Cancer of the Head and Neck
Paul B. Romesser, Nadeem Riaz, Alan L. Ho, Richard J. Wong and Nancy Y. Lee
• In the United States it was estimated that in the year 2012, head and neck cancer would account for 3.2% of all new cancer cases and 2% of all cancer deaths.
• The three major risk factors for head and neck cancer are tobacco, human papillomavirus infection, and alcohol.
• Management of head and neck cancers requires a multidisciplinary approach with effective integration of multiple specialties to achieve the desired goals of cure and functional organ preservation.
• Staging of head and neck cancer should be comprehensive and include a complete physical examination, fiberoptic laryngoscopy, computed tomography and/or magnetic resonance imaging of the head and neck, and a positron emission tomography scan for advanced stage disease to assess nodal involvement and distant metastases.
• The treatment of head and neck cancer is dictated by the primary site.
• Management of paranasal sinus malignancies is primarily surgical, with adjuvant radiation and possibly chemotherapy for advanced lesions. In unresectable and nonoperative cases, definitive radiotherapy should be offered.
• Surgery is generally considered the preferred initial treatment modality for oral cavity lesions. Adjuvant radiotherapy with or without chemotherapy is indicated for patients with high-risk features on surgical pathology. Definitive radiotherapy is reserved for unresectable tumors, nonoperable tumors, and tumors in which surgical resection would result in significant functional impairment.
• Most oropharyngeal cancers in the United States are now due to human papillomavirus and as such have an improved prognosis. Early-stage oropharynx cancers can effectively be treated with surgery or radiation therapy. The standard of care for locally advanced disease is chemoradiation; however, other modes of treatment are under active investigation.
• In persons with larynx cancer, the goal of first-line therapy should be to preserve the function of the larynx, without sacrificing tumor control. Early-stage laryngeal cancer can effectively be treated with either surgery or radiotherapy. In early-stage disease, the anatomic location and extent of disease will dictate if surgery is feasible. The treatment of choice in most locally advanced larynx cancers is concurrent chemotherapy and radiation. Because the Veterans Affairs (VA) larynx study demonstrated poor outcomes for persons with T4a disease, total laryngectomy is also a consideration for these patients.
• Surgical resection is the mainstay of management in salivary gland cancer, whether it arises from the parotid, submandibular, sublingual, or minor salivary glands. Combined therapy is recommended for high-grade malignancies of the salivary gland because postoperative irradiation has been shown to improve local-regional control in patients with positive surgical margins; high-risk features such as advanced stage, high-grade, skin/nerve invasion; and adenoid cystic carcinoma.
• The management of locally recurrent head and neck cancer is technically challenging and should be performed at centers where personnel have experience in treating these patients. Surgery is typically preferred and offered for resectable lesions in the absence of unacceptable functional sequelae. The roles of adjuvant radiotherapy (or reirradiation) and chemotherapy must be determined on a case-by-case basis.
• Metastatic head and neck squamous cell carcinoma carries a poor prognosis with a median survival of months. Systemic therapies are indicated for widespread disease.
Introduction
In the year 2012, it was estimated that head and neck cancer (HNC) would account for 3.2% of all new cancer cases and 2% of all cancer deaths in the United States.1 Because these tumors are relatively uncommon, misdiagnosis along with patient neglect contributes to an advanced stage at presentation and limited survival.2 Despite the relatively low numbers of HNCs in clinical practice, research and management of HNCs continue to receive significant emphasis because of the rich anatomic and functional complexity of this body site, which is critical to issues of self-esteem, communication, and social integration. Although squamous cell carcinomas (SCCs) constitute the majority of adult histopathological patterns in HNCs, the variation in histopathological types and the differing features of the possible anatomic subsites of involvement result in tremendous variability in the natural course of the disease for this small anatomic region.3
Tobacco, alcohol, and human papillomavirus (HPV) infection are the three major risk factors for head and neck squamous cell carcinoma (HNSCC) in developed countries. Current smokers have an approximate tenfold higher risk of HNC than do lifelong nonsmokers.4 Historically, approximately 80% to 90% of all head and neck carcinomas were attributable to tobacco consumption, particularly cigarette smoking, although currently the attribution is thought to be less given an increase in HPV-associated HNC.4 HPV-positive HNC incidence has been steadily increasing and is expected to surpass the incidence of HPV-negative HNC, which traditionally has been associated with tobacco and alcohol.5 It remains to be seen if current HPV vaccination recommendations will result in a decrease in HPV-associated HNC.
Clinical Presentation and Patient Evaluation
Staging Investigations
Currently the American Joint Committee on Cancer (AJCC) staging system, which uses a unidimensional tumor size and local anatomic invasion (T category), nodal involvement (N category), and presence of metastatic disease (M category), is the most widely accepted and applied prognostic system relating to cancer.6,7 Generally, T1, T2, and T3 represent increasing tumor size, whereas T4 is defined by invasion of a surrounding structure (i.e., skin, nerve, vessel, and cartilage). The node (N) category is classified largely by size and location (ipsilateral vs. contralateral) of involved lymph nodes. The absence or presence of distant metastases is defined as M0 if absent or M1 if present. The T, N, and M categories are combined into overall AJCC stages that are presented in Table 68-1. Because the natural history of HNC varies somewhat according to specific anatomic location of the primary disease and because stages III and IV include a large number of different T and N stages, it is customary to refer to specific head and neck cancers by their individual T, N, and M stage and the primary site.
Table 68-1
Overall Group Staging of Head and Neck Cancer: Tumor-Node-Metastasis (TNM) Classification
| Stage | Grouping | ||
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T3 | N0 | M0 |
| T1 | N1 | M0 | |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| IVA | T4a | N0 | M0 |
| T4a | N1 | M0 | |
| T1 | N2 | M0 | |
| T2 | N2 | M0 | |
| T3 | N2 | M0 | |
| T4a | N2 | M0 | |
| IVB | T4b | Any N | M0 |
| Any T | N3 | M0 | |
| IVC | Any T | Any N | M1 |
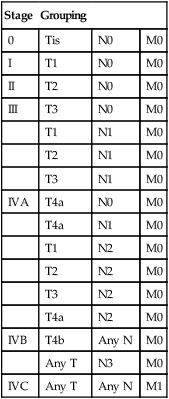
From American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Follow-up Program
After completion of initial treatment, patients are carefully assessed for response to treatment. For advanced-stage cancers treated with chemoradiation therapy (CRT), CT, positron emission tomography (PET), and/or MRI scans are obtained to assess radiographic disease response approximately 12 weeks after completion of treatment. Thereafter patients are seen at gradually increasing intervals for surveillance examinations. Chest radiographs may be obtained intermittently as indicated for surveillance for metastatic disease or secondary primary malignancies, particularly for patients with a history of smoking. Thyroid-stimulating hormone levels should be checked every 12 months because 20% to 25% of patients who have had neck irradiation experience hypothyroidism, necessitating thyroid hormone supplementation.8 After 5 years, patients may be examined yearly.
Treatment Overview
Defining Treatment Algorithms—Primary Site
The single-modality principle for treatment of early-stage disease attempts to minimize toxicity and emphasizes the importance of patient and tumor selection. When postoperative RT (PORT) appears likely, definitive RT may be more appropriate, particularly for small-volume disease, because it is unclear that combined modality therapy is superior to RT alone. This consideration is particularly relevant when initial definitive en bloc resection may, in combination with PORT, further complicate treatment toxicity and organ function. Not uncommonly, these issues arise with more extensive early-stage HNSCC or in cases in which the anatomic location limits achieving adequate surgical margins. For unresectable locally advanced disease, conventionally fractionated RT has been traditionally recommended. Suboptimal local-regional disease control in the absence of surgical options has forced investigation into various modalities of treatment intensification, including neoadjuvant chemotherapy, concurrent CRT, and altered fractionated RT.9,10
Although the AJCC staging system is the most widely accepted and applied prognostic system in persons with cancer, much attention has been given to its inability to identify patients with HNC who are at high risk of treatment failure.7 As such, various radiographic biomarkers have been studied to help improve our ability to prognosticate outcomes for individual patients.
During the past decade, interest has expanded in the use of PET-CT, specifically given its ability to be an accurate and sensitive imaging modality for the pre- and posttreatment evaluation of patients with HNC compared with clinical examination and CT alone.11,12 Controversy has plagued studies evaluating the prognostic and predictive utility of the standardized uptake value, essentially the metabolic tumor activity. The success of metabolic-based radiographic biomarkers rests on the ability to standardize protocols allowing interinstitutional comparisons.
Similarly, some volumetric indices have been explored as potential prognostic and predictive indices. Multiple studies have demonstrated that the gross tumor volume correlates with local-regional control and survival in patients with HNSCC who are undergoing surgery,13 RT,16–16 or CRT treatments in various HNC sites.17–21 Further incorporation of metabolic volume metrics in defining tumor volume, such as the metabolic tumor volume, has defined yet another prognostic radiologic biomarker.7,22,23 Unfortunately, no volumetric cut points have been prospectively validated.
Defining Treatment Algorithms—Management of the Neck
Typically, combined-modality strategies are used for the treatment of neck disease. In a neck with negative clinical findings, when surgical resection has been elected for the primary site management, elective neck dissection may be omitted for most subsites if preoperative evaluation determines a high risk of requiring PORT and the risk of occult nodal metastasis is sufficiently high to warrant elective management. When RT has been selected for management of the primary site, neck dissection in a neck with negative clinical findings is not indicated. In fact, pre-RT neck dissection may alter the lymphatic flow of the neck, necessitating larger volumes of the neck to be irradiated. Pre-RT neck dissection also may contribute to delays in the delivery of RT, which have been reported in a retrospective analysis to contribute to an adverse overall survival when compared with post-RT neck dissection.24,25
When conventionally fractionated RT alone has been used for locally advanced HNSCC, suboptimal regional control rates coupled with the morbidity and the limited success of subsequent salvage neck dissection have prompted the incorporation and general acceptance of a planned neck dissection. This approach, in the presence of residual adenopathy after RT, also has been selectively applied to patients with adverse risk factors such as large nodal size (typically 3 cm or greater). This risk stratification is based on RT series demonstrating an inverse relationship between nodal size and control rate.26,27 In a study of RT alone for treatment of HNSCC in 1251 patients, Dubray and colleagues27 noted 3-year neck control rates, by maximum nodal size, of 77% for 0.5 cm, 67% for 2 cm, 60% for 4 cm, 52% for 6 cm, 37% for 8 cm, and 7% for 10 cm. Multivariate analysis revealed that regional relapses independently increased with increased nodal size (P = .0001), decreased radiation dose (P = .0001), T4 primary disease (P = .0001), node fixation (P = .02), bilateral neck disease (P = .03), and geographic miss (P = .0001).
Controversy continues, however, regarding the benefit of a planned neck dissection in the setting of a complete clinical response in the neck, particularly in large pretreatment lymph nodes, after completion of RT.30–30 Treatment with conventionally fractionated RT has demonstrated that the prognosis of large neck nodes with a complete response is associated with a prognosis comparable with that of smaller nodal metastases, and that the risk of relapse is low.26,31 This finding suggests that perhaps the subgroup of patients with a complete response in the neck may have more radiosensitive disease and may not require a neck dissection. A neck dissection continues to be favored for persons with advanced neck disease (especially stage N3), because the likelihood of achieving a complete response with RT alone is limited. In addition, assessment of disease response by PET/CT allows a more objective evaluation of patients who would likely benefit most from a neck dissection.
Second Primary Tumors
Management of patients with HNSCC is further complicated by the risk for the development of second primary carcinomas and relapses within an aerodigestive tract that may have been extensively exposed to prior treatment. An irradiated aerodigestive tract not only limits reirradiation but also may preclude an effective surgical salvage, because concerns of residual microscopic disease often exist in cases that would otherwise warrant use of PORT. When a second primary or relapse occurs within a previously irradiated field, surgical resection should be the primary treatment option.32 Experiences with various repeat external beam radiotherapy (EBRT) strategies, including the integration of chemotherapy, have unfortunately demonstrated limited success, often at the risk of significant toxicities.32,33 The exception appears to be nasopharyngeal carcinomas (NPCs) that are more radiosensitive. Even then, significant late complications may arise but may need to be considered in view of the limited surgical options.
Surgery
Neck Dissection
A variety of different types of neck dissections may be performed (Fig. 68-1). The radical neck dissection is a procedure wherein the lymph nodes from all five levels of the neck, the sternocleidomastoid muscle, the internal jugular vein, and the spinal accessory nerve are all removed en bloc. This procedure is indicated in the setting of high-volume neck metastases involving these nonlymphatic structures.
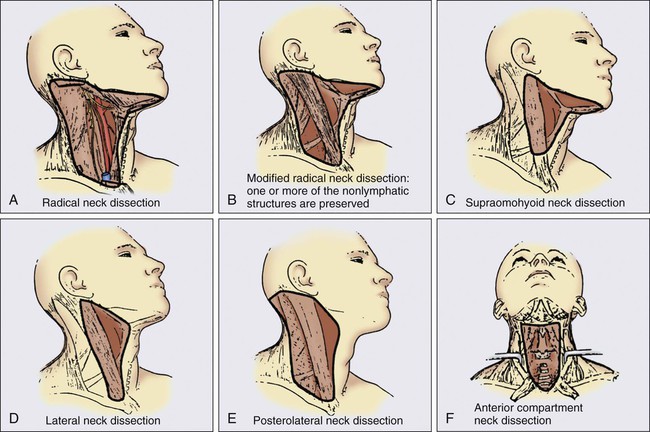
A modified radical neck dissection removes lymph nodes from all five levels of the neck but spares one or more of the nonlymphatic structures. Three types of modified radical neck dissection are type I (which spares one structure, most commonly the spinal accessory nerve), type II (which spares two structures, most commonly the spinal accessory nerve and the internal jugular vein), and type III (which spares all three structures—the spinal accessory nerve, internal jugular vein, and sternocleidomastoid muscle). A type III dissection also is termed a functional or Bocca neck dissection.34 Of these three structures, removal of the accessory nerve is generally considered to result in the most clinically significant functional deficit from shoulder dysfunction.
Selective neck dissections involve the resection of fewer than all five levels of lymph nodes, usually involving three or four levels according to the site of the primary cancer. These neck dissections are commonly performed in the setting of necks classified as N0, but they may be performed in selected cases of N+ nodal disease.37–37
Radiotherapy
RT has a long and successful history in the treatment of primary head and neck malignancies. Historical experiences with EBRT have demonstrated that acute treatment-limiting RT-induced dermatitis may be limited by fractionation and the use of higher energy RT. Accordingly, current standard RT practices have evolved to utilize a fractionated RT prescription using modern linear accelerators that can produce a spectrum of beam energies. With fractionation, issues of patient immobilization and treatment set-up reproducibility become important considerations. For the head and neck, critical normal tissue structures such as the spinal cord and optic chiasm often are in close proximity to the irradiated target. For these reasons, a prerequisite for treatment simulation is that patients are immobilized with various devices such as a custom-made face mask and frame, with the setup referenced to a laser light coordinate system in the treatment rooms (Figs. 68-2 and 68-3).
Immobilization addresses the issue of the precision of the treatment delivery as a strategy to optimize the therapeutic ratio. Historically, patients were treated with two-dimensional RT, which did not permit significant sparing of normal tissues and resulted in significant toxicity. In these cases, the oncologist would outline the areas to irradiate on a radiograph of the head and neck. Modern treatment with intensity-modulated RT (IMRT) involves obtaining axial CT images and delineating the tumor and normal tissue on each CT slice. IMRT allows the modulation of the RT beam to achieve exquisite dose conformality, which means that the tumor receives the full dose of RT while minimizing the dose delivered to critical normal structures. Multiple randomized trials have now demonstrated significantly reduced toxicity with IMRT compared with two-dimensional RT.40–40
Given the improved conformality, the success of IMRT is dependent on the ability to identify anatomic sites that harbor subclinical disease. Currently, the basis for this determination is derived from surgical and clinical documentation of disease extension that is often unique to each head and neck subsite. During the past decade interest has been expanding in PET/CT, specifically given its ability to be an accurate and sensitive imaging modality for the pre- and posttreatment evaluation of patients with HNC compared with clinical examination and CT alone.11,12 Currently, the radiation oncologist must incorporate data from the clinical examination, CT, PET/CT, and often MRI when designing the radiation plan.
The identification of nodal groups typically at risk for harboring subclinical nodal metastases has proven more difficult. To aid in this identification, several reports have defined axial anatomic structures that may be used to delineate the various nodal groups (Table 68-2).41 The incidence of nodal metastases has been summarized by site and can aid the radiation oncologist (Table 68-3). Of note, however, when prior treatment to the neck has occurred, altered flow of lymphatics is a concern. As with the head and neck surgeon, judgment must be exercised with these precise treatment techniques.
Table 68-2
Recommendation for the Radiologic Boundaries of the Neck Node Levels
| Level | Anatomic Boundary | |||||
| Cranial | Caudal | Anterior | Posterior | Lateral | Medial | |
| Ia | Geniohyoid m. | Platysma m. | Symphysis menti; platysma m. | Body of hyoid bone | Medial edge of anterior belly of digastric m. | NA* |
| Ib | Mylohyoid m., cranial edge of submandibular gland or caudal edge of medial pterygoid m. | Platysma m. | Symphysis menti | Body of hyoid bone; posterior edge of submandibular gland | Basilar edge of mandible; platysma m. | Lateral edge of anterior belly of digastric m. |
| II | Bottom edge of the body of CI | Bottom edge of the body of hyoid bone | Posterior edge of submandibular gland; posterior edge of posterior belly of digastric m. | Posterior border of sternocleidomastoid m. | Medial edge of sternocleidomastoid m. | Internal edge of internal carotid artery, paraspinal (levator scapulae) m. |
| III | Bottom edge of the body of hyoid bone | Bottom edge of cricoid cartilage | Posterolateral edge of sternohyoid m. | Posterior edge of sternocleidomastoid m. | Medial edge of sternocleidomastoid m. | Internal edge of carotid artery, paraspinal (scalenus) m. |
| IV | Bottom edge of cricoid cartilage | Cranial border of clavicle | Posterolateral edge of sternohyoid m. | Posterior edge of sternocleidomastoid m. | Medial edge of sternocleidomastoid m. | Internal edge of internal carotid artery, paraspinal (scalenus) m. |
| V | Skull base | Cranial border of clavicle | Posterior edge of sternocleidomastoid m. | Anterior border of trapezius m; scalenus m. | Platysma m; skin | Paraspinal (levator scapulae, splenius capitis) m. |
| VI | Bottom edge of the body of hyoid bone | Sternal manubrium | Skin; platysma m. | Posterolateral edge of sternohyoid m. | Medial edge of common carotid artery, skin and anterior-medial edge of sternocleidomastoid m. | NA |
| Retropharyngeal | Base of skull | Cranial edge of the body of hyoid bone | Levator veli palatini m. | Prevertebral m. (longus colli, longus capitis) | Medial edge of internal carotid artery | Midline |
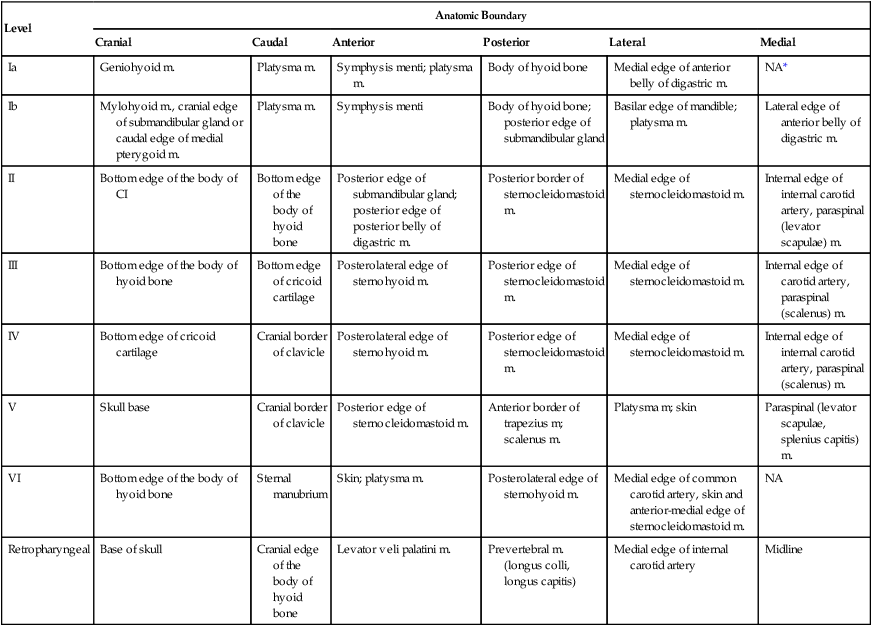
*Midline structure lying between the medial borders of the anterior belly of the digastric muscle.
From Grégoire V, Coche E, Cosnard G, et al: Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000;56:135–50.
Table 68-3
Distribution of Clinical Metastatic Neck Nodes from Head and Neck Squamous Cell Carcinomas
| Distribution of Metastatic Lymph Nodes per Level (% of Node-Positive Patients) | |||||||
| Tumor Size | Patients with N+ (%) | I | II | III | IV | V | Other* |
| Oral cavity (N = 787) | 36 | 42/3.5† | 79/8 | 18/3 | 5/1 | 1/0 | 1.4/0.3 |
| Oropharynx (N = 1479) | 64 | 13/2 | 81/24 | 23/5 | 9/2.5 | 13/3 | 2/1 |
| Hypopharynx (N = 847) | 70 | 2/0 | 80/13 | 51/4 | 20/3 | 24/2 | 3/1 |
| Supraglottic larynx (N = 428) | 55 | 2/0 | 71/21 | 48/10 | 18/7 | 15/4 | 2/0 |
| Nasopharynx (N = 440) | 80 | 9/5 | 71/56 | 36/32 | 22/15 | 32/26 | 15/10 |
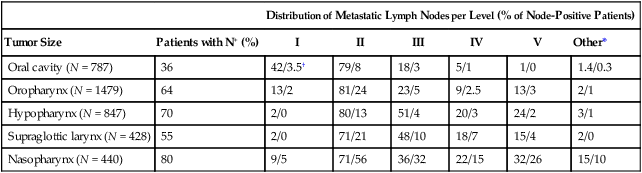
†Ipsilateral/contralateral nodes.
From Grégoire V, Coche E, Cosnard G, et al: Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000;56:135–50.
Conventional or daily RT fractionation (typically, daily 1.8- to 2-Gy fractions to a total dose of 70 Gy) has permitted the delivery of high RT doses, which is currently limited by normal tissue radiation dose tolerance levels, such as in the mandible, parotid glands, brainstem, and lens. Conceptually, RT failures may result from insufficient doses of RT relative to the number of tumor clonogens or may be due to cellular mechanisms of radioresistance. The former has proved to be more amenable to therapeutic manipulation with the use of altered fractionation schedules, which may be generalized into two groups: hyperfractionation and accelerated fractionation.42
A hyperfractionation schedule, or the use of lower doses per treatment fraction, has been hypothesized to reduce the risk of late RT-induced complications associated with an increase in the total RT dose. Typically, the dose per fraction is reduced to 1.2 to 1.5 Gy and exploits a differential radiosensitivity between normal late-responding tissues and most HNSCC. To ensure that the overall treatment time is not adversely protracted, fractions often are delivered twice a day, with an interfraction period of 6 hours. European Organization for Research and Treatment of Cancer (EORTC) trial 22791 compared hyperfractionation (1.5 Gy twice daily to a total dose of 80.5 Gy) with conventional fractionation (2 Gy once daily to a total dose of 70 Gy) in patients with stage T2-3N0-1 oropharyngeal cancer (excluding the base of the tongue). This trial demonstrated a statistically significant improvement in local control of the hyperfractionation arm (38% vs. 56%, P = .01) at 5 years with no increase in late complications.43
Alternatively, an accelerated fractionation RT schedule attempts to deliver the prescribed total dose over a shorter treatment duration. This strategy was founded on observations of adverse local-regional control rates with protracted treatment durations (with conventional fractionated schedules) such that higher total doses are required to maintain the same probability of tumor control. These results have been interpreted to be consistent with a model whereby tumor clonogens surviving each daily RT fraction undergo an accelerated rate of repopulation. As a consequence, a larger tumor burden would be expected with increasing duration of treatment interruptions. It has been rationalized that by reducing the overall treatment time, the opportunity and impact of accelerated tumor repopulation would be minimized. As the severity of acute toxicities is increased, some accelerated schedules studies have attempted to modify the risk of unacceptable acute toxicities by modifying either the dose per fraction or the total dose as a strategy to achieve an acceptable therapeutic ratio. EORTC trial 22851 compared accelerated fractionation (1.6 Gy three times daily to a total dose of 72 Gy) with conventional fractionation (1.8- to 2-Gy once daily to a total dose of 70 Gy) in patients with intermediate to advanced HNCs, excluding the hypopharynx. A significantly improved 5-year local-regional control rate (P = .02) was noted, but acute and late toxicities were increased in the accelerated fractionation arm.44
To date, no single fractionation schedule has proved optimal. RTOG 90-03, a phase 3 randomized study comparing hyperfractionation, two variants of accelerated fractionation, and standard fractionation, reported that both accelerated fractionation with a concomitant boost and hyperfractionation were associated with improved local-regional control and disease-free survival compared with standard fractionation, but acute toxicity was increased.10,45
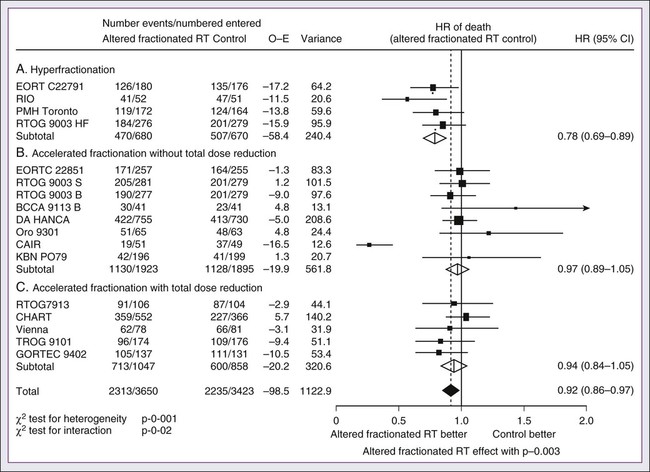
The metaanalysis of RT in carcinomas of the head and neck collaborative group pooled 15 HNSCC randomized trials for a total of 6515 patients and reported that altered fractioned schedules, hyperfractionated or accelerated RT, had a 5-year absolute survival benefit of 3.4% (hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.86-0.97; P = .003) and an absolute local control benefit of 6.4% (P < .0001) when compared with standard fractionation (Fig. 68-4).46 It is important to note that the benefit was significantly higher for younger patients aged <50 years. Since RTOG 01-29 and GORTEC 99-02 both demonstrated no significant improvement in concurrent fractionated CRT versus altered fractionated CRT, altered fractionated therapy is typically reserved for patients with locally advanced disease who are not candidates for systemic therapy.47,48
Postoperative Radiotherapy
For more advanced primary lesions (typically defined as stage T3 and T4 disease), increased treatment-related toxicities generally have been accepted because of their inferior local control rates. Accordingly, a combined-modality approach typically has been used. Traditionally, this approach has included surgery and RT with either preoperative or postoperative conventionally fractionated RT. A postoperative protocol generally has been favored because the RT can be delayed until more accurate delineation of the tumor extent of disease and histopathological stratification of patients requiring PORT.49
PORT has been demonstrated to provide superior outcomes in a subset of patients with HNSCC compared with preoperative RT. The indications for PORT are based on factors associated with an increased risk of local-regional recurrence after surgery, including advanced tumor stage (T3/T4), presence of a positive margin, extracapsular spread outside lymph nodes, lymphovascular invasion, perineural invasion, presence of a lymph node greater than 3 cm, and multiple positive lymph nodes. When evaluated in a randomized trial in 277 patients with supraglottic larynx and hypopharynx carcinomas (Radiation Therapy Oncology Group [RTOG] trial 73–03), PORT demonstrated superior local-regional control rates compared with preoperative RT, although the results were confounded by use of a higher dose delivered in the postoperative setting.50
Poor risk factors such as the presence of extracapsular extension or positive margins require the addition of chemotherapy to PORT. In a study of 334 patients with tumors possessing high-risk pathological features and who underwent surgery with curative intent, EORTC trial 22931, patients were randomly assigned to postoperative RT alone (66 Gy) versus PORT with concurrent cis-diamminedichloroplatinum (CDDP; cisplatin) (with a dose of 100 mg/m2 on days 1, 22, and 43 of the RT regimen).51 Postoperative concurrent CRT (POCRT) significantly improved progression-free survival (HR 0.75; 95% CI 0.56-0.99) and overall survival (HR 0.70; 95% CI 0.52-0.95) and reduced the cumulative incidence of local-regional recurrence in patients receiving POCRT compared with RT alone. Similarly, RTOG 95-01, which randomized 459 patients with high-risk pathological features to receive PORT alone (60 to 66 Gy in 30 to 33 fractions) or RT plus concurrent cisplatin (100 mg/m2 on days 1, 22, and 43), reported that POCRT yielded significantly improved rates of local-regional control (HR 0.61; 95% CI 0.41 to 0.91) and disease-free survival (HR 0.78; 95% CI 0.61 to 0.99). Not surprisingly, CRT resulted in greater grade 3 or greater acute toxicity compared with RT alone (77% vs. 34%, P < .001). Pooled analysis of these two trials suggested that PORT with cisplatin should be considered for patients with either a positive margin or extracapsular extension.52
Recent randomized trials have permitted the identification of prognostic factors adversely influencing the outcome, particularly in high-risk patients.53,54 Of greater significance, it is now clear that the time from surgery to the start of PORT and the overall treatment time of PORT are important determinants of local-regional control.55
Brachytherapy
Although brachytherapy previously had a significant role in the management of HNSCCs, its current role in the initial management of HNSCC has decreased in the modern era. With the advent of IMRT and the routine use of concurrent chemotherapy, brachytherapy has become less used in the upfront setting, although this is not true in the recurrent setting. It is important to note that studies have demonstrated good outcomes with brachytherapy implants in the definitive setting for several tumor sites, including the tonsil and soft palate,56–61 oral tongue,62–65 base of tongue,66–73 and lip.74,75
Neoadjuvant and Induction Chemotherapy
Induction chemotherapy, which has been studied for more than 3 decades, has been repeatedly associated with significant tumor shrinkage and possibly with a decrease in the risk of distant metastases.76 Unfortunately, an improvement in overall survival for induction chemotherapy over standard treatment approaches has not been definitively demonstrated.
Nonrandomized phase 2 trials in the 1970s used single-agent chemotherapy based on strategies used in the recurrent and metastatic setting. These single-agent trials reported 30% to 40% response rates. Induction strategies subsequently evolved to include multiple chemotherapy regimens. The first reported trials by Wittes and colleagues77 demonstrated a 71% response rate with complete responses noted in 21% of patients using cisplatin and continuous infusion bleomycin in 21 patients. Other studies using cisplatin/bleomycin with other drugs such as hydroxyurea followed and revealed increased toxicity with no improvement in response rates or survival.78 Investigators from Wayne State University reported the first trial using neoadjuvant cisplatin with infusional 5-fluorouracil (5-FU), with an overall response rate of 88% and a complete response rate of 54%.79 Trials using carboplatin with 5-FU have also produced good rates of response.80
In 1985, the increasing interest in laryngeal preservation coupled with disappointing experiences with upfront radiotherapy for advanced disease laid the foundation for the Veterans Affairs Cooperative Studies Program to initiate a multiinstitutional randomized trial of neoadjuvant chemotherapy as an organ preservation strategy.81 Patients with previously untreated, locally advanced, but potentially resectable stage III (T2-3N1 or T3N0) or stage IV (T1-3N2-3 or T4N0-1) disease of the supraglottic or glottic larynx were randomized either to receive neoadjuvant chemotherapy or to undergo surgical resection. The chemotherapy regimen was cisplatin (100 mg/m2) with continuous-infusion 5-FU (1000 mg/m2) for 5 days every 21 days with response after two cycles used to stratify patients to either continue with an additional cycle of chemotherapy followed by RT or, for nonresponders, salvage surgery followed by PORT. In total, 332 patients were enrolled: 216 patients with T3 disease, 85 with T4 disease, and 240 patients with N0-1 disease. Laryngeal preservation was achieved in 64% of patients enrolled in the chemotherapy arm. The local failure rate was significantly higher in the chemotherapy plus irradiation arm, but the distant failure rate was significantly lower in this arm. The estimated 2-year overall survival rate was 68% in each arm. This trial demonstrated that laryngeal conservation was achievable in a significant proportion of patients with advanced laryngeal carcinoma without sacrificing overall survival. However, the incremental value of neoadjuvant chemotherapy over RT alone was not answered in this trial.
These findings led to the RTOG 91-11 trial, which was conducted in patients with stage III or IV resectable disease of the larynx.82 Patients were randomized to three treatment groups: chemotherapy (cisplatin plus 5-FU) followed by RT, concurrent CRT with high-dose cisplatin as the radiosensitizer, or standard fractionated EBRT. The rate of laryngeal preservation at a median follow-up of 3.8 years was significantly higher among patients who received RT with concurrent cisplatin (84%) than in those who received induction chemotherapy followed by RT (72%, P = .005) or RT alone (67%; P < .001). Both chemotherapy regimens suppressed distant metastases and resulted in better disease-free survival than did RT alone. Despite these differences, the overall survival rates were similar in all three groups. As expected, the rate of high-grade toxic effects was greater with the chemotherapy-based regimens compared with RT alone. This study demonstrated that concurrent CRT was the preferred approach for laryngeal preservation.
Efforts continue to improve on the clinical efficacy of neoadjuvant chemotherapy in hopes of achieving significant activity to yield consistent survival benefits.83 Largely based on evidence from two European studies (TAX 323 and TAX 324), the U.S. Food and Drug Administration (FDA) has approved induction chemotherapy with docetaxel (Taxotere), cisplatin (Platin), and 5-FU (TPF) in patients with inoperable and operable HNSCC.76 The TAX 323 trial was a multicenter, randomized, phase 3 trial of 358 patients with previously untreated, unresectable, locally advanced stage III and IV tumors who were randomized to cisplatin and 5-FU with or without docetaxel (TPF and PF regimens, respectively).84 Four to 7 weeks after chemotherapy, patients who did not have progressive disease underwent RT. Patients treated with TPF had a reduction in their risk of death (27%, P = .02) and improved progression-free survival (HR 0.72; P = .007). The TAX 324 trial randomly assigned 501 patients with stage III or IV HNSCC to receive either PF or TPF as induction chemotherapy followed by CRT with concurrent carboplatin (sequential therapy) in patients who did not have progressive disease. In this trial, patients who received TPF had improved overall survival (HR 0.70, P = .006) and local-regional control (P = .004).85 Benefits with TPF did come at the cost of higher rates of toxicity. Notably, neither of these trials had a control arm with standard CRT alone.
The metaanalysis of chemotherapy on HNC (MACH-NC) from Pignon and colleagues86 included 31 induction chemotherapy trials for a total of 5311 patients over a median follow-up of 6.1 years. Overall, induction chemotherapy had a nonsignificant absolute survival benefit of 2.4% at 5 years (HR 0.96, 95% CI 0.90-1.02: P = .18; Fig. 68-5). No significant variation (P = .23) of the effect according to the type of chemotherapy was found. These investigators concluded that no clear evidence existed of a differential effect of induction chemotherapy on survival according to age, sex, performance, stage, or tumor site. Without randomized data demonstrating the benefit of induction chemotherapy compared with CRT alone, the use of induction chemotherapy remains an investigational approach.
Concurrent and Concomitant Chemoradiotherapy
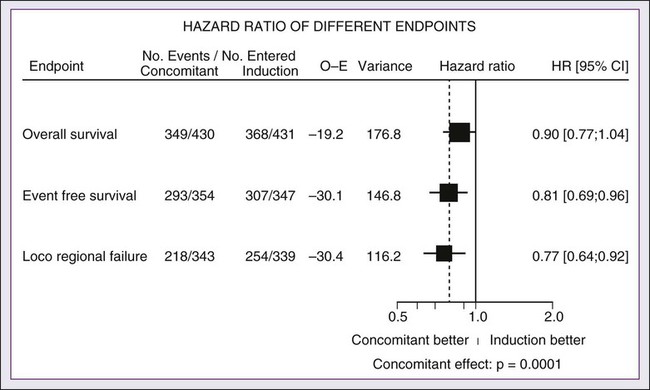
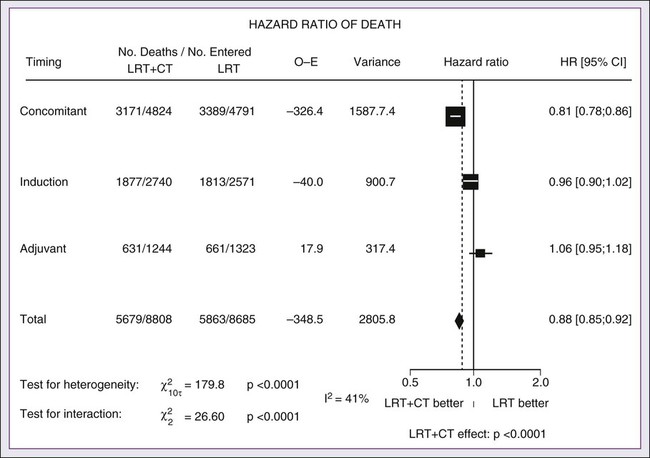
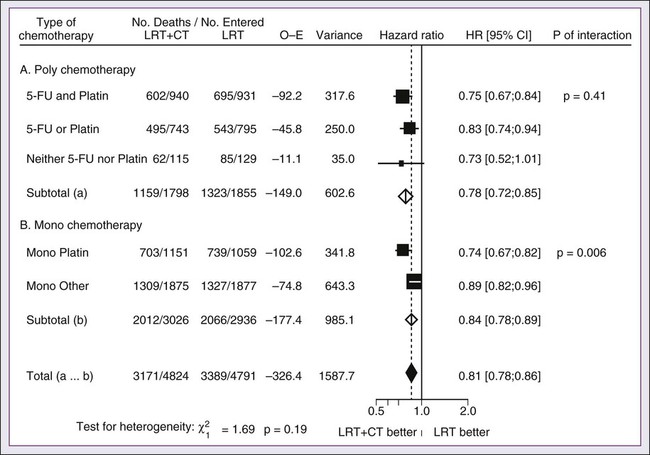
A significant body of literature exists, including numerous randomized trials of concurrent chemotherapy, systematically analyzed by several investigators.87–90 These independent reviews have consistently favored the concurrent integration of chemotherapy with RT. Pignon and colleagues86 from the MACH-NC have reported the largest and most recently updated of these metaanalyses. A patient-based metaanalysis of 9615 patients who underwent concomitant CRT was derived from 50 randomized trials and confirmed an absolute 5-year survival benefit of 6.5% (HR 0.81; 95% CI 0.78-0.86; Fig. 68-5). The benefit of chemotherapy was due to its effect on deaths related to HNC (HR 0.78; 95% CI 0.73-0.84), because no benefit was seen for noncancer deaths (HR 0.96; 95% CI 0.82-1.12). Similarly, an absolute benefit of 6.2% was seen in event-free survival (HR 0.79; 95% CI 0.76-0.83). This benefit was largely restricted to patients 70 years of age and younger because a statistically significant decreasing effect of chemotherapy on survival with increasing age was found (P = .003). Importantly, no significant difference was seen between monochemotherapy and polychemotherapy regimens (P = .19), and no difference was noted between different polychemotherapy subgroups (Fig. 68-6). In patients receiving monochemotherapy, the effect was significantly higher with platinum agents than with other types of monochemotherapies (Fig. 68-6).
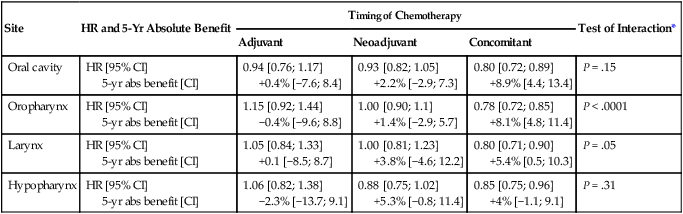
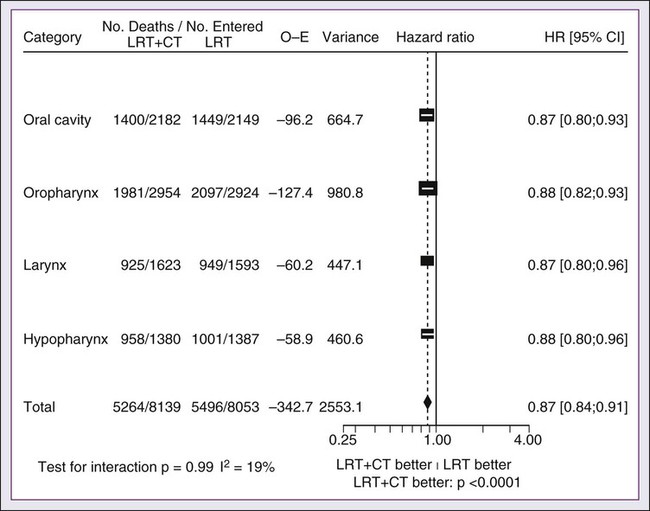
In its most recent update, the MACH-NC collaborative group published a comprehensive analysis by tumor site.91 This analysis included 4331 patients with cancer of the oral cavity, 5878 patients with oropharyngeal cancer, 3216 patients with laryngeal cancer, and 2767 patients with hypopharyngeal cancer. Overall, the addition of chemotherapy resulted in a reduction of the risk of death by 13% (Fig. 68-7), with the greater benefit for tumor sites associated with concomitant administration. The 5-year absolute overall survival benefits associated with concomitant chemotherapy were 8.9%, 8.1%, 5.4%, and 4% for oral cavity, oropharynx, larynx, and hypopharynx tumors, respectively (Table 68-4). Similarly, the 5-year absolute event-free survival benefits associated with concomitant chemotherapy are 6.9%, 8.4%, 5.4%, and 3.2% for oral cavity, oropharynx, larynx, and hypopharynx tumors, respectively. In a direct comparison of concomitant versus induction chemotherapy, MACH-NC analyzed six trials with a total of 861 patients over a median follow-up of 10.9 years.86 Patients who underwent concomitant CRT had a nonsignificant improvement in overall survival with an absolute benefit of 3.5%, significantly decreased local-regional failure, and significantly improved event-free survival compared with patients who were treated with induction chemotherapy (Fig. 68-8).
Table 68-4
Hazard Ratios of Death and 5-year Absolute Benefit (Overall Survival) Associated with the Use of Chemotherapy According to Tumor Site and Chemotherapy Timing
| Site | HR and 5-Yr Absolute Benefit | Timing of Chemotherapy | Test of Interaction* | ||
| Adjuvant | Neoadjuvant | Concomitant | |||
| Oral cavity | HR [95% CI] 5-yr abs benefit [CI] |
0.94 [0.76; 1.17] +0.4% [−7.6; 8.4] |
0.93 [0.82; 1.05] +2.2% [−2.9; 7.3] |
0.80 [0.72; 0.89] +8.9% [4.4; 13.4] |
P = .15 |
| Oropharynx | HR [95% CI] 5-yr abs benefit [CI] |
1.15 [0.92; 1.44] −0.4% [−9.6; 8.8] |
1.00 [0.90; 1.1] +1.4% [−2.9; 5.7] |
0.78 [0.72; 0.85] +8.1% [4.8; 11.4] |
P < .0001 |
| Larynx | HR [95% CI] 5-yr abs benefit [CI] |
1.05 [0.84; 1.33] +0.1 [−8.5; 8.7] |
1.00 [0.81; 1.23] +3.8% [−4.6; 12.2] |
0.80 [0.71; 0.90] +5.4% [0.5; 10.3] |
P = .05 |
| Hypopharynx | HR [95% CI] 5-yr abs benefit [CI] |
1.06 [0.82; 1.38] −2.3% [−13.7; 9.1] |
0.88 [0.75; 1.02] +5.3% [−0.8; 11.4] |
0.85 [0.75; 0.96] +4% [−1.1; 9.1] |
P = .31 |
abs, Absolute; CI, 95% confidence interval; HR, hazard ratio.
Cetuximab, a monoclonal IgG1 antibody against the ligand-binding domain of the epidermal growth factor receptor (EGFR), has been studied as a single agent or in combination with cisplatin in patients with HNC with promising results.92,93 Bonner and colleagues94 randomized 424 patients with stage III/IV HNC to treatment with high-dose RT alone (n = 213) or high-dose RT plus weekly cetuximab (n = 211) at an initial dose of 400 mg/m2 followed by weekly 250 mg/m2 doses for the duration of RT. RT with concurrent cetuximab resulted in a significant 32% reduction in the risk of local-regional progression, increased overall survival (HR for death 0.74, P = .03), and prolonged progression-free survival (HR for disease progression or death 0.70, P = .006). This overall survival advantage was further validated in a subsequent report on the updated 5-year analysis (HR 0.73, P = .018).95 Unfortunately, in the MACH-NC metaanalysis, concurrent CRT trials with cetuximab were limited, preventing definitive conclusions.
Nutritional Considerations
The proximity of HNCs to the oral and esophageal mucosa results in an increased risk of treatment toxicity. Approximately 50% of patients undergoing concurrent CRT experience severe dysphagia, and the majority experience significant mucositis throughout treatment, both of which result in decreased oral intake and resultant malnutrition.96,97
Prophylactic feeding tubes were routinely used in patients with HNC who were undergoing CRT because it was thought that by providing adequate nutritional support, one could decrease dehydration, malnutrition, fatigue, and other treatment-related complications and improve overall treatment outcomes by limiting medically indicated radiation treatment breaks, which historically have been associated with worse outcomes.55 Although multiple single-institution reports have reported decreased weight loss in patients with HNC who underwent percutaneous endoscopic gastrostomy (PEG) placement,98 none has demonstrated improved clinically outcomes.
The routine use of prophylactic feeding tubes has recently been called into question in a recent report from Memorial Sloan-Kettering Cancer Center (MSKCC). Placement of a prophylactic percutaneous gastrostomy (pPEG) in patients with oropharyngeal HNC who were undergoing definitive CRT failed to significantly improve overall survival, increase albumin levels, decrease acute toxicity rates (e.g., dysphagia, mucositis, and xerostomia), decrease chronic dysphagia, and affect treatment duration compared with patients who refused pPEG placement.99 Given these data, pPEG likely has a role in the management of patients with HNC, but it remains to be defined who will benefit most, and we recommend placement on a case-by-case basis. Current recommendations, from our institution and the National Comprehensive Cancer Network, are to consider the prophylactic placement of PEG in patients who have severe weight loss prior to treatment or are at high risk of weight loss and dehydration that would require interventions that could potentially delay treatment.
Specific Anatomic Sites
Nasopharyngeal Carcinoma
Anatomy
Local spread may include extension anteriorly through the submucosa including the nasal cavity, superiorly through the foramen lacerum, located superior to Rosenmüller’s fossa, into the base of skull, laterally to the parapharyngeal space, and inferiorly into the oropharynx. In patients with skull-base involvement, two cranial neuropathies have commonly been described. The petrosphenoidal syndrome, that is, superior invasion into the base of skull, involves cranial nerves III, IV, V, and VI, and patients commonly describe facial pain with V2 involvement with or without ocular muscle and efferent papillary reflex deficits. The retroparotidian syndrome, that is, lateral invasion into the parapharyngeal space, involves cranial nerves IX, X, XI, and XII. Metastatic spread to adjacent upper cervical lymph nodes is seen in 80% to 90% of patients at the time of presentation, with more than 50% presenting with bilateral neck disease, most commonly involving the retropharyngeal lymph nodes though other major routes of lymphatic drainage, including jugular chain and spinal accessory chain pathways. Hematogenous dissemination, most commonly to bone and thereafter to the lung and liver, is present in 3% to 6% of patients at time of presentation, most commonly in patients with advanced neck node metastases, especially with low-neck involvement.100–105
Epidemiology
Nasopharyngeal cancer exhibits regional bias with age-adjusted incidence rates (per 100,000 people per year) among men ranging from 0.6 in the United States and Japan, 5.4 in Algeria, 11.0 in Singapore, 17.2 among Eskimos, to 26.9 in Hong Kong and Guangdong province in Southern China.108–108 This discriminative geographic distribution is likely of a multifactorial etiology related to geographic virus infectivity rates, genetic associations, and regional environmental causes. The high consumption of salted fish in Southern China has been implicated, largely as a result of high concentrations of dimethylnitrosamine, a suspected human carcinogen.109–112 Genome-wide association studies have identified HLA A2, B46, and B17 as associated with an increased risk of developing nasopharyngeal carcinoma.113–116 Cigarette smoke, alcohol consumption, and exposure to formaldehyde have also been associated with increased risk of NPC.117,118 Epstein-Barr virus (EBV), one of the seven classified oncoviruses discussed in Chapter 11, has been associated with NPC, specifically the nonkeratinizing type, irrespective of ethnic or geographic origin.119,120 Increased immunoglobulin (Ig)-A antibodies to EBV have been reported to appear months to years before the clinical onset of NPC and can be used to define populations at high risk of EBV-associated epithelial cancer.121
Histology/Pathology
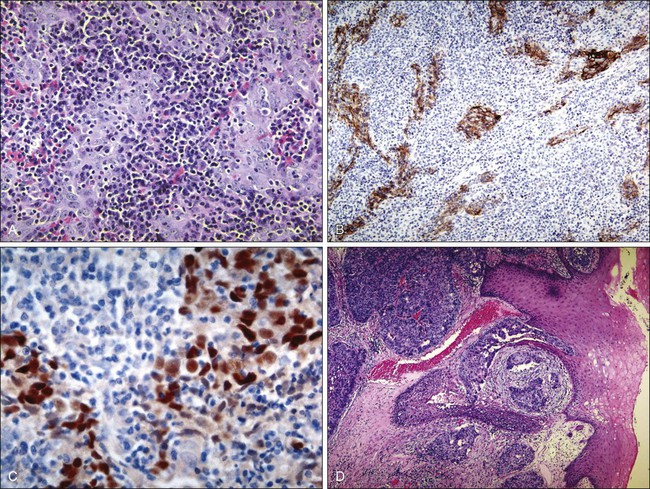
NPC constitutes 80% to 95% of nasopharyngeal cancers, with the remaining 5% to 20% consisting of lymphoma (approximately 5%) and less commonly adenocarcinoma, sarcoma, plasmacytoma, and melanoma. The 2005 World Health Organization pathological classification includes three classifications: keratinizing, nonkeratinizing (differentiated or undifferentiated), and basaloid SCC (Fig. 68-9).122 Keratinizing and nonkeratinizing SCC in the nasopharynx is morphologically similar to lesions found in the larynx and oral cavity. The nonkeratinizing forms are often EBV-associated and may also take the appearance of transitional epithelium—hence the designation of transitional type. Basaloid SCC is a rare and highly aggressive variant characterized by cells infiltrating in small to large nests with prominent central comedo-type necrosis. At the edges of the nests, the tumor cells form an organized palisade. In addition, variable areas of the tumor show malignant squamous cell morphology with keratinization. Cytologically, the tumor cells show marked nuclear pleomorphism with single cell necrosis and high mitotic rates. Histologic differences between these three classifications are not distinct, and many NPCs are histologic hybrids.
Diagnostic/Staging Workup
Pretreatment diagnostic evaluations include a comprehensive medical history, complete physical examination with added focus on palpation of cervical and supraclavicular neck nodes, cranial nerve testing, abdominal palpation for hepatomegaly and/or splenomegaly, and a spine/bone examination to assess for tenderness, fiberoptic endoscopy, and otologic assessment with baseline audiologic testing (recommended). Laboratory tests should include a complete blood cell count, basic metabolic panel, liver function tests, urinalysis, and EBV-specific serologic tests including antibody titers (IgA, IgM, and IgG), antiviral capsule antigen titers, and serum EBV-DNA levels. Radiographic studies are required to assess local-regional disease with preference for MRI, but CT remains an acceptable alternative.125–125 A chest radiograph should be obtained for all patients. Further radiographic studies to define distant metastases are required in patients with local-regional advanced disease (e.g., stage N3 disease) with initial preference given to PET and chest radiography, but further recommendations include CT of the chest and abdomen in patients with abnormal liver function tests or clinical suspicion of lung or liver metastases and a bone scan in patients with advanced local-regional disease, elevated alkaline phosphatase, and/or symptoms suggestive of bone metastases.
Prognostic Factors
Current staging of NPC is based on the 2010 AJCC staging system (Table 68-5). Updates to the 2010 publication included some changes in the staging of NPC worth noting. Former stage T2a lesions were downstaged to T1 lesions, and hence former stage IIA is now stage I, in an effort to separate tumors confined to the nasopharynx or with extension into the oropharynx or nasal cavity without parapharyngeal involvement versus those with parapharyngeal involvement, which are now classified as stage T2 (previously stage T2b). Hence the previous stage IIB is now stage II. The nodal classification was slightly updated as retropharyngeal lymph nodes, regardless of laterality, are now considered stage N1 lesions. These changes are important to consider when reviewing literature prior to and after the implementation of the seventh edition of the AJCC. Advanced T category is associated with worse local control, whereas advanced N category is associated with an increased risk of distant metastasis and inferior survival. Metastatic disease is associated with a poor prognosis, and treatment options generally focus on palliation. A worse prognosis is associated with cranial nerve involvement, bone erosion, and lower lymph node level disease.126–129 The presence of EGFR expression is associated with inferior rates of survival.132–132
Table 68-5
American Joint Committee on Cancer Nasopharyngeal Carcinoma Staging
| Stage | Staging Criteria |
| T CATEGORY | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor confined to the nasopharynx, or tumor extends to nasal cavity* and/or oropharynx† without parapharyngeal extension‡ |
| T2 | Tumor with parapharyngeal extension‡ |
| T3 | Tumor involves bony structures and/or paranasal sinuses |
| T4 | Tumor with intracranial extension, involvement of cranial nerves, hypopharynx, orbit, or infratemporal fossa/masticator space§ |
| N CATEGORY | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Unilateral metastasis in cervical lymph node(s), ≤6 cm in greatest dimension, above the supraclavicular fossa, and/or unilateral or bilateral retropharyngeal lymph node(s), ≤6 cm in greatest dimension |
| N2 | Bilateral metastasis in cervical lymph node(s), ≤6 cm in greatest dimension, above the supraclavicular fossa |
| N3 | Metastasis in lymph node(s) >6 cm and/or to supraclavicular fossa |
| N3a. >6 cm in dimension | |
N3b. Extension to the supraclavicular fossa |
|
| M CATEGORY | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
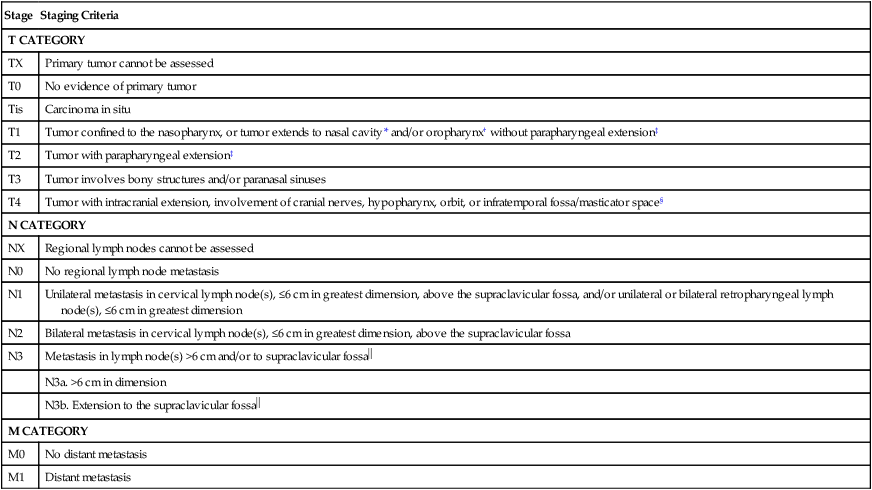
*Nasal cavity: anterior extension beyond the posterior margins of the choanal orifices.
†Oropharynx: inferior extension beyond the level of the free border of the soft palate. The junction at C1/C2 level is recommended as a more consistent radiologic landmark (37).
‡Parapharyngeal extension: posterolateral infiltration beyond the pharyngobasilar fascia.
§Masticator space and infratemporal fossa: Extension beyond the anterior surface of the lateral pterygoid muscle, or lateral extension beyond the posterolateral wall of the maxillary antrum, and the pterygomaxillary fissure.
 Supraclavicular fossa: Triangular region defined by the superior margin of the sternal end of the clavicle, the superior margin of the lateral end of the clavicle, and the point where the neck meets the shoulder.
Supraclavicular fossa: Triangular region defined by the superior margin of the sternal end of the clavicle, the superior margin of the lateral end of the clavicle, and the point where the neck meets the shoulder.
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010, and Sobin L. International Union Against Cancer: TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009.
EBV, which is strongly associated with development of NPC, has been demonstrated to be an important biomarker because the presence of serum EBV has a high sensitivity (96%) and specificity (93%) for detecting NPC. Circulating EBV-DNA levels have been demonstrated to correlate with tumor burden, and pretreatment EBV-DNA levels were demonstrated to be an independent prognostic variable in NPC, allowing the dichotomization into high-risk (≥4000 copies/mL) and low-risk (<4000 copies/mL) cohorts.133,134 More recently, two studies have demonstrated that the clearance rate of plasma EBV-DNA during the first month of salvage chemotherapy may predict tumor response and overall survival in patients with metastatic/recurrent NPC; an undetectable level after the first cycle indicated significantly a better rate of survival.135,136
Treatment Strategy Test
Because of the anatomic location of the nasopharynx, surgical resection typically has not been recommended as a result of the inherent surgical complication rates in this area, including the inability to achieve tumor-free margins. Accordingly, RT with or without concurrent chemotherapy is the treatment of choice. For early-stage disease, RT alone may be used with excellent results, with 3- to 4-year overall survival rates ranging from 70% to 100% and 65% to 100% for stage I and II disease, respectively.129,137–139
IMRT has replaced conventional RT as the standard of care in the treatment of persons with NPC.39,40 The intensity of the radiation beams can be modulated to deliver a high dose to the tumor with superior target volume coverage while significantly limiting the dose to surrounding normal tissues.140–145 Currently a dose-painting approach is being used in some modern IMRT plans, allowing different fraction doses to be simultaneously delivered to tumor volumes and elective nodal regions in a single course of RT.
Prophylactic neck radiation is usually recommended in patients with stage N0 disease given the high incidence of occult neck node involvement with a risk of 40% neck relapse if untreated, which is associated with a significantly higher incidence of distant failure (21% versus 6%) despite successful nodal salvage.104,146 With appropriate doses of radiation, that is, 50 to 60 Gy, the probability of relapse is less than 5%. In a patient with positive clinical findings of the neck, doses of 60 Gy or greater are typically recommended, with average regional control rates of 90% (range, 86% to 96%) and 75% (ranges, 71% to 87%) for neck nodes 3 cm or less and greater than 6 cm, respectively.147
Studies have demonstrated improved local control in patients with NPC who received >70 Gy,148 but despite this dose-response relationship, local control for T3/T4 tumors remained below 55% when treated with >70 Gy.148,149 The results of the Intergroup 0099 study (IG0099) have made concurrent CRT followed by adjuvant chemotherapy the standard of care for persons with stage III and IV NPC.150 The investigators reported on 147 of the registered 193 patients who were randomly assigned to receive radiation alone (1.8 to 2 Gy per fraction to a total dose of 70 Gy) or radiation (as described) with concurrent cisplatin (100 mg/m2 on days 1, 22, and 43), followed by three cycles of adjuvant cisplatin (80 mg/m2 on day 1) and 5-FU (1000 mg/m2/d on days 1-4) every 4 weeks for three cycles. The trial was closed after an interim analysis demonstrated a significantly improved 3-year progression-free survival (69% vs. 24%; P < .001) and overall survival (78% vs. 47%, P = .001) for the arm that received chemotherapy compared with the arm that received radiation alone.
Although these data were received with much debate, subsequent studies have confirmed the benefit of concurrent cisplatin-based chemotherapy in persons with stage III/IV NPC. Wee and colleagues151 reported on 221 patients with stage III/IV NPC who were randomized to receive either RT alone (70 Gy in 2 Gy/fraction) or CRT, which consisted of a slightly modified version of the IG0099 regimen: cisplatin (25 mg/m2 on days 1-4) for three cycles every 3 weeks, followed by adjuvant cisplatin (20 mg/m2 on days 1-4) and 5-FU (1000 mg/m2/d on days 1-4) for three cycles. Concurrent CRT reduced the incidence of distant metastasis by 17% at 2 years (P = .003) and had a superior 3-year overall survival rate (85% vs. 65%, P = .006), respectively. Further supportive data came from a metaanalysis reported by Langendijk and colleagues152 of 10 trials that randomized patients with NPC to conventional RT or CRT. Subgroup analysis confirmed that patients treated with CRT had an overall survival benefit (HR for death of 0.48) and absolute survival benefit of 20% at 5 years.
Less evidence exists for treatment with CRT for persons with stage II NPC, yet given a substantial risk of distant metastasis, CRT is generally recommended. Chen and colleagues153 reported a phase 3 trial of 230 patients with T1-2N1M0 or T2N0M0 disease with parapharyngeal space involvement who were randomized to RT alone or RT with concurrent cisplatin (30 mg/m2); they found that patients treated with CRT had improved overall survival (94.5% vs. 85.8%, P = .007), progression-free survival (87.9% vs. 77.8%, P = .017), and distant metastasis-free survival (94.8% vs. 83.9%, P = .007). However, no statistically significant difference was found in the 5-year local-regional relapse-free survival rate (93.0% vs. 91.1%, P = .29). Importantly, multivariate analysis demonstrated that the number of chemotherapy cycles delivered was the only independent factor associated with survival and distant control. These findings are controversial because according to the updated 2010 AJCC staging system, the disease of 13% of these patients was restaged as stage III.
In almost all CRT studies an increased rate of acute toxicities has been reported, with an increase in late-occurring toxicities being reported in many studies as well. Chen and colleagues153 reported that the addition of cisplatin to RT was associated with a significant increase in acute grade 3 or 4 leukopenia/neutropenia (12.9% vs. 0%, P < .001), nausea/vomiting (8.6% vs. 0%, P = .001), and mucositis (46% vs. 33%, P = .04) compared with RT alone. In the IG0099 trial only 63% of patients completed all three cycles of concurrent chemotherapy, and only 55% were able to receive all three courses of adjuvant therapy.150 Multiple studies have demonstrated that prolonged treatment breaks correlated with inferior outcomes, with investigators estimating that local-regional relapses increased 3.3% per day of treatment interruption.154,155
In an effort to minimize toxicities associated with concurrent CRT, trials have been designed to evaluate lower but more frequent doses of chemotherapy. Chan and colleagues156 reported a phase 3 randomized trial of concurrent cisplatin weekly (40 mg/m2) with EBRT in 350 patients. Seventy-eight percent of patients in the CRT arm received at least four cycles of cisplatin, and patients treated with CRT had superior 5-year overall survival (70.3% vs. 58.6%), which just reached significance on multivariate analysis (HR 0.71, 95% CI 0.5- 1.0, P = .049). Although its use has been validated by other studies, weekly doses of cisplatin have never been directly compared with high-dose cisplatin.157,158
Studies to evaluate less toxic chemotherapeutic regimens in an effort to identify gentler regimens without sacrificing treatment efficacy have been undertaken. Cisplatin is dose-limited by ototoxicity, irreversible peripheral neuropathy, nephrotoxicity, myelotoxicity, and intractable nausea. As such, carboplatin, which carries a more favorable toxicity profile, was studied as a substitute for cisplatin, with comparable efficacy, in a noninferiority trial reported by Chitapanarux et al.159 Two hundred six patients were randomized to either concurrent cisplatin with adjuvant cisplatin/5-FU (with the same dosage used in the IG0099 study) or concurrent weekly carboplatin (100 mg/m2 on days 1, 8, 15, 22, 29, and 36) with adjuvant carboplatin (area under the curve 5 on day 1) and 5-FU (1000 mg/m2 over 96 hours) every 4 weeks with no significant difference in 3-year rates of disease-free survival (63.4% vs. 60.9%, P = .9613, respectively) or overall survival (77.7% vs. 79.2%, P = .9884). Importantly, the treatment regimen was better tolerated in the carboplatin group, with 70% (the major dose-limiting factor was thrombocytopenia) completing adjuvant treatment compared with 42% of the patients in the cisplatin arm. Hence, although cisplatin-based treatment should be prioritized, patients with locally advanced NPC who are not candidates for cisplatin may be considered for CRT with carboplatin followed by adjuvant carboplatin/5-FU as an acceptable alternative regimen.159,160 Similarly, a regimen of weekly oxaliplatin (70 mg/m2) was evaluated in a phase 3 trial of 115 patients randomized to CRT versus RT alone and was reported to be well tolerated with good efficacy, but to date no studies have directly compared this regimen to cisplatin-based regimens, and it is not currently recommended.161
Newer targeted therapies are increasingly being investigated in persons with NPC. Ma and colleagues162 reported a phase 2 clinical trial of 30 patients with stage III/IV disease who were treated with an initial dose of cetuximab (400 mg/m2) 7 to 10 days prior to initiation of weekly cisplatin (30 mg/m2) and weekly cetuximab (250 mg/m2). Overall, these investigators reported a tolerable adverse effect profile and good efficacy when compared with published reports with 2-year overall, local-regional progression-free, distant metastasis-free, and progression-free survival rates of 89.9%, 93.0%, 82.8%, and 86.5%, respectively. Similarly, bevacizumab, a VEGF inhibitor that is overexpressed in approximately 66% of patients with NPC, has been studied in a multiinstitutional phase 2 trial (RTOG 06-15) of 44 patients to evaluate the toxicity and efficacy of bevacizumab when added to the standard CRT CDDP regimen.163
Although CRT is considered the standard of care, the additional benefit provided by adjuvant chemotherapy remains under investigation. Chen and colleagues164 reported a phase 3 randomized trial at seven institutions in China in which concurrent CRT (66 Gy in 2.0- to 2.27-Gy per fraction with 40 mg/m2 cisplatin weekly for 7 weeks) was compared with concurrent CRT (as described) and adjuvant chemotherapy (cisplatin/5-FU) in 508 patients with stage III/IV disease. No significant differences were noted in failure-free survival (P = .13), distant failure-free survival (P = .12), or overall survival (P = .32), but data interpretation was limited because 18% of patients randomized to the adjuvant arm did not receive any adjuvant chemotherapy and 49% required dose reduction. Further, this trial was not designed as a noninferiority trial. Given the increasing rates of distant metastases with current treatment regimens, adjuvant chemotherapy remains the standard of care. Currently, the role of circulating EBV-DNA after concurrent CRT is being investigated to select patients who may benefit the most from adjuvant chemotherapy.133
The addition of induction chemotherapy to concurrent CRT is an active area of interest. A pooled analysis of the two largest phase 2 studies to date demonstrated that the addition of cisplatin-based induction chemotherapy to RT was associated with a modest but significant decrease in 5-year relapse-free survival (50.9% vs. 42.7%, P = .014) and improvement in 5-year disease-specific survival (63.5% vs. 58.1%, P = .029) in persons with advanced stage NPC.165 The incidence of local-regional failure and distant metastases was reduced by 18.3% and 13.3% at 5 years, respectively. However, no improvement in overall survival occurred (61.9% vs. 58.1%, P = .092). Induction chemotherapy in this setting remains investigational, and patients are currently being recruited for several phase 3 trials.
Various strategies have been used in attempts to improve the survival rates, particularly for locally advanced disease. The first strategy is to optimize local-regional control, not only because it is a major pattern of relapse in this group of patients but also for the potential impact on the risk of distant metastases. The second strategy is the integration of various chemotherapy agents with definitive radiotherapy. Because a dose-response relationship exists for local control166 and the overall treatment time155 also appears to have an adverse impact on treatment outcome, several institutions have reported their results using altered fractionation for locally advanced NPC with or without concurrent chemotherapy.167
Despite the improved outcomes resulting from CRT for intermediate stage and locally advanced NPC, a proportion of patients continue to have persistent disease. An important distinction exists between persistent disease (i.e., tumors that do not completely regress after primary treatment) and recurrent disease (i.e., tumors that reemerge after initial complete regression) because the prognoses and therapeutic considerations are different, with better survival and control rates for persistent disease.168 Early detection of local-regional failure is crucial for a better chance of salvage, and regular follow-up after completion of primary treatment is recommended. Frequently used methods include manual palpation, rigid nasopharyngeal endoscopy and nasopharyngeal biopsies, imaging techniques (e.g., CT, PET, and MRI), and serologic tests (e.g., anti-EBV titers).
Therapeutic options for persistent disease have generally been centered around further irradiation or observation. Studies have demonstrated that further irradiation in early-stage disease that is amendable to intracavitary and interstitial techniques may result in local control rates comparable to those achieved in patients demonstrating a prompt complete response.169 Hence dose escalation may be adequate in compensating for tumors that demonstrate a low radioresponsiveness. The optimal dose schedule remains to be determined, with both 60 Gy low dose rate and 22.5 to 25 Gy high dose rate schedules having been reported. Stereotactic radiosurgery has been used, with preliminary data suggesting that up to 70% of patients with organ-confined recurrences may achieve local control for up to 2 years.170 Late-appearing toxicity does not appear to be increased. In light of the poorer local control rates and survival rates in patients who have local recurrences, a brachytherapy implant or stereotactic radiosurgery may be considered in the management of patients demonstrating persistent disease.
Because NPCs are radiosensitive, locally recurrent carcinomas, they may be amenable to reirradiation. The risk of late-appearing complications including soft tissue and brain necrosis and neuropathies is highly dose and volume dependent with reirradiation, and thus the general strategy has been to incorporate conformal RT techniques for the boost component of the reirradiation regimen. As with treatment for persistent disease, this treatment may take the form of either brachytherapy or a stereotactic radiosurgery boost.171 In a series of more than 891 patients undergoing reirradiation, the extent of the recurrence was prognostic. Overall, approximately 30% achieved local disease control when a repeat radiation dose of 60 Gy or greater was delivered.172 The selection of small EBRT fraction size and the use of a brachytherapy implant were associated with a reduced risk of late-appearing complications. Several other institutional series have reported sustained local control rates of 20% to 60%, with the variability due to the extent of initial disease at presentation and at recurrence and the dose of reirradiation.169,173,174 In selected series treating only disease confined to the nasopharynx amenable to management by either intracavitary or interstitial implant, sustained local control rates of 50% to 60% may be realized. These series also have demonstrated a significant risk of developing late-appearing radiation-related complications, including soft tissue and bone necrosis, trismus, fistula formation, and neurologic complications such as radiation myelitis and temporal lobe necrosis.
Many of the recent salvage radiation studies have included cisplatin-based chemotherapy for advanced-stage disease.175–178 Using gemcitabine and cisplatin as induction chemotherapy followed by reirradiation with IMRT in 20 patients (95% of whom had stage rT3-4 disease), Chua et al.176 reported a 1-year local salvage rate of 75%. In a study of 35 patients (66% of whom had stage rT3-4 disease), Poon et al.175 reported a 1-year event-free survival rate of 42% with concurrent cisplatin and adjuvant cisplatin and 5-FU. Further study regarding the efficacy and safety of adding chemotherapy to reirradiation approaches is needed.
Salvage surgery is occasionally performed for NPC in select patients. In patients with persistent nodal disease, a radical neck dissection is the preferred treatment if no evidence of distant metastases exists, with at least one study reporting a 5-year nodal control rate of 66% and a 5-year disease-free survival rate of 37%.179
Surgical management of primary site recurrences is difficult given the complex anatomy and space constraints. Although it is controversial, salvage surgery is being performed at certain centers with limited data published as small retrospective studies, which have demonstrated feasibility and success in local control.180,181 Hsu and colleagues181 studied 60 patients who underwent salvage surgery and showed that patients who underwent surgical resection demonstrated slightly improved local control and overall survival compared with patients who underwent high-dose reirradiation for local relapse, with fewer late complications. These investigators favored salvage surgery for rT1-2 and limited rT3 disease because of the lower complication rate. Access to the pharyngeal recess is of critical importance in the surgical management of recurrent NPC, given the high rate of recurrence in this area. Wei and Sham182 demonstrated an anterolateral approach or maxillary swing approach for localized recurrence in the nasopharynx. In this approach the maxilla is swung laterally as an osteocutaneous anterior check flap, exposing the nasopharynx. Wei et al.183 reported on 161 patients who underwent salvage nasopharyngectomy with use of this approach at Queen Mary Hospital (Hong Kong) for recurrent NPC after primary treatment by radical RT. Twelve patients had prior brachytherapy as a salvage procedure. All patients had recurrent stage T1 disease, with 78% of these patients achieving negative tumor resection margins, confirmed by frozen section; the remaining patients had positive microscopic margins at the internal carotid artery or the skull base. All patients recovered from this anterolateral approach and were discharged. Significant surgical morbidities included trismus (60%) and palatal fistula (25%). Postoperative reirradiation is recommended for patients with positive surgical margins and/or advanced disease,186–186 whereas its potential benefit in patients with negative surgical margins remains to be determined.187
Treatment-Related Toxicities
Given the anatomic complexity of the nasopharynx with its proximity to critical structures combined with the need for high radiation doses, the risks of acute and late-appearing treatment-related toxicities are significant. The overall complication rate from conventional treatment ranges from 31% to 66%, with severe sequelae including temporal lobe necrosis, hearing loss, xerostomia, neck fibrosis, cranial nerve dysfunction, carotid artery injury/rupture, endocrine dysfunction, soft tissue necrosis, osteonecrosis, and transverse radiation myelitis.101,188,189 As mentioned previously, IMRT is now considered the standard of care for NPC given the improved toxicity profile as exemplified by Lee et al.,144 who demonstrated that IMRT is superior in the preserving salivary function, a finding subsequently confirmed by others.190,191 Reirradiation carries a significantly greater risk of the development of treatment-related toxicities and should largely be performed at specialized centers.
Nasal Cavity and Paranasal Sinus Cancer
Among the different subsites within the paranasal sinus, SCCs occur most commonly in the maxillary sinus.192 The second most common location is the nasal cavity, and the remainder originate in the ethmoid sinus, which is also the more frequent location for the histologic variants adenocarcinoma and esthesioneuroblastoma. Cancer rarely arises in the frontal and sphenoid sinuses.192
Paranasal sinus malignancies demonstrate a male predominance (4 : 1 male:female), with risk factors including occupational exposures, air pollution, tobacco smoke, and HPV infection. The prognosis for maxillary and ethmoid lesions is based on the 2010 (seventh edition) AJCC staging system (Table 68-6) and, in the case of maxillary sinus malignancies, on their relationship to Ohngren’s line, a theoretical plane that extends from the medial canthus of the eye to the angle of the mandible. Tumors anteromedial to Ohngren’s line are thought to have a considerably better prognosis, whereas tumors posterolateral to the line carrier a worse prognosis. No standard staging system has been developed for frontal or sphenoid sinus tumors.
Table 68-6
American Joint Committee on Cancer Paranasal Sinus Staging
| Stage | Staging Criteria |
| T CATEGORY | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| MAXILLARY SINUS | |
| T1 | Tumor limited to the maxillary sinus mucosa with no erosion or destruction of bone |
| T2 | Tumor causing bone erosion or destruction including extension into the hard palate and/or middle nasal meatus, except extension to posterior wall of maxillary sinus and pterygoid plates |
| T3 | Tumor invades any of the following: bone of the posterior wall of maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses |
| T4a | Tumor invades anterior orbital contents, skin of cheek, pterygoid plates, infratemporal fossa, cribriform plate, sphenoid or frontal sinuses |
| T4b | Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V2), nasopharynx, or clivus |
| NASAL CAVITY AND ETHMOID SINUS | |
| T1 | Tumor restricted to any one subsite, with or without bony invasion |
| T2 | Tumor invading two subsites in a single region or extending to involve an adjacent region within the nasoethmoidal complex, with or without bony invasion |
| T3 | Tumor extends to invade the medial wall or floor of the orbit, maxillary sinus, palate, or cribriform plate |
| T4a | Tumor invades any of the following: anterior orbital contents, skin of nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid or frontal sinuses |
| T4b | Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than V2, nasopharynx, or clivus |
| N CATEGORY | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastases in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2 | |
| N2a | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
| N2b | Metastasis in a multiple ipsilateral lymph nodes ≤6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes ≤6 cm in greatest dimension |
| N3 | Metastasis in lymph node(s) >6 cm |
| M CATEGORY | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
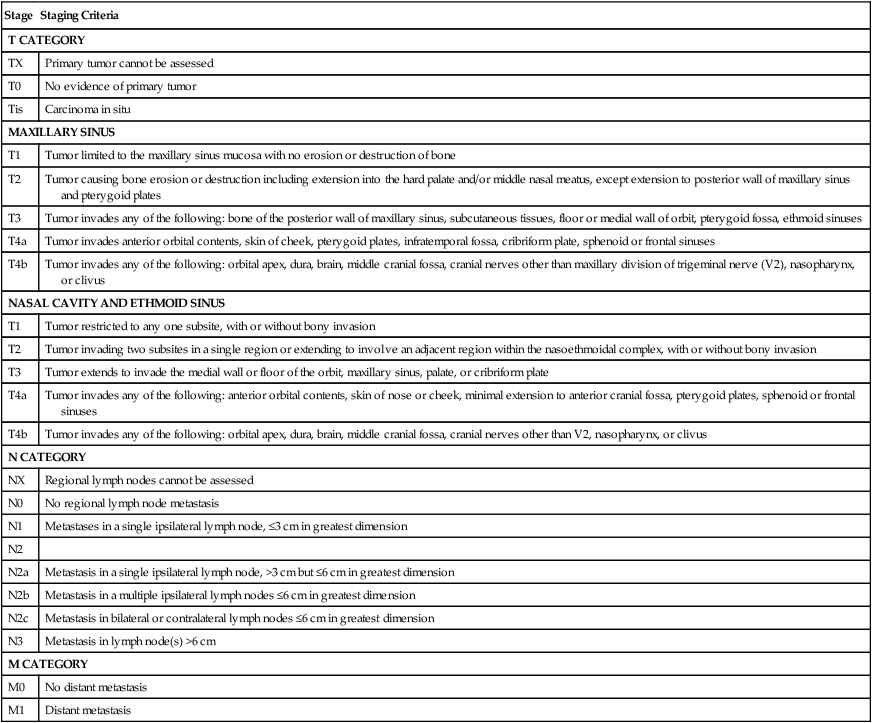
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Most malignancies are advanced at presentation and commonly involve one or more adjacent structures (Fig. 68-10). Orbital invasion often occurs early with cancers of the maxillary and of the ethmoid sinuses, whereas it often is a late event for nasal cavity tumors. Malignancies beginning in the anterolateral infrastructure of the maxilla often erode through the inferolateral wall and extend into the oral cavity, with involvement of the maxillary gingival or the adjacent gingivobuccal sulcus.
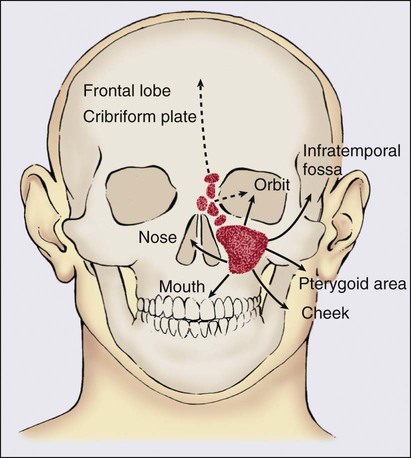
The maxillary sinuses have a poor lymphatic supply. The retropharyngeal nodes are the first echelon lymphatic drainage for sinus malignancies, with other commonly involved lymph nodes being the periparotid and level IB nodes. The risk of cervical node metastases is low unless the tumor has progressed to involve mucosal surfaces with abundant lymphatics, such as the oral cavity. Nodal recurrences, which are reported in approximately 3% of patients, are more frequent for SCC, neuroendocrine tumors, and rhabdomyosarcoma of the paranasal sinus.193
Treatment Strategy
Management of paranasal sinus malignancies is primarily surgical, with adjuvant radiation and possibly chemotherapy for advanced lesions. Newer advanced endoscopic procedures are being used and evaluated for their potential use in the surgical management of sinus tumors, especially for localized T1-T2 disease. Multiple single-institution reports have concluded that disease control with endoscopic surgery may be equivalent to standard external approaches in selected patients with excellent functional outcomes.193
Tumors that invade the ethmoid sinuses may require a craniofacial resection for surgical removal because of the proximity to the skull base. This procedure requires not only a facial incision approach to the ethmoid area, but also a neurosurgical craniotomy approach to access the anterior skull base. Orbital exenteration should be considered if the tumor involves the periorbital fat or extraocular muscles. Orbital preservation may still be considered in cases of limited periorbital invasion, even with medial wall or orbital floor invasion. The decision regarding orbital preservation versus exenteration may be made intraoperatively, because clinical examination and imaging studies may not definitively predict orbital invasion.194
Paranasal sinus cancer has a significant chance for local failure even in cases of gross total resection. PORT is successful in decreasing the incidence of local recurrence.193 In view of the proximity of these cancers to many critical normal structures, IMRT, stereotactic radiosurgery, fractionated stereotactic RT, or even proton-based therapy are commonly recommended in an effort to improve the therapeutic ratio. These techniques are important considerations, especially given the studies reporting a potential dose-response relationship, with doses greater than 65 Gy recommended.195 For patients who underwent a successful complete total resection, adjuvant RT to 60 Gy is generally recommended, whereas in patients with positive margins, the prescribed doses range between 66 Gy and 70 Gy administered daily as 2 Gy per fraction. Although studies have reported the difference in outcomes between patients treated with surgery and adjuvant RT versus definitive RT, comparing the outcomes of these modalities is flawed because it must be assumed that the patients selected for nonsurgical therapy were more likely to have unresectable or borderline unresectable disease.193
In unresectable and nonoperative cases, definitive RT should be offered. In addition, RT may be considered in patients who request an organ preservation strategy to avoid orbital exenteration. In this capacity, it may be used definitively, reserving surgery for salvage or with preoperative radiation as a strategy to downstage the tumor.196
Although neck dissection and PORT is recommended for all patients with cervical lymph node involvement either at presentation or in the recurrent setting, the role of elective neck management remains unclear. Prophylactic neck RT has been demonstrated to be successful in the management of patients with N0 disease. Bristol and colleagues197 reported that in a cohort of patients with maxillary sinus cancer with N0 disease, among those who did not receive prophylactic management, nodal failure developed in 36% compared with only 7% who received prophylactic neck irradiation (P < .001). This difference in nodal recurrence rates translated into a significant reduction in distant metastases (20% vs. 3%) and an increase in relapse-free survival (45% vs. 67%) for electively treated patients.193 Hoppe and colleagues198 reported their experience with 85 patients and concluded that elective ipsilateral neck irradiation translated to clinically superior outcomes. Because prophylactic neck irradiation increases treatment morbidity, many centers have decided to omit it and defer to prophylactic unilateral selective neck dissection. This decision provides additional information for staging and prognostication, and an ipsilateral prophylactic neck dissection is routinely recommended for patients with T3 or T4 disease. Postoperative neck irradiation is recommended for patients with T3/T4 disease who have a squamous cell or undifferentiated carcinoma histologic variant, and in any histology with positive margins or evidence of extracapsular extension. In patients with unresectable T3/T4 disease or inoperable tumors as a result of a patient’s medical comorbidities and are recommended to undergo definitive RT, the general consensus is to prophylactically treat the ipsilateral neck with N0 disease, whereas close observation and monitoring is generally recommended for those patients with T1/2N0 disease. It is important to reiterate that in all patients with nodal disease diagnosed at presentation or after a prophylactic neck dissection, PORT should be recommended.
Treatment Outcomes and Related Toxicities
Given the complex anatomy and confined location of paranasal sinus malignancies, it is not surprising that treatment-related morbidity is high. Mendenhall et al.199 reported that 26% of patients who underwent surgery followed by PORT experienced severe complications, including ipsilateral blindness (4/56), postoperative infection necessitating hospitalization (1/56), graft failure, frontal bone osteoradionecrosis, frontal lobe necrosis, intracranial bleeding, postoperative meningitis, cerebrospinal fluid leak, nasal bone necrosis, bilateral blindness, and fatal infected bone flap. Chen and colleagues200 demonstrated that over time the observed incidence of grade III/IV late-occurring toxicity decreased from 53% to 45% to 39% to 28% to 16% among patients treated in the 1960s, 1970s, 1980s, 1990s, and 2000s, respectively. They concluded that improvements in the therapeutic ratio were responsible for the decreasing incidence of compilations treated throughout these decades.
Oral Cavity
It was estimated that in 2012 approximately 26,740 new cases of pharyngeal carcinoma would be diagnosed, with approximately 5,520 deaths attributable to oral cavity carcinoma.201 When grouped together with pharyngeal cancer, cancer of the oral cavity is the sixth most common cancer in the world.202 Signs and symptoms associated with oral cavity tumors include bleeding, dysphagia, dysarthria, and halitosis. In addition, the patient may report ill-fitting dentures that previously fit properly. At presentation, oral cavity tumors generally present with local invasion, tissue destruction, and lymph node metastases, but distant metastatic disease is uncommon.202
The oral cavity (Fig. 68-11) is composed of the lip, anterior two thirds of the tongue (oral tongue), floor of mouth, buccal mucosa, gingiva, hard palate, and retromolar trigone. The floor of the mouth is bounded by the lower alveolar ridge anteriorly and laterally and the ventral tongue surface and anterior tonsillar pillar posteriorly. The tongue is divided into an oral component, which lies anterior to the circumvallate papillae, and the base of tongue, which is a part of the oropharynx and lies posterior to the circumvallate papillae. The buccal mucosa overlies the buccinator muscle, is bounded superiorly and inferiorly by the gingiva, and extends posteriorly to the retromolar trigone. The gingiva is the soft tissue overlying the alveolar ridges of the mandible and maxilla. The hard and soft palates form the roof of the mouth. The retromolar trigone mucosa overlies the mandibular ramus and is bounded anteriorly by the buccal mucosa and posteriorly by the anterior tonsillar pillar. The oral cavity has a rich lymphatic supply, and as such, nodal metastases to levels I to III are common. A multidisciplinary approach is critical given the important role that oral cavity structures play, including cosmesis, mastication, deglutition, and speech articulation.
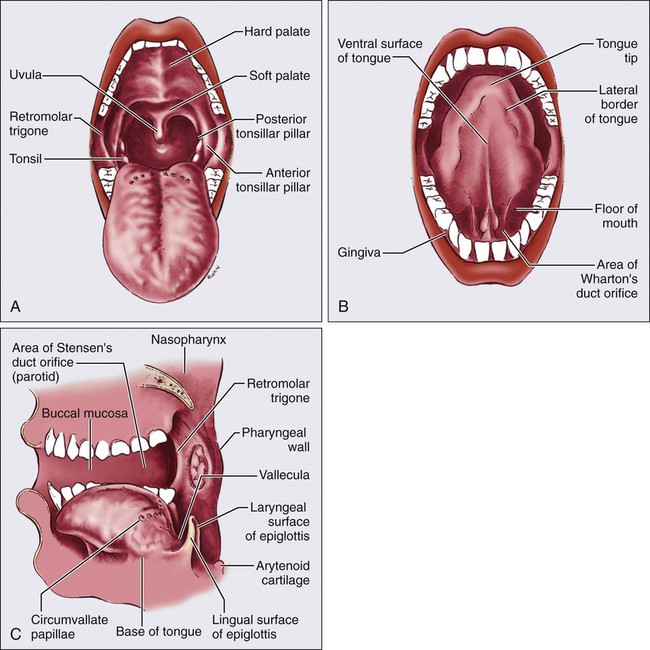
Histology/Pathology
As elsewhere in the head and neck, SCC is the most common histology, accounting for approximately 95% of all malignant tumors in the oral cavity, except in the hard palate, where most tumors originate in the minor salivary glands. Other malignancies involving the oral cavity include malignant mucosal melanoma, lymphoma, and sarcoma. Although SCC can occur anywhere in the oral cavity, the most common locations include the floor of the mouth and tongue. In the earliest recognizable stage, SCC appears as firm, pearly plaques or as irregular, roughened, or verrucous areas of mucosal thickening, which can be mistaken for leukoplakia, a premalignant lesion. Larger lesions seldom are mistaken for leukoplakia and form either exophytic masses (Fig. 68-12, A) or endophytic lesions, often with associated ulceration and heaped-up edges (Fig. 68-12, B). Histologic examination frequently reveals an association with in situ lesions, sometimes with surrounding areas of epithelial dysplasia of varying degrees (Fig. 68-12, C). These tumors range from well-differentiated keratinizing neoplasms to poorly differentiated SCC without keratinization to anaplastic and sometimes sarcomatoid growth patterns (Fig. 68-12, D). Less common patterns include verrucous carcinoma.
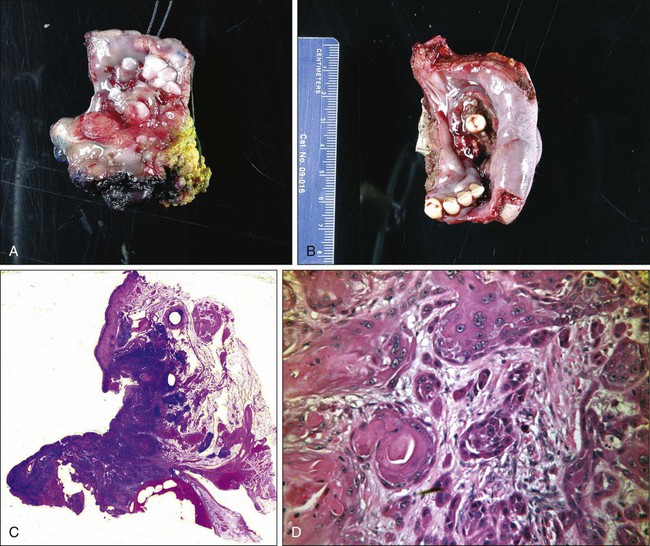
Diagnostic/Staging Workup
Advanced oral cavity tumors can invade bone and adjacent soft tissue, and thus pretreatment imaging studies are necessary. As with other sites, CT with contrast is effective in visualizing bone invasion and cervical nodal status, MRI provides superior visualization of soft tissue involvement and perineural spread, and PET/CT can help further aid in the delineation of the primary tumor and may help in the identification of metastatic disease. At minimum a chest radiograph should be obtained, but CT or PET/CT should be considered to evaluate for distant metastatic disease in persons with more advanced-stage lesions. For staging of cancers of the oral cavity, the AJCC classification system is used (Table 68-7).
Table 68-7
American Joint Committee on Cancer Oral Cavity and Oropharyngeal Staging
| Stage | Staging Criteria |
| T CATEGORY | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤2 cm in greatest dimension |
| T2 | Tumor >2 cm but ≤4 cm in greatest dimension |
| T3 | Tumor >4 cm in greatest dimension or extension to lingual surface or epiglottis |
| T4a | Tumor invades the larynx, extrinsic muscles of tongue, medial pterygoid, hard palate, or mandible* |
| T4b | Tumor invades lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, or skull base or encases carotid artery |
| N CATEGORY | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastases in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2 | |
| N2a | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
| N2b | Metastasis in a multiple ipsilateral lymph nodes ≤6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes ≤6 cm in greatest dimension |
| N3 | Metastasis in lymph node(s) >6 cm |
| M CATEGORY | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
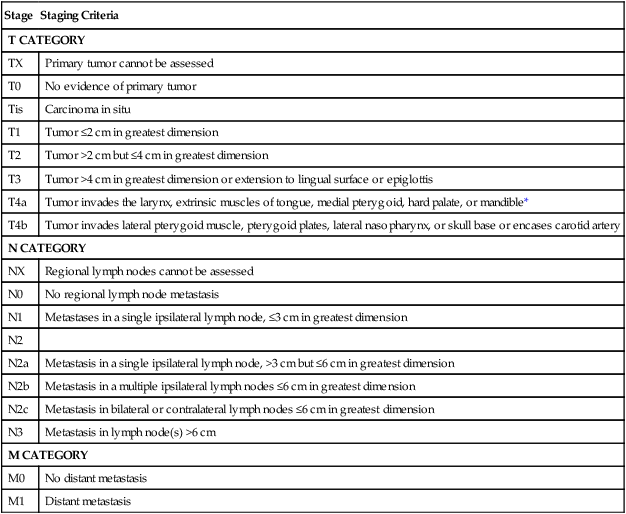
*Mucosal extension to lingual surface of epiglottis from primary tumors of the base of tongue and vallecula does not constitute invasion of larynx.
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Treatment Strategy
Lip
SCC and basal cell carcinoma (BCC) commonly originate in the lip. Cancers of the lower lip are more commonly SCC. Tumor thickness, at its maximal point, is a predictor of metastatic lymph node involvement for SCC.203 Conversely, BCC is the more common histology of the upper lip, and it rarely metastasizes. Interestingly, SCC of the upper lip, as well as the oral commissure, carries a worse prognosis than SCC that originates in the lower lip, because it has a tendency to metastasize earlier.204
Surgical management of SCC of the lip is complex because of the challenges in reconstruction. Although secondary to the complete resection of the lesion, the preservation of speech, oral competence, and cosmesis must be considered. Surgical therapy is considered to be equal in effectiveness to irradiation for the treatment of early T1 or T2 lesions.205–208 Small lesions of the lip can be managed surgically by means of wide local excision, with optimal margins being ≥5 mm, and primary closure for lesions that involve less than two thirds of the lip. Treatment morbidity associated with this approach often is less than that experienced with irradiation. With larger lesions that require resection of more than two thirds of the lip, local flap reconstruction is typically necessary. These flaps involve the mobilization of remaining lip tissue or the use of tissue from the opposite lip. PORT with prophylactic neck treatment is indicated for T1-2N0 disease with positive/close margins, when reresection is not feasible, or with pathological upstaging to T3-T4, perineural invasion, or lymphovascular invasion.
Metastases from lip carcinoma are relatively rare, with the incidence reported at 12% or less.209,210 In patients with N0 disease in the neck, occult metastases are estimated to occur in 5% to 10% of cases, and thus elective neck dissection is not routinely performed in patients with N0 disease of the neck. Neck dissections are generally performed when cervical metastases are clinically or radiographically apparent.
Predictably, the results of these procedures depend on the extent of disease. The 5-year survival rate for T1 and T2 cancers of the lip is greater than 90%, whether they are treated with surgery or irradiation.206 For larger cancers, especially those that have metastasized, the cure rates are in the range of 40% to 50%.204,206 Thus for stage III and IV cancers, a combined approach with surgery and postoperative radiation is indicated. Patients with advanced disease (stage T3-T4) but without nodal involvement (stage N0) should undergo surgical resection of the primary tumor if feasible with an ipsilateral or bilateral (if midline lesions) selective neck dissection. Patients with local-regionally advanced disease (i.e., any T stage with N1-3 stage) should undergo surgical resection of the primary tumor, if possible, with at minimum an ipsilateral neck dissection and consideration of a contralateral neck dissection for midline lesions. Patients with bilateral nodal metastases should undergo a bilateral neck dissection. Patients with pathologically confirmed N2/N3 nodal disease with adverse features (e.g., positive margins or extracapsular nodal extension) should undergo POCRT. Patients with pathologically confirmed N1 disease without adverse features should be considered for PORT, which is the preference at the MSKCC.
RT can be used for definitive treatment and can consist of RT with or without brachytherapy boost or concurrent CRT. In general, doses of 66 to 74 Gy are required for tumor sterilization in the definitive setting, with recommendation for 60 to 66 Gy in the adjuvant setting. In both the definitive and adjuvant settings, the neck is treated in patients with adverse features such as positive margins, perineural invasion, or lymphovascular invasion.211
Oral Tongue
Oral tongue carcinomas represent approximately 25% of oral cavity carcinomas. These lesions are characterized by early infiltration into the underlying tongue musculature with an early and high risk for regional metastases. Although it has been shown that irradiation and surgery are effective in treating early lesions, a review of 332 patients revealed that disease-free survival was better with surgery alone than irradiation alone.212,213 In general, surgery is often the treatment of choice for early-stage lesions because of the ease of surgical access, excellent reconstruction options, and quick treatment time.
Surgical or radiation treatment of the neck is indicated in most cases of oral tongue SCC. The rates of occult cervical metastases exceed 30% for lesions that are stage T2 and greater.62,63,204,214 Therefore, for a patient with stage N0 disease in the neck, a selective neck dissection, of levels I, II, III, and IV, is indicated in T2 to T4 lesions, and T1 lesions with depth of invasion greater than 3 to 4 mm.216–216 Patients with clinical or radiographic evidence of cervical metastasis may require more radical procedures depending on the number and size of nodal involvement. Depth of invasion is a key risk factor for lymph node involvement.217
Floor of the Mouth
Surgical management of the neck must take into consideration that occult metastases occur frequently in cancers located in the floor of the mouth. The incidence is between 23% and 35% of lesions.62,63,218,219 Therefore most investigators recommend treatment of the node-negative neck in all patients with T2 or higher grade lesions.220–220 For stage T1 lesions, tumors greater than 2 to 4 mm in thickness have been found to have a high rate of metastasis, and treatment of the neck is also recommended.221,222 If surgical rather than radiation treatment is pursued, a selective neck dissection that includes the first-echelon nodes (level I and upper level II regions of the neck) should be performed. Unless the lesion is clearly unilateral, a bilateral neck dissection is recommended. Treatment of the floor of the mouth generally calls for a supraomohyoid neck dissection with resection of levels I, II, and III. Level IV is often included in a dissection for cancers of the oral tongue.223 The cure rates with surgical therapy for floor of the mouth cancers have been reported as 95% for stage I cancers and 86% for stage II cancers.224 However, another study showed that surgery and radiation are equally effective in treating early lesions and that stage III and stage IV lesions should be treated with combined therapy.225 In general, the early lesions of the floor of the mouth are best managed surgically unless a contraindication exists, with combined therapy reserved for larger lesions or locally advanced lesions.
Oropharynx
Anatomy
The oropharynx anteriorly connects to the oral cavity and joins the nasopharynx with the larynx/hypopharynx. Anteriorly, the oropharynx begins at the base of the tongue and extends posteriorly to the pharyngeal wall (Fig. 68-11). It extends superiorly from the top of the soft palate to its inferior border, the top of the hyoid bone. The four subsites contained within the oropharynx are the base of tongue, the palantine tonsils and tonsillar pillars, the soft palate, and the pharyngeal wall.
Lymph node drainage from the base of tongue and the palantine tonsils is typically to levels II to IV and the lateral retropharyngeal nodes.36,226,227 The most common site of lymph node metastasis is to the jugulodigastric nodes. Occasionally, levels Ib and V can become involved.228 Level Ib is at increased risk of involvement with significant invasion of the oral tongue. Midline tumors, such as base of the tongue tumors, are at risk for bilateral lymph node metastasis. Small lateralized tonsillar tumors without midline extension have less risk of contralateral lymphadenopathy.229
Epidemiology
In 2012 approximately 13,510 new cases of pharyngeal carcinoma were diagnosed, and approximately 2330 deaths were attributable to pharyngeal carcinoma.201 Although these statistics of pharyngeal carcinoma are not limited to the oropharynx, most pharyngeal carcinomas originate in the oropharynx. Ninety percent to 95% of tumors in the oropharynx are SCCs, whereas others are minor salivary gland tumors, melanomas, sarcomas, lymphomas, or other rarer histologies.
Until recently, smoking and alcohol consumption were the main risk factors for development of oropharyngeal cancer in the United States.230,231 Molecular evidence suggests a role for HPV, specifically HPV-16, in the pathogenesis of a subgroup of HNSCC. HPV viral oncogenes E6 and E7 are frequently overexpressed in the oropharynx.76 Interestingly, exposure to HPV has an increased association with oropharyngeal cancer, regardless of tobacco and alcohol use, suggesting two distinct pathways of oncogenesis.76 At present HPV infection is the main etiology; it was responsible for 70% of oropharyngeal cancer between 2000 and 2004, compared with only 16.3% between 1984-1989.5 The epidemic of HPV-related head and neck malignancies will likely lead to oropharyngeal cancer becoming the most common subsite in the pharynx, surpassing laryngeal carcinoma.
Presentation, Workup, and Staging
The physical examination should include a complete assessment of the head and neck, a thorough examination of the bilateral cervical and supraclavicular lymph node chains, a detailed cranial nerve examination, and fiberoptic nasopharyngoscopy or indirect mirror examination. Examination of the oral cavity should include a bimanual examination including palpation of the base of tongue. Sometimes visualization of lesions at the base of the tongue can be difficult; however, an abnormality can be detected on palpation. A lack of tongue mobility may indicate that cancer has invaded the deep musculature of the tongue. The retromolar trigone should be visualized, because SCCs of the tonsil can often extend anteriorly into it. Examination of the primary tumor should include a measurement of the lesion’s size and a description of whether the lesion is exophytic or infiltrative. To help in visualizing the vallecula on fiberoptic examination, the patient should be asked to protrude his or her tongue. Determining the mucosal or superficial extent of disease by examination is important, because the extent of the disease is often not well represented on imaging studies.232
Additional tests include biopsy, chest imaging, and cross-sectional imaging of the neck and primary site with either a contrast-enhanced CT or an MRI scan. CT scans are often readily available and can adequately assess lymph node involvement and bone invasion and estimate the primary tumor size. MRI provides superior soft-tissue contrast relative to CT, is less susceptible to dental artifact, and offers multiplanar imaging to get a better understanding of the superior-inferior extent of disease. T2 sequences and T1 postcontrast fat-saturated sequences can often help delineate the extent of tumor.233 The role of PET/CT in the workup of disease is not fully defined, but it may help detect lymph nodes that are not positive by CT criteria but are fluorodeoxyglucose avid and most likely involved.
The staging of oropharyngeal cancers is displayed in Table 68-7. Lymph node staging is similar to that of the majority of other subsites in the head and neck. Primary tumor size is staged by size, except for locally advanced disease.
Treatment
Early-Stage Disease
Definitive RT can be delivered with conventional fractionation or accelerated fractionation. The RTOG 90-03 trial examined the role of altered fractionation and included 11 patients with stage II disease. It concluded that a concomitant boost strategy for stage T2 disease is acceptable.10 The RTOG 02-22 trial examined treatment of early-stage oropharynx cancers with accelerated hypofractionated RT with IMRT and found a locoregional failure rate of 9%.234 For well-lateralized tumors, treating only the ipsilateral neck can spare morbidity, with a low rate of contralateral neck recurrences.229
Studies evaluating the prognosis of patients after the surgical removal of early-stage tumors have found good outcomes. Cosmidis and colleagues235 reported on 53 patients with early-stage oropharynx cancers that were surgically resected (81% transorally) and reported a 5-year local-regional control rate of 88.7%. Walvekar et al.236 reported on 49 patients with early-stage disease who underwent surgery, of whom 72% did not receive adjuvant RT, and found a crude recurrence rate of 28.6%.
Advanced Stage
National guidelines outline three different treatment options for patients with locally advanced disease: (1) definitive CRT, (2) surgery with or without PORT as appropriate, and (3) induction chemotherapy followed by definitive CRT.237 At MSKCC, the preferred approach for locally advanced disease is CRT, because it has the highest level of evidence and the longest history of results with good functional outcomes. The other two options for treatment are areas of active clinical research, and the role of the two treatment options is likely to evolve in the near future.
Historically, the Veterans Affairs (VA) larynx study, RTOG 91-11, and large metaanalyses, including the MACH-NC, established CRT as more effective than RT alone for locally advanced HNCs. These trials also suggested that upfront surgery could be deferred without sacrificing outcomes.81,82 A French trial that included patients with oropharyngeal carcinoma demonstrated that concurrent CRT was superior to RT alone for the treatment of oropharynx cancer.238 The trial consisted of 226 patients randomized to either concurrent carboplatin and 5-FU for three cycles with RT (70 Gy in 35 fractions) or RT alone (70 Gy in 35 fractions). The 5-year local-regional control and disease-free survival were both superior in the concurrent chemotherapy arm: 48% versus 25% (P = .002) and 27% versus 15%, respectively (P = .01). The 5-year overall survival was also significantly improved with combined modality therapy: 22% versus 16% (P = .05). Combined modality treatment was associated with an increase in acute grade 3 or 4 toxicities (56% vs. 30%), although this finding was not statistically significant (P = .12). Further, a metaanalysis of all randomized controlled trials with or without concurrent chemotherapy in HNC demonstrated that oropharyngeal cancers had an 8% overall survival advantage with concurrent chemotherapy compared with RT alone.91 As a single agent, cisplatin appears to be the most effective concurrent agent and is typically what we recommend.239
Modern retrospective institutional series have demonstrated significantly improved outcomes with IMRT with or without chemotherapy (Table 68-8).234,240–243 IMRT allows for the sparing of normal structures while simultaneously increasing the dose to gross disease (especially lymph nodes) compared with conventional techniques. At 2 years, local and regional control rates have been greater than 90%, and overall survival has been greater than 80%. Part of the improvement in outcomes in these series is attributed to the use of concurrent chemotherapy and improved targeting of disease, although it may be that some of the improvement may be due to the changing prevalence of HPV-related disease during this period.5
Table 68-8
Representative Large Oropharynx Series with the Majority of Patients Receiving Definitive Intensity-Modulated Radiation Therapy
| Study | N | Median Follow-up (Mo) | Stage III-IV (%) | Definitive (%) | Chemotherapy (%) | Local Control and/or Regional Control (%) | Overall Survival (%) |
| Garden et al., 2007 | 51 | 45 | 84 | 100 | 10 | LC: 96 (2 yr) LRC: 93 (2 yr) |
3 yr: 87 |
| Yao et al, 2006 | 66 | 27 | 92 | 94 | 70 | LRC 99 (3 yr) | 3 yr: 78 |
| Sanguineti et al., 2008 | 50 | 33 | 88 | 10 | 0 | LC: 94 (3 yr) RC: 85 (3 yr) |
NA |
| Huang et al., 2008 | 71 | 33 | 100 | 100 | 100 | LC: 94 (3 yr) RC: 94 (3 yr) |
3 yr: 83 |
| Daly et al., 2009 | 107 | 29 | 96 | 79 | 87 | LRC: 92 (3 yr) | 3 yr: 83 |
| Eisbruch et al., 2010* | 69 | 34 | 43† | 100 | 0 | LRC: 91 (2 yr) | 2 yr: 96 |
| Setton et al., 2012 | 442 | 36 | 95 | 93 | 91.4 | LC: 94.6 (3 yr) RC: 94.4(3 yr) |
3 yr: 85 |
LC, local control; LRC, local-regional control.
An alternative to concurrent chemotherapy for patients with advanced disease is concurrent biological therapy with an EGFR inhibitor. Cetuximab, a monoclonal antibody against EGFR, has been shown to improve overall survival and disease-free survival compared with RT alone.94 In subgroup analysis, patients with oropharyngeal disease appeared to derive benefit. At present, no head-to-head comparison of concurrent biological therapy versus concurrent chemotherapy has been completed; however, single-institution retrospective studies suggest that concurrently administered cetuximab may be inferior to concurrently administered cisplatin.244 Randomized studies comparing these two agents are under way, but until they are completed, at MSKCC we typically prefer to use concurrently administered cisplatin.
Historically, after treatment with definitive RT, patients with advanced nodal disease would undergo a planned neck dissection. Multiple studies have demonstrated the increased risk of nodal relapse with N2 or N3 disease with RT alone.247–247 However, in the modern era, the risk of nodal relapse has decreased significantly. Many institutions now only proceed with a neck dissection if patients do not have a complete response by 12 weeks or have radiographic evidence of residual disease on their first follow-up scan. Thariat et al.248 found a 5-year regional control rate of 92% in patients who had a complete response after definitive treatment and did not undergo planned neck dissection. In patients with N3 disease, fewer data are available on the safety of omitting a neck dissection, and omission is more controversial.249
The role of brachytherapy in the management of the initial presentation of SCCs of the oropharynx has decreased in the modern era. Historically, a brachytherapy boost could be delivered after 45 to 54 Gy of EBRT to base of tongue tumors or tonsillar tumors.252–252 With the advent of IMRT, the routine use of concurrent chemotherapy, and the changes in the biology of oropharynx cancers, local-regional control rates are now greater than 90%, and it is unlikely that a brachytherapy boost will have a clinically significant benefit. In the recurrent setting or in the setting of previous head and neck irradiation, a brachytherapy boost as a component of treatment may play a more important role.
Because combined modality treatment now allows for organ preservation, open surgery is usually reserved as a salvage treatment instead of as part of upfront management. Although no randomized studies have directly compared definitive radiotherapy with surgery in oropharyngeal cancers, in other SCCs of the head and neck, no difference in outcomes has occurred.81,82 Parsons et al.253 reviewed 51 studies (mostly retrospective) comprising approximately 6400 patients with oropharyngeal carcinoma who were treated with either definitive surgery with or without PORT or with definitive RT with or without neck dissection. They found no difference in local control between surgery versus definitive radiation in local control (79% vs. 76%, P = .09), local-regional control (65% vs. 69%, P = .1), or 5-year overall survival (47% vs. 43%, P = .2). However, a significant increase in the rate of severe complications with surgery (23% vs. 6%, P < .001) and fatal complications 3.2% vs. 0.8% (P < .001) occurred. Severe complications in this review for the RT arm consisted of permanent gastrostomy, soft tissue necrosis, severe osteoradionecrosis, radiation-induced sarcoma, serious neurologic injury, and severe edema. Severe complications from surgery included wound breakdown or fistula requiring surgical repair, osteomyelitis, permanent gastrostomy, permanent tracheostomy, chronic aspiration, carotid artery rupture, or several cardiopulmonary or central nervous system events.
In an attempt to improve functional outcomes, transoral and robotic techniques have become increasingly popular in the past several years.254 In 2006, O’Malley and colleagues reported on the first series of three patients with base of the tongue tumors resected with transoral robotic surgery.255 Subsequently, multiple other institutions demonstrated the feasibility of this approach.258–258 However, long-term oncologic outcomes and functional outcomes from these types of surgical resections are limited.
Weinstein et al.259 reported on 47 patients with stage III or IV advanced oropharyngeal carcinoma with a mean follow-up of 26.6 months. They reported a local control rate of 98% and a regional control rate of 96%. Eighty-five percent of patients received either adjuvant RT or CRT. Moore and colleagues260 reported on 66 patients with SCC of the oropharynx (88% with stage III or IV disease) and a minimum follow-up period of 2 years or longer. They reported local control and regional control rates of 97% and 94%, respectively. In this cohort, 21.2% received adjuvant RT and 62.1% received adjuvant CRT. Three patients had long-term gastrostomy tube use, and one patient required long-term use of a tracheostomy.
The role of robotic surgery in the management of locally advanced oropharyngeal carcinomas is an area of active investigation and is likely to evolve in the near future as more experience accrues. Pathologic information from surgery may help decrease the need for adjuvant chemotherapy; however, exactly how to tailor adjuvant therapy for patients treated with robotic surgery is also an area that will likely evolve and perhaps depend on HPV status.254
Major and Minor Salivary Gland Cancer
Salivary gland tumors constitute a rare and interesting heterogeneous group of tumors that represent approximately 6% to 8% of head and neck tumors.261 The vast majority of salivary gland tumors are benign and develop in the parotid gland.261 Tumors in the minor salivary gland are more likely to be malignant (50% to 60%), as are sublingual salivary gland tumors (80% to 90%), in comparison with major salivary gland and parotid tumors (20% to 30%) and submandibular tumors (30% to 40%).
The large majority of patients with major salivary gland cancer present with a painless mass or swelling in the parotid, submandibular, or sublingual gland. Patients with minor salivary gland cancer often present with ulcerations or a submucosal mass on the palate, lips, buccal mucosa, or nasal cavity/maxillary sinus. Similar to nasopharyngeal cancer, minor salivary gland cancer of the nasopharynx usually presents at an advanced stage with invasion of skull base, intracranial extension, or involvement of cranial nerves. The presence of neurologic signs and symptoms is almost always indicative of a malignant rather than benign tumor and is negatively prognostic.262 Additional features suggestive of a malignant parotid tumor include pain, rapid growth, skin involvement, and cervical lymphadenopathy. Negative findings of FNA does not exclude the possibility of malignancy, and pursuing additional biopsies or a parotidectomy may be indicated.
Histology/Pathology
Benign and malignant salivary gland tumors are classified according to the 2005 World Health Organization system.263
Mucoepidermoid Carcinoma
Mucoepidermoid carcinomas vary in size and lack a well-defined capsule. Microscopically, the tumor shows a punching or infiltrative border. Tumors vary in appearance from white to gray and frequently contain small to microscopic mucinous cysts (Fig. 68-13, A). Microscopically, the tumor is composed of cells arranged in cords, nests, and sheets with varying amounts of squamous, mucous, and intermediate cells. These tumors can vary from well differentiated to highly aggressive, poorly differentiated histopathology. Various grading schemes to account for these differences cluster tumors into low-, intermediate-, and high-grade categories based on the amount of mucinous cells, solid squamous nests, mitotic rate, necrosis, and pleomorphism.264,265 These tumor grades also correlate with patient outcome. Low-grade tumors (Fig. 68-13, B) rarely metastasize, often are locally infiltrative, and recur in 10% to 15% of cases and therefore have an excellent 5-year survival rate of greater than 90%. By contrast, high-grade tumors (Fig. 68-13, C), are highly infiltrative, recur in 30% to 40% of cases, and have metastasized in 30% to 40% of cases at presentation, producing a 5-year survival rate of only 50%.268–268 Grading appears to have less prognostic importance when the submandibular glands are involved. Involvement of this site appears to be associated with an increased risk of distant relapse regardless of grade.
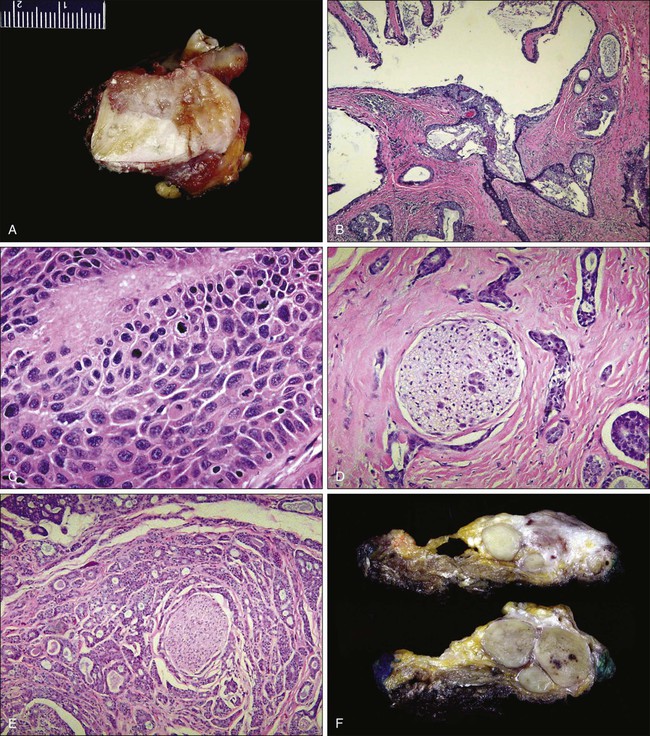
Adenoid Cystic Carcinomas
Adenoid cystic carcinomas account for some 20% of malignant salivary gland tumors. They are frequently located in the minor salivary glands (in 40% to 50% of cases) and less often in the parotid gland (in 20% to 30% of cases). The most common histologic pattern is the classic cribriform type or “Swiss cheese” pattern, characterized by neoplastic cells that form oval or circular spaces or nests. Within these nests is dark, eosinophilic, hyaline-like basement membrane material. The amount of hyaline material can distort the cell nests and produce a small acinar or cordlike appearance to the tumor nests (Fig. 68-13, D). Perineural invasion is almost invariably observed in these tumors (Fig. 68-13, E), and a diagnosis of adenoid cystic carcinoma without finding perineural invasion should be carefully reconsidered. Adenoid cystic carcinomas are graded into low-, intermediate-, and high-grade tumors based on the amount of solid growth, mitoses, necrosis, and pleomorphism; as with mucoepidermoid carcinoma, the degree of differentiation is correlated with long-term survival.
Malignant Mixed Tumor (Carcinoma Ex Pleomorphic Adenoma)
Malignant mixed tumors of the salivary gland are predominantly tumors that started as pleomorphic adenomas in which the epithelial tumor component subsequently underwent malignant transformation. This transformation may be confined to the adenoma, resulting in the noninvasive carcinoma ex pleomorphic adenoma. A majority of carcinomas that arise out of pleomorphic adenomas, however, are infiltrative and aggressive lesions (Fig. 68-13, F and G). The transformation to a malignant phenotype is heralded by a change in the behavior of the patient’s underlying pleomorphic adenoma that is characterized by sudden enlargement of an otherwise stable adenoma. Morphologically, the malignant transformation can take on any malignant epithelial salivary tumor phenotype, the most common being “adenocarcinoma not otherwise specified.” Occasionally, multiple recognizable histologic patterns may be present. These tumors are highly aggressive, with 5-year survival rates as low as 25%. Another form of the malignant mixed tumor is the true carcinosarcoma, which again often arises from a pleomorphic adenoma background. These tumors have both malignant epithelial and malignant stromal elements.
Staging
Major salivary gland cancer is staged according to the AJCC seventh edition (Table 68-9), whereas tumors arising in the minor salivary glands are staged according to their anatomic site of origin.
Table 68-9
American Joint Committee on Cancer Salivary Cancer Staging
| Stage | Staging Criteria |
| T CATEGORY | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤2 cm in greatest dimension without extraparenchymal extension* |
| T2 | Tumor >2 cm but ≤4 cm in greatest dimension without extraparenchymal extension* |
| T3 | Tumor >4 cm in greatest dimension and/or tumor having extraparenchymal extension* |
| T4a | Tumor invades the skin, mandible, ear canal, and/or facial nerve |
| T4b | Tumor invades skull base and/or pterygoid plates and/or encases carotid artery |
| N CATEGORY | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastases in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2 | |
| N2a | Metastasis in a single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
| N2b | Metastasis in a multiple ipsilateral lymph nodes ≤6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes ≤6 cm in greatest dimension |
| N3 | Metastasis in lymph node(s) >6 cm |
| M CATEGORY | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
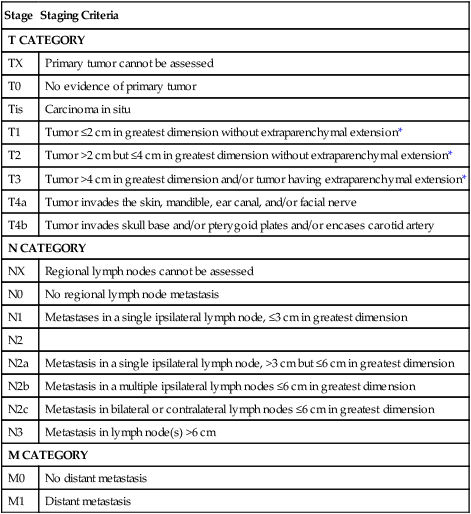
*Extraparenchymal extension is clinical or macroscopic evidence of invasion of soft tissues. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Treatment Strategy
Combined therapy usually is recommended for high-grade malignancies of the salivary gland, such as high-grade mucoepidermoidal carcinoma. Postoperative irradiation has been shown to improve local-regional control in several studies, especially for patients with positive surgical margins, high-risk features such as advanced stage, high-grade, skin/nerve invasion, and adenoid cystic carcinoma.269,270
In one study, PORT compared with surgery alone reduced the incidence of local recurrence from 54% to 14% in patients with microscopic or close surgical margins.271 Similarly, in a separate study, the use of PORT in patients with locally advanced disease improved the 5-year local control rate from 17% to 51% and the 5-year cause-specific survival rate from 10% to 51%.272 Generally, adjuvant RT to a total dose of 70 Gy in 2 Gy per fraction is recommended for patients with stage T2 or greater high-grade tumors, locally advanced T3/T4 tumors, positive regional lymph nodes, positive surgical margins, perineural, vascular, or lymphatic invasion, and extracapsular extension. In nonoperable and unresectable tumors, definitive RT should be considered.
Nodal metastases are present in approximately 20% of patients at presentation. A selective neck dissection is indicated in the event of known cervical metastasis with PORT. The management of a clinically negative neck is still being debated. The incidence of occult metastases has been reported to be 12% to 45% in patients with clinically negative findings in their necks. Hence some centers recommend elective neck dissection for all patients with malignant pathology,273 whereas other centers have reserved elective neck dissection for patients deemed to be at high risk for occult nodal disease, including those with high-grade tumors, locally advanced disease, facial nerve paralysis or weakness, and advanced age.274
Larynx
Anatomically, the larynx is divided into three subsites: the supraglottic, glottic, and subglottic larynx (Fig. 68-14). The supraglottic larynx extends from above the tip of the epiglottis to the laryngeal ventricle and includes the epiglottis, the aryepiglottic folds, the arytenoids, and the false vocal cords. During swallowing, the epiglottis covers the entrance of the larynx and prevents food from entering the trachea. The epiglottis itself is divided into a suprahyoid and infrahyoid portion. The aryepiglottic folds connect the epiglottis to the arytenoids. The arytenoids are paired pyramidal-shaped cartilages that sit on top of the cricoid cartilage and serve as the posterior attachments for the true vocal cords. The false vocal cords lie superior and laterally to the true vocal cords.
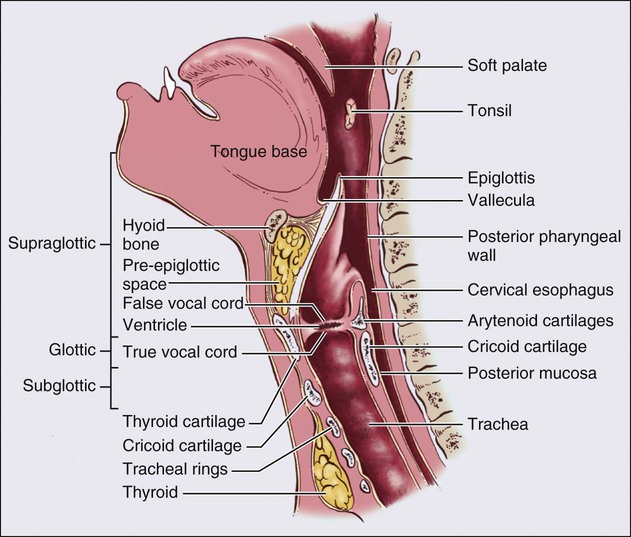
Early T-stage glottic larynx cancers are very unlikely to drain to lymph nodes because of poor lymphatics in the true vocal cords.275 Locally advanced lesions, including T3 and T4 lesions, have a higher propensity for lymph node spread, because the primary tumor extends outside of the glottis itself. These lesions may spread to levels II to IV, the pretracheal delphian nodes, and to a lesser extent, level V.36,226 On the other hand, the supraglottic larynx has a rich network of lymphatics, and primary lesions arising here are at risk for bilateral lymph node metastasis, because the supraglottic larynx is a midline structure. Up to 55% of patients present with positive nodes and 16% of patients present with bilateral nodes.
Epidemiology
It was estimated that in 2012 there would be an estimated 12,360 cases of laryngeal carcinoma and 3650 deaths due to the cancer. Eighty percent of cases were expected to occur in men. Similar to other head and neck cancers, the majority of cases are related to smoking, alcohol consumption, or consumption of betel nuts. In laryngeal cancers, 89% of the population attributable risk appears to be due to smoking, alcohol, or a combination of the two.276 The 5-year overall survival rate is between 60% and 65%;277 this rate does not have appear to have improved within the past 30 years.278 Glottic cancers are roughly two times more common than supraglottic cancers, whereas subglottic cancers are rare and consist of fewer than 5% of laryngeal malignancies.
Laryngeal cancers represent about 25% of all head and neck malignancies and historically were the most common subsite in the pharynx among patients in the United States. However, a decline in smoking has led to a reduction in laryngeal cancer incidence,278 and laryngeal cancers will soon be displaced by the rising epidemic of HPV-associated oropharyngeal cancers. HPV infections have also been found in laryngeal cancers; however, the clinical significance of these infections and the impact on prognosis are not as clear as with oropharynx cancers.279
Presentation, Workup, and Staging
The physical examination should include a careful examination of the bilateral cervical and supraclavicular lymph node chains. As previously discussed, lymph node metastasis is rare in early glottic cancers but occurs in more than 50% of supraglottic laryngeal cancers. Tenderness or a mass over the thyroid cartilage may suggest invasion of the cartilage. Loss of the laryngeal click on palpation of the thyroid cartilage suggests invasion into the postcricoid area. Fiberoptic examination or an indirect mirror examination is required. For glottic cancers, determining the location of the lesion—whether it extends above or below the glottis—and alterations in vocal cord mobility will alter the AJCC stage (Table 68-10). For both supraglottic and glottic laryngeal cancers, careful attention to the mucosal spread of disease is important, because this development is often only poorly captured on cross-sectional imaging.
Table 68-10
American Joint Committee on Cancer T Staging System for Larynx Cancers
| Stage | Staging Criteria |
| SUPRAGLOTTIC LARYNX T STAGE | |
| T1 | Tumor limited to one subsite of the supraglottis with normal vocal cord mobility |
| T2 | Tumor invades mucosa of more than one adjacent subsite of the supraglottis or glottis or region outside of the supraglottis (e.g., mucosa of the base of tongue, vallecula, medial wall of pyriform sinus) without fixation of the larynx |
| T3 | Tumor limited to larynx with vocal cord fixation and/or invades any of the following: postcricoid area, preepiglottic space, paraglottic space, and/or inner cortex of thyroid cartilage |
| T4a | Moderately advanced local disease; tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx (e.g., trachea, soft tissues of neck, including deep extrinsic muscles of the tongue, strap muscles, thyroid, or esophagus) |
| T4b | Very advanced local disease; tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| GLOTTIC LARYNX T STAGE | |
| T1 | Tumor limited to the vocal cord(s) may involve anterior or posterior commissure) with normal mobility |
| T1a | Tumor limited to one vocal cord |
| T1b | Tumor limited to both vocal cords |
| T2 | Tumor extends to supraglottis and/or subglottis, and/or with impaired vocal cord mobility |
| T3 | Tumor limited to the larynx with vocal cod fixation and/or invasion of paraglottic space, and/or inner cortex of thyroid cartilage |
| T4a | Moderately advanced local disease; tumor invades the outer cortex of the thyroid cartilage and/or invades tissues beyond the larynx (e.g., trachea, soft tissues of neck including deep extrinsic muscle of tongue, strap muscles, thyroid, or esophagus) |
| T4b | Very advanced local disease; tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| SUBGLOTTIS T STAGE | |
| T1 | Tumor limited to the subglottis |
| T2 | Tumor extends to vocal cord(s) with normal or impaired mobility |
| T3 | Tumor limited to larynx with vocal cord fixation |
| T4a | Moderately advanced local disease. Tumor invades cricoid or thyroid cartilage and/or invades tissues beyond the larynx (e.g., trachea, soft tissues of neck including deep extrinsic muscles of the tongue, strap muscles, thyroid, or esophagus) |
| T4b | Very advanced local disease. Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
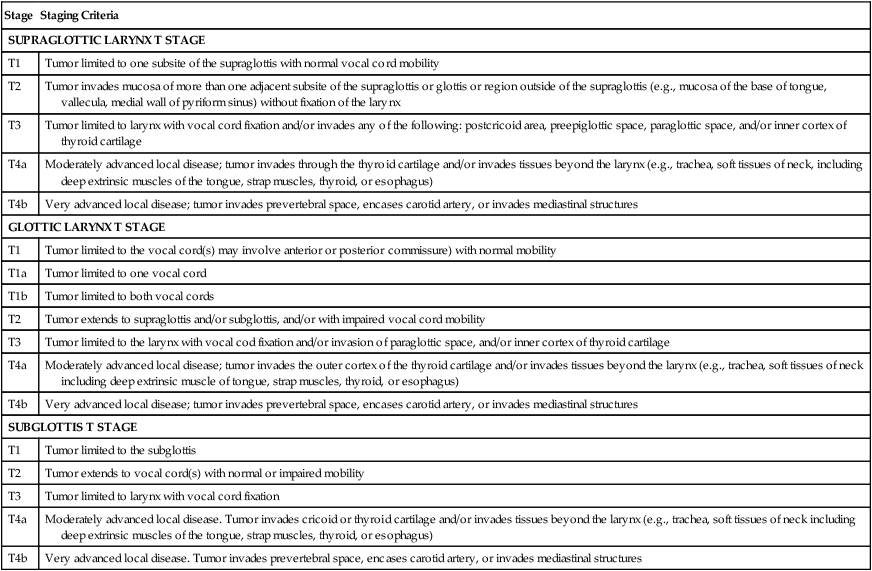
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
The National Comprehensive Cancer Network recommends cross-sectional imaging to evaluate the primary site and the neck for either glottic or supraglottic cancers, although these studies may not be necessary for patients with Tis or T1 glottic cancers. Endoscopic and clinical examination alone tends to underestimate invasion of the laryngeal cartilages and extralaryngeal soft tissues, compared with pathologic results.280 Axial imaging allows for evaluation of paraglottic invasion, thyroid cartilage invasion, extralaryngeal disease, preepiglottic invasion, and nodal metastasis. Whether CT or MRI is optimal for laryngeal cancers remains controversial, and each modality has its own set of advantages and disadvantages. CT scans can be acquired quickly and are not susceptible to artifact from swallowing or breathing, as are MRI scans.281 However, MRI is more sensitive for cartilage invasion, although it is less specific.280 At MSKCC, we typically prefer to perform a CT scan as the initial imaging study. PET/CT may be helpful in more advanced stages to detect nodal or distant metastasis. Additional studies to complete the workup include chest imaging and a biopsy of the primary site.
Treatment
Total laryngectomy is a highly effective operation in removing even advanced-stage laryngeal cancer, but it is associated with significant morbidity, including permanent loss of voice and stomal respiration. One study demonstrated that 25% of healthy persons were willing to decrease their absolute chance of survival by 20% in order to preserve their voice.282 In the past few decades, advances have been made in the ability to preserve the function of the larynx with additional surgical techniques and a variety of combined modality protocols. Early-stage disease is typically treated with either surgery or RT alone, whereas advanced disease is typically treated with a combined modality approach. Before beginning treatment, patients should be referred for a speech and swallow evaluation when appropriate, a dental evaluation (if he or she is to be treated with RT), and a hearing evaluation (for patients treated with chemotherapy).
In persons with early glottic cancer, typically T1-T2N0 disease, either surgery or RT are acceptable options.283,284 Unfortunately, no randomized trials have been performed to compare limited surgical procedures to RT. However, a wealth of retrospective studies demonstrate excellent local control with either modality. A Cochrane review comparing the evidence between surgery and RT concluded that evidence is insufficient to decide between therapies.285 The choice between modalities will depend on the resectability of the lesion with adequate local control, as well as patient preference.
Multiple surgical procedures are available for early-stage disease, including endoscopic CO2 laser excision, open cordectomy, and partial laryngectomy.286,287 Transoral endoscopic approaches appear to have local control rates that are similar to those of open surgical procedures.288 RT is typically preferred when bilateral vocal cord involvement is present. Some persons have suggested that voice quality is worse after surgery; however, this topic is controversial, with conflicting studies in the literature.289,290
Multiple retrospective studies have demonstrated excellent local control rates with fractionated RT for early glottic cancers.291–295 T1 tumors have local control rates ranging between 83% and 94% (Table 68-11). T2 tumors have worse local control rates, in the range of 70% to 80%, but they still have outcomes similar to that of surgery.284,296 Ultimate local control after surgical salvage for recurrence is greater than 95% and 90% for T1 and T2 glottic tumors, respectively. A randomized study from Japan demonstrated that hypofractionated RT improves local control compared with conventional fractionation.297 Yamazaki and colleagues297 randomly assigned 180 patients with early glottic cancer to either treatment with 2 Gy per fraction versus 2.25 Gy per fraction (hypofractionated treatment). They reported an increase from 77% to 92% in the 5-year local control rate but no difference in overall survival.
Table 68-11
Select Radiotherapy Series for Early Glottic Carcinoma
| Author | N | T1 Local Control* (after X-ray Therapy) (%) | T2 Local Control* (after X-ray Therapy) (%) | Control Rates after Surgical Salvage (%) |
| Mendenhall et al.291 | 519 | 93-94 | 72-80 | >95 |
| Smee et al.292 | 522 | 83 | 72 | 85-95 |
| Cellai et al.293 | 831 | 84 | NA | 95 |
| Le et al.294 | 398 | 85 | 70 | 92-97 |
| Johansen et al.295 | 358 | 83 | NA | 94 |
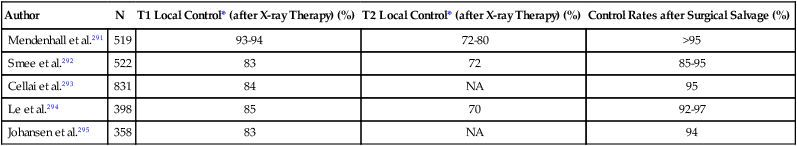
*Local control rates before surgical salvage. Most local failures are surgically salvageable.
Historically, in locally advanced disease, total laryngectomy followed by PORT was the treatment of choice. The VA Larynx study definitively established the paradigm of organ preservation.81 In this landmark trial, 322 patients with stage III or IV larynx cancer were randomly assigned to either total laryngectomy and PORT (control arm) or to induction chemotherapy followed by radiation (experimental arm). In the experimental arm, patients who did not have at least a partial response after two cycles of chemotherapy went on to undergo a total laryngectomy, whereas those who responded received a third cycle of chemotherapy before RT. The chemotherapy consisted of 5-FU and cisplatin. Of note, patients with T1N1 disease and those with unresectable disease were excluded.
In the experimental arm, 85% of patients had at least a partial response at the primary site and hence continued forward with treatment. The 2-year overall survival was 68% in both arms. The larynx preservation rate in the induction chemotherapy arm was 64%. Although an increase in local-regional failure occurred in the CRT arm, this did not impact overall survival, because these laryngectomy was still an option. Subgroup analysis revealed that 56% of patients with T4 cancers required salvage laryngectomy compared with 29% of patients with smaller primary lesions (P = .001). This trial suggested that an organ-preservation approach did not decrease survival, which was a finding confirmed in a similar trial by the EORTC in the hypopharynx.298
This study naturally led to RTOG 91-11, which was a three-arm randomized trial designed to find the optimal organ preservation regimen.82 It compared (1) induction chemotherapy followed by radiation, (2) concurrent chemotherapy with radiation, and (3) RT alone. Based on the results of the VA larynx study, patients with large-volume T4 disease were excluded (i.e., “tumor penetrating through cartilage or extending more than 1 cm into the base of tongue”). At 2 years the concurrent CRT had a statistically superior larynx preservation rate of 88%. No difference in overall survival was found between the groups.
Hypopharynx
Hypopharynx cancers are more often asymptomatic compared with their laryngeal counterparts. The most common symptoms are a neck mass, sore throat, and odynophagia. Workup is similar to cancers of the larynx and includes a thorough history and physical examination, fiberoptic examination, and axial imaging (CT scan or MRI). PET/CT should be considered for locally advanced disease. Staging is reviewed in Table 68-12.
Table 68-12
American Joint Committee on Cancer T Staging System for Hypopharynx Cancers
| Hypopharynx T Stage | Staging Criteria |
| T1 | Tumor limited to one subsite of hypopharynx and/or 2 cm or less in greatest dimension |
| T2 | Tumor invades more than one subsite of hypopharynx or an adjacent site, or measures more than 2 cm, but no more than 2 cm in greatest dimension without fixation of hemilarynx |
| T3 | Tumor more than 4 cm in greatest dimension or with fixation of hemilarynx or extension to esophagus |
| T4a | Moderately advanced local disease; tumor invades thyroid/cricoid cartilage, hyoid bone, thyroid gland, or central compartment soft tissue |
| T4b | Very advanced local disease; tumor invades prevertebral fascia, encases carotid artery, or involves mediastinal structures |
From Edge SB, Byrd DR, Compton CC, et al., editors. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Early-stage disease is infrequent, but patients with T1-T2/N0 disease typically can be treated with either conservative surgery or definitive RT. Locally advanced disease is treated in a similar manner to larynx cancer, with an organ preservation approach with combined CRT for most patients. The EORTC conducted a study of induction chemotherapy followed by RT versus surgical resection and PORT in cancers of the hypopharynx.299 The rates of local-regional failure in the two arms was similar; however, fewer distant failures occurred in the arm with chemotherapy. Overall survival was better in the chemotherapy arm. This trial is historically important because it confirms the results of the VA larynx trial, namely, that organ preservation as an upfront strategy does not jeopardize overall survival.