Chapter 30 Natural history of gallstones and asymptomatic gallstones
History
Gallstones have been recognized since antiquity and have even been found during autopsies of Egyptian mummies. The Greek physician Trallianus described calculi within radicles of a human liver (Glenn & Grafe, 1966). Vesalius and Fallopius described gallstones in the gallbladders of dissected human bodies (Schwartz, 1981), and in 1882, Langenbuch performed the first successful cholecystectomy, setting the path for therapeutic intervention in cholelithiasis.
Epidemiology
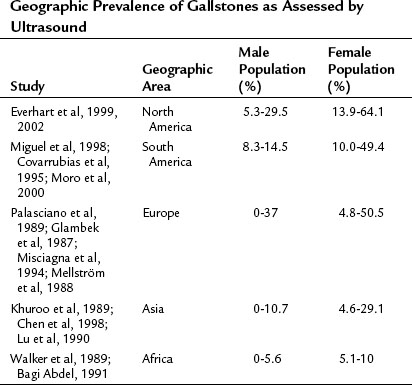
Gallstones represent a sizeable problem for the contemporary health care system in both developed and developing countries alike. In the year 2000, there were 2,624,111 admissions and 778,632 outpatient visits for gallstones in the United States. With 6.3 million affected men and 14.2 million affected women between the ages of 20 and 74 years, and at an annual cost of $6.5 billion, cholelithiasis is the most expensive digestive tract disorder today in the United States (Shaffer, 2006). Incidence of gallstones and gallstone-related operations in developed countries appears to be increasing. With an overall prevalence of 10% to 15% in developed countries, geographic-specific prevalence is from 0% to 10% in Africa and up to 60% to 70% in certain populations, such as Pima Indians; this likely reflects combined differences in environmental, dietary, and genetic factors (Shaffer, 2006). The most common type of gallstones in developed countries are composed primarily of cholesterol, and the highest incidence is in the Native American population; moderate prevalence is seen in Hispanics, and the lowest prevalence is seen in black Americans, East Asians, and sub-Saharan Africans. Increased incidence of brown-pigmented stones are noted in East Asians, reflecting a higher rate of biliary tract infections (Shaffer, 2006; see Chapter 43). More detailed information about geographic disparities in the prevalence of gallstone disease can be found in Table 30.1.
Risk Factors for Gallstones Development
Gallstone disease clearly results from a complex interaction of genetic and environmental factors. This is confirmed by an altered incidence of gallstone detection when people from diverse geographical areas travel and settle in areas with a different dietary regimen, level of physical activity, and hygiene. The common mechanisms of gallstone formation include cholesterol hypersecretion, alteration in intestinal bile salt/cholesterol absorption, and gallbladder hypokinesia, which leads to bile cholesterol supersaturation and nucleation (see Chapter 7). Bacterial infection and an increase in bilirubin load play a role in the development of black and brown gallstones. The following is a quick overview of the most common risk factors for the development of gallstones.
Age
The incidence of gallstones increases with age across all ethnic groups, and it is very low among infants and children (Chen et al, 1998). Some specific populations such as Pima Indians, mentioned previously, have an increased incidence of gallstones (up to 70%) by 30 years of age, implicating hereditary metabolic factors (Sampliner et al, 1970). Pediatric populations with chronic hemolytic conditions, such as sickle cell anemia, also represent another distinct group in whom gallstones develop early.
Gender
Female gender is a risk factor for developing gallstones, surpassing males in the incidence of gallstones and the chance of having surgery by 2 : 1 or 3 : 1 in most studies (see Table 30.1). Pregnancy is associated with up to a 30% risk of developing biliary sludge, and low-dose estrogen therapy in postmenopausal women increases the risk of gallstone formation (Maringhini, et al, 1993; Hulley et al, 1998).
Obesity, Diet, Physical Activity, and Weight Loss
Obesity is a well-established major risk factor for the development of gallstones. The association with simple obesity is important in females; in males it is mostly associated with intraabdominal (central) obesity and metabolic syndrome (Maclure et al, 1989; Heaton et al, 1991; De Santis et al, 1997; Tsai et al, 2004).
The frequency of gallstones has almost doubled in the last 60 years, in parallel with the changed composition of stones from pigment to cholesterol. In humans, most cholesterol found in gallstones is from the diet, since de novo hepatic synthesis is only about 20% (Paigen & Carey, 2002). This emphasizes the importance of high caloric and carbohydrate intake as risk factors for the development of gallstones in modern society.
Bariatric patients are at a high risk for the development of cholelithiasis after weight-reduction surgery. Sludge and gallstones form in up to 35% of patients following bariatric procedures, with weight loss more than 1.5 kg/week being a risk factor (Shiffman, et al, 1991; Weinsier et al, 1995).
Patients receiving total parenteral nutrition (TPN) are another group with a high incidence of gallstone and sludge development. After 3 months of TPN therapy, approximately 45% of patients will develop gallstones; 100% of patients will develop sludge within 6 weeks (Shaffer, 2001; Murray et al, 1992). Low levels of physical activity are associated with development of gallstones in large observational and population studies also related to obesity and diet (Leitzmann et al, 1999). Finally, patients receiving long-term somatostatin analogue therapy for various disorders (acromegaly, functional neuroendocrine tumors) are at increased risk for gallstone formation as a result of reduced gallbladder motility and reduced bile flow (Bornschein et al, 2009).
Genetics
Population studies indicate a 30% genetic component in the development of gallstone disease. Hepatic cholesterol hypersecretion, resulting in bile supersaturation and cholesterol stone formation, may be caused by increased hepatic uptake and synthesis of cholesterol, decreased hepatic synthesis of bile salts, decreased hepatic synthesis of cholesterol esters for inclusion in very-low-density lipoprotein (VLDL), and derangements in the intestinal absorption of cholesterol and bile salts. Genes controlling hepatic cholesterol metabolism have been implicated in the pathogenesis of gallstone formation in transgenic/knockout mouse models and in humans (Table 30.2). Excess cholesterol load in smooth muscles of the gallbladder can affect signaling via the cholecystokinin A (CCK-A) receptor and contribute to the development of gallstones by inducing bile stasis. Intestinal cholesterol/bile salt absorption is tightly controlled and genes responsible for cholesterol absorption (NPC1L1, Niemann-Pick C1-like protein) or cholesterol efflux (ABCG5/G8) have been implicated in the development of gallstones in certain populations because of intestinal bile salt loss. Single-gene mutations result in hemolysis and increase the incidence of black stones because of an excess of unconjugated bile acids forming polymerized calcium bilirubinate stones. A summary of genes found to be involved in gallstone pathogenesis can be found in Table 30.2 (Grünhage & Lammert, 2006).
| Genes and Encoded Proteins | Mechanism | Significance in Humans |
|---|---|---|
| ABCB4, ATP binding cassette transporter B4 | Decreased biliary phospholipid secretion | + |
| ABCB11, ATP binding cassette transporter B11 | Decreased bile salts secretion | + |
| APOB, apolipoprotein B | Decreased hepatic VLDL secretion | +/? |
| APOE, apolipoprotein E | Increased intestinal cholesterol absorption, decreased bile acid secretion | +/? |
| CCKAR, cholecystokinin A receptor | Gallbladder hypomotility | ? |
| CETP, cholesteryl ester transfer protein, plasma | Increased HDL metabolism | + |
| CFTR, cystic fibrosis transmembrane conductance regulator | Increased biliary bilirubin secretion and bile salt excretion in feces, decreased bile pH | + |
| ANK1, EPB42, SPTA1, SPTB, SLC4A1, associated with hereditary spherocytosis | Increased bilirubin load | + |
| HBB, hemoglobin-β, associated with sickle cell anemia and thalassemia | Increased bilirubin load | + |
| AK1, G6PD, GPI, GSR, PGK1, PKLR, associated with erythrocyte enzyme deficiencies | Increased bilirubin load | + |
| CYP39A1, oxysterol 7-alpha-hydroxylase | Induced hypercholesterolemia | + |
| UGT1A1, Gilbert syndrome–associated protein | Increased bilirubin load | + |
| NPC1L1, NPC1 (Niemann-Pick disease, type C1, gene)–like protein 1 | Reduced cholesterol intestinal absorption | + |
| ABCG5/ABCG8, ATP binding cassette G5/G8, cholesterol transporter | Increased cholesterol intestinal efflux | ? |
ATP, adenosine triphosphate; HDL, high-density lipoprotein; VLDL, very-low-density lipoprotein; +, clear evidence; ?, potentially related but no definitive evidence
Other Risk Factors
Certain medical conditions and medications can cause gallstones by multiple mechanisms. Cirrhosis can induce decreased bile salt synthesis and bile salt malabsorption together with chronic hemolysis and a hyperestrogenic state. Inflammatory bowel disease (Crohn disease) or ileal resection can also interfere with bile salt absorption, estrogen can increase biliary cholesterol secretion, and progesterone can impair gallbladder motility. In addition to causing gallbladder hypomotility, octreotide increases the amount of reabsorbed deoxycholic acid and results in cholesterol supersaturation. Ceftriaxone is excreted in bile in significant amounts and can cause biliary pseudolithiasis and sludge, which usually disappears after antibiotic therapy is discontinued (Lambou-Gianoukos & Heller, 2008).
Natural History of Gallstones
Gallstones do not cause any symptoms in up to 80% of carriers. Within 5 years, 10% to 20% became symptomatic. The risk of further symptoms is approximately 2% per year with an overall risk of biliary complications (acute pancreatitis and symptomatic choledocholithiasis) in asymptomatic patients of 0.3% per year; the risk of gallbladder cancer is 0.02% per year (Gibney, 1990; Thistle et al, 1984; Attili et al, 1995; Gracie & Ransohoff, 1982; Friedman, 1993; McSherry et al, 1985). Half of the patients with symptoms develop a second attack within a year, but in 30% of all cases, there is only one symptomatic attack. The analysis of the natural history of gallstones started with a landmark study by Gracie and Ransohoff (1982), which followed 123 Michigan University faculty members—110 men and 13 women—with asymptomatic gallstones at screening for 15 years. At 5, 10, and 15 years of follow-up, 10%, 15%, and 18% had become symptomatic, respectively. None of the subjects developed complications before the onset of typical symptoms. The approximate rate at which the subjects developed biliary pain was 2% per year for the first 5 years with a subsequent decrease over time. Three patients in this study developed biliary complications, all of which were preceded by biliary pain. The investigators concluded that, based on these results, prophylactic cholecystectomy for asymptomatic gallstones could not be recommended (Gracie & Ransohoff, 1982).
Attili and colleagues (1995) also followed 151 subjects with gallstones over 10 years. Of those subjects, 33 had symptoms, and 118 were asymptomatic at the beginning of the study. The cumulative probability of developing biliary colic was 12% at 2 years, 17% at 4 years, and 26% at 10 years. The cumulative probability of developing complications after 10 years was 3% in the initial asymptomatic group and 7% in the symptomatic group. The authors concluded that the natural history of gallstone disease might not be as benign as previously thought.
The results of several other natural history studies have been reported. In a Japanese study, Wada and Imamura (1993) found one third of 1850 patients with cholelithiasis to be symptomatic. Of the remaining 680 asymptomatic patients, 20% became symptomatic over a median follow-up of 13 years. Patients older than 70 years were more likely to become symptomatic than patients younger than 70 years. McSherry and colleagues (1985) followed 135 asymptomatic men and women with gallstones who were subscribers to the Health Insurance Plan of Greater New York. Of these subjects, 10% developed symptoms, and 7% required cholecystectomy over a median follow-up of 46 months. A placebo group of 193 asymptomatic patients who were part of a chemical dissolution trial were followed for 24 months (Thistle et al, 1984). In this study, 31% of patients eventually developed biliary pain, which is high but perhaps related to the intense surveillance or to the requirement that participants have no symptoms only for the 12 months preceding the trial. Cucchiaro and colleagues (1990) followed 125 asymptomatic patients for 5 years. Fifteen patients developed symptoms during that time, and 2 patients required emergent surgery for gallstone complications; 54 patients died during that period because of malignancies, cardiovascular disease, or renal insufficiency, but none died from gallstone disease. Friedman and coworkers (1989) followed 123 asymptomatic patients for 20 years and found that 6% of patients developed severe gallstone-related symptoms during the first 5 years after diagnosis. Halldestamm and colleagues (2004) followed 120 asymptomatic patients, 14 of whom required treatment for symptoms or gallstone-related complications over a median observation period of 87 months. The authors calculated that the cumulative risk of requiring treatment during the first 5 years after detection of gallstones was 7.6%. A summary of the natural history of gallstones as reported by various authors is shown in Table 30.3.
In a Marcov model, simulation investigators from the Netherlands attempted to predict the effect of cholecystectomy on adverse events during a 10-year period (Venneman et al, 2005). In this particular model, 3.2% of the small gallstone carriers developed acute cholecystitis, 1.8% developed symptomatic choledocholithiasis, and up to 3.4% developed acute pancreatitis. There was no clear cost benefit and no life-years were gained from prophylactic cholecystectomy, indicating no clear-cut advantages of prophylactic cholecystectomy in asymptomatic cholelithiasis. As a result, in patients with asymptomatic gallstones, expectant management is recommended.
Patients with diabetes may have a higher incidence of gallstones from the indirect effects of the metabolic syndrome, obesity, and a family history of gallstones. No data show worse evolution of asymptomatic cholelithiasis in diabetics, and prophylactic cholecystectomy in asymptomatic gallstones carriers with diabetes is not recommended (Sleisenger & Fordtran, 1993).
The size of gallstones is thought to be linked to a higher risk for the development of gallbladder cancer, but this is controversial (see Chapter 49). Cholecystectomy is possibly indicated in some populations carrying a high risk of gallbladder cancer (Chilean Hispanics, Mapuche Indians, and Maoris from Easter Island), even when associated with asymptomatic cholelithiasis (Randi et al, 2006; Grimaldi et al, 1993). Prophylactic cholecystectomy also could be indicated in patients with active hemolytic anemias, before or during renal transplantation or during bariatric surgery (Bonatsos et al, 2001; Graham et al, 1995; Shiffman et al, 1993).
Gallstones, Porcelain Gallbladder, and Gallbladder Carcinoma (See Chapter 49)
Gallstones are closely associated with gallbladder cancer (level V evidence, descriptive studies only), and it is speculated that gallstones larger than 3 cm, family history of gallbladder cancer, and duration of cholelithiasis are all potential risk factors for the development of gallbladder cancer (Miyazaki et al, 2008). Gallstones are present in 60% to 90% of gallbladder cancer cases (Goldin & Roa, 2009), although no direct causal relationship between gallstones and gallbladder cancer has been proven. The presence of large gallstones could simply reflect the duration of cholelithiasis rather than causality, and a significant difference in prevalence of gallbladder cancer worldwide points to the influence of other factors. The risk of developing carcinoma is estimated to be 1% of calculous gallbladders 20 years after the initial diagnosis of gallstones, with the risk increased mainly in men (Maringhini et al, 1987); however, given the high incidence of gallstones and the rarity of gallbladder cancer, this estimate would seem to be high. In some series, no patients with gallstones developed gallbladder cancer over a 10-year period. As a result, cholecystectomy to prevent the development of gallbladder cancer in patients with gallstones is not recommended. Some populations such as Pima Indians, Chileans, and Pakistani women with gallstones have an unusually high incidence of gallbladder cancer (12 to 21 per 100,000; Goldin & Roa, 2009). In these unique populations, prophylactic cholecystectomy to prevent the development of gallbladder cancer is probably justified.
Calcification of the gallbladder is encountered in up to 0.8% of all gallbladder pathology specimens. Porcelain gallbladder or diffuse gallbladder calcification was long considered to be a significant risk factor for the presence of gallbladder cancer, ranging from 12% to 62% (Stephen & Berger, 2001; Towfigh et al, 2001; Cunningham & Alexander, 2007). Recently, two studies from the United States analyzed a large database of cholecystectomy specimens and reported much lower incidence rates of cancer in porcelain gallbladder than in previous studies (Stephen & Berger, 2001; Towfigh et al, 2001). Diffuse calcification appeared to confer little or no increased risk of cancer; by contrast, the incidence of cancer in gallbladders with selective, not diffuse, calcifications was much higher at 7% (Stephen & Berger, 2001). Based on this data, at least in Western populations, the incidence of cancer in porcelain gallbladder should be considered to be increased but not as high as reported previously. Taking into account that older studies from other populations (Japan and South America) are citing a much higher association of porcelain gallbladder with gallbladder cancer, and because gallbladder cancer is a highly lethal disease, careful decision making should be undertaken when assessing individual risk of cancer in patients with a calcified gallbladder.
Angelico F, et al. Ten-year incidence and natural history of gallstone disease in a rural population of women in central Italy. The Rome Group for the Epidemiology and Prevention of Cholelithiasis (GREPCO). Ital J Gastroenterol Hepatol. 1997;29:249-254.
Attili AF, et al. The natural history of gallstones: the GREPCO experience. The GREPCO Group. Hepatology. 1995;21(3):655-660.
Bagi Abdel M, et al. Prevalence of gallbladder disease in Sudan: first sonographic study in adult population [abstract]. Gastroenterology. 1991;100:A307.
Bonatsos G, et al. Laparoscopic cholecystectomy in adults with sickle cell disease. Surg Endosc. 2001;15(8):816-819.
Bornschein J, Drozdov I, Malfertheiner P. Octreotide LAR: safety and tolerability issues. Expert Opin Drug Saf. 2009;8(6):755-768.
Chen CY, et al. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing. 1998;27(4):437-441.
Covarrubias C, et al. The role of ethnicity and other risk factors for cholelithiasis in a highly prevalent area: cross-sectional and cohort studies in Chilean and Amerindian Araucanos. Gastroenterology. 1995;108:A1053.
Cucchiaro G, et al. Clinical significance of ultrasonographically detected coincidental gallstones. Dig Dis Sci. 1990;35:417-421.
Cunningham SC, Alexander HR. Porcelain gallbladder and cancer: ethnicity explains a discrepant literature? Am J Med. 2007;120(4):e17-e18.
De Santis A, et al. Gallstones and diabetes: a case-control study in a free-living population sample. Hepatology. 1997;25(4):787-790.
Everhart JE, et al. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117(3):632-639.
Everhart JE, et al. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology. 2002;35(6):1507-1512.
Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165(4):399-404.
Friedman GD, et al. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol. 1989;42:127-136.
Gibney EJ. Asymptomatic gallstones. Br J Surg. 1990;77(4):368-372.
Glambek I, et al. Prevalence of gallstones in a Norwegian population. Scand J Gastroenterol. 1987;22(9):1089-1094.
Glenn F, Grafe WRJr. Historical events in biliary tract surgery. Arch Surg. 1966;93:848-852.
Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55(2):218-229.
Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med. 1982;307(13):798-800.
Graham SM, et al. The utility of prophylactic laparoscopic cholecystectomy in transplant candidates. Am J Surg. 1995;169(1):44-48. discussion 48-49
Grimaldi CH, et al. Increased mortality with gallstone disease: results of a 20-year population-based survey in Pima Indians. Ann Intern Med. 1993;118(3):185-190.
Grünhage F, Lammert F. Gallstone disease: pathogenesis of gallstones—a genetic perspective. Best Pract Res Clin Gastroenterol. 2006;20(6):997-1015.
Halldestamm I, et al. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg. 2004;91:734-738.
Heaton KW, et al. Why do men get gallstones? Role of abdominal fat and hyperinsulinaemia. Eur J of Gastroenterol Hepatol. 1991;3:745-751.
Hulley S, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605-613.
Juhasz ES, et al. Incidental cholecystectomy during colorectal surgery. Ann Surg. 1994;219:467-472.
Khuroo MS, et al. Prevalence of biliary tract disease in India: a sonographic study in adult population in Kashmir. Gut. 1989;30(2):201-205.
Lambou-Gianoukos S, Heller SJ. Lithogenesis and bile metabolism. Surg Clin North Am. 2008;88(6):1175-1194. vii
Leitzmann MF, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777-784.
Lu SN, et al. Risk factors for gallstones among Chinese in Taiwan: a community sonographic survey. J Clin Gastroenterol. 1990;12(5):542-546.
Maclure KM, et al. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med. 1989;321(9):563-569.
Maringhini A, et al. Gallstones, gallbladder cancer, and other gastrointestinal malignancies: an epidemiologic study in Rochester, Minnesota. Ann Intern Med. 1987;107:30-35.
Maringhini A, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med. 1993;119(2):116-120.
McSherry CK, et al. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg. 1985;202(1):59-63.
Mellström D, Asztély M, Svanvik J. Gallstones and previous cholecystectomy in 77- to 78-year-old women in an urban population in Sweden. Scand J Gastroenterol. 1988;23(10):1241-1244.
Miguel JF, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115(4):937-946.
Misciagna G, et al. Epidemiology of cholelithiasis in southern Italy. Eur J Gastroenterol Hepatol. 1994;6:937-941.
Miyazaki M, et al. Risk factors for biliary tract and ampullary carcinomas and prophylactic surgery for these factors, Japanese Association of Biliary Surgery, Japanese Society of Hepato-Pancreatic Surgery, Japan Society of Clinical Oncology. J Hepatobiliary Pancreat Surg, 15. 2008;1:15-24.
Moro PL, et al. Gallstone disease in Peruvian coastal natives and highland migrants. Gut. 2000;46(4):569-573.
Murray FE, Stinchcombe SJ, Hawkey CJ. Development of biliary sludge in patients on intensive care unit: results of a prospective ultrasonographic study. Gut. 1992;33(8):1123-1125.
Paigen B, Carey MC. Gallstones. In: King, RA, Rotter, JF, Motulsky, AG. The Genetic Basis for Common Diseases. London: Oxford University Press; 2002:298-335.
Palasciano G, et al. Gallstone prevalence and gallbladder volume in children and adolescents: an epidemiological ultrasonographic survey and relationship to body mass index. Am J Gastroenterol. 1989;84(11):1378-1382.
Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118(7):1591-1602.
Sampliner RE, et al. Gallbladder disease in Pima Indians: demonstration of high prevalence and early onset by cholecystography. N Engl J Med. 1970;283(25):1358-1364.
Schwartz SI. Sequence of stones. Contemp Surg. 1981;18:9.
Shaffer EA. Gallbladder sludge: what is its clinical significance? Curr Gastroenterol Rep. 2001;3(2):166-173.
Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20(6):981-996.
Shiffman ML, et al. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86(8):1000-1005.
Shiffman ML, et al. Gallstones in patients with morbid obesity: relationship to body weight, weight loss and gallbladder bile cholesterol solubility. Int J Obes Relat Metab Disord. 1993;17(3):153-158.
Sleisenger, MH, Fordtran, JS. Gastrointestinal Disease. Philadelphia: Saunders. 1993:973-984.
Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129(6):699-703.
Thistle JL, et al. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med. 1984;101(2):171-175.
Towfigh S, et al. Porcelain gallbladder is not associated with gallbladder carcinoma. Am Surg. 2001;67(1):7-10.
Tsai CJ, et al. Prospective study of abdominal adiposity and gallstone disease in U.S. men. Am J Clin Nutr. 2004;80(1):38-44.
Wada K, Imamura T. Natural course of asymptomatic gallstone disease. Nippon Rinsho. 1993;51:1737-1743.
Walker AR, et al. Prevalence of gallstones in elderly black women in Soweto, Johannesburg, as assessed by ultrasound. Am J Gastroenterol. 1989;84(11):1383-1385.
Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med. 1995;98(2):115-117.
Venneman NG, et al. Small gallstones are associated with increased risk of acute pancreatitis: potential benefits of prophylactic cholecystectomy? Am J Gastroenterol. 100(11), 2005. 2540–2450