Postoperative and Chronic Pain
Systemic and Regional Analgesic Techniques
Pamela Flood MD, Pedram Aleshi MD
Chapter Outline
NONPHARMACOLOGIC INTERVENTIONS
Mechanisms and Prevalence of Pain
Pregnancy is associated with increased excitability of mechanosensitive afferent nerve fibers innervating the uterine cervix and lower uterine corpus. This change in sensitivity of the nerve fibers is likely due, at least in part, to elevated estrogen levels during pregnancy.1,2 Whether skin and visceral nociception are transmitted by different nerve fiber subgroups or discharge patterns is controversial.3 The uterine (visceral) afferent fibers stimulated by pressure and vasoconstriction primarily include C fibers and some A-delta fibers. By contrast, the majority of afferent fibers that relay nociceptive stimuli from the skin are A-delta fibers.
Postoperative pain results from direct trauma to tissue and subsequent inflammation. Local and systemic inflammatory cytokines act to sensitize the peripheral nerves and enhance pain perception.4 Inflammation likely plays a significant role in pain after delivery because inflammatory cytokines are elevated as a part of the normal labor and delivery process.5,6 Additionally, after cesarean delivery, cytokines are present in the wound; their concentration is positively correlated with analgesic drug consumption.7
Multimodal therapy has long been advocated for postoperative analgesia in nonobstetric patients, and it clearly provides benefit to obstetric patients. Local anesthetics, nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, gabapentin, and epidural clonidine are efficacious adjuvants to opioid analgesia after cesarean delivery.8–13
Pain is less severe immediately after vaginal delivery than after cesarean delivery (Figure 27-1).14 In one multicenter study, patients reported an average pain score of 3.3 of a possible 10 during the first 24 hours after vaginal delivery and an average score of 4.7 after cesarean delivery; however, there was significant variability in both groups. Forceps-assisted vaginal delivery was associated with more pain than spontaneous vaginal delivery, likely owing to local tissue damage from the forceps or factors related to the indication for forceps delivery. Mode of delivery was not associated with risk for persistent pain 8 weeks after delivery.14
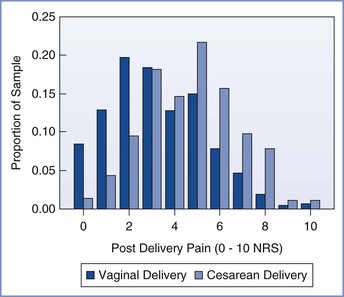
FIGURE 27-1 Distribution of numerical rating scale (NRS) scores (0, no pain; 10, worst pain imaginable) of average pain for the first 24 hours after vaginal and cesarean delivery. (From Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain 2008; 140:87-94.)
Intrapartum and postpartum pain was previously considered a reality of life that was not discussed as a complication of childbirth. The idea that acute pain during and immediately after delivery may have long-term consequences was not evaluated until the early 2000s, when investigators noted that severe pain after some types of surgery was associated with a high incidence of persistent chronic pain.14,15
Pain after Cesarean Delivery
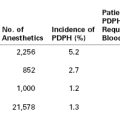
In a prospective study, the prevalence of persistent pain 8 weeks after cesarean delivery was 9.2%; the risk for experiencing pain at 8 weeks was associated with the severity of acute postpartum pain.14 In a retrospective survey, the incidence of persistent pain 1 year after delivery was 10% after vaginal delivery and 18% after cesarean delivery.16 Another retrospective study reported a 6% incidence of daily or almost daily pain 6 to 18 months after cesarean delivery.17 In these studies,16,17 the incidence of persistent pain after cesarean delivery was comparable to that reported 4 months after a hysterectomy.18 By contrast to the results of retrospective studies,16,17 a prospective study observed that the incidence of persistent pain 1 year after either cesarean or vaginal delivery was less than 1%.19
Pain after Vaginal Delivery
In the absence of analgesia, almost all patients experience severe pain at some point during labor and vaginal delivery (see Chapter 20).20,21 The severity of acute pain after vaginal delivery is highly variable.14 Most patients experience some degree of cramping pain from uterine involution. Severe pain may result from episiotomy, perineal lacerations, and/or perineal hematoma. This pain is transmitted primarily via the pudendal and iliohypogastric nerves and the sacral and lumbar plexuses. Such pain should be treated aggressively because it significantly distracts from the activities of daily living for a new mother. First-line treatment of pain after vaginal delivery typically consists of NSAIDs, supplemented by opioids if necessary. In a prospective study, the prevalence of persistent pain 8 weeks after vaginal delivery was 10%.14
Predictors of Pain
Intrinsic Patient Factors
The likelihood of developing chronic pain after childbirth may be influenced by multiple factors. There is evidence for heritability in labor pain. Studies of nonpregnant patients have shown that patients who carry a common polymorphism in the μ-opioid receptor gene (OPRM1: A118G [substitution of guanine for adenine at nucleotide position 118; Rs 1799971]) have an altered response to exogenous opioids.22 Landau et al.23 studied the ED50 (median effective dose) of intrathecal fentanyl for labor analgesia in women who were homozygous for the wild-type allele compared with women who carried the A118G polymorphism. Women who carried at least one copy of the G-allele had a lower ED50 and requested analgesia at a greater cervical dilation than women with two copies of the wild-type allele.23 However, in another study, the duration of intrathecal fentanyl analgesia did not vary according to the OPRM1 genotype.24
In contrast to the findings of Landau et al.,23 in which women with the G-allele had a lower ED50 for intrathecal fentanyl for labor analgesia, Sia et al.25 observed that women who carried the G-allele self-administered a higher dose of morphine to treat breakthrough pain after intrathecal morphine administration for postcesarean analgesia. The reasons for these seemingly disparate results have yet to be elucidated.26
Elevated concentrations of endorphins and enkephalins are found in the plasma and cerebrospinal fluid (CSF) of parturients, and opioid antagonists abolish pregnancy-induced analgesia to visceral stimulation in experimental animals (see Chapter 2). Given our current knowledge of the influence of genetic variability on response to exogenous opioids, it would not be surprising if some of the variability in labor pain and acute postpartum pain is a result of differing responses to endogenous and exogenous opioids. Other genes may also play a role. β2-Adrenergic receptor polymorphisms have been associated with preterm labor27 and labor progress.28,29 Slower development of pain during labor may be associated with slower labor progress.28
Long-standing psychological factors and mental preparation for labor influence labor pain or its expression during labor. Catastrophizing (i.e., the unfounded belief that something will be worse than it is) has been associated with labor pain and request for treatment.30,31 There is evidence that even the way that the practitioner asks about pain influences the response. When the negative word “pain” was used in a postcesarean visit, 54% of women reported pain. By comparison, when patients were asked, “Are you comfortable,” only 28% reported pain directly or indirectly (P < .001).32 Preexisting depression and anxiety impact the outcome after surgery; affected patients report more severe pain and are at increased risk for analgesic drug abuse.33
Women who abuse illegal drugs are at increased risk for adverse pregnancy outcomes.34 These patients have particularly high risk for inadequate treatment of intrapartum and postpartum pain. Although a large proportion of pregnant women who abuse drugs deny doing so,35 it is important to identify these patients antenatally to devise an analgesia plan. Buprenorphine and methadone are the most common drugs used for treatment of opioid addiction during pregnancy. Both drugs are effective and are not associated with respiratory depression in the neonate, although these infants usually require treatment of opioid withdrawal.36 Women who use opioids, cocaine, and/or amphetamines during pregnancy require more analgesia during labor than nonusers.37 Patients on opioid maintenance programs should receive their normal maintenance dose of opioid and should receive additional medication to treat postpartum pain. A multimodal analgesia strategy that includes NSAIDs is beneficial in this setting.
Women with preexisting chronic pain syndromes may have significant anxiety regarding childbirth pain. Anxiety itself is a predictor of labor pain.38 Patients with chronic pain syndromes who are treated with chronic opioid therapy are likely to develop opioid tolerance similar to patients who use opioids recreationally. Thus, patients with chronic pain syndromes should be maintained on their therapeutic regimen throughout the course of labor, delivery, and recovery; additional medication will likely be necessary to treat postpartum pain.
Environmental Factors
Sleep deprivation accentuates responses to noxious stimuli. Research subjects who sleep less than 6.5 hours per day have greater areas of secondary hyperalgesia in response to a heat test after capsaicin treatment. Additionally, those deprived of sleep have a lower threshold for pressure pain.39 Sleep deprivation accentuates pain, and pain interrupts sleep. This association creates a vicious cycle that commonly accentuates pain after cesarean delivery. Prolonged labor may precede cesarean delivery. Afterward, care of the newborn requires frequent interruptions in sleep. It is important to facilitate sleep as a therapeutic intervention as much as possible by decreasing environmental stimuli and reducing light during the evening.
Stress is also an important factor that can worsen postoperative pain and can facilitate conversion to chronic pain. Antepartum stressors induced by fear of surgery, parenting, or other changes that are associated with childbirth and cesarean delivery may accentuate postoperative pain. In patients undergoing back surgery, preoperative report of worry or intrusive memories was associated with chronic preoperative pain, failed back syndrome, and chronic postoperative pain.40 Further, biochemical evidence of preoperative stress, as measured by abnormal reactivity of the hypothalamic-pituitary-adrenal axis, was also associated with chronic pain after surgery.40 Evidence specific to obstetric surgery is limited, but there is no reason to expect that the common stressors that accompany a significant life change such as childbirth, as well as specific individual stressors, would not have a similar impact on postcesarean pain and conversion to chronic pain.
In summary, the presence of intrinsic and extrinsic factors predicts severe acute pain after cesarean delivery. Similarly, conversion to chronic pain is associated with the presence of these factors and the presence of severe acute postoperative pain. Patients may have an intrinsic tendency toward severe pain in response to injury. This tendency may be inherited as a genetic or epigenetic phenomenon, or it may be acquired through life experiences. Assessment of these predictive factors with validated scales may identify a subset of women for whom aggressive multimodal treatment of acute postoperative pain may prevent conversion of acute to chronic pain.41
Depression, anxiety, sleep deprivation, and disability are also predictable consequences of severe pain, thus creating a positive reinforcing cycle. Interruption of this cycle by predicting and treating severe postcesarean pain may help prevent the development of chronic pain. In addition, identification and treatment of coexisting psychological distress and sleep disorders may help prevent conversion of acute pain to chronic pain.
In 2001, The Joint Commission announced new pain management standards.42 These standards recognize the right of patients to receive appropriate assessment and management of pain. The standards require accredited organizations to screen patients for pain during the initial assessment, to reassess pain periodically, and to educate patients about pain management options. Fulfillment of these requirements in the obstetric setting is challenging because pain is expected during childbirth. Nonetheless, the mandate for pain assessment is not controversial. As occurs with all other types of surgery, obstetric patients should be assessed for postpartum pain at regular intervals and offered treatment. Multimodal pharmacologic and nonpharmacologic treatment for pain is the optimal approach and should be offered whenever feasible and medically indicated.
Systemic Opioid Analgesia
Neuraxial opioid administration currently represents the “gold standard” for providing effective postcesarean analgesia. Intrathecal and epidural opioid administration provide analgesia that is superior to intramuscular opioid and intravenous opioid patient-controlled analgesia (PCA) after cesarean delivery (see Chapter 28, Figure 28-1).43–45 A 2010 systematic review found that a single bolus dose of epidural morphine provides better analgesia than parenteral opioids after cesarean delivery.46 In the United States, most women who undergo cesarean delivery with neuraxial anesthesia receive neuraxial opioids for postcesarean analgesia. Multimodal analgesic strategies are used to augment the analgesic effect of neuraxial opioids in this setting. Some women may have breakthrough pain using this analgesia strategy and may require augmentation with systemic opioids.
Although pain after vaginal delivery is usually less severe than pain after cesarean delivery, its severity is highly variable. A large episiotomy with a third- or fourth-degree perineal laceration may warrant administration of a single dose of a neuraxial opioid for postpartum analgesia.47
The analgesic effect of opioids can vary significantly among patients; one study observed a fivefold difference in the maximum blood drug concentration still associated with pain (MCP).48 The MCP decreases over time after a surgical procedure. The goal of opioid administration is to achieve a minimum effective analgesic concentration (MEAC) with minimal side effects.49 Because both the MEAC and the MCP vary greatly among patients, an individualized approach to pain control is required. There are no maximum allowable doses for specific opioids; the primary limiting factor is the occurrence of side effects (Table 27-1).
TABLE 27-1
Management of Opioid Side Effects*
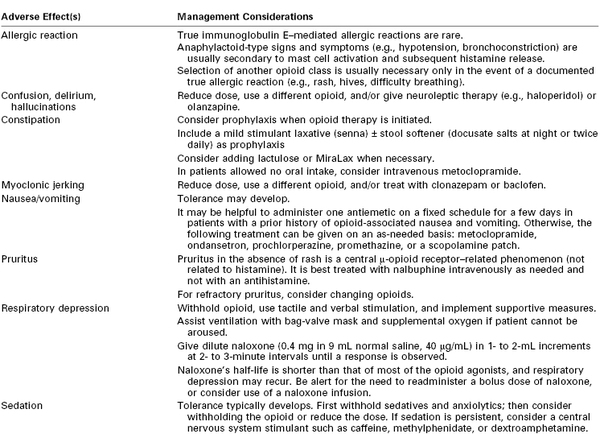
* Nonopioid analgesic options should be considered to limit opioid-related side effects. All adverse events should be carefully evaluated to rule out other potential causes.
Courtesy of the Dana Farber Cancer Institute Pain and Palliative Care Program and the Brigham and Women’s Hospital Pain Committee. Modified with permission from Bridget C. Fowler, Pharm. D., Clinical Pharmacy Manager, Dana Farber Cancer Institute.
Patients with hepatic or renal dysfunction (which may occur with severe preeclampsia), morbid obesity, and/or obstructive sleep apnea are particularly susceptible to the respiratory depressant effects of opioids. Patients who have never received opioids may be especially prone to the occurrence of side effects. It is important to monitor the respiratory rate and sedation level before giving an additional dose or adjusting the bolus dose that the patient can self-administer. After adjusting the dose, the clinician must document the respiratory rate and pattern, sedation level, and analgesic response. The use of a multimodal analgesic approach helps provide adequate analgesia while limiting opioid-related side effects (see later discussion).
Intravenous Patient-Controlled Analgesia
In the past, opioids were commonly administered intramuscularly or subcutaneously; these simple routes of administration do not require the postoperative return of bowel activity or the use of sophisticated equipment. Intramuscular and subcutaneous medications are inexpensive, easy to administer, and associated with a long history of safety. Disadvantages of this approach include the need for repeated painful injections, a delayed (and sometimes erratic) absorption of drug, and an inconsistent analgesic response due to variations in plasma opioid concentrations.
A number of studies have compared intravenous opioid PCA to traditional nurse-administered parenteral analgesia. A 2006 meta-analysis concluded that intravenous PCA provided better postoperative analgesia and greater patient satisfaction than conventional nurse-administered opioid analgesia.50 Although the use of intravenous PCA was associated with greater opioid use and a higher incidence of pruritus, the incidence of other side effects was not different between groups.50 The American Society of Anesthesiologists (ASA) Task Force for Acute Pain Management in the Perioperative Setting51 recommends that “these modalities [epidural or intrathecal opioids, systemic opioid PCA, and peripheral regional techniques] should be used in preference to intramuscular opioids ordered ‘as needed.’ ”
PCA has been used with intravenous and epidural routes of administration after cesarean delivery. In a study of intravenous versus epidural meperidine PCA, higher pain scores with rest and movement, greater sedation, and lower patient satisfaction were observed with the intravenous route of administration.52 Moreover, plasma meperidine and normeperidine concentrations were almost double with the intravenous route.52 Similarly, another study that compared intravenous versus epidural fentanyl PCA after cesarean delivery reported higher pain scores and greater fentanyl consumption with the intravenous route, although patient satisfaction ratings were similar in the two groups.53 Another study compared intravenous versus epidural hydromorphone PCA after cesarean delivery.54 Hydromorphone requirements were threefold to fourfold higher in the intravenous group. The two groups had similar pain and sedation scores, but patients in the intravenous group reported more frequent drowsiness and less pruritus.54
Several studies have compared intravenous morphine PCA to single-shot epidural morphine for postcesarean analgesia.45,55,56 Although the incidence of pruritus was higher with epidural morphine than with intravenous morphine PCA, analgesia and patient satisfaction were better with epidural morphine.45,55,56 The incidence of nausea was not different between groups45,55,56; sedation was greater in the intravenous PCA group.45
Choice of Opioid
Overall, factors that affect the choice of opioid are speed of onset, duration of action, overall efficacy, and the type and frequency of side effects (Table 27-2). If side effects prevent adequate analgesia, other opioids or nonopioid adjuvants should be used. Patient preferences based on past experiences and desired analgesia should also be considered.
TABLE 27-2
Opioid Characteristics
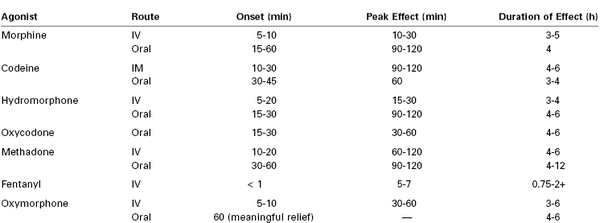
IM, intramuscular; IV, intravenous.
Courtesy of the Dana Farber Cancer Institute Pain and Palliative Care Program and the Brigham and Women’s Hospital Pain Committee. Modified with permission from Bridget C. Fowler, PharmD, Clinical Pharmacy Manager, Dana Farber Cancer Institute.
Historically, meperidine has been a popular opioid for postoperative analgesia. It is the least potent of the opioids used clinically, and it has a long-acting active metabolite, normeperidine, that is excitotoxic to the central nervous system. In the past two decades a concerted effort has been made to decrease the use of meperidine for postoperative analgesia.57 In 1999, the American Pain Society stated that “meperidine should not be used for more than 48 hours for acute pain…[it] should be reserved for brief courses in otherwise healthy patients who have demonstrated an unusual reaction or allergic response to other opioids.”58 The American College of Obstetricians and Gynecologists (ACOG)59 has discouraged the use of meperidine because of the accumulation of normeperidine in the neonate and its subsequent effect on neurobehavioral scores.
Safety
Health professionals who use PCA should (1) be able to evaluate candidates for PCA (e.g., mental state, level of consciousness, patient understanding); (2) know drug selection criteria, dosing schedules, lockout periods, and infusion devices; (3) be able to provide patient education on pain management and the use of PCA; (4) understand when to alter PCA settings and when to give or withhold additional (rescue) doses of medications; and (5) be able to respond to side effects and adverse events.
In December 2004, The Joint Commission60 issued a Sentinel Event Alert on PCA “by proxy” (i.e., when other individuals, including family members, become involved in drug administration). The Joint Commission acknowledged that PCA is a safe and effective method of controlling pain when used as prescribed; however, serious adverse events, including oversedation, respiratory depression, and death, can result when analgesia is delivered “by proxy.” The Joint Commission60 made the following recommendations: (1) develop criteria for selecting appropriate candidates for PCA, (2) carefully monitor patients, (3) teach patients and family members about the proper use of PCA and the dangers of others’ pressing the button for the patient, (4) alert staff to the dangers of administering a dose outside a prescribed protocol, and (5) consider placing warning tags on all PCA delivery pendants stating, “Only the patient should press this button.”
When intravenous PCA is used for postoperative analgesia, guidelines for safe administration should be employed and documented. At many institutions two registered nurses must verify all pump settings when they are entered or changed and during communications associated with all patient transfers or nurse shift changes. The amount of opioid administered is recorded from the pump every 2 hours and when a drug cartridge is changed. The PCA settings (drug, demand dose, lockout interval, 4-hour limit, and the rate of continuous infusion, if used) are documented on a flow sheet. Any changes in PCA settings are clearly documented. In our institution, the use of a continuous background infusion is discouraged except in patients who were taking opioids preoperatively or in patients in whom nonstandard dosing requirements have already been demonstrated.
Infusion Pump Settings
A number of PCA parameters must be considered, including drug choice, incremental dose, maximum dose, and lockout interval (Table 27-3). Owen et al.48,61–63 performed a number of investigations of PCA in patients undergoing abdominal surgery. In an assessment of PCA morphine demand bolus doses (0.5, 1, or 2 mg with a 5-minute lockout interval), more patients in the 0.5-mg group had inadequate pain relief, whereas those in the 2-mg group had more side effects, including respiratory depression (i.e., respiratory rate < 10 breaths/min).62 These results correlated with the total dose of self-administered morphine. Although the role of the lockout interval was not addressed in this study, the investigators suggested that inadequate analgesia could be produced by lockout intervals that were too long or demand doses that were too small. By contrast, larger doses or shorter lockout intervals might lead to more opioid-related side effects. Therefore, longer lockout intervals typically require larger bolus doses, whereas smaller bolus doses typically require shorter lockout intervals.
TABLE 27-3
General PCA Dosing in Opioid-Naive Patients

Recorded as: PCA bolus dose/lockout interval/4-hour limit/continuous infusion rate. A continuous background infusion is typically avoided except in selected cases (e.g., opioid tolerance).
IV, intravenous; PCA, patient-controlled analgesia.
Courtesy of the Dana Farber Cancer Institute Pain and Palliative Care Program and the Brigham and Women’s Hospital Pain Committee. Modified with permission from Bridget C. Fowler, PharmD, Clinical Pharmacy Manager, Dana Farber Cancer Institute.
Although patients who experience inadequate analgesia would be expected to make more PCA demands, this is often not the case.62 Patients may be afraid to administer too much opioid or anticipate more severe side effects. Additionally, it has been suggested that patients are discouraged by an inadequate analgesic effect or they may expect a delayed response.62 For this reason, a hydrophilic opioid (e.g., morphine) with a longer latency may be preferred over a lipophilic opioid (e.g., fentanyl).
The amount of opioid delivered in a continuous basal (background) infusion may or may not alter the analgesic efficacy of patient-controlled bolus doses. One study compared PCA bolus doses of morphine (0.4, 0.7, or 1.0 mg) combined with a continuous infusion of morphine at 1.5 mg/h after gynecologic surgery.61 The number of demand bolus doses and the quality of analgesia did not vary among groups despite the overall higher use of morphine in the group that received the largest bolus dose. In addition, there were no differences among groups in patient satisfaction or side effects. In an earlier study, Owen et al.63 observed that the patient-administered morphine dose did not differ in patients randomized to receive patient-administered bolus morphine compared with patient-administered bolus morphine with a continuous basal infusion (morphine 1.5 mg/h). Moreover, the quality of analgesia was similar in the two groups despite a twofold higher total dose of morphine in the group receiving a continuous infusion.
Controversy exists regarding the use of a continuous basal infusion during administration of intravenous opioid PCA. Studies of patients undergoing gynecologic surgery do not support the use of a continuous background infusion to provide better postoperative analgesia. Parker et al.64 evaluated 230 women who had undergone an abdominal hysterectomy; one group received a demand bolus dose of morphine (1 to 2 mg) without a continuous infusion, and the other three groups received a continuous infusion of morphine (0.5, 1, or 2 mg/h) in addition to the demand bolus dose. No differences in the number of demand or delivered doses per hour, pain scores, or overall morphine consumption were observed, except that an overall higher dose of morphine was administered in the 2 mg/h continuous infusion group than in the demand bolus–only group. A subsequent study by the same investigators in a similar patient population compared a group receiving an intravenous PCA morphine (2 mg demand bolus dose) regimen with a group receiving the same intravenous PCA regimen and a nighttime continuous infusion of morphine (1 mg/h).65 There were no differences between groups in postoperative pain, sleep patterns, demand or delivered bolus doses per hour, opioid consumption, or recovery from surgery. The investigators reported that the use of a continuous infusion resulted in six errors during the programming of the device and that three patients required discontinuation of the continuous infusion because of significant oxyhemoglobin desaturation.65
Thus, a continuous basal infusion is often avoided in opioid-naive patients because of concern about the risks of oversedation and respiratory depression. The ASA Task Force on Acute Pain Management51 concluded that intravenous PCA with a continuous background infusion of morphine results in use of a larger total dose of analgesic drug than intravenous PCA without a background infusion; the findings were equivocal regarding comparisons of quality of analgesia and the incidence of nausea, vomiting, pruritus, and sedation.51 The Task Force concluded that special caution should be taken when a continuous infusion is used, owing to the potential for adverse effects from opioid accumulation.51 The American Pain Society has also urged caution in the use of a continuous basal infusion in opioid-naive patients receiving intravenous opioid PCA.66
With the use of PCA, the ratio of patient demands to bolus doses delivered appears to be a good measure of analgesia and is strongly correlated with pain scores.67 A high ratio is likely to reflect patient misunderstanding or inadequate analgesia, but a ratio close to 1 signifies adequate pain relief. Patients use PCA bolus demands for different reasons, including worsening pain at rest, pain with movement or coughing, and anticipation of an activity that is likely to produce pain.61 Inadequate analgesia most likely can be improved by an increase in the demand bolus dose, a shorter lockout interval, or a change of opioid. Patients also should be encouraged to use demand bolus doses before movement or activity.
A commonly neglected but important guideline is the achievement of adequate analgesia and the initiation of intravenous PCA before the patient is discharged from the postanesthesia care unit (PACU). Although there is significant variation in individual perception of pain and response to analgesia, the assessment of pain scores can facilitate evaluation of the adequacy of analgesia. In a study of postoperative patients after general anesthesia (the surgical procedures were not disclosed), Aubrun et al.68 demonstrated that the relationship between visual analog pain scores (VAPS, 0 to 100 scale) and morphine requirements was represented by a sigmoid curve; they observed one plateau below a score of 40 and another plateau above a score of 80. Although patients with a VAPS of 70 or higher were considered to have severe pain and required a larger total dose of morphine to obtain a VAPS lower than 50, a smaller total dose of morphine was required to reduce a VAPS of 50 to a score of 30 or less.68 This study suggests that pain scores are slow to change when severe pain exists but that the scores decline rapidly once pain begins to improve. Other investigators have identified relevant VAPS thresholds associated with changes in intravenous PCA dose requirements after intra-abdominal surgery as 30 or less, 31 to 70, and more than 70.69 Only 4% of patients with a VAPS of 30 or less requested an increase in analgesic dose, whereas 80% of patients with a VAPS higher than 70 requested a higher dose. Among patients with a VAPS between 31 and 70, those whose scores improved at least 10 points were less likely to request additional analgesia than those with little change or an increase in VAPS.69 Thus, a patient’s perception of pain improvement may depend, in part, on her initial pain score.
Severe pain most likely antagonizes the sedative and respiratory depressant side effects of opioids, a possibility that becomes increasingly important in patients requesting higher doses of opioids. Therefore, a transition from an opioid-only to a multimodal, balanced analgesia approach should be employed to optimize pain control and minimize opioid-related side effects. The morbidity associated with high doses of opioids should invoke the application of algorithms for pain assessment, management, and monitoring. At many institutions, postcesarean patients are assessed every 4 hours for respiratory rate and quality, oxygen saturation, pain score, and sedation level. Patients are asked whether their level of pain relief is acceptable; and if not, an adjustment of analgesic dose is performed and documented. The pain score is reassessed within 15 to 30 minutes before additional interventions are made. High-risk patients, including those with hepatic or renal dysfunction, obstructive sleep apnea, and/or morbid obesity, are assessed every 2 hours for 12 hours after initiation of intravenous PCA therapy and then every 4 hours if the respiratory rate is 8 breaths/minute or higher, oxygenation is stable, and sedation level is minimal. Patients are assessed every 30 minutes for the first hour after the intravenous PCA dose is increased; thereafter, if pain relief is adequate and the clinical condition is unchanged, patients are assessed every 4 hours as previously described.
Acute postoperative pain is limited in duration, so a plan should be devised for the transition from intravenous opioids to oral agents when the patient’s pain is controlled and she is able to take medication by mouth. Table 27-4 lists the equi-analgesic doses for commonly used opioids.
TABLE 27-4
Opioid Equi-analgesic Doses
| Drug | Oral (mg) | Subcutaneous/Intravenous (mg) |
| Morphine | 30 | 10 |
| Oxycodone | 20 | NA |
| Hydrocodone | 20 | NA |
| Hydromorphone | 7.5 | 1.5 |
| Fentanyl | NA A 25-µg/h transdermal patch is equi-analgesic to ≈50 mg of oral morphine per day |
0.1 (100 µg) |
| Oxymorphone | 10 | 1 |
NA, not applicable.
Courtesy of the Dana Farber Cancer Institute Pain and Palliative Care Program and the Brigham and Women’s Hospital Pain Committee. Modified with permission from Bridget C. Fowler, Pharm. D., Clinical Pharmacy Manager, Dana Farber Cancer Institute.
Oral Opioid Analgesia
Some investigators have advocated the use of oral analgesics rather than intravenous PCA for postcesarean pain.70–73 Advantages of this approach are cost-effectiveness, facilitation of early mobility by discontinuation of the intravenous catheter and other equipment, and, perhaps, greater patient satisfaction.
In a randomized controlled comparison of oral oxycodone-acetaminophen and intravenous morphine PCA, patients randomized to the oral regimen experienced less pain, nausea, and drowsiness at 6 hours after cesarean delivery.73
Multimodal Analgesia
Multimodal analgesia, also known as “balanced analgesia,” has been used effectively in treatment of cancer and chronic pain for many decades. The rationale behind its use is the optimization of additive and/or synergistic analgesic effects of different modes of analgesia or drug classes, while reducing doses and minimizing the side effects of each drug.74 A primary goal of multimodal analgesia is to provide adequate analgesia with minimum maternal side effects. Important secondary goals include minimizing transfer of drugs to breast milk and reducing maternal side effects that may interfere with breast-feeding or caring for the neonate. Various combinations of opioids, NSAIDs, acetaminophen, and local anesthetics, among other drugs, have been used with varying degrees of success.75 Several analgesic regimens have been shown to reduce opioid use and opioid-induced side effects when used to treat postcesarean pain.
Nonsteroidal Anti-inflammatory Drugs
All NSAIDs have opioid-sparing activity. Ibuprofen is one of the most widely recognized NSAIDs that is available without prescription. It nonselectively inhibits cyclooxygenase-1 and cyclooxygenase-2 (COX) enzymes. As such, in addition to its anti-inflammatory, analgesic, and antipyretic properties, ibuprofen inhibits platelet adhesion and causes renal artery vasoconstriction and gastrointestinal irritation; therefore, its use in patients at risk for hemorrhage and renal failure warrants caution. Nonetheless, in most parturients without risk factors for hemorrhage or renal failure, its use is safe. There is limited transfer of drug to breast milk, making it particularly beneficial for lactating mothers. In a small study (oral ibuprofen 400 mg every 6 hours for 24 hours), less than 1 mg of ibuprofen was excreted in breast milk in a 36-hour period.76 Many centers administer 600 to 800 mg every 8 hours as a standard dose. In a study comparing oral naproxen 500 mg every 12 hours to placebo after elective cesarean delivery,77 the authors found a considerable reduction in incision pain with sitting (mean ± SD VAPS of 38 ± 26 and 51 ± 26 in the treatment and placebo groups, respectively) that was accompanied by a decrease in opioid consumption (Figure 27-2). Similar to other analgesics, fixed-interval dosing of NSAIDs provides more effective analgesia and results in better patient satisfaction than on-demand dosing.78
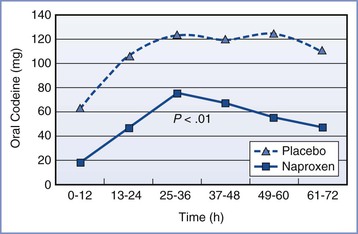
FIGURE 27-2 Effect of naproxen on requirement for oral codeine after cesarean delivery: oral codeine use in milligram equivalents (expressed as mean) over time by group. (From Angle PJ, Halpern SH, Leighton BL, et al. A randomized controlled trial examining the effect of naproxen on analgesia during the second day after cesarean delivery. Anesth Analg 2002; 95:741-5.)
Diclofenac has also been extensively studied and found to be effective for postcesarean analgesia. Diclofenac rectal suppositories (100 mg twice a day) decreased morphine consumption (14 mg versus 22 mg in 32 hours) compared with placebo in patients who had undergone cesarean delivery.79 A single dose of diclofenac rectal suppository 100 mg prolonged the mean time to first analgesic administration by more than 5 hours in patients who received intrathecal morphine for postcesarean analgesia.80 Patients who received intrathecal morphine doses as small as 25 µg (0.025 mg) required no rescue analgesic when intramuscular diclofenac 75 mg was administered every 8 hours.81
Parenteral ketorolac is another NSAID that is useful in the treatment of postpartum pain. In a randomized, controlled study, Lowder et al.82 provided evidence that ketorolac decreased pain scores at 2, 3, 4, 6, 12, and 24 hours after cesarean delivery and also significantly decreased opioid consumption. Unlike other NSAIDs, the U.S. Food and Drug Administration (FDA) has a mandated black box warning that states that ketorolac is “contraindicated in nursing mothers because of the potential adverse effects of prostaglandin-inhibiting drugs on neonates.”83 This warning was mandated in spite of a study showing minimal to undetectable ketorolac levels in breast milk.84 The American Academy of Pediatrics (AAP) considers ketorolac safe for nursing mothers.85
Selective Cyclooxygenase-2 Inhibitors
COX-2 specific inhibitors have a potential benefit compared with traditional nonselective NSAIDS in that they have minimal effects on platelet adhesion and thus are less likely to interfere with blood clot formation and contribute to hemorrhage. However, concerns about the potential to increase the risk for cardiovascular and thrombotic events, combined with the baseline elevated risk for these events during pregnancy and postpartum, have prevented COX-2 inhibitors from playing a major role in postpartum analgesia. Celecoxib is the only widely available COX-2 selective inhibitor in the United States. The breast milk content of valdecoxib and its pro-drug precursor parecoxib was studied after a single 40-mg intravenous dose of parecoxib following cesarean delivery. The breast milk concentration was very low, and neonatal neurologic and adaptive scores were normal.86
Acetaminophen
Despite widespread popularity, few published data support the use of acetaminophen (paracetamol) for postcesarean analgesia. A 2012 meta-analysis identified four randomized controlled trials evaluating acetaminophen and its effect on opioid consumption after major surgery; only one study included obstetric patients.87 The meta-analysis revealed that acetaminophen was less effective than NSAIDs in decreasing opioid consumption and postoperative nausea and vomiting.87 A comparison of intravenous acetaminophen with oral ibuprofen in postcesarean patients failed to show an advantage in pain scores, opioid consumption, or patient satisfaction.88 The combination of NSAIDs and acetaminophen is synergistic in human experimental pain studies.89
Intravenous acetaminophen has gained popularity owing to reports of slower absorption of oral acetaminophen in patients treated with morphine90 and higher peak plasma and CSF levels of acetaminophen after intravenous administration.91 However, in patients with a functioning gastrointestinal system, there is no documented analgesic advantage in using intravenous rather than oral administration.
α2-Adrenergic Receptor Agonists
α2-Adrenergic agonists have been used for the treatment of acute and chronic pain in nonobstetric patients.92 Intravenous dexmedetomidine has been used as an adjunct to opioids for labor analgesia and as a component of general anesthesia for cesarean delivery.93 Dexmedetomidine may be preferred over clonidine for intrapartum intravenous administration because an isolated, perfused human placenta model suggested that less dexmedetomidine is transferred across the placenta to the fetus. The investigators noted that more dexmedetomidine was retained in the placenta, which they attributed to its higher lipophilicity.94
Neuraxial clonidine has been used for labor analgesia but is not commonly used for analgesia after cesarean delivery. The epidural formulation of clonidine carries the following “black box” warning from the U.S. FDA95:
Clonidine hydrochloride injection (epidural clonidine) is not recommended for obstetrical, postpartum or perioperative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients. However in a rare obstetrical, postpartum or perioperative patient, potential benefits may outweigh the possible risks.
The combination of intrathecal clonidine (150 µg) and bupivacaine compared with sufentanil combined with bupivacaine has been shown to reduce peri-incisional hyperalgesia but does not alter pain scores or opioid consumption after elective cesarean delivery.96 These findings suggest that intrathecal clonidine might be beneficial in a patient who is at risk for the development of chronic pain after cesarean delivery. Another randomized trial compared intrathecal clonidine 75 µg combined with hyperbaric bupivacaine to saline combined with bupivacaine.97 Clonidine prolonged the duration of analgesia, but there was no difference in the need for rescue analgesia. Epidural clonidine (75 µg and 150 µg) added to a mixture of morphine, bupivacaine, and epinephrine prolonged the duration of analgesia and reduced morphine consumption without a significant increase in the incidence of side effects in patients who had undergone cesarean delivery.98 However, given the availability of other agents and the lack of overwhelming evidence in favor of α2-agonists, routine use of these agents in the postpartum period should be restricted to patients in whom other agents are contraindicated or in those with risk factors for chronic pain.
Magnesium Sulfate
Indications for peripartum magnesium sulfate therapy include tocolysis of preterm labor, seizure prophylaxis in preeclamptic women, and fetal neuroprotection in women at risk for preterm delivery.99 A 2013 meta-analysis of trials of intravenous magnesium for the treatment of acute postoperative pain after nonobstetric surgery performed with general anesthesia concluded that magnesium sulfate administration resulted in a trivial reduction in pain scores and a more substantial reduction in opioid use; however, the incidence of nausea and vomiting was not reduced.100 Epidural magnesium sulfate 500 mg (off-label route of administration) added to bupivacaine was more effective than bupivacaine alone in decreasing pain scores and opioid consumption but was not as effective as combining bupivacaine with morphine 1.5 mg.101 Albrecht et al.102 reviewed 18 trials of the efficacy and safety of neuraxial magnesium sulfate for postoperative analgesia. They observed an overall increase in the interval to first analgesic request (mean difference of 40 minutes [P = .0009] after intrathecal administration and mean difference of 110 minutes [P = .02] after epidural administration). However, the authors concluded that there were not enough patients (n = 140) and trials to evaluate the risk for neurologic complications.
Gabapentin
Gabapentin is an anticonvulsant that has analgesic properties, particularly in the setting of neuropathic pain. Although extensively studied in the management of chronic pain, its mechanism of action is uncertain. As part of a multimodal analgesic regimen in patients undergoing cesarean delivery, a preoperative dose of oral gabapentin 600 mg was associated with significantly lower pain scores with movement and at rest; however, the incidence of sedation was greater in the gabapentin group than in the placebo group (19% versus 0%).8 In a follow-up study, placebo was compared with two doses of gabapentin (300 mg and 600 mg) in the hope of finding an efficacious dose associated with less sedation. Unfortunately the trial failed to show efficacy in either gabapentin group.103 Limited evidence of efficacy and concerns about excessive sedation limit enthusiasm for routine use of gabapentin for postcesarean analgesia.
Ketamine and Dextromethorphan
Ketamine, an N-methyl-D-aspartate (NMDA) antagonist, has analgesic properties and may play a role in the treatment of acute postoperative pain and prevention or reversal of central sensitization.104 It also is used as an intraoperative adjuvant to neuraxial anesthesia in patients undergoing cesarean delivery; small intravenous doses provide significant analgesia with minimal respiratory depression. An evaluation of low-dose ketamine for postcesarean analgesia compared intravenous ketamine 0.15 mg/kg, intrathecal fentanyl 10 µg, and placebo.105 The study demonstrated a prolonged duration of analgesia in both the fentanyl and ketamine groups compared with the placebo group (time to first analgesic request: 145 minutes in the placebo group, 165 minutes in the fentanyl group, 199 minutes in the ketamine group). Ketamine was also superior to fentanyl and placebo in reducing pain scores both at 90 and 180 minutes, and reducing analgesic requirements in the first 24 hours, but not in the second 24 hours, after cesarean delivery.105 In another study,106 women undergoing cesarean delivery were randomized to receive intravenous ketamine 10 mg or placebo shortly after delivery as part of a multimodal regimen of intrathecal morphine and regular NSAID therapy. Oral acetaminophen/hydrocodone was administered for rescue analgesia. The authors were unable to demonstrate a difference in breakthrough pain, time to first analgesic request, or cumulative rescue analgesic requirements.106 However, they demonstrated lower pain scores in the ketamine group 2 weeks after the surgery.106
Dextromethorphan, which is another NMDA antagonist, has been evaluated for its effect on postoperative analgesia, with mixed results.107 After cesarean delivery, the addition of oral dextromethorphan 60 mg to various doses of intrathecal morphine failed to produce a significant change in pain scores.11 Given the current level of evidence, routine use of NMDA antagonists for postcesarean analgesia is not recommended. However, low-dose ketamine may be useful in patients who suffer from superimposed chronic pain.
Non-Neuraxial Regional Analgesic Techniques
Transversus Abdominis Plane Block
The transversus abdominis plane (TAP) block is a regional anesthetic technique in which local anesthetic is injected between the internal oblique and the transversus abdominis muscle layers to block the plexus of nerves supplying the anterior abdominal wall (Figure 27-3). In a trial in patients undergoing elective cesarean delivery, women randomized to receive a TAP block with 0.75% ropivacaine required significantly less morphine, had a longer time before first analgesic request, and had lower pain scores than women who received a block procedure with saline.108 In another trial, bilateral TAP block with ropivacaine 0.5% was compared with saline; the ropivacaine group consumed less morphine (ropivacaine group, 18 mg; saline group, 32 mg; P < .05) and reported greater patient satisfaction.109
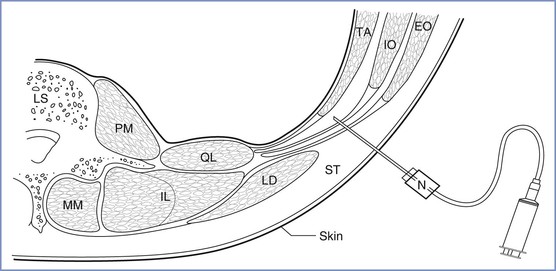
FIGURE 27-3 Line drawing of a transverse section through the abdominal wall at the level of the lumbar triangle of Petit (TOP). The floor of the triangle is composed, from superficial to deep, of the fascial extensions of external oblique, internal oblique, and transversus abdominis, respectively, and the peritoneum. The needle is inserted through the triangle, using the loss-of-resistance technique. The needle is shown in the transversus abdominis plane, and the fascial layers have separated as a result of the injection of local anesthetic. LS, lumbar spine; LD, latissimus dorsi; PM, psoas major; QL, quadratus lumborum; MM, multifidus muscle; IL, longissimus, iliocostalis; TA, transversus abdominis; IO, internal oblique; EO, external oblique; N, 50-mm blunt-tipped needle; ST, subcutaneous tissue. (From McDonnell JG, Curley G, Carney J, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg 2008; 106:186-91.)
Several trials have compared intrathecal morphine analgesia to a TAP block after cesarean delivery.110,111 In one such trial, patients who received intrathecal morphine 0.2 mg had a longer time to first analgesic request (8 hours versus 4 hours; P = 0.005) and decreased analgesic requirements in the first 12 hours compared with those who received a TAP block.110 However, the incidence of nausea, vomiting, and pruritus was greater in the intrathecal morphine group.110 Similarly, Loane et al.111 randomized patients to receive intrathecal morphine 0.1 mg or a TAP block; intrathecal morphine resulted in lower opioid consumption and pain scores but was associated with a greater incidence of nausea, vomiting, and pruritus.111 Finally, Costello et al.112 found that the combination of intrathecal morphine and TAP block did not provide better postcesarean analgesia than intrathecal morphine alone.112 Thus, TAP block represents a reasonable alternative for patients who are unable to receive neuraxial morphine, but TAP block should not replace neuraxial opioid analgesia as the gold standard for postcesarean analgesia.
Technique
The TAP block can be performed blindly using surface landmarks or with ultrasonographic guidance.113 Continuous infusion of local anesthetic has been described, but the block is usually performed as a single-shot technique. The originally described landmark/loss-of-resistance technique involves introduction of the needle into the triangle of Petit.114 Using ultrasonographic guidance, an anterior oblique-subcostal approach, a midaxillary approach, and a posterior approach have been described.114 The pattern of local anesthetic spread within the transversus abdominis plane may vary depending on the site of injection; thus, the block characteristics, including extent of analgesia, may also vary depending on the specific technique.114
The classic, midaxillary, ultrasonography-guided TAP block is performed with the patient in the supine position. An area of skin on the lateral abdominal wall between the subcostal margin and the iliac crest is prepared with antiseptic solution. Using a high-frequency (8 to 12 MHz) linear array ultrasound probe, the abdominal wall is scanned between the anterior and posterior axillary line (Figure 27-4). The muscle layers are identified, and the needle (usually a 21- to 22-gauge, 80- to 100-mm short-bevel needle with extension tubing attached to a syringe with saline solution) is inserted in-plane from medial to lateral. Using ultrasonographic guidance, the needle tip is advanced through the muscle layers until it is positioned in the fascial plane between the internal oblique and transversus abdominis muscles. A small amount of saline is injected to verify hydrodissection of the plane. After correct identification of the plane, 15 to 20 mL of local anesthetic solution is incrementally injected into the plane, again with ultrasonographic guidance.
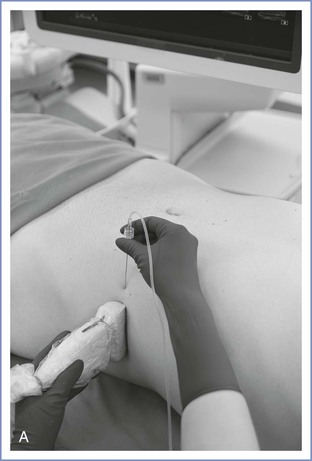
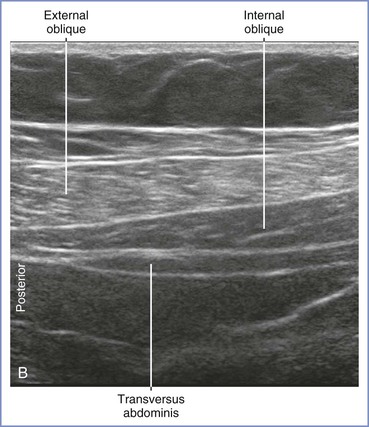
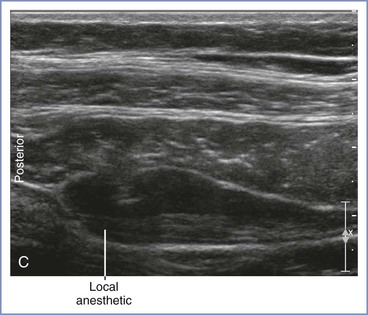
FIGURE 27-4 Midaxillary transversus abdominis plane (TAP) block. A, With the patient in the supine position, the transducer is placed near the midaxillary line between the costal margin and pelvic brim for a transverse imaging plane. B, Transverse ultrasonographic image of the anterolateral abdominal wall defining the borders of the muscle layers. The underlying muscles are the external oblique, internal oblique, and transversus abdominis. The transversus abdominis plane lies between the internal oblique and transversus abdominis muscles. C, Ultrasonographic image illustrating the local anesthetic injection. Visualization of an elliptical distribution of the local anesthetic with well-defined margins provides evidence for the proper injection of the solution into the plane between the internal oblique and transversus abdominis muscles. (From Gray AT. Atlas of Ultrasound-Guided Regional Anesthesia. 2nd edition. Philadelphia, Elsevier-Saunders, 2013.)
Complications of the block include intravascular injection resulting in local anesthetic systemic toxicity (LAST) and bowel perforation. The local anesthetic dose/volume/concentration−response relationships have not been well studied. Griffiths et al.115 performed bilateral TAP blocks with a total ropivacaine dose of 2.5 mg/kg (diluted with 0.9% saline to a total volume of 40 mL) in women who had spinal anesthesia for elective cesarean delivery. (This dose corresponds to 20 mL of 0.5% ropivacaine on each side for an 80-kg patient.) The mean (± SD) peak plasma ropivacaine concentration (30 minutes after injection) was 1.82 ± 0.69 µg/mL. Although this concentration is below the reported threshold for systemic toxicity (2.2 µg/mL), 12 of 30 patients had peak concentration measurements above this threshold (maximum, 3.76 µg/mL), and 3 patients had symptoms of LAST (perioral tingling, metallic taste).115 These findings suggest that ropivacaine doses less than 2.5 mg/kg should be used and that patients should be observed for at least 30 minutes after the block is performed.
Wound Infusion Catheters
Wound infusion catheters have gained popularity for pain relief after general and orthopedic surgery as ambulatory disposable pumps have become widely available. However, data are conflicting regarding the efficacy of these catheters. In a study comparing pain control provided by wound infusion and epidural levobupivacaine, the epidural group had less pain only at the 4-hour time point. There were no significant differences between groups in opioid consumption.9 In another study comparing intrathecal morphine and wound infusion with ropivacaine or saline,116 more oxycodone was required in the ropivacaine wound infusion group than in the intrathecal morphine group during the first 24 hours (48 mg versus 26 mg; P = .004); there was no difference in oxycodone use between the ropivacaine wound infusion and saline-control groups.116 By contrast, another group of investigators found that wound infusion with ropivacaine 0.2% at 5 mL/h provided superior analgesia and a lower incidence of nausea and vomiting compared with epidural morphine 2 mg administered every 12 hours.117 Carvalho et al.118 added ketorolac to bupivacaine for wound infusion; compared with infusion of bupivacaine alone, pain scores and opioid consumption were decreased, as were local inflammatory mediators collected from the wound.118 The addition of hydromorphone to bupivacaine did not alter these outcomes.118
Some of the differences in outcomes among these studies may be due to differences in catheter insertion technique, local anesthetic concentration, and rate of infusion; however, current evidence does not support the superiority of local anesthetic wound infusion over neuraxial opioid administration. Although some centers use wound infusion catheters, the additional cost of the pump and the inconvenience may be prohibitive.
Local Infiltration
Local infiltration of the wound is a relatively simple component of multimodal postoperative analgesia. In a study of patients who did not receive neuraxial morphine for postcesarean analgesia, infiltration of 0.25% bupivacaine with epinephrine (40 mL) before wound closure was associated with decreased opioid requirements in the first 12 hours compared with saline-placebo.119 However, wound infiltration with 30 mL bupivacaine 0.5% did not provide additional analgesia when combined with neuraxial opioid administration.120
Ilioinguinal-Iliohypogastric Block
Ilioinguinal-iliohypogastric block is useful for postoperative analgesia after lower abdominal surgery. Similar to TAP blocks, these blocks can be performed with ultrasonographic guidance. Evidence is inconsistent as to whether ilioinguinal-iliohypogastric blocks improve analgesia provided by neuraxial morphine.121,122 In a study of women who did not receive neuraxial morphine, bilateral, multilevel ilioinguinal-iliohypogastric blocks were associated with lower systemic opioid use, but a decrease in opioid side effects was not observed.123
Nonpharmacologic Interventions
Nonpharmacologic interventions may play a role as adjuvant treatments in the management of postcesarean pain, but these interventions have not been subjected to rigorous investigation. Acupuncture has been used to treat pain after cesarean delivery. In one trial, patients treated with acupuncture used 30% less opioid in 24 hours and reported lower pain scores at 2 hours.124 Foot and hand massage has been reported to reduce reported pain scores and the need for rescue pain medication.125
Impact of Pain and Analgesic Treatment on Breast-Feeding
The AAP strongly supports breast-feeding because of its benefits to the infant, mother, and society. However, patients may discontinue breast-feeding when taking analgesic medications because of incomplete or incorrect information regarding neonatal health. The AAP Committee on Drugs recommends that physicians take the following steps before prescribing medications to breast-feeding women: (1) determination of the reasons for the medication, (2) identification of the safest available medication in a particular category, and (3) measurement of blood concentrations of drug in those neonates for whom the drug presents a significant risk.85 The AAP has also suggested that the best time for the mother to take a medication is immediately after breast-feeding or just before the infant takes a nap.85
The two most important items needed to determine the potential neonatal risks of maternal drug administration are the amount of medication excreted in breast milk and the therapeutic effect of that medication when given to the infant.126 Some investigators have arbitrarily defined a breast milk concentration lower than 10% of the therapeutic infant plasma concentration (or a breast milk dose less than 10% of the weight-normalized maternal dose) as a safe level of exposure. In breast-feeding infants, the estimated absolute infant drug dose is calculated by multiplying the average infant breast milk intake by the average concentration of the drug in the milk (area under the pharmacokinetic curve). The absolute infant drug dose is then expressed as a percentage of the maternal dose, normalized by weight (mg/kg/day), to yield the relative infant dose. If the relative infant dose is high and the drug has potential to harm the infant, or if side effects attributed to the drug are detected in the infant, clinicians should consider discontinuing the drug, modifying the dose, or changing to a different drug.
Persistent pain is associated with sympathetic nervous system activation, vasoconstriction, and decreased breast milk production in animal models.127 Better pain management after cesarean delivery is associated with more episodes of breast feeding.128 Clearly, pain requires treatment after cesarean delivery, and the optimal method or methods to prevent adverse neonatal effects through breast milk transfer is an important consideration. Because significantly smaller total opioid doses are required in women who receive neuraxial opioids, less opioid is available for transfer to breast milk. In a parturient treated with continual intrathecal morphine for chronic pain, minimal morphine concentrations were detected in the neonate, and neurobehavioral scores were normal.129 A relatively small amount (1.6%) of the maternal dose of hydrocodone is transferred to the neonate as hydromorphone in breast milk.130 In general, the small amounts of analgesic drugs that are transferred to breast milk in the postpartum period are not harmful to the neonate.
Standard references, including the AAP list of drugs that are usually compatible with breast-feeding,85 should be consulted before prescribing drugs to breast-feeding women.131
Maternal Anesthesia and Breast-Feeding
Surgeons and breast-feeding mothers often have questions about whether breast-feeding should be interrupted when the mother undergoes anesthesia for a surgical procedure. Short-term administration of other potent medications commonly used for sedation and analgesia (e.g., midazolam, propofol, fentanyl) has been found to result in breast milk doses less than 1.25% of the weight-normalized maternal dose.132 Thus, women do not have to “pump and dump” because of concerns of infant exposure to anesthetic agents.
References
1. Liu B, Tong C, Eisenach JC. Pregnancy increases excitability of mechanosensitive afferents innervating the uterine cervix. Anesthesiology. 2008;108:1087–1092.
2. Yan T, Liu B, Du D, et al. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth Analg. 2007;104:1246–1250.
3. Brierley SM. Molecular basis of mechanosensitivity. Auton Neurosci. 2010;153:58–68.
4. Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140:441–451.
5. Smith R. Parturition. N Engl J. Med 2007;356:271–283.
6. Unal ER, Cierny JT, Roedner C, et al. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol. 2011;204:223 e1–5.
7. Carvalho B, Clark DJ, Angst MS. Local and systemic release of cytokines, nerve growth factor, prostaglandin E2, and substance P in incisional wounds and serum following cesarean delivery. J Pain. 2008;9:650–657.
8. Moore A, Costello J, Wieczorek P, et al. Gabapentin improves postcesarean delivery pain management: a randomized, placebo-controlled trial. Anesth Analg. 2011;112:167–173.
9. Ranta PO, Ala-Kokko TI, Kukkonen JE, et al. Incisional and epidural analgesia after caesarean delivery: a prospective, placebo-controlled, randomised clinical study. Int J Obstet Anesth. 2006;15:189–194.
10. Paech MJ, Pavy TJ, Orlikowski CE, et al. Postcesarean analgesia with spinal morphine, clonidine, or their combination. Anesth Analg. 2004;98:1460–1466.
11. Choi DM, Kliffer AP, Douglas MJ. Dextromethorphan and intrathecal morphine for analgesia after Caesarean section under spinal anaesthesia. Br J Anaesth. 2003;90:653–658.
12. Halpern SH, Walsh VL. Multimodal therapy for post-cesarean delivery pain. Reg Anesth Pain Med. 2001;26:298–300.
13. Rosaeg OP, Lui AC, Cicutti NJ, et al. Peri-operative multimodal pain therapy for caesarean section: analgesia and fitness for discharge. Can J Anaesth. 1997;44:803–809.
14. Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94.
15. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625.
16. Kainu JP, Sarvela J, Tiippana E, et al. Persistent pain after caesarean section and vaginal birth: a cohort study. Int J Obstet Anesth. 2010;19:4–9.
17. Nikolajsen L, Sorensen HC, Jensen TS, Kehlet H. Chronic pain following Caesarean section. Acta Anaesthesiol Scand. 2004;48:111–116.
18. Brandsborg B, Dueholm M, Nikolajsen L, et al. A prospective study of risk factors for pain persisting 4 months after hysterectomy. Clin J Pain. 2009;25:263–268.
19. Eisenach JC, Pan P, Smiley RM, et al. Resolution of pain after childbirth. Anesthesiology. 2013;118:143–151.
20. Conell-Price J, Evans JB, Hong D, et al. The development and validation of a dynamic model to account for the progress of labor in the assessment of pain. Anesth Analg. 2008;106:1509–1515.
21. Debiec J, Conell-Price J, Evansmith J, et al. Mathematical modeling of the pain and progress of the first stage of nulliparous labor. Anesthesiology. 2009;111:1093–1110.
22. Kasai S, Ikeda K. Pharmacogenomics of the human µ-opioid receptor. Pharmacogenomics. 2011;12:1305–1320.
23. Landau R, Kern C, Columb MO, et al. Genetic variability of the mu-opioid receptor influences intrathecal fentanyl analgesia requirements in laboring women. Pain. 2008;139:5–14.
24. Wong CA, McCarthy RJ, Blouin J, Landau R. Observational study of the effect of mu-opioid receptor genetic polymorphism on intrathecal opioid labor analgesia and post-cesarean delivery analgesia. Int J Obstet Anesth. 2010;19:246–253.
25. Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109:520–526.
27. Landau R, Xie HG, Dishy V, et al. Beta2-adrenergic receptor genotype and preterm delivery. Am J Obstet Gynecol. 2002;187:1294–1298.
28. Reitman E, Conell-Price J, Evansmith J, et al. Beta2-adrenergic receptor genotype and other variables that contribute to labor pain and progress. Anesthesiology. 2011;114:927–939.
29. Miller RS, Smiley RM, Daniel D, et al. Beta2-adrenoceptor genotype and progress in term and late preterm active labor. Am J Obstet Gynecol. 2011;205:137.e1–137.e7.
30. Veringa I, Buitendijk S, de Miranda E, et al. Pain cognitions as predictors of the request for pain relief during the first stage of labor: a prospective study. J Psychosom Obstet Gynaecol. 2011;32:119–125.
31. Flink IK, Mroczek MZ, Sullivan MJ, Linton SJ. Pain in childbirth and postpartum recovery: the role of catastrophizing. Eur J Pain. 2009;13:312–316.
32. Chooi CS, Nerlekar R, Raju A, Cyna AM. The effects of positive or negative words when assessing postoperative pain. Anaesth Intensive Care. 2011;39:101–106.
33. Zieger M, Schwarz R, Konig HH, et al. Depression and anxiety in patients undergoing herniated disc surgery: relevant but underresearched—a systematic review. Cent Eur Neurosurg. 2010;71:26–34.
34. Bell J, Harvey-Dodds L. Pregnancy and injecting drug use. BMJ. 2008;336:1303–1305.
35. Ludlow JP, Evans SF, Hulse G. Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. Aust N Z J Obstet Gynaecol. 2004;44:302–306.
36. Jones HE, Finnegan LP, Kaltenbach K. Methadone and buprenorphine for the management of opioid dependence in pregnancy. Drugs. 2012;72:747–757.
37. Ludlow J, Christmas T, Paech MJ, Orr B. Drug abuse and dependency during pregnancy: anaesthetic issues. Anaesth Intensive Care. 2007;35:881–893.
38. Lang AJ, Sorrell JT, Rodgers CS, Lebeck MM. Anxiety sensitivity as a predictor of labor pain. Eur J Pain. 2006;10:263–270.
39. Okifuji A, Hare BD. Do sleep disorders contribute to pain sensitivity? Curr Rheumatol Rep. 2011;13:528–534.
40. Geiss A, Rohleder N, Kirschbaum C, et al. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–117.
41. White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008;9:76–82.
42. Phillips DM. JCAHO pain management standards are unveiled. JAMA. 2000;284:428–429.
43. Cohen SE, Subak LL, Brose WG, Halpern J. Analgesia after cesarean delivery: patient evaluations and costs of five opioid techniques. Reg Anesth. 1991;16:141–149.
44. Lim Y, Jha S, Sia AT, Rawal N. Morphine for post-caesarean section analgesia: intrathecal, epidural or intravenous? Singapore Med J. 2005;46:392–396.
45. Eisenach JC, Grice SC, Dewan DM. Patient-controlled analgesia following cesarean section: a comparison with epidural and intramuscular narcotics. Anesthesiology. 1988;68:444–448.
46. Bonnet MP, Mignon A, Mazoit JX, et al. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain. 2010;14:894.e1–894.e9.
47. Macarthur A, Imarengiaye C, Tureanu L, Downey K. A randomized, double-blind, placebo-controlled trial of epidural morphine analgesia after vaginal delivery. Anesth Analg. 2010;110:159–164.
48. Owen H, Brose WG, Plummer JL, Mather LE. Variables of patient-controlled analgesia. 3. Test of an infusion-demand system using alfentanil. Anaesthesia. 1990;45:452–455.
49. Mather LE, Owen H. The scientific basis of patient-controlled analgesia. Anaesth Intensive Care. 1988;16:427–436.
50. Hudcova J, McNicol E, Quah C, et al. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006;4.
51. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting. Anesthesiology. 2012;116:248–273.
52. Paech MJ, Moore JS, Evans SF. Meperidine for patient-controlled analgesia after cesarean section: intravenous versus epidural administration. Anesthesiology. 1994;80:1268–1276.
53. Ngan Kee WD, Lam KK, Chen PP, Gin T. Comparison of patient-controlled epidural analgesia with patient-controlled intravenous analgesia using pethidine or fentanyl. Anaesth Intensive Care. 1997;25:126–132.
54. Parker RK, White PF. Epidural patient-controlled analgesia: an alternative to intravenous patient-controlled analgesia for pain relief after cesarean delivery. Anesth Analg. 1992;75:245–251.
55. Harrison DM, Sinatra R, Morgese L, Chung JH. Epidural narcotic and patient-controlled analgesia for post-cesarean section pain relief. Anesthesiology. 1988;68:454–457.
56. Rapp-Zingraff N, Bayoumeu F, Baka N, et al. Analgesia after caesarean section: patient-controlled intravenous morphine vs epidural morphine. Int J Obstet Anesth. 1997;6:87–92.
57. Gordon DB, Jones HD, Goshman LM, et al. A quality improvement approach to reducing use of meperidine. Jt Comm J Qual Improv. 2000;26:686–699.
58. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 4th edition. American Pain Society: Glenview, IL; 1999.
59. American College of Obstetricians and Gynecologists. Obstetric analgesia and anesthesia. ACOG Practice Bulletin No. 36. Washington, DC, July 2002 (reaffirmed 2012). Obstet Gynecol. 2002;100:177–191.
60. JCAHO alert gives new recommendations for PCA. Hosp Peer Rev. 2005;30:24–25.
61. Owen H, Kluger MT, Plummer JL. Variables of patient-controlled analgesia. 4. The relevance of bolus dose size to supplement a background infusion. Anaesthesia. 1990;45:619–622.
62. Owen H, Plummer JL, Armstrong I, et al. Variables of patient-controlled analgesia. 1. Bolus size. Anaesthesia. 1989;44:7–10.
63. Owen H, Szekely SM, Plummer JL, et al. Variables of patient-controlled analgesia. 2. Concurrent infusion. Anaesthesia. 1989;44:11–13.
64. Parker RK, Holtmann B, White PF. Patient-controlled analgesia: does a concurrent opioid infusion improve pain management after surgery? JAMA. 1991;266:1947–1952.
65. Parker RK, Holtmann B, White PF. Effects of a nighttime opioid infusion with PCA therapy on patient comfort and analgesic requirements after abdominal hysterectomy. Anesthesiology. 1992;76:362–367.
66. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 6th edition. American Pain Society: Glenview, IL; 2009.
67. McCoy EP, Furness G, Wright PM. Patient-controlled analgesia with and without background infusion: analgesia assessed using the demand:delivery ratio. Anaesthesia. 1993;48:256–260.
68. Aubrun F, Langeron O, Quesnel C, et al. Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology. 2003;98:1415–1421.
69. Bodian CA, Freedman G, Hossain S, et al. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology. 2001;95:1356–1361.
70. Jakobi P, Solt I, Tamir A, Zimmer EZ. Over-the-counter oral analgesia for postcesarean pain. Am J Obstet Gynecol. 2002;187:1066–1069.
71. Bloomfield SS, Cissell GB, Mitchell J, et al. Analgesic efficacy and potency of two oral controlled-release morphine preparations. Clin Pharmacol Ther. 1993;53:469–478.
72. Jakobi P, Weiner Z, Solt I, et al. Oral analgesia in the treatment of post-cesarean pain. Eur J Obstet Gynecol Reprod Biol. 2000;93:61–64.
73. Davis KM, Esposito MA, Meyer BA. Oral analgesia compared with intravenous patient-controlled analgesia for pain after cesarean delivery: a randomized controlled trial. Am J Obstet Gynecol. 2006;194:967–971.
74. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056.
76. Townsend RJ, Benedetti TJ, Erickson SH, et al. Excretion of ibuprofen into breast milk. Am J Obstet Gynecol. 1984;149:184–186.
77. Angle PJ, Halpern SH, Leighton BL, et al. A randomized controlled trial examining the effect of naproxen on analgesia during the second day after cesarean delivery. Anesth Analg. 2002;95:741–745.
78. Jakobi P, Solt I, Tamir A, Zimmer EZ. Over-the-counter oral analgesia for postcesarean pain. Am J Obstet Gynecol. 2002;187:1066–1069.
79. Dahl V, Hagen IE, Sveen AM, et al. High-dose diclofenac for postoperative analgesia after elective caesarean section in regional anaesthesia. Int J Obstet Anesth. 2002;11:91–94.
80. Dennis AR, Leeson-Payne CG, Hobbs GJ. Analgesia after caesarean section: the use of rectal diclofenac as an adjunct to spinal morphine. Anaesthesia. 1995;50:297–299.
81. Cardoso MM, Carvalho JC, Amaro AR, et al. Small doses of intrathecal morphine combined with systemic diclofenac for postoperative pain control after cesarean delivery. Anesth Analg. 1998;86:538–541.
82. Lowder JL, Shackelford DP, Holbert D, Beste TM. A randomized, controlled trial to compare ketorolac tromethamine versus placebo after cesarean section to reduce pain and narcotic usage. Am J Obstet Gynecol. 2003;189:1559–1562.
83. Ketorolac Tromethamin Injection, USP. Package insert. Hospira. [Available at] http://www.hospira.com/Images/EN-2958_32-91429_1.pdf [Accessed May 2013] .
84. Wischnik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521–524.
85. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–789.
86. Paech MJ, Salman S, Ilett KF, et al. Transfer of parecoxib and its primary active metabolite valdecoxib via transitional breastmilk following intravenous parecoxib use after cesarean delivery: a comparison of naive pooled data analysis and nonlinear mixed-effects modeling. Anesth Analg. 2012;114:837–844.
87. Rawlinson A, Kitchingham N, Hart C, et al. Mechanisms of reducing postoperative pain, nausea and vomiting: a systematic review of current techniques. Evid Based Med. 2012;17:75–80.
88. Alhashemi JA, Alotaibi QA, Mashaat MS, et al. Intravenous acetaminophen vs oral ibuprofen in combination with morphine PCIA after Cesarean delivery. Can J Anaesth. 2006;53:1200–1206.
89. Ing Lorenzini K, Besson M, Daali Y, et al. A randomized, controlled trial validates a peripheral supra-additive antihyperalgesic effect of a paracetamol-ketorolac combination. Basic Clin Pharmacol Toxicol. 2011;109:357–364.
90. Petring OU, Dawson PJ, Blake DW, et al. Normal postoperative gastric emptying after orthopaedic surgery with spinal anaesthesia and i.m. ketorolac as the first postoperative analgesic. Br J Anaesth. 1995;74:257–260.
91. Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12:523–532.
92. Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2-agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322.
93. Palanisamy A, Klickovich RJ, Ramsay M, et al. Intravenous dexmedetomidine as an adjunct for labor analgesia and cesarean delivery anesthesia in a parturient with a tethered spinal cord. Int J Obstet Anesth. 2009;18:258–261.
94. Ala-Kokko TI, Pienimaki P, Lampela E, et al. Transfer of clonidine and dexmedetomidine across the isolated perfused human placenta. Acta Anaesthesiol Scand. 1997;41:313–319.
95. Duraclon (clonidine hydrochloride injection). Package insert. Bioniche Pharma. [Available at] http://www.bionichepharmausa.com/pdf/Duraclon_Xanodyne_PI.pdf [Accessed May 2013] .
96. Lavand’homme PM, Roelants F, Waterloos H, et al. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008;107:948–955.
97. van Tuijl I, van Klei WA, van der Werff DB, Kalkman CJ. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain and morphine requirements after Caesarean section: a randomized controlled trial. Br J Anaesth. 2006;97:365–370.
98. Capogna G, Celleno D, Zangrillo A, et al. Addition of clonidine to epidural morphine enhances postoperative analgesia after cesarean delivery. Reg Anesth. 1995;20:57–61.
99. Pryde PG, Mittendorf R. Contemporary usage of obstetric magnesium sulfate: indication, contraindication, and relevance of dose. Obstet Gynecol. 2009;114:669–673.
100. De Oliveira GSJ, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2013.
101. Sun J, Wu X, Xu X, et al. A comparison of epidural magnesium and/or morphine with bupivacaine for postoperative analgesia after cesarean section. Int J Obstet Anesth. 2012;21:310–316.
102. Albrecht E, Kirkham KR, Liu SS, Brull R. The analgesic efficacy and safety of neuraxial magnesium sulphate: a quantitative review. Anaesthesia. 2013;68:190–202.
103. Short J, Downey K, Bernstein P, et al. A single preoperative dose of gabapentin does not improve postcesarean delivery pain management: a randomized, double-blind, placebo-controlled dose-finding trial. Anesth Analg. 2012;115:1336–1342.
104. Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–125.
105. Sen S, Ozmert G, Aydin ON, et al. The persisting analgesic effect of low-dose intravenous ketamine after spinal anaesthesia for caesarean section. Eur J Anaesthesiol. 2005;22:518–523.
106. Bauchat JR, Higgins N, Wojciechowski KG, et al. Low-dose ketamine with multimodal postcesarean delivery analgesia: a randomized controlled trial. Int J Obstet Anesth. 2011;20:3–9.
107. Duedahl TH, Romsing J, Moiniche S, Dahl JB. A qualitative systematic review of peri-operative dextromethorphan in post-operative pain. Acta Anaesthesiol Scand. 2006;50:1–13.
108. McDonnell JG, Curley G, Carney J, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2008;106:186–191.
109. Belavy D, Cowlishaw PJ, Howes M, Phillips F. Ultrasound-guided transversus abdominis plane block for analgesia after Caesarean delivery. Br J Anaesth. 2009;103:726–730.
110. Kanazi GE, Aouad MT, Abdallah FW, et al. The analgesic efficacy of subarachnoid morphine in comparison with ultrasound-guided transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2010;111:475–481.
111. Loane H, Preston R, Douglas MJ, et al. A randomized controlled trial comparing intrathecal morphine with transversus abdominis plane block for post-cesarean delivery analgesia. Int J Obstet Anesth. 2012;21:112–118.
112. Costello JF, Moore AR, Wieczorek PM, et al. The transversus abdominis plane block, when used as part of a multimodal regimen inclusive of intrathecal morphine, does not improve analgesia after cesarean delivery. Reg Anesth Pain Med. 2009;34:586–589.
113. Sharkey A, Finnerty O, McDonnell JG. Role of transversus abdominis plane block after caesarean delivery. Curr Opin Anaesthesiol. 2013;26:268–272.
114. Carney J, Finnerty O, Rauf J, et al. Studies on the spread of local anaesthetic solution in transversus abdominis plane blocks. Anaesthesia. 2011;66:1023–1030.
115. Griffiths JD, Le NV, Grant S, et al. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110:996–1000.
116. Kainu JP, Sarvela J, Halonen P, et al. Continuous wound infusion with ropivacaine fails to provide adequate analgesia after caesarean section. Int J Obstet Anesth. 2012;21:119–124.
117. O’Neill P, Duarte F, Ribeiro I, et al. Ropivacaine continuous wound infusion versus epidural morphine for postoperative analgesia after cesarean delivery: a randomized controlled trial. Anesth Analg. 2012;114:179–185.
118. Carvalho B, Lemmens HJ, Ting V, Angst MS. Postoperative subcutaneous instillation of low-dose ketorolac but not hydromorphone reduces wound exudate concentrations of interleukin-6 and interleukin-10 and improves analgesia following cesarean delivery. J Pain. 2013;14:48–56.
120. Pavy T, Gambling D, Kliffer P, et al. Effect of preoperative skin infiltration with 0.5% bupivacaine on postoperative pain following cesarean section under spinal anesthesia. Int J Obstet Anesth. 1994;3:199–202.
121. Vallejo MC, Steen TL, Cobb BT, et al. Efficacy of the bilateral ilioinguinal-iliohypogastric block with intrathecal morphine for postoperative cesarean delivery analgesia. Scientific World Journal. 2012;2012:107316.
122. Wolfson A, Lee AJ, Wong RP, et al. Bilateral multi-injection iliohypogastric-ilioinguinal nerve block in conjunction with neuraxial morphine is superior to neuraxial morphine alone for postcesarean analgesia. J Clin Anesth. 2012;24:298–303.
123. Bell EA, Jones BP, Olufolabi AJ, et al. Iliohypogastric-ilioinguinal peripheral nerve block for post-Cesarean delivery analgesia decreases morphine use but not opioid-related side effects. Can J Anaesth. 2002;49:694–700.
124. Wu HC, Liu YC, Ou KL, et al. Effects of acupuncture on post-cesarean section pain. Chin Med J (Engl). 2009;122:1743–1748.
125. Abbaspoor Z, Akbari M, Najar S. Effect of foot and hand massage in post-cesarean section pain control: a randomized control trial. Pain Manag Nurs. 2013.
126. Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118–126.
127. Morales T, Shapiro E, Marina N, Mena F. Sympathetic innervation of mammary glands mediates suckling-induced reflex inhibition of milk yield in rats. Physiol Behav. 2001;74:37–43.
128. Woods AB, Crist B, Kowalewski S, et al. A cross-sectional analysis of the effect of patient-controlled epidural analgesia versus patient controlled analgesia on postcesarean pain and breastfeeding. J Obstet Gynecol Neonatal Nurs. 2012;41:339–346.
129. Oberlander TF, Robeson P, Ward V, et al. Prenatal and breast milk morphine exposure following maternal intrathecal morphine treatment. J Hum Lact. 2000;16:137–142.
130. Sauberan JB, Anderson PO, Lane JR, et al. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol. 2011;117:611–617.
131. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 9th edition. Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia; 2011.
132. Nitsun M, Szokol JW, Saleh HJ, et al. Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin Pharmacol Ther. 2006;79:549–557.