Nicotine Dependence
Current Treatments and Future Directions
Maher Karam-Hage, Vance Rabius, Paul M. Cinciripini, Jason D. Robinson and Ellen R. Gritz
Prevalence of Tobacco Use and Nicotine Dependence in Patients with Cancer
• Approximately one in five American adults is a current smoker.
• Smoking accounts for one third of all cancer deaths.
• Patients with cancer in general have a higher dependence on nicotine and are more likely to be smokers or ex-smokers.
• Approximately 15.1% of adult cancer survivors are current smokers.
Biological Characteristics and Genetics
• The reward pathway and dopamine are involved in the use, abuse, and dependence of all such substances, including nicotine.
• Pharmacogenetics research has the premise to tailor treatment to enhance efficacy and reduce toxicity.
• Several instruments measure nicotine dependence; among them are the Fagerström Test for Nicotine Dependence and the Wisconsin Smoking Withdrawal Scale. However, the evaluation and final recommendation are clinical, and the treatment plan must be individualized.
Current Treatment Recommendations
• Nonpharmacologic treatments include behavioral counseling (e.g., identifying triggers and managing withdrawal), quitlines, and self-help material (e.g., booklets and videos).
• Nonpharmacologic approaches are popular but yield relatively low quit rates.
• Pharmacologic treatments approved by the U.S. Food and Drug Administration, including nicotine replacement therapies (e.g., transdermal patch, gum, nasal spray, inhaler, and lozenge) and bupropion, have been shown to double smoking cessation rates compared with placebo. Varenicline is the newest agent, with efficacy superior to that of nicotine replacement therapies and bupropion.
• About half of patients with cancer continue to smoke after diagnosis, even though tobacco use complicates cancer treatment, reduces survivorship rates, increases the risk for a second primary tumor, and diminishes quality of life.
• Few studies have examined predictors of continued smoking among patients with cancer, but some studies have reported on such factors as nonsmoking-related cancers, comorbid depression, and poor prognosis.
• Several retrospective studies have shown the detrimental effect of continuing to smoke on cancer treatment outcomes. A limited number of randomized controlled trials of smoking cessation treatments have been conducted among patients with cancer. These reports suggest that a combination of medications and a behavioral approach are needed to make a difference.
• Patients with cancer who use tobacco should be treated according to evidence-based treatment guidelines with particular attention to tailoring education about their disease-tobacco link, pharmacotherapy, comorbid medical and psychiatric disorders, and family/household tobacco use.
Barriers to Cessation Treatment in the Oncology Setting
• Health care providers have limited time and expertise to address smoking among patients with cancer, and patients may have comorbid substance use/dependence or other emotional and mental disorders that undermine their ability to quit smoking.
• Systems-level changes and tailored treatment approaches are thought to be needed to lower the rate of persistent tobacco use and recidivism among patients with cancer.
Introduction
Tobacco use has become a worldwide epidemic, and cigarettes are the most common form of tobacco consumed. Smoking cigarettes constitutes the most sophisticated system of drug delivery (nicotine) to the brain, resulting in diseases that are among the most common and the deadliest and yet the most preventable of those involving human behavior: respiratory diseases, cardiovascular diseases, and cancer.1 Tobacco use accounts for at least 30% of all cancer deaths in the United States, and smoking has been causally linked to 18 different cancers, including lung, head and neck, esophageal, pancreatic, bladder, kidney, cervical, endometrial, and gastric cancers and acute myeloid leukemia.2 The scare of a diagnosis of cancer often impels smokers to quit, and although some people resume use of tobacco once their cancer is in remission, others manage to avoid a relapse.
The high recidivism rates could be attributed to comorbid conditions and to the neurobiology of tobacco addiction. Tobacco addiction is a complex phenomenon. Nicotine receptors are spread throughout most areas of the brain, and nicotine consumption activates the reward pathway, like all other substances of addiction.3 In addition, several other neurotransmitter systems are implicated in the process of establishing an addiction to nicotine.4 After years of tobacco consumption, complex neuronal involvements and adaptations develop, rendering the extinguishing of tobacco addiction a challenging task. However, pharmacologic and behavioral treatments have proven to be essential and highly effective in comparison with other medical treatments of chronic diseases.5 In recent years, individualized treatment has become the gold standard, with hopes that advancement in genetics will allow us to eventually match the best treatment to each individual profile.6 Unfortunately, multiple hurdles and obstacles remain in the way of extending basic treatment to all smokers and tobacco users, including those diagnosed with cancer and cancer survivors.7 In this chapter we summarize these aspects of tobacco use and their implication for patients with cancer.
Epidemiology and Tobacco Use in Patients with Cancer
Tobacco use, in particular smoking cigarettes, began an upward trend around the beginning of the twentieth century. This trend has been mostly attributed to James Buchanan “Buck” Duke, who introduced the mechanized manufacturing of cigarettes and used aggressive marketing techniques,8 in addition to other societal forces and circumstances such as the free cigarettes provided with daily rations to all enlisted American soldiers during World Wars I and II.9 That upward trend continued until a pivotal moment: the release of the landmark first Surgeon General’s report on smoking and health in 1964,10 at which point the evidence from more than 7000 scientific publications on harm from tobacco smoke was put together and received the endorsement of Surgeon General Luther Terry, the highest officer for public health within the United States government. That report had an enduring effect on public beliefs and attitudes about tobacco. As an example, a Gallup Survey conducted in 1958 found that only 44% of Americans believed smoking caused cancer, whereas 70% believed so by 1969, 5 years after the report was published. The belief that smoking causes cancer continued to rise, and the rate of belief reached 94% by 1990.11 Furthermore, since 1964 a gradual and almost annual decrease in smoking rates has occurred in the United States, with the rate reaching a plateau of around 20% in the past 5 years. This lower rate nonetheless encompasses 60 million Americans who, almost 50 years after the landmark report, continue to use tobacco despite the clear knowledge of harm from its consumption. Other Surgeon General reports subsequently solidified the case against tobacco consumption, and to date 33 of those reports have been dedicated to tobacco, focusing on specific topics such as nicotine addiction, secondhand smoke, minorities, women, and youth.12 Although these efforts have improved the public health, unfortunately, disparities in health care extend to tobacco consumption, as evidenced by higher smoking rates among American Indians/Alaskan Natives, persons with diagnosis of mental disorders, those who are under the poverty level of income, and the unemployed. It is of importance that level of education is inversely related to smoking rates.13
Although the trend for smoking among patients older than 45 years and who are recovering from cancer has decreased to about the same as for the general population, the prevalence among survivors in the younger age group is still high. Around 40.4% of the persons 18 to 44 years of age in the survivors cohort still smoke cigarettes compared with 24.7% in the general population of the same age range.14 Nevertheless, one potential confounding factor contributing to these statistics is the high mortality rate in patients with smoking-related tumors. Therefore smokers who would survive less lethal cancers are counted within the younger age group.15
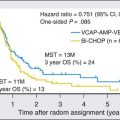
Smoking cessation rates in the cancer population seem highly dependent on the disease (tumor) site and its relationship to smoking.16 As an example, around 80% of patients with lung cancer and 65% of patients with head and neck cancer were reported to stop smoking in the year after their treatment17,18 compared with 31% of patients with bladder cancer.19 Other factors come into play in improving smoking cessation among patients with cancer, such as having a good prognosis, having spent a long time in the hospital (a smoke-free environment), and having treatment-related adverse effects. In particular, adverse effects of treatment such as nausea, difficulty breathing, facial surgery, or difficulty swallowing may make it harder or more difficult to smoke. Consequently, physicians should pay attention to the particular smoking-cessation pattern in patients with cancer. Typically, cancer survivors have an increased chance of a delayed relapse to smoking 1 to 6 months after treatment,20 in contrast to persons in the general population, who generally relapse within the first week or so of cessation. Unfortunately, it has been reported that patients with lung cancer who have already abstained from smoking prior to their diagnosis tend to return to smoking once they are informed of their cancer.21 Many studies have been published on the benefits of smoking cessation in patients with cancer; many of the studies focus in particular on cancer treatment–related outcome (Table 24-1). Unfortunately, almost all published studies to that effect have methodologic limitations such as retrospective analyses, nonstandardized tobacco assessment, no biochemical confirmation, and no tracking of behavior after diagnosis. Addressing smoking behavior at the time of diagnosis and throughout treatment and recovery is thus essential for persons with cancer, constituting a continuum of potential teachable moments as described by McBride et al.22
Table 24-1
Continued Smoking Versus Cessation As It Relates to Cancer Treatment Outcomes in Retrospective Studies
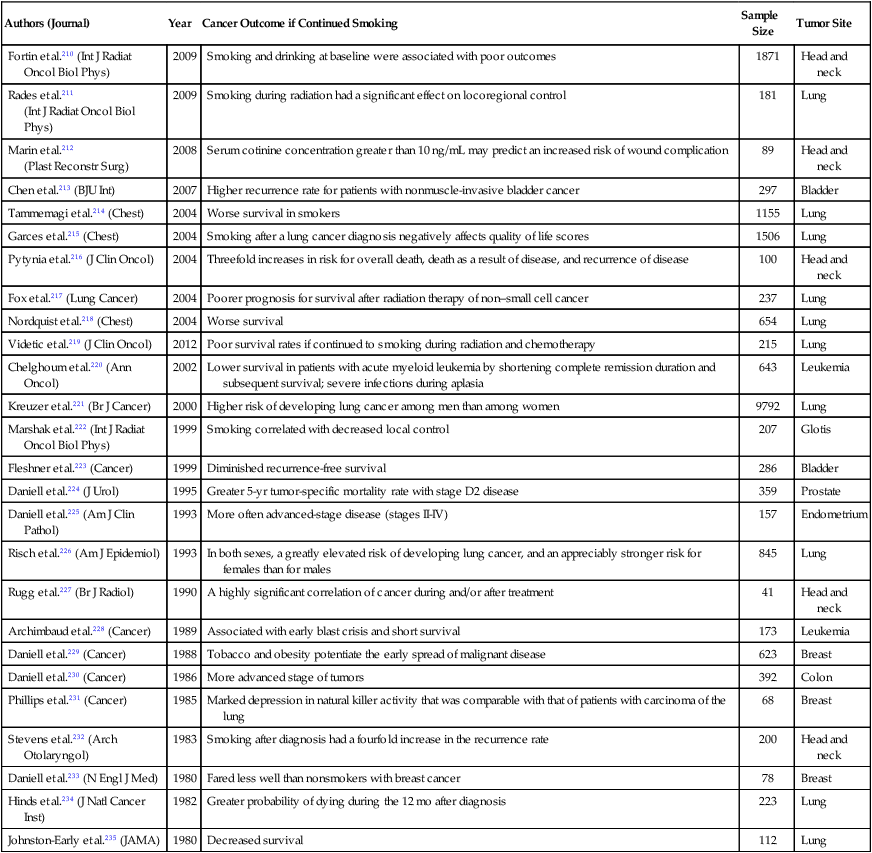
| Authors (Journal) | Year | Cancer Outcome if Continued Smoking | Sample Size | Tumor Site |
| Fortin et al.210 (Int J Radiat Oncol Biol Phys) | 2009 | Smoking and drinking at baseline were associated with poor outcomes | 1871 | Head and neck |
| Rades et al.211 (Int J Radiat Oncol Biol Phys) |
2009 | Smoking during radiation had a significant effect on locoregional control | 181 | Lung |
| Marin et al.212 (Plast Reconstr Surg) |
2008 | Serum cotinine concentration greater than 10 ng/mL may predict an increased risk of wound complication | 89 | Head and neck |
| Chen et al.213 (BJU Int) | 2007 | Higher recurrence rate for patients with nonmuscle-invasive bladder cancer | 297 | Bladder |
| Tammemagi et al.214 (Chest) | 2004 | Worse survival in smokers | 1155 | Lung |
| Garces et al.215 (Chest) | 2004 | Smoking after a lung cancer diagnosis negatively affects quality of life scores | 1506 | Lung |
| Pytynia et al.216 (J Clin Oncol) | 2004 | Threefold increases in risk for overall death, death as a result of disease, and recurrence of disease | 100 | Head and neck |
| Fox et al.217 (Lung Cancer) | 2004 | Poorer prognosis for survival after radiation therapy of non–small cell cancer | 237 | Lung |
| Nordquist et al.218 (Chest) | 2004 | Worse survival | 654 | Lung |
| Videtic et al.219 (J Clin Oncol) | 2012 | Poor survival rates if continued to smoking during radiation and chemotherapy | 215 | Lung |
| Chelghoum et al.220 (Ann Oncol) | 2002 | Lower survival in patients with acute myeloid leukemia by shortening complete remission duration and subsequent survival; severe infections during aplasia | 643 | Leukemia |
| Kreuzer et al.221 (Br J Cancer) | 2000 | Higher risk of developing lung cancer among men than among women | 9792 | Lung |
| Marshak et al.222 (Int J Radiat Oncol Biol Phys) | 1999 | Smoking correlated with decreased local control | 207 | Glotis |
| Fleshner et al.223 (Cancer) | 1999 | Diminished recurrence-free survival | 286 | Bladder |
| Daniell et al.224 (J Urol) | 1995 | Greater 5-yr tumor-specific mortality rate with stage D2 disease | 359 | Prostate |
| Daniell et al.225 (Am J Clin Pathol) | 1993 | More often advanced-stage disease (stages II-IV) | 157 | Endometrium |
| Risch et al.226 (Am J Epidemiol) | 1993 | In both sexes, a greatly elevated risk of developing lung cancer, and an appreciably stronger risk for females than for males | 845 | Lung |
| Rugg et al.227 (Br J Radiol) | 1990 | A highly significant correlation of cancer during and/or after treatment | 41 | Head and neck |
| Archimbaud et al.228 (Cancer) | 1989 | Associated with early blast crisis and short survival | 173 | Leukemia |
| Daniell et al.229 (Cancer) | 1988 | Tobacco and obesity potentiate the early spread of malignant disease | 623 | Breast |
| Daniell et al.230 (Cancer) | 1986 | More advanced stage of tumors | 392 | Colon |
| Phillips et al.231 (Cancer) | 1985 | Marked depression in natural killer activity that was comparable with that of patients with carcinoma of the lung | 68 | Breast |
| Stevens et al.232 (Arch Otolaryngol) | 1983 | Smoking after diagnosis had a fourfold increase in the recurrence rate | 200 | Head and neck |
| Daniell et al.233 (N Engl J Med) | 1980 | Fared less well than nonsmokers with breast cancer | 78 | Breast |
| Hinds et al.234 (J Natl Cancer Inst) | 1982 | Greater probability of dying during the 12 mo after diagnosis | 223 | Lung |
| Johnston-Early et al.235 (JAMA) | 1980 | Decreased survival | 112 | Lung |
Reward Pathway and Biological Characteristics of Addiction
The Reward Pathway
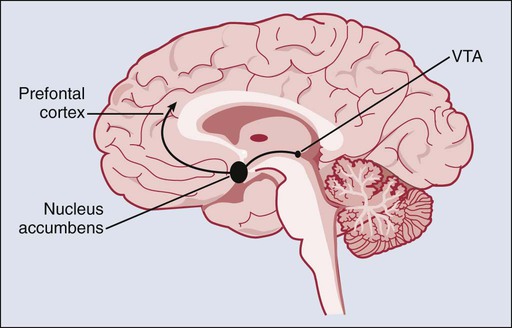
Like most drugs associated with abuse and dependence, nicotine use stimulates its receptors in the brain. These receptors are spread throughout most areas of the brain. Nicotine use leads to a rapid increase in dopamine release in the nucleus accumbens and the ventral tegmental area. This stimulation typically occurs within 10 seconds of smoking a cigarette.23,24 It has been established that natural rewards such as food consumption, social affiliation, and sexual activity, which are linked to survival of the individual or species, normally activate these two central areas of the reward pathway within the brain. Furthermore, the reward pathway projects from the nucleus accumbens and ventral tegmental area to the prefrontal cortex, the amygdala, and the olfactory tubercle (Figure 24-1), leading to a complex interaction between the reward, its saliency, and the ability to inhibit the intake of a drug. Several other brain systems (neurotransmitters and pathways) are thought to be involved in the process of addiction to a substance, whereas dopamine appears to be the final common neurotransmitter in the reward pathway. These other pathways are thought to be involved via neuroadaptation (the brain adjusting to the new conditions). The γ-aminobutyric acid, glutamate, serotonin, norepinephrine, cannabinoid, opioid, and cholinergic systems have all been shown to be involved in one way or another in the brain neuroadaptation process that results in addiction to nicotine and other substances.27–27 However, it is important to note that these major neurotransmitter systems in the brain are in constant and dynamic interplay, possibly resulting in different types of neuroadaptations that lead to and maintain the addiction to a substance.4
Neuronal Adaptation
A pleasurable sensation from the activation of the reward pathway is associated with the acute use of an addictive substance such as nicotine. However, repeated administration of a substance, in this case nicotine, over months or years is likely to lead to increased tolerance, which in turn produces a state of withdrawal in the absence of nicotine. Tolerance and withdrawal are the physiological hallmarks of dependence (addiction) and are thought to be the result of neuroadaptive effects occurring within the brain.28 Interestingly, the chronic use of drugs of abuse and dependence (including nicotine) seems to result in a generalized decrease in dopaminergic neurotransmission. Although this decrease can be a prodrome for vulnerability to addiction, it is likely to be a homeostatic response to the intermittent yet repetitive increases in dopamine induced by the frequent and sustained use of such drugs.29 Another facet of neuroadaptation is the increase in levels of corticotropin-releasing factor induced by the use of substances; this increase is associated with the activation of central stress pathways. Sensitization and counteradaptation are two of the major neuroadaptive models that have been proposed to explain how changes that start in the reward pathway may result in the development of substance dependence. Sensitization can be thought of as the gradual increase in “wanting” a drug after intermittent but repeated use, which then can facilitate the transition from occasional use to chronic use and tolerance.29,30 The counteradaptation model postulates that the initial positive feelings of reward (resulting from the use of a drug) are followed by an opposing rather than synchronous development of tolerance, as the brain’s homeostatic (counterbalance) response to the exposure. Finally, tolerance is often manifested by the appearance of withdrawal symptoms associated with the lack of the substance.31 Other models of nicotine addiction are based on mechanisms associated with capacity for or the lack of cognitive control and the development of reinforcement learning,32 in particular the negative reinforcement associated with withdrawal from nicotine, which builds up with the reduction in negative affect from smoking a cigarette.33
Genetics
Genes Associated with Smoking, Nicotine Dependence, and Lung Cancer
Several recent genome-wide association (GWA) and candidate gene studies have revealed significant associations between smoking, nicotine dependence (ND), and lung cancer in a region of interest on the long arm of chromosome 15 (15q24/15q25.1), encompassing the nicotinic acetylcholine receptor CHRNA3-A5-B4 subunit cluster. Neuronal nicotinic acetylcholine receptors are pentameric ligand-gated channels composed of α and β subunits,34 several of which contribute to nicotine-stimulated dopamine release in the striatum, a region associated with reward learning and vital to the development of substance dependence.35 The CHRNA3-A5-B4 subunit cluster demonstrates extensive linkage disequilibrium, and the single nucleotide polymorphisms on that cluster are frequently correlated. Some of the statistically strongest associations with ND in persons of European or African ancestry have been obtained for the nonsynonymous CHRNA5 single nucleotide polymorphism rs16969968.36–41 Other gene variants in this region (CHRNA3-A5-B4) identified by GWA and candidate gene studies as being significant contributors to the risk of smoking, ND, and lung cancer include the CHRNA3 rs1051730,41–45 the CHRNA3 rs578776,36,38 and the AGPHD1 rs8034191.36,42,44 Finally, candidate gene studies have identified associations between ND and genetic markers associated with dopamine expression (ANKK1,44,46–48 DRD2,48,49 and SLC6A349–52), serotonin expression (SLC6A4),55–55 and nicotine metabolism (CYP2A6).45
An increasing amount of work has been focused on the investigation of genes that inform brain regions related to nicotine dependence. The CHRNA5 rs16969968 risk allele was found to be associated with decreased resting state connectivity strength in a dorsal anterior cingulate-ventral striatum/extended amygdala circuit associated with smoking.56 A small functional magnetic resonance imaging study that investigated the role of CHRNA5 rs16969968 on brain reactivity to smoking cues in nicotine-dependent women found that women homozygous for the G allele had greater functional magnetic resonance imaging reactivity to smoking cues in the hippocampus and dorsal striatum, which are areas related to memory and habitual behavior.57 Smokers identified as fast nicotine metabolizers, as indicated by the CYP2A6 gene, had greater brain responses to smoking cues in the amygdala, cingulate cortex, hippocampus, insula, and striatum compared with slow metabolizers.58
Candidate Gene Studies Predicting Treatment Outcome
A growing body of candidate gene studies have examined genetic predictors of responsiveness to nicotine replacement therapies (NRTs) and bupropion smoking-cessation therapies (see references 59, 60, and 61 for reviews). Cessation outcome for NRT has been found to be associated with several polymorphisms in the D2 receptor gene (DRD2), including the C957T rs6277,62 −141C ins/del rs1799732,62 and ANKK1 (rs1800497).65–65 Other markers that influence dopamine reuptake and expression, including the COMT rs468066,67 and a VNTR in the DRD4 C-521T,68 have been shown to predict cessation outcome for NRT. Genes associated with the opioid (OPRM1),71–71 serotonergic (SLC6A4),69,72,73 cannabinoid,74,75 and CHRNA3-A5-B476 pathways have also been associated with NRT outcome.
Similarly, variants of many of these same markers have been associated with successful smoking cessation treatment using bupropion, including ANKK1,79–79 DRD2 −141C ins/del,62 COMT,80 DRD4,81 SLC6A3,82 CYP2B6,83 and the CHRNA3-A5-B4 subunit cluster.86–86 Varenicline, the most recently approved smoking cessation pharmacotherapy, is the next target for pharmacogenetic inquiry. A recent analysis of 1175 smokers who received either varenicline, bupropion, or placebo found that abstinence was associated with multiple CHRNB2, CHRNA7, and CHRNA4 subunit genes for persons given varenicline and with the CYP2B6 for persons given bupropion.6
Comorbid Conditions
The smoking rates among persons with no mental illness, past-month mental illness, and lifetime mental illness have been reported to be 22%, 34%, and 41%, respectively, which means that having a current or past mental disorder effectively doubles the likelihood of being a smoker. Furthermore, in a nationally representative sample, it was reported that smokers with a mental disorder in the past month consume 44% of all cigarettes smoked.87 The evidence that smoking is more prevalent in and closely linked with several psychiatric comorbidities, including abuse of other substances, suggests a shared biological pathway between nicotine dependence and these psychiatric and substance use conditions. For example, several studies have demonstrated a positive correlation between smoking and alcohol use, abuse, or dependence, substance abuse or dependence, and other psychiatric disorders.87–94 When looked at from the opposite direction, the prevalence of lifetime alcohol dependence and/or drug abuse with the adult smoker population is estimated to be between 23% to 30%.95,96 Similarly, among current smokers (whether tobacco-dependent or nondependent), the lifetime rates of mood and anxiety disorders have been reported to range from 33% to 46% and from 12% to 26%, respectively.95 Furthermore, among current smokers who are tobacco-dependent, even the 12-month prevalence of any mood or anxiety disorder is reported to be 22%, a relatively high proportion.97 Adding support to the idea of a possible causal link between smoking and mental health disorders is the fact that smokers also have an elevated risk of a first onset of major depression, panic disorder, or generalized anxiety disorder.98–102 Cognitive dysfunction has also been highly linked to smoking, as evidenced by the odds ratio comparing persons who have ever smoked with persons who have never smoked, and smoking is positively related to the number of attention deficit hyperactivity disorder (ADHD) symptoms. There seems to be an effect of early onset on the severity of ADHD or vice versa, because lifetime regular smoking has an inverse relationship between the number of ADHD symptoms and the age at onset. In addition, a positive relationship between the number of symptoms and the number of cigarettes smoked has been observed.103 The aforementioned evidence of co-occurrence supports the importance of screening and treating mental health disorders among smokers, whether the relationship is causal or a simple correlation. Treating these disorders may increase the ability to focus on quitting smoking, or at least reduce the impact of negative affect or comorbid mental disorders on a person’s ability to quit using tobacco, and may have an impact on their resilience with regard to maintaining tobacco abstinence. Treating these disorders is of particular importance among patients with cancer and cancer survivors, because higher levels of confidence about the ability to quit smoking were found to be related to lower depression scores and lower tumor stage.104
Patient Assessment
In the absence of specific biological markers, the diagnosis of nicotine addiction remains a clinical one. The Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR),105 uses universal criteria for all substance dependence (traditionally referred to as addiction), including nicotine. Because of their universality, the DSM-IV-TR criteria are not particularly specific to nicotine and therefore do not capture many of the peculiar aspects of tobacco use and ND (addiction). This lack of specificity has led to the development of specific scales to quantify ND. Traditionally, the Fagerström Test of Nicotine Dependence (FTND) has been used, and more recently the Wisconsin Inventory of Nicotine Dependence Motives (WISDM) has become more accepted as a more comprehensive scale.
Despite their limitations, the DSM-IV criteria offer ease of use for clinicians because of their universality for all substances of dependence. According to DSM-IV-TR, the diagnosis is made when someone meets three or more of the seven criteria for at least a year. As previously mentioned, some criteria are more pertinent than others to nicotine. For instance, after prolonged smoking, the user develops nicotine tolerance and begins to exhibit withdrawal symptoms when nicotine is absent. That withdrawal can happen overnight with nicotine’s half-life in blood being 1 to 2 hours.106 Having tolerance and/or withdrawal is referred to as physiological dependence in the DSM-IV-TR. Nicotine may also be responsible for three other criteria for dependence: loss of control over smoking (e.g., not being able to reduce or stop smoking, or smoking more than intended), compulsive smoking (e.g., smoking all day long and giving up important events or activities if there is no place to smoke), and continued smoking despite adverse consequences (e.g., heart attack, emphysema, or cancer). The newer DSM-5 criteria (planned to be released in 2013) are proposed with a similar universal applicability to all substances; however, they expand the “use despite consequences” criteria and incorporate “craving for a substance” as an important new dimension, and only two of those criteria would suffice to have the diagnosis of “substance use disorder,” a new nomenclature. The more criteria that are met, the more severe is the disorder, which will effectively replace the term “dependence,” which in past versions of the manual had been introduced to replace the term “addiction.”107
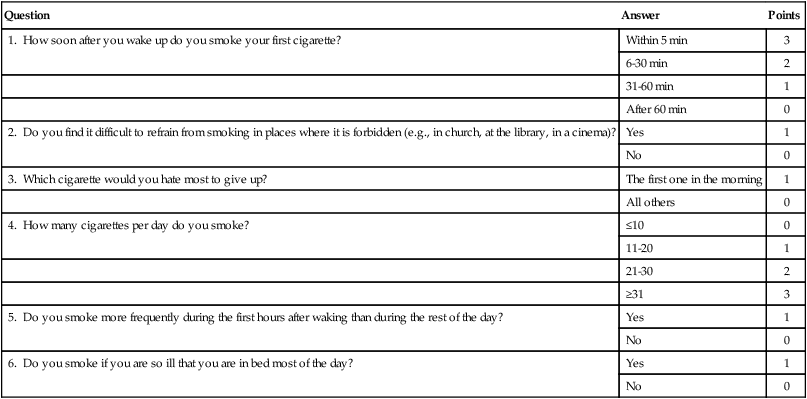
The FTND (Table 24-2) is one of the most widely recognized and used scales for nicotine dependence. This tool, and in particular its first item (“How soon after you wake up do you smoke your first cigarette?”), measures physiological dependence reliably well (i.e., tolerance and withdrawal). However, it does not measure well the psychosocial and behavioral dimensions of ND.108 A person with three points or more out of a possible maximum of 10 points on this scale is customarily considered to be ND.109,110
Table 24-2
Items and Scoring for Fagerström Test for Nicotine Dependence236
| Question | Answer | Points |
| 1. How soon after you wake up do you smoke your first cigarette? | Within 5 min | 3 |
| 6-30 min | 2 | |
| 31-60 min | 1 | |
| After 60 min | 0 | |
| 2. Do you find it difficult to refrain from smoking in places where it is forbidden (e.g., in church, at the library, in a cinema)? | Yes | 1 |
| No | 0 | |
| 3. Which cigarette would you hate most to give up? | The first one in the morning | 1 |
| All others | 0 | |
| 4. How many cigarettes per day do you smoke? | ≤10 | 0 |
| 11-20 | 1 | |
| 21-30 | 2 | |
| ≥31 | 3 | |
| 5. Do you smoke more frequently during the first hours after waking than during the rest of the day? | Yes | 1 |
| No | 0 | |
| 6. Do you smoke if you are so ill that you are in bed most of the day? | Yes | 1 |
| No | 0 |
The WISDM111 is a more recently developed multidimensional scale of ND. This comprehensive scale includes measures of cognitive enhancement, negative reinforcement, positive reinforcement, automaticity, affiliative attachment, loss of control, behavioral choice/amelioration, craving, cue exposure/associative processes, social/environmental goals, taste/sensory processes, weight control, and tolerance. The WISDM has been validated and found to be reliable in measuring ND, and it has been reported to have internal consistency along a 13-factor model.112
1. The Patient Health Questionnaire113 is a multiple-choice self-report inventory that is used as a screening and diagnostic tool for mental health disorders of depression, anxiety, alcohol, eating, and somatoform.
2. The Center for Epidemiological Studies on Depression114 tool is a 20-item instrument that was developed by the National Institute of Mental Health to screen for major or clinical depression in adolescents and adults. This instrument has four separate factors: depressive affect, somatic symptoms, positive affect, and interpersonal relations.
3. The Sleep Problems Questionnaire115 is a four-item brief survey (each item scored from 0 to 5 based on the last 4-week period) that considers difficulty falling asleep, difficulty staying asleep, waking up multiple times, and waking up feeling tired.
4. The FTND109 has previously been described.
5. The Wisconsin Smoking Withdrawal Scale116 is a nicotine withdrawal scale that contains seven factors: anger, anxiety, sadness, concentration, craving, sleep, and hunger.
6. The Positive Affect and Negative Affect Schedule117 is a psychometric scale developed to measure the largely independent constructs of positive and negative affect both as states and traits. Positive and negative affect have been shown to relate to other personality states and traits, such as anxiety.
7. The Alcohol Use Disorders Identification Test118 is a simple 10-question test developed by the World Health Organization to determine if a person’s alcohol consumption may be harmful. The test was designed to be used internationally and has been validated in different settings; originally it was validated in a study using patients from six countries. Questions 1 to 3 deal with alcohol consumption, questions 4 to 6 relate to alcohol dependence, and questions 7 to 10 consider alcohol-related problems.
Treatment of Tobacco Use
Tobacco treatment among patients with cancer needs to be as intensive and tailored as possible. Hence if treatment programs are to be comprehensive and to make a difference, they ought to provide at a minimum behavioral modalities and medications.119 Comprehensive treatments by definition would need to address the biological, psychological, and social aspects of ND. Moreover, these approaches ought to be longitudinal, because tobacco addiction is a chronic disease that often is characterized by multiple relapses before reaching full remission.120 The main components of a biopsychosocial treatment are behavioral therapies and social support in conjunction with medications. Among the various therapies, motivational interviewing, skill-building, problem-solving, and cognitive behavioral therapies have been empirically supported.5 Although the main first-line medications that are approved by the Food and Drug Administration (FDA) are NRTs, bupropion (Zyban), and varenicline (Chantix), the second-line medications clonidine and nortriptyline have been used off-label and tested in clinical trials with similar positive results.
Among several other medications that have been or are being researched, three have shown promising preliminary results but have faced different challenges: topiramate, rimonabant, and nicotine vaccines. Although a care provider may prescribe any available medications off-label, to date, none of the aforementioned three medications is FDA-approved for smoking cessation purposes, and only topiramate is available in the United States. However, their importance lies in their mechanisms of action, which may lead to improved or similar agents in the future. Topiramate, a voltage-dependent sodium channel blocker, augments γ-aminobutyric acid, antagonizes glutamate, and inhibits carbonic anhydrase activity; it has FDA label indication as an adjunct treatment for seizure disorders and migraine headaches.121 Topiramate is reported to have a beneficial effect in the treatment of alcohol dependence and ND but has several adverse effects that limit its use.122 Rimonabant is a selective CB1 endocannabinoid receptor antagonist reported to have beneficial effects for the treatment of obesity123 and ND124; it was shown to be relatively efficacious compared with placebo (odds ratio 1.6)125 in large multinational trials. It was available in Europe from mid 2006 until January 2009; then the European Agency for the Evaluation of Medicinal Projects recommended its withdrawal because of neuropsychiatric adverse effects (i.e., depression and several reports of suicides).126 Although rimonabant is currently available in other countries, it did not receive FDA approval in the United States because of similar concerns about neuropsychiatric adverse effects. Several vaccines against nicotine have been under study for some years and have shown initial promising results in animal and early human trials. The most advanced stage III clinical multisite human trial on one of the nicotine vaccines did not find it to be different from placebo as a treatment for smoking cessation. However, the vaccine concept is important and merits further investigation, because it may have a role in relapse prevention and as part of multifaceted approach to smoking cessation that includes behavioral and pharmacologic intervention.127
The Clinical Practice Guideline for Treating Tobacco Use and Dependence (CPG-TTUD), which provides a comprehensive review of all tobacco treatment–related literature, was last updated in 2008. The key points are presented in an executive summary in Box 24-1. We summarize and discuss these approaches in the following section, specifically their applicability to the cancer population.
Pharmacologic Interventions
Some persons are not interested in or lack the motivation to quit smoking, and some persons are not ready for change. Even in these instances, pharmacologic treatments have been shown to increase the odds of reducing the number of cigarettes per day by half.5 This decrease in cigarettes smoked can in return positively influence patients, increasing their self-efficacy and motivation to quit.128
The FDA has approved the use of several NRTs, among them the patch, nasal spray, buccal inhaler, gum, and lozenges. The abstinence results for NRTs are compelling and largely encouraging based on hundreds of studies.128 Despite that, recent debate over their long-term efficacy is taking place, in particular now that they are available over the counter.129 However, data on using a combination of a patch with other intermittent NRTs show that combining NRTs is more effective than any single pharmacologic treatment, including varenicline.130
Nicotine is the key ingredient in NRTs, with the premise that replacing the addictive substance in cigarettes helps the user to quit. This nicotine is at a much lower dose and is delivered through the venous system, compared with a higher dose from smoking a cigarette with arterial delivery to the brain. The intent is to reduce cravings and the withdrawal effects of tobacco cessation.131 Interestingly and despite some controversies, NRTs have been found to be effective with and without the aid of a therapeutic support.132 However, one should not forget that management of expectations and patient education before initiating pharmacologic treatment are key to the success of smoking cessation interventions. In addition, NRTs are relatively safe to use regardless of the individual’s smoking status.133 In other words, NRTs may be used before the individual abstains from smoking. Most studies did not identify any noteworthy nicotine toxicity or adverse events; however, the results of other studies showed that NRT used before quitting helped reduce the number of cigarettes per day by half even in persons who were not interested in quitting.134–137 It has been suggested that some smoker characteristics (e.g., the metabolism rate of nicotine) can affect response to NRTs, suggesting that the tailored use of NRT for subgroups of smokers could improve NRT efficacy.138 For example, the level of nicotine dependence affects the response to NRTs, with more dependent smokers showing higher rates of quitting when they receive higher doses of NRTs.139,140 NRTs are an important treatment option for patients with cancer and have been reported to have similar efficacy to that among other populations of smokers.141 However, some animal and laboratory cell culture studies in normal and cancer tissue have shown that nicotine promotes tumor growth, whereas others report it to also have a tumor-promoting effect.142,143 Thus far human studies have not found a significant causal relationship between NRTs and cancer genesis,144 even after 5 years of NRT use.145,146
Bupropion
Bupropion was first introduced in the market as an antidepressant (Wellbutrin) and was then found to be effective in the treatment of tobacco dependence. This medication was consequently approved for and is currently used by smokers with or without depression (Zyban).147 To date, bupropion is the only antidepressant approved for smoking cessation on the market; however, no controlled studies have been published that examine the role of depression as a moderator of the response to bupropion in depressed smokers. Bupropion is a relatively well-tolerated medication with dry mouth, insomnia, and fine tremor as the most common adverse effects. A metaanalysis of 31 controlled clinical trials showed bupropion to be twice as effective as placebo (odds ratio, 1.96) in reaching end-of-treatment abstinence (8 weeks), as well as in long-term cessation (6 and 12 months).148
Unfortunately, certain patients with cancer may be unable to use bupropion, because it is known to lower the seizure threshold; therefore, it is contraindicated in persons with active seizures or a history of seizures, head trauma, elevated liver enzymes, bulimia, or anorexia nervosa. It would be considered risky to give bupropion to patients with cancer who have a low threshold for seizures, such as those with metastases to the brain or with low appetite or body weight. However, bupropion has been reported to be effective in populations with mental disorders such as schizophrenia,149 depression150 and posttraumatic stress disorder.151 Moreover, research has shown that bupropion limits the weight gain commonly associated with smoking cessation152 and restores sexual functioning.151 These added advantages can increase its usefulness among cancer survivors with fatigue from chemotherapy or radiation or those with comorbid disorders, in addition to persons who are overweight or have sexual dysfunction.
Varenicline
Varenicline, marketed as Chantix in the United States and Champix in other countries, is the most recently (in 2006) introduced nonnicotine-based medication for smoking cessation. Varenicline’s unique properties as a partial agonist (traditionally referred to as a mixed agonist-antagonist) provide some relief from withdrawal symptoms (agonist property) usually associated with tobacco cessation, while diminishing the pleasurable sensation from smoking (antagonist property). Clinical trials of varenicline showed that when it was used at 1 mg twice per day, it was twice as effective as bupropion and three times more effective than placebo at the end of treatment (12 weeks and at 1-year follow-up).155–155 In a continuation of treatment study, an additional 12 weeks of randomized varenicline or placebo therapy (for a total of 24 weeks) was provided in a double-blind design to patients who had abstained from smoking at some point during the first 3 months of open-label varenicline; 70% of those randomly assigned to varenicline stayed abstinent at the end of the continuation phase of 3 months (a total of 6 months of treatment with varenicline) compared with 50% of persons randomly assigned to placebo after having quit smoking while taking varenicline. Furthermore, the 1-year follow-up abstinence rate (after medication treatment) was double that of patients who received only 3 months of varenicline (25% and 12%, respectively).156 The most common adverse event reported was nausea in almost 30% of the persons using this medication. Although the nausea was mild or moderate in the majority of cases, this adverse effect limits its use among patients with cancer undergoing treatment. Other commonly reported adverse effects were vivid dreams and flatulence. Less common were the neuropsychiatric events among the general population reported in the postmarketing phase to the FDA via the voluntary reporting tool “MedWatch,” with these events consisting mainly of difficulty with coordination, depressive symptoms, aggression, irritability, and (more rarely) suicidal ideations.157 In light of these reports, the FDA has commissioned the manufacturer to deepen their data analyses and conduct postmarketing studies to directly compare psychiatric versus nonpsychiatric populations to determine the magnitude of these adverse events and their potential link to varenicline. One of those studies was recently published, reporting on varenicline’s efficacy and adverse effect profile among stable patients with schizophrenia. Although the quit rates were somewhat lower than expected (19% with varenicline vs. 5% with placebo), no exacerbation of their schizophrenia symptoms occurred, and varenicline was well tolerated.158 Varenicline has an added advantage for heavy drinkers who are smokers, in that it helps decrease alcohol consumption159; this finding is of particular importance, because alcohol and smoking are common comorbidities among patients with head and neck cancer.
A recent Cochrane review of partial agonists on nicotine receptors considered a number of studies. Two recent studies found that cytisine (a product based on natural ingredients) was effective for smoking cessation (pooled relative risk [RR] of 3.98), whereas another trial found that dianicline (a synthetic product similar to varenicline) was not effective (RR 1.2). Fifteen trials concluded that varenicline at the standard dose (2 mg/day) increased the chances of quitting more than twofold compared with placebo (RR 2.27); however, low-dose varenicline (1 mg/day) roughly doubled the chances of quitting (RR 2.09) and reduced the number and severity of adverse effects. In the same review, varenicline was reported to be better than bupropion in three comparison trials (pooled RR 1.52); however, two other trials did not show a clear benefit of varenicline over the nicotine patch.160
Another important factor in an era of cost containment is the cost-effectiveness of a new treatment, and in a recent review, varenicline seemed to dominate over bupropion in most cost-effectiveness models utilized.161
Combinations of First-Line Medications
Combining medications is becoming a trend, in particular for such traditionally hard-to-treat diseases as tuberculosis, gastric ulcers, human immunodeficiency virus, and others. Tobacco dependence is not an exception, as combination therapy is reported to be better than monotherapy for smoking cessation (Table 24-3).162 It has been recently suggested that all smokers (except light smokers and those who live with a smoker) may respond better to combination pharmacotherapy than to monotherapy.163 Before varenicline was available, the options for combination were adding NRTs to bupropion or using a patch with episodic NRTs (e.g., lozenges and gums).112,164 Studies designed to compare these smoking cessation medications alone or in combination reported that certain combinations were superior: specifically, bupropion plus lozenges and the patch plus lozenges resulted in higher cessation rates than did monotherapy bupropion, patch, or lozenges.112,133,164,165 Now that varenicline is available, combining this medication with bupropion has gained attention as another option. This combination is of particular interest because it combines two effective medications that have different mechanisms of action, with the possibility of potentiating each other. A particular advantage of this combination might be the beneficial effect of bupropion as an antidepressant in the mitigation of negative affect and neuropsychiatric effects resulting from smoking cessation; those neuropsychiatric effects have been linked to varenicline use mostly among patients with a psychiatric diagnosis.157 A single-arm, open-label pilot study at the Mayo Clinic tested the effectiveness of varenicline-bupropion combination in 38 smokers. The point prevalence smoking abstinence rates were as high as 70% at 3 months follow-up and almost 60% at 6 months follow-up.166 Other controlled trials are underway to systematically test the efficacy and safety of this combination.
Table 24-3
Metaanalysis (in CPG-TTUD 2008)*: Effectiveness and Abstinence Rates for Various Medications and Medication Combinations Compared with Placebo 6 Months after Quitting (N = 83 Studies)5
| Medication | No. of Arms | Estimated Odds Ratio (95% CI) | Estimated Abstinence Rate (95% CI) |
| Placebo | 80 | 1.0 | 13.8 |
| Monotherapies | |||
| Varenicline (2 mg/day) | 5 | 3.1 (2.5-3.8) | 33.2 (28.9-37.8) |
| Nicotine nasal spray | 4 | 2.3 (1.7-3.0) | 26.7 (21.5-32.7) |
| High-dose nicotine patch (>25 mg) (these patches included both standard or long-term duration) | 4 | 2.3 (1.7-3.0) | 26.5 (21.3-32.5) |
| Long-term nicotine gum (>14 wk) | 6 | 2.2 (1.5-3.2) | 26.1 (19.7-33.6) |
| Varenicline (1 mg/day) | 3 | 2.1 (1.5-3.0) | 25.4 (19.6-32.2) |
| Nicotine inhaler | 6 | 2.1 (1.5-2.9) | 24.8 (19.1-31.6) |
| Clonidine | 3 | 2.1 (1.2-3.7) | 25.0 (15.7-37.3) |
| Bupropion SR | 26 | 2.0 (1.8-2.2) | 24.2 (22.2-26.4) |
| Nicotine patch (6-14 wk) | 32 | 1.9 (1.7-2.2) | 23.4 (21.3-25.8) |
| Long-term nicotine patch (>14 wk) | 10 | 1.9 (1.7-2.3) | 23.7 (21.0-.6) |
| Nortriptyline | 5 | 1.8 (1.3-2.6) | 22.5 (16.8-29.4) |
| Nicotine gum (6-14 wk) | 15 | 1.5 (1.2-1.7) | 19.0 (16.5-21.9) |
| Combination therapies | |||
| Patch (long-term; >14 wk) + ad lib NRT (gum or spray) | 3 | 3.6 (2.5-5.2) | 36.5 (28.6-45.3) |
| Patch + bupropion SR | 3 | 2.5 (1.9-3.4) | 28.9 (23.5-35.1) |
| Patch + nortriptyline | 2 | 2.3 (1.3-4.2) | 27.3 (17.2-40.4) |
| Patch + inhaler | 2 | 2.2 (1.3-3.6) | 25.8 (17.4-36.5) |
| Patch + second generation antidepressants (e.g., paroxetine, venlafaxine) | 3 | 2.0 (1.2-3.4) | 24.3 (16.1-35.0) |
| Medications not shown to be effective | |||
| Selective serotonin reuptake inhibitors | 3 | 1.0 (0.7-1.4) | 13.7 (10.2-18.0) |
| Naltrexone | 2 | 0.5 (0.2-1.2) | 7.3 (3.1-16.2) |
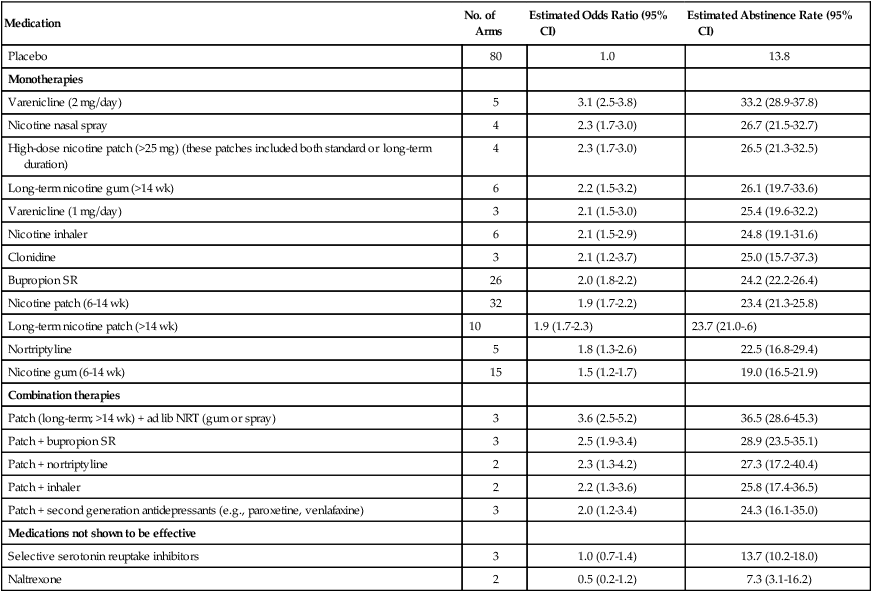
*The articles in this metaanalysis can be found at www.surgeongeneral.gov/tobacco/gdlnrefs.htm.
Second-Line Medications
Smokers who cannot use or tolerate first-line medications are usually referred to second-line medications such as clonidine or nortriptyline.148,167,168 Although several clinical trials have already proven their efficacy, these agents do not have FDA approval because they are older medications available as generics. Specifically, clonidine has shown superiority to placebo in two metaanalyses, with odds ratios ranging between 2.0 and 2.4.167 Nevertheless, it has three times–per-day dosing and a considerable adverse effect profile (dry mouth, dizziness, somnolence, and orthostatic hypotension); therefore the use of clonidine remains narrow even as a second-line treatment. Clinical trials of nortriptyline found it to be effective, with an odds ratio of 2.1 compared with placebo.169 However, the potential lethality in an overdose, as well as anticholinergic and cardiac adverse effects, limits its use in the general and cancer population.
Psychosocial Interventions
The Centers for Disease Control and Prevention emphasized the need to screen all patients at all visits in all health care settings for tobacco use, advise them to quit, and refer them to or offer them treatment—in other words, establish tobacco treatment programs where applicable.170 Gritz et al.171 postulated that the cancer population is at the heart of the concern. Smoking rates in certain patients with cancer are higher than average, and mounting evidence links smoking to outcomes related to cancer treatment success, survival, and quality of life.172–177 In patients receiving advanced treatments such as stem cell transplantation, those who never smoked, or recent quitters were recently reported to have almost double survivorship rates and about half as many hospitalization days in comparison with smokers.178 Therefore particular attention must be given to the oncology setting.18,179–181 In general, patients with cancer were found to be more motivated to quit smoking than were persons in the general population.18,182 This phenomenon can be seen as an advantage and an opportunity for care providers to intervene and promote smoking cessation while the tobacco user is motivated by the immediacy of the consequences of continued use on their disease treatment and recovery.
Certain issues are particular to the application of smoking cessation programs among patients with cancer. On the one hand, patients with cancer may be susceptible to guilt and blame attributions183; on the other hand, the clinician must be knowledgeable and alert to comorbid disorders, oncologic treatment adverse effects, and medical contraindications for medications when treating patients undergoing treatment for cancer. The CPG-TTUD, starting in 2000, recommended that all physicians and health care providers advise their patients to quit.184 This guideline included the five necessary steps in tobacco-dependence treatment, also known as the five A’s: ask, advice, assess, assist, and arrange. Fiore et al.185 suggested adding smoking to the vital signs checkup of every patient to ensure proper documentation, systematic screening, and ongoing monitoring of tobacco use status. Furthermore, electronic health records can be used to increase the identification of smokers and facilitate referrals to treatment.186,187 Interestingly, research has shown that even short interventions (3 minutes or less) would have an effect on abstinence. However, as the number of sessions increases, in particular for face-to-face interventions, abstinence rates rise as well.5 Therefore the CPG-TTUD recommends at least a minimal intervention of brief support in addition to medications, in particular if the patient does not have access to or will not ultimately receive a full tobacco treatment intervention.
Quitlines
Quitlines provide physicians with an alternative easy-access treatment, which can be especially helpful for physicians who are not affiliated with hospitals or work in hospitals that do not have a tobacco treatment program. Quitlines are a good option for patients who are unable or unwilling to travel to have an in-person appointment. In addition, Quitlines provide telephone-based, individual counseling for smoking cessation and are recommended as effective by the CPG-TTUD5 and the Cochrane Review.188 A recent review referred to Quitlines as an underrecognized success story189 that has been effective for both young adult smokers and older smokers190 and for African Americans as well as white smokers.191
In the United States, each state offers telephone-based quitline counseling for smoking cessation. Many quitlines also offer Internet-based assistance. Tobacco users can access telephone quitline counseling in all 50 states, the District of Columbia, Guam, and Puerto Rico by calling the national quitline portal 1-800-QUIT-NOW. Callers are routed to their appropriate state quitline based on the area code of the landline they are calling from or the location of the cell phone tower that routes the call. For more general information about quitlines, see http://www.smokefree.gov/quitlines-faq.aspx and http://www.naquitline.org/?page=whatisquitline.
Most state quitlines also have health care provider referral programs, where patients can be referred to quitline services by their physician or other health care provider via fax or e-mail, or as part of an electronic health record. Quitlines then make proactive outbound calls to referred tobacco users to enroll them in quitline services and provide feedback to the referring clinic. Research indicates that patients referred to a quitline by their health care provider are more likely to quit smoking successfully than are those who self-refer.192
Once enrolled in quitline services, tobacco users also have access to free NRTs or prescription medications (bupropion or varenicline) in 70% of states. Information about each state quitline, including specific services offered, hours of operations, and contact information for fax and e-referral programs, can be found on the North American Quitline Association Web site at http://map.naquitline.org.
Self-Help Materials
Self-help smoking cessation interventions are significantly better than no intervention at all; they are reported to produce quit rates in the range of 4% to 11%.193 An important new vehicle is the Internet, through which self-help smoking cessation interventions can be delivered effectively.194,195 Although few studies have evaluated the efficacy of Internet-based cessation approaches, in one study, a tailored Internet-based cessation intervention resulted in a quit rate of 24% compared with 5% in the control condition at 3 months follow-up.196,197 This finding is consistent with other preliminary work showing the efficacy of Internet-based cessation interventions.198 More controlled trials of Internet-based approaches are needed for this medium to be recommended for the delivery of tobacco and ND treatment.
Cessation Treatments for Patients with Cancer: Availability and Challenges
Cancer treatments and their contraindications impose certain limitations for clinicians who treat smokers who wish to quit. Particular attention should be given to the type of pharmacologic or adjuvant treatment given to smokers and any interaction with the current cancer type and cancer treatment type, in addition to psychiatric comorbidity (e.g., alcohol dependence and depression).174,199 More research is needed to identify proper and tailored smoking cessation treatments for the cancer population.120
Gritz et al.171 have identified several intervention studies that addressed smoking cessation among patients with cancer. Two studies have assessed the role and efficacy of physician-patient interventions.200,201 In the first study, the authors compared patients with cancer who were randomly assigned to full physician advice, tailored booklets, and booster advice sessions to patients who were offered minimal advice sessions, as a control. In the second study, the authors compared intensive cognitive behavioral interventions and patient education/advice. Both studies found no significant difference between the two conditions; however, in the first study, up to 70% of the participants remained abstinent at 1-year follow-up, and in the latter study about 40% remained abstinent at 3-months follow-up. Even though no single treatment was found to be superior in helping patients with cancer to quit smoking, assistance and advice in general generated good abstinence rates. The findings thus suggest incorporating some level of advice that could be tailored to the patient and type of cancer diagnosis.
Other studies examined nurse-delivered interventions in smoking cessation across various cancer diagnoses. A single-session intervention had lower cessation rates and multisession interventions had the highest rates.202,203 Although the samples differed greatly in size and composition, these authors believed that these results were promising and that nurse-based interventions should be explored further. Moreover, a retrospective study by Garces et al.204 emphasized the need for early interventions. The analysis showed higher abstinence rates among patients with cancer who were treated within 3 months of diagnosis versus those treated later than 3 months after diagnosis.
Finally, a recent trial assessed peer-based counseling versus self-help intervention in young adults who survived childhood cancer.205 Higher rates of smoking cessation were found in the counseling group at 12-month follow-up. Another study comparing a mixed group of patients with cancer who received motivational interviewing–based intervention versus those who received usual care consisting of brief smoking cessation advice did not find them to be significantly different.206
The combination of psychosocial therapies and medication doubles the odds of patients quitting smoking in general, despite the multiple types and levels of intensity for the psychosocial therapies (Table 24-3). A recent review of controlled trials among patients with cancer suggested that the combination of medication and therapy is needed to make a significant difference in smoking cessation in the oncology setting.119 Furthermore, the American Society of Addiction Medicine has described several possible levels of care and different types of treatment programs for all addictions, with those levels of care and types of programs based on whether concurrent psychiatric disorders are diagnosed and treated.207
Challenges and Future Directions
Many challenges remain to be overcome in treating tobacco dependence, in particular in the oncology setting. For example, geographic distance has often been a major obstacle for highly specialized and quality interventions. However, with advances in videoconferencing and telemedicine and their availability in remote locations, this problem can be solved.208 MD Anderson Cancer Center is in the process of expanding the TTP to the four regional care centers currently in operation. The plan is to use videoconferencing to take advantage of the current infrastructure and to expand where needed (with a fast Intranet connection and a new generation of video cameras and software). In addition, the TTP is currently piloting delivery of follow-up sessions by Web camera to the patient’s home, using a special software with a high security standard to safeguard the patient’s confidentiality. This approach is in lieu of the usual telephone counseling that currently takes place for follow-up when patients are outside our metro area or simply not able to come to the medical center regularly.
As mentioned in the genetics section, a major challenge and yet an opportunity to advance our current pharmacologic treatments for smoking cessation is to match a person’s genetic profile6 both to a successful pharmacologic intervention and to a lower side effect profile within clinical practice and clinical trials guidelines.
Single medication trials have led to around 40% cessation rates by the end of treatment. However, another 40% of participants have a partial response (they reduce their smoking but do not achieve total abstinence). Therefore combining medications is being suggested as a strategy for all hard-core smokers.163 This approach can be implemented by augmentation (i.e., adding another medication/agent to the one already in place for partial responders), which is a natural and logical approach for a patient who is engaged in treatment and is motivated to quit. Another strategy is prescribing a combination of medications to all or certain smokers from the beginning of cessation efforts. However, further studies are needed in this area to determine whether one strategy is superior.
The traditional approach and the vast majority of pharmacotherapy clinical trials ask participants to choose a quit date within 1 to 2 weeks of starting medications. However, that practice was born out of the practical need to test a hypothesis in a study within a short 8- to 12-week period. Allowing patients to choose their own interval for a quit date or not to set a quit date but rather to reduce gradually and then quit has been suggested in some studies209; however, these options have not been systematically tested against the strategy of setting a fixed quit date. Therefore more work is needed to clarify this important area.