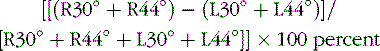Chapter 8 Vertigo
Physiologic Basis of Balance
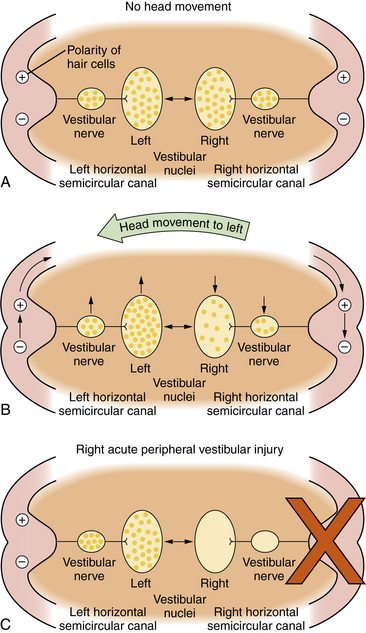
The oculovestibular reflex is a mechanism by which a head movement automatically results in an eye movement that is equal and opposite to the head movement so that the visual axis of the eye stays on target: that is, a leftward head movement is associated with a rightward eye movement and vice versa. Another feature of the oculovestibular reflex is that the two vestibular nuclear complexes on either side of the brainstem cooperate with one another in such a way that, for the horizontal system, when one nucleus is excited, the other is inhibited. The central nervous system responds to differences in neural activity between the two vestibular complexes. When there is no head movement, the neural activity, i.e., the resting discharge, is symmetrical in the two vestibular nuclei. The brain detects no differences in neural activity and concludes that the head is not moving (Figure 8-1A). When the head moves, e.g., to the left, endolymph flow produces an excitatory response in the labyrinth on the side toward which the head moves, e.g., on the left, and an inhibitory response on the opposite side, e.g., on the right. Thus, neural activity in the vestibular nerve and nuclei, e.g., on the left and right, increases and decreases, respectively (Figure 8-1B). The brain interprets this difference in neural activity between the two vestibular complexes as a head movement and generates appropriate oculovestibular and postural responses. This reciprocal push-pull balance between the two labyrinths is disrupted following labyrinthine injury.
An acute loss of peripheral vestibular function unilaterally, e.g., on the right, causes a loss of resting neural discharge activity in that vestibular nerve and the ipsilateral nucleus (Figure 8-1C). Since the brain responds to differences between the two labyrinths, this will be interpreted by the brain as a rapid head movement toward the healthy labyrinth, i.e. vertigo. “Corrective” eye movements are produced toward the opposite side, resulting in nystagmus, with the slow component moving toward the abnormal side, e.g., the right, and the quick components of nystagmus moving toward the healthy labyrinth, e.g., the left.
Evaluation of Patients with Dizziness
Physical Examination
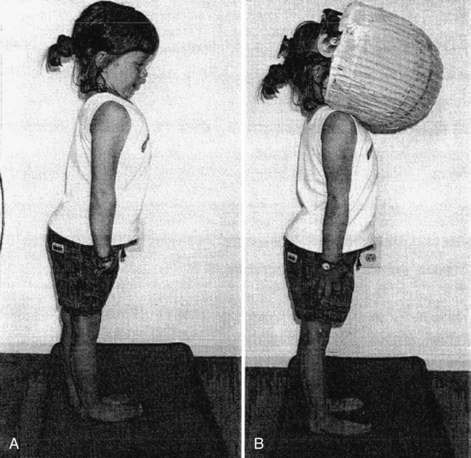
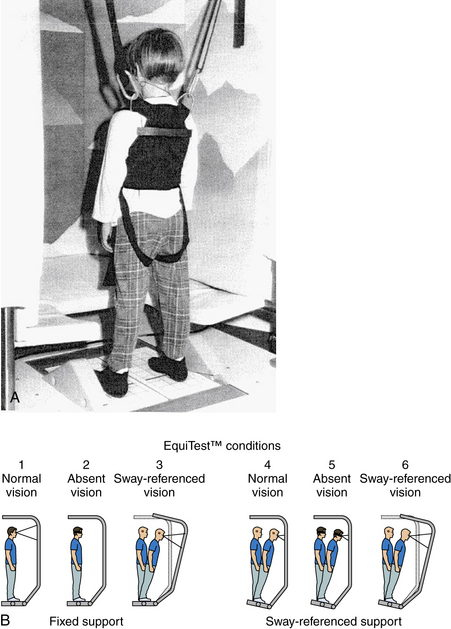
In addition to a complete neurologic examination, the child should also be observed when walking or running for incoordination of movements, i.e., ataxia. Also, an assessment of nystagmus is especially important. Spontaneous nystagmus is an involuntary, rhythmic movement of the eyes not induced by any external stimulation. Spontaneous nystagmus has two components: slow and fast. Nystagmus is named by the fast component, which is easily identified. Spontaneous nystagmus is tested by having the patient look straight ahead with and without fixation. Gaze-evoked nystagmus is assessed by having the patient deviate the eyes laterally (no greater than 30 degrees) with fixation. Positional testing is performed with the use of maneuvers that may produce nystagmus or vertigo. Static positional nystagmus is assessed by placing the patient in each of the following six positions: sitting, supine, supine with the head turned to the right, supine with the head turned to the left, and right and left lateral positions. Positional nystagmus presents as soon as the patient assumes the position and persists for as long as the patient remains in the provocative position. Assessment of vestibulospinal function with a foam pad (Figure 8-2) should be performed with or without a visual conflict dome.
Videonystagmography
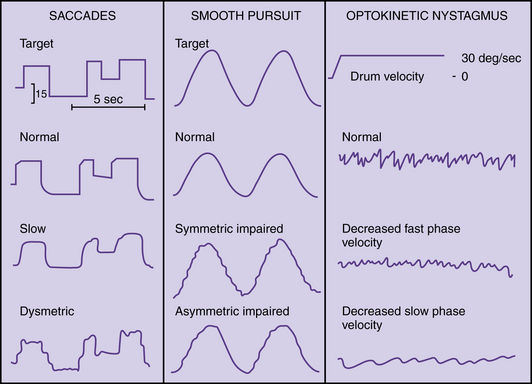
Videonystagmography (VNG) is currently the most widely used method of recording eye movements; it uses infrared light. Ocular motor testing, positional testing, and caloric testing constitute a common test battery that requires about 1 hour. Sedatives and vestibular suppressant medications should be discontinued for 2 days prior to testing. Ocular motor testing evaluates neural motor output independent of the vestibular system (Figure 8-3). Abnormalities in the ocular motor system may cause misleading conclusions from vestibular testing that relies on eye movements. Testing saccades uses a computer-controlled sequence of target jumps. Saccade abnormalities are defined as overshooting the target (hypermetric saccades) and undershooting the target (hypometric saccades). Disorders in the saccadic system suggest a central nervous system abnormality. Spontaneous nystagmus and gaze-evoked nystagmus are recorded with and without fixation (closing the eyes or darkness) (Figure 8-4), and by asking the patient to look 30 degrees to the right and left. Spontaneous nystagmus present in darkness without fixation, which decreases or resolves with visual fixation, suggests a peripheral vestibular disorder. However, spontaneous nystagmus that is present with fixation and does not significantly decrease with loss of fixation is most likely a central nervous system abnormality. Ocular pursuit involves asking the patient to follow a moving target back and forth along a slow pendular path. Normal subjects can follow a target smoothly without interruption. Abnormalities of pursuit tracking are caused by lesions in the central nervous system. Laboratory testing of optokinetic nystagmus uses black-and-white stripes moving left and right. Abnormalities include asymmetries or absence of responses, which suggest a central nervous system abnormality.
Caloric Testing
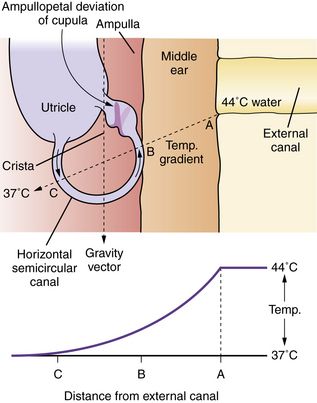
Caloric testing aims to assess each labyrinth separately by producing nystagmus via thermal stimulation of the vestibular system. The patient is placed in a position in which the horizontal semicircular canals lie in the vertical plane (head elevated 30 degrees). Caloric stimulation causes a convection current in the horizontal semicircular canal that causes a deflection of the cupula (into which the hairs of the hair cells are embedded) and a change in activity of the vestibular nerve. Cold irrigation produces a fast nystagmus component away from the irrigated ear; warm irrigation produces a fast nystagmus component toward the irrigated ear (Figure 8-5). Binaural bithermal caloric testing uses stimuli of 30°C and 44°C, and each canal is irrigated for 30 seconds with 250 mL of water. There is a rest period of 5 minutes between irrigations. The most common method of measuring the caloric response is to compute the peak slow-component velocity of the nystagmus induced by the thermal stimulus, which reflects the intensity of the vestibular response. To compare the responsiveness of one ear to the other ear, it is established practice to use Jongkees’ formula to compute a percentage of “reduced vestibular response”:
Computerized Dynamic Platform Posturography
Computerized dynamic posturography, known commercially as EquiTest™ (NeuroCom International, Inc.), consists of a floor and a visual scene that can move (Figure 8-6A). By combining visual and floor conditions, six different sensory conditions can be used to assess the patient’s ability to use combinations of sensory inputs (Figure 8-6B). Conditions 5 and 6 assess how patients use vestibular information when it is the only available sense providing reliable information; reduced or distorted sensory information from the visual system and somatosensory system forces patients to rely on their vestibular sensations to maintain upright balance.
Posturography and Vestibular Disorders – Results from the Medical Literature
Several studies have suggested that, after successful vestibular compensation, posturography test results normalize and patients lose their “5,6 pattern” (i.e., their abnormal response to conditions 5 and 6 on posturography testing) and may, in fact, have normal postural sway [Furman, 1995]. Thus, posturography may provide valuable information regarding the status of compensation for a peripheral vestibular deficit.
Vestibular-Evoked Myogenic Potentials
Vestibular-evoked myogenic potentials (VEMPs) refer to electrical activity recorded from neck muscles in response to intense auditory clicks [Colebatch and Halmagyi, 1992; Murofushi et al., 1998]. VEMPs provide information about the status of the sacculus and inferior vestibular nerve. A limitation of VEMPs is that it requires normal middle ear function when performed using air-conducted stimuli. VEMPs have been performed successfully in children [Kelsch et al., 2006; Brantberg et al., 2007; Valente, 2007]. Children as young as age 3 can tolerate testing [Kelsch et al., 2006].
Disorders Producing Vertigo
Vertigo in children can be divided into three broad categories:
A recent study of 2000 children found that vertigo in children was caused by: a migrainous equivalent, 25 percent; paroxysmal benign vertigo of childhood, 20 percent; head trauma, 10 percent; ocular disorders, 10 percent; inner ear malformations, 10 percent; vestibular neuronitis, 5 percent; labyrinthitis, 5 percent; and posterior fossa tumors, less than 1 percent [Wiener-Vacher, 2008].
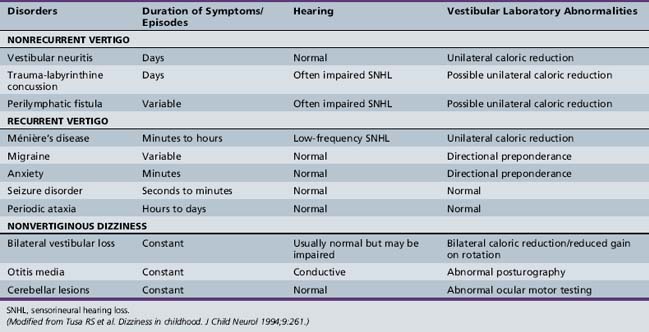
Acute Nonrecurring Spontaneous Vertigo
Head Trauma
Head trauma can cause an acute episode of vertigo via a labyrinthine concussion. The mechanism of injury in labyrinthine concussion is poorly understood but may relate to pressure waves transmitted to the labyrinth. Other mechanisms of vertigo after head trauma include injury of the CNS, specifically, a brainstem or cerebellar contusion, or a temporal bone fracture. Another diagnostic consideration for a patient with head trauma followed by vertigo or nonspecific dizziness is that of perilymphatic fistula, i.e., an anomalous connection between the inner ear and middle ear spaces that has been well documented in children [Supance and Bluestone, 1983].
Vestibular Neuritis
Vestibular neuritis is rarely seen in children younger than 10 years old. It should be considered when a viral syndrome is followed by symptoms suggestive of an acute unilateral peripheral vestibular loss [Sekitani et al., 1993]. It presents with acute severe vertigo, nystagmus, nausea, and vomiting. The vertigo is worsened by head movements, and patients often prefer to lie down, usually with the affected ear up. There is no hearing loss or tinnitus. Management is supportive and symptomatic, with early ambulation. Vestibular suppressants such as meclizine may be given, but only for a short course, as they may delay long-term recovery.
Recurrent Vertigo
Migraine-Related Dizziness
Migraine is probably the most common cause of recurrent vertigo in children. Whereas migraine typically presents as headache in adults, other manifestations of migraine, including recurrent vertigo and dysequilibrium, are more common in children. Benign paroxysmal vertigo of childhood, which is likely to be of migrainous origin, as well as paroxysmal torticollis of infancy, can present with recurrent vertigo in children. Nonvertiginous symptoms of vestibular dysfunction can also be related to migraine. The manifestations of migraine in childhood are quite varied [Balkany and Finkel, 1986].
Benign paroxysmal vertigo of childhood was first described by Basser [Basser, 1964]. Vertigo occurs in isolation, without tinnitus and hearing loss. The age of onset is usually by 4 years, but can be as late as 12 years [Blayney and Colman, 1984]. Vertigo usually lasts less than 1 minute but may last only seconds. Vertigo may occur while sitting, standing, or lying. Pallor, nausea, sweating, and occasionally vomiting occur. Consciousness is not impaired and the child can recall the episode. There may be no pain or headache associated with the attacks. Immediately after the attack, the child resumes normal activities. The interval between the attacks varies from weekly to every 6 months. Vertigo attacks usually cease spontaneously after a few years. Physical examination, including a neurologic evaluation, is normal, as is imaging of the skull and temporal bones. Basser reported a moderate or complete canal paresis on caloric testing [Basser, 1964]. However, the response to bithermal caloric testing has been found to be highly variable [Dunn and Snyder, 1976; Finkelhor and Harker, 1987; Mira et al., 1984]. Other testing is normal. Children with benign paroxysmal vertigo of childhood often have a positive family history of migraine, and migraine headaches may develop in later years [Koehler, 1980; Lanzi et al., 1994] and may respond positively to antimigraine treatment. The initial treatment of migrainous vertigo in children is dietary restrictions of foods known to provoke migraine [Constantine and Scott, 1994]. If this is unsuccessful, the next step is symptomatic treatment with a vestibular suppressant, such as meclizine, during episodes. However, the episodes are usually very brief. If the spells are frequent and especially if they impair school performance, use of a prophylactic antimigraine agent, such as propranolol, should strongly be considered [Cass et al., 1997].
Ménière’s Disease
Ménière’s disease, a syndrome presumably caused by endolymphatic hydrops, can occur spontaneously or as a delayed sequela of a previous insult from trauma or viral infection. The disorder rarely occurs in children [Filipo and Barbara, 1985; Hausler et al., 1987; Meyerhoff et al., 1978]. Ménière’s disease is characterized by a combination of dizziness, unilateral hearing loss, and unilateral tinnitus, which are usually preceded by a feeling of fullness in the affected ear. Following episodes, children are more likely to recover auditory function than are adults. Ménière’s disease can be bilateral. Also, with time, a reduction in the responsiveness of the involved peripheral vestibular system occurs. Management of endolymphatic hydrops in children includes reassurance and explanation of the condition to the parents, in addition to salt restriction and a diuretic [Cyr et al., 1985].
Seizure Disorders
Seizure disorders are often accompanied by some sense of dizziness and dysequilibrium, although seizures are not frequently associated with true vertigo. However, the term “tornado epilepsy” has been used to describe seizures that are associated with a sense of spinning that can mimic the symptoms of a peripheral vestibular ailment [Eviatar and Eviatar, 1977].
Familial Periodic Ataxia
Familial periodic ataxia is a rare syndrome with autosomal-dominant inheritance, and is characterized by episodes of dizziness, dysequilibrium, and gait instability that may last for several hours. At least two types of the syndrome have been identified [Farmer and Mustian, 1963; Parker, 1946], and genetic testing is available. These syndromes differ in the duration of the paroxysms of ataxia.
Nonvertiginous Dysequilibrium
Bilateral Peripheral Vestibular Loss
Bilateral peripheral vestibular loss can be either congenital and due to inner ear malformations, or acquired from meningitis, ototoxicity, and autoimmune disease of the inner ear. Regardless of etiology, bilateral vestibular loss, if severe, is called Dandy’s syndrome. Dandy’s syndrome is characterized by two specific symptoms: namely, oscillopsia (i.e., jumbling of the visual surround during head motion) and severe gait instability in darkness [Dandy, 1941]. Children with bilateral vestibular loss often learn to use alternative sensory inputs, such as vision and proprioception. Also, they modify strategies of eye movements. Environments and tasks that require vestibular function, such as ambulating in dimly lit spaces or trying to maintain stable vision during walking, are extremely challenging for individuals with bilateral vestibular loss.
References
The complete list of references for this chapter is available online at www.expertconsult.com.
Balkany T.J., Finkel R.S. The dizzy child. Ear Hear. 1986;7(3):138-142.
Basser L. Benign paroxysmal vertigo of childhood. Brain. 1964;87:141-152.
Blayney A.W., Colman B.H. Dizziness in childhood. Clin Otolaryngol Allied Sci. 1984;9(2):77-85.
Brantberg K., Granath K., Schart N. Age-related changes in vestibular evoked myogenic potentials. Audiol Neurootol. 2007;12(4):247-253.
Cass S.P., et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol. 1997;106(3):182-189.
Colebatch J.G., Halmagyi G.M. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42(8):1635-1636.
Constantine L., Scott S.. Migraine: The complete Guide. 1994.
Cyr D.G., et al. Vestibular evaluation of infants and preschool children. Otolaryngol Head Neck Surg. 1985;93(4):463-468.
Dandy W.E. The Surgical Treatment of Intracranial Aneurysms of the Internal Carotid Artery. Ann Surg. 1941;114(3):336-340.
Dunn D.W., Snyder C.H. Benign paroxysmal vertigo of childhood. Am J Dis Child. 1976;130(10):1099-1100.
Eviatar L., Eviatar A. Vertigo in children: differential diagnosis and treatment. Pediatrics. 1977;59(6):833-838.
Farmer T.W., Mustian V.M. Vestibulocerebellar ataxia. A newly defined hereditary syndrome with periodic manifestations. Arch Neurol. 1963;8:471-480.
Filipo R., Barbara M. Juvenile Meniere’s disease. J Laryngol Otol. 1985;99(2):193-196.
Finkelhor B.K., Harker L.A. Benign paroxysmal vertigo of childhood. Laryngoscope. 1987;97(10):1161-1163.
Furman J.M. Role of posturography in the management of vestibular patients. Otolaryngol Head Neck Surg. 1995;112(1):8-15.
Hausler R., et al. Meniere’s disease in children. Am J Otolaryngol. 1987;8(4):187-193.
Kelsch T.A., Schaefer L.A., Esquivel C.R. Vestibular evoked myogenic potentials in young children: test parameters and normative data. Laryngoscope. 2006;116(6):895-900.
Koehler B. Benign paroxysmal vertigo of childhood: a migraine equivalent. Eur J Pediatr. 1980;134(2):149-151.
Lanzi G., et al. Benign paroxysmal vertigo of childhood: a long-term follow-up. Cephalalgia. 1994;14(6):458-460.
Meyerhoff W.L., Paparella M.M., Shea D. Meniere’s disease in children. Laryngoscope. 1978;88(9 Pt 1):1504-1511.
Mira E., et al. Benign paroxysmal vertigo in childhood. Diagnostic significance of vestibular examination and headache provocation tests. Acta Otolaryngol Suppl. 1984;406:271-274.
Murofushi T., Matsuzaki M., Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1998;124(5):509-512.
Parker H. Periodic ataxia. Collect Papers Mayo Clinic Mayo Found. 1946;38:642-645.
Sekitani T., et al. Vestibular neuronitis: epidemiological survey by questionnaire in Japan. Acta Otolaryngol Suppl. 1993;503:9-12.
Supance J.S., Bluestone C.D. Perilymph fistulas in infants and children. Otolaryngol Head Neck Surg. 1983;91(6):663-671.
Valente M. Maturational effects of the vestibular system: a study of rotary chair, computerized dynamic posturography, and vestibular evoked myogenic potentials with children. J Am Acad Audiol. 2007;18(6):461-481.
Wiener-Vacher S.R. Vestibular disorders in children. Int J Audiol. 2008;47(9):578-583.