Malignant Effusions
Lola A. Fashoyin-Aje and Julie R. Brahmer
• Malignant ascites is a common complication of cancer.
• Malignant ascites accounts for 10% of all cases of ascites.
• Malignant ascites most commonly occurs in patients with ovarian cancer, gastrointestinal malignancies, and carcinoma of unknown primary location.
• Malignant ascites is rarely life threatening, but occurrence signals advanced cancer.
• A history and physical examination are important in evaluation, although the sensitivity and specificity of a physical examination are variable.
• Diagnostic paracentesis with cytology is the gold standard for diagnosis.
• Monitor the situation if the ascites volume is small and/or asymptomatic.
• Consider the patient’s performance status and likelihood of response to systemic therapy in choosing a treatment approach.
• Treatment approaches include use of diuretics, drainage or diversion of fluid, or use of intracavitary therapies.
Malignant Pericardial Effusion
• The most common cause of pericardial effusion in the Western world is malignancy.
• Malignant pericardial effusion is most commonly associated with lung and breast carcinomas.
• The appearance of malignant pericardial effusion portends a poor prognosis.
• Diagnosis more commonly follows a cancer diagnosis.
• The differential diagnosis of pericardial effusion in patients with cancer also includes treatment-related adverse effects such as radiation.
• Fluid sampling for cytology is needed for definitive diagnosis but has low sensitivity; a pericardial biopsy may increase sensitivity.
• Two-dimensional echocardiography provides valuable information about the location, size, and effect on heart function.
• Patients should be evaluated for clinical signs of cardiac tamponade (e.g., hypotension, signs of low cardiac output, and abnormal pulsus paradoxus).
• Patients with small and/or asymptomatic effusions may be monitored.
• Patients with cardiac tamponade require urgent pericardiocentesis to drain fluid.
• Treatment depends on symptoms of effusion and performance status of the patient.
• Treatment options include subxiphoid pericardiostomy, instillation of sclerosants in the pericardial space, and pericardial window.
• Malignant pleural effusion is a common complication of cancer.
• Malignant pleural effusion is a frequent occurrence in carcinomas of the lung and breast and in persons with lymphoma.
• Fluid sampling with evaluation of cytology is required for definitive diagnosis.
• Exudative effusions are more common; chylous effusions are more common in persons with lymphoma.
• Thoracentesis under ultrasound guidance improves diagnostic yield.
• Asymptomatic effusions can be monitored.
• Systemic chemotherapy can be effective in stable patients.
• For symptomatic patients, effective options include systemic therapy for patients with very responsive disease (e.g., hematologic malignancies, germ cell tumors, breast cancer, and small cell lung cancer) or repeated thoracentesis.
• For patients with rapidly recurring effusions, options include pleurodesis with talc, long-term intrapleural drainage catheters, or video-assisted thoracoscopic surgery with mechanical abrasion.
Malignant Ascites
Ascites is a pathologic accumulation of fluid within the peritoneal cavity. The word ascites is derived from the Greek askites and askos, meaning bladder, bag, or belly. In adults, the most common cause of ascites is parenchymal liver disease with cirrhosis, which accounts for 85% of cases.1 The next most common cause of ascites is malignancy, which accounts for 10% of cases. Malignant ascites occurs as a result of various primary abdominal and extraabdominal neoplasms, the most common of which are gastric, uterine, ovarian, breast, lung, lymphoma, and pancreatic.2 Malignant ascites is more common in women because of its incidence in patients with ovarian cancer. Malignant ascites develops in up to 15% of patients with gastrointestinal cancers at some stage in their disease. Additionally, it is estimated that up to 20% of cases of malignant ascites occur without an identifiable primary tumor.3 Regardless of its cause, with the exception of ovarian cancer, where median survival is longer than in gastrointestinal tract malignancies, the appearance of malignant ascites usually heralds the onset of advanced disease and portends a poor prognosis. Management of malignant ascites focuses on palliation of symptoms.
Etiology and Pathogenesis
The peritoneal membrane is a single-layer mesothelial tissue comprising a surface area of approximately 7500 cm2 and totaling about 90 µm in thickness (five layers of connective tissue of a basal membrane). It covers the abdominal and pelvic spaces and is comprised of capillaries that filter the plasma by exchanging substances and cells with the abdominal cavity through a large number of channels called “foramina” distributed along the peritoneal squamous epithelium. Peritoneal fluid is drained by the open ends of lymphatic channels, which are called “stomata,” found on the serosal layer.4,5 Under normal conditions, approximately 100 mL of free fluid lubricates the serosal surfaces. Factors influencing fluid balance include the portal pressure, oncotic pressure, the sodium-water equilibrium, and the permeability of vascular channels for cells and macromolecules. Two thirds of peritoneal fluid is reabsorbed by lymphatic system and ultimately drains into the right subclavian vein.6
Proposed mechanisms for the development of malignant ascites include increased permeability of tumor vessels, increased production and release of peritoneal fluid, and decreased resorption of fluid.9–9 Vascular endothelial growth factor (VEGF) has been implicated in the increase in permeability of small blood vessels, as well as the uncontrolled angiogenesis of tumor mass.12–12 When VEGF is upregulated, it acts by helping to activate tyrosine kinase through linkage to the receptor vascular permeability factor. Carcinomatosis has also been implicated in the development of malignant ascites through marked neovascularization of the peritoneum and increased production of glycoproteins, which also increase the vascular permeability of small vessels.13 Other methods of increased vascular permeability include peritumoral inflammation and the production of matrix metalloproteinases, which are thought to disrupt tissue matrices during cancer spread.14 Interleukin-2, tumor necrosis factor, and interferon-alpha have also been implicated.15,16 Another mechanism that contributes to the formation of malignant ascites is the activation of the renin-angiotensin-aldosterone system with resultant sodium retention occurring as a result of decreased circulating blood volume that results from the presence of ascites.17 The aforementioned hypotheses mainly explain increased production of malignant ascites, but decreased resorption of fluid also plays a significant role. Tumor growth in the abdominal cavity leads to obstruction through micro- and macroinvasion of lymphatic channels. With subsequent obstruction of these channels, fluid drainage is compromised.6
Diagnosis and Evaluation
History and Physical
Patients with malignant ascites often report increased abdominal girth, abdominal pain, nausea, fatigue, and early satiety.2 In instances in which a large volume of fluid is present that may increase pressure on the diaphragm and reduce lung expansion, a patient may report dyspnea. Depending on the quantity of fluid present, the physical examination may reveal bulging flanks, a fluid wave, and a protuberant abdomen. Signs such as jaundice, spider angiomata, collateral abdominal veins, and palmar erythema are more common in ascites caused by primary liver disease but may be present with some gastrointestinal malignancies or if there is significant disease burden in the liver. However, it should be noted that the physical examination is notoriously variable in its sensitivity and specificity with regard to reliably diagnosing ascites (50% to 94% sensitivity and 29% to 82% specificity).18
Imaging Studies
Ascites fluid often layers in the dependent regions of the abdomen such as the hepatorenal recess or Morison pouch and the pelvic cul-de-sac, and it can be easily seen on ultrasound. Although this imaging modality is limited in patients who are obese or have complex, loculated ascites, it is a sensitive test in most patients and is the gold standard for diagnosing malignant ascites.19 It also helps to decrease risk of injury to viscera when used during fluid drainage. Malignant ascites can also be detected by plain radiography, computed tomography (CT), and magnetic resonance imaging (MRI). These studies are not usually used in the first-line evaluation of malignant ascites because it is often found incidentally when patients are undergoing imaging for other reasons. However, imaging studies are very useful in situations in which the presence of ascites precedes a diagnosis of malignancy and when a primary site of tumor in the liver or peritoneal metastasis may be present. When a gynecologic cancer is suspected, an ultrasound may be useful in helping make the diagnosis and should be performed in all women who present with malignant ascites without any evident primary tumor.
Diagnostic Paracentesis
Diagnostic paracentesis is an expedient, inexpensive, and relatively safe procedure that can provide a lot of information about the etiology of ascites. In most cases, malignant ascites follows a diagnosis of malignancy. However, in up to 20% of cases, the primary tumor cannot be identified, and as such, analysis of the ascites fluid becomes critical in the evaluation of these patients. The appearance of the fluid can provide some clues as to the etiology of the ascites.20 Clear fluid is usually associated with cirrhosis. Infected fluid is cloudy. Milky fluid can indicate chylous ascites and should be sent for triglyceride evaluation. Such fluid often has triglyceride levels greater than 200 mg/dL and often as high as 1000 mg/dL. Some studies have demonstrated that the most common cause for chylous ascites is malignancy, although others have found cirrhosis as the primary cause.1,21 Fluid obtained during this procedure is submitted for chemical, microscopic, and cytologic evaluation, including cell count and differential, albumin concentration, lactate dehydrogenase (LDH), and cultures; the latter should involve immediate inoculation of the culture bottles at the bedside to maximize culture growth.22,23 Fibronectin may also be used as a marker of tumor-related ascites, although its use is controversial.24,25 Submission for cytology is also very important, and the sample is analyzed for the presence of tumor cells in the fluid. In approximately 50% of cases, the presence of ascites in patients with known neoplasms heralds the presence of peritoneal carcinomatosis.22,23 In these patients, malignant cells can be detected in the fluid up to 97% of the time, making this test very sensitive and the gold standard for diagnosing peritoneal carcinomatosis.22 In contrast, positive cytology findings are variable in patients with hepatic metastases, lymphoma, or hepatocellular carcinoma. Performing immunohistochemical analysis for S100, carcinoembryonic antigen (CEA), human melanoma black 45, leukocyte common antigen, cytokeratin, vimentin, and other tumor markers can also be a useful adjunct to the workup.26,27
Surgical Approaches
Despite all the efforts previously outlined, on occasion the diagnostic workup confirms the presence of cancer but does not reveal a primary tumor even in the setting of positive cytology. In these cases, if radiographic imaging proves fruitless, as may be the case with small tumors of gynecologic or peritoneal origin, then maneuvers such as laparoscopy or laparotomy should be pursued for tissue diagnosis. These procedures, which enable biopsy samples to be obtained from the ovaries and other intraabdominal structures, may also facilitate placement of catheters for future therapy in the peritoneum. These procedures should be performed by experienced practitioners to minimize risk of procedure-related tumor spread and infection.28,29
Management
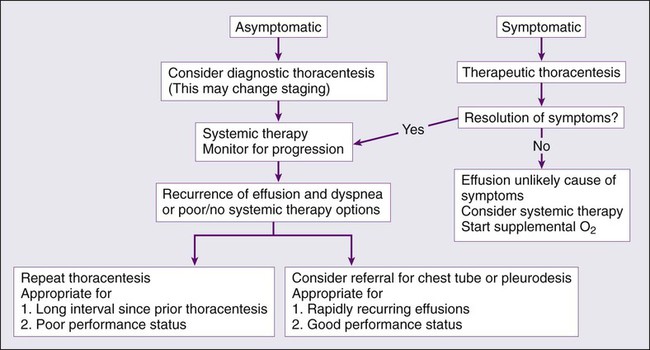
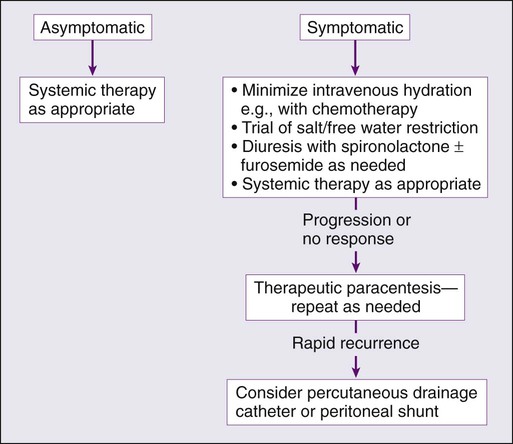
It is important to determine whether it is appropriate to treat malignant ascites. Small amounts of ascites are very well tolerated and often do not require any ascites-specific treatment beyond diagnosis. However, once malignant ascites has become symptomatic causing severe pain, respiratory compromise, or other clinical problems, the benefits and risks of treatment must be assessed in conjunction with the clinical status of the patient. A strategy must be implemented promptly to improve quality of life. Figure 54-1 outlines the management approach to malignant ascites. Management strategies include both medical and surgical treatments as will be outlined.
Diuretics
The first-line approach to managing ascites usually involves the use of diuretics. Currently, no randomized controlled trials have been performed that assess the efficacy of diuretics in managing malignant ascites. Retrospective analyses have shown that diuretic therapy is effective in controlling malignant ascites and its symptoms in approximately 40% of patients with malignant ascites who have peritoneal carcinomatosis.30 This finding may be due to the manner in which diuretics work, via effects on the renin-angiotensin-aldosterone system, which is not prominent in the development of malignant ascites; the exception is malignant ascites occurring in the context of hepatic metastases or hepatocellular carcinoma, where portal hypertension is more commonly seen.23,31 Nevertheless, diuretic therapy is one of the most commonly used interventions to manage malignant ascites, second only to large-volume paracentesis.30 Generally, aldosterone antagonists such as spironolactone and loop diuretics such as furosemide or bumetanide are used with varying doses and duration. The use of diuretics is associated with complications, although the degree of adverse effects due to this approach is not known. Common complications include systemic blood volume depletion, hypotension, electrolyte imbalances, and renal dysfunction, which may already occur as a result of cancer treatment itself. Despite their frequent use, however, they are generally not very effective in managing most cases of malignant ascites, and other approaches often need to be implemented.30
Large-Volume Paracentesis
Large-volume paracentesis is the most frequently used and most effective means of managing malignant ascites. A clear benefit in achieving symptom control has been shown in patients. Large-volume paracentesis provides prompt relief of symptoms such as dyspnea in about 90% of patients.17 However, its effects are short-lived, with many patients experiencing recurrence of symptoms within days of fluid evacuation.30,32 Repeat procedures must be performed, but frequent large-volume paracentesis can cause systemic volume losses that result in electrolyte imbalances, hypotension, dehydration, protein loss, and renal dysfunction. The use of colloidal volume expansion products such as albumin has been shown to reduce the risk of hemodynamic and renal compromise, although no survival benefit has been demonstrated.20,33–35 Although the mortality rate of paracentesis is low (0.16% to 0.39%), repeated procedures increase the risk of bleeding, pain, bowel perforation, and secondary peritonitis.36,37 The use of ultrasound guidance and sterile technique can mitigate these risks.
Drainage Catheters
Large-volume paracentesis is an effective but short-lived strategy in the management of malignant ascites because of reaccumulation of ascites. In patients for whom paracentesis must be repeated more frequently than every 7 days, placement of a permanent catheter is usually needed to achieve long-term symptom relief.6 These catheters provide the flexibility and ease of self-drainage, thus eliminating the discomfort, risks, and frequent hospital visits of repeated paracenteses. Common catheters used for these purpose include the tunneled Tenckhoff, Pleurx, and Port-a-Cath catheters and the nontunneled Pleurx and Cope-type loop catheters.38 Tunneled catheters are preferred because of their greater stability and lower infection rates when compared with nontunneled catheters. Infections are the most common complications of catheters, with one study reporting that 47% of patients had positive surveillance cultures of their fluid and 12% experienced clinically significant infections necessitating catheter removal.39 Other complications include catheter occlusion, leakage at the insertion site, and cellulitis.40 Prior abdominal surgery and adhesions, the presence of carcinomatosis, and loculated peritoneal effusions can complicate placement of these catheters and increase the risks of complications. Ultrasound-guided catheter placement is used to reduce placement-associated complications. Recently, CT-guided placement has been used for patients with more complicated anatomy.41 Once the catheter is placed, patients should be counseled regarding the appropriate drainage techniques and frequency. Additionally, routine electrolyte monitoring and replacement may need to be implemented. These catheters may remain in place for several months.38
Peritoneovenous Shunting
A peritoneovenous shunt is a tubal system that has a pressure-activated one-way valve that mimics physiologic mechanisms to return ascites fluid to the venous system by enabling ascites fluid to flow from the peritoneum to the vena cava. The goal of a peritoneovenous shunt is to achieve symptomatic relief while minimizing the losses of fluid, protein, and electrolytes that occur with repeated large-volume paracentesis and/or catheter drainage. LeVeen introduced the first shunt in 1974 for the surgical management of ascites related to alcoholic liver disease, and the peritoneovenous shunt has since been used to manage malignant ascites.42 The peritoneovenous shunt has not been shown to have a survival advantage compared with large-volume paracentesis, although it has been shown to be comparable in its effectiveness at controlling ascites (62% to 88% of the time).45–45 Early studies had demonstrated that up to two thirds of patients with a peritoneovenous shunt experienced pump failures, but more recent studies have demonstrated lower rates of occlusion, particularly when the following conditions are met prior to placement:46,47
• Expected survival is 3 months or longer
• Rapid rate of fluid reaccumulation after large-volume paracentesis
The data suggest that longer shunt patency occurs if the shunt placed in patients with ascites that is cytologically negative for tumor cells.48,49 Historically, different types of shunts have been used—the Hyde, the LeVeen, and the Denver—with the last two being most commonly used.50 The Denver shunt features a compressible pump chamber that enables the physician or patient to clear the shunt to overcome the frequent complication of shunt occlusion that occurs with the LeVeen shunt. However, neither shunt was shown to be more effective or to have a lower complication rate.
The operative risk of mortality of a peritoneovenous shunt ranges between 10% and 20%. Approximately 20% of patients undergoing placement of a peritoneovenous shunt experience a complication such as transient periprocedure fevers, disseminated intravascular coagulation, infection, and tumor embolization to extraabdominal sites.48,49 In patients with advanced heart or renal failure, the large fluid shifts that occur immediately after the procedure can precipitate volume overload and respiratory compromise. Increased risk of disseminated intravascular coagulation appears to be associated with placement of a peritoneovenous shunt in cirrhotic patients with poor hepatic function but not clearly in patients with malignant ascites. This phenomenon is thought to occur as a result of the introduction of soluble collagen into the circulation as a result of the reinfusion of ascites fluid; the latter causes dilution of coagulation factors.51,52 This complication can be averted by removing two thirds of the ascites fluid prior to placing a shunt. Infection risk is also a concern, although the incidence of shunt-induced peritonitis appears to be highest in patients with cirrhosis-related ascites, which is likely due to the higher levels of protein and immunoglobulin in the ascites of cirrhotic patients.53 Additionally, some studies have demonstrated that patients with breast and ovarian cancer had the best response to a peritoneovenous shunt, whereas patients with gastrointestinal tract cancers did poorly.47 As such, it has been suggested that in the case of malignant ascites management, placement of a peritoneovenous shunt should not be performed in patients with gastrointestinal cancers. A review of 31 published series totaling 968 peritoneovenous shunts found that 70% were effective in palliating symptoms.47
Intraperitoneal Therapy
Cytotoxic agents delivered intraperitoneally have been used to treat intraabdominal malignancies and malignant ascites with variable success. The rationale for locoregional administration of chemotherapy is that it increases the local concentration of the chemotherapeutic agent at the site of action, thus reducing systemic toxicities. Malignant ascites resolution with these drugs occurs from the death of tumor cells that promote ascites formation and obstruct lymphatic vessels with subsequent decreased reabsorption of the fluid. The chemotherapy also induces a diffuse tissue fibrosis that results in decreased inflow of plasma from the tumor and peritoneal vessels. Furthermore, tissue fibrosis and sclerosis in the abdominal cavity leads to adhesion formation with reduction in space available for fluid to accumulate in a fashion akin to pleurodesis in the lung.47 The limitations with this method of drug administration include nonuniform distribution of the drug, limited penetration into approximately 1 mm of tumor, and complications resulting from accessing the peritoneal cavity.56–56 This therapy is therefore best suited for patients with minimal intraperitoneal disease burden.
Various chemotherapeutic drugs have been used with varying remission rates depending on the agent used and the tumor type. In the early 1940s, thiotepa and mechlorethamine were the first drugs used for this purpose, and a temporary and partial response was seen in 30% to 60% of patients.57,58 These responses, however, were associated with serious complications such as bowel obstruction and peritoneal irritation.59,60 Today, cisplatin appears to be one of the most effective agents in the treatment of intraabdominal malignancies, particularly cancer of ovarian origin, although other agents such as mitomycin, 5-fluorouracil (5-FU), bleomycin, and Adriamycin have also been used.61–65 Overall, when used to treat malignant ascites, response rates of 50% have been observed with the use of cisplatin to treat gastric cancer and peritoneal mesothelioma.61,62 5-FU alone or in combination with cisplatin seems to yield similar outcomes. 56,63 In general, the literature suggests that treatment of persons who have gastrointestinal malignancies with intraperitoneal chemotherapy is not as favorable as treating persons with breast and gynecologic cancers. 62,64,65 The administration of intraperitoneal chemotherapy has been modified over time to optimize its efficacy in treating intraabdominal malignancies and peritoneal carcinomatosis and to palliate or prevent development of malignant ascites. This modification has included the use of cytoreductive surgery combined with intraperitoneal chemotherapy either in the intraoperative setting with hyperthermia, known as HIPEC, or in the postoperative period, known as early postoperative intraperitoneal chemotherapy or EPIC. These studies have generally been small studies. 66–72 Results have been more favorable for gynecologic tumors than other tumor types. 73 Nevertheless, these treatment modalities are not universally used in the treatment of malignant ascites because their use is limited to centers with expertise in these therapies.
Biological Therapy
Immunotherapy
The use of immune stimulatory therapies to treat malignant ascites dates back three decades when studies in animal models showed that interferon, tumor necrosis factor, and the streptococcal preparation OK-432 were efficacious in resolving malignant ascites.75–75 These agents have been demonstrated to be effective in palliating malignant ascites in humans as well. The mechanism of cancer cell destruction is thought to be through cell-mediated immunity and cytokine activity. The use of OK-432 was associated with a reduction in ascites volume in 60% of patients and a reduction in tumor burden in 20% of patients. 75 Adverse effects are usually mild and include malaise, chills, fever, nausea and vomiting, and fatigue. However, the use of these agents is uncommon.
Targeted Therapy
The central role of VEGF in the development of malignant ascites has made it a viable option for targeted therapies. VEGF has been shown to play a role in the development of malignant ascites in several tumor types, including ovarian, colorectal, pancreatic, and gastric carcinomas.11 As such, drugs targeting VEGF, such as anti-VEGF antibodies, anti-VEGF receptor antibodies, and metalloproteinase inhibitors, have been shown in small series to decrease ascites fluid formation and provide symptom relief.14,76 In addition to anti-VEGF therapies, the intraperitoneal administration of the trifunctional antibody catumaxomab has been shown to bind tumor cells with immune cells (natural killer cells, T cells, dendritic cells, and macrophages) using epithelial cell adhesion molecule, CD-3, and FcyRs receptors,77,78 which has resulted in the reduction in malignant ascites formation and the need for paracentesis. Common adverse effects of this drug are fever and gastrointestinal upset. Its clinical application in the treatment of malignant ascites, like other biological therapies, is yet to be widely adopted.
Radioisotopes
The use of radioisotopes in the treatment of peritoneal carcinomatosis and malignant ascites has been limited by the side effect profile of this mode of therapy. In the half century that radioisotopes have been used in managing peritoneal carcinomatosis and malignant ascites, two major isotopes have shown some response.79 The first of the radioisotopes to be used was a gold isotope administered intraperitoneally.80 A dose response was observed, but adverse effects such as intestinal obstruction prohibited its use. A phosphate radioisotope has also been developed. It too has shown good response in gynecologic cancers, but challenges with uneven distribution within the intraabdominal cavity, particularly in the presence of adhesions, has been identified as a potential adverse effect. 81,82 Like the biological therapies, radioisotope use has yet to be broadly adopted in the management of malignant ascites.
Malignant Pericardial Effusions
The most common cause of pericardial effusion in the Western world is cancer.83,84 A cancer-related pericardial effusion can result from metastatic disease to the pericardium, a primary pericardial or cardiac tumor, or complications from treatment. 85,86 In an autopsy series, 10% of patients who died of cancer were found to have cardiac metastases.87 Primary tumors of the pericardium (e.g., malignant mesothelioma, fibrosarcomas, hemangiomas, teratomas, neurofibromas, lipomas, and lymphangiomas) are very rare, but malignant pericardial disease has been estimated to occur in 10% to 21% of patients with cancer based on autopsy series.88,89 Not all cases of pericardial effusion are clinically evident prior to a patient’s death, and even when metastases are present in the pericardium, effusion is present only 12% to 15% of the time and even fewer patients experience cardiac tamponade.90 When diagnosed, the presence of pericardial involvement heralds advanced cancer and a poor prognosis. Metastatic involvement of the pericardium usually occurs in the form of a malignant pericardial effusion but can also occur as a solid metastatic tumor. Although most malignant tumors can metastasize to the heart and cause malignant pericardial effusion, the most common tumor types associated with malignant pericardial effusions include lung, breast, and hematologic cancers (i.e., leukemias and lymphomas).87,91–94 Melanomas, malignant germ cell tumors, esophageal tumors, and sarcomas are also frequent culprits. In approximately 4% of cases, pericardial involvement is the initial indication that a malignancy is present.95
Etiology and Pathogenesis
The pericardium is a double-layered membrane that measures 0.8 to 2.5 mm in thickness. It consists of an outer fibrous cover and an inner serous sac that has both a visceral layer that directly overlies the heart and great vessels and a parietal layer that lines the inside of the fibrous cover. The pericardium helps anchor the heart in the thorax by its attachments to the sternum and the diaphragm. The space between the visceral and parietal layers of the serous sac normally contains 50 to 100 mL of a straw-colored serous ultrafiltrate of plasma with lower protein concentrations than are seen in plasma. Pericardial effusion is the accumulation of increased fluid in this space due to infections, cancer, hemorrhage, autoimmune disorders, and other medical conditions, or fluid retention. It can also occur as a result of radiation therapy.86 The rate of fluid accumulation often dictates the development of symptoms. Symptoms that develop as a result of the effusion are due to the membrane’s limited capacity to stretch, particularly when fluid accumulation is rapid. The effusion may occupy the entire sac or be loculated. Malignant pericardial effusions develop as a result of the presence of tumor cells within the pericardium that are deposited there either by direct extension due to proximity of primary tumor, such as is the case in lung and mediastinal tumors, or by metastatic spread through the hematogenous (leukemia, lymphoma, melanoma) or lymphatic (breast, lung) routes, with the latter occurring through invasion through retrograde lymphatic flow in the setting of obstruction of regional lymph nodes.96–99
Evaluation and Diagnosis
A thorough evaluation of patients is critical in determining which patients may require an urgent or emergent procedure to resolve the pericardial effusion and which patients may need less acute interventions (Box 54-1). The severity of a patient’s symptoms may be more reflective of the rate at which the fluid has accumulated than of the amount that has accumulated. Therefore the chronicity of the effusion along with the symptoms that it causes are factors that help guide decisions about how, when, and how quickly to intervene. Also to be considered are tumor type, likelihood of response to systemic therapy, and the patient’s clinical status.
History and Physical
Patients with malignant pericardial effusion, like other patients with a pericardial effusion, may be asymptomatic and the effusion may be discovered during routine imaging for purposes of cancer diagnosis or staging. More often than not, the presence of a malignant pericardial effusion is revealed at autopsy. For the vast majority of patients for whom malignant pericardial effusion is diagnosed during life, a diagnosis of malignancy usually precedes its development. On rare occasions, a symptomatic pericardial effusion including pericardial tamponade is the first manifestation of cancer, and often by this time, the cancer is in its advanced stages. Symptoms reported by patients with malignant pericardial effusion include dyspnea, chest pain, cough, palpitations, edema, and orthopnea. They may also report symptoms that are suggestive of malignancy, such as unintentional weight loss, night sweats, fevers, and fatigue. On physical examination, these patients may be tachycardic, have new-onset arrhythmias, or have signs of low cardiac output such as cool extremities, hypotension, and diaphoresis. They may also have distant heart sounds, jugular venous distension, narrowed pulse pressure, pericardial rub, and a pulsus paradoxus. On electrocardiography, low voltage defined as less than 0.5 mV in the limb leads and electrical alternans of the QRS complex are pathognomonic for pericardial tamponade, but this scenario occurs in fewer than 3% of cases. 84 Tamponade occurs when pericardial pressures exceed intracardiac pressure, resulting in compression of the cardiac chambers, compromising cardiac filling and cardiac output; this has the potential to cause hemodynamic instability. In a series of 98 patients with symptomatic pericardial effusions, 57% had acute onset symptoms, and the remainder presented with chronic symptoms.100 It is important to recognize that not all pericardial effusions occurring in patients with cancer are due to cancer. In a series by Posner et al.,101 58% of patients had a malignant pericardial effusion and the remainder had idiopathic or radiotherapy-induced pericarditis.
Pericardiocentesis and Fluid Analysis
Pericardiocentesis is usually performed with a 16- to 22-gauge needle (often a spinal needle) attached to a syringe and inserted at roughly a 45-degree angle below the xiphoid process cephalad toward the tip of the left scapula. The needle is attached to an electrocardiograph machine during the procedure, and advancement into the myocardium usually reveals an injury pattern on the electrocardiogram. Although this procedure is usually performed semielectively, pericardiocentesis occasionally must be performed in an emergent setting, where removal of as little as 50 mL of fluid can improve hemodynamic status.102 Complications of this procedure range from 5% to 20% and include ventricular perforation, arrhythmias, and pneumothoraxes. These complications occur less frequently (2%) when echocardiography is used to delineate the size and location of the fluid with respect to normal cardiac structures. Fluid sampling is critical in the evaluation of malignant pericardial effusion because the definitive diagnosis is made by a positive cytologic examination of the pericardial fluid. Pericardiocentesis is the most common maneuver used to obtain fluid for cytologic confirmation. The reported sensitivity of cytology is 67% to 92%, with lower sensitivity in patients with mesothelioma.105–105 Fluid obtained from pericardiocentesis should be analyzed for cell count, microbiological cultures, cytology, flow cytometry if a hematologic malignancy is suspected, and biochemical parameters, including adenosine deaminase if tuberculous pericarditis is suspected. Viral cultures and polymerase chain reaction may also be requested. A high white blood cell count with a high number of polymorphonuclear neutrophils is usually suggestive of an inflammatory process such as a rheumatologic disorder or infection, whereas high monocytes are usually associated with malignancy or hypothyroidism.106
Pericardiocentesis may also be used to obtain tissue as an adjunct to establishing the etiology of the effusion. Tissue may be obtained through blind biopsy of the pericardium or through the use of pericardioscopy, which allows direct visualization of the pericardial space and biopsy of the epicardium at the time of drainage of the malignant pericardial effusion; the latter has a sensitivity of 97% for the diagnosis of malignancy compared with 56% to 65% for blind biopsy.89,107 The patient undergoes deep sedation, and after evacuation of the effusion, an introductory sheath is used to introduce a flexible endoscope. Epicardial and pericardial biopsies are then obtained under direct visualization to ensure that biopsies are taken from the most prominent lesions. Combining the cytologic fluid analysis with histologic assessment of tissue obtained through biopsies is superior to cytology alone.
In the setting of cytologically negative samples or when the evaluation is equivocal, tumor markers can be helpful in determining the etiology of the effusion. Although cutoff values for common markers are not established, markers such as alpha fetoprotein, CEA, serum cytokeratin 19 fragments (CYFRA 21-1), neuron-specific enolase, and cancer antigens CA 72-4, CA 125, CA 15-3, and CA 19-9 in the effusion may be helpful in the setting of solid tumors and should be checked.108 Specificity is thought to be high for some markers and tumors (among carcinomas: 80% to 100% for CEA, 80% to 97% for neuron-specific enolase, and 70% to 100% for CYFRA), and the combination of two or more tumor markers leads to a higher diagnostic value. 108,109
Imaging
Various imaging modalities can be used in the evaluation of a patient with a malignant pericardial effusion. Depending on the volume of fluid, chest radiography may show widening of the cardiac silhouette if the effusion is greater than 200 mL in size. Two-dimensional echocardiography is the modality of choice in the imaging of cardiac metastases and may provide valuable information about the effusion such as its location, size, and effect on the heart function. It can also help guide drainage of fluid. The demonstration on echocardiogram of right ventricular collapse in early diastole and right atrial collapse in late diastole that lasts a third or more of the cardiac cycle are characteristic of tamponade. MRI and CT are also useful in elucidating malignancy in the pericardium but are not typically the front-line imaging modalities used when evaluating a malignant pericardial effusion. Contrast-enhanced helical CT helps elucidate the etiology of the effusion.110,111 Pericardioscopy aids direct visualization of the pericardium during pericardiocentesis and targeted biopsy of the epicardium and pericardium. Although it does not replace cytology, pericardioscopy enables macroscopic inspection that helps to differentiate a malignant effusion from a nonmalignant effusion.
Management
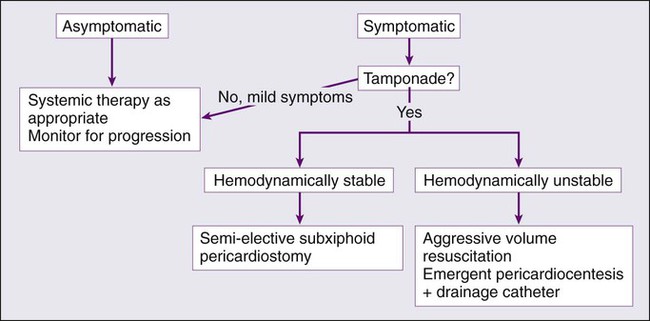
The current management of malignant pericardial effusion includes the use of several modalities, which include instillation of cytostatic and/or sclerosing agents into the pericardial space, use of systemic chemotherapy or radiotherapy, and the use of surgical procedures such as pericardiotomy. However, none has been established through randomized controlled studies as the optimal initial therapeutic approach. Furthermore, the inclusion of patients with cytologically positive and negative pericardial fluid further complicates the application of the findings of studies that are available. For example, a retrospective study comparing simple drainage, systemic chemotherapy, and local treatment showed a survival advantage in the group receiving chemotherapy.112 The goal of treatment is usually to achieve resolution of the symptoms, prevent recurrence, and treat discrete metastatic deposits in the pericardial space or tumors that infiltrate the pericardium and the lymphatic vessels. Treatment is also very dependent on the hemodynamic stability of the patient, because patients with acute symptoms require emergent measures to prevent a poor outcome. Patients with more chronic onset of symptoms that are mild to moderate in severity may be monitored over time, and other strategies may be considered for treatment. A general approach to managing pericardial effusions is outlined in Figure 54-2. When new-onset arrhythmias such as atrial fibrillation are associated with pericardial effusion, a rhythm control strategy may be implemented to prevent thromboembolism and avoid anticoagulation.106 Finally, a recurrent malignant pericardial effusion may require second-line therapies, although no good data are available to guide the choice of therapy.
Pericardiocentesis
Pericardiocentesis is indicated in both the diagnosis and treatment of malignant pericardial effusions, particularly when cardiac tamponade is present. This procedure alone is generally not considered a definitive management strategy because only a third of effusions are controlled in this manner, with 90% of effusions recurring within 90 days.115–115 However, in the acute setting, it is the most expedient therapeutic maneuver to provide relief to patients with cardiac tamponade. It is the most commonly performed procedure for patients with a malignant pericardial effusion regardless of acuity of symptoms. Patients who have cardiac tamponade and are hemodynamically unstable require prompt drainage with supportive measures such as volume expansion when appropriate to ensure adequate systemic perfusion. Pericardiocentesis is an effective singular measure to manage these effusions if they are not likely to recur or if the patient is in an advanced enough stage in their disease that palliation of symptoms is the goal and the risks of more invasive procedures outweigh the benefit. In patients with cancer and a pericardial effusion, positive cytology has been shown to be predictive of a poor outcome.83
Catheter-based pericardiocentesis using the subxiphoid route is the technique most often used. This procedure is usually performed in the cardiac catheterization laboratory with electrocardiographic and hemodynamic monitoring.116 After drainage, the catheter is left in place with suctioning for a few days with the goal of reducing the likelihood of recurrence; it is thought that leaving the catheter in place enables the formation of adhesions and subsequent obliteration of the pericardial space. In contrast, removal of the catheter soon after evacuation of the fluid carries a 40% risk of recurrence.117 Therefore removal of the catheter is recommended after a few days when drainage is less than 20 to 30 mL/24 hours.
Intrapericardial Therapies
The use of local therapies to treat malignant pericardial disease is thought to provide a higher local concentration of the drug with fewer systemic side effects; the drug is also thought to relieve lymphatic obstruction when it is reabsorbed.116,118–121 Agents such as bleomycin, doxycycline, and tetracycline have been used to induce inflammation and cause subsequent sclerosis. The most commonly used purely sclerosing agent is bleomycin.122 However, a randomized study failed to show a statistically significant difference in the control of effusion between observation alone after drainage versus instillation of bleomycin after drainage.123 The adverse effects associated with the use of sclerosants include fever, pain, paroxysmal atrial fibrillation, and the development of constrictive pericarditis; the latter can increase the risk of tamponade should fluid reaccumulate even in small amounts as a result of reduced compliance. Pericarditis may also preclude repeat drainage because of the risk of hemodynamic impairment should a loculated effusion be present.124
In addition to using sclerosant treatment, the instillation of various chemotherapeutic agents has been used to treat malignant pericardial effusions.116,118–121 These therapies are delivered after evacuation of the effusion using pericardiocentesis. Platinums are more often used. Cisplatin in particular has demonstrated efficacy in management of malignant pericardial effusion due to various tumor types.127–127 Lestuzzi et al.128 reported the median effusion-free days in their series to be 223 days with 58%, 52%, 33%, and 16% of patients being effusion free at 1, 2, 6, and 12 months, respectively. Given the risks associated with sclerosing treatment and the low complication rate of instillation of cytotoxic drugs, some physicians favor locally administered chemotherapy rather than sclerosis.128
Surgical Procedures
First performed in 1829, the subxiphoid pericardial window is the most common surgical procedure used to relieve a malignant pericardial effusion.129 Currently, alternate surgical techniques such as video-assisted thoracoscopy (VATS) or open thoracotomy are used. The goal of such approaches is to provide a conduit to another reabsorptive cavity such as the pleural cavity or the peritoneum, to which the fluid can drain.130–134 Although no randomized trial to date has confirmed this, a pericardial window is generally more efficient than performing repeated pericardiocentesis because the malignant effusion tends to recur.135,136 Before considering a surgical approach such as subxiphoid surgical pericardial windowing in managing malignant pericardial effusion, the patient’s clinical status and life expectancy should be considered.
Malignant Pleural Effusions
The first published account of malignant cells in effusion occurred in 1874.137 Although there have been no epidemiologic studies to confirm the incidence of malignant pleural, in the United States it is estimated that there are 150,000 to 200,000 cases each year.138,139 In 30% of patients, a malignant pleural effusion can be the first indication of the presence of malignancy.140 Malignant pleural effusions occur as a result of invasion of malignant cells into the pleural membranes. This phenomenon can occur as a result of primary pleural tumors such as malignant mesothelioma, which account for approximately 10% of cases, or as result of secondary tumors, the two most common being lung and breast cancer, which together account for approximately 50% to 60% of cases.141 In order of decreasing frequency, lymphoma, ovarian cancer, and gastric cancer are the next most common, accounting for a combined 15% to 25% of cases.142 In addition, an estimated 7% to 10% of malignant pleural effusions occur in the setting of malignancy of an unknown primary cancer.139,143 The onset of malignant pleural effusion often represents advanced cancer with a median survival in the order of months. In persons with lung cancer, for example, the presence of malignant pleural effusion often signals incurability. Prognosis is particularly poor in patients with lung, gastric, and ovarian cancer, with a median survival of 4 to 6 months, whereas patients with breast cancer tend to fare better, with median survival in the order of months to years; persons with lymphoma have intermediate survival.144,145 More often than not, the focus of treatment is to prevent and/or relieve effusion-related symptoms to improve quality of life.
Etiology and Pathogenesis
Pleural effusions occur commonly in patients with cancer (Box 54-2). These effusions can be malignant or paramalignant (defined as presence of pleural effusion in a patient known to have cancer but without cytologic and/or histologic evidence of malignancy in the fluid or the pleural surfaces).143 These effusions can occur as a result of local effects of the tumor (e.g., lymphatic obstruction, parapneumonic effusion from postobstructive pneumonia, or superior vena cava syndrome), complications from treatment (e.g., adverse effects of chemotherapy, radiation pleuritis, or a trapped lung), or systemic effects of the tumor (e.g., pulmonary embolism or hypoalbuminemia).146 A malignant pleural effusion can result from virtually any cancer type with the presence of tumor in the pleural membranes and/or cavity occurring via direct extension from surrounding structures in the thorax or by hematogenous spread from extrathoracic sites.139,143 Various hypotheses describe the pathogenesis of malignant pleural effusion. The presence of carcinomatous infiltration of the hilar and/or mediastinal lymph nodes has been associated with malignant pleural effusion, suggesting that obstruction of lymphatic channels may play a role in the accumulation of fluid.147 However, other mechanisms may be involved because the malignant pleural effusion can be transudative in nature; furthermore, the presence of mediastinal/pleural membrane carcinomatosis does not always result in malignant pleural effusion, because up to 60% of patients with malignant pleural disease do not experience a malignant pleural effusion.137,142,147 As with malignant ascites, evidence suggests that upregulation of vascular endothelial growth factor (VEGF) may play a significant role in the pathogenesis of malignant pleural effusion.148
Evaluation and Diagnosis
Determining the etiology of the effusion is critical because it has enormous implications for prognosis and for management strategies. Once the history and physical examination are completed, an invasive diagnostic procedure is warranted to confirm the diagnosis. Ultrasound-guided thoracentesis is usually the initial procedure of choice and can provide important information that may further elucidate the etiology of the effusion, such as the presence of nodules or masses. It is important to consider that up to 50% of patients with pleural effusion known to have an underlying malignancy have nonmalignant effusions and that these generally indicate a better prognosis.149 (See Table 54-1 for a list of malignancies commonly associated with a pleural effusion.) Therefore an accurate diagnosis is critical to providing the appropriate care to patients with cancer who have pleural effusions.
Table 54-1
Malignant Neoplasms Associated with Pleural Effusion*
| Cause | No. | Percent |
| Total malignant effusions | 1283 | 100 |
| Lung cancer | 450 | 35 |
| Breast cancer | 256 | 20 |
| Lymphomas and leukemia | 256 | 20 |
| Unknown primary (adenocarcinoma) | 154 | 12 |
| Unknown primary (all types) | 95 | 7 |
| Gastrointestinal tract | 90 | 7 |
| Reproductive tract | 70 | 5 |
| Genitourinary tract | 66 | 5 |
| All other† | 39 | 3 |
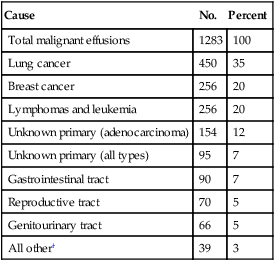
*Data from references 137, 142, 214–218.
†Includes causes of malignant effusion (each less than 1%): endocrine, head and neck cancer, mesothelioma, soft tissue sarcoma, bone cancer, and myeloma.
History and Physical
The physical examination alone may suggest the presence of an effusion in a patient complaining of progressive dyspnea with exertion, which is the most frequent sign of malignant pleural effusion.150 Dyspnea may occur as a result of tumor-related reduced compliance of the diaphragm, chest wall, and lung parenchyma.143 There may also be a shift in the trachea, causing shortness of breath. Patients may also report cough and chest pain. The physical examination may reveal dullness to percussion and decreased breath sounds and vocal fremitus. In addition, because of the often advanced stage of disease, patients may also report unintentional weight loss and may exhibit other signs of chronic illness. Still, up to 25% of patients may be completely asymptomatic.142
Imaging Studies
Imaging is an important component in the evaluation of pleural effusion in a patient. Imaging can help elucidate the cause of the effusion. Chest radiography may reveal ipsilateral effusions in case of lung and breast cancers, whereas bilateral effusions are common in most other cancers. When there is complete or near complete opacification of the hemithorax, 70% of patients will have a malignancy.151 Evidence indicates that the size of the effusion has prognostic value, with larger effusions having a worse prognosis than smaller ones.152 Although chest radiography can detect pleural fluid on lateral and posterior-anterior views, CT is more sensitive and specific in detecting malignant pleural disease.153 Characteristics of malignant pleural effusion on CT include nodularity and parietal pleural thickening of more than 1 cm and mediastinal pleural involvement.154 MRI can demonstrate chest wall and diaphragmatic invasion, whereas positron emission tomography often demonstrates higher standardized uptake values (SUVs) in malignant pleural effusion.155,156 The combination of positron emission tomography with CT improves sensitivity.157
Diagnostic Thoracentesis and Pleural Fluid Analysis
Despite the high sensitivity and specificity of imaging studies at detecting malignant pleural effusion, pleural fluid analysis is a required component to evaluate pleural effusion and to confirm that the fluid is malignant. A diagnostic thoracentesis is often the first invasive procedure done to obtain fluid. Performing this procedure under ultrasound guidance not only improves its safety but also improves its diagnostic yield, particularly when initial cytology is negative. This fluid is then assessed as follows: chemical analysis (glucose, pH, LDH, albumin, total protein, cholesterol, and amylase), cell count, and microbiological studies if infection is suspected.160–160 The fluid is also sent for cytology and flow cytometry, particularly if lymphoma is suspected. In general, it is recommended that no less than 60 mL of fluid be obtained for this purpose.161 There is great variability in the diagnostic yield of cytologic analysis, with studies suggesting a range of 62% to 90%.164–164 Distinguishing between the different cell types (e.g., mesothelioma, lymphomas, reactive mesothelial cells, and adenocarcinoma) can be challenging, and in these cases, immunohistochemical analyses and determining the presence of tumor markers such as CEA, CA 15-3, CYFRA 21-1, and pleural fluid mesothelin can be helpful.167–167 However, occasions may occur when even immunohistochemistry is insufficient in characterizing the cells that are present in the effusion, and in these cases, flow cytometry can be a useful additional method. Studies have shown that when performed together, these tests have sensitivity and specificity of 100% and 94% to 100%, respectively. For example, the presence of B cells on flow cytometry in a patient with an effusion may indicate the presence of lymphoma, because the vast majority of lymphomas are B-cell lymphomas. Malignant pleural effusions are usually exudates by Light criteria and tend to have low pH, glucose <60 mg/dL, lymphocyte predominance (50% to 70% range but less than that observed in tuberculous effusions [>90%]), and are eosinophilic in some cases.168,169 Malignant pleural effusion is usually exudative in nature, but it should be noted that approximately 5% of malignant pleural effusion are transudates.142,150,170 Protein levels range between 1.5 and 8.0 g/dL, and effusions are often low in pH and glucose. Studies have shown that results of the biochemical tests correlate with prognosis. For example, low pH, low glucose, and high LDH are associated with poor survival.141,158,159 Likewise, elevated levels of the aforementioned tumor markers may represent predictors of poor survival, although this supposition has not been validated in studies.165 Lymphoma accounts for the most common cause of chylothorax caused by malignancy.143
Pleural Biopsy
When cytology fails to be diagnostic and there remains a strong suspicion for a malignant effusion, a pleural biopsy is sometimes necessary to determine the etiology of the effusion. Blind percutaneous biopsies of the parietal pleura have proven to have comparable diagnostic yield as cytologic evaluation, particularly in the setting of low disease burden in the pleura. However, image-guided pleural biopsy improves sensitivity and specificity (76% to 86% and 100%, respectively) and is now most commonly performed.166,171,172 Thoracoscopic pleural biopsy through medical thoracoscopy or through VATS has a diagnostic yield of 90% to 100%.173
Management
Despite our current therapies, the presence of a malignant pleural effusion precludes cure, with the goal of treatment being palliation of symptoms. The approaches to managing patients with malignant pleural effusion are variably successful and risky. Each case must be individualized, with consideration given to the presence and severity of symptoms, expected survival, performance status, characteristics of the malignancy responsible for the effusion, and the overall treatment plan for the underlying malignancy. In most cases, this is best achieved by a multidisciplinary team with expertise in the various approaches. Figure 54-3 provides an overview of a general approach to managing pleural effusion in patients with cancer. Evidence indicates that malignant pleural effusions due to breast cancer, small cell lung cancer, and lymphoma may be responsive to systemic chemotherapy and radiotherapy. However, in most cases the management of malignant pleural effusions requires local therapy in the form of drainage and pleurodesis. Other approaches include therapeutic thoracentesis, placement of drainage catheters, tube thoracostomy, pleurodesis, pleuroperitoneal shunting, pleurectomy, and decortication. The overarching goal is always to ensure that the treatment that is selected is appropriate for the patient’s overall clinical status and prognosis. None of the management approaches to malignant pleural effusion have demonstrated prolongation of survival, but its management is tantamount to preventing significant morbidity that may have a negative impact on quality of life and/or shorten life.
Therapeutic Large-Volume Thoracentesis
Thoracentesis is not only important as a diagnostic tool but is also usually the first step in treating a malignant pleural effusion. The frequency of thoracentesis helps to separate patients who are likely to respond to intermittent drainage from patients who require continuous drainage. Additionally, the procedure itself helps to rule out other potential etiologies of dyspnea in the patient with cancer such as lymphangitic spread, pulmonary embolism, or malignant airway obstruction. Finally, in patients with limited life expectancy and/or who are unable to tolerate more invasive procedures or infrequent reaccumulation of fluid, this option may good even if it is performed repeatedly. Generally, it is recommended that no more than 1.0 to 1.5 L of fluid be removed at a time or that pleural pressures not fall below −20 cmH2O and that the procedure be aborted once the patients experiences chest discomfort, dyspnea, or cough. These symptoms could be an indication of reexpansion pulmonary edema, which is a complication of the procedure.176–176 Larger volumes may be removed if there is evidence of mediastinal shift as a result of the effusion. Standard practice requires real-time ultrasound guidance to reduce risk of procedure-related pneumothoraces.174,177,178 Ultimately, nearly all patients with malignant pleural effusion have recurrence of fluid and symptoms within 30 days of therapeutic drainage.179 Therefore this approach is often reserved for patients who have slow fluid accumulation, are expected to have resolution of the effusion with systemic and/or local therapies, have short life expectancies, or are not fit enough to undergo more invasive procedures.
Pleurodesis
The rationale for pleurodesis is to incite irritation of the two-layered pleural lining, which is thought to activate the pleural coagulation cascade, resulting in fibrin deposition, the evolution of pleural symphysis, and subsequent obliteration of the pleural space.180 Pleurodesis can be performed at the bedside using chest thoracostomy or pleuroscopy or VATS. When a chest tube thoracostomy is performed, pleurodesis is initiated after evacuation of the fluid is confirmed by chest radiography and satisfactory apposition of the parietal and visceral pleura is achieved.183–183 The patient then has the sclerosing agent of choice instilled in the pleural space, usually after pleural drainage decreases to <150 to 200 mL/day, followed by chest tube clamping for 1 to 2 hours.149,184 The chest tube is then reconnected to suction. Some physicians, however, support immediate instillation of the sclerosant after drainage.183,185–188
Chemical pleurodesis involves the use of various sclerosing agents to cause inflammation as previously described, but some may also have direct antitumor effect as well. A variety of chemicals have been used, including bacterial products, chemotherapy, talc, and antibiotics such as tetracycline, doxycycline, and minocycline.189 Approximately 80% of the effusion resolves with even higher rates achieved with talc pleurodesis. Although there have been no direct comparisons between the various sclerosing agents, metaanalyses and reviews suggest that talc, either by poudrage or slurry, results in better efficacy for achieving pleurodesis than other agents. In one review comprising 723 patients, a 91% success rate was achieved using varying doses of talc.190 No consensus exists regarding which method of talc administration is superior. Although an earlier small study showed better outcomes with talc poudrage compared with slurry,191 a subsequent larger study conducted by Dresler and colleagues192 showed no difference in recurrence of malignant pleural effusion between the two methods; however, a subgroup analysis revealed that patients with breast and lung cancer did better with talc poudrage than with slurry., The most common complications are pain, fever, respiratory complications such as acute respiratory distress syndrome, atelectasis, pneumonia, and arrhythmias.139,193,194
Mechanical pleurodesis is a process whereby mechanical abrasion of the visceral and parietal pleura is achieved via thoracoscopy; the abrasion induces petechial bleeding, which results in diffuse inflammation, leading to fibrin deposition in a fashion similar to the chemical sclerosants. Although no head to head comparisons between chemical and mechanical pleurodesis have been conducted, a study by Crnjac and colleagues195 demonstrated similar outcomes to pleurodesis with talc slurry.
Ultimately, pleurodesis is generally an effective means of palliating malignant pleural effusion, but it is important to recognize that up to 30% of patients referred for pleurodesis are inappropriate candidates for the procedure.192 The following reasons preclude pleurodesis: extensive intrapleural deposition of tumor tissue, multiple pleural loculations, trapped lungs, and airway obstruction from an endobronchial tumor.196 Successful pleurodesis is generally achieved 71% to 97% of the time, and morbidity and mortality are estimated at 3% to 26% and <1%, respectively.199–199
Indwelling Pleural Catheters
In 1997 the tunneled indwelling PleurX catheter was approved for management of malignant pleural effusions. Since then, intrapleural catheters have been shown to be safe and efficacious in the management of malignant pleural effusion.199–202 These catheters offer the advantages of outpatient management and patient control over symptoms, with immediate relief of dyspnea in 94% to 100% of patients and spontaneous pleurodesis in 40% to 70% of patients.199–204 Patients who are more likely to achieve improvement in their symptoms include those with gynecologic or breast tumors, absence of chest wall irradiation, positive cytology, and complete reexpansion of the underlying lung after drainage. Placement of the catheters rarely interrupts ongoing cancer treatment, and they are deemed superior for managing trapped lung and refractory chylothorax, which is known to respond poorly to talc pleurodesis.
Although guidelines recommend chest tube insertion and pleurodesis as first-line treatment of malignant pleural effusions, with talc being the most effective pleurodesis agent, the 30-day failure rate of talc pleurodesis defined as recurrence of effusion requiring further intervention is estimated at 30%. Complications associated with these catheters include infection, displacement, catheter obstruction with tension pleural effusion, skin breakdown, and catheter-track metastases. Until recently, no studies had compared intrapleural catheter use with pleurodesis and talc slurry in first-line management of malignant pleural effusion. However, in a randomized controlled trial comparing the use of intrapleural catheters and talc pleurodesis in controlling dyspnea in patients with malignant pleural effusions, no difference in dyspnea was found 42 days after either procedure, but improvement in dyspnea in the intrapleural catheter group was found at 6 months.205 Quality of life was the same in both groups. More patients in the talc group required further pleural procedures, and the procedure was associated with longer hospital stay. The intrapleural catheter group had more adverse events compared with the talc group, but no difference in the occurrence of serious adverse events was found between the two groups.
Pleuroperitoneal Shunts
Pleuroperitoneal shunts help manually pump fluid out of the pleural space into the peritoneum. Although they have mostly fallen out of favor because of their complications and burdensome management, they are especially useful in the management of chylous effusions, which are not successfully managed with pleurodesis or intrapleural catheter drainage. Additionally, they serve as a viable alternative to managing patients with trapped lung, for which pleurodesis is precluded by failure of the lung to reexpand.206 Data from a study published by Genc et al.207 revealed that this strategy achieved effective palliation of symptoms of malignant pleural effusion in 95% of patients with a shunt complication rate of 14.8% due largely in part to shunt occlusion and infection.
Pleurectomy
Pleurectomy is associated with significant morbidity and mortality; operative mortality is 10% to 19%, and as such, it currently does not constitute a mainstay option in the treatment of malignant pleural effusion.208 It is most often reserved for the fittest patients with expected long survivals for whom chemical pleurodesis has failed to control the effusion or for patients with malignant pleural mesothelioma.209 In that setting, it has been shown to decrease malignant pleural effusion. It is usually performed through VATS. Significant complications include hemorrhage, empyema, and cardiorespiratory failure.
Systemic Chemotherapy and Radiotherapy
In general, systemic chemotherapy is ineffective in treating malignant pleural effusion. However, there are some exceptions. Evidence indicates that malignant pleural effusion due to lymphoma, breast cancer, and small cell lung cancer may respond reasonably well to combination chemotherapy, with one study demonstrating a 58% response rate.139,210,211 Testicular and other germ cell neoplasms are not only responsive but also very curable, even at an advanced state, and thus early systemic treatment should be used if at all possible.212 Even patients with advanced germ cell tumors have good responses and should be considered for aggressive systemic therapy. Breast cancer might respond well to hormonal therapy or chemotherapy, particularly among patients who have not received prior systemic therapy. Mesothelioma is a unique tumor that arises directly from pleural or peritoneal surfaces. Effusions are often associated with the tumor and are not included in the staging system. It should be noted that the effusion may initially increase in size at the initiation of chemotherapy, but this increase does not reflect failure of the treatment, and therefore the chemotherapy should be continued. Patients amenable for aggressive local therapy, even with an effusion, should be considered for such therapy. However, because patients with solid-organ tumors and advanced disease have relatively poor responses to chemotherapy overall, early direct management of the effusion among patients with dyspnea should be considered at the outset for patients who are unlikely to achieve rapid responses to chemotherapy.
Radiotherapy provides some benefit, particularly when mediastinal lymph nodes are involved.213 However, irradiating the lungs for purposes of treating a malignant effusion carries the risk of radiation pneumonitis.