Chapter 47 Tumors of the biliary tree
Pathologic features
Overview
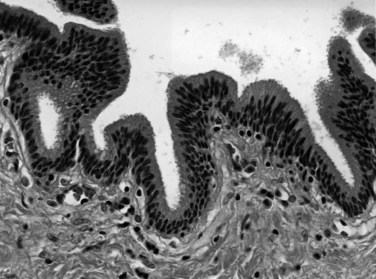
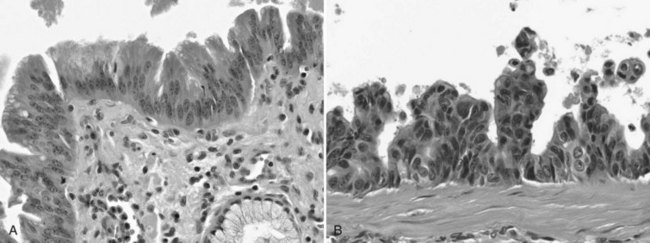
The biliary tract is lined by simple columnar epithelial cells of foregut origin (Fig. 47.1; Frierson, 1989; 1997). Most biliary neoplasms are related to this cell type and hence show major similarities to other foregut tumors, particularly those of pancreatic ductal origin. By far the most common neoplasm of the biliary tract is adenocarcinoma, which is referred to collectively with pancreatic ductal adenocarcinoma as a pancreatobiliary-type adenocarcinoma (Figs. 47.2 through 47.5), reflecting the similarities between these two neoplasms.
Tumors arising from the gallbladder and the different segments of the bile ducts have overlapping histologic characteristics; therefore their pathologic classification is also very similar (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). On the other hand, the risk factors, clinical findings, and biologic behavior may vary (see Chapters 49, 50A, and 50B). For instance, although gallstones are the main risk factor for gallbladder adenocarcinoma, adenocarcinomas of proximal bile ducts have a much stronger association with primary sclerosing cholangitis (PSC) (see Chapter 41) or anomalous pancreatobiliary duct junction (see Chapter 46). This variability also is partially reflected in the molecular alterations, which although not specific for the histologic subtype of the tumor, may affect the management and prognosis (see Chapter 8B). The different presentations of biliary adenocarcinoma also influence the mode by which a specimen is obtained for pathologic examination. For example, many gallbladder adenocarcinomas are currently identified in “routine” cholecystectomy specimens in primary care facilities, performed with a preoperative diagnosis of chronic cholecystitis or cholelithiasis, whereas extrahepatic bile duct lesions are seldom resected without a strong preoperative suspicion of carcinoma, and this is usually performed in tertiary care facilities.
This chapter discusses the pathologic aspects of biliary tract neoplasia, with special emphasis on adenocarcinomas. The tumor types are discussed by viewing the biliary system as a whole; site-specific characteristics are mentioned only when pertinent to pathologic classification. For the details of clinical findings, risk factors, and site-specific management, the reader is referred to other chapters of this book (see Chapters 49, 50A, and 50B).
Invasive Carcinomas Of The Biliary Tract
Most “biliary tract cancers” are conventional adenocarcinomas (pancreatobiliary type), which are morphologically very similar to pancreatic ductal adenocarcinoma (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). This tumor is referred to as gallbladder adenocarcinoma in the gallbladder, cholangiocarcinoma in the intrahepatic biliary tract, and adenocarcinoma of the extrahepatic bile ducts in the extrahepatic biliary tract. As in adenocarcinomas of other organs, these are usually tumors of the elderly. The association of biliary adenocarcinomas with preceding chronic inflammation has been well established (Herzog & Goldblum, 1996; Sheth et al, 2000), mostly by epidemiologic data showing the high incidence of gallbladder cancer in areas with a high prevalence of gallstones and by the pathologic observation that many individual carcinomas have associated gallstones or cholecystitis (see Chapter 49). Also, the risk of adenocarcinoma is relatively high in patients with PSC and indirectly in those with ulcerative colitis (see Chapter 41). The association of biliary cancers with parasites (see Chapter 45; Carriaga & Henson, 1995) and choledochal cysts (see Chapter 46) is also presumably related to the potential for chronic inflammation from constant epithelial injury and repair that leads to neoplastic alterations (Parkin et al, 1993).
Growth Patterns and Macroscopic Features
Based on their macroscopic growth pattern, biliary carcinomas have been divided into four types: 1) polypoid, 2) nodular (nodular-sclerosing), 3) schirrous-constricting, and 4) diffusely infiltrative (Albores-Saavedra et al, 2000a; Todoroki et al, 1980; Van Heerden et al, 1967; Weinbren & Mutum, 1983). Polypoid growth typically is found in carcinomas with an associated component of intraductal papillary neoplasm (IPN), previously designated noninvasive papillary carcinoma (Albores-Saavedra et al, 2010), which are associated with a better prognosis. The nodular and schirrous types have a propensity to infiltrate surrounding tissues and are therefore difficult to resect. The diffusely infiltrating type tends to spread linearly along the ducts. The constricting and diffusely infiltrative patterns may be difficult to differentiate from chronic inflammatory conditions, especially PSC and autoimmune cholangitis (Corvera et al, 2005). Significant histologic overlaps occur among these different growth patterns, so their utility in tumor classification is limited.
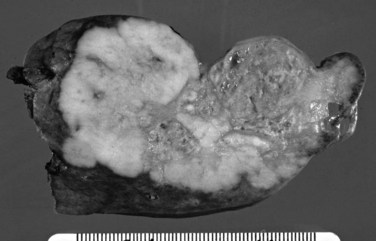
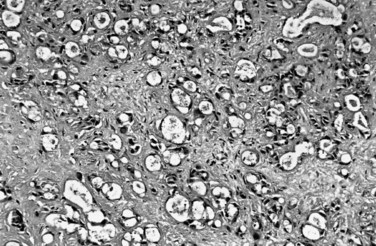
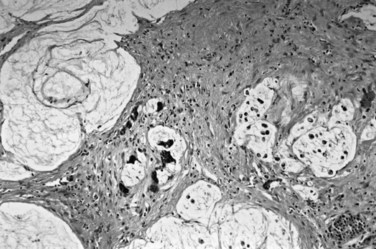
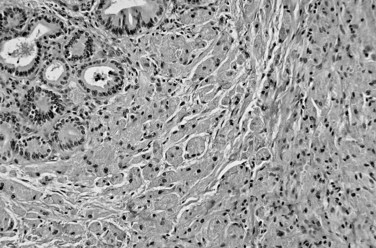
On cut sections, the intraluminal components of biliary carcinomas, especially those with a polypoid gross appearance, may appear more friable, soft, and tan; this reflects the presence of intraductal papillary neoplasm elements growing into the lumen, and ulceration and necrosis may be evident in larger tumors. The infiltrating components of adenocarcinomas are more schirrous (scarlike) with a firm, white, gritty appearance (see Fig. 47.2) because of the abundance of desmoplasia, a fibrotic tissue reaction associated with infiltrating carcinoma. Necrosis may also be evident.
When adenocarcinoma invades into the adjacent liver, the growth pattern often becomes more expansile, and the liver-carcinoma interface appears deceptively well demarcated. This allows easier detection of the boundaries of these carcinomas in hepatic resections. In contrast, the boundaries of carcinomas invading the hilar soft tissue are typically poorly defined and difficult to appreciate. Gallbladder carcinomas are often associated with gallstones. In patients with porcelain gallbladder (Stephen & Berger, 2001), the wall of the gallbladder may be entirely calcified.
Microscopic Features
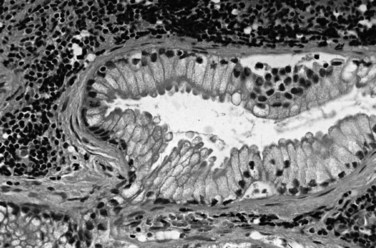
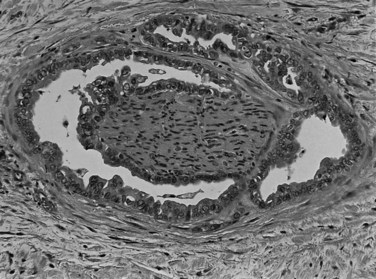
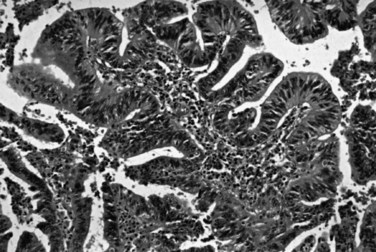
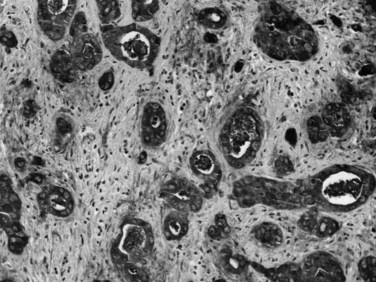
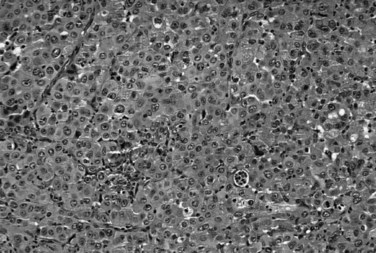
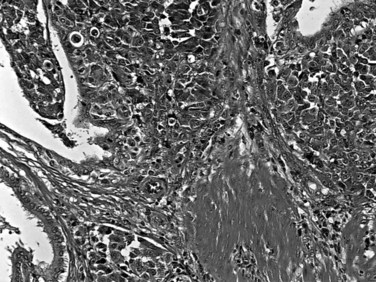
Most adenocarcinomas of gallbladder and bile ducts show the characteristic features of a pancreatobiliary-type adenocarcinoma (see Figs. 47.3 to 47.5): both simple and complex, irregularly shaped glands mixed with small clusters of cells, often with associated stromal desmoplasia (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). The glands often are well formed, lined by cuboidal cells, and show dilated lumina. Often the nuclear grade is unexpectedly high for the degree of glandular differentiation, and marked variation in nuclear size, shape, and intracellular location occurs between different cells within the individual glands. The cytoplasm may be acidophilic and granular in some cases and pale or clear in others. Variable amounts of intracytoplasmic and intraluminal mucin are present; in some cases, mucin is readily evident by routine histologic examination; in others, it is demonstrable by special stains.
Perineural (see Fig. 47.4) and vascular invasion are common, and carcinoma cells may have a deceptively well-differentiated appearance, even when they invade these structures. In fact, the distinction of a well-differentiated adenocarcinoma from a benign reactive process in this region is one of the more challenging differential diagnoses in surgical pathology.
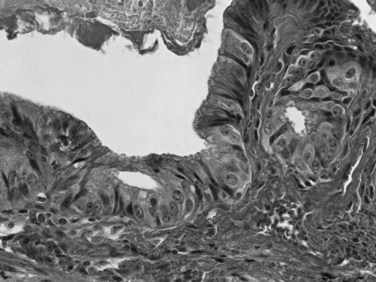
Dysplasia, or biliary intraepithelial neoplasia (BilIN; Fig. 47.6), is often present in the adjacent biliary epithelium (Zen et al, 2007). Sometimes the intraepithelial component comprises a papillary neoplasm (Fig. 47.7). In such cases, the noninvasive and invasive components of the tumor should be evaluated separately, and the extent of invasion should be quantified because those with “minimal invasion” have a better prognosis (Albores-Saavedra et al, 2000b).
Anatomic Variations
For therapeutic and prognostic purposes, tumors of the extrahepatic biliary tract are separated by their anatomic distribution into upper third (above the cystic duct junction, including the both hepatic ducts, common hepatic duct, and the cystic duct), middle third (upper half of the common bile duct [CBD]), and lower third (distal half of the CBD) (Klatskin, 1965; Tompkins et al, 1981; Van Heerden et al, 1967; Weinbren & Mutum, 1983). The distinctive clinical characteristics of adenocarcinomas occurring in different segments of the biliary tract are discussed in other chapters of this book, and only a few issues pertinent to pathologic aspects are mentioned here. Most carcinomas arise in the upper third of the bile ducts and tend to be the schirrous-constricting and diffusely infiltrative types. Recent studies have shown that many originate within 5 mm of the cystic duct junction or within the cystic duct itself. Hilar carcinoma, located at the confluence of the right and left hepatic ducts, sometimes referred to as a Klatskin tumor (Bosma, 1990; Klatskin, 1965), has distinctive clinical features. Klatskin tumors usually grow into the liver rather than distally toward the duodenum (see Chapter 50B; Hayashi et al, 1994), and the component that invades the liver is often well demarcated. Carcinomas in the middle third tend to be the nodular-sclerosing type—thickened along a long segment, with a narrow lumen and inflammatory changes in the surrounding tissues; hence these are difficult to differentiate from sclerosing cholangitis. They have a very high propensity for perineural invasion and involvement of radial surfaces, making curative resection difficult (Bhuiya et al, 1993). Those in the distal third have the best prognosis, partly because of their resectability by pancreatoduodenectomy and partly because many, especially those close to the ampullary region, are composed predominantly of noninvasive papillary neoplasmic elements (Adsay, 2004; Albores-Saavedra et al, 2000a; Lack, 2003).
Pathologic Differential Diagnosis
The difficulty at the clinical level of distinguishing biliary adenocarcinomas from benign inflammatory conditions, such as sclerosing cholangitis, is also problematic at the microscopic level (Ludwig, 1989; Ludwig et al, 1992). Reactive changes in the accessory biliary ductules in the wall of the bile ducts can mimic adenocarcinomas, although nonneoplastic ductules may retain a lobular configuration, and they lack the density of cellularity of a carcinoma. As also discussed in Chapter 56, pancreatobiliary adenocarcinomas can be deceptively benign appearing, composed of well-formed glandular elements lined by fairly organized, cytologically bland glandular cells (see Figs. 47.3 and 47.4). The distinction of reactive changes in the surface epithelium from dysplasia (BilIN) often proves to be even more challenging, especially because any injury to the biliary epithelium, including instrumentation and stent placement, has a tendency to induce marked cytologic atypia that mimics the appearance of high-grade dysplasia (Fig. 47.8). Marked nuclear enlargement or irregularity, hyperchromasia, loss of polarity, mitotic figures, apoptotic cells, and intraluminal necrosis are findings in favor of a neoplastic process (see Fig. 47.6); however, overlaps are common, and at times this distinction may not be possible on the basis of biopsies or frozen sections.
Biliary carcinomas that grow into the liver must be distinguished from primary hepatocellular carcinomas (see Chapter 78). The presence of true glandular elements and mucin are common findings in biliary carcinomas and typically are lacking in hepatocellular carcinomas. In contrast, hepatocellular carcinomas may have intracellular bile and generally lack significant stromal fibrosis and desmoplasia. Other distinctive features of hepatocellular carcinomas—the solid and trabecular growth pattern, centrally located nuclei with prominent nucleoli, and abundant eosinophilic cytoplasm—usually are identifiable; immunohistochemical demonstration of hepatocellular differentiation with markers such as hepatocyte-1 or glypican-3 can be used in problematic cases.
Biliary carcinomas metastatic to other sites may mimic the primary tumors of those organs. In particular, metastases to the ovary often become cystic and are mistaken for primary ovarian mucinous cystic neoplasms (Young & Hart, 1989), and lung metastases can resemble mucinous bronchioloalveolar carcinomas. Metastases to the peripheral liver from biliary carcinomas can be nearly impossible to distinguish from metastatic pancreatic ductal adenocarcinoma.
Immunohistochemical and Molecular Characteristics
By immunohistochemistry, biliary adenocarcinomas typically express CK7, CK19, CA19-9, CEA , MUC1 (Fig. 47.9), and MUC5AC (Adsay, 2004; Albores-Saavedra et al, 2000a; Lack, 2003). On occasion, these markers can be helpful in distinguishing certain other carcinomas that do not express these markers, such as hepatocellular carcinoma. However, none of these markers is specific enough to prove biliary origin for an adenocarcinoma when a metastasis from another organ is under consideration. Biliary adenocarcinomas generally lack expression of CK20 and CDX2 typically found in intestinal adenocarcinomas; TTF1 and napsin, found in pulmonary primaries; and hormone receptors. Mutations at codon 12 of the KRAS oncogene, seen in more than 90% of pancreatic ductal adenocarcinomas, are much less common in biliary adenocarcinomas (Rashid et al, 2002); the frequency of KRAS mutation appears to decrease from distal to proximal along the biliary tree. Similarly, loss of SMAD4 is also less common in biliary than pancreatic adenocarcinomas (Argani et al, 2001). More than half of the cases have abnormal expression of TP53 and loss of heterozygosity at 8p, 9q, and 18q; amplification of ERBB2 is also reported in more than half.
Other Types of Carcinomas in the Biliary Tract
Other carcinomas of glandular epithelial origin, in the gallbladder and biliary tract are classified separately from pancreatobiliary adenocarcinomas (Adsay, 2004; Albores-Saavedra et al, 1996, 2000a, 2010; Lack, 2003). Intestinal-type adenocarcinomas (Albores-Saavedra et al, 1986) are morphologically similar to their counterparts in the gastrointestinal tract. Signet ring cell carcinomas are characterized by a diffusely infiltrative pattern of individual cells, often with signet ring morphology because of intracellular mucin; a cordlike growth pattern may also occur in the biliary tract but is exceedingly uncommon. Mucinous adenocarcinomas may be seen in some cases, with extensive mucin production associated with stromal mucin deposition (Fig. 47.10), usually mixed with conventional adenocarcinomas; this subtype can occur with intraductal papillary neoplasms. Some studies suggest that the prognosis of mucinous adenocarcinomas may be more favorable than that of conventional pancreatobiliary-type adenocarcinoma (Bosma, 1990). Adenosquamous carcinomas (Nishihara et al, 1994) are rare tumors in which a mixture of glandular and squamous differentiation is seen in variable amounts. Clear-cell carcinomas (Vardaman & Albores-Saavedra, 1995) are described, in which the morphologic features resemble those of renal cell carcinoma.
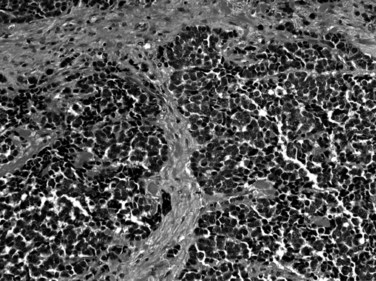
Some patterns of biliary carcinoma lack gland, mucin, or papilla formation. Undifferentiated (Fig. 47.11) and sarcomatoid carcinomas (Appelman & Coopersmith, 1970; Nishihara & Tsuneyoshi, 1993; Suster et al, 1987) probably represent the least differentiated end of the spectrum of adenocarcinomas, in which glandular differentiation is no longer detectable; these patterns can coexist with conventional adenocarcinomas, pointing to the close relationship of these tumor types. In sarcomatoid carcinomas the cells acquire mesenchymal characteristics, including a spindle shape; in some cases “heterologous” elements, such as bone and cartilage, occur. In the absence of a more epithelioid or glandular component or preinvasive neoplasia within the bile ducts, these tumors may be difficult to distinguish from true sarcomas. Some examples of undifferentiated carcinomas are associated with abundant osteoclast-like giant cells and are referred to as undifferentiated carcinoma osteoclastic-like giant cells (Haratake et al, 1992; Husek, 1990; Ito et al, 1992). It has been well documented that these giant cells are in fact of histiocytic origin, and the malignant cells in this tumor are the mononuclear cells in the background.
Small-cell carcinoma (Komminoth et al, 2010; Maitra et al, 2001; van der Wal et al, 1990) also occurs in the region of biliary tree, predominantly in the gallbladder or distal CBD. Small-cell carcinomas (Fig. 47.12) are defined by the same histologic criteria applied to their counterparts in the lung, including high nucleus/cytoplasm ratio, molding of nuclei, diffuse chromatin pattern, and absence of nucleoli. Small-cell carcinomas are high-grade neuroendocrine carcinomas; therefore neuroendocrine markers such as chromogranin, synaptophysin, and CD56 are often detectable by immunohistochemistry. Large-cell neuroendocrine carcinomas may also occur in this region (Komminoth et al, 2010; Papotti et al, 2000).
Pathologic Evaluation of Biliary Specimens with Carcinomas
Clinically Relevant Pathologic Parameters in Biliary Carcinomas
Tumor Type
Whether the tumor is a conventional pancreatobiliary invasive adenocarcinoma or another type of neoplasm is important (Bivins et al, 1975). For instance, intraductal papillary neoplasms with focal microinvasion have a significantly better prognosis, and undifferentiated carcinoma has a worse outcome.
Pathologic Stage
The size and depth of the tumor are the most important aspects of pathologic staging of biliary carcinomas (Choi et al, 2010; Greene et al, 2002; Hong et al, 2007). There are, however, pitfalls in establishing the depth of the tumor in some parts of the biliary tract, where unlike other sites, such as the gastrointestinal tract, the layers that constitute the duct walls are not well defined. In particular, in parts of the CBD the interface of the mucosa with the muscular layer and the interface of the muscle with the perimuscular tissue are highly irregular, which hinders the accurate evaluation of tumor depth. Similarly, in the intrapancreatic segment of the common bile duct, the demarcation of the common bile duct from pancreatic lobules is highly variable, and how to evaluate invasion into the pancreas is controversial (Hong et al, 2005).
Grading
The grading scheme advocated by the World Health Organization (WHO) (Albores-Saavedra et al, 2000c) is based on the percent of the tumor showing glandular differentiation, specifically, tubule formation. If more than 95% of the tumor is composed of tubules, it is well differentiated; 40% to 95% is moderately differentiated, and 5% to 39% is poorly differentiated. Those without any glandular differentiation are regarded as undifferentiated.
Assessment of Surgical Margins
Proper orientation of the specimen with identification of the margins by the surgical team using sutures or dyes often proves very helpful for accurate evaluation of the surgical margins, especially in complex specimens. The status of the resection margins is an important factor predictive of recurrence (Weber et al, 2001). Bile duct margins typically are amputated from the specimen and submitted en face, whereas hepatic and soft tissue margins are inked and sections are submitted perpendicular to the margin.
Noninvasive Epithelial Neoplasia
Dysplasia (Biliary Intraepithelial Neoplasia)
Dysplasia, also called biliary intraepithelial neoplasia (BilIN; see Fig. 47.6), can be seen in mucosa adjacent to invasive biliary carcinomas and, on occasion, as an incidental finding in specimens obtained for other reasons (Albores-Saavedra et al, 1980; Laitio, 1983; Ojeda et al, 1985; Suzuki et al, 1989; Yamagiwa, 1989; Zen et al, 2007). In general, it is a radiographically and grossly occult process characterized by cytoarchitectural atypia of the biliary epithelium, including nuclear enlargement, irregularities, loss of polarity, and mitotic activity. Based on the degree of atypia, dysplasia is graded as low, intermediate, or high grade or BilIN1, BilIN2, and BilIN3, respectively (Zen et al, 2007); the high-grade category has been historically referred to as carcinoma in situ. Among biliary organs, dysplasia is most common and best studied in the gallbladder, where high-grade dysplasia has been reported in up to 3.5% of routine cholecystectomy specimens (Chan, 1988; Ojeda et al, 1985); this figure is reported to be higher in geographic regions with a higher prevalence of gallbladder carcinoma. The incidence of dysplasia in surgical specimens in the remainder of the biliary tract appears to be much lower. However, when hepatic explants for cirrhosis resulting from alcohol abuse or hepatitis C are systematically evaluated, dysplasia—particularly the low to intermediate grade—is found in more than half of the cases (Wu et al, 2009). However, the diagnostic criteria for dysplasia are highly subjective, and reactive atypia in the biliary epithelium as a result of inflammation or stenting is very difficult to distinguish from dysplasia, rendering the intraoperative frozen section evaluation of bile duct margins challenging. On the other hand, in patients with invasive carcinoma, dysplasia may be difficult to distinguish from retrograde mucosal involvement by invasive carcinoma, a phenomenon referred to as colonization of the surface epithelium. For these reasons, the true frequency of dysplasia in the biliary tract is difficult to determine. It should also be noted that the concept of BilIN, proposed in analogy to pancreatic intraepithelial neoplasia (PanIN) in the pancreas (Zen et al, 2007), is thus far largely based purely on morphologic findings in the biliary epithelium. Molecular alterations supporting the neoplastic nature of all these lesions, analogous to the frequent clonal mutations in the KRAS oncogene found in PanINs, have yet to be documented.
In the gallbladder, dysplasia associated with invasive carcinomas appears to have no bearing on the prognosis. Cases of gallbladder dysplasia detected incidentally in the absence of invasive carcinoma appear to be clinically silent and have no documented clinical consequences, provided that the gallbladder has been extensively examined histologically to exclude occult foci of invasive carcinoma. However, data from the Surveillance Epidemiology and End Results (SEER) program of the National Cancer Institute show that one third of the patients with high-grade dysplasia (carcinoma in situ) of the gallbladder died of carcinoma in 10 years, although all were alive at 5 years, suggesting that either a small invasive carcinoma was missed or the patients developed a second malignant neoplasm elsewhere in the biliary system (Albores-Saavedra et al, 2000a). Extrapolating from these observations, a “field effect” phenomenon may occur in some cases, and patients with high-grade gallbladder dysplasia, especially if it is extensive, probably should have some surveillance to screen for subsequent invasive biliary carcinoma. In the bile ducts, high-grade dysplasia rarely is detected in the absence of invasive carcinoma, so the clinical significance of isolated dysplasia is unclear. Such patients would presumably also have an increased risk of developing invasive bile duct carcinomas, and careful examination of the biliary tree to exclude a synchronous, discontinuous focus of invasive carcinoma would seem prudent.
Mass-Forming Intraepithelial Neoplasms
Exophytic, mass-forming neoplasms that project into the lumina of the gallbladder or bile ducts include a family of related entities that have been designated by different terms depending on the location of the tumor, its architecture, and the cell types it comprises. Most of these neoplasms are predominantly formed of papillary structures or tubular glands and some cases have a mixture of both. In the gallbladder, the term adenoma is used for localized, generally small tumors that may be tubular, papillary, or tubulopapillary and are further subdivided into pyloric gland, intestinal, foveolar, and biliary types based on the morphology of the epithelium (O’Shea et al, 2002). Pyloric gland adenoma (tubular adenoma, pyloric gland type) is by far the most common type and typically is found as an incidental polyp less than 1 cm composed of tightly packed, cytologically bland pyloric-type cells in routine cholecystectomy specimens (Albores-Saavedra et al, 2010). Exophytic gallbladder neoplasms composed of complex biliary-type epithelium with more marked atypia, a predominantly papillary architecture, and more diffuse involvement of the gallbladder mucosa are regarded as intracystic papillary neoplasms (Albores-Saavedra et al, 2010). Most have high-grade dysplasia, which should be graded on the basis of the most severe focus. These tumors have also been designated noninvasive papillary carcinoma when high-grade dysplasia is present (Albores-Saavedra et al, 2000a).
Adenomas are uncommon in the bile ducts, and most exophytic bile duct tumors are collectively designated intraductal papillary neoplasms, including cases previously reported as papillomatosis (Albores-Saavedra et al, 2010; Gouma et al, 1984; Sagar et al, 1993; Taguchi et al, 1993). Most intraductal papillary neoplasms of the bile ducts have high-grade dysplasia (see Fig. 47.7) and were previously designated noninvasive papillary carcinoma (Albores-Saavedra et al, 2000b). In contrast to dysplasia or BilIN, intraductal papillary neoplasms can be identified grossly as soft, polypoid tumors within the bile duct lumen. Most are relatively localized within a segment of the bile duct, although multicentric or diffuse involvement can occur, as in so-called papillomatosis (Gouma et al, 1984; Sagar et al, 1993; Taguchi et al, 1993). Cystic dilation of the bile ducts may occur (Zen et al, 2006), and some cases exhibit mucin hypersecretion (Shibahara et al, 2004). The epithelial cell types in intraductal papillary neoplasms include pancreatobiliary, gastric, intestinal, and oncocytic (Albores-Saavedra et al, 2010; Ji et al, 2008; Martin et al, 2002; Shibahara et al, 2004; Tanaka et al, 2009), similar to the spectrum of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. Some authors have referred to these tumors as biliary IPMNs (Abraham et al, 2003; Chen et al, 2001; Kim et al, 2000; Shibahara et al, 2004; Tamada et al, 2002). However, differences in the frequency and types of associated invasive carcinoma, as well as the morphology and staining, are significant (Klimstra, 2002). Accumulating data suggest regional geographic differences in the proportion of intraductal papillary neoplasms with the different cell types, perhaps related to varying etiologic factors in different parts of the world (Shibahara et al, 2004). In Asian patients, for example, an association of intraductal papillary neoplasms with Clonorchis infection has been reported (see Chapter 45; Jang et al, 2008).
Exophytic intraepithelial neoplasms of the biliary tree are biologically benign when no invasive carcinoma is present, so the detection of a component of invasive carcinoma is key to the pathologic evaluation of these tumors. Small gallbladder adenomas may display high-grade dysplasia, but invasive carcinomas are very rare. In contrast, intracystic papillary neoplasms of the gallbladder and intraductal papillary neoplasms of the bile ducts have associated invasive carcinomas in more than half of all cases (Choi et al, 2010; Paik et al, 2008; Shipov & Aizikov, 1992). Conversely, in some patients with invasive adenocarcinomas, a focal, residual, intraductal papillary neoplasm component is identifiable; in fact, many adenocarcinomas with a polypoid gross appearance represent this latter group. Intraductal/intracystic papillary neoplasms have an excellent prognosis if they can be completely resected, and patients with minimally invasive carcinoma also have a favorable prognosis (Albores-Saavedra et al, 2000b). Once there is a significant amount of invasive carcinoma, however, the prognosis approaches that of biliary adenocarcinomas that do not arise from papillary precursors. Most invasive carcinomas arising in intraductal papillary neoplasms are conventional tubular adenocarcinomas, similar to other bile duct carcinomas. Some cases have a mucinous (colloid) pattern, usually when the intraductal neoplasm is of the intestinal type. Undifferentiated carcinoma and high-grade neuroendocrine carcinoma have also been reported (Albores-Saavedra et al, 2010).
One lesion to be distinguished from intraductal papillary neoplasms of the bile ducts is biliary mucinous cystic neoplasm, also know as hepatobiliary cystadenoma (Albores-Saavedra et al, 2010; Devaney et al, 1994; Wheeler & Edmondson, 1985). Biliary mucinous cystic neoplasms are analogous to mucinous cystic neoplasms of the pancreas and are also regarded as a type of mass-forming preinvasive neoplasm. They form multilocular cystic lesions that occur predominantly in adult women and exhibit pathognomonic hormone receptor–expressing ovarian-type subepithelial stroma (Grayson et al, 1996). The lining epithelium is composed of cuboidal to columnar cells, sometimes with abundant apical mucin. Papillary projections may be identified in the cyst lumen. Although most biliary mucinous cystic neoplasms show benign cytoarchitectural features, some may harbor foci of high-grade dysplasia or invasive carcinoma. Carcinoma can be focal; for this reason, thorough histologic examination is warranted.
Neuroendocrine Neoplasms
Carcinoid tumors (Fig. 47.13), also known as well-differentiated neuroendocrine tumors, may occur in any part of the biliary tract but tend to be more common in the gallbladder and CBD (Barron-Rodriguez et al, 1991; Ferrone et al, 2007; Komminoth et al, 2010; Maitra et al, 2000; Modlin & Sandor, 1997). They are seen predominantly in young or middle-aged adults. Most cases are nonfunctioning and patients present with signs and symptoms of biliary obstruction. Rare examples may be associated with von Hippel–Lindau syndrome (Sinkre et al, 2001). Grossly, carcinoid tumors form relatively well-demarcated nodules and may be polypoid and mucosa covered. They are fleshy, firm, and homogeneous on cut sections. Microscopically, carcinoid tumors are characterized by distinct nests separated by a fibrovascular stroma and strands of cells with round, uniform nuclei, coarsely granular chromatin, and abundant cytoplasm. Rarely, the cytoplasm may exhibit clear-cell, oncocytic, or signet ring–like changes, and a goblet cell variant has also been described. In general, carcinoid tumors are low-grade malignancies with an indolent clinical course. Rare carcinoid tumors mixed with adenocarcinoma (composite adenocarcinoma-carcinoid; Olinici & Vasiu, 1991) behave more like standard adenocarcinomas.
Focal neuroendocrine differentiation and carcinoid-like patterns may be seen in other neoplasms of the biliary tract that should not be classified as carcinoids. Rarely, paraganglioma (Caceres et al, 2001), a tumor that also displays neuroendocrine differentiation, may occur in the biliary tract.
Other Tumors
Mesenchymal Tumors
Among the extremely rare mesenchymal tumors of the biliary tree (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003), the one that warrants specific attention is embryonal (botyroid) rhabdomyosarcoma (Davis et al, 1969; Lack et al, 1981) because it is relatively more common and well characterized in this region. These are predominantly seen in children 3 to 4 years old and constitute 1% of all rhabdomyosarcomas. The tumor consists of a conglomerate of soft, mucosa-covered polyps filling the lumen. Sizes range from 3 to 14 cm, and the most common site is the CBD. Beneath the surface layer of flattened biliary epithelium is a dense zone of primitive spindle cells, representing the cambium layer. Cytoplasmic cross-striations may be seen, and skeletal muscle differentiation is demonstrable by immunohistochemical staining for actin, desmin, or myoD1. Although the prognosis is poor, multimodal therapy has resulted in long-term survival in some cases. Metastases occur in 40% of cases, but death usually is caused by the local effects of the tumor.
Granular cell tumor (Fig. 47.14) is another mesenchymal tumor that occurs in this region (Eisen et al, 1991), predominantly in the CBD. It is characterized by cells containing abundant acidophilic granular cytoplasm with occasional larger globules. This tumor is of uncertain origin, but most evidence, including expression of S-100 protein, points toward a relationship to Schwann cells. Although it has an infiltrative appearance, it is a benign tumor with only minimal recurrence potential, even when incompletely excised.
A variety of other mesenchymal tumors have been documented in the biliary tree, both benign (neurofibroma, hemangioma, lipoma, lymphangioma, leiomyoma, ganglioneuroma, myofibroblastic tumors) and malignant (peripheral nerve sheath tumor, leiomyosarcoma, Kaposi sarcoma, angiosarcoma, fibrous histiocytoma, etc.), mostly as case reports (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). Before a case is classified as sarcoma, the possibility of a sarcomatoid carcinoma must be carefully considered.
Secondary Tumors
The biliary tree may be involved by a variety of carcinomas originating in other organs—especially the pancreas, stomach, colon, kidney, and breast—either by metastasis or direct invasion (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). Among these, metastatic renal cell carcinoma is notorious for mimicking a primary tumor because it may form a polypoid lesion, and the history of the primary tumor may be too remote to be appreciated in the preoperative clinical evaluation. Similarly, melanomas (Fig. 47.15) may also form polypoid lesions and mimic primary tumors, and often the history of melanoma may not be apparent. Metastases from primary colorectal cancer to the biliary epithelium have also been described (Povoski et al, 2000).
Hematopoietic Malignancies
The biliary tree may be involved by hematopoietic malignancies (lymphoma, myeloma, or leukemia) as a part of systemic disease; rarely, this may be the initial presentation (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). Primary tumors of the mucosa-associated lymphoid tissue type also have been reported.
Tumorlike Lesions
In addition to sclerosing cholangitis, a few other nonneoplastic conditions of the biliary tract may present as tumors (see Chapter 42A, Chapter 42B, Chapter 48 ). Rarely, heterotopic tissue, especially pancreatic tissue (Inceoglu et al, 1993), may form a mass. Traumatic, or “amputation,” neuroma (Sano et al, 1985), which is an exuberant regenerative proliferation of transected nerves, may form a tumor-like nodule, typically in the cystic duct stump, that may mimic a carcinoma. These are very rare and may be seen with obstruction-related signs and symptoms, sometimes several years after the intervention.
Certain types of tumorlike lesions occur rather frequently in the gallbladder but not often in the remainder of the biliary tree. Nonneoplastic polyps of various kinds may be seen, including cholesterolosis, lymphoid polyps, inflammatory (fibrous, granulation tissue) polyps, and hamartomatous polyps (Albores-Saavedra et al, 1993). Cystic change in Rokitansky-Aschoff sinuses and adenomyomas (Levesque et al, 1964) also commonly form pseudotumors in the gallbladder; however, these are not seen in the bile ducts.
Autoimmune sclerosing disorders related to autoimmune pancreatitis may affect the bile ducts and gallbladder. As in the pancreas, these are characterized by a dense, subepithelial lymphoplasmacytic inflammatory infiltrate; dense fibrosis, often with a storiform pattern; and obliterative venulitis. There is an association with elevated levels of immunoglobulin G4 in the serum, and immunoglobulin G4–expressing plasma cells can be found in large numbers within the lesions by immunohistochemistry (Kamisawa & Okamoto, 2008). These processes form a tumorlike mass that can be mistaken for carcinoma on imaging studies (Corvera et al, 2005). Some cases are associated with autoimmune pancreatitis (Wang et al, 2009), whereas others present with disease limited to the gallbladder or bile ducts, sometimes called autoimmune cholangitis (Deshpande et al, 2009).
Abraham SC, et al. Molecular and immunohistochemical analysis of intraductal papillary neoplasms of the biliary tract. Hum Pathol. 2003;34(9):902-910.
Adsay NV. Gallbladder, extrahepatic biliary tree and ampulla. In: Mills, SE, et al. Sternberg’s Diagnostic Surgical Pathology. Philadelphia: Lippincott, Williams & Wilkins; 2004:1775-1829.
Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of the gallbladder, extrahepatic bile ducts and ampulla of Vater. In: Rosai, J, editor. Atlas of Tumor Pathology. Washington DC: Armed Forces Institute of Pathology, 2000.
Albores-Saavedra J, Molberg K, Henson DE. Unusual malignant epithelial tumors of the gallbladder. Semin Diagn Pathol. 1996;13:326-338.
Albores-Saavedra J, Nadji M, Henson DE. Intestinal-type adenocarcinoma of the gallbladder: a clinicopathologic and immunohistochemical study of seven cases. Am J Surg Pathol. 1986;10:19-25.
Albores-Saavedra J, Vardaman CJ, Vuitch F. Non-neoplastic polypoid lesions and adenomas of the gallbladder, Path Annu Rev, 1993.
Albores-Saavedra J, et al. The precursor lesions of invasive gallbladder carcinoma: hyperplasia, atypical hyperplasia and carcinoma in situ. Cancer. 1980;45:919-927.
Albores-Saavedra J, et al. Noninvasive and minimally invasive papillary carcinomas of the extrahepatic bile ducts. Cancer. 2000;89(3):508-515.
Albores-Saavedra J, et al. Tumors of the gallbladder and extrahepatic bile ducts. In: Hamilton, SR, Aaltonen, LA. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Digestive System. Lyon: IARC Press, 2000.
Albores-Saavedra J, et al. Carcinoma of the gallbladder and extrahepatic bile ducts. In: Bosman, FT, et al. WHO Classification of Tumors of the Digestive System. Lyon: International Agency for Research on Cancer; 2010:266-273.
Appelman HD, Coopersmith N. Pleomorphic spindle-cell carcinoma of the gallbladder: relation to sarcoma of the gallbladder. Cancer. 1970;25:535-541.
Argani P, et al. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer. 2001;91(7):1332-1341.
Barron-Rodriguez LP, et al. Carcinoid tumor of the common bile duct: evidence for its origin in metaplastic endocrine cells. Am J Gastroenterol. 1991;86(8):1073-1076.
Bhuiya MR, et al. Clinicopathologic factors influencing survival of patients with bile duct carcinoma: multivariate statistical analysis. World J Surg. 1993;17(5):653-657.
Bivins BA, Meeker WR, Griffen WOJr. Importance of histologic classification of carcinoma of the gallbladder. Am Surg. 1975;41(3):121-124.
Bosma A. Surgical pathology of cholangiocarcinoma of the liver hilus (Klatskin tumor). Semin Liver Dis. 1990;10:85-90.
Caceres M, et al. Paraganglioma of the bile duct. Southern Med J. 2001;94(5):515-518.
Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:175-190.
Chan KW. Review of 253 cases of significant pathology in 7,910 cholecystectomies in Hong Kong. Pathology. 1988;20(1):20-23.
Chen TC, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34(4 Pt 1):651-658.
Choi SC, et al. The clinicopathological features of biliary intraductal papillary neoplasms according to the location of tumors. J Gastroenterol Hepatol. 2010;25(4):725-730.
Corvera CU, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201(6):862-869.
Davis GL, Kissane JM, Ishak KG. Embryonal rhabdomyosarcoma (sarcoma botryoides) of the biliary tree: report of five cases and a review of the literature. Cancer. 1969;24(2):333-342.
Deshpande V, et al. IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod Pathol. 2009;22(10):1287-1295.
Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma: a light microscope and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078-1091.
Eisen RN, Kirby WM, O’Quinn JL. Granular cell tumor of the biliary tree: a report of two cases and a review of the literature. Am J Surg Pathol. 1991;15:460-465.
Ferrone CR, et al. Extrahepatic bile duct carcinoid tumors: malignant biliary obstruction with a good prognosis. J Am Coll Surg. 2007;205(2):357-361.
Frierson HFJr. The gross anatomy and histology of the gallbladder, extrahepatic bile ducts, Vaterian system, and minor papilla. Am J Surg Pathol. 1989;13(2):146-162.
Frierson HF. Gallbladder and Extrahepatic Biliary Tree: Histology for Pathologists. New York: Raven Press; 1997. pp 593-611
Gouma DJ, et al. Intrahepatic biliary papillomatosis. Br J Surg. 1984;71(1):72-74.
Grayson W, et al. Immunohistochemical demonstration of progesterone receptor in hepatobiliary cystadenoma with mesenchymal stroma. Histopathology. 1996;29(5):461-463.
Greene FL, et al. AJCC Cancer Staging Manual. New York: Springer-Verlag, 2002.
Haratake J, et al. Giant cell tumor-like cholangiocarcinoma associated with systemic cholelithiasis. Cancer. 1992;69(10):2444-2448.
Hayashi S, et al. Invasive growth patterns of hepatic hilar ductal carcinoma: a histologic analysis of 18 surgical cases. Cancer. 1994;73:2922-2929.
Herzog K, Goldblum JR. Gallbladder adenocarcinoma, acalculous chronic lymphoplasmacytic cholecystitis, ulcerative colitis. Mod Pathol. 1996;9:194-198.
Hong SM, et al. Superficial vs deep pancreatic parenchymal invasion in the extrahepatic bile duct carcinomas: a significant prognostic factor. Mod Pathol. 2005;18(7):969-975.
Hong SM, et al. Measurement of the invasion depth of extrahepatic bile duct carcinoma: an alternative method overcoming the current T classification problems of the AJCC staging system. Am J Surg Pathol. 2007;31(2):199-206.
Husek K. Anaplastic osteoclastic carcinoma of the gallbladder [in Czeck]. Cesk Patol. 1990;26(3):138-141.
Inceoglu R, et al. An unusual cause of hydropic gallbladder and biliary colic—heterotopic pancreatic tissue in the cystic duct: report of a case and review of the literature. Surg Today. 1993;23(6):532-534.
Ito M, et al. Osteoclast-like giant cell tumour of the gallbladder. Virchows Arch A Pathol Anat Histopathol. 1992;420(4):359-366.
Jang KT, et al. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008;453(6):589-598.
Ji Y, et al. Intraductal papillary neoplasms of bile duct: a distinct entity like its counterpart in pancreas. Histol Histopathol. 2008;23(1):41-50.
Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008;14(25):3948-3955.
Kim HJ, et al. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32(5):389-393.
Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: an unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241-256.
Klimstra DS. Comparative pathology of biliary, ampullary, and pancreatic neoplasms. Histopathology. 2002;41:267-273.
Komminoth P, et al. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. In: Bosman, FT, et al. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer; 2010:247-276.
Lack EE. Gallbladder and extrahepatic biliary tract. In: Lack, EE, Albores-Saavedra, J. Pathology of the Pancreas, Gallbladder, Extrahepatic Biliary Tract, and Ampullary Region. New York: Oxford University Press; 2003:3-391.
Lack EE, Perez-Atayde AR, Schuster SR. Botryoid rhabdomyosarcoma of the biliary tract. Am J Surg Pathol. 1981;5(7):643-652.
Laitio M. Histogenesis of epithelial neoplasms of the gallbladder. I. Dysplasia. Pathol Res Pract. 1983;178:51-56.
Levesque HP, et al. Adenoma of the gallbladder. Anatomic and radiological findings [in French]. Union Med Can. 1964;93:1487-1506.
Ludwig J. Surgical pathology of the syndrome of primary sclerosing cholangitis. Am J Surg Pathol. 1989;13(1):43-49.
Ludwig T, et al. Papillary bile duct dysplasia in primary sclerosing cholangitis. Gastroenterology. 1992;102:2134-2138.
Maitra A, et al. Carcinoid tumors of the extrahepatic bile ducts: a study of seven cases. Am J Surg Pathol. 2000;24(11):1501-1510.
Maitra A, et al. Small cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and molecular pathology study of 12 cases. Am J Surg Pathol. 2001;25(5):595-601.
Martin RC, et al. Hepatic intraductal oncocytic papillary carcinoma. Cancer. 2002;95(10):2180-2187.
Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79(4):813-829.
Nishihara K, Tsuneyoshi M. Undifferentiated spindle cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and flow cytometric study of 11 cases. Hum Pathol. 1993;24:1298-1305.
Nishihara K, et al. Adenosquamous carcinoma of the gallbladder: a clinicopathological, immunohistochemical and flow-cytometric study of twenty cases. Jpn J Cancer Res. 1994;85(4):389-399.
Ojeda VJ, Shilkin KB, Walters MNI. Premalignant epithelial lesions of the gallbladder: a prospective study of 120 cholecystectomy specimens. Pathology. 1985;17:451-454.
Olinici CD, Vasiu R. Composite endocrine cell, typical adenocarcinoma and signet ring carcinoma of the gallbladder. Rom J Morphol Embryol. 1991;37(3-4):171-173.
O’Shea M, Fletcher HS, Lara JF. Villous adenoma of the extrahepatic biliary tract: a rare entity. Am Surg. 2002;68(10):889-891.
Paik KY, et al. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97(6):508-512.
Papotti M, et al. Large cell neuroendocrine carcinoma of the gallbladder: report of two cases. Am J Surg Pathol. 2000;24(10):1424-1428.
Parkin DM, et al. Cholangiocarcinoma: epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev. 1993;2(6):537-544.
Povoski SP, et al. Recognition of intrabiliary hepatic metastases from colorectal adenocarcinoma. HPB Surg. 2000;11(6):383-390.
Rashid A, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8(10):3156-3163.
Sagar PM, Omar M, Macrie J. Extrahepatic biliary papillomatosis occurring after removal of a dysplastic gallbladder. HPB Surg. 1993;6(3):219-221.
Sano T, et al. Polypoid traumatic neuroma of the gallbladder. Arch Pathol Lab Med. 1985;109(6):574-576.
Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol. 2000;95(6):1402-1410.
Shibahara H, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28(3):327-338.
Shipov AA, Aizikov GS. Physical state, equilibrium function and vestibulo-motor reflexes in the rats after space flights on board biosatellites of Cosmos series. Physiologist. 1992;35(1 Suppl):S206-S207.
Sinkre PA, et al. Clear cell carcinoid tumor of the gallbladder: another distinctive manifestation of von Hippel–Lindau disease. Am J Surg Pathol. 2001;25(10):1334-1339.
Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129(6):699-703.
Suster S, et al. Adenosquamous carcinoma of the gallbladder with spindle cell features: a light microscopic and immunocytochemical study of a case. Histopathology. 1987;11(2):209-214.
Suzuki M, et al. The development and extension of hepatohilar bile duct carcinoma: a three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphics computer system. Cancer. 1989;64:658-666.
Taguchi J, et al. Intrahepatic and extrahepatic biliary papillomatosis. Arch Pathol Lab Med. 1993;117:944-947.
Tamada S, et al. Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int. 2002;52(11):713-723.
Tanaka M, et al. Intraductal oncocytic papillary neoplasm of the bile duct: clinicopathologic and immunohistochemical characteristics of 6 cases. Hum Pathol. 2009;40(11):1543-1552.
Todoroki T, et al. Gross appearance of carcinoma of the main hepatic duct and its prognosis. Surg Gynecol Obstet. 1980;150(1):33-40.
Tompkins RK, et al. Prognosis factors in bile duct carcinoma: analysis of 96 cases. Ann Surg. 1981;194:447-457.
van der Wal AC, van Leeuwen DJ, Walford. Small cell neuroendocrine (oat cell) tumour of the common bile duct. Histopathology. 1990;16:398-400.
Van Heerden JA, Judd ES, Dockerty MB. Carcinoma of the extrahepatic bile ducts: a clinicopathologic study. Am J Surg. 1967;113(1):49-56.
Vardaman CJ, Albores-Saavedra. Clear cell carcinomas of the gallbladder and extrahepatic bile ducts. Am J Surg Pathol. 1995;19(1):91-99.
Wang WL, et al. Autoimmune pancreatitis–related cholecystitis: a morphologically and immunologically distinctive form of lymphoplasmacytic sclerosing cholecystitis. Histopathology. 2009;54(7):829-836.
Weber SM, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384-391.
Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139:217-238.
Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts: a clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56(6):1434-1445.
Wu TT, et al. Biliary intraepithelial neoplasia in patients without chronic biliary disease: analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer. 2009;115(19):4564-4575.
Yamagiwa H. Mucosal dysplasia of the gallbladder: isolated and adjacent lesions to carcinoma. Jpn J Cancer Res. 1989;80:238-243.
Young RH, Hart WR. Metastases from carcinomas of the pancreas simulating primary mucinous tumors of the ovary: a report of seven cases. Am J Surg Pathol. 1989;13(9):748-756.
Zen Y, et al. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19(9):1243-1254.
Zen Y, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20(6):701-709.