Reproductive Complications
Lynda Kwon Beaupin, Tracey O’Connor and Donald L. Trump
• Reproductive complications resulting from cancer or its treatment are expected to increase as the number of cancer survivors increases.
• The risks of infertility related to cancer therapy and the available fertility preservation options should be discussed with all patients of reproductive age before cancer therapy begins.
• Oligospermia is present in more than 50% of patients with Hodgkin lymphoma and testicular cancer.
• Prostatectomy and other pelvic surgeries are associated with erectile dysfunction; retroperitoneal dissection is associated with retrograde ejaculation.
• Erectile dysfunction occurs within 2 years after treatment in 60% to 80% of patients with prostate cancer who are treated with external beam radiation.
• The use of sildenafil has reestablished potency in a large number of patients with surgery- or radiation-induced erectile dysfunction.
• Radiation can affect testicular spermatogenesis after doses as low as 15 cGy. Ovarian function is more resistant, but the effects are age related.
• Gonadal shielding and ovarian transposition ameliorate the effects of radiation on gonadal function.
• Gynecologic surgery can have a direct impact on sexual function by altering the normal female genital anatomy.
• Alkylating agents are associated with the highest rates of infertility in men and women.
• Doses and duration of chemotherapy agents are directly associated with the risk of infertility.
• The return of menses does not indicate preservation of ovarian function.
• Cancer complicates 1 in every 1000 pregnancies.
• The highest risk of congenital abnormalities is associated with chemotherapy or radiation exposure during the first trimester of pregnancy.
• It has not yet been determined whether the use of gonadotropin-releasing hormone analogs protects ovarian function and preserves spermatogenesis.
Reproductive Physiology
Gonadal Form and Function
The ovary produces mature fertilizable eggs, sex steroids, and reproductive/gonadal peptides. These activities are carried out in an integrated manner within the follicle. Humans have approximately one million follicles at birth. During the reproductive years, typical cyclic follicular recruitment, selection, and dominance eventually deplete the ovary of follicles, leading to cessation of ovarian function and menopause.1
In males, the testes secrete androgenic hormones and produce mature spermatozoa. These two processes are highly interrelated and regulated by multiple factors. Much like the ovary, compartments of the testes serve different functions. Spermatogenesis occurs in Sertoli cells within the seminiferous tubules. The interstitial or Leydig cells are essential for testosterone synthesis. Testosterone is transported from the Leydig cells to the seminiferous tubules, where it enhances spermatogenesis.2
Hypothalamic-Pituitary-Gonadal Axis
The main regulators of testicular and ovarian function are the gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). The biosynthesis and secretion of gonadotropins are modulated by an interplay of hypothalamic factors: gonadotropin-releasing hormone (GnRH), intrapituitary factors (pituitary peptides—activin and follistatin), and feedback by gonadal factors.1,2 Depending on the reproductive stage, estrogens can either increase or decrease gonadotropin production. Increased levels of estrogen in females or testosterone in males downregulate gonadotropin secretion. However, increased levels of estrogens at the time of LH surge exert a positive feedback effect. The gonadal proteins activin, inhibin, and follistatin also modulate the release of FSH.3 Inhibin decreases and activin stimulates gonadotropin function. Follistatin also inhibits FSH but is less potent than inhibin.
Regulatory effects of gonadotropins on the ovaries and testes are similar. Activation of gonadotropin receptors on the plasma membranes of Leydig cells and granulosa and theca cells induces the regulation and production of testosterone and of female steroid hormone production and follicular maturation, respectively.1,2
Direct Effects of Cancer on Reproductive Function
Central nervous system damage by primary or metastatic cancer or indirectly through paraneoplastic involvement can have a considerable impact on reproductive function. Pituitary prolactin-secreting adenomas are commonly associated with impotence in men and amenorrhea and galactorrhea in women. Other pituitary adenomas can affect reproductive function by destroying LH- and FSH-producing gonadotropes. Metastatic disease that affects the hypothalamus-pituitary axis also leads to reproductive dysfunction. Paraneoplastic syndromes are an infrequent but well-documented cause of reproductive dysfunction.4
Although men with Hodgkin lymphoma may have pretreatment impairment of spermatogenesis, no well-defined predictors of semen quality at the time of diagnosis have been identified. Some studies have indicated a correlation between elevated erythrocyte sedimentation rate and semen quality.5,6 In other studies, no correlation has been found between semen abnormalities and disease stage or systemic symptoms.7 A recent study of patients with early-stage Hodgkin lymphoma reported that 90% had good or intermediate sperm quality.8 In this study of 474 patients, no relation was found between sperm quality and age, clinical stage, or smoking. However, B symptoms (fever, weight loss, and night sweats) had a statistically negative effect on sperm quality, especially fever and night sweats. No association has been found between sperm quality and pretreatment FSH levels.
The association between testicular cancer and abnormalities of spermatogenesis is even more pronounced. The degree of spermatogenic abnormalities in these patients is greater than can be attributable to local tumor effect or the degree of systemic involvement. A recent study found that total sperm count was lower in men with testicular germ cell cancer (median, 29 × 106/mL vs. 162 × 106/mL) compared with healthy men.9 Histologic investigations have revealed a high prevalence of dysfunctional spermatogenesis even in the contralateral testicle that is uninvolved with cancer.10 An increased risk of testicular cancer has also been observed in men with an abnormal semen analysis and infertility. Infertile men with abnormal semen analyses have a twentyfold higher incidence of testicular cancer compared with the general population.11 The specific links between the pathological events that cause infertility and testicular cancer remain unclear.
Effects of Cancer Therapy on Sexual and Reproductive Function
Surgery
Prostate Cancer
Erectile dysfunction in patients undergoing radical prostatectomy commonly occurs after surgery.12–15 Steineck and colleagues16 randomly assigned 376 patients to radical prostatectomy versus watchful waiting; the incidence of erectile dysfunction was significantly higher in the surgical group (80%) compared with the observation group (45%). Bilateral nerve-sparing surgeries are considerably more effective in allowing the patient to maintain an erection compared with unilateral nerve-sparing surgeries. Potency rates after bilateral sparing surgery at 3 years were 76% compared with 30% with unilateral nerve-sparing surgery in previously potent patients younger than 60 years. The rates of potency are lower in older patients and in patients with known erectile dysfunction before surgery.17 A recent study also indicated that incontinence and sexual dysfunction remain high with robotic-assisted laparoscopic radical prostatectomy; patients should not expect fewer adverse effects with this newer method.18
Testicular Cancer
Retroperitoneal lymph node dissection (RPLND) frequently damages the sympathetic nerves that innervate the seminal vesicles and the bladder neck, which leads to loss of seminal vesicle emission or emission without bladder neck closure (retrograde ejaculation).19,20 In a recent report on long-term survivors of testicular cancer, when comparing different treatment modalities, only RPLND was associated with sexual dysfunction related to ejaculatory dysfunction.21 A selective RPLND, as described by Donohue and colleagues,22 results in the sparing of a unilateral sympathetic chain and preservation of antegrade ejaculation. Jacobsen and colleagues23 reported preserved antegrade ejaculation in 89% of patients undergoing an RPLND after chemotherapy.
Rectal Cancer
Conventional rectal surgery is associated with high rates of impotence and retrograde ejaculation, likely because of the damage of the pelvic autonomic parasympathetic and sympathetic nerves by blunt dissection. However, the introduction of total mesorectal excision (TME) has been associated with a lower rate of local recurrence and a higher rate of potency preservation. A comparison of sexual outcomes of patients with conventional surgery and TME showed that the ability to have intercourse dropped from 75% to 13% in the conventional surgery arm compared with a drop from 67% to 29% in the TME group.24 A more recent study demonstrated earlier recovery of normal voiding and sexual function for patients undergoing robotic-assisted TME compared with patients who underwent laparoscopic TME.25 Limited information is available about sexual dysfunction in women with rectal cancer. One study reported that 39% of sexually active women and 62% of all women treated for rectal cancer had Female Sexual Function Index scores that were considered abnormal despite the use of nerve-sparing surgery at the reporting institution.26
Gynecologic Surgeries
Gynecologic surgeries can alter sexual function directly by affecting the anatomy of the female genital tract. In one study of 50 women who underwent pelvic surgery for vulvar, cervical, or endometrial cancer, 83% reported sexual problems compared with 20% of the control group.27 They also reported decreased sexual desire and impaired vaginal lubrication. A recent study found that in 179 women with a history of invasive cervical cancer, the majority of patients were sexually active. Women who had hysterectomies less often reported a lack of interest in or desire for sexual activity compared with those who had not had hysterectomies.28 Among women with breast cancer, several prospective studies show no difference in quality of life outcomes or sexual functioning on the basis of surgical treatment.29,30 In younger women with breast cancer (≤50 years), sexual problems were significantly greater immediately after surgery, and although these problems decreased over time, they were still greater at 1 year after surgery than before diagnosis.31
Radiation Therapy
Central Nervous System Effects on Reproductive Function
Pituitary dysfunction as a result of irradiation is attributed to a disturbance in the hypothalamic-pituitary axis. In both men and women, it has been shown that cranial irradiation of 35 to 40 Gy or greater disturbs the hypothalamic-pituitary axis and increases the risk of infertility.32 A study of 593 long-term survivors of childhood acute lymphoblastic leukemia disclosed an increased rate of infertility among those treated with whole-brain radiation.33
Radiation Effects on Testicular Function
The testis is one of the most radiosensitive tissues; very low doses of radiation cause significant impairment of testis function. Permanent Leydig cell dysfunction occurs with a dose of 2000 to 3000 cGy. Therapeutic irradiation (2400 cGy) to the testes in patients with acute leukemia causes Leydig cell dysfunction, which is manifested by low testosterone levels or a poor testosterone response to gonadotropins.34,35 Radiation doses as low as 15 cGy transiently suppress spermatogenesis, and doses higher than 600 cGy permanently destroy the germinal elements.35 Berthelsen36 evaluated the effects of adjuvant irradiation for seminoma on gonadal function. Retroperitoneal and ipsilateral iliac irradiation resulted in an estimated 200 to 1300 cGy scatter to the unaffected contralateral testicle. Azoospermia developed in two thirds of patients, and it took a median of 540 days from the end of treatment before spermatozoa were again found in semen samples. A median of 1250 days passed before the pretreatment sperm count was reached. Sperm counts were low (median: 6 × 106 per ejaculate) up to 5 years after treatment, and serum FSH was elevated (median: 61 IU/L). No evidence of an increase in posttreatment congenital abnormalities was found, and the posttreatment conception rate was 60% to 70%.
Radiation Effects on Ovarian Function
Human oocytes are sensitive to the effects of radiation, and it has been suggested by Wallace et al.37 that the LD50 (the radiation dose that is required to kill 50% of oocytes) is less than 2 Gy. In a review of the risk of premature menopause because of cancer treatment, it was found that younger ovaries are less sensitive to the effects of irradiation. Although a radiation dose of 6 Gy is sufficient to result in permanent ovarian failure in women older than 40 years, higher doses in the range of 10 to 20 Gy result in permanent ovarian failure in the majority of patients who are treated in childhood.38
Pelvic Radiation as a Cause of Reproductive Dysfunction
Radiation therapy is commonly used as definitive treatment for patients with localized or locally advanced prostate cancer. Although the etiology of erectile dysfunction after definitive radiation therapy for prostate cancer is likely to be multifactorial, the arteriogenic mechanism appears to be more significant than the cavernosal mechanism. Maintenance of normal erection requires both vasodilation of penile arteries (arteriogenic element) and concomitant relaxation of the corporal smooth muscles (cavernosal element). Duplex ultrasonography can assess arteriogenic function by measuring peak penile blood flow and cavernosal function by measuring distension of the corpora cavernosa in the setting of normal penile flow.39 In patients with prostate cancer who have radiation therapy–induced erectile dysfunction, duplex ultrasonography confirmed a 63% rate of arteriogenic dysfunction. By contrast, with prostatectomy-induced erectile dysfunction, only 32% of patients had arteriogenic dysfunction and 52% had cavernosal dysfunction.39 Erectile dysfunction is frequently seen after external beam radiation for prostate cancer and increases in frequency with time. In 290 patients with prostate cancer who were treated with radiation, 62% and 41% of those who were potent before treatment maintained potency at 12 and 24 months, respectively, and potency rates dropped further with time.40 Conformal radiation therapy limits the radiation field while delivering a high dose of radiation to the prostate and may be associated with a lower degree of impotence.41,42 Mantz and colleagues42 described a 5-year potency rate of 53% among 287 patients with prostate cancer who were treated with 6000 to 7200 cGy conformal radiation therapy.42 The use of brachytherapy in the treatment of prostate cancer has also been associated with a lower incidence of impotence. Prostate brachytherapy as monotherapy was associated with 5- and 6-year potency rates of 76% and 52%, respectively, among previously potent patients. Furthermore, the addition of external beam radiation or antiandrogen therapy to brachytherapy decreases the rates of potency substantially.43,44 The use of intensity-modulated radiation therapy for nonmetastatic prostate cancer has increased significantly in the past decade, and although patients receiving this therapy are less likely than those receiving conformal radiation therapy to undergo additional cancer treatments, there is an increased association with erectile dysfunction 45.
Pelvic radiotherapy for cervical carcinoma is associated with vaginal atrophy, shortening, or agglutination, making intercourse difficult or impossible for women who receive this therapy.46 At 2 years of follow-up, Jensen and colleagues47 reported that 85% of women had no interest in sex, 55% had dyspareunia, and 50% had vaginal shortening. These problems were very significant in comparison with the women’s own premorbid sexual function and age-matched control subjects.
Abdominal and pelvic radiation therapy may be part of therapy for management of Wilms tumor, pelvic rhabdomyosarcoma, and Ewing sarcoma of the pelvis or spine. Young patients exposed to flank radiation (20 to 30 Gy) may have preservation of ovarian function. Women who do conceive after treatment with this degree of abdominal radiation have a significant risk of preterm delivery, low birth weight infants, and infants who are small for gestational age compared with control subjects.48 Other data also found a particularly high risk of preterm delivery and low birth weight but no congenital malformations in women who conceive within 1 year after completion of irradiation. These data imply that uterine or hormonal defects are the cause of these abnormalities.49
Hormonal Therapy
Gonadotropin-Releasing Hormone Agonists and Antagonists
Leuprolide and goserelin are two potent GnRH analogs that are commercially available in the United States. These two analogs are much more potent in stimulating gonadotropin release than is GnRH. Initial treatment with GnRH agonists results in an LH and FSH surge with resultant gonadal steroid synthesis stimulation. However, after 10 to 14 days of continuous exposure to GnRH analogs, GnRH receptors on gonadotropin cells in the pituitary are downregulated, resulting in inhibition of LH/FSH release and gonadal suppression. After prolonged GnRH analog therapy, testosterone and estrogen levels are suppressed to castrate levels. In men, this is usually associated with substantial loss of sexual desire and a marked decrease in frequency, magnitude, duration, and rigidity of nocturnal erections.50 Treatment for longer than 2 years results in atrophic testes, which might not recover even if GnRH is discontinued.51 In women, the use of GnRH in the adjuvant treatment of breast cancer is associated with an increased rate of sexual dysfunction, but the symptoms are usually reversible upon discontinuation of therapy.52
Antiandrogens
Antiandrogens bind to and block the activity of androgen receptors. Androgen receptor blockage is associated with a rise in FSH/LH and a resultant rise in serum testosterone.53 Antiandrogens, such as flutamide, bicalutamide, or nilutamide, are commonly used in the management of prostate cancer either with luteinizing hormone-releasing hormone (LHRH) analogs or after LHRH agonist/antagonist failure. A recent prospective study combining flutamide and leuprolide demonstrated a decrease in libido, sexual activity, and perceptions of masculinity during therapy.54 Once therapy ended, these levels improved during a 2-year period, although not to baseline levels. Erectile function, however, did return to baseline. High-dose bicalutamide has been evaluated as monotherapy in patients with advanced prostate cancer. Although some studies suggest comparable clinical activity, significantly less impotence and loss of libido occur with antiandrogen monotherapy.55 The ability to maintain potency while receiving antiandrogen monotherapy is limited. A study evaluating flutamide as monotherapy in 147 previously untreated patients with prostate cancer resulted in 22% preservation of sexual activity and 20% preservation of morning erection at 2 to 6 years from the start of therapy.56 The median time to loss of morning erections and sexual activity was 12.9 and 13.7 months, respectively.
Endocrine Therapy and Breast Cancer
In a randomized study of standard (n = 45) versus high-dose (n = 53) tamoxifen, patients experienced hot flashes (85%), vaginal dryness and/or dyspareunia (47%), and decreased sexual desire (44%).57 Decreased sexual interest correlated significantly with vaginal dryness and/or dyspareunia. These symptoms decreased significantly after discontinuation of tamoxifen. Other reports have shown that tamoxifen does not make a significant contribution to sexual dysfunction in women older than 50 years,58 and in a randomized study that examined only premenopausal women, patients receiving tamoxifen alone did not report decreased sexual function.59
Aromatase inhibitors are increasingly used as the standard hormonal therapy for postmenopausal women with breast cancer and are successful in increasing distant and overall disease-free survival, as well as preventing contralateral breast cancer, compared with tamoxifen. Aromatase inhibition results in a marked decrease in estrogen synthesis, leading to minimal levels of circulating estrogen. In a recently published report of the quality-of-life measurements of postmenopausal women participating in the “Arimidex, Tamoxifen, Alone or in Combination” (ATAC) trial, patients reported diminished libido (34% vs. 26%) and dyspareunia (17% vs. 8%) significantly more frequently with anastrozole (Arimidex) treatment than with tamoxifen treatment.60
Chemotherapy
Effects in Men
Many chemotherapeutic agents are gonadotoxic. Temporary infertility among men is not uncommon during cancer treatment as a result of cytotoxic damage to rapidly differentiating spermatogonia.61,62 Fertility in men may be evaluated through semen analysis and assessment of sperm concentration, motility, and morphology. Among chemotherapeutic agents, higher dose alkylating agents (e.g., cyclophosphamide, chlorambucil, procarbazine, and busulfan) and cisplatin are most commonly associated with prolonged or permanent infertility.61,62 Table 60-1 summarizes commonly used cancer therapy regimens and the degree of risk of azoospermia among men. Combination chemotherapies that contain alkylating agents impair fertility far more than do nonalkylating combinations. The majority of men with Hodgkin lymphoma who are treated with mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) become severely oligospermic or azoospermic, and testicular biopsies confirm germinal aplasia.63,64 Comparison of MOPP with the Adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) regimen revealed a considerably higher azoospermia with MOPP (100%) than with ABVD (35%), and men treated with MOPP rarely resumed spermatogenesis.65 Azoospermia also occurred considerably less often in patients receiving two cycles of MOPP compared with six cycles.66
Table 60-1
| Degree of Risk | Treatment | Common Usage |
| HIGH RISK | TBI | BMT/SCT |
| Prolonged azoospermia after treatment | Testicular radiation dose >2.5 Gy in men | Testicular cancer, ALL, NHL |
| Testicular radiation dose ≥ 6 Gy in boys | ALL, NHL, sarcoma, germ cell tumors | |
| Protocols containing procarbazine: COPP, MOPP, MVPP, ChIVPP, ChIVPP/EVA, MOPP/ABVD, COPP/ABVD | Hodgkin lymphoma | |
| Alkylating chemotherapy for transplant conditioning (cyclophosphamide, busulfan, melphalan) | BMT/SCT | |
| Any alkylating agent (e.g., procarbazine, nitrogen mustard, cyclophosphamide) + TBI, pelvic radiation, or testicular radiation | Testicular cancer, BMT/SCT, ALL, NHL, sarcoma, neuroblastoma, Hodgkin lymphoma | |
| Cyclophosphamide >7.5 g/m2 | Sarcoma, NHL, neuroblastoma, ALL | |
| Cranial/brain radiation ≥40 Gy | Brain tumor | |
| INTERMEDIATE RISK | BEP × 2-4 cycles | Testicular cancer |
| Prolonged azoospermia not common at standard dose | Cumulative cisplatin dose <400 mg/m2 | Testicular cancer |
| Cumulative carboplatin dose ≤2 g/m2 | Testicular cancer | |
| Testicular radiation dose 1-6 Gy (due to scatter from abdominal/pelvic radiation) | Wilms tumor, neuroblastoma | |
| LOW RISK | Nonalkylating chemotherapy: ABVD, OEPA, NOVP, CHOP, COP | Hodgkin lymphoma, NHL |
| Temporary azoospermia after treatment | Testicular radiation dose 0.2-0.7 Gy | Testicular cancer |
| VERY LOW/NO RISK | Testicular radiation dose <0.2 Gy | Multiple cancers |
| No effects on sperm production | Interferon-α | Multiple cancers |
| Radioactive iodine | Thyroid | |
| UNKNOWN RISK | Irinotecan | Colon |
| Bevacizumab (Avastin) | Colon, non–small cell lung | |
| Cetuximab (Erbitux) | Colon, head and neck | |
| Erlotinib (Tarceva) | Non–small cell lung, pancreatic | |
| Imatinib (Gleevec) | CML, GIST |
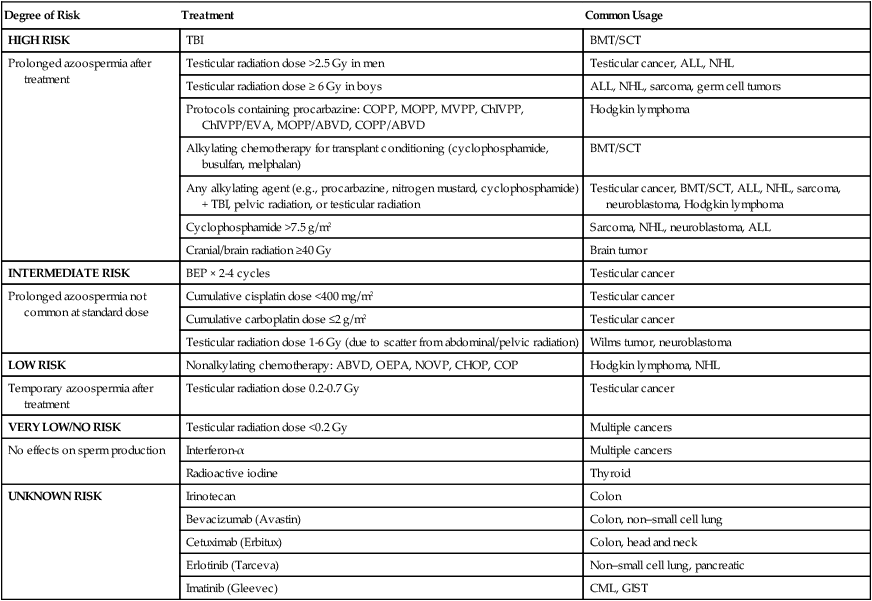
Adapted from www.fertilehope.org.
In men with testicular cancers, combination chemotherapy is associated with FSH elevation and oligospermia. These findings are complicated further by the fact that the majority of patients with testicular cancer have abnormal spermatogenesis before the initiation of therapy. In 89 patients who were normospermic before chemotherapy, the postchemotherapy count was normospermic in 64%, oligospermic in 16%, and azoospermic in 20%. Clear evidence for recovery beyond 1 year was found, and the probability of spermatogenesis increased to 48% at 2 years and 80% by 5 years.67 Patients with pretreatment oligospermia or persistent FSH elevation 2 years after treatment are unlikely to recover normal spermatogenesis after treatment.
Effects in Women
Follicular growth and maturation are affected by chemotherapy through adverse effects on DNA function and prevention of cell division within ovarian cells. The risk of infertility is dependent on patient age, drug dose, and the duration and type of chemotherapy administered. Histologic evaluation of ovaries among women who have been treated with cytotoxic chemotherapy shows fibrosis and follicular destruction.68,69 Table 60-2 summarizes the risk of amenorrhea from commonly used cancer therapy regimens. Alkylating agents are strongly associated with ovarian dysfunction.70,71 Most series report a 50% or higher incidence of amenorrhea within 1 month of starting cyclophosphamide.72 Younger women are more tolerant of the effects of chemotherapy and have a better chance of resuming menstruation after completing chemotherapy.73 In one study among patients 30 to 40 years of age, a mean dose of 9.3 g of cyclophosphamide was associated with amenorrhea, whereas patients older than 40 years required a median dose of 5.2 g.74 A median of 20.4 g was required before the onset of amenorrhea in patients younger than 30 years.75 Menses resumed in 50% of patients younger than 40 years, and it rarely occurred in women older than 40 years.76 Other nonalkylating chemotherapies, such as antimetabolites, bleomycin, vinca alkaloids, and daunorubicin, are not a frequent cause of amenorrhea.
Table 60-2
| Degree of Risk | Treatment Protocol | Common Usage |
| HIGH RISK | Whole abdominal or pelvic radiation doses ≥6 Gy in adult women Whole abdominal or pelvic radiation doses ≥15 Gy in prepubertal girls ≥10 Gy in postpubertal girls |
Multiple cancers |
| (>80% of women experience amenorrhea after treatment) | Wilms tumor, neuroblastoma, sarcoma, Hodgkin lymphoma | |
| TBI radiation doses | BMT/SCT | |
| CMF, CEF, CAF × 6 cycles in women 40+ yr | Breast cancer | |
| Cyclophosphamide 5 g/m2 in women 40+ yr | Multiple cancers | |
| Cyclophosphamide 7.5 g/m2 in girls <20 yr | NHL, neuroblastoma, ALL, sarcoma | |
| Alkylating chemotherapy (e.g., cyclophosphamide, busulfan, melphalan) conditioning for transplant | BMT/SCT | |
| Any alkylating agent (e.g., cyclophosphamide, ifosfamide, busulfan, BCNU, CCNU) + TBI or pelvic radiation | BMT/SCT, ovarian cancer, sarcoma, neuroblastoma, Hodgkin lymphoma | |
| Protocols containing procarbazine: MOPP, MVPP, COPP, ChIVPP, ChIVPP/EVA, BEACOPP, MOPP/ABVD, COPP/ABVD | Hodgkin lymphoma | |
| Cranial/brain radiation ≥40 Gy | Brain tumor | |
| INTERMEDIATE RISK | CMF or CEF or CAF × 6 cycles in women 30-39 yr | Breast cancer |
| (~30%-70% of women experience amenorrhea after treatment) | AC in women 40+ yr | Breast cancer |
| Whole abdominal or pelvic radiation 10 to <15 Gy in prepubertal girls | Wilms tumor | |
| Whole abdominal or pelvic radiation 5 to <10 Gy in postpubertal girls | Wilms tumor, neuroblastoma | |
| Spinal radiation ≥25 Gy | Spinal tumor, brain tumor, neuroblastoma, relapsed ALL or NHL | |
| LOW RISK | AC in women 30-39 yr | Breast cancer |
| (<20% of women experience amenorrhea after treatment) | CMF, CEF, or CAF × 6 cycles in women under 30 yr | Breast cancer |
| Nonalkylating chemotherapy: ABVD, CHOP, COP | Hodgkin lymphoma, NHL | |
| AC | AML | |
| Multiagent therapies | ALL | |
| VERY LOW/NO RISK | MF | Breast cancer |
| (Negligible effect on menses) | Vincristine (used in multiagent therapies) | Leukemia, Hodgkin lymphoma, NHL, neuroblastoma, rhabdomyosarcoma, Wilms tumor, Kaposi sarcoma |
| Radioactive iodine | Thyroid cancer | |
| UNKNOWN RISK | Paclitaxel, docetaxel (taxanes used in AC protocols) | Breast cancer |
| Oxaliplatin | Ovarian cancer | |
| Irinotecan | Colon cancer | |
| Bevacizumab (Avastin) | Colon, non–small cell lung | |
| Cetuximab (Erbitux) | Colon, head and neck | |
| Trastuzumab (Herceptin) | Breast cancer | |
| Erlotinib (Tarceva) | Non–small cell lung, pancreatic | |
| Imatinib (Gleevec) | CML, GIST |
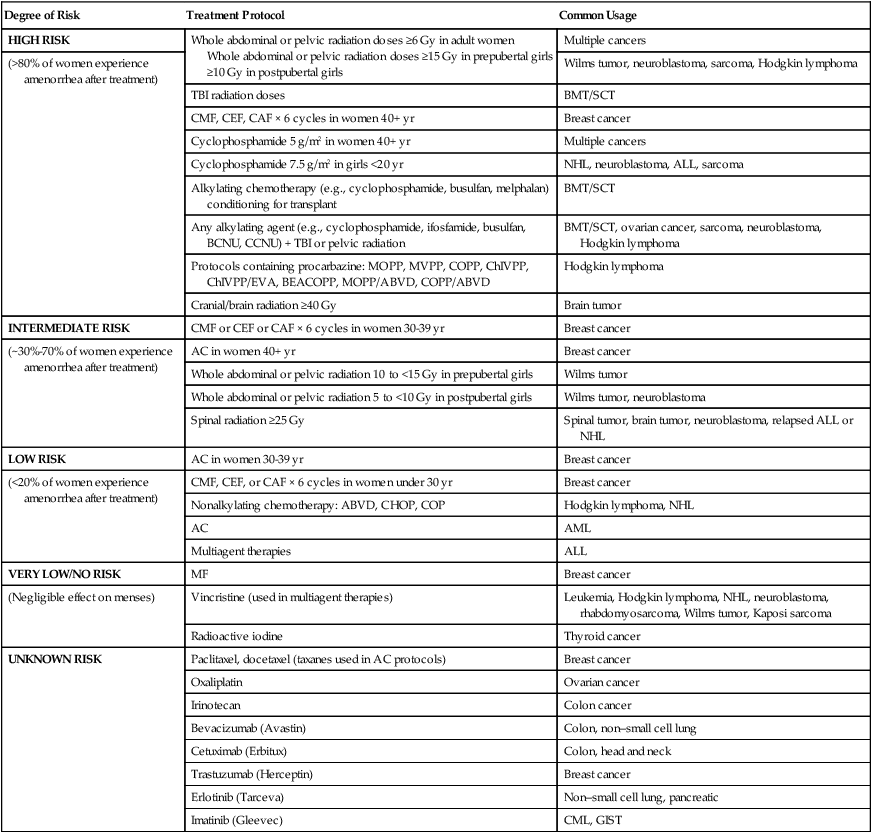
Adapted from www.fertilehope.org.
The duration of combination chemotherapy has a direct influence on the induction of amenorrhea. Treatment of premenopausal women with a combination of cyclophosphamide, methotrexate, fluorouracil, vincristine, and prednisone resulted in amenorrhea in 55% of patients who were treated for 12 weeks and in 83% of patients who were treated for 36 weeks.76
It is important to note that resumption of normal menses after chemotherapy is not a reliable assessment of ovarian function.77 Despite regular menses upon completion of chemotherapy, reduced follicle numbers are assessed by ultrasound, and alterations in FSH, anti-Mullerian hormone, inhibin B, and LH levels are found. 78
High-Dose Chemotherapy (Stem Cell Transplantation)
Effects in Women
Gonadal dysfunction after high-dose chemotherapy is dependent on age, sex, type of conditioning regimen, and previous therapy. In women, increased age and treatment with an alkylating conditioning regimen including total body irradiation (TBI) result in a high rate of ovarian failure. In 144 women who underwent stem cell transplantation for leukemia after TBI and cyclophosphamide, amenorrhea was present for 3 years after transplantation in all women. Only nine patients eventually recovered their ovarian function.79 TBI appears to be the most toxic conditioning treatment, with most women experiencing gonadal failure. Gonadal damage is dependent on total irradiation dose such that ovarian failure is highly likely with a single nonfractionated 10-Gy dose of versus with a fractionated 12-Gy total dose.80 In one study, ovarian function was restored in 70% of women who did not undergo irradiation, compared with 22% of patients who underwent TBI.81 With chemotherapy-only conditioning regimens, busulfan has been reported to be more gonadotoxic, whereas different doses of cyclophosphamide have been associated with impaired ovarian function.82
Stem cell transplantation may also be complicated by graft-versus-host disease. When severe, graft-versus-host disease can cause vaginal strictures and adhesions that interfere with intercourse.80
Effects in Males
The majority of men who undergo stem cell transplantation with or without TBI have marked elevations in their LH and FSH. Testosterone levels may be depressed but are usually maintained in the normal range, reflecting adequate compensatory reaction by Leydig cells to the rise in LH. Infertility is the rule in men receiving high-dose chemotherapy with or without TBI, reflecting the relative sensitivity of the germinal component to chemotherapy and/or radiation therapy relative to Leydig cells. After stem cell transplantation (SCT), testosterone levels were in the low-normal range in a study of 24 men with features of hypogonadism and erectile dysfunction.83 Testosterone supplementation resulted in an improvement in libido, yet there was no clear beneficial effect on erectile dysfunction.83 Doppler studies in these patients confirmed evidence of cavernosal arterial insufficiency, which was strongly correlated with prior TBI therapy.83,84
Chemotherapy and Radiation during Pregnancy
Cancer complicates 1 in 1000 pregnancies.85 The likelihood of conceiving after cancer therapy depends on the type of cancer, age at diagnosis, duration and type of gonadotoxic agents used, and other fertility issues the person may have. Radiation and chemotherapy may induce DNA damage in oocytes that may increase the risk of malformations and genetic defects among offspring. Among adult cancer survivors, in 678 reported pregnancies, no increased risk of congenital malformations was found (odds ratio [OR] 0.6), although pregnancies more often resulted in preterm delivery (OR 2.8), low birth weight (OR 2.5), and caesarean delivery (OR 2.3).86
Fetal Stage of Development and Pregnancy Outcome
The most important factor influencing fetal outcome in patients with cancer who are treated during pregnancy is the stage of fetal development upon initiation of treatment. The first trimester is the most susceptible period. Exposure to chemotherapy during the first trimester may result in a malformation rate of 10% to 20% compared with an estimated rate of 3% in the general population.87 By the thirteenth week of gestation, all organs have developed, with the exception of the brain and gonads.88 Exposure to chemotherapy during the second and third trimesters is thus unlikely to result in major birth defects but may result in fetal growth retardation.89 Treatment of patients with hematologic malignancies and breast cancer during the second and third trimesters has not been associated with any increase in the rate of congenital anomalies.90,91
Effects of Different Classes of Chemotherapy on Pregnancy Outcome
Different classes of chemotherapy have varying teratogenic potential. Alkylating agents and antimetabolites appear to have a greater potential of causing a detrimental effect than do antitumor antibiotics, platinum analogs, and vinca alkaloids.92
Alkylating Agents
Fetal abnormalities have been reported from first-trimester exposure to cyclophosphamide, chlorambucil, and busulfan.93–96 No definite causal relationship is available with other agents, such as thiotepa, melphalan, and dacarbazine, but exposure data in the first trimester for these compounds are limited.
Antimetabolites
Methotrexate is known for its teratogenic effects and has been used as an abortifacient. Congenital anomalies have been described with first trimester use. Malformations include severe skull abnormalities, heart defects such as dextroposition, and digital anomalies.98,99 The drug 5-fluorouracil may be similarly associated with congenital anomalies when administered in the first trimester. One case of multiple congenital anomalies including radial dysplasia, absent digits, and hypoplasia of multiple organs has been reported in a first-trimester exposure.100 Reports of congenital malignancies resulting from first-trimester exposure of cytarabine have been conflicting, although its use in second- and third-trimester pregnancies in women with hematologic malignancies appears safe.103–103 Sporadic reports on the use of gemcitabine in both the first and second trimesters have not resulted in any congenital malformations.104,105
Vinca Alkaloids
Definite fetal anomalies resulting from use of vinca alkaloids have not been reported. Sporadic anomalies have been reported in patients receiving combination therapy. Several pregnant women who were exposed to vinca alkaloids during the first trimester of pregnancy have had normal neonates.89,107
Platinum Analogs
Information on the use of platinum analogs during pregnancy is limited. In one report of 10 pregnant women with cancer who received cisplatin during the second or third trimester, none of the neonates demonstrated any congenital anomalies, but fetal growth was restricted in 50% of pregnancies.108
Taxanes
Data on the use of taxanes during pregnancy are limited. In 2010, 40 case reports of pregnancies with taxane exposure were reviewed (paclitaxel in 21 cases, docetaxel in 16 cases, and both drugs in 3 cases). Neither spontaneous abortions nor intrauterine deaths were reported. This review supported the notion that taxane administration in the second and third trimesters was safe.109 However, it was recently reported that a 35-year-old woman with triple-negative breast cancer who tolerated dose-dense doxorubicin with cyclophosphamide during her second trimester experienced oligohydramnios with weekly administration of paclitaxel in her third trimester. She was induced at 36 weeks and gave birth to a healthy baby.110
Topoisomerase II Inhibitors
Etoposide has not been reported to cause congenital malformations. However, fetal marrow suppression manifesting as severe neonatal anemia and leukopenia has been reported in a patient treated for leukemia.111
In general, an increased risk of congenital anomalies has been associated with treatment in the first trimester, and termination of the pregnancy should be considered if systemic cytotoxic therapy is indicated.112 Delay in chemotherapy until it can be given more safely in the second and third trimesters should be considered if the outcome for the mother is not at risk. The long-term effects on progeny have not been adequately evaluated for different chemotherapeutic agents. Anecdotal data suggest that most offspring who are exposed in utero exhibit normal physical and mental development. Eighty-four children who were born to patients with hematologic malignancies and were exposed to chemotherapeutic agents in utero were followed up for a median of 18.7 years.113 In all the children who were studied, the learning and educational performances were normal, and no congenital, neurologic, or psychological abnormalities were observed. No increase in malignancies was apparent. Some of these persons became parents during the period of follow-up. Twelve second-generation offspring were evaluated, and all of them were normal.
Targeted Therapy
More recently developed therapeutic agents target specific growth factor receptors, antigens, or kinases that are essential for cell growth and development. Of 11 reports of trastuzumab administered during pregnancy, the outcome was oligohydramnios in five cases and anhydramnios in two cases.114 The literature includes one case report on lapatinib and pregnancy.115 Use of lapatinib was discontinued after 11 weeks, and a healthy newborn was delivered. A 29-year-old patient with diffuse large cell lymphoma was reported to have been treated during her second and third weeks of pregnancy with a combination of rituximab and cyclophosphamide, hydroxydaunomycin, Oncovin, and prednisone (CHOP) therapy.116 She delivered a healthy female newborn at 36 weeks who had normal B-lymphocyte levels by 4 months of age.
Imatinib is now a standard therapy for patients with chronic myeloid leukemia. There have been sporadic reports on pregnancy outcomes with exposure to imatinib. Although no harmful effects seem to occur in offspring of male patients who are treated with imatinib, some rare congenital malformations have been reported.117
Immunomodulators
Limited data are available on the effects of interferons and interleukins on pregnancy. More than 20 case reports of pregnancy during interferon-α treatment in all three trimesters have been reported.118,119 None of the cases were associated with anomalies, but growth retardation and premature births were more frequent than expected.119
Effects of Radiation Therapy on Pregnancy Outcome
Human data regarding the effects of radiation on fetal outcome are limited to accidental exposure and to nuclear disaster victims. The most comprehensive review of the clinical effects of pelvic radiation therapy was reported by Dekaban.120 Pelvic irradiation as long as 3 weeks after conception did not result in severe congenital anomalies, although a considerable number of embryos may have been resorbed or aborted. Irradiation between weeks 4 and 11 led to the development of severe congenital anomalies in many organs. Exposure between weeks 11 and 16 led to anomalies of the eye, skeleton, and genital organs, stunted growth, microcephaly, and mental retardation. Exposure between 16 to 20 weeks was associated with mild microcephaly, mental retardation, and stunted growth. Later exposures were unlikely to cause structural abnormalities.121
Data from survivors of the atomic bombs in Hiroshima and Nagasaki suggest a dose-dependent effect of radiation on congenital anomalies, with a dose of 50 cGy resulting in a 40% risk of microcephaly. Doses in excess of 10 cGy may result in cognitive impairment, and higher exposures result in further exacerbation of mental retardation. In utero radiation exposure also results in an increased risk of carcinogenesis, with an estimated 6% risk of cancer by age 15 years per Gy of exposure.122
Prevention
Unfortunately, most cancer diagnoses have limited options for treatment, and the choice of a regimen with a low potential for gonadal toxicity is often not feasible. Spermatogenesis and follicular growth and maturation are particularly sensitive to the effects of chemotherapy because of their high mitotic rate. GnRH analogs have been shown to inhibit spermatogenesis in various animals and in humans. The use of GnRH analogs has been reported to protect rat testes from chemotherapy and radiation.123,124 However, treatment of patients who have testicular cancer and Hodgkin lymphoma with LHRH analogs has failed to show any protective effects against the development of azoospermia.125,126 Effective inhibition of spermatogenesis may require several weeks of hormonal manipulation with GnRH analogs. Treatment with GnRH analogs for several weeks before initiation of chemotherapy is often not appropriate clinically and may account for the failure of previous studies to show a protective effect on gonadal function.127
Similar attempts to protect the ovaries from cytotoxic chemotherapy by suppressing cycling through GnRH analogs and oral contraceptives have been made. One study retrospectively analyzed the outcomes of 100 women with high-risk early breast cancer who received a GnRH analog for 12 months and adjuvant chemotherapy.128 Although all women younger than 40 years had resumed their menses, only 56% of patients older than 40 years did so, and only three pregnancies were reported. In a phase 3 prospective study, 281 patients with stage I through III breast cancer were randomly assigned to receive chemotherapy with or without a GnRH analog.129 The rate of menopause was 25.9% in the chemotherapy-alone group compared with 8.9% in the chemotherapy + GnRH analog (triptorelin). Two recent studies demonstrated that GnRH analogs did not preserve ovarian function among women undergoing a stem cell transplant130 or for those with early-stage breast cancer undergoing chemotherapy.131 Further studies are needed in this area because it is still unclear whether GnRH analogs have a definitive effect on protecting ovarian function.
Other means of protection from radiation effects include gonadal shielding. In addition, transposition of the ovaries may be an alternative to avoid radiation damage. However, spontaneous pregnancy rates after ovarian transposition are low, presumably because of the distorted tubo-ovarian anatomy resulting from the procedure itself or the local therapy (radiation) to the pelvic area.132
Treatment
Hormonal Replacement
Premature ovarian failure results in the sudden onset of menopausal symptoms as a result of an abrupt decrease in estrogen levels. Sexual symptoms related to ovarian failure include vaginal atrophy, thinning of vulvar tissue and the vagina, decreased vaginal lubrication and elasticity, mood swings and irritability, and hot flashes. Estrogen replacement therapy (in combination with progesterone in patients without hysterectomy) can reverse most of these symptoms and should be discussed with all patients with iatrogenic ovarian failure. Risks, including an increased rate of cardiovascular and cerebral accidents, and benefits, such as osteoporosis prevention, should be addressed before initiation of therapy.133 Patients who are not candidates for hormone replacement can be treated symptomatically with vaginal moisturizers (e.g., Replens) and water-based lubricants (e.g., K-Y liquid). Vaginal-directed estrogen therapy with an Estring vaginal ring, estrogen creams, or Vagifem tablets have also resulted in improvements in symptoms of vaginal dryness and dyspareunia and a decrease in the incidence of urinary tract infections.
Male hypogonadism as a result of chemotherapy and radiation therapy is associated with loss of libido, hot flashes, and impotence. Testosterone replacement as a depot injection or in a transdermal formulation may restore sexual function in those instances.134,135
Management of Erectile Dysfunction
Sildenafil leads to successful intercourse in patients with prostate cancer who have erectile dysfunction after prostatectomy, external beam radiation, or brachytherapy. Response rates typically range between 70% and 80%.136–139 The ease of oral administration and the efficacy of this agent have made it the most commonly prescribed agent for erectile dysfunction.140 Alternative therapies include penile injections, vacuum devices, or intraurethral suppositories, which are more cumbersome to the patient and are associated with high dropout rates.141 In refractory situations, surgical intervention with penile implants may be considered.
Fertility Preservation and Assisted Reproductive Technologies
Once germinal testicular aplasia or premature ovarian failure occurs as a result of cancer therapy, the damage might be irreversible. Unless sperm or embryonic banking is performed before treatment, these patients will not be able to parent their own biological children. This issue has not been given adequate attention, and its importance to patients has long been overlooked. To help health care professionals inform their patients about fertility preservation, guidelines have been published by several national organizations, including the President’s Cancer Panel (2004), the American Society of Reproductive Medicine Ethics Committee (2005), and the American Society of Clinical Oncology (2006).142
Several advances in reproductive technologies allow for fertility preservation in patients who are undergoing gonadal toxic therapies. Intrauterine insemination is accomplished by selecting washed sperm with high motility and injecting them directly into the uterus at the time of ovulation. This procedure requires cryopreservation of 5 to 10 million normal sperm. In vitro fertilization with embryo transfer involves culturing the aspirated oocytes and spermatozoa in vitro, followed by the transcervical replacement of the embryo into the uterine cavity. With in vitro fertilization with embryo transfer, the number of sperm required is 0.5 to 1 million.143 Intracytoplasmic sperm injection (ICSI) involves the injection of a single sperm into the cytoplasm of the oocyte with transcervical placement of the embryo into the uterine cavity. ICSI reduces the criteria for sperm cryopreservation theoretically to the presence of one motile sperm, which makes almost any male patient with cancer who is not completely azoospermic a candidate for sperm cryopreservation. Even in patients with complete ejaculatory azoospermia, testicular sperm extraction followed by ICSI and embryo cryopreservation might represent an option for fertility preservation.144,145 Testicular sperm extraction is typically achieved by obtaining open biopsies of testicular tissues with or without microdissection.146 In patients for whom sperm is stored or extracted successfully, successful pregnancy rates in the range of 30% are expected.147,148 Transrectal electroejaculation is yet another viable method for sperm collection for the purpose of cryopreservation or in vitro fertilization in patients with retrograde ejaculation.149 Other options of sperm collection in patients with retrograde ejaculation are insemination with use of sperm-rich urine (after masturbation) or bladder washings. Successful reports of insemination with use of these collection methods have been reported, with techniques ranging from intrauterine insemination to ICSI.149
For female patients, fertility preservation techniques are mostly investigational and include embryo cryopreservation, oocyte cryopreservation, and ovarian tissue banking. Embryo cryopreservation before initiation of treatment is quite successful, with survival rates per thawed embryo ranging from 35% to 90% and implantation rates between 8% to 30%.150 However, this process requires ovarian stimulation (which can take weeks to months) and available sperm, neither of which is feasible for many young women with a newly diagnosed cancer. Recent advances in the technique of oocyte cryopreservation have improved success rates from thawed oocytes, and more than 500 live births have been achieved with this technique.151 Ovarian tissue cryopreservation is a novel technique that is under investigation. The procedure involves oophorectomy and cryopreservation before the initiation of cancer treatments. Upon completion of cancer-directed therapy and when conception is planned, the frozen banked ovarian tissue is thawed and autotransplanted in the patient. Successful ovulation after autotransplantation has been reported, and the procedure continues to be under investigation.151 For patients who are unable to conceive as a result of uterine or cervical abnormalities attributed to the cancer or its treatment, in vitro fertilization with implantation in a surrogate has been described.152
An overview of fertility preservation options for men and women are provided in Tables 60-3 and 60-4. Fertility preservation options are costly, and with cryopreservation, annual storage fees apply. Many insurance companies do not cover fertility-preservation procedures. The Sharing Hope program, available through Fertile Hope (http://www.livestrong.org/fertilehope), provides financial assistance for sperm cryopreservation and embryo and oocyte cryopreservation through negotiated discounted rates and with fertility centers and sperm banks across the United States.
Table 60-3
Fertility Preservation Options for Males
| Option | Sperm Banking (Masturbation) | Sperm Banking (Alternative Collection Methods) | Radiation Shielding of Gonads | Testicular Tissue Freezing | Testicular Sperm Extraction |
| Medical status | Standard | Experimental | Standard | Experimental | Standard |
| Definition | Sperm is obtained through masturbation, then frozen | Sperm obtained through testicular extraction or electroejaculation under sedation | Use of shielding to reduce the dose of radiation delivered to the testes | Tissue obtained through biopsy and frozen for future use | Use of biopsy to obtain individual sperm from testicular tissue |
| Pubertal status | After puberty | After puberty | Before and after puberty | Before and after puberty | After puberty |
| Time requirement | Outpatient procedure | Outpatient procedures | In conjunction with radiation treatments | Outpatient procedure | Outpatient procedure |
| Success rates | Generally high; the most established technique for men | If sperm is obtained, similar to standard sperm banking | Possible with select radiation fields and anatomy | No available human success rates | 30%-70% in postpubescent patients |
| Cost | Approx. $1500 for 3 samples; storage fees average $500/yr | Varies greatly based on collection method | Generally included in the cost of radiation treatments | $500-$2500 for surgery; $300-$1000 for freezing; $500/yr for storage | $4000-$16,000 (in addition to costs for in vitro fertilization) |
| Timing | Before treatment | Before treatment | During treatment | Before treatment | Before or after treatment |
| Special considerations | Deposits can be made every 24 hours | Can be considered if male cannot ejaculate | Expertise required; does not protect against effects of chemotherapy | May be only option for prepubescent boys | Center should be able to freeze sperm found at time of biopsy |
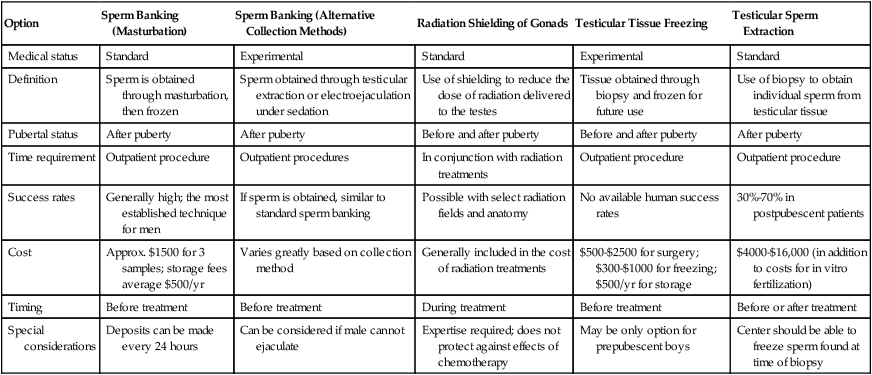
Adapted from www.fertilehope.com.
Table 60-4
Fertility Preservation Options Among Females
| OPTION | Embryo Freezing | Egg Freezing | Ovarian Tissue Freezing | Radiation Shielding of Gonads | Ovarian Transposition | Radical Trachelectomy | Ovarian Suppression |
| Medical status | Standard | Experimental | Experimental | Standard | Standard | Standard | Experimental |
| Definition | Harvesting eggs, in vitro fertilization, and freezing of embryos for later implantation | Harvesting and freezing of unfertilized eggs | Freezing of ovarian tissue and reimplantation after cancer treatment | Use of shielding to reduce scatter radiation to the reproductive organs | Surgical repositioning of ovaries away from the radiation field | Surgical removal of the cervix with preservation of the uterus | Gonadotropin- releasing hormone analogs or antagonists used to suppress ovaries |
| Pubertal status | After puberty | After puberty | Before or after puberty | Before or after puberty | Before or after puberty | After puberty | After puberty |
| Time requirement | 10-14 days from menses; outpatient surgical procedure | 10-14 days from menses; outpatient surgical procedure | Outpatient surgical procedure | In conjunction with radiation treatments | Outpatient procedure | Inpatient surgical procedure | In conjunction with chemotherapy |
| Success rates | Approximately 40% per transfer; varies by age and center; thousands of babies born | Approximately 21.6% per embryo transfer; 200+ live births | Case reports of two live births | Only possible with selected radiation fields and anatomy | Approximately 50% because of altered blood flow and scattered radiation | No evidence of higher cancer recurrence rates in appropriate candidates | Unknown; conflicting results reported; larger randomized trials in progress |
| Cost | Approx. $12,000/cycle; storage fees and pregnancy costs additional | Approx. $12,000/cycle; storage fees and pregnancy costs additional | $12,000 for procedure; storage fees and reimplantation costs additional | Generally included in cost of radiation | Unknown; may be covered by insurance | Generally included in the cost of cancer treatment | $500/month |
| Timing | Before or after treatment | Before or after treatment | Before or after treatment | During treatment | Before treatment | During treatment | During treatment |
| Special considerations | Need partner or donor sperm | May be attractive to single women or those opposed to embryo creation | Not suitable if high risk of ovarian metastases; only preservation option for prepubescent girls | Expertise required; does not protect against effects of chemotherapy | Expertise required | Limited to early stage cervical cancer; offered at a limited number of centers | Does not protect from radiation effects |
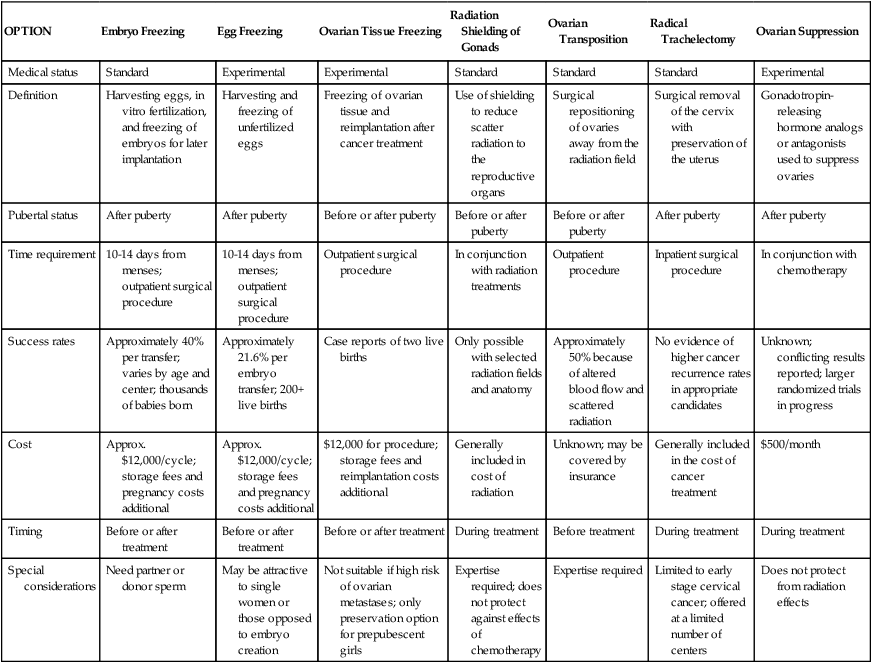
Adapted from www.fertilehope.org.






