Neonatal Assessment and Resuscitation
Susan W. Aucott MD
Chapter Outline
The transition from intrauterine to extrauterine life represents the most important adjustment that a neonate will make. Occurring uneventfully after most deliveries, this transition is dependent on the anatomic and physiologic condition of the infant, the ease or difficulty of the delivery, and the extrauterine environmental conditions. When the transition is unsuccessful, prompt assessment and supportive care must be initiated immediately.
At least one person skilled in neonatal resuscitation should be present at every delivery.1 The resuscitation team may include personnel from the pediatrics, anesthesiology, obstetrics, respiratory therapy, and nursing services. The composition of the team varies among institutions, but some form of 24-hour coverage should be present within all hospitals that provide labor and delivery services.1 A multidisciplinary team should participate in the process of ensuring that appropriate personnel and equipment are available for neonatal resuscitation.1
All personnel working in the delivery area should receive basic training in neonatal resuscitation to ensure prompt initiation of care before the arrival of the designated resuscitation team. The 2010 American Heart Association (AHA) Conference on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care led to the publication of updated guidelines for neonatal resuscitation.2 Changes in these guidelines reflected a review of scientific evidence by members of the American Academy of Pediatrics (AAP), the AHA, and the International Liaison Committee on Resuscitation. These guidelines have been incorporated into the Neonatal Resuscitation Program (NRP), which is the standardized training and certification program administered by the AAP. The NRP, which was originally sponsored by the AAP and the AHA in 1987, is designed to be appropriate for all personnel who attend deliveries. To ensure the implementation of current guidelines for neonatal resuscitation, the AAP recommends that at least one NRP-certified practitioner attend every delivery.3,4
Both the American Society of Anesthesiologists (ASA) and the American College of Obstetricians and Gynecologists (ACOG) have published specific goals and guidelines for neonatal resuscitation (Box 9-1).5 The ASA has emphasized that a single anesthesiologist should not be expected to assume responsibility for the concurrent care of both the mother and her child. Rather, a second anesthesia provider or a qualified individual from another service should assume responsibility for the care of the neonate, except in an unforeseen emergency.
In clinical practice, anesthesiologists often are involved in neonatal resuscitation.6,7 Heyman et al.7 observed that anesthesia personnel were involved in neonatal resuscitation in 99 (31%) of 320 selected Midwestern community hospitals. In 13.4% of these hospitals, the individual who administered anesthesia to the mother was also responsible for the care of the neonate; in 6.8% of these institutions, a second anesthesia provider typically assumed primary responsibility for the neonate. In a larger survey of obstetric anesthesia workforce patterns within the United States, Bucklin et al.6 found that fewer anesthesiologists were involved in neonatal resuscitation in 2001 than in 1981, with this practice occurring in less than 5% of cesarean deliveries.
Despite this relatively low incidence of primary involvement, the anesthesiologist is often asked to provide assistance in cases of difficult airway management or when members of the neonatal resuscitation team have not yet arrived. The anesthesiologist should be prepared to provide assistance, provided that such care does not compromise the care of the mother. A study of University of Pennsylvania anesthesiology residency program graduates from 1989 to 1999 revealed that, despite a desire to be certified in neonatal resuscitation, most anesthesiologists were not.8
In a 1991 review of the ASA Closed-Claims Database, 13% of obstetric anesthesia malpractice claims were related to neonatal resuscitation, including delayed or failed tracheal intubation and an unrecognized esophageal intubation.9 Another review of obstetric anesthesia–related lawsuits from 1985 to 1993 demonstrated that 12 (17%) of the 69 cases involved claims of inadequate neonatal resuscitation by anesthesia personnel7; 10 of these 12 cases resulted in payment to the plaintiff. Written hospital policies should identify the personnel responsible for neonatal resuscitation; obstetric anesthesia providers should also maintain a high level of skill in neonatal resuscitation.
Transition From Intrauterine to Extrauterine Life
Circulation
At birth, the circulatory system changes from a fetal circulation pattern (which is in parallel), through a transitional circulation, to an adult circulation pattern (which is in series) (Figure 9-1).10 In the fetus, blood from the placenta travels through the umbilical vein and the ductus venosus to the inferior vena cava and the right side of the heart. The anatomic orientation of the inferior vena caval–right atrial junction favors the shunting (i.e., streaming) of this well-oxygenated blood through the foramen ovale to the left side of the heart. This well-oxygenated blood is pumped through the ascending aorta, where branches that perfuse the upper part of the body (e.g., heart, brain) exit proximal to the entrance of the ductus arteriosus.11 Desaturated blood returns to the heart from the upper part of the body by means of the superior vena cava. The anatomic orientation of the superior vena caval–right atrial junction favors the streaming of blood into the right ventricle. Because fetal pulmonary vascular resistance is higher than systemic vascular resistance (SVR), approximately 90% of the right ventricular output passes through the ductus arteriosus and enters the aorta distal to the branches of the ascending aorta and aortic arch; therefore, less well-oxygenated blood perfuses the lower body, which consumes less oxygen than the heart and brain.
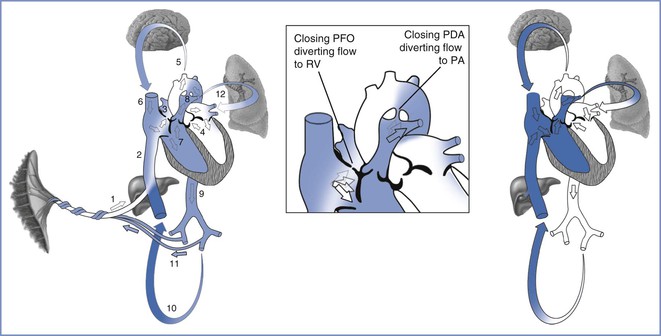
FIGURE 9-1 Modification of blood flow patterns from the fetal (left), via the transitional (center), to the neonatal (right) circulation. In the fetal circulation, oxygenated blood (white) from the placenta travels through the umbilical vein (1) into the ductus venosus and the inferior vena cava (2). The majority of oxygenated blood passes through the patent foramen ovale (PFO) from the right atrium to the left atrium (3) and ventricle (4), and distributes this blood to the brain (5). The deoxygenated blood (blue) from the brain and upper extremities enters the superior vena cava, mixing with a small portion of the oxygenated blood in the right atrium, before entering the right ventricle (RV, 7). The mostly deoxygenated blood is transported into the pulmonary artery where the majority is diverted through the patent ductus arteriosus (PDA, 8) into the descending aorta (9), thereby bypassing the lungs. Some blood enters the lower body (10), but the majority returns to the placenta via the umbilical arteries (11). A small amount of blood from the pulmonary artery enters the lungs (12). During the transitional circulation, which occurs over a few days, the PFO closes, diverting blood from the right atrium to the right ventricle. Closure of the PDA diverts deoxygenated blood through the pulmonary arteries to the lungs. The neonatal circulation separates the oxygenated and deoxygenated blood flow pathways. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
At the time of birth and during the resulting circulatory transition, the amount of blood that shunts through the foramen ovale and ductus arteriosus diminishes and the flow becomes bidirectional. Clamping the umbilical cord (or exposing the umbilical cord to room air) results in increased SVR. Meanwhile, expansion of the lungs and increased alveolar oxygen tension and pH result in decreased pulmonary vascular resistance and subsequently greater flow of pulmonary artery blood through the lungs.12,13 Increased pulmonary artery blood flow results in improved oxygenation and higher left atrial pressure; the latter leads to a diminished shunt across the foramen ovale. Increased PaO2 and SVR and decreased pulmonary vascular resistance result in a constriction of the ductus arteriosus.14,15 Together, these changes in vascular resistance result in functional closure of the foramen ovale and the ductus arteriosus. This process does not occur instantaneously, and arterial oxygen saturation (SaO2) remains higher in the right upper extremity (which is preductal) than in the left upper extremity and the lower extremities until blood flow through the ductus arteriosus is minimal.16 Differences in SaO2 are usually minimal by 10 minutes and absent by 24 hours after birth. Provided that there is no interference with the normal drop in pulmonary vascular resistance, both the foramen ovale and the ductus arteriosus close functionally, and the infant develops an adult circulation (which is in series).
Persistent fetal circulation—more correctly called persistent pulmonary hypertension of the newborn—can occur when the pulmonary vascular resistance remains elevated at the time of birth. Factors that may contribute to this problem include hypoxia, acidosis, hypovolemia, and hypothermia.13,17 Maternal use of nonsteroidal anti-inflammatory drugs may also cause premature constriction of the ductus arteriosus in the fetus and thus predispose to persistent pulmonary hypertension of the newborn.18
Respiration
Fetal breathing movements have been observed in utero as early as 11 weeks’ gestation. These movements increase with advancing gestational age but undergo a marked reduction within days of the onset of labor. They are stimulated by hypercapnia and maternal smoking and are inhibited by hypoxia and central nervous system (CNS) depressants (e.g., barbiturates). Under normal conditions, this fetal breathing activity results only in the movement of pulmonary dead space.19
The fetal lung contains a liquid composed of an ultrafiltrate of plasma, which is secreted by the lungs in utero20; the volume of this lung liquid is approximately 30 mL/kg. Partial reabsorption of this liquid occurs during labor and delivery, and approximately two thirds is expelled from the lungs of the term neonate by the time of delivery.21 Small preterm infants and those requiring cesarean delivery may have a greater amount of residual lung liquid after delivery. These infants experience less chest compression at delivery than infants who are larger or delivered vaginally; this difference can lead to difficulty in the initiation and maintenance of a normal breathing pattern. Retained fetal lung liquid is the presumed cause of transient tachypnea of the newborn (TTN).22
The first breath occurs approximately 9 seconds after delivery. Air enters the lungs as soon as the intrathoracic pressure begins to fall. This air movement during the first breath is important, because it establishes the neonate’s functional residual capacity (Figure 9-2).
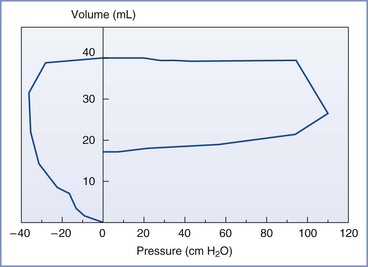
FIGURE 9-2 Typical pressure-volume loop of the first breath. The intrathoracic pressure falls to −30 to −40 cm H2O, drawing air into the lungs. The expiratory pressure is much greater than the inspiratory pressure. (Modified from Milner AD, Vyas H. Lung expansion at birth. J Pediatr 1982; 101:881.)
Lung inflation is a major physiologic stimulus for the release of lung surfactant into the alveoli.23 Surfactant, which is necessary for normal breathing, is present within the alveolar lining cells by 20 weeks’ gestation24 and within the lumen of the airways by 28 to 32 weeks’ gestation. However, significant amounts of surfactant do not appear in terminal airways until 34 to 38 weeks’ gestation unless surfactant production has been stimulated by chronic stress or maternal corticosteroid administration.25
Stress during labor and delivery can lead to gasping efforts by the fetus, which may result in the inhalation of amniotic fluid into the lungs.26 This event can produce problems if the stress causes the fetus to pass meconium into the amniotic fluid before gasping.
Catecholamines
Transition to extrauterine life is associated with a catecholamine surge, which may be necessary for the process to be successful. In chronically catheterized sheep, catecholamine levels begin to rise a few hours before delivery and may be higher at the time of delivery than at any other time during life.27 Catecholamines have an important role in the following areas: (1) the production and release of surfactant, (2) the mediation of preferential blood flow to vital organs during the period of stress that occurs during every delivery, and (3) thermoregulation of the neonate.
Thermal Regulation
Thermal stress challenges the neonate in the extrauterine environment. Neonates raise their metabolic rates and release norepinephrine in response to cold; this response facilitates the oxidation of brown fat, which contains numerous mitochondria. The oxidation results in nonshivering thermogenesis, the major mechanism for neonatal heat regulation.28 This process may lead to significant oxygen consumption, especially if the neonate has not been dried off and kept in an appropriate thermoneutral environment, such as a radiant warmer. Thermal stress is an even greater problem in infants with low fat stores, such as preterm infants or infants who are small for gestational age. An alternative method to eliminate heat loss from evaporation is to provide an occlusive wrap rather than drying the infant. For infants born at less than 28 weeks’ gestation, the use of polythene wraps or bags is recommended to minimize heat loss.29,30 The maintenance of a neutral thermal environment (i.e., 34° to 35° C) is recommended. However, in the neonate with a perinatal brain injury, mild hypothermia therapy through selective head or whole body cooling is initiated in the first 6 hours of life and may be neuroprotective in the setting of hypoxia-ischemia.31,32 Hyperthermia may worsen neurologic outcomes and should be avoided.2,33 Hypothermia therapy, via selective head cooling or whole body hypothermia, is continued for 72 hours after initiation. Consequently, if an infant is delivered at a center where hypothermia therapy is unavailable, passive cooling can be initiated by turning the radiant warmer off while awaiting infant transfer.
Administration of epidural analgesia during labor is associated with an increase in maternal and fetal temperature.34 Concern has been expressed that the temperature elevation associated with intrapartum epidural analgesia might result in an increase in the frequency of neonatal sepsis evaluations.34,35 However, a number of variables (e.g., preeclampsia/hypertension, gestational age, birth weight, meconium aspiration, respiratory distress at birth, hypothermia at birth, and group B beta-hemolytic streptococcal colonization of the maternal birth canal) have been observed to be strong predictors of the performance of neonatal sepsis evaluations, whereas maternal fever and epidural analgesia have not.36 Confounding variables may influence the findings of these types of association studies; patients who choose either to receive or not receive epidural analgesia may be inherently different. The incidence of actual neonatal sepsis is not different in term infants whose mothers either did or did not receive epidural analgesia.
Antenatal Assessment
Approximately 10% of neonates require some level of resuscitation.2 The need for resuscitation can be predicted before labor and delivery with approximately 80% accuracy on the basis of a number of antepartum factors (Box 9-2).
Preterm delivery increases the likelihood that the neonate will require resuscitation. When a mother is admitted with either preterm labor or premature rupture of membranes, plans should be made for neonatal care in the event of delivery. The antenatal assessment of gestational age is based on the presumed date of the last menstrual period, the fundal height, and ultrasonographic measurements of the fetus. Unfortunately, it may be difficult to assess gestational age accurately, because menstrual dates may be unknown or incorrect, the fundal height may be affected by abnormalities of fetal growth or amniotic fluid volume, and ultrasonographic assessment of fetal age is less precise after mid pregnancy. The assessment of gestational age is most accurate in patients who receive prenatal care in early pregnancy. An accurate approximation of gestational age enables the health care team to plan for the needs of the neonate and to counsel the parents regarding neonatal morbidity and mortality. These plans and expectations must be formulated with caution and flexibility, because the antenatal assessment may not accurately predict neonatal size, maturity, and/or condition at delivery.
A variety of intrauterine insults can impair the fetal transition to extrauterine life. For example, neonatal depression at birth can result from acute or chronic uteroplacental insufficiency or acute umbilical cord compression. Chronic uteroplacental insufficiency, regardless of its etiology, may result in fetal growth restriction. Fetal hemorrhage, viral or bacterial infection, meconium aspiration, and exposure to opioids or other CNS depressants also can result in neonatal depression. Although randomized trials have not confirmed that fetal heart rate (FHR) monitoring improves neonatal outcome, a nonreassuring FHR tracing is considered a predictor of the need for neonatal resuscitation.37
Studies have evaluated the use of fetal pulse oximetry for the evaluation of fetal well-being during labor. This technique involves the transcervical insertion of a flexible fetal oxygen sensor until it rests against the fetal cheek. A randomized trial found that use of the fetal pulse oximeter in conjunction with FHR monitoring led to a reduction in the number of cesarean deliveries performed due to a nonreassuring FHR tracing.38 However, this decrease was offset by an increased number of cesarean deliveries performed due to dystocia, raising the concern that the presence of the probe might predispose to dystocia. As a consequence, the ACOG has recommended further study before fetal pulse oximetry is used routinely in clinical practice.39 A meta-analysis of five trials concluded that there was some benefit to fetal pulse oximetry in the presence of a nonreassuring FHR tracing, but the use of fetal pulse oximetry did not lead to an overall reduction in the cesarean delivery rate.40
Infants with congenital anomalies (e.g., tracheoesophageal fistula, diaphragmatic hernia, CNS and cardiac malformations) may need resuscitation and cardiorespiratory support. Improved ultrasonography allows for the antenatal diagnosis of many congenital anomalies and other fetal abnormalities (e.g., nonimmune hydrops). Obstetricians should communicate knowledge or suspicions regarding these entities to those who will provide care for the neonate in the delivery room to allow the resuscitation team to make specific resuscitation plans.
In the past, infants born by either elective or emergency cesarean delivery were considered more likely to require resuscitation than infants delivered vaginally. Evidence suggests that repeat cesarean deliveries and those performed for dystocia—in patients without FHR abnormalities—result in the delivery of infants at low risk for neonatal resuscitation, especially when the cesarean deliveries are performed with neuraxial anesthesia.3,4,41 Of interest, infants born by elective repeat cesarean delivery are at higher risk for subsequent respiratory problems (e.g., transient tachypnea of the newborn) than similar infants born vaginally. In addition, infants born by cesarean delivery after a failed trial of labor are at a higher risk for neonatal sepsis than similar infants born vaginally.42 Emergency cesarean delivery is considered a risk factor for the need for neonatal resuscitation.
Neonatal Assessment
Apgar Score
Resuscitative efforts typically precede the performance of a thorough physical examination of the neonate. Because NRP instructions require simultaneous assessment and treatment, it is important that the neonatal assessment be both simple and sensitive. In 1953, Dr. Virginia Apgar, an anesthesiologist, described a simple method for neonatal assessment that could be performed while care was being delivered.43 She suggested that this standardized and relatively objective scoring system would differentiate between infants who require resuscitation and those who need only routine care.44
The Apgar score is based on five parameters that are assessed at 1 and 5 minutes after birth. Further scoring at 5- or 10-minute intervals may be done if initial scores are low. The parameters are heart rate, respiratory effort, muscle tone, reflex irritability, and color. A score of 0, 1, or 2 is assigned for each of these five entities (Table 9-1). A total score of 8 to 10 is normal; a score of 4 to 7 indicates moderate impairment; and a score of 0 to 3 signals the need for immediate resuscitation. Dr. Apgar emphasized that this system does not replace a complete physical examination and serial observations of the neonate for several hours after birth.45
TABLE 9-1
Apgar Scoring System
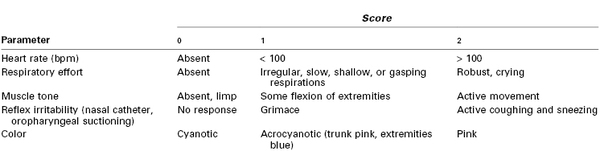
bpm, beats per minute.
Modified from Tabata BK. Neonatal resuscitation. In Rogers MC, editor. Current Practice in Anesthesiology. 2nd edition. St. Louis, Mosby, 1990:368.
The Apgar score is widely used to assess neonates, although its value has been questioned. The scoring system may help predict mortality and neurologic morbidity in populations of infants, but Dr. Apgar cautioned against the use of the Apgar score to make these predictions in an individual infant. She noted that the risk for neonatal mortality was inversely proportional to the 1-minute score.45 In addition, the 1-minute Apgar score was a better predictor of mortality within the first 2 days of life than within 2 to 28 days of life.
Several studies have challenged the notion that a low Apgar score signals perinatal asphyxia. In a prospective study of 1210 deliveries, Sykes et al.46 noted a poor correlation between the Apgar score and the umbilical cord blood pH. Other studies, including those of low-birth-weight infants, have found that a low Apgar score is a poor predictor of neonatal acidosis, although a high score is reasonably specific for excluding the presence of severe acidosis.47–53 By contrast, the fetal biophysical profile has a good correlation with the acid-base status of the fetus and the neonate (see Chapter 6).54 The biophysical profile includes performance of a nonstress test and ultrasonographic assessment of fetal tone, fetal movement, fetal breathing movements, and amniotic fluid volume.54
Additional studies have suggested that Apgar scores are poor predictors of long-term neurologic impairment.55,56 The Apgar score is more likely to predict a poor neurologic outcome when the score remains 3 or less at 10, 15, and 20 minutes. However, when a child has cerebral palsy, low Apgar scores alone are not adequate evidence that perinatal hypoxia was responsible for the neurologic injury.
The ACOG Task Force on Neonatal Encephalopathy and Cerebral Palsy published criteria for defining an intrapartum event sufficient to cause cerebral palsy.57 An Apgar score of 0 to 3 beyond 5 minutes of age is not included in the list of “essential criteria”; rather, it is one of five criteria that “collectively suggest an intrapartum timing (within close proximity to labor and delivery…) but are nonspecific to asphyxial insults.”57–62
In a retrospective analysis of 151,891 singleton infants born at 26 weeks’ gestation or later between 1988 and 1998, Casey et al.63 examined the relationship between Apgar scores and neonatal death rates during the first 28 days of life. The highest relative risk for neonatal death was observed in infants with an Apgar score of 3 or less at 5 minutes of age. The 5-minute Apgar score was a better predictor of neonatal death than the umbilical arterial blood pH. In term infants, the relative risk for neonatal death was eight times higher in infants with a 5-minute Apgar score of 3 or less than in those with an umbilical arterial blood pH of 7.0 or less.63,64 In preterm infants, lower 5-minute Apgar scores were associated with younger gestational ages (i.e., mean score 6.6 ± 2.1 for infants born at 26 to 27 weeks’ and 8.7 ± 0.8 for infants born at 34 to 36 weeks’ gestation).63,64 Similarly, earlier studies found that preterm infants were more likely than term infants to have low 1- and 5-minute Apgar scores, independent of neonatal oxygenation and acid-base status. Respiratory effort, muscle tone, and reflex irritability are the components of the score that are most influenced by gestational age.65
The earlier the gestational age, the greater the likelihood of a low Apgar score, even in the presence of a normal umbilical cord blood pH. Preterm infants often require active resuscitation efforts immediately after delivery, and these manipulations may affect the components of the Apgar score. For example, pharyngeal and tracheal stimulation may cause a reflex bradycardia, which affects the heart rate score.49 In addition, it is difficult to judge respiratory effort during suctioning or endotracheal intubation.
During cases of active neonatal resuscitation, the Apgar scores often are not assigned at the appropriate times; rather, these scores may be assigned retrospectively. In these situations, the individual must rely on recall of the infant’s condition at earlier times, introducing inaccuracy. Even if the scores are assigned at the appropriate times, there may be disagreement among the several individuals who are providing care for the infant. To avoid bias, Dr. Apgar recommended that someone not involved in the care of the mother assign the score.
Although there is some appeal to the use of objective measurements (i.e., SaO2, heart rate) rather than subjective observations, it should not be inferred that the subjective components of the Apgar score (e.g., muscle tone) are less important. There are some practical limitations that may make objective measurements difficult to obtain (e.g., movement artifact with pulse oximetry).16 However, newer-generation pulse oximeters provide more accurate estimations of SaO2 (see later discussion).66
In summary, the usefulness of the Apgar score is still being debated more than 50 years after its inception.63,64 The Apgar scoring system is used throughout the world, but its limitations must be kept in mind. Low Apgar scores alone do not provide sufficient evidence of perinatal asphyxia; rather, Apgar scores can be low for a variety of reasons. Preterm delivery, congenital anomalies, neuromuscular diseases, antenatal drug exposure, manipulation at delivery, and subjectivity and error may influence the Apgar score.
Umbilical Cord Blood Gas and pH Analysis
Umbilical cord blood gas and pH measurements reflect the fetal condition immediately before delivery and can be obtained routinely after delivery or measured only in cases of neonatal depression. These measurements may be a more objective indication of a neonate’s condition than the Apgar score. However, there is a delay between obtaining the samples and completing the analysis; during this interval, decisions must be made on the basis of clinical assessment. The ACOG67 has recommended that cord blood gas measurements be obtained in circumstances of cesarean delivery for fetal compromise, low 5-minute Apgar score, severe growth restriction, abnormal FHR tracing, maternal thyroid disease, intrapartum fever, and/or multiple gestations.
The fetus produces carbonic acid (from oxidative metabolism) and lactic and beta-hydroxybutyric acids (primarily from anaerobic metabolism). Carbonic acid, which is often called respiratory acid, is cleared rapidly by the placenta as carbon dioxide when placental blood flow is normal. However, metabolic clearance of lactic and beta-hydroxybutyric acids requires hours; thus, these acids are called metabolic or fixed acids. In the fetus, metabolic acidemia is more ominous than respiratory acidemia because the former reflects a significant amount of anaerobic metabolism.
The measured components of umbilical cord blood gas analysis are pH, PCO2, PO2, and HCO3−. Bicarbonate (HCO3−) is a major buffer in fetal blood. The measure of change in the buffering capacity of umbilical cord blood is reflected in the delta base, which is also known as the base excess or deficit; this value can be calculated from the pH, PCO2, and HCO3−. Ideally, blood samples from both the umbilical artery and vein are collected. Umbilical artery blood gas measurements represent the fetal condition, whereas umbilical vein measurements reflect the maternal condition and uteroplacental gas exchange. Unfortunately, it may be difficult to obtain blood from the umbilical artery, especially when it is small, as it is in very low-birth-weight (VLBW) infants. Caution should be used in the interpretation of an isolated umbilical venous blood pH measurement, which can be normal despite the presence of arterial acidemia.
Proper blood sampling and handling are necessary. The measurements should be accurate, provided that (1) the umbilical cord is double clamped immediately after delivery68–70; (2) the samples are drawn, within 15 minutes of delivery,71 into a syringe containing the proper amount of heparin72; and (3) the samples are analyzed within 30 to 60 minutes.71,73 The PO2 measurement is more accurate if residual air bubbles are removed from the syringe.
Historically, a normal umbilical cord blood pH measurement was believed to be 7.2 or higher.74 However, investigators have challenged the validity of this number, given its lack of distinction between umbilical arterial and venous blood despite clear differences in their normal measurements.75 One study noted that the median umbilical arterial blood pH in vigorous infants (those with 5-minute Apgar scores of 7 or higher) was 7.26, with a measurement of 7.10 representing the 2.5th percentile.76 Published studies suggest that the lower limit of normal umbilical arterial blood pH measurements may range from 7.02 to 7.18 (Table 9-2).46,77–86 A number of factors may also influence the umbilical arterial blood pH measurement. A fetus subjected to the stress of labor has lower pH measurements than one born by cesarean delivery without labor.83 Offspring of nulliparous women tend to have a lower pH than offspring of parous women, a difference that is likely related to a difference in the duration of labor.87
TABLE 9-2
Studies Reporting Umbilical Cord Arterial Blood Gas Measurements*
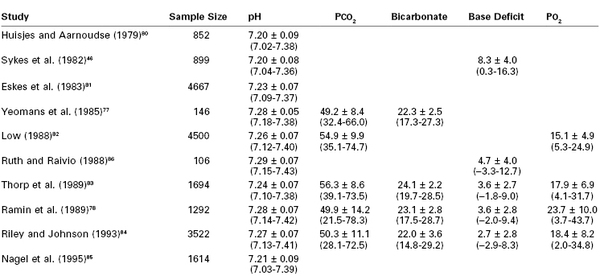
* Data are presented as mean ± 1 SD and (−2 to +2 SD). Sample size pertains to cord arterial pH and not necessarily to other parameters.
Modified from Thorp JA, Dildy BA, Yeomans ER, et al. Umbilical cord blood gas analysis at delivery. Am J Obstet Gynecol 1996; 175:517-22.
Some studies have suggested that preterm infants have a higher incidence of acidemia; however, later studies have observed that term and preterm infants have similar umbilical cord blood gas and pH measurements.78,79,87 Preterm infants often receive low Apgar scores despite the presence of normal umbilical cord blood gas and pH measurements; therefore, the assessment of umbilical cord blood may be especially helpful in the evaluation of preterm neonates.
Physicians should use strict definitions when interpreting umbilical cord blood gas and pH measurements. Terms such as birth asphyxia should be avoided in most cases.57 Acidemia refers to an increase in the hydrogen ion concentration in the blood. Acidosis occurs when there is an increased hydrogen ion concentration in tissue. Asphyxia is a clinical situation that involves hypoxia (i.e., a decreased level of oxygen in tissue), damaging acidemia, and metabolic acidosis.
When acidemia is present, the type—respiratory, metabolic, or mixed—must be identified (Table 9-3). Metabolic acidemia is more likely to be associated with acidosis than respiratory acidemia and is clinically more significant. Similarly, mixed acidemia with a high PCO2, an extremely low HCO3−, and a high base deficit is more ominous than a mixed acidemia with a high PCO2 but only a slightly reduced HCO3− and a low base deficit. Mixed or metabolic acidemia (but not respiratory acidemia) is associated with an increased incidence of neonatal complications and death.87 In their study of 3506 term neonates, Goldaber et al.88 noted that an umbilical arterial blood pH measurement less than 7.00 was associated with a significantly higher incidence of neonatal death. All neonatal seizures in their study occurred in infants with an umbilical arterial blood pH less than 7.05. By contrast, a short-term outcome study failed to show a good correlation between arterial blood pH and the subsequent health of an infant.53 In the previously discussed large study reported by Casey et al.,63 an umbilical arterial blood pH of 7.0 or less was a poorer predictor of the relative risk for neonatal death during the first 28 days of life than a 5-minute Apgar score of 3 or less. However, 6264 infants were excluded from their study because umbilical arterial blood gas measurements could not be obtained, and these infants had a higher incidence of neonatal death than those for whom blood gas measurements were available (4.5 per 1000 versus 1.2 per 1000, respectively). In a separate review of 51,519 term deliveries, Yeh et al.89 found an increased risk for adverse outcomes in infants with a pH less than 7.10, with the lowest risk in infants with a pH between 7.26 and 7.30; however, 75% of infants with neurologic morbidity had a normal pH. Thus, it is important to remember that neonates may suffer multiorgan system damage, including neurologic injury, even in the absence of low pH and Apgar scores.
TABLE 9-3
Criteria Used to Define Types of Acidemia in Neonates with an Umbilical Arterial pH Measurement Less Than 7.20

* Means ± SD given in parentheses.
From the American College of Obstetricians and Gynecologists. Assessment of fetal and newborn acid-base status. ACOG Technical Bulletin No. 127. Washington, DC, April 1989.
According to the ACOG Task Force, an umbilical arterial blood pH less than 7.0 and a base deficit greater than or equal to 12 mmol/L at delivery are considered one part of the definition of an acute intrapartum hypoxic event sufficient to cause cerebral palsy.57 The base deficit and bicarbonate (the metabolic component) values are the most significant factors associated with morbidity in neonates with an umbilical arterial blood pH less than 7.0. Ten percent of infants with an umbilical arterial base deficit of 12 to 16 mmol/L have moderate to severe complications, which increases to 40% when the deficit is greater than 16 mmol/L.67
Abnormal FHR patterns and umbilical cord blood gas measurements are not consistently correlated with poor neonatal outcomes.37 In a longitudinal study that evaluated outcomes at 6.5 years of age, Hafstrom et al.90 found that infants with an umbilical arterial blood pH less than 7.05 but a normal examination at birth had outcomes that did not differ from those for matched infants with a normal umbilical arterial blood pH.
As Dr. Apgar emphasized in 1962, the most important components of neonatal assessment are a careful physical examination and continued observation for several hours.45 Additional information can be gained from the antenatal history, Apgar scores, and umbilical cord blood gas and pH measurements, provided that clinicians are aware of the proper methods of interpretation as well as the limitations of these methods of assessment.
Respiration and Circulation
There are some similarities between the initial assessment of the neonate and the initial assessment of an adult who requires resuscitation. In both situations, the physician should give immediate attention to the ABCs of resuscitation (i.e., airway, breathing, circulation).
The normal neonatal respiratory rate is between 30 and 60 breaths per minute. Breathing should begin by 30 seconds and be regular by 90 seconds of age. Failure of the neonate to breathe by 90 seconds of age represents either primary or secondary apnea, based on the neonatal rhesus monkey asphyxia model.91 In this model, gasping motions were observed for approximately 1 minute immediately after delivery; this was followed by a 1-minute period of apnea (primary apnea), then 5 minutes of gasping motions, and a final period of apnea (secondary or “terminal” apnea). During primary apnea, but not secondary apnea, tactile stimulation of the newborn monkey initiated breathing efforts. In addition, although heart rate was low with both periods of apnea, a reduction in blood pressure was observed only during secondary apnea. With the onset of secondary apnea (approximately 8 minutes after birth), the pH was 6.8 and the PaO2 and PaCO2 measurements were less than 2 and 150 mm Hg, respectively.
This experimental model illustrates two important points. First, distinguishing primary from secondary apnea is not possible unless blood pressure and/or blood gases and pH are measured. Second, by the time secondary apnea has begun, blood gas measurements have deteriorated significantly. Therefore, during evaluation of the apneic neonate, aggressive resuscitation must be initiated promptly if tactile stimulation does not result in the initiation of spontaneous breathing.
Assessment of the adequacy of respiratory function requires comprehensive observation for signs of neonatal respiratory distress. These signs include cyanosis, grunting, flaring of the nares, retracting chest motions, and unequal breath sounds. The adequacy of respiratory function can also be assessed by the estimation of SaO2. The reliability of pulse oximetry for the assessment of neonatal SaO2 was questioned initially because of concerns about the accuracy of spectrophotometric assessments of fetal hemoglobin and the difficult signal detection caused by the rapidity of the neonate’s heart rate.92,93 The newer generation of pulse oximetry monitors, which employ signal extraction and averaging techniques, are able to provide more reliable measurements, especially in the presence of poor perfusion, patient movement, and ambient light artifacts.66,94
Pulse oximetry provides accurate estimates of SaO2 during periods of stability but may overestimate values during rapid desaturation.95 In addition, the SaO2 (SpO2) measurements may fluctuate in the delivery room as a result of the ongoing transition from the fetal to the neonatal circulation, and it may take more than 10 minutes to achieve a preductal SaO2 greater than 95% in a healthy term infant. Overall, the newer-generation pulse oximeters reliably provide continuous noninvasive SaO2 measurements and are useful for neonatal monitoring.96–98
The pulse oximeter sensor should be applied to the neonate’s right upper extremity, which receives preductal blood flow (see earlier discussion); because CNS blood flow is also preductal, right upper extremity SaO2 measurements provide a more accurate assessment of CNS oxygenation.16 Sensor placement can be difficult on skin that is wet and covered with vernix caseosa; therefore, it may be easier to place the sensor over the right radial artery, especially in preterm infants.94
Neonatal arterial blood sampling is technically difficult and thus rarely obtained in the delivery room. Cannulation of the umbilical artery is useful in infants who will require frequent blood sampling. This procedure often requires the use of microinstruments (especially in preterm and VLBW infants) and the ability to monitor the infant when obscured from view by surgical drapes; therefore, this procedure is usually performed in the neonatal intensive care unit (NICU).
The normal neonatal heart rate may be greater than 160 beats per minute (bpm) in the very early preterm neonate, but it should be within the range of 120 to 160 bpm by 28 weeks’ gestational age. The heart rate can be determined in several ways. The clinician can lightly grasp the base of the umbilical cord and feel the arterial pulsations. (This method cannot be used in situations in which the pulsations become difficult to feel, such as in an infant with a low cardiac output.) Alternatively, the clinician can listen to the apical heartbeat. When either of these two methods is used, the evaluator should tap a hand with each heartbeat so that other members of the resuscitation team are aware of the rate. By contrast, the use of a pulse oximeter provides an audible heart rate, the additional benefit of SaO2 monitoring, and the ability to eliminate the need for an additional team member.
Measurement of arterial blood pressure is not a priority during the initial assessment and resuscitation of the neonate.2 However, observation for signs of abnormal circulatory function is considered essential. These signs include cyanosis, pallor, mottled coloring, prolonged capillary refill time, and weakness or absence of pulses in the extremities. One of the causes of abnormal circulatory function is hypovolemia, which should be anticipated in cases of bleeding from the umbilical cord or the fetal side of the placenta or whenever a neonate does not respond appropriately to resuscitation. The hypovolemic neonate may exhibit not only signs of abnormal circulatory function but also tachycardia and tachypnea. (Neonatal hypovolemia usually does not accompany placental abruption, which may cause maternal bleeding or other conditions associated with fetal asphyxia.)
Neurologic Status
The initial neonatal neurologic assessment requires only simple observation. The neonate should demonstrate evidence of vigorous activity, including crying and active flexion of the extremities. Signs of possible neurologic abnormalities include apnea, seizures, hypotonia, and unresponsiveness. Neonates should be assessed for physical signs of hypoxic-ischemic encephalopathy (Table 9-4). The stages of hypoxic-ischemic encephalopathy are associated with different outcomes: stage I, good; stage II, moderate; and stage III, poor.99 Although detailed neurologic assessment is performed after the neonate is transferred to the NICU, assessment of tone, baseline heart rate, respirations, and reflex activity is part of both the Apgar scoring system and the assessment for hypoxic-ischemic encephalopathy and is made initially in the delivery room.
TABLE 9-4
Stages of Neonatal Hypoxic-Ischemic Encephalopathy
| Stage I | Stage II | Stage III |
| Irritable | Lethargic/obtunded | Coma |
| Normal respirations | Depressed respirations | Apnea |
| Hypertonic | Hypotonic | Flaccid |
| Increased reflexes | Decreased reflexes | Absence of reflexes |
| No seizures | Occasional seizures | Status epilepticus or nearly isoelectric electroencephalogram |
| Good outcome | Moderate outcome | Poor outcome |
Modified from Eicher DJ, Wagner C. Update on neonatal resuscitation. J S C Med Assoc 2002; 98:115.
Gestational Age
When assessing a very small neonate whose gestational age appears to be lower than that of viability, the evaluator must consider whether it is appropriate to initiate and maintain resuscitation efforts. The neonatal gestational age is often assessed with the use of the scoring systems described initially by Dubowitz et al.100 and subsequently modified by Ballard et al.101 The Dubowitz system makes use of an external score based on physical characteristics, described previously by Farr et al.,102,103 and a neurologic score. The Ballard system uses simplified scoring criteria to assess gestational age. Ballard et al.101 eliminated certain physical criteria such as edema and skin color because of the unreliability of these criteria in some clinical conditions. In addition, they abbreviated the neurologic criteria on the basis of observations by Amiel-Tison.104
The Dubowitz and Ballard scores are most accurate when used to estimate gestational age at 30 to 42 hours, rather than during the first several minutes, after birth. These scoring systems are also less accurate in very small preterm infants. In one study of 100 preterm infants with birth weights less than 1500 g, agreement among antenatal measures of gestational age (e.g., last menstrual period, ultrasonography determination) and postnatal measures (e.g., Dubowitz and Ballard scores) was poor.105 Both scoring systems overestimated gestational age in this subset of VLBW infants. Ballard et al.106 refined their scoring system to provide a more accurate estimate of gestational age in preterm infants (Figure 9-3). The new Ballard score assesses physical criteria, such as eyelid fusion, breast tissue, lanugo hair, and genitalia, and neurologic criteria, such as wrist “square window.” (The square window assessment is performed by flexing the infant’s wrist on the forearm and noting the angle between the hypothenar eminence and the ventral aspect of the forearm.) Although the new Ballard score may be more accurate than the older score for the assessment of preterm infants, inconsistencies occur with all of these methods. Of particular interest is the observation that fetuses of different racial origin appear to mature at different rates (i.e., black fetuses mature faster than white fetuses).107
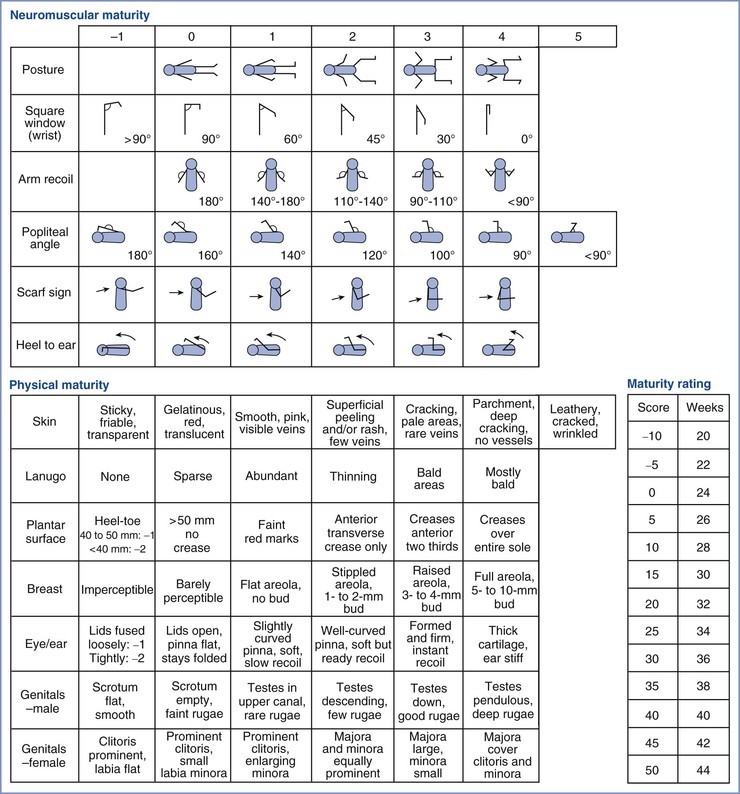
FIGURE 9-3 Modified Ballard scoring system for clinical assessment of maturation in neonates. This scoring system was expanded to include extremely preterm infants, and it was refined to improve the accuracy of assessment of more mature infants. (Modified from Ballard JL, Khoury JC, Wedig K, et al. New Ballard score, expanded to include extremely premature infants. J Pediatr 1991; 119:418.)
Another commonly used criterion for the estimation of gestational age is birth weight. Normal values for birth weight are published and readily available.108 Although birth weight may help physicians estimate the gestational age of an otherwise healthy preterm infant, physicians cannot rely on birth weight to provide an accurate estimate of gestational age in an infant who suffered from intrauterine growth restriction or who is large for gestational age.
Because of the potential for inaccurate gestational age estimation in the delivery room, it is best not to use these scoring systems to guide decisions regarding the initiation or continuation of neonatal resuscitation immediately after delivery. In most circumstances, the neonate’s response to resuscitative efforts is the best indicator as to whether further intervention is warranted.
Neonatal Resuscitation
The equipment and medications needed for neonatal resuscitation are listed in Box 9-3. Equipment, supplies, and medications should be checked regularly to ensure that all components are available and functional.
Although previously published guidelines recommended suctioning of the mouth and nose after delivery of the head, the guidelines published in 2010 do not recommend routine intrapartum oropharyngeal and nasopharyngeal suctioning for infants born with either clear or meconium-stained amniotic fluid.2
Timing of cord clamping may vary by the gestational age and vigor of the infant. Current evidence suggests that a delay in cord clamping for 1 minute after the delivery of vigorous term infants improves iron stores throughout early infancy.109 In vigorous preterm infants, a brief delay in cord clamping (30 seconds to 3 minutes) is associated with improved blood pressure and a lower incidence of intraventricular hemorrhage110; no alterations in Apgar scores or need for delivery room resuscitation have been observed with this practice.111 In nonvigorous infants, regardless of gestational age, the benefits of delayed cord clamping may be outweighed by the need to promptly initiate resuscitation.
After delivery is complete, the neonate is transferred to the resuscitation area. The availability of sterile blankets allows the individual performing the delivery to remain sterile while transferring the infant; this issue is especially important during cesarean deliveries. The timing of delivery should be noted, assessment and appropriate resuscitative measures should be continued, and Apgar scores should be assigned at the appropriate intervals (Figure 9-4).
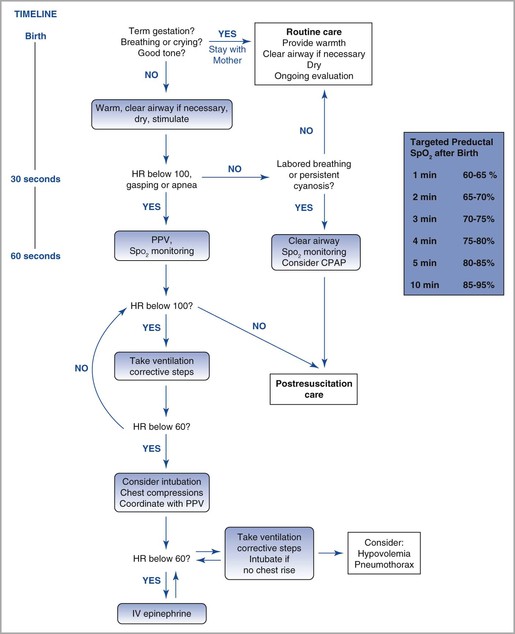
FIGURE 9-4 Algorithm for resuscitation of the newly born infant. HR, heart rate; PPV, positive-pressure ventilation; SpO2, oxygen saturation. (Modified from Textbook of Neonatal Resuscitation. 6th edition. Elk Grove Village, IL, American Academy of Pediatrics and American Heart Association, 2011:216. Figure by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
The physician or nurse should place the infant beneath an overhead radiant warmer and promptly dry the skin of infants delivered at greater than 28 weeks’ gestation. The infant who is delivered preterm at less than 28 weeks’ gestation should be placed in a polythene bag or wrapping to prevent heat loss.29,30 Hypothermia can result in increased oxygen consumption and metabolic acidosis112 and leads to a significantly higher mortality rate among preterm infants.113
Selective cerebral hypothermia114 or whole-body hypothermia31,32 may protect against brain injury in the asphyxiated infant. The use of intentional hypothermia therapy requires an NICU with defined protocols and multidisciplinary support. When assessing an infant for hypothermia therapy, the radiant warmer can be turned off to allow passive cooling. With further assessment, if the criteria for hypothermia therapy are not met, the infant can be warmed. Hyperthermia should be avoided in all infants.2
The neonate should be positioned in a way that allows the airway to remain open, with the head in the “sniffing” position (the neck flexed on the chest and the head extended on the neck, thereby aligning the oropharynx, pharynx, and hypopharynx). Suctioning of the mouth and nose with a bulb syringe may be necessary if secretions accumulate.
The neonate with a normal respiratory pattern, heart rate, and color requires no further intervention. Often the neonate has a normal respiratory pattern and heart rate but may not be pink. Acrocyanosis often persists for several minutes after delivery and does not require intervention. However, an evaluation for choanal atresia can be performed at this time with the gentle insertion of a small suction catheter through each nostril into the nasopharynx. Vigorous nasal suctioning should be avoided because it can cause trauma to the nasal mucosa and result in progressive edema and airway obstruction. The neonate is an obligate nasal breather; thus, choanal atresia is a potentially lethal anomaly that requires immediate attention. If this anomaly is present (as evidenced by failure of nasal passage of the catheter), the neonate should have an oral airway or endotracheal tube inserted and an evaluation performed for repair of the obstruction. The classic clinical presentation for choanal atresia is an infant with cyanosis and respiratory distress at rest who becomes pink when crying.
Tactile stimulation should be used if the neonate does not breathe immediately; this consists of gently rubbing the back and flicking the soles of the feet. Tactile stimulation does not trigger respiratory efforts during secondary apnea in the neonate. Therefore, if the neonate does not begin to breathe spontaneously after tactile stimulation, the evaluator should begin positive-pressure mask ventilation. If the neonate has an abnormally slow heart rate (i.e., less than 100 bpm), positive-pressure ventilation should be performed until the heart rate rises to the normal range. Overzealous tactile stimulation (e.g., slapping the back) is not useful; it provides no advantage over the more moderate methods and can cause traumatic injury. Infants with labored or persistent cyanosis may benefit from continuous positive airway pressure.
High concentrations of oxygen (as opposed to ambient air) can raise production of oxygen free radicals, which have been linked to hypoxia-reoxygenation injury.115 Additionally, an association between neonatal oxygen supplementation and childhood cancer has been noted with supplemental oxygen exposure for 3 minutes or longer.116 In two studies, term or near-term infants were randomly assigned to receive neonatal resuscitation with either room air or 100% oxygen; no major outcome differences were observed.117,118 Subsequently a pooled meta-analysis of five trials, consisting of 1032 term or near-term infants, showed a significantly lower mortality rate with no evidence of harm when resuscitation was performed initially with room air rather than 100% oxygen.119 The current guidelines for neonatal resuscitation for term infants recommend the use of room air for assisted ventilation. In preterm infants, assisted ventilation should be initiated with an inspired oxygen concentration (FIO2) of 30% to 90% and should be guided by the response to resuscitation and the use of pulse oximetry to assess oxygenation. The FIO2 should be lowered as soon as possible to minimize the risk for retinopathy of prematurity and pulmonary toxicity. SaO2 measurements of 85% to 92% are thought to be adequate and appropriate for neonates of less than 34 weeks’ gestation.117,118,120,121 A meta-analysis detected no significant differences in neurodevelopmental outcomes at 12 to 24 months of age between infants resuscitated with either room air or 100% oxygen.122
Positive-pressure ventilation must be performed correctly to ensure that it is effective and does not cause barotrauma. A ventilation bag with a volume of 250 to 500 mL may be used. The circuit must contain a safety pop-off pressure valve (e.g., at 35 cm H2O), a visible pressure gauge, or both. An oxygen flow rate of 5 to 10 L/min is adequate. Alternatively, a T-piece, which is a valved mechanical device, may be used; it allows more consistent delivery of target inflation pressures and long inspiratory times. The mask must be of appropriate size and shape to ensure a good seal around the nose and mouth. A variety of masks should be available to accommodate infants of all sizes and gestational ages. For the infant with excessive occipital scalp edema (e.g., caput succedaneum), placing a small roll under the shoulders to alleviate hyperflexion of the neck may be helpful.
During the first assisted breath, positive pressure at 30 cm H2O in term infants should be maintained for 4 to 5 seconds at the end of inspiration to overcome the surface tension of the lungs and open the alveoli.123 The neonatal response to a large, rapid inflation of the lungs is a sharp inspiration of its own (Head’s paradoxical reflex).124 Subsequent breaths should be delivered at a rate of 40 to 60 breaths per minute, with intermittent inspiratory pauses to prevent the development of atelectasis. The maximum pressure generated should range between 20 and 30 cm H2O, with an inspiration-to-expiration ratio of approximately 1 : 1. In preterm infants, whose lungs may be more easily injured, initial inflation pressures of 20 to 25 cm H2O may be adequate. If mask ventilation is needed for longer than 2 to 3 minutes, the stomach should be emptied with an orogastric catheter. Distention of the stomach with air can compromise respiratory function in the neonate. This maneuver should be performed with care, because pharyngeal stimulation can result in arrhythmias and apnea.125
The adequacy of respiratory resuscitation can be monitored from observation of its effect on heart rate; an increase in heart rate is the first consistently reliable sign of effective oxygenation. By contrast, changes in color occur slowly, are difficult to assess, and are a relatively poor index of successful resuscitation.
When the neonate’s heart rate is higher than 100 bpm, positive-pressure ventilation can be stopped, and the infant can be reevaluated for spontaneous respiratory effort. If the neonate does not begin to breathe and if an opioid effect is the suspected etiology, administration of naloxone is not recommended. Naloxone can worsen the neurologic damage caused by asphyxia126,127 and can precipitate acute neonatal opioid withdrawal, including seizure activity in cases of maternal opioid abuse. Assisted ventilation should be continued until resolution of the opioid effect rather than attempting to reverse it with naloxone.
If positive-pressure mask ventilation does not improve oxygenation (as reflected by an increase in heart rate), prompt tracheal intubation is indicated. Tracheal intubation must be performed gently to avoid damage to the delicate neonatal neck and airway. The size of the neonate’s head is large relative to that of its body; therefore, the neonate is in the optimal position when it lies supine. In most cases, it is not necessary to elevate or hyperextend the neonate’s head during laryngoscopy. The neonatal larynx is more anterior than that of the adult, and visualization often is easier when cricoid pressure is applied. The practitioner should hold the laryngoscope and apply cricoid pressure with the same hand. The thumb and first two fingers hold the base of the laryngoscope, the third finger rests on the mandible, and the fourth finger applies cricoid pressure. This technique promotes gentleness during airway manipulation. The distance from the gums to the larynx often is surprisingly short. A common mistake is to advance the laryngoscope blade too deeply—past the larynx and into the esophagus. When this error occurs, the larynx falls into view if the laryngoscope blade is withdrawn slowly to allow a second attempt.
The diameter of the endotracheal tube should be large enough to allow adequate ventilation and insertion of a suction catheter (if needed) but small enough to avoid causing trauma and subsequent subglottic stenosis. The ratio of internal diameter to gestational age should be less than 0.1 (e.g., 3.0 mm tube/35 weeks’ gestation = 0.09).128,129
After tracheal intubation, positive-pressure ventilation should be resumed by means of an appropriate circuit, as described earlier for mask ventilation. Assessment of proper tube placement is accomplished by listening for breath sounds in both axillae. Exhaled CO2 detection is the recommended method for confirming placement of the tube in the trachea.2 False-negative results can occur in situations in which the infant is correctly intubated, with the tube in the trachea, but pulmonary blood flow is poor or absent. This may lead to unnecessary extubation in critically ill infants. As noted previously, the FIO2 should be reduced as soon as possible, especially in the preterm neonate. The addition of a pulse oximeter and an oxygen blender allows more targeted delivery of supplemental oxygen to the preterm infant immediately after birth. If the neonate is to remain intubated, a chest radiograph should be obtained to confirm the exact position of the endotracheal tube. The skill and experience required for correct tracheal intubation and effective bag-and-mask ventilation may be lacking in providers who are inexperienced with neonatal resuscitation; as a consequence, the laryngeal mask airway (LMA) has been evaluated as a potential alternative airway device for neonatal resuscitation.130–132 The LMA is blindly inserted into the pharynx, and a cuff is inflated to provide a low-pressure seal around the larynx. When evaluated in term infants requiring resuscitation at delivery, use of the LMA was found to be highly successful and without complications.130,131 The revised neonatal resuscitation guidelines state that the LMA is an acceptable alternative means of establishing an airway in infants born at 34 weeks’ gestation and greater and weighing over 2000 g; it can be used by appropriately trained providers when bag-and-mask ventilation is ineffective or attempts at tracheal intubation have been unsuccessful.2
One cause of unequal breath sounds and eventual circulatory collapse is a tension pneumothorax. Some physicians have recommended that providers of neonatal resuscitation be skilled in needle aspiration of a tension pneumothorax.1 This maneuver is accomplished by placement of a 22- or 25-gauge needle in the second intercostal space in the midclavicular line (on the side where no breath or heart sounds are heard). Air will rush out of the needle hub, thereby reducing the tension pneumothorax.
In the vast majority of resuscitations, the neonate responds to ventilatory support. Chest compressions are needed in only 0.03% of deliveries.133 Chest compressions are indicated when the heart rate is less than 60 bpm despite adequate ventilation with supplemental oxygen for 30 seconds.2
The preferred method for providing chest compressions is with the thumbs of both hands and the hands encircling the chest.2,134 Pressure is applied over the sternum just below an imaginary line drawn between the nipples; pressure applied over the lower part of the sternum or xiphoid can injure the abdomen. The sternum should be compressed to approximately one third the anteroposterior dimension of the chest, and the compression depth must be adequate to produce a palpable pulse.2,135–137 The compression time should be slightly shorter than the release time, particularly to improve blood flow in the very young infant.138 Ventilation is compromised if the chest is compressed simultaneously with the administration of positive-pressure ventilation. The recommended ratio of compressions to breaths is 3 : 1.139,140 This pattern is given at a rate of 120 events per minute, with 90 chest compressions and 30 breaths administered each minute. Respirations, heart rate, and color should be rechecked every 30 seconds. Compressions should be resumed until the heart rate is 60 bpm or higher. Positive-pressure ventilation with supplemental oxygen titrated to SaO2 should be continued until the heart rate is higher than 100 bpm.
Medications are rarely required during neonatal resuscitation because most neonates who require resuscitative measures respond well to satisfactory oxygenation and ventilation alone.141 However, a variety of pharmacologic agents should be available in the delivery room (see Box 9-3). Epinephrine (0.01 to 0.03 mg/kg or 0.1 to 0.3 mL/kg of a 1 : 10,000 solution) should be administered if the heart rate remains lower than 60 bpm after 30 seconds of adequate ventilation and chest compressions.2 Intravenous administration is the preferred route (via an umbilical venous line). While intravenous access is being established, intratracheal administration through an endotracheal tube may be considered; however, a larger dose of epinephrine (0.05 to 0.1 mg/kg) may be required. Administration of epinephrine is especially important if the heart rate is zero. Epinephrine raises the heart rate (the major determinant of neonatal cardiac output) and restores coronary and cerebral blood flow.142
Sodium bicarbonate is used infrequently during resuscitation. Because of its high osmolarity, this agent can cause hepatic injury at any gestational age and cerebral hemorrhage in the preterm infant143,144; it may also compromise myocardial and cerebral function.145,146 It should be given only during prolonged resuscitation and when adequate ventilation and circulation have been established. Arterial blood gas measurements and serum chemistry determinations should guide the use of sodium bicarbonate. The current recommended dose is 1 to 2 mEq/kg of a 0.5 mEq/mL solution given over at least 2 minutes by slow intravenous push.
Atropine is not recommended for use during neonatal resuscitation. Epinephrine is considered the drug of choice for the treatment of bradycardia.
Calcium administration is not recommended for neonatal resuscitation, unless it is given specifically to reverse the effect of magnesium (which may have crossed the placenta from the mother to the fetus). Evidence suggests that calcium administration causes cerebral calcification and decreases survival in stressed neonates.147
Volume expanders must be given strictly according to recommended dosage. A continuous infusion is dangerous in the neonate, because it can easily result in the administration of an excessive fluid volume. Fluid overload can cause hepatic capsular rupture, brain swelling in the asphyxiated infant, or intracranial hemorrhage in the preterm infant. Fluids and medications can be administered either intravenously (most commonly through the umbilical vein) or, if necessary, intraosseously.
The cannulation of the umbilical vein involves insertion of a soft catheter into the cut end of the vein (Figure 9-5). The catheter is advanced until blood return is noted, but no more than 2 cm past the abdominal surface. If ongoing vascular access is required during the neonate’s hospital course, the soft umbilical catheter can be advanced through the ductus venosus into the inferior vena cava. Care must be taken to avoid leaving the tip in an intermediate location because of possible hepatic damage if a high-osmolarity substance (e.g., improperly diluted sodium bicarbonate) were injected. Other complications of umbilical venous catheterization are hemorrhage and sepsis. The prolonged absence of vascular access in critically ill neonates can lead to hypoglycemia, which in association with hypoxia, can increase the risk for adverse neonatal outcomes.148
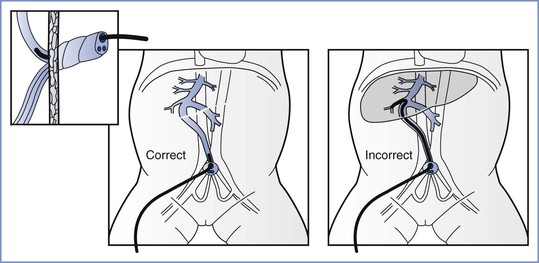
FIGURE 9-5 Cannulation of the umbilical vein. A 3.5F or 5F umbilical catheter with a single end-hole and a radiopaque marker should be used. For emergency use, the catheter should be inserted into the vein of the umbilical stump until the tip of the catheter is just below the skin level but free flow of blood is present. If the catheter is inserted farther, there is a risk for infusing solutions into the liver and possibly causing damage. (Adapted from Textbook of Neonatal Resuscitation. 6th edition. Elk Grove Village, IL, American Academy of Pediatrics and American Heart Association, 2011:216.)
Intraosseous access is accomplished by insertion of a 20-gauge needle into the proximal tibia approximately 1 cm below the tibial tuberosity.149 This technique may be easier to perform for practitioners who have little experience with intravenous or umbilical neonatal catheterization. Absorption from the neonatal bone marrow into the general circulation occurs almost immediately.150,151 This rapid absorption results from the preponderance of red bone marrow over yellow bone marrow; yellow bone marrow is less vascular and is the dominant form of marrow after 5 years of age. Complications related to this technique are rare and include tibial fracture (which occurs more often in older children)152 and osteomyelitis. The risk for infection is proportional to the duration of intraosseous infusion153–155; therefore, the needle should be removed after 1 to 2 hours and, if necessary, a more conventional route of access should be established. Current guidelines state that intraosseous access should be used for medication administration or volume expansion when venous access is difficult to achieve.2
Volume expanders should be considered when the infant demonstrates signs of shock, such as pale skin, poor perfusion, and weak pulse, or has not shown adequate response to other resuscitative measures. Normal saline and lactated Ringer’s solution are the preferred volume expanders, given initially at 10 mL/kg over 5 to 10 minutes, with doses repeated as necessary after reassessment for ongoing hypovolemia. Intravascular volume should be assessed through evaluation of heart rate, capillary refill time, and color. If heavy blood loss is suspected, O-negative packed red blood cells may be used according to the same dosage regimen.2 Red blood cells replete the oxygen-carrying capacity as well as the intravascular volume. O-negative blood should be available at all times for emergency use during neonatal resuscitation. Placental blood has been used for neonatal volume expansion,156 but this practice is discouraged in most institutions because of the risks of infection or transfusion of clotted blood. Albumin administration is no longer recommended, because it carries a risk for infectious disease and has been associated with higher mortality.157
Special Resuscitation Circumstances
Meconium Aspiration
There has been significant interest in the management of the neonate whose airway has been exposed to meconium-containing amniotic fluid. Meconium is present in the intestinal tract of the fetus after approximately 31 weeks’ gestation. Meconium-stained amniotic fluid is present in 10% to 15% of all pregnancies; the incidence is higher in post-term pregnancies. Intrapartum passage of meconium may be associated with fetal stress and hypoxia.158,159
Meconium aspiration syndrome (MAS) is defined as respiratory distress in a neonate whose airway was exposed to meconium and whose chest radiographic study exhibits characteristic findings, including pulmonary consolidation and atelectasis.160 Treatment of MAS often involves the use of positive-pressure ventilation and is associated with a 5% to 20% incidence of pneumothorax from pulmonary air leaks.161 In one study of 176,790 infants born between 1973 and 1987, the annual death rate from MAS was as high as 6 per 10,000 live infants.162 Extracorporeal membrane oxygenation (ECMO) and inhaled nitric oxide have been used for the treatment of pulmonary hypertension associated with MAS and have been observed to reduce mortality rates.163–165
Neonatologists have attempted to determine whether peripartum suctioning of the neonate’s airway reduces the risk for developing MAS. Gregory et al.162 published the original study of 80 meconium-exposed neonates who were born either vaginally or by cesarean delivery. All infants underwent tracheal intubation and suctioning after delivery. In 34 infants, no meconium was observed below the vocal cords; none of these infants demonstrated MAS. Meconium was noted below the cords in the remaining 46 infants, and MAS developed in a total of 16 (35%) of these infants. These investigators concluded that “all infants born through thick, particulate, or ‘pea soup’ meconium should have the trachea aspirated immediately after birth.”162 Subsequent studies documented similar findings for all infants born through meconium-stained fluid,166 with a suggestion that earlier suctioning could decrease the incidence of MAS.167 However, additional investigators documented that airway suctioning at birth does not prevent MAS and its associated mortality168,169; these studies indicated that MAS was primarily a result of intrauterine events such as asphyxia or sepsis. Hypoxia induces pathologic changes in the pulmonary vasculature, which results in pulmonary hypertension and respiratory distress after birth. The pulmonary damage is independent of meconium aspiration; therefore, it is not prevented by the suctioning of meconium.
Murphy et al.170 examined the lungs of 11 neonates who had MAS and died within 4 days of birth. Ten of these neonates also had a diagnosis of persistent pulmonary hypertension, and all had evidence of excessive muscularization of the intra-acinar arteries, which is an abnormal finding in the fetus or neonate.171,172 Meyrick and Reid173 demonstrated that chronic hypoxia (i.e., at least 4 weeks’ duration), but not acute hypoxia, results in pulmonary vascular muscularization in an animal model. Murphy et al.170 concluded that the pathologic findings in the 1- to 4-day-old human lung could not be explained by the postdelivery effects of meconium aspiration; a more likely origin was the intrauterine maldevelopment of the pulmonary vasculature. They suggested a potential link between greater intestinal motility, passage of meconium, and precocious muscularization of the intra-acinar arteries.
A prospective study designed to assess the efficacy of routine tracheal suctioning of meconium to prevent MAS indicated little or no benefit to this practice.174 Among the infants who underwent tracheal suctioning, four experienced MAS and two had laryngeal stridor. By contrast, none of the infants who did not undergo suctioning had complications, suggesting that vigorous neonates who have begun breathing before transfer to the resuscitation table may derive little or no benefit from tracheal suctioning and, in fact, may suffer some harm.
A subsequent review of studies published between 1980 and 1999 found that most cases of severe MAS were not causally related to meconium aspiration but rather resulted from intrauterine stress.175 The authors concluded that severe MAS is a misnomer because, in most cases, much more than meconium aspiration has contributed to the lung damage. The implication is that when severe MAS occurs, inadequate suctioning at delivery or during resuscitation should not be considered the cause; therefore, other causes of intrauterine lung damage should be investigated.
Amnioinfusion—the instillation of saline into the amniotic cavity—has been used successfully for reduction of cord compression in the presence of oligohydramnios during labor. It has also been proposed as a potential treatment to reduce the incidence of MAS in infants born to women with thick meconium staining of the amniotic fluid. Potential benefits include (1) the reduction of cord compression, thus alleviating fetal compromise and acidemia that contribute to MAS, and (2) the dilution or washing out of the meconium in the amniotic fluid. A large multicenter randomized trial found no difference in rates of MAS or other neonatal disorders with the use of amnioinfusion.176,177 Thus, the routine practice of amnioinfusion for meconium-stained fluid alone is not recommended.178
Current guidelines do not recommend routine intrapartum oropharyngeal and nasopharyngeal suctioning before delivery of the infant’s head,2,179 given that a large multicenter randomized trial showed no benefit to this practice in term-gestation infants.180 After stabilization of the infant, meconium may be gently cleared from the mouth and nose by means of a bulb syringe or a large suction catheter (e.g., 12F to 14F).
Preterm Infant
The preterm neonate, especially the VLBW infant, is at higher risk for problems with multiple organ systems simply because of immaturity. During resuscitation, the physician should give special attention to the effect of prematurity on the lungs and the brain. Before the addition of surfactant and high-frequency ventilation to the therapeutic armamentarium of the neonatologist, pulmonary hyaline membrane disease (also known as neonatal respiratory distress syndrome) was the overwhelming obstacle to the attempted salvage of the very preterm infant.
Between 1970 and 2005, the proportion of infants weighing less than 1500 g at delivery rose from 1.17% to 1.5%; in 2009, the proportion stabilized at 1.45%.181 The survival rate of these 500- to 1500-g infants has increased to approximately 85%.181 Of these, 5% to 10% have what is characterized as cerebral palsy and 25% to 50% exhibit behavioral and cognitive deficits that lead to important school problems (see Chapter 10).182,183 These VLBW infants constitute a tiny proportion of the birth population, but they are at the highest risk for development of cerebral palsy; infants weighing less than 1500 g at birth account for 25% of cases of this disorder.58
Markers for brain injury affecting preterm infants are germinal matrix intraventricular hemorrhage (IVH) and periventricular leukomalacia. The brain injury may occur either as a consequence of the IVH and its sequelae or as an associated finding. The incidence of germinal matrix IVH in preterm infants declined from 35% to 50% in the late 1970s and early 1980s to approximately 15% in the mid 1990s.184 Despite the decreased incidence of germinal matrix IVH, which is directly related to prematurity,183 the overall burden of disability has sharply increased in recent years due to the proportion of very preterm infants who are surviving.184 Periventricular leukomalacia, which is the classic neuropathology associated with hypoxic-ischemic cerebral white matter injury in the preterm infant, commonly accompanies IVH.185
The fragility of the immature subependymal germinal matrix predisposes the preterm infant to the development of IVH. The hemorrhage originates from the endothelial cell–lined vessels that course through the germinal matrix in free communication with the venous circulation (i.e., the capillary–venule junction). The mechanism of damage to these endothelial cells and to the integrity of these capillaries has been investigated in animal models186 and in human neonates by means of Doppler velocimetry.187
Volpe,188,189 who has reviewed the theories of the pathogenesis of germinal matrix IVH, has concluded that the pathogenesis is multifactorial; different combinations of factors are relevant in different patients. The three major categories in the pathogenesis of IVH are intravascular, vascular, and extravascular. Intravascular factors include fluctuating cerebral blood flow (CBF), which can result from respiratory disturbances in the ventilated preterm infant with neonatal respiratory distress syndrome187,190; increases in CBF186,191; increases in cerebral venous pressure192; decreases in CBF followed by reperfusion; and platelet and coagulation disturbances.193 Vascular factors include the tenuousness of the capillary integrity of the germinal matrix and the vulnerability of the matrix capillaries to hypoxic-ischemic injury.194 Extravascular factors include deficient vascular support, excessive fibrinolytic activity, and a possible postnatal decrease in extravascular tissue pressure.195
Of special interest in the discussion of antepartum and intrapartum care and neonatal resuscitation are the possible interventions that may prevent or lessen the severity of IVH. The best way to prevent germinal matrix IVH is to prevent preterm birth. Infection and inflammation are the most common identified causes of preterm birth at the lowest relevant gestational age.196 Antenatal treatment of infections has not been proved to prevent preterm labor or premature rupture of membranes58; however, prevention of infection, if possible, may be an important way to reduce the risk for IVH. Another intervention that lowers the incidence of IVH is the transportation of the preterm mother while the fetus is still in utero to a center that specializes in the care of high-risk neonates.1
Various antenatal pharmacologic interventions have been evaluated for the prevention of IVH. Clinical trials of antenatal maternal administration of phenobarbital197,198 and vitamin K199,200 have yielded conflicting results, and their routine use is not currently recommended.58
Corticosteroids are currently the most beneficial antenatal pharmacologic intervention for the prevention of IVH. This effect was first noticed when obstetricians began giving betamethasone and dexamethasone to pregnant women to help accelerate fetal lung maturity. The mechanism behind this protection is thought to be improved neonatal cardiovascular stability, which results in less hypotension and less need for blood pressure treatment in these infants.201 Antenatal betamethasone administration leads to lower placental vascular resistance and higher placental blood flow.202 This improvement in placental blood flow may decrease impairment of the preterm infant’s cerebral autoregulation. In addition, corticosteroids may stimulate the maturation of the germinal matrix. There is consensus regarding the efficacy of a single course of corticosteroids in patients at risk for preterm delivery, but the risks and benefits of multiple courses of corticosteroids for women who remain undelivered 7 days after the initial dose are still controversial. Obstetricians must balance the possible benefits of these agents against their potentially deleterious effects on neuronal and organ growth (see Chapter 34).
Multiple studies have demonstrated a lower incidence of cerebral palsy in infants of mothers given magnesium sulfate for the treatment of preeclampsia or for tocolysis203,204; subsequent studies have observed a similar benefit when magnesium has been given specifically for fetal neuroprotection.205–207 An ACOG committee opinion208 now recommends the administration of magnesium sulfate to mothers in preterm labor. Maternal magnesium sulfate administration does not result in a decreased incidence of IVH, although the incidence of high-grade (grade III or IV) lesions may be reduced.209 Although some investigators have suggested that antenatal exposure to magnesium sulfate results in a higher risk for adverse neonatal outcomes,210 others have observed no association between umbilical cord blood magnesium concentration and the need for delivery room resuscitation when magnesium was administered for neuroprotection in anticipation of a preterm birth.211
Postnatal interventions that may prevent IVH include the avoidance of overly rapid infusion of volume expanders or hypertonic solutions such as sodium bicarbonate.143,212 The establishment of adequate ventilation is the most beneficial immediate intervention that helps preserve cerebrovascular autoregulation in the preterm infant. The prevention of hypoxemia and hypercarbia is essential, because they are both linked to pressure-passive cerebral circulation, which in turn leads to the development of IVH.212
Among infants who exhibit fluctuating CBF velocity, Pearlman et al.213 found that treatment with pancuronium bromide, which corrects this fluctuation, reduced both the incidence and severity of IVH.213 Other clinical trials have evaluated the efficacy of other pharmacologic agents for the correction of fluctuating hemodynamic disturbances. Studies of meperidine214 and fentanyl215 have shown some benefit, but the side effects and need for prolonged ventilation associated with these agents must be weighed against any potential benefits.
If the use of antepartum and intrapartum pharmacologic prophylaxis against IVH becomes part of preterm delivery management, the practice of obstetric anesthesia for preterm patients will be directly affected. For example, the conventional wisdom is that preterm infants are more sensitive than term infants to the effects of maternally administered agents such as analgesics216 and that this effect is inherently deleterious. However, if this effect is found to protect the preterm infant brain from factors that may lead to IVH (e.g., hemodynamic instability), perhaps obstetric anesthesia providers will no longer attempt to avoid the placental transfer of pharmacologic agents but will deliberately administer these agents to the mother with the intent that they reach the fetus.
Congenital Anomalies
Occasionally, neonatal resuscitation is complicated by congenital anomalies of the airway or diaphragm. These anomalies may manifest as respiratory distress, which resolves only when appropriate resuscitation techniques are used. For example, neonates are obligatory nose breathers. The diagnosis and management of choanal stenosis and atresia include placement of an oral airway or endotracheal tube until a definitive surgical procedure can be performed.
Other congenital anomalies that cause upper airway obstruction include (1) micrognathia, as in Pierre Robin sequence; (2) macroglossia, as in Beckwith-Wiedemann syndrome or glycogen storage disease type II; (3) laryngeal webs; (4) laryngeal atresia; (5) stenosis or paralysis at the level of the vocal cords; (6) subglottic stenosis; (7) subglottic webs; (8) tracheal agenesis; and (9) tracheal rings. Obstruction also can occur as a result of tumors such as subglottic hemangiomas. The presence of a cleft palate may lead to difficulty with manual ventilation. In an infant with micrognathia or macroglossia, airway patency may be maintained if the neonate is kept in the prone position, which reduces posterior movement of the tongue into the pharynx. If macroglossia is extreme, use of an oral airway or a small nasogastric or orogastric suction catheter may be necessary to prevent complete obstruction of the pharynx by the tongue.
When respiratory distress and difficulty with bag-and-mask ventilation are encountered, laryngoscopy should be performed. The cause of the obstruction may be evident if it is supraglottic in location. Some supraglottic entities (e.g., laryngeal webs) may be treated successfully by passing an endotracheal tube through the obstruction and into the trachea. Subglottic lesions may require tracheostomy. The help of an otolaryngologist may be invaluable during resuscitation of a neonate with congenital airway obstruction. If there is antepartum evidence of such a condition (e.g., laryngeal stenosis), it is best to have an otolaryngologist present at the time of delivery.217 If obstruction is discovered after delivery, the resuscitator should not hesitate to call for surgical assistance.
Fetal neck masses such as cervical teratoma and lymphangioma can lead to extrinsic airway compression. The resulting distortion of the airway can result in airway obstruction, and it may be difficult—if not impossible—to secure an airway in a timely fashion at delivery. These masses often are diagnosed before delivery because of the associated occurrence of polyhydramnios resulting from esophageal compression. In these rare cases, a multidisciplinary team should be assembled before delivery to assist in securing the airway. Leichty et al.218 described a way of providing the time necessary to secure an airway, known as the ex utero intrapartum treatment (EXIT) procedure (see Chapter 7). An EXIT procedure delivers the fetal head and shoulders, but keeps the lower torso and umbilical cord intact within the uterus, thereby maintaining placental perfusion and oxygenation. The fetus can be given additional agents intramuscularly (fentanyl, vecuronium, and atropine) to provide fetal analgesia and to prevent movement and breathing. The FHR and SaO2 are monitored continuously via a pulse oximeter probe attached to the fetal hand. The pediatric surgeon can then perform direct laryngoscopy, rigid bronchoscopy, or tracheostomy if necessary. After establishment of the airway, the delivery of the infant is completed.
The EXIT procedure has been considered an option for fetuses with a number of congenital anomalies.219,220 A common indication for the EXIT procedure is an intrinsic airway obstruction. Intrinsic airway obstruction of the larynx or upper trachea (e.g., laryngeal web, subglottic cyst, tracheal atresia) can lead to retention of bronchial secretions and subsequent pulmonary distention; this constellation of findings is often classified as congenital high airway obstruction syndrome (CHAOS).219 Use of the EXIT procedure resulted in the first long-term survival of a child with this syndrome.221
The EXIT procedure also may be useful in conditions such as severe congenital heart disease, in which the need for emergency ECMO at birth is anticipated. The EXIT procedure allows for the placement of arterial and venous cannulas before umbilical cord clamping, thereby avoiding an unstable period between the termination of placental perfusion and the institution of ECMO. 219 Other possible indications for the EXIT procedure include the resection of congenital cystic adenomatoid malformations and as a first step in separation procedures for conjoined twins with cardiovascular involvement.
Noah et al.222 compared the short-term maternal outcomes of 34 patients who underwent the EXIT procedure between 1994 and 1999 with those in a control group who underwent nonemergency primary cesarean delivery. The EXIT procedure group had a higher estimated blood loss, but there was no difference in the postoperative hematocrit or duration of hospital stay. The EXIT procedure group also had a higher rate of superficial wound infection (15% versus 2%), but the incidence of endometritis was not different. A review of fetal and maternal outcomes after performance of an EXIT procedure in 12 infants with a giant neck mass found that 11 infants survived and 10 had normal development. All of the six mothers who desired future pregnancies subsequently had uncomplicated deliveries.223
Anesthetic considerations for the mother during an EXIT procedure include those relevant to general anesthesia for the mother undergoing cesarean delivery or other surgical procedures during pregnancy (see Chapters 7, 17, and 26). Several volatile halogenated agents have been used for the EXIT procedure, including isoflurane, desflurane, and sevoflurane.219 The anesthetic management for an EXIT procedure differs from that for a routine cesarean delivery in the following ways: (1) general anesthesia is used much more often than neuraxial anesthesia, (2) a greater depth of anesthesia is achieved and maintained, (3) maximum uterine relaxation is desirable, (4) warm fluid is occasionally instilled into the uterus, and (5) a second anesthesiologist provides care for the fetus.220
George et al.224 described an alternative approach for the EXIT procedure with the use of combined spinal-epidural anesthesia (1.5 mL of bupivacaine 0.75%, fentanyl 15 µg, and morphine 0.15 mg, administered intrathecally, followed by placement of a multiorifice epidural catheter). Supplemental oxygen was provided through a face mask at 6 L/min. Immediately before uterine incision, the patients were given intravenous nitroglycerin 50 to 100 µg, followed by an infusion of nitroglycerin (0.5 to 1.5 µg/kg/min), allowing adequate uterine relaxation for partial delivery of the infant’s head. Maternal hypotension occurred in two of the three women and required vasopressor administration. After the infant’s airway was secured and the infant’s delivery was completed, the nitroglycerin was discontinued at the time of umbilical cord clamping.
Esophageal atresia and tracheoesophageal fistula occur in 1 of every 3000 births.225 There are many variations of these anomalies, the most common being esophageal atresia with a distal tracheoesophageal fistula (80% to 90% of cases). Neonates with a tracheoesophageal fistula are at increased risk for the pulmonary aspiration of gastric contents through the fistula into the lung. When the presence of a tracheoesophageal fistula is not known antepartum, it should be suspected if bubbling secretions are observed during spontaneous or bag-and-mask ventilation. Once a tracheoesophageal fistula is suspected, bag-and-mask ventilation should be discontinued, because its use may contribute to overdistention of the gastrointestinal tract with air, possibly leading to difficulty in ventilation from impingement of the enlarged stomach on the diaphragm. A suction catheter should be placed in the esophageal pouch to facilitate the removal of oral secretions. If mechanical ventilation is necessary, an endotracheal tube should be inserted with the tip distal to the entrance of the fistula. This positioning can be accomplished by performing an intentional right mainstem bronchial intubation followed by slowly withdrawing the tube until breath sounds are auscultated on the left; a lack of breath sounds over the stomach should then be confirmed. Percutaneous gastrostomy placement may be necessary during resuscitation to facilitate decompression of the gastrointestinal tract.
Congenital diaphragmatic hernia (CDH) occurs in approximately 1 in 3000 live births.226 The mortality rate from CDH is 30% to 60%. In 80% to 90% of cases the CDH occurs on the left side and is the result of herniation of the gut through the posterolateral defect of Bochdalek. During formation of the lung, herniation of the gut into the thoracic cavity results in hypoplasia of the lung tissue and pulmonary vasculature. This hypoplasia may be unilateral, but often it is bilateral because of the shift in mediastinal structures to the other side. CDH should be suspected when a neonate has respiratory difficulty and a scaphoid abdomen; this abnormal abdominal shape results from the presence of abdominal contents in the thorax.
During resuscitation of the neonate with CDH, bag-and-mask ventilation is contraindicated because it allows further distention of the gut, which would further impinge on the lung. Tracheal intubation is recommended, followed by the placement of a nasogastric or orogastric tube to ensure decompression of the gastrointestinal tract. Ventilation should consist of low-positive-pressure breaths to decrease the risk for causing a pneumothorax on the side contralateral to the CDH. If a pneumothorax does occur, it must be evacuated promptly. In the neonate, evacuation is accomplished initially by placement of a 22-gauge needle into the second intercostal space in the midclavicular line and aspiration of air with an attached stopcock and syringe. Severe pulmonary hypertension often accompanies CDH. Maintenance of euthermia, normoxia, and adequate systemic blood pressure promotes pulmonary artery blood flow.
Whenever congenital anomalies of the respiratory tract are noted, the presence of other anomalies should be suspected. It is important to evaluate the neonate promptly for cardiac malformations, especially if appropriate resuscitative efforts are not successful. Echocardiography is used to evaluate cardiac structures and function.
Ethical Considerations
The current neonatal resuscitation guidelines address the ethical considerations of non-initiation or discontinuation of resuscitation in the delivery room.2 Extremes of prematurity (< 23 weeks’ confirmed gestation) and severe congenital anomalies (e.g., anencephaly, confirmed trisomy 13 or 18) are examples of circumstances when non-initiation of resuscitation is considered appropriate. Because intrapartum confirmation of pertinent information may not be possible, it is recognized that initiation of resuscitation may occur and that its discontinuation may then be appropriate after further information has been obtained and discussion with family has occurred. In some cases, a trial of therapy may be appropriate, which does not always mandate continued support. In situations or conditions in which there is a high rate of survival and acceptable morbidity (i.e., ≥ 25 weeks’ gestation and most congenital malformations), resuscitation is generally indicated. For those situations with a poor prognosis, including unlikely survival and potentially high morbidity (i.e., 23 to 25 weeks’ gestation), the parents’ desires as to initiation of resuscitation should be supported (Table 9-5).227
TABLE 9-5
Guidelines for Withholding or Discontinuing Resuscitation
| Conditions with high survival, acceptable risk for morbidity | ≥ 25 weeks’ gestational age Most congenital malformations |
Resuscitation nearly always indicated |
| Conditions with poor prognosis, high risk for morbidity | 23 to 25 weeks’ gestational age | Parental desires about initiating/continuing resuscitation should be supported |
| Conditions with almost certain death or unacceptably high morbidity | < 23 weeks’ gestational age Birth weight < 400 g Anencephaly Chromosomal abnormalities incompatible with life (e.g., trisomy 13) |
Resuscitation not indicated |
Modified from Neonatal Resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010; 126:e1400-1413.
Discontinuation of resuscitation of an infant with cardiopulmonary arrest may be appropriate if spontaneous circulation has not occurred in 15 minutes. After 10 minutes of asystole, survival itself and survival without severe disabilities are very unlikely.227–232
Neurobehavioral Testing
It is difficult to detect subtle neurobehavioral differences among neonates during the assignment of Apgar scores or the performance of the initial neurologic examination; therefore, investigators have developed and studied methods of documenting neonatal neurobehavioral status (Table 9-6). In the past, the neonate was considered incapable of exhibiting higher cortical function. However, investigators have noted that the term neonate is able to sense and respond to a variety of stimuli in a well-organized fashion.233–235
In 1973, Brazelton236 described the Neonatal Behavioral Assessment Scale (NBAS) with the following four variables as key determinants of neonatal neurobehavior: (1) various prenatal influences (e.g., infection); (2) the maturity of the infant, especially its CNS; (3) the effects of analgesics and anesthetics administered to the mother before and during delivery; and (4) the effects of difficulties encountered during delivery (e.g., trauma). The NBAS was developed as a tool to detect neurobehavioral abnormalities that resulted from any of these four variables.
This scale consists of 47 individual tests with 27 evaluating behavior and 20 evaluating elicited or provoked responses. The 47 tests can be completed in approximately 45 minutes. The NBAS evaluates the ability of the neonate to perform complex motor behaviors, to alter the state of arousal, and to suppress meaningless stimuli. The goal is to provide an extensive evaluation of neonatal cortical function and to detect subtle differences among groups of infants. Habituation (i.e., the ability to suppress the response to meaningless, repetitive stimuli) is considered an excellent indicator of normal early cortical function.233
In 1974, Scanlon et al.237 described the Early Neonatal Behavioral Scale (ENNS), which consisted of tests that were easy to perform and score quantitatively during the neonatal period. The ENNS was developed primarily for the evaluation of the effects of maternal medications (e.g., analgesic and anesthetic agents) on neonatal neurobehavior. The ENNS consists of (1) 15 observations of muscle tone and power, reflexes (e.g., rooting, sucking, Moro), and response to stimuli (e.g., light, sound, pinprick); (2) 11 observations of the infant’s state of wakefulness; (3) an assessment of the ability of the neonate to habituate to repetitive stimuli; and (4) an overall general assessment of neurobehavioral status. This test can be performed in 6 to 10 minutes.
In 1982, Amiel-Tison et al.238 described the Neurologic and Adaptive Capacity Score (NACS) to differentiate neonatal depression secondary to maternally administered drugs from depression due to asphyxia, birth trauma, or neurologic disease. Whereas the ENNS concentrates on the infant’s habituation ability, the NACS emphasizes motor tone as a key indicator of drug-induced abnormal neurobehavior. The basis for this emphasis on neonatal motor tone is explained as follows: unilateral or upper body hypotonus may occur as a result of either birth trauma or anoxia, but global motor depression is more likely a result of anesthetic- or analgesic-induced depression. A total of 20 criteria are tested in the areas of adaptive capacity, passive tone (e.g., scarf sign), active tone (e.g., assessment of the flexor and extensor muscles of the neck), primary reflexes (e.g., Moro), and alertness. The total possible score is 40, and a score of 35 to 40 is considered normal. The NACS can be performed in 3 to 4 minutes.
Amiel-Tison et al.238 examined inter-observer reliability and assessed the correlation of results between the NACS and ENNS. The inter-observer reliability was 93% for the NACS and 88% for the ENNS. Approximately 92% of infants with high scores on the ENNS scored equally well on the NACS. However, the reliability of NACS has been questioned239,240; Halpern et al.241 examined 200 healthy term infants with the NACS and found poor inter-observer reliability. In contrast, in 2002 Amiel-Tison242 reported her later experience with the NACS and documented good inter-observer reliability.
Anesthesiologists have used neurobehavioral testing to document the effects of analgesic and anesthetic agents and techniques on neonatal neurobehavior (see Table 9-6); the American Academy of Pediatrics243 and the U.S. Food and Drug Administration244,245 have recommended that these investigations be performed. A number of studies have demonstrated transient, serum concentration–dependent depression of neonatal neurobehavior with the maternal administration of systemic agents (e.g., meperidine, diazepam).246–248 However, in a NBAS examination that controlled for patient and labor and delivery characteristics, only decreased habituation was observed in neonates born to mothers who had received intravenous meperidine.249 Similarly, maternal administration of intravenous fentanyl appears to minimally affect neonatal NACS examinations.250
As is the case with many studies of systemic agents, studies of epidural anesthesia are often confounded by variables that are difficult to control, such as different patient populations, varied durations of labor, and multiple drug administrations. Scanlon et al.237 introduced the ENNS in a study of the effect of maternal epidural anesthesia on neonatal neurobehavior. The researchers concluded that epidural anesthesia was associated with lower ENNS scores because of decreased muscle strength and tone. In this study, all patients who had received epidural anesthesia were considered part of one group, although 9 patients had received lidocaine and 19 had received mepivacaine. Further investigation showed that epidural lidocaine, even when administered in larger doses for cesarean delivery, does not affect ENNS scores.251 The difference in ENNS scores between the epidural and nonepidural groups noted in the earlier study237 was most likely related to the use of mepivacaine rather than lidocaine.252 As was observed with lidocaine, epidurally administered bupivacaine, 2-chloroprocaine, and etidocaine—when given for cesarean delivery—do not affect ENNS scores.251,253 Kuhnert et al.254 assessed NBAS scores in a group of infants exposed to either epidural lidocaine or 2-chloroprocaine. Although the investigators observed subtle changes in neurobehavior in the group of infants whose mothers had received lidocaine, they concluded that other variables (e.g., mode of delivery) are more likely to affect performance on neurobehavioral testing.
Sepkoski et al.255 compared NBAS scores between two groups of vaginally delivered infants. In one group, the mothers had received epidural bupivacaine, and in the other group, the mothers had received no anesthesia or analgesia. The infants in the epidural group showed less alertness, less orientation ability, and less motor function maturity than the infants in the control group. However, variables such as duration of labor, incidence of oxytocin administration, and incidence of instrumental delivery were not similar in the two groups. Earlier, Abboud et al.256 performed ENNS examinations on vaginally delivered infants whose mothers had received epidural bupivacaine. In this study, epidural administration of bupivacaine did not affect the ENNS scores. The maternal doses of epidural bupivacaine and the maternal venous and umbilical cord blood bupivacaine concentrations were similar to those noted by Sepkoski et al.255 Abboud et al.256 also noted normal ENNS scores for infants whose mothers had received epidural lidocaine or 2-chloroprocaine.
Critics of the ENNS and NACS claim that the evaluations are unable to demonstrate subtle differences in neurobehavior that would be detected by the more comprehensive NBAS.257 However, although some differences have been observed in NBAS performance among groups of infants exposed or not exposed to local anesthetics, confounding variables have prevented clear conclusions as to cause and effect.
Hodgkinson et al.258 observed that the subarachnoid administration of tetracaine for cesarean delivery did not adversely affect ENNS performance. Other studies have indicated that NACS performance is not significantly affected by the maternal epidural administration of opioids259–264 or epinephrine (in combination with a local anesthetic).265–268
The effects of general anesthetic agents on neonatal neurobehavior have been evaluated by the ENNS and NACS. In a prospective, randomized study, Abboud et al.269 assessed NACS performance at 15 minutes, 2 hours, and 24 hours of age in infants whose mothers received general, epidural, or spinal anesthesia for cesarean delivery. Women who underwent general anesthesia received thiopental 4 mg/kg followed by enflurane 0.5% with nitrous oxide 50% in oxygen. Although the NACS was lower at both 15 minutes and 2 hours of age in the infants in the general anesthesia group than in the infants in the neuraxial anesthesia groups, no difference in NACS results was noted at 24 hours of age.
Hodgkinson et al.258 used the ENNS to evaluate outcomes among three groups of infants, all of whom were delivered by elective cesarean delivery. One group of women received general anesthesia with thiopental 4 mg/kg followed by 50% nitrous oxide. A second group received general anesthesia with ketamine 1 mg/kg followed by 50% nitrous oxide. A third group received spinal anesthesia with tetracaine 6 to 8 mg. The ENNS evaluations were conducted at 4 to 8 hours of age and again at 24 hours. During the 4- to 8-hour examination, infants in the spinal anesthesia group scored significantly higher on multiple components of the ENNS than did infants in either of the general anesthesia groups. At 24 hours, infants in the spinal anesthesia group scored significantly higher than those in the thiopental group in alertness, total decrement score, and overall assessment. Similarly, infants in the spinal anesthesia group scored higher than those in the ketamine group in alertness and overall assessment. No significant differences existed between the scores of the thiopental group infants and the ketamine group infants.258 Palahniuk et al.270 observed similar results in a study that compared groups of infants whose mothers received either epidural anesthesia or general anesthesia for elective cesarean delivery. Infants whose mothers had received thiopental and nitrous oxide scored significantly lower in the alertness component of the ENNS than infants whose mothers had received epidural lidocaine with epinephrine.
Stefani et al.271 observed that subanesthetic maternal doses of enflurane or nitrous oxide did not affect neonatal neurobehavior (as assessed by ENNS and NACS) at 15 minutes, 2 hours, and 24 hours of age. Abboud et al.272 obtained similar results from NACS examinations of infants whose mothers had received subanesthetic doses of isoflurane.
The long-term effects of perinatal exposure to either general or neuraxial anesthesia at the time of cesarean delivery compared with vaginal delivery appear limited. In a population-based birth cohort, Sprung et al.273 found that children exposed to either general or regional anesthesia during cesarean delivery were not more likely to develop learning disabilities than children who were delivered vaginally.
In summary, subtle changes in neonatal neurobehavior may result from factors such as antepartum maternal drug exposure. Parent-infant bonding and the ability of the infant to breast-feed may be adversely affected by these neurobehavioral changes.233 These transient effects may seem trivial to some observers but important to others. With regard to the long-term neurologic outcome of individual infants, performance during neurobehavioral assessment may aid the observer in the formulation of a prognosis. However, as demonstrated with Apgar scores, the prognostic value of an isolated test score is likely to be lower than the prognostic value of multiple factors considered together during the overall assessment of an individual infant.
References
1. American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 6th edition. American Academy of Pediatrics and American College of Obstetrics and Gynecology: Elk Grove, IL; 2007.
2. Perlman JM, Wyllie J, Kattwinkel J, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S516–S538.
3. Gordon A, McKechnie EJ, Jeffery H. Pediatric presence at cesarean section: justified or not? Am J Obstet Gynecol. 2005;193:599–605.
4. Atherton N, Parsons SJ, Mansfield P. Attendance of paediatricians at elective Caesarean sections performed under regional anaesthesia: is it warranted? J Paediatr Child Health. 2006;42:332–336.
5. American College of Obstetricians and Gynecologists Committee on Obstetric Practice and American Society of Anesthesiologists Committee on Obstetric Anesthesia. Optimal goals for anesthesia care in obstetrics. [ACOG Committee Opinion No. 433] Obstet Gynecol. 2009;113:1197–1199.
6. Bucklin BA, Hawkins JL, Anderson JR, Ullrich FA. Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005;103:645–653.
7. Heyman HJ. Neonatal resuscitation and anesthesiologist liability. Anesthesiology. 1994;81:783.
8. Gaiser R, Lewin SB, Cheek TG, Guttsche BB. Anesthesiologists’ interest in neonatal resuscitation certification. J Clin Anesth. 2001;13:374–376.
9. Chadwick HS, Posner K, Caplan RA, et al. A comparison of obstetric and nonobstetric anesthesia malpractice claims. Anesthesiology. 1991;74:242–249.
10. Rudolph AM, Heyman MA. Fetal and neonatal circulation and respiration. Annu Rev Physiol. 1974;36:187–207.
11. Rudolph AM. The changes in the circulation after birth: their importance in congenital heart disease. Circulation. 1970;41:343–359.
12. Cassin S, Dawes GS, Mott JC, et al. The vascular resistance of the foetal and newly ventilated lung of the lamb. J Physiol. 1964;171:61–79.
13. Rudolph AM, Yuan S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J Clin Invest. 1966;45:399–411.
14. Boreus LO, Malmfors T, McMurphy DM, Olson L. Demonstration of adrenergic receptor function and innervation in the ductus arteriosus of the human fetus. Acta Physiol Scand. 1969;77:316–321.
15. Assali NS, Morris JA, Smith RW, Manson WA. Studies on ductus arteriosus circulation. Circ Res. 1963;13:478–489.
16. Dimich I, Singh PP, Adell A, et al. Evaluation of oxygen saturation monitoring by pulse oximetry in neonates in the delivery system. Can J Anaesth. 1991;38:985–988.
17. Walsh-Sukys MC. Persistent pulmonary hypertension of the newborn: the black box revisited. Clin Perinatol. 1993;20:127–143.
18. Alano MA, Ngougmna E, Ostrea EM Jr, Konduri GG. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics. 2001;107:519–523.
19. Adams FH, Moss AJ, Fagan L. The tracheal fluid in the fetal lamb. Biol Neonat. 1963;5:151–158.
20. Ross BB. Comparison of foetal pulmonary fluid with foetal plasma and amniotic fluid. Nature. 1963;199:1100.
21. Karlberg P. The adaptive changes in the immediate postnatal period, with particular reference to respiration. J Pediatr. 1960;56:585–604.
22. Usher RH, Allen AC, McLean FH. Risk of respiratory distress syndrome related to gestational age, route of delivery, and maternal diabetes. Am J Obstet Gynecol. 1971;111:826–832.
23. Lawson EE, Birdwell RL, Huang PS, Taeusch HW Jr. Augmentation of pulmonary surfactant secretion by lung expansion at birth. Pediatr Res. 1979;13:611–614.
24. Platzker AC, Kitterman JA, Mescher EJ, et al. Surfactant in the lung and tracheal fluid of the fetal lamb and acceleration of its appearance by dexamethasone. Pediatrics. 1975;56:554–561.
25. Smrcek JM, Schwartau N, Kohl M, et al. Antenatal corticosteroid therapy in premature infants. Arch Gynecol Obstet. 2005;271:26–32.
26. Turbeville DF, McCaffree MA, Block MF, Krous HF. In utero distal pulmonary meconium aspiration. South Med J. 1979;72:535–536.
27. Lagercrantz H, Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatr Res. 1977;11:889–893.
28. Dahm LS, James LS. Newborn temperature and calculated heat loss in the delivery room. Pediatrics. 1972;49:504–513.
29. Cramer K, Wiebe N, Hartling L, et al. Heat loss prevention: a systematic review of occlusive skin wrap for premature neonates. J Perinatol. 2005;25:763–769.
30. Vohra S, Roberts RS, Zhang B, et al. Heat Loss Prevention (HeLP) in the delivery room: a randomized controlled trial of polyethylene occlusive skin wrapping in very preterm infants. J Pediatr. 2004;145:750–753.
32. Papile LA. Systemic hypothermia—a “cool” therapy for neonatal hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1619–1620.
33. Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64.
34. Philip J, Alexander JM, Sharma SK, et al. Epidural analgesia during labor and maternal fever. Anesthesiology. 1999;90:1271–1275.
35. Lieberman E, Lang JM, Frigoletto F Jr, et al. Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics. 1997;99:415–419.
36. Kaul B, Vallejo M, Ramanathan S, Mandell G. Epidural labor analgesia and neonatal sepsis evaluation rate: a quality improvement study. Anesth Analg. 2001;93:986–990.
37. Huddleston JF. Intrapartum fetal assessment: a review. Clin Perinatol. 1999;26:549–568.
38. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049–1058.
39. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Fetal pulse oximetry. September 2001. [ACOG Committee Opinion No. 258] Obstet Gynecol. 2001;98:523–524.
40. East CE, Chan FY, Colditz PB, Begg LM. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database Syst Rev. 2007;(2).
41. Algert CS, Bowen JR, Giles WB, et al. Regional block versus general anaesthesia for caesarean section and neonatal outcomes: a population-based study. BMC Med. 2009;7:20.
42. Hook B, Kiwi R, Amini SB, et al. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics. 1997;100:348–353.
43. Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. 1953;32:260–267.
44. Apgar V. The newborn (Apgar) scoring system: reflections and advice. Pediatr Clin North Am. 1966;13:645–650.
45. Apgar V, James LS. Further observations on the newborn scoring system. Am J Dis Child. 1962;104:419–428.
46. Sykes GS, Molloy PM, Johnson P, et al. Do Apgar scores indicate asphyxia? Lancet. 1982;1:494–496.
47. Lauener PA, Calame A, Janecek P, et al. Systematic pH-measurements in the umbilical artery: causes and predictive value of neonatal acidosis. J Perinat Med. 1983;11:278–285.
48. Suidan JS, Young BK. Outcome of fetuses with lactic acidemia. Am J Obstet Gynecol. 1984;150:33–37.
49. Fields LM, Entman SS, Boehm FH. Correlation of the one-minute Apgar score and the pH value of umbilical arterial blood. South Med J. 1983;76:1477–1479.
50. Boehm FH, Fields LM, Entman SS, Vaughn WK. Correlation of the one-minute Apgar score and umbilical cord acid-base status. South Med J. 1986;79:429–431.
51. Page FO, Martin JN, Palmer SM, et al. Correlation of neonatal acid-base status with Apgar scores and fetal heart rate tracings. Am J Obstet Gynecol. 1986;154:1306–1311.
52. Luthy DA, Shy KK, Strickland D, et al. Status of infants at birth and risk for adverse neonatal events and long-term sequelae: a study in low birth weight infants. Am J Obstet Gynecol. 1987;157:676–679.
53. Josten BE, Johnson TR, Nelson JP. Umbilical cord blood pH and Apgar scores as an index of neonatal health. Am J Obstet Gynecol. 1987;157:843–848.
54. Vintzileos AM, Gaffney SE, Salinger LM, et al. The relationships among the fetal biophysical profile, umbilical cord pH, and Apgar scores. Am J Obstet Gynecol. 1987;157:627–631.
55. Drage JS, Kennedy C, Berendes H, et al. The Apgar score as an index of infant morbidity. A report from the collaborative study of cerebral palsy. Dev Med Child Neurol. 1966;8:141–148.
56. Drage JS, Kennedy C, Schwarz BK. The Apgar score as an index of neonatal mortality. A report from the collaborative study of cerebral palsy. Obstet Gynecol. 1964;24:222–230.
57. American College of Obstetrics and Gynecologists, American Academy of Pediatrics. Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis & Pathophysiology. American College of Obstetrics and Gynecologists: Washington, DC; 2003.
58. Freeman JM, Nelson KB. Intrapartum asphyxia and cerebral palsy. Pediatrics. 1988;82:240–249.
59. Gilstrap LC 3rd, Leveno KJ, Burris J, et al. Diagnosis of birth asphyxia on the basis of fetal pH, Apgar score, and newborn cerebral dysfunction. Am J Obstet Gynecol. 1989;161:825–830.
60. Nelson KB, Ellenberg JH. Antecedents of cerebral palsy: multivariate analysis of risk. N Engl J Med. 1986;315:81–86.
61. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. The Apgar score. [ACOG Committee Opinion No. 333] Obstet Gynecol. 2006;107:1209–1212.
62. Finster M, Wood M. The Apgar score has survived the test of time. Anesthesiology. 2005;102:855–857.
63. Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344:467–471.
64. Papile LA. The Apgar score in the 21st century. N Engl J Med. 2001;344:519–520.
65. Catlin EA, Carpenter MW, Brann BIV, et al. The Apgar score revisited: influence of gestational age. J Pediatr. 1986;109:865–868.
66. Urschitz MS, Von EV, Seyfang A, Poets CF. Use of pulse oximetry in automated oxygen delivery to ventilated infants. Anesth Analg. 2002;94:S37–S40.
67. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Umbilical cord blood gas and acid-base analysis. [ACOG Committee Opinion No. 348] Obstet Gynecol. 2006;108:1319–1322.
68. Lievaart M, de Jong PA. Acid-base equilibrium in umbilical cord blood and time of cord clamping. Obstet Gynecol. 1984;63:44–47.
69. Ackerman BD, Sosna MM, Ullrich JR. A technique for serial sampling of umbilical artery blood at birth. Biol Neonate. 1972;20:458–465.
70. Chou PJ, Ullrich JR, Ackerman BD. Time of onset of effective ventilation at birth. Biol Neonate. 1974;24:74–81.
71. White CR, Mok T, Doherty DA, et al. The effect of time, temperature and storage device on umbilical cord blood gas and lactate measurement: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25:587–594.
72. Kirshon B, Moise KJ Jr. Effect of heparin on umbilical arterial blood gases. J Reprod Med. 1989;34:267–269.
73. Strickland DM, Gilstrap LC, Hauth JC, Widmer K. Umbilical cord pH and PCO2: effect of interval from delivery to determination. Am J Obstet Gynecol. 1984;148:191–194.
74. Gilstrap LC, Hauth JC, Hankins GD, Beck AW. Second-stage fetal heart rate abnormalities and type of neonatal acidemia. Obstet Gynecol. 1987;70:191–195.
75. Miller JM Jr, Bernard M, Brown HL, et al. Umbilical cord blood gases for term healthy newborns. Am J Perinatol. 1990;7:157–159.
76. Helwig JT, Parer JT, Kilpatrick SJ, Laros RK Jr. Umbilical cord blood acid-base state: what is normal? Am J Obstet Gynecol. 1996;174:1807–1814.
77. Yeomans ER, Hauth JC, Gilstrap LC, Strickland DM. Umbilical cord pH, PCO2, and bicarbonate following uncomplicated term vaginal deliveries. Am J Obstet Gynecol. 1985;151:798–800.
78. Ramin SM, Gilstrap LC, Leveno KJ, et al. Umbilical artery acid-base status in the preterm infant. Obstet Gynecol. 1989;74:256–258.
79. Thorp JA, Dildy GA, Yeomans ER, et al. Umbilical cord blood gas analysis at delivery. Am J Obstet Gynecol. 1996;175:517–522.
80. Huisjes HJ, Aarnoudse JG. Arterial or venous umbilical pH as a measure of neonatal morbidity? Early Hum Dev. 1979;3:155–161.
81. Eskes TK, Jongsma HW, Houx PC. Percentiles for gas values in human umbilical cord blood. Eur J Obstet Gynecol Reprod Biol. 1983;14:341–346.
82. Low JA. The role of blood gas and acid-base assessment in the diagnosis of intrapartum fetal asphyxia. Am J Obstet Gynecol. 1988;159:1235–1240.
83. Thorp JA, Sampson JE, Parisi VM, Creasy RK. Routine umbilical cord blood gas determinations? Am J Obstet Gynecol. 1989;161:600–605.
85. Nagel HT, Vandenbussche FP, Oepkes D, et al. Follow-up of children born with an umbilical arterial blood pH < 7. Am J Obstet Gynecol. 1995;173:1758–1764.
86. Ruth VJ, Raivio KO. Perinatal brain damage: predictive value of metabolic acidosis and the Apgar score. BMJ. 1988;297:24–27.
87. Vintzileos AM, Egan JF, Campbell WA, et al. Asphyxia at birth as determined by cord blood pH measurements in preterm and term gestations: correlations with neonatal outcome. J Matern Fetal Med. 1992;1:7–13.
88. Goldaber KG, Gilstrap LC, Leveno KJ, et al. Pathologic fetal acidemia. Obstet Gynecol. 1991;78:1103–1107.
89. Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. 2012;119:824–831.
90. Hafstrom M, Ehnberg S, Blad S, et al. Developmental outcome at 6.5 years after acidosis in term newborns: a population-based study. Pediatrics. 2012;129:e1501–e1507.
91. Adamsons K Jr, Behrman R, Dawes GS, et al. The treatment of acidosis with alkali and glucose during asphyxia in foetal Rhesus monkeys. J Physiol. 1963;169:679–689.
92. Zwart A, Buursma A, Oeseburg B, Zijlstra WG. Determination of hemoglobin derivatives with IL 282 CO-oximeter as compared with a manual spectrophotometric five-wavelength method. Clin Chem. 1981;27:1903–1907.
93. Huch R, Huch A, Tuchschmid P, et al. Carboxyhemoglobin concentration in fetal cord blood. Pediatrics. 1983;71:461–462.
94. Kopotic RJ, Lindner W. Assessing high-risk infants in the delivery room with pulse oximetry. Anesth Analg. 2002;94:S31–S36.
95. Jennis MS, Peabody JL. Pulse oximetry: an alternative method for the assessment of oxygenation in newborn infants. Pediatrics. 1987;79:524–528.
96. Severinghaus JW, Naifeh KH. Accuracy of response of six pulse oximeters to profound hypoxia. Anesthesiology. 1987;67:551–558.
97. Kamlin CO, Dawson JA, O’Donnell CP, et al. Accuracy of pulse oximetry measurement of heart rate of newborn infants in the delivery room. J Pediatr. 2008;152:756–760.
98. Dawson JA, Kamlin CO, Wong C, et al. Oxygen saturation and heart rate during delivery room resuscitation of infants <30 weeks’ gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed. 2009;94:F87–F91.
99. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705.
100. Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77:1–10.
101. Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95:769–774.
102. Farr V, Mitchell RG, Neligan GA, Parkin JM. The definition of some external characteristics used in the assessment of gestational age in the newborn infant. Dev Med Child Neurol. 1966;8:507–511.
103. Farr V, Kerridge DF, Mitchell RG. The value of some external characteristics in the assessment of gestational age at birth. Dev Med Child Neurol. 1966;8:657–660.
104. Amiel-Tison C. Neurological evaluation of the maturity of newborn infants. Arch Dis Child. 1968;43:89–93.
105. Sanders M, Allen M, Alexander GR, et al. Gestational age assessment in preterm neonates weighing less than 1500 grams. Pediatrics. 1991;88:542–546.
106. Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423.
107. Alexander GR, de Caunes F, Hulsey TC, et al. Ethnic variation in postnatal assessments of gestational age: a reappraisal. Paediatr Perinat Epidemiol. 1992;6:423–433.
108. Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–163.
109. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;(2).
110. Rabe H, Reynolds G, Diaz-Rossello J. A systematic review and meta-analysis of a brief delay in clamping the umbilical cord of preterm infants. Neonatology. 2008;93:138–144.
111. Kaempf JW, Tomlinson MW, Kaempf AJ, et al. Delayed umbilical cord clamping in premature neonates. Obstet Gynecol. 2012;120:325–330.
112. Schubring C. Temperature regulation in healthy and resuscitated newborns immediately after birth. J Perinat Med. 1986;14:27–33.
113. Hazan J, Maag U, Chessex P. Association between hypothermia and mortality rate of premature infants—revisited. Am J Obstet Gynecol. 1991;164:111–112.
114. Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670.
115. Solberg R, Andresen JH, Escrig R, et al. Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidation. Pediatr Res. 2007;62:559–563.
116. Spector LG, Klebanoff MA, Feusner JH, et al. Childhood cancer following neonatal oxygen supplementation. J Pediatr. 2005;147:27–31.
117. Vento M, Asensi M, Sastre J, et al. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–647.
118. Saugstad OD, Rootwelt T, Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics. 1998;102:e1.
119. Davis PG, Tan A, O’Donnell CP, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet. 2004;364:1329–1333.
120. Weinberger B, Laskin DL, Heck DE, Laskin JD. Oxygen toxicity in premature infants. Toxicol Appl Pharmacol. 2002;181:60–67.
121. Escrig R, Arruza L, Izquierdo I, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics. 2008;121:875–881.
122. Saugstad OD, Vento M, Ramji S, et al. Neurodevelopmental outcome of infants resuscitated with air or 100% oxygen: a systematic review and meta-analysis. Neonatology. 2012;102:98–103.
123. Vyas H, Milner AD, Hopkin IE, Boon AW. Physiologic responses to prolonged and slow-rise inflation in the resuscitation of the asphyxiated newborn infant. J Pediatr. 1981;99:635–639.
124. Thach BT, Taeusch HW Jr. Sighing in newborn human infants: role of inflation-augmenting reflex. J Appl Physiol. 1976;41:502–507.
125. Cordero L Jr, Hon EH. Neonatal bradycardia following nasopharyngeal stimulation. J Pediatr. 1971;78:441–447.
126. Young RS, Hessert TR, Pritchard GA, Yagel SK. Naloxone exacerbates hypoxic-ischemic brain injury in the neonatal rat. Am J Obstet Gynecol. 1984;150:52–56.
127. Chernick V, Manfreda J, De Booy V, et al. Clinical trial of naloxone in birth asphyxia. J Pediatr. 1988;113:519–525.
128. Sherman JM, Lowitt S, Stephenson C, Ironson G. Factors influencing acquired subglottic stenosis in infants. J Pediatr. 1986;109:322–327.
129. Laing IA, Cowan DL, Ballantine GM, Hume R. Prevention of subglottic stenosis in neonatal ventilation. Int J Pediatr Otorhinolaryngol. 1986;11:61–66.
130. Singh R, Mohan CVR, Taxak SMC. Controlled trial to evaluate the use of LMA for neonatal resuscitation. J Anaesth Clin Pharmacol. 2005;21:303–306.
131. Trevisanuto D, Micaglio M, Pitton M, et al. Laryngeal mask airway: is the management of neonates requiring positive pressure ventilation at birth changing? Resuscitation. 2004;62:151–157.
132. Zanardo V, Weiner G, Micaglio M, et al. Delivery room resuscitation of near-term infants: role of the laryngeal mask airway. Resuscitation. 2010;81:327–330.
133. Jain L, Vidyasagar D. Cardiopulmonary resuscitation of newborns: its application to transport medicine. Pediatr Clin North Am. 1993;40:287–302.
134. David R. Closed chest cardiac massage in the newborn infant. Pediatrics. 1988;81:552–554.
135. Orlowski JP. Optimum position for external cardiac compression in infants and young children. Ann Emerg Med. 1986;15:667–673.
137. Finholt DA, Kettrick RG, Wagner HR, Swedlow DB. The heart is under the lower third of the sternum: implications for external cardiac massage. Am J Dis Child. 1986;140:646–649.
138. Dean JM, Koehler RC, Schleien CL, et al. Age-related effects of compression rate and duration in cardiopulmonary resuscitation. J Appl Physiol. 1990;68:554–560.
139. Fitzgerald KR, Babbs CF, Frissora HA, et al. Cardiac output during cardiopulmonary resuscitation at various compression rates and durations. Am J Physiol. 1981;241:H442–H448.
140. Babbs CF, Tacker WA, Paris RL, et al. CPR with simultaneous compression and ventilation at high airway pressure in 4 animal models. Crit Care Med. 1982;10:501–504.
141. Burchfield DJ. Medication use in neonatal resuscitation. Clin Perinatol. 1999;26:683–691.
142. Schleien CL, Dean JM, Koehler RC, et al. Effect of epinephrine on cerebral and myocardial perfusion in an infant animal preparation of cardiopulmonary resuscitation. Circulation. 1986;73:809–817.
143. Simmons MA, Adcock EW 3rd, Bard H, Battaglia FC. Hypernatremia and intracranial hemorrhage in neonates. N Engl J Med. 1974;291:6–10.
144. Papile LA, Burstein J, Burstein R, et al. Relationship of intravenous sodium bicarbonate infusions and cerebral intraventricular hemorrhage. J Pediatr. 1978;93:834–836.
145. Kette F, Weil MH, Gazmuri RJ. Buffer solutions may compromise cardiac resuscitation by reducing coronary perfusion pressure. JAMA. 1991;266:2121–2126.
146. Kette F, Weil MH, von Planta M, et al. Buffer agents do not reverse intramyocardial acidosis during cardiac resuscitation. Circulation. 1990;81:1660–1666.
147. Changaris DG, Purohit DM, Balentine JD, et al. Brain calcification in severely stressed neonates receiving parenteral calcium. J Pediatr. 1984;104:941–946.
148. Tam EW, Haeusslein LA, Bonifacio SL, et al. Hypoglycemia is associated with increased risk for brain injury and adverse neurodevelopmental outcome in neonates at risk for encephalopathy. J Pediatr. 2012;161:88–93.
149. Fiser DH. Intraosseous infusion. N Engl J Med. 1990;322:1579–1581.
150. Hodge D III, Delgado-Paredes C, Fleisher G. Intraosseous infusion flow rates in hypovolemic “pediatric” dogs. Ann Emerg Med. 1987;16:305–307.
151. Redmond AD, Plunkett PK. Intraosseous infusion. Arch Emerg Med. 1986;3:231–233.
152. La Fleche FR, Slepin MJ, Vargas J, Milzman DP. Iatrogenic bilateral tibial fractures after intraosseous infusion attempts in a 3-month-old infant. Ann Emerg Med. 1989;18:1099–1101.
153. Rosetti VA, Thompson BM, Miller J, et al. Intraosseous infusion: an alternative route of pediatric intravascular access. Ann Emerg Med. 1985;14:885–888.
154. Quilligan JJ Jr, Turkel H. Bone marrow infusion and its complications. Am J Dis Child. 1946;71:457–465.
155. Heinild S, Sondergaard T, Tudvad F. Bone marrow infusion in childhood; experiences from a thousand infusions. J Pediatr. 1947;30:400–412.
156. Golden SM, O’Brien WF, Metz SA. Anticoagulation of autologous cord blood for neonatal resuscitation. Am J Obstet Gynecol. 1982;144:103–104.
157. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Cochrane Injuries Group Albumin Reviewers. BMJ. 1998;317:235–240.
158. Brown CA, Desmond MM, Lindley JE, Moore J. Meconium staining of the amniotic fluid; a marker of fetal hypoxia. Obstet Gynecol. 1957;9:91–103.
159. Matthews TG, Warshaw JB. Relevance of the gestational age distribution of meconium passage in utero. Pediatrics. 1979;64:30–31.
160. Yeh TF, Harris V, Srinivasan G, et al. Roentgenographic findings in infants with meconium aspiration syndrome. JAMA. 1979;242:60–63.
161. Wiswell TE, Tuggle JM, Turner BS. Meconium aspiration syndrome: have we made a difference? Pediatrics. 1990;85:715–721.
162. Gregory GA, Gooding CA, Phibbs RH, Tooley WH. Meconium aspiration in infants—a prospective study. J Pediatr. 1974;85:848–852.
163. Short BL, Miller MK, Anderson KD. Extracorporeal membrane oxygenation in the management of respiratory failure in the newborn. Clin Perinatol. 1987;14:737–748.
164. Truog WE. Inhaled nitric oxide: a tenth anniversary observation. Pediatrics. 1998;101:696–697.
165. Wessel DL, Adatia I, Van Marter LJ, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics. 1997;100:E7.
166. Ting P, Brady JP. Tracheal suction in meconium aspiration. Am J Obstet Gynecol. 1975;122:767–771.
167. Carson BS, Losey RW, Bowes WA Jr, Simmons MA. Combined obstetric and pediatric approach to prevent meconium aspiration syndrome. Am J Obstet Gynecol. 1976;126:712–715.
168. Davis RO, Philips JB 3rd, Harris BA Jr, et al. Fatal meconium aspiration syndrome occurring despite airway management considered appropriate. Am J Obstet Gynecol. 1985;151:731–736.
169. Falciglia HS, Henderschott C, Potter P, Helmchen R. Does DeLee suction at the perineum prevent meconium aspiration syndrome? Am J Obstet Gynecol. 1992;167:1243–1249.
170. Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr. 1984;104:758–762.
171. Hislop A, Reid L. Intra-pulmonary arterial development during fetal life-branching pattern and structure. J Anat. 1972;113:35–48.
172. Hislop A, Reid L. Pulmonary arterial development during childhood: branching pattern and structure. Thorax. 1973;28:129–135.
173. Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation: an ultrastructural study. Lab Invest. 1978;38:188–200.
174. Linder N, Aranda JV, Tsur M, et al. Need for endotracheal intubation and suction in meconium-stained neonates. J Pediatr. 1988;112:613–615.
175. Ghidini A, Spong CY. Severe meconium aspiration syndrome is not caused by aspiration of meconium. Am J Obstet Gynecol. 2001;185:931–938.
176. Fraser WD, Hofmeyr J, Lede R, et al. Amnioinfusion for the prevention of the meconium aspiration syndrome. N Engl J Med. 2005;353:909–917.
177. Ross MG. Meconium aspiration syndrome—more than intrapartum meconium. N Engl J Med. 2005;353:946–948.
178. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Amnioninfusion does not prevent meconium aspiration syndrome. [ACOG Committee Opinion No. 346] Obstet Gynecol. 2006;108:1053.
179. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Management of delivery of a newborn with meconium-stained amniotic fluid. [ACOG Committee Opinion No. 379] Obstet Gynecol. 2007;110:739.
180. Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597–602.
181. Kochanek KD, Kirmeyer SE, Martin JA, et al. Annual summary of vital statistics: 2009. Pediatrics. 2012;129:338–348.
182. Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev Med Child Neurol. 1999;41:94–109.
183. Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics. 2000;105:1216–1226.
184. Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5:135–151.
185. Chen CH, Shen WC, Wang TM, Chi CS. Cerebral magnetic resonance imaging of preterm infants after corrected age of one year. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1995;36:261–265.
186. Goddard J, Lewis RM, Armstrong DL, Zeller RS. Moderate, rapidly induced hypertension as a cause of intraventricular hemorrhage in the newborn beagle model. J Pediatr. 1980;96:1057–1060.
188. Volpe JJ. Neurologic outcome of prematurity. Arch Neurol. 1998;55:297–300.
189. Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol. 1997;24:567–587.
190. Perlman JM, Volpe JJ. Are venous circulatory abnormalities important in the pathogenesis of hemorrhagic and/or ischemic cerebral injury? Pediatrics. 1987;80:705–711.
191. Goldberg RN, Chung D, Goldman SL, Bancalari E. The association of rapid volume expansion and intraventricular hemorrhage in the preterm infant. J Pediatr. 1980;96:1060–1063.
192. Nakamura Y, Okudera T, Fukuda S, Hashimoto T. Germinal matrix hemorrhage of venous origin in preterm neonates. Hum Pathol. 1990;21:1059–1062.
193. van de Bor M, Briet E, Van Bel F, Ruys JH. Hemostasis and periventricular-intraventricular hemorrhage of the newborn. Am J Dis Child. 1986;140:1131–1134.
194. Goldstein GW. Pathogenesis of brain edema and hemorrhage: role of the brain capillary. Pediatrics. 1979;64:357–360.
195. Gould SJ, Howard S. An immunohistochemical study of the germinal layer in the late gestation human fetal brain. Neuropathol Appl Neurobiol. 1987;13:421–437.
196. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507.
197. Shankaran S, Woldt E, Nelson J, et al. Antenatal phenobarbital therapy and neonatal outcome. II. Neurodevelopmental outcome at 36 months. Pediatrics. 1996;97:649–652.
198. Shankaran S, Cepeda E, Muran G, et al. Antenatal phenobarbital therapy and neonatal outcome. I. Effect on intracranial hemorrhage. Pediatrics. 1996;97:644–648.
199. Morales WJ, Angel JL, O’Brien WF, et al. The use of antenatal vitamin K in the prevention of early neonatal intraventricular hemorrhage. Am J Obstet Gynecol. 1988;159:774–779.
200. Thorp JA, Parriott J, Ferrette-Smith D, et al. Antepartum vitamin K and phenobarbital for preventing intraventricular hemorrhage in the premature newborn: a randomized, double-blind, placebo-controlled trial. Obstet Gynecol. 1994;83:70–76.
201. Moise AA, Wearden ME, Kozinetz CA, et al. Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics. 1995;95:845–850.
202. Wallace EM, Baker LS. Effect of antenatal betamethasone administration on placental vascular resistance. Lancet. 1999;353:1404–1407.
203. Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics. 1995;95:263–269.
204. Paneth N, Jetton J, Pinto-Martin J, Susser M. Magnesium sulfate in labor and risk of neonatal brain lesions and cerebral palsy in low birth weight infants. The Neonatal Brain Hemorrhage Study Analysis Group. Pediatrics. 1997;99:E1.
205. Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676.
206. Marret S, Doyle LW, Crowther CA, Middleton P. Antenatal magnesium sulphate neuroprotection in the preterm infant. Semin Fetal Neonatal Med. 2007;12:311–317.
207. Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905.
208. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Magnesium sulfate before anticipated preterm birth for neuroprotection. [ACOG Committee Opinion No. 455] Obstet Gynecol. 2010;115:669–671.
209. Hirtz DG, Nelson K. Magnesium sulfate and cerebral palsy in premature infants. Curr Opin Pediatr. 1998;10:131–137.
210. Mittendorf R, Dambrosia J, Pryde PG, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–1118.
211. Johnson LH, Mapp DC, Rouse DJ, et al. Association of cord blood magnesium concentration and neonatal resuscitation. J Pediatr. 2012;160:573–577.
212. Yanowitz TD. Cerebrovascular autoregulation among very low birth weight infants. J Perinatol. 2011;31:689–691.
213. Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312:1353–1357.
214. Miall-Allen VM, Whitelaw AG. Effect of pancuronium and pethidine on heart rate and blood pressure in ventilated infants. Arch Dis Child. 1987;62:1179–1180.
215. Saarenmaa E, Huttunen P, Leppaluoto J, et al. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. J Pediatr. 1999;134:144–150.
216. Myers RE, Myers SE. Use of sedative, analgesic, and anesthetic drugs during labor and delivery: bane or boon? Am J Obstet Gynecol. 1979;133:83–104.
217. Richards DS, Yancey MK, Duff P, Stieg FH. The perinatal management of severe laryngeal stenosis. Obstet Gynecol. 1992;80:537–540.
218. Liechty KW, Crombleholme TM, Flake AW, et al. Intrapartum airway management for giant fetal neck masses: the EXIT (ex utero intrapartum treatment) procedure. Am J Obstet Gynecol. 1997;177:870–874.
219. MacKenzie TC, Crombleholme TM, Flake AW. The ex-utero intrapartum treatment. Curr Opin Pediatr. 2002;14:453–458.
220. Garcia PJ, Olutoye OO, Ivey RT, Olutoye OA. Case scenario: anesthesia for maternal-fetal surgery: the Ex Utero Intrapartum Therapy (EXIT) procedure. Anesthesiology. 2011;114:1446–1452.
221. Crombleholme TM, Sylvester K, Flake AW, Adzick NS. Salvage of a fetus with congenital high airway obstruction syndrome by ex utero intrapartum treatment (EXIT) procedure. Fetal Diagn Ther. 2000;15:280–282.
222. Noah MM, Norton ME, Sandberg P, et al. Short-term maternal outcomes that are associated with the EXIT procedure, as compared with cesarean delivery. Am J Obstet Gynecol. 2002;186:773–777.
223. Lazar DA, Olutoye OO, Moise KJ Jr, et al. Ex-utero intrapartum treatment procedure for giant neck masses—fetal and maternal outcomes. J Pediatr Surg. 2011;46:817–822.
224. George RB, Melnick AH, Rose EC, Habib AS. Case series: combined spinal epidural anesthesia for Cesarean delivery and ex utero intrapartum treatment procedure. Can J Anaesth. 2007;54:218–222.
225. Shaw-Smith C. Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet. 2006;43:545–554.
226. Clark RH, Hardin WD Jr, Hirschl RB, et al. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009.
227. Tyson JE, Parikh NA, Langer J, et al. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358:1672–1681.
228. Wyckoff MH, Salhab WA, Heyne RJ, et al. Outcome of extremely low birth weight infants who received delivery room cardiopulmonary resuscitation. J Pediatr. 2012;160:239–244.
229. Jain L, Ferre C, Vidyasagar D, et al. Cardiopulmonary resuscitation of apparently stillborn infants: survival and long-term outcome. J Pediatr. 1991;118:778–782.
230. Laptook AR, Shankaran S, Ambalavanan N, et al. Outcome of term infants using Apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619–1626.
231. Yeo CL, Tudehope DI. Outcome of resuscitated apparently stillborn infants: a ten year review. J Paediatr Child Health. 1994;30:129–133.
232. Casalaz DM, Marlow N, Speidel BD. Outcome of resuscitation following unexpected apparent stillbirth. Arch Dis Child Fetal Neonatal Ed. 1998;78:F112–F115.
233. Brazelton TB, Scholl ML, Robey JS. Visual responses in the newborn. Pediatrics. 1966;37:284–290.
234. Ball W, Tronick E. Infant responses to impending collision: optical and real. Science. 1971;171:818–820.
235. Kearsley RB. The newborn’s response to auditory stimulation: a demonstration of orienting and defensive behavior. Child Dev. 1973;44:582–590.
237. Scanlon JW, Brown WU Jr, Weiss JB, Alper MH. Neurobehavioral responses of newborn infants after maternal epidural anesthesia. Anesthesiology. 1974;40:121–128.
238. Amiel-Tison C, Barrier G, Shnider SM, et al. A new neurologic and adaptive capacity scoring system for evaluating obstetric medications in full-term newborns. Anesthesiology. 1982;56:340–350.
239. Brockhurst NJ, Littleford JA, Halpern SH. The Neurologic and Adaptive Capacity Score: a systematic review of its use in obstetric anesthesia research. Anesthesiology. 2000;92:237–246.
240. Camann W, Brazelton TB. Use and abuse of neonatal neurobehavioral testing (editorial). Anesthesiology. 2000;92:3–5.
241. Halpern SH, Littleford JA, Brockhurst NJ, et al. The neurologic and adaptive capacity score is not a reliable method of newborn evaluation. Anesthesiology. 2001;94:958–962.
242. Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol. 2002;27:196–212.
243. American Academy of Pediatrics. Committee on Drugs. Effect of medication during labor and delivery on infant outcome. Pediatrics. 1978;62:402–403.
244. U.S. Food and Drug Administration. Guidelines for the clinical evaluation of general anesthetics. U.S. Department of Health and Human Services, Public Health Service: Rockville, MD; 1977.
245. U.S. Food and Drug Administration. Guidelines for the clinical evaluation of local anesthetics. U.S. Department of Health and Human Services, Public Health Service: Rockville, MD; 1977.
246. Brackbill Y, Kane J, Manniello RL, Abramson D. Obstetric meperidine usage and assessment of neonatal status. Anesthesiology. 1974;40:116–120.
247. Dailey PA, Baysinger CL, Levinson G, Shnider SM. Neurobehavioral testing of the newborn infant: effects of obstetric anesthesia. Clin Perinatol. 1982;9:191–214.
248. Hodgkinson R, Bhatt M, Wang CN. Double-blind comparison of the neurobehaviour of neonates following the administration of different doses of meperidine to the mother. Can Anaesth Soc J. 1978;25:405–411.
249. Lieberman BA, Rosenblatt DB, Belsey E, et al. The effects of maternally administered pethidine or epidural bupivacaine on the fetus and newborn. Br J Obstet Gynaecol. 1979;86:598–606.
250. Rayburn WF, Smith CV, Leuschen MP, et al. Comparison of patient-controlled and nurse-administered analgesia using intravenous fentanyl during labor. Anesthesiol Rev. 1991;18:31–36.
251. Kileff ME, James FM 3rd, Dewan DM, Floyd HM. Neonatal neurobehavioral responses after epidural anesthesia for cesarean section using lidocaine and bupivacaine. Anesth Analg. 1984;63:413–417.
252. Brown WU, Bell GC, Lurie AO, et al. Newborn blood levels of lidocaine and mepivacaine in the first postnatal day following maternal epidural anesthesia. Anesthesiology. 1975;42:698–707.
253. Datta S, Corke BC, Alper MH, et al. Epidural anesthesia for cesarean section: a comparison of bupivacaine, chloroprocaine, and etidocaine. Anesthesiology. 1980;52:48–51.
254. Kuhnert BR, Harrison MJ, Linn PL, Kuhnert PM. Effects of maternal epidural anesthesia on neonatal behavior. Anesth Analg. 1984;63:301–308.
255. Sepkoski CM, Lester BM, Ostheimer GW, Brazelton TB. The effects of maternal epidural anesthesia on neonatal behavior during the first month. Dev Med Child Neurol. 1992;34:1072–1080.
256. Abboud TK, Khoo SS, Miller F, et al. Maternal, fetal, and neonatal responses after epidural anesthesia with bupivacaine, 2-chloroprocaine, or lidocaine. Anesth Analg. 1982;61:638–644.
257. Tronick E. A critique of the neonatal Neurologic and Adaptive Capacity Score (NACS). Anesthesiology. 1982;56:338–339.
258. Hodgkinson R, Bhatt M, Kim SS, et al. Neonatal neurobehavioral tests following cesarean section under general and spinal anesthesia. Am J Obstet Gynecol. 1978;132:670–674.
259. Hughes SC, Rosen MA, Shnider SM, et al. Maternal and neonatal effects of epidural morphine for labor and delivery. Anesth Analg. 1984;63:319–324.
260. Preston PG, Rosen MA, Hughes SC, et al. Epidural anesthesia with fentanyl and lidocaine for cesarean section: maternal effects and neonatal outcome. Anesthesiology. 1988;68:938–943.
261. Murakawa K, Abboud TK, Yanagi T, et al. Clinical experience of epidural fentanyl for labor pain. J Anesth. 1987;1:93–95.
262. Cohen SE, Tan S, Albright GA, Halpern J. Epidural fentanyl/bupivacaine mixtures for obstetric analgesia. Anesthesiology. 1987;67:403–407.
263. Abboud TK, Afrasiabi A, Zhu J, et al. Epidural morphine or butorphanol augments bupivacaine analgesia during labor. Reg Anesth. 1989;14:115–120.
264. Abboud TK, Zhu J, Afrasiabi A, et al. Epidural butorphanol augments lidocaine sensory anesthesia during labor. Reg Anesth. 1991;16:265–267.
265. Abboud TK, David S, Nagappala S, et al. Maternal, fetal, and neonatal effects of lidocaine with and without epinephrine for epidural anesthesia in obstetrics. Anesth Analg. 1984;63:973–979.
266. Abboud TK, Sheik-ol-Eslam A, Yanagi T, et al. Safety and efficacy of epinephrine added to bupivacaine for lumbar epidural analgesia in obstetrics. Anesth Analg. 1985;64:585–591.
267. Abboud TK, DerSarkissian L, Terrasi J, et al. Comparative maternal, fetal, and neonatal effects of chloroprocaine with and without epinephrine for epidural anesthesia in obstetrics. Anesth Analg. 1987;66:71–75.
268. Abboud TK, Afrasiabi A, Zhu J, et al. Bupivacaine/butorphanol/epinephrine for epidural anesthesia in obstetrics: maternal and neonatal effects. Reg Anesth. 1989;14:219–224.
269. Abboud TK, Nagappala S, Murakawa K, et al. Comparison of the effects of general and regional anesthesia for cesarean section on neonatal neurologic and adaptive capacity scores. Anesth Analg. 1985;64:996–1000.
270. Palahniuk RJ, Scatliff J, Biehl D, et al. Maternal and neonatal effects of methoxyflurane, nitrous oxide and lumbar epidural anaesthesia for Caesarean section. Can Anaesth Soc J. 1977;24:586–596.
271. Stefani SJ, Hughes SC, Schnider SM, et al. Neonatal neurobehavioral effects of inhalation analgesia for vaginal delivery. Anesthesiology. 1982;56:351–355.
272. Abboud TK, Gangolly J, Mosaad P, Crowell D. Isoflurane in obstetrics. Anesth Analg. 1989;68:388–391.
273. Sprung J, Flick RP, Wilder RT, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–310.

 or
or  inch
inch