Figure 18-2 The steps of tumor invasion Tumor invasion involves the loss of cell-cell adhesions (cadherins represented by green bars), alterations in cell-matrix adhesion (integrins represented by ovals), proteolysis of the extracellular matrix (blue matrix, degradation demonstrated by clearing of matrix mediated by proteinases represented by scissors), and motility involving alterations in the actin cytoskeleton (intracellular black and gray lines).
Loss of function of cell-cell adhesion molecules other than E-cadherin is associated with the ability of tumor cells to invade and metastasize. Neural cell adhesion molecule (NCAM), a member of the immunoglobulin-like cell adhesion molecule Ig-CAM family, is downregulated in several tumor types, and NCAM loss results in an increased ability of tumor cells to disseminate.
8 Other Ig-CAMs, such as DCC (deleted in colorectal carcinoma), CEACAM1 (carcinoembryonic antigen CAM1), and Mel-CAM (melanoma-CAM), also demonstrate reduced expression in specific cancer types. However, not all cell-cell adhesion molecules can be viewed as potential invasion suppressors. N-cadherin promotes motility in some cell types, and Ig-CAMs such as L1, CEA (carcinoembryonic antigen), and ALCAM (activated leukocyte CAM) are often overexpressed in advanced cancers and have functions associated with cancer progression. This complexity may be explained by signaling functions for these molecules, either direct or indirect, that are distinct from their role in cell-cell adhesion. The interrelatedness of tumor growth and tumor invasion, and limitations of experimental model systems, often does not allow a distinction between growth effects that influence the appearance of an invasive phenotype and an effect on cellular invasion per se.
The extracellular matrix (ECM) provides a scaffold for the organization of cells and spatial cues that dictate cell behavior.
9 The ECM is composed of proteins, primarily triple-helical collagens, glycoproteins such as laminins and fibronectin, and proteoglycans. The basement membrane is an organized ECM that separates polarized epithelial, endothelial, and muscle cells from the underlying tissue. Interstitial matrix provides the structure characteristic of connective tissues. The molecular composition of the ECM varies between tissues and organs and provides contextual information to cellular constituents. In addition, the ECM serves as a repository for secreted regulatory proteins and growth factors. Finally, ECM proteins themselves can be active signaling molecules, activities that frequently are only revealed after proteolysis reveals cryptic sites. Thus, the interaction of cells with ECM molecules determines their capacity for survival, growth, differentiation, and migration.
Cells adhere to ECM via integrins, a family of transmembrane glycoproteins assembled as specific combinations of 18
α and 8 β subunits.
5 Integrins bind to distinct but overlapping subsets of ECM components. During tumor progression, cancer cells tend to undergo a switch in their integrin expression pattern, downregulating the integrins that mediate adhesion and maintain a quiescent, differentiated state, and expressing integrins that promotes survival, migration, and proliferation.
10 Although there is a cell-type dependency on integrin function, in general integrins α
2β
1 and α
3β
1 are viewed as suppressors of tumor progression, whereas α
vβ
3, αβ
6, and α
6β
4 promote cellular proliferation and migration. Integrins mediate both “outside-in” and “inside-out” signaling, so that changes in cellular adhesion can alter cellular phenotype, and changes in intracellular signaling pathways can modulate cellular adhesion. A well-described and important mechanism whereby integrin-ECM interactions modulate cell function is by cooperative signaling with different growth factor receptors. Many of the cellular responses induced by activation of tyrosine kinase growth factor receptors are dependent on the cells being able to adhere to an ECM substrate in an integrin-dependent fashion. Signaling in response to ECM ligation usually activates focal adhesion kinase (FAK) and nonreceptor tyrosine kinases of the
src family.
Matrix Degradation
Disruption of basement membrane is a hallmark of malignancy. Degradative enzymes produced by the tumor cells, and by resident and infiltrating cells as a response to the tumor, contribute to matrix degradation and facilitate tumor cell invasion. Proteolytic enzymes of many classes have been implicated in tumor cell invasion, including the serine proteinases plasmin, plasminogen activator, seprase, hepsin, several kallikreins, the cysteine proteinase cathepsin-B, the aspartyl proteinase cathepsin-D, and metal-dependent proteinases of the matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase (ADAM) families. Other matrix-degrading enzymes such as heparanase, which cleaves heparin sulfate proteoglycans, and hyaluronidase cleavage of its substrate hyaluronic acid have also been causally associated with tumor progression and invasion.
Liotta and colleagues observed that metastatic potential correlates with the degradation of type IV basement membrane collagen and focused attention on the metal-dependent gelatinases.
11 These enzymes are now recognized as MMP2 and MMP9, and many of the 23 members of the MMP family of matrix-degrading metalloproteinases have been associated with tumor progression. Elevated MMP levels correlate with invasion, metastasis, and poor prognosis in many cancer types, and animal models provide evidence for a causal role for MMP activity in cancer progression.
12 The plasminogen activator/plasmin system has also been causally implicated in cancer invasion,
13 and urokinase plasminogen activator (uPA) and plasminogen activator inhibitor-1
(PAI-1) are validated prognostic and predictive markers for breast cancer.
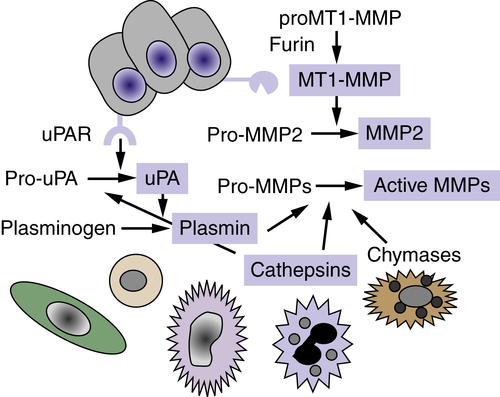
14 The regulation of matrix proteolysis is complex and can involve the concerted action of multiple proteinases and proteinase classes from both tumor cells and adjacent resident and infiltrating cells (
Figure 18-3 ). The conversion of pro-MMP2 to active MMP2 requires membrane-type MT1-MMP (MMP14), a transmembrane MMP that is activated intracellularly by the proprotein convertase family member, furin. There is evidence for a cascade of cathepsin-D–cathepsin-B–uPA–plasmin–MMP activation that results in activated enzymes capable of degrading all components of the ECM. Proteolysis is also regulated by the production of specific endogenous protease inhibitors, including the tissue inhibitors of metalloproteinases (TIMPs), serine proteinase inhibitors (serpins), and cysteine protease inhibitors (cystatins). These inhibitory activities are produced and secreted by tumor or stromal cell types, and some proteinase inhibitors are stored in high concentrations in the ECM. Proteinase activity cascades can function via proteolytic degradation of some of these proteinase inhibitors in addition to activation of other proteinases.
The original view that proteolytic enzymes function predominantly to remove physical ECM barriers has been expanded with the realization that proteolysis is a key regulator of multiple steps of tumor progression. For example, MMP substrates in the matrix or on the cell surface that modulate cellular growth, differentiation, apoptosis, angiogenesis, chemotaxis, and migration have been identified.
12 The abundant evidence for a role for MMPs in tumor progression led to the design and testing of synthetic MMP inhibitors for cancer therapy.
15 These inhibitors proved to be ineffective in clinical trials, results that have been explained by problems with inhibitor or clinical trial design and a lack of understanding of the broad range of MMP activities resulting in both cancer-promoting and cancer-inhibitory effects.
Figure 18-3 Proteolytic cascades Extracellular proteinases are made by tumor cells as well as by stromal fibroblasts and inflammatory cells. Proteolytic cascades result in the conversion of proenzymes to their active form. Enzymes in blue boxes are capable of degrading components of the extracellular matrix (ECM). In many cases, proteolytic cascades are localized to the surface of tumor cells. The urinary plasminogen activator receptor (uPAR) is expressed by many tumor cells and initiates and localizes the conversion of pro-urokinase plasminogen activator (pro-uPA) to its active form, which then converts the serum protein plasminogen into the active serine proteinase, plasmin. The membrane type 1-matrix metalloproteinase (MT1-MMP) is a transmembrane protein that is activated intracellularly by the proprotein convertase furin. MT1-MMP converts pro-MMP2 to its active form, MMP-2. Enzymes of many classes convert pro-MMPs to their active form.
Motility
Cellular locomotion occurs as the result of coordinated polymerization and depolymerization of the actin cytoskeleton to extend a pseudopod at the leading edge of the cell, followed by contraction associated with disassembly of cell-matrix adhesive contacts at the trailing edge.
16 Lamellipodial protrusions at the leading edge are nucleated by a branched actin network involving the Arp2/3 complex and its regulators, the WASp (Wiskott-Aldrich syndrome protein) family, cortactin, and the GTPase Rac. Actin contractility is regulated by myosin light-chain kinase and upstream small GTPases, in particular Rho and its effector Rho-kinase (ROCK). Single cells migrate with a spindle-shaped morphology, referred to as
mesenchymal migration, or with the less adhesive ellipsoid shape used by leukocytes and
Dictyostelium termed
amoeboid migration (
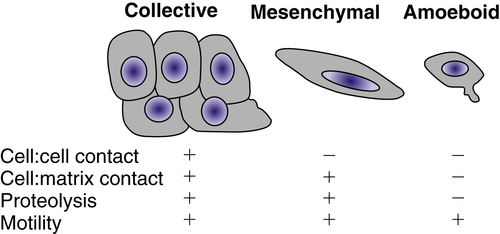
Figure 18-4 ). Collective migration can occur when the cells retain cell-cell junctions and clusters of cells move in single file through a tissue.
Tumor cells can secrete factors that stimulate motility in an autocrine fashion. Tumor cell–produced lysophospholipase D (autotaxin) stimulates motility, as does lysophosphatidic acid (LPA), which can be produced by lysophospholipase D
activity on lysophosphatidylcholine. Hepatocyte growth factor/scatter factor (HGF/SF) interacts with its receptor,
c-met, to induce chemokinetic activity of epithelial cells, resulting in an invasive phenotype. Directional motility is a chemotactic or haptotactic effect in response to a gradient of soluble or localized factors, respectively. Chemotaxis is often the result of growth factors such as IGF, and chemokines of the CCR and CXC family. Haptotaxis is characterized as a response to gradients of ECM components such as laminin-5 and fibronectin and can be modulated positively or negatively by proteolysis.
Figure 18-4 Types of cellular invasion Cells can move through matrix barriers as collectives, in which multiple cells remain attached and move together, or as single cells with mesenchymal or amoeboid characteristics. Epithelial-derived tumor cells undergoing collective migration retain cell-cell adhesions, whereas those undergoing mesenchymal or amoeboid movement have reduced or absent cadherin-mediated adhesions. Mesenchymal motility requires proteolysis and integrin-mediated cell-matrix adhesion. In the absence of proteolysis and extracellular matrix (ECM) adhesions, tumor cells can move through ECM using amoeboid movement, similar to that displayed by infiltrating leukocytes. Amoeboid movement is characterized by elevated actin cytoskeleton activity mediated by the small GTPase Rho and its regulator Rho-kinase.
Coordination of Cancer Invasion
The coordination of cell-cell and cell-matrix adhesion, matrix degradation, and cytoskeletal activity is required for cellular invasion. The type of cell migration (i.e., collective, mesenchymal, or amoeboid) is influenced by the relative levels of adhesion mediated by cadherins and integrins, proteolytic activity, and actin contractility. Modulation of any of these factors can convert one type of motility into another.
16 Invadopodia is the name that has been given to structures identified in invading cells that represent the physical convergence of the adhesive, proteolytic, and motility components of invasion (
Figure 18-5 ).
4 Invadopodia are actin-rich organelles that protrude from the plasma membrane and contact and locally degrade the ECM. Invadopodia contain adhesion molecules, including several β1 integrins and CD44, the serine proteinases seprase and dipeptidyl dipeptidase IV, and several MMP and ADAM metalloproteinases. Inside the plasma membrane, invadopodia contain actin and actin assembly molecules and multiple signaling molecules including focal adhesion kinase (FAK),
src-associated proteins such as p130Cas and Tks5/FISH (tyrosine kinase substrate 5/five SH3 domains), and the small GTPases cdc42, Arf1, and Arf6. Thus, invadopodia are implicated as key cellular structures that are used to coordinate and regulate the various components of the process of cancer invasion.
The Metastatic Cascade
Although invasion is required for metastasis, the ability to invade is not sufficient for metastasis (see
Figure 18-1). Some tumors are highly aggressive, forming secondary lesions with high frequency (e.g., small-cell carcinoma of the lung,
melanoma, pancreatic carcinoma), whereas others are rarely metastatic despite being locally invasive (e.g., basal cell carcinomas of the skin, glioblastoma multiforme). Fidler and colleagues have proposed an analogy regarding metastasis that is highly illustrative. Metastatic cells are likened to athletes participating in the decathlon. Each cell must be capable of completing every step of the metastatic cascade. If a cell cannot complete any step, it cannot go on to subsequent steps and cannot form a metastasis.
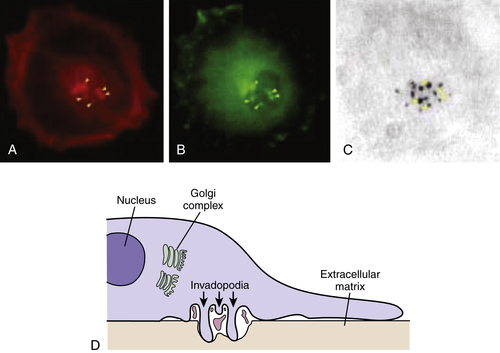
Figure 18-5 Invadopodia Confocal laser image showing triple immunofluorescence labeling of A375MM melanoma cells plated on tetramethylrhodamine isothiocyanate (TRITCJ)-conjugated gelatin. (A) Invadopodial structures marked by actin-binding phalloidin-Alexa 546. (B) Invadopodial structures marked by Alexa 633-conjugated anti-phosphotyrosine antibodies. (C) Degradation areas on the underlying Alexa 488-conjugated gelatin. Image shows colocalization between actin, phosphotyrosine, and patches of degraded extracellular matrix, fulfilling the criteria for the definition of invadopodia. (D) Schematic diagram of the invadopodial complex based on correlative light-electron microscopy reconstructions. Spatial relationships with the nucleus and the Golgi complex are shown. Invadopodial protrusions originate from profound invaginations of the ventral surface of the plasma membrane; within the area delimited by the large invagination, large fragments of gelatin can often be seen. From Ayala I, Baldassarre M, Caldieri G, et al. Invadopodia: a guided tour. Eur J Cell Biol. 2006;85:159-164, with permission.
Metastasis is primarily thought of as developing via dissemination in the bloodstream, although other routes of spread occur. Carcinoma cells tend to escape and spread initially to draining lymph nodes, becoming trapped and proliferating. The thoracic duct links the lymphatic system to the bloodstream, connecting lymphatic to hematogenous spread. Metastases can also develop by spreading across body cavities. For example, ovarian carcinoma cells most frequently establish secondary tumors by dissemination in the peritoneum while rarely forming metastases via hematogenous spread. Other routes of spread also exist but are far less common (e.g., dissemination of melanoma cells along the space between endothelium and basement membrane or perineural spread in pancreatic and prostatic carcinomas). Thus, the route of dissemination is not inherent to a definition of metastasis.
Intravasation
How tumor cells enter the bloodstream is not clearly understood. The growth of a tumor exerts a hydrostatic pressure, and studies imply that tumor-cell invasive cords follow lines of least resistance. Angiogenesis is likely to be a prerequisite for metastasis, but this has not been formally proven (see
Chapter 17). Tumor cell entry into intact blood vessels is an active process that requires serine and metalloproteinase activity in an experimental model of intravasation.
17 Tumor blood vessels, however, are highly abnormal, with fewer pericytes and increased permeability compared with normal vessels, and presumably provide an easier route for direct entry into the bloodstream.
18 Lymphatic vessels are also abnormal, but their role in intravasation is unknown. Regardless of the route, tumor cells enter the circulation in great numbers: Estimates are 3 to 4 million cells/day/g of tumor.
19 The number of tumor cells in the peripheral blood, however, does not necessarily predict whether the patient will develop metastases.
20 In contrast, the detection of disseminated tumor cells in lymph nodes and bone marrow does correlate with metastatic relapse, suggesting that, at least in breast cancer, the properties that allow the cells to find their way to these tissues and survive are the same properties that permit distant metastases.
Transport
Once tumor cells enter a circulatory compartment, they can move actively by motility mechanisms or passively, carried or pushed along with fluid flow. Injection of radiolabeled cells directly into circulation reveals that a substantial proportion is lost during the transport phase of the metastatic cascade. Many tumor cells are eliminated by natural killer (NK) cells or monocytes before arrival in a secondary site. Tumor cells that escape immune recognition are frequently killed by exposure to hemostatic shear forces.
21 Bioassays in the lungs, liver, heart, and muscle have been performed following intravenous injection of tumor cells. It is noted that by the time it takes to remove the tissues for assay (2 to 3 minutes), most cells are dead due to mechanical trauma.
21 The average tumor cell diameter ranges from 20 to 30 μm but must navigate through vessels significantly smaller (e.g., 6- to 7-μm capillaries). Even if tumor cells have the ability to deform and squeeze through the passages, they are subjected to significant hydrostatic pressures. Depending on the tumor type and biophysical parameters such as membrane fluidity, cellular elasticity, and cytoskeletal organization, the cells will remain intact or be broken by shear. Deformability is also affected by the pressures found within various tissues. In contrast to the shear forces usually encountered in the vasculature, blood flow in bone sinusoids is sluggish (about 30-fold less than in capillaries and postcapillary venules), and diameter is not a concern.
22 During transport, the behavior of tumor cells is often determined by their presence as single cells or as emboli. Embolization can be homotypic (tumor cell–tumor cell) or heterotypic (tumor cell–leukocyte, tumor cell–platelet, tumor cell–fibrin). The association of tumor cells with blood cells can be the result of altered cell surface glycosylation and expression of sialyl Lewis X/A on the tumor cell that permits interaction with a class of vascular adhesion molecules found on normal leukocytes and endothelium, the selectins. Alterations in the adherence of tumor cells to endothelium via E-selectin, platelets via P-selectin, and leukocytes via L-selectin alter the metastatic potential in animal models.
23 Embolus size can also contribute to protection of the tumor cells from biophysical forces or immune attack. In essence, encapsulation of tumor cells helps to protect them. As a result of the consequence of embolus formation, heparin, an inhibitor of selectin/glycan interactions, has been considered as an antimetastatic agent.
Visualization of tumor cells in the circulation during transport indicates that the cells roll rather than float in a manner analogous to leukocytes. Nonetheless, during this time, tumor cells are weakly adherent and subject to anoikis, a specialized type of apoptosis in which cells that are
anchorage dependent are induced to die.
24 In general, metastatic cells are more resistant to anoikis than nonmetastatic cells and are frequently referred to as being anchorage independent. This is somewhat misleading, because some tumor cells will induce apoptosis even if firmly attached to a substrate if that substrate is not the preferred one for the type of cell. It is possible, then, that circulating tumor cells receive sufficient signals from the ECM, other cells, and/or serum proteins to limit their susceptibility to anoikis.
Arrest
It is important to discriminate between the physical trapping and arrest of circulating cells in the microvasculature and selective adhesion to the walls of the microvasculature. Both processes have been observed, and the relative importance of these mechanisms in specific organs is debated.
There are three types of endothelial structures found in higher vertebrates: continuous, discontinuous, and fenestrated. Most endothelial cells form tight junctions with their neighbors and have a continuous, unbroken basement membrane beneath them. However, in certain organs, such as liver and spleen, the endothelial cells and the basement membrane have gaps, or discontinuities, in their structure. In the kidney, a fenestrated endothelium, there are gaps between endothelial cells, but a membrane-like structure connects them and the entire structure overlaps in a continuous basement membrane. The structure of these endothelial/basement membrane barriers contributes to the normal function of the tissues and forms different barriers through which tumor cells must pass.
Adhesion of circulating tumor cells to organ microvessel endothelial cells represents one of the more important steps in metastasis, especially organ-specific metastasis. In general, higher rates of tumor cell–endothelial adhesion correlate well with metastatic potential. In vivo and in vitro kinetic studies indicate that initial attachment of cancer cells occurs preferentially at endothelial cell junctions.
25 Frequently, tumor cells adhere at sites where inflammation is taking place and is most likely related to alterations in cell surface components of endothelial cells at these sites. Tumor cells use many of the same mechanisms to attach to and traverse endothelium as inflammatory cells, including glycan/selectin interactions.
Once tumor cells bind to the endothelium, they induce the endothelial cells to retract and eventually overlap the tumor cell. During this time, there is no loss of electrical resistance, suggesting that tight junction integrity is maintained. Tumor cells then adhere to subendothelial basement membrane components, and a higher rate of tumor cell adhesion to subendothelial basement membrane correlates with metastatic potential. In the case of HT1080 fibrosarcoma cells, the attachment of circulating tumor cells to the lung vasculature is mediated by tumor α
3β
1 integrin ligation to laminin-5 in the basement membrane.
26 Patches of exposed basement membrane were found to be preexisting using intravital microscopy techniques in isolated, perfused lungs.
Arrested tumor cells can undergo rapid apoptosis. It is envisioned that in some cases this is the result of the lack of suitable survival signals and the initiation of anoikis. In addition, the attachment of tumor cells to endothelium can release nitric oxide (NO) produced by endothelial nitric oxide synthase.
27 NO can induce apoptosis of tumor cells, indicating an active process that contributes to tumor cell loss and metastatic inefficiency.
Extravasation
Extravasation is the process of tumor cells invading from the interior of a vessel into the organ parenchyma. Extravasation was viewed as a rate-limiting step for metastasis formation, but intravital microscopy studies have indicated that extravasation can be a remarkably efficient process, at least in some situations. For example, 87% of B16F1 murine melanoma cells that were injected through the mesenteric vein into the liver were arrested in the liver 90 minutes after injection, and 83% of the injected cells were found in the liver parenchyma by 3 days, indicating that more than 95% of the arrested cells extravasated.
28 The molecular mechanisms underlying extravasation are viewed as being identical to those involved in invasion, and in vitro assays for extravasation reveal a contribution of cellular adhesion molecules, proteinases, and motility factors.
There is controversy as to whether extravasation is required for the formation of metastases. In the case of some pulmonary metastases, there is evidence that tumor cells can attach to the lung endothelium, survive, and grow intravascularly.
29 Extravasation occurs in this model only when the intravascular foci outgrow the vessel.
Colonization
Colonization, the formation of clusters of tumor cells at ectopic sites, represents a highly inefficient step in the metastatic cascade. In the model of B16F1 cells injected into the liver vasculature, only
2% of the injected cells formed micrometastases, and only 0.02% formed lesions that persisted,
grew progressively, and threatened the life of the animal.
28 The formation of micrometastatic lesions requires that the tumor cell must first survive and then grow in the foreign environment. In some tumor types (i.e., breast and melanoma), metastases can arise decades after the treatment of the primary tumor, indicating that tumor cells can survive in a state of dormancy for long periods. Tumor cells can persist as solitary cells, or they can grow to a size of several hundred cells in which the rate of growth is balanced by the rate of apoptosis. Conversion to a clinically detectable metastatic lesion requires the subsequent initiation of angiogenesis (see
Chapter 17). The growth of the cells is dependent on factors, primarily soluble growth factors, present at the site of colonization. Although it is natural to focus on factors that promote the growth of tumor cells in selective sites, there is ample experimental evidence showing that some tissues are hostile to tumor cells.
A tumor cell’s ability to establish a metastatic lesion is very much dependent on the microenvironment (see
Chapter 16). A prime example of this effect is the role of the “vicious cycle” in the propensity for breast carcinoma to metastasize to bone (
Figure 18-6 ).
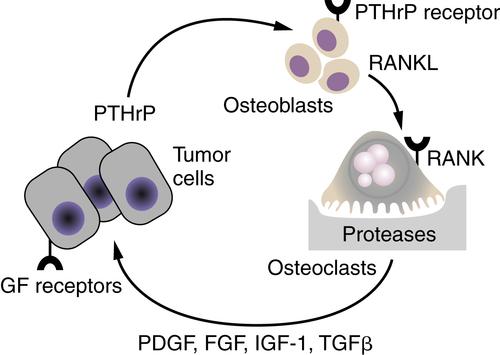
30 The mammary carcinoma cells produce parathyroid hormone–related peptide (PTHrP), which during pregnancy would function to release calcium from bone stores. Using the same molecular pathways, tumor cell-produced PTHrP acts on its receptors on osteoblasts to release the tumor necrosis factor-α (TNF-α) family member, receptor activator of nuclear factor-κB ligand (RANKL). RANKL interacts with its receptor RANK on osteoclasts and activates them to degrade mineralized bone. The bone matrix contains an abundance of growth factors, including PDGF, FGFs, IGF-1, and TGF-β/bone morphogenetic protein family members, which are released during the osteolytic process. It is the release of these growth factors that stimulates the breast cancer cells to grow and to continue to secrete PTHrP and fuel the “vicious cycle.” The colonization of breast cancer cells in the bone is thus facilitated by specific characteristics of the bone microenvironment that promote the growth of breast cancer cells.
Figure 18-6 The vicious cycle of host-tumor interactions in breast cancer metastasis to bone Breast cancer cells produce parathyroid hormone–related protein (PTHrP), which stimulates bone osteoblasts to express the tumor necrosis factor-α (TNF-α) family member receptor activator of nuclear factor-κB ligand (RANKL). RANKL interacts with its receptor RANK on osteoclast precursors to differentiate into active osteoclasts, resulting in the release of proteases and bone degradation. Growth factors such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF-1), and transforming growth factor-β (TGF-β), which are stored in the bone matrix, are released and stimulate the growth of receptor-containing tumor cells. An increase in tumor cells results in an increase in PTHrP release, leading to a vicious cycle of tumor cell growth and bone degradation.
Organ Selectivity of Metastasis
There is a clear tendency for primary tumors to form metastatic lesions in specific organ sites (
Table 18-1 ). Common regional metastatic involvements can often be attributed to anatomic or mechanical considerations (e.g., efferent venous circulation or lymphatic drainage) and explained by arrest of tumor cells in the first capillary bed or lymph node encountered.
31 Because most tumor cells enter the vasculature in small veins or capillaries, the most common site of metastasis is lung and liver. However, distant metastasis patterns are typically more site specific. In 1889, Paget analyzed postmortem data of women who died of breast cancer and noticed a higher frequency of metastasis to skeleton than would be expected based solely on cardiac output to each organ.
32 He concluded that the pattern of organ distribution of metastases was not simply a matter of chance and suggested that metastases develop only when the “seed” (tumor cells with metastatic ability) and the “soil” (organs or tissues providing growth advantages to seeds) are compatible. Importantly, the mechanical theory and the “seed and soil” hypothesis are not mutually exclusive, and both contribute to metastatic dissemination.
Table 18-1
Common Sites of Metastasis
| Primary Tumor Site |
Most Common Sites of Metastases |
| Breast |
Axillary RLN, contralateral breast via lymphatics, lung, pleura, liver, bone, brain, adrenal, spleen, ovary |
| Colon |
RLN, liver, lung, direct extension into urinary bladder or stomach |
| Kidney |
Lung, liver, bone |
| Lung |
RLN, pleura, diaphragm by direct extension, liver, bone, brain, kidney, adrenal, thyroid, spleen |
| Ovary |
Peritoneum, RLN, lung, liver |
| Pancreas |
Liver, stomach by direct extension, colon, peritoneum |
| Prostate |
Bones of spine and pelvis, RLN |
| Stomach |
RLN, liver, lung, bone |
| Testis |
RLN, lung, liver |
| Urinary bladder |
Direct extension into rectum, colon, prostate, ureter, vagina, bone, RLN, lung, peritoneum, pleura, liver, brain |
| Uterine endometrium |
RLN, lung, liver, ovary |
RLN, Regional lymph nodes.
Experimental data supporting the seed and soil hypothesis include preferential invasion and growth of B16 melanoma metastases in specific organs.
33 In addition, palliative treatment of women with advanced ovarian carcinoma has provided an opportunity to test this theory in humans. These patients often have a large ascites burden, but seldom present with disease outside the peritoneal cavity. Tarin and colleagues treated patients with potentially lethal malignant ascites by introducing a tube that drains the peritoneal ascites into the vena cava.
34 In doing so, tumor cells in the ascites were given direct entry into the circulation. Despite continuous entry of billions of viable tumor cells into the circulation, metastases to the lung (i.e., the first capillary bed encountered) were rare. This single clinical observation highlights the inefficiency of the metastatic process and, more important, demonstrates that merely seeding cells in different tissues is not adequate to develop metastases.
The mechanisms responsible for organ selectivity in tissues can be attributed to the arrest and the colonization steps of the metastatic cascade in particular. Tumor cells adhere more selectively to organ-derived microvascular endothelial cells than to large-vessel endothelial cells, and variants of the B16 melanoma previously selected for metastases to brain, lung, ovary, or liver adhere at a more rapid rate to brain, lung, ovary, or liver endothelial cells, respectively.
35 Using phage-display technology, endothelial cells in different tissues have been demonstrated to express unique markers, and tumor cells recognize the molecular “addresses” to adhere to in a selective manner.
35 Tumor cells are also able to recognize subendothelial basement membrane differences. In vitro studies demonstrate the selective growth of tumor cells in organ-derived soluble growth factors or cells.
36 In vivo, breast tumor cells that express the chemokine receptor CXCR4 preferentially metastasized to tissues that expressed the ligand SDF1/CXCL12.
37 There is a concept that tumor cells colonize in a premetastatic niche initiated in target organs by tumor cell–generated soluble factors that induce the expression of fibronectin by resident fibroblast-like cells.
38 Bone marrow–derived cells that express the vascular endothelial cell growth factor receptor 1 and the integrin α
4β
1 selectively adhere to these regions, produce the proteinase MMP9 and the chemokine SDF1/CXCL12, and provide a permissive niche for colonization by tumor cells.
Although the data strongly support the notion that there are soluble factors produced in different tissues to which tumor cells can respond, the process of homing has not been validated. Strictly speaking, homing would require directed movement throughout the transit of tumor cells as they leave the primary tumor. Rather, tumor cells distribute according to circulatory patterns initially but may “home” once they are more proximate. Many of the mechanisms used by lymphocytes to home to peripheral lymph nodes or sites of inflammation are apparently shared by tumor cells.
Some of the strongest evidence supporting organ selectivity of cancer cells comes from data showing selection of variants that colonize different tissues. The first selections were done by repetitive isolation of lung metastases from the B16 melanoma followed by reinjection and recolonization.
33 Similar approaches have been used for other tumors, most recently using a human breast carcinoma cell line with selection of metastases to bone, lung, and adrenal gland. Using these breast carcinoma cell lines coupled with a comparison by cDNA microarray has highlighted the requirement for coordinated expression of multiple genes for metastasis.
39 Transcriptomes were compared between parental and bone-selective variants, and over- and underexpressed genes were identified. Among the overexpressed genes in the bone metastasis signature were a matrix metalloproteinase, MMP1; the ECM component osteopontin; the cytokine interleukin-11; the chemokine receptor, CXCR4; and connective tissue–derived growth factor. Subpopulations within the parental population expressed one or more of the bone signature genes, but only a few expressed all of them. Transfection of individual cDNAs only modestly increased bone metastatic efficiency, whereas cotransfection of gene combinations into the parental cells resulted in populations as efficient at bone colonization as the bone-selective variants. Similar studies with a lung-selective variant revealed a lung metastasis signature that overlapped only minimally with the bone metastasis signature.
40 These data highlight that there are specific genes that control metastasis in an organ-specific fashion, and coordinated expression of multiple genes is required.
Metastatic Progression
The journey of a metastatic cancer cell involves several steps from the time it leaves the primary site to the moment it reaches a distant organ. So how long does this journey take to commence, and what is the path that these cells take? Are these metastatic cancer cells or “seeds” disseminated early or late in the life of an evolving primary tumor? These questions are important to understand, because they are linked to the functional consequences of genetic and epigenetic changes that are accrued by metastatic cancer cells when compared to their originating primary tumor. If metastatic progression
occurs early and takes place in parallel to the primary tumor, the genomic landscape of the primary tumor and metastasis can be predicted to be significantly different. On the other hand, if metastatic progression occurs late through clonal evolution within the primary tumor and the cancer cells that left the tumor early became dead ends, then one can expect the primary tumor and metastases to look genetically similar. These questions have direct clinical implications because several drugs that target the primary tumor fail to treat advanced metastatic disease in the clinic. If the metastases were indeed genetically distinct from the primary tumors, it could be envisioned that drugs that target oncogenic changes in the primary tumor would not work for metastases.
Deciphering both the similarities and distinctions between the primary tumor and the metastases may be important in devising strategies to successfully treat metastatic cancer. What is also necessary is gaining an understanding of the regional differences and heterogeneity within the tumor and metastases. A large part of the intratumoral heterogeneity and metastatic diversity is shaped by the distinct tumor microenvironment in which the cancer cells reside. Therefore it can be envisioned that microenvironment-linked selective pressures influence the genetic and epigenetic heterogeneity of tumors and determine the evolutionary trajectory of cancer cells during metastatic progression. Recognition and characterization of such genetic and phenotypic diversity is therefore key for designing rational therapeutic interventions.
The advent of major technological advances over the past decade in the field of sequencing and high-resolution analysis of disseminated cancer cells has made it possible to begin to address such complex biological questions. Deciphering metastatic progression and phylogeny of metastases is an emerging goal as sequencing data from different cancers accumulate, with several hypotheses and models being discussed and debated. Two hypotheses that tower over the rest in explaining the origin of metastatic cells are the linear progression and clonal evolution model on one hand and the parallel progression model on the other. The two models are not mutually exclusive and could be at play to different degrees, depending on the tumor type and its oncogenetic composition.
Linear Progression Model
Pioneering work by the group of Isaiah Fidler has shown that only a subset of preexisting cells within a heterogeneous primary tumor is competent to metastasize.
41 The linear progression paradigm has been the prevalent model to explain this observation. According to this model, cancer cells undergo multiple rounds of mutation and selection in the primary tumor, and only a small subset of these malignant clones acquire the genetic and epigenetic alterations necessary for metastasis.
42,43 A clinical correlation between tumor size and frequency of metastasis is in line with this model.
44 The model also suggests that larger tumors growing over time would have a higher likelihood of containing metastasis-competent clones within their heterogeneous population. The reduced probability of metastasis in cases where primary tumors of less than 2 cm in size are surgically resected in the clinic lends support to this model. New insights into genomic evolution during tumor progression came from Aparicio and colleagues, who sequenced a breast cancer metastasis and its corresponding primary tumor that was removed 9 years earlier.
45 Eleven of the 30 mutations detected in the metastatic lesion were present in the primary tumor. This study revealed that preexisting mutations in the primary tumor do get selected in the metastases, but there is also considerable genomic evolution that occurs during the metastatic process. Comparing the frequency of these somatic mutations in the primary tumors and metastases revealed two discernible trends. Five of the 11 shared mutations were prevalent in the primary tumor, whereas 6 were present in low frequencies. In contrast, all these mutations were prevalent in the metastases, indicating less heterogeneity than the primary tumors. These data are compatible with the clonal expansion model and suggest that a clone from the heterogeneous primary tumor is selected, expands, and generates the distant metastasis. More recently, analysis of tumor evolution using single-cell sequencing technology in two breast cancer cases, by Michael Wigler and colleagues, suggested that a single clonal expansion both formed the primary tumor and seeded the metastasis in the examined cases.
46 It remains to be seen how general these findings are as more matched primary tumors and their metastases in different cancers are sequenced.
In the context of metastasis, what is the time line of metastatic progression? The linear progression model would predict metastatic spread to be a late event in tumor progression, given the time required for the accumulation of sufficient genetic and epigenetic alterations within the primary tumor that permits metastasis. Also implicit in the assumptions from this model and discussed earlier, primary tumors and metastasis are expected to be genetically similar, and a majority of the mutations detected in metastasis should preexist in the primary tumor. Indeed, new insights from genomic sequencing of pancreatic cancer patients suggest that metastasis is a late event in the genetic evolution of pancreatic cancer.
47 Clonal populations that give rise to metastasis preexist within the primary tumor but are more genetically evolved than the original parental, nonmetastatic clone. Using mathematical modeling, Yachida and colleagues showed that a decade passes between the occurrence of an
initiating mutation and the birth of a parental, nonmetastatic cancer cell clone in a pancreatic tumor. Thereafter, at least 5 years pass before metastatic ability is gained in these clones and an average of 2 years elapses until the patient’s death. In an analogous manner, colorectal cancer progression was calculated to be 17 years between the birth of an adenoma founder cell and advanced carcinoma; however, only a further 1.8 years pass until the evolution of a metastatic founder cell appears.
48 These studies provide several key insights into the time scale of evolution of metastases in the setting of pancreatic and colon cancer. They suggest that clones in certain advanced cancer already harbor most of the mutations needed for metastatic competence and it takes a relatively short time to develop into metastases. We have to keep in mind that the temporal course of acquisition of metastatic traits might vary greatly between different types of cancers. It is thought that in the case of estrogen receptor–positive breast cancer, there is usually a prolonged latency period, often up to a decade, from gaining infiltration potential and seeding until competence to colonize and outgrow in a distant organ is gained. However, in the case of lung cancer, there is a relatively short interval (often measured in months) between gaining infiltration ability and successful colonization.
49 Large-scale sequencing efforts are under way to reveal the genetic landscape of primary tumors and their metastases in other cancer types, and this could provide insights into the generality of these observations.
Parallel Progression Model
Although there is supportive evidence for the linear progression model, single-cell comparative genomic hybridization analysis of isolated disseminated tumor cells (DTCs) in the bone marrow and primary breast tumors provides an alternative explanation. Christoph Klein and colleagues observed that the genetic changes in the DTCs in the bone marrow did not resemble those in their corresponding primary breast tumor, which led to the conception of the “parallel progression model.” According to this model, quasinormal cells disseminate relatively early in the course of tumor progression and evolve independently from the cells in the primary tumor.
50 As a result, genetic and epigenetic evolution occurs through multiple rounds of genetic diversification and clonal selection mostly at the distant organ site(s), which ultimately gives rise to overt metastasis. Parallel evolution thus predicts divergence for many mutations and genetic alterations for the selected cells at the distant sites.
51 Based on the mathematical modeling of tumor growth rates, the parallel progression model questions how large sizes of metastases can be explained by the linear model if they arise only at the advanced stages of tumor progression. Work emerging from several laboratories has shown that tumor cells can indeed disseminate early at the pre-invasive stages in breast cancer progression, both in human breast cancer patients and in experimental mammary tumor models.
52–54 Moreover, Podsypanina and colleagues
54 showed that even untransformed mouse mammary epithelial progenitor cells are able to extravasate and survive at the distant site for prolonged periods and start to grow again on oncogene induction. Collectively, these findings in breast cancer lend support to the parallel progression model and underscore the need for a deeper understanding of metastatic progression in different cancers.
Tumor Self-Seeding
Self-seeding is a recent paradigm that addresses the directionality of tumor seeding by experimental modeling and provides new insights into tumor progression.
55 The concept of self-seeding is not in conflict with either of the models discussed earlier but is complementary to both and provides deeper insights into how the disease can progress. According to this model, cancer dissemination is a bidirectional process in which cancer cells not only seed distant sites but some of these cells reenter the circulation and are attracted back to the original primary tumor.
55 Having egressed to distant organs and having gained increased metastatic abilities, many of these aggressive circulating tumor cells (CTCs) also return to their birthplace using the acquired metastatic abilities. In experimental tumor models, self-seeders are attracted back to the primary tumor by cytokines such as interleukins IL-6 and IL-8. Moreover, seeder cells express high levels of the proteolytic enzyme matrix metalloproteinase 1 (MMP1) and the actin crosslinking protein of invadopodia, Fascin-1, to aid in their infiltration back to the primary tumor. Once back in the primary tumors, self-seeders clonally expand, enriching the tumor population with aggressive clones. Self-seeders may also promote tumor growth using a number of genes, including the chemokine CXC motif ligand1 (CXCL-1) that recruits leukocytes to the tumor microenvironment. Therefore this model predicts that the process of tumor self-seeding can select for aggressive CTCs that can accelerate primary tumor growth and in the process also selects for aggressive subpopulations that are primed for metastasis. Local growth of the primary tumor can therefore be promoted by returning metastatic cells, which in turn can act as a reservoir for breeding aggressive clones through the process of “self-seeding.”
In summary, the recent studies on linear progression, parallel progression, and DTC dynamics are clearly bellwethers that have provided new molecular insights into tumor progression. However, it has to be kept in mind that these
efforts represent only early steps of decoding metastatic evolution with several open questions. Though there has been a plethora of supportive evidence for the linear progression model over the past decades, the parallel progression model is intriguing and deserves careful consideration. It is becoming increasingly evident that quasinormal cancer cells can be detected early in circulation during the course of tumor progression, but whether these disseminated cells give rise to pathologically detectable, overt metastasis remains to be shown. None of the models of metastatic progression alone can explain all the phenomena in their entirety in all cancers.
Colonization and Interactions with the Tumor Microenvironment
One of the most challenging and rate-limiting steps of the metastasis cascade is the final step of colonization. On arrival in distant organs, cancer cells need not only to survive in a new and unfamiliar microenvironment but also to grow out into overt metastasis. A large proportion of cancer cells die soon after extravasation; among the few that survive, some remain singly and some in clusters known as micrometastases. A vast majority of these cells will stay in a dormant state and never form clinically detectable metastases. However, some of these cells may successfully withstand these unfavorable conditions, or metastatic stress, and are able to reinitiate tumor growth, resulting in macrometastases. It is not difficult to envision that the cancer cells that can give rise to metastatic lesions are one of the most aggressively selected populations and are often refractory to most standard therapies.
It is now well recognized that tumors grow in a complex environment composed of multiple cell types and supporting structures together known as the tumor microenvironment. Tumor cells interact with their neighbors in this microenvironment, composed of ECM, immune cells, and blood vessels, which influences metastatic success. Recent examples of interactions of metastatic cells with components of the tumor microenvironment are discussed next.
Extracellular Matrix
A major component of the metastasis niche or tumor microenvironment is the ECM, a network of proteins, proteoglycans, glycoproteins, and polysaccharides that constitutes the scaffold and milieu in which all cells sit and migrate. The diverse functions of ECM have been well characterized in primary tumors, where this molecular meshwork provides physical support and serves as a base for cell anchorage. The ECM is also critical in determining polarity and acts as a substrate for migration. Adding to its versatile role, the ECM also acts as a reservoir for bound factors that can be released as bioactive ligands. As a tumor grows with the disruption of tissue organization and remodeling, the mechanical state of the tumor in relation to force, tension, and stiffness of the matrix constantly changes and evolves. ECM remodeling is a highly dynamic process in which the biomechanical cues and ECM alterations influence cell growth, migration, and survival in various stages of tumor progression.
56 For example, lysyl oxidase (LOX) activity is upregulated in several cancers and is tightly linked to the biomechanical properties of the ECM. LOX acts as a crosslinking enzyme that increases ECM stiffness to promote tumor cell invasion and progression in breast cancer models.
57 LOX has been also shown to promote primary tumor growth and metastasis in colon cancer models.
58 Several studies have shed light on new roles of ECM proteins in premetastatic and metastatic niches. ECM molecules have been shown to be important components of stem cell niches. Perturbation of ECM function by either loss of ECM contact or inhibition of ECM receptor integrins or other ECM binding components leads to reduction of stem cells.
56 Furthermore, ECM proteins can provide anchorage for stem cells that is essential for maintaining their stem-cell characteristics.
59 An interesting example is that of ECM proteins such as fibronectin and osteopontin, which can modulate recruitment of bone marrow–derived cells in the distant microenvironment before the arrival of tumor cells.
60, 61 Such interactions in the premetastatic niche can dictate the pattern and success of colonization of metastatic tumor cells.
The function of ECM proteins as niche components in metastasis is becoming increasingly appreciated. New studies suggest that the ECM provides essential support for metastasis-initiating cells. Recent reports elucidate how two ECM components, tenascin-C (TNC) and periostin, that coexist in the metastatic niche maintain the viability of metastasis-initiating cells. TNC and periostin bind to each other tightly to form scaffolding. TNC enhances metastatic colonization by promoting the survival and fitness of metastasis-initiating cells.
62 Interestingly, stem cell–like breast cancer cells express the ECM component TNC, which enhances their response to Wnt and Notch signaling pathways. TNC expression therefore can help metastasis-initiating cells to thrive in harsh microenvironments and promote metastatic outgrowth. In the lung parenchyma, TGFβ3 stimulates myofibroblast cells that produce the other ECM protein, periostin, which then binds to stromal Wnt factors.
63 Periostin thus concentrates Wnt ligands and presents them to cancer cells, which results in their enhanced ability for lung colonization. Moreover, the cancer cells that benefit from the periostin-Wnt axis are thought to be stem cell–like, metastasis-initiating cells.
Immune Cells
Myeloid cells are the most abundant nucleated hematopoietic cells in the body, consisting of several types of cells with diverse functions. Among myeloid cells, tumor-associated macrophages (TAMs) represent one of the most abundant immune infiltrates in a tumor, and their presence is clinically correlated with poor patient outcome.
64 TAMs belong to a subcategory of macrophages that is associated with immunosuppressive cytokines and pro-angiogenic factors.
65 Several tumor growth-promoting factors and paracrine interactions between cancer cells and macrophages have been characterized in the past few years. Pollard, Condeelis, and colleagues have elucidated how TAMs increase the invasiveness of breast cancer cells via the paracrine interaction between cancer cells and TAMs.
66 Cancer cells express colony-stimulating factor (CSF)1, a critical cytokine for macrophage maturation and activation. CSF1 is also a potent chemoattractant for TAMs that express the CSF1R receptor. TAMs, for their part, express epidermal growth factor that enhances the migration and invasiveness of breast cancer cells expressing the EGF receptor (EGFR). These studies showed how the density of TAMs influences the efficiency of the intravasation of tumor cells. An effector of the CSF1 signaling pathway is the transcription factor Ets2. Deletion of Ets2 in TAMs decreases tumor angiogenesis and lung metastasis in breast tumor models.
67 Yet another interesting interaction is between TAMs and breast cancer cells in the lung involving the cell adhesion molecule vascular cell adhesion molecule 1 (VCAM-1) that promotes cell survival of metastatic cells in the lung.
68 VCAM-1 expressed on breast cancer cells binds to α
4 integrin on TAMs. Thus engaged, VCAM1 activates the adaptor protein Ezrin, which enhances PI3K/Akt signaling in the cancer cells to suppress apoptosis.
Recent studies have shed new light on novel interactions between cells of the adaptive immune system and TAMs that promote metastatic progression. The cross talk between helper T cells and TAMs serves as an example. A large portion of the literature has been focused on T-cell responses in mediating anti-tumor immunity. T-cell activation pathways can be categorized into Th1, Th2, and Th17 responses based on the three different known subsets of helper T cells.
69 Th1 effector cells are characterized by producing γ-interferon, Th2 cells by IL-4 and IL-13 production, and Th17 by IL-17A production. With current advances in understanding the adaptive immune system, it is becoming clear that the presence of CD8
+ T cells with markers of Th1 response overall signify good prognosis in solid tumors. Recent studies have revealed that IL-4–expressing CD4
+ T cells promote lung metastasis in breast cancer models by affecting macrophage phenotype and effector functions. This prometastatic Th2 CD4 response is mediated by a subset of macrophages that are dependent on IL-4 and IL-13. Such macrophages (referred to as M2 macrophages) produce a number of cytokines, including TGF-β that suppresses anti-tumor immune responses and EGFR ligands that promote tumor growth. IL-4–producing T cells therefore are able to program macrophages toward a prometastatic phenotype that enhances both tumor invasion and metastasis. Another mechanism by which IL-4 regulates growth-promoting functions of TAM in metastasis is by inducing cathepsin protease activity.
70 Cathepsin proteases are a class of proteolytic enzymes that have been associated with increased motility of tumor cells through the matrix and vasculature. Studies in prostate cancer suggest that T cell and macrophage-derived factor RANKL promotes metastasis.
71 This effect is mediated through the activation of inhibitor of nuclear factor kappa-B kinase subunit alpha (IKK-α). Upon activation, IKK alpha represses a metastasis suppressor gene called
maspin.
72 Myeloid-derived suppressor cells, or MDSCs, have become the focus of intense study in cancer biology over the past few years in the context of tumor progression. MDSCs are a heterogeneous population of myeloid cells composed of immature myeloid cells and progenitor cells. These cells are usually not abundant under normal physiological conditions. In healthy individuals, immature myeloid cells differentiate into mature granulocytes or macrophages. However, in individuals with cancer, these bone marrow–derived cells have a partial block in differentiation that results in an expansion of the immature population. The ability of the MDSC population to suppress immune function has major implications for cancer progression. The MDSCs can be categorized into two distinct groups, granulocytic/polymorphonuclear MDSCs (G/PMN-MDSCs) and monocytic MDSCs (M-MDSCs), based on their phenotype.
73 Besides phenotypic differences, the two subsets of MDSCs also differ in the mechanisms they use to suppress immune functions. G-MDSCs use reactive oxygen species (ROS) for immunosuppression, whereas M-MDSCs use inducible nitric oxide synthase (iNOS) and arginase for suppressing immune functions.
74 G/PMN-MDSCs are immature neutrophils that express abnormally high ROS, myeloperoxidase, and lysosomal enzymes compared to differentiated neutrophils (PMN). G/PMN-MDSCs are also less phagocytic than neutrophils. MDSCs isolated from cancer patients with the phenotype LIN
− HLA
− DR
− CD33
+CD11b
+ share properties of granulocyte precursors or progranulocytes. The abundance of these cells correlates with poor prognosis and radiographic progression of disease in breast and colorectal cancers. In animal models, MDSCs are shown to infiltrate at the invasive front of tumors, where they contribute to metastasis through enhanced metalloproteinase activity.
75 MDSCs are also responsible for the refractoriness of anti-angiogenic VEGF
inhibitors at least in part by promoting tumor angiogenesis bypassing the VEGF requirement.
76 Recently, G/PMN-MDSCs have been shown to play a critical role in mediating both chemoresistance and metastasis in breast cancer through a network of paracrine signals between carcinoma, endothelial cells, and G/PMN-MDSCs.
77 M-MDSCs in metastatic sites express versican, an ECM proteoglycan that promote metastasis in animal models.
78 Platelets are specialized blood cells that are produced from megakaryocytes in the bone marrow. Their main function in physiology is to prevent hemorrhage from injury. In the mid-19th century, Trousseau first documented that excessive clotting was related to occult cancer in the body, thus proposing a link between the hemostatic system and malignancy. Since then it has been well recognized that clinical signs of thrombosis or aberrant platelet activation and aggregation are often present in cancer patients with advanced metastatic disease.
79 High platelet counts are associated with poor prognosis in breast, lung, and pancreatic cancers. It has been also shown that depletion of platelets or inhibition of platelet aggregation indeed reduces experimental metastasis in animals.
80 Experimental insights from several laboratories suggest that several functions of platelets might be at play in promoting metastatic progression.
81 Early studies showed that platelets might be functioning in shielding tumor cells from the immune system, in particular from NK cell–mediated tumor lysis.
82 Interestingly, TGF-β released from platelets can also diminish NK function by affecting their granule mobilization and cytotoxicity. Recently Labelle and colleagues showed that platelets could actively signal to tumor cells in transit outside the primary microenvironment.
83 A transient contact between platelets and tumor cells can induce an EMT phenotype by synergistic action of both TGF-β/Smad and NFκB pathways that can increase the invasiveness and metastatic potential in tumor cells. Ablation of TGF-β in platelets or inhibition of NFκB in tumor cells significantly reduces lung metastasis. However, activation of neither the TGF-β/Smad nor the NFκB pathway alone in this context is sufficient to promote lung metastasis. What this study suggests is that tumor cells that have intravasated without losing their epithelial properties might become more mesenchymal and invasive on interactions with platelets in circulation. Although our understanding of platelet function advances, the pro-metastatic functions of platelets in cancer progression remain to be fully explored. It is interesting that platelets can release the neurotransmitter serotonin on activation and thus modulate vascular tone.
84 Future research is necessary to determine how platelets support metastatic growth by altering blood vessel permeability. Given the promising mechanistic studies on platelet function in metastasis, the clinical correlation of platelet activities and cancer, and the potential for antimetastatic therapy, it is imperative to understand platelet involvement in metastatic progression.
In summary, as advances in genomics usher us into an era of personalized diagnostics and treatment development, combinatorial targeting approaches of inhibiting both cancer cell intrinsic and microenvironment-linked pathways are much needed. A deeper understanding of the biology of metastases will be gained by comparing the genetic and phenotypic characteristics of the primary tumor and associated metastases in different cancers and by predicting the course of the development of metastases.
Challenges and Opportunities in Studying the Biology of Disseminated Cancer Cells
New insights into the process of metastasis are pointing to new clinical opportunities for prognosis and intervention and have paved the way for future research. Beyond this, it is becoming increasingly recognized that genetic variations in individual tumors fueled by gene amplification, specific mutations, and single-nucleotide polymorphisms greatly affect the relative efficacy of anticancer treatment. These observations stimulate the consideration of personalized medicine, where treatment would ultimately be determined by each tumor’s unique molecular and genomic features. A critical question for oncologists is to define which cancer patients are at a higher risk of metastatic relapse so that treatments can be tailored to those patients most likely to benefit. Research is currently geared toward developing new technologies for detecting cancer cells in the blood (CTCs) and in various organs (DTCs) and to correlate the presence of these cells with the risk of metastatic relapse.
Advanced molecular, cytometric and immunological approaches have improved the ability to detect, monitor, and analyze single DTCs in the bone marrow and CTCs in the blood of cancer patients. Detecting these cells early could provide insights into the biology of metastatic spread and serve as a diagnostic resource for monitoring the efficacy of current cancer therapy. CTC numbers in several cancers have been shown to be prognostic of disease recurrence.
85 For example, CTC numbers before treatment and at the first follow-up visit after initiation of therapy were found to be independently associated with progression-free and overall survival in patients with metastatic breast cancer.
86 Clinical evidence also exists for the association between the presence of DTCs at the time of tumor resection and postoperative metastatic relapse.
87–89 Several new techniques based on cytometric/immunological and molecular approaches have been developed for the detection of CTCs/DTCs over the
past few years. For example, the commercially available CellSearch system (Johnson & Johnson, USA), which consists of an automated enrichment and immunostaining device, was approved by the FDA for the detection of CTCs in patients with breast, colon, and prostate cancer.
90 Other examples include EPISPOT (epithelial immunospot) for the detection of viable DTCs and CTCs and the microfluid platform called the
CTC chip that captures CTCs from unfractionated blood under controlled laminar flow. Molecular polymerase chain reaction (PCR)-based techniques are also under development. In the future, analysis of CTCs and perhaps DTCs could also be helpful when estimating the efficacy of therapy. However, as is the case with developing new technologies, there are several hurdles to be overcome. At present, a major limitation is that CTCs can be detected in many more patients than ever experience relapse. Other hurdles include tumor heterogeneity, lack of expression of uniform surface markers, limit of detection, and repeated need for bone marrow sampling in cancer patients, to name only a few. Nonetheless, these efforts represent steps in the right direction that could provide critical tools for diagnosis and guiding personalized treatment of metastatic disease in the future.
1. Heppner G.H. , Miller F.R. The cellular basis of tumor progression . Int Rev Cytol . 1988 ; 177 : 1 – 56 .
2. Welch D.R. , Tomasovic S.P. Implications of tumor progression on clinical oncology . Clin Exp Metastasis . 1985 ; 3 : 151 – 158 .
3. REFERENCE DELETED IN PROOFS
4. Ayala I. , Baldassarre M. , Caldieri G. et al. Invadopodia: a guided tour . Eur J Cell Biol . 2006 ; 85 : 159 – 164 .
5. Hynes R.O. Integrins: bidirectional, allosteric signaling machines . Cell . 2002 ; 110 : 673 – 687 .
6. Chambers A.F. , Matrisian L.M. Changing views of the role of matrix metalloproteinases in metastasis . J Natl Cancer Inst . 1997 ; 89 : 1260 – 1270 .
7. Thiery J.P. Epithelial-mesenchymal transitions in tumour progression . Nat Rev Cancer . 2002 ; 2 : 442 – 454 .
8. Cavallaro U. , Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer . Nat Rev Cancer . 2004 ; 4 : 118 – 132 .
9. Boudreau N. , Bissell M.J. Extracellular matrix signaling: integration of form and function in normal and malignant cells . Curr Opin Cell Biol . 1998 ; 10 : 640 – 646 .
10. Guo W. , Giancotti F.G. Integrin signalling during tumour progression . Nat Rev Mol Cell Biol . 2004 ; 5 : 816 – 826 .
11. Liotta L.A. , Tryggvason K. , Garbisa S. et al. Metastatic potential correlates with enzymatic degradation of basement membrane collagen . Nature . 1980 ; 284 : 67 – 68 .
12. Egeblad M. , Werb Z. New functions for the matrix metalloproteinases in cancer progression . Nat Rev Cancer . 2002 ; 2 : 161 – 174 .
13. Danø K. , Behrendt N. , Høyer-Hansen G. et al. Plasminogen activation and cancer . Thromb Haemost . 2005 ; 93 : 676 – 681 .
14. Duffy M.J. The urokinase plasminogen activator system: role in malignancy . Curr Pharmaceut Biotechnol . 2004 ; 10 : 39 – 49 .
15. Coussens L.M. , Fingleton B. , Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations . Science . 2002 ; 295 : 2387 – 2392 .
16. Wolf K. , Friedl P. Molecular mechanisms of cancer cell invasion and plasticity . Br J Dermatol . 2006 ; 154 ( suppl 1 ) : 11 – 15 .
17. Kim J. , Yu W. , Kovalski K. et al. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semi-quantitative PCR-based assay . Cell . 1998 ; 94 : 353 – 362 .
18. Jain R.K. , Munn L.L. , Fukumura D. Dissecting tumour pathophysiology using intravital microscopy . Nat Rev Cancer . 2002 ; 2 : 266 – 276 .
19. Butler T.P. , Gullino P.M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma . Cancer Res . 1975 ; 35 : 512 – 516 .
20. Pierga J.Y. , Bonneton C. , Vincent-Salomon A. et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients . Clin Cancer Res . 2004 ; 10 : 1392 – 1400 .
21. Weiss L. Metastatic inefficiency . Adv Cancer Res . 1990 ; 54 : 159 – 211 .
22. Welch D.R. , Harms J.F. , Mastro A.M. et al. Breast cancer metastasis to bone: evolving models and research challenges . J Musculoskelet Neuronal Interact . 2003 ; 3 : 30 – 38 .
23. Varki N.M. , Varki A. Heparin inhibition of selectin-mediated interactions during the hematogenous phase of carcinoma metastasis: rationale for clinical studies in humans . Semin Thromb Hemost . 2002 ; 28 : 53 – 66 .
24. Stupack D.G. , Teitz T. , Potter M.D. et al. Potentiation of neuroblastoma metastasis by loss of caspase-8 . Nature . 2006 ; 439 : 95 – 99 .
25. Nicolson G.L. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites . Cancer Metastasis Rev . 1988 ; 7 : 143 – 188 .
26. Wang H. , Fu W. , Im J.H. et al. Tumor cell alpha3beta1 integrin and vascular laminin-5 mediate pulmonary arrest and metastasis 1 . J Cell Biol . 2004 ; 164 : 935 – 941 .
27. Qiu H. , Orr F.W. , Jensen D. et al. Arrest of B16 melanoma cells in the mouse pulmonary micro circulation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells . Am J Pathol . 2003 ; 162 : 403 – 412 .
28. Chambers A.F. , Groom A.C. , MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites . Nat Rev Cancer . 2002 ; 2 : 563 – 572 .
29. Wong C.W. , Song C. , Grimes M.M. et al. Intravascular location of breast cancer cells after spontaneous metastasis to the lung . Am J Pathol . 2002 ; 161 : 749 – 753 .
30. Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities . Nat Rev Cancer . 2002 ; 2 : 584 – 593 .
31. Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy . Clin Exp Metastasis . 1992 ; 10 : 191 – 199 .
32. Paget S. The distribution of secondary growths in cancer of the breast . Lancet . 1889 ; 133 : 571 – 573 .
33. Fidler I.J. Selection of successive tumour lines for metastasis . Nat New Biol . 1973 ; 242 : 148 – 149 .
34. Tarin D. , Price J.E. , Kettlewell M.G. et al. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts . Cancer Res . 1984 ; 44 : 3584 – 3592 .
35. Ruoslahti E. Vascular zip codes in angiogenesis and metastasis . Biochem Soc Trans . 2004 ; 32 : 397 – 402 .
36. Nicolson G.L. , Dulski K.M. Organ specificity of metastatic tumor colonization is related to organ-selective growth properties of malignant cells . Int J Cancer . 1986 ; 38 : 289 – 294 .
37. Muller A. , Homey B. , Soto H. et al. Involvement of chemokine receptors in breast cancer metastasis . Nature . 2001 ; 410 : 50 – 56 .
38. Kaplan R.N. , Riba R.D. , Zacharoulis S. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche . Nature . 2005 ; 438 : 820 – 827 .
39. Kang Y. , Siegel P.M. , Shu W. et al. A multigenic program mediating breast cancer metastasis to bone . Cancer Cell . 2003 ; 3 : 537 – 549 .
40. Minn A.J. , Gupta G.P. , Siegel P.M. et al. Genes that mediate breast cancer metastasis to lung . Nature . 2005 ; 436 : 518 – 524 .
41. Fidler I.J. , Kripke M.L. Metastasis results from preexisting variant cells within a malignant tumor . Science . 1977 ; 197 : 893 – 895 .
42. Cairns J. Mutation selection and the natural history of cancer . Nature . 1975 ; 255 : 197 – 200 .
43. Fearon E.R. , Vogelstein B. A genetic model for colorectal tumorigenesis . Cell . 1990 ; 61 : 759 – 767 .
44. Koscielny S. , Tubiana M. , Le M.G. et al. Breast cancer: relationship between the size of the primary tumour and the probability of metastatic dissemination . Br J Cancer . 1984 ; 49 : 709 – 715 .
45. Shah S.P. , Morin R.D. , Khattra J. et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution . Nature . 2009 ; 461 : 809 – 813 .
46. Navin N. , Kendall J. , Troge J. et al. Tumour evolution inferred by single-cell sequencing . Nature . 2011 ; 472 : 90 – 94 .
47. Yachida S. , Jones S. , Bozic I. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer . Nature . 2010 ; 467 : 1114 – 1117 .
48. Jones S. , Chen W.D. , Parmigiani G. et al. Comparative lesion sequencing provides insights into tumor evolution . Proc Natl Acad Sci U S A . 2008 ; 105 : 4283 – 4288 .
49. Nguyen D.X. , Bos P.D. , Massagué J. Metastasis: from dissemination to organ-specific colonization . Nat Rev Cancer . 2009 ; 9 : 274 – 284 .
50. Klein C.A. Parallel progression of primary tumours and metastases . Nat Rev Cancer . 2009 ; 9 : 302 – 312 .
51. Schmidt-Kittler O. , Ragg T. , Daskalakis A. et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression . Proc Natl Acad Sci U S A . 2003 ; 100 : 7737 – 7742 .
52. Husemann Y. , Geigl J.B. , Schubert F. et al. Systemic spread is an early step in breast cancer . Cancer Cell . 2008 ; 13 : 58 – 68 .
53. Nagrath S. , Sequist L.V. , Maheswaran S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology . Nature . 2007 ; 450 : 1235 – 1239 .
54. Podsypanina K. , Du Y.C. , Jechlinger M. , Beverly L.J. , Hambardzumyan D. , Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung . Science . 2008 ; 321 : 1841 – 1844 .
55. Kim M.Y. , Oskarsson T. , Acharyya S. et al. Tumor self-seeding by circulating cancer cells . Cell . 2009 ; 139 : 1315 – 1326 .
56. Lu P. , Weaver V.M. , Werb Z. The extracellular matrix: a dynamic niche in cancer progression . J Cell Biol . 2012 ; 196 : 395 – 406 .
57. Levental K.R. , Yu H. , Kass L. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling . Cell . 2009 ; 139 : 891 – 906 .
58. Baker A.M. , Cox T.R. , Bird D. et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer . J Natl Cancer Inst . 2011 ; 103 : 407 – 424 .
59. Li L. , Xie T. Stem cell niche: structure and function . Annu Rev Cell Dev Biol . 2005 ; 21 : 605 – 631 .
60. McAllister S.S. , Gifford A.M. , Greiner A.L. et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin . Cell . 2008 ; 133 : 994 – 1005 .
61. Psaila B. , Lyden D. The metastatic niche: adapting the foreign soil . Nat Rev Cancer . 2009 ; 9 : 285 – 293 .
62. Oskarsson T. , Acharyya S. , Zhang X.H. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs . Nat Med . 2011 ; 17 : 867 – 874 .
63. Malanchi I. , Santamaria-Martinez A. , Susanto E. et al. Interactions between cancer stem cells and their niche govern metastatic colonization . Nature . 2012 ; 481 : 85 – 89 .
64. Joyce J.A. , Pollard J.W. Microenvironmental regulation of metastasis . Nat Rev Cancer . 2009 ; 9 : 239 – 252 .
65. Ruffell B. , Affara N.I. , Coussens L.M. Differential macrophage programming in the tumor microenvironment . Trends Immunol . 2012 ; 33 : 119 – 126 .
66. Wyckoff J. , Wang W. , Lin E.Y. et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors . Cancer Res . 2004 ; 64 : 7022 – 7029 .
67. Zabuawala T. , Taffany D.A. , Sharma S.M. et al. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis . Cancer Res . 2010 ; 70 : 1323 – 1333 .
68. Chen Q. , Zhang X.H. , Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs . 123 Cell . 2011 ; 20 : 538 – 549 .
69. Hanahan D. , Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment . Cancer Cell . 2012 ; 21 : 309 – 322 .
70. Gocheva V. , Wang H.W. , Gadea B.B. et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion . Genes Dev . 2010 ; 24 : 241 – 255 .
71. Tan W. , Zhang W. , Strasner A. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling . Nature . 2011 ; 470 : 548 – 553 .
72. Luo J.L. , Tan W. , Ricono J.M. et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin . Nature . 2007 ; 446 : 690 – 694 .
73. Gabrilovich D.I. , Ostrand-Rosenberg S. , Bronte V. Coordinated regulation of myeloid cells by tumours . Nat Rev Immunol . 2012 ; 12 : 253 – 268 .
74. Youn J.I. , Gabrilovich D.I. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity . Eur J Immunol . 2010 ; 40 : 2969 – 2975 .
75. Yang L. , Huang J. , Ren X. et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis . Cancer Cell . 2008 ; 13 : 23 – 35 .
76. Shojaei F. , Wu X. , Malik A.K. et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells . Nat Biotechnol . 2007 ; 25 : 911 – 920 .
77. Acharyya S. , Oskarsson T. , Vanharanta S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis . Cell . 2012 ; 150 : 165 – 178 .
78. Gao D. , Joshi N. , Choi H. et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition . Cancer Res . 2012 ; 72 : 1384 – 1394 .
79. Honn K.V. , Tang D.G. , Crissman J.D. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev . 1992 ; 11 : 325 – 351 .
80. Kim Y.J. , Borsig L. , Varki N.M. , Varki A. P-selectin deficiency attenuates tumor growth and metastasis . Proc Natl Acad Sci U S A . 1998 ; 95 : 9325 – 9330 .
81. Gay L.J. , Felding-Habermann B. Contribution of platelets to tumour metastasis . Nat Rev Cancer . 2011 ; 11 : 123 – 134 .
82. Nieswandt B. , Hafner M. , Echtenacher B. , Mannel D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets . Cancer Res . 1999 ; 59 : 1295 – 1300 .
83. Labelle M. , Begum S. , Hynes R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis . Cancer Cell . 2011 ; 20 : 576 – 590 .
84. Skolnik G. , Bagge U. , Blomqvist G. , Dahlstrom A. , Ahlman H. Involvement of platelet-released 5-HT in tumor cell lodgement . J Surg Res . 1985 ; 38 : 559 – 567 .
85. Pantel K. , Brakenhoff R.H. , Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells . Nat Rev Cancer . 2008 ; 8 : 329 – 340 .
86. Cristofanilli M. , Budd G.T. , Ellis M.J. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer . N Engl J Med . 2004 ; 351 : 781 – 791 .
87. Aguirre-Ghiso J.A. Models, mechanisms and clinical evidence for cancer dormancy . Nat Rev Cancer . 2007 ; 7 : 834 – 846 .
88. Hermanek P. , Sobin L.H. , Wittekind C. How to improve the present TNM staging system . Cancer . 1999 ; 86 : 2189 – 2191 .
89. Singletary S.E. , Greene F.L. , Sobin L.H. Classification of isolated tumor cells: clarification of the 6th edition of the . American Joint Committee on Cancer Staging Manual. Cancer . 2003 ; 98 : 2740 – 2741 .
90. Pantel K. , Alix-Panabieres C. , Riethdorf S. Cancer micrometastases . Nat Rev Clin Oncol . 2009 ; 6 : 339 – 351 .
The Molecular Basis of Cancer Expert Consult - Online