Chapter 86 Regional chemotherapy for liver tumors
Systemic Chemotherapy
Responses to systemic chemotherapy (SYS) vary with the type of tumor; for example, patients with liver metastases from a breast primary may have high response rates with SYS (Harris et al, 1997), in contrast to metastases from a gastric or pancreatic primary tumor. Despite 20% response rates and median survivals less than 1 year, fluoropyrimidines represented the standard of care for metastatic CRC for decades (Thirion et al, 2004). More recently, patients with metastatic colon carcinoma have had response rates greater than 30%. With the development of newer drugs, such as irinotecan and oxaliplatin, response rates have increased to 35% to 40% with median survivals of 15 to 19 months (Goldberg et al, 2002; Saltz et al, 2000). The use of targeted agents, such as bevacizumab and cetuximab (Cunningham et al, 2004), has further improved outcomes, and bevacizumab demonstrated overall survival of 20 months (Hurwitz et al, 2004). Many chemotherapy trials do not differentiate patients with liver-only metastases to show how these patients respond to chemotherapy. In tumors with high response rates, such as breast carcinoma, a reasonable response to SYS is found even in patients with liver metastases, although the response is lower with liver metastases than with soft-tissue metastases (George & Hoogstraten, 1978). In patients with CRC, the liver is the most common site of dissemination, and 60% of patients (Daly & Kemeny, 1986) develop liver metastases during the course of their disease.
Rationale for Hepatic Arterial Chemotherapy
1 Liver metastases are perfused almost exclusively by the hepatic artery, whereas normal hepatocytes derive their blood supply from the portal vein and the hepatic artery (Breedis & Young, 1954). After injection of floxuridine (FUDR) into either the hepatic artery or the portal vein, mean liver concentrations of the drug do not differ based on the route of injection; however, mean tumor FUDR levels are significantly increased (15-fold) when the drug is injected via the hepatic artery (Sigurdson et al, 1987).
2 The use of drugs that are largely extracted by the liver during first-pass metabolism results in high local concentrations of drug with minimal systemic toxicity. Ensminger and colleagues (1978) showed that 94% to 99% of FUDR is extracted by the liver during the first pass compared with 19% to 55% of 5-fluorouracil (5-FU). FUDR is therefore an ideal drug for hepatic arterial infusion (HAI), with a 400-fold increase in hepatic exposure with FUDR. The pharmacologic advantage of various chemotherapeutic agents for HAI is summarized in Table 86.1 (Ensminger & Gyves, 1983).
3 Drugs with a steep dose-response curve are more useful when given by the intrahepatic route, because small increases in the concentration of administered drug result in a large improvement in response. FUDR follows linear kinetics and is not saturated at high dose rates.
4 Drugs with a high total body clearance are more useful for hepatic infusion. The area under the concentration-versus-time curve is a function not only of drug clearance but also of hepatic arterial flow. Because hepatic arterial blood flow has a high regional exchange rate (100 to 1500 mL/min), drugs with a high clearance rate are needed (Collins, 1984). If a drug is not cleared rapidly, recirculation through the systemic circulation mitigates the advantage of intraarterial therapy over systemic therapy (Collins, 1986).
5 Another rationale for hepatic arterial chemotherapy, especially for patients with metastatic CRC, is the concept of a stepwise pattern of metastatic progression (Weiss, 1989; Weiss et al, 1986). This theory states that hematogenous spread occurs first via the portal vein to the liver, then from the liver to the lungs, and then to other organs. Aggressive treatment, either resection or hepatic infusion, of metastases confined to the liver yields prolonged survival for some patients.
Table 86.1 Estimated Increase in Hepatic Exposure for Drugs Given by Hepatic Arterial Infusion
| Estimated Increase by Drug | Half-Life (min) | Hepatic Arterial Exposure |
|---|---|---|
| Fluorouracil | 10 | 5- to 10-fold |
| Floxuridine | 10 | 100- to 400-fold |
| Bis-chloroethyl-nitrosourea | 5 | 6- to 7-fold |
| Mitomycin C | 10 | 6- to 8-fold |
| Cisplatin | 20 to 30 | 4- to 7-fold |
| Doxorubicin | 60 | 2-fold |
| Dichloromethotrexate | — | 6- to 8-fold |
The development of an implantable infusion pump allowed for the safe administration of hepatic arterial chemotherapy in the outpatient setting (Blackshear et al, 1972). Early trials using an implantable pump and continuous FUDR therapy produced a median response rate of 47% (Johnson & Rivkin, 1985) and a median survival of 17 months (Weiss et al, 1983). To further show that HAI had a therapeutic benefit, several randomized studies were conducted.
First-Line Therapy in Unresectable Liver Metastases
One of the first randomized trials was conducted at Memorial Sloan-Kettering Cancer Center (MSKCC) (Kemeny et al, 1987). Before randomization, patients were stratified for extent of liver involvement by tumor and baseline lactate dehydrogenase level, two factors that have been shown to be important prognostic indicators of survival (Table 86.2; Kemeny & Braun, 1983; Kemeny et al, 1989). This prospective randomized trial compared HAI with SYS using the same chemotherapeutic agent (FUDR), drug schedule (a 14-day continuous infusion), and method of administration (internal pump) in both groups.
Table 86.2 Randomized Studies of Hepatic Arterial Infusion (HAI) vs. Systemic Chemotherapy (SYS) for Unresectable Hepatic Metastases
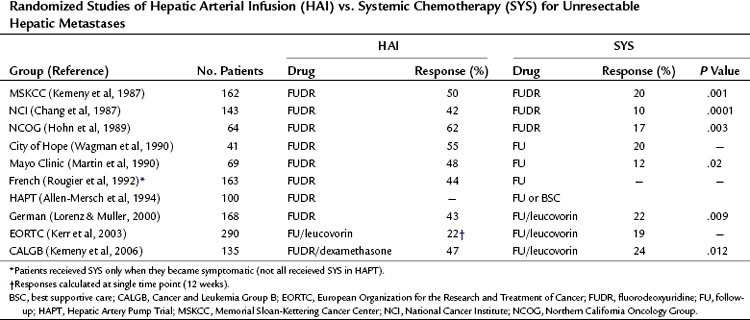
Two subsequent European randomized trials using HAI therapy in this setting have been published. The first was conducted by the Medical Research Council (MRC) and European Organization for the Research and Treatment of Cancer (EORTC) groups, which compared HAI 5-FU/leucovorin (LV) with intravenous 5-FU/LV. Crossover from the intravenous to the HAI arm was not allowed. Of 290 patients randomized, only 66% on the HAI arms received treatment. Response rates were 22% for HAI and 19% for intravenous 5-FU/LV. This trial used subcutaneous ports rather than implantable pumps and had significant catheter-related problems (36% of HAI patients) (Kerr et al, 2003). The median survival was 14.7 months and 14.8 months in the HAI and SYS groups, respectively (P = .79).
The second trial was by a German cooperative group that randomized 168 patients with unresectable colorectal liver metastases to HAI of FUDR, HAI of 5-FU/LV, or intravenous 5-FU/LV (Lorenz & Muller, 2000). Response rates were higher in the two HAI arms, with no significant differences in time to progression, the primary end point, or overall survival (OS) between the arms. Only 70% of patients on the HAI arms actually received assigned treatment. The study also used ports instead of pumps.
The Cancer and Leukemia Group B (CALGB) (Kemeny et al, 2006) completed trial 9481, which compared systemic 5-FU/LV via the Mayo Clinic regimen, considered standard of care at the time of trial design, with HAI of FUDR, LV, and dexamethasone, a regimen that had produced high response rates (78%) and lower toxicity (3% biliary sclerosis) in a phase II study (Kemeny et al, 1994). An earlier trial randomized patients to HAI of FUDR with or without dexamethasone, which demonstrated less biliary toxicity and a trend toward median OS (23 months vs. 15 months, respectively, P = .06) in the dexamethasone-containing arm (Kemeny et al, 1992). In CALGB 9481, no crossover was permitted, and a total of 134 patients were randomized. Most patients (70%) had greater than 30% liver involvement, synchronous metastases (78%), and were chemotherapy naive (97%). Response rates were higher in the HAI group (47% vs. 24%; P = .012), although time to progression was not significantly different (5.3 months vs. 6.8 months; P = .8); time to hepatic progression was better in the HAI arm (9.8 months vs. 7.3 months; P =.017) and in the systemic arm (7.7 months vs. 14.8 months; P = .029). Median OS was significantly better in the HAI arm (24.4 months vs. 20 months; P = .0034; Kemeny et al, 2006). Resource use, quality of life, and molecular markers of prognosis, such as thymidylate synthase (TS) and p21 gene expression, were examined prospectively in this study, and final analysis of these factors were presented. At 3- and 6-month follow-up, physical functioning was improved in the HAI group. TS levels correlated with survival in HAI patients (24 months if TS >4, 14 months if TS ≤4), but these differences were not significant (P = .17).
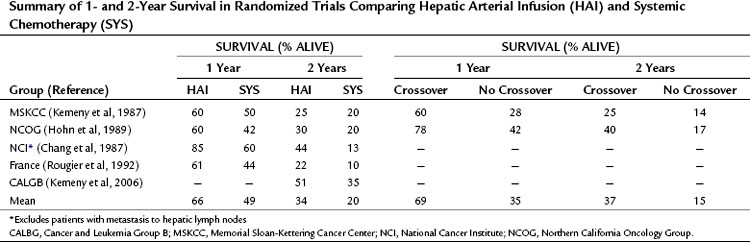
A total of 10 randomized phase III trials have been done, most of which demonstrate a higher response rate with HAI versus SYS in patients with hepatic metastases from CRC (see Table 86.2). Whether this increase in response rate translates into increased survival is controversial. Several factors complicate this issue. Importantly, most of the trials contain relatively few patients; therefore the power to observe differences in survival rates is low. Also, because of early successes with intrahepatic infusion, some of these studies allowed patients in the systemic arm to cross over to intrahepatic therapy after tumor progression on systemic therapy. This crossover may have negated any difference in survival between the two groups. The studies show a survival advantage for those who received subsequent HAI, with a mean 1-year survival of 69% for the patients who had crossed over from SYS to HAI versus 35% for the patients who did not cross over (Table 86.3). Additionally, some trials included patients with extrahepatic disease, complicating survival analyses in the absence of systemic treatment. Other factors to consider are that some patients randomized to HAI did not receive it but were included in the survival analysis, and an absence of modern toxicity-based dose-titration schema may have resulted in fewer cycles of treatment.
Table 86.3 Summary of 1- and 2-Year Survival in Randomized Trials Comparing Hepatic Arterial Infusion (HAI) and Systemic Chemotherapy (SYS)
Two meta-analyses of the original seven trials were conducted and included more than 600 patients. The Meta-Analysis Group in Cancer (MAGIC, 1996) confirmed the increased response rates seen with HAI over SYS (41% vs. 14%); however, survival differences were deemed only statistically significant in the trials that included a control group treated only when symptoms arose. With the statistical methodology used, the authors were unable to include the data from Hohn and colleagues (1989); however, a second meta-analysis published the same year did incorporate the results from that study and found an absolute survival difference of 12.5% at 1 year (P = .002) and 7.5% at 2 years (P = .026) in favor of HAI (Harmantas et al, 1996). The authors omitted the trial by Allen-Mersch and colleagues (1994) given the fact that most patients in the SYS arm of that study received best supportive care only. Furthermore, they analyzed their results to show that, when omitting the data from Rougier and colleagues (1992)—because, like the study from Allen-Mersh and others (1994), only half of the patients in the SYS arm received chemotherapy—survival was only statistically significantly higher at the 1-year time point. Nonetheless, factoring only those six studies without crossover, the survival differences at 1 and 2 years were accentuated, and actually both significantly favor of HAI.
As mentioned above, CALGB 9481 permitted no crossover, and with the incorporation of dexamethasone, it demonstrated survival with HAI that compares well to published results with modern SYS regimens; however, a recent and well-orchestrated third meta-analysis that accounted for the design flaws discussed above examined all 10 randomized trials to date and failed to demonstrate a survival benefit with HAI (Mocellin et al, 2007). This report and the published commentary that followed demonstrated that, although there may be no clear survival benefit in using HAI instead of SYS, combining HAI and SYS has led to high response rates with acceptable toxicity profiles.
Toxicity of Intrahepatic Therapy
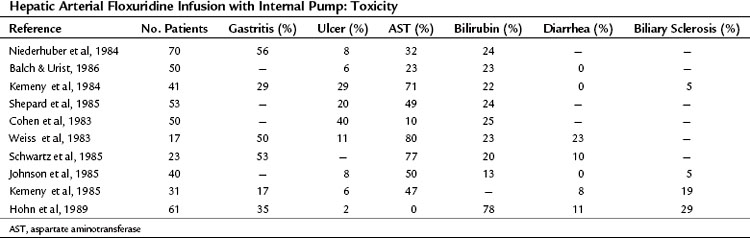
Table 86.4 summarizes the GI toxicities noted by investigators using the implantable pump. The side effects of SYS are almost never observed with HAI, and myelosuppression does not occur with intrahepatic FUDR (Kemeny et al, 1984). Although intrahepatic mitomycin C or bis-chloroethyl-nitrosourea may depress platelet counts, the absolute depression and frequency of depression are less than with systemic administration. Nausea, vomiting, and diarrhea do not occur with HAI of FUDR. If diarrhea does occur, shunting of the chemotherapeutic agent to the bowel should be suspected (Gluck et al, 1985).
The most common problems with HAI are ulcer disease and hepatic toxicity (Hohn et al, 1985; Kemeny et al, 1984). Severe ulcer disease results from inadvertent perfusion of the stomach and duodenum with drug via small, unligated branches from the hepatic artery, but this can be prevented by meticulous dissection at the time of pump placement (Hohn et al, 1985); however, even without radiologically visible perfusion of the stomach or duodenum, mild gastritis and duodenitis can occur. This toxicity can be reduced by careful dose reductions when any GI symptoms arise. Hepatobiliary toxicity is the most problematic toxicity seen with HAI. Although some evidence of hepatocellular necrosis and cholestasis has been found on liver biopsy specimens (Doria et al, 1986), most studies point to a combined ischemic and inflammatory effect on the bile ducts as the most important cause of this toxicity. The bile ducts are particularly sensitive to HAI; this is because the bile ducts derive their blood supply almost exclusively from the hepatic artery, similar to hepatic tumors (Northover & Terblanche, 1979).
In patients with severe toxicity, endoscopic retrograde cholangiopancreatography (ERCP) shows lesions resembling primary sclerosing cholangitis (Kemeny et al, 1985). Because the ducts are sclerotic and nondilated, ultrasound usually is not helpful. In some patients, the strictures are more focal, and they are usually worse at the bifurcation, and drainage procedures by endoscopic or by percutaneous transhepatic intubation may be helpful. Duct obstruction from metastases should be excluded first by computed tomography (CT) of the liver.
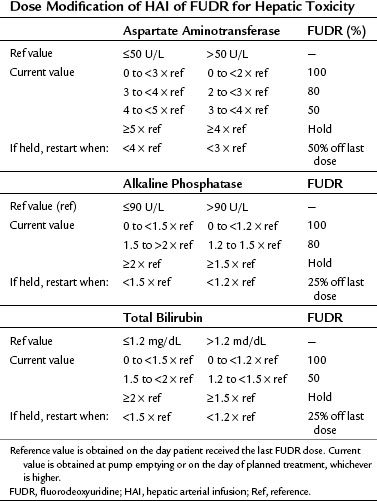
Close monitoring of liver function tests is necessary to avoid biliary sclerosis. If the serum bilirubin becomes elevated, no further treatment should be given until the bilirubin returns to normal, and then treatment should proceed with only a small test dose (0.05 mg/kg/day). In patients who cannot tolerate even a low dose for 2 weeks, it may be possible to continue treatment by giving the FUDR infusion for 1 week rather than the usual 2 weeks. At MSKCC, we modify treatment as outlined in Table 86.5.
In older trials, cholecystitis occurred in 33% of patients receiving HAI (Kemeny et al, 1986a). In more recent series, the gallbladder was removed at the time of catheter placement to prevent this complication and to avoid the confusion of these symptoms with other hepatic side effects of chemotherapy.
Surgical Technique
Hepatic Artery Pump Placement
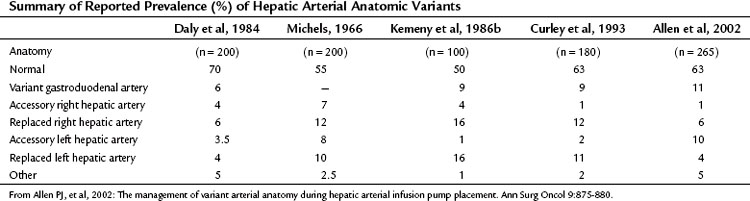
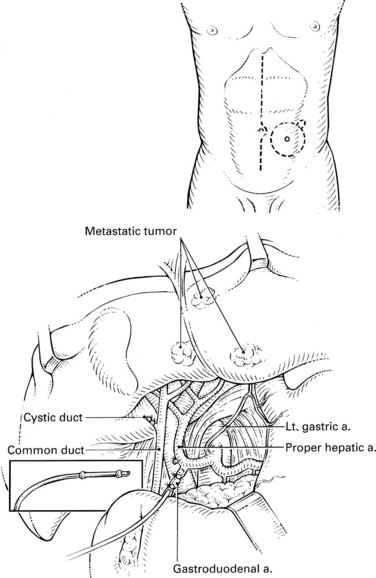
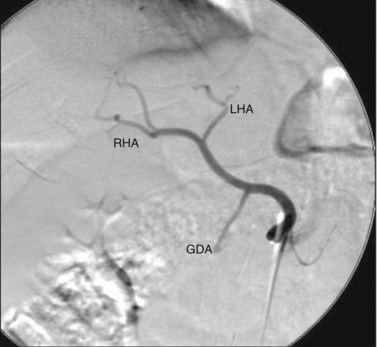
The arterial anatomy of the liver varies (Table 86.6), and a normal anatomy is present in only about two thirds of patients (Allen et al, 2002; see Chapter 1B). Before consideration of pump placement, it is imperative to carry out a careful review of the arteriogram with the radiologist and formulate a plan for the management of aberrant anatomy. In the past, direct arteriography was required, but excellent definition now can be ascertained from CT angiography. In most cases, a pump with a single catheter is adequate to provide access to the entire hepatic arterial inflow. It is best not to place the catheter directly into the hepatic artery, which risks thrombosis of the vessel; instead, it should be placed into an accessible side branch. The gastroduodenal artery (GDA) is the preferred conduit for the catheter and provides the most reliable method of catheter implantation (Allen et al, 2002). As previously mentioned, cholecystectomy should be performed to prevent chemotherapy-induced cholecystitis. Numerous incisions have been employed for this operation with success, including an upper midline incision, right subcostal incision, or limited right subcostal hockey-stick incision. Preoperative intravenous antibiotics close to the time of incision are imperative.
For patients with unresectable disease, a staging laparoscopy is advisable to rule out occult extrahepatic disease, which in our experience occurs in approximately one third of patients (Grobmyer et al, 2004). A thorough examination of the abdomen is performed at laparoscopy and at laparotomy to look for extrahepatic disease. The most common sites of extrahepatic metastases are the peritoneum and portal lymph nodes; therefore a biopsy should be done if the lymph nodes look suspicious, because their involvement generally would preclude the use of the pump. The extent of liver involvement should be assessed and documented. Any radiographically occult hepatic tumors should be noted, and the potential for future resection should be specifically addressed in the operative note.
Standard Hepatic Arterial Anatomy
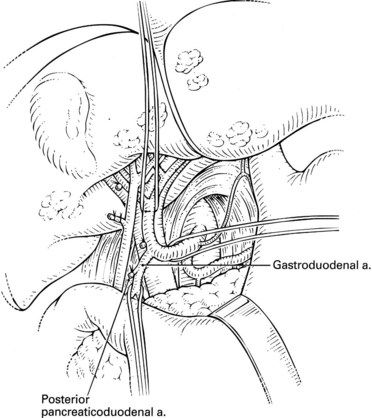
A standard cholecystectomy is performed, and the hepatic artery and its branches are circumferentially dissected. The common hepatic artery and the GDA are palpable superior to the body of the pancreas and the first portion of the duodenum. The GDA runs parallel to and lies immediately to the left of the common bile duct, and it is advisable to start by dissecting the common hepatic artery to minimize the risk of injuring the bile duct. The right gastric artery is ligated and divided. The distal common hepatic artery, the entire GDA, and the proximal proper hepatic artery are dissected away from their attachments. It is important to mobilize the full length of the extrapancreatic GDA to facilitate insertion of the catheter. Suprapyloric side branches of the GDA are often encountered and must be ligated. Frequently, branches to the pancreas and duodenum arise from many of these dissected vessels, and it is essential to identify and ligate these branches to avoid perfusion of the pancreas, stomach, or duodenum. The common hepatic artery is mobilized 1 cm proximally, and the proper hepatic artery is mobilized about 2 cm distally from the origin of the GDA. Branches to the retroperitoneum from the right or left hepatic artery are common and should be ligated. Review of preoperative angiography to look specifically for these branches is important, because they are often found in retrospect. At this point, a complete circumferential dissection of the common hepatic artery, GDA, and proper hepatic artery should be ensured such that no vessels to the pancreas, stomach, or duodenum remain (Fig. 86.1). The GDA should be temporarily occluded with palpation of the proper hepatic artery to rule out retrograde flow to the liver through the GDA secondary to celiac stenosis. No attempt at dissection of the common bile duct is necessary, because this risks devascularization and ischemic stricturing.
An arteriotomy is made in the distal GDA, and the catheter is inserted up to, but not beyond, the junction with the hepatic artery (Fig. 86.2). If the catheter protrudes into the common hepatic artery, turbulence of blood flow can lead to thrombosis of the vessel. Failure to pass the catheter to the junction leaves a short segment of the GDA exposed to full concentrations of FUDR without the diluting effect of blood flow, potentially resulting in sclerosis, thrombosis, or late dislodgment. When positioned, the catheter should be secured two or three times with nonabsorbable ties proximal to the tying rings on the catheter. Perfusion of both lobes of the liver and lack of extrahepatic perfusion is confirmed by infusing 2 to 3 mL of half-strength fluorescein through the pump and visualizing it with a Woods lamp. Half-strength methylene blue injection is an alternative method of ensuring proper perfusion. After the perfusion test, the catheter is flushed with heparinized saline, and the wounds are closed. Antibiotic coverage should be continued for 2 to 3 days postoperatively because of the seriousness of a pump infection. Any sign of erythema indicates a wound infection postoperatively and should be treated immediately and aggressively.
Aberrant Hepatic Arterial Anatomy (See Chapter 1B)
As discussed earlier, aberrant hepatic arterial anatomy is common, and numerous variations occur. Each anatomic situation is specifically addressed here, but first, general principles in managing variant anatomy are discussed. In analyses of our extensive experience with this operation, the factor most consistently associated with catheter-related complications and decreased durability of the catheter is cannulation of a vessel other than the GDA. The overall preferred technique is placement of the catheter in the GDA with ligation of the isolated variant vessel. This method relies on intrahepatic collateral development and cross-perfusion to the liver fed by the ligated vessel. Although concerns have been raised over incomplete hepatic perfusion with this technique, it is rare; in our published experience with this operation for variant anatomy, incomplete hepatic perfusion occurred once in 52 cases. This cross-perfusion sometimes can take 4 weeks to occur, and early perfusion scans may be abnormal initially; however, these should be rechecked in a few weeks to assess for the cross-perfusion, because most normalize (Allen et al, 2002; Curley et al, 1993). The only exception to this rule may be patients with central tumors so large that they impede cross-collateralization. Lastly, although cross-perfusion after ligation of aberrant vessels is highly reliable, it has not been proven that this results in equal blood flow for chemotherapy delivery.
Aberrant Origin of the Gastroduodenal Artery
The GDA can arise from the right or left hepatic artery, or there can be a trifurcation in which the GDA, right hepatic artery, and left hepatic artery all arise simultaneously from the common hepatic artery; this anomaly occurs 6% to 11% of the time (Allen et al, 2002). In general, it is preferable to place the catheter into the GDA and ligate vessels that are not receiving catheter-directed flow, because this is the technique associated with the lowest rate of catheter-related complications (Allen et al, 2002, 2005). In the case of a trifurcation, the catheter should be placed in the GDA, and perfusion should be tested with fluoroscein or methylene blue. If bilobar perfusion is adequate, ligation of vessels is unnecessary. If no perfusion of one lobe of the liver is seen at testing, the hepatic artery to that lobe should be ligated, most commonly the left hepatic artery, thereby relying on cross-perfusion of the left liver.
Replaced Left Hepatic Artery
A replaced left hepatic artery arises from the left gastric artery and supplies the left liver; no native left hepatic artery is found, an abnormality present in 4% to 16% of patients. Once again, the preferred technique is to place the catheter in the GDA and ligate the replaced left hepatic artery. Initial reports on this specific situation suggested rates of incomplete cross-perfusion of 40% (Cohen et al, 1987). More recent reports, including our experience, show that incomplete cross-perfusion is uncommon in this situation and occurred in only 1 of 10 of our patients at last analysis (Allen et al, 2002; Curley et al, 1993). Other techniques, such as placing catheters in the GDA and in a branch of the replaced left hepatic artery, can be considered in patients with bulky disease in the left liver or in those with a large central tumor that may impede cross-perfusion.
Replaced Right Hepatic Artery
A replaced right hepatic artery originates from the superior mesenteric artery, runs in the portacaval space, and supplies the whole right liver. No branches to the right liver originate from the proper hepatic artery, an anatomic situation that occurs 6% to 16% of the time. If the surgeon ligates the replaced right hepatic artery, cross-perfusion from the left hepatic artery occurs almost uniformly (Allen et al, 2002; Cohen et al, 1987; Curley et al, 1993). The catheter should be placed in the GDA, and the replaced right hepatic artery should be ligated. Other techniques have been described, such as placing a second catheter in the replaced right hepatic artery, either directly through a small arteriotomy, as there are no significant side branches from this vessel, or using a vascular graft, but these are rarely if ever indicated. This technique requires that the catheter be trimmed flush just beyond the tying ring and that it be placed such that the ring lies just inside the vessel, and the arteriotomy is closed over the ring.
Postoperative Assessment
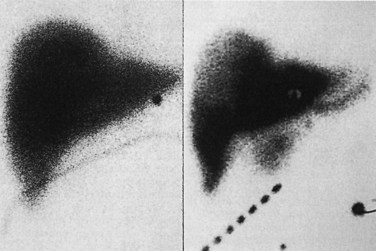
After surgery and before administering chemotherapy, the distribution of arterial perfusion is assessed by a radionuclide pump flow study (see Chapter 15). A baseline technetium 99m (99mTc)-sulfur colloid scan is obtained to identify the liver contour. Pump perfusion is assessed by infusing 99mTc-labeled macroaggregated albumin (MAA) through the bolus port of the pump and into the hepatic artery. When the MAA scan produces an image that matches the sulfur colloid liver scan, the pump is perfusing the entire liver as intended. Perfusion of extrahepatic tissues produces 99mTc signals outside the liver image (Fig. 86.3). Incomplete perfusion of the liver produces an incomplete image on the MAA scan. Misperfusion is discovered in 5% to 7% of cases and often can be corrected by surgical or angiographic intervention to occlude additional hepatic vessels missed at operation (Campbell et al, 1993). If no additional vessels are seen on angiography for embolization, it is worth repeating the scan in a few weeks to allow sufficient time for intrahepatic collaterals and cross-perfusion to develop (Allen et al, 2002).
Technical Complications of Hepatic Artery Infusion Pump Placement
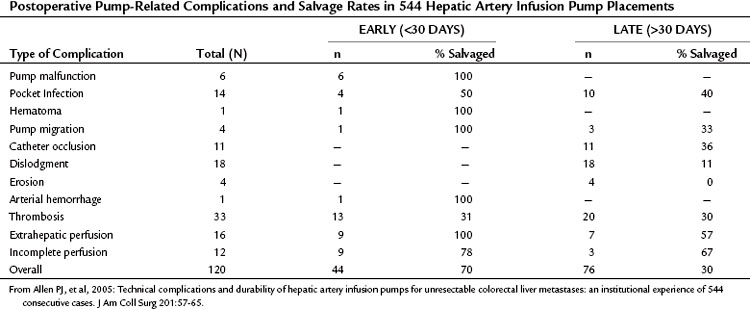
Surgical and technical complications of pump placement occur, and the spectrum and frequency of these complications must be understood by the surgeon, the medical oncologist, and the patient before initiating treatment. To assess the risks of insertion and the use of an implantable pump for hepatic artery chemotherapy, we reviewed the charts of all patients who underwent pump insertion for unresectable metastases over a 15-year period (1986 to 2001) at MSKCC (Allen et al, 2005). During this period, 544 infusion pumps were inserted by several different surgeons for HAI of FUDR alone or in combination with other drugs for patients with isolated unresectable colorectal hepatic metastases. Variant arterial anatomy was present in 205 patients (38%), most of which (82%) involved a single vessel. A colectomy was performed in addition to the pump placement in 136 patients (25%).
Pump-related morbidity occurred in 120 patients (22%) and is summarized in Table 86.7. Most of these complications (63%) occurred more than 30 days after pump placement. The most common complications (51%) were related to the hepatic arterial system and included thrombosis, hemorrhage, or perfusion abnormalities. Early (<30 days) pump-related morbidity occurred in only 8% of cases, and all of the catheter-related complications occurred after 30 days. Overall, the pumps were salvaged from these complications 45% of the time; however, salvage was much more common with early complications (70% if <30 days) than with late complications (30% if >30 days). In a multivariate analysis, surgeon experience and the use of any vessel other than the GDA were independently associated with pump-related morbidity and pump survival. Pump failure was generally low—5% at 6 months, 9% at 1 year, 16% at 2 years, and 26% at 3 years—and HAI was discontinued for pump-related complications in only 9% of cases. The performance of a concomitant colorectal resection was not associated with a higher rate of complications.
Table 86.7 Postoperative Pump-Related Complications and Salvage Rates in 544 Hepatic Artery Infusion Pump Placements
Extrahepatic Perfusion
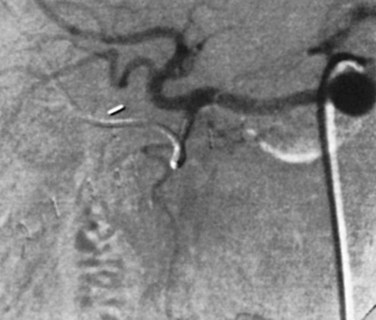
If extrahepatic perfusion is seen on the 99mTc MAA scan, the next step is to perform an angiogram by bolus injection through the pump; the angiogram usually shows the vessel responsible for the extrahepatic perfusion. This occasionally is unsuccessful, and a transfemoral celiac or superior mesenteric artery study is needed to identify the problem (Fig. 86.4). More recently, we have proceeded directly to transfemoral angiograms for assessment of patients in whom the perfusion scan shows obvious extrahepatic perfusion. This approach simplifies the process, because one procedure can diagnose the problem and potentially treat it. In our review of 544 cases, 9 patients (2%) were found to have extrahepatic perfusion on the postoperative scan. The cause was identified arteriographically, and in 7 cases, it was corrected with transarterial embolization or surgical ligation; these patients went on to receive HAI without complication (Allen et al, 2005).
Arterial or Catheter Thrombosis
Complete dissection and thrombosis of the hepatic artery intraoperatively has been described during pump insertion, but this condition is rare. In our series, 13 cases (2%) of acute postoperative arterial thrombosis were noted, but the technical problem leading to thrombosis was uncertain in most of these cases. Of these 13 cases of early thrombosis, 31% were salvaged with anticoagulation or lytic therapy. We also observed that delayed thrombosis of the hepatic artery (20 cases) was more common than acute thrombosis (13 cases). The vasculitis caused by infusional chemotherapy is thought to contribute to late arterial thrombosis. Often, arterial thrombosis manifests as extrahepatic perfusion on 99mTc MAA scan (Fig. 86.5). Catheter thrombosis was seen only as a late complication, and it occurred in 11 cases (2%) and was more likely to be related to technical errors, such as not filling the pump frequently or allowing back bleeding into the catheter during side-port manipulation.
Laparoscopic Placement of Hepatic Arterial Infusion Pumps
Published experience with laparoscopic placement of HAI pumps is largely limited to small cases series (Cheng et al, 2003, 2004; Feliciotti et al, 1996; Urbach & Hansen, 2000; Urbach et al, 2001). The largest series to date was published by Cheng and colleagues and describes their experience in 38 patients (Cheng et al, 2004), of whom 24 had additional radiofrequency ablation (RFA), 2 had a laparosopic partial hepatectomy, and 1 was converted to laparotomy. The median time in the operating room was 5.5 hours, and the overall results were excellent: only one postoperative cardiac-related mortality was reported but no pump-related morbidity, and the median hospital stay was 3 days.
Nonoperative Placement of Hepatic Arterial Infusion Pumps
HAI pump placement can be done safely by laparotomy with an acceptable morbidity. Poor overall prognosis and the requirement for a laparotomy have deterred many surgeons and oncologists from using this modality, however. Robotic-assisted laparoscopic pump placement has recently been described (Hellan & Pigazzi, 2008) that carries the potential to improve on perioperative outcomes but warrants surgical expertise with new technology. Several groups have worked on alternate placement methods to eliminate the laparotomy “hurdle.”
Percutaneous techniques to catheterize the hepatic artery for delivery of intrahepatic chemotherapy have been developed using a variety of peripheral arteries for access (Table 86.8). Most of these techniques have been abandoned or are used only by a few specialized groups for the following reasons:
1 The insertion is technically difficult and sometimes cumbersome. The technique described by Arai and colleagues (Arai, 1988; Arai et al, 1997; Inaba et al, 1998) requires embolization of the GDA to anchor the catheter. The tip of the catheter remains anchored in the GDA, while perfusion is achieved through a sidehole in the catheter.
2 The insertion site is in a mobile part of the body, such as the arm or the leg, resulting in risk of catheter dislocation or migration (Oberfield et al, 1979).
3 The artery used for access is “essential” for limb perfusion, and any thrombotic complication is a potentially limb-threatening complication (see Table 86.8).
Table 86.8 Peripheral Arteries Used for Catheter Placement in the Hepatic Artery
| Artery | References |
|---|---|
| Femoral artery | Matsumada et al, 1997 |
| Brachial artery | Cohen et al, 1980 |
| Axillary artery | Arai, 1988; Cohen et al, 1983 |
| Subclavian artery | Arai, 1988; Arai et al, 1997; Inaba et al, 1998 |
| Hypogastric artery | Arai et al, 1997, 1988; Inaba et al, 1998 |
| Intercostal artery | Castaing et al, 1998; Saldinger & Sandhu, 2004 |
Castaing and colleagues (1998) tried to address these shortfalls by using an intercostal artery as conduit. A cutdown is performed over the tenth left rib. The intercostal artery is catheterized and the catheter is advanced, under fluoroscopic control, across the aorta into the ostium of the celiac axis and ultimately into the proper hepatic artery, just beyond the takeoff of the GDA. The catheter is connected to an implantable port located in the left upper quadrant. The study included 35 patients with metastatic lesions to the liver, mainly from CRC, and 30 patients (86%) underwent a successful placement. Placement was not feasible in five patients because of an unsuitable artery. No procedure-associated deaths were reported, no thrombotic complications in either the aorta or the hepatic artery resulted, and no dislocations or migrations of the catheter occurred. Follow-up was brief because most of these patients died from their disease, although all of the patients had not responded to previous standard treatments and had advanced disease at the time of catheter placement.
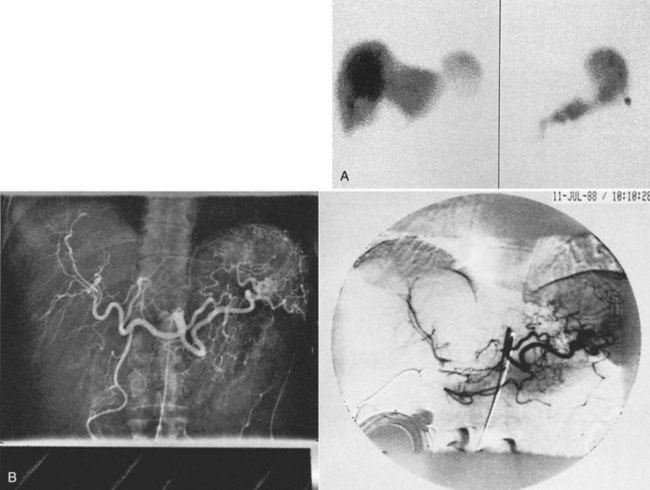
Our group modified Castaing’s method to address these points (Saldinger & Sandhu, 2004). A peripherally inserted 3-Fr central catheter was chosen as the indwelling arterial catheter (Arrow International, Reading, PA). We used a 15-mL Codman 3000 constant-flow implantable pump for access and drug delivery (Codman, Raynham, MA). The pump catheter and arterial catheter were connected via a special connector (Arrow International, Walpole, MA; Fig. 86.6). Our method uses largely the same techniques as described by Castaing, but with a few differences:
1 A preoperative angiogram is obtained for mapping of the hepatic vessels (Fig. 86.7). The angiogram is saved and used during the procedure as a guide. Accessory hepatic arteries are embolized to optimize pump perfusion, and if the distance between the GDA and the hepatic bifurcation is deemed too short, the GDA is embolized; that is, if it poses a risk for nondelivery of the target drug.
2 The intercostal artery is accessed by micropuncture, followed by introduction of a sheath. This sheath allows repeated introduction of wires, should the situation require it. A guidewire is introduced and placed into the hepatic artery (Fig. 86.8). The 3-Fr catheter is advanced into the hepatic artery, the wire is withdrawn, and the catheter is secured (Fig. 86.9). An on-table angiogram is obtained through the catheter to confirm proper placement. We confirm catheter placement and exclude extrahepatic perfusion the next day with a perfusion scan. The pump is filled the same day with chemotherapy.
Approaches to Decrease Hepatic Toxicity
New approaches to decrease the hepatic toxicity induced by HAI of FUDR have been studied. Because portal triad inflammation may lead to ischemia and stricturing of the bile ducts, the hepatic arterial administration of dexamethasone decreases biliary toxicity. As previously mentioned, a prospective double-blind randomized study of intrahepatic FUDR with dexamethasone versus FUDR alone was conducted at MSKCC (Kemeny et al, 1992). The response rate in 49 evaluable patients was 71% for those who received FUDR plus dexamethasone versus 40% for those given FUDR alone (P = .03). Survival also favored the FUDR-dexamethasone group: 23 months versus 15 months for FUDR alone (P = .06). In addition, a trend was noted toward decreased bilirubin elevation in patients receiving FUDR plus dexamethasone compared with the group receiving FUDR alone (9% vs. 30%; P = .07).
A second method to decrease hepatic toxicity is the use of circadian modification of hepatic intraarterial FUDR infusion. In a retrospective nonrandomized study (Hrushesky et al, 1990), a comparison of constant (flat) infusion versus circadian-modified HAI of FUDR was conducted in 50 patients. Over nine courses of treatment, the patients with circadian-modified infusion tolerated almost twice the daily dose of FUDR. Circadian-modified infusion resulted in 46% of patients having no hepatic toxicity versus 16% after flat FUDR infusion. The authors did not present information on response rates achieved in both groups.
Another approach to decrease toxicity from HAI is to alternate intraarterial FUDR with intraarterial 5-FU. A weekly intraarterial bolus of 5-FU has similar activity to intraarterial FUDR and does not cause hepatobiliary toxicity; however, it frequently produces treatment-limiting systemic toxicity or arteritis with the pharmacokinetic differences previously discussed. Stagg and colleagues (1991) used an alternating regimen of HAI of FUDR and HAI of 5-FU, with a response rate of 51% and median survival of 22.4 months. In contrast to the experience with single-agent HAI of FUDR, no patient had treatment terminated because of drug toxicity. Another report described 57 CRC patients with unresectable liver metastases treated with an alternating HAI schedule of FUDR and 5-FU (Davidson et al, 1996), of which 2 patients (3.5%) developed biliary sclerosis, and 12 (21.1%) had mild transient liver function abnormalities.
Methods to Increase Response Rate
Based on the fact that systemic combination chemotherapy regimens are more effective than single agents, the potential benefit of multidrug HAI has been evaluated. In an early study using mitomycin C, bis-chloroethyl-nitrosourea, and FUDR, Cohen and associates (1985) produced a 69% partial response rate. In a randomized trial at MSKCC comparing this three-drug regimen with FUDR alone (Kemeny et al, 1993), the response rates were 45% for the three-drug regimen and 32% for FUDR alone in the 67 patients who entered this trial. The median survivals from the initiation of HAI were 18.9 months and 14.9 months. Other trials using FUDR and LV have produced response rates up to 62% (Hohn et al, 1989).
Methods to Treat Extrahepatic Disease: Combined Hepatic Arterial Infusion and Systemic Chemotherapy
Extrahepatic disease develops in 40% to 70% of patients who undergo HAI. Such metastases can occur even when the patient’s hepatic tumors are responding. In many patients, extrahepatic disease is the cause of death. Safi and colleagues (1989) studied whether concomitant SYS reduces the development of extrahepatic metastases in patients who receive HAI. Ninety-five patients were randomized to intraarterial FUDR (0.2 mg/kg/day for 14 of 28 days) or a combination of intraarterial FUDR (0.21 mg/kg/day) and intravenous FUDR (0.09 mg/kg/day) given concurrently for 14 of 28 days (intraarterial/intravenous). The response rates were 60% for both arms of the study; however, the incidence of extrahepatic disease was significantly less in patients who received the intraarterial/intravenous treatment (56%) compared with intraarterial treatment (79%; P < .01). No significant difference in survival was found between the two groups (P = .08). The results of this trial, as well as the development of improved SYS regimens, have underscored the need for further study of combined systemic/intraarterial regimens. Results of some of these studies are presented in Table 86.9, and SYS irinotecan and oxaliplatin are discussed in detail below.
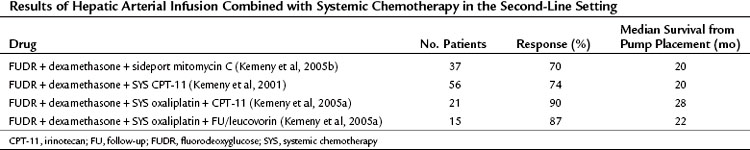
Table 86.9 Results of Hepatic Arterial Infusion Combined with Systemic Chemotherapy in the Second-Line Setting
Irinotecan is a topoisomerase I inhibitor with proven efficacy in first-line and second-line treatment of metastatic CRC. The activity of irinotecan is not inhibited by high thymidylate synthase activity (Saltz et al, 1998); combining systemic irinotecan with HAI therapy may therefore result in improved control of extrahepatic disease. In a Phase I study at MSKCC, 46 patients with unresectable liver metastases, 8 of whom underwent cryosurgery, received HAI of FUDR/dexamethasone and systemic irinotecan in escalating doses; all 38 patients who did not undergo cryosurgery were previously treated, and 16 had prior therapy with irinotecan. The regimen was well tolerated, with dose-limiting toxicities of diarrhea and myelosuppression. The response rate was 74%, median time to progression was 8.1 months, and 13 of 16 patients who had previously received irinotecan had partial responses (Kemeny et al, 2001).
Another nonrandomized study used HAI of FUDR with systemic irinotecan as “adjuvant” therapy after cytoreduction (therefore not “true adjuvant”) of unresectable hepatic CRC metastases. The cytoreduction was defined as using cryosurgery, RFA, partial resection, or some combination to treat all identifiable sites of disease. Seventy-one patients received “adjuvant” therapy and were compared with a historic control group that received cytoreduction alone. Time to progression was 19 months versus 10 months, and median survival was 30.6 months versus 20 months, for HAI versus control groups (Bilchik et al, 2000). A Japanese group examined HAI of 5-FU and SYS irinotecan in previously treated patients and demonstrated response rates of 76.5%, with median OS of 20 months (Shiatra et al, 2006).
Oxaliplatin is a cytotoxic agent with a mechanism of action similar to that of other platinum derivatives, but it has a different spectrum of activity and toxicity. When combined with 5-FU/LV, clinical response rates have been greater than 50%, with median survival of 16.2 months in untreated patients with metastatic CRC (de Gramont et al, 2000; Tournigand et al, 2004). Preliminary studies using systemic oxaliplatin-based regimens combined with HAI of FUDR demonstrated the feasibility and safety of this approach and revealed promising early results (Pancera et al, 2002). In a two-grouped MSKCC Phase I study, 36 patients with unresectable hepatic metastases, 89% of whom were previously treated, received HAI of FUDR/dexamethasone and SYS oxaliplatin plus either SYS irinotecan or SYS 5-FU/LV. Both regimens were well tolerated, and response rates for the two groups were 90% and 88% (see Table 86.9; Kemeny et al, 2005a). This high response rate for second-line therapy with HAI plus SYS, as well as conversion to resectability, was demonstrated in a recent MSKCC pooled analysis of phase I data in 49 patients with unresectable liver metastases from CRC (Kemeny et al, 2009a). Patients received HAI of FUDR/dexamethasone, SYS irinotecan, and SYS oxaliplatin, and 98% had bilobar disease, whereas 85% had tumors bordering vasculature. A partial or complete response was reported in 92% (84% partial, 8% complete), and 47% of patients were resectable, three with pathologic complete responses. With a median follow-up of 26 months from time of pump placement, OS was 51 months for untreated patients: all 23 responded to treatment, and 57% had resection. OS was 35 months for previously treated patients: 85% responded to treatment, and 38% had resection. This revealed that even in heavily pretreated patients with significant hepatic metastasis, HAI of FUDR can increase response and resection rates when combined with modern SYS. Future randomized trials are needed to assess the impact that HAI specifically has in these outcomes.
Adjuvant Treatment after Liver Resection of Colorectal Metastases
The rationale for the use of adjuvant chemotherapy after resection of liver metastases from CRC is based on the observation that, although some patients have long-term disease-free survival (DFS) after resection, many patients’ disease recurs. Recurrence may be solely in the liver, it may be extrahepatic and intrahepatic, or it may be solely extrahepatic. An effective adjuvant treatment may have a substantial impact on recurrence and survival. The use of SYS without HAI has been formally tested in this setting, but only to a limited extent, with one randomized study (Portier et al, 2006) that met its primary end point of 5-year DFS—33.5% for the chemotherapy group and 26.7% for the control group (P = .028)—but failed to show a survival benefit with 6 months of adjuvant 5-FU/folinic acid. Because this trial and another Phase III study (EORTC) both closed early because of slow accrual, their results were pooled and reported together (Mitry et al, 2009), demonstrating both a lack of DFS and OS statistical significance for 5-FU adjuvant therapy in this setting. When adjusting for poor characteristics in a multivariate analysis, however, an increase in DFS was reported for the group treated with chemotherapy. In EORTC 40983, reversible complications occurred in 25% of the patients receiving perioperative 5-FU plus leucovorin and oxaliplatin (FOLFOX) compared with 16% in the surgery-alone group (P =.04) (Nordlinger et al, 2008). The Japanese are conducting a multicenter randomized Phase II/III trial of adjuvant modified FOLFOX versus hepatic metastasectomy alone (Kanemitsu et al, 2009).
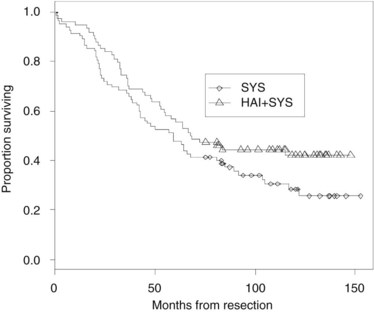
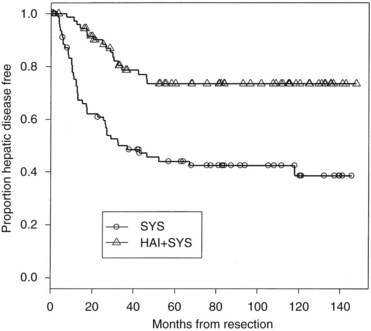
Because combining HAI and SYS may be useful in decreasing recurrence, four relatively large randomized trials have been conducted in this group of patients (Table 86.10). In the MSKCC study (Kemeny et al, 1999), 156 patients with resected hepatic metastases were randomized to 6 months of systemic 5-FU/LV or systemic 5-FU/LV plus HAI with FUDR. Primary end points were 2-year overall and progression-free survival. Forty percent of patients received prior adjuvant chemotherapy after resection of their primary CRC, and 15% had received prior chemotherapy as treatment for metastatic disease. Randomization was performed intraoperatively after complete resection of metastases, and patients were stratified based on number of metastases and prior treatment history. Of enrolled patients, 92% received treatment as assigned; 2-year survival was 86% in the combined therapy group versus 72% for those who received systemic therapy alone (P = .03), with median survivals of 72.2 months and 59.3 months, respectively. In an updated analysis, 10-year survival was 41% in the HAI plus SYS group, with 27% alive in the SYS-alone group still alive (Fig. 86.10; Kemeny & Gonen, 2005). Two-year hepatic progression-free survival was 90% for the combined group and 60% for the monotherapy group (P < .001). Updated hepatic progression-free survival at 10 years was 70% and 40%, respectively (P = .0001; Fig. 86.11), and overall progression-free survival was 40% and 20%, respectively, at 10 years (P = .001). If patients with poor clinical risk scores are considered (Fong et al, 1999), their survival is still improved with HAI compared with SYS alone (Fig. 86.12).
Table 86.10 Randomized Trials of Adjuvant Hepatic Arterial Infusion After Resection of Liver Metastases
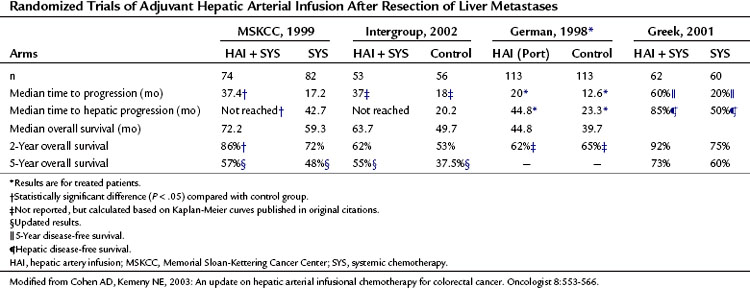
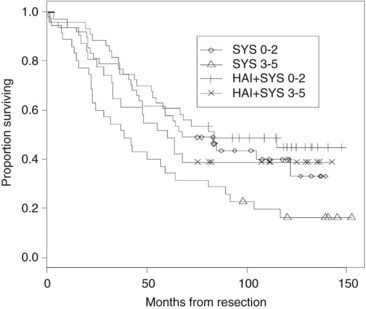
FIGURE 86.12 Survival stratified by clinical risk score. A score ranging from 0 to 5 was calculated by adding 1 point for the following clinical variables: tumor size >5 cm, tumor number >1, serum CEA >200 ng/mL, node-positive primary tumor, and disease-free interval >12 months (Fong et al, 1999). SYS, systemic therapy; HAI, hepatic anterial infusion.
The intergroup study (Kemeny et al, 2002) randomized 109 patients to resection alone or resection followed by four cycles of HAI with FUDR and infusional systemic 5-FU, followed by eight more cycles of systemic 5-FU. Patients with more than three liver metastases or extrahepatic disease at laparotomy were taken off the study; only 80 of 109 patients were actually included in the study, which was designed to examine the effect of adjuvant therapy on DFS. When patients were analyzed as treated (n = 75), the 4-year DFS (46% vs. 25%; P = .04) and 4-year hepatic DFS (67% vs. 43%; P = .03) favored HAI over the control groups. Based on an intention-to-treat analysis, no difference was reported in median or 4-year OS between the groups. Currently, the 5-year survival is 55% versus 37.5% favoring adjuvant therapy (Kemeny M, 2005); however, this study was not designed to look at OS.
In the German cooperative multicenter study (Lorenz et al, 1998), 226 patients were randomized to resection alone or resection plus 6 months of HAI with 5-FU/LV given as a 5-day continuous infusion every 28 days. The study was terminated early, because an interim analysis suggested a very low chance of showing a survival benefit with adjuvant therapy. The impact of HAI in this study is difficult to assess because only 74% of patients assigned to HAI received this treatment, and only 30% completed it. No difference in time to progression, time to hepatic progression, and median OS was noted in an intention-to-treat analysis. When patients were analyzed “as treated,” time to hepatic progression (45 months vs. 23 months) and time to progression or death (20 months vs. 12.6 months) were improved in the HAI arm. Grade 3 or 4 toxicity—including stomatitis, nausea, and vomiting—was noted in 63% of patients who received adjuvant therapy. These findings reflect significant systemic absorption of 5-FU when given via HAI.
A study conducted in Greece on 122 patients used mitomycin C, 5-FU, and interleukin (IL)-2 by both HAI and the intravenous (IV) route versus the IV route alone. The 2-year survival was 92% versus 75%, and 5-year survival was 73% versus 60% for the HAI plus SYS group versus the SYS-alone group, respectively. DFS was significantly longer for the HAI plus SYS group: 60% at 5 years versus 20% for the intravenous group (P = .0012); hepatic DFS was 85% versus 50% (P = .0001; Lygidakis et al, 2001).
The two American studies showed a reduction in hepatic and extrahepatic recurrence for combined HAI plus systemic therapy after surgical resection. Of the two European studies, one showed a significant increase in survival and DFS, whereas the other did not. In the MSKCC study, 2-year survival after liver resection was significantly improved with combined therapy: 86% compared with 72% with systemic therapy alone. Historic 2-year survivals (Chang et al, 1987; Fegiz et al, 1991; Jamison et al, 1997; Scheele et al, 1990) for patients treated with resection alone range from 55% to 70%.
Nonrandomized studies of modern chemotherapy regimens combined with HAI of FUDR in the post–liver metastasis resection (adjuvant) setting have been conducted and suggest improved outcomes. Phase I and II trials of adjuvant SYS irinotecan combined with FUDR/dexamethasone in 96 patients showed a 2-year survival of 89% at a median follow-up of 26 months. The dose-limiting toxicities were diarrhea and neutropenia, and all 27 patients treated at the maximum-tolerated dose were alive at publication (Kemeny et al, 2003). A phase I trial of adjuvant HAI of FUDR/dexamethasone with escalating doses of SYS oxaliplatin/5-FU/LV in 35 patients revealed 4-year survival and progression-free survival of 88% and 50%, respectively, at a median follow-up of 43 months. Dose-limiting toxicities of diarrhea and elevated bilirubin were each 8.5% (Kemeny et al, 2009b).
A multivariate analysis performed as a part of a recent review of over 1000 patients showed that post–liver metastasis resection HAI was one of the significant factors to improve survival. Median OS was 50 months in those who did not receive HAI and 68 months in those who did (P = .0001) (Ito et al, 2008). Another MSKCC retrospective review described 612 patients who underwent liver resection from 1985 to 1994. The 10-year OS was 38% for patients who received HAI of FUDR and 15% for those who did not (P < .0001) (Tomlinson et al, 2007). Finally, colleagues at our institution retrospectively compared outcomes from 125 metastatic CRC patients who received postoperative SYS (FOLFOX or 5-FU plus leucovorin and irinotecan [FOLFIRI]) with HAI to 125 similar patients who received only SYS. The 5-year hepatic progression-free survival was 75% in the HAI group and 52% in the SYS group (P = .0005) with a median follow-up of 43 months; OS at 5 years was 72% and 52%, respectively (House et al, 2011). These data underscore the need for randomized studies of modern HAI plus SYS compared with modern SYS alone.
Neoadjuvant Hepatic Arterial Infusion
Neoadjuvant treatment is a strategy increasingly used in solid-tumor oncology, given the multifaceted rationale that it may enable downsizing and subsequent resectability of tumor burden, and more systemic treatment may be tolerated, or given at all, than in postoperative settings, where complications may limit or preclude administration of chemotherapy. In addition, neoadjuvant HAI may prevent unnecessary operations in patients who have clinically and biologically declared themselves to have inoperable metastatic disease. In the context of converting CRC patients with unresectable liver metastases to operable candidates, some neoadjuvant approaches have been discussed above. Combining SYS with HAI agents besides FUDR has been studied in the neoadjuvant setting. Numerous reports have been made that HAI of oxaliplatin, for instance, has shown benefit in response and resectability rates when combined with SYS 5-FU (Boige et al, 2008; Del Freo et al, 2006; Ducreux et al, 2005).
Hepatic Arterial Infusion for Noncolorectal Liver Metastases and Primary Liver Cancer (See Chapters 50A, 80, and 81C)
Although liver metastases from noncolorectal carcinoma are less commonly treated with regional therapy, HAI chemotherapy has been used in cases of breast cancer, HCC, melanoma (Melichar et al, 2009), bronchogenic carcinoma, and pancreas cancer. Studies in breast cancer have employed numerous HAI agents including FUDR, mitomycin/FU/doxorubin, vinblastine/cisplatin, and cyclophosphamide/etoposide. One trial of 15 patients undergoing systemic chemotherapy for refractory metastatic breast cancer tested HAI of FUDR and mitomycin and demonstrated a partial response rate of 53% and OS of 18 months after HAI was begun (Maral et al, 1985). FUDR alone and in combination with various cytotoxic agents has also been evaluated in HCC (see Chapter 80), including treatment with mitomycin/interferon/FUDR (Atiq el al, 1992) and LV/doxorubicin/cisplatin/FUDR (Patt el al, 1994) in 27 patients. In the latter, response rates were 41% to 54%, and median OS was 15 months.
More recent experience with HAI for HCC and intrahepatic cholangiocarcinoma (ICC; see Chapter 50A) at MSKCC was published, and it demonstrates the correlative and predictive utility of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in 34 patients with unresectable disease treated with HAI of FUDR/dexamethasone. Partial responses were seen in 47%, with median OS of 30 months (Jarnagin et al, 2009). One patient in this study responded sufficiently to undergo resection and was found to have had a complete pathologic response. The median duration of response was 1 year, and median time to progression was 7 months. Median follow-up at time of publication was 35 months, and 1- and 2-year survival was 88% and 67%, respectively. Therapy was well tolerated, and DCE-MRI data showed that pretreatment and posttreatment tumor perfusion and permeability parameters correlated with median survival, therefore these may serve as a biomarker of treatment outcome.
Allen PJ, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol. 2002;9:875-880.
Allen PJ, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57-65.
Allen-Mersch TG, et al. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. 1994;344:1255-1260.
Arai Y. The study of catheter placement into the hepatic artery via the left subclavian artery for hepatic infusion therapy. J Nagoya City Univ Med Assoc. 1988;39:453-464.
Arai Y, et al. Interventional techniques for hepatic arterial infusion chemotherapy. In: Castaneda-Zuniga WR, editor. Interventional Radiology, vol 1. Philadelphia: Williams & Wilkins; 1997:192-205.
Atiq OT, et al. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992;69:920-924.
Balch CM, Urist MM. Intraarterial chemotherapy for colorectal liver metastases and hepatomas using a totally implantable drug infusion pump. Recent Results Cancer Res. 1986;100:123-147.
Bilchik AJ, et al. Cryosurgery ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657-662.
Blackshear PJ, et al. The design and initial testing of an implantable infusion pump. Surg Gynecol Obstet. 1972;134:51-56.
Boige V, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15:219-226.
Breedis C, Young C. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969.
Campbell KA, et al. Regional chemotherapy devices: effect of experience and anatomy on complications. J Clin Oncol. 1993;11:822-826.
Castaing D, et al. Implantable hepatic arterial infusion device: placement without laparotomy via an intercostal artery. J Am Coll Surg. 1998;187:565-568.
Chang AE, et al. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685-693.
Cheng J, et al. Laparoscopic radiofrequency ablation and hepatic artery infusion pump placement in the evolving treatment of colorectal hepatic metastases. Surg Endosc. 2003;17:61-67.
Cheng J, et al. Laparoscopic placement of hepatic artery infusion pumps: technical considerations and early results. Ann Surg Oncol. 2004;11:558-559.
Cohen AM, et al. Transbrachial hepatic arterial chemotherapy using an implanted infusion pump. Dis Colon Rectum. 1980;23:223-227.
Cohen AM, et al. Regional hepatic chemotherapy using an implantable drug infusion pump. Am J Surg. 1983;145:529-533.
Cohen AM, et al. Treatment of colorectal cancer hepatic metastases by hepatic artery chemotherapy. Dis Colon Rectum. 1985;28:389-393.
Cohen AM, et al. Effect of ligation of variant hepatic arterial structures on the completeness of regional chemotherapy infusion. Am J Surg. 1987;153:378-380.
Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2:498-504.
Collins JM. Pharmacologic rationale for hepatic arterial therapy. Recent Results Cancer Res. 1986;100:140-148.
Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345.
Curley SA, et al. Technical considerations and complications associated with the placement of 180 implantable hepatic arterial infusion devices. Surgery. 1993;114:928-935.
Daly J, Kemeny N. The therapy of colorectal hepatic metastases. In: DeVita, VT, et al. Important Advances in Oncology. Philadelphia: Lippincott; 1986:251-268.
Daly J, et al. Long-term hepatic arterial infusion chemotherapy. Arch Surg. 1984;119:936-941.
Davidson, et al. Alternating floxuridine and 5-fluorouracil hepatic arterial chemotherapy for colorectal liver metastases minimizes biliary toxicity. Am J Surg. 1996;172:244-247.
de Gramont A, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947.
Del Freo A, et al. Hepatic arterial chemotherapy with oxaliplatin, folinic acid and 5-fluorouracil in pre-treated patients with liver metastases from colorectal cancer. In Vivo. 2006;20:743-746.
Doria MIJr, et al. Liver pathology following hepatic arterial infusion chemotherapy: hepatic toxicity with FUDR. Cancer. 1986;58:855-861.
Ducreux M, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: a trial of the gastrointestinal group of the Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 2005;23:4881-4887.
Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10:176-182.
Fegiz G, et al. Hepatic resections for colorectal metastases: the Italian multicenter experience. J Surg Oncol Suppl. 1991;2:1441-1454.
Feliciotti F, et al. Laparoscopic intraarterial catheter implantation for regional chemotherapy of liver metastasis. Surg Endosc. 1996;10:449-453.
Fong Y, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-318.
George SL, Hoogstraten B. Prognostic factors in the initial response to therapy by patients with advanced breast cancer. J Natl Cancer Inst. 1978;60:731-736.
Gluck WL, et al. A reversible enteropathy complicating continuous hepatic artery infusion chemotherapy with 5-fluoro 2-deoxyuridine. Cancer. 1985;56:2424-2427.
Goldberg R, et al. Oxaliplatin (Oxal) or CPT-11 + 5-fluorouracil (5FU)/leucovorin (LV) or oxal + CPT-11 in advanced colorectal cancer (CRC). Proc Am Soc Clin Oncol. 2002;21:511.
Grobmyer SR, et al. Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139:1326-1330.
Harmantas A, et al. Regional versus systemic chemotherapy in the treatment of colorectal carcinoma metastatic to the liver: is there a survival difference? Meta-analysis of the published literature. Cancer. 1996;78:1639-1645.
Harris J, et al. Malignant tumors of the breast. In: DeVita, VT, et al. Cancer: Principles and Practice of Oncology. Philadelphia, Lippincott: Williams & Wilkins; 1997:1557-1616.
Hellan M, Pigazzi A. Robotic-assisted placement of a hepatic artery infusion catheter for regional chemotherapy. Surg Endosc. 2008;22:548-551.
Hohn DC, et al. Avoidance of gastroduodenal toxicity in patients receiving hepatic arterial 5-fluoro-2′-deoxyuridine. J Clin Oncol. 1985;3:1257-1260.
Hohn DC, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: the Northern California Oncology Group trial. J Clin Oncol. 1989;7:1646-1654.
House M, et al. Comparison of adjuvant systemic chemotherapy with or without hepatic arterial infusional chemotherapy after hepatic resection for metastatic colorectal cancer. Ann Surg. 2011;254:851-856.
Hrushesky W, et al. Circadian-shaped infusions of floxuridine for progressive metastatic renal cell carcinoma. J Clin Oncol. 1990;8:1504-1513.
Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
Inaba Y, et al, 1998: Unnecessity of surgical approach for hepatic arterial catheter placement: minimally invasive procedures using angiographic techniques. Presented at the ASCO annual meeting, Los Angeles, 1998 (abstract 1125).
Ito H, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;6:994-1002.
Jamison RL, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505-511.
Jarnagin WR, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589-1595.
Johnson LP, Rivkin SE. The implanted pump in metastatic colorectal cancer of the liver: risk versus benefit. Am J Surg. 1985;149(5):595-598.
Kanemitsu Y, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol. 2009;39:406-409.
Kemeny M: Personal communication, 2005.
Kemeny MM, et al. Sclerosing cholangitis after continuous hepatic artery infusion of FUDR. Ann Surg. 1985;202:176-181.
Kemeny MM, et al. Results of a prospective randomized trial of continuous regional chemotherapy and hepatic resection as treatment of hepatic metastases from colorectal primaries. Cancer. 1986;57:492-498.
Kemeny MM, et al. Continuous hepatic artery infusion with an implantable pump: problems with hepatic artery anomalies. Surgery. 1986;99:501-504.
Kemeny MM, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy—an intergroup study. J Clin Oncol. 2002;20:1499-1505.
Kemeny N, Braun DW. Prognostic factors in advanced colorectal carcinoma: the importance of lactic dehydrogenase, performance status, and white blood cell count. Am J Med. 1983;74:786-794.
Kemeny N, et al. Hepatic artery pump infusion toxicity and results in patients with metastatic colorectal carcinoma. J Clin Oncol. 1984;2:595-600.
Kemeny N, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. Ann Intern Med. 1987;107:459-465.
Kemeny N, et al. Prognostic variables in patients with hepatic metastases from colorectal cancer: importance of medical assessment of liver involvement. Cancer. 1989;63:742-747.
Kemeny N, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasone versus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69:327-334.
Kemeny N, et al. Randomized trial of hepatic arterial floxuridine, mitomycin and carmustine versus floxuridine alone in previously treated patients with liver metastases from colorectal cancer. J Clin Oncol. 1993;11:330-335.
Kemeny N, et al. Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 1994;12:2288-2295.
Kemeny N, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039-2048.
Kemeny N, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001;19:2687-2695.
Kemeny N, et al. Phase I/II study of hepatic arterial therapy with floxuridine and dexamethasone in combination with intravenous irinotecan as adjuvant treatment after resection of hepatic metastases from colorectal cancer. J Clin Oncol. 2003;21:3303-3309.
Kemeny N, et al. A Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23:4888-4896.
Kemeny N, et al. Hepatic arterial infusion of floxuridine and dexamethasone plus high-dose mitomycin C for patients with unresectable hepatic metastases from colorectal carcinoma. J Surg Oncol. 2005;91:97-101.
Kemeny N, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395-1403.
Kemeny N, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465-3471.
Kemeny N, et al. Phase I trial of adjuvant hepatic arterial infusion (HAI) with floxuridine (FUDR) and dexamethasone plus systemic oxaliplatin, 5-fluorouracil and leucovorin in patients with resected liver metastases from colorectal cancer. Ann Oncol. 2009;20:1236-1241.
Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734-735.
Kerr DJ, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomized trial. Lancet. 2003;361:368-373.
Lorenz M, Muller H. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243-254.
Lorenz M, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer: German Cooperative on Liver Metastases (Arbeitsgruppe Lebermetastasen). Ann Surg. 1998;228:756-762.
Lygidakis NJ, et al. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection: results of a prospective randomized study. Hepatogastroenterology. 2001;48:1685-1691.
Maral J, et al. Intra-arterial chemotherapy for hepatic metastases: experience of the Pitie-Salpetriere Hospital Group. Ann Gastroenterol Hepatol (Paris). 1985;21:99-101.
Martin JKJr, et al. Intra-arterial floxuridine vs systemic fluorouracil for hepatic metastases from colorectal cancer: a randomized trial. Arch Surg. 1990;125:1022-1027.
Matsumada T, et al. Laparotomy versus interventional radiological procedures for implantation of arterial infusion devices. Surg Today. 1997;27:398-402.
Melichar B, et al. Liver metastases from uveal melanoma: clinical experience of hepatic arterial infusion of cisplatin, vinblastine and dacarbazine. Hepatogastroenterology. 2009;56:1157-1162.
Meta-Analysis Group in Cancer. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst. 1996;88:252-258.
Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966;112:337-347.
Mitry E, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2009;26:4906-4911.
Mocellin S, et al. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol. 2007;25:5649-5654.
Niederhuber JE, et al. Regional chemotherapy of colorectal cancer metastatic to the liver. Cancer. 1984;53:1336.
Nordlinger B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016.
Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379-384.
Oberfield RA, et al. Prolonged and continuous percutaneous intra-arterial hepatic infusion chemotherapy in advanced metastatic liver adenocarcinoma from colorectal primary cancer. Cancer. 1979;44:414-423.
Pancera G, et al. A feasibility study of combined hepatic arterial infusion (HAI) with FUDR and systemic chemotherapy (SYS) with FOLFOX-4 in advanced colorectal cancer (CRC). Proc Am Soc Clin Oncol. 2002;21:105b. (abstract 2233)
Patt YZ, et al. Hepatic arterial infusion of floxuridine, leucovorin, doxorubicin, and cisplatin for hepatocellular carcinoma: effects of hepatitis B and C viral infection on drug toxicity and patient survival. J Clin Oncol. 1994;12:1204-1211.
Portier G, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982.
Rougier P, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10:1112-1118.
Safi F, et al. Regional chemotherapy for hepatic metastases of colorectal carcinoma (continuous intra-arterial versus continuous intra-arterial/intravenous therapy). Cancer. 1989;64:379-387.
Saldinger PF, Sandhu F. Minimally invasive/transintercostal placement of hepatic arterial infusion pump. J Gastrointest Surg. 2004;8:167A.
Saltz L, et al. High thymidylate synthase (TS) expression does not preclude activity of CPT-11 in colorectal cancer (CRC). Proc Am Soc Clin Oncol. 1998;17:281a. abstract 1080
Saltz LB, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914.
Scheele J, et al. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246.
Schwartz SI, et al. Assessment of treatment of intrahepatic malignancies using chemotherapy via an implantable pump. Ann Surg. 1985;201:560-567.
Shepard KV, et al. Therapy for metastatic colorectal cancer with hepatic artery infusion chemotherapy using a subcutaneous implanted pump. J Clin Oncol. 1985;3:161-169.
Shiatra K, et al. Combination chemotherapy with hepatic arterial infusion of 5-fluorouracil (5-FU) and systemic irinotecan (CPT-11) in patients with unresectable liver metastases from colorectal cancer. Gan To Kagaku Ryoho. 2006;33:2033-2037.
Sigurdson ER, et al. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J Clin Oncol. 1987;5:1936-1940.
Stagg RJ, et al. Alternating hepatic intra-arterial floxuridine and fluorouracil: a less toxic regimen for treatment of liver metastases from colorectal cancer. J Natl Cancer Inst. 1991;83:423-428.
Thirion P, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775.
Tomlinson JS, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-4580.
Tournigand C, et al. FOLFIRI followed by FOLFOX6 versus FOLFOX or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237.
Urbach DR, Hansen PD. Laparoscopic placement of a continuous hepatic artery infusion pump. Semin Laparosc Surg. 2000;7:140-147.
Urbach DR, et al. Laparoscopic hepatic artery infusion pump placement. Arch Surg. 2001;136:700-704.
Wagman LD, et al. A prospective, randomized evaluation of the treatment of colorectal cancer metastatic to the liver. J Clin Oncol. 1990;8:1885-1893.
Weiss GR, et al. Long-term hepatic arterial infusion of 5-fluorodeoxyuridine for liver metastases using an implantable infusion pump. J Clin Oncol. 1983;1:337-344.
Weiss L. Metastatic inefficiency and regional therapy for liver metastases from colorectal carcinoma. Reg Cancer Treat. 1989;2:77-81.
Weiss L, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203.