Testicular Cancer
Terence W. Friedlander, Charles J. Ryan, Eric J. Small and Frank Torti
• One percent of all male malignancies, accounting for 6000 to 8000 new cases a year in the United States
• Most common malignancy among men aged 15 to 35
• Germ cell tumors: 95% of all testicular cancers
• Pure seminoma: 40% of all germ cell tumors
• Nonseminoma: 60% of all germ cell tumors, with embryonal elements most frequent
• Testicular torsion, hydrocele, varicocele, spermatocele, epididymitis (can coexist with germ cell tumors)
• Other malignancies: lymphoma, metastases from prostate cancer, lung cancer, or melanoma (generally in older age group)
Diagnosis and Staging Evaluation
• Complete history and physical examination
• Bilateral testicular ultrasonography
• Tumor serum markers (lactate dehydrogenase [LDH], β-human chorionic gonadotropin [β-hCG], and α-fetoprotein [AFP])
• Complete blood count, chemistry studies including renal function
• Computed tomography (CT) of chest, abdomen, and pelvis
• Additional imaging studies as appropriate (e.g., imaging of brain in patient with pure choriocarcinoma)
• Radical (inguinal) orchiectomy (transscrotal biopsy or orchiectomy should be avoided)
• Localized disease is curable with orchiectomy alone. Both adjuvant chemotherapy and adjuvant low-dose radiotherapy to lymph nodes can reduce the risk of relapse.
• Locally advanced disease (stage IIA/IIB) is curable in more than 90% of patients with orchiectomy plus radiotherapy to involved nodal areas or with combination chemotherapy.
• Metastatic disease (stage III) or bulky locally advanced disease (stage IIB/IIC) is curable in 90% with combination chemotherapy
• Postchemotherapy retroperitoneal lymphadenectomy can prevent subsequent relapse in select patients
• Clinically localized disease is curable with orchiectomy alone in 60% to 87% of patients
• Risk of relapse is decreased with both retroperitoneal lymphadenectomy as well as with adjuvant combination chemotherapy, although in selected patients, surveillance without surgery is a possible option
• Adjuvant combination chemotherapy after lymphadenectomy may further decrease risk of relapse, but does not impact survival
• No bulky locally advanced (stage IIA) disease is cured with retroperitoneal lymphadenectomy alone in up to 65%. Adjuvant chemotherapy after surgery can reduce the risk of relapse for these patients.
• Moderate to bulky nodal disease (stage IIB/C) requires combination chemotherapy
• Metastatic disease (stage III), or bulky locally advanced disease is curable in 80% of patients with combination chemotherapy
• Postchemotherapy retroperitoneal lymphadenectomy can prevent subsequent relapse in selected patients
Effective Second- and Third-Line Therapies
• Chemotherapy salvages about 80% of patients who have failed surgical or radiation therapeutic treatments
• Second-line chemotherapy for patients who have failed prior chemotherapy curative in 20% of patients
• Subsequent high-dose therapy with autologous bone marrow transplant (ABMT)/peripheral stem cell transplant (PSCT) possibly curative in 15% to 20% of those who have failed second-line therapy
Epidemiology
Incidence
GCTs account for 1% of all male malignancies, and it is estimated that 8590 new cases were diagnosed in the United States in the year 2011.1 Despite its overall curability, approximately 360 men in the United States died in the year 2011 as a consequence of germ cell tumors. Although GCT is an uncommon malignancy, it is the most common malignancy among men aged 15 to 35. Seminoma accounts for half of all germ cell tumors, whereas nonseminoma germ cell tumors (NSGCTs) account for the remaining half. Although occasionally GCT occurs in children (generally yolk sac tumors), and in men over 70 (generally spermatocytic seminoma), by and large it is a malignancy of early adulthood, with the incidence of seminoma peaking in the 25- to 45-year-old group; the highest incidence of nonseminoma occurs in a slightly younger group of men (15- to 30-year-old group).2 Although the incidence of germ cell neoplasms in African American men is one fourth that of white men, African Americans have a higher disease stage at diagnosis than white men.3 Bilateral tumors occur in 2% to 4% of patients.4
Etiology
Although risk factors for the development of this disease are largely unknown, a history of cryptorchidism appears to be related to the development of GCT. The risk of developing GCT is 10- to 40-fold higher in cryptorchid testes, and it is anticipated that 12% of all GCTs arise in cryptorchid testes. Conversely, from 1% to 5% of boys with a history of an undescended testicle will go on to develop GCT. The risk is highest (at approximately 5%) when a cryptorchid testis is retained intraabdominally, falls to 1% if retained in the inguinal canal, and appears to fall further if the undescended testis is surgically placed in the scrotum (orchiopexy) before 6 years of age. Despite reducing risk, orchiopexy does not completely eliminate the elevated risk of testis cancer in this population, as evidenced by the fact that one-fourth of GCTs arising in patients with a history of cryptorchidism occur in the normal, descended testicle, suggesting that systemic sequelae of cryptorchidism (i.e., testicular atrophy) is of greater etiologic importance than local or anatomic abnormalities.5,6 Further, males exposed to tobacco smoke in utero have a higher incidence of cryptorchidism,7 although a direct link between tobacco smoke and testis cancer has not been observed.
In patients with testicular feminization syndrome and intraabdominally retained gonads, a 40-fold increase of GCT is seen. In phenotypically female but genotypically male patients, this syndrome may be mistaken for ovarian cancer.8
Approximately 1% to 3% of men with GCT have a family history, pointing to a possible genetic contribution. Brothers or sons of affected patients have between a 6- to 10-fold increased risk of developing the disease in their lifetime.9
Although the association between cryptorchidism and the development of GCT is indisputable, nearly 90% of men with GCT have no history of cryptorchidism. The contribution of orchitis, testicular trauma, or irradiation to the genesis of GCT is unknown, but it has been postulated that the final pathway common to all of these associations is testicular atrophy with increased follicle-stimulating hormone (FSH) drive. There is also growing support for the concept of transplacental damage to the fetal gonad by maternal estrogen levels as a contributing causative agent of germ cell cancer.10
Extragonadal GCTs appear to arise as a consequence of the malignant transformation of residual midline germinal elements, usually in the mediastinum or retroperitoneum, but occasionally in other locations such as the sacrococcygeal region and the pineal gland.11 Whether these residual germinal elements are a consequence of abnormal germ cell migration is not known, and other factors that may contribute to the development of extragonadal GCT have not been identified.
Molecular Biology
The cytogenetic and molecular biology of GCT has only recently begun to be understood. Most interest has focused on changes involving chromosome 12. The isochromosome of the short arm of chromosome 12, i(12p), has been reported in up to 90% of GCT patients,12,13 and cases in which the isochromosome is not seen, excess genetic material is typically found on the p arm of chromosome 12.14 Although it is occasionally found in gastric cancer, i(12p) is nearly a pathognomic feature of GCT of all histologic types, whether of gonadal or extragonadal origin. This cytogenetic abnormality has been reported in carcinoma in situ tissue, suggesting it is an early marker, if not a cause, of germ cell tumorigenesis.15 In patients with mediastinal GCT, in whom there is an increased incidence of hematologic malignancies, often acute myeloid leukemia i(12p) can be found in both the mediastinal GCT tissue and in the leukemic cells,16 suggesting a common clonal origin for both. Similarly, i(12p) is found in malignant tissue of diverse histologies that has developed from the malignant transformation of teratoma, which is a component of nonseminomatous germ cell cancers. The presence of i(12p) has been utilized diagnostically in patients with midline carcinomas of unknown origin,17 allowing, in one series, a definitive diagnosis of GCT in 28% of patients and also serving as a marker of chemotherapy sensitivity (within the group of patients with midline malignancies of uncertain histogenesis).18 The presence of three or more copies of i(12p) has been correlated with poor prognosis GCT.19 Other changes in chromosome 12 are seen in GCT. Deletions of the terminal portion of 12q have been observed in up to 44% of patients with GCT, as well as in several GCT cell lines, suggesting the possibility of a tumor suppressor gene in this area.20
The gene or genes on i(12p) that may be involved in carcinogenesis have not been identified, although the oncogene KRAS, which is located on i(12p), has been implicated in GCT cell lines.21 Cyclin D2, encoded by the gene CCND2, has received much attention in normal testicular development as well as in the pathogenesis of GCT. It has an important role in cellular proliferation and its expression is tightly regulated throughout the cell cycle. It facilitates passage of cells though the G1 cell-cycle checkpoint. The gene is located on the short arm of chromosome 12 and it is overexpressed in nearly all GCTs. CCND2 is therefore a candidate GCT oncogene.20 Activated mutations in the protooncogene KIT have also been isolated from seminoma specimens.22 KIT encodes a transmembrane receptor tyrosine kinase that appears to have a role in normal spermatogenesis, and it is expressed in early fetal germ cells up to 12 weeks of gestation, but not beyond. In addition, it has also been detected in carcinoma in situ and seminoma cells reflecting a possible role in GCT oncogenesis.22,23 Epidermal growth factor receptor (EGFR) has also been found to be overexpressed in approximately 25% of the β-human chorionic gonadotropin (β-hCG)–expressing component of mixed GCTs.24 EGFR is a membrane protein that after binding to its ligand activates protein tyrosine kinase activity. This leads to activation of a cascade of biochemical and physiological responses that are involved in the mitogenic signal transduction of normal as well as malignant cells.
Histology and Natural History
Overview of Histology
A commonly used histologic classification of testicular neoplasms is derived from the Armed Forces Institute of Pathology classification schema of Dixon and Moore,25 which recognizes pure seminoma, as well as four other categories, each of which may occur with or without seminoma elements: (1) embryonal carcinoma, (2) teratoma, (3) teratoma with foci of embryonal carcinoma and choriocarcinoma (also termed teratocarcinoma), and (4) choriocarcinoma with and without embryonal elements.
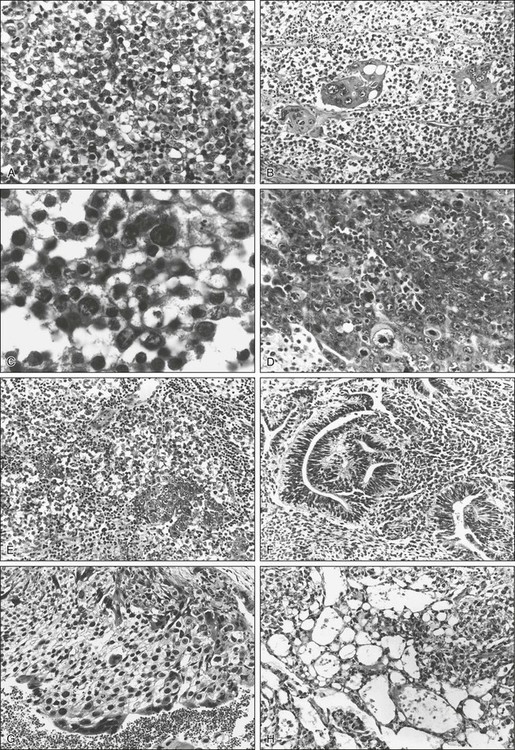
The World Health Organization (WHO) international classification divides tumors into those of single histologic type (seminoma, spermatocytic seminoma, embryonal carcinoma, choriocarcinoma, teratoma, and yolk sac tumors) and those of more than one type, in which the listing and estimation of relative proportion of each type is required.26 The major important differences between these two schemas are the recognition by the WHO schema of yolk sac tumors (endodermal sinus tumors) and spermatocytic seminoma as distinct categories (Table 86-1). Representative photomicrographs of various germ cell tumors can be seen in Figure 86-1.
Table 86-1
Histologic Classification of Testicular Tumors*
| WHO* | British Tumor Board† |
| Seminoma | Seminoma |
| Typical (classic) | |
| Anaplastic | |
| Embryonal carcinoma | Malignant teratoma, undifferentiated |
| Teratoma | Malignant teratoma, differentiated |
| Mature | |
| Immature | |
| With malignant differentiation | |
| Choriocarcinoma | Malignant teratoma, trophoblastic |
| Yolk sac tumor | Yolk sac tumor |
| Mixed germ cell tumors (specify components) | Malignant teratoma, intermediate |
WHO, World Health Organization.
*Data from Mostofi FK, Sesterhenn IA. Revised international classification of testicular tumors. In Jones WG, Harnden P, Appleyard I, editors. Germ Cell Tumors III. Oxford: Pergamon; 1994. p. 153.
†Pugh RCB: Pathology of the Testis. Oxford: Blackwell; 1976.
From Bosl GJ, Bajorin DF, Sheinfeld J, et al. Cancer of the testes. In Devita VT, editor. Principles and Practice of Oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
Testicular cancers that are of nongerminal origin include specialized gonadal stromal neoplasms as well as sarcomas. These, in addition to adenocarcinoma (of the rete testis) and secondary (nonprimary) malignancies such as acute leukemia, lymphoma, carcinoma, and melanoma, comprise less than 5% of testicular neoplasms. The frequency and natural history of specific histologic subtypes are discussed below and summarized in Table 86-2.
Table 86-2
Frequency and Age Distribution of Germ Cell Tumors
| All GCTs (%) | Peak Age | |
| HISTOLOGIC SUBTYPE | ||
| Seminoma | 40 | 25–45 |
| Classic and anaplastic | 35 | 25–45 |
| Spermatocytic | 5 | Average 65 |
| Nonseminoma | 60 | 15–35 |
| Embryonal ± seminoma | 24 | 20–30 |
| Teratoma ± seminoma | 5 | |
| Teratoma + embryonal, choriocarcinoma, or both | 25 | |
| Choriocarcinoma (pure) | <1 | |
| Yolk sac tumor (pure) | <1 | |
| SITE OF ORIGIN | ||
| Gonadal | 95–99 | As above |
| Extragonadal | 1–5 | 20–35 |
Overview of Natural History
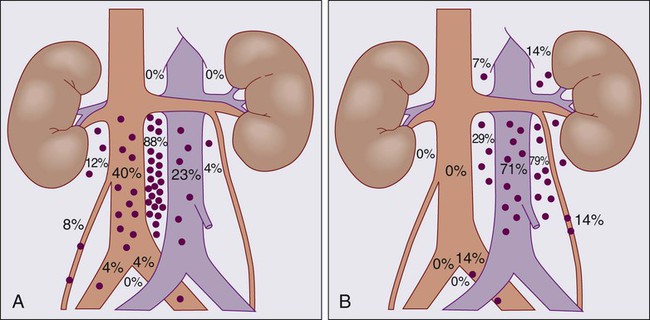
The distribution of retroperitoneal lymph node metastases in nonseminomatous GCT has been described by Ray et al.27 and Donohue et al.,28 and they provide the basis for the specific surgical and therapeutic approaches described below. Figure 86-2 diagrams the anatomy and distribution of retroperitoneal lymph node involvement in GCT. A right-sided testicular primary is most frequently found to have interaortocaval nodal metastases, followed (in order of decreasing frequency) by the precaval and preaortic nodes. Contralateral nodal involvement occurs in 15% of patients, but in virtually every case with contralateral involvement, ipsilateral nodes were also involved. Left testis tumors most frequently have nodal spread to the left paraaortic, preaortic, and interaortocaval nodes, in that order. Suprahilar nodal involvement does not occur in patients with microscopic or low burden infrahilar disease (stage B1; see below), whereas 25% of patients with gross infrahilar disease have been found to have positive suprahilar nodes.27 Other nodal metastases are rare, but can occur in the external iliac and obturator nodes if the primary tumor invades the epididymis or extends up the spermatic cord, or in inguinal lymph nodes if the tumor extends through the tunica vaginalis to involve the scrotum, or if transscrotal exploration has been used.29
Seminoma
Seminomas account for 40% of all GCTs. The three distinct histologic patterns that have historically been described are classic seminoma, anaplastic seminoma, and spermatocytic seminoma. However, a clinical distinction can only be made between classic seminoma and spermatocytic seminoma, and the histologic definition of anaplastic seminoma (more than five mitotic figures per high-power field, cellular anaplasia, and tissue disruption) is of historic interest only, as neither response to therapy nor survival are adversely affected by the presence of anaplastic features.30
Classic seminoma usually manifests in the fourth or fifth decade. It is localized to the testes (stage I) in approximately 70% of patients and is metastatic to lymph nodes (generally stage II) in 25%.31 Metastases to lymph nodes occur in an orderly sequential fashion along draining lymph node chains. Visceral metastases are present at presentation in less than 5% of patients, and in general occur late in the course of the disease. Seminomas tend to appear homogeneous, with little necrosis or hemorrhage on gross inspection. Microscopically, they consist of sheets of uniform cells with large central hyperchromatic nuclei and clear or granular cytoplasm, which are divided by thin fibrous septations. Although lymphocytic infiltration and occasional giant cells may be seen, neoplasms in which any teratomatous or embryonal elements are seen are by definition not considered to be pure seminomas.25 Spermatocytic seminoma represents approximately 5% of all seminomas, and warrants special consideration. It generally occurs in the sixth decade. Although it is more likely to be bilateral than typical seminoma (6% vs. 2%), it is nonetheless a fairly indolent malignancy in which metastatic events are distinctly uncommon.32 Spermatocytic seminoma can be histologically distinguished from classic seminoma by the relative lack of compartmentalization of sheets of cells by fibrous septae, by the marked variation in cell size, and by the absence of lymphocytic infiltration.
Embryonal Carcinoma
Pure embryonal carcinomas account for more than 60% of NSGCTs, although foci of embryonal elements may be found in a large majority of NSGCTs. Embryonal carcinoma occurs in 20- to 30-year-olds and is a highly malignant tumor characterized by rapid and bulky growth. In addition to lymphatic spread, these tumors are characterized by hematogenous spread of cancer cells, particularly to lung and liver. More than 60% of patients with embryonal carcinoma have metastases at presentation and the likelihood of occult nodal metastases in clinical stage I (confined to testis) tumors is a function of the proportion of the tumor that is composed of embryonal carcinoma.33 Furthermore, embryonal carcinoma has been reported to have the highest rate of venous invasion, lymphatic invasion, and tunica (capsular) invasion in both stage I and II tumors.30 Embryonal carcinomas exhibit focal necrosis and hemorrhage and microscopically are quite variable. Cellular features of embryonal carcinoma correlate with its more aggressive behavior and include anaplastic cells with embryoid features. Large pleomorphic nuclei, mitotic figures, and multinucleation are common as well. Stroma varies from loose to thick and fibrous.25
Teratoma and Teratocarcinoma
Mature teratoma has elements of one or more of the three germinal layers that are fully differentiated. Pure teratoma is uncommon and comprises less than 5% of GCT in adults. More than 75% of nonseminomas have been reported to have variable amounts of teratomatous elements, so that “pure” teratomas must be sampled carefully to exclude undifferentiated foci. The term teratocarcinoma refers to teratomas in combination with other elements, although some pathologists reserve the term for the combination of teratoma and embryonal carcinoma. When a teratoma has cellular and active stroma with mitotic figures, it is referred to as immature teratoma. Teratomas and teratocarcinomas are composed of solid and cystic spaces on cut surface, with areas of hemorrhage and necrosis. A histologic mix of fully differentiated cartilage, muscle, or epithelial tissue and malignant embryonal elements is seen. Predominant teratomatous features account for one-third of teratocarcinomas, whereas approximately two-thirds are mostly composed of nonteratomatous elements.25 Mature teratoma is the least aggressive of the nonseminoma GCTs, although up to 30% of adult patients with clinical stage I teratoma treated with orchiectomy alone will subsequently relapse, suggesting that pure teratomas should not be exempted from the usual clinical and pathological staging of GCT or from the usual subsequent therapeutic interventions.34 The natural history of teratocarcinomas lies somewhere between that of mature teratoma and embryonal carcinoma, but for practical (diagnostic and therapeutic) purposes, teratocarcinomas can be grouped with embryonal cell carcinomas. The likelihood that residual or recurrent masses after treatment are composed of mature teratoma is increased in patients with more extensive teratomatous elements at presentation and is discussed below.
Choriocarcinoma
Choriocarcinoma is the most aggressive of the nonseminoma GCTs, with early hematogenous dissemination to lungs, liver, brain, and other visceral sites. Pure choriocarcinoma is exceedingly rare, accounting for less than 0.5% of all testicular malignancies, but focal areas of choriocarcinoma are seen in approximately 12% of embryonal and teratocarcinomas.35 Because advanced-stage disease at diagnosis is common, pure choriocarcinoma has a particularly poor prognosis. If pure choriocarcinomas are excluded, the specific histologic subtype of NSGCT does not have an influence on survival. The microscopic diagnosis of choriocarcinoma requires the presence of two cell types, syncytiotrophoblastic cells (giant cells with multiple hyperchromatic nuclei and abundant eosinophilic cytoplasm) and cytotrophoblasts (sheets of cells with single nuclei, abundant clear cytoplasm, and well-defined borders) arranged in papillary or pseudovillous patterns.25 Serum hCG is usually elevated in patients with choriocarcinoma; however, choriocarcinoma does not produce alpha-fetoprotein.
Yolk Sac Tumors
Also known as endodermal sinus tumors, pure yolk sac tumors occur rarely, accounting for 1% of GCTs in adults. More commonly, yolk sac tumors in adults are found in combination with other tumor types, occurring in up to 70% of GCTs. In one series of 459 patients with stage I and II testicular cancer, the most common histology observed was a mixed one consisting of embryonal carcinoma plus yolk sac tumor and teratoma. Furthermore, the presence of teratoma and yolk sac tumor appeared to be associated with a large primary tumor size.35 Although pure yolk sac tumors are conventionally considered to be more aggressive with early hematogenous distribution,30 recent histologic reviews have suggested that patients in whom yolk sac tumor elements are present are at lower risk of relapse than patients in whom they are absent. However, the relatively high frequency with which yolk sac tumors and embryonal carcinoma are found together (50% of all tumors in some series) and the extremely rare occurrence of pure yolk sac tumors (1%) make this a difficult conclusion to substantiate.36 Yolk sac tumors occur more commonly in children, in whom they appear to be a less aggressive histologic subtype.37 The histology of yolk sac tumors, though characteristic, may be observed in several common patterns. These include a papillary pattern in which Schiller-Duval bodies (a fibrovascular core with a circle of cells around it, vaguely reminiscent of a glomerulus) can be seen, as well as microcystic, glanduloalveolar, and solid patterns. Nearly all patients with yolk sac tumors have elevations in serum AFP. In contrast to choriocarcinoma, yolk sac tumors do not produce AFP.
Stromal Cell Tumors
Derived from the stromal and supporting cells surrounding germ cells, stromal cell tumors consist of Leydig cell tumors, Sertoli cell tumors, and granulosa cell tumors. As a group, they account for 3% to 4% of primary testicular tumors but constitute nearly 20% of childhood testicular tumors. Although by and large they are benign, up to 10% may metastasize.38 Histologic features appear to be useful in predicting the risk of disseminated disease, and management generally consists of orchiectomy and clinical staging with computed tomographic (CT) scan, without use of RPLND. These tumors may be estradiol secreting; gynecomastia occurs in 30% of Sertoli cell tumors and 15% of Leydig cell tumors.
Secondary (Metastatic) Neoplasms
Although the large majority of testicular neoplasms in young men are germ cell tumors, in men more than 60 years of age, only 25% of malignancies will be of germinal origin. Testicular malignancies in this age group are predominantly composed of lymphomas,39 although metastases to the testicles, primarily from prostatic adenocarcinoma, lung carcinoma, and melanoma primary tumors must also be considered. Chemotherapeutics may not penetrate the blood–testis barrier efficiently; thus special consideration must be given to the treatment and subsequent surveillance of metastatic tumors (usually lymphomas) in the testis.
Clinical Manifestations
The most common presentation of testicular cancer is testicular swelling (73% in one series of 450 patients).35 A commonly held misconception is that testicular cancers are by and large painless and that painful testicular masses need not be evaluated for malignancy. In fact, testicular pain is a manifesting feature of 18% to 46% of patients with GCT.35 Acute pain may be associated with torsion of the neoplasm, infarction or bleeding in the tumor, as well as with epididymitis. Signs and symptoms indistinguishable from acute epididymitis have been observed in up to one-fourth of patients with testicular neoplasms. Less commonly presenting symptoms will include gynecomastia in hCG-producing tumors such as choriocarcinoma (10%), back or flank pain from metastatic disease (10%), and infertility in less than 5% of tumors. Approximately 25% of patients with advanced disease have symptoms referable to their metastases, such as back pain.40 This is frequently the manifesting symptom in patients with primary retroperitoneal GCT. Pulmonary symptoms including shortness of breath, chest pain, and hemoptysis are rare but can occur in patients with advanced pulmonary disease or primary mediastinal GCT.
The constellation of elicited symptoms and physical examination findings can offer a clue to the histology of a testicular mass. For example, it has been reported that pain more commonly occurred in patients who had embryonal carcinoma elements in their tumors than in those without that histologic feature (56% vs. 37%; P < 0.02). The same study reported a significantly higher incidence of testicular swelling when the primary tumors contained teratoma or yolk sac tumor elements.35 Primary tumors from patients with seminoma tend to be larger and more homogeneous, with diffuse involvement of the testicle, whereas embryonal and teratomatous elements tend to from smaller, discrete masses. Rapidly growing tumors, particularly those with extranodal dissemination, should raise the possibility of choriocarcinoma. These observations are of course no substitute for the appropriate clinical staging and histopathological evaluation.
Evaluation of the Patient: Diagnosis, Clinical Staging, and Risk Assessment
Diagnosis: Testicular Ultrasonography
The initial evaluation of a testicular mass is ultrasonographic evaluation. Testicular ultrasonography is a sensitive and specific test that can discriminate between a testicular neoplasm and nonmalignant processes included in the differential diagnosis such as testicular torsion, hydrocele, varicocele, spermatocele, and epididymitis. Thus the demonstration of hypoechoic or heterogeneous masses on ultrasonographic examination should prompt a thorough subsequent evaluation as described below. Similarly, a negative ultrasonogram fairly reliably establishes the absence of intrascrotal GCT.41 Both testes should be examined carefully, as 2% to 4% of patients will harbor bilateral lesions.42 Extragonadal GCTs are a distinct clinical entity described below, constituting from 1% to 5% of all GCTs; in these patients, scrotal ultrasonography will be negative.
Diagnosis: Orchiectomy
Any patient with a testicular mass or abnormal ultrasonography, or both, should have testicular carcinoma ruled out by a unilateral radical transinguinal orchiectomy. Orchiectomy is the definitive procedure for both pathological diagnosis and local control of the primary tumor and in some cases may be a curative procedure. Furthermore, important predictive factors can be identified with routine histologic techniques that are crucial to risk assessment strategies used in the clinical management of these patients.34,35,43–46 The removal of suspicious testicles by inguinal orchiectomy should be undertaken even in patients in whom a diagnosis of disseminated GCT has been made by biopsy of a metastatic site, as the testes appear to be chemotherapy sanctuaries for cancer cells.47
Orchiectomy delay for up to two cycles of chemotherapy is occasionally indicated, when control of metastatic disease must be obtained urgently. There appears to be a blood–testis barrier, so that viable GCTs can persist in the testis despite complete eradication of cancer from metastatic deposits by chemotherapy. Orchiectomy should therefore always be performed once control of metastatic disease is achieved.48,49 Patients with a diagnosis of extragonadal GCT with normal testicular examinations and ultrasonograms need not undergo orchiectomy. Transscrotal orchiectomies or needle biopsies are absolutely contraindicated, as they have been associated with up to 24% incidence of local recurrence or spread to inguinal lymph nodes.29
Tumor Markers
HCG is a glycoprotein of 45,000 MW. It consists of two covalently linked subunits, an α-subunit shared by luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid-stimulating hormone, and a unique β-subunit (β-hCG) that can be readily measured in serum. Although it is normally produced at high levels by the placenta, low levels are detectable in normal nonpregnant adults, including some men. HCG elevations are seen in neoplasms other than GCTs, including prostate, bladder, ureteral, and renal cancers.50 Spurious elevations have been noted in patients using marijuana.51 Some patients that have undergone systemic therapy develop cis-platinum–induced testicular atrophy in the remaining testis, resulting in lower levels of testosterone, with a compensatory hypersecretion of LH to stimulate Leydig cell secretion of testosterone. Although hCG and LH are not immunologically cross-reactive, very high levels of LH may spuriously elevate measured hCG levels. When necessary, measurement of hCG levels 2 weeks after the administration of testosterone cypionate (Depo-Testosterone) should rule out this possibility. The biological half-life of serum hCG is approximately 24 hours. In germ cell tumors, hCG is produced by syncytiotrophoblastic cells in embryonal carcinoma, choriocarcinoma, and seminoma. Extremely high levels of hCG are generally associated with choriocarcinoma. Although seminomas are generally marker negative, the incidence of hCG-positive seminoma varies from 5% to 40%. In general, these hCG elevations do not exceed a level of 100 mIU/mL, and probably do not connote a worse prognosis.52–56
AFP is a glycoprotein that is the major serum protein of the fetus. It is also an oncofetal protein, found not only in patients with nonseminomatous GCT but in pregnant women (fetal hepatic production), in patients with hepatocellular carcinoma, and occasionally, at somewhat lower levels, in patients with cirrhotic livers demonstrating nodular regeneration or in patients with hepatitis. Its biological half-life is 4 to 6 days. AFP elevations are most commonly seen in embryonal carcinoma and yolk sac tumors, with reports of up to 89% of tumors with yolk sac elements demonstrating elevated AFP.35 Pure seminomas and pure choriocarcinomas do not produce AFP. Thus in the absence of hepatitis or other causes of AFP, by definition the presence of an elevated AFP in a histologically pure seminoma identifies the presence of nonseminomatous elements, and mandates that the management proceeds accordingly.
LDH is a nonspecific marker that appears to be related to tumor burden and that can serve as both an indicator of response to treatment and as a predictor of prognosis. Interestingly, the gene for LDH isoenzyme 1 maps to chromosome 12, and the serum level of LDH isoenzyme 1 has been shown to correlate with the number of copies of i(12p) in the tumor, a fairly specific genetic marker of germ cell malignancies. Furthermore, the presence of three or more copies of i(12p) has been correlated with a worse prognosis.19
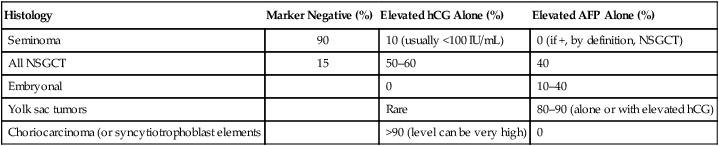
Overall, approximately 85% of nonseminomas will have an elevation of either AFP or β-hCG.50,57,58 Approximately 15% are marker negative. AFP elevation alone is elevated in 40% of nonseminomatous GCTs, and β-hCG elevation alone is seen in 50% to 60%. It is important to note that up to 30% of patients with early-stage nonseminomatous GCT will have normal serum markers, so the absence of marker elevation should not influence the decision to perform an orchiectomy. The incidence of serum marker elevation as a function of tumor histology is shown in Table 86-3.
Table 86-3
Germ Cell Tumors and Serum Markers
| Histology | Marker Negative (%) | Elevated hCG Alone (%) | Elevated AFP Alone (%) |
| Seminoma | 90 | 10 (usually <100 IU/mL) | 0 (if +, by definition, NSGCT) |
| All NSGCT | 15 | 50–60 | 40 |
| Embryonal | 0 | 10–40 | |
| Yolk sac tumors | Rare | 80–90 (alone or with elevated hCG) | |
| Choriocarcinoma (or syncytiotrophoblast elements | >90 (level can be very high) | 0 |
AFP, α-Fetoprotein; hCG, human chorionic gonadotropin; NSGCT, nonseminoma germ cell tumor.
Even though most seminomas and up to 30% of nonseminomatous GCTs are marker negative, AFP, LDH, and β-hCG levels should always be checked pre- and postorchiectomy. The correct interpretation of serum marker levels requires that the biological half-life of AFP and hCG be taken into account. Thus the persistence of elevated AFP or β-hCG levels higher than predicted by the half-lives of these glycoproteins after orchiectomy implies residual (occult) disease. The converse, however, is not true: normalization of markers after orchiectomy does not ensure the absence of occult disease. In fact, up to 60% of patients with nonseminomatous GCT felt on clinical grounds to be confined to the testis (stage I) with normalization of markers after orchiectomy will have involvement of retroperitoneal lymph nodes with tumor.34,44,45,59 The management of clinical stage I cancers reflects this fact and is discussed below.
In patients with advanced disease, serum markers can also be used as a measure of response to therapy. Patients with retroperitoneal adenopathy who have been treated by lymphadenectomy should normalize their markers; failure to do so implies residual or recurrent disease. The response of advanced GCT to chemotherapy can also be monitored by a fall in serum markers. However, in this situation, unlike the postorchiectomy or post-RPLND setting, tumor debulking is not instantaneous. Thus the use of known half-lives to calculate the expected decay of levels of serum tumor markers is more difficult. A 10-fold decrease in the hCG level over a 3-week period has been empirically observed to be consistent with disease eradication. Other investigators have shown that a durable complete response (CR) to chemotherapy could be predicted with a high degree of accuracy by the ratio of the hCG level measured on day 22 (after one cycle of chemotherapy) to the day 1 hCG level. In this schema, if the hCG level failed to normalize after one cycle of chemotherapy (day 22), and if the day-22/day-1 ratio was greater than 1 : 200 (0.005), an incomplete response could be predicted in 94% of patients. Conversely, if the day-22 hCG value was within normal range, or if the day-22/day-1 ratio was less than 1 : 200, a durable CR could be predicted in 91% of patients.60 Other reports have noted that observed half-life decays of greater than 7 days for AFP, and greater than 4 days for hCG after treatment with chemotherapy, are associated with poor outcome and probably correlate with the emergence of drug-resistant disease. Likewise, reappearance of serum marker elevation after therapy is an invaluable method of detecting early relapse, often predating any radiologic evidence of relapse or recurrence. On occasion, tumor lysis with chemotherapy will result in transient “flares” or rises in serum markers followed by a subsequent decline. The prognostic implications of such a flare are not well understood.
Radiologic Evaluation
The standard method of staging retroperitoneal disease in a patient with testicular cancer consists of abdominal and pelvic CT scanning. In patients with nonseminomatous GCT, abdominopelvic CT understaging (e.g., false-negatives) occurs in as high as 50% of patients, whereas overstaging (e.g., false positives) occur in approximately 10% of patients.44,59,45 The sensitivity and specificity of abdominal CT for detecting lymph node metastases in patients with clinical stage I disease (testis-only disease) has been evaluated.61 Using a cutoff of 10 mm or larger, a sensitivity of 37% and a specificity of 100% were observed, whereas using a 4-mm cutoff enhances sensitivity to 93% but decreases specificity to 58%.
The exact incidence of occult retroperitoneal lymph node metastases in patients with seminoma who have normal CT is not known because these patients are typically treated with radiation therapy, and do not undergo surgical exploration. Nonetheless, an estimated incidence of occult nodal metastases of 10% to 25% is generally accepted.43,62
More recently, 18F-labeled deoxyglucose positron emission tomography (FDG-PET) has been used to identify viable cancer in residual postchemotherapy masses for patients with pure seminoma. Sensitivity and specificity of 88% and 95%, respectively, have been reported, along with high positive and negative predictive values (90% and 96%) in two multicenter trials.62 By contrast, the usefulness of FDG-PET in the initial staging of GCT patients is less clear. Several reports comprising more than 110 patients evaluated the accuracy of FDG-PET compared with CT scan staging in patients with stage I and II GCTs. Sensitivity and specificity were reported as 73% to 94% and 40% to 78%, respectively. FDG-PET was unable to detect mature teratomas as well as lesions smaller than 5 mm in diameter.63,64 Similarly, the absence of PET-avid disease in men with high-risk stage I disease does not imply a low likelihood of recurrence; in a study of 87 men with no evidence of disease by FDG-PET, 33 patients (38%) relapsed within 1 year.65 Because of these data, FDG-PET is not routinely used or recommended as initial staging for stage I-II GCTs.
Other imaging modalities such as head CT and radionuclide bone scan are not routinely undertaken except as warranted by symptoms. One exception to this rule is for choriocarcinoma, in which an increased incidence of brain metastases mandates a CT or magnetic resonance imaging (MRI) of the brain prior to proceeding with chemotherapy.66
Staging
Several schemas are incorporated in the current staging of GCT. Because lymphadenectomy is almost never undertaken as a primary therapeutic modality in seminoma, it is staged clinically and radiographically. Until recently, the standard treatment of nonseminomatous tumors has included an RPLND, so that most patients with nonseminomatous GCT were pathologically staged. Because up to 80% of patients with carefully staged clinical stage I nonseminomatous GCT tumors have been found to be free of retroperitoneal disease, many patients with clinical stage I nonseminomatous GCT are offered observation in lieu of lymphadenectomy (see below). Data have shown that histologic predictors including the presence of lymphovascular invasion in primary testicular nonseminomatous tumors may be used to help risk-stratify patients for further therapy or for surveillance. A clear distinction must be made in these and all patients with nonseminomatous GCT, between clinical stage and pathological stage. The development of an international consensus GCT risk classification schema (see Advanced Disease section later) has allowed the use of prognostic groupings as the basis of a revised staging classification. This revised (1997) TNM American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) classification (Table 86-4) continues to rely on anatomic disease extent, allows for the pathological classification of lymph nodes, but also takes into account serum tumor markers. Patients who have only serologic marker evidence of disease after orchiectomy are classified as stage I-S.
Table 86-4
TNM Classification of Testis Tumors
| PRIMARY TUMOR (pT) | ||||
| The extent of primary tumor is classified after radical orchiectomy. | ||||
| pT | Primary tumor cannot be assessed (if no radical orchiectomy has been performed TX is used). | |||
| pT0 | No evidence of primary tumor (e.g., histologic scar in testis) | |||
| pTis | Intratubular germ cell neoplasia (carcinoma in situ) | |||
| pT1 | Tumor limited to testis and epididymis without vascular/lymphatic invasion | |||
| Tumor may invade into tunica albuginea but not tunica vaginalis. | ||||
| pT2 | Tumor limited to testis and epididymis with vascular/lymphatic invasion, or tumor extending through tunica albuginea with involvement of tunica vaginalis. | |||
| pT3 | Tumor invades spermatic cord with or without vascular/lymphatic invasion. | |||
| pT4 | Tumor invades scrotum with or without vascular/lymphatic invasion. | |||
| REGIONAL LYMPH NODES (N) | ||||
| Clinical | ||||
| NX | Regional lymph nodes cannot be assessed. | |||
| N0 | No regional lymph node metastasis | |||
| N1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension; or multiple lymph nodes, none more than 2 cm in greatest dimension | |||
| N2 | Metastasis with a lymph node mass more than 2 cm or less than 5 cm in greatest dimension; or multiple lymph nodes, any one mass more than 2 cm but not more than 5 cm in greatest dimension | |||
| N3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | |||
| PATHOLOGICAL CLASSIFICATION OF REGIONAL LYMPH NODES (pN) | ||||
| pNX | Regional lymph nodes cannot be assessed. | |||
| pN0 | No regional lymph node metastasis | |||
| pN1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension and 5 or fewer positive nodes, none more than 2 cm in greatest dimension | |||
| pN2 | Metastasis with a lymph node mass, more than 2 cm but not more than 5 cm in greatest dimension; or more than 5 nodes positive, none more than 5 cm; or evidence of extranodal extension of tumor | |||
| pN3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension | |||
| DISTANT METASTASIS (M) | ||||
| MX | Distant metastasis cannot be assessed. | |||
| M0 | No distant metastasis | |||
| M1 | Distant metastasis | |||
| M1a | Nonregional lymph node or pulmonary metastasis | |||
| M1b | Nonpulmonary visceral metastasis | |||
| SERUM TUMOR MARKERS (S) | ||||
| SX | Marker studies not available or not performed | |||
| S0 | Marker study levels within normal limits | |||
| S1 | LDH < 1.5 × normal and hCG (mIU/mL) < 5000 and AFP (ng/mL) < 1000 | |||
| S2 | LDH 1.5–10 × normal or hCG (mIU/mL) 5000–50,000 or AFP (ng/mL) 1000–10,000 | |||
| S3 | LDH > 10 × normal or hCG (mIU/mL) > 50,000 or AFP (ng/mL) > 10,000 | |||
| N indicates the upper limit of normal for the LDH assay | ||||
| STAGING GROUPING | ||||
| Stage 0 | pTis | N0 | M0 | S0, SX |
| Stage I | pT1–4 | N0 | M0 | SX |
| Stage IA | pT1 | N0 | M0 | S0 |
| Stage IB | pT2 | N0 | M0 | S0 |
| pT3 | N0 | M0 | S0 | |
| pT4 | N0 | M0 | S0 | |
| Stage IS | Any pT/Tx | N0 | M0 | S1–3 |
| Stage II | Any pT/Tx | N1–3 | M0 | SX |
| Stage IIA | Any pT/Tx | N1 | M0 | S0 |
| Any pT/Tx | N1 | M0 | S1 | |
| Stage IIB | Any pT/Tx | N2 | M0 | S0 |
| Any pT/Tx | N2 | M0 | S1 | |
| Stage IIC | Any pT/Tx | N3 | M0 | S0 |
| Any pT/Tx | N3 | M0 | S1 | |
| Stage III | Any pT/Tx | Any N | M1, M1a | SX |
| Stage IIIA | Any pT/Tx | Any N | M1, M1a | S0 |
| Any pT/Tx | Any N | M1, M1a | S1 | |
| Stage IIIB | Any pT/Tx | N1–3 | M0 | S2 |
| Any pT/Tx | Any N | M1, M1a | S2 | |
| Stage IIIC | Any pT/Tx | N1–3 | M0 | S3 |
| Any pT/Tx | Any N | M1, M1a | S3 | |
| Any pT/Tx | Any N | M1b | Any S | |
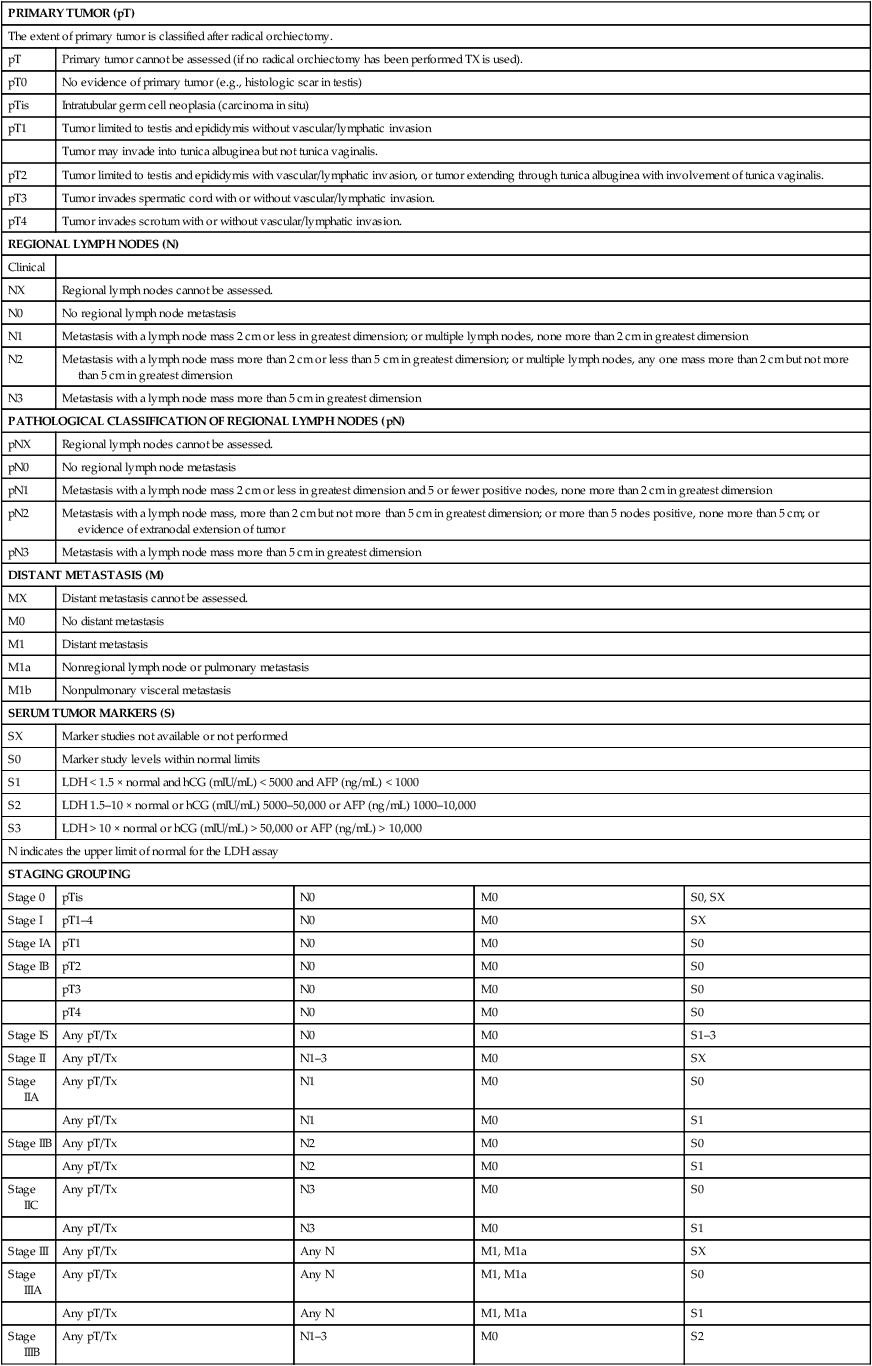
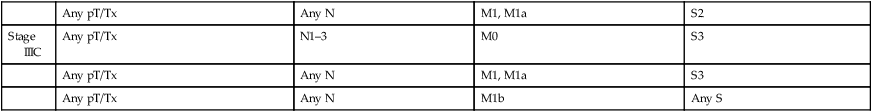
From AJCC: Testis. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010, pp 469-78.
In general, stage I (or A) refers to tumors confined to the testis, with no evidence of nodal or pulmonary parenchymal involvement. Stage II (or B) denotes tumors with retroperitoneal lymph node metastases. Stage II patients are further subdivided by the relative tumor burden. In seminoma patients this is clinically defined by the size of nodal involvement detected by imaging studies, whereas in nonseminomatous GCT this is usually but not always a pathological diagnosis. Stage IIA (or B1) implies minimal but definite nodal involvement, generally of a microscopic nature; IIB (or B2) refers to macroscopic disease, or a larger number of microscopic metastases, whereas IIC (or B3) indicates bulky retroperitoneal nodal disease. Stage III (or C) refers to disease that has spread beyond the retroperitoneum, usually to supradiaphragmatic lymph nodes, pulmonary parenchyma, or other sites including liver, bone, or brain. Some staging schemas, particularly those for seminoma, distinguish between stage III (supradiaphragmatic lymph nodes) and stage IV (any extranodal metastases, including pulmonary parenchyma, bone, and brain) (Table 86-5). The distinction of hepatic, central nervous system, and osseous metastases is not without merit, as patients with metastases to these regions clearly have a worsened prognosis.
Table 86-5
Royal Marsden Clinical Staging Schema for Seminoma
| Stage | Definition |
| I | Confined to testis |
| IIA | Abdominal nodal disease: <2 cm |
| IIB | Abdominal nodal disease: 2–5 cm |
| IIC | Abdominal nodal disease: >5 cm |
| III | Supradiaphragmatic nodal disease |
| IV | Extranodal disease |
From American Joint Committee on Cancer. AJCC Manual for Staging of Cancer. 5th ed. Philadelphia: JB Lippincott; 1997, with permission.
Management of Low-Stage Disease
Clinical Stage I Seminoma
Risk Assessment
Prognostic factors that have been postulated as predictors of outcome include DNA ploidy status, mitotic rate, DNA S-phase percentage, presence of syncytiotrophoblasts, degree of lymphocytic infiltration of the primary tumor, and the expression of β-hCG and low-molecular-weight keratin on immunohistochemical analyses. However, none of these factors has been confirmed in prospective trials.30,67,68 Pure seminoma does not make AFP; therefore an elevated AFP level should raise concern for nonseminomatous elements even when only seminoma is found at initial diagnosis. In contrast, 10% of pure seminoma patients will have an elevated β-hCG level at diagnosis. Preorchiectomy elevations of β-hCG do not connote a worse prognosis in stage I seminoma patients.53–56,68
Less than 20% of unselected patients with stage I seminoma who are managed with active surveillance will relapse (see Treatment section). A pooled analysis of data from 4 surveillance series identified size of primary tumor (>4 cm) and rete testis invasion as independent predictors of relapse, with approximately one-third of patients with both of these risk factors relapsing within 5 years of diagnosis. Other data sets suggest that the risk of relapse in patients who lack the adverse prognostic features of age >34 years, tumors ≥6 cm, and small vessel invasion have a relapse rate of approximately 6%.69 Although previous studies suggested that patients <30 years old were at higher risk of relapse, this was not observed in this pooled analysis. An increased incidence of pelvic nodal involvement is also seen in patients who have had previous inguinal surgery, or in whom the primary tumor has invaded scrotal skin. Scrotal skin involvement also predisposes to relapse in the hemiscrotum.31,70 Finally, tumor invasion of the spermatic cord increases the risk of relapse in the inguinal orchiectomy scar.71
Treatment
Radiation therapy to the paraaortic and pelvic (retroperitoneal) lymph nodes has been traditionally for adjuvant therapy for stage I seminoma, although its use has declined in recent years as more men choose active surveillance or adjuvant chemotherapy.72 Conventional radiation fields for clinical stage I seminoma treat the paraaortic nodes from the diaphragm at the level of the T10 vertebral body down to the lower border of the L4 vertebral body, including the ipsilateral common iliac and external iliac nodes. If pelvic nodes may be involved, the field is extended to include the ipsilateral inguinofemoral lymph nodes. Spermatic cord involvement necessitates a radiation field that covers the entire inguinal orchiectomy scar, whereas scrotal skin involvement mandates radiation to the hemiscrotum.71
A trial conducted by the MRC in Great Britain in conjunction with the EORTC randomized 625 patients with stage I seminoma to therapy with 20 Gy in 10 fractions over 2 weeks or 30 Gy in 15 fractions during 3 weeks after orchiectomy. Interim results suggest that short-term morbidity (lethargy and decreased work capacity) is slightly higher in the patients receiving 30 Gy, although all differences resolve by 12 weeks. At 61 months of follow-up, there was no difference in relapse rates.73 These data suggest that 20 Gy may be reasonable in this setting.
Because of the concern about risks of secondary malignancies associated with radiation therapy, adjuvant chemotherapy has been explored as an option for men with stage I seminoma. In particular, two major studies have been conducted to determine the efficacy of single-agent carboplatin administered as an adjuvant therapy for stage I seminoma. The first is a large randomized phase III comparative study conducted by the Medical Research Council (MRC) in Great Britain that compared adjuvant radiotherapy with a single dose of adjuvant carboplatin.74 In total, 1477 patients with stage I seminoma (excluding those with T4 primary tumors) were randomized to therapy with radiation (N = 904) administered in a dog-leg or paraaortic field at a dose of 20 to 30 Gy versus a single dose of carboplatin (N = 573) administered at an area under the curve (AUC) of 7. With a median follow-up of 6.5 years, the relapse-free survival rates were similar for patients receiving radiation versus chemotherapy, with 96% versus 94.7% of patients relapse-free at 5 years, respectively. Although rare, patients who received carboplatin were significantly less likely to develop secondary primary germ cell tumors. Criticisms of this study include a lack of stratification by risk factors among patients as well as the lack of an arm that included observation, the inclusion of which would have given insight into the margin of benefit for any adjuvant therapy versus none.
In the second study, a “risk adapted” approach was used in which carboplatin (AUC of 7 for two cycles) was administered to patients with either rete testis invasion (11%) or a primary tumor size ≥4 cm (42%), or both (16%). Patients without these risk factors underwent surveillance. A total of 314 patients were enrolled and 214 (36%) received carboplatin. Of the 100 patients undergoing surveillance, 6 (6%) relapsed compared to 7 (3.3%) of those who received carboplatin. Interestingly, 9% of the patients with rete testis invasion relapsed compared to 0.8% of those with tumor size ≥4 cm. All patients who relapsed were salvaged with standard etoposide plus cisplatin chemotherapy.75
Several large studies have evaluated the role of surveillance in patients with stage I seminoma, with similar results.76–80 The cumulative risk of relapse within the first year is 5% to 10%, 10% to 15% in the second year, and 15% to 20% by the third year. As noted above, two adverse prognostic factors can identify patients at high risk of relapse: tumor size >4 cm and rete testis involvement. In a pooled analysis, the 5-year relapse-free survival for patients with 0 or 1 risk factors was 83% to 88%, whereas patients with 2 adverse factors had a 5-year relapse-free survival of 69%.81 These data have allowed an estimation of the frequency of occult nodal metastases in clinical stage I seminoma. The most common site of relapse has been the paraaortic lymph nodes. At relapse, salvage therapy with either radiation or systemic therapy is extremely successful, resulting in a cause-specific survival identical to historical experience with conventional radiotherapy (95% to 100%). There is no consensus regarding the optimal follow-up protocol for seminoma patient undergoing surveillance. In general, patients are seen at 3- to 4-month intervals for 3 to 4 years, every 6 months for another 2–3 years, and then annually. At each visit, a clinical assessment, chest x-ray or chest CT, and serum markers are obtained. CT scan of the abdomen and pelvis is typically obtained at intervals of every 4 months for the first 2 to 3 years, although ongoing studies seek to determine if a lower frequency of scans or the use of MRI may be equivalently effective.79 Because most seminomas are marker-negative, serial tumor marker surveillance has a lower usefulness than in men with NSGCT.
Clinical Stage I Nonseminoma
Risk Assessment
Controversy over the optimal management of clinical stage I nonseminomatous GCT has prompted a search for prognostic factors that can be used to predict the risk of occult nodal involvement. Histologic subtype, local tumor extension, and vascular or lymphatic invasion have all been shown to correlate with occult nodal metastases (pathological stage IIA) or a high risk of relapse (summarized in Table 86-6).

*See text for findings of individual studies.
Several studies have identified histologic evidence of vascular or lymphatic invasion in the primary tumor as a predictor of either relapse or occult nodal involvement in clinical stage I patients who were followed after orchiectomy with surveillance, or treated with RPLND. In patients with clinical stage I NSGCT, the Testicular Cancer Intergroup Study observed venous or lymphatic invasion in 23.6% and 9.2% of specimens, respectively, compared with 55% and 45%, respectively, of specimens from patients with clinical stage II disease. Not only was vascular invasion more common in node-positive patients than in node-negative patients, but in node-negative patients treated with orchiectomy and RPLND alone, relapse was noted in 10 of 168 (6%) patients who demonstrated no vascular invasion, compared with 12 of 62 (19.4%) if vascular invasion were present.82 Other studies36,83 have confirmed the significance of vascular or lymphatic invasion as a predictor of occult nodal metastases or relapse, either alone or in combination with other variables. A British study of 259 clinical stage I nonseminomatous GCT patients identified invasion of veins or lymphatics, along with two histologic features (presence of embryonal carcinoma and absence of yolk sac tumor) as four factors most predictive of relapse. If three or four features were present, the likelihood of relapse or occult nodal involvement was 58%; patients with two positive risk factors had a 24% chance of relapse, whereas patients with zero or one risk factor relapsed only 9% of the time.49 A Swedish/Norwegian study found vascular invasion, absence of AFP before orchiectomy, or high T stage as predictive factors that identified high-risk clinical stage I nonseminomatous GCT patients.84 In a Danish study, patients with vascular invasion had a risk of relapse of 36%, compared with patients with no vascular invasion, whose relapse rate was 13%. A more recent prospective study identified 13.5% relapse-free survival for men with stage I disease with vascular invasion.85
Several series have demonstrated an association between the presence of embryonal carcinoma in the primary tumor and an increased risk of lymph node metastasis. In 321 patients with either stage I or II nonseminomatous GCT who were treated with orchiectomy and lymphadenectomy, the Testicular Cancer Intergroup Study demonstrated that the risk of relapse remained low until the percentage of embryonal carcinoma exceeded 30% to 40% (Figure 86-3). However, in this series, the percentage of embryonal carcinoma was not found to be a significant prognostic factor after adjustment for either vascular invasion or nodal stage.48 Another large series of 292 patients with clinical stage I NSGCT from Indiana University suggested that embryonal carcinoma–predominant tumors were both more likely to have occult positive lymph nodes (32% vs. 15.6%) and were also considerably more likely to relapse after RPLND (21% vs. 3%).33
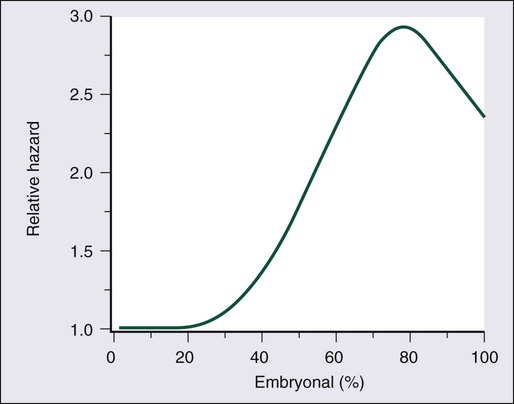
The absence of yolk sac elements is felt to be a marker of poor prognosis in patients with stage I NSGCT. The confirmation by the Testicular Cancer Intergroup Study that AFP production is linked to yolk sac histology suggests that the identification of AFP negativity as a marker of poor prognosis in stage I NSGCT is a reflection of the fact that yolk sac tumor histology appears to confer some benefit to these patients.34
Local extension of tumor into paratesticular structures also appears to be predictive of higher relapse rates. Although some studies have noted higher relapse rates in patients with invasion of any structures outside the testis (rete testis, tunica albuginea, epididymis, or spermatic cord), others have noted that tumor involvement of only certain paratesticular structures carried an increased risk of relapse.86 Thus Sesterhenn et al.35 report a 42% versus 16% relapse rate after RPLND in stage I and II patients with and without tunica albuginea involvement. Other studies have suggested that involvement of the rete and epididymis, but not the tunica albuginea or spermatic cord, were associated with a higher risk of relapse. Recent studies suggest that negative immunohistochemical staining with an antibody against the Ki-67 receptor, a marker of tumor proliferation, identifies a good risk population with a negative predictive value for occult nodal involvement of 88%, although this has not been confirmed by others.87
A variety of other factors have been shown to have no prognostic value. Interestingly, increased levels of preorchiectomy β-hCG and AFP, the size of the primary (in contrast to seminoma) or the side of the primary (right vs. left) are not generally predictive of the risk of relapse after orchiectomy.86
Treatment
Retroperitoneal lymph node dissection
After RPLND, clinical stage I NSGCT patients are restaged as either pathological stage I, or pathological stage IIA (microscopic nodal involvement). In the pre–cis-platinum era, survival rates were 93% for pathological stage I and 75% for pathological stage IIA patients. Since the advent of cis-platinum–based chemotherapy, which is used in this setting as either salvage or adjunctive therapy (see below), the long-term survival rate approaches 100% for patients with pathological stage I tumors, and 96% for patients with pathological stage IIA tumors.88
A large prospective trial by the Testicular Cancer Intergroup Study, enrolled 195 pathological stage II NSGCT patients (including 64 patients with pathological stage IIA) who were randomized after RPLND to observation or immediate adjuvant chemotherapy.88 For patients with pathological stage I NSGCT, at a median follow-up of 45 months, 28 patients (10.5%) had recurrent disease, and 6 had died, 2 of drug-resistant cancer. At the time of report, 98% of patients were alive and disease-free.
Patients with pathological stage IIA disease who are treated with orchiectomy and RPLND only will have a relapse rate as high as 30% to 40%,88 although some investigators have reported relapse rates in the 10% range in carefully selected pathological stage IIA patients. Regardless of surgical cure rate, in the cis-platinum era, the overall survival of patients with pathological stage IIA NSGCT treated initially with orchiectomy and RPLND is in the 96% to 99% range.91–91 The integration of chemotherapy into treatment schemas for stage II NSGCT is discussed below.
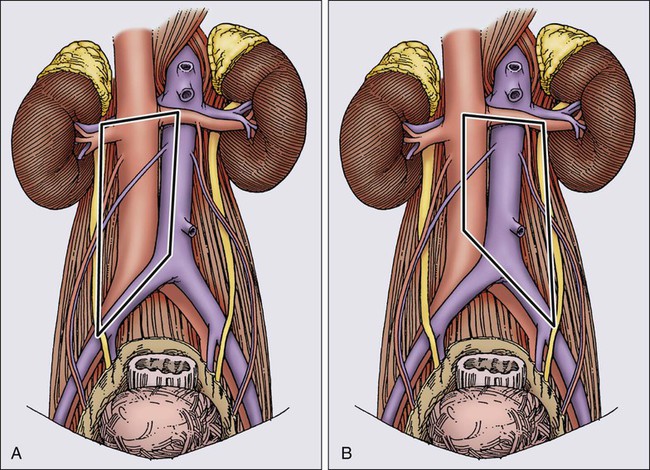
In experienced hands, a full RPLND is associated with low morbidity and mortality, but loss of ejaculatory function occurs in 65% to 100% of patients.28 It has been stressed that disruption of these fibers results in retrograde ejaculation, but not in loss of potency, libido, or ability to have an orgasm.124 The high incidence of ejaculatory dysfunction after full RPLND, coupled with an understanding of the pattern of nodal involvement in patients with low-volume disease, has led investigators to modify the traditional full RPLND. Extensive experience with full RPLND has allowed accurate mapping of nodal involvement in patients with low- and moderate-volume disease.42 As described above, this work has shown that (1) suprahilar metastases are exceedingly rare in patients with low-volume retroperitoneal disease and (2) patients with minimal-volume retroperitoneal disease almost always have unilateral retroperitoneal disease. Patients with left-sided primaries characteristically have nodal metastases to the upper left periaortic zone, whereas patients with right-sided primaries have involvement of the interaortocaval and precaval regions. These observations led to modifications of the traditional full RPLND in patients with low-volume retroperitoneal disease, whereby a suprahilar dissection was eliminated, and a limited dissection template was utilized, depending on the side of the primary. For right-sided lesions, dissection is limited on the left by the lateral margin of the aorta to the bifurcation, and for left-sided tumors, the right margin is the right renal hilum and the vena cava to the bifurcation. The templates for right and left modified RPLND are illustrated in Figure 86-4. The benefits of a modified RPLND in this group of patients were shorter operative time, shorter postoperative ileus, and a significant reduction in ejaculatory dysfunction (30% to 40% dysfunction after a right modified RPLND, and 60% to 70% dysfunction after left modified RPLND). Furthermore, modification of the traditional full RPLND in patients with minimal retroperitoneal disease does not have an adverse effect on outcome, yielding surgical cure rates of approximately 90% in pathological stage I patients, and 70% in pathological stage IIA patients and an overall long-term survival rate approaching 100%.92
The most recent modification in technique for RPLND in patients with low-stage NSGCT is the nerve-sparing dissection described by Donohue et al.93 In this procedure, postganglionic sympathetic fibers from lumbar ganglia are prospectively identified and preserved prior to lymphadenectomy, with the intent to stage and treat in a standard fashion, while preserving normal ejaculatory function. One hundred percent of 73 patients with clinical stage I patients who underwent a nerve-sparing lymphadenectomy at Indiana University had normal postoperative ejaculation. The distribution of pathological stages and relapse pattern was no different from what has been described with full or modified RPLND. Of 73 clinical stage I patients, 14 (20%) were found to have occult nodal metastases. The relapse rate was 7% in the 61 pathological stage I patients, and 28% in the 14 pathological stage IIA patients.94 All patients who suffered a relapse were salvaged with platinum-based chemotherapy. (The characteristic relapse pattern after RPLND is discussed below, and contrasted with the relapse pattern in similar patients who are not treated with an RPLND.) Thus, the nerve-sparing RPLND appears to be diagnostically and therapeutically as efficacious as more morbid full and modified retroperitoneal lymphadenectomies. Overall, approximately 30% of clinical stage I NSGCTs are found to have occult nodal involvement at RPLND, and are classified as pathological stage IIA. The use of adjuvant chemotherapy in these patients is discussed below in the section on the treatment of stage II nonseminoma.
Alternatives to RPLND
Adjuvant Radiation Therapy.
Some investigators have suggested that clinical stage I NSGCT patients felt to be at high risk of relapse could potentially be treated with adjuvant chemotherapy or radiation therapy rather than RPLND.29 Although NSGCT is less radiosensitive than seminoma and requires considerably higher radiation doses (45 to 55 Gy), radiation therapy nonetheless may occasionally be required under unusual circumstances. The results of radiation therapy alone in clinical stage I NSGCT have been reviewed. Although overall survival rates from 70% to 90% have been reported, the advent of highly effective chemotherapy has largely obviated the role of radiation therapy in the treatment of clinical stage I NSGCT.
Adjuvant Chemotherapy.
Up to 50% of patients with high-risk clinical stage I NSGCT are subsequently found to have pathological nodal involvement. As discussed below, some of these patients will go on to receive chemotherapy after RPLND or in the salvage setting. It has therefore been suggested that chemotherapy might serve as effective primary adjunctive therapy after orchiectomy, thereby sparing patients an RPLND. A phase III study explored whether adjuvant chemotherapy was superior to RPLND in men with stage I NSGCT.95 In this study, 382 men were randomly assigned to receive RPLND or one cycle of bleomycin, etoposide, and cisplatin (BEP) chemotherapy. Notably, there was no surveillance arm on this study. At a median follow-up of 4.7 years, fewer recurrences had occurred in the chemotherapy arm, with 2-year relapse-free survival rates of 99.5% for patients treated with adjuvant chemotherapy, compared to 91.9% for patients treated with RPLND. Most relapses in men who underwent RPLND occurred outside of the RPLND template. Men who received 2 cycles of adjuvant BEP did not relapse.
Because the majority of men with stage I NSGCT will never relapse, surgery or adjuvant chemotherapy results in the overtreatment of a substantial number of men. Thus, more recent studies have evaluated a risk-adapted approach, aiming to spare good prognosis patients the potential toxicities of chemotherapy or surgery. In one large Spanish study, a risk-adapted approach was applied to the selection of patients for chemotherapy following orchiectomy.75 Patients with vascular invasion or invasion of local structures received 2 cycles of BEP whereas those without these risks underwent surveillance. A relapse proportion of <1% in the first 2 years, compared with a relapse rate of 19% (71 of 358) in the patients undergoing surveillance suggests that the use of chemotherapy in this setting prevents relapse, but does not address specifically the question of the proportion of patients who were unnecessarily exposed to the risks of chemotherapy.
The most compelling of the risk-adapted studies included 745 men with stage I NSGCT enrolled in the Sweden and Norway Testicular Cancer (SWENOTECA) Management program.85 Patients with lymphovascular invasion underwent chemotherapy with one or two cycles of BEP chemotherapy, and patients without lymphovascular invasion were followed with surveillance or one course of BEP. No other criteria were used to stratify patients for treatment. The relapse rate at a median of 5 years for patients without lymphovascular invasion on surveillance was 13.5%, with the majority of relapses occurring within the first year. One cycle of BEP reduced this relapse rate in the lymphovascular invasion–negative patients to 1.3%. In line with earlier studies, the relapse rate for the small number of men with lymphovascular invasion who did not receive any adjuvant therapy was 41.7%. One cycle of adjuvant BEP reduced this rate to 3.2%, and no patients relapsed with two cycles of adjuvant BEP with a median follow-up of 4.8 years. Based on these results, adjuvant chemotherapy with one cycle of BEP should be considered a standard of care for men with stage I NSGCT with lymphovascular invasion. This avoids the problems of overtreating a significant number of men who would otherwise never relapse.
Surveillance.
Surveillance programs for patients with a negative initial staging evaluation have been evaluated primarily in nonrandomized studies.96,97 A summary of published results from nearly 600 patients who underwent surveillance after orchiectomy revealed a relapse rate of approximately 30%, but an overall disease-free survival rate of more than 95%.98 The Medical Research Council (UK) has reported on a prospective evaluation of surveillance in 373 clinical stage I NSGCT patients. Although the 5-year freedom from relapse rate was 73%, salvage surgical and chemotherapeutic interventions yielded an overall 5-year survival of 98%.99 These data have been interpreted to suggest that the strategy of surveillance yields equivalent results to RPLND in carefully selected clinical stage I NSGCT patients.100 As discussed above, risk-adapted approaches to surveillance using lymphovascular invasion status85 have been gaining acceptance.
Successful surveillance requires strict adherence to selection criteria and surveillance methodologies. Most centers rely on serum markers, physical examinations, and chest radiographs obtained every 1 to 2 months, as well as CT scan of the abdomen and pelvis every 3 or 4 months the first year, every 6 months the second year, and yearly thereafter. Eligibility criteria must include a compliant and motivated patient, in whom rigorous clinical staging (negative examination, serum markers, chest radiograph or CT, and abdominal/pelvic CT) has been undertaken. Most studies have excluded patients with locally advanced tumors (e.g., spermatic cord, scrotal sac involvement), and it has been suggested that patients at high risk for nodal metastases based on the risk assessment schemas outlined above should not undergo surveillance alone. Patient compliance with intensive surveillance programs remains a recognized problem, and successful surveillance mandates rigorous patient selection.97
Characteristically, relapses after RPLND for clinical stage I (pathological stage I or IIA) NSGCT are either serologic or occur in the chest, although some series have reported from 4% to 6% of relapses occurring in the retroperitoneum.97,100 Isolated retroperitoneal nodal relapses in the absence of elevated markers or other sites of relapse are exceedingly rare. Furthermore, relapses more than 2 years after RPLND are extraordinarily uncommon.88 By contrast, most relapses in clinical stage I patients who have selected orchiectomy alone followed with surveillance are identified by tumor markers and by and large occur in the retroperitoneum. Although some investigators have made the observation that the majority of these retroperitoneal relapses occur with bulky disease,97 there is no evidence to suggest that overall survival is affected. Additionally, surveillance patients appear to be at risk for relapse for a longer period than patients treated with RPLND. Even though most relapses occur within the first year of instituting surveillance (median, 3 to 4 months), late relapses in the third to fifth year do occur. These patterns of relapse, along with consideration of the morbidity involved with each therapeutic alternative, have formed the basis of debate regarding the relative merits of surveillance versus immediate RPLND in clinical stage I NSGCT patients.
Stage II Seminoma: Treatment and Results
The Royal Marsden Hospital clinical staging system (Table 86-5) divides stage II (abdominal) seminoma patients into subgroups on the basis of tumor bulk, as follows: IIA, smaller than 2 cm; IIB, from 2 to 5 cm; and IIC, larger than 5 cm. Historically, radiation therapy has been used for all stages of seminoma, allowing a retrospective analysis of failure rates and survival rates as a function of tumor mass. More recent series generally report better outcomes, perhaps because the era of CT scanning has allowed more precise definition of nodal margins and tumor volume.101 Reviews of recent collected series have found recurrence rates generally less than 5% in patients with masses less than 5 cm in size (stages IIA and IIB); 80% to 90% will be cured with standard abdominal radiation therapy alone. The use of salvage chemotherapy or radiation therapy (or both) in patients with masses smaller than 5 cm who relapse after initial radiotherapy results in a cause-specific survival of 95% to 100%.43 Radiation therapy is therefore recommended for small-volume (<5 cm) retroperitoneal disease. The radiation field is generally limited to the infradiaphragmatic lymph nodes as in stage I disease. Relapses, when they occur, have largely been reported to occur outside the radiation field,102 although relapse in the mediastinum or supraclavicular nodes is a distinctly unusual event,103 and when it occurs, salvage with radiation therapy or chemotherapy is successful.81 Thus supradiaphragmatic radiotherapy is not warranted in these patients.
Chemotherapy for stage IIA seminoma may allow patients to avoid some of the toxicity of radiation therapy. Single-agent carboplatin, though effective in preventing relapse for men with stage I seminoma, does not appear to be as effective as radiation therapy for IIA disease. This was illustrated in a study including 51 men with stage IIA/B disease, all of whom received 3 doses of carboplatin AUC 7.104 Although a CR was observed in 90% of patients, an unacceptably high number of men (16%) eventually experience a relapse. A second study explored combination chemotherapy with three cycles of the BEP regimen for men with stage IIA/B disease.105 Though effective (only 6/54 patients with IIB disease relapsed and 5-year overall survival was 95%), the toxicity of three cycles of BEP must be weighed against that of radiation therapy. A similar study from the SWENOTECA group confirmed good outcomes for men with stage IIA/B seminoma treated with chemotherapy.106 For most men with stage IIA/B disease, radiation therapy is considered the standard of care, with combination cisplatin-based chemotherapy an alternative for patients in whom radiation therapy is not feasible.
By contrast, failure rates of around 10% are reported in patients with masses between 5 and 10 cm, although some series have reported relapse rates as high as 40%.107 The relapse rate after radiation therapy alone in patients with masses greater than 10 cm is unacceptably high, at approximately 35%.43 Cure rates with radiation therapy alone for patients with IIC seminoma range from 30% to 60%, although an overall cause-specific survival of up to 90% can be expected with salvage chemotherapy.107 Two major problems are associated with the use of radiotherapy alone for stage IIC seminoma. The first is a risk of mediastinal or supraclavicular relapse in up to 20% of patients.103 In general, the degree of marrow compromise in patients receiving infra- and supradiaphragmatic radiation makes supradiaphragmatic radiation therapy an impractical solution to the problem. Secondly, it has been suggested that the radiation fields required for treating large masses, even if shrinking field techniques are used, make renal toxicity difficult to avoid.108 As a consequence, it is generally recommended that stage IIC seminoma patients (masses >5 cm), and especially patients with masses larger than 10 cm be initially treated with chemotherapy. This approach is discussed below, and results in cure rates from 85% to 95%.107,108
Stage II Nonseminoma
Risk Assessment
The identification of factors uniformly associated with a higher risk of relapse in node-positive NSGCT patients after RPLND has not been possible. The Testicular Cancer Intergroup Study randomized 195 patients with completely resected retroperitoneal disease to observation or two cycles of adjuvant-based chemotherapy. Among the patients randomized to observation, approximately 50% subsequently relapsed.109 Recurrence rates were higher among patients with advanced nodal stage: 40% for patients with microscopically positive nodes, 53% for nodes less than 2 cm, and 60% for nodes larger than 2 cm. Although these differences were not statistically significant, this study was likely underpowered to detect clinically significant differences. Other series have supported an association between greater nodal involvement and higher relapse rates. Patients with substantial retroperitoneal nodal involvement or extracapsular extension treated with RPLND but no adjuvant chemotherapy have a greater than 50% risk of relapse.86 By contrast, some series report significantly lower relapse rates (from 0% to 20%) than reported in the Williams trial, for patients with minimal nodal involvement who received no adjuvant therapy.112–112 The impact of histologic type on risk of relapse after RPLND in stage II NSGCT was confirmed by the Testicular Cancer Intergroup Study pooled data from stage I and II patients, which demonstrated that the presence of embryonal carcinoma clearly increased the risk of recurrence, although histology was not retained as a significant predictor of relapse after correction for vascular invasion or nodal involvement.88 Although earlier reports from the same group had suggested that vascular invasion was not a predictor of relapse in stage II NSGCT, a later study35 reported that in node-positive patients, 24% of patients without vascular invasion relapsed, compared with a 63.5% relapse rate among patients with documented vascular invasion.
Treatment of Clinical Stage II Patients
RPLND followed by adjuvant chemotherapy
Before the advent of cisplatinum-based therapy, the observed high rate of relapse after RPLND in patients who were lymph node–positive was the basis of the use of adjuvant chemotherapy in these patients. Before the late 1970s, this treatment included drugs such as bleomycin, vinblastine, and dactinomycin.113 Although it was acknowledged that more than 50% of patients would be treated unnecessarily, the poor prognosis at relapse mandated this approach. Improvements in chemotherapy for systemic disease led to the use of platinum-based therapy in the adjuvant setting.114 An international study by Williams et al.113 was published in 1987 in which 195 patients with completely resected retroperitoneal disease (108 patients with pathological stage II NSGCT) were randomized to either immediate adjuvant chemotherapy consisting of two cycles of cisplatinum, vinblastine, and bleomycin (PVB) or vinblastine, dactinomycin, bleomycin, cisplatinum, and cyclophosphamide (VAB-6) or no adjuvant therapy followed by chemotherapy at the time of relapse. With a median follow-up of 4 years, 49% of the patients in the observation arm relapsed, compared with 6% in the adjuvant therapy arm. Patients in the observation arm who relapsed were treated with salvage chemotherapy. Because of effective salvage therapy, the overall survival (97%) was the same in both arms. Two conclusions arose from this study: one, that the deferral of adjuvant treatment was appropriate, and two, that a brief course of cisplatinum-based therapy can prevent relapse. Although this report included patients with a mixed group of stage II tumors, ranging from microscopic nodal involvement (N1) to grossly involved lymph nodes greater than 2 cm (N2b) and nodes with capsular penetration (N3b), no subgroup was identified in which adjuvant chemotherapy was mandatory, or with so favorable an outcome that adjuvant therapy was not necessary. Nevertheless, as noted, and despite the results of this study, nodal stage is felt by some to have value in predicting relapse.112,114 For this reason, most centers recommend adjuvant chemotherapy over surveillance in patients with moderate retroperitoneal lymph node involvement (i.e., stage IIB disease), but an option for patients with minimal microscopic disease is surveillance after RPLND, because true pathological stage IIA patients are at a very low risk of relapse (0% to 10%). Other reports support these data.100 A more recent series reported 11 relapses (22%) in 50 NSGCT patients with low-volume nodal metastases who were managed expectantly after RPLND. Most relapses consisted of marker elevation. Five of the patients who suffered relapse had elevated markers before RPLND. The relative risk of relapse in patients with persistent marker elevation before RPLND (8.0) suggests that chemotherapy, and not RPLND, should be the treatment of choice in such patients.108 These data support earlier observations of a high relapse rate of patients with rising markers following orchiectomy (stage I S disease).115
Nonrandomized series have confirmed the high overall disease-free survival rate (98% to 100%) observed with adjuvant chemotherapy administered after RPLND. Earlier studies used either PVB or one of the VAB regimens. However, studies in the late 1980s demonstrated that the substitution of etoposide for vinblastine produced equivalent survival, with substantially less toxicity when used for patients with advanced disease.116 Consequently, several centers have tested the substitution of etoposide for vinblastine in the adjuvant setting as well. Bleomycin was omitted in two of these studies.119–119 Survival in all these series was 99% to 100%, with follow-up ranging from 30 to 72 months.
Taken together, these data demonstrate that excellent long-term survival can be achieved with RPLND followed by either observation (with salvage chemotherapy at relapse) or adjuvant chemotherapy. The disadvantage of adjuvant therapy is that it unnecessarily subjects approximately 50% of the patients (those who would have been cured with RPLND alone) to chemotherapy. Surveillance after RPLND avoids this problem, but it requires one half the patients to endure the psychological trauma of recurrent disease and further requires that patients adhere to a frequent follow-up schedule, which is often difficult in this population of patients. (In the Williams study,113 for example, 25% of the patients made 6 or fewer follow-up visits.) Finally, patients who do not receive adjuvant therapy will likely require more extensive chemotherapy for relapsed disease than had they been treated in the adjuvant setting. The excellent results of Motzer and colleagues119 suggest that two cycles of etoposide and cisplatin may be adequate in the adjuvant setting, yet a chemotherapy-naive relapsed patient would probably receive either four cycles of etoposide and cisplatin or three cycles of BEP.
Primary chemotherapy
Patients who have clinical stage IIC NSGCT have traditionally been grouped with those who have stage III disease when therapeutic options are being considered, and they are usually treated with systemic chemotherapy. The rationale for this approach includes the excellent results of chemotherapy in “good risk” disseminated disease, which includes patients with clinical stage IIC NSGCT (see below), as well as the increased morbidity associated with RPLND for patients with bulky disease.120
The excellent response of advanced disease to systemic therapy on the one hand, and the possibility of cure without an RPLND in selected clinical stage I patients on the other, are the basis for the experimental use of systemic chemotherapy without an RPLND in patients with clinical stage II NSGCT.121 Two centers, the M.D. Anderson Cancer Center (MDA) and the RMH, have reported on the use of primary chemotherapy in clinical stage II NSGCT patients.121,122 Postchemotherapy RPLND was required in only 22% to 30% of these patients. Analysis of the clinical and histologic features of the 50 MDA patients suggested that patients with tumors greater than 5 cm had the highest frequency of postchemotherapy RPLND (33%), although this number did not reach statistical significance. At the RMH, 17% of 58 stage IIA patients (nodes <2 cm) required RPLND, compared with 39% of 64 stage IIB patients. In the MDA series, the most important predictor of the need for postchemotherapy RPLND was the presence of teratomatous elements in the primary tumor (36% incidence of need for RPLND in these patients, compared with only 8% of patients with embryonal with or without seminoma; P = 0.014). A 96% CR rate with primary chemotherapy followed selectively by an RPLND was reported by MDA, whereas the 5-year actuarial survival probability for the RMH patients was 95%, with a median follow-up of 5.5 years. These series suggest that primary chemotherapy may be a therapeutic option for stage II NSGCT, obviating the need for surgery in 70% to 80% of patients. Patients particularly suited for this approach appear to be those with masses less than 2 cm in size, and with no teratomatous elements in their primary orchiectomy specimens. The risks of persistent disease or progression of residual teratoma for up to 10 years after apparent clinical eradication of stage II GCT (or both) have been cited as reasons for using this approach with caution.123,124 The degree of retroperitoneal lymphadenopathy at which patients are directed toward primary chemotherapy rather than primary RPLND varies among institutions and is, in part, based on the experience of the treating surgeon. The approach at the University of California, San Francisco (UCSF), is to perform primary RPLND in all patients with clinical stage IIA and in most patients with stage IIB disease. For some stage IIB patients with more extensive and/or bilateral retroperitoneal disease, which would not be amenable to a modified or nerve-sparing RPLND (generally masses >3 to 5 cm in size), primary chemotherapy is offered with a “good risk” (see below) regimen such as BEP for three cycles or PE for four cycles. The role of adjunctive surgery after chemotherapy in disseminated disease is discussed below.