Postpartum Headache
Alison Macarthur BMSc, MD, FRCPC, MSc
Chapter Outline
Postpartum headache is the complaint of cephalic, neck, or shoulder pain occurring during the first 6 weeks after delivery. The incidence of postpartum headache throughout the 6-week postpartum period has not been followed in a prospective manner. However, information is available from an evaluation of women during the first week postpartum,1 from a database that recorded symptoms during pregnancy and in the first week after delivery,2 and from a survey of women at 5 months and 1 year postpartum.3 Goldszmidt et al.1 evaluated 985 women during the first week postpartum and found a 38.7% incidence of headache. The median time to onset of symptoms was 2 days, and the median duration of headache was 4 hours. Benhamou et al.2 examined information collected on pregnant women who delivered at their institution during a 2-year period; exclusion criteria included recognized dural punctures, preterm deliveries, multiple gestation, and/or elective cesarean delivery. Headache was reported by 12% of 1058 patients who had epidural anesthesia without dural puncture and by 15% of 140 patients who delivered without epidural anesthesia. Saurel-Cubizolles et al.3 surveyed 1,286 postpartum women and found the incidence of headache was 22% and 42% at 5 and 12 months, respectively.
Post–dural puncture headache (PDPH) is one of the most common postpartum complications of neuraxial anesthesia. However, physicians and nurses should be aware that a dural puncture is only one of many causes of postpartum headache (Table 31-1). Most headaches are benign or do not require immediate attention; however, the timely diagnosis of some headaches (e.g., cortical vein thrombosis, subdural hematoma) is critical to good outcomes. Knowledge of both benign and nonbenign headaches is important for the anesthesiologist who is frequently the first physician to evaluate the patient with postpartum headache. Difficult diagnostic problems may require the opinion of a neurologist. The purpose of this chapter is to discuss the differential diagnosis of postpartum headache and PDPH in particular.
TABLE 31-1
Differential Diagnosis of Postpartum Headache
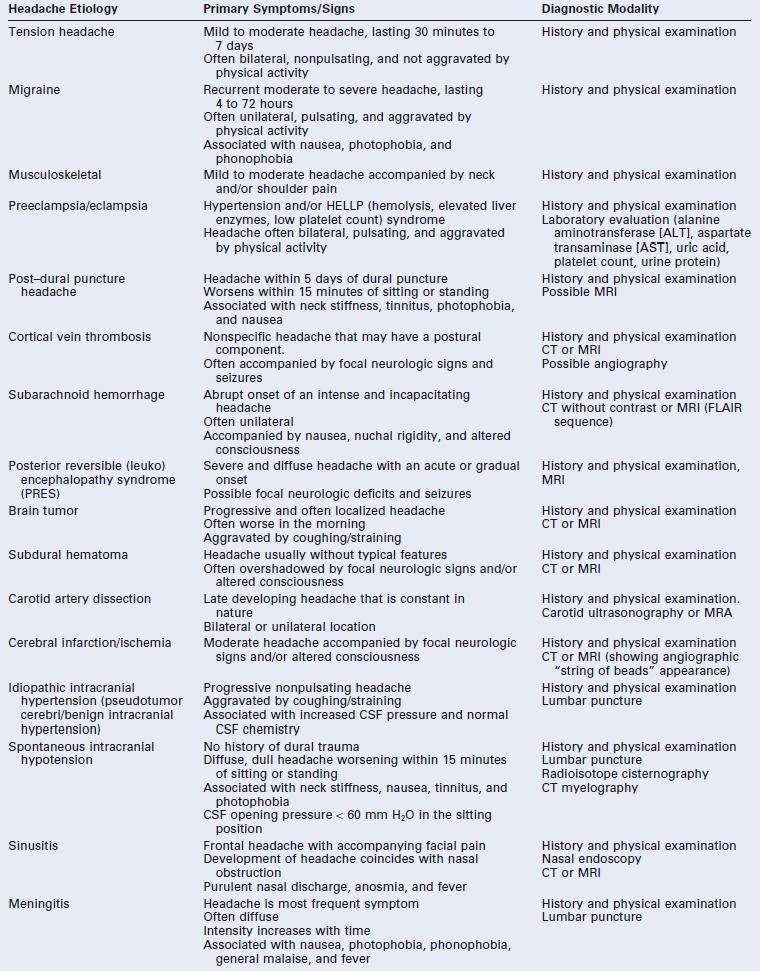
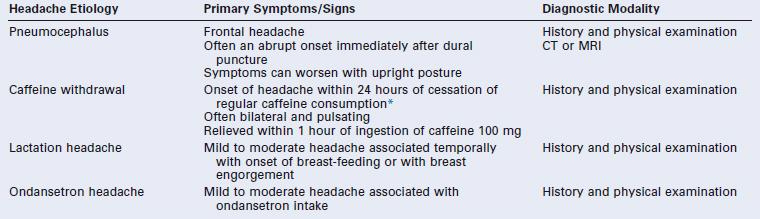
* The International Classification of Headache Disorders-II criteria state that caffeine-withdrawal headache occurs on cessation of ≥ 200 mg daily caffeine consumption for more than 2 weeks.4 However, others have suggested that caffeine-withdrawal headache may occur after as little as 3 days’ exposure to 300 mg/day or 7 days’ exposure to 100 mg/day.35
CSF, cerebrospinal fluid; CT, computed tomography; FLAIR, fluid-attenuated inversion recovery; MRA, magnetic resonance angiogram; MRI, magnetic resonance imaging.
Differential Diagnosis of Postpartum Headache
The classification of headaches follows the International Classification of Headache Disorders (ICHD), created in 1988 by the Headache Classification Subcommittee of the International Headache Society. This classification system, which has been updated (ICHD-II), identifies two broad categories of headaches: primary and secondary (Box 31-1).4 Primary headaches are classified as migraine, tension-type headache, cluster headache and trigeminal autonomic cephalalgia, or other. Secondary headaches are attributable to a specific underlying pathologic process. Primary headaches are 20 times more common than secondary headaches among women in the first week postpartum.1
After delivery, women frequently suffer from headache. Stella et al.5 retrospectively reviewed 5 years of hospital records to identify women who had postpartum headaches between 24 hours and 6 weeks after delivery. Ninety-five women met these criteria, and although the incidence of postpartum headache could not be calculated, the study did identify some important features of postpartum headache. Most women (82%) were still in the hospital at time of onset of the headache. Additionally, the demographics of the study population largely reflected the general obstetric population; the mean age was 25 years, 87% of the women had received some type of neuraxial analgesia/anesthesia, and 29% had a cesarean delivery.
Primary Headaches
The postpartum patient can present with a recurrence of her known primary disorder or with the first manifestation of a primary condition. Patients with a history of headache disorders typically are diagnosed with one of the four major types of primary headaches. The most common postpartum headaches are tension-type and migrainous headaches, which account for almost two thirds of headaches during this period.1,2 Tension-type headaches are often circumferential and constricting, can be associated with scalp tenderness, and are usually of mild to moderate severity.
Migraine
Migrainous headaches (or migraines) are defined as recurring cranial pain lasting 4 to 72 hours, often with typical features such as pulsating pain in a unilateral location, nausea, and photophobia.4 Headache with aura is a subtype of migraine that is characterized by focal neurologic symptoms preceding the headache. The prevalence of migraine is approximately 17% in the female population (three times more common than in men) and is more common in patients between 30 and 50 years or age.6 Pregnancy has an ameliorating effect on migraine frequency in the majority of sufferers. However, symptoms may recur soon after delivery, with reports of 34% within the first week postpartum and 55% within the first month.7 Generally, the symptoms are similar to their typical pattern, although often milder and less often unilateral. It is rare for a migraine to manifest for the first time during the postpartum period. There appears to be an association between migraines and preeclampsia, which may reflect an underlying predisposition to cerebral ischemic injury.8
Secondary Headaches
A common secondary headache in the postpartum period is the musculoskeletal headache, exacerbated by the maternal physical exertion of labor and associated sleep deprivation. This headache has accompanying neck and shoulder pain without a history of dural puncture. Approximately 11% to 14% of postpartum headaches are diagnosed as musculoskeletal.1 Other causes of secondary headache are discussed in the following paragraphs.
Hypertension
Hypertensive disorders of pregnancy, including preeclampsia, are commonly associated with headaches. Eclampsia is a form of hypertensive encephalopathy that includes headache, visual disturbances, nausea, vomiting, seizures, stupor, and sometimes coma. Seizures may occur in the absence of severe hypertension. Headache is a serious premonitory sign, being present in over 50% of women in whom eclampsia develops.9 Other hypertensive disorders, with or without superimposed preeclampsia, are also associated with headaches both antepartum and postpartum and may lead to encephalopathy. The diagnosis can be complex if the parturient’s labor and delivery course is complicated by a dural puncture.10
Posterior Reversible Leukoencephalopathy Syndrome
Posterior reversible (leuko)encephalopathy syndrome (PRES) was described in 1996 after recognition of consistent symptom presentation and brain magnetic resonance imaging (MRI) findings in a diverse group of patients. Conditions associated with PRES include preeclampsia, uremia, hemolytic-uremic syndrome, and exposure to immunosuppressant drugs.11 Approximately 25% of cases of PRES occur during pregnancy or in the immediate postpartum period. PRES symptoms include headache, seizures, altered mental status, visual changes, and, occasionally, focal neurologic deficits.12
The neuroradiologic features of PRES include symmetric areas of cerebral edema, predominantly involving the white matter regions of the posterior circulation (occipital lobes, posterior parietal and temporal lobes) (Figure 31-1). The pathophysiology of PRES is similar to that of hypertensive encephalopathy in that altered cerebrovascular regulation causes loss of blood-brain barrier integrity. The accompanying vasogenic edema can be reversed by prompt recognition and supportive therapy (e.g., cessation of provocative medications, aggressive treatment of hypertension, seizure prophylaxis). However, irreversible cytotoxic edema with permanent neurologic damage can occur if the initial disorder is not diagnosed early. This syndrome often manifests in the postpartum period, frequently in conjunction with preeclampsia. Distinguishing PRES from preeclampsia or eclampsia is complex because headache, seizures, and focal neurologic deficits, such as temporary loss of vision, are common symptoms of both disorders.13,14 Typical features that distinguish PRES from other postpartum headaches include seizures and focal neurologic deficits, such as temporary loss of vision. Definitive diagnosis is made with MRI.
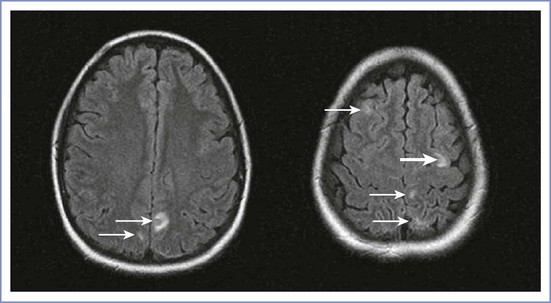
FIGURE 31-1 Posterior reversible (leuko)encephalopathy syndrome (PRES). Axial fluid-attenuated inversion-recovery (FLAIR) images demonstrate foci of abnormally increased T2 signal (arrows) in the subcortical white matter and cortex of the frontal and parietal lobes involving both anterior and posterior circulations. (From Long TR, Hein BD, Brown MJ, et al. Posterior reversible encephalopathy syndrome during pregnancy: seizures in a previously healthy parturient. J Clin Anesth 2007; 19:145-8.)
Cortical Vein Thrombosis
The incidence of cerebral cortical vein thrombosis is increased during pregnancy and in the puerperium, and is estimated to be 10 to 20 per 100,000 deliveries in developed countries. The incidence appears higher in developing countries (e.g., 450 per 100,000 deliveries in India).15 Often it is difficult to distinguish cortical vein thrombosis from PDPH, because the headache of cortical vein thrombosis may have a postural component. Preceding dural punctures have been reported, and it has been hypothesized that the reductions in cerebrospinal fluid (CSF) pressure and cerebral vasodilation that accompany dural puncture predispose to thrombosis development.16 Associated features may include focal neurologic signs, seizures, and coma. Cerebral infarction may ensue if diagnosis is delayed. Diagnosis is best confirmed by MRI, because computed tomography (CT) appears to identify only one third of cases.17 Treatment of cortical vein thrombosis largely is symptomatic, with the aim of preventing seizures. Anticoagulation therapy is commonly used to treat these patients, with observational and randomized trial studies indicating better outcomes.18
Cerebral Infarction/Ischemia
Cerebral arterial insufficiency is one cause of stroke in pregnancy with an estimated incidence of 4 to 11 per 100,000 deliveries.19 Approximately half of the events occur in the peripartum period, and the clinical presentation often comprises a sudden onset of a nonpostural thunderclap headache, vomiting, seizures, and focal neurologic deficits. Postpartum cerebral angiopathy has been detected with the aid of cerebral angiography, in which a characteristic “arterial beading” pattern indicative of arterial spasm is evident.20 Initial CT and MRI findings are often normal, and intracranial Doppler or angiographic investigations may be necessary to diagnose the arterial ischemia or infarct. Treatment is supportive and may include vasodilator therapy.
Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) occurs secondary to aneurysmal or nonaneurysmal causes. The risk for SAH due to nonaneurysmal causes appears to be increased during pregnancy. A 2012 U.S. population–based study estimated an overall incidence of peripartum SAH at 5.8 per 100,000 deliveries.21 The classic presentation consists of the sudden onset of a severe headache that is unlike any previous headache. Associated symptoms may be present, such as decreased level of consciousness and focal deficits. Pregnancy may increase the risk for bleeding because of increased blood volume and hormonal changes that affect arterial integrity. Two thirds of all cases in pregnancy occur in the postpartum period, but maternal mortality appears to be less than in nonpregnant patients.21 Other factors associated with SAH during pregnancy include advanced maternal age, African American race, hypertensive disorders, smoking history, alcohol abuse, and intracranial venous thrombosis. Suspicion of SAH necessitates urgent investigation by CT scan, because nonsurgical therapies (e.g., endovascular ablation) are available and long-term sequelae can be minimized.
Subdural Hematoma
In rare instances, dural puncture is associated with the subsequent development of a subdural hematoma (see later discussion). In several case reports, the identification of the subdural hematoma was preceded by symptoms of a PDPH.22 Dural puncture results in leakage of CSF and decreased intracranial pressure (ICP). Presumably, the reduction in ICP causes stress on bridging cerebral vessels, which may precipitate bleeding. Neurologic signs of subdural hematoma are variable but include evidence of increased ICP (e.g., headache, somnolence, vomiting, confusion) and focal abnormalities.
Carotid Artery Dissection
A rare, vascular cause of postpartum headache is spontaneous carotid artery dissection. Borelli et al.23 reviewed the 19 known published cases of postpartum carotid artery dissection. The mean interval from delivery to headache onset was 9.3 days. The headaches were constant in character and both unilateral and bilateral. Stricken woman appeared older (mean age, 35 years) than the average parturient. Diagnosis was made after carotid vessel ultrasonography or magnetic resonance angiography.
Brain Tumor
Intracranial tumors may manifest as postpartum headache. Headache that is dull rather than throbbing may be an early feature of a brain tumor. Nausea, vomiting, seizures, and/or focal neurologic signs may be present. Neurologic examination may reveal evidence of increased ICP. Case reports suggest that atypical presentation of the headache, either with persisting headache symptoms in the supine position or exacerbation after epidural blood patch, should prompt further neuroradiologic investigations.24,25
Idiopathic Intracranial Hypertension
Parturients with idiopathic intracranial hypertension (i.e., increased ICP in the absence of a mass lesion, also known as pseudotumor cerebri or benign intracranial hypertension) have headache and visual disturbances, usually in the antepartum period. The features of the postpartum headache of pseudotumor cerebri mimic the usual chronic headache symptoms experienced by the patient. The diagnosis largely is one of exclusion (see Chapter 49). Treatment involves reduction of CSF pressure, either with glucocorticoids, carbonic anhydrase inhibitors, diuretics, or serial lumbar punctures. Case reports describe the use of an intrathecal catheter for labor analgesia26 and administration of epidural blood patch for PDPH in patients with idiopathic intracranial hypertension.27
Spontaneous Intracranial Hypotension
Spontaneous intracranial hypotension develops because of CSF leakage secondary to dural tears. The tears usually occur at the thoracic spinal level and are not associated with prior spinal intervention.28 Diagnosis requires radioisotope cisternography and CT myelography, which may also identify the level of the leak. Presentation of this disorder is identical to that of PDPH, because the pathophysiology is the same. The only difference is the lack of a prior neuraxial procedure in spontaneous intracranial hypotension. One case report has described the development of a postural headache 4 days after a spontaneous vaginal delivery without neuraxial anesthesia.29 The patient was found to have a cervicothoracic dural leak.
Pneumocephalus
The subdural or subarachnoid injection of air used for identification of the epidural space may be associated with the sudden onset of severe headache, sometimes accompanied by neck pain, back pain, or changes in mental status.30 Headache symptoms can mimic PDPH in that they are worse in the sitting position and may be relieved by lying down. Radiologic studies confirm the presence of intracranial air; the headache typically resolves over the first week. Administration of oxygen by nasal cannula or face mask may hasten resorption of the air and speed recovery, although this has yet to be proven with pneumocephalus after neuraxial anesthesia.31
Meningitis
The severe headache of meningitis typically manifests within the first several postpartum days (see Chapter 32). It is accompanied by fever, nuchal rigidity, and the presence of Kernig and Brudzinski signs. Lethargy, confusion, vomiting, seizures, and a rash also may occur. The most common pathogen is Streptococcus viridans.32,33 These organisms are found in the upper airway and vagina; the causative organism has been linked to the oropharyngeal flora of the proceduralist in a number of reports of iatrogenic meningitis after dural puncture procedures. Aseptic technique during the neuraxial procedure, including donning of a mask by the proceduralist, is of paramount importance. The diagnosis is confirmed by examination and CSF culture.
Sinusitis
Headache caused by inflamed paranasal sinuses is associated with purulent nasal discharge and, occasionally, fever. Pain may be unilateral or bilateral, depending on the extent of the disease, and the skin over the affected sinus may be tender. Frontal sinus infection causes headache in the frontal region. Ethmoidal and sphenoidal sinus infections cause periorbital pain, and maxillary sinus infection may cause diffuse facial discomfort. The sinuses fill overnight, and pain typically is worse on awakening. Pain improves in the upright position, which assists drainage.34
Caffeine Withdrawal
The withdrawal of caffeine may lead to headache, increased fatigue, and anxiety. Caffeine withdrawal headaches may occur after just 3 days’ exposure to 300 mg/day or 7 days’ exposure to 100 mg/day of caffeine.35 Normal-sized caffeinated drinks usually contain 50 to 100 mg of caffeine per serving. Although caffeine withdrawal headache has not been documented as a cause of postpartum headache, if the parturient has been drinking beverages with caffeine during the pregnancy the diagnosis should be considered.
Lactation Headache
Askmark and Lundberg36 reported episodes of intense headache during periods of breast-feeding in a woman known to suffer from migraine. Onset of headaches occurred within the first few minutes of breast-feeding, and the headaches resolved after cessation of nursing. The headaches were associated with an increase in plasma vasopressin concentration. Headaches have also been described in women with breast engorgement who either have elected not to breast-feed or have reduced the frequency of breast-feeding.37
Ondansetron
Sharma and Panda38 reported a case in which a woman received ondansetron for nausea and vomiting after uneventful spinal anesthesia for cesarean delivery. Several hours later she developed a severe frontal headache that was worse in the upright position and in the morning and evening hours. The symptoms abruptly stopped after the discontinuation of ondansetron.38 Headache is a common side effect of ondansetron (incidence, 3% to 17%), owing to its antagonism of serotonin 5-HT3 receptors, and should be considered in the differential diagnosis of postpartum headache.
Post–Dural Puncture Headache
Incidence
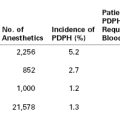
PDPH may occur after intentional dural puncture with a spinal needle or unintentional dural puncture with an epidural or other needle. A meta-analysis of studies of PDPH in obstetric patients (n = 328,769) calculated a pooled risk for unintentional dural puncture with any epidural needle of 1.5% (95% confidence interval [CI], 1.5% to 1.5%).39 After a dural puncture with an epidural needle, the risk for PDPH was 52.1% (95% CI, 51.4% to 52.8%) (Figure 31-2). The rate of PDPH after dural puncture with spinal needles ranged between 1.5% and 11.2%, depending on the needle size and type of needle (see later discussion) (Table 31-2).39–43 Although PDPH is often considered a “minor” complication of dural puncture, it was the cause of 14% of obstetric claims in the American Society of Anesthesiologists (ASA) Closed-Claims Project database (see Chapter 33).
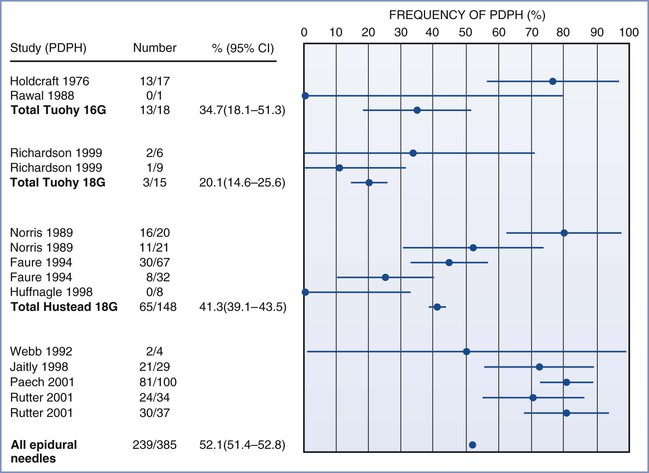
FIGURE 31-2 Meta-analysis of post–dural puncture headache (PDPH) frequency for epidural needles in the obstetric population. The dots represent the percentages of patients experiencing the event. The horizontal lines represent the 95% confidence interval (CI). (From Choi PT, Galinski SE, Takeuchi L, et al. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetric studies. Can J Anaesth 2003; 50:460-9.)
TABLE 31-2
Frequency of Post–Dural Puncture Headache in Obstetric Patients According to Spinal Needle Design
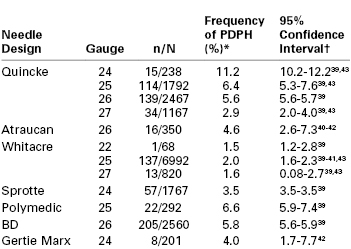
* Estimates based on binomial probability estimation.
† Superscript numbers indicate reference citations at the end of the chapter.
n, number of headaches; N, total number of procedures; PDPH, post–dural puncture headache.
Symptoms
Patients typically experience headache pain in the frontal and occipital regions, but location is not diagnostic. Pain often radiates to the neck, which may be stiff. Some women have a mild headache that permits full ambulation. In others, pain is severe and incapacitating. Symptoms are worse in the upright position and are usually relieved in the horizontal position. Abdominal compression may relieve pain in some patients. The ICHD-II defines PDPH as occurring within 15 minutes of moving to an upright position (sitting or standing) and resolving within 15 minutes of moving to the supine position, and requires one of the following symptoms to be present: headache, neck stiffness, tinnitus, hypacusis, photophobia, or nausea.4
Lybecker et al.44 reported the incidence of these symptoms in a prospective study of 75 nonobstetric patients with PDPH (Table 31-3). Cranial nerve palsy, thought to be secondary to nerve traction due to low CSF volume, is occasionally associated with PDPH. The sixth cranial nerve is most susceptible to traction during its long intracranial course. The traction results in failure of the involved eye to abduct, and patients may have diplopia. Hearing loss is usually in the low-frequency range and may be secondary to endolymph and perilymph imbalance and alteration of hair cell position in the inner ear.45 The risk for hearing loss appears to be increased with advanced age (> 40 years) and dural puncture with larger-gauge needles. Other rare symptoms associated with PDPH include seizures,46,47 vertigo,48 bilateral forearm pain,49 abdominal pain, and diarrhea.50 In all these case reports the headache and associated symptoms cleared after therapy with an epidural blood patch.
TABLE 31-3
Symptoms Associated with Post–Dural Puncture Headache
| Symptom | Incidence (%) |
| Nausea | 60 |
| Vomiting | 24 |
| Neck stiffness | 43 |
| Ocular* | 13 |
| Auditory† | 12 |
* Ocular symptoms include photophobia, diplopia, and difficulty in accommodation.
† Auditory symptoms include hearing loss, hypacusis, and tinnitus.
Data from Lybecker H, Djernes M, Schmidt JF. Postdural puncture headache (PDPH): onset, duration, severity, and associated symptoms. An analysis of 75 consecutive patients with PDPH. Acta Anaesthesiol Scand 1995; 39:605-12.
Onset and Duration
Headache typically occurs on the first or second day after dural puncture; by ICHD-II criteria, it must appear within 5 days of dural puncture.4 However, Choi et al.39 found that PDPH can occur up to 7 days after dural puncture, and one case report identified a woman who developed a PDPH 12 days after labor neuraxial analgesia.51 Ninety-five percent of PDPH headaches last less than a week. The National Obstetric Anaesthesia Database project of the Obstetric Anaesthetists’ Association demonstrated that 75% of 975 women with PDPH had difficulty with performing activities of daily living.52 Rarely, symptoms may persist for months or even years.53 Webb et al.54 found that 18% of women who sustained a dural puncture with a 17-gauge Tuohy needle suffered from chronic headaches compared with 5% of women in a matched cohort who did not sustain dural puncture with an epidural needle. These findings require confirmation, but they suggest that there may be long-term sequelae from dural puncture with a large-gauge needle.
Imaging
Imaging investigations are not routinely recommended for the postpartum patient with a PDPH unless the symptoms suggest other diagnoses or the diagnosis of PDPH is in doubt. Contrast-enhanced MRI is the method of choice to study the meninges and has revealed characteristic findings of PDPH.55,56 These findings include (1) marked, diffuse pachymeningeal thickening and enhancement; (2) compression of the ventricles; (3) caudal displacement of the brain, brainstem, and optic chiasm; (4) cerebellar ectopia; (5) pituitary enlargement and enhancement; (6) and expansion of the superior sagittal sinus. The enlarged venous sinus may represent compensatory venous expansion in response to low CSF pressure. The presence of intracranial air on CT imaging (after using a loss-of-resistance to air technique to identify the epidural space) may help differentiate headache due to pneumocephalus from a low-CSF pressure headache.57
Pathophysiology
Debate continues regarding the precise etiology of PDPH symptoms. The original theory was that pain-sensitive nerve fibers were stimulated by a downward shift of the brain secondary to a loss of CSF volume. German surgeon August Bier58 is credited with the first description of successful spinal anesthesia and PDPH after his pioneering work on spinal anesthesia with cocaine. Bier and his assistant, Hildebrandt, performed spinal anesthesia on each other; using blows to the shin with an iron hammer and application of a burning cigar to the skin they demonstrated adequate sensory blockade.58 Both experienced severe PDPH. The assistant forced himself to work the next day, but Bier stayed home for 9 days. Bier suggested the PDPH might be caused by CSF loss. Today there is no doubt that leakage of CSF initiates the syndrome. Kunkle et al.59 consistently produced PDPH by draining 20 mL of CSF from volunteers. Symptoms were immediately relieved by subarachnoid injection of saline to restore initial CSF pressure.
Total CSF volume is estimated to be 150 mL, and the production rate is approximately 0.35 mL/min or a daily rate of 500 mL. The rate of CSF leakage through a dural hole may exceed the rate of CSF production. If this occurs, low CSF pressure results in a loss of the cushioning effect provided by intracranial fluid.
CSF pressure during labor is normal between contractions but increases significantly during painful contractions and expulsive efforts. Effective epidural analgesia attenuates this increase in CSF pressure.60 In a study of five women with unintentional dural puncture, epidural pressures were normal preceding the development of headache.61 However, with development of headache symptoms, the mean epidural pressure measurements were found to decrease significantly.
Not all patients with PDPH symptoms have decreased CSF pressure, and not all patients with a significant CSF leak experience symptoms. The pain of PDPH may be caused, in part, by an increase in cerebral blood flow (and cerebral vasodilation) as a consequence of low CSF pressure or volume. This phenomenon has been observed in animals.62,63 The inverse relationship between intracranial blood volume and CSF volume reflects the body’s effort to maintain a constant intracranial volume.64 The lumbar CSF compartment is a dynamic structure and acts as a reservoir for intracranial CSF volume adjustment.65 The occurrence of cerebral vasodilation may explain the relief of headache symptoms with vasoconstrictors such as caffeine, theophylline, and sumatriptan.
Risk Factors
In a classic study of 10,098 spinal anesthetics published in 1956, Vandam and Dripps66 noted that three patient factors influenced the incidence of PDPH: age, gender, and pregnancy. The analysis did not allow determination as to whether these factors were independent risk factors. Subsequently, other risk factors for development of PDPH have been identified.
Age
Extensive evidence supports the observation that PDPH is uncommon in patients older than 60 years of age and is most common in patients who are younger than 40 years of age.67 In the elderly, the dura may be inelastic and less likely to gape after puncture. CSF leakage may be impeded by adhesions and calcification. The cerebrovascular system also may be less reactive in older patients. Further, this group is less active physically and older patients may be less likely to complain.
Gender
Vandam and Dripps66 observed a twofold higher incidence of PDPH after spinal anesthesia in women than in men (14% versus 7%, respectively). This difference may be related to differences in cerebrovascular reactivity, because it is well known that migraine headaches occur predominantly in females and are influenced by hormonal changes. Women may have enhanced vascular reactivity, or perhaps changes in cerebral blood flow are more likely to produce pain in women than in men. A meta-analysis of randomized clinical trials identified a twofold higher risk for PDPH in nonpregnant females than in males.68
Vaginal Delivery
Vandam and Dripps66 also observed a high incidence of PDPH (22%) in women who received spinal anesthesia for vaginal delivery. This high incidence may be a result of the mechanical consequences of expulsive efforts during the second stage of labor and/or postpartum hormonal changes in cerebrovascular reactivity. Expulsive efforts in the second stage may increase CSF leakage. This possibility has prompted some physicians to restrict maternal pushing after dural puncture and to use forceps to shorten the second stage of labor. In a 20-year retrospective review of 460 parturients who experienced unintentional dural puncture during labor at the Birmingham Maternity Hospital in the United Kingdom, Stride and Cooper69 did not identify a lower incidence of PDPH in women who underwent prophylactic forceps delivery than in those who had spontaneous vaginal delivery. In contrast, in a retrospective review of the records of 33 laboring women who had experienced unintentional dural puncture, Angle et al.70 found that women who were allowed to push were much more likely to develop PDPH (relative risk [RR], 7.4; 95% CI, 1.1 to 48.2) and also were more likely to require an epidural blood patch.
Morbid Obesity
In a retrospective study, Faure et al.71 found that morbidly obese patients are less susceptible to PDPH and are also less likely to receive an epidural blood patch for treatment of PDPH, suggesting either a reduced severity of PDPH or anesthesia provider reticence to perform an epidural blood patch in this patient population. Possible but unproven explanations of a lower incidence and severity of PDPH in obese patients include increased abdominal pressure (which may reduce the extent of CSF leakage) and/or reduced physical activity in these patients. Other confounding factors, such as differences in the mode of delivery (higher rate of cesarean delivery) and neuraxial opioid administration, may also play a role. In contrast to these findings, Webb et al.54 found that the median body mass index was greater in women who developed PDPH after unintentional dural puncture with a Tuohy needle than in women who did not develop PDPH.
History of Previous Post–Dural Puncture Headache
A history of PDPH after previous spinal anesthesia is associated with the development of PDPH with subsequent spinal anesthesia.72 A cohort of nonobstetric women with a history of previous spinal anesthesia was monitored prospectively after a second spinal anesthesia procedure. Those with a previous history of PDPH were 2.3 times more likely (95% CI, 1.0 to 5.1) to have a second PDPH than women without a history of headache (24.2% versus 10.6%, respectively). This finding suggests that certain individuals are predisposed to the development of PDPH.
Multiple Dural Punctures
Seeberger et al.73 found that multiple dural punctures significantly increased the risk for PDPH. Surgical patients who received a second spinal injection owing to failure of the initial spinal puncture had a 4.2% incidence of PDPH compared with a 1.6% incidence among patients who had a single dural puncture.
Neuraxial Anesthetic Technique
Technical factors related to the neuraxial technique influence the incidence of PDPH.
Epidural Needle Size/Design.
The high rate of PDPH after unintentional dural puncture with an epidural needle has led investigators to alter the epidural needle design or size in an attempt to reduce headache incidence or severity. Data on the success of this endeavor are conflicting. In an in vitro study using cadaver dura, no differences were found in fluid leak rate among punctures made with Hustead, Tuohy, Crawford, and Sprotte epidural needles.74 In contrast, in an in vivo study, Morley-Forster et al.75 observed a lower incidence of PDPH with the use of an 18-gauge Sprotte needle than with the use of the standard 17-gauge Tuohy needle, despite no difference in the unintentional dural puncture rate. A pilot study has examined the use of a 19-gauge Tuohy needle with 23-gauge epidural catheter.76
Spinal Needle Design.
Historically, the beveled Quincke needle (Figure 31-3) has been the most widely used needle for dural puncture for both diagnostic and anesthetic purposes (see Chapter 12). A modification of the Quincke needle, the Atraucan needle, has a cutting tip and a double bevel, which is intended to cut a small dural hole and then dilate it.40 Clinical experience with the Atraucan needle has been generally good,40,41 although studies appear to suggest that the use of this needle is associated with a higher risk for PDPH than the use of a non-cutting, pencil-point needle (see Table 31-2).42
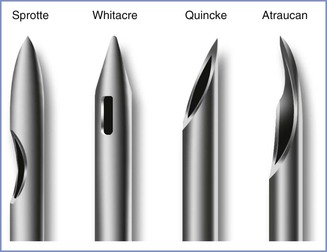
FIGURE 31-3 Designs of spinal needle tips (not to scale). (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
In 1951, Hart and Whitacre77 introduced a solid-tipped, pencil-point spinal needle with a lateral injection port, which is now known as the Whitacre design (see Figure 31-3). They believed that their new needle would stretch and separate rather than cut the dural fibers and result in a lower incidence of PDPH. Currently, both 25- and 27-gauge Whitacre needles are very popular, and studies have confirmed the anticipated low incidence of PDPH.39 A randomized comparison of 27-gauge Quincke and Whitacre needles in outpatients undergoing spinal anesthesia found a lower incidence of PDPH in the Whitacre needle group.78 In vitro evidence suggests that fluid leak through the dural puncture site is lower after use of a pencil-point needle than after use of a beveled needle.79 With the recognition that pencil-point spinal needle tips reduce the incidence of PDPH, other tip designs have appeared. In 1987, Sprotte et al.80 reported experience with the use of a new needle that was designed to reduce the risk for neural and dural trauma (see Figure 31-3). The Sprotte needle has a solid oval tip and a longer orifice than the Whitacre needle. The incidence of PDPH was 0.02% with its use in a heterogeneous patient population of almost 35,000 patients.80 Subsequent studies have shown the incidence of PDPH with the Sprotte needle is lower than that with Quincke needles of smaller gauge (see Table 31-2).39 There are currently many other non-cutting spinal needle products available and all are associated with a low PDPH rate.
Spinal Needle Size.
With the Quincke needle, the incidence and severity of PDPH are directly related to the size of the needle. The incidence of PDPH appears to be lower with a 27-gauge needle than with 25- and 26-gauge needles (see Table 31-2). A similar relationship may exist with pencil-point needles. When needles smaller than 27-gauge are used, the incidence of PDPH is very low, but technical problems with needle insertion and failure to produce adequate anesthesia are more common.81 Locating the epidural space before insertion of the spinal needle (e.g., using the epidural needle as an introducer needle) may improve the success rate with these fine-gauge needles.
The current popularity of spinal anesthesia in obstetric patients is largely a result of new needle technology, which has led to a reduction in the incidence of PDPH. Because of the morbidity associated with PDPH, every effort should be made to use a needle associated with a low incidence of PDPH (e.g., a small-gauge, non-cutting needle).82 There are times when urgency or body habitus will dictate the use of a larger needle, but there is seldom justification for using a Quincke needle larger than 27-gauge.
Direction of Bevel of the Quincke Needle.
Studies have confirmed that puncturing the dura mater with a Quincke needle bevel parallel to the long axis of the spine reduces the incidence of PDPH by 70% compared with a perpendicular orientation.83 An early study by Franksson and Gordh84 demonstrated that orientation of the bevel of a Quincke spinal needle parallel to the long axis of the spine produced less dural trauma than occurred when the bevel was inserted perpendicularly. These investigators thought that the dural fibers were predominantly longitudinal in direction. Electron microscopy has revealed that the dural structure is far more complex than originally proposed. Fink and Walker85 noted that the dura consists of multidirectional interlacing collagen fibers with both transverse and longitudinal elastic fibers. They suggested that the insertion of the needle with the bevel parallel to the long axis of the spine most likely results in less tension on the dural hole and, therefore, a smaller aperture with less CSF leak. In vitro studies of bevel orientation and fluid leak have provided conflicting results.86–88 Despite confusing anatomic evidence, clinical experience strongly supports insertion of the Quincke needle with the bevel parallel to the long axis of the spine.83
Direction of the Bevel of the Tuohy Needle.
Norris et al.89 examined two groups of women who received epidural anesthesia with a Tuohy needle. In one group the bevel was kept perpendicular to the long axis of the spine. In the other group the needle entered the epidural space with the bevel parallel to the long axis and the needle was then rotated 90 degrees before insertion of the catheter. The authors observed a decreased incidence of PDPH in the latter group. However, some anesthesiologists argue that rotation of the needle within the epidural space may increase the risk for unintentional dural puncture. Richardson and Wissler90 randomized laboring women to a cephalad or lateral orientation of the Tuohy bevel during epidural needle insertion. The needle was not rotated before insertion of the epidural catheter. There was no difference in dural puncture or PDPH rates, but catheter insertion was easier with a cephalad orientation of the bevel.
Midline or Paramedian Approach.
There is conflicting evidence as to whether the spinal needle approach affects the incidence of PDPH. Haftalvi91 reported no cases of PDPH in a retrospective survey of 4465 spinal anesthesia procedures. This investigator used a paramedian approach with a 20-gauge Quincke needle, and the skin was punctured 3 cm from the midline. He suggested that tangential dural puncture creates a dural flap and prevents PDPH. In contrast, Viitanen et al.,92 prospectively monitoring obstetric patients after administration of single-shot spinal analgesia for labor (27-gauge Quincke needle), observed PDPH in 3 of 85 (3.5%) patients in whom the midline approach was used, compared with 15 of 127 (11.8%) patients in whom the paramedian approach was used. Using a rigid paper cylinder model of the dura, Kempen and Mocek93 studied median and paramedian punctures with a 22-gauge Quincke needle in different orientations. With midline punctures, all entry and exit holes were of uniform size regardless of bevel orientation and no dural flaps were seen. After paramedian punctures, flaps formed when the needle bevel faced the cylinder surface with a near-tangential angle of perforation, suggesting it may be beneficial to insert the needle via the paramedian approach to reduce the incidence of PDPH. Currently, data are insufficient to recommend either the median or paramedian approach in regard to subsequent PDPH.
Skin Preparation.
In a nonrandomized, nonblinded study reported in a letter, Gurmarnik94 found that the removal of dried povidone-iodine solution from the skin before placement of the spinal needle was associated with a lower incidence of PDPH (6% versus 0%). This investigator recommended removal of the povidone-iodine solution before insertion of the spinal needle and suggested that chemical meningismus resulting from povidone-iodine introduced into the intrathecal space by the spinal needle contributed to PDPH. This finding has not been confirmed by other investigators. Simsa95 suggested that using an introducer needle so the spinal needle does not touch the povidone-iodine solution may be preferable and accomplish the same goal. (Editors’ note: We do not recommend removal of povidone-iodine [or other solutions used for skin antisepsis] before initiating neuraxial analgesia, because povidone-iodine works by desiccating bacteria, and removing the povidone-iodine reduces its antibacterial effect. Rather, the solution should be allowed to dry on the skin.)
Air versus Saline Method of Locating the Epidural Space.
The medium (air or saline) used for the loss-of-resistance technique to identify the epidural space has not clearly been shown to influence the incidence of PDPH. Many anesthesia providers have adopted the loss-of-resistance-to-saline technique in the belief that it is associated with a lower incidence of unintentional dural puncture and PDPH than the use of air.96 However, the data are inconsistent, and not all studies have found a difference.97,98 In a meta-analysis of prospective randomized studies comparing air to saline and the incidence of both unintentional dural puncture and PDPH, no difference in either outcome was found.99 Segal and Arendt100 suggested that a randomized study of air versus saline may not be the best approach to study headache rate; the study design may lead to overestimation of the difference between air and saline, should one exist, because it is impossible to blind the anesthesia provider to technique and it forces the provider to use a less preferred technique. They performed a retrospective study comparing air with saline; the rate of unintentional dural puncture was not different between groups. However, among anesthesia care providers who had a preference for one technique, use of their preferred technique was associated with fewer unintentional dural punctures. Thus, current data do not support a difference in the incidence of PDPH between air and saline used for the loss-of-resistance technique to identify the epidural space, and one should certainly not change techniques hoping it may decrease headache rates.
Choice of Local Anesthetic Drug for Spinal Anesthesia.
Naulty et al.101 reported that the use of bupivacaine-glucose or lidocaine-glucose for spinal anesthesia was associated with a higher incidence of PDPH than the use of tetracaine-procaine. They postulated that osmotic, cerebral irritant, and/or cerebrovascular effects of the glucose could be responsible for these findings. Whether this finding is applicable to other non–glucose-containing local anesthetic preparations, such as plain bupivacaine, is unknown.
Continuous Spinal Anesthesia.
A multicenter trial published in 2008 reexamined the safety and use of 28-gauge microcatheters for spinal labor analgesia.102 There was no difference in the incidence of PDPH (9% versus 4%, respectively) or epidural blood patch (5% versus 2%, respectively) between women randomly assigned to receive an intrathecal catheter and those who received an epidural catheter; however, the study was insufficiently powered to assess these outcomes. Spinal microcatheters are not currently commercially available in North America.
A 23-gauge spinal catheter inserted using a catheter-over-needle technique is available in North America. Three case series have evaluated this catheter for labor analgesia or cesarean delivery.103–105 The headache rate ranged from 9% to 29% in these series. This rate is greater than that reported in other studies using traditional epidural or combined spinal-epidural techniques.
Combined Spinal-Epidural Anesthesia.
The combined spinal-epidural (CSE) technique is widely used for both labor analgesia and cesarean delivery. Intuitively, it seems that the incidence of PDPH should be identical to, or greater than, that observed after single-shot spinal anesthesia with the same size and type of needle. However, the available evidence, primarily from observational studies, suggests that the risk for PDPH is not increased with the CSE technique.106–108 PDPH rates with the CSE technique in these three studies were 1.7%, 0.43%, and 1.4%, respectively, compared with 1.6%, 0.45%, and 0.8% for the epidural technique. Initial placement of the epidural needle facilitates precise dural puncture, and the subsequent increase in epidural space pressure after the epidural injection of local anesthetic may reduce CSF leakage. If the anesthesia provider is in doubt about correct epidural needle placement, a needle-through-needle dural puncture might resolve the issue and prevent unintentional dural puncture with a large-gauge epidural needle.
Complications
The immediate problems associated with post–dural puncture headaches include (1) the inability to perform activities of daily living, such as providing care for the newborn; (2) an extended duration (almost one full day) of hospitalization; and (3) a higher number of emergency department visits, with almost 40% of patients returning for more than one visit.109 Although these complications are very bothersome to the patient, they are short-lived and do not result in long-term morbidity. However, rare but serious complications may occur after dural puncture and PDPH.
Zeidan et al.110 reviewed the published reports of subdural hematoma after dural puncture. They found that subdural hematoma was associated with new neurologic symptoms in addition to changing headache characteristics. The proposed mechanism of subdural hematoma development is ongoing intracranial hypotension leading to caudal movement of the brain and rupture of fragile, bridging subdural veins. These cases have been managed expectantly, with an epidural blood patch, as well as with neurosurgical decompression.
Dural sinus thrombosis has been documented after unintentional dural puncture and treatment of PDPH with an epidural blood patch.111 Responsible factors may be cerebral venous dilation (associated with decreased ICP) and the hypercoagulability that occurs during pregnancy. Therapy may include anticoagulation.18
Diplopia or hearing loss after dural puncture, secondary to cranial nerve dysfunction, may be permanent, even after successful treatment of the PDPH with an epidural blood patch.112 A review of 95 cases of neurapraxia or axonotmesis of the ocular cranial nerves (oculomotor, trochlear, and abducens nerves) concluded that symptoms may last from 2 weeks to 8 months but that almost 90% of patients recover.113
Three studies have focused on the long-term morbidity arising from unintentional dural puncture or spinal anesthesia in obstetric patients.54,114,115 Women delivering between 1978 and 1985,114 between 1991 to 1996,115 and between 2009 to 201054 were asked to recall symptoms beginning after their deliveries, including back pain and headache. The responses of women with unintentional dural punctures or spinal anesthesia were compared with women who had uneventful procedures. The first study found that 18% of women with unintentional dural puncture had complaints of frequent headaches or neck ache compared with only 7% of women experiencing uneventful neuraxial procedures.114 The second study, from the same labor and delivery unit in Birmingham, England, did not confirm an increased risk for headache with prior unintentional dural puncture but instead found a higher rate of backache symptoms.115 The third and most recent study compared patients with unintentional dural puncture (identified from a database and contacted at 12 to 24 months after delivery) with 40 women who received neuraxial techniques without an unintentional dural puncture.54 Using validated pain questionnaires, the investigators found that 11 of 40 (28%) women with an unintentional dural puncture had chronic headaches compared with 2 of the 40 (5%) women in the control group.
Prevention
Many practices and maneuvers have been used in an attempt to reduce the incidence of PDPH after unintentional dural puncture, most with limited success. A 2005 survey of British obstetric anesthesiologists quantified the frequency of such practices.116 These include encouraging postpartum fluid intake (91%), regular analgesia (83%), caffeine administration (30%), and using an intrathecal catheter (15%) at the time of unintentional dural puncture. Older practices, such as avoiding pushing during the second stage, restricting postpartum mobility, abdominal binders, and prophylactic epidural administration of saline or autologous blood, appear to be declining in use. Panadero et al.117 suggest it may be prudent to restrict air travel after discharge from the hospital. Presumably, the headache can be precipitated by a change in the gradient between the subarachnoid space and the epidural space due to decreased atmospheric pressure. A 2010 systematic review of prevention strategies summarized the evidence from comparative studies and concluded that studies were heterogeneous and that “no clinical recommendation can be made until the superiority of one preventative intervention over another has been unequivocally proven in a definitive multicenter RCT [randomized controlled trial].”118
Posture
In a systematic review, Sudlow and Warlow119 reviewed the evidence for reducing PDPH by use of bed rest rather than early mobilization (usually within 6 hours of dural puncture). The review included eleven trials with 1723 patients, and the results were consistent across all patient types; there was no benefit to bed rest compared with early mobilization (PDPH incidence 31% versus 27%, respectively). It is important to encourage early ambulation during the puerperium. Pregnant women are hypercoagulable and at increased risk for deep vein thrombosis and pulmonary embolism, and immobility increases this risk (see Chapter 39).
Hydration
Despite the widespread practice of encouraging women to increase oral fluid intake after unintentional dural puncture, there is little evidence that greater hydration prevents PDPH. A 2002 systematic review identified only one randomized trial in 100 nonobstetric patients. There was no difference in the incidence of PDPH in patients randomly assigned to receive 3 L or 1.5 L of fluid per day.119
Caffeine
Two clinical trials in nonobstetric patients have evaluated the efficacy of oral caffeine to prevent PDPH, but neither study showed a reduction in the incidence of headache.120,121 Based on these results, prophylactic caffeine is not advocated for prevention of PDPH.
Cosyntropin
Hakim122 evaluated whether cosyntropin, an adrenocorticotropic hormone (ACTH) analogue, was effective in reducing the incidence of PDPH after unintentional dural puncture in parturients. Patients were randomized to receive intravenous cosyntropin 1 mg or placebo 30 minutes after delivery. The incidence of PDPH was significantly lower in the cosyntropin group (33% versus 69%). The mechanism is unknown but may be related to an aldosterone-stimulating effect on volume expansion, modulation of pain perception via central endorphin-like action, or increased CSF production by enhanced sodium ion transport. Adequate dose-response and safety studies have yet to be performed.
Neuraxial Opioids
Earlier studies suggested that prophylactic neuraxial administration of a hydrophilic or lipophilic opioid does not reduce the incidence of PDPH after spinal anesthesia or unintentional dural puncture.123,124 However, in a randomized, blinded trial published in 2008, 50 parturients with unintentional dural puncture and subsequent epidural analgesia were randomized to receive epidural morphine 3 mg or saline-placebo after delivery and again 24 hours later, before removal of the epidural catheter.125 The incidence of PDPH was 48% in the saline-placebo group and 12% in the morphine group. Although no complications were reported, we would caution against routine administration of epidural morphine in these circumstances until this finding is confirmed and further safety studies are undertaken. The movement of morphine across the dura is increased by the presence of a large-gauge needle puncture, possibly increasing the risk for respiratory depression.126
Intrathecal Catheters
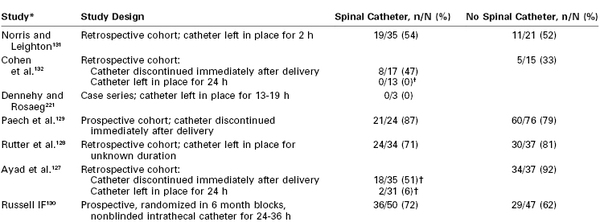
Placing a 19- or 20-gauge epidural catheter into the intrathecal space after an unintentional dural puncture with an epidural needle has become an increasingly popular technique.127–129 The immediate benefits of an intrathecal catheter are reliable, low-dose local anesthetic labor analgesia and rapid-onset surgical anesthesia should it be required. Some experts have speculated that the intrathecal catheter might reduce the immediate CSF leak into the epidural space by a mechanical obstruction and induce an inflammatory fibrous reaction in the dura, thus facilitating closure of the puncture after removal of the catheter. Most studies are retrospective and observational and lack rigorous outcome definitions and follow-up. Data from these studies are conflicting but suggest that intrathecal catheters do not significantly reduce the incidence of PDPH unless they are left in place for at least 24 hours after delivery (Table 31-4).127–133 Russell130 conducted a prospective, nonblinded, quasi-randomized multicenter study of parturients who had an unintentional dural puncture during epidural needle placement. Women were randomized in 6-month blocks to a repeat epidural procedure or placement of a spinal catheter through the dural puncture and leaving the catheter in situ for 24 to 36 hours. The incidence of PDPH was not significantly different between the epidural (62%) and spinal (72%) catheter groups. Given the current evidence, intrathecal catheters do not appear to reduce the incidence of PDPH, but further study is required before a definitive conclusion can be reached.
Prophylactic Epidural/Intrathecal Saline
Trivedi et al.134 randomly assigned patients with unintentional dural puncture to receive either a prophylactic epidural saline bolus (40 to 60 mL) or blood patch (15 mL) given just before epidural catheter removal or conservative therapy without a saline bolus or blood patch. The incidence of PDPH was not different between the saline and control groups (88% versus 67%, respectively). Shah61 studied 17 patients who received an epidural saline infusion (at a rate of approximately 40 mL/h) for 24 to 36 hours after unintentional dural puncture. Four patients complained of severe intrascapular pain, which resolved when the infusion rate was reduced. Severe PDPH developed in 47% of patients after the infusion was stopped. In contrast, in a nonrandomized, nonblinded study of patients with unintentional dural puncture, Charsley and Abram135 reported that intrathecal injection of 10 mL of saline immediately before needle or catheter withdrawal resulted in a lower incidence of PDPH (32%) than in a control group who did not receive the saline (62%), as well as a less frequent need for an epidural blood patch. The intrathecal injection of saline after unintentional dural puncture deserves further study. Intrathecal saline should not be administered until residual local anesthetic effects have resolved.
Prophylactic Blood Patch
Interest in the use of prophylactic epidural blood patch prior to the removal of a labor epidural catheter arose after early observational studies suggested that the incidence of PDPH was lower with such treatment.136 A 2010 systematic review137 identified five studies of obstetric patients involving 221 women; four studies found a reduction in the headache rate and one did not.138 Scavone et al.,138 in the largest study included in the review, reported a double-blind trial in which 64 parturients with unintentional dural puncture were randomly assigned to receive 20 mL of autologous blood (prophylactic epidural blood patch) or a sham procedure. Both groups had a 56% incidence of PDPH; however, the duration of headache was shorter in the prophylactic blood patch group. The systematic review highlighted the difficulty in estimating the risks and benefits of prophylactic epidural blood patch. Because of the low incidence of unintentional dural puncture, studies are small; therefore, reliable conclusions about the technique cannot be made based on current evidence.137
Because unintentional dural puncture with a 16- or 18-gauge epidural needle results in a high incidence of PDPH, some anesthesiologists believe that prophylactic blood patch is always justified. Others argue that with such an approach, a significant number of patients would receive unnecessary treatment and that a blood patch is not devoid of complications. These latter anesthesia providers call attention to the potential for epidural catheters to become contaminated after prolonged use. The injection of blood through a contaminated epidural catheter may be associated with a higher risk for infection than injection through an epidural needle placed de novo for a therapeutic blood patch. A case report of a parturient who received a prophylactic epidural blood patch and was subsequently diagnosed with streptococcal septicemia highlights the potential risk for maternal infection during the puerperium.139
If performed, a prophylactic blood patch should be delayed until resolution of residual neuroblockade. The occurrence of pain during injection is a signal for the anesthesia provider to stop the injection of blood. If the patient has sensory blockade, she will not perceive pain. Additionally, evidence suggests that lidocaine may inhibit coagulation.140 Finally, Leivers141 reported a case of total spinal anesthesia after the epidural injection of 15 mL of blood before epidural anesthesia had regressed. The investigator speculated that residual lidocaine in the lumbar CSF was transferred to the brain as a consequence of an increase in lumbar CSF pressure and reduced volume produced by the patch. (Editors’ note: One of us [DHC] has observed one case of transient, total blindness after the rapid, bolus injection of 30 mL of epidural saline after vaginal delivery in a patient who had experienced unintentional dural puncture during labor. The blindness resolved after 15 to 20 minutes, and subsequent ophthalmologic and neurologic examinations were normal. The etiology of the transient blindness was unclear. Nonetheless, it seems prudent to delay administration of prophylactic epidural saline or blood until the block has regressed and to avoid rapid epidural administration of blood or saline at any time.)
It is important to avoid the direct intrathecal injection of blood. Aldrete and Brown142 reported a case of intrathecal hematoma and arachnoiditis with prolonged neurologic sequelae after prophylactic blood patch. Nineteen milliliters of blood were injected through an epidural catheter that, in retrospect, was positioned in the subarachnoid space. There was considerable resistance to injection of the blood, and severe lower back pain with tinnitus accompanied the procedure.
Prophylactic Dextran Patch
Salvador et al.143 reported the prophylactic epidural injection of 20 mL of dextran-40 in 17 patients who had experienced unintentional dural puncture with a 17- or 18-gauge needle. Three of the patients were parturients, and none of the 17 patients experienced PDPH. This injection was performed before regression of the local anesthetic effect. No additional studies of this technique have been reported; its safety and efficacy remain unclear.
Treatment
Early treatment of PDPH is indicated. Not only does this avert the vicious cycle of immobility, weakness, and depression, but it also may help prevent the rare case of subdural hematoma or cranial nerve palsy in the patient with persistent PDPH.
Psychologic Support
The patient is aware that PDPH is an iatrogenic problem, and she may be angry and resentful as well as depressed and tearful. It is imperative to include disclosure of the risk during the preoperative interview. Headache makes it more difficult to care for the newborn and to interact with other family members. Severe PDPH may delay discharge from the hospital and have economic consequences.109 Unlike patients who have PDPH after nonobstetric surgery, these patients typically are healthy and do not expect to feel ill. Two patients have eloquently described their own miserable experiences with postpartum PDPH.144,145 Not surprisingly, a retrospective study of 43 obstetric patients with PDPH showed that this complication leads to a negative attitude toward epidural anesthesia.146
It is essential that anesthesia providers visit the patient at least once daily to explain symptoms and prognosis, give support, and offer therapeutic options. If feasible, the patient’s partner should attend these discussions. Nurses can help the patient by ensuring adequate analgesics are given on a regular schedule and by teaching alternative breast-feeding techniques, such as the lateral horizontal position.
The anesthesia provider and nurses should write detailed notes in the patient’s record. After discharge, follow-up telephone conversations should be documented. Headache associated with neuraxial anesthesia was the third most common reason for litigation among obstetric cases in the American Society of Anesthesiologists (ASA) Closed-Claims database (see Chapter 33), after maternal death and neonatal brain damage.147 This fact should dispel any notion that postpartum PDPH is a trivial complaint.
Posture
The diagnosis of PDPH requires demonstration of a postural component. Significant relief should occur when the patient assumes the horizontal position. However, there is no evidence that remaining supine for a prolonged period of time treats or shortens the duration of the headache.119 The prone position relieves PDPH in some patients, presumably because increased intra-abdominal pressure results in an increase in CSF pressure. Unfortunately, this position is not comfortable for many patients, especially those who had a cesarean delivery.
Hydration
Enhanced oral hydration remains a popular therapy initiated by most anesthesiologists for parturients with PDPH, but there is no evidence that vigorous hydration has any therapeutic benefit in a patient with normal fluid intake. However, no patient with PDPH should be allowed to become dehydrated, because of the increased fluid demands of breast milk formation and CSF production.119
Pharmacologic Treatment
In the past, a variety of drugs have been used to treat PDPH, including steroids, vasopressin, alcohol, and ergotamine. A safe and effective oral drug therapy for PDPH would be very useful, even if relief is transient. Blood patch therapy is not appropriate or effective in all patients.
Caffeine.
Caffeine has been used to treat PDPH for many years, despite lack of clear evidence of its efficacy. A systematic review of the literature148 identified a single randomized trial performed by Camann et al.149 Forty postpartum women with PDPH received a one-time dose of oral caffeine or placebo. Headache pain scores (visual analog scale [VAS], 0 to 100 mm) were lower in the caffeine group at 4 hours than the control group (33 ± 6 versus 49 ± 7 mm, respectively), but there was no difference at 24 hours. It appears that the beneficial effect of caffeine is transient.
The caffeine content of a 150-mL cup of drip coffee is approximately 150 mg. Caffeine is a cerebral vasoconstrictor, and one study has demonstrated a reduction of cerebral blood flow after intravenous administration of caffeine sodium benzoate for the treatment of PDPH.150 Caffeine is also a potent central nervous system stimulant. There are published case reports of seizures after intravenous151 and oral administration152 of caffeine for the treatment of PDPH in postpartum patients.
Caffeine appears in breast milk in very small amounts.153 To our knowledge, there are no reports of adverse effects on the infant after maternal administration of one or two doses of caffeine for the treatment of PDPH. The risk-to-benefit ratio to mother and newborn of multiple doses of caffeine has not been addressed. Until such studies are available it seems wise to restrict the prescription of oral caffeine to 600 mg in 24 hours, given as 300-mg doses at least 8 hours apart. Long-term caffeine therapy cannot be recommended. Another methylxanthine, theophylline, is also a cerebral vasoconstrictor, and is available in long-acting preparations. Some investigators have found that intravenous theophylline is more effective than placebo in the treatment of PDPH, but it has not become a popular therapy.154
Sumatriptan.
Sumatriptan is a serotonin receptor agonist that affects predominantly type 1D receptors. Possessing cerebral vasoconstrictor properties, this agent is used in the treatment of migraine headaches. It is given by subcutaneous injection. Side effects include pain at the injection site and, uncommonly, chest tightness. Sumatriptan may cause coronary artery vasospasm and should not be used in those with Prinzmetal’s angina or known coronary artery disease. Carp et al.155 reported that the administration of sumatriptan 6 mg resulted in complete resolution of PDPH in four of six patients, and Paech et al.129 reported relief of PDPH in one of seven patients treated with sumatriptan. Connelly et al.156 studied 10 patients with severe PDPH scheduled for epidural blood patch who were randomized to receive either sumatriptan 6 mg or placebo. After 1 hour, only one patient in each group had significant relief, and the authors concluded that sumatriptan was of no value.
Adrenocorticotrophic Hormone.
The use of ACTH for the treatment of PDPH was first reported in a 1994 letter to the editor.157 Subsequently, anecdotal reports have described different regimens of either intramuscular or intravenously administered ACTH or the synthetic drugs cosyntropin or tetracosactrin acetate. Mood elevation, anti-inflammatory effects, increased endorphin levels, and augmented intravascular volume are postulated as possible modes of action in the relief of headache with ACTH. Kshatri and Foster158 described a curative response to ACTH in two patients with PDPH. The ACTH dose was 1.5 units/kg in 250 mL of normal saline, infused intravenously over 30 minutes. Gupta and Agrawal159 assessed the effect of ACTH 60 units intramuscularly in 48 patients with PDPH. Complete and permanent relief was obtained in 40 patients, without any side effects. Carter and Pasupuleti160 reported the management of a patient with intractable PDPH who had already received three epidural blood patches; cosyntropin 0.5 mg in 1 L lactated Ringer’s solution was infused over 8 hours, and the headache was completely and permanently relieved.
To date, the only randomized clinical trial of ACTH treatment involved 18 postpartum women who had PDPH after spinal anesthesia or unintentional dural punctures.161 There was no difference in the severity of headache or the requirement for epidural blood patch between women receiving tetracosactrin acetate 1 mg intramuscularly and those who received saline-placebo.
Oliver and White162 described three patients who experienced PDPH after administration of epidural analgesia during labor and who subsequently had seizures. All had received two or three doses of tetracosactrin acetate (1 mg intramuscularly), and one had also received sumatriptan. No epidural blood patch procedures were performed. CT scans showed or suggested infarction in two of these subjects. The investigators stated that before the occurrence of these three cases ACTH had been used regularly to treat PDPH in their institution but that subsequently they had discontinued the administration of ACTH as a therapy for PDPH. No conclusion can be drawn about the possible contribution of ACTH therapy to the observed seizure activity. With the evidence to date, it appears that ACTH therapy cannot be recommended for first-line treatment of PDPH but may be considered for cases that are not amenable to epidural blood patch therapy.
Miscellaneous Medications.
Other agents evaluated for their effectiveness in reducing PDPH symptoms include pregabalin, methylergonovine, gabapentin, and hydrocortisone. Randomized clinical trials have been conducted with intravenous hydrocortisone163 and oral pregabalin.164 Noyan Ashraf et al.163 evaluated 60 parturients who developed PDPH after spinal anesthesia and were randomly assigned to receive intravenous hydrocortisone (200 mg loading dose followed by 100 mg three times daily for 2 days) or conventional therapy (bed rest, hydration, and scheduled acetaminophen with meperidine). Patients who received hydrocortisone had a 50% reduction in headache severity as assessed by VAS scores at 6 to 48 hours. A criticism of this study is the lack of blinding on the part of the study participants, which may have influenced their perception of headache pain. Huseyinoglu et al.164 randomized 40 patients after lumbar puncture and PDPH to receive pregabalin (150 mg daily for 2 days followed by 300 mg for 3 days) or placebo; pain scores and oral analgesic requirements were lower in the treatment group.
Use of gabapentin or methylergonovine has been reported only in case series.165 Efficacy and side effects (maternal and neonatal) are unclear. All four drugs require further study before they can be recommended for therapy for PDPH.
Epidural Morphine
Eldor et al.166 reported six nonobstetric patients whose PDPH headaches were successfully treated with epidural morphine 3.5 to 4.5 mg. Further study is required before conclusions can be reached. However, this therapy should be used with caution because epidural morphine injected in the presence of a large dural puncture site may pass readily into the CSF and predispose to respiratory depression.
Epidural/Intrathecal Saline
The use of epidural or intrathecal fluids to treat PDPH preceded the use of epidural blood patches. Intrathecal injection of fluid was first described by Jacobeus et al.167 in 1923, and epidural injection of saline was reported by Rice and Dabbs in 1950.168 These first reports were in conjunction with research attempting to understand the pathophysiology of PDPH, and they demonstrated transient elevations of CSF pressure after fluid injection. Subsequently there has been sporadic interest in using injection of fluids (other than blood) into the neuraxial space to treat PDPH.
Usubiaga et al.169 injected 10 to 30 mL of saline through a lumbar epidural catheter in 11 nonobstetric patients with a PDPH after spinal anesthesia in whom 48 hours of conservative therapy had failed. Immediate relief of headache was observed in 10 patients, and the relief was permanent in 8 patients. However, the investigators did not comment whether other therapies (e.g., supine posture, abdominal binder, analgesics) were continued. In a quasi-randomized trial, 43 parturients with PDPH after unintentional dural puncture during an epidural procedure or after spinal anesthesia with a 25-gauge needle were assigned to receive a 30-mL epidural saline bolus in the lumbar region or a 10-mL lumbar epidural blood patch.170 Forty-two patients had dramatic relief of their symptoms in the first hour after the intervention; however, 12 of the 21 (57%) patients who had received saline had return of the PDPH in the next 24 hours.
Prolonged epidural saline infusion may provide better therapy for PDPH symptoms than therapy with a single bolus.171,172 Two case reports described the use of epidural saline infusion for parturients with an unintentional dural puncture, whose PDPH symptoms returned after epidural blood patch therapy. The rate of infusion (15 to 25 mL/h) was limited by the onset of pain in the back, legs, and eyes. A comparative study of epidural saline bolus versus infusion to treat PDPH is needed to determine whether either modality continued over 24 hours would provide better results than conservative therapy. Although both saline techniques seem to only offer temporary relief, these options might be considered for patients who have a contraindication to epidural blood patch therapy.
Epidural Blood Patch
Efficacy.
The epidural blood patch is regarded by many as the gold standard therapy for PDPH. Although reported in third person, Gormley, in 1960, is credited with performing the first successful epidural blood patch.173 He described relief of PDPH symptoms in seven patients after epidural administration of only 2 to 3 mL of blood. However, this report was largely ignored until 1970, when DiGiovanni and Dunbar174 described the immediate and permanent cure of PDPH in 41 of 45 patients in whom 10 mL of autologous blood was injected into the epidural space. Their success led to the widespread adoption of this technique for the relief of PDPH. An excellent review of the history of PDPH and development of the blood patch was written by Harrington.175
In early case series, the reported success rate of epidural blood patch therapy for PDPH series was between 89% and 91%.174,176 Subsequent studies have not confirmed this high rate of success. Taivainen et al.177 studied 81 patients with PDPH after spinal needle dural puncture. Initially, symptoms were relieved in 88% to 96% of patients; however, a permanent cure was achieved in only 61%. Safa-Tisseront et al.178 reviewed their experience with epidural blood patch therapy over a 12-year period (n = 504 patients, including 78 obstetric patients). Complete relief of PDPH was obtained in 75%, partial relief occurred in 18%, and treatment failed in 7% of patients. The investigators noted a significantly higher failure rate of epidural blood patch after large-gauge needle puncture of the dura. The difference in early reports and more modern audits of PDPH and epidural blood patch success may be related to differences in the duration of follow-up or perhaps to other differences in management after blood patch therapy, such as delayed mobilization.
In studies limited to obstetric patients, the published success rates of epidural blood patch have been even less encouraging. Stride and Cooper69 noted complete and permanent relief of PDPH in 64% of patients after one blood patch procedure. Williams et al.179 reported only 33% of their patients obtained complete and permanent relief from the first blood patch. The authors suggested that this high failure rate might be related, in part, to their frequent performance of the blood patch procedure within 24 hours of dural puncture. Banks et al.180 prospectively monitored 100 patients with unintentional dural puncture. Fifty-eight received a therapeutic blood patch; the treatment completely failed in 3 patients, and 17 patients had recurrence of moderate or severe headache requiring further therapy. These observational studies also describe the use of repeat epidural blood patch procedures for parturients with a recurrence of PDPH.
A 2010 systematic review137 identified three randomized trials comparing epidural blood patch to either a sham procedure or conservative treatment. All three studies found a reduction in headache rate in the treatment group. Seebacher et al.181 randomized 12 heterogeneous patients with PDPH to receive an epidural blood patch with 10 to 20 mL of blood or a sham patch procedure. Five of six patients receiving a blood patch had complete relief of headache symptoms at 24 hours, and none of the sham procedure patients did. Sandesc et al.182 described a randomized trial of 32 obstetric and nonobstetric patients with PDPH symptoms for a minimum of 24 hours; subjects were randomly assigned to receive conservative therapy or an epidural blood patch. The primary outcome was headache VAS scores at 2 and 24 hours. At 2 hours the mean VAS score for the conservative therapy group was 8.2 ± 1.4 cm compared with 1.0 ± 0.2 cm for the group receiving a blood patch (P < .001). This difference remained at 24 hours. In the largest trial to date, van Kooten et al.183 randomized 40 subjects with PDPH for 1 to 7 days to receive either conservative therapy or an epidural blood patch using 15 to 20 mL of autologous blood. The primary outcome was headache 24 hours after intervention, but patients were observed for 1 week after therapy. The incidence of headache at 24 hours was 58% in the blood patch group compared with 90% in the conservative therapy group (RR, 0.64; 95% CI, 0.43 to 0.96). At 1 week the difference widened, with 16% incidence of headache in the blood patch group compared with 86% in the conservative group. In summary, administration of an autologous epidural blood patch, although not perfect, often dramatically relieves this debilitating condition and, at present, it is the therapy with the greatest likelihood of success.
Volume.
The optimal volume of injected blood remains controversial. Szeinfeld et al.184 used a gamma camera to observe the epidural spread of technetium-labeled red blood cells during and after epidural blood patch. They injected blood until pain occurred in the back, buttocks, or legs. The mean ± SD volume injected was 14.8 ± 1.7 mL of blood, and the mean ± SD spread was 9.0 ± 2.0 spinal segments. Blood spread more readily in the cephalad than in the caudad direction. The blood patch relieved the headache in all 10 patients. The investigators concluded that 12 to 15 mL of blood should be a sufficient patch volume for most patients.
The best evidence to date is an international, multicenter study by Paech et al.,185 who randomized 121 women after unintentional dural puncture with a Tuohy needle (16- or 18-gauge) to receive an epidural blood patch with 15, 20, or 30 mL of blood. Partial or complete relief of headache (the primary outcome) occurred in 61%, 73%, and 67% of those who received 15, 20, or 30 mL of blood, respectively. However, complete relief of headache was achieved by only 10%, 32%, and 26% of women in these same groups. The rate of complete headache relief was greater in the 20-mL than the 15-mL group; there was no difference between the 20- and 30-mL groups. Thus, the investigators concluded that 20 mL of autologous blood is the optimal volume for an epidural blood patch. A 2011 survey demonstrated that most North American anesthesiologists inject 20 mL of blood.186
Beards et al.187 performed MRI studies after the performance of an epidural blood patch (18 to 20 mL) in five patients. Similar to Szeinfeld et al.,184 they noted that the injected blood spread over three to five segments in a predominantly cephalad direction. All patients had an extensive hematoma in subcutaneous fat, and some also had displacement of nerve roots and/or evidence of intrathecal blood. A thick layer of mature clot had formed by 7 hours, which had broken up into smaller clots by 18 hours. These findings may help explain the back pain and occasional nerve root pain that occur after blood patch therapy.
In another MRI study, Vakharia et al.188 noted compression of the thecal sac and a mean spread over 4.6 segments after the lumbar epidural injection of 20 mL of blood (Figure 31-4). Djurhuus et al.189 employed CT epidurography in four patients immediately and 24 hours after an 18-mL blood patch. Initial images showed adherence of clot to the dura in three patients as well as dural compression in two patients, but there was no evidence of compression at 24 hours.
FIGURE 31-4 A, Pre–blood patch magnetic resonance imaging using sagittal spin-echo proton density. The dural puncture has been performed at L2 to L3, which local static fluid collection (arrows) affirms; 3, denotes L3 vertebral body. B, Post–blood patch magnetic resonance imaging of cerebrospinal fluid flow in systole shows the long length of the compression of the thecal sac posteriorly (arrows). (From Vakharia SB, Thomas PS, Rosenbaum AE, et al. Magnetic resonance imaging of cerebrospinal fluid leak and tamponade effect of blood patch in postdural puncture headache. Anesth Analg 1997; 84:585-90.)
Using a goat model, DiGiovanni et al.190 examined the microscopic appearance of the dura as late as 6 months after dural puncture. Some study animals received a 2-mL blood patch in addition to dural puncture. The investigators concluded that the blood patch acted as a gelatinous tampon that produced no harmful tissue reaction.
Mechanism of Action.
The mechanism of action of epidural blood patch for relief of PDPH is unclear. Pain relief is often rapid, but CSF volume is not restored immediately. Thus, there must be another explanation for the immediate relief of headache besides “patching” of the dural puncture. Carrie191 hypothesized that epidural injection of blood increases lumbar CSF pressure, an action that restores intracranial CSF pressure and decreases symptoms. Increased CSF pressure also may result in reflex cerebral vasoconstriction. Coombs and Hooper192 demonstrated that epidural blood patch resulted in a threefold increase in lumbar CSF pressure. Further, they noted that 15 minutes later, lumbar CSF pressure was sustained at greater than 70% of the peak pressure observed after the injection of blood. Ultrasonographic examination of the optic nerve sheath diameter (a noninvasive measurement that correlates with intracranial pressure) in 10 patients with PDPH demonstrated increased measurements after epidural blood patch.193 The sheath diameter increased 10 minutes after an epidural blood patch with 17 to 26 mL of blood and was sustained over the 20-hour study period. The only patient whose blood patch failed to successfully relieve the PDPH did not have the same increase in optic nerve sheath diameter.
MRI and CT studies have shown that the epidural blood is largely resorbed or broken up 18 to 24 hours after the procedure.187,188 It is unlikely that the increase in CSF pressure is sustained or that the blood acts as a mechanical plug to block CSF leak for a prolonged duration. The blood applied to the hole in the dura may initiate an inflammatory reaction that facilitates puncture site repair and closure. It is possible, and even likely, that an epidural blood patch ameliorates PDPH by several mechanisms.
Timing.
The optimal timing for administration of a blood patch has not been adequately studied. Observational studies suggest that failure is more likely if the blood patch is performed within 24178 or 48 hours185 of the dural puncture. It is unclear, however, whether this finding is a result of selection bias. Early-onset PDPH (often resulting from dural punctures with large-gauge needle) is likely to be more severe and more difficult to treat. Alternatively, a large CSF leak may displace the clot. Partial healing of the dura may have already occurred if a blood patch is delayed, a possibility that may explain the better outcome of a delayed patch procedure.
Technique.
The anesthesia provider should thoroughly explain the risks and benefits of the blood patch procedure to the patient, and the patient should give consent for the procedure. An epidural blood patch can be accomplished on an outpatient basis. Ideally, the environment for the procedure is one conducive to postpartum patients who may have accompanying family and a newborn. Contraindications to the administration of an epidural blood patch are related to complications of placing a needle in the central neuraxis or the injection of blood into the epidural space; they include (1) known coagulopathy, (2) local cutaneous infection or untreated systemic infection, (3) increased ICP due to a space-occupying lesion, and (4) patient refusal. Transient bradycardia has been observed after administration of an epidural blood patch, and some anesthesia providers may choose to establish intravenous access and monitor the electrocardiogram in selected patients.194
The blood patch procedure should employ sterile measures equivalent to those used for the administration of any neuraxial procedure. The lateral position is usually more comfortable than the sitting position for patients with severe PDPH. If the anesthesia provider is uncertain about the location of the dural puncture, the more caudad interspace should be chosen. The epidural space is identified in the usual manner. Using meticulous sterile technique (including skin preparation and draping, and donning of a mask and sterile gloves), an assistant withdraws the desired volume of blood (usually 10 to 25 mL) into a syringe. This autologous blood is injected slowly into the epidural space through the Tuohy needle; the injection is terminated if severe back, leg, or neck pain or pressure occurs. Sometimes slowing the injection rate leads to resolution of the back pain. Jehovah’s Witness patients may accept a blood patch procedure if a technique is used that keeps blood in continuity with the circulation.195
Occasionally a few drops of CSF are encountered on entering the epidural space, leading to doubt about correct needle placement.196 One can either repeat the epidural needle placement, or a small test dose of a local anesthetic agent can be administered, sufficient to cause a rapid onset of spinal anesthesia. If no block results, the blood patch can be performed. Two case reports have described the use of real-time ultrasound-guided and fluoroscopically guided epidural blood patch.197,198 The usefulness of these modalities to assist in localizing deposition of epidural blood requires further study.
After the procedure the patient should rest quietly in the supine position for 1 to 2 hours.199 Subsequently, the patient may resume ambulation, but she should avoid vigorous physical activity for several days. It would be wise for the patient to avoid the Valsalva maneuver and heavy lifting. A stool softener should be considered. Most patients report almost instantaneous relief of headache symptoms, although relief is delayed for 6 to 8 hours in some patients. The patient may continue to have neck and back fullness over the next 24 hours.185 The back pain can continue up to 5 days after the procedure. Patients should be counseled to immediately report fever, severe back pain, or radiating lower extremity pain. The anesthesia provider should contact the patient after the blood patch procedure.
The blood patch may be repeated if the initial patch fails to relieve pain. Often the second patch is successful. Although not adequately studied, it seems reasonable to wait 24 hours before repeating an epidural blood patch procedure to allow the first procedure adequate time to work. The diagnosis should be reconsidered if headache persists after two failed blood patch procedures. Consultation by a neurologist may be appropriate when a PDPH fails to respond to two blood patches and should definitely be requested if there is any doubt about the diagnosis. Imaging of the head should be considered to exclude other causes of headache.
Complications.
Ong et al.200 reported that the success of neuraxial anesthesia/analgesia was impaired in women with a prior history of unintentional dural puncture with or without epidural blood patch therapy. However, this conclusion has been refuted by follow-up studies in both obstetric201 and nonobstetric patients.202 In both of these retrospective studies, a history of blood patch therapy had no apparent effect on the quality of subsequent epidural anesthesia.
Although epidural blood patch therapy is the most reliable method of relieving PDPH symptoms, adverse outcomes are associated with the procedure. These adverse events can be categorized into two broad groups: infectious/hematologic and neurologic.
Infectious/Hematologic Complications.
Conventional wisdom holds that the patient should be afebrile at the time of the blood patch procedure. Many anesthesia providers believe that it is wise to avoid the epidural injection of blood in the presence of systemic infection. Meningitis has been reported after a blood patch procedure.203 After conservative measures have failed, the optimal treatment of a febrile patient with severe, persistent PDPH is controversial. Epidural infusion of saline involves the use of an indwelling epidural catheter for many hours, which also may be undesirable in a febrile patient. A patch using dextran-40 may be an alternative in febrile patients, but further experience is needed in healthy patients before this technique can be recommended. The presence of high fever and/or other evidence of sepsis contraindicate the performance of a blood patch procedure. However, we do not believe that a low-grade fever of known etiology is an absolute contraindication to epidural blood patch, provided the patient is receiving appropriate antibiotic therapy. Management should be individualized, and the known benefits of blood patch should be weighed against the unknown risk for infection.
The risks of epidural blood patch therapy in the presence of human immunodeficiency virus (HIV) infection have been debated (see Chapter 45). However, the central nervous system is infected with HIV at the time of primary infection; therefore, it seems unlikely that the injection of autologous blood into the epidural space would alter progression of the disease. There are published reports of the successful use of blood patch therapy, without sequelae, in patients with acquired immunodeficiency syndrome (AIDS) or who are HIV positive.204,205
The incidence of cancer during pregnancy is increasing, likely secondary to advancing maternal age. The development of PDPH in this population has raised a theoretical concern about seeding the neuraxial space with neoplastic cells if a blood patch procedure is performed. This concern should be discussed with the patient and her oncologist before the procedure. Bucklin et al.206 reported the conservative management of a woman with acute leukemia and PDPH; the investigators discussed the therapeutic options for this immunocompromised patient. The use of epidural fibrin glue was reported in a nonobstetric patient with metastatic breast cancer and PDPH,207 whereas a blood patch was performed for PDPH in a young woman with rhabdomyosarcoma.208
Neurologic Complications.
Serious or permanent problems after epidural blood patch therapy are rare. Diaz and Weed209 wrote a review of case reports of adverse neurologic complications after epidural blood patch procedures. These authors identified 26 reports published between the years of 1966 and 2004 and stratified the complications into neurologic, neurovascular, or inflammatory events. The events occurring in obstetric patients included lumbovertebral syndrome (defined as low back pain with neurologic impairment of the lower extremities), subdural hematoma, arachnoiditis, radicular back pain, pneumocephalus, seizures, and acute meningeal irritation. Compressive complications (e.g., lumbovertebral syndrome, subdural hematoma, cauda equina syndrome) were associated with a larger blood patch volume (mean of 35 mL) than the noncompressive complications (17 mL). Cranial nerve palsy symptoms that were present before blood patch administration did not uniformly resolve. The delay in performing the blood patch may have been the significant factor. Two patients who were treated within 4 days of the onset of PDPH symptoms recovered within 6 weeks, whereas three patients treated on days 9 to 11 had palsy that persisted for 3 to 4 months. Two obstetric patients experienced new facial nerve palsies, which manifested as facial weakness after administration of an epidural blood patch.
Abouleish et al.176 reported the results of the long-term evaluation of 118 patients who had received an epidural blood patch. Back pain was the most common complication; it occurred during the first 48 hours in 35% of patients and persisted in 16% of patients, with a mean duration of 27 days. These investigators also noted cases of neck pain, lower extremity radicular pain, and transient temperature elevation.
Epidural space scarring is also a possibility after placement of a blood patch. Although subsequent epidural analgesia is generally successful (see earlier discussion),201,202 Collier210 reported two cases of unsuccessful epidural analgesia related to suspected scarring of the epidural space. In both women, the initial epidural catheter placement was complicated by unintentional dural puncture and PDPH treated with epidural blood patch. During a subsequent pregnancy, epidural catheter placement was complicated by inadequate analgesia, and an epidurogram revealed limited spread of the contrast media, suggesting epidural space scarring.
The development of an inflammatory reaction to epidural blood can cause acute arachnoiditis, an entity that can present several days after epidural blood patch.211 This phenomenon is believed to be secondary to free radical damage to spinal root nerves in the intrathecal space after hemoglobin degradation. There are case reports of obstetric patients with this entity who required analgesic therapy for prolonged periods.212 The diagnosis is made with a presenting history of severe back pain, often with radicular pain, and characteristic MRI findings such as nerve root clumping in the intrathecal space and adhesions between nerve roots.
The occurrence of new neurologic symptoms appearing after an epidural blood patch should prompt consideration of the presence of other intracranial pathology. Such symptoms may include (1) mental deterioration due to increased ICP from an intracranial tumor24 and (2) seizures due to late-onset eclampsia.213
Diaz214 described a woman in whom permanent paraparesis and cauda equina syndrome developed after an epidural blood patch with 30 mL of blood injected slowly and without symptoms. Low back pain and leg pain developed after the blood patch procedure, and later the patient also experienced incontinence. Twelve days after the procedure a subdural hematoma at L2-L4 was diagnosed and surgically treated. Six months later the patient still had marked symptoms. Although a larger volume of blood than usual was injected, the technique appears to have been within normal practice standards. Other long-term sequelae reported in obstetric patients include a postpartum cerebral ischemic event after two blood patches that resulted in permanent hemianopsia215 and a calcified epidural blood patch leading to chronic back pain.216
Alternatives to Epidural Blood Patch
Alternatives to an epidural blood patch may be considered if a blood patch is contraindicated or fails. In extreme cases, surgery may be needed to close the dural tear.
Dextran/Gelatin Patch.
Dextran-40 and gelatin-based solutions, including Gelfoam and Plasmion, have been substituted for blood in epidural patches.143,217 These solutions were chosen as alternatives to blood owing to relative contraindications to injection of blood. The use of these agents appears to be more common in countries outside North America. In an observational study of 56 patients, Barrios-Alarcon et al.218 reported that epidural administration of 20 to 30 mL of dextran-40 was safe and effective for the relief of PDPH; all headaches were relieved permanently. The only side effect was a transient discomfort or burning sensation at the time of injection in 6 patients. Some physicians have treated intractable PDPH successfully by performing a dextran-40 patch followed by epidural infusion of dextran at 3 mL/h for 5 to 12 hours.219,220
Information on neurotoxicity of these materials is scant; Chanimov et al.221 did not observe neurotoxicity after infusion of dextran-40 or polygeline, a gelatin powder, into the rat intrathecal space. However, further information is needed before these materials can be widely adopted for epidural administration in humans. From MRI studies in patients with an epidural blood patch, we can anticipate that some dextran will enter the subarachnoid space. The small but definite risk for anaphylaxis after the injection of dextran also must be considered, although the risk appears minimal with dextran-40.
Fibrin Sealant Patch.
Fibrin sealant is composed of fibrinogen and thrombin. Several commercial products are prepared from human pooled plasma. Products may also contain antifibrinolytics, such as animal aprotinin.222 When injected, these products form a firm, nonretractable fibrin clot. Epidural injection of fibrin glue in rats produces a sustained rise in CSF pressure comparable to the increase that occurs after injection of blood.223 Fibrin sealant has been evaluated for its effectiveness in preventing dural leaks after spinal surgery.224 An epidural fibrin glue patch has been used successfully to treat recurrent PDPH,225 spontaneous intracranial hypotension,226 and CSF leak after long-term intrathecal catheterization.227 In the future, fibrin glue may have a role in patients with intractable PDPH, but further study is required before it can be recommended for routine use.
Surgery.
There are rare reports of curative surgical closure of a dural rent for intractable PDPH. In one case, the interval between dural puncture and surgery was 5 years.228
Summary of Treatment
The parturient with PDPH should be actively managed with scheduled analgesics and should receive psychological support as she cares for her newborn and manages her symptoms. If the headache is severe, the physician can consider additional agents, such as caffeine or sumatriptan, or proceed directly to an epidural blood patch. Epidural administration of fluids other than blood, such as saline or dextran, typically is not first-line therapy but may be considered when there are contraindications to the epidural injection of autologous blood or if an epidural blood patch procedure fails. The accuracy of PDPH diagnosis must always be considered when atypical symptoms present or when therapy fails.
Unanswered Questions
Important information about PDPH is still lacking. A large, detailed prospective study of PDPH, with and without epidural blood patch, with a long follow-up period (e.g., 1 year) is needed in obstetric patients. What are the long-term effects of both PDPH and blood patch therapy? How common are residual back pain, neurologic symptoms, and auditory/visual symptoms, and do they interfere with everyday life? Answers to these questions are needed to give our patients reliable information, a sound basis for informed consent, and the best possible care. The Obstetric Anaesthetists’ Association and the Association of Anaesthetists of Great Britain and Ireland229 have recommended that each facility providing obstetric anesthesia services should have institution-specific protocols for the management of PDPH to facilitate the identification of parturients with this complication and to provide consistent care.
References
1. Goldszmidt E, Kern R, Chaput A, Macarthur A. The incidence and etiology of postpartum headaches: a prospective cohort study. Can J Anaesth. 2005;52:971–977.
2. Benhamou D, Hamza J, Ducot B. Post partum headache after epidural analgesia without dural puncture. Int J Obstet Anesth. 1995;4:17–20.
3. Saurel-Cubizolles MJ, Romito P, Lelong N, Ancel PY. Women’s health after childbirth: a longitudinal study in France and Italy. BJOG. 2000;107:1202–1209.
4. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160.
5. Stella CL, Jodicke CD, How HY, et al. Postpartum headache: is your work-up complete? Am J Obstet Gynecol. 2007;196:318 e1–7.
7. Sances G, Granella F, Nappi RE, et al. Course of migraine during pregnancy and postpartum: a prospective study. Cephalalgia. 2003;23:197–205.
8. Adeney KL, Williams MA. Migraine headaches and preeclampsia: an epidemiologic review. Headache. 2006;46:794–803.
9. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402–410.
10. Katzin LW, Levine M, Singhal AB. Dural puncture headache, postpartum angiopathy, pre-eclampsia and cortical vein thrombosis after an uncomplicated pregnancy. Cephalalgia. 2007;27:461–464.
11. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.
12. Pande AR, Ando K, Ishikura R, et al. Clinicoradiological factors influencing the reversibility of posterior reversible encephalopathy syndrome: a multicenter study. Radiat Med. 2006;24:659–668.
13. Torrillo TM, Bronster DJ, Beilin Y. Delayed diagnosis of posterior reversible encephalopathy syndrome (PRES) in a parturient with preeclampsia after inadvertent dural puncture. Int J Obstet Anesth. 2007;16:171–174.
14. Long TR, Hein BD, Brown MJ, et al. Posterior reversible encephalopathy syndrome during pregnancy: seizures in a previously healthy parturient. J Clin Anesth. 2007;19:145–148.
15. Lockhart EM, Baysinger CL. Intracranial venous thrombosis in the parturient. Anesthesiology. 2007;107:652–658.
16. Ghatge S, Uppugonduri S, Kamarzaman Z. Cerebral venous sinus thrombosis following accidental dural puncture and epidural blood patch. Int J Obstet Anesth. 2008;17:267–270.
17. Bousser MG. Cerebral venous thrombosis: diagnosis and management. J Neurol. 2000;247:252–258.
18. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43:e41–e42.
19. Feske SK. Stroke in pregnancy. Semin Neurol. 2007;27:442–452.
20. Fugate JE, Ameriso SF, Ortiz G, et al. Variable presentations of postpartum angiopathy. Stroke. 2012;43:670–676.
21. Bateman BT, Olbrecht VA, Berman MF, et al. Peripartum subarachnoid hemorrhage: nationwide data and institutional experience. Anesthesiology. 2012;116:324–333.
22. Gomez-Rios MA, Kuczkowski KM. Bilateral subdural intracranial hematoma after accidental dural puncture. Anesthesiology. 2012;117:646.
23. Borelli P, Baldacci F, Nuti A, et al. Postpartum headache due to spontaneous cervical artery dissection. Headache. 2011;51:809–813.
24. Eede HV, Hoffmann VL, Vercauteren MP. Post-delivery postural headache: not always a classical post-dural puncture headache. Acta Anaesthesiol Scand. 2007;51:763–765.
25. Su TM, Lan CM, Yang LC, et al. Brain tumor presenting with fatal herniation following delivery under epidural anesthesia. Anesthesiology. 2002;96:508–509.
26. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anaesthesia. 2007;62:178–181.
27. Lussos SA, Loeffler C. Epidural blood patch improves postdural puncture headache in a patient with benign intracranial hypertension. Reg Anesth. 1993;18:315–317.
28. Mokri B. Spontaneous low cerebrospinal pressure/volume headaches. Curr Neurol Neurosci Rep. 2004;4:117–124.
29. Albayram S, Tuzgen S, Gunduz A, et al. Spontaneous intracranial hypotension after labor without spinal intervention. Eur J Neurol. 2008;15:91–93.
30. Smarkusky L, DeCarvalho H, Bermudez A, Gonzalez-Quintero VH. Acute onset headache complicating labor epidural caused by intrapartum pneumocephalus. Obstet Gynecol. 2006;108:795–798.
31. Gore PA, Maan H, Chang S, et al. Normobaric oxygen therapy strategies in the treatment of postcraniotomy pneumocephalus. J Neurosurg. 2008;108:926–929.
32. Hebl JR. The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med. 2006;31:311–323.
33. Reynolds F. Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008;26:23–52.
34. Sobol SE, Frenkiel S, Nachtigal D, et al. Clinical manifestations of sinonasal pathology during pregnancy. J Otolaryngol. 2001;30:24–28.
35. Shapiro RE. Caffeine and headaches. Neurol Sci. 2007;28(Suppl 2):S179–S183.
36. Askmark H, Lundberg PO. Lactation headache—a new form of headache? Cephalalgia. 1989;9:119–122.
37. Thorley V. Lactational headache: a lactation consultant’s diary. J Hum Lact. 1997;13:51–53.
38. Sharma R, Panda A. Ondansetron-induced headache in a parturient mimicking postdural puncture headache. Can J Anaesth. 2010;57:187–188.
39. Choi PT, Galinski SE, Takeuchi L, et al. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth. 2003;50:460–469.
40. Sharma SK, Gambling DR, Joshi GP, et al. Comparison of 26-gauge Atraucan and 25-gauge Whitacre needles: insertion characteristics and complications. Can J Anaesth. 1995;42:706–710.
41. Pan PH, Fragneto R, Moore C, Ross V. Incidence of postdural puncture headache and backache, and success rate of dural puncture: comparison of two spinal needle designs. South Med J. 2004;97:359–363.
42. Vallejo MC, Mandell GL, Sabo DP, Ramanathan S. Postdural puncture headache: a randomized comparison of five spinal needles in obstetric patients. Anesth Analg. 2000;91:916–920.
43. Shaikh JM, Memon A, Memon MA, Khan M. Post dural puncture headache after spinal anaesthesia for caesarean section: a comparison of 25 g Quincke, 27 g Quincke and 27 g Whitacre spinal needles. J Ayub Med Coll Abbottabad. 2008;20:10–13.
44. Lybecker H, Djernes M, Schmidt JF. Postdural puncture headache (PDPH): onset, duration, severity, and associated symptoms: an analysis of 75 consecutive patients with PDPH. Acta Anaesthesiol Scand. 1995;39:605–612.
45. Erol A, Topal A, Arbag H, et al. Auditory function after spinal anaesthesia: the effect of differently designed spinal needles. Eur J Anaesthesiol. 2009;26:416–420.
46. Shearer VE, Jhaveri HS, Cunningham FG. Puerperal seizures after post-dural puncture headache. Obstet Gynecol. 1995;85:255–260.
47. Vercauteren MP, Vundelinckx GJ, Hanegreefs GH. Postpartum headache, seizures and bloodstained C.S.F.: a possible complication of dural puncture? Intensive Care Med. 1988;14:176–177.
48. Vazquez R, Johnson DW, Ahmed SU. Epidural blood patch for postdural puncture positional vertigo. Pain Med. 2011;12:148–151.
49. Schabel JE, Wang ED, Glass PS. Arm pain as an unusual presentation of postdural puncture intracranial hypotension. Anesth Analg. 2000;91:910–912.
50. Yang CP, Lee CH, Borel CO, et al. Postdural puncture headache with abdominal pain and diarrhea. Anesth Analg. 2005;100:879–881.
51. Reamy BV. Post-epidural headache: how late can it occur? J Am Board Fam Med. 2009;22:202–205.
52. Chan TM, Ahmed E, Yentis SM, Holdcroft A. Postpartum headaches: summary report of the National Obstetric Anaesthetic Database (NOAD) 1999. Int J Obstet Anesth. 2003;12:107–112.
53. Klepstad P. Relief of postural post dural puncture headache by an epidural blood patch 12 months after dural puncture. Acta Anaesthesiol Scand. 1999;43:964–966.
54. Webb CA, Weyker PD, Zhang L, et al. Unintentional dural puncture with a Tuohy needle increases risk of chronic headache. Anesth Analg. 2012;115:124–132.
55. Bakshi R, Mechtler LL, Kamran S, et al. MRI findings in lumbar puncture headache syndrome: abnormal dural-meningeal and dural venous sinus enhancement. Clin Imaging. 1999;23:73–76.
56. Corbonnois G, O’Neill T, Brabis-Henner A, et al. Unrecognized dural puncture during epidural analgesia in obstetrics later confirmed by brain imaging. Ann Fr Anesth Reanim. 2010;29:584–588.
57. Somri M, Teszler CB, Vaida SJ, et al. Postdural puncture headache: an imaging-guided management protocol. Anesth Analg. 2003;96:1809–1812.
58. Bier A. Versuche ueber cocainisirung des rueckenmarkes. Dtsch Zeitschr Chir. 1899;51:361–369.
60. Marx GF, Zemaitis MT, Orkin LR. Cerebrospinal fluid pressures during labor and obstetrical anesthesia. Anesthesiology. 1961;22:348–354.
61. Shah JL. Epidural pressure and postdural puncture headache in the parturient. Int J Obstet Anesth. 1993;2:187–189.
62. Boezaart AP. Effects of cerebrospinal fluid loss and epidural blood patch on cerebral blood flow in swine. Reg Anesth Pain Med. 2001;26:401–406.
63. Hattingh J, McCalden TA. Cerebrovascular effects of cerebrospinal fluid removal. S Afr Med J. 1978;54:780–781.
64. Grant R, Condon B, Patterson J, et al. Changes in cranial CSF volume during hypercapnia and hypocapnia. J Neurol Neurosurg Psychiatry. 1989;52:218–222.
65. Martins AN, Wiley JK, Myers PW. Dynamics of the cerebrospinal fluid and the spinal dura mater. J Neurol Neurosurg Psychiatry. 1972;35:468–473.
66. Vandam LD, Dripps RD. Long-term follow-up of patients who received 10,098 spinal anesthetics; syndrome of decreased intracranial pressure (headache and ocular and auditory difficulties). JAMA. 1956;161:586–591.
67. Amorim JA, Gomes de Barros MV, Valenca MM. Post-dural (post-lumbar) puncture headache: risk factors and clinical features. Cephalalgia. 2012;32:916–923.
68. Wu CL, Rowlingson AJ, Cohen SR, et al. Gender and post-dural puncture headache. Anesthesiology. 2006;105:613–618.
69. Stride PC, Cooper GM. Dural taps revisited: a 20-year survey from Birmingham Maternity Hospital. Anaesthesia. 1993;48:247–255.
70. Angle P, Thompson D, Halpern S, Wilson DB. Second stage pushing correlates with headache after unintentional dural puncture in parturients. Can J Anaesth. 1999;46:861–866.
71. Faure E, Moreno R, Thisted R. Incidence of postdural puncture headache in morbidly obese parturients. Reg Anesth. 1994;19:361–363.
72. Amorim JA, Valenca MM. Postdural puncture headache is a risk factor for new postdural puncture headache. Cephalalgia. 2008;28:5–8.
73. Seeberger MD, Kaufmann M, Staender S, et al. Repeated dural punctures increase the incidence of postdural puncture headache. Anesth Analg. 1996;82:302–305.
74. Angle PJ, Kronberg JE, Thompson DE, et al. Dural tissue trauma and cerebrospinal fluid leak after epidural needle puncture: effect of needle design, angle, and bevel orientation. Anesthesiology. 2003;99:1376–1382.
75. Morley-Forster PK, Singh S, Angle P, et al. The effect of epidural needle type on postdural puncture headache: a randomized trial. Can J Anaesth. 2006;53:572–578.
76. Angle PJ, Hussain K, Morgan A, et al. High quality labour analgesia using small gauge epidural needles and catheters. Can J Anaesth. 2006;53:263–267.
77. Hart JR, Whitacre RJ. Pencil-point needle in prevention of postspinal headache. JAMA. 1951;147:657–658.
78. Santanen U, Rautoma P, Luurila H, et al. Comparison of 27-gauge (0.41-mm) Whitacre and Quincke spinal needles with respect to post-dural puncture headache and non-dural puncture headache. Acta Anaesthesiol Scand. 2004;48:474–479.
79. Westbrook JL, Uncles DR, Sitzman BT, Carrie LE. Comparison of the force required for dural puncture with different spinal needles and subsequent leakage of cerebrospinal fluid. Anesth Analg. 1994;79:769–772.
80. Sprotte G, Schedel R, Pajunk H. An “atraumatic” universal needle for single-shot regional anesthesia: clinical results and a 6 year trial in over 30,000 regional anesthesias. Reg Anaesth. 1987;10:104–108.
81. Lesser P, Bembridge M, Lyons G, Macdonald R. An evaluation of a 30-gauge needle for spinal anaesthesia for caesarean section. Anaesthesia. 1990;45:767–768.
82. Halpern S, Preston R. Postdural puncture headache and spinal needle design: metaanalyses. Anesthesiology. 1994;81:1376–1383.
83. Richman JM, Joe EM, Cohen SR, et al. Bevel direction and postdural puncture headache: a meta-analysis. Neurologist. 2006;12:224–228.
84. Franksson C, Gordh T. Headache after spinal anesthesia and a technique for lessening its frequency. Acta Chir Scand. 1946;94:443–454.
85. Fink BR, Walker S. Orientation of fibers in human dorsal lumbar dura mater in relation to lumbar puncture. Anesth Analg. 1989;69:768–772.
86. Ready LB, Cuplin S, Haschke RH, Nessly M. Spinal needle determinants of rate of transdural fluid leak. Anesth Analg. 1989;69:457–460.
87. Cruickshank RH, Hopkinson JM. Fluid flow through dural puncture sites: an in vitro comparison of needle point types. Anaesthesia. 1989;44:415–418.
88. Dittmann M, Schafer HG, Ulrich J, Bond-Taylor W. Anatomical re-evaluation of lumbar dura mater with regard to postspinal headache: effect of dural puncture. Anaesthesia. 1988;43:635–637.
89. Norris MC, Leighton BL, DeSimone CA. Needle bevel direction and headache after inadvertent dural puncture. Anesthesiology. 1989;70:729–731.
90. Richardson MG, Wissler RN. The effects of needle bevel orientation during epidural catheter insertion in laboring parturients. Anesth Analg. 1999;88:352–356.
91. Hatfalvi BI. Postulated mechanisms for postdural puncture headache and review of laboratory models: clinical experience. Reg Anesth. 1995;20:329–336.
92. Viitanen H, Porthan L, Viitanen M, et al. Postpartum neurologic symptoms following single-shot spinal block for labour analgesia. Acta Anaesthesiol Scand. 2005;49:1015–1022.
93. Kempen PM, Mocek CK. Bevel direction, dura geometry, and hole size in membrane puncture: laboratory report. Reg Anesth. 1997;22:267–272.
94. Gurmarnik S, Kandror KV. Postdural puncture headache: the Betadine factor. Reg Anesth. 1996;21:375–376.
95. Simsa J. How to reduce the risk for contamination of the central nervous system by spinal needles. Reg Anesth. 1997;22:297.
96. Gleeson CM, Reynolds F. Accidental dural puncture rates in UK obstetric practice. Int J Obstet Anesth. 1998;7:242–246.
97. Evron S, Sessler D, Sadan O, et al. Identification of the epidural space: loss of resistance with air, lidocaine, or the combination of air and lidocaine. Anesth Analg. 2004;99:245–250.
98. Beilin Y, Arnold I, Telfeyan C, et al. Quality of analgesia when air versus saline is used for identification of the epidural space in the parturient. Reg Anesth Pain Med. 2000;25:596–599.
99. Schier R, Guerra D, Aguilar J, et al. Epidural space identification: a meta-analysis of complications after air versus liquid as the medium for loss of resistance. Anesth Analg. 2009;109:2012–2021.
100. Segal S, Arendt KW. A retrospective effectiveness study of loss of resistance to air or saline for identification of the epidural space. Anesth Analg. 2010;110:558–563.
101. Naulty JS, Hertwig L, Hunt CO, et al. Influence of local anesthetic solution on postdural puncture headache. Anesthesiology. 1990;72:450–454.
102. Arkoosh VA, Palmer CM, Yun EM, et al. A randomized, double-masked, multicenter comparison of the safety of continuous intrathecal labor analgesia using a 28-gauge catheter versus continuous epidural labor analgesia. Anesthesiology. 2008;108:286–298.
103. Alonso E, Gilsanz F, Gredilla E, et al. Observational study of continuous spinal anesthesia with the catheter-over-needle technique for cesarean delivery. Int J Obstet Anesth. 2009;18:137–141.
104. Dresner M, Pinder A. Anaesthesia for caesarean section in women with complex cardiac disease: 34 cases using the Braun Spinocath spinal catheter. Int J Obstet Anesth. 2009;18:131–136.
105. Tao W, Nguyen AP, Ogunnaike BO, Craig MG. Use of a 23-gauge continuous spinal catheter for labor analgesia: a case series. Int J Obstet Anesth. 2011;20:351–354.
106. Norris MC, Fogel ST, Conway-Long C. Combined spinal-epidural versus epidural labor analgesia. Anesthesiology. 2001;95:913–920.
107. Van de Velde M, Teunkens A, Hanssens M, et al. Post dural puncture headache following combined spinal epidural or epidural anaesthesia in obstetric patients. Anaesth Intensive Care. 2001;29:595–599.
108. Miro M, Guasch E, Gilsanz F. Comparison of epidural analgesia with combined spinal-epidural analgesia for labor: a retrospective study of 6497 cases. Int J Obstet Anesth. 2008;17:15–19.
110. Zeidan A, Farhat O, Maaliki H, Baraka A. Does postdural puncture headache left untreated lead to subdural hematoma? Case report and review of the literature. Middle East J Anesthesiol. 2010;20:483–492.
111. Stocks GM, Wooller DJ, Young JM, Fernando R. Postpartum headache after epidural blood patch: investigation and diagnosis. Br J Anaesth. 2000;84:407–410.
112. Lybecker H, Andersen T, Helbo-Hansen HS. The effect of epidural blood patch on hearing loss in patients with severe postdural puncture headache. J Clin Anesth. 1995;7:457–464.
113. Nishio I, Williams BA, Williams JP. Diplopia: a complication of dural puncture. Anesthesiology. 2004;100:158–164.
114. MacArthur C, Lewis M, Knox EG. Accidental dural puncture in obstetric patients and long term symptoms. BMJ. 1993;306:883–885.
115. Jeskins GD, Moore PA, Cooper GM, Lewis M. Long-term morbidity following dural puncture in an obstetric population. Int J Obstet Anesth. 2001;10:17–24.
116. Baraz R, Collis RE. The management of accidental dural puncture during labour epidural analgesia: a survey of UK practice. Anaesthesia. 2005;60:673–679.
117. Panadero A, Bravo P, Garcia-Pedrajas F. Postdural puncture headache and air travel after spinal anesthesia with a 24-gauge Sprotte needle. Reg Anesth. 1995;20:463–464.
118. Apfel CC, Saxena A, Cakmakkaya OS, et al. Prevention of postdural puncture headache after accidental dural puncture: a quantitative systematic review. Br J Anaesth. 2010;105:255–263.
119. Sudlow C, Warlow C. Posture and fluids for preventing post-dural puncture headache. Cochrane Database Syst Rev. 2002;(2).
120. Yucel A, Ozyalcin S, Talu GK, et al. Intravenous administration of caffeine sodium benzoate for postdural puncture headache. Reg Anesth Pain Med. 1999;24:51–54.
121. Esmaoglu A, Akpinar H, Ugur F. Oral multidose caffeine-paracetamol combination is not effective for the prophylaxis of postdural puncture headache. J Clin Anesth. 2005;17:58–61.
122. Hakim SM. Cosyntropin for prophylaxis against postdural puncture headache after accidental dural puncture. Anesthesiology. 2010;113:413–420.
123. Devcic A, Sprung J, Patel S, et al. PDPH in obstetric anesthesia: comparison of 24-gauge Sprotte and 25-gauge Quincke needles and effect of subarachnoid administration of fentanyl. Reg Anesth. 1993;18:222–225.
124. Abboud TK, Zhu J, Reyes A, et al. Effect of subarachnoid morphine on the incidence of spinal headache. Reg Anesth. 1992;17:34–36.
125. Al-metwalli RR. Epidural morphine injections for prevention of post dural puncture headache. Anaesthesia. 2008;63:847–850.
126. Bernards CM, Kopacz DJ, Michel MZ. Effect of needle puncture on morphine and lidocaine flux through the spinal meninges of the monkey in vitro: implications for combined spinal-epidural anesthesia. Anesthesiology. 1994;80:853–858.
127. Ayad S, Demian Y, Narouze SN, Tetzlaff JE. Subarachnoid catheter placement after wet tap for analgesia in labor: influence on the risk of headache in obstetric patients. Reg Anesth Pain Med. 2003;28:512–515.
128. Rutter SV, Shields F, Broadbent CR, et al. Management of accidental dural puncture in labour with intrathecal catheters: an analysis of 10 years’ experience. Int J Obstet Anesth. 2001;10:177–181.
129. Paech M, Banks S, Gurrin L. An audit of accidental dural puncture during epidural insertion of a Tuohy needle in obstetric patients. Int J Obstet Anesth. 2001;10:162–167.
130. Russell IF. A prospective controlled study of continuous spinal analgesia versus repeat epidural analgesia after accidental dural puncture in labour. Int J Obstet Anesth. 2012;21:7–16.
131. Norris MC, Leighton BL. Continuous spinal anesthesia after unintentional dural puncture in parturients. Reg Anesth. 1990;15:285–287.
132. Cohen S, Amar D, Pantuck EJ, et al. Decreased incidence of headache after accidental dural puncture in caesarean delivery patients receiving continuous postoperative intrathecal analgesia. Acta Anaesthesiol Scand. 1994;38:716–718.
133. Dennehy KC, Rosaeg OP. Intrathecal catheter insertion during labour reduces the risk of post-dural puncture headache. Can J Anaesth. 1998;45:42–45.
134. Trivedi NS, Eddi D, Shevde K. Headache prevention following accidental dural puncture in obstetric patients. J Clin Anesth. 1993;5:42–45.
135. Charsley MM, Abram SE. The injection of intrathecal normal saline reduces the severity of postdural puncture headache. Reg Anesth Pain Med. 2001;26:301–305.
136. Colonna-Romano P, Shapiro BE. Unintentional dural puncture and prophylactic epidural blood patch in obstetrics. Anesth Analg. 1989;69:522–523.
137. Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010;(1).
138. Scavone BM, Wong CA, Sullivan JT, et al. Efficacy of a prophylactic epidural blood patch in preventing post dural puncture headache in parturients after inadvertent dural puncture. Anesthesiology. 2004;101:1422–1427.
139. Allen DL, Berenguer JV, White JB. A potential complication of early blood patching following inadvertent dural puncture. Int J Obstet Anesth. 1993;2:202–203.
140. Tobias MD, Pilla MA, Rogers C, Jobes DR. Lidocaine inhibits blood coagulation: implications for epidural blood patch. Anesth Analg. 1996;82:766–769.
141. Leivers D. Total spinal anesthesia following early prophylactic epidural blood patch. Anesthesiology. 1990;73:1287–1289.
142. Aldrete JA, Brown TL. Intrathecal hematoma and arachnoiditis after prophylactic blood patch through a catheter. Anesth Analg. 1997;84:233–234.
143. Salvador L, Carrero E, Castillo J, et al. Prevention of post dural puncture headache with epidural-administered dextran 40. Reg Anesth. 1992;17:357–358.
144. Magides AD. A personal view of postdural puncture headache. Anaesthesia. 1991;46:694.
145. Weir EC. The sharp end of the dural puncture. BMJ. 2000;320:127.
146. Costigan SN, Sprigge JS. Dural puncture: the patients’ perspective. A patient survey of cases at a DGH maternity unit 1983-1993. Acta Anaesthesiol Scand. 1996;40:710–714.
147. Davies JM, Posner KL, Lee LA, et al. Liability associated with obstetric anesthesia: a closed claims analysis. Anesthesiology. 2009;110:131–139.
148. Halker RB, Demaerschalk BM, Wellik KE, et al. Caffeine for the prevention and treatment of postdural puncture headache: debunking the myth. Neurologist. 2007;13:323–327.
149. Camann WR, Murray RS, Mushlin PS, Lambert DH. Effects of oral caffeine on postdural puncture headache: a double-blind, placebo-controlled trial. Anesth Analg. 1990;70:181–184.
150. Mathew RJ, Wilson WH. Caffeine induced changes in cerebral circulation. Stroke. 1985;16:814–817.
151. Cohen SM, Laurito CE, Curran MJ. Grand mal seizure in a postpartum patient following intravenous infusion of caffeine sodium benzoate to treat persistent headache. J Clin Anesth. 1992;4:48–51.
152. Paech M. Unexpected postpartum seizures associated with post-dural puncture headache treated with caffeine. Int J Obstet Anesth. 1996;5:43–46.
153. Ryu JE. Effect of maternal caffeine consumption on heart rate and sleep time of breast-fed infants. Dev Pharmacol Ther. 1985;8:355–363.
154. Ergün U, Say B, Ozer G, et al. Intravenous theophylline decreases post-dural puncture headaches. J Clin Neurosci. 2008;15:1102–1104.
155. Carp H, Singh PJ, Vadhera R, Jayaram A. Effects of the serotonin-receptor agonist sumatriptan on postdural puncture headache: report of six cases. Anesth Analg. 1994;79:180–182.
156. Connelly NR, Parker RK, Rahimi A, Gibson CS. Sumatriptan in patients with postdural puncture headache. Headache. 2000;40:316–319.
157. Foster P. ACTH treatment for post-lumbar puncture headache. Br J Anaesth. 1994;73:429.
158. Kshatri AM, Foster PA. Adrenocorticotropic hormone infusion as a novel treatment for postdural puncture headache. Reg Anesth. 1997;22:432–434.
159. Gupta S, Agrawal A. Postdural puncture headache and ACTH. J Clin Anesth. 1997;9:258.
161. Rucklidge MW, Yentis SM, Paech MJ. Synacthen Depot for the treatment of postdural puncture headache. Anaesthesia. 2004;59:138–141.
162. Oliver CD, White SA. Unexplained fitting in three parturients suffering from postdural puncture headache. Br J Anaesth. 2002;89:782–785.
163. Noyan Ashraf MA, Sadeghi A, Azarbakht Z, et al. Evaluation of intravenous hydrocortisone in reducing headache after spinal anesthesia: a double blind controlled clinical study [corrected]. Middle East J Anesthesiol. 2007;19:415–422.
164. Huseyinoglu U, Huseyinoglu N, Hamurtekin E, et al. Effect of pregabalin on post-dural-puncture headache following spinal anesthesia and lumbar puncture. J Clin Neurosci. 2011;18:1365–1368.
165. Erol DD. The analgesic and antiemetic efficacy of gabapentin or ergotamine/caffeine for the treatment of postdural puncture headache. Adv Med Sci. 2011;56:25–29.
166. Eldor J, Guedj P, Cotev S. Epidural morphine injections for the treatment of postspinal headache. Can J Anaesth. 1990;37:710–711.
167. Jacobaeus HC, Frumerie K. About the leakage of spinal fluid after lumbar puncture and its treatment. Acta Med Scand. 1923;58:102–108.
168. Rice GG, Dabbs CH. The use of peridural and subarachnoid injections of saline solution in the treatment of severe postspinal headache. Anesthesiology. 1950;11:17–23.
169. Usubiaga JE, Usubiaga LE, Brea LM, Goyena R. Effect of saline injections on epidural and subarachnoid space pressures and relation to postspinal anesthesia headache. Anesth Analg. 1967;46:293–296.
170. Bart AJ, Wheeler AS. Comparison of epidural saline placement and epidural blood placement in the treatment of post-lumbar-puncture headache. Anesthesiology. 1978;48:221–223.
171. Baysinger CL, Menk EJ, Harte E, Middaugh R. The successful treatment of dural puncture headache after failed epidural blood patch. Anesth Analg. 1986;65:1242–1244.
172. Stevens RA, Jorgensen N. Successful treatment of dural puncture headache with epidural saline infusion after failure of epidural blood patch: case report. Acta Anaesthesiol Scand. 1988;32:429–431.
173. Cullen S. Current concept. Anesthesiology. 1960;21:564–568.
174. DiGiovanni AJ, Dunbar BS. Epidural injections of autologous blood for postlumbar-puncture headache. Anesth Analg. 1970;49:268–271.
175. Harrington BE. Postdural puncture headache and the development of the epidural blood patch. Reg Anesth Pain Med. 2004;29:136–163.
176. Abouleish E, Vega S, Blendinger I, Tio TO. Long-term follow-up of epidural blood patch. Anesth Analg. 1975;54:459–463.
177. Taivainen T, Pitkanen M, Tuominen M, Rosenberg PH. Efficacy of epidural blood patch for postdural puncture headache. Acta Anaesthesiol Scand. 1993;37:702–705.
178. Safa-Tisseront V, Thormann F, Malassine P, et al. Effectiveness of epidural blood patch in the management of post-dural puncture headache. Anesthesiology. 2001;95:334–339.
179. Williams EJ, Beaulieu P, Fawcett WJ, Jenkins JG. Efficacy of epidural blood patch in the obstetric population. Int J Obstet Anesth. 1999;8:105–109.
180. Banks S, Paech M, Gurrin L. An audit of epidural blood patch after accidental dural puncture with a Tuohy needle in obstetric patients. Int J Obstet Anesth. 2001;10:172–176.
181. Seebacher J, Ribeiro V, LeGuillou JL, et al. Epidural blood patch in the treatment of post dural puncture headache: a double blind study. Headache. 1989;29:630–632.
182. Sandesc D, Lupei MI, Sirbu C, et al. Conventional treatment or epidural blood patch for the treatment of different etiologies of post dural puncture headache. Acta Anaesthesiol Belg. 2005;56:265–269.
183. van Kooten F, Oedit R, Bakker SL, Dippel DW. Epidural blood patch in post dural puncture headache: a randomised, observer-blind, controlled clinical trial. J Neurol Neurosurg Psychiatry. 2008;79:553–558.
184. Szeinfeld M, Ihmeidan IH, Moser MM, et al. Epidural blood patch: evaluation of the volume and spread of blood injected into the epidural space. Anesthesiology. 1986;64:820–822.
185. Paech MJ, Doherty DA, Christmas T, Wong CA. The volume of blood for epidural blood patch in obstetrics: a randomized, blinded clinical trial. Anesth Analg. 2011;113:126–133.
186. Baysinger CL, Pope JE, Lockhart EM, Mercaldo ND. The management of accidental dural puncture and postdural puncture headache: a North American survey. J Clin Anesth. 2011;23:349–360.
187. Beards SC, Jackson A, Griffiths AG, Horsman EL. Magnetic resonance imaging of extradural blood patches: appearances from 30 min to 18 h. Br J Anaesth. 1993;71:182–188.
188. Vakharia SB, Thomas PS, Rosenbaum AE, et al. Magnetic resonance imaging of cerebrospinal fluid leak and tamponade effect of blood patch in postdural puncture headache. Anesth Analg. 1997;84:585–590.
189. Djurhuus H, Rasmussen M, Jensen EH. Epidural blood patch illustrated by CT-epidurography. Acta Anaesthesiol Scand. 1995;39:613–617.
190. DiGiovanni AJ, Galbert MW, Wahle WM. Epidural injection of autologous blood for postlumbar-puncture headache. II. Additional clinical experiences and laboratory investigation. Anesth Analg. 1972;51:226–232.
191. Carrie LE. Epidural blood patch: why the rapid response? Anesth Analg. 1991;72:129–130.
192. Coombs DW, Hooper D. Subarachnoid pressure with epidural blood patch. Reg Anesth Pain Med. 1979;4:3–6.
193. Dubost C, Le Gouez A, Zetlaoui PJ, et al. Increase in optic nerve sheath diameter induced by epidural blood patch: a preliminary report. Br J Anaesth. 2011;107:627–630.
194. Andrews PJ, Ackerman WE, Juneja M, et al. Transient bradycardia associated with extradural blood patch after inadvertent dural puncture in parturients. Br J Anaesth. 1992;69:401–403.
195. Brimacombe J, Clarke G, Craig L. Epidural blood patch in the Jehovah’s Witness. Anaesth Intensive Care. 1994;22:319.
196. Cucchiara RF, Wedel DJ. Finding cerebrospinal fluid during epidural blood patch: how to proceed. Anesth Analg. 1984;63:1121–1123.
197. Kawaguchi M, Hashizume K, Watanabe K, et al. Fluoroscopically guided epidural blood patch in patients with postdural puncture headache after spinal and epidural anesthesia. J Anesth. 2011;25:450–453.
198. Khayata I, Lance Lichtor J, Amelin P. Ultrasound-guided epidural blood patch. Anesthesiology. 2011;114:1453.
199. Martin R, Jourdain S, Clairoux M, Tetrault JP. Duration of decubitus position after epidural blood patch. Can J Anaesth. 1994;41:23–25.
200. Ong BY, Graham CR, Ringaert KR, et al. Impaired epidural analgesia after dural puncture with and without subsequent blood patch. Anesth Analg. 1990;70:76–79.
201. Blanche R, Eisenach JC, Tuttle R, Dewan DM. Previous wet tap does not reduce success rate of labor epidural analgesia. Anesth Analg. 1994;79:291–294.
202. Hebl JR, Horlocker TT, Chantigian RC, Schroeder DR. Epidural anesthesia and analgesia are not impaired after dural puncture with or without epidural blood patch. Anesth Analg. 1999;89:390–394.
203. Harding SA, Collis RE, Morgan BM. Meningitis after combined spinal-extradural anaesthesia in obstetrics. Br J Anaesth. 1994;73:545–547.
204. Parris WC. Post-dural puncture headache and epidural blood patch in an AIDS patient. J Clin Anesth. 1997;9:87–88.
205. Tom DJ, Gulevich SJ, Shapiro HM, et al. Epidural blood patch in the HIV-positive patient: review of clinical experience. San Diego HIV Neurobehavioral Research Center. Anesthesiology. 1992;76:943–947.
206. Bucklin BA, Tinker JH, Smith CV. Clinical dilemma: a patient with postdural puncture headache and acute leukemia. Anesth Analg. 1999;88:166–167.
207. Decramer I, Fuzier V, Franchitto N, Samii K. Is use of epidural fibrin glue patch in patients with metastatic cancer appropriate? Eur J Anaesthesiol. 2005;22:724–725.
208. Scher CS, Amar D, Wollner N. Extradural blood patch for post-lumbar puncture headaches in cancer patients. Can J Anaesth. 1992;39:203–204.
209. Diaz JH, Weed JT. Correlation of adverse neurological outcomes with increasing volumes and delayed administration of autologous epidural blood patches for postdural puncture headaches. Pain Pract. 2005;5:216–222.
211. Rice I, Wee MY, Thomson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth. 2004;92:109–120.
212. Riley CA, Spiegel JE. Complications following large-volume epidural blood patches for postdural puncture headache. Lumbar subdural hematoma and arachnoiditis: initial cause or final effect? J Clin Anesth. 2009;21:355–359.
213. Marfurt D, Lyrer P, Ruttimann U, et al. Recurrent post-partum seizures after epidural blood patch. Br J Anaesth. 2003;90:247–250.
214. Diaz JH. Permanent paraparesis and cauda equina syndrome after epidural blood patch for postdural puncture headache. Anesthesiology. 2002;96:1515–1517.
215. Mercieri M, Mercieri A, Paolini S, et al. Postpartum cerebral ischaemia after accidental dural puncture and epidural blood patch. Br J Anaesth. 2003;90:98–100.
216. Willner D, Weissman C, Shamir MY. Chronic back pain secondary to a calcified epidural blood patch. Anesthesiology. 2008;108:535–537.
217. Ambesh SP, Kumar A, Bajaj A. Epidural gelatin (Gelfoam) patch treatment for post dural puncture headache. Anaesth Intensive Care. 1991;19:444–447.
218. Barrios-Alarcon J, Aldrete JA, Paragas-Tapia D. Relief of post-lumbar puncture headache with epidural dextran 40: a preliminary report. Reg Anesth. 1989;14:78–80.
219. Aldrete JA. Persistent post-dural-puncture headache treated with epidural infusion of dextran. Headache. 1994;34:265–267.
220. Reynvoet ME, Cosaert PA, Desmet MF, Plasschaert SM. Epidural dextran 40 patch for postdural puncture headache. Anaesthesia. 1997;52:886–888.
221. Chanimov M, Berman S, Cohen ML, et al. Dextran 40 (Rheomacrodex) or Polygeline (Haemaccel) as an epidural patch for post dural puncture headache: a neurotoxicity study in a rat model of Dextran 40 and Polygeline injected intrathecally. Eur J Anaesthesiol. 2006;23:776–780.
222. Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion (Paris). 2008;48:1502–1516.
223. Kroin JS, Nagalla SK, Buvanendran A, et al. The mechanisms of intracranial pressure modulation by epidural blood and other injectates in a postdural puncture rat model. Anesth Analg. 2002;95:423–429.
224. Nakamura H, Matsuyama Y, Yoshihara H, et al. The effect of autologous fibrin tissue adhesive on postoperative cerebrospinal fluid leak in spinal cord surgery: a randomized controlled trial. Spine. 2005;30:E347–E351.
225. Crul BJ, Gerritse BM, van Dongen RT, Schoonderwaldt HC. Epidural fibrin glue injection stops persistent postdural puncture headache. Anesthesiology. 1999;91:576–577.
226. Kamada M, Fujita Y, Ishii R, Endoh S. Spontaneous intracranial hypotension successfully treated by epidural patching with fibrin glue. Headache. 2000;40:844–847.
227. Gerritse BM, van Dongen RT, Crul BJ. Epidural fibrin glue injection stops persistent cerebrospinal fluid leak during long-term intrathecal catheterization. Anesth Analg. 1997;84:1140–1141.
228. Harrington H, Tyler HR, Welch K. Surgical treatment of post-lumbar puncture dural CSF leak causing chronic headache: case report. J Neurosurg. 1982;57:703–707.
229. Obstetric Anaesthetists’ Association and the Association of Anaesthetists of Great Britain and Ireland. OAA-AAGBI guidelines for obstetric anesthetic services revised edition. [Available at] http://www.aagbi.org/sites/default/files/obstetric05.pdf; 2005 [Accessed March 2013] .