Chapter 106 Pain Management and Palliative Care
Pain Management
Introduction
Pain has been defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [IASP, 1979]. The stimulus can be thermal (heat or cold), chemical (acid or alkali), or mechanical (torsion, stretch, pinch, or prick). Nociception, or pain perception, is the series of electrochemical events following the tissue damage or injury, excluding any emotional correlates of the noxious sensation. Acute pain occurs in a wide variety of pediatric medical encounters, and chronic pain, in one form or another, affects an estimated 15–20 percent of children [Goodman and McGrath, 1991]. Pain can be effectively relieved in 90 percent of patients; nevertheless, only 20 percent of patients achieve effective relief of chronic pain [Ferrell and Rhiner, 1991]. Many studies now demonstrate that neonates, infants, and children can receive analgesia and anesthesia safely for acute pain [Taddio, 2002; Shah and Ohlsson, 2002; Polaner, 2001; Stevens et al., 2000].
Historical Background
Studies from the 1970s into the 1990s documented under-treatment of pediatric patients compared to adults undergoing uncomfortable diagnostic procedures (e.g., lumbar puncture) and even for major surgical procedures (e.g., amputations, nephrectomies, and atrial septal defect repairs) [Schechter, 1989]. Despite an increased number of studies documenting adverse effects of pain over the past two decades, misinformation about pain and nociception in very young children and neonates has prevailed until very recently. Contrary to prevalent myth, newborn infants have the neurophysiologic basis necessary to experience and remember pain, as well as an intact and functional opiate receptor system [Lee et al., 2005]. Fortunately, pain assessment and treatment of painful symptoms are now standards of care for patients of all ages [Joint Commission on Accreditation of Healthcare Organizations, 2001].
Physiology
The physiology of pain perception has been reviewed previously [Anand and Carr, 1989]. Nociceptors present in mucosal membranes, cornea, subcutaneous tissue, bone and teeth, joints, and muscle detect noxious or potentially noxious thermal and mechanical stimuli, and chemical agents. A noxious stimulus is transduced into electrical sensory nerve activity, and the sensory information is projected to the central nervous system via several types of fibers. A-beta (moderately myelinated, fast-conducting) fibers carry impulses for the perception of pressure/position; A-delta (moderately myelinated, fast-conducting) fibers carry impulses from high-threshold mechanoreceptors and polymodal peripheral sensory neuron, which respond to pressure and temperature; and C-fibers (unmyelinated, slow-conducting) carry impulses from cutaneous and deep, low-threshold mechanoreceptors, chemoreceptors, and thermoreceptors. A-delta fibers carry sharp, spatially distinct pain signals, while C-fibers carry diffuse pain signals. A-beta fibers signal nonpainful touch and pressure that can compete with nociception. The majority of peripheral sensory neurons have cell bodies in the dorsal root ganglion, with some afferent input transmitted through the ventral root. Entering through the dorsal root, these fibers separate into A and C fiber bundles in the medial and lateral divisions of the dorsolateral fasciculus, and form synapses within the laminae of spinal cord gray matter. Neurons within laminae V, VII, and X of the spinal cord relay information to the long ascending tracts to various portions of the brain. The long ascending tracts make monosynaptic or polysynaptic reflex connections with lower motor neurons in the ventral horns and have projections to the locus ceruleus, thalamus, hypothalamus, anterior and posterior cingulate gyrus, amygdala, insular cortex, and somatosensory cortex, forming what has been termed the “pain matrix” [May, 2007]. The components of the nociceptive system mature at different rates [Lee et al., 2005]. Sensory signals can be amplified by neuronal activation in the periphery or the spinal cord by inflammatory mediators (bradykinin, cytokines, catecholamines, and substance P), or they can be attenuated by competitive spinal cord stimulation of A-beta fibers. Afferent impulses can be amplified in the spinal cord by substance P or neurokinin A, or by central processes such as “wind-up” with secondary hyperalgesia, or central sensitization; they can be attenuated by endogenous opioids (endorphins, dynorphins, and enkephalins), and serotonergic, noradrenergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic compounds via dorsolateral cortex-mediated mechanisms. Opioid receptors present in the cortex, spinal cord, limbic system, midbrain respiratory centers, and the periphery respond to stereospecific endogenous opioids to produce analgesia (mu), as well as side effects such as euphoria/dysphoria, (kappa) respiratory depression, decreased gastrointestinal motility, bradycardia/tachycardia, and dependency. Several anatomical structures contribute to the inhibition of nociceptive afferent input. They include the cortex, thalamus, periaqueductal gray matter, medulla, and dorsolateral funiculus via inhibitory effects of norepinephrine, serotonin, endogenous opioids, GABA, and acetylcholine. Most of the inhibition occurs at the level of the dorsal horn.
Developmental Differences
Fetal stress responses to pain have been documented as early as 18 weeks’ gestation, with peripheral, spinal, and supraspinal capacity for afferent pain transmission by 26 weeks’ gestation [Lee et al., 2005]. At this age, newborns can mount behavioral, autonomic, and metabolic stress responses to tissue injury. Neonates demonstrate characteristic facial expressions, aversive body movements, alterations in cardiac activity, and changes in cry in response to painful stimuli. Nociceptive nerve endings (receptors) are fewer in number in children than in adults; so tissue damage must be more significant before a pain response is elicited. Pain impulses travel over nonmyelinated nerve fibers, potentially delaying behavioral responses to pain. Young infants may perceive pain more intensely than older children or adults since descending inhibitory pathways that modulate and reduce the pain develop later than afferent excitatory pathways [Franck et al., 2000]. Inadequately treated pain in premature infants is associated with adverse short-term effects (e.g., more postoperative complications) [Lee et al., 2005]; the accompanying physiologic changes (increased heart rate, blood pressure, cardiac variability, hypoxemia, changes in autonomic tone, increased venous pressure, increased cerebral blood flow, and increased intracranial pressure) may augment risks of intraventricular hemorrhage or white-matter injury in the immature brain. Inadequately treated repetitive pain in the developing brain may accentuate neuronal apoptosis [Bhutta and Anand, 2002], fosters wind-up (in which unmodified afferent transmission enhances N-methyl-d-aspartate (NMDA) receptors and facilitates hyperalgesia over time) [Taddio et al., 2009], fosters hyper-reactive pain responses to subsequent stimuli [Weisman et al., 1998], promotes allodynia [Anand et al., 2001], causes long-term behavioral changes [Grunau et al., 2009], and influences the future development of pain syndromes [Jones et al., 2009].
Clinical Assessment
Pain is a subjective and variable experience, with no direct relationship between “pain experience,” physical pathology, and pain intensity [Derbyshire et al., 2004; Danziger and Willer, 2005]. Responses to pain vary widely, depending on gestational age, chronologic age, developmental state, initial behavioral state, prior experience of pain, cognitive state, emotional state, and the ability to respond to and habituate to sensory stimuli. There is no single uniform, standard technique for assessing pain in neonates or children. Biochemical responses to pain, (elevations in cortisol, catecholamines, beta-endorphins, insulin, glucagon, renin-aldosterone, growth hormone, and prolactin) are rarely useful to clinicians. Infants and children demonstrate a variety of nonspecific but consistent behavioral, physiologic, and autonomic responses to pain (Box 106-1) [Anand and Carr, 1989]. These signs have been used by clinicians to recognize pain in nonverbal infants and to quantify severity of the pain experience.
Box 106-1 Responses to Painful Stimuli
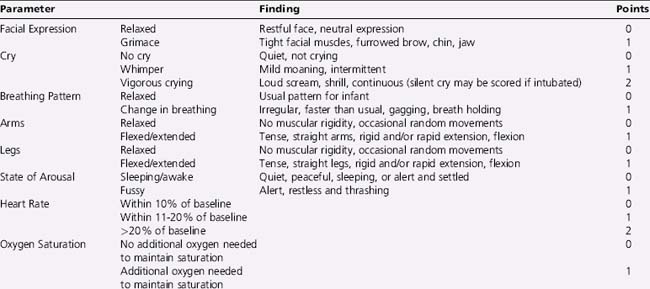
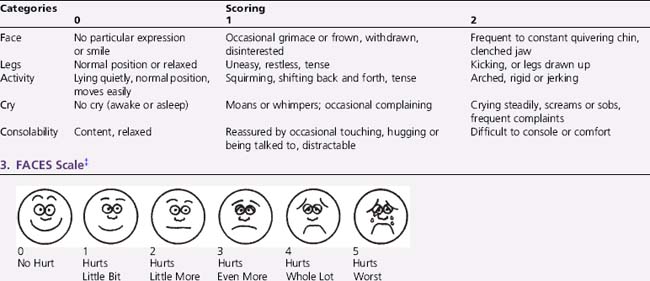
Self-report, usually by linear analog scale, is regarded as most reliable, but only for children who have attained the cognitive capacity to use it. Infants and preverbal children cannot self-report pain quality, quantify its severity, nor inform providers as to the efficacy of analgesic treatments. For preverbal patients, pain assessment is best achieved using multidimensional scales, including behavioral, physiologic, and autonomic responses (Box 106-2). Behavioral observational scales are used for pain assessment in neonates, children under the age of 4, or cognitively impaired children. Neonatal scales used include the Neonatal Infant Pain Scale (NIPS), Premature Infant Pain Profile (PIPP), and pain assessment score sheet [Franck et al., 2000]. Toddlers and preschool children who cannot communicate pain quality or intensity effectively can often use structured questioning or standardized semiquantitative pain assessment tools (poker chips in increasing numbers, cartoon drawings of faces, a pain thermometer, and colors or words) to rate pain intensity consistently and reliably. Children of 8 years or older are able to communicate pain intensity and quality effectively using questionnaires or visual analog scales designed for adults. Behavioral assessment tools, such as the FLACC tool, have been used recently to evaluate children with developmental delay at particular risk of undiagnosed or untreated pain, either acute or chronic [McGrath et al., 1999; Voepel-Lewis et al., 2002; Oberlander et al., 1999].
Box 106-2 Examples of Pain Assessment Scales for Differing Developmental Ages
* For preterm and term infants; scored on a 0–10 point system.
† For infants 4 months of age to children 3 years of age. Each of the five categories – (F) Face; (L) Legs; (A) Activity; (C) Cry; (C) Consolability – is scored from 0 to 2, which results in a total score between 1 and 10.
‡ For children 3–11 years of age.
(Example 1, Neonatal Infant Pain Scale (NIPS), modified from Lawrence J, et al: The development of the tool to assess neonatal pain, Neonatal Netw 6:59–66, 1993. Example 2, FLACC Scale, from Merkel SI, et al: The FLACC: A behavioral scale for scoring postoperative pain in young children, Pediatr Nurs 23:293–297, 1997. Example 3, FACES Scale, from Wong DL, et al: Wong’s Essentials of Pediatric Nursing, ed 6, St. Louis, 2001, Mosby, p 1301.)
Management
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) now requires hospital policies to address four standards of pain management in the accreditation process: management as a patient right, regular and comprehensive pain assessment, patient education, and performance monitoring [JCAHO, 2005]. The American Academy of Pediatrics, the American Pain Society, and the National Association of Neonatal Nurses all provide standards of pain management [AAP, 2000, 2001].
A four-step approach to painful conditions, initially intended as a model for cancer pain management but applicable to other diseases, has been advocated by the World Health Organization [Schug et al., 1990]. Pain, acute or chronic, should be assessed regularly and treated aggressively with nonpharmacologic methods and appropriate analgesic therapies. Effective pain management schemes should utilize the simplest effective pharmacologic regimen, dose around the clock for on-going painful conditions, and tailor the regimen to fit individual patient needs. A variety of nonpharmacologic techniques may be effective in managing mild to moderate pain. In neonates, non-nutritive sucking (with or without sucrose), distraction, breastfeeding, swaddling, kangaroo care, music therapy, and multisensorial stimulation have been utilized to mitigate the severity of procedural pain [Golianu et al., 2007]. Massage and acupuncture have also been suggested for the treatment of pain in neonates [Golianu et al., 2007]. For older infants and children, nonpharmacologic interventions should be age-appropriate for the patient’s developmental stage [Khan and Weisman, 2007].
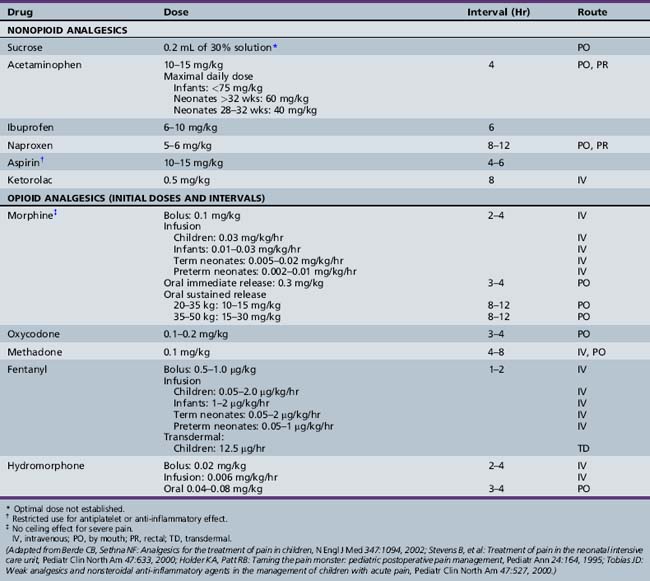
Sucrose (given orally as a 24 or 30 percent solution) is effective in reducing mild procedural pain (such as circumcision and immunizations) in neonates and infants; however, an association between poor neurodevelopmental outcomes with repeated sucrose analgesia has been reported [Johnston et al., 2002].
Mild pain unresponsive to cognitive techniques, or sucrose in neonates, can be treated with weak (nonopiate) analgesics (e.g., non-steroidal anti-inflammatory drugs [NSAIDs] or acetaminophen). For moderate pain, oral “weaker” opioids, or combinations of opioids and NSAIDs or acetaminophen, can be used. Severe pain requires more aggressive parenteral opioids, such as morphine or fentanyl. Intractable pain or unacceptable toxicities of pain medications may require invasive interventions, such as nerve blocks or intraspinal anesthetic infusions. A multimodal approach to pain relief allows drug synergy or potentiation and can reduce single drug doses, minimizing side effects [Galloway and Yaster, 2000]. Common side effects of opiates, such as drowsiness, constipation, nausea, respiratory distress, and pruritus, can be anticipated and treated before or when they occur, without reducing analgesic dosing.
Types of Pain Medications
Aspirin, Acetaminophen, and NSAIDs
Except for specific indications in rheumatologic diseases and for inhibition of platelet adhesion, aspirin is used less commonly as an analgesic in pediatrics because of its association with Reye’s syndrome. NSAIDs are most effective as pre-emptive agents, or for treatment of mild to moderate pain of somatic origin. Acetaminophen is used widely for minor pain and discomfort. Dosage guidelines for aspirin and other analgesics are summarized in Table 106-1. As excess dosing can cause hepatic failure in infants and children, maximal daily doses are also listed. Other NSAIDs, most commonly ibuprofen and naproxen, have gained wide acceptance in pediatric use, and are equally effective. For children who cannot tolerate oral dosing, ketorolac is available. NSAIDs provide a weak analgesia effect that tends to be more effective for pain that has an inflammatory component, as with postoperative pain. The concurrent use of NSAIDs can reduce opioid dosing needed for effective pain management. The use of more than one NSAID concurrently offers little therapeutic advantage and increases risks for side effects, such as gastrointestinal ulceration and bleeding. NSAIDs used in conjunction with corticosteroids augment risks of serious gastrointestinal complications.
Opioids
Opioids are the mainstay of treatment of severe pain, operative procedures, postoperative pain relief, and management of chronic painful medical conditions in neonates and children [Davies et al., 2008b; Berde and Sethna, 2002; Golianu et al., 2000; Stevens et al., 2000]. Opioids bind to brain and spinal cord opiate receptors to block neurotransmitter production. Mu-receptor agonists, such as morphine, codeine, fentanyl, hydrocodone, and hydromorphone, are effective drugs for all pain intensities and are first-line choices for pain management. Agonist–antagonist drugs are not recommended, as they have an analgesic ceiling, may precipitate withdrawal if given concomitantly with full agonists, and have limited routes of administration. Suggested guidelines for opiate-naive patients are shown in Table 106-1. As opiate agonists have no therapeutic ceiling, dosages should be adjusted to meet the patient’s individual needs. The overall safety and efficacy of opioids are well established. In children, as in adults, the risks of addiction after receiving opioids for pain are low; thus, clinicians should not hesitate to treat pain aggressively. However, increased risks for respiratory depression in infants less than 6 months of age require close cardiorespiratory and saturation monitoring in settings in which emergent airway management is feasible. Respiratory depression is seen more commonly when sedatives are used. Methadone, a long-acting opioid agonist, has been utilized in combination therapy for children with cancer pain, as it adds to the opioid analgesia and prevents or reverses a portion of the opioid tolerance [ Davies et al., 2008b; Gold et al., 2006]. Fentanyl is the drug most often used for short procedures because of its rapid onset (1–2 minutes), peak effect (3–5 minutes), and short duration of action (2–4 hours). Morphine has a somewhat slower onset (5 minutes), with a peak response at 10–30 minutes, and longer duration of action (3–8 hours).
Intravenous bolus or continuous infusions allow more rapid and reliable onset of analgesia that can be titrated easily and reversed quickly by narcotic antagonists. Patient-controlled analgesia can be used in children (generally over age 7 years), but younger children should be under parent or nurse control. Opioids also may be administered subcutaneously, orally, rectally, transdermally, or intranasally. For some procedures, oral transmucosal fentanyl citrate provides an alternative for conscious sedation, although emesis is a frequent side effect. Transdermal fentanyl patches with a drug-release rate of 12.5 μg/hour match the lower dose requirements for pediatric cancer pain control [Zernikow et al., 2009]. Rectal administration can be utilized when oral dosing is contraindicated; intramuscular injection should be used only as a last resort. Adverse effects of opioids include respiratory depression, sedation, vasodilatation, hypotension, bradycardia (fentanyl), muscle rigidity, urinary retention, ileus, seizures, and rigid chest (fentanyl given rapidly). Tolerance may develop more rapidly with continuous infusions and with use of synthetic opioids. Characteristic withdrawal symptoms, including irritability, hypertonicity, diaphoresis, fever, and emesis, can occur when opioids are discontinued abruptly after several days’ exposure.
Procedural Sedation and Analgesia
Most minor procedures that are minimally invasive can be performed using a mild analgesic and sedation. Nonpharmacologic pain management strategies may also decrease the need for pharmacologic treatment. Among those demonstrated as effective are perioperative preparation, relaxation training, distraction, guided imagery, cognitive interventions, and hypnosis [Khan and Weisman, 2007]. More uncomfortable procedures require deeper sedation, more appropriately performed by anesthesiologists, intensivists, or emergency physicians trained in advanced life-support and experienced in deep sedation techniques and monitoring. The American Academy of Pediatrics has defined two levels of sedation less deep than general anesthesia [AAP, 2002]. Conscious sedation is a medically controlled state of depressed consciousness that:
Deep sedation is a medically controlled state of depressed consciousness or unconsciousness from which the patient is not easily aroused, loses protective airway reflexes, and cannot maintain an airway or respond purposefully to physical stimulation or verbal command. Guidelines for sedating children undergoing therapeutic or diagnostic procedures have been reviewed [Krauss and Green, 2000]. A recent review details procedural pain management in neonates [Lago et al., 2009].
Sedation
Benzodiazepines are the most commonly used drugs for sedating children, particularly in pediatric intensive care. Midazolam is the preferred drug for procedural sedation. It has a rapid onset of action (1–5 minutes) and a short half-life (1–12 hours). Available for intravenous, intramuscular, intrathecal, or enteral routes, it provides potent sedation, some muscle relaxation, memory loss, and anxiolysis. Oral or intravenous routes are preferable for children; nasal administration causes an intense irritant response. As it has shorter duration than diazepam or lorazepam, midazolam is a more appropriate choice for brief procedural sedation. Oral diazepam avoids the pain of intravenous injection and the variable absorption of intramuscular doses. For painful procedures, benzodiazepine sedation should be accompanied by opioid analgesia, and the patient should be monitored for hypoxia or respiratory depression. Midazolam can be reversed by flumazenil. Barbiturates are regarded as the sedatives of choice for diagnostic imaging in children under 3 years of age, but provide no analgesia. Barbiturates lower the pain threshold, especially when pain is already present. Intravenous pentobarbital, rectal methohexital, and thiopental are the drugs most extensively used for procedural sedation. Chloral hydrate, an acceptably safe alternate sedative without analgesic efficacy, can be used for nonpainful procedures; the oral or rectal dose is 25–100 mg/kg (maximum dose, 2 g). Its use is largely restricted to sedation for electroencephalograms and diagnostic imaging in young children under 3 years of age. Its extremely long half-life in neonates mitigates against repeated administration [Mayer et al., 1991], and other concerns have been raised about potential carcinogenicity and genotoxicity in mice, even when it is given as a single low dose [Salmon et al., 1995]. Given at subanesthetic doses, intravenous ultra-short-acting agents (etomidate, methohexital, propofol, remifentanil, and thiopental) can be used for procedural pain management, but should be used only by certified practitioners experienced in dealing with the potential for over-sedation or rapid swings in consciousness.
Types of Pain
Neuropathic Pain
A variety of neuromuscular and neurodegenerative diseases can cause both nociceptive pain (e.g., contractures or osteoarthritis) and neuropathic pain (e.g., nerve injury or entrapment) [Galloway and Yaster, 2000]. Neuropathic pain is seldom responsive to opioids, and often requires multiple modalities for effective pain relief. Infiltration or compression of nerves by tumors responds, to varying extent, to palliative radiation, steroids, surgical decompression, adjuvant drugs (tricyclics, antiepileptics), neurolytic nerve blocks, epidural or intrathecal blocks, and opioids (which are often ineffective) [Galloway and Yaster, 2000]. Phantom pain after amputation may respond to pre-emptive regional anesthesia, antiepileptics, tricyclic antidepressants, calcitonin, topical agents, or intrathecal medication. Nerve trauma may be treated with injected local anesthetics, steroids, neurolysis, antiepileptics, tricyclic antidepressants, or mexiletine. Neuropathies after chemotherapy can be treated with antiepileptics, tricyclic antidepressants, or mexiletine.
Tricyclic antidepressants (most commonly, amitriptyline, doxepin, or sertraline) are used as adjuvants to inhibit norepinephrine and serotonin reuptake, thereby increasing neurotransmitter tone at the cortical, brainstem, and spinal cord level. Pain relief is achieved more quickly than relief of depression. Given the quinidine-like effects of tricyclics on cardiac conduction, baseline and periodic electrocardiograms are recommended. Antiepileptic agents (carbamazepine and gabapentin) have been used, to a limited extent, in children to treat lancinating pain from peripheral nerve compression or injury and for peripheral neuropathies [Galloway and Yaster, 2000]. Mexiletine, an oral antiarrhythmic with potent analgesic effects, may be useful for some neuropathic pain resistant to tricyclics or antiepileptics. Since it can enhance the efficacy of other agents, it is usually used in combination with other drugs.
Pain in Children with Significant Neurological Impairment
Children with cerebral palsy or developmental delays experience pain, despite some reports of blunted pain responses or insensitivity to pain [Oberlander et al., 1999]. Patients with neurodevelopmental impairments have a variety of conditions that may cause pain: splinting and casting, dislocations and joint contractures, spasticity, pressure sores, feeding tubes, constipation, and diagnostic and therapeutic procedures. Pain from spasticity and muscle spasms can be treated with diazepam, dantrolene, oral baclofen, or botulinum toxin. Oral analgesia is least invasive and is therefore the preferred route; intramuscular injections may be more painful in children with decreased muscle mass. Subcutaneous and transdermal administration can be used for chronic administration.
Patients with neurologic impairment are more likely to have pain discounted or denied, and thus may have pain treated ineffectively. Providers believe that cognitively impaired children manifest more sensitivity to pain than children without impairment, and that children with mild cognitive impairment may over-react to pain [Breau et al., 2003]. In patients too impaired to communicate pain levels, behavioral responses should be regarded as surrogate measures of pain and the pain should be treated appropriately. To avoid over- or under-treatment when self-report is uncertain, it is important to choose a measure appropriate to the individual patient that can be measured over time. For children with significant neurologic impairments, the need for analgesia for acute procedural pain must be balanced by the recognition that systemic opioids may have undesirable side effects, such as respiratory depression or constipation.
Migraine and Headache
Symptomatic pain relief can be by oral, rectal, or intravenous routes, which have been reviewed elsewhere [Annequin et al., 2000]. NSAIDs or weak analgesics can be given in adequate doses for mild to moderate pain from tension headaches and many children with migraine attacks. Practitioners should be aware of the possible development of medication overuse headache when NSAIDS or opiates are used chronically. Practice parameters have been published for the evaluation and treatment of migraine headache in children and adolescents [Lewis et al., 2002, 2004]. With intractable migraine, dihydroergotamine and sumatriptan have been used in preference to opioids, due to the potential for abuse or dependence in patients with recurrent attacks. For migraine, the hallmark of effective control is treatment early in an attack. Intranasal administration of dihydroergotamine may be preferable due to the nausea and vomiting associated with some migraine headaches. Children with tension headaches improve with simple analgesia, and relief of any precipitating stressors. Once a chronic pattern is established, cognitive-behavioral techniques, such as biofeedback and relaxation therapy, may help alleviate headaches.
Summary
Pain in pediatric patients is often under-recognized and under-treated. Relief of pain is one of the most important aspects of ethical medical care, especially in young children and children with neurological handicaps who cannot communicate their experience of pain [Oberlander et al., 1999]. The success of nonpharmacologic means in reducing anxiety or fear should not dissuade physicians from giving pediatric patients appropriate analgesics.
Palliative Care
Introduction
Over 50,000 children die each year in the United States from trauma, lethal congenital conditions, extreme prematurity, heritable disorders, or acquired illness [Himelstein et al., 2004; Catlin and Carter, 2002]. At least 500,000 children suffer life-threatening medical conditions that put them at high risk for not surviving into adulthood [Himelstein et al., 2004]. Nearly half of pediatric deaths occur in the hospital, and over 70 percent of deaths from chronic complex conditions are in hospital; of these, nearly half occur in an intensive care setting [Feudtner et al., 2002]. Although children younger than 1 year of age have a higher death rate than any other pediatric age group [Guyer et al., 1999], it is estimated that only 5000–7000 children (1 percent of dying children) receive hospice care or formal palliative care services. It is thought that another 10,000–15,000 children could benefit from hospice services [WHO, 1998]. Currently, approximately 60 percent of pediatric intensive care unit deaths and nearly 75 percent of neonatal intensive care unit deaths follow limitation of care or withdrawal of life-sustaining treatments when therapy appears to offer no benefits or when quality of life appears unacceptable [Garros et al., 2003; Wall and Partridge, 1997]. Recent population-based studies have documented that 15 percent of pediatric hospital deaths are from neurologic or neuromuscular diseases [Feudtner et al., 2002]. Neuromuscular disorders cause 3–4 percent of childhood deaths in all age groups, but account for 18 percent of deaths of children with complex chronic conditions [Feudtner et al., 2007]. A variety of severe neurologic diagnoses fit into four broad categories of life-limiting conditions for which palliative care may be indicated (Box 106-3) [Dangel, 2002].
Box 106-3 Examples of Neuromuscular Conditions that may be Appropriate for Pediatric Palliative Care
Deficiencies in provision of end-of-life care have been well documented [Wolfe et al., 2000] in other subspecialty fields. Although children with neuromuscular disorders tend to be referred for palliative care earlier than those with other chronic diseases (cancer, human immunodeficiency virus, cystic fibrosis, sickle cell disease, or chronic lung disease after prematurity), 30–35 percent of children with metabolic diseases, muscular dystrophy, severe cerebral palsy, and post-traumatic brain injury are referred for palliative care only at the end of life [Thompson et al., 2009]. Some neurologists may feel they lack the training or experience to manage the complex needs of dying children, treat pain or other end-of-life symptoms, or communicate bad news to young patients and their families.
Historical Background
Palliative care as an adjunct to modern medical care began with the founding of St. Christopher’s Hospice by Cecily Saunders in 1967. Although the first pediatric hospices started in 1982, it is only recently that widespread institution of pediatric palliative care has been recommended, including in intensive care settings [AAP, 1996, 2000; WHO, 1998].
Pediatric palliative care offers a cost-effective means to care for dying children [Pierucci et al., 2001]. It decreases the number of procedures patients receive, increases the use of support services, shifts deaths to less intensive settings, and increases the incidence of withholding or withdrawing aggressive measures [Pierucci et al., 2001]. Increasing emphasis on understanding patients’ and families’ experience of death and dying can improve the care of critically ill children [Wolfe et al., 2000]. More recently, end-of-life care has become a focus for national quality improvement initiatives.
Definitions of Palliative Care
Typically provided by a multidisciplinary team, palliative care seeks to ensure the quality of living and of dying for children for whom curative efforts are not (or are no longer) considered appropriate (Box 106-4) [Catlin and Carter, 2002]. The primary focus is to provide comprehensive, compassionate, and developmentally appropriate care that meets the physical, psychological, emotional, social, and spiritual needs of the child as the disease progresses [IOM, 1997; WHO, 1998; AAP, 2000]. Additionally, palliative care optimally provides support and respite for parents through illness, death, and bereavement in ways appropriate to their values, upbringing, religion, culture and community. The intent is a decent or good death, free from avoidable distress and suffering for patients, families, or caregivers.
Components of Palliative Care
Identifying the Need
Given that there is often a significant degree of prognostic uncertainty, a series of questions can facilitate a shift in goals of care [Feudtner, 2007]. First, what has been the child’s health status and quality of life previously? Second, how likely is the child to survive the current life-threatening condition? Third, what degree of suffering is entailed in the recovery process if the child survives in the short term? Fourth, what is the anticipated quality of life in the future and how long is it expected to endure? Fifth, how likely are future crises or deteriorations? Answers to these questions can help inform the counseling process and facilitate later decisions about the appropriateness of further interventions.
Providers should allow time for the family to nurture their child, while recognizing the difficult emotional stresses that parents face seeing the acute change in their child’s condition or coming to grips with a certainty of neurologic compromise or death in the future. Providers should attempt to give parents and, when appropriate, children some sense of control when they often feel helpless and overwhelmed. Even when critically ill or dying, children benefit from being with and comforted by their parents. While to an extent remaining strangers at the bedside, physicians and nurses often become powerful and meaningful sources of support to parents [Meyer et al., 2002].
Transition in Goals of Care
Palliative care for children does not need to be restricted to those expected to die soon when therapeutic interventions fail or are withdrawn. Some neurologic conditions are associated with a predictably shortened life span (trisomy 13 and 18, neurodegenerative diseases, anencephaly or severe hydrocephalus, and many inborn errors of metabolism are examples), and the primary focus of care is limited to optimizing quality of life when a cure is not possible. Other diseases make prediction of life expectancy and quality of life more difficult (for example, traumatic or hypoxic brain injury, some central nervous system or spinal tumors, congenital myopathies and neuropathies, muscular dystrophy, or storage diseases). As it can be difficult to tell who will die early on in many central nervous system and neuromuscular disease processes, it seems appropriate to offer palliative care to children with progressive conditions at an early stage, transitioning from curative efforts to palliative care in response to their worsening condition. Accordingly, palliative care efforts can, and should, coincide with therapies aimed to cure the disease or limit its progression. With children old enough to express their preferences, it is important to address future choices for care before neurologic status deteriorates to the extent that they are no longer able to participate in health-care decisions. Parents must be given the information necessary to decision-making, but must also understand the limits of our ability to prognosticate treatment efficacy and outcome. Mention of palliative therapies, supportive care, or levels of non-intervention early in the course of a chronic or progressive life-threatening disease can soften the impact of non-intervention discussions later in the disease trajectory. An essential step is to clarify, and re-assess periodically, the goals of continuing, rather than limiting, on-going interventions as the chances for cure lessens (Figure 106-1).
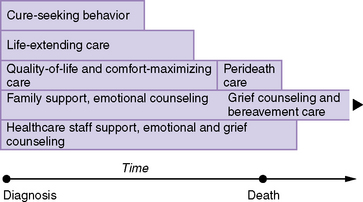
Fig. 106-1 Model for concurrent components of palliative care.
(Modified from Michelson KN, Steinhorn DM: Pediatric end-of-life issues and palliative care, Clin Pediatr Emerg Med 8[3]:212, 2007; Feudtner C: Collaborative communication in pediatric palliative care: a foundation for problem-solving and decision-making, Pediatr Clin N Am 54:583, 2007.)
Providers should facilitate the transition from curative/life-extending care to supportive efforts intended to improve quality of life. They may need to initiate discussions about changing treatment goals when parents have difficulty admitting that end-of-life care is needed [Callahan, 2000], particularly when parents have adapted to the demands of caring for children with life-threatening chronic conditions and to the diminished quality of life associated with many of these conditions (e.g., spinal muscular atrophy, unresponsive central nervous system tumors, or states of minimal consciousness). Acute brain injury (such as perinatal asphyxia, and other sudden catastrophic deteriorations in neurologic status) can make the transition to palliative care difficult, as physicians often cannot offer clear prognostic certainty on survival or later neurodevelopmental functioning. With emergent conditions, there is often little time for difficult decisions or coping with complex emotions, and the transition from curative interventions to palliative care can seem abrupt [Frager, 1996; Sahler et al., 2000].
Levels of Care
The shift in treatment goals, however, does not imply that on-going medical interventions aimed at treating or curing the child’s disease (such as blood product administration, oxygen, or antibiotics) must be abandoned, as they typically are in adult hospice care. Some potentially beneficial treatments entail significant burdens, and it becomes important to clarify specific measures that will continue to be utilized, as well as what level of non-intervention appears most appropriate. An order proscribing all or selected resuscitative measures in the event of a cardiac or respiratory arrest (a “Do not resuscitate” [DNR] or “Do not attempt resuscitation” [DNAR] order) is often the first step. This order represents a selective non-intervention order in the event of a respiratory or cardiac arrest, not a half-hearted resuscitation (“slow code”) of a child deemed likely to die. When providers and parents perceive that further medical therapy offers no benefit, that on-going interventions are inappropriate, or that the present quality of life is untenable, more extensive discussions about withholding or withdrawing specific life-sustaining therapies may be indicated [Wall and Partridge, 1997]. Withholding of life-sustaining measures involves an agreement not to institute specific medical measures that would otherwise be indicated medically. This level of non-intervention is reserved more often for children with a grim prognosis or whose quality of life would not be improved by more aggressive interventions. Such a level of non-intervention might be deemed appropriate for infants with trisomy 13 or 18, cerebral or spinal tumors, severe central nervous system malformations, or severe hydrocephalus. Interventions deemed futile are not required and should be discontinued [Paris et al., 1999] – for example, in patients with brain death, anencephaly, lethal neuropathies or myopathies, or untreatable inborn errors of metabolism. Withdrawal of life-sustaining measures involves the discontinuation of medical interventions currently in use but deemed either futile or no longer appropriate to the child’s best interests, accepting the possibility that death may be hastened. While ethically equivalent, withholding and withdrawing often feel different at the bedside and can raise emotional responses for parents or providers. Whenever support is withheld or withdrawn, it is appropriate for the primary physician to discuss DNAR status with parents and the health-care team before writing the order.
Communication
Patterns of communication sensitive to the child’s developmental age and neurological status and to the parents’ emotional state are critical to good palliative care for children. The process by which collaborative communication in pediatric palliative care can set the foundation for problem-solving and decision-making has been reviewed in detail elsewhere [Feudtner, 2007]. The degree to which the child can participate in decision-making increases as children develop increasing cognitive capacity (over the age of 7). The use of pictures, stories, toys, music, or other family rituals can help younger children communicate their preferences. On the other hand, infections of the nervous system, hypoxia-ischemia, neurodegenerative diseases, or traumatic brain injury can diminish older children’s decision-making capacity. Whenever the child has the cognitive capacity to participate, it is important for providers to allow the child the time to express hopes, dreams, and fears [Back et al., 2003].
Discussions about treatment options should review the child’s current status, estimate the presumed disease trajectory, characterize the quality of life that each treatment choice would entail, and, finally, assist the child and parents in selecting which measures should and should not be done. Given the frequency of prognostic uncertainty in many pediatric conditions, it may be helpful to ask a series of questions to provide the best information on which to base decisions [Feudtner, 2007] (Figure 106-2). As the disease progresses, it may be necessary to reframe the situation by shifting from a goal of curing the disease to a goal of caring for the patient. The primary focus is to determine what is important to the child and the family: longer life, pain-free, time with family members, specific desires, environment and setting, time to say goodbye. The next step is to devise a collaborative care plan that respects the patient’s emotional and spiritual needs, incorporates family values, and supports family involvement in health-care decisions. When time allows, such conversations are best held by continuity providers over a period of time, giving cognitively capable patients or parents time for questions, second-opinion consultation, talks among family members, reflection, deliberation, and informed decision-making. Repeated discussions empower informed decision-making, and help minimize the guilt some parents feel about their decisions after their child dies.
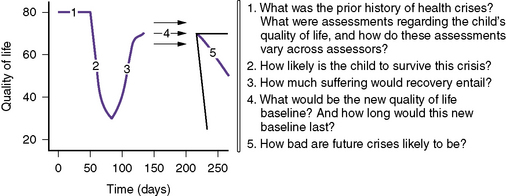
Fig. 106-2 Questions to address prognostic uncertainty in palliative care decisions for children.
(From Feudtner C: Collaborative communication in pediatric palliative care: a foundation for problem-solving and decision-making, Pediatr Clin N Am 54:583, 2007.)
A structured approach can help physicians deliver difficult news and negotiate goals of care (Box 106-5). Critical aspects of the communication process include simple, direct, clear, and honest explanations that relate the expected outcomes of each treatment option without prejudicing or constraining the choices. It is also important that the information neither overwhelms the parents nor leaves them in despair. Providers should avoid promising outcomes that they do not believe are reasonably feasible. Neurologists play a key role in communicating the expected neurologic outcome after a life-threatening illness; neurology consultation can help parents understand expected functional status when there is little chance for an acceptable outcome. To minimize the sense of giving up or abandoning their child, it is critical that parents understand that the goal of care will be to do everything that should be done, rather than everything that can be done.
Conflicting perspectives occasionally complicate end-of-life discussions. Attempts to understand the perspectives of all of the parties in the decision-making process better may result in a more collaborative process [Feudtner, 2007]. This may require providers to explore their own attitudes, as well as clarifying those of the patient and family. The use of open, direct, and supportive language is critical to consensus-building and resolution of potential conflict [Feudtner, 2007].
Health Care Decision-Making
Parents (or the competent adolescent) determine the quantity of information necessary for them to make an informed decision. If decisions seem not to reflect a full understanding of the disease, the goals of care, or expected outcomes, further discussions facilitate review of the information and reflection on treatment options. Decisions that do not concur with the provider’s choices should be respected when taken in the child’s best interests. Advanced directives regarding care preferences may have no legal relevance in pediatric patients [Pierucci et al., 2001], although the process may afford older children a more participatory role in making end-of-life decisions [Zinner, 2009]. In general, parents are assumed to be the primary proxy decision-makers for young children; however, their discretion is not limitless. When consensus is difficult to reach, ethics committee consultation can help clarify impediments to decision-making. In rare situations in which parents’ judgment may be questionable or the child’s best interests remain uncertain, providers may need to advocate for the child or even refer to child welfare services. A process of guided autonomy can support the child’s and the parents’ involvement in treatment decisions while preserving some autonomy and minimizing the undue emotional strain of making decisions to limit care. Once the goals are clarified and decisions have been made, the care plan should be disseminated to the entire health-care team, and any specific limitations to medical interventions clearly documented in the patient’s chart. It is critical that decisions to limit the invasiveness of care not lead to a “do nothing” environment [Feudtner, 2007].
Persistent Vegetative State
A small proportion of children who survive critical illnesses continue for varying periods of time in a persistent vegetative or a minimally conscious state. While the exact incidence is unknown, the estimated 6000 infants and children that live in this state present a special challenge to clinicians and ethicists alike [Ashwal et al., 1992]. Although there is no consensus as to whether children in a persistent vegetative state experience pain and suffering, it seems prudent to treat signs or symptoms in children in a persistent vegetative state to minimize pain and suffering. Decisions to withdraw or withhold life-sustaining measures should be made in collaboration with parents only when neurodiagnostic testing and a sufficient period of observation suggest there is no chance for a meaningful recovery. Decisions to forego supportive care, especially those involving the withholding of fluids or hydration, remain controversial, even when deemed appropriate responses to the child’s medical situation. Such decisions present complex emotional and ethical challenges to clinicians and family members [Carter and Leuthner, 2003].
Environment for Death and Dying
Some parents may prefer continued hospital care where skilled nursing and medical care offsets the limitations to privacy and the inhospitable ambience of most inpatient settings. A private space for families to gather before and after the child’s death is recommended (e.g., a screen for privacy or, better, a separate comfort care room) and should not impede nursing care or prompt medical attention to pain and symptoms. Other inpatient options include transfer to a lower level of care or to a hospice, although some parents may feel abandoned by changes in care providers late in their child’s disease. Death at home is preferred by a significant proportion of children and parents [Pierucci et al., 2001]. An increasing proportion (approximately 25 percent) of deaths from neuromuscular disorders occur in the home setting, second only to deaths from malignancy [Feudtner et al., 2007]. Parents who opt for home care must be supported adequately in managing terminal care for the child outside the hospital. Plans for a death at home require a continuity provider skilled in managing pain and suffering, and able to provide 24-hour coverage from discharge through the child’s death and the parents’ bereavement.
Assessment and Treatment of Symptoms
Pain should be treated aggressively with analgesics. When there is severe or unremitting pain at the end of life, terminal sedation with opioids is used to alleviate suffering, recognizing that the opioids may suppress respiratory drive in debilitated patients and may thereby hasten death. The risk of death is justified because there is no alternative that alleviates suffering while lessening the risk of death. Fears of addiction to opioids, the mainstay of pain management in end-of-life care, on the part of providers or family members are unwarranted. Paralytics are not appropriate when life support is being withdrawn [Pierucci et al., 2001]. Anxiolytics may help terminal agitation and perhaps air hunger experienced in the terminal phases. Many neurologists will feel comfortable treating seizures, insomnia, and depression in dying patients, but may prefer to have primary care pediatricians or palliative care specialists manage other frequently encountered end-of-life symptoms (dyspnea, anxiety, fatigue, fever, bleeding, nausea, skin breakdown, anorexia, dehydration, and constipation). Despite shared concerns for patient well-being, treatment of other symptoms experienced by dying patients has been inconsistent [Pierucci et al., 2001; Wolfe et al., 2000; Partridge and Wall, 1997]. Attempts should be made to organize a care plan allowing a death without suffering (either at home or in hospital) for any child in need, regardless of socioeconomic circumstances.
Developmental, Emotional and Spiritual Concerns
Care for a dying child requires candor, openness, and an emotional availability to the child’s experience of illness and imminent death. Children need to come to understand death and dying in terms of their developmental age. Research indicates that they come to understand it as a change of being by age 3, and as a universal fact of living by age 5–6, and have gained a concept of their personal mortality by age 8–9 [Himelstein et al., 2004]. Spirituality is one way that patients and families cope with death and dying, whether or not they adhere to any specific religion. Approaches that respect the children’s spiritual nature at varying developmental stages will allow providers to explore issues of love, hope, security, loneliness, and concepts of the legacy that the child will leave to surviving parents, siblings, friends, and schoolmates. Family routines and rituals add familiar structure and a sense of normality as the medical condition worsens, and should be encouraged whenever possible.
Bereavement
Children are aware of dying and disease; they understand much more about death and dying than adults often realize. Parents who sense that the child is aware of imminent death more often regret not having talked with the child than do parents who do not sense this awareness in the child, and typically parents who talk with their child about death do not regret doing so [Kreicbergs et al., 2004]. Children grieve as much as adults, although in different ways. Dying children may grieve about their impending death, the loss of functional status, or the inability to participate in current or future events. They may fear how their family members will cope after their death. Dying children and their siblings do not need to be protected from death and dying as much as they need help making it understandable. When heritable neuromuscular disorders pose a future risk to affected siblings, particular care is necessary to deal with parents’ sense of guilt, parents’ and siblings’ grief, concerns about subsequent pregnancies, or family fears of illness in the future.
Barriers to Palliative Care
The remarkable success of medicine in treating many medical conditions can make it difficult to abandon attempts to cure the primary disease. Providers may encourage treatments overtly or tacitly, with little or no hope of a favorable outcome, abandoning them only when death seems inevitable. In pediatrics, a late transition from curative care to palliative care is a frequent problem. Some providers may conceive of palliative care as a second-string treatment regimen, to be offered and used only in people who cannot be cured and only very near the end of life [Pierucci et al., 2001]. For many pediatric neurologic diagnoses, it is difficult to predict the course of the disease, which children will die from their disease, or the quality of life as the disease progresses [Davies et al., 2008a]. Parents’ recognition that death is inevitable often lags behind their understanding of the diagnosis. Both parents and providers may feel the death of a child is inexplicable, unnatural, something that should not happen. Palliative care requires increased time and staff to provide for the many needs of the dying patient and of family members [Davies et al., 2008a].
Summary
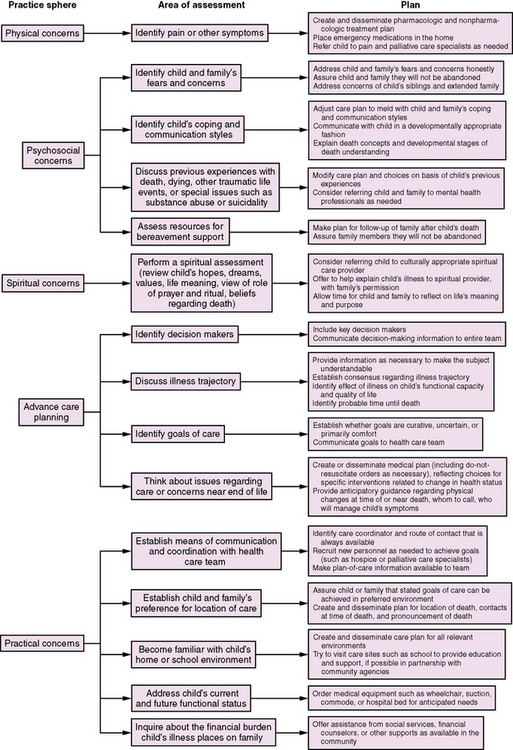
Neurologists can offer children and their families many things when a cure for a life-threatening neuromuscular disease is not expected: the best medical information on diagnosis, current status, and expected outcome; adequate analgesia and treatment of other symptoms; medical and emotional support; perspective and referrals; assistance in the decision-making partnership; open discussion about ways to “optimize” the dying process; follow-up meetings; and opportunities/referrals for grief counseling. Palliative care offers many ways to improve children’s and families’ experience of end-of-life care (Figure 106-3). Primary-care neurologists should develop expertise in the palliative care of children with life-threatening neuromuscular conditions. Consultant neurologists should coordinate with primary pediatric providers or palliative care specialists to meet their patients’ needs for end-of-life care. With an interdisciplinary team approach and the use of medical technology only when benefits outweigh burdens, providers and parents ensure the best possible life while the child lives, and a death that is comfortable, peaceful, and dignified.
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
American Academy of Pediatrics Committee on Bioethics. Ethics and the care of critically ill infants and children. Pediatrics. 1996;98:149.
American Academy of Pediatrics Committee on Bioethics and Committee on Hospital Care. Palliative care for children. Pediatrics. 2000;106:351.
American Academy of Pediatrics. Policy statement: the assessment and management of acute pain in infants, children, and adolescents. Pediatrics. 2001;108:793.
Anand K.J.S., the International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173.
Anand K.J.S., Carr D.B. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. 1989;36:795.
Annequin D., Tourniare B., Massiou H. Migraine and headache in childhood and adolescence. Pediatr Clin North Am. 2000;47:617.
Ashwal S., Bale J.F., Coulter D.L., et al. The persistent vegetative state in children: report of the Child Neurology Society Ethics Committee. Ann Neurol. 1992;32:570.
Back A.L., Arnold R.M., Quill T.E. Hope for the best, and prepare for the worst. Ann Intern Med. 2003:439.
Berde C.B., Sethna N.F. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347:1094.
Bhutta A.T., Anand K.J. Vulnerability of the developing brain. Neuronal mechanisms. Clin Perinatol. 2002;29:357.
Breau L.M., MacLaren J., McGrath P.J., et al. Caregivers’ beliefs regarding pain in children with cognitive impairment: relation between pain sensation and reaction increases with severity of impairment. Clin J Pain. 2003;19:335.
Callahan D. Death and the research imperative. N Engl J Med. 2000;342:654-656.
Carter B.S., Leuthner S.R. The ethics of withhold/withdrawing nutrition in the newborn. Semin Perinatol. 2003;27:480.
Catlin A., Carter B. Creation of a neonatal end-of-life palliative care protocol. J Perinatol. 2002;22:184.
Committee on Drugs. American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 2002;110:836.
Dangel T. The status of pediatric palliative care in Europe. J Pain Symptom Manage. 2002;24:160.
Danziger N., Willer J.C. Tension-type headache as the unique pain experience of a patient with congenital insensitivity to pain. Pain. 2005;117:478.
Davies B., Sehring S.A., Partridge J.C., et al. Barriers to palliative care for children: perceptions of pediatric health care providers. Pediatrics. 2008;121:282.
Davies D., DeVlaming D., Haines C. Methadone analgesia for children with advanced cancer. Pediatr Blood Cancer. 2008;51:393.
Derbyshire S.W., Whalley M.G., Stenger V.A., et al. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23:392.
Ferrell B.R., Rhiner M. High-tech comfort: ethical issues in cancer pain management for the 1990s. J Clin Ethics. 1991;2:108.
Feudtner C. Collaborative communication in pediatric palliative care: a foundation for problem-solving and decision-making. Pediatr Clin North Am. 2007;54:583.
Feudtner C., Christakis D.A., Zimmerman F.J., et al. Characteristics of deaths occurring in children’s hospitals: implications for supportive services. Pediatrics. 2002;109:887.
Feudtner C., Feinstein J.A., Satchell M., et al. Shifting place of death among children with complex chronic conditions in the United States, 1989-2003. JAMA. 2007;297:2725.
Frager G. Pediatric palliative care: building the model, bridging the gaps. J Palliat Care. 1996;12:9.
Franck L.S., Greenberg C.S., Stevens B. Pain assessment in infants and children. Pediatr Clin North Am. 2000;47:487.
Galloway K.S., Yaster M. Pain and symptom control in terminally ill children. Pediatr Clin North Am. 2000;47:711.
Garros D., Rosychuk R.J., Cox P.N. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics. 2003;112:e371.
Gold J.I., Townsend J., Jury D.L., et al. Current trends in pediatric pain management: from preoperative to the postoperative bedside and beyond. Semin Anesth Periop Med Pain. 2006;25:159.
Golianu B., Krane E.J., Galloway K.S., et al. Pediatric acute pain management. Pediatr Clin North Am. 2000;47:559.
Golianu B., Krane E., Seybold J., et al. Non-pharmacologic techniques for pain management in neonates. Semin Perinatol. 2007;31:318.
Goodman J.E., McGrath P.J. The epidemiology of pain in children and adolescents: a review. Pain. 1991;46:247.
Grunau R.E., Whitfield M.F., Petrie-Thomas J., et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138.
Guyer B., Hoyert D.L., Martin J.A., et al. Annual summary of vital statistics – 1998. Pediatrics. 1999;104:1229.
Himelstein B.F., Hilden J.M., Boldt A.M., et al. Pediatric palliative care. N Engl J Med. 2004;350:1752.
Institute of Medicine. Committee on Care at the End of Life. Approaching death: improving care at the end of life. Washington, DC: National Academy Press; 1997.
International Association for the Study of Pain. Subcommittee on Taxomony. Pain terms: a list with definitions and notes on usage: Recommended by the IASP Subcommittee on Taxonomy. Pain. 1979;6:249-252.
Johnston C.C., Filion F., Snider L., et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics. 2002;110:523.
Joint Commission on Accreditation of Healthcare Organizations. Hospital accreditation standards. Oakbrook Terrace, IL: Joint Commission Resources, Inc; 2005.
Joint Commission on Accreditation of Healthcare Organizations. Pain assessment and management standards – hospitals. Oakbrook Terrace, IL: Joint Commission Resources, Inc; 2001.
Jones G.T., Power C., MacFarlane G.J. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92.
Khan K.A., Weisman S.J. Nonpharmacologic pain management strategies in the pediatric emergency department. Pediatr Emerg Med. 2007;8:240.
Krauss B., Green S.M. Sedation and analgesia for procedures in children. N Engl J Med. 2000;342:938.
Kreicbergs U., Valdimarsdottir U., Onelov E., et al. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175.
Lago P., Garetti E., Merazzi, et al. Guidelines for procedural pain in the newborn. Acta Paediatr. 2009;98:932.
Lee S.J., Ralston H.J., Drey E.A., et al. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947.
Lewis D.W., Ashwal S., Dahl G., et al. Practice parameter: evaluation of children and adolescents with recurrent headaches: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;59:490.
Lewis D.W., Ashwal S., Hershey A., et al. Practice parameter: pharmacologic treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215.
May A. Neuroimaging: Visualizing the brain in pain. Neurol Sci. 2007;28:S101-S107.
Mayer D., Hindmarsh K.W., Sankaran K., et al. Chloral hydrate disposition following singe dose administration to critically ill neonates and children. Dev Pharmacol Ther. 1991;16:71.
McGrath P.J., Rosmus C., Camfield C., et al. Behaviors caregivers use to determine pain in non-verbal cognitively impaired children. Dev Med Child Neurol. 1999;40:340.
Meyer E.C., Burns J.P., Griffith J.L., et al. Parental perspective on end-of-life care in the pediatric intensive care unit. Crit Care Med. 2002;30:226.
National Association of Neonatal Nurses. Pain assessment and management: guideline for practice. 2001. Document 1222:1
Oberlander T.F., Gilbert C.A., Chambers C.T., et al. Biobehavioral responses to acute pain in adolescents with a significant neurologic impairment. Clin J Pain. 1999;15:201.
Paris J.J., Singh J., Schreiber M.D., et al. Unilateral do-not-resuscitate order in the neonatal intensive care unit. J Perinatol. 1999;19:383.
Partridge J.C., Wall S.N. Analgesia for dying infants whose life support is withdrawn or withheld. Pediatrics. 1997;99:76.
Pierucci R.L., Kirby R.S., Leuthner S.R. End-of-life care for neonates and infants: the experience and effects of a palliative care consultation service. Pediatrics. 2001;108:653.
Polaner D.M. Sedation-analgesia in the pediatric intensive care unit. Pediatr Clin North Am. 2001;48:695.
Sahler O.J., Frager G., Levetown M., et al. Medical education about end-of-life care in the pediatric setting: principles, challenges, and opportunities. Pediatrics. 2000;105:575.
Salmon A.G., Kizer K.W., Zeise L., et al. Potential carcinogenicity of chloral hydrate – a review. J Toxicol Clin Toxicol. 1995;33:115.
Schechter N.L. The undertreatment of pain in children: an overview. Pediatr Clin North Am. 1989;36:781.
Schug S.A., Zech D., Dorr U. Cancer pain management according to WHO analgesic guidelines. J Pain Symptom Manage. 1990;5:27.
Shah V., Ohlsson A. The effectiveness of premedication for endoctracheal intubation in mechanically ventilated neonates. A systematic review. Clin Perinatol. 2002;29:535.
Singer P.A., Martin D.K., Kelner M. Quality end-of-life care: patients’ perspectives. JAMA. 1999;281:163.
Stevens B., Gibbins S., Franck L.S. Treatment of pain in the neonatal intensive care unit. Pediatr Clin North Am. 2000;47:633.
Taddio A. Opioid analgesia for infants in the neonatal intensive care unit. Clin Perinatol. 2002;29:493.
Taddio A., Shah A.E., Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain. 2009;144:43.
Thompson L.A., Knapp C., Madden V., et al. Pediatricians’ perceptions of and preferred timing for pediatric palliative care. Pediatrics. 2009;123:e777.
Voepel-Lewis T., Merkel S., Tait A.R., et al. The reliability and validity of the Face, Legs, Activity, Cry, Consolability observational tools as a measure of pain in children with cognitive impairment. Anesth Analg. 2002;95:1224.
Wall S.N., Partridge J.C. Death in the intensive care nursery: physician practice of withdrawing and withholding life support. Pediatrics. 1997;99:64.
Weisman S.J., Bernstein B., Schechter N.L. Consequences of inadequate analgesia during painful procedures in children. Arch Pediatr Adolesc Med. 1998;152:147.
Wolfe J., Grier H.E., Klar N., et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342:326.
World Health Organization. Cancer pain relief and palliative care in children. Geneva, Switzerland: World Health Organization; 1998.
Zernikow B., Michel E., Craig F., et al. Pediatric palliative care: use of opioids for the management of pain. Paediatr Drugs. 2009;11:129.
Zinner S.E. The use of pediatric advance directives: a tool for palliative care physicians. Am J Hosp Palliat Med. 2009;25:427.