Neurologic and Neuromuscular Disease
Angela M. Bader MD, MPH
Chapter Outline
Cutaneous Angiomatosis with Central Nervous System Abnormalities
The choice of anesthetic technique for pregnant women with neurologic disease requires knowledge of the pathophysiology of the disorder and an understanding of controversies involved in the diagnosis and management of the disease. If a patient’s neurologic condition deteriorates postpartum, the cause may be unclear and the anesthetic technique may be blamed unfairly. There are limited published data on specific neurologic and neuromuscular disorders in pregnant women. However, few of these disorders contraindicate the use of neuraxial anesthesia. In most cases, the obstetrician should obtain early antepartum consultation from an anesthesiologist. Early consultation allows accurate antepartum documentation of the extent and pattern of the neurologic deficit as well as discussion and formulation of the anesthetic plan with the patient, her obstetrician, and a neurologist or neurosurgeon.
Because patients with a wide variety of neurologic disorders will present for preoperative evaluation, the following thought process will assist the clinician with completing a proper evaluation and formulating an anesthetic plan.
What is the basic pathophysiology of the particular neurologic disorder? Neurologic disorders may be stable, progressive, or relapsing/recurrent. It is important to understand the common disease patterns. The potential for progression of the disease after delivery will depend on the pattern of progression and underlying pathophysiology and on the effect of pregnancy on disease progression.
What is the patient’s history and current findings after neurologic examination? A history should include the onset date and current course of the disorder. Symptoms related to neurologic issues should be documented (e.g., seizure type and frequency, deficits after cerebrovascular events, cognitive deficits). A basic physical examination should be conducted to document existing deficit patterns, including cognitive dysfunction (e.g., ability to understand and cooperate), deficits involving vision, hearing, speech, and swallowing; respiratory symptoms; and weakness and sensory deficits in the head and neck, trunk, and extremities. Motor and sensory deficits are classified as mild, moderate, or severe, with a description of the affected area. Special attention should be directed to limitations in ambulatory ability (e.g., bed-bound, wheelchair, walking with assistance) or positioning.
What are the current treatments and what testing results are available? Documentation of medical and nonmedical therapies is essential. For some disorders (e.g., myasthenia gravis), documentation of the timing of treatment is also critical. In most cases, specific laboratory testing will not influence management and outcome. However, pulmonary function testing should be considered in patients with neurologic disorders that result in significant respiratory compromise; the findings may assist the anesthesiologist in making decisions about anesthetic management.
What is the impact of the neurologic disorder on other organ systems (e.g., cardiac, respiratory, airway)? The patient’s neurologic disease may affect organ systems that are relevant to the anesthetic plan. For example, central core disease is associated with a risk for malignant hyperthermia. In addition, progressive neurologic disorders may significantly compromise the patient’s respiratory status, thereby increasing the risks associated with neuraxial and general anesthesia.
What are the potential impacts, risks, or benefits of particular anesthetic options based on the disease’s pathophysiology, symptoms, and treatment? Can treatment be initiated antepartum or before delivery that will improve outcome? For most rare neurologic disorders there is limited evidence on which to base decisions about anesthetic management. In these cases, the anesthesiologist should consider the disease’s basic pathophysiology and its possible direct and indirect interactions with specific anesthetic techniques. Encouraging the obstetrician to send these patients for early antepartum consultation will enable the anesthesiologist to obtain formal input from a neurologist or other consultant if necessary. A multidisciplinary discussion that includes the patient may be necessary to weigh the risks and benefits of specific obstetric and anesthesia plans.
In all cases, accurate documentation of the responses to the previous questions will greatly assist the team providing analgesic or anesthetic care for these patients. Some of the more common neurologic conditions are addressed in this chapter, and the existing literature is surveyed relative to the peripartum management of these patients. This knowledge allows the anesthesiologist an opportunity to formulate a safe and rational anesthetic plan as well as enable an appropriate discussion with the patient regarding the risks and benefits of particular anesthetic options.
Multiple Sclerosis
Multiple sclerosis is a major cause of neurologic disability in young adults. The prevalence of the disorder varies with the population. Recent data suggest that the prevalence of the disease is increasing, especially in females, and may be as high as 300 per 100,000 in some parts of North America.1 Both environmental and genetic factors appear to play a role in the incidence and prevalence of disease.
The disease is characterized by variable neurologic disabilities with two general patterns of presentation: (1) exacerbating remitting, which accounts for 85% of cases, in which attacks appear abruptly and resolve over several months, and (2) chronic progressive, in which continued deterioration occurs over time.2 The relapse rate varies significantly among patients, averaging approximately 0.4 attacks per year; this rate reflects the large proportion of patients with relapsing/remitting disease. The deficits tend to become more progressive and debilitating over time. Environmental factors (e.g., stress, infection, increased body temperature) may provoke a relapse. Most relapses reproduce previously experienced neurologic deficits, which can manifest as pyramidal, cerebellar, or brainstem symptoms.
The etiology remains unclear. There is a clinically significant heritable component, and alleles in the HLA locus have been identified as risk factors for multiple sclerosis.3 Pathologic findings include inflammation and loss of myelin in the central nervous system (CNS). It is possible that the disease results from a yet undetermined combination of genetic predisposition and exposure to specific environmental factors.
The more common symptoms include motor weakness, impaired vision, ataxia, bladder and bowel dysfunction, and emotional lability. Cerebrospinal fluid (CSF) immunoglobulin and lymphocyte concentrations are increased, and magnetic resonance imaging (MRI) studies demonstrate white matter plaques. Lesions may be documented by the demonstration of prolonged evoked potentials in areas of involvement.
There is no cure. Immunosuppressive therapies may hasten recovery from a relapse, but no evidence suggests that these agents influence the progressive course of the disease. Administration of interferon-beta may significantly reduce the relapse rate and retard disability; however, an increased risk for fetal loss and low birth weight (LBW) has been observed with the use of this therapy during the first trimester of pregnancy.4 In contrast, administration of intravenous immunoglobulin may reduce the risk for relapse and has no known adverse effects on pregnancy outcome.5 Acute relapses during pregnancy can be treated with intravenous corticosteroids, although their use may be associated with maternal glucose intolerance and neonatal adrenal suppression.6
Interaction with Pregnancy
Evidence regarding the effect of multiple sclerosis on pregnancy is conflicting. In one cohort study that compared 198 affected women with 1584 healthy women, the number of maternal complications was not higher in women with multiple sclerosis.7 However, infants delivered of women with multiple sclerosis appear to be at greater risk for meconium aspiration, even though the presence of moderate to heavy meconium is not significantly increased.7 This finding may reflect an intrauterine environment in patients with multiple sclerosis that is more susceptible to acute hypoxic events.7 A subsequent cohort study of 649 pregnancies in women with multiple sclerosis concluded that infants of these women were more likely to be small for gestational age; this outcome was also attributed to a suboptimal intrauterine environment.8 Moreover, this study found that mothers with multiple sclerosis were more likely to undergo induction of labor and operative delivery, possibly as a result of neuromuscular weakness and spasticity.
In a 2011 meta-analysis of reports of pregnant women with multiple sclerosis, the relapse rate was lower during pregnancy than before or after pregnancy.9 It is unclear whether the prevalence of cesarean deliveries, spontaneous abortions, preterm births, and LBW neonates is higher in women with multiple sclerosis than in healthy women, although the rates did not reach levels that would warrant great concern.
Data from prospective studies suggest that the rate of relapse increases during the first 3 months postpartum in comparison with the year before pregnancy.10 Relapses during this period were more likely in women who had higher relapse rates in the year before pregnancy or during pregnancy. Stress, exhaustion, infection, the loss of antenatal immunosuppression, and the postpartum decline in concentrations of reproductive hormones may account for the higher postpartum relapse rate. Treatment with immunologically active agents (e.g., interferon-beta) may result in a decreased postpartum relapse rate, but data are limited.10
Pregnancy does not negatively affect the long-term outcome of multiple sclerosis. Rather, at least one study has suggested that parturition may have a slightly favorable effect on long-term disease activity.11 Data are conflicting as to whether exclusive breast-feeding is associated with a lower risk for relapse than partial or no breast-feeding.12,13
Anesthetic Management
The anesthesiologist should assess the patient’s level of compromise, document the pattern of deficits, and give special attention to respiratory involvement. Historically, the optimal route of anesthesia in patients with multiple sclerosis has been controversial. Most anesthesia providers have considered general anesthesia to be safe, although published data are limited.14,15 Many anesthesia providers have been reluctant to administer neuraxial anesthesia because the effect of local anesthetic drugs on the course of the disease is unclear. Some anesthesiologists have expressed concern that neuraxial anesthesia may expose demyelinated areas of the spinal cord to potentially neurotoxic effects of local anesthetic agents. Several animal studies have investigated the histologic effects of local anesthetic agents on the normal spinal cord. In one study, subarachnoid injection of small doses of a local anesthetic agent produced no histologic changes in the spinal cord or meninges.16 Injection of very large doses caused reversible inflammatory and degenerative changes, but all changes resolved within 14 days of injection.
Diagnostic lumbar puncture is not associated with a higher rate of relapse.17 Two small reports have implicated spinal anesthesia in the exacerbation of multiple sclerosis.15,18 Bamford et al.15 described one case of relapse after the administration of spinal anesthesia in 9 patients, and Stenuit and Marchand18 identified two cases of relapse after the administration of spinal anesthesia in 19 patients. The relationship of these relapses to spinal anesthesia or other postoperative conditions (e.g., stress, infection, hyperpyrexia) known to exacerbate multiple sclerosis is unclear.
There are few published data on the use of epidural anesthesia in patients with multiple sclerosis. Warren et al.19 reported minor exacerbations after the administration of epidural anesthesia for two separate vaginal deliveries in one patient. Crawford et al.20 reported one postoperative relapse in 50 nonobstetric and 7 obstetric patients who received epidural analgesia. Confavreux et al.21 reported a study of 269 pregnancies in 254 women with multiple sclerosis, of whom 42 received epidural analgesia. They noted that epidural analgesia did not have an adverse effect on the rate of relapse or on the progression of disability in these patients. Bader et al.22 retrospectively evaluated 32 pregnancies in women with multiple sclerosis; they observed that women who received epidural anesthesia for vaginal delivery did not have a higher incidence of relapse than those who received only local infiltration anesthesia. In a prospective study of 227 women who had multiple sclerosis for at least 1 year before conception, of whom 42 received epidural analgesia during labor, no adverse effect of epidural analgesia on the rate of relapse or the progression of disability was identified.10
Bader et al.22 observed that all of the women who experienced a relapse after epidural anesthesia had received a concentration of bupivacaine greater than 0.25%. The concentration of local anesthetic in the CSF progressively increases during prolonged administration of epidural anesthesia, and the authors suggested that the higher concentration may overwhelm the protective effect of dilution within the CSF. An alternative explanation is that women who require a higher concentration of neuraxial local anesthetic may have more stressful labor. However, these observations suggest that anesthesia providers should use a dilute solution of local anesthetic for epidural analgesia during labor, when possible.
The addition of an opioid reduces the total dose of local anesthetic required for epidural analgesia during labor. Berger and Ontell23 reported the administration of intrathecal morphine, which was added to low-dose tetracaine for surgical anesthesia and postoperative analgesia, and observed no exacerbation of multiple sclerosis at 1 and 6 months after surgery. Leigh et al.24 described the successful use of intrathecal diamorphine for postoperative analgesia in a patient with multiple sclerosis who underwent a laparotomy.
The administration of neuraxial anesthesia for cesarean delivery is controversial. Because the operation is of limited duration, multiple doses of local anesthetic are typically not needed, so a progressive increase in CSF concentration of local anesthetic over time is less likely. In light of the significant benefits of neuraxial techniques for intraoperative anesthesia and postoperative analgesia, either spinal or epidural anesthesia is the principal anesthetic technique used for cesarean delivery in patients with multiple sclerosis in many institutions, including my own.
In summary, published data do not contraindicate the use of neuraxial anesthetic techniques for labor analgesia or operative anesthesia. The patient should be aware that there is a higher incidence of relapse during the postpartum period, even without the use of neuraxial analgesia or anesthesia. In addition, when anesthetic techniques are used, the type of anesthesia selected does not appear to influence the relapse rate. Neither pregnancy nor anesthesia appears to have a negative influence on the long-term course of the disease. The willingness of anesthesiologists to use neuraxial techniques in pregnant patients with multiple sclerosis is reflected in a survey of obstetric anesthesiologists published in 2006.25 The majority (91%) of respondents had seen fewer than 10 cases of multiple sclerosis in the past 10 years; 79% and 98% of anesthesiologists indicated they would perform a neuraxial anesthetic technique for labor and elective cesarean delivery, respectively.
Headache during Pregnancy
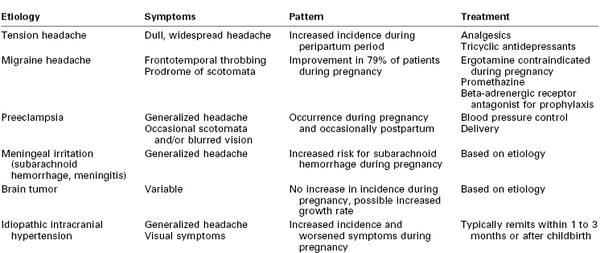
Headaches are among the most frequently observed neurologic symptoms during pregnancy (Table 49-1). Tension headaches, migraine headaches, and headaches associated with hypertension in pregnancy, including preeclampsia, are commonly observed during pregnancy. A pregnant patient with a history of chronic headaches who reports new or different symptoms should be closely evaluated to exclude serious etiologies such as preeclampsia, tumor, or intracranial vascular malformation. Symptoms of concern include sudden onset, intense severity, altered mental status, meningeal signs, fever, vomiting, and any localizing or lateralizing abnormality.
Tension Headache
Tension or muscle contraction headaches are the most common type of headache observed during pregnancy.26 The symptoms typically consist of dull, persistent pain that extends over the entire head. The onset is usually gradual, but the symptoms may persist for long periods. Although the etiology is unknown, this type of headache is believed to be associated with stress rather than hormonal changes. These headaches are more common in women, are frequently associated with anxiety, and may be a symptom of postpartum depression.27
Treatment
In the nonpregnant patient, treatment of tension headaches may involve acetaminophen, aspirin, opioids, tricyclic antidepressants, and benzodiazepines. In the pregnant patient, acetaminophen should be used as a first-line analgesic. Caffeine may be contained in combination analgesic products (e.g., Fioricet, Fiorinal). The American College of Obstetricians and Gynecologists (ACOG) has stated that, at the current time, there is no clear evidence that caffeine exposure increases the risk for fetal growth restriction (also known as intrauterine growth restriction).28 Because a final conclusion regarding risk of high caffeine intake and miscarriage cannot be made, the ACOG recommends moderate caffeine intake (< 200 mg/d) during pregnancy. Limited data suggest that butalbital is not associated with congenital anomalies.29 Ergot alkaloids (e.g., ergotamine) are contraindicated during pregnancy; these agents may cause marked increases in uterine tone, which may compromise placental perfusion and fetal oxygenation.29 Use of nonsteroidal anti-inflammatory drugs (NSAIDs) should be limited during the third trimester because of concerns about their association with premature closure of the fetal ductus arteriosus and prolongation of pregnancy. Although a 2013 review did not find evidence that first-trimester exposure to benzodiazepines is associated with an increased risk for congenital malformations,30 these drugs are not usually used to treat headache during pregnancy. Opioids and tricyclic antidepressants have a long record of safe use during pregnancy; one study suggested that tricyclic antidepressants do not have detrimental effects on the neurodevelopment of children exposed in utero.31
Obstetric and Anesthetic Management
Pregnancy is not likely to reduce the frequency or severity of tension headaches because they are not hormonally mediated. Obstetric and anesthetic management are rarely affected by the presence of tension headaches, although a history of chronic tension headaches has been associated with an increased risk for placental abruption (adjusted odds ratio, 1.60).32
Migraine Headache
Migraine headaches are classically described as unilateral, throbbing headaches sometimes accompanied by nausea and vomiting. The duration varies from hours to days. Visual disturbances (e.g., scotomata) typically precede the onset of these headaches, and focal neurologic symptoms (e.g., aphasia, hemiplegia) may also occur. Most investigators favor neurovascular vasospasm, followed by cerebral vasodilation, as a cause of these headaches; a primary vascular disorder or a disturbance in the noradrenergic nervous system also may be involved. Patients appear to be more susceptible to symptoms when serotonin levels are low.
The 1-year period prevalence of migraine headache in the United States is 3.9% for men and 5.1% for women.33 Prevalence is higher in middle life, between the ages of 30 and 59 years. Hormonal influences have a strong association with these headaches; estrogen withdrawal is associated with an exacerbation of symptoms.34 After delivery, the reduction in hormonal concentrations coincides with an increase in migraine symptoms.35 In a prospective study of 208 Japanese women,36 85% of women had headache regression during pregnancy; no patient had worsening of headache symptoms during pregnancy. More than 50% of women experienced recurrence of migraine headache in the first postpartum month; breastfeeding was protective against the recurrence of headache.
Treatment
In nonpregnant patients, therapy often involves ergotamine tartrate, typically in combination with caffeine (e.g., Cafergot, Migergot). However, ergot alkaloids are contraindicated during pregnancy because of associated uterotonic effects and possible (but unproven) teratogenic effects.29,35,37 In general, acetaminophen is the first-line treatment during pregnancy. Combination therapy with agents containing caffeine and/or butalbital can be used with caution; the caffeine component should be limited to a dose less than 200 mg/day (see earlier discussion). Use of NSAIDs should be limited during the third trimester because of concerns about their association with premature closure of the ductus arteriosus, oligohydramnios, and prolongation of pregnancy. Beta-adrenergic receptor antagonists (e.g., propranolol) may be used for prophylaxis; however, owing to their ability to cross the placenta, these agents should be used only when a patient’s symptoms are severe. Occasionally, calcium entry–blocking agents are used. The use of sumatriptan or other selective serotonin agonists is controversial. A higher incidence of congenital anomalies has been observed after administration of high doses of sumatriptan in animals37; however, in a review of human studies, no evidence of any specific adverse effect of sumatriptan on pregnancy outcome was found.38
Obstetric and Anesthetic Management
Women with a history of migraines have a higher risk for developing gestational hypertension or preeclampsia (adjusted odds ratio, 2.85).37,39 In addition, patients with a lifetime history of migraine have been reported to have a twofold increased risk for placental abruption.
Cerebral ischemia has been reported after the administration of terbutaline in pregnant patients with migraine. Rosene et al.40 recommended that physicians avoid the administration of terbutaline in pregnant women with a history of vascular headache.
There are no published data on the relationship between intrapartum anesthesia and postpartum migraine headaches.
Spinal Cord Injury
Worldwide, there are large geographic differences in the incidence, prevalence, and lethality of spinal cord injuries.41 In the United States, traumatic spinal cord injuries occur with an incidence of 23.7 to 77.0 per million population per year; the prevalence per million inhabitants is 473 to 1800.41 Improved handling and stabilization of victims at the site of an accident and the availability of extensive rehabilitation services have resulted in a higher number of women who present for obstetric care after spinal cord injury than in the past.
Patient disability and residual function depend on the anatomic location of the injury.42 Cord injuries below S2 involve mainly bladder, bowel, and sexual functions. Affected patients have relaxed perineal muscles, and women with such injuries experience pain during labor. Women with a lesion above T10 do not experience labor pain. Patients with a lesion above T6 have varying levels of respiratory compromise and are at risk for autonomic hyperreflexia (see later discussion).
Spinal shock, defined as transient sensorimotor dysfunction resolving in less than 24 hours, may develop in about half of spinal cord–injured patients.43 Neurogenic shock consists of hemodynamic and sensorimotor abnormalities and is characterized by flaccid paralysis with loss of tendon and autonomic reflexes for weeks to months.43 Patients with neurogenic shock lose vasomotor tone, temperature regulation, sweating, and piloerection in the parts of the body below the lesion. Pulmonary edema, hemodynamic instability, and circulatory collapse can develop in the absence of brainstem regulation of vasomotor tone. Patients are at risk for aspiration, infection, and other pulmonary complications. Paraplegic patients may have a compensatory tachycardia, whereas quadriplegic patients may have bradycardia due to unopposed vagal tone.
After a variable period, the patient progresses to a chronic stage in which reflex activity is regained. In most cases, this return of reflex activity occurs within 1 to 6 weeks after the injury; rarely, return of reflex activity may take several months. This stage is characterized by disuse atrophy, flexor spasms, and an exaggeration of reflexes. The mass motor reflex results from the absence of central inhibitory mechanisms. A stimulus that normally would cause the contraction of a few muscle units leads to the widespread spasm of entire muscle groups. The mass motor reflex can occur with any level of spinal cord injury. It may occur with autonomic hyperreflexia in a patient with a lesion above T6.44
Approximately 85% of patients with chronic spinal cord injuries at or above T6 experience the syndrome of autonomic hyperreflexia.43 This is a life-threatening complication that results from the absence of central inhibition on the sympathetic neurons in the cord below the injury. Noxious stimuli, bladder or bowel distention, and uterine contractions result in afferent transmission by means of the dorsal spinal root (Figure 49-1).45 These afferent neurons synapse with sympathetic neurons, and the impulse is propagated both cephalad and caudad in the sympathetic chain, without central inhibition. The propagation results in extreme sympathetic hyperactivity and severe systemic hypertension secondary to vasoconstriction below the level of the lesion. In response, the reflex arcs involving the baroreceptors of the aortic and carotid bodies lead to bradycardia and vasodilation above the level of the lesion. In patients with lesions of T6 and above, these compensatory mechanisms are insufficient to compensate for the severe hypertension. Intracranial hemorrhage, arrhythmias, and myocardial infarction occur in some cases. A variety of agents have been used for control of the hypertension of autonomic hyperreflexia (Figure 49-2).
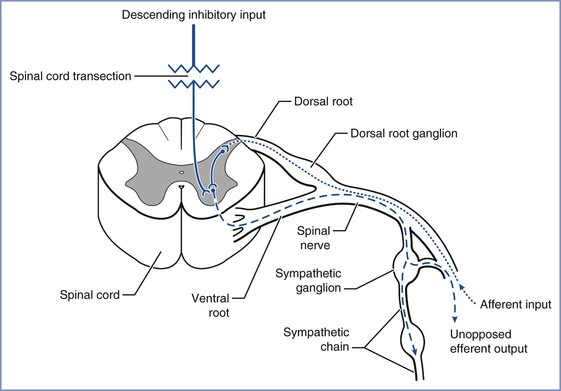
FIGURE 49-1 Noxious stimuli enter the dorsal horn of the spinal cord through the dorsal spinal root (dotted line). These afferent neurons synapse either directly or by means of interneurons (solid line) with sympathetic neurons in the intermediolateral columns of the lateral horns, which then project through the anterior roots to the paraspinal sympathetic chain (dashed line). The impulse is propagated peripherally at that spinal level and also travels both cephalad and caudad in the sympathetic chain, exiting at multiple thoracic and lumbar levels (dashed line) and resulting in sympathetic hyperactivity. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
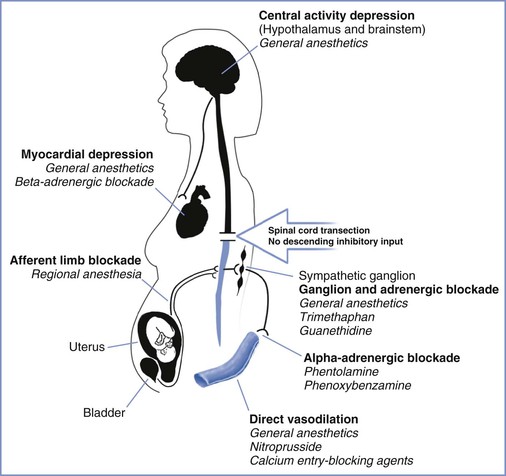
FIGURE 49-2 Sites of action for agents used in the control of hypertension associated with autonomic hyperreflexia. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Obstetric Management
Pregnancy may aggravate many of the medical complications of spinal cord injury (Box 49-1).45 The loss of both functional residual capacity and expiratory reserve volume during pregnancy may increase the likelihood of respiratory compromise associated with spinal cord injury. Pregnancy increases the risks for thromboembolic phenomena and urinary tract infection. Loss of sympathetic tone below the level of the lesion renders pregnant patients with spinal cord injury particularly prone to orthostatic hypotension, which may result in a decrease in uteroplacental perfusion. Uterine contractions can stimulate autonomic hyperreflexia, and the resultant vasoconstriction can result in fetal hypoxia and bradycardia. In pregnant women, autonomic hyperreflexia occurs most commonly during labor.
Women with a lesion above T11 may have a higher risk for preterm labor.42 Because these women do not experience labor pain, obstetric management includes weekly cervical examinations during the third trimester. Vaginal delivery is preferred. The use of assisted vaginal delivery may be necessary because of the parturient’s inability to push.42 In a study of 52 pregnancies in spinal cord–injured women, 9 of 12 patients with lesions above T5 had symptoms of autonomic hyperreflexia. The cesarean delivery rate was 47% for women with lesions above T5 and 26% for women with lesions at T5 or below.46 Preterm delivery occurred in 19% of patients. Autonomic hyperreflexia may affect uteroplacental blood flow, necessitating careful monitoring of the fetal heart rate (FHR).
Anesthetic Management
Women with spinal cord lesions at or above T6 are at risk for autonomic hyperreflexia. This syndrome can be distinguished from other causes of intrapartum hypertension by the occurrence of cyclic hypertension (i.e., blood pressure increases during contractions and decreases between contractions). The ACOG47 recommends continuous hemodynamic monitoring during labor for all patients at risk for autonomic hyperreflexia.
Administration of neuraxial anesthesia is the most common method for prevention or treatment of autonomic hyperreflexia during labor and delivery. Spinal anesthesia has effectively controlled blood pressure in paraplegic patients undergoing general surgical procedures.48 Although some anesthesiologists contend that distortion of the vertebral column in paraplegic patients makes it more difficult to predict and control the level of spinal anesthesia, published data do not lend support to this argument.48 If spinal anesthesia is chosen, insertion of an intrathecal catheter and use of a continuous technique may be appropriate; this approach may allow careful titration of the resulting neuroblockade.
Most obstetric anesthesiologists prefer the use of epidural analgesia for the prevention or treatment of autonomic hyperreflexia during labor and delivery. Consideration also should be given to providing epidural analgesia after vaginal delivery to minimize the possibility of autonomic hyperreflexia, which has been reported to occur in response to pain as late as 5 days after delivery.49
Case reports have described the successful epidural administration of 0.25% or 0.5% bupivacaine or the administration of combined spinal-epidural (CSE) anesthesia for the mitigation of autonomic hyperreflexia.50–52 Baraka53 reported the successful use of epidural meperidine, an opioid with local anesthetic qualities, in avoiding the signs of autonomic hyperreflexia. Abouleish et al.54 observed that epidural fentanyl alone did not effectively treat the hypertension of autonomic hyperreflexia, but the addition of 0.25% bupivacaine led to a decrease in blood pressure to baseline levels. Maehama et al.55 described the successful use of magnesium sulfate for management of autonomic hyperreflexia during labor.
Patients with spinal cord injury often have a low baseline blood pressure and some hemodynamic instability. Placement of an intra-arterial catheter before induction of anesthesia allows the continuous assessment of blood pressure.
Positioning for neuraxial block may be difficult; the anesthesiologist should consider performing the block with the patient in a lateral position because the sitting position may cause hypotension from venous pooling in the lower body. Therapeutic doses of a local anesthetic agent should be administered cautiously with the understanding that the cephalad level of the sensory block can be fully assessed only if it is higher than the level of the spinal cord lesion. As a result, the typical epidural test dose may not identify unintentional subarachnoid injection in a patient with spinal cord injury. Neuraxial blockade can be partially assessed by evaluating segmental reflexes below the level of the lesion. For example, the anesthesiologist can lightly stroke each side of the abdomen above and below the umbilicus, looking for contraction of the abdominal muscles and deviation of the umbilicus toward the stimulus. Reflexes are absent below the level of the block. In some patients with spastic paresis at baseline, the level of anesthesia may be confirmed by the conversion of spastic paresis to flaccid paresis.50 A decline in blood pressure may also herald the onset of neuraxial blockade. Using a nerve stimulator connected to a saline-filled, wire-reinforced epidural catheter was found to be a reliable and relatively simple method of confirming catheter placement in the epidural space.56
Alternative means of treating autonomic hyperreflexia should be available if neuraxial anesthesia is not successful. Antihypertensive medications such as magnesium sulfate or arteriolar vasodilators may be effective, recognizing that hypotension can result in decreased uterine blood flow.46 Careful titration of nitroprusside, noting the potential for fetal/neonatal cyanide intoxication, or beta-adrenergic receptor blockade, may also be useful. The anesthesiologist should recognize that increased vagal activity during autonomic hyperreflexia can result in electrocardiographic changes including first- and second-degree atrioventricular block and sinus arrest.57
If cesarean delivery is necessary, epidural or spinal anesthesia can be administered. Spinal anesthesia is generally associated with a more rapid onset and a more unpredictable level of neuroblockade and can lead to significant hypotension.58 The effect of neuraxial blockade on respiratory function may be less severe with epidural anesthesia than with spinal anesthesia.
Severe respiratory insufficiency or technical difficulties with neuraxial anesthesia may necessitate the use of general anesthesia.59 If general anesthesia is required, a depolarizing muscle relaxant such as succinylcholine should not be given during the period of denervation injury. By a conservative definition, this period begins 24 hours after the injury and lasts for 1 year. The use of succinylcholine during this period of denervation injury may cause severe hyperkalemia60; therefore, a nondepolarizing muscle relaxant should be used to facilitate laryngoscopy and tracheal intubation.
Myasthenia Gravis
Myasthenia gravis is an autoimmune disorder characterized by episodes of muscle weakness that are made worse by activity. Its prevalence is 50 to 125 cases per million. Women are twice as likely to have the disease as men, and the onset is earlier (second or third decade in women versus the sixth or seventh decade in men).61 Myasthenia gravis has been classified according to severity as follows61:
II. Mild generalized myasthenia; may include ocular, oropharyngeal, and respiratory involvement
III. Moderate generalized disease
IV. Severe generalized weakness
V. Defined by requirement for tracheal intubation, with or without mechanical ventilation
Myasthenia gravis results from an abnormality in autoimmune regulation, which leads to the production of antibodies against the nicotinic acetylcholine receptor on the neuromuscular end plate of skeletal muscle. The result is receptor destruction as well as antibody-induced blockade of the remaining acetylcholine receptors.62 Smooth muscle and cardiac muscle are not affected. Thymic hyperplasia is common, and thymic tumors occur in approximately 10% of patients. There is an association between myasthenia gravis and other autoimmune disorders, such as rheumatoid arthritis and polymyositis. In general, an early age at onset and an extended duration of purely ocular myasthenia are good prognostic signs.
Medical Management
Treatment involves a thymectomy, administration of anticholinesterase medications and/or immunosuppressive agents, and plasmapheresis. A thymectomy improves the disease course in approximately 96% of patients; 46% of these patients undergo complete remission, and an additional 50% are asymptomatic or experience improvement with therapy.63 In addition, a thymectomy appears to exert a favorable influence on the outcome of pregnancy.64 One study noted decreased maternal and perinatal morbidity as well as less frequent clinical exacerbations in patients who had undergone thymectomy.64
Anticholinesterase drugs, which inhibit the breakdown of acetylcholine, are the mainstay of therapy. Decreased muscle weakness within minutes of administering an intravenous dose of edrophonium (10 mg) confirms the diagnosis of myasthenia gravis. Physostigmine crosses the blood-brain barrier and is not used for long-term therapy. Neostigmine and pyridostigmine are quaternary ammonium compounds that do not cross the blood-brain barrier. These drugs may be administered orally or intravenously. In general, pyridostigmine is preferred because it has less severe muscarinic side effects.65
Corticosteroids and azathioprine have been used with some success. Plasmapheresis can be especially helpful for patients in crisis. One study noted that preoperative plasmapheresis resulted in less need for mechanical ventilation and less time in the intensive care unit postoperatively.66
Myasthenia gravis can manifest in two types of crises. A cholinergic crisis results from an excess of the muscarinic effects of anticholinesterase medications combined with a poor response to anticholinesterase therapy. Symptoms include muscle weakness, respiratory difficulty or failure, increased sweating, salivation, bronchial secretions, and miosis. In contrast, a myasthenic crisis results from a worsening of the disease; its symptoms include more severe muscle weakness, including the respiratory muscles. These two crises can be distinguished by the administration of edrophonium. The symptoms do not improve if the crisis is cholinergic. In contrast, improvement indicates a myasthenic crisis and the need for a higher dose of anticholinesterase medication.
Many drugs can cause a worsening of myasthenic symptoms. These patients are extremely sensitive to drugs that potentiate muscle weakness.67 These agents include neuromuscular blocking agents, quinidine, propranolol, aminoglycoside antibiotics, and tocolytic agents such as magnesium sulfate68,69 and terbutaline. One case report noted worsened symptoms after the maternal administration of betamethasone.70
Obstetric Management
The course of myasthenia gravis during pregnancy varies. In general, approximately 29% of cases improve, 41% worsen, and 30% show no change.71 Approximately 30% of patients experience a relapse postpartum. The highest chance of exacerbations occurs in the first trimester and in the acute postpartum period.72
Myasthenia gravis increases the rates of pregnancy wastage, preterm labor, and maternal mortality and morbidity.72,73 In 1991 Plauché71 estimated that maternal mortality is approximately 40 per 1000 live births and perinatal mortality is approximately 68 per 1000 births. Maternal mortality risk is inversely proportional to the duration of myasthenia gravis, with the highest risk occurring in the first year; consequently, myasthenic women are sometimes counseled to delay childbirth for the first few years after diagnosis.73
The maternal physiologic changes of pregnancy, including alterations in drug absorption, increases in blood volume, and changes in renal clearance, may require adjustments in the doses of anticholinesterase drugs. However, in the presence of a myasthenic crisis, aggressive intravenous therapy is essential, even during labor. Anticholinesterase agents are quaternary ammonium compounds that undergo minimal placental transfer but have known uterotonic effects73; thus, uterine activity should be monitored during the administration of these drugs. Each patient should be monitored carefully for progressive respiratory compromise secondary to diaphragmatic elevation during pregnancy. Vital capacity can be measured to monitor fatigue during labor. The treatment of the myasthenic patient with preeclampsia or preterm labor is problematic because the use of magnesium sulfate for maternal seizure prophylaxis or fetal neuroprotection may be associated with a significant increase in maternal and fetal muscle weakness.72
The uterus consists of smooth muscle; therefore, myasthenia gravis should not affect the first stage of labor. However, the second stage of labor often requires the use of striated muscle and consequently an assisted (e.g., vacuum or forceps) vaginal delivery may be required.
Maternal antibodies to the acetylcholine receptor are transferred across the placenta. Neonatal myasthenia gravis occurs in approximately 16% of infants of mothers with myasthenia gravis.64,71 Physiologic variations in the levels of alpha-fetoprotein, which can block the binding of the antibody to the acetylcholine receptor, can alter the clinical course of myasthenia during pregnancy.74 The rapid decrease in alpha-fetoprotein concentrations in the neonate after birth may be responsible for transient symptoms of myasthenia (e.g., feeding problems, hypotonia, respiratory difficulty) within the first 4 days of life.72 The symptoms abate as the antibodies are metabolized, with resolution typically occurring within 2 to 4 weeks; however, anticholinesterase therapy may be required during the interim.
Anesthetic Management
Myasthenia gravis patients should undergo early antepartum consultation with an anesthesiologist. This evaluation should assess the extent of bulbar and respiratory involvement and overall baseline muscle strength. Pulmonary function testing should be performed in patients with evidence of respiratory compromise. In a study of surgical patients, the presence of bulbar symptoms, a preoperative serum level of antiacetylcholine receptor antibody greater than 100 nmol/L, and intraoperative blood loss greater than 1000 mL were risk factors for having a postoperative myasthenic crisis.75
Patients with respiratory compromise may be more susceptible to opioid-induced respiratory depression, and consideration should be given to minimizing or avoiding opioids when possible. Neuraxial techniques are the preferred method for labor analgesia in patients with myasthenia gravis, given their association with low pain scores and high maternal satisfaction, even without the addition of opioids.76 The use of anticholinesterase drugs may prolong the half-life of ester local anesthetic agents.
Neuraxial anesthetic techniques are preferred for cesarean delivery unless the patient has significant bulbar involvement or respiratory compromise. The use of bilevel positive airway pressure for ventilatory support in patients with moderate respiratory compromise may improve the safety of neuraxial anesthesia.77
In the patient with severe bulbar involvement or respiratory compromise, it may be prudent to secure the airway before surgery. Sodium thiopental, ketamine, and propofol have been used successfully for the induction of general anesthesia in patients with myasthenia gravis.73,77,78 Depolarizing muscle relaxants (e.g., succinylcholine) have an unpredictable effect in these patients, with affected and unaffected muscles being more sensitive and resistant to these agents, respectively.63 However, the commonly administered dose of succinylcholine (1 to 1.5 mg/kg), which is three to five times the effective dose in 95% of normal patients, will most likely provide adequate relaxation even for resistant muscles. Anticholinesterase agents and plasmapheresis cause decreases in the activity of plasma cholinesterase and may cause delays in succinylcholine hydrolysis.
Myasthenic patients are extremely sensitive to nondepolarizing muscle relaxants. If a nondepolarizing muscle relaxant must be given, the anesthesia provider should administer a small amount of an agent with a short half-life (e.g., rocuronium, atracurium, vecuronium).63 Mivacurium is metabolized via plasma pseudocholinesterase, which may be inhibited by pyridostigmine. In general, greater disease severity corresponds with enhanced sensitivity to nondepolarizing muscle relaxants, necessitating the use of clinical judgment and neuromuscular monitoring to determine the amount and timing of drug doses. Myasthenia may prevent a full-strength contraction with nerve stimulation; therefore, a control train-of-four stimulus test should be performed before paralysis for later comparison. For nondepolarizing agents, approximately 50% of the standard dose may be adequate, and a prolonged recovery should be anticipated. Small doses of neostigmine may be given cautiously for the reversal of neuromuscular blockade.
After delivery, fluid shifts and decreased maternal alpha-fetoprotein concentrations may necessitate an adjustment of anticholinesterase drug doses. Some patients who receive general anesthesia require postoperative ventilation. The following factors are predictive of an increased risk for postoperative ventilation: (1) female gender, (2) FEF25%-75% (forced expiratory flow during the middle half of the forced vital capacity) less than 3.3 L/sec and less than 85% of that predicted, (3) FVC (forced vital capacity) less than 2.6 L/sec and less than 78% of that predicted, and (4) MEF50% (maximal expiratory flow at 50% of expired vital capacity) less than 3.9 L/sec and less than 80% of that predicted.79
Epilepsy
Epilepsy is a condition characterized by recurrent seizure activity in the absence of metabolic disorders or acute brain disease. The classification scheme for epileptic seizures is evolving; however, most seizures are grouped into the two major types: partial and generalized.80,81 In partial seizures, the excess neuronal discharge is thought to originate in one region of the cerebral cortex; in generalized seizures, the discharge occurs bilaterally and involves the entire cortex.
Medical Management
A variety of antiepileptic agents are used for seizure therapy, depending on the type of seizure and clinical response (Table 49-2). A variety of adverse effects have been reported with these agents, including early-onset events (e.g., somnolence, dizziness, hypersensitivity, rash, gastrointestinal symptoms) and late-onset events (e.g., depression, leukopenia, aplastic anemia, thrombocytopenia, megaloblastic anemia, hyponatremia).81
TABLE 49-2
Epilepsy Drugs
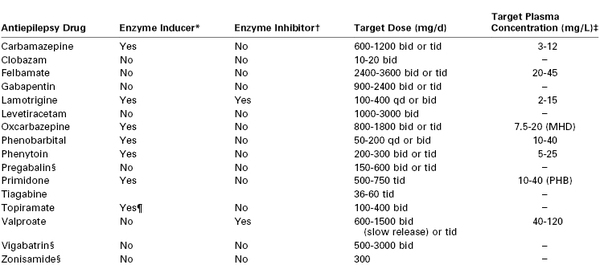
* Enzyme inducer of the CYP cytochrome P450 system.
† Enzyme inhibitor of the CYP cytochrome P450 and uridine diphosphate glucuronyl transferase systems.
‡ Dash (−) indicates not relevant.
§ Dose should be reduced in patients with renal dysfunction.
¶ For doses > 200 mg/d.
MHD, monohydroxy derivative; PHD, phenobarbital.
From Elger CE, Schmidt D. Modern management of epilepsy: a practical approach. Epilepsy Behav 2008; 12:501-39.
Prognosis for medical control of seizures is good for patients with generalized seizure disorders; as many as 2% to 40% of newly diagnosed epilepsy patients become seizure-free without or with minimal antiepileptic drug therapy.82 Two of three newly treated epilepsy patients will eventually enter long-term remission (5 years or more without a seizure).82 However, about one third of patients will have an intermittent pattern (early remission with late recurrence or late remission). Finally, the standard mortality ratio for patients with epilepsy (observed number of deaths in the study compared with the general population) is consistently increased in the first several years after the diagnosis of epilepsy.
Interaction with Pregnancy
Three to five births per thousand occur in women with epilepsy.83 A 2009 systematic review by the American Academy of Neurology and American Epilepsy Society concluded that there is insufficient evidence to determine whether seizure frequency changes during pregnancy.84 Women who are free of seizures for at least 9 months to 1 year before pregnancy have a probability of 84% to 92% of remaining seizure-free during pregnancy.84
Optimizing antiseizure therapy before pregnancy is critical. Because of the teratogenic aspects of many antiepileptic agents, some physicians consider withdrawal of these drugs after 2 years without seizures and recommend waiting at least 6 additional months after withdrawal before attempting to conceive. Substituting new antiepileptic agents after conception is not recommended.
A variety of causes have been proposed for the increase in seizure frequency observed in some pregnant women (Table 49-3). Higher estrogen concentrations in pregnancy lower the seizure threshold.85 Greater sodium and water retention, alkalosis secondary to hyperventilation, sleep deprivation, and increased stress and anxiety also have been suggested as mechanisms.86 In addition, anticonvulsant drug levels can decrease during pregnancy, often despite the administration of a larger dose87; this may be partially explained by the decreased plasma protein binding and greater drug clearance observed during pregnancy.88 The American Academy of Neurology and American Epilepsy Society concluded that monitoring of lamotrigine, carbamazepine, and phenytoin levels should be considered during pregnancy. Monitoring of levetiracetam and oxcarbazepine (and its active metabolite, monohydroxy derivative) may be considered, and monitoring of other antiepileptic agents should not be discouraged despite limited data regarding their pharmacokinetic behavior during pregnancy.89
TABLE 49-3
Possible Causes of Increased Seizure Frequency during Pregnancy
| Mechanism | Examples |
| Hormonal | Changes in levels of estrogen (proconvulsant) and progesterone (anticonvulsant) |
| Metabolic | Increased water and sodium retention |
| Psychological | Stress, sleep deprivation |
| Pharmacokinetics | Increase in liver metabolism, renal clearance, or volume of distribution |
| Physiologic | Decreased gastrointestinal absorption |
Maternal seizures can have devastating consequences. Hypoxia and acidosis that occur during a generalized seizure can result in fetal compromise or intrauterine fetal death. During the past three decades, the overall risk for obstetric complications in epileptic women has declined. Although some studies have suggested an increased risk for hypertension in pregnancy (including preeclampsia), bleeding, and preterm birth, a systematic review concluded that evidence is inconclusive.84 However, women with epilepsy may have a moderately increased risk for cesarean delivery.84
Fetuses and neonates of women with epilepsy are approximately twice as likely to have adverse pregnancy outcomes, including intrauterine fetal death, cesarean delivery, 1-minute Apgar score less than 7, neonatal and perinatal death, LBW, and abnormal development.90 Antiepilepsy drugs taken in the first trimester of pregnancy are associated with an increased risk for major congenital malformations.83,90,91 Data are insufficient to judge whether in utero exposure to antiepileptic agents in general increases the risk for cognitive impairment in the offspring of women with epilepsy, although there is some evidence that the risk may be increased for specific drugs.83,91 Specifically, in utero exposure to valproate has been associated with maladaptive childhood behavior and autism.91
The risk for congenital malformations in women with epilepsy receiving antiepileptic drug monotherapy is 4% to 6%90,91 Malformations have been associated with all currently used therapeutic modalities; those most often observed are cleft lip and palate and cardiac, neural tube, and urogenital defects (hypospadias).91
Certain drugs have been associated with a higher relative risk for congenital defects than others. Data from prospective studies indicate that valproate in particular is associated with significantly higher rates of major malformations.83,91 Animal studies suggest that newer agents (e.g., lamotrigine, gabapentin, felbamate, topiramate, tiagabine, levetiracetam, pregabalin) have less teratogenic effect in animals, but adequate human studies have not been performed.91 Lamotrigine may be less teratogenic in humans than other antiepileptic agents, although neonates with orofacial clefts have been reported in association with its use.92 Collaborative international registries are collecting more information regarding the dose-dependent effects of antiepileptic agents during pregnancy, particularly information regarding the newer drugs.93 Several studies suggest that maternal folic acid supplementation before conception may decrease the risk for major congenital abnormalities in the offspring of women with epilepsy on antiepileptic therapy.89
Tomson and Battino91 reviewed data from the International Registry of Antiepileptic Drugs and Pregnancy (EURAP) and made the following suggestions: (1) before conception select the most appropriate agent for the woman’s type of epilepsy, (2) select the drug with the lowest teratogenic potential, (3) aim for monotherapy with the lowest effective dose, (4) whenever possible avoid valproate, and (5) if possible avoid valproate at doses of 700 mg/d and higher.
Neonates of mothers undergoing long-term antiepileptic therapy may be at risk for deficiencies in vitamin K–dependent clotting factors or other coagulation defects, despite the absence of clinically evident maternal coagulation abnormalities.89 Enzyme-inducing antiepileptic agents (e.g., phenytoin, phenobarbital, carbamazepine) can cross the placenta and may increase the rate of oxidative degradation of vitamin K in the fetus. Affected infants are at risk for neonatal hemorrhage and respond to vitamin K (1 mg) given intramuscularly at birth. The administration of prenatal vitamin K to epileptic women with long-term exposure to these particular antiepileptic agents has not been conclusively shown to reduce the risk for neonatal hemorrhage.89
Anesthetic Management
There are significant interactions between antiepileptic drugs and anesthetic agents.94 Carbamazepine, phenytoin, phenobarbital, and primidone are potent inducers of the cytochrome P450 enzymes in hepatic metabolism (see Table 49-2), which may result in decreased plasma concentrations of many medications, including beta-adrenergic receptor antagonists and calcium entry–blocking agents.81
Serum levels of antiepileptic drugs should be checked if therapeutic levels are known (see Table 49-2). Drug doses should not be missed during the peripartum period. If the patient experiences a seizure during labor, airway protection and support of ventilation are essential. Small doses of a benzodiazepine, propofol, or sodium thiopental arrest most seizures. Fetal bradycardia may necessitate immediate delivery.
Oral antiepileptic therapies should be continued whenever possible throughout the peripartum period. Unfortunately many of the agents are not available in parenteral forms. If oral agents cannot be taken, conversion to a parenteral agent such as phenytoin may be required. In general, antiepileptic agents have sedating properties and some are known to induce liver enzymes; this feature could potentially lead to more rapid breakdown of anesthetic agents that are metabolized by the liver.
The presence of epilepsy is not a contraindication to the administration of neuraxial analgesia or anesthesia. In a retrospective review of 100 epileptic obstetric patients, 19 received general anesthesia, 48 received spinal anesthesia, 21 received epidural or caudal anesthesia, and 12 received pudendal nerve block.95 Of the 5 women who had a postpartum seizure, 4 had received spinal anesthesia and 1 had received general anesthesia with enflurane. No seizures occurred in patients who received epidural or caudal anesthesia. Although antiepileptic drugs have been associated with adverse effects on the coagulation system, Manohar et al.96 observed no abnormal clotting parameters or platelet counts preoperatively in a series of patients with epilepsy undergoing surgery.
If general anesthesia is necessary, it seems prudent to avoid drugs such as ketamine and meperidine, which may lower the seizure threshold.86,97 Sevoflurane has stronger epileptogenic properties than isoflurane, but co-administration of nitrous oxide and hyperventilation both counteract this effect.98 Low doses of propofol also have been shown to cause activation of the electrocorticogram in epileptic patients, but at higher doses burst suppression was induced.99 The highest incidence of seizure activity with induction of anesthesia is believed to occur with etomidate, followed by thiopental, methohexital, and propofol. Ketamine may also facilitate seizures at low dosages, but at high doses each of these induction agents acts as an anticonvulsant.94 Induction of general anesthesia can be performed with propofol or sodium thiopental and succinylcholine, and anesthesia may be maintained with a mixture of oxygen, nitrous oxide, and isoflurane. One study noted that some patients who receive phenytoin are resistant to vecuronium but not to atracurium.100
Myotonia and Myotonic Dystrophy
Myotonia is the general term used to describe a prolonged contraction of certain muscles after stimulation, which is followed by a delay in relaxation. Myotonic dystrophies are a genetically and phenotypically heterogeneous group of neuromuscular disorders caused by expansion defects in nucleotide sequences, principally on chromosome 19.101 Based on clinical ascertainment, the estimated prevalence of myotonic dystrophy is about 1 in 8000; however, prevalence estimates vary widely.101 As the most common form of myotonic disorders, myotonic dystrophies manifest in two distinct forms with different nucleotide sequences, DM1 and DM2. Both DM1 and DM2 are multisystem disorders characterized by skeletal muscle weakness and myotonia, cardiac conduction abnormalities, cataracts, hypogammaglobulinemia, and insulin resistance. DM1, also known as Steinert’s disease, is generally more severe and exists in congenital, juvenile, and adult forms, whereas only an adult form has been identified for DM2.101
Myotonias can involve specific muscles, typically the hand, facial, masseter, and pretibial muscles, which become dystrophic or wasted. The disorder is slowly progressive with continual deterioration and gradual involvement of pharyngeal and laryngeal muscles, proximal limb muscles, and the diaphragm. Uterine smooth muscle is affected, and cardiac conduction abnormalities are often present. Patients typically succumb to either pulmonary or cardiac failure.
Congenital myotonic dystrophy is a severe form of myotonic dystrophy (DM1) that manifests early in infancy with hypotonia and feeding difficulties.102 Myotonia becomes apparent during the first few years of life. In most cases the mother has myotonic dystrophy.
Myotonia congenita is a milder familial disorder characterized by myotonia of the skeletal muscles; multisystem involvement does not occur.103 Unlike myotonic dystrophy, cardiac abnormalities are not present and smooth muscles are not affected. In some cases, muscle hypertrophy rather than wasting occurs. This disorder can be compatible with long life. It is distinguished from DM1 and DM2 by characteristic clinical features and an absence of significant histopathology in the muscle biopsy specimen. Myotonia congenita is characterized by dysfunction of the chloride channel.
Central core disease is a rare disorder in which muscle biopsies demonstrate the absence of oxidative enzyme activity in the longitudinal axis of the muscle fiber (i.e., the “central core”). Affected individuals have proximal muscle weakness and often scoliosis. This disease is caused by mutations in the skeletal muscle ryanodine receptor gene (RYR1) at chromosome 19q13.1, which has been associated with malignant hyperthermia.104 Many patients with central core disease test positive for the malignant hyperthermia susceptibility trait on the caffeine-halothane contracture test (in vitro contracture test) (see Chapter 47); these patients should be considered at risk for malignant hyperthermia when exposed to triggering agents (i.e., succinylcholine, volatile halogenated agents).104 Some patients with multi-minicore and nemaline rod myopathy may also be at risk for malignant hyperthermia.105
Drugs such as quinine and mexiletine are most commonly used to relieve myotonic symptoms.101–103 Corticosteroids, phenytoin, and tocainide also have been prescribed.
Obstetric Management
In patients with myotonic dystrophy, symptoms of weakness and myotonia usually remain unchanged during pregnancy; however, in a minority of women, symptoms worsen during pregnancy but generally resolve after delivery.106 Antepartum evaluation should include pulmonary function testing, to assess the severity of restrictive lung disease due to muscle wasting, and an electrocardiogram, which may reveal conduction abnormalities.
There may be a higher risk for preterm labor in patients with myotonic dystrophy. Other complications of pregnancy include polyhydramnios (secondary to reduced fetal swallowing) and an increased risk for placenta previa.107 Magnesium sulfate has been reported to cause respiratory compromise.108 Poor uterine contractions may result in prolonged labor, uterine atony, and an increased risk for postpartum hemorrhage.109,110 Muscle weakness may result in a prolonged second stage of labor and a higher incidence of operative delivery.107 The neonate also may have respiratory distress if affected by congenital myotonic dystrophy.
There are reports of patients with myotonia congenita who experience temporary worsening of symptoms during pregnancy.111 Obstetric problems have not been described, most likely because this disease involves skeletal muscle only; uterine smooth muscle is not affected in these patients.
Anesthetic Management
Patients with myotonic disorders may be especially sensitive to the respiratory depressant effects of opioid analgesic and general anesthetic agents.112 Sedative-hypnotic agents should be used with caution; in some cases, opioids or sedatives may precipitate apnea. Thus, neuraxial anesthesia is preferred for labor and vaginal or cesarean delivery. Both spinal and epidural anesthesia have been used successfully in patients with myotonic dystrophy.113–115 Although the clinical characteristics of myotonic dystrophy DM2 are generally more benign than DM1, anesthesiologists should be aware that both may be associated with dysphagia, cardiomyopathy, and cardiac conduction abnormalities.116
The prolonged contractions witnessed in patients with myotonia are due to an intrinsic muscle disorder that is not relieved by spinal or epidural anesthesia; however, infiltration with a local anesthetic agent may partially release contractions. Cold external temperatures and shivering are known triggers of myotonia, so the patient should be kept warm. Some anesthesiologists recommend the cautious administration of intrathecal or epidural opioids for their anti-shivering effect.113 Patients with myotonic dystrophy have a high incidence of pulmonary complications after general anesthesia.117
If general anesthesia is required, it is prudent to limit the use of opioids and carefully titrate muscle relaxants to mitigate the risk for postoperative pulmonary complications.118 Depolarizing agents such as succinylcholine should be avoided because fasciculations may trigger myotonia,119 thereby making ventilation and tracheal intubation difficult. By contrast, patients with myotonic dystrophy appear to have a normal response to nondepolarizing muscle relaxants. Regardless, careful neuromuscular monitoring is essential, particularly in those with significant baseline muscle weakness. Patients receiving quinine may require a smaller dose of a nondepolarizing muscle relaxant. In a review of dystrophic myotonias and their possible association with malignant hyperthermia, Parness et al.120 concluded that susceptibility to malignant hyperthermia in this group of patients is similar to that of the general population. Patients with central core disease should be assumed to be susceptible to malignant hyperthermia.104
Muscular Dystrophy
Muscular dystrophy is a group of disorders characterized by a progressive degeneration of skeletal muscle with intact innervation.121 Research on the subsarcolemmal muscle fiber protein dystrophin has led to a reclassification of these disorders. Analysis of dystrophin quality and quantity can be used diagnostically before and during pregnancy and can identify carriers in some cases.
Duchenne and Becker muscular dystrophies are transmitted as X-linked recessive disorders and occur almost exclusively in males. The most common muscular dystrophies affecting females are fascioscapulohumeral dystrophy and limb-girdle dystrophies. Fascioscapulohumeral dystrophy is an autosomal dominant, slowly progressive disorder that primarily involves the muscles of the shoulders and face.121 Over time the pelvic and pretibial muscles may be affected. Tachycardia and arrhythmias have been infrequently reported. Limb-girdle dystrophies involve slow degeneration of the shoulder and pelvic muscles.121 The inheritance pattern and severity of these diseases are variable. Cardiac conduction disorders and cardiomyopathies occur in some affected patients.
Obstetric Management
The classification of the muscular dystrophies is defined by DNA and dystrophin analysis. The presentations of these dystrophinopathies are variable, and the overall management is guided by the presence and severity of symptoms. If significant weakness is present, pulmonary function testing should be obtained to assess the extent of restrictive disease. An antepartum electrocardiogram and echocardiogram should also be considered. Pregnant women with muscular dystrophies may have an increased incidence of operative delivery; the presence of severe pelvic wasting may necessitate an instrumental vaginal or cesarean delivery.122,123 In a series of pregnant women with fascioscapulohumeral dystrophy, increased rates of LBW infants and operative deliveries were observed; the disorder worsened in 24% of these pregnancies and generally did not resolve after delivery.122 A larger study of the course of pregnancy in women with hereditary neuromuscular disorders reported a high rate (27%) of abnormal fetal presentation in women with limb-girdle muscular dystrophy, especially in chair-bound patients.106 About half of patients with limb-girdle muscular dystrophy reported a deterioration of symptoms during and after pregnancy.
Anesthetic Management
Limb-girdle muscular dystrophy is associated with various cardiac abnormalities, including cardiomyopathies and conduction abnormalities. Reduced lung function and respiratory compromise can be exacerbated by the physiologic changes of pregnancy; one report of a parturient with limb-girdle muscular dystrophy noted the requirement of noninvasive positive-pressure ventilation during the third trimester of pregnancy for progression of severe restrictive pulmonary disease.124 Neuraxial techniques are preferred for labor analgesia and cesarean delivery anesthesia. Severe disease may result in both airway abnormalities and spinal deformities, which may complicate the administration of either general or neuraxial anesthesia. Severe kyphoscoliosis during pregnancy can prevent adaptive hyperventilation and gradually result in respiratory insufficiency.125
Whereas most females are asymptomatic carriers of the abnormal gene for muscular dystrophies, approximately 2.5% of female carriers have symptoms of the disease—although usually in milder forms than those witnessed in men.126 There are reported cases of muscular dystrophy associated with “malignant hyperthermia.” In a systematic review, Gurnaney et al.127 summarized reported cases of patients with muscular dystrophy who developed hyperthermia, tachycardia, rhabdomyolysis, and hyperkalemia after exposure to succinylcholine and/or volatile anesthetic agents. However, none of these patients had other classic signs of malignant hyperthermia or evidence of hypermetabolism. The mechanism for this response is not well understood but may be related to the ability of these agents to exacerbate instability and permeability of dystrophin-deficient muscle membranes.127 Although the authors concluded that muscular dystrophy patients are unlikely to be at increased risk for malignant hyperthermia, they recommended that volatile anesthetic agents be used cautiously because of the risk for severe rhabdomyolysis. Succinylcholine may lead to hyperkalemia because of up-regulation of extrajunctional acetylcholine receptors or as a result of rhabdomyolysis. Thus, succinylcholine should not be administered to patients with known muscular dystrophy. In general, these patients have a normal response to nondepolarizing muscle relaxants, but careful neuromuscular monitoring is needed, especially in patients with severe muscle wasting.
The Phakomatoses (Neurocutaneous Syndromes)
The phakomatoses are congenital disorders that manifest as CNS and cutaneous abnormalities. Structures of ectodermal origin such as skin, nervous system, and eyes are commonly affected.128 The diseases are classified into three main groups: neurofibromatoses, tuberous sclerosis, and angiomatoses with CNS abnormalities (Box 49-2). The most common phakomatoses are neurofibromatosis types 1 and 2, tuberous sclerosis, Sturge-Weber disease, and von Hippel-Lindau disease. Abnormalities of the brain and spinal cord can have significant implications for anesthetic management.
Neurofibromatosis
Neurofibromatosis occurs as a result of excessive proliferation of neural crest elements such as Schwann cells, melanocytes, and fibroblasts. Clinical manifestations include hyperpigmented lesions (café-au-lait spots) accompanied by a variety of cutaneous and subcutaneous tumors. This disorder is now believed to exist in two distinct forms with gene abnormalities on two different chromosomes. Neurofibromatosis type 1, the “classic” form, has an incidence of approximately 1 in 3000. The severity and progression of the disease are variable, with the neurologic symptoms depending on the location of the tumors. Intracranial tumors and paraspinal neurofibromas are a cause of concern and may require surgical excision. The risk for pheochromocytoma is greater in these patients.129 Neurofibromatosis type 2 is a less common, more recently discovered, form of the disease with fewer cutaneous lesions. Acoustic neuromas as well as other cranial or spinal neurofibromas, meningiomas, and gliomas may be present.
Obstetric Management
Pregnancy may exacerbate the disease by increasing tumor growth.130 Regression occurs after delivery in some women. Although early, small studies suggested an increased risk for obstetric complications in women with type 1 neurofibromatosis, a review of 247 pregnancies did not confirm a higher rate of preeclampsia, preterm delivery, fetal growth restriction, spontaneous abortion, or perinatal mortality compared with healthy women.130 The high cesarean delivery rate (36%) was associated with pregnancy-related complications of the disease, including the compression of the birth canal by pelvic neurofibromas.130 The presence of intracranial masses may be problematic during labor and vaginal delivery, particularly with the increased intracranial pressure (ICP) that occurs with the Valsalva maneuver during the second stage of labor.
Anesthetic Management
An anesthesiologist should thoroughly assess the patient’s current symptoms and known lesions, particularly if they involve neck and laryngeal tumors; these tumors are common, particularly in patients with neurofibromatosis type 1.131
Neuraxial anesthetic techniques can be used for labor analgesia and operative anesthesia in most patients with the disorder. However, severe kyphoscoliosis owing to the presence of paraspinal tumors may complicate the administration of neuraxial anesthesia. The presence of asymptomatic paraspinal and intracranial tumors has prompted some anesthesiologists to suggest that neuraxial anesthesia should be administered only after careful clinical and radiographic evaluations.132
The use of muscle relaxants in these patients is controversial, because both increased and decreased sensitivity to succinylcholine has been reported; increased sensitivity to nondepolarizing agents has been reported as well.133–135 However, a number of investigators observed only minimal alterations in dose response to both depolarizing and nondepolarizing muscle relaxants in these patients and have recommended no alterations in the dose of drug.133
Tuberous Sclerosis
Tuberous sclerosis is a phakomatosis characterized by epilepsy, mental retardation, and adenoma sebaceum.136 The brain shows abnormal growth of glial cells in hamartomas called tubers. Hamartomatous tumors can occur in multiple organs, including the heart, kidneys, liver, and lungs. The inheritance pattern is autosomal dominant with a variable expression, and the disease is slowly progressive.
Obstetric and Anesthetic Management
There are few reports of pregnancy in women with tuberous sclerosis. The obstetrician and anesthesiologist should know the locations of lesions in an individual patient. Hemorrhage into the tumors, renal failure, and hypertension may complicate pregnancy.137 Renal involvement appears to represent an important prognostic factor during pregnancy, and spontaneous rupture of a renal angiomyolipoma has been reported.138 Published reports have included several patients who required cesarean delivery.137,138 Factors that could potentially impact anesthetic management of these patients include the presence of cardiac and renal tumors (angiomyolipomas), spinal and intracranial tubers, epilepsy, pharyngeal tumors, and pulmonary involvement.139 Cardiac rhabdomyosarcomas have been reported to occur in over 60% of children with this disorder. These tumors generally regress with age, but arrhythmias and cardiac failure from ventricular obstruction may occur. In the presence of known elevated ICP from cerebral lesions, some anesthesiologists believe that neuraxial anesthesia is contraindicated. Imaging should be considered before administration of neuraxial blockade if intracranial or spinal lesions are suspected. In addition, the airway should be assessed closely for the presence of oral tubers, which have been described in approximately 15% of these patients.
Cutaneous Angiomatosis with Central Nervous System Abnormalities
One group of phakomatoses consists of disorders in which a cutaneous vascular anomaly is accompanied by CNS abnormalities (see Box 49-2).140 There are few reports of pregnancy in patients with these disorders. Patients may have neurologic problems related to hemangiomas of the CNS. Cesarean delivery with epidural anesthesia has been reported in a patient with spinal hemangiomas.141 The presence of widespread varicosities in these disorders may result in a chronically low ventricular preload; if a significant increase in preload occurs during the peripartum period, cardiac overload and peripartum cardiomyopathy may occur.142
Acute Idiopathic Polyneuritis (Guillain-Barré Syndrome)
Acute idiopathic polyneuritis, also known as Guillain-Barré syndrome, is an inflammatory demyelinating illness with a reported incidence of approximately 1 case per 100,000 persons per year.143 In 60% of patients, a viral illness precedes neurologic symptoms by 1 to 3 weeks. Cases also have occurred after the administration of antirabies and influenza vaccines.
Patients with this disorder initially have weakness in the limbs, followed by the trunk, neck, and facial muscles. Loss of reflexes, total motor paralysis, and respiratory failure can occur. Sensory loss typically is not detectable. Symptoms peak at 2 to 3 weeks. The majority of patients recover completely; approximately 10% of patients have severe residual disability, and in 3% the syndrome is fatal.143
Slowing of nerve conduction occurs. Pathologic changes include lymphoid cellular infiltration and areas of demyelination that most likely result from a cell-mediated immunologic reaction against peripheral nerves. Autonomic nervous system involvement and dysfunction may occur.
Treatment is largely supportive and may include mechanical ventilatory support. Plasmapheresis reduces the duration of illness when instituted during the evolution phase and has been used safely during pregnancy.143,144
Obstetric Management
The incidence of this syndrome appears to be lower in pregnant women than in nonpregnant women. Using data from several nationwide registries, Jiang et al.145 found that the age-adjusted relative risk of Guillain-Barré syndrome appears to be decreased during pregnancy but increases in the first 3 postpartum months. In severe cases, the risk for preterm labor is increased and neurologic deterioration may occur after delivery.146 Termination of pregnancy does not appear to improve the course of the disease, but induction of labor may be indicated if autonomic dysfunction occurs. Instrumental vaginal delivery may be necessary.146
Anesthetic Management
Anesthetic management depends on patient status at the time of delivery; epidural, spinal, and CSE techniques have been described in patients with Guillain-Barré syndrome.147–149 However, some anesthesiologists have expressed concern regarding the use of neuraxial techniques in these patients, citing the theoretical potential for neurologic changes as a result of anesthetic toxicity or immunologic modulation. Steiner et al.150 implicated epidural anesthesia as a trigger of Guillain-Barré syndrome in four patients; Wiertlewski et al.148 reported the immediate worsening of neurologic status after delivery in a pregnant patient with Guillain-Barré syndrome who had received epidural anesthesia. These reports did not establish a causal relationship between the disease and neuraxial anesthesia techniques, nor did they properly acknowledge the increased frequency of Guillain-Barré syndrome in the postpartum period.
If general anesthesia is necessary in a patient with Guillain-Barré syndrome, succinylcholine most likely should be avoided because of the risk for hyperkalemia in patients with acute muscle wasting. Careful titration of nondepolarizing muscle relaxants is also necessary.
The parturient with a history of remote Guillain-Barré syndrome may have persistent diminished respiratory reserve, even in the absence of obvious residual disability.151 Pulmonary evaluation should be considered before the administration of anesthesia. Approximately 5% of patients experience a relapse, with a small number of cases progressing to a chronic disorder.
Poliomyelitis
Poliomyelitis is a disease caused by a picornavirus that is transmitted by the fecal-oral route. Most cases are asymptomatic or are accompanied by mild systemic symptoms. More severe symptoms and nervous system involvement occur in approximately 1% of patients.152 Motor neurons in the cerebral cortex, brainstem, and spinal cord are affected. Asymmetric flaccid paralysis develops over several days. Bulbar paralysis is more common in young adults. The CSF findings are consistent with viral meningitis. Recovery occurs 3 to 4 months after onset, most likely from motor axon terminal sprouting that reinnervates the previously denervated muscle fibers; however, residual deficits often persist.
A slowly progressive syndrome called postpoliomyelitis muscular atrophy may develop as many as 40 years after the acute illness. Klingman et al.153 speculated that the increased functional demands on the surviving neurons or the motor axon terminal sprouts eventually result in their death. Others believe that this syndrome results from a reactivation of the initial viral infection.154
Obstetric Management
Currently, polio is a cause for concern only in countries with ineffective vaccination programs. Although the poliovirus vaccine has been available since the 1950s, the last phase of poliomyelitis eradication has been difficult; as of 2011, transmission of the disease continues in countries such as such as Nigeria, India, Pakistan, and Afghanistan.155 The oral form of the vaccination does not appear to have harmful effects on fetal development and can be used if vaccination is required during pregnancy.156 In the past, a history of poliomyelitis was believed to affect labor and delivery only if residual deficits resulted in pelvic asymmetry or an inability to push effectively157; however, more recent data suggest a higher incidence of preeclampsia, maternal renal dysfunction, LBW infants, perinatal death, and cesarean delivery in poliomyelitis survivors.158 Some of these adverse outcomes may be related to chronic pulmonary issues or mechanical obstruction during labor.
Anesthetic Management
A complete preanesthetic evaluation should be performed for the presence of respiratory impairment, sleep apnea, swallowing difficulties, and other neurologic and motor deficits in all parturients with a history of poliomyelitis.152 Some anesthesiologists have feared that administration of neuraxial anesthesia in patients with a history of poliomyelitis might cause reactivation of the virus or postpoliomyelitis and muscular atrophy. However, there is no evidence that neuraxial analgesia or anesthesia worsens symptoms in these patients. Crawford et al.147 reported the successful use of epidural analgesia with no adverse complications in patients with a history of poliomyelitis. Rezende et al.159 reported a series of 123 patients with a history of poliomyelitis undergoing 162 surgical procedures and observed postoperatively for 22 months; neuraxial blockade was used in 64% of cases, with no patients exhibiting worsening of neurologic symptoms. Anesthetic considerations in these patients should include assessment for pulmonary restrictive disease as well as anatomic issues that may make neuraxial techniques difficult.160,161 A study from India reported that about one third of pregnancies complicated by kyphoscoliosis occurred in patients with a history of poliomyelitis in infancy.161 Radiographic imaging has been successfully used to guide spinal needle placement in these patients.
Suneel et al.162 reported a patient with a history of poliomyelitis who developed muscle weakness after general anesthesia that responded to additional neostigmine, suggesting that these patients may have a low threshold for the effects of neuromuscular blocking agents. For the patient with poliomyelitis in whom general anesthesia is needed, some anesthesiologists have suggested the use of a decreased dose of a short-acting nondepolarizing muscle relaxant in lieu of succinylcholine, which may provoke severe acute hyperkalemia.163
Brain Neoplasms
Intracranial neoplasms vary in incidence, histology, clinical presentation, and prognosis (Table 49-4).164 Brain neoplasms in pregnant women appear to occur with the same relative frequency as in nonpregnant women; however, the physiologic alterations that occur during pregnancy can have profound implications for symptomatology and management.
TABLE 49-4
Classification of Brain Tumors in Women
| Histologic Type | Percentage of all Diagnosed Tumors |
| Benign | |
| Meningioma | 35 |
| Schwannoma | 7 |
| Pituitary neoplasms | 7 |
| Malignant | |
| Gliomas: | |
| Low-grade astrocytoma | 3 |
| Glioblastoma multiforme (plus high-grade astrocytoma) | 23 |
| Other astrocytoma | 8 |
| Other | 5 |
| Lymphoma | 2 |
| Medulloblastoma | 2 |
| Other brain neoplasms | 8 |
Modified from Swensen R, Kirsch W. Brain neoplasms in women: a review. Clin Obstet Gynecol 2002; 45:904-27.
Gliomas are the most common intracranial neoplasms, accounting for approximately 39% of all primary intracranial tumors.165 These tumors, which result from anaplasia of astrocytes, exhibit diversity in invasive potential and include glioblastoma multiforme, astrocytomas, ependymomas, and oligodendrocytomas. Glioblastoma multiforme is the most lethal, whereas oligodendrocytomas have a better prognosis.
Meningiomas account for approximately one third of all primary brain tumors.165 These benign tumors originate from the dura mater or arachnoid. Surgery typically is curative.
Pituitary adenomas account for 7% of diagnosed primary brain neoplasms, but postmortem studies suggest a significantly higher incidence.165 Only a small fraction of these tumors cause symptoms (e.g., visual field deficits). These tumors may secrete prolactin, growth hormone, or adrenocorticotropic hormone. Growth of pituitary tumors is physically limited by the sella turcica of the sphenoid bone and, in a cephalad orientation, the hypothalamus. Compression of the hypothalamus or pituitary may result in respective decreases in the production or release of vasopressin, leading to diabetes insipidus. Bromocriptine often provides effective medical therapy for prolactin-secreting adenomas. Irradiation and surgery also represent effective therapies, and the prognosis is generally good.
Schwannomas, also called neurinomas, account for 7% of all brain tumors.165 These lesions originate in the Schwann cells surrounding the nerve. Clinical presentation depends on the location of the tumor. Acoustic neuromas result when the eighth cranial nerve is involved; these lesions are often seen in patients with neurofibromatosis. The treatment is surgical excision.
Metastatic carcinomas account for a significant number of brain neoplasms.165 Common primary cancers include those of the lung, breast, and colon. Prognosis and therapy depend on the tumor of origin.
Brain tumors share several pathophysiologic features. Neurologic deficits can result from a mass effect or increased ICP, even if the tumor is benign. Brain edema, which may result from a combination of vasogenic and cytotoxic mechanisms, is a prominent feature of cerebral neoplasms.
The potential for herniation must be considered in any patient with a mass lesion. The brain is divided into three basic compartments. The falx cerebri separates the cerebrum into right and left halves, and the tentorium isolates the cerebellum. High pressure from a mass can cause shifts from one compartment to another with devastating effects.
Obstetric Management
The incidence of primary brain tumors first manifesting in pregnancy does not appear to be greater than that in aged-matched, nonpregnant women.165 Approximately 9% of patients with choriocarcinoma have brain metastases at the time of diagnosis.166 In one epidemiologic study, patients with primary brain tumors had a higher incidence of spontaneous abortion, possibly because of hormonal factors.167 A 2012 study using a retrospective cohort from the National Inpatient Sample reported an increased rate of maternal mortality, cesarean delivery, and preterm labor in patients with malignant brain tumors, and an increased rate of preterm labor and cesarean delivery in patients with benign brain tumors.168 Pregnancy complications were significantly more likely to occur in patients having a neurosurgical procedure during their admission.
Although pregnancy does not affect the incidence of brain tumors, some of these lesions appear to grow faster during pregnancy. Visual field defects from pituitary adenomas worsen as a result of tumor enlargement during pregnancy, and symptoms have been observed to improve during the postpartum period.169 Edema and the increased blood volume may account for some of these symptoms. Pregnancy-induced hormonal changes also may play a role because estrogen and progesterone receptors are present in meningiomas and some gliomas.170
Diagnosis during pregnancy requires intracranial imaging. In general, MRI is preferred because it avoids the use of ionizing radiation. MRI may require the use of gadolinium-based contrast agents. Gadolinium has the advantage compared with other contrast agents of not containing iodine, and studies demonstrating adverse fetal effects are lacking in humans. However, gadolinium appears rapidly in the fetal bladder and amniotic fluid, from where it may be swallowed by the fetus and absorbed from the gastrointestinal tract. Its fetal half-life is unknown. The American College of Radiology has stated that the “decision to administer a gadolinium-based MR contrast agent to pregnant patients should be accompanied by a well-documented and thoughtful risk-benefit analysis.”171
Management during pregnancy depends on the nature of the tumor. Surgery for benign tumors (e.g., meningiomas) with mild symptoms can often be delayed until after delivery. Women with more aggressive, malignant tumors or with tumors causing seizures or severe visual impairment may require urgent surgery during pregnancy to avoid acute neurologic deterioration. Delivery also may be recommended as soon as reasonable fetal survival can be expected, sometimes by cesarean delivery immediately before neurosurgery. For women with pregnancies far from fetal viability, radiation therapy or stereotactic radiosurgery can be considered. Cranial radiation therapy is generally administered as a first therapeutic procedure to reduce the size of the mass in cases of aggressive neoplasm. However, radiation therapy, and particularly systemic chemotherapy, can pose significant hazards to the fetus, especially when administered during the first trimester.165 Some women may opt for surgery after an elective abortion.
In the normal parturient, CSF pressure may increase significantly with painful uterine contractions.172 In patients with an intracranial mass lesion, this situation could result in an increased risk for herniation. The location and size of the tumor should be assessed in the individual patient so that an appropriate delivery plan can be developed with multidisciplinary input. In general, either a pain-free second stage (with instrumental vaginal delivery to avoid pushing) or cesarean delivery may be appropriate.173
Anesthetic Management
The optimal anesthetic technique for labor analgesia and cesarean delivery anesthesia in the patient with an intracranial tumor is controversial. Epidural analgesia prevents the increase in ICP that can result with pushing during the second stage of labor.174 Several published reports have described the successful use of labor epidural analgesia in women with intracranial neoplasms174,175; in addition, the use of spinal anesthesia for an emergency cesarean delivery in a patient with a glioblastoma has been described.176 However, in pregnant women with increased ICP, an unintentional dural puncture associated with an attempted epidural catheter placement can result in a fatal brain herniation.177 As a consequence, many anesthesiologists favor general anesthesia for cesarean delivery in the patient with a brain neoplasm178; however, potential disadvantages of general anesthesia include (1) the loss of verbal and motor responses that facilitate neurologic assessment and (2) the risks of increased ICP with tracheal intubation and extubation.
Wang and Paech179 have reviewed specific elements in the anesthetic management of the pregnant patient undergoing neurosurgery, many of which are also relevant to the patient with an intracranial tumor undergoing cesarean delivery. The induction of general anesthesia may consist of the administration of an induction dose of propofol or sodium thiopental and either a depolarizing or a rapid-acting nondepolarizing neuromuscular blocking agent. Some anesthesiologists avoid succinylcholine because it may cause a transient increase in ICP, but others consider this effect to be clinically insignificant.179 A combination of a volatile halogenated agent (sevoflurane or isoflurane), nitrous oxide, and an opioid is commonly used for maintenance of anesthesia. The FHR should be monitored during intracranial surgery when possible.
To preserve cerebral and uteroplacental perfusion, hemodynamic stability should be maintained through appropriate fluid administration, avoidance of aortocaval compression, the prophylactic or early use of vasopressor drugs, and intra-arterial blood pressure monitoring instituted before induction of anesthesia.179 In general, blood pressure should be kept close to baseline measurements; in the setting of an emergency neurosurgical procedure in a patient with increased ICP, a drop in blood pressure may compromise cerebral perfusion. Fluid management for intracranial surgery should involve administration of isonatremic, isotonic, and glucose-free intravenous solutions to reduce the risk for cerebral edema and hyperglycemia.179 Mannitol administered to a pregnant woman slowly accumulates in the fetus, leading to fetal hyperosmolality and the subsequent physiologic changes of reduced fetal lung fluid production, decreased fetal urine production, and increased fetal plasma sodium concentrations180,181; however, mannitol in doses of 0.25 to 0.5 mg/kg has been reported in individual cases and appears to be associated with good maternal and fetal outcomes.179 Furosemide is an alternative diuretic that also should be administered cautiously.
There may be some conflict between maternal and fetal interests in the patient with increased ICP. Moderate mechanical hyperventilation may be used to reduce the increased ICP that occurs in nonpregnant patients with a brain tumor or brain injury. Minute ventilation increases during normal pregnancy, resulting in a maternal PaCO2 of 28 to 32 mm Hg; additional hyperventilation and hypocapnia may cause uterine artery vasoconstriction and a leftward shift in the maternal oxyhemoglobin dissociation curve (see Chapter 2). For pregnant women with an acute increase in ICP, Wang and Paech179 have suggested a target PaCO2 range of 25 to 30 mm Hg; however, data are currently insufficient to support evidence-based recommendations specific to pregnant women undergoing intracranial surgery. In pregnant patients with increased ICP, we recommend maintenance of maternal PaCO2 in the middle or at the lower end of the normal range for pregnancy. Management should be individualized according to the clinical setting.
When the decision is made to perform a cesarean delivery and brain tumor resection sequentially during a single anesthetic, hypertension should be avoided to prevent deleterious effects from tumor expansion. To avoid this complication during induction, some authors have used a combination of a high dose of fentanyl, thiopental, or propofol with succinylcholine.
A recent report of a patient with an unresected astroglioma undergoing cesarean delivery describes the postoperative use of transversus abdominis plane block to provide analgesia and reduce opioid requirements, thus reducing the risk for postoperative respiratory depression and potential exacerbation of ICP.182 In cases for which it is considered optimal to initially perform neurosurgery and then allow completion of the pregnancy, neuraxial techniques may be considered for delivery. The patient should be assessed to determine if ICP is elevated because this may preclude the use of a neuraxial technique. After neurosurgery, a reduction in ICP from a CSF leak may cause intracranial hypotension and may make confirmation of correct spinal needle placement by identification of CSF difficult.179
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension, previously referred to as pseudotumor cerebri or benign intracranial hypertension, is defined as an increase in ICP with a normal CSF composition in the absence of hydrocephalus or a mass lesion.183 The disorder most often occurs in obese women of childbearing age, suggesting that hormonal factors may play a role in the pathophysiology. The majority of patients have a headache, and in some cases visual symptoms occur. Over time the disorder generally improves, but there is a small risk for recurrence.
Traditional therapies have varied in efficacy; they include serial lumbar punctures and the administration of a carbonic anhydrase inhibitor and/or corticosteroid. Lumboperitoneal shunting may be required in severe cases with visual symptoms. Weight loss appears to improve the condition.
Interaction with Pregnancy
Symptoms of idiopathic intracranial hypertension worsen during pregnancy in 50% of cases and typically improve after delivery.184 However, in the presence of severe maternal symptoms, the placement of an intracranial shunt can result in clinical improvement and normal perinatal outcomes.185 Overall, this disorder does not seem to adversely affect maternal and perinatal outcomes.186
Anesthetic Management
Deliberate lumbar puncture represents a common form of treatment for idiopathic intracranial hypertension. Cerebellar tonsillar herniation does not occur because of the uniform, global increase in ICP. Paruchuri et al.187 noted that there are only two published cases of cerebellar tonsillar herniation after diagnostic lumbar puncture in patients with this disorder. Both patients had severe headache, neck pain exacerbated by movement, and focal neurologic deficits. In the absence of these signs and symptoms, the anesthesia provider can provide neuraxial analgesia or anesthesia.188
Some anesthesiologists recommend the administration of general anesthesia for cesarean delivery in patients with lumboperitoneal shunt. They contend that local anesthetic agents that reach the subarachnoid space may escape into the peritoneum, making it difficult to achieve adequate anesthesia. Moreover, the performance of neuraxial anesthesia may result in trauma to the shunt catheter. Bédard et al.189 reported the successful administration of epidural anesthesia in a preeclamptic patient with a lumboperitoneal shunt that had been placed for the treatment of idiopathic intracranial hypertension. Preoperative radiographic examination may help the anesthesia provider avoid needle placement near the catheter, although such imaging was not used in this published case. Provision of neuraxial anesthesia with an intrathecal catheter has been performed for both vaginal and cesarean delivery in parturients with idiopathic intracranial hypertension and a lumboperitoneal shunt; in one case, the intrathecal catheter provided both labor analgesia and temporary control of ICP.190,191 Questions regarding the functional status of an in situ subarachnoid shunt or the possible (very rare) use of the shunt for the administration of spinal anesthesia should be discussed with neurologic or neurosurgical consultants.192
Maternal Hydrocephalus with Shunt
Hydrocephalus results from a variety of conditions. The most common are intracranial hemorrhage in preterm infants, fetal and neonatal infections, the Arnold-Chiari malformation, aqueductal stenosis, and the Dandy-Walker syndrome.193 The Arnold-Chiari malformation consists of extension of a portion of cerebellar tissue into the cervical canal, with progressive hydrocephalus. The Dandy-Walker syndrome occurs with failure of development of the midline of the cerebellum, with resultant hydrocephalus of the fourth ventricle.
Ventriculoatrial or ventriculoperitoneal shunt catheters are placed for the treatment of many of these disorders. Because of advances in neonatal and neurosurgical care, hydrocephalic women with CSF shunt catheters are reaching childbearing age in increasing numbers.
Obstetric Management
Obstetric management depends on the presence of other medical and neurologic conditions. In general, although maternal shunt dependency carries a relatively high risk for complications for some patients, proper management can lead to normal pregnancy and delivery.194 Neurologic complications may occur in as many as 76% of pregnant women with preexisting shunts, including severe headache, shunt obstruction, and increased ICP.195 Most symptoms resolve postpartum.
Most pregnant women with intracranial shunt catheters can undergo labor and vaginal delivery; elective cesarean delivery is recommended only in the presence of severe neurologic symptoms or instability.
Anesthetic Management
Anesthetic management of the patient with hydrocephalus may depend on the location of the shunt. There has been concern that some of the local anesthetic agent entering the CSF may escape into the atrium or peritoneum, resulting in inadequate analgesia. However, both epidural and spinal anesthesia have been used in patients with lumboperitoneal, ventriculoatrial, and ventriculoperitoneal shunts (as discussed earlier).189–191 The anesthetic technique chosen for an urgent sequential ventriculoperitoneal shunt revision and cesarean delivery must balance the needs of the maternal neurologic condition and the pregnancy.
Because of the risk for shunt infection, some physicians recommend preoperative use of a prophylactic antibiotic regimen similar to that used to prevent bacterial endocarditis.196
Intracerebral Hemorrhage
Cerebrovascular disease during pregnancy can result from three major mechanisms—hemorrhage, arterial infarction, and venous thrombosis. Intracerebral hemorrhage is most commonly associated with an arteriovenous malformation or aneurysm (Figure 49-3). Using data from the National Inpatient Sample (1995 to 2008), the prevalence of subarachnoid hemorrhage was 5.8 per 100,000 deliveries in women aged 15 to 44 years.197 The ratio of hemorrhage from an arteriovenous malformation to that from an aneurysm was significantly higher in pregnant than in nonpregnant patients. The mortality rate was 10.6%; subarachnoid hemorrhage was associated with 4.1% of all pregnancy-related deaths.
FIGURE 49-3 Relative probability of major causes of subarachnoid hemorrhage for women stratified by age. (From Donaldson JO. Neurology of Pregnancy. 2nd edition. London, WB Saunders, 1989:139.)
Data are conflicting as to whether the aneurysm bleeding rate is higher during pregnancy.198 Some authors have reported a progressive increase in the incidence of aneurysm bleeding throughout gestation and up to 6 weeks postpartum.199 This interval corresponds with the period of physiologic increase in blood volume. In general, unruptured aneurysms diagnosed during pregnancy should be treated if they are symptomatic or enlarging.200 The treatment of ruptured aneurysms during pregnancy should mimic treatment of aneurysms in nonpregnant patients.200 The American Heart Association (AHA) guidelines for the management of aneurysmal subarachnoid hemorrhage include monitoring and controlling blood pressure (balancing the risk for stroke, rebleeding, and maintenance of cerebral perfusion pressure) and early surgical clipping or endovascular coiling.201 Controversy exists as to whether endovascular coiling results in better outcomes than clipping. Although the AHA has suggested that “endovascular coiling can be beneficial,” some experts argue that several factors may alter the risk-benefit ratio in pregnancy. These factors include (1) the need for exposure to ionizing radiation for coil placement, (2) the possible need for anticoagulation or use of antifibrinolytic agents, and (3) the increased rate of incomplete aneurysm occlusion associated with coiling compared with clipping.196,202 Endovascular coiling has not been specifically studied in the pregnant population, although several cases of successful endovascular treatment of ruptured intracranial aneurysms in pregnant women have been reported.200,203
Bleeding from arteriovenous malformations has been reported to occur with equal or greater frequency with advancing gestational age.196,199 The risk for hemorrhage from an arteriovenous malformation in pregnant women does not appear to differ from that in the general population, although the risk for bleeding appears greater during the second half of pregnancy and the first 6 postpartum weeks, corresponding to the period of high cardiac output.196 The management of arteriovenous malformations in pregnancy does not differ from standard care of the nonpregnant patient. As with aneurysms, a multidisciplinary decision-making process allows planning based on the location of the lesion, the duration of pregnancy, and the relative risks of interventional and noninterventional methods of management. Management of arteriovascular malformations, including those that present during pregnancy, has increasingly shifted over the past decade from a surgical to an endovascular approach.
Obstetric Management
If the lesion has been treated surgically, the patient requires no special care during labor and delivery. For an untreated aneurysm or arteriovenous malformation, the hemodynamic stress occurring during labor and delivery should be minimized. Current data do not demonstrate a definite advantage of cesarean delivery over assisted vaginal delivery.204 The decision about the method of delivery should be based on the individual patient and her pregnancy history. For labor and vaginal delivery, neuraxial analgesia and low outlet forceps or vacuum assistance may be used to shorten the second stage of labor and attenuate fluctuations in blood pressure.
Anesthetic Management
If the parturient has undergone surgical repair of either an aneurysm or arteriovenous malformation, anesthetic management need not differ from that for other obstetric patients. Hypertension should be avoided in the parturient with an untreated lesion. If vaginal delivery is planned, epidural or CSE analgesia should be considered. For cesarean delivery, either epidural or spinal anesthesia can be used. Some anesthesiologists contend that epidural anesthesia or sequential CSE anesthesia (e.g., initial use of a lower intrathecal drug dose, followed by block augmentation with epidural injection of local anesthetic) provides greater hemodynamic stability and is thus preferred for cesarean delivery. Interdisciplinary planning is important.196,205
In some cases, the neurosurgeon may ligate or excise the vascular lesion during pregnancy, before delivery. The anesthesiologist should consider the general principles of anesthetic management for pregnant women undergoing nonobstetric surgery (see Chapter 17) as well as the special considerations for pregnant women undergoing neurosurgery (as discussed earlier).179 The risks for hypertension and intracranial bleeding, as well as the risk for aspiration should be considered during induction of anesthesia. It is critical to maintain stable blood pressure during induction of anesthesia, laryngoscopy, tracheal intubation, and extubation. The patient should receive adequate sedation before and after arrival in the operating room. Placement of an intra-arterial catheter is mandatory. The anesthesiologist may attenuate the hypertensive response to laryngoscopy and tracheal intubation by intravenous administration of esmolol, labetalol, lidocaine, nitroglycerin, nitroprusside, and/or an opioid (e.g., remifentanil). Succinylcholine can be used for tracheal intubation. Regardless of the choice of muscle relaxant, it is critical that laryngoscopy and tracheal intubation not be performed until the patient is anesthetized adequately.
The anesthesiologist may maintain anesthesia with nitrous oxide and modest doses of isoflurane and an opioid. Aggressive maternal hyperventilation may result in decreased uterine blood flow.196 However, the anesthesiologist may use modest hyperventilation (e.g., PaCO2 of 28 to 30 mm Hg) as needed to reduce maternal ICP. The anesthesiologist should maintain left uterine displacement in patients beyond 20 weeks’ gestation. Intraoperative FHR monitoring allows assessment of the fetal response to maternal general anesthesia and hyperventilation. At many institutions, including my own, intraoperative FHR monitoring is used beginning at 24 weeks’ gestation, which corresponds to the onset of extrauterine neonatal viability. Typically, an obstetric nurse monitors the FHR tracing during surgery and requests obstetric consultation if needed. An adverse change in the FHR tracing should prompt the anesthesiologist to ensure adequate maternal oxygenation, ventilation, and perfusion.
Use of deliberate hypotension may compromise uteroplacental perfusion, although its safe use has been reported during neurovascular intracranial surgery in pregnant women. There is no consensus regarding an acceptable or safe level of hypotension, or the ideal method for achieving hypotension, in these patients. The prolonged administration of large doses of nitroprusside may result in fetal cyanide toxicity, although short-term administration appears safe. Intraoperative FHR monitoring allows assessment of the fetal response to deliberate hypotension. Endovascular treatment with general anesthesia avoids the need for craniotomy and deliberate hypotension.
In some cases, the obstetrician and neurosurgeon may perform a combined procedure (e.g., a cesarean delivery followed by ligation or excision of the neurovascular lesion). Principles of anesthetic management are similar to those described earlier for intracranial neurovascular surgery during pregnancy.
Rarely, anesthesiologists may provide care for pregnant women who are receiving extended somatic support after brain death. Powner and Bernstein206 reviewed 11 reports of 10 cases of brain death during pregnancy, in which somatic support was provided until successful delivery. Intracranial hemorrhage was the cause of maternal brain death in 6 of the 10 patients. The longest period of support was 107 days, from 15 to 32 weeks’ gestation. All 10 infants survived. The authors concluded that preservation of uteroplacental blood flow is the most important priority during extended somatic support, but they acknowledged that this goal is difficult to achieve because of hemodynamic instability, the high prevalence of infection, and other adverse consequences (e.g., diabetes insipidus) associated with brain death.
Cerebral Vein Thrombosis
Thrombosis of the cerebral veins and sinuses most often affects young adults and children; approximately 75% of the adult patients are women.207 Thromboses commonly involve the cavernous sinus, lateral sinus, sagittal sinus, or cortical veins. Thrombosis of the cerebral veins causes venous obstruction with local effects, whereas thrombosis of the major sinuses causes intracranial hypertension. A prothrombotic risk factor or a direct cause can be identified in approximately 85% of patients. Pregnancy may be a precipitating factor for sinus thrombosis in a person with a genetically increased risk.207
Primary cerebral cortical vein thrombosis is the type of thrombosis most often seen in pregnancy. The estimated incidence of cerebral vein thrombosis during pregnancy is 12 cases per 100,000 deliveries in developed countries208; the incidence appears to be higher in some developing countries. Cerebral vein thrombosis occurs more frequently during the last trimester of pregnancy and in the second and third postpartum weeks.208 Although the etiology is unclear, pregnancy may predispose patients to this condition because of at least two factors.209 First, traumatic damage to the endothelial lining of vessels may occur during the second stage of labor. Second, pregnancy is a hypercoagulable state (see Chapters 2 and 39). Mechanical causes of sinus thrombosis may include head injury and lumbar puncture.210 It has been postulated that low CSF pressure after a lumbar puncture causes the brain to shift downward, resulting in traction on the cortical veins and sinuses.
Patients with cerebral vein thrombosis may have headache, nausea and vomiting, and blurred vision. In more severe cases, lateralizing neurologic signs, lethargy, and seizures may occur. In severe cases, transtentorial herniation due to a focal mass effect can occur.
Care should be taken to differentiate cerebral vein thrombosis from post–dural puncture headache (PDPH).209 In general, the headache associated with cerebral vein thrombosis is more diffuse in location. Earlier teaching suggested that the headache does not vary with position, but a 2007 review concluded that the nature of the headache may change over time and often manifests “as a positional headache that overlaps the usual timing…and treatment of PDPH in the parturient.”210
Diagnosis can be confirmed by magnetic resonance (MR) venography. The American Heart Association/American Stroke Association published a set of management guidelines in 2011; the guidelines recommend full anticoagulation with unfractionated heparin (titrated to an activated partial thromboplastin time two times normal) or weight-adjusted low-molecular-weight heparin, continued for a minimum of 6 months’ duration.208
Some patients with cerebral vein thrombosis may require anticonvulsant therapy. In some cases, residual neurologic deficits and seizures may persist.
Obstetric and Anesthetic Management
Cerebral vein thrombosis rarely occurs before delivery, although such an occurrence may prompt an urgent delivery if associated with maternal neurologic instability and fetal deterioration. Maternal anticoagulation contraindicates the administration of neuraxial anesthesia. The anesthesia provider should avoid systemic hypotension, which may reduce cerebral perfusion pressure and blood flow to injured areas already subjected to marginal perfusion. If the patient has an asymmetric cerebral hematoma, dural puncture may precipitate herniation of the brainstem. Thus, it seems preferable to administer general anesthesia for cesarean delivery, with special attention to the treatment of increased ICP. Cerebral venous thrombosis is a rare, but reported, complication after spinal and epidural anesthesia, presumably due to intracranial hypotension.210
Motor Neuron Disorders
Motor neuron diseases are a group of disorders characterized by progressive muscular weakness and atrophy. These disorders may affect motor function alone or in conjunction with sensory deficits. There are few data on the course of these disorders in pregnant women. This discussion focuses on three of these disorders, amyotrophic lateral sclerosis and primary spinal muscular atrophy, which are pure motor neuron disorders, and peroneal muscular atrophy, which involves both motor and sensory degeneration. Currently there is no cure for any of these degenerative disorders.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis involves progressive degeneration of anterior horn cells with progressive atrophic weakness and hyperreflexia. Patients typically succumb to respiratory failure within 6 years of diagnosis.
This disease is seen more often in patients older than 50 years, but there are several reports of this disorder in pregnant women.211,212 Physicians should assess and frequently monitor the patient’s respiratory compromise throughout the peripartum period. Epidural analgesia and anesthesia have been used in these patients without evidence of worsened neurologic function postoperatively.213,214 Patients with amyotrophic lateral sclerosis may be sensitive to the effects of nondepolarizing muscle relaxants.215 The physiologic changes during late stages of pregnancy may worsen marginal respiratory status in these patients, and early cesarean delivery may be warranted.216
Spinal Muscular Atrophy
Like amyotrophic lateral sclerosis, primary spinal muscular atrophy involves degeneration of anterior horn cells. However, affected patients tend to be younger, and this disorder progresses more slowly. Some types are hereditary. Spinal muscular atrophy mainly involves the spinal cord, without involvement of the corticospinal tract. Marked kyphoscoliosis combined with truncal and limb weakness, especially involving the proximal musculature, can occur and result in significant ventilatory limitations.
Spinal muscular atrophy may be associated with an increased incidence of preterm labor.217 One series noted that pregnancy was associated with an exacerbation of muscle weakness in 8 of 12 patients.217 Epidural and spinal analgesia and anesthesia have been used successfully in patients with this disorder.218,219 In children with this rare disorder, both general and regional anesthesia have been successfully used; special attention should be paid to postoperative respiratory function.220
Peroneal Muscular Atrophy
Peroneal muscular atrophy, also known as Charcot-Marie-Tooth disease, includes several inherited peripheral motor and sensory neuropathies; it is one of the most common inherited neuromuscular diseases.221 It involves a progressive sensory and motor degeneration of peripheral nerves and roots. The peroneal nerve is affected early. The disorder progresses to involve all the nerves and muscles of the legs and finally the hands. Paresthesias are typically present. Restrictive pulmonary impairment, phrenic nerve dysfunction, diaphragmatic dysfunction, thoracic cage abnormalities, and sleep apnea have been described in association with peroneal muscular atrophy. Vocal cord dysfunction, possibly due to laryngeal nerve involvement, can also be present. Assessment of peripartum respiratory function is essential. Approximately 30% of patients with this disorder report deterioration in overall function during pregnancy, with approximately 20% indicating persistent postpartum deficits.106
A review of 108 deliveries found that women with this disorder have higher rates of abnormal fetal presentation, emergency operative delivery, and postpartum bleeding.222 Both neuraxial and general anesthesia have been used for delivery.223 Careful titration of muscle relaxants is essential if general anesthesia is employed.
Isolated Mononeuropathies during Pregnancy
Pregnancy is associated with an increased incidence of several specific mononeuropathies: Bell’s palsy, carpal tunnel syndrome, and meralgia paresthetica.
Bell’s Palsy
Bell’s palsy is a syndrome of acute-onset paralysis of the facial nerve; it tends to present during the third trimester and the first few postpartum weeks. The incidence during pregnancy is approximately 3.3 times higher than that in nonpregnant women, which in turn is 2 to 4 times higher than that in men.224 Some studies have suggested an association with preeclampsia, which may be based on increased interstitial edema.225 Interestingly, Maloney226 proposed that the smile of the famed portrait “The Mona Lisa” was the result of Leonardo da Vinci’s anatomically precise rendering of a new mother affected by Bell’s palsy during her pregnancy.
One study noted that pregnant patients whose symptoms progressed to complete facial paralysis within 10 days of onset were less likely to experience satisfactory recovery than a comparison group of nonpregnant patients.227 Patients may benefit from a short course of prednisone.228
Dorsey and Camann229 retrospectively reviewed 36 cases of Bell’s palsy associated with pregnancy; 25 women experienced symptoms during the third trimester, and the remaining 11 had symptoms during the first week postpartum. Of the 36 women, 27 received spinal or epidural analgesia or anesthesia. There were no differences in incidence or progression of the Bell’s palsy or maternal and fetal outcomes in relation to the type of anesthesia given; therefore, neuraxial analgesia or anesthesia does not appear to be contraindicated in patients with Bell’s palsy.
Carpal Tunnel Syndrome
Carpal tunnel syndrome is common during pregnancy; in a systematic review, the reported incidence ranged from 0.8% to 70%.230 The disorder results from compression of the median nerve in the flexor retinaculum at the wrist. Patients typically report paresthesias and weakness in the median nerve distribution, with symptoms worse in the morning on awakening from sleep. Symptoms have been reported to persist in approximately 50% and 30% of patients after 1 year and 3 years, respectively.230 Patients may be treated with splinting of the wrists, although in severe cases, surgery may be required. In many cases, symptoms resolve spontaneously within the first 2 months postpartum and appear to correlate with losing the weight gained during pregnancy.231
Meralgia Paresthetica
Meralgia paresthetica involves sensory loss and paresthesias in the lateral thigh stemming from compression of the lateral femoral cutaneous nerve. Obesity and the exaggerated lordosis of pregnancy can stretch the nerve. Symptoms of meralgia paresthetica typically resolve within 3 months of delivery. This peripheral nerve palsy and other neurologic deficits are discussed more fully in Chapter 32.
References
1. Koch-Henriksen N, Sorensen PS. Why does the north-south gradient of incidence of multiple sclerosis seem to have disappeared on the northern hemisphere? J Neurol Sci. 2011;311:58–63.
2. Kurtzke JF. Patterns of neurologic involvement in multiple sclerosis. Neurology. 1989;39:1235–1238.
3. Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862.
4. Boskovic R, Wide R, Wolpin J, et al. The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology. 2005;65:807–811.
5. Achiron A, Kishner I, Dolev M, et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004;251:1133–1137.
6. Ferrero S, Esposito F, Pretta S, Ragni N. Fetal risks related to the treatment of multiple sclerosis during pregnancy and breastfeeding. Expert Rev Neurother. 2006;6:1823–1831.
7. Mueller BA, Zhang J, Critchlow CW. Birth outcomes and need for hospitalization after delivery among women with multiple sclerosis. Am J Obstet Gynecol. 2002;186:446–452.
8. Dahl J, Myhr KM, Daltveit AK, et al. Pregnancy, delivery, and birth outcome in women with multiple sclerosis. Neurology. 2005;65:1961–1963.
9. Finkelsztejn A, Brooks JB, Paschoal FM Jr, Fragoso YD. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG. 2011;118:790–797.
10. Vukusic S, Hutchinson M, Hours M, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127:1353–1360.
11. Roullet E, Verdier-Taillefer MH, Amarenco P, et al. Pregnancy and multiple sclerosis: a longitudinal study of 125 remittent patients. J Neurol Neurosurg Psychiatry. 1993;56:1062–1065.
12. Portaccio E, Ghezzi A, Hakiki B, et al. Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology. 2011;77:145–150.
13. Langer-Gould A, Huang SM, Gupta R, et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol. 2009;66:958–963.
14. Baskett PJ. Anaesthetics problems in multiple sclerosis: are certain agents contraindicated? Anaesthesia. 1970;25:397–401.
15. Bamford C, Sibley W, Laguna J. Anesthesia in multiple sclerosis. Can J Neurol Sci. 1978;5:41–44.
16. Tui A, Preiss A, Barcham I, Nevin M. Local nervous tissue changes following spinal anesthesia in experimental animals. J Pharmacol Exp Ther. 1944;81:209–217.
17. Schapira K. Is lumbar puncture harmful in multiple sclerosis? J Neurol Neurosurg Psychiatry. 1959;22:238.
18. Stenuit J, Marchand P. Sequelae of spinal anesthesia. Acta Neurol Psychiatr Belg. 1968;68:626–635.
19. Warren TM, Datta S, Ostheimer GW. Lumbar epidural anesthesia in a patient with multiple sclerosis. Anesth Analg. 1982;61:1022–1023.
20. Crawford J, James F, Nolte H, et al. Regional analgesia for patients with chronic neurological disease and similar conditions. Anaesthesia. 1981;36:821.
21. Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291.
22. Bader AM, Hunt CO, Datta S, et al. Anesthesia for the obstetric patient with multiple sclerosis. J Clin Anesth. 1988;1:21–24.
23. Berger JM, Ontell R. Intrathecal morphine in conjunction with a combined spinal and general anesthetic in a patient with multiple sclerosis. Anesthesiology. 1987;66:400–402.
24. Leigh J, Fearnley SJ, Lupprian KG. Intrathecal diamorphine during laparotomy in a patient with advanced multiple sclerosis. Anaesthesia. 1990;45:640–642.
25. Drake E, Drake M, Bird J, Russell R. Obstetric regional blocks for women with multiple sclerosis: a survey of UK experience. Int J Obstet Anesth. 2006;15:115–123.
26. Marcus DA. Headache in pregnancy. Curr Pain Headache Rep. 2003;7:288–296.
27. Stein G, Morton J, Marsh A, et al. Headaches after childbirth. Acta Neurol Scand. 1984;69:74–79.
29. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 9th edition. Lippincott Williams & Wilkins: Philadelphia; 2011.
30. Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry. 2013;35:3–8.
31. Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262.
32. Sanchez SE, Williams MA, Pacora PN. Risk of placental abruption in relation to migraines and headaches. BMC Women’s Health. 2010;10:30.
33. Silberstein S, Loder E, Diamond S, et al. Probable migraine in the United States: results of the American Migraine Prevalence and Prevention (AMPP) study. Cephalalgia. 2007;27:220–229.
34. Somerville BW. The influence of progesterone and estradiol upon migraine. Headache. 1972;12:93–102.
35. Chen TC, Leviton A. Headache recurrence in pregnant women with migraine. Headache. 1994;34:107–110.
36. Hoshiyama E, Tatsumoto M, Iwanami H, et al. Postpartum migraines: a long-term prospective study. Intern Med. 2012;51:3119–3123.
37. Nappi RE, Albani F, Sances G, et al. Headaches during pregnancy. Curr Pain Headache Rep. 2011;15:289–294.
38. Fox AW, Chambers CD, Anderson PO, et al. Evidence-based assessment of pregnancy outcome after sumatriptan exposure. Headache. 2002;42:8–15.
39. Marcoux S, Berube S, Brisson J, Fabia J. History of migraine and risk of pregnancy-induced hypertension. Epidemiology. 1992;3:53–56.
40. Rosene KA, Featherstone HJ, Benedetti TJ. Cerebral ischemia associated with parenteral terbutaline use in pregnant migraine patients. Am J Obstet Gynecol. 1982;143:405–407.
41. Hagen EM, Rekand T, Gilhus NE, Gronning M. Traumatic spinal cord injuries—incidence, mechanisms and course. Tidsskr Nor Laegeforen. 2012;132:831–837.
42. Pereira L. Obstetric management of the patient with spinal cord injury. Obstet Gynecol Surv. 2003;58:678–687.
43. Kang AH. Traumatic spinal cord injury. Clin Obstet Gynecol. 2005;48:67–72.
44. Marshall J. Observations on reflex changes in the lower limbs in spastic paraplegia in man. Brain. 1954;77:290–304.
45. Crosby E,St, Jean B, Reid D, Elliott RD. Obstetrical anaesthesia and analgesia in chronic spinal cord–injured women. Can J Anaesth. 1992;39:487–494.
46. Westgren N, Hultling C, Levi R, Westgren M. Pregnancy and delivery in women with a traumatic spinal cord injury in Sweden, 1980-1991. Obstet Gynecol. 1993;81:926–930.
47. American College of Obstetricians and Gynecologists. Obstetric management of patients with spinal cord injuries. [ACOG Committee Opinion No. 275. Washington, DC (Reaffirmed 2005)] Obstet Gynecol. 2002;100:625–627.
48. Hambly PR, Martin B. Anaesthesia for chronic spinal cord lesions. Anaesthesia. 1998;53:273–289.
49. Cross LL, Meythaler JM, Tuel SM, Cross AL. Pregnancy, labor and delivery post spinal cord injury. Paraplegia. 1992;30:890–902.
50. Stirt JA, Marco A, Conklin KA. Obstetric anesthesia for a quadriplegic patient with autonomic hyperreflexia. Anesthesiology. 1979;51:560–562.
51. Agostoni M, Giorgi E, Beccaria P, et al. Combined spinal-epidural anaesthesia for Caesarean section in a paraplegic woman: difficulty in obtaining the expected level of block. Eur J Anaesthesiol. 2000;17:329–331.
52. Kobayashi A, Mizobe T, Tojo H, Hashimoto S. Autonomic hyperreflexia during labour. Can J Anaesth. 1995;42:1134–1135.
53. Baraka A. Epidural meperidine for control of autonomic hyperreflexia in a paraplegic parturient. Anesthesiology. 1985;62:688–690.
54. Abouleish E, Hanley E, Palmer S. Can epidural fentanyl control autonomic hyperreflexia in a quadriplegic parturient? Anesth Analg. 1989;68:523–526.
55. Maehama T, Izena H, Kanazawa K. Management of autonomic hyperreflexia with magnesium sulfate during labor in a woman with spinal cord injury. Am J Obstet Gynecol. 2000;183:492–493.
56. Riazi S, Niazi AU, Tumber PS, Peng P. Confirmation of epidural catheter placement in a quadriplegic patient using a nerve stimulator. Can J Anaesth. 2010;57:276–277.
57. Erickson RP. Autonomic hyperreflexia: pathophysiology and medical management. Arch Phys Med Rehabil. 1980;61:431–440.
58. Ng K, Parsons J, Cyna AM, Middleton P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev. 2004;(2).
59. Ahmed AB, Bogod DG. Anaesthetic management of a quadriplegic patient with severe respiratory insufficiency undergoing caesarean section. Anaesthesia. 1996;51:1043–1045.
60. Stone WA, Beach TP, Hamelberg W. Succinylcholine—danger in the spinal-cord–injured patient. Anesthesiology. 1970;32:168–169.
61. Stafford IP, Dildy GA. Myasthenia gravis and pregnancy. Clin Obstet Gynecol. 2005;48:48–56.
62. Richman DP, Agius MA. Acquired myasthenia gravis: immunopathology. Neurol Clin. 1994;12:273–284.
63. Blichfeldt-Lauridsen L, Hansen BD. Anesthesia and myasthenia gravis. Acta Anaesthesiol Scand. 2012;56:17–22.
64. Hoff JM, Daltveit AK, Gilhus NE. Myasthenia gravis in pregnancy and birth: identifying risk factors, optimising care. Eur J Neurol. 2007;14:38–43.
65. Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810.
66. d’Empaire G, Hoaglin DC, Perlo VP, Pontoppidan H. Effect of prethymectomy plasma exchange on postoperative respiratory function in myasthenia gravis. J Thorac Cardiovasc Surg. 1985;89:592–596.
67. Barrons RW. Drug-induced neuromuscular blockade and myasthenia gravis. Pharmacotherapy. 1997;17:1220–1232.
68. Cohen BA, London RS, Goldstein PJ. Myasthenia gravis and preeclampsia. Obstet Gynecol. 1976;48:35S–37S.
69. Bashuk RG, Krendel DA. Myasthenia gravis presenting as weakness after magnesium administration. Muscle Nerve. 1990;13:708–712.
70. Catazanite VA, McHargue AM, Sandberg EC, Dyson DC. Respiratory arrest during therapy for premature labor in a patient with myasthenia gravis. Obstet Gynecol. 1984;64:819–822.
71. Plauché WC. Myasthenia gravis in mothers and their newborns. Clin Obstet Gynecol. 1991;34:82–99.
72. Ciafaloni E, Massey JM. The management of myasthenia gravis in pregnancy. Semin Neurol. 2004;24:95–100.
73. Daskalakis GJ, Papageorgiou IS, Petrogiannis ND, et al. Myasthenia gravis and pregnancy. Eur J Obstet Gynecol Reprod Biol. 2000;89:201–204.
74. Hatada Y, Munemura M, Matsuo I, et al. Myasthenic crisis in the puerperium: the possible importance of alpha-fetoprotein. Case report. Br J Obstet Gynaecol. 1987;94:480–482.
75. Watanabe A, Watanabe T, Obama T, et al. Prognostic factors for myasthenic crisis after transsternal thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg. 2004;127:868–876.
76. D’Angelo R, Gerancher JC. Combined spinal and epidural analgesia in a parturient with severe myasthenia gravis. Reg Anesth Pain Med. 1998;23:201–203.
77. Warren J, Sharma S. Ventilatory support using bilevel positive airway pressure during neuraxial blockade in a patient with severe respiratory compromise. Anesth Analg. 2006;102:910–911.
78. O’Flaherty D, Pennant JH, Rao K, Giesecke AH. Total intravenous anesthesia with propofol for transsternal thymectomy in myasthenia gravis. J Clin Anesth. 1992;4:241–244.
79. Naguib M, el Dawlatly AA, Ashour M, Bamgboye EA. Multivariate determinants of the need for postoperative ventilation in myasthenia gravis. Can J Anaesth. 1996;43:1006–1013.
80. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501.
81. Elger CE, Schmidt D. Modern management of epilepsy: a practical approach. Epilepsy Behav. 2008;12:501–539.
82. Schmidt D, Sillanpaa M. Evidence-based review on the natural history of the epilepsies. Curr Opin Neurol. 2012;25:159–163.
83. Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50:1237–1246.
85. Ramsay RE. Effect of hormones on seizure activity during pregnancy. J Clin Neurophysiol. 1987;4:23–25.
86. Yerby MS. Pregnancy and epilepsy. Epilepsia. 1991;32(Suppl 6):S51–S59.
87. Thomas SV. Management of epilepsy and pregnancy. J Postgrad Med. 2006;52:57–64.
88. Nau H, Kuhnz W, Egger HJ, et al. Anticonvulsants during pregnancy and lactation: transplacental, maternal and neonatal pharmacokinetics. Clin Pharmacokinet. 1982;7:508–543.
89. Harden CL, Pennell PB, Koppel BS, et al. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review): III. Vitamin K, folic acid, blood levels, and breast-feeding: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50:1247–1255.
90. Borthen I, Gilhus NE. Pregnancy complications in patients with epilepsy. Curr Opin Obstet Gynecol. 2012;24:78–83.
91. Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11:803–813.
92. Cunnington M, Tennis P. Lamotrigine and the risk of malformations in pregnancy. [International Lamotrigine Pregnancy Registry Scientific Advisory Committee] Neurology. 2005;64:955–960.
93. Rudzinski LA, Meador KJ. Epilepsy: five new things. Neurology. 2011;76:S20–S25.
94. Perks A, Cheema S, Mohanraj R. Anaesthesia and epilepsy. Br J Anaesth. 2012;108:562–571.
95. Aravapalli R, Abouleish E, Aldrete JA. Anesthetic implications in the parturient epileptic patient (abstract). Anesth Analg. 1988;67:266.
96. Manohar C, Avitsian R, Lozano S, et al. The effect of antiepileptic drugs on coagulation and bleeding in the perioperative period of epilepsy surgery: the Cleveland Clinic experience. J Clin Neurosci. 2011;18:1180–1184.
97. Modica PA, Tempelhoff R, White PF. Pro- and anticonvulsant effects of anesthetics (Part I). Anesth Analg. 1990;70:303–315.
98. Iijima T, Nakamura Z, Iwao Y, Sankawa H. The epileptogenic properties of the volatile anesthetics sevoflurane and isoflurane in patients with epilepsy. Anesth Analg. 2000;91:989–995.
99. Smith M, Smith SJ, Scott CA, Harkness WF. Activation of the electrocorticogram by propofol during surgery for epilepsy. Br J Anaesth. 1996;76:499–502.
100. Ornstein E, Matteo RS, Schwartz AE, et al. The effect of phenytoin on the magnitude and duration of neuromuscular block following atracurium or vecuronium. Anesthesiology. 1987;67:191–196.
101. Udd B, Krahe R. The myotonic dystrophies: molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891–905.
102. Modoni A, Silvestri G, Pomponi MG, et al. Characterization of the pattern of cognitive impairment in myotonic dystrophy type 1. Arch Neurol. 2004;61:1943–1947.
103. Heatwole CR, Moxley RT 3rd. The nondystrophic myotonias. Neurotherapeutics. 2007;4:238–251.
104. Jungbluth H. Central core disease. Orphanet J Rare Dis. 2007;2:25.
105. Klingler W, Rueffert H, Lehmann-Horn F, et al. Core myopathies and risk of malignant hyperthermia. Anesth Analg. 2009;109:1167–1173.
106. Awater C, Zerres K, Rudnik-Schoneborn S. Pregnancy course and outcome in women with hereditary neuromuscular disorders: comparison of obstetric risks in 178 patients. Eur J Obstet Gynecol Reprod Biol. 2012;162:153–159.
107. Argov Z, de Visser M. What we do not know about pregnancy in hereditary neuromuscular disorders. Neuromuscul Disord. 2009;19:675–679.
108. Catazanite V, Gambling D, Bird LM, et al. Respiratory compromise after MgSO4 therapy for preterm labor in a woman with myotonic dystrophy: a case report. J Reprod Med. 2008;53:220–222.
109. Arulkumaran S, Rauff M, Ingemarsson I, et al. Uterine activity in myotonia dystrophica: case report. Br J Obstet Gynaecol. 1986;93:634–636.
110. Blumgart CH, Hughes DG, Redfern N. Obstetric anaesthesia in dystrophia myotonica. Anaesthesia. 1990;45:26–29.
111. Gilchrist JM. Muscle disease in the pregnant woman. Adv Neurol. 1994;64:193–208.
112. Russell SH, Hirsch NP. Anaesthesia and myotonia. Br J Anaesth. 1994;72:210–216.
113. Camann WR, Johnson MD. Anesthetic management of a parturient with myotonia dystrophica: a case report. Reg Anesth. 1990;15:41–43.
114. Campbell AM, Thompson N. Anaesthesia for caesarean section in a patient with myotonic dystrophy receiving warfarin therapy. Can J Anaesth. 1995;42:409–414.
115. Cherng YG, Wang YP, Liu CC, et al. Combined spinal and epidural anesthesia for abdominal hysterectomy in a patient with myotonic dystrophy: case report. Reg Anesth. 1994;19:69–72.
116. Weingarten TN, Hofer RE, Milone M, Sprung J. Anesthesia and myotonic dystrophy type 2: a case series. Can J Anaesth. 2010;57:248–255.
117. Mathieu J, Allard P, Gobeil G, et al. Anesthetic and surgical complications in 219 cases of myotonic dystrophy. Neurology. 1997;49:1646–1650.
118. Owen PM, Chu C. Emergency caesarean section in a patient with myotonic dystrophy: a case of failed postoperative extubation in a patient with mild disease. Anaesth Intensive Care. 2011;39:293–298.
119. Paterson IS. Generalized myotonia following suxamethonium: a case report. Br J Anaesth. 1962;34:340–342.
120. Parness J, Bandschapp O, Girard T. The myotonias and susceptibility to malignant hyperthermia. Anesth Analg. 2009;109:1054–1064.
121. O’Neill GN. Inherited disorders of the neuromuscular junction. Int Anesthesiol Clin. 2006;44:91–106.
122. Ciafaloni E, Pressman EK, Loi AM, et al. Pregnancy and birth outcomes in women with facioscapulohumeral muscular dystrophy. Neurology. 2006;67:1887–1889.
123. Rudnik-Schoneborn S, Glauner B, Rohrig D, Zerres K. Obstetric aspects in women with facioscapulohumeral muscular dystrophy, limb-girdle muscular dystrophy, and congenital myopathies. Arch Neurol. 1997;54:888–894.
124. Allen T, Maguire S. Anaesthetic management of a woman with autosomal recessive limb-girdle muscular dystrophy for emergency caesarean section. Int J Obstet Anesth. 2007;16:370–374.
125. Gamzu R, Shenhav M, Fainaru O, et al. Impact of pregnancy on respiratory capacity in women with muscular dystrophy and kyphoscoliosis: a case report. J Reprod Med. 2002;47:53–56.
126. Molyneux MK. Anaesthetic management during labour of a manifesting carrier of Duchenne muscular dystrophy. Int J Obstet Anesth. 2005;14:58–61.
127. Gurnaney H, Brown A, Litman RS. Malignant hyperthermia and muscular dystrophies. Anesth Analg. 2009;109:1043–1048.
128. Lin DD, Barker PB. Neuroimaging of phakomatoses. Semin Pediatr Neurol. 2006;13:48–62.
129. Galan SR, Kann PH. Genetics and molecular pathogenesis of pheochromocytoma and paraganglioma. Clin Endocrinol (Oxf). 2013;78:165–175.
130. Dugoff L, Sujansky E. Neurofibromatosis type 1 and pregnancy. Am J Med Genet. 1996;66:7–10.
131. Hirsch NP, Murphy A, Radcliffe JJ. Neurofibromatosis: clinical presentations and anaesthetic implications. Br J Anaesth. 2001;86:555–564.
132. Spiegel JE, Hapgood A, Hess PE. Epidural anesthesia in a parturient with neurofibromatosis type 2 undergoing cesarean section. Int J Obstet Anesth. 2005;14:336–339.
133. Richardson MG, Setty GK, Rawoof SA. Responses to nondepolarizing neuromuscular blockers and succinylcholine in von Recklinghausen neurofibromatosis. Anesth Analg. 1996;82:382–385.
134. Mitterschiffthaler G, Maurhard U, Huter O, Brezinka C. Prolonged action of vecuronium in neurofibromatosis (von Recklinghausen’s disease). Anaesthesiol Reanim. 1989;14:175–178.
136. Kohrman MH. Emerging treatments in the management of tuberous sclerosis complex. Pediatr Neurol. 2012;46:267–275.
137. Petrikovsky BM, Vintzileos AM, Cassidy SB, Egan JF. Tuberous sclerosis in pregnancy. Am J Perinatol. 1990;7:133–135.
138. Forsnes EV, Eggleston MK, Burtman M. Placental abruption and spontaneous rupture of renal angiomyolipoma in a pregnant woman with tuberous sclerosis. Obstet Gynecol. 1996;88:725.
139. Causse-Mariscal A, Palot M, Visseaux H, et al. Labor analgesia and cesarean section in women affected by tuberous sclerosis: report of two cases. Int J Obstet Anesth. 2007;16:277–280.
140. Ropper AH, Samuels MA. Adam’s and Victor’s Principles of Neurology. 9th edition. McGraw-Hill: New York; 2009.
141. Ogasawara KK, Ogasawara EM, Hirata G. Pregnancy complicated by von Hippel-Lindau disease. Obstet Gynecol. 1995;85:829–831.
142. Carta G, De Lellis V, Di Nicola M, Kaliakoudas D. Peripartum cardiomyopathy and Klippel-Trenaunay syndrome. Clin Exp Obstet Gynecol. 2010;37:155–157.
143. Douglas MR, Winer JB. Guillain-Barré syndrome and its treatment. Expert Rev Neurother. 2006;6:1569–1574.
144. Gautier PE, Hantson P, Vekemans MC, et al. Intensive care management of Guillain-Barré syndrome during pregnancy. Intensive Care Med. 1990;16:460–462.
145. Jiang GX, de Pedro-Cuesta J, Strigard K, et al. Pregnancy and Guillain-Barré syndrome: a nationwide register cohort study. Neuroepidemiology. 1996;15:192–200.
146. Rockel A, Wissel J, Rolfs A. Guillain-Barré syndrome in pregnancy—an indication for caesarian section? J Perinat Med. 1994;22:393–398.
147. Wipfli M, Arnold M, Luginbuhl M. Repeated spinal anesthesia in a tetraparetic patient with Guillain-Barre syndrome. J Clin Anesth. 2013;pii.
148. Wiertlewski S, Magot A, Drapier S, et al. Worsening of neurologic symptoms after epidural anesthesia for labor in a Guillain-Barré patient. Anesth Analg. 2004;98:825–827.
149. Vassiliev DV, Nystrom EU, Leicht CH. Combined spinal and epidural anesthesia for labor and cesarean delivery in a patient with Guillain-Barré syndrome. Reg Anesth Pain Med. 2001;26:174–176.
150. Steiner I, Argov Z, Cahan C, Abramsky O. Guillain-Barré syndrome after epidural anesthesia: direct nerve root damage may trigger disease. Neurology. 1985;35:1473–1475.
151. Sibert KS, Sladen RN. Impaired ventilatory capacity after recovery from Guillain-Barré syndrome. J Clin Anesth. 1994;6:133–138.
152. Lambert DA, Giannouli E, Schmidt BJ. Postpolio syndrome and anesthesia. Anesthesiology. 2005;103:638–644.
153. Klingman J, Chui H, Corgiat M, Perry J. Functional recovery: a major risk factor for the development of postpoliomyelitis muscular atrophy. Arch Neurol. 1988;45:645–647.
154. Sharief MK, Hentges R, Ciardi M. Intrathecal immune response in patients with the post-polio syndrome. N Engl J Med. 1991;325:749–755.
155. Bhutta ZA. The last mile in global poliomyelitis eradication. Lancet. 2011;378:549–552.
156. Harjulehto-Mervaala T, Aro T, Hiilesmaa VK, et al. Oral polio vaccination during pregnancy: lack of impact on fetal development and perinatal outcome. Clin Infect Dis. 1994;18:414–420.
157. Daw E, Chandler G. Pregnancy following poliomyelitis. Postgrad Med J. 1976;52:492–496.
158. Veiby G, Daltveit AK, Gilhus NE. Pregnancy, delivery and perinatal outcome in female survivors of polio. J Neurol Sci. 2007;258:27–32.
159. Rezende DP, Rodrigues MR, Costa VV, et al. Patients with sequelae of poliomyelitis: does the anesthetic technique impose risks? Rev Bras Anestesiol. 2008;58:210–219.
160. Costello JF, Balki M. Cesarean delivery under ultrasound-guided spinal anesthesia [corrected] in a parturient with poliomyelitis and Harrington instrumentation. Can J Anaesth. 2008;55:606–611.
161. Chopra S, Adhikari K, Agarwal N, et al. Kyphoscoliosis complicating pregnancy: maternal and neonatal outcome. Arch Gynecol Obstet. 2011;284:295–297.
162. Suneel PR, Sinha PK, Unnikrishnan KP, Abraham M. Anesthesia for craniotomy in a patient with previous paralytic polio. J Clin Anesth. 2008;20:210–213.
163. Connelly N, Abbott T. Successful use of succinylcholine for cesarean delivery in a patient with postpolio syndrome. Anesthesiology. 2008;108:1151–1152.
164. Swensen R, Kirsch W. Brain neoplasms in women: a review. Clin Obstet Gynecol. 2002;45:904–927.
165. Stevenson CB, Thompson RC. The clinical management of intracranial neoplasms in pregnancy. Clin Obstet Gynecol. 2005;48:24–37.
166. Soper JT, Spillman M, Sampson JH, et al. High-risk gestational trophoblastic neoplasia with brain metastases: individualized multidisciplinary therapy in the management of four patients. Gynecol Oncol. 2007;104:691–694.
167. Choi NW, Schuman LM, Gullen WH. Epidemiology of primary central nervous system neoplasms: II. Case-control study. Am J Epidemiol. 1970;91:467–485.
168. Terry AR, Barker FG 2nd, Leffert L, et al. Outcomes of hospitalization in pregnant women with CNS neoplasms: a population-based study. Neuro Oncol. 2012;14:768–776.
169. Enoksson P, Lundberg N, Sjostedt S, Skanse B. Influence of pregnancy on visual fields in suprasellar tumors. Acta Psychiatr Scand. 1961;36:524–538.
170. Isla A, Alvarez F, Gonzalez A, et al. Brain tumor and pregnancy. Obstet Gynecol. 1997;89:19–23.
171. Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447–1474.
172. Marx GF, Zemaitis MT, Orkin LR. Cerebrospinal fluid pressures during labor and obstetrical anesthesia. Anesthesiology. 1961;22:348–354.
173. Finfer SR. Management of labour and delivery in patients with intracranial neoplasms. Br J Anaesth. 1991;67:784–787.
174. Kepes ER, Andrews IC, Radnay PA, et al. Conduct of anesthesia for delivery with grossly raised cerebrospinal fluid pressure. N Y State J Med. 1972;72:115–156.
175. Goroszeniuk T, Howard RS, Wright JT. The management of labour using continuous lumbar epidural analgesia in a patient with a malignant cerebral tumour. Anaesthesia. 1986;41:1128–1129.
176. Atanassoff PG, Alon E, Weiss BM, Lauper U. Spinal anaesthesia for caesarean section in a patient with brain neoplasm. Can J Anaesth. 1994;41:163–164.
177. Su TM, Lan CM, Yang LC, et al. Brain tumor presenting with fatal herniation following delivery under epidural anesthesia. Anesthesiology. 2002;96:508–509.
178. Chang L, Looi-Lyons L, Bartosik L, Tindal S. Anesthesia for cesarean section in two patients with brain tumours. Can J Anaesth. 1999;46:61–65.
179. Wang LP, Paech MJ. Neuroanesthesia for the pregnant woman. Anesth Analg. 2008;107:193–200.
180. Burns PD, Linder RO, Drose VE, Battaglia F. The placental transfer of water from fetus to mother following the intravenous infusion of hypertonic mannitol to the maternal rabbit. Am J Obstet Gynecol. 1963;86:160–167.
181. Lumbers ER, Stevens AD. Changes in fetal renal function in response to infusions of a hyperosmotic solution of mannitol to the ewe. J Physiol. 1983;343:439–446.
182. French JL, McCullough J, Bachra P, Bedforth NM. Transversus abdominis plane block for analgesia after caesarean section in a patient with an intracranial lesion. Int J Obstet Anesth. 2009;18:52–54.
183. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495.
184. Koontz WL, Herbert WN, Cefalo RC. Pseudotumor cerebri in pregnancy. Obstet Gynecol. 1983;62:324–327.
185. Bagga R, Jain V, Das CP, et al. Choice of therapy and mode of delivery in idiopathic intracranial hypertension during pregnancy. Med Gen Med. 2005;7:42.
186. Digre KB, Varner MW, Corbett JJ. Pseudotumor cerebri and pregnancy. Neurology. 1984;34:721–729.
187. Paruchuri SR, Lawlor M, Kleinhomer K, et al. Risk of cerebellar tonsillar herniation after diagnostic lumbar puncture in pseudotumor cerebri. Anesth Analg. 1993;77:403–404.
188. Karmaniolou I, Petropoulos G, Theodoraki K. Management of idiopathic intracranial hypertension in parturients: anesthetic considerations. Can J Anaesth. 2011;58:650–657.
190. Kaul B, Vallejo MC, Ramanathan S, et al. Accidental spinal analgesia in the presence of a lumboperitoneal shunt in an obese parturient receiving enoxaparin therapy. Anesth Analg. 2002;95:441–443.
191. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anaesthesia. 2007;62:178–181.
192. Heckathorn J, Cata JP, Barsoum S. Intrathecal anesthesia for cesarean delivery via a subarachnoid drain in a woman with benign intracranial hypertension. Int J Obstet Anesth. 2010;19:109–111.
193. Paciorkowski AR, Greenstein RM. When is enlargement of the subarachnoid spaces not benign? A genetic perspective. Pediatr Neurol. 2007;37:1–7.
194. Liakos AM, Bradley NK, Magram G, Muszynski C. Hydrocephalus and the reproductive health of women: the medical implications of maternal shunt dependency in 70 women and 138 pregnancies. Neurol Res. 2000;22:69–88.
195. Wisoff JH, Kratzert KJ, Handwerker SM, et al. Pregnancy in patients with cerebrospinal fluid shunts: report of a series and review of the literature. Neurosurgery. 1991;29:827–831.
196. Ng J, Kitchen N. Neurosurgery and pregnancy. J Neurol Neurosurg Psychiatry. 2008;79:745–752.
197. Bateman BT, Olbrecht VA, Berman MF, et al. Peripartum subarachnoid hemorrhage: nationwide data and institutional experience. Anesthesiology. 2012;116:324–333.
198. Kim YW, Neal D, Hoh BL. Cerebral aneurysms in pregnancy and delivery: pregnancy and delivery do not increase the risk of aneurysm rupture. Neurosurgery. 2013;72:143–150.
199. Dias MS, Sekhar LN. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery. 1990;27:855–866.
200. Tarnaris A, Haliasos N, Watkins LD. Endovascular treatment of ruptured intracranial aneurysms during pregnancy: is this the best way forward? Case report and review of the literature. Clin Neurol Neurosurg. 2012;114:703–706.
201. Bederson JB, Connolly ES Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
202. Marshman LA, Aspoas AR, Rai MS, Chawda SJ. The implications of ISAT and ISUIA for the management of cerebral aneurysms during pregnancy. Neurosurg Rev. 2007;30:177–180.
203. Piotin M, de Souza Filho CB, Kothimbakam R, Moret J. Endovascular treatment of acutely ruptured intracranial aneurysms in pregnancy. Am J Obstet Gynecol. 2001;185:1261–1262.
204. Trivedi RA, Kirkpatrick PJ. Arteriovenous malformations of the cerebral circulation that rupture in pregnancy. J Obstet Gynaecol. 2003;23:484–489.
205. Le LT, Wendling A. Anesthetic management for cesarean section in a patient with rupture of a cerebellar arteriovenous malformation. J Clin Anesth. 2009;21:143–148.
206. Powner DJ, Bernstein IM. Extended somatic support for pregnant women after brain death. Crit Care Med. 2003;31:1241–1249.
207. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352:1791–1798.
208. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192.
209. Wilder-Smith E, Kothbauer-Margreiter I, Lammle B, et al. Dural puncture and activated protein C resistance: risk factors for cerebral venous sinus thrombosis. J Neurol Neurosurg Psychiatry. 1997;63:351–356.
210. Lockhart EM, Baysinger CL. Intracranial venous thrombosis in the parturient. Anesthesiology. 2007;107:652–658.
211. Leveck DE, Davies GA. Rapid progression of amyotrophic lateral sclerosis presenting during pregnancy: a case report. J Obstet Gynaecol Can. 2005;27:360–362.
212. Jacka MJ, Sanderson F. Amyotrophic lateral sclerosis presenting during pregnancy. Anesth Analg. 1998;86:542–543.
213. Hara K, Sakura S, Saito Y, et al. Epidural anesthesia and pulmonary function in a patient with amyotrophic lateral sclerosis. Anesth Analg. 1996;83:878–879.
214. Kochi T, Oka T, Mizuguchi T. Epidural anesthesia for patients with amyotrophic lateral sclerosis. Anesth Analg. 1989;68:410–412.
215. Rosenbaum KJ, Neigh JL, Strobel GE. Sensitivity to nondepolarizing muscle relaxants in amyotrophic lateral sclerosis: report of two cases. Anesthesiology. 1971;35:638–641.
216. Sarafov S, Doitchinova M, Karagiozova Z, et al. Two consecutive pregnancies in early and late stage of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:483–486.
217. Pugh CP, Healey SK, Crane JM, Young D. Successful pregnancy and spinal muscular atrophy. Obstet Gynecol. 2000;95:1034.
218. Weston LA, DiFazio CA. Labor analgesia and anesthesia in a patient with spinal muscular atrophy and vocal cord paralysis: a rare and unusual case report. Reg Anesth. 1996;21:350–354.
219. Harris SJ, Moaz K. Caesarean section conducted under subarachnoid block in two sisters with spinal muscular atrophy. Int J Obstet Anesth. 2002;11:125–127.
220. Graham RJ, Athiraman U, Laubach AE, Sethna NF. Anesthesia and perioperative medical management of children with spinal muscular atrophy. Paediatr Anaesth. 2009;19:1054–1063.
221. Aboussouan LS, Lewis RA, Shy ME. Disorders of pulmonary function, sleep, and the upper airway in Charcot-Marie-Tooth disease. Lung. 2007;185:1–7.
222. Hoff JM, Gilhus NE, Daltveit AK. Pregnancies and deliveries in patients with Charcot-Marie-Tooth disease. Neurology. 2005;64:459–462.
223. Greenwood JJ, Scott WE. Charcot-Marie-Tooth disease: peripartum management of two contrasting clinical cases. Int J Obstet Anesth. 2007;16:149–154.
224. Cohen Y, Lavie O, Granovsky-Grisaru S, et al. Bell palsy complicating pregnancy: a review. Obstet Gynecol Surv. 2000;55:184–188.
225. Shmorgun D, Chan WS, Ray JG. Association between Bell’s palsy in pregnancy and pre-eclampsia. QJM. 2002;95:359–362.
226. Maloney WJ. Bell’s palsy: the answer to the riddle of Leonardo da Vinci’s ‘Mona Lisa. J Dent Res. 2011;90:580–582.
227. Gillman GS, Schaitkin BM, May M, Klein SR. Bell’s palsy in pregnancy: a study of recovery outcomes. Otolaryngol Head Neck Surg. 2002;126:26–30.
228. Worster A, Keim SM, Sahsi R, Pancioli AM. Do either corticosteroids or antiviral agents reduce the risk of long-term facial paresis in patients with new-onset Bell’s palsy? J Emerg Med. 2010;38:518–523.
229. Dorsey DL, Camann WR. Obstetric anesthesia in patients with idiopathic facial paralysis (Bell’s palsy): a 10-year survey. Anesth Analg. 1993;77:81–83.
230. Padua L, Di Pasquale A, Pazzaglia C, et al. Systematic review of pregnancy-related carpal tunnel syndrome. Muscle Nerve. 2010;42:697–702.
231. Finsen V, Zeitlmann H. Carpal tunnel syndrome during pregnancy. Scand J Plast Reconstr Surg Hand Surg. 2006;40:41–45.