Chapter 4 Liver blood flow
Physiology, measurement, and clinical relevance
Physiology
Liver Blood Supply
The liver normally receives about one quarter of the total cardiac output despite constituting only 2.5% of total body weight; it obtains its blood supply from two main sources, the hepatic artery and the portal vein. Approximately 75% to 80% of the blood entering the liver is partially deoxygenated portal venous blood drained from the stomach, intestine, spleen, and pancreas. The remainder is well-oxygenated blood from the aorta, carried by the hepatic artery. Mixing of arterial and portal blood occurs in the sinusoids, which are drained by the hepatic venous system into the inferior vena cava. Hepatic blood volume accounts for 10% to 15% of total blood volume with 40% of hepatic blood held in large vessels such as the hepatic arteries, portal vein, and hepatic veins; the remaining 60% is held in the sinusoids (Lautt, 1977; Greenway & Stark, 1971).
Hepatic Artery
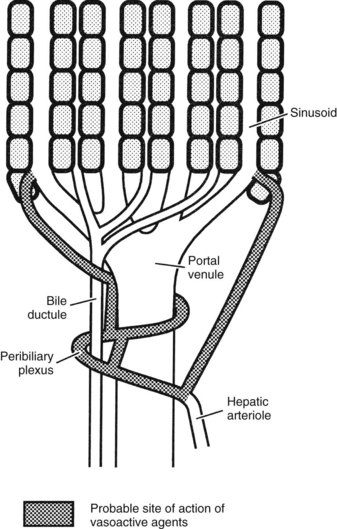
The hepatic arterioles empty directly or via a peribiliary plexus into the sinusoids and terminal portal venules. Direct artery-to-hepatic vein connections do not usually exist but may arise in liver disease. Reduction of pressure in the arterial system toward that existing in the portal circulation is achieved mainly by 1) the presinusoidal arteriolar resistance in the peribiliary plexus and 2) the intermittent closure of the arterioles, which protect the portal bloodstream from arterial pressure (Rappaport, 1973).
In the event of hepatic arterial occlusion, numerous small arteries provide a source for the formation of a collateral circulation. Upon interruption of hepatic arterial flow, collaterals can arise from 1) the inferior phrenic arteries, which can develop connections with hepatic arteries within the liver, and 2) from the gastroduodenal arteries, which derive blood flow from the superior mesenteric artery and supply the liver via the peribiliary arterial plexus around the intrahepatic bile ducts (Rappaport & Schneiderman, 1976). The precise nature of the functional collateral supply after hepatic arterial ligation depends on the site of occlusion. If the common hepatic artery is interrupted, revascularization occurs through both major routes; if only the right or left hepatic artery is interrupted, intrahepatic translobar anastomoses reestablish arterial flow in the ligated system (Mays & Wheeler, 1974). Ligation of the proper hepatic arteries leads to revascularization mainly via a hypertrophied inferior phrenic circulation (Jefferson et al, 1956). Complete long-term dearterialization of the liver by any form of arterial vascular occlusion is difficult to achieve.
Portal Vein
The inferior and superior mesenteric veins join with the splenic vein to form the portal vein, and jointly they collect the venous outflow from the entire prehepatic splanchnic vascular bed (the intestinal tract from the lower esophagus to the rectum plus the pancreas and spleen). This valveless system is a low-pressure/low-resistance system regulated by the degree of constriction of mesenteric and splanchnic arterioles coupled with the intrahepatic vascular resistance. The portal vein normally carries about 75% of the total blood flow to the liver, or 90 mL/min per 100 g of liver weight; normal portal pressure is 5 to 8 mm Hg. Portal blood is postcapillary and partly deoxygenated, but because of its large volume flow rate, it may supply 50% to 70% of the liver’s normal oxygen requirement. During fasting states, the oxygen saturation in the portal blood approaches 85%, which is greater than other systemic veins. Hepatic oxygen supply is diminished if portal blood flow is significantly reduced, but the effect is minimized by an increase in oxygen extraction from the hepatic arterial blood and not by increasing flow. In fact, isovolemic hemodilution or upregulation of hepatic enzymes leading to oxygen deprivation does not result in hepatic artery dilatation, refuting the concept that arterial flow is regulated by liver cell metabolism (Lautt et al, 1987).
Hepatic Veins
The hepatic venous system is the systemic drainage tract of the entire splanchnic circulation. A total liver blood flow (LBF) of 1.5 L/min is considered the normal value in the average male, but the range can be quite wide (1 to 2 L/min). Although the free pressure in a hepatic vein is 1 to 2 mm Hg, the wedged hepatic venous pressure, which is useful for estimating sinusoidal pressure, may be an indicator of portal venous pressure (Boyer et al, 1977). The sinusoidal pressure is estimated to be slightly higher than that of the vena cava, or 2 to 4 mm Hg (Eipel et al, 2010). Hepatic venous blood is normally about two-thirds saturated with oxygen, but this may be markedly reduced during periods of low delivery of oxygen to the liver, when oxygen is extracted by hepatocytes. In resting states, the liver accounts for approximately 20% of the total oxygen consumption of the body.
Control of Liver Blood Flow
Some controversy surrounds the autoregulation of hepatic arterial vasculature. Although some studies have suggested nonlinear pressure-regulated changes in arterial resistance in denervated dogs (Hanson et al, 1966), others have demonstrated a more direct relationship (Torrance, 1961). The pressure-flow dynamic in the portal system has been found to have little to no autoregulation, as evidenced by partial collapse of the portal vascular bed in response to reduced portal pressure (Condon et al, 1962).
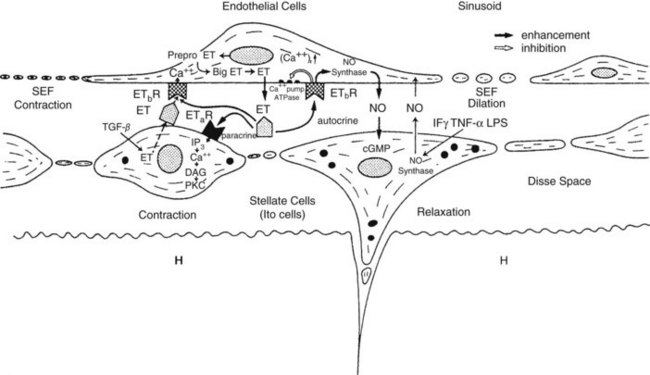
Intrahepatic Vascular Resistance in Health
Intrahepatic vascular resistance (IHVR) seems to be located at the level of the portal venules (presinusoidal) or sinusoids (Sherman et al, 1996; Shibayama & Nakata, 1985; Zhang et al, 1994, 1995). Knisley suggested the presence of sphincter-like structures at the entrance and exit of sinusoids that maintain tremendous portal venous pressure gradients, but these may be species dependent (Knisley et al, 1957). In dogs and cats, IHVR is located at the level of hepatic venules (postsinusoidal) under resting conditions (Lautt et al, 1986, 1987), whereas it may be shifted to presinusoidal sites during neural stimulation (Greenway & Lautt, 1970; Legare & Lautt, 1987). Sinusoidal contraction in response to the vasoconstrictor endothelin (ET) 1 has been observed despite the lack of smooth muscle (Bauer et al, 1994, 1995; Okumura et al, 1994; Zhang et al, 1994, 1995). This contractility is mainly attributed to hepatic stellate cells—also called Ito cells, lipocytes, and fat-storing cells—which are distinguished by autofluorescence derived from their intracellular vitamin A (Suematsu et al, 1995; Zhang et al, 1994). ET-1 causes reversible and graded contraction of isolated stellate cells in tissue culture and results in narrowing of sinusoidal lumens in perfused livers (Pinzani et al, 1992; Rockey & Weisiger, 1996; Rockey et al, 1992; Zhang et al, 1994). Hepatic stellate cells have been compared to pericytes, because they contain smooth muscle–specific intermediate desmin-like and actin-like filaments that encircle neighboring sinusoids (Greenwel et al, 1991; Martinez-Hernandez, 1985; Martinez-Hernandez & Amenta, 1993; see Chapter 6).
Studies using intravital fluorescent microscopy showed that the sites of sinusoidal dilation and constriction is colocalized with that of an autofluorescent vitamin A substance (Suematsu et al, 1995, 1996; Zhang et al, 1994, 1995). Hepatic stellate cells acting as liver-specific pericytes (Friedman, 1997; Martinez-Hernandez, 1985; Martinez-Hernandez & Amenta, 1993) may play a crucial role in modulating IHVR and blood flow, especially at the sinusoidal level (Bauer et al, 1994; Okumura et al, 1994; Pannen et al, 1996; Zhang et al, 1994, 1995). Endothelium-derived nitric oxide (NO) in the hepatic sinusoids may modulate portal resistance under physiologic circumstances (Bauer et al, 1997; Shah et al, 1997). Electron microscopy studies have also demonstrated the presence of smooth muscle cells in the sublobar veins (Aharinejad et al, 1997), as well as terminal hepatic venules (Sasse et al, 1976), which may contribute to IHVR.
Relationship between Hepatic Artery and Portal Vein Blood Flow
Individual control of the hepatic arterial and portal venous circulations is complicated by the existence of a complex interaction between the two systems within the liver. Studies have shown an increase in hepatic arterial blood flow following reduction of portal blood flow (Kock et al, 1972; Mathie et al, 1980a; Schenk et al, 1962); however, a compensatory increase in portal flow following decreased arterial perfusion has rarely been observed (Mathie, 1997). Intraoperative measurements of hepatic artery and portal vein flow in anesthetized patients demonstrated a significant increase in hepatic arterial flow, up to 30% of baseline, with temporary occlusion of the portal vein and no effect on portal flow on occlusion of the hepatic artery (Jakab et al, 1995).
The ability of the hepatic artery to respond acutely to changes in portal flow is referred to as the hepatic arterial buffer response (Lautt, 1981). Highlighting its early embryonic origin, in experiments measuring fetal hepatic arterial blood flow, it has been shown that this regulatory process is even present prenatally (Ebbing et al, 2008). Experimental studies support the view that adenosine, accumulating in the liver as a result of reduced hepatic outflow or released from the walls of blood vessels in response to partial tissue hypoxia, has a significant role in the regulation of the buffer response (Lautt & Legare, 1985; Mathie & Alexander, 1990). This adenosine washout hypothesis is supported by the fact that portal infusion of adenosine increases hepatic arterial blood flow by one half to one third the amount of direct arterial instillation (Lautt et al, 1987). The demonstration of receptors mediating dilation to adenosine in the hepatic arterial vascular bed reinforces this view (Mathie et al, 1991; Fig. 4.1).
Metabolism
Hepatic arterial blood flow has not been traditionally linked to liver metabolism (Lautt, 1983; Mathie & Blumgart, 1983a). It has been shown that neither altered oxygen supply nor bile secretion causes a dependent change in arterial flow (Lautt, 1983); instead, the physiologic role of the buffer system is to maintain hepatic clearance in the face of reduced portal flow and to maintain adequate tissue oxygenation. This is despite the fact that the liver receives more oxygen than it requires and can therefore extract more by hepatocyte metabolism if needed. Therefore arterial flow is independent of oxygenation and is secondary to the nutrient and hormonal needs of the liver.
Blood Gas Tensions
Experimental studies have attempted to clarify the role of arterial blood gas tensions and pH on the hepatic circulation. Hypercarbia (Paco2 > 70 mm Hg) increases portal venous flow and decreases hepatic arterial flow in dogs (Hughes et al, 1979a), whereas hypocarbia (Paco2 < 30 mm Hg) decreases both (Hughes et al, 1979b). Systemic hypoxia (Pao2 < 70 mm Hg) causes a decrease in arterial flow but has no effect on the contribution from the portal vein (Hughes et al, 1979c). The response to metabolic acidosis is similar to that induced by hypercarbia, whereas metabolic alkalosis has essentially no significant effect (Hughes et al, 1980b). The sympathetic nervous system is thought to be responsible for the hepatic arterial vasoconstriction observed in hypercarbia and hypoxia (Mathie & Blumgart, 1983b).
Sympathetic Nervous System
Denervation experiments have shown that the sympathetic nervous system is not involved in basal arterial tone in the liver (Mathie & Blumgart, 1983b). Hepatic sympathetic nervous stimulation causes hepatic arterial vasoconstriction and reduced blood flow, but this is secondary to the autoregulatory response (Greenway & Stark, 1971). Sensory denervated rats and pigs have a diminished arterial buffer response on partial occlusion of the portal vein (Ishikawa, 1995). Portal pressure increases as a result of an increase in portal venous resistance, but portal flow does not decrease, unless there is a decrease in intestinal or splenic blood flow caused by simultaneous sympathetic stimulation of these vascular beds. The liver is a significant blood reservoir, and 50% of its blood volume may be mobilized by nerve stimulation (Greenway & Lautt, 1989).
Although the hepatic artery contains both α-adrenergic and β-adrenergic receptors, the portal venous system is believed to contain only α-receptors (Richardson & Withrington, 1981). At low doses, epinephrine causes hepatic and mesenteric arterial vasodilation, whereas at high doses, vasoconstriction occurs in the hepatic arterial and portal venous vascular beds and in the mesenteric circulation (Greenway & Stark, 1971; Richardson & Withrington, 1981).
Other Endogenous Vasoactive Agents
Hepatic blood flow is profoundly increased by glucagon as a consequence of its strong vasodilatory action on the mesenteric vasculature, but insulin has little hemodynamic effect on the hepatic circulation. Histamine causes hepatic arterial dilation and, in the dog only, hepatic venous constriction. Bradykinin is a potent hepatic arterial vasodilator that has little effect on the portal venous system. The hepatic arterial vascular bed is dilated by most prostaglandins; however, prostacyclin does not affect hepatic arterial flow but increases portal blood flow through a vasodilator effect on the prehepatic vascular bed. NO causes vasodilation in the hepatic arterial and mesenteric vascular beds as discussed earlier. Each of the gut hormones—gastrin, secretin, cholecystokinin, and vasoactive intestinal peptide—causes vasodilation of the hepatic artery. In addition, antagonists of calcitonin gene–related peptide and neurokinin significantly reduce hepatic arterial blood flow, suggesting the presence of their receptors on the arterial vasculature (Biernat et al, 2005).
Angiotensin decreases hepatic arterial and portal blood flow and is one of the few substances to produce a significant vasoconstrictor effect on the hepatic artery. However, the ETs that can exert a powerful and prolonged generalized systemic constriction (Miller et al, 1989; Zhang et al, 1994) also have a direct effect on the hepatic blood flow. ETs reduce hepatic perfusion (Kurihara et al, 1992), increase portal pressure (Bauer et al, 1994; Isales et al, 1993; Tanaka et al, 1994; Tran-Thi et al, 1993), and reduce sinusoidal diameter (Bauer et al, 1994; Okumura et al, 1994; Zhang et al, 1994). Vasopressin decreases portal flow and pressure by mesenteric arterial vasoconstriction but has variable effects on the hepatic artery. Serotonin is believed to mediate vasoconstriction of portal radicles and has been targeted as a substance involved in the maintenance of portal hypertension, which will be discussed later. In contrast, hydrogen sulfide either endogenously or exogenously can reverse the norepinephrine-induced vasoconstriction in an NO-independent fashion (Fiorucci et al, 2005). Intraportal administration of exogenous vasoactive agents affects hepatic arterial resistance (Lautt et al, 1984); the mechanisms underlying this intrahepatic transvascular effect are not understood, but it is likely that the close anatomic association between arterioles and venules (see Fig. 4.1) could permit this and may be a means by which hepatic arterial blood flow is finely controlled by endogenous agents such as gut hormones.
Anesthetic Agents
Hepatic arterial and portal venous blood flow decreases passively in parallel with cardiac output during halothane inhalation, with little change in vascular resistance (Hughes et al, 1980a; Thulin et al, 1975). Hepatic oxygen consumption is not diminished by halothane because of a marked increase in the oxygen extraction rate from the reduced blood supply (Andreen et al, 1975). Enflurane has been found to have similar effects to those of halothane, although there is a decrease in hepatic arterial vascular resistance as part of a generalized decrease in peripheral vascular resistance (Hughes et al, 1980a). Cyclopropane and methoxyflurane reduce LBF, mainly by increasing the mesenteric vascular resistance (Batchelder & Cooperman, 1975). NO in concentrations of 30% to 70% reduces hepatic artery and portal vein flow, possibly as a result of a generalized stimulatory action on α-adrenergic receptors (Thomson et al, 1982). Isoflurane seems to have minimal effects on hepatic arterial and portal venous flows, and the intravenous agent fentanyl may have little effect on prehepatic splanchnic blood flow (Nagano et al, 1988); thiopentone in low doses vasoconstricts the hepatic arterial and mesenteric vascular beds (Thomson et al, 1986).
Measurement of Liver Blood Flow
The earliest methods of measuring LBF involved direct invasive techniques, such as intravascular devices or venous outflow collection (Burton-Opitz, 1910, 1911; MacLeod & Pearce, 1914). Indirect determination of blood flow by the use of a variety of indicator-clearance techniques were subsequently developed but were confounded by the presence of liver disease. The available methods are discussed under three broad headings: 1) flow in single blood vessels, 2) total LBF, and 3) hepatic tissue perfusion. The techniques currently employed for experimental and clinical investigations are listed in Table 4.1.
Table 4.1 Summary of Methods Currently Used for Measuring Liver Blood Flow
| Flow in Single Vessels |
Flow in Single Vessels
Electromagnetic Flowmeter
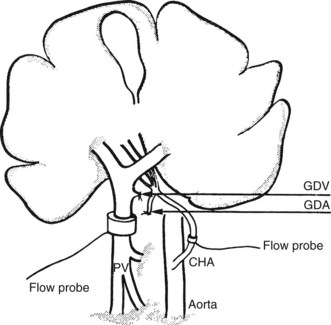
The direct and continuous measurement of hepatic arterial and portal venous blood flow with electromagnetic flow probes remains the best available means of assessing individual vessel flow. Although the technique has found widespread application in experiments using large animals, its use in clinical situations has been limited by the relatively extensive vascular dissection required for placement of the probes and by the overestimation of true hepatic tissue blood flow, which occurs in the presence of portosystemic shunts. In addition, relative movements of the probes can give rise to errors and the need for repetitive calibrations. Total LBF in anesthetized subjects was determined to be approximately 1 L/min, of which about 25% was supplied by the hepatic artery (Schenk et al, 1962). Electromagnetic probes have been used intraoperatively to assess the hemodynamic status of the liver in cirrhosis (Ohnishi et al, 1987) after liver surgery or transplantation (Takaoka et al, 1990). A typical experimental preparation using electromagnetic flow probes is illustrated in Fig. 4.2.
Doppler Ultrasound
Invasive
Because the ultrasound probes are fitted directly to the vessel, such systems have been used successfully in the intraoperative (Henderson et al, 1991) and postoperative measurement of portal venous and hepatic arterial blood flow in liver transplant patients (Payen et al, 1990).
Noninvasive
In experiments performed on anesthetized dogs, good correlation was found between portal venous flow measured by a transcutaneous Doppler duplex system and electromagnetic flow probes fitted to the portal vein (Dauzat & Layrargues, 1989). However, the clinical application of transcutaneous Doppler ultrasound to obtain quantitative hepatic blood flow measurements in the portal vein have been more difficult (Burns et al, 1987). This technique has been utilized to assess postoperative hemodynamic consequences of portosystemic shunt procedures, such as transjugular intrahepatic portosystemic stent shunt (TIPS) (see Chapter 96; Fung et al, 1998), and for diagnosis of cirrhosis and portal hypertension (Iwao et al, 1997). In normal subjects, portal blood flow of 600 to 900 mL/min was reported (Brown et al, 1989; Moriyasu et al, 1986), whereas blood flow in the common hepatic artery was reported to be approximately 250 mL/min (Nakamura et al, 1989). The use of color Doppler has improved the suitability of the Doppler method for routine clinical use (Rosemurgy et al, 1997). The availability of ultrasound echo-enhancing materials, such as the galactose-based microbubble agent Levovist (Ernst et al, 1996), may further improve the precision of hepatic blood flow measurement and imaging using the Doppler technique.
Total Liver Blood Flow
Clearance Techniques
The rate of disappearance from the bloodstream of an indicator substance exclusively cleared by the liver is proportional to LBF. First applied to humans by Bradley and colleagues in 1945, indirect clearance methods of LBF measurement rely on the Fick equation. The flow measurement obtained by Bradley’s group depended on the fact that intravenously injected bromosulfophthalein is removed from the bloodstream and into the bile entirely by hepatocytes. They derived a value for the rate of hepatic bromosulfophthalein removal indirectly by determining the rate of intravenous infusion of dye that maintained the arterial concentration at a constant level, and by measuring the arteriovenous concentration difference of bromosulfophthalein, they were able to calculate total hepatic blood flow. The mean value obtained in a group of normal subjects was 1.5 L/min.
Other substances dependent on hepatocyte extraction into bile have also been applied, such as indocyanine green (Caesar et al, 1961). None of these substances achieve complete hepatic removal, and hepatic vein cannulation is necessary to allow calculation of the true extraction efficiency. Many investigators now use a simplified version of the original method, in which indocyanine green is administered as a bolus, instead of as an infusion, and hepatic extraction efficiency is determined from an analysis of the clearance curve derived from blood samples taken from a peripheral vein, instead of from the hepatic vein (Grainger et al, 1983). Limitations of this technique include extrahepatic shunting, which is common in liver disease and results in an underestimation of blood flow. Other hepatic clearance techniques have been used, such as colloidal clearance by the hepatic Kupffer cells (Dobson & Jones, 1952) and hepatocyte removal of galactose (Keiding, 1988), sorbitol (Zeeh et al, 1988), rose bengal (Combes, 1960), or propranolol (George, 1979). The more complete hepatic extraction of these substances overcomes the need to cannulate a hepatic vein in patients with normal liver function.
A modification of the colloid extraction method developed in the 1980s allows the derivation of the ratio of hepatic arterial to total LBF, termed the hepatic perfusion index. The basis of the technique is the ability to determine by dynamic scintigraphy the temporal separation of accumulating hepatic activity from the arterial and portal supplies after the intravenous administration of a bolus of technetium-99m–sulfur colloid (Fleming et al, 1981; Parkin et al, 1983).
Indicator Dilution
The indicator dilution method relies on the application of the Stewart-Hamilton principle, also derived from the Fick equation (Stewart, 1897). In principle, the hepatic blood flow is proportional to the amount of hepatic blood that has diluted an introduced indicator. This method involves the injection into the hepatic artery and portal vein of a labeled substance that is not removed by the liver; changes in hepatic vein concentration are measured by blood sampling or by monitoring the hepatic isotope activity with an external detector. Such a method is therefore independent of hepatocellular function and reliable, provided the indicator remains in the vascular space and is not excreted prior to sampling. In addition, sampling must not be done in such a manner that affects hepatic circulation, or not more than 30 mL/min in dogs (Cohn et al, 1969).
Portal vein flow may be determined separately using a modification of this technique by sampling portal blood after splenic vein or superior mesenteric artery injection (Chiandussi et al, 1968; Huet et al, 1973). Hepatic artery flow may be calculated as the difference between total hepatic flow and portal flow. In addition, a modified thermal dilution technique has been used to measure portal blood flow in humans (Biber et al, 1983). Indicator dilution methods overestimate true blood flow to hepatic tissue when intrahepatic or extrahepatic shunts are present, although it is possible to measure azygos blood flow by thermal dilution in patients with cirrhosis (Bosch & Groszmann, 1984).
Indicator Fractionation
The measurement of regional blood flow by fractional distribution of cardiac output was first described by Sapirstein in 1956. Briefly, a known amount of radioactive microspheres is injected into the left ventricle, and a reference sample is withdrawn from a peripheral artery at a known rate. The microspheres are then extracted from the various vascular beds, where they have lodged in proportion to the cardiac output. The hepatic arterial blood flow is determined directly by this method, but the portal flow contribution is found indirectly by addition of the flow values in the prehepatic splanchnic organs. Examination of the intrahepatic distribution of microspheres provides a means of assessing the pattern of arterial flow in different regions of the liver (Greenway & Oshiro, 1972). Because the microsphere method requires the postmortem removal of the organs of interest for radioactivity or colorimetric measurement, the additional determination of tissue weight enables flow per gram (i.e., tissue perfusion) to be calculated. Microspheres may be employed to determine the extent of portosystemic shunts: the fractional distribution in the liver may be measured with respect to systemic (lung) activity after portal vein injection, or it may be estimated by injecting a second radioactive microsphere directly into the splenic or mesenteric venous system (Groszmann et al, 1982).
Hepatic Tissue Perfusion
Inert Gas Clearance
By exploiting the fact that radioactive gases such as krypton (85Kr) and xenon (133Xe) distribute equally between tissue and blood according to a specific partition coefficient, the rate of clearance of such gases can be measured after their injection into the hepatic blood supply. After injection and rapid diffusion throughout the liver, the gas clears from the tissue into the blood and is almost completely eliminated from the body after a single passage through the lungs. The clearance rate is proportional to hepatic tissue perfusion, which may be calculated using a standard formula (Leiberman et al, 1978). Beta-emissions of 85Kr are recorded by a Geiger-Müller tube or semiconductor (silicon) detector placed on or immediately above the exposed liver surface, while the γ-emissions of 133Xe are monitored transcutaneously by a single scintillation crystal or a γ-camera; the latter device allows simultaneous measurement of hepatic tissue perfusion in many regions of interest.
Inert gas techniques involve minimal trauma to the patient, and their accuracy is not markedly affected by the presence of hepatic cellular disease or nonperfusion shunts; however, some variability does occur, even within the same subject, when multiple studies are performed. The first to use the inert gas method in the hepatic circulation were Aronsen and colleagues (1966), who recorded the γ-emissions of 133Xe after the injection of a saline solution of the isotope into the portal vein.
Laser Doppler Flowmetry
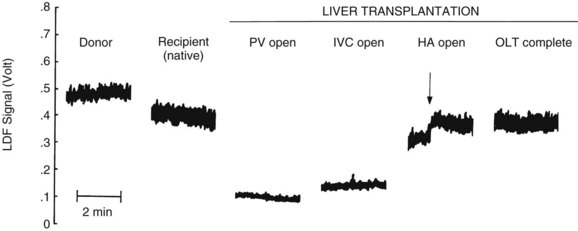
Laser Doppler flowmetry (LDF) is a more recent but established technique for the real-time measurement of microvascular red blood cell perfusion in the liver. By illuminating the tissue with low-power laser light and capturing the backscattered light with independent photodetectors, the Doppler shift of moving cells can be transmitted as an electrical signal. Linearity of the LDF signal from the liver with total organ perfusion has been shown (Almond & Wheatley, 1992; Shepherd et al, 1983, 1987), and the technique has been shown to be sensitive to rapid changes in organ flow (Almond & Wheatley, 1992). In a rat model, the technique provided a good measure of hepatic perfusion in vivo (Wheatley et al, 1993a; Fig. 4.3). LDF has also been used successfully to assess LBF during shock and resuscitation (Wang et al, 1995) and during drug-induced changes in LBF in the rat (Kurihara et al, 1992), and the technique has been applied successfully to measure LBF during liver transplantation in humans (Seifalian et al, 1997). A major drawback of the technique is that, owing to the small volume of tissue interrogated by the laser, the LDF signal can only be used to measure arbitrary, instead of absolute, blood perfusion in a single area. The development of laser Doppler perfusion imaging devices that estimate tissue perfusion by scanning an area of tissue using a laser may overcome the problem of signal calibration (Wardell et al, 1993).
In Vivo Fluorescent Microscopy
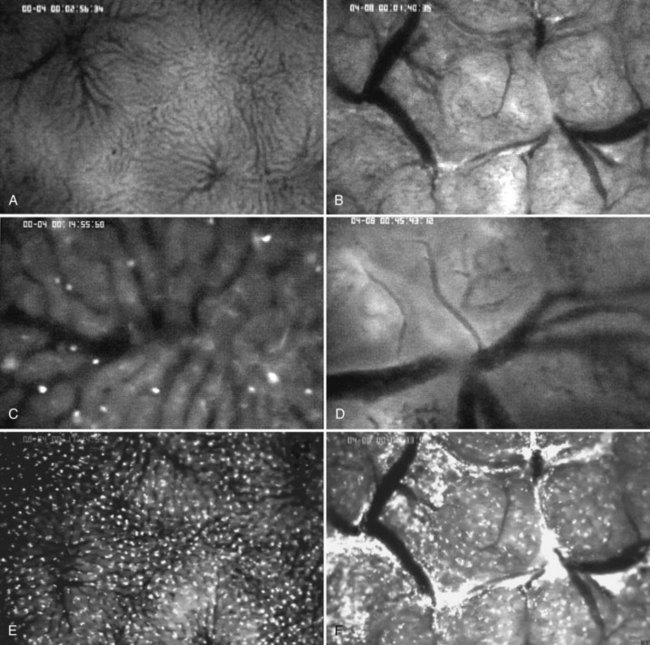
Intravital microscopy was first described in the microvessels of the frog tongue by Waller in 1846. Using this technique, individual sinusoids and terminal venules can be visualized, and changes in their diameters and the velocities with which erythrocytes pass through them can be seen (Menger & Messmer, 1991; Fig. 4.4). The introduction of fluorescent dyes has broadened the spectrum of in vivo microscopy in the liver from morphologic analysis to the study of pathologic events. However, from a hemodynamic point of view, intravital fluorescent microscopy has problems of interpretation (Sherman et al, 1990). In perfused liver, a 2.5-fold increase in portal venous blood flow has been found to be associated with only a 22% increase in sinusoidal red blood cell velocity, suggesting that changes in portal venous blood flow have only a minor effect on the capillary transit time (Sherman et al, 1996). In the regenerating liver; however, a 50% increase in portal venous blood flow resulted in a similar (66%) increase in sinusoidal red blood cell velocity (Wheatley et al, 1996; Zhang et al, 1997).
Near-Infrared Spectroscopy
Near-infrared spectroscopy is a new noninvasive technique that utilizes light transmission and absorption to measure hemoglobin and mitochondrial oxygenation. In contrast to visible light, which can only penetrate a few millimeters, near-infrared light (700 to 1000 nm) can be detected through up to 80 mm of tissue. The application of this technology to monitor liver oxygenation has been validated in models of endotoxic shock in pigs (Nadhum, 2006) and by intraoperative quantification of congestion and mitochondrial redox during hepatic vein occlusion in living-donor transplantation (Ohdan, 2003).
New and Future Developments
Orthogonal polarized spectral imaging has been used to obtain images of the liver microcirculation comparable to those of fluorescence microscopy but without the need for fluorescent dyes (Langer, 2001). This system relies on the absorbance of hemoglobin and can discriminate a single red blood cell in an individual capillary with a diameter of 5 microns. Studies with hand-held devices using this technology have been applied to healthy living donors to obtain sinusoidal red blood cell velocity and columetric blood flow (Puhl et al, 2003). A recent successor to spectral imaging is sidestream dark field imaging, whereby a light guide surrounded by diodes can detect both red and white blood cells in the microcirculation without the surface reflections (Czerny et al, 2009). Portal venous blood flow measured by noninvasive magnetic resonance imaging (MRI) has been found to correlate well with flow measured by Doppler ultrasound flow probes (Pelc et al, 1992); subsequently, the technique has been used to measure portal venous blood flow in human liver transplantation candidates (Kuo et al, 1995). Further methodology improvements in MRI (e.g., dynamic contrast enhancement) and in other techniques, such as positron emission tomography, may lead to their increasing use in the study of hepatic hemodynamics in humans (Chow et al, 2003).
Clinical Relevance
Hemorrhagic Shock, Hypoperfusion, and Ischemia-Reperfusion Injury
Total LBF decreases approximately in relation to the degree of the hemorrhage, and portal venous blood flow decreases in parallel to cardiac output; but similar to the coronary, pulmonary, and cerebral circulations, hepatic arterial flow does not decrease until extremely low blood pressures are reached. As a result, the hepatic oxygen supply tends to be maintained, although oxygen extraction greatly increases to preserve normal total oxygen consumption (Smith et al, 1979). Hepatic blood volume can increase significantly in cardiac failure and can compensate up to 25% of hemorrhage from its large capacitance vessels (Lautt, 2007).
The clinical entity known as shock liver has long been recognized, typically related to cardiogenic or hemorrhagic shock (Birgens et al, 1978; Bynum et al, 1979). Hepatic dysfunction caused by hypoperfusion is manifested pathologically by centrilobular necrosis and clinically by abdominal pain, cholestatic jaundice, and marked elevation of serum transaminases. Three phases of liver injury attributed to ischemia were proposed by Champion, whereby the initial hepatic dysfunction would resolve as long as no additional insults (e.g., sepsis) were incurred (Champion et al, 1976). Gottlieb and colleagues (1983) showed that hepatic dysfunction in humans after trauma was related to a reduced hepatic blood flow rate up to 70% of resting levels. Hepatic blood flow was markedly reduced after injury, and although total splanchnic oxygen delivery was decreased, oxygen consumption remained normal as a result of increased hepatic extraction.
In the ischemic state, upregulation of acute-phase proteins (C-reactive protein, fibrinogen, ceruloplasmin, haptoglobin) is prioritized over production of other hepatic proteins such as albumin and transferrin (Sganga et al, 1985).
More recently, hemorrhagic shock has been recognized to result in generalized vascular endothelial dysfunction and impaired endothelial biosynthesis of NO. Endothelial NO that continues to be expressed by the liver during ischemia is believed to protect against the initial hepatic injury arising from severe hemorrhage. By contrast, more prolonged hemorrhagic shock (>6 hours’ duration) induces greatly increased production of NO owing to activation of an inducible NO synthase enzyme in hepatocytes and Kupffer cells (Peitzman et al, 1995).
Survival has been demonstrated after portal vein ligation to control hemorrhage after traumatic injury (Pachter et al, 1979) or after temporary portal occlusion during difficult biliary tract surgery. Similarly, survival after hepatic artery ligation for trauma, aneurysm, hemobilia, or neoplasms has been described (Rappaport & Schneiderman, 1976). In the transplantation era, the consequences of ischemia and reperfusion for the liver have been increasingly investigated and understood. The damage to the hepatic endothelium and parenchyma that results from postischemic reperfusion is caused by numerous interrelated phenomena, including the action of locally liberated oxygen-derived free radicals and excess formation of vasoconstrictor agents (Wendon, 1999). Endogenous NO tends to protect the liver in the early reperfusion period after hepatic ischemia (Shimamura et al, 1996; Wang et al, 1995). Ischemia is also the primary signal for heat-shock protein production in liver tissue. Experimental studies of heat-shock protein preconditioning through intermittent portal clamping demonstrated attenuated transaminase elevations and improved bile production after ischemia (Terajima et al, 1999).
Liver Atrophy
Liver atrophy results from a significant reduction of LBF containing hepatotrophic substances (see Chapter 5). The degree of atrophy depends on the degree of blood flow deprivation and may be distributed according to the source of deprivation, including portal venous or hepatic artery blood flow or their combination.
Atrophy and fatty degeneration of the canine liver after total portal diversion through an Eck fistula initially was reported more than a century ago (Hahn et al, 1893). Partial or complete diversion of portal vein blood flow from the liver results in atrophy. Complete portal venous flow diversion with interruption of all portal venous collaterals results in more profound liver atrophy than the partial deviation of portal venous flow resulting from side-to-side portacaval anastomoses (Bollman, 1961). A precise relationship between the degree of liver atrophy and the reduction of portal venous blood flow is still to be determined, primarily because of the relative composition and contribution of portal flow and resultant compensatory hepatic arterial flow.
Liver atrophy after portal diversion is not believed to be the result of a decrease in absolute volume flow but instead is due to the effective loss of hepatotrophic substances in the portal blood. Rats subjected to portal flow diversion with portacaval transposition had a decrease in relative liver weight (Guest et al, 1977) despite the effective preservation of portal perfusion from the inferior vena cava (Ryan et al, 1978). Dogs with “partial portacaval transposition” (Marchioro et al, 1967) or “splanchnic flow division” (Starzl et al, 1973, 1975) with diversion of pancreaticogastroduodenosplenic blood had atrophy in liver lobes, although normal tissue perfusion was shown in all regions of the liver (Mathie et al, 1979). Starzl hypothesized that insulin was the factor responsible for preventing atrophy.
The fate of the liver after ligation of the hepatic artery depends largely on the extent of a functional collateral arterial circulation (Rappaport & Schneiderman, 1976). If limited collaterals are present, liver infarction and necrosis may occur after hepatic artery ligation, resulting in death. However, hepatic artery ligation results only in transient ischemic changes in the periphery of the hepatic acinus (zone 3) in the presence of adequate collaterals. Atrophy after hepatic artery ligation can occur in liver segments that have compensatory collateral supply to prevent necrosis. The effects of hepatic artery flow absence are magnified by the presence of low portal venous blood flow, limited oxygen saturation, and superimposed infection (Rappaport & Schneiderman, 1976). Histologically, arterial obstruction results in ischemic changes such as mitochondrial swelling, cell membrane disruption, platelet aggregation, and widening of the spaces of Disse (Mallet-Guy et al, 1972).
Liver Resection and Regeneration
As exemplified in Greek mythology by the myth of Prometheus, the adult liver exhibits a remarkable potential to restore its cellular mass in response to injury through hepatocyte hyperplasia. After the removal of two thirds of liver mass in the rat by partial hepatectomy, liver mass and function are fully recovered by 2 to 3 weeks. In the early stages of regeneration, as a consequence of hepatocyte division, clusters of hepatocytes develop with a reduced distribution of sinusoids (Martinez-Hernandez & Amenta, 1995). Hepatic regeneration of the normal liver remnant proceeds rapidly after partial hepatic resection (see Chapters 4 and 80; Aronsen et al, 1970; Blumgart et al, 1971).
Major resection is associated with a significant and persistent increase in portal pressure (Lee et al, 1987; Morsiani et al, 1995; Wu et al, 1993). Partial liver resection without devascularization normally would be expected to produce little change in total blood flow to the liver. This occurs because the major contributor to total flow, the portal vein, is affected less by events occurring within the liver than by control mechanisms in the arterial resistance vessels of the prehepatic splanchnic bed. On the other hand, the failure of the liver to directly control its portal venous flow may have the result of portal hyperperfusion of a reduced parenchymal mass. Because essentially the same total blood flow is redistributed to a smaller mass of liver tissue, a corresponding increase in tissue perfusion (mL/min per unit tissue weight) would be anticipated in the in situ remnant. Experimental studies support these expectations; an increase in hepatic tissue perfusion was observed in rats immediately after two-thirds hepatectomy (Rice et al, 1977; Wheatley et al, 1993b; Wu et al, 1993). This increase in hepatic perfusion is due primarily to portal venous inflow (Wu et al, 1993), because hepatic arterial blood flow is low, and hepatic arterial resistance is high even 24 hours after partial hepatectomy in rats (Wheatley et al, 1996). Similar studies in patients showed an immediate increase in tissue perfusion of approximately 120% in the remnant (Mathie & Blumgart, 1982). Recent experimental evidence has suggested that intrahepatic shear stress from increased portal flow is a regulator of liver regeneration (Schoen et al, 2001). The significance of blood flow in relation to liver regeneration has been debated frequently in the years since Mann (1944) suggested that regenerative hyperplasia of the liver after partial resection was a function of portal blood flow, and that the process could be prevented by portal flow diversion. However, regenerative hyperplasia normally occurs after partial liver resection in portacavally transposed animals, in which there is no direct supply of portal blood nor the usual posthepatectomy increase in hepatic tissue perfusion. A similar response is seen during portal vein embolization for preoperative expansion of the liver remnant (see Chapter 93A, Chapter 93B ).
Blood Flow in Hepatic Tumors
It has been demonstrated that tumors of the liver, whether primary or secondary, are perfused almost exclusively with arterial blood (Breedis & Young, 1954). Several investigators have attempted to achieve differential diagnosis of patients by measuring the proportion of the hepatic arterial contribution to total flow, adopting the hepatic perfusion index obtained by scintigraphy (Leveson et al, 1985) or the Doppler perfusion index obtained by ultrasound (Leen et al, 1991a). Such measurements have shown an increased hepatic arterial contribution in patients with confirmed liver metastases compared with normal subjects. Patients who have colorectal cancer with no observed liver metastases were found to have altered hemodynamics that overlapped those of the other groups, suggesting the presence of occult hepatic disease (Leen et al, 1991b). The Doppler perfusion index has been shown to be more sensitive than computed tomography, conventional ultrasound, or laparotomy alone in the detection of colorectal liver metastases (Leen et al, 1995).
Taking advantage of this phenomenon, the application of the vasoconstrictors epinephrine, phenylephrine, or angiotensin has improved cytotoxic drug delivery to tumor-bearing areas in human and animal models (Bloom et al, 1987; Goldberg et al, 1991; Hemingway et al, 1991). This approach is feasible because the neovasculature of tumor tissue lacks smooth muscle and therefore does not respond to vasoconstrictor agents, enabling increased delivery of chemotherapeutic drugs. Biodegradable microspheres also have been used for enhancing targeted therapy; they cause temporary blood flow interruption, resulting in improved uptake of chemotherapeutic agents in tumor tissue and consequent reduced systemic toxicity (Ball, 1991). Multiple drugs have been utilized in the palliative setting for percutaneous intrarterial instillation, and these can be loaded into the two current commercially available microsphere systems, DC Bead and QuadraSphere (Liapi et al, 2010).
Bile Duct Obstruction
Bile duct obstruction can affect hepatic hemodynamics significantly (see Chapter 7). In general, LBF is reduced in the presence of chronic biliary obstruction, leading to hepatic dysfunction. Conversely, acute increases in bile duct pressure from early obstruction result in a reflexive increase in LBF, which attempts to maintain adequate flow in the face of an increased pressure gradient opposing secretion and excretion of bile. Most evidence suggests that the hemodynamic response of the liver to biliary obstruction is related, directly or indirectly, to changes in bile duct pressure. Given the limited space of Mall in the portal triad, it is conceivable that increased biliary pressures may compress the portal capacitance vessels, leading to increased arterial flow (Kanda et al, 1996). Nagorney and colleagues (1982) showed that acute serial increases in bile duct pressure in dogs with complete bile duct obstruction increased hepatic arterial blood flow by 250% but did not affect portal venous blood flow.
Chronic bile duct obstruction is associated with a decrease in total LBF. In addition, dilation of the sinusoids and elevation of portal pressure in dogs was noted after 4 weeks of complete bile duct obstruction (Ohlsson et al, 1970). Relief of long-term obstruction does not result in the return of normal hemodynamics, suggesting irreversible intrahepatic vascular damage (Aronsen et al, 1969). Furthermore a 23% reduction in effective LBF persisted for 1 to 5 years after operative decompression in patients with choledocholithiasis and jaundice for more than 2 weeks preoperatively (Aronsen, 1968). Hunt (1979) serially measured LBF daily for 1 week after bile duct ligation in rats, using the 133Xe clearance technique to document the early hemodynamic response. Total LBF decreased steadily after the first postoperative day to a plateau level of approximately 50% of the preoperative value 5 days after operation. Mathie and colleagues (1988) confirmed the decrease in total LBF after bile duct ligation by measuring the individual portal venous and hepatic arterial components of LBF. Using electromagnetic flowmeters in dogs with complete bile duct ligation, hepatic arterial and portal venous blood flow were observed to decrease by 36% and 44%, respectively; they also showed a 200% increase in intrahepatic portal resistance but a lesser increase in hepatic arterial resistance. Similarly, Bosch and others (1983) showed that dogs with chronic bile duct ligation had decreased portal venous flow and had developed sinusoidal portal hypertension and extensive portosystemic shunting.
The precise mechanism for reduction in LBF after chronic bile duct obstruction is unknown. Although increased portal vascular resistance is the accepted underlying cause, the primary site of this resistance change has been considered to be presinusoidal (Reuter & Chuang, 1976), sinusoidal (Bosch et al, 1983), or postsinusoidal (Tamakuma et al, 1975). Bosch and colleagues (1983) also showed the development of significant portosystemic shunting with an inverse correlation between shunting and portal venous blood flow, suggesting that the site of shunting is predominantly extrahepatic (Ohlsson, 1972).
Chronic biliary obstruction results in two hemodynamic consequences: portal hypertension associated with secondary biliary fibrosis and shock after biliary tract decompression. Approximately 20% of patients with prolonged biliary obstruction develop clinically significant portal hypertension (Adson & Wychulis, 1968; Blumgart et al, 1984; Sedgwick et al, 1966). The operative risk of biliary decompression in these patients is significant. Technical difficulties of stricture repair—dense fibrous adhesions, hilar ductal involvement, and infection—are complicated by the risk of hemorrhage from subhepatic and periductal varices and potential postoperative liver failure. Portosystemic shunts may be performed before biliary decompression in patients with bleeding esophageal varices or previous intraoperative hemorrhage that precluded successful stricture repair (Adson & Wychulis, 1968; Sedgwick et al, 1966).
In addition to the hemodynamic consequences of chronic bile duct obstruction, sudden decompression of the obstructed biliary tree also may have profound hemodynamic effects. Tamakuma and colleagues (1975) studied the significance of clinical shock after biliary decompression and noted that hypotension and shock could develop in the immediate postoperative period. They showed that biliary decompression resulted in an abrupt decrease in wedged hepatic vein pressure, portal vein pressure, and arterial pressure within 30 minutes of decompression. Similarly, Steer and colleagues (1968) reported that rapid needle decompression of an obstructed biliary tree in jaundiced dogs induced a decreased arterial pressure, central venous pressure, and portal venous pressure within 1 hour; they concluded that sudden decompression of chronic biliary obstruction leads to sequestration of fluid within the liver, resulting in a decrease in the effective circulating plasma volume and subsequent hypotension.
Portal Hypertension (See Chapter 70A, Chapter 70B, Chapter 74 )
Hemodynamics
Portal hypertension is a state of sustained increase in the intraluminal pressure of the portal vein and its collaterals. A mean pressure greater than 12 mm Hg is the accepted target, as variceal bleeding does not occur at lower pressures than this (Garcia-Tsao et al, 1985). Hemodynamic factors that influence portal hypertension are best understood by application of the flow-resistance principle that applies to the portal venous system. Portal pressure depends on two basic components: portal blood flow and hepatic portal vascular resistance. Portal hypertension may result from a significant increase in hepatic portal inflow from the prehepatic splanchnic vasculature, an increase in intrahepatic portal resistance, or both. Although simple in concept, multiple factors may influence both the components of the system and the pathophysiology of portal hypertension.
Increased portal pressure, diminished hepatic portal blood flow, and an extensive extrahepatic collateral venous network supplied by a hyperdynamic splanchnic and systemic circulation characterize the hemodynamics of portal hypertension (e.g., in cirrhosis). The traditional view of the source of increased portal pressure is fibrotic encroachment around portal radicles, leading to increased intrahepatic portal resistance. In cirrhotic patients, extrahepatic shunts may account for at least 50% of the portal flow, whereas 80% of portal flow actually reaching the liver has been observed to bypass the sinusoidal vascular bed via intrahepatic shunts (Okuda et al, 1977). The magnitude of extrahepatic shunt flow in patients with cirrhosis was measured directly by thermal dilution assessment of azygos blood flow; a value 300 mL/min greater than in patients without portal hypertension was noted (Bosch & Groszmann, 1984). The hepatic artery probably provides a greater relative contribution to the total LBF in patients with cirrhosis than in normal subjects, although it also was shown that 33% of the arterial blood may flow through intrahepatic shunts to the systemic venous circulation (Groszmann et al, 1977).
The direction of portal blood flow has been proposed as a contributor to the pathophysiology of portal hypertension. The progression of intrahepatic disease and increasing sinusoidal pressure has been postulated as contributing to reversal of flow in the portal vein, which further aggravates the injury by depriving the liver of nutrients (Warren & Muller, 1959). However, reversed, or hepatofugal, portal vein flow has rarely been documented. Hemodynamic information from 273 cirrhotic patients collected from the literature in the 1970s revealed no case of spontaneous flow reversal (Moreno et al, 1975), and Gaiani and colleagues (1991) reported an incidence of only 3.1% in a sample of 228 patients.
The hypothesis that the low peripheral vascular resistance in portal hypertension may be caused by the stimulated production of NO induced by endotoxemia was proposed by Vallance and Moncada in 1991. The initial experimental evidence was provided by Pizcueta and others (1992), who showed an increase in systemic and splanchnic vascular resistance in cirrhotic rats after the administration of an NO inhibitor. Although endotoxemia is likely responsible for the decompensation in end-stage cirrhosis, the hemodynamic alterations are due to NO (Lee et al, 1995; Niederberger et al, 1995). Constitutive NO synthase seems to be upregulated in discrete anatomic locations, such as in the endothelium of the mesenteric artery and in the esophageal, gastric, and jejunal mucosa (Mathie, 1999).
Intrahepatic Vascular Resistance in Liver Cirrhosis
Liver cirrhosis occurs in response to chronic liver injury and is characterized primarily by a disruption of hepatic architecture, with the development of fibrous septa and abnormal nodules and circulation, leading to a sustained increase in portal vascular resistance and portal pressure. In addition, afferent (portal venules and hepatic arterioles) and efferent vessels (hepatic venules) can be found within the fibrous septa (Kelty et al, 1950). The pathogenesis of cirrhosis involves initial hepatocyte necrosis and inflammation with subsequent transformation of hepatic stellate cells into myofibroblasts (see Chapter 6). Microscopically, excessive accumulation of collagen leads to the collagenization of the space of Disse and to Ito cell activation (Greenwel et al, 1991; Martinez-Hernandez, 1985; Martinez-Hernandez & Amenta, 1993). Capillarization of sinusoidal endothelial cells occurs by defenestration or loss of endothelial cell pores and the appearance of a basement membrane (Varin & Huet, 1985). In the cirrhotic liver, the sites of vascular resistance are still unclear; however, because portal and hepatic venules can be found within the fibrous septa, constriction or distortion of portal venules, hepatic venules, or both may be involved (Kelty et al, 1950). As portal venous blood flow progressively decreases in cirrhosis, arterial resistance may decrease through an increase in arterial flow, suggesting an intact buffer response. Studies in cirrhotic rats have demonstrated higher hepatic arterial flows compared with normal controls under baseline conditions (Richter et al, 2000). This finding was confirmed using intraoperative measurements in patients with end-stage cirrhosis undergoing living-donor liver transplantation (Aoki et al, 2005).
Until recently, it was thought that the elevated IHVR in cirrhosis was irreversible. Evidence now suggests that it can be reduced pharmacologically with vasodilators (Bhathal & Grossman, 1985; Reichen & Le, 1986). IHVR can be marginally reduced by prostaglandin E2 (Ballet, 1991) and isoprenaline and more substantially by nitroprusside, papaverine, and verapamil (Ballet, 1991; Bhathal & Grossman, 1985; Reichen & Le, 1986). Groszmann (1990) reported that intravenous nitroglycerin caused a 24% decrease in IHVR in cirrhotic patients.
In alcoholic patients, a significant correlation is seen between the extent of hepatic stellate cell activation and the level of portal vascular resistance (Rockey & Chung, 1995). Several factors have been implicated in stellate cell activation such as inflammatory mediators, cytokines, and growth factors (Friedman, 1993, 1997; Gandhi et al, 1994; Greenwel et al, 1991) and ET (Pinzani et al, 1996; Rockey & Weisiger, 1996; Rockey et al, 1998). ET-induced contractility of isolated hepatic stellate cells increases in proportion to the degree of hepatic stellate cell activation (Rockey & Weisiger, 1996). Overexpression of ET-1 has been found in patients with cirrhosis (Housset et al, 1993; Rockey & Weisiger, 1996), which probably contributes to the elevated IHVR, probably by increased hepatic stellate cell contraction (Housset et al, 1993; Rockey & Weisiger, 1996). Microscopically, ET-1 receptors (ETa and ETb) are detected on all cell types in rat liver, especially Ito cells, as well as in cirrhotic human subjects (Leivas et al, 1995; Pinzani et al, 1996; Rockey et al, 1998). ET-1-induced hepatic sinusoidal contractility is enhanced in ethanol-induced fatty liver but not in cirrhotic liver, indicating that ET-1 may be involved in the regulation of sinusoidal flow in the early stage of liver cirrhosis in rats (Bauer et al, 1995).
Decreased endothelial NO synthesis and release by the liver (Gupta et al, 1998; Rockey & Chung, 1998) coupled with an associated increase in portal vascular resistance may potentiate by the effect of increased ET-1 expression by hepatic endothelial and hepatic stellate cells (Pinzani et al, 1996). The interplay between NO, ET, and other local regulatory mechanisms controlling sinusoidal hemodynamics is depicted in Figure 4.5. Groszmann (1990) suggested that the increased mesenteric blood flow in the hyperdynamic stage of portal hypertension may be relatively less important than the elevated intrahepatic portal resistance in maintaining increased portal pressure. Clinically, the vasodilaton of the splanchnic circulation likely serves to increase flow in the extrahepatic collateral circulation, leading to variceal hemorrhage.
Treatment (See Chapter 70A, Chapter 70B, Chapter 74 )
Medical and surgical management strategies for portal hypertension strive to improve patient survival by the reduction of pressure and flow in extrahepatic variceal vessels, mainly esophageal and gastric vessels, while preserving adequate portal flow to the liver. Portosystemic shunting and pharmacologic reduction of portal flow can provide effective decompression, but both deprive the liver of portal flow. Surgical treatment of portal hypertension may be performed by one of the many portosystemic shunt procedures (see Chapter 76B); the initial clinical application of the portacaval shunt was reported 50 years after its description by Eck (Whipple, 1945). The hemodynamic consequences of shunt surgery depend on the particular shunt performed, the nature and severity of the disease, and the hemodynamic condition of the patient. End-to-side portacaval shunts divert all portal blood flow away from the liver, whereas less complete diversions reduce portal flow in proportion to the degree to which the shunt reduces portal pressure. The hepatic artery flow may increase by 100%, but even a maximal flow increase can usually only partly compensate for loss of portal flow (Mathie & Blumgart, 1983a). Hepatic oxygen consumption tends to be maintained by increased oxygen extraction from the available arterial supply.
Total portacaval shunts are very effective in reducing portal pressure and preventing bleeding from esophageal varices. However, because of bypass of the hepatic circulation, liver failure and encephalopathy are common complications of the operation (see Chapter 76A). Therefore, partial shunts—such as the side-to-side (see Chapter 76B), mesocaval (see Chapter 76D), and proximal or distal splenorenal shunts (see Chapter 76C)—have gained popularity. Transjugular intrahepatic portosystemic shunting (TIPS), first reported by Rössle and colleagues (1989), is currently the treatment of choice for recurrent variceal bleeding in patients who are refractory to conservative medical management (see Chapters 75A and 76E; Iannitti & Henderson, 1997).
Pharmacologic reduction of portal hypertension (see Chapter 75B) initially was based largely on an attempt to diminish hepatic portal inflow from the mesenteric vascular bed by the use of vasoconstrictor agents. At a dose that decreases the heart rate by 25%, the β-adrenoceptor antagonist propranolol significantly reduced the risk of rebleeding in cirrhotic patients who were otherwise in good condition (Lebrec et al, 1981). Propranolol exerts its action by two mechanisms: decreased cardiac output as a result of β1-adrenergic cardiac receptor blockade and antagonism of β2-adrenoceptors in the splanchnic vasculature that leaves the vasoconstrictive influence of α-adrenergic receptors unopposed, resulting in a decrease portal flow and pressure. Vasopressin causes generalized peripheral vasoconstriction (Bosch et al, 1988), whereas the effect of somatostatin is specific to the splanchnic vascular bed (Kravetz et al, 1984) and is the result of an inhibition of glucagon release and direct vasoconstriction. Somatostatin is at least as effective as vasopressin in controlling bleeding, and with fewer side effects; it may be the agent of choice in acute management of variceal hemorrhage. Serotonin may play a significant role in maintaining increased portal pressure, and smooth muscle serotonin-receptor antagonists have been shown to lower the pressure in cirrhosis (Hadengue et al, 1987; Mastaï et al, 1989).
The nitrovasodilators isosorbide dinitrate and isosorbide mononitrate were observed to lower the portal pressure in portal hypertensive animals (Blei & Gottstein, 1986) and to increase hepatic (but not azygos) blood flow in cirrhotic patients (Navasa et al, 1989), suggesting that they may act by reducing intrahepatic portal vascular resistance. Application of nitroglycerin by transdermal tape to cirrhotic patients resulted in a reduction in portal pressure without affecting hepatic blood flow (Iwao et al, 1991).
These experiments support the previous discussion of NO and ET-1 control of hepatic sinusoidal tone. An ET receptor antagonist was reported to reduce portal pressure in cirrhotic rats by nearly 30% (Reichen et al, 1998). The combined effects of the long-acting nitrovasodilator molsidomine plus propranolol have been found by some (Hori et al, 1996) but not others (Garcia-Pagán et al, 1996) to be a more effective portal hypotensive regimen than propanolol alone.
Hemodynamic Studies and Human Liver Transplantation
Orthotopic liver transplantation (OLT) (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E, Chapter 98A, Chapter 98B, Chapter 98C ) of a normal donor organ does not normalize the splanchnic and systemic hemodynamic alterations of end-stage liver disease (Henderson, 1993). In fact, total hepatic blood flow remains elevated 6 months after OLT (Hadengue et al, 1993; Henderson et al, 1989; Navasa et al, 1993) mainly because of portal venous blood flow (Henderson et al, 1992a, 1992b; Henderson, 1993; Paulsen & Klintmalm, 1992). Azygos flow also remains elevated, and other portosystemic shunts have been documented up to 4 years after OLT (Navasa et al, 1993). Ligation of these portosystemic collateral pathways has been shown to increase portal venous blood flow (Fujimoto et al, 1995). Cardiac output data have been conflicting, with one group reporting persistently elevated values (Hadengue et al, 1993) and others showing decreases 2 weeks and 2 months after OLT (Navasa et al, 1993). Gadano and colleagues (1995) emphasized that factors such as anemia and sepsis may account for the deranged hemodynamics after OLT.
Effect of Liver Transplantation on Liver Blood Flow
Henderson and others (1989) demonstrated that LBF remains elevated for a prolonged period after liver transplantation, suggesting that baseline LBF may be under direct sympathetic control, which is lost after OLT, leading to an unopposed rise. The hemodynamic consequences of OLT in human patients are difficult to interpret for several reasons: 1) the causes of liver failure in end-stage transplant candidates are diverse; 2) immunosuppressive drugs are used, such as cyclosporine, which causes arterial hypertension; and 3) to control systemic hypertension after OLT, patients may also be given vasodilators such as hydralazine, which can cause persistently increased cardiac output.
The hepatic artery buffer reponse is conserved following OLT despite denervation. This was demonstrated in a series of experiments by Payen and colleagues (1990), who serially clamped the portal vein every 12 hours for 7 days following OLT and reported reciprocal increases in hepatic arterial flow.
The advent of living-donor partial liver transplantation has introduced the phenomenon of small-for-size syndrome, whereby the pressure of the full portal flow traveling through a small liver remnant leads to decreased arterial flow, presumably through an intact hepatic arterial buffer response (Michalopoulos, 2010). The reduced hepatic arterial flow is hypothesized to be diverted to the splenic and gastroduodenal circulation. Prospective studies have demonstrated that ligation or embolization of the splenic artery leads to improved hepatic arterial inflow and improved graft function (Lo et al, 2003; Umeda et al, 2007).
Effect of Laparoscopy on Liver Blood Flow
The use of a carbon dioxide pneumoperitoneum in laparoscopic donor surgery has been demonstrated to substantially reduce portal venous flow in parallel with the intraperitoneal pressure (Schilling et al, 1997; Jakimowicz et al, 1998). The hepatic artery buffer response is usually preserved during laparoscopy, however, conflicting data exist in support of the maintenance of this effect in high-pressure pneumoperitoneum. Rat models using fluorescent microspheres supported preserved hepatic arterial flow during decreased portal venous flow (Yokoyama et al, 2002), but this was not supported by others (Richter et al, 2001), who saw a parallel decrease in arterial and portal venous flows during laparoscopy. Some groups have suggested the avoidance of head-up positioning and pressures greater than 15 mm Hg during laparoscopy to preserve LBF (Junghans et al, 1997; Klopfenstein et al, 1998).
Acknowledgement
Thank you to Drs. Blumgart, Wheatley, and Mahie for their previous contributions to this chapter.
Adson MA, Wychulis AR. Portal hypertension in secondary biliary cirrhosis. Arch Surg. 1968;96:604-612.
Aharinejad S, et al. Sphincters of canine hepatic sublobular veins respond to endothelin-1 and 3. Anat Embryol. 1997;196:299-309.
Almond NE, Wheatley AM. Measurement of hepatic perfusion in the rat by laser Doppler flowmetry. Am J Physiol. 1992;262:G203-G209.
Andreen M, et al. The effect of controlled halothane anaesthesia on splanchnic oxygen consumption in the dog. Acta Anaesth Scand. 1975;19:238-244.
Aoki T, et al. Intraoperative direct measurement of hepatic arterial buffer response in patients with and without cirrhosis. Liver Transpl. 2005;11:684-691.
Aronsen KF. Late effects of biliary stasis on the effective liver blood flow. Acta Chir Scand. 1968;134:278-281.
Aronsen KF, et al. The clearance of 133Xenon from the liver after intraportal injection in man. Nucl Med. 1966;5:241-245.
Aronsen KF, et al. Liver blood flow studies during and after various periods of total biliary obstruction in the dog. Acta Chir Scand. 1969;135:55-59.
Aronsen KF, et al. Evaluation of hepatic regeneration by scintillation scanning, cholangiography and angiography in man. Ann Surg. 1970;171:567-574.
Ball ABS. Regional chemotherapy for colorectal hepatic metastases using degradable starch microspheres: a review. Acta Oncol. 1991;30:309-313.
Ballet F. Hepatic resistance in isolated perfused normal and cirrhotic liver. In: Ballet, F, Thurman, RG. Research in Perfused Liver. London, INSERM/John Libbey; 1991:339-360.
Batchelder BM, Cooperman LH. Effects of anesthetics on splanchnic circulation and metabolism. Surg Clin North Am. 1975;55:787-794.
Bauer C, et al. Role of nitric oxide in the regulation of the hepatic microcirculation in vivo. J Hepatol. 1997;27:1089-1095.
Bauer M, et al. ET-1 induced alterations of hepatic microcirculation: sinusoidal and extrasinusoidal sites of action. Am J Physiol. 1994;267:G143-G149.
Bauer M, et al. Chronic ethanol consumption increases hepatic sinusoidal contractile response to endothelin-1 in the rat. Hepatology. 1995;22:1565-1576.
Bhathal PS, Grossman HJ. Reduction of the increased portal vascular resistance of the isolated perfused cirrhotic rat liver by vasodilators. J Hepatol. 1985;1:325-337.
Biber B, et al. Portal blood flow in man during surgery, measured by a modification of the continuous thermodilution method. Scand J Gastroenterol. 1983;18:233-239.
Biernat J, et al. Role of afferent nerves and sensory peptides in the mediation of hepatic artery buffer response. J Physiol Pharmacol. 2005;56:133-145.
Birgens HS, et al. The shock liver. Acta Med Scand. 1978;204:417-421.
Blei AR, Gottstein J. Isosorbide dinitrate in experimental portal hypertension: a study of factors that modulate the hemodynamic response. Hepatology. 1986;6:107-111.
Bloom ND, et al. Enhancement of tumor blood flow and tumoricidal effect of doxorubicin by intraportal epinephrine in experimental liver metastasis. Arch Surg. 1987;122:1269-1272.
Blumgart LH. Liver atrophy, hypertrophy and regenerative hyperplasia in the rat: the relevance of blood flow. In Ciba Foundation Symposium 55 (new series): Hepatotrophic Factors. Amsterdam: Elsevier Excerpta Medica; 1978. pp 181-215
Blumgart LH, et al. Observations on liver regeneration after right hepatic lobectomy. Gut. 1971;12:922-928.
Blumgart LH, et al. Benign bile duct stricture following cholecystectomy: critical factors in management. Br J Surg. 1984;71:836-843.
Bollman JL. The animal with an Eck fistula. Physiol Rev. 1961;41:607-621.
Bosch J, Groszmann RJ. Measurement of azygous venous blood flow by a continuous thermal dilution technique: an index of blood flow through gastroesophageal collaterals in cirrhosis. Hepatology. 1984;4:424-429.
Bosch J, et al. Chronic bile duct ligation in the dog: hemodynamic characterization of a portal hypertensive model. Hepatology. 1983;3:1002-1007.
Bosch J, et al. Effects of vasopressin on the intravariceal pressure in patients with cirrhosis: comparison with the effects on portal pressure. Hepatology. 1988;8:861-865.
Boyer TD, et al. Direct transhepatic measurement of portal vein pressure using a thin needle: comparison with wedged hepatic vein pressure. Gastroenterology. 1977;72:584-589.
Bradley SE, et al. The estimation of hepatic blood flow in man. J Clin Invest. 1945;24:890-897.
Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-985.
Brown HS, et al. Measurement of normal portal venous blood flow by Doppler ultrasound. Gut. 1989;30:503-509.
Burns P, et al. Doppler flowmetry and portal hypertension. Gastroenterology. 1987;92:824-826.
Burton-Opitz R. The vascularity of the liver: I. The flow of blood in the hepatic artery. Q J Exp Physiol. 1910;3:297-313.
Burton-Opitz R. The vascularity of the liver: IV. The magnitude of the portal inflow. Q J Exp Physiol. 1911;4:113-125.
Bynum TE, et al. Ischaemic hepatitis. Dig Dis Sci. 1979;24:129-135.
Caesar J, et al. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961;21:43-57.
Chaland P, et al. Orthotopic liver transplantation with hepatic artery anastomoses. Transplantation. 1990;49:675-678.
Champion HR, et al. Clinicopathological study of hepatic dysfunction following shock. Surg Gynecol Obstet. 1976;142:657-663.
Chezmar JL, et al. Persistence of portal systemic collaterals and splenomegaly on CT scan after orthotopic liver transplantation. Am J Roentgenol Radium Ther Nucl Med. 1992;159:317-320.
Chiandussi L, et al. Estimation of hepatic arterial and portal venous blood flow by direct catheterisation of the vena porta through the umbilical cord in man. Acta Hepatosplenol. 1968;15:166-171.
Chow P, et al. The measurement of liver blood flow: a review of experimental and clinical methods. J Surg Res. 2003;112:1-11.
Cohn JN, et al. Intrahepatic distribution of hepatic arterial and portal venous flows in the dog. Am J Physiol. 1969;216:285.
Combes B. Estimation of hepatic blood flow in man and dogs by I131-labelled rose bengal. J Lab Clin Med. 1960;56:537.
Condon RE, et al. Hepatic arterial and portal venous pressure–flow relationships in isolated, perfused liver. Am J Phys. 1962;202:1090-1094.
Czerny V, et al. In situ assessment of the liver microcirculation in mechanically ventilated rats using sidestream dark-field (SDF) imaging. Physiol Res. 2009;58:49-55.
Dauzat M, Layrargues GP. Portal vein blood flow measurements using pulsed Doppler and electromagnetic flowmetry in dogs: a comparative study. Gastroenterology. 1989;96:913-919.
Dobson EL, Jones HB. The behaviour of intravenously injected particulate material: its rate of disappearance from the blood stream as a measure of liver blood flow. Acta Med Scand Suppl. 1952;273:1-71.
Ebbing C, et al. Hepatic artery hemodynamics suggest operation of a buffer response in the human fetus. Reprod Sci. 2008;15:166-178.
Eipel C, et al. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046-6057.
Ernst H, et al. Color Doppler ultrasound of liver lesions: signal enhancement after intravenous injection of the ultrasound contrast agent Levovist. J Clin Ultrasound. 1996;24:31-35.
Fiorucci S, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539-548.
Fleming JS, et al. In vivo assessment of hepatic-arterial and portal-venous components of liver perfusion: concise communication. J Nucl Med. 1981;22:18-21.
Friedman SL. The cellular basis of hepatic fibrosis: mechanisms and treatment strategies. N Engl J Med. 1993;119:1828-1835.
Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424-430.
Fujimoto M, et al. Influence of spontaneous portosystemic collateral pathways on portal hemodynamics in living-related liver transplantation in children: Doppler ultrasonographic study. Transplantation. 1995;60:41-45.
Fung Y, et al. Portal vein velocities measured by ultrasound: usefulness for evaluating shunt functioning following TIPS. Abdom Imaging. 1998;23:511-514.
Gadano A, et al. Hemodynamics after orthotopic liver transplantation: study of associated factors and long-term effects. Hepatology. 1995;22:458-465.
Gaiani S, et al. Prevalence of spontaneous, hepatofugal portal flow in liver cirrhosis. Gastroenterology. 1991;100:160-167.
Gandhi CR, et al. Endothelins: biochemistry and pathophysiologic actions. Anesthesiology. 1994;80:892-905.
Garcia-Pagán JC, et al. Propranolol plus molsidomine vs propranolol alone in the treatment of portal hypertension in patients with cirrhosis. J Hepatol. 1996;24:430-435.
Garcia-Tsao G, et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419-424.
George CF. Drug kinetics and hepatic blood flow. Clin Pharmacokinet. 1979;4:433-448.
Goldberg JA, et al. The use of angiotensin II as a potential method of targeting cytotoxic microspheres in patients with intrahepatic tumour. Br J Cancer. 1991;63:308-310.
Gottlieb ME, et al. Hepatic perfusion and splanchnic oxygen consumption in patients post injury. J Trauma. 1983;23:836-843.
Grainger SL, et al. Clearance and non-invasive determination of the hepatic extraction of indocyanine green in baboons and man. Clin Sci. 1983;64:207-212.
Greenway CV, Lautt WW. Effects of hepatic venous pressure on transsinusoidal fluid transfer in the liver of the anesthetized cat. Circ Res. 1970;26:697-703.
Greenway CV, Lautt WW. Hepatic circulation. In: Schultz, SG, et al. Handbook of Physiology: The Gastrointestinal System, vol 1. New York: American Physiological Society, Oxford University Press; 1989:1519-1564.
Greenway CV, Oshiro G. Intrahepatic distribution of portal and hepatic arterial blood flows in anaesthetized cats and dogs and the effects of portal occlusion, raised venous pressure and histamine. J Physiol (Lond). 1972;227:473-485.
Greenway CV, Stark RD. Hepatic vascular bed. Physiol Rev. 1971;51:23-65.
Greenway CV, et al. The effects of stimulation of the hepatic nerves, infusions of noradrenaline and occlusion of the carotid arteries on liver blood flow in the anaesthetised cat. J Physiol. 1967;192:21-41.
Greenwel P, et al. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers: differences in the production of interleukin-6. Lab Invest. 1991;65:644-653.
Groszmann RJ. Pathophysiology of portal hypertension. In: Gentilini, P, et al. Liver Diseases and Renal Complications. New York: Raven Press; 1990:165-173.
Groszmann RJ, et al. Intrahepatic arteriovenous shunting in cirrhosis of the liver. Gastroenterology. 1977;73:201-204.
Groszmann RJ, et al. Splanchnic hemodynamics in portal-hypertensive rats: measurement with gamma-labeled microspheres. Am J Physiol. 1982;242:G156-160.
Groth CG, et al. Studies of blood flow and ultrastructural changes in rejecting and nonrejecting canine orthotopic liver homografts. Surgery. 1968;63:658-668.
Guest J, et al. Portacaval transposition and subsequent partial hepatectomy in the rat: effects on liver atrophy, hypertrophy and regenerative hyperplasia. Br J Exp Pathol. 1977;58:140-146.
Gupta TK, et al. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926-931.
Hadengue A, et al. Beneficial hemodynamic effects of ketanserin in patients with cirrhosis: possible role of serotoninergic mechanisms in portal hypertension. Hepatology. 1987;7:644-647.
Hadengue A, et al. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993;17:175-178.
Hahn M, et al. Die eckische Fistel zwischen der Unteren Hohlvene und der Pfortader und ihre Folgen für den. Organismus. Arch Exp Pathol Pharmacol. 1893;32:161-210.
Hanson KM, et al. Local control of hepatic arterial and portal venous flow in the dog. Am J Phys. 1966;211:712-720.
Hemingway DM, et al. The effects of intra-arterial vasoconstrictors on the distribution of a radiolabelled low molecular weight marker in an experimental model of liver tumour. Br J Cancer. 1991;63:495-498.
Henderson JM. Abnormal splanchnic and systemic hemodynamics of end-stage liver disease: what happens after liver transplantation? Hepatology. 1993;17:514-516.
Henderson JM, et al. Increased galactose clearance after liver transplantation: a measure of increased blood flow through the denervated liver? Hepatology. 1989;10:288-291.
Henderson JM, et al. Hepatic artery flow increases intraoperatively during liver transplantation in response to portal vein flow reduction. Hepatology. 1991;14:48A.
Henderson JM, et al. Hemodynamics during liver transplantation: the interactions between cardiac output and portal venous and hepatic arterial blood flows. Hepatology. 1992;16:715-718.
Henderson JM, et al. High cardiac output of advanced liver disease persists after orthotopic liver transplantation. Hepatology. 1992;15:258-262.
Hori N, et al. Haemodynamic effects of combined treatment with molsidomine and propranolol on portal hypertension in conscious and unrestrained cirrhotic rats. J Gastroenterol Hepatol. 1996;11:985-992.
Housset C, et al. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci U S A. 1993;90:9266-9270.
Huet PM, et al. Simultaneous estimation of hepatic and portal blood flows by an indicator dilution technique. J Lab Clin Med. 1973;82:836-846.
Hughes RL, et al. The effect of hypercarbia on hepatic blood flow and oxygen consumption in the greyhound. Br J Anaesth. 1979;51:289-296.
Hughes RL, et al. Liver blood flow and oxygen consumption during hypocapnia and IPPV in the greyhound. J Appl Physiol. 1979;47:290-295.
Hughes RL, et al. Systemic hypoxia and hyperoxia, and liver blood flow and oxygen consumption in talc greyhound. Pflugers Arch. 1979;381:151-157.
Hughes RL, et al. Effects of enflurane and halothane on liver blood flow and oxygen consumption in the greyhound. Br J Anaesth. 1980;52:1079-1086.
Hughes RL, et al. Liver blood flow and oxygen consumption during metabolic acidosis and alkalosis in the greyhound. Clin Sci. 1980;60:355-361.
Hunt DR. Changes in liver blood flow with development of biliary obstruction in the rat. Aust N Z J Surg. 1979;49:733-737.
Iannitti DA, Henderson JM. Surgery in portal hypertension. Baillieres Clin Gastroenterol. 1997;11:351-364.
Isales CM, et al. Endothelin-1 induces cholestasis, which is mediated by an increase in portal pressure. Biochem Biophys Res Commun. 1993;191:1244-1251.
Ishikawa M, et al. Hemodynamic changes in blood flow through the denervated liver in pigs. J Invest Surg. 1995;8:95-100.
Iwao T, et al. Hemodynamic study during transdermal application of nitroglycerin tape in patients with cirrhosis. Hepatology. 1991;13:124-128.
Iwao T, et al. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012-1017.
Jakab F, et al. The interaction between hepatic arterial and portal venous blood flows; simultaneous measurement by transit time ultrasonic volume flowmetry. Hepatogastroenterology. 1995;42:18-21.
Jakimowicz J, et al. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12:129-132.
Jefferson NC, et al. Formation of effective collateral circulation following excision of hepatic artery. Am J Physiol. 1956;184:589-592.
Junghans T, et al. Does pneumoperitoneum with different gases, body position, and intraperitoneal pressures influence renal and hepatic blood flow? Surgery. 1997;121:206-211.
Kanda H, et al. Hepatic blood flow after acute biliary obstruction and drainage in conscious dogs. Hepatogastroenterology. 1996;43:235-240.
Keiding S. Galactose clearance measurements and liver blood flow. Gastroenterology. 1988;94:477-481.
Kelty RH, et al. The relation of the regenerated liver nodule to the vascular bed in cirrhosis. Gastroenterology. 1950;15:285-295.
Klopfenstein CE, et al. Effects of abdominal CO2 insufflation and changes in position on hepatic blood flow in anesthetized pigs. Am J Physiol. 1998;275:H900-H905.
Knisley MH, et al. Hepatic sphincters: brief summary of present-day knowledge. Science. 1957;125:1023-1026.
Kock NG, et al. Interaction between portal venous and hepatic arterial blood flow: an experimental study in the dog. Surgery. 1972;72:414-419.
Kravetz D, et al. Comparison of intravenous somatostatin and vasopressin infusions in treatment of acute variceal hemorrhage. Hepatology. 1984;4:442-446.
Kuo PC, et al. Magnetic resonance imaging and hepatic hemodynamics: correlation with metabolic function in liver transplantation candidates. Surgery. 1995;117:373-379.
Kurihara T, et al. Relationship between endothelin and thromboxane A2 in rat liver microcirculation. Life Sci. 1992;51:PL281-PL285.
Kuznetsova LV, et al. Effect of orthotopic liver transplantation on systemic and splanchnic hemodynamics in conscious rat. Am J Physiol. 1995;269:G153-G159.
Langer S, et al. Orthogonal polarization spectral imaging as a tool for the assessment of hepatic microcirculation: a validation study. Transplantation. 2001;71:1249-1256.
Lautt WW. Hepatic vasculature: a conceptual review. Gastroenterology. 1977;73:1163-1169.
Lautt WW. Effect of stimulation of hepatic nerves on hepatic O2 uptake and blood flow. Am J Physiol. 1977;232:H652-H656.
Lautt WW. Hepatic nerves: a review of their functions and effects. Can J Physiol Pharmacol. 1980;58:105-123.
Lautt WW. Role and control of the hepatic artery. In: Lautt, WW, editor. Hepatic Circulation in Health and Disease. New York: Raven Press; 1981:203-226.
Lautt WW. Relationship between hepatic blood flow and overall metabolism: the hepatic arterial buffer response. Fed Proc. 1983;42:1662-1666.
Lautt WW. Regulatory processes interacting to maintain hepatic blood flow constancy: vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, escape from vasoconstriction. Hepatol Res. 2007;37:891-903.
Lautt WW, Legare DJ. The use of 8-phenyltheophylline as a competitive antagonist of adenosine and an inhibitor of the intrinsic regulatory mechanism of the hepatic artery. Can J Physiol Pharmacol. 1985;63:711-722.
Lautt WW, et al. The comparative effects of substances via the hepatic artery or portal vein on hepatic arterial resistance, liver blood volume and hepatic extraction in cats. Hepatology. 1984;4:927-932.
Lautt WW, et al. Localization of intrahepatic portal vascular resistance. Am J Physiol. 1986;251:G375-G381.
Lautt WW, et al. Conceptual review of the hepatic vascular bed. Hepatology. 1987;7:952-963.
Lautt WW, et al. Effect of hepatic nerves, norepinephrine, angiotensin, and elevated central venous pressure on postsinusoidal resistance sites and intrahepatic pressures in cats. Microvasc Res. 1987;33:50-61.
Lebrec D, et al. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371-1374.
Lee FY, et al. Peripheral endotoxemia and hyperdynamic circulation of rats with intrahepatic or extrahepatic portal hypertension. Hepatology. 1995;22:257A.
Lee S, et al. Reduction of intrahepatic vascular space in the pathogenesis of portal hypertension. Gastroenterology. 1987;93:157-161.
Leen E, et al. The use of Duplex sonography in the detection of colorectal hepatic metastases. Br J Cancer. 1991;63:323-325.
Leen E, et al. Detection of hepatic metastases using duplex/color Doppler sonography. Ann Surg. 1991;214:599-604.
Leen E, et al. Detection of colorectal liver metastases: comparison of laparotomy, CT, US, and Doppler perfusion index and evaluation of postoperative follow-up results. Radiology. 1995;195:113-116.
Legare DJ, Lautt WW. Hepatic venous resistance site in the dog: localization and validation of intrahepatic pressure measurements. Can J Physiol Pharmacol. 1987;65:352-359.
Leiberman DP, et al. The hepatic arterial and portal venous circulations of the liver studied with a krypton-85 clearance technique. J Surg Res. 1978;25:154-162.
Leivas A, et al. Endothelin-1 does not play a major role in the homeostasis of arterial pressure in cirrhotic rats with ascites. Gastroenterology. 1995;108:1842-1848.
Leveson SH, et al. Deranged liver blood flow patterns in the detection of liver metastases. Br J Surg. 1985;72:128-130.
Liapi E, et al. Chemoembolization for primary and metastatic liver cancer. Cancer J. 2010;16:156-162.
CM Lo, et al. Portal hyperperfusion injury as the cause of primary nonfunction in a small-for-size liver graft: successful treatment with splenic artery ligation. Liver Transpl. 2003;9:626-628.
MacLeod JJR, Pearce RG. The outflow of blood from the liver as affected by variations in the condition of the portal vein and hepatic artery. Am J Physiol. 1914;35:87-105.
Mallet-Guy Y, et al. Note sur les lésions ultrastructurales immédiates du foie après clampage expérimental de l’artère hépatique. Lyon Chir. 1972;68:170-175.
Mann FC. The William Henry Welch Lectures. II. Restoration and pathologic reactions of the liver. J Mt Sinai Hosp. 1944;11:65-74.
Marchioro TL, et al. The effect of partial portacaval transposition on the canine liver. Surgery. 1967;61:723-732.
Martinez-Hernandez A. The hepatic extracellular matrix: II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985;53:166-186.
Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix: I. Components and distribution in normal liver. Virchow Arch A Pathol Anat Histopathol. 1993;423:1-11. (editorial)
Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401-1410.
Mastaï R, et al. Serotonin blockade in conscious, unrestrained cirrhotic dogs with portal hypertension. Hepatology. 1989;9:265-268.
Mathie RT. Hepatic haemodynamics during and after brief occlusion of the hepatic artery. Hepatol Res. 1997;8:198-206.
Mathie RT. The hepatic haemodynamic effects of nitric oxide. In: Mathie, RT, Griffith, TM. The Haemodynamic Effects of Nitric Oxide. London: Imperial College Press; 1999:292-317.
Mathie RT, Alexander B. The role of adenosine in the hyperaemic response of the hepatic artery to portal venous occlusion (the “buffer response. Br J Pharmacol. 1990;100:626-630.
Mathie RT, Blumgart LH. Hepatic tissue perfusion studies during partial hepatectomy in man. Surg Gastroenterol. 1982;1:297-302.
Mathie RT, Blumgart LH. The hepatic haemodynamic response to acute portal venous blood flow reductions in the dog. Pflugers Arch. 1983;399:223-227.
Mathie RT, Blumgart LH. Effect of denervation on the hepatic haemodynamic response to hypercapnia and hypoxia in the dog. Pflugers Arch. 1983;397:152-157.
Mathie RT, et al. The role of blood flow in the control of liver size. J Surg Res. 1979;27:139-144.
Mathie RT, et al. The hepatic arterial blood flow response to portal vein occlusion in the dog: the effect of hepatic denervation. Pflugers Arch. 1980;386:77-83.
Mathie RT, et al. Hepatic tissue perfusion studies during distal splenorenal shunt. Am J Surg. 1980;140:384-386.
Mathie RT, et al. Hepatic hemodynamics after chronic obstruction of the biliary tract in the dog. Surg Gynecol Obstet. 1988;166:125-130.
Mathie RT, et al. Adenosine-induced dilatation of the rabbit hepatic arterial vasculature is mediated by A2-purinoceptors. Br J Pharmacol. 1991;103:1103-1107.
Mays ET, Wheeler CS. Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med. 1974;290:993-996.
Menger MD, Messmer K. In vivo fluorescent microscopy for quantitative analysis of the hepatic microcirculation in hamsters and rats. Eur Surg Res. 1991;23:158-169.
Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2-13.
Miller WL, et al. Integrated cardiac, renal, and endocrine actions of endothelin. J Clin Invest. 1989;83:317-320.
Moreno AH, et al. Spontaneous reversal of portal blood flow: the call for and against its occurrence in patients with cirrhosis of the liver. Ann Surg. 1975;181:346-358.
Moriyasu F, et al. Clinical application of an ultrasonic Duplex system in the quantitative measurement of portal blood flow. J Clin Ultrasound. 1986;14:579-588.
Morsiani E, et al. Increased sinusoidal wall permeability and liver fatty change after two-thirds hepatectomy: an ultrastructural study in the rat. Hepatology. 1995;21:539-544.
Nadhum E, et al. Correlation of transcutaneous hepatic near-infrared spectroscopy readings with liver surface readings and perfusion parameters in a piglet endotoxemic shock model. Liver Int. 2006;26:1277-1282.
Nagano K, et al. Hepatic oxygen supply–demand relationship during anaesthesia in the pig. Anesthesiology. 1988;69:A440.
Nagorney DM, et al. Bile duct pressure as a modulator of liver blood flow after common bile duct obstruction. Surg Forum. 1982;33:206-208.
Nakamura T, et al. Quantitative measurement of abdominal arterial blood flow using image-directed Doppler ultrasonography: superior mesenteric, splenic and common hepatic arterial blood flow in normal adults. J Clin Ultrasound. 1989;17:261-268.
Navasa M, et al. Reduction of portal pressure by isosorbide-5-mononitrate in patients with cirrhosis. Gastroenterology. 1989;96:1110-1118.
Navasa M, et al. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993;17:355-360.
Niederberger M, et al. Normalization of nitric oxide production corrects arterial vasodilation and hyperdynamic circulation in cirrhotic rats. Gastroenterology. 1995;109:1624-1630.
Ohdan H, et al. Intraoperative near-infrared spectroscopy for evaluating hepatic venous outflow in living-donor right lobe liver. Transplantation. 2003;76:791-797.
Ohlsson EG. The arterial circulation in the liver after total biliary obstruction in dogs. Acta Chir Scand. 1972;138:1-58.
Ohlsson EG, et al. Changes in portal circulation after biliary obstruction in dogs. Am J Surg. 1970;120:16-22.
Ohnishi K, et al. Portal hemodynamics in idiopathic portal hypertension (Banti’s syndrome). Gastroenterology. 1987;92:751-758.
Okuda IC, et al. Percutaneous transhepatic catheterization of the portal vein for the study of portal hemodynamics and shunts: a preliminary report. Gastroenterology. 1977;73:279-284.
Okumura S, et al. Vasoactive effect of endothelin-1 on rat liver in vivo. Hepatology. 1994;19:155-161.
Orrego H, et al. Correlation of intrahepatic pressure with collagen in the Disse space and hepatomegaly in humans and in the rat. Gastroenterology. 1981;80:546-556.
Pachter HL, et al. Traumatic injuries of the portal vein: the role of acute ligation. Ann Surg. 1979;189:383-385.
Pannen BHJ, et al. Endotoxin pretreatment enhances portal venous contractile response to endothelin-1. Am J Physiol. 1996;270:H7-H15.
Parkin A, et al. Liver perfusion scintigraphy-method, normal range and laparotomy correlation in 100 patients. Nucl Med Commun. 1983;4:395-402.
Paulsen AW, Klintmalm GBG. Direct measurement of hepatic blood flow in native and transplanted organs, with accompanying systemic hemodynamics. Hepatology. 1992;16:100-111.
Payen DM, et al. Portal and hepatic arterial blood flow measurements of human transplanted liver by implanted Doppler probes: interest for early complications and nutrition. Surgery. 1990;107:417-427.
Peitzman AB, et al. Hemorrhagic shock. Curr Prob Surg. 1995;32:925-1002.
Pelc LC, et al. Arterial and venous blood flow: non-invasive quantitation with MR imaging. Radiology. 1992;185:809-812.
Pinzani M, et al. Fat-storing cells as liver-specific pericytes: spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992;90:642-646.
Pinzani M, et al. Endothelin-1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534-548.
Pizcueta P, et al. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology. 1992;103:1909-1915.
Puhl G, et al. Noninvasive in vivo analysis of the human hepatic microcirculation using orthogonal polarization spectral imaging. Transplantation. 2003;75:756-761.
Rappaport AM. The microcirculatory hepatic unit. Microvasc Res. 1973;6:212-228.
Rappaport AM, Schneiderman JH. The function of the hepatic artery. Rev Physiol Biochem Pharmacol. 1976;76:129-175.
Reichen J, Le M. Verapamil favorably influences hepatic microvascular exchange and function in rats with cirrhosis of the liver. J Clin Invest. 1986;78:448-455.
Reichen J, et al. The effect of endothelin and its antagonist Bosentan on hemodynamics and microvascular exchange in cirrhotic rat liver. J Hepatol. 1998;28:1020-1030.
Reuter SR, Chuang VP. The location of increased resistance to portal blood flow in obstructive jaundice. Invest Radiol. 1976;11:54-59.
Rice GC, et al. Liver tissue blood flow measured by 85Kr clearance in the anaesthetized rat before and after partial hepatectomy. Br J Exp Pathol. 1977;58:243-250.
Richardson PDI, Withrington PG. Liver blood flow: II. Effects of drugs and hormones on liver blood flow. Gastroenterology. 1981;81:356-375.
Richter S, et al. Impact of intrinsic blood flow regulation in cirrhosis: maintenance of hepatic arterial buffer response. Am J Gastrointest Liver Physiol. 2000;279:G454-G462.
Richter S, et al. Loss of physiologic hepatic blood flow control (“hepatic aterial buffer response”) during CO2 pneumoperitoneum in the rat. Anesth Analg. 2001;93:872-877.
Rocheleau B, et al. Hepatic artery buffer response following left portal vein ligation: its role in liver tissue homeostasis. Am J Physiol. 1999;277:G1000-G1007.
Rockey DC, Chung JJ. Inducible nitric oxide synthase in rat hepatic lipocytes and the effect of nitric oxide on lipocyte contractility. J Clin Invest. 1995;95:1199-1206.
Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351.
Rockey DC, Weisiger RA. Endothelin-induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233-240.
Rockey DC, et al. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193-203.
Rockey DC, et al. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472-480.
Rosemurgy AS, et al. Differential effects on portal and effective hepatic blood flow: a comparison between transjugular intrahepatic portosystemic shunt and small diameter H-graft portacaval shunt. Ann Surg. 1997;225:607-608.
Rössle M, et al. New non-operative treatment for variceal haemorrhage. Lancet. 1989;2:153.
Ryan CJ, et al. Liver blood flow measurement in the portacavally transposed rat before and after partial hepatectomy. Br J Exp Pathol. 1978;59:111-115.
Sapirstein LA. Fractionation of the cardiac output of rats with isotopic potassium. Circ Res. 1956;4:689-692.
Sasse D, et al. Electron microscopic evidence for a muscle layer in the wall of the terminal hepatic venule in the rat. Cell Tissue Res. 1976;165:391-396.
Schenk WG, et al. Direct measurement of hepatic blood flow in surgical patients: with related observations on hepatic flow dynamics in experimental animals. Ann Surg. 1962;156:463-469.
Schilling MK, et al. Splanchnic microcirculatory changes during CO2 laparoscopy. J Am Coll Surg. 1997;184:378-382.
Schoen JM, et al. Shear stress–induced nitric oxide release triggers the liver renegeration cascade. Nitric Oxide. 2001;5:453-464.
Sedgwick CE, et al. Management of portal hypertension secondary to bile duct strictures: review of 18 cases with splenorenal shunt. Ann Surg. 1966;163:949-953.
Seifalian AM, et al. Hepatic microcirculation during human orthotopic liver transplantation. Br J Surg. 1997;84:1391-1395.
Sganga G, et al. Reprioritization of hepatic plasma protein release in trauma and sepsis. Arch Surg. 1985;120:187-199.
Shah V, et al. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J Clin Invest. 1997;100:2923-2930.
Shepherd AP, et al. Laser Doppler measurements of blood flow within the intestinal wall and on the surface of the liver. In: Koo, A, et al. Microcirculation of the Alimentary Tract. Singapore: World Scientific Publishing Company; 1983:115-129.
Shepherd AP, et al. Evaluation of an infrared laser-Doppler blood flowmeter. Am J Physiol. 1987;252:G832-G839.
Sherman IA, et al. Hepatic microvascular changes associated with development of liver fibrosis and cirrhosis. Am J Physiol. 1990;258:H460-H465.
Sherman IA, et al. Dynamics of arterial and portal venous flow interactions in perfused rat liver: an intravital microscopic study. Am J Physiol. 1996;271:G201-G210.
Shibayama Y, Nakata K. Localization of increased hepatic vascular resistance in liver cirrhosis. Hepatology. 1985;5:643-648.
Shimamura T, et al. Nitric oxide down-regulates endothelin production during ischemia and reperfusion of canine livers. Hepatology. 1996;24:579A.
Smith A, et al. Effect of haemorrhagic hypotension on liver blood flow and oxygen consumption in the dog. Gut. 1979;20:A454.
Starzl TE, et al. The origin, hormonal nature and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179-199.
Starzl TE, et al. The effect of diabetes mellitus on portal blood hepatotrophic factors in dogs. Surg Gynecol Obstet. 1975;140:549-562.
Steer ML, et al. Chronic biliary obstruction: hemodynamic effects of decompression. Surg Forum. 1968;19:342-344.
Stewart GN. Researches on the circulation time and on the influences which affect it. IV. The output of the heart. J Physiol. 1897;22:159.
Suematsu M, et al. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest. 1995;96:2431-2437.
Suematsu M, et al. Gaseous monoxides: a new class of microvascular regulator in the liver. Cardiovasc Res. 1996;32:679-686.
Takaoka F, et al. Intraoperative evaluation of Eurocollins and University of Wisconsin preservation solutions in patients undergoing hepatic transplantation. Transplantation. 1990;49:544-547.
Tamakuma S, et al. Relationship between hepatic hemodynamics and biliary pressure in dogs: its significance in clinical shock following biliary decompression. Jpn J Surg. 1975;5:255-268.
Tanaka A, et al. Endothelin-1 stimulates bile acid secretion and vesicular transport in the isolated perfused rat liver. Am J Physiol. 1994;266:G324-G329.
Terajima H, et al. Reduction of hepatic microcirculatory failure caused by normothermic ischemia/reperfusion-induced injury by means of heat shock preconditioning. Shock. 1999;12:329-334.
Thomson IA, et al. Effects of nitrous oxide on liver haemodynamics and oxygen consumption in the greyhound. Anaesthesia. 1982;37:548-553.
Thomson IA, et al. Effects of certain IV anaesthetics on liver blood flow and hepatic oxygen consumption in the greyhound. Br J Anaesth. 1986;58:69-80.
Thulin L, et al. Effect of controlled halothane anaesthesia on splanchnic blood flow and cardiac output in the dog. Acta Anaesth Scand. 1975;19:146-153.
Torrance HB. The control of the hepatic arterial circulation. J Physiol. 1961;158:39-49.
Tran-Thi TA, et al. Regulation of endothelin-1 action on the perfused rat liver. FEBS Lett. 1993;318:353-357.
Umeda Y, et al. Preoperative proximal splenic artery embolization: a safe and efficacious portal decompression technique that improves the outcome of live donor liver transplantation. Transpl Int. 2007;20:947-955.
Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776-778.
Varin F, Huet PM. Hepatic microcirculation in the perfused cirrhotic rat liver. J Clin Invest. 1985;76:1904-1912.
Waller A. Microscopic examination of some of the principal tissues of the animal tongue, as observed in the tongue of the living frog and toad. Philos Mag J Sci. 1846;29:397-405.
Wang Y, et al. Inhibition of nitric oxide synthesis aggravates reperfusion injury after hepatic ischemia and endotoxemia. Shock. 1995;4:282-288.
Wardell K, et al. Laser Doppler perfusion imaging by dynamic light scattering. IEEE Trans Biomed Eng. 1993;40:309-316.
Warren WD, Muller WH. A classification of some hemodynamic changes in cirrhosis and their surgical significance. Ann Surg. 1959;150:413-427.
Wendon JA. Local consequences of reperfusion in the liver. In: Grace, PA, Mathie, RT. Ischaemia-Reperfusion Injury. Oxford: Blackwell Science; 1999:56-64.
Wheatley AM, Hickman R. The influence of flow and hematocrit on the laser Doppler flux signal from the surface of the perfused pig liver. Microcirculation. 1995;2:19-25.
Wheatley AM, Zhao D. Intraoperative assessment by laser Doppler flowmetry of hepatic perfusion during orthotopic liver transplantation in the rat. Transplantation. 1993;56:1315-1318.
Wheatley AM, et al. Interpretation of the laser Doppler signal from the liver of the rat. Microvasc Res. 1993;45:290-301.
Wheatley AM, et al. Effect of orthotopic liver transplantation and chemical denervation of the liver on hepatic hemodynamics in the rat. J Hepatol. 1993;19:442-450.
Wheatley AM, et al. Altered hemodynamics during hepatic regeneration in the adult rat. Hepatology. 1996;24:144A.
Whipple AO. The problem of portal hypertension in relation to the hepatosplenopathies. Ann Surg. 1945;122:449-475.
Wu Y, et al. Hormonal and splanchnic hemodynamic alterations following hepatic resection. J Surg Res. 1993;55:44-48.
Yokoyama Y, et al. Hepatic vascular response to elevated intraperitoneal pressure in the rat. J Surg Res. 2002;105:86-94.
Zeeh J, et al. Steady-state extrarenal sorbitol clearance as a measure of hepatic plasma flow. Gastroenterology. 1988;95:749.
Zhang JX, et al. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624-G632.
Zhang JX, et al. Vessel- and target cell-specific actions of endothelin-1 and endothelin-3 in rat liver. Am J Physiol. 1995;269:G269-G277.
Zhang XY, et al. Involvement of endothelin in changes to the hepatic microcirculation in the regenerating rat liver. Hepatology. 1997;26:276A.