Spinal Cord Compression
Daniel M. Sciubba, Ali A. Baaj and Ziya L. Gokaslan
• Spinal cord compression is diagnosed in more than 30% of all patients with disseminated cancer, and roughly 5% to 10% of all patients experience cord dysfunction.
• Back pain is the most common presenting complaint and almost always precedes neurologic dysfunction.
• Spinal cord compression has a devastating effect on quality of life and is frequently diagnosed late.
• Compression results from an epidurally located tumor or from bony fragments generated by pathologic fractures.
• Constant compression leads to spinal cord arterial compromise, venous occlusion, vasogenic edema, and demyelination with subsequent clinical deterioration.
• Any new or worsening back pain in a patient with cancer mandates urgent radiographic evaluation.
• Any evidence of myelopathy may signal impending catastrophic loss of cord function.
• Magnetic resonance imaging of the entire spine is the most rapid and cost-effective means of diagnosis and should be the first procedure performed.
• Corticosteroids, conventional radiation therapy, and decompressive surgery are the mainstays of treatment.
• Although treatment of metastatic spinal lesions is considered palliative, aggressive medical and surgical treatment can significantly improve pain, neurologic function, and quality of life.
Introduction
Spinal metastases have long been a significant source of morbidity in patients with systemic cancer. Regional pain, pathological compression fractures, deformity, and spinal cord compression with ensuing neurologic compromise are problems that commonly must be managed in both patients with advanced cancer and patients with isolated metastatic vertebral disease. In regard to metastatic epidural spinal cord compression (MESCC), data from the 1970s from Gilbert and colleagues1 and from Greenberg and colleagues2 showed that a significant proportion of patients entering the hospital in an ambulatory state left with varying degrees of paresis and paralysis. Specialists from that era emphasized the need for earlier diagnosis and improved treatment.3 Unfortunately, proposed diagnostic algorithms relied heavily on the only available diagnostic tool at the time: myelography.4 Early opposition existed for use of such an invasive test on neurologically intact patients with cancer who had new-onset back pain; however, Rodichok and colleagues4,5 demonstrated that roughly 60% of patients with new back pain, abnormal findings on a plain spinal radiograph, and normal findings at neurologic examination who underwent myelography were shown to have epidural cord compression. Since that time, diagnosing spinal cord compression before clinically apparent neurologic dysfunction has remained paramount in the management of metastatic vertebral disease. Early diagnosis and early, aggressive treatment are the hallmarks of current treatment.
Currently, approximately 30% of patients with cancer experience symptomatic spinal metastases during the course of their illness, and as many as 90% of patients with cancer have metastatic lesions within the spine at the time of death.6 With advances in the treatment of systemic oncologic disease, patient survival rates have increased during the past few decades. This increase, combined with improved imaging modalities of the neurologic and musculoskeletal systems (e.g., magnetic resonance imaging [MRI] and bone scintigraphy), will undoubtedly increase the incidence of spinal metastases encountered by physicians. As a result, it is essential that persons with cancer and those caring for them understand not only the myriad ways in which spinal metastases present but also the means by which they are currently diagnosed and managed. In this chapter, we attempt to address these issues by using an evidence-based approach from the most recently compiled literature. In addition, we propose a simple algorithm to aid in deciding which patients are best treated with medication and which patients could benefit from surgery.
Epidemiology
More than 1.4 million new cases of cancer are diagnosed annually in the United States,7 and roughly 500,000 persons die annually from metastatic disease.8 Metastases to the skeletal system occur third in frequency behind metastases to the lungs and liver.9,10 Within the skeletal system, the spinal column is the most commonly affected site. Cadaver studies have shown that as many as 30% to 90% of patients with terminal cancer have metastatic disease to the spine.11–15 Although it is estimated that only roughly 10% of patients with cancer experience symptomatic spinal metastasis,16 the prevalence of spinal metastases is likely to increase as duration or length of survival duration for many patients with cancer continues to improve.
The highest incidence of clinically detected spinal metastases occurs during midlife (40 to 65 years of age), which corresponds to the period of increased cancer risk.17 Primary breast, lung, and prostate cancers represent the most common histologies that are metastatic to the spine, reflecting both their higher prevalences and their tendencies to metastasize to bone.18,19 A slightly higher incidence in men is related to the slightly higher incidence of prostate cancer compared with breast cancer.17 However, as adjuvant treatment for breast cancer improves overall survival duration, this dissimilarity could vanish.
Pathophysiology
Figure 49-1 shows the relevant anatomy of the spinal cord, spine, and associated structures and the location of metastatic lesions in these areas. Lesions that cause spinal cord compression usually first invade the epidural space, most often as direct extension of metastatic disease from the vertebral body. Spine metastases are thought to arise by various mechanisms, often predicated on the biological behavior of the primary cancer.
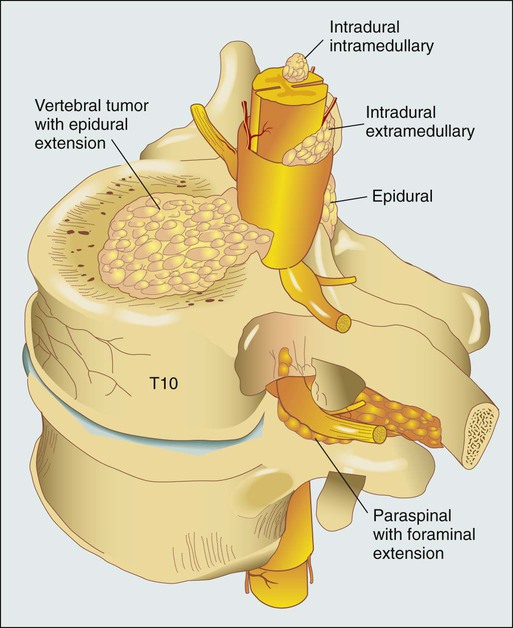
• Hematogenous spread. As the most common means of malignant tumor spread to the skeleton, hematogenous spread likely occurs by both venous and arterial routes. Batson’s venous plexus is the longitudinal network of valveless veins that course parallel and juxtaposed to the spinal column. This plexus communicates with multiple venous systems (e.g., the spine, vena cava, portal, azygous, intercostals, pulmonary, and renal), and the flow direction within the plexus may be variable because of changes in intrathoracic and intraabdominal pressures. In this way, tumors in multiple sites of the major body cavities could deposit tumor cells in the spine.20 Because of the significant blood flow to the vertebral bodies, the arterial system also is effectively able to deliver tumor cells to these well-perfused bones.21 Although hematogenous dissemination of tumor cells is less common, it may also lead to direct metastasis to the cord (intramedullary) or to the epidural space itself.
• Direct extension. Primary tumors located in paravertebral soft tissues can often extend into the vertebral column via intervertebral foramina. Lung cancer, for instance, may become locally aggressive and invade the thoracic spine, whereas prostate, bladder, and colon cancer may invade the lumbar and sacral spine.20
• Cerebrospinal fluid spread. Cerebrospinal fluid spread is usually conferred by “shedding” of tumor cells from cerebral or cerebellar metastatic lesions, often after surgical manipulation of brain metastases.22
After a malignant lesion has begun to grow in the epidural space, it is unclear how or at what point spinal cord compression (as seen on MRI or myelogram) leads to clinically apparent spinal cord compromise. Animal models demonstrate that at least three mechanisms may be at play.25–25 First, direct pressure on the neural structures of the cord may result in demyelination. Second, compression may lead to arterial insufficiency with subsequent neural degeneration. Finally, compression may cause venous blockage with secondary vasogenic edema as a result of disruption of the blood–spinal cord barrier. Interestingly, results of various animal experiments have shown that the spinal cord can tolerate prolonged pressure if the onset of the pressure is slow and the neural degeneration is not irreversible.28–28 Along these lines, Rades and colleagues found that slower clinical progression of neurologic symptoms before diagnosis led to improved outcome,29 emphasizing again that early diagnosis is a key to successful management.
Clinical Evaluation
Back Pain
Significant back pain will likely affect more than 80% of Americans at some point in their lives.30 Because the overwhelming majority of such patients have noncancerous musculoskeletal pain, the presenting complaint of back pain may lead many physicians to ignore these complaints from patients who will ultimately harbor underlying spinal metastatic disease. However, in roughly 10% of patients with developing cancer, symptomatic spinal metastases may be the initial presentation.31 In addition, when vertebral lesions are discovered, pain is the most common presenting symptom, occurring in approximately 83% to 95% of patients.3,18,32 As a result, a patient with cancer who reports back strain from a particular event still needs very close attention and follow-up care.33
Three classic pain syndromes affect patients with spinal metastases: local, mechanical, and radicular pain. Patients often present with a combination of these pain syndromes. Local pain is usually described by patients as a persistent “gnawing” or “aching” pain emanating from the region of the spine that is affected by metastatic disease. It is hypothesized that growth of the metastatic tumor, most commonly located in the posterior vertebral body, leads to periosteal stretching and/or a local inflammatory process that stimulates the pain fibers within the spinal periosteum. In such patients, percussion over the spinous process may elicit local tenderness. Such pain usually responds well to steroid administration.34
Mechanical pain, also known as axial back pain, is aggravated by movement, activity, or simply increasing weight-bearing forces on the spinal segment affected. Spinal metastases that result in vertebral body damage (e.g., deformity or fracture) may result in spinal instability, which likely results in muscle, tendon, ligament and/or joint capsule strain and ensuing symptoms of mechanical pain. Unfortunately, such discomfort is usually refractory to narcotics and steroids but responds to recumbency, bracing, and/or internal stabilization. Radicular pain may occur when spinal lesions compress or irritate an exiting nerve root, yielding pain in the dermatomal distribution of the involved root that is often described as “sharp,” “shooting,” or “stabbing.” Interestingly, dysesthetic/neuropathic pain may also arise when patients have intradural extramedullary disease, creating pain that may be described as an “intense, burning” sensation.22
Motor and Autonomic Dysfunction
The second most common presenting complaint of patients with vertebral metastases is motor dysfunction, which can manifest as myelopathy and/or radiculopathy. Although it is less common than the presentation of pain alone, roughly 60% to 85% of patients with MESCC are weak at the time of diagnosis.2,3,18 In addition, patients often have some level of bladder, bowel, and/or sexual dysfunction related to the MESCC that they will not readily report to the physician unless an inquiry is made. Bladder dysfunction is the most common autonomic finding and commonly correlates with the degree of motor dysfunction.8 Although the rate of clinical progression is variable, patients with motor dysfunction inevitably progress to complete paralysis in the absence of intervention.35 In addition, severe autonomic dysfunction with urinary retention, constipation, and loss of control of bowel or bladder function is a late and particularly ominous finding because full paraplegia can follow within hours.33
Neurologic status at the time of diagnosis, particularly motor function, has been shown to correlate with prognosis from MESCC,21,36 thus reinforcing the concept that diagnosis before the development of a neurologic deficit is of paramount importance. Unfortunately, because complaints of chronic back pain in the general population are extremely common,30 it is likely that a delay in diagnosis of vertebral metastasis occurs frequently in patients who report merely new-onset back or neck pain. In a study of 319 patients with cancer, Levack and colleagues37 reported that a median of 2 months passed from the onset of pain, as reported to their primary care providers, until the diagnosis of metastatic spinal cord compression. For this reason, new-onset back or neck pain in a patient with known cancer must be considered to be spinal metastatic disease until proven otherwise. Moreover, thoracic pain is less common than is pain originating from the mobile cervical and lumbar regions, where degenerative disease is the more common precipitating cause of pain; thus pain in the thoracic region should raise a high level of suspicion for the likelihood of cancer.
Diagnosis
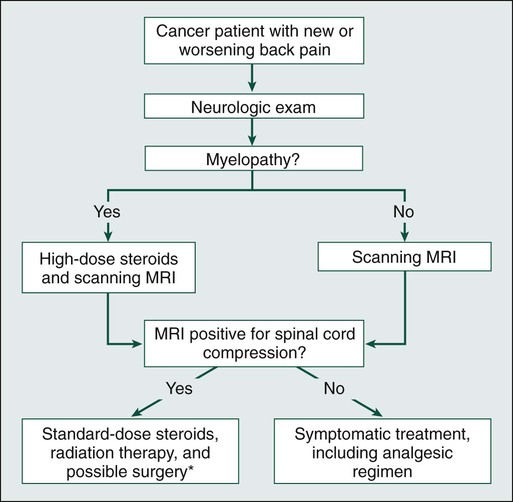
In patients with known or suspected malignancy who have back pain, the first step is a neurologic examination (Fig. 49-2). If the presence of an abnormal finding is in question, assistance should be sought from a neurologist. Any sign of myelopathy should lead to a prompt MRI of the entire spine with institution of high-dose dexamethasone therapy before the patient is sent to the MRI suite. Because a single bolus of steroid is unlikely to cause any significant adverse effects, waiting for confirmation of spinal cord compression before instituting administration of steroids is almost never warranted.38
Plain Films
Plain radiographs have long been the first course of imaging obtained for patients with spine pain, mainly because of ease of use and relatively low cost. They serve mainly as a screening test by revealing lytic or sclerotic areas of bone, pathological compression fractures, deformity, and/or paraspinal masses. The major proportion of spinal metastatic lesions are osteolytic, but as much as 50% of the bone must be eroded before there is a noticeable change on plain radiographs.39 Notably, osteoblastic/sclerotic lesions most often result from carcinoma of the breast or prostate.40
Bone Scan
Bone scanning or nuclear scintigraphy is sensitive for identifying increased metabolic activity throughout the entire skeletal system. Thus whereas plain films might not detect tumor-induced radiographic changes until 30% to 50% or more of the vertebral medullary space has been replaced,41 bone scans may reveal the lesion at an earlier stage.42 However, such scans may detect increased metabolic activity associated with spinal inflammation and infection, leading to overall decreased specificity. In addition, nuclear scintigraphy currently has poor imaging resolution, necessitating correlation with CT or MRI to exclude benign processes and to plan possible operative intervention.43 PET scanning with 18F-fluorodeoxyglucose is now more commonly used for whole-body metastatic surveys and as a staging technique in patients with known systemic cancer.44 However, as with nuclear scintigraphy, poor spatial resolution in PET necessitates concomitant use of CT or MRI.
Magnetic Resonance Imaging
MRI is considered the gold standard imaging modality for assessing spinal metastatic disease.47–47 MRI is more sensitive than standard radiographs, CT, and bone scans in detecting primary malignant bone tumors and metastatic lesions in the spine.48,49 Such sensitivity is due to the fact that MRI allows for superior resolution of soft-tissue structures such as intervertebral discs, spinal cord and nerve roots, meninges, and paraspinal musculature. Moreover, MRI provides clarity at the osseous–soft-tissue interface, yielding accurate anatomic detail of bony compression or invasion of neural and paraspinal structures.
When a patient has either local or radicular back pain with no evidence of myelopathy, a scanning MRI should be ordered.50 This study involves a series of T1-weighted sagittal images extending from the cervical spine to the sacrum. If metastatic lesions of bone are found, the procedure is converted to a more comprehensive set of images. This method is a faster and more cost-effective approach to early diagnosis of spinal cord compression.50 The comprehensive MRI protocol should include T1- and T2-weighted images and contrast-enhanced studies that provide axial, sagittal, and coronal reconstructions.51 Because of the high signal intensity of fat within bone marrow on T1-weighted images, fat suppression techniques are useful in evaluating osseous lesions that enhance with contrast material.51 Diffusion-weighted imaging may be helpful in distinguishing between benign and pathological compression fractures.52
Angiography
Angiography is an advanced imaging modality that is ordered by the neuroradiologist and/or neurosurgeon once the diagnosis of a spine tumor has been made. In patients with spinal metastases that are suspected to result from highly vascular primary tumors, such as renal cell carcinoma, thyroid carcinoma, angiosarcoma, leiomyosarcoma, hepatocellular carcinoma, and neuroendocrine tumors (e.g., pheochromocytoma and paraganglioma),53 angiography may provide diagnostic information and have therapeutic benefit. If the lesions are considered for surgery, preoperative knowledge of the vascular supply may prove invaluable. In addition, angiography might allow preoperative embolization of the spinal lesion, leading to decreased blood loss with operative resection.54 Such intraoperative blood loss can influence the surgeon’s ability to visualize an adequate view of the surgical field, thus affecting achievement of complete resection. In addition, significant blood loss may be associated with life-threatening hemorrhage intraoperatively, longer operating times, intraoperative complications, postoperative hematomas, and wound breakdown. In patients who are not candidates for surgery, embolization may be effective as primary treatment for the lesion.55
Percutaneous Spine Biopsy
Although imaging modalities have improved vastly during the past two decades, acquisition of tissue is not uncommonly needed for diagnosis. If surgery is not indicated up front and no other systemic lesions can be found, percutaneous image-guided biopsy may be indicated. Improved CT fluoroscopy and needle biopsy systems provide relatively easy access to most lesions, with success rates approaching 90%. The majority of such procedures are now performed in the outpatient setting.56,57
Treatment
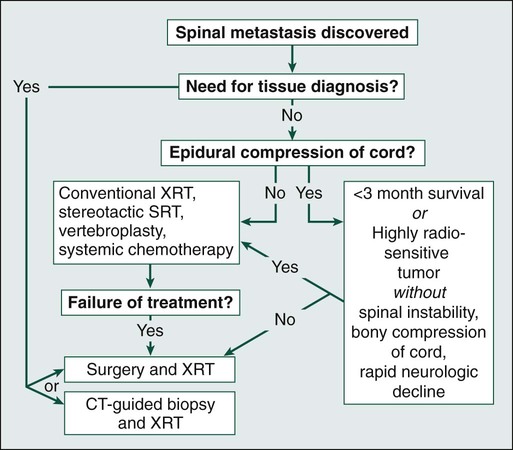
Treatment of spinal metastases is primarily palliative. As a result, goals of treatment are centered on pain relief, maintenance or restoration of spinal stability, and preservation of neurologic function. Although patients may be cured with radical surgical excision in rare circumstances (e.g., solitary renal cell carcinoma spinal metastasis),58 patient variables, including age, tumor burden, life expectancy, and functional status, overwhelmingly influence the choice of therapeutic options. The three traditional mainstays of therapy have been corticosteroids, radiation therapy, and surgery.59 In the following paragraphs, we will provide the most current treatment paradigms involving each of these modalities and describe how technologic advances (e.g., bisphosphonates, radiosurgery, percutaneous vertebroplasty, and aggressive decompressive surgery) have increased the armamentarium against metastatic spine disease and have consequently improved outcomes (Fig. 49-3).
Hormonal Therapy, Chemotherapy, and Medical Therapy
Chemotherapeutic agents can be classified into antitumor drugs and drugs that minimize the secondary effects of the tumor.60 Except in cases of chemosensitive tumors, such as Ewing sarcoma and neuroblastoma, antitumor drugs continue to have a limited role in the treatment of spinal metastases. On the other hand, drugs that are used to prevent or ameliorate the effects of spinal tumors (e.g., corticosteroids, bisphosphonates, and analgesics) are widely accepted.
The three most common primary tumors that metastasize to the spine are breast, prostate, and lung cancers.18,19 Unfortunately, although lung cancer is still the leading cause of cancer mortality in the United States, the median survival of patients with lung cancer metastatic to bone is reportedly between 3 and 9 months. As a result, most patients die of progressive disease before skeletal complications become a large problem.60 In most prostate and breast cancers, on the other hand, lesions may be sensitive to hormonal manipulation.60,62 Estrogen agonists/antagonists, such as tamoxifen, and aromatase inhibitors, such as letrozole, have been shown to be effective for breast cancer. For prostate cancer, androgen deprivation is the mainstay of treatment with GnRH agonists and/or flutamide.
Chemotherapy is usually administered to patients with prostate and breast cancer when hormonal agents become ineffective. Breast cancer is often sensitive to chemotherapy; the most effective agents include doxorubicin and the taxanes. Although prostate cancer is considered less responsive to chemotherapy, some agents have demonstrated effectiveness, such as mitoxantrone, prednisone, vinblastine, and the taxanes. Multiple myeloma responds to combinations of melphalan and prednisone, as well as infused vincristine and adriamycin.60
Corticosteroids
Corticosteroids are the mainstay of pharmacologic therapy for pain associated with vertebral metastases and for the acute neurologic deterioration that often accompanies MESCC. Corticosteroids decrease tumor-associated inflammation (providing an analgesia effect), decrease spinal cord edema (improving short-term neurologic function), and may be directly oncolytic, as with lymphoma, multiple myeloma, and breast cancer.3 Experimental animal models have confirmed the clinical observations that animals treated with dexamethasone have improvement in motor function faster than in untreated control subjects.65–65 Memorial Sloan-Kettering Cancer Center has popularized a high-dose regimen: a loading dose of 100 mg dexamethasone followed by 6 mg given four times a day (total dose: 24 mg).2,38,66 This regimen is based on studies demonstrating a dose-response benefit of dexamethasone.64 However, no optimal dosing regimen for corticosteroids used with MESCC currently exists, and no consensus data are available to recommend high-dose steroids (96 mg/day) versus low-dose steroids (16 mg/day). For instance, Vecht and colleagues67 compared initial doses of a 10-mg intravenous (IV) bolus versus a 100-mg IV bolus but showed no outcome differences regarding pain, ambulation, or bladder function.
As was stated previously, however, if a metastatic spine lesion is suspected, starting high-dose dexamethasone in anticipation of an MRI scan has minimal drawbacks. In patients without obvious myelopathy, lower doses of steroids may be initiated, such as 4 mg every 6 hours or a bolus of 10 mg, while awaiting MRI.38 Whatever dose is initiated, however, tapering of the steroid should begin during the course of radiation therapy.68 By reducing the dose by one third every 3 to 4 days, the steroid course will be almost completely tapered by the end of radiation treatment.33 Long-term use of corticosteroids, on the other hand, may be fraught with serious clinical adverse effects. Higher doses of steroids are rarely needed beyond the first 48 to 72 hours or even beyond the initial bolus. Therefore if neurologic symptoms continue to progress with either the standard or high-dose regimen, prompt surgical intervention may be indicated.33 In addition, recrudescence of symptoms during the steroid taper may be an indication for increasing the steroid dose or considering surgical decompression.
Bisphosphonates
Bisphosphonates suppress bone resorption by inhibiting osteoclastic activity. In this way, bisphosphonates may reduce the risk of pathologic fracture,69 relieve local pain resulting from lytic lesions,70 and lower malignancy-associated hypercalcemia. Although most of the clinical trials on bisphosphonates have been conducted in patients with metastatic breast cancer and multiple myeloma, studies with small numbers of patients who have other metastatic carcinomas have demonstrated benefit.60
Analgesia
Poorly managed cancer pain contributes to depression, anxiety, and fatigue.71 Unfortunately, undertreatment of pain in patients with cancer persists despite significant efforts by clinicians to improve delivery of analgesics. In 1995 the American Pain Society Quality of Care Committee Consensus Statement delineated the high prevalences of unrelieved pain across clinical settings with specific regard for pain relating to cancer.72 The World Health Organization has outlined a three-step system for the treatment of progressively severe pain that uses nonopioid analgesics, opioid analgesics, and adjuvant medications.73 First-line agents should be nonopioid analgesics, such as acetaminophen, aspirin, and other nonsteroidal antiinflammatory drugs, which are often efficacious for bone pain. If pain is not controlled, an opioid analgesic should be added. If pain remains uncontrolled, a strong opioid analgesic should then be prescribed. At all levels of this ladder, adjuvant drugs may be added to treat specific types of pain.74 Specifically, corticosteroids have been shown to be effective for use in patients with pain relating to spine tumors. In addition, neuropathic pain may respond to anticonvulsant medications (e.g., gabapentin, lamotrigine, and carbamazepine), the 5% lidocaine patch, opioid analgesics, and tricyclic antidepressants.75
Conventional Radiation Therapy
XRT is typically administered to spinal lesions during a 5- to 14-day course with a total radiation dose of 20 to 50 Gy.78–78 The historical standard radiation portal involves the diseased level with a 5-cm margin, which effectively includes two vertebral bodies above and below the target.79 With better imaging, treating one vertebral body above and below the lesion is now also an accepted approach. Local vertebral tumor control is related to the radiation dose delivered to the area and the dose per fraction. The primary factor that limits radiation to the spine is the relatively low tolerance of the spinal cord for radiation damage. Unfortunately, conventional XRT lacks the precision to deliver large single-fraction doses of radiation to the spine near radiosensitive structures such as the spinal cord; therefore, the actual treatment dose that is delivered is often below the optimal therapeutic dose.82–82 Use of greater radiation doses to reach just the specifically targeted treatment volume, as is the potential with stereotactic radiosurgery, may increase tumor control while minimizing the risk of spinal cord injury.
Surgical Approaches and Techniques
As a result of improvements in chemotherapy, radiation therapy, and hormonal therapy with concurrent increases in survival time for many patients with metastatic spine disease, many clinicians have advocated aggressive surgical decompression and stabilization for symptomatic lesions.83 Effective techniques to achieve dorsal and ventral spinal stabilization have become more widely available in the past two decades, allowing surgeons to achieve adequate decompression and tumor resection with acceptable morbidity. The ability to correctly identify appropriate candidates for surgical intervention requires an understanding of the anatomy of the tumor and its surrounding structures, the biomechanical changes created by vertebral metastases, and the characteristics of the specific histopathology that may affect surgical resection. When the patient has a high degree of epidural cord compression with myelopathy or gross spinal instability, surgery precedes radiation therapy. If the tumor is radiosensitive (e.g., lymphoma or multiple myeloma) with no gross instability or myelopathy, then XRT is used first. In the case of solid tumors with no instability or myelopathy, radiation therapy in the form of sterotactic radiosurgery is recommended.
Biomechanical Considerations
Biomechanical studies have shown that the vertebral body supports as much as 80% of the axial load from above.84 Because the vertebral body is the most common site of metastatic growth, destructive lesions in this area can have a significant impact on the load-bearing capacity of the spine. Such capacity is directly associated with tumor size, cross-sectional area of the remaining intact body, and bone mineral density. Windhagen and colleagues85 have shown that pathological fracture can be accurately predicted (in cadaveric spines) by calculating the product of the remaining intact vertebral cross-sectional area and the bone mineral density. Taneichi and colleagues86 studied 53 patients with osteolytic metastases and found that impending collapse was predicted by 50% to 60% involvement of the vertebral body in the thoracic spine and 35% to 40% involvement in the thoracolumbar/lumbar spine. As a result, axial loading most commonly creates a compression fracture or a burst fracture with structurally significant lesions. Conversely, invasion and disruption of the posterior spinal elements, such as the facet joints, may predispose patients to dislocation and translational deformity. However, because these dorsal elements are less commonly affected by metastases, such structural pathology is unusual.
Surgical Anatomy and Histopathology Affect Resection
Metastatic tumors that cause epidural cord compression typically arise from the vertebral body and extend dorsally; thus anterior approaches commonly provide the greatest ability to decompress the spinal cord. The cervical spine can be approached easily both anteriorly and posteriorly, and the accompanying reconstructive instrumentation is familiar to most surgeons. In the thoracic spine, the upper segments (T1 to T4) may be particularly challenging to access, possibly requiring a combination of an anterolateral cervical approach with a sternotomy and/or a thoracotomy to decompress the anterior cord.87 To avoid encountering the great vessels and aortic arch, thoracic levels T5 to T10 are ideally approached via a right-sided thoracotomy unless the bulk of the extravertebral tumor is on the left side.88 Approaches to the thoracolumbar junction (T11 to L1) may require a combined thoracotomy and retroperitoneal approach, whereas decompression at the L2 to L4 levels can occur via a retroperitoneal or transperitoneal/transabdominal approach. Disease that is limited to L5 is most commonly treated with posterior decompression and stabilization. Sacral lesions may require posterior alone approaches or transperitoneal entry into the pelvis for anterior-posterior approaches.
Adequate decompression of the spinal cord from a lone posterior approach is challenging but possible. Several authors have advocated bilateral transpedicular or costotransversectomy approaches to decompress the ventral aspect of the spinal cord.89–94 Reconstruction of the anterior and middle columns of the spine may then be achieved with polymethylmethacrylate (PMMA) secured with Steinman pins90,91 or a chest tube,89 titanium mesh cages,95 or expandable titanium cages.94–94 One potential criticism of such approaches is that the spine is severely destabilized from such procedures. Specifically, because the normal posterior elements are generously resected to remove the diseased anterior elements, there is the potential for increased surgical morbidity and a lower chance of successful biological osseous fusion as a result of the large gap that is created between neighboring bone surfaces.
In general, vertebral body resection for tumor with successive reconstruction/stabilization is now most commonly performed via an anterior approach with subsequent vertebral column reconstruction (with a distractible mesh titanium cage) and anterolateral plating. Supplemental posterior stabilization with pedicle screw instrumentation is advocated in patients with significant damage to the posterior elements from tumor or from tumor removal surgery, significant kyphosis, or lesions at the thoracolumbar junction and in patients with two or more adjacent vertebrectomies (Fig. 49-4). Lone posterior approaches should likely be reserved for patients with contraindications to anterior approaches (e.g., the transthoracic approach in patients with severe lung disease) because of the severely destabilizing characteristics of such procedures.
Metastatic Epidural Spinal Cord Compression Treatment
Metastatic epidural spinal cord compression (MESCC) is defined as radiographic evidence of an epidural metastatic lesion that is causing displacement of the spinal cord from its normal position in the spinal canal. This condition occurs in roughly 5% to 10% of patients with cancer and in as many as 40% of patients who have concurrent nonspinal bone metastases.98–98 The result is approximately 25,000 annual cases of symptomatic MESCC in the United States.8 Traditionally, treatments for patients with MESCC have included corticosteroids, surgery, and XRT. Historically, surgical options were limited to decompressive laminectomy. Unfortunately, laminectomy procedures frequently did not address the primary site of spinal cord compression, which is the ventral vertebral body. Tumor in this area often causes intrinsic instability of the anterior column of the spine. Laminectomy procedures, which are destructive to the posterior spinal elements, may thus further destabilize the spine and worsen a patient’s pain and neurologic status.89,99 For this reason, combined with improved spinal instrumentation, aggressive surgical decompression with instrumented stabilization has become mainstream.
A review of the literature from 1964 to 2005 by Witham and colleagues100 regarding articles related to radiation therapy and surgical management for MESCC has shown improved outcomes concurrent with evolution toward more aggressive decompressive surgery and more extensive surgical stabilization (Table 49-1). Review of articles investigating neurologic function after XRT alone in patients with MESCC revealed that a mean of 36% of patients improved and a mean of 17% worsened. Review of articles investigating neurologic function in patients treated with laminectomy with or without XRT showed results similar to those of the XRT-alone series, with improved neurologic function in a mean of 42% of patients and worsened function in a mean of 13% of patients. Notably, surgical morbidity and mortality (a mean surgical mortality of 6%) did occur in the laminectomy series compared with the nonsurgical XRT series. Interestingly, in the series of articles involving patients undergoing stabilization procedures performed in conjunction with posterior decompressive procedures, functional outcomes were improved, with a mean motor improvement in 64% of patients. In this series, pain relief was achieved a high percentage of the time (mean: 88%) with a mean mortality rate that was comparable with that of laminectomy alone (5%). Moreover, in the series of articles involving patients undergoing stabilization procedures concurrent with anterior decompression to the spinal cord, functional neurologic improvement was even more remarkable (mean: 75%), with only a slight increase in surgical mortality (mean: 10%).
Table 49-1
Review of the Literature for Treatment of Metastatic Epidural Spinal Cord Compression
| Improved Neurologic Function, % (Mean) | Surgical Mortality, % (Mean) | |
| XRT alone | 36 | N/A |
| Laminectomy ± XRT | 42 | 6 |
| Laminectomy and spinal stabilization ± XRT | 64 | 5 |
| Anterior decompression and spinal stabilization ± XRT | 75 | 10 |
N/A, Not applicable; XRT, radiation therapy.
Data from Witham TF, Khavkin YA, Gallia GL, et al. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol 2006;2:87–94.
Recently a randomized, prospective clinical trial of direct decompressive surgical resection and XRT versus XRT alone for patients with MESCC was conducted by Patchell and colleagues101 (Table 49-2). Both groups of patients received a total radiation dose of 30 Gy delivered in 10 fractions. The intent of surgery in all cases was spinal cord decompression, removal of as much tumor as possible, and spinal stabilization. Results showed a statistically significant difference in the posttreatment ambulatory rate in the surgery group of 84% (42 of 50) compared with the XRT-alone group 57% (29 of 51) (P = .001). In addition, posttreatment ambulatory patients retained the ability to walk significantly longer in the surgery group than in the XRT group (median of 122 days versus 13 days, P = .003). Regarding patients who were ambulatory before treatment, 94% (32 of 34) in the surgery group continued to be ambulatory compared with 74% (26 of 35) in the XRT group (P = .024). In addition, 62% (10 of 16) of the patients in the surgery group who were nonambulatory before treatment regained the ability to ambulate compared with only 19% (3 of 16) in the XRT group (P = .012).
Table 49-2
Results of a Randomized, Prospective Trial Comparing Radiation Therapy Alone with Surgery and Radiation Therapy Combined
| Posttreatment Ambulatory Rate (%) | Posttreatment Retention of Ambulation (Days) | Patient Who Were Ambulatory before Treatment Who Maintained Ambulation (%) | Patients Who Were Nonambulatory before Treatment Who Regained Ambulation (%) | Mean Survival (Days) | |
| XRT alone | 57 | 13 | 74 | 19 | 100 |
| Surgery and XRT | 84 | 122 | 94 | 62 | 122 |

Data from Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643–648.
Most important, Patchell and colleagues101 demonstrated that survival time, maintenance of continence, muscle strength (American Spinal Injury Association scores), and functional ability (Frankel scores) were all significantly improved in the surgery group relative to the XRT-alone group. Specifically, median survival in the surgery group was 126 days relative to 100 days in the XRT group (P = .033). Thus Patchell and colleagues concluded that selected patients with MESCC treated with surgery and XRT possess a modest improvement in survival time, maintain the ability to ambulate longer, and recover the ability to ambulate more frequently than those treated with XRT alone.
On the basis of such results, basic recommendations can be made regarding indications for XRT alone versus surgery and XRT. Indications for XRT alone include MESCC resulting from highly radiosensitive tumors (e.g., lymphoma, myeloma, and small cell lung carcinoma) without spinal instability, significant bony compromise of the spinal canal, or rapidly progressive neurologic decline. XRT alone could also be indicated in patients with life expectancy less than 3 months. Indications for surgery include MESCC resulting from radio-resistant tumors or those that recur despite XRT, rapid neurologic deterioration during XRT, spinal instability, epidural cord compression from bone, or a need for tissue diagnosis (see Fig. 49-3). Solitary lesions with indolent courses, such as renal cell carcinoma in the absence of additional systemic metastases, may be considered for en bloc tumor resection with total spondylectomy in hope of a cure.90,102–107
Spinal Stereotactic Radiosurgery
Although spinal stereotactic radiosurgery (SRS) is a relatively new radiation treatment option for spinal metastases and therefore has not undergone rigorous, long-term investigation, it may have advantages over conventional XRT for the treatment of MESCC. The most common delivery systems are modified linear accelerators (Novalis, BrainLAB Inc., Chicago, IL) and a robotic linear accelerator (CyberKnife, Accuray, Sunnyvale, CA). In these systems, stereotactic localization provides precise convergence of multiple radiation beams onto the targeted lesion. Because the use of multiple radiation beams allows some of the normal tissues to be out of the radiation field part of the time, tolerance to the radiation exposure is optimized. Spinal SRS and intensity-modulated radiotherapy thus permit accurate delivery of radiation while minimizing radiation exposure to normal tissues.76 Total doses typically range from 8 to 18 Gy, and SRS can be administered in one or two sessions in an outpatient setting.
A number of studies involving SRS for MESCC have provided encouraging results. Degen and colleagues108 presented the outcomes of patients with MESCC who were treated with CyberKnife to treat 58 spinal metastatic lesions that were documented as durable at 1-year follow-up. Of the patients with a neurologic deficit, 31% improved, 47% were unchanged, and 22% worsened. In this same study, a significant improvement in pain was noted, and quality of life was maintained with relatively limited treatment morbidity. Interestingly, 100% tumor control was achieved for metastatic lesions treated with SRS that had not undergone previous irradiation. In another recent study by Gerszten and colleagues109 involving CyberKnife radiosurgery for spinal lesions, axial and radicular pain improved in 74 of 79 patients who were symptomatic before treatment. Potential limitations of SRS include the inability to address spinal instability or deformity as a result of metastatic spine disease and the inability to treat large lesions safely because of its associated highly concentrated radiation dose. In addition, SRS is still largely viewed as a developing treatment modality for MESCC. Nonetheless, spinal SRS could prove to be an effective and indispensable weapon in the arsenal against metastatic spine disease.
Percutaneous Vertebroplasty and Kyphoplasty
Traditionally, extensive, multifocal metastatic disease of the spinal column has been treated by conventional XRT combined with conservative measures such as bed rest, bracing, corticosteroids, and analgesia. However, successful XRT often does not provide pain relief for as long as 2 weeks, and bone strengthening is not seen for as long as 4 months if it occurs at all.110 Although originally developed for the treatment of painful vertebral hemangiomas, percutaneous vertebroplasty/kyphoplasty has now become an effective treatment for the painful pathologic fractures of metastatic spine disease.
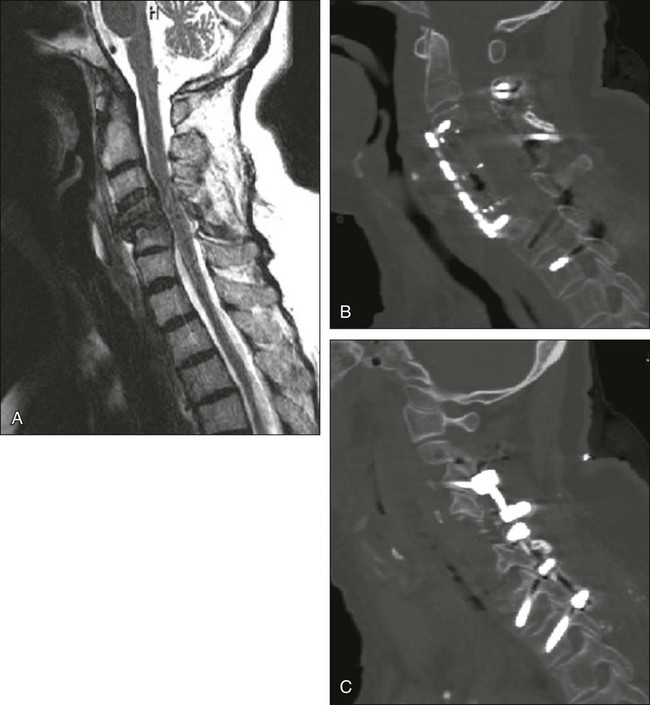
Percutaneous injection of PMMA cement into a pathologically collapsed vertebral body has been shown to be effective for the treatment of pain.111 Although it is assumed that such techniques minimize mechanical pain via cement-augmented structural support, it is possible that the cement itself possesses analgesic properties within the vertebral body.112 Vertebroplasty is characterized by direct injection of PMMA into the vertebral body (Fig. 49-5). Kyphoplasty, on the other hand, involves first placing an expandable balloon into the vertebral body. Once inflated, the balloon creates a cavity for the subsequent injection of PMMA. It is postulated that kyphoplasty might actively improve kyphotic deformity of the spine with such balloon expansion.
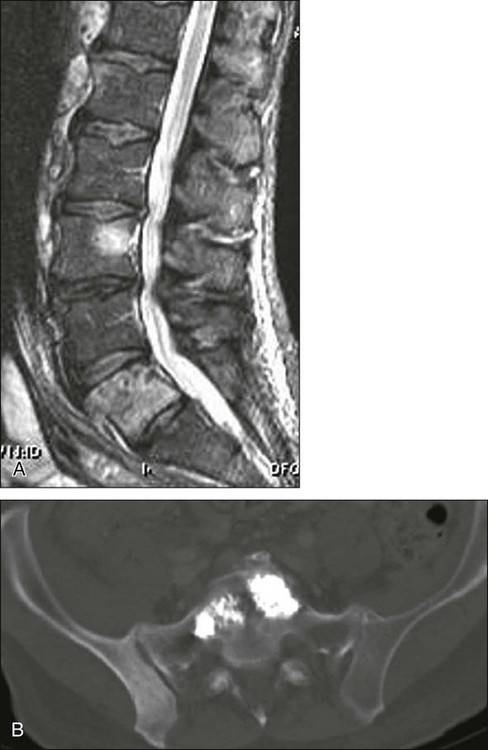
Exact indications for vertebroplasty/kyphoplasty in relationship to metastatic spinal disease are evolving. The technique is safe and effective for treating intractable pain resulting from vertebral fractures. A relative contraindication to vertebroplasty/kyphoplasty is epidural spinal cord compression; however, when combined with XRT, such procedures can result in dramatic pain relief for poor surgical candidates. For instance, Fourney and colleagues111 presented 97 procedures in patients with intractable pain resulting from pathological vertebral body fractures and noted moderate to complete pain relief in 84%. Although rare, complications of such procedures are related to leakage or misdirection of PMMA outside the confines of the vertebral body, leading to spinal cord/root compression or PMMA pulmonary emboli through cement injection into the spinal venous plexus.113