Chapter 26 Quality of life and hepatopancreatobiliary tumors
Quality of Life
Quality of life (QoL) is a clinically and biologically meaningful outcome for the oncologic patient. Historically, surgical, procedural, and interventional outcomes have been measured in clinical and objective terms using end points such as morbidity, mortality, and disease-free survival. In the 1990s, there was increased recognition of the need to evaluate the patient’s subjective experiences, how they felt and functioned. Measurement of a patient’s QoL throughout the clinical course should be standard practice. A surgeon often manages the care of a hepatopancreatobiliary cancer patient beyond the surgical procedure alone. Management includes orchestrating diagnostic tests, biliary interventions, and percutaneous ablative therapies. For any of these procedures, the goal of the physician is to minimize the impact on a patient’s function and to improve overall QoL (Langenhoff et al, 2001; Miner et al, 2002; Blazeby & Vickery, 2001; Sajid et al, 2008; Pallis & Mouzas, 2004; Sun et al, 2008).
Surgical resection of malignant tumors of the liver, bile ducts, gallbladder, and pancreas is performed for both curative and palliative reasons. Hepatopancreatobiliary surgical outcomes have continued to improve, because advancements in presurgical imaging and surgical techniques have resulted in better patient outcomes, fewer complications, and higher survival rates. After recovery from surgery, while a patient remains disease free, QoL is usually excellent. For those patients who are not candidates for a curative resection, palliation is the major treatment goal (Sun et al, 2008; Abraham et al, 2002; Singhal et al, 2005). Indications for palliative surgical resection include locoregional disease control and surgical bypass to relieve biliary or gastrointestinal obstruction (Nakakura & Warren, 2007; Koninger et al, 2007). Resolution of symptoms such as pruritus, anorexia, fatigue, and pain and improvement of QoL are the major treatment goals.
Definition of Quality of Life
In 1947, the World Health Organization (WHO) broadened the definition of health to include not only the absence of disease but also “the complete physical, mental, and social well-being of a person” (WHO, 1947). Karnofsky and Burchenal (1949) subsequently outlined basic criteria for evaluation of new chemotherapeutic agents, describing a simple scale to measure the effect of nitrogen mustard on patients with lung cancer. In the years following, numerous anticancer therapies emerged that increased survival, but toxicities were significant. Izsak and Medalie (1971) sought to identify the “costs of survival” among cancer patients. Noting that a growing number of patients with cancer were surviving longer, they began looking at the lives of the survivors from multiple angles, namely the psychological, behavioral, and environmental aspects. This new insight into patient health care caused a dramatic increase in interest in the new field of QoL research (Aaronson, 1988). One event that placed QoL end points in the spotlight was the controversial approval of gemcitabine for the treatment of pancreatic cancer on the basis of a beneficial clinical response. The “surrogate end point” of clinical benefit was likened to improvement in QoL based on the parameters of pain intensity, analgesic consumption, performance status, and weight gain (Burris et al, 1997). The concept of clinical benefit has increased dialogue about pancreatic cancer and QoL and undoubtedly has raised awareness of the importance of QoL assessment in palliative care settings.
In an effort to better describe clinical benefit, the term health-related quality of life emerged and has come to include the aspects of QoL that are most influenced by health and health care interventions (Kassa & Loge, 2002). The terms quality of life and health-related quality of life are often used interchangeably, but no single definition of either exists. There is consensus, however, that QoL is multidimensional and at a minimum encompasses physical symptoms, treatments, functional ability, family factors, emotional well-being, intimacy, and social aspects (Cella, 1998).
The broad overuse of the term quality of life has led to the misleading notion that measurements of performance status (e.g., Karnofsky and Eastern Cooperative Oncology Group, 1949), clinical indicators (e.g., bilirubin level), or symptom checklists adequately reflect QoL (Blazeby & Vickery, 2001). Other clinical factors—including laboratory values, postoperative complications, and time spent in the hospital—also have been translated into QoL results. Although these assessments have value in clinical care and research, care must be taken not to mistake or substitute them for proper QoL measurement.
Applications of Quality of Life Assessments in Surgery
In the past, informal feedback from patients regarding symptoms and functional status combined with the clinician’s objective assessment was considered sufficient for making judgments about patients’ QoL. Over the past few decades, a demand for more information about the broader effects of treatment and illness has required more formal definitions and evaluations of QoL (Blazeby & Vickery, 2001; Gouma et al, 1999; Barbarisi et al, 2001). In addition to defining the patient’s physical, psychological, and functional problems, QoL assessments also evaluate surgical outcomes, compare treatment options, aid preoperative selection of patients, predict prognosis, and monitor quality of care. In the case of neurosurgical measurements of QoL, the standard remains the Karnofsky Performance Status (KPS) set by Karnofsky and Burchenal (1949), which only addresses three variables of QoL: 1) work, 2) activity, and 3) self-care (Meyers & Weitzner, 1995).
Quality of Life as an Outcome
Outcomes research is a new and interesting field in clinical research and in daily clinical practice. In 1996, the American Society of Clinical Oncology published a special article to clarify effectiveness of cancer treatments (ASCO, 1996). Outcomes were divided into cancer outcomes (response rate) and patient outcomes (survival and QoL). As a surgical outcome, QoL has become an issue of increasing importance. The American College of Surgeons has dedicated a section of its Surgical Forum to QoL and outcome studies since 1996 (Tassinari et al, 2003; Velanovich, 2001). More holistic patient outcomes can be given priority over cancer outcomes in several situations. Examples of surgical trials in which consideration should be given to QoL data are 1) adjuvant trials in which a moderate risk of disease recurrence is present; 2) trials in which survival is not expected to differ between treatment arms, but QoL might be affected; 3) trials in which the primary intent is palliative; and 4) equivalency trials in which lesser surgical procedures are done with the expectation that survival and tumor control will be equal (Whalen & Ferrans, 2001).
Psychological Screening
For many patients with newly diagnosed cancer, the psychological trauma can be quite intense. This is particularly true for patients with hepatopancreatobiliary cancer, most of whom face an uncertain future, with much of their remaining time consumed by oncologic treatment. The psychological impact of receiving such a diagnosis and living with the disease is profound. Depression and anxiety have been reported in 50% of patients with pancreatic cancer before their diagnosis, and several studies have suggested hormonal and neuropsychiatric links to explain this phenomenon (Holland et al, 1986; Passik & Breitbart, 1996; Passik & Roth, 1999). Attention to the emotional sequelae of hepatopancreatobiliary cancer may improve QoL and treatment adherence significantly. QoL questionnaires and/or psychological questionnaires may be used early in the diagnosis to identify problems that may not otherwise be detected during routine evaluation. Linden and colleagues (2005) found the Psychological Screen for Cancer to be an important tool in screening and for “following changes in patient distress throughout the cancer care trajectory.” Early identification of depression or anxiety allows for prompt evaluation by a mental health professional. Subsequent intervention with psychotherapy and/or medication may result in better psychological outcome for many patients.
Prognostic Indicators
QoL data have been shown to have prognostic value in several chemotherapeutic trials in patients with advanced metastatic colorectal cancer. Earlam and colleagues (1996) reported physical symptom scores were better predictors of survival than tumor size. In three large studies, QoL scores were independent predictors of survival; QoL was a stronger predictor of survival than clinician-rated performance status in two of these studies (Earlam et al, 1996; Schoffski et al, 1996; Maisey et al, 2001). One author concluded that QoL measurement may help stratify patients in clinical trials and may facilitate comparisons between studies (Blazeby & Vickery, 2001; Conroy & Blazeby, 2003). Pretreatment QoL was found by de Graeff and others (2001) to be a strong predictive indicator in the assessment of cancer survival in which performance status, physical functioning, and well-being were the QoL variables. For many patients with primary liver cancer or metastatic neuroendocrine cancer in the liver, treatment is often palliative and multimodal in nature, consisting of surgical, hormonal, ablative, and chemotherapeutic approaches. QoL assessment as a prognostic indicator and stratification tool may be useful for treatment algorithms and may help patients with poor performance status avoid futile treatments.
Physician-Patient Communication and Decision Making
The surgical oncologist is responsible for explaining surgical and ablative treatment options and for discussing treatment side effects, potential for cure, and impact of treatment on QoL. Physicians should have open discussions with patients as they advise them in these important treatment decisions. The physician opens the door for better communication by first trying to understand the value each patient places on issues such as survival, toxicity, disease-free interval, and impact on functional status; by matching treatment options to the patient’s value system, the physician can become more effective. Research has shown that patients differ greatly in how they view risk/benefit ratios. A study evaluating treatment options for elderly patients found that meetings with a facilitator along with written information allowed the patient and the family to make decisions better suited to their QoL needs (Matulonis, 2004). Knowledge of and effective communication of QoL results may lead to better informed, shared decision making and ultimately may improve the physician–patient relationship and the outcome (D’Angelica et al, 1998).
Quality of Life After hepatopancreatobiliary Surgery
Over the past 10 years, hepatopancreatobiliary operations have moved from high morbidity and high mortality procedures to procedures that are safe and effective in an experienced surgeon’s hands. When procedures are performed with the intent to cure, patients are usually accepting of a short-term decline in QoL as a part of recovery from surgery or as a surgical or procedural complication (Perez et al, 1997). When consenting for a procedure, patients usually assume the risks of the surgery willingly as the price of a cure. In this setting, assessment of QoL improves understanding of the impact of a surgery. On the other hand, for both procedural and surgical palliative interventions, patients are less forgiving of postprocedure complications that cause a decline in QoL in the face of an imminently diminishing life span. Therefore QoL becomes an important outcome to include when evaluating a palliative intervention.
Miner and others (2002) offer insight into outcomes after palliative surgeries for advanced malignancy. Their study, limited by a small number of subjects (n = 36) and early mortality (median survival, 108 days), found that 54% of the patients who underwent palliative operations for unresectable disease experienced no clinical improvement after surgery, and these patients died significantly sooner than those who experienced a benefit. For those who did experience an improvement in QoL, duration of improvement was a median of 3.4 months. The surgeon’s preprocedure predictions regarding ability to relieve symptoms were correct 78% of the time for those with positive outcomes, while he or she only mistakenly thought they could ameliorate 1 (7%) of the 14 patients who did not improve. The reasons for the surgeon proceeding with the surgery in the belief of a poor clinical outcome were not discussed. Prior to surgery, 26% of patients expressed fear that they did not expect to survive the surgery; patients also reported a strong relationship with their surgeon before the surgery, and this did not change significantly afterward.
Unfortunately, the study found that 46% of patients undergoing palliative surgery expressed hope that their physician would find no evidence of disease at the time of surgery (Miner et al, 2002). This belief cannot simply be attributed to optimism, hope, or a delusional process; however, it is likely that these patients harbored misconceptions regarding the procedure that were not corrected during the consenting process. It is critical that patients understand that a palliative procedure is being done only to improve QoL; a cure cannot be achieved, and there will still be evidence of disease. Furthermore, patients should have a realistic expectation as to whether the goal of palliation is achievable. Surgeons should trust their clinical judgment regarding their ability to bring about a favorable clinical outcome as well as the potential for futility. These opinions should be discussed with patients, and such discussions are likely to be in depth and difficult; but the end goal of more realistic patient expectations should not be undervalued, and this is an important topic in palliative medicine that should be further explored.
Measurement and Interpretation of Quality of Life End Points
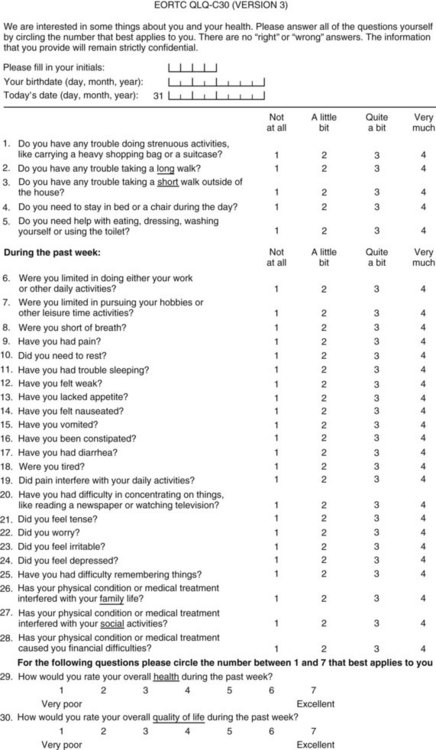
It has been established that QoL is an important outcome that should be incorporated into surgical oncology, but the problem remains: How should it be measured? Specifically, what should be measured? First, the concept needs to be clearly defined, because a great deal of variability surrounds the way QoL might be measured in a clinical trial. In a review of QoL studies in six major surgical journals, Velanovich (2001) found that many studies made errors in conceptually defining QoL and in choosing QoL questionnaires. Passik and Kirsch (2000) suggested a “levels of evidence” approach to understanding QoL end points in clinical trials. Such an approach is based on the type and sophistication of the questionnaire chosen for the trial and the conclusions one can make from the resulting data. This approach may be helpful in interpreting QoL conclusions made by authors and in aiding investigators in trial design (Table 26.1). The European Organization for the Research and Treatment of Cancer (EORTC) has placed the evaluation of QoL on questionnaires such as the QLQ-C30, SF-36, and the Rotterdam Symptom Checklist and on modules specific to the disease and treatment (Giannakopoulos et al, 2005). Better understanding of the QoL concept would help the investigator design a study, and it would help to make the published results more meaningful (Koller & Lorenz, 2002; Sprangers, 2002).
Table 26.1 Levels of Evidence for the Determination of QoL in Clinical Trials
| Level of Evidence | Approach Taken | Explanation |
|---|---|---|
| Low | Single items | This approach includes the use of face-valid items only. Typically, these are designed by researchers for a particular study and have not been piloted or validated by any formal psychometric means. |
| Middle (A) | Conversion of existing tools | This approach is highlighted by the adaptation of tools from traditional psychology and psychiatry to oncology and assuming that they will be valid and reliable indicators for cancer patients (e.g., Ways of Coping population questionnaire). |
| Middle (B) | Assessment of a singular domain of QoL | The middle level of evidence also may focus on measuring a single domain of QoL. Typically, the focus is placed on physical well-being and measurement of side effects of treatment (e.g., toxicity ratings of treatments). |
| High | Multidimensional assessment of QoL | The highest level of evidence requires a multidimensional approach. This should cover the domains of physical, psychological, social, and functional well-being (e.g., FACT, FLIC, CARES). |
QoL, Quality of life; FACT, Functional Assessment of Cancer Therapy; FLIC, Functional Living Index-Cancer; CARES, Cancer Rehabilitation Evaluation System
From Passik S, Kirsch K, 2000: The importance of quality-of-life endpoints in clinical trials to the practicing oncologist. Hematol Oncol Clin North Am 14:877-886.
Types of Quality of Life Instruments
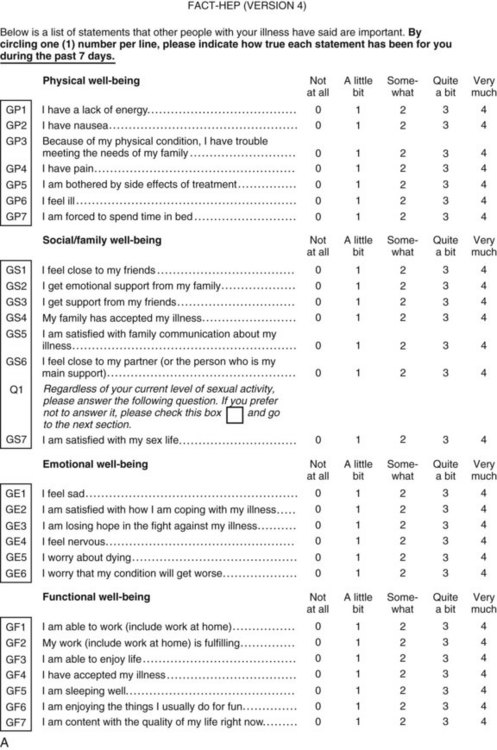
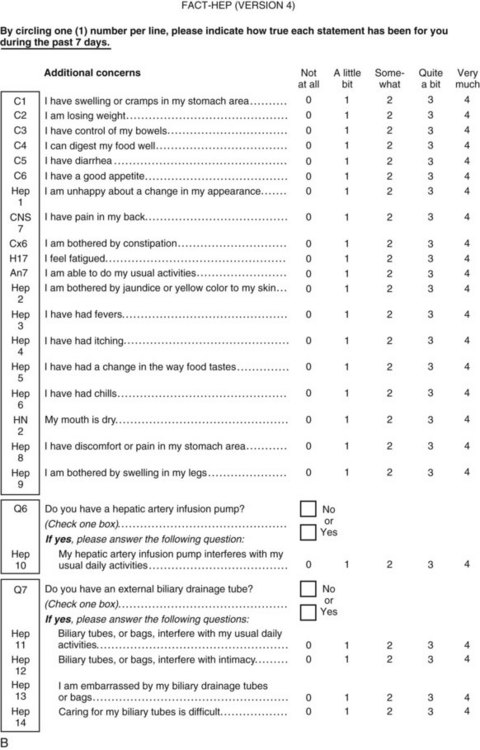
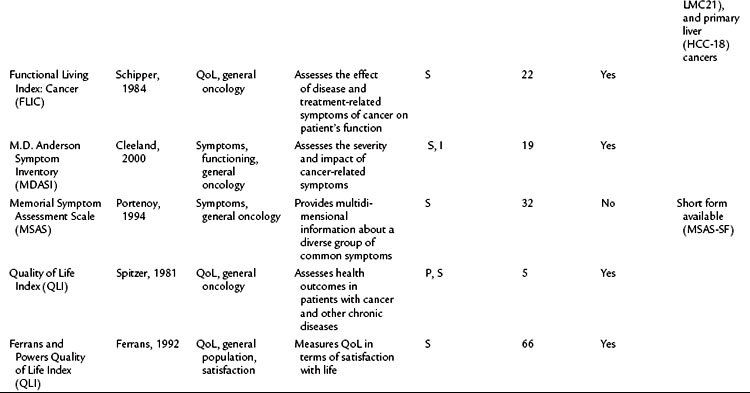
Perhaps the gold standard of QoL instruments for cancer patients is the modular questionnaire. Two examples are the Functional Assessment of Cancer Therapy (FACT), also known as the Functional Assessment of Chronic Illness Therapy (FACIT), and the EORTC QLQ-C30. They combine assessment of overall QoL domains—physical, social, functional, and emotional well-being—with disease-specific subscales or “modules.” This approach often eliminates the need for multiple instruments yet results in general and disease-specific or treatment-specific QoL information. The FACT and QLQ-C30 are multilingual and are widely used around the world. They are considered by many experts to be the most valid and standardized instruments for QoL assessment in cancer patients, and each has disease-specific subscales developed for use in patients with hepatopancreatobiliary cancer, such as the FACT-HEP (Blazeby et al, 2004; Fitzsimmons et al, 1999; Heffernan et al, 2002; Kavadas et al, 2003; Pallis & Mouzas, 2004). The FACT-HEP is shown in Figure 26.1, and the QLQ-C30 is shown in Figure 26.2. Disease-specific subscales for the QLQ-C30 include the QLQ-LMC21 (metastatic colorectal cancer), PAN-26 (pancreatic cancer), and HCC-18 (hepatocellular carcinoma).
Questionnaire Selection
The first step is to decide if QoL is an end point and if changes in QoL are expected. Groenvold (1999) presented four criteria that can be used in the evaluation of QoL as an end point in clinical trials: 1) inclusion of “relevant QoL issues in the questionnaire,” 2) large sample size “to detect meaningful differences,” 3) timing, and 4) the questionnaire must be free of missing data. A trial meeting these four criteria produces high-quality data, otherwise the results of that trial may be invalid (Groenvold, 1999).
Questionnaire selection and study design depend on the research question being asked. An excellent resource that is dedicated to helping investigators with QoL research is the Mapi Research Institute. This institute has developed a patient-reported outcome and QoL instruments database, which contains valuable information regarding patient-reported outcomes research and an index of QoL questionnaires (www.PROQOLID.org).
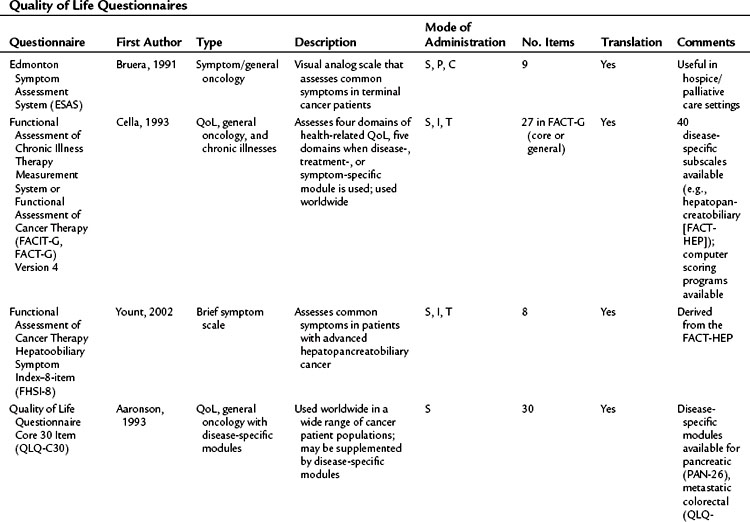
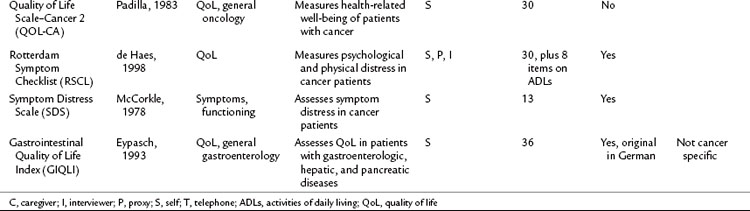
Table 26.2 lists basic issues to consider or questions to ask when designing a study with QoL as an outcome, modified from a combination of two sources: the minimum standard checklist from Efficace and colleagues (2003) and the Gill and Feinstein Criteria for Assessment of Quality of Life studies (Gill & Feinstein, 1994). Table 26.3 lists questionnaires that are relevant for use in hepatopancreatobiliary patients, limited to QoL and symptom questionnaires for patients with cancer or those that address the symptoms associated with diseases of the hepatopancreatobiliary tract. The list is not exhaustive, and questionnaire selection depends on study design and the research question being asked (Mapi Research Trust, QoL Instrument Database; www.PROQOLID.org).
| Conceptual |
Is there a rationale for using a specific questionnaire?
Is the questionnaire valid, reliable, and sensitive to change?
Was it validated for the study population you intend to use it for?
Does it cover at least the main dimensions relevant for cancer patients?
Is it relatively short and simple?
Is it completed by the patient?
Is it available in other languages relevant to the study population?
In what setting will the study be conducted (inpatient, outpatient, home)?
Is a baseline assessment included?
Will timing of assessments reflect change in health-related QoL owing to disease or treatment?
How will it be administered (self-completion with/without research staff’s assistance, mailed questionnaires, or phone completion)?
Will proxy completion be allowed?
Is there a research nurse or research assistant responsible for collecting data?
QoL, quality of lift
Modified from Efficace F, et al, 2003: Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials—does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol 21(18):3502-3511.
Quality of Life Studies in Hepatopancreatobiliary Cancer
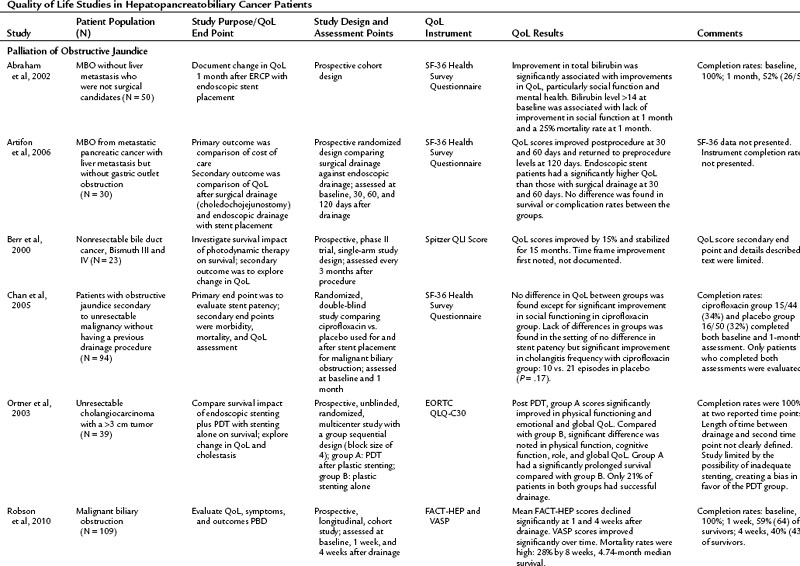
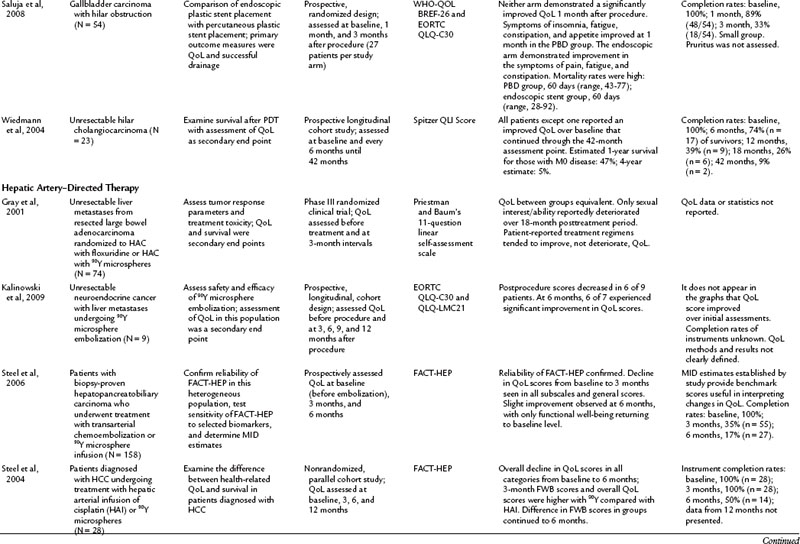
The literature of QoL studies in hepatopancreatobiliary cancer, and particularly pancreatic cancer, has grown significantly since the turn of the century. In the past, authors of research articles discussed QoL in terms of a practitioner’s subjective observations, but these conclusions were not grounded in evidence supported by a validated instrument. The discussion of QoL studies in specific disease states that follows is limited to those studies that utilized a validated instrument. The results of these studies are summarized in Table 26.4.
Pancreas Resection
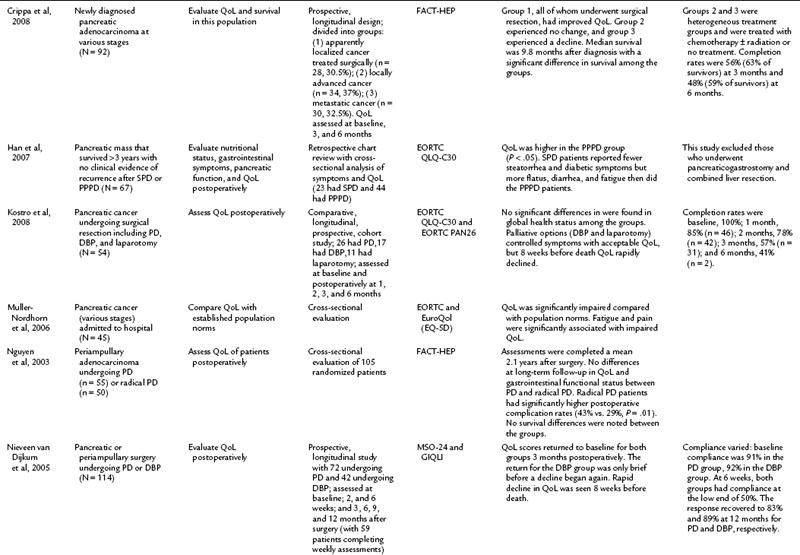
It is commonly known that patients with pancreatic cancer, who experience symptoms of pain and fatigue among others, have impaired QoL compared with the norms in a population (Muller-Nordhorn et al, 2006; see Chapter 58B). Crippa and others (2008) demonstrated that surgical intervention for pancreatic cancer has a favorable impact on QoL. The literature on QoL after pancreatic resection is the most robust collection of all the subgroup analyses, and the group of studies in Table 26.4 include two randomized pancreatic cancer studies, which is comparatively more than for any of the other disease subsets discussed in this chapter (Van Heek et al, 2003; Nguyen et al, 2003). For many other studies in this group, comparisons were made in a nonrandomized setting, which minimizes the scientific rigor of the comparison (Scheingraber et al, 2005).
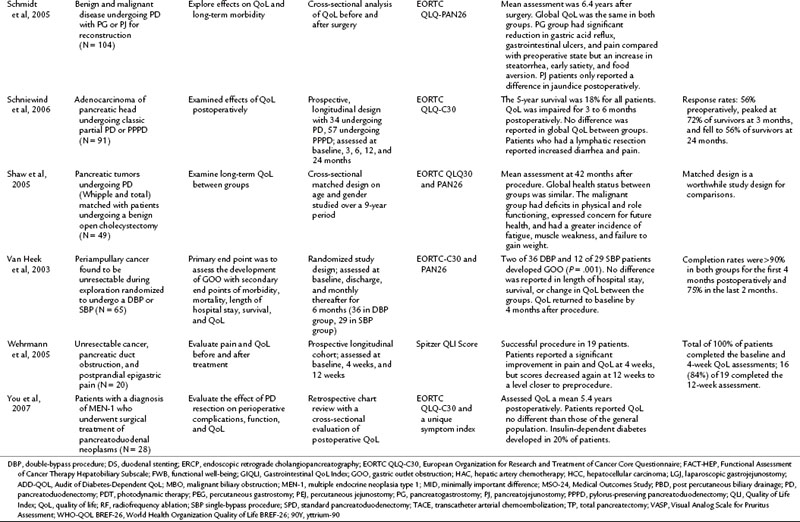
Many studies have compared the various surgical approaches to pancreatoduodenectomy (PD) including the pylorus-preserving PD (PPPD), Whipple procedure, standard PD (SPD), classical partial PD (CPPD), and total PD (TPD; see Chapter 62A). For patients undergoing surgical resection with a curative intent, an initial decline from baseline was seen in the first 3 postoperative months (Schniewind et al, 2006; Nieveen van Dijkum et al, 2005). Various surgical approaches were compared, but only Nguyen and colleagues (2003) evaluated randomized patients postoperatively. The authors found no differences in QoL and gastrointestinal function between PD and radical PD at a mean of 2.2 years after resection. In related cross-sectional studies, similar global QoL scores were found in patients who had PD with pancreatogastrostomy or pancreatojejunostomy reconstruction and those who underwent PD for multiple endocrine neoplasia type 1, compared with the general population (You et al, 2007; Schmidt et al, 2005).
Total pancreatectomy (TP) generally has been avoided, because it creates an iatrogenic chronic disease process in which patients are dependent on pancreatic enzymes and high doses of insulin. Billings and colleagues (2005) compared 27 TP patients at a mean of 7.5 years after resection with a national database. TP patients experienced an impact on their QoL and health status compared with the general population (Billings et al, 2005). Prior to undergoing a TP, it is advised that patients be thoroughly advised regarding not only the procedure but the expected outcomes, as well as being meticulously educated, prudently selected, and ascertained of adequate medical support in the future.
Palliation of unresectable pancreatic cancer is a worthwhile area of QoL exploration, because the goal of palliation is impacted by a major surgical procedure (see Chapter 63B). Van Heek and colleagues (2003) found in a randomized study that double bypass—hepaticojejunostomy and a retrocolic gastrojejunostomy—reduced the need for reoperation and creation of a gastrojejunostomy by 18%, and it significantly lowered the risk of postoperative gastric outlet obstruction over a single bypass (hepaticojejunostomy; Van Heek et al, 2003). No difference in postoperative QoL was reported between the groups. These QoL results were similar to the results of Shaw and others (2005), who compared PD to open cholecystectomy and found no difference in global QoL between the two groups, although differences were seen in subscales and item analysis (see Table 26.4; Shaw et al, 2005). In a different area of palliative care, Wehrmann and colleagues (2005) found that pancreatic duct stenting for obstruction relieved pain symptoms by 4 weeks, lasting for 12 weeks, and improved QoL at the 4-week time point before returning to baseline levels at 12 weeks.
Hepatic Resection
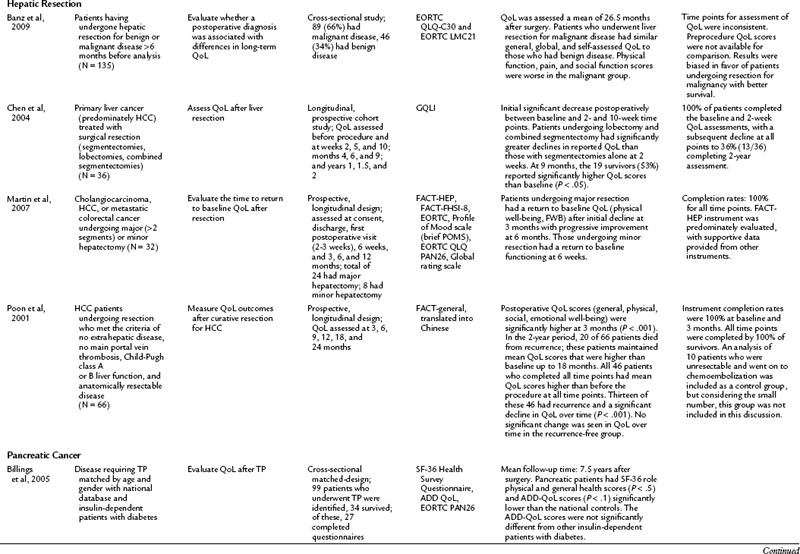
QoL after hepatic resection has been understudied and underreported in the literature despite the frequency that hepatic resections are performed and the improved outcomes (see Chapter 80, Chapter 81A, Chapter 81B, Chapter 81C ). Available studies do demonstrate consistent findings among the population. For those who have a survival duration beyond several months, an improvement is seen in QoL. Poon and colleagues (2001) reported on 66 patients who underwent surgical resection of hepatocellular carcinoma (HCC). Although 33 patients had recurrence, mean QoL scores were higher for all patients at 3 months after surgery than at baseline. For those who remained free of recurrence over the 2-year study, QoL measures demonstrated a sustained improvement. This finding was echoed by Chen and others (2004), who demonstrated that after a surgical resection for a primary liver cancer, an initial decline in QoL was seen for the first 10 weeks. After that, an improvement over baseline scores was noted among survivors. The patients who underwent a major hepatectomy experienced a greater decline in QoL immediately following the surgery. Similarly, other studies have reported the return to baseline physical and functional well-being after surgical resection to be 6 weeks for a minor hepatectomy (two segments or less) and 3 months for a major hepatectomy (Martin et al, 2007; Eid et al, 2006). Banz and colleagues (2009) compared those who underwent hepatectomy for benign and malignant disease and found no difference in general and global QoL. Unlike many other populations discussed in this chapter, the surgical procedure, not the disease burden, has the most significant impact on QoL.
Hepatic Artery–Directed Therapies
Regardless of the population treated or the method—transarterial chemoembolization (TACE), bland embolization, or use of yttrium 90 microspheres (90Y)—hepatic artery–directed treatments (see Chapters 83 and 84A) have not been well investigated from a QoL standpoint. Since 2000, only four studies have been published investigating QoL in patients undergoing hepatic artery embolization (HAE) (Gray et al, 2001; Steel et al, 2004, 2006; Kalinowski et al, 2009). All of these studies involve the use of 90Y, they had limited subjects, and the conclusions were divergent. In overlapping populations, Steel and colleagues (2004, 2006) found a decline in QoL for those undergoing TACE or 90Y at 3 months postprocedure, but the directionality diverged at 6 months between the two studies. Gray and colleagues (2001) used a little-researched tool, and Kalinowski and colleagues (2009) reported on only nine patients, so their findings of improvement of QoL postprocedure at 18 and 6 weeks, respectively, are limited.
Although not a traditional QoL assessment, symptom assessment offers value in understanding the impact of a treatment on a population. The Carcinoid Symptom Severity Score (CSSS) was developed to quantify symptoms of patients with neuroendocrine tumors. Neuroendocrine cancer patients have slowly progressing disease, but the symptoms caused by their tumor can negatively impact their QoL. The CSSS categorizes symptom presence, frequency, and effects on lifestyle into five different levels of symptoms (Schell et al, 2002; Wessels & Schell, 2001). Schell and others (2002) demonstrated an improvement in symptoms after HAE with lipiodol/Gelfoam utilizing the CSSS. Current clinical practice and research endeavors would benefit from use of this CSSS tool to consistently and succinctly summarize the impact of symptoms on this population.
Treatment of Gastric Outlet Obstruction
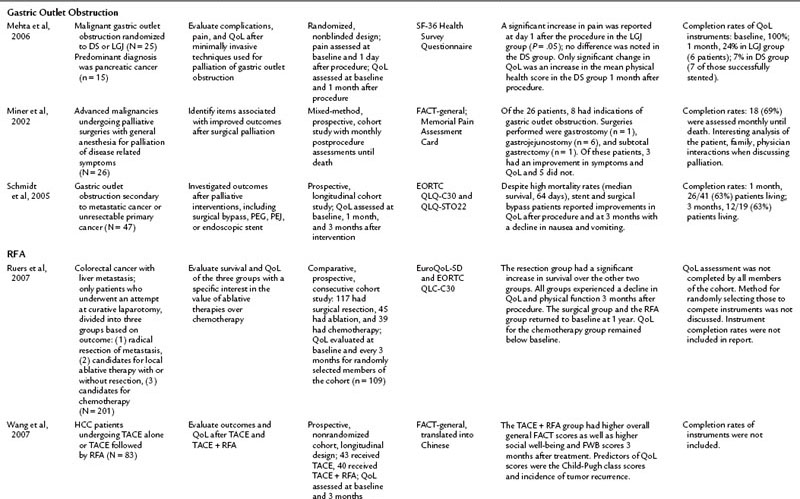
Gastric outlet obstruction (GOO) is a debilitating problem for those with advanced malignancies, particularly pancreatic and gastric cancers. Palliative interventions that relieve the symptoms of dysphagia, nausea, emesis, reflux, abdominal pain, and impaired eating stand to improve QoL for this population. Currently, the palliative treatment options are surgical bypass—gastrojejunostomy or laproscopic gastrojejunostomy (LGJ)—percutaneous gastrostomy, percutaneous jejunostomy, or duodenal stenting (DS). Only three reports include a QoL component, and study populations are small, but the initial results are promising for minimally invasive techniques. DS was shown to improve QoL and to decrease nausea and emesis for up to 3 months afterward, although success rates varied from 71% to 90% among the groups (Schmidt et al, 2005; Mehta et al, 2006; Kim et al, 2001). Schmidt and colleagues (2005) reported an improvement in QoL in their surgical bypass group as well. Miner and others (2002) presented data for a heterogenous group of patients undergoing surgical palliations; eight in that group underwent a palliative surgery for GOO, and three of eight patients experienced an improvement in all measured parameters; five did not. LGJ was not shown to be superior to DS in one small, randomized study (Mehta et al, 2006).
Palliation of Malignant Biliary Obstruction
In most cases, relief of malignant biliary obstruction (MBO) is a palliative intervention (see Chapter 18, Chapter 27, Chapter 28, Chapter 29, Chapter 49, Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D, Chapter 63A, Chapter 63B ). Endoscopic stenting (ES), surgical bypass, and percutaneous biliary drainage (PBD) are all capable of relieving jaundice; however, the topic has not been sufficiently explored to offer clear direction regarding which procedure is best suited for the spectrum of diseases and presentations. Advances have been made, and today palliative surgical bypass is infrequently performed because of the availability and technical successes of the less invasive ES and PBD.
Current literature is restricted to a handful of articles discussing QoL in patients with MBO who have undergone ES, PBD, surgical bypass, or photodynamic therapy (Berr et al, 2000; Abraham et al, 2002; Ortner et al, 2003; Wiedmann et al, 2004; Chan et al, 2005; Artifon et al, 2006; Saluja et al, 2008; Robson et al, 2010). Saluja and colleagues (2008) investigated QoL as a primary end point; the other authors assessed QoL secondarily. Beyond the common indication of relief of MBO, the populations in these studies varied, from those with specific cancers—pancreatic, gallbladder, and bile duct carcinomas—to more general malignant populations with MBO. For that reason it is difficult to make generalized conclusions from these studies. In addition, all of the discussed studies struggle with confounding variables from advancing disease and low response rates as a result of morbidity and mortality, which limit the ability to draw meaningful conclusions.
Patients were evaluated either longitudinally, to assess change in QoL after an intervention, or they were randomized for treatment comparisons. In one longitudinal study, Abraham and others (2002) found that hyperbilirubinemia and weight loss were predictive of poor QoL prior to ES, and ES was found to bring about an improved QoL in this population 1 month after ES placement, especially for those whose initial bilirubin was less than 13 mg/dL. Artifon and colleagues (2006) took evaluation of ES a step further and compared patients who had ES to those who underwent surgical resection. Both groups experienced an improvement in QoL scores at 30 and 60 days postprocedure with a decrease to below preprocedure levels at 120 days. The improvement at 30 and 60 days was significantly greater in the endoscopic group when compared with the surgical group (P = .05).
In another longitudinal study, Robson and colleagues (2010) studied the largest cohort in this subset (n = 109), looking at patients with MBO who presented for PBD who were not ES candidates. The patients reported a significant decline in QoL in the overall FACT-HEP scale over the 4-week assessment period but noted a significant improvement in pruritus during this time. The authors concluded that PBD did not improve QoL in the population in the face of a high rate of mortality (28% at 8 weeks postdrainage) (Robson et al, 2010). Saluja and colleagues (2008) randomized gallbladder carcinoma with hilar obstruction patients to either a percutaneously placed plastic stent or an endoscopically placed plastic stent. Neither group had a significant improvement in QoL, but PBD offered better technical success, and the ES group had a lower incidence of cholangitis over the PBD group. In light of these studies, the role of drainage, either ES or PBD, in decreasing the rate of clinical decline in these populations should be considered.
Improvement of QoL after photodynamic therapy (PDT) with ES placement is an emerging theme in the literature. In both prospective and randomized trials, patients experienced an improved QoL after undergoing PDT (Berr et al, 2000; Ortner et al, 2003; Wiedmann et al, 2004). Ortner and colleagues (2003) reported an associated improvement in survival over the stenting group. These reports offer the most positive outcomes from the prospective of improving QoL in a population.
Radiofrequency Ablation
Radiofrequency ablation (RFA) can be done intraoperatively and percutaneously (see Chapter 85C). Percutaneous RFA programs have expanded dramatically over the past 10 years. The percutaneous approach avoids complications related to an open or laparoscopic surgical procedure while still treating the disease and its symptoms, but these benefits have not been fully evaluated from a QoL perspective in the literature (Cho et al, 2010). Current literature on RFA is limited to two studies evaluating QoL in the setting of HCC or colorectal cancer with liver metastasis, the former utilizing percutaneous RFA, the latter operative RFA (Wang et al, 2007; Ruers et al, 2007). In the HCC study, Wang and others (2007) found a higher sustained QoL after transcatheter arterial embolization (TACE) with RFA than with TACE alone. They also found Child-Pugh class and rates of tumor recurrence were predictors of higher QoL scores.
Ruers and colleagues (2007) evaluated QoL after laparotomy for attempted resection of colorectal liver metastases. Expectedly, those patients who had successful resection of the metastasis had a better survival than those who could not be resected and instead had an intraoperative RFA or were closed and given postoperative chemotherapy. After an initial decline in all groups, QoL returned to baseline at 1 year for the resection and RFA groups, but the chemotherapy group’s QoL remained low. This study design was complex and compared heterogenous populations. These results will be clarified better when the Belgium CLOCC trial (EORTC 40004, chemotherapy and local ablation versus chemotherapy) is published. That trial compares RFA with chemotherapy and chemotherapy alone in patients with liver metastases from colorectal cancer. These studies offer promising results: that percutaneous RFA may have the potential to improve QoL over standard treatment options. As minimally invasive RFA applications expand, it is essential that QoL outcomes be evaluated along with clinical outcomes to further substantiate this procedure as a valid therapeutic option in palliative and curative settings.
Challenges in Quality of Life Research
QoL has become a field of research unto itself and has drawn attention to the human side of cancer (Passik & Kirsh, 2000). It is far from a perfect science and comes with its own unique set of challenges. Aside from methodologic issues such as proxy completion, missing data, and attrition in terminal or very sick populations of cancer patients, many obstacles exist in the interpretation and applicability of QoL data. One problem faced by QoL researchers and clinicians is the translation of statistically significant changes in QoL scores to clinically meaningful changes. Many tests for QOL assessment have come under harsh criticism, as they tend to rely on mathematical data that “offer such narrow insights into a complex disease and into life when facing cancer” (Corner, 1999). A recently described dilemma that can complicate data integrity is the response-shift phenomenon. This occurs when a patient reports stable QoL over time, when clinically they are sicker, because the patient’s internal standard adapts to the illness (Sprangers, 2002). The new “surrogate end points” in which clinical benefit is claimed from certain cancer therapies without the use of properly validated instruments poses yet another challenge.
Conclusion
There is still more work to be done. As Levine and Ganz (2002) reported, despite the fact that thousands of patients have had QoL measured by multiple instruments in clinical trials, there are only a few examples of outcomes that have influenced individual patients’ decision-making or treatment policies. The bridge across the gap between QoL research and clinical practice is the challenge of the future. The conversion of a number into a true understanding of a patient experience is not realistic, but there are merits to utilizing scores to evaluate a patient against established population norms or to deepen the understanding of a person’s clinical presentation.
Aaronson NK. Quality of life: What is it? How should it be measured? Oncology. 1988;2(5):69-76.
Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376.
Abraham NS, et al. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56(6):835-841.
American Society of Clinical Oncology. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol. 1996;4(2):671-679.
Artifon EL, et al. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. Am J Gastroenterol. 2006;101(9):2031-2037.
Banz VM, et al. Long-term quality of life after hepatic resection: health is not simply the absence of disease. World J Surg. 2009;33(7):1473-1480.
Barbarisi A, et al. Impact of surgical treatment on quality of life of patients with gastrointestinal tumors. Ann Oncol. 2001;12(Suppl 3):S27-S30.
Berr F, et al. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31(2):291-298.
Billings BJ, et al. Quality of life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9(8):1059-1066.
Blazeby JM, Vickery CW. Quality of life in patients with cancers of the upper gastrointestinal tract. Expert Rev Anticancer Ther. 2001;1(2):269-276.
Blazeby JM, et al. Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer. 2004;40(16):2439-2444.
Bruera E, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
Burris HA, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
Cella DF, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579.
Cella DF. Quality of life. In: Holland, JC, editor. Psycho-oncology. New York: Oxford University Press; 1998:1135-1146.
Chan G, et al. The role of ciprofloxacin in prolonging polyethylene biliary stent patency: a multicenter, double-blinded effectiveness study. J Gastrointest Surg. 2005;9(4):481-488.
Chen L, et al. Quality of life in patients with liver cancer after operation: a 2-year follow-up study. Hepatoobiliary Pancreat Dis Int. 2004;3(4):530-533.
Cho YK, et al. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284-1290.
Cleeland CS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634-1646.
Conroy T, Blazeby JM. Health-related quality of life in colorectal cancer patients. Expert Rev Anticancer Ther. 2003;3(4):493-504.
Corner J. Interface between research and practice in psycho-oncology. Acta Oncol. 1999;8(6):703-707.
Crippa S, et al. Quality of life in pancreatic cancer: analysis by stage and treatment. J Gastrointest Surg. 2008;12(5):783-793.
D’Angelica M, et al. Surgeon-patient communication in the treatment of pancreatic cancer. Arch Surg. 1998;133(9):962-966.
de Graeff A, et al. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37(3):332-339.
de Haes JC, Olschewski M. Quality of life assessment in a cross-cultural context: Use of the Rotterdam Symptom Checklist in a multinational randomised trial comparing CMF and Zoladex (Goserlin) treatment in early breast cancer. Ann Oncol. 1998;9(7):745-750.
Earlam S, et al. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol. 1996;14(1):171-175.
Efficace F, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials—does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003;21(18):3502-3511.
Eid S, et al. Assessment of symptom experience in patients undergoing hepatic resection or ablation. Cancer. 2006;107(11):2715-2722.
Eypasch E, et al. Gastrointestinal Quality of Life Index: Development, validation and application of a new instrument. Br J Surg. 1995;82(2):216-222.
Ferrans CE, Powers MJ. Psychometric assessment of the Quality of Life Index. Res Nurs Health. 1992;15(1):29-38.
Fitzsimmons D, et al. Development of a disease-specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. Eur J Cancer. 1999;35(6):939-941.
Giannakopoulos X, et al. Quality of life survey in patients with advanced prostate cancer. Exp Oncol. 2005;27(1):13-17.
Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272(8):619-626.
Gouma DJ, et al. Surgical palliative treatment in bilio-pancreatic malignancy. Ann Oncol 10. 1999;Suppl 4:269-272.
Gray B, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12(12):1711-1720.
Groenvold M. Methodological issues in the assessment of health-related quality of life in palliative care trials. Acta Anaesthesiol Scand. 1999;43(9):948-953.
Han SS, et al. A comparison of the long-term functional outcomes of standard pancreatoduodenectomy and pylorus-preserving pancreatoduodenectomy. Hepatogastroenterology. 2007;54(78):1831-1835.
Heffernan N, et al. Measuring health-related quality of life in patients with hepatoobiliary cancers: the functional assessment of cancer therapy: hepatobiliary questionnaire. J Clin Oncol. 2002;20(9):2229-2239.
Holland JC, et al. Comparative psychological disturbance in patients with pancreatic and gastric cancer. Am J Psychiatry. 1986;43(8):982-986.
Izsak FC, Medalie JH. Comprehensive follow-up of carcinoma patients. J Chronic Dis. 1971;24(2):179-191.
Kalinowski M, et al. Selective internal radiotherapy with yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single-center study. Digestion. 2009;79(3):137-142.
Karnofsky DA, Burchenal J. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. New York: Columbia University Press; 1949.
Kassa S, Loge JH. Quality-of-life assessment in palliative care. Lancet Oncology. 2002;3:175-182.
Kavadas V, et al. Development of an EORTC disease-specific quality of life questionnaire for use in patients with liver metastases from colorectal cancer. Eur J Cancer. 2003;39(9):1259-1263.
Kim JH, et al. Self-expanding coil stent with a long delivery system for palliation of unresectable malignant gastric outlet obstruction: a prospective study. Endoscopy. 2001;33(10):838-842.
Koller M, Lorenz W. Survival of the quality of life concept. Br J Surg. 2002;90(10):1175-1177.
Koninger J, et al. Surgical palliation in patients with pancreatic cancer. Langenbecks Arch Surg. 2007;392(1):13-21.
Kostro J, Sledziski Z. Quality of life after surgical treatment of pancreatic cancer. Acta Chir Belg. 2008;108(6):679-684.
Langenhoff BS, et al. Quality of life as an outcome measure in surgical oncology. Br J Surg. 2001;88(5):6436-6452.
Levine MN, Ganz PA. Beyond the development of quality-of-life instruments: where do we go from here? J Clin Oncol. 2002;20(9):2215-2216.
Linden W, et al. Development and validation of a psychosocial screening instrument for cancer. Health Qual Life Outcomes. 2005;3:54.
Maisey NR, et al. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2001;38(10):1351-1357.
Martin RC, et al. Health-related quality of life: return to baseline after major and minor liver resection. Surgery. 2007;142(5):676-684.
Matulonis UA. End of life issues in older patients. Semin Oncol. 2004;31(2):274-281.
McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1(5):373-378.
Mehta S, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20(2):239-242.
Meyers CA, Weitzner MA. Neurobehavioral functioning and quality of life in patients treated for cancer of the central nervous system. Curr Opin Oncol. 1995;7(3):197-200.
Miner TJ, et al. A prospective evaluation of patients undergoing surgery for the palliation of an advanced malignancy. Ann Surg Oncol. 2002;9(7):696-703.
Muller-Nordhorn J, et al. Health-related quality of life in patients with pancreatic cancer. Digestion. 2006;74(2):118-125.
Nakakura EK, Warren RS. Palliative care for patients with advanced pancreatic and biliary cancers. Surg Oncol. 2007;16(4):293-297.
Nguyen T, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7(1):1-9.
Nieveen van Dijkum EJ, et al. Quality of life after curative or palliative surgical treatment of pancreatic and periampullary carcinoma. Br J Surg. 2005;92(4):471-477.
Ortner MEJ, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125(5):1355-1363.
Padilla GV, et al. Quality of life index for patients with cancer. Res Nurs Health. 1983;6(3):117-126.
Pallis AG, Mouzas IA. Instruments for quality of life assessment in patients with gastrointestinal cancer. Anticancer Res.. 2004;24(3B):2117-2121.
Passik SD, Breitbart WS. Depression in patients with pancreatic carcinoma. Diagnostic and treatment issues. Cancer. 1996;78(Suppl 3):615-626.
Passik SD, Kirsch KL. The importance of quality-of-life endpoints in clinical trials to the practicing oncologist. Hematol Oncol Clin North Am. 2000;14(4):877-886.
Passik SD, Roth AJ. Anxiety symptoms and panic attacks preceding pancreatic cancer diagnosis. Psychooncology. 1999;8(3):268-272.
Perez DJ, et al. A comparison of time trade-off and quality of life measures in patients with advanced cancer. Qual Life Res. 1997;6(2):133-138.
Poon RT, et al. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 2001;136(6):693-699.
Portenoy RK, et al. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
Robson PC, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol. 2010;17(9):2303-2311.
Ruers TJ, et al. Comparison between local ablative therapy and chemotherapy for non-resectable colorectal liver metastases: a prospective study. Ann Surg Oncol. 2007;14(3):1161-1169.
Sajid M, et al. Use of health-related quality of life tools in hepatobiliary surgery. Hepatoobiliary Pancreat Dis Int. 2008;7(2):135-137.
Saluja SS, et al. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. 2008;6(8):944-950.
Scheingraber S, et al. Comparison between a general and a disease-specific health-related quality-of-life questionnaire in patients after pancreatic surgery. J Hepatoobiliary Pancreat Surg. 2005;12(4):290-297.
Schell SR, et al. Hepatic artery embolization for control of symptoms, octreotide requirements, and tumor progression in metastatic carcinoid tumors. Gastrointest Surg. 2002;6(5):664-670.
Schipper H, et al. Measuring the quality of life of cancer patients: The Functional Living Index-Cancer: Development and validation. J Clin Oncol. 1984;2(5):472-483.
Schmidt U, et al. Quality of life and functional long-term outcome after partial pancreatoduodenectomy: pancreatogastrostomy versus pancreatojejunostomy. Ann Surg Oncol. 2005;12(6):467-472.
Schniewind B, et al. Quality of life after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head. Br J Surg. 2006;93(9):1099-1107.
Schoffski P, et al. Quality of life predicts for both response and survival in patients treated for metastatic colorectal cancer: results of a randomized phase III study. Proc Am Soc Clin Oncol. 1996;5:213.
Shaw C, et al. Long-term quality of life following pancreaticoduodenectomy. Hepato-gastroenterology. 2005;52(63):927-932.
Singhal D, et al. Palliative management of hilar cholangiocarcinoma. Surg Oncol. 2005;14(2):59-74.
Spitzer WO, et al. Measuring the quality of life of cancer patients: A concise QL-index for use by physicians. J Chronic Dis. 1981;34(12):585-597.
Sprangers MA. Quality-of-life assessment in oncology: achievements and challenges. Acta Oncol. 2002;41(3):229-237.
Steel J, et al. Quality of life in patients diagnosed with primary hepatocellular carcinoma: hepatic arterial infusion of cisplatin versus 90-yttrium microspheres (Therasphere). Psychooncology. 2004;13(2):73-79.
Steel JL, et al. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatoobiliary carcinoma. Ann Oncol. 2006;17(2):304-312.
Sun V, et al. Symptom concerns and quality of life in hepatoobiliary cancers. Oncol Nurs Forum. 2008;35(3):45-52.
Tassinari D, et al. Surrogate outcomes in quality-of-life research: where will we end up? J Clin Oncol. 2003;9:1894-1895.
Van Heek NT, et al. The need for a prophylactic gastrojejunostomy for unresectable periampullary cancer: a prospective randomized multicenter trial with special focus on assessment of quality of life. Ann Surg Oncol. 2003;238(6):894-902.
Velanovich V. The quality of quality of life studies in general surgical journals. J Am Coll Surg. 2001;193(3):288-296.
Wang YB, et al. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res. 2007;16(3):389-397.
Wehrmann T, et al. Endoscopic debridement of paraesophageal, mediastinal abscesses: a prospective case series. Gastrointest Endosc. 2005;62(3):344-349.
Wessels FJ, Schell SR. Radiofrequency ablation treatment of refractory carcinoid hepatic metastases. J Surg Res. 2001;95(1):8-12.
Whalen GF, Ferrans CE. Quality of life as an outcome in clinical trials and cancer care: a primer for surgeons. J Surg Oncol. 2001;77(4):270-276.
Wiedmann M, et al. Photodynamic therapy in patients with non-resectable hilar cholangiocarcinoma: 5-year follow-up of a prospective phase II study. Gastrointest Endosc. 2004;60(1):68-75.
World Health Organization, 1947: Constitution, World Health Organization, 1-29.
You YN, et al. Pancreatoduodenal surgery in patients with multiple endocrine neoplasia type 1: operative outcomes, long-term function, and quality of life. Surgery. 2007;142(6):829-836.
Yount S, et al. Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancers: The FACT hepatobiliary symptom index. J Pain Symptom Manage. 2002;24(1):32-44.