Nonobstetric Surgery During Pregnancy
Marc Van de Velde MD, PhD
Chapter Outline
Estimates of the frequency of nonobstetric surgery performed during pregnancy range from 0.3% to 2.2%.1,2 Thus, as many as 93,000 and 110,000 pregnant women in the United States and the European Union, respectively, may require a surgical or anesthetic intervention each year. These numbers are likely to be an underestimation, because pregnancy may be unrecognized at the time of operation. The reported incidence of positive pregnancy tests in women of childbearing age ranged from 0.002% in women presenting for orthopedic surgery3 to 0.3% in women presenting to an ambulatory surgery center4 to 2.6% in women scheduled to undergo elective sterilization procedures.5
The practice of routine pregnancy testing for all women of childbearing age presenting for elective surgery and anesthesia is controversial. The American Society of Anesthesiologists Task Force on Preanesthesia Evaluation states “… the literature is inadequate to inform patients or physicians on whether anesthesia causes harmful effect in early pregnancy. Pregnancy testing may be offered to female patients of childbearing age and for whom the result would change the patient’s management.”6 In 2000, the U.K. National Institute for Health and Clinical Excellence (NICE) issued preoperative screening guidelines recommending routine pregnancy testing for “female patients who say that it is possible they may be pregnant.”7 There was uncertainty among the Guideline Development Group as to whether women should undergo pregnancy testing if the last menstrual period was documented or if women stated that it was not possible that they were pregnant.7 This guideline was poorly followed, resulting in 42 serious incidents, 3 pregnancy losses, and several cases of litigation between 2003 and 2009.8 In 2010, the U.K. National Patient Safety Agency stated that women should be offered a pregnancy test “if there is any possibility that a woman could be pregnant.”8 In certain populations, medical history alone may be an unreliable means of excluding the possibility of pregnancy.6,7 Thus, some institutions routinely perform pregnancy tests on all women of childbearing age who present for elective surgery.3
Surgery may be necessary during any stage of pregnancy. Among 5405 Swedish women who had operations during pregnancy, 42% occurred during the first trimester, 35% during the second trimester, and 23% during the third trimester.1 Laparoscopy for gynecologic indications was the most common first-trimester procedure (34%), whereas appendectomy was the most common procedure during the remainder of pregnancy. Indications for pregnancy-related surgery include cervical incompetence, the presence of ovarian cysts, and conditions amenable to fetal surgery (see Chapter 7). Indications for non–pregnancy-related surgery include the presence of acute abdominal disease (most commonly appendicitis and cholecystitis), malignancies, and trauma.
When caring for pregnant women undergoing nonobstetric surgery, anesthesia providers must consider both the mother and the fetus. Standard anesthetic procedures may have to be modified to accommodate pregnancy-induced maternal physiologic changes and the presence of the fetus. The Confidential Enquiries into Maternal and Child Health in the United Kingdom demonstrate that, even in early pregnancy, mothers die of hemorrhage, sepsis, thromboembolism, and anesthesia; substandard care is often present.9 Erekson et al.10 analyzed 2005 to 2009 data from the American College of Surgeons National Surgical Quality Improvement Program database. The rate of major complications (e.g., infections, reoperation, wound problems, respiratory complications, venous thromboembolism, blood transfusion, maternal death) for antenatal nonobstetric surgery was approximately 6%.10
Possible risks to the fetus of antenatal surgery include (1) the effects of the disease process itself, or related therapy; (2) the teratogenicity of anesthetic agents or other drugs administered during the perioperative period; (3) intraoperative perturbations of uteroplacental perfusion and/or fetal oxygenation; and (4) the risk for abortion or preterm delivery.
Maternal Safety: Altered Maternal Physiology
During pregnancy, profound changes in maternal physiology result from increased concentrations of various hormones, mechanical effects of the gravid uterus, greater metabolic demand, and the hemodynamic consequences of the low-pressure placental circulation. Hormonal changes are likely responsible for most of the changes that occur during the first trimester. Mechanical effects become apparent when the uterus emerges from the pelvis during the second half of gestation (see Chapter 2).
Respiratory System and Acid-Base Balance Changes
Alveolar ventilation increases by 30% or more by mid pregnancy. This increase results in chronic respiratory alkalosis with a PaCO2 of 28 to 32 mm Hg, a slightly alkaline pH (approximately 7.44), and decreased levels of bicarbonate and buffer base. Although oxygen consumption is increased, PaO2 usually increases only slightly or remains within the normal range. Functional residual capacity (FRC) diminishes by approximately 20% as the uterus expands, resulting in decreased oxygen reserve and the potential for airway closure. When FRC is decreased further (e.g., from morbid obesity; perioperative intra-abdominal distention; placement of the patient in the supine, Trendelenburg, or lithotomy position; or induction of anesthesia), airway closure may be sufficient to cause hypoxemia.
Weight gain during pregnancy and capillary engorgement of the respiratory tract mucosa lead to more frequent problems with mask ventilation and tracheal intubation (see Chapter 30). Failed intubation (a leading cause of anesthesia-related maternal death) is as much a risk during early pregnancy with nonobstetric surgery as it is during cesarean delivery.9
Decreased FRC, increased oxygen consumption, and diminished buffering capacity result in the rapid development of hypoxemia and acidosis during periods of hypoventilation or apnea. Moreover, induction of inhalation anesthesia occurs more rapidly during pregnancy because alveolar hyperventilation and decreased FRC allow faster equilibration of inhaled agents. In addition, induction of anesthesia is accelerated owing to the 30% to 40% decrease in the minimum alveolar concentration (MAC) for volatile anesthetic agents that occurs even during early gestation.11 The anesthesia provider must be especially vigilant when administering subanesthetic concentrations of analgesic and anesthetic agents to the pregnant patient, in whom unconsciousness can occur quickly and unexpectedly.
Cardiovascular System Changes
Cardiac output increases by up to 50% during pregnancy because of increases in heart rate and stroke volume; both systemic and pulmonary vascular resistances decrease, but myocardial contractility is unaffected. Early in pregnancy (i.e., by 6 weeks’ gestation), significant cardiovascular alterations are present.12 By 8 weeks’ gestation, 57% of the increase in cardiac output, 78% of the increase in stroke volume, and 90% of the decrease in systemic vascular resistance that are typically achieved by 24 weeks’ gestation have occurred.13
During the second half of gestation, the uterus compresses the inferior vena cava when the mother lies supine; the compression reduces venous return and cardiac output by approximately 30%. Although upper extremity blood pressure may be maintained by compensatory vasoconstriction and tachycardia, uteroplacental perfusion is jeopardized whenever the mother lies supine. In some women, frank hypotension may occur in the supine position, especially when neuraxial or general anesthesia attenuates or abolishes normal compensatory mechanisms. For these reasons, it is essential to displace the uterus laterally during any operation performed after 18 to 20 weeks’ gestation. Vena caval compression also results in distention of the epidural venous plexus, which increases the likelihood of intravascular injection of local anesthetic during the administration of epidural anesthesia. The reduced capacity of the epidural space most likely contributes to the enhanced spread of epidural local anesthetic solution that is observed during pregnancy.
Changes in Blood Volume and Blood Constituents
Blood volume expands in the first trimester and increases 30% to 45% by term. Dilutional anemia occurs as a result of the smaller increase in red blood cell volume than in plasma volume. Although moderate blood loss is well tolerated during pregnancy, preexisting anemia decreases the patient’s reserve when significant hemorrhage occurs. Pregnancy is associated with benign leukocytosis; consequently, the white blood cell count is an unreliable indicator of infection. In general, pregnancy induces a hypercoagulable state, with increases in fibrinogen; factors VII, VIII, X, and XII; and fibrin degradation products. Pregnancy is associated with enhancement of platelet turnover, clotting, and fibrinolysis; there is a wide range in the normal platelet count. Thus, pregnancy represents a state of accelerated but compensated intravascular coagulation. Although thrombocytopenia may be present in some pregnant women, these patients may still be hypercoagulable. During the postoperative period, pregnant surgical patients are at high risk for thromboembolic complications; thus, thromboembolism prophylaxis is recommended.14
Gastrointestinal System Changes
Incompetence of the lower esophageal sphincter and distortion of gastric and pyloric anatomy during pregnancy increase the risk for gastroesophageal reflux, despite similar gastric emptying rates in pregnant and nonpregnant patients15; thus the pregnant woman is at risk for regurgitation of gastric contents and aspiration pneumonitis. It is unclear at what stage during pregnancy this risk becomes significant. Although lower esophageal sphincter tone is impaired early in pregnancy (especially in patients with heartburn),16 the mechanically induced factors do not become relevant until later in pregnancy. It seems prudent to consider any pregnant patient as having a higher risk for aspiration after mid gestation; some anesthesia providers contend that pregnant women are at increased risk for aspiration from the beginning of the second trimester onward.16
Altered Responses to Anesthesia
In addition to the decrease in MAC for inhaled anesthetic agents, thiopental requirements begin to decrease early in pregnancy.17 The effects of pregnancy on propofol requirements are conflicting.18,19 Higuchi et al.18 found that the median effective dose for loss of consciousness (ED50) was unchanged in pregnancy, whereas Mongardon et al.19 reported that lower propofol doses were required to achieve loss of consciousness in early pregnancy compared with the nonpregnant state. This effect appeared unrelated to increased progesterone levels. More extensive neural blockade is attained with epidural and spinal anesthesia in pregnant patients than in nonpregnant patients (see Chapter 12). Pregnancy also enhances the response to peripheral neural blockade.
Plasma cholinesterase levels decrease by approximately 25% from early in pregnancy until the seventh postpartum day. Fortunately, prolonged neuromuscular blockade with succinylcholine is uncommon, because the larger volume available for drug distribution offsets the impact of decreased drug hydrolysis.20 Nonetheless, the dose of succinylcholine should be controlled carefully in the pregnant patient, and the anesthesia provider should monitor neuromuscular blockade with a nerve stimulator to ensure adequate reversal before extubation.
Decreased protein binding associated with lower albumin and alpha-glycoprotein concentrations during pregnancy may result in a larger fraction of unbound drug, with the potential for greater drug toxicity during pregnancy.21 Pregnant surgical patients may require drugs that are infrequently used during pregnancy; limited information may exist on the effects of pregnancy on the response to these drugs. Cautious administration of such agents is advisable, because their pharmacokinetic and pharmacodynamic profiles may differ from those in nonpregnant patients. Pregnancy-associated changes in volume of distribution (due to changes in body composition and/or plasma protein binding capacity), metabolic activity, and hepatic or renal elimination (due to changes in glomerular filtration rate and tubular transport processes) may contribute to changes in drug effects and metabolism.22 For example, in pregnant women, cefazolin clearance is approximately twice as high between 20 and 40 weeks’ gestation and acetaminophen clearance is increased, compared with nonpregnant women.23,24
Fetal Considerations
Risk for Teratogenicity
Although maternal catastrophes that cause severe maternal hypoxia or hypotension pose the greatest risk to the fetus, considerable attention has focused on the role of anesthetic agents as abortifacients and teratogens. Teratogenicity has been defined as any significant postnatal change in function or form in an offspring after prenatal treatment. Concern about the potential harmful effects of anesthetic agents stems from their known effects on mammalian cells, which include reversible decreases in cell motility, prolongation of DNA synthesis, and inhibition of cell division. Despite these concerns, no data specifically link any of these cellular events with teratogenic changes. Unfortunately, prospective clinical studies of the teratogenic effects of anesthetic agents are impractical; such studies would require huge numbers of patients exposed to the drug under investigation. Therefore, investigations of anesthetic agents have taken one of the following directions: (1) studies of the reproductive effects of anesthetic agents in small animals, (2) epidemiologic surveys of operating room personnel constantly exposed to subanesthetic concentrations of inhalation agents, and (3) studies of pregnancy outcome in women who have undergone surgery while pregnant.
Principles of Teratogenicity
A number of important factors influence the teratogenic potential of a substance, including species susceptibility, dose of the substance, duration and timing of exposure, and genetic predisposition. Like other toxicologic phenomena, the effects of teratogens are dose dependent (Figure 17-1).25 Most teratologists accept the principle that any agent can be teratogenic in an animal provided that enough is given at the right time. Thus, the finding of teratogenesis of an agent after the single administration of a high dose, or the long-term administration of a low dose, does not imply that a single, short exposure (e.g., during a typical anesthetic) would incur similar risk. The interaction between dose and timing is also critical. A small dose of a teratogen may cause malformations or death in the susceptible early embryo, whereas much larger doses may prove harmless to the fetus,25 as was shown with thalidomide. Most studies have used small animals (e.g., chick embryos, mice, rats), and their results cannot necessarily be extrapolated to other species, especially humans. Of the more than 2200 agents listed in Shepard’s Catalog of Teratogenic Agents,26 approximately 1200 are teratogenic in animals, but only about 30 of these are known to cause defects in humans.
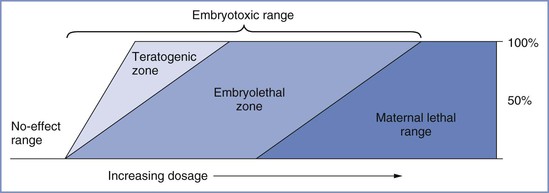
FIGURE 17-1 Toxic manifestations with increasing dosage of a teratogen. A no-effect range of dosage occurs below the threshold, at which embryotoxic effects abruptly appear. Teratogenesis and embryolethality often have similar thresholds and may increase at roughly parallel rates as dosage increases to a point at which all conceptuses are affected. Increasing dosage causes increased embryolethality, but teratogenicity appears to decrease, because many defective embryos die before term. A further increase in dosage reaches the maternal lethal range. (Modified from Wilson JG. Environment and Birth Defects. New York, Academic Press, 1973:31.)
Manifestations of teratogenicity include death, structural abnormality, growth restriction, and functional deficiency.25 Depending on when it occurs, death is referred to as abortion, fetal death, or stillbirth in humans and as fetal resorption in animals. Structural abnormalities can lead to death if they are severe, although death may occur in the absence of congenital anomalies. Growth restriction is considered a manifestation of teratogenesis and may relate to multiple factors, including placental insufficiency and genetic and environmental factors. Functional deficiencies include a number of behavioral and learning abnormalities, the study of which is called behavioral teratology. The stage of gestation at which exposure occurs determines the target organs or tissues, the types of defects, and the severity of damage. Most structural abnormalities result from exposure during the period of organogenesis, which extends from approximately day 31 to day 71 after the first day of the last menstrual period. Figure 17-2 shows the critical stages of development and the related susceptibility of different organs to teratogens. Functional deficiencies are usually associated with exposure during late pregnancy or even after birth, because the central nervous system (CNS) continues to mature during this period.
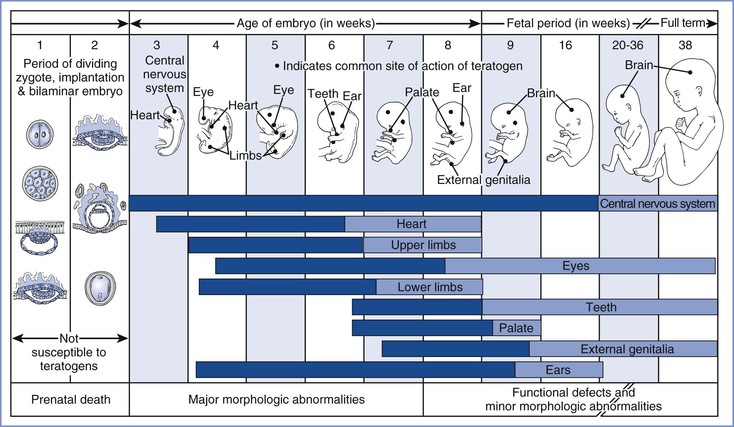
FIGURE 17-2 Critical periods in human development. During the first 2 weeks of development, the embryo typically is not susceptible to teratogens. During these predifferentiation stages, a substance either damages all or most cells of the embryo, resulting in its death, or damages only a few cells, allowing the embryo to recover without development of defects. The dark bars denote highly sensitive periods, whereas the light bars indicate periods of lesser sensitivity. The ages shown refer to the actual ages of the embryo and fetus. Clinical estimates of gestational age represent intervals beginning with the first day of the last menstrual period. Because fertilization typically occurs 2 weeks after the first day of the last menstrual period, the reader should add 14 days to the ages shown here to convert to the estimated gestational ages that are used clinically. (Redrawn from Moore KL. The Developing Human. 4th edition. Philadelphia, WB Saunders, 1993:156.)
Consideration of the possible teratogenicity of anesthetic agents must be viewed against the naturally high occurrence of adverse pregnancy outcomes. Roberts and Lowe27 estimated that as many as 80% of human conceptions are ultimately lost; many are lost even before pregnancy is recognized. The incidence of congenital anomalies among humans is approximately 3%, most of which are unexplained. Indeed, exposure to drugs and environmental toxins accounts for only 2% to 3% of such defects (Table 17-1).25 Shepard26 has listed several criteria for determining that an agent is a human teratogen, including the following: (1) proven exposure to the agent at the critical time of development; (2) consistent findings in two or more high-quality epidemiologic studies; (3) careful delineation of the clinical cases, ideally with the identification of a specific defect or syndrome; and (4) an association that “makes biological sense.” Documentation of teratogenicity in experimental animals is important but not essential. The list of agents or factors that are proven human teratogens does not include anesthetic agents (which are listed as “unlikely teratogens”) or any drug routinely used during the course of anesthesia (Box 17-1).
TABLE 17-1
Etiology of Human Developmental Abnormalities
| Causes of Developmental Defects in Humans | Percentage |
| Genetic transmission | 20 |
| Chromosomal aberration | 3-5 |
| Environmental causes: | |
| Radiation | < 1 |
| Infection | 2-3 |
| Maternal metabolic imbalance | 1-2 |
| Drugs and environmental chemicals | 2-3 |
| Unknown | 65-70 |
| TOTAL | 100 |
Modified from Wilson JG. Environmental and Birth Defects. New York, Academic Press, 1973:49.
Nondrug Factors Encountered in the Perioperative Period
Derangements of Normal Physiology.
Anesthesia and surgery can cause derangements of maternal physiology that may result in hypoxia, hypercapnia, stress, and abnormalities of temperature and carbohydrate metabolism. These states may be teratogenic themselves, or they may enhance the teratogenicity of other agents.26 Severe hypoglycemia and prolonged hypoxia and hypercarbia have caused congenital anomalies in laboratory animals,26,28 but there is no evidence to support teratogenicity after brief episodes in humans. The chronic hypoxemia experienced by mothers at high altitudes results in the delivery of infants with lower birth weights but with no increase in the rate of congenital defects.26 Maternal stress and anxiety are teratogenic in animals,29 but their significance as human teratogens remains questionable; supporting epidemiologic studies are lacking. Hypothermia is not teratogenic, whereas hyperthermia is teratogenic in both animals and humans.26 Congenital anomalies, especially involving the CNS, have repeatedly been associated with maternal fever during the first half of pregnancy. It must be remembered that fetal temperature is on average 0.5° C to 1° C higher than maternal temperature. Embryonic oxidative stress from reactive oxygen species has been implicated as one of the mechanisms involved in teratogenicity of many agents.30
Diagnostic Procedures.
Ionizing radiation is a human teratogen that can result in an increased, dose-related risk for malignant disease, genetic disease, congenital anomalies, and/or fetal death.26,31 The effects of radiation are often classified as being deterministic or stochastic. Deterministic effects are dose related and are observed above a certain threshold dose (e.g., pregnancy loss, growth restriction, mental retardation, organ malformation). In contrast, stochastic effects are possible at any level of exposure with no minimum threshold but with the likelihood of worsening effects at higher doses. An increased risk for childhood cancer is a stochastic effect when fetuses are exposed to ionizing radiation in utero. The type and severity of effects vary with the radiation exposure to the uterus and fetus and with the gestational age of the fetus.
Radiation is expressed as grays (Gy) or milligrays (mGy) (1 Gy = 100 rad) and is evaluated as cumulative dose (i.e., background radiation and medical diagnostic radiation) throughout the entire pregnancy. Background radiation during gestation is 1.3 to 5.8 mGy.31 There is no evidence that radiation exposure less than 50 mGy is associated with a teratogenic effect in either humans or animals.31 The absorbed fetal dose for all conventional radiographic imaging procedures outside the abdomen and pelvis is negligible and is well below 50 mGy (Table 17-2). However, direct radiographic examination of the abdomen and pelvis (e.g., abdominal computed tomography) and abdominal imaging studies that include fluoroscopy (e.g., barium enema) may result in more significant fetal radiation exposure, with a dose that may approach 100 mGy.31,32 Helical computed tomography for suspected pulmonary embolism results in a lower fetal radiation dose than ventilation perfusion scanning. Interventional radiologic procedures (e.g., cerebral angiography, cerebral embolization, endoscopic retrograde cholangiopancreatography) are frequently complex and prolonged, particularly when surgery is not an option. These procedures, especially the abdominal interventions, expose the fetus to a significant radiation dose; thus, a number of societies have developed guidance for the use of these technologies during pregnancy (Table 17-3).33
TABLE 17-2
Fetal Radiation Exposure for Common Diagnostic Procedures
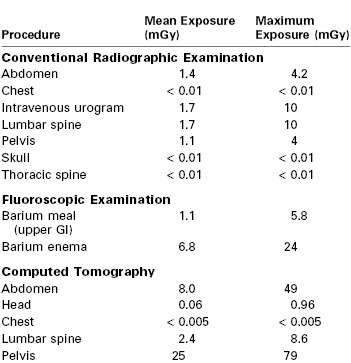
GI, gastrointestinal.
From Valentin J. Pregnancy and medical radiation. ICRP Publication 84. Ann ICRP 2000; 30:1-43.
TABLE 17-3
Estimated Fetal Radiation Exposure for Common Interventional Radiologic Procedures
| Procedure | Estimate (mGy) | Range (mGy) |
| Cardiac catheter ablation | 0.15-0.6* | |
| ERCP | 3.1 | 0.01-55.9 |
| TIPS creation | 5.5 | |
| Pulmonary angiography | 0.02-0.46 | |
| Uterine fibroid embolization | 42 | |
| Cerebral angiography | 0.06 |
* Depending on procedure duration.
ERCP, endoscopic retrograde cholangiopancreatography; TIPS, transjugular intrahepatic portosystemic shunt.
Modified from Dauer LT, Thornton RH, Miller DL, et al. Radiation management for interventions using fluoroscopic or computed tomographic guidance during pregnancy. J Vasc Interv Radiol 2012; 23:19-32.
In contrast to the negligible risk for teratogenicity, observational studies suggest that there is a slightly higher risk for childhood cancer at radiation doses greater than or equal to 10 mGy.32 The relative risk for childhood malignancy after maternal abdominal radiation exposure has been estimated to be 2.28 (95% confidence interval, 1.31 to 3.97).31 Nonetheless, if the mother’s condition necessitates diagnostic testing and radiation exposure, and no other acceptable imaging modality is available, testing should not be withheld if the benefits to the mother (and, by extension, the fetus) are judged to outweigh the risks. Delayed diagnosis of a critical condition may result in significant harm to the mother and, by extension, to the fetus. The radiographer should adhere to the ALARA principle (as low as reasonably achievable) and take measures to minimize the absorbed dose of radiation, including abdominal shielding, reduced scan length, and milliampere modulation.
Intravenous iodinated contrast media crosses the placenta to the fetus when administered in the usual clinical doses and can potentially cause neonatal hypothyroidism; however, the use of radiopaque (iodide containing) contrast media during pregnancy is acceptable.34
Diagnostic ultrasonography during pregnancy has long been considered to be devoid of embryotoxic effects. In animals, ultrasound intensities up to 20 W/cm2 have been found to be safe.35,36 However, when higher intensities (> 30 W/cm2) have been used, or when repeated exposure has occurred during early pregnancy, postnatal neurobehavioral effects have been described.36,37 Ultrasound waves also increase the fetal temperature, and hyperthermia is a known teratogen. Modern diagnostic ultrasound equipment is capable of inducing significant increases in temperature, especially when imaging is prolonged. Miller et al.38 concluded that these temperature increments were within the range of temperatures that could be teratogenic. Epidemiologic human data are reassuring; no biologic effects have been documented from diagnostic ultrasonographic examinations in the pregnant patient, despite widespread use over several decades. Nonetheless, it must be stressed that these epidemiologic studies were conducted in an era when ultrasound equipment was less potent and ultrasound-related increases in temperature were lower. Doppler interrogation emits significantly more acoustic intensity than pulse-echo imaging equipment; thus, more heat is generated. Therefore, Doppler technology should be used judiciously, keeping the exposure time and acoustic output to the lowest level possible.
No adverse effects on pregnancy and neonatal outcome were noted in a small series of pregnant patients (n = 26) exposed to gadolinium (paramagnetic contrast medium).39 However, gadolinium-based MRI contrast agents readily cross the placenta, enter the fetal circulation, and are excreted by the fetal kidneys into the amniotic fluid, where they may be swallowed by the fetus or resorbed by the mother. Although no toxic effects have been described after gadolinium administration, the long-term consequences remain unknown. The American College of Radiology recommends that intravenous gadolinium be avoided during pregnancy and used only if absolutely essential.40
Systemic Agents
Animal Studies.
Early studies documented teratogenicity in rodents after a variety of neurotropic agents (e.g., opioids, tricyclic antidepressants).41,42 The fetuses exhibited a characteristic group of CNS malformations as well as skeletal abnormalities and growth restriction. Whether these investigations truly reflect the teratogenic potential of opioids is questionable, however, because the respiratory depression and impaired feeding that accompany large bolus injections of opioids also may be teratogenic. In a study designed to avoid such problems, Fujinaga and Mazze43 maintained clinically relevant concentrations of morphine throughout most of pregnancy in rats by means of continuously implanted osmotic mini-pumps. Structural anomalies were not observed at any morphine dose, although fetal growth restriction was present, and mortality was increased among the offspring. Using the same methodology, Fujinaga et al.44,45 found fentanyl, sufentanil, and alfentanil completely devoid of teratogenic effects. Additional animal studies have confirmed the absence of teratogenicity with other opioids.46
Many tranquilizers and anxiolytics taken by pregnant women have been investigated less systematically than opioids. Animal studies have demonstrated structural or behavioral teratogenesis after exposure to some of the barbiturate, phenothiazine, and tricyclic antidepressant agents.47,48 The reader is referred to standard teratology reference sources for animal data related to specific drugs.26,49 The package insert provided by a drug’s manufacturer typically describes unpublished in-house studies related to the drug’s reproductive effects.
Studies in rodents and nonhuman primates indicate that exposure of the immature brain to anesthetic agents such as propofol, thiopental, and ketamine (i.e., agents classified as N-methyl-D-aspartate [NMDA] antagonists and gamma-aminobutyric acid [GABA] agonists)] are associated with brain cell apoptosis and functional learning deficits.50,51 Whether this effect occurs in humans or other species remains to be determined (see Chapter 10). These observed changes may represent indirect effects, such as hypoxia or hypoglycemia, as well as direct effects of the anesthetic agent on the developing brain.
Human Studies.
Teratogenesis has not been associated with the use of any of the commonly used induction agents—including the barbiturates, ketamine, and the benzodiazepines—when they were administered in clinical doses during anesthesia.26 Similarly, no evidence supports the teratogenicity of opioids in humans; there is no increase in the incidence of congenital anomalies among offspring of mothers who use morphine or methadone during pregnancy.26,49
Although human data relating to long-term tranquilizer therapy have raised questions about the possible teratogenicity of some agents, most studies are retrospective and suffer from a variety of methodologic flaws. Benzodiazepine therapy became controversial after an association between maternal diazepam ingestion during the first trimester and infants with cleft palate, with or without cleft lip, was reported.52 Subsequently, prospective work in 854 women who ingested diazepam during the first trimester did not demonstrate a higher risk associated with benzodiazepine therapy.53 Although the present consensus among teratologists is that diazepam is not a proven human teratogen,26 it is appropriate to consider the risk-to-benefit ratio before initiating long-term benzodiazepine therapy during the first trimester. No evidence suggests that a single dose of a benzodiazepine (e.g., midazolam) during the course of anesthesia is harmful to the fetus.
Local Anesthetics
Procaine, lidocaine, and bupivacaine cause reversible cytotoxic effects in cultures of hamster fibroblasts.54 However, no evidence supports morphologic or behavioral teratogenicity associated with lidocaine administration in rats,55 and no evidence supports teratogenicity associated with any local anesthetic used clinically in humans.26 Maternal cocaine abuse is associated with adverse reproductive outcomes, including abnormal neonatal behavior and, in some reports, a higher incidence of congenital defects of the genitourinary and gastrointestinal tracts.26 The greatest risk to the fetus most likely results from the high incidence of placental abruption associated with maternal cocaine use (see Chapter 54).
Muscle Relaxants
Testing muscle relaxants for teratogenicity using standard in vivo animal studies either is complicated by maternal respiratory depression and the need for mechanical ventilation (a complex undertaking in rats or mice) or requires the administration of very low doses of the drug. Fujinaga et al.56 used the whole-embryo rat culture system to investigate the reproductive toxicity of high doses of D-tubocurarine, pancuronium, atracurium, and vecuronium. Although dose-dependent toxicity was manifested, these effects occurred only at concentrations 30-fold greater than those encountered in clinical practice. These findings are consistent with earlier studies demonstrating no toxicity with small doses of muscle relaxants.57 Given that fetal blood concentrations of muscle relaxants are only 10% to 20% of maternal concentrations, these drugs appear to have a wide margin of safety when administered to the mother during organogenesis.
Whether their administration later in gestation has adverse effects is unclear. Prolonged disturbance of normal muscular activity by muscle relaxants has caused axial and limb deformities in the chick but has seldom been seen in other experimental animals. Although one case report described arthrogryposis (i.e., persistent joint flexure) in the infant of a woman with tetanus who received D-tubocurarine for 19 days beginning at 55 days’ gestation, the patient also was hypoxic and received multiple other drugs.58 Many women have received muscle relaxants for several days during late gestation without adverse effect on the neonate.
Inhalation Anesthetics
Animal Studies.
Volatile Agents.
Many studies have shown that, under certain conditions, the volatile halogenated anesthetic agents can produce teratogenic changes in chicks or small rodents. Basford and Fink59 observed skeletal abnormalities but no increase in fetal loss when rats were exposed in utero to 0.8% halothane for 12 hours on days 8 and 9 of pregnancy (i.e., the “critical period” in the 21-day rat gestation). Long-term exposure to subanesthetic concentrations of halothane caused fetal growth restriction in rats but no increase in the incidence of congenital anomalies,60 whereas isoflurane had no adverse effects.61
More significant reproductive effects have occurred with greater exposures to anesthetic agents. Fetal skeletal abnormalities or death followed repeated or prolonged maternal exposure of mice to anesthetic concentrations of volatile anesthetic agents.62 However, teratogenicity in these studies most likely was caused by the physiologic changes (e.g., profound hypothermia, hypoventilation) associated with anesthesia rather than by the anesthetic agent itself. Moreover, some strains of mice are especially likely to demonstrate anomalies such as cleft palate. Mazze et al.63 exposed rats to 0.75 MAC of halothane, isoflurane, or enflurane, or 0.55 MAC of nitrous oxide for 6 hours daily on 3 consecutive days at various stages of pregnancy. The animals remained conscious throughout the study, and normal feeding and sleep patterns were preserved. Under these conditions, no teratogenic effects were associated with any of the volatile agents. The only positive finding was a threefold increase in the rate of fetal resorption with nitrous oxide. No evidence has suggested reproductive toxicity with either sevoflurane or desflurane in clinical concentrations.
Nitrous Oxide.
In contrast to the volatile halogenated agents, nitrous oxide is a weak teratogen in rodents under certain conditions, even when normal homeostasis is maintained. Rats continually exposed to 50% to 70% nitrous oxide for 2 to 6 days (starting on day 8 of gestation) had an increased incidence of congenital abnormalities.63,64 To exclude the possibility that adverse effects were a consequence of the anesthetic state, Lane et al.65 exposed rats to 70% nitrous oxide or to a similar concentration of xenon (a slightly more potent anesthetic devoid of biochemical effects) for 24 hours on day 9 of gestation; abnormalities occurred only in the nitrous oxide group. With the exception of one study in which extremely prolonged exposure to a low concentration of nitrous oxide had some minor effects,66 at least 50% nitrous oxide has been required to consistently produce anomalies.64 The threshold exposure time has not been rigorously determined, although exposure for at least 24 hours typically was necessary.
In vivo and embryo culture studies in rats have confirmed that nitrous oxide has several adverse reproductive effects, each of which results from exposure at a specific period of susceptibility.67,68 Fetal resorptions occurred after exposure on days 8 and 11 of gestation, skeletal anomalies after exposure on day 8 or 9, and visceral anomalies (including situs inversus) only when exposure occurred on day 8.69
Initially, teratogenicity associated with nitrous oxide was thought to result from its oxidation of vitamin B12, which interferes with its function as a coenzyme for methionine synthase.70 Transmethylation from methyltetrahydrofolate to homocysteine to produce tetrahydrofolate (THF) and methionine is catalyzed by methionine synthase (Figure 17-3). Thus, methionine synthase inhibition could cause a decrease in THF (with a resultant decrease in DNA synthesis) and lower methionine levels (with resultant impairment of methylation reactions). Nitrous oxide rapidly inactivates methionine synthase in both animals71 and humans.72 Prolonged human exposure to nitrous oxide leads to neurologic and hematologic symptoms, the latter probably resulting from diminished DNA synthesis.70 The hematologic—but not the neurologic—changes are prevented by the co-administration of folinic acid (5-formyl THF) with nitrous oxide, with the goal of restoring DNA synthesis.
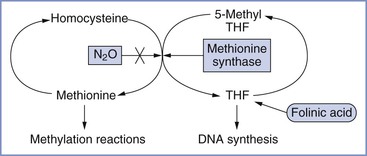
FIGURE 17-3 Pathway showing the inhibition of methionine synthase by nitrous oxide (N2O) and its potential metabolic consequences (e.g., decreased DNA synthesis and impaired methylation reactions). THF, tetrahydrofolate. (Courtesy M. Fujinaga, Palo Alto, CA.)
Considerable evidence indicates that methionine synthase inhibition and a consequent lack of THF are not solely responsible for the teratogenic effects of nitrous oxide. First, maximal inhibition of methionine synthase activity occurs at concentrations of nitrous oxide that are much lower than those required to produce teratogenic effects.73 Second, folinic acid, which bypasses the effect of methionine synthase inhibition on THF formation, partially prevents only one of the structural abnormalities (i.e., minor skeletal defects) produced by nitrous oxide.74 Third, the administration of isoflurane or halothane with nitrous oxide prevents almost all of the teratogenic effects but does not prevent the decrease in methionine synthase activity.75,76 Fourth, studies using an in vitro whole-embryo rat culture system have shown that supplementation of nitrous oxide with methionine (but not with folinic acid) almost completely prevents growth restriction and all malformations with the exception of situs inversus.77 Additional studies have implicated α1-adrenergic receptor stimulation in the production of situs inversus by nitrous oxide.68,78,79 Postulated mechanisms by which sympathetic stimulation might have adverse reproductive effects include a decrease in uterine blood flow and overstimulation of G protein–dependent membrane signal transduction pathways.80 There is also evidence that nitrous oxide may cause neuronal apoptosis in rats.81
In summary, evidence suggests that the etiology of nitrous oxide teratogenicity in rats is complex and multifactorial. Determination of the relative roles of methionine deficiency and sympathetic stimulation or other mechanisms awaits performance of further studies. Although nitrous oxide is considered a weak teratogen in rats and mice, reproductive effects occur only after prolonged exposure to high concentrations that are unlikely to be encountered in humans during clinical anesthesia. Whether nitrous oxide administration is associated with neuronal apoptosis and learning impairments in humans remains to be determined.
Human Studies.
Occupational Exposure to Waste Anesthetic Agents.
Epidemiologic surveys dating from the 1960s and 1970s suggested that reproductive hazards (e.g., spontaneous abortion, congenital anomalies) were associated with operating room and dental surgery work.82–86 These hazards were attributed to exposure to trace concentrations of anesthetic agents, principally nitrous oxide. Critical reviews of these studies questioned their conclusions. The reviewers noted that response bias, inappropriate control groups, lack of verification of medical data, and exposure to multiple environmental factors made definitive conclusions impossible.87–89
The most consistent risk associated with occupational exposure was spontaneous abortion, which carried a relative risk ratio of 1.3. The ratio for congenital anomalies (1.2) had borderline statistical significance.87,89 These relative risks are well within the range that might be explained by bias or uncontrolled variables.89 For example, the relative risk of second-trimester abortion among women who drink one or two alcoholic drinks per day is 1.98; this risk increases to 3.53 with more than three drinks daily.90 Similarly, cigarette smoking carries a relative risk of 1.8 for spontaneous abortion.91
Shirangi et al.92 conducted a questionnaire-based survey of 2028 female veterinarians; information on 744 pregnancies regarding the risk for preterm birth (< 37 weeks) was collected. The prevalence of preterm birth in women exposed to unscavenged anesthetic gases was 7.3%, compared with 5.7% in the general population. The identification of the specific gas used was not requested; however, halothane, nitrous oxide, enflurane, and methoxyflurane were the most commonly used anesthetic gases during the study period. A hazards model predicted a 2.5-fold increase in preterm delivery risk in women exposed to unscavenged gas 1 or more hours per week compared with an unexposed group. Of note, a 3.7-fold increased risk was observed in veterinarians working greater than 45 hours a week compared with fewer than 45 hours.
Other studies have not confirmed an association between operating room work and higher reproductive risk.93,94 Pregnancy outcomes were comparable in exposed and nonexposed operating room nurses when questionnaire information was matched with objective data obtained from medical records and registries of abortions, births, and congenital anomalies.93 Similarly, in a 10-year prospective survey of all female physicians in the United Kingdom, Spence94 found no differences in reproductive outcome when anesthesiologists were compared with other working female physicians. Although these studies may have missed a higher incidence of very early abortion, their data do not support a statistically demonstrable reproductive hazard resulting from operating room exposure to anesthetic agents.
It is possible that the higher waste levels of nitrous oxide encountered in dentists’ offices pose a reproductive risk.85,95 In 1980, Cohen et al.85 reported a doubling of the spontaneous abortion rate among exposed female chair-side assistants and the wives of exposed male dentists. The incidence of birth defects among the children of exposed dental assistants was slightly higher than that for nonexposed assistants. However, the validity of this finding is doubtful; the incidence of anomalies among the offspring of nonexposed dentists was similar to that of the exposed assistants. Moreover, the expected dose-response relationship did not exist. In another study,95 reduced fertility was reported among female dental assistants working with nitrous oxide in an unscavenged environment for more than 5 hours per week. However, because the affected group consisted of only 19 individuals, it is difficult to draw firm conclusions from these data. Overall, the epidemiologic data do not support an increased risk for congenital anomalies with long-term exposure to nitrous oxide.
Studies of Operations Performed during Pregnancy.
In 1963, Smith96 reviewed the obstetric records of 18,493 pregnant women. Sixty-seven (0.36%) had had an operation during pregnancy; only 10 procedures occurred during the first trimester. Fetal mortality was 11.2%, with the poorest survival occurring after operations for appendiceal abscess and cervical incompetence. In 1965, Shnider and Webster97 examined the records of 9073 obstetric patients; 147 (1.6%) of this group had had operations during pregnancy. Preterm delivery followed operation in 8.8% of patients, and the incidences of perinatal mortality and low-birth-weight (LBW) infants were increased in patients who had surgery during pregnancy. Brodsky et al.2 surveyed 12,929 pregnant dental assistants and wives of male dentists, 2% of whom had operations during gestation. Spontaneous abortions were more common in the surgical group than in the control group (8% versus 5.1% during the first trimester and 6.9% versus 1.4% during the second trimester, respectively). None of these three studies reported a higher incidence of congenital anomalies among infants of women who underwent surgery during pregnancy. Two additional studies98,99 that focused on the risks associated with nitrous oxide anesthesia during early pregnancy found no increase in the incidence of congenital abnormalities or spontaneous abortions.
Duncan et al.100 used health insurance data to study the entire Manitoba, Canada, population between 1971 and 1978, matching 2565 women who had operations during pregnancy with similar controls who did not undergo surgery. Type of anesthesia was classified as nil (18%), general (57%), spinal/nerve block (2%), or local (24%). Although the incidence of congenital anomalies was similar in the surgical and control groups, spontaneous abortion was more common among women who had general anesthesia for surgery during the first or second trimester. This was true for both gynecologic procedures (relative risk [RR], 2.00) and nongynecologic procedures (RR, 1.58). Unfortunately, no conclusions regarding the relationship between anesthetic technique and fetal loss could be drawn, because most of the gynecologic and other major procedures were performed with general anesthesia. As in most studies, it is difficult to separate the effects of the anesthetic technique from those of the surgical procedure.
In the largest study to date, Mazze and Källén1 linked data from three Swedish health care registries—the Medical Birth Registry, the Registry of Congenital Malformations, and the Hospital Discharge Registry—for the years 1973 to 1981. Among the population of 720,000 pregnant women, 5405 (0.75%) had nonobstetric surgery, including 2252 who had procedures during the first trimester. (Cervical cerclage was excluded from analysis.) Of the women who had surgery, 54% received general anesthesia, which included nitrous oxide in 97% of cases. The researchers examined the following adverse outcomes: (1) congenital anomalies, (2) stillbirths, (3) neonatal death within 7 days, and (4) LBW or very low-birth-weight (VLBW) infants. There was no difference between surgical and control patients with regard to the incidence of stillbirth or the overall incidence of congenital anomalies (Figure 17-4). Although the overall rate of anomalies among infants of women who had first-trimester operations was not higher, this group did have a higher-than-expected incidence of neural tube defects (6.0 observed versus 2.5 expected).101 Five of the 6 women whose infants had these defects were among the 572 women who had had surgery during gestational weeks 4 to 5, which is the period of neural tube formation; the researchers cautioned that this finding could have been a chance association.101 However, if a true causal relationship exists between neural tube defects and anesthesia at this stage of gestation, it could represent an eightfold to ninefold increase in the risk for this anomaly (i.e., an absolute risk of almost 1%). Other positive findings were a higher incidence of LBW and VLBW infants in the surgical group, which resulted from both preterm delivery and fetal growth restriction (also known as intrauterine growth restriction).1 A predictable consequence of preterm delivery was a higher number of deaths of live-born infants within the first 7 days of life. Finally, no anesthetic technique or operation was associated with a significantly higher number of adverse outcomes.
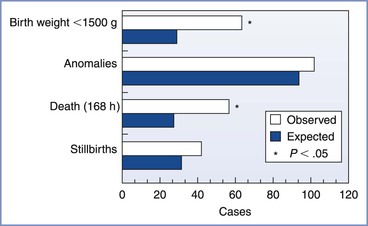
FIGURE 17-4 Total number of observed and expected adverse outcomes among women having nonobstetric operations during pregnancy. Incidences of infants with a birth weight of less than 1500 g and of infants born alive who died within 168 hours of birth were significantly increased. (Modified from Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol 1989; 161:1178.)
A case-control study of infants born in Atlanta between 1968 and 1980 gathered information regarding first-trimester exposure to general anesthesia from the mothers of 694 infants with major CNS defects and 2984 control mothers.102 A striking association was found between general anesthesia exposure and hydrocephalus in conjunction with another major defect (the strongest association was with hydrocephalus and eye defects). Limitations of this study include its retrospective nature and a lack of information about the types of surgery, the anesthetic agents used, and the presence or absence of complications. The investigators cautioned that further studies are necessary to confirm their observations.
In summary, although anesthesia and surgery are associated with a higher incidence of abortion, fetal growth restriction, and perinatal mortality, these adverse outcomes can often be attributed to the procedure, the site of surgery (e.g., proximity to the uterus), and/or the underlying maternal condition. Evidence does not suggest that anesthesia during pregnancy results in an overall increase in congenital abnormalities, and there is no evidence of a relationship between outcome and type of anesthesia.
Behavioral Teratology
It is well known that some teratogens produce enduring behavioral abnormalities without any observable morphologic changes. The CNS may be especially sensitive to such influences during the period of major myelination, which in humans extends from the fourth intrauterine month to the second postnatal month (see Chapter 10). Several studies have shown that brief intrauterine exposure to halothane adversely affects postnatal learning behavior and causes CNS degeneration and decreased brain weight in rats.103–105 The fetal nervous system in the rat is most susceptible to the effects of halothane during the second trimester.103 Maternal administration of systemic drugs—including barbiturates, meperidine, and promethazine—has also resulted in behavioral changes in offspring,106–108 whereas no effect has been noted with the administration of lidocaine.55 In humans, investigations of the effects of maternally administered analgesics at delivery have revealed transient, dose-related depression of neonatal behavior.
Currently used general anesthetic agents act by one of two principal mechanisms, (1) the potentiation of GABAA receptors (benzodiazepines, volatile halogenated agents, and barbiturates) or (2) the antagonism of NMDA receptors (nitrous oxide and ketamine). Drugs that act by either of these mechanisms appear to induce widespread neuronal apoptosis in the developing rat brain when administered during the period of synaptogenesis (i.e., the brain growth-spurt period).81,109–111 Jevtovic-Todorovic et al.81 observed that the administration of a general anesthetic “cocktail” (midazolam, isoflurane, and nitrous oxide), in doses sufficient to maintain general anesthesia for 6 hours in 7-day-old infant rats, resulted in widespread apoptotic neurodegeneration in the developing brain, deficits in hippocampal synaptic function, and persistent memory/learning impairments. They concluded that these deficits are “subtle enough to be easily overlooked” but may persist into adolescence and adulthood.81 Ikonomidou et al.110 described neurodegeneration in rat pups after NMDA receptor antagonism. These data suggest that prolonged exposure to anesthetic agents at a critical period in brain development may accelerate the normal developmental process of apoptotic neurodegeneration, potentially resulting in prolonged behavioral deficits.112 Thus, the safety of providing anesthesia during early life has been questioned.113
The implications, if any, for the human fetus during the maternal administration of general anesthesia are unknown because there are methodologic issues with these animal studies. Surgery may result not only in exposure to anesthetic agents but also in derangements in maternal physiology (e.g., hypoxia, stress, hypoglycemia) that can lead to apoptosis during the critical period of neuronal development.114 Also, it should be noted that in experimental studies the rats typically were exposed to large doses of anesthetic agents for prolonged periods. Hayashi et al.115 demonstrated that a single dose of ketamine did not lead to apoptosis but that its repeated administration for several hours caused neuronal degeneration. It is also important to remember that painful stimuli per se can cause long-term behavioral changes.116 Finally, the species model may be important. For example, McClain et al.117 did not observe any histologic or functional effects of exposure to general anesthesia in fetal lambs. Anand and Soriano113 concluded:
Clearer understanding of the mechanisms by which exposures to pain/stress or prolonged anesthesia in the perinatal period can alter the survival or development of immature neurons and glia may prevent some long-term neurobehavioral abnormalities in humans. In the meantime, clinicians should administer anesthetic agents to newborn infants or pregnant mothers but avoid prolonged periods of anesthetic exposure. … Alleviation of pain and stress during the perinatal period should remain an essential clinical goal until further research defines the clinical importance of [experimental] results [observed in animals].
After a review of the data and a public hearing in March 2007, the Anesthetic and Life Support Drugs Advisory Committee of the U.S. Food and Drug Administration stated that currently “there are not adequate data to extrapolate the animal findings to humans.”118
Fetal Effects of Anesthesia
Maintenance of Fetal Well-Being
The most serious fetal risk associated with maternal surgery during pregnancy is that of intrauterine asphyxia. Because fetal oxygenation depends on maternal oxygenation, maintenance of normal maternal arterial oxygen tension, oxygen-carrying capacity, oxygen affinity, and uteroplacental perfusion are critical to fetal well-being.
Maternal and Fetal Oxygenation.
Transient mild to moderate decreases in maternal PaO2 are well tolerated by the fetus, because fetal hemoglobin has a high affinity for oxygen. Severe maternal hypoxemia results in fetal hypoxia and, if persistent, may cause fetal death. Any complication that causes profound maternal hypoxemia (e.g., difficult intubation, esophageal intubation, pulmonary aspiration, total spinal anesthesia, systemic local anesthetic toxicity) is a potential threat to the fetus.
Studies of isolated human placental vessels have suggested that hyperoxia might cause uteroplacental vasoconstriction, with potential impairment of fetal oxygen delivery.119 This fear has proved to be unfounded, because studies in pregnant women have demonstrated better fetal oxygenation with increasing maternal PaO2.120 Fetal PaO2 never exceeds 60 mm Hg, even when maternal PaO2 increases to 600 mm Hg, because of a large maternal-fetal oxygen tension gradient (see Chapter 4). Thus, intrauterine retrolental fibroplasia and premature closure of the ductus arteriosus cannot result from high levels of maternal PaO2. McClain et al.117 observed that the maternal administration of general anesthesia for 4 hours in gravid ewes produced an initial—but not sustained—increase in fetal systemic oxygenation, which was accompanied by a sustained increase in fetal cerebral oxygenation. The investigators hypothesized that the increase in fetal cerebral oxygenation resulted from greater cerebral perfusion, lower cerebral metabolic rate, or both. Histologic examination found no evidence of neurotoxicity.
Maternal Carbon Dioxide and Acid-Base Status.
Maternal hypercapnia can cause fetal acidosis, because fetal PaCO2 correlates directly with maternal PaCO2. Although mild fetal respiratory acidosis is of little consequence, severe acidosis can cause fetal myocardial depression and hypotension. Maternal hyperventilation with low maternal PaCO2 and high pH can adversely affect fetal oxygenation by means of several mechanisms.121–123 Respiratory or metabolic alkalosis can compromise maternal-fetal oxygen transfer by causing umbilical artery constriction121 and by shifting the maternal oxyhemoglobin dissociation curve to the left.122 In addition, hyperventilation, independent of changes in PaCO2, may reduce uterine blood flow and cause fetal acidosis.123 This decrease most likely is a consequence of mechanical ventilation, whereby increased intrathoracic pressure reduces venous return and cardiac output, which in turn decreases uteroplacental perfusion. Thus, hyperventilation should be avoided in the pregnant surgical patient. Rather, the PaCO2 should be kept in the normal range for pregnancy.
Uteroplacental Perfusion.
Maternal hypotension from any cause can jeopardize uteroplacental perfusion and cause fetal asphyxia. The most common causes of hypotension in the pregnant patient undergoing surgery include (1) deep levels of general anesthesia, (2) sympathectomy with high levels of spinal or epidural blockade, (3) aortocaval compression, (4) hemorrhage, and (5) hypovolemia. In monkeys, prolonged hypotension (i.e., systolic blood pressure < 75 mm Hg) caused by deep halothane anesthesia resulted in fetal hypoxia, acidosis, and hypotension.124 After experiencing as much as 5 hours of severe partial asphyxia in utero (pH < 7.00 for at least 1 hour), neonatal monkeys were depressed and experienced seizures. Postnatal survival was poor, and pathologic brain changes included swelling, necrosis, and hemorrhage. The clinical course and neuropathologic findings in these animals resembled those in infants known to have suffered severe intrauterine asphyxia and who died within a few days of birth. Despite these alarming data, case reports have described good outcome after deliberate induction of moderate degrees of hypotension during pregnancy, usually to facilitate performance of a neurosurgical procedure.125 Fetal and neonatal outcomes were unaffected when maternal systolic blood pressure was kept between 70 to 80 mm Hg, even when pressures as low as 50 mm Hg were briefly permitted. In such circumstances, the risk to the fetus must be balanced against the risk for uncontrolled maternal bleeding or stroke.
The multiple factors that influence uteroplacental blood flow are discussed in detail in Chapter 3. Of particular relevance to the pregnant surgical patient are drugs that cause uterine vasoconstriction. Preoperative anxiety and light anesthesia increase circulating catecholamines, possibly impairing uterine blood flow.126 Drugs that cause uterine hypertonus (e.g., ketamine in early pregnancy in doses higher than 2 mg/kg,127 toxic doses of local anesthetics128) may increase uterine vascular resistance, decreasing uteroplacental perfusion.
New evidence has challenged the historic view that the mixed-adrenergic agonist ephedrine is preferred to the alpha-adrenergic agonist phenylephrine for the treatment of hypotension during the administration of neuraxial anesthesia in obstetric patients (see Chapter 26).129,130 A meta-analysis of randomized controlled trials comparing ephedrine with phenylephrine for the treatment of hypotension during spinal anesthesia for cesarean delivery resulted in the following conclusions: (1) there was no difference between phenylephrine and ephedrine for the prevention and treatment of maternal hypotension, (2) maternal bradycardia was more likely to occur with phenylephrine than with ephedrine, (3) women given phenylephrine had neonates with higher umbilical arterial blood pH measurements than those given ephedrine, and (4) there was no difference between the two vasopressors in the incidence of true fetal acidosis (i.e., umbilical arterial blood pH < 7.20).129 Cooper et al.130 randomly assigned 147 patients to receive phenylephrine, ephedrine, or both for the maintenance of maternal arterial pressure during spinal anesthesia for elective cesarean delivery. Fetal acidosis was more common in the women who received ephedrine. The investigators speculated that “increased fetal metabolic rate, secondary to ephedrine-induced beta-adrenergic stimulation, was the most likely mechanism for the increased incidence of fetal acidosis in the ephedrine group.”130 Ngan Kee et al.131 demonstrated that placental transfer was greater for ephedrine than phenylephrine and fetal metabolism was greater for phenylephrine than ephedrine. They also observed increased fetal concentrations of lactate, glucose, and catecholamines with ephedrine compared with phenylephrine, thus supporting the hypothesis that the lower fetal pH observed with ephedrine is related to metabolic effects secondary to stimulation of fetal beta-adrenergic receptors. Therefore, the use of phenylephrine to treat maternal hypotension is acceptable and may be preferable to ephedrine. However, the potential for phenylephrine to decrease maternal cardiac output and alter uteroplacental perfusion, despite treating hypotension, should be further studied.132
Fetal Effects of Inhalation Agents
The volatile halogenated anesthetic agents can affect the fetus directly (by depressing the fetal cardiovascular system or CNS) or indirectly (by causing maternal hypoxia or hypotension). Studies in gravid ewes have shown minimal fetal effects with maternal administration of moderate concentrations of a volatile agent.133 Uterine perfusion was maintained during the inhalation of 1.0 and 1.5 MAC halothane or isoflurane, because uterine vasodilation compensated for small decreases in maternal blood pressure. Higher concentrations (e.g., 2.0 MAC) given for prolonged periods induced marked maternal hypotension. Consequently, reduced uteroplacental blood flow resulted in fetal hypoxia, diminished fetal cardiac output, and fetal acidosis.134
The effects of anesthesia on the stressed fetal lamb remain unclear. In one study, the administration of 1% halothane to the mothers of asphyxiated fetal lambs caused severe fetal hypotension, worsening of fetal acidosis, and decreases in cerebral blood flow and oxygen delivery.134 In other studies, acidosis that was less severe or of a shorter duration was associated with the maintenance of fetal cardiac output and a preservation of the balance between oxygen supply and demand.135–137 The protective compensatory mechanisms that exist during asphyxia may be abolished by high but not low concentrations of volatile agents.
The relevance of these data to the human mother undergoing surgery during pregnancy is not clear. Clinical experience does not support avoidance of volatile agents, provided that maternal hypotension is prevented. Indeed, the depressant effect of these agents on myometrial contractility may be beneficial. If intraoperative fetal heart rate (FHR) monitoring reveals signs of fetal compromise, it may be advisable to discontinue the volatile agent until the fetal condition improves.
Fetal Effects of Systemic Drugs
Opioids and induction agents decrease FHR variability, possibly to a greater extent than the inhalation agents.138,139 This finding most likely signals the presence of an anesthetized fetus and is not a cause for concern in the absence of maternal hypotension or other abnormalities. Fetal respiratory depression is relevant only if cesarean delivery is to be performed at the same time as the surgical procedure. Even then, high-dose opioid anesthesia need not be avoided when it is indicated for maternal reasons (e.g., anesthesia for patients with cardiac disease). The pediatrician should be informed of maternal drug administration so that preparations can be made to support neonatal respiration. Some data indicate that remifentanil may result in less neonatal depression than longer-acting opioids.140,141
Maternal administration of muscle relaxants and reversal agents typically has not proved to be problematic for the fetus. It has been suggested that rapid intravenous injection of an anticholinesterase agent might stimulate acetylcholine release, which might cause increased uterine tone and thus precipitate preterm labor.142 Although this concern is unproven, slow administration of an anticholinesterase (after prior injection of an anticholinergic agent) is recommended. Atropine rapidly crosses the placenta and, when given in large doses, causes fetal tachycardia and loss of FHR variability.143 Although neither atropine nor glycopyrrolate significantly affects FHR when standard clinical doses are administered,144 glycopyrrolate is often recommended because it crosses the placenta less readily and may be a more effective antisialagogue. Although limited transplacental passage of neostigmine is expected, significant transfer occasionally may occur. One case report described mild fetal bradycardia when neostigmine was administered with glycopyrrolate during emergence from general anesthesia at 31 weeks’ gestation.145 This problem did not occur during the administration of a second general anesthetic to the same patient 4 days later, when atropine was administered with neostigmine, presumably because atropine undergoes greater placental transfer than glycopyrrolate. Because the effects of reversal agents are unpredictable, the monitoring of FHR during maternal drug administration is suggested.
Sodium nitroprusside and esmolol have been used during pregnancy to induce hypotension during surgical procedures. Standard doses of nitroprusside have proved to be safe for the fetus146; the risk for fetal cyanide toxicity appears to be low, provided that tachyphylaxis does not occur and the total dose is limited. The use of esmolol during pregnancy remains controversial. Ostman et al.147 observed minimal fetal effects after the administration of esmolol in gravid ewes, whereas Eisenach and Castro148 reported significant decreases in FHR and blood pressure as well as a modest reduction in fetal PaO2. Fetal effects dissipated rapidly in the first study but persisted for 30 minutes or more in the second. Two case reports have described small decreases in FHR but no morbidity when esmolol was administered with nitroprusside during neurosurgical procedures.149,150 In contrast, severe fetal compromise followed the administration of esmolol at 38 weeks’ gestation to correct maternal supraventricular tachycardia.151 Because fetal tachycardia preceded the onset of severe bradycardia in this case, the authors speculated that fetal compromise resulted from reduced maternal cardiac output rather than fetal beta-adrenergic receptor blockade.
Prevention of Preterm Labor
Most epidemiologic studies of nonobstetric surgery during pregnancy have reported a higher incidence of abortion and preterm delivery.1,97,98,152 It is unclear whether the surgery, manipulation of the uterus, or the underlying condition is responsible. In a study of 778 women who underwent appendectomy during pregnancy, Mazze and Källén152 found that 22% of women who underwent surgery between 24 and 36 weeks’ gestation delivered in the week after surgery. In the women in whom pregnancy continued beyond a week after surgery there was no further increase in the rate of preterm birth. Although this study’s database was unsuitable for determining the incidence of preterm delivery in women who had surgery before 24 weeks’ gestation, a similar increase appeared likely. Second-trimester procedures and operations that do not involve uterine manipulation carry the lowest risk for preterm labor.
Although the volatile agents depress myometrial irritability and thus are theoretically advantageous for abdominal procedures, evidence does not show that any one anesthetic agent or technique positively or negatively influences the risk for preterm labor. Published evidence does not support the routine use of prophylactic tocolytic agents.153 Monitoring for uterine contractions may be performed intraoperatively with an external tocodynamometer (when technically feasible) and for several days postoperatively, allowing tocolytic therapy to be instituted, if appropriate. Additional surveillance is necessary in patients who receive potent postoperative analgesics, who may be unaware of mild uterine contractions.
A relatively new class of tocolytic agents—oxytocin receptor antagonists (e.g., atosiban)—has been investigated.154 Atosiban selectively blunts the calcium influx in the myometrium and thus inhibits myometrial contractility. However, atosiban, in laboratory conditions, blocks the protective effects of oxytocin in fetal/neonatal neurons after hypoxic-ischemic insults.155 Whether greater surveillance and early tocolytic therapy will reduce the risk for preterm delivery after surgery during pregnancy is not known.
Magnesium sulfate is among the most common drugs used in pregnancy as a tocolytic, anticonvulsant, or fetal neuroprotective agent. Antenatal magnesium sulfate has been shown to reduce the incidence and severity of cerebral palsy after very preterm birth (see Chapter 10).156 However, magnesium has concurrent effects that are relevant to the delivery of anesthesia, including an increase in the rate of onset of neuromuscular blockade,157 the reestablishment of neuromuscular blockade in patients recovering from a nondepolarizing muscle relaxant,158 a reduction in general anesthetic requirements,159 and possible impairment in coagulation (as a calcium antagonist).160
Practical Considerations
Timing of Surgery
Elective surgery should not be performed during pregnancy. When possible, surgery should be avoided during the first trimester, especially during the period of organogenesis. The second trimester is the optimal time to perform surgery, because the risk for preterm labor is lowest at that time. Urgent operation is often indicated for abdominal emergencies, some malignancies, and neurosurgical and cardiac conditions. The management and timing of most acute surgical procedures should mimic that for nonpregnant patients.161,162 The risk for perinatal loss is increased when maternal appendicitis is advanced.162 Appendiceal perforation may be more common in pregnant patients than in nonpregnant patients because diagnostic difficulties may delay performance of surgery. Generalized peritonitis may also be more likely because increased steroid levels during pregnancy may suppress the normal inflammatory response and prevent the “walling off” of the appendix by the omentum.162
In the event of a serious maternal illness, the remote fetal risks associated with anesthesia and surgery are of secondary importance. The primary goal is to preserve the life of the mother. Hypothermia,163 induced hypotension,125 cardiopulmonary bypass,164 and liver transplantation165 have been associated with successful neonatal outcomes. The decision to perform simultaneous cesarean delivery depends on a number of factors, including the gestational age, the risk to the mother of a trial of labor at a later date, and the presence of intra-abdominal sepsis. Cesarean delivery may be performed immediately before the surgical procedure to avoid fetal risks associated with special patient positioning (e.g., the sitting or prone position),166 prolonged anesthesia, major intraoperative blood loss, maternal hyperventilation, deliberate hypotension, or cardiopulmonary bypass.164
Abdominal Emergencies
Acute abdominal disease occurs in 1 in 500 to 1 in 635 pregnancies.167,168 Accurate diagnosis, especially of an acute abdominal crisis (e.g., appendicitis, cholecystitis), can be very difficult during pregnancy.162 Box 17-2 lists some of the conditions that must be considered in the differential diagnosis of abdominal pain during pregnancy. Nausea, vomiting, constipation, and abdominal distention are common symptoms of both normal pregnancy and abdominal pathology. Abdominal tenderness may be indistinguishable from ligamentous or uterine contraction pain. The expanding uterus makes a physical examination of the abdomen difficult. For example, the appendix rotates counterclockwise; thus, as term approaches, the tip typically lies over the right kidney (Figure 17-5).169 Because the white blood cell count in normal pregnancy may reach 15,000/mm3, it must be markedly elevated to be diagnostically helpful. Additional delay results from the reluctance to perform necessary imaging studies involving radiation. In 1991, Mazze and Källén152 reported the misdiagnosis of appendicitis during pregnancy in 36% of cases, with a lower rate (23%) during the first trimester than during the last two trimesters (43%). More reassuringly, using 2005 to 2009 data from the American College of Surgeons (ACS) National Surgical Quality Improvement Program, Silvestri et al.170 compared surgical outcomes in a large cohort of pregnant and nonpregnant women (857 pregnant and 20,029 nonpregnant cases of appendectomy, and 436 pregnant and 32,915 nonpregnant cases of cholecystectomy); no difference was noted in postoperative 30-day morbidity and mortality.
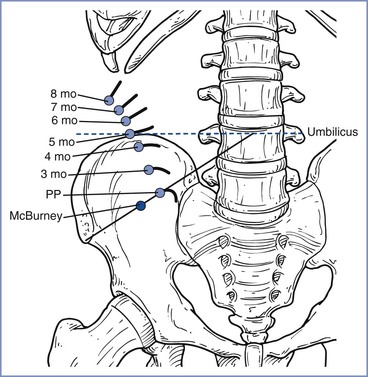
FIGURE 17-5 Changes in position and direction of the appendix during pregnancy. PP, pulse pressure. (Redrawn from Baer JL, Reis RA, Arens RA. Appendicitis in pregnancy with changes in position and axis of normal appendix in pregnancy. JAMA 1932; 98:1359.)
Sometimes, the correct diagnosis is determined only at operation. The selection of the procedure and choice of incision are influenced by the stage of gestation, the nature of the surgical problem, the certainty of the probable diagnosis, and the experience of the surgeon. Laparoscopy is performed during pregnancy for both diagnostic and therapeutic indications with increasing frequency. Laparotomy continues to be performed for many abdominal conditions that occur during the later stages of pregnancy.
Laparoscopy
Concerns exist about the effects of laparoscopy on fetal well-being, especially the risks for (1) uterine or fetal trauma, (2) fetal acidosis from absorbed carbon dioxide, and (3) decreased maternal cardiac output and uteroplacental perfusion resulting from an iatrogenic increase in intra-abdominal pressure. In some animal studies, maternal and fetal acidosis and tachycardia have occurred during intra-abdominal insufflation, perhaps because maternal ventilation was guided by measurements of end-tidal rather than arterial carbon dioxide levels.171 In a study in gravid ewes, a marked increase in PaCO2 to end-tidal CO2 gradient developed during CO2 insufflation, suggesting that PaCO2 should be used to guide ventilation if maternal and fetal acidosis are to be avoided.171 Uteroplacental perfusion decreased by 61% in one study in which gravid ewes were subjected to a CO2 pneumoperitoneum at a pressure of 20 mm Hg (although there were no adverse fetal consequences).172 It is unclear whether the severity of acidosis and decrement in uteroplacental perfusion are related to insufflation pressure.171
Many practitioners believe, however, that the potential benefits of laparoscopic surgery compared with open abdominal surgery outweigh the risks. Potential benefits include (1) shorter hospitalization, (2) less postoperative pain, (3) decreased risk for thromboembolic and wound complications, and (4) faster return to normal activities, including earlier return of normal gastrointestinal function, less uterine irritability, and less fetal depression.173 Laparoscopy is being performed with growing frequency in pregnant women.173–176 In a 1994 survey of laparoendoscopic surgeons, Reedy et al.174 obtained data from 413 laparoscopic procedures performed during pregnancy and reviewed an additional 55 previously published cases. Among the procedures surveyed, 48% were cholecystectomies, 28% were adnexal operations, 16% were appendectomies, and 8% were diagnostic procedures. Thirty-two percent of operations were performed in the first trimester, 54% in the second, and 13% in the third. Several principally retrospective trials comparing open and laparoscopic interventions reported no maternal and fetal outcome differences.175,176
Human clinical studies and clinical experience suggest that the fetal effects of the CO2 pneumoperitoneum and increased intra-abdominal pressure are limited. In one clinical study, there were no differences in the maternal pH, PaCO2, or arterial to end-tidal CO2 pressure gradients before, during, or after termination of the pneumoperitoneum during laparoscopy.177 Steinbrook and Bhavani-Shankar178 used thoracic electrical bioimpedance cardiography to measure changes in cardiac output in four pregnant women undergoing laparoscopic cholecystectomy. They observed hemodynamic changes similar to those that typically occur during laparoscopic surgery in nonpregnant patients (i.e., decrease in cardiac index with concurrent increases in mean arterial pressure and systemic vascular resistance).
Reported clinical experiences with laparoscopy during pregnancy generally have been favorable; complications such as intraoperative perforation of the uterus with the Veress needle have occurred rarely.174 Nonetheless, Amos et al.179 urged caution in a case series study in which they reported fetal death after four of seven laparoscopic procedures. Because of concerns about the fetal effects of CO2 pneumoperitoneum, some practitioners have suggested using a gasless laparoscopic technique.180,181
Careful surgical and anesthetic techniques are critical to avoid problems associated with pregnancy and the special hazards of laparoscopic surgery. The surgeon should be experienced with the technique, and the anesthesia provider must be aware of the accompanying cardiorespiratory changes. In 2007, the Society of American Gastrointestinal Endoscopic Surgeons182 issued “Guidelines for Diagnosis, Treatment, and Use of Laparoscopy for Surgical Problems during Pregnancy” (Box 17-3). According to these guidelines, indications for laparoscopic surgery in pregnant patients do not differ from those for nonpregnant patients and the procedure may be performed during any trimester of pregnancy.
General anesthesia has been used in the vast majority of laparoscopic procedures, although the use of epidural anesthesia has also been described.174 Steinbrook et al.183 described their anesthetic technique for 10 cases of laparoscopic cholecystectomy during pregnancy. They administered general anesthesia with a rapid-sequence induction followed by tracheal intubation and positive-pressure ventilation to maintain end-tidal CO2 between 32 and 36 mm Hg. Anesthesia was maintained with a nondepolarizing muscle relaxant, an opioid, and a volatile halogenated agent, but nitrous oxide was avoided to prevent bowel distention and to allow administration of a higher concentration of inspired oxygen. The pneumoperitoneum resulted in increased peak airway pressure (Figure 17-6) and decreased total lung compliance, changes that were progressively greater with advancing gestation.
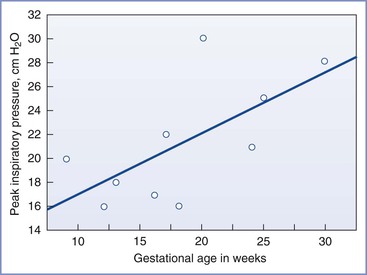
FIGURE 17-6 Peak inspiratory pressure during laparoscopic cholecystectomy during pregnancy as a function of gestational age. The best-fit line by linear regression is shown (y = 12.1 + 0.5x; R2 = 0.43). Peak inspiratory pressure tends to increase with advancing gestation. (Modified from Steinbrook RA, Brooks DC, Datta S. Laparoscopic cholecystectomy during pregnancy. Surg Endosc 1996; 10:511-5.)
The Trendelenburg position exacerbates decreases in FRC, and hypoxemia from airway closure may occur with this position. Hyperventilation, which may be necessary to maintain normal maternal PaCO2, may reduce uteroplacental perfusion and affect fetal oxygenation. Hypotension may result from pneumoperitoneum, aortocaval compression, or use of the reverse Trendelenburg position, and a vasopressor may be needed to maintain maternal blood pressure during laparoscopy.183 As with open surgery, fetal well-being is best preserved by maintaining maternal oxygenation, acid-base status, and hemodynamic parameters within normal pregnancy limits. The FHR and uterine tone should be monitored both before and after surgery (see later discussion).
Electroconvulsive Therapy
Psychiatric disease is an important cause of maternal morbidity and mortality during pregnancy.9 The treatment of major psychiatric disorders during pregnancy, including electroconvulsive therapy, is discussed in Chapter 51. As with the treatment of other diseases during pregnancy, withholding treatment is rarely justified, although the benefits of the treatment should be weighed against the risk for harm to the mother and fetus.
Direct-Current Cardioversion
Direct-current (DC) cardioversion may be necessary during pregnancy (see Chapter 42). It is safe in all stages of pregnancy. The electrical current that reaches the fetus is small.184 Careful FHR monitoring during the procedure is required, as is left uterine displacement to avoid aortocaval compression. The risk for pulmonary aspiration of gastric contents associated with sedation (with an unprotected airway) should be weighed against the risk for general anesthesia with tracheal intubation. Regardless of whether sedation or general anesthesia is selected, a nonparticulate oral antacid should be administered; administration of a histamine-2 receptor antagonist to increase gastric pH should also be considered.
Maternal Cardiac Arrest and Resuscitation
Initiation of treatment for maternal cardiac arrest differs little from that in the nonpregnant patient. Standard Basic Life Support (BLS) and Advanced Cardiac Life Support (ACLS) principles apply to these patients. However, anatomic and physiologic changes of pregnancy require several specific modifications to the resuscitation protocol (see Chapters 42 and 55).185 Left uterine displacement should be maintained during resuscitation, and hand position should be 1 to 2 cm higher on the sternum because of the upward shift of the diaphragm (see Boxes 42-11 and 55-5). Perimortem cesarean delivery is an essential aspect of maternal resuscitation for women in the second half of gestation.186 The primary purpose of the hysterotomy is to improve the chance of maternal survival, but early delivery will also improve the likelihood of fetal survival.185,186 Thus, if initial resuscitative efforts are unsuccessful, perimortem cesarean delivery should be initiated within 4 minutes of the arrest with the goal of delivering the fetus within 5 minutes.185 The reversible causes of cardiac arrest during pregnancy are similar to those in nonpregnant patients. Additional causes specific to pregnancy include amniotic fluid embolism, eclampsia, placental abruption, and hemorrhage.
Fetal Monitoring during Surgery
Continuous FHR monitoring (using transabdominal Doppler ultrasonography) is feasible beginning at 18 to 20 weeks’ gestation.187 However, technical problems may limit the use of continuous FHR monitoring between 18 and 22 weeks’ gestation. Transabdominal monitoring may not be possible during abdominal procedures or when the mother is very obese; thus, the intraoperative use of use of transvaginal Doppler ultrasonography may be considered in selected cases. The American College of Obstetricians and Gynecologists (ACOG) has stated that “the decision to use [intraoperative] fetal monitoring should be individualized and, if used, should be based on gestational age, type of surgery, and facilities available.188 Responses to a survey sent to members of the Association of Professors of Gynecology and Obstetrics indicated that only 43% routinely used intraoperative FHR monitoring.189
FHR variability, which typically is a good indicator of fetal well-being, is present by 25 to 27 weeks’ gestation. Changes in the baseline FHR and FHR variability caused by anesthetic agents or other drugs must be distinguished from changes that result from fetal hypoxia. Persistent severe fetal bradycardia typically indicates true fetal compromise.
Intraoperative FHR monitoring requires the presence of a provider who can interpret the FHR tracing. In addition, a plan should be in place that addresses how to proceed in the event of persistent nonreassuring fetal status, including whether to perform emergency cesarean delivery. The greatest value of intraoperative FHR monitoring is that it allows for the optimization of the maternal condition if the fetus shows signs of compromise. In one case, for example, decreased FHR variability was associated with maternal hypoxia, and the pattern resolved when maternal oxygenation improved (Figure 17-7).139 An unexplained change in FHR mandates the evaluation of maternal position, blood pressure, oxygenation, and acid-base status and the inspection of the surgical site to ensure that neither surgeons nor retractors are impairing uteroplacental perfusion.
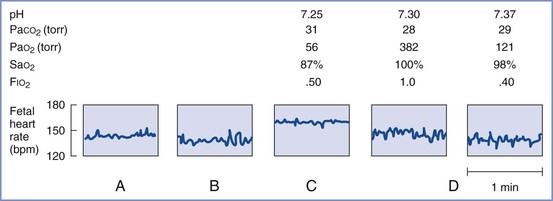
FIGURE 17-7 Serial samples of the fetal heart rate tracing and corresponding maternal arterial blood gas measurements in a patient undergoing eye surgery. A and B, Baseline fetal heart rate of 140 bpm with normal variability. C, Fetal tachycardia and decrease in variability during inadvertent maternal hypoxemia (maternal PaO2 = 56 mm Hg). D, After correction of maternal ventilation, baseline fetal heart rate and variability return. (Redrawn from Katz JD, Hook R, Barash PG. Fetal heart rate monitoring in pregnant patients undergoing surgery. Am J Obstet Gynecol 1976; 125:267.)
Anesthetic Management
Preoperative Management
Premedication may be necessary to allay maternal anxiety. Pregnant women are at increased risk for acid aspiration after 18 to 20 weeks’ gestation (see earlier discussion). Pharmacologic precautions against acid aspiration may include preanesthetic administration of a histamine receptor antagonist, metoclopramide, and a clear nonparticulate antacid such as sodium citrate.
Choice of Anesthesia
Maternal indications and consideration of the site and nature of the surgery should guide the choice of anesthesia. No study has found an association between improved fetal outcome and any specific anesthetic technique, except for a single retrospective medical record analysis in which the use of general anesthesia was associated with a significantly lower birth weight despite similar gestational age at delivery.190 When possible, however, local or regional anesthesia (with the exception of paracervical block) is preferred, because it permits the administration of drugs with no laboratory or clinical evidence of teratogenesis. In addition, maternal respiratory complications occur less frequently with local and regional anesthetic techniques. These techniques are suitable for cervical cerclage and urologic or extremity procedures. Most abdominal operations require general anesthesia, because the incision typically extends to the upper abdomen. This situation may create an unacceptably high risk for aspiration in a pregnant patient with an unprotected airway.
Prevention of Aortocaval Compression
Beginning at 18 to 20 weeks’ gestation, the pregnant patient should be transported on her side, and the uterus should be displaced leftward when she is positioned on the operating table.
Monitoring
Maternal monitoring should include noninvasive or invasive blood pressure measurement, electrocardiography, pulse oximetry, capnography, temperature monitoring, and the use of a peripheral nerve stimulator. The FHR and uterine activity should be monitored both before and after surgery. Intraoperative FHR monitoring may be considered when technically feasible, depending on the ease of monitoring, the type and site of surgery, and gestational age (see earlier discussion).
Anesthetic Technique
General anesthesia mandates tracheal intubation beginning at 18 to 20 weeks’ gestation or if the stomach is full. Denitrogenation (i.e., preoxygenation) should precede the induction of anesthesia. Although rapid-sequence induction with the application of cricoid pressure has been a long-standing practice for the induction of general anesthesia, some experts have argued that it is unnecessary in fasted pregnant women undergoing elective surgery.191 Drugs with a history of safe use during pregnancy include thiopental, propofol, morphine, fentanyl, succinylcholine, and the nondepolarizing muscle relaxants.
A commonly used technique employs a high concentration of oxygen, a muscle relaxant, and an opioid and/or a moderate concentration of a volatile halogenated agent. Scientific evidence does not support avoidance of nitrous oxide during pregnancy,192 particularly after the sixth week of gestation. Omission of nitrous oxide may increase fetal risk if inadequate anesthesia results or if a high dose of a volatile agent results in maternal hypotension. A cautious approach would restrict nitrous oxide administration to a concentration of 50% or less and would limit its use in extremely long operations. Hyperventilation should be avoided; rather, end-tidal CO2 should be maintained in the normal range for pregnancy.
Rapid intravenous infusion of 500 mL of crystalloid immediately before or during the initiation of spinal or epidural anesthesia seems prudent, although the anesthesia provider should not assume that this measure will prevent maternal hypotension. Some anesthesia providers argue that colloids are more effective than crystalloids in preventing hypotension. Vasopressors should be available to treat hypotension if it occurs. Maternal hypotension should be treated aggressively. The usual precautions must be taken to guard against a high neuraxial blockade and systemic local anesthetic toxicity.
Regardless of the anesthetic technique, steps to avoid hypoxemia, hypotension, acidosis, and hyperventilation are the most critical elements of anesthetic management.
Postoperative Management
The FHR and uterine activity should be monitored during recovery from anesthesia. Adequate analgesia should be ensured with systemic or neuraxial opioids, acetaminophen, or neural blockade. Nonsteroidal inflammatory agents may be used until the second half of pregnancy, at which time they should be used with caution. Prophylaxis against venous thrombosis should be considered, especially if patients are immobilized.
References
1. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161:1178–1185.
2. Brodsky JB, Cohen EN, Brown BW Jr, et al. Surgery during pregnancy and fetal outcome. Am J Obstet Gynecol. 1980;138:1165–1167.
3. Kahn RL, Stanton MA, Tong-Ngork S, et al. One-year experience with day-of-surgery pregnancy testing before elective orthopedic procedures. Anesth Analg. 2008;106:1127–1131.
4. Manley S, de Kelaita G, Joseph NJ, et al. Preoperative pregnancy testing in ambulatory surgery: incidence and impact of positive results. Anesthesiology. 1995;83:690–693.
5. Kasliwal A, Farquharson RG. Pregnancy testing prior to sterilisation. BJOG. 2000;107:1407–1409.
6. American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation. Anesthesiology. 2012;116:522–538.
7. National Institute for Health and Clinical Excellence. Preoperative tests: the use of routine preoperative tests for elective surgery. [Available at] www.nice.org.uk/nicemedia/pdf/preop_Fullguideline.pdf [Accessed April 2013] .
8. Lamont T, Coates T, Mathew D, et al. Checking for pregnancy before surgery: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c3402.
9. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
10. Erekson EA, Brousseau EC, Dick-Biascoechea MA, et al. Maternal postoperative complications after nonobstetric antenatal surgery. J Matern Fetal Neonatal Med. 2012;25:2639–2644.
11. Gin T, Chan MT. Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology. 1994;81:829–832.
12. Spaanderman ME, Meertens M, van Bussel M, et al. Cardiac output increases independently of basal metabolic rate in early human pregnancy. Am J Physiol Heart Circ Physiol. 2000;278:H1585–H1588.
13. Capeless EL, Clapp JF. Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol. 1989;161:1449–1453.
14. Duhl AJ, Paidas MJ, Ural SH, et al. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromboembolism and adverse pregnancy outcomes. Am J Obstet Gynecol. 2007;197:457.
15. Macfie AG, Magides AD, Richmond MN, Reilly CS. Gastric emptying in pregnancy. Br J Anaesth. 1991;67:54–57.
16. Brock-Utne JG, Dow TG, Dimopoulos GE, et al. Gastric and lower oesophageal sphincter (LOS) pressures in early pregnancy. Br J Anaesth. 1981;53:381–384.
17. Gin T, Mainland P, Chan MT, Short TG. Decreased thiopental requirements in early pregnancy. Anesthesiology. 1997;86:73–78.
18. Higuchi H, Adachi Y, Arimura S, et al. Early pregnancy does not reduce the C(50) of propofol for loss of consciousness. Anesth Analg. 2001;93:1565–1569.
19. Mongardon N, Servin F, Perrin M, et al. Predicted propofol effect-site concentration for induction and emergence of anesthesia during early pregnancy. Anesth Analg. 2009;109:90–95.
20. Leighton BL, Cheek TG, Gross JB, et al. Succinylcholine pharmacodynamics in peripartum patients. Anesthesiology. 1986;64:202–205.
21. Tsen LC, Tarshis J, Denson DD, et al. Measurements of maternal protein binding of bupivacaine throughout pregnancy. Anesth Analg. 1999;89:965–968.
22. Feghali MN, Mattison DR. Clinical therapeutics in pregnancy. J Biomed Biotechnol. 2011;2011:783528.
23. Allegaert K, van Mieghem T, Verbesselt R, et al. Cefazolin pharmacokinetics in maternal plasma and amniotic fluid during pregnancy. Am J Obstet Gynecol. 2009;200:170.
24. Allegaert K, Kulo A, Verbesselt R, et al. The pharmacokinetics of a high intravenous dose of paracetamol after caesarean delivery: the effect of gestational age. Eur J Anaesthesiol. 2012;29:484–488.
25. Wilson JG. Environment and Birth Defects. Academic Press: New York; 1973.
26. Shepard TH, Lemire RJ. Catalog of Teratogenic Agents. 13th edition. Johns Hopkins University Press: Baltimore; 2010.
27. Roberts CJ, Lowe CR. Where have all the conceptions gone? Lancet. 1975;305:498–499.
28. Haring OM. Effects of prenatal hypoxia on the cardiovascular system in the rat. Arch Pathol. 1965;80:351–356.
29. Geber WF. Developmental effects of chronic maternal audiovisual stress on the rat fetus. J Embryol Exp Morphol. 1966;16:1–16.
30. Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24:31–41.
31. Lowe SA. Diagnostic radiography in pregnancy: risks and reality. Aust N Z J Obstet Gynaecol. 2004;44:191–196.
32. International Commission on Radiological Protection. Pregnancy and medical radiation. Ann ICRP. 2000;30:1–43.
33. Dauer LT, Thornton RH, Miller DL, et al. Radiation management for interventions using fluoroscopic or computed tomographic guidance during pregnancy: a joint guideline of the Society of Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2012;23:19–32.
34. American College of Obstetricians and Gynecologists. Guidelines for diagnostic imaging during pregnancy. ACOG Committee Opinion No. 299. September 2004 (reaffirmed 2009). (Obstet Gynecol 2004; 104:647-51.).
35. Fisher JE Jr, Acuff-Smith KD, Schilling MA, et al. Teratologic evaluation of rats prenatally exposed to pulsed-wave ultrasound. Teratology. 1994;49:150–155.
36. Vorhees CV, Acuff-Smith KD, Schilling MA, et al. Behavioral teratologic effects of prenatal exposure to continuous-wave ultrasound in unanesthetized rats. Teratology. 1994;50:238–249.
37. Hande MP, Devi PU. Teratogenic effects of repeated exposures to X-rays and/or ultrasound in mice. Neurotoxicol Teratol. 1995;17:179–188.
38. Miller MW, Nyborg WL, Dewey WC, et al. Hyperthermic teratogenicity, thermal dose and diagnostic ultrasound during pregnancy: implications of new standards on tissue heating. Int J Hyperthermia. 2002;18:361–384.
39. De Santis M, Straface G, Cavaliere AF, et al. Gadolinium periconceptional exposure: pregnancy and neonatal outcome. Acta Obstet Gynecol Scand. 2007;86:99–101.
40. Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530.
41. Jurand A, Martin LV. Teratogenic potential of two neurotropic drugs, haloperidol and dextromoramide, tested on mouse embryos. Teratology. 1990;42:45–54.
42. Ciociola AA, Gautieri RF. Evaluation of the teratogenicity of morphine sulfate administered via a miniature implantable pump. J Pharm Sci. 1983;72:742–745.
43. Fujinaga M, Mazze RI. Teratogenic and postnatal developmental studies of morphine in Sprague-Dawley rats. Teratology. 1988;38:401–410.
44. Fujinaga M, Stevenson JB, Mazze RI. Reproductive and teratogenic effects of fentanyl in Sprague-Dawley rats. Teratology. 1986;34:51–57.
46. Martin LV, Jurand A. The absence of teratogenic effects of some analgesics used in anaesthesia: additional evidence from a mouse model. Anaesthesia. 1992;47:473–476.
47. Finnell RH, Shields HE, Taylor SM, Chernoff GF. Strain differences in phenobarbital-induced teratogenesis in mice. Teratology. 1987;35:177–185.
48. Tonge SR. Permanent alterations in catecholamine concentrations in discrete areas of brain in the offspring of rats treated with methylamphetamine and chlorpromazine. Br J Pharmacol. 1973;47:425–427.
49. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: a Reference Guide to Fetal and Neonatal Risk. 9th edition. Lippincott Williams & Wilkins: Philadelphia; 2011.
50. Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436.
51. Nikizad H, Yon JH, Carter LB, Jevtovic-Todorovic V. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann N Y Acad Sci. 2007;1122:69–82.
52. Safra MJ, Oakley GP Jr. Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet. 1975;2:478–480.
53. Shiono PH, Mills JL. Oral clefts and diazepam use during pregnancy. N Engl J Med. 1984;311:919–920.
54. Sturrock JE, Nunn JF. Cytotoxic effects of procaine, lignocaine and bupivacaine. Br J Anaesth. 1979;51:273–281.
55. Fujinaga M, Mazze RI. Reproductive and teratogenic effects of lidocaine in Sprague-Dawley rats. Anesthesiology. 1986;65:626–632.
56. Fujinaga M, Baden JM, Mazze RI. Developmental toxicity of nondepolarizing muscle relaxants in cultured rat embryos. Anesthesiology. 1992;76:999–1003.
57. Jacobs RM. Failure of muscle relaxants to produce cleft palate in mice. Teratology. 1971;4:25–29.
58. Jago RH. Arthrogryposis following treatment of maternal tetanus with muscle relaxants. Arch Dis Child. 1970;45:277–279.
59. Basford AB, Fink BR. The teratogenicity of halothane in the rat. Anesthesiology. 1968;29:1167–1173.
60. Wharton RS, Mazze RI, Baden JM, et al. Fertility, reproduction and postnatal survival in mice chronically exposed to halothane. Anesthesiology. 1978;48:167–174.
61. Mazze RI. Fertility, reproduction, and postnatal survival in mice chronically exposed to isoflurane. Anesthesiology. 1985;63:663–667.
62. Mazze RI, Wilson AI, Rice SA, Baden JM. Fetal development in mice exposed to isoflurane. Teratology. 1985;32:339–345.
63. Mazze RI, Fujinaga M, Rice SA, et al. Reproductive and teratogenic effects of nitrous oxide, halothane, isoflurane, and enflurane in Sprague-Dawley rats. Anesthesiology. 1986;64:339–344.
64. Mazze RI, Wilson AI, Rice SA, Baden JM. Reproduction and fetal development in rats exposed to nitrous oxide. Teratology. 1984;30:259–265.
65. Lane GA, Nahrwold ML, Tait AR, et al. Anesthetics as teratogens: nitrous oxide is fetotoxic, xenon is not. Science. 1980;210:899–901.
66. Vieira E, Cleaton-Jones P, Austin JC, et al. Effects of low concentrations of nitrous oxide on rat fetuses. Anesth Analg. 1980;59:175–177.
67. Fujinaga M, Baden JM, Mazze RI. Susceptible period of nitrous oxide teratogenicity in Sprague-Dawley rats. Teratology. 1989;40:439–444.
68. Fujinaga M, Baden JM. Critical period of rat development when sidedness of asymmetric body structures is determined. Teratology. 1991;44:453–462.
69. Baden JM, Fujinaga M. Effects of nitrous oxide on day 9 rat embryos grown in culture. Br J Anaesth. 1991;66:500–503.
70. Chanarin I. Cobalamins and nitrous oxide: a review. J Clin Pathol. 1980;33:909–916.
71. Koblin DD, Watson JE, Deady JE, et al. Inactivation of methionine synthetase by nitrous oxide in mice. Anesthesiology. 1981;54:318–324.
72. Koblin DD, Waskell L, Watson JE, et al. Nitrous oxide inactivates methionine synthetase in human liver. Anesth Analg. 1982;61:75–78.
73. Baden JM, Rice SA, Serra M, et al. Thymidine and methionine syntheses in pregnant rats exposed to nitrous oxide. Anesth Analg. 1983;62:738–741.
74. Keeling PA, Rocke DA, Nunn JF, et al. Folinic acid protection against nitrous oxide teratogenicity in the rat. Br J Anaesth. 1986;58:528–534.
75. Mazze RI, Fujinaga M, Baden JM. Halothane prevents nitrous oxide teratogenicity in Sprague-Dawley rats; folinic acid does not. Teratology. 1988;38:121–127.
76. Fujinaga M, Baden JM, Yhap EO, Mazze RI. Reproductive and teratogenic effects of nitrous oxide, isoflurane, and their combination in Sprague-Dawley rats. Anesthesiology. 1987;67:960–964.
77. Fujinaga M, Baden JM. Methionine prevents nitrous oxide-induced teratogenicity in rat embryos grown in culture. Anesthesiology. 1994;81:184–189.
78. Fujinaga M, Baden JM. Evidence for an adrenergic mechanism in the control of body asymmetry. Dev Biol. 1991;143:203–205.
79. Fujinaga M, Maze M, Hoffman BB, Baden JM. Activation of alpha-1 adrenergic receptors modulates the control of left/right sidedness in rat embryos. Dev Biol. 1992;150:419–421.
80. Fujinaga M, Baden JM, Suto A, et al. Preventive effects of phenoxybenzamine on nitrous oxide-induced reproductive toxicity in Sprague-Dawley rats. Teratology. 1991;43:151–157.
81. Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882.
82. Cohen EN, Bellville JW, Brown BW Jr. Anesthesia, pregnancy, and miscarriage: a study of operating room nurses and anesthetists. Anesthesiology. 1971;35:343–347.
83. Knill-Jones RP, Rodrigues LV, Moir DD, Spence AA. Anaesthetic practice and pregnancy: controlled survey of women anaesthetists in the United Kingdom. Lancet. 1972;1:1326–1328.
84. American Society of Anesthesiologists Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room Personnel. Occupational disease among operating room personnel: a national study. Anesthesiology. 1974;41:321–340.
85. Cohen EN, Gift HC, Brown BW, et al. Occupational disease in dentistry and chronic exposure to trace anesthetic gases. J Am Dent Assoc. 1980;101:21–31.
86. Spence AA, Cohen EN, Brown BW Jr, et al. Occupational hazards for operating room-based physicians: analysis of data from the United States and the United Kingdom. JAMA. 1977;238:955–959.
87. Buring JE, Hennekens CH, Mayrent SL, et al. Health experiences of operating room personnel. Anesthesiology. 1985;62:325–330.
88. Tannenbaum TN, Goldberg RJ. Exposure to anesthetic gases and reproductive outcome: a review of the epidemiologic literature. J Occup Med. 1985;27:659–668.
89. Mazze RI, Lecky JH. The health of operating room personnel. Anesthesiology. 1985;62:226–228.
90. Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2:173–176.
91. Kline J, Stein ZA, Susser M, Warburton D. Smoking: a risk factor for spontaneous abortion. N Engl J Med. 1977;297:793–796.
92. Shirangi A, Fritschi L, Holman CD. Associations of unscavenged anesthetic gases and long working hours with preterm delivery in female veterinarians. Obstet Gynecol. 2009;113:1008–1017.
93. Ericson HA, Källén AJ. Hospitalization for miscarriage and delivery outcome among Swedish nurses working in operating rooms 1973-1978. Anesth Analg. 1985;64:981–988.
94. Spence AA. Environmental pollution by inhalation anaesthetics. Br J Anaesth. 1987;59:96–103.
95. Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327:993–997.
96. Smith BE. Fetal prognosis after anesthesia during gestation. Anesth Analg. 1963;42:521–526.
98. Crawford JS, Lewis M. Nitrous oxide in early human pregnancy. Anaesthesia. 1986;41:900–905.
99. Aldridge LM, Tunstall ME. Nitrous oxide and the fetus: a review and the results of a retrospective study of 175 cases of anaesthesia for insertion of Shirodkar suture. Br J Anaesth. 1986;58:1348–1356.
100. Duncan PG, Pope WD, Cohen MM, Greer N. Fetal risk of anesthesia and surgery during pregnancy. Anesthesiology. 1986;64:790–794.
101. Källén B, Mazze RI. Neural tube defects and first trimester operations. Teratology. 1990;41:717–720.
102. Sylvester GC, Khoury MJ, Lu X, Erickson JD. First-trimester anesthesia exposure and the risk of central nervous system defects: a population-based case-control study. Am J Public Health. 1994;84:1757–1760.
103. Smith RF, Bowman RE, Katz J. Behavioral effects of exposure to halothane during early development in the rat: sensitive period during pregnancy. Anesthesiology. 1978;49:319–323.
104. Levin ED, Bowman RE. Behavioral effects of chronic exposure to low concentrations of halothane during development in rats. Anesth Analg. 1986;65:653–659.
105. Chalon J, Hillman D, Gross S, et al. Intrauterine exposure to halothane increases murine postnatal autotolerance to halothane and reduces brain weight. Anesth Analg. 1983;62:565–567.
106. Armitage SG. The effects of barbiturates on the behavior of rat offspring as measured in learning and reasoning situations. J Comp Physiol Psychol. 1952;45:146–152.
107. Chalon J, Walpert L, Ramanathan S, et al. Meperidine-promethazine combination and learning function of mice and of their progeny. Can Anaesth Soc J. 1982;29:612–616.
108. Hoffeld DR, McNew J, Webster RL. Effect of tranquilizing drugs during pregnancy on activity of offspring. Nature. 1968;218:357–358.
109. Palanisamy A, Baxter MG, Keel PK, et al. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–528.
110. Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74.
111. Ishimaru MJ, Ikonomidou C, Tenkova TI, et al. Distinguishing excitotoxic from apoptotic neurodegeneration in the developing rat brain. J Comp Neurol. 1999;408:461–476.
112. Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297.
113. Anand KJ, Soriano SG. Anesthetic agents and the immature brain: are these toxic or therapeutic? Anesthesiology. 2004;101:527–530.
114. Bhutta AT, Anand KJ. Vulnerability of the developing brain: neuronal mechanisms. Clin Perinatol. 2002;29:357–372.
115. Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr Anaesth. 2002;12:770–774.
116. Ruda MA, Ling QD, Hohmann AG, et al. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631.
117. McClaine RJ, Uemura K, de la Fuente SG, et al. General anesthesia improves fetal cerebral oxygenation without evidence of subsequent neuronal injury. J Cereb Blood Flow Metab. 2005;25:1060–1069.
118. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. [Anesthetic and Life Support Drugs Advisory Committee Meeting, Rockville, MD, March 29, 2007. Available at] www.fda.gov/ohrms/dockets/ac/07/transcripts/2007-4285t1.pdf [Accessed May 2013] .
119. Panigel M. Placental perfusion experiments. Am J Obstet Gynecol. 1962;84:1664–1683.
120. Khazin AF, Hon EH, Hehre FW. Effects of maternal hyperoxia on the fetus. I. Oxygen tension. Am J Obstet Gynecol. 1971;109:628–637.
121. Motoyama EK, Rivard G, Acheson F, Cook CD. The effect of changes in maternal pH and PCO2 on the PO2 of fetal lambs. Anesthesiology. 1967;28:891–903.
122. Kambam JR, Handte RE, Brown WU, Smith BE. Effect of normal and preeclamptic pregnancies on the oxyhemoglobin dissociation curve. Anesthesiology. 1986;65:426–427.
123. Levinson G, Shnider SM, DeLorimier AA, Steffenson JL. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974;40:340–347.
124. Brann AW Jr, Myers RE. Central nervous system findings in the newborn monkey following severe in utero partial asphyxia. Neurology. 1975;25:327–338.
125. Newman B, Lam AM. Induced hypotension for clipping of a cerebral aneurysm during pregnancy: a case report and brief review. Anesth Analg. 1986;65:675–678.
126. Shnider SM, Wright RG, Levinson G, et al. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology. 1979;50:524–527.
127. Oats JN, Vasey DP, Waldron BA. Effects of ketamine on the pregnant uterus. Br J Anaesth. 1979;51:1163–1166.
128. Greiss FC Jr, Still JG, Anderson SG. Effects of local anesthetic agents on the uterine vasculatures and myometrium. Am J Obstet Gynecol. 1976;124:889–899.
129. Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–926.
130. Cooper DW, Carpenter M, Mowbray P, et al. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2002;97:1582–1590.
131. Ngan Kee WD, Khaw KS, Tan PE, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
132. Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–765.
133. Palahniuk RJ, Shnider SM. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462–472.
134. Palahniuk RJ, Doig GA, Johnson GN, Pash MP. Maternal halothane anesthesis reduces cerebral blood flow in the acidotic sheep fetus. Anesth Analg. 1980;59:35–39.
135. Cheek DB, Hughes SC, Dailey PA, et al. Effect of halothane on regional cerebral blood flow and cerebral metabolic oxygen consumption in the fetal lamb in utero. Anesthesiology. 1987;67:361–366.
136. Yarnell R, Biehl DR, Tweed WA, et al. The effect of halothane anaesthesia on the asphyxiated foetal lamb in utero. Can Anaesth Soc J. 1983;30:474–479.
137. Baker BW, Hughes SC, Shnider SM, et al. Maternal anesthesia and the stressed fetus: effects of isoflurane on the asphyxiated fetal lamb. Anesthesiology. 1990;72:65–70.
138. Johnson ES, Colley PS. Effects of nitrous oxide and fentanyl anesthesia on fetal heart-rate variability intra- and postoperatively. Anesthesiology. 1980;52:429–430.
139. Liu PL, Warren TM, Ostheimer GW, et al. Foetal monitoring in parturients undergoing surgery unrelated to pregnancy. Can Anaesth Soc J. 1985;32:525–532.
140. Van de Velde M, Teunkens A, Kuypers M, et al. General anaesthesia with target controlled infusion of propofol for planned caesarean section: maternal and neonatal effects of a remifentanil-based technique. Int J Obstet Anesth. 2004;13:153–158.
141. Heesen M, Klohr S, Hofmann T, et al. Maternal and foetal effects of remifentanil for general anaesthesia in parturients undergoing caesarean section: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2013;57:29–36.
142. McNall PG, Jafarnia MR. Management of myasthenia gravis in the obstetrical patient. Am J Obstet Gynecol. 1965;92:518–525.
143. Hellman LM, Johnson HL, Tolles WE, Jones EH. Some factors affecting the fetal heart rate. Am J Obstet Gynecol. 1961;82:1055–1063.
144. Abboud T, Raya J, Sadri S, et al. Fetal and maternal cardiovascular effects of atropine and glycopyrrolate. Anesth Analg. 1983;62:426–430.
146. Naulty J, Cefalo RC, Lewis PE. Fetal toxicity of nitroprusside in the pregnant ewe. Am J Obstet Gynecol. 1981;139:708–711.
147. Ostman PL, Chestnut DH, Robillard JE, et al. Transplacental passage and hemodynamic effects of esmolol in the gravid ewe. Anesthesiology. 1988;69:738–741.
148. Eisenach JC, Castro MI. Maternally administered esmolol produces fetal beta-adrenergic blockade and hypoxemia in sheep. Anesthesiology. 1989;71:718–722.
149. Larson CP Jr, Shuer LM, Cohen SE. Maternally administered esmolol decreases fetal as well as maternal heart rate. J Clin Anesth. 1990;2:427–429.
150. Losasso TJ, Muzzi DA, Cucchiara RF. Response of fetal heart rate to maternal administration of esmolol. Anesthesiology. 1991;74:782–784.
151. Ducey JP, Knape KG. Maternal esmolol administration resulting in fetal distress and cesarean section in a term pregnancy. Anesthesiology. 1992;77:829–832.
152. Mazze RI, Källén B. Appendectomy during pregnancy: a Swedish registry study of 778 cases. Obstet Gynecol. 1991;77:835–840.
153. Ferguson JE, Albright GA, Ueland K. Prevention of preterm labor following surgery. Baden JM, Brodsky JB. The Pregnant Surgical Patient. Futura Publishing: Mount Kisco, NY; 1985:223–246.
154. Shim JY, Park YW, Yoon BH, et al. Multicentre, parallel group, randomised, single-blind study of the safety and efficacy of atosiban versus ritodrine in the treatment of acute preterm labour in Korean women. BJOG. 2006;113:1228–1234.
155. Ceanga M, Spataru A, Zagrean AM. Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neurosci Lett. 2010;477:15–18.
156. Doyle LW. Antenatal magnesium sulfate and neuroprotection. Curr Opin Pediatr. 2012;24:154–159.
157. Kim MH, Oh AY, Jeon YT, et al. A randomised controlled trial comparing rocuronium priming, magnesium pre-treatment and a combination of the two methods. Anaesthesia. 2012;67:748–754.
158. Hans GA, Bosenge B, Bonhomme VL, et al. Intravenous magnesium re-establishes neuromuscular block after spontaneous recovery from an intubating dose of rocuronium: a randomised controlled trial. Eur J Anaesthesiol. 2012;29:95–99.
159. Olgun B, Oguz G, Kaya M, et al. The effects of magnesium sulphate on desflurane requirement, early recovery and postoperative analgesia in laparascopic cholecystectomy. Magnes Res. 2012;25:72–78.
160. Na HS, Chung YH, Hwang JW, Do SH. Effects of magnesium sulphate on postoperative coagulation, measured by rotational thromboelastometry (ROTEM). Anaesthesia. 2012;67:862–869.
161. McKellar DP, Anderson CT, Boynton CJ, Peoples JB. Cholecystectomy during pregnancy without fetal loss. Surg Gynecol Obstet. 1992;174:465–468.
162. Cherry SH. The pregnant patient: need for surgery unrelated to pregnancy. Mt Sinai J Med. 1991;58:81–84.
163. Stånge K, Halldin M. Hypothermia in pregnancy. Anesthesiology. 1983;58:460–461.
164. Strickland RA, Oliver WC Jr, Chantigian RC, et al. Anesthesia, cardiopulmonary bypass, and the pregnant patient. Mayo Clin Proc. 1991;66:411–429.
165. Merritt WT, Dickstein R, Beattie C, et al. Liver transplantation during pregnancy: anesthesia for two procedures in the same patient with successful outcome of pregnancy. Transplant Proc. 1991;23:1996–1997.
166. Buckley TA, Yau GH, Poon WS, Oh T. Caesarean section and ablation of a cerebral arterio-venous malformation. Anaesth Intensive Care. 1990;18:248–251.
167. Coleman MT, Trianfo VA, Rund DA. Nonobstetric emergencies in pregnancy: trauma and surgical conditions. Am J Obstet Gynecol. 1997;177:497–502.
168. Augustin G, Majerovic M. Non-obstetrical acute abdomen during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2007;131:4–12.
169. Babaknia A, Parsa H, Woodruff JD. Appendicitis during pregnancy. Obstet Gynecol. 1977;50:40–44.
170. Silvestri MT, Pettker CM, Brousseau EC, et al. Morbidity of appendectomy and cholecystectomy in pregnant and nonpregnant women. Obstet Gynecol. 2011;118:1261–1270.
171. Cruz AM, Southerland LC, Duke T, et al. Intraabdominal carbon dioxide insufflation in the pregnant ewe: uterine blood flow, intraamniotic pressure, and cardiopulmonary effects. Anesthesiology. 1996;85:1395–1402.
172. Barnard JM, Chaffin D, Droste S, et al. Fetal response to carbon dioxide pneumoperitoneum in the pregnant ewe. Obstet Gynecol. 1995;85:669–674.
173. Fatum M, Rojansky N. Laparoscopic surgery during pregnancy. Obstet Gynecol Surv. 2001;56:50–59.
174. Reedy MB, Galan HL, Richards WE, et al. Laparoscopy during pregnancy: a survey of laparoendoscopic surgeons. J Reprod Med. 1997;42:33–38.
175. Buser KB. Laparoscopic surgery in the pregnant patient: results and recommendations. JSLS. 2009;13:32–35.
176. Corneille MG, Gallup TM, Bening T, et al. The use of laparoscopic surgery in pregnancy: evaluation of safety and efficacy. Am J Surg. 2010;200:363–367.
177. Bhavani-Shankar K, Steinbrook RA, Brooks DC, Datta S. Arterial to end-tidal carbon dioxide pressure difference during laparoscopic surgery in pregnancy. Anesthesiology. 2000;93:370–373.
178. Steinbrook RA, Bhavani-Shankar K. Hemodynamics during laparoscopic surgery in pregnancy. Anesth Analg. 2001;93:1570–1571.
179. Amos JD, Schorr SJ, Norman PF, et al. Laparoscopic surgery during pregnancy. Am J Surg. 1996;171:435–437.
180. Tanaka H, Futamura N, Takubo S, Toyoda N. Gasless laparoscopy under epidural anesthesia for adnexal cysts during pregnancy. J Reprod Med. 1999;44:929–932.
181. Melgrati L, Damiani A, Franzoni G, et al. Isobaric (gasless) laparoscopic myomectomy during pregnancy. J Minim Invasive Gynecol. 2005;12:379–381.
182. Guidelines Committee of the Society of American Gastrointestinal Endoscopic Surgeons. Guidelines for diagnosis, treatment, and use of laparoscopy for surgical problems during pregnancy. Surg Endosc. 2008;22:849–861.
183. Steinbrook RA, Brooks DC, Datta S. Laparoscopic cholecystectomy during pregnancy: review of anesthetic management, surgical considerations. Surg Endosc. 1996;10:511–515.
184. Adamson DL, Nelson-Piercy C. Managing palpitations and arrhythmias during pregnancy. Heart. 2007;93:1630–1636.
185. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S829–S861.
186. Einav S, Kaufman N, Sela HY. Maternal cardiac arrest and perimortem caesarean delivery: evidence or expert-based? Resuscitation. 2012;83:1191–1200.
187. Biehl DR. Foetal monitoring during surgery unrelated to pregnancy. Can Anaesth Soc J. 1985;32:455–459.
188. American College of Obstetricians and Gynecologists. Nonobstetric surgery during pregnancy. ACOG Committee Opinion No. 474. (Obstet Gynecol 2011; 117:420-1.).
189. Kilpatrick CC, Puig C, Chohan L, et al. Intraoperative fetal heart rate monitoring during nonobstetric surgery in pregnancy: a practice survey. South Med J. 2010;103:212–215.
190. Jenkins TM, Mackey SF, Benzoni EM, et al. Non-obstetric surgery during gestation: risk factors for lower birthweight. Aust N Z J Obstet Gynaecol. 2003;43:27–31.
191. de Souza DG, Doar LH, Mehta SH, Tiouririne M. Aspiration prophylaxis and rapid sequence induction for elective cesarean delivery: time to reassess old dogma? Anesth Analg. 2010;110:1503–1505.
192. Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: a mechanistic and toxicologic review. Anesthesiology. 2008;109:707–722.