Cancer Prevention, Screening, and Early Detection
Therese B. Bevers, Powel H. Brown, Karen Colbert Maresso and Ernest T. Hawk
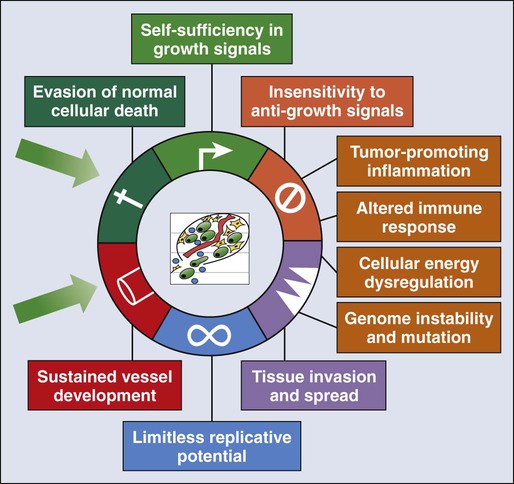
• The six hallmarks, or cardinal derangements, characterizing all epithelial cancers are sustained proliferative signaling, evasion of growth suppressors, activation of invasion and metastasis, replicative immortality, induction of angiogenesis, and resisting cell death. These six hallmarks tend to occur in a permissive context characterized by four features: suppressed immune surveillance, tumor-promoting inflammation, cellular dysregulation, and genome instability and mutation.
• Tobacco, which accounts for 30% of all cancers and 90% of lung cancers, is the greatest modifiable risk factor for cancer. The use of tobacco is on the rise in developing nations, and declines in smoking prevalence in the United States have recently begun to slow. Tobacco is likely to remain an important public health issue in the United States and globally for the foreseeable future unless tobacco control strategies can be more fully implemented and sustained.
• After tobacco, obesity has the highest attributable cancer mortality. Recent evidence suggests that obesity, resulting at least in part from excess caloric intake, is a key driver in cancer development. Diet, physical inactivity, infections, and sun exposure contribute to cancer risk as well.
• Data related to the role of nutrition in cancer risk is more persuasive for specific foods, rather than for specific nutrients or other food constituents. A few factors have been “convincingly” associated with an increased risk of cancer, as classified by the American Institute for Cancer Research, but none has been “convincingly” associated with a decreased risk.
• Cancer incidence is set to double by 2030 as a result of a growing and aging population.
• Nearly two thirds of all cancer deaths are attributable to tobacco, poor diet, physical inactivity, and obesity.
• We can prevent approximately half of all cancers occurring today by implementing tools and knowledge we already have.
• Thirteen chemopreventive agents have been approved by the U.S. Food and Drug Administration for treatment of precancerous lesions or to reduce the risk of invasive cancer, nearly all of which are for accessible organs.
• The identification and eradication of a number of infectious, oncogenic agents can yield significant cancer preventive benefits as well. Globally, about 18% of cancers have an infectious etiology. In addition to the use of the human papillomavirus vaccines, vaccination for hepatitis B, “triple therapy” for Helicobacter pylori, and treatment of chronic hepatitis B and C can yield a marked reduction in the cancer burden in regions where these agents are endemic, although these medical interventions are not labeled for a cancer preventive indication.
• Because chemoprevention focuses on healthy individuals in whom cancer may never develop, balancing the risks and benefits of any chemopreventive intervention is central to their development.
• In the future, chemopreventive trials must be smaller, faster, cheaper, and focused on high-risk cohorts and drug combinations. Integrative assessments of the full range of benefits and risks of chemopreventive agents across cancer sites and diseases will also be important.
• Population-based screening tests are available for the following cancers: cervical (Papanicolaou test), colon (colonoscopy, fecal occult blood testing, flexible sigmoidoscopy, and double-contrast barium enema), breast (mammography), and prostate (prostate-specific antigen test).
• The National Lung Screening Trial study demonstrated that low-dose helical computed tomography screening can reduce lung cancer mortality by 20%.
• Numerous genomics-based and proteomics-based approaches are attempting to identify biomarkers that can aid in the risk assessment and early detection of various cancers.
Introduction
The International Agency for Research on Cancer (IARC) estimates a doubling of cancer incidence worldwide by 2030 because of a growing and aging population.1 Nearly two thirds of all cancer deaths are attributable to the modifiable risk factors of tobacco, poor diet, physical inactivity, and obesity.2 Although rates of these risk factors have slowed or leveled off within the United States, they are increasing in many parts of the world, particularly as persons in developing countries adopt westernized lifestyles. Such trends are not sustainable. Efforts directed toward reducing these risk factors and improving rates of screening in the population can substantially mitigate the cancer burden. Within the clinic, enhanced risk stratification and novel preventive strategies targeted toward high-risk groups are needed. In sum, the need for safe and effective preventive strategies has never been more urgent.
Carcinogenesis
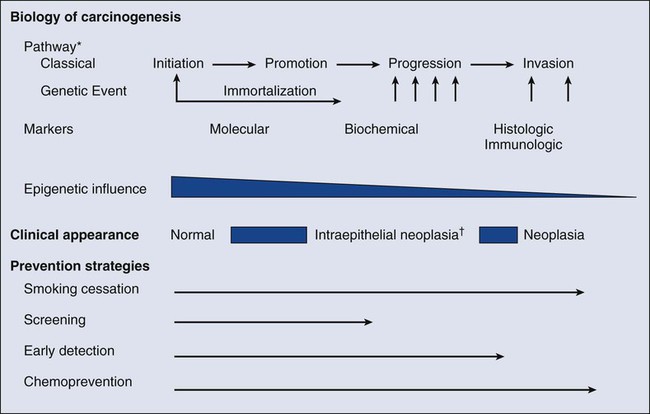
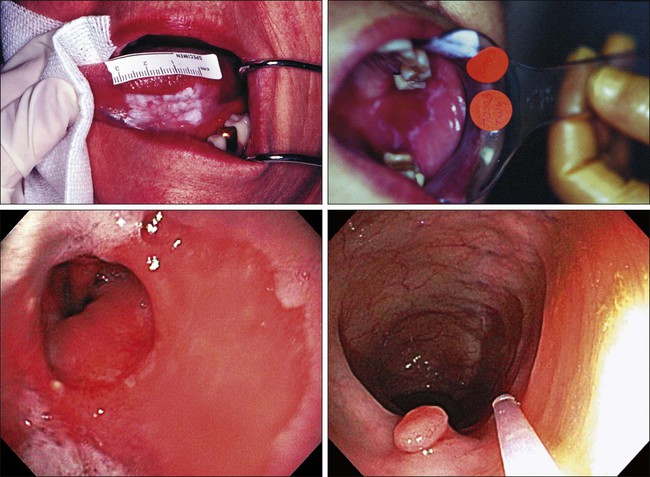
Carcinogenesis, a multistep, chronic process occurring over decades, is characterized by a progression of genetic changes affecting cellular identity and growth that ultimately culminates in cancer (Fig. 23-1). Our understanding of this process has emerged from the study of cancer development in carcinogen-driven or genetically driven animal models and human specimens. Conceptually, carcinogenesis can be thought of as occurring in three steps: initiation, which is the irreversible alteration of a cancer-related gene, such as an oncogene or tumor suppressor gene; promotion, the clonal expansion of the initiated cell, which is reversible if detected; and progression, the final stage, which is characterized by the transformation of a benign mass of cells into a malignant tumor, driven by the acquisition of additional mutations. The order of acquired mutations in carcinogenesis is not as important as their overall accumulation, and although the particular affected pathway(s) and acquired mutations vary across the different cancer sites, the paradigm of carcinogenesis and the six typical cardinal derangements (i.e., evasion of cell death, self-sufficiency in growth signals, insensitivity to antigrowth signals, tissue invasion and spread, limitless replicative potential, and sustained vessel development) and four contextual influences (i.e., tumor-promoting inflammation, altered immune response, cellular energy dysregulation, and genome instability and mutation) characterizing epithelial cancers are universal (Fig. 23-2).3 The cumulative genetic and epigenetic aberrations are typically reflected in a series of cytomorphologic and histopathological derangements termed “preinvasive neoplastic lesions,” or more simply, precancerous lesions (Fig. 23-3). These lesions are characterized by seven morphologic features: increased nuclear size, abnormal nuclear shape, increased nuclear stain uptake, nuclear pleomorphism (i.e., increased cell-to-cell variation in size, shape, and staining), increased mitoses, abnormal mitoses, and disordered or absent differentiation.4
Risk Modeling
A number of risk prediction models have been developed during the past 10 to 20 years in an effort to more accurately and reliably assess cancer risks in average- and high-risk individuals. These models are important as a foundation of risk : benefit discussions between a practitioner and patient and also to aid research and developmental clinical trials in support of both devices and interventions. The National Cancer Institute (NCI) maintains an important bibliography of peer-reviewed models relating to both risk of gene carrier status and absolute cancer risk prediction, as well as online risk prediction tools and calculators, on its Web site at http://epi.grants.cancer.gov/cancer_risk_prediction/.
Prevention
General Prevention/Lifestyle Intervention
Although the number of new cancer cases is expected to rise by more than 50% within the United States and more than double worldwide by 2030,1 it is estimated that more than half of all cancers can be prevented by applying what we already know.5 In 1981 Doll and Peto6 published their landmark estimates of the relative contributions of the avoidable causes of cancer and concluded that up to three fourths of all cancers occurring in the United States during 1970 theoretically may have been avoided. This estimate as to the “preventability” of cancer was based on their approximations of the relative contribution of individual cancer risk factors: 30% for tobacco, 2% to 4% for alcohol, 35% for diet, 1% to 10% for infectious agents, 2% for pollution, and 4% for occupational exposures.6 Subsequent epidemiological data have largely supported these estimates and have identified obesity and lack of physical activity as additional cancer risk factors, causing a slight redistribution of the percentage of cancer mortality attributable to the various lifestyle risk factors. A review summarizing more recent data on this topic states that obesity accounts for 15% to 20% of cancers, physical inactivity accounts for approximately 5%, and the estimates of diet have decreased to 5%, much less than the initial estimate of 35% by Doll and Peto (Table 23-1).2 Although there are substantial limitations to estimates of the contributions of individual risk factors to cancer mortality, and although the estimated fraction of cancer that can be prevented may be a best-case scenario that is difficult to achieve in reality, such studies not only demonstrate a need to better understand the interplay among lifestyle factors but highlight the potential impact of prevention and underscore its urgency.
Table 23-1
Relative Contribution of Individual Cancer Risk Factors to Overall Cancer Mortality
| Risk Factor | % Estimate |
| Biological | |
| Reproductive factors | 5 |
| Viruses | 8 |
| Medication | 5 |
| Total biological contribution | 18 |
| Social/behavioral | |
| Tobacco | 30 |
| Diet | 5 |
| Alcohol | 4 |
| Total social/behavioral contribution | 39 |
| Physical environmental | |
| Obesity | 20 |
| Sedentary lifestyle | 5 |
| Environment | 4 |
| Sun/radiation | 2 |
| Total physical environmental contribution | 31 |
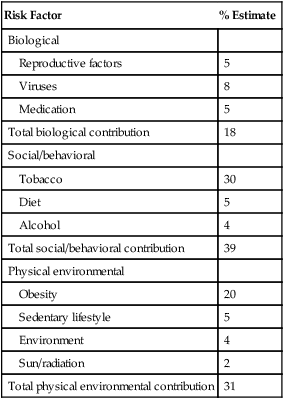
Data from Colditz GA, Wei EK. Preventability of cancer: the relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu Rev Public Health 2012;33:137–56.
Tobacco accounts for approximately one third of all cancer deaths, more than any other risk factor. In the United States, although the prevalence of smoking has been declining for decades, this decline has recently begun to slow and has not been observed in all population subgroups.7 Based on such data, the Centers for Disease Control and Prevention (CDC) state that tobacco is likely to remain an important public health issue in the United States for the foreseeable future unless tobacco control strategies can be more fully implemented and sustained.8 Some recent federal tobacco control initiatives include provisions in the Affordable Care Act (Table 23-2) that allow for coverage of evidence-based smoking cessation treatments and the Family Smoking Prevention and Tobacco Control Act, which grants the U.S. Food and Drug Administration (FDA) the authority to regulate the manufacturing, sale, and marketing of tobacco products. Worldwide, tobacco use is on the rise in developing nations, which harbor a majority of the world’s population, and where tobacco-attributable deaths are expected to significantly increase by 2050.9,10 In an effort to combat global tobacco use, the World Health Organization’s Framework Convention on Tobacco Control, the first international public health treaty, outlines tobacco control and regulation strategies for ratifying countries.11
Table 23-2
Affordable Care Act Provisions that Advance Cancer Prevention in the Public
| Provision | Details |
| Elimination of cost-sharing for clinical preventive services | For all new plans, Medicare |
| Covered services include those with an “A” or “B” rating from the USPSTF | |
| Does not apply to Medicaid | |
| New funding for community preventive services | Provides funds for community preventive services |
| Three different provisions will provide grants to communities to implement and develop preventive interventions | |
| Examples of such services include tobacco cessation programs, disease management programs, educational initiatives, taxes and new laws | |
| Provisions are not automatically appropriated; appropriations are left to Congress | |
| Work-site wellness programs | Evidence for effectiveness of work-site wellness programs is strong |
| ACA expands financial incentives by allowing employers to discount health insurance premiums for employees who satisfy a health standard, up to 50% | |
| Small businesses eligible for $200 million in new grants for development of work-site wellness programs | |
| CDC commissioned to study such programs to determine what does and does not work |
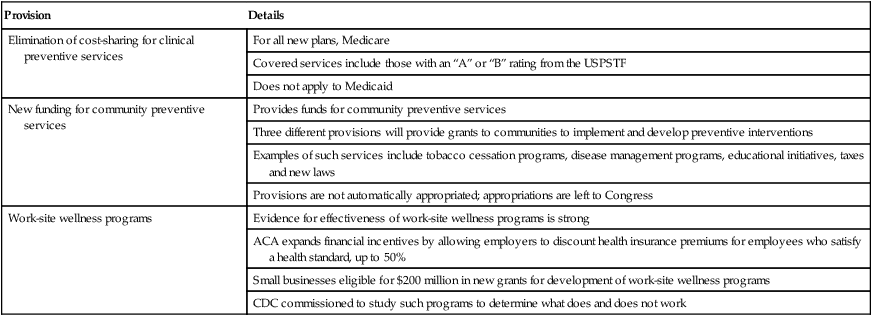
Data from Preston CM, Alexander M. Prevention in the United States Affordable Care Act. J Prev Med Public Health 2010;43:455–8.
According to the most recent data, obesity is now the risk factor with the highest attributable cancer mortality (14% to 20%) after tobacco.2,12 During the past 30 years, the United States has experienced an epidemic of obesity, with its prevalence more than doubling between the periods of 1976-1980 and 2003-2006.13 In 2011, nearly two thirds of the U.S. population was overweight or obese, and higher prevalences are observed in various subgroups, such as racial and ethnic minorities, where the proportion of overweight or obese individuals can exceed 70%.14 The United States is not alone in this epidemic, as other developed nations have also experienced increasing proportions of overweight and obese individuals.13 The mechanisms of how excess body weight influences cancer risk and progression is an area of active research. The strongest empirical data suggest that this increased risk may, at least in part, be mediated by the effect of the various peptides and steroid hormones produced by adipose tissue on other cell types along the neoplastic continuum (Fig. 23-4).13,15 Overweight and obesity have now been definitively associated with an increased risk of six cancers—postmenopausal breast, colorectal, esophagus, pancreas, endometrial, and kidney—and possibly others, including prostate and ovarian.16 In addition, higher body mass index (BMI) has been associated with increased death rates in breast, endometrial, colorectal, esophageal, pancreatic, kidney, gastric, cervical, uterine, ovarian, and prostate cancers; multiple myeloma; and non-Hodgkin lymphoma.12,17,18 American Cancer Society (ACS) recommendations that affect this modifiable risk factor include balancing caloric intake with physical activity, avoiding excessive weight gain throughout life, and, if overweight or obese, achieving and maintaining a healthy weight.19
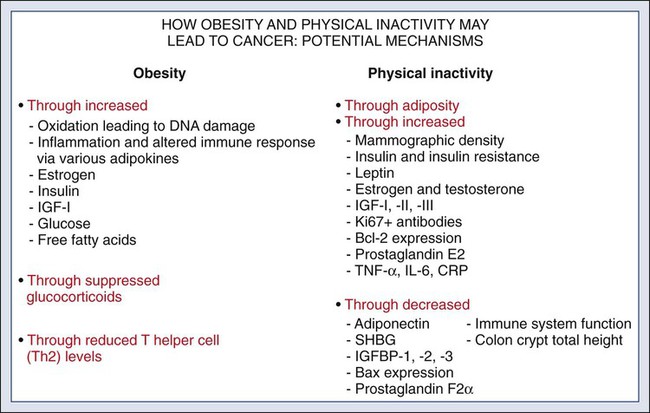
Evidence for the protective effect of physical activity with regard to cancer risk continues to grow. It is important to note that physical activity has independent protective effects beyond its indirect effect on cancer risk through body weight. The protective effect of physical activity has been most convincingly established for breast and colorectal cancers, but some studies have also demonstrated similar effects on endometrial and lung cancers.20 As with body weight, the effects of physical activity are not limited to the development of cancer but may be observed across the cancer continuum, with some studies suggesting a beneficial effect on survival in patients with breast and colorectal cancers.17,21–24 Although the exact mechanisms of the protective effects of physical activity on cancer are unclear, current evidence suggests that they may result from reductions in circulating concentrations of various hormones and by enhancements to overall energy metabolism (Fig. 23-4).20,25 Recommendations from the ACS relating to physical activity include getting 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity physical activity each week and limiting sedentary behavior.19
Independent of obesity, certain foods, drinks, vitamins or other nutrients, and particular dietary patterns may increase or decrease the risk of various cancers. The association of diet with risk of cancer has been extensively studied, although few specific dietary components have been convincingly shown to either increase or decrease risk. This situation is due in large part to challenges in the accuracy and reliability of long-term dietary measurements and the likely long-term follow-up required to observe effects of any single dietary component, combination, or pattern. Most recently, in the first study to examine multivitamin use over several years of follow-up in a large cohort, Gaziano et al.26 report that daily multivitamin use among male physicians over a median of 11.2 years of follow-up modestly but significantly reduced the risk of total cancer (hazard ratio [HR], 0.92; 95% confidence interval [CI], 0.86-0.998; P = .04).26 Although there is no single ideal way to categorize the evidence on food and nutrition, the World Cancer Research Fund/American Institute for Cancer Research publishes the most comprehensive and systematic assessment of epidemiological evidence regarding diet in cancer prevention.16 Based on this source, a few generalizations can be made. First, the evidence is more persuasive for specific foods rather than for specific nutrients or food constituents. Second, although a few factors have been “convincingly” associated with an increased risk of cancer, none has been “convincingly” associated with a decreased risk. Foods and particular food constituents with evidence for increasing cancer risk deemed as “convincing” by the American Institute for Cancer Research include aflatoxins in persons with liver cancer, beta-carotene supplements in smokers and arsenic in drinking water for persons with lung cancer, red or processed meats in persons with colorectal cancer, and alcoholic drinks in persons with colorectal, esophageal, oropharynx, and both pre- and postmenopausal breast cancers.16 Although currently no evidence exists for any food, dietary constituent, or pattern deemed as “convincing” for decreasing cancer risk, nonstarchy vegetables and fruits display the most consistent evidence, deemed as “probable,” for a protective effect on the risk of oropharynx, esophageal, stomach, and lung cancers.16 In addition, garlic, milk, calcium, and foods containing fiber demonstrate “probable” evidence for decreasing the risk of colorectal cancer, as does folate for pancreatic cancer and lycopene, selenium, and diets high in calcium for prostate cancer.16 Based on these findings, the ACS recommends a diet based on plant foods and whole grains, with limited intake of red or processed meats and alcohol.19
Infectious agents are an additional root cause of cancer and represent a significant cause of cancer in the developing world, where the medical care and resources necessary to prevent and control their transmission are scarce. Worldwide, it is estimated that approximately 18% of cancers have an infectious etiology.27 Adequate prevention or treatment of infections may be associated with a substantially reduced risk of several cancers, including liver (hepatitis B and C); cervical, oral, anal, and head and neck (human papillomavirus [HPV]); Kaposi sarcoma and non-Hodgkin lymphoma (human immunodeficiency virus [HIV]/AIDS); bladder cancer (schistosomiasis); gastric cancer (Helicobacter pylori); nasopharyngeal cancer (Epstein-Barr virus [EBV]); and adult T-cell leukemia/lymphoma (human T-cell lymphotropic virus–1). Currently, the only therapies for treating infectious agents that have been formally FDA-approved with the indication of cancer risk reduction are the HPV vaccines Gardasil, approved in 2006, and Cervarix, approved in 2009, although hepatitis B vaccine and treatment of hepatitis C, HIV, H. pylori, and schistosomiasis infections are believed, if not actually demonstrated, to result in cancer risk reductions as well (Table 23-3).
Table 23-3
Strategies for Probable Cancer Risk Reduction in the Setting of an Infectious Agent
| Infectious Agent | Associated Cancer | Intervention |
| Hepatitis B virus | Hepatocellular carcinoma | Hepatitis B vaccine, interferon therapy, nucleoside analogues |
| Hepatitis C virus | Hepatocellular carcinoma | Interferon therapy, nucleoside analogues |
| Human immunodeficiency virus | Kaposi sarcoma and non-Hodgkin lymphoma | Antiretroviral therapies |
| Helicobacter pylori | Gastric/stomach cancer | Antibiotics—“triple therapy” |
| Schistosomiasis | Bladder cancer | Antischistosomal agents—Praziquantel and Metrifonate |
Sun and radiation exposure, the final modifiable risk factors, are estimated to account for 2% of preventable cancers.2 Skin cancer is the most common form of cancer, and its incidence is highest among non-Hispanic white persons. In recent years, the incidence of skin cancer has been rising, particularly for melanoma among white persons, for whom incidence has been increasing by 3% annually since 2004.7 Some possible factors contributing to this rise include a decrease in the protective ozone layer, changing patterns of dress favoring more skin exposure, more leisure time spent in the sun, and increased use of tanning beds.28 Prevention of skin cancer is dependent upon “sun-protective” behaviors, including covering up skin or avoiding the sun during its peak hours, seeking shade, applying sunscreen, and avoiding use of tanning beds.28
Chemoprevention
• an abnormal level of expression in early, preinvasive human neoplasia relative to the normal epithelium from which such lesions are derived;
• a clear biological contribution to the initiation, maintenance, or progression of a preinvasive neoplasm in a model system rather than just a bystander role;
• pharmacologic accessibility, with its modulation resulting in reductions in neoplastic incidence, histopathological grade, cancer mortality, and/or other convincing pathological evidence of improvement; and finally,
• specificity for neoplasia, rather than the normal tissue from which cancers arise.
Although use of the term “chemoprevention” is generally limited to the oncology field, it is nevertheless a well-established concept in both chronic and infectious disease prevention. The use of lipid-lowering and antihypertensive medications to reduce the risk of coronary artery disease, myocardial infarction, and stroke or the use of vaccines and various antibiotics to prevent infectious diseases are excellent examples of effective “chemoprevention” regimens that are well established and well accepted in clinical medicine. A major hurdle to the establishment and acceptance of chemoprevention within cancer medicine relates to a lack of accurate, reliable, and serially accessible surrogate end point biomarkers (SEBs) that reliably correlate with risk of disease.4 Biomarkers that occur early in the carcinogenic process and that can substitute for the true end point of cancer incidence or mortality—in that they strongly correlate with their risk—are urgently needed. Much as blood pressure and low-density lipoprotein cholesterol levels predict the risk of cardiovascular and cerebrovascular diseases and have served as focal points of preventive efforts in cardiovascular medicine, clinical oncology still requires these types of biomarkers that correlate with risk and that can serve as targets for cancer preventive efforts. Despite this challenge, we have seen some success in the setting of intraepithelial neoplasia when it is used as an SEB. A number of potential chemopreventive agents have entered clinical trials, and 13 have received FDA approval specifically for treatment of precancerous lesions or to reduce the risk of invasive cancer (Table 23-4).29 An evaluation of the end points in clinical trials that have resulted in the regulatory approval of these preventive agents reveals that nearly all have been approved for indications relating to intraepithelial neoplasia treatment, particularly in accessible organs, as opposed to prevention, per se.
Table 23-4
Approved Agents for Treatment of Precancerous Lesions or Cancer Risk Reduction, 2012
| Disease | Intervention |
| Breast cancer | Tamoxifen |
| Raloxifene | |
| Cervical intraepithelial neoplasia and cancer Vulvovaginal/anal intraepithelial neoplasia and cancer |
Human papillomavirus vaccines: Gardasil and Cervarix |
| Esophageal dysplasia | Photofrin + photodynamic therapy |
| Colonic adenomas | Celecoxib* |
| Bladder dysplasia | Bacillus Calmette-Guérin |
| Valrubicin | |
| Actinic keratoses | Fluorouracil |
| Diclofenac sodium | |
| 5-aminolevulinic acid + photodynamic therapy | |
| Masoprocol† | |
| Ingenol mebutate |
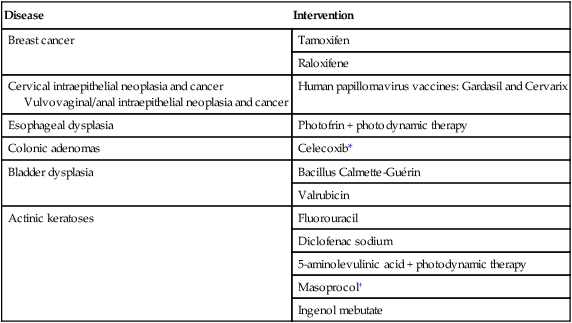
*Food and Drug Administration labeling voluntarily withdrawn by Pfizer, February 2011.
The development of chemopreventive compounds proceeds through a phased process of clinical trials akin to other areas of therapeutic development, but with somewhat greater attention to safety (Table 23-5).30 Phase I studies are designed to quickly evaluate an acceptable dose or dose range in a small number of healthy volunteers, whereas phase II trials generate preliminary, short-term efficacy data based on changes in any number of biomarkers believed to relate to cancer risk and confirm safety data in the context of a specific neoplastic condition. Phase II trials can be divided into phase IIa and phase IIb, where phase IIa trials establish preliminary drug effects in an uncontrolled manner and phase IIb trials compare the experimental agent with a placebo or the standard of care against scientifically or clinically justified biomarkers of neoplasia. Phase III trials typically involve hundreds to thousands of carefully selected subjects observed over years and typically randomly assign participants to a preventive agent or agent combination versus placebo or the standard of care to reduce clinically relevant neoplasia. Phase IV trials are undertaken after the drug has been approved by the FDA and marketed. The goal of this phase is to monitor the long-term safety and effectiveness of the agent or agent combination in a less controlled and more clinically relevant, “real-world” setting.
Table 23-5
Characteristics of Clinical Trial Phases
| Phase | Agent Dosing | Duration | Sample Size and Allocation | Control Group | Goals |
| I | Escalation | Weeks to months | <25; nonrandomized or randomized | Occasionally | Pharmacokinetics; dose finding based on short-term, mild to moderate toxicity |
| IIa | Deescalation | Months | <50; nonrandomized | Never | Dose finding based on reliable biomarker modulation |
| IIb | Stable | Months to a year | <100-200; randomized | Standard care* | Biomarker modulation (e.g., dysplasia regression) vs. standard care* |
| III | Stable | Years | 100 to >1000; randomized | Standard care* | Definitive efficacy to complement or replace standard care (e.g., reduce dysplasia/cancer incidence) |
| IV | Stable | Unspecified | General marketing population | N/A | Long-term safety in target population |
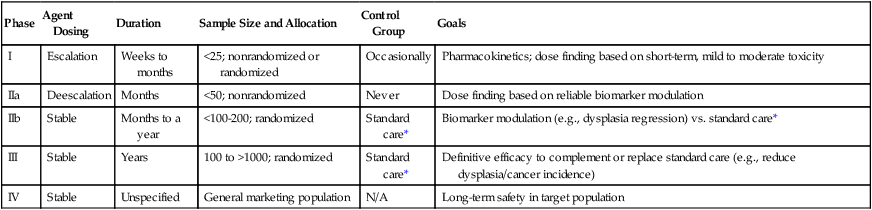
*In cancer chemoprevention, placebo may represent the standard of care.
Reprinted from Viner JL, Richmond E, Hawk ET, Lawrence J. Chemoprevention: the cancer handbook. Hoboken, NJ: John Wiley & Sons; 2005.
Central to the development of chemopreventive agents is the imperative to balance risks and benefits of an applied intervention, because “healthy,” or at least asymptomatic, populations are the focus of chemoprevention, thus necessitating a higher level of vigilance against potential toxicities and harms associated with an agent. The FDA has stated that because strategies to prevent cancer expose healthy people in whom cancer may never develop to a drug and its potential harms, a high level of certainty associated with benefits and harms of any potential intervention must exist.31 Consequently, the need exists to find better ways to balance risks and benefits to achieve acceptable therapeutic indices of chemopreventive agents. In this regard, agents need to be prioritized and tested with more attention to the relevance of model systems to the clinical context in which agents will be placed and their proposed mechanism(s) of action. Additionally, given that chemopreventive agents are often administered orally, yielding systemic exposure with the potential for broad efficacy across organs, integrative assessments of the risks and benefits of agents across multiple cancers and multiple diseases (e.g., cancer and cardiovascular disease) may be needed to tip the risk-benefit ratio in favor of their acceptance.
In conclusion, chemoprevention represents an important part of the future of cancer medicine, and the identification of chemopreventive agents holds tremendous promise in reducing the burden of cancer. Challenges facing the field that will significantly affect its future development relate to the need for early-stage biomarkers and acceptable SEBs and the need to improve upon the risk-benefit ratio of agents. Trials must be smaller, faster, cheaper, and focused on high-risk cohorts, as such cohorts that offer more power over a shorter time frame, are more likely to be tolerant of adverse effects, and have more motivation to adhere to a given intervention. In addition, the pharmaceutical industry is faced with many business disincentives when it comes to investing in cancer prevention; consequently, new business models and incentives are needed to stimulate private investment and motivate pharmaceutical companies to truly invest in chemoprevention.32 Finally, there is a strong interest in novel therapeutic approaches such as synthetic lethality, which targets early molecular derangements in cancer development,33 and chemopreventive combinations such as the coadministration of eflornithine and sulindac, which demonstrates that synergy between agents can lead to improved efficacy (up to 70% to 90% reductions in recurrent precancerous lesions) at lower doses, resulting in fewer and/or less severe toxicities.34
Screening and Early Detection
Technological advances in screening and early detection are essential for progress in both the prevention and treatment of cancer, but incorporation of such advances into useful clinical practice is often challenging and requires careful consideration of all potential risks and benefits. For any screening test to be useful, three criteria must be met: first, a test must exist that will detect the disease earlier than routine methods; second, evidence must exist that earlier treatment leads to improved outcomes; and third, benefits of screening must outweigh the risks associated with any subsequent diagnostic and therapeutic treatments. Observational data may suggest the benefits of screening tests, but they have the potential to mislead as well as to inform because of at least two important potential biases: lead-time and length biases (Fig. 23-5). Because the intent of screening is to advance the date of diagnosis, lead time refers to the amount of time between screen-detected versus symptom-detected diagnosis. This lead time in diagnosis appears to prolong survival in screened individuals, although mortality in this group may not actually be delayed, creating a lead-time bias.35 Length bias refers to the tendency of screening to detect cancers that are indolent and slower growing because of their longer detectable preclinical phase compared with faster-growing, more aggressive forms of cancer. Consequently, in a group of screened individuals, this phenomenon creates the appearance that screening is extending survival, when in fact extended survival is due to the more indolent nature of the cancers found in this group and not necessarily to the screening itself.35 To avoid these biases, development of effective screening tests should culminate in well-conceived and well-conducted randomized, controlled trials assessing a mortality end point.
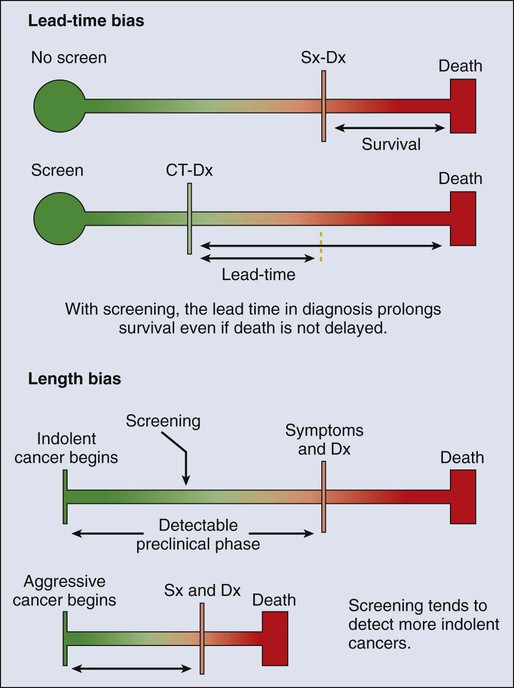
In any situation in which a screenable disease is prevalent but potentially indolent, the potential exists for overscreening, overdiagnosis, and overtreatment, resulting in a range of personal and social costs that may ultimately outweigh the intended benefits. According to Croswell et al.,35 overdiagnosis is an extreme form of length-biased sampling and occurs when a lesion is diagnosed that would otherwise never have caused symptoms. Overscreening refers to the same phenomenon, where cancers are detected by screening that would otherwise never go on to cause problems for the individual. An excellent example of these issues that challenge screening efforts involves the use of the prostate-specific antigen (PSA) test for prostate cancer screening, wherein enthusiasm for PSA testing to reduce cancer mortality appears to have exceeded the actual data. In addition, the routine use of screening mammography in women between the ages of 40 and 50 years of age has become a topic of debate, given concerns over the ability of the test to detect lesions (i.e., ductal carcinoma in situ) that may never progress to invasive cancer. Such issues have challenged the overall science of screening and have led to an increased reliance on disease-specific versus all-cause mortality and risk-benefit measures within screening trials.36,37
Currently, population-based screening tests are available for the following cancers: cervical (Papanicolaou [Pap] test), colon (colonoscopy, fecal occult blood testing [FOBT], flexible sigmoidoscopy, and double-contrast barium enema), breast (mammography), and prostate (PSA test). However, based on systematic reviews, only tests for breast, cervical, and colon cancers are currently recommended in average-risk populations by the U.S. Preventive Services Task Force (USPSTF) (Table 23-6). An examination of Behavioral Risk Factor Surveillance System data from recent years demonstrates that mammography use has not substantially changed since 2000, with 75% of women aged 40 years or older reporting having had a mammogram with the past 2 years.38 However, this same study documented that colorectal cancer screening rates continue to lag behind those of other cancer screening tests, with 65% of persons aged 50 to 75 years reporting having had one of the screening tests recommended by the USPSTF.39 Various research efforts are directed at making more cancers amenable to screening, including the application of novel imaging modalities and the development of early-stage biomarkers. Research into imaging modalities has advanced in the area of lung cancer, where low-dose helical computed tomography (CT) screening has been recently shown to significantly reduce lung cancer mortality by 20%.40 In addition, numerous genomics- and proteomics-based approaches are attempting to identify biomarkers that can aid in the risk assessment and early detection of various cancers, such as recent proteomic work to identify plasma antibodies predictive of triple-negative breast cancer41 and the recent mapping of colorectal susceptibility loci through genome-wide association studies.42 Although we have established screening tools for some cancers—and aside from the challenges associated with the development and implementation of new tests for others—a significant challenge to the field will be to apply all recommended screening tests in an equitable manner to all persons who need them.
Table 23-6
Most Recent United States Preventive Services Task Force Classification of the Evidence for Various Cancer Screening Tests in Average-Risk Individuals*
| Certainty of Net Benefit | Magnitude of Net Benefit | |||
| Substantial | Moderate | Small | Zero/Negative | |
| High | A Cervical: Pap smear, 3 yr, ages 21-65 yr Pap smear and HPV cotesting, 5 yr, ages 30-65 yr Colon†: FOBT, flexible sigmoidoscopy, colonoscopy, ages 50-75 yr |
B | C | D |
| Moderate | B | B Breast: Biennial mammography ages 50-74 yr |
C Breast‡: Biennial mammography ages 40-49 yr |
D Prostate§: PSA testing|| |
| Low | Insufficient evidence to make a recommendation:
Colon: CT colonography, fecal DNA testing (both are recommended by ACS as screening tests for colorectal cancer) Lung: Low-dose computerized tomography, chest x-ray, sputum cytology, or a combination of these Skin¶: Whole-body skin examination by a primary-care physician or patient skin self-examination |
|||
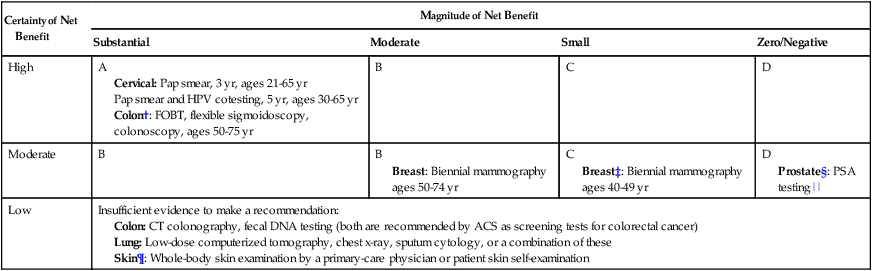
*Each cell within the table corresponds to a USPSTF recommendation grade, which is based on the magnitude and certainty of the net benefit deriving from the screening test. Only the tests receiving an “A” or “B” classification are recommended to the general public and are reimbursed by Medicare under the Affordable Care Act.
†ACS recommendations for colorectal screening also include CT colonography and fecal DNA testing, in addition to FOBT, flexible sigmoidoscopy, and colonoscopy.
‡ACS recommends annual mammography for all women 40+ years.
§ACS recommends PSA testing for men 50+ after careful discussion of benefits and harms of test. This discussion should occur at age 45 years for high-risk men.
||The USPSTF recommendation regarding prostate cancer screening does not apply to PSA testing as part of surveillance after diagnosis or treatment of prostate cancer.
¶Recommended by the ACS as part of a periodic cancer-related check-up.
Lung Cancer
Lung cancer is the most common cause of cancer-related death and the second most diagnosed cancer in the United States for both men and women. Estimated figures for 2012 alone exceed 225,000 new cases and 160,000 deaths, with men carrying roughly a 5% greater risk than women, and African American men at highest risk.7 On a global scale, these figures escalate, with lung cancer leading both worldwide cancer cases (more than 1.3 million diagnoses) and cancer-related mortality.45–45 Although American lung cancer incidence and death rates have been declining in men for more than a decade, the first statistically significant decrease in these rates among women was recently reported for the 2003-2007 period, after several decades of successive increases in incidence and death rates among women46 (see Chapter 72).
Risk Factors/Etiology
As the major cause of lung cancer in the United States, smoking contributes to lung cancer–related deaths at a rate of approximately 90% in men and 80% in women,47,48 with the variation due to gender-biased decades of peak cigarette use.46 Additional risk factors associated with smoking include (1) amount smoked (pack-years), (2) exposure to secondhand smoke, (3) age at smoking onset, (4) type of product smoked (e.g., filtered/unfiltered and tar/nicotine content), and (5) depth of inhalation. In addition to tobacco, other risk factors include exposure to alternative toxins, such as asbestos, metals, radon, air pollution, or paint, which can be exacerbated by a family history of lung cancer or tobacco exposure.7,49
Risk Modeling/Assessment
Current risk assessment for lung cancer is based primarily on smoking history, and although established clinical applications of risk modeling have existed for more than a decade for breast cancer50,51 and cardiovascular disease,54–54 models assessing risk of lung cancer have only recently been developed, and include Bach, Spitz, Liverpool Lung Project, and van Klaveren risk models.55–60 These models, based on epidemiological risk factors alone or in combination with DNA repair biomarkers,59 have been designed for limited use in the general60 and high-risk55 lung cancer populations.
The Bach model was initially developed in 2003 based on data from 18,172 participants of the Carotene and Retinol Efficacy Trial (CARET).55,57 Model inputs include basic demographic and exposure data from each participant (i.e., age, sex, exposure history to asbestos, and smoking history, including duration and packs per day and/or duration of abstinence), and the model estimates individual absolute risk of diagnosis of lung cancer within 10 years. Spitz et al.61 then constructed and validated individual lung cancer risk models for current and former smokers and persons who never smoked using epidemiological data from 1851 patients with lung cancer at MD Anderson Cancer Center and 2001 matched control subjects.61 The model’s predictive precision was subsequently improved by incorporating two biomarkers for DNA repair,59 leading to improved concordance statistics of 0.73 (current smokers) and 0.70 (former smokers). Like the Spitz model, the Liverpool Lung Project model originated as an epidemiological model based on five risk factors and DNA genotyping data, including smoking duration, prior diagnosis of pneumonia, occupational exposure to asbestos, prior diagnosis of malignant tumor, family history of lung cancer, and a single-nucleotide polymorphism (SNP) within the seizure 6–like (SEZ6L) gene.56,62 In an effort to develop a more comprehensive risk assessment model for lung cancer, Maisonneuve et al.58 investigated another strategy that combines both epidemiological and clinical risk factors. By using data from the COSMOS trial to recalibrate the Bach model and then incorporating low-dose CT (LD-CT) findings at baseline screening, these investigators have identified a potential model that assists with determination of the time interval to subsequent screening for persons in a high-risk lung cancer population. Furthermore, this model appears to stratify individuals according to their risk of lung cancer diagnosis at repeat screening scans. Although these results exhibit the potential to support large-scale screening programs, validation of these results remains to be proved.
With the more recent data concerning risk of cancer, both biological and genetic, lung cancer risk models are currently heading toward an integrative strategy incorporating epidemiological risk factors with clinical risk factors.63,64 Ultimately, by adding to this bilateral approach the additional aspects of molecular epidemiology (e.g., SNPs and genome-wide studies) and both lung and nonlung biomarker assessments, lung cancer risk assessment will take its next step.63
Prevention
Lung cancer primary prevention strategies include smoking avoidance and cessation and eliminating exposure to other toxins, such as asbestos and radon. In support of this, alternative nicotine replacement therapy (NRT) approaches have been developed, including the nicotine patch, nicotine gum, and nicotine inhalers. These therapies represent the most common form of pharmacotherapy currently in use65 and have been shown to increase smoking cessation by 50% to 70%.66 In addition, studies are currently in progress to investigate smoking cessation after administration of nicotine vaccines (e.g., NicVAX and NIC002), which reduce the amount of nicotine that reaches the brain.
First- and second-line pharmacologic treatments now provide additional strategies for smoking cessation. These alternative agents include antidepressants, such as the norepinephrine and dopamine reuptake inhibitors bupropion (e.g., Wellbutrin and Zyban) and nortriptyline (e.g., Aventyl and Pamelor), as well as the second-line pharmacotherapy alpha-agonist hypotensive agent clonidine (e.g., Catapres, Dixarit, and Kapvay).67 In 1997, bupropion hydrochloride (Zyban), an antidepressant not associated with sexual dysfunction,68–75 was the first nonnicotine pharmacotherapy to be approved by the FDA as a smoking cessation drug.65 Many studies have since reported on the effectiveness (doubled the odds of smoking cessation versus placebo) and safety of bupropion.76,77
However, the nicotine receptor partial agonist varenicline (Chantix) has been shown to be more effective for smoking cessation than both NRTs and nicotine agonists78,79 and three times more effective than placebo when administered with an established fixed quit date.82–82 In a phase III clinical trial, treatment with varenicline tartrate (1 mg twice a day) resulted in 43.9% participant abstinence (n = 344) compared with 29.8% abstinence (n = 342) with bupropion (Zyban, 150 mg twice a day) and 17.5% abstinence with placebo (n = 341).78 This increased efficacy with varenicline has been demonstrated in other clinical trials as well, such as the study by Rennard et al.82 Varenicline is potentially the most effective smoking cessation pharmacotherapy to date. As a result, studies have been and continue to be conducted by Gritz and others to maximize the positive translation of this benefit through physician- and pharmacist-based training and counseling into routine practice.84–84 In 2008, an updated guideline report was issued by the U.S. Public Health Service providing specific recommendations for brief and intensive tobacco cessation clinical interventions, as well as system-level changes designed to promote the assessment and treatment of tobacco dependence.85
Chemoprevention
Vitamins and Minerals
Potential use of the carotenoid beta-carotene as an effective chemopreventive strategy for lung cancer was investigated in two randomized trials, the Alpha-Tocopherol, Beta-Carotene Cancer (ATBC) trial and CARET. Unfortunately, results of these studies showed that beta-carotene significantly increased risk of lung cancer in current smokers rather than decreasing it.86,87 In a secondary prevention setting of 1166 patients with stage I non–small cell lung cancer (SCLC), the Lung Intergroup Trial demonstrated no reduction in rates of SPTs, recurrences, or mortality in persons treated with isotretinoin and further identified, in both secondary subset analyses and a 6.2-year follow-up, increased overall and cancer-related mortality in current smokers receiving isotretinoin.88,89
Clark and colleagues90 conducted a phase III selenium skin cancer prevention study with secondary end points of other cancers. Although they identified no reduction in skin cancer associated with selenium treatment, secondary analyses suggested a decrease in both prostate and lung cancer. This information led to the development of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) for the prevention of prostate cancer. A secondary end point was incidence of lung cancer.91 Contrary to the findings by Clark et al., the results of this study identified no decrease in prostate or lung cancer associated with selenium treatment.92 Another phase III trial testing selenium for lung cancer prevention was conducted through the cooperative groups. Results from this trial did not identify a reduction in lung cancer recurrence or in second primary lung cancer associated with selenium supplements.93
Other Novel Agents
Many drugs have been shown to prevent lung cancer in preclinical studies.94 To overcome the difficulties associated with inhalation of tobacco smoke in animal models, Witschi95 has described a reproducible tobacco smoke carcinogenesis model using strain A/J mice, which develop lung tumors after exposure to suspended tobacco smoke particulates, and identifies the preventive potential of a mixture of myo-inositol and dexamethasone. Among the agents that have been tested in animal models are indole-3-carbinol,98–98 Kava,99 8-methyoxypsoralen,102–102 myo-inositol,103–106 PEITC,107–113 Polyphenon E,114–122 rapamycin,121 and silibinin.125–125
After preclinical studies, a phase I clinical study was conducted to establish the chemopreventive potential, maximum tolerated dose, and toxicity of myo-inositol in smokers with bronchial dysplasia.126 Results from this dose escalation (12-30 g/day) study showed that 3-month treatment with myo-inositol increased regression of preexisting dysplastic lesions by more than 40% compared with placebo. In addition, myo-inositol (18 g/day) was found to be safe and well tolerated.
Veronesi et al.127 layered a phase IIb clinical trial of inhaled budesonide within a phase III LD-CT screening trial currently underway at the European Institute of Oncology. Participants of the phase II chemoprevention trial included 202 of the 5203 current and former smokers who had been recruited for the phase III screening trial. Results recently published demonstrated that budesonide had no significant effect on reduction of lung nodule size compared with placebo (2% versus 1% effect, respectively). However, both nonsolid and partially solid nodules (the types most likely to represent adenocarcinoma precursors) exhibited reduction in size, suggesting the potential for effective treatment within this defined patient population.
Another promising chemopreventive agent for lung cancer is iloprost, a prostaglandin analogue often used to treat current and former smokers experiencing pulmonary hypertension. Results reported recently from a 6-year multicenter phase II clinical trial conducted by Keith et al.128 demonstrated significant improvement of endobronchial histology in former smokers treated with iloprost. Treatment was dose-escalated from 50 mg/day to 150 mg/day iloprost clathrate within the first 2 months, then maintained at 150 mg/day for the duration of treatment. Participants underwent two bronchoscopies, one before randomization and one after the end of treatment. The primary end point of the study was the difference between before treatment and after treatment bronchoscopy average histologic scores. Keith et al.128 identified significant histologic improvement in former smokers (58.6%) compared with placebo (28.6%), and treatment was reasonably well tolerated. This improvement was not observed within current smokers, suggesting a need to conduct individual studies for these two patient populations. However, because pulmonary hypertension is common among patients with chronic obstructive pulmonary disease, treatment of persons who have chronic obstructive pulmonary disease with iloprost could have a twofold benefit by decreasing the degree of pulmonary hypertension and reducing the risk of lung cancer. Based on the results of this study, a large phase III clinical trial is currently in development.
Other potential preventive agents for lung cancer currently supported for use by the Division of Cancer Prevention at the NCI include epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (e.g., erlotinib and gefitinib), polyphenols (e.g., green tea catechins and grape seed polyphenols), retinoids (e.g., all-trans-retinoic acid tretinoin, 9-cis-retinoic acid alitretinoin, 13-cis-retinoic acid isotretinoin, 4-hydroxy(phenyl)retinamide, and (4-HPR)-fenretinide), prostacyclin agonists, selenium compounds (e.g., selenized yeast, selenomethionine, and selenomethylsysteine), and antioxidants containing sulfur (e.g., anethole trithione).129 In addition, the NCI is currently funding a phase II biomarker prevention study targeting the effects of phenethyl isothiocyanate in the prevention of SCLC and non-SCLC.130
Lung carcinogenesis can encompass 20 to 30 years of a person’s life.131 Therefore effective strategies focused on prevention rather than on the treatment of established lung cancer have become the focus of many studies. Primary prevention methods, such as smoking avoidance and cessation, in combination with effective chemoprevention, particularly within high-risk populations, could provide the means to significantly decrease lung cancer development in the future.131
Screening
Screening trials for lung cancer were initiated in the 1970s and were based on chest radiography and sputum cytology. However, given the lack of evidence that current screening methods, including chest radiographs and sputum cytology, are associated with any significant decrease in lung cancer mortality, an effective strategy for lung screening has yet to be identified.132,133 Furthermore, these methods produce a significant number of false-positive tests, resulting in unnecessary invasive diagnostic treatments and procedures.
With improved prognosis directly dependent on early detection, new strategies targeting early detection of lung cancer remain critical. Other potential screening methods are therefore being developed, including LD-CT scans, which have recently been reported to be associated with improved survival rates compared with standard chest radiographs.40 Likewise, positron emission tomography scans provide an alternative method of early detection, although they are expensive and are associated with significant radiation exposure. Ultimately, future strategies targeting the prevention of lung cancer must emphasize primary prevention, secondary prevention, and prevention of SPTs. A summary of lung cancer screening trials is provided in Table 23-7.
Table 23-7
Select Lung Cancer Screening Studies
| Trial | Patient Population | Study Design: Screen vs. Control | Results | Reference |
| PLCO | 154,901 men and women from 10 U.S. sites; 55-74 yr of age | Screen: standard chest radiograph Control: usual care |
7-yr and 13-yr follow-ups showed no reduction in mortality | 133 |
| NLST | 53,454 men and women from 33 U.S. sites; 50-74 yr of age | Screen: 3 annual LD-CT screenings Control: 3 annual vs. chest radiography screenings |
Preliminary results identified a 20% reduction in mortality, resulting in early termination of the study | 40; 135 |
| DANTE | 3206 men from Milan, Bergamo, and Catania, Italy; 60-75 yr of age | Screen: 5 annual LD-CT screenings Control: baseline chest x-ray followed by usual care |
3-yr results show minimal reduction (1.9%) in lung cancer incidence and no reduction in lung cancer mortality | 137 |
| ItaLung | 3206 men from Tuscany, Italy; 55-69 yr of age | Screen: 4 annual LD-CT screenings Control: usual care |
Pending | 141 |
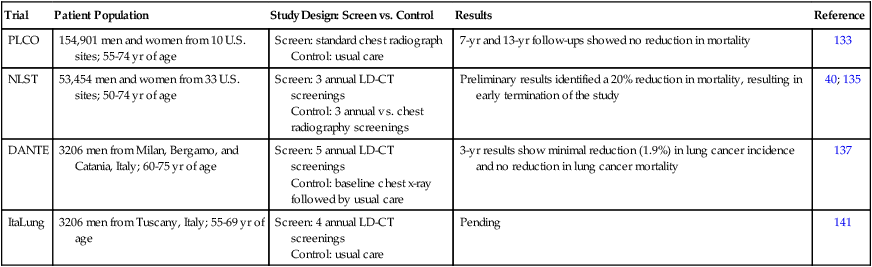
The Prostate, Lung, Colorectal and Ovarian133 cancer screening trial enrolled 154,901 men and women (55 to 74 years of age) at 10 screening centers within the United States from 1993 to 2001.134 With regard to lung cancer, participants received either annual screening with a chest radiograph (n = 77,445) or usual medical care (n = 77,456) and were followed up for lung cancer mortality as a primary end point. In 2011, the 13-year follow-up report identified no reduction in lung cancer mortality associated with annual chest radiograph screening compared with usual care.133
The National Lung Screening Trial compared the effectiveness of LD-CT versus standard chest radiography in the early detection of lung cancer, also with a primary end point of lung cancer mortality. From 2002 to 2004, 53,454 participants were accrued from 33 centers across the United States.40 Participant recruitment was limited to high-risk patients (persons 50 to 74 years of age with extensive smoking histories [30 pack-years or more]) who quit smoking within the last 15 years and have received LD-CT. Three screenings were conducted, one at baseline and two at annual follow-up examinations, and quality of life and cost-effectiveness data were collected; study follow-up extended through 2009. Recently published results identified a 20% reduction in lung cancer mortality in high-risk patients, who also showed a 7% lower overall mortality rate.40 These findings arguably represent the most significant and impactful result in lung cancer management to date, other than tobacco prevention and cessation. However, the percentage of false-positive screens identified in the study was relatively high in comparison with other screening analyses.135 Specifically, of the 75,126 total CT screens, 18,146 (24.2%) tested positively, whereas only 649 (0.9%) led to diagnoses of lung cancer, representing a false-positive screening rate of 23.3%.40 The percentage of false-positive results was lower but remained significant for chest radiograph screening with a 6.5% false-positive rate.
The Detection and Screening of Early Lung Cancer trial is also currently underway in Italy.136 This trial enrolled 3206 male smokers, with the screening group receiving five annual LD-CT screenings and the control group receiving only baseline chest radiographs followed by usual care for the duration of the study. The 3-year results identified a nominal reduction in lung cancer incidence (1.9%) in participants receiving annual LD-CT screening versus the control group, and no reduction (0.1%) in lung cancer death.137,138
The ItaLung trial, part of the European Union–United States Collaborative Spiral CT working group,139 accrued 3206 smokers or former smokers who were 55 to 69 years of age for a study investigating the efficacy of LD-CT in reducing lung cancer mortality.140 Participants were randomly assigned to receiving LD-CT annually for 4 years (n = 1613) or to receiving usual care only with no screening (n = 1593). Results from the study are currently pending, but baseline screening was comparable with that observed with other studies.141
Head and Neck Cancers
Head and neck squamous cell carcinomas (HNSCCs) account for approximately 3% of all cancers in the United States (40,000 new diagnoses are estimated for 2012142), are the sixth most common cancers worldwide, and appear at rates twice as high in men as in women. American incidence rates are highest in non-Hispanic white persons, but mortality rates are highest in the African American population.143 Although the incidence and mortality rates of head and neck cancers have been decreasing during the past two decades, the 5-year survival rate for advanced oral cancers remains just under 30% (accounting for an estimated 7500 deaths within the United States in 2012142). Unfortunately, two thirds of HNSCCs are diagnosed at an advanced stage (see Chapter 68).
Risk Factors/Etiology
Anatomic sites of head and neck cancers include the nasal and oral cavities, sinuses, salivary glands, tongue, throat, and larynx. Because of the mucosal epithelium that is common to much of this region, malignancies in these organs are particularly vulnerable to tobacco-mediated malignant transformation144,145; synergistic effects on carcinogenesis can be seen when alcohol is consumed concomitantly, especially in persons with particular polymorphisms of the alcohol dehydrogenase gene.148–148 In fact, combined heavy use of both tobacco products and alcohol has been shown to increase risk in excess of thirtyfold.146 In addition to these two major HNSCC risk factors, HPV has become an additional well-established etiologic factor, with more than 50% of oropharyngeal tumors and just under 100% of laryngeal tumors testing HPV positive.149,150 Currently, incidence rates are increasing for U.S. non-Hispanic white persons, in both men and women. In addition to HPV, the EBV has been implicated in HNSCCs and nasopharyngeal carcinomas (NPCs).151–154 Although several models have been suggested, the specific course of events in the path from EBV infection to EBV-associated NPCs and HNSCCs remains unclear.157–157 However, a recent study by Groma and colleagues identifies CD44-mediated signaling (important in cell-to-cell interactions, cell adhesion and migration) as a potentially critical mechanism in these malignancies158 by enabling efficient transfer of EBV infection to normal and premalignant cells.159 Prognosis for patients with NPCs appears to be significantly and negatively associated with increased CD44 expression and later stages of tumor development at diagnosis.162–162
Risk Modeling/Assessment
To date, a major component of HNSCC risk assessment includes assessment of (1) tobacco use, (2) alcohol use, (3) HPV status, and (4) a history of head and neck or lung cancer. However, the trifold incorporation of epidemiological, clinical, and genetic data in recent and ongoing studies is enabling future HNSCC risk modeling to shift toward a higher-benefit personalized chemoprevention capable of more effectively addressing the needs of high-risk persons. As an example of such a study, Hildebrandt et al.163 recently examined 137 (SNPs across 20 components of the P13K/PTEN/AKT/mTOR pathway, of which 22 demonstrated an association with increased risk of SPT/recurrence, with a gene-dosage effect resulting from combined analysis of these SNPs.163 These results identify the relevance of genetic variations within the P13K/PTEN/AKT/mTOR pathway, and specifically of these high-risk loci, in predicting high-risk patients. Incorporation of these high-risk loci into an HNSCC risk model could provide a more effective and personalized chemopreventive strategy in the future. A second study has demonstrated that patients with squamous cell carcinoma (SCC) of the head and neck had significantly lower mean DNA repair capacity (DRC) for removing tobacco-induced DNA adducts compared with control subjects, although no association was found for DRC with tumor characteristics.164 DRC significantly improved the sensitivity of the risk prediction model in ROC analysis.164 These results suggest that DRC is an independent susceptibility biomarker for SCC of the head and neck risk.
Prevention
Among the primary preventive interventions for head and neck cancer are smoking cessation programs promoted by physicians (e.g., nicotine replacement products, bupropion, and varenicline); smokeless tobacco cessation programs (targeting oral cancers); and awareness/intervention-based programs targeting increased risk of cancers that are alcohol-related (head and neck). Vaccines developed to induce binding of nicotine within the blood, thereby preventing transportation across the blood-brain barrier and blocking access to the nicotine receptor,165,166 may become routine for the immunologic prevention of head and neck cancers in the future. Initial studies of the nicotine vaccine as a strategy for smoking cessation have been clinically promising and showed immunotherapy to be well tolerated (e.g., NicVAX developed by Nabi Pharmaceuticals, Rockville, MD; Nicotine-Q-beta (NIB002) by Cytos AG [Cytos Biotechnology/Novartis], Schlieren, Switzerland167; and TA-NIC by Celtic Pharma168). However, trial design problems in more than one study (e.g., assessment of primary end point months after loss of antinicotine antibody therapeutic levels) have resulted in repeated failure to increase abstinence within vaccinated populations.167,169–171 Other nicotine immunotherapy trials still underway include the phase II TA-NIC study by Celtic Pharma, which is complete and awaiting the release of clinical results.171,172 The NCI is currently supporting three primary prevention clinical trials of both head and neck and lung cancers that target smoking-associated malignancies and are studying such strategies as NRT (e.g., varenicline and the nicotine patch) and preventive measures for environmental tobacco smoke exposure.130
Viral-based prevention of head and neck cancer includes HPV-related cancers (oropharyngeal, non–tobacco-related) and EBV-related cancers. Both triplet-dose HPV vaccines licensed by the FDA for use in women (Cervarix, produced by GlaxoSmithKline, Brentford, Middlesex, United Kingdom, and Gardasil, produced by Merck & Co., Inc., Whitehouse Station, NJ) protect against HPV types 16 and 18, prevent cervical cancers, and are well tolerated.173 Gardasil has the additional benefits of protecting against HPV types 6 and 11, preventing cancers of the vulva, vagina, and anus, and has been approved by the FDA for use in males.173 Vaccine-based prevention targeting EBV at present is focused on testing for CD44v3 and/or EBV DNA (nasopharyngeal cancers). However, the EBV vaccine (gp350), although currently unavailable, has been studied in multiple phases I and I/II clinical trials, resulting in mixed effects on the gp350-specific immune response, and has been recommended for use in a phase III trial.174
Chemoprevention
Chemopreventive efforts in head and neck cancers have focused on retinoids. Waun Ki Hong and co-investigators were the first to demonstrate the relevance of chemoprevention in the prevention/delay of cancer. Dr. Hong’s research demonstrated that short-term high-dose treatment with 13-cis-retinoic acid (13-cRA) prevents second primary cancers in persons with previous head and neck cancer.175 However, the toxic effects with high-dose 13-cRA were significant, resulting in dose reduction in 70% of the study population and discontinuation of treatment for 25% of the patients. Consequently, a low-dose phase-III 13-cRA (isotretinoin) study was initiated, the Retinoid Head and Neck Second Primary (HNSP) Trial.176 In 2006, after 3 years of treatment with 30 mg/day of low-dose isotretinoin and an additional 4 years of follow-up, the HNSP reported no significant difference in SPT/recurrence among patients with stages I and II HNSCC who were treated with low-dose 13-cRA compared with placebo.177 However, subsequent exploratory, post-hoc pharmacogenetic analysis in 450 HNSP trial participants has identified 13 loci that represent the majority of patients who both responded favorably to 13-cRA and were at high-risk of SPT/recurrence.178 Persons with common retinoid X receptor SNPs were found to have a 76% reduction in SPT/recurrence after 13-cRA treatment.178 Such work suggests that a pharmacogenetics-based approach to 13-cRA chemoprevention might improve its therapeutic index.
Among the agents currently being tested in head and neck chemoprevention trials are peroxisome proliferator activated receptor gamma agonists (pioglitazone and rosiglitazone) and retinoids (all-trans-retinoic acid, 9-cis-retinoic acid, and 13-cis-retinoic acid). Numerous additional clinical trials focused on the prevention of head and neck cancers are currently supported by the NCI. Among the non–smoking-related prevention-based trials currently active are studies examining the effects of the EGFR inhibitor erlotinib hydrochloride alone and in combination with Polyphenon E derived from green tea extract, EGFR and cyclooxygenase-2 (COX-2) inhibitor combined therapy, a pan-COX inhibitor sulindac therapy, and the vascular endothelial growth factor receptor antagonist vandetanib.179 Conversely, several dietary/supplement-based trials are targeting, among other things, the soy protein extract Bowman-Birk Inhibitor Concentrate, lyophilized black raspberries, and polyphenols obtained from strawberries, which have been shown to decrease risk of gum disease.179
Screening
Initial screening for oropharyngeal cancers is generally through patient observation or physical examination by the dentist and/or physician. Because prognosis of these cancers is directly related to tumor stage at diagnosis, high-risk patients (e.g., smokers, smokeless tobacco users, heavy alcohol consumers, sexually active individuals, and EBV-positive individuals) should receive routine, thorough inspections of the oral cavity for HNSCCs and NPCs.180 Furthermore, upon identification, biopsy specimens should be analyzed for lesions at all stages (preneoplastic lesions, leukoplakia, and erythroplakia) to determine relative prognosis.
Premalignant alterations can now also be detected through autofluorescent imaging techniques. By enabling detection at earlier stages of tumor development than that associated with visual detection of these cancers, improved prognosis would be expected for these persons. Optical diagnostic methods enable minimally invasive in vivo or ex vitro differentiation of clinically similar characteristics.181 Among these strategies are confocal spectroscopy, deferential path-length spectroscopy, elastic scattering spectroscopy, fluorescence imaging, microendoscopy, near-infrared spectroscopy, optical coherence tomography, and Raman spectroscopy.181 Recently, EBV oncoproteins have been detected in both premalignant and malignant oropharyngeal ex vivo tissues by polymerase chain reaction.182–186 In addition, plasma EBV DNA detection is now poised as one of the most promising molecular markers for nasopharyngeal malignancies.187
HPV screening has been incorporated into the battery of screening strategies for head and neck cancer for high-risk patient populations. An international effort initiated by Rebecca Richards-Kortem and Ann Gillenwater at Rice University and MD Anderson Cancer Center, respectively, with the Tata Memorial Hospital, Mambai, India, is evaluating novel devices for use in screening for oral cancer.188 In addition, Dr. Richards-Kortem has developed and is testing the efficacy of optically active contrast agents and molecular imaging systems capable of supporting the early detection of cancer.189 Their research has already identified a tenfold enhancement in normal versus precancerous image contrast associated with these molecular-specific contrast agents compared with the fluorescent dyes currently in use. The development of high-resolution imaging has led to its incorporation into clinical trials, enabling the imaging of architectural, morphologic, and targeted biomarkers.
Esophageal Cancer
Esophageal carcinoma occurs as two predominant histopathological types: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Through the 1970s, SCC predominated in the United States, as it still does in most of the developing world.190 However, after this point, adenocarcinoma incidence rates rose sharply in the United States and many other Western countries, and it now accounts for at least half of all cases. Although treated similarly, the two diseases are distinguished by their histopathologies, patterns of occurrence, and primary risk factors.
Risk Factors/Etiology
In most parts of the world, ESCC occurs more frequently in men than women by 2 to 3 : 1. Its global distribution is also markedly heterogenous, occurring at very high rates in certain regions of China, Iran, and Africa, regardless of gender. Risk factors for ESCC include tobacco smoking, excessive alcohol consumption, HPV exposure (often arising in association with sexual contact), dietary deficiencies such as low intakes of fruits and vegetables, achalasia (an esophageal motility disorder), various foodborne fungi and molds, and low socioeconomic status.191 ESCC typically arises through a series of precursor dysplastic lesions associated with threefold (mild dysplasia) to thirtyfold (severe dysplasia) risk for progression to ESCC.192
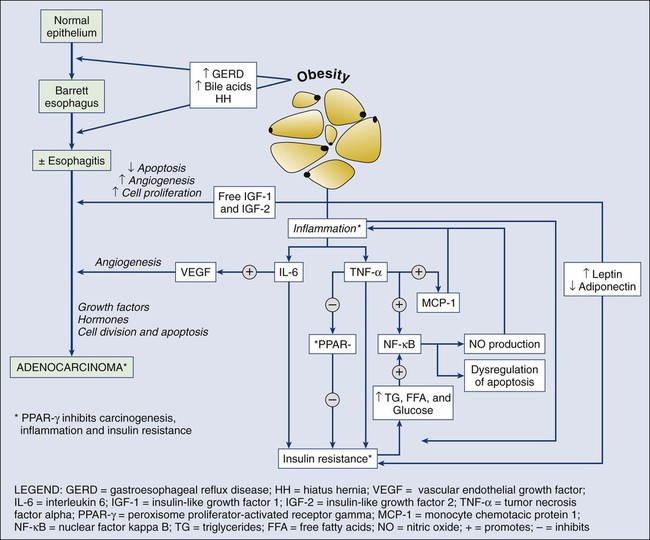
EAC has the fastest rising incidence of all cancers in Western countries,193 which directs attention to its neoplastic precursor, Barrett esophagus (BE). BE arises from a process of intestinal metaplasia, in which columnar epithelium replaces normal squamous epithelium of the lower esophagus. EAC typically occurs in the distal esophagus, occurs more frequently in white men, and is often associated with a pathogenesis involving abdominal obesity, which predisposes to chronic gastroesophageal reflux disease (GERD) by increasing intraabdominal pressure. GERD increases the risk of developing BE. Estimated conversion rates of BE into EAC are approximately 0.27% to 0.5% per annum. Although in most persons with BE the condition does not progress to cancer, there is no accurate way to determine in which patients BE will progress. Moreover, the vast majority of patients with cancer do not have a prior diagnosis of BE.
Obesity’s role in driving esophageal neoplastic progression is probably multifold (Fig. 23-6). It may promote overt reflux of acid and bile, directly damaging the esophageal mucosa and causing local inflammation, but it may also alter metabolic profiles driving cell cycle and genetic abnormalities through systemic inflammation, insulin resistance, and adipokine receptor expression.194 Thus risk factors for EAC include obesity with central adiposity, GERD, and BE, as well as tobacco smoking, achalasia, and the absence of H. pylori in the stomach.191
Risk Modeling/Assessment
Risk models could be useful at several stages of clinical management in persons at risk for esophageal cancer. With regard to either histopathology, risk models could help identify high-risk persons who should undergo endoscopic screening to identify either precursor lesions or cancer and to assess the risk of progression to invasive cancer from any intermediate neoplastic stage (e.g., BE, squamous cell, or BE-related dysplasia).195 Current health economic data do not support broad, population-based endoscopic screening,196 but no specific risk model exists for ESCC or EAC. A model was recently developed to assist in identifying patients with GERD who should be referred for endoscopic screening to identify BE.197 The final risk model was well calibrated and included terms for age, sex, smoking status, BMI, highest level of education, and frequency of acid suppressant medications and achieved moderate discrimination in an external dataset (area under the ROC curve = 0.61, 95% CI = 0.56-0.66). It has the potential to be an effective and useful clinical tool to identify persons who should be referred for endoscopic screening. Additionally, a group has developed a nonendoscopic cytosponge used to collect cells for immunohistochemistry for trefoil factor 3 as a screening modality. It is undergoing further evaluation in studies in Australia and the United Kingdom (UK) now.198 The best marker of neoplastic progression in the context of patients with BE remains histopathological dysplasia. Limitations of its usefulness relate to challenges in obtaining wholly representative mucosal sampling in the context of highly heterogeneous clonal evolution199 and the substantial inter- and intraobserver variability in assessing and grading dysplasia.200,201
Prevention
ESCC
Most ESCC prevention trials have focused on high-risk populations, such as persons in Linxian, China, a region with some of the highest rates of ESCC in the world. These randomized controlled trials (RCTs) have focused on various vitamins, minerals, and/or herbs and results are generally encouraging; however, none of the approaches has translated into established or recommended regimens at this point.202–208
A particularly large phase III trial involving a complex fractional factorial design administering four different vitamin/mineral combinations at doses one to two times the U.S. recommended dietary allowance for 5 years to nearly 30,000 participants revealed that a combination of selenium, beta-carotene, and vitamin E supplements resulted in a statistically significant 13% reduction in total cancer deaths, a significant 21% reduction in gastric cancer deaths, and a 4% nonsignificant reduction in esophageal cancer deaths, compared with participants not receiving this combination.209 More recently, a placebo-controlled 2×2 factorial RCT of selenomethionine (200 mcg per day) and celecoxib (200 mg twice a day) was conducted in patients with mild or moderate squamous dysplasia in Linxian. Whereas celecoxib exhibited no effect, selenomethionine improved mild, but not moderate, dysplasia.210 These results suggest a modest role for such interventions against ESCC in high-risk populations.
In sum, these studies reveal the preventive potential of various vitamin-mineral combinations in high-risk, potentially nutritionally compromised populations, but because of interpretive complexities related to the agents, populations, or subsets most likely to benefit and potential ambiguities or conflicts in weighing various end points, they have yet to be translated into recommended clinical regimens. Agents demonstrating promise in in vivo models of ESCC but that have not yet been tested clinically include ellagic acid, diallyl sulfide, various tea-related theaflavins, curcumin, resveratrol, irinotecan, various isothiocyanates, and COX inhibitors.211
EAC
A key limitation in identifying and developing chemopreventive strategies for EAC has been the lack of a convincing animal model of the disease that could be used to explore the efficacy of potential agents. Therefore mechanistic targets and empiric human observational data have guided most human studies. Given the prominent role of GERD and chronic inflammation in the pathogenesis of EAC, it is logical that proliferation, Mcm2, nuclear factor-κB, cytosolic phospholipase, and COX-2 have been proposed as the most compelling preventive targets for EAC, and acid suppressives, antioxidants, and antiinflammatory agents have been proposed as the most promising chemopreventive agents.212
Proton pump inhibitors provide symptomatic relief of acid reflux, but their efficacy in regressing BE or preventing cancer remains unproven.213,214 Observational studies suggest reduced risks of dysplasia or adenocarcinoma,215,216 and long-term observational studies suggest that proton pump inhibitors are very safe,217 but RCTs are required to confirm the patient population and specific regimens (e.g., agent, dose, and duration) needed to prevent EAC.
With regard to nonsteroidal anti-inflammatory drugs (NSAIDs), in vitro studies and a small early-phase trial of a COX-2 inhibitor suggest that they may reduce proliferation or induce apoptosis in EAC and its precursors,218,219 and two metaanalyses of a large body of observational work suggest that aspirin or other NSAIDs significantly reduce EAC by as much as 30% to 40%.220,221 An RCT of celecoxib, 200 mg twice daily, versus placebo in 100 patients with Barrett dysplasia found no difference in dysplasia regression from baseline but a reduction in the total Barrett surface area at 1 year.222,223 The Aspirin Esomeprazole Chemoprevention Trial is a phase III multicenter, randomized, 2×2 factorial, open-label, controlled trial evaluating whether esomeprazole at two doses (20 vs. 80 mg/day) with or without aspirin (300 mg/day) can reduce all-cause mortality or EAC or high-grade dysplasia.224 As of 2009, more than 85% of randomized patients continued to take their medication without dose changes.225 Interim results are anticipated in 2013. To date, aspirin (or NSAIDs) seem to be effective in reducing risk of EAC, but the optimal dosing regimen and duration are unclear.
Observational studies suggest that diets rich in fruits and vegetables may reduce EAC risk by 40% to 50%,226 but dietary adjustments have not been rigorously tested in trials. Retinoid supplements227 telomerase inhibitors,228 and antioxidants229 have been suggested as promising in in vitro studies, but none has been tested in human trials. Ongoing or recent trials not yet reporting results are evaluating esomeprazole, difluoromethylornithine (DFMO), ursodeoxycholic acid, Polyphenon E, erlotinib, and sorafenib.230
Another strategy to reduce cancer risks of Barrett-associated dysplasia involves ablative therapies through photodynamic therapy or radiofrequency ablation. For example, in 2003 the FDA approved a photosensitizing porphyrin mixture (Photofrin) in conjunction with photodynamic therapy (PDT) (Axcan Pharma Inc., Quebec, Canada) to treat high-grade dysplasia in patients with BE who cannot or choose not to undergo esophagectomy. The FDA approval of Photofrin-based PDT provided an important therapeutic option for patients with high-grade esophageal dysplasia, but more recently, a competing ablative strategy for BE or dysplasia has gained favor because of its improved efficacy and safety results. HALO radiofrequency ablation (RFA) uses either a balloon-based catheter or an electrode-tipped gastroscope with an energy generator for either circumferential or focal ablations. Studies have demonstrated its efficacy in patients with nondysplastic BE, as well as in persons with low- and high-grade dysplasia.233–233 A recent multicenter, sham-controlled, randomized trial involving 127 patients with Barrett dysplasia showed complete remission in 90.5% versus 22.7% among persons with low-grade disease and complete remission of 81% versus 19% in the high-grade disease group.234 Additionally, treated patients had significantly greater complete remission of intestinal metaplasia (77.4% vs. 2.3%), less disease progression (3.6% vs. 16.3%), and fewer cancers (1.2% vs. 9.3%) compared with control subjects. RFA is also associated with few complications and greater patient acceptance, making RFA with endoscopic mucosal resection a current endoscopic standard of care.235
Screening
Both esophageal cancer types are associated with dysplastic precursors identifiable by cytologic or histopathological analysis. However, because no screening strategy has been rigorously tested through carefully conducted RCTs confirming safety, effectiveness, or cost-effectiveness, none is currently recommended in Western populations.236
Regular endoscopic “surveillance” for EAC in patients with BE is recommended, but this recommendation is due to differences in 5-year survival rates of persons identified with early stage versus metastatic cancer, rather than data from RCTs suggesting tangible clinical benefits. Therefore lead time and length biases may be operative as well.237,238 Currently the American College of Gastroenterology recommends two endoscopies with biopsy within 1 year, followed by endoscopy every 3 years.239 The Seattle biopsy protocol, which involves four-quadrant biopsies at 1-cm intervals using jumbo forceps and sampling of any mucosal abnormality, is recommended as well.240 Confirmation of dysplasia requires at least two expert pathologists, and if dysplasia is diagnosed, repeat endoscopy with biopsy occurs after 6 to 12 months in persons with low-grade dysplasia, followed by yearly endoscopy in the absence of progression. High-grade dysplasia management is controversial, with some persons advocating serial surveillance and others recommending esophagectomy. Decision making is individualized based on age, comorbidities, and informed preferences. The U.S. American Gastroenterological Association has offered surveillance and management guidelines for BE as well.238 Standard white-light endoscopy has a sensitivity of ~82% for BE,236 but visualization of dysplasia is unreliable, and thus extensive biopsies are required in current guidelines. Endoscopy with biopsy is the gold standard for BE with or without dysplasia. However, adherence to national surveillance guidelines averages only 51% in recent studies, and thus the potential exists for many cases of missed progressive neoplasia.241
A variety of newer imaging techniques are under development to improve the quality of surveillance for progressive neoplasia or dysplasia in at-risk populations.236 High-definition endoscopy with chromoendoscopy using vital stains boosts sensitivity and specificity to 95% and 97%, respectively,242 and may help to direct endoscopic biopsies to mucosal areas of particular concern.243 Narrow-band imaging (NBI) offers another well-studied approach to dysplasia detection in BE. NBI uses interference filters to illuminate esophageal mucosa with narrow bands of light, allowing visualization of glandular structures and vascular architecture.244 A recent meta-analysis of NBI estimated NBI’s overall sensitivity and specificity for high-grade dysplasia to be 96% and 94%, respectively.245 Other imaging technologies under development for surveillance include confocal endomicroscopy, which may help target biopsies more specifically and successfully, and endoscopic ultrasound, which can help distinguish high-grade dysplasia from cancer246 and provides more accurate presurgical staging of Barrett-associated cancers.247
Colorectal Cancer
In the United States, colorectal cancer (CRC) is the third most commonly diagnosed cancer and the third most common cause of cancer death within each gender.248 Incidence and mortality rates are highest among African Americans and lowest among Asian Americans, although incidence and mortality rates have been declining among all ethnic groups for both men and women during the past 10 to 20 years248 (see Chapter 77).
Risk Factors/Etiology
Risk factors for CRC include both modifiable lifestyle factors and nonmodifiable genetic factors. Modifiable risk factors are obesity, smoking, alcohol consumption, and red or processed meat consumption. The nonmodifiable risk factors include a personal or family history of colorectal cancer or adenomatous polyps, including the inherited conditions of familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HNPCC), and a personal history of chronic inflammatory bowel disease. Ecological studies demonstrate substantial variation in incidence rates by country and world region, and migration studies indicate that migrant populations generally assume the risk for CRC of their adopted country within one to two generations.249 The presence of adenomas upon screening is a strong predictor of CRC risk.
Risk Modeling/Assessment
At least two models have been developed to assess risks of colorectal adenomas or cancer, drawing from the risk factors previously outlined. The Cleveland Clinic “Score Against Colon Cancer” risk model is available online. The Freedman risk model is based on similar variables.250
Prevention
Chemoprevention efforts can also affect CRC incidence and mortality in conjunction with an effective screening program. Adenomas are the accepted intermediate measure generally used to assess the efficacy of potential agents. The use of adenomas as end points in chemopreventive trials is supported by multiple lines of evidence, including epidemiological, preclinical, and molecular data.251–255 Adenomas also have a long history of being used as end points in chemopreventive agent efficacy trials, and they were the focus of the U.S. FDA’s preliminary approval of celecoxib in FAP256 before celecoxib’s labeled indication was sacrificed in early 2011.
Results from epidemiological studies on the effects of selenium in CRC risk or adenoma formation have been mixed.257–260 A recent nested case-control study within the Women’s Health Initiative (WHI) found no protective effect of selenium on CRC among women.261 An RCT by Clark et al.90 tested selenium supplementation as a secondary end point in persons with a history of skin cancer and suggested a 58% reduction in colorectal cancer incidence. A more recent report by Lippman et al.92 from the SELECT trial did not find any significant difference in CRC incidence among men taking either oral selenium, vitamin E, or both after approximately 7 years of follow-up. Additional RCTs are ongoing (clinicaltrials.gov identifiers: NCT00078897, NCT00706121, and NCT01211561).
In addition to significant biological plausibility for the role of calcium in the prevention of CRC,264–264 data from observational studies are consistent, suggesting an inverse association between calcium intake and recurrent polyps265,266 or CRC risk.267–270 Two RCTs of calcium supplements conducted in persons with a history of adenomas demonstrated an approximately 20% to 30% reduction in adenoma recurrence.271,272 A recent RCT examined calcium plus vitamin D on the risk of CRC in postmenopausal women within the WHI and concluded that after a follow-up period of 7 years, daily supplementation of calcium with vitamin D did not have an effect on CRC incidence among postmenopausal women.273 However, the follow-up period of this trial may not have been long enough to observe an effect. Follow-up of participants within this trial is still ongoing. The Vitamin D/Calcium Polyp Prevention Study is another ongoing trial that will examine the effects of calcium and vitamin D on adenoma recurrence (clincaltrials.gov identifier: NCT00153816 ).
Observational data supporting the use of postmenopausal HRT to reduce the risk of CRC and adenoma recurrence is extensive and largely consistent,274–277 although the WHI observational study and the European Prospective Investigation into Cancer and Nutrition study failed to document a significant effect of either unopposed estrogen therapy or estrogen plus progestin therapy on the risk of CRC.278,279 Findings from randomized trials, including those within the WHI, do not currently support the use of either unopposed estrogen therapy or combined estrogen plus progestin therapy in colorectal risk reduction.280–285 Although a number of observational studies suggest a protective effect of HRT, it remains unclear which formulations and dosing regimens would be optimal or the duration of treatment and observation needed to observe benefits, if they occur.
Perhaps the most well-established chemopreventive agents for CRC and adenoma recurrence are NSAIDs. Data supporting their chemopreventive use across the range of colorectal neoplasia is highly consistent across different agents. Regarding aspirin, a total of 10 RCTs focused on colorectal neoplasia have been carried out in populations with varying levels of risk (Table 23-8). The most recent trials have tested aspirin in high-risk (FAP or HNPCC) or intermediate-risk (history of adenomas or CRC) populations. One trial in a high-risk HNPCC cohort found that 600 mg per day of aspirin for a mean of 25 months significantly reduced CRC incidence by 37% after 55.7 months,286 although another trial in persons with FAP using the same aspirin dose did not observe this effect.287 A total of three RCTs have been carried out in persons with a history of adenomas290–290 and one in CRC survivors.291 All studies had adenoma recurrence as their primary end point, and treatment and follow-up durations ranged from 1 to 3 years. All four studies reported a significant ~20% to 40% reduction in either the risk of any recurrent adenoma, the risk of advanced adenoma, or both in persons taking aspirin compared with placebo. One study recently provided an update on findings after 4 years of follow-up and found that the protective effects identified after 1 year of aspirin treatment did not remain after the 4-year follow-up period.292 Four RCTs have tested aspirin in the setting of CRC prevention within the general population. Two of these studies, the Women’s Health Study293 and the Physician’s Health Study,294 used relatively low, intermittent doses of aspirin and did not find a significant effect of aspirin on incidence of CRC. The combined analysis of two smaller trials using higher doses over a longer time frame, the UK Transient Ischemic Attack Aspirin Trial and the British Doctors’ Aspirin Trial, suggests a significant ~25% to 40% reduction in CRC incidence, with a greater reduction in risk if aspirin treatment lasted 5 years or more; and this effect was observed only after a latency of 10 years.295 It is important to note that these trials were established to test aspirin in the setting of cardiovascular outcomes and not CRC. These findings are consistent with much of the observational data, in which higher doses administered over longer intervals result in CRC protection. A 2009 international consensus statement calls for more studies to define aspirin’s lowest effective dose, the most appropriate age at which to begin therapy, the optimum duration of therapy, and the population subsets in which benefits might outweigh potential harms.296
Table 23-8
Randomized Controlled Trials with Colorectal Cancer or Adenoma Incidence Demonstrating Positive Effects for Aspirin, by Population (Average Risk vs. Increased Risk)
| Study | Intervention | Cohort | Primary Result |
| Average risk populations | |||
| Gann et al., 1993 | 325 mg aspirin or placebo every other day × 5 yr | 22,071 male physicians aged 40-84 yr | CRC incidence: RR, 1.15 (0.80-1.65) For in-situ cancers and polyps: RR, 0.86 (0.68-1.10) |
| Cook et al., 2005 | 100 mg aspirin or placebo administered every other day × 10 yr | 39, 876 U.S. women aged ≥45 yr and initially without history of cancer, CVD, or other major chronic illness | CRC incidence: RR, 0.97 (0.77-1.24) |
| Flossman et al., 2007 (pooled analysis of data from the UK-TIA and British Doctors’ Aspirin Trials) | UK-TIA: 300-500 mg/day vs. placebo × 1-7 yr British Doctors’ Aspirin Trial: 300-1500 mg/day vs. no aspirin × 5-6 yr |
UK-TIA: 2449 persons, mean age of 60.3 yr, 73% male; British Doctors’ Aspirin Trial: 5139 persons, mean age 61.6 yr | CRC incidence: HR, 0.74 (0.56-0.97), P = .02; effect seen after a latency of 10 yr |
| Increased risk populations | |||
| Baron et al., 2003 | Aspirin 81 mg/day vs. 325 mg/day vs. placebo × 3 yr | 1211 patients with prior adenoma | 81 mg/day, adenoma incidence: RR, 0.81 (0.69-0.96) Advanced adenoma incidence: RR, 0.59 (0.38-0.92) 325 mg/day Adenoma incidence: RR, 0.96 (0.81-1.13) Advanced adenoma incidence: RR, 0.83 (0.55-1.23) |
| Sandler et al., 2003 | Aspirin 325 mg/day vs. placebo × 3 yr | 635 patients with prior resected early-stage CRC | Adenoma incidence: RR, 0.65 (0.46-0.91); prolonged time to first adenoma |
| Benamouzig et al., 2003 (APACC trial) Benamouzig et al., 2012 (4-yr follow-up of APACC) |
Lysine acetylsalicylate 160-300 mg/day vs. placebo × 4 yr | 291 patients with prior adenomas | Recurrent adenoma: RR, 0.73 (0.52-1.04), P = .08 Recurrent adenoma >5 mm in diameter: RR, 0.44 (0.24-0.82), P = .01 4-yr follow-up: Proportion of patients with at least 1 recurrent adenoma: 41% in aspirin-treated group vs. 40% in placebo, NS Polyp burden: 3.1 ± 5.8 mm vs. 3.4 ± 6.2 mm, NS Proportion with at least 1 advanced recurrent adenoma: 10% vs. 7%, NS |
| Logan et al., 2008 | Aspirin 300 mg/day and folate supplements 0.5 mg/day vs. placebo in 2×2 factorial design | 939 patients with prior adenomas, mean age of 57.8 yr (range, 27.6-74.6) | Adenoma recurrence: RR, 0.79 (0.63-0.99); Advanced adenoma: RR, 0.63 (0.43-0.91) |
| Burn et al., 2011 CAPP-1 |
Aspirin 600 mg/day and/or resistant starch (30 g/day) vs. placebo × 1-12 yr in 2×2 factorial design | 206 FAP patients, aged 10-21 yr | Polyp count: aspirin – RR, 0.77 (0.54-1.10) Size of largest observed polyp: mean size of 3.0 mm in aspirin-treated group vs. 6.0 mm in placebo group, P = .02 |
| Burn et al., 2011 CAPP-2 |
600 mg aspirin or aspirin placebo or 30 g resistant starch or starch placebo, for up to 4 yr | 937 carriers of Lynch syndrome | Time to first CRC: HR, 0.63 (0.35-1.13), P = .12 For those completing 2 yr of intervention, HR, 0.41 (0.19-0.86), P = .02; IRR, 0.37 (0.18-00.78), P = .008 |
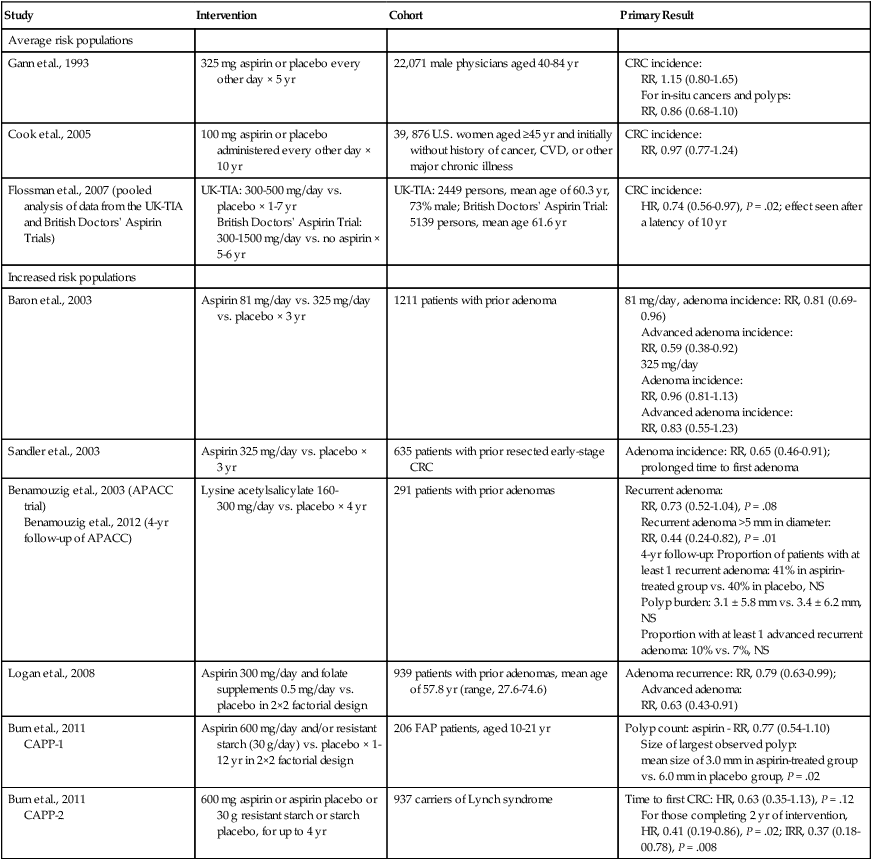
Other NSAIDs that have demonstrated at least some efficacy in RCTs of CRC chemoprevention are selective COX-2 inhibitors and sulindac. Celecoxib was initially tested in an RCT of 83 patients with FAP, demonstrating significant reductions in the number of polyps and in polyp burden after 6 months of treatment.297 These data led to an interim FDA approval of celecoxib as an adjunct to usual endoscopic and surgical management of colorectal adenoma burdens in patients with FAP. It was subsequently tested in the Adenoma Prevention with Celecoxib and Prevention of Colorectal Sporadic Adenomatous Polyps trials of individuals with a history of adenomas,298,299 and although significant protective effects were observed, the Adenoma Prevention with Celecoxib trial identified a dose-dependent two- to threefold higher risk of cardiovascular events among persons taking celecoxib. Subsequent data have shown that the increased risk of cardiovascular events due to celecoxib is largely confined to persons with a high baseline risk of cardiovascular disease.300,301 Nevertheless, because of a shared set of risk factors in people at risk for CRC and cardiovascular disease, celecoxib cannot currently be recommended. However, a recent phase I dose-escalation trial of celecoxib in children (ages 10-14 years) with FAP demonstrated that it is safe, well tolerated, and reasonably effective, significantly reducing the number of colorectal polyps in these patients.302
Various small trials have tested sulindac in high- and intermediate-risk settings of FAP. In a primary prevention context, sulindac was not shown to prevent adenoma development or affect the number and size of polyps after 4 years of treatment in carriers of the FAP genotype.303 Yet in a secondary prevention context in patients with FAP who had adenomas, studies have generally shown protective effects of sulindac with regard to the number and size of polyps and polyp regression.306–306 One small trial in 44 persons with a prior history of polyps did not demonstrate significant effects on polyp regression after 4 months of sulindac treatment.307 It is important to note that results of these trials are based on very limited sample sizes, ranging from just 10 to 44 patients with FAP.
Combinations of agents may demonstrate more potent effects than either agent alone, and often at lower doses. The trial of sulindac therapy given along with DFMO in persons with sporadic adenomas is a prime example of this concept. This trial was stopped early because its end points were met sooner than anticipated. Results demonstrated a remarkable ~70% reduction in recurrent adenomas and 90%+ reductions in multiple and advanced adenomas, with low doses of each drug given in combination with no significant difference in adverse effects between the treatment and placebo groups.34 Additional trials of sulindac and DFMO are ongoing in persons with FAP (clinicaltrials.gov identifier: NCT01483144 and NCT01349881), and results of RCT testing of other combinations of agents, such as DFMO along with aspirin or celecoxib in high-risk CRC populations (clinicaltrials.gov identifier: NCT00983580 and NCT00033371), are anticipated.
Finally, and most recently, an RCT in 55 patients with FAP demonstrated a significant 22% reduction in polyp number and a nearly 30% significant reduction in the sum of polyp diameters after 6 months of treatment with the omega-3 fatty acid eicosapentaenoic acid.308 However, epidemiological data are inconsistent, and a recent systematic review of 20 different cohorts spanning 11 different cancer sites identified just one estimate of decreased risk for CRC due to omega-3 fatty acids and 17 estimates showing no association of omega-3 fatty acids with CRC.309
A promising new chemopreventive strategy involves intermittent administration of chemopreventive combinations inducing synthetic lethality in APC-deficient cells.33
Screening
Certain characteristics of CRC make it highly amenable to screening. It has a long detectable, preclinical phase, and safe and accurate diagnostic tests are available. In addition, evidence is strong that screening and earlier treatment improves CRC outcomes and survival. Currently, 5-year survival rates are 90% for localized disease, 70% for regional disease, and just 12% for distant disease.248 Such figures provide a strong rationale for screening programs to identify early-stage colorectal cancers and polyps that can be removed to reduce cancer risk. Numerous screening tests are available that are safe and cost-effective, although 2010 data from the CDC’s behavioral risk factor surveillance system indicate that more than one third of persons older than age 50 years have never had a sigmoidoscopy or colonoscopy and just 17% report having had an FOBT within the previous 2 years.14
The five screening tests currently in use are the FOBT, double-contrast barium enema (DCBE), flexible sigmoidoscopy, colonoscopy, and CT colonography (Fig. 23-7). Of these tests, the FOBT has the strongest direct evidence supporting its use as a CRC screening test. A 2007 metaanalysis of four RCTs demonstrated a 16% to 25% reduction in CRC mortality.310 Because the FOBT is both inexpensive and noninvasive, it is the most commonly used screening test worldwide, although it can have a high false-positive rate and patient compliance is often low because of the test’s dietary restrictions.
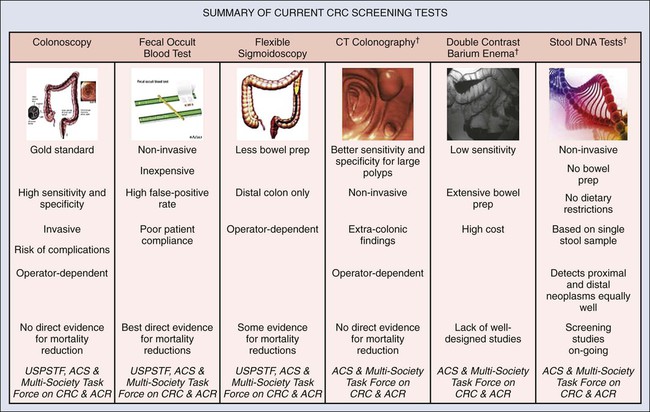
DCBE has a relatively low sensitivity for the detection of both CRC and adenomas and is also a labor-intensive procedure requiring extensive bowel preparation.311 There is a paucity of well-designed studies assessing its effectiveness on mortality and incidence, and since the introduction of CT colonography, DCBE is rarely used.
Direct evidence from three RCTs demonstrate significant reductions in both CRC incidence and mortality using flexible sigmoidoscopy.314–314 However, a substantial limitation of this test is that it only evaluates the distal colon, creating the possibility of significant false-negative screening examinations.
Because of its high sensitivity and specificity for the identification of polyps and CRC, as well as its ability to concomitantly diagnose and reduce risks associated with biopsied precancerous lesions, colonoscopy is considered the gold standard against which other screening tests are compared in the United States and other high-resource settings in which the test is both available and affordable. Yet evidence supporting the use of colonoscopy in screening is largely indirect. No RCTs to date have specifically demonstrated the effectiveness of colonoscopy in reducing CRC incidence or mortality. A large RCT is currently underway, but results are not expected until 2026315 (clinicaltrials.gov identifier: NCT00883792). Drawbacks of colonoscopy include its invasiveness, risk of serious complications, expense, and operator dependence.
As with colonoscopy, no direct evidence currently establishes the effectiveness of CT colonography in reducing CRC incidence and mortality. Metaanalyses have shown it to have high sensitivity and specificity for the detection of polyps ≥10 mm but reduced sensitivity and specificity for smaller polyps.316,317 Importantly, although this test is relatively noninvasive, it can result in the identification of extracolonic findings that may or may not be clinically important. In addition, advanced, multimaker stool tests have emerged as another possible screening strategy, with multicenter screening studies currently underway to assess their accuracy. The USPSTF currently states that the evidence to assess the benefits and harms of CT colonography and DNA stool tests is currently insufficient and therefore it cannot make a recommendation for their use in screening average-risk populations for CRC at this time.318
For persons at average risk of CRC, the 2002 USPSTF screening recommendations were updated in 2008 based on the availability of newer screening technologies, as well as on new population models estimating the optimal ages at which to start and stop screening.318 Although the 2002 recommendations applied to anyone older than 50 years, with no upper age limit on screening, the USPSTF now recommends discontinuing screening at age 75 years because of a decreased likelihood of early detection yielding a mortality benefit in this age group, given the long average time between adenoma development and cancer diagnosis. The USPSTF continues to recommend screening with high-sensitivity FOBT annually, or sigmoidoscopy every 5 years with high-sensitivity FOBT every 3 years, or colonoscopy every 10 years. More aggressive screening guidelines exist for persons at high risk for CRC because of the hereditary syndromes of FAP and HNPCC or because of a history of polyps or other risk factors.319
Hepatocellular Cancer
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer (more than 700,000 cases in 2008) and the third most frequent cause of cancer-associated mortality worldwide.143 The global distribution of HCC is driven by the heterogeneity of major risk factors across populations, including viruses, environmental toxins, and alcohol.320 During the past three decades, HCC incidence has risen sharply in many industrialized countries. In the United States, the twofold increase in HCC incidence between 1975 and 1995 probably reflects the latency of viral carcinogenesis after the hepatitis B virus/hepatitis C virus (HBV/HCV) epidemics of the 1970s and 1980s.321 The influx of American immigrants from regions endemic for hepatitis viruses also contributes to this concerning trend. HCC is an aggressive disease with very poor survival rates, and thus enhanced risk assessment modalities, screening/surveillance, and chemoprevention offer appealing strategies to improve HCC management and control (see Chapter 80).
Risk Factors/Etiology
Major risk factors for HCC include chronic infectious HBV or HCV, alcohol, aflatoxin B, diabetes, and tobacco, with several notable adverse synergies in persons harboring multiple risk factors.322 Approximately 85% of HCC cases occur in eastern Asia or sub-Saharan Africa because of the prevalence of chronic HBV, which may be spread by mother-child transmission or among siblings,323 and/or aflatoxin contamination of foods.320 The lifetime relative risk for HCC in HBV carriers is vastly elevated at 15 to 20, with incidence associated with duration of infection, viral load, co-infections with HCV or HIV, and environmental exposures to alcohol or aflatoxin B.325–325 Most HBV-related cancer occurs on a backdrop of chronic liver disease, with cirrhosis having been diagnosed in 70% to 90% of persons.326
In Japan, Europe, and North America, HCV infections associated with past contaminated blood transfusions or needle sharing327 and alcohol use are primary risk factors.328 HCV is thought to increase the risk for HCC by inducing liver fibrosis and cirrhosis, which occurs in 15% to 35% of cases at ~30 years from the time of initial infection.329 The annual incidence of HCC in persons with HCV-associated cirrhosis is 3% to 5%.330 A recent metaanalysis of HCV-infected persons reported a doubling of HCC risks with concomitant use of alcohol,331 but other reports suggest that alcohol use increases risks in hepatitis-infected persons regardless of the specific infection.330 Tobacco use increases risks of HCC in HBV and HCV carriers through additive or multiplicative interactions, respectively, according to a recent metaanalysis.332
Metabolic syndrome and diabetes are also associated with an increased HCC risk.12 A recent review of 49 observational studies reported a pooled HCC risk estimate of 2.2 to 2.4 among patients with diabetes.333 Some studies have identified an association between diabetes and HCC, regardless of concomitant hepatitis infections.334 Given the current obesity epidemic in many industrialized countries, obesity-related liver disease, especially in persons with nonalcoholic steatohepatitis, is expected to rise, and with it, rates of HCC associated with obesity.335 Minor increases in HCC risk (i.e., less than twofold increases) have been described, with several host genetic factors involving tumor necrosis factor–α and glutathione S-transferase variants.323
Risk Modeling/Assessment
HCC screening by biannual ultrasonography and serum alpha-fetoprotein concentrations was associated with substantial improvements in survival in a single RCT involving 18,816 Chinese patients with HBV.336 Despite suboptimal adherence to the test procedures, the trial reported increased survival rates for screened versus unscreened participants at 1, 3, and 5 years; improved stage shift, including smaller lesions; improved rates of surgical resection; and an overall significant 37% reduction in HCC-associated mortality by biannual screening. Whether these same benefits would accrue to other populations of at-risk patients or in other settings is unclear, but the question may never be definitively answered, given the great perceived benefit of screening demonstrated in this trial.337
However, there is a significant need to improve the selection of patients entering into HCC screening, which depends on their inherent risk of HCC and their ability to withstand a recommended treatment (e.g., tumor ablation/resection, chemoembolization, or chemotherapy), which, in turn, depends on comorbid conditions and their liver’s estimated posttherapeutic functional reserves.322
To address the first of these issues—assisting with HCC risk estimates—several predictive scoring schemes have been advanced recently, drawing on data from large Asian cohorts.338–341 The various risk models rely on a variety of factors including age, gender, various components of liver function (e.g., serum bilirubin, albumin, or alanine aminotransferase levels; a diagnosis of cirrhosis), and HBV DNA concentrations. A more recent model by Wu et al. uses commonly available clinical parameters to estimate risks (Wen and Wu, Journal of the National Cancer Institute, in press). Although presumably useful among Asian populations, their relevance to American or European populations with chronic HBV or HCV infections is unclear. It is hoped that these clinical risk models will prove useful in targeting ultrasound screening only for persons most likely to benefit and least likely to be harmed. Besides cost efficiencies, such a strategy might be used to implement screening earlier or with more sensitive technologies in persons at greatest risk.342
Prevention
Because cirrhosis can be considered a premalignant condition regardless of its specific etiology, anything that will prevent or slow its progression can be considered a potentially effective HCC preventive strategy.343 Elimination of cocarcinogens such as alcohol, tobacco, or dietary aflatoxins in a patient with chronic hepatitis of any cause is important.344 Alcohol education, alcoholism screening, and treatment are logical preventive strategies, although data supporting the effectiveness of these approaches are limited.345 Similarly, type 2 diabetes is an independent risk factor for HCC, raising risks approximately two- to fourfold334; therefore effective screening, prevention, and management of the condition are anticipated to reduce HCC rates. Healthy food choices and increased physical activity would be expected to prevent HCC and promote health more broadly.346 In the setting of morbid obesity or refractory obesity, bariatric surgery may even be contemplated, although specific data related to its impact on HCC incidence are lacking.347 Avoiding aflatoxin contamination of foods through improved food storage and economic development should help significantly in previously endemic regions of the world.348
A range of dietary enhancements or supplements including beta-carotene, selenium, vitamin C, antioxidant phytochemicals, ellagic acid, curcumin, lycopene, coenzyme Q10, green tea or tea extracts, resveratrol, and N-acetyl cysteine have been proposed as potential HCC preventives, based on mechanistic, in vitro, or observational data. However, a recent comprehensive review concluded that it is very difficult to offer compelling recommendations for any of these dietary agents because of strongly conflicting results and the paucity of high-quality clinical trial data.349
Vaccination against cancer-causing organisms represents one of the most effective preventive strategies available. Vaccinations against HBV became available in the mid 1980s. Thereafter, universal HBV vaccination of infants has drastically reduced HBV carrier rates350 and HCC incidence in endemic regions, such as Taiwan.353–353 Similar success would be anticipated after the development and dissemination of a vaccine against HCV,354 but these efforts have been more challenging because of HCV’s genomic diversity and high rates of mutation, its tendency to escape immunologic surveillance, and the short-lived nature of HCV neutralizing antibodies.355 However, at least three different HCV vaccine candidates have emerged in recent years, offering hope for the future of this strategy.356
Other effective HCC prevention strategies include therapy for persons harboring chronic HBV or HCV infections in an attempt to slow or block disease progression. A randomized, placebo-controlled trial of interferon (IFN) in 101 Taiwanese men with chronic HBV infections revealed a substantial reduction in HCC incidence in treated versus untreated men (1.5% vs. 12%) after up to 11.5 years of follow-up.357 Similarly, a recent metaanalysis of RCTs of IFN treatment in patients with HBV-related cirrhosis revealed a significant 41% reduction in HCC incidence.358 Effects of IFN on HCC incidence in patients with chronic HCV infections are similarly suggestive of preventive benefits in responding patients with a sustained virologic response or persistently normalized alanine aminotransferase levels,359–363 although the data are not from randomized trials. In addition, lamivudine, a nucleoside analog, reduced the incidence of HCC by 51% in an RCT of persons with chronic HBV infections,364 and results were similarly promising in a large, retrospective Japanese analysis.365 In addition, combinations of pegylated IFN and another nucleoside analog, ribavirin, reduce HCC risk in patients with HCV infection.366 Indeed, a range of nucleoside analogues are now available with low rates of viral resistance and long-term HBV DNA suppression, resulting in reduced risk of HCC.367
The availability of effective therapies for HBV and HCV infections suggests an important preventive strategy. Recently, the CDC promulgated evidence-based recommendations to systematically identify persons infected with HCV. Currently, adults born from 1945 to 1965 should be screened for HCV without prior ascertainment of HCV risk so that any identified infected person may be counseled regarding alcohol intake and referred to appropriate care for HCV infection.368 This recommendation is intended to reduce the rate and severity of adverse outcomes, such as HCC, associated with HCV infection. A program to link gastroenterology specialists with expertise in HCV management to community practitioners has proved extremely effective in achieving sustained viral responses, reducing treatment-related adverse effects, and improving effectiveness and efficiency of care of persons with chronic HCV infections.369
Finally, a licorice extract, glycyrrhizin, was tested in IFN-resistant HCV-infected persons via a retrospective analysis and was reported to reduce HCC risks.370 Other promising chemopreventive approaches involve phase-2 enzyme inducers that may boost aflatoxin detoxification,371 NSAIDs/COX-2 inhibitors, and behavioral approaches to decrease risk among persons with alcohol-related cirrhosis.
Screening
After identifying a high-risk HBV population and deeming them healthy enough to benefit from screening and early detection strategies, several groups, including the American Association for the Study of Liver Diseases, the Asia Pacific Association for the Study of the Liver, and a U.S. group, have provided guidelines for programmatic HCC screening.372–376
The best experimental data regarding the effectiveness of HCC screening comes from the large RCT referenced earlier by Zhang et al.,336 which demonstrated substantial survival benefits among screened individuals.
The preferred imaging approach for screening is ultrasonography every 6 months because it is well tolerated, widely available, and has good sensitivity (60% to 80%) and specificity (~90%).377 More frequent imaging every 3 months has been shown to increase detection of small nodules, but it has not been shown to improve survival.378
Stomach Cancer
Gastric cancer (GC) is the fourth most common cancer and the second most common cause of cancer-related death worldwide.379 This situation exists despite significant reductions in both incidence and mortality, which have occurred during the past several decades. The exact cause of the observed incidence reduction is unknown, but improvements in food preservation and storage, as well as improved hygiene associated with less crowding, are probably important contributors. Its distribution across the globe is also extremely heterogeneous, with up to tenfold variations in incidence, although the reason(s) underlying these differences is not entirely clear. Rates in high-risk regions of the world such as East Asia, Eastern Europe, and Central/South America exceed 20 cases per 100,000. By contrast, low-risk regions such as Southern Asia, North/East Africa, North America, Australia, and New Zealand report rates less than 10 cases per 100,000 on average. Importantly, even in low-risk regions, very high-risk subsets of the population may be found, as exemplified by Koreans living in the United States.380 As with many other solid tumors, incidence rises with age, with a 2 : 1 predilection for men compared with women. Survival is strongly stage-related because the foundation of effective therapy is surgical excision, creating a compelling need for effective screening and prevention strategies (see Chapter 75).
Risk Factors/Etiology
GC has been associated with a variety of environmental factors such as diets high in fatty, pickled, smoked, or salty foods; diets low in fresh fruits and vegetables; and gastric mucosal infection with H. pylori.381 H. pylori infection of the stomach is one of the strongest and most clearly documented risk factors. Weighing all available evidence, the IARC classified H. pylori infection as a definite carcinogen in 1994.382 It is estimated that 60% to 75% of gastric cancers worldwide are associated with H. pylori infection.27 Epidemiological studies have estimated a six- to twentyfold increased risk of distal stomach cancer associated with H. pylori infection.383,384 Recent reviews suggest that H. pylori may even be considered a “necessary, but insufficient” risk factor for GC.
Migrant studies demonstrate the importance of environmental risk factors in GC, because immigrants typically retain the risks of their homeland; however, their children have the GC risks of the new country’s population.385 Some of the important risk factors for GC include tobacco use,386 alcohol use,387 consumption of foods containing high amounts of salt or nitrites,388 and diets characterized by either limited fruit/vegetable intakes389,390 or high red meat and processed meat intakes.391,392 Obesity in men was associated with a doubling of GC risk in a recent large cohort study.12
A family history of GC has been reported to increase risk for GC,393 but it is unknown whether this finding is due to shared genetic or environmental factors, especially early in life.394 A very rare form of early-onset GC follows from inheritance of an autosomal-dominant mutation in the E-cadherin gene, CDH1.395 Other hereditary syndromes associated with GC development include Lynch syndrome,396 Peutz-Jeghers syndrome,397 Li-Fraumeni syndrome,398 and Cowden syndrome.399
Risk Assessment/Screening/Prevention
Current GC risk and prevention algorithms are focused on population subsets at high risk based on population rates of GC. It is recommended that high-risk (≥20 cases/100,000) and possibly intermediate-risk (10 to 19.9 cases/100,000) populations undergo H. pylori screening400,401 by a locally validated H. pylori serology.400 H. pylori serologies identifying IgG antibodies to H. pylori antigens are widely available, inexpensive, and associated with a diagnostic accuracy of 80% to 90%+, depending on local validation, which is important to account for differing strains across geographies.402 H. pylori screening is most often recommended to begin 10 to 20 years before the initial GC incidence starts to rise in the local population. H. pylori–positive patients are then treated with the most effective therapeutic combination with attention to compliance to minimize antibiotic resistance, again based on local or national treatment guidelines. These regimens typically involve a week of “triple therapy” (i.e., antibiotics and proton pump inhibitors) such as clarithromycin, amoxicillin, and a proton pump inhibitor.403,404 This treatment is recommended because of an evidence base that includes RCTs demonstrating significant reductions in precancerous lesions and incident cancers after treatment.
RCTs of H. pylori eradication have been conducted in high-risk populations, and most trials have shown nonsignificant reductions in GC incidence.407–407 Interpretations have been difficult because some study results have only been reported in a preliminary manner, and longer follow-up is expected. Nevertheless, a meta-analysis of the trial data reveals a nonsignificant 35% reduction in GC incidence after H. pylori eradication.408,409 These data led to an Asia-Pacific consensus conference to recommend population-based screening and treatment for H. pylori in high-risk population settings.400 After this recommendation was made, Talley et al.401 suggested that these recommendations may be applicable to other populations at high risk and possibly moderate risk for GC, but H. pylori screening and treatment is not recommended in low-risk settings because of costs. More recently, Ma et al.410 reported that H. pylori treatment reduced GC diagnoses by a significant 40% and GC-related deaths by a nonsignificant 33% after 14.7 years of follow-up.
Beyond H. pylori eradication, several other agents have been proposed as possible GC chemopreventives. Early studies explored antioxidants because of the protective association between diets high in fruits and vegetables and reduced GC risk. Antioxidants such as vitamin C, beta-carotene, vitamin E, or selenium could offer protection against GC by reducing free radical damage, inhibiting tumor growth, stimulating cancer suppressor genes or blocking angiogenesis.411 Observational data have been conflicting. Several trials209,405,407,412 have examined the effects of antioxidant supplements, but as is typical in complex, multifactorial trials, making interpretations regarding specific supplements is challenging.408 Currently no convincing or consistent data suggest GC preventive benefits of antioxidant supplements.413
The most promising class of agents for GC chemoprevention has been aspirin and NSAIDs. Numerous observational studies, both retrospective and prospective in design, have suggested protective benefits for GC in NSAID users. Although inconsistencies certainly exist, when looking across all observational studies of aspirin/NSAIDs and GC, it appears that regular use is associated with a ~40% reduced GC risk.414 More recent data examining cancer outcomes in RCTs of aspirin use in cardiovascular risk reduction have confirmed protective effects of aspirin against GC with a hazard ratio of 0.42.415 Broadly speaking, evidence is most compelling for current users, for persons using the agents for several years, and for noncardiac gastric cancers. Exact details regarding specific agents within the class (e.g., aspirin vs. other NSAIDs), along with factors such as dose, duration, and patients most likely to respond, must be addressed in future trials.
To date, the only randomized trial examining COX inhibitors as GC preventives was performed by Wong et al.416 The trial involved 1024 patients with precancerous gastric lesions who were randomly assigned in a factorial design to H. pylori eradication for 1 week, celecoxib for 24 months, both, or neither. Results revealed that both celecoxib and H. pylori eradication alone had beneficial effects on regression of advanced gastric lesions, but the combination was not statistically significantly better than placebo. A large four-arm trial of esomeprazole at 20 mg or 80 mg per day with or without 300 mg per day of aspirin (the Aspirin Esomeprazole Chemoprevention Trial) is ongoing to examine rates of all-cause mortality and esophageal adenocarcinoma. Secondary end points will no doubt include incident cancers, and therefore some additional data should be forthcoming in the next decade (http://clinicaltrials.gov/ct2/show/NCT00357682).
Pancreatic Cancer
Despite some recent therapeutic improvements for pancreatic cancer, the prognosis of affected patients generally remains dismal, with annual incidence and mortality rates mirroring one another.417 However, important new insights into the etiology and molecular/histopathological pathogenesis of pancreatic tumor progression,420–420 identification of several high-risk cohorts,421 and new genetically engineered mouse models that more faithfully recapitulate human disease422,423 offer great hope for improved risk assessment and, possibly, prevention (see Chapter 81).
Risk Factors/Etiology
The pathogenesis of pancreatic cancer development has been carefully examined by Hruban and colleagues, resulting in a molecular and histopathological model of tumor development and progression.420–420 The disease is generally thought to arise from ductal epithelial cells through a process of cumulative molecular derangements involving activation of KRAS, inactivation of several tumor suppressor genes including p16, SMAD4, p53, and overexpression of EGF, HGF, and IGF-1.424,425 Table 23-9 describes features of common precursor lesions in the pancreas.
Table 23-9
Common Precursor Lesions in the Pancreas
| Feature | Mucinous Cystic Neoplasm | Intraductal Papillary Mucinous Neoplasm | Pancreatic Intraepithelial Neoplasia |
| Predominant age | 40-50 yr | In the 60s | Increases with age |
| Gender | Female > male | Male > female | Male = female |
| Head vs. body/tail | Body/tail | Head | Head > body/tail |
| Relation of cysts to large ducts | Usually not connected | Always connected | N/A |
| Cyst contents | Mucoid | Mucoid | N/A |
| Mucin oozing from ampulla | No | Yes | No |
| Stroma | Ovarian type | Collagen rich | Collagen rich |
| Multifocal disease | Very rare | In 20%-30% | Often |
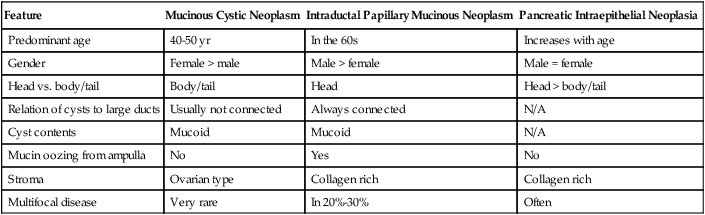
Reprinted from Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am 2007;36(4)836–49 with permission from Elsevier.
Risk factors associated with pancreatic cancer development in the general population include aging, tobacco use, heavy alcohol use, diabetes, and obesity. The median age of pancreatic cancer development is 72 years, with development of the disease occurring in fewer than 10% before the age of 50 years.426 Tobacco use is estimated to double the risk of pancreatic cancer, with population estimates of attributable risk reaching as high as 25% because of the frequency of smoking.427,428 Despite the complex interrelationships between the use of alcohol and tobacco, a recent metaanalysis suggests that heavy alcohol use is associated with a 22% increased risk of pancreatic cancer.429 Observational data also suggest a strong relationship between type 1 diabetes,430 long-standing type 2 diabetes,431 and abnormal glucose tolerance432 and pancreatic cancer risk. Finally, metaanalyses have identified a strong and consistent relationship between obesity or BMI and pancreatic cancer risk.433
High-risk families and germline mutation carriers with elevated risks for pancreatic cancer have been identified. At least six such genetic syndromes are known, and several are associated with a characteristic histomorphology suggesting the syndrome when it is encountered.421,434 Genetic syndromes associated with pancreatic cancer include Lynch syndrome, FAP, hereditary breast-ovarian cancer syndrome, hereditary pancreatitis, Peutz-Jeghers syndrome, and the familial atypical multiple mole melanoma syndrome.421
Prevention
Preclinical data suggest that NSAIDs, green tea catechins, vitamin D, vitamin E, beta-carotene, Perillyl alcohol, isothiocyanates, genistein, resveratrol, capsaicin, erlotinib, or metformin may be effective in various cell- or animal-based systems.435
Observational data suggest that antiinflammatory agents such as aspirin may be associated with a 40% reduced risk of pancreatic cancer,436 but this finding has not been consistently reported in other studies. Indeed, another large observational study reported a 58% increased risk of pancreatic cancer among women who have used regular aspirin for more than 20 years.437 Inconsistent effects have been seen with studies of green tea, beta-carotene, and vitamin D as well. Observational data from the ATBC study actually suggested a trend toward an increased risk of pancreatic cancer among patients receiving vitamin E supplements.438 More recently, a study of metformin use in patients with type 2 diabetes revealed a 62% lower risk of pancreatic cancer.439
Currently, the best recommendations for a preventive strategy against pancreatic cancer include steps to reduce known modifiable risk factors, including tobacco and alcohol use, eat a proper diet including a variety of fruits and vegetables, exercise regularly, and maintain a normal body weight throughout life.440,441 Future efforts are anticipated to focus on likely aberrant molecular defects in high-risk subsets, although the question of an accurate, reliable, and accessible intermediate efficacy biomarker remains as a substantial barrier in chemoprevention trial design and conduct.
Risk Modeling/Assessment/Screening
By contrast, high-risk individuals with a strong family history or specific germline mutations (e.g., familial atypical multiple mole melanoma with p16 mutations and at least one case of pancreatic cancer in the family; hereditary pancreatitis; Peutz-Jeghers syndrome with a strong family history of pancreatic cancer; or BRCA1 or BRCA2 mutation carriers with at least one case of pancreatic cancer in the family) estimated to raise lifetime risks by greater than tenfold have been recommended to undergo periodic endoscopic screening.421 Screening has also been advocated for persons with individual risk factors such as intraductal papillary mucinous neoplasm (IPMN), pancreatic cysts, ductal ectasia, diabetes mellitus, or chronic pancreatitis combined with hereditary factors, but such screening is more controversial.
The optimal screening method is unclear, but screening of high-risk cohorts is generally started at 40 years of age or when one is 10 years younger than the age of the youngest relative with pancreatic cancer.442 Many institutions favor endoscopic ultrasonography, but CT or magnetic resonance imaging (MRI)-based strategies may be effective, too.440,442,443 In addition to image analysis, some sites recommend serum tumor markers (e.g., CEA or CA19-9) as well.444,445 Generally, screening examinations are conducted annually until an abnormality is identified, at which time intervals may close to every 3 to 6 months. With IPMNs, screening is recommended every 3 to 6 months by an international consensus panel.446 The most important, yet controversial, clinical preventive measure is identifying which patients should be recommended for prophylactic pancreatectomy. Ideally, prophylactic resection would be recommended in patients with PanIN3, IPMN, mucinous cystic neoplasms with high-grade atypia, or minimally invasive cancer.447,448 The extent of resection is also controversial because patients’ quality of life may be severely hampered by a total resection, yet removal of all cancerous and precancerous tissue is necessary to achieve effective risk reduction.
Prostate Cancer
In men, prostate cancer is second in incidence only to skin cancer, and is second in death only to lung cancer. The 2012 estimated figures for prostate cancer include more than 240,000 new diagnoses and 28,000 deaths in U.S. men.7 Racial disparity due to as yet unknown reasons is significant, with the African American population accounting for 60% more than the white population in incidence rates and >100% more in death rates in 2008.7 Further complicating matters, prostate cancer incidence rates have fluctuated in the past in part because of shifts in screening methods, including development and use of the PSA blood test (see Chapter 84).
Local/regional-stage diagnosis is now typical for more than 90% of prostate cases, resulting in 5-year survival rates just under 100%, having improved 30% during the past several decades.7 In addition, prostate cancer progression is typically slow, often allowing time for effective treatment.
Risk Modeling/Assessment
Racial background (African American or African Jamaican), genetic predisposition (family history of prostate cancer), and age (older than 50 years) represent the three widely recognized risk factors for prostate cancer.7 Smoking and obesity are also associated with an increased risk of dying from prostate cancer.7
The NCI, through its Early Detection Research Network, has been actively investigating strategies targeting the early detection and screening of prostate cancer. These efforts include the development of a proteomic-based platform for the detection of prostate cancer and the Prostate Cancer Risk Calculator, a collaborative effort with investigators of the Prostate Cancer Prevention Trial (PCPT) focused on prostate cancer risk assessment. Age, race/ethnicity, digital rectal examination (DRE) results, history of prostate cancer (both a prior negative biopsy and familial), and PSA level are integrated by the risk calculator, providing individualized risk assessment for prostate cancer and for high-grade disease.449 Multiple studies, both national and international, were conducted to validate the predictive potential of this risk calculator, which were mostly supportive.450–454 To improve the predictive value of this model, PCPT investigators incorporated the prostate cancer gene–3 into the risk calculator, which resulted in improved diagnostic accuracy (area under the curve of 0.696).455
Prevention
Strategies for targeting primary prevention of prostate cancer focus primarily on the identification of nutritional factors that influence development of the disease, as well as of susceptibility genes known to place persons at increased risk. Potential nutritional factors include dietary fat, fruit, meat, micronutrients, minerals, phytoestrogens, vegetables, and vitamins.456 Natural dietary supplements that have been the focus of studies include vitamins such as C, D, and E, carotenoids and retinoids, minerals including both calcium and selenium, and phytoestrogens such as flavonoids, isoflavonoids, and lignans. In addition, regular assessment of the BMI to determine and properly control total energy intake has been shown to be associated with decreased risk of cancer.457 Although such nutritional interventions remain promising, no studies to date have conclusively shown that nutritional, dietary, or behavioral changes reduce prostate cancer incidence. Potentially the most prudent intervention at this time is to maintain a healthy weight and BMI, adhere to a healthy diet rich in fruits and vegetables, and maintain a vigorous exercise program.
Chemoprevention
Epidemiological and experimental data have led to numerous potential agents being suggested and/or tested as prevention agents for prostate cancer. Among these interventions are specialized diets (e.g., consumption of cruciferous vegetables [isothiocyanates], fish, legumes, low-fat protein, and soy/isoflavonoids), consumption of red-colored fruits and vegetables (lycopene), and use of dietary supplements such as vitamins (e.g., A, C, D, E, folic acid, and multivitamins), minerals and essential fatty acids (e.g., calcium, selenium, omega-3 fatty acid, and omega-6 fatty acid), green tea/polyphenols, and therapeutic drugs (e.g., 5-alpha-reductase inhibitors; antioxidants and glutathione precursors such as N-acetylcysteine; COX-2 inhibitors; DMFO; glutathione S-transferase inducers such as oltipraz; NSAIDS; selective estrogen receptor modulators [SERMs]; and statins).458 Table 23-10 provides a summary of prostate chemoprevention studies.
Table 23-10
Prostate Cancer Screening Studies
| Trial | Patient Population | Study Design: Screen vs. Control | Results | Reference |
| LUPCSP | 46,193 men 45-80 yr | Screen: PSA and DRE at onset and annual PSA test Control: no PSA or DRE |
7-yr follow-up: 67.1% reduction in prostate cancer mortality 11-yr follow-up: 62% reduction in prostate cancer mortality | 463; 464 |
| PLCO | 76,685 men 55-74 yr | Screen: 6 annual PSA test and 4 annual DREs Control: usual care |
7-yr and 13-yr follow-up showed no reduction in mortality | 466; 467 |
| ERSPC | 182,160 men 50-74 yr | Screen: 4 annual PSA tests Control: no testing |
9-yr median follow-up showed 20% reduction in prostate cancer mortality and 41% reduction in metastasis NNS : NNT = 1410 : 48 at 9 yr and 503 : 18 at 12 yr7 High risk of overdiagnosis |
468 |
| ERSPC-Göteborg | 20,000 men 50-64 yr | Screen: biennial PSA tests to upper age limit (median 69 yr, range 67-71 yr) reached Control: no testing |
14-yr follow-up (Swedish arm of ERSPC): 44% reduction in prostate cancer mortality, 58% reduction in prostate cancer incidence NNS : NNT = 293 : 12 High risk of overdiagnosis |
469 |
| ERSPC-Spain | 4278 men 45-70 yr | Screen: 4 annual PSA tests Control: no testing |
15-year follow-up (Spanish arm of ERSPC): no reduction in mortality | 470 |
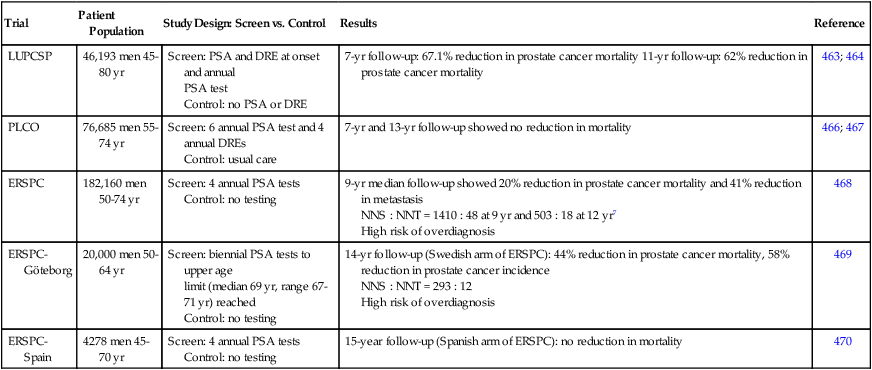
Two trials have targeted the 5-alpha-reductase enzyme for prostate cancer prevention: the PCPT and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial. These antiandrogenic agents inhibit the 5-alpha-reductase enzyme preventing the conversion of testosterone into the biologically active 5-dihydrotestosterone. The third large-scale phase III prostate cancer prevention trial, the SELECT trial, investigated the cancer prevention activity of the dietary supplements selenium and vitamin E (Table 23-11).
Table 23-11
Prostate Cancer Large Scale (Phase III) Chemoprevention Studies with Outcomes
| Prostate Cancer | |||||||
| Trial | Patients | Agent | Dose | Relative Risk | Absolute Risk | AR: High Grade | Reference |
| PCPT | 18,882 | Finasteride | 5 mg/d | 25% decrease | 6% decrease | 0.6% increase | 459 |
| REDUCE | 8,231 | Dutasteride | 0.5 mg/d | 23% decrease | 5% decrease | ≤0.5 % increase | 460 |
| SELECT | 35,533 | Selenium vitamin E Selenium + vitamin E 400 IU/d |
200 µg/d 400 IU/d 200 µg/d 400 IU/d |
9% increase 17% increase 5% increase |
0.8% increase 1.6% increase 0.4% increase |
No known effect No known effect No known effect |
91; 92 |
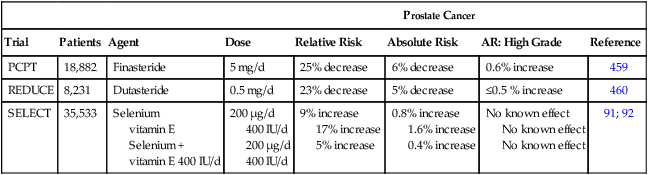
The PCPT was the first large phase III prostate prevention clinical trial459 that investigated the effects of finasteride treatment (5 mg/day) compared with placebo with a primary end point of the presence or absence of biopsy-proven prostate cancer. Results demonstrated a 25% reduction in relative risk of prevalent prostate cancer after finasteride treatment versus placebo. However, the study also identified an unexpected increase of 0.6% in high-grade (Gleason score 8 to 10) prostate cancer, although subsequent studies suggest that this increase may have been due to increased detection of high-grade disease in prostate glands whose size was reduced by finasteride.460 Nevertheless, concern about the induction of high-grade prostate cancer has lessened enthusiasm for use of this drug in prostate cancer prevention.31
The REDUCE trial studied dutasteride-induced reduction of diagnosed prostate cancer in healthy men and demonstrated a 23% decrease in biopsy-detectable prostate cancers with dutasteride treatment (0.5 mg/day) versus placebo.460 Although no increase in high-grade prostate cancer was seen with use of dutasteride, concerns about its risk-benefit ratio have kept the FDA from approving dutasteride for prostate cancer prevention.31,460,461
A recent reanalysis by Pinsky et al.462 of the PCPT and REDUCE trials demonstrated, at best, a potential decrease in the treatment arms for both trials, and at worst, only a minor increase in prostate cancer mortality, suggesting that previous risk assessment analyses were biased.
In the SELECT trial, African American men 50 years and older and other men 55 years and older with PSA levels ≤4 ng/mL were treated with vitamin E (400 IU/day), selenium (200 µg/day), vitamin E + selenium (400 IU/day and 200 µg/day, respectively), or placebo. Although initial study results failed to identify prevention of prostate cancer associated with any one of the three treatment arms, follow-up results identified an increase in risk of both prostate cancer and heart disease associated with dietary supplements of vitamin E in healthy men.92
Screening
The ability of the PSA test, which measures levels of a prostate epithelial cell–secreted glycoprotein, to reduce prostate-cancer–specific mortality when used in a screening context has been examined in a number of large trials, both within the United States and Europe (Table 23-10). Although the Laval University Prostate Cancer Screening Program conducted in Quebec, Canada, from 1988 to 1996 identified a 67.1% decrease in cause-specific mortality associated with screening and early treatment,463 with an 11-year follow-up analysis still identifying a 62% reduction in prostate cancer–related mortality,464 issues with both the study design and data analysis have resulted in speculation concerning the reported findings.465 Results of the U.S.-based Prostate, Lung, Colorectal, and Ovarian Screening Trial134,466 did not identify any significant benefit from screening on prostate cancer incidence or mortality at either 7 or 13 years follow-up.466,467 In addition, the 13-year follow-up also reported a lack of association between age, baseline prostate or overall morbidity, or PSA pretrial testing.467 By contrast, the European Randomized Study of Screening for Prostate Cancer (ERSPC),468 a collaborative effort involving nine European countries (Belgium, Finland, France, Italy, the Netherlands, Portugal, Spain, Sweden, and Switzerland), identified a 20% reduction in cause-specific mortality as a result of PSA-based screening. However, these results demonstrated a high risk of overdiagnosis of prostate cancer in subjects lacking clinical symptoms. The 14-year follow-up results from the Swedish arm of the ERSPC trial found enhanced benefits of PSA screening, with cancer-specific mortality reduced by almost half.469 Conversely, the 15-year follow-up results from the Spanish arm of the ERSPC study failed to demonstrate any effect of PSA-based screening on overall or cancer-specific mortality.470
Results from the population-based Tyrol Prostate Cancer Demonstration Project conducted in Tyrol, Austria, identified a reduction in prostate cancer–specific mortality.471 However, because of changes in PSA, DRE, and transrectal ultrasonography screenings throughout the life of the study, and because PSA velocity was incorporated into the standard diagnostic evaluations, results are questioned.
Over time, the PSA test has oscillated in use as a screening strategy for the early detection of prostate cancer. Guidelines published earlier this year by the USPSTF recommend against routine use of the PSA for prostate cancer screening in average-risk populations (Table 23-12). This decision was based on clinical evidence of a poor risk-benefit ratio associated with the test. With results from the Prostate, Lung, Colorectal, and Ovarian134 screening trial unable to demonstrate any reduction in prostate cancer mortality associated with PSA screening466,467 and the ERSPC trial identifying only 1 in 1000 prostate cancer–specific deaths prevented through PSA screening,468 the benefit of the test was considered minimal for the general population. In addition, the test carries a high false-positive rate due to other prostatic abnormalities and dysfunctions associated with PSA elevations or to low-risk prostate cancers that might remain asymptomatic for the remainder of the individual’s life (leading to overdiagnosis). Because of the current inability to effectively distinguish indolent from lethal tumors, these false-positive tests often result in interventions leading to complications (e.g., bleeding, bowel control, erectile dysfunction, fever, infection, pain, urinary incontinence, and death) as a result of overtreatment through surgery, radiation, and hormone therapy (HT). Follow-up reports from the Prostate, Lung, Colorectal, and Ovarian (PLCO) and ERSPC clinical trials identified a rate of 17% to 50% overdiagnosis resulting from false-positive PSA screening.472 Furthermore, it has been shown that long-term erectile dysfunction and urinary incontinence occur in ≥20% to 30% of prostate radiotherapy and surgery cases and that 40% of localized prostate cancers treated with androgen deprivation therapy also result in erectile dysfunction.473,474
Table 23-12
Current Prostate Cancer Prostate-Specific Antigen Screening Recommended Guidelines
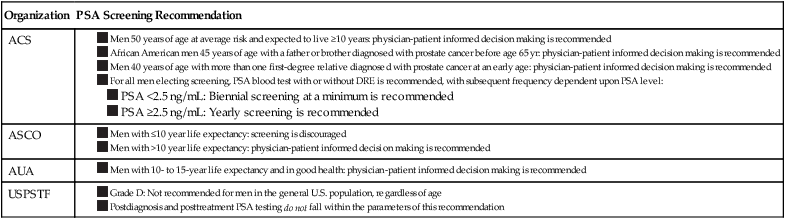
Despite the potential for overdiagnosis and overtreatment, PSA continues to be used as a serum marker for prostate cancer screening by many practitioners. However, screening guidelines remain controversial, with the ACS providing age- and risk-based recommendations.465 The current ACS recommendations include physician-patient discussions of the potential risks and benefits of PSA-based screening at the age of 50 years for average-risk men with ≥10-year life expectancies, at the age of 45 years for high-risk men (i.e., in persons of African descent or those having positive family history), and at the age of 40 years for very high-risk men (i.e., persons having a strong family history of prostate cancer).7 In addition, a DRE is recommended (although optional) by the ACS for men at average risk of prostate cancer beginning at the age of 50 years.7
Early Detection Biomarkers
Because in general PSA screening is no longer broadly recommended for average-risk men, a major effort to develop more sensitive and specific biomarkers for early detection of prostate cancer has recently commenced.7 Autoantibodies are being investigated extensively for use as potential prostate cancer biomarkers. These efforts include both single biomarker studies and numerous combinatorial biomarker studies.475–478 Although the current list of potential biomarkers is expansive, among those suggested for use in prostate cancer or currently under investigation are cysteine-rich secretory protein 3 (CRISP-3); enhancer of zest homolog 2 (EZH2); insulin-like growth factors and associated binding proteins (IGFs and IGFBPs); prostate cancer gene expression marker 1 (PCGEM-1); prostate-derived Ets factor (PDEF); prostate secretory protein 94 (PSP94); and prostate stem cell antigen (PSCA). Studies involving multiple biomarkers include investigations targeting AMACR + EZH2 + hespin + sarcosine and transmembrane protease, serine 2-ETS transcription factor gene fusion (TMPRSS2-ERG, also known as TMPRSS2-ETS and T2-erg), as well as other studies targeting combinations of bone morphogenetic protein 6 (BMP6) + noggin + sclerostin (SOST)479 and AMACR + Ets-related gene (ERG) + prostate cancer antigen 3 (PCA3, also known as DD3).480
Currently, nutritional and dietary supplements lack sufficient evidence to support their use as a prevention strategy for prostate cancers.481 In addition, the three large-scale prevention trials targeting this disease have produced controversial results. Clearly, a critical need remains for effective preventive strategies for prostate cancer. Ultimately, the reduction of morbidity and mortality associated with prostate cancer rests on the development and implementation of novel, effective paradigms for each aspect of early disease progression, including risk assessment, primary prevention, screening, early detection and chemoprevention.
Breast Cancer
It is predicted that in 2012, more than 226,000 breast cancers will be diagnosed and more than 39,000 women will die of this disease in the United States.7 The number of newly diagnosed breast cancers has been decreasing recently, but it still represents a large number of potentially preventable cancers. Risk factors for this cancer include a family history of breast or ovarian cancer, a history of premalignant breast lesions (such as atypical hyperplasias), radiation exposure, moderate to heavy alcohol use, exogenous estrogen plus progesterone exposure (such as in postmenopausal hormone replacement), and various endocrinologic features (such as early age of menarche, late age of menopause, and nulliparity or late age of pregnancy).482 These endocrinologic risk factors and the responsiveness of breast cancer to antiestrogen drugs have led to the use of antiestrogen strategies for the prevention of breast cancer. Many of these strategies can dramatically reduce breast cancer incidence, but drug-based preventive strategies have not been widely used because of concerns about adverse effects (see Chapter 91).
Risk Modeling/Assessment
Breast cancer risk assessment is relatively sophisticated compared with other cancers. The “Gail model,” a multivariable model to assess population risk of breast cancer that is based on age, family history, the presence of premalignant lesions, and various other endocrinologic features, is now typically used by primary care doctors and specialists to predict a woman’s breast cancer risk.50,51 This model is often used to assist in decision making for recommending cancer preventive therapy with antiestrogen drugs, such as tamoxifen or raloxifene. However, although this model is generally accurate for populations, its ability to accurately predict an individual’s risk is limited. Thus many groups are now working to develop more accurate risk modeling systems. These more recent risk assessment models include the English model, which uses many of the variables used in the Gail model, but also takes mammographic density into account.485–485
For women with a strong family history of breast cancer, other models may be more accurate. Models developed for genetically high-risk individuals include the Berry-Parmigiani-Aguilar (BRCA-Pro),488–488 the Claus,489 the Couch,490 the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm,493–493 and the Tyrer-Cuzick models.485–485 These models more accurately predict breast cancer risk in women with a strong family history, including those with paternal family histories of breast cancer. Several of these models predict the likelihood of women carrying breast cancer susceptibility gene mutations (such as BRCA1 or BRCA2 mutations). It is now typical for geneticists and physicians specializing in cancer risk assessment to use many of these models to guide genetic testing decisions, as well as recommendations for breast screening and risk reduction interventions.
Primary Prevention
Primary breast cancer prevention is typically achieved by avoiding radiation and limiting exposure to exogenous estrogen. In addition, many physicians recommend exercise and a healthy diet high in fruits and vegetables and low in red meat; however, data conclusively showing that such behavioral interventions prevent breast cancer is lacking. Researchers have a high degree of interest in identifying other environmental carcinogens relevant for breast cancer so that prevention could be achieved by avoiding such environmental carcinogens. The Institute of Medicine (IOM) released a Consensus Report in December 2011 entitled “Breast Cancer and the Environment: A Life Course Approach”494 in which the scientific evidence for exogenous carcinogens relevant for breast cancer was evaluated and presented. As stated in this report, “…the IOM concludes that women may have some opportunities to reduce their risk of breast cancer through personal actions, such as avoiding unnecessary medical radiation throughout life, avoiding use of estrogen-progestin HT, avoiding smoking, limiting alcohol consumption, increasing physical activity, and, for postmenopausal breast cancer, minimizing weight gain.”494 Although the IOM did not find conclusive evidence that other environmental exposures cause breast cancer, they did recommend that it would be prudent to avoid possible carcinogens such as those in plastic or other chemicals such as benzene, 1,3-butadiene, and ethylene oxide.494
Another form of prevention that is being used by more and more women is prophylactic bilateral mastectomies. This drastic surgery is often chosen by women at extremely high risk of breast cancer, those who carry BRCA1 or BRCA2 mutations. Such prophylactic surgery has been shown to reduce one’s risk of breast cancer by 90%.495 Although such drastic surgery is certainly not appropriate for most women at risk of breast cancer, with more comprehensive genetic testing, the number of prophylactic mastectomies being performed is increasing.496
Screening
Breast cancer screening is now typically performed using digital mammography in the general population. Various guidelines are currently available to guide breast cancer screening in those at average risk, including guidelines from the ACS, the USPSTF, the National Comprehensive Cancer Network (NCCN), and the American Congress of Obstetricians and Gynecologists groups.497 Recently, the use of mammography in women younger than 50 years has been questioned.498 However, in the United States, guidelines most often used recommend annual mammography beginning at age 40 years and continuing every 1 to 2 years until age 50 years, after which time mammograms are performed annually. European guidelines and the USPSTF guidelines recommend beginning mammograms after age 50 years and performing them every 2 years (up to age 74 years in the USPSTF guidelines).
Women who have a high risk of breast cancer (because of a family history, a previous breast cancer, or a premalignant lesion) should be more aggressively screened. For very high-risk groups (those who have a greater than 20% lifetime risk of breast cancer as assessed by any of the models previously mentioned under Risk Assessment), screening using mammograms in combination with breast MRI scans should be considered.499 Although the optimal frequency and timing of breast MRI scans is not well established, it is frequent practice to perform both annual bilateral mammograms and breast MRI scans (often staggered by 6 months) because these imaging modalities are complementary. Breast MRI scanning is more sensitive in detecting breast masses in dense breasts (such as in young women), whereas breast mammograms are more sensitive in detecting breast calcifications. Thus both are used to obtain optimal breast screening. Other screening tests such as breast ultrasound and breast tomography are beginning to be used, but these modalities have not yet been routinely used for screening even in high-risk groups.
Whereas the aforementioned imaging tests are clinically available, research is being performed to identify even more effective screening approaches, which include the use of circulating tumor cells,500,501 serum biomarkers,502,503 breast duct fluid,504 and even biomarkers in saliva.505 These strategies offer great hope in improving early detection of breast cancer, but they are not currently available for clinical use. It is anticipated that in the next few years, blood or biofluid-based early detection tests will be used in conjunction with more standard imaging screening tests.
Chemoprevention
Selective Estrogen Receptor Modulators
During the past 20 years, there has been a focused effort to develop drugs that will prevent the development of breast cancer. These efforts were stimulated by results from trials of the antiestrogen drug tamoxifen for the treatment of early-stage breast cancer; in women who took tamoxifen, approximately 50% fewer contralateral (or new) primary breast cancers developed than in women taking placebo.508–508 This observation, together with a variety of preclinical data, led to the development of many phase III breast cancer prevention trials using tamoxifen as preventive therapy in women without breast cancer (Table 23-13). The largest of these trials was the National Surgical Adjuvant Breast and Bowel Project P-1 trial.509 In this trial, women at moderately high risk of breast cancer (both pre- and postmenopausal women) were treated with tamoxifen or placebo for up to 5 years.509,510 This study was stopped early when initial results showed that tamoxifen use was associated with a 49% reduction in breast cancer and a similar reduction in noninvasive breast cancer.509 These results led to FDA approval of tamoxifen for breast cancer risk reduction in high-risk women. Several other similar trials were conducted (the UK study511 and the Italian study512) that also demonstrated that tamoxifen reduced the risk of breast cancer by at least 30%.513–516 Although tamoxifen prevented many breast cancers, this cancer preventive effect was limited to estrogen receptor (ER)–positive breast cancers. Each of these clinical trials showed that tamoxifen did not prevent the development of ER-negative breast cancers.510,514–516
Table 23-13
Select Estrogen Receptor Modulator (SERM) Breast Cancer Prevention Studies
| Trial | Patient Population | Study Design | Results | Reference |
| TAMOXIFEN | ||||
| Royal Marsden | 2494 high-risk women, 30-70 yr | Tamoxifen (20 mg/day) or placebo for 8 yr | No difference initially; with longer follow-up, reductions in invasive and ER-positive breast cancer incidence; no significant change in ER-negative or overall breast cancer incidence | 511; 515 |
| NSABP-P1 (BCPT) | 13,388 high-risk women, >35 yr | Tamoxifen (20 mg/day) or placebo for 5 yr | Reductions in invasive, noninvasive, and ER-positive breast cancer incidence; no significant change in ER-negative tumors | 509; 510 |
| Italian | 5408 normal-risk women with a hysterectomy, 35-70 yr | Tamoxifen (20 mg/day) or placebo for 5 yr | Reductions in invasive and ER-positive breast cancer incidence; no significant change in ER-negative tumors | 512; 516 |
| IBIS-I | 7154 high-risk women, 35-70 yr | Tamoxifen (20 mg/day) or placebo for 5 yr | Reductions in invasive, noninvasive (DCIS), ER-positive, and overall breast cancer incidence; no significant change in ER-negative tumors | 513; 514 |
| NSABP-P2 (STAR) | 19,747 high-risk, postmenopausal women, >35 yr | Tamoxifen (20 mg/day) or raloxifene (60 mg/day) for 5 yr | Reductions in invasive and noninvasive ER-positive tumors less effective (but with decreased toxicity) with raloxifene than tamoxifen | 519; 520 |
| RALOXIFENE | ||||
| MORE | 7705 postmenopausal women with low BMD, <80 yr | Raloxifene (60 or 120 mg/day) or placebo for 4 yr | Reductions in invasive, ER-positive, and overall breast cancer incidence; no significant change in ER-negative tumors | 517 |
| CORE | 4011 postmenopausal women with low BMD (reconsented from MORE trial), <80 yr | Raloxifene (60 mg/day) or placebo for an additional 4 yr after 4 yr of raloxifene on MORE trial | Reductions in invasive, ER-positive, and overall breast cancer incidence; no significant change in noninvasive of ER-negative tumors | 636 |
| RUTH | 10,101 postmenopausal women with CHD, >35 yr | Raloxifene (60 mg/day) or placebo for a median of 5.6 yr | Reduction in invasive ER-positive tumors | 637 |
| LASOFOXIFENE | ||||
| PEARL | 8556 women with low BMD, 59-80 yr | Lasofoxifene (0.25 mg or 0.5 mg/day) or placebo | Reduction in invasive ER-positive tumors | 521; 638 |
| ARZOXIFENE | ||||
| Generations | 9354 women with low BMD, 60-85 yr | Arzoxifene (20 mg/day) or placebo for 4 yr | Reduction in invasive ER-positive tumors | 522; 639 |
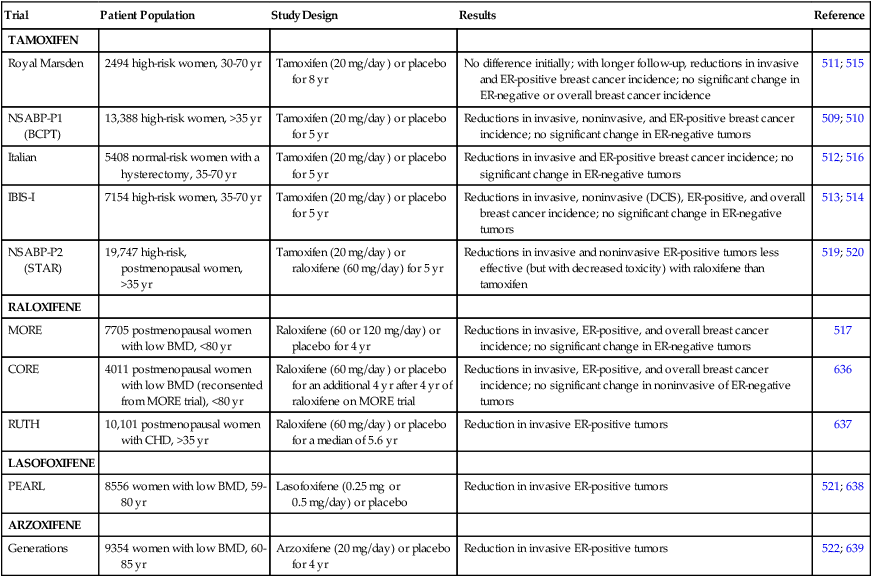
Another SERM, raloxifene, has been shown to effectively prevent many breast cancers. Raloxifene was initially developed and later approved as a drug for the treatment of osteoporosis to prevent bone fractures. However, the Multiple Outcomes of Raloxifene Evaluation clinical trial tested this drug in postmenopausal women with osteoporosis for its ability to prevent breast cancer.517 The results of this study demonstrated that women taking raloxifene had 72% fewer breast cancers than were seen in women taking placebo.518 These promising results led to the development and conduction of the Study of Tamoxifen and Raloxifene (STAR) trial that compared the ability of raloxifene or tamoxifen to prevent breast cancer in high-risk postmenopausal women. The initial results of the STAR trial showed that both tamoxifen and raloxifene had similar preventive activity.519 Each of these drugs prevented approximately 50% of the expected breast cancers; however, raloxifene use was ineffective in preventing preinvasive breast cancers and was associated with significantly fewer uterine cancers, thrombotic events, and hot flushes.519 Recently, the 81-month follow-up results of the STAR trial were released.520 These results showed that the breast cancer preventive activity of raloxifene decreased after 3 years, whereas the cancer preventive effect of tamoxifen persisted. After 81 months of follow-up, raloxifene retained 76% of tamoxifen’s ability to prevent invasive breast cancer. However, although raloxifene was slightly less effective than tamoxifen, it continued to show fewer adverse effects, with fewer uterine cancers, thrombotic events, and hot flushes.
Two other SERMs, lasofoxifene and arzoxifene, have been tested for their ability to prevent breast cancer. Both of these drugs were shown to reduce the risk of ER-positive breast cancer. In the Postmenopausal Evaluation and Risk-reduction with Lasofoxifene trial, lasofoxifene (0.25 mg/day and 0.5 mg/day) reduced the incidence of all invasive breast cancers by 79% and reduced ER-positive breast cancers by 83%.521 Like raloxifene, lasofoxifene use was associated with increased thromboembolic events, leg cramps, and hot flushes, but it was not associated with an increased risk of endometrial hyperplasias or endometrial cancers. As with the other SERMs, lasofoxifene had no effect on the development of ER-negative breast cancers. In the Generations trial, arzoxifene (20 mg/day) was compared with placebo to determine whether it reduced bone fractures and breast cancer in postmenopausal women with osteoporosis.522 Primary end points were new vertebral bone fractures and invasive breast cancer. After a follow-up period of 36 months (for bone fracture assessment) or 48 months (for breast cancer assessment), treatment with arzoxifene was associated with a 2.3% fewer vertebral bone fractures (a 41% relative risk reduction of vertebral bone fractures, with an absolute reduction of 2.3% fewer vertebral fractures) and a 56% relative risk reduction of invasive breast cancers (representing 1.3% fewer breast cancers after 4 years). The specific effect on ER-positive and ER-negative breast cancers was not described in this first report. Arzoxifene caused significantly more thromboembolic events, hot flushes, and vaginal discharge but did not significantly increase uterine cancers or endometrial hyperplasias.522 These results show that many different SERMs (Table 23-13) can prevent ER-positive breast cancers. The most effective SERM to date has been lasofoxifene.
Cuzick et al.523 have recently published a metaanalysis of these trials demonstrating that these antiestrogen drugs can prevent the development of invasive ER-positive breast cancer but not ER-negative breast cancer. In a recent consensus statement by Cuzick et al.,524 the authors suggest that women at high risk of breast cancer, such as those with a strong family history of breast cancer, high mammographic density, and atypical hyperplastic premalignant lesions, are good candidates for SERM preventive therapy.
Aromatase Inhibitors
Because aromatase inhibitor (AI) drugs (e.g., anastrozole, letrozole, and exemestane) have been shown to be more effective than SERMs at treating breast cancer (reviewed in Litton et al.525), interest has been strong in using these drugs for the prevention of breast cancer. The first indication that AI drugs would be effective for breast cancer prevention came from the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial testing anastrozole (Arimidex), tamoxifen, or the combination for the treatment of early-stage breast cancer.526 Results from the ATAC trial showed that anastrozole alone was more effective than tamoxifen in preventing the development of second primary breast cancers. 527 Women treated with anastrozole had 58% fewer breast cancers than those treated with tamoxifen when the ATAC trial data were initially reported (odds ratio [OR] = 0.42; P = .007).526 With the report of the 10-year follow-up data, the results showed that in women taking anastrozole, 32% fewer contralateral total breast cancers developed than in women taking tamoxifen (HR = 0.68; P = .01 for all breast cancers), and 38% fewer ER-positive breast cancers developed (HR = 0.62; P = .003).527 These provocative results led to the development of many clinical trials testing the ability of AI drugs for the prevention of breast cancer in women at increased risk (Table 23-14). Of these studies, the Mammary Prevention Trial-.3 (MAP.3) study has been completed and the results reported.528 The MAP.3 study compared exemestane to placebo in healthy postmenopausal women at high risk of breast cancer. The results showed that women who took exemestane had 65% fewer invasive breast cancers than did those taking a placebo (HR = 0.35; P = .002) and 73% fewer ER-positive breast cancers (HR = 0.27; P < .001). Women taking exemestane had significantly more adverse symptoms, including hot flushes, joint and muscle pain, and gastrointestinal symptoms, than did women taking a placebo. However, no significant differences in serious adverse effects, including skeletal fractures, thromboembolic events, cardiovascular events, or other cancers, were found between women taking exemestane or a placebo. These results indicate that exemestane may be particularly useful in preventing ER-positive breast cancers and may be tolerable in high-risk women, particularly those who are not at high risk of osteoporosis. Although the MAP.3 results demonstrate a significant benefit in postmenopausal women at risk of breast cancer, the exact role of AIs in managing breast cancer risk will await longer term results from this trial to document the long-term adverse effects of AI use in healthy women, as well as the results of the other breast cancer prevention trials shown in Table 23-14. However, it is clear that along with SERMs, the AI drugs offer a promising strategy to prevent ER-positive breast cancer.
Table 23-14
Select Aromatase Inhibitor (AI) Breast Cancer Prevention Studies
| Trial | Patient Population | Study Design | Primary End Point | Reference |
| DCIS Trials | ||||
| NSABP B-35 | Planned: 3000 women Postmenopausal with ER ± PR + DCIS |
Anastrozole (1 mg/day) vs. tamoxifen (20 mg/day) for 5 yr | Ipsilateral/contralateral breast cancer incidence | ClinicalTrials.gov, NCT00053898 |
| IBIS-II (DCIS) | Planned: 4000 women Postmenopausal with DCIS |
Anastrozole (1 mg/day) vs. tamoxifen (20 mg/day) for 5 yr | Ipsilateral/contralateral breast cancer incidence | 640 |
| PREVENTION TRIALS | ||||
| NCIC-MAP.3 | 4560 women accrued Postmenopausal, high-risk ≥35 yr |
Exemestane (25 mg/day) vs. placebo for 5 yr | Invasive breast cancer incidence | 528 |
| IBIS-II | Planned: 6000 women Postmenopausal, high-risk |
Anastrozole (1 mg/day) vs. placebo | Invasive breast cancer incidence | 640 |
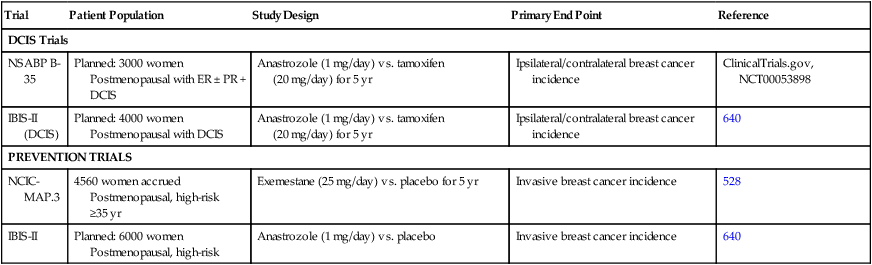
Other Drugs to Prevent ER-Negative Breast Cancer
SERMs and AI drugs are very effective at suppressing the development of ER-positive breast cancer. However, they have no ability to prevent the 30% to 40% of breast cancers that are ER negative, and thus a major unmet need is the development of drugs or strategies to prevent these particularly aggressive breast cancers. A variety of drugs have been studied in preclinical models to determine preventive efficacy on ER-negative breast cancer (Table 23-15). These agents include celecoxib, retinoid X receptor–selective retinoids (called “rexinoids”), vitamin D analogs, and EGFR and HER2 inhibitors (reviewed by Johnson and Brown529). Each of these molecularly targeted drugs has been shown to delay or prevent ER-negative mammary tumor formation in transgenic mouse models. Several drugs have also been tested in early-phase breast cancer prevention trials, including the rexinoid bexarotene,530 metformin,531 epigallocatechin gallate, the active agent in green tea,532 and the anti-HER2 drug trastuzumab.533 Other agents that are being tested in ongoing trials including the anti-EGFR/HER2 drug, lapatinib; the COX-2 inhibitor, celecoxib; and the 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitor, atorvastatin. With the completion of these early-phase trials, clinical investigators will be poised to test the most promising of these agents in phase III cancer prevention trials. Ultimately, it is anticipated that combination preventive therapy using drugs that target the ER in combination with drugs that target drivers of ER-negative breast tumorigenesis will be required to prevent all forms of breast cancer.
Table 23-15
Breast Cancer Prevention Studies Using Other Novel Agents
| Trial | Phase | Patient Population | Study Design | Results | Reference |
| Bexarotene | II | 87 high-risk women Postmenopausal |
Bexarotene (200 mg/m2) or placebo for 28 days | Reduction in cyclin D1 RNA, but not in Ki67 protein | 530 |
| Metformin | II | 200 nondiabetic women with operable breast cancer | Metformin (850 mg, bid) or placebo for 28 days | No difference in Ki67 protein, but different insulin-resistance-based effects (in luminal B tumors) | 531 |
| EGCG | I | 40 women with a history of stage I to III ER-negative breast cancer | EGCG (200, 600, 800 mg, bid) or placebo for 6 mo | The maximum tolerated dose for Poly E is 600 mg, bid | 532 |
| Trastuzumab | Pilot | 69 women with DCIS | Trastuzumab (8 mg/kg over 90-min intravenous infusion) vs. untreated | No significant antiproliferative or apoptotic changes, but augmented antibody-dependent cell mediated cytotoxicity with single-dose trastuzumab monotherapy | 533 |
| Lapatinib | II | Planned: 40 women with ER-positive or HER-positive DCIS | Lapatinib (1000 mg) vs. placebo | Currently in progress | ClinicalTrials.gov, NCT00555152 |
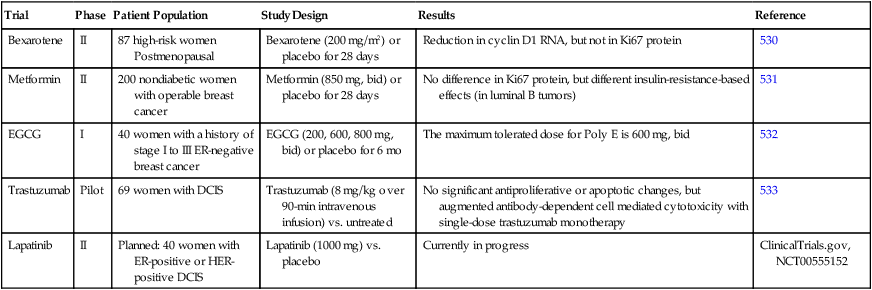
Other Strategies to Prevent Breast Cancer
Although traditional targeted drug therapy offers significant potential to reduce breast cancer incidence, researchers have a strong interest in developing other more effective and safer strategies for breast cancer prevention. A highly promising approach is to develop vaccines for breast cancer prevention. Although the development of vaccines has been a goal for many decades, recent results demonstrate the promise of a vaccine targeting the HER2 protein. In addition, recent studies by Mittendorf et al.534 have shown that a vaccine to the HER2 or ErbB2 protein can induce antitumor immunity against HER2-overexpressing breast cancers. These investigators have conducted phases I and II therapeutic trials testing the E75 peptide, a human leukocyte antigen A2/A3-restricted HER-2/neu (HER2) peptide that induced significant anti-HER2 immunity and caused an improvement in disease-free survival in women with early breast cancer. This vaccine is now being tested in phase III cancer treatment trials, and if effective, it would be a potentially promising strategy to prevent some, but not all, HER2-positive breast cancers. The results with this vaccine offer great promise for the development of other antitumor vaccines that may ultimately be most useful for the prevention of breast cancer.