Superior Vena Cava Syndrome
Janessa Laskin, Anthony J. Cmelak, Steven Meranze, John Yee and David H. Johnson
• Superior vena cava (SVC) syndrome is usually due to a neoplastic process, predominantly primary lung carcinoma, with a disproportionate number of patients having small cell histology; non-Hodgkin lymphoma and metastatic tumors are the next most common.
• SVC syndrome can be iatrogenic; it is sometimes seen as a complication of a central venous line or cardiac surgery.
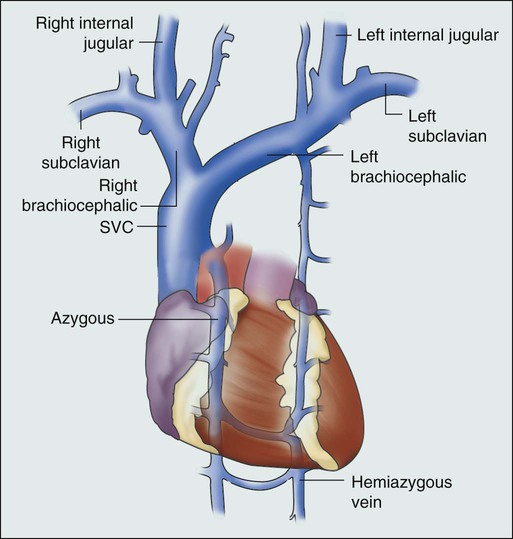
• The junction of the brachiocephalic veins forms the thin-walled, low-pressure SVC, which is subjected to obstruction from a variety of mediastinal components.
• External compression often precedes direct tumor invasion or thrombus formation.
• The SVC has an extensive collateral network.
• Usual symptoms are head “fullness,” dyspnea, cough, and chest pain, typically with insidious onset.
• More severe symptoms are infrequent, and life-threatening neurologic symptoms are rare.
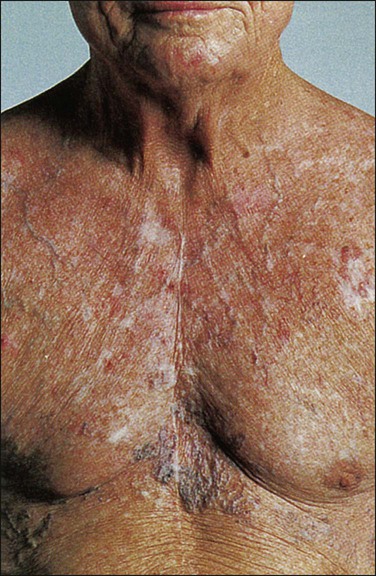
• Diagnosis is based on clinical findings such as dilated chest veins and facial edema.
• A chest radiograph typically shows mediastinal widening; a mass is often seen in the region of the SVC.
• Small-dose cavograms can be safely accomplished to define the exact location and routes of collateral flow.
• Computed tomography scanning identifies the mass and collateral flow and is the most helpful study to guide treatment.
• Treatment of an identified mass before histologic diagnosis is rarely justified unless prior diagnosis is established.
• Methods used to define histology are sputum cytology, endobronchial ultrasound with and without bronchoscopy, lymph node biopsy, thoracentesis, percutaneous biopsy, and video-assisted mediastinoscopy or thoracotomy; these techniques are considered quite safe.
• Radiation therapy with or without chemotherapy is the preferred treatment in most malignant causes of SVC obstruction, particularly in treatment-sensitive cancers such as small cell lung cancer.
• Chemotherapy would be the initial treatment of choice if a definitive diagnosis of lymphoma has been made.
• The radiation therapy fractionation schedule depends on tumor histology, stage, prognosis, the patient’s general condition, and whether the obstruction is acute or subacute.
• Surgery is usually reserved for select patients with benign causes of obstruction and consists of a bypass procedure.
• Percutaneously placed, self-expanding intravascular wire stents provide an option or adjunct to other procedures in the palliative treatment of patients (usually with malignant disease).
Introduction
Obstruction of the superior vena cava (SVC) may occur as an acute or subacute process producing a syndrome with characteristic features including facial edema and plethora, dilation of chest wall and neck veins, mild to moderate respiratory difficulty, and, less commonly, conjunctival edema, central nervous system complaints such as headache, or, more rarely, visual disturbances and signs of altered states of consciousness.1,2 The first recorded description of SVC obstruction (SVCO) occurred in 1757 when William Hunter described the entity in a patient with a syphilitic aortic aneurysm.3 For nearly two centuries thereafter, nonmalignant processes such as aortic aneurysms, syphilitic aortitis, or chronic mediastinitis due to tuberculosis were the predominant etiologic factors.1,2,4 However, these diseases are now quite rare, and cancer has become the leading cause of SVCO primarily because of the rapid increase in the incidence of bronchogenic carcinoma after World War II.1,2,5,6 Although SVCO was once considered a medical emergency, it is now well established that patients with SVCO rarely experience immediate, life-threatening complications.4,7–9 Consequently, in cases in which a diagnosis is not known, it is appropriate to first proceed with a biopsy to establish the underlying cause, because optimal management is dependent on etiology.10
Anatomy and Pathophysiology
The SVC is formed by the junction of the brachiocephalic veins, which in turn are formed by the joining of the internal jugular and subclavian veins. Thus the SVC represents the major drainage system of venous blood from the head, neck, arms, and upper thorax. The right and left brachiocephalic veins join at about the level of the sternal angle to form the SVC. The SVC descends on the right side of the ascending aorta and empties into the right atrium, with its distal 2 cm lying within the pericardial sac (Fig. 48-1).
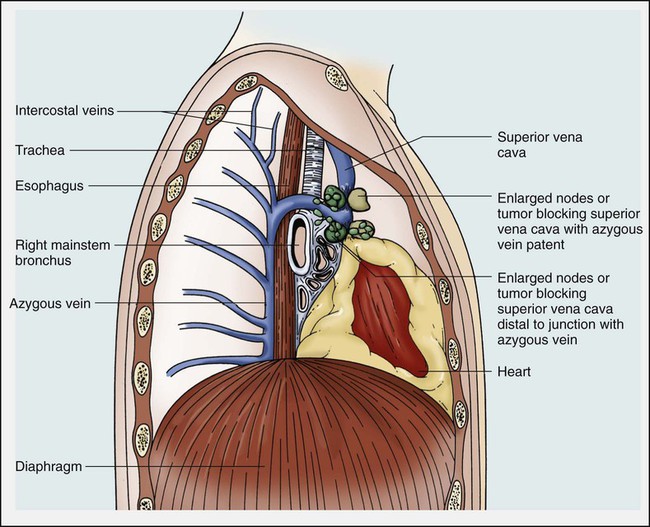
Because of its mediastinal location and because it is surrounded by several rigid structures including the sternum, trachea, pulmonary artery, right main-stem bronchus, and numerous lymph nodes, the SVC is particularly vulnerable to obstruction. Despite being a relatively large vessel, its thin vascular walls and low intravascular pressure contribute to the ease with which the SVC can be obstructed. SVCO can be caused by external compression due to tumor or by lymph nodes enlarged by inflammation or metastases (Fig. 48-2). SVCO also can be caused by direct tumor invasion or by a thrombus. Secondary thrombus is reported to occur in up to 50% of cases and may contribute to the lack of response to appropriate therapeutic maneuvers.
Etiology
Since the middle part of the twentieth century, cancer has been the principal cause of SVCO, with bronchogenic carcinoma accounting for up to 85% of cases (Table 48-1).1,2,7,11,12 The two most frequent lung cancer histologic types associated with SVCO are small cell and squamous cell carcinoma.1 Although small cell lung cancer (SCLC) accounts for just 15% to 20% of newly diagnosed lung cancers, it is the underlying cause of up to 65% of all cases of SVCO.4,13 The tendency of SCLC to occur centrally within the lung, as well as its high incidence of mediastinal lymph node metastases, most likely accounts for this consistent observation. Although lung cancer is the leading cause of SVCO, the incidence of this syndrome in patients with lung cancer is relatively low.
Table 48-1
Causes of Superior Vena Cava Syndrome
| Parish et al.2 | Yellin et al.12 | Rice et al.11 | Armstrong et al.7 | Bell et al.1 | |
| Total No. of patients | 86 | 63 | 78 | 125 | 159 |
| Lung cancer | 45 | 30 | 36 | 99 | 129 |
| Non–small cell lung cancer | 33 | 26 | 19 | 57 | 64 |
| Small cell lung cancer | 12 | 4 | 17 | 42 | 65 |
| Lymphoma | 8 | 13 | 6 | 18 | 3 |
| Metastases | 12 | 4 | 2 | 8 | 4 |
| Thymoma/thyroid | 2 | 4 | – | – | – |
| Benign | 19 | 11 | 31 | – | 2 |
| Biopsy not done | – | – | – | – | 21 |
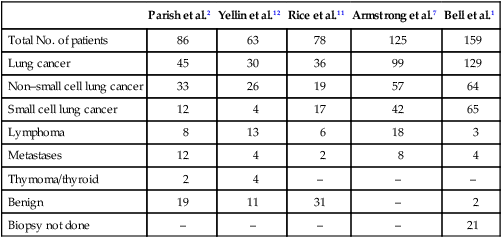
Non-Hodgkin lymphoma is the second most common cause of SVCO.2,4,14 Perez-Soler and colleagues14 identified 36 cases among 915 patients with lymphoma who were treated at the M.D. Anderson Cancer Center (University of Texas, Houston). SVCO was most commonly observed with diffuse large cell and lymphoblastic lymphomas. The frequency of mediastinal presentations with the latter histologic types may account for this association, because up to 65% of patients with lymphoblastic lymphomas are first seen with a mediastinal mass. The incidence of SVCO in these categories of non-Hodgkin lymphoma is reported to be 7% and 20%, respectively.14
Metastatic cancers account for approximately 5% to 10% of SVCOs (see Table 48-1).1,2,7,11,12 In approximate order of frequency, the most common primary tumor sites are breast cancer, germ cell malignancies, and gastrointestinal cancers. Less common primary sites include sarcomas (including primary sarcomas of the great vessels), transitional cell carcinoma, prostate cancer, and melanomas. However, virtually any cancer capable of metastasizing to the mediastinum can result in SVCO.
Nonmalignant causes of SVCO account for up to 5% of cases. An increasingly common benign cause of SVCO is central venous catheter–induced thrombosis, which may occur with cardiac pacemakers, LaVeen shunts, hyperalimentation lines, and Swan-Ganz catheters, as well as those used for chemotherapy administration.5,11,15 SVCO seems to be more common when the tip of the catheter is placed in the left subclavian vein in the upper part of the vena cava.16 Although it has been the subject of many randomized trials, no convincing evidence supports the routine use of thromboprophylactic agents in patients with central venous catheters, as demonstrated by a systemic review and metaanalysis reviewing outcomes with heparin or warfarin; the role for newer anticoagulants is less well defined.17 Additional rare benign causes of SVCO include chronic mediastinitis as a result of histoplasmosis, retrosternal goiters, Nocardia infection, and congestive heart failure.2
In children, SVCO is most frequently related to iatrogenic causes resulting from cardiovascular surgery for congenital heart disease or ventriculoatrial shunts for hydrocephalus. The most common malignant causes of SVCO in children are non-Hodgkin lymphoma, acute lymphoblastic leukemia, Hodgkin disease, neuroblastomas, and yolk sac tumors.18,19
Clinical Features
Although the duration of symptoms may range from a few days to several weeks, a majority of patients have symptoms of 4 weeks’ duration or less.1 The physical findings accompanying SVCO such as dilated chest veins and facial edema are diagnostic (Fig. 48-3). Patients frequently complain of a sense of head “fullness,” mild dyspnea, cough, chest pain, and occasionally dysphagia (Table 48-2).1,2,7,12,20 Less frequently, arm edema, stridor, upper body cyanosis, and neurologic symptoms (e.g., headaches or lethargy) may occur. All symptoms may be aggravated by positional changes, particularly those associated with lowering of the head—for example, bending to put on shoes.
Table 48-2
Signs and Symptoms of Superior Vena Cava Obstruction
| Total Patients (N = 489) |
Frequency (%) | |
| Edema/suffusion | 290 | 59 |
| Dyspnea | 254 | 52 |
| Cough | 100 | 20 |
| Pain | 60 | 12 |
| Dysphagia | 30 | 6 |
| Syncope | 12 | 2 |
| Arm edema | 104 | 21 |
| Stridor | 2 | 0.4 |
| Neurologic | 2 | 0.4 |
| Hemoptysis | 5 | 1 |
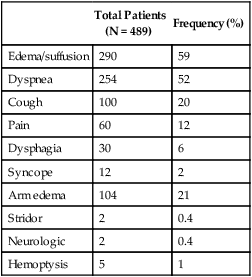
Although the clinical pattern is readily described and recognized, it was only recently that a grading or classification system was proposed based on the Common Terminology Criteria for Adverse Events that is widely used in oncology clinical trials.21 If this system were to be widely adopted, it would help streamline data collection.
The prospect of catastrophic neurologic events has led to the characterization of SVCO as an “oncologic emergency.”13,19 However, recent reviews have clearly demonstrated that although the clinical presentation can be dramatic, life-threatening neurologic symptoms such as seizures, syncope, or coma rarely occur.4,5,8,10
Radiographic Findings and Diagnostic Studies
Imaging Studies
A standard chest radiograph is the first radiographic procedure performed when SVCO is suspected, with the most common abnormality being mediastinal widening. Typically, a mass is found in the superior mediastinum, right hilum or perihilar region, or right upper lobe; however, normal findings of a chest radiograph do not rule out the diagnosis of SVCO.2
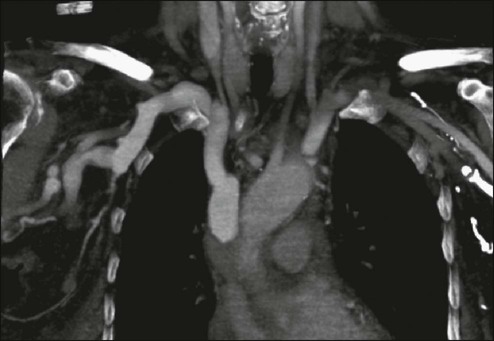
A contrast-enhanced chest computed tomography (CT) scan provides visualization of extravascular and intravascular tumor, as well as thrombus formation within the SVC, and also demonstrates collateral flow. A CT diagnosis depends on diminished or absent contrast opacification of central venous structures such as the innominate vein or the SVC inferior to the obstruction, and opacification of collateral venous routes, especially anterior subcutaneous collateral veins (Fig. 48-4).22 Because dilution of the contrast medium by unopacified blood or the displacement of blood by laminar flow may simulate an intraluminal filling defect, both criteria must be present for the diagnosis of SVCO to be made.22 The anatomy defined by a CT scan may help to guide a fine-needle aspiration biopsy or another diagnostic procedure if a histologic diagnosis has not been previously established. The current-generation helical CT scans also have been used to diagnose SVCO with results that correlate well with regular contrast CT scans. In addition, helical scans can potentially reveal more information regarding the site and extent of disease and the collateral pathways involved, as well as define soft-tissue abnormalities.
Diagnostic Approach
In the absence of a known cause of SVCO, every effort should be made to obtain a histologic diagnosis before the initiation of any therapy for two reasons.4,5,8,10 First, a definitive diagnosis is necessary to plan therapy, and second, even a brief course of radiation therapy before establishing a diagnosis can make histologic diagnosis difficult or even impossible.10,23
The least invasive diagnostic technique should be performed initially, followed by more invasive procedures as necessary (Box 48-1). Procedures commonly used to establish a tissue diagnosis include sputum cytology, bronchoscopy with or without endobronchial ultrasound-guided biopsies of the lung or mediastinum, peripheral or mediastinal lymph node biopsy, and video-assisted thoracotomy and mediastinoscopy, although the diagnosis is sometimes obtained through other means such as thoracentesis and percutaneous lung biopsy with or without ultrasound guidance.4,24–26 The complication rate of invasive procedures in the face of SVCO is fairly modest. Schraufnagel and associates8 reviewed the outcome of 93 invasive procedures in 62 patients with diagnostic problems, and none of the procedures, including bronchoscopies and mediastinoscopies, was associated with a fatal outcome. Yellin and coworkers12 performed 27 invasive diagnostic procedures in 63 patients with SVCO. No mortality or major bleeding episodes were observed, and diagnostic material was obtained in 89% of patients. Ahmann4 reported that complications of bronchoscopy and lymph node biopsies are virtually nonexistent and that contrast studies, such as nuclear medicine venography, are remarkably safe in the presence of SVCO. Of the various invasive procedures used to obtain tissue in patients with SVCO, mediastinoscopy seems to be the most risky. However, even this procedure has a relatively low complication rate. Mineo and colleagues24 reviewed the outcome of 80 patients who underwent diagnostic mediastinoscopy for SVCO by a single surgeon over a 23-year period. Five patients had significant bleeding, but only one required an urgent sternotomy, and no perioperative mortality was recorded. A definitive diagnosis was made in all of the patients; therefore mediastinoscopy should be considered if less invasive procedures are unsuccessful in determining a diagnosis. Recent advances in imaging technology allow for easier and safer investigation of the mediastinal structures using either endobronchial ultrasound or video-assisted mediastinoscopy or thoracoscopy; when available, either of these techniques would be the preferred diagnostic methodology when an invasive study is required to establish a diagnosis.25,26
The diagnostic yield from various noninvasive and invasive procedures ranges from approximately 20% for cytologic studies to virtually 100% with thoracotomy and mediastinoscopy.26–26 Although these findings may reflect publication bias, the existing data argue against the need for immediate irradiation without a histologic diagnosis in most cases. In rare circumstances, a definitive tissue diagnosis cannot be made in a timely manner. In such cases, particularly when the symptoms are severe or life threatening, it is appropriate to proceed with placement of an endovascular stent if available or radiation therapy if a stent is not possible, because either therapy is effective for most underlying causes of SVCO. Prudence dictates that prolonged attempts to establish a histologic diagnosis should be discouraged in the presence of severe dyspnea due to tracheal compression or rapidly progressive neurologic symptoms. Furthermore, in circumstances in which SVCO occurs in an individual with an established diagnosis of cancer or other known cause, an attempt to reestablish the histology of the underlying cause clearly is not a productive exercise.13
Treatment
Radiotherapy
Treatment Intent
The vast majority of patients presenting with SVCO will receive radiotherapy as part of their treatment, either to alleviate symptoms or as prophylaxis to prevent deterioration of quality of life from local progressive disease. With an efficacy rate of 80% across tumor types, localized radiation is effective at reducing tumor mass effect, is antiedematous and antiinflammatory, is antisecretory, produces relatively rapid responses, and is cost-effective.27
Dose Fractionation
Historically, all patients with SVCO were treated palliatively, and considerable controversy is found in the radiation oncology literature during the past 40 years regarding fractionation in patients with SVCO. Before 1960, treatment of SVCO generally consisted of low-dose (i.e., 50 to 100 cGy) fractions to avoid inducing “radiation edema,” which was assumed to cause further compromise of SVC patency and worsen associated symptoms. In reality, however, the fear of producing further SVC compromise by radiation-induced edema was largely unfounded, and symptoms previously ascribed to radiation edema were predominantly due to inadequately treated tumor, causing progressive obstruction.28
Rubin and associates28 undertook a retrospective comparison of a high-fraction regimen (400 cGy/day) with historical control subjects treated with lower fraction schedules (200 cGy/day). They noted more prompt relief of facial swelling after an initial high-dose-per-fraction schedule. The apparent superiority of high-dose-per-fraction regimens in providing faster symptom relief has been corroborated in several other retrospective nonrandomized studies.1,8,10 In contrast, Perez-Soler and colleagues14 found the high-dose-per-fraction regimen to be only slightly better than conventional fractions. It is our belief that for the management of patients who have the potential to be cured, 300-cGy fractions for the first 2 to 3 days is reasonable therapy for those experiencing rapidly progressive and distressing SVCO. Thereafter, the daily fraction size can be safely reduced and the total dose adjusted to compensate for the number of initial high-dose fractions. Patients with subacute SVCO treated with curative intent should be managed with conventional (e.g., 200 cGy) fractions throughout their treatment course. Effects on surrounding normal tissues treated with tolerance doses are more predictable with standard fractions. Appropriate oblique fields with spinal cord shielding can later be used to boost the target volume.
Total Dose
In the person with SVCO, the planned total radiation therapy dose should take into account the specific type and extent of underlying malignancy and normal tissue tolerance. Other considerations include prognosis and performance status, the speed at which SVCO symptoms developed, and whether chemotherapy is to be included in the treatment program. The radiation oncologist must also decide if the goal of treatment is curative or palliative and treat accordingly. If radiation alone is to be used for the curative treatment of lymphoma in adults, 3600 to 4400 cGy is commonly recommended. More commonly, however, combined-modality treatment is given, and total radiation dose can then be reduced. In persons with SCLC, typically 4500 cGy twice daily or 6000 cGy given daily with concomitant chemotherapy is recommended,29 and for persons with non-SCLC, 6000 to 7000 cGy is usually used sequentially or, more commonly, simultaneously with chemotherapy.
Field Size
Radiation field size is determined by the extent of disease, baseline pulmonary reserve as determined by pulmonary function tests and split perfusion-ventilation scans, and the type of chemotherapy, if any, the patient will receive. Toxicity of treatment, in particular radiation pneumonitis, pericarditis, and fibrosis, can carry significant morbidity and mortality if severe. Therefore efforts to minimize radiation field size carry considerable importance in the treatment of SVCO. Risk of pneumonitis is a function of many patient- and treatment-related factors: pretreatment performance status, gender (more likely in women than in men), forced expiratory volume in 1 second, low Pao2 (<80 torr), mean lung dose, and volume of lung irradiated to 20 Gy.30 To reduce the risk of pulmonary and cardiac toxicity, some groups have used induction chemotherapy followed by thoracic irradiation with a reduced treatment volume. With bronchogenic carcinoma, it has been demonstrated that the postchemotherapy tumor volume can be treated without significantly compromising local control or survival. In addition, treatment planning based on CT scans, particularly with intensity-modulated radiation therapy, and on-board imaging while the patient is in the treatment position, provides better assurance of the target volume and allows a reduction in the lung volumes irradiated. Volumes should be constricted if high total doses are used (>4000 cGy) or if methotrexate, mitomycin C, doxorubicin, or bleomycin is used concomitantly.
Response to Radiotherapy
Clinical response of SVCO signs and symptoms to various radiotherapeutic dose-fractionation schedules is high (Table 48-3).7,12,20 Radiographic and postmortem pathological studies, however, indicate that reestablishment of vena cava patency is rare, and therefore collateral flow rather than therapeutic intervention may be responsible for symptomatic improvement. Ahmann4 reported the response to radiation therapy for SVCO based on a literature review of more than 90 publications since 1934. Overall, approximately 50% to 70% of treated patients achieve symptomatic improvement within 2 weeks of the initiation of therapy. In circumstances in which serial venograms were obtained, normal venous flow through the SVC was rarely observed after completion of radiation therapy.31 In some instances, complete obstruction remained despite clinical improvement in the patient’s signs and symptoms.31
Table 48-3
Clinical Response of Superior Vena Cava Obstruction to Radiation Therapy
| Author | No. of Patients | Response Rate (%) | Recommendation |
| Yellin et al.12 | 23 | 78 | SVCO not emergency; obtain biopsy before RT |
| Armstrong et al.7 | 125 | 78 to 83 | Use initial 4-Gy fractions |
| Maddox et al.20 | 14 | 64 | Chemotherapy and RT are equally effective |
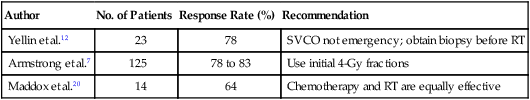
RT, Radiation therapy; SVCO, superior vena cava obstruction.
SVC patency after radiation also has been assessed in autopsy studies.4 After radiotherapy, the SVC usually is not sufficiently patent to allow adequate blood flow through the vessel. Ahmann’s literature review found that among 99 postmortem examinations, of which most patients had achieved symptomatic relief after radiation therapy, only 14 patients had complete or partial SVC patency. Although autopsy studies may be less reliable than venography obtained in living patients, these results are entirely consistent with reported radiographic data. Although radiotherapy is associated with improvement or relief of clinical signs and symptoms in most patients, the majority of patients do not actually achieve any measurable increase in vena cava blood flow. The development of collateral blood flow probably contributes to the clinical improvement in some patients and is the sole reason for improvement in others. Thus the literature does not support the traditional dogma that emergency irradiation is needed in all patients with SVCO.
Chemotherapy
Small Cell and Non–Small Cell Lung Cancer
SVCO has been reported to occur in 7% to 12% of patients with SCLC at diagnosis.20,32,33 With rare exceptions,20 the literature indicates that the presence of SVCO has little impact on the prognosis of patients with SCLC, provided that therapy is appropriately instituted.32,34 Even in the presence of SVCO, chemotherapy (with or without thoracic radiotherapy) is the preferred initial treatment of SCLC. The choice of treatment is dictated by the patient’s stage and performance status. Patients with limited-stage disease, good performance status, and no contraindication to cisplatin are best treated with cisplatin plus etoposide and concurrent chest irradiation.29 In extensive-stage disease, it is reasonable to proceed with chemotherapy alone—either cisplatin or carboplatin-based therapy—because there are toxicity and tolerability differences but little difference in terms of efficacy.35 A majority of patients will experience partial or complete resolution of signs and symptoms within 7 days of initiation of treatment, and in most series, complete resolution of symptoms has occurred within 2 weeks. Although the rapidity of symptom resolution suggests that a direct treatment effect on tumor regression is the principal cause of symptom improvement, the development of collateral drainage undoubtedly plays an equally significant role, as noted earlier. Thoracic radiotherapy does not cause transient worsening of symptoms. Although SVCO recurs in approximately 25% of patients, reinstitution of therapy with salvage chemotherapy alone, radiotherapy alone, or a combination of these modalities has resulted in the prompt resolution of symptoms in most cases.
Chemotherapy alone also has been used for the treatment of SVCO in patients with non-SCLC with positive results.36 However, because of their innate chemotherapy resistance, this approach is less effective with non–small cell tumors, and thus radiotherapy or combined-modality therapy remains the preferred initial treatment. Unlike in persons with SCLC, the presence of SVCO in persons with non-SCLC seems to have a negative impact on the prognosis of patients with locally advanced disease.36
Non-Hodgkin Lymphoma
Although symptoms can be well controlled with radiotherapy, chemotherapy alone also can effectively palliate the symptoms of SVCO in persons with non-Hodgkin lymphoma.14 Furthermore, because lymphoma is usually a systemic process and death is rarely due to localized disease, chemotherapy is usually the modality of choice for first-line therapy, and radiotherapy should never be used in isolation except when dealing with recurrent disease. Local recurrences tend to occur primarily in patients with large mediastinal masses and large cell histologic types.14 Therefore local consolidation with radiotherapy after a few cycles of chemotherapy can be justified in patients with large cell lymphoma and mediastinal masses larger than 10 cm. Conversely, in persons with lymphoblastic lymphoma, recurrences are uniformly systemic, obviating the need for radiotherapy in this histologic type of non-Hodgkin lymphoma.14 The optimal chemotherapy regimen for treatment for lymphoma-associated SVCO should be dictated by the underlying histologic type, further underscoring the need to establish a tissue diagnosis before institution of therapy. It should be noted that a fine-needle aspiration is insufficient for a complete diagnosis and characterization of a lymphoma and should not be relied on as the sole diagnostic procedure.
Surgery
Surgery generally plays a limited role in the management of patients with SVCO, although reconstruction of the obstructed SVC may be indicated in highly selected patients.37,38 Surgical intervention is reserved most often for patients with SVCO due to a benign cause such as granulomatous disease, aortic aneurysm, or retrosternal goiter.39 When symptomatic malignant obstruction is refractory to radiotherapy, chemotherapy, or both, and when anticipated survival approaches 6 months, surgery may be considered. Surgical intervention also may prove beneficial in the setting of recurrent SVCO after chemotherapy and radiation, and when caval thrombosis is the primary problem and fails to improve symptomatically with anticoagulant drugs or thrombolytic therapy.40
Surgical bypass of the obstructed SVC may be accomplished with synthetic grafts (Dacron or Gore-Tex), autologous pericardium, or autogenous vein graft, with preference for the later because of a better potential for long-term patency.37,39 The spiral vein graft, initially described by Doty and associates,38,39 is constructed by using the saphenous vein, which is slit longitudinally and wrapped around a stent (usually a chest tube) of the desired diameter. The edges of the vein are joined by using continuous suture, resulting in a large-bore conduit. The bypass is constructed between the brachiocephalic or left internal jugular vein and the right atrial appendage. Long-term relief of symptoms may be achieved. When SVCO is the result of a primary tumor of the SVC or right atrium, resection (with cardiopulmonary bypass) and reconstruction is recommended. These tumors may be relatively slow-growing sarcomas (angiosarcomas or leiomyosarcomas), and long-term palliation is sometimes possible.41
Stents
Percutaneously placed, self-expanding intravascular wire stents can offer an attractive adjunct to other procedures in the palliative treatment of patients with SVCO in the presence or absence of an underlying malignant disease.42 These devices have been placed before radiotherapy, during the course of radiotherapy or chemotherapy, and after maximum-tolerance radiation therapy.43–46 This percutaneous treatment option offers the ability to increase the lumen diameter of the SVC and does not usually require general anesthesia (Fig. 48-5). Complete symptom relief is often obtained within 24 to 48 hours in 75% to 95% of patients, depending on the underlying cause of SVCO and the type of stent deployed. Dyspnea seems to be the least likely symptom to improve, whereas facial and upper body edema improve within 48 hours in virtually all patients.44 Possible complications include acute thrombosis, retroperitoneal hemorrhage, and death due to cardiac arrhythmias; in addition, reocclusion may occur (especially in the absence of anticoagulant therapy).45 Because all currently available expandable stents have a metallic composition, their presence may preclude or limit serial follow-up with magnetic resonance imaging. This point should be taken into account before this course of treatment is chosen.
Venous access is accomplished through a femoral, jugular, or subclavian vein. It is necessary to traverse the obstruction with a guide wire to allow deployment of the stent, and in many cases, a preplacement venous angioplasty is indicated. The presence of acute thrombosis superimposed on the chronic SVCO may indicate the need for preprocedure thrombolytic therapy. Although thrombolytic therapy is associated with a known risk of hemorrhage, it may minimize the risk of thromboembolization and permit greater accuracy of stent placement.42,46 Careful consideration should be given to a preprocedure cerebral CT scan to help rule out the presence of cerebral metastases before the use of thrombolytic therapy.
Whereas a variety of metallic stents is currently available, most reports address one or more of three general device designs: the Gianturco-Rösch (sometimes referred to as the “Z-stent”; Cook, Bloomington, IN), the Wallstent (Schneider, Plymouth, MN), and the Palmaz stent (Johnson & Johnson Interventional Systems, Warren, NJ). No rigorous comparisons of outcomes have been made among the various stent designs. Each has particular qualities with respect to flexibility and radial strength, which may make one or another particularly well suited under certain clinical circumstances. Although the Wallstent and Z-stent are “self-expanding” devices, balloon dilatation may be required after stent insertion to provide a clinically adequate effect.47 Newer designs with the same self-expanding properties have been developed using nickel titanium alloy (Nitinol). These stents have the advantage of easier placement and greater radial strength than the Wallstent and are compatible with magnetic resonance imaging.48,49 The use of a Nitinol stent-graft, which is a self-expanding nitinol stent covered with Polytetrafluoroethylene, has the potential to limit ingrowth of tumor through the stent, but the experience is too limited to draw any conclusions at this time.50
Technical and clinical success and long-term venous patency have been demonstrated by several trials, which are summarized in Table 48-4.42,43,46,51–53 The overall complication rate is relatively low, although considerable variation is found in the reporting of complications, in that some studies include reocclusion as a complication. The majority of the more serious complications involve graft migration. Many patients require more than one stent either to maintain SVC confluence with adjacent vessels or because the vascular lesion is too extensive to be covered by a single stent. Close follow-up is required to monitor venous patency, and stent occlusion can generally be alleviated by a second procedure.
Table 48-4
Evaluation of Intravascular Stents
| Author | No. of Patients | Primary Patency Rate (%) | Clinical Success (%) | Overall Complication Rate (%) |
| Kee et al.42 | 51 | 95 | 78 | 10 |
| Chatziioannou et al.52 | 18 | 100 | 89 | 0 |
| Lanciego et al.51 | 52 | 92 | 100 | 25 |
| de Gregorio Ariza et al.43 | 82 | 93 | 95 | 0 |
| Nicholson et al.46 | 76 | 100 | 90 | 9 |
| Urruticoechea et al.53 | 52 | 100 | 90 | 10 |
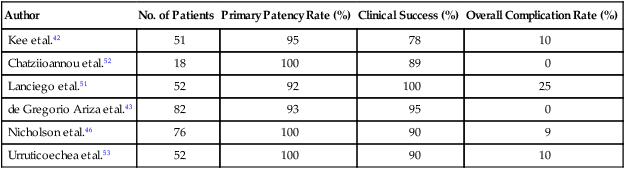
A discrepancy exists in the literature regarding balloon dilation before stent placement. Some studies advocate routine balloon dilation before stent insertion, whereas others limit such dilations to situations necessitated by very tight stenoses.42,43,51 This variation in practice may relate to the type of stent used.
Considerable variation also exists regarding routine anticoagulation after stent placement. Chatziioannou and coworkers52 recommend oral anticoagulation therapy with coumarin “for life.” Kee and colleagues42 recommend continuous oral anticoagulation for all of their patients with an underlying malignancy. In both the studies of Lanciego and associates51 and Chacon Lopez-Muniz et al.,44 patients were initially given anticoagulation with heparin and warfarin; however, over time their standard of practice changed to recommend antiplatelet agents alone, with seemingly no adverse effects. This shift in practice may reflect parallel studies of anticoagulation for coronary artery stents. Because no standard recommendations exist now, the risks and benefits of full anticoagulation versus antiplatelet agents must be weighed for individual patients.
A report by Wilson and colleagues54 highlights the difficulty of randomized trials for the treatment of SVCO. They designed a randomized trial to study primary radiation therapy versus stenting plus radiation therapy and were unable to enroll a single patient after 1 year, despite the fact that the study was being performed at a large comprehensive cancer center. However, although no randomized trials have been done or likely will be done, two studies have directly compared the results of endovascular stenting with radiation with or without chemotherapy for malignant SVCO. Tanigawa and associates55 reported the results of 33 patients treated with radiation therapy or stents. These patients were selected for a specific therapy and were not randomly assigned to a therapy. Of the 23 patients who underwent a stenting procedure, 11 had undergone ineffective radiation therapy for their obstructions. The majority of patients in the stent group and all 10 of the patients who received radiation therapy had a primary lung cancer. Symptom relief was seen in 78% of the stent group overall: 75% of those who had not had previous therapy and 82% of those who had received prior radiation. Although the rate of improvement (80%) in clinical signs and symptoms in patients who were treated with radiation therapy was similar, the median time to respond was longer at 5.5 days compared with 24 to 48 hours after stenting. No difference was found in the overall survival between the two treatment groups. These authors suggested that stenting be considered first-line therapy and stated that it is certainly indicated after an ineffective trial of radiation. Nicholson and colleagues46 reported their experience with malignant SVCO of 76 patients treated with stent insertion and compared the results retrospectively with those of 25 similar patients who had been treated with radiation therapy for malignant SVCO. Of the 76 patients, 26 were treated with stents alone, and the remainder were treated with chemotherapy or radiation before or after stent placement. The report of Nicholson and colleagues46 confirms the more rapid resolution of clinical symptoms in the stent group versus the group that received radiation alone; however, it should be noted that the radiation group was examined retrospectively. The mean asymptomatic time was significantly longer in the stent group at 21.8 weeks compared with 11.7 weeks in the radiation-only group. Although 83% of patients treated with a stenting procedure continued to have minor symptoms of swelling or venous distention, major symptoms were relieved in all 76 patients. In addition, more than 90% of patients in the stent group died without a recurrence of their SVCO symptoms, compared with only 12% in the radiation-only group. The figures for the stent group include the patients who were treated with additional therapy, although the trends are the same for the 26 patients who only underwent stent insertion. Poststent venography was performed for 19 patients. Interestingly, although they were all asymptomatic, seven of these patients had a reoccluded SVC, suggesting that collateral vessels play a significant role in symptom relief. This finding is consistent with the reocclusion after radiation therapy noted in autopsy studies.4,8
Although a definite role exists for stents in persons with malignant SVCO, the results of endothelial stent placement for benign causes are more mixed, and few long-term follow-up data are available.47 Life expectancy for patients with malignant disease is often limited, but the same may not be true in persons with benign SVCO, and therefore in these patients, long-term patency is a critical consideration. The underlying cause of the initial SVCO and the likelihood of recurrence may be more relevant in these situations. Technical success and symptom relief have been reported, primarily for venous catheter–induced SVCO.43 The gold standard for benign SVCO remains surgical bypass, although, given the variability and rarity of this condition, no studies have been performed comparing these two modalities.
Although endovascular stents may provide more rapid relief of symptoms, they are not designed to treat the underlying cause of the obstruction, whereas radiation, chemotherapy, and surgery may function both palliatively and therapeutically. As mentioned, no randomized trials are likely to compare treatments for SVCO, because the goals of each therapeutic modality are somewhat different. However, many studies report that patients who undergo stent insertion experience a rapid and almost complete resolution of the symptoms associated with SVCO.43,44,51,55 For this reason, several recent studies advocated stent placement as first-line therapy for patients with malignant causes of SVCO.46,53,55 Stent insertion should not interfere with subsequent radiation or chemotherapy and may allow symptom relief while further therapy is being planned and initiated, and thus for patients with severe symptoms, stent placement should be at the forefront of a treatment strategy.
Supportive Measures
Few medical measures are of proven benefit in the management of SVCO, other than therapy directed at the underlying cause. General recommendations include bed rest, elevation of the head of the bed, and administration of oxygen.12,13 These therapies are designed to reduce venous pressure and cardiac output. Diuretics also have been advocated without clear evidence of efficacy, although they are thought to lessen edema.13 Steroids also are sometimes administered without good documentation as to their efficacy. These agents have been advocated for their putative benefit in lessening the likelihood of radiation-induced edema. However, in practice, little inflammation or edema has been observed with irradiation. Anticoagulation and thrombolytic therapy may be of benefit when the underlying cause is an indwelling catheter, but otherwise they have not been shown to alter the course of recovery. Furthermore, because anticoagulation may impair diagnostic efforts, it should be avoided until a clear indication for its use is identified.
Summary
SVCO is usually not a true oncologic emergency, except in rare situations in which a patient experiences respiratory compromise as a result of tracheal obstruction. Often the effect of the SVC obstruction itself may be difficult to separate out from the impact of a mass exerting pressure on other local structures. Yu and colleagues21 recently published a practical treatment algorithm that is guided by the severity of the manifesting symptoms and the requirement for a tissue diagnosis. In general, administration of irradiation before histologic confirmation of the underlying cause can lead to inappropriate therapy and should be discouraged. Excessive concern about the risk of invasive procedures in the face of SVCO is likewise without foundation. Numerous reports have demonstrated the relative safety of bronchoscopy, thoracentesis, lymph node biopsy, and similar invasive procedures, particularly with the introduction of endobronchial ultrasound and video-assisted technologies to guide mediastinal sampling. In the past 5 years, numerous studies have demonstrated the palliative benefit of endovascular stent placement, particularly for malignant causes of SVCO. Stent placement does not preclude the use of additional therapy such as chemotherapy or radiation. In experienced hands, the procedure has a low rate of complications and often provides rapid and lasting relief of symptoms. Depending on availability, endovascular stenting should be considered early in the treatment of SVCO and certainly when a different primary therapy has failed. In many institutions, radiotherapy remains the treatment of choice, with exceptions made for causes known to be relatively sensitive to chemotherapy (e.g., SCLC or non-Hodgkin lymphoma).