Chapter 25 Postoperative complications requiring intervention, diagnosis, and management
Overview
The literature regarding postoperative adverse events is difficult to interpret, because most reports are retrospective in nature, and the definitions of adverse events vary among institutions. Complication rates may vary by how hard one looks and by what definition an individual surgeon uses to declare a postoperative event as a complication rather than normal postoperative recovery. This is particularly true for retrospective analyses, which likely overlook many events. Over the past decade these difficulties have been addressed through the development of more carefully defined systems for documenting and grading perioperative complications to improve reporting and provide more uniform methods for comparing outcomes across institutions and countries (Bassi et al, 2005a; Clavien et al, 2009; DeOliveira et al, 2006; Grobmyer et al, 2007; Vin et al, 2008). These systems of standardized grading and reporting of adverse events have become the norm in medical oncology trials and are becoming so in prospective surgical trials. Such standardization should improve our ability to define the morbidity of a procedure and to compare morbidity among approaches and institutions.
Over the last 15 years, major hepatic and pancreatic resections have been performed with increasing frequency (Allen, 2007; Brennan et al, 2004; Cameron et al, 2006; Jarnagin et al, 2002; Nguyen et al, 2009; Riediger et al, 2007; Balcom et al, 2001). This increase has been most apparent at high-volume institutions and may be secondary to a trend in specialization. Multiple reports have shown a link between high-volume surgeons and institutions and decreased postoperative mortality for both pancreatic and liver resection (Begg et al, 1998; Birkmeyer et al, 2003; Eppsteiner et al, 2008, 2009; Lieberman et al, 1995). Despite the observed decrease in postoperative mortality, morbidity remains statistically unchanged over time with most large series reporting a 35% to 45% major complication rate following either pancreatic or hepatic resection (Ho et al, 2005; Simons et al, 2009; Turaga et al, 2008).
This apparent improvement in postoperative mortality without an observed decrease in morbidity suggests an improved ability to successfully rescue patients who experience significant postoperative complications. Management of serious complications following pancreatectomy and liver resection has shifted from operative reexploration to interventional or endoscopic procedures (Vin et al, 2008). High-quality imaging and increasing familiarity with the specific perioperative events has led to earlier detection and intervention and a subsequent reduction in the need for reoperation. Percutaneous drainage procedures are now the typical approach to postpancreatectomy leak or abscess, and they can be performed with minimal sedation and low morbidity.
Pancreatectomy
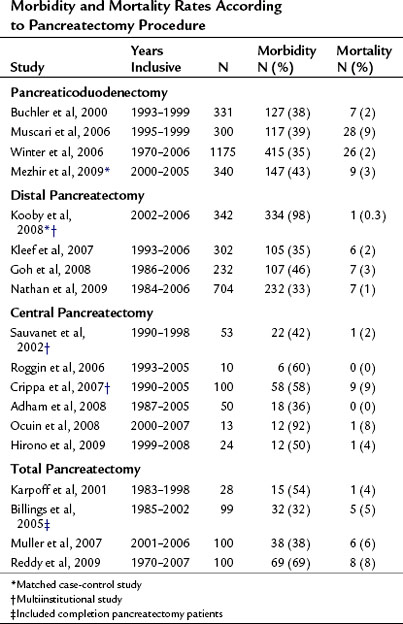
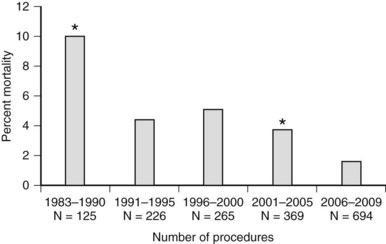
The mortality associated with pancreatectomy has significantly improved over the last 20 years; however, the morbidity has remained essentially unchanged. Operative mortality following pancreatic resection in large series reported since 2000 is presented in Table 25.1. The overall perioperative mortality has ranged from 2% to 9% for pancreaticoduodenectomy (PD), and reported morbidity rates are between 35% and 43% (Buchler et al, 2000; Mezhir et al, 2009, Muscari et al, 2006; Winter et al, 2006a). Recent studies show significantly improved mortality rates compared with reports from the 1980s and 1990s. This trend is illustrated in perioperative mortality data over a span of more than 25 years at Memorial Sloan-Kettering Cancer Center (MSKCC; Fig. 25.1). In a 5-year period between 1983 and 1990, the mortality rate was 10% in 125 pancreatic resections; between 2006 and 2009, the operative mortality rate was 1.7% in 694 pancreatic resections. The operative mortality rates presented in Table 25.1 are remarkably similar despite the fact that these data are from different institutions around the world.
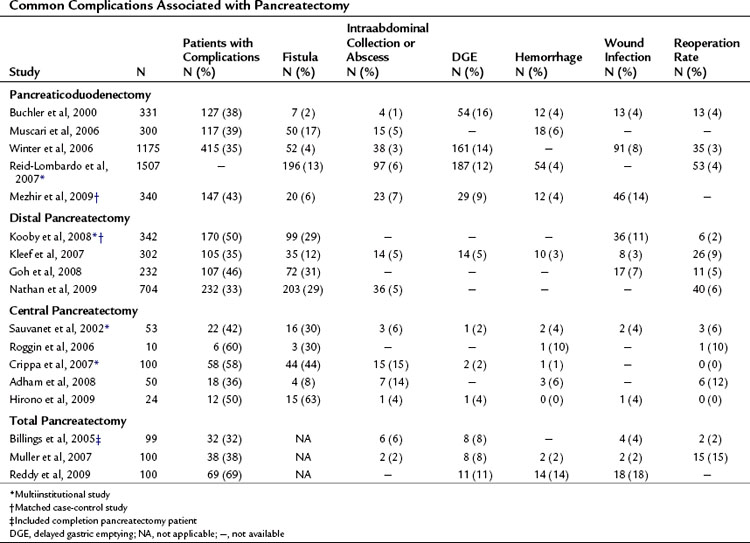
Complications may occur at the operative site (hemorrhage, leak, obstruction) or remotely (deep venous thrombosis, cardiac arrhythmia, pneumonia). Despite efforts to optimally manage comorbid conditions preoperatively, cardiac and pulmonary events occur following major surgeries. In addition, the combination of prolonged operative time, fluid volume, and underlying malignancy may increase the risk of certain complications unrelated to technique, such as pulmonary embolus, atrial dysrhythmia, and pneumonia. The most common severe complications following pancreatic and hepatic resection are directly related to the technical aspects of the operation itself. The frequency of the most commonly reported major complications following pancreatectomy are listed in Table 25.2. These include delayed gastric emptying (DGE), intraabdominal fluid collection or abscess, hemorrhage, wound infection, and anastomotic fistula. A comprehensive list of complications taken from a series of 204 consecutive pancreaticoduodenectomies at MSKCC from 2001 to 2003 is presented in Table 25.3 (Grobmyer et al, 2007).
| Complication | N | % |
|---|---|---|
| Anastomotic leak, pancreas | 24 | 12 |
| Wound infection | 22 | 11 |
| Delayed gastric emptying | 14 | 7 |
| Hemorrhage | 10 | 5 |
| Intraabdominal abscess | 8 | 4 |
| Fascial dehiscence or evisceration | 7 | 3 |
| Supraventricular arrhythmia | 7 | 3 |
| Urinary tract infection | 6 | 3 |
| Anastomotic leak, biliary | 6 | 3 |
| Hypotension, shock | 5 | 2 |
| Cellulitis | 3 | 1.5 |
| Clostridium difficile colitis | 3 | 1.5 |
| Congestive heart failure, left ventricular dysfunction | 3 | 1.5 |
| Myocardial infarction | 3 | 1.5 |
| Renal failure | 3 | 1.5 |
| Apnea or hypoxia | 2 | 1 |
| Atelectasis | 2 | 1 |
| Catheter-related infection | 2 | 1 |
| Deep venous thrombosis | 2 | 1 |
| Dehydration | 2 | 1 |
| Anastomotic leak, intestinal | 2 | 1 |
| Gastrointestinal bleeding | 2 | 1 |
| Pleural effusion | 2 | 1 |
| Pneumonitis | 2 | 1 |
| Sepsis | 2 | 1 |
| Small bowel obstruction | 1 | 0.5 |
| Angina, cardiac ischemia | 1 | 0.5 |
| Aspiration | 1 | 0.5 |
Modified from Grobmyer SR, et al, 2007: Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg 204(3):356-364.
Pancreatic leak/fistula continues to be one of the most common and most difficult complications following pancreatectomy. Multiple studies have reported pancreatic fistula to be more common following central or distal pancreatectomy compared with pancreaticoduodenectomy (Pratt et al, 2006; Goh et al, 2008; Kleeff et al, 2007; Kooby et al, 2008; Nathan et al, 2009; Ferrone et al, 2008; Reid-Lombardo et al, 2007). The series reported in Table 25.1 also demonstrate that central pancreatectomy (Adham et al, 2008; Crippa et al, 2007; Hirono et al, 2009; Ocuin et al, 2008; Roggin et al, 2006; Sauvanet et al, 2002) and total pancreatectomy (Billings et al, 2005; Karpoff et al, 2001; Muller et al, 2007; Reddy et al, 2009) are procedures associated with slightly higher overall morbidity than PD and distal pancreatectomy, and total pancreatectomy is associated with a slightly higher mortality rate than PD.
Defining and Grading Complications After Pancreatectomy
Accurate identification and reporting of perioperative complications are vital to improve quality of care and to compare outcomes among different institutions and time periods. Without a standardized system for complication recording, there is a significant risk of inaccuracy and underreporting. Recently, several institutions have implemented specific classification and grading systems for surgical complications (Clavien et al, 1992, 2009; DeOliveira et al, 2006; Grobmyer et al, 2007). These systems have developed simplified definitions of postoperative complications and a grading system for severity using therapy-oriented grading schema.
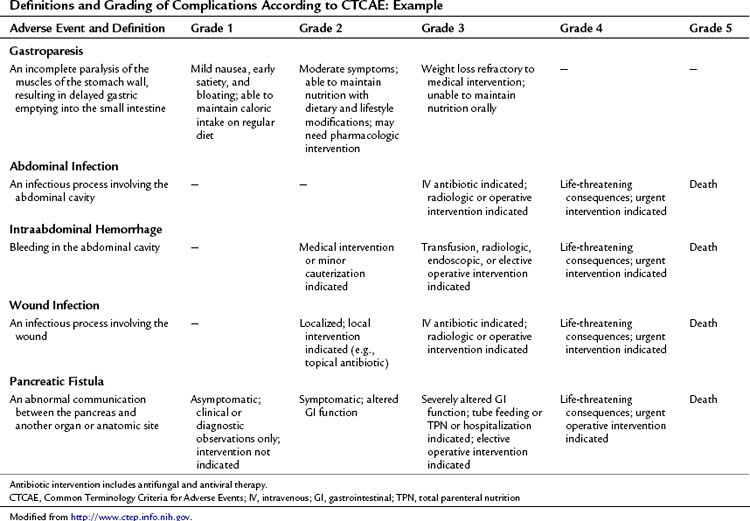
This approach to defining and grading adverse events has been well established in medical oncology. The Common Terminology Criteria for Adverse Events (CTCAE) developed by the National Institutes of Health Cancer Therapy Evaluation Program (CTEP) has been utilized in clinical trials since 1982 (National Cancer Institute’s Cancer Therapy Evaluation Program). CTCAE is an example of a universally employed terminology system for complication reporting that has been utilized across medical disciplines. This system also includes a limited schema for postoperative complications. Table 25.4 demonstrates an example of the grading system for common complications following pancreatectomy. The grade of the complication increases with the invasiveness of the treatment required.
Among the complications listed, pancreatic fistula is one of the most severe that can occur after pancreatectomy. The reporting of this complication is arguably the most problematic, as definitions have varied. Pancreatic fistula often precedes other complications, such as abscess and hemorrhage. Inherent to its cause, complications from pancreatic leak, or any anastomotic leak for that matter, often overlap (see Management of Fistula, Leak, and Abscess below). When a postoperative patient has fever, tachycardia, and leukocytosis and cross-sectional imaging reveals a perianastomotic fluid collection, the etiology of this collection is likely an anastomotic leak. When the collection is drained, if it is infected, it may be recorded or defined as an abscess. If the fluid is amylase rich, one investigator may report it as a pancreatic fistula and another may report it as a pancreatic leak. This ambiguity is reflected in reports that reflect rates of intraabdominal abscess for pancreatectomy ranging from 1% to 15%. Moreover, the incidence of fistula ranges from 2% to 27% for PD and 12% to 31% for distal pancreatectomy in part as a result of the inconsistent definitions utilized in studies from different institutions.
Toward a Unifying System for Reporting Pancreatic Fistula
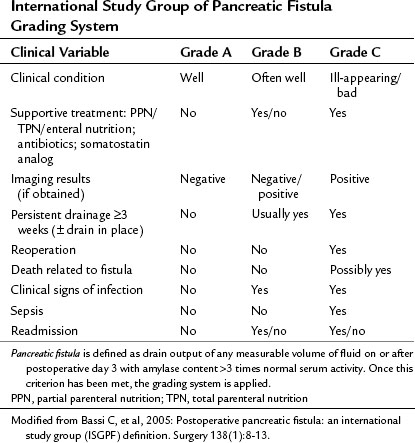
Pancreatic fistula has been recently defined by the International Study Group of Pancreatic Fistula (ISGPF; Bassi et al, 2005a). This system has served as a foundation for defining and reporting pancreatic fistula among numerous studies and is an example of the efforts to unify complication reporting. The study group comprised an international panel of pancreatic surgeons working in high-volume centers, and it aimed to develop a simplified and universal definition of pancreatic fistula with a grading system based on clinical impact. Fistula was defined as “drain output of any measurable volume of fluid on or after postoperative day 3 with amylase content greater than three times that of normal serum amylase.” Three grades were applied according to clinical impact, from grade A (none) to grade C (significant; Table 25.5). As demonstrated in Table 25.6, many institutions have implemented this system for reporting the rates and outcomes of pancreatic fistula.
Independent prospective validation of the ISGPF definition and grading system has been reported. Pratt and others (2006) prospectively analyzed postoperative complications in 176 consecutive patients after pancreaticoduodenectomy. There were 53 patients with confirmed fistulae (30% incidence), categorized in the various grades: 26 patients (15%) were grade A, 21 (12%) were grade B, and 6 (3%) were grade C. No deaths were related to fistula in this study. Patients with grade A fistulas had shorter hospital stays and fewer secondary complications than patients with grade B and C fistulas, although the overall rate of secondary complications between grade B and C fistulas was not significantly different (76% vs. 100%, respectively, P = .2). Patients with grade C fistulas had a higher frequency of intensive care unit (ICU) transfer and blood transfusions and longer hospital stays compared with those who had grade B fistulas, and five of the six patients with a grade C fistula were discharged to a rehabilitation facility, significantly more than patients with grade A and B fistulae.
Randomized Controlled Trials Aimed at Reducing Complications
Multiple prospective randomized controlled trials have been performed to evaluate technique and complications following pancreatic resection. Selected prospective trials published since 2000 are presented in Table 25.7. In general, these studies have reported conflicting outcomes with no specific technique demonstrating superiority. The interpretation of surgical technique trials is difficult, as most trials randomize patients between “the standard technique” at a given institution and a “new,” perhaps less familiar technique. These studies are often underpowered, and it is therefore not surprising that equivalency is concluded. Fortunately, none of the reported techniques have been shown to be inferior from an oncologic standpoint, and therefore any of these reported techniques may be considered acceptable in the appropriately selected patient.
Table 25.7 Prospective Randomized, Controlled Trials Aimed at Reducing Perioperative Complications After Pancreatectomy
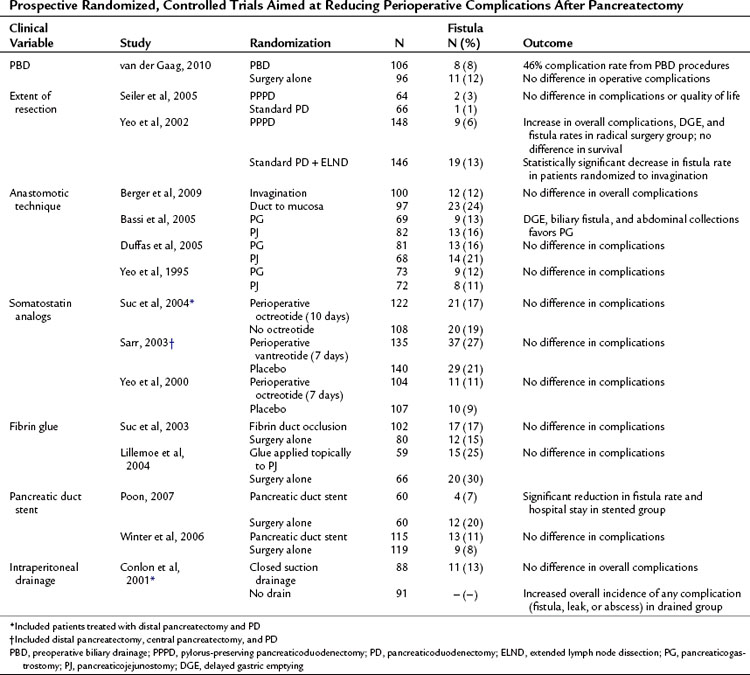
Routine preoperative biliary drainage (PBD) has been evaluated in multiple prospective randomized trials. These trials have evaluated both percutaneous external and endoscopic internal drainage (see Chapters 27 and 28). These studies, as well as multiple retrospective case-matched reports, have documented an increase in perioperative complications in patients undergoing PBD (Mezhir et al, 2009; Povoski et al, 1999). A recent prospective, controlled clinical trial randomized patients with serum bilirubin levels between 2.3 and 14.6 mg/dL to PBD or no PBD prior to pancreaticoduodenectomy (van der Gaag et al, 2010). The authors reported 39% significant complications in the no PBD group compared with 74% in the PBD group (P < .001). These data support previous prospective and retrospective studies and do not support routine PBD in patients with moderately elevated bilirubin.
Pylorus preservation has been evaluated as a technique to decrease the morbidity of PD (see Chapter 62A). Standard PD with partial gastrectomy versus pylorus-preserving PD (PPPD), with or without extended lymphadenectomy, has also been investigated in randomized trials. As demonstrated in Table 25.7, no benefit was demonstrated for either approach in regard to operative or long-term oncologic outcomes, but extended lymphadenectomy was associated with increased perioperative morbidity, including DGE and pancreatic fistula (Yeo et al, 2002; Seiler et al, 2005).
Pancreatic fistula rates have been assessed according to anastomotic technique in an attempt to reduce postoperative pancreatic fistula. Randomized comparisons have been made between pancreaticojejunal invagination to direct duct-mucosa anastomosis and pancreaticojejunostomy (PJ) to pancreaticogastrostomy (PG). Berger and colleagues (2009) performed a two-institution randomized controlled trial comparing pancreatic invagination to duct–mucosa anastomosis with patients stratified based on the texture of the pancreas. A significant reduction in fistula rates were seen when using the invagination technique (12%) compared with duct-mucosa anastomosis (24%), and leak rates were significantly higher overall in soft glands. Bassi and colleagues (2005b) showed a decrease in DGE, biliary fistula, and abdominal collections with PG; however, these findings have not been replicated by other groups (Duffas et al, 2005; Yeo et al, 1995). No study to date has demonstrated convincingly an optimal method for pancreatic stump closure following distal pancreatectomy (Knaebel et al, 2005); the results of an ongoing, large, multiinstitutional randomized trial may provide some guidance (Diener et al, 2008).
Other adjuncts to reduce pancreatic leak have included somatostatin analogs, stents, and tissue glues. The perioperative use of somatostatin analogs was found to reduce fistula rates in several European trials (Montorsi et al, 1995; Buchler et al, 1992; Friess et al, 1995). However, these findings have not been reproduced in recent large, single, and multiinstitutional studies performed in the United States (Sarr, 2003; Suc et al, 2004; Yeo et al, 2000). Fibrin glue has been applied in two settings to determine its role for reducing pancreatic fistula, applied in a perianastomotic fashion (Lillemoe et al, 2004) or as a plug into the pancreatic duct prior to anastomosis (Suc et al, 2003). Neither study showed a reduction in fistula rates. Two prospective trials evaluated pancreatic stent placement at the time of anastomosis; in one study, a pancreatic duct stent significantly reduced the rate of pancreatic fistula (Poon et al, 2007), but the other study did not demonstrate any difference in fistula rates or overall complications (Winter et al, 2006b).
The routine use of intraperitoneal drains following elective pancreatic surgery remains an area of debate. Conlon and colleagues (2001) randomized 179 patients after pancreatic resection (PD or distal pancreatectomy) to placement of intraperitoneal drains or no drains, demonstrating no benefit to routine drainage. Moreover, patients in the drainage group were more likely to have an intraabdominal abscess, collection, or fistula.
Management of Postpancreatectomy Complications
Pancreatic Leak, Fistula, and Abscess
Vin and colleagues (2008) reported a series of 908 pancreatic resections and the rate and management of pancreatic fistula, leak, and abscess. As demonstrated in Figure 25.2, a significant overlap was found among these three separately defined complications in the 158 patients in which they occurred. This further highlights the difficulty in defining discrete complications, the crossover between different definitions, and the clinical relevance of complication grading. In this study, a pancreatic fistula, defined as clinical signs or symptoms with amylase-rich drainage greater than 50 mL/day beyond postoperative day 10, was identified in 92 patients; however, in 63 of these (69%), the fluid that was drained was culture positive. These patients could therefore have been defined as having either or both a pancreatic fistula and postoperative abscess. Regardless of the definition, patients who develop a pancreatic fistula, leak, or abscess will often evidence similar findings and be treated in a similar fashion. Importantly, the type of complication (fistula, leak, or abscess) was not associated with presentation (fever, leukocytosis) or overall morbidity (duration of drainage, length of stay, ICU admission); however, these factors were similar in patients who had a similar grade of complication.
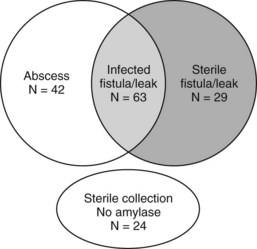
(Modified from Vin Y, et al, 2008: Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg 207[4]:490-498.)
Vin and colleagues (2008) reported a 90-day mortality rate of 5% in patients who developed fistula, leak, or abscess. These complications were more common in men and in patients undergoing treatment for bile duct, ampullary, and duodenal tumors (63 [31%] of 206) compared with pancreatic tumors (95 [14%] of 702; P < .001). Type of resection (PD vs. distal pancreatectomy), tumor histopathologic subtype, and PBD were not associated with occurrence of fistula, leak, or abscess. Surgical drains were placed in 88 patients with these complications. The surgical drain alone, without additional interventional radiologic management, was successful for treatment in 16 (18%) of 88 patients, and 133 patients were managed with percutaneous drainage alone.
Delayed Gastric Emptying
Delayed gastric emptying (DGE) after PD is common (see Table 25.2) and is typically reported in 9% to 16% of patients. A review of randomized controlled trials for treatment of DGE has been recently published (Traverso & Hashimoro, 2008), examining the literature for level I evidence, and four trials were identified that focused on the prevention of DGE. The largest study from Yeo and others (1993) randomized patients to erythromycin or control and found no reduction in the incidence of DGE. In a separate study, erythromycin did significantly reduce postoperative DGE, but the study was very small, and DGE occurred in 57% of patients in the control arm (Ohwada et al, 2001). Tani and colleagues (2006) randomized patients to retrocolic or antecolic gastrojejunostomy and found a significantly lower rate of DGE with the antecolic approach.
Although DGE and pylorus preservation have been a concern, to date no study has found a difference in the incidence of DGE following conventional compared with pylorus-preserving procedures (Diener et al, 2007). As discussed earlier in this chapter, and as highlighted in Table 25.7, the reported incidence of DGE was lower in two studies analyzing anastomotic technique. Bassi and colleagues (2005b) found a statistically significant reduction in DGE in patients who had PG compared to PJ. In the study by Yeo and others (2002), comparing radical with standard PD, an increase in DGE was seen in the radical group. It is important to note, however, that neither study had DGE as the primary end point.
Among these studies, significant variability was observed in the range of reported incidence of DGE. As with pancreatic fistula, part of the variability in incidence across studies was from the variation in definition. The ISGPF recently devised a formal grading system for DGE based on duration of nasogastric tube placement and the timing of patients’ tolerance of solid food (Wente et al, 2007).
Hemorrhage
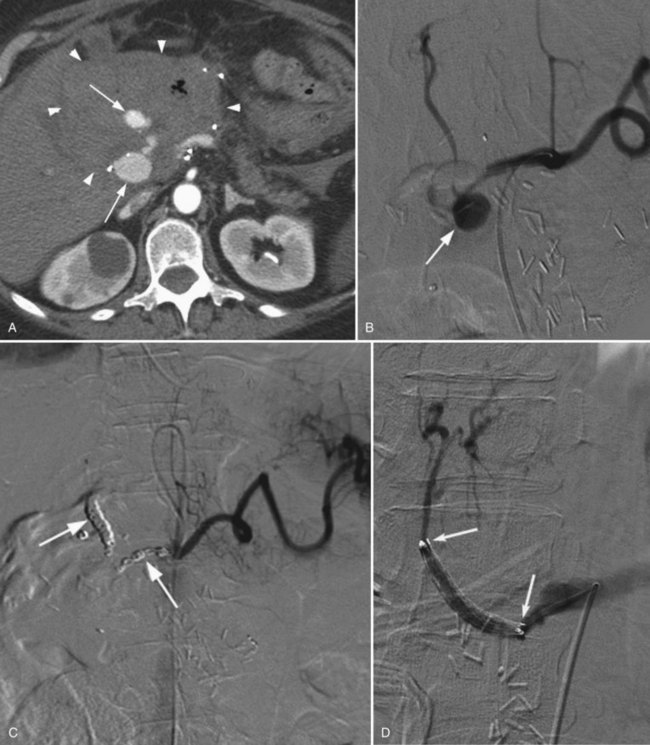
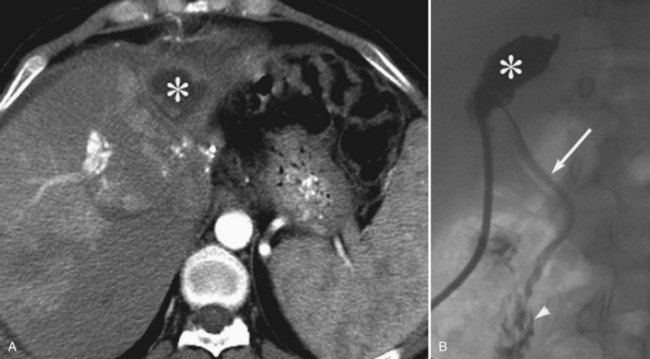
Postoperative hemorrhage is a potentially lethal complication after pancreatectomy, and it can occur in the immediate postoperative period (<7 days) or in a delayed fashion (>14 days). Management is often guided first by the patient’s hemodynamic stability. Any unstable patient should be transfused and taken for reexploration. Patients who can be easily stabilized may be candidates for endoscopic or interventional radiologic management. Intraluminal bleeding is most commonly from the gastrojejunal anastomosis and can often be managed with endoscopic therapy. Extraluminal bleeding from arterial pseudoaneurysm (Fig. 25.3) or vascular erosion from a pancreatic fistula typically occur later, following operation, and these can be approached via angiographic embolization in stable patients. A high index of suspicion is critical, as mortality from late postoperative hemorrhage is significant (Brodsky & Turnbull, 1991).
Yekebas and others (2007) analyzed the treatment and outcome of 87 patients who had postoperative hemorrhage in a series of 1669 pancreatic resections in 1524 patients (overall hemorrhage rate, 6%). Hemorrhage was classified as mild in 36 patients (41%) and severe in 51 patients (59%); 29 patients (33%) had a sentinel bleed. The bleeding site was extraluminal in 51 (59%) and intraluminal in 36 (41%); 17 patients had what appeared to be extraluminal hemorrhage, but further evaluation confirmed bleeding from the transected pancreas or resection cavity; and 34 patients (39%) had pancreatic fistula that preceded hemorrhage.
This and other studies have demonstrated that significant morbidity and mortality are associated with postoperative hemorrhage, especially when delayed beyond postoperative day 5 (Choi et al, 2004). These patients often require reoperation or interventional radiology procedures to halt life-threatening hemorrhage. Furthermore, patients who experience a postoperative hemorrhage often have underlying complications that require treatment, such as pancreatic fistula. If angiographic embolization successfully treats hemorrhage, cross-sectional imaging should be performed to assess for the presence of leak, fistula, or abscess that would require drainage.
Imaging and Interventional Therapy of Complications after Pancreatic Surgery
Imaging of Complications After Pancreatic Surgery
Imaging plays an important role in identifying complications and directing therapy in patients who have recently undergone pancreatic resection. The observation that 22% of patients undergoing a PD over a 12-year period required percutaneous interventions highlights the importance of imaging and image-guided interventions in the postoperative management of these patients (Zink et al, 2009). Transabdominal ultrasound (US) is a commonly available imaging tool that enables detection of focal, encapsulated, or free intraperitoneal fluid collections, but gastrointestinal gas and/or postoperative pneumoperitoneum superimposition represents the major disadvantage of US in the evaluation of the pancreas (see Chapter 13).
Computed tomography (CT) is the most effective imaging modality for evaluation of the pancreas after surgery (Johnson et al, 2002; see Chapter 16). It allows detection of the normal features as well as postoperative complications such as bleeding, anastomotic leakage, abscess, or fistula formation (Johnson et al, 2002; Scialpi et al, 2005). CT is performed when there is clinical or biochemical suspicion of complications in the early postoperative period (Scialpi et al, 2005). The data sets obtained with modern multidetector-row CT scanners provide voxels that are both submillimeter in dimension and isotropic, enabling reformations in any desired plane with spatial resolution similar to that in the transverse plane. Ideally the multiphase scan protocol includes good oral contrast with unenhanced CT as well as intravenous contrast-enhanced CT, including images acquired during the arterial phase and portal venous phase.
One potential advantage of MRI over CT imaging is the option to perform both contrast-enhanced MRI and magnetic resonance cholangiopancreatography (MRCP) using thick-slab and thin-slab, highly T2-weighted imaging (see Chapter 17). Ishizu and colleagues (1997) reported visualizing the pancreatic duct with MRCP in 91% of patients (69% complete duct visualization and 23% partial duct visualization) who had previous pancreatic surgery. This feature may enable improved management of leaks; however, the use of magnetic resonance imaging (MRI) must be balanced by its lower spatial resolution and reduced accessibility compared with CT.
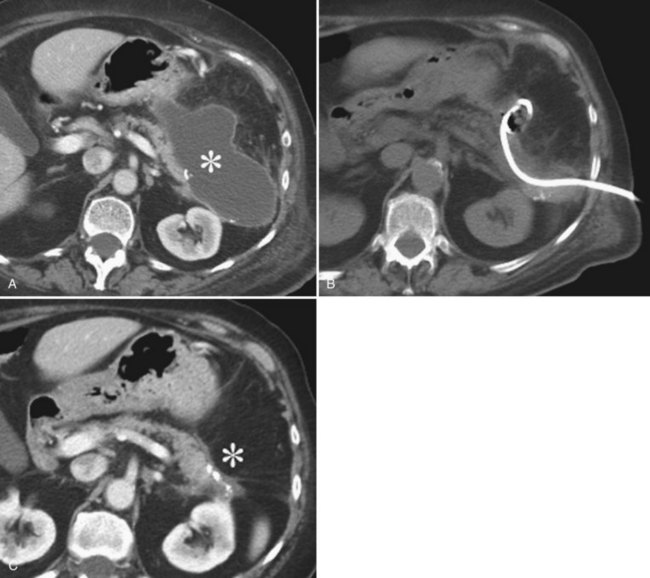
On imaging studies, postoperative fluid collections are the most commonly seen complication of pancreatic surgery. An infected collection (abscess) is characterized by a complex fluid collection, representing pus and detritus, surrounded by a thick rim of contrast enhancement, representing inflammatory tissue (Fig. 25.4). Septations and gas inclusions may also be present. However, a clear differentiation between uninfected postoperative fluid collections with surrounding granulation tissue and abscess cannot be reliably done by imaging, and fine needle aspiration (FNA) is necessary when infection is suspected. Fluid collections after pancreatic surgery may occur in either retroperitoneal or intraperitoneal locations. The location of the fluid collection may suggest its origin. It has been observed that fluid collections around the PJ site were significantly more frequently associated with a pancreatic fistula. Fluid collections around the pancreas have shown elevated amylase values compatible with leakage in 82% of cases, in contrast to perihepatic collections, which have elevated fluid amylase values in only 46% of the collections (Zink et al, 2009; Hashimoto et al, 2007).
One of the most challenging imaging interpretations in the PD patient is distinguishing the jejunal anastomotic loop from a postsurgical fluid collection. The course of the normal loop can usually be followed from its origin to the pancreatic and biliary anastomoses in the right upper quadrant. Rarely, oral contrast refluxes into the loop, which can help identify its presence. In some cases, needle puncture of the possible collection with subsequent contrast injection may be necessary to differentiate a postsurgical fluid collection from the normal postsurgical jejunal loop (Gervais et al, 2001).
A fluid-containing pancreatic fistula presents as a tubular tract communicating with the main pancreatic duct, often surrounded by a rim of contrast enhancement. At MRCP, a fistula often shows a highly hyperintense T2 signal because of the pancreatic juice. Fistula can often be demonstrated with CT and MRI/MRCP. It has been reported that with CT, the site of pancreatic fistula, may be identified in 75% of patients compared with 93% with MRI and MRCP (O’Toole et al, 2007).
Finally, hematomas and pseudoaneurysms are important imaging findings after pancreatic surgery. Hematomas may appear as dense collections on CT, and active bleeding may be seen as contrast extravasation. Pseudoaneurysms may be the result of direct injury related to the dissection or to pancreatic fluid eroding a vessel; often they do not occur until several days to months after surgery (Sugimoto et al, 2001). Imaging with CT, Doppler ultrasound (US), or MRI may identify these pseudoaneurysms and help guide intervention (see Chapter 19; see Figure 25.3).
Interventional Radiology Techniques for the Postpancreatic Surgery Patient
Interventional radiology (IR) techniques have become the first-line management choice for many postpancreatectomy complications. Image-guided drainage of intraabdominal fluid collections, percutaneous biliary drainage (PBD), and angiography with vessel embolization permit a less invasive approach to postsurgical complications and frequently mitigate the need for reoperation. Sohn and colleagues (2003) reported the use of postoperative IR procedures in 12% of 1061 patients undergoing PD. And according to Baker and others (2008), these postoperative IR procedures were for intraabdominal abscess drainage (72%), PBD (18%), and angiography with or without embolization (10%).
Image-Guided Abdominal Drainage
One of the major advances in the management of postoperative fluid collections and abscesses over the past 2 decades has been the introduction of image-guided percutaneous drainage procedures, which have changed the management of patients previously requiring an urgent reexploration. Small fluid collections at the resection site are common in the immediate postprocedural period and usually resolve spontaneously. These collections generally do not require an interventional procedure, but they can be aspirated with a needle if there is clinical suspicion for infection (Gervais et al, 2001). Abscess drainage can be performed with US but is done more often with CT guidance (see Fig. 25.4). The technical success rate has been reported to be 96% to 100% (Betsch et al, 2002; Lagan et al, 2008; Zink et al, 2009). The complication rate of interventional drainage of fluid collections is low, however, bleeding and bowel perforation have been reported (Lambiase et al, 1992; Betsch et al, 2002; Lagan et al, 2008; Zink et al, 2009).
There are two widely used techniques of imaging-guided abscess drainage: the trocar technique and the Seldinger technique. The trocar technique is performed by direct puncture of the abscess using a trocar needle, which is covered by the catheter, and by removal of the trocar needle after the catheter is in position. The Seldinger technique is performed by puncture of the abscess using a coaxial needle, through which a guidewire is inserted into the fluid collection. The needle is then removed, leaving the guidewire in situ. The needle tract is serially dilated by exchange of dilators and guidewires. Finally, a drainage catheter is inserted into the abscess over the guidewire. The advantage of the Seldinger technique is that it is less traumatic to the surrounding tissue because of the initial use of small needles and the subsequent dilation with dilators, until the size of the catheter is reached. The advantage of the trocar technique is that this procedure is easier and faster to perform. Independent of the technique, a wide range of drainage catheters is available, ranging from 6 to 14 Fr in diameter (Gerzof et al, 1978; Goletti et al, 1993; vanSonnenberg et al, 1984). Although no difference has been seen comparing 7 and 14 Fr drainage (Roethlin et al, 1998), the diameter of the catheter may be chosen on the basis of viscosity of the content.
Percutaneous drainage of abdominal fluid collections has been reported to be as effective as operative drainage. No significant differences have been reported in mortality or morbidity, and the duration of hospital stay has been similar when patients were matched for age, sex, diagnosis, and abscess etiology and location (Hemming et al, 1991; Olak et al, 1986).
The earliest experiences with CT-guided percutaneous drainage of abdominal abscesses were reported in the late 1970s (Gerzof et al, 1978). Since then, the efficacy of the procedure has been confirmed in several studies, in which success rates of drainage of abdominal fluid collection or abscesses were 62% to 96% (Zink et al, 2009; Betsch et al, 2002; Lagan et al, 2008; Lambiase et al, 1992; Gerzof et al, 1978; vanSonnenberg et al, 1984; Cantasdemir et al, 2003; Cinat et al, 2002; Freeny et al, 1988). Multiple drainage procedures may be necessary in the same patient to achieve clinical success (Zink et al, 2009; Cinat et al, 2002).
A similar high rate of successful imaging-guided drainage can be achieved in patients with accompanying pancreatic fistula. The reported success rates in patients with accompanying fistulas have been 67% to 89% (Zink et al, 2009; Aassar et al, 1999; Kerlan et al, 1985; Singh et al, 2006). One important prognostic factor associated with decreased success of percutaneous drainage is the presence of yeast in the abscess fluid (Cinat et al, 2002).
Although studies have shown that fluid collections with pancreatic fistulae have similar drainage success compared with fluid collections without an active pancreatic leak, fluid collections with pancreatic fistulae require longer drainage. Zink and others (2009) reported the median duration of drainage in patients with abscess was 29 days compared with 11 days in patients with a sterile fluid collection. Patients with a pancreatic leak had median drain duration of 30 days (Zink et al, 2009).
Percutaneous Biliary Drainage
Biliary obstruction or leak is another example of a complication that can occur after pancreatic surgery that can be managed using nonoperative techniques. Endoscopic management is possible but frequently difficult because of the alteration in the anatomy as a result of surgery. Malignant strictures can be palliated with a biliary drainage catheter, and metallic stents may be useful, but they frequently occlude in less than a year (Dahlstrand et al, 2009). Therefore, the decision to pursue metallic stenting often hinges upon a patient’s life expectancy. Benign strictures can be relieved with balloon dilation but may also recur and require repeated dilations (Kocher et al, 2007). Bile leaks at the hepaticojejunostomy usually require both drainage of the bile fluid collection and PBD to divert bile and allow for the leak to heal (Gervais et al, 2001; Dorico et al, 2008).
Angiography and Embolization (See Chapter 19)
Postoperative bleeding pseudoaneurysms usually occur relatively late after surgery and are associated with a high mortality rate (Rumstadt et al, 1998). In a report by Otah and colleagues (2002), the average presentation was 19 days after surgery, and the majority of patients were already discharged home. All patients in this report first had a sentinel bleed with minimal blood seen in drains or in the GI tract. Sentinel bleeds were defined as the appearance or paradoxical increase in the amount of blood from the abdominal drains or GI tract combined with a decrease in hemoglobin of 2 g/dL at least 3 days after surgery (Tien et al, 2008). This has led some to take a very aggressive approach to the presence of even minimal bleeding beyond 3 days after resection. CT angiography can sometimes identify the vessel of interest, however, this potential benefit must be weighed with the additional contrast dose needed to perform CT. Given that the likely bleeding source will be identified on angiography, the CT may not be beneficial, especially in patients with borderline renal function. Many believe angiography with possible embolization should be pursued in the presence of a sentinel bleed, with reoperation reserved only for the presence of active, massive bleeding or failed embolization (Otah et al, 2002; Blanc et al, 2007). The success rate is approximately 85% (Tsai et al, 2007).
Pseudoaneurysms after pancreatic surgery occur in the gastroduodenal artery most commonly, followed by the hepatic artery, superior mesenteric artery, and splenic artery (Tien et al, 2008). Selective coil embolization across the pseudoaneurysm is successful in approximately 85% of patients (Tsai et al, 2007). More recently, however, some have reported the use of covered stents, instead of coils, to exclude the pseudoaneurysm while preserving flow to distal structures supplied by the injured vessel (Narumi et al, 2007; Suzuki et al, 2009; Wallace et al, 2007). A demonstration of these images and techniques is shown in Figure 25.3.
Drain Management
Imaging method depends on physician preference: some prefer a fluoroscopic tube injection; others prefer a CT scan, and others still prefer both studies. The fluoroscopic contrast injection will show the remaining cavity size and the presence of fistula. The CT can also show the cavity size but not necessarily a fistula. If the CT shows a collection remaining around the catheter, a malfunctioning tube must be suspected, as functional tubes should have no collection around the tube. If imaging demonstrates nondraining locules, some prefer to try an intracavitary tissue-type plasminogen activator (TPA). One report showed an 89% success rate using TPA to drain abscess refractory to simple catheter drainage (Beland et al, 2008). The risk of bleeding should be carefully considered prior to the use of TPA in a postpancreatectomy patient, and this should be utilized only in carefully selected patients.
Postpancreatic drains may be present for several weeks. The study by Vin and colleagues (2008) demonstrated a mean duration of 38 days (range, 3 to 228 days). Pancreatic drains ideally require an active participation by the patient or patient’s caregivers.
Supportive Management of Pancreatic Fistula
Patients with infected fluid collections are typically treated with broad-spectrum antibiotic therapy until clinical signs and symptoms of infection resolve. To date, no strong clinical evidence is available to show that somatostatin analogs enhance fistula closure (Stojadinovic et al, 2003). A recent prospective trial randomized patients with pancreatic or enterocutaneous fistulae to intramuscular injection of 30 mg of a sustained-release somatostatin analog, lanreotide, or placebo (Gayral et al, 2009). The investigators found a significant reduction in fistula output within 72 hours of injection (primary end point), however, no difference was found in fistula closure rates (secondary end point).
Reoperation
Improvements in interventional and endoscopic techniques have reduced the need for reoperation in many patients after pancreatic resection. Reoperation is still necessary, however, in 3% to 5% of patients following pancreatic or liver resection, and it should be considered in patients with clinical deterioration from hemorrhage, abscess, leak/fistula, or intestinal ischemia. Reoperation rates, as seen in Table 25.2, range from 4% to 15% and vary according to type of pancreatic resection. Standop and others (2009) reported their series of 31 patients (11% of 285 consecutive PDs) who required reoperation. The most common indications for reoperation were hemorrhage (29%) and intraabdominal abscess (23%). Among patients who required reoperation, 45% required a second operative procedure. The mortality rate among patients requiring reoperation was 13% compared with 2% for the cohort of patients that did not require reoperation. Significant mortality in patients requiring reoperation is as high as 60% in some series, indicating the critically ill nature of these patients (Hoshal et al, 2004).
Hepatectomy
A large series of hepatic resection reveals that the morbidity associated with open resection ranges from 14% to 45%, and the reported mortality ranges from 0% to 3% (Table 25.8; Jarnagin et al, 2002; Adam et al, 2006; Cescon et al, 2009; Huang et al, 2009; Imamura et al, 2003, Palavecino et al, 2010; Poon et al, 2004; Rees et al, 2008; Koffron et al, 2007). The outcome following hepatic resection has significantly improved over the last 20 years. In 1977, Foster and Berman first reported a multicenter analysis of 621 hepatic resections. In this report, the overall mortality was 13%, and it was more than 20% in patients who had major hepatic resection. The most common cause of operative mortality was hemorrhage. Large series from high-volume centers published in the late 1990s have reported mortality rates of less than 5% (Fan et al, 1999; Gayowski et al, 1994). The significant improvements are multifactorial and include a better understanding of hepatic anatomy, modern cross-sectional imaging, improved anesthetic techniques, improved patient selection, and the use of parenchymal-sparing resections. These innovations have significantly reduced the incidence of massive hemorrhage, which is no longer the dominant cause of death after hepatic resection. The perioperative mortality rate over the last 20 years at MSKCC reflects this trend, with operative mortality rate decreasing from 4.3% from 1996 to 1997 to 1.3% in 2000 to 2001 (P < .001). From September 2001 to September 2009, we performed 1877 hepatic resections, and the 90-day mortality rae remained at 2.1%. Over the past 8 years, median estimated blood loss was slightly lower (300 mL), but mean hospital stay remained the same (9 days). With this reduction in hemorrhage, a shift has been seen toward hepatic failure and infectious complications being the leading causes of death after hepatic resection.
Table 25.8 Morbidity and Mortality Rates After Hepatic Resection from Large, Single-Institution Series

The most commonly reported complications following hepatectomy are presented in Table 25.8. In the series of 1803 patients reported by Jarnagin and others (2002), the most common complications arose from the liver and biliary tract that included perihepatic abscess, hepatic failure, sterile perihepatic fluid collection, and bile leak or biloma. Pulmonary complications were the second most frequent complication and included symptomatic pleural effusion, pneumonia, and atelectasis. These findings are similar to other large series that have carefully documented complications by system; these are presented in Table 25.9 (Cescon et al, 2009; Imamura et al, 2003; Jarnagin et al, 2002; Poon et al, 2004).
Factors associated with the development of perioperative complications after hepatectomy have been examined in large series. In the series reported by Jarnagin and colleagues (2002), one of the patterns noted over time was a significant increase in parenchymal-sparing segmental resections and a decrease in the number of hepatic segments resected. This corresponded to a decrease in blood loss, decreased use of blood products, and shortened hospital stay (Table 25.10). On multivariate analysis, two factors predictive of morbidity and mortality included the number of hepatic segments resected (Fig. 25.5) and operative blood loss. Retrospective data from MSKCC, as well as those from other institutions, have shown that a parenchymal-sparing approach is associated with less perioperative morbidity without compromising oncologic outcome in appropriately selected patients (Gold et al, 2008; de Haas et al, 2008). Independent predictors of morbidity following hepatic resection in the series from Jarnagin and colleagues (2002) included major concomitant biliary procedure or vascular resection, lower preoperative albumin, and elevated preoperative creatinine. Additional factors associated with increased mortality included elevated preoperative bilirubin, complex hepatic resection, a lower preoperative platelet count, and increased age.
Table 25.10 Trends in Operative Results After Hepatectomy at Memorial Sloan-Kettering Cancer Center (1983–2001)

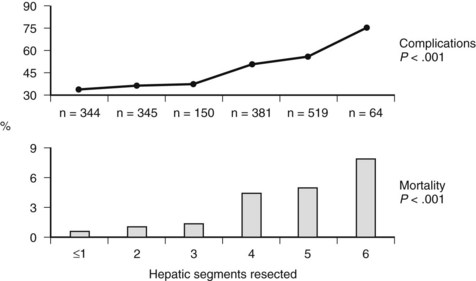
FIGURE 25.5 Perioperative complications and mortality rate stratified by the number of hepatic segments resected.
(Reproduced from Jarnagin WR, et al, 2002: Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236[4]:397-406.)
Attempts to reduce complications following hepatic resection have been aimed at techniques to reduce blood loss, operative time, and the requirement for transfusion (see Chapter 22). One major advance in liver surgery has been the concept of low central venous pressure (CVP) anesthesia during resection. It has been demonstrated from retrospective (Melendez et al, 1998) and prospective randomized controlled trials (Wang et al, 2006) that maintaining a low CVP (<5 mm Hg) results in reduced blood loss, lower morbidity, and increased preservation of renal function after hepatic resection.
Techniques for parenchymal transection and their association with postoperative complications have been extensively studied (Pamecha et al, 2009; Poon, 2007). Techniques for parenchymal division include crush clamping, stapling, vessel sealing systems (i.e., LigaSure, Covidien, Boulder, CO), and multiple options for ultrasonic dissection with or without intermittent inflow occlusion. Among the numerous techniques studied in randomized trials, there does not appear to be a single “best” option. Since most studies are performed by high-volume, experienced liver surgeons, it may be difficult to show small differences among techniques (Palavecino et al, 2010; Arita et al, 2005; Ikeda et al, 2009; Patrlj et al, 2010). A recent Cochrane review analyzed seven randomized controlled trials comparing methods for parenchymal division, which included 556 patients (Pamecha et al, 2009). The authors concluded that the crush-clamp technique was the procedure of choice, because the parenchymal transection devices offered no improvement in time or reduction in blood loss compared with crush clamping. In addition, the crush-clamp technique was faster in many of the studies examined. In one positive randomized controlled trial, Lesurtel and colleagues (2005) found that the crush-clamp technique with intermittent vascular inflow occlusion was more effective in terms of reduced time and blood loss and more cost efficient than ultrasonic dissection devices.
An additional technique to potentially reduce the need for perioperative blood and plasma transfusion is intraoperative acute normovolemic hemodilution (ANH), which has recently been studied in a prospective randomized trial at MSKCC (Jarnagin et al, 2008), where 136 patients undergoing major hepatectomy (three or more segments) were randomized to ANH or standard low-CVP anesthesia techniques. Patients randomized to the hemodilution arm had a reduction in the overall perioperative transfusion rate, but this did not reach statistical significance (50% vs. 25% in the standard arm; P = .067). ANH patients did have a statistically significant decrease in intraoperative transfusions and had higher postoperative hemoglobin levels compared with the control arm. Furthermore, where intraoperative blood loss was 800 mL or greater, hemodilution patients had a statistically significant decrease in both red cell and plasma transfusion rates. Hemodilution was safe and effective, yet the ideal patients for routine use are currently not defined.
In an effort to better predict the need for intraoperative transfusion, Sima and colleagues (2009) developed a nomogram for perioperative transfusion risk. Factors such as number of segments in the planned resection, malignant versus benign diagnosis, anticipation of extrahepatic resection, and decreased preoperative hemoglobin and platelet count predict the risk for potential transfusion, and blood conservation techniques such as acute normovolemic hemodilution may be planned preoperatively.
The need for routine intraperitoneal drains following hepatic resection has been studied in a prospective manner. Fong and colleagues (1996) randomized 120 patients to drain or no drain following hepatic resection; patients were stratified based on extent of resection, and no difference was found in overall morbidity, perioperative mortality, or requirement for percutaneous drainage between groups. The authors reported that in the 50 subsequent hepatic resections following completion of the trial, only four patients (8%) required percutaneous drainage. These results were concordant with a recent Cochrane review, which demonstrated no evidence for routine placement of intraperitoneal drains following elective hepatectomy (Gurusamy et al, 2007). Currently, it is our practice to drain selectively, and we consider routine drainage only in those patients who have ongoing biliary leakage from the hepatic transection surface and/or a bilioenteric anastomosis created at the time of resection.
Imaging and Interventional Therapy of Bile Leaks and Abscesses After Hepatectomy
Imaging
US, helical CT, and MRI/MRCP are the noninvasive diagnostic procedures widely applied to the liver and biliary system. Ordinary postoperative fluid collections, bilomas, hematomas, and abscesses can be detected with these modalities. Acute and subacute hemorrhage usually shows a typical high density in CT and can therefore be reliably diagnosed with this modality. In addition, active bleeding can be demonstrated by contrast media extravasation on contrast-enhanced CT. In particular, the typical appearance of biloma on imaging is that of an encapsulated mass with space-occupying and space-conforming characteristics contiguous with the liver resection plane or biliary structure (Vujic & Brock, 1982). Repeated imaging may be necessary to clearly differentiate between uninfected postoperative fluid collections and abscesses because of overlapping. In addition, an ordinary postoperative fluid collection may progress to become an abscess. FNA may be necessary to rule out infection when a patient’s condition is suspicious for infection and imaging shows an equivocal fluid collection.
Interventional Procedures for Treating Posthepatectomy Patients
Hepatic Abscess Drainage
Percutaneous drainage of a hepatic abscess is a minimally invasive, image-guided procedure that carries lower morbidity and is less expensive compared with surgical treatment (Ferraioli et al, 2008). Preparation for the procedure includes evaluation for coagulopathy, choosing the imaging modality for guidance during the procedure (US or CT), and choosing the appropriate route of access. Single abscesses may be drained with US guidance, whereas multiple abscesses may require CT guidance and placement of multiple catheters (vanSonnenberg et al, 2001).
The technical success rate of US- and CT-guided hepatic catheter drainage has been reported to be 94% to 96% (Liu et al, 2009). Abscesses located near the dome of the diaphragm may be technically more difficult to drain without transgressing the pleura, but clinical success rates for percutaneous drainage of intrahepatic abscesses of 70% to 100% have been reported (Akinci et al, 2005; Attar et al, 1986; Dondelinger et al, 1990; Gerzof et al, 1985; Giorgio et al, 2006; Rajak et al, 1998; Mezhir et al, 2010). We reported that yeast in culture and the presence of an obstructed biliary tree were independent predictors of failure of percutaneous drainage of pyogenic hepatic abscess (Mezhir et al, 2010). When biliary obstruction is present, relief of the obstructed biliary tree is mandatory for successful abscess treatment (Fig. 25.6). Size and the presence of loculations did not preclude successful percutaneous drainage in this study.
Although single, small abscesses are generally adequately treated by percutaneous drainage, debate continues on the optimal treatment of large, multiloculated abscesses. Nonetheless, similar clinical success rates of 82% to 92% have been reported for single and multiple abscesses and for single multiloculated and multiple multiloculated abscesses (Liu et al, 2009; Ogawa et al, 1999). Other authors have reported higher success rates for unilocular abscesses and have proposed a treatment algorithm, with small abscesses being treated with antibiotics alone; large, unilocular abscess with percutaneous drainage plus antibiotics; and large, multiloculated abscessed treated with surgical therapy (Hope et al, 2008). It has been reported that repeated catheter drainage improves the success rate (Hope et al, 2008). The clinical success rate for treatment of unilocular abscesses was 83% if a single drainage was performed and 100% if repeated drainages were performed. The success rate for treatment of multilocular abscesses was 33% for single drainage and 50% if repeated drainages were performed (Hope et al, 2008). Some suggest use of intracavitary TPA to aid with multiloculated collections refractory to simple percutaneous drainage (Beland et al, 2008).
Several studies have shown the superiority of percutaneous catheter drainage over needle aspiration. For abscesses, needle aspiration was successful in 60% to 92% of patients, whereas percutaneous catheter drainage was successful in 97% to 100% of patients (Giorgio et al, 2006; Rajak et al, 1998; Singh et al, 2009; Zerem & Hadzic, 2007). Among the successfully treated patients, the average times for clinical improvement, hospital stay, and total resolution of the abscess were similar in the two treatment groups (Giorgio et al, 2006; Rajak et al, 1998; Singh et al, 2009; Zerem & Hadzic, 2007). In one study no significant difference in the success rate between aspiration and drainage was reported, but multiple aspirations were required in the aspiration group to achieve success (Yu et al, 2004).
Interventional Management of Bilomas and Bile Leaks
Hepatic tumor surgery may also be complicated by biliary leak. It is known that a large number of ordinary postsurgical bile leaks heal spontaneously (Czerniak et al, 1988), but many perihepatic bilomas require percutaneous drainage, particularly those that become infected (Christoforidis et al, 2007). In cases of persistence of a fistula, patients may have prolonged hospital stays (Czerniak et al, 1988). Endoscopic transpapillary drainage or PBD may serve to decompress the biliary system and divert bile flow from the defect in the bile ducts, which may lead to healing of the fistula without need for repeat surgery.
Percutaneous transhepatic biliary drainage has been proven as a low-risk procedure that provides a considerable reduction in repeat laparotomy (Cozzi et al, 2006; Funaki et al, 1999; Kaufman et al, 1985; Vaccaro et al, 1991). It has been reported that percutaneous transhepatic bile drainage was successful in patients with bile leaks in 81% to 90% (Cozzi et al, 2006, Funaki et al, 1999, Ernst et al, 1999) and that technical success was achieved in 90% to 100% when the bile ducts were not dilated (Cozzi et al, 2006; Funaki et al, 1999). Several authors have reported that the success rate of percutaneous or endoscopic treatment depends on bile leak location: success rates are higher when bile leaks are located at the resection plane of the liver, and they are lower when bile leaks occur in the area of the main bile ducts (Fragulidis et al, 2008; Lo et al, 1998).
Aassar OS, et al. Percutaneous management of abscess and fistula following pancreaticoduodenectomy. Cardiovas Intervent Radiol. 1999;22(1):25-28.
Adam R, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244(4):524-535.
Adham M, et al. Central pancreatectomy: single-center experience of 50 cases. Arch Surg. 2008;143(2):175-180. discussion 180-181
Akinci D, et al. Percutaneous drainage of 300 intraperitoneal abscesses with long-term follow-up. Cardiovasc Intervent Radiol. 2005;28(6):744-750.
Allen PJ. Pancreatic adenocarcinoma: putting a hump in survival. J Am Coll Surg. 2007;205(Suppl 4):S76-S80.
Arita J, et al. Randomized clinical trial of the effect of a saline-linked radiofrequency coagulator on blood loss during hepatic resection. Br J Surg. 2005;92(8):954-959.
Attar B, et al. CT-guided percutaneous aspiration and catheter drainage of pyogenic liver abscesses. Am J Gastroenterol. 1986;81(7):550-555.
Baker TA, et al. Role of interventional radiology in the management of complications after pancreaticoduodenectomy. Am J Surg. 2008;195(3):386-390.
Balcom JH, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136(4):391-398.
Bassi C, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8-13.
Bassi C, et al. Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg. 2005;242(6):767-771. discussion 771-773
Begg CB, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747-1751.
Beland MD, et al. Complex abdominal and pelvic abscesses: efficacy of adjunctive tissue-type plasminogen activator for drainage. Radiology. 2008;247(2):567-573.
Berger AC, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208(5):738-747. discussion 747-749
Betsch A, et al. CT-guided percutaneous drainage of intra-abdominal abscesses: APACHE III score stratification of 1-year results. Acute Physiology, Age, Chronic Health Evaluation. Eur Radiol. 2002;12(12):2883-2889.
Billings BJ, et al. Quality of life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9(8):1059-1066. discussion 1066-1067
Birkmeyer JD, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117-2127.
Blanc T, et al. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194(1):3-9.
Brennan MF, et al. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240(2):293-298.
Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy: the “sentinel bleed.”. Arch Surg. 1991;126(8):1037-1040.
Buchler M, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163(1):125-130. discussion 130-131
Buchler MW, et al. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87(7):883-889.
Cameron JL, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10-15.
Cantasdemir M, et al. Percutaneous drainage for treatment of infected pancreatic pseudocysts. South Med J. 2003;96(2):136-140.
Cescon M, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249(6):995-1002.
Choi SH, et al. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199(2):186-191.
Christoforidis E, et al. A single-center experience in minimally invasive treatment of postcholecystectomy bile leak, complicated with biloma formation. J Surg Res. 2007;141(2):171-175.
Cinat M, et al. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. 2002;137(7):845-849.
Clavien PA, et al. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(5):518-526.
Clavien PA, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196.
Conlon KC, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234(4):487-493. discussion 493-494
Cozzi G, et al. Percutaneous transhepatic biliary drainage in the management of postsurgical biliary leaks in patients with nondilated intrahepatic bile ducts. Cardiovasc Intervent Radiol. 2006;29(3):380-388.
Crippa S, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246(1):69-76.
Czerniak A, et al. The management of fistulas of the biliary tract after injury to the bile duct during cholecystectomy. Surg Gynecol Obstet. 1988;167(1):33-38.
Dahlstrand U, et al. Primary patency of percutaneously inserted self-expanding metallic stents in patients with malignant biliary obstruction. HPB (Oxford). 2009;11(4):358-363.
de Haas RJ, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248(4):626-637.
DeOliveira ML, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244(6):931-937. discussion 937-939
Diener MK, et al. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg. 2007;245(2):187-200.
Diener MK, et al. DISPACT trial: a randomized controlled trial to compare two different surgical techniques of DIStal PAnCreaTectomy—study rationale and design. Clin Trials. 2008;5(5):534-545.
Dondelinger RF, et al. Percutaneous treatment of pyogenic liver abscess: a critical analysis of results. Cardiovasc Intervent Radiol. 1990;13(3):174-182.
Dorico R, et al. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl. 2008;14(5):611-615.
Duffas JP, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189(6):720-729.
Eppsteiner RW, et al. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008;12(10):1709-1716. discussion 1716
Eppsteiner RW, et al. Surgeon volume impacts hospital mortality for pancreatic resection. Ann Surg. 2009;249(4):635-640.
Ernst O, et al. Biliary leaks: treatment by means of percutaneous transhepatic biliary drainage. Radiology. 1999;211(2):345-348.
Fan ST, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229(3):322-330.
Ferraioli G, et al. Percutaneous and surgical treatment of pyogenic liver abscesses: observation over a 21-year period in 148 patients. Dig Liv Dis. 2008;40(8):690-696.
Ferrone CR, et al. Pancreatic fistula rates after 462 distal pancreatectomies: staplers do not decrease fistula rates. J Gastrointest Surg. 2008;12(10):1691-1697. discussion 1697-1698
Fong Y, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171(1):158-162.
Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1-342.
Fragulidis G, et al. Managing injuries of hepatic duct confluence variants after major hepatobiliary surgery: an algorithmic approach. World J Gastroenterol. 2008;14(19):3049-3053.
Freeny PC, et al. Infected pancreatic fluid collections: percutaneous catheter drainage. Radiology. 1988;167(2):435-441.
Friess H, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg. 1995;82(9):1270-1273.
Funaki B, et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts. Am J Roentgenol. 1999;173(6):1541-1544.
Gayowski TJ, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116(4):703-710. discussion 710-711
Gayral F, et al. Randomized, placebo-controlled, double-blind study of the efficacy of lanreotide 30 mg PR in the treatment of pancreatic and enterocutaneous fistulae. Ann Surg. 2009;250(6):872-877.
Gervais DA, et al. Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics. 2001;21(3):673-690.
Gerzof SG, et al. Computed tomography in the diagnosis and management of abdominal abscesses. Gastrointest Radiol. 1978;3(3):287-294.
Gerzof SG, et al. Intrahepatic pyogenic abscesses: treatment by percutaneous drainage. Am J Surg. 1985;149(4):487-494.
Giorgio A, et al. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. Am J Roentgenol. 2006;187(6):1585-1590.
Goh BK, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008;143(10):956-965.
Gold JS, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg. 2008;247(1):109-117.
Goletti O, et al. Percutaneous ultrasound-guided drainage of intra-abdominal abscesses. Br J Surg. 1993;80(3):336-339.
Grobmyer SR, et al. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. J Am Coll Surg. 2007;204(3):356-364.
Gurusamy KS, et al, 2007: Routine abdominal drainage for uncomplicated liver resection. Cochrane Database Syst Rev (3):CD006232.
Hashimoto M, et al. CT features of pancreatic fistula after pancreaticoduodenectomy. Am J Roentgenol. 2007;188(4):W323-W327.
Hemming A, et al. Surgical versus percutaneous drainage of intra-abdominal abscesses. Am J Surg. 1991;161(5):593-595.
Hirono S, et al. A central pancreatectomy for benign or low-grade malignant neoplasms. J Gastrointest Surg. 2009;13(9):1659-1665.
Ho CK, et al. Complications of pancreatic surgery. HPB (Oxford). 2005;7(2):99-108.
Hope W, et al. Optimal treatment of hepatic abscess. Am Surg. 2008;74(2):178-182.
Hoshal VLJr, et al. Personal experience with the Whipple operation: outcomes and lessons learned. Am Surg. 2004;70(2):121-125. discussion 126
Huang ZQ, et al. Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl). 2009;122(19):2268-2277.
Ikeda M, et al. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg. 2009;250(2):199-203.
Imamura H, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138(11):1198-1206. discussion 1206
Ishizu H, et al. The role of magnetic resonance cholangiopancreatography (MRCP) after resection of the pancreas. Surg Today. 1997;27(3):285-287.
Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397-406. discussion 407
Jarnagin WR, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248(3):360-369.
Johnson P, et al. Spiral CT following the Whipple procedure: distinguishing normal postoperative findings from complications. J Computer Assist Tomo. 2002;26(6):956-961.
Karpoff HM, et al. Results of total pancreatectomy for adenocarcinoma of the pancreas. Arch Surg. 2001;136(1):44-47. discussion 48
Kaufman S, et al. Percutaneous transhepatic biliary drainage for bile leaks and fistulas. Am J Roentgenol. 1985;144(5):1055-1058.
Kerlan RK, et al. Abdominal abscess with low-output fistula: successful percutaneous drainage. Radiology. 1985;155(1):73-75.
Kleeff J, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245(4):573-582.
Knaebel HP, et al. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92(5):539-546.
Kocher M, et al. Percutaneous treatment of benign bile duct strictures. Eur J Radiol. 2007;62(2):170-174.
Koffron AJ, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246(3):385-392. discussion 392-394
Kooby DA, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248(3):438-446.
Lagan D, et al. Image-guided percutaneous treatment of abdominal-pelvic abscesses: a 5-year experience. Radiol Med. 2008;113(7):999-1007.
Lambiase RE, et al. Percutaneous drainage of 335 consecutive abscesses: results of primary drainage with 1-year follow-up. Radiology. 1992;184(1):167-179.
Lesurtel M, et al. How should transection of the liver be performed? A prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242(6):814-822. discussion 822-823
Lieberman MD, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222(5):638-645.
Lillemoe KD, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8(7):766-772. discussion 772-774
Liu C-H, et al. Percutaneous hepatic abscess drainage: do multiple abscesses or multiloculated abscesses preclude drainage or affect outcome? J Vasc Interven Radiol. 2009;20(8):1059-1065.
Lo CM, et al. Biliary complications after hepatic resection: risk factors, management, and outcome. Arch Surg. 1998;133(2):156-161.
Melendez JA, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187(6):620-625.
Mezhir JJ, et al. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13(12):2163-2169.
Mezhir JJ, et al. Current management of pyogenic liver abscess: surgery is now second-line treatment. J Am Coll Surg. 2010;210(6):975-983.
Montorsi M, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery. 1995;117(1):26-31.
Muller MW, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246(6):966-974. discussion 974-975
Muscari F, et al. Risk factors for mortality and intra-abdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006;139(5):591-598.
Narumi S, et al. Endovascular treatment of life-threatening pseudoaneurysm of the hepatic artery after pancreaticoduodenectomy. Hepatogastroenterology. 2007;54(79):2152-2154.
Nathan H, et al. Risk factors for pancreatic leak after distal pancreatectomy. Ann Surg. 2009;250(2):277-281.
National Cancer Institute Cancer Therapy Evaluation Program. Available online at http://ctep.cancer.gov
Nguyen KT, et al. World review of laparoscopic liver resection: 2,804 patients. Ann Surg. 2009;250(5):831-841.
Ocuin LM, et al. Comparison of central and extended left pancreatectomy for lesions of the pancreatic neck. Ann Surg Oncol. 2008;15(8):2096-2103.
Ogawa T, et al. The role of percutaneous transhepatic abscess drainage for liver abscess. J Hepatobiliary Pancreat Surg. 1999;6(3):263-266.
Ohwada S, et al. Low-dose erythromycin reduces delayed gastric emptying and improves gastric motility after Billroth I pylorus-preserving pancreaticoduodenectomy. Ann Surg. 2001;234(5):668-674.
Olak J, et al. Operative vs. percutaneous drainage of intraabdominal abscesses: comparison of morbidity and mortality. Arch Surg. 1986;121(2):141-146.
Otah E, et al. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137(1):55-59.
O’Toole D, et al. Diagnosis and management of pancreatic fistulae resulting in pancreatic ascites or pleural effusions in the era of helical CT and magnetic resonance imaging. Gastroenterol Clin Biol. 2007;31(8-9):686-693.
Palavecino M, et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery. 2010;147(1):40-48.
Pamecha V, et al. Techniques for liver parenchymal transection: a meta-analysis of randomized controlled trials. HPB (Oxford). 2009;11(4):275-281.
Patrlj L, et al. Combined blunt-clamp dissection and ligasure ligation for hepatic parenchyma dissection: postcoagulation technique. J Am Coll Surg. 2010;210(1):39-44.
Poon RT. Current techniques of liver transection. HPB (Oxford). 2007;9(3):166-173.
Poon RT, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240(4):698-708. discussion 710
Poon RT, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;246(3):425-433. discussion 433-435
Povoski SP, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230(2):131-142.
Pratt W, et al. Postoperative pancreatic fistulas are not equivalent after proximal, distal, and central pancreatectomy. J Gastrointest Surg. 2006;10(9):1264-1278. discussion 1278-1279
Rajak CL, et al. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. Am J Roentgenol. 1998;170(4):1035-1039.
Reddy S, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250(2):282-287.
Rees M, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125-135.
Reid-Lombardo KM, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11(11):1451-1458. discussion 1459
Riediger H, et al. Long-term outcome after resection for chronic pancreatitis in 224 patients. J Gastrointest Surg. 2007;11(8):949-959. discussion 959-960
Roethlin MA, et al. Percutaneous drainage of abdominal abscesses: are large-bore catheters necessary? Eur J Surg. 1998;164(6):419-424.
Roggin KK, et al. Central pancreatectomy revisited. J Gastrointest Surg. 2006;10(6):804-812.
Rumstadt B, et al. Hemorrhage after pancreatoduodenectomy. Ann Surg. 1998;227(2):236-241.
Sarr MG. The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg. 2003;196(4):556-564. discussion 564-565; author reply 565
Sauvanet A, et al. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 2002;132(5):836-843.
Scialpi M, et al. Imaging evaluation of post pancreatic surgery. Eur J Radiol. 2005;53(3):417-424.
Seiler CA, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92(5):547-556.
Sima CS, et al. Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg. 2009;250(6):914-921.
Simons JP, et al. National complication rates after pancreatectomy: beyond mere mortality. J Gastrointest Surg. 2009;13(10):1798-1805.
Singh A, et al. Imaging-guided catheter drainage of abdominal collections with fistulous pancreaticobiliary communication. Am J Roentgenol. 2006;187(6):1591-1596.
Singh O, et al. Comparative study of catheter drainage and needle aspiration in management of large liver abscesses. Indian J Gastroenterol. 2009;28(3):88-92.
Sohn TA, et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointestinal Surg. 2003;7(2):209-219.
Standop J, et al. Operative re-intervention following pancreatic head resection: indications and outcome. J Gastrointest Surg. 2009;13(8):1503-1509.
Stojadinovic A, et al. An evidence-based approach to the surgical management of resectable pancreatic adenocarcinoma. J Am Coll Surg. 2003;196(6):954-964.
Suc B, et al. Temporary fibrin glue occlusion of the main pancreatic duct in the prevention of intra-abdominal complications after pancreatic resection: prospective randomized trial. Ann Surg. 2003;237(1):57-65.
Suc B, et al. Octreotide in the prevention of intra-abdominal complications following elective pancreatic resection: a prospective, multicenter randomized controlled trial. Arch Surg. 2004;139(3):288-294. discussion 295
Sugimoto M, et al. Secondary aortoduodenal fistula mimicking submucosal tumor. Endoscopy. 2001;33(1):101.
Suzuki K, et al. Stent-graft treatment for bleeding superior mesenteric artery pseudoaneurysm after pancreaticoduodenectomy. Cardiovasc Intervent Radiol. 2009;32(4):762-766.
Tani M, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243(3):316-320.
Tien YW, et al. Angiography is indicated for every sentinel bleed after pancreaticoduodenectomy. Ann Surg Oncol. 2008;15(7):1855-1861.
Traverso LW, Hashimoro Y. Delayed gastric emptying: the state of the highest level of evidence. J Hepatobiliary Pancreat Surg. 2008;15(3):262-269.
Tsai CC, et al. Transcatheter arterial coil embolization of iatrogenic pseudoaneurysms after hepatobiliary and pancreatic interventions. Hepatogastroenterology. 2007;54(73):41-46.
Turaga K, et al. In hospital outcomes after pancreatectomies: an analysis of a national database from 1996 to 2004. J Surg Oncol. 2008;98(3):156-160.
Vaccaro JP, et al. Treatment of biliary leaks and fistulae by simultaneous percutaneous drainage and diversion. Cardiovasc Intervent Radiol. 1991;14(2):109-112.
van der Gaag NA, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362(2):129-137.
vanSonnenberg E, et al. Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part I: results, failures, and complications. Radiology. 1984;151(2):337-341.
vanSonnenberg E, et al. Percutaneous abscess drainage: update. World J Surg. 2001;25(3):362-369.
Vin Y, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207(4):490-498.
Vujic I, Brock JG. Biloma: aspiration for diagnosis and treatment. Gastrointest Radiol. 1982;7(3):251-254.
Wallace MJ, et al. Superior mesenteric artery pseudoaneurysm following pancreaticoduodenectomy: management by endovascular stent-graft placement and transluminal thrombin injection. Cardiovasc Intervent Radiol. 2007;30(3):518-522.
Wang WD, et al. Low central venous pressure reduces blood loss in hepatectomy. World J Gastroenterol. 2006;12(6):935-939.
Wente MN, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761-768.
Winter JM, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10(9):1199-1210. discussion 1210-1211
Winter JM, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10(9):1280-1290. discussion 1290
Yekebas E, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246(2):269-280.
Yeo CJ, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy: a prospective, randomized, placebo controlled trial. Ann Surg. 1993;218(3):229-237.
Yeo CJ, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222(4):580-588. discussion 588-592
Yeo CJ, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232(3):419-429.
Yeo CJ, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236(3):355-366. discussion 366-368
Yu SCH, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39(4):932-938.
Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. Am J Roentgenol. 2007;189(3):W138-W142.
Zink S, et al. Pancreaticoduodenectomy: frequency and outcome of post-operative imaging-guided percutaneous drainage. Abdominal Imaging. 2009;34(6):767-771.