndrome is recommended.47,58 Given the frequent aortic abnormalities in these patients, preconception MRI is also recommended. Cesarean delivery is frequently required in these patients because of cephalopelvic disproportion.58
Management
Given the inherent risk for aortic dissection, parturients with aortic disease should deliver in a center that has immediate access to a cardiothoracic surgeon with expertise in aortic endovascular repair techniques.59 Current guidelines are based on expert opinion, case reports, and current standard of care (level C evidence) (Box 42-5; Table 42-2). The 2010 American College of Cardiology (ACC) Foundation/American Heart Association (AHA) guidelines recommend the following for pregnant women with chronic aortic dilation: (1) strict blood pressure control; (2) monthly or bimonthly echocardiographic measurement of aortic dimension; (3) cesarean delivery in women with significant aortic enlargement, dissection, or severe aortic valve regurgitation; and (4) prophylactic surgery in the setting of progressive aortic dilation and/or advancing aortic valve regurgitation.47
TABLE 42-2
Anesthetic Considerations for Specific Cardiovascular Pathologic Processes


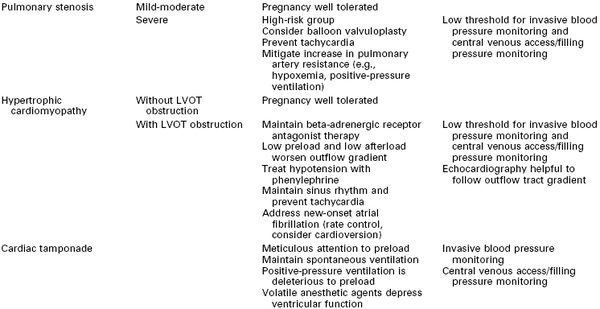
All patients with cardiovascular disease should labor in the lateral position to decrease aortocaval compression. Central venous pressure monitoring should be performed above the diaphragm.
ASD, atrial septal defect; LV, left ventricle; LVOT, left ventricular outflow tract; PAP, pulmonary artery pressure; RV, right ventricle; SVR, systemic vascular resistance; VSD, ventricular septal defect.
All patients with Marfan syndrome should receive beta-adrenergic receptor antagonist therapy throughout pregnancy to decrease the rate of aortic dilation.47,60 The risk for major aortic complications during pregnancy appears low if the aortic root diameter is less than 4.0 cm.47 In the event of a type A aortic dissection in the first or second trimester, surgical repair should be performed with the knowledge of fetal risk during hypothermic circulatory arrest. If the dissection occurs in the third trimester and the fetus is deemed viable, an urgent cesarean delivery followed by aortic surgery may be performed.
Both neuraxial61,62 and general anesthesia63 may be safely performed in these patients, with emphasis on meticulous blood pressure stability and control. Invasive blood pressure monitoring is recommended to facilitate tight hemodynamic control. Dural ectasia and scoliosis may complicate neuraxial anesthetic techniques in parturients with Marfan syndrome.64 The increase in lumbar cerebrospinal fluid volume associated with dural ectasia may cause unpredictable and inadequate spread of intrathecal local anesthetic solutions; thus, it may be prudent to obtain lumbar spine MRI before planned pregnancy.
Congenital Heart Disease
Atrial Septal Defect
Patients with an atrial septal defect may remain asymptomatic until the fourth decade of life. Not infrequently, women with an atrial septal defect may become symptomatic during pregnancy. The most common defect is the secundum-type atrial septal defect (80%), whereas the primum, sinus venosus, and coronary sinus types of atrial septal defect are less common. Right ventricular overload leads to pulmonary hypertension and Eisenmenger syndrome in less than 5% of patients with an atrial septal defect. In women with both an atrial septal defect and Eisenmenger syndrome, pregnancy carries a significant risk for both maternal and fetal mortality, and is not recommended.54,65 In the absence of pulmonary hypertension, pregnancy is overwhelmingly well tolerated in women with an atrial septal defect.
Cardiac complications are similar in women with unrepaired and repaired atrial septal defects. Preeclampsia, fetal demise, and small-for-gestational-age infants are more common in pregnant women with an unrepaired atrial septal defect than in the general obstetric population.66 Pregnant women with an atrial septal defect are more likely to develop supraventricular and ventricular arrhythmias than women who are not pregnant.67
The risk for paradoxical embolism is increased in patients with an unrepaired atrial septal defect. It is critically important to ensure that intravenous catheters are de-aired.54 Transesophageal echocardiography demonstrates the presence of microbubbles in the right-sided cardiac chambers within 15 seconds of the epidural injection of air or fluid.68 Therefore, it seems prudent to avoid using the loss-of-resistance-to-air technique to identify the epidural space (see Table 42-2). Both neuraxial and general anesthesia are appropriate for patients with a repaired or unrepaired atrial septal defect.
Ventricular Septal Defect
There are four types of ventricular septal defects; the most common type is a perimembranous ventricular septal defect. Pregnancy is well tolerated in women with a repaired ventricular septal defect or a small ventricular septal defect in the absence of pulmonary hypertension. An unrepaired ventricular septal defect with Eisenmenger syndrome is associated with a high risk for maternal cardiac complications (see later discussion). Pregnancy is not recommended in patients with a ventricular septal defect and Eisenmenger syndrome.54,65 Preeclampsia is encountered more frequently in women with an unrepaired ventricular septal defect.69 Echocardiography allows assessment of right-sided pressures and shunt fraction.
Patent Ductus Arteriosus
Pregnancy is well tolerated in patients with patent ductus arteriosus, and complications are rare.70 A left-to-right shunt may cause pulmonary hypertension. Pregnancy is not recommended in women with patent ductus arteriosus with Eisenmenger syndrome.54,65
Coarctation of the Aorta
Women with repaired coarctation of the aorta tolerate pregnancy well. Systemic arterial hypertension is often observed during labor. The coarctation may be associated with a bicuspid aortic valve in more than half the patients.71 Prepregnancy evaluation of the coarctation, including the residual degree of obstruction72 and associated anomalies (e.g., bicuspid aortic valve), is recommended.54
Vaginal delivery with neuraxial anesthesia is the preferred mode of delivery; cesarean delivery is reserved for obstetric indications.73–75 Epidural anesthesia has been successfully administered in a patient with an uncorrected coarctation.76 One report described the use of remifentanil to control blood pressure during administration of general anesthesia for cesarean delivery.77
Fontan Repair
The Fontan repair is a surgical procedure that establishes blood flow from the venous system to the pulmonary artery by bypassing the right ventricle (Figure 42-1). It can be performed for valvular defects such as tricuspid or pulmonic atresia, or other anomalies with a single ventricle. Because there is no functional right ventricle, blood flow from the periphery to the lungs occurs at very low pressure gradients. Owing to the presence of surgical scar tissue in the atrium, patients with a Fontan repair are prone to supraventricular and, less commonly, ventricular arrhythmias.78,79 The Fontan repair is associated with the highest prevalence of arrhythmias during pregnancy of any congenital heart condition.39 Deterioration in the NYHA functional status during pregnancy can occur.79
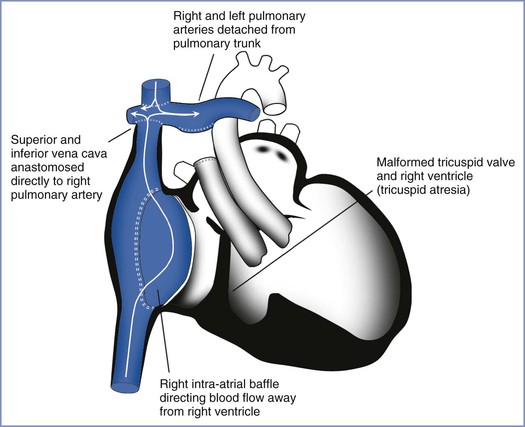
FIGURE 42-1 Schematic depiction of a Fontan repair. There is no functional right ventricle. The white lines with arrows represent the pathway of venous blood returning to the heart. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Administration of neuraxial analgesia/anesthesia for labor and vaginal delivery80 and emergency cesarean delivery81 has been described.82 Administration of neuraxial anesthesia, with meticulous attention to maintenance of normal intravascular preload, appears to be the preferred anesthetic technique for cesarean delivery. Use of neuraxial anesthesia avoids the adverse effects of myocardial depression and positive-pressure ventilation on the Fontan circulation, which lacks a functioning right ventricle (see Table 42-2).80,81
Transposition of the Great Arteries
Transposition of the great arteries comprises two distinct groups: complete transposition of the great arteries (d-transposition) and congenitally corrected transposition of the great arteries (l-transposition). In both conditions, the aorta originates from the right ventricle and the pulmonary artery originates from the left ventricle. Complete transposition of the great arteries manifests as neonatal cyanosis.
Pregnant women born with d-transposition of the great arteries have undergone surgical correction—traditionally an atrial switch procedure (Senning or Mustard) or the more contemporary arterial switch procedure (Jatene or Rastelli). In the atrial switch procedure, the right ventricle functions as the systemic ventricle. Atrial arrhythmias, right ventricular (systemic ventricle) dysfunction, tricuspid regurgitation (systemic atrioventricular valve), atrial baffle obstruction or leaks, and pulmonary hypertension are some of the long-term complications of the traditional atrial switch surgical repair of d-transposition of the great arteries.65 The advantage of the Jatene and Rastelli procedures is that the left ventricle functions as the systemic ventricle. However, myocardial ischemia may occur after arterial switch operations, because the coronary arteries are reimplanted during these procedures.83
All women with repaired d-transposition of the great arteries require detailed echocardiography and, ideally, CMR imaging before planned pregnancy.54,65 Owing to the propensity for arrhythmias in these patients,39,84 continuous telemetry monitoring during labor and delivery seems appropriate. Women who have undergone the Mustard operation tolerate pregnancy well85,86; however, there is a risk for right ventricular dysfunction84,87 that may be irreversible.88 The physiologic changes of pregnancy and/or the natural progression of disease result in an increased right ventricular volume in pregnant women who have undergone a Mustard procedure.85,88 There are limited data about pregnancy in patients who have undergone an arterial switch operation. Overall, pregnancy outcomes after arterial switch operations appear favorable.89
Patients with congenitally corrected transposition (l-transposition) of the great arteries tolerate pregnancy well.90–92 Thorough echocardiographic evaluation before and throughout pregnancy is advisable.54,65,83
Both neuraxial and general anesthesia appear to be reasonable options for parturients with surgically corrected and congenitally corrected transposition of the great arteries (see Table 42-2). Successful cesarean delivery with general anesthesia has been reported in a parturient with d-transposition of the great arteries corrected with a Jatene procedure.93
Ebstein’s Anomaly
In Ebstein’s anomaly the tricuspid valve is displaced toward the apex of the right ventricle, which results in severe tricuspid regurgitation and right atrial enlargement (Figures 42-2 and 42-3). It is commonly associated with an atrial septal defect and preexcitation syndromes. Accessory pathways result in arrhythmias in approximately 30% of patients; the most commonly observed arrhythmias include atrial tachycardia, atrial flutter, and atrial fibrillation. Pregnancy appears to be well tolerated in patients with Ebstein’s anomaly, especially in women with preserved ventricular function (see Table 42-2).65,94,95 Patients with a concomitant atrial septal defect and cyanosis have an increased risk for fetal loss and low infant birth weight.54
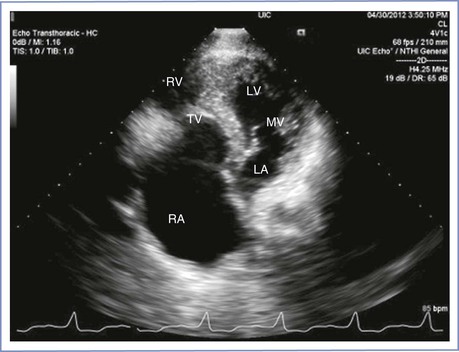
FIGURE 42-2 Echocardiographic image of Ebstein’s anomaly. The right atrium is markedly enlarged. RA, right atrium; RV, right ventricle; TV, tricuspid valve; LA, left atrium; MV, mitral valve; LV, left ventricle. (Courtesy Dr. Mayank Kansal, University of Illinois at Chicago, Chicago, IL.)
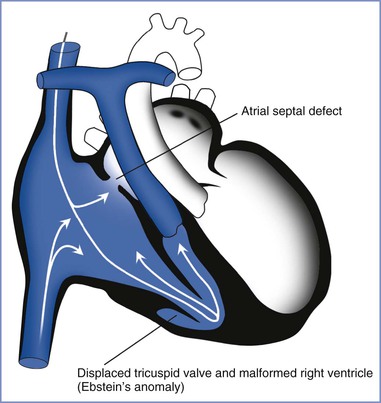
FIGURE 42-3 Schematic depiction of Ebstein’s anomaly and atrial septal defect. An atrial septal defect is present in more than one third of patients with Ebstein’s anomaly. The white lines with arrows represent the pathway of venous blood returning to the heart. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Tetralogy of Fallot
Unrepaired tetralogy of Fallot consists of a ventricular septal defect, an aorta that overrides the ventricular septal defect, and right ventricular outflow tract obstruction (infundibular, valvular, or both), with resulting right ventricular hypertrophy.54,65 Most women born with tetralogy of Fallot in the United States now present in pregnancy with surgically repaired tetralogy of Fallot. Unrepaired tetralogy of Fallot is rarely seen in developed countries; pregnancy is associated with significant risk in patients with unrepaired defects and is not recommended.54,65
During surgical repair the ventricular septal defect is closed and the right ventricular outflow obstruction is repaired. After surgery, important considerations include residual pulmonic valve insufficiency and resulting right ventricular dilation and dysfunction. The preanesthesia evaluation of these patients should include detailed echocardiographic evaluation of cardiac structure and function. Preferably, preconception CMR imaging should be performed because it provides superior imaging of the right-sided chambers. Patients with repaired tetralogy of Fallot are at risk for atrial and ventricular arrhythmias. Sudden cardiac death has been observed late after repair.96
Women with repaired tetralogy of Fallot and well-compensated hemodynamic function tolerate pregnancy well (see Table 42-2).97–99 However, the presence of pulmonary hypertension, right ventricular dysfunction, right ventricular dilation, and/or pulmonic regurgitation predisposes these patients to adverse peripartum complications such as arrhythmias and right-sided heart failure.65,99,100
The safe management of neuraxial analgesia/anesthesia for labor and vaginal or cesarean delivery has been described in parturients with repaired tetralogy of Fallot and a wide range of residual pathologic processes.101 In patients with unrepaired tetralogy of Fallot or tetralogy of Fallot with residual pathology, the anesthesiologist should avoid a decrease in SVR, which worsens the severity of the right-to-left shunt. It is also important to maintain adequate intravascular volume and venous return. In the presence of right ventricular compromise, high filling pressures are needed to enhance right ventricular performance and ensure adequate pulmonary blood flow. Administration of neuraxial analgesia during early labor (low-dose local anesthetic-opioid epidural analgesia or opioid-only intrathecal analgesia) may attenuate the surge of catecholamines and subsequent hemodynamic instability. It may be advisable to choose a titratable neuraxial technique for cesarean delivery (epidural or low-dose sequential spinal-epidural anesthesia [see Chapter 26]) to avoid the abrupt decrease in SVR associated with single-shot spinal anesthesia.
Pulmonary Hypertension
Box 42-6 outlines the current classification of pulmonary hypertension. Group 1 (pulmonary arterial hypertension) includes idiopathic pulmonary arterial hypertension and pulmonary hypertension due to congenital heart disease.102 Other clinical conditions associated with group 1 pulmonary hypertension include connective tissue disease, human immunodeficiency virus (HIV) infection, portal hypertension, drugs/toxins (e.g., anorectics, methamphetamine, cocaine), and hemoglobinopathies (e.g., sickle cell disease).102–104
All of these conditions are associated with exceptionally high maternal mortality. Older studies reported maternal mortality rates as high as 56%105; however, more contemporary studies have demonstrated some improvement in maternal mortality, with rates ranging from 17% to 33%.106 Nonetheless, pregnancy should be discouraged in women with pulmonary hypertension.
Normal mean pulmonary artery pressure (PAP) at rest is 14 ± 3 mm Hg (upper limit of normal = 20 mm Hg). Pulmonary arterial hypertension is defined as a mean PAP greater than 25 mm Hg with a normal pulmonary artery occlusion pressure (PAOP) (≤ 15 mm Hg) and pulmonary vascular resistance greater than 3 Wood* units.103,104 In contrast, in “postcapillary” pulmonary hypertension due to left-sided heart failure (group 2), PAOP is elevated (> 15 mm Hg). Increased pulmonary vascular resistance results in right ventricular overload, which is followed by right ventricular hypertrophy. Right ventricular hypertrophy progresses to dilation of the right-sided chambers, eventually leading to right ventricular failure and death.104
A loud pulmonic heart sound (P2) can be heard on physical examination. The second heart sound is widely split owing to delayed closure of the pulmonic valve resulting from high right-sided pressures (pulmonary valve closes after the aortic valve). A right-sided holosystolic murmur of tricuspid regurgitation is frequently appreciated. Palpation of the precordium demonstrates the classic right ventricular heave. The most commonly encountered ECG findings in patients with pulmonary hypertension are right-axis deviation and right ventricular hypertrophy.
Echocardiography helps establish the diagnosis and prognosis of pulmonary hypertension because it demonstrates the degree of right ventricular hypertrophy and allows estimation of pulmonary artery pressure by assessing the velocity of the tricuspid regurgitant jet (Figure 42-4). Echocardiography also allows assessment of right ventricular function.
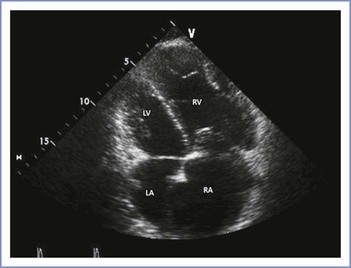
FIGURE 42-4 Echocardiographic image of pulmonary hypertension with severe right-chamber enlargement. The RV basal measurement is 55 mm (upper limit of normal 42 mm); the RA minor axis dimension is 70 mm (upper limit of normal 44 mm). RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle. (Courtesy Dr. Mayank Kansal, University of Illinois at Chicago, Chicago, IL.)
Echocardiographic assessment of right-sided pressures may be inaccurate; therefore, invasive right-sided—and frequently left-sided—heart catheterization is required for the definitive diagnosis of pulmonary arterial hypertension. Right-sided heart catheterization allows pressure measurements, thermodilution and Fick cardiac output determination, and vasoreactivity testing. Vasoreactivity testing is usually performed with inhaled nitric oxide or intravenous infusion of sodium nitroprusside, epoprostenol, or adenosine in the cardiac catheterization laboratory. Patients who achieve a decrease in mean PAP of 10 mm Hg or more and achieve a mean PAP of 40 mm Hg or less, without a decrease in cardiac output, are “positive acute responders.”103,104 Patients with a positive response have a better prognosis and may respond to oral therapy with a calcium entry–blocking agent.103
Eisenmenger Syndrome
In patients with an anatomic shunt between the systemic and pulmonary circulations at the atrial, ventricular, or aortopulmonary artery level, a left-to-right shunt initially causes increased pulmonary blood flow. Over time, pulmonary vascular resistance increases and pulmonary hypertension develops. The development of pulmonary hypertension results, at least in part, from endothelial dysfunction and vascular remodeling of the pulmonary vascular bed. This increase in pulmonary vascular resistance causes reversal of the shunt (from left-to-right to right-to-left), which results in hypoxemia and cyanosis. This anatomic and physiologic scenario is referred to as Eisenmenger syndrome.
Maternal mortality in women with Eisenmenger syndrome is exceptionally high (i.e., 30% to 50%).105,106 The cardiovascular physiologic changes of pregnancy present a significant hemodynamic challenge for women with pulmonary hypertension and may lead to development of right ventricular failure. The peripartum period, with its rapid fluid shifts and increased oxygen demand, is particularly challenging. Death usually occurs peripartum or postpartum. In a systematic review of case reports of pulmonary hypertension associated with congenital heart disease published between 1997 and 2007 (n = 29),106 all eight maternal deaths occurred postpartum (range, 0 to 24 days after delivery).
Women with Eisenmenger syndrome often cannot respond to the increased oxygen demands of pregnancy. The normal pregnancy-related decrease in pulmonary vascular resistance does not occur because pulmonary vascular resistance is fixed. In addition, the normal pregnancy-associated decrease in SVR tends to exacerbate the severity of the right-to-left shunt. These changes, together with the normal pregnancy-associated decrease in functional residual capacity, predispose women with Eisenmenger syndrome to hypoxemia. Maternal hypoxemia leads to a high incidence of fetal growth restriction and fetal demise. With contemporary drug therapy, cardiac imaging, and collaborative care, successful pregnancy has been described in patients with Eisenmenger syndrome.106
Pulmonary arterial hypertension due to congenital heart disease can be caused by a number of unrepaired congenital heart defects with a left-to-right shunt. Eisenmenger syndrome is associated with ventricular septal defect, atrial septal defect, patent ductus arteriosus, and atrioventricular septal defect (also referred to as endocardial cushion defect). More rarely encountered congenital heart defects (e.g., partial or total anomalous pulmonary venous return, transposition of the great arteries) may also lead to pulmonary arterial hypertension and Eisenmenger syndrome.54
Medical and Obstetric Management
Pregnant women with pulmonary hypertension should receive multidisciplinary care in a referral center. Diuretics are frequently needed to manage volume overload in patients with pulmonary arterial hypertension. Diuretics may be particularly helpful in the immediate postpartum period, when uterine contraction and autotransfusion cause an increase in ventricular preload. Dobutamine infusion may help improve right ventricular function. It is unclear whether all of these patients should receive thromboprophylaxis. Both hemorrhage and thromboembolism are causes of maternal mortality.106
Therapy for pulmonary arterial hypertension includes general supportive measures, assessment of vasoreactivity, and administration of vasoactive drugs.104 Inhaled nitric oxide selectively dilates the pulmonary vasculature. Case reports have described the successful use of nitric oxide for vaginal and cesarean deliveries in parturients with pulmonary arterial hypertension.107–109 Epoprostenol, treprostinil, and iloprost are prostacyclins used in the treatment of pulmonary hypertension. Successful pregnancy has been described in patients with pulmonary arterial hypertension treated with epoprostenol.110–113 Similarly, sildenafil, a phosphodiesterase type-5 inhibitor, has been successfully used in pregnant women with pulmonary arterial hypertension.114,115 Endothelin receptor antagonists (e.g., bosentan, ambrisentan) are likely teratogenic, and therefore their use is contraindicated during pregnancy.
The optimal mode of delivery in patients with pulmonary hypertension is unknown. In a systematic review that included reports from 1978 to 1996,105 operative delivery was an independent risk factor for maternal mortality. In contrast, the mode of delivery was not identified as a risk factor for maternal death in a systematic review that included more recent cases.106 Cesarean delivery is associated with larger changes in intravascular volume, more bleeding complications and blood loss, and a greater risk for thromboembolism; therefore, it seems reasonable to reserve cesarean delivery for obstetric indications.
Anesthetic Management
The primary goals of anesthetic management are (1) maintenance of adequate SVR; (2) maintenance of intravascular volume and venous return; (3) avoidance of aortocaval compression; (4) prevention of pain, hypoxemia, hypercarbia, and acidosis, which may increase pulmonary vascular resistance; and (5) avoidance of myocardial depression during general anesthesia (see Table 42-2).
Current evidence on choice of anesthetic technique for patients with pulmonary arterial hypertension is based on case reports and series from high-volume referral centers. The use of both general113,116 and epidural anesthesia117,118 has been reported.119 Neuraxial (epidural and combined spinal-epidural) anesthesia with use of pulmonary vasodilators in highly specialized centers appears to be associated with favorable overall outcomes.120,121 In published case reports, cautious administration of epidural anesthesia did not affect the shunt flow in parturients with Eisenmenger syndrome. Slowly titrated epidural or combined spinal-epidural anesthesia eliminates the undesirable effects of myocardial depression and positive-pressure ventilation (with its associated decrease in preload) associated with general anesthesia.
Intravascular volume assessment in patients with pulmonary arterial hypertension is of utmost importance. It is likely best achieved with central venous pressure monitoring; pulmonary artery catheterization without the use of fluoroscopy is technically challenging owing to the frequent presence of tricuspid regurgitation and right-sided chamber enlargement in these patients. Pulmonary artery catheterization has not been shown to improve outcome. Both transthoracic and transesophageal echocardiography are very helpful, and invasive blood pressure monitoring is indispensable. Because patients with pulmonary arterial hypertension frequently require systemic anticoagulation, the choice of anesthesia in these patients is best determined by a multidisciplinary team.
Because of its ease of administration, nitric oxide can be readily administered in the urgent setting. Nitric oxide has been administered during epidural anesthesia for emergency cesarean delivery using a noninvasive ventilation device.122 Successful cesarean delivery with inhaled iloprost and slowly titrated epidural anesthesia has been reported.123,124
Given the high rate of postpartum mortality, patients should be monitored in an intensive care setting for a number of days postpartum.
Infective Endocarditis
Endocarditis during pregnancy is rare. It is most frequently associated with intravenous drug use or preexisting structural heart and valve abnormalities (e.g., rheumatic valvular disease, congenital heart disease). Maternal and fetal mortality rates are both high (range, 15% to 25%, respectively).125
Antibiotic Prophylaxis
Currently, the ACC, the AHA, and the American College of Obstetricians and Gynecologists (ACOG)126 do not recommend antibiotic prophylaxis during vaginal or cesarean delivery in the absence of structural heart disease.54,127,128 In patients with congenital heart disease and the highest risk for adverse outcomes, it is reasonable to administer antibiotic prophylaxis against infective endocarditis before vaginal delivery at the time of membrane rupture.54 High-risk patients include those with one or more of the following: (1) a prosthetic cardiac valve or a valve repaired with prosthetic material, (2) unrepaired or palliated cyanotic congenital heart disease, and (3) surgically constructed palliative shunts and conduits (Box 42-7). In patients with one of these high-risk conditions who have an established infection (e.g., chorioamnionitis), the underlying infection should be treated. Additional antibiotics specific to endocarditis prophylaxis are not recommended.126 Mitral valve prolapse is not an indication for antibiotic prophylaxis. It is important to note that the level of evidence for these recommendations is C (consensus opinion of experts, case studies, standard of care). In young women of childbearing age, there is no difference in the rate of prosthetic valve endocarditis with mechanical or bioprosthetic valves.
Diagnosis and Treatment
The diagnosis of endocarditis rests on a very high index of suspicion, physical examination, laboratory findings, and cardiac imaging. The modified Duke criteria are the most widely accepted criteria for the diagnosis of endocarditis (Box 42-8). Patients with endocarditis may have negative blood cultures. Therefore, absence of bacterial growth in blood cultures does not automatically rule out endocarditis.
In addition to systemic antibiotic therapy, valve replacement may be required in pregnant women with endocarditis. Alternatively, successful treatment with aggressive antibiotic therapy has been described.
Neuraxial Anesthesia in Patients with Systemic Infection
The safety of neuraxial anesthesia in patients with systemic infection has been debated for many years (see Chapter 37).129 Neurologic complications after spinal or epidural anesthesia are rare in large observational series130,131; however, there is general agreement that patients with untreated systemic infection should not receive neuraxial anesthesia.
Published data and clinical experience suggest that spinal anesthesia may be safely administered in patients who have received antibiotic treatment and are responding to treatment at the time of dural puncture. Similarly, it is likely that epidural anesthesia may be safely administered to patients with treated systemic infection.129 In patients with endocarditis, bacteremia may seed the epidural space and cause epidural abscess in the absence of neuraxial anesthesia. Similarly, meningitis is a recognized neurologic complication of endocarditis. Therefore, it may be difficult to determine whether the neuraxial procedure contributed to the development of the infection if meningitis or epidural abscess should develop in a patient with endocarditis receiving neuraxial anesthesia.
Implantable Cardiac Devices
Permanent and Temporary Pacemakers
Permanent pacemakers implanted before pregnancy are occasionally encountered. Pregnancy and labor and delivery are generally well tolerated in these patients.132,133
Advanced second-degree (two or more nonconducted P waves) or third-degree atrioventricular block is rare in pregnant women and is most commonly seen in patients with congenital heart disease. Recommendations are inconsistent as to whether temporary pacing is required for labor and delivery.134–138 Some of the principal indications for pacemaker placement (e.g., symptomatic bradycardia, periods of asystole greater than 3 seconds, escape rhythms below the atrioventricular node with rates < 40 beats per minute) also appear to be appropriate indications for parturients; the decision to electively place either a temporary or permanent device should be made by a multidisciplinary team. Patients who develop hemodynamic instability due to bradycardia should receive a temporary venous pacemaker. Transcutaneous pacing is an attractive alternative, but it is uncomfortable for prolonged use. Because the majority of patients requiring temporary pacing have underlying congenital heart disease, it is critically important to understand the cardiac anatomy before placing the venous pacemaker to minimize complications such as perforation or valve injury.
Implantable Cardioverter-Defibrillators
Pacemaker and implantable cardioverter-defibrillator (ICD) implantation during pregnancy may be performed with echocardiographic guidance, thus reducing fetal radiation exposure. A wearable automatic defibrillator is an attractive option for pregnant women, because it may allow ICD implantation to be postponed until after delivery. In the future, ICDs that are placed entirely within a subcutaneous pocket may be offered to pregnant women and eliminate the need for fetal radiation exposure.
Women with previously placed ICDs usually tolerate pregnancy and delivery well. Pregnancy does not increase the risk for ICD-related complications, and it does not increase the number of ICD discharges. Rather, the severity of underlying structural heart disease determines the overall complication rate.139 Case reports suggest that pregnant women tolerate ICD shocks as well as nonpregnant women.139,140
Peripartum Management
Pacemakers and ICDs should be interrogated before or during pregnancy and before and after labor and vaginal or cesarean delivery (Figure 42-5). The type of device, manufacturer, and model should be documented. The indication for the device, the patient’s underlying rhythm, and whether the patient is pacemaker dependent should be identified. Given the complexity of contemporary pacing and ICD devices, it is imperative to perform this evaluation in collaboration with an electrophysiologist or a cardiologist familiar with device management. It is helpful to obtain a 12-lead ECG before and after delivery and before and after any change in device programming.
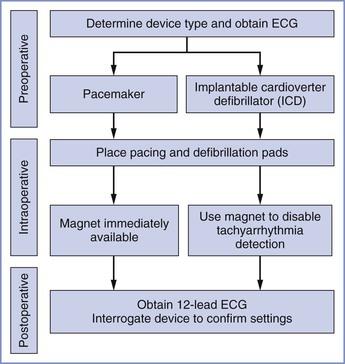
FIGURE 42-5 A suggested perioperative approach to the management of implantable cardiac devices during cesarean delivery. ECG, electrocardiogram.
The response of the device to magnet placement and removal should be known. It is important to recognize that different devices may respond quite differently to magnet placement. Most pacemakers will pace in an asynchronous mode after application of an external magnet. For most ICDs, the magnet will disable tachycardia detection (i.e., the device will not deliver a shock for ventricular tachycardia or ventricular fibrillation), but the magnet will not alter the pacing mode and rate settings. Magnets do not change bradycardia pacing settings for ICDs.
Because of the variability of pacemaker design from different manufacturers, and the highly sophisticated programming settings, peripartum device management should be individualized. Management is best planned before labor and delivery using a collaborative approach. For operative obstetric procedures, electromagnetic interference is unlikely given the distance of the site of surgery from the implanted device. Because pacemaker electrical activity can occur without resulting ventricular contraction, monitoring patients with an implantable cardiac device includes continuous ECG together with either plethysmography (pulse oximetry) or invasive blood pressure monitoring to ascertain the presence of a pulse.
For all procedures in patients with a pacemaker, a magnet should be immediately available. Immediate availability of external pacing and defibrillation capability is mandatory for all patients regardless of the type of device and urgency of the surgery. For most obstetric surgeries, it is recommended that devices not be reprogrammed and that ICDs not be deactivated by reprogramming. If deactivation of an ICD is necessary, the patient must remain in a monitored setting with external defibrillation pads placed on the patient until it is confirmed that the ICD has been reactivated. If the ICD is not deactivated by reprogramming, a magnet should be placed over the device intraoperatively to disable tachyarrhythmia detection. Removal of the magnet will restore previous ICD settings.
In patients with a pacemaker or an ICD, central intravenous catheter placement in the upper body needs to be performed with extreme caution so as not to damage or entangle device leads; this is particularly important in leads that were recently inserted (< 3 months earlier).
Adult Arrhythmias
The incidence of arrhythmias is increased during pregnancy in patients with and without structural heart disease. The mechanisms have been attributed to atrial16 and ventricular stretch40 due to increased intravascular volume as well as the increase in resting heart rate. Additionally, autonomic and hormonal changes of pregnancy have been proposed as putative mechanisms.
Palpitations are frequent during pregnancy, and Holter monitoring often reveals premature atrial and ventricular contractions. The frequency of ectopy decreases after delivery. Interestingly, there is no correlation between symptomatic palpitations and frequency of underlying arrhythmias.141 Therefore, no treatment is needed in asymptomatic patients with premature atrial contractions or premature ventricular contractions. Substances such as caffeine, alcohol, and cocaine should be discontinued, and treatment with a beta-adrenergic receptor antagonist may be considered in symptomatic patients.
Women who have been diagnosed with an arrhythmia before pregnancy frequently develop an exacerbation of arrhythmia during pregnancy. Recurrence of a preexisting arrhythmia is associated with adverse fetal events.142
Supraventricular Arrhythmias
Overall, supraventricular tachycardia (SVT) during pregnancy is rare, with an estimated 24 episodes/100,000 pregnancies.143 It is unclear whether pregnancy increases the risk for new-onset supraventricular tachycardia,144,145 although the first onset of paroxysmal supraventricular tachycardia during pregnancy is unusual. Symptoms of supraventricular tachycardia may be exacerbated during pregnancy.144
Premature atrial contractions are frequently encountered during pregnancy. Their frequency generally decreases in the postpartum period.141 Premature atrial contractions are overwhelmingly benign and rarely cause significant palpitations. Symptomatic patients can be treated with a low dose of a beta-adrenergic receptor antagonist.
Atrial fibrillation is encountered rarely during pregnancy. Rate and/or rhythm control along with prevention of thromboembolism are the mainstays of treatment of atrial fibrillation. The ventricular rate can be successfully controlled with digoxin, a beta-adrenergic receptor antagonist, or a nondihydropyridine calcium entry−blocking agent.146 Alternatively, quinidine, sotalol, flecainide, or amiodarone may be used to control rapid ventricular response. These drugs also allow for pharmacologic cardioversion of atrial fibrillation. Although restoration of sinus rhythm reduces the risk for thromboembolism and may provide considerable short-term hemodynamic advantages in certain patients, the impact of a rhythm-control treatment strategy on long-term outcome is unclear.
Because atrial fibrillation during pregnancy most frequently results from underlying structural heart disease (rheumatic mitral stenosis or congenital heart disease), systemic anticoagulation during pregnancy is recommended. There is extensive published experience with the use of unfractionated heparin and warfarin during pregnancy; limited data are available with low-molecular-weight heparin (LMWH).
Use of the CHADS2 score147 facilitates risk stratification for predicting stroke and thromboembolism associated with atrial fibrillation. This risk stratification provides guidance for choice of anticoagulation.146 Points are assigned for the presence of six risk factors: Congestive heart failure (1 point), Hypertension (1 point), Age 75 years or older (1 point), Diabetes mellitus (1 point), and prior Stroke/transient ischemic accident and thromboembolism (2 points). Patients at low risk for stroke generally receive aspirin, whereas those at high risk receive oral anticoagulation with warfarin or a newer oral anticoagulant (e.g., dabigatran, apixiban, rivaroxaban). There are no published reports of the use of these newer oral anticoagulants in pregnant women.
Atrial flutter is an organized macro-reentrant arrhythmia rarely seen during pregnancy. The atrial rate is usually 300 beats per minute; ventricular rate control may be difficult to achieve medically. Electric cardioversion is recommended for unstable patients, although pharmacologic cardioversion with ibutilide during pregnancy has been reported.148
Atrial tachycardia most frequently results from increased automaticity of atrial cells. Incessant atrial tachycardia has been successfully ablated during pregnancy.