Uteroplacental Blood Flow
Warwick D. Ngan Kee BHB, MBChB, MD, FANZCA, FHKCA, FHKAM
Chapter Outline
Uteroplacental blood flow is responsible for the delivery of oxygen and nutrients to the fetus. A normal uteroplacental circulation is essential for healthy fetal growth and development. Acute reduction in uteroplacental blood flow may rapidly threaten fetal viability. Chronic reduction in uteroplacental blood flow, as may occur from abnormal development of the placental vasculature, leads to gestational pathologic processes such as preeclampsia and fetal growth restriction (also known as intrauterine growth restriction) and may even predispose the fetus to developing cardiovascular disease during subsequent adulthood.1 The uteroplacental circulation undergoes circadian changes2 and may be affected by parturition, disease, and anesthetic techniques and drugs. An understanding of the regulation of uteroplacental circulation is an important foundation for the safe provision of obstetric anesthesia and assists in the management of many pregnancy-related diseases. Research in this area is active but complicated by ethical considerations. Much of the available knowledge comes from studies in animals, particularly sheep but also nonhuman primates and other species. It is important to consider possible interspecies differences and to critically examine the methodology and context of animal research when extrapolating findings into recommendations for clinical care.
Anatomy and Structure
The blood supply to the uterus is derived mainly from the uterine arteries (Figure 3-1) with a smaller, variable contribution from the ovarian arteries. Although the pelvic vasculature shows anatomic variation,3 the uterine artery arises bilaterally from the anterior division of the internal iliac (hypogastric) artery, whereas the ovarian artery arises from the anterolateral abdominal aorta below the renal arteries. The uterine artery passes medially to the side of the uterus, where it supplies branches to the cervix and vagina and ascends between the two layers of the broad ligament, yielding arcuate arteries that supply the body of the uterus to the junction with the fallopian tubes. During pregnancy, flow may differ between the right and left uterine arteries; Konje et al.4 estimated that vessel diameter was approximately 11% greater and blood flow was approximately 18% greater in the uterine artery on the same side as the placenta compared with the contralateral artery. Anastomoses are formed with the contralateral uterine artery, the vaginal arteries, and the ovarian arteries. The arcuate arteries give rise to small branches that supply the myometrium and large radial arteries that branch deeply and enter the endometrium to form the convoluted spiral arteries. During gestation, trophoblastic invasion of the spiral arteries results in loss of smooth muscle and loss of contractile ability, leading to vasodilation with decreased resistance and increased blood flow. Abnormal or inadequate trophoblastic invasion is integral to the pathophysiology of preeclampsia (see Chapter 36).5
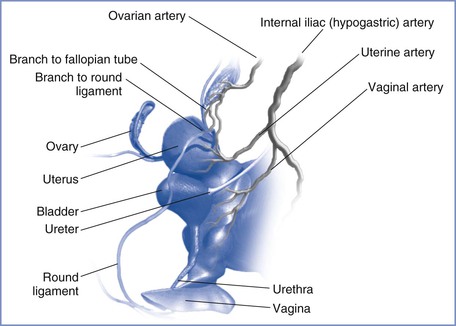
FIGURE 3-1 Arterial supply to the female reproductive tract. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
From the spiral arteries, oxygenated maternal blood enters the intervillous space in fountain-like jets. Blood traveling toward the chorionic plate bathes the villi, permitting the exchange of oxygen, nutrients, and wastes between maternal and fetal blood. Maternal blood then returns to the basal plate and drains into multiple collecting veins. Venous drainage of the uterus occurs via the uterine veins to the internal iliac veins and also via the ovarian veins (utero-ovarian plexus) to the inferior vena cava on the right and the renal vein on the left.6 The uterine artery and other branches of the anterior division of the internal iliac artery, as well as the ovarian artery, may be targeted during angiographic embolization procedures for treatment of obstetric and gynecologic hemorrhage3 and for the treatment of uterine fibroids.7
Changes and Function during Pregnancy
Pregnancy-Induced Changes
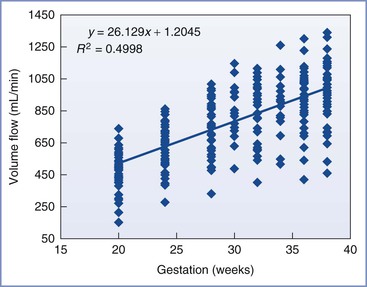
Uterine blood flow increases dramatically during pregnancy, rising from 50 to 100 mL/min before pregnancy to 700 to 900 mL/min at term, depending on the method of measurement (Figure 3-2). Studies in sheep have shown that increases in uterine blood flow can be divided into three phases.8 An initial phase, most likely controlled by the ovarian hormones estrogen and progesterone, occurs before and during implantation and early placentation. A second phase results from the growth and remodeling of the uteroplacental vasculature to support further placental development. A third and final phase results from progressive uterine artery vasodilation to meet the markedly increased nutrient requirements of the rapidly growing fetus. When expressed in terms of uterine weight, however, uterine flow per gram of tissue is particularly high in early gestation, and this ratio decreases as pregnancy progresses.8 In comparison, umbilical blood flow, expressed as a function of fetal weight, is relatively constant throughout most of pregnancy and is estimated to be 110 to 120 mL/min/kg.9 Uterine blood flow is increased in twin pregnancy, but the flow per unit of estimated fetal weight is similar to that in a singleton pregnancy.10 The progressive increase in uteroplacental blood flow during pregnancy is matched by a concurrent increase in blood flow on the fetal side (fetoplacental blood flow). However, despite suggestions of the possibility of intrinsic flow matching, it is believed that these circulations are independently regulated.11
Distribution of Blood Flow
Uterine blood flow at term represents a greater proportion of cardiac output (approximately 12%) than in early pregnancy (approximately 3.5%).12 Regional distribution of blood flow within the pelvis also changes during gestation. Palmer et al.13 observed that increases in common iliac artery blood flow during pregnancy were associated with corresponding increases in uterine artery blood flow but also with decreases in external iliac artery blood flow. This pattern effectively constitutes a “steal” phenomenon, in which blood flow in the pelvis is preferentially redistributed toward the uterus (Figure 3-3).
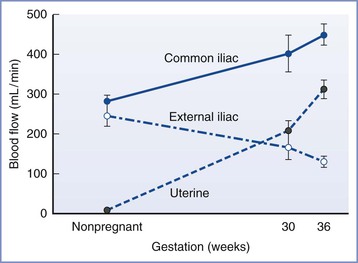
FIGURE 3-3 Redistribution of blood flow in pelvic blood vessels during pregnancy determined unilaterally by Doppler ultrasonography. Blood flow increased in the common iliac and uterine arteries but decreased in the external iliac artery, indicating that redistribution of flow favors uterine perfusion. Data are mean ± SEM. (Adapted from Palmer SK, Zamudio S, Coffin C, et al. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 1992; 80:1000-6.)
Primate studies have shown that 80% to 90% of total uterine blood flow perfuses the placenta at term, with the remainder supplying the myometrium and nonplacental endometrium.14 The placental and nonplacental vasculatures are anatomically and functionally distinct, and regulation of perfusion through these vascular beds differs.14 Therefore, it is important to differentiate studies that measure total uteroplacental blood flow versus placental blood flow.
Functional Classification
Placental vascular function varies among species. The human multivillous model is commonly believed to function as a “venous equilibrator,” in which oxygen tension in the umbilical vein approximates that in the uterine veins. In contrast, the placenta in some species (e.g., rodents) functions as a countercurrent exchanger. The more efficient function of the latter is reflected by the higher fetoplacental weight ratio in rodents (20 : 1) than in humans (6 : 1).15
Autoregulation
Studies of pressure-flow relationships suggest that the nonpregnant uterine circulation exhibits autoregulation, alternately vasoconstricting or vasodilating in response to a number of different stimuli.16 In contrast, the pregnant uterine circulation is complicated by the properties of both the placental and nonplacental circulations. Animal studies have demonstrated that the uteroplacental circulation is a widely dilated, low-resistance system with perfusion that is largely pressure dependent.17,18 However, a study in pregnant rabbits found that uteroplacental blood flow was relatively constant over a wide range of perfusion pressures.19 During hemorrhage in pregnant rats, uterine vascular resistance increased as systemic blood pressure and uterine blood flow decreased, thereby demonstrating an absence of autoregulation. Moreover, although the uteroplacental circulation is often considered to be maximally vasodilated with little or no ability for autoregulation,17 further vasodilation has been observed in response to systemically administered estrogen, prostacyclin, bradykinin, and acetylcholine.20–22 These discrepancies may be explained by changes in the nonplacental uterine vasculature, which accounts for a small fraction of total uteroplacental blood flow but appears to have similar autoregulatory responses during pregnant and nonpregnant states; this feature contrasts with the limited autoregulatory ability of the placental circulation.23 Laird et al.18 found that reducing arterial pressure by 22% with an inflatable aortic occluder in pregnant rabbits produced a reduction in total uteroplacental and placental blood flow but no significant change in myoendometrial blood flow. Clinically, limited autoregulation means that placental blood flow may diminish with reductions in maternal blood pressure (e.g., during neuraxial anesthesia).
Margin of Safety
Studies in animals have demonstrated that, in normal physiologic conditions, uterine blood flow exceeds the minimum required to satisfy fetal oxygen demand.24 Although this feature confers a margin of safety that protects the fetus from fluctuations in uterine blood flow,25 decreases in fetal PO2 and progressive metabolic acidosis can occur with reductions in uteroplacental blood flow, depending on the magnitude and duration.26 However, the relationship between uterine blood flow and oxygen transfer appears nonlinear and suggests that uteroplacental blood flow can decrease by as much as 50% for limited periods before fetal oxygen uptake decreases and metabolic acidosis occurs.24
Studies in sheep have shown that although uterine blood flow varies over a wide range, fetal oxygen uptake remains relatively constant, suggesting that the efficiency of oxygen extraction is greater when perfusion decreases.27 Using an inflatable balloon occluder around the terminal aorta to reduce uterine blood flow in sheep, Wilkening and Meschia24 found that at high levels of oxygen delivery, fetal oxygen uptake was not significantly affected by variations in uterine blood flow; moreover, fetal oxygen uptake became flow dependent only when uterine oxygen delivery was reduced to less than half the baseline value. Boyle et al.,28 investigating the effects of acute uterine arterial embolization with microspheres in sheep, found a linear decrease in fetal aortic oxygen tension as uterine blood flow decreased. However, uterine oxygen consumption did not decrease and fetal hydrogen ion concentration did not increase until uterine blood flow had decreased to approximately 50% of the baseline value. As uterine blood flow diminished, a reduction in uterine venous oxygen content and a greater arteriovenous oxygen content difference were observed, indicating an increase in oxygen extraction. Gu et al.29 reported comparable findings with the compression of the common uterine artery by an inflatable occluder in sheep.
Although the preceding experiments were conducted in sheep, the same principles may apply to humans. The human placenta, like the sheep placenta, is a relatively inefficient oxygen exchanger. Thus, in humans and sheep, the transfer rate of oxygen is affected less by decreases in placental perfusion than the transfer rate in animals with more efficient placentas, such as the rabbit and guinea pig. Of interest, this difference may afford some protection in humans, because alterations in placental perfusion in animals with more efficient placentas frequently result in spontaneous abortion.30 Animal data would also suggest the presence of a significant physiologic buffer that protects the fetus during transient fluctuations in uteroplacental perfusion (e.g., changes in endogenous vasoconstrictor levels, uterine contractions, and parturition).31 This may partially explain why clinical studies have failed to demonstrate fetal acidosis when alpha-adrenergic agonists are used to maintain maternal blood pressure during neuraxial anesthesia,32 despite experimental data showing that these agents reduce uteroplacental perfusion in laboratory animals.33 These observations are based on an assumption of normal physiology; the presence of pathology likely diminishes any margin of safety.
Changes during Parturition
With the onset of the uterine contractions of labor, uteroplacental perfusion undergoes cyclical changes. During uterine contractions, a decrease in perfusion occurs that is inversely related to the strength of the contraction and the increase in intrauterine pressure.31 Conversely, during uterine relaxation, there is a period of hyperemia when perfusion is increased. Placental perfusion is believed to be more sensitive to these contraction-induced changes than myometrial or endometrial blood flow.34 Within the first few hours of parturition, uterine blood flow in sheep decreases on average by 50% or more, although there is notable inter-individual variation.35
Clinical Determinants of Uterine Blood Flow
In the acute setting, uterine blood flow is related to perfusion pressure (the difference between uterine arterial pressure and uterine venous pressure) and vascular resistance, as represented in the following equation:
 (1)
(1)
Thus, there are several ways that uterine blood flow can decrease (Box 3-1). First, uterine blood flow may decline with reductions in perfusion pressure because of decreased uterine arterial pressure—for example, during systemic hypotension from hemorrhage, aortocaval compression, or sympathetic blockade during neuraxial anesthesia. Second, uterine blood flow may decline with reductions in perfusion pressure caused by increased uterine venous pressure—for example, with vena caval compression, increased intrauterine pressure during uterine contractions, drug effects (e.g., oxytocin, cocaine), and Valsalva maneuvers that accompany maternal expulsive efforts during the second stage of labor. Third, uterine blood flow may decline because of increased uterine vascular resistance, which may be caused by a number of factors, including endogenous vasoconstrictors that are released in response to stress, exogenous vasoconstrictors, and compression of endometrial spiral arterioles with uterine contractions.34
Mechanisms of Vascular Changes and Regulation
Vascular Changes during Pregnancy
Because mean arterial pressure decreases slightly during pregnancy, the increase in uteroplacental blood flow is dependent on a substantial decrease in uterine vascular resistance (Figure 3-4), together with increased cardiac output and intravascular volume. The main factors contributing to the decrease in vascular resistance include vascular remodeling, changes in vascular reactivity, and the development of the widely dilated placental circulation.
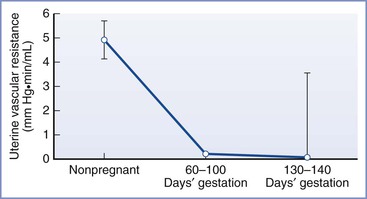
FIGURE 3-4 Changes in uterine vascular resistance with gestation. Data are mean ± SE. (Adapted from Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol 1977; 232:H231-5.)
Vascular remodeling of arteries in the uterus during pregnancy is believed to include increases in both vessel diameter and vessel length. In humans, both vessel lengthening and straightening of coiled vessels may occur.12 According to Poiseuille’s law, vascular resistance is decreased in proportion to the fourth power of the radius, whereas resistance is increased in proportion to the first power of vessel length; as such, the effects of changes in vessel diameter dominate, resulting in an overall decrease in resistance. Palmer et al.,13 using serial Doppler studies during pregnancy, observed that uterine artery diameter is doubled by 21 weeks’ gestation, whereas there is no change in the diameter of the common iliac or external iliac arteries. These investigators also showed that uterine artery mean flow velocity increased progressively during pregnancy to values eight times greater than those of nonpregnancy. In parallel with arterial changes, there is also structural remodeling of uterine veins in pregnancy. This includes increases in diameter and distensibility and decreases in elastin content.36 Although blood viscosity decreases during pregnancy and also contributes to reduced uterine vascular resistance, this is considered a relatively minor effect compared with vascular changes.37
Changes in vascular reactivity during pregnancy include a vasodilatory response that is meditated at endothelial and vascular smooth muscle levels.38 The growth of the placenta creates a low-resistance vascular pathway by eliminating the intramyometrial microcirculation and creating an intervillous space.39 This has functional characteristics of an arteriovenous shunt.15
The mechanisms underlying the vascular changes during pregnancy are incompletely understood. Contributing factors include steroid hormones, decreased response to vasoconstrictors, endothelium-derived vasodilators, increased shear stress, and venoarterial exchange.
Steroid Hormones
Steroids play an integral role in the development and regulation of the uteroplacental circulation. Estrogen and progesterone are especially important, and there is evidence that cortisol may also contribute.
Estrogen has a fundamental role in the short- and long-term uterine vascular changes during pregnancy. Plasma concentrations of estrogen, initially derived from the ovaries and later predominantly from the placenta, rise concomitantly with the increase in uterine blood flow during pregnancy. Exogenously administered estrogen causes uterine vasodilation and a marked rise in uterine blood flow, independent of systemic effects.40 Angiogenic and vasodilatory effects of estrogen are meditated via estrogen receptors ER-α and ER-β, which are structurally and functionally distinct. The majority of these receptors are located in the nucleus and mediate genomic effects by regulating transcription of genes that are particularly responsible for the longer-term uterine angiogenic responses. There are also membrane receptors that mediate nongenomic effects by up-regulating endothelial production of nitric oxide through the activation of endothelial nitric oxide synthase (eNOS) and the augmentation of eNOS protein expression.41
Progesterone modulates the effect of estrogen on uterine blood flow. In a nonpregnant sheep model, exogenous progesterone administered alone had no uterine vasodilatory effect but had an inhibitory effect when combined with estrogen.38 Progesterone down-regulates expression of estrogen receptors.42 An increase in the estrogen-progesterone ratio parallels the increase in uterine blood flow in late pregnancy in many species.43
Plasma cortisol levels approximately double during pregnancy. Cortisol has both systemic and local effects on uterine blood flow. Systemically, cortisol contributes to regulation of uterine blood flow by increasing plasma volume. Although cortisol is believed to decrease eNOS protein expression and decrease nitric oxide release, it potentiates the response to vasoconstrictor agents including angiotensin II, vasopressin, and norepinephrine. Attenuation of these effects occurs during pregnancy.38
Decreased Response to Vasoconstrictors
In pregnancy, there is a generalized reduction in response to endogenous and exogenous vasoconstrictors, including angiotensin II, endothelin, thromboxane, epinephrine, norepinephrine, phenylephrine, serotonin, thromboxane, and arginine vasopressin.44–46 The relative refractoriness of the systemic and uterine circulations varies for different agents, which has important implications for the regulation and maintenance of uteroplacental blood flow.
During pregnancy, concentrations of angiotensin II in maternal blood are increased twofold to threefold47; however, the vasopressor response to angiotensin II is attenuated.48 This refractoriness is diminished in patients in whom preeclampsia develops.48 The uterine circulation is less responsive to angiotensin II than the systemic circulation. Thus, infusion of physiologic doses of angiotensin II has been shown to have minimal effect on uteroplacental blood flow while increasing systemic blood pressure.49 The difference in sensitivity of the uterine and systemic circulations to angiotensin II is considered an important physiologic adaptation during pregnancy that contributes to the redistribution of cardiac output, the increase in uterine blood flow, and possibly the maintenance of uterine blood flow during normal fluctuations in blood pressure.50
Sensitivity to vasoconstrictors such as epinephrine, norepinephrine, and phenylephrine is attenuated during pregnancy.51 However, in contrast to the responses to angiotensin II, the uterine circulation is more responsive to these agents than the systemic circulation.51 Thus, during hemorrhage or other major stresses that result in large catecholamine release, it is unlikely that uteroplacental perfusion will be preferentially preserved above essential maternal perfusion.52
The mechanism underlying the difference in vascular sensitivity between the uterine and systemic circulations is unclear, but distribution of receptor subtypes is believed to be important.53 There are two distinct subtypes of angiotensin II receptors: AT1R and AT2R. In most tissues, including systemic vascular smooth muscle, AT1R receptors are predominant and mediate vasoconstriction. However, AT2R receptors, which do not mediate smooth muscle contraction, account for 75% to 90% of angiotensin II binding in uterine artery and myometrium.54,55
Vasodilators
The greater synthesis and higher circulating concentrations of endothelial-derived vasodilators during pregnancy are believed to modulate systemic and uterine vascular responses to angiotensin II and other vasoconstrictors.56 Uterine vascular production of prostacyclin is greater than systemic vascular production, which probably contributes to maintaining uteroplacental blood flow in opposition to the effects of circulating vasoconstrictors.57 An enhanced response to angiotensin II during pregnancy has been demonstrated with the systemic and local infusion of indomethacin (which blocks prostacyclin production).58 However, inhibition of prostaglandin synthesis by an infusion of indomethacin induces only a transient decrease in uteroplacental blood flow, indicating that uteroplacental blood flow is not solely dependent on the continued production of prostacyclin.56
Nitric oxide is synthesized from arginine in vascular endothelial cells and stimulates soluble guanylate cyclase in vascular smooth muscle, resulting in vascular relaxation through increases in cyclic guanosine monophosphate. Synthesis of nitric oxide is an important mechanism underlying changes in systemic and uterine vascular resistance, attenuated responses to vasoconstrictors, and vascular effects of estrogen during pregnancy.59 During pregnancy, uterine arteries have increased eNOS activity, higher levels of eNOS messenger ribonucleic acid and eNOS protein, and increased biosynthesis of nitric oxide and cyclic guanosine monophosphate.59,60 Removal of the vascular endothelium diminishes or eliminates the refractoriness of the uterine artery to vasoconstrictors45 and inhibition of nitric oxide synthesis by N-nitro-L-arginine methyl ester (L-NAME) decreases uterine blood flow and also reverses refractoriness to vasoconstrictors.61 Long-term inhibition of nitric oxide synthase causes hypertension and fetal growth restriction in rats.62
Other Vasoactive Substances
Atrial and brain natriuretic peptides attenuate the response to angiotensin II, and intravenous infusion of atrial natriuretic peptide reduces blood pressure while increasing uterine blood flow in preeclamptic women.63 Protein kinase C activity is decreased in uterine, but not systemic, arteries of pregnant sheep and may cause vasodilation and an increase in uterine blood flow; this may have a regulatory effect on local ovarian and placental estrogen production.43 Studies in rats have shown a decrease in endogenous endothelin-dependent vasoconstrictor tone in uteroplacental vessels, which may contribute to the increase in placental blood flow in late gestation.64 Uterine vascular resistance in early pregnancy may be increased by relaxin, which may have a role in modulating the effects of estrogen and progesterone.65
Shear Stress
Shear stress, the frictional forces on the vessel wall from blood flow, is believed to be an important stimulus for uteroplacental vasodilation and remodeling.66 The reduction in downstream resistance resulting from the formation of the placenta would be expected to increase the upstream flow velocity and thus shear stress.39 Nitric oxide is considered an important mediator of this effect because increases in eNOS expression and nitric oxide production are witnessed with shear stress and because stripping the endothelium or pretreatment with L-NAME reduces or abolishes flow-induced vasodilation.66 Studies in vitro have shown that shear stress also increases endothelial production of prostacyclin.
Venoarterial Signaling
It has been postulated that growth factors or signal substances secreted by the placenta and/or myometrium could pass from uterine veins to adjacent uterine arteries; this may provide a system whereby the uterus and placenta regulate their own perfusion.39 Possible candidates for signal substances include vascular endothelial growth factor and placental growth factor. Confirmation of whether this mechanism is important in humans is awaited.
Methods of Measurement of Uteroplacental Blood Flow
Many techniques have been used to measure uteroplacental blood flow in animals and humans. The approaches used in different studies have varied according to the nature of the experimental question, the existing state of technology, and ethical considerations and limitations. All methods have an inherent potential for error.
Many past studies of uterine artery flow have measured flow in only one uterine artery, which may not be an accurate representation of total flow, depending on the location of the placenta (see earlier discussion). The parameter of greatest clinical interest is placental perfusion, but this is not always differentiated from total uterine blood flow, from which it may vary independently. However, in most circumstances, the measurement of intervillous blood flow provides a close approximation of functional placental blood flow. Ovarian arterial blood flow is generally not measured, although studies in primates suggest it may contribute as much as one sixth of placental perfusion.67
Early studies of uteroplacental blood flow involved a number of substances that could affect maternal hemodynamics (e.g., nitrous oxide) or myometrial activity (e.g., 4-amino-antipyrine) and relied on the Fick principle. This principle, which calculates blood flow by the measurement of a marker substance entering and leaving an organ, is subject to error in the uterus, where a number of veins are responsible for collecting venous effluent.68 In animals, placental perfusion can also be measured by the injection of radioactive microspheres. This method allows for the separate calculation of placental and myometrial blood flows but only provides information from a single point in time. Total uterine arterial blood flow can also be measured (or estimated) with the use of surgically implanted electromagnetic or Doppler flow probes.
In humans, placental perfusion can be measured by the injection of trace amounts of radioactive substances, typically xenon-133.69 During the washout phase, the rapid decrease in measured radioactivity over the placenta is calculated as a biexponential or triexponential process. The most rapid decay constant is ascribed to intervillous perfusion. Alternatively, radioactively tagged proteins (e.g., albumin) can be injected and measured by scintigraphy over the placenta.70 Although the accuracy of these methods for determining absolute flow is limited, their ability to measure relative change over time is adequate in most cases.
In humans, the most common method of clinically assessing uterine blood flow is Doppler ultrasonography.71 The uterine artery is identified after it crosses the external iliac artery, before it divides into branches. Color flow aids vessel identification. Blood flow can be quantified by measuring the mean flow velocity and vessel cross-sectional area.
Flow velocity is calculated by applying the principle of Doppler shift. A pulsed ultrasound signal from a stationery transducer is directed toward the vessel with an angle of insonation (θ) less than 60 degrees. Reflections scattered from the red blood cells are received. Because the red blood cells are moving, the frequency of the received signal differs from the transmitted frequency (f0) by an amount known as the Doppler shift (Δf). This shift is proportional to the red blood cell flow velocity (VRBC) according to the following equation:
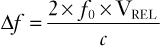 (2)
(2)
where c is the speed of sound propagation in tissue and VREL is the vector component of the velocity of flow relative to the direction of the transducer. The latter takes into account the difference between the direction of the ultrasound signal from the direction of flow according to θ (Figure 3-5). With the use of basic trigonometry, VRBC is related to the relative velocity of flow in the direction of the probe (VREL) according to the following equation:
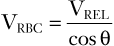 (3)
(3)
Combining equations 2 and 3 gives the following equation:
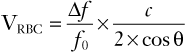 (4)
(4)
Thus, the flow velocity is estimated from the ratio of the Doppler shift frequency to the transmitted frequency, multiplied by the speed of sound propagation, and divided by two times the cosine of the insonation angle.
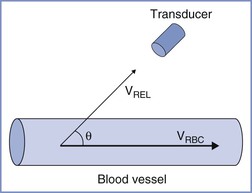
FIGURE 3-5 Principles of use of Doppler ultrasonography to estimate blood flow. Blood flow is calculated as the product of blood vessel cross-sectional area and mean flow velocity in the vessel (VRBC). The latter is derived from the measured flow velocity relative to the direction of the probe (VREL) and requires precise determination of the angle of insonation (θ).
An estimation of the volume of blood flow (Q) can be made by multiplying mean velocity by the vessel cross-sectional area (A), which is estimated with two-dimensional (B-mode) ultrasonography:
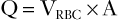 (5)
(5)
However, measurement of absolute flow using this technique is prone to difficulty and error, both from inaccurate measurement of vessel cross-sectional area (e.g., arteries pulsate during the cardiac cycle) and from inaccurate measurement of flow (e.g., from inaccuracies in measurement of θ). Therefore, for diagnostic purposes, a number of indices related to vascular impedance can be derived from the flow velocity waveform that are independent of θ.72 These rely on the fact that the uterine vascular bed normally has low resistance with flow continuing during diastole. If distal resistance is increased, for example during the development of preeclampsia or fetal growth restriction, diastolic velocity decreases relative to systolic velocity resulting in a waveform showing greater pulsatility. Commonly derived indices (Figure 3-6) are:
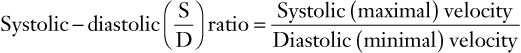 (6)
(6)
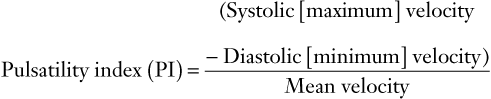 (7)
(7)
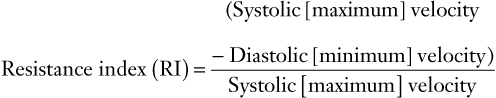 (8)
(8)
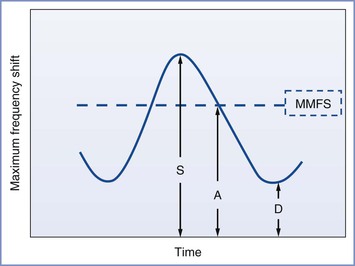
FIGURE 3-6 Schematic diagram showing elements of typical Doppler waveform of the uterine artery. S, peak systolic frequency shift (maximum velocity); D, end-diastolic frequency shift (minimum velocity); A, temporal averaged frequency shift (mean velocity) averaged over one cardiac cycle; MMFS, mean maximum frequency shift. Derived indices include systolic/diastolic (S/D) ratio = S/D, pulsatility index (PI) = (S − D)/A, and resistance index (RI) = (S − D)/S.
In addition, the waveform can be described or categorized according to features such as the absence of end-diastolic flow and the presence of post-diastolic notches. Examples of normal and abnormal uterine artery Doppler tracings are shown in Figure 3-7.73 Doppler velocimetry can be applied to the umbilical vessels for antepartum fetal assessment (see Chapter 6).
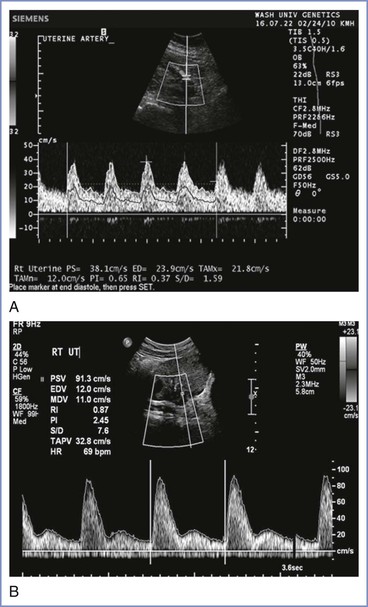
FIGURE 3-7 Normal (A) and abnormal (B) uterine artery Doppler waveforms. The normal waveform has no notching and normal pulsatility. The abnormal waveform shows notching and increased pulsatility. (From Tuuli M, Odibo AO. The role of serum markers and uterine artery Doppler in identifying at-risk pregnancies. Clin Perinatol 2001; 38:1-19.)
There are several potential sources of error in Doppler measurements of absolute flow with regard to both accuracy and reproducibility of measurements. For example, small errors in the estimation of θ can result in blood flow measurement errors as large as 30%.13 Thus, the methods used in any clinical study that employs Doppler ultrasonography to assess uterine artery blood flow should be examined critically.
Neuraxial Anesthesia
The effect of neuraxial anesthesia on uteroplacental blood flow depends on the complex interaction of many factors (Box 3-2). Pain and stress during labor may reduce uteroplacental blood flow through sympathetic stimulation and the release of circulating catecholamines. Shnider et al.74 observed that acute stress increased plasma norepinephrine concentrations by 25% and decreased uterine blood flow by 50% in gravid ewes. In laboring women, stress is associated with increased plasma epinephrine concentrations and abnormal fetal heart rate patterns. Effective pain relief with neuraxial analgesia decreases circulating concentrations of catecholamines75 and reduces hyperventilation and therefore may help protect uteroplacental blood flow. In the absence of hypotension, epidural anesthesia does not change uteroplacental blood flow in pregnant sheep.76 Results from human studies are variable, partly because of differences in study design, techniques used, and clinical circumstances. However, most studies have shown no change or an increase in uteroplacental blood flow after administration of epidural analgesia.77–80 Some studies have shown an increase in uterine vascular resistance indices,81,82 but with no effect on neonatal outcomes. There is evidence that in women with preeclampsia, epidural analgesia using a plain local anesthetic may reduce uterine artery resistance78 and increase intervillous blood flow.83 Ginosar et al.84 reported that antenatal continuous epidural infusion of ropivacaine in preterm patients with preeclampsia reduced uterine artery resistance. Further work is required to determine whether this might have therapeutic potential for short-term prolongation of pregnancy.
Fetal bradycardia is sometimes observed after combined spinal-epidural techniques and has been attributed to decreases in uteroplacental blood flow; the mechanism for this association is unclear. Although alterations in uteroplacental blood flow have been primarily attributed to maternal hypotension and respiratory depression,85 another postulated mechanism is uterine tachysystole (hypertonus) caused by a rapid decrease in circulating catecholamine concentrations (see Chapter 23).86 Additional studies are needed to evaluate the relationship between neuraxial anesthetic techniques, uteroplacental blood flow, and fetal bradycardia.
Hypotension
Hypotension occurring during neuraxial blockade, depending on its magnitude and duration, may decrease uteroplacental blood flow for several reasons—reduction in perfusion pressure,17 reflex release of endogenous vasoconstrictors, diversion (steal) of blood to the lower limbs,87 and response to administered vasopressors.33 The rapid and extensive sympathetic blockade during spinal anesthesia, and some of the methods used to treat hypotension, may account for the observation that umbilical arterial blood pH is lower with spinal anesthesia than with epidural or general anesthesia for cesarean delivery.88
Intravenous Fluid Loading
Studies of the effect of intravenous fluid boluses used in conjunction with assessment of the uteroplacental circulation have had mixed results. Most Doppler studies have shown that fluid preload before the initiation of neuraxial analgesia does not change vascular resistance indices,89 although a decrease has been reported.90
Vasopressors
The effects of vasopressors on uteroplacental blood flow and the resulting implications for clinical drug selection are controversial. Animal and in vitro studies have observed that uteroplacental blood flow was better maintained using ephedrine versus alpha-adrenergic agonists such as phenylephrine, metaraminol, and methoxamine,33 which likely reflects the predominant beta-adrenergic effects of ephedrine. In addition, in vitro studies in pregnant sheep evaluating the effects of ephedrine on blood vessels have demonstrated enhanced vasoconstrictor activity on the femoral versus uterine vessels and decreased uterine vasoconstriction as a result of nitric oxide release.91 In contrast, an increase in the uterine arteriolar vasoconstrictor response to phenylephrine has been observed during pregnancy.92 However, in clinical studies, umbilical arterial blood pH and base excess have been observed to be greater with the use of alpha-adrenergic agonists in comparison to ephedrine to maintain maternal blood pressure during spinal anesthesia for cesarean delivery (Figure 3-8).93–100 A comparison of different infusion regimens of phenylephrine, titrated to keep maternal systolic blood pressure near baseline, observed no depression of fetal pH and base excess despite very large total doses (up to 2500 µg) before delivery.32 In contrast, large doses of ephedrine administered to maintain blood pressure during spinal anesthesia for cesarean delivery depressed umbilical arterial blood pH and base excess in a dose-dependent manner.101
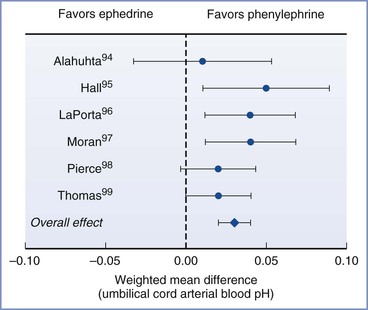
FIGURE 3-8 Results from a meta-analysis of trials comparing phenylephrine and ephedrine for the management of hypotension during spinal anesthesia for cesarean delivery. The chart shows the effect of choice of vasopressor on umbilical cord arterial pH. Data are mean difference with 95% confidence intervals. (Modified from Lee A, Ngan Kee WD, Gin T. A quantitative systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg 2002; 94:920-6.)
The explanation for the discrepancy between experimental and clinical data is complex and incompletely determined. Animal studies are not always appropriate models for clinical situations. Under clinical conditions, Doppler studies have shown some evidence that uterine vascular resistance is increased by alpha-adrenergic agonists,94 but this finding has not been consistent.100 Although data suggest that alpha-adrenergic agonists increase uterine vascular resistance more than systemic vascular resistance, the difference may be primarily due to an effect in the myometrium, with relative sparing of the vessels that perfuse the placenta.102 In addition, uteroplacental blood flow in humans has a margin of safety that appears to allow modest decreases in uterine blood flow (caused by clinically appropriate doses of alpha-adrenergic agonists) to occur without compromising oxygen transfer. Finally, the propensity of ephedrine to worsen fetal acid-base status may be related less to its effects on uteroplacental blood flow and more to direct beta-adrenergic receptor–mediated fetal metabolic effects. When compared with phenylephrine, ephedrine has been observed to cross the placenta to a greater extent and be associated with higher fetal levels of lactate, glucose, epinephrine, and norepinephrine.103
Thus, when considering the choice of vasopressor for clinical use, the anesthesiologist should take into account the sum effect on fetal oxygen supply and demand balance rather than the isolated effects on uteroplacental blood flow. In this respect, clinical studies do not favor the use of ephedrine. In addition, the slow onset and long duration of action of ephedrine make it more difficult to titrate than phenylephrine. Conversely, the use of phenylephrine is commonly associated with a reflex slowing of heart rate and a corresponding decrease in cardiac output. At modest doses, this decrease reflects a normalization of the cardiac output that is elevated secondary to decreased afterload after the initiation of spinal anesthesia; at larger doses, alpha-adrenergic agents can cause cardiac output to decrease below baseline.104 The implications of these findings on uteroplacental blood flow are controversial because the relative importance of maintaining cardiac output versus maintaining uterine perfusion pressure is unknown. Overall, to date, studies comparing ephedrine and other vasopressors in humans have not demonstrated differences in clinical neonatal outcome.
Limited data are available for the comparison of vasopressors in the presence of fetal compromise or placental insufficiency. Erkinaro et al.105,106 developed a sheep model to compare the effects of phenylephrine and ephedrine after a period of experimental fetal hypoxia. Hypotension was induced by epidural anesthesia and then corrected with either phenylephrine or ephedrine. In an initial study, ephedrine was associated with better restoration of uterine artery blood flow, but no differences in fetal acid-base measurements or lactate concentration were observed.105 However, in a second study, these investigators embolized the placenta with microspheres to model placental insufficiency and found that phenylephrine and ephedrine had similar effects on uterine blood flow, fetal pH, and base excess as found in the initial study, with the exception that fetal lactate concentration was greater in the phenylephrine group.106 Although the investigators speculated that this exception might reflect impaired fetal clearance of lactate, the placental embolization may have narrowed the margin of safety for uteroplacental blood flow and increased fetal lactate production in the phenylephrine group. Ngan Kee et al.107 compared phenylephrine and ephedrine for maintaining blood pressure in patients receiving spinal anesthesia for nonelective cesarean delivery, 24% of whom had evidence of fetal compromise. The results showed that although umbilical arterial and venous blood lactate concentrations were lower in the phenylephrine group, umbilical arterial blood pH and base excess values were similar in the two groups. However, umbilical arterial and venous blood PO2 measurements were lower in the phenylephrine group, suggesting that although phenylephrine may have caused some reduction in uteroplacental perfusion, adequate oxygen supply was likely maintained by increased oxygen extraction.
In summary, ephedrine and phenylephrine both continue to be used clinically for maintaining maternal blood pressure during the administration of neuraxial anesthesia. Although most experimental data suggest that uteroplacental perfusion is likely to be better maintained with ephedrine than with alpha-adrenergic agonists, this advantage may be outweighed by other considerations, such as differences in efficacy for maintaining blood pressure and direct fetal effects that occur from the placental transfer of the drug.
Local Anesthetics
Studies in vitro have shown that local anesthetics constrict arteries directly and inhibit endothelium-mediated vasodilation.108 High concentrations of local anesthetic can decrease uteroplacental blood flow by stimulating vasoconstriction and myometrial contractility.109,110 A comparative study in pregnant sheep showed that bupivacaine was more potent than either lidocaine or 2-chloroprocaine in decreasing uterine blood flow.110 However, the adverse effects of local anesthetics were seen only at concentrations in excess of those observed clinically, with two possible exceptions: (1) the unintentional intravenous injection of local anesthetic and (2) the use of local anesthetics for a paracervical block. At clinically relevant doses, no adverse effect on uteroplacental blood flow was reported.111 Although initially the inherent vasoconstrictor properties of ropivacaine were a matter of concern, studies in animals111 and humans112 have not shown that administration of ropivacaine results in a reduction in uterine blood flow.
Epinephrine and α2-Adrenergic Agonists
Epinephrine is often combined with local anesthetic agents in obstetric anesthesia. Wallis et al.76 found that the epidural injection of 1.5% 2-chloroprocaine with epinephrine (10 µg/mL) produced a small, brief reduction in uterine blood flow in pregnant sheep. In contrast, Alahuhta et al.113 reported that epidural bupivacaine with epinephrine (5 µg/mL) had no effect on intervillous blood flow in women undergoing cesarean delivery. Studies have not shown a reduction in uteroplacental blood flow as a result of the absorption of epinephrine from local anesthetic solutions given epidurally to healthy women during labor.114 However, one study observed that the addition of epinephrine (85 to 100 µg) to epidural bupivacaine increased Doppler indices of uteroplacental vascular resistance in hypertensive parturients with chronic fetal asphyxia.115 Therefore, some anesthesia providers avoid epidural administration of epinephrine-containing local anesthetic solutions to women with preeclampsia. Commonly, epinephrine (10 to 15 µg) is included in the epidural test dose. Marcus et al.116 reported that repeated epidural injections of epinephrine (10 to 15 µg) did not decrease uterine blood flow in pregnant sheep; however, the same dose injected intravenously reduced uterine blood flow, with a maximum decrease of 43% observed at 1 minute.
The epidural and intrathecal administration of α2-adrenergic agonists (e.g., clonidine, dexmedetomidine) has been a subject of clinical investigations. Intravenous, but not epidural, administration of clonidine decreased uterine blood flow in gravid ewes.117,118
Opioids
Opioids are often combined with local anesthetic agents for epidural and intrathecal analgesia during labor and the peripartum period. Intrathecal opioids have been implicated as contributing to a greater risk for fetal bradycardia when used for labor analgesia compared with non-intrathecal opioid neuraxial analgesic techniques.119 The mechanism for this effect has been postulated as an increase in uterine tone and a resulting decrease in uteroplacental blood flow, although further research is needed. Craft et al.120,121 observed that neither epidural fentanyl nor morphine had a significant effect on uterine blood flow in gravid ewes. Alahuhta et al.122 reported that epidural sufentanil 50 µg did not alter uterine artery blood flow velocity waveform indices in laboring women. Intrathecal meperidine and sufentanil, however, may be associated with hypotension that may potentially decrease uterine blood flow.123,124
General Anesthesia
Induction Agents
Available data suggest that the commonly used induction agents have minimal or no direct adverse effect on uteroplacental blood flow. Allen et al.125 found that thiopental inhibited the response of human myometrial arteries to contractile agents in vitro but had no effect on relaxation induced by prostacyclin. Alon et al.126 reported that uterine blood flow did not change significantly during induction and maintenance of propofol anesthesia in pregnant sheep. Craft et al.127 reported that uterine tone increased but uterine blood flow remained constant after an intravenous bolus of ketamine in pregnant sheep. Similarly, Strümper et al.128 reported that neither racemic nor S+-ketamine affected uterine perfusion in pregnant sheep. Few data are available on the direct effects of etomidate on uteroplacental blood flow.
During the intravenous induction of general anesthesia, uteroplacental perfusion may be affected by indirect mechanisms such as blood pressure changes and the sympathetic response to laryngoscopy and endotracheal intubation. Jouppila et al.129 reported that intervillous blood flow decreased by 22% to 50% during induction of general anesthesia for cesarean delivery with thiopental 4 mg/kg, succinylcholine 1 mg/kg, and endotracheal intubation. Gin et al.130 compared thiopental 4 mg/kg and propofol 2 mg/kg in patients undergoing elective cesarean delivery. These investigators found that venous plasma concentrations of epinephrine and norepinephrine increased after endotracheal intubation in both groups, but maximum norepinephrine concentrations were lower in the propofol group. No differences in neonatal outcomes were observed. Levinson et al.131 found that intravenous ketamine increased blood pressure with a concomitant rise in uterine blood flow in pregnant sheep. Addition of a rapid-acting opioid (e.g., alfentanil, remifentanil) during induction of general anesthesia may minimize the increase in circulating catecholamines that occurs after laryngoscopy and endotracheal intubation.132,133 Although the use of such opioids might attenuate any decrease in uterine blood flow, the potential for neonatal respiratory depression should be considered.
Inhalational Agents
Studies in pregnant sheep have shown that usual clinical doses (i.e., 0.5 to 1.5 minimum alveolar concentration) of the volatile anesthetic agents, including isoflurane, desflurane, and sevoflurane, have little or no effect on uterine blood flow, although deeper planes of anesthesia are associated with reductions in cardiac output, maternal blood pressure, and uterine blood flow.134,135 Nonetheless, high concentrations of inhalational agents (approximately 2 minimum alveolar concentration) have been used during ex utero intrapartum treatment procedures without evidence of impaired fetal gas exchange.136 A dose-dependent reduction in uterine tone caused by inhalational agents would be expected to increase uterine blood flow in clinical circumstances in which tone is increased (e.g., hyperstimulation with oxytocin, cocaine overdose, placental abruption). Overall, there is little reason to choose one inhalational agent over another on the basis of an agent’s effects on uterine blood flow.
Ventilation
Although moderate levels of hypoxemia and hypercapnia do not affect uteroplacental blood flow,137 marked alterations may reduce blood flow indirectly by mechanisms most likely involving sympathetic activation and catecholamine release. The effect of hypocapnia on uteroplacental blood flow is controversial. Some investigators have noted that hyperventilation with hypocapnia caused fetal hypoxia and metabolic acidosis in animals,138 whereas others have found no effect.139 Levinson et al.140 observed that positive-pressure ventilation decreased uterine blood flow in pregnant sheep; however, because the addition of carbon dioxide did not improve uterine blood flow, the reduction in blood flow was attributed to the mechanical hyperventilation rather than the hypocapnia. In general, most authorities recommend that hyperventilation be avoided in pregnancy, in part because of concerns about uterine blood flow.
Effects of Other Drugs
Magnesium Sulfate
Magnesium sulfate increases uterine blood flow in normotensive and hypertensive pregnant sheep.141,142 Although hypermagnesemia was found to exacerbate maternal hypotension during epidural anesthesia in pregnant sheep, no reduction in uterine blood flow was observed.141 In women in preterm labor143 and with severe preeclampsia,144 magnesium sulfate caused a modest decrease in Doppler indices of uterine vascular resistance. Infusion of magnesium caused an increase in uterine blood flow, which was associated with an improvement in red blood cell deformability in women with preeclampsia or fetal growth restriction.145
Antihypertensive Agents
In patients with pregnancy-induced hypertension, the effects of antihypertensive drugs on uteroplacental perfusion depend on the interaction of their effects on uterine vascular resistance and systemic maternal blood pressure. In animal models of pharmacologically induced hypertension, hydralazine reduced maternal blood pressure but increased uteroplacental blood flow, reflecting a decrease in uterine vascular resistance.146,147 Similar studies with labetalol have had varying results, showing increased,148 decreased,149 and no change150 in uteroplacental blood flow. A study in preeclamptic women observed an increase in uterine artery resistance indices after hydralazine but not labetalol.151 However, previous studies have generally demonstrated no significant change in uteroplacental blood flow with either drug,152–155 indicating that other considerations are probably more important for guiding drug selection. Studies of methyldopa in patients with preeclampsia have found either a reduction156 or no change157 in indices of uterine and placental vascular resistance.
Calcium Entry–Blocking Agents
Verapamil 0.2 mg/kg was shown to decrease maternal blood pressure and uterine blood flow in pregnant sheep.158 Studies with nifedipine have yielded conflicting results. Some animal studies have shown that nifedipine decreases uteroplacental blood flow and worsens the fetal condition, whereas human studies have shown either no change in uteroplacental blood flow or vascular resistance159 or a decrease in vascular resistance.160
Vasodilators
Nitroglycerin was shown to relax human uterine arteries in vitro.161 In women with abnormal uterine artery blood flow at 24 to 26 weeks’ gestation, infusion of intravenous nitroglycerin decreased uterine resistance indices.162 Similarly, transdermal nitroglycerin administered for 3 days to patients with preeclampsia and fetal growth restriction decreased uterine resistance indices.163 However, Grunewald et al.164 reported that an infusion of nitroglycerin in women with severe preeclampsia did not change the pulsatility index of the uterine artery. When interpreting such studies, clinicians should remember that increases in total uterine blood flow do not necessarily result in enhanced placental perfusion.165 Further work is required to define the utility of systemic vasodilators for improving uteroplacental blood flow in clinical practice.
Inotropic Drugs
Positive inotropic drugs are rarely indicated in obstetric patients. On the basis of studies of normal pregnant sheep, milrinone and amrinone may increase uterine blood flow, whereas dopamine and epinephrine may diminish it.166,167 The choice of an inotropic agent should be based primarily on the desired efficacy (i.e., maternal considerations) rather than the potential direct effects on uterine blood flow. This is especially important during maternal resuscitation or cardiac arrest, when maternal welfare is the overriding priority and standard resuscitation drugs should be given. Restoration of spontaneous circulation and adequate uterine perfusion pressure is far more important than avoidance of uterine vasoconstriction.
References
1. Couzin J. Quirks of fetal environment felt decades later. Science. 2002;296:2167–2169.
2. Harbert GM Jr. Circadian changes in uteroplacental blood flow. Rosenfeld CR. The Uterine Circulation. Perinatology Press: Ithaca, NY; 1989:157–173.
3. Pelage JP, Le Dref O, Soyer P, et al. Arterial anatomy of the female genital tract: variations and relevance to transcatheter embolization of the uterus. AJR Am J Roentgenol. 1999;172:989–994.
4. Konje JC, Kaufmann P, Bell SC, Taylor DJ. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am J Obstet Gynecol. 2001;185:608–613.
5. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191.
6. Cicinelli E, Einer-Jensen N, Galantino P, et al. The vascular cast of the human uterus: from anatomy to physiology. Ann N Y Acad Sci. 2004;1034:19–26.
7. Hickey M, Hammond I. What is the place of uterine artery embolisation in the management of symptomatic uterine fibroids? Aust NZ J Obstet Gynaecol. 2008;48:360–368.
8. Rosenfeld CR, Morriss FH Jr, Makowski EL, et al. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5:252–268.
9. Gill RW, Kossoff G, Warren PS, Garrett WJ. Umbilical venous flow in normal and complicated pregnancy. Ultrasound Med Biol. 1984;10:349–363.
10. Rigano S, Boito S, Maspero E, et al. Absolute uterine artery blood flow volume is increased in twin human pregnancies compared to singletons. Ultrasound Obstet Gynecol. 2007;30:506.
11. Flo K, Wilsgaard T, Acharya G. Relation between utero-placental and feto-placental circulations: a longitudinal study. Acta Obstet Gynecol Scand. 2010;89:1270–1275.
12. Thaler I, Manor D, Itskovitz J, et al. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162:121–125.
13. Palmer SK, Zamudio S, Coffin C, et al. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80:1000–1006.
14. Novy MJ, Thomas CL, Lees MH. Uterine contractility and regional blood flow responses to oxytocin and prostaglandin E2 in pregnant rhesus monkeys. Am J Obstet Gynecol. 1975;122:419–433.
15. Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol. 1994;102:122–134.
16. Greiss FC Jr, Anderson SG. Pressure-flow relationship in the nonpregnant uterine vascular bed. Am J Obstet Gynecol. 1974;118:763–772.
17. Greiss FC Jr. Pressure-flow relationship in the gravid uterine vascular bed. Am J Obstet Gynecol. 1966;96:41–47.
18. Laird MR, Faber JJ, Binder ND. Maternal placental blood flow is reduced in proportion to reduction in uterine driving pressure. Pediatr Res. 1994;36:102–110.
19. Venuto RC, Cox JW, Stein JH, Ferris TF. The effect of changes in perfusion pressure on uteroplacental blood flow in the pregnant rabbit. J Clin Invest. 1976;57:938–944.
20. Rosenfeld CR, Morriss FH Jr, Battaglia FC, et al. Effect of estradiol-17 β on blood flow to reproductive and nonreproductive tissues in pregnant ewes. Am J Obstet Gynecol. 1976;124:618–629.
21. Rosenfeld CR, Worley RJ, Gant N Jr. Uteroplacental blood flow and estrogen production following dehydroisoandrosterone infusion. Obstet Gynecol. 1977;50:304–307.
22. Still JG, Greiss FC Jr. The effect of prostaglandins and other vasoactive substances on uterine blood flow and myometrial activity. Am J Obstet Gynecol. 1978;130:1–8.
23. Greiss FC Jr. Uterine blood flow: an overview since Barcroft. Rosenfeld CR. The Uterine Circulation. Perinatology Press: Ithaca, NY; 1989:3–15.
24. Wilkening RB, Meschia G. Fetal oxygen uptake, oxygenation, and acid-base balance as a function of uterine blood flow. Am J Physiol. 1983;244:H749–H755.
25. Meschia G. Safety margin of fetal oxygenation. J Reprod Med. 1985;30:308–311.
26. Skillman CA, Plessinger MA, Woods JR, Clark KE. Effect of graded reductions in uteroplacental blood flow on the fetal lamb. Am J Physiol. 1985;249:H1098–H1105.
27. Clapp JF III. The relationship between blood flow and oxygen uptake in the uterine and umbilical circulations. Am J Obstet Gynecol. 1978;132:410–413.
28. Boyle JW, Lotgering FK, Longo LD. Acute embolization of the uteroplacental circulation: uterine blood flow and placental CO diffusing capacity. J Dev Physiol. 1984;6:377–386.
29. Gu W, Jones CT, Parer JT. Metabolic and cardiovascular effects on fetal sheep of sustained reduction of uterine blood flow. J Physiol. 1985;368:109–129.
30. Faber JJ. Review of flow limited transfer in the placenta. Int J Obstet Anaesth. 1995;4:230–237.
32. Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–474.
33. Ralston DH, Shnider SM, deLorimier AA. Effects of equipotent ephedrine, metaraminol, mephentermine, and methoxamine on uterine blood flow in the pregnant ewe. Anesthesiology. 1974;40:354–370.
34. Rosenfeld CR. Changes in uterine blood flow during pregnancy. Rosenfeld CR. The Uterine Circulation. Perinatology Press: Ithaca, NY; 1989:135–156.
35. Caton D, Wilcox CJ, Kalra PS. Correlation of rate of uterine blood flow and plasma steroid concentrations at parturition in sheep. J Reprod Fertil. 1980;58:329–337.
36. Page KL, Celia G, Leddy G, et al. Structural remodeling of rat uterine veins in pregnancy. Am J Obstet Gynecol. 2002;187:1647–1652.
37. Oosterhof H, Wichers G, Fidler V, Aarnoudse JG. Blood viscosity and uterine artery flow velocity waveforms in pregnancy: a longitudinal study. Placenta. 1993;14:555–561.
38. Chang K, Zhang L. Steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15:336–348.
39. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda). 2009;24:58–71.
40. Magness RR, Rosenfeld CR. Local and systemic estradiol-17β: effects on uterine and systemic vasodilation. Am J Physiol. 1989;256:E536–E542.
41. Pastore MB, Jobe SO, Ramadoss J, Magness RR. Estrogen receptor-alpha and estrogen receptor-beta in the uterine vascular endothelium during pregnancy: functional implications for regulating uterine blood flow. Semin Reprod Med. 2012;30:46–61.
42. Chen JZJ, Sheehan PM, Brennecke SP, Keogh RJ. Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol Cell Endocrinol. 2012;349:138–144.
43. Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260:E464–E470.
44. Weiner CP, Martinez E, Chestnut DH, Ghodsi A. Effect of pregnancy on uterine and carotid artery response to norepinephrine, epinephrine, and phenylephrine in vessels with documented functional endothelium. Am J Obstet Gynecol. 1989;161:1605–1610.
45. Weiner CP, Thompson LP, Liu KZ, Herrig JE. Endothelium-derived relaxing factor and indomethacin-sensitive contracting factor alter arterial contractile responses to thromboxane during pregnancy. Am J Obstet Gynecol. 1992;166:1171–1178.
46. Yang D, Clark KE. Effect of endothelin-1 on the uterine vasculature of the pregnant and estrogen-treated nonpregnant sheep. Am J Obstet Gynecol. 1992;167:1642–1650.
47. Wier RJ, Brown JJ, Fraser R, et al. Relationship between plasma renin, renin-substrate, angiotensin II, aldosterone and electrolytes in normal pregnancy. J Clin Endocrinol Metab. 1975;40:108–115.
48. Chesley LC, Talledo E, Bohler CS, Zuspan FP. Vascular reactivity to angiotension II and norepinephrine in pregnant women. Am J Obstet Gynecol. 1965;91:837–842.
49. Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest. 1981;68:468–474.
50. Rosenfeld CR. Consideration of the uteroplacental circulation in intrauterine growth. Semin Perinatol. 1984;8:42–51.
51. Magness RR, Rosenfeld CR. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol. 1986;155:897–904.
52. Bruce NW. Effects of acute maternal hemorrhage on uterine blood flow in the pregnant rat. J Appl Physiol. 1973;35:564–569.
53. Rosenfeld CR, DeSpain K, Word RA, Liu XT. Differential sensitivity to angiotensin II and norepinephrine in human uterine arteries. J Clin Endocrinol Metab. 2012;97:138–147.
54. Rosenfeld CR. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1025–R1040.
55. Cox BE, Rosenfeld CR, Kalinyak JE, et al. Tissue specific expression of vascular smooth muscle angiotensin II receptor subtypes during ovine pregnancy. Am J Physiol. 1996;271:H212–H221.
56. Magness RR, Mitchell MD, Rosenfeld CR. Uteroplacental production of eicosanoids in ovine pregnancy. Prostaglandins. 1990;39:75–88.
57. Magness RR, Rosenfeld CR, Hassan A, Shaul PW. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am J Physiol. 1996;270:H1914–H1923.
58. Magness RR, Rosenfeld CR, Faucher DJ, Mitchell MD. Uterine prostaglandin production in ovine pregnancy: effects of angiotensin II and indomethacin. Am J Physiol. 1992;263:H188–H197.
59. Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol. 1997;21:367–380.
60. Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–R463.
61. Miller SL, Jenkin G, Walker DW. Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am J Obstet Gynecol. 1999;180:1138–1145.
62. Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993;169:1316–1320.
63. Grunewald C, Nisell H, Jansson T, et al. Possible improvement in uteroplacental blood flow during atrial natriuretic peptide infusion in preeclampsia. Obstet Gynecol. 1994;84:235–239.
64. Ajne G, Nisell H, Wolff K, Jansson T. The role of endogenous endothelin in the regulation of uteroplacental and renal blood flow during pregnancy in conscious rats. Placenta. 2003;24:813–818.
65. Jauniaux E, Johnson MR, Jurkovic D, et al. The role of relaxin in the development of the uteroplacental circulation in early pregnancy. Obstet Gynecol. 1994;84:338–342.
66. Li Y, Zheng J, Bird IM, Magness RR. Effects of pulsatile shear stress on signaling mechanisms controlling nitric oxide production, endothelial nitric oxide synthase phosphorylation, and expression in ovine fetoplacental artery endothelial cells. Endothelium. 2005;12:21–39.
67. Wehrenberg WB, Chaichareon DP, Dierschke DJ, et al. Vascular dynamics of the reproductive tract in the female rhesus monkey: relative contributions of ovarian and uterine arteries. Biol Reprod. 1977;17:148–153.
68. Meschia G. Techniques for the study of the uteroplacental circulation. Rosenfeld CR. The Uterine Circulation. Perinatology Press: Ithaca, NY; 1989:35–51s.
69. Jouppila R, Jouppila P, Hollmén A, Kuikka J. Effect of segmental extradural analgesia on placental blood flow during normal labour. Br J Anaesth. 1978;50:563–567.
70. Skjöldebrand A, Eklund J, Johansson H, et al. Uteroplacental blood flow measured by placental scintigraphy during epidural anaesthesia for caesarean section. Acta Anaesthesiol Scand. 1990;34:79–84.
71. Boote EJ. AAPM/RSNA physics tutorial for residents: topics in US: Doppler US techniques: concepts of blood flow detection and flow dynamics. Radiographics. 2003;23:1315–1327.
72. Urban G, Vergani P, Ghidini A, et al. State of the art: non-invasive ultrasound assessment of the uteroplacental circulation. Semin Perinatol. 2007;31:232–239.
73. Tuuli M, Odibo AO. The role of serum markers and uterine artery Doppler in identifying at-risk pregnancies. Clin Perinatol. 2001;38:1–19.
74. Shnider SM, Wright RG, Levinson G, et al. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology. 1979;50:524–527.
75. Shnider SM, Abboud TK, Artal R, et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am J Obstet Gynecol. 1983;147:13–15.
76. Wallis KL, Shnider SM, Hicks JS, Spivey HT. Epidural anesthesia in the normotensive pregnant ewe: effects on uterine blood flow and fetal acid-base status. Anesthesiology. 1976;44:481–487.
77. Jouppila R, Jouppila P, Kuikka J, Hollmén A. Placental blood flow during caesarean section under lumbar extradural analgesia. Br J Anaesth. 1978;50:275–279.
78. Ramos-Santos E, Devoe LD, Wakefield ML, et al. The effects of epidural anesthesia on the Doppler velocimetry of umbilical and uterine arteries in normal and hypertensive patients during active term labor. Obstet Gynecol. 1991;77:20–26.
80. Patton DE, Lee W, Miller J, Jones M. Maternal, uteroplacental, and fetoplacental hemodynamic and Doppler velocimetric changes during epidural anesthesia in normal labor. Obstet Gynecol. 1991;77:17–19.
81. Chen LK, Lin CJ, Huang CH, et al. The effects of continuous epidural analgesia on Doppler velocimetry of uterine arteries during different periods of labour analgesia. Br J Anaesth. 2006;96:226–230.
82. Fratelli N, Prefumo F, Andrico S, et al. Effects of epidural analgesia on uterine artery Doppler in labour. Br J Anaesth. 2011;106:221–224.
83. Jouppila P, Jouppila R, Hollmén A, Koivula A. Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol. 1982;59:158–161.
84. Ginosar Y, Nadjari M, Hoffman A, et al. Antepartum continuous epidural ropivacaine therapy reduces uterine artery vascular resistance in pre-eclampsia: a randomized, dose-ranging, placebo-controlled study. Br J Anaesth. 2009;102:369–378.
85. Lu JK, Manullang TR, Staples MH, et al. Maternal respiratory arrests, severe hypotension, and fetal distress after administration of intrathecal, sufentanil, and bupivacaine after intravenous fentanyl. Anesthesiology. 1997;87:170–172.
86. Friedlander JD, Fox HE, Cain CF, et al. Fetal bradycardia and uterine hyperactivity following subarachnoid administration of fentanyl during labor. Reg Anesth. 1997;22:378–381.
87. Baumann H, Alon E, Atanassoff P, et al. Effect of epidural anesthesia for cesarean delivery on maternal femoral arterial and venous, uteroplacental, and umbilical blood flow velocities and waveforms. Obstet Gynecol. 1990;75:194–198.
88. Reynolds F, Seed PT. Anaesthesia for Caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia. 2005;60:636–653.
89. Gogarten W, Struemper D, Gramke HF, et al. Assessment of volume preload on uteroplacental blood flow during epidural anaesthesia for Caesarean section. Eur J Anaesthesiol. 2005;22:359–362.
90. Giles WB, Lah FX, Trudinger BJ. The effect of epidural anaesthesia for caesarean section on maternal uterine and fetal umbilical artery blood flow velocity waveforms. Br J Obstet Gynaecol. 1987;94:55–59.
91. Tong C, Eisenach JC. The vascular mechanism of ephedrine’s beneficial effect on uterine perfusion during pregnancy. Anesthesiology. 1992;76:792–798.
92. Wang SY, Datta S, Segal S. Pregnancy alters adrenergic mechanisms in uterine arterioles of rats. Anesth Analg. 2002;94:1304–1309.
93. Lee A, Ngan Kee WD, Gin T. A quantitative systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–926.
94. Alahuhta S, Räsänen J, Jouppila P, et al. Ephedrine and phenylephrine for avoiding maternal hypotension due to spinal anaesthesia for caesarean section: effects on uteroplacental and fetal haemodynamics. Int J Obstet Anaesth. 1992;1:129–134.
95. Hall PA, Bennett A, Wilkes MP, Lewis M. Spinal anaesthesia for Caesarean section: comparison of infusions of phenylephrine and ephedrine. Br J Anaesth. 1994;73:471–474.
96. LaPorta RF, Arthur GR, Datta S. Phenylephrine in treating maternal hypotension due to spinal anaesthesia for caesarean delivery: effects on neonatal catecholamine concentrations, acid base status and Apgar scores. Acta Anaesthesiol Scand. 1995;39:901–905.
97. Moran DH, Perillo M, LaPorta RF, et al. Phenylephrine in the prevention of hypotension following spinal anesthesia for cesarean delivery. J Clin Anesth. 1991;3:301–305.
98. Pierce ET, Carr DB, Datta S. Effects of ephedrine and phenylephrine on maternal and fetal atrial natriuretic peptide levels during elective cesarean section. Acta Anaesthesiol Scand. 1994;38:48–51.
99. Thomas DG, Robson SC, Redfern N, et al. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:61–65.
100. Ngan Kee WD, Lau TK, Khaw KS, Lee BB. Comparison of metaraminol and ephedrine infusions for maintaining arterial pressure during spinal anesthesia for elective cesarean section. Anesthesiology. 2001;95:307–313.
101. Ngan Kee WD, Khaw KS, Lee BB, et al. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2000;90:1390–1395.
102. Greiss FC Jr. Differential reactivity of the myoendometrial and placental vasculatures: adrenergic responses. Am J Obstet Gynecol. 1972;112:20–30.
103. Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
104. Stewart A, Fernando R, McDonald S, et al. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–1237.
105. Erkinaro T, Makikallio K, Kavasmaa T, et al. Effects of ephedrine and phenylephrine on uterine and placental circulations and fetal outcome following fetal hypoxaemia and epidural-induced hypotension in a sheep model. Br J Anaesth. 2004;93:825–832.
106. Erkinaro T, Kavasmaa T, Pakkila M, et al. Ephedrine and phenylephrine for the treatment of maternal hypotension in a chronic sheep model of increased placental vascular resistance. Br J Anaesth. 2006;96:231–237.
107. Ngan Kee WD, Khaw KS, Lau TK, et al. Randomized double-blinded comparison of phenylephrine versus ephedrine for maintaining blood pressure during spinal anaesthesia for non-elective Caesarean section. Anaesthesia. 2008;63:1319–1326.
108. Johns RA. Local anesthetics inhibit endothelium-dependent vasodilation. Anesthesiology. 1989;70:805–811.
109. Greiss FC Jr, Still JG, Anderson SG. Effects of local anesthetic agents on the uterine vasculatures and myometrium. Am J Obstet Gynecol. 1976;124:889–899.
110. Fishburne JI Jr, Greiss FC Jr, Hopkinson R, Rhyne AL. Responses of the gravid uterine vasculature to arterial levels of local anesthetic agents. Am J Obstet Gynecol. 1979;133:753–761.
111. Santos AC, Arthur GR, Roberts DJ, et al. Effect of ropivacaine and bupivacaine on uterine blood flow in pregnant ewes. Anesth Analg. 1992;74:62–67.
112. Alahuhta S, Räsänen J, Jouppila P, et al. The effects of epidural ropivacaine and bupivacaine for cesarean section on uteroplacental and fetal circulation. Anesthesiology. 1995;83:23–32.
113. Alahuhta S, Räsänen J, Jouppila R, et al. Effects of extradural bupivacaine with adrenaline for caesarean section on uteroplacental and fetal circulation. Br J Anaesth. 1991;67:678–682.
114. Albright GA, Jouppila R, Hollmén AI, et al. Epinephrine does not alter human intervillous blood flow during epidural anesthesia. Anesthesiology. 1981;54:131–135.
115. Alahuhta S, Räsänen J, Jouppila P, et al. Uteroplacental and fetal circulation during extradural bupivacaine-adrenaline and bupivacaine for caesarean section in hypertensive pregnancies with chronic fetal asphyxia. Br J Anaesth. 1993;71:348–353.
116. Marcus MA, Gogarten W, Vertommen JD, et al. Haemodynamic effects of repeated epidural test-doses of adrenaline in the chronic maternal-fetal sheep preparation. Eur J Anaesthesiol. 1998;15:320–323.
117. Eisenach JC, Castro MI, Dewan DM, et al. Intravenous clonidine hydrochloride toxicity in pregnant ewes. Am J Obstet Gynecol. 1989;160:471–476.
118. Eisenach JC, Castro MI, Dewan DM, Rose JC. Epidural clonidine analgesia in obstetrics: sheep studies. Anesthesiology. 1989;70:51–56.
119. Mardirosoff C, Dumont L, Boulvain M, Tramer MR. Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematic review. BJOG. 2002;109:274–281.
120. Craft JB Jr, Coaldrake LA, Bolan JC, et al. Placental passage and uterine effects of fentanyl. Anesth Analg. 1983;62:894–898.
121. Craft JB Jr, Bolan JC, Coaldrake LA, et al. The maternal and fetal cardiovascular effects of epidural morphine in the sheep model. Am J Obstet Gynecol. 1982;142:835–839.
122. Alahuhta S, Räsänen J, Jouppila P, et al. Epidural sufentanil and bupivacaine for labor analgesia and Doppler velocimetry of the umbilical and uterine arteries. Anesthesiology. 1993;78:231–236.
124. D’Angelo R, Anderson MT, Philip J, Eisenach JC. Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology. 1994;80:1209–1215.
125. Allen J, Svane D, Petersen LK, et al. Effects of thiopentone and chlomethiazole on human myometrial arteries from term pregnant women. Br J Anaesth. 1992;68:256–260.
126. Alon E, Ball RH, Gillie MH, et al. Effects of propofol and thiopental on maternal and fetal cardiovascular and acid-base variables in the pregnant ewe. Anesthesiology. 1993;78:562–576.
127. Craft JB Jr, Coaldrake LA, Yonekura ML, et al. Ketamine, catecholamines, and uterine tone in pregnant ewes. Am J Obstet Gynecol. 1983;146:429–434.
128. Strümper D, Gogarten W, Durieux ME, et al. The effects of S+-ketamine and racemic ketamine on uterine blood flow in chronically instrumented pregnant sheep. Anesth Analg. 2004;98:497–502.
129. Jouppila P, Kuikka J, Jouppila R, Hollmén A. Effect of induction of general anesthesia for cesarean section on intervillous blood flow. Acta Obstet Gynecol Scand. 1979;58:249–253.
130. Gin T, O’Meara ME, Kan AF, et al. Plasma catecholamines and neonatal condition after induction of anaesthesia with propofol or thiopentone at Caesarean section. Br J Anaesth. 1993;70:311–316.
131. Levinson G, Shnider SM, Gildea JE, deLorimier AA. Maternal and foetal cardiovascular and acid-base changes during ketamine anaesthesia in pregnant ewes. Br J Anaesth. 1973;45:1111–1115.
132. Gin T, Ngan Kee WD, Siu YK, et al. Alfentanil given immediately before the induction of anesthesia for elective cesarean delivery. Anesth Analg. 2000;90:1167–1172.
133. Ngan Kee WD, Khaw KS, Ma KC, et al. Maternal and neonatal effects of remifentanil at induction of general anesthesia for cesarean delivery: a randomized, double-blind, controlled trial. Anesthesiology. 2006;104:14–20.
134. Palahniuk RJ, Shnider SM. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462–472.
135. Stein D, Masaoka T, Wlody D, et al. The effects of sevoflurane and isoflurane in pregnant sheep: uterine blood flow and fetal well-being. Anesthesiology. 2007;75:851.
136. Dahlgren G, Tornberg DC, Pregner K, Irestedt L. Four cases of the ex utero intrapartum treatment (EXIT) procedure: anesthetic implications. Int J Obstet Anesth. 2004;13:178–182.
137. Makowski EL, Hertz RH, Meschia G. Effects of acute maternal hypoxia and hyperoxia on the blood flow to the pregnant uterus. Am J Obstet Gynecol. 1973;115:624–631.
138. Motoyama EK, Rivard G, Acheson F, Cook CD. Adverse effect of maternal hyperventilation on the foetus. Lancet. 1966;1:286–288.
139. Lumley J, Renou P, Newman W, Wood C. Hyperventilation in obstetrics. Am J Obstet Gynecol. 1969;103:847–855.
140. Levinson G, Shnider SM, deLorimier AA, Steffenson JL. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974;40:340–347.
141. Vincent RD Jr, Chestnut DH, Sipes SL, et al. Magnesium sulfate decreases maternal blood pressure but not uterine blood flow during epidural anesthesia in gravid ewes. Anesthesiology. 1991;74:77–82.
142. Dandavino A, Woods JR Jr, Murayama K, et al. Circulatory effects of magnesium sulfate in normotensive and renal hypertensive pregnant sheep. Am J Obstet Gynecol. 1977;127:769–774.
143. Keeley MM, Wade RV, Laurent SL, Hamann VD. Alterations in maternal-fetal Doppler flow velocity waveforms in preterm labor patients undergoing magnesium sulfate tocolysis. Obstet Gynecol. 1993;81:191–194.
144. Souza AS, Amorim MM, Coutinho IC, et al. Effect of the loading dose of magnesium sulfate (MgSO4) on the parameters of Doppler flow velocity in the uterine, umbilical and middle cerebral arteries in severe preeclampsia. Hypertens Pregnancy. 2010;29:123–134.
145. Schauf B, Mannschreck B, Becker S, et al. Evaluation of red blood cell deformability and uterine blood flow in pregnant women with preeclampsia or IUGR and reduced uterine blood flow following the intravenous application of magnesium. Hypertens Pregnancy. 2004;23:331–343.
146. Ring G, Krames E, Shnider SM, et al. Comparison of nitroprusside and hydralazine in hypertensive pregnant ewes. Obstet Gynecol. 1977;50:598–602.
147. Pedron SL, Reid DL, Barnard JM, et al. Differential effects of intravenous hydralazine on myoendometrial and placental blood flow in hypertensive pregnant ewes. Am J Obstet Gynecol. 1992;167:1672–1678.
148. Eisenach JC, Mandell G, Dewan DM. Maternal and fetal effects of labetalol in pregnant ewes. Anesthesiology. 1991;74:292–297.
149. Morgan MA, Silavin SL, Dormer KJ, et al. Effects of labetalol on uterine blood flow and cardiovascular hemodynamics in the hypertensive gravid baboon. Am J Obstet Gynecol. 1993;168:1574–1579.
150. Ahokas RA, Mabie WC, Sibai BM, Anderson GD. Labetalol does not decrease placental perfusion in the hypertensive term-pregnant rat. Am J Obstet Gynecol. 1989;160:480–484.
151. Baggio MR, Martins WP, Calderon AC, et al. Changes in fetal and maternal Doppler parameters observed during acute severe hypertension treatment with hydralazine or labetalol: a randomized controlled trial. Ultrasound Med Biol. 2011;37:53–58.
152. Jouppila P, Kirkinen P, Koivula A, Ylikorkala O. Effects of dihydralazine infusion on the fetoplacental blood flow and maternal prostanoids. Obstet Gynecol. 1985;65:115–118.
153. Janbu T, Nesheim BI. The effect of dihydralazine on blood velocity in branches of the uterine artery in pregnancy induced hypertension. Acta Obstet Gynecol Scand. 1989;68:395–400.
154. Jouppila P, Kirkinen P, Koivula A, Ylikorkala O. Labetalol does not alter the placental and fetal blood flow or maternal prostanoids in pre-eclampsia. Br J Obstet Gynaecol. 1986;93:543–547.
155. Lunell NO, Nylund L, Lewander R, Sarby B. Acute effect of an antihypertensive drug, labetalol, on uteroplacental blood flow. Br J Obstet Gynaecol. 1982;89:640–644.
156. Günenç O, Çiçek N, Görkemli H, et al. The effect of methyldopa treatment on uterine, umbilical and fetal middle cerebral artery blood flows in preeclamptic patients. Arch Gynecol Obstet. 2002;266:141–144.
157. Montan S, Anandakumar C, Arulkumaran S, et al. Effects of methyldopa on uteroplacental and fetal hemodynamics in pregnancy-induced hypertension. Am J Obstet Gynecol. 1993;168:152–156.
158. Murad SH, Tabsh KM, Shilyanski G, et al. Effects of verapamil on uterine blood flow and maternal cardiovascular function in the awake pregnant ewe. Anesth Analg. 1985;64:7–10.
159. Moretti MM, Fairlie FM, Akl S, et al. The effect of nifedipine therapy on fetal and placental Doppler waveforms in preeclampsia remote from term. Am J Obstet Gynecol. 1990;163:1844–1848.
160. Guclu S, Gol M, Saygili U, et al. Nifedipine therapy for preterm labor: effects on placental, fetal cerebral and atrioventricular Doppler parameters in the first 48 hours. Ultrasound Obstet Gynecol. 2006;27:403–408.
161. Toda N, Kimura T, Yoshida K, et al. Human uterine arterial relaxation induced by nitroxidergic nerve stimulation. Am J Physiol. 1994;266:H1446–H1450.
162. Ramsay B, De Belder A, Campbell S, et al. A nitric oxide donor improves uterine artery diastolic blood flow in normal early pregnancy and in women at high risk of pre-eclampsia. Eur J Clin Invest. 1994;24:76–78.
163. Cacciatore B, Halmesmaki E, Kaaja R, et al. Effects of transdermal nitroglycerin on impedance to flow in the uterine, umbilical, and fetal middle cerebral arteries in pregnancies complicated by preeclampsia and intrauterine growth retardation. Am J Obstet Gynecol. 1998;179:140–145.
164. Grunewald C, Kublickas M, Carlstrom K, et al. Effects of nitroglycerin on the uterine and umbilical circulation in severe preeclampsia. Obstet Gynecol. 1995;86:600–604.
165. Landauer M, Phernetton TM, Rankin JH. Maternal ovine placental vascular responses to adenosine. Am J Obstet Gynecol. 1986;154:1152–1155.
166. Fishburne JI Jr, Dormer KJ, Payne GG, et al. Effects of amrinone and dopamine on uterine blood flow and vascular responses in the gravid baboon. Am J Obstet Gynecol. 1988;158:829–837.
167. Santos AC, Baumann AL, Wlody D, et al. The maternal and fetal effects of milrinone and dopamine in normotensive pregnant ewes. Am J Obstet Gynecol. 1992;166:257–262.