Oral Complications
Kostandinos Sideras, Christopher L. Hallemeier and Charles L. Loprinzi
• Mucositis, a major dose-limiting toxic effect of chemotherapy for solid tumors, develops in 5% to 40% of patients.
• Mucositis develops in 70% to 100% of patients who receive high doses of chemotherapy with bone marrow rescue.
• Mucositis is the most troublesome acute reaction for patients undergoing radiation therapy directed at the oral cavity.
• Radiation therapy directed at the oral cavity frequently causes a host of other oral complications including xerostomia, dental caries, tissue necrosis, and taste alterations.
• Oxidative stress caused by cytotoxic chemotherapy and radiation therapy leads to upregulation and subsequently amplification of multiple inflammatory pathways in a complex process. This subsequently leads to mucosal ulceration.
• Secondary infections occur as a result of treatment-induced immunosuppression.
• The importance of instituting oral hygiene protocols in patients receiving chemotherapy is well established.
• Cryotherapy is the most conventional and easy to use preventative method, at least for 5-fluorouracil–based bolus therapy, and it appears to have implications for other chemotherapeutic regimens as well, such as edatrexate and high-dose melphalan therapy.
• Keratinocyte growth factor has been approved by the Food and Drug Administration for use with high-dose chemotherapeutic regimens associated with high rates of mucositis and has shown promise in other settings as well.
• Low-level laser therapy has shown promise, but its use is limited for now to centers that are able to support its use.
• Pretreatment dental care, good oral hygiene, and sophisticated treatment planning is recommended for patients receiving radiation therapy.
• Overall, evidence is lacking regarding the efficacy of various agents in promoting healing of the oral mucosa after mucositis is established.
• Systemic analgesic therapy of mucositis pain with narcotic medications is well established and recommended.
• Antibiotics and/or antifungal medications should be given to patients with evidence of infection.
• In the palliative setting, various mouthwashes are widely used in clinical practice based on provider preference and experience. These mouthwashes most frequently contain combinations of diphenhydramine, viscous lidocaine, magnesium hydroxide/aluminum hydroxide, nystatin, and corticosteroids. The efficacy of these measures has not been adequately evaluated to date.
• Baking soda mouth rinses appear to be the most economical solution, although efficacy is not clearly established.
• Low-level laser therapy has also shown promise in treating established mucositis lesions.
Introduction
The oral cavity is a common site for chemotherapy- and radiation-induced toxicity. Manifestations of this toxicity include alimentary tract mucositis, secondary infectious complications induced by bacteria, fungi, and viruses, and graft-versus-host disease in patients receiving allogeneic bone marrow transplants. Although alimentary tract mucositis can involve the entire gastrointestinal tract,1 it is most frequently manifested in the oral cavity as ulceration, pain, and bleeding. Mucositis leads to significant patient morbidity and a decline in quality of life, and it limits the use of additional chemotherapeutic treatment. Moreover, the economic burden of this frequent oncologic complication is also considerable.2,3 This chapter discusses the etiology, incidence, risk factors, prevention, and treatment of oral toxic effects of standard chemotherapy, intensive marrow-ablative chemotherapy, and radiation therapy. The prevention and treatment of graft-versus-host disease is beyond the scope of this chapter.
Pathophysiology of Mucosal Injury and Clinical Manifestations
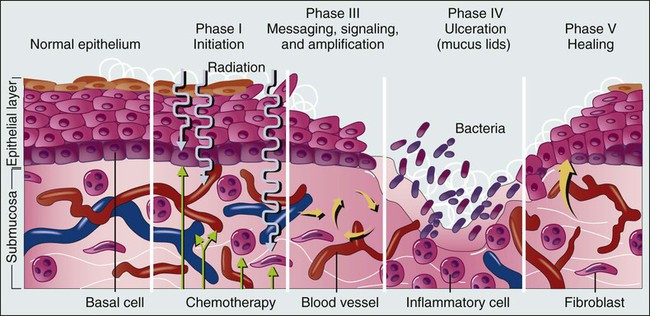
It is currently accepted that the process of mucosal injury and subsequent healing is not limited to the epithelium alone but involves all layers of the mucosa, including the extracellular matrix. A five-stage process has been postulated to explain the complex molecular, cellular, and histologic events associated with chemotherapy-induced mucosal injury (Fig. 43-1).4 Oxidative stress related to chemotherapy is thought to be responsible for the first phase of mucosal injury (the initiation phase). The second phase involves the upregulation of transcription factors and the generation of messenger signals (primary damage response phase). In this stage, upregulation of nuclear factor (NF)–κB is thought to play an important role in the subsequent upregulation of multiple proinflammatory cytokines, such as tumor necrosis factor (TNF)–α, interleukin (IL)-1b, and IL-6. NF-κB is also thought to upregulate cyclooxygenase-2, which in turn is implicated in the upregulation of matrix metalloproteinase.5 In addition, activation of the ceramide pathway, fibronectin breakup, and macrophage activation are other complex events that take place, leading to further mucosal injury and apoptosis. The third phase of mucosal injury involves additional signaling and amplification of the aforementioned pathways through biological feedback, leading to the generation of additional proinflammatory cytokines (the signaling and amplification phase). Until this point the biologically altered mucosa appears anatomically intact, but epithelial proliferation halts. The fourth phase consists of the symptomatic phase of mucositis, involving erythema, mucosal ulceration, plaque formation, pain, and bleeding (the ulceration phase). Immune cell infiltration occurs in this phase. Microbial superinfection and reduction in salivary gland function can complicate and amplify the mucosal injury. Oral candidiasis and herpes simplex virus (HSV) infections are particularly common, constituting the majority of the infectious complications seen in patients with mucositis, although the increased risk of bacteremia in patients with mucositis also points to the role of bacterial superinfections of the ulcerative lesions. It is important to note that although microbial superinfections can influence the duration and severity of mucositis, they are not believed to be involved in the pathogenesis of mucositis. This belief is supported by multiple studies that have failed to show any benefit of antimicrobial agents in the prevention of mucositis. The fifth and final phase involves the healing of the mucosa, a process that depends on angiogenesis and increased biological activity in the extracellular matrix (the healing phase). In patients undergoing myeloablative chemotherapy, the healing phase may not begin until leukocyte recovery. It is important to understand that these phases do not necessarily follow a linear progression but may occur simultaneously at different locations.6
Oral Complications From Chemotherapy, Including Myeloablative Chemotherapy
Incidence and Risk Factors
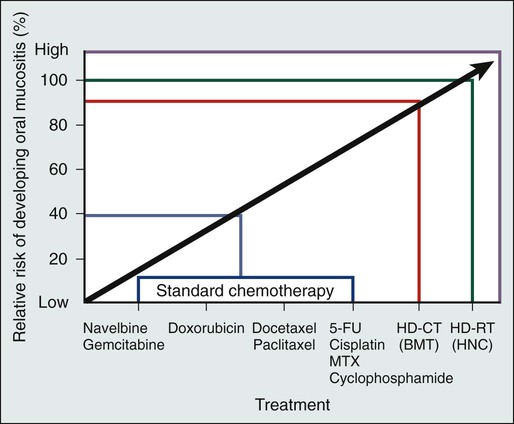
The type of chemotherapeutic agents used, the specific dose, route, and frequency of administration, and whether the chemotherapy is given as monotherapy or in combination with other agents and modalities of treatment significantly affects the degree of mucosal injury (Fig. 43-2).
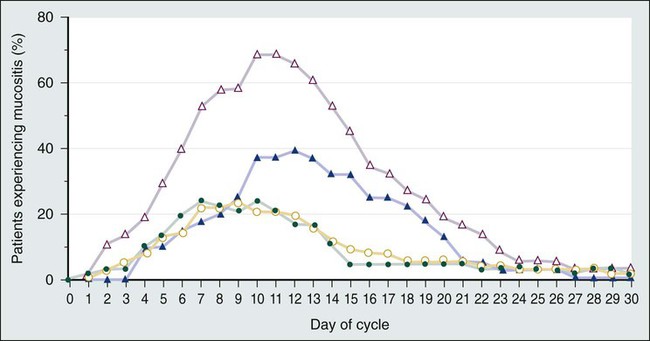
Regarding the time course of mucosal injury, 5-FU–induced mucositis is usually first noticed from 3 to 7 days after initiation of therapy. Incidence peaks at 7 to 12 days and diminishes by around 2 to 3 weeks (Fig. 43-3). With myeloablative chemotherapy for hematologic malignancies, mucositis severity can peak up to 18 days after initiation of therapy.8
Chemotherapy for the treatment of solid tumors leads to the development of mucositis in 5% to 40% of patients (5% to 15% for patients with grade 3-4 mucositis).9 Many modern treatment approaches are still associated with this problem and, because of their aggressiveness, may be more toxic.10 A vast experience exists with 5-FU, the drug most commonly used to treat gastrointestinal malignancies. Although it has long been believed that continuous administration of 5-FU carries a higher risk of mucositis than does bolus administration, a metaanalysis of trials failed to support this association.11
When myeloablative chemotherapy is used, the incidence of mucositis increases to 70% to 100% (21% to 67% for patients with grade 3-4 mucositis).8,12 The increase in the incidence of mucositis appears to be especially true for induction regimens containing high-dose melphalan.8 When chemotherapy is used in combination with radiation therapy to treat cancer of the head and neck, the mucositis rate approaches 90% to 100% (43% for patients with grade 3-4 mucositis).13
The role of age and gender in the development of mucositis has not yet been clearly defined.14 Evidence is also conflicting about whether the type of bone marrow transplant (autologous vs. allogeneic), the use of total body irradiation in the conditioning regimen, the use of granulocyte colony-stimulating factor (G-CSF), or the baseline nutritional status are related to the risk of mucositis.14 On the other hand, poor oral hygiene, dental caries, periodontal disease, high titers of HSV, and positive cultures for Candida tropicalis are generally accepted to be risk factors.14
Finally, interpersonal variability in the development of mucositis has been observed for years. This variability may be due to the differences in metabolism of the chemotherapeutic drugs from person to person. A prime example is the drug methotrexate, which results in much higher degrees of mucositis in patients with an inability to metabolize this drug. Another example involves polymorphisms in the genes for the glutathione-S-transferases, which are important detoxification enzymes. Patients with reduced activity of these enzymes have nearly double the risk of developing mucositis.15–16
Biological Therapies
Multiple targeted therapies, such as epidermal growth factor receptor (EGFR) inhibitors, vascular endothelial growth factor inhibitors, mammalian target of rapamycin inhibitors, and other drugs have emerged in the treatment of various human cancers, either as part of standard clinical practice or as part of investigational clinical studies. These newer therapies can also have adverse oral effects. However, oral complications with targeted therapies are less frequent and generally milder and only rarely lead to treatment discontinuation. Mucosal ulcerations appear to be more consistent with aphthous ulcers than frank mucositis.17 The mammalian target of rapamycin inhibitors are perhaps the targeted therapies with the highest incidence of mucositis. Everolimus and temsirolimus are reported to produce a 20% to 40% incidence of grade 1 to 2 mucositis and a 3% to 4% incidence of aphthous ulcerations but only a 1% to 3% incidence of grade 3 complications.18 As single agents, the EGFR inhibitors panitumumab and erlotinib, known primarily for causing a characteristic skin rash, are responsible for approximately a 20% incidence of grade 1 to 2 mucositis, but only 1% of patients experience grade 3 mucositis or require treatment discontinuation for this reason.18 Mucosal inflammation without overt mucositis has been reported in 14% of patients with everolimus. With regard to other tyrosine kinase inhibitors, imatinib and sunitinib have been reported to cause between a 10% and 38% incidence of mucositis, but only 2% to 3% of patients have mucositis of grade 3 or higher.18 Oral adverse effects with drugs such as bevacizumab, trastuzumab, or lapatinib are very rare. It should be kept in mind that underreporting of oral adverse effects of biological therapies has been identified as a possible reason for the low incidence reported in some trials.
When these newer therapies are combined with traditional chemotherapeutics, they can increase the usual severity of the individual chemotherapeutic drugs. Although some major studies have failed to reveal an increase in the incidence of mucositis when EGFR inhibitors are combined with radiation,19 a metaanalysis has shown that there is indeed such an increased risk (hazard ratio 1.76, P < .001).20
Oral Care Protocols and Oral Hygiene
Although evidence is insufficient to support a particular approach,21 the institution of comprehensive oral care protocols for patients receiving chemotherapy for solid tumors is generally recommended.22,23 Multiple oral care protocols have demonstrated feasibility and tolerability, and some have produced a reduction in the severity of mucositis and an improvement in patients’ ability to cope with their symptoms.24 Such oral care protocols are usually implemented by nursing staff and involve various degrees of patient education. These protocols can include cavity screening and dental consultations, basic oral care with soft tooth brushing, flushing, and rinsing, regular inspection of the oral cavity, and avoidance of smoking, alcohol, and spices.25,26 The cross-study differences seen in two similar treatment arms illustrated in Figure 43-3 may be related to the use of nurse-directed oral care recommendations in the second study that were not used in the first study.
Regarding the oral care of candidates for myeloablative chemotherapy, current guidelines that were drafted in 2009 by multiple organizations in a global effort recommend a formal dental evaluation and performance of any needed dental work before institution of conditioning regimens.27,28 This dental work includes appropriate treatment of caries, proper fitting of dental prostheses, and extraction of teeth with significant periodontal disease. These interventions should ideally be performed 10 to 14 days before any conditioning therapy. During therapy, oral hygiene should be maintained; rinses should be performed four to six times a day with use of either sterile water, normal saline solution, or sodium bicarbonate solutions, and patients should brush their teeth at least twice daily with a soft or ultra-soft toothbrush or a Toothette (i.e., a foam swab on a stick). Use of toothpaste is optional, and daily dental flossing should be performed by patients experienced in the technique, if it can be done without trauma. Orthodontic appliances and space maintainers can be removed during therapy, although, if good tissue integrity and satisfactory daily oral hygiene is maintained, their use can continue during the initial conditioning phase.27
In a retrospective review of 140 patients undergoing autologous bone marrow transplantation at a single institution, patients received professional oral health care after the initiation of local institutional guidelines in 2005. Patients treated after the institution of these guidelines (2005 to 2009) had a 66% incidence of mucositis compared with a 93% incidence for patients who did not receive professional oral health care in prior years (2002 to 2005).29
Antimicrobial and Antiseptic Interventions
In the past, microorganisms were hypothesized to play a central role in the pathogenesis of mucositis. This hypothesis is no longer believed to be true, in part because both topical and systemic antibiotics have failed to significantly change the incidence and severity of mucositis, and as a result, they are currently not recommended for routine use.21,23,30
One explanation for the general failure of antimicrobial agents in this setting may be their inability to significantly eradicate microbes from the oral cavity, or the possibility that alterations in the microbial flora by antimicrobial agents might cause more harm than good.30,31 Another explanation is that microorganisms probably play a more complex and possibly a lesser role than initially thought. It is currently suggested that instead of being involved in the initiation phase, microorganisms probably intensify the inflammatory process associated with the later phases of mucosal injury.4 However, an association exists between the severity of mucositis and the incidence of sepsis, which suggests that microbes may use the already damaged mucosa as a portal of entry.
Of all the antiseptics, chlorhexidine has been most extensively studied, with the results of nine randomized controlled trials being mixed. All of these trials ultimately provide no evidence for the benefit of chlorhexidine, and as a result, its use is not recommended.23–23 Iseganan (a naturally occurring peptide with broad antimicrobial spectrum) and a topical povidone-iodine preparation have also failed to show benefit after each compound was tested in two randomized clinical trials.21
Reactivation of HSV, which frequently manifests in patients as oral ulceration, can be a significant complication for patients receiving myeloablative chemotherapy. Nonetheless, HSV is thought to have a marginal role in causing frank oral mucositis.24 HSV infection should be suspected if mucositis persists or appears to worsen 2 or more weeks after transplantation. Guidelines for testing, prophylaxis, and treatment of HSV infection are well established.27
Cryotherapy
Oral cryotherapy during administration of chemotherapy is hypothesized to work by cooling oral mucosal tissues and thereby causing local vasoconstriction during periods of peak chemotherapy blood concentration, thus decreasing the delivery chemotherapy to the oral mucosa. It is currently recommended in three specific clinical settings and is considered to be investigational in several others.23–23
5-FU–Based Chemotherapy
A North Central Cancer Treatment Group randomized clinical trial demonstrated that oral cryotherapy could inhibit the development of bolus 5-FU–induced mucositis.32 This result has been independently validated by multiple other investigators.33–36 The therapy is administered by having the patient suck on crushed ice, starting 5 minutes before 5-FU administration and continuing for a total of 30 minutes. Longer duration of oral cryotherapy (60 minutes) does not appear to provide any additional benefit in this setting.37
Edatrexate
Four small nonrandomized phase 1 and 2 trials have used 20 to 30 minutes of oral cryotherapy for prevention of mucositis in patients receiving edatrexate, a methotrexate analogue with improved preclinical antitumor activity. Three out of the four trials have shown good tolerability of edatrexate when used with oral cryotherapy,40–40 with one study showing high toxicity despite this preventative strategy.41 Current guidelines recommend cryotherapy as an attempt to decrease mucositis in patients treated with bolus edatrexate.23–23
High-Dose Melphalan
At least five small prospective, nonrandomized studies in patients receiving high-dose melphalan therapy have tested the efficacy of oral cryotherapy compared with historic control subjects. Grade 3 mucositis developed in only 0% to 11% of patients treated with oral cryotherapy compared with a greater than 70% incidence of mucositis in historic control subjects.42–46 In the only randomized, placebo-controlled study, in which room-temperature normal saline solution was used as a placebo, 40 patients were treated with cryotherapy or placebo for 30 minutes before and 6 hours after chemotherapy with high-dose melphalan. Grade 3 mucositis was experienced in 14% of patients receiving cryotherapy compared with 74% of patients receiving normal saline solution.47 Although the need for such prolonged administration of cryotherapy is questionable because of patient noncompliance and the probably equivalent efficacy of shorter administration, cryotherapy appears to be a promising strategy in lowering mucositis in patients receiving high-dose melphalan therapy, and it is currently recommended in this setting.22 One randomized study in patients receiving methotrexate did not show a benefit.48 Overall, these data point to a possible role for cryotherapy in more diverse settings than previously thought.
Antioxidants, Anticholinergics, and Coating Agents
In general, insufficient evidence exists regarding the effectiveness of antioxidant compounds, such as all-trans retinoic acid and vitamin E, to prevent oral mucositis in patients receiving chemotherapy.14 Insufficient evidence also exists to support the prophylactic use of propantheline, an anticholinergic drug that is thought to reduce the amount of etoposide secreted into the saliva.21
Regarding sucralfate, a coating agent, at least nine randomized clinical studies have provided mixed results. However, the most recent metaanalysis suggests a 33% reduction in severe mucositis with this agent.21 Most of the studies are in patients treated with radiation therapy. Nonetheless, sucralfate is not currently recommended by any set of guidelines.22,23
Antiinflammatory Agents
Because inflammatory mediators appear to play a central role in mucositis development, the use of antiinflammatory agents has been proposed as a method to prevent mucositis. Pentoxifylline, a TNF-α and IL-2 inhibitor, misoprostol (an analog of prostaglandin-E1), and prostaglandin-E2 have failed to show benefit in randomized controlled trials.21
Amino Acids
Glutamine is a nitrogen-rich nonessential amino acid with a critical role in nucleotide synthesis, muscle function, and overall metabolic homeostasis. However, during periods of stress it becomes a conditionally essential amino acid, and its stores can be significantly depleted, as is the case in patients with cancer. Multiple trials to date have attempted to investigate the beneficial potential of different glutamine preparations through both the parenteral and oral routes, with mixed results. Glutamine had been administered parenterally, as part of total parenteral nutrition, or as an IV infusion mixed with normal saline solution. It has also been administered as an oral supplement and in “swish and swallow” mouthwash preparations. Overall, these trials have been small, and conclusions have been difficult to draw; in the transplant setting, the possibility of harm has been raised.21,49 None of these preparations is currently recommended, but further research is ongoing.22,23 An oral suspension form of L-glutamine (AES-14) was shown in a randomized controlled study of 326 patients with breast cancer who were undergoing chemotherapy to reduce the rate of grade 2 and higher oral mucositis by 11% (50% to 39%: P = .03) and grade 3 and higher mucositis by 5% (1.2% vs. 6.7%; P = .005).50
Growth Factors
Growth factors, systemically or topically, are hypothesized to prevent oral mucositis because of their potential to improve healing. Although the use of subcutaneous G-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF) has been associated with reduced mucositis in certain randomized trials, the data are inconclusive.23–23 However, one large study randomly assigned patients to prophylactic G-CSF or placebo; this study involved 195 patients undergoing chemotherapy with cyclophosphamide, doxorubicin, and etoposide for small-cell lung cancer.51 Patients treated with G-CSF had a significantly lower rate of oral mucositis (70% vs. 53%). In addition, a study of adjuvant Taxotere (docetaxel), Adriamycin (doxorubicin), and cyclophosphamide (TAC) had to be amended after 116 patients with breast cancer were enrolled because of the high incidence of febrile neutropenia. As a result, 423 additional patients who were treated with TAC received prophylactic G-CSF. The addition of G-CSF, along with reducing the rate of febrile neutropenia, was also associated with a reduced rate of grade 2 or higher stomatitis, from 32% to 23% (P = 0.01).52 In conclusion, subcutaneous injections of G-CSF appear to reduce the rates of oral mucositis. However, this intervention is not recommended for mucositis reduction alone, and its use is restricted to patients with currently approved indications, such as high risk of neutropenic fever.
Topical keratinocyte growth factor (KGF), which is secreted by injured mucosal epithelium, was hypothesized to have efficacy in mucositis prevention. One such preparation, Palifermin, has been approved by the Food and Drug Administration (FDA) for use in preventing mucositis induced by myeloablative chemotherapy. The mechanism of action of KGF is thought to be complex, with effects both on stimulation of epithelial proliferation and antiinflammatory properties.53 The recommendation for approval was based on a study of 212 patients randomly assigned to receive intravenous (IV) KGF or placebo for 3 consecutive days immediately before the initiation of conditioning therapy.54 Grade 3 or 4 mucositis developed in 63% of patients in the KGF group and in 98% of patients in the placebo group. KGF has been tested in at least five more trials.21 Two of these studies were in patients undergoing myeloablative chemotherapy, two were in patients with solid tumors (colorectal cancer and sarcoma), and one was in patients undergoing chemoradiation for head and neck cancer. In all trials except for the last (where a weekly instead of a daily dose was used), a benefit has been suggested.21 KGF is currently recommended for patients undergoing myeloablative chemotherapy but not in patients being treated for solid tumors, because further studies are needed.22,23 Although effective, its use constitutes an expensive option for mucositis prevention.
Low-Level Laser Therapy
Accumulating evidence suggests that low-level laser therapy (LLIT) has the ability to promote wound healing and reduce pain and inflammation. Several trials have suggested a benefit for laser therapy for the prevention of oral mucositis. A recent metaanalysis of randomized trials found 11 randomized studies, 9 of which were in patients undergoing chemotherapy for solid tumors or hematologic malignancies.55 The results of these trials have been impressive, with a relative risk reduction of developing mucositis of 2.03 (95% confidence interval [CI], 1.11 to 3.69), a shortening of the duration of grade 2 or higher mucositis by 4.38 days (95% CI, 3.35 to 5.40), and significant reductions in mucositis severity and pain.55 A Cochrane review included five of these trials and also suggested significant benefit in mucositis prevention.21 However, the expense and the need for specialized training and equipment limit the widespread applicability of this approach. Guidelines recommend the use of this technology in specialized centers that have the appropriate expertise; further studies are needed.22,23 However, it is true that the expense and availability of the technology is significantly improving and the technical specifications are being standardized, and thus it is possible that widespread clinical use may soon become a reality.
Other Interventions
Amifostine, a cytoprotective agent, has been primarily tested in patients undergoing head and neck chemoradiation. However, in at least three studies, amifostine has been tested in patients undergoing high-dose chemotherapy or myeloablative chemotherapy with positive results.21 Although the newer subcutaneous formulation appears to address the toxicity and administration difficulties associated with daily intravenously administered amifostine, further studies are needed. An oral spray of intestinal trefoil factor, a peptide secreted by goblet cells and involved in mucosal protection, was tested in one randomized study of 99 patients undergoing 5-FU–based chemotherapy for colorectal cancer. A 75% to 81% reduction in the risk of mucositis was found, and further studies with the agent are under way.56 In two studies in patients undergoing bone marrow transplantation, a benefit has been suggested for a neutral supersaturated calcium phosphate rinse (Caphosol).57,58
Allopurinol mouthwashes, despite constituting “standard clinical practice” at some institutions in the early 1990s, are currently not recommended because several randomized clinical trials have convincingly shown that they have no benefit.21,59,60 Traumeel S, a homeopathic remedy, and chamomile mouthwash have also failed to show any benefit.
Treatment of Chemotherapy-Induced Oral Mucositis
Scant information is available regarding the effective treatment of chemotherapy-induced mucositis, despite a plethora of prescribed remedies. Hence initial treatment of established mucositis varies significantly among institutions, and different providers often prescribe remedies based on their individual experience and preference.61 Among the many proposed treatments are various mouthwashes, coating agents, topical anesthetics or analgesics, antiinflammatory agents, systemic narcotics, and topical and systemic growth factors.24 These treatments are aimed at promoting the healing of the injured oral mucosa and thus limiting the severity and duration of ulcerations, as well as palliating the symptoms of oral mucositis.
Mouthwashes and Coating Agents
For patients with established mucositis, one of the first therapeutic measures frequently used consists of having patients rinse their mouths every 2 to 4 hours with a salt and baking soda solution (½ tsp salt plus ½ tsp baking soda in an 8-oz glass of warm water). This rinse is often soothing and is thought to be cleansing. Some centers use baking soda alone, because the addition of salt is thought to be too drying to the mucosa. In one multicenter study, 200 patients receiving standard chemotherapy who followed a carefully planned oral hygiene protocol (PRO-SELF) were randomly assigned to one of three different mouthwashes; salt and soda, chlorhexidine, or “magic” mouthwash (lidocaine, Benadryl, and Maalox). No significant differences were observed in time to cessation of the signs and symptoms of mucositis among the three regimens, with salt and soda being the least costly.62
Mouthwashes, often referred to as “magic” or “miracle mouthwash,” are topical preparations of analgesic, anesthetic, and coating agents, the composition of which varies across institutions. The most common ingredients are diphenhydramine, viscous lidocaine, magnesium hydroxide/aluminum hydroxide, nystatin, and corticosteroids.63 Other ingredients include benzocaine, milk of magnesia, chlorhexidine, kaolin, and pectin.24 Despite their widespread use, the general lack of evidence supporting their efficacy and tolerability does not support the inclusion of any of these preparations in formal guidelines.22,23 Further study of these palliative mixtures, however, is strongly encouraged given their overall availability, ease of administration, frequent use, and low cost. Chlorhexidine mouth washes are not recommended, because studies have failed to support the use of this agent for the treatment of established mucositis.22,23
Coating agents such as sucralfate have also failed to show any benefit for the treatment of established chemotherapy-induced mucositis when tested in small randomized trials.64 Other coating agents include Gelclair and MuGard, which are oral lubricating gels and are used by some institutions based on anecdotal reports. No well-conducted trials exist, and Gelclair did not show promise in small preliminary studies.65,66
Systemic Analgesics
Oral mucositis pain can be severe and significantly interfere with the quality of life of patients receiving chemotherapy. Management should follow the same aggressive guidelines used to treat pain in patients with cancer in general.68 The use of patient-controlled anesthesia is effective and well established for treating mucositis pain, especially in the hematopoietic stem cell transplantation setting. Although it is not superior to continuous infusion delivery in controlling pain, the use of patient-controlled anesthesia allows for a lesser quantity of narcotics to be used.64
Laser Therapy
Low-level laser therapy (LLLT) has also shown promise in treating established mucositis lesions.64 In a randomized trial, 37 patients with hematologic malignancies who were undergoing myeloablative chemotherapy and who had established grade 1 or 2 oral mucositis were randomly assigned to LLLT or sham illumination.69 Progression to grade 3 mucositis occurred in 16 of 18 sham-treated patients and in only 3 of 16 laser-treated patients. In another study, 21 children and adolescents undergoing high-dose chemotherapy were randomly assigned to laser therapy versus sham treatment.70 Patients were treated for 5 consecutive days after mucositis was first diagnosed. After 7 days, only 1 of 9 patients in the treatment group had oral lesions versus 9 of 12 patients in the sham group. As discussed in the prevention section, this technology is awaiting further development.
Oral Complications From Radiation Therapy
Mucositis
Etiology of Mucositis
Clinical and radiobiological evidence shows that the protraction of overall treatment time adversely influences the radiocurability of certain human tumors, particularly squamous cell carcinomas of the head and neck region. The additional dose needed to compensate for a protracted course of radiation therapy has been attributed to an accelerated tumor clonogenic growth rate. Randomized clinical trials have demonstrated improved local control and survival when altered fractionation schemes that deliver conventional or higher doses of radiation therapy are used over a shorter than conventional period.71 Therefore a break in radiation therapy because of mucositis may lead to treatment failure.
Prevention of Mucositis
Radiotherapy Technique
Sophisticated radiation therapy treatment planning can help in limiting the volume of normal tissues irradiated, thereby reducing the severity of normal tissue reactions. Normal tissue reactions can be reduced in a substantial number of patients with head and neck cancer with the use of computed tomography–based target delineation, intensity-modulated radiation therapy, and simple, custom-made, intraoral devices designed to exclude uninvolved tissues from the treatment portals or to provide shielding of tissues within the treatment area.72,73 Patients with primary cancers of the oral cavity, oropharynx, paranasal sinuses, and salivary glands are the best candidates for the use of such devices. These intraoral stents can be very useful in excluding the mucosa of the tongue and the floor of the mouth when hard palate, nasal cavity, and paranasal sinus malignancies are being treated. These same stents can be useful in excluding the palate mucosa during treatment of the tongue or floor of the mouth. Shielding stents made with a lead alloy may be useful in treatment of well-lateralized tumors of the oral cavity, parotid gland, lip, and skin of the cheek. These shielding stents can decrease the amount of radiation delivered to the contralateral mucosa. More frequent use of electron-beam and/or sophisticated three-dimensional conformal, intensity-modulated, wedged-pair, or oblique treatment plans also help exclude or minimize the radiation dose to uninvolved mucosa. Packing gauze between metallic dental restorations and mucosa of the lateral tongue and buccal area may be very beneficial in minimizing the dose from scattered radiation.
Growth Factors
Results of pilot studies suggested that G-CSF may be effective in the prevention and treatment of radiation-induced oral mucositis; however, prospective randomized clinical trials revealed no definite evidence that subcutaneously administered G-CSF or GM-CSF reduce the severity of radiation-induced mucositis.76–76
A single randomized, double-blinded, placebo-controlled trial showed that a topically applied recombinant human epidermal growth factor spray was effective in reducing the incidence of severe mucositis in patients undergoing head and neck radiotherapy.77 Confirmatory studies are needed.
Early murine and human clinical data suggested that keratinocyte growth factor may be efficacious in the prevention and treatment of radiation-induced mucositis. Recently, two large multiinstitutional randomized controlled trials demonstrated that the keratinocyte growth factor palifermin has efficacy in preventing severe oral mucositis in patients with head and neck squamous cell carcinoma who are receiving concurrent chemotherapy and radiotherapy.78,79 Le et al.79 reported a study of 188 patients receiving definitive radiotherapy and concurrent cisplatin who were randomly assigned to palifermin (180 µg/kg) or placebo IV bolus infusion weekly during radiotherapy. The primary end point of incidence of World Health Organization (WHO) grade 3 to 4 oral mucositis was significantly lower in patients receiving palifermin versus placebo (54% vs. 69%, P = .041). Henke et al.78 reported a study of 186 patients receiving postoperative radiotherapy and concurrent cisplatin who were randomly assigned to receive palifermin (120 µg/kg) or placebo IV bolus infusion weekly during radiotherapy. Patients receiving palifermin had a significantly lower incidence of the primary end point of WHO grade 3 to 4 oral mucositis than did those receiving placebo (51% vs. 67%, P = .027). However, in both of these trials, no statistically significant difference was found in patient-reported mouth pain, opioid analgesic use, or radiotherapy compliance, and thus it is not clear whether the modest benefit in prevention of severe radiotherapy-induced oral mucositis associated with palifermin justifies the added costs.
Low-Level Laser Therapy
LLLT has been investigated as a potential noninvasive technique for the prevention of oral mucositis in patients undergoing head and neck radiotherapy. LLLT involves daily local treatment of the oropharyngeal mucosa with a monochromatic light source. The exact mechanism of action of LLLT is unknown. Initial phase 3, randomized, placebo-controlled trials evaluating the efficacy of LLLT in the prevention of radiation-induced mucositis have demonstrated some degree of benefit, although further trials are needed to define the optimal laser treatment parameters, dosing, and schedule.82–82
Antibiotics and Probiotics
It has been reported that the oropharyngeal flora may contribute to radiation therapy–induced mucositis. Several controlled clinical trials have evaluated the combination of relatively nonabsorbable antibiotics (e.g., tobramycin, polymyxin E, and amphotericin B or bacitracin, clotrimazole, and gentamicin or iseganan) for patients undergoing radiation therapy directed at the oral cavity.83–92 In total, these trials do not provide convincing data of sufficient clinical magnitude to recommend use of antimicrobial mouthwashes, lozenges, or paste as part of standard practice.
Recently a large single-institution, randomized, double-blind, placebo-controlled trial demonstrated the safety and efficacy of a probiotic lozenge in reducing chemoradiotherapy-related oral mucositis in patients with head and neck cancer.93 Two hundred patients receiving definitive radiotherapy and concurrent cisplatin were randomly assigned to receive Lactobacillus brevis or placebo lozenges administered every 2 to 3 hours for a total of six lozenges per day during radiotherapy. The primary end point of WHO grade 3 to 4 oral mucositis was observed in 52% of patients in the L. brevis arm versus 77% of patients in the placebo arm (P < .001). Secondary end points of patient-reported quality of life, radiotherapy completion rate, and freedom from narcotic analgesic use were improved in the L. brevis arm compared with the placebo arm. Confirmatory studies are needed before probiotics can be recommended for the prevention of radiotherapy-induced oral mucositis.
Benzydamine Hydrochloride
An oral rinse containing 0.15% benzydamine hydrochloride is a nonsteroidal drug possessing analgesic, anesthetic, antiinflammatory, and antimicrobial properties. Three randomized trials have demonstrated the efficacy of benzydamine hydrochloride for prophylactic treatment of radiation-induced oral mucositis, although further confirmatory studies are needed before this intervention can be recommended as part of routine practice.85,94,95
Sucralfate
Sucralfate suspension has been hypothesized to provide a protective barrier and may also have a cytoprotective effect. The latter may be mediated through prostaglandin release, resulting in increased mucosal blood flow, increased mucus production, increased mitotic activity, and a surface migration of cells. However, results from small double-blind, placebo-controlled, randomized prospective trials are contradictory.96–102 Thus the randomized trials of sucralfate for therapy-induced mucositis do not establish a role for sucralfate in clinical practice.
Amifostine
The radioprotector amifostine has been evaluated as a means of preventing radiation-induced acute mucositis. One small randomized clinical trial reported that amifostine reduced the severity of acute mucositis.103 Unfortunately, larger randomized trials evaluating intravenously or subcutaneously administered amifostine in patients receiving radiation therapy alone or combined with chemotherapy have revealed conflicting results.104–108 Nausea, vomiting, hypotension, and allergic and injection site reactions are common adverse effects of this drug. The questionable efficacy and significant toxicity are associated with a remarkably high cost.109 In total, present data are insufficient to recommend amifostine at the current dose and schedules to prevent mucositis associated with radiation therapy.110
Other Interventions
Other interventions for the prevention of radiotherapy-induced mucositis have demonstrated initial efficacy in pilot studies and/or small randomized controlled trials. Three small placebo-controlled randomized trials demonstrated that honey was effective in reducing the severity of radiotherapy-induced mucositis.113–113 Zinc sulfate, alpha-tocopherol, indigo wood root, and IV L-alanyl-L-glutamine were found to be effective in decreasing the severity of radiation-induced mucositis and oral discomfort in small placebo-controlled prospective trials.114–117 Maciejewski and colleagues118 reported that painting the buccal mucosa with a 2% silver nitrate solution for several days before radiation therapy stimulates normal mucosa repopulation during radiation therapy, producing a significantly less severe mucosal reaction and faster mucosal healing after completion of radiation therapy. A double-blind, placebo-controlled, randomized trial of treatment with 40 mg of prednisone, beginning on day 8 of an accelerated course of radiation therapy, did not show a reduction in the intensity or duration of mucositis; however, there was a trend favoring prednisone in terms of shorter treatment interruptions and a significant reduction in overall treatment time.119 These approaches to the problem of acute mucositis deserve further study.
Treatment of Established Mucositis
Concurrent Oral Mucosa Infection
In patients experiencing oral mucositis while undergoing radiation therapy, the oral cavity should be examined at least once a week, and antibiotic or antifungal medications should be prescribed as infections are documented. Clotrimazole troches, one dissolved in the mouth five times a day for 14 days, generally work well for oral candidiasis. However, if significant mucositis has developed, the troches may not be tolerated. In this situation, nystatin oral suspension or fluconazole in tablet or liquid form is often effective. Fluconazole is more effective than nystatin and may need to be given at a higher dose and/or for an extended period of time in patients receiving combined chemotherapy and radiation therapy because of infections with resistant species.120,121
Analgesics
When discomfort develops, topical anesthetic agents can be used. As the pain progresses, use of systemic analgesics, including acetaminophen with codeine suspension or an oral morphine sulfate elixir, may become necessary. Suspensions are preferred to elixirs because they are formulated without alcohol. Many narcotic pain medications come in a liquid formulation that is relatively easy to swallow or can be administered through a feeding tube. Fentanyl patches are also very effective for patients who cannot swallow. A topical morphine mouthwash may be a more effective treatment for mucositis pain than a commonly used “magic mouthwash” (lidocaine, diphenhydramine, and magnesium aluminum hydroxide).122
Daily Nursing Evaluation
Janjan and colleagues123 reported improved pain management in patients undergoing radiation therapy for head and neck cancer with a daily nursing intervention consisting of instructions on the use of mouthwashes and a three-step analgesic protocol consisting of acetaminophen, acetaminophen with codeine suspension, and liquid morphine for relief of mild, moderate, and severe pain, respectively. The physician promptly changed the prescribed analgesic regimen when the patient’s symptoms changed. Patients who received a daily nursing intervention reported fewer days of moderate and severe pain, had less pain throughout the day, and noted less disturbance in sleep, eating, and energy level. Daily review of a symptom survey by a radiation oncology nurse combined with a well-defined strategy for mouth care and analgesics appeared to improve pain management of radiation-induced oropharyngeal mucositis because of prompt attention to patient needs.
Other Interventions
The following agents have all been evaluated with mixed results: viscous lidocaine with 1% cocaine and dyclonine hydrochloride 1.0%; a mixture of kaolin-pectin solution, diphenhydramine, and saline; morphine; tricyclic antidepressants; a mucosa-adhesive water-soluble polymer film containing topical anesthetics and antibiotics; oral aloe vera; capsaicin lozenges; orgotein; and a sodium-sucrose octasulfate oral rinse.124–130
Xerostomia
Etiology of Xerostomia
With the use of modern, computed tomography–based treatment planning and multibeam treatment techniques, such as three-dimensional conformal and intensity-modulated radiotherapy, investigators have examined relationships between partial volume radiation dose to the parotid glands and subsequent development of xerostomia. A linear correlation is found between postradiotherapy flow ratio and parotid gland dose (5% loss of function per 1 Gy of mean dose) and a strong parotid volume dependency.131 Chao and coworkers132 observed a correlation between mean parotid dose and the fractional reduction of stimulated saliva output at 6 months after the completion of radiation therapy. They also noted that responses to quality-of-life questions regarding eating and speaking functions were significantly correlated with stimulated and unstimulated saliva flow at 6 months. Additionally, Eisbruch and colleagues133 found that the degree of xerostomia was related to the degree of preradiation therapy xerostomia, the time since radiation therapy, and the mean dose to the major salivary glands (most notably the submandibular gland) and to the oral cavity. Recovery of parotid function can be demonstrated at 6 months, 12 months, and 5 years after radiation therapy.134,135 For a detailed review of dose-volume parameters and salivary gland function, the reader is referred to an article by Deasy et al.136
Prevention of Xerostomia
Radiotherapy Technique
Given the clear dose-response relationship regarding radiation dose to the salivary glands and subsequent development of xerostomia, advanced technologies have been developed with the goal of reducing the dose to the salivary glands. In prospective uncontrolled and controlled phase 3 clinical trials, three-dimensional conformal and intensity-modulated radiation therapy have been shown to preserve parotid salivary flow and improve quality of life by reducing the dose to the parotid glands.105,132,133,137–139 Blanco and colleagues131 and Eisbruch and coworkers133 suggested that a mean parotid gland dose of 26 Gy or less should be a planning goal if substantial sparing of the gland function is desired. Murdoch-Kinch et al.140 suggested that a mean submandibular gland dose of less than 39 Gy should be targeted to preserve gland function. Sparing of the oral mucosa with its minor salivary glands should be a goal in treatment planning, as well to reduce the severity of radiation-induced xerostomia.133 It should be emphasized that attempts to reduce dose to the salivary glands should only be pursued if doing so will not compromise coverage of tissues at risk for harboring tumor clonogens.
Altered fractionation radiation therapy schedules may also help to preserve salivary function. Leslie and Dische141 evaluated the function of parotid glands in patients treated with three different radiation therapy schedules, 9 or more months after completion of treatment. Twelve parotid glands that had received conventionally fractionated radiotherapy to a dose of 60 to 66 Gy showed a mean percentage flow of 20% and a significant decrease in saliva pH. Six glands that had received continuous hyperfractionated accelerated radiation therapy showed mean percentage flows of 65% with only slight and nonsignificant decreases in saliva pH. These results were attributed to the lower dose per fraction used, with subsequent greater repair of sublethal damage between treatment fractions.
Amifostine
Amifostine appears to protect the salivary glands from the effects of radiation therapy and may prove to be helpful in preventing or minimizing the effects of xerostomia and the loss of taste.103,104,106,108,142,143 Several studies have reported significant reduction in the severity of acute and chronic radiation-induced xerostomia with prophylactic use of amifostine, as determined by nonplacebo-controlled clinical trials.103,104,106,140,141 No evidence was found that amifostine interfered with the antitumor effects of radiation therapy as measured by local or regional control and overall survival. Nausea and emesis are common adverse effects of amifostine, with 28% of patients discontinuing the amifostine before the end of radiotherapy in one study.143 Subcutaneous administration of amifostine may be equally effective as intravenous administration with less severe nausea, vomiting, and hypotension but more frequent cutaneous toxicity.144,145 A placebo-controlled phase 3 trial failed to confirm the radioprotective benefit of amifostine on salivary function after chemoradiotherapy.107 Thus the use of amifostine may be considered to decrease the incidence of acute and late xerostomia in patients undergoing fractionated radiation therapy in the head and neck region that includes the salivary glands.110 Amifostine has been approved by the FDA for use in the postoperative adjuvant setting.
Pilocarpine
One small retrospective study and two small double-blind, placebo-controlled, randomized trials suggested that pilocarpine (5 mg given orally four times a day), started the day before or on the same day as radiation therapy and given concurrently with radiation therapy and for 3 months after radiation therapy, results in a lower frequency of oral symptoms and xerostomia during treatment and afterward.148–148 However, three large placebo-controlled clinical trials failed to confirm these initial findings and did not demonstrate any reduction in the incidence or severity of radiation-induced xerostomia with prophylactic use of pilocarpine.151–151 Therefore pilocarpine is not recommended for the prevention of xerostomia.
Salivary Gland Transfer
In selected patients with cancers in the oropharynx, hypopharynx, or larynx, it has been reported that surgical transfer of a submandibular gland into the submental space can be successfully accomplished.152 If patients require postoperative radiation therapy, the submandibular gland can more readily be excluded from the irradiated volume, thus preserving some saliva production. This process requires validation by controlled clinical trials.
Acupuncture
Recently, small randomized controlled trials demonstrated that acupuncture was an effective prophylactic treatment for reducing the severity of xerostomia compared with no acupuncture or sham treatment for patients undergoing radiotherapy for nasopharyngeal cancer.153,154 Confirmatory studies are needed before acupuncture can be recommended as a routine preventive measure for xerostomia.
Treatment of Xerostomia
Oral Lubricants
Patients should be advised to take frequent sips of water and suck on ice chips. Because chewing stimulates the flow of saliva, patients with residual salivary function may be helped by chewing sugarless gum. Patients with xerostomia are highly susceptible to dental caries and should not use foods containing sugar or acidic foods or beverages to stimulate salivary flow. Commercial nonprescription solutions used to lubricate the oral tissues may be the only effective treatment for patients without functioning salivary gland parenchyma or for those whose salivary glands do not respond to stimulation. Virtually all lubricants can provide some short-term relief for patients with xerostomia. Some studies have indicated that salivary substitutes containing carboxymethylcellulose or hydroxymethyl cellulose are more effective in relieving dryness than water- or glycerin-based solutions. Some patients prefer mucopolysaccharide solutions. Xialine, a xanthan gum–based saliva substitute, has been shown to be no better than placebo in decreasing the effects of xerostomia, although a trend was seen in favor of Xialine for improving problems with speech and senses.155
Muscarinic Receptor Agonists
Pilocarpine and cevimeline are parasympathomimetic muscarinic secretagogues that have been investigated for the treatment of postirradiation xerostomia. Two large randomized, double-blind, placebo-controlled, multicenter clinical trials have documented the efficacy of oral pilocarpine (5 mg given orally three times a day) in relieving oral dryness; improving salivary flow, mouth comfort, and ability to speak; and reducing the need for oral comfort agents after head and neck irradiation.158–158 Adverse reactions were minimal, with the most common being mild to moderate sweating, which is dose related. Best results may require continuous treatment for more than 8 weeks. Most patients report significant relief of symptoms of xerostomia and improvement in quality of life that do not appear to be dependent on previous radiotherapy dose/volume parameters, suggesting that oral pilocarpine acts primarily by stimulating ectopic salivary glands and can be of benefit for a whole range of patients with xerostomia of varying severity. Topical pilocarpine administration has shown results similar to those achieved with systemic treatment but with improved patient tolerance.159 Two large double-blinded placebo-controlled trials demonstrated similar efficacy of cevimeline in treatment of postirradiation xerostomia.160
Acupuncture
Various reports have suggested that acupuncture and acupuncture-like transcutaneous electric nerve stimulation can subjectively (according to patient-completed xerostomia inventories) and objectively (per measurement of unstimulated and stimulated salivary flow rates) reduce symptoms of xerostomia and improve salivary flow rates.161–164 Large confirmatory studies comparing these treatments with sham acupuncture are ongoing.
Dental Caries
Etiology of Dental Caries
Prevention and Treatment of Dental Caries
After radiation therapy, patients should be seen every 3 months for dental checkups. There is no concern regarding the additional x-ray exposure of dental films, because the dose is insignificant compared with the therapeutic dose given for the cancer therapy. All routine dental procedures can be performed without unusual precautions after a course of radiation therapy, except radical periodontal treatment and extractions, which may lead to osteoradionecrosis if they are not performed with special care. When extractions are required after a course of radiation therapy, it is best to remove one tooth at a time with as little trauma to adjacent tissues as possible and to wait until healing is complete before proceeding to further extractions. Prophylactic antibiotic coverage should be started 1 day before extraction and continued until the site is completely healed. Some institutions favor the use of hyperbaric oxygen before extraction.165 Primary closure of the wound should be carried out over a smooth bony surface so that no sharp spicules or ridges are left beneath the mucosa. Postradiation therapy tooth extractions carried out in this manner have a good chance of complete healing without the development of necrosis. When extreme root sensitivity occurs after radiation therapy, brushing fluoride onto the exposed root surface and using specially formulated, commercially available toothpaste appears to decrease the sensitivity to some extent.
Soft Tissue Necrosis
Etiology of Soft Tissue Necrosis
Treatment of Soft Tissue Necrosis
More than 90% of soft tissue necroses will heal with conservative treatment, although, in some instances, it may take many months. A small trial (consisting of 12 patients with 15 sites of late radiation necrosis of the soft tissues) has been conducted to evaluate the effect of pentoxifylline on healing radiation soft tissue necrosis.166 The average duration of nonhealing before treatment with pentoxifylline was 30.5 weeks. With the institution of pentoxifylline (400 mg given orally three times a day), 13 of 15 necroses healed completely and one partially healed an average of 9 weeks after treatment was started. All patients had pain relief. Additional case reports and small clinical trials have suggested that the combination of pentoxifylline, tocopherol, and clodronate may be beneficial in healing radiation-induced ulcerated fibrosis, soft tissue necrosis, and mucosal necrosis.169–169 These results support further study of pentoxifylline in patients in whom soft tissue necrosis develops after a course of radiation therapy.
Osteoradionecrosis
Etiology of Osteoradionecrosis
Treatment of Osteoradionecrosis
Recent data suggest that pentoxifylline and tocopherol may be efficacious in treating refractory osteoradionecrosis. In a small phase 2 trial involving 18 patients with refractory osteoradionecrosis, combination treatment with pentoxifylline and tocopherol resulted in significant healing in all patients.170 A phase 2 trial investigated the safety and efficacy of long-term treatment with a combination regimen called PENTOCLO, consisting of 800 mg pentoxifylline, 1000 IU vitamin E, and 1600 mg clodronate 5 days per week alternating with 20 mg prednisone and 1000 mg ciprofloxacin 2 days per week.171 Fifty-four patients with refractory osteoradionecrosis were treated with PENTOCLO for a median of 16 months. This regimen was associated with significant bone healing and symptom improvement in all patients. Further studies of this treatment approach are warranted.
Taste Alterations
Loss of taste occurs rapidly early in the course of radiation therapy directed at the oral cavity. Most patients report that the sense of taste is essentially nonexistent by the third or fourth week of treatment. After the completion of radiation therapy, most patients report some taste improvement within 1 to 2 months. Full recovery of taste usually requires 2 to 4 months. In some patients, taste never returns to normal, at least in part because of xerostomia. Although some studies have suggested that zinc therapy may be useful in improving taste acuity, a randomized clinical trial did not show any benefit for zinc compared with a placebo.172 Amifostine may protect against taste loss caused by irradiation.103,106
Trismus
Etiology of Trismus
Prevention and Treatment of Trismus
High-energy x-ray beams and sophisticated multiple field techniques such as intensity-modulated radiotherapy should be used whenever possible to reduce the dose of radiation therapy to the temporal-mandibular joint and to the muscles of mastication. Patients treated with both surgery and radiation therapy have a greater risk for trismus than patients treated with either modality alone. Patients at high risk for trismus and those in whom trismus has developed before treatment should perform jaw-stretching exercises daily in an attempt to increase the inter-arch or inter-incisor distance. A number of techniques are used, including commercially available jaw-stretching tools and less expensive stacked tongue blades, tapered corks, or clothespins. These devices are inserted between the teeth to increase the interincisor distance until slight pain is encountered. The exercises should be done for about 30 seconds every 2 hours. Additional tongue blades can be added or a thicker aspect of the cork can be placed between the teeth every few days to increase the inter-incisor distance and stretch the muscles of mastication. Results from a pilot study suggested that pentoxifylline may be effective in treating trismus caused by radiotherapy.173
Malignancy
The carcinogenic effect of ionizing radiation has long been recognized. The latent interval between radiation therapy and the development of cancer varies from several to many years. Kogelnik and colleagues174 reviewed charts of 1163 patients treated for head and neck cancer at the M.D. Anderson Cancer Center who had survived a minimum of 5 years after treatment without having recurrent cancer. The incidence of new cancers in the primary tumor site (1.8% vs. 2.7%), within the immediate vicinity of the primary tumor (4.2% vs. 3.1%), or at sites remote from the primary tumor but still within the oral cavity or pharynx (4.7% vs. 5.7%) was very similar for patients treated with surgery alone versus patients treated with irradiation with or without surgery, respectively. It was concluded that moderate or high-dose radiation therapy did not produce any new squamous cell carcinomas of the mucous membranes. Rusthoven et al.175 analyzed 27,985 patients in the Surveillance, Epidemiology, and End Results database with localized squamous cell carcinoma of the head and neck who were treated with radiotherapy (44%) or without radiotherapy (56%). The 15-year risk of second malignancy of the head and neck was 7.7% in those treated with radiotherapy versus 10.5% in those not treated with radiotherapy (P < .001). The authors suggest that radiotherapy may eradicate microscopic foci of second primary head and neck cancer.