Miscellaneous pediatric disorders
VASCULAR DISEASES
Central nervous system vascular pathology is uncommon in children, but there are rare forms of vasculopathy unique to this age group, as well as presentations in childhood of some disorders that are more commonly seen in older individuals (Table 7.1).
Table 7.1
| Fetal presentation | Postnatal onset | |
| Vascular malformation | Proliferative vasculopathy and hydranencephaly–hydrocephaly Meningocerebral angiodysplasia and renal agenesis |
Aneurysm of great vein of Galen Arteriovenous malformation Cavernous angioma |
| Aneurysm | Saccular Fusiform (basilar) Infectious (mycotic) Dissecting |
|
| Vasculitis | Kawasaki disease Takayasu’s arteritis |
|
| Coagulopathy | Hemolytic–uremic syndrome Hemorrhagic shock and encephalopathy syndrome |
PROLIFERATIVE VASCULOPATHY AND HYDRANENCEPHALY–Hydrocephaly (Fowler Syndrome)
MACROSCOPIC APPEARANCES
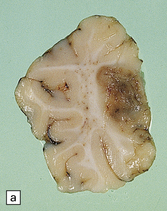
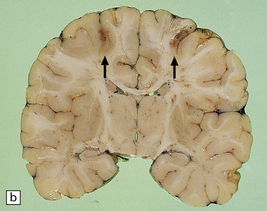
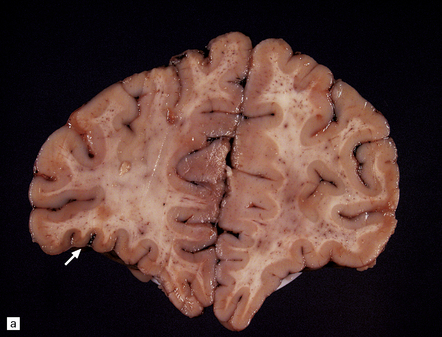
Arthrogryposis, pterygia, muscular hypoplasia, and pulmonary hypoplasia are found at necropsy (Fig. 7.1a). Bubble-like hemispheres that are macroscopically indistinguishable from those seen in encephaloclastic hydranencephaly have a diaphanous pallium studded with calcifications (Fig. 7.1b).
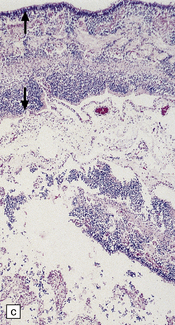
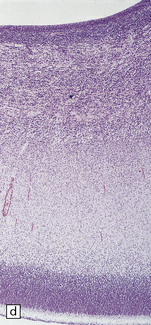
7.1 Proliferative vasculopathy and hydranencephaly–hydrocephaly in a 17-week fetus.
(a) Arthrogryposis and webbing of the elbow joint. (b) Lateral aspect of the cystic cerebral hemisphere demonstrated under water. (c) The extremely thin pallium (between arrows) is barely one-third of the normal. (d) Normal age-matched control for comparison with (c).
MICROSCOPIC APPEARANCES
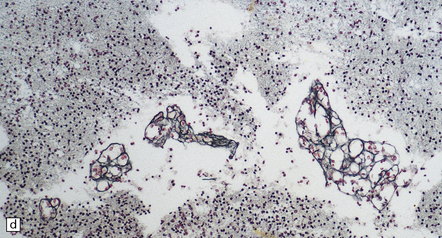
The thin, disorganized pallium has a narrow immature cortical plate. Glomeruloid endothelial vasculopathy, which is unique to this disorder, is prominent throughout the CNS (Fig. 7.2). Endothelial cells in the glomeruloid structures have PAS-positive intracytoplasmic inclusions, which appear on electron microscopy as homogeneous granular material surrounded by rough endoplasmic reticulum (Fig. 7.3).
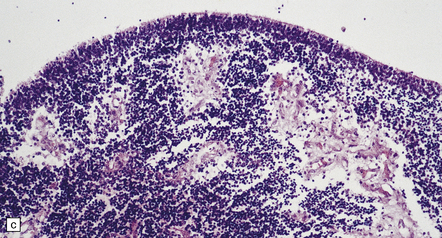
7.2 Proliferative vasculopathy and hydranencephaly–hydrocephaly in a fetus.
Glomeruloid vascular structures are widespread in the brain and spinal cord. (a) Glomeruloid vasculopathy in the marginal zone and cortical plate. (b) Prominent vascular expansion involving the intermediate and subventricular zones. (c) Vasculopathy is also visible in the germinal eminence. (d) Glomeruloid structure in the cerebellum (reticulin). (e) Glomeruloid vascular expansion in the anterior horn of the spinal cord.
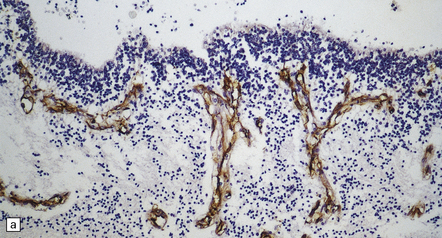
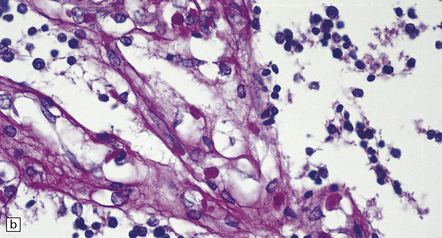
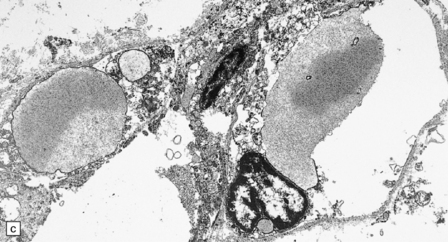
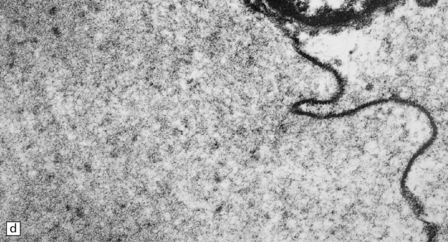
7.3 Proliferative vasculopathy and hydranencephaly–hydrocephaly.
(a) The glomeruloid vascular structures have many small lumina lined by endothelial cells, as demonstrated by lectin histochemistry with Ulex europaeus agglutinin. (b) Some endothelial cells contain PAS-positive intracytoplasmic inclusions. (c) Electron microscopy reveals endothelial inclusions of very varied size comprising moderately electron-dense granular material surrounded by rough endoplasmic reticulum. (d) Close-up of the surrounding rough endoplasmic reticulum.
MENINGOCEREBRAL ANGIODYSPLASIA AND RENAL AGENESIS
This sporadic disorder causes stillbirth or premature delivery with minimal survival.
MICROSCOPIC APPEARANCES
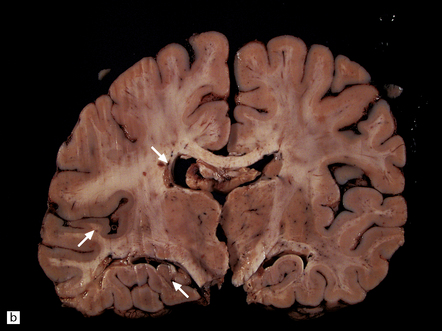
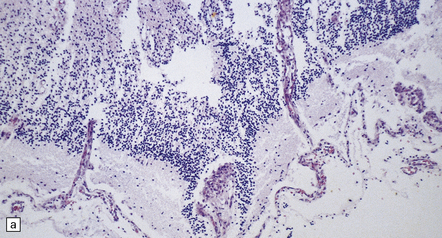
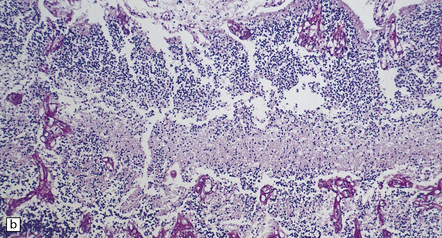
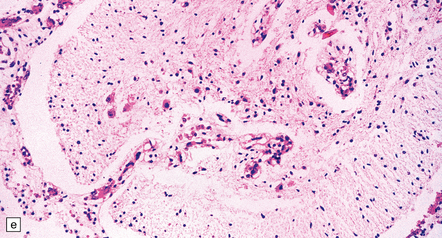
There is diffuse angiodysplasia, consisting of ectatic capillaries and veins. These are present in the leptomeninges, cerebral cortex, cerebellum, and brain stem. The angiodysplasia is complicated by thrombosis, infarction, hemorrhage, hemosiderin-laden macrophages, gliosis and calcification in the cerebral cortex and white matter (Fig. 7.4).
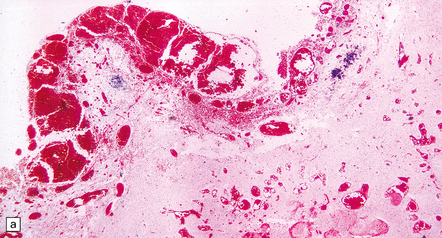
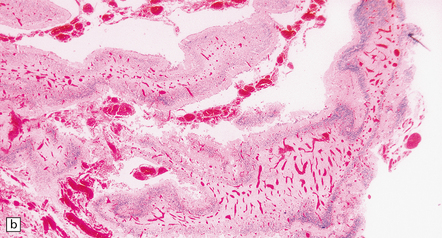
7.4 Meningocerebral angiodysplasia and renal agenesis.
(a) Engorged and markedly ectatic thin-walled vessels are seen within the pia–arachnoid and also in the underlying partially necrotic cerebral parenchyma. Note also the vascular thrombosis and focal calcification. (b) A similar process also affects the cerebellum.
ANEURYSM OF THE VEIN OF GALEN
MACROSCOPIC AND MICROSCOPIC APPEARANCES
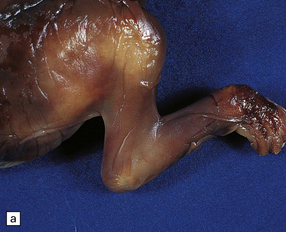
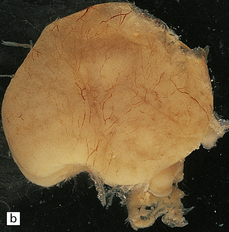
The vascular malformation is fed by one or both of the posterior cerebral arteries or their branches resulting in dilatation of the vein of Galen and enlargement of the cerebral venous system and sinuses (Fig. 7.5). The enlarging vascular mass may compress the aqueduct causing hydrocephalus, or may undergo calcification or thrombosis. Microscopically, veins show the typical changes of ‘arterialization’, e.g. hyperplasia and hypertrophy of the intima and muscularis, that result from increased intraluminal pressure.
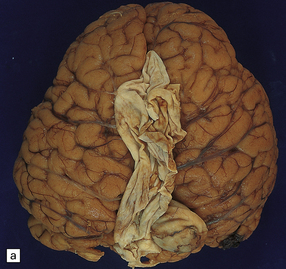
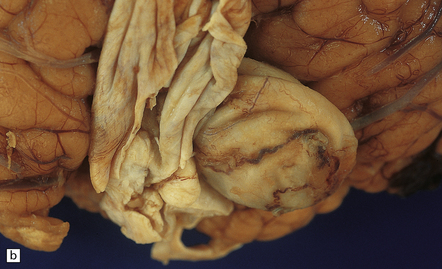
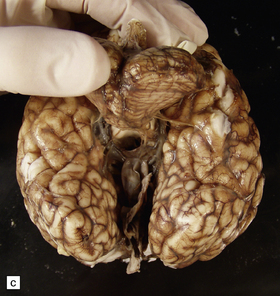

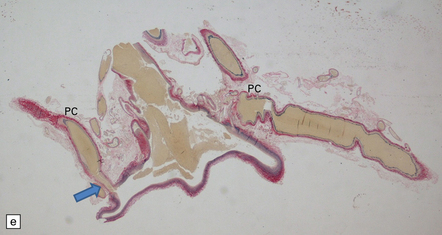
7.5 Vein of Galen aneurysm (arteriovenous fistula).
(a,b) The patient is a 15-month-old child who died secondary to extensive encephalomalacia produced by a ‘steal’ of blood from normal brain through the shunt. (c,d) This aneurysm is fed by both posterior cerebral arteries, the right is duplicated. (e) Microscopic section of the aneurysm and its feeding posterior cerebral arteries (PC) with loss of elastic at the fistulous connexion (arrow).
TAKAYASU’S ARTERITIS
MACROSCOPIC AND MICROSCOPIC APPEARANCES
Lymphocytic infiltration of the media and destruction of the elastica of affected vessels are associated with giant cells and followed by fibrosis and thickening. The blood vessels are further narrowed by superimposed intimal proliferation and atheroma. Thromboembolic disease leads to cerebral ischemia. Only rarely does the arteritic process directly involve cerebral vessels (Fig. 7.6).
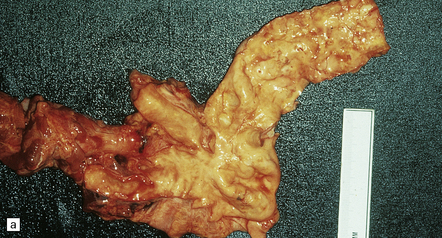
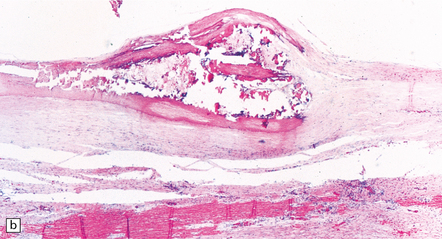
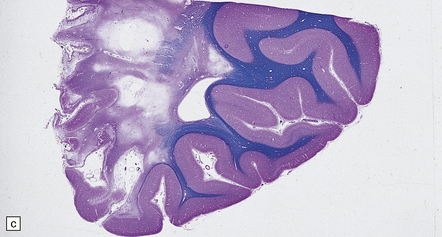
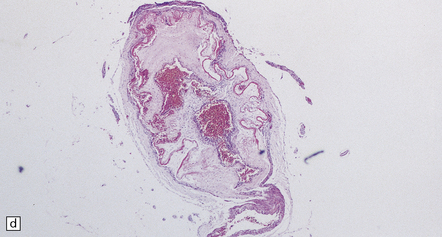
7.6 Takayasu’s arteritis in a 14-year-old girl.
(a) Severe complicated atheroma in the aorta. (b) Histologically, the atheroma consists of calcified and atheromatous thickening of the intima and a mononuclear infiltrate disrupting the media. (c) Old infarction centered on the parieto-occipital vascular boundary zone. (d) All the vessels of the circle of Willis were affected by a chronic arteritic process with intimal thickening, elastic fragmentation, medial atrophy and mononuclear infiltration. The middle cerebral arteries were occluded and recanalized.
KAWASAKI DISEASE (MUCOCUTANEOUS LYMPH NODE SYNDROME)
HEMOLYTIC–UREMIC SYNDROME
EPILEPSY IN CHILDHOOD
Generalized epileptic seizures in childhood may be precipitated by many infective, metabolic, or toxic disorders, and by space-occupying lesions such as a hematoma or neoplasm. In addition, there is an array (Fig. 7.10) of more restricted structural lesions associated with focal or unilateral seizures, which are now amenable to surgical treatment. The aim of such treatment is to resect the seizure focus, or, if removal is impossible, to disconnect the focus from the rest of the brain. Most of these disorders are considered in other chapters.
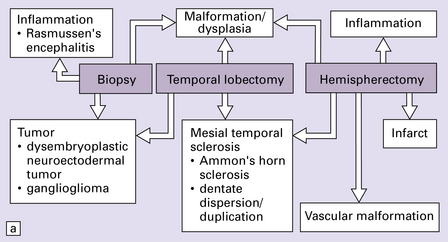
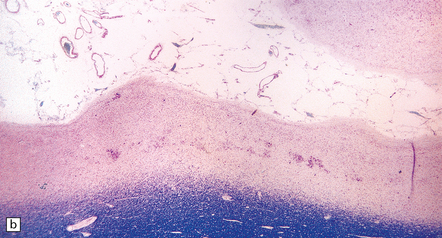
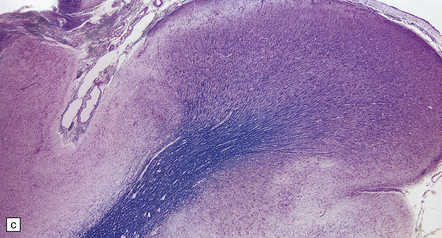
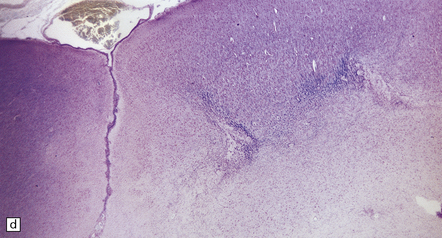
7.10 Epilepsy in childhood.
(a) A diagrammatic outline of the types of specimen and range of pathology encountered following surgery for pediatric epilepsy. (b) Hypoxic–ischemic lesions around the time of birth can leave a large scar, commonly in the territory of the middle cerebral artery, which becomes a focus for seizure activity. This resection contains areas of completely destroyed cortex, with a thin gliotic or cystic remnant including mineralized debris. (c) In some places the tips of the gyri are preserved, giving a ulegyric appearance. (d) At the edge of the infarcted area there is aberrant myelination: état fibromyélinique.
RASMUSSEN’S ENCEPHALITIS
This is a progressive subacute unilateral and intractable seizure disorder with histologic features reminiscent of a chronic viral encephalitis (see also Chapter 13).
MACROSCOPIC AND MICROSCOPIC APPEARANCES
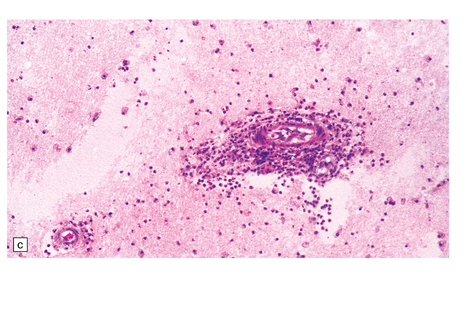
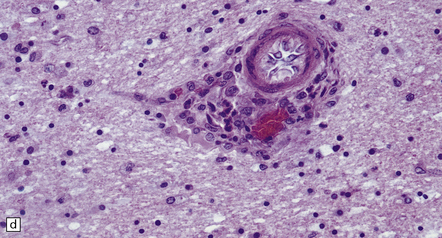
Histologic changes (Fig. 7.11) are usually much more widespread than suspected macroscopically and include patchy chronic leptomeningitis and chronic inflammatory changes in the neocortex and hippocampus, subjacent white matter, and sometimes the basal ganglia. The principal features are:
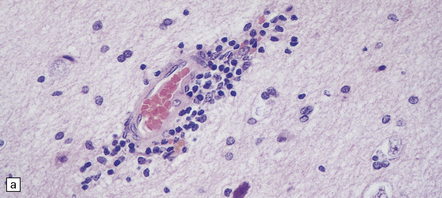
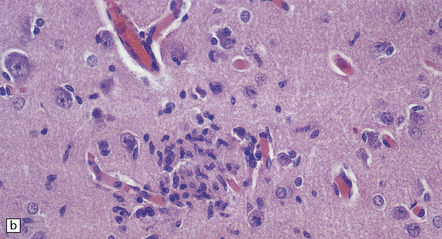
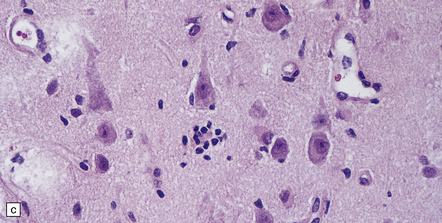
7.11 Rasmussen’s encephalitis.
The typical lesions resemble an indolent viral encephalitis and include the following. (a) Mononuclear perivascular cuffs in the cerebral cortex. (b) Clusters of lymphocytes and microglial cells in the cortex. (c) Neuronophagia.
 cuffs of lymphocytes around blood vessels
cuffs of lymphocytes around blood vessels
 lymphocytes, and microglial cells, either scattered across the parenchyma or in clusters (‘microglial nodules’)
lymphocytes, and microglial cells, either scattered across the parenchyma or in clusters (‘microglial nodules’)
In nearly all cases these changes are unilateral (Fig. 7.12). Progressive neuronal loss can lead to an end-stage appearance of a spongy gliotic and cystic cortical remnant devoid of nerve cells.
HEMICONVULSIONS–HEMIPLEGIA– EPILEPSY (HHE) SYNDROME (ACUTE POSTCONVULSIVE HEMIPLEGIA)
Delay in treating febrile convulsions or prolonged status epilepticus in childhood can lead to an acute postconvulsive hemiplegia, which is flaccid at first with notable facial involvement, but is later spastic. Imaging shows unilateral hemispheric edema followed by widespread atrophy. Recovery can be complete, but the usual prognosis is dismal with residual epilepsy, mental retardation, and persistent weakness. Better acute treatment for epilepsy has made the syndrome a rather rare event, but occasional hemispherectomy specimens show the classic features of extensive neocortical neuronal loss, amounting in places to full-thickness destruction with residual cysts (Fig. 7.13).
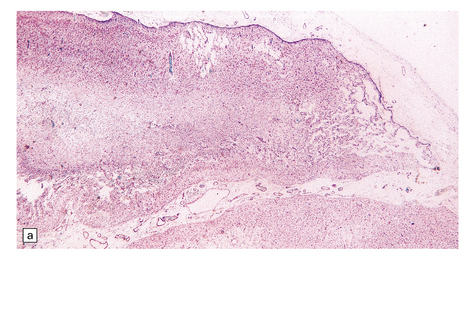
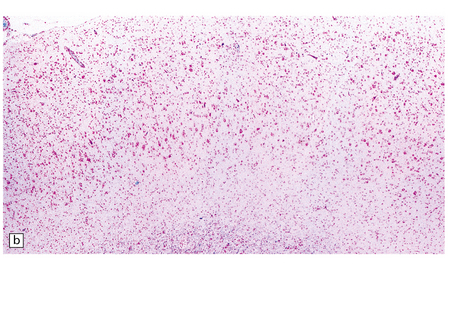
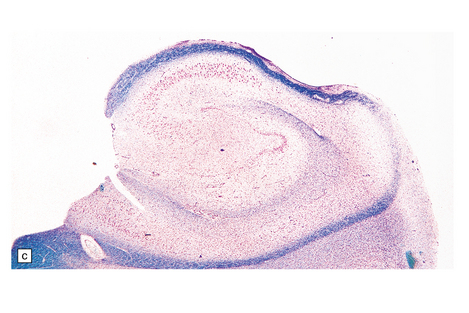
7.13 Hemiconvulsions–hemiplegia–epilepsy (HHE) syndrome.
(a) In the hemispherectomy specimen, there are large areas of devastated cystic cortex and subjacent gliotic white matter. (b) Other areas of remaining cortex show severe neuronal fallout. (c) The hippocampus shows typical Ammon’s horn sclerosis.
PROGRESSIVE MYOCLONIC EPILEPSIES
Disorders with other neurologic features but in which progressive myoclonic epilepsy is a consistent, and often the principal, manifestation
Disorders in which progressive myoclonic epilepsy is a frequent, but not the principal, manifestation
 Many lysosomal storage diseases (see Chapter 23).
Many lysosomal storage diseases (see Chapter 23).
 Familial encephalopathy with neuroserpin inclusion bodies (see Chapter 31).
Familial encephalopathy with neuroserpin inclusion bodies (see Chapter 31).
 Several other pediatric degenerative diseases of gray and white matter, such as Alpers disease, Canavan’s disease, and Alexander’s disease (see Chapters 5 and 6).
Several other pediatric degenerative diseases of gray and white matter, such as Alpers disease, Canavan’s disease, and Alexander’s disease (see Chapters 5 and 6).
 Some chronic/subacute viral infections of the CNS, particularly subacute sclerosing panencephalitis (see Chapter 13).
Some chronic/subacute viral infections of the CNS, particularly subacute sclerosing panencephalitis (see Chapter 13).
 Creutzfeldt–Jakob disease (see Chapter 32).
Creutzfeldt–Jakob disease (see Chapter 32).
 The late stages of most other neurodegenerative dementing diseases (see Chapter 31).
The late stages of most other neurodegenerative dementing diseases (see Chapter 31).
Other causes of myoclonus, with or without progressive neurologic deterioration, are considered in Chapter 30.
MACROSCOPIC AND MICROSCOPIC APPEARANCES
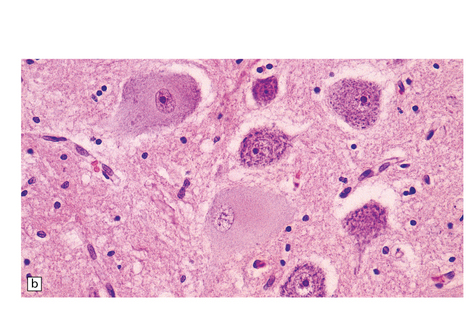
The brain usually appears macroscopically normal. Histology reveals numerous Lafora bodies in the cytoplasm of neurons and astrocytes in the cerebral cortex (Fig. 7.14), basal ganglia (especially the globus pallidus), thalamus, substantia nigra, cerebellar cortex, and dentate nucleus. Fewer inclusions are present in the brain stem and they are sparse in the spinal cord.
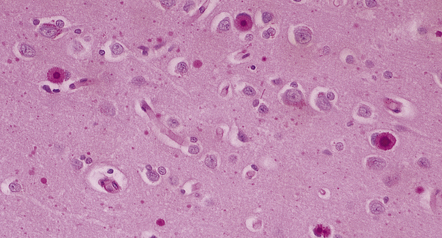
7.14 Lafora body disease.
Lafora bodies ranging from 1–2 mm to about 25 mm in diameter are visible within neurons and astrocytes in the cerebral cortex.The section has been stained with PAS reagent, with which the Lafora bodies react strongly. The Lafora bodies have a central deeply stained core with a spiculated outline, and a surrounding zone of less intensely stained material.
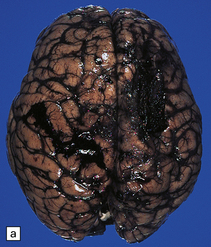
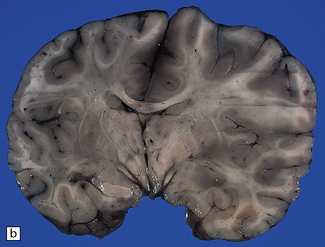
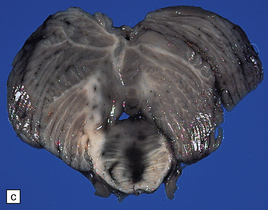
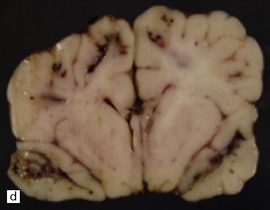
7.15 Traumatic cranial injuries.
The child’s brain is exquisitely sensitive to injury, hypoxia, or surgical manipulation, and may swell very rapidly. (a) In this patient subdural hemorrhage followed a road traffic accident. (b) This led very rapidly to unilateral brain swelling and midline shift. (c) It also led very rapidly to transtentorial herniation, coning, and Duret hemorrhages. (d) Hemorrhagic tears are a characteristic finding in young children suffering closed head injury. These so-called contusional tears are especially common in orbitofrontal or temporal white matter.
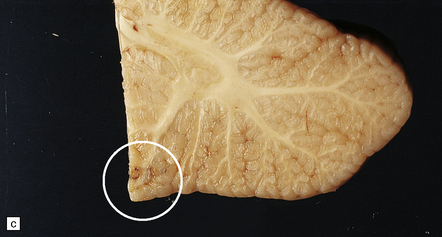
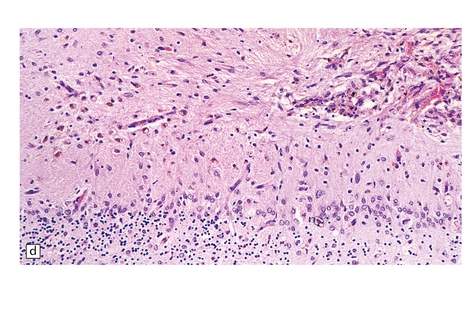
7.16 Non-accidental traumatic CNS injuries in child abuse cases.
Multiple traumatic incidents are the rule. (a) In this baby the necropsy disclosed an acute cord hemorrhage presumably related to the fatal injury. (b) There was also chromatolysis in anterior horn cells, which suggests previous recent injury. (c) An old herniation contusion (circle) of the cerebellar tonsil (similar to that seen in a boxing injury) was also evident. (d) Microscopically the tonsillar lesion contained hemosiderin-laden macrophages.
AN APPROACH TO THE NEUROPATHOLOGY OF FATAL PEDIATRIC TRAUMA
REFERENCES
Blümcke, I., Thom, M., Aronica, E., et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174.
Brett, E.M., Neville, B.G.R. Epilepsy and convulsions; the surgical treatment of epilepsy in childhood. In: Brett E.M., ed. Paediatric neurology. 3 rd ed. New York: Churchill Livingstone; 1997:333–406.
Buchhalter, J.R. Inherited epilepsies of childhood. J Child Neurol.. 1994;9:S9–S12.
Delgado-Escueta, A.V., Serratosa, J.M., Liu, A., et al. Progress in mapping human epilepsy genes. Epilepsia.. 1994;35:S29–S40.
Harding, B.N., Ramani, P., Thurley, P. The familial syndrome of proliferative vasculopathy and hydranencephaly–hydrocephaly: immunocytochemical and ultrastructural evidence for endothelial proliferation. Neuropathol Appl Neurobiol.. 1995;21:61–67.
Geddes, J.F., Whitwell, H.L. Head injury in routine and forensic pathological practice. Curr Top Pathol.. 2001;95:101–124.
Geddes, J.F., Vowles, G.H., Hackshaw, A.K., et al. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain.. 2001;124:1299–1306.
Kalimo, H., Kaste, M., Haltia, M. Vascular diseases. In: Graham D.I., Lantos P.I., eds. Greenfield’s neuropathology. London: Arnold; 1997:315–396.
Leestma, J. Forensic neuropathology. In: Duckett S., Malvern P.A., eds. Paediatric neuropathology. Baltimore: Williams and Wilkins; 1995:243–283.
Levin, M., Walters, S. Infections of the nervous system. In: Brett E.M., ed. Paediatric neurology. 3 rd ed. New York: Churchill Livingstone; 1997:621–690.
Meyer, E., Ricketts, C., Morgan, N.V., et al. Mutations in FLVCR2 are associated with proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome (Fowler syndrome). Am J Hum Genet. 2010;86:471–478.
Online Mendelian Inheritance in Man, OMIM (TM). McKusick-Nathans Institute for Genetic Medicine, Johns Hopkins University (Baltimore, MD) and National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD). 2000. http://www.ncbi.nlm.nih.gov/omim/.
Robitaille, Y. Neuropathologic aspects of chronic encephalitis. In: Andermann F., ed. Chronic encephalitis and epilepsy: Rasmussen’s syndrome. London: Butterworth-Heinemann; 1991:79–110.
Serratosa, J.M., Delgado-Escueta, A.V., Posada, I., et al. The gene for progressive myoclonus epilepsy of the Lafora type maps to chromosome 6q. Hum Mol Genet.. 1995;4:1657–1663.
Valdivieso, E.M.B., Scholtz, C.L. Diffuse meningocerebral angiodysplasia. Pediatr Pathol.. 1986;6:119–126.
Virtaneva, K., D’Amato, E., Miao, J., et al. Unstable minisatellite satellite expansion causing recessively inherited myoclonus epilepsy, EPM 1. Nat Genet.. 1997;15:393–396.