Nausea and Vomiting
• Most common chemotherapy-associated toxicities
• More frequent and severe with repetitive doses of chemotherapy
• Significant impact on quality of life and can influence patient compliance with treatment
• Acute nausea and vomiting often mediated by activation of serotonin type 3 receptors in the gastrointestinal tract
• Mechanism of delayed nausea and vomiting (more than 24 hours after chemotherapy) unknown
• Risk factors, including type of chemotherapy (drugs, doses, schedule), age, sex, and prior alcohol use should be assessed before treatment
• Episodes of vomiting should be recorded (number, duration, and time to onset); severity of nausea can be graded with a visual analog scale
• Optimal treatment provides complete control of acute nausea and vomiting in the majority of patients receiving chemotherapy; only 15% of patients receiving highly emetogenic chemotherapy regimens have severe nausea and vomiting
• Treatment of delayed nausea and vomiting has improved with the introduction of aprepitant and palonosetron; this complication can now be completely controlled in two thirds of patients
Introduction
Nausea and vomiting are common adverse effects associated with systemic chemotherapy and are among the adverse effects most feared by patients.1,2 Although these complications of treatment are usually self-limiting and are seldom life-threatening, the deleterious effects on nutritional status and quality of life can be substantial. Many recently introduced therapeutic antibodies and oral agents have less potential to produce nausea and vomiting. However, combination chemotherapy continues to be the cornerstone of treatment for many types of cancer, ensuring that antiemetic therapy will continue to be an integral aspect of supportive care.
Antiemetic therapy has improved dramatically during the past 20 years. With optimum treatment, most patients receiving highly emetogenic chemotherapy do not experience any nausea or vomiting during the 24 hours after treatment.3–11 However, delayed symptoms are more common and are often underestimated by treating physicians and nurses.12 Accurate assessment of delayed nausea and emesis is essential in providing maximal intervention with recently available agents.
Physiology of the Vomiting Reflex
The pioneering work of Borison and Wang13 more than 50 years ago provided the basis for understanding the vomiting reflex. In studies using ablative techniques and electrical stimulation with microelectrodes (primarily in decerebrate cats), these investigators proposed the existence of two distinct sites in the brainstem believed to be critical for the control of emesis. The first of the sites, the so-called vomiting center, was thought to be located in the lateral reticular formation of the medulla. Electrical stimulation of this site triggered the vomiting reflex, whereas ablation prevented the vomiting induced by a variety of stimuli. The vomiting center was thought to be located adjacent to the other structures involved in the coordination of vomiting, including the respiratory, vasomotor, and salivary centers, and cranial nerves VIII and X. More recent studies have suggested that the “vomiting center” is actually not anatomically discrete but that the initiation of the vomiting reflex is controlled by a complex system of networks located in the brainstem, including the parvocellular reticular formation, the Bötzinger complex, and the nucleus tractus solitarius.14,15 The networks in this area coordinate complex patterns of motor activity such as the vomiting reflex and are more accurately described as “central pattern generators.”
Although these concepts have been retained and are integral to the current understanding of the vomiting reflex, several other important components have also been recognized. Input from the gastrointestinal tract, predominantly through afferent vagal fibers, is critical in initiating the vomiting reflex after ingestion of noxious substances.16 Incoming vagal afferents connect with the vomiting center directly; an intact CTZ is not essential when vomiting is initiated by this mechanism. It is now known that in addition to ingested substances, some blood-borne substances, including chemotherapeutic agents, can trigger the vomiting reflex through activation of the vagal afferent mechanism.
Two additional components of this complex system involve the vestibular apparatus and the higher brainstem and cortical structures. The vestibular system is involved primarily in initiating the vomiting reflex in persons with motion sickness. Input from higher cortical centers seems to be critical in a variety of conditions, including anticipatory emesis seen in patients who have previously experienced chemotherapy-induced emesis. The various components of the vomiting reflex are illustrated diagrammatically in Figure 42-1, along with the clinical situations in which they are operative. Given the complexity of this system, it is not surprising that different pharmacologic approaches are necessary to control vomiting of different etiologies.
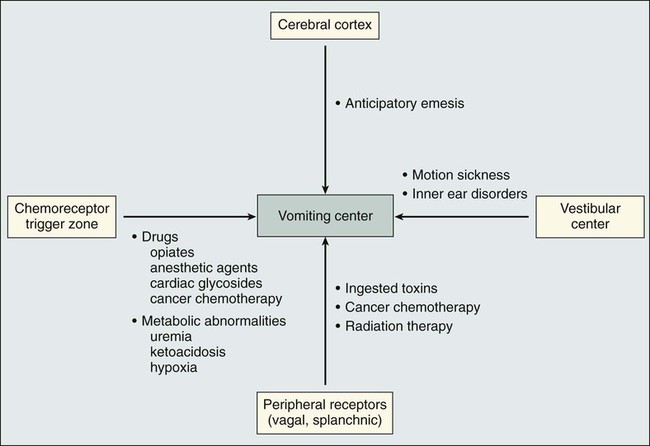
Improved understanding of the neurochemistry of the emetic reflex has been important in developing antiemetic agents with new mechanisms of action. The initial focus of such investigation was the area postrema, where receptors for a large number of neuroactive agents have been identified.19–19 Many of these neurotransmitters (e.g., dopamine, histamine, acetylcholine, norepinephrine, and substance P) are in themselves emetogenic agents. The development of pharmacologic agents that block specific sets of receptors (e.g., dopamine and neurokinin-1 [NK1]) has resulted in the identification of valuable antiemetic drugs, and it is likely that continued efforts in this area will yield additional valuable agents in the future.
In addition to neurotransmitters located in the CTZ, type 3 serotonin (5-hydroxytryptamine 3 [5-HT3]) receptors are present in large quantities on vagal and splanchnic afferents within the gastrointestinal tract.20 These peripheral receptors are pivotal in the initiation of the acute nausea and vomiting caused by cisplatin and other strongly emetogenic chemotherapeutic agents; inhibition of this pathway by specific 5-HT3 receptor antagonists results in highly effective antiemetic therapy.21
Clinical Features of Chemotherapy-Induced Emesis
Clinical Syndromes
Acute Nausea and Vomiting
Acute nausea and vomiting after the administration of chemotherapy occur within 24 hours after the chemotherapy dose. The nausea and vomiting are the most severe during this phase, hence the emphasis on therapeutic intervention at this stage. With most chemotherapeutic agents, acute nausea and vomiting begin 1 to 2 hours after intravenous (IV) administration. This delay in onset argues against a direct effect at the CTZ, which would be expected to produce emesis within minutes of IV drug administration. A peripherally mediated vomiting reflex, probably serotonin release from small intestinal mucosa, offers a better explanation of the delayed onset of emesis.22 The onset of nausea and vomiting after the IV administration of cyclophosphamide is delayed even longer than with other agents, typically occurring 9 to 18 hours after administration of the drug.23 The mechanism of cyclophosphamide-induced nausea and vomiting is unclear; the difference in the time of onset suggests that the mechanism might differ from that of other agents.
Delayed Nausea and Vomiting
Delayed nausea and vomiting occur 24 or more hours after administration of chemotherapy. Although the severity is decreased in comparison with acute nausea and vomiting, the course can be more protracted, resulting in significant difficulties with hydration, nutrition, and performance status. Delayed emesis is most severe and frequent after administration of high-dose cisplatin; most patients treated with this drug experience some degree of delayed emesis, with onset most frequently occurring 24 to 72 hours after chemotherapy.24 In some patients, onset can occur as late as 4 to 5 days after treatment and can persist for several days. Patients who have poor control of acute nausea and vomiting are more likely to experience delayed nausea and vomiting as well; however, delayed emesis can occur among patients who have complete emetic control during the first 24 hours after administration of chemotherapy.
Anticipatory Nausea and Vomiting
Anticipatory nausea and vomiting often occur among patients who have experienced poor control of emesis during previous courses of chemotherapy.25 The onset can occur before or during administration of chemotherapy. Because this response is conditioned, certain associations with chemotherapy administration, such as the hospital environment or the oncologist’s office, might trigger the onset of emesis.
Prognostic Factors
Multiple clinical factors that are important in determining the incidence and severity of chemotherapy-induced nausea and vomiting have been identified. These factors include the type of chemotherapy administered, certain patient characteristics, and the antiemetic regimen used (Box 42-1).
Chemotherapeutic Agents
A four-level classification of IV antineoplastic agents (high, moderate, low, and minimal) is widely accepted and is used to produce recommendations for antiemetic therapy.26,27 Table 42-1 summarizes the emetogenic potential of commonly used IV antineoplastic agents. Drugs in the high-risk category produce emesis in more than 90% of patients and require maximum antiemetic prophylaxis, whereas drugs with minimum risk produce emesis in fewer than 10% of patients and require no routine prophylaxis. The drugs that cause emesis most frequently also cause the most severe emesis. Emesis is most severe during the first 8 hours after onset, but with strongly emetogenic drugs, patients are often ill throughout the 24-hour period after administration.
Table 42-1
Emetogenic Potential of Commonly Used Intravenous Antineoplastic Agents
| Risk | Frequency of Emesis (%) (without Prophylaxis) | Agent |
| High | >90 | Carmustine |
| Cisplatin | ||
| Cyclophosphamide ≥1500 mg/m2 | ||
| Dacarbazine | ||
| Mechlorethamine | ||
| Streptozocin | ||
| Moderate | 30-90 | Alemtuzumab |
| Arsenic trioxide | ||
| Azacitidine | ||
| Bendamustine | ||
| Carboplatin | ||
| Clofarabine | ||
| Cyclophosphamide <1500 mg/m2 | ||
| Cytarabine ≥1 g/m2 | ||
| Doxorubicin | ||
| Epirubicin | ||
| Eribulin | ||
| Idarubicin | ||
| Ifosfamide | ||
| Irinotecan | ||
| Melphalan ≥50 mg/m2 | ||
| Methotrexate ≥250 mg/m2 | ||
| Mitoxantrone | ||
| Oxaliplatin | ||
| Low | 10-30 | Bortezomib |
| Cabazitaxel | ||
| Cytarabine <1 g/m2 | ||
| Cetuximab | ||
| Docetaxel | ||
| Doxorubicin (liposomal) | ||
| Etoposide | ||
| 5-Fluorouracil | ||
| Gemcitabine | ||
| Yttrium-90 ibritumomab tiuxetan | ||
| Ixabepilone | ||
| Methotrexate <250 mg/m2 | ||
| Mitomycin | ||
| Paclitaxel | ||
| Panitumumab | ||
| Pemetrexed | ||
| Temsirolimus | ||
| Topotecan | ||
| Tositumomab/iodine-131 tositumomab | ||
| Minimal | <10 | Asparaginase |
| Bevacizumab | ||
| Bleomycin | ||
| Cladribine | ||
| Fludarabine | ||
| Gemtuzumab ozogamicin | ||
| Ofatumumab | ||
| Pentostatin | ||
| Rituximab | ||
| Trastuzumab | ||
| Vinblastine | ||
| Vincristine | ||
| Vinorelbine |
Adapted from Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21(Suppl. 5):v232–43.
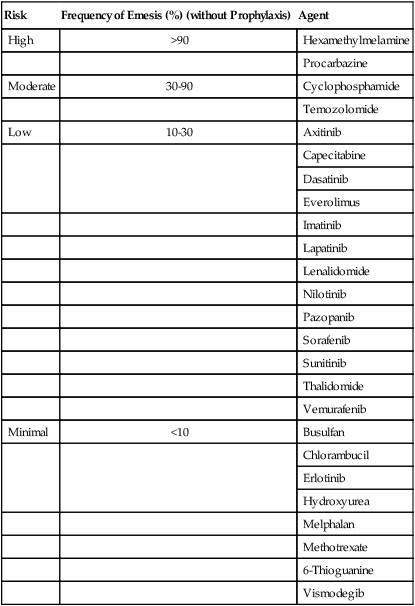
Oral agents are increasingly common in the treatment of cancer. Most of these agents have a lower risk of emesis than agents administered intravenously. However, antiemetic therapy can be problematic because of the chronic administration schedules of most oral agents; with prolonged administration, even low-level nausea can become a significant problem. The emetogenic risk of commonly used oral agents is summarized in Table 42-2. In general, the estimates of risk are based on the risk during an entire course of therapy, rather than a single dose. Because nausea or emesis can first occur several days of dosing, it is likely that this problem has been underreported for some of the oral agents.
Table 42-2
Emetogenic Potential of Commonly Used Oral Antineoplastic Agents
| Risk | Frequency of Emesis (%) (without Prophylaxis) | Agent |
| High | >90 | Hexamethylmelamine |
| Procarbazine | ||
| Moderate | 30-90 | Cyclophosphamide |
| Temozolomide | ||
| Low | 10-30 | Axitinib |
| Capecitabine | ||
| Dasatinib | ||
| Everolimus | ||
| Imatinib | ||
| Lapatinib | ||
| Lenalidomide | ||
| Nilotinib | ||
| Pazopanib | ||
| Sorafenib | ||
| Sunitinib | ||
| Thalidomide | ||
| Vemurafenib | ||
| Minimal | <10 | Busulfan |
| Chlorambucil | ||
| Erlotinib | ||
| Hydroxyurea | ||
| Melphalan | ||
| Methotrexate | ||
| 6-Thioguanine | ||
| Vismodegib |
Adapted from Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21(Suppl. 5):v232–43.
The use of chemotherapeutic agents in combination increases the emetogenic potential of a treatment regimen.28 The information contained in Tables 42-1 and 42-2 can be used to predict the emetic potential of a combination regimen. The following guidelines can be used:
• Identify the emetic risk of each drug.
• Combinations of moderate-risk agents result in high emetic risk.
• Low-risk agents + moderate-risk agents usually result in high-risk combinations.
• Combinations containing only low-risk agents have moderate risk.
• The addition of minimal-risk agents does not change the emetic potential of the other drugs.
Patient Characteristics
Age
Data are conflicting regarding the effect of patient age on the severity of chemotherapy-induced nausea and vomiting. However, increasing evidence indicates that chemotherapy-induced emesis occurs more frequently in younger patients.21,22,27 Fortunately, the best current antiemetic agents are effective and well tolerated by patients of all ages.
Gender
Data from large prospective studies indicate that female patients have more severe and frequent chemotherapy-induced nausea and vomiting than do male patients, even after controlling for the chemotherapy regimen.29,30 In one study, all patients received regimens containing cisplatin and were treated with ondansetron. More women who received high-dose cisplatin with either 5-fluorouracil or etoposide for lung cancer or head and neck carcinomas had poor control of emesis than did men who received these same regimens (49% vs. 29%, respectively).30
History of Alcohol Intake
Patients with a history of chronic alcohol intake (four to five mixed drinks per day) have more effective control of chemotherapy-induced nausea and vomiting when optimal antiemetic agents are used.31,32 In a prospective study of 52 patients receiving high-dose cisplatin with combination antiemetic therapy, 93% of those with a high alcohol intake experienced no emesis, versus 61% of patients without this history.31 It is important to emphasize, however, that the administration of highly emetogenic chemotherapy to these patients in the absence of appropriate antiemetic therapy still results in a high incidence of severe acute nausea and vomiting. The mechanism of the alcohol effect is unclear; it is possible, however, that various receptor sites are less sensitive in patients with a history of alcohol intake and that blockade of these receptors is relatively easy with appropriate antiemetic agents.
Previous Chemotherapy
Patients who have experienced poor control of emesis during previous chemotherapy are more likely to have unsatisfactory results with subsequent antiemetic drugs.30 The development of an anticipatory component to the nausea and vomiting is certainly one influence; whether additional influences are also involved is unknown.
Treatment of Chemotherapy-Induced Nausea and Vomiting
Acute Nausea and Vomiting
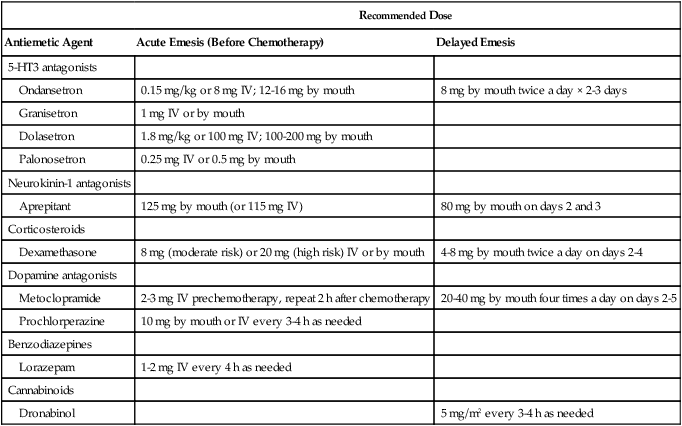
Several families of drugs with antiemetic activity have been identified. Table 42-3 lists the classes of antiemetic agents in current use, in approximate order of antiemetic potency. Only the 5-HT3 receptor antagonists, the substituted benzamides, and the NK1 receptor antagonists show marked activity against highly emetogenic chemotherapy. Because no single agent is ideal, combination antiemetic regimens have been developed, which have further improved efficacy. Increased understanding of the mechanism of action of the various compounds has led to more rational development of combination regimens; in general, the most effective regimens use agents with different mechanisms of action.
Table 42-3
Antiemetic Agents: Recommended Dosing
| Recommended Dose | ||
| Antiemetic Agent | Acute Emesis (Before Chemotherapy) | Delayed Emesis |
| 5-HT3 antagonists | ||
| Ondansetron | 0.15 mg/kg or 8 mg IV; 12-16 mg by mouth | 8 mg by mouth twice a day × 2-3 days |
| Granisetron | 1 mg IV or by mouth | |
| Dolasetron | 1.8 mg/kg or 100 mg IV; 100-200 mg by mouth | |
| Palonosetron | 0.25 mg IV or 0.5 mg by mouth | |
| Neurokinin-1 antagonists | ||
| Aprepitant | 125 mg by mouth (or 115 mg IV) | 80 mg by mouth on days 2 and 3 |
| Corticosteroids | ||
| Dexamethasone | 8 mg (moderate risk) or 20 mg (high risk) IV or by mouth | 4-8 mg by mouth twice a day on days 2-4 |
| Dopamine antagonists | ||
| Metoclopramide | 2-3 mg IV prechemotherapy, repeat 2 h after chemotherapy | 20-40 mg by mouth four times a day on days 2-5 |
| Prochlorperazine | 10 mg by mouth or IV every 3-4 h as needed | |
| Benzodiazepines | ||
| Lorazepam | 1-2 mg IV every 4 h as needed | |
| Cannabinoids | ||
| Dronabinol | 5 mg/m2 every 3-4 h as needed | |
5-HT3 Receptor Antagonists
The selective 5-HT3 receptor antagonists are the most effective family of antiemetic agents in the treatment of acute emesis. These agents block the serotonin type 3 receptors and are thought to exert their antiemetic activity primarily through peripheral blockade in the small intestine (see the preceding discussion). The first three agents in this class (“first-generation” agents) available in the United States were ondansetron, granisetron, and dolasetron. All three of these drugs proved superior to high-dose metoclopramide (the previous standard) in the prophylaxis of cisplatin-induced emesis,33–38 and all were superior to standard agents when used to prevent emesis associated with cyclophosphamide-based regimens.41–41 Dose-ranging studies with all drugs demonstrated an efficacy plateau,44–44 and multiple large randomized trials comparing these agents showed equivalent efficacy.3–11 For patients receiving high-dose cisplatin therapy, use of appropriate doses of any one of these three agents results in complete control of emesis in 50% to 70% of patients during the first 24 hours after administration of chemotherapy. Complete control of emesis ranges from 70% to 80% among patients receiving moderately emetogenic regimens, which usually are cyclophosphamide based (Box 42-2).
Palonosetron, a “second-generation” 5-HT3 receptor antagonist, is approved for the prophylaxis of acute and delayed chemotherapy-induced nausea and vomiting. Compared with other 5-HT3 receptor antagonists, palonosetron has a higher receptor binding affinity and a longer half-life (approximately 40 hours). In two phase 3 randomized clinical trials that included patients who received moderately emetogenic chemotherapy, palonosetron provided superior control of acute and delayed nausea and vomiting when compared with either ondansetron or dolasetron.45,46 Because both of these trials were designed as noninferiority trials, and because most patients did not receive concurrent dexamethasone, the superiority of palonosetron was not universally accepted, and additional trials were performed. Two additional randomized trials have largely confirmed the results of earlier trials.47,48 In the most recent, palonosetron plus dexamethasone was superior to granisetron plus dexamethasone in the complete control of emesis (56.8% vs. 44.5%; P < .0001) in patients receiving highly emetogenic chemotherapy (either cisplatin or cyclophosphamide/anthracycline combinations).48 The superiority of palonosetron was primarily in the control of delayed emesis. Therefore the results from these individual studies and a recent metaanalysis support the superiority of palonosetron when compared with the first-generation 5-HT3 antagonists.49
Recommended IV and oral doses of the 5-HT3 receptor antagonist and other antiemetics are outlined in Table 42-3. For prophylaxis of acute nausea and vomiting, a single dose during the first 24 hours is as effective as multiple doses. Therefore the use of additional doses of these agents for “breakthrough” vomiting during this time is to be discouraged, and the writing of “as needed” orders for these agents should be avoided. Oral preparations are available for all 5-HT3 receptor antagonists and have shown equivalent efficacy.49–53
NK1 Receptor Antagonists
The NK1 receptor is a component of the centrally mediated vomiting reflex. Interaction of the NK1 receptor and substance P, a tachykinin contained in vagal afferents innervating the area postrema and nucleus tractus solitarii in the brainstem, stimulates the vomiting reflex.19 Several selective NK1 receptor antagonists have shown antiemetic activity,56–56 and one of these, aprepitant, is currently approved by the Food and Drug Administration for the prevention of acute and delayed chemotherapy-induced nausea and vomiting.57–62 The most substantial contribution of this drug has been in the management of delayed emesis. In the pivotal randomized phase 3 study, patients treated with high-dose cisplatin received prophylaxis with either granisetron/dexamethasone or granisetron/dexamethasone plus aprepitant.57 Patients who received aprepitant more frequently had no vomiting during the first 24 hours (93% vs. 67%; P < .001) and also during days 2 through 5 (82% vs. 33%; P < .001). Similar improvements have subsequently been demonstrated in patients receiving cyclophosphamide/anthracycline-based regimens.60 In these patients, the addition of aprepitant to ondansetron/dexamethasone improved the complete control during a 5-day period (50.8% vs. 42.5%). Adverse effects with aprepitant are mild and infrequent and include fatigue, constipation, hiccoughs, and headache.
The contribution of aprepitant to the antiemetic prophylaxis of other moderately emetogenic regimens is less clear. In a recent phase 3 trial including patients who received a variety of moderately emetogenic regimens, the addition of aprepitant improved the overall complete control rate.62 However, retrospective subset analysis indicated that the improvement was limited to patients receiving cyclophosphamide/anthracycline combinations. Until more evidence is available, current antiemetic guidelines do not include aprepitant as part of routine prophylaxis for moderately emetogenic regimens except cyclophosphamide/anthracycline combinations.
Corticosteroids
The antiemetic mechanism of action of the corticosteroids is unclear. Unlike most other antiemetic agents, there is no current evidence that neurotransmitter blockade is involved. The antiemetic activity of the corticosteroids has been confirmed in several trials, predominantly for patients receiving moderately emetogenic chemotherapy.63,64 Corticosteroids that have been used as antiemetic agents include dexamethasone, methylprednisolone, and occasionally prednisone. Although no obvious differences in efficacy have been demonstrated among the corticosteroids, dexamethasone currently is almost universally used. Because of their moderate antiemetic efficacy, corticosteroids should be used as single agents only in the prophylaxis of mildly emetogenic chemotherapy. However, corticosteroids consistently add to the effect of other antiemetic agents when used in combination (see the discussion later in this chapter), presumably because of their different mechanism of action. Randomized dose-finding studies have identified optimal dexamethasone doses for highly emetogenic (cisplatin) and moderately emetogenic regimens to be 20 mg and 8 mg, respectively.65,66
Substituted Benzamides
High-dose metoclopramide was the first drug to demonstrate substantial antiemetic activity among patients treated with high doses of cisplatin.67 The mechanism of action was initially attributed to dopamine receptor antagonism. However, high-dose metoclopramide also inhibits the 5-HT3 receptors, which probably is a more important mechanism.68 Today, high-dose metoclopramide is rarely used as a result of the higher efficacy and lower toxicity of the selective 5-HT3 receptor antagonists.
Benzodiazepines
Lorazepam is the only benzodiazepine that has found widespread use in antiemetic therapy. Direct antiemetic effects of lorazepam are minor; however, the sedative, anxiolytic, and amnesic effects have made this drug attractive for use in combination regimens for patients with high levels of anxiety.69 The use of IV lorazepam often causes marked sedation lasting several hours, which limits its use in the outpatient setting. In addition, some patients experience confusion, amnesia, and transient enuresis.
Butyrophenones
The butyrophenones, haloperidol and droperidol, have antiemetic activity as a result of specific dopamine receptor blockade.70,71 Common adverse effects of the butyrophenones include sedation, dystonic reactions, and akathisia; in addition, hypotension is occasionally encountered. Because the butyrophenones are less efficacious than the 5-HT3 receptor antagonists, the NK1 antagonists, and high-dose metoclopramide, their use should be reserved for the occasional patient who has poor results with standard antiemetic treatment.
Cannabinoids
Anecdotal reports of reduced emesis among patients who smoke marijuana during chemotherapy stimulated interest during the early 1980s in the effectiveness of cannabinoids. Several cannabinoids have been evaluated as antiemetic agents; at present, dronabinol is the only commercially available agent in this class. This drug has antiemetic activity for patients receiving moderately emetogenic chemotherapy; in this setting, it has been more effective than prochlorperazine.72 The mechanism of action of the cannabinoids is incompletely defined; a central nervous system site of action has been postulated because of the marked psychoactive properties of these agents. Clinical use of cannabinoids is limited because of their unfavorable toxicity profile in some patients. Frequent toxicities include dysphoria, hallucinations, vertigo, dry mouth, sedation, and disorientation. These adverse effects are more common in elderly patients. The cannabinoids should not be considered for first-line antiemetic therapy, because more effective and better-tolerated agents exist. They should be considered for the occasional patient with mild to moderate nausea and vomiting who has either poor tolerance or poor response to other antiemetic drugs.73
Combination Antiemetic Therapy
The corticosteroids have been most extensively evaluated as “second drugs” in combination regimens because of their ease of administration, minimal toxicity, and different mechanism of action. When combined with the 5-HT3 receptor antagonists, dexamethasone has consistently improved antiemetic efficacy among patients receiving moderately or highly emetogenic regimens.76–76 In a recent metaanalysis of randomized trials comparing various regimens with or without dexamethasone, complete protection from emesis was increased by 16% when dexamethasone was added to a 5-HT3 receptor antagonist.77 Therefore a corticosteroid should be included in the prophylactic treatment of all patients receiving moderately or highly emetogenic chemotherapy, unless specific contraindications exist. A single dexamethasone dose before therapy (8 mg for moderately emetogenic regimens and 20 mg for highly emetogenic regimens) is optimum.65,66
Aprepitant is also an agent with proven efficacy in combination regimens, usually as a third drug (added to a 5-HT3/dexamethasone combination). The addition of aprepitant reduces acute and delayed emesis in patients receiving moderately or highly emetogenic chemotherapy.57–62 Aprepitant should be included in the prophylactic antiemetic treatment of all patients receiving highly emetogenic chemotherapy and in patients receiving cyclophosphamide/anthracycline combinations.59–62 The routine inclusion of aprepitant in other moderately emetogenic regimens has not been sufficiently investigated. However, if aprepitant is not initially included in the prophylaxis of patients receiving moderately emetogenic therapy, it should be added during subsequent cycles if the control of emesis is suboptimal.
Anticipatory Nausea and Vomiting
Because anticipatory nausea and vomiting are conditioned responses, effective control of chemotherapy-induced nausea and vomiting prevents the development of this reflex and is therefore the best strategy for preventing this problem.25,78 The highly effective combination regimens in current use have greatly decreased the prevalence of this problem. For patients in whom anticipatory nausea and vomiting develop, treatment with anxiolytic agents such as lorazepam is sometimes effective.69 In addition, various nonpharmacologic approaches, including hypnosis and behavioral modification, have shown some benefit for these patients.25,79,80
Delayed Nausea and Vomiting
Even after the first-generation 5-HT3 antagonists had dramatically improved control of acute chemotherapy-induced nausea and vomiting, effective control of delayed nausea and vomiting remained problematic. The first-generation 5-HT3 antagonists, as well as dexamethasone, metoclopramide, and phenothiazines, had limited efficacy.83–83 High rates of delayed nausea (59%) and vomiting (32%) continued to occur in patients receiving moderately or highly emetogenic chemotherapy, and 23% of patients with complete control during the first 24 hours experienced subsequent nausea or vomiting.84
The introduction of palonosetron and aprepitant improved the treatment of delayed nausea and vomiting substantially. Because both of these agents are highly effective in the acute and delayed phases, their use also blurs the distinction between these entities in terms of clinical management. Palonosetron is more effective than either ondansetron or dolasetron in controlling delayed nausea and vomiting.45–49 In patients receiving moderately emetogenic chemotherapy, a single dose of palonosetron (0.25 mg) provided complete control of delayed nausea and vomiting in 48%, compared with 36% for dolasetron, 100 mg.45 Similar results occurred when palonosetron, 0.25 mg, was compared with ondansetron, 32 mg (74% vs. 55% control of delayed emesis; P < .001).46 The differences between palonosetron and the other 5-HT3 receptor antagonists are probably related to the longer half-life of palonosetron and to the improved control of acute emesis.
The addition of aprepitant, the first NK1 antagonist, has been even more important in controlling delayed nausea and vomiting.57–62 When added to granisetron/dexamethasone in patients receiving cisplatin-based chemotherapy, aprepitant reduced the incidence of delayed emesis from 67% to 18% (P < .001).57 Improvement also occurred when aprepitant was added to ondansetron/dexamethasone in patients receiving cyclophosphamide/anthracycline chemotherapy (complete control improved from 42.5% to 50.8%; P = .019).60
Radiation-Induced Nausea and Vomiting
Radiation-induced nausea and vomiting are common with some types of radiation therapy and are related to the size of the radiation portal, the dose delivered, and the site of radiation. Radiation-induced emesis occurs acutely in more than 90% of patients receiving total-body irradiation. Among patients receiving conventional daily doses of radiotherapy (2 Gy/fraction), emesis develops within 2 to 3 weeks in about 50% of patients receiving an upper abdominal portal.85 Low-risk areas (i.e., 10% to 30% incidence) include the thoracic region, pelvis, and brain (radiosurgery), whereas areas with minimal risk include the head and neck, extremities, breast, and chest wall. The mechanism of radiation-induced emesis remains unclear, but release of serotonin from the gastrointestinal enterochromaffin cells and subsequent involvement of the gastrointestinal 5-HT3 receptors and vagal afferent fibers is most likely.
Most of the antiemetic agents found to be active against chemotherapy-induced nausea and vomiting also have some activity against radiation-induced emesis; however, few randomized trials have been performed to identify an optimal regimen. Given the postulated mechanism of radiation-induced emesis and the available clinical data, it seems likely that the 5-HT3 receptor antagonists are the most active antiemetic agents in this setting. Dexamethasone or ondansetron, used as single agents, were superior to placebo in patients receiving upper abdominal irradiation86,87 or total-body irradiation.88 With multifractionated courses, the use of daily dexamethasone is problematic; however, the addition of dexamethasone (4 mg orally daily, during the first 5 days only) to ondansetron (8 mg orally twice daily) improved results in patients receiving upper abdominal radiation therapy.89
Summary of Recommendations for Combination Antiemetic Therapy
Optimal antiemetic therapy is selected on the basis of the emetic potential of the planned chemotherapy regimen, as well as consideration of patient-related risk factors. The information presented in Tables 42-1 and 42-2 should be used to assign an emetic level to the chemotherapy agent or regimen prescribed for the patient. Optimal doses of all antiemetic agents are contained in Table 42-3.
With the antiemetic agents currently available, the goal of prophylactic therapy for each patient should be complete control (i.e., no nausea or vomiting). Table 42-4 contains a summary of currently recommended prophylactic antiemetic regimens, based on the anticipated emetogenic potential of chemotherapy. Patients with incomplete control should have modifications to their prophylactic regimen during subsequent cycles so they receive the antiemetic agents recommended for higher risk chemotherapy.
Table 42-4
Summary of Recommended Antiemetic Prophylaxis for Patients Receiving Intravenous Chemotherapy
| Risk of Emesis | Day 1 (Prechemotherapy) | After Day 1 |
| High | 5-HT3 receptor antagonist (consider palonosetron) + dexamethasone + aprepitant | Aprepitant on days 2 and 3 ± dexamethasone on days 2-4 |
| Moderate | ||
| Cyclophosphamide/anthracycline | 5-HT3 receptor antagonist + dexamethasone + aprepitant | Aprepitant on days 2 and 3 |
| Other | 5-HT3 receptor antagonist + dexamethasone | Dexamethasone on days 2 and 3 |
| Low | Dexamethasone | None |
| Minimal | As needed | None |






