Chapter 36 Stones in the bile duct
Endoscopic and percutaneous approaches
Historical Overview
In the 1970s and 1980s, endoscopic retrograde cholangiopancreatography (ERCP) transformed the diagnostic approach to suspected biliary disease and jaundice (see Chapters 18 and 27). Similarly, in the years since it was first performed in humans (Classen & Demling, 1974; Kawai et al, 1974), endoscopic sphincterotomy (ES) has had a dramatic impact on the management of biliary disease, specifically in the treatment of common bile duct (CBD) stones. Approximately 150,000 endoscopic biliary sphincterotomies are performed annually in the United States, and the availability of this procedure undoubtedly influences surgical decision making, when CBD stones are diagnosed on imaging or suspected, and when ERCP and ES are readily available. The surgical community initially resisted the introduction of ERCP and ES into CBD stone management, until their roles became established when they were shown to reduce the mortality rate significantly in a range of situations (Carr-Locke & Cotton, 1985; Cotton, 1984). Interest in ERCP and endoscopic sphincterotomy as definitive therapy for CBD stones grew in the 1990s after the introduction of laparoscopic cholecystectomy. Patient-related factors, clinical judgment, availability of expertise, and current evidence from clinical trials must be combined to decide on an endoscopic, percutaneous, or surgical approach. Although ERCP as a diagnostic modality has been replaced by noninvasive imaging modalities such as magnetic resonance cholangiopancreatography (MRCP; see Chapter 17), it remains the major nonoperative tool to manage biliary diseases such as CBD stones and obstructive jaundice.
Indications for Endoscopic Therapy
Patients with CBD stones may be seen initially with asymptomatic stones on noninvasive imaging or direct cholangiography or with a variety of clinical problems alone or in combination, such as cholestasis, pain, cholangitis, and pancreatitis (see Chapter 30). In the early days of ES—at a time when few endoscopy centers could offer the technique, and criticisms by surgical experts were common—it was considered justifiable only in elderly postcholecystectomy patients with recurrent or retained bile duct stones who were at high risk of serious complications from open surgical CBD exploration or reexploration (Blumgart & Wood, 1978). The impressive successes of ES in this group combined with expanded availability, a low rate of complications, and strong patient preference led many centers to widen their indications for the procedure to include younger and fitter postcholecystectomy patients and those with a gallbladder in situ. This change initially occurred in the absence of comparative trial data, and there was such enthusiasm for ES that the establishment of randomized trials was difficult, although nevertheless essential to clarify relative morbidity and mortality risks, as different groups of patients were likely to be treated empirically by endoscopic or surgical means and were not comparable (Cotton, 1984).
1 Acute cholangitis regardless of gallbladder status
2 Acute gallstone pancreatitis regardless of gallbladder status
4 Postcholecystectomy, stone shown on intraoperative cholangiogram
5 Postcholecystectomy, retained stone, early presentation
6 Postcholecystectomy, late presentation
7 Gallbladder in situ, variable risk factors for surgery, possible need for subsequent cholecystectomy
Endoscopic Techniques (See Chapter 27)
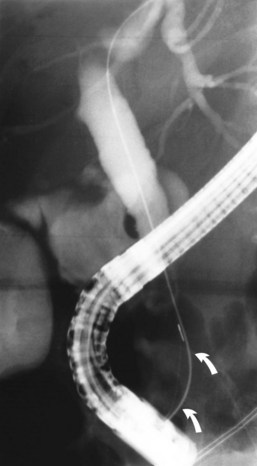
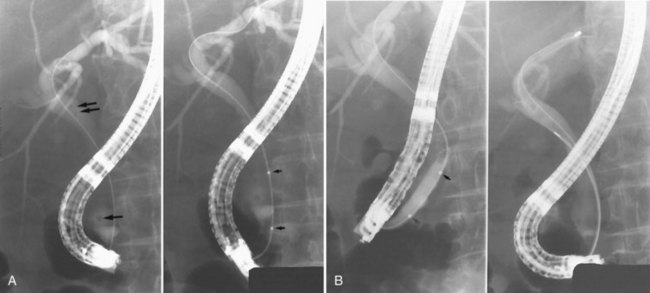
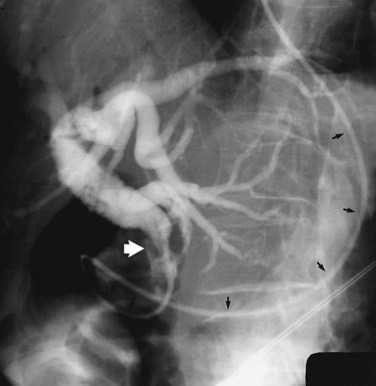
ES is usually the first therapeutic step and immediately follows diagnostic ERCP, which delineates the problem to be treated and allows accurate placement of instruments within the CBD. Standard pull-type sphincterotomes allow a vertical incision to be made from the papillary orifice in a cephalad direction along the intramural course of the CBD for a variable length (average, 10 to 15 mm) depending on local anatomy, the degree of CBD dilation, and the size of stone to be removed (Fig. 36.1). The incision is produced by the controlled application of monopolar electrocautery delivered by a generator specifically designed for endoscopic use, which does not exceed 150 W output. It is fundamental to good ES technique that complete control of wire tension and electrocautery be maintained at all times, whether the ES incision is made as a single continuous movement or in incremental steps. “Smart” generators incorporate a pulsed generator (Erbe, Tubingen, Germany; ConMed Endoscopic Technologies, Billerica, MA) with feedback-controlled power output, and there is potential for increased safety in avoiding a “zipper effect” and reducing pancreatitis and bleeding.
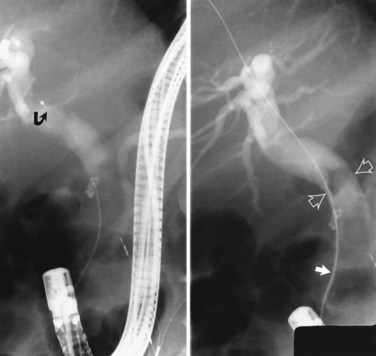
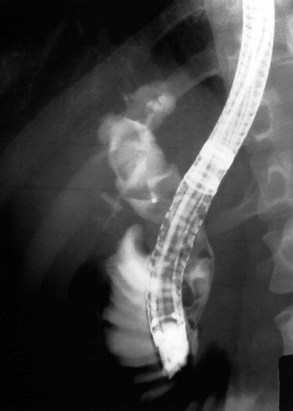
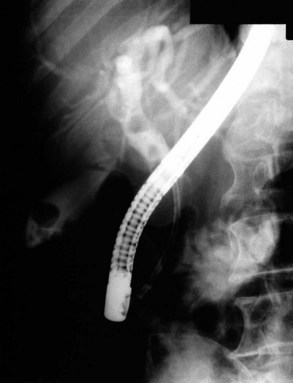
Radiographic confirmation of correct sphincterotome placement within the CBD is mandatory to avoid pancreatic trauma. Occasionally, a precut papillotomy, more appropriately called an access papillotomy, is needed to initiate ES when the standard instrument cannot be inserted deeply. This incision is often needed when cannulation has been prevented by an impacted stone. The needle-knife is more useful in this situation, because the intramural CBD is usually grossly distended and easily incised, starting from the papilla and extending cephalad. Needle-knife fistulotomy is a variant of this technique; the incision is begun above the papilla to form a choledochoduodenal fistulotomy. This technique is similar in efficacy to needle-knife papillotomy, but more often it requires mechanical lithotripsy, although it may have a lower rate of pancreatitis (Mavrogiannis et al, 1999). Patients with Billroth II partial gastrectomy (Fig. 36.2) and Roux-en-Y bypass operations present special problems to the endoscopist, and numerous methods have been described to obtain successful cannulation (Lin et al, 1999; Wright et al, 2002) and removal of CBD stones (Bergman et al, 2001).
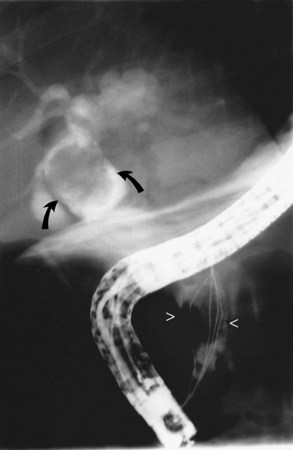
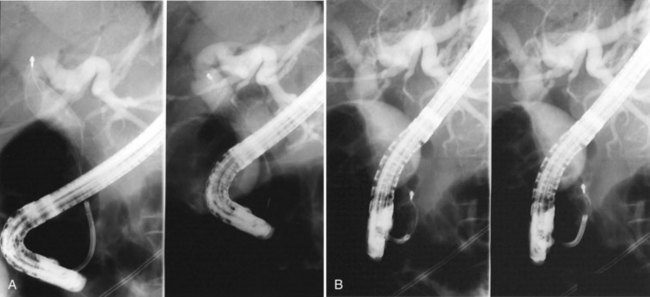
It is standard practice to attempt stone extraction from the CBD immediately after ES. The two accessory instruments used most commonly for this are the Dormia basket (Fig. 36.3) and the Fogarty balloon (Fig. 36.4), which are greater than 90% successful in clearing the CBD. It also is possible to extract stones without a preliminary ES using a balloon dilator (Fig. 36.5) (Bergman et al, 1997). Randomized trials of balloon dilation of the papilla compared with ES have shown similar outcomes for successful stone removal but an increased need for lithotripsy, and although the overall complication rates were similar, there was a higher rate of post-ERCP pancreatitis after balloon dilation if sphincterotomy was not performed beforehand (Baron & Harewood, 2004; Bergman et al, 1997; Disario et al, 2004). Large-diameter balloon dilation after sphincterectomy has become a popular and safe technique (Attasaranya et al, 2008).
Difficult Stones
The most difficult circumstances encountered during endoscopic stone removal result from technical difficulties with achieving deep biliary cannulation or in performing ES. These difficulties are sometimes because of an inaccessible papilla related to aberrant anatomy or unfavorable duodenal or papillary structures, such as a periampullary diverticulum, or prior surgery, such as Billroth II or Roux-en-Y reconstruction. Techniques have been described for the unique challenge of selective bile duct cannulation in a patient with a Billroth II partial gastrectomy (Lin et al, 1999). The performance of ES is also a challenge, because the visualized anatomy is inverted. In especially difficult cases, needle-knife sphincterotomy with a stent, nasobiliary drain, or guidewire used as a guide for cutting may be an option, or specially designed reverse-direction accessories. The literature on techniques of cannulation and sphincterotomy in Roux-en-Y reconstructions is limited (Wright et al, 2002), but some success has been reported with balloon enterosocopy or overtube-assisted ERCP (Koornstra et al, 2008, Kikuyama et al, 2009).
When ES has been successfully performed, extraction may be hindered by a variety of stone factors—including size, number, consistency, shape, and location of stones—ductal factors such as contour and diameter at the level of and distal to the stones, and the presence of coexisting pathology (e.g., stricture or tumor). Stones that are likely to be more difficult to extract and may require adjuvant techniques to remove them are those that appear larger than the endoscope on radiographic imaging (usually >15 mm); stones that are numerous or hard in consistency; stones that are square, piston shaped, or faceted that tightly fit the bile duct or that are packed against each other; intrahepatic stones; or stones located proximal to a stricture or narrowed distal bile duct or in a sigmoid-shaped duct. Techniques that have been developed to reduce stone size and facilitate endoscopic removal include mechanical lithotripsy, extracorporeal shock-wave lithotripsy (ESWL), intracorporeal lithotripsy with laser or electrohydraulic probes, and chemical contact dissolution therapy. Treatment options must be discussed jointly by the endoscopist, surgeon, and interventional radiologist when difficulties are encountered (Fig. 36.6; see Chapter 27).
Mechanical Lithotripsy
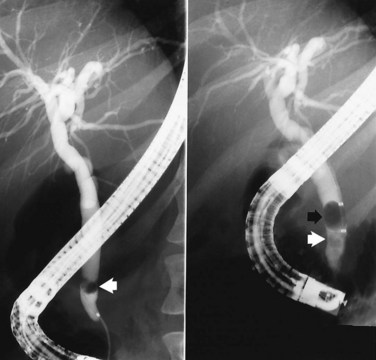
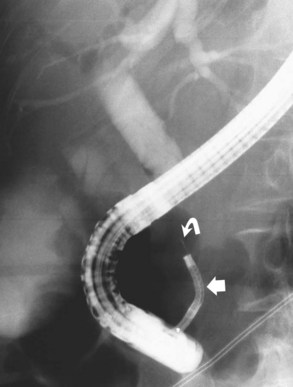
Removal of large CBD stones is a challenge for the most skilled endoscopist. Mechanical lithotripsy (see Chapter 27; Leung & Tu, 2004; Leung et al, 2001) remains the best initial option for stones that cannot be removed by conventional techniques, because it can be applied safely and effectively during the initial endoscopic procedure. Standard retrieval baskets contained within a polytef (Teflon) sheath may fail to crush large stones, because most exceed the breaking strength of the catheter. Standard-type, lithotripsy-compatible, and lithotripsy-convertible baskets are commercially available. Mechanical lithotripters are modifications of standard Dormia baskets and possess great tensile strength (Fig. 36.7). The reinforced basket is opened in the CBD, and the stone is entrapped within the braided wires. This procedure can be performed through the endoscope instrumentation channel, or it can be done after the endoscope has been removed from the patient and a metal sheath has been extended over the inner Teflon catheter. The end of the metal sheath is attached to a winding mechanism, which retracts the basket when cranked and impales the stone against the rigid distal end of the metal sheath leading to stone fracturing. The stone fragments can be removed with the same basket or a standard retrieval basket or balloon. In experienced centers, this technique allows removal of more than 90% of difficult bile stones that are refractory to standard extraction techniques (Chang et al, 2005; Shaw et al, 1993; Van Dam & Sivak, 1993).
Other Lithotripsy Modalities
For the 5% of patients with biliary stones resistant to ES and mechanical lithotripsy, other methods are available, including intracorporeal techniques (laser or electrohydraulic probes) and ESWL (Adamek et al, 1996). The choice between these methods or surgery depends largely on availability and local expertise.
Laser Lithotripsy
The first-generation laser systems using continuous wave energy neodymium:yttrium-aluminum-garnet (Nd:YAG) devices were ineffective at stone fragmentation and carried a high risk of thermal bile duct injury (Ell et al, 1988). Second-generation devices that have gained acceptance are based on high-energy, flashlamp, pulsed-dye laser technology. The application of the laser pulse leads to rapid expansion and collapse of a plasma on the stone surface, resulting in a mechanical shock wave. Initially, the only means to ensure laser-stone apposition was through the use of “mother and baby” dual endoscope systems (Cotton et al, 1990; Neuhaus et al, 1992a; Ponchon et al, 1991), but more recently, laser lithotripsy has been possible under fluoroscopic guidance with the use of devices that recognize the difference between stone and tissue. Two randomized trials comparing the xenon flashlamp pulsed rhodamine 6G laser with an integrated stone-tissue detection system with ESWL showed similar or improved efficacy in stone clearance, requiring fewer fragmentation and endoscopic sessions with use of the laser (Jakobs et al, 1997; Neuhaus et al, 1998).
Electrohydraulic Lithotripsy
Since its development during the 1950s in the former Soviet Union as a method to fragment rocks during mining, electrohydraulic lithotripsy has been adapted for medical use in the treatment of nephrolithiasis and, more recently, biliary tract calculi. The electrohydraulic probe consists of two coaxially isolated electrodes at the tip of a flexible catheter, which is capable of delivering electric sparks in short, rapid pulses leading to sudden expansion of the surrounding liquid environment and generating pressure waves that result in stone fragmentation (Picus, 1990). Stone contact with the electrode is achieved through the use of basket or balloon catheter systems (Siegel et al, 1990; Arya et al, 2004) or under direct visual targeting (Fig. 36.8) through the working channel of a daughter endoscope (Hixson et al, 1992), minimizing the risk of CBD injury and perforation. Reports document complete stone clearance after multiple sessions in 86% of patients (Adamek et al, 1996; Hixson et al, 1992; Siegel et al, 1990; Arya et al, 2004), and in a prospective nonrandomized trial, electrohydraulic lithotripsy was comparable to ESWL in stone clearance (Adamek et al, 1996). Its main advantages over laser lithotripsy are its lower cost and increased portability.
Extracorporeal Shock-Wave Lithotripsy
ESWL with a variety of lithotripsy machines is now an accepted alternative to endoscopic fragmentation of difficult bile duct stones. In contrast to intracorporeal techniques, direct contact with the stone is unnecessary. Most centers localize stones with fluoroscopic focusing during contrast perfusion of the bile duct through an endoscopically placed nasobiliary catheter or percutaneous drain (Gordon et al, 1991; White et al, 1998). Ponchon and colleagues (1990) reported ESWL success with an ultrasound localization system, although it was less effective when multiple stones were present. Several large series (Bland et al, 1989; Gilchrist et al, 1997; Sackman et al, 2001; Sauerbruch & Stern, 1989) indicated success rates for ESWL stone fragmentation of 53% to 91% and duct clearance in 58% to 90%. Minor complications are common and include hematuria, biliary pain, hemobilia, transient liver function test elevations, and cutaneous petechiae. Overall, with the use of endoscopic techniques such as mechanical lithotripsy, electrohydraulic lithotripsy, laser lithotripsy, and ESWL, one report showed successful stone removal in 98% of 217 patients, with only five patients requiring surgery (Schumacher et al, 1998).
Dissolution Therapy
Contact chemical dissolution of stones (see Chapter 28) has been attempted by perfusing the CBD with solvents administered via an indwelling nasobiliary tube, percutaneous transhepatic catheter, cholecystostomy tube, or an existing T-tube. The initial results with these agents were disappointing because of incomplete stone dissolution and complications. A semisynthetic vegetable oil, monooctanoin, composed of 70% glycerol-1-monooctanoate and 30% glycerol-1,2-dioctanoate, was used experimentally for the dissolution of CBD stones beginning in 1977. Results collected from 222 clinicians treating 343 patients with CBD stones between 1977 and 1983 reported a success rate for complete stone dissolution of only 25.6% and an additional partial success rate of 28% (Palmer & Hoffmann, 1986). Serious adverse effects leading to discontinuation of treatment occurred in 5% of patients, including hemorrhage from duodenal ulceration, acute pancreatitis, jaundice, pulmonary edema, acidosis, anaphylaxis, septicemia, and leukopenia, but no deaths were reported. The use of organic solvents, such as the aliphatic ether methyl tert-butyl ether (MTBE) (Allen et al, 1985), also has been disappointing, with complete stone dissolution achieved in only 30% to 45% and an unacceptable complication rate related to systemic absorption from spillover of solvent into the duodenum and intrahepatic bile ducts (Brandon et al, 1988; Diaz et al, 1992; Kaye et al, 1990; Murray et al, 1988; Neoptolemos et al, 1990). Sophisticated computer-controlled two-way pump systems may reduce complications and improve efficacy, but total success for large CBD stones using cholesterol solvents is unlikely, because these stones are composed primarily of bile pigments with small concentrations of cholesterol. Expectations of developing a solvent-chelating agent (ethylenediaminetetraacetic acid) for pigment stones have not been realized. As a result of its low efficacy and morbidity, contact dissolution therapy has not assumed an important role in patients with refractory CBD stones, and newer agents with better methods for instillation are awaited.
Endoprosthesis Placement
In the few situations in which stone extraction is incomplete or impossible because of stone size, local anatomy, bleeding, or technical difficulty leading to incomplete ES, a nasobiliary tube (Fig. 36.9) or endoprosthesis (Fig. 36.10) must be inserted to provide biliary decompression and prevent stone impaction in the distal CBD. This is a temporizing therapy to allow the patient’s clinical condition to improve, until complete stone clearance is achieved via additional endoscopic maneuvers or surgery. In patients with uncertain duct clearance, a follow-up cholangiogram can be obtained via a nasobiliary tube without the need for repeat ERCP.
Nasobiliary tubes are rarely tolerated beyond a few days. The primary problem with tube placement has been accidental dislodgment, and this has led to the alternative therapy of temporary biliary endoprosthesis placement (Kiil et al, 1989; Rustgi & Schapiro, 1991). In a poor-risk surgical patient, ES and long-term placement of a biliary endoprosthesis has been proposed as a nonsurgical alternative (Cotton et al, 1987; Foutch et al, 1989; Nordback, 1989; Soomers et al, 1990). Stent patency is not of major clinical importance in this situation, because the sphincterotomy typically provides an adequate conduit for bile flow around the prosthesis, which then serves principally to prevent stone impaction. The use of oral ursodeoxycholic acid in these patients has resulted in CBD stone disappearance, but additional studies are necessary to determine the true efficacy in this setting (Johnson et al, 1991). Of 84 patients intentionally treated with permanent stents for endoscopically irretrievable stones and followed for a mean of 3 years, 49 (58%) developed biliary complications, and nine died as a result of complications. Most of the patients had a long, symptom-free interval, however, before complications developed, supporting stenting as effective short-term treatment (Bergman et al, 1995; Maxton et al, 1995). In a randomized study with a shorter mean follow-up (1.5 years) that compared placement of a plastic biliary stent with ES as definitive therapy with stone clearance by means of the basket, balloon, or mechanical lithotripter, the patients with stents had a significantly greater rate of cholangitis (36%) than the patients managed by a conventional endoscopic duct clearance approach (14%). The high risk of long-term complications does not support the concept of permanent stent therapy except for patients with severe comorbidity and a short life expectancy.
Results of Endoscopic Therapy
Successful endoscopic treatment of CBD stones requires an adequate ES, which is now achieved in greater than 90% of attempts in most reported series, with noticeable improvement as experience increases (Blumgart & Wood, 1978; Cotton, 1984; Cotton & Vallon, 1981; Geenen et al, 1981; Leese et al, 1985a; Schumacher et al, 1998; Seifert et al, 1982; Siegel, 1981). Although success rates for achieving ES are fairly uniform, rates for complete clearance of the CBD vary because not all endoscopists use extraction methods routinely, and follow-up ERCP may be incomplete. Most experts now would expect to extract stones in at least 90% of successful sphincterotomies. Failure to extract or pass stones may be due to the size or number of stones within the duct or unfavorable duct diameter, usually in its retropancreatic segment. Stones 10 mm in diameter do not give rise to many problems, but in general, with size greater than 15 to 20 mm, the chance of retention increases. Interpretation of success rates necessitates care, because centers with greater expertise are more likely to be referred difficult cases that may be failures from attempts elsewhere, and this would bias some results. Patient groups also vary considerably from unit to unit and country to country, reflecting different referral patterns, selection of patients, and attitudes toward endoscopic therapy. Results from centers around the world (Cotton, 1984; Cotton & Vallon, 1981; Freeman et al, 1996; Geenen et al, 1981; Leese et al, 1985a; Nakajima et al, 1979; Reiter et al, 1978; Safrany, 1978; Schumacher et al, 1998; Seifert et al, 1982; Sherman et al, 1991; Siegel, 1981; Vaira et al, 1989) with individual and collected series of 430 to 9041 patients range from 75% to 96% for duct clearance with a median value of 91%.
Complications of Endoscopic Therapy
Early complications of ES have been well documented, and despite the disparate indications and selection of patients among centers, the incidence seems to be remarkably consistent at 5% to 10% (Cotton, 1984; Cotton & Vallon, 1981; Freeman et al, 1996; Geenen et al, 1981; Leese et al, 1985a; Masci et al, 2001; Seifert et al, 1982; Siegel, 1981; Vandervoort et al, 1996). The expected higher complication rate during early experience with ES is reflected in a single-center series comparing the results of the first 394 procedures (Leese et al, 1985a), which carried an overall morbidity rate of 10.4%, with a subsequent consecutive group of 300 sphincterotomies, in which this rate decreased to less than 6%. However, the respective proportions of individual complications remain similar in most reports: acute hemorrhage from the sphincterotomy site, 2% to 2.9%; acute pancreatitis, 1.5% to 5.4%; cholangitis, 1% to 2.7%; and retroperitoneal perforation, 0.3% to 1%, with small numbers of other problems, such as impacted basket, gallstone ileus, and acute cholecystitis overall accounting for 1.1%. Emergency surgery was required in 1% to 2.5% of cases for bleeding, cholangitis, perforation, and pancreatitis in descending order of frequency. Complication rates must be interpreted with caution, because definitions of hemorrhage, acute pancreatitis, cholangitis, and perforation often differ and are not always given.
A consensus conference provided a set of standards for defining complications and their severity (Cotton et al, 1991a). We have always included any episode of overt bleeding (hematemesis or melena) and a decrease in hemoglobin of 2 g/dL or more after ES as significant hemorrhage, although some authors have included only episodes requiring transfusion. Use of aspirin or related drugs in the usual doses does not seem to increase the incidence of hemorrhage (Freeman et al, 1996). The diagnosis of pancreatitis and cholangitis must depend on the presence of clinically recognizable syndromes rather than asymptomatic hyperamylasemia or transient elevation of temperature alone. There does not seem to be a significant influence on the rate or type of complication based on the initial presentation of the CBD stone (pain alone, jaundice alone, pancreatitis, a combination of these), except that cholangitis and cholecystitis are more likely after ES if cholangitis is preexisting. Present evidence does not suggest that complications are more likely in older patients or after previous biliary surgery (Clarke et al, 2001).
Statistically significant risk factors for complications include difficulty in cannulation, access (precut) papillotomy, suspected sphincter of Oddi dysfunction, more than two pancreatic cannulations, acinarization of the pancreas, failed biliary access or drainage, and technical skill of the endoscopist; the age and general medical condition of the patient were not statistically significant (Freeman et al, 1996; Neoptolemos et al, 1989; Vandervoort et al, 2002). Duodenal diverticula, although sometimes rendering ERCP and ES technically more difficult, do not seem to add any further risk. Many series do not include nonendoscopic complications occurring after ES, such as cardiovascular, cerebrovascular, or respiratory events. In addition, although surgery for the treatment of complications is usually documented, surgery for failed endoscopic therapy is often not documented. These factors are important if comparative data from the surgical literature are to be interpreted correctly.
Management of Complications
The management of complications by centers performing ES is well standardized, but many patients are referred from other hospitals to which they are returned after the procedure, where experience in managing complications may be limited. Of all complications, hemorrhage usually requires surgical intervention, but this occurs only in a few cases. In the rare major arterial hemorrhage, endoscopic view of the papillary area is often obscured completely by blood, and further endoscopic therapy may be impossible. The sphincterotomy usually is converted to a formal surgical sphincteroplasty, which includes the likely bleeding artery. Immediate methods for hemostasis include balloon tamponade, direct bipolar electrocautery, injecting or washing the area with 1 : 10,000 epinephrine solution, application of hemostatic clips, laser coagulation, superselective arterial catheterization and embolization, and infiltration with sclerosant (Grimm & Soehendra, 1983; Leung et al, 1995; Wilcox et al, 2004).
Acute pancreatitis is managed in the standard fashion (see Chapters 53 and 54), and although many attacks are mild and self-limited, clinicians should not be complacent, because some attacks are more severe and should be graded and treated appropriately. Wire-guided cannulation decreases the risk of post-ERCP pancreatitis when compared to the contrast-injection method (Cennamo et al, 2009). There is no evidence that pre-ES or post-ES administration of aprotinin (Trasylol) or glucagon influences the incidence or severity of pancreatitis. In contrast to hemorrhage, the onset of pancreatitis may be delayed for several hours or, rarely, for 1 or 2 days. Gabexate, a synthetic protease inhibitor, and somatostatin have been shown in randomized, double-blind controlled trials to reduce the extent of enzyme elevations and the incidence of pancreatitis after ERCP (Cavallini et al, 1996; Masci et al, 2001; Poon et al, 2003). Gabexate was infused for 30 to 90 minutes before and for 6.5 to 12 hours after ERCP, whereas somatostatin was administered either as a bolus infusion or in a manner similar to gabexate (Bordas et al, 1998; Poon et al, 1999, 2003). A trial suggested diclofenac administered as a rectal suppository after ERCP reduced the incidence of post-ERCP pancreatitis (Murray et al, 2003). Trials using secretin are in progress.
Cholangitis is confined almost completely to patients in whom CBD clearance has not been achieved, and measures should be directed at providing adequate bile drainage (e.g., by nasobiliary catheter or endoprosthesis) and administering parenteral antibiotics (see Chapter 43). Emergency surgery for cholangitis carries high risk but is indicated in patients who do not improve within 24 hours.
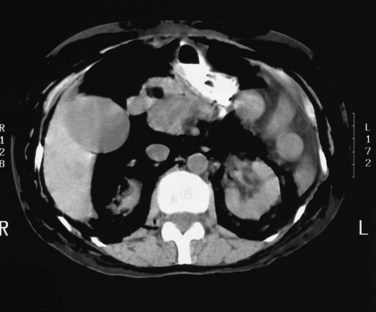
Perforation may be asymptomatic and noticed only as retroperitoneal gas (Fig. 36.11) or extravasation of radiographic contrast material, but even in a symptomatic patient, conservative treatment is often effective, with spontaneous resolution and avoidance of potentially difficult surgery. Occasionally, this complication presents late after ES with a retroperitoneal collection of bile or pus in the flank or inguinal region (Neoptolemos et al, 1984a; Leese et al, 1985a) and requires percutaneous or surgical drainage.
Mortality
Death after ES has not been reported in a standardized way. Deaths directly attributable to the procedure are fairly constant at 0.8% to 1.5% (Cotton, 1984; Cotton & Vallon, 1981; Geenen et al, 1981; Leese et al, 1985a; Nakajima et al, 1979; Reiter et al, 1978; Safrany, 1978; Seifert et al, 1982; Siegel, 1981; Sivak, 1989), with almost equal distribution of causes between hemorrhage, pancreatitis, cholangitis, and perforation. Most of the deaths are postprocedural. Mortality rate as a proportion of complications ranges from 7% to 17%, which presumably reflects the comprehensive reporting of all complications by some authors, but reporting of only more severe complications by others. Using the accepted method of reporting 30-day surgical mortality, mortality within 1 month of ES was 3.3% compared with 0.8% immediate postprocedural mortality (Leese et al, 1985a). The additional deaths were due to a variety of vascular, respiratory, renal, infectious, and malignant conditions in a group whose mean age was 79 years. Another group of 59 elderly patients considered unfit for surgery by surgeons underwent ES (Neoptolemos et al, 1984b) with only one death (1.7%).
Long-Term Morbidity
Long-term follow-up of 1 to 15 years after ES in postcholecystectomy patients (Cotton, 1984; Escourrou et al, 1984; Hawes et al, 1990; Ikeda et al, 1988; Rosch et al, 1981; Seifert et al, 1982; Sivak, 1989) shows more than 90% of patients to be well on symptomatic review and 7% to 11% have significant symptoms secondary to recurrent stones (5%), with or without stenosis of the ES site (1.5% to 3%) and cholangitis (2%). One study with long-term follow-up of 15 years after ES found an early complication rate of 15% with a high incidence of late complications, including recurrent stones and pancreatitis in 24% (Bergman et al, 1996); however, this study included only procedures performed between 1976 and 1980. A higher rate of asymptomatic stones and stenosis might result from the use of plain abdominal radiography for detecting pneumobilia, barium studies for detecting duodenobiliary reflux, radionuclide scanning, and repeat ERCP, which do not seem justified outside clinical trials. Most of these long-term complications are amenable to further endoscopic treatment.
Percutaneous Approach
In the 5% to 10% of patients for whom the endoscopic approach is unsuccessful at clearing the CBD of stones, two nonsurgical approaches are available: a rendezvous procedure and a complete percutaneous procedure (see Chapters 27 and 28). If the papillary region can be reached endoscopically, the rendezvous procedure may be used. This involves a two-stage procedure with the introduction of a percutaneous guidewire through the bile ducts and papilla into the duodenum in the first stage followed by an ERCP (Dowsett et al, 1989; Martin, 1994; Verstandig et al, 1993). In one report of rendezvous procedures for choledocholithiasis, 0.9% (15 of 1753) of ERCPs were unsuccessful at biliary cannulation, usually owing to duodenal diverticula or Billroth II anatomy (Calvo et al, 2001). Three patients underwent surgery, and 93% of the remaining patients underwent a successful rendezvous procedure. There was one complication with a retroperitoneal perforation that required surgical management, and during follow-up, only one patient developed recurrent choledocholithiasis requiring a repeat rendezvous procedure. In the multicenter prospective trial of endoscopic biliary sphincterotomy complications (Freeman et al, 1996), the combined endoscopic-percutaneous approach was a risk factor for the development of complications, with a high rate of complications at 22.6% with 6.5% classified as severe.
The complete percutaneous approach is labor intensive and typically requires multiple sessions. It has been established as treatment for hepatolithiasis (Yeh et al, 1995; see Chapters 39 and 44), but it may be used for choledocholithiasis. The procedure involves initial establishment of a transhepatic fistula, followed by stone extraction under fluoroscopy or cholangioscopy 7 to 8 days after the fistula forms. In a series of 31 patients with failed endoscopic procedures, percutaneous biliary access was achieved in all patients, and stone clearance was complete in 87% after a mean of 5.6 sessions (Van der Velden et al, 2000). All patients underwent balloon dilation of the papilla, and most patients required additional means of stone fragmentation, including mechanical lithotripsy, electrohydraulic lithotripsy, and ESWL. Complications including pancreatitis and bacteremia occurred in 9.7% and did not require surgical intervention. The four patients who did not respond to percutaneous management underwent surgery. Bile duct access by endoscopic ultrasonography has largely removed the need for percutaneous approaches (see Chapter 14).
Complications, Mortality Rates, and Long-Term Results of Bile Duct Surgery
Open Bile Duct Exploration (See Chapters 29 and 35)
Morbidity and mortality after open bile duct exploration have been well documented (Allen et al, 1981; Blamey et al, 1983; Dixon et al, 1983; Doyle et al, 1982; Girard & Legros, 1981; Glenn, 1974, 1975; Kune, 1972; McSherry & Glenn, 1980; Pitt et al, 1981; Vellacott & Powell, 1979; Way et al, 1972), with examination of specific risk factors (Blamey et al, 1983; Dixon et al, 1983; Pitt et al, 1981) that allow some prediction of the likelihood of complications and perhaps predict the need for preoperative biliary drainage. Direct comparison with the endoscopic data is not valid, because extremely diverse groups of patients are being treated by these modalities. This situation highlights the concept of “apples and oranges” succinctly stated by Cotton (1984), but mention must be made of published surgical figures to enable some clinical judgments to be made in the absence of randomized trials. It is immediately apparent that, in contrast to endoscopic therapy, surgical morbidity and mortality are determined by patient age, comorbidities (Glenn, 1975; McSherry & Glenn, 1980; Vellacott & Powell, 1979), hemotologic and biochemical factors (Blamey et al, 1983; Dixon et al, 1983; Pitt et al, 1981), and whether the operation is elective (Houghton & Donaldson, 1983; Sullivan et al, 1982) or emergent (Boey & Way, 1980; Thompson et al, 1982). Mortality rates range from 1% in relatively fit younger patients to 28% in unfit and elderly patients and 12% to 14% in younger patients undergoing emergency surgery for cholangitis. These figures nearly all refer to primary bile duct operations and should be compared only with results of ES when the gallbladder is in situ. Equivalent results for secondary bile duct explorations are less well recorded, but mortality rates of 2% are possible for elective surgery (Girard & Legros, 1981; McSherry & Glenn, 1980). Consideration also must be given to biliary drainage operations, which avoid problems of stone retention, although this may be at the expense of an increased postoperative morbidity and mortality. Average mortality rates are 1% to 5% for choledochoduodenostomy and transduodenal sphincteroplasty (Capper, 1961; Jones, 1978; Lygidakis, 1981; Madden et al, 1970; Speranza et al, 1982; Stuart & Hoerr, 1972). A review of 246 such operations (Baker et al, 1985) in which one of these drainage procedures was used revealed a mortality rate of 5.4% for each of the two types of operations and major morbidity rates of 12% for choledochoduodenostomy and 21% for transduodenal sphincteroplasty. The results stated here should be read in conjunction with those reported in Chapters 29 and 35.
Long-Term Follow-up of Surgically Explored and Reexplored Bile Ducts
Long-term follow-up data after open bile duct exploration are lacking. The wide variation in reported recurrent stone rates has been mentioned, but morbidity and the need for further surgery have been found in 5% after 5 years (Larson et al, 1966), 10% after 12 years (Peel et al, 1975), and 21% after 6 to 11 years (Lygidakis, 1983) when exploration without biliary drainage has been performed. After choledochoduodenostomy (see Chapter 29), there was no morbidity in a 6- to 11-year follow-up (Lygidakis, 1983) with similar results for transduodenal sphincteroplasty (Degenshein, 1964; Stuart & Hoerr, 1972). Follow-up of 90% of the survivors of one series of 246 patients 1 to 12 years postoperatively (mean, 4.4 years) revealed that complications had been treated in 3% of the choledochoduodenostomy group (mainly sump syndrome) and 6% of the transduodenal sphincteroplasty group (mainly cholangitis), with additional symptoms discovered on interview in 8% of the choledochoduodenostomy and 5% of the transduodenal sphincteroplasty groups.
Laparoscopic Common Bile Duct Exploration
Laparoscopic CBD exploration (see Chapter 34) was first performed in the early 1990s and is now used increasingly in experienced tertiary referral centers (Khoo et al, 1996; Martin et al, 1998; Rhodes et al, 1995). Ductal exploration may be accomplished through the cystic duct or directly through a choledochotomy. The transcystic route is the least invasive and generally does not require any ductal manipulation or drainage procedure, whereas choledochotomy requires either closure of the duct over a T-tube or primary closure of the choledochotomy with or without a biliary stent placed in an antegrade fashion without need for a T-tube (Huang et al, 1996; Rhodes et al, 1995). Bile duct clearance rates average 90%, with a median rate of conversion to open operation of 4% (Tranter & Thompson, 2002). Complication rate is 2.5%, with a median mortality rate of 1% (Strasberg et al, 1995).
In a randomized trial of laparoscopic CBD exploration versus postoperative ERCP for CBD stones (Rhodes et al, 1998), the clearance rates at the first intervention were the same (75%). With subsequent treatment, predominantly in the form of repeated ERCP, duct clearance rates approached 100% in both groups. In a similar study sponsored by the European Association of Endoscopic Surgeons (Cuschieri et al, 1996), patients were randomized to preoperative ERCP followed by laparoscopic cholecystectomy or laparoscopic cholecystectomy with laparoscopic CBD exploration. Duct clearance was similar in the two groups, but there was a higher rate of conversion to open surgery and decreased length of stay with single-stage surgical treatment. Our group has shown that therapeutic ERCP can be performed safely as an outpatient procedure without need for hospitalization (Tham et al, 1997). To minimize hospital stay, the outpatient ERCP would need to be coupled with laparoscopic cholecystectomy, which requires good coordination between the surgeon and the endoscopist.
Specific Clinical Problems
Postcholecystectomy with T-Tube In Situ (See Chapter 29)
CBD stones of less than 10 mm in diameter detected in the early postoperative period on T-tube cholangiography may pass spontaneously or with hydrostatic pressure from flushing or perfusing the T-tube. Most of these stones require additional mechanical manipulation, however. Increased morbidity, mortality, and retained stone rate after secondary bile duct explorations stimulated the development of alternative techniques, including hydraulic T-tube irrigation with or without pharmacologic relaxation of the sphincter of Oddi with glucagon or nitrates (Tritapepe et al, 1988), T-tube infusion of cholesterol solvents (Tritapepe et al, 1988), T-tube tract choledochoscopy and lithotripsy (Josephs & Birkett, 1992), and percutaneous extraction of stones through a mature T-tube tract. Success rates with the last technique have ranged from 77% to 96%, but multiple sessions are often required and the technique may be complicated by sepsis, biliary trauma, and biliary leakage in 4% to 8% of patients (Burhenne, 1980; Caprini, 1988; Cotton, 1990; Mason, 1980; Nussinson et al, 1991; Tritapepe et al, 1988). Delays of 4 to 6 weeks are required before manipulation to allow maturation of the T-tube tract.
Alternatively, early ES can be applied safely and effectively after stone detection without the need for T-tube maturation, allowing timely discharge from hospital. The results from eight endoscopic series totaling 337 patients indicate an overall endoscopic success rate of 90% with a morbidity rate of 7% and mortality rate of 0.6% (Bickerstaff et al, 1988; Danilewitz, 1989; Lambert et al, 1988; Nussinson et al, 1991; O’Doherty et al, 1986; Simpson et al, 1985; Soehendra et al, 1981; Tandon et al, 1990). The choice between these two techniques depends on local expertise, because direct comparisons have not been made in controlled trials.
Postcholecystectomy Without T-Tube
The incidence of retained CBD stones is approximately 2% to 5% after conventional and laparoscopic cholecystectomy and 5% to 15% after CBD exploration. ES remains the treatment of choice for elderly patients seen days to years after cholecystectomy (Danilewitz, 1989), because these patients are at higher risk from further abdominal surgery (Cranley & Logan, 1980; Sheridan et al, 1987). ES also is recommended for choledocholithiasis in young, fit postcholecystectomy patients, even those with normal diameter ducts (Cotton et al, 1998).
Pregnancy
Endoscopic treatment can be performed safely with a complication rate comparable to that in nonpregnant patients (Jamidar et al, 1995; Kahaleh et al, 2004; Tham et al, 2003, Simmons et al, 2004). We reviewed our experience with 15 pregnant patients who underwent ERCP (Tham et al, 2003). Precautions were taken to minimize fetal exposure to radiation, which included covering the pelvis of all patients with an abdominal lead apron and minimizing fluoroscopy. No adverse outcomes occurred to the mother or the fetus, and the average fetal radiation dose was 310 mrad, which is below the 5- to 10-rad level considered teratogenic. These patients should undergo cholecystectomy on completion of pregnancy to prevent recurrence.
Patients with Gallbladder In Situ
In elderly patients or in patients with significant comorbidity, a deliberate decision often is made to leave the gallbladder in situ after ES and CBD stone removal. The short-term and long-term results and complications of ES in patients with gallbladders do not differ from those in postcholecystectomy patients (Kaw et al, 2002). One randomized study of high-risk surgical patients reported a higher rate of recurrent biliary symptoms in the ES group compared with patients who underwent open cholecystectomy with operative cholangiography (Targarona et al, 1996); however, details of the endoscopic procedure were not reported in the article, and the failure rate for ES was high at 20%. In view of their shorter life expectancy, high-risk patients can be managed expectantly, unless symptoms dictate otherwise. Careful follow-up for 5 to 10 years in patients with intact gallbladders indicates that approximately 10% develop gallbladder symptoms or complications sufficient to warrant cholecystectomy, and most occur in the first year (Davidson et al, 1988; Escourrou et al, 1984; Hill et al, 1991; Rosseland & Solhaug, 1984; Siegel et al, 1988; Tanaka et al, 1987). The risk for developing gallbladder symptoms is similar to that of patients with asymptomatic cholelithiasis (Gracie & Ransohoff, 1982). Most patients requiring cholecystectomy can be treated electively.
The risk of developing biliary symptoms seems to depend on the continuing presence of stones in the gallbladder. In two reports (Siegel et al, 1988; Tanaka et al, 1987), none of the patients with acalculous gallbladders at initial ES developed symptoms or complications that subsequently required cholecystectomy. In patients with gallstones, there were no reliable predictors to identify patients with cholelithiasis who were likely to develop gallbladder complications after ES. Nonfilling of the gallbladder with radiographic contrast material at ERCP (Worthley & Toouli, 1988) or with radiotracer by nuclear scintigraphy (Holbrook et al, 1991) may confer a higher likelihood of developing symptoms, although results from other series do not concur (Davidson et al, 1988; Hill et al, 1991). These results suggest that the low risk of subsequent gallbladder complications after ES and bile duct clearance, when patients are kept under supervision, outweighs the known higher mortality rate of cholecystectomy in high-risk patients and mitigates against routine cholecystectomy in this group. Few physicians would recommend this approach in a fit patient, in whom the expectation for long-term survival and development of symptoms necessitating cholecystectomy are greater. The risk of elective cholecystectomy is probably smaller than the risk of subsequently developing gallbladder symptoms in this group, so elective cholecystectomy would seem most appropriate. This idea was confirmed by a study of 120 patients with known gallstones who underwent successful ES with bile duct clearance; the study excluded patients who were not surgical candidates, and patients were randomized to laparoscopic cholecystectomy within 6 weeks after ES or a wait-and-see policy with median 2.5-year follow-up. Nearly half the patients in the wait-and-see group developed biliary-related problems, including biliary pain and cholecystitis, which led to cholecystectomy or repeat ERCP or both, whereas none of the patients in the cholecystectomy group experienced biliary-related problems. The wait-and-see group also had a higher rate of conversion to an open procedure at the time of surgery (Boerma et al, 2002).
The place of preoperative ES in young, low-risk surgical patients with suspected choledocholithiasis has been examined to determine whether preoperative ES confers an advantage in terms of morbidity, mortality, and length of hospital stay compared with a conventional approach of open cholecystectomy, operative cholangiography, and CBD exploration. Two prospective, randomized controlled trials (Neoptolemos et al, 1987; Stiegmann et al, 1992) have addressed this question with the conclusion that no advantage is conferred by initial endoscopic bile duct clearance before cholecystectomy. High-risk surgical patients were excluded from these studies, and both studies were conducted in the era of open cholecystectomy, before the use of high-quality MRCP.
Laparoscopic Cholecystectomy and Endoscopic Retrograde Cholangiopancreatography
Laparoscopic cholecystectomy (see Chapter 34) has replaced open cholecystectomy as the procedure of choice for gallbladder removal in most patients with symptomatic cholelithiasis. Optimal management of patients with suspected or documented CBD stones varies according to the expertise available in a particular institution. Laparoscopic transcystic CBD exploration (Hunter, 1992) or direct choledochotomy (Jacobs et al, 1991) can be performed at the time of laparoscopic cholecystectomy, but these procedures require special expertise and equipment. Laparoscopic transcystic choledochoscopy is hindered by the small size and tortuosity of the cystic duct, making instrument passage and stone lithotripsy and removal potentially difficult.
In patients with suspected CBD stones, MRCP has replaced ERCP as the diagnostic modality of choice to identify the presence or absence of CBD stones (see Chapter 17). A need for endoscopic management has evolved, with ERCP playing a central role in preoperative and postoperative CBD stone removal. Initially, we and others (Cotton et al, 1991b) found it useful to stratify patients preoperatively into low, intermediate, and high likelihood for CBD stones (Table 36.1) based on established clinical and biochemical indicators, including elevated liver function tests, a dilated CBD (>8 mm on ultrasound), and a history of jaundice or pancreatitis (Del Santo et al, 1985; Hauer-Jensen et al, 1985; Lacaine et al, 1980). In the absence of clinical or biochemical indicators, CBD stones are present in only 2% to 3% of individuals (Neuhaus et al, 1992b). Small asymptomatic stones may be of minor clinical relevance, because their natural history is unknown, and conceivably many if not most of these calculi would pass spontaneously without ever coming to clinical attention. We have favored selective evaluation of the CBD only for patients with risk factors for choledocholithiasis.
Table 36.1 Cholangiography Stratification for Risk of Choledocholithiasis
| High Likelihood (Preoperative ERCP) |
ERCP, endoscopic retrograde cholangiopancreatography; CBD, common bile duct; IOC, intraoperative cholangiography
We have proposed three general strategies to manage patients with suspected or documented CBD stones (Tham et al, 1998). Algorithm 1 offers the advantage of complete surgical management at a single procedure without the need for additional postoperative stone extraction. The major disadvantage is the high rate of conversion to an open procedure, which is not in keeping with the desire to maintain a minimally invasive approach to therapy, but it is required in some patients (Cuschieri et al, 1999; Huang et al, 1996). This strategy has not gained widespread acceptance, but it would be a reasonable approach in centers incapable of providing adequate endoscopic therapy, or when techniques are achieved for performing laparoscopic CBD exploration. Algorithm 2 focuses on endoscopic cholangiography and stone clearance before performance of laparoscopic cholecystectomy. This approach seems justified in a patient with a high likelihood of a CBD stone; it would avoid prolonging the operative procedure with intraoperative cholangiography while maintaining the minimally invasive approach and reducing the need for conversion to an open CBD exploration for laparoscopic failures (Tham et al, 1998; Wright et al, 2002). It is often possible to remove small stones (3 mm in diameter) during ERCP through the intact papilla, sparing the patient the potential short-term and long-term risks of ES (Cotton, 1993; Ibuki et al, 1992; Staritz et al, 1985). Algorithm 3 uses laparoscopic transcystic cholangiography to detect CBD stones and postoperative ERCP to remove stones in patients with positive cholangiograms. This strategy relies heavily on ERCP expertise, because failed stone removal necessitates referral to a more experienced endoscopist or a second open operative procedure. The choice between these strategies should be strongly influenced by the surgical and endoscopic expertise available. It should be stressed that noninvasive imaging of the CBD with MRCP has replaced ERCP as the diagnostic modality of choice for patients with suspected CBD stones.
At our institution, in a prospective series of 1847 consecutive laparoscopic cholecystectomies (Table 36.2), preoperative ERCP (algorithm 2) was performed in 135 (7.3%) patients considered highly likely to have CBD stones based on a total bilirubin greater than 2 mg/dL, alkaline phosphatase greater than 150 U/L, a dilated CBD on ultrasound, or a recent history of jaundice or pancreatitis. ERCP was successful in 133 (98.5%) patients, and 43 (32%) were found to have CBD stones. All stones were removed after sphincterotomy, with nine complications: six cases of pancreatitis (three mild, three moderate), two of hemorrhage (one mild, one moderate), and one fever with negative blood cultures. Aberrant ductal anatomy was present in two ERCPs, Mirizzi syndrome (see Chapter 42A, Chapter 42B ) was present in one, and 83 were normal. Using stricter criteria for preoperative ERCP selection, we increased the preoperative detection rate of CBD stones from 32% to 56% predicted.
Table 36.2 Endoscopic Retrograde Cholangiopancreatography in 1847 Laparoscopic Cholecystectomies at Brigham & Women’s Hospital (1990–1992)
| Preoperative ERCP | 135 (7.3%) |
| Normal | 83 (62%) |
| Stones | 43 (32%) |
| Mirizzi syndrome | 1 (0.7%) |
| Aberrant anatomy | 2 (1.5%) |
| Failed ERCP | 2 (1.5%) |
| Intraoperative cholangiography | 87 (5%) |
| Successful | 39 (45%) |
| Stones | 2 (2%) |
| Not attempted | 1625/1847 (88%) |
ERCP, endscopic retrograde cholangiopancreatography
Acute Cholangitis
Acute cholangitis (see Chapter 43) resulting from CBD stones traditionally was managed by supportive measures and parenteral antibiotics, followed by early surgery if improvement was slow or absent. The mortality from emergency surgery ranges from 12% to 16% with higher rates for elderly patients (Boey & Way, 1980; Cotton, 1984; Thompson et al, 1982). In the 1980s, we analyzed the treatment outcomes of 82 patients with severe acute cholangitis and CBD stones admitted over a 7-year period during which ES was available (Leese et al, 1985b). Mean age was 71 years (range, 19 to 88 years), with 87% older than 60 years and 23% older than 80 years. Overall 30-day mortality rate was 14.6% but varied considerably with different modes of therapy. Eleven patients received conservative treatment only; of these, seven responded to antibiotics alone, but four (36.4%) were moribund and died before any treatment could be instituted. Seventy-one patients underwent early biliary decompression either surgically (n = 28) or endoscopically (n = 43). Of the 11 postcholecystectomy patients, four had early surgery with biliary-digestive bypass, with two deaths within 30 days (50%), and seven had early ES with no deaths. Of the 60 patients with gallbladders, 24 were treated surgically (mean age, 62 years) with a mortality rate of 16.7%; 13 had an ES followed by elective cholecystectomy (mean age, 64 years) with no deaths; and 23 had an ES with gallbladders in situ. Of these, six died from unrelated causes, and two required surgery for empyema of the gallbladder—at 19 days after ES in one and after recurrence of cholangitis at 5 months in the other. Complications after ES occurred in 10 patients: five hemorrhages, three exacerbations of cholangitis, one mild acute pancreatitis, and one gallstone ileus requiring surgical intervention. Bile duct clearance was achieved in 40 (93%) of 43 patients. Of the three failures, one patient died without further therapy, one underwent surgery 4 weeks later, and one remained asymptomatic, having declined further treatment. The 30-day mortality rate for patients treated by early surgery was 21.4% (six of 28), and 30-day mortality rate for patients treated by early ES, regardless of subsequent treatment, was 4.7% (two of 43). We concluded that patients not responding to standard initial therapy within 24 hours should be offered endoscopic biliary decompression and bile duct clearance if this is locally available. The only randomized trial of emergency endoscopic versus surgical management of severe calculous cholangitis (Lai et al, 1992) showed a threefold difference in mortality rate (10% vs. 32%; P < .03) in favor of ERCP.
Gallstone Pancreatitis
Acute pancreatitis (see Chapters 53 and 54) resulting from impaction of gallstones in the ampulla of Vater was first reported by Opie in 1901. From his observations in this study, an “obstructive theory” was derived to explain the mechanism responsible for gallstone pancreatitis. Stone impaction in the common channel of the pancreatic duct and CBD is postulated to result in obstruction of the two systems with resultant reflux of bile into the pancreatic duct (Moody et al, 1993). In support of the obstructive theory, gallstones can be recovered from the feces of 85% to 95% of patients (Acosta & Ledesma, 1974; Kelly, 1980a, 1980b), and the incidence of CBD stones is 80% in patients undergoing urgent operative or endoscopic intervention compared with a 5% to 30% incidence when the procedure is delayed (Acosta et al, 1978; Kelly, 1980a, 1980b; Ranson, 1979; Stone et al, 1981).
The Ranson, Imrie, Glasgow, and Acute Physiology, Age, and Chronic Health Evaluation (APACHE II) assessments provide well-established criteria for assessing the severity of pancreatitis and predicting local adverse events—such as necrosis, hemorrhage, infection, and pseudocyst formation—and systemic complications of acute respiratory distress syndrome, disseminated intravascular coagulation, distant fat necrosis, and renal failure (see Chapters 52 to 54; Banks, 1991). Stratifying patients by severity based on these criteria has been helpful in directing appropriate management. Most patients experience mild pancreatitis resulting from transient impaction of a stone in the ampulla followed by spontaneous migration into the duodenum. These patients do well with conservative therapy alone and are unlikely to benefit from urgent intervention. In contrast, it has been proposed that more severe cases of pancreatitis result from persistent stone impaction or choledocholithiasis with infected bile, suggesting the possibility that early stone extraction by surgical or endoscopic techniques would halt progression of the acute event and prevent the development of future attacks in the short term.
Early surgical therapy in cases of acute gallstone pancreatitis has been challenged owing to the high operative morbidity and mortality. Results and conclusions from numerous series comparing early and late surgical therapy in gallstone pancreatitis are difficult to interpret. The mortality rates range from 2% to 67%, studies are retrospective with frequent comparisons to historical controls, and stratification for severity of illness has not been used (Acosta et al, 1978; Kim & Bosner, 1988; Osborne et al, 1981; Ranson, 1979). In one series, Kelly and Wagner (1988) prospectively randomized 165 patients with gallstone pancreatitis to early or delayed surgery. In the group with severe pancreatitis, mortality was 48% after urgent operative intervention compared with 11% mortality rate in patients in whom surgery for gallstones was delayed for more than 48 hours. In contrast, patients with mild pancreatitis had mortality rates of 3.3% and 0%, respectively. Another study of moderate to severe gallstone pancreatitis with peripancreatic fluid collections confirmed these results, with complications in 44% of patients in the early surgery group compared with 6% in the delayed surgery group (Nealon et al, 2004). These results would favor avoidance of early operative intervention in the acute phase of biliary pancreatitis, unless local complications from necrotizing disease dictate otherwise.
An endoscopic approach to gallstone pancreatitis offers the theoretical advantage of immediate relief of ampullary obstruction and ductal clearance without the risks of general anesthesia or surgery. There was initial reluctance to perform ERCP in patients with acute pancreatitis because of concerns that the procedure would exacerbate the illness and lead to death. The Leicester investigators (Neoptolemos et al, 1986) were the first to perform a controlled study to evaluate the efficacy of ERCP in biliary pancreatitis. A group of 121 patients with suspected gallstone pancreatitis was randomized to urgent (within 72 hours of hospitalization) ERCP or conventional nonendoscopic management. ES was performed if bile duct stones were present on ERCP. CBD stones were found in 63% of the patients in the predicted severe group and in only 26% of patients with predicted mild attacks. Patients with mild pancreatitis had favorable outcomes regardless of treatment strategy, with a similar incidence of complications and no deaths. In the group of patients with severe pancreatitis defined by modified Glasgow criteria, urgent ES significantly reduced the morbidity (local and systemic complications) from 61% to 24% and reduced the length of hospitalization from 17 days to 9.5 days compared with conservatively managed patients. Mortality was lower in the ERCP group (4% vs. 18%), but the difference did not reach statistical significance.
A second study published by the Hong Kong group (Fan et al, 1993) prospectively randomized 195 consecutive patients with pancreatitis of any cause to urgent (within 24 hours of hospital admission) ERCP with papillotomy or initial conservative treatment with selective ERCP only if the clinical condition deteriorated. In this region of the world, where biliary lithiasis is frequently responsible for acute pancreatitis, emergency ERCP resulted in a reduction of biliary sepsis from 12% in the conservatively treated patients to 0% in patients undergoing endoscopic papillotomy and stone extraction. Overall, no significant differences were found in the incidences of either local (10.3% vs. 12.2%) or systemic (10.3% vs. 14.3%) complications. In the subset of patients with severe pancreatitis and CBD stones, urgent ES resulted in a decrease in the combined incidence of local and systemic complications to 21% and mortality rate to 5.3% compared with the conservatively managed group, in which the rates were 68.8% and 25%, respectively.
A third randomized trial (Nowak et al, 1998) had a different design from the two preceding studies. All 280 patients with acute gallstone pancreatitis in the study underwent urgent duodenoscopy within 24 hours of onset of their disease; nearly 25% were found to have impacted stones at the papilla, which were treated by immediate sphincterotomy. The remaining 205 patients were randomized to conventional treatment or sphincterotomy regardless of the cholangiographic findings. Compared with conventional treatment, the authors showed a significant advantage for patients treated endoscopically with respect to morbidity (17% vs. 36%; P < .001) and mortality (2% vs. 13%; P < .001). Both rates also were more strikingly reduced the earlier the endoscopic intervention was undertaken. Because all patients seem to benefit regardless of severity, the conclusion was that emergency ERCP and sphincterotomy were indicated in all patients with acute gallstone pancreatitis, but further analysis of the data is required when the full report is available.
A much criticized fourth randomized multicenter study in Germany (Folsch et al, 1997) showed that endoscopic intervention in gallstone pancreatitis in the absence or low likelihood of bile duct obstruction or cholangitis did not show benefit, but increased morbidity and mortality rates were reported in the ERCP group that were not previously found in other trials. The study has been criticized because it randomized significantly fewer patients with severe pancreatitis, and 19 of 22 centers contributed fewer than two patients per year to the study.
These studies show that ERCP and endoscopic stone extraction can be performed safely in the setting of acute biliary pancreatitis. Urgent ERCP and ES can be performed with good rationale in patients with severe or progressive pancreatitis, owing to the resultant reduction in major local peripancreatic complications and the increasingly recognized coexistence of acute cholangitis, potentially translating to reduced mortality. Subsequent elective cholecystectomy should be considered, although in a high-risk patient the gallbladder may be left in situ. In a patient with mild pancreatitis, no immediate intervention is advised. If a patient later is proved to have gallstones or microlithiasis (Lee et al, 1992; Targarona et al, 1995), elective laparoscopic cholecystectomy with preoperative ERCP or intraoperative cholangiography should be performed. In a postcholecystectomy patient, endoscopic therapy is definitive. The role of ES in lieu of cholecystectomy for a high-risk patient with an intact gallbladder and gallstone pancreatitis in the absence of choledocholithiasis requires further evaluation (May & Shaffer, 1991).
Acosta JM, Ledesma CI. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484-487.
Acosta JM, et al. Early surgery for acute gallstone pancreatitis: evaluation of a systematic approach. Surgery. 1978;83:367-370.
Adamek HE, et al. Management of retained bile duct stones: a prospective open trial comparing extracorporeal and intracorporeal lithotripsy. Gastrointest Endosc. 1996;44:40-47.
Allen B, et al. Management of recurrent and residual common duct stones. Am J Surg. 1981;142:41-47.
Allen MJ, et al. Rapid dissolution of gallstones by methyl-tert-butyl ether. N Engl J Med. 1985;312:217-220.
Arya N, et al. Electrohydraulic lithotripsy in 111 patients: a safe and effective therapy for difficult bile duct stones. Am J Gastroenterol. 2004;99:2330-2334.
Attasaranya S, et al. Large diameter biliary orifice balloon dilation to aid in endoscopic bile duct stone removal: A multicenter series. Gastrointest Endosc. 2008;67:1046-1052.
Baker AR, et al. Sump syndrome following choledochoduodenostomy and its endoscopic management. Br J Surg. 1985;72:433-435.
Banks PA. Predictors of severity in acute pancreatitis. Pancreas. 1991;6(Suppl 1):7-12.
Baron TH, Harewood GC. Endoscopic balloon dilation of the biliary sphincter compared to endoscopic biliary sphincterotomy for removal of CBD stones during ERCP: a meta-analysis of randomized, controlled trials. Am J Gastroenterol. 2004;99:1455-1460.
Bergman JJ, et al. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile duct stones: report on 117 patients. Gastrointest Endosc. 1995;42:195-201.
Bergman JJ, et al. Long-term follow-up after endoscopic sphincterotomy for bile duct stones in patients younger than 60 years of age. Gastrointest Endosc. 1996;44:643-649.
Bergman JJ, et al. Randomized trial of endoscopic balloon dilation versus endoscopic sphincterotomy for removal of bile duct stones. Lancet. 1997;349:1124-1129.
Bergman JJ, et al. A randomized trial of endoscopic balloon dilation and endoscopic sphincterotomy for removal of bile duct stones in patients with a prior Billroth II gastrectomy. Gastrointest Endosc. 2001;53:19-26.
Bickerstaff KI, et al. Early postoperative endoscopic sphincterotomy for retained biliary stones. Ann R Coll Surg Engl. 1988;70:350-351.
Blamey SL, et al. Prediction of risk in biliary surgery. Br J Surg. 1983;70:535-538.
Bland KI, et al. Extracorporeal shock wave lithotripsy of bile duct calculi. Ann Surg. 1989;209:743-755.
Blumgart LH, Wood CB. Endoscopic treatment of biliary tract diseases. Lancet. 1978;2:1249. (letter)
Boerma D, et al. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile duct stones: a randomized trial. Lancet. 2002;360:761-765.
Boey JH, Way LW. Acute cholangitis. Ann Surg. 1980;190:264-270.
Bordas JM, et al. Effects of bolus somatostatin in preventing pancreatitis after endoscopic pancreatography: results of a randomized study. Gastrointest Endosc. 1998;47:230-234.
Brandon JC, et al. Common bile duct calculi: updated experience with dissolution with ethyl-tertiary-butyl-ether. Radiology. 1988;166:665-667.
Burhenne JH. Percutaneous extraction of retained biliary tract stones. Am J Roentgenol. 1980;134:888-898.
Calvo MM, et al. The rendezvous technique for the treatment of choledocholithiasis. Gastrointest Endosc. 2001;54:511-513.
Capper WM. External choledochoduodenostomy, an evaluation of 125 cases. Br J Surg. 1961;49:292-300.
Caprini JA. Biliary stone extraction. Am J Surg. 1988;54:343-346.
Carr-Locke DL, Cotton PB. Endoscopic surgery: biliary tract and pancreas. Br Med Bull. 1985;42:257-264.
Cavallini G, et al. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy—Italian Group. N Engl J Med. 1996;335:919-923.
Cennamo V, et al. Can a wire-guided cannulation technique increase bile duct cannulation rate and prevent post-ERCP pancreatitis? A meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104(9):2343-2350.
Chang WH, et al. Outcome of simple use of mechanical lithotripsy of difficult common bile duct stones. World J Gastroenterol. 2005;11:593-596.
Clarke GA, et al. The indications, utilization and safety of gastrointestinal endoscopy in an extremely elderly patient cohort. Endoscopy. 2001;33:580-584.
Classen M, Demling L. Endoscopische Sphinkterotomie der Papilla Vater. Dtsch Med Wochenschr. 1974;99:496-497.
Cotton PB. Endoscopic management of bile duct stones (apples and oranges). Gut. 1984;25:587-597.
Cotton PB. Retained bile duct stones: T-tube in place, percutaneous or endoscopic management? Am J Gastroenterol. 1990;85:1075-1078.
Cotton PB. Removing duct stones without sphincterotomy. Gastrointest Endosc. 1993;39:312.
Cotton PB, Vallon AG. British experience with duodenoscopic sphincterotomy for removal of bile duct stones. Br J Surg. 1981;68:373-375.
Cotton PB, et al. Endoscopic stenting for long-term treatment of large bile duct stones: 2- to 5-year follow up. Gastrointest Endosc. 1987;33:411-412.
Cotton PB, et al. Endoscopic laser lithotripsy of large bile duct stones. Gastroenterology. 1990;99:1128-1133.
Cotton PB, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393.
Cotton PB, et al. Laparosopic cholecystectomy and the biliary endoscopist. J Gastrointest Endosc. 1991;37:94-97.
Cotton PB, et al. Endoscopic sphincterotomy for stones by experts is safe, even in younger patients with normal ducts. Ann Surg. 1998;227:201-243.
Cranley B, Logan H. Exploration of the common bile duct: the relevance of the clinical picture and the importance of pre-operative cholangiography. Br J Surg. 1980;67:869-872.
Cuschieri A, et al. EAES ductal stone study: preliminary findings of multi-center prospective randomized trial comparing two-stage versus single-stage management. Surg Endosc. 1996;10:1130-1135.
Cuschieri A, et al. EAES multicenter prospective randomized trial comparing two-stage vs single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc. 1999;13:952-957.
Danilewitz MD. Early postoperative endoscopic sphincterotomy for retained common bile duct stones. Gastrointest Endosc. 1989;35:298-299.
Davidson BR, et al. Endoscopic sphincterotomy for common bile duct calculi in patients with gallbladder in situ considered unfit for surgery. Gut. 1988;29:114-120.
Degenshein GA. Choledocho-duodenostomy: an 18-year study of 175 consecutive cases. Surgery. 1964;76:316-324.
Del Santo P, et al. Prediction of operative cholangiography in patients undergoing elective cholecystectomy with routine liver function chemistries. Surgery. 1985;98:7-11.
Diaz D, et al. Methyl tert-butyl ether in the endoscopic treatment of common bile duct radiolucent stones in elderly patients with nasobiliary tube. Dig Dis Sci. 1992;37:97-100.
Disario JA, et al. Endoscopic balloon dilation compared with sphincterotomy for extraction of bile duct stones. Gastroenterology. 2004;127:1291-1299.
Dixon JM, et al. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983;24:845-852.
Dowsett JF, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96:1180-1186.
Doyle PJ, et al. The value of routine peroperative cholangiography: a report of 4000 cholecystectomies. Br J Surg. 1982;69:617-619.
Ell C, et al. Laser lithotripsy of common bile duct stones. Gut. 1988;29:746-751.
Escourrou J, et al. Early and late complications after endoscopic sphincterotomy for biliary lithiasis, with and without the gallbladder “in situ.”. Gut. 1984;25:598-602.
Fan ST, et al. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232.
Folsch UR, et al. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997;336:237-242.
Foutch HPG, et al. Endoscopic placement of biliary stents for the treatment of high-risk geriatric patients with common duct stones. Am J Gastroenterol. 1989;84:527-529.
Freeman ML, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918.
Geenen JE, et al. Resumé of a seminar on endoscopic retrograde sphincterotomy (ERS). Gastrointest Endosc. 1981;27:31-38.
Gilchrist AM, et al. Extracorporeal shockwave lithotripsy for common bile duct stones. Br J Surg. 1997;84:29-32.
Girard RM, Legros G. Retained and recurrent bile duct stones: surgical or non-surgical removal? Ann Surg. 1981;193:150-154.
Glenn F. Retained calculi within the biliary ductal system. Ann Surg. 1974;179:528-539.
Glenn F. Trends in surgical treatment of calculus disease of the biliary tract. Surg Gynecol Obstet. 1975;140:877-884.
Gordon SJ, et al. Successful shockwave lithotripsy of bile duct stones using ultrasound guidance. Dig Dis Sci. 1991;36:1102-1109.
Gracie W, Ransohoff D. The natural history of silent gallstones. N Engl J Med. 1982;307:798-800.
Grimm H, Soehendra N. Unterspritzung zur Behandlung der Papillotomie-Blutung. Dtsch Med Wochenschr. 1983;108:1512-1514.
Hauer-Jensen M, et al. Predictive ability of choledocholithiasis indicators. Ann Surg. 1985;202:64-68.
Hawes RH, et al. Follow-up 6 to 11 years after duodenoscopic sphincterotomy for stones in patients with prior cholecystectomy. Gastroenterology. 1990;98:1008-1012.
Hill J, et al. Risk of leaving the gallbladder in situ after endoscopic sphincterotomy for bile duct stones. Br J Surg. 1991;78:554-557.
Hixson LJ, et al. Peroral cholangioscopy with intracorporeal electrohydraulic lithotripsy for choledocholithiasis. Am J Gastroenterol. 1992;87:296-299.
Holbrook RF, et al. Biliary patency imaging after endoscopic retrograde sphincterotomy with gallbladder in situ: clinical impact of nonvisualization. Arch Surg. 1991;126:738-742.
Houghton PJW, Donaldson LA. Elective biliary surgery: a safe procedure. Geriatr Med. 1983;13:814-816.
Huang SM, et al. An alternative approach of choledocholithotomy via laparoscopic choledochotomy. Arch Surg. 1996;13:407-411.
Hunter JG. Laparoscopic transcystic common bile duct exploration. Am J Surg. 1992;163:53-56.
Ibuki Y, et al. Endoscopic retrograde extraction of common bile duct stones with drip infusion of isosorbide dinitrate. Gastrointest Endosc. 1992;38:178-180.
Ikeda S, et al. Endoscopic sphincterotomy: long-term results in 408 patients with complete follow-up. Endoscopy. 1988;20:13-17.
Jacobs M, et al. Laparoscopic choledocholithotomy. J Laparoendosc Surg. 1991;1:79-82.
Jakobs R, et al. Fluoroscopically guided laser lithotripsy versus extracorporeal shock wave lithotripsy for retained bile duct stones: a prospective randomized study. Gut. 1997;40:678-682.
Jamidar PA, et al. Endoscopic retrograde cholangiopancreatography in pregnancy. Am J Gastroenterol. 1995;90:1263-1267.
Johnson G, et al. Treatment of non-extractable common bile duct stones with combination ursodeoxycholic acid (UDCA) plus endoprosthesis. Gastrointest Endosc. 1991;37:253.
Jones SA. The prevention and treatment of recurrent bile duct stones by transduodenal sphincteroplasty. World J Surg. 1978;2:473-485.
Josephs LF, Birkett DH. Laser lithotripsy for the management of retained stones. Arch Surg. 1992;127:603-605.
Kahaleh M, et al. Safety and efficacy of ERCP in pregnancy. Gastrointest Endosc. 2004;60:287-292.
Kaw M, et al. Management of gallstone pancreatitis: cholecystectomy or ERCP and endoscopic sphincterotomy. Gastrointest Endosc. 2002;56:61-65.
Kawai K, et al. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20:148-151.
Kaye GL, et al. Methyl-tert-butyl ether dissolution therapy for common bile duct stones. J Hepatol. 1990;10:337-340.
Kelly TR. Gallstone pancreatitis: the timing of surgery. Surgery. 1980;88:34-35.
Kelly TR. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery. 1980;104:600-605.
Kelly TR, Wagner DS. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery. 1988;104:600-605.
Khoo DE, et al. Laparoscopic common bile duct exploration: evolution of a new technique. Br J Surg. 1996;83:341-346.
Kiil J, et al. Large bile duct stones treated by endoscopic biliary drainage. Surgery. 1989;105:51-56.
Kikuyama M, et al. ERCP after Roux-en-Y reconstruction can be carried out using an oblique-viewing endoscope with an overtube. Digestive Endoscopy. 2009;21(3):180-184.
Kim U, Bosner B. Timing of surgery for acute gallstone pancreatitis. Am J Surg. 1988;156:393-396.
Koornstra JJ, Fry L, Monkemuller K. ERCP with the balloon-assisted enteroscopy technique: a systematic review. Dig Dis. 2008;26(4):324-329.
Kune GA. Current Practice of Biliary Surgery. Boston: Little, Brown; 1972.
Lacaine F, et al. Preoperative evaluation of the risk of common bile duct stones. Arch Surg. 1980;115:1114-1116.
Lai EC, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586.
Lambert ME, et al. Endoscopic removal of retained stones after biliary surgery. Br J Surg. 1988;75:896-898.
Larson RE, et al. The early and long-term results of 500 consecutive explorations of the common duct. Surg Gynecol Obstet. 1966;122:744-750.
Lee SP, et al. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589-593.
Leese T, et al. Successes, failures, early complications and their management following endoscopic sphincterotomy: results in 394 consecutive patients from a single centre. Br J Surg. 1985;72:215-219.
Leese T, et al. Management of acute cholangitis and the impact of endoscopic sphincterotomy. Gut. 1985;26:A553.
Leung JW, Tu R. Mechanical lithotripsy for large bile duct stones. Gastrointest Endosc. 2004;59:688-690.
Leung JW, et al. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factors and the role of epinephrine injection. Gastrointest Endosc. 1995;42:550-554.
Leung JW, et al. Mechanical lithotripsy in the common bile duct. Endoscopy. 2001;33:800-804.
Lin LF, et al. ERCP in post–Billroth II gastrectomy patients: emphasis on technique. Am J Gastroenterol. 1999;94:144-148.
Lygidakis NJ. Choledochoduodenostomy in calculous biliary tract disease. Br J Surg. 1981;68:762-765.
Lygidakis NJ. Surgical approaches to recurrent choledocholithiasis. Am J Surg. 1983;145:633-639.
Madden JL, et al. Choledochoduodenostomy, an unjustly maligned surgical procedure? Am J Surg. 1970;119:45-54.
Martin DF. Combined percutaneous and endoscopic procedures for bile duct obstruction. Gut. 1994;35:1011-1012.
Martin LJ, et al. Towards T-tube free laparoscopic bile duct exploration: a methodologic evolution during 300 consecutive procedures. Ann Surg. 1998;228:29-34.
Masci E, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417-423.
Mason R. Percutaneous extraction of retained gallstones via the T-tube tract: British experience of 131 cases. Clin Radiol. 1980;1:497-499.
Mavrogiannis C, et al. Needle-knife fistulotomy versus needle-knife precut papillotomy for the treatment of common bile duct stones. Gastrointest Endosc. 1999;50:334-339.
Maxton DG, et al. Retained common bile duct stones after endoscopic sphincterotomy: temporary and long-term treatment with biliary stenting. Gut. 1995;36:446-449.
May GR, Shaffer EH. Should elective endoscopic sphincterotomy replace cholecystectomy for the treatment of high-risk patients with gallstone pancreatitis? J Clin Gastroenterol. 1991;13:125-128.
McSherry CK, Glenn F. The incidence and causes of death following surgery for non-malignant biliary tract disease. Ann Surg. 1980;191:271-275.
Moody FG, et al. Another challenge to the Opie myth. Gastroenterology. 1993;104:927-931.
Murray B, et al. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786-1791.
Murray WR, et al. Choledocholithiasis: in vivo stone dissolution using methyl tertiary butyl ether (MTBE). Gut. 1988;29:143-145.
Nakajima M, et al. Five years experience of endoscopic sphincterotomy in Japan: a collective study from 25 centers. Endoscopy. 1979;2:138-141.
Nealon WH, et al. Appropriate timing of cholecystectomy in patients who present with moderate to severe gallstone-associated acute pancreatitis with peripancreatic fluid collections. Ann Surg. 2004;239:741-751.
Neoptolemos JP, et al. Abdominal wall staining and “biliscrotum” after retroperitoneal perforation following endscopic sphincterotomy. Br J Surg. 1984;71:684.
Neoptolemos JP, et al. The management of common bile duct calculi by endoscopic sphincterotomy in patients with gallbladders in situ. Br J Surg. 1984;71:69-71.
Neoptolemos JP, et al. A prospective study of ERCP and endoscopic sphincterotomy in the diagnosis and treatment of gallstone acute pancreatitis: a rational and safe approach to management. Arch Surg. 1986;121:697-702.
Neoptolemos JP, et al. Prospective randomized study of preoperative endoscopic sphincterotomy versus surgery alone for common bile duct stones. Br Med J. 1987;294:470-474.
Neoptolemos JP, et al. A multivariate analysis of preoperative risk factors in patients with common bile duct stones: implications for treatment. Ann Surg. 1989;209:157-161.
Neoptolemos JP, et al. Methyl-tert-butyl-ether for treating bile duct stones: the British experience. Br J Surg. 1990;77:32-35.
Neuhaus H, et al. Laser lithotripsy of pancreatic and biliary stones via 3.4 mm and 3.7 mm miniscopes: first clinical results. Endoscopy. 1992;24:208-214.
Neuhaus H, et al. Prospective evaluation of the use of endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy. Endoscopy. 1992;24:745-749.
Neuhaus H, et al. Randomized study of intracorporeal laser lithotripsy versus extracorporeal shock-wave lithotripsy for difficult bile duct stones. Gastrointest Endosc. 1998;47:327-334.
Nordback I. Management of unextractable bile duct stones by endoscopic stenting. Ann Chir Gynaecol. 1989;78:290-292.
Nowak A, et al. Biliary pancreatitis needs endoscopic retrograde cholangiopancreatography with endoscopic sphincterotomy for cure. Endoscopy. 1998;30:A256-A259.
Nussinson E, et al. A 10-year single centre experience of percutaneous and endoscopic extraction of bile duct stones with T tube in situ. Gut. 1991;32:1040-1043.
O’Doherty DP, et al. Endoscopic sphincterotomy for retained common bile duct stones in patients with T-tube in situ in the early post-operative period. Br J Surg. 1986;73:454-456.
Opie EL. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp. 1901;12:182-188.
Osborne DH, et al. Biliary surgery in the same admission for gallstone-associated pancreatitis. Br J Surg. 1981;68:758-761.
Palmer KR, Hoffmann AF. Intraductal mono-octanoin for the direct dissolution of bile duct stones: experience in 343 patients. Gut. 1986;27:196-202.
Peel ALG, et al. How should the common bile duct be explored? Ann R Coll Surg Engl. 1975;56:124-134.
Picus D. Intracorporeal biliary lithotripsy. Radiol Clin North Am. 1990;28:1241-1249.
Pitt HA, et al. Factors affecting mortality in biliary tract surgery. Am J Surg. 1981;141:66-72.
Ponchon T, et al. Extracorporeal lithotripsy of bile duct stones using ultrasonography for stone localization. Gastroenterology. 1990;98:726-732.
Ponchon T, et al. Pulsed-dye laser lithotripsy of bile duct stones. Gastroenterology. 1991;100:1730-1736.
Poon R, et al. Prophylactic effect of a somatostatin on post-ERCP pancreatitis: a randomized controlled study. Gastrointest Endosc. 1999;49:593-598.
Poon R, et al. Intravenous bolus somatostatin after diagnostic cholangiopancreatography reduces the incidence of pancreatitis associated with therapeutic endoscopic retrograde cholangiopancreatography procedures: a randomized controlled trial. Gut. 2003;52:1768-1773.
Ranson JHC. The timing of biliary surgery in acute pancreatitis. Ann Surg. 1979;189:654-662.
Reiter JJ, et al. Results of endoscopic papillotomy: a collective experience from nine endoscopic centers in West Germany. World J Surg. 1978;2:505-511.
Rhodes M, et al. Laparoscopic exploration of the common bile duct: lessons learned from 129 consecutive cases. Br J Surg. 1995;82:666-668.
Rhodes M, et al. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet. 1998;351:159-161.
Rosch W, et al. Long-term follow-up after endoscopic sphincterotomy. Endoscopy. 1981;13:152-153.
Rosseland AR, Solhaug JH. Early or delayed endoscopic papillotomy (EPT) in gallstone pancreatitis. Ann Surg. 1984;199:165-167.
Rustgi AK, Schapiro RH. Biliary stents for common bile duct stones. Gastrointest Endosc Clin N Am. 1991;1:79-91.
Sackman M, et al. Extracorporeal shock wave lithotripsy for clearance of bile duct stones resistant to endoscopic extraction. Gastrointest Endosc. 2001;53:27-32.
Safrany L. Endoscopic treatment of biliary tract diseases. Lancet. 1978;2:983-985.
Sauerbruch T, Stern M. Fragmentation of bile duct stones by extracorporeal shock waves: a new approach to biliary calculi after failure of routine endoscopic measures. Gastroenterology. 1989;96:146-152.
Schumacher B, et al. Endoscopic treatment of symptomatic choledocholithiasis. Hepatogastroenterology. 1998;45:672-676.
Seifert E, et al. Langzeitresultate nach endoskopischer Sphinkterotomie: follow-up-Studie aus 25 Zentren in der Bundesrepublik. Dtsch Med Wochenschr. 1982;107:610-614.
Shaw MJ, et al. Results of a multicenter trial using a mechanical lithotripter for the treatment of large bile duct stones. Am J Gastroenterol. 1993;88:730-733.
Sheridan WG, et al. Morbidity and mortality of common bile duct exploration. Br J Surg. 1987;74:1095-1099.
Sherman S, et al. Complications of endoscopic sphincterotomy: a prospective series with emphasis on the increased risk associated with sphincter of Oddi dysfunction and nondilated bile ducts. Gastroenterology. 1991;101:1068-1075.
Siegel J, et al. Endoscopic electrohydraulic lithotripsy. Gastrointest Endosc. 1990;36:134-136.
Siegel JH. Endoscopic papillotomy in the treatment of biliary tract disease: 258 procedures and results. Dig Dis Sci. 1981;26:1057-1064.
Siegel JH, et al. Duodenoscopic sphincterotomy in patients with gallbladders in situ: reports of a series of 1272 patients. Am J Gastroenterol. 1988;83:1255-1258.
Simmons DC, et al. Endoscopic retrograde cholangiopancreatography (ERCP) in pregnancy without the use of radiation. Am J Obstet Gynecol. 2004;190:1467.
Simpson CJ, et al. Early endoscopic sphincterotomy for retained common bile duct stones. J R Coll Surg Edinb. 1985;30:288-289.
Sivak MV. Endoscopic management of bile duct stones. Am J Surg. 1989;158:228-240.
Soehendra N, et al. Early postoperative endoscopy after biliary tract surgery. Endoscopy. 1981;13:113-117.
Soomers AJ, et al. Endoscopic placement of biliary endoprostheses in patients with endoscopically unextractable common bile duct stones. Endoscopy. 1990;22:24-26.
Speranza V, et al. Transduodenal papillostomy as a routine procedure in managing choledocholithiasis. Arch Surg. 1982;117:875-878.
Staritz M, et al. Endoscopic removal of common bile duct stones through the intact papilla after medical sphincter dilation. Gastroenterology. 1985;88:1807-1811.
Stiegmann GV, et al. Precholecystectomy endoscopic cholangiography and stone removal is not superior to cholecystectomy, cholangiography, and common duct exploration. Am J Surg. 1992;163:227-230.
Stone HH, et al. Gallstone pancreatitis: biliary tract pathology in relation to time of operations. Ann Surg. 1981;194:305-312.
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101.
Stuart M, Hoerr SO. Late results of side-to-side choledochoduodenostomy and of transduodenal sphincterotomy for benign disorders. Am J Surg. 1972;123:67-72.
Sullivan DM, et al. Biliary tract surgery in the elderly. Am J Surg. 1982;143:218-220.
Tanaka M, et al. The long-term fate of the gallbladder after endoscopic sphincterotomy. Am J Surg. 1987;154:505-509.
Tandon RK, et al. Management of retained common bile duct stones in patients with T-tube in situ: role of endoscopic sphincterotomy. Am J Gastroenterol. 1990;85:1126-1131.
Targarona EM, et al. Laparoscopic treatment of acute biliary pancreatitis. Int Surg. 1995;80:365-368.
Targarona EM, et al. Randomised trial of endoscopic sphincterotomy with gallbladder left in situ versus open surgery for common bile duct calculi in high-risk patients. Lancet. 1996;347:926-929.
Tham TC, et al. Therapeutic ERCP in outpatients. Gastrointest Endosc. 1997;45:225-230.
Tham TC, et al. Role of endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis in patients undergoing laparoscopic cholecystectomy. Gastrointest Endosc. 1998;47:50-56.
Tham TC, et al. Safety of ERCP during pregnancy. Am J Gastroenterol. 2003;98:308-311.
Thompson JE, et al. Factors in management of acute cholangitis. Ann Surg. 1982;195:137-145.
Tranter SE, Thompson MH. Comparison of endoscopic sphincterotomy and laparoscopic exploration of the common bile duct. Br J Surg. 2002;89:1495-1504.
Tritapepe R, et al. Non-invasive treatment for retained common bile duct stones in patients with T tube in situ: saline washout after intravenous ceruletide. Br J Surg. 1988;75:144-146.
Vaira D, et al. Endoscopic sphincterotomy in 1000 consecutive patients. Lancet. 1989;2:431-434.
Van Dam J, Sivak MV. Mechanical lithotripsy of large common bile duct stones. Cleve Clin J Med. 1993;60:38-42.
Van der Velden JJ, et al. Percutaneous treatment of bile duct stones in patients treated unsuccessfully with endoscopic retrograde procedures. Gastrointest Endosc. 2000;51:418-422.
Vandervoort J, et al. Prospective analysis of risk factors for pancreatitis after diagnostic and therapeutic ERCP. Gastrointest Endosc. 1996;43:400.
Vandervoort J, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652-656.
Vellacott KD, Powell PH. Exploration of the common bile duct: a comparative study. Br J Surg. 1979;66:389-391.
Verstandig AG, et al. Combined transhepatic and endoscopic procedures in the biliary system. Postgrad Med J. 1993;69:384-388.
Way LW, et al. Management of choledocholithiasis. Ann Surg. 1972;176:347-359.
White DM, et al. Extracorporeal shock-wave lithotripsy for bile duct calculi. Am J Surg. 1998;175:10-13.
Wilcox CM, et al. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Am J Gastroenterol. 2004;99:244-248.
Worthley CS, Toouli J. Gallbladder non-filling: an indication for cholecystectomy after endoscopic sphincterotomy. Br J Surg. 1988;75:796-798.
Wright BE, et al. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest Endosc. 2002;56:225-232.
Yeh YH, et al. Percutaneous trans-hepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995;42:13-18.