Obesity
Ashraf S. Habib MBBCh, MSc, MHSc, FRCA, Robert D’Angelo MD
Chapter Outline
Obesity is a worldwide health problem, with the prevalence in the general population growing at an alarming rate and reaching epidemic proportions. Data from the National Center for Health Statistics show that in 2010, 69% of Americans were overweight and 36% were obese; 56% of women of reproductive age (20 to 39 years old) were overweight, and 32% were obese.1 Although no definition of obesity specific to pregnancy exists, a pregnant woman is generally considered overweight when her body mass index (BMI) is 25.0 to 29.9 kg/m2, and obese when her BMI is 30 kg/m2 or greater. The World Health Organization defines three grades of obesity: class I (BMI 30.0 to 34.9 kg/m2), class II (BMI 35.0 to 39.9 kg/m2), and class III (BMI 40 kg/m2 or greater).
Obesity is associated with an increased risk for maternal morbidity and mortality. The care of obese parturients poses significant challenges to the anesthesia provider as a result of common comorbidities, an increased cesarean delivery rate, and technical difficulties associated with both neuraxial and general anesthesia. Understanding the pathophysiologic changes and comorbidities associated with obesity and pregnancy is crucial for the safe conduct of anesthesia in these high-risk patients.
Physiologic Changes of Obesity
Pulmonary Changes
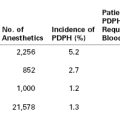
Obesity increases the demands on the pulmonary system. As energy expenditure increases proportionate to the increase in body mass,2 oxygen consumption and carbon dioxide (CO2) production also increase proportionate to the increase in work performed.3 Minute ventilation is increased owing to the elevated respiratory demand, except in the 5% to 10% of patients with Pickwickian syndrome, who display a reduced sensitivity to CO2.4 Obesity affects the body’s ability to meet these demands by changing pulmonary mechanics, altering lung volumes, and impairing oxygen consumption. The combined effects of obesity and pregnancy on the respiratory system are summarized in Table 50-1.
TABLE 50-1
Physiologic Changes in the Respiratory System Induced by Pregnancy and Obesity
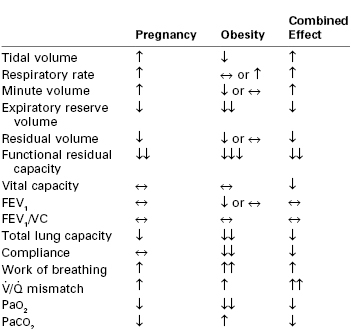
↑, increase; ↓, decrease, ↔, no change; more than one arrow represents the degree of intensity; FEV, forced expiratory volume in 1 sec; VC, vital capacity;  , ventilation/perfusion.
, ventilation/perfusion.
Modified from Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia 2006; 61:36-48.
Pulmonary Mechanics
Obesity increases the weight of the chest wall; thus, greater energy expenditure is required during ventilation to move this greater mass. Several prospective studies have demonstrated that morbidly obese patients, in comparison with controls, expend a disproportionately high percentage of total oxygen consumption on respiratory work, even during quiet breathing.5 The weight gain associated with pregnancy further increases the work of breathing in obese patients. In obese individuals, frequent shallow respirations may represent a more efficient breathing pattern than a pattern characterized by large tidal volumes. This pattern of frequent shallow respirations contrasts to the increased tidal volumes that typically accompany pregnancy. Although the PaCO2 in most morbidly obese pregnant women is not different from that in nonobese pregnant women, pulmonary reserve is reduced.
Lung Volumes
Greater abdominal weight restricts diaphragm movement, especially in the supine or Trendelenburg position, thus encouraging smaller tidal volumes. Functional residual capacity (FRC) decreases at the expense of expiratory reserve volume and may be less than closing capacity. In morbidly obese patients, this difference can result in airway closure during tidal ventilation. Similarly, expiratory reserve volume, vital capacity, inspiratory capacity, total lung capacity, and maximum minute ventilation all decrease in morbidly obese patients. Both chest wall and lung compliance decrease, but airway resistance increases.6,7
Pregnancy also alters lung volumes, and these changes may modify some of the normal effects of obesity on respiratory function. In nonobese pregnant women, expiratory reserve volume and FRC both decline 20% to 25% by term. Eng et al.,8 examining a series of pregnant women whose estimated prepregnancy weights ranged from 50% to 140% above normal, measured lung volumes during the third trimester and again at 2 months postpartum. With the exception of FRC, the lung volume changes resembled those that occur in nonobese pregnant women. However, FRC decreased less in obese pregnant women than in nonobese pregnant women.
Oxygenation
Pulmonary diffusion typically remains normal in most women with morbid obesity. Decreased chest wall compliance and greater abdominal weight promote airway closure in the dependent portion of the lung.9 Ventilation preferentially occurs in the more compliant, nondependent portion of the lung. In contrast, pulmonary blood flow preferentially occurs in the dependent portion of the lung, resulting in ventilation-perfusion mismatch and hypoxemia.9
Consistent with the positional deterioration of lung volumes, oxygenation worsens in obese persons in the supine and Trendelenburg positions. Both term pregnant and obese patients are prone to rapid oxygen desaturation during induction of general anesthesia.10 Although oxygenation does not necessarily correlate linearly with weight,11 massive weight loss improves PaO2 and expiratory reserve volume. Weight loss does not, however, improve forced expiratory volume in 1 second, forced vital capacity, or maximum mid-expiratory flow.12
Cardiovascular Changes
Cardiovascular changes associated with pregnancy and obesity are summarized in Table 50-2. Both obesity and pregnancy increase blood volume and cardiac output. The latter increases by 30 to 50 mL/min for every 100 g of fat.13 This change occurs as a result of increases in both stroke volume and heart rate. In addition to the elevated preload observed in obese parturients, left ventricular afterload is also increased owing to the high peripheral resistance and greater arterial wall stiffness. These changes result in both eccentric and concentric left ventricular hypertrophy. Messerli et al.14 documented a 30-fold increase in premature ventricular contractions in obese patients with eccentric left ventricular hypertrophy in comparison with lean subjects. The increase in heart rate limits the time available for diastolic filling. Diastolic relaxation is impaired, leading to diastolic dysfunction.15 In contrast, ventricular systolic function is usually normal in obese individuals.16
TABLE 50-2
Physiologic Changes in the Cardiovascular System Induced by Pregnancy and Obesity
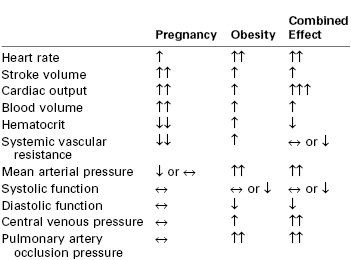
↑, increase; ↓, decrease, ↔, no change; more than one arrow represents the degree of intensity.
Modified from Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia 2006; 61:36-48.
Pulmonary blood volume increases in proportion to increases in cardiac output and total blood volume. Pulmonary hypertension can occur and may be position dependent. Paul et al.17 observed an 11% increase in oxygen consumption and a 44% increase in pulmonary capillary wedge pressure when morbidly obese patients were placed in a supine position. Hypoxemia, if present, increases pulmonary vascular resistance. Airway obstruction may also increase pulmonary artery pressure.
Hypertension occurs more frequently among obese pregnant women than lean women. A BMI of 30 kg/m2 or more is associated with a threefold higher incidence of hypertension during pregnancy than a BMI less than 30 kg/m2.18 BMI and left ventricular mass are directly related, even after controlling for age and blood pressure, especially in patients with a BMI greater than 30 kg/m2.19 Among morbidly obese pregnant women, left atrial size, left ventricular thickness, interventricular septal thickness, and left ventricular mass are increased compared with nonobese pregnant women.13 Fatty infiltration of the heart can occur, especially in the right ventricle and perhaps in the conduction system.20
Aortocaval compression of the great vessels in the supine position may be greater in obese parturients, particularly those with a large fat panniculus.21 Tsueda et al.22 reported two cases of cardiac arrest in morbidly obese patients who had been placed in the supine position. The authors speculated that the sudden circulatory changes associated with this change in position accounted for the sudden death of these patients.
Gastrointestinal Changes
It is unclear whether obesity in pregnancy is associated with an increase in gastric volume and a decrease in gastric pH. Vaughan et al.23 observed that 88% of obese nonpregnant patients presenting for surgery had a gastric pH less than 2.5 and 86% had a gastric volume exceeding 25 mL. These findings resemble those in a cohort of healthy pregnant women who presented for elective cesarean delivery.24 Roberts and Shirley25 reported that gastric volumes aspirated from obese laboring women undergoing cesarean delivery were significantly higher than those obtained from lean controls.
Other studies, however, did not confirm these findings and reported conflicting evidence regarding gastric volume and pH in obese patients.26,27 Similarly, studies have reported conflicting data regarding gastric emptying in the obese population; studies have reported delayed, unchanged, or more rapid rates of gastric emptying in obese subjects compared with lean subjects.28 In a nonobstetric obese population, Maltby et al.29 reported that drinking 300 mL of clear fluid 2 hours before surgery had no effect on gastric fluid volume and pH compared with fasting after midnight. Similarly, Wong et al.30 found that gastric emptying in obese, nonlaboring term pregnant volunteers was not delayed after ingestion of 300 mL of water compared with ingestion of 50 mL of water. The gastric volume was similar to baseline 60 minutes after ingestion of water. It should be remembered, however, that obesity is a risk factor for diabetes,18 which may cause delayed gastric emptying.
Both gastroesophageal reflux and hiatal hernia are more common in obese than in nonobese patients.31 Obesity is also associated with a higher risk for difficult airway management, which is a known risk factor for aspiration.32 Therefore, it seems likely that morbidly obese patients are at higher risk for pulmonary aspiration of gastric contents.
Coagulation Changes
Obesity is associated with a higher risk for thromboembolic complications.33 Venous thromboembolism was the leading cause of direct maternal mortality in the United Kingdom from 1985 to 2005.34 Although the most recent report of the Confidential Enquiries into Maternal Deaths in the United Kingdom covering the triennium 2006 to 2008 showed a reduction in deaths from venous thromboembolism, 12 of the 16 women who died were obese.34 Obesity is associated with changes in coagulation, venous stasis, and endothelial injury that contribute to the pathogenesis of venous thromboembolism. For instance, adipose tissue secretes the following: (1) adipokines such as plasminogen activator inhibitor-1 (PAI-1), which results in impaired fibrinolysis; (2) leptin, which promotes platelet aggregation; and (3) interleukin-6, which stimulates the liver to produce coagulation factors.35,36 C-reactive protein levels are also elevated in obese women, leading to platelet activation.37 Venous stasis is compounded in obese women by increased intra-abdominal pressure, which leads to increased iliofemoral venous pressure.38 Endothelial injury may also be increased in obese patients; obesity was shown to be associated with endothelial dysfunction in the nonpregnant population.39 Therefore, all risk factors that contribute to the pathogenesis of thromboembolic complications are likely to be exacerbated by obesity.
Endocrine Changes
Gestational diabetes and diabetes mellitus occur more frequently in obese patients.18 The pathologic process is attributed to the following: (1) peripheral insulin resistance as a result of augmentation of free fatty acids by visceral obesity,40 (2) increased proinflammatory cytokine levels,41 (3) relative gonadotropin resistance, and (4) a low sex hormone–binding globulin concentration, which leads to hyperandrogenism and decreased insulin sensitivity.42 The concentration of adiponectin, an adipokine with insulin-sensitizing properties, is also decreased in obesity, which leads to decreased insulin sensitivity.43
CoMorbidities Associated with Obesity
Sleep Apnea
Obesity is a significant risk factor for obstructive sleep apnea (OSA), which is characterized by repeated episodes of complete or partial upper airway collapse, leading to hypoxemia and hypercarbia. Those repeated periods of hypoxemia and reoxygenation lead to significant endocrine and metabolic disturbances, which result in an increased risk for hypertension, myocardial infarction, stroke, diabetes, and metabolic syndrome.44 There is no consensus on the definition of OSA in pregnancy, and therefore the prevalence is unknown.
The changes of pregnancy may both worsen and protect against OSA. For instance, weight gain45 and estrogen-induced hyperemia and edema of nasal mucosa46 might promote OSA, whereas sleeping in the lateral position,47 reduced rapid eye movement (REM) sleep, and the progesterone-induced increase in minute ventilation might protect against it. Obesity does not seem to be as strongly correlated with OSA in pregnancy as in the nonpregnant population.45
The risks for gestational hypertension, preeclampsia, and gestational diabetes are increased with OSA.48,49 Some studies have examined the potential impact of maternal OSA on poor perinatal outcomes, but most of the studies have been small with conflicting results. For instance, Sahin et al.50 simultaneously performed polysomnography and a nonstress test to assess the impact of hypoxemia due to OSA on the fetus. OSA was found in 4 of the 35 women evaluated; in 3 of these women fetal heart rate (FHR) decelerations accompanied maternal oxyhemoglobin desaturation. Olivarez et al.51 performed simultaneous polysomnography and at least 3 hours of continuous FHR monitoring on 100 pregnant women (including 19 with a diagnosis of OSA), and they found no association between FHR abnormalities and OSA parameters. Louis et al.52 reported an increased risk for preeclampsia (odds ratio [OR], 3.54; 95% confidence interval [CI], 1.26 to 9.92), neonatal intensive care unit admission (OR, 3.39; 95% CI, 1.23 to 9.32), and cesarean delivery (OR, 3.04; 95% CI, 1.14 to 8.1) among obese pregnant women with OSA compared with those with no OSA after adjusting for age, race, and BMI.
Other Comorbidities
Obesity is associated with an increased risk for a number of disease states compared with lean controls (Table 50-3).31,33,53 These comorbidities complicate the care of obese parturients.
TABLE 50-3
Relative Risk or Odds Ratio of Comorbidities in Obese Women
| Comorbidity | Relative Risk | 95% CI of Relative Risk |
| Type 2 diabetes* | 12.41 | 9.03, 17.06 |
| Hypertension* | 2.42 | 1.95, 3.67 |
| Coronary artery disease* | 3.10 | 2.81, 3.43 |
| Congestive heart failure* | 1.78 | 1.07, 2.95 |
| Pulmonary embolism* | 3.51 | 2.61, 4.73 |
| Stroke* | 1.49 | 1.27, 1.74 |
| Asthma* | 1.78 | 1.36, 2.32 |
| Gallbladder disease* | 2.32 | 1.17, 4.57 |
| Osteoarthritis* | 1.96 | 1.88, 2.04 |
| Chronic back pain* | 2.81 | 2.27, 3.48 |
| Odds Ratio | 95% CI of Odds Ratio | |
| Depression† | 1.55 | 1.22. 1.98 |
| Gastroesophageal reflux disease‡ | 1.89 | 1.70, 2.09 |
* Data from Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9:88.
† Data from Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67:220-9.
‡ Data from Eslick GD. Gastrointestinal symptoms and obesity: a meta-analysis. Obes Rev 2012; 13:469-79.
CI, confidence interval.
Impact of Obesity on Pregnancy
Maternal and Fetal Complications
Obesity results in greater use of health care resources. Chu et al.54 reported that obese pregnant women receive significantly more prenatal tests, ultrasonographic examinations, medications, and prenatal visits with a physician, and they are at greater risk for having a high-risk pregnancy, cesarean delivery, and prolonged hospitalization than pregnant women of normal weight.
Obesity is associated with a significantly increased incidence of maternal, fetal, and neonatal complications. These include a higher risk for spontaneous abortion (miscarriage), thromboembolic complications, gestational diabetes, hypertensive disorders of pregnancy, dysfunctional labor, shoulder dystocia, operative vaginal delivery, cesarean delivery, postpartum hemorrhage, wound infection, fetal macrosomia, fetal congenital anomalies, stillbirth, and neonatal death.18,55,56 In a prospective multicenter cohort study of more than 16,000 unselected pregnant women in the United States, Weiss et al.18 assessed obstetric complications in 1473 obese women (BMI 30.0 to 34.9 kg/m2), 877 morbidly obese women (BMI ≥ 35 kg/m2), and 3752 lean controls (BMI < 30.0 kg/m2). The odds of gestational diabetes, gestational hypertension, preeclampsia, macrosomia, preterm delivery, and operative vaginal and cesarean delivery were all greater in morbidly obese pregnant women compared with lean women (Table 50-4).
TABLE 50-4
Obstetric Complications in Obese and Morbidly Obese Women
| Outcome | Odds Ratio (95% Confidence Interval) | |
| OBESE VERSUS CONTROL | MORBIDLY OBESE VERSUS CONTROL | |
| Gestational diabetes | 2.6 (2.1, 3.4) | 4.0 (3.1, 5.2) |
| Gestational hypertension | 2.5 (2.1, 3.0) | 3.2 (2.6, 4.0) |
| Preeclampsia | 1.6 (1.1, 2.3) | 3.3 (2.4, 5.5) |
| Birth weight > 4500 g | 2.0 (1.4, 3.0) | 2.4 (1.5, 3.8) |
| Birth weight > 4000 g | 1.7 (1.4, 2.0) | 1.9 (1.5, 2.3) |
| Preterm delivery | 1.1 (0.9, 1.5) | 1.5 (1.1, 2.1) |
| Operative vaginal delivery | 1.0 (0.8, 1.3) | 1.7 (1.2, 2.2) |
| Cesarean delivery* | 1.7 (1.4, 2.2) | 3.0 (2.2, 4.0) |
* Nulliparous women.
Data from Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol 2004; 190:1091-7.
Most importantly, obesity increases the risk for death during pregnancy. The latest report of Confidential Enquiries into Maternal Deaths in the United Kingdom for the 2006 through 2008 triennium showed that 49% of women who died were overweight or obese.34 Similarly, in the previous report that covered the 2003-2005 triennium, 52% of the mothers who died were overweight or obese.57 The impact of obesity on maternal mortality was even greater among women who died of thromboembolism or cardiac disease; of the mothers who died of these two disease entities, 78% and 61%, respectively, were overweight or obese.34 Because of the high risk for thromboembolism in obese pregnant women and the significant probability of subtherapeutic anticoagulation with fixed-dose low-molecular-weight heparin regimens, the Royal College of Obstetricians and Gynaecologists (RCOG) added weight-based recommendations for thromboprophylaxis to their 2009 guidelines.58
Obesity has also been identified as a risk factor for anesthesia-related maternal mortality. Six of the 13 direct maternal deaths attributed to anesthesia in the last two triennial reports from the United Kingdom occurred in obese parturients.34,57 In the United States, Mhyre et al.59 reported that six of eight pregnant women who died of anesthesia-related deaths in Michigan between 1985 and 2003 were obese. Because of the increased risk for complications, in 2010 the United Kingdom Centre for Maternal and Child Enquiries (CMACE) and the RCOG published joint guidelines on the management of women with obesity in pregnancy.60 Both these guidelines and the American College of Obstetricians and Gynecologists (ACOG) guidelines61 recommend a multidisciplinary approach to the care and treatment of obese pregnant women, including (1) evaluation of all women for obesity by calculating BMI, (2) offering preconception counseling to obese women, (3) screening for gestational diabetes, (4) providing guidelines for prenatal weight gain, and (5) referring obese women for antepartum consultation with an anesthesiologist.
Progress of Labor and Method of Delivery
The progress of labor appears to be impacted by BMI. A large multicenter study involving 118,978 patients, whose labor management reflected current practice in the United States, reported that labor progressed more slowly with increasing BMI for both nulliparous and parous women.62 The median time to progress from 4 to 10 cm cervical dilation increased from 5.4 hours to 7.7 hours for lean and morbidly obese nulliparous women, respectively, and from 4.6 hours to 5.4 hours for lean and morbidly obese parous women, respectively. These findings were independent of gestational age and induction of labor. Entry into the active phase of labor was also delayed in parous women as a function of BMI. Possible explanations include increased fetal size, higher induction rates, and/or decreased responsiveness to oxytocin.62 Poor uterine contractility has also been demonstrated in obese parturients. Zhang et al.63 found that myometrium obtained from obese women at cesarean delivery contracted with less force and frequency and had less calcium flux than that from normal-weight women. This observation may be attributable to the inhibitory effect of cholesterol63 and/or adipokines (e.g., leptin, ghrelin, apelin), which were shown to inhibit human uterine contractility in vitro.64
Obesity is also associated with a higher risk for failed medical induction of labor. In a secondary analysis of data from a large labor induction trial involving 1273 patients (in which patients were stratified according to BMI), the duration of labor, oxytocin requirements, and cesarean delivery rates were significantly higher in women with a greater BMI.65 In a large series from Sweden involving 233,887 deliveries, Cedergren66 found a fourfold increase in the risk for cesarean delivery in parturients with a BMI greater than 40 kg/m2, primarily because of failed or obstructed labor, despite attempts at augmentation.
Operative vaginal delivery, with its associated maternal and fetal morbidity, is more likely in the obese parturient.18 One study examining maternal anthropometric parameters associated with shoulder dystocia reported a 2.7-fold increase in risk for shoulder dystocia in obese compared with lean parturients after adjustment for potential confounders such as macrosomia and diabetes.67 Risk for fetal macrosomia is also higher with obesity, which, in addition to increasing the risk for shoulder dystocia and its associated birth trauma, predisposes to perineal lacerations, newborn infant injury, and postpartum hemorrhage.18,67,68
Higher BMI, increased prepregnancy weight, and excessive maternal weight gain increase the risk for both elective and emergency cesarean delivery.69,70 This risk is further increased by obesity-related pregnancy complications such as macrosomia, fetal growth restriction (also known as intrauterine growth restriction), diabetes mellitus, and hypertensive disorders of pregnancy.55,71 In a meta-analysis of 33 trials,56 the unadjusted odds ratios (95% CI) of cesarean delivery were 1.46 (1.34 to 1.60), 2.05 (1.86 to 2.27), and 2.89 (2.28 to 3.79) among overweight, obese, and severely obese women, respectively, compared with normal-weight pregnant women.
Anesthetic Management
The high incidence of comorbid conditions among obese pregnant women necessitates early, careful preanesthetic assessment. There are also a number of technical matters that should be considered when caring for an obese parturient.
An appropriate-sized blood pressure cuff must be used for noninvasive blood pressure measurements. Unless the length of the sphygmomanometer cuff exceeds the circumference of the arm by 20%, systolic and diastolic blood pressure measurements may overestimate true maternal blood pressure. Forearm blood pressure measurement is sometimes used if an appropriate-sized blood pressure cuff is not available or if the upper arm cuff continues to slide from its position owing to the shape of the obese patient’s upper arm. There is a good correlation between upper arm and forearm noninvasive measurements, but forearm pressures exceed upper arm pressures by 10 ± 10 mm Hg (mean ± SD).72 In selected cases, invasive monitoring of blood pressure with an intra-arterial catheter may be desirable.57
Intravenous access may be difficult in some obese patients. Ultrasonographic guidance may be useful; however, if peripheral intravenous access remains unsuccessful, central venous cannulation may be necessary.
Appropriately sized labor beds, transportation gurneys, and operating tables, and sufficient personnel to assist with patient transport, are imperative. Although standard operating tables are generally rated for persons weighing up to 500 pounds (227 kg), this rating may be insufficient for morbidly obese patients, especially when the table is articulated. Regardless of the weight rating of the table, it is critical that the obese patient be centered over the operating table pedestal at all times. Special equipment for positioning the patient, and longer spinal/epidural needles, may be needed (see later discussion).
Labor and Vaginal Delivery
Options for analgesia are the same as those for nonobese patients. Using the McGill pain questionnaire, Melzack73 reported a positive correlation between BMI and the severity of labor pain. A later study, however, did not confirm these findings.74
Many of the options for labor analgesia have limitations in the obese parturient. For example, obese parturients with OSA may be more susceptible to the respiratory depressant effect of systemic opioids, leading to episodes of apnea and oxyhemoglobin desaturation. Pudendal nerve block may be more difficult technically in obese patients. Inhalation analgesia is useful in some patients; however, nitrous oxide has limited effectiveness and is not available in many birthing rooms. Further, inhalation analgesia may lead to loss of consciousness, which can be very dangerous in an obese woman with a difficult airway.
Neuraxial analgesia represents the best option for pain relief and is particularly desirable in the obese parturient. Given the greater risks for fetal macrosomia and shoulder dystocia in obese patients, adequate analgesia is often needed to facilitate an atraumatic vaginal delivery. The use of epidural analgesia during labor allows the anesthesia provider to extend epidural analgesia to surgical anesthesia for cesarean delivery and thus avoid the need for general anesthesia with its associated risks. Given the increased likelihood for cesarean delivery and the greater risks of general anesthesia in the obese parturient, the early administration of neuraxial labor analgesia is recommended in the obese parturient.
When performing a neuraxial anesthetic technique in the obese parturient, technical difficulties may include (1) inability to palpate the spinous processes or identify the midline75; (2) greater depth of the epidural space,76 which may exaggerate minor needle directional errors and increase the likelihood of identifying a lateral portion of the epidural space77; and (3) the presence of fat pockets as well as hormonal softening of the ligaments, which may result in a false loss of resistance and/or a higher risk for unintentional dural puncture.78 In the majority of obese parturients, however, the epidural space can be identified with a standard-length epidural needle.79 Therefore, it seems prudent to use a standard-length needle first, which allows the provider better control, before switching to a longer needle.
Observing the prominence of the seventh cervical vertebra and the gluteal cleft can facilitate identification of the midline. Asking the parturient about the perceived location of the needle during block placement (relative to the midline) can also facilitate identification of the midline. Marroquin et al.80 reported that 77% of morbidly obese parturients who were questioned about the position of the needle during a difficult labor epidural needle placement provided useful feedback to the anesthesia provider. Probing the subcutaneous tissue with a needle can also help identify the spinous processes and help identify a lumbar interspace.81 More objectively, ultrasonographic guidance can be used to identify the midline, image the epidural space, and measure the distance from the skin to the epidural space. Grau et al.82 reported that prepuncture ultrasonographic imaging significantly reduced the number of puncture sites and attempts and facilitated the performance of labor epidural analgesia. Similarly, Vallejo et al.83 reported that prepuncture ultrasonography (performed to determine the midline, correct needle direction, and distance from the skin to the epidural space) reduced the number of attempts and need for catheter replacements when inexperienced trainees performed the blocks. Balki et al.84 found a strong correlation between the depth of the epidural space measured by ultrasonography and that measured by the epidural needle in obese parturients. However, ultrasonographic imaging is more difficult in obese individuals; the same group of investigators reported that they were able to identify the midline but not measure the depth of the epidural space in a morbidly obese parturient with a BMI of 70 kg/m2.78 Furthermore, not all anesthesia providers are proficient in this technique.
Placing the patient in the sitting position facilitates identification of the midline and is preferred by many anesthesia providers when initiating a neuraxial anesthetic procedure in obese parturients. In the lateral position, gravity may cause lateral fat to sag down and obscure the midline. Bahar et al.85 reported that the risk for epidural venous cannulation was lower in the lateral head-down position than in the sitting position and that there was no difference between the two positions in the number of attempts required to locate the epidural space. However, the average BMI of subjects in their study was approximately 37 kg/m2.85 Further, the distance from the skin to the epidural space is minimized when the patient is in the sitting flexed position.86,87
Care is needed to avoid dislodgement of the epidural catheter after insertion. Hamilton et al.87 demonstrated that patient movement from the sitting-flexed to the lateral decubitus position causes redistribution of the soft tissue of the back. The distance from the skin to the epidural space increases, and an unsecured catheter will appear to be drawn inward by as much as 1.0 to 2.5 cm.87 An epidural catheter secured with tape to the back of a patient in the sitting flexed position can be unintentionally dislodged from the epidural space when the patient moves from the sitting to the lateral decubitus position. Movement of the epidural catheter relative to the skin is most striking in obese patients. Therefore, these investigators recommended that the patient assume the lateral position before the epidural catheter is secured to the skin.
Numerous authors have documented technical difficulties with neuraxial techniques in obese parturients. Hood and Dewan88 reported that 94% of patients who weighed more than 300 lb (136.4 kg) experienced adequate analgesia for delivery, compared with 98% of controls. More attempts were required to identify the epidural space in obese women, there was a significantly higher initial failure rate (42% versus 6%), and placement of a second or third epidural catheter was more often required. Similarly, Dresner et al.89 reported an increased risk for labor epidural block failure and need for catheter replacement with increasing BMI (2.4% for BMI < 25 kg/m2 versus 6.6 % for BMI > 40 kg/m2). Perlow et al.90 reported that 74.4% of morbidly obese parturients needed more than one attempt and 14% needed more than three attempts for successful epidural catheter placement. In contrast, Bamgdade et al.91 reported a significantly higher number of attempts to perform neuraxial anesthesia in obese parturients, but they observed no difference in the rate of failure of neuraxial anesthetic techniques for cesarean delivery in obese parturients. Although some investigators have reported a higher incidence of unintentional dural puncture in morbidly obese parturients than in lean parturients,92 others did not confirm those findings.74
It is not known if epidural local anesthetic dose requirements are altered in morbidly obese parturients. In 1980, Hodgkinson and Husain93 administered 20 mL of 0.75% epidural bupivacaine at the L3 to L4 interspace to women undergoing elective or emergency cesarean delivery over a period of 40 seconds. The patients remained supine for 40 minutes after drug injection. Twenty-seven percent of the patients with a BMI less than 28 kg/m2 needed supplementation of the block at 30 minutes, whereas none of the patients whose BMI exceeded 28 kg/m2 required additional local anesthetic to achieve surgical anesthesia. The cephalad extent of neuroblockade was associated with patient BMI and weight but not with height. Similarly, using an up-down sequential allocation study design to estimate the median effective epidural bupivacaine dose (administered in a volume of 20 mL), Panni and Columb94 found that obese women required significantly less epidural bupivacaine for initiation of labor analgesia than lean parturients.94 In contrast, Milligan et al.95 observed that neither patient position nor obesity affected the extent of sensory blockade when 12 mL of 0.25% bupivacaine was administered for epidural analgesia during labor.95 Duggan et al.96 found that obesity had only a weak effect in enhancing the spread of epidural local anesthetics; this effect was only observed with the largest volume and concentration used in the study (15 mL of bupivacaine 0.75%), but not with a lower volume (10 mL) and concentration (0.5%) of bupivacaine.
The goals of epidural labor analgesia should be the provision of excellent pain relief with minimal motor block. Epidural administration of a dilute solution of bupivacaine with fentanyl provides analgesia for labor while minimizing adverse effects such as hypotension and motor blockade. The neuroblockade provided to obese parturients in labor should be bilateral and near perfect. Otherwise, the epidural catheter should be removed and replaced because an inadequate block with a frequent need for top-up doses may lead to failure of extending the block for cesarean delivery.97 Regular evaluation of the parturient’s neuraxial block is essential. In a study of morbidly obese parturients, the initial administration of local anesthetic through the epidural catheter resulted in failure of analgesia/anesthesia in 42% of the women, a rate that was seven times higher than that in control parturients.88 However, careful evaluation of the epidural block and early replacement of a malpositioned catheter resulted in a high rate of success, so that only 1 of 55 cesarean deliveries attempted with epidural anesthesia required conversion to general anesthesia because of inadequate anesthesia.
The combined-spinal epidural (CSE) technique provides a rapid onset of excellent pain relief. However, the epidural catheter remains untested until the spinal block has worn off, and therefore it might fail to provide adequate anesthesia if it is malpositioned and a need for emergency cesarean delivery arises. For this reason, some anesthesia providers prefer to use a standard epidural technique when providing labor analgesia in morbidly obese parturients. On the other hand, a number of studies have reported a higher success rate for epidural catheters inserted as part of a CSE technique compared with those inserted as part of a standard epidural technique98,99; presumably, obtaining backflow of cerebrospinal fluid (CSF) through the spinal needle indirectly confirms correct epidural needle position.
In cases of unintentional dural puncture, continuous spinal analgesia can be used to provide labor analgesia. In addition to providing reliable labor analgesia, continuous spinal analgesia may be converted to spinal anesthesia for emergency cesarean delivery. It is crucial, however, that the catheter be clearly labeled and all personnel are made aware of its intrathecal location because unintentional administration of an epidural dose of a local anesthetic through the spinal catheter markedly increases the risk for high spinal block and subsequent respiratory arrest. The risk for post–dural puncture headache may be lower in the obese parturient. The higher intra-abdominal pressure that results from a large abdominal panniculus may contribute to reduced CSF leak through the dural puncture site.92
Cesarean Delivery
A thorough preanesthetic evaluation is critical to the safe care of the obese parturient. Of particular importance is a thorough airway assessment. Large breasts, the greater anteroposterior diameter of the chest, airway edema, and reduced chin-to-chest distance increase the likelihood of difficult laryngoscopy and failed tracheal intubation in obstetric patients.100 Obesity exaggerates many of the anatomic changes of pregnancy. Increased fat in the neck and shoulders increases the difficulty of positioning the patient for laryngoscopy and tracheal intubation. Excess fat deposition may also cause distorted anatomy, such as an enlarged tongue and redundant pharyngeal and palatal soft tissue. Further, the fat pads on the back of the shoulders often restrict the range of motion of the neck, exacerbating the difficulty of mask ventilation, laryngoscopy, and tracheal intubation.
All morbidly obese parturients undergoing cesarean delivery should be placed in a ramped position with left uterine displacement, regardless of the planned anesthetic technique. This position was shown to improve laryngoscopic view in morbidly obese patients undergoing elective bariatric surgery.101 Folded blankets or a padded ramp designed for this purpose are placed under the chest and head to achieve horizontal alignment between the external auditory meatus and the sternal notch (Figure 50-1).102 This position aligns the oral, pharyngeal, and tracheal axes to facilitate tracheal intubation and has been shown to improve hemodynamic and respiratory parameters during laparoscopic gastric bypass surgery.103 Modern surgical tables can also be flexed at a number of angles, and this feature can be used to optimize the patient position for laryngoscopy and tracheal intubation.
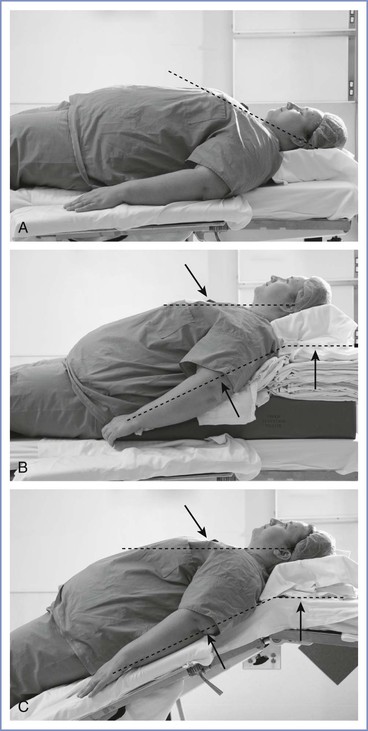
FIGURE 50-1 Positioning the obese patient to facilitate intubation of the trachea. A, Patient in the supine position using standard head support. Note that the dashed line (compare with B) is not parallel to the floor. B, Elevating the patient’s head with neck and shoulder supports so that an imaginary line drawn through the external auditory meatus and the sternal notch (upper dashed line) is parallel to the floor may facilitate tracheal intubation. C, Similar positioning achieved by repositioning the operating table. Note the similarity of the upper and lower dashed lines with use of the second and third positioning techniques.
It may be difficult to position the obese patient appropriately and safely. The protuberant abdomen may shift markedly with left uterine displacement. The patient must be secured to the operating table before the table is tilted leftward; however, it is important to initiate left uterine displacement as soon as possible. Tsueda et al.22 described two obese patients who experienced acute cardiovascular collapse after assuming the supine position.
The anesthesia provider should confirm that the patient’s weight does not exceed the weight limits of the operating table, consider use of lateral table extenders, and use foam or blankets to ensure that the shoulders and arms are positioned in a horizontal plane. Correct arm position will maximize patient comfort, improve stability, and avoid neurologic injury to the upper extremity.104
The anesthesia care team may be asked to participate in cephalad retraction of the large panniculus by tethering retractors to an object such as the ether screen. Both the obstetrician and the anesthesia provider must remain cognizant of the risks of hypotension, difficulty with ventilation, and fetal compromise during cephalad retraction of the panniculus in morbidly obese patients. Hodgkinson and Husain105 reported an intraoperative fetal death in a morbidly obese patient who had received epidural anesthesia for cesarean delivery. The death was attributed to prolonged hypotension associated with cephalad retraction of a large panniculus. A vertical and cephalad suspension of the panniculus has been suggested to avoid maternal hypotension and hypoxemia.78
Pharmacologic aspiration prophylaxis is crucial in this patient population. Oral administration of 30 mL of a 0.3 M solution of sodium citrate effectively increases gastric pH within 5 minutes.106 Administration of a histamine-2 (H2)-receptor antagonist and metoclopramide provide additional protection.107 The anesthesia provider must be aware that the patient remains at risk for aspiration at the end of surgery; the efficacy of sodium citrate wanes 45 to 60 minutes after administration.24
Neuraxial Techniques
Neuraxial anesthesia is the anesthetic technique of choice in the obese parturient. Single-shot spinal anesthesia provides a reliable, fast onset and dense neuroblockade, and it is a common anesthetic technique in nonobese parturients undergoing planned cesarean delivery. However, concerns about the use of single-shot spinal anesthesia in obese patients include technical difficulties, appropriate dosing, and insufficient duration of anesthesia.
Spinal anesthesia is technically feasible in morbidly obese pregnant women, although a longer spinal needle may be required. The distribution of adipose tissue varies among obese patients. Spinal needle placement can be uneventful in women who do not have excessive adipose tissue over the midline of the back. However, in others with excessive adipose tissue at the needle placement site, identification of the intrathecal space with a small-gauge spinal needle can be very challenging. Identification of the epidural space with a large-gauge epidural needle is often technically easier, and a needle-through-needle CSE technique may be easier to perform than a single-shot spinal technique in the morbidly obese parturient. Furthermore, the duration of surgery is often prolonged in the obese parturient.108 Therefore, single-shot spinal anesthesia may not have sufficient duration. Because intraoperative induction of general anesthesia is undesirable and potentially hazardous in morbidly obese pregnant women, a continuous neuraxial technique such as a CSE or epidural technique that allows the maintenance of neuraxial anesthesia with the epidural catheter is preferable to a single-shot spinal technique. Epidural anesthesia is preferred if the patient has a well-functioning epidural catheter in situ. Otherwise, a CSE technique may be preferable because it combines the reliability of the spinal block and the flexibility of epidural anesthesia. Anesthesia can be initiated with a low dose of intrathecal local anesthetic combined with an opioid (see Chapter 26). Insufficient extent of cephalad neuroblockade can be treated by administering additional local anesthetic via the epidural catheter. Continuous spinal anesthesia with a spinal catheter is also an option. Some anesthesiologists have suggested that this technique should be considered in the setting of emergency cesarean delivery for obese parturients because it may be technically easier to rapidly identify the spinal space with a large-gauge epidural needle than with a spinal needle.79
Local Anesthetic Dosing for Neuraxial Techniques.
The choice of local anesthetic dose in morbidly obese parturients in controversial. It has been a long-held belief that neuraxial local anesthetic doses should be reduced in obese patients because of fear of an unpredictable and exaggerated spread of local anesthetic, resulting in a high block. Magnetic resonance imaging (MRI) has confirmed that obese patients have a reduced lumbar CSF volume.109 Engorgement of the epidural veins secondary to compression of the inferior vena cava by the gravid uterus and abdominal panniculus, as well as inward movement of soft tissue through the intervertebral foramina as a result of increased abdominal pressure, may be responsible for the reduced CSF volume in these patients. A separate study using MRI demonstrated an inverse correlation between the cephalad extent of neuroblockade and lumbar CSF volume110; this finding suggests that reduced CSF volume in obese patients increases the risk for a high spinal block. Additionally, Greene111 has suggested that excess adipose tissue in the buttocks may result in relative Trendelenburg positioning of the vertebral column in the patient in the supine position, which can lead to an exaggerated cephalad spread of anesthesia.
A number of studies, however, do not seem to support the concerns of exaggerated cephalad spread of spinal anesthesia in morbidly obese women undergoing cesarean delivery. Norris112 and Hartwell et al.113 administered hyperbaric bupivacaine 12 mg intrathecally and found no correlation between height, weight, BMI, and the extent of spinal anesthesia. However, morbidly obese patients were not specifically studied, so these results may not be applicable to heavier parturients. In an up-down sequential allocation dose-finding study, Lee et al.114 reported that the estimated ED95 (effective dose in 95% of patients) for hyperbaric bupivacaine was similar between obese and nonobese patients; no patient had an excessively high cephalad block with bupivacaine doses up to 12 mg. More recently, Carvalho et al.115 estimated the ED50 (median effective dose) and ED95 for hyperbaric spinal bupivacaine for cesarean delivery in 42 morbidly obese parturients who were randomized to receive doses that ranged from 5 to 11 mg as part of a CSE technique. They reported that the ED50 and ED95 in these morbidly obese parturients were similar to those in nonobese parturients enrolled in a previous study performed by the same authors using similar methodology. Although it was common to obtain a satisfactory initial sensory level even with the lowest doses studied, few of the low-dose blocks were adequate for surgery and many required supplementation via the epidural catheter. Thus, the findings of these studies suggest that reducing the dose of intrathecal bupivacaine is not justified in morbidly obese patients and might increase the risk for inadequate anesthesia.
Results of studies of epidural local anesthetic dosing in morbidly obese parturients are also inconsistent (see earlier discussion). Unlike single-shot spinal anesthesia in which titration of the dose is not possible, careful titration of epidural anesthesia is recommended to achieve the desired dermatomal level of neuroblockade.
General Anesthesia
Difficult mask ventilation and tracheal intubation may be associated with more rapid oxyhemoglobin desaturation during apnea in obese patients than in lean individuals. The association between obesity and a short neck can make tracheal intubation difficult in this population.116 In a series of patients who received general anesthesia for cesarean delivery, the incidence of difficult tracheal intubation was 33% among women who weighed more than 300 lb (136.4 kg).88 Lee et al.117 reported their experience with difficult tracheal intubation in 284 morbidly obese patients who underwent gastric bypass surgery. The incidence of difficult tracheal intubation was 2.4% among patients between 1.5 and 1.75 times the ideal weight and tripled to 7.3% in patients whose weight was 1.75 to 2.0 times the ideal. In a meta-analysis of 35 studies (with 50,760 patients), Shiga et al.118 reported that obese patients had a threefold higher risk for difficult tracheal intubation than nonobese patients. In an analysis of 91,332 patients undergoing general anesthesia and direct laryngoscopy, a BMI of 35 kg/m2 or more was associated with a greater risk for difficult tracheal intubation (OR, 1.34; 95% CI, 1.19 to 1.51).119
The potential for failed tracheal intubation and difficult mask ventilation in the obese patient underscores the need for an experienced assistant during induction of general anesthesia. The primary anesthesia provider may fatigue rapidly with attempted mask ventilation of an obese patient. Further, the jaw-thrust maneuver may require the use of both hands, and additional personnel will be required to provide positive-pressure ventilation and cricoid pressure. A short-handled laryngoscope, assorted laryngoscope blades, various sizes of endotracheal tubes and supraglottic airway devices (e.g., laryngeal mask airways), a video laryngoscope, a fiberoptic intubation device, and equipment for percutaneous cricothyrotomy and transtracheal jet ventilation should be readily available.120 A failed tracheal intubation algorithm should be initiated and help should be called immediately in the event of failed tracheal intubation (see Chapter 30). Supraglottic airway devices may be lifesaving in these situations.
Awake tracheal intubation, via either video laryngoscopy or direct fiberoptic laryngoscopy, is an alternative method of securing the anticipated difficult airway. However, women requiring urgent performance of cesarean delivery may not be ideal candidates for awake tracheal intubation because of the lack of adequate time to optimally prepare the patient’s airway.
When preanesthetic assessment suggests that tracheal intubation will not be difficult, a rapid-sequence induction is indicated. The administration of general anesthesia begins with effective pulmonary denitrogenation (so-called preoxygenation). During apnea, pregnant women become hypoxemic more rapidly than nonpregnant women.121 Similarly, during apnea, obese patients become hypoxemic more rapidly than nonobese patients.122 Therefore, adequate denitrogenation is essential before the administration of general anesthesia in obese pregnant women.
One study demonstrated that four maximal inspirations of 100% oxygen within 30 seconds provide benefit similar to that provided by 3 minutes of tidal-volume breathing of 100% oxygen before rapid-sequence induction of general anesthesia for cesarean delivery.123 However, another study reported a more rapid onset of hypoxemia in patients who underwent four maximal inspirations of 100% oxygen than in similar patients who underwent 3 minutes of tidal-volume breathing of 100% oxygen.124 Goldberg et al.107 evaluated the use of both techniques in morbidly obese nonpregnant patients undergoing gastric bypass surgery. The techniques provided similar increases in PaO2, but patients who used the 3-minute technique showed evidence of a slight retention of CO2. The investigators speculated that a blunted ventilatory response to CO2 contributed to the increase in PaCO2. In contrast, other studies have suggested that obese patients have a normal ventilatory response to CO2.4 Later data from a study in 20 pregnant volunteers (at 36 to 38 weeks’ gestation) compared 3 minutes of tidal-volume breathing with four deep breaths in 30 seconds (4 DB) or eight deep breaths in 1 minute (8 DB) of 100% oxygen by measuring end-tidal fractional oxygen concentration (FETO2) after preoxygenation.125 An FETO2 value of 90% or greater was achieved in 76% of women after either the 3-minute or the 8-DB method, compared with only 18% of women after the 4-DB method of preoxygenation.125 The investigators concluded that for emergency cesarean delivery using general anesthesia, the 8-DB method was as effective as the 3-minute tidal volume method of preoxygenation and was more quickly performed.
It is wise to apply a tight-fitting face mask and administer 100% oxygen as soon as the patient is moved onto the operating table; this maneuver helps achieve denitrogenation while other preparations are being made. It then seems reasonable to let circumstances dictate the selected method of denitrogenation. When the 3-minute tidal volume breathing technique is selected, the anesthesia provider should encourage the patient to take several deep breaths. In urgent situations, such as emergency cesarean delivery for maternal hemorrhage, the patient should be instructed to take eight deep breaths (the 8-DB method) to extend the safe interval before oxyhemoglobin desaturation occurs. When time allows, the 8-DB method is preferred over the 4-DB method.
The dose of intravenous induction agents should not be based on total body weight. Rather, the induction dose of propofol or thiopental in the obese parturient should be based on the lean body weight.126 This practice will avoid excessive doses of drug with subsequent adverse effects. Propofol 2 to 2.8 mg/kg, thiopental 4 to 5 mg/kg, or other induction agents can be used according to the clinical circumstances and the anesthesia provider preference. All these agents will cross the placenta and can affect the neonate.
Succinylcholine remains the muscle relaxant of choice for rapid-sequence induction, and doses of 1.0 to 1.5 mg/kg are commonly used for general anesthesia and tracheal intubation in obese parturients. Lemmens et al.127 compared the efficacy of succinylcholine in dosing regimens of 1 mg/kg based on ideal body weight, lean body weight, and total body weight in morbidly obese nonpregnant patients. The third regimen (1 mg/kg total body weight) was superior for providing complete neuromuscular paralysis and predictable laryngoscopic conditions in almost every patient, whereas intubating conditions were poor in one third of the patients dosed according to ideal body weight. The investigators also noted that “none of these dosing regimens will provide…a safe (short) duration of apnea.”
Rocuronium at a dose of 1 to 1.2 mg/kg provides equivalent intubating conditions to succinylcholine 1 mg/kg. However, this dose range is associated with a significantly prolonged duration of neuromuscular blockade.128 In obese patients, the dose of rocuronium should be based on ideal body weight.129 Sugammadex is a cyclodextrin molecule that has been available in Europe since 2008. Sugammadex will effectively reverse high-dose rocuronium (1.2 mg/kg) in 2 minutes when used in a dose of 12 mg/kg in surgical patients.130 Recent small series in obstetric patients have reported the use of sugammadex after rocuronium doses of 0.6 mg/kg,131 1 mg/kg,132 and 1.2 mg/kg,133 with no major adverse maternal or neonatal effects. Two studies in nonpregnant obese patients suggested that sugammadex dosing can be based on ideal body weight plus 40%134 or on corrected body weight (ideal body weight + 0.4[total body weight − ideal body weight]).135 However, its use has not been specifically studied in obese parturients and its safety in this patient population remains to be confirmed.
Maintenance of anesthesia is usually achieved with a volatile halogenated agent with or without nitrous oxide. No evidence suggests that obesity alters the minimum alveolar concentration (MAC) of volatile halogenated anesthetic agents in pregnant women. In theory, increased body fat serves as a reservoir for inhalational and intravenous agents. Likewise, the body fat reservoir could increase the threat of biotransformation of volatile halogenated agents, which would increase the risk for organ toxicity. Isoflurane is an appropriate choice for morbidly obese parturients owing to its limited biotransformation.136 Desflurane and sevoflurane are associated with a shorter time to extubation than isoflurane in obese patients, although the difference may not be clinically relevant.137,138
Morbidly obese patients may require administration of a higher inspired oxygen concentration and may not tolerate usual concentrations of nitrous oxide. Moreover, general anesthesia reduces FRC. The supine and Trendelenburg positions further decrease FRC, thus increasing the risk for intraoperative hypoxemia. The following strategies have been recommended to reduce the risk for small airway closure, atelectasis, and hypoxemia in obese patients: (1) use of inspired oxygen concentration of less than 0.8, (2) ventilation with tidal volumes ranging from 6 to 10 mL/kg ideal body weight, (3) increasing the respiratory rate to maintain physiologic PaCO2, (4) use of manual or automated periodic lung inflation (i.e., a recruitment maneuver), and (5) application of positive end-expiratory pressure.139
Emergence from general anesthesia is a critical period. Indeed, maternal deaths from hypoventilation and airway obstruction have been reported during emergence and recovery from general anesthesia.59 It is imperative that the patient is fully awake with complete reversal of neuromuscular blockade before tracheal extubation, which preferably should be performed in the semi-upright position to minimize diaphragmatic compression by abdominal viscera. Trained personnel should provide postoperative care before the discharge of the patient to the ward.
Postoperative Complications
Obesity increases the risk for postoperative complications such as endometritis, urinary tract infection, wound infection, wound dehiscence, peripheral nerve injury, hemorrhage, deep vein thrombosis, pulmonary thromboembolism, atelectasis, pneumonia, respiratory depression, hypoxemia, tracheal reintubation, sleep apnea, myocardial infarction, cardiac arrest, and maternal death.79,140
The combination of obesity, OSA, general anesthesia, and opioid administration may increase the risk for opioid-induced respiratory depression. In a report on maternal mortality in Michigan, Mhyre et al.59 found that all anesthesia-related maternal deaths from airway obstruction or hypoventilation occurred during emergence and recovery, with obesity being identified as a significant risk factor for these complications. The American Society of Anesthesiologists (ASA) has issued Practice Guidelines for the Perioperative Management of Patients with Obstructive Sleep Apnea, which include recommendations for the preoperative, intraoperative, and postoperative management of these patients.141 Although the published literature is often insufficient to establish definite relationships between interventions and outcomes, the Task Force consultants made the recommendations listed in Box 50-1. These recommendations are not intended specifically for pregnant women, but they do provide some guidance for the care of the obese parturient with OSA undergoing cesarean delivery. Morbidly obese patients at risk for OSA who have undergone cesarean delivery should be monitored with continuous pulse oximetry after being discharged from the postanesthesia care unit.
Postoperative Care
Postoperative Analgesia
Adequate postoperative analgesia is crucial to facilitate early mobilization, thereby reducing the risk for thromboembolic and pulmonary complications. However, the ideal analgesic regimen in the obese parturient remains unclear. Neuraxial morphine has been shown to provide superior analgesia to that provided by parenteral and oral opioids after cesarean delivery, at the expense of increased opioid-related side effects such as pruritus and nausea.142,143 There is a concern, however, that morbidly obese patients might be at higher risk for respiratory depression after neuraxial opioid administration, although there are few data addressing this issue. In a series of 856 patients who received intrathecal morphine 0.2 mg added to hyperbaric bupivacaine for cesarean delivery, Abouleish144 reported that respiratory depression, defined as an SaO2 of 85% or less and/or a respiratory rate of 10 breaths per minute or less, occurred in 8 patients, all of whom were obese. In patients who have received general anesthesia, postoperative opioids are best administered using an intravenous patient-controlled analgesia (PCA) system. With all routes of opioid administration, vigilance and appropriate postoperative monitoring are essential in the obese patient who might have undiagnosed OSA and may be at a higher risk for opioid-induced respiratory depression.
A multimodal analgesic regimen including regular administration of a nonsteroidal anti-inflammatory drug and acetaminophen may contribute to opioid sparing and improve postoperative analgesia (see Chapter 27). Local anesthetic techniques including wound infiltration and transversus abdominis plane (TAP) block may also be valuable for postoperative analgesia. A systematic review of randomized controlled trials (including both obstetric and nonobstetric patients) found that continuous infusion of local anesthetic through catheters placed at the incisional site led to an improvement in analgesia and patient satisfaction as well as a reduction in opioid use, side effects, and duration of hospital stay, in comparison with administration of systemic opioids alone.145 However, the value of this technique combined with neuraxial administration of a long-acting opioid is unclear.
Similarly, TAP block was found to produce opioid sparing and reduce pain scores and opioid-related side effects in women who received general anesthesia or did not receive neuraxial morphine as part of their neuraxial anesthetic technique. However, it did not enhance analgesia in patients who received neuraxial morphine and produced analgesia inferior to that produced by neuraxial morphine.146 Further, performance of TAP block may be technically challenging in the obese parturient with a large abdominal panniculus. Patient-controlled epidural analgesia (PCEA) with ropivacaine provides analgesia comparable to that provided by epidural morphine,147 but with more motor block, thus delaying patient mobilization.147 PCEA techniques have not been specifically studied in obese parturients.
Thromboprophylaxis
The American College of Chest Physicians published guidelines for antithrombotic therapy in the parturient.148 They used major and minor risk factors to identify women at increased risk for venous thromboembolism after cesarean delivery, and they classified obesity (BMI > 30 kg/m2) as a minor risk factor. Thromboprophylaxis was recommended in the presence of one major risk factor (risk for thromboembolism > 3%) or two minor risk factors (combined risk > 3%) (see Box 39-4). In the setting of emergency cesarean delivery, one minor risk factor results in a risk greater than 3%, and, therefore, mechanical or pharmacologic thromboprophylaxis is recommended. In the United Kingdom, the RCOG guidelines58 recommend thromboprophylaxis with low-molecular-weight heparin for all obese parturients undergoing either emergency or elective cesarean delivery. Further, the RCOG guidelines58 recommend thromboprophylaxis after vaginal delivery in all morbidly obese women.
References
1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497.
2. Lafortuna CL, Agosti F, Galli R, et al. The energetic and cardiovascular response to treadmill walking and cycle ergometer exercise in obese women. Eur J Appl Physiol. 2008;103:707–717.
3. Dempsey JA, Reddan W, Balke B, Rankin J. Work capacity determinants and physiologic cost of weight-supported work in obesity. J Appl Physiol. 1966;21:1815–1820.
4. Lourenco RV. Diaphragm activity in obesity. J Clin Invest. 1969;48:1609–1614.
5. Babb TG, Ranasinghe KG, Comeau LA, et al. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008;178:116–123.
6. Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–210.
7. Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–660.
8. Eng M, Butler J, Bonica JJ. Respiratory function in pregnant obese women. Am J Obstet Gynecol. 1975;123:241–245.
9. Holley HS, Milic-Emili J, Becklake MR, Bates DV. Regional distribution of pulmonary ventilation and perfusion in obesity. J Clin Invest. 1967;46:475–481.
10. Tanoubi I, Drolet P, Donati F. Optimizing preoxygenation in adults. Can J Anaesth. 2009;56:449–466.
11. Lee J, Larsen R, Buckley J, Roberts R. Pulmonary function and its correlation to the degree of obesity of 294 patients. Anesthesiol Rev. 1981;8:28–32.
12. Vaughan RW, Cork RC, Hollander D. The effect of massive weight loss on arterial oxygenation and pulmonary function tests. Anesthesiology. 1981;54:325–328.
13. Veille JC, Hanson R. Obesity, pregnancy, and left ventricular functioning during the third trimester. Am J Obstet Gynecol. 1994;171:980–983.
14. Messerli FH, Nunez BD, Ventura HO, Snyder DW. Overweight and sudden death: increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987;147:1725–1728.
15. Vasan RS. Cardiac function and obesity. Heart. 2003;89:1127–1129.
16. de Simone G, Devereux RB, Mureddu GF, et al. Influence of obesity on left ventricular midwall mechanics in arterial hypertension. Hypertension. 1996;28:276–283.
17. Paul DR, Hoyt JL, Boutros AR. Cardiovascular and respiratory changes in response to change of posture in the very obese. Anesthesiology. 1976;45:73–78.
18. Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and Cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol. 2004;190:1091–1097.
19. Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236.
20. Pantanowitz L. Fat infiltration in the heart. Heart. 2001;85:253.
21. Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61:36–48.
22. Tsueda K, Debrand M, Zeok SS, et al. Obesity supine death syndrome: reports of two morbidly obese patients. Anesth Analg. 1979;58:345–347.
23. Vaughan RW, Bauer S, Wise L. Volume and pH of gastric juice in obese patients. Anesthesiology. 1975;43:686–689.
24. Dewan DM, Floyd HM, Thistlewood JM, et al. Sodium citrate pretreatment in elective Cesarean section patients. Anesth Analg. 1985;64:34–37.
25. Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859–868.
26. Harter RL, Kelly WB, Kramer MG, et al. A comparison of the volume and pH of gastric contents of obese and lean surgical patients. Anesth Analg. 1998;86:147–152.
27. Lam AM, Grace DM, Penny FJ, Vezina WC. Prophylactic intravenous cimetidine reduces the risk of acid aspiration in morbidly obese patients. Anesthesiology. 1986;65:684–687.
28. Wisen O, Hellstrom PM. Gastrointestinal motility in obesity. J Intern Med. 1995;237:411–418.
29. Maltby JR, Pytka S, Watson NC, et al. Drinking 300 mL of clear fluid two hours before surgery has no effect on gastric fluid volume and pH in fasting and non-fasting obese patients. Can J Anaesth. 2004;51:111–115.
30. Wong CA, McCarthy RJ, Fitzgerald PC, et al. Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007;105:751–755.
31. Eslick GD. Gastrointestinal symptoms and obesity: a meta-analysis. Obes Rev. 2012;13:469–479.
32. Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–284.
33. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
34. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
35. Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19.
36. Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85–101.
37. Davi G, Guagnano MT, Ciabattoni G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014.
38. Arfvidsson B, Eklof B, Balfour J. Iliofemoral venous pressure correlates with intraabdominal pressure in morbidly obese patients. Vasc Endovascular Surg. 2005;39:505–509.
39. Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289.
40. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143.
41. Farah N, Hogan AE, O’Connor N, et al. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine. 2012;60:96–99.
42. Fedorcsak P, Dale PO, Storeng R, et al. The impact of obesity and insulin resistance on the outcome of IVF or ICSI in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:1086–1091.
43. Worda C, Leipold H, Gruber C, et al. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191:2120–2124.
44. Fung AM, Wilson DL, Barnes M, Walker SP. Obstructive sleep apnea and pregnancy: the effect on perinatal outcomes. J Perinatol. 2012;32:399–406.
45. Maasilta P, Bachour A, Teramo K, et al. Sleep-related disordered breathing during pregnancy in obese women. Chest. 2001;120:1448–1454.
46. Bende M, Gredmark T. Nasal stuffiness during pregnancy. Laryngoscope. 1999;109:1108–1110.
48. Perez-Chada D, Videla AJ, O’Flaherty ME, et al. Snoring, witnessed sleep apnoeas and pregnancy-induced hypertension. Acta Obstet Gynecol Scand. 2007;86:788–792.
49. Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36:849–855.
50. Sahin FK, Koken G, Cosar E, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet. 2008;100:141–146.
51. Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202:552 e1–7.
52. Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085–1092.
53. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229.
54. Chu SY, Bachman DJ, Callaghan WM, et al. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008;358:1444–1453.
55. Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224.
56. Chu SY, Kim SY, Schmid CH, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8:385–394.
57. Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mother’s lives: reviewing maternal deaths to make motherhood safer -2003-2005. [The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom] CEMACH: London; 2007.
58. Royal College of Obstetricians and Gynaecologists. Reducing the risk of thrombosis and embolism during pregnancy and the puerperium. [Green-top Guideline No. 37a. Available at] http://www.rcog.org.uk/womens-health/clinical-guidance/reducing-risk-of-thrombosis-greentop37a [Accessed March 2013] .
59. Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
60. Modder J, Fitzsimons KJ. The Centre for Maternal and Child Enquiries (CMACE) and the Royal College of Obstetricians and Gynaecologists (RCOG). CMACE/RCOG Joint Guideline. Management of Women with Obesity in Pregnancy. [Available at] http://www.oaa-anaes.ac.uk/assets/_managed/editor/File/Reports/2010_CMACE-RCOG_guideline_obesity_in_pregnancy.pdf [Accessed March 2013] .
61. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. [ACOG Committee Opinion No. 315. Washington, DC, September 2005] Obstet Gynecol. 2005;106:671–675.
62. Kominiarek MA, Zhang J, Vanveldhuisen P, et al. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205:244 e1–8.
63. Zhang J, Bricker L, Wray S, Quenby S. Poor uterine contractility in obese women. BJOG. 2007;114:343–348.
64. Hehir MP, Morrison JJ. The adipokine apelin and human uterine contractility. Am J Obstet Gynecol. 2012;206:359 e1–5.
65. Pevzner L, Powers BL, Rayburn WF, et al. Effects of maternal obesity on duration and outcomes of prostaglandin cervical ripening and labor induction. Obstet Gynecol. 2009;114:1315–1321.
66. Cedergren MI. Non-elective caesarean delivery due to ineffective uterine contractility or due to obstructed labour in relation to maternal body mass index. Eur J Obstet Gynecol Reprod Biol. 2009;145:163–166.
67. Mazouni C, Porcu G, Cohen-Solal E, et al. Maternal and anthropomorphic risk factors for shoulder dystocia. Acta Obstet Gynecol Scand. 2006;85:567–570.
68. Johnson JW, Longmate JA, Frentzen B. Excessive maternal weight and pregnancy outcome. Am J Obstet Gynecol. 1992;167:353–370.
69. Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182.
70. Crane SS, Wojtowycz MA, Dye TD, et al. Association between pre-pregnancy obesity and the risk of Cesarean delivery. Obstet Gynecol. 1997;89:213–216.
71. LaCoursiere DY, Bloebaum L, Duncan JD, Varner MW. Population-based trends and correlates of maternal overweight and obesity, Utah 1991-2001. Am J Obstet Gynecol. 2005;192:832–839.
72. Pierin AM, Alavarce DC, Gusmao JL, et al. Blood pressure measurement in obese patients: comparison between upper arm and forearm measurements. Blood Press Monit. 2004;9:101–105.
73. Melzack R, Kinch R, Dobkin P, et al. Severity of labour pain: influence of physical as well as psychologic variables. Can Med Assoc J. 1984;130:579–584.
74. Ranta P, Jouppila P, Spalding M, Jouppila R. The effect of maternal obesity on labour and labour pain. Anaesthesia. 1995;50:322–326.
75. Ellinas EH, Eastwood DC, Patel SN, et al. The effect of obesity on neuraxial technique difficulty in pregnant patients: a prospective, observational study. Anesth Analg. 2009;109:1225–1231.
76. D’Alonzo RC, White WD, Schultz JR, et al. Ethnicity and the distance to the epidural space in parturients. Reg Anesth Pain Med. 2008;33:24–29.
77. Clinkscales CP, Greenfield ML, Vanarase M, Polley LS. An observational study of the relationship between lumbar epidural space depth and body mass index in Michigan parturients. Int J Obstet Anesth. 2007;16:323–327.
78. Whitty RJ, Maxwell CV, Carvalho JC. Complications of neuraxial anesthesia in an extreme morbidly obese patient for Cesarean section. Int J Obstet Anesth. 2007;16:139–144.
79. Soens MA, Birnbach DJ, Ranasinghe JS, van Zundert A. Obstetric anesthesia for the obese and morbidly obese patient: an ounce of prevention is worth more than a pound of treatment. Acta Anaesthesiol Scand. 2008;52:6–19.
80. Marroquin BM, Fecho K, Salo-Coombs V, Spielman FJ. Can parturients identify the midline during neuraxial block placement? J Clin Anesth. 2011;23:3–6.
81. Maitra AM, Palmer SK, Bachhuber SR, Abram SE. Continuous epidural analgesia for Cesarean section in a patient with morbid obesity. Anesth Analg. 1979;58:348–349.
82. Grau T, Leipold RW, Conradi R, et al. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth. 2002;14:169–175.
83. Vallejo MC, Phelps AL, Singh S, et al. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19:373–378.
84. Balki M, Lee Y, Halpern S, Carvalho JC. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg. 2009;108:1876–1881.
85. Bahar M, Chanimov M, Cohen ML, et al. The lateral recumbent head-down position decreases the incidence of epidural venous puncture during catheter insertion in obese parturients. Can J Anaesth. 2004;51:577–580.
86. Hamza J, Smida M, Benhamou D, Cohen SE. Parturient’s posture during epidural puncture affects the distance from skin to epidural space. J Clin Anesth. 1995;7:1–4.
87. Hamilton CL, Riley ET, Cohen SE. Changes in the position of epidural catheters associated with patient movement. Anesthesiology. 1997;86:778–784.
88. Hood DD, Dewan DM. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210–1218.
89. Dresner M, Brocklesby J, Bamber J. Audit of the influence of body mass index on the performance of epidural analgesia in labour and the subsequent mode of delivery. BJOG. 2006;113:1178–1181.
90. Perlow JH, Morgan MA. Massive maternal obesity and perioperative cesarean morbidity. Am J Obstet Gynecol. 1994;170:560–565.
91. Bamgbade OA, Khalaf WM, Ajai O, et al. Obstetric anaesthesia outcome in obese and non-obese parturients undergoing caesarean delivery: an observational study. Int J Obstet Anesth. 2009;18:221–225.
92. Faure E, Moreno R, Thisted R. Incidence of postdural puncture headache in morbidly obese parturients. Reg Anesth. 1994;19:361–363.
93. Hodgkinson R, Husain FJ. Obesity and the cephalad spread of analgesia following epidural administration of bupivacaine for Cesarean section. Anesth Analg. 1980;59:89–92.
94. Panni MK, Columb MO. Obese parturients have lower epidural local anaesthetic requirements for analgesia in labour. Br J Anaesth. 2006;96:106–110.
96. Duggan J, Bowler GM, McClure JH, Wildsmith JA. Extradural block with bupivacaine: influence of dose, volume, concentration and patient characteristics. Br J Anaesth. 1988;61:324–331.
97. Bauer ME, Kountanis JA, Tsen LC, et al. Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: a systematic review and meta-analysis of observational trials. Int J Obstet Anesth. 2012;21:294–309.
98. Norris MC. Are combined spinal-epidural catheters reliable? Int J Obstet Anesth. 2000;9:3–6.
99. Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveries. Int J Obstet Anesth. 2004;13:227–233.
100. Honarmand A, Safavi MR. Prediction of difficult laryngoscopy in obstetric patients scheduled for Caesarean delivery. Eur J Anaesthesiol. 2008;1–7.
101. Brodsky JB, Lemmens HJ, Brock-Utne JG, et al. Anesthetic considerations for bariatric surgery: proper positioning is important for laryngoscopy. Anesth Analg. 2003;96:1841–1842.
102. Brodsky JB, Lemmens HJ, Brock-Utne JG, et al. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–736.
103. Artuso D, Wayne M, Cassaro S, et al. Hemodynamic changes during laparoscopic gastric bypass procedures. Arch Surg. 2005;140:289–292.
104. Brunette KE, Hutchinson DO, Ismail H. Bilateral brachial plexopathy following laparoscopic bariatric surgery. Anaesth Intensive Care. 2005;33:812–815.
105. Hodgkinson R, Husain FJ. Caesarean section associated with gross obesity. Br J Anaesth. 1980;52:919–923.
106. O’Sullivan GM, Bullingham RE. Noninvasive assessment by radiotelemetry of antacid effect during labor. Anesth Analg. 1985;64:95–100.
107. Goldberg ME, Norris MC, Larijani GE, et al. Preoxygenation in the morbidly obese: a comparison of two techniques. Anesth Analg. 1989;68:520–522.
108. Butwick A, Carvalho B, Danial C, Riley E. Retrospective analysis of anesthetic interventions for obese patients undergoing elective cesarean delivery. J Clin Anesth. 2010;22:519–526.
109. Hogan QH, Prost R, Kulier A, et al. Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology. 1996;84:1341–1349.
110. Carpenter RL, Hogan QH, Liu SS, et al. Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology. 1998;89:24–29.
111. Greene NM. Distribution of local anesthetic solutions within the subarachnoid space. Anesth Analg. 1985;64:715–730.
112. Norris MC. Height, weight, and the spread of subarachnoid hyperbaric bupivacaine in the term parturient. Anesth Analg. 1988;67:555–558.
113. Hartwell BL, Aglio LS, Hauch MA, Datta S. Vertebral column length and spread of hyperbaric subarachnoid bupivacaine in the term parturient. Reg Anesth. 1991;16:17–19.
114. Lee Y, Balki M, Parkes R, Carvalho JC. Dose requirement of intrathecal bupivacaine for cesarean delivery is similar in obese and normal weight women. Rev Bras Anestesiol. 2009;59:674–683.
115. Carvalho B, Collins J, Drover DR, et al. ED50 and ED95 of intrathecal bupivacaine in morbidly obese patients undergoing cesarean delivery. Anesthesiology. 2011;114:529–535.
116. Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;77:67–73.
117. Lee J, Larson R, Buckley J, Roberts R. Airway maintenance in the morbidly obese. Anesthesiol Rev. 1980;7:33–36.
118. Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology. 2005;103:429–437.
119. Lundstrøm LH, Møller AM, Rosenstock C, et al. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology. 2009;110:266–274.
120. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia. Anesthesiology. 2007;106:843–863.
121. Byrne F, Oduro-Dominah A, Kipling R. The effect of pregnancy on pulmonary nitrogen washout: a study of pre-oxygenation. Anaesthesia. 1987;42:148–150.
122. Berthoud MC, Peacock JE, Reilly CS. Effectiveness of preoxygenation in morbidly obese patients. Br J Anaesth. 1991;67:464–466.
123. Norris MC, Dewan DM. Preoxygenation for Cesarean section: a comparison of two techniques. Anesthesiology. 1985;62:827–829.
124. Gambee AM, Hertzka RE, Fisher DM. Preoxygenation techniques: comparison of three minutes and four breaths. Anesth Analg. 1987;66:468–470.
125. Chiron B, Laffon M, Ferrandiere M, et al. Standard preoxygenation technique versus two rapid techniques in pregnant patients. Int J Obstet Anesth. 2004;13:11–14.
126. Ingrande J, Lemmens HJM. Dose adjustment of anaesthetics in the morbidly obese. Br J Anaesth. 2010;105(Suppl 1):i16–i23.
127. Lemmens HJ, Brodsky JB. The dose of succinylcholine in morbid obesity. Anesth Analg. 2006;102:438–442.
128. Perry JJ, Lee JS, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2008;(2).
129. Meyhoff CS, Lund J, Jenstrup MT, et al. Should dosing of rocuronium in obese patients be based on ideal or corrected body weight? Anesth Analg. 2009;109:787–792.
130. Puhringer FK, Rex C, Sielenkamper AW, et al. Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points: an international, multicenter, randomized, dose-finding, safety assessor-blinded, phase II trial. Anesthesiology. 2008;109:188–197.
131. Puhringer FK, Kristen P, Rex C. Sugammadex reversal of rocuronium-induced neuromuscular block in Caesarean section patients: a series of seven cases. Br J Anaesth. 2010;105:657–660.
132. Nauheimer D, Kollath C, Geldner G. Modified rapid sequence induction for Caesarean sections: case series on the use of rocuronium and sugammadex. Anaesthesist. 2012.
133. Williamson RM, Mallaiah S, Barclay P. Rocuronium and sugammadex for rapid sequence induction of obstetric general anaesthesia. Acta Anaesthesiol Scand. 2011;55:694–699.
134. Van Lancker P, Dillemans B, Bogaert T, et al. Ideal versus corrected body weight for dosage of sugammadex in morbidly obese patients. Anaesthesia. 2011;66:721–725.
135. Gaszynski T, Szewczyk T, Gaszynski W. Randomized comparison of sugammadex and neostigmine for reversal of rocuronium-induced muscle relaxation in morbidly obese undergoing general anaesthesia. Br J Anaesth. 2012;108:236–239.
136. Carpenter RL, Eger EI 2nd, Johnson BH, et al. The extent of metabolism of inhaled anesthetics in humans. Anesthesiology. 1986;65:201–205.
137. Torri G, Casati A, Comotti L, et al. Wash-in and wash-out curves of sevoflurane and isoflurane in morbidly obese patients. Minerva Anestesiol. 2002;68:523–527.
138. Juvin P, Vadam C, Malek L, et al. Postoperative recovery after desflurane, propofol, or isoflurane anesthesia among morbidly obese patients: a prospective, randomized study. Anesth Analg. 2000;91:714–719.
139. Pelosi P, Gregoretti C. Perioperative management of obese patients. Best Pract Res Clin Anaesthesiol. 2010;24:211–225.
140. Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109:419–433.
141. Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.
142. Bonnet MP, Mignon A, Mazoit JX, et al. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain. 2010;14:894 e1–9.
143. McDonnell NJ, Paech MJ, Browning RM, Nathan EA. A randomised comparison of regular oral oxycodone and intrathecal morphine for post-caesarean analgesia. Int J Obstet Anesth. 2010;19:16–23.
144. Abouleish E, Rawal N, Rashad MN. The addition of 0.2 mg subarachnoid morphine to hyperbaric bupivacaine for cesarean delivery: a prospective study of 856 cases. Reg Anesth. 1991;16:137–140.
146. Mishriky BM, George RB, Habib AS. Transversus abdominis plane block for analgesia after Cesarean delivery: a systematic review and meta-analysis. Can J Anaesth. 2012;59:766–778.
147. Chen LK, Lin PL, Lin CJ, et al. Patient-controlled epidural ropivacaine as a post-Cesarean analgesia: a comparison with epidural morphine. Taiwan J Obstet Gynecol. 2011;50:441–446.
148. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e691S–736S.