Tumor Lysis Syndrome
• Tumor lysis syndrome can occur in any patient with newly diagnosed or relapsed cancer, and thus all patients should undergo risk stratification and management according to their risk for clinical tumor lysis syndrome.
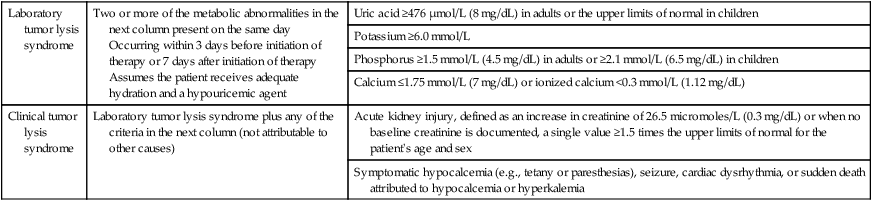
• Laboratory tumor lysis syndrome is defined as the presence of two or more of the following abnormalities present on the same day: hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia as a result of hyperphosphatemia.
• Clinical tumor lysis syndrome is defined as laboratory tumor lysis syndrome plus acute kidney injury, symptomatic hyperkalemia, or symptomatic hypocalcemia, and it should be prevented whenever possible.
• The incidence of clinical tumor lysis syndrome depends on the number of risk factors present at presentation and on the management of patients potentially at risk.
• Risk factors for clinical tumor lysis syndrome include a large cancer mass, high cell lysis potential (chemosensitivity), and patient factors (e.g., preexisting nephropathy, dehydration, acidosis, hypotension, and nephrotoxin exposure).
• Management depends on the risk of the development of clinical tumor lysis syndrome:
 Negligible risk—no prophylaxis, no monitoring
Negligible risk—no prophylaxis, no monitoring
 Low risk (1% risk for clinical tumor lysis syndrome)—hyperhydration, allopurinol, and daily laboratory evaluation
Low risk (1% risk for clinical tumor lysis syndrome)—hyperhydration, allopurinol, and daily laboratory evaluation
 Intermediate risk—hyperhydration, rasburicase, inpatient monitoring, and laboratory evaluation every 8 to 12 hours
Intermediate risk—hyperhydration, rasburicase, inpatient monitoring, and laboratory evaluation every 8 to 12 hours
 High risk—hyperhydration, rasburicase, cardiac monitoring on the inpatient ward, laboratory evaluation every 6 to 8 hours, and rapid access to hemodialysis
High risk—hyperhydration, rasburicase, cardiac monitoring on the inpatient ward, laboratory evaluation every 6 to 8 hours, and rapid access to hemodialysis
 Established clinical tumor lysis syndrome at presentation—hyperhydration, rasburicase, cardiac monitoring in the intensive care unit, laboratory evaluation every 4 to 6 hours, and rapid access to hemodialysis
Established clinical tumor lysis syndrome at presentation—hyperhydration, rasburicase, cardiac monitoring in the intensive care unit, laboratory evaluation every 4 to 6 hours, and rapid access to hemodialysis
Epidemiology and Definition
Tumor lysis syndrome is a potentially fatal metabolic condition that occurs in patients with rapidly proliferating, bulky, or chemosensitive tumors (Table 38-1).1 It has been most commonly reported in patients with high-grade non-Hodgkin lymphomas (NHL) and acute leukemias, but it can occur in persons with virtually any type of cancer when a large cancer cell mass is present and the cancer is sensitive to initial therapy.
Table 38-1
Modified Cairo-Bishop Classification of Tumor Lysis Syndrome
| Laboratory tumor lysis syndrome | Two or more of the metabolic abnormalities in the next column present on the same day Occurring within 3 days before initiation of therapy or 7 days after initiation of therapy Assumes the patient receives adequate hydration and a hypouricemic agent |
Uric acid ≥476 µmol/L (8 mg/dL) in adults or the upper limits of normal in children |
| Potassium ≥6.0 mmol/L | ||
| Phosphorus ≥1.5 mmol/L (4.5 mg/dL) in adults or ≥2.1 mmol/L (6.5 mg/dL) in children | ||
| Calcium ≤1.75 mmol/L (7 mg/dL) or ionized calcium <0.3 mmol/L (1.12 mg/dL) | ||
| Clinical tumor lysis syndrome | Laboratory tumor lysis syndrome plus any of the criteria in the next column (not attributable to other causes) | Acute kidney injury, defined as an increase in creatinine of 26.5 micromoles/L (0.3 mg/dL) or when no baseline creatinine is documented, a single value ≥1.5 times the upper limits of normal for the patient’s age and sex |
| Symptomatic hypocalcemia (e.g., tetany or paresthesias), seizure, cardiac dysrhythmia, or sudden death attributed to hypocalcemia or hyperkalemia |
Data from reference 1.
Etiology and Pathogenesis
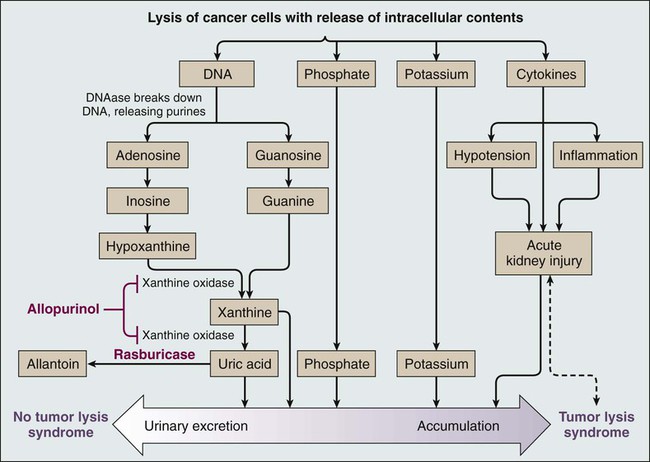
Tumor lysis syndrome occurs most commonly after treatment with cytotoxic therapy, but it can also occur spontaneously in patients with highly proliferative tumors. By releasing tumor cellular components into the bloodstream, tumor lysis syndrome results in metabolic abnormalities including hyperphosphatemia, hyperkalemia, hypocalcemia, hyperuricemia, and azotemia (Fig. 38-1). Acute kidney injury, seizures, cardiac arrhythmias, nausea, and vomiting may occur as a result of these metabolic abnormalities. To reduce morbidity and mortality, early diagnosis and identification of patients at risk for tumor lysis syndrome are of the utmost importance.1,2 The catabolism of nucleic acids ultimately results in the production of uric acid.3 Purines are first degraded into hypoxanthine, then xanthine, and finally into uric acid through the action of xanthine oxidase.4,5 Hyperuricemia leads to the deposition of uric acid crystals in the renal tubules because of the poor solubility of uric acid and can result in acute kidney injury.4 Hyperphosphatemia can also cause acute kidney injury. Phosphates combine with calcium, generating calcium phosphate salts that deposit in the renal tubules. The binding of calcium by phosphate leads to hypocalcemia, which in turn can cause vomiting, muscle cramps, tetany, paresthesias, seizures, and cardiac dysrhythmias.6 Hyperkalemia from cellular lysis can lead to cardiac dysrhythmias, ventricular tachycardia, fibrillations, or cardiac arrest.6
Risk Factors and Incidence of Tumor Lysis Syndrome
Several risk factors for tumor lysis syndrome have been identified, including tumor-related factors, individual patient characteristics, and the type of chemotherapy used.7 Certain tumor types have historically been associated with an increased risk for the development of tumor lysis syndrome, including Burkitt leukemia, acute lymphoblastic leukemia (ALL), acute myeloid leukemia (especially those with inv(16) chromosomal translocation), and NHL (particularly Burkitt lymphoma)10–10; patients with these tumor types were the most frequently enrolled in compassionate-use trials of hypouricemic agents.11,12 However, tumor lysis syndrome can also develop in patients with chronic lymphocytic leukemia (CLL) and chronic myelogenous leukemia.13 Acute leukemias and high-grade lymphomas often have a high proliferative rate, a large tumor burden, and are particularly sensitive to chemotherapy; the combination of high tumor bulk and sensitivity to therapy make tumor lysis syndrome more likely.14 The high nucleic acid, phosphorus content, and increased activity of purine metabolism in these tumors predispose patients to tumor lysis syndrome.7 In a study of 102 patients with high-grade NHL who received prophylactic hyperhydration and allopurinol, the incidence of clinically significant tumor lysis syndrome was 6%, and one patient required dialysis.15 A retrospective study of 755 patients with NHL or acute leukemia who received rasburicase for prevention or treatment of tumor lysis syndrome documented a 5.3% incidence of tumor lysis syndrome (including both laboratory and clinical tumor lysis syndrome).16 Seven patients in this study died from tumor lysis syndrome (0.9% of all patients and 17.5% of patients with tumor lysis syndrome).16,17 In a multinational study of 235 pediatric patients with advanced-stage, B-cell NHL who were treated with the same chemotherapy regimen, 27% and 15% of U.S. patients (who all received allopurinol) experienced tumor lysis syndrome and required dialysis, respectively, whereas 11% and 3% of French patients (who all received rasburicase) experienced tumor lysis syndrome and required dialysis, respectively.18 Hence the risk for tumor lysis syndrome depends not only on tumor-related risk factors but also on the supportive care used (Fig. 38-1).
In addition to tumor-related risk factors for tumor lysis syndrome, patient-related factors affect tumor lysis syndrome risk, including leukocytosis, hyperuricemia, elevated serum lactate dehydrogenase, elevated serum creatinine, renal insufficiency, dehydration, acidic urine, and decreased urinary flow at the time of presentation.7,14 Finally, specific cytotoxic agents have been associated with tumor lysis syndrome in particular diseases, such as fludarabine and lenalidomide in patients treated for CLL.7,13,19,20 Because the risk of clinical tumor lysis syndrome is proportional to the rapidity of response to therapy, patients whose tumors respond quickly to a new therapeutic agent have an increased risk for the development of tumor lysis syndrome. Patients with diagnoses not typically associated with tumor lysis syndrome (e.g., metastatic colon cancer) can also be affected by tumor lysis syndrome when the treatment includes new, highly active agents, such as cetuximab.21 To successfully treat and prevent tumor lysis syndrome, it is necessary to identify patients at risk and tailor prevention according to risk, as described in the next section.6
Prevention and Management of Tumor Lysis Syndrome
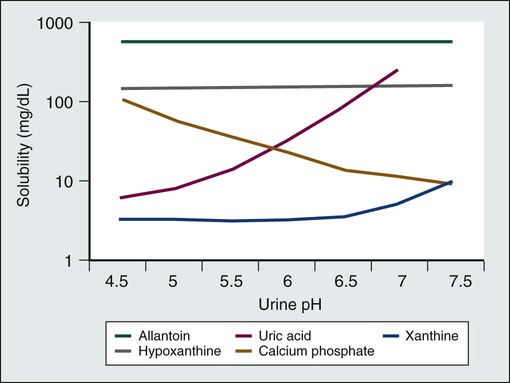
Published guidelines for the diagnosis, prevention, and management of tumor lysis syndrome (Table 38-2) differ in many details. However, all guidelines agree that patients at risk for tumor lysis syndrome should undergo risk stratification and management on the basis of their risk. Clinical tumor lysis syndrome is the outcome that should be prevented, because by definition it is associated with morbidity. Standard prophylaxis includes close monitoring, hyperhydration to increase urine output and facilitate renal excretion of uric acid and phosphorus, and administration of a hypouricemic agent, such as allopurinol or rasburicase, to prevent the formation of uric acid crystals in the kidney, as well as administration of phosphate binders to reduce calcium phosphate precipitation in the kidneys.1,2,4,22 Both allopurinol and rasburicase have been used successfully to reduce the incidence of tumor lysis syndrome in pediatric and adult patients.1,23 Historically, urine has been alkalinized by the administration of bicarbonate to improve the solubility of uric acid (Fig. 38-2)21,24; however, this step is not currently recommended, because the solubility of calcium phosphate decreases at higher pH values (Fig. 38-2), and therefore precipitation in the renal tubules may be exacerbated. As an alternative to urine alkalinization, the prophylactic administration of rasburicase has been shown to effectively reduce uric acid levels.7
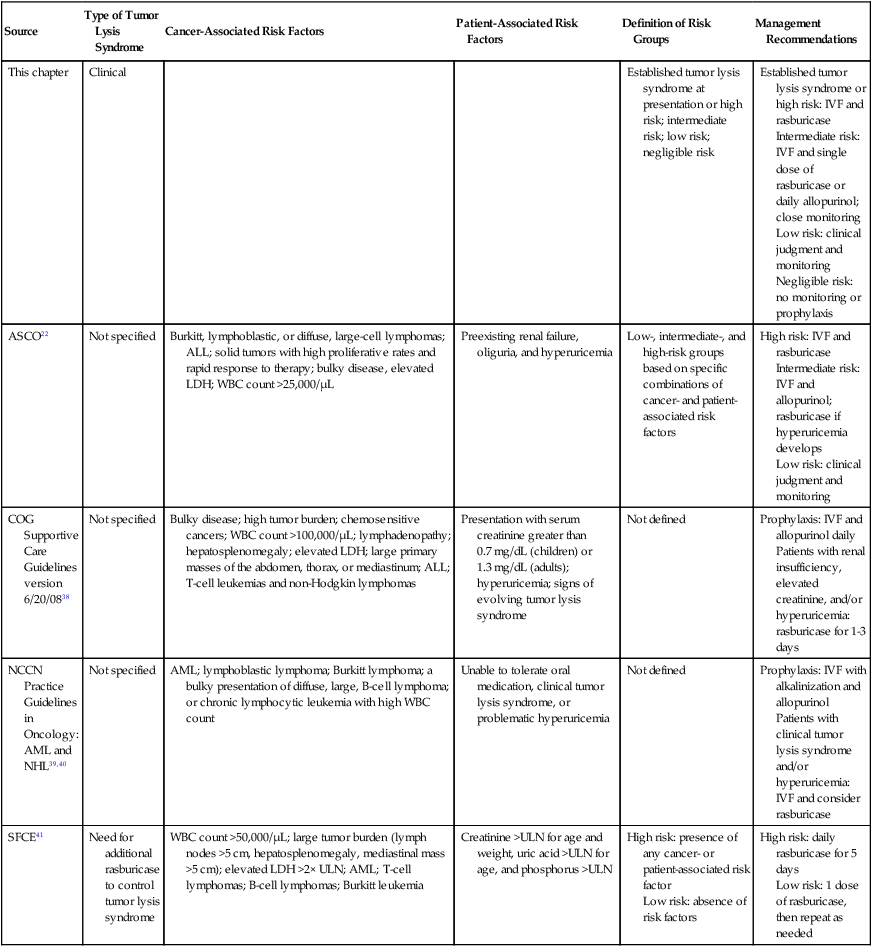
Table 38-2
Published Guidelines for Risk Classification and Management of Tumor Lysis Syndrome
| Source | Type of Tumor Lysis Syndrome | Cancer-Associated Risk Factors | Patient-Associated Risk Factors | Definition of Risk Groups | Management Recommendations |
| This chapter | Clinical | Established tumor lysis syndrome at presentation or high risk; intermediate risk; low risk; negligible risk | Established tumor lysis syndrome or high risk: IVF and rasburicase Intermediate risk: IVF and single dose of rasburicase or daily allopurinol; close monitoring Low risk: clinical judgment and monitoring Negligible risk: no monitoring or prophylaxis |
||
| ASCO22 | Not specified | Burkitt, lymphoblastic, or diffuse, large-cell lymphomas; ALL; solid tumors with high proliferative rates and rapid response to therapy; bulky disease, elevated LDH; WBC count >25,000/µL | Preexisting renal failure, oliguria, and hyperuricemia | Low-, intermediate-, and high-risk groups based on specific combinations of cancer- and patient-associated risk factors | High risk: IVF and rasburicase Intermediate risk: IVF and allopurinol; rasburicase if hyperuricemia develops Low risk: clinical judgment and monitoring |
| COG Supportive Care Guidelines version 6/20/0838 | Not specified | Bulky disease; high tumor burden; chemosensitive cancers; WBC count >100,000/µL; lymphadenopathy; hepatosplenomegaly; elevated LDH; large primary masses of the abdomen, thorax, or mediastinum; ALL; T-cell leukemias and non-Hodgkin lymphomas | Presentation with serum creatinine greater than 0.7 mg/dL (children) or 1.3 mg/dL (adults); hyperuricemia; signs of evolving tumor lysis syndrome | Not defined | Prophylaxis: IVF and allopurinol daily Patients with renal insufficiency, elevated creatinine, and/or hyperuricemia: rasburicase for 1-3 days |
| NCCN Practice Guidelines in Oncology: AML and NHL39,40 | Not specified | AML; lymphoblastic lymphoma; Burkitt lymphoma; a bulky presentation of diffuse, large, B-cell lymphoma; or chronic lymphocytic leukemia with high WBC count | Unable to tolerate oral medication, clinical tumor lysis syndrome, or problematic hyperuricemia | Not defined | Prophylaxis: IVF with alkalinization and allopurinol Patients with clinical tumor lysis syndrome and/or hyperuricemia: IVF and consider rasburicase |
| SFCE41 | Need for additional rasburicase to control tumor lysis syndrome | WBC count >50,000/µL; large tumor burden (lymph nodes >5 cm, hepatosplenomegaly, mediastinal mass >5 cm); elevated LDH >2× ULN; AML; T-cell lymphomas; B-cell lymphomas; Burkitt leukemia | Creatinine >ULN for age and weight, uric acid >ULN for age, and phosphorus >ULN | High risk: presence of any cancer- or patient-associated risk factor Low risk: absence of risk factors |
High risk: daily rasburicase for 5 days Low risk: 1 dose of rasburicase, then repeat as needed |
All patients at intermediate or high risk for clinical tumor lysis syndrome should receive hyperhydration with intravenous fluids at 2500 mL/m2/day or higher with a goal of very high urine output to reduce the risk of uric acid and calcium-phosphate precipitation in renal tubules. If urine output is inadequate even after hyperhydration, a diuretic may be added to increase urine output, but only after the patient is very well hydrated.1 Patients at intermediate or high risk should be monitored in the hospital with serum electrolytes and uric measurement every 8 to 12 hours (intermediate risk) or 4 to 6 hours (high risk). Patients who present with tumor lysis syndrome should be connected to a cardiac monitor and observed in an intensive care or step-down unit.1
Allopurinol
Allopurinol inhibits xanthine oxidase and prevents metabolism of hypoxanthine and xanthine into uric acid, thus reducing the formation of uric acid and the incidence of tumor lysis syndrome when used prior to the initiation of chemotherapy (Fig. 38-3).4,25 However, allopurinol has several limitations. Allopurinol therapy is most effective when it is used 24 to 48 hours before the start of chemotherapy, which may cause a delay in the initiation of cytotoxic therapy.22 Furthermore, it does not remove existing uric acid, which is a significant problem in patients who present with significant hyperuricemia. Finally, treatment with allopurinol increases xanthine concentrations in the urine, which can cause xanthine crystal formation and nephropathy.26 Hande and colleagues27 examined purine excretion in 11 patients with bulky lymphomas. All patients received allopurinol 2 to 5 days before chemotherapy. Although urinary concentrations of uric acid and hypoxanthine remained below solubility in all patients, concentrations of xanthine exceeded solubility in 6 patients (55%). Purine metabolism was studied in 19 children with ALL who received allopurinol for the prevention of tumor lysis syndrome before starting chemotherapy.28 Xanthine exceeded urine solubility in 16 patients (84%). Because xanthine is rarely measured, its contribution to tumor lysis syndrome remains unknown,29 but its low solubility at all urine pH values and high concentrations in the urine of patients treated with allopurinol suggest that xanthine nephropathy may occur commonly in patients with a high cancer cell mass, who produce large amounts of purines after initiation of chemotherapy.
Rasburicase
Rasburicase converts uric acid into allantoin, a highly soluble metabolite that is excreted by the kidneys (see the light green line in Fig. 38-2). Unlike most other mammals, humans lack a functional urate oxidase enzyme because of a nonsense mutation in the genetic sequence. Rasburicase lowers serum uric acid levels quickly and has few adverse effects (Fig. 38-1).1,23,30 However, rasburicase is contraindicated in patients with glucose-6-phosphate dehydrogenase deficiency because of the high risk of the development of methemoglobinemia and hemolytic anemia after receiving these agents, because of the breakdown of uric acid into hydrogen peroxide.1
In an effort to reduce costs, some clinicians administer lower doses of rasburicase than the 0.15 to 0.2 mg/kg approved by the U.S. Food and Drug Administration (FDA), in the hope that the lower dose will effectively prevent or manage tumor lysis syndrome. Although no clinical trial results have validated the efficacy of this approach, several retrospective studies and case reports have been published in which lower doses of rasburicase were used successfully. Lee and colleagues31 used a 4.5-mg fixed dose to treat three children with ALL. All three patients had a rapid reduction in uric acid, but when the weight-adjusted dose was determined for the 4.5-mg dose, it was found that one patient received a higher quantity than the FDA-approved dose (0.26 mg/kg), one patient received the approved dose (0.17 mg/kg); and one patient received 50% of the approved dose (0.08 mg/kg). Another case series examined 11 adults with hematologic malignancies at risk for tumor lysis syndrome.32 Eight patients had renal impairment as a result of tumor lysis syndrome at the time of presentation, and all were treated with a 6-mg, single dose of rasburicase (corresponding to a median weight-based dose of 0.08 mg/kg). In 10 of the 11 patients, single-dose rasburicase lowered and maintained normal uric acid levels; the mean pretreatment uric acid level, 11.7 mg/dL, was reduced to 2.0 mg/dL after treatment. One morbidly obese patient required a second dose of rasburicase (12 mg; 0.046 mg/kg, based on actual body weight) to control his uric acid levels. Among the eight patients with renal impairment, three had a return to baseline renal function after receiving rasburicase, one required hemodialysis, and four had no subsequent renal function data reported. Although the authors concluded that use of a fixed, 6-mg dose of rasburicase appeared to be safe and effective, the fact that one patient required a large second dose and one required dialysis implies inadequate uric acid control, especially when compared with the 98% to 100% efficacy reported in large studies using the FDA-approved dose.11 Another report describes an obese woman with chronic myelomonocytic leukemia in blast crisis who was treated with 11 mg of rasburicase (a dose based on her ideal body weight), which adequately controlled her uric acid.33
Two retrospective studies of reduced-dose rasburicase have been reported recently.34,35 A fixed rasburicase dose of 3 mg, with repeated doses as needed based on subsequent uric acid levels, was administered to 43 adult patients undergoing stem cell transplantation (51%) or receiving chemotherapy (49%).34 The total doses of rasburicase administered were 3 mg (n = 37), 4.5 mg (n = 2), or 6 mg (n = 4), and uric acid levels were all within normal limits 48 hours after the first dose. Although three patients were already receiving dialysis at the time of the study, no additional patients required dialysis. What is not clear from this study is whether the patients were at risk for tumor lysis syndrome. Many patients undergoing stem cell transplantation already have a low bulk of disease and would not be expected to have tumor lysis syndrome, and the disease status of the patients in the study cohort was not described. The second cohort in which use of a reduced-dose of rasburicase was evaluated included 46 adults with hematologic cancers and four with solid tumors.35 Patients were eligible to receive rasburicase if they had bulky disease, an elevated white blood cell count, elevated lactate dehydrogenase in addition to elevated uric acid, or a history of tumor lysis syndrome after a prior course of chemotherapy. Rasburicase dosing was at the discretion of the treating clinician, and the initial dose ranged from 1.5 to 16.5 mg. Nine patients had uric acid levels above the normal range after the initial rasburicase dose, despite a mean decrease of 41% from their baseline levels. Because of the heterogeneous cohort of patients (diagnoses included ALL, acute myeloid leukemia, CLL, myeloma, solid tumors, and both high- and low-grade lymphomas) and the wide range of rasburicase doses used, it is difficult to derive specific treatment recommendations from the data presented. However, the results do suggest that using doses of rasburicase that are lower than recommended may be effective for some patients.
The use of reduced doses of rasburicase should be studied in defined cohorts of patients at an intermediate risk for the development of clinical tumor lysis syndrome to determine the optimal dose (i.e., the dose at which no patient experiences acute kidney injury or clinical tumor lysis syndrome and at which the least amount of rasburicase is used). In patients receiving low-dose rasburicase to prevent tumor lysis syndrome, serum uric acid levels must be measured precisely. Blood samples must be collected into chilled tubes, placed on ice immediately, and assayed promptly to avoid ex vivo breakdown of uric acid by rasburicase, which produces artificially low levels of uric acid.36
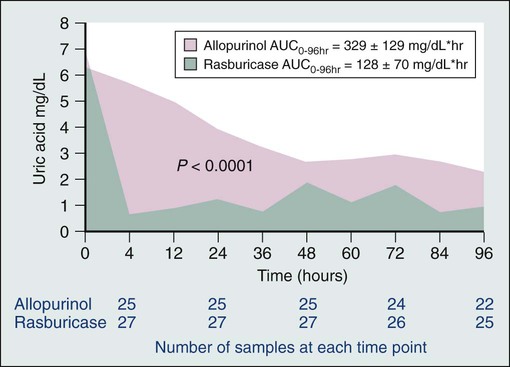
Although the optimal treatment for patients at intermediate risk for clinical tumor lysis syndrome has not been determined by randomized clinical trials, a prudent approach at present is the administration of one standard dose of rasburicase (0.15 mg/kg), especially if hyperuricemia or laboratory tumor lysis syndrome develops. Patients at high risk for the development of tumor lysis syndrome should receive at least one standard dose of rasburicase. Repeat doses of rasburicase should be given to patients with elevated uric acid levels, and all patients with laboratory tumor lysis syndrome should receive at least one standard dose of rasburicase (0.15 mg/kg) to prevent progression to clinical tumor lysis syndrome. Although this strategy has not been validated by a prospective randomized trial, the one randomized trial comparing rasburicase to allopurinol documented improved creatinine during the first 4 days of therapy in patients receiving rasburicase but not in patients receiving allopurinol.37