Second Malignant Neoplasms
• Subsequent malignancies after successful treatment for cancer occur in a meaningful minority of persons.
• Etiology is often multifactorial and includes known hereditary conditions, unrecognized genetic predisposition, primary cancer, therapeutic exposures, age, and lifestyle practices.
• Guidelines for screening are available for some but not all situations; health care providers and patients should be aware of such guidelines.
• Further research is needed to identify persons at highest risk so more useful preventive strategies can be developed.
Introduction
The American Cancer Society estimated that 1,638,910 cases of new cancer would be diagnosed in the United States in 2012, a figure that excludes cancer in situ and nonmelanoma skin cancer. In the same time period, 577,190 persons were expected to die from cancer. Because of the rising incidence of and survival from cancer, an estimated 12.8 million survivors of cancer are now alive in the United States, with 5-year survival rates of 67% for adults and 82% for children now being diagnosed with cancer, according to the National Cancer Institute (NCI) Surveillance Epidemiology and End Results (SEER). With a growing cohort of cancer survivors now living, efforts have focused on decreasing the risk for adverse long-term health-related outcomes, the most significant of which are subsequent malignant neoplasms (SMNs). According to current data from SEER, SMNs represent 16% (1 in 6) of reported cancers.1
The most recent monograph from the NCI on subsequent malignancies, published in 2006, does not reflect the current percentage because it reports on the period from 1973 to 2000,2 expanding on the prior report issued more than 20 years earlier.3 However, this monograph represents the largest overview of subsequent malignancies that includes data on both adult and pediatric cancers. In this report, among 2 million patients with cancer who survived at least 2 months with close to 11 million person-years at risk, cancer survivors had a 14% higher risk of having a new malignancy than would have been expected in the general SEER population (observed/expected = 1.14). A total of 185,407 new primary cancers were observed compared with the expected number of 162,602 which translates to an excess absolute risk (EAR) of 21 per 10,000 person-years. However, the risk of SMN compared with the general population was highly dependent on the age of the patient at first cancer diagnosis, because risk for cancer rises with increased attained age in the general population. For example, for persons younger than 18 years at initial diagnosis, the relative risk (RR) was 6.13 and the EAR was 15 per 10,000 person-years. The RR and EAR for persons 70 to 79 years at initial diagnosis dropped to their lowest values, 1.02 and 4, respectively. The highest EARs of 32 to 39 were noted in young to middle-age adults ages 30 to 59 years at initial cancer diagnosis. Gender also influenced risk, with a slightly increased risk for females (RR = 1.17) compared with males (RR = 1.11), a pattern that remained even when gender-specific malignancies were excluded from the analyses. Blacks also had elevated RR and EAR (1.31 and 46, respectively) compared with whites (1.12 and 20, respectively). Overall, an SMN developed in 14% of SEER patients by 25 years of follow-up. Although RRs were highest in the first 5 years from diagnosis, cumulative incidence increased by years of follow-up: 5.0%, 8.4%, 10.8%, and 13.7% at 5, 10, 15, and 25 years, respectively. More than 80% of multiple primary cancers reported arose in separate or independent organ systems. New malignancies that occurred in the same site or organ as the first primary cancer accounted for 13.2%, with the most common sites being the female breast, colon, lung, and skin (melanoma). Another 3.8% of new malignancies originated in neighboring tissues or organs.2 Table 62-1 shows the significant relative and excess absolute risks by primary cancer site. As is obvious, risk is highest for survivors of childhood cancer. However, for adult-onset cancers, although detailed lifestyle or environmental data were clearly not obtained for this analysis, the EAR for cancers occurring at tobacco- or alcohol-related sites, such as the oral cavity, pharynx, esophagus, larynx, and lung, was 114 cases per 10,000 person-years and overall accounted for 10,000 excess subsequent cancers.2
Table 62-1
Risk Factors for Subsequent Malignancy in the United States by Site*
| Primary Cancer | Observed Versus Expected | Excess Absolute Risk/10,000 Person Years |
| Lip | 1.16 | 34 |
| Oral cavity | 2.45 | 231 |
| Nasopharynx | 1.49 | 47 |
| Salivary gland | 1.25 | 644 |
| Colon | 1.07 | 13 |
| Anal | 1.24 | 36 |
| Lung | 1.36 | 65 |
| Larynx | 1.71 | 150 |
| Nasal | 1.44 | 68 |
| Breast | 1.18 | 23 |
| Cervix | 1.32 | 24 |
| Vulva | 1.12 | 16 |
| Ovary | 1.18 | 15 |
| Testis | 1.62 | 21 |
| Bladder | 1.17 | 34 |
| Kidney | 1.13 | 23 |
| Renal pelvis/ureter | 1.29 | 55 |
| Bone sarcoma | 1.24 | 13 |
| Soft tissue sarcoma | 1.19 | 20 |
| Melanoma of skin | 1.24 | 27 |
| Ocular melanoma | 1.16 | 24 |
| Other ocular (nonretinoblastoma) | 1.35 | 51 |
| Brain and central nervous system | 1.11 | 4 |
| Thyroid | 1.11 | 8 |
| Hodgkin lymphoma | 2.22 | 49 |
| Non-Hodgkin lymphoma | 1.14 | 14 |
| Chronic lymphocytic leukemia | 1.19 | 38 |
| Chronic myeloid leukemia | 1.16 | 19 |
| Acute lymphocytic leukemia | 1.55 | 5 |
| Childhood cancer | 6.07 | 15 |
| Retinoblastoma | 14.71 | 24 |
| Ewing sarcoma | 14.84 | 44 |
| Hodgkin lymphoma | 9.55 | 39 |
| Central nervous system peripheral neuroectodermal | 12.54 | 26 |
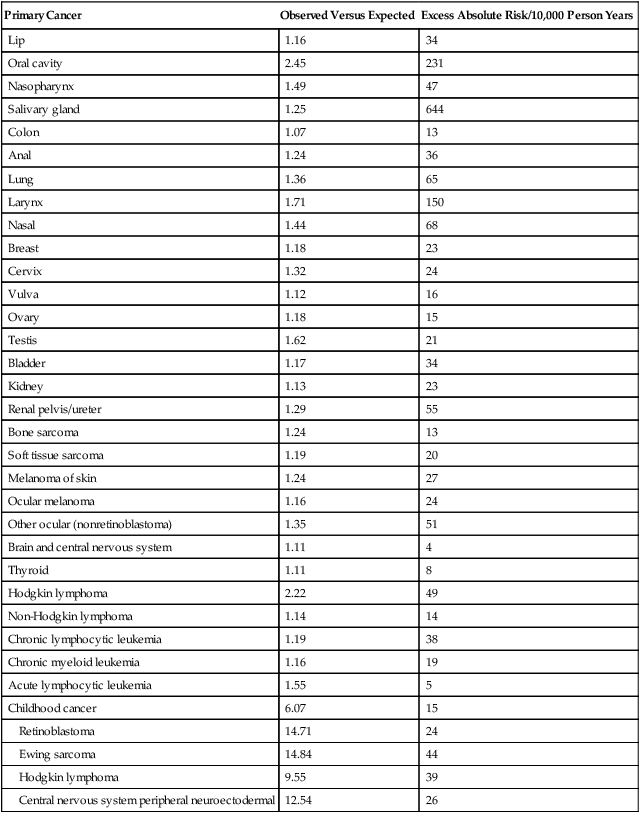
*Adapted from National Cancer Institute. New malignancies among cancer survivors: SEER cancer registries, 1973-2000, <http://seer.cancer.gov/publications/mpmono/>; 2013 [accessed 25.04.13].
The etiology of SMNs is multifactorial. Host and environmental factors clearly play a role, but defining those contributions can be difficult. Superimposed on this general population risk for second and subsequent primary cancers are treatment-associated malignancies, where chemotherapy and radiotherapy for the primary cancer can increase risk for a subsequent cancer. This risk is then modified by host and environmental factors that affect risk of primary cancer, such as lifestyle (e.g., use of tobacco and alcohol, sun exposure, and diet), workplace and home exposures to carcinogens, viruses, age, gender, genetics, infection, immune function, hormone levels, and interactions of all of these factors. It is difficult to evaluate disease separate from known genetic associations with those diseases (e.g., breast, colon, and retinoblastoma) or separate from exposure to specific therapeutic modalities used to treat those malignancies (e.g., chemotherapy and radiation therapy). Table 62-2 summarizes common risk factors for SMNs in these general categories, but categories are not fully exclusive of one another.
Table 62-2
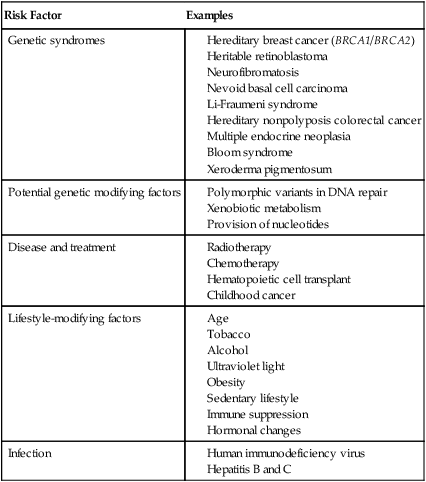
Genetic Risks for Subsequent Malignancy
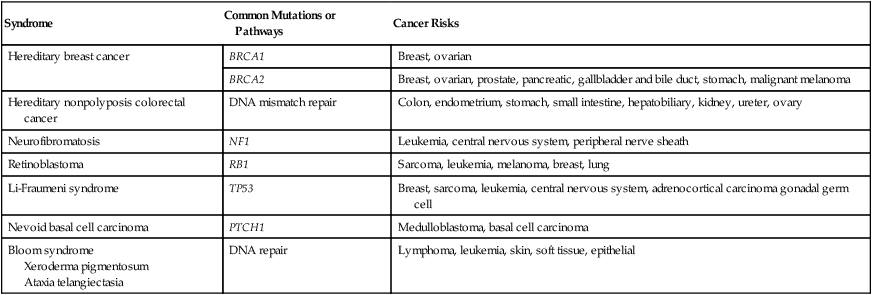
When considering the risk of SMNs associated with genetic predisposition, one must consider genetic disorders with high risk for both primary and subsequent malignancy, as well as less well-defined gene-environment interactions. The former category, which accounts for a small minority of SMNs, includes hereditary breast cancer (BRCA1/BRCA2), heritable retinoblastoma, neurofibromatosis, nevoid basal cell carcinoma (BCC), Li-Fraumeni syndrome, hereditary nonpolyposis colorectal cancer (Lynch syndrome), multiple endocrine neoplasia, Bloom syndrome, xeroderma pigmentosum, and other syndromes.4,5 Table 62-3 summarizes these syndromes and common sites for primary and subsequent neoplasms; in addition, several are discussed here.
Table 62-3
Common Hereditary Cancer Syndromes
| Syndrome | Common Mutations or Pathways | Cancer Risks |
| Hereditary breast cancer | BRCA1 | Breast, ovarian |
| BRCA2 | Breast, ovarian, prostate, pancreatic, gallbladder and bile duct, stomach, malignant melanoma | |
| Hereditary nonpolyposis colorectal cancer | DNA mismatch repair | Colon, endometrium, stomach, small intestine, hepatobiliary, kidney, ureter, ovary |
| Neurofibromatosis | NF1 | Leukemia, central nervous system, peripheral nerve sheath |
| Retinoblastoma | RB1 | Sarcoma, leukemia, melanoma, breast, lung |
| Li-Fraumeni syndrome | TP53 | Breast, sarcoma, leukemia, central nervous system, adrenocortical carcinoma gonadal germ cell |
| Nevoid basal cell carcinoma | PTCH1 | Medulloblastoma, basal cell carcinoma |
| Bloom syndrome Xeroderma pigmentosum Ataxia telangiectasia |
DNA repair | Lymphoma, leukemia, skin, soft tissue, epithelial |
Mutations in BRCA1 and BRCA2 are associated with high risk for secondary breast cancer.6,7 In a study of 810 women with a stage I or II primary breast cancer in the setting of BRCA1 or BRCA2, a contralateral breast cancer developed in 149 subjects (18.4%), with a 15-year actuarial risk of 36.1% and 28.5%, respectively, for women with a BRCA1 or BRCA2 mutation. Younger age (<50 years) at primary breast cancer diagnosis and having two or more first-degree relatives with breast cancer also increased risk. A reduction of risk was noted with oophorectomy (RR = 0.47).6 Among 396 women with stage I or stage II primary breast cancer in the setting of a BRCA1 or BRCA2 mutation, the 5-year actuarial risk of ipsilateral breast cancer was 5.8%, and the 10-year risk was 12.9%. Receipt of chemotherapy and radiotherapy both reduced risk (RR = 0.45 and 0.28, respectively). Oophorectomy was associated with a significant risk reduction (RR = 0.33). On average, after a diagnosis of breast cancer, the annual risk of ipsilateral breast cancer risk in BRCA mutation carriers is 1.2% per year.7 Of course, in addition to these risks, patients with BRCA1 and BRCA2 also have an increased risk for primary ovarian cancer,8 and patients with BRCA2 have an increased risk for prostate cancer, pancreatic cancer, gallbladder and bile duct cancer, stomach cancer, and malignant melanoma.9 Of note, mutations in BRCA1, BRCA2, or TP53 are not thought to be responsible for the increased incidence of SMN among Hodgkin lymphoma survivors. In a study of 44 patients who experienced one or more SMNs, mutations in the TP53 gene were examined in all 44 patients and BRCA1 and BRCA2 were examined in 19 female patients in whom one or more secondary breast cancer developed. One of 44 patients tested for TP53 harbored a novel homozygous germline abnormality, and one of 19 patients tested for BRCA2 carried a previously described heterozygous inactivating mutation; no germline BRCA1 mutations were identified.10
Mutations in mismatch repair genes in persons with hereditary nonpolyposis colorectal cancer can lead to the more site-specific colorectal cancer syndrome associated with other gastrointestinal cancers (Lynch syndrome type I) or the family cancer syndrome, in which early-onset endometrial and stomach cancers are seen in addition to colorectal cancer (Lynch syndrome type II).11,12
Neurofibromatosis type 1 (NF1), which results from a mutation in the NF1 gene at chromosome 11q12, is not associated with malignancy in the majority of patients with this syndrome. However, patients (and particularly children) with NF1 have an increased risk for leukemias, central nervous system (CNS) tumors, and peripheral nerve sheath tumors.13,14 In a report from the Late Effects Study Group, NF1 was second only to retinoblastoma among children with a predisposition to SMN.15
Retinoblastoma, which is associated with germline mutation of the RB1 gene at chromosome 13q14, is the most common hereditary syndrome associated with SMN after childhood cancer.18–18 Survivors of retinoblastoma have a significantly increased risk of sarcomas both inside and outside the radiotherapy field, along with leukemia, melanoma, and epithelial tumors such as breast and lung cancers. In a cohort of 1604 patients treated from 1914 to 1984, the cumulative incidence of SMN was 51% and 5% at 50 years for heritable and nonheritable retinoblastoma survivors, respectively.19 A follow-up 9 years later found 50-year cumulative incidences to be 36% and 5.7%, with standardized incidence ratios (SIRs) of observed/expected SMNs of 19 and 1.2, respectively, for persons with heritable disease versus nonheritable disease. Radiotherapy increased SMN risk in heritable patients 3.1-fold.20 The cumulative probability of death from SMN was 25.5% for bilateral disease survivors, and the increased risk extended beyond 40 years after retinoblastoma diagnosis.21
Li-Fraumeni syndrome, which is associated with germline mutation in the TP53 gene at chromosome 17q13, is characterized by increased risk for breast cancer, sarcoma, leukemia, brain tumors, adrenocortical carcinoma, and gonadal germ cell tumors.22,23 Several small studies have reported an excess of multiple primary tumors in patients with Li-Fraumeni syndrome, but this phenomenon has not been systematically studied.10,24,25
Nevoid BCC syndrome associated with mutation of the PTCH1 gene on chromosome 9 is associated with BCC and medulloblastoma, and many BCCs can develop during a patient’s lifetime. Although multiple BCCs are reported in survivors of childhood cancer, it is likely that only persons who experience these along the track of radiotherapy within a very short latency of 6 months have this genetic predisposition. In other persons, including those treated with total body irradiation (TBI) for stem cell transplant or radiotherapy after childhood cancer, early-onset nonmelanoma skin cancers are noted, although environmental and lifestyle factors have not been systematically collected and represent an area of future research.26,27
Bloom syndrome, xeroderma pigmentosum, and ataxia telangiectasia are all rare disorders of DNA repair that can lead to multiple primary cancers, including leukemia and lymphoma, skin cancer, soft tissue sarcomas, and epithelial tumors. However, these syndromes have well-recognized phenotypes and thus are not an occult cause of SMN.28–32
Treatment-Associated Risks for Subsequent Malignancies
Radiation Therapy
Data on the carcinogenic potential of ionizing radiation exposure from cancer treatment, other disease treatment, and environmental causes have been available for several decades. These data have shown that organs differ in their sensitivity for SMN and the dose needed to induce malignancy and that latency and age at time of exposure remain important risk factors.33–41 Risk of SMN from radiation therapy appears to be proportional to the number of premalignant stem cells created and the number of cells that survive radiation therapy, which in turn are related to cellular killing, cellular repopulation occurring between fractions and after the last fraction, and the ratio of proliferation rate for premalignant to normal cells.42 Furthermore, the type and energy of radiation and the time course during which exposure occurs all affect risk for SMNs. Low-linear-energy transfer radiation, such as x-rays and gamma rays, is sparsely ionizing and is generally less efficient in tumor induction than densely ionizing high-linear-energy transfer radiation (e.g., α particles and neutrons), for which the carcinogenic effectiveness is not diminished at high doses and may be increased with fractionation and protraction. Risk is related to age at treatment; generally, younger age is associated with increased risk, total dose, and mode of radiation therapy delivery. Latency periods are long, and risk appears to rise without a clear plateau for decades after radiation therapy is conducted.43–49
Many of the data on SMN in childhood cancer come from the Childhood Cancer Survivor Study (CCSS), which evaluates long-term outcomes in childhood cancer survivors treated between 1970 and 1987 who survived at least 5 years after the initial diagnosis. In this cohort, risk for SMN was examined with respect to therapeutic exposures, which were well documented through chart abstraction for each participant.50,51 In addition, radiation dosimetry was evaluated for some in-depth analyses of radiation risk.52 In the most recent report on subsequent neoplasms from the CCSS, among 14,359 survivors, 1402 individuals had a total of 2703 subsequent neoplasms, which included 802 second malignant neoplasms, 159 nonmalignant meningiomas, 169 benign or in-situ neoplasms, and 1574 nonmelanoma skin cancers. In multivariable analysis, risk of any subsequent neoplasm was increased 2.7-fold with radiotherapy exposure. Risk related to radiotherapy for malignant neoplasms other than nonmelanoma skin cancer was increased 2.6-fold, risk of meningioma was increased 16.6-fold, and risk of nonmelanoma skin cancer was increased 4.4-fold.46 Dose-response relationships also appear to differ between organs with respect to risk of SMN in childhood cancer survivors, as is evidenced by work done within the CCSS and reviewed by Armstrong.53 Among 116 survivors with a subsequent CNS neoplasm, compared with control subjects who did not have a subsequent CNS neoplasm, radiation therapy was associated with an increased risk for any subsequent CNS malignant neoplasm, and specifically for subsequent glioma (odds ratio [OR] = 6.78) and meningioma (OR = 9.94). Furthermore, linear dose-response relationships between radiation dose and secondary glioma and meningioma were identified. The excess RR per Gy, equal to the dose of the linear response function, was 0.33 per Gy for glioma and 1.06 per Gy for meningioma. With increasing length of follow-up, the number of new glioma cases declined, but the incidence of meningioma continued to increase with longer length of follow-up.54 In analyses of secondary thyroid cancer, risk increased with radiation dose to the thyroid gland for doses as high as 29 Gy and decreased for doses greater than 30 Gy, with this linear exponential dose most pronounced in persons younger than 10 years at the time of exposure.55,56 Risk of breast cancer after chest radiation therapy is now well established. SIR (evaluating observed/expected) for breast cancer was 24.7 in women treated with chest radiotherapy in the CCSS compared with an SIR of 4.8 for women who were not treated with chest radiation therapy.46,57 Notably, receipt of ovarian radiation reduced rates of breast cancer (RR, 0.6). In Hodgkin lymphoma survivors who were treated with chest radiation, the cumulative incidence of breast cancer at age 40 years was 12.9%.57
To evaluate the dose-response relationship between chest radiation therapy and the development of breast cancer, 120 confirmed breast cancer cases were matched with control subjects, and a linear relationship was identified between risk of developing breast cancer and radiation dose. At a dose of 40 Gy to the breast, an elevenfold increased risk for the development of breast cancer was found. The slope of the dose-response curve was altered when the radiation dose to the ovaries was considered, because ovarian radiation exposure of 5 Gy or more decreased the risk of breast cancer.58
Risk of SMN related to radiotherapy among adults treated for cancer clearly differs from that among children. In a report from SEER, in patients ≥20 years who were diagnosed with a primary cancer between 1973 and 2002, an analysis compared risk for SMN among those treated with or without radiotherapy. In this group of 647,672 patients with cancer, a second solid organ malignancy developed in 60,271 (9%). For each of the first cancer sites, the RR of developing an SMN associated with radiotherapy was elevated and was highest for organs that typically received more than 5 Gy. As seen in studies of childhood cancer survivors, risk decreased with increasing age at diagnosis and increased with time since diagnosis. It was estimated that 3266 excess solid organ SMNs could be related to radiotherapy, and by 15 years after diagnosis, there were five excess cancers per 1000 patients treated with radiotherapy.59
However, survivors of adult-onset malignancies who were treated with radiotherapy are clearly at some increased risk for SMN, as evidenced by several large population-based studies of SMNs. Travis and colleagues60 identified 2285 second solid cancers in 40,576 1+-year survivors of testicular cancer who were treated between 1943 and 2001. Statistically significant increased risks of solid cancers were observed among patients treated with radiotherapy alone (RR = 2.0) or in combination with chemotherapy (RR = 2.9).60 In contrast, among 42,722 survivors treated between 1943 and 2002, secondary leukemia developed in 89 patients (EAR = 10.8 per 100,000 person-years). In multivariate analyses, leukemia risk was higher among patients whose initial management included chemotherapy compared with those receiving radiotherapy alone.61 Among 104,760 one-year survivors of cervical cancer, 12,496 SMNs were found overall (SIR = 1.33). Compared with the general population, patients with cervical cancer who were treated with radiotherapy, but not patients who did not receive radiotherapy, were at increased risk for all second cancers, particularly at the sites of the colon, rectum/anus, urinary bladder, ovary, and genitalia. The association of radiotherapy with second cancer risk was modified by age at cervical cancer diagnosis for the rectum/anus, genital sites, and urinary bladder, with higher hazard ratios for second cancer at younger ages of diagnosis of cervical cancer.62 In a cohort of 32,941 patients treated for Hodgkin lymphoma between 1935 and 1994, SMNs occurred in 2153 and the risk for solid tumors was elevated 2.3-fold in patients who were treated with radiation alone, 1.7-fold in those treated with chemotherapy alone, and 3.1-fold with combined modality therapy.63 In a cohort of 770 female patients who had been diagnosed with Hodgkin lymphoma before age 41 years, breast cancer developed in 48. Risk of breast cancer increased statistically significantly with radiation dose: patients who received 38.5 Gy or more had an RR 4.5 times that of patients who received less than 4 Gy. Patients who received combined modality therapy had a statistically significantly lower risk than those treated with radiotherapy alone (RR = 0.45). Breast cancer risk increased with increasing radiation dose among patients who received radiotherapy alone at doses ≥38.5 Gy (RR = 12.7).64
The most common second cancers associated with radiotherapy exposure are shown in Table 62-4, together with the most common primary cancers for which the radiotherapy was administered.
Table 62-4
Common Radiotherapy-Associated Cancers
| Radiation Field | Common Primary Malignancy Treated | Common Subsequent Malignancies |
| Brain | Central nervous system, leukemia | Central nervous system |
| Neck | Hodgkin lymphoma | Thyroid |
| Thoracic | Hodgkin lymphoma, Wilms tumor, sarcoma, breast | Breast, lung |
| Abdomen | Sarcoma, Wilms, Hodgkin, gastrointestinal | Colon |
| Pelvis | Gonadal | Gonadal |
| Soft tissue | Sarcoma | Sarcoma |
| Bone | Sarcoma, retinoblastoma | Sarcoma |
Chemotherapy
The relationship between chemotherapy and SMN is likely more complex than that with RT, because agents appear to have different oncogenic potential, and a number of different mechanisms are associated with SMN development. Table 62-5 summarizes some of the common chemotherapeutic agents and subsequent malignancies with which they are associated.
Table 62-5
Common Chemotherapy-Associated Cancers
| Chemotherapy | Common Primary Cancers | Subsequent Malignancies |
| Alkylating agents | Sarcoma Lymphoma |
Myelodysplastic syndrome, leukemia |
| Etoposide | Sarcoma Hodgkin lymphoma |
Leukemia |
| Anthracyclines | Sarcoma Lymphoma Breast cancer |
Myelodysplastic syndrome, leukemia |
| Platinum | Ovarian cancer | Leukemia |
Chemotherapy-related SMNs have been studied for several decades. Acute myeloid leukemia, acute lymphoblastic leukemia, and myelodysplastic syndrome are largely associated with alkylating agents often, but not exclusively, after Hodgkin lymphoma. In fact, Hodgkin lymphoma serves as a model for the study of balancing efficacy with toxicity when considering primary treatment. Hodgkin lymphoma is a primary malignancy that is associated with a high cure rate, but the very therapy that resulted in that cure rate shares the etiologic limelight for risk of subsequent leukemia. This phenomenon reflects the use of chemotherapy agents that unfortunately also have leukemogenic potential, such as mechlorethamine (nitrogen mustard) and procarbazine. To better quantify the risk of leukemia associated with alkylating agents, Meadows and colleagues65 developed an alkylating agent score to describe the number of different alkylating agents administered and the duration of therapy for any patient. With use of this model, the risk of subsequent leukemia was found to increase with alkylating agent score.65 Replacement of mechlorethamine by cyclophosphamide in Hodgkin lymphoma protocols and/or use of doxorubicin, bleomycin, vinblastine, and dacarbazine instead of or to replace some of the alkylating agent have both led to reductions in subsequent leukemia risk.66–69
Alkylating agents cause unstable positively charged ions that participate in covalent bonds with electron-rich sites on DNA bases. Resultant adduct formation can induce apoptosis or mutagenesis. Interstrand cross-links and breaks also occur. DNA adducts may lead to configurational changes in the base and subsequent mismatch. Bulky DNA adducts or strand breaks that are large or occur at critical areas can result in disruption of DNA replication. Point mutations or deletions may result in either activation of an oncogene or mutation of a tumor suppressor gene. Alkylating agent–associated leukemia or MDS is typically characterized by abnormalities of chromosomes 5q or 7q, where putative tumor suppressor genes are postulated. Accompanying mutations in RAS oncogenes are also present in a number of cases.70–74
Topoisomerase II inhibitors are another class of chemotherapy agents associated with subsequent leukemia. Topoisomerase II is a nuclear enzyme that catalyzes and regulates both DNA cleavage and ligation and is essential for DNA replication, transcription, and repair. Topoisomerase II inhibitors form a complex between the topoisomerase II and the DNA, and the ligase activity for DNA repair is inhibited; in addition, illegitimate recombination events occur. Lastly, active metabolites can lead to DNA adduct or free radical formation, leading to mutagenesis.75,76 Most of the translocations involve the MLL gene at 11q23 and fuse MLL with a wide variety of partners. However, subsequent leukemia with other chromosomal translocations such as 8;21, 3;21, inv16,8;16, 15;17, and 9;22 are reported.77–81 Epipodophyllotoxins, which are strong topoisomerase II inhibitors, specifically etoposide, and in the past teniposide, have been associated with subsequent leukemia after treatment for leukemia and a number of solid tumors. Risk appears related to the schedule of administration, cumulative dose, and concomitant administration of either alkylating agents such as cyclophosphamide or other topoisomerase inhibitors such as doxorubicin.48,82–89 In a case-control study of 61 patients with secondary leukemia who were matched with 196 control subjects, two factors were found to increase the risk of leukemia in multivariate analysis, namely, the type of the first tumor, with an excess risk in patients with Hodgkin lymphoma (RR = 6.4) or osteosarcoma (RR = 5), and exposure to epipodophyllotoxins and anthracyclines. The risk of leukemia increased regularly with the cumulative dose of etoposide. Patients who received between 1.2 and 6 g/m2 of epipodophyllotoxins or more than 170 mg/m2 of anthracyclines had a sevenfold increased risk of leukemia.90 In the more recent era of chemotherapy for Hodgkin lymphoma, etoposide has been incorporated in a number of upfront regimens. In these regimens, doxorubicin was also used, and dexrazoxane was used for cardioprotection, resulting in the administration of three topoisomerase II inhibitors in a relatively short period. In an analysis of two such Hodgkin lymphoma studies, 239 patients were assigned randomly to receive dexrazoxane for cardioprotection and 239 did not receive this agent. SMNs developed in 10 patients. Six of eight patients in whom both leukemia and solid tumors (osteosarcoma and papillary thyroid carcinoma) developed were recipients of dexrazoxane. The SIR for all SMNs was 41.86 versus 10.08 with and without dexrazoxane, respectively (P = .02).91
In the most recent comprehensive analysis of SMNs from the CCSS, in a multivariable model, risk of any subsequent neoplasm was increased by female sex (RR = 1.5), older age at primary childhood cancer diagnosis (RR = 1.3), radiation therapy exposure (RR = 2.7), and primary diagnosis of Hodgkin lymphoma (RR = 1.5), but not by chemotherapy. However, secondary breast cancer was associated with increased anthracycline exposure, together with primary diagnosis of Hodgkin lymphoma and radiation therapy. Sarcomas, which represented 15% of the subsequent malignancies, were associated with alkylating agent and anthracycline exposure, as well as radiotherapy and primary sarcoma diagnosis.46 These data highlight the complexity of risk factor analysis in the era of multimodality therapies.
Chemotherapy-associated leukemia is not limited to treatment of childhood cancer or Hodgkin lymphoma. In a case-control study of 96 women with secondary leukemia and 272 control subjects in a population-based cohort of 28,971 women in North America and Europe who were treated for ovarian cancer between 1980 and 1993, among the women who received platinum-based combination chemotherapy for ovarian cancer, the RR of leukemia was 4.0 (6.5 for carboplatin and 3.3 for cisplatin), with evidence of a dose-response relation, with RRs reaching 7.6 at doses of 1000 mg or more of platinum.92 Similar to data from Hodgkin lymphoma treatment, in a cohort study of 11,386 two-year survivors of non-Hodgkin lymphoma, 35 case patients with secondary leukemia were matched with 140 control subjects. Leukemia risk was elevated with exposure to prednimustine (RR = 13.4), regimens containing mechlorethamine and procarbazine (RR = 12.6), and chlorambucil with cumulative doses of ≥1300 mg (RR = 6.5). Cyclophosphamide regimens were associated with a small, nonsignificant increased risk.93
Modifications of Treatment-Related Effects on SMN Risk
The ABCB1 gene product, P-glycoprotein, is a membrane efflux pump that extrudes chemotherapeutic agents by active transport, and the presence of P-glycoprotein in the plasma membrane confers a multidrug-resistant phenotype. Another membrane protein pump that is important for some chemotherapeutic agents is multidrug resistance–associated protein, and doxorubicin, which is associated with secondary leukemia, is one of its substrates. One may hypothesize that such a transport pump may act as a host factor, influencing SMN risk from doxorubicin by modulation of the intracellular accumulation.94
Although there are known disorders of DNA repair that infer high individual attributable risks for cancer, such as Fanconi anemia,95 they are uncommon in the general population. However, polymorphic variants of a number of DNA repair genes likely exist that may contribute to a gene-environment interaction, whereby both the polymorphic variant and the exposure (e.g., chemotherapy or radiotherapy) increase risk for SMN. This interaction might explain some of the interindividual variability in treatment-associated SMN. Mismatch repair abnormalities have been associated with treatment-associated myelodysplasia and leukemia, wherein patients are found to have microsatellite instability and methylation of MLH1.98–98 In the homologous repair pathway, a polymorphism of RAD51 has been shown to be overrepresented in treatment-associated myelodysplasia and leukemia, with further increased risk conferred by the presence of a polymorphism in XRCC3, a gene in the base excision repair pathway.99 However, even within these repair pathways, variation of effect by gene occurs, as shown by a protective role of a variant of XRCC1 for treatment-associated myelodysplasia and leukemia and BCC.100 An identified variant in the nucleotide excision pathway, ERCC2, has also been associated with increased risk for treatment-associated myelodysplasia and leukemia.
Phase I metabolism is primarily performed by the cytochrome P450 (CYP) family of enzymes and involves activation of substrates into highly reactive electrophilic intermediates that can damage DNA. Phase II pathways, such as glutathione S-transferase or arylamine N-acetyltransferases and NAD (p) H : quinone oxidoreductase-1, participate in inactivation or detoxification of genotoxic substrates.103–103 However, as was seen with the DNA-repair enzymes and SMN risk, associations with variants in these metabolizing genes are modest, and although many studies have attempted to demonstrate associations between polymorphic variants in these DNA repair pathways and SMN, results are mixed or inconclusive. Thus research in this area is ongoing.104–110
Environmental Exposures
Although it is clear that for many SMNs, particularly solid organ tumors, risk continues to rise with ongoing time since treatment, that period is also marked by lifestyle and environmental exposures such as tobacco, alcohol, ultraviolet light, obesity, a sedentary lifestyle, immune suppression, and hormonal changes, all of which have a role in primary cancers. The intersection of such factors in the etiology of SMN is difficult to determine, because one would need to simultaneously evaluate genetic risks, therapeutic exposures from the primary cancer, and many environmental exposures in the same cohort, which would be difficult to assemble and for which it would be difficult to provide adequate statistical power. However, tobacco and alcohol are both risk factors for multiple malignancies involving the lung, gastrointestinal system, biliary system, oral mucosa and cavity, pharynx, and upper aerodigestive track, where the effect is synergistic. In the most recent SEER monograph on SMN, it was estimated that in terms of absolute excess risk, tobacco- and alcohol-related cancer sites account for 35% of SMNs.1,111
The role of diet and nutrition in SMN risk is much less well defined. Associations between colorectal cancer, ovarian cancer, endometrial cancer, and breast cancer are well known,112 as are data suggesting some role in decreased recurrence or mortality risk in breast cancer survivors who made dietary changes that included increased soy, decreased fat, and increased fruits and vegetables; however, results are not fully consistent.115–115 No studies have been performed to investigate the role of dietary or exercise intervention specifically to reduce the risk of SMN.
Similarly, sun exposure increases the risk for primary cutaneous melanoma and BCC. Both of these cancers are now seen after primary cancer treatment, particularly after childhood cancer and hematopoietic cell transplantation.27 However, the role of sun exposure remains relatively unknown because these malignancies in both the childhood cancer and stem cell populations are clearly related to the receipt of radiation therapy. Whether sun exposure creates an additive risk is unclear, but cancer survivors are advised to avoid excess sun exposure and use sunscreen. It is plausible to hypothesize that the radiation therapy from cancer therapy causes sensitization such that excess ultraviolet exposure from the sun has an additive effect.
High-Risk Populations for Subsequent Malignancies
Childhood Cancer Survivors
In the most recent analysis from the CCSS referenced earlier, the cumulative incidence at 30 years after the childhood cancer diagnosis was 20.5% for all subsequent neoplasms, 7.9% for second malignant neoplasms (excluding nonmelanoma skin cancer), 9.1% for nonmelanoma skin cancer, and 3.1% for meningioma. Excess risk was evident for all primary diagnoses, with the highest being for Hodgkin lymphoma and Ewing sarcoma. In the Poisson multivariable analysis, female sex, older age at diagnosis, earlier treatment era, diagnosis of Hodgkin lymphoma, and treatment with radiation therapy were associated with increased risk of a subsequent neoplasm.46
In two analyses from the CCSS on subsequent thyroid cancer, more defined risks related to radiation dose and concomitant alkylating agent therapy were elicited. The 30-year cumulative incidence of thyroid cancer is 1.3% for women and 0.6% for men. Thyroid cancer risk increased linearly with radiation dose up to approximately 20 Gy, where the relative risk peaked at 14.6-fold, after which a downturn in the dose-response relationship was observed. A higher radiation risk was found with younger age, among females, and with longer time since exposure. Among patients with thyroid radiation doses of 20 Gy or less, treatment with alkylating agents was associated with a significant 2.4-fold increased risk of thyroid cancer. Chemotherapy-related risk was evident for thyroid radiation doses more than 20 Gy.116,117
Moreover, childhood cancer survivors are at risk for carcinomas that are uncommon in young persons, as well as multiple subsequent neoplasms. In another report from the CCSS, at a median age of 27 years and a median elapsed time of 15 years, 71 carcinomas were diagnosed in the genitourinary system (35%), head and neck area (32%), gastrointestinal tract (23%), and other sites (10%). Fifty-nine patients (83%) had received radiotherapy, and a second malignant neoplasm developed in a previous radiotherapy field in 42 patients (59%).118 In a recent CCSS analysis of 1382 survivors with one subsequent neoplasm, a second subsequent neoplasm developed in 27.9%, and of those, 39.6% experienced more than two subsequent neoplasms. Radiation increased risk for more than one subsequent neoplasm, and persons with nonmelanoma skin cancer had a 15-year cumulative incidence of SMN (excluding nonmelanoma skin cancer) of 20.3% compared with only 10.7% for persons exposed to radiation and whose first subsequent neoplasm was an invasive SMN.119
In another large cohort of childhood cancer survivors (a combined cohort of 8884 North American, 2893 British, and 1574 Nordic subjects with Wilms tumor diagnosed before 15 years of age during 1960-2004), 174 solid tumors (exclusive of BCCs) and 28 leukemias were ascertained in 195 subjects. The cumulative incidence of solid tumors at age 40 years was 6.7%. Leukemia risk was highest during the first 5 years after Wilms tumor diagnosis. Standardized incidence ratios for solid tumors and leukemias were 5.1 and 5.0, respectively.120
Sarcomas
Sarcomas of the bone and soft tissues (osteogenic sarcoma, Ewing sarcoma, and soft tissue sarcoma) are associated with increased risk for SMN, and similarly, sarcomas are common second malignancies following childhood cancer. In a report from the CCSS, Henderson and colleagues121 reported that 108 of 14372 participants had secondary sarcomas, diagnosed a median of 11 years after the diagnosis of childhood cancer, with a risk ninefold that of the general population. Risk was elevated with radiation therapy (RR = 3.1), primary diagnosis of sarcoma (RR = 10.1), history of other secondary neoplasms (RR = 2.2,), and treatment with anthracyclines (RR = 2.3) or alkylating agents (RR = 2.2).121 In the CCSS, the SIRs for SMN after primary soft tissue, Ewing sarcoma, and osteosarcoma were 5.8, 8.5, and 4.2, respectively. Risks of secondary bone or soft tissue sarcoma were 19.0-fold and 8.1-fold that expected in the general population.46 Of particular interest is the risk of SMN after diagnosis and treatment of Ewing sarcoma. In an analysis of 1166 patients with Ewing sarcoma in the SEER registry who were treated between 1973 and 2005, an SMN developed in 35 patients. The estimated risks of SMN at 5, 10, and 20 years after original diagnosis were 2.1%, 4.4%, and 8%, respectively. The risk of SMN was higher in patients diagnosed before the age of 20 years in the earlier years of the cohort (though 1985) and in those treated with radiation therapy.122 In a report from the CCSS, 36 SMNs were reported in 34 of 403 patients who had Ewing sarcoma, with a cumulative incidence of 9%. The SIR in all Ewing sarcoma survivors, those treated with radiation, and those not treated with radiation was 5.9, 6.6, and 3.3, respectively. Notably, 13 breast cancers developed in 11 women, 7 of which were in the radiation field. The SIR for subsequent breast cancer after whole-lung radiation was 36.0.123 In a review from St. Jude Children’s Research Hospital, among 237 patients treated for Ewing sarcoma, the cumulative incidence of SMN was 3% and 4.7% at 5 and 10 years, respectively, translating to a SIR of 17.8.124
Hodgkin Lymphoma Survivors
Several large cohort studies have established the high risk of SMN after diagnosis and treatment of Hodgkin lymphoma. Among 35,511 survivors from 14 population-based cancer registries in Nordic countries and North America during 1970-2001, 217 were diagnosed with leukemia, whereas it was expected that 10.8 would be diagnosed. Excess absolute risk was highest during the first 10 years after Hodgkin lymphoma diagnosis but remained elevated thereafter. Risk was higher for those whose Hodgkin lymphoma was diagnosed at ≥35 years of age than in those diagnosed before 35 years of age. The risk declined after 1984, which may be associated with modifications in chemotherapy.125
In an international population-based cohort of 3817 female 1-year survivors diagnosed from 1965 to 1994, for survivors who were treated at ≤25 years of age with a chest radiation dose of at least 40 Gy without alkylating agents, the estimated cumulative absolute risks of breast cancer by ages 35, 45, and 55 years were 1.4%, 11.1%, and 29.0%, respectively. Cumulative absolute risks were lower in women who were treated with alkylating agents.126 In a case-control study from this cohort, women who received a ≥5-Gy radiation dose to the breast had a 2.7-fold increased breast cancer risk, compared with those given less than a 5-Gy dose.127
In another cohort of 18,862 5-year survivors from 13 population-based cancer registries in North America and Europe, 1490 SMNs were identified, with 850 estimated to be in excess. For most cancer sites, risk decreased with increasing age at Hodgkin lymphoma diagnosis and showed strong dependencies on attained age. Thirty-year cumulative risks of solid organ SMNs for men and women diagnosed at 30 years were 18% and 26%, respectively, compared with 7% and 9%, respectively, in the general population. For young patients, risks of breast and colorectal cancers were elevated 10 to 25 years before the age when routine screening would be recommended in the general population.128
Within a population-based cohort of 19,046 patients diagnosed from 1965 through 1994, a case-control study of lung cancer was conducted, comparing 222 patients in whom lung cancer developed and 444 matched control subjects. Treatment with alkylating agents without radiotherapy was associated with increased lung cancer risk, as was radiation dose to the lung of 5 Gy or more without alkylating agents. Risk increase was associated with both an increasing number of cycles of alkylating agents and increasing radiation dose. Excess risk after treatment with radiotherapy began 5 years after treatment and persisted for more than 20 years. Tobacco use increased lung cancer risk more than twentyfold.129 In the Late Effects Study Group cohort of 1380 children diagnosed with Hodgkin lymphoma from 1955 to 1986 in patients aged 16 years or younger, with 212 SMNs reported, the cohort was at an 18.5-fold increased risk of the development of SMNs compared with the general population. The cumulative incidence of any second malignancy was 10.6% at 20 years, increasing to 26.3% at 30 years, and the cumulative evidence of solid malignancies was 7.3% at 20 years, increasing to 23.5% at 30 years. Breast cancer was the most common solid malignancy, but other commonly occurring solid malignancies included thyroid cancer, bone tumors, and colorectal and gastric cancers. Third neoplasms developed in 32 patients of the 212 SMNs, with the cumulative incidence approaching 21% at 10 years from diagnosis of second malignancy.130
In the CCSS, 277 invasive SMNs have occurred among 257 five-year survivors, with the median time to first SMN being 18.7 years from diagnosis (range, 5.0-33 years). The 30-year cumulative incidence was 10.9% among men and 26.1% among women, driven by the incidence of invasive breast cancer. The risk was highest for bone, thyroid, and breast cancer. The cumulative incidence of nonmelanoma skin cancer was also high among Hodgkin lymphoma survivors at 16.7% at 30 years after diagnosis.131
Hematopoietic Cell Transplant Survivors
In a multiinstitutional cohort of 28,874 allogeneic transplant recipients, 189 solid malignancies occurred, with an observed-to-expected ratio 2.1-fold and threefold among patients followed up for 15 years or more after transplantation.132 Although a substantial increase in SMN occurs with the use of chemotherapy alone, the addition of TBI clearly causes an increase in second tumor formation. In a cohort of 8079 patients who had undergone hematopoietic cell transplantation (HCT) at the Fred Hutchinson Cancer Research Center and 5806 patients who had survived beyond 100 days, 381 SMNs were reported. Risk factors included treatment with TBI and, furthermore, use of TBI in a single dose. In addition, as the dose of fractionated TBI increased, the hazard ratio increased. Patients with TBI exposure had a 25-year incidence of SMN of 21%, compared with 10% for patients not exposed to TBI. Risk continued to rise with elapsed time since transplantation, without an obvious plateau. Of particular interest is that for patients younger than 10 years at HCT, those with TBI exposure had a 25-year incidence of SMN of 19%, compared with 5% for patients not exposed to TBI. For patients older than 10 years at hematopoietic stem cell transplantation, those with TBI exposure had a 25-year incidence of SMN of 21%, compared with 11% for patients not exposed to TBI. The effect of TBI in the entire cohort appeared to be due to younger patients without TBI having a very low incidence of SMN and older patients without TBI having a somewhat higher cumulative incidence of SMN.45
In another report from the Fred Hutchinson Cancer Research Center, among 4810 patients who received allogeneic HCT and survived for at least 100 days, 237 experienced at least one BCC or squamous cell carcinoma (SCC) of the skin or mucosal surfaces (BCC, n = 158; SCC, n = 95). Twenty-year cumulative incidences of BCC and SCC were 6.5% and 3.4%, respectively. For BCC, TBI was a significant risk factor, most strongly among patients younger than 18 years at the time of HCT. Acute graft-versus-host disease increased the risk of SCC (P = .02), whereas chronic graft-versus-host disease increased the risk of both BCC and SCC.27 Among 3337 female 5-year survivors who underwent allogeneic HCT at the Fred Hutchinson Cancer Research Center or at one of 82 centers reporting to the European Bone Marrow Transplant Registry, breast cancer developed in 52 survivors with a standardized incidence ratio of 2.2 and 25-year cumulative incidence of 11%. In multivariable analysis, increased risk was associated with longer time since transplantation, use of TBI, and younger age at transplantation.133
Impact of Subsequent Malignancies
Mortality
Prevention and Early Detection of Subsequent Malignancies
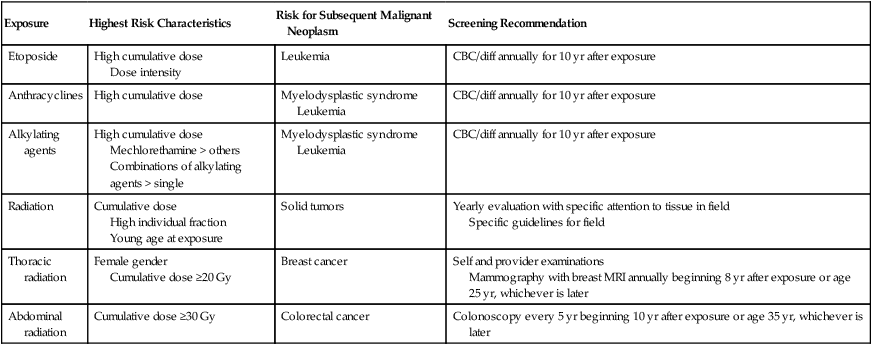
No clear evidence exists for strategies for primary prevention of SMN, although changes in therapy that limit exposures to alkylating agents, topoisomerase II inhibitors, and radiotherapy will serve to decrease risk. However, the significant advances in survival from pediatric and adult cancers during the past few decades should not be overshadowed by the risk of SMN. Although the balance of efficacy with short- and long-term toxicity should always be considered in the choice of therapy for a primary cancer, curative therapeutic options should not be withheld, even if they produce risk for SMNs. Survivors who are at increased risk for SMNs should certainly avoid additional known carcinogenic environmental exposures but should also be educated about their risk for SMNs and any specific screening that is recommended (Table 62-6). The Institute of Medicine has recognized the need for a plan of surveillance and monitoring for adverse long-term effects of cancer and its therapy among survivors of childhood and adult-onset cancers, including SMN. Recommendations also include enhanced communication between oncologists and primary care physicians and an interdisciplinary model of care, but no consensus has been reached about how such care should be delivered, in what setting, by what providers, and at what intervals.134,135 Most childhood cancer institutions now have survivorship clinics to follow-up patients and advise or conduct specific screening for subsequent cancers. A number of cancer centers have similar programs for survivors of adult-onset cancer and/or adult survivors of childhood cancer. Guidelines and recommendations that can inform screening and follow-up are found in some of the disease-specific guidelines of the National Comprehensive Cancer Network and the American Cancer Society, and more detailed consensus-based screening guidelines based on exposure are available from the Children’s Oncology Group, and for those treated with stem cell transplant, from the National Marrow Donor Program. Table 62-7 provides a brief summary of common sites for subsequent cancers, risk factors, and screening or prevention, adapted from the Children’s Oncology Group. In a recent review on SMN, Wood and colleagues111 have provided some suggestions for survivors of breast, colon, ovarian, endometrial, skin, lung and prostate cancer. Table 62-8 provides other sources for follow-up recommendations for cancer survivors in greater detail, where information is regularly updated.
Table 62-6
| Exposure | Highest Risk Characteristics | Risk for Subsequent Malignant Neoplasm | Screening Recommendation |
| Etoposide | High cumulative dose Dose intensity |
Leukemia | CBC/diff annually for 10 yr after exposure |
| Anthracyclines | High cumulative dose | Myelodysplastic syndrome Leukemia |
CBC/diff annually for 10 yr after exposure |
| Alkylating agents | High cumulative dose Mechlorethamine > others Combinations of alkylating agents > single |
Myelodysplastic syndrome Leukemia |
CBC/diff annually for 10 yr after exposure |
| Radiation | Cumulative dose High individual fraction Young age at exposure |
Solid tumors | Yearly evaluation with specific attention to tissue in field Specific guidelines for field |
| Thoracic radiation | Female gender Cumulative dose ≥20 Gy |
Breast cancer | Self and provider examinations Mammography with breast MRI annually beginning 8 yr after exposure or age 25 yr, whichever is later |
| Abdominal radiation | Cumulative dose ≥30 Gy | Colorectal cancer | Colonoscopy every 5 yr beginning 10 yr after exposure or age 35 yr, whichever is later |
CBC/diff, Complete blood cell count/differential; MRI, magnetic resonance imaging.
*Adapted from the Children’s Oncology Group. Refer to guidelines for updated and detailed information at www.survivorshipguidelines.org.
Table 62-7
Common Subsequent Cancers: Sites, Exposures, and Screening
| Site | Risk Factor | Type of Subsequent Malignant Neoplasm | Prevention or Screening |
| Brain | Radiation therapy | Glioma Meningioma |
Routine examinations |
| Oral cavity/pharynx Upper aerodigestive |
Radiation Tobacco Alcohol Human papillomavirus |
Carcinoma | Routine examinations Tobacco cessation Human papillomavirus vaccine for appropriate age groups |
| Thyroid | Radiation enhanced by alkylating agents | Carcinoma | Routine examinations |
| Breast | Radiation Genetic |
Carcinoma | Mammography Breast magnetic resonance imaging Self and provider examination |
| Lung | Radiation Tobacco |
Carcinoma | For high-risk smokers, low-dose lung computed tomography Tobacco cessation |
| Gastrointestinal | Radiation Genetic |
Carcinoma | Colonoscopy |
| Genitourinary | Radiation Genetic Human papillomavirus |
Carcinoma | Routine examination Papanicolaou smear (female) Prostate examination (male) Human papillomavirus vaccine for appropriate age groups |
| Soft tissue and Bone | Radiation enhanced by alkylating agents | Sarcoma | Routine examination |
| Bone marrow | Radiation Alkylating agents Etoposide Anthracyclines |
Myelodysplasia Leukemia |
Complete blood cell count |
| Skin | Radiation Ultraviolet light Immune compromise Human papillomavirus |
Melanoma Basal cell carcinoma Squamous cell carcinoma |
Sunscreen Routine skin examination |
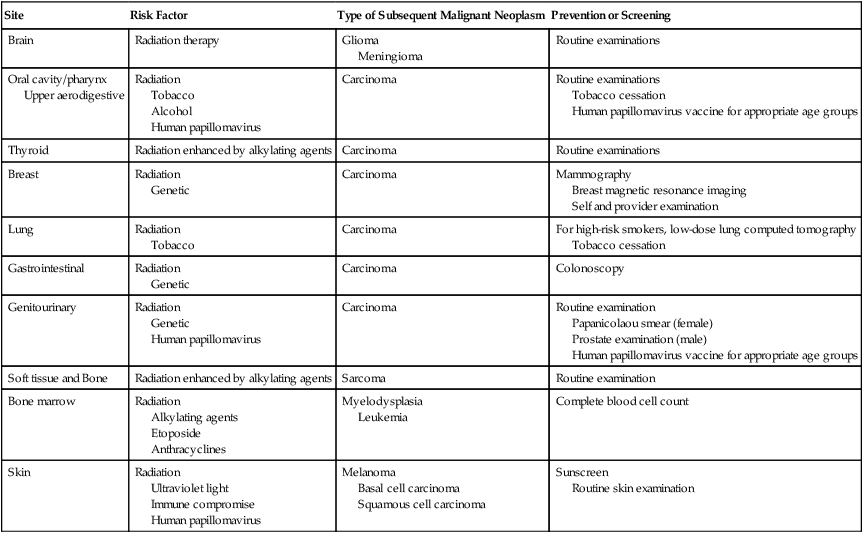
Table 62-8
Sources for Cancer Survivorship Follow-up Guidelines
| Patient Population | Organization | Web Site |
| Childhood, adolescent, and young adult cancer | Children’s Oncology Group | www.survivorshipguidelines.org |
| Adult-onset cancers | American Cancer Society | http://www.cancer.org/acs/groups/cid/documents/webcontent/002043-pdf.pdf |
| Adult-onset cancers disease- specific | National Comprehensive Cancer Network | www.nccn.org |
| Hematopoietic cell transplant | National Marrow Donor Program | http://marrow.org/Physicians/Post-Transplant_Care/11008_Recommended_Post-Transplant_Care_MAY2012_PDF.aspx |