Chapter 9 Liver immunology
Fundamentals of Immunology
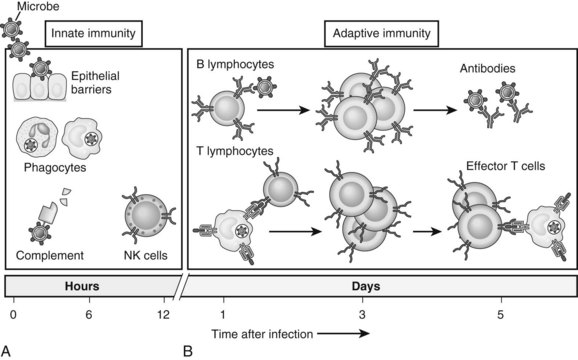
The immune system is composed of two major components: innate and adaptive immunity (Fig. 9.1; Janeway, 2001). Innate immune cells are armed with hard-wired detection systems that recognize common structures found on pathogens or changes in surface molecules of host cells that signal danger. Such pattern recognition receptors (PRRs) account for the rapid response of innate immune cells to infection or host injury. A variety of PRRs exist, of which the best characterized is the Toll-like receptor (TLR) family. In humans, 11 TLRs are currently known that recognize various structurally conserved components of bacteria and viruses in addition to endogenous intracellular ligands. Engagement of PRRs by their respective ligands causes activation of innate immune cells, such as neutrophils, monocytes, macrophages, natural killer (NK) cells, and dendritic cells (DCs). The ensuing response results in destruction of the invading pathogen or tumor via phagocytosis or release of various cytotoxic or inflammatory agents.
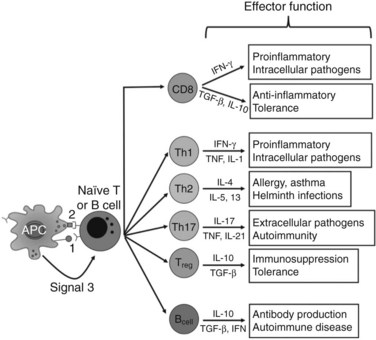
In contrast, adaptive immunity refers to the arm of the immune system that is involved in antigen-specific responses, which occur either later in a particular immune response or rapidly after repeat exposure to a particular pathogen. The adaptive immune system is essentially comprised of T and B cells that circulate within the blood, lymphatic tissues, and organs. These lymphocytes interact with various antigen-presenting cells (APCs) of the innate immune system, such as DCs and macrophages. Activation occurs in the presence of specific signals between APCs and T or B cells. These signals include 1) stable antigen presentation within the context of major histocompatibility complex (MHC) molecules MHC-I or MHC-II, 2) appropriate costimulation by APCs and the corresponding receptor on T or B cells, and 3) cytokine-mediated signals that can modulate the overall response (Fig. 9.2). Innate and adaptive immune responses provide protection against a vast array of pathogens, preserving normal cells and tissues from attack. Failure of the immune system’s ability to correctly recognize foreign or abnormal cells may facilitate the development or spread of infection or malignancy. In contrast, inability to distinguish self from foreign antigens may result in autoimmune disease.
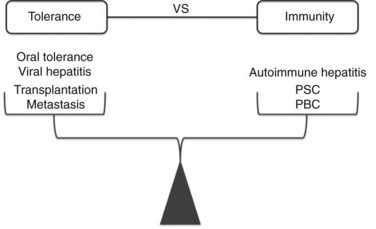
Modern clinical and experimental work has shown that the liver is particularly important in immunity (Fig. 9.3). First, the fact that the liver is one of the most common sites for metastatic disease suggests that it may have an increased propensity toward immunologic acceptance. Second, allogeneic liver transplantation can be accomplished in certain strains of mice without the need for immunosuppression, and in humans it often requires less immunosuppression compared with kidney or other solid organ transplants. Third, the liver is home to chronic viral infection in the form of hepatitis B and C. Additionally, oral ingestion or portal vein injection of foreign proteins can lead to tolerance in animal models. Conversely, the liver is the site of several autoimmune processes, including primary sclerosing cholangitis and primary biliary sclerosis. Despite the significant role that the liver plays in altering the balance between tolerance and immunity, the study of liver immunology remains in its infancy. This chapter discusses our current understanding of the function of liver immune cells and their role in disease.
Anatomic Considerations
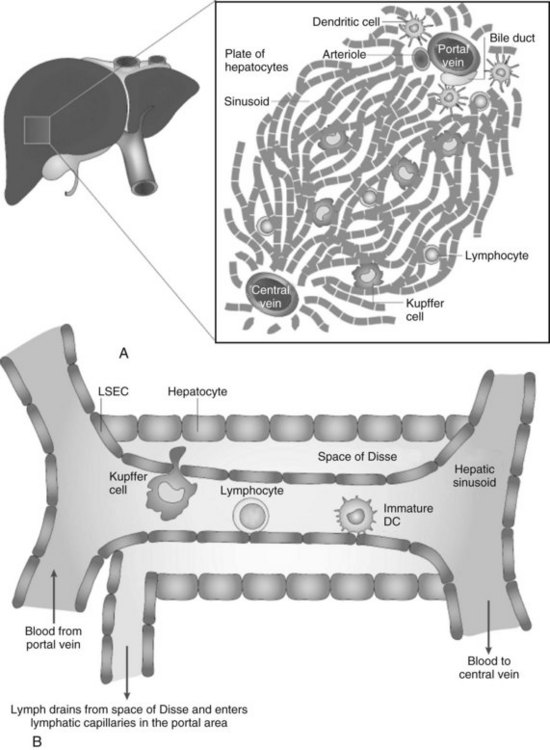
Because the vascular supply of the liver derives principally from the portal venous system draining the gut, a heavy antigen load is delivered to the liver, particularly after meals. Portal venous blood flows slowly through the vast network of hepatic sinusoids, which are discontinuously lined by fenestrated endothelium lacking a basement membrane (Fig. 9.4; see also Chapter 6). The sluggish flow of blood allows for the efficient capture of antigens by leukocytes traveling in the blood within the sinusoids and by the endothelial cells lining the sinusoids. The microscopic anatomy of the liver also favors the ability of blood-borne leukocytes to interact with hepatic parenchymal cells and resident immune cells of the liver.
Tolerance Versus Immunity
One of the most intriguing aspects of liver immunology is the propensity of antigens passing through the liver to produce tolerance rather than immunity. In experimental animal models and clinical studies of humans undergoing liver transplantation, a greater propensity for graft acceptance has been noted compared with transplantation of other solid organs. A liver transplant protects a kidney allograft transplanted simultaneously from the same donor (Creput et al, 2003). Trying to decipher the reasons for the liver’s inherent propensity for tolerance has been a particularly active area of research in the field of transplantation and cancer immunobiology.
Multiple competing theories to explain the cause of liver tolerance have been advanced. One postulate is that, as in central thymic tolerance, clonal deletion of antigen-specific T cells occurs in the liver. Another theory is that liver allografts release large amounts of soluble MHC class I molecules that potentially could function to neutralize donor-specific antibodies or cytolytic T cells. Kupffer cells (KCs) and natural killer (NK) T cells have been implicated as being important in the development of hepatic tolerance, because the depletion of either has been associated with a loss of oral tolerance. Liver DCs also have been suggested to play a role in liver tolerance, because as a group they have been shown to be less immunogenic than DCs from lymphoid organs such as the spleen (Bamboat et al, 2009). In addition, the numbers of several unusual cell types are elevated in the liver, and more recent studies have shown that multiple lineages of intrahepatic leukocytes are unique in function and phenotype compared with their counterparts from lymphoid organs. Controversy exists, however, as to whether these differences are due to de novo development, conditioning within the liver, or chemotactic signals in the liver that attract a unique population of cells. Regardless, the differences in composition and function are likely to play an important role in defining the nature of liver immunology. We devote a portion of this chapter to the major populations of liver immune cells and their role in various liver diseases.
Liver Immune Cells
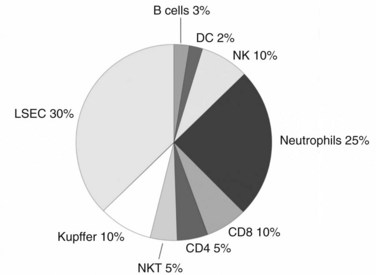
Although the liver is primarily a nonlymphoid organ, it contains numerous nonparenchymal cells (NPCs; see Chapter 6), one quarter of which are leukocytes. The composition of the intrahepatic leukocyte population is markedly different from that seen in other organs (Fig. 9.5). As in the rest of the body, however, the liver contains most of the cellular components of innate and adaptive immunity. Advances in technology have allowed for the precise delineation and isolation of cell types based on the expression of a distinct set of surface markers.
Antigen-Presenting Cells
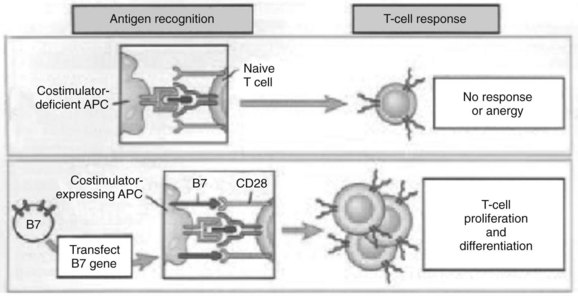
The experimental observation that antigens passing through the liver can lead to tolerance makes the understanding of antigen presentation in the liver particularly germane (Li et al, 2004). Antigen-presenting cells (APCs) play a crucial role in determining the nature of adaptive immune responses. The manner in which an APC presents any particular antigen can alter dramatically the response of antigen-specific T cells. Specifically, when antigen presentation occurs in conjunction with the appropriate costimulatory molecules, T cells proliferate and develop an immunogenic phenotypic and functional profile (Fig. 9.6). In contrast, antigens presented in the absence of costimulation lead to anergy or activation-induced T-cell death, two of the mechanisms of peripheral tolerance induction and maintenance.
Dendritic Cells
The body of literature studying the function of DCs grown in vitro from murine bone marrow progenitors or human peripheral blood mononuclear cells is vast. DCs isolated directly from the spleen, lymph nodes, or thymus also have been well studied. In contrast, because of the rarity of DCs in the liver, few studies have shown the phenotype or function of liver DCs. Initial studies focused on DC progenitors grown from liver nonparenchymal cells cultured with various cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF). These early studies showed that liver DC progenitors are relatively immature cells with poor immunostimulatory ability that can promote immunologic tolerance and prolong solid organ transplants in animal models (Lu et al, 1994, 2001; Lau & Thomson, 2003).
To gain a better understanding of liver DCs as they function in situ, recent focus has been on studying freshly isolated liver DCs. In our initial studies, we wished to increase the number of liver DCs to facilitate their isolation. Because GM-CSF had been used to grow DC progenitors in culture from liver nonparenchymal cells, and because we had previously shown that in vivo GM-CSF overexpression increases the number of myeloid DCs in the spleen (Miller et al, 2002a), we suspected that it might expand liver DCs as well. We found that overexpression of GM-CSF using an adenoviral vector led to a dramatic increase in the number of highly immunostimulatory liver DCs (Pillarisetty et al, 2003). We also showed that a large proportion of the cells recruited to the liver by GM-CSF were DC precursor cells that develop into CD11c+ DCs in culture, which suggests that DC development can occur in the liver. We used immunohistochemistry to locate both types of recruited cells. The liver DCs were concentrated around the central veins, whereas the DC precursors were distributed diffusely throughout the liver parenchyma.
Our experience with expanding liver DCs sheds light on in situ liver DC development, but we wished to understand the phenotype and function of liver DCs in normal, untreated mice. When our ability to isolate liver DCs from normal mice improved, we began to study them in depth. We found that compared with the relatively well-studied DCs from the spleen, CD11c+ liver DCs were immature and only weakly immunostimulatory (Pillarisetty et al, 2004). We also noticed that, in contrast to spleen DCs, liver DCs are heterogeneous in their expression of MHC class II and costimulatory molecules. We further separated CD11c+ liver DCs based on their expression of the myeloid marker CD11b and the lymphoid marker CD8α+, which have been commonly used to define murine DC subtypes. Myeloid (CD11b+) and lymphoid (CD8α+) liver DCs, which each comprise approximately 10% of the total population of DCs in the liver, were as able to activate T cells as were their splenic counterparts. The bulk of the remaining cells, which had low to no expression of CD11b and CD8α, were only poorly immunostimulatory. This study showed that the presence of these atypical DCs was primarily responsible for the weakly activating nature of liver DCs on the whole. More recently, we discovered that despite having multiple APCs, the CD11chi subset of DCs within the liver are required for effective presentation of soluble protein (Plitas et al, 2008). Using a transgenic mouse in which CD11chi DCs can be depleted selectively, we found that activation of antigen-specific CD8+ T cells in the liver only occurred in the presence of CD11chi DCs.
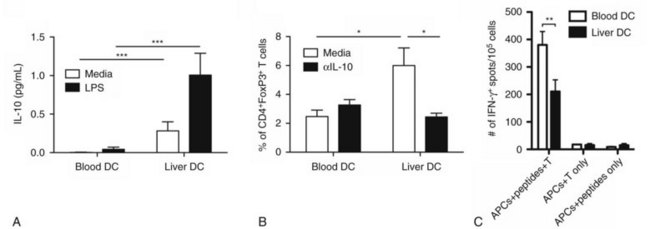
Our investigation into liver DCs has now been extended into humans. We found that freshly isolated DCs from human liver exhibit tolerogenic properties when compared with autologous blood DCs. Liver DCs are weaker stimulators of T cells and favor the production of antiinflammatory IL-10, which induces the differentiation of naïve CD4+ T cells into regulatory T cells with suppressive function (Fig. 9.7; Bamboat et al, 2009a).
Kupffer Cells
Liver macrophages, referred to as Kupffer cells (KCs), have long been believed to be the primary phagocytic cells of the liver. KCs represent the largest pool of macrophages in the body, derived from monocytic precursors in the blood. They are typically found in the hepatic sinusoids; however, they also can migrate through the space of Disse to interact with hepatocytes (see Fig. 9.4). KCs have been thought to play a major role in antigen presentation and have been implicated in portal venous tolerance. Evidence also suggests that KCs may regulate T-cell responses to antigens and induce immune tolerance in the setting of liver allografts (Sun et al, 2003).
Liver Sinusoidal Endothelial Cells
Several studies have shown that in addition to serving as the building blocks lining the sinusoids, LSECs are immune cells with the ability to capture and present antigen and activate T cells (Knolle & Limmer, 2001). As with KCs, considerable controversy surrounds the immunologic function of LSECs. In contrast to earlier work, we have shown more recently that although LSECs are highly capable of capturing various antigens in vivo and in vitro, they lack the ability to activate T cells in the absence of exogenous costimulation (Katz et al, 2004). The differences in results may derive from the use of more specific methods of cell isolation in the latter study. The finding that LSECs are not independently capable of triggering a T cell–mediated immune response does not, however, exclude the possibility that LSECs, in concert with DCs or KCs, play an important role in antigen presentation in the liver.
Effector Cells
T Cells
Liver T cells are divided into the same types of cells, as are the cells of lymphoid organs, however, evidence shows differences in the proportions of T-cell subtypes found within the liver. The interaction between CD4+ and CD8+ cells is clearly important, and more recent studies have suggested that the ratio between these two cell types—which is approximately 2 : 1 in the spleen, lymph nodes, and peripheral blood—is reversed in the liver (Crispe, 2003). Preliminary murine data from our laboratory, using more inclusive isolation techniques, have shown that the liver has a similar CD4+ to CD8+ T-cell ratio of approximately 1.5 : 1 as found in the spleen and node (Katz et al, 2005). Prior studies have shown that unconventional T cells are highly represented in the liver compared with the spleen or lymph nodes, and our data are in agreement with these findings.
γδ T cells
The γδ T-cell receptor is relatively invariant, but it can recognize multiple nonpeptide antigens without the need for MHC presentation. γδ T cells represent 10% of liver T cells, whereas they comprise only a small proportion (< 5%) of T cells in the blood or lymphoid organs. γδ T cells also are highly represented in the skin and at mucosal interfaces and are thought to play a regulatory role through their secretion of activating and modulatory cytokines as part of an early response to atypical bacterial and viral pathogens. They can influence the activation of γδ T cells and also have been shown to be crucial in tumor immunity through their early secretion of interferon γ (IFN-γ) (Gao et al, 2003). The high proportion of γδ T cells in the liver suggests that they have an important immunologic role, but further investigation is required. Similarly, both human and mouse livers are enriched with a large proportion of memory T cells. Although some investigators believe this is because the liver serves as a reservoir for activated T cells just before they die, other theories include that the liver secretes chemokines that attract CD8+ T cells.
Natural Killer T Cells
NK T cells constitute a relatively large proportion of T cells found in the liver compared with other organs. In addition, an expansion of NK T cells is seen in the liver in several models of liver injury, such as partial hepatectomy. NK T cells have been shown to play a role in the mechanisms of inflammatory diseases and clearance of infection in the liver. Depletion of NK T cells has been shown to abrogate the effects of an experimentally induced hepatitis in a mouse model, and mice lacking NK T cells are susceptible to a host of viral and bacterial infections. NK T cells are now also thought to play a part in tumor surveillance in the liver. Data from murine primary and metastatic tumor models have shown that NK T cells can mediate tumor rejection or suppression, in part because of their ability to secrete IFN-γ (Miyagawa et al, 2004).
Natural Killer Cells
NK cells have been shown to be a major component of murine and human liver lymphocytes (see Fig. 9.5) and are partially responsible for the inflammatory reactions seen in viral and autoimmune hepatitis. In addition, NK cells serve as the first line of defense against tumors in the liver. We recently discovered that bulk human liver NK cells possess weaker lytic capabilities when compared with autologous blood NK cells (Burt et al, 2009), because the liver has a greater proportion of NK cell subtypes, with weaker cytolytic function when compared with blood NK cells.
Cytokines
Cytokines (see also Chapter 10) are small signaling molecules produced by immune cells. They are proteins that typically act on nearby cells, but they also may have systemic effects. Cytokines can be roughly divided into two categories, those of innate and those of adaptive immunity (Table 9.1). Cytokines also can be distinguished based on whether they are activating or inhibitory, however, these distinctions are not always consistent. Additionally, the same cytokine can have different effects, depending on the setting and the particular cell on which it is acting. Although hundreds of cytokines have been described, we limit our discussion here to a select few that have been shown to be produced in, and have important effects on, the liver.
Table 9.1 Cytokines Involved in Innate and Adaptive Immunity
| Cytokine | Principal Source | Principal Target: Biologic Effects |
|---|---|---|
| Innate Immunity | ||
| TNF-α | Macrophages, T cells, DCs | |
IFN-β: fibroblasts
TNF, Tumor necrosis factor; DCs, dendritic cells; IL, interleukin; NK, natural killer; IFN, interferon; MHC, major histocompatability complex; Ig, immunoglobulin.
Modified from Abbas AK, 2003: Cellular and molecular immunology. Philadelphia, Saunders Elsevier.
Interleukin-6
Interleukin-6 (IL-6) is a pleiotropic cytokine that is highly expressed by the liver and has effects on innate and adaptive immunity. It is synthesized by DCs, macrophages, T cells, B cells, endothelial cells, fibroblasts, and keratinocytes. IL-6 is produced as an early response to TNF-α and is thought to amplify the inflammatory response in the liver through the induction of acute-phase protein production by hepatocytes. As part of its promotion of the early immune response, IL-6 also stimulates the differentiation of neutrophils from bone marrow precursors. Additionally, experimental models have shown that absence of IL-6 in cultures favors the differentiation of DCs; however, IL-6–knockout mice have normal numbers and types of DCs in their spleens and livers (Bleier et al, 2004). IL-6 also is known to play an important role in liver regeneration after trauma or hepatectomy.
Type I Interferons (Interferon-α and Interferon-β)
IFN-α refers to a family of structurally similar cytokines that are crucial in the immune system’s defenses against viral infection. IFN-α is secreted by immune cells, whereas IFN-β is secreted by multiple cell types, such as fibroblasts, in response to their own viral infection. IFN-α was once thought to be produced by macrophages; however, more recent evidence has shown that plasmacytoid DCs are its primary producers (Siegal et al, 1999). Type I IFNs have direct antiviral action by triggering virally infected cells to produce enzymes that interfere with viral RNA or DNA replication. They also lead to the increased expression of MHC class I molecules on the surface of virally infected cells, increasing their likelihood of being killed by cytolytic CD8+ T cells. Additionally, type I IFNs inhibit cellular proliferation. IFN-α has been used clinically in the treatment of viral hepatitis for decades.
Interferon-γ
IFN-γ is a type II IFN. It activates macrophages and plays a crucial role in bridging innate and adaptive immunity by increasing antigen presentation through the MHC class I and II pathways (Schroder et al, 2004). It is produced by T cells, B cells, NK T cells, DCs, and NK DCs. IFN-γ has many functions that are desirable for tumor immunotherapy, including a direct antiproliferative effect. Although not as crucial as the type I IFNs, the production of IFN-γ also is important in the clearance of viral infections within the liver.
Immune System in Liver Diseases
Transplantation
Transplant immunology has shed significant insight into the unique properties of the liver as an immunologic organ (see Chapter 96). Unlike other organs, the liver can be accepted across MHC barriers in animal models of transplantation (Qian et al, 1994). In addition, allograft recipients often develop systemic, donor-specific tolerance (Kamada et al, 1981). In humans, liver transplants confer protection from other organs from the same donor (Rasmussen et al, 1995), and recipients can often be weaned off immunosuppression altogether (Mazariegos et al, 1997). The comparative immune privilege of the liver highlights its role as a tolerogenic organ.
The development of mouse models of orthotopic liver transplantation have provided an opportunity to gain mechanistic insight into the regulation of immune responses in the liver (Qian et al, 1991). Of the factors that are thought contribute to hepatic tolerance (Table 9.2), microchimerism and induction of activated T-cell apoptosis have been the most intensively studied and widely accepted. Defined as the persistence of donor cells in recipients that accept their allografts, microchimerism has been postulated to be a prerequisite for organ allograft acceptance (Starzl et al, 1992, 1996). The high antigenic load derived from the donor liver coupled with persistence of donor APCs within lymphoid tissues of the recipient may provide a continuing source of allostimulation (Suzuki et al, 1988; Sriwatanawongsa et al, 1995). The persistent activation of alloreactive recipient T cells is thought to result in exhaustive deletion and induction of systemic tolerance. The role of specific donor-derived liver APCs, such as DCs and KCs, in contributing to chimerism-induced tolerance remains unclear. Although freshly isolated liver DCs have been shown to exhibit tolerogenic potential via the production of IL-10 (Bamboat et al, 2009a), others have shown that donor-derived liver DCs migrate into recipients’ circulation and prime recipient T cells to differentiate into proinflammatory subtypes that promote rejection (Bosma et al, 2009).
MHC, Major histocompatability complex; KCs, Kupffer cells; DCs, dendritic cells; NK, natural killer; LSECs, liver sinusoidal endothelial cells; APCs, antigen-presenting cells
Modified from Gershwin ME, et al, 2003: Liver immunology. Philadelphia, Hanley and Belfus, Inc.
The immunoregulatory role of KCs has also been extensively investigated. KCs have been implicated in the induction of oral tolerance and in lymphocyte apoptosis, which has been a proposed mechanism of immunosuppression in liver transplantation (Callery et al, 1989). More recently, KCs have been shown to play a crucial role in induction of antigen-specific T-cell tolerance (Breous et al, 2009) and are thought to suppress T-cell response via the production of prostaglandins (You et al, 2008). Despite evidence of the tolerogenic potential of KCs, the use of gadolinium chloride to suppress KC function has not been shown to impact survival of liver allografts in mouse models of liver transplantation. In addition, hepatocyte transplantation-induced liver inflammation has been shown to result in cytokines and chemokines associated with KCs (Krohn et al, 2009).
Activated T cells and their subsequent apoptosis within the liver are thought to be another critical component in the induction of hepatic tolerance and the acceptance of liver allografts. Donor-derived APCs deliver normal activating signals to recipient T cells in lymphoid tissues. These activated T cells subsequently migrate to the liver, secondary to inflammatory cytokines, where they encounter a large antigenic load that causes restimulation and apoptotic cell death (Huang et al, 1994; Crispe et al, 2000). The overall bias of intrahepatic T-cell responses toward tolerance and apoptosis might account for the survival of liver allografts (Crispe, 2003).
From a more clinical perspective, patients transplanted for end-stage liver disease secondary to hepatitis B or C often experience higher viral loads after transplantation secondary to the immunosuppressive regimen required to prevent rejection. Recurrent disease in the transplanted liver is inevitable and is therefore one of the most common reasons for allograft failure (Brown, 2005). Recent work using adoptive immunotherapy of donor lymphocytes provides some encouraging insight into dealing with recurrent viral disease in transplant recipients. Lymphocytes enriched in NK and NK T cells harvested from donor livers at the time of explanation were primed ex vivo for 2 days prior to reinjection into allograft recipients. Hepatitis C viral loads were found to be significantly lower at 1 month posttransplantation in patients treated with adoptive therapy versus noninjected controls (Ohira et al, 2009). Using human-hepatocyte chimeric mice, the authors showed that adoptive transfer of activated donor lymphocytes conferred protection via the production of IFN-γ. This makes sense, as the liver is enriched in cells capable of producing IFN-γ, such as NK, NK T, and CD8+ T cells. Other groups have also shown that antigen-specific CD8+ T cells are thought to be important in preventing recurrence of hepatitis in transplant recipients via the production of IFN-γ (Jo et al, 2009).
Hepatitis
The viruses hepatitis B (HBV) and hepatitis C (HCV) are noncytopathic and hepatotropic, and they cause acute and chronic liver disease (see Chapter 64). The worldwide burden of disease is immense, with an estimated 500 million people infected with either of these two viruses (World Health Organization, http://www.who.int/mediacentre/factsheets). The cost to society is magnified when the increased risk of developing cirrhosis and liver cancer in those infected with HBV and HCV are taken into account. Immune-mediated destruction of liver parenchymal cells is a common final pathway in viral hepatitis. The presence of large numbers of KCs and DCs in the liver, which produce TNF-α in response to viral antigens, leads to high local levels of inflammatory cytokines and untoward host cell damage. Similarly, T cells, NK T cells, and NK cells in the liver produce IFN-γ, which also serves to amplify the local immune response by activating KCs and DCs. Consistent with this, chronic active hepatitis is morphologically identified by piecemeal necrosis associated with a predominantly mononuclear cell infiltrate (Ferrari et al, 1999). Therefore to achieve successful viral control and limit collateral damage, a sustained, antigen-specific immune response is necessary (Gerlach et al, 1999; Maini et al, 2000).
The tolerogenic predisposition of the liver outlined in the transplantation section may account in part for the persistence of viral infection. A number of additional host parameters have been shown in experimental models to alter the immune response to viral hepatitis infection. Age can influence the outcome of infection, as vertical transmission to neonates generally results in persistent infection throughout life. The reason for persistent infection in younger hosts is thought to be related to neonatal tolerance and inability of the immune system to mount an effective immune response early in the course of infection, when these viruses are thought to be most vulnerable. In line with this hypothesis, chronic viral infection in animal models of hepatitis occurs more often when the initial insult fails to elicit an acute inflammatory response. In woodchucks, the absence of an acute infection following viral inoculation correlated with an indolent and chronic course of disease (Nakamura et al, 2001).
The precise mechanisms that account for persistent infection in hosts who are infected during adulthood are less clear. Immune-specific parameters that correlate with control of viral load include a coordinated helper and cytotoxic T-cell response against a number of viral antigens. Recognition of multiple viral epitopes by the host is also advantageous, as it theoretically minimizes the emergence of mutants that escape the immune system. Although significant strides have been made in understanding the immunopathogenesis of viral hepatitis over the past two decades, a cure remains elusive. Emerging research tools that will undoubtedly shed more light on the immune mechanisms underlying viral persistence and resistance to treatment include the development of chimeric mice that are reconstituted with human hepatocytes and immune cells (Manz & Di Santo, 2009). The transplantation of human hepatocytes into immunodeficient mice will allow for large-scale screening therapeutics against human hepatitis viruses. In addition, chimeric murine models will enable researchers to study the in vivo effects of human hepatotropic viruses on human immune responses to infection for the first time.
Cancer
The immune response to neoplastic cells has been shown to be a potential determinant of survival in several human malignancies including hepatocellular carcinoma (HCC) (Fu et al, 2007; Gao et al, 2007; Sasaki et al, 2008). In addition, recently published landmark studies revealed that the density, location, and functional capacity of intratumoral T cells can serve as a powerful predictor of outcome in primary colorectal cancer (Pages et al, 2005; Galon et al, 2006). The high prevalence of metastatic disease to the liver is likely due to multiple factors; however, evidence suggests that particular characteristics of the liver immune system may play an important role in this propensity. As delineated earlier, the primary mediators of innate and adaptive immunity found in the liver, DCs and T cells, are unique in their function and distribution of subtypes (Pillarisetty et al, 2004; Katz et al, 2005). In addition, freshly isolated liver DCs may promote the development of tolerance to antigens and have been found to be less immunogenic overall than their splenic and peripheral blood counterparts (Bosma et al, 2006; Bamboat et al, 2009a).
Although many investigators have attempted to manipulate the immune system for the treatment of cancer (Hunder et al, 2008), few attempts have been made to directly target intrahepatic immune cells. A report by Wagner and colleagues (2008) showed that intrahepatic T cells appear to be selectively activated in patients with colorectal liver metastases. Further evidence on the importance of the hepatic immune system in malignancy comes from recent data by our group, which reveal that 1) when compared with autologous blood, human liver specimens from patients with malignancy contain a disproportionately higher percentage of NK cells with reduced antitumor function (Burt et al, 2009), and 2) the hepatic T cell infiltrate predicts survival following surgery in patients with metastatic colorectal liver cancer (Katz et al, 2009). The hepatic immune system may therefore play an important role in explaining the liver’s propensity for harboring metastatic disease.
The primary goal of cancer immunotherapy, particularly for liver tumors, is to reverse the tendency toward tolerance to tumor antigens. This reversal has been accomplished in animal models by activating DCs, T cells, NK cells, and monocytes. We previously showed the importance of IL-12 production by DCs in activating NK cells toward protection against tumor in a mouse melanoma liver metastasis model (Miller et al, 2002b). Since then, work by others has revealed that patients with low numbers of mature DCs within metastatic liver tumors have a worse prognosis (Miyagawa et al, 2004). Despite having less lytic potential than blood NK cells, we found that human liver NK cells do have the capacity to become potent antitumor cells when activated in the presence of KCs and TLR3 ligands (Burt et al, 2009).
In addition to DCs, recent work on the role of peritumoral monocytes in patients with HCC has garnered much attention among immunologists and hepatologists alike. Activated monocytes in the peritumoral stroma of HCC have been shown to foster a state of tolerance and promote tumor progression via the expression of an immunosuppressive antigen, programmed death ligand 1 (PDL-1) (Kuang et al, 2009). In addition, tumor expression of PDL-1 was recently shown to serve as a predictor of recurrence in patients with resected HCC (Gao et al, 2009). Blocking antibodies to molecules such as PDL-1 are currently in development for human use. Modulating the peritumoral interface may be a key step in harnessing the immune system to fight hepatic malignancies.
Autoimmune Hepatitis
Autoimmune hepatitis is an idiopathic disorder that leads to cirrhosis. In contrast to the preceding sections that highlighted disease states associated with hepatic tolerance, autoimmune hepatitis is a manifestation of an overactive hepatic immune system. It has a female predominance and is characterized by elevated levels of immunoglobulin G (IgG) autoantibodies and increased responsiveness to immunosuppression (Obermayer-Straub et al, 2000). An experimental model of autoimmune hepatitis is based on the treatment of mice with concanavalin A (ConA), a plant lectin known to activate T cells in vitro. A single intravenous injection of ConA leads to severe liver damage and is associated with the activation of CD4+ T cells and the production of TNF-α and IFN-γ. Further studies showed that NK T cells are the most important subset of CD4+ T cells involved in mediating ConA hepatitis. The early activation of NK T cells also is associated with IL-4 production, which contributes to triggering autoimmune hepatitis. The importance of NK T cells in mediating autoimmune liver damage also is supported by the observation that injection of α-galactosylceramide, an activator of NK T cells through CD1d binding, leads to a similar form of injury as that seen in ConA hepatitis.
Primary Biliary Cirrhosis
Primary biliary cirrhosis is an autoimmune liver disease that leads to the destruction of intrahepatic bile ducts and subsequent cholestasis and cirrhosis (Bergasa et al, 2004; Lindor et al, 2009). Most cases of primary biliary cirrhosis (95%) are associated with antimitochondrial antibodies directed against specific enzymes located on the inner mitochondrial membrane. Antinuclear antibodies—usually associated with systemic lupus erythematosus, rheumatoid arthritis, and several other autoimmune diseases—are present in 50% of patients with primary biliary cirrhosis. Helper T cells and cytolytic T lymphocytes also are thought to be important in the pathogenesis of primary biliary cirrhosis. This is supported by the findings of lymphoid infiltration of the portal tracts and aberrant expression of MHC class II on biliary epithelial cells. Because biliary epithelial cells are the primary target of injury in primary biliary cirrhosis, their expression of MHC molecules and cytokines is likely to play an important role in the pathogenesis of this disease. In addition to increased MHC class II molecules, biliary epithelial cells from patients with primary biliary cirrhosis have elevated levels of intercellular adhesion molecule (ICAM) 1, and they attract mononuclear cells via the chemokine CX3CL-1 and overexpress the inflammatory cytokines IL-6 and TNF-α (Shimoda et al, 2009).
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) results from fibrosis of the intrahepatic and extrahepatic bile ducts (Lee & Jonas, 2007; see Chapter 41). The pathogenesis is thought to result from immune activation within the liver after bacteria gain access to the portal circulation via a diseased colon. The end result is biliary cirrhosis and a high risk of developing cholangiocarcinoma. Most cases of PSC are associated with underlying inflammatory bowel disease, specifically ulcerative colitis. In contrast to most autoimmune diseases, which predominantly affect women, PSC affects men twice as often as it does women. PSC is also associated with a particular MHC class II haplotype associated with other autoimmune disorders believed to be mediated by T cells. Although specific evidence that PSC is an autoimmune disorder remains to be elucidated, the disease is associated with T cells infiltrating the portal tracts. In addition, an increase in production of circulating proinflammatory cytokines has been shown to correlate with disease progression.
Ischemia/Reperfusion Injury
Liver ischemia/reperfusion (I/R) injury is a well-recognized consequence of trauma, circulatory shock, partial hepatectomy, and liver transplantation. It contributes to the shortage of organs available for transplantation and is a major determinant of postoperative allograft dysfunction and morbidity (Clavien et al, 1992). During liver I/R, the release of endogenous molecules signal danger to the host by activating innate immune cells through their interaction with TLRs (Lotze et al, 2007). Such “danger signals,” or danger-associated molecular patterns (DAMPs), can be classified as intracellular proteins, nucleic acids, or components of the extracellular matrix that are released following host cell injury. The ensuing host immune response results in untoward collateral tissue damage that culminates in hepatocyte death and a systemic inflammatory response. The injury stems from the inability of TLRs on innate immune cells to distinguish infectious ligands from DAMPs released by autologous tissue. The need for effective approaches to manage patients with I/R-induced organ damage is highlighted by the fact that current treatment is merely supportive care.
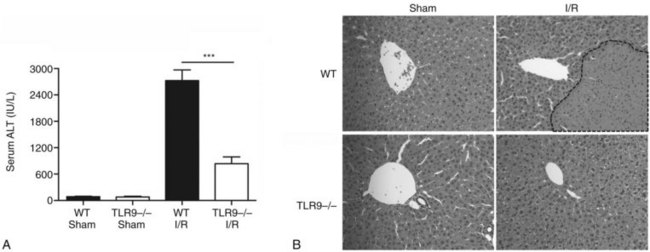
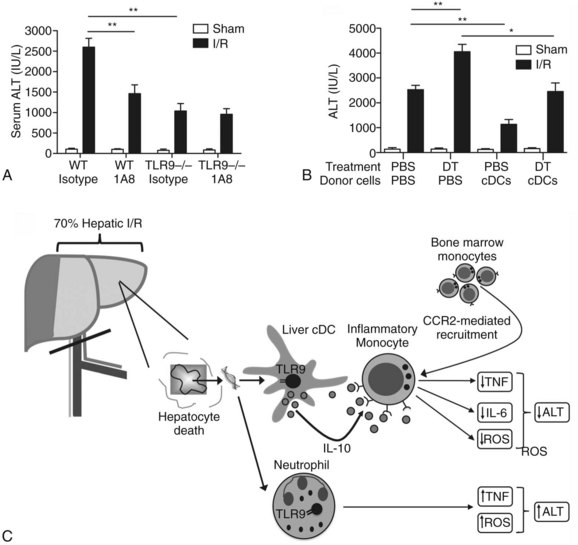
Using a murine model of segmental liver I/R, we have recently discovered that hepatic DCs and TLR-9 play critical roles in modulating the host immune response during liver I/R (Bamboat et al, 2009b). Hepatocyte DNA released during ischemia binds TLR-9 on a variety of liver immune cells that cause liver damage. Using TLR-9–knockout mice, we found that the global absence of TLR-9 signaling confers protection from liver I/R injury (Fig. 9.8). Interestingly, the immune effects of TLR-9 activation appear to be cell specific. In neutrophils, host DNA binds TLR-9 and exacerbates injury through production of inflammatory cytokines and reactive oxygen species (ROS). In contrast, DCs respond to host DNA by curtailing injury via production of antiinflammatory IL-10, which confers protection by suppressing the function of inflammatory monocytes that migrate to the liver from the bone marrow (Fig 9.9).
Partial Hepatectomy
Unlike most other mammalian organs, the liver has the capacity to regenerate after resection or traumatic injury (see Chapter 90A). This unique property not only allows for partial liver transplantation from live donors but also enables surgeons to perform major hepatic resections for malignancy. The early response to hepatectomy involves the activation of NF-κB in liver NPCs and hepatocytes, which causes additional injury through the production of ROS, TNF, and other inflammatory cytokines. Over time, IL-6 and IL-10 production increases, favoring a more antiinflammatory state that limits immune-mediated damage and promotes hepatocyte regeneration. Liver DCs have been reported to increase in number and function after partial hepatectomy in mouse models (Castellaneta et al, 2006). DCs produce more IL-10 and promote the development of antiinflammatory T cells from naïve precursors. In addition, increasing DC numbers via the administration of the growth factor fms-like tyrosine kinase 3 ligand (Flt3L) enhanced liver regeneration after partial hepatectomy. These data suggest that inherent tolerogenic properties of hepatic DCs may serve to promote hepatocyte regeneration after hepatectomy.
Like liver I/R, partial hepatectomy results in the release of a variety of danger signals that bind PRRs on innate immune cells, triggering a cascade of signaling events that modulate the rate of parenchymal regeneration. Deficiency in the intracellular signaling molecule, MyD88, which acts downstream of almost all TLRs (except TLR3), results in poorer outcome in mouse models of partial hepatectomy (Seki et al, 2005). In contrast, the absence of TLR3, which signals in a MyD88-independent manner, or blocking signaling of another PRR, the receptor for advanced glycation end products (RAGE), promotes liver regeneration and survival after massive hepatectomy (Cataldegirmen et al, 2005; Zorde-Khvalevsky et al, 2009). These data suggest that a balance exists between various TLRs and RAGE that governs the net effect of partial hepatectomy. Identification of mechanisms that limit regeneration after massive injury holds the key to expanding the limits of liver transplantation and salvaging livers and hosts overwhelmed by carcinoma and toxic insults.
Drug-Induced Liver Disease
The prevalence of liver failure as a result of drug-induced toxicity is increasing at an alarming rate and is the most common cause of acute liver failure in the developed world (Lee, 2007). The injury is characterized by toxin-induced hepatocyte death and activation of the innate immune system. This triggers a cascade of proinflammatory signals that result in further hepatocyte death and ultimately in liver failure. Through the use of mouse models of acetaminophen-induced liver injury, the roles of various components of the hepatic immune system have been well characterized. LSECs are thought to mediate liver injury by detecting hepatocyte DNA via TLR-9, resulting in production of pro–IL-1 and pro–IL-18, which are cleaved into their active forms by an intracellular protein complex called the inflammasome (Imaeda et al, 2009). Widespread inflammation ensues, resulting in additional parenchymal injury. The authors went on to show that administration of aspirin blocks inflammasome activation and the generation of active IL-1 and IL-18 and limits acetaminophen-induced liver injury.
In another set of elegant experiments using the same mouse model of liver injury, Chen and colleagues discovered how immune cells differentiate host danger signals from pathogenic ligands (Chen et al, 2009). An immune cell antigen, CD24, limits pathologic tissue damage by blocking PRR signaling from ligands released during sterile host cell damage. It fails, however, to block signaling from pathologic ligands that bind to the same PRR during infection. By understanding how immune cells distinguish between ligands released during infection and those encountered during sterile inflammation, researchers can develop approaches to curtail the progression of autoimmune diseases that are triggered by the release of host-derived danger signals.
Bacterial and Parasitic Liver Disease
Hepatic tolerance not only appears to be involved in the hyporesponsiveness to liver allografts but may also play a role in the vulnerability of the liver to various bacterial and parasitic infections (see Chapters 11, 66, 67, and 68). Pyogenic liver abscesses have increased in incidence as a result of more aggressive management of hepatobiliary diseases. The placement of stents for malignant biliary obstruction is the most common cause of hepatic abscesses. The host response to bacteria within the liver results in formation of an abscess in an effort to curtail the spread of infection. Neutrophils are recruited to the site of infection via chemoattractant signals released by the invading bacteria and activation of complement. Neutrophils then release cytokines and reactive oxygen species that promote inflammation and kill bacteria.
Helminthic infections of the liver such as schistosomiasis, echinococcus, and ascariasis have a hepatic phase in their life cycles. Animal models of schistosomiasis have helped in understanding the relationship between the hepatic immune system and the parasite. Eggs that are laid by adult worms in the portal vein get trapped within the hepatic sinusoids and trigger an immune response that results in granuloma formation. The early immune response mediated by eosinophils and neutrophils is proinflammatory. Later on, the cytokine profile within the liver becomes antiinflammatory and continues for the course of disease, ultimately resulting in liver fibrosis (Burke et al, 2009). KCs, IL-4, and IL-13 are thought to play a major role in shifting the cytokine profile during the course of schistosomiasis infection (Hayashi et al, 1999).
Bamboat ZM, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182(4):1901-1911.
Bamboat ZM, et al. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2009;51(2):621-632.
Bergasa NV, et al. Primary biliary cirrhosis: report of a focus study group. Hepatology. 2004;40(4):1013-1020.
Bleier JI, et al. Increased and long-term generation of dendritic cells with reduced function from IL-6-deficient bone marrow. J Immunol. 2004;172(12):7408-7416.
Bosma BM, et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transpl. 2006;12(3):384-393.
Bosma BM, et al. Migration of allosensitizing donor myeloid dendritic cells into recipients after liver transplantation. Liver Transpl. 2010;16(1):12-22.
Breous ES, et al. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50(2):612-621.
Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436(7053):973-978.
Burke ML, et al. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31(4):163-176.
Burt BM, et al. The lytic potential of human liver NK cells is restricted by their limited expression of inhibitory killer Ig-like receptors. J Immunol. 2009;183(3):1789-1796.
Callery MP, et al. Kupffer cell blockade inhibits induction of tolerance by the portal venous route. Transplantation. 1989;47(6):1092-1094.
Castellaneta AA, et al. Functional modification of CD11c+ liver dendritic cells during liver regeneration after partial hepatectomy in mice. Hepatology. 2006;43(4):807-816.
Cataldegirmen G, et al. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005;201(3):473-484.
Chen GY, et al. CD24 and Siglec-10 selectively repress tissue damage–induced immune responses. Science. 2009;323(5922):1722-1725.
Clavien PA, et al. Preservation and reperfusion injuries in liver allografts: an overview and synthesis of current studies. Transplantation. 1992;53(5):957-978.
Creput C, et al. Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation. Am J Transplant. 2003;3(3):348-356.
Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3(1):51-62.
Crispe IN, et al. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47-62.
Ferrari C, et al. Immunopathogenesis of hepatitis C virus infection. J Hepatol. 1999;31(Suppl 1):31-38.
Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328-2339.
Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960-1964.
Gao Q, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586-2593.
Gao Q, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971-979.
Gao Y, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198(3):433-442.
Gerlach JT, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117(4):933-941.
Hayashi N, et al. Kupffer cells from Schistosoma mansoni-infected mice participate in the prompt type 2 differentiation of hepatic T cells in response to worm antigens. J Immunol. 1999;163(12):6702-6711.
Huang L, et al. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1(9):741-749.
Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698-2703.
Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119(2):305-314.
Janeway CAJr. How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci U S A. 2001;98(13):7461-7468.
Jo J, et al. Analysis of CD8+ T-cell–mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology. 2009;136(4):1391-1401.
Kamada N, et al. Reversal of transplantation immunity by liver grafting. Nature. 1981;292(5826):840-842.
Katz SC, et al. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173(1):230-235.
Katz SC, et al. Conventional liver CD4 T cells are functionally distinct and suppressed by environmental factors. Hepatology. 2005;42(2):293-300.
Katz SC, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16(9):2524-2530.
Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22(8):432-437.
Krohn N, et al. Hepatocyte transplantation-induced liver inflammation is driven by cytokines-chemokines associated with neutrophils and Kupffer cells. Gastroenterology. 2009;136(5):1806-1817.
Kuang DM, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327-1337.
Lau AH, Thomson AW. Dendritic cells and immune regulation in the liver. Gut. 2003;52(2):307-314.
Lee CK, Jonas MM. Pediatric hepatobiliary disease. Curr Opin Gastroenterol. 2007;23(3):306-309.
Lee WM. Acetaminophen toxicity: changing perceptions on a social/medical issue. Hepatology. 2007;46(4):966-970.
Li W, et al. Role of the liver in peripheral tolerance: induction through oral antigen feeding. Am J Transplant. 2004;4(10):1574-1582.
Lindor KD, et al. Primary biliary cirrhosis. Hepatology. 2009;50(1):291-308.
Lotze MT, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60-81.
Lu L, et al. A novel subset of dendritic cells propagated from the liver promotes differentiation of T regulatory cells and enhances allograft survival. Transplant Proc. 2001;33(1-2):229.
Lu L, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179(6):1823-1834.
Maini MK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191(8):1269-1280.
Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 2009;10(10):1039-1042.
Mazariegos GV, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63(2):243-249.
Miller G, et al. Endogenous granulocyte–macrophage colony-stimulating factor overexpression in vivo results in the long-term recruitment of a distinct dendritic cell population with enhanced immunostimulatory function. J Immunol. 2002;169(6):2875-2885.
Miller G, et al. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell–mediated tumor protection. Cancer Res. 2002;62(18):5260-5266.
Miyagawa S, et al. Prognostic significance of mature dendritic cells and factors associated with their accumulation in metastatic liver tumors from colorectal cancer. Hum Pathol. 2004;35(11):1392-1396.
Nakamura I, et al. Pathogenesis of experimental neonatal woodchuck hepatitis virus infection: chronicity as an outcome of infection is associated with a diminished acute hepatitis that is temporally deficient for the expression of interferon gamma and tumor necrosis factor-alpha messenger RNAs. Hepatology. 2001;33(2):439-447.
Obermayer-Straub P, et al. Autoimmune hepatitis. J Hepatol. 2000;32(1 Suppl):181-197.
Ohira M, et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119(11):3226-3235.
Pages F, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654-2666.
Pillarisetty VG, et al. GM-CSF expands dendritic cells and their progenitors in mouse liver. Hepatology. 2003;37(3):641-652.
Pillarisetty VG, et al. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172(2):1009-1017.
Plitas G, et al. Dendritic cells are required for effective cross-presentation in the murine liver. Hepatology. 2008;47(4):1343-1351.
Qian S, et al. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19(4):916-924.
Qian SG, et al. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52(3):562-564.
Rasmussen A, et al. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59(6):919-921.
Sasaki A, et al. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34(2):173-179.
Schroder K, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163-189.
Seki E, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. 2005;41(3):443-450.
Shimoda S, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51(2):567-575.
Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835-1837.
Sriwatanawongsa V, et al. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med. 1995;1(5):428-432.
Starzl TE, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339(8809):1579-1582.
Starzl TE, et al. Liver transplants contribute to their own success. Nat Med. 1996;2(2):163-165.
Sun Z, et al. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003;9(5):489-497.
Suzuki G, et al. Antigen-induced suppression of the proliferative response of T cell clones. J Immunol. 1988;140(5):1359-1365.
Wagner P, et al. Detection and functional analysis of tumor infiltrating T-lymphocytes (TIL) in liver metastases from colorectal cancer. Ann Surg Oncol. 2008;15(8):2310-2317.
You Q, et al. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48(3):978-990.
Zorde-Khvalevsky E, et al. Toll-like receptor 3 signaling attenuates liver regeneration. Hepatology. 2009;50(1):198-206.