Lung Metastases
R. Taylor Ripley and Valerie W. Rusch
• Lungs are the second most common site of metastases.
• Lungs are the sole site of metastasis in 80% of patients with sarcoma and in 2% to 10% of patients with carcinoma.
• Lung metastases occur via hematogenous spread.
• Lymphangitic spread in carcinomas can occur early or late in the natural progression of all cancers.
• It is not well understood why lung metastases take several years to develop.
• Few lung metastases are symptomatic; only 15% to 20% of patients report having a cough or pain. All patients with isolated pulmonary metastasis from an extrathoracic malignancy should be evaluated for the possibility of resection.
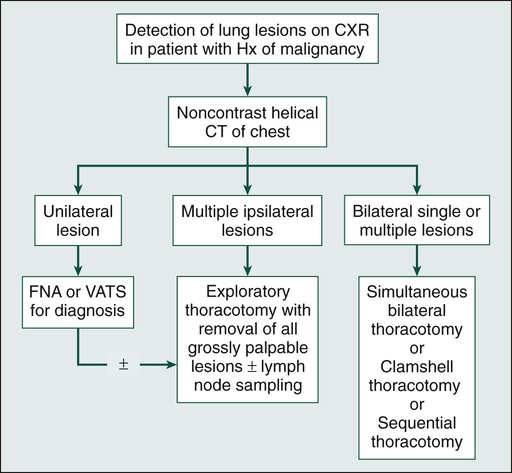
• Initial imaging studies should consist of a computed tomographic (CT) examination to predict resectability. Integrated fluorine-18 fluorodeoxyglucose positron emission tomography–CT may be substituted for CT alone. Magnetic resonance imaging has a limited role.
• CT is unable to distinguish reliably between malignant and benign lesions.
• CT differs from the final pathology report in 42% of cases.
• CT underestimates the number of malignant lesions in 25% to 35% of cases.
• The accuracy of radiologic imaging is only 37%, underestimating the number of lesions by 39% and overestimating them by 25%, for patients undergoing bilateral exploration.
• Prognostic factors include number of metastases, disease-free interval, and histology/organ site of the primary tumor.
Pulmonary Metastasis for Specific Tumor Types
• From 10% to 25% of patients with primary colorectal tumors have detectable metastases at the time of primary tumor diagnosis.
 Some form of metastasis develops in 50% of all patients with colorectal cancer.
Some form of metastasis develops in 50% of all patients with colorectal cancer.
 Approximately 2% to 7% of patients with colon cancer have isolated lung metastases; patients with rectal cancer have about double that number.
Approximately 2% to 7% of patients with colon cancer have isolated lung metastases; patients with rectal cancer have about double that number.
• Metastatic disease develops in 25% to 70% of patients with localized bone and soft tissue sarcoma; 10% will present with metastasis at the time of primary tumor diagnosis.
 Isolated lung metastases occur in up to 20% of patients with sarcoma during the course of their disease, with the lung being the sole site of failure after treatment in up to 90% of cases.
Isolated lung metastases occur in up to 20% of patients with sarcoma during the course of their disease, with the lung being the sole site of failure after treatment in up to 90% of cases.
• Patients with metastatic melanoma have an especially poor prognosis, with isolated lung metastasis occurring in 2% to 11% of patients.
• In 50% of patients who have a radical nephroureterectomy, pulmonary metastases later develop; however, only 16% have metastatic disease confined to the lung.
• Head and neck tumors, especially squamous cell cancers, tend to metastasize to the lung; however, 10% to 40% of lung nodules in these patients are actually second cancers manifesting as primary lung tumors.
• Pulmonary metastasis upon presentation occurs in approximately 50% of patients with retroperitoneal germ cell tumors.
• The first case of pulmonary metastasectomy was described by Weinlechner in 1882.
• Alexander and Haight described the first series of patients; 12 patients remained disease free for 1 to 12 years.
• The following general guidelines should be met before undertaking a resection:
 Control of the primary tumor or ability to resect the primary tumor
Control of the primary tumor or ability to resect the primary tumor
 Ability to resect metastatic disease completely
Ability to resect metastatic disease completely
 Ability of the patient to withstand the extent of pulmonary resection required to remove all gross tumor
Ability of the patient to withstand the extent of pulmonary resection required to remove all gross tumor
 Absence of extrathoracic metastasis
Absence of extrathoracic metastasis
 Absence of better alternative treatment
Absence of better alternative treatment
• The location of metastases determined the extent and type of resection:
 Peripheral metastases: parenchymal sparing
Peripheral metastases: parenchymal sparing
 Central metastasis: lobectomy or pneumonectomy
Central metastasis: lobectomy or pneumonectomy
 Solitary endobronchial metastasis: lobectomy, sleeve lobectomy, or pneumonectomy
Solitary endobronchial metastasis: lobectomy, sleeve lobectomy, or pneumonectomy
• All grossly palpable tumors must be resected with clear margins.
• More radical resection (e.g., a lobectomy or pneumonectomy) does not increase survival rates.
• Bilateral metastases and recurrence of pulmonary metastases are not contraindications to resection and should not deter resection in lesion(s) that can be removed completely. Two therapeutic options are emerging as potentially effective alternatives to resection: stereotactic body radiation therapy and radiofrequency ablation.
Introduction
The first described case of pulmonary metastasectomy was reported in 1882 by a German surgeon named Weinlechner, who removed two incidental pulmonary nodules during a chest wall resection for sarcoma.1 In 1938 Barry and Churchill2 reported the first long-term survivor from pulmonary metastasectomy, a patient with metastatic renal cell cancer. Their patient survived 23 years after surgery. Subsequently, Alexander and Haight described the first series of patients undergoing pulmonary metastasectomy and its correlation to survival.3 Twelve patients in their study remained free of disease for 1 to 12 years. Most important, from this early study came the first generally accepted criteria for pulmonary metastasectomy:
1. The primary tumor should be completely removed.
2. There should be no evidence of extrapulmonary disease.
3. The patient should be able to tolerate the planned operation from the standpoint of his or her overall medical condition.
Subsequently these criteria were modified by other authors to reflect our improved understanding of the management of pulmonary metastases. Current additional criteria include:4
1. Control of the primary tumor, or ability to resect the primary tumor completely simultaneous with resection of metastasis
2. Ability to resect metastatic disease completely
3. Ability of the patient to tolerate the extent of pulmonary resection required to remove all gross tumor
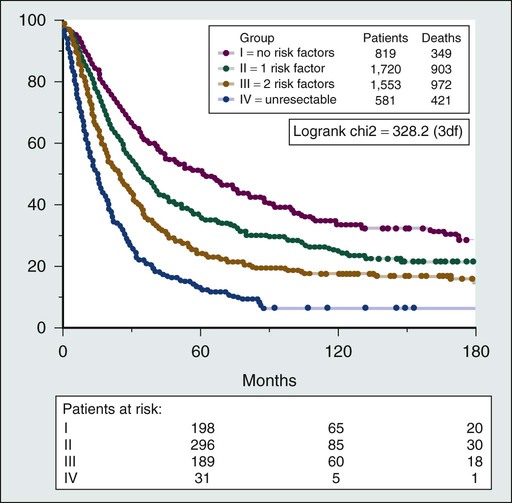
Several prognostic factors that are not universal across all tumor histologies have been reported to affect outcome after pulmonary metastasectomy (Box 52-1). The largest series of pulmonary metastasectomy reported to date is from the International Registry of Lung Metastasis, which analyzed 5206 cases.5 The overall 5-year survival after pulmonary metastasectomy without stratifying for tumor type was 36%. Factors associated with better prognosis included a long disease-free interval (DFI), complete resection, and a small number of lung nodules. A staging system based on these prognostic factors was proposed (Fig. 52-1).
The role of surgery in the treatment of pulmonary metastases will continue to evolve as better systemic therapies become available. Currently, only a minority of patients with metastatic disease from any source are candidates for pulmonary metastasectomy; however, improved imaging studies and the widespread use of computed tomography (CT) might detect more patients who have small-volume pulmonary metastases and are therefore candidates for metastasectomy. Recently, the European Society of Thoracic Surgeons formed the Lung Metastasectomy Working Group to review the evidence and produce guidelines.6 They concluded that the level of evidence is relatively low and randomized controlled trials are absent in this field. Despite these limitations, long-term survival does exist for patients with multiple histologies who rarely have long-term survival with systemic therapy alone. A current perspective of the evaluation and treatment of patients with isolated lung metastases is presented in this chapter.
Diagnosis
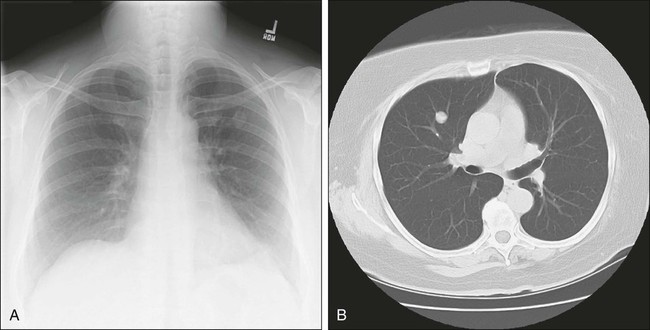
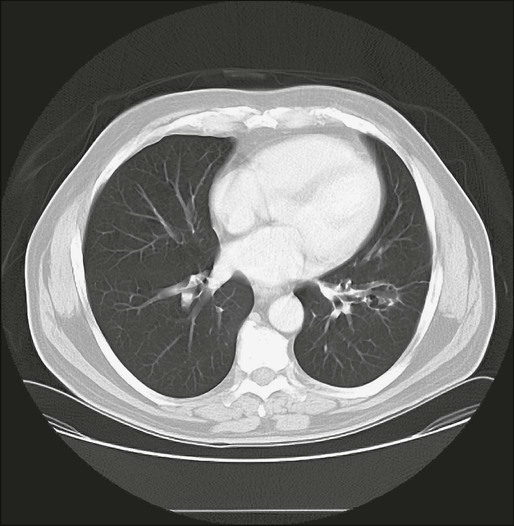
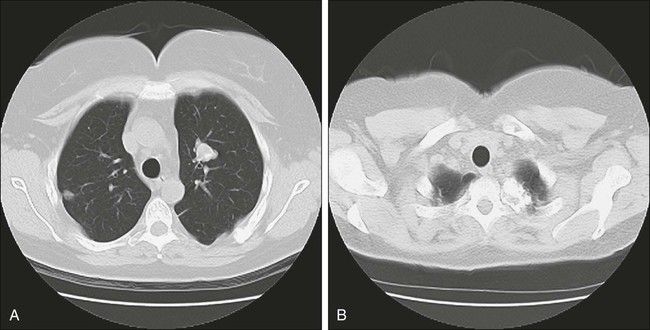
Few patients with pulmonary metastasis are symptomatic. It is estimated that only 15% to 20% of patients present with a cough or nonspecific chest pain and even fewer still with hemoptysis. Traditionally, a chest radiograph has been the most commonly used and cost-effective modality for screening patients for metastasis from extrathoracic malignancy, but CT scans have rapidly replaced the chest radiograph as the most useful tool. The most frequent radiographic appearance of a pulmonary metastasis is a peripherally located, well-circumscribed nodule (Fig. 52-2).7,8 Several less common radiographic characteristics have also been described. Cavitating lesions are associated with a differential diagnosis that includes benign, infectious, and malignant causes (Fig. 52-3). When cavitary lesions are malignant, they are usually squamous cell carcinomas. The frequency of cavitation in metastatic nodules is approximately 4%; however, squamous cell malignancy is responsible for 69% of these lesions. Spontaneous pneumothorax also can occur with metastatic lung lesions and is thought to be caused by cavitation and erosion into a bronchiole wall. Spontaneous pneumothorax is most frequently seen in patients with sarcoma. It is said that a spontaneous pneumothorax in a patient with a history of sarcoma should prompt an evaluation for possible occult metastatic lung lesions. Calcification of pulmonary nodules is usually related to a benign process such as a hamartoma; however, metastatic lesions of many types (especially osteogenic sarcoma) are known to produce calcification (Fig. 52-4).7,8 Calcification in metastatic lesions is thought to be produced by several processes in different tumor types, including bone formation in osteogenic sarcoma, mucinous calcification of adenocarcinomas, or dystrophic calcification of lesions such as synovial sarcoma or giant cell tumors of the bone.7 Hemorrhage around lung nodules is also seen more frequently in benign lung lesions (e.g., fungal or mycobacterial infections) and is visualized as a halo around the lung nodule. This appearance can also be seen in metastatic lesions and should raise the suspicion for metastasis in patients with a prior history of malignancy.
Computed Tomography
The use of CT has supplanted chest radiography as the primary mode of imaging pulmonary metastases. CT is able to visualize more lesions than chest radiographs as noted in several reports.5,9–18 Chang and colleagues12 reported that when compared with conventional radiography, CT was able to visualize nearly twice as many nodules.19 This change has been facilitated by the development of high-speed, multislice scanners. These scanners are available up to 256 slices, but no data exist to indicate that these extremely high-resolution scanners detect more lesions than 5-mm thin-slice scanners.20 CT is not able to distinguish reliably between malignant and benign lesions, however. McCormack and coworkers9 retrospectively studied 144 patients who had both a chest radiograph and a CT scan to identify metastatic lesions. The CT results differed from the final pathology reports in 42% of cases, with CT scans underestimating the number of malignant nodules in 25% of patients. Pastorino and associates5 reported results of imaging on 2988 patients undergoing pulmonary metastasectomy. The overall accuracy of radiologic assessment of the number of metastatic nodules was 61%, underestimating metastasis in 25% of patients. Interestingly, in the patients (1134) who had bilateral exploration, the accuracy of imaging was only 37%, underestimating the number of lesions in 39% and overestimating the number in 25%. Cerfolio and associates21 prospectively assessed the incidence of nonimaged malignant nodules at thoracotomy among patients who had a 64-slice helical CT scan with 5-mm slices.21 Fifty-one patients of 152 (34%) had 57 pulmonary nodules that were not detected preoperatively. Thirty-two of the 57 nodules (56%) were malignant. Ellis and associates22 reported a similar conclusion. Significantly more nodules were palpated at thoracotomy than were detected preoperatively despite using a multidetector helical CT scanner with 5-mm slices (mean 3.24 vs. 2.12). In 19 of 54 thoracotomies for osteosarcoma (35%), Kayton and associates23 reported additional metastatic lesions not imaged by 5-mm CT scanners. The accuracy and sensitivity of CT also depend on the size of the lesions (Table 52-1): the larger the lesion, the greater the sensitivity and accuracy. Munden and colleagues,15 who reported on the clinical significance of pulmonary lesions less than 1 cm in diameter, found malignant pulmonary lesions in 81% of patients with a history of prior malignancy. Multiple authors have described the ability of CT to detect a greater number of pulmonary nodules, while acknowledging a decreasing specificity of identifying malignant nodules with this diagnostic tool.12,16 Therefore not all small pulmonary nodules in patients with a history of cancer can be assumed to represent metastatic disease. Currently no established guidelines exist for routine screening for pulmonary metastases, but CT scanning has become the sole screening tool for identifying pulmonary metastases in recent reports.12,16
Table 52-1
Detection of Pulmonary Metastasis by Computed Tomography
| Year | Author | No. Nodules | Sensitivity for Small Lesions (%) | Sensitivity for Larger Lesions (%) |
| 1998 | Waters et al.*19 | 144 | 44 (≤5 mm) | 91 (>5 mm) |
| 1999 | Diederich et al.10 | 90 | 69 (≤6 mm) | 95 (>6 mm) |
| 2002 | Margaritora et al.†11 | 188 | 48 (≤6 mm) | 87 (>6 mm) |

Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) provides the benefits of reduced radiation exposure (of particular interest for cases involving younger patients) and the ability to detect lesions at lung-mediastinal interfaces. MRI has not gained wide acceptance as a screening tool, however, mainly because of its increased time constraints and cost. Further unfavorable technical considerations include motion-related artifacts and an inability to detect calcified lesions. Kersjes and coworkers24 performed a study comparing MRI and helical CT in the detection of pulmonary metastasis and showed that MRI has an overall accuracy of 84%. For lesions smaller than 5 mm, however, the sensitivity of MRI was only 36%. The routine use of MRI is currently not advocated as a screening tool for patients with pulmonary metastasis.
Nuclear Imaging
Imaging with fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) is being used frequently to assist in the staging of primary tumors. This modality is used most often at the time of diagnosis to rule out distant metastasis, but occasionally it is used to assess response to therapy. Currently FDG-PET is not the standard for identifying pulmonary metastasis, but it is increasingly used in this role.25,26 Pastorino and associates27 report that 19 of 86 patients (21%) were excluded from pulmonary metastasectomy because of multiple histologies on the basis of the results of FDG-PET. Dalrymple-Hay and associates25 reported that 9% of patients who were evaluated for pulmonary metastasectomy for metastatic melanoma were excluded because of FDG-PET results. Unfortunately, the number of patients screened was unclear in this study. Other authors suggest that FDG-PET is not superior to CT in the identification of pulmonary metastasis. Lucas and colleagues28 studied 62 patients with soft tissue sarcoma who had FDG-PET during initial evaluation. The sensitivity of FDG-PET in detecting lung metastasis was 86.7% compared with 100% for CT. FDG-PET may not be superior to conventional imaging techniques in identifying pulmonary metastasis from bone and soft tissue sarcomas.28,29 The value of FDG-PET alone may be an obsolete argument with the increased use of integrated PET-CT. The data regarding this modality are limited, but integrated scanners may optimize the advantages of both studies. In the previously mentioned prospective study by Cerfolio and associates,21 preoperative identification of pulmonary metastasis was performed by helical CT scans, but 132 of 152 patients (87%) had integrated PET-CT. Despite use of this technology, 34% of patients had lesions identified at thoracotomy that were not detected preoperatively. In addition, FDG-PET, whether integrated or not, does not reliably detect subcentimeter lesions. Reinhardt and associates30 report that the false-negative rate for detecting 6 to 10 mm lesions is 41%, and for lesions less than 5 mm, it is 100%. Integrated PET-CT may have a role in detection of unsuspected extrathoracic disease prior to pulmonary metastasectomy, but strong recommendations for this modality cannot be made at this time.
Surgical Approaches to Lung Metastasis
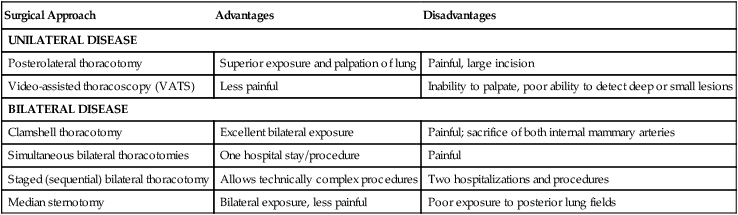
The goal of pulmonary metastasectomy is to achieve complete resection of all visible and palpable tumor in the lung. The surgical approach is dictated by the extent and location of disease and by the patient’s performance status. Several approaches are available depending on the size, number, and location of the lesions. The advantages and disadvantages of these approaches are outlined in Table 52-2.
Table 52-2
Surgical Approaches to Resection of Pulmonary Metastases
| Surgical Approach | Advantages | Disadvantages |
| UNILATERAL DISEASE | ||
| Posterolateral thoracotomy | Superior exposure and palpation of lung | Painful, large incision |
| Video-assisted thoracoscopy (VATS) | Less painful | Inability to palpate, poor ability to detect deep or small lesions |
| BILATERAL DISEASE | ||
| Clamshell thoracotomy | Excellent bilateral exposure | Painful; sacrifice of both internal mammary arteries |
| Simultaneous bilateral thoracotomies | One hospital stay/procedure | Painful |
| Staged (sequential) bilateral thoracotomy | Allows technically complex procedures | Two hospitalizations and procedures |
| Median sternotomy | Bilateral exposure, less painful | Poor exposure to posterior lung fields |
Video-assisted thoracoscopy (VATS) for pulmonary metastasectomy has been fairly extensively described during the past 15 years. This approach remains controversial, however.31–35 McCormack and coworkers32 performed a prospective study evaluating the role of VATS to treat pulmonary metastasis. Eighteen patients had preoperative CT followed by VATS resection of all visible and CT-detected lesions. All patients then immediately underwent thoracotomy with resection of any additional lung nodules. Additional malignant lesions were found in 56% of patients after attempted VATS resection. VATS is dependent on preoperative imaging to detect metastatic lesions. Since this study by McCormack and colleagues was reported in 1996, improvements in helical scanning and the expanded role of integrated PET-CT have occurred, as noted in the previous section. Despite these improvements, several authors report similar results of missed lesions based on preoperative imaging.21,22 As mentioned previously, Cerfolio and associates,21 Ellis and colleagues,22 and Kayton and associates23 reported missed lesions by preoperative imaging among patients who underwent modern helical CT scanning with 5-mm slices. Despite these advances in technology, a significant number of metastases are detected by palpation at open thoracotomy that would be missed by VATS resection.
Some authors have advocated the use of VATS for patients with a solitary pulmonary metastasis. Mutsaerts and associates31,35 described their experience with 20 patients undergoing either VATS or thoracotomy for resection of a single pulmonary metastasis. The 5-year survival and recurrence rates appeared to be similar to the rates seen with thoracotomy.35 Gossot and colleagues36 reviewed the resection of one or two metastases from sarcoma by comparing 31 patients treated by VATS with 29 patients who underwent thoracotomy but were deemed appropriate for VATS. These investigators concluded that VATS yielded similar survival rates for patients with one or two nodules from sarcoma. Other authors have described localization methods using radiotracer injection to help identify small or deeply located lesions during VATS resections.33,37
Despite clear evidence that additional lesions are discovered at thoracotomy compared with preoperative evaluation, a survival advantage has not been shown. Nakas and associates38 report no difference in actuarial survival among 27 patients undergoing VATS resection compared with open resection of colorectal cancer with a median follow-up of 22 months. Of note, 32% of patients in the VATS group and 37% in the open group had hepatic metastases, and therefore extrathoracic disease may be as responsible for mortality as pulmonary disease. Carballo and associates report a retrospective review of 186 patients, of whom 135 were treated by thoracotomy and 36 by VATS for multiple histologies.194 With a median follow-up of 26 months, they report an insignificant difference in both 5-year actuarial and median survivals. Nakajima and associates37 reported significantly more recurrent nodules after open thoracotomy (27/43, 62.8%) versus VATS (27/79, 34.2%) at 2 years after the operations. In addition, they reported a significantly lower recurrence-free survival after open thoracotomy.
The approach to bilateral disease is more variable, but the principles remain the same. A median sternotomy, “clamshell” thoracotomy (i.e., a bilateral anterior thoracotomy with a transverse sternotomy), sequential bilateral thoracotomies, and simultaneous bilateral posterolateral thoracotomies are used as standard surgical approaches to the resection of bilateral metastases.41–41 In general, resection of bilateral metastases is preferably done as a single operation. Sequential thoracotomies are performed only when the anatomic location of a lesion requires a complex or extensive operation or when the patient’s comorbidities dictate a more conservative approach to management. Members of the European Society of Thoracic Surgeons were surveyed by Internullo and associates42 to determine the current clinical practices for pulmonary metastasectomy. Sixty-five percent considered palpation of the lung necessary for adequate metastasectomy. Of those who performed open thoracotomy for bilateral disease, two out of three perform bilateral staged thoracotomies. Median and clamshell sternotomies were performed less frequently. Regardless of the favored approach, the majority of surgeons altered their approaches based on tumor location, lung function, the number of metastases, and the performance status of the patient.
In contrast to primary lung cancers, pulmonary metastases require only a local excision with a surrounding rim (1 to 2 cm) of benign lung tissue. The European Society of Thoracic Surgeons Practice Pattern Survey revealed that local excision is performed in 92% of cases.42 This technique is accomplished most frequently by wedge resection performed by precision electrocautery or with a stapling instrument (Fig. 52-5). Segmentectomy, lobectomy, or pneumonectomy are used less commonly. It is important to note that survival after metastasectomy is not increased by a more radical resection such as lobectomy or pneumonectomy. These procedures might be required technically to remove the lesion, however, and should be applied to do so if needed. The most important principle of these techniques is a clear margin of resection.
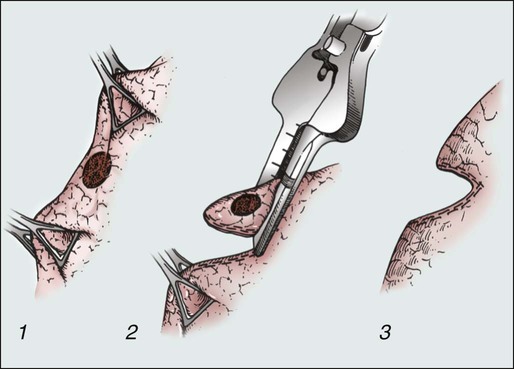
The preoperative evaluation for these patients is similar to that for patients undergoing lung resection for any other cause (Fig. 52-6). Pulmonary function testing should be obtained for all patients to ensure that the volume of lung resection will not compromise overall respiratory function. Thorough evaluation of underlying cardiovascular disease should also be undertaken, with preoperative stress testing as clinically indicated.
Pulmonary Metastasectomy for Specific Tumor Types
Colorectal Cancer
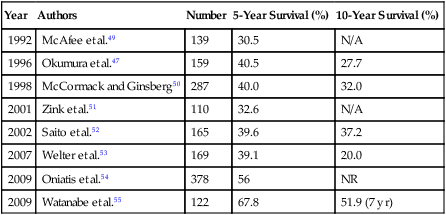
It is estimated that 10% to 25% of patients with primary colorectal tumors will have detectable metastases at the time of diagnosis.43 Despite advances in adjuvant therapy and surgery, 50% of all patients with colorectal cancer will develop some form of metastasis during their lifetimes.44 In approximately 15% of patients having curative resection of their primary colorectal tumor, distant metastasis will develop, including metastasis to the lung.45 Isolated pulmonary metastases occur in about 2% to 7% of patients with colon cancer and about double that number for patients with rectal cancer.46 Pulmonary metastasis can occur even with favorable primary tumor characteristics. Okumura and associates47 reported that 26% of pulmonary metastectomies were performed in patients with Duke A or B primary colorectal cancer. Since Blalock48 reported the first pulmonary metastasectomy for colorectal cancer in 1944, several authors have reported their experience regarding overall survival and prognostic factors. The 5- and 10-year survival rates range from 30% to 68% and 20% to 37%, respectively (Table 52-3).49–55 It is clear from the literature that pulmonary metastasectomy for colorectal cancer is associated with long-term survival, especially when a complete resection is performed. McCormack and colleagues56 reviewed 144 patients who underwent pulmonary metastasectomy for colorectal cancer and showed that survival for patients who underwent complete resection was approximately 40% at 5 years, whereas incomplete resection was associated with a poor prognosis. More recently, Melloni and associates57 reported the same conclusion with a 5-year survival rate of 44% for complete resection versus 0% for incomplete resection.
Table 52-3
Survival of Patients Undergoing Pulmonary Metastasectomy for Colorectal Cancer*
| Year | Authors | Number | 5-Year Survival (%) | 10-Year Survival (%) |
| 1992 | McAfee et al.49 | 139 | 30.5 | N/A |
| 1996 | Okumura et al.47 | 159 | 40.5 | 27.7 |
| 1998 | McCormack and Ginsberg50 | 287 | 40.0 | 32.0 |
| 2001 | Zink et al.51 | 110 | 32.6 | N/A |
| 2002 | Saito et al.52 | 165 | 39.6 | 37.2 |
| 2007 | Welter et al.53 | 169 | 39.1 | 20.0 |
| 2009 | Oniatis et al.54 | 378 | 56 | NR |
| 2009 | Watanabe et al.55 | 122 | 67.8 | 51.9 (7 yr) |
As for other primary tumors, studies evaluating the DFI, the number of lesions, and the presence of lymph node involvement have shown that these prognostic factors are significant after pulmonary metastasectomy for colorectal cancer.47,49–52,56,58–63 Recently Oniatis and associates54 reported that more than three metastases, DFI less than 1 year, age older than 65 years, and male sex were adverse prognostic factors on multivariate analysis. Colorectal cancer is also unique in that the serum tumor marker carcinoembryonic antigen (CEA), which is used as a marker for follow-up, has been shown to be a prognostic indicator for patients with pulmonary metastases.49–52,55,56,58–62,64,65 Also relatively unique to colorectal cancer is that pulmonary metastasectomy with either synchronous or metachronous resected hepatic metastasis is another reported adverse prognostic factor.66
The presence of bilateral metastases was previously thought to be a contraindication to metastasectomy, but the significant prognostic factor is probably the number of metastases, not the laterality. Several authors have reported that the survival for patients with bilateral lesions is not significantly reduced compared with patients who have multiple ipsilateral lesions.50,59 Although a solitary metastasis might be associated with a better prognosis than multiple metastases (either unilateral or bilateral), the main criteria used to select patients with multiple lesions is whether removal of these lesions is technically feasible and whether removal of the volume of lung parenchyma does not appear to compromise the patient’s lung function to a significant extent.
CEA levels are elevated in 40% to 70% of patients prior to pulmonary metastasectomy. Most authors report that elevated CEA levels are an adverse prognostic factor.51,52,60,61,67 The reason for this observation is unclear, although it has been postulated that the presence of CEA may promote adhesion or attachment of tumors cells or could be due to undetected extrathoracic metastasis.68 Although an elevated CEA level is not used currently to exclude patients from resection, it could be useful to consider this value in the context of other known prognostic factors. Multiple studies have reported cutoff values for CEA levels that predict a poor prognosis.55,64,65 Lee and associates64 report 5-year survival rates of 86.9% for patients with normal CEA levels versus 23.4% for patients with CEA levels greater than 5 ng/mL. Saito and associates52 report similar results with a 4-year survival of 42.7% versus 15.1% for patients with less than or greater than 10 ng/mL of CEA, respectively. Kanemitsu and associates65 reported that prethoracotomy CEA levels were one of the strongest prognostic factors for adverse outcomes. CEA displayed the strongest relationship with death at 3 years on multivariate analysis. This relationship is strong enough that increasing CEA was associated with decreased survival in a preoperative nomogram. These data reveal that CEA is an important prognostic factor, but it doesn’t necessarily exclude patients from resection. A useful approach may be to recommend that a patient with a persistently or rapidly increasing CEA after primary resection be treated initially with chemotherapy rather than directly undergoing pulmonary metastasectomy.
Hilar or mediastinal lymph node metastases occur in 1% to 49% of patients with colorectal pulmonary metastasis.47,52,54,56,58,59,61 Oniatis and colleagues54 reported that of 378 patients, 175 (46%) had N0 disease, 131 (35%) had N1 disease, and 54 (14%) had N2 disease, with only 5% unknown. Saito and associates52 reported that the 5-year survival was 48.5% for the patients without hilar or mediastinal lymph node metastasis versus 6.2% at 4 years for the patients with lymph node metastasis. Welter and colleagues53 reported that 28 of 169 patients undergoing resection of colorectal pulmonary metastases had intrapulmonary, hilar, or mediastinal nodal metastases, with 5-year actuarial survivals at 78, 0, and 0%, respectively. The 5-year survival rates were 42% versus 19% without or with any lymph node involvement, respectively. This study reveals that any lymph node involvement and increasing station of involvement adversely affect overall survival. Okumura and colleagues47 performed systematic lymph node dissection for 100 patients with colorectal pulmonary metastasis. Fifteen of these patients had positive lymph nodes with a 5-year survival of 6.7% compared with a 50% survival rate for patients with negative lymph nodes, indicating that complete lymph node dissection does not increase survival. The routine use of mediastinal lymph node dissection in patients with pulmonary metastases appears to be the exception rather than the norm. The presence of malignant lymph nodes could indicate a group of patients otherwise thought to be disease-free who might benefit from additional adjuvant therapy. We favor mediastinal lymph node sampling or dissection in these patients. Although these procedures might not have a therapeutic effect, they certainly provide important prognostic information.
Previously, the presence of both liver and lung metastases from colorectal cancer was thought to be a contraindication to metastasectomy, but during the past decade, authors of several studies have reported long-term survival with combined pulmonary and hepatic metastatsectomy.66,69–71 Synchronous or metachronous lung and liver metastasis occurs in approximately 5% of patients with colorectal cancer. Headrick and coworkers72 reported 5- and 10-year survivals of 30% and 16%, respectively, for 58 patients who underwent resection of both liver and lung metastases from colorectal cancer. Zabaleta and associates66 reported 3- and 5-year survival rates of 50% and 39% after pulmonary metastasectomy among 17 patients who had previously undergone hepatic metastasectomy for colorectal carcinoma. Decreasing morbidity and mortality rates for liver resection now make resection of both lung and liver metastases a viable option in carefully selected patients.
Bone and Soft Tissue Sarcoma
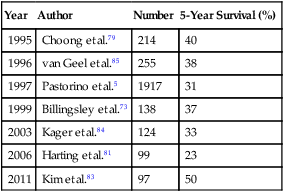
Bone and soft tissue sarcomas constitute a histologically diverse group of tumors accounting for 1% of all adult malignancies, with approximately 6600 cases occurring in the United States annually.73,74 Metastasis will occur in 25% to 70% of patients with localized disease, and 10% will present with metastasis upon diagnosis.74 Isolated pulmonary metastases occur in up to 20% of patients with sarcoma during the course of their disease, with the lung being the site of failure after treatment in up to 90% of cases.75,76 Inability to control thoracic disease is the most common cause of death.77 Factors associated with an increased risk of pulmonary metastasis include high tumor grade, primary tumor size greater than 5 cm, lower extremity site, and histologic subtype.75,78 The lack of effective systemic therapy for most soft tissue sarcomas makes surgical resection the best treatment for pulmonary metastases. Several studies have shown that the complete resection of pulmonary metastases is associated with long-term survival.5,73,75,78–83 Billingsley and associates73 reported that the 3-year actuarial survival was 46% in patients who had complete resection compared with 17% (P < .001) for patients with incomplete resection of pulmonary metastasis. Kager and colleagues84 reported similar findings with 5-year actuarial survival of 44% in patients with complete resection versus 0% with incomplete resection. The completeness of surgical resection was one of only two prognostic factors that was significant on multivariate analysis. The cumulative 5-year survival for patients with bone and soft tissue sarcomas after pulmonary metastasectomy ranges from 23% to 50% (Table 52-4).5,73,79,83–87
Table 52-4
Survival of Patients Undergoing Pulmonary Metastasectomy for Bone and Soft Tissue Sarcomas
| Year | Author | Number | 5-Year Survival (%) |
| 1995 | Choong et al.79 | 214 | 40 |
| 1996 | van Geel et al.85 | 255 | 38 |
| 1997 | Pastorino et al.5 | 1917 | 31 |
| 1999 | Billingsley et al.73 | 138 | 37 |
| 2003 | Kager et al.84 | 124 | 33 |
| 2006 | Harting et al.81 | 99 | 23 |
| 2011 | Kim et al.83 | 97 | 50 |
The histologic subtype of sarcoma influences both the development of pulmonary metastasis and overall survival. High-grade, undifferentiated, and alveolar soft part sarcomas produce pulmonary metastasis in approximately 60% of patients.73 High-grade tumors and histologic variants such as liposarcoma, malignant fibrous histiocytoma, and malignant peripheral nerve tumor have all been reported to be unfavorable prognostic factors.73,75,88 Longer DFI, fewer lesions, and unilaterality have been reported to be favorable prognostic factors.84
Recently, Stephens and associates89 reported that progression of disease after neoadjuvant chemotherapy prior to pulmonary metastasectomy is a novel adverse prognostic factor. The 5-year actuarial survival was 32% for patients without progressive disease versus 0% for patients with progressive disease. These results suggest that as multimodality therapy evolves, the response to systemic therapy can aid in appropriate patient selection for metastasectomy. The timing and role of metastasectomy in the setting of multimodality therapy warrants further study and is rapidly becoming a standard treatment strategy.
The incidence of hilar or mediastinal lymph node metastasis in patients with lung metastasis from sarcoma is 2%.5 Veronesi and associates reported that 1of 17 patients had lymph node involvement from sarcoma.90 Pfannschmidt and associates91 reported that 9 of 69 patients with sarcoma had N1 disease but no N2 involvement. This low propensity for lymph node involvement is also a feature of primary sarcomas. Therefore it seems that routine mediastinal lymph node sampling or dissection is unlikely to offer significant prognostic information or therapeutic benefit.
The rate of recurrence after pulmonary metastasectomy for sarcoma ranges from 45% to 83%; however, the role of repeat resection of pulmonary metastases and resection of synchronous and metachronous extrathoracic disease have been studied by a few authors.75,86,88,92–95 Weiser and colleagues96 reported experience from Memorial Sloan Kettering in 86 patients who underwent reresection of pulmonary metastases for soft tissue sarcoma. The 5-year survival after undergoing at least two operations for pulmonary metastasectomy was 36%. Patients who had a complete reresection had a median survival of 51 months, compared with 6 months in patients in whom complete reresection was not possible. Poor prognostic indicators for reresection included three or more nodules, lesions greater than 2 cm in size, and high-grade primary tumors. This study strongly suggested a benefit for repeat or multiple procedures for clearance of pulmonary disease in carefully selected patients. Burt and associates94 reported repeated pulmonary metastasectomy in nearly 50% of patients with pulmonary recurrences, but they did not specifically state the long-term survival of this group of patients. Blackmon and associates95 reported the experience at MD Anderson Cancer Center of pulmonary resection with extrathoracic metastases. The median survivals were 36, 38, and 14 months for patients with pulmonary metastasectomy only, pulmonary and extrapulmonary metastasectomy, and pulmonary and extrapulmonary lesions that were not resected. This study suggests that if complete resection is feasible, some patients may benefit from aggressive surgical resection of sarcoma despite multiple sites of disease.
Pulmonary metastases from osteosarcoma occur most frequently in pediatric patients and respond better to chemotherapy than do soft tissue sarcomas. Pulmonary metastasectomy within the context of a multimodality therapy program is a well-accepted approach to treatment and is associated with a 5-year survival of approximately 30%. Response to chemotherapy, the DFI from resection of the primary tumor, and the number of metastases and complete resection are reported to influence overall survival.81,87,97 Harting and associates81 report that tumor necrosis after neoadjuvant therapy was a characteristic associated with long-term survival.
Melanoma
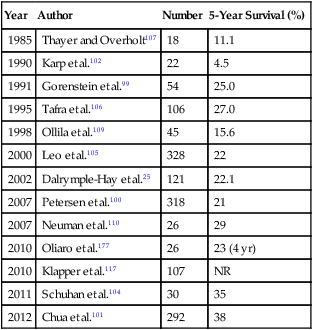
Patients with metastatic melanoma have an especially poor prognosis. The most common sites of metastasis from melanoma are the lungs, distant subcutaneous tissue, and distant lymph nodes, with isolated lung metastasis occurring in 1.9% to 11% of patients.98,99 Petersen and associates100 reviewed 1720 patients with pulmonary metastases. The significant predictors of survival were nodular histologic type, number of metastases, extrathoracic metastases, and performance of pulmonary metastasectomy. A survival advantage was noted for the 318 patients who underwent resection, but an interaction was found between metastasectomy, DFI, and extrathoracic metastasis. If patients had a DFI greater than 5 years, surgery revealed a survival advantage of 19 versus 7 months. Surgery had a survival advantage of 18 versus 8 months for patients without extrathoracic metastasis. Also, they report a significant 5-year survival advantage of 21% versus 13% for complete versus incomplete resection, respectively. Chua and colleagues101 reported that among 1737 patients diagnosed with metastatic lung melanoma, 292 patients underwent pulmonary metastasectomy. Two hundred thirty-four (80%) of these patients underwent a complete resection. On multivariate analysis, an involved surgical margin was associated with a significantly worse survival (P = .001). Other authors also have reported that complete resection is associated with prolonged survival.100–105 Overall, the 5-year survival rates after resection of pulmonary metastasis from malignant melanoma vary from 4.5% to 38% (Table 52-5).25,99,102,106–109 This wide range of values probably reflects the relatively small numbers of patients included in some studies.
Table 52-5
Survival of Patients Undergoing Pulmonary Metastasectomy for Malignant Melanoma
| Year | Author | Number | 5-Year Survival (%) |
| 1985 | Thayer and Overholt107 | 18 | 11.1 |
| 1990 | Karp et al.102 | 22 | 4.5 |
| 1991 | Gorenstein et al.99 | 54 | 25.0 |
| 1995 | Tafra et al.106 | 106 | 27.0 |
| 1998 | Ollila et al.109 | 45 | 15.6 |
| 2000 | Leo et al.105 | 328 | 22 |
| 2002 | Dalrymple-Hay et al.25 | 121 | 22.1 |
| 2007 | Petersen et al.100 | 318 | 21 |
| 2007 | Neuman et al.110 | 26 | 29 |
| 2010 | Oliaro et al.177 | 26 | 23 (4 yr) |
| 2010 | Klapper et al.117 | 107 | NR |
| 2011 | Schuhan et al.104 | 30 | 35 |
| 2012 | Chua et al.101 | 292 | 38 |
Several prognostic factors have been reported for survival after pulmonary metastasectomy. 25,98,99,102,103,106–109 Chua and associates101 report that metastasis greater than 2 cm, positive surgical margin, and more than one metastasis were predictors of poor long-term survival. Other reports confirm that solitary pulmonary metastasis, complete resection, and DFI are associated with improved survival.103–105,110 Harpole and associates103 performed an analysis of the subset of patients who had a solitary metastasis and found that patients who underwent resection had a significantly better median survival than did patients managed nonsurgically. These data suggest that appropriately selected patients with metastatic melanoma confined to the lungs can benefit from pulmonary metastasectomy.
Survival among patients with metastatic melanoma is poor even in the highly selected group of patients who undergo metastasectomy, but multimodality therapy may be possible. Recently, the highly-selective BRAF inhibitors, the anti-CTLA-4 antibody ipilimumab, and immune-based therapies work by novel mechanisms that have improved survival.111–116 In addition, Klapper and associates117 report thoracic metastasectomy for procurement of tumor-infiltrating lymphocytes to provide a cell-mediated immune-based approach for the treatment of refractory metastatic melanoma.117,118 These advances provide hope for a disease that has had minimal options and dismal outcomes. The role of metastasectomy in combination with these therapies is currently unknown but provides a strategy that may improve survival of patients with metastatic melanoma.
Renal Cell Carcinoma
Barry and Churchill2 performed the first pulmonary metastasectomy in a patient with renal adenocarcinoma in 1938. Approximately 30% of patients with renal cell carcinoma will present with metastasis, and distant metastases will develop in approximately 30% to 50% of patients with initially localized tumors.119 In half of patients who have a radical nephroureterectomy, pulmonary metastases will develop later, and only 16% of these patients will have disease confined to the lung.120,121 The 5-year survival of patients with unresected metastasis is approximately 2.7%.122 The 5-year survival after resection of isolated pulmonary metastasis is reported to range from 36% to 53% (Table 52-6).121–127 Several prognostic factors for survival have been identified, including complete resection, the DFI between primary tumor treatment and metastasis, the number of metastases, and the presence or absence of lymph node metastases.121,122,124,126 Recently, Meimarakis and associates127 used the prognostic factors determined by multivariate analysis of complete metastasectomy, metastasis greater than 3 cm, positive nodes of primary tumor, mediastinal nodes, synchronous metastasis, and pleural infiltration to develop a scoring system to stratify patients into low-, intermediate-, and high-risk groups. These factors may help determine which patients may benefit from both metastasectomy and adjuvant therapy after metastasectomy. The role of adjuvant therapy in this setting is largely unexplored.
Table 52-6
Survival of Patients Undergoing Pulmonary Metastasectomy for Renal Cancer*
| Year | Author | Number | 5-Year Survival (%) |
| 1994 | Cerfolio et al.125 | 96 | 36 |
| 1997 | Fourquier et al.121 | 50 | 44 |
| 1999 | Friedel et al.123 | 77 | 39 |
| 2002 | Pfannschmidt et al.122 | 149 | 42 |
| 2002 | Piltz et al.124 | 105 | 40 |
| 2005 | Hofmann et al.126 | 64 | 33.40 |
| 2006 | Marulli et al.178 | 59 | 53 |
| 2011 | Meimarakis et al.127 | 202 | 39 |
| 2011 | Alt et al.128 | 224 | 73.6† |
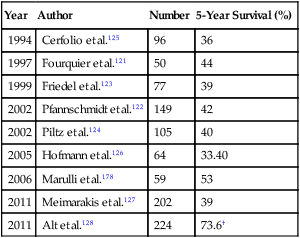
*Studies represent analysis of 50 patients or more.
†Complete pulmonary metastasectomy. Incomplete resections were not reported.
In the aforementioned series, Meimarakis and associates127 reported on 202 patients who underwent pulmonary metastasectomy for renal cell cancer. They found that complete resection was possible in 87% of patients and that these patients had 5- and 10-year survival rates of 45% and 31%. No patients with either R1 or R2 incomplete resections survived for 5 years. Alt and associates128 reported a series of 887 patients with metastatic renal cell carcinoma, of whom 224 had lung-only metastasis. They reported a cancer-specific 5-year survival of 73.6% for patients who had undergone complete pulmonary metastasectomy versus 19% for patients with incomplete resection. The overall survival of the entire surgical group was not reported. Also, the patients who underwent complete metastasectomy were more likely to have initially localized disease, lung-only metastasis, and one or two metastatic lesions. Regardless, these results suggest that when complete resection is technically feasible and the patient can tolerate the operation, a substantial number of patients will experience long-term survival.
Hilar and mediastinal lymph node metastases are seen in 22% to 30% of patients with metastatic renal cell cancer.121,122 Fourquier and colleagues,121 Pfannschmidt and associates,122 and Meimarakis and colleagues127 performed systematic mediastinal lymph node dissection on 50, 191, and 91 patients, respectively. Each study found that the presence of lymph node involvement was associated with a poorer survival. Although it is unknown whether lymph node dissection is therapeutic, it offers important prognostic information and should probably be performed in patients with metastatic renal cell cancer.
Synchronous metastases are traditionally thought to be a relative contraindication to resection. The true incidence of synchronous versus metachronous metastases in renal cell cancer is unclear, although in reported series of patients who underwent resection, synchronous lesions are less frequent and are thought to indicate a worse prognosis.121,122 On the other hand, Fourquier and colleagues121 examined survival in patients with synchronous lung metastases who underwent complete resection. Although the overall survival rate was lower in patients with synchronous lesions (48% vs. 20%), this finding was not statistically significant and again appeared to offer a survival benefit relative to no resection at all. Alt and associates128 reported a 5-year cancer-specific survival of 32.5% for patients who underwent resection of multiple sites versus 12.4% for patients who did not undergo resection (P < .001). Hofmann and colleagues126 report that synchronous metastases are an adverse prognostic factor, but only five patients in this group underwent resection. These patients should be individualized, but the presence of synchronous metastasis should not be an absolute contraindication to resection because some patients do experience long-term survival.
Renal cell carcinoma has been resistant to standard chemotherapeutic agents, but in the past decade multiple targeted agents have shown efficacy in this disease. Examples of these drugs include the monoclonal antibody against vascular endothelial growth factor, bevacizumab; the small molecule inhibitors, sorafenib and sunitinib; and the mammalian target of rapamycin inhibitors, temsirolimus and everolimus. Although these developments are significant, the end points of most of these studies are progression-free survival, and the overall response rates are relatively low (0-47%).129 Although the role of metastasectomy in the setting of these newer agents is relatively unknown, as in other diseases, the response to systemic therapy may prove to be an important prognostic factor that could help select patients for whom resection is an option. This strategy is an evolving area of cancer management, and the impact of these drugs on pulmonary metastasectomy warrants study.
Head and Neck Cancer
Approximately 60,000 new cases of head and neck cancer occur each year in the United States.130 The potential for metastatic spread is dependent on the stage of the primary tumor, with the rate of lung metastasis ranging from 4.3% to 25.1%.131 Head and neck tumors and especially squamous cell cancers have a predilection for metastasizing to the lung, which is often the only site of metastasis.132 The diagnosis of lung nodules in these patients is made even more challenging by the fact that 10% to 40% of lung nodules in these patients are actually second primary lung tumors.133 Few effective systemic therapy options are available for the treatment of lung metastasis from head and neck tumors, leaving surgical resection as the most viable option. The estimated 5-year survival after pulmonary metastasectomy in these patients ranges from 21% to 59% (Table 52-7).130,131,133–136 The wide range of survival rates may be related to the heterogeneous histologic groups reported in most series.
Table 52-7
Survival of Patients Undergoing Pulmonary Metastasectomy for Head and Neck Cancer
| Year | Author | Number | 5-Year Survival (%) |
| 1992 | Finley et al.*133 | 18 | 29 |
| 1996 | Wedman et al.134 | 21 | 59 |
| 1997 | Nibu et al.131 | 32 | 32 |
| 1999 | Liu et al.130 | 83 | 50 |
| 2008 | Winter et al.136 | 80 | 20.9 |
| 2009 | Shiono et al.135 | 114 | 26.5 |
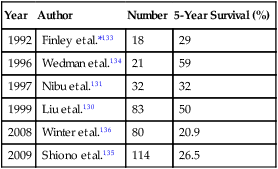
*Included only patients with squamous cell carcinoma metastasis.
Squamous cell tumors of head and neck origin have a worse prognosis than their glandular counterparts such as thyroid, adenoid cystic, and mucoepidermoid tumors.130,131,134,137 Liu and coworkers130 reported on 83 patients undergoing pulmonary metastasectomy as a result of head and neck tumors. In their series the 5-year overall survival for squamous cell tumors was 34% compared with 64% for tumors of glandular origin (P = .14). Shiono and associates135 reported that the 5-year survival of 114 patients with squamous cell carcinoma was 26.5%. They reported that oral cavity cancers, lymph node metastasis, a DFI less than 24 months, and incomplete resection were adverse prognostic factors. Neither the size, the number, nor the bilaterality of metastasis affected survival. Bilateral metastasis and recurrence of metastasis are not contraindications to resection and have not been shown to be adverse prognostic factors at this time.
Germ Cell Tumors
Germ cell tumors constitute only 1% of cancers but are the most common neoplasm in men aged 15 to 35 years. The vast majority of these tumors arise in the testis, with an annual incidence of 5 cases per 100,000.138 The survival of patients with germ cell tumors has increased dramatically during the past 35 years as a result of cisplatin-based chemotherapy.138 Monitoring of treatment and recurrence has been made possible by the use of sensitive tumor markers, including alpha fetoprotein and beta human chorionic gonadotropin.
Pulmonary metastasis at the time of presentation is common in these patients, approaching 50% in patients with retroperitoneal disease.139 Residual masses after chemotherapy are present in approximately 50% of patients and could contain viable malignancy, mature teratoma, or only fibrosis and/or necrosis.139,140 Surgical resection of these lesions is crucial to identify which of the aforementioned components is present, to predict outcome, and to determine if any further therapy is warranted. The estimated 5-year survival rate after pulmonary metastasectomy ranges from 59% to 94% (Table 52-8).138,139,141–144
Table 52-8
Survival after Pulmonary Metastasectomy for Germ Cell Tumors
| Year | Author | Number | 5-Year Survival (%) |
| 1998 | Cagini et al.139 | 141 | 77 |
| 1994 | Anyanwu et al.141 | 104 | 59 |
| 1998 | Liu et al.138 | 157 | 68 |
| 2005 | Kesler et al.145 | 134 | 42.3 |
| 2010 | Pfannschmidt et al.140 | 52 | 76 |
| 2009 | Besse et al.143 | 71 | 94 |
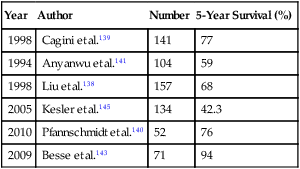
The most significant prognostic factor is the presence of viable tumor cells in the resected specimen. Over a 28-year period, Liu and associates138 reviewed the experience of 157 patients who were undergoing pulmonary metastasectomy for germ cell tumors at Memorial Sloan-Kettering Cancer Center. After resection, a viable tumor was found in 70 patients (44.5%), necrosis was found in 47 patients (29.9%), and a mature teratoma was found in 40 patients (25.4%). Survival was significantly poorer in patients with viable tumor cells (43% over 10 years) compared with patients who had necrosis/fibrosis (86% 10-year survival) or mature teratoma (84% 10-year survival).138 The presence of a mature teratoma in a specimen did not significantly worsen prognosis. Kesler and colleagues145 reported the results of salvage resection in 134 patients with either lung or mediastinal metastases. The overall survival at 5 years was 42.3%. Fizazi and associates142 report a 5-year survival of 61% among 211 patients who underwent complete resection of viable tumor cells. Other prognostic factors include older age, lung rather than mediastinal metastases, and increasing number of metastases. The inability to make an accurate determination of the presence or absence of viable tumor cells in all residual lesions after treatment for germ cell tumors mandates that all of these lesions be resected to determine overall prognosis and the potential for further therapy.
Breast Cancer
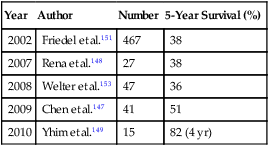
Breast cancer is the most prevalent cancer among women in the United States, with approximately 100,000 cases occurring annually.146 Approximately 15% to 25% of patients with metastatic disease will have their disease confined to the thorax. The data regarding pulmonary metastasectomy is controversial, with most studies analyzing the outcome of small groups of patients treated over several decades. Chen and associates,147 Rena and colleagues,148 and Yhim and associates149 report 5-year survival rates of 51%, 38%, and 82% (4-year), respectively (Table 52-9). Although these results are encouraging, the number of patients who are appropriate candidates for resection is extremely small relative to the number of patients with metastatic disease.149–149 McDonald and associates150 reported the Mayo Clinic experience, which revealed that only 0.4% of patients had isolated breast cancer metastasis to the lungs. The largest series reported to date is by Friedel and colleagues151 from the International Registry of Lung Metastases. They reported on 467 patients undergoing pulmonary metastasectomy for breast cancer. Complete resection of all metastasis was possible in 84% of patients. The 5-, 10-, and 15-year overall survival rates were 38%, 22%, and 20% in patients with complete resection, compared with a 5-year survival of 18% in patients with incomplete resection (P = .0009). A longer DFI and fewer lesions were associated with a longer survival in this group.151 McDonald and associates150 reported on 60 patients undergoing pulmonary metastasectomy for breast cancer and failed to show a survival benefit for surgical management. Estrogen receptor status has also been shown to be a prognostic factor.152,153 Because breast cancer also frequently progresses to extrathoracic disease and is sensitive to current systemic therapies, pulmonary metastasectomy is rarely an appropriate treatment option. Indeed, breast cancer is an example of the evolution of the role of pulmonary metastasectomy. Before the advent of effective hormonal and chemotherapy, pulmonary metastasectomy was commonly performed for breast cancer with metastases confined to the lungs. Surgery is now infrequently considered for treatment.
Miscellaneous Histologies
Experience with resection of other histologies is limited, but a few reports exist for gastric, esophageal, hepatocellular, cervical, urothelial transitional cell, and adrenocortical carcinoma. Kemp and associates154 reviewed case reports and series of 48 pulmonary resections in 43 patients with gastric cancer. Most patients had isolated lesions, and the 5-year survival was 33%. Shiono and colleagues155 reported 57 patients who underwent resection of pulmonary metastasis from esophageal cancer. The 5-year survival was 30%, and a DFI less than 12 months was a significant predictor of adverse outcomes. Yoon and associates156 reported the experience with pulmonary resection for hepatocellular carcinoma. The 5-year survival was 37%, and favorable prognostic factors were first recurrence in the lungs, a longer DFI, and a solitary lesion.156,157 Clavero and associates158 reported that pulmonary resection of gynecologic cancers had a 5-year survival of 46.8%. Again, a longer DFI was a favorable prognostic factor. Matsuguma and associates159 reported that pulmonary metastasectomy for urinary transitional cell carcinoma resulted in 5-year survival of 50% among 32 patients. The majority of patients had a single metastasis, and size of metastasis greater than 3 cm was an adverse prognostic factor. Kemp and colleagues160 reported on 26 patients undergoing 60 metastasectomies for adrenocortical carcinoma. The 5-year survival was 41%, and significant predictors of survival were DFI and T stage.
Nonsurgical Approaches to Lung Metastasis
Two therapeutic options are emerging as potentially effective alternatives to resection in selected patients with lung metastases: stereotactic body radiation therapy (SBRT) and radiofrequency ablation (RFA). SBRT is a method for delivering focused radiation to a tumor while excluding tissues not grossly involved with the tumor. It is usually delivered as high-dose hypofractionated treatment over just a few days and is therefore an attractive option for patients who have advanced disease or who may also require chemotherapy within a short interval of local therapy. Several small series now report excellent local tumor control rates with low toxicity (Table 52-10). Small, solitary, peripheral tumors have been considered the ideal target for SBRT, although some series include patients with larger tumors, multiple lesions, or tumors that include the midline and the hilum.161 More recently, prospective phase I-II trials have been reported.162,163 Rusthoven and associates162 reported a prospective phase I-II trial with dose escalation, one to three metastases, and lesions smaller than 7 cm. The primary end point was local control among patients with 6 months of radiographic follow-up. They report actuarial local control rates of 100% and 96% at 1 and 2 years, respectively. Eight of 38 patients died rapidly and were not radiologically assessed, and therefore they were not included in the local control calculations. In addition, local control was determined by recurrence in the planned target volume, whereas lesions close to that area were considered distant metastasis even if they were in the same lobe. Based on these patients and definitions, local control rates may be less than reported. Median and 2-year survival rates were 19 months and 39%, respectively. The length of follow-up after SBRT is relatively short in most series, and no randomized trials have been conducted to compare SBRT to surgical pulmonary metastasectomy. Le and colleagues163 reported a dose-escalation phase I trial from 15 to 30 Gy of 32 patients with lung tumors; 11 of those patients had metastases targeted. Only patients receiving more than 25 Gy experienced any toxicity. Rusthoven and associates162 reported dose escalation from 48 to 60 Gy with 3 of 38 patients experiencing grade 3 toxicity. They reported no grade 4 or 5 toxicity. Despite these trials, the optimal total radiation dose and dose per fraction and the need for respiratory gating during treatment are not yet fully defined.164
Table 52-10
Published Treatment Concepts and Results of Stereotactic Radiotherapy of Targets in Thorax
| Authors, Year | No. Patients | No. Targets | Average No. Targets/Patient | Median Lesion Volume (Range) | Central Tumor Dose (Gy)/No. Fractions | Isodose Treated (%) | Crude Local Control (%) | Median Follow-Up Time in Months (Range) | Acute Toxicity (Grade 1-2) (%) | Acute Toxicity (Grade 3-5) (%) |
| Blomgren et al., 1998179 | 13 | 17 | 1.3 | 48 mL (3-198) | 21-66/1-3 | 66 | 94 | 8.2 (3.5-25) | NA | NA |
| Uematsu et al., 1998180 | 45 | 66 | 1.5 | 2.5 cm (0.8-4.8) | 38-94/5-15 | 80 | 97 | 11 (3-31) | 11 | 0 |
| Wulf et al., 2001181 | 26 | 27 | 1 | 57 mL (5-277) | 45/3 | 65 | 85 | 8 (2-33) | 22 | 8 (grade 5) |
| Nakagawa et al., 2000182 | 15 | 22 | 1.5 | Chest wall 40 mL (5-126); central lung 4.5 mL (0.8-13) | *15-45/1*18-25/1 | NA | 95 | 10 (1-82) | 100 (grade 1) | 0 |
| Uematsu et al., 2001183 | 50 | 50 | 1 | 3.2 cm (0.8-5) | 38-75/5-10 | 80† | 94 | 36 (22-66) | —‡ | 0 |
| Nagata et al., 2002184 | 40 | 43 | 1.1 | 12.6 mL (0.5-39) | 40-48/4 | 100 | 94 | 18 (3-29) | 95 (grade 1) | 0 |
| Hara et al., 2002185 | 19 | 23 | 1.2 | 4 mL (1-16) | 23-36/1 | 100 | 79 | 13 (3-24) | 5 (grade 2) | 5 (grade 3) |
| Onimaru et al., 2003186 | 45 | 57 | 1.3 | 2.6 cm (0.6-6) | 48-60/8 | 100 | 88 | 18 (2-44) | 2.2 (grade 2) | 2.2 (grade 5) |
| Hof et al., 2003187 | 10 | 10 | 1 | 12 mL (5-19) | 19-26/1 | 100 | 80 | 15 (8-30) | 70 (grade 1) | 0 |
| Lee et al., 2003188 | 28 | 34 | 1.2 | 41.4 mL (4.4-230) | 30-40/3-4 | 100 | 91 | 18 (7-35) | 100 (grade 1) | 0 |
| Timmerman et al., 2003189 | 37 | 37 | 1 | 22.5 mL (1.5-157) | 24-60/3 | 100 | 84 | 15.2 (2-30) | 95 (grade 1-2) | 5 (grade 3) |
| Wulf et al., 2004190 | 61 | 71 | 1.2 | 17 mL (1-277) | 33-56/1-3 | 66-80 | 92 | 9-11 (2-61) | 7 | 0 |
| Okunieff et al., 2006161 | 49 | 125 | 2.6 | 4.7 mL (0.1-125) | 50/10 | 100 | 94 | 18.7 (4-61) | 41 (grade 1-2) | 2 (grade 3) |
| Ernst-Strecken et al., 2006191§ | 18 | 36 | 2.0 | 2.89 mL (0.15-67.94) | 8/5|| | 90 | 70 | NA | 100% (grade 1) | 1 (grade 3) |
| Le et al., 2006163§¶ | 11 | 11 | 1.0 | 17.1 mL (2-103) | 30/1|| | NA | 54/91# | NA | 12.5% (grade 2) | 4 (grade 3-5) |
| Norihisa et al., 2008192 | 34 | 43 | 1.3 | NA | 12/4-5 | 100 | 90 | 27 | 12% (grade 2) | 1 (grade 3) |
| Guckenberger et al., 2009193 | 124 | 159 | 1.3 | 8 mL (1-196) | 6-26/1-8 | 65 | 82 | 14 | 17.8% (grade 2) | 2 (grade 3) |
| Rusthoven et al., 2009162§ | 38 | 63 | 1.7 | 4.2 mL (0.2-52.3) | 20/3|| | 80-90 | 100 | 15.4 | 13.1% (grade 1-2) | 3 (grade 3) |
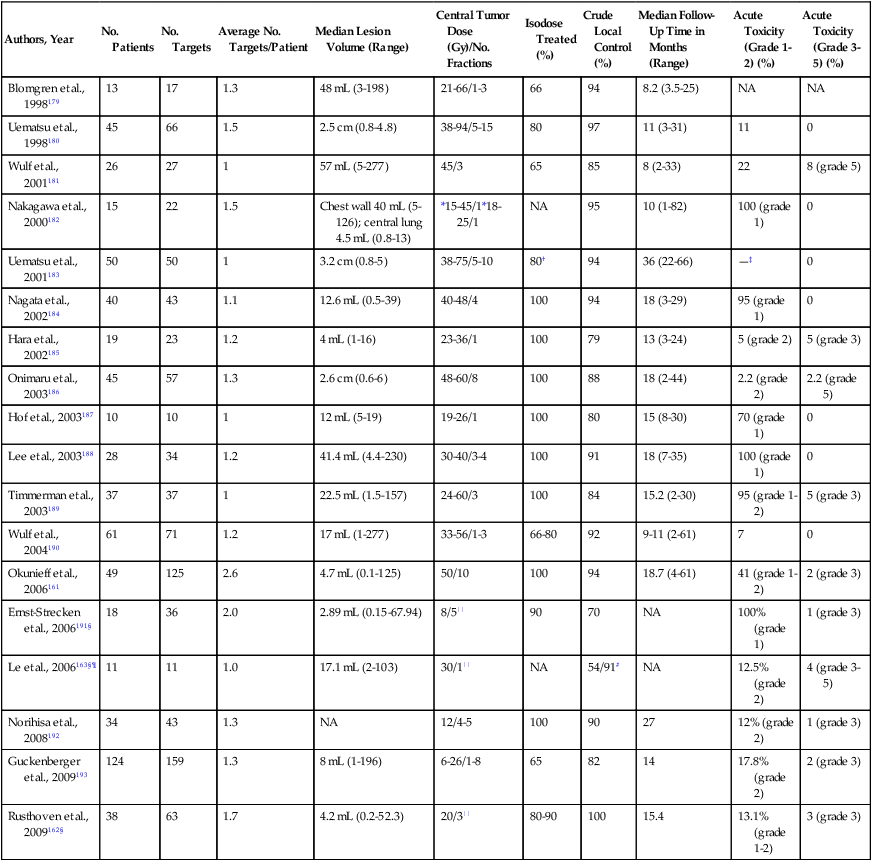
*Only tumor peripheral dose reported. All but one patient also received 20-40 Gy fractionated radiation.
†Prescribed doses were 30-60 Gy. The isodose line used varied and for this table was assumed to be 80% based on the examples published.
‡“Most” patients had grade 1. No patients experienced toxicity >grade 1.
§Prospective phase I-II stereotactic body radiotherapy trial.
||Highest dose in phase I escalation trial.
¶A total of 11/32 patients had metastatic lesions.
#Local control for <20 or >20 Gy during dose escalation.
Adapted with permission from Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol 2006;45:808–17.
RFA is a thermal energy system delivered via a percutaneous needle that is placed under imaging guidance into a tumor or via open thoracotomy in lesions difficult to resect without significant parenchymal loss. RFA causes tumor destruction by coagulation necrosis. There is even less experience with RFA than with SBRT for the treatment of pulmonary metastases. Currently most institutions limit the use of RFA to peripheral tumors that are less than 5 cm in size. However, local recurrence rates of up to 50% are reported. Serious complications including hemorrhage, pneumothorax, severe pleuritic pain, and effusions occur with RFA. Steinke and associates165 reported a 30% morbidity and 0.4% mortality in an international survey that included 493 patients. The most common complication was pneumothorax. They stress that every surgical resection requires tube thoracostomy, and thus draining the chest after RFA is not significantly different than surgery.
Long-term follow-up data in large numbers of patients are lacking, and most studies report a combination of histologies.166–169 Pennathur and associates170 reported the outcomes of 22 patients with mixed histologies. Six of these patients underwent RFA as an adjunct to open thoracotomy based on the locations of lesions. They reported a 2-year survival rate of 68%, a complete response rate of 9%, a partial response rate of 36%, and a significantly better survival with lesions less than 3 cm. Yan and colleagues171 presented one of the few reports with a single histology. They reported that for colorectal pulmonary metastasis, the local recurrence rate was 38%, and estimated 1-, 2- and 3-year survival rates were 85%, 64%, and 46%, respectively. Similar to the study by Pennathur, metastatic lesions greater than 3 cm were associated with worse survival. Both of these studies treated patients who were medically inoperable at least for complete resection. They report that in this patient group, RFA may have a role, but surgical resection is the preferred therapy if feasible.
Other Investigational Approaches to Lung Metastases
Two additional approaches have been investigated for the treatment of unresectable pulmonary metastases: isolated lung perfusion (ILP) and transpulmonary chemoembolization.172,173 ILP is an extension of the technique of isolated organ perfusion originally developed for limb perfusion of patients with malignant melanoma or sarcoma. First developed by Johnston and colleagues174 in 1983, ILP requires surgical exploration via thoracotomy or median sternotomy, isolation, and cannulation of the pulmonary artery and veins and chemotherapeutic perfusion of the isolated lung. The possibility of performing this procedure percutaneously may evolve as catheters are designed that adequately isolate the pulmonary arteries and veins.175 However, clinical trials in humans have shown that ILP can be associated with significant pulmonary toxicity. Also, all of the trials have been phase I trials. The technical complexity and potential morbidity of this approach to treatment have prevented widespread acceptance of ILP. It remains an investigational treatment modality that should only be used within the context of clinical trials.172
Transpulmonary chemoembolization is a method of delivering chemotherapy to the lung without surgery. Selective percutaneous image-guided catheterization of segmental pulmonary arteries is performed with a balloon catheter that occludes blood inflow. Chemotherapy is injected into the segmental pulmonary artery, which is then occluded by a second injection of microspheres. In an updated trial involving 52 patients, Vogl and coworkers173,176 performed transpulmonary embolization of 106 lung metastases. Tumor regression was observed in 16 patients and tumor stabilization in 11 patients. Treatment was well tolerated, with only minor complications. Although clearly an investigational approach, transpulmonary chemoembolization appears to warrant further study for patients with unresectable pulmonary metastases.