Chapter 38 Postcholecystectomy problems
Overview
Cholecystectomy for gallbladder diseases has become the most common abdominal operation in Westernized countries. Since it was first performed in 1987, laparoscopic cholecystectomy (LC) has surpassed the open approach as the gold standard operation, largely because of significant decreases in operative time, patient pain, hospital stay, and costs (Begos & Modlin, 1994; see Chapter 34). At present, approximately 750,000 LCs are performed annually in the United States, accounting for roughly 90% of all cholecystectomies (Khan et al, 2007). This minimally invasive approach has revolutionized the treatment of patients with gallbladder disease and improved outcome, and it has permitted a liberalization of indications. Patients who would have otherwise been turned away from open cholecystectomy (OC) are now undergoing LC, and the open operation is now reserved for the most complicated scenarios (Khan et al, 2007).
Although LC has some advantages for both patients and surgeons, a new set of complications has arisen. The popularity of LC, combined with its innovative technology and the decreased threshold to whom it is offered, has resulted in novel postoperative problems, such as dropped stones, port-site hernias, and unique forms of common bile duct (CBD) injuries (see Chapter 42A, Chapter 42B ). More disturbing is that despite the widespread acceptance of LC and increasing experience with the procedure, it has a higher serious complication rate than that of the open approach. Furthermore, just as with open cholecystectomy, patients with abdominal pain that masquerades as gallbladder disease may undergo LC yet continue to be persistently symptomatic after the operation. This chapter addresses the causes of postcholecystectomy problems, both biliary and nonbiliary, along with optimal diagnostic and treatment strategies. Henceforth, we will refer to postcholecystectomy problems (PCPs) as persistent symptoms after surgical removal of the gallbladder regardless of etiology.
Indications for Cholecystectomy (See Chapters 30 and 31)
Gallstones that cause symptoms of epigastric and/or right upper quadrant (RUQ) pain, usually occurring after fatty meals and associated with dyspepsia and sometimes excessive flatulence, are most commonly treated with cholecystectomy. LC is successful in eliminating pain in 85% of patients with this constellation of presurgical symptoms (Tantia et al, 2008). Furthermore, patients with acute cholecystitis manifested by fevers, RUQ pain, elevated white blood count (WBC), and nausea and vomiting are also treated with LC.
A more contentious indication for cholecystectomy falls into the category of functional biliary disorders. Patients with persistent episodes of prolonged RUQ pain who lack a defined organic etiology should be evaluated for a functional biliary disorder. This condition, commonly referred to as biliary dyskinesia, affects approximately 2.4% of patients with biliary pain in the absence of cholelithiasis and results from the impaired physiologic contraction of the gallbladder, leading to insufficient emptying and pain (Barbara et al, 1987). Various studies suggest that patients with motility disorders of the gallbladder may benefit from LC (Westlake et al, 1990; Chen et al, 2001). However, if the physiologic derangement is downstream of the cystic duct, pain may persist even after removal of the gallbladder. This nebulous condition is termed sphincter of Oddi dysfunction, and it will be discussed in more detail later in this chapter. Finally, carcinoma of the gallbladder is an uncommon problem—only 9250 new cases of gallbladder cancer occurred in the United States in 2008—that is optimally treated with cholecystectomy and en bloc partial hepatectomy; but it is associated with a poor prognosis, with 5-year survival rates between 5% and 10% and an overall median survival of 3 to 6 months from diagnosis (Hueman et al, 2009). An in-depth discussion of these indications for cholecystectomy is found in Chapters 30, 31, and 49.
Complications of Cholecystectomy
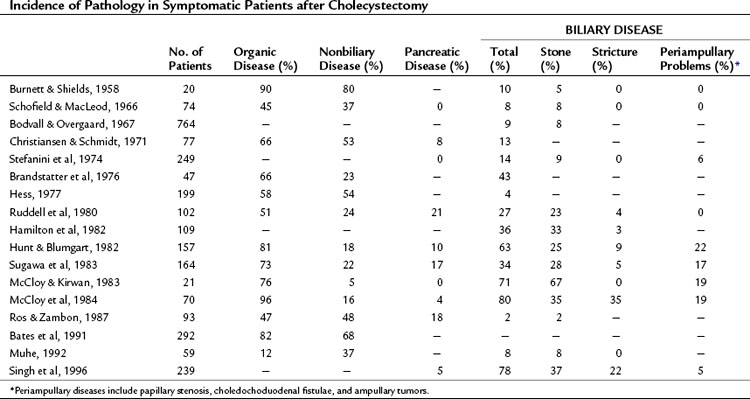
Postsurgical complications may be classified by biliary and nonbiliary causes. Furthermore, biliary complications may be subdivided into early, late, and mechanical (Table 38.1). Despite the advantages in care offered by the laparoscopic approach, cholecystectomy should still be recognized and referred to as a major operation. This is emphasized by the fact that mortality rates after cholecystectomy exceed many elective operations felt to be far more significant in scope, such as cardiac bypass, colonic resection, and even pancreatectomy. In a multivariate comparison of postcholecystectomy complications studied by Jatzko and colleagues (1995), open cholecystectomy was associated with higher morbidity (7.7% vs. 1.9%) and mortality rates (5% vs. 1%) than those of LC. The incidence of pathology seen in patients who are symptomatic after having a cholecystectomy is summarized in Table 38.2.
Table 38.1 Classification of Causes of Postcholecystectomy Symptoms
| Biliary |
| Early |
Early Complications
Biliary Injury
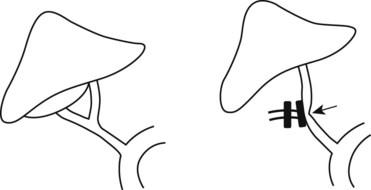
Despite the advantages of LC over OC, common bile duct injuries are seen more frequently in the laparoscopic approach (0.2% to 0.7%) than in OC (0.1% to 0.04%) (Konsten et al, 1993; Nuzzo et al, 2005; Soderlund et al, 2005; Ali et al, 2007; Karvonen et al, 2007). Retrospective analyses of CBD injuries reveal that the two most common factors associated with injury are acute cholecystitis, wherein the normal gallbladder and biliary anatomy is obscured or distorted because of indurated and/or edematous tissues, and misperceptions or inexperience on the part of the surgeon. A learning curve is also implicated. In a series of 350 LCs reviewed by Huang and colleagues, CBD injuries occurred more often in the first 10 to 15 operations performed by a surgeon (Huang et al, 1993). In addition to the classic direct CBD injuries caused by misidentification of the cystic and common bile ducts, clipping of the “tented” CBD may occur in cases specific to LC when applying the first clip on the cystic duct by excessive traction of the CBD into the clip itself (Fig. 38.1). These injuries can lead to stricture and obstruction of the CBD and may be avoided by placing the first clip with visualized distance from the cysticocholedochal junction (Duca et al, 2003). In a retrospective review of 10,000 patients undergoing LC in a single center by four separate surgeons with no documented CBD injuries, it was found that surgical experience, preoperative imaging, precise operative procedures, and conversion from LC to OC when needed are necessary prophylactic measures to prevent CBD injuries (Ou et al, 2009). In addition, regular reliance on the “critical view” technique, along with abandonment of the infundibular dissection approach, can optimize a safer dissection (Strasberg et al, 2000; Strasberg, 2002).
Minor biliary leaks from the bed of the gallbladder, so-called Luschka leaks, may also be troublesome intraoperatively. Often, such leaks occur as a result of errant dissection into a deep plane of hepatic parenchyma. Accessory bile ducts found in the gallbladder bed may be controlled by ligation when transection and spillage is identified, but if these leaks are not identified in the operating room, postoperative bilomas and even bilious ascites may ensue. If the drainage of these “minor” leaks exceeds 500 mL over a 24-hour period, endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy and transampullary stenting is useful in decompressing the biliary system and preventing reoperation (Pinkas & Brady, 2008; Pawa & Al-Kawas, 2009).
Hemorrhage
In a retrospective analysis of 9542 consecutive LCs by Duca and colleagues (2003), intraoperative hemorrhage occurred in 224 patients for an incidence of 2.3%. Bleeding was most commonly from the gallbladder bed and was treated with a fibrin-collagen patch. Conversion to an open procedure with suturing of the gallbladder peritoneum was required in five patients, however. It was noted that such hemorrhage from the gallbladder bed was encountered most commonly in patients with acute cholecystitis or cirrhosis. Tangential lesions of the cystic artery or, less commonly, total sectioning of the cystic artery occurred in 95 cases. In the majority of cases, the intraoperative bleeding was controlled laparoscopically with hemostatic clips, but conversion to an open procedure occurred in one patient. Damage to the hepatic artery occurred in one patient and required immediate open conversion. Finally, bleeding from the greater omentum was seen in 18 patients and was controlled laparoscopically in 16 patients.
Another source of massive intraoperative blood loss is from inadvertent incursion into a deep plane of hepatic parenchyma, where distal tributaries of the middle hepatic vein may be encountered. In fact, 10% of patients harbor large branches of the middle hepatic vein directly adjacent to the gallbladder fossa, which may lead to significant hemorrhage in instances of even minimal parenchymal dissection (Ball et al, 2006). Furthermore, postoperative hemorrhage may occur from trocar placement, or the bleeding may be from the cystic artery itself, albeit less than 1% of the time (Marakis et al, 2007). Finally, it should be recognized that management of profuse bleeding during LC, particularly when unexpected, can be fraught with significant ramifications that include major vascular ligation (hepatic artery, portal vein) and/or bile duct injury. This occurs from haphazard clip application or aggressive use of cautery in a poorly visualized field, thus conversion to open laparotomy is often the more prudent approach.
Dropped Stones
In 6% to 40% of LC procedures perforation of the gallbladder with subsequent spillage of gallstones occurs (Helme et al, 2009). These stones are usually retrieved under direct visualization, and the Morison pouch can be copiously irrigated; however, in 13% to 32% of patients these stones may be “lost” (Helme et al, 2009). This scenario contributes to postoperative morbidity 0.1% to 6% of the time, and the effects may vary in presentation and timing (Hand et al, 2006). Although such complications are infrequent, the patient may have symptoms such as fever, chronic abdominal pain, and/or intestinal obstruction. Furthermore, a meta-analysis by Zehetner and colleagues (2007) revealed that complications from “dropped stones” may present months to years after LC, most commonly as subcutaneous or intraabdominal abscesses or fistulas to less frequent manifestations such as liver abscesses or bacteremia. Additionally, stones have also been reported to erode into the chest cavity, causing empyema and broncholithiasis with expectoration (Zehetner et al, 2007). Perforation of the gallbladder during LC often occurs as the organ is dissected off of the liver by excessive retraction, direct puncture, or during omental adhesiolysis, but it may also occur as the gallbladder is being removed through trocar sites. Regular use of a retrieval bag may decrease complications from removal through trocar sites. Iatrogenic perforation of the gallbladder occurs more frequently in LC compared with OC; furthermore, spilled stones are arguably better visualized and more readily retrieved during open operations.
In a large retrospective review of more than 10,000 LCs, Schafer and colleagues reported a 6% incidence of gallbladder perforation with complications from dropped stones in 0.08% (Schafer et al, 1998). Furthermore, a 3-year prospective study by Rice and colleagues (1997) of 1059 patients who underwent LC at the Mayo Clinic revealed a perforation rate of 29% with an 11% dropped-stone rate. In this study, 2% of patients with perforated gallbladders developed subhepatic abscesses, and patients with intact gallbladders extracted had abscesses (Rice et al, 1997). In experimental studies using rabbit, rat, and dog models, the intraperitoneal introduction of gallstones resulted in a predominantly benign course without long-term complications (Welch et al, 1991; Sax & Adams, 1993; Cline et al, 1994; Cohen et al, 1994; Johnston et al, 1994; Zisman et al, 1995; Tzardis et al, 1996). Therefore, when gallbladder perforation and dropped stones are encountered, efforts should be made to retrieve stones with suctioning or forceps, and the subhepatic fossa should be copiously irrigated. Conversion to an open procedure is not necessary owing to a low rate of complications; however, it is a good policy to document the event and to inform the patient that stone spillage occurred.
Insufficient Cholecystectomy
In patients with severe acute inflamed cholecystitis, visualization of the cystic duct can be difficult, and historically this was treated with subtotal cholecystectomy. This approach has been proven to be effective and safe in open procedures. Recent reports suggest that when a critical view is impossible to obtain, laparoscopic subtotal cholecystectomy is also relatively safe (Hobbs et al, 2006; Ji et al, 2006; Sharp et al, 2009). When the triangle of Calot cannot be safely dissected, transection and oversewing of the gallbladder neck with resection of the anterior wall of the gallbladder, leaving a small strip of posterior gallbladder wall, can be an option.
Although this is a viable albeit rare approach, PCPs requiring an invasive reintervention still occur (Pernice & Andreoli, 2009). In a study from the United Kingdom of 26 cases of laparoscopic subtotal cholecystectomy over a 3-year period, five patients underwent postoperative ERCP for persistent biliary leak (n = 4) or retained common bile duct stones (n = 1). One patient developed a subphrenic abscess and underwent reoperative laparotomy, and another patient who was critically ill before surgery died postoperatively. A completion LC was required for one patient with a gallstone in a retained pouch, and two patients developed port-site hernias (Philips et al, 2008). In cases mandating laparoscopic subtotal cholecystectomy, the procedure must be performed by experienced surgeons, a drain should be left at the level of transection, and if a posterior wall is retained, it should be coagulated. A late manifestation of “partial” cholecystectomy is a remnant gallbladder pouch with potential for retained stones or cholecystitis.
Infections
Postoperative infection after cholecystectomy is a rare event. In a 7-year study conducted by the Centers for Disease Control and Prevention, out of 54,504 patients, the rate of surgical site infections (SSIs) was significantly lower in the laparoscopic approach when compared with open surgery (0.62% vs 1.82%, P = .001); however, the pathogens that caused SSIs were similar in both groups (Richards et al, 2003). The pathogens identified included Enterobacter species, Escherichia coli, and Staphylococcus aureus. The majority of infections in the open approach were seen in superficial spaces; in the laparoscopic approach, the majority of infections were intraabdominal. As expected, the SSI rates were higher in patients taken to the operating room emergently or with an ASA of 3 or higher (Richards et al, 2003). That said, prophylactic antibiotics given preoperatively to any low-risk patient undergoing elective LC are not indicated as evidenced by several reported studies (Chang et al, 2006; Tocchi et al, 2000; Uludag et al, 2009).
Late Postoperative Complications
Months to years after undergoing a cholecystectomy, patients may be seen with abdominal pain that differs from the symptoms felt before surgery. The incidence of pain from abdominal incisions is higher in OC (9%) versus LC (5%) over a 6-month period as reported by Vander Velpen and colleagues (1993). Bile duct neuromas have also been reported as a cause of pain up to 45 years after cholecystectomy (Paquette et al, 2009). A randomized controlled study performed by Halm and colleagues with 1-year follow-up in the prelaparoscopic era demonstrated that incisional hernias complicated 2% (1 of 60) of patients undergoing OC with a transverse incision compared with 14% (9 of 63) with a midline incision (Halm et al, 2009). Muhe (1992) reported that 12% of patients developed incisional hernias or pain after OC compared with 2% after LC.
Strictures (See Chapter 42A, Chapter 42B )
Benign stenosis of the CBD is more common after LC, and 95% of the time, it is related to iatrogenic injury in the form of partial or total sectioning, clipping, or ligation (Kassab et al, 2006). The incidence of patients developing strictures after LC varies from 0% to 2.7% compared with 0.2% to 0.5% after OC (Deziel et al, 1993; Strasberg et al, 1996). Postsurgical benign strictures may also result from delayed thermal injury or intraoperative ischemic devascularization (Genest et al, 1986; Deziel et al, 1993). Additionally, choledochoduodenal fistula formation tends to manifest more commonly after such stricture formation. In the postoperative period, the problems caused by strictures may result in abdominal pain, fever, and jaundice. Cholestasis may lead to choledocholithiasis and recurrent cholangitis (Kassab et al, 2006). Malignant strictures after cholecystectomy should also be considered, as rare cases have been reported (Sharma et al, 2008). This indicates a misdiagnosis prior to the cholecystectomy.
Biliary strictures have historically been treated by surgical reconstruction via hepaticojejunostomy, although many studies have recently reported the safety and efficacy of endoscopic balloon dilation and/or stenting as a noninvasive method to treat such problems (Kassab et al, 2006; Gouma, 2007). Surgery is therefore reserved for major bile duct injuries or failure of endoscopic therapy. A more detailed discussion of both benign and malignant strictures can be found in Chapter 42A, Chapter 42B, Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D .
Retained Stones
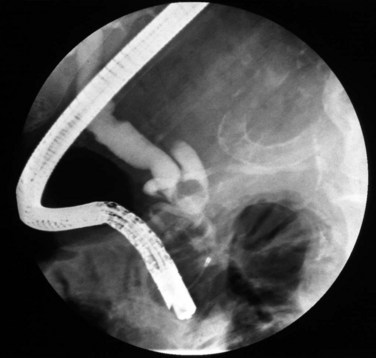
The remnant of the cystic duct or gallbladder (in subtotal cholecystectomy) has historically been implicated as the source of pain, nausea, and vomiting in postcholecystectomy patients (Bodvall, 1973). Several theories as to the etiology of such symptoms, characterized as the cystic duct stump syndrome, have been hypothesized (Garlock & Hurwitt, 1951). An increase in choledochal pressure resulting in cystic stump distension, inflammation or stone obstruction within the remnant of the cystic duct or gallbladder, and an increase in the sphincter of Oddi pressure have all served as causes of postcholecystectomy problems (Bodvall, 1973). However, various studies have confirmed that symptomatic improvement only occurs when a stone is present in the CBD or cystic duct and is subsequently removed endoscopically or via operative reintervention (Hopkins et al, 1979; Mergener et al, 1999; Tantia et al, 2008). In general, persistent symptoms following a cholecystectomy in which no cholangiogram was performed warrants analysis of the liver function profile and/or noninvasive imaging with either ultrasound or MRCP to assess for retained stones. If CBD stones are present, therapeutic ERCP is suggested (Fig. 38.2). The comprehensive management of common bile duct stones is discussed in Chapters 35 through 37.
Papillary Disorders
The apparatus responsible for directing the flow of biliary and pancreatic secretions is the sphincter of Oddi, which consists of three segments of smooth muscle: 1) the CBD segment, which is about 10 mm in length; 2) the pancreatic segment, which is roughly 6 mm in length; and 3) the segment of the confluence of both the bile duct and pancreatic duct, which is intraduodenal and close to 6 mm in length (Bistritz & Bain, 2006). When constricted, this sphincter impairs forward flow of both bile and pancreatic juices.
Sphincter of Oddi dysfunction (SOD) continues to be a diagnostic dilemma, and its management is controversial. Many entities have been reported describing this phenomenon, including papillary stenosis, sclerosing papillitis, and biliary spasm. The pathogenesis of this disorder is incompletely understood but is thought to result from an increase in basal sphincter pressure resulting in an increase in CBD pressure and stasis from obstructed biliary and pancreatic flow (Funch-Jensen et al, 2006). Microlithiasis causing transient obstruction has also been implicated as a result of stasis in the CBD from sphincter dysfunction, but this has been shown in several studies to be of little consequence in postcholecystectomy pain (Quallich et al, 2002; Rashdan et al, 2003; Bistritz & Bain, 2006). Uncoordinated spasm of the sphincter of Oddi has also been shown to produce the classic symptoms of RUQ colic with elevated liver function tests (LFTs) and has been characterized as biliary dyskinesia (Chuttani & Carr-Locke, 1993), therefore SOD may be described as two separate mechanisms: stenosis and dyskinesia.
Sphincter of Oddi Stenosis
Sphincter of Oddi stenosis refers to a fixed constriction or narrowing of part or all of the anatomic structures of the sphincter of Oddi. The stenosis may result from the trauma of recurrent passing gallstones, repeated attacks of pancreatitis, the effects of iatrogenic instrumentation, or other nonspecific causes. These insults result in fibrotic changes that cause a noncompliant orifice and elevated resting pressures, correlating to symptoms that fall into the category of type I biliary SOD (Bistritz & Bain, 2006). Ampullary biopsies of 43% of patients with SOD show evidence of fibrosis and inflammation (Ponchon et al, 1995). In the postcholecystectomy patient, the resulting backpressure of “papillary stenosis” is compounded by the lack of a gallbladder, which could normally serve as a reservoir for increased pressure in the CBD (Bistritz & Bain, 2006).
Sphincter of Oddi Dyskinesia
Uncoordinated contraction and relaxation of the sphincter of Oddi results in transient obstruction of biliary and/or pancreatic secretions. This leads to an elevation of intraluminal pressure with a concurrent elevation of LFTs. An increase in frequency of phasic contractions and a paradoxical response to the administration of cholecystokinin (failure of relaxation) may also occur in patients with dyskinesia (Tanaka, 2002). Interestingly, there is a significant overlap in patients with biliary dyskinesia and other intestinal motility disorders such as irritable bowel syndrome or hypertensive lower esophageal sphincter disorder (Evans et al, 1995, 1997; Chan et al, 1997).
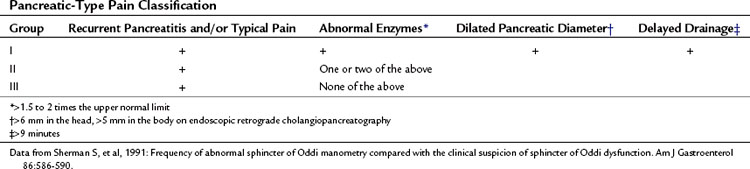
Clinically, SOD presents in 1% of patients after cholecystectomy and in 14% to 23% of patients presenting with the postcholecystectomy syndrome (Bar-Meir et al, 1984; Meshkinpour & Mollot, 1992; Drossman et al, 1993). SOD can also manifest as recurrent pancreatitis or with recurrent acalculous RUQ pain in patients with an intact gallbladder. Patients with SOD may be classified into three types based on presentation, laboratory results, and ERCP findings. Specifically, two classification systems, shown in Tables 38.3 and 38.4, stratify patients into three clinical groups for both biliary and pancreatic symptoms and predict the outcomes of endoscopic versus surgical treatment (Hogan & Geenen, 1988; Sherman et al, 1991). Patients with type I disease have biliary pain, elevated LFTs, an increase in the diameter of the CBD (>12 mm) with a delay in contrast drainage of more than 45 minutes. Patients with type II disease present with biliary pain and one or two of the remaining criteria delineated above. Patients with type III disease are seen initially with the sole criteria of biliary-type pain (Hogan & Geenen, 1988).
When patients present with the constellation of symptoms that fall under SOD, including pain that lasts at least 30 minutes, other organic causes of RUQ pain must be ruled out with laboratory tests that include complete blood counts and LFTs with amylase and lipase measurements. Transabdominal or endoscopic ultrasonography along with nuclear hepatobiliary scans and cholangiopancreatography may aid in narrowing the diagnostic differential. Often evidence of a dilated common bile duct is seen in the setting of type I SOD and is most amenable to treatment with sphincterotomy (Rolny et al, 1993; Sugawa et al, 2001). Although no specific test is available to assess for SOD, ampullary manometry may be utilized to define hypertensive sphincters (>40 mm Hg) or to detect an increase in the frequency of phasic contractions as well as a paradoxical response to cholecystokinin (Geenen et al, 1989; Thune et al, 1991; Smithline et al, 1993). A further discussion of the diagnostic tools and management strategies used for SOD ensues later in this chapter.
Nonbiliary Causes of Postcholecystectomy Problems
In 70% to 80% of patients seen with postcholecystectomy pain, the etiology is attributed to nonbiliary gastrointestinal causes including gastroesophageal reflux, peptic ulcer disease, gastritis, irritable bowel syndrome, or diverticular disease (Burnett & Shields, 1958; Bates et al, 1991). These may have been the original source of symptoms in poorly chosen patients for cholecystectomy. Discomfort in the epigastrum and RUQ is often reproduced in patients with gastric, esophageal, pancreatic, hepatic, duodenal, and even pulmonary sources and therefore must be investigated in patients with postcholecystectomy symptoms. In addition, patients with flatulent dyspepsia prior to surgery often continue to have persistent symptoms after removal of the gallbladder. Patients with RUQ pain and gallstones are often immediately offered a cholecystectomy, although other gastrointestinal sources of pain or discomfort may exist and persist subsequent to surgery, presenting as a PCP.
Postcholecystectomy diarrhea (PCD), defined as three or more loose stools per day, can occur long term in up to 12% of patients after cholecystectomy (O’Donnell, 1999). At least 5% to 8% of patients report a change in their bowel habits after cholecystectomy (O’Donnell, 1999). The etiology of PCD is incompletely understood but may relate to the malabsorption of bile acids (Merrick et al, 1985). Three categories of bile acid malabsorption have been defined. In type I malabsorption, patients have previously undergone a terminal ileal (TI) resection, as bile acids are normally reabsorbed by the TI, or they may have an ileal mucosal abnormality. Type II malabsorption is considered idiopathic, and patients with type III bile acid malabsorption have either undergone a vagotomy or cholecystectomy (Fromm & Malavolti, 1986). The pathogenesis of bile acid–induced diarrhea in patients after cholecystectomy revolves around the premise that the storage depot of bile, the gallbladder, is absent, and bile acids have the ability to flow with more ease into the colon, which allows for osmotic and electrolyte shifts as well as increased transit time. The use of diagnostic studies including a synthetic radiolabeled bile acid 23-selena-25 homocholytaurine (SeHCAT) assay may be useful in narrowing the etiology of diarrhea in patients after cholecystectomy; the retention of SeHCAT at 7 days after oral administration demonstrates normal absorption of bile acids (Ford et al, 1992). The diagnostic accuracy of SeHCAT is not convincing because of studies that reveal normal levels of fecal bile acids in patients with PCD (Fromm et al, 1987). The use of bile acid binding agents such as cholestyramine have varied effects as reported in the literature (Sciarretta et al, 1986; Arlow et al, 1987; Fromm et al, 1987), however, traditional antidiarrheals may also be used in the treatment of PCD.
Postcholecystectomy pancreatic disease may be another cause of PCPs and is often concurrent and undiagnosed, with patient complaints having led to the cholecystectomy. Some studies show, however, that cholelithiasis itself may cause pancreatic changes as seen on patients undergoing ERCP (Axon et al, 1979). Whether these concurrent pancreatic changes are the cause of pain is uncertain, as not all patients with pancreatic disease after cholecystectomy underwent ERCP at the time of cholecystectomy. Furthermore, reports of patients with pancreatic disease at the time of cholecystectomy who do not manifest postoperative problems also exist (Schofield & Macleod, 1966).
Finally, after all other organic causes of PCPs are ruled out, the diagnosis of psychosomatic pain must be entertained. Various studies have revealed a higher incidence of anxiety, depression, psychosomatic disorders, and use of psychotropic drugs in patients presenting with abdominal complaints after cholecystectomy (Hansky & Dreiling, 1963; Christiansen & Schmidt, 1971; Kakizaki et al, 1976; Talley et al, 1986; Haug et al, 1995; McMahon et al, 1995). In particular, patients with type III SOD (biliary pain only) have been shown to exhibit increased rates of depression, anxiety, and obsessive-compulsive traits (Desautels et al, 1999). However, care should be taken to thoroughly evaluate patients for biliary or other abdominal causes of pain before the diagnoses of psychosomatic disorders are entertained.
Diagnostic Studies in the Evaluation of Postcholecystectomy Problems
Ultrasonography (See Chapter 13)
The first imaging modality used to evaluate patients with RUQ pain is often ultrasonography, because it is inexpensive, quick, and noninvasive; however, it is highly operator dependent. With experienced technicians, ultrasonography can be used to assess for retained stones and CBD diameter and, less commonly, for sphincter of Oddi dysfunction (Funch-Jensen et al, 2006). In the early postoperative period, transabdominal ultrasonography (TUS) is particularly useful in identifying fluid collections such as bilomas, abscesses, or hemorrhage. If fluid collections are large, ultrasound-guided aspiration and culture should be performed; if the character of the collection is bilious or purulent, drainage catheters are left in place. Bile duct imaging with TUS is difficult, and the sensitivity of ultrasonography to detect stones in the distal CBD is low (Dewbury & Smith, 1983; Filip et al, 2009). Visualizing the pancreatic duct is also difficult with ultrasonography because of overlying gas from the stomach or intestines, which often obscures the acoustic windows.
The use of TUS in the detection of SOD has also been investigated. Measurements of the CBD (upper limits of normal <8 to 10 mm) can be taken after the infusion of cholecystokinin or 45 minutes after the ingestion of a fatty meal, which in the face of distal obstructive physiology, such as that of SOD, will demonstrate dilation of the diameter of the CBD over baseline (Rosenblatt et al, 2001). These diagnostic tools have not gained popularity in the assessment of SOD because of low sensitivity rates and low reproducibility (Simeone et al, 1982; Darweesh et al, 1988; Rosenblatt et al, 2001).
Endoscopic ultrasonography (EUS) is another alternative to more accurately assess the presence of CBD stones and has the ability to better visualize the stomach, duodenum, and pancreas to rule out other organic causes of postcholecystectomy syndrome (see Chapter 14). EUS is also useful in the diagnosis of chronic pancreatitis and in the detection and staging of pancreatic, ampullary, and bile duct carcinoma. In patients with suspected choledocholithiasis, EUS may also be used prior to more invasive testing, such as ERCP. In EUS the ampulla itself is not cannulated, and although not therapeutic, this method has lower complication and failure rates. Additionally, the initial use of EUS may obviate the need for ERCP in one half to two thirds of patients (Filip et al, 2009; Petrov & Savides, 2009). Limitations of EUS include the need for sedation and limited availability, and it may not be conclusive in the presence of duodenal stenosis and previous sphincterotomy and/or biliary stenting in which pneumobilia may obscure a clear view (Stewart et al, 2001). Therefore patients with an elevated serum bilirubin, with or without an elevation of alkaline phosphatase, and a CBD measurement greater than 8 to 10 mm on TUS may be candidates for EUS which, if results are negative for stones or other pathology, may obviate the need for further invasive testing such as with ERCP, thereby decreasing the risk of radiation exposure, postprocedural pancreatitis, and postsphincterotomy bleeding or perforation.
Cholangiopancreatography (See Chapter 18)
Cholangiopancreatography provides direct visualization of the biliary tree, papilla, and pancreatic duct and is highly sensitive and specific in diagnosing the causes of PCPs. The use of magnetic resonance cholangiopancreatography (MRCP; see Chapter 17) provides a noninvasive method to evaluate various biliary and pancreatic disease processes including obstructive stones. Although there is no therapeutic value to MRCP, patients with abnormal LFTs and dilated CBDs (>10 mm) after cholecystectomy with no evidence of stones or other pathology on TUS may be candidates for MRCP (Terhaar et al, 2005). MRCP has been reported to be 95% to 100% sensitive and 88% to 97% specific in detecting biliary obstructions, although it is less sensitive in detecting stones less than 6 mm in size (Terhaar et al, 2005; Tse et al, 2006).
MRCP with secretin (MRCP-S) allows for functional testing of the pancreatic duct (Mariani et al, 2003). Long-term results of MRCP-S reveal diagnostic accuracy comparable to that of ERCP and with excellent positive predictive value; however, when the test showed abnormalities, radiologists were unable to distinguish between the biliary and the pancreatic segment dysfunction of the sphincter of Oddi. Additionally, the negative predictive value of MRCP-S was poor at 64% (Testoni et al, 2008). MRCP and EUS are diagnostic tools that may be used in patients with abnormal but inconclusive first-line testing (laboratory values and TUS) to obviate the need for further invasive options. The gold standard diagnostic and therapeutic procedure in patients with biliary obstruction is ERCP. It carries a sensitivity and specificity of more than 95% in the detection of obstruction and, where pathology is seen, it may also be therapeutic, allowing for sphincterotomy, stent placement, and tissue collection (Snady et al, 1992; Palazzo et al, 1995; Prat et al, 1996; Buscarini et al, 2003). However, ERCP carries a significant risk of complications. Acute pancreatitis is the most common complication and occurs in 5% to 40% of patients. Additionally, pancreatic necrosis, duodenal or biliary tree perforation, multiorgan failure, and death may occur, albeit less than 1% of the time (Dancygier & Nattermann, 1994; Masci et al, 2001; Barthet et al, 2002; Lieb & Draganov, 2007).
In patients with postcholecystectomy pain where SOD is suspected, ERCP can also allow for concurrent sphincter of Oddi manometry (SOM), which is considered the gold standard for detection of SOD (Khashab et al, 2009). A triple-lumen catheter connected to external transducers is positioned in the sphincter of Oddi segment of both the CBD and pancreatic duct separately, and baseline pressure and phasic wave amplitude, duration, and frequencies are measured with video recording for subsequent analysis (Csendes et al, 1979; Geenen et al, 1980). SOD is a dynamic process, and a single, normal endoscopic evaluation may not be sufficient in patients with persistent symptoms (Varadarajulu et al, 2003; Khashab et al, 2009). In a study of 5352 patients who underwent SOM over a 13-year period, 1037 had SOM readings within normal limits (Table 38.5). Of these patients, 30 had repeat ERCP for persistent symptoms, and 60% of these were given the diagnosis of SOD (Khashab et al, 2009).
The most common cause of abdominal pain occurring after cholecystectomy is residual or recurrent choledocholithiasis (see Chapter 35), seen in 42% of patients undergoing ERCP in one study of more than 1000 patients with PCPs (Tondelli & Gyr, 1983; Zhou et al, 2003). ERCP findings of biliary or pancreatic strictures are second to stones in frequency in patients with PCPs (Goenka et al, 1996; Singh et al, 1996). Furthermore, in various studies in which ERCP was performed on patients with severe PCPs manifesting as pain and/or jaundice, the diagnosis was made in over half of the patients; CBD stones comprised the majority (Ruddell et al, 1980; Hamilton et al, 1982; Carlson et al, 1992).
As noted above, bile leak from CBD injury or inadequate cystic duct stump ligation are serious complications of cholecystectomy. In this scenario, intervention via ERCP with the use of endoprosthetic stents with or without sphincterotomy is an important diagnostic and therapeutic tool (Katsinelos et al, 2006). In some cases, endoscopic access to the biliary tree is limited, such as in patients who have had diverting gastric bypass surgery, and percutaneous transhepatic cholangiography (PTC) is an alternative approach. However, a majority of patients with biliary injuries following cholecystectomy may be successfully managed initially with ultrasound or computed tomography (CT) guided percutaneous drainage of biliary collections. Patients with persistent leaks or those who present with jaundice should be considered for ERCP as a first-line diagnostic and therapeutic option (Zerem & Omerovic, 2009).
Hepatobiliary Scintigraphy (See Chapter 15)
In patients who have undergone cholecystectomy, nuclear scanning reveals rapid flow of intravenously administered 99mTc-n-(2,6,dimethylphenylcarbamoylmethyl) iminodiacetic acid (99mTc-HIDA) from the hepatocytes to the biliary canaliculi then to the CBD and ultimately to the duodenum via the sphincter of Oddi. In patients with SOD a marked delay in transpapillary transit times is seen with stasis in the intrahepatic and extrahepatic biliary system. This sort of quantitative cholescintigraphy may aid in the diagnosis of patients suspected to have SOD following cholecystectomy (Shaffer et al, 1986; Corazziari et al, 1994; Madacsy et al, 1999; Corazziari et al, 2003). It is also useful in confirming the presence of a biliary leak after cholecystectomy, although discrimination of the precise location is often not possible.
Treatment Strategies in Patients with Postcholecystectomy Problems
Early Postcholecystectomy Problems
In the scenario of a biliary leak presenting as a biloma, the initial management should be aimed at percutaneous drainage to control local sepsis; however, endoscopic management may ultimately be necessary. In minor injuries, ERCP and sphincterotomy will eliminate the transpapillary pressure gradient, allowing for uninhibited flow across the papilla, thereby diminishing the extent of the leak, until it eventually ceases (De Palma et al, 2002). The endobiliary stent may also seal off the flow of bile through a physical apposition against the defect in the biliary tree, although this is less likely. External drainage of bile usually dissipates immediately, within a few days; the stent may be removed a month later, if the cholangiogram shows resolution of the leak. If external drainage does not resolve, or if it recurs in the early period, the next approach would be the serial placement of multiple endobiliary stents in a “bunched” fashion, or even deployment of a temporary covered metal stent. Should these maneuvers fail, operative repair is required and usually calls for a bilioenteric bypass. Major damage to the biliary tree can usually be salvaged by stents, but massive bile ascites causing generalized peritonitis may require an operative washout and drainage; this can sometimes be achieved laparoscopically, realizing that an open approach may ultimately be required to definitively address the damage.
Late Postcholecystectomy Problems
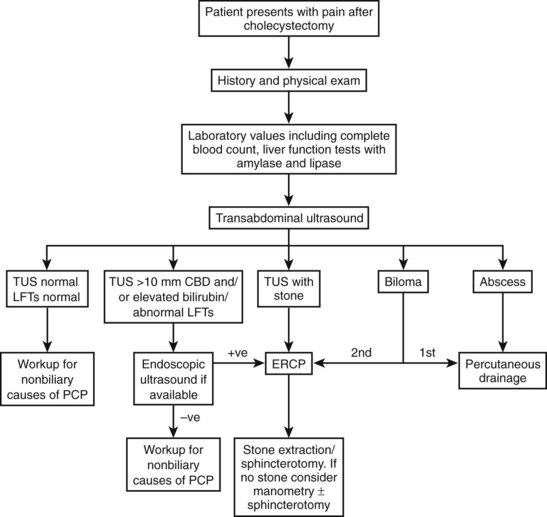
When patients come to the hospital months to years after having a cholecystectomy with symptoms of PCPs, a complete laboratory and diagnostic workup will guide the surgeon’s management decisions. In the case of abnormal LFTs, with or without leukocytosis, and abnormal TUS findings, the patient should proceed directly to ERCP. If, however, a postcholecystectomy patient’s LFTs are abnormal, but the TUS is equivocal or normal, it is reasonable to proceed with EUS or MRCP prior to ERCP. The various endoscopic management strategies regarding choledocholithiasis, strictures, and fistulae are discussed elsewhere in this textbook (see Chapter 35, Chapter 42A, Chapter 42B ).
Medical treatment of SOD is often attempted as a first-line management option, but results are usually poor. The utility of calcium channel blockers and oral nitrates have been plagued with poor outcomes secondary to tachyphylaxis and side effects of the medications (Staritz et al, 1985; Fullarton et al, 1992; Khuroo et al, 1992; Sand et al, 1993; Craig & Toouli, 2002; Wu et al, 2005b; Bistritz & Bain, 2006). Although controversial and underexamined, some studies reveal a decrease in sphincter of Oddi basal pressures with the use of intravenous somatostatin and its analogues (Fazel et al, 2002; Wu et al, 2005a).
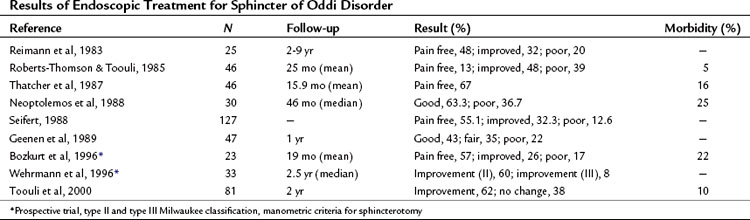
Endoscopic treatment of SOD utilizes the ability to decrease transpapillary pressures with sphincterotomy. Patients with a functional obstruction resulting in SOD, as seen in type I disease in particular, benefit from sphincterotomy even when manometry may not reveal hypertension. Symptomatic relief has been achieved at more than 2-year follow-up in patients with type I SOD with or without manometric findings of hypertension (Rolny et al, 1993; Sugawa et al, 2001). Based on these and other findings that corroborate these results, sphincterotomy without manometry is advised in all patients with type I SOD. In contrast, patients with type II SOD only benefit from sphincterotomy when basal sphincter pressures are elevated. Therefore, manometry should precede endoscopy in this patient population (Geenen et al, 1989; Toouli & Craig, 1999). In patients without objective evidence of biliary obstruction (type III), the response to sphincterotomy does not correlate well with manometric findings, thus it has been suggested that patients who carry a diagnosis of type III SOD should undergo hepatobiliary scintigraphy to gather additional objective data prior to invasive manometry or sphincterotomy (Wehrmann et al, 1996). In this patient population, botulinum toxin may also be used to avoid sphincterotomy and its resultant complications such as bleeding, pancreatitis, and perforated viscera (Wehrmann et al, 1998). Recent studies suggest that phosphodiesterase type 5 inhibitors such as vardenafil (Levitra) infused into the duodenum with endoscopy may reduce basal sphincter of Oddi pressures without significant adverse events (Cheon et al, 2009). These and other agents are under active investigation as alternatives to the more invasive approach of sphincterotomy.
Endoscopic stenting for SOD in patients without an elevated basal sphincter pressure has been attempted with poor response and a high associated risk of stent-induced pancreatitis (Goff, 1995). Additionally, stent placement has also been advocated to predict which patients would respond to sphincterotomy; although in some studies, these trials were able to detect a population of patients who would respond, this method did not gain popularity because of the risks involved and the lack of compelling evidence (Bistritz & Bain, 2006). Therefore the use of biliary stenting in the setting of SOD is not advisable.
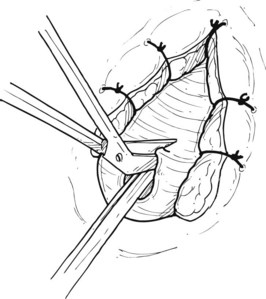
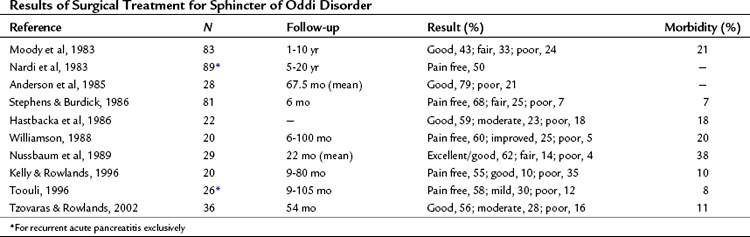
Surgical intervention for SOD was developed before the advent of ERCP (Madura et al, 2005). Transduodenal sphincteroplasty with transampullary septectomy (TDS-TAS) was the procedure of choice at that time (Fig. 38.3). Prior to the advent of ERCP, no objective studies could accurately diagnose patients with SOD; subjective intraoperative methods, such as palpation of the sphincter through the wall of the duodenum, were used to select patients for TDS-TAS. As time progressed, various studies were developed to improve patient selection, such as the morphine–neostigmine evocative test (Nardi & Acosta, 1966). Expertise in endoscopy also continued to progress, until endoscopic sphincterotomies were standardized by the mid-1970s (Kawai et al, 1974). Advantages of the surgical approach include accurate apposition of the duodenal and ductal mucosa, as well as access to the transampullary septum, which cannot always be accomplished with ERCP (Madura et al, 2005). Endoscopic transampullary septotomy is rare, and drainage of the pancreatic duct is inadequate (Fuji et al, 1989; Rolny et al, 1989). In a series of 17 patients, pain from an unsuccessful sphincteroplasty was relieved by excision of the septum in 12 patients (Moody et al, 1990). These studies and others demonstrate the importance of a septotomy; however, both surgical and endoscopic results are not optimal owing to the vague nature of this disease (Tables 38.6 and 38.7). Patients with a suspected diagnosis of SOD should be treated by experienced surgeons and endoscopists working in concert. A treatment algorithm for patients presenting with PCP is illustrated in Figure 38.4.
Potential Pitfalls in Modern Approaches to Cholecystectomy
The field of hepatobiliary surgery has progressed in various ways over the years, but one of the most fascinating leaps in modern surgery is the pace at which minimally invasive methods are supplanting open operative interventions. From single incision laparoscopic surgery (SILS) to natural orifice translumenal endoscopic surgery (NOTES), the approach to cholecystectomy appears poised for further changes. To date, scant data are available regarding the outcomes of such novel approaches, but there is no doubt that these innovative methods will introduce a host of new PCPs. In fact, an early outcomes report of 39 patients undergoing NOTES cholecystectomy, both transvaginal and transgastric, revealed NOTES-related complications such as gastric hematomas and esophageal and colonic perforations (Salinas et al, 2009). Because of safety concerns, NOTES procedures still have to be further refined prior to widespread dissemination, although SILS techniques seem to be embraced with more enthusiasm because of the ability to use existing instrumentation and the improved clinical outcomes. Clearly, additional studies are essential for the further evolution of these contemporary techniques (Kuon Lee et al, 2009).
Conclusions
Symptoms resembling those encountered prior to cholecystectomy may recur in 10% to 40% of patients undergoing either laparoscopic or traditional open cholecystectomy (Pernice & Andreoli, 2009). This occurrence is disappointing and sometimes vexing for both the patient and surgeon, therefore it is imperative that the surgeon caring for these patients rule out the most serious complications first, namely, bile duct injury, bleeding, and retained choledocholithiasis. The use of diagnostic tools and therapeutic options and the systematic approach outlined in this chapter should provide the caregiver with enough data to accurately diagnose and treat the etiology of a patient’s PCPs. The vast majority of the time, these problems will be organic in nature and therefore treatable. However, we have described the diagnostic dilemma that SOD presents, and it is imperative that a stepwise, logical methodology also be applied to these patients whose outcomes are less gratifying.
Ali U, et al. Iatrogenic bile duct injuries from biliary tract surgery. Hepatobiliary Pancreat Dis Int. 2007;6(3):326-329.
Anderson TM, Pitt HA, Longmire WPJr. Experience with sphincteroplasty and sphincterotomy in pancreatobiliary surgery. Ann Surg. 1985;201(4):399-406.
Arlow FL, et al. Bile acid-mediated postcholecystectomy diarrhea. Arch Intern Med. 1987;147(7):1327-1329.
Axon AT, et al. Pancreatogram changes in patients with calculous biliary disease. Br J Surg. 1979;66(7):466-470.
Ball CG, et al. Hepatic vein injury during laparoscopic cholecystectomy: the unappreciated proximity of the middle hepatic vein to the gallbladder bed. J Gastrointest Surg. 2006;10(8):1151-1155.
Bar-Meir S, et al. Frequency of papillary dysfunction among cholecystectomized patients. Hepatology. 1984;4(2):328-330.
Barbara L, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology. 1987;7(5):913-917.
Barthet M, et al. Complications of endoscopic sphincterotomy: results from a single tertiary referral center. Endoscopy. 2002;34(12):991-997.
Bates T, et al. Influence of cholecystectomy on symptoms. Br J Surg. 1991;78(8):964-967.
Begos DG, Modlin IM. Laparoscopic cholecystectomy: from gimmick to gold standard. J Clin Gastroenterol. 1994;19(4):325-330.
Bistritz L, Bain VG. Sphincter of Oddi dysfunction: managing the patient with chronic biliary pain. World J Gastroenterol. 2006;12(24):3793-3802.
Bodvall B. The postcholecystectomy syndromes. Clin Gastroenterol. 1973;2(1):103-126.
Bodvall B, Overgaard B. Computer analysis of postcholecystectomy biliary tract symptoms. Surg Gynecol Obstet. 1967;124(4):723-732.
Bozkurt T, et al. Long-term clinical outcome of post-cholecystectomy patients with biliary-type pain: results of manometry, non-invasive techniques and endoscopic sphincterotomy. Eur J Gastroenterol Hepatol. 1996;8(3):245-249.
Brandstatter G, et al. Die diagnostische Bedeutung der endokopisch retrograden Cholangiopankreatikographie: beim sogenannten postcholezystektomiesyndrom. Wien Klin Wochenschr. 1976;88:806-810.
Burnett W, Shields R. Symptoms after cholecystectomy. Lancet. 1958;1(7027):923-925.
Buscarini E, et al. EUS for suspected choledocholithiasis: do benefits outweigh costs? A prospective, controlled study. Gastrointest Endosc. 2003;57(4):510-518.
Carlson GL, et al. Role of endoscopic retrograde cholangiopancreatography in the investigation of pain after cholecystectomy. Br J Surg. 1992;79(12):1342-1345.
Chan YK, et al. Discordance of pressure recordings from biliary and pancreatic duct segments in patients with suspected sphincter of Oddi dysfunction. Dig Dis Sci. 1997;42(7):1501-1506.
Chang WT, et al. The impact of prophylactic antibiotics on postoperative infection complication in elective laparoscopic cholecystectomy: a prospective randomized study. Am J Surg. 2006;191(6):721-725.
Chen PF, et al. The clinical diagnosis of chronic acalculous cholecystitis. Surgery. 2001;130(4):578-581. discussion 581-583
Cheon YK, et al. Effects of vardenafil, a phosphodiesterase type-5 inhibitor, on sphincter of Oddi motility in patients with suspected biliary sphincter of Oddi dysfunction. Gastrointest Endosc. 2009;69(6):1111-1116.
Christiansen J, Schmidt A. The postcholecystectomy syndrome. Acta Chir Scand. 1971;137(8):789-793.
Chuttani R, Carr-Locke DL. Pathophysiology of the sphincter of Oddi. Surg Clin North Am. 1993;73(6):1311-1322.
Cline RW, et al. An assessment of potential complications caused by intraperitoneal gallstones. Am Surg. 1994;60(5):303-305.
Cohen RV, et al. Is the retrieval of lost peritoneal gallstones worthwhile? Surg Endosc. 1994;8(11):1360.
Corazziari E, et al. Hepatoduodenal bile transit in cholecystectomized subjects: relationship with sphincter of Oddi function and diagnostic value. Dig Dis Sci. 1994;39(9):1985-1993.
Corazziari E, et al. Scintigraphic assessment of SO dysfunction. Gut. 2003;52(11):1655-1656.
Craig AG, Toouli J. Slow-release nifedipine for patients with sphincter of Oddi dyskinesia: results of a pilot study. Intern Med J. 2002;32(3):119-120.
Csendes A, et al. Pressure measurements in the biliary and pancreatic duct systems in controls and in patients with gallstones, previous cholecystectomy, or common bile duct stones. Gastroenterology. 1979;77(6):1203-1210.
Dancygier H, Nattermann C. The role of endoscopic ultrasonography in biliary tract disease: obstructive jaundice. Endoscopy. 1994;26(9):800-802.
Darweesh RM, et al. Efficacy of quantitative hepatobiliary scintigraphy and fatty-meal sonography for evaluating patients with suspected partial common duct obstruction. Gastroenterology. 1988;94(3):779-786.
De Palma G, et al. Leaks from laparoscopic cholecystectomy. Hepatogastroenterology. 2002;49(46):924-925.
Desautels SG, et al. Postcholecystectomy pain syndrome: pathophysiology of abdominal pain in sphincter of Oddi type III. Gastroenterology. 1999;116(4):900-905.
Dewbury KC, Smith CL. The misdiagnosis of common bile duct stones with ultrasound. Br J Radiol. 1983;56(669):625-630.
Deziel DJ, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165(1):9-14.
Drossman DA, et al. U.S. householder survey of functional gastrointestinal disorders: prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569-1580.
Duca S, et al. Laparoscopic cholecystectomy: incidents and complications—a retrospective analysis of 9542 consecutive laparoscopic operations. HPB (Oxford). 2003;5(3):152-158.
Evans PR, et al. Abnormal sphincter of Oddi response to cholecystokinin in postcholecystectomy syndrome patients with irritable bowel syndrome: the irritable sphincter. Dig Dis Sci. 1995;40(5):1149-1156.
Evans PR, et al. Small bowel dysmotility in patients with postcholecystectomy sphincter of Oddi dysfunction. Dig Dis Sci. 1997;42(7):1507-1512.
Fazel A, et al. Octreotide relaxes the hypertensive sphincter of Oddi: pathophysiological and therapeutic implications. Am J Gastroenterol. 2002;97(3):612-616.
Filip M, et al. Postcholecystectomy syndrome—an algorithmic approach. J Gastrointest Liver Dis. 2009;18(1):67-71.
Ford GA, et al. Use of the SeHCAT test in the investigation of diarrhoea. Postgrad Med J. 1992;68(798):272-276.
Fromm H, Malavolti M. Bile acid–induced diarrhea. Clin Gastroenterol. 1986;15(3):567-582.
Fromm H, et al. Absence of significant role of bile acids in diarrhea of a heterogeneous group of postcholecystectomy patients. Dig Dis Sci. 1987;32(1):33-44.
Fuji T, et al. Endoscopic pancreatic sphincterotomy—technique and evaluation. Endoscopy. 1989;21(1):27-30.
Fullarton GM, et al. Controlled study of the effect of nicardipine and ceruletide on the sphincter of Oddi. Gut. 1992;33(4):550-553.
Funch-Jensen P, et al. Evaluation of the biliary tract in patients with functional biliary symptoms. World J Gastroenterol. 2006;12(18):2839-2845.
Garlock JH, Hurwitt ES. The cystic duct stump syndrome. Surgery. 1951;29(6):833-841.
Geenen JE, et al. Intraluminal pressure recording from the human sphincter of Oddi. Gastroenterology. 1980;78(2):317-324.
Geenen JE, et al. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter of Oddi dysfunction. N Engl J Med. 1989;320(2):82-87.
Genest JF, et al. Benign biliary strictures: an analytic review (1970 to 1984). Surgery. 1986;99(4):409-413.
Goenka MK, et al. Endoscopic retrograde cholangiopancreatography in postcholecystectomy syndrome. J Assoc Physicians India. 1996;44(2):119-122.
Goff JS. Common bile duct sphincter of Oddi stenting in patients with suspected sphincter dysfunction. Am J Gastroenterol. 1995;90(4):586-589.
Gouma DJ. Stent versus surgery. HPB (Oxford). 2007;9(6):408-413.
Halm JA, et al. Incisional hernia after upper abdominal surgery: a randomised controlled trial of midline versus transverse incision. Hernia. 2009;13(3):275-280.
Hamilton I, et al. Endoscopic retrograde cholangiopancreatography in the investigation and management of patients after acute pancreatitis. Br J Surg. 1982;69(9):504-506.
Hand AA, et al. Abdominal wall abscess formation two years after laparoscopic cholecystectomy. JSLS. 2006;10(1):105-107.
Hansky J, Dreiling DA. The postcholecystectomy syndrome: psychogenic and physiologic factors. Geriatrics. 1963;18:721-724.
Hästbacka J, et al. Results of sphincteroplasty in patients with spastic sphincter of Oddi. Predictive value of operative biliary manometry and provocation tests. Gastroenterol. 1986;21(5):516-520.
Haug TT, et al. Life events and stress in patients with functional dyspepsia compared with patients with duodenal ulcer and healthy controls. Scand J Gastroenterol. 1995;30(6):524-530.
Helme S, et al. Complications of spilled gallstones following laparoscopic cholecystectomy: a case report and literature overview. J Med Case Reports. 2009;3:8626.
Hess W. Nachoperationen an den Gallenwegen, Part 91. Stuttgart, Enke. Praktische Chirugi. 1977.
Hobbs MS, et al. Surgeon experience and trends in intraoperative complications in laparoscopic cholecystectomy. Br J Surg. 2006;93(7):844-853.
Hogan WJ, Geenen JE. Biliary dyskinesia. Endoscopy. 1988;20(Suppl 1):179-183.
Hopkins SF, et al. The problem of the cystic duct remnant. Surg Gynecol Obstet. 1979;148(4):531-533.
Huang SM, et al. Bile duct injury and bile leakage in laparoscopic cholecystectomy. Br J Surg. 1993;80(12):1590-1592.
Hueman MT, et al. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16(8):2011-2015.
Hunt DR, Blumgart LH. Endoscopic abnormalities in patients with postcholecystectomy symptoms. Surg Gastroenterol. 1982;1:155-158.
Jatzko GR, et al. Multivariate comparison of complications after laparoscopic cholecystectomy and open cholecystectomy. Ann Surg. 1995;221(4):381-386.
Ji W, et al. Role of laparoscopic subtotal cholecystectomy in the treatment of complicated cholecystitis. Hepatobiliary Pancreat Dis Int. 2006;5(4):584-589.
Johnston S, et al. The need to retrieve the dropped stone during laparoscopic cholecystectomy. Am J Surg. 1994;167(6):608-610.
Kakizaki G, et al. Postbiliary surgery complaints: psychosomatic aspects. Am J Gastroenterol. 1976;66(1):62-68.
Karvonen J, et al. Bile duct injuries during laparoscopic cholecystectomy: primary and long-term results from a single institution. Surg Endosc. 2007;21(7):1069-1073.
Kassab C, et al. Endoscopic management of post-laparoscopic cholecystectomy biliary strictures: long-term outcome in a multicenter study. Gastroenterol Clin Biol. 2006;30(1):124-129.
Katsinelos P, et al. The role of endoscopic treatment in postoperative bile leaks. Hepatogastroenterology. 2006;53(68):166-170.
Kawai K, et al. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974;20(4):148-151.
Kelly SB, Rowlands BJ. Transduodenal sphincteroplasty and transampullary septectomy for papillary stenosis. HPB Surg. 1996;9(4):199-207.
Khan MH, et al. Frequency of biliary complications after laparoscopic cholecystectomy detected by ERCP: experience at a large tertiary referral center. Gastrointest Endosc. 2007;65(2):247-252.
Khashab MA, et al. Frequency of sphincter of Oddi dysfunction in patients with previously normal sphincter of Oddi manometry studies. Endoscopy. 2010;42(5):369-374.
Khuroo MS, et al. Efficacy of nifedipine therapy in patients with sphincter of Oddi dysfunction: a prospective, double-blind, randomized, placebo-controlled, crossover trial. Br J Clin Pharmacol. 1992;33(5):477-485.
Konsten J, et al. Long-term follow-up after open cholecystectomy. Br J Surg. 1993;80(1):100-102.
Kuon Lee S, et al. Single-port transumbilical laparoscopic cholecystectomy: a preliminary study in 37 patients with gallbladder disease. J Laparoendosc Adv Surg Tech A. 2009;19(4):495-499.
Lieb JGII, Draganov PV. Early successes and late failures in the prevention of post endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2007;13(26):3567-3574.
Madacsy L, et al. Comparison of the dynamics of bile emptying by quantitative hepatobiliary scintigraphy before and after cholecystectomy in patients with uncomplicated gallstone disease. Clin Nucl Med. 1999;24(9):649-654.
Madura JA, et al. Surgical sphincteroplasty in 446 patients. Arch Surg. 2005;140(5):504-511. discussion 511-513
Marakis GN, et al. Major complications during laparoscopic cholecystectomy. Int Surg. 2007;92(3):142-146.
Mariani A, et al. Secretin MRCP and endoscopic pancreatic manometry in the evaluation of sphincter of Oddi function: a comparative pilot study in patients with idiopathic recurrent pancreatitis. Gastrointest Endosc. 2003;58(6):847-852.
Masci E, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96(2):417-423.
McCloy RF, et al. Endoscopy and postcholecystectomy problems. In: Salmon PR, editor. Gastrointestinal Endoscopy: Advances in Diagnosis and Therapy, vol 1. London: Chapman & Hall; 1984:199-206.
McCloy RF, Kirwan M, 1983: unpublished data.
McMahon A, et al. Symptomatic outcome 1 year after laparoscopic and minilaparotomy cholecystectomy: a randomized trial. Br J Surg. 1995;82(10):1378-1382.
Mergener K, et al. A stone in a grossly dilated cystic duct stump: a rare cause of postcholecystectomy pain. Am J Gastroenterol. 1999;94(1):229-231.
Merrick MV, et al. Is bile acid malabsorption underdiagnosed? An evaluation of accuracy of diagnosis by measurement of SeHCAT retention. Br Med J (Clin Res Ed). 1985;290(6469):665-668.
Meshkinpour H, Mollot M. Sphincter of Oddi dysfunction and unexplained abdominal pain: clinical and manometric study. Dig Dis Sci. 1992;37(2):257-261.
Moody FG, Becker JM, Potts JR. Transduodenal sphincteroplasty and transampullary septectomy for postcholecystectomy pain. Ann Surg. 1983;197(5):627-636.
Moody FG, et al. Stenosis of the sphincter of Oddi. Surg Clin North Am. 1990;70(6):1341-1354.
Muhe E. Long-term follow-up after laparoscopic cholecystectomy. Endoscopy. 1992;24(9):754-758.
Nardi GL, Acosta JM. Papillitis as a cause of pancreatitis and abdominal pain: role of evocative test, operative pancreatography and histologic evaluation. Ann Surg. 1966;164(4):611-621.
Nardi GL, Michelassi F, Zannini P. Transduodenal sphincteroplasty. 5-25 year follow-up of 89 patients. Ann Surg. 1983;198(4):453-461.
Neoptolemos JP, et al. ERCP findings and the role of endoscopic sphincterotomy in acute gallstone pancreatitis. Br J Surg. 1988;75(10):954-960.
Nussbaum MS, et al. Transduodenal sphincteroplasty and transampullary septotomy for primary sphincter of Oddi dysfunction. Am J Surg. 1989;157(1):38-43.
Nuzzo G, et al. Bile duct injury during laparoscopic cholecystectomy: results of an Italian national survey on 56,591 cholecystectomies. Arch Surg. 2005;140(10):986-992.
O’Donnell LJ. Post-cholecystectomy diarrhoea: a running commentary. Gut. 1999;45(6):796-797.
Ou ZB, et al. Prevention of common bile duct injury during laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2009;8(4):414-417.
Palazzo L, et al. Value of endoscopic ultrasonography in the diagnosis of common bile duct stones: comparison with surgical exploration and ERCP. Gastrointest Endosc. 1995;42(3):225-231.
Paquette IM, et al. Neuroma of the bile duct: a late complication after cholecystectomy. J Gastrointest Surg. 2009;13(8):1517-1519.
Pawa S, Al-Kawas FH. ERCP in the management of biliary complications after cholecystectomy. Curr Gastroenterol Rep. 2009;11(2):160-166.
Pernice LM, Andreoli F. Laparoscopic treatment of stone recurrence in a gallbladder remnant: report of an additional case and literature review. J Gastrointest Surg. 2009;13(11):2084-2091.
Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96(9):967-974.
Philips JA, et al. The use of laparoscopic subtotal cholecystectomy for complicated cholelithiasis. Surg Endosc. 2008;22(7):1697-1700.
Pinkas H, Brady PG. Biliary leaks after laparoscopic cholecystectomy: time to stent or time to drain. Hepatobiliary Pancreat Dis Int. 2008;7(6):628-632.
Ponchon T, et al. Biopsies of the ampullary region in patients suspected to have sphincter of Oddi dysfunction. Gastrointest Endosc. 1995;42(4):296-300.
Prat F, et al. Prospective controlled study of endoscopic ultrasonography and endoscopic retrograde cholangiography in patients with suspected common bile duct lithiasis. Lancet. 1996;347(8994):75-79.
Quallich LG, et al. Bile duct crystals do not contribute to sphincter of Oddi dysfunction. Gastrointest Endosc. 2002;55(2):163-166.
Rashdan A, et al. Frequency of biliary crystals in patients with suspected sphincter of Oddi dysfunction. Gastrointest Endosc. 2003;58(6):875-878.
Rice DC, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg. 1997;1(1):85-90. discussion 90-91
Richards C, et al. Does using a laparoscopic approach to cholecystectomy decrease the risk of surgical site infection? Ann Surg. 2003;237(3):358-362.
Riemann JF, et al. Long-term results after endoscopic papillotomy. Endoscopy. 1983;15:165-168.
Roberts-Thomson IC, Toouli J. Is endoscopic sphincterotomy for disabling biliary-type pain after cholecystectomy effective? Gastrointest Endosc. 1985;31(6):370-373.
Rolny P, et al. Clinical significance of manometric assessment of both pancreatic duct and bile duct sphincter in the same patient. Scand J Gastroenterol. 1989;24(6):751-754.
Rolny P, et al. Post-cholecystectomy patients with “objective signs” of partial bile outflow obstruction: clinical characteristics, sphincter of Oddi manometry findings, and results of therapy. Gastrointest Endosc. 1993;39(6):778-781.
Ros E, Zambon D. Postcholecystectomy symptoms. A prospective study of gall stone patients before and two years after surgery. Gut. 1987;28(11):1500-1504.
Rosenblatt ML, et al. Comparison of sphincter of Oddi manometry, fatty meal sonography, and hepatobiliary scintigraphy in the diagnosis of sphincter of Oddi dysfunction. Gastrointest Endosc. 2001;54(6):697-704.
Ruddell WS, et al. Endoscopic retrograde cholangiography and pancreatography in investigation of post-cholecystectomy patients. Lancet. 1980;1(8166):444-447.
Salinas G, et al. Early experience in human hybrid transgastric and transvaginal endoscopic cholecystectomy. Surg Endosc. 2010;24(5):1092-1098.
Sand J, et al. Nifedipine for suspected type II sphincter of Oddi dyskinesia. Am J Gastroenterol. 1993;88(4):530-535.
Sax HC, Adams JT. The fate of the spilled gallstone. Arch Surg. 1993;128(4):469.
Schafer M, et al. Spilled gallstones after laparoscopic cholecystectomy: a relevant problem? A retrospective analysis of 10,174 laparoscopic cholecystectomies. Surg Endosc. 1998;12(4):305-309.
Schofield GE, Macleod RG. Sequelae of cholecystectomy. Br J Surg. 1966;53(12):1042-1045.
Sciarretta G, et al. Use of 23-selena-25-homocholyltaurine to detect bile acid malabsorption in patients with ileal dysfunction or diarrhea. Gastroenterology. 1986;91(1):1-9.
Seifert E. Long-term follow-up after endoscopic sphincterotomy (EST). Endoscopy. 1988;20(suppl 1):232-235.
Shaffer EA, et al. Cholescintigraphic detection of functional obstruction of the sphincter of Oddi: effect of papillotomy. Gastroenterology. 1986;90(3):728-733.
Sharma A, et al. Post-cholecystectomy biliary strictures: not always benign. J Gastroenterol Hepatol. 2008;23(7 Pt 2):e63-e66.
Sharp CF, et al. Partial cholecystectomy in the setting of severe inflammation is an acceptable consideration with few long-term sequelae. Am Surg. 2009;75(3):249-252.
Sherman S, et al. Frequency of abnormal sphincter of Oddi manometry compared with the clinical suspicion of sphincter of Oddi dysfunction. Am J Gastroenterol. 1991;86(5):586-590.
Simeone JF, et al. Sonography of the bile ducts after a fatty meal: an aid in detection of obstruction. Radiology. 1982;143(1):211-215.
Singh V, et al. Postcholecystectomy problems and the role of endoscopic retrograde cholangiopancreatography. Br J Clin Pract. 1996;50(4):183-186.
Smithline A, et al. Sphincter of Oddi manometry: interobserver variability. Gastrointest Endosc. 1993;39(4):486-491.
Snady H, et al. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peri-pancreatic mass. Gastrointest Endosc. 1992;38(1):27-34.
Soderlund C, et al. Bile duct injuries at laparoscopic cholecystectomy: a single-institution prospective study. Acute cholecystitis indicates an increased risk. World J Surg. 2005;29(8):987-993.
Staritz M, et al. Effect of glyceryl trinitrate on the sphincter of Oddi motility and baseline pressure. Gut. 1985;26(2):194-197.
Stefanini P, et al. Factors influencing the long term results of cholecystectomy. Surg Gynecol Obstet. 1974;139(5):734-738.
Stephens RV, Burdick GE. Microscopic transduodenal sphincteroplasty and transampullary septoplasty for papillary stenosis. Am J Surg. 1986;152(6):621-627.
Stewart CJ, et al. Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol. 2001;54(6):449-455.
Strasberg SM. Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9(5):543-547.
Strasberg SM, et al. Laparoscopic surgery of the bile ducts. Gastrointest Endosc Clin N Am. 1996;6(1):81-105.
Strasberg SM, et al. The “hidden cystic duct” syndrome and the infundibular technique of laparoscopic cholecystectomy—the danger of the false infundibulum. J Am Coll Surg. 2000;191(6):661-667.
Sugawa C, et al. Endoscopic sphincterotomy for stenosis of the sphincter of Oddi. Surg Endosc. 2001;15(9):1004-1007.
Sugawa C, Clift D, Walt AJ. Endoscopic retrograde cholangiopancreatography after cholecystectomy. Surg Gynecol Obstet. 1983;157(3):247-251.
Talley NJ, et al. Association of anxiety, neuroticism, and depression with dyspepsia of unknown cause: a case-control study. Gastroenterology. 1986;90(4):886-892.
Tanaka M. Advances in research and clinical practice in motor disorders of the sphincter of Oddi. J Hepatobiliary Pancreat Surg. 2002;9(5):564-568.
Tantia O, et al. Post cholecystectomy syndrome: role of cystic duct stump and re-intervention by laparoscopic surgery. J Minim Access Surg. 2008;4(3):71-75.
Terhaar OA, et al. Imaging patients with “post-cholecystectomy syndrome”: an algorithmic approach. Clin Radiol. 2005;60(1):78-84.
Testoni PA, et al. MRCP-secretin test–guided management of idiopathic recurrent pancreatitis: long-term outcomes. Gastrointest Endosc. 2008;67(7):1028-1034.
Thatcher BS, et al. Endoscopic sphincterotomy for suspected dysfunction of the sphincter of Oddi. Gastrointest Endosc. 1987;33(2):91-95.
Thune A, et al. Reproducibility of endoscopic sphincter of Oddi manometry. Dig Dis Sci. 1991;36(10):1401-1405.
Tocchi A, et al. The need for antibiotic prophylaxis in elective laparoscopic cholecystectomy: a prospective randomized study. Arch Surg. 2000;135(1):67-70. discussion 70
Tondelli P, Gyr K. Biliary tract disorders: postsurgical syndromes. Clin Gastroenterol. 1983;12(1):231-254.
Toouli J, et al. Division of the sphincter of Oddi for treatment of dysfunction associated with recurrent pancreatitis. Br J Surg. 1996;83(9):1205-1210.
Toouli J, et al. Manometry based randomised trial of endoscopic sphincterotomy for sphincter of Oddi dysfunction. Gut. 2000;46(1):98-102.
Toouli J, Craig A. Clinical aspects of sphincter of Oddi function and dysfunction. Curr Gastroenterol Rep. 1999;1(2):116-122.
Tse F, et al. Nonoperative imaging techniques in suspected biliary tract obstruction. HPB (Oxford). 2006;8(6):409-425.
Tzardis PJ, et al. Septic and other complications resulting from biliary stones placed in the abdominal cavity. Experimental study in rabbits. Surg Endosc. 1996;10(5):533-536.
Tzovaras G, Rowlands BJ. Transduodenal sphincteroplasty and transampullary septectomy for sphincter of Oddi dysfunction. Ann R Coll Surg Engl. 2002;84(1):14-19.
Uludag M, et al. The role of prophylactic antibiotics in elective laparoscopic cholecystectomy. JSLS. 2009;13(3):337-341.
Vander Velpen GC, et al. Outcome after cholecystectomy for symptomatic gallstone disease and effect of surgical access: laparoscopic vs open approach. Gut. 1993;34(10):1448-1451.
Varadarajulu S, et al. Determination of sphincter of Oddi dysfunction in patients with prior normal manometry. Gastrointest Endosc. 2003;58(3):341-344.
Wehrmann T, et al. Do patients with sphincter of Oddi dysfunction benefit from endoscopic sphincterotomy? A 5-year prospective trial. Eur J Gastroenterol Hepatol. 1996;8(3):251-256.
Wehrmann T, et al. Endoscopic injection of botulinum toxin for biliary sphincter of Oddi dysfunction. Endoscopy. 1998;30(8):702-707.
Welch N, et al. Gallstones in the peritoneal cavity: a clinical and experimental study. Surg Laparosc Endosc. 1991;1(4):246-247.
Westlake PJ, et al. Chronic right upper quadrant pain without gallstones: does HIDA scan predict outcome after cholecystectomy? Am J Gastroenterol. 1990;85(8):986-990.
Williamson RC. Pancreatic sphincteroplasty: indications and outcome. Ann R Coll Surg Engl. 1988;70(4):205-211.
Wu SD, et al. Effects of somatostatin analogues on human sphincter of Oddi pressure. Hepatobiliary Pancreat Dis Int. 2005;4(2):302-305.
Wu SD, et al. Nitroester drug’s effects and their antagonistic effects against morphine on human sphincter of Oddi motility. World J Gastroenterol. 2005;11(15):2319-2323.
Zehetner J, et al. Lost gallstones in laparoscopic cholecystectomy: all possible complications. Am J Surg. 2007;193(1):73-78.
Zerem E, Omerovic S. Minimally invasive management of biliary complications after laparoscopic cholecystectomy. Eur J Intern Med. 2009;20(7):686-689.
Zhou PH, et al. Endoscopic diagnosis and treatment of post-cholecystectomy syndrome. Hepatobiliary Pancreat Dis Int. 2003;2(1):117-120.
Zisman A, et al. The fate of long-standing intraperitoneal gallstone in the rat. Surg Endosc. 1995;9(5):509-511.