Liver Metastases
Karen L. Sherman and David M. Mahvi
• Metastatic liver disease is common in most gastrointestinal malignancies because of hepatic portal venous drainage from the gastrointestinal tract.
Diagnostic and Preoperative Evaluation
• Contrast-enhanced computed tomography is the most widely used imaging modality for detection of liver metastases, operative planning, and postoperative surveillance, with a sensitivity of 93% and a specificity of 100%.
• Percutaneous liver biopsy is rarely indicated.
• Underlying liver function and preoperative chemotherapy influence the minimum size of the future liver remnant after resection.
• Contraindications to resection of liver metastases have evolved such that tumor size and number are less important than obtaining an R0 resection. Resection may also be considered for patients with resectable extrahepatic metastases.
Management of Colorectal Liver Metastases
• Improvements in surgical technique and perioperative management have allowed for safer hepatic resections.
• Hepatic resection is the first-line treatment of liver metastases, with 5-year survival rates between 25% and 58%.
• Survival after resection of liver metastases is influenced by the following risk factors: node-positive primary cancer, disease-free interval, tumor number, tumor size, number of metastases, and preoperative carcinoembryonic antigen level.
• Preoperative systemic therapy, although helpful in identifying patients most likely to benefit from resection of liver metastases, is associated with hepatotoxicity and may affect postresection hepatic regeneration.
• Fluorouracil, oxaliplatin, and irinotecan-based perioperative chemotherapy regimens are most commonly used in the adjunctive treatment of liver metastases.
• In approximately 20% of patients with initially unresectable liver metastases, the metastases may become resectable after administration of neoadjuvant chemotherapy.
• Portal vein embolization and the two-staged hepatectomy are also strategies for improving the resectability of initially unresectable liver metastases.
• Unresectable liver metastases can be managed with systemic therapy and/or a variety of liver-directed techniques such as radiofrequency ablation, microwave ablation, cryotherapy, hepatic artery infusion, or yttrium-90 radioembolization.
Management of Noncolorectal Liver Metastases
• Surgical debulking of neuroendocrine liver metastases is widely accepted to improve symptoms and prolong survival but is associated with high recurrence rates.
• Noncolorectal nonneuroendocrine liver metastases are increasingly being resected, but outcomes are generally poorer than for colorectal liver metastases.
Introduction
Metastatic liver disease is common in most gastrointestinal malignancies, presumably because of hepatic portal venous drainage from the gastrointestinal tract. Disease isolated to the liver is a unique feature of colorectal carcinoma. Isolated liver metastases are less commonly associated with breast cancer, lung cancer, pancreatic cancer, gastric cancer, neuroendocrine malignancies, and melanoma. The unusual biology of colorectal cancer has made the liver a tempting target for local therapies such as surgical resection. When colorectal metastases are isolated in the liver, surgical resection affords an opportunity for cure, yielding 5-year survival rates from 20% to 50%.1,2 Indications for surgical resection have become broader as surgical techniques have improved. Surgical resection of liver metastases is, however, limited by tumor location and hepatic reserve. Despite a more aggressive approach to management of liver metastases, only 15% to 25% of patients are able to undergo curative surgical resection.
Diagnostic and Preoperative Evaluation
Evaluation of patients with liver metastases is initially focused on evaluating criteria for surgical resection. Unresectable extrahepatic metastatic disease is a contraindication to hepatic resection. However, patients with synchronous lung disease or an intact primary cancer may benefit from hepatectomy in highly selected cases. The number of metastases and location of metastatic disease are critical in surgical decision making, but hard and fast numbers (e.g., the number of lesions and their size and location) cannot easily be defined. More subtle factors such as tumor proximity to major vasculature and biliary structures, hepatic functional reserve, and the impact of prior chemotherapy also must be considered.3
Computed Tomography
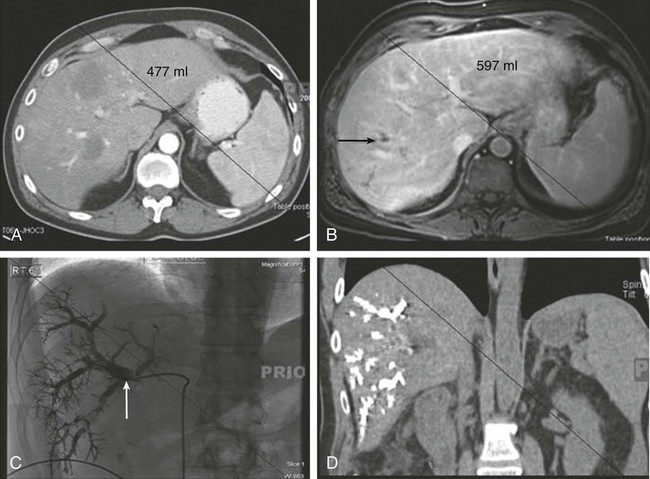
Computed tomography (CT) is the most widely used imaging modality for rapid survey of liver metastases and estimation of liver tumor burden. Contrast-enhanced CT has high sensitivity (93%) and specificity (100%) for detecting liver metastases. In most cases, portal-venous phase imaging is sufficient; however, for patients with highly vascular primary tumors such as renal cell carcinoma, neuroendocrine tumors, or melanoma, the addition of an arterial phase can improve detection of liver metastases.4 Newer developments in imaging technique allow for the creation of three-dimensional models that can more accurately estimate the areas at risk for devascularization or venous congestion after resection of liver metastases. These details allow for precise operative planning and allow for preservation of as little as 20% of liver volume provided there is normal hepatic function and adequate arterial and portal venous inflow, as well as venous and biliary outflow.3
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) provides information on the morphologic features of the tumor and the presence of satellite nodules or chronic liver disease and is frequently used for long-term follow-up.3,5 MRI is also the imaging study of choice for detection of hepatic metastases in patients with iodine allergy or renal insufficiency where radiographic contrast is contraindicated. Additionally, MRI provides better visualization of liver metastases in the presence of hepatic steatosis.4 A pooled metaanalysis comparing preoperative imaging modalities found that MRI had superior sensitivity (86%) when compared with CT (70%), fluorodeoxyglucose-positron emission tomography FDG-PET (55%), and PET-CT (51%).5
Positron Emission Tomography
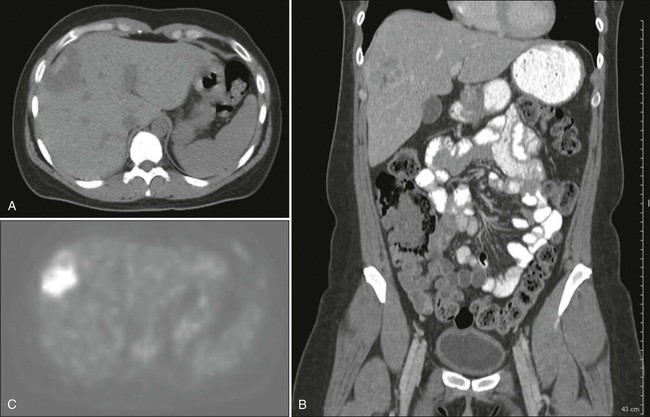
Combined PET and CT provides excellent anatomic localization of FDG-avid “hot spots” for further evaluation (Fig. 53-1). However, its use is limited by availability and in the detection of small liver metastases because of the heterogeneity of hepatic uptake of FDG. PET can detect extrahepatic metastatic disease that is difficult to detect on CT, such as nonenlarged lymph nodes with metastatic foci.4 Engledow et al.6 demonstrated that the addition of PET/CT led to changes in cancer stage (31% upstaged and 3% downstaged) and ultimate management in patients with colorectal liver metastases. Ruers and colleagues7 demonstrated that in 150 patients with colorectal liver metastases, the addition of PET/CT compared with CT alone led to a reduction in futile laparotomies from 45% to 28%. Wiering et al.,8 in a study of 150 patients randomly assigned to PET/CT or CT alone, also demonstrated a 38% reduction in futile laparotomies. Because chemotherapy decreases the metabolic rate of the tumor cells, patients with preoperative chemotherapy showed decreased FDG uptake and less efficient detection of metastatic lesions.9 The cost of preoperative evaluation, however, must be a factor in the decision to obtain additional imaging. Currently the use of PET is not standard of care, and its use should be individualized.
Intraoperative Ultrasound
Intraoperative ultrasound is the final diagnostic evaluation of the liver before resection. All patients undergoing liver resection must undergo evaluation of the liver by ultrasound before resection. Intraoperative ultrasound identifies occult liver metastases indicating incurable disease in 25% to 48% of patients.10,11 In the past, intraoperative ultrasound was used in conjunction with bimanual palpation of liver parenchyma with a reported sensitivity for detection of liver metastases of 82%.12 As laparoscopic techniques emerged, use of laparoscopic intraoperative ultrasound increased. Studies investigating the benefit of contrast-enhanced intraoperative ultrasound have mixed results.10,13
Percutaneous Biopsy
The use of percutaneous biopsy for definitive tissue diagnosis of liver metastases raises concern about the potential for tumor seeding along a percutaneous biopsy tract, particularly in patients who would otherwise be good surgical candidates. Much of the literature on this topic is focused on tract seeding after percutaneous biopsy of primary liver tumors, and very few cases of tract seeding after fine-needle aspiration have been reported. Tract seeding has been described as rare, but several small series report rates of 9% to 19% after percutaneous biopsy of liver metastases.14–17 Given the accuracy of liver imaging in the diagnosis of liver metastases, percutaneous biopsy is rarely indicated.
Evaluation of Future Liver Remnant
Evaluation of the future liver remnant (FLR) has become a more critical part of preoperative planning as the use of preoperative systemic therapy has increased. The importance of measurement of the FLR is focused on the volume of the liver remnant, but the volume is only a surrogate for estimation of the function of the FLR after resection. FLR is generally expressed in terms of the percentage of the total liver volume remaining after resection. FLR can be measured by using three-dimensional CT reconstruction and then normalized based on body surface area.18
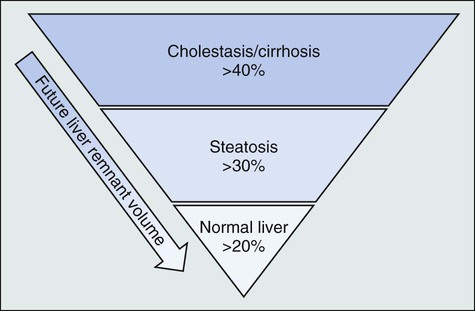
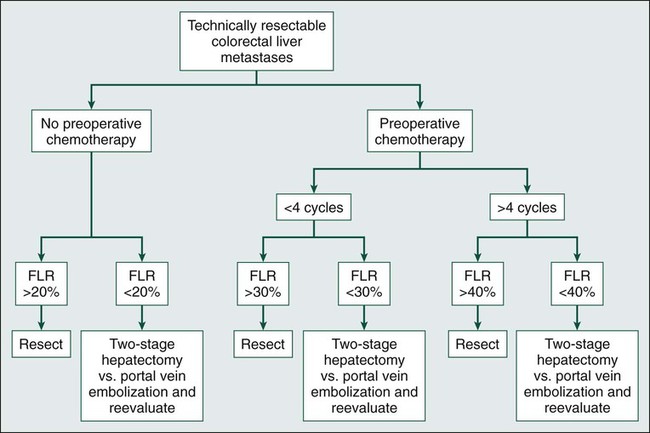
The limits for safe liver resection depend on a variety of factors, including underlying hepatic function, the receipt of preoperative chemotherapy, and FLR volume and function (Fig. 53-2). Many persons suggest that patients with underlying hepatic disease require an FLR in excess of 40%, whereas those with chemotherapy-induced steatosis require an FLR of at least 30%. Patients with normal liver function can be left with an FLR as small as 20% provided major hepatic vessels and biliary structures can be preserved.19–22 Figure 53-3 depicts an algorithm for approaching resectable liver metastases in the setting of normal hepatic function.
Patients with substantial chemotherapy-induced hepatoxicity require a future liver remnant of at least 30%.20 Narita et al.23 established limits on safe hepatic resections for colorectal liver metastases in a study of 101 patients with colorectal liver metastases who underwent preoperative chemotherapy. These investigators found that the safe FLR threshold for predicting overall morbidity, sepsis, and liver failure were 44.8%, 43.1%, and 37.7%, respectively. Chemotherapy-induced steatosis has been shown to impede liver regeneration, but the degree of its influence on postoperative morbidity and mortality has been debated. A review of 406 patients with colorectal liver metastases, 61% of whom had preoperative chemotherapy consisting primarily of either fluorouracil alone or in combination with irinotecan or oxaliplatin, demonstrated that irinotecan was associated with more steatohepatitis compared with no chemotherapy (18.9% vs. 1.9%, P < .001). Patients with steatohepatitis had an increased 90-day mortality rate (14.7% vs. 1.6%, P = .001).24
Routine hepatic biochemical tests such as bilirubin, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels are an essential component of the preoperative hepatic function assessment. A coagulation panel and platelet count also provides information on hepatic function. Hyaluronic acid concentration can provide information on the degree of hepatic fibrosis and sinusoidal endothelial damage.25 Many dynamic tests have been described to evaluate hepatic functional reserve but are largely used for patients with hepatocellular carcinoma or cirrhosis.21 The presence of underlying hepatic dysfunction suggests that the role of surgical resection may be more limited than in patients with normal hepatic reserve. These patients either are not candidates for resection, require more limited resection, or should be allowed to recover from any insult to their liver more fully before resection.
Diagnostic Laparoscopy
Despite extensive preoperative imaging, almost 20% of patients have unresectable colorectal liver metastases at the time of surgery. Roughly half have extensive intrahepatic disease that is underestimated on preoperative imaging, and the other half have occult extrahepatic metastases.11 Diagnostic laparoscopy can be used to detect occult metastases and thereby prevent futile laparotomy, but it is reserved for patients at highest risk of having occult metastases. Mann et al.26 reported a series of 200 patients with colorectal liver metastases, in which 39 of 67 (58%) patients were identified as having incurable disease on diagnostic laparoscopy, leading to a change in management in 19.5% of patients. Fong and associates27 and Jarnagin and colleagues28 suggest using a clinical risk score to estimate the risk of occult metastatic disease based on five preoperative criteria (node positivity, relapse-free interval <12 months, more than one liver metastasis, carcinoembryonic antigen level >200 ng/mL [within 1 month of surgery], and tumor size <5 cm). When more than two of these factors were present, laparoscopy detected occult metastatic disease in 42% of patients. Diagnostic laparoscopy is also helpful in patients with suspicious findings on preoperative imaging. These criteria were established before neoadjuvant treatment, and it remains unclear how previous therapy factors into these treatment algorithms.
Management of Colorectal Liver Metastases
Liver metastases develop in half of patients with colorectal cancer; unfortunately, approximately 80% of these patients have unresectable disease at the time of presentation.29,30 Untreated, the median survival for patients with unresectable liver metastasis from colorectal cancer is generally poor, with roughly a 30% 1-year survival rate and between a 0% and 5% 5-year survival rate.30,31 Several studies have demonstrated that patients with resectable liver metastases that go untreated fare slightly better, with 1-year survival rates ranging between 20% and 80% and median survival rate ranging from 10.6 to 21 months (Table 53-1). A retrospective analysis of 484 patients with untreated colorectal liver metastases identified six independent determinants of survival rate on multivariable analysis. Although the percentage of metastatic liver tumor burden was the strongest predictive factor, primary tumor grade, extrahepatic disease, mesenteric lymph node involvement, serum carcinoembryonic antigen, and age were also independent predictors of survival.31 Data on cancer-specific survival rates of patients after resection are robust and should be discussed with all patients. Factors that are unknown preoperatively are risk factors for recurrence (e.g., incomplete tumor resection), but they do not assist in preoperative decision making.
Table 53-1
Natural History of Resectable Liver Metastases in Patients Who Did Not Undergo Resection
| n | Overall Survival Rate | Median Survival (Mo) | |||
| Study | 1-Yr (%) | 3-Yr (%) | 5-Yr (%) | ||
| Wood, 1976 | |||||
| Solitary | 15 | 60 | 13.3 | 8 | 16.7* |
| Multiple Unilobar | 11 | 27 | 9.9 | 10.6* | |
| Wanebo, 1978 | 18 | 72 | 17 | 19 | |
| Wagner, 1984 | |||||
| Solitary | 39 | 20 | 21 | ||
| Multiple Unilobar | 31 | 15 | |||
| Scheele, 1990 | 10 | 14.2 | |||
| Norstein, 1997 | 10 | 80 | 0 | 0 | 18 |
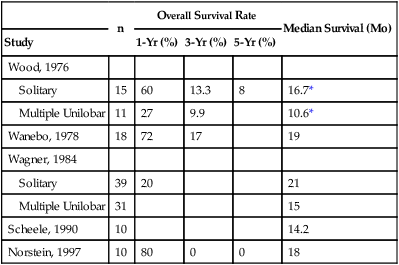
Adapted from Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg 1997;1(5):398–407.
Prediction of Survival Rate after Surgical Resection
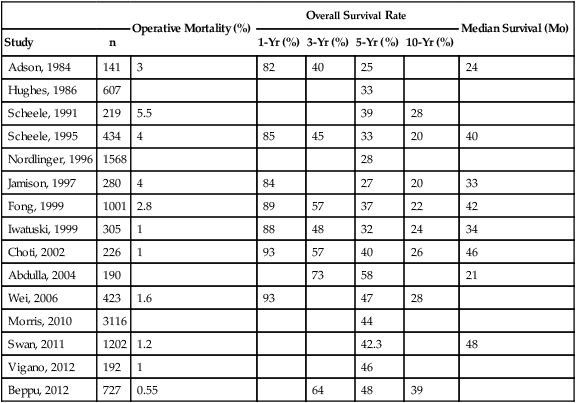
Before the era of surgical resection for liver metastases, survival rates were poor. However, as the approach shifted from palliative to curative and surgery became the mainstay for treatment of liver metastases, long-term survival and even cure became possible. As surgical management of liver metastases became more aggressive, survival rates and operative mortality improved. Ten-year survival rates reported during the past two decades have ranged between 20% and 39% after resection of liver metastases, and 5-year survival rates ranged between 25% and 58% (Table 53-2).19,27,32–37
Table 53-2
Operative Mortality and Survival Rates After Resection of Colorectal Liver Metastases
| Operative Mortality (%) | Overall Survival Rate | Median Survival (Mo) | |||||
| Study | n | 1-Yr (%) | 3-Yr (%) | 5-Yr (%) | 10-Yr (%) | ||
| Adson, 1984 | 141 | 3 | 82 | 40 | 25 | 24 | |
| Hughes, 1986 | 607 | 33 | |||||
| Scheele, 1991 | 219 | 5.5 | 39 | 28 | |||
| Scheele, 1995 | 434 | 4 | 85 | 45 | 33 | 20 | 40 |
| Nordlinger, 1996 | 1568 | 28 | |||||
| Jamison, 1997 | 280 | 4 | 84 | 27 | 20 | 33 | |
| Fong, 1999 | 1001 | 2.8 | 89 | 57 | 37 | 22 | 42 |
| Iwatuski, 1999 | 305 | 1 | 88 | 48 | 32 | 24 | 34 |
| Choti, 2002 | 226 | 1 | 93 | 57 | 40 | 26 | 46 |
| Abdulla, 2004 | 190 | 73 | 58 | 21 | |||
| Wei, 2006 | 423 | 1.6 | 93 | 47 | 28 | ||
| Morris, 2010 | 3116 | 44 | |||||
| Swan, 2011 | 1202 | 1.2 | 42.3 | 48 | |||
| Vigano, 2012 | 192 | 1 | 46 | ||||
| Beppu, 2012 | 727 | 0.55 | 64 | 48 | 39 | ||
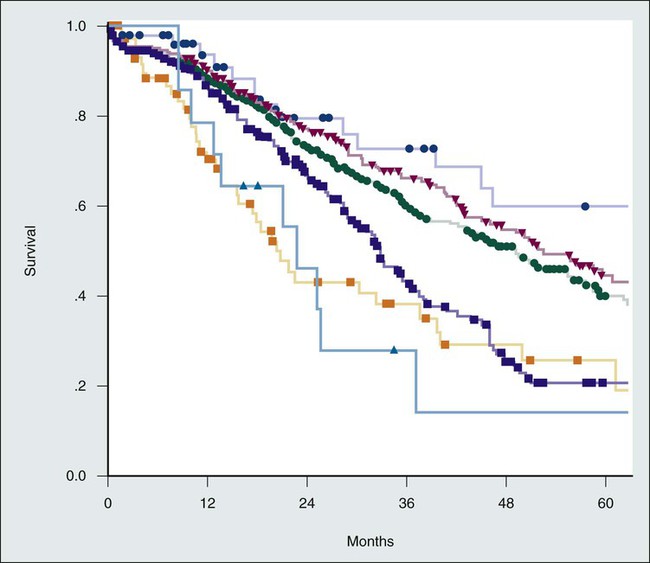
Fong et al.27 proposed seven independent predictors of poor long-term outcomes after resection of colorectal metastases: positive margins, extrahepatic disease, node-positive primary cancer, disease-free interval from primary cancer to metastases <12 months, number of hepatic tumors, and carcinoembryonic antigen level >200 ng/mL. When the latter five criteria were used in a preoperative scoring system in which one point was assigned for each criterion, the total score was highly predictive of survival (Table 53-3). The survival rate, stratified by clinical risk score, after liver resection is shown in Figure 53-4.
Table 53-3
Fong’s Clinical Risk Score for Tumor Recurrence
| Overall Survival Rate (%) | |||||||
| Score | 1-Yr | 2-Yr | 3-Yr | 4-Yr | 5-Yr | Median (Mo) | |
| Risk Factors* for tumor recurrence are node-positive primary, disease-free interval <12 mo, >1 tumor, preoperative carcinoembryonic antigen >200 ng/mL, and tumor size >5 cm | 0 | 93 | 79 | 72 | 60 | 60 | 74 |
| 1 | 91 | 76 | 66 | 54 | 44 | 51 | |
| 2 | 89 | 73 | 60 | 51 | 40 | 47 | |
| 3 | 86 | 69 | 42 | 25 | 20 | 33 | |
| 4 | 70 | 45 | 38 | 29 | 25 | 20 | |
| 5 | 71 | 45 | 27 | 14 | 14 | 22 | |

*Each risk factor is given one point to generate a Clinical Risk Score for tumor recurrence.
Adapted from Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230(3):309–18; discussion 318–21.
Management of Surgically Resectable Liver Metastasis
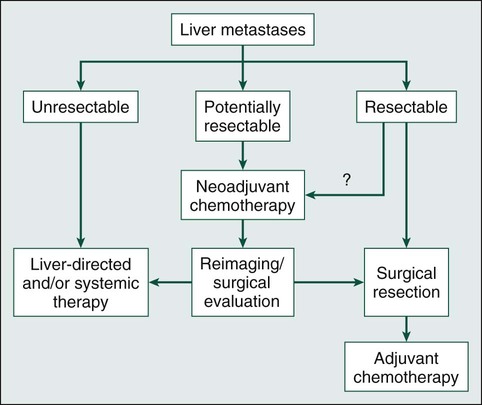
The general approach to patients with liver metastases begins with determination of surgical resectability. As shown in Figure 53-5, if the disease is resectable, no data exist that give absolute guidance to the clinician. Patients with initially resectable disease but a higher rate of recurrence after hepatic resection (Fong’s Clinical Risk Score 4 to 5) may undergo neoadjuvant chemotherapy as a means of identifying patients who experience other disease during systemic therapy and thus are less likely to benefit from surgical resection.38–41 Other potential advantages to neoadjuvant chemotherapy include earlier treatment of micrometastases and avoidance of local therapy in patients with early disease progression.42 However, neoadjuvant chemotherapy is associated with liver injury and may lead to a missed opportunity for resection if disease progression occurs with chemotherapy.43 All patients should be evaluated by an experienced liver surgeon before the initiation of systemic therapy for isolated liver disease.
Surgical Resection of Liver Metastases
Patient Selection
Surgical resection clearly improves survival in patients with colorectal liver metastases. It is the treatment of choice in patients who are deemed surgical candidates. However, fewer than 25% of patients with isolated liver metastases are amenable to resection.44 Traditional contraindications to resection of colorectal liver metastases include more than four liver metastases, extrahepatic disease, and inability to achieve 1-cm resection margins.45 More recently, patient selection has been liberalized to include all patients for whom all metastatic disease can be resected with a negative margin, leaving adequate hepatic functional reserve. An international consensus statement defines absolute criteria for unresectability, including untreatable extrahepatic disease, unfitness for surgery, or involvement of more than 70% of the liver or six segments.46 Resectability may also depend on hepatic artery, major bile duct, main portal vein, or celiac/paraaortic involvement.47 Although once considered an absolute contraindication to resection of liver metastases, select patients with limited extrahepatic disease, such as pulmonary metastases, are now being considered for resection because it may improve long-term survival.44,48 Table 53-4 summarizes the evolving indications for surgical resection of liver metastases.
Table 53-4
Evolving Indications for Surgical Resection of Liver Metastases
| Characteristics | Conventional Indications | Current Approach |
| Tumor number | <4 metastases | Any |
| Lobar involvement | Unilobar | Unilobar or bilobar |
| Tumor size | <5 cm | Any |
| Extrahepatic disease | None | Untreatable extrahepatic disease |
| Future liver remnant | Adequate remnant liver | Increase FLR with PVE |
| Resection | R0 | RFA for R1 |
| Synchronicity | Metachronous only | Either synchronous or metachronous |
| Lymph nodes | No hepatic pedicle nodal metastases | No celiac axis involvement |
| Venous involvement | No vena cava/hepatic vein invasion | Vein resection/reconstruction |
FLR, Future liver remnant; PVE, portal vein embolism; RFA, radiofrequency ablation.
Adapted from Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 2005;23(33):8490–9.
Anatomic Considerations
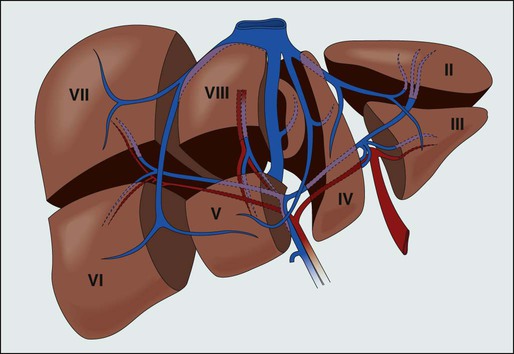
Hepatic resection requires a detailed understanding of liver anatomy. Historically, four common liver resections were performed, including formal left or right hepatic lobectomy, lateral segmentectomy, or trisegmentectomy (extended right hepatic lobectomy).49 As knowledge of functional anatomy has improved, liver resections have evolved. Couinaud defined eight liver segments as being related to branches of the portal and hepatic veins, where segment I is the caudate lobe, segments II to IV comprise the left lobe, and segments V to VIII comprise the right lobe (Fig. 53-6). Although these segments cannot be seen on the surface of the liver, intraoperative ultrasound allows for identification of segmental anatomy. In addition to individual segmentectomy, anatomic liver resection options therefore include right hepatectomy (segments V to VIII), left hepatectomy (segments II to IV), right anterior sectionectomy (segments V to VIII) right posterior sectionectomy (segments VI to VII), left medial sectionectomy (segment IV), left lateral sectionectomy (segments II and III), right trisectionectomy (also known as an extended right hepatectomy; segments IV to VIII), and left trisectionectomy (also known as an extended left hepatectomy; segments II to V and VIII).50
Current Surgical Controversies
Does the Number of Tumors Matter?
Several recent studies have suggested that resection of liver metastases should not be limited by the number of liver metastases. Although Ekberg et al.45 reported no survivors at 3 years among patients with four or more colorectal liver metastases in a small series in 1986, more recent literature has shown evidence to the contrary. In a series of 600 hepatic resections for liver metastases, no survival difference was found between patients with one to three liver metastases and patients with four or more metastases, provided R0 resection was achieved.51 Pawlik et al.52 reported a series of 159 patients with four or more colorectal liver metastases who underwent resection with a 22% 5-year disease-free survival rate and a 51% 5-year overall survival rate. Thus larger, more contemporary reports suggest that the number of liver metastases is less important than obtaining an R0 resection.
Does Tumor Size Matter?
The evidence regarding the importance of metastatic tumor size on prognosis is conflicting. Although some studies suggest that tumor size is an important prognostic factor,27,53 other studies suggest it is not important.34,54 Given mixed results, large tumor size alone should not be considered a factor in the resectability of liver metastases. However, large tumors may compromise the surgeon’s ability to achieve negative margins or leave an adequate hepatic remnant, thus contributing to unresectability.55
Is Anatomic Resection Superior to Nonanatomic Resection?
In anatomic liver resections, the lines of resection match Couinaud’s functional segments of the liver, whereas in nonanatomic resection, these lines are crossed to minimize parenchymal loss. Nonanatomic resection is increasingly used in patients with colorectal liver metastases, with results comparable to anatomic resections. In several reports of patients with colorectal liver metastases who underwent anatomic versus nonanatomic resection, no differences in positive margin rates, recurrence patterns, or overall survival were demonstrated.56,57 Thus anatomic resections are not superior, and nonanatomic resection remains an excellent strategy for minimization of liver parenchymal loss in patients with liver metastases.
Does the Surgical Margin Matter?
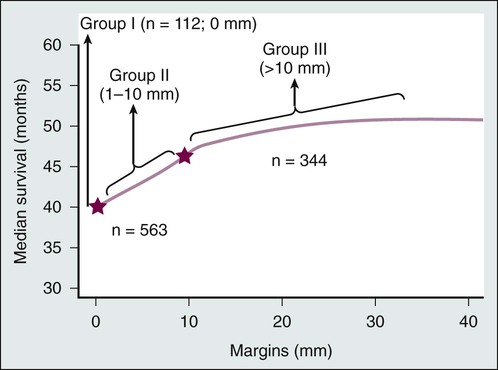
As with any oncologic resection, careful attention should be given to obtaining negative margins; however, conflicting results have been reported with regard to the width of surgical margins in the resection of liver metastases. Are et al.58 reported on the impact of margins on outcomes after resection of colorectal liver metastases in 1019 patients. They demonstrated a stepwise improvement in median survival as margins increased from involved, or 0 mm margins (group I), to 1 to 10 mm (group II) to more than 1 cm (group III) (Fig. 53-7), suggesting that although 1-cm margins are associated with improved survival, subcentimeter margins are also associated with favorable outcomes and should not preclude resection in an otherwise appropriate surgical candidate.58 Another study of 523 patients with nonpositive hepatic resection margins for colorectal liver metastases determined that subcentimeter resection margins are not associated with local recurrence.59 Muratore et al.60 confirmed that the width of the resection margin (greater or less than 1 cm) was not a prognostic factor for recurrence-free survival. For colorectal liver metastases, tumor biology plays a more important role than margin width because these metastases tend to be well circumscribed with minimal satellitosis, Glisson sheath extension, and rare micrometastases.61 When surgical margins are positive or close and further resection is impossible, edge cryotherapy can be used to treat margins.62,63
Can Simultaneous Colectomy Be Performed Safely?
The optimal timing for surgical resection in patients with synchronous colorectal liver metastases is poorly defined. Although simultaneous resection of the primary and liver metastases results is clearly more desirable from the patient’s perspective, the increased morbidity and mortality associated with a simultaneous approach may outweigh the potential benefits in select patients. In a series of 610 patients who underwent simultaneous (n = 135) or staged (n = 475) liver resection for colorectal liver metastases, Reddy et al.64 report that the extent of hepatic resection is an important factor for both severe morbidity and mortality. Patients who had simultaneous resections that included minor hepatic resection (fewer than three segments) had similar severe morbidity and mortality when compared with those who had staged resections with minor hepatic resections (severe morbidity, 14.1% vs. 12.5%; mortality, 1.0% vs. 0.5%), although overall morbidity was higher in the simultaneous group. However, for patients requiring major hepatic resections (more than three segments), those with simultaneous resections had increased overall morbidity (44% vs. 27%), severe morbidity (36.1% vs. 15.1%), and mortality (8.3% vs. 1.4%).64 Results from other studies are similar (Table 53-5). Thus for patients who require a minor hepatic resection, simultaneous resection may be reasonably considered without additional mortality risk and at least similar, if not improved, morbidity. Because the results for major hepatic resection are mixed, no definitive guideline has been established.
Table 53-5
Comparison of Simultaneous Versus Staged Resection of Primary Colorectal Cancer and Liver Metastases
| Study | n | Major Resection* (%) | Morbidity (%) | Perioperative Mortality (%) | Median Hospital Days | |
| Capussotti, 2007 | ||||||
| Staged major | 48 | 100 | 56† | 0 | 10.4† | |
| Simultaneous major | 31 | 100 | 33† | 3.20 | 13.9† | |
| Reddy, 2007 | ||||||
| Staged major | 291 | 100 | 27† | 1.4† | 7† | |
| Simultaneous major | 36 | 100 | 44† | 8.3† | 9† | |
| Staged minor | 184 | 0 | 20† | 0.5 | 6† | |
| Simultaneous minor | 99 | 0 | 33† | 1.0 | 8.5† | |
| Martin, 2009 | ||||||
| Staged | 160 | 32 | 55 | 1.0 | 18† | |
| Simultaneous | 70 | 33 | 56 | 0.0 | 10† | |
| Slupski, 2009 | ||||||
| Staged | 61 | 48 | 13 | 1.60 | 9 | |
| Simultaneous | 28 | 28 | 14 | 0 | 12 | |
| de Haas, 2010 | ||||||
| Staged minor | 173 | 0 | 25† | 0.6 | NR | |
| Simultaneous minor | 55 | 0 | 11† | 0.0 | NR | |
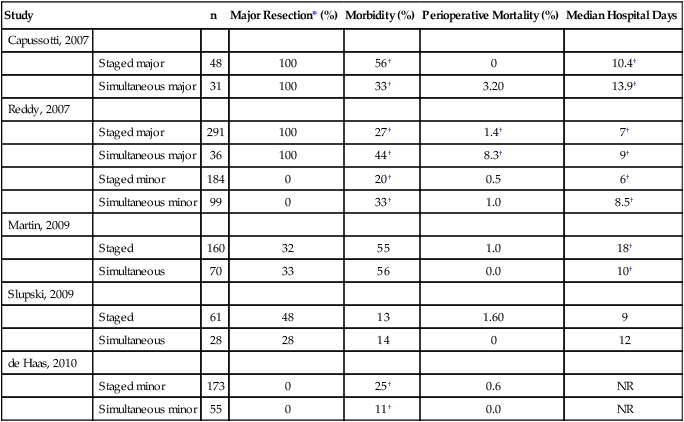
*Major resection refers to three or more segments. NR, not reported.
†Signifies a statistically significant difference with P < .05.
Is Extrahepatic Disease a Contraindication to Liver Resection?
Technically, resectable extrahepatic disease is not an absolute contraindication to resection. Concurrent resection of extrahepatic disease in highly selected patients is associated with poorer 5-year survival rates (26%) when compared with patients without extrahepatic disease (49%), with a 95% recurrence rate.65 In an international review of 1629 patients who underwent resection of colorectal liver metastases, 171 patients underwent concurrent resection of intra- and extrahepatic disease. The authors reported 16% survival at the median follow-up of 26 months. The 5-year survival rate was 26% compared with 58% for patients without extrahepatic disease, and the recurrence rate was 84%.66 However, select patients with limited extrahepatic disease, such as pulmonary metastases, are now being considered for resection because it may improve long-term survival.44,48 Concurrent resection of extrahepatic disease in selected patients allows for improved long-term survival but is associated with a high risk of recurrence.
Does New Technology Make Parenchymal Dissection Safer?
The standard technique for parenchymal dissection is based on crushing the parenchyma and selectively ligating vessels and bile ducts. This technique has been reported with either finger fracture or surgical clamps. Newer dissection techniques make use of emerging technology such as ultrasonic dissection (Cavitron Ultrasonic Surgical Aspirator [CUSA]), pressurized water jet (hydrojet), or bipolar radiofrequency energy dissection (e.g., LigaSure or Habib 4X). Ultrasonic instruments allow for parenchymal dissection without disturbing blood vessels or bile ducts, which are subsequently controlled with bipolar coagulation, surgical clips, or suture ligation. Controlled bipolar radiofrequency energy leads to coagulative tissue necrosis, which seals small bile ducts and blood vessels and allows for essentially bloodless parenchymal dissection in the absence of vascular inflow occlusion.69–69 Another common strategy is a hybrid of the crush-clamp technique, which uses a vascular stapler for sealing of vasculature and bile ducts.69
A randomized clinical trial of three hepatic parenchymal dissection techniques (crush-clamping, ultrasonic dissection, and use of a bipolar device) demonstrated similar outcomes in terms of blood loss, transfusion requirements, postoperative complications, and mortality.70 Thus the choice of dissection technique is a matter of device availability and surgeon preference. A second randomized study of hepatic transection techniques compared the following techniques: crush-clamping with use of the Pringle maneuver, ultrasonic dissection, pressurized water dissection, and radiofrequency sealer without use of the Pringle maneuver. Crush-clamping was the most cost-effective and also the most efficient technique in terms of resection time, blood loss, and transfusion requirements.71
Can Blood Loss Be Safely Controlled?
Bleeding during liver resection is most difficult to control when it emanates from the hepatic veins. Maintenance of a low central venous pressure (CVP) less than 4 during hepatic resections is associated with decreased hepatic venous bleeding and reduced blood loss.72,73 Multiple techniques can effectively lower the CVP. Limitation of intravenous fluid before resection combined with vasodilation from epidural anesthetic agents typically will result in a CVP of 4 or less. If this approach is not effective, blood can be removed immediately before resection and then transfused again postoperatively.74
Blood flow from the inflow vessels also contributes to blood loss. Vascular inflow occlusion, known as the Pringle maneuver, involves clamping the portal vein and the hepatic artery within the hepatic pedicle. Prolonged occlusion carries a risk of postischemic reperfusion injury, which may compromise postoperative hepatic function, and therefore intermitting clamping is recommended.72 Hypothermic perfusion and ischemic preconditioning have been used to prolong ischemic intolerance, although the safe time for total vascular inflow occlusion is uncertain.75,76
Does Laparoscopic Resection of Liver Metastases Compromise Surgical Principles?
Advances in laparoscopic techniques and devices for hepatic resection have led to a safe and minimally invasive approach for surgical resection of liver metastases.77 Robinson et al.78 reported a series of 37 laparoscopic liver resections performed by hepatobiliary surgeons, demonstrating that although there is a learning curve, once it is overcome, major laparoscopic liver resections can be safely performed. A study of 117 patients with colorectal liver metastases who underwent laparoscopic (n = 20) or open (n = 97) major liver resection demonstrated similar median procedure duration (P = .23), median blood loss (P = .88), rates of postoperative complications (P = 1.0), and disease-free and overall survival rates in both groups (P = .64 and P = .87, respectively).79 Thus laparoscopic resection is feasible and results in similar outcomes. This technique has not demonstrated significant advantage in postoperative morbidity or length of stay and has not been subjected to prospective evaluation.
Is There an Optimal Type and Frequency of Surveillance?
No conclusive evidence has shown that close surveillance of patients with colorectal liver metastases after treatment improves outcomes. However, as the morbidity of liver resection has improved and adjuvant strategies have become more effective, identification of recurrent disease may allow for additional surgical or medical treatment. Guidelines put forth by the National Comprehensive Cancer Network (NCCN) recommend clinical evaluation and CT of the chest, abdomen, and pelvis every 3 to 6 months for 2 years and then every 6 to 12 months for 5 years (Fig. 53-8). Carcinoembryonic antigen (CEA) levels should be obtained every 3 to 6 months for 2 years and then every 6 to 12 months for 3 to 5 years. PET imaging may be obtained in the setting of a rising CEA level to assess for occult recurrent metastatic disease. In addition, patients should undergo surveillance colonoscopy to assess for local recurrence.80
Systemic Therapy
Systemic Therapy for Resectable Disease
In the setting of resectable liver metastases, neoadjuvant chemotherapy can be administered as a means of identifying patients most likely to benefit from surgical resection. Patients without disease progression during neoadjuvant chemotherapy have been shown to have improved survival postoperatively.81 A prospective randomized analysis compared surgery alone with perioperative treatment (i.e., three cycles preoperatively and three cycles postoperatively) with FOLFOX (folinic acid, fluorouracil, and oxaliplatin) in 364 patients. Three-year progression-free survival increased by 9% (from 33.2% to 42.4%, P = .025) in patients who underwent resection. These investigators demonstrated no increase in perioperative mortality and a 9% increase in reversible postoperative complications with the addition of perioperative chemotherapy to surgical resection.38 These studies suggest that perioperative FOLFOX chemotherapy is beneficial in patients who undergo curative resection of their colorectal liver metastases.
Based on the current available evidence for patients with resectable colorectal liver metastases, the NCCN panel recommends perioperative (preoperative and postoperative) chemotherapy during a period of 6 months. Recommended options include FOLFOX or CAPOX (capecitabine and oxaliplatin) or FOLFIRI (irinotecan, fluorouracil, folinic acid) plus cetuximab in patients with wild-type K-ras and FOLFOX or CAPOX or FOLFIRI with or without bevacizumab for all other patients.80 Currently, no definitive evidence supports routine use of preoperative chemotherapy for resectable colorectal liver metastases.
Chemotherapy-induced liver injury is an important consideration for patients undergoing neoadjuvant chemotherapy. Hepatoxicity is most problematic for patients who require major hepatic resections, because they will require significant hepatic regeneration. The most commonly used chemotherapeutic agents, such as oxaliplatin, irinotecan, and fluorouracil, are all individually associated with hepatoxicity. Administration of these agents in combination can lead to synergistic hepatoxicity.25 Chemotherapy-induced hepatotoxicity can be broadly categorized into two main types: steatohepatitis and sinusoidal injury. Steatohepatitis is seen in 30% to 47% of patients treated with fluorouracil and in 12% to 25% of patients treated with irinotecan. Sinusoidal injury occurs in 19% to 78% of patients treated with oxaliplatin.20 The ideal interval between neoadjuvant chemotherapy and surgery balances the reduction in hepatotoxicity over time with the risk of interval disease progression. Chemotherapy-induced hepatotoxicity can be minimized by limiting the duration of chemotherapy to 2 to 3 months.20,80
Unresectable Metastases: Conversion to Resectability
The treatment of initially unresectable liver metastases has progressed substantially during the past 15 years with advances in the surgical approach to liver metastases and the development of effective chemotherapy protocols. Strategies aimed at improving resectability include those that increase the hepatic volume/functional reserve, such as portal vein embolization or two-stage hepatectomy, combined surgical resection with ablation, and the use of neoadjuvant chemotherapy to decrease tumor volume. Because surgical debulking of colorectal metastases has not been found to be beneficial, identifying patients in whom an R0 resection can be obtained is essential.82 Because surgical resection of liver metastases is the only treatment modality that offers a chance of long-term survival, applying these strategies to potentially convert to resectability can have a significant impact on survival.
Neoadjuvant Chemotherapy
For patients with metastases isolated to the liver that initially are deemed anatomically unresectable, neoadjuvant chemotherapy permits complete resection by shrinking tumors in 12.5% to 30% of patients.83–86 In patients with initially unresectable colorectal liver metastases who then undergo resection, the survival rate at 5 years (30% to 35%) approaches the survival rate of patients who undergo upfront hepatic resection for initially resectable disease.85,86 In a small study of 40 patients that evaluated the efficacy of neoadjuvant irinotecan and fluorouracil and folinic acid, it was demonstrated that 47.5% responded to chemotherapy and 33% underwent conversion to resectable disease after undergoing chemotherapy.87 Additionally, the administration of neoadjuvant chemotherapy can improve selection of patients for surgical resection. Several studies have demonstrated poorer outcomes in patients who experience progressive disease while undergoing neoadjuvant chemotherapy. A retrospective review of 131 patients who underwent resection of colorectal liver metastases demonstrated that the patients whose tumors progressed while undergoing neoadjuvant chemotherapy had a 5-year survival rate of 8% compared with 37% in patients who responded to chemotherapy.81 Another review of 305 patients with colorectal liver metastases who underwent neoadjuvant oxaliplatin or irinotecan-based chemotherapy showed improved 5-year survival among those with a complete response (75%) compared with those with a major response (56%) or a minor response (33%).88 Therefore poor response to neoadjuvant chemotherapy may identify patients with more aggressive tumor biology who are less likely to benefit from surgical resection.
The addition of cetuximab to proven chemotherapy for initially unresectable colorectal liver metastases has been favorable. A multicenter trial of 114 patients with colorectal liver metastases who were randomly assigned to receive cetuximab plus FOLFOX or FOLFIRI demonstrated partial or complete response in 68% of patients treated with cetuximab plus FOLFOX compared with 57% of those treated with cetuximab plus FOLFIRI. Retrospective analysis revealed that combined treatment with cetuximab and FOLFOX or FOLFIRI leads to increased resectability rates (from 32% to 60%) in patients with wild-type K-ras tumors.83 A double-blinded randomized controlled trial of 1401 patients with metastatic colorectal cancer that evaluated the benefit of adding bevacizumab to the capecitabine plus oxaliplatin or FOLFOX regimen demonstrated no survival benefit to the addition of bevacizumab.89 Studies investigating long-term outcomes in patients with colorectal cancer and initially unresectable liver metastases who underwent neoadjuvant chemotherapy demonstrate that 12.5% to 47% of patients proceeded to surgical resection with a 23% to 33% 10-year survival rate.3
Patients who undergo neoadjuvant chemotherapy before surgical resection must be evaluated for liver injury preoperatively because such injury may affect the postoperative residual function. Liver injury after neoadjuvant chemotherapy can take the form of steatosis, steatohepatitis, or sinusoidal obstruction syndrome.3 Increased postoperative complication rates were associated with patients who underwent six or more chemotherapy cycles and those who waited less than 5 weeks prior to undergoing resection.90,91 For patients with colorectal liver metastases who undergo neoadjuvant chemotherapy and in whom the expected future liver remnant is 30% or less, minimization of parenchymal loss and portal vein embolization are important strategies to minimize postoperative morbidity.3
Two-Stage Hepatectomy
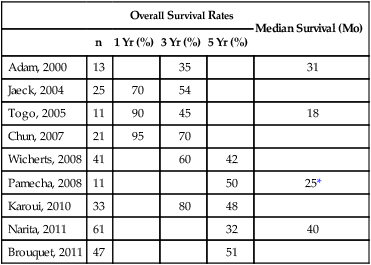
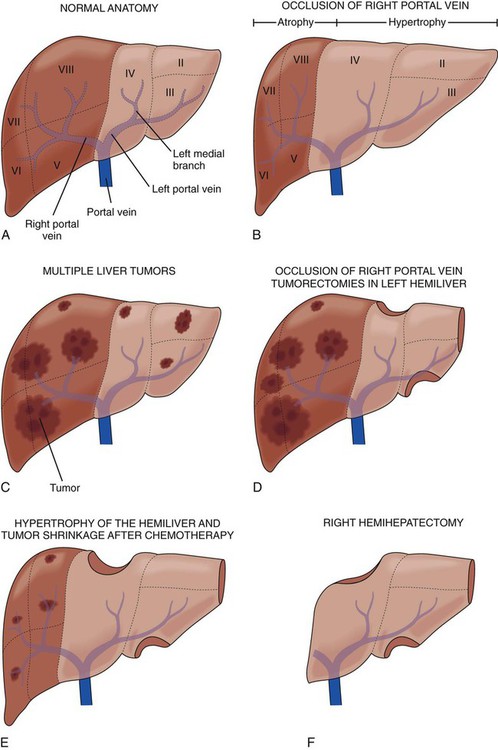
Bilobar liver metastases historically were a contraindication to surgical resection. With improvements in surgical techniques and chemotherapy regimens and other adjuncts, operative criteria have expanded to include patients with bilobar metastases. If it is impossible to resect all liver metastases in a single operation, a staged resection with or without portal vein embolism can still offer a chance of cure. The goal of this technique is to minimize the risk of postoperative liver failure resulting from an inadequate liver remnant after resection of all liver metastases. The first stage hepatectomy involves nonanatomic wedge resection of all metastases from the future liver remnant, typically the left hemiliver (Fig. 53-9).21 Intraoperative radiofrequency ablation is often used as an adjunctive strategy to minimize local recurrence. Next, a combined strategy of right portal vein occlusion with either concomitant systemic or intraarterial chemotherapy is used to decrease tumor size and increase liver volume via atrophy of the right hemiliver and hypertrophy of the left future liver remnant. Systemic therapy regimens are variable but commonly include fluorouracil-based therapy with or without oxaliplatin, irinotecan, or bevacizumab.21 The second strategy involves en-bloc resection of the remaining metastases either through a right hemihepatectomy (segments V to VIII) or through an extended right hemihepatectomy (segments IV to VIII). Several reports on two-stage hepatectomy have demonstrated survival similar to that in patients with colorectal liver metastases who are treated with a one-stage approach (Table 53-6).
Table 53-6
Two-Staged Resection—Patients Who Underwent Both Stages for Bilobar Metastases
| Overall Survival Rates | Median Survival (Mo) | ||||
| n | 1 Yr (%) | 3 Yr (%) | 5 Yr (%) | ||
| Adam, 2000 | 13 | 35 | 31 | ||
| Jaeck, 2004 | 25 | 70 | 54 | ||
| Togo, 2005 | 11 | 90 | 45 | 18 | |
| Chun, 2007 | 21 | 95 | 70 | ||
| Wicherts, 2008 | 41 | 60 | 42 | ||
| Pamecha, 2008 | 11 | 50 | 25* | ||
| Karoui, 2010 | 33 | 80 | 48 | ||
| Narita, 2011 | 61 | 32 | 40 | ||
| Brouquet, 2011 | 47 | 51 | |||
Portal Vein Embolization
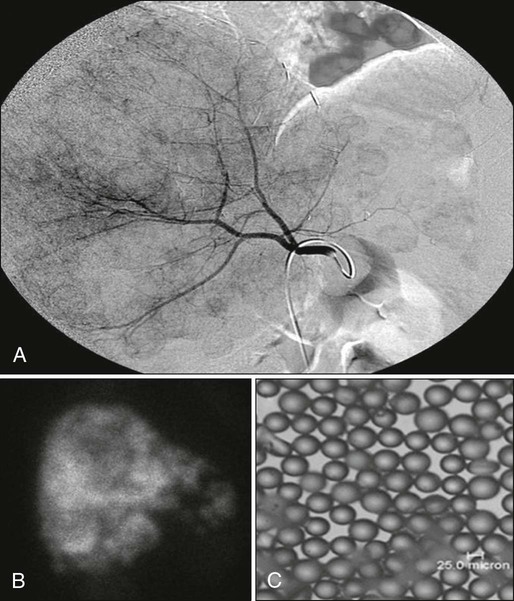
The regenerative potential of the liver can be exploited to improve the resectability of liver metastases via portal vein embolization (PVE). PVE is a minimally invasive percutaneous approach that uses ultrasonography and fluoroscopic guidance where portal branches leading to the tumor-containing segment are occluded, thereby redistributing portal flow to areas of the liver that will remain after resection. As a result of increased portal flow to these segments, the size of the future liver remnant increases and liver function improves.21,85 The PVE technique is shown in Figure 53-10.
The indications for PVE depend on liver function and adjuvant therapy. PVE is indicated when the FLR is ≤20% in patients with an otherwise normal liver. In patients who underwent neoadjuvant chemotherapy, PVE is indicated for FLR of 30% or less. Patients who have underlying hepatic dysfunction such as hepatic fibrosis or cirrhosis and an FLR of ≤40% may also benefit. Absolute contraindications to PVE include FLR above the aforementioned volume thresholds and tumor invasion of the portal vein. Relative contraindications include tumor extension to the FLR, uncorrectable coagulopathy, and biliary dilatation in the FLR, along with portal hypertension and renal failure. Repeat imaging 3 to 4 weeks after PVE is indicated to reassess liver volume and hypertrophy.85 PVE can also be used as a preoperative test of hepatic regenerative capacity.21
Management of Unresectable Liver Metastases
Chemotherapy for Unresectable Liver Metastases
Systemic chemotherapy for colorectal liver metastases includes several agents used alone or in combination. The most commonly used regimens include oxaliplatin, irinotecan, folinic acid/fluorouracil, and biological agents such as capecitabine, cetuximab, and panitumumab. The choice of therapy depends largely on the goals of therapy, the timing of adjunctive therapy, and the various toxicities of each agent. The NCCN Panel recommends the following five options for first-line chemotherapy: FOLFOX, FOLIFIRI, CAPOX, infusional 5-FU/LV (5-fluorouracil/leucovorin), and capecitabine or FOLFOXIRI (folinic acid, fluorouracil, oxaliplatin, and irinotecan). In general, infusional 5-FU is less toxic than any bolus regimen and should be given in conjunction with irinotecan or oxaliplatin biweekly.80
Radiofrequency Ablation
RFA is the most widely used modality of hepatic-directed therapy for patients with unresectable liver metastases. Metal probes are inserted into the liver parenchyma under ultrasound guidance, and alternating radiofrequency current (5000 to 9000 MHz) is applied. Heat generated by the radiofrequency current leads to coagulative tissue necrosis. RFA can be performed by using an open, laparoscopic, or percutaneous approach under image guidance, although tumors located near the inferior liver edge should not be treated percutaneously because of the potential injury of adjacent organs. RFA is well tolerated and is associated with low mortality (0% to 2%) and low serious morbidity (6% to 9%) in most studies.92 A series of 312 patients who underwent RFA in the treatment of 582 hepatic tumors (of which 80% were metastatic tumors) reported five deaths (three from portal vein thrombosis, one from bowel perforation, and another from liver failure) and a 10.6% serious complication rate (seven with liver abscess, five with pleural effusion, five with skin burns, four with hypoxemia, three with pneumothorax, two with subcapsular hematoma, and one each with acute renal insufficiency, hemoperitoneum, and needle tract seeding).93
Advances in RFA technology during the past 15 years have led to more powerful RFA probes that ablate larger areas and require shorter ablation times. In addition, new probes are associated with lower rates of margin recurrence (5.2% vs. 17.4%) and improved disease-free survival (16 months vs. 8 months).94 Despite these advances, RFA has limitations. RFA is less efficacious in large tumors because of the limited area of heat generation surrounding the probe and the lack of current conductance through charred tissue and the difficulties with three-dimensional overlapping ablation zones. RFA efficacy is also limited by proximity of major vascular structures (>3 mm) that serve as heat sinks, essentially cooling the surrounding liver parenchyma and preventing uniform tissue coagulative necrosis.97–97 The same principle also may be responsible for patchy tissue destruction near smaller vessels throughout the liver parenchyma. Upon pathological review, studies have reported nests of viable tumor cells near large vessels after tumor ablation, potentially contributing to high local recurrence rates.97,98
The American Society of Clinical Oncology convened a panel to evaluate the literature regarding RFA in the treatment of colorectal liver metastases, and it was concluded that the data were insufficient to establish practice guidelines. No prospective randomized trials comparing RFA to other modalities have been performed to date, and retrospective studies show great variability in patient selection. However, retrospective studies have demonstrated that RFA improved overall survival, particularly in patients with resectable metastases limited to the liver. The 5-year survival rates (14% to 55%) and local tumor recurrence rates (3.6% to 60%) varied widely.92 A systematic review of RFA demonstrated significantly improved overall survival after RFA versus chemotherapy alone and after RFA plus chemotherapy versus RFA alone. The median progression-free survival ranged from 6 to 13 months.99 A retrospective series of patients with unresectable disease who were treated with RFA versus chemotherapy demonstrated mildly improved survival in those treated with RFA.19 The role of RFA in patients with unresectable liver metastases and extrahepatic disease is still unclear, and currently not enough evidence exists to justify its use.92 Randomized trials evaluating the use of RFA in the treatment of liver metastases are necessary, however, based on the current body of evidence; RFA confers a survival benefit in patients with unresectable liver metastases compared with systemic therapy alone.
Microwave Ablation
MWA has several potential additional advantages when compared with RFA. High-frequency microwaves (900 to 2500 MHz) are emitted by an antenna to agitate water molecules, create friction, and therefore create heat, which leads to coagulative tissue necrosis.100 MWA actively transmits microwaves over a relatively large volume of liver as opposed to passive conduction in RFA, and thus heat is generated more quickly and over a larger area.97,101 MWA is less susceptible to the heat sink phenomenon and is not affected by charring. MWA has been used extensively in Asia and Europe for hepatocellular carcinoma, but its use in the United States and for liver metastases is still limited.100 The largest randomized study comes from Japan, in which 30 patients with potentially resectable colorectal liver metastases underwent laparoscopic MWA or surgical resection. Two-year (56% vs. 57%) and 3-year (14% vs. 23%) survival rates, as well as median survival (25 vs. 27 months), were similar (Fig. 53-11).101 In another study of 100 patients who underwent 270 ablations for liver tumors, half of which were for borderline resectable liver metastases, a 2% local recurrence rate and 37% intrahepatic recurrence rate were reported at nonablated sites at 3-years follow-up.102
Hepatic Artery Infusion
Because liver metastases derive their blood supply directly from the hepatic artery, hepatic artery infusion (HAI) was developed to target chemotherapy directly to liver metastases via a port or pump placed directly into the hepatic artery at the time of surgical resection. The ability to almost entirely extract a chemotherapeutic agent (typically floxuridine [FUDR]) during its first pass through the liver made this treatment option attractive. Although initial small reports evaluating HAI were encouraging,103 subsequent studies in patients with resectable liver disease were not encouraging. A study of 226 patients that evaluated the use of HAI with 5-FU plus leukovorin versus surgical resection alone was closed prematurely because of poorer outcomes with HAI. The group treated with HAI demonstrated poorer median survival (35 vs. 41 months) and similar median time to disease progression (14.2 months vs. 13.7 months).104
HAI was then evaluated as an adjunct to surgical resection in a randomized trial from the Intergroup. Seventy-five patients who underwent resection of colorectal liver metastases were randomly assigned to receive postoperative HAI with FUDR plus systemic fluorouracil versus surgical resection alone. Combined HAI and systemic therapy was associated with improved 4-year liver recurrence–free survival (67% vs. 43%) and overall recurrence-free survival (46% vs. 25%) but no difference in median overall survival.105 Hepatotoxicity associated with HAI-directed FUDR also limited its use after liver resection. Although several trials have shown improvement in time to disease progression with HAI, few studies have demonstrated a survival benefit. A few recent studies have evaluated combined HAI with systemic irinotecan or oxaliplatin, although to date no randomized trials have been conducted.106,107 National guidelines from NCCN suggest that HAI with or without systemic therapy be considered in selected patients at institutions with extensive experience in HAI.80 Improvements in systemic therapy have resulted in diminished interest in HAI during the past decade.
Yttrium-90 Radioembolization
Another liver-directed treatment modality for patients with unresectable liver metastases is the hepatic artery infusion of 90Y glass or resin microspheres. A form of brachytherapy, the 90Y microspheres deliver high doses of radiation to liver metastases while sparing normal liver parenchyma. Hepatic arteriograms are used to identify all extrahepatic vessels, which must be embolized before 90Y administration to prevent extrahepatic microsphere delivery. A technetium-99 macroaggregated albumin scan is performed to estimate the extent of extrahepatic spread and pulmonary shunting (Fig. 53-12). A study of 72 patients with unresectable colorectal liver metastases treated with 90Y microspheres reported acceptable toxicity (fatigue, 16%; nausea, 21%; abdominal pain, 25%; and grade 3/4 bilirubin toxicity, 12.6%), a tumor response rate of 40%, a median time to disease progression of 15.4 months, and a median survival after 90Y treatment of 14.5 months. Asymptomatic patients with normal liver function benefited most from 90Y treatment.108 A prospective randomized phase 3 trial of 44 patients with unresectable colorectal liver metastases who did not respond to chemotherapy underwent systemic administration of fluorouracil or systemic administration of fluorouracil plus 90Y radioembolization. It was demonstrated that patients who received combined systemic therapy with 90Y radioembolization had longer time to disease progression (2.1 months vs. 4.5 months, P = .03).109 90Y radioembolization may be considered in highly selected patients who are not surgical candidates at institutions with experience in this procedure.80
Cryotherapy
Cryotherapy involves destruction of tumors with rapid freezing and thawing, with subsequent ice crystals causing cellular damage and subsequent death. A metal cooled cryoprobe is inserted under ultrasound guidance directly into the tumor, producing a sphere of ice surrounding each metastasis. Cryotherapy can lead to cracking of the hepatic parenchyma in 5% to 28% of patients. Cryotherapy, when used in conjunction with surgical resection and hepatic arterial chemotherapy in the treatment of multiple liver metastases, has been shown to improve survival.110 In a few studies reporting long-term outcomes after cryosurgery used in the management of suboptimal margins, lower local recurrence rates and improved survival were demonstrated.62,63,111 The use of cryotherapy, however, has diminished as newer techniques such as RFA have been developed.
Percutaneous Ethanol Injection
Percutaneous intratumoral injection of absolute ethanol (98%) is infrequently used in the United States. The technique involves injection of as much as 30 mL of ethanol under ultrasound guidance to induce cellular necrosis. Percutaneous ethanol injection in small hepatocellular cancers has been shown to be beneficial, but liver metastases have not responded to this technique and thus it has been abandoned.112,113
Chemotherapy after Resection
The role of systemic chemotherapy after resection of liver metastases is not nearly as well defined as the role of surgical resection. NCCN guidelines for systemic therapy for colorectal liver metastases are similar to those for patients with stage III colon cancer. The role of perioperative systemic therapy has been evaluated in two major randomized trials. First, the European Organization for Research and Treatment of Cancer trial compared perioperative FOLFOX (six cycles preoperatively and six cycles postoperatively) with surgery alone in patients who had resectable colorectal liver metastases. When restricted to eligible patients who underwent surgical resection, the patients demonstrated improved 3-year progression-free survival.38 Another multicenter study of 321 patients with resectable colorectal liver metastases randomly assigned patients to receive FOLFIRI or 5-FU/FA with or without irinotecan. No difference was demonstrated in the median disease-free survival between patients treated with irinotecan and patients not treated with irinotecan (25 months vs. 22 months, respectively).114
Treatment of Recurrent Disease
Recurrence after resection presents a significant challenge in the management of patients with liver metastases. Recurrent metastases are isolated to the liver in 35% to 40% of patients. Repeat curative intent surgery is more often performed for patients with recurrent colorectal liver metastases with outcomes comparable to primary resection of liver metastases.115 Preoperative assessment of the future liver remnant is difficult in reoperative candidates because of the altered intrahepatic vascular and biliary system after the prior resection. Three-dimensional reconstructive imaging is again helpful when evaluating the future liver remnant after a second resection.116 In a series of 1706 patients who underwent resection of liver metastases, recurrent liver metastases developed in 38% and 14% underwent repeat resection of liver metastases. Additionally, 3% underwent a third resection and 0.5% underwent a fourth liver resection with curative intent. The reported 5-year survival rates after the first, second, and third resection were 47%, 33%, and 24%, respectively.117 In another series of 705 patients who underwent resection of colorectal liver metastases, 57% recurred within 2 years and 22% underwent repeat liver resection, with a 31% 5-year survival rate compared with a 3.9% 5-year survival rate in patients managed palliatively.118 Third and even fourth resections for recurrent liver metastases have been described with similar survival to primary and secondary resections.119 Thus in selected patients with small recurrent tumor burden and adequate hepatic functional reserve, repeat liver resection can prolong survival.
Management of Noncolorectal Liver Metastases
As the morbidity and mortality of hepatic resections has improved, patients with noncolorectal liver metastases are increasingly undergoing surgical resection. Although noncolorectal liver metastases are generally more ominous and associated with poorer outcomes compared with colorectal liver metastases, several studies have demonstrated a benefit to surgical resection as part of a multimodality approach.120–124
Neuroendocrine
Liver metastases from neuroendocrine tumors constitute approximately 10% of all liver metastases and occur in 20% to 90% of patients with neuroendocrine tumors. Neuroendocrine liver metastases are well suited for aggressive surgical resection because of their poor response to chemotherapy and long tumor doubling time, which result in slow disease progression. In addition, their size and production of hormones frequently make these tumors symptomatic.125 Thus surgical debulking of neuroendocrine liver metastases is widely accepted to improve symptoms and prolong survival (61% at 5 years) but is associated with high recurrence (84% at 5 years).126 Other therapies such as RFA, octreotide, iodine-131 metaiodobenzylguanidine treatment, or targeted chemoradiation have all been shown to improve outcomes in patients who are not surgical candidates.125
Noncolorectal, Nonneuroendocrine Liver Metastases
Increasingly, patients with noncolorectal, nonneuroendocrine liver metastases are being offered surgical resection, yet outcomes are poorer when compared with colorectal or neuroendocrine liver metastases (Table 53-7). The approach to noncolorectal, nonneuroendocrine liver metastases is dependent on the primary lesion, and treatment modalities include surgery and systemic therapy regimens targeted to the primary source.
Table 53-7
Survival After Resection of Noncolorectal, Nonneuroendocrine Liver Metastases
| Overall Survival | Median (Mean) Survival (Mo) | Operative Mortality (%) | |||
| n | 3 Yr (%) | 5 Yr (%) | |||
| Harrison, 1997 | 96 | 45 | 37 | 32 | — |
| Lendoire, 2007 | 106 | 34 | 19 | — | 1.8 |
| O’Rourke, 2008 | 102 | 56 | 39 | 42 | — |
| Ercolani, 2009 | 134 | 57 | 40 | — | 3 |
| Schmelzle, 2010 | 44 | 29 | 20 | −21 | — |

Liver metastases in persons with breast cancer are common, although only 5% of patients have metastases isolated to the liver.127 Small studies of highly selected patients who underwent resection of breast cancer metastases to the liver report a median survival ranging from 26 to 58 months.123,128–131 Reported 5-year survival rates after resection of breast cancer–associated liver metastases range from 6% to 57%.123,128–131 Patients with estrogen receptor–positive disease who underwent resection of breast cancer liver metastases had improved survival compared with those with estrogen receptor–negative disease.132 Thus surgical resection may be of considerable benefit compared with systemic therapy alone and should be considered as part of a multimodality approach to patients with breast cancer metastases to the liver.
Conclusions
Liver metastases, which once were considered untreatable, are now potentially curable. New imaging technology has resulted in early detection of lesions and more accurate assessment of surgical resectability. Moreover, the indications for surgical resection have liberalized to incorporate a more aggressive approach toward treatment of persons with more advanced metastatic disease. Advancements in surgical technique during the past several decades have made hepatectomy safer with less blood loss and lower perioperative morbidity and mortality. Systemic and local chemotherapeutic delivery has led to improved outcomes when used in conjunction with surgical resection or for unresectable disease. Local ablative techniques including RFA, MWA, and cryotherapy are being used as surgical adjuncts and for unresectable disease, but the role of ablation in the management of liver metastases is still being investigated. Less frequently, patients with unresectable disease can be treated with 90Y irradiation via the hepatic artery, although this treatment has not been as effective as for primary liver malignancies. Ongoing investigations into the optimal combination and sequence of the various systemic and local adjunctive options will elucidate the ideal approach for patients with unresectable liver metastases.