Renal Disease
Yaakov Beilin MD
Chapter Outline
“Children of women with renal disease used to be born dangerously or not at all—not at all if their doctors had their way.”1 This statement describes early experiences with maternal renal disease and pregnancy outcome. It remains true that renal disease, either preexisting or occurring during gestation, may impair maternal and fetal health. Experience and investigations during the past three decades have significantly improved the outcome for pregnant women with renal disease.2
Physiologic Changes in Pregnancy
A review of the renal physiologic changes that occur during normal pregnancy is helpful to understand and evaluate coexisting renal disorders (see Chapter 2). Early in gestation, increased intravascular volume leads to renal enlargement. Hormonal changes result in dilation of the renal pelvis and ureters; dilation often is accompanied by decreased ureteral peristalsis. Dilated uterine and ovarian veins, and the gravid uterus, may obstruct ureter drainage at the pelvic brim. Together, these changes predispose pregnant women to vesicoureteric reflux and ascending infection. Alterations in glomerular hemodynamics and tubular function also occur. Increased cardiac output and decreased intrarenal vascular resistance cause an 80% increase in renal blood flow and a 50% increase in glomerular filtration rate (GFR) during pregnancy. These changes are somewhat less pronounced near term. Because of the increased GFR, a serum creatinine concentration greater than 0.6 to 0.8 mg/dL and a blood urea nitrogen (BUN) concentration greater than 8 to 9 mg/dL (upper limit of normal for the pregnant patient) suggest renal insufficiency in the pregnant woman. Tubular sodium reabsorption and osmoregulation are reset, allowing a “physiologic hypervolemia” during gestation. Modest proteinuria, up to 300 mg in 24 hours, also occurs during pregnancy.3
Urinary tract infections (see Chapter 37) and renal dysfunction associated with hypertensive disorders of pregnancy (see Chapter 36) are discussed elsewhere in this text.
Renal Parenchymal Disease
Definition and Pathophysiology
Renal parenchymal disease consists of two general groups of disorders, glomerulopathies and tubulointerstitial disease. Glomerulopathies are further subdivided into disorders that involve inflammatory or necrotizing lesions—the nephritic syndromes—and disorders that involve abnormal permeability to protein and other macromolecules—the nephrotic syndromes. More than 20 specific glomerulopathies exist. The nomenclature for these glomerulopathies is complex, and specific diseases are not discussed in detail here.
Tubulointerstitial diseases are disorders characterized by abnormal tubular function. They result in abnormal urine composition and concentration but are not characterized by decreased GFR until late in the disease course. The disorders in this category include interstitial nephritis, renal cystic disease, renal neoplasia, and functional tubular defects.
Patients with renal parenchymal disorders may remain asymptomatic for years, and they may exhibit only proteinuria and microscopic hematuria, with little if any evidence of reduced renal function. Spontaneous recovery or improvement with treatment occurs with many glomerulopathies. However, other patients exhibit progressive nephropathy, hypertension, and renal insufficiency. The incidence of kidney disease in pregnancy is approximately 0.12%.4 In two thirds of these patients, the disorder results from glomerulopathy, and in one third, from tubulointerstitial disease.5
Diagnosis
Women with preexisting disease may choose to become pregnant without the counsel of their nephrologist. When such patients become pregnant, the obstetrician and nephrologist seek to define the extent of renal involvement. Serial blood pressure measurements are obtained to define the severity of hypertension and the efficacy of current antihypertensive therapy. Creatinine clearance and the level of proteinuria should be determined. Urinalysis yields information about the presence of renal casts and bacteriuria. The determination of serum creatinine and BUN concentrations defines the extent of renal insufficiency. A serum creatinine concentration greater than 0.8 mg/dL, which may be normal in the nonpregnant woman, may represent significant renal insufficiency during pregnancy. Alternatively, the obstetrician may first detect renal dysfunction through routine prenatal screening tests. If proteinuria, hematuria, or azotemia is detected, a complete biochemical evaluation should be performed.
Both preeclampsia and renal disease may manifest as hypertension, proteinuria, and edema. The distinction between the two disorders is often unclear, especially after 20 weeks’ gestation. Fisher et al.6 evaluated 176 renal biopsy specimens obtained from hypertensive women immediately postpartum, most of whom had a clinical diagnosis of preeclampsia. The clinicopathologic correlation was poor. Histologic evidence of preeclampsia (e.g., glomerular endotheliosis without hypercellularity) was present in only 65% of these hypertensive women. Primary renal disease was present in 20%, and hypertensive nephrosclerosis occurred in 11%. Nulliparous women (84%) were more likely to have a correct diagnosis of preeclampsia than parous women (38%).
Renal tissue biopsy is often used to establish a diagnosis in nonpregnant patients. Chen et al.7 reported a series of 15 percutaneous renal biopsies performed in 15 pregnant women with onset of renal dysfunction of unknown cause during pregnancy. All patients underwent biopsy before 30 weeks’ gestation. A biopsy-related complication occurred in only one patient who experienced gross hematuria. The authors judged that histologic results provided useful clinical guidance that facilitated successful fetal outcome in 14 of the pregnancies. More recently, Day et al.8 performed renal biopsy in 20 pregnant women; the biopsy results led to altered management in 9 of 20. One patient had minor hematuria that resolved spontaneously. In contrast, Kuller et al.9 reviewed 18 renal biopsies performed during pregnancy (n = 15) or in the immediate postpartum period (n = 3) in 15 women with elevated blood pressure. After the biopsy, renal hematoma was identified in 7 (39%) women, and 2 (11%) women required blood transfusion. Four intrauterine fetal deaths occurred, although none was a direct result of the biopsy. It is possible that women with elevated blood pressure are at a greater risk for postbiopsy complications. Because renal biopsy exposes the pregnant woman to potential complications, many perinatologists recommend biopsy only when sudden deterioration in renal function or symptomatic nephrotic syndrome occurs before 28 weeks’ gestation, at which time definitive diagnosis may guide appropriate treatment. For problems beyond 28 weeks’ gestation, the recommendation is to delay biopsy until the postpartum period.8,9
Effect of Pregnancy on Preexisting Kidney Disease
The extent to which pregnancy affects preexisting renal disease depends on the level of renal insufficiency before pregnancy. Among women with mild antenatal renal insufficiency, pregnancy does not substantially alter the natural course of renal disease.10,11 Jungers et al.10 evaluated the effect of pregnancy on renal function among 360 women with primary glomerulonephritis. During the study period, 171 (48%) women became pregnant. All study subjects had normal renal function at the time of entry into this study, and all of the patients who became pregnant had normal renal function at conception. In this case-controlled study, pregnancy was not identified as a risk factor for progression to end-stage renal failure. Limardo et al.11 evaluated 223 women with biopsy-documented IgA nephropathy who had a serum creatinine level greater than 1.2 mg/dL, 136 of whom became pregnant. Women were observed for a minimum of 5 years (median, 10 years), and pregnancy did not seem to affect long-term outcome of kidney disease or the onset of proteinuria or hypertension.
In contrast, Jones and Hayslett12 analyzed the outcome of 82 pregnancies in 67 women with preexisting moderate or severe renal insufficiency (i.e., serum creatinine level > 1.4 mg/dL before pregnancy or at the first antepartum visit). The mean ± SD serum creatinine concentration increased from 1.9 ± 0.8 mg/dL in early pregnancy to 2.5 ± 1.3 mg/dL in the third trimester. The prevalence of hypertension rose from 28% at baseline to 48% during late pregnancy. Pregnancy-related deterioration of maternal renal function occurred in 43% of cases. Purdy et al.13 also found that greater than 40% of women with moderate to severe kidney disease had deterioration in renal function due to pregnancy. Women with a serum creatinine concentration greater than 2.0 mg/dL who became pregnant had a one-in-three chance of developing dialysis-dependent end-stage renal disease during or shortly after pregnancy.14
The pathophysiology by which pregnancy exacerbates renal disease is unknown. One hypothesis is that increased glomerular perfusion, which normally accompanies pregnancy, paradoxically causes further injury to the kidneys in patients with preexisting impairment of function. However, this hypothesis is unsupported by published data, which demonstrate no evidence of hyperfiltration (i.e., an initial decline in serum creatinine concentration) during early pregnancy in patients with renal disease.15 An alternative hypothesis is that preexisting renal disease may induce a cascade of platelet aggregation, microvascular fibrin thrombus formation, and endothelial dysfunction that leads to microvascular injury in the already tenuous kidneys.14
Effect on the Mother and Fetus
Pregnant women with chronic kidney disease are at an increased risk for maternal and fetal complications. Nevis et al.16 systematically reviewed all published observational studies of women with chronic kidney disease that included a control group for comparison, excluding retrospective studies. They identified 13 studies that included at least five women between 1966 and 2010. Maternal complications included gestational hypertension, preeclampsia/eclampsia, and maternal mortality. Adverse fetal outcomes included preterm births, fetal growth restriction (also known as intrauterine growth restriction), small-for-gestational-age infants, neonatal mortality, stillbirths, and low birth weight. Adverse maternal outcomes were found in 12 studies, and the incidence was five times greater than in women without kidney disease. Adverse fetal outcomes were identified in nine studies, and the incidence was two times greater than in the normal healthy women.
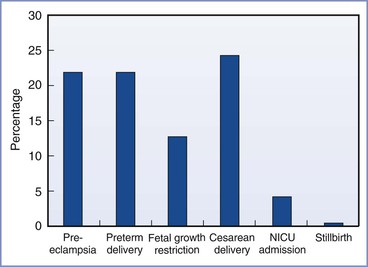
The incidence of obstetric complications is proportional to the extent of preexisting maternal renal disease and preexisting hypertension.17 Bar et al.18 evaluated maternal and neonatal outcomes in 38 women (46 pregnancies) with primary renal disease. They observed an increase in complications compared with healthy women (Figure 52-1). In a logistic regression model, only preexisting hypertension and a high preconception serum uric acid level predicted worse outcome. Other factors (e.g., degree of preexisting renal impairment) were not significant predictors, but, of note, 90% of the cohort had mild disease. In a study of women with moderate to severe renal disease, Jones and Hayslett found the complication rate was much greater.12 The incidence of preterm birth was 59%, the incidence of fetal growth restriction was 37%, and the cesarean delivery rate was 59%. Ramin et al.19 reviewed the literature for studies of renal disease and maternal and fetal outcome. Overall, fetal survival ranged from 64% to 98% depending on the extent of renal insufficiency and the presence or absence of hypertension.
Medical and Obstetric Management
During pregnancy, the nephrologist and the obstetrician monitor maternal renal function, blood pressure, and fetal development at frequent intervals. Monthly determination of serum creatinine concentration, creatinine clearance, and proteinuria allows the recognition of renal deterioration. An antepartum consultation with the anesthesiologist should also be considered.
Some glomerulopathies respond to corticosteroids, and corticosteroid therapy should be continued during pregnancy. Rapid deterioration of renal function that occurs before 28 weeks’ gestation may require renal biopsy to exclude rapidly progressive glomerulopathies that require treatment. Antihypertensive therapy should be instituted as needed (see Chapter 36). Recombinant human erythropoietin improves maternal anemia during pregnancy.20 Protein restriction places the fetus at risk for growth restriction and is not used. Deterioration of maternal renal function, the onset of preeclampsia, or evidence of fetal compromise may necessitate preterm delivery.
Hemodialysis and Long-Term Ambulatory Peritoneal Dialysis
When renal disease has progressed to end-stage renal failure (i.e., GFR < 5 mL/min), fertility is suppressed and conception and pregnancy are rare. Less than 10% of premenopausal patients undergoing dialysis have regular menses. Luteinizing hormone and follicle-stimulating hormone concentrations assume an anovulatory pattern, which causes 40% of affected women to be amenorrheic. Half of all female patients undergoing dialysis exhibit hyperprolactinemia because of reduced clearance and hypothalamic disturbances.21 Toma et al.22 surveyed 2504 dialysis units in Japan and reported only 172 pregnancies among 38,889 women who were undergoing dialysis.
There are two modalities of dialysis: extracorporeal hemodialysis and intracorporeal peritoneal dialysis. Hemodialysis necessitates vascular access and the need for anticoagulation of the extracorporeal circuit and may be complicated by cardiovascular instability, large fluid and electrolyte shifts, and the risk for hepatitis. Hypotension may compromise uteroplacental perfusion and cause fetal compromise. Even when hypotension and major fluid shifts are avoided, Doppler ultrasonographic examination of uterine and umbilical artery flow during hemodialysis suggests the occurrence of a redistribution of arterial flow away from the uteroplacental vascular bed.23 Fetal heart rate monitoring is recommended during dialysis.24 Rapid removal of maternal solutes and reduced oncotic pressure with attendant free-water diffusion into the amniotic cavity may lead to polyhydramnios. Hemodynamic consequences are minimized by more frequent but shorter dialysis runs. Long-term ambulatory peritoneal dialysis allows less hemodynamic trespass, a more stable fetal environment, and the freedom to undergo dialysis at home. However, peritoneal dialysis may not be associated with greater fetal survival. Complications of this modality include peritonitis and catheter difficulties.25
Published reports have noted a wide range of successful pregnancies in dialysis-dependent pregnant women, regardless of the modality of dialysis. Toma et al.22 reported that 90 (52%) of 172 pregnancies in women undergoing long-term hemodialysis were successful. More recently, because of improvement in maternal-fetal care, the success rate appears to be improving. Chou et al.26 pooled data from 10 published case series and 12 case reports and found that 71% of women undergoing hemodialysis and 64% of women undergoing peritoneal dialysis had a successful delivery. Similarly, Piccoli et al.27 pooled data from 10 studies that included 90 conceptions in 78 women and reported successful delivery can be achieved 75% of the time.
Maternal complications include malnutrition, anemia, and hypertension. Fetal complications include fetal growth restriction, fetal death, and preterm labor. BUN levels should be kept below 50 mg/dL before dialysis and below 30 mg/dL after dialysis.25 At birth, azotemia in the newborn is similar to that in the mother, but this quickly corrects because the newborn has normal kidney function. The long-term effects of intrauterine azotemia on newborn cognitive development are unknown.28
Patients undergoing hemodialysis have a high prevalence of viral hepatitis, a greater frequency of active tuberculosis, and a higher rate of infection with vancomycin-resistant enterococci, human immunodeficiency virus (HIV), and methicillin-resistant Staphylococcus aureus. The risk for hepatitis C virus (HCV) infection is particularly of concern, with reported rates as high as 36%. However, with improvement in aseptic technique and more attention to hand washing, the decline in the reuse of dialysis equipment, and the use of dedicated isolated dialysis machines for HCV-seropositive patients, the rates of infection and seroconversion can be markedly reduced.29,30
Anesthetic Management
Anesthetic management is influenced by the extent of renal dysfunction and hypertension. The parturient with stable renal disease, mild to moderate renal insufficiency, well-controlled hypertension, and euvolemia requires minimal special consideration. In contrast, the dialysis patient with end-stage renal failure presents many anesthetic challenges because renal disease may affect almost every organ system (Box 52-1). Poorly controlled hypertension leads to left ventricular hypertrophy and dysfunction. Symptoms of cardiovascular compromise should prompt echocardiography to evaluate ventricular function. An intra-arterial catheter also may aid the management of the parturient with poorly controlled hypertension. Uremic pericarditis, cardiomyopathy, and accelerated atherosclerosis are rarely seen until advanced uremia has been present for several years.
Normochromic, normocytic anemia secondary to impaired erythropoietin production, chronic gastrointestinal bleeding, and vitamin deficiency are common findings. Typically, the anemia is well tolerated and does not require transfusion, unless excessive surgical bleeding occurs. Uremic toxins may cause functional platelet defects; these abnormalities are reversed by dialysis. Thrombocytopenia may also occur as a result of increased peripheral destruction of platelets. Generalized coagulopathy may result from the anticoagulation used during the dialysis process.31 A full coagulation profile and careful bleeding history should be performed, especially before the initiation of neuraxial anesthesia. Hemodialysis fistulas should be padded carefully to prevent thrombosis. Blood pressure cuffs should not be placed on these extremities.
Neuraxial Anesthesia
Neuraxial anesthesia is the preferred technique for both labor analgesia and cesarean delivery, but there are some unique considerations in the parturient with renal disease. Uremic patients may be hypervolemic or hypovolemic, depending on the time elapsed since their last dialysis session. Hypovolemia and autonomic neuropathy may lead to profound hypotension during the initiation of sympathetic blockade. Intravascular volume should be assessed before induction of anesthesia. Assessment of clinical signs (e.g., skin turgor, mucous membranes, tachycardia) is generally sufficient. Central venous pressure monitoring or transthoracic echocardiography may be useful when the volume status remains unclear. Although there are no studies in the renal transplant patient, a role for intravenous prehydration to prevent hypotension is unlikely because this modality is not efficacious in the healthy parturient.32 There is insufficient evidence to recommend spinal versus epidural techniques for the patient with renal disease. Frequent monitoring of blood pressure and immediate treatment of hypotension is suggested.33 Preexisting peripheral neuropathy should be documented before the administration of neuraxial anesthesia.
Local anesthetic systemic toxicity (LAST) after bupivacaine brachial plexus blockade has been reported in patients with chronic renal failure.34 Whether LAST is related to toxic levels of local anesthetic unique to the renal failure patient is unclear. Rice et al.35 found no significant difference in the pharmacokinetic profile of bupivacaine after brachial plexus blockade in a group of uremic patients and in patients with normal renal function. There are no published data on the pharmacokinetics of epidurally administered local anesthetic agents in patients with chronic renal failure.
Orko et al.36 administered spinal anesthesia with plain bupivacaine 22.5 mg to 20 nonpregnant patients with chronic renal failure and 20 control patients. Maximal segmental anesthesia occurred more rapidly in the patients with renal disease (21 versus 35 minutes), but the duration was reduced. Further, the extent of sensory blockade was two segments higher in the patients with renal disease. There were no untoward effects in any of the patients.
General Anesthesia
Patients with chronic uremia exhibit delayed gastric emptying and hyperacidity, which may increase the risk for aspiration pneumonitis. In addition to sodium citrate, when time allows, the anesthesia provider also should consider administering a histamine-2 (H2)-receptor antagonist and metoclopramide. Recommended single doses for patients with renal failure are ranitidine 50 mg and metoclopramide 10 mg given intravenously. Weir and Chung37 suggested that patients with chronic renal failure present greater difficulties with tracheal intubation than otherwise healthy patients; however, an objective analysis of airway difficulty has not been performed in this population.
All the standard induction agents are safe in patients with renal failure. Etomidate may have an advantage because it supports the circulation better than other induction agents. Propofol exhibits normal volume of distribution and elimination in patients with renal failure and is also commonly used. Protein binding of propofol is unaffected by renal failure.38 Uremia increases blood-brain barrier permeability to many drugs.39 These changes may warrant a small reduction in the dose of propofol or thiopental for induction. The serum potassium concentration should be determined before induction of anesthesia. If the potassium concentration is greater than 5.5 mEq/L, dialysis should be performed before an elective procedure. Succinylcholine will cause a 0.5 to 0.7 mEq/L increase in potassium concentration, which is similar to the increment that occurs in patients without renal disease.40 If the patient is already hyperkalemic, this mild elevation may be sufficient to precipitate cardiac dysrhythmias. Plasma cholinesterase concentrations are normal, even after dialysis, and the duration of action of succinylcholine is not prolonged.41
Neuromuscular blockade should be maintained with an agent that does not rely on renal elimination. Cisatracurium undergoes Hofmann degradation, and therefore the duration of action is not prolonged in patients with renal failure. Hypermagnesemia, commonly found in patients with kidney disease, may potentiate neuromuscular blockade.42 Although anticholinesterase agents undergo renal elimination and have a prolonged duration in patients with renal insufficiency, the volume of distribution remains the same and standard doses are used for the reversal of neuromuscular blockade.
Postoperative Analgesia
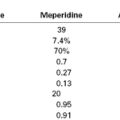
The principles of postoperative analgesia for the woman with renal disease are the same as for healthy woman, with some important considerations because drug clearance can be altered for opioids and their metabolites (see Chapters 27 and 28). Morphine is generally safe as a single dose, but with longer-term use its metabolite, morphine-6-glucuronide, may accumulate. Meperidine is of particular concern because its active metabolite, normeperidine, is neurotoxic and is renally excreted. Hydromorphone and oxycodone, and their metabolites hydromorphone-3-glucuronide and α- and β-oxycodol, respectively, are also renally excreted and may accumulate with prolonged use. Methadone does not accumulate in patients with renal disease and may be a useful long-term analgesic. Fentanyl and sufentanil are only minimally excreted in the urine, and because they are short-acting drugs they may be particularly useful. Remifentanil is metabolized by blood and tissue esterases and is not dependent on the kidney for excretion, making it safe as well for use in patients with renal failure.43 The safest approach may be to use neuraxial opioids because small doses are administered. Alternative techniques that avoid opioids such as transversus abdominis plane (TAP) block may also be considered, but the efficacy of TAP block for analgesia after cesarean delivery has been questioned (see Chapter 27).44
Acute Renal Failure
Definition and Epidemiology
Acute renal failure (ARF) is an uncommon but serious complication of pregnancy. Rapid deterioration of renal function leads to an accumulation of fluid and nitrogenous waste products with impaired electrolyte regulation. In the mid-20th century, nearly a fourth of all cases of ARF were obstetric. Fortunately, during the past five decades, the incidence of ARF in developed countries has decreased significantly.45,46 Stratta et al.45 reported a steady decline in the incidence of ARF from 1 in 3000 to 1 in 18,000 pregnancies from 1958 to 1994. With respect to all ARF cases, the proportion related to pregnancy decreased from 43% to 0.5%. This progress has resulted from improved obstetric care and fewer septic abortions.
Pathophysiology and Diagnosis
ARF is suggested by a sharp increase in the plasma creatinine (> 0.8 mg/dL) and BUN (> 13 mg/dL) concentrations. In complete renal failure, the serum creatinine concentration increases at the rate of 0.5 to 1.0 mg/dL/day. Urine output typically decreases to less than 400 mL/day (oliguria), but some patients may be nonoliguric. ARF is subdivided according to underlying cause (i.e., prerenal, postrenal, and intrarenal) (Box 52-2). The inciting disorders vary throughout the world. In developing countries septic abortion is the leading cause of pregnancy-related ARF.47,48 In developed countries, severe preeclampsia/eclampsia, acute pyelonephritis of pregnancy, and bilateral renal cortical necrosis are the most common underlying disorders.45,46
Prerenal Causes
The most common prerenal causes of ARF—hyperemesis gravidarum and obstetric hemorrhage—lead to hypovolemia and inadequate renal perfusion.49,50 Urinary indices show urinary osmolality greater than 500 mOsm/kg water, urine sodium less than 20 mEq/L, fractional sodium excretion less than 1%, and a urinary-to-plasma creatinine ratio greater than 40.51 Concealed uterine hemorrhage from placental abruption may remain unrecognized until hypotension and renal failure ensue.52 Women with preeclampsia may be more likely to develop ARF after hemorrhage because of preexisting intravascular contraction and widespread maternal endothelial dysfunction.53 Women who developed preeclampsia during pregnancy, with or without renal failure, are more likely to develop renal failure later in life.54
Intrarenal Causes
An intrarenal cause is diagnosed once prerenal and postrenal causes of ARF have been excluded. In general, oliguric intrarenal ARF is not easily reversed and must run its course. Causes include acute tubular necrosis, interstitial nephritis, and acute glomerulonephritis as well as a few causes unique to pregnancy. These include renal cortical necrosis, acute pyelonephritis, severe preeclampsia/eclampsia, acute fatty liver of pregnancy, and idiopathic postpartum renal failure. A thorough history, review of medications, and urinalysis typically help determine the specific initiating factor.55
Acute tubular necrosis results from nephrotoxic drugs, amniotic fluid embolism, rhabdomyolysis, intrauterine fetal death, and prolonged renal ischemia secondary to hemorrhage or septic shock. Urinalysis demonstrates dirty brown epithelial cell casts and coarse granular casts. Urinary indices show urine osmolality less than 350 mOsm/kg water, urine sodium concentration greater than 40 mEq/L, fractional sodium excretion greater than 1%, and a urinary-to-plasma creatinine ratio less than 20.55
Acute interstitial nephritis is caused by nonsteroidal anti-inflammatory drugs (NSAIDs) and various antibiotics. Patients typically have fever, rash, eosinophilia, and urine eosinophils.
Acute glomerulonephritis is rare during pregnancy. It is suggested by hematuria, red cell casts, and proteinuria. Urinary indices of acute glomerulonephritis are similar to those of prerenal ARF.
Bilateral renal cortical necrosis, which is rarely observed in the nonobstetric patient, is responsible for 10% to 38% of cases of obstetric ARF.47,56–58 It may occur during early or late pregnancy. Hemorrhage is the most common precipitating event. The pathogenesis of this disorder is unclear but may involve renal hypoperfusion or endothelial damage by endotoxins imposed on the normal hypercoagulable state of pregnancy.59 A single dose of endotoxin may precipitate bilateral renal cortical necrosis in pregnant animals and has led some investigators to view this disorder as a clinical analogue of the experimental Sanarelli-Shwartzman reaction.60 Extensive microthrombi are found within the glomeruli and renal arterioles. Diagnosis is made by selective renal arteriography, which reveals absence or patchiness of blood flow in the cortex. Renal biopsy may also be performed in the absence of active coagulopathy.9
Acute pyelonephritis is one of the most common infectious complications of pregnancy (see Chapter 37). Although acute pyelonephritis rarely leads to ARF in the nongravid patient, it accounts for 5% of cases of ARF among pregnant women.53 The reason for this greater susceptibility is unclear. Whalley et al.61 noted that acute pyelonephritis causes a marked reduction of GFR in pregnant women. In contrast, pyelonephritis causes little reduction in GFR in nonpregnant patients. The kidney may be more sensitive to bacterial endotoxins during pregnancy.
Severe preeclampsia/eclampsia may be responsible for 20% of cases of obstetric ARF in developed countries53 and as much as 35% of cases in developing countries.62 Renal failure is generally associated with severe preeclampsia or HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets); in these patients the incidence may be as high as 36%.63 However, many cases of renal dysfunction and failure may only mimic preeclampsia and may actually result from other factors.6 Other causes of ARF should be considered before preeclampsia is considered to be the basis of renal failure.
Sibai and Ramadan64 reported 32 cases of ARF associated with HELLP syndrome. The majority of patients had derangement of multiple organ systems and other obstetric complications (e.g., placental abruption, intrauterine fetal death, disseminated intravascular coagulation, postpartum hemorrhage, sepsis). Renal histology in a woman with HELLP syndrome and renal failure demonstrated thrombotic microangiopathy and acute tubular necrosis, suggesting a possible pathogenesis of acute renal failure associated with HELLP syndrome.65 In the Sibai and Ramadan64 report, 4 (13%) parturients died, and 10 (31%) required dialysis. The perinatal mortality rate was 34%, with 72% of deliveries occurring preterm. Of interest, Flynn et al.66 reported the successful use of cadaveric kidneys procured from a parturient who died after HELLP syndrome and ARF. Both recipients had acceptable graft function 2 years after transplantation.
Acute fatty liver of pregnancy, a rare but life-threatening disorder of pregnancy, is associated with a 60% to 100% incidence of ARF. Specific clinical features of acute fatty liver of pregnancy are discussed in Chapter 46.
The syndrome of idiopathic postpartum renal failure was initially described in 1968 by Robson et al.67 Subsequently, approximately 200 cases have been reported. This syndrome is characterized by ARF, microangiopathic hemolytic anemia, and thrombocytopenia occurring 2 days to 10 weeks after an uncomplicated delivery. It appears closely related to the hemolytic-uremic syndrome. Idiopathic postpartum renal failure is typically preceded by a viral upper respiratory tract or gastrointestinal syndrome that rapidly progresses to ARF. The use of ethinyl estradiol as a contraceptive may also be causally related to this syndrome.68 Spontaneous bleeding, congestive heart failure, hypertension, and seizures have been reported with this syndrome.69 Some investigators believe that this syndrome represents a clinical analogue to the generalized Shwartzman reaction, a condition induced in laboratory animals by two successive injections of endotoxin, which results in factor XII activation, thrombin generation, and fibrin deposition.60 Others consider the platelet deposition to be the primary event that leads to microvascular thrombi.69
Management involves plasma exchange transfusion, dialysis, and antiplatelet therapy. The role of heparin therapy in idiopathic postpartum renal failure is controversial. The morbidity and mortality among affected patients vary by study. Reports from 1979 and 1988 suggested a mortality rate of approximately 50%.70,71 Although survival has improved with prompt diagnosis and aggressive treatment, morbidity is still high. Shrivastava et al.72 reported three patients who had postpartum hemolytic-uremic syndrome and had initial recovery with immediate exchange transfusion; long-term follow-up was not reported. Dashe et al.73 reported 10 patients with peripartum or postpartum hemolytic-uremic syndrome and followed their course for a mean of 9 years. Although all 10 initially survived, 1 subsequently died and all had major morbidity, including recurrence of renal failure, hypertension and seizures.
Postrenal Causes
The postrenal causes of ARF include nephrolithiasis and ureteral obstruction by the gravid uterus.74 The latter complication is more likely in pregnant women with polyhydramnios or multiple gestation.75 Preexisting ureteral dilation and impaired peristalsis increase the risk for obstructive uropathy during pregnancy. Flank pain and decreased urine output during late gestation should alert the clinician to this possibility. Courban et al.76 reported an unusual case of obstructive uropathy leading to ARF in a pregnant woman with multiple uterine leiomyomas.
Effect on the Mother and Fetus
Maternal mortality from ARF has decreased significantly in the past 40 years. Stratta et al.45 reviewed their experience with ARF in pregnancy from 1958 to 1995. In the early period from 1956 to 1957 the maternal mortality rate was 31% but decreased to zero from 1988 to 1994. They hypothesized that this was probably due to improvement in medical and obstetric care, especially of women with preeclampsia. Although maternal prognosis has improved significantly in developed countries, mortality ranges between 20% and 30% in developing countries.48,62 The prognosis for the fetus is worse than for the mother. Reported fetal mortality is as high as 40% to 50%,77,78 although outcomes may be improving. Two groups reported much lower mortality rates (0% to 13%) with the use of aggressive hemodialysis (six times per week with the goal of maintaining the blood urea nitrogen level < 50 mg/dL).79,80
Medical and Obstetric Management
Management is directed toward rapid recognition of the underlying abnormality. Reversible disorders such as hypovolemia, concealed uterine hemorrhage, urinary tract infection, ureteral obstruction, and drug-induced ARF must be excluded. The urine-to-plasma osmolality ratio is a useful laboratory test to identify reversible prerenal causes. Intravascular volume should be optimized. Electrolyte and acid-base status should be monitored carefully. Hypertension and preeclampsia must be managed aggressively. Many obstetric causes of ARF also may cause disseminated intravascular coagulation; therefore, coagulation abnormalities should be excluded in pregnant women with ARF.45
Because urea and other metabolic products cross the placenta, hemodialysis or peritoneal dialysis should be directed toward maintaining the postdialysis BUN concentration at or below 30 mg/dL. Fluid shifts during hemodialysis should be minimized by short but frequent periods of dialysis. If the fetus is mature, delivery should be accomplished when the maternal condition is stabilized. The pediatrician must be alerted to the presence of high fetal BUN levels, which may lead to an osmotic diuresis and neonatal dehydration. Ertürk et al.81 reported the first known delivery of a healthy infant during a hemodialysis session.
Anesthetic Management
A multidisciplinary approach involving anesthesiologists, obstetricians, and nephrologists should be employed to optimize the maternal condition before the induction of labor or performance of cesarean delivery in a woman with ARF. The level of azotemia, electrolyte balance, and hematologic status should be assessed. If the BUN level is greater than 80 mg/dL or the serum potassium concentration greater than 5.5 mEq/L, dialysis should be performed before elective vaginal or cesarean delivery. Neuraxial anesthesia may be administered in the absence of coagulopathy, thrombocytopenia, and severe hypovolemia. Volume status is difficult to assess. In the past, it was common to place a central line or pulmonary artery catheter to assess volume status, but this is now rarely done. Intravenous fluid without potassium (e.g., 0.9% saline) should be administered. Occult uterine hemorrhage should be excluded, and hypertension, if present, controlled. Both spinal and epidural analgesia/anesthesia are safe and preferred to general anesthesia. As the sympathetic blockade dissipates, the mother should be monitored for evidence of volume overload and pulmonary edema. General anesthesia may be required for urgent cesarean delivery or in patients with coagulopathy or hemorrhage.
Renal Transplantation
Although pregnancy is uncommon in women undergoing long-term dialysis,22 fertility is improved within months of transplantation. The first recorded live birth to a woman with a kidney transplant was reported in 1958.82 In a review of female renal transplant patients in the United States, a pregnancy rate of 20 per 1000 was estimated in transplanted patients in the year 2000 compared with 100 per 1000 in the general population.83
Although a successful obstetric outcome can be anticipated in more than 95% of kidney transplant recipients, they are at greater risk for both maternal and fetal complications than healthy women. In a systematic review of articles published between the years 2000 and 2010, Deshpande et al.84 reported pregnancy-related outcome data in kidney transplant recipients. Fifty studies representing 4706 pregnancies in 3570 recipients met inclusion criteria. The incidences of preeclampsia (27%), gestational diabetes (8%), cesarean delivery (56.9%), and preterm delivery (45.6%) were greater than in the general population.
Effect of Pregnancy on the Renal Allograft
When a kidney is removed from a donor and transplanted into an anephric recipient, it undergoes a process of hyperfiltration. This is a maladaptive response that, in the short term, attempts to bring the GFR toward the rate of a bi-nephric system. In the long term, this hyperfiltration may lead to glomerular sclerosis and loss of renal function if it is associated with increased glomerular or capillary pressure.85 In normal pregnancy, the GFR increases by 30% to 50% during the first and second trimesters and subsequently decreases somewhat during the third trimester. Theoretically, this additional hyperfiltration of pregnancy predisposes the patient to a loss of renal function.
Baylis et al.86 allayed many of these concerns by demonstrating that gestational hyperfiltration is not associated with increased glomerular pressure because of matching afferent and efferent arteriolar vasodilation. They produced hyperfiltration in rodent kidneys by performing uninephrectomy, maintaining the animals on a high-protein diet, and subjecting them to five consecutive pregnancies. The investigators observed no functional impairment or renal histologic changes in this animal model. In addition, they demonstrated that glomerular pressure is lower in female rats than in male rats 10 months after uninephrectomy.87 Similar sex advantage has been seen in humans after uninephrectomy.88
There have been a number of studies assessing graft function after pregnancy. Most studies suggest that there is no adverse effect provided renal function is normal before conception and there is no evidence of hypertension.89–93 Levidiotis et al.90 analyzed 40 years of outcome data from the Australian and New Zealand Dialysis and Transplant Registry and did not find any impact of pregnancy on 20-year graft or patient survival.
Rahamimov et al.91 compared long-term graft survival, kidney function, and patient survival between women who became pregnant after renal transplantation (n = 39; 55 births) with those who did not (n = 117). Each pregnant woman was matched with three nonpregnant women for 12 factors that may affect graft survival. Graft (61.6%) and patient survival (84.8%) in the pregnant women did not differ from the matched nonpregnant group (68.7% graft and 78.8% patient survival) during the 15-year follow-up study.
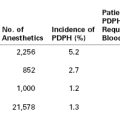
Sturgiss and Davison92 performed a case-control study of 36 renal transplant recipients, of whom 18 became pregnant and 18 did not. Groups were matched according to age, early rejection episodes, primary renal function, interval since transplantation, and extent of histocompatibility. The investigators noted no significant difference between the two groups in plasma creatinine concentration, GFR, mean arterial blood pressure, or the number who suffered graft loss or chronic rejection over a mean follow-up period of 12 years (Table 52-1).
TABLE 52-1
Effect of Pregnancy on Long-Term Function of Renal Allografts*
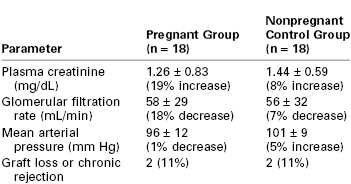
* Percentage increase or decrease represents change from initial assessment to end of follow-up. No statistically significant differences were noted.
From Sturgiss SN, Davison JM. Effect of pregnancy on long-term function of renal allografts. Am J Kidney Dis 1992; 19:167-72.
Kashanizadeh et al.93 also compared graft survival, allograft function, and patient survival between transplant recipients who conceived (n = 86) and those who did not (n = 125). They, too, did not find a difference in 5-year graft or patient survival between groups. Interestingly, they noted a smaller increase in creatinine levels in women who had conceived, suggesting that pregnancy might exert a protective effect, but this finding has not been confirmed by others.94
Effect on the Fetus
Although pregnancy seems to have minimal effect on maternal health or allograft survival in renal transplant recipients, fetal outcome is less favorable. The Toronto Renal Transplant Program reviewed 44 consecutive pregnancies in 26 women who had undergone renal transplantation.95 Of these, 12 (27%) pregnancies ended with abortion (two elective, four spontaneous) or intrauterine death, and 32 (73%) pregnancies resulted in live-born infants. The mean birth weight in this group was 2540 g, versus 3590 g in a control group. In Singapore, Tan et al.96 reported abortion or stillbirth among 13 (31%) of 42 pregnancies after renal transplantation. The remaining successful pregnancies were complicated by preterm delivery (45%) and fetal growth restriction (86%). Toma et al.22 surveyed 194 pregnancies in renal transplant recipients. Spontaneous or elective abortion occurred in 28 (14%) of these gestations, and successful delivery of surviving infants occurred in 159 (82%). Deshpande et al.,84 in their large systematic review, found a 73.5% live birth rate in renal transplant patients, which is similar to that found in the individual studies.22,95,96
Most post-transplantation protocols consist of a primary immunosuppressant (cyclosporine or tacrolimus) and one or two adjunctive agents (azathioprine, mycophenolate mofetil, sirolimus, and/or corticosteroids).97 Despite transplacental exposure to immunosuppressant drugs, congenital anomalies and other adverse effects are not greater than in the general population, but none of the studies included patients who were receiving mycophenolate mofetil.96,98 There have been reports of congenital defects with mycophenolate mofetil, including cleft lip and palate, microtia, absence of auditory canals, brachydactyly of the fifth finger, and hypoplastic toenails; therefore, its use has to be weighed against the risk for allograft rejection.99
Intrauterine exposure to cyclosporine impairs development and function of T, B, and NK lymphocytes in neonates. This effect, as well as depressed levels of serum immunoglobulin, persists during the first year of life.100 These factors place the infant at risk for a suboptimal immunologic response after administration of classic vaccines and for adverse effects after administration of live, attenuated vaccines.
Transplant recipients may become infected with cytomegalovirus (CMV) at the time of transplantation, or they may experience reactivation secondary to immunosuppression. Active CMV infection during pregnancy is associated with congenital anomalies (e.g., cerebral cysts, microcephaly, mental retardation). In addition, active neonatal CMV infection may lead to serious illness or death.
After renal transplantation, residual impairment of renal function may lead to false-positive results of biochemical screening for trisomy 21. Karidas et al.101 demonstrated a significant correlation between free β-subunit human chorionic gonadotropin and serum urea and creatinine concentrations. Similar alterations in alpha-fetoprotein levels were not observed. In this setting, the double-marker biochemical test may be interpreted inaccurately. In patients with altered serum urea and creatinine concentrations, first-trimester nuchal translucency measurement—in combination with second-trimester ultrasonography—may be a more useful screening regimen (see Chapter 6).
Medical and Obstetric Management
Discontinuation of immunosuppressant therapy, even years after transplantation, may lead to acute rejection. Thus, the renal transplant recipient’s immunosuppressant regimen must be continued during pregnancy unless toxicity results, although some practitioners discontinue mycophenolate mofetil. Cyclosporine requirements increase during pregnancy, most likely because of enhanced metabolism.102 The pregnant patient must be intensively monitored for any evidence of acute or chronic allograft rejection, infection, ureteral and renal artery obstruction, impaired renal function, hypertension, fluid volume disturbances, anemia, or any combination of these symptoms. Recombinant human erythropoietin (darbepoetin) has been successfully used to treat anemia during pregnancy.103
Initial laboratory studies in pregnant renal transplant patients include (1) complete blood cell count, (2) renal function tests, (3) serum electrolyte and glucose concentrations, and (4) viral serologic testing for CMV, hepatitis B virus, HCV, and HIV. Serial ultrasonographic assessments allow the recognition of fetal anomalies and the evaluation of fetal growth.
Cultures of the lower genital tract should be obtained in women with lesions suggestive of herpes simplex virus infection. A patient who presents in labor and with evidence of active genital herpes simplex virus infection should undergo cesarean delivery (see Chapter 37).
Vaginal examinations are minimized and always performed in a strict aseptic manner. The renal allograft is typically implanted in the extraperitoneal iliac fossa and does not impair vaginal delivery. Prophylactic antibiotics and stress-dose corticosteroids are indicated in patients who undergo cesarean delivery.
Anesthetic Management
In the absence of renal dysfunction and hypertension, anesthetic management of the parturient with a renal transplant is similar to that of the healthy parturient. Strict aseptic technique is maintained during the placement of intravascular catheters and the performance of neuraxial anesthetic techniques. Sowter et al.104 reported an epidural abscess that occurred 23 days after epidural anesthesia in a nonpregnant patient receiving corticosteroid therapy for rheumatoid arthritis. Fortunately, this complication is exceedingly rare. In the absence of systemic infection, immunosuppression itself should not be considered a contraindication to administration of epidural or spinal anesthesia.
Urolithiasis
Definition and Epidemiology
Urolithiasis is characterized by the abnormal formation of calculi within the renal calyces or pelvis. Calculi may lodge within the ureters or bladder. Most stones are calcium oxalate (70%) or calcium phosphate (10%). The disorder affects 1% to 5% of the general U.S. population, but it is more common in the southeastern “stone belt” and mountainous regions. Symptomatic urolithiasis occurs during 1 in 240 to 1 in 3300 pregnancies and is more common among whites than African-Americans.105 This incidence approximates that observed among nonpregnant young women, suggesting that pregnancy does not affect the rate of urolithiasis.
Pathophysiology
The presence of urolithiasis presumes an underlying physiologic abnormality that leads to persistent supersaturation of the particular minerals involved. During pregnancy, an elevated plasma 1,25-dihydroxyvitamin D level causes greater intestinal absorption of calcium, net mobilization of calcium from bone, and a state of absorptive hypercalciuria.106 Ultimately, these changes provide calcium for the fetal skeleton. Because pregnant women do not develop urolithiasis at a rate greater than that in the general population, it would appear that the occurrence of other physiologic changes during pregnancy offsets this stone-forming factor. Calcium stone inhibitors such as citrate, magnesium, and glycoprotein are excreted in the urine to a greater extent during pregnancy.107
Diagnosis
Urolithiasis most commonly manifests during the second or third trimester. Only 20% of affected pregnant women recount a prior history of renal calculi. More than 80% of cases of gestational urolithiasis are diagnosed in parous women, possibly reflecting the higher incidence of this disease with advanced age.108 Similar to that seen in the nonpregnant woman, stones occur with equal frequency on the right and left sides.109 The signs and symptoms of urolithiasis during pregnancy must be differentiated from that of ectopic pregnancy, preterm labor, appendicitis, pyelonephritis, and benign hematuria of pregnancy. A history of previous urolithiasis, recurrent urinary tract infections, or urologic surgery is suggestive. Symptoms include flank and abdominal pain, urgency, dysuria, nausea, and fever. Examination reveals costovertebral tenderness, abdominal tenderness, pyuria, and hematuria. Urolithiasis must be considered in patients with pyelonephritis who remain febrile or have continued bacteriuria despite 48 hours of parenteral antibiotics.
The initial imaging modality for the evaluation of urolithiasis during pregnancy is transabdominal ultrasonography. Transabdominal ultrasonography is diagnostic in about 60% of cases and does not expose the mother or fetus to radiation.110 Color Doppler ultrasonography allows the identification of ureteral jets; the asymmetry or absence of these jets indicates the presence of urinary calculi. Transvaginal ultrasonography may augment suboptimal transabdominal ultrasonographic images.111 Combining ultrasound evaluation with assessment of the intrarenal artery resistive index increases the accuracy of ultrasonography to greater than 70%.112
If urinary calculi are not successfully visualized with ultrasonography and clinical suspicion for urolithiasis remains high, magnetic resonance (MR) urography should be considered because it does not use ionizing radiation or iodinated contrast media (see Chapter 17).113 If the diagnosis is still unclear and the patient has persistent flank pain after both these tests, some experts recommend low-dose computed tomography114 whereas others recommend intravenous pyelography.108
Effect of Pregnancy on Urolithiasis
In an effort to determine any effect of pregnancy on the natural history of urolithiasis, Coe et al.115 reviewed the records of 58 pregnancies in women with the preexisting diagnosis of urolithiasis. The stone recurrence rate in this group was 0.49 stone per patient-year, which was not significantly different from the rate of 0.44 stone per patient-year in the general population. The authors concluded that pregnancy does not alter the activity or severity of stone disease.
Effect on the Mother and Fetus
In a retrospective cohort study, Swartz et al.116 compared pregnant women with nephrolithiasis (n = 2339) with randomly selected women without nephrolithiasis (n = 6729). The investigators found that women with nephrolithiasis had an almost twofold higher rate of preterm delivery. However, these women were not at increased risk for other adverse pregnancy outcomes, including premature rupture of the membranes, low birth weight, and infant death. The etiology of preterm labor and perhaps delivery is unclear but may be related to urinary tract infections that occur with a greater frequency in those with urolithiasis.117 Honoré118 suggested that there is a higher incidence of renal stones among women who have a spontaneous abortion. He hypothesized that abnormalities of calcium hemostasis may lead to myometrial hyperirritability or abnormal hormonal secretion by the corpus luteum, the placenta, or both. Rare cases of ureteral rupture119 and obstructed labor caused by a bladder calculus120 have been reported.
Urologic and Obstetric Management
Women with a history of urolithiasis should increase their intake of fluids. Calcium supplementation through prenatal vitamins should be avoided in women with recurrent urolithiasis. During pregnancy, 70% of calculi pass spontaneously with conservative management (e.g., hydration, antibiotics if the patient is febrile, bed rest, analgesia). More aggressive therapy will be required if conservative management is not successful. The decision to move beyond conservative therapy should be taken on a case-by-case basis. Infected hydronephrosis, especially with impaired renal function or urosepsis, is an indication for more aggressive therapy.121
Medical management, commonly used for treatment of urolithiasis,122 is limited during pregnancy by fetal concerns. Medical expulsive therapy with alpha-adrenergic receptor blocking agents has been used successfully to increase the rate of stone passage and decrease pain associated with expulsion by relaxing ureteral smooth muscle.123 However, there are no published reports of its use during pregnancy. Other medical treatments that are used to treat urolithiasis, including thiazide diuretics, xanthine oxidase inhibitors, and D-penicillamine, are contraindicated during pregnancy owing to possible effects on the fetus.124,125
Urologic intervention is indicated in the patient with persistent pyelonephritis, deterioration of renal function, massive hydronephrosis, persistent pain, or sepsis. Ureteral stent placement with ureteroscopy and ultrasonographic guidance, or percutaneous nephrostomy, should be considered because either can be performed without the need for anesthesia or radiation exposure.105 Holmium:yttrium-aluminum-garnet (YAG) laser lithotripsy, using state-of-the-art ureteroscopes, is an emerging technique for stone management in pregnancy.126 Extracorporeal lithotripsy should be avoided during pregnancy because the shockwaves may be harmful to the fetus.127
The following conditions may raise suspicion of the presence of primary hyperparathyroidism in a pregnant woman: (1) urolithiasis with or without pancreatitis, (2) hyperemesis beyond the first trimester, (3) a history of recurrent spontaneous abortion or intrauterine fetal death, (4) neonatal hypocalcemia or tetany, and (5) a total serum calcium concentration greater than 10.1 mg/dL during the second trimester or greater than 8.8 mg/dL during the third trimester.128
Anesthetic Management
Anesthesiologists are occasionally asked to provide analgesia for patients with renal or ureteral stones. The ureters receive sensory innervation through the renal, ovarian, and hypogastric plexuses (T11 to L1 spinal segments). During conservative management of urolithiasis, epidural analgesia provides the patient with significant pain relief and facilitates the passage of the calculus, possibly through decreased ureteral spasm.129,130 Ready and Johnson129 reported the use of epidural analgesia in a patient with severe renal colic at 23 weeks’ gestation. Analgesia that was maintained for 16 hours allowed the passage of the stone. Neuraxial analgesia avoids the use of systemic opioids, which impair normal peristalsis in ureteric smooth muscle. Improved maternal pain control may also decrease endogenous catecholamine release and improve uteroplacental blood flow.
References
1. Pregnancy and renal disease. Lancet. 1975;2:801–802.
2. Fischer MJ. Chronic kidney disease and pregnancy: maternal and fetal outcomes. Adv Chronic Kidney Dis. 2007;14:132–145.
3. Jeyabalan A, Lain KY. Anatomic and functional changes of the upper urinary tract during pregnancy. Urol Clin North Am. 2007;34:1–6.
4. Fischer MJ, Lehnerz SD, Hebert JR, Parikh CR. Kidney disease is an independent risk factor for adverse fetal and maternal outcomes in pregnancy. Am J Kidney Dis. 2004;43:415–423.
5. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180.
6. Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore). 1981;60:267–276.
7. Chen HH, Lin HC, Yeh JC, Chen CP. Renal biopsy in pregnancies complicated by undetermined renal disease. Acta Obstet Gynecol Scand. 2001;80:888–893.
8. Day C, Hewins P, Hildebrand S, et al. The role of renal biopsy in women with kidney disease identified in pregnancy. Nephrol Dial Transplant. 2008;23:201–206.
9. Kuller JA, D’Andrea NM, McMahon MJ. Renal biopsy and pregnancy. Am J Obstet Gynecol. 2001;184:1093–1096.
10. Jungers P, Houillier P, Forget D, et al. Influence of pregnancy on the course of primary chronic glomerulonephritis. Lancet. 1995;346:1122–1124.
11. Limardo M, Imbasciati E, Ravani P, et al. Pregnancy and progression of IgA nephropathy: results of an Italian multicenter study. Am J Kidney Dis. 2010;56:506–512.
12. Jones DC, Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1996;335:226–232.
13. Purdy LP, Hantsch CE, Molitch ME, et al. Effect of pregnancy on renal function in patients with moderate-to-severe diabetic renal insufficiency. Diabetes Care. 1996;19:1067–1074.
14. Epstein FH. Pregnancy and renal disease. N Engl J Med. 1996;335:277–278.
15. Baylis C. Glomerular filtration rate in normal and abnormal pregnancies. Semin Nephrol. 1999;19:133–139.
16. Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598.
17. Lindheimer MD, Katz AI. Gestation in women with kidney disease: prognosis and management. Baillieres Clin Obstet Gynaecol. 1994;8:387–404.
18. Bar J, Orvieto R, Shalev Y, et al. Pregnancy outcome in women with primary renal disease. Isr Med Assoc J. 2000;2:178–181.
19. Ramin SM, Vidaeff AC, Yeomans ER, Gilstrap LC 3rd. Chronic renal disease in pregnancy. Obstet Gynecol. 2006;108:1531–1539.
20. McGregor E, Stewart G, Junor BJ, Rodger RS. Successful use of recombinant human erythropoietin in pregnancy. Nephrol Dial Transplant. 1991;6:292–293.
21. Lim VS. Reproductive function in patients with renal insufficiency. Am J Kidney Dis. 1987;9:363–367.
22. Toma H, Tanabe K, Tokumoto T, et al. Pregnancy in women receiving renal dialysis or transplantation in Japan: a nationwide survey. Nephrol Dial Transplant. 1999;14:1511–1516.
23. Krakow D, Castro LC, Schwieger J. Effect of hemodialysis on uterine and umbilical artery Doppler flow velocity waveforms. Am J Obstet Gynecol. 1994;170:1386–1388.
24. Malone FD, Craigo SD, Giatras I, et al. Suggested ultrasound parameters for the assessment of fetal well-being during chronic hemodialysis. Ultrasound Obstet Gynecol. 1998;11:450–452.
25. Reddy SS, Holley JL. Management of the pregnant chronic dialysis patient. Adv Chronic Kidney Dis. 2007;14:146–155.
26. Chou CY, Ting IW, Lin TH, Lee CN. Pregnancy in patients on chronic dialysis: a single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol. 2008;136:165–170.
27. Piccoli GB, Conijn A, Consiglio V, et al. Pregnancy in dialysis patients: is the evidence strong enough to lead us to change our counseling policy? Clin. J Am Soc Nephrol. 2010;5:62–71.
28. Brem AS, Singer D, Anderson L, et al. Infants of azotemic mothers: a report of three live births. Am J Kidney Dis. 1988;12:299–303.
29. Agarwal SK, Dash SC, Gupta S, Pandey RM. Hepatitis C virus infection in haemodialysis: the ‘no-isolation’ policy should not be generalized. Nephron Clin Pract. 2009;111:c133–c140.
30. Scheithauer S, Eitner F, Mankartz J, et al. Improving hand hygiene compliance rates in the haemodialysis setting: more than just more hand rubs. Nephrol Dial Transplant. 2012;27:766–770.
31. Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006;19:317–322.
32. Rout CC, Akoojee SS, Rocke DA, Gouws E. Rapid administration of crystalloid preload does not decrease the incidence of hypotension after spinal anaesthesia for elective caesarean section. Br J Anaesth. 1992;68:394–397.
33. Tighe KE, Smith ID, Bogod DG. Caesarean section in chronic renal failure. Eur J Anaesthesiol. 1995;12:185–187.
34. Lucas LF, Tsueda K. Cardiovascular depression after brachial plexus block in two diabetic patients with renal failure. Anesthesiology. 1990;73:1032–1035.
35. Rice AS, Pither CE, Tucker GT. Plasma concentrations of bupivacaine after supraclavicular brachial plexus blockade in patients with chronic renal failure. Anaesthesia. 1991;46:354–357.
36. Orko R, Pitkanen M, Rosenberg PH. Subarachnoid anaesthesia with 0.75% bupivacaine in patients with chronic renal failure. Br J Anaesth. 1986;58:605–609.
37. Weir PH, Chung FF. Anaesthesia for patients with chronic renal disease. Can Anaesth Soc J. 1984;31:468–481.
38. Costela JL, Jimenez R, Calvo R, et al. Serum protein binding of propofol in patients with renal failure or hepatic cirrhosis. Acta Anaesthesiol Scand. 1996;40:741–745.
39. Freeman RB, Sheff MF, Maher JF, Schreiner GE. The blood-cerebrospinal fluid barrier in uremia. Ann Intern Med. 1962;56:233–240.
40. Miller RD, Way WL, Hamilton WK, Layzer RB. Succinylcholine-induced hyperkalemia in patients with renal failure? Anesthesiology. 1972;36:138–141.
41. Ryan DW. Preoperative serum cholinesterase concentration in chronic renal failure: clinical experience of suxamethonium in 81 patients undergoing renal transplant. Br J Anaesth. 1977;49:945–949.
42. Ghoneim MM, Long JP. The interaction between magnesium and other neuromuscular blocking agents. Anesthesiology. 1970;32:23–27.
44. Loane H, Preston R, Douglas MJ, et al. A randomized controlled trial comparing intrathecal morphine with transversus abdominis plane block for post-cesarean delivery analgesia. Int J Obstet Anesth. 2012;21:112–118.
45. Stratta P, Besso L, Canavese C, et al. Is pregnancy-related acute renal failure a disappearing clinical entity? Ren Fail. 1996;18:575–584.
46. Machado S, Figueiredo N, Borges A, et al. Acute kidney injury in pregnancy: a clinical challenge. J Nephrol. 2012;25:19–30.
47. Prakash J, Kumar H, Sinha DK, et al. Acute renal failure in pregnancy in a developing country: twenty years of experience. Ren Fail. 2006;28:309–313.
48. Bentata Y, Housni B, Mimouni A, et al. Acute kidney injury related to pregnancy in developing countries: etiology and risk factors in an intensive care unit. J Nephrol. 2012;25:764–775.
49. Hill JB, Yost NP, Wendel GD Jr. Acute renal failure in association with severe hyperemesis gravidarum. Obstet Gynecol. 2002;100:1119–1121.
50. Hassan I, Junejo AM, Dawani ML. Etiology and outcome of acute renal failure in pregnancy. J Coll Physicians Surg Pak. 2009;19:714–717.
51. Albright RC Jr. Acute renal failure: a practical update. Mayo Clin Proc. 2001;76:67–74.
52. Krane NK. Acute renal failure in pregnancy. Arch Intern Med. 1988;148:2347–2357.
53. Grunfeld JP, Pertuiset N. Acute renal failure in pregnancy: 1987. Am J Kidney Dis. 1987;9:359–362.
54. Suzuki H, Watanabe Y, Arima H, et al. Short- and long-term prognosis of blood pressure and kidney disease in women with a past history of preeclampsia. Clin Exp Nephrol. 2008;12:102–109.
55. Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation—a systematic review. Int Urol Nephrol. 2010;42:425–433.
56. Chugh KS, Jha V, Sakhuja V, Joshi K. Acute renal cortical necrosis—a study of 113 patients. Ren Fail. 1994;16:37–47.
57. Ventura JE, Villa M, Mizraji R, Ferreiros R. Acute renal failure in pregnancy. Ren Fail. 1997;19:217–220.
58. Prakash J, Vohra R, Wani IA, et al. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant. 2007;22:1213–1217.
59. Khan RZ, Badr KF. Endotoxin and renal function: perspectives to the understanding of septic acute renal failure and toxic shock. Nephrol Dial Transplant. 1999;14:814–818.
60. Grunfeld JP, Pertuiset N. Acute renal failure in pregnancy. Adv Exp Med Biol. 1987;212:245–250.
61. Whalley PJ, Cunningham FG, Martin FG. Transient renal dysfunction associated with acute pyelonephritis of pregnancy. Obstet Gynecol. 1975;46:174–177.
62. Prakash J, Niwas SS, Parekh A, et al. Acute kidney injury in late pregnancy in developing countries. Ren Fail. 2010;32:309–313.
63. Celik C, Gezginc K, Altintepe L, et al. Results of the pregnancies with HELLP syndrome. Ren Fail. 2003;25:613–618.
64. Sibai BM, Ramadan MK. Acute renal failure in pregnancies complicated by hemolysis, elevated liver enzymes, and low platelets. Am J Obstet Gynecol. 1993;168:1682–1687.
65. Abraham KA, Kennelly M, Dorman AM, Walshe JJ. Pathogenesis of acute renal failure associated with the HELLP syndrome: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2003;108:99–102.
66. Flynn MF, Power RE, Murphy DM, et al. Successful transplantation of kidneys from a donor with HELLP syndrome-related death. Transpl Int. 2001;14:108–110.
67. Robson JS, Martin AM, Ruckley V, Macdonald MK. Irreversible post-partum renal failure: a new syndrome. Q J Med. 1968;37:423–435.
68. Hayslett JP. Current concepts. Postpartum renal failure. N Engl J Med. 1985;312:1556–1559.
69. Sun NC, Johnson WJ, Sung DT, Woods JE. Idiopathic postpartum renal failure: review and case report of a successful renal transplantation. Mayo Clin Proc. 1975;50:395–401.
70. Li PK, Lai FM, Tam JS, Lai KN. Acute renal failure due to postpartum haemolytic uraemic syndrome. Aust N Z J Obstet Gynaecol. 1988;28:228–230.
71. Segonds A, Louradour N, Suc JM, Orfila C. Postpartum hemolytic uremic syndrome: a study of three cases with a review of the literature. Clin Nephrol. 1979;12:229–242.
72. Shrivastava M, Modi G, Singh RK, Navaid S. Early diagnosis and management of postpartum hemolytic uremic syndrome with plasma exchange. Transfus Apher Sci. 2011;44:257–262.
73. Dashe JS, Ramin SM, Cunningham FG. The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancy. Obstet Gynecol. 1998;91:662–668.
74. Khanna N, Nguyen H. Reversible acute renal failure in association with bilateral ureteral obstruction and hydronephrosis in pregnancy. Am J Obstet Gynecol. 2001;184:239–240.
75. Brandes JC, Fritsche C. Obstructive acute renal failure by a gravid uterus: a case report and review. Am J Kidney Dis. 1991;18:398–401.
76. Courban D, Blank S, Harris MA, et al. Acute renal failure in the first trimester resulting from uterine leiomyomas. Am J Obstet Gynecol. 1997;177:472–473.
77. Holley JL, Reddy SS. Pregnancy in dialysis patients: a review of outcomes, complications, and management. Semin Dial. 2003;16:384–388.
78. Asamiya Y, Otsubo S, Matsuda Y, et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217–1222.
79. Luders C, Castro MC, Titan SM, et al. Obstetric outcome in pregnant women on long-term dialysis: a case series. Am J Kidney Dis. 2010;56:77–85.
80. Haase M, Morgera S, Bamberg C, et al. A systematic approach to managing pregnant dialysis patients—the importance of an intensified haemodiafiltration protocol. Nephrol Dial Transplant. 2005;20:2537–2542.
81. Ertürk S, Akar H, Uçkuyu A, et al. Delivery of healthy infant during hemodialysis session. J Nephrol. 2000;13:75–77.
82. Murray JE, Merrill JP, Harrison JH. Kidney transplantation between seven pairs of identical twins. Ann Surg. 1958;148:343–359.
83. Gill JS, Zalunardo N, Rose C, Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant. 2009;9:1541–1549.
84. Deshpande NA, James NT, Kucirka LM, et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11:2388–2404.
85. Terasaki PI, Koyama H, Cecka JM, Gjertson DW. The hyperfiltration hypothesis in human renal transplantation. Transplantation. 1994;57:1450–1454.
86. Baylis C, Reckelhoff JF. Renal hemodynamics in normal and hypertensive pregnancy: lessons from micropuncture. Am J Kidney Dis. 1991;17:98–104.
87. Baylis C, Wilson CB. Sex and the single kidney. Am J Kidney Dis. 1989;13:290–298.
88. Goldfarb DA, Matin SF, Braun WE, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047.
89. Crowe AV, Rustom R, Gradden C, et al. Pregnancy does not adversely affect renal transplant function. QJM. 1999;92:631–635.
90. Levidiotis V, Chang S, McDonald S. Pregnancy and maternal outcomes among kidney transplant recipients. J Am Soc Nephrol. 2009;20:2433–2440.
91. Rahamimov R, Ben-Haroush A, Wittenberg C, et al. Pregnancy in renal transplant recipients: long-term effect on patient and graft survival. A single-center experience. Transplantation. 2006;81:660–664.
92. Sturgiss SN, Davison JM. Effect of pregnancy on long-term function of renal allografts. Am J Kidney Dis. 1992;19:167–172.
93. Kashanizadeh N, Nemati E, Sharifi-Bonab M, et al. Impact of pregnancy on the outcome of kidney transplantation. Transplant Proc. 2007;39:1136–1138.
94. Miranda CT, Melaragno C, Camara NO, et al. Adverse effects of pregnancy on renal allograft function. Transplant Proc. 2002;34:506–507.
95. Sgro MD, Barozzino T, Mirghani HM, et al. Pregnancy outcome post renal transplantation. Teratology. 2002;65:5–9.
97. Gaston RS. Maintenance immunosuppression in the renal transplant recipient: an overview. Am J Kidney Dis. 2001;38:S25–S35.
98. Bar J, Stahl B, Hod M, et al. Is immunosuppression therapy in renal allograft recipients teratogenic? A single-center experience. Am J Med Genet A. 2003;116A:31–36.
99. Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698–1702.
100. Schen FP, Stallone G, Schena A, et al. Pregnancy in renal transplantation: immunologic evaluation of neonates from mothers with transplanted kidney. Transpl Immunol. 2002;9:161–164.
101. Karidas CN, Michailidis GD, Spencer K, Economides DL. Biochemical screening for Down syndrome in pregnancies following renal transplantation. Prenat Diagn. 2002;22:226–230.
102. Biesenbach G, Zazgornik J, Kaiser W, et al. Cyclosporin requirement during pregnancy in renal transplant recipients. Nephrol Dial Transplant. 1989;4:667–669.
103. Goshorn J, Youell TD. Darbepoetin alfa treatment for post-renal transplantation anemia during pregnancy. Am J Kidney Dis. 2005;46:e81–e86.
104. Sowter MC, Burgess NA, Woodsford PV, Lewis MH. Delayed presentation of an extradural abscess complicating thoracic extradural analgesia. Br J Anaesth. 1992;68:103–105.
105. Cormier CM, Canzoneri BJ, Lewis DF, et al. Urolithiasis in pregnancy: current diagnosis, treatment, and pregnancy complications. Obstet Gynecol Surv. 2006;61:733–741.
106. Gertner JM, Coustan DR, Kliger AS, et al. Pregnancy as state of physiologic absorptive hypercalciuria. Am J Med. 1986;81:451–456.
107. Loughlin KR, Ker LA. The current management of urolithiasis during pregnancy. Urol Clin North Am. 2002;29:701–704.
108. Butler EL, Cox SM, Eberts EG, Cunningham FG. Symptomatic nephrolithiasis complicating pregnancy. Obstet Gynecol. 2000;96:753–756.
109. Parulkar BG, Hopkins TB, Wollin MR, et al. Renal colic during pregnancy: a case for conservative treatment. J Urol. 1998;159:365–368.
110. Isen K, Hatipoglu NK, Dedeoglu S, et al. Experience with the diagnosis and management of symptomatic ureteric stones during pregnancy. Urology. 2012;79:508–512.
111. Laing FC, Benson CB, DiSalvo DN, et al. Distal ureteral calculi: detection with vaginal US. Radiology. 1994;192:545–548.
112. Andreoiu M, MacMahon R. Renal colic in pregnancy: lithiasis or physiological hydronephrosis? Urology. 2009;74:757–761.
113. Spencer JA, Chahal R, Kelly A, et al. Evaluation of painful hydronephrosis in pregnancy: magnetic resonance urographic patterns in physiological dilatation versus calculous obstruction. J Urol. 2004;171:256–260.
114. White WM, Zite NB, Gash J, et al. Low-dose computed tomography for the evaluation of flank pain in the pregnant population. J Endourol. 2007;21:1255–1260.
115. Coe FL, Parks JH, Lindheimer MD. Nephrolithiasis during pregnancy. N Engl J Med. 1978;298:324–326.
116. Swartz MA, Lydon-Rochelle MT, Simon D, et al. Admission for nephrolithiasis in pregnancy and risk of adverse birth outcomes. Obstet Gynecol. 2007;109:1099–1104.
117. Lewis DF, Robichaux AG 3rd, Jaekle RK, et al. Urolithiasis in pregnancy: diagnosis, management and pregnancy outcome. J Reprod Med. 2003;48:28–32.
118. Honoré LH. The increased incidence of renal stones in women with spontaneous abortion: a retrospective study. Am J Obstet Gynecol. 1980;137:145–146.
119. Eaton A, Martin PC. Ruptured ureter in pregnancy—a unique case? Br J Urol. 1981;53:78–79.
120. Keepanasseril A, Nanjappa B, Prasad GV, et al. Vesical calculus: an unusual cause of labour dystocia. J Obstet Gynaecol. 2012;32:596–597.
121. Biyani CS, Joyce AD. Urolithiasis in pregnancy. II. Management. BJU Int. 2002;89:819–823.
122. Spernat D, Kourambas J. Urolithiasis—medical therapies. BJU Int. 2011;108(Suppl 2):9–13.
123. Parsons JK, Hergan LA, Sakamoto K, Lakin C. Efficacy of alpha-blockers for the treatment of ureteral stones. J Urol. 2007;177:983–987.
124. Gray MJ. Use and abuse of thiazides in pregnancy. Clin Obstet Gynecol. 1968;11:568–578.
125. Corcoran R, Castles WJ. Penicillamine therapy and teratogenesis. Br Med J. 1977;1:838.
126. Watterson JD, Girvan AR, Beiko DT, et al. Ureteroscopy and holmium:YAG laser lithotripsy: an emerging definitive management strategy for symptomatic ureteral calculi in pregnancy. Urology. 2002;60:383–387.
127. Asgari MA, Safarinejad MR, Hosseini SY, Dadkhah F. Extracorporeal shock wave lithotripsy of renal calculi during early pregnancy. BJU Int. 1999;84:615–617.
128. Schnatz PF, Curry SL. Primary hyperparathyroidism in pregnancy: evidence-based management. Obstet Gynecol Surv. 2002;57:365–376.
129. Ready LB, Johnson ES. Epidural block for treatment of renal colic during pregnancy. Can Anaesth Soc J. 1981;28:77–79.
130. Romagnoli A. Letter: Continuous epidural block in the treatment of impacted ureteric stones. Can Med Assoc J. 1973;109:968.