Intrapartum Fetal Assessment and Therapy
Elizabeth G. Livingston MD
Chapter Outline
The value of intrapartum obstetric interventions to assess and maintain fetal well-being has been scrutinized in recent years. Old technologies have been reassessed, and new ones have been introduced. A rising cesarean delivery rate, renewed interest in home birth, persistent cases of fetal/neonatal neurologic injury, and excessive litigation have prompted an ongoing search for methods to identify and intervene on behalf of the fetus at risk during labor and delivery.
Fetal Risk during Labor
Historical epidemiologic data suggest that the fetus is at increased risk for morbidity and mortality during labor and delivery. In 1963, the British Perinatal Mortality Survey reviewed autopsy data for 1400 stillborn infants and concluded that slightly more than 30% of these losses resulted from intrapartum asphyxia.1 Neonatal outcomes in the industrialized world have improved over the past 40 years. A Canadian database identified approximately 80 intrapartum stillbirths per 120,000 live births from 1981 to 2002, a rate of 0.67/1000; some 11 of the infants were considered viable (i.e., not severely preterm or anomalous), which indicated a preventable death rate of 0.09/1000.2 A Scottish study indicated that perinatal deaths due to intrapartum anoxia in term, singleton, cephalic infants declined significantly from 5.7 to 3.0/10,000 births between 1988 and 2007.3 Intrapartum stillbirths are rare in developed countries, constituting less than 10% of all stillbirths. By contrast, in some developing countries, as many as 50% of stillbirths occur intrapartum.4 According to a World Health Organization report, intrapartum-related neonatal deaths account for almost 10% of deaths in children younger than 5 years of age.5,6
Experimental models lend support to the hypothesis that intrapartum events can have long-term neurologic sequelae. Fetal monkeys subjected to hypoxia in utero suffer neurologic injuries similar to those seen in children who presumably suffered asphyxia in utero.7–9 Work with rodent models has shown similar patterns of damage.10 Hankins et al.11 suggested that performance of elective cesarean delivery at 39 weeks’ gestation (and avoidance of a trial of labor) might result in an 83% reduction in risk for moderate or severe neonatal encephalopathy. Altogether, epidemiologic and experimental data suggest that the fetus is at significant risk during labor and delivery.
Some fetuses appear to be at greater risk for adverse intrapartum events than others. Older studies report that high-risk mothers constitute 20% of the pregnant population, but their offspring represent 50% of the cases of perinatal morbidity and mortality.12 Various schemes to identify high-risk pregnancies have been published.13,14 High-risk pregnancies include, but are not limited to, women with (1) medical complications (e.g., hypertension, preeclampsia, diabetes, autoimmune disease, hemoglobinopathy); (2) fetal complications (e.g., fetal growth restriction [also known as intrauterine growth restriction], nonlethal anomalies, prematurity, multiple gestation, postdatism, hydrops); and (3) intrapartum complications (e.g., abnormal vaginal bleeding, maternal fever, meconium-stained amniotic fluid, oxytocin augmentation of labor). Owing to inadequate sensitivity, poor positive predictive values, and the inability to modify outcomes related to risk factors, scoring systems for identifying high-risk fetuses have not been shown to improve pregnancy outcomes.15 Lo et al.16 indicated that more than half of infants with asphyxia had no clinical risk factors. However, for low-risk parturients who wish to avoid continuous monitoring during labor, scoring systems may demonstrate some benefit.15 One strategy used in European centers for identifying high-risk parturients is fetal heart rate (FHR) tracing analysis at the time of admission to the labor and delivery suite; if the FHR tracing is abnormal, patients receive more intensive monitoring.17 A meta-analysis of this approach observed an increase in cesarean delivery rates but failed to demonstrate improvements in fetal and neonatal outcomes.18
The magnitude of risk for intrapartum fetal neurologic injury is a matter of some dispute. In 2003, the American College of Obstetricians and Gynecologists (ACOG) Task Force on Neonatal Encephalopathy and Cerebral Palsy concluded that 70% of these types of fetal neurologic injuries result from events that occur before the onset of labor.19–21 Examples of antepartum events that may cause fetal neurologic injury include congenital anomalies, chemical exposure, infection, and fetal thrombosis/coagulopathy. Only 4% of cases of neonatal encephalopathy result solely from intrapartum hypoxia, an incidence of approximately 1.6/1000.19,20,22 The Task Force identified criteria that define an acute intrapartum hypoxic event sufficient to cause cerebral palsy (Box 8-1).19,20,23 A recent study found that fetuses that underwent a sudden and sustained deterioration of the FHR and that subsequently had a diagnosis of cerebral palsy demonstrated characteristics consistent with the criteria developed by the ACOG Task Force.24 An additional case series of sentinel events during labor (e.g., uterine rupture, cord prolapse, placental abruption, amniotic fluid embolus) resulted in a high rate of hypoxic-ischemic encephalopathy in surviving infants.25 Approximately 25% of fetuses may have antepartum and intrapartum risk factors for neurologic injury.
The ability of contemporary obstetricians to recognize and treat pregnancies at risk for hypoxia during labor is an evolving science. With the current understanding of pathophysiology and the technology in current clinical use, the extent to which obstetricians can prevent intrapartum injury is unclear.26 It is hoped that the development of standardized, clearer definitions of intrapartum injury, and new strategies and interventions that can correct reversible pathophysiology, will lead to more precise identification and optimization of fetuses at risk.
Efforts to understand placental physiology and pathophysiology are central to the efforts to support the health of the pregnant woman and her fetus, both antepartum and intrapartum. The fetus depends on the placenta for the diffusion of nutrients and for respiratory gas exchange. Many factors affect placental transfer, including concentration gradients, villus surface area, placental permeability, and placental metabolism (see Chapter 4). Maternal hypertensive disease, congenital anomalies, and intrauterine infection are examples of conditions that may impair placental transfer.
One of the most important determinants of placental function is uterine blood flow.27 A uterine contraction results in a transient decrease in uteroplacental blood flow. A placenta with borderline function before labor may be unable to maintain gas exchange adequate to prevent fetal asphyxia during labor. The healthy fetus may compensate for the effects of hypoxia during labor.28,29 The compensatory response includes (1) decreased oxygen consumption, (2) vasoconstriction of nonessential vascular beds, and (3) redistribution of blood flow to the vital organs (e.g., brain, heart, adrenal glands, placenta).30,31 Humoral responses (e.g., release of epinephrine from the adrenal medulla, release of vasopressin and endogenous opioids) may enhance fetal cardiac function during hypoxia.27 Prolonged or severe hypoxia overwhelms these compensatory mechanisms, resulting in fetal injury or death.
Intrapartum Fetal Assessment
Electronic Fetal Heart Rate Monitoring
An optimal, yet practical method for assessing fetal health during labor and delivery has not been developed. Most contemporary methods include assessment of the FHR. The FHR can be monitored intermittently with a simple DeLee stethoscope. Alternatively, either Doppler ultrasonography or a fetal electrocardiographic (ECG) electrode can be used to monitor the FHR intermittently or continuously.
Experimental models have provided insight into the regulation of the FHR. Both neuronal and humoral factors affect the intrinsic FHR. Parasympathetic outflow by means of the vagus nerve decreases the FHR, whereas sympathetic activity increases FHR and cardiac output.27 Baroreceptors respond to increased blood pressure and chemoreceptors respond to decreased PaO2 and increased PaCO2 to modulate the FHR through the autonomic nervous system. Cerebral cortical activity and hypothalamic activity affect the FHR through their effects on integrative centers in the medulla oblongata (Figure 8-1).27 Both animal studies and clinical observations have helped establish a correlation between FHR and perinatal outcome.
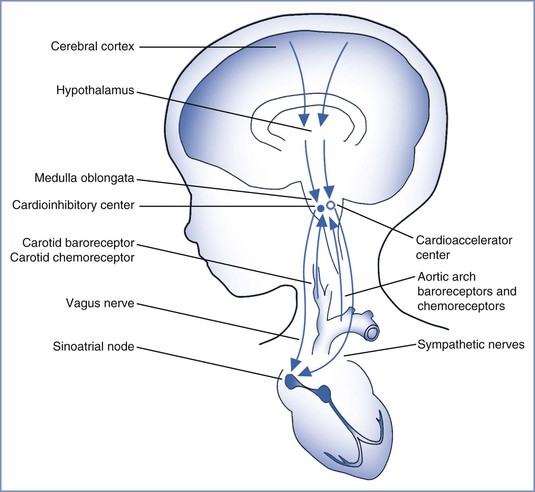
FIGURE 8-1 Regulation of fetal heart rate. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
An electronic monitor simultaneously records the FHR and uterine contractions. Use of an electronic monitor allows determination of the baseline rate and patterns of the FHR and their relationship to uterine contractions. External or internal techniques can assess the FHR and uterine contractions (Figure 8-2). Doppler ultrasonography detects the changes in ventricular wall motion and blood flow in major vessels during each cardiac cycle. The monitor calculates the FHR by measuring the intervals between fetal myocardial contractions. Alternatively, an ECG lead attached to the fetal scalp enables the cardiotachometer to calculate the FHR by measuring each successive RR interval. Both external and internal methods allow continuous assessment of the FHR.
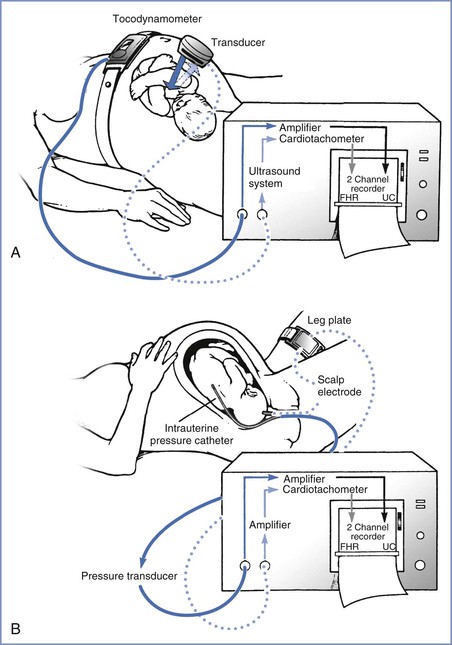
FIGURE 8-2 Electronic fetal monitoring apparatus. A, Instrumentation for external monitoring. Contractions are detected by the pressure-sensitive tocodynamometer, amplified, and then recorded. The fetal heart rate (FHR) is monitored with the Doppler ultrasound transducer, which emits and receives the reflected ultrasound signal that is then counted and recorded. B, Techniques used for direct monitoring of FHR and uterine contractions (UC). Uterine contractions are assessed with an intrauterine pressure catheter connected to a pressure transducer. This signal is then amplified and recorded. The fetal electrocardiogram (ECG) is obtained by direct application of the scalp electrode, which is then attached to a leg plate on the mother’s thigh. The signal is transmitted to the monitor, where it is amplified, counted by the cardiotachometer, and recorded. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
The FHR is superimposed over the uterine contraction pattern. Uterine contractions can be monitored externally with a tocodynamometer or internally with an intrauterine pressure catheter. The tocodynamometer allows determination of the approximate onset, duration, and offset of each uterine contraction. A normal pattern of uterine contractions in labor is five or less in a 10-minute period averaged over 30 minutes; tachysystole is defined as more than five uterine contractions within a 10-minute period.32 An intrauterine pressure catheter may be used to measure the strength of uterine contractions. In some cases, an intrauterine pressure catheter is placed to determine the precise onset and offset of each uterine contraction. Such information may be used to distinguish among early, variable, and late FHR decelerations. In a nonblinded, randomized controlled trial, Bakker et al.33 observed no significant differences in adverse neonatal outcomes with internal tocodynamometry as compared with external monitoring of uterine contractions in women in whom oxytocin was used for induction or augmentation of labor.
The following features of the FHR pattern can be assessed: (1) baseline measurements, (2) variability (the extent to which the rate changes both instantaneously and over longer periods), (3) accelerations, and (4) decelerations and their association with uterine contractions.
Baseline Fetal Heart Rate
A normal baseline FHR is defined as 110 to 160 beats per minute (bpm) and is determined by assessing the mean heart rate over a 10-minute period rounded to increments of 5 bpm.32,34 In general, term fetuses have a lower baseline FHR than preterm fetuses because of greater parasympathetic nervous system activity. Laboratory studies suggest that bradycardia (caused by increased vagal activity) is the initial fetal response to acute hypoxemia. After prolonged hypoxemia, the fetus may experience tachycardia as a result of catecholamine secretion and sympathetic nervous system activity.28 Changes in baseline FHR may also be caused by fetal anatomic or functional heart pathology, maternal fever and/or intrauterine infections, or maternally administered medications, such as beta-adrenergic receptor agonists (e.g., terbutaline) or the anticholinergic agent atropine.
Fetal Heart Rate Variability
Fetal heart rate variability is the fluctuation in the FHR of 2 cycles or greater per minute.32,34 Previously, FHR variability was divided into short term (from one beat, or R wave, to the next) and long term (occurring over the course of 1 minute), but this distinction is no longer made because in clinical practice variability is visually assessed as a unit (Figure 8-3). The presence of normal FHR variability reflects the presence of normal, intact pathways from—and within—the fetal cerebral cortex, midbrain, vagus nerve, and cardiac conduction system (see Figure 8-1).27 Variability is greatly influenced by the parasympathetic tone, by means of the vagus nerve; maternal administration of atropine, which readily crosses the placenta, can eliminate some variability. In humans, the sympathetic nervous system appears to have a lesser role in influencing variability.27 Maternal administration of the beta-adrenergic receptor antagonist propranolol has little effect on FHR variability.27
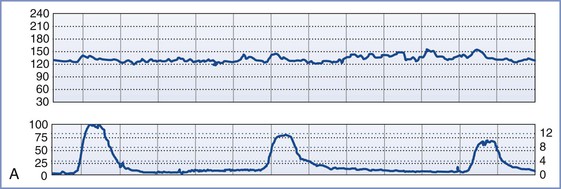
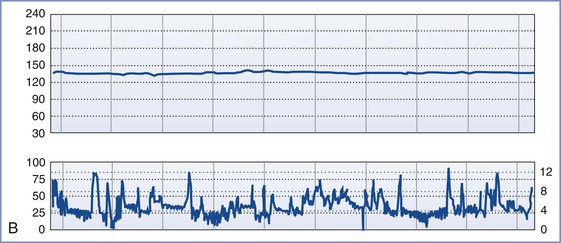
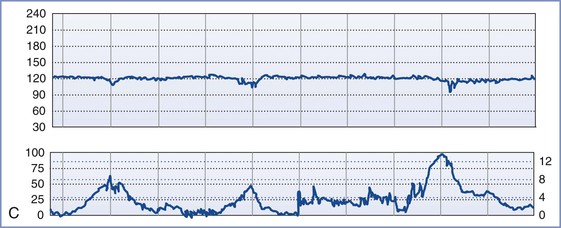
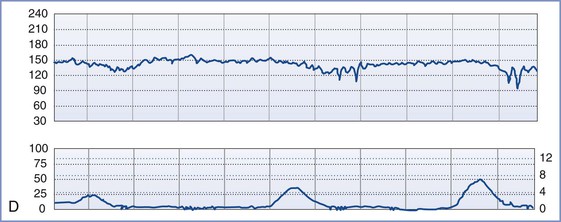
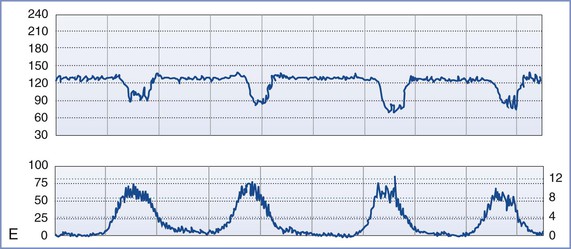
FIGURE 8-3 A, Normal intrapartum fetal heart rate (FHR) tracing. The infant had Apgar scores of 8 and 8 at 1 and 5 minutes, respectively. B, Absence of variability in a FHR tracing. Placental abruption was noted at cesarean delivery. The infant had an umbilical arterial blood pH of 6.75 and Apgar scores of 1 and 4, respectively. C, Early FHR decelerations. After a normal spontaneous vaginal delivery, the infant had Apgar scores of 8 and 8, respectively. D, Late FHR decelerations. The amniotic fluid surrounding this fetus was meconium stained. Despite the late FHR decelerations, the variability remained acceptable. The infant was delivered by cesarean delivery and had an umbilical venous blood pH of 7.30. Apgar scores were 9 and 9, respectively. E, Variable FHR decelerations. A tight nuchal cord was noted at low-forceps vaginal delivery. The infant had Apgar scores of 6 and 9, respectively. Numerical scales: Left upper panel margin, FHR in beats per minute; left lower panel margin, uterine pressure in mm Hg; right lower panel margin, uterine pressure in kilopascal (kPa).
During hypoxemia, myocardial and cerebral blood flows increase to maintain oxygen delivery.35,36 However, in severe hypoxemia, blood flow cannot increase sufficiently to maintain oxygen delivery and a loss of FHR variability is observed.27 The absence of FHR variability in an anencephalic fetus indicates the influence of an intact central nervous system (CNS) in producing these patterns. In animal models, perfusion of the CNS with calcium results in depolarization of electroencephalographic (EEG) activity, which abolishes FHR variability.
Clinically, the presence of normal FHR variability predicts early neonatal health, as defined by an Apgar score of greater than 7 at 5 minutes.37,38 In a case series of monitored fetal deaths, no fetus had normal variability immediately before demise.27 The differential diagnosis of decreased variability includes fetal hypoxia, fetal sleep state, fetal neurologic abnormality, and decreased CNS activity that results from exposure to drugs such as opioids.
Accelerations
Accelerations are abrupt changes in the FHR above the baseline. Beyond 32 weeks’ gestation, an acceleration is defined by a peak of at least 15 bpm above the baseline, lasting at least 15 seconds. (Before 32 weeks’ gestation, a peak of 10 bpm above the baseline, lasting at least 10 seconds, is required.34) A prolonged acceleration extends for at least 2 minutes; however, when greater than 10 minutes in duration, it is considered a change in baseline.34 Antepartum FHR accelerations can occur in response to fetal movement and typically are a sign of fetal well-being; their presence indicates a reactive nonstress test. During the intrapartum period, the significance of FHR accelerations is less clear.28,38 Although the presence of accelerations generally precludes the existence of significant fetal metabolic acidosis, in some cases intrapartum accelerations may indicate a vulnerable umbilical cord.
Decelerations
Decelerations are typically classified as early, late, or variable. Early decelerations occur simultaneously with uterine contractions and usually are less than 20 bpm below baseline. The onset and offset of each deceleration coincides with the onset and offset of the uterine contraction (see Figure 8-3). In animal models, head compression can precipitate early decelerations.28 In humans, early decelerations are believed to result from reflex vagal activity secondary to mild hypoxia. Early decelerations are not ominous.
Late decelerations begin 10 to 30 seconds after the beginning of uterine contractions and end 10 to 30 seconds after the end of uterine contractions. Late decelerations are smooth and repetitive (i.e., they occur with each uterine contraction). Animal studies suggest that late decelerations represent a response to hypoxemia. The delayed onset of the deceleration reflects the time needed for the chemoreceptors to detect decreased oxygen tension and mediate the change in FHR by means of the vagus nerve.28,39 Late decelerations may also result from decompensation of the myocardial circulation and myocardial failure. Unfortunately, clinical and animal studies suggest that late decelerations may be an oversensitive indication of fetal asphyxia.28 However, the combination of late decelerations and decreased or absent FHR variability is an accurate, ominous signal of fetal compromise.29,40,41
Variable decelerations vary in depth, shape, and duration. They often are abrupt in onset and offset. Variable decelerations result from baroreceptor- or chemoreceptor-mediated vagal activity. Experimental models and clinical studies suggest that umbilical cord occlusion, either partial or complete, results in variable decelerations. During the second stage of labor, variable decelerations may result from compression of the fetal head. In this situation, dural stimulation leads to increased vagal discharge.42 The healthy fetus can typically tolerate mild to moderate variable decelerations (not below 80 bpm) without decompensation. With prolonged, severe variable decelerations (< 60 bpm) or persistent fetal bradycardia, it is difficult for the fetus to maintain cardiac output and umbilical blood flow.42
Sinusoidal and saltatory patterns are two unusual FHR tracing results that may indicate fetal compromise. The sinusoidal FHR pattern is a regular, smooth, wavelike pattern that may signal fetal anemia.27 Occasionally, maternal administration of an opioid can lead to a sinusoidal FHR pattern. The saltatory pattern consists of excessive alterations in variability (> 25 bpm) and may signal the occurrence of acute fetal hypoxia; there is a weak association between this pattern and low Apgar scores.27
Limitations of Electronic Fetal Heart Rate Monitoring
Despite laboratory and clinical data suggesting that FHR monitoring accurately reflects fetal health, controversy exists regarding the ability of this assessment tool to improve fetal and neonatal outcomes. First described more than 40 years ago, the use of continuous electronic FHR monitoring increased dramatically to encompass 45% and 85% of the monitored deliveries by 1980 and 2002, respectively.32 Retrospective reports of continuous FHR monitoring associate its use with a lower incidence of intrauterine fetal demise, neonatal seizures, and neonatal death.43–45 By contrast, the only consistent finding from multiple case-control studies and more than a dozen prospective, randomized trials of electronic FHR monitoring (with control arms that employed intermittent FHR auscultation46–48) is an increased rate of operative delivery. In a meta-analysis of these trials, which included more than 50,000 women from several continents, the incidence of 1-minute Apgar scores less than 4 and neonatal seizures was decreased with the use of continuous FHR monitoring.46–49 These results appear to suggest a correlation between abnormal FHR tracings and fetal acidemia.41,50 An evaluation of the 2004 United States birth cohort data suggested that the use of electronic FHR monitoring was associated with a significant decrease in early neonatal and infant mortality, a decreased risk for 5-minute APGAR scores less than 4 in low-risk pregnancies, and a lower rate of neonatal seizures in high-risk pregnancies.51
It remains unclear why prospective studies have not confirmed greater benefit of the use of continuous electronic FHR monitoring during labor; the intensity of intrapartum assessment and care may be partially responsible. In prospective trials, women randomly assigned to receive intermittent FHR auscultation were monitored by dedicated nursing staff who provided intensive intrapartum care. By contrast, the historical cohort studies compared patients who received continuous electronic FHR monitoring and intensive intrapartum care with patients who had intermittent FHR auscultation with nonintensive nursing care. There are no published studies that randomly assigned a group of patients to receive no FHR monitoring; however, the continued high rate of intrapartum stillbirth in unmonitored births in the developing world suggests that FHR assessment may be beneficial.
Consistent with the findings of the prospective trials, the ACOG endorses the use of either intermittent auscultation or continuous electronic FHR monitoring during labor. In high-risk patients, the ACOG guidelines recommend that the obstetrician or nurse review the electronic FHR tracing every 15 minutes during the first stage of labor and every 5 minutes during the second stage. For low-risk patients, the intervals may be lengthened to 30 minutes for the first stage and 15 minutes for the second stage.52 The optimal interval for intermittent FHR monitoring has not been studied, but the intervals suggested within the ACOG guidelines have some indirect support.53 Adherence to these standards for intermittent auscultation may be difficult to achieve in the clinical setting; in one study, only 3% of parturients met these standards.54
Several hypotheses to account for the apparent failure of intrapartum FHR monitoring to reduce the incidence of cerebral palsy have been proposed and include (1) a large proportion of the asphyxial damage begins before the onset of labor; (2) catastrophic events (e.g., cord prolapse, placental abruption, uterine rupture) may not allow sufficient time for intervention before neurologic damage occurs; (3) a larger proportion of very low-birth-weight infants survive and thus contribute to the numbers with cerebral palsy; (4) infection is associated with abnormal FHR patterns and the subsequent development of cerebral palsy, and it is unclear that early intervention offers any benefit in such cases; and (5) the amount of asphyxia required to cause permanent neurologic damage approximates the amount that causes fetal death, leaving a narrow window for intervention.55 The number of patients in whom cerebral palsy develops from intrapartum asphyxia is probably quite small.55
Limitations of FHR monitoring include a poor positive predictive value in distinguishing between abnormal FHR tracings and abnormal outcomes. Because of this imprecision, the ACOG recommended that abnormal FHR tracings be described with the term nonreassuring fetal status rather than fetal distress or birth asphyxia.56 In one population-based study of California children with cerebral palsy, FHR tracings were retrospectively reviewed and compared with those of neurologically normal children (i.e., control subjects). A markedly higher incidence of tracings with late decelerations and decreased variability was found in children with cerebral palsy than in the control subjects. However, of the estimated 10,791 monitored infants weighing 2500 g or more who had these FHR abnormalities, only 21 (0.19%) had cerebral palsy, representing a false-positive rate of 99.8%.57 Later case-control studies have yielded similar results.16,58 A 2006 meta-analysis of 12 trials including 37,000 women suggested that electronic FHR monitoring has resulted in a decrease in the occurrence of seizures but no change in the incidence of neonatal mortality or cerebral palsy.49 The authors suggested that electronic FHR monitoring resulted in one cesarean delivery for every 58 women monitored and that 661 women would need to be monitored to prevent one neonatal seizure.49 Therefore, use of electronic FHR monitoring in combination with clinical and laboratory assessments has been proposed to enhance the prediction and perhaps the prevention of severe asphyxia, but with poor specificity.16
Further limitations of continuous FHR monitoring include (1) the poor intra-observer and inter-observer agreement despite the use of trained observers, (2) the required continual presence of a nurse or physician to assess the FHR tracing, (3) the inconvenience for the patient (e.g., confinement to bed and the application of monitor belts or a scalp electrode), and (4) the need to archive the FHR tracings as legal documents.59–61
Despite little evidence for its efficacy, Parer and King62 have noted that obstetricians continue to rely heavily on FHR monitoring for at least the following three reasons: (1) professional obstetric organizations (e.g., the ACOG) advise some form of monitoring during labor, (2) electronic FHR monitoring is logistically easier and less expensive than one-on-one nursing care during labor, and (3) individual (often anecdotal) experiences cause “many obstetricians [to] believe that in their own hands FHR monitoring is … efficacious.”62
In 2008 the National Institute of Child Health and Human Development sponsored a workshop that resulted in the publication of updated definitions, interpretation, and research guidelines for intrapartum electronic FHR monitoring.34 The published report proposed a three-tier system for the categorization of FHR patterns (Box 8-2).34 The ACOG described this system in subsequent 2009 practice bulletins with suggested options for management32,63:
In a multicenter review of 48,444 patients, Jackson et al.64 observed that category I, II, and III FHR tracings were present 78%, 22%, and 0.004% of the time, respectively. Only 0.2% of newborns of women whose last 2 hours of labor had exclusively category I FHR tracings had low 5-minute Apgar scores followed by admission to a neonatal intensive care unit (NICU); in contrast, when more than 75% of the last 2 hours of labor showed category II FHR tracings, the incidence of low 5-minute Apgar scores with NICU admission increased to 0.7%.
A significant difficulty with the current three-tier system is the number and heterogeneity of fetuses with category II tracings.65 A five-tier system has been proposed.66,67 However, Elliott et al.68 used specialized computer software to distinguish the five tiers among 2472 FHR tracings of near-term fetuses with and without known neonatal encephalopathy and blood gas abnormalities, and they found that a lack of specificity still occurred. A correlation between the frequency and duration of FHR abnormalities with worse neonatal outcome was observed; however, the electronic FHR patterns that identified 75% of the neonatal encephalopathy group also included 29% of the normal neonates.68
Methods for Improving the Efficacy of Electronic Fetal Heart Rate Monitoring
Several technologies have been employed to enhance the value of electronic FHR monitoring. To facilitate continual FHR assessment, many labor-and-delivery units transmit the tracings from the bedside to the nurses’ station. Presumably, this practice facilitates a rapid response to worrisome FHR tracings.
Computerized algorithms may assist in the interpretation of FHR tracings. Although some studies have suggested that computerized analysis may be more accurate than traditional methods in identifying pregnancies with a pathologic neonatal outcome, others have not confirmed this finding.69–73 As a result, none of these computerized methods has achieved widespread use.
Continuous FHR monitoring requires the patient to wear FHR and uterine contraction monitoring devices and remain within several feet of the monitor. An alternative is the use of telemetry, which transmits the FHR from the patient to the monitor and consequently allows ambulation. The low-risk patient who wishes to ambulate probably does not require continuous electronic FHR monitoring.
Electronic archiving allows for the electronic storage and retrieval of FHR tracings and eliminates the need for long-term storage of the paper record. The FHR tracing is a medicolegal document, and if it is lost, the plaintiff’s lawyer may allege that the tracing was discarded intentionally because it was detrimental to the defendant.74
Supplemental Methods of Fetal Assessment
Electronic FHR monitoring is more than 99% accurate in predicting a 5-minute Apgar score greater than 7. Unfortunately, this monitoring also suffers from a lack of specificity; an abnormal FHR tracing has a false-positive rate of more than 99%.52 As a consequence, clinicians have sought additional fetal assessment tools to assist in the identification of the compromised fetus with greater specificity.
Fetal scalp blood pH determination is an older method used to confirm or exclude the presence of fetal acidosis when FHR monitoring suggests the presence of fetal compromise. Suggested indications include the presence of decreased or absent FHR variability or persistent late or variable FHR decelerations.27 The obstetrician inserts an endoscope into the vagina, makes a small laceration in the fetal scalp (or buttock), and uses a capillary tube to collect a sample of fetal capillary blood (Figure 8-4). The technical challenges of obtaining the sample and having readily available instrumentation to conduct the test have led most U.S. centers to abandon this technique. Although early studies suggested that fetal scalp sampling may decrease the cesarean delivery rate,75 a 1994 study reported that there was no change in the rate of cesarean delivery or perinatal asphyxia when the technique was abandoned.76 A similar, alternative method used in Europe evaluates the fetal blood sample for the presence of lactate. When compared with pH determination, an interpretable result is obtained more frequently with lactate determination primarily owing to a smaller volume of blood required; however, no differences in fetal/neonatal/infant outcomes have been observed in trials comparing the two methods.77
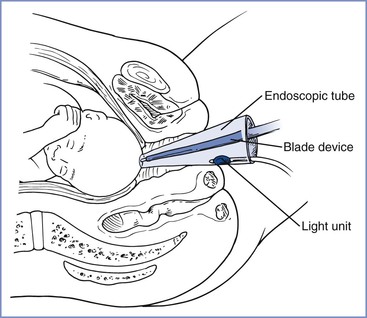
FIGURE 8-4 Technique of obtaining fetal scalp blood during labor. (Redrawn from Creasy RK, Parer JT. Perinatal care and diagnosis. In Rudolph AM, editor. Pediatrics. 16th edition. New York, Appleton-Century-Crofts, 1977:121.)
Fetal scalp stimulation can be performed during a vaginal examination either digitally or with an Allis clamp. The heart rate of a healthy, nonacidotic fetus accelerates in response to scalp stimulation; FHR acceleration is associated with a fetal pH of at least 7.19.78,79
Advocates of vibroacoustic stimulation contend that the application of an artificial larynx to the maternal abdomen results in an FHR acceleration in a healthy fetus and improves the specificity of FHR monitoring.80 Vibroacoustic stimulation is used primarily for antepartum fetal assessment. A 2005 Cochrane review concluded that there is insufficient evidence from randomized clinical trials on which to make conclusions and recommendations regarding the use of vibroacoustic stimulation for the evaluation of fetal well-being during labor in the presence of a nonreassuring FHR tracing.81 Nonetheless, direct fetal scalp stimulation and/or vibroacoustic stimulation have largely replaced the use of fetal scalp blood pH determination in most centers. A meta-analysis of intrapartum stimulation tests (i.e., fetal scalp blood pH determination, Allis clamp and digital fetal scalp stimulation, and vibroacoustic stimulation) found the tests to be equivalent in predicting fetal acidemia, with digital fetal scalp stimulation having the greatest ease of use.82
The intrapartum use of umbilical artery velocimetry has been used as an adjunct to FHR monitoring to assess fetal well-being, with mixed results (see Chapter 6).83,84 The biophysical profile (BPP), which is composed of four ultrasonographic assessments and a nonstress test, has been observed antenatally to decrease the false-positive rate of a positive nonstress test (see Chapter 6); however, its value during intrapartum management is still under investigation.85,86
The presence of meconium-stained amniotic fluid has long been associated with an increased risk for depression at birth. Moderate to thick meconium is associated with lower Apgar scores, lower umbilical arterial blood pH, an increased incidence of neonatal seizures, and higher rates of cesarean delivery and admission to an intensive care nursery.87–89 Although 5% to 20% of all deliveries are complicated by meconium-stained amniotic fluid, few of these infants experience neonatal depression. The odds ratio for complications is increased with meconium, but the majority of infants with neonatal complications have clear fluid.87 Meconium-stained fluid has a poor positive predictive value and poor sensitivity in predicting adverse neonatal outcomes.87 The physiology associated with the passage of meconium is incompletely understood. Ultrasonographic imaging suggests that the fetus regularly passes rectal contents into the amniotic fluid throughout gestation.87 However, meconium-stained amniotic fluid is more common in pregnancies complicated by postdatism or fetal growth restriction. Putative triggers for the passage of meconium include umbilical cord compression and hypoxia. The presence of meconium combined with an abnormal FHR tracing or another risk factor (e.g., fetal growth restriction, postdatism) appears to be associated with an increased likelihood of neonatal depression.88,89
Among the pregnancies with meconium-stained amniotic fluid, approximately 5% develop significant neonatal respiratory compromise termed meconium aspiration syndrome.90–92 Antenatal risk factors for this syndrome include moderate or thick meconium (suggesting recent passage and lower amniotic fluid volume) and abnormal FHR tracings.91 The lung injury likely originates from intrapartum fetal hypoxia.91,93,94 Oropharyngeal suctioning at delivery has not proved beneficial; randomized controlled trials have suggested that vigorous newborns do not need aggressive airway cleansing with tracheal intubation (see Chapter 9).91,95 Aggressive obstetric management of postdate pregnancies (i.e., avoidance of postdatism) has led to a substantial decrease in the incidence of meconium-stained amniotic fluid and meconium aspiration syndrome.92
New Technologies for Fetal Assessment
Because FHR monitoring provides only an indirect measure of fetal oxygenation and acid-base status, alternative technologies, such as transcutaneous PO2, PCO2, and pH monitors have been developed to provide a more direct assessment.96–98 However, the use of these monitors has been limited by technical difficulties in the application of the probe(s), drift in the baseline readings, artifactual measurements, and difficulties in establishing diagnostic criteria for intervention.
ST waveform analysis of fetal electrocardiography is a technique proposed to enhance intrapartum fetal assessment. Fetal hypoxia induces changes in the ECG morphology of the ST segment and T wave. ST waveform analysis enhances the specificity of FHR tracings,99,100 but not the sensitivity.101 A 2006 meta-analysis of randomized controlled trials, accounting for almost 10,000 deliveries, of automatic ST waveform analysis (the STAN S21 system, Neoventa Medical, Göteborg, Sweden) demonstrated that a combination of FHR monitoring and ST waveform analysis reduced the risk for severe fetal acidosis and the incidence of neonates with encephalopathy.102–104 No differences in the rates of cesarean delivery, 5-minute Apgar scores less than 7, or admission to the NICU were observed.100,102,103,105,106 In a recent trial of 5600 parturients randomized to fetal observation with and without ST waveform analysis, Westerhuis et al.107 observed a lower rate of acidosis in the ST waveform analysis group but no other differences in outcome (e.g., operative deliveries or hypoxic ischemic encephalopathy). An unmasked, randomized controlled trial of ST waveform analysis is currently being conducted to assess neonatal outcomes in 11,000 parturients within the U.S. Maternal-Fetal Medicine Network.
Reflectance pulse oximetry has been adapted for assessment of fetal oxygenation. The U.S. Food and Drug Administration (FDA) approved the Nellcor N-400 fetal pulse oximeter (Nellcor, Puritan Bennett, Pleasanton, CA) for use in the setting of a term, singleton fetus at more than 36 weeks’ gestation with a vertex presentation and a nonreassuring FHR pattern after rupture of membranes.108,109 The most commonly used probes are held in place against the fetal head or cheek with pressure from the cervix. A reliable pulse oximetry signal can be obtained in 60% to 70% of cases; however, environmental factors and physiologic events (e.g., fetal scalp congestion, thick fetal hair, vernix caseosa, uterine activity, movement artifacts) may affect the accuracy.109 The saturation measurements are averaged every 45 seconds,108 and the human fetus typically demonstrates an oxygen saturation of 35% to 65%. Animal and human data suggest that metabolic acidosis does not occur until the oxygen saturation has fallen below 30% for at least 10 minutes when measured with this device.110,111 Pulse oximetry does not predict acidosis accurately in fetuses with severe variable decelerations during the second stage of labor.112 Moreover, the accuracy of fetal pulse oximetry readings lower than 30% in human fetuses has been challenged.108,113 Fetal pulse oximetry used in conjunction with FHR monitoring appears to reduce the rate of cesarean delivery for a nonreassuring FHR tracing; however, this reduction is offset by an increased rate of cesarean delivery for dystocia.114–116 The absence of an effect on the overall cesarean delivery rate prompted the ACOG to withhold an endorsement of fetal pulse oximetry pending further investigation of its use.109 A Spanish trial randomly allocated 180 women with nonreassuring intrapartum FHR tracings to receive further evaluation with either ST waveform analysis or pulse oximetry; the investigators observed a lower cesarean delivery rate in the ST waveform analysis group, with fewer low 1-minute APGAR scores and improved umbilical cord venous blood pH measurements.117
Proton magnetic resonance spectroscopy (1H MRS) can obtain metabolic information from human and animal brains, and early investigations suggest an ability to assess fetal brain oxygenation.118–120 1H MRS has proved useful in the evaluation of hypoxic-ischemic encephalopathy and metabolic disorders in pediatric patients. This technique can also measure levels of the metabolites lactate, N-acetylaspartate, creatine, choline, and inositol in fetal and neonatal neural tissue. It has been applied experimentally in a case series of fetuses with fetal growth restriction.121 Although these measurements can be correlated with the level of tissue oxygenation, the clinical use of this technique as a means of fetal assessment remains unclear.122
Near-infrared spectroscopy (NIRS) has the potential to directly measure fetal tissue oxygenation.123 Transabdominal NIRS has been used in research settings to assess placental oxygenation.124 NIRS has gained some acceptance in assessing the effects of clinical interventions on neonatal organ oxygenation at the bedside. NIRS can detect changes in the ratio of reduced to oxygenated cytochrome-c oxidase in the brain and in the ratio of oxygenated to deoxygenated hemoglobin in the blood perfusing the brain; the technique can also measure the total amount of hemoglobin in the tissue, thereby allowing an estimation of tissue blood perfusion.123 In theory, NIRS offers the opportunity to determine whether neurons are at risk for hypoxic damage; currently, the technique is being correlated with other measures of fetal well-being, such as periodic FHR changes and umbilical cord blood pH measurements at delivery. However, similar to fetal pulse oximetry, NIRS technology is limited by a frequent (approximately 20%) inability to obtain interpretable measurements and the need to correlate the measurements with long-term neurodevelopmental outcomes. As a consequence, NIRS remains a research rather than a clinical tool at this time.123
Intrapartum Fetal Therapy
The ACOG has suggested intrapartum management be based on the three-tier evaluation framework.63 A category I tracing requires only periodic reevaluation, whereas a category III tracing should prompt preparation for delivery if intrauterine resuscitative measures do not result in improvement of the FHR in a timely manner (Table 8-1). Category II tracings should be evaluated for the presence of moderate variability and spontaneous or provoked accelerations, which suggest a nonacidotic fetus (see earlier discussion). The identification of potential intrapartum fetal compromise with a category II or III tracing should prompt a careful assessment of maternal, placental, and fetal factors. Clinical history, physical findings, laboratory findings, and fetal monitoring (e.g., FHR, ultrasonography) should be evaluated in an attempt to identify an etiology for poor tracings. If intrauterine resuscitative measures do not result in an improved category II tracing, or if the FHR tracing progresses to category III, then the obstetrician should consider prompt delivery.63
TABLE 8-1
Various Intrauterine Resuscitative Measures for Category II or Category III Fetal Heart Rate (FHR) Tracings or Both
| Goal | Associated Fetal Heart Rate Abnormality* | Potential Intervention(s)† |
| Promote fetal oxygenation and improve uteroplacental blood flow | Recurrent late decelerations Prolonged decelerations or bradycardia Minimal or absent FHR variability |
Initiate lateral positioning (either left or right) Administer supplemental maternal oxygen Administer intravenous fluid bolus Reduce uterine contraction frequency |
| Reduce uterine activity | Tachysystole with category II or III tracing | Discontinue oxytocin or cervical ripening agents Administer tocolytic medication (e.g., terbutaline) |
| Alleviate umbilical cord compression | Recurrent variable decelerations Prolonged decelerations or bradycardia |
Initiate maternal repositioning Initiate amnioinfusion If prolapsed umbilical cord is noted, elevate the presenting fetal part while preparations are underway for operative delivery |
* Evaluation for the underlying suspected cause(s) is also an important step in management of abnormal FHR tracings.
† Depending on the suspected underlying cause(s) of FHR abnormality, combining multiple interventions simultaneously may be appropriate and potentially more effective than doing individually or serially (Simpson KR, James DC. Efficacy of intrauterine resuscitation techniques in improving fetal oxygen status during labor. Obstet Gynecol 2005;105:1362-8).
From American College of Obstetricians and Gynecologists. Management of intrapartum fetal heart rate tracings. ACOG Practice Bulletin No. 116, November 2010. (Obstet Gynecol 2010; 116:1232-40.)
Correctable maternal factors that may contribute to fetal compromise include pathologic states that result in hypoxemia or decreased oxygen delivery to the placenta. Respiratory failure due to long-standing diseases (e.g., asthma) can be determined from the history and physical findings, whereas additional laboratory measurements may be necessary to diagnose pneumonia or pulmonary edema as an underlying cause. Decreased oxygen delivery to the placenta may result from acute (e.g., sepsis, hypotension) or chronic conditions. Decreased uteroplacental perfusion can result from reduced maternal cardiac output (e.g., cardiovascular disease) or chronic vascular disease (e.g., chronic hypertension, diabetes). Dehydration from prolonged labor is a more subtle cause of diminished uteroplacental perfusion.
Attention to the etiology of fetal hypoxemia and institution of appropriate treatments may mitigate fetal compromise. Administration of supplemental oxygen may enhance fetal oxygenation, even in the previously normoxemic mother; however, whether maternal oxygen therapy improves fetal outcome remains unclear.125–128
Uterine hypertonus or frequent uterine contractions (tachysystole), which may result in decreased uteroplacental perfusion, are known risks of oxytocin or prostaglandin compounds used for the induction of labor. Uterine contractions constrict the uterine spiral arteries, decreasing oxygen delivery to the placenta. A rare cause of fetal compromise is uterine rupture, which may result from uterine hyperstimulation, particularly in the setting of a uterine scar. Placental abruption, which may result in a partial or complete cessation of oxygen transfer to the fetus, can be associated with chronic or acute diseases. Long-standing vascular diseases produced by chronic hypertension or smoking, as well as acute factors such as cocaine abuse and abdominal trauma, can precipitate a placental abruption.
The treatment of uteroplacental causes of fetal compromise includes correction of uterine hypertonus or tachysystole by cessation of oxytocin infusion and the removal of cervical ripening agents (see Table 8-1). Oxytocin has a plasma half-life of 1 to 6 minutes; consequently, it may take several minutes for the hypertonus to be relieved. Alternatively, a tocolytic agent (e.g., terbutaline, nitroglycerin) may be administered. Normal maternal circulation should be maintained by avoiding aortocaval compression, expanding intravascular volume, and giving a vasopressor (e.g., phenylephrine, ephedrine) for treatment of hypotension.129
Fetal factors may contribute to fetal hypoxemia and acidosis. Umbilical cord prolapse through the cervix causes cord compression and often results in sudden fetal bradycardia. In the vast majority of circumstances, treatment of a prolapsed cord consists of manual elevation of the fetal head until emergency cesarean delivery can be accomplished. Only rarely should the umbilical cord be returned into the uterus and expectant care attempted.130 Reports from the developing world indicate that, in some cases, expeditious vaginal delivery may produce acceptable neonatal outcome.131 Alternative methods to decompress a prolapsed umbilical cord include the use of the Trendelenburg position or an infusion of 500 to 700 mL of 0.9% saline into the maternal bladder until an expedited delivery can occur.130,132,133
Uterine contractions represent a much more common cause of umbilical cord compression and can manifest as variable FHR decelerations or bradycardia. Oligohydramnios is a risk factor for this type of cord compression, and a change in maternal position or the use of saline amnioinfusion may be therapeutic. Amnioinfusion has been observed to reduce the frequency of severe variable FHR decelerations and the incidence of cesarean delivery and to increase the umbilical cord blood pH in women with preterm premature rupture of membranes, oligohydramnios, or variable FHR decelerations during labor.134,135 Systematic reviews have produced different conclusions as to whether prophylactic intrapartum amnioinfusion in patients with oligohydramnios is superior to therapeutic amnioinfusion in patients with both oligohydramnios and FHR abnormalities.136,137
Initial studies suggested that in patients with thick, meconium-stained amniotic fluid, amnioinfusion might decrease the incidence of meconium aspiration syndrome and fetal acidosis.138–140 However, meta-analyses of studies suggest no benefit of amnioinfusion in the setting of meconium unless decelerations due to oligohydramnios are present.141–145
Saline amnioinfusion requires a dilated cervix, ruptured membranes, and the placement of an intrauterine catheter. Equipment that allows simultaneous saline amnioinfusion and measurement of intrauterine pressure is preferred. Either normal saline or lactated Ringer’s solution may be infused as a bolus or as a continuous infusion.146 The ideal rate of infusion has not been determined, but a commonly used regimen consists of a bolus of as much as 800 mL (infused at a rate of 10 to 15 mL/min) followed by either a continuous infusion at a rate of 3 mL/min or repeated boluses of 250 mL, as needed.146 The necessity of either an infusion pump or a fluid warmer has not been demonstrated.146 Alleviation of abnormal FHR patterns generally requires 20 to 30 minutes.146
Although most studies suggest that amnioinfusion is safe for the mother and fetus, some complications have been reported. Overdistention of the uterus and a higher rate of maternal infections and maternal respiratory distress, including cases of fatal amniotic fluid embolism, have occurred.143,147–149 A causal relationship between amniotic fluid embolism and amnioinfusion has yet to be determined. Overdistention of the uterus may be controlled with proper documentation of fluid loss from the uterus during infusion, the provision of amnioinfusion by gravity instead of an infusion pump, and the use of ultrasonography to evaluate the fluid volume.147
Maternal fever may increase fetal oxygen consumption. Obstetricians should treat maternal fever with acetaminophen, a cooling blanket, and antibiotics as indicated to maintain maternal and fetal euthermia. Hyperglycemia also increases fetal oxygen consumption, so administration of a large bolus of a glucose-containing solution is contraindicated.
Fetal cardiac failure results in inadequate umbilical blood flow and fetal hypoxemia and acidosis. Fetal anemia due to maternal isoimmunization, fetal hemoglobinopathy, or fetal hemorrhage results in diminished fetal oxygen-carrying capacity. There are few options for the treatment of fetal cardiac failure or anemia during labor.
Standardization of FHR tracing interpretation and staged levels of intervention based on likely etiology will assist obstetric management. If intrapartum assessment suggests the presence of fetal compromise and fetal therapy is unsuccessful, the obstetrician should effect an expeditious, atraumatic delivery.
References
1. Bonham DG, Butler NR. Perinatal mortality: the first report of the 1958 British Perinatal Mortality Survey. [Edinburgh, Livingstone] 1963.
2. Mattatall FM, O’Connell CM, Baskett TF. A review of intrapartum fetal deaths, 1982 to 2002. Am J Obstet Gynecol. 2005;192:1475–1477.
3. Pasupathy D, Wood AM, Pell JP, et al. Rates of and factors associated with delivery-related perinatal death among term infants in Scotland. JAMA. 2009;302:660–668.
4. McClure EM, Wright LL, Goldenberg RL, et al. The global network: a prospective study of stillbirths in developing countries. Am J Obstet Gynecol. 2007;197:247.e1–247.e5.
5. Lawn JE, Bahl R, Bergstrom S, et al. Setting research priorities to reduce almost one million deaths from birth asphyxia by 2015. PLoS Med. 2011;8(1):e1000389.
6. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161.
8. Juul SE, Aylward E, Richards T, et al. Prenatal cord clamping in newborn Macaca nemestrina: a model of perinatal asphyxia. Dev Neurosci. 2007;29:311–320.
9. Jacobson Misbe EN, Richards TL, McPherson RJ, et al. Perinatal asphyxia in a nonhuman primate model. Dev Neurosci. 2011;33:210–221.
10. Bernert G, Hoeger H, Mosgoeller W, et al. Neurodegeneration, neuronal loss, and neurotransmitter changes in the adult guinea pig with perinatal asphyxia. Pediatr Res. 2003;54:523–528.
11. Hankins GD, Clark SM, Munn MB. Cesarean section on request at 39 weeks: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. Semin Perinatol. 2006;30:276–287.
12. Nesbitt RE Jr, Aubry RH. High-risk obstetrics. II. Value of semiobjective grading system in identifying the vulnerable group. Am J Obstet Gynecol. 1969;103:972–985.
13. Gomez JL, Young BK. A weighted risk index for antenatal prediction of perinatal outcome. J Perinat Med. 2002;30:137–142.
14. Knox AJ, Sadler L, Pattison NS, et al. An obstetric scoring system: its development and application in obstetric management. Obstet Gynecol. 1993;81:195–199.
15. Koong D, Evans S, Mayes C, et al. A scoring system for the prediction of successful delivery in low-risk birthing units. Obstet Gynecol. 1997;89:654–659.
16. Low JA, Pickersgill H, Killen H, Derrick EJ. The prediction and prevention of intrapartum fetal asphyxia in term pregnancies. Am J Obstet Gynecol. 2001;184:724–730.
17. Gourounti K, Sandall J. Admission cardiotocography versus intermittent auscultation of fetal heart rate: effects on neonatal Apgar score, on the rate of caesarean sections and on the rate of instrumental delivery—a systematic review. Int J Nurs Stud. 2007;44:1029–1035.
18. Devane D, Lalor JG, Daly S, et al. Cardiotocography versus intermittent auscultation of fetal heart on admission to labour ward for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2012;(2).
19. American College of Obstetricians and Gynecologists. Neonatal encephalopathy and cerebral palsy: executive summary. Obstet Gynecol. 2004;103:780–781.
20. American College of Obstetricians and Gynecologists Task Force on Neonatal Encephalopathy and Cerebral Palsy. Neonatal encephalopathy and cerebral palsy: defining the pathogenesis and pathophysiology. American College of Obstetricians and Gynecologists: Washington, DC; 2003.
21. Badawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47:293–298.
22. Perlman JM. Intrapartum asphyxia and cerebral palsy: is there a link? Clin Perinatol. 2006;33:335–353.
23. Strijbis EM, Oudman I, van Essen P, MacLennan AH. Cerebral palsy and the application of the international criteria for acute intrapartum hypoxia. Obstet Gynecol. 2006;107:1357–1365.
24. Phelan JP, Korst LM, Martin GI. Application of criteria developed by the Task Force on Neonatal Encephalopathy and Cerebral Palsy to acutely asphyxiated neonates. Obstet Gynecol. 2011;118:824–830.
25. Martinez-Biarge M, Madero R, Gonzalez A, Quero J, Garcia-Alix A. Perinatal morbidity and risk of hypoxic-ischemic encephalopathy associated with intrapartum sentinel events. Am J Obstet Gynecol. 2012;206:148.e1–148.e7.
26. Miller R, Depp R. Minimizing perinatal neurologic injury at term: is cesarean section the answer? Clin Perinatol. 2008;35:549–559.
27. Parer JT. Handbook of fetal heart rate monitoring. 2nd ed. WB Saunders: Philadelphia; 1997.
28. Court DJ, Parer JT. Experimental studies of fetal asphyxia and fetal heart rate interpretation. Research in Perinatal Medicine. Perinatology Press: Ithaca, NY; 1984:113–169. Nathanielsz PW, Parer JT. Research in Perinatal Medicine. Vol I.
29. Parer JT, Livingston EG. What is fetal distress? Am J Obstet Gynecol. 1990;162:1421–1425.
30. Peeters LL, Sheldon RE, Jones MD Jr, et al. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135:637–646.
31. Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824.
32. American College of Obstetricians and Gynecologists. Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. ACOG Practice Bulletin No. 106. Obstet Gynecol. 2009;114:192–202.
33. Bakker JJ, Verhoeven CJ, Janssen PF, et al. Outcomes after internal versus external tocodynamometry for monitoring labor. N Engl J Med. 2010;362:306–313.
34. Macones GA, Hankins GD, Spong CY, et al. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112:661–666.
35. Jones M Jr, Sheldon RE, Peeters LL, et al. Fetal cerebral oxygen consumption at different levels of oxygenation. J Appl Physiol. 1977;43:1080–1084.
36. Fisher DJ, Heymann MA, Rudolph AM. Fetal myocardial oxygen and carbohydrate consumption during acutely induced hypoxemia. Am J Physiol. 1982;242:H657–H661.
37. Hammacher K, Huter KA, Bokelmann J, Werners PH. Foetal heart frequency and perinatal condition of the foetus and newborn. Gynaecologia. 1968;166:349–360.
38. Krebs HB, Petres RE, Dunn LJ, et al. Intrapartum fetal heart rate monitoring. I. Classification and prognosis of fetal heart rate patterns. Am J Obstet Gynecol. 1979;133:762–772.
39. Westgate JA, Wibbens B, Bennet L, et al. The intrapartum deceleration in center stage: a physiologic approach to the interpretation of fetal heart rate changes in labor. Am J Obstet Gynecol. 2007;197:236.e1–236.e11.
40. Williams KP, Galerneau F. Intrapartum fetal heart rate patterns in the prediction of neonatal acidemia. Am J Obstet Gynecol. 2003;188:820–823.
41. Sameshima H, Ikenoue T. Predictive value of late decelerations for fetal acidemia in unselective low-risk pregnancies. Am J Perinatol. 2005;22:19–23.
42. Ball RH, Parer JT. The physiologic mechanisms of variable decelerations. Am J Obstet Gynecol. 1992;166:1683–1688.
43. Renou P, Chang A, Anderson I, Wood C. Controlled trial of fetal intensive care. Am J Obstet Gynecol. 1976;126:470–476.
44. Paul RH, Huey JR Jr, Yaeger CF. Clinical fetal monitoring: its effect on cesarean section rate and perinatal mortality: five-year trends. Postgrad Med. 1977;61:160–166.
45. Yeh SY, Diaz F, Paul RH. Ten-year experience of intrapartum fetal monitoring in Los Angeles County/University of Southern California Medical Center. Am J Obstet Gynecol. 1982;143:496–500.
46. Vintzileos AM, Nochimson DJ, Guzman ER, et al. Intrapartum electronic fetal heart rate monitoring versus intermittent auscultation: a meta-analysis. Obstet Gynecol. 1995;85:149–155.
47. Thacker SB, Stroup DF, Peterson HB. Efficacy and safety of intrapartum electronic fetal monitoring: an update. Obstet Gynecol. 1995;86:613–620.
48. Graham EM, Petersen SM, Christo DK, Fox HE. Intrapartum electronic fetal heart rate monitoring and the prevention of perinatal brain injury. Obstet Gynecol. 2006;108:656–666.
49. Alfirevic Z, Devane D, Gyte GM. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2006;(3).
50. Sameshima H, Ikenoue T, Ikeda T, et al. Unselected low-risk pregnancies and the effect of continuous intrapartum fetal heart rate monitoring on umbilical blood gases and cerebral palsy. Am J Obstet Gynecol. 2004;190:118–123.
51. Chen HY, Chauhan SP, Ananth CV, et al. Electronic fetal heart rate monitoring and its relationship to neonatal and infant mortality in the United States. Am J Obstet Gynecol. 2011;204:491.e1–491.e10.
52. American College of Obstetricians and Gynecologists. Intrapartum fetal heart rate monitoring. ACOG Practice Bulletin Nol 7, December 2005. Obstet Gynecol. 2005;106:1453–1460.
54. Morrison JC, Chez BF, Davis ID, et al. Intrapartum fetal heart rate assessment: monitoring by auscultation or electronic means. Am J Obstet Gynecol. 1993;168:63–66.
55. Freeman RK. Problems with intrapartum fetal heart rate monitoring interpretation and patient management. Obstet Gynecol. 2002;100:813–826.
56. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Inappropriate use of the terms fetal distress and birth asphyxia. ACOG Committee Opinion. No. 197, February 1998. Int J Gynaecol Obstet. 1998;61:309–310.
57. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med. 1996;334:613–618.
58. Larma JD, Silva AM, Holcroft CJ, et al. Intrapartum electronic fetal heart rate monitoring and the identification of metabolic acidosis and hypoxic-ischemic encephalopathy. Am J Obstet Gynecol. 2007;197:301.e1–301.e8.
59. Blix E, Oian P. Interobserver agreements in assessing 549 labor admission tests after a standardized training program. Acta Obstet Gynecol Scand. 2005;84:1087–1092.
60. Figueras F, Albela S, Bonino S, et al. Visual analysis of antepartum fetal heart rate tracings: inter- and intra-observer agreement and impact of knowledge of neonatal outcome. J Perinat Med. 2005;33:241–245.
61. Palomaki O, Luukkaala T, Luoto R, Tuimala R. Intrapartum cardiotocography—the dilemma of interpretational variation. J Perinat Med. 2006;34:298–302.
62. Parer JT, King T. Fetal heart rate monitoring: is it salvageable? Am J Obstet Gynecol. 2000;182:982–987.
63. American College of Obstetricians and Gynecologists. Management of intrapartum fetal heart rate tracings. ACOG Practice Bulletin No. 116, November 2010. (Obstet Gynecol. 2010;116:1232–1240.
64. Jackson M, Holmgren CM, Esplin MS, et al. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118:803–808.
65. Parer JT, Ikeda T, King TL. The 2008 National Institute of Child Health and Human Development report on fetal heart rate monitoring. Obstet Gynecol. 2009;114:136–138.
66. Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197:26.e1–26.e6.
67. Coletta J, Murphy E, Rubeo Z, Gyamfi-Bannerman C. The 5-tier system of assessing fetal heart rate tracings is superior to the 3-tier system in identifying fetal acidemia. Am J Obstet Gynecol. 2012;206:226.e1–226.e5.
68. Elliott C, Warrick PA, Graham E, Hamilton EF. Graded classification of fetal heart rate tracings: association with neonatal metabolic acidosis and neurologic morbidity. Am J Obstet Gynecol. 2010;202:258.e1–258.e8.
69. Tongsong T, Iamthongin A, Wanapirak C, et al. Accuracy of fetal heart-rate variability interpretation by obstetricians using the criteria of the National Institute of Child Health and Human Development compared with computer-aided interpretation. J Obstet Gynaecol Res. 2005;31:68–71.
70. Giannubilo SR, Buscicchio G, Gentilucci L, et al. Deceleration area of fetal heart rate trace and fetal acidemia at delivery: a case-control study. J Matern Fetal Neonatal Med. 2007;20:141–144.
71. Georgoulas G, Stylios CD, Groumpos PP. Predicting the risk of metabolic acidosis for newborns based on fetal heart rate signal classification using support vector machines. IEEE Trans Biomed Eng. 2006;53:875–884.
72. Agrawal SK, Doucette F, Gratton R, et al. Intrapartum computerized fetal heart rate parameters and metabolic acidosis at birth. Obstet Gynecol. 2003;102:731–738.
73. Salamalekis E, Hintipas E, Salloum I, et al. Computerized analysis of fetal heart rate variability using the matching pursuit technique as an indicator of fetal hypoxia during labor. J Matern Fetal Neonatal Med. 2006;19:165–169.
74. Phelan JP. Confronting medical liability. Contemp Obstet Gynecol. 1991;36:70–81.
75. Young DC, Gray JH, Luther ER, Peddle LJ. Fetal scalp blood pH sampling: its value in an active obstetric unit. Am J Obstet Gynecol. 1980;136:276–281.
76. Goodwin TM, Milner-Masterson L, Paul RH. Elimination of fetal scalp blood sampling on a large clinical service. Obstet Gynecol. 1994;83:971–974.
77. East CE, Leader LR, Sheehan P, et al. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2010;(3).
78. Clark SL, Gimovsky ML, Miller FC. The scalp stimulation test: a clinical alternative to fetal scalp blood sampling. Am J Obstet Gynecol. 1984;148:274–277.
79. Rathore AM, Ramji S, Devi CB, et al. Fetal scalp stimulation test: an adjunct to intermittent auscultation in non-reassuring fetal status during labor. J Obstet Gynaecol Res. 2011;37:819–824.
80. Smith CV, Nguyen HN, Phelan JP, Paul RH. Intrapartum assessment of fetal well-being: a comparison of fetal acoustic stimulation with acid-base determinations. Am J Obstet Gynecol. 1986;155:726–728.
81. East CE, Smyth R, Leader LR, et al. Vibroacoustic stimulation for fetal assessment in labour in the presence of a nonreassuring fetal heart rate trace. Cochrane Database Syst Rev. 2005;(2).
82. Skupski DW, Rosenberg CR, Eglinton GS. Intrapartum fetal stimulation tests: a meta-analysis. Obstet Gynecol. 2002;99:129–134.
83. Ogunyemi D, Stanley R, Lynch C, et al. Umbilical artery velocimetry in predicting perinatal outcome with intrapartum fetal distress. Obstet Gynecol. 1992;80:377–380.
84. Dawes GS, Moulden M, Redman CW. Short-term fetal heart rate variation, decelerations, and umbilical flow velocity waveforms before labor. Obstet Gynecol. 1992;80:673–678.
85. Tongprasert F, Jinpala S, Srisupandit K, Tongsong T. The rapid biophysical profile for early intrapartum fetal well-being assessment. Int J Gynaecol Obstet. 2006;95:14–17.
86. Kim SY, Khandelwal M, Gaughan JP, et al. Is the intrapartum biophysical profile useful? Obstet Gynecol. 2003;102:471–476.
87. Greenwood C, Lalchandani S, MacQuillan K, et al. Meconium passed in labor: how reassuring is clear amniotic fluid? Obstet Gynecol. 2003;102:89–93.
88. Nathan L, Leveno KJ, Carmody TJ 3rd, et al. Meconium: a 1990s perspective on an old obstetric hazard. Obstet Gynecol. 1994;83:329–332.
89. Berkus MD, Langer O, Samueloff A, et al. Meconium-stained amniotic fluid: increased risk for adverse neonatal outcome. Obstet Gynecol. 1994;84:115–120.
90. Halliday HL. Endotracheal intubation at birth for preventing morbidity and mortality in vigorous, meconium-stained infants born at term. Cochrane Database Syst Rev. 2000;(2).
91. Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000;105:1–7.
92. Yoder BA, Kirsch EA, Barth WH, Gordon MC. Changing obstetric practices associated with decreasing incidence of meconium aspiration syndrome. Obstet Gynecol. 2002;99:731–739.
93. Ahanya SN, Lakshmanan J, Morgan BL, Ross MG. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv. 2005;60:45–56.
94. Ross MG. Meconium aspiration syndrome—more than intrapartum meconium. N Engl J Med. 2005;353:946–948.
95. Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597–602.
96. Nickelsen C, Thomsen SG, Weber T. Continuous acid-base assessment of the human fetus during labour by tissue pH and transcutaneous carbon dioxide monitoring. Br J Obstet Gynaecol. 1985;92:220–225.
97. Mueller-Heubach E, Caritis SN, Edelstone DI, et al. Comparison of continuous transcutaneous PO2 measurement with intermittent arterial PO2 determinations in fetal lambs. Obstet Gynecol. 1981;57:248–252.
99. Kwee A, van der Hoorn-van den Beld CW, Veerman J, et al. STAN S21 fetal heart monitor for fetal surveillance during labor: an observational study in 637 patients. J Matern Fetal Neonatal Med. 2004;15:400–407.
100. Luttkus AK, Noren H, Stupin JH, et al. Fetal scalp pH and ST analysis of the fetal ECG as an adjunct to CTG: a multi-center, observational study. J Perinat Med. 2004;32:486–494.
101. Westerhuis ME, Kwee A, van Ginkel AA, et al. Limitations of ST analysis in clinical practice: three cases of intrapartum metabolic acidosis. BJOG. 2007;114:1194–1201.
102. Ojala K, Vaarasmaki M, Makikallio K, et al. A comparison of intrapartum automated fetal electrocardiography and conventional cardiotocography—a randomised controlled study. BJOG. 2006;113:419–423.
103. Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst Rev. 2006;(3).
104. Noren H, Amer-Wahlin I, Hagberg H, et al. Fetal electrocardiography in labor and neonatal outcome: data from the Swedish randomized controlled trial on intrapartum fetal monitoring. Am J Obstet Gynecol. 2003;188:183–192.
105. Vayssiere C, David E, Meyer N, et al. A French randomized controlled trial of ST-segment analysis in a population with abnormal cardiotocograms during labor. Am J Obstet Gynecol. 2007;197:299.
106. Su LL, Chong YS, Biswas A. Use of fetal electrocardiogram for intrapartum monitoring. Ann Acad Med Singapore. 2007;36:416–420.
107. Westerhuis ME, Visser GH, Moons KG, et al. Cardiotocography plus ST analysis of fetal electrocardiogram compared with cardiotocography only for intrapartum monitoring: a randomized controlled trial. Obstet Gynecol. 2010;115:1173–1180.
108. Stiller R, von Mering R, Konig V, et al. How well does reflectance pulse oximetry reflect intrapartum fetal acidosis? Am J Obstet Gynecol. 2002;186:1351–1357.
109. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Fetal pulse oximetry. ACOG Committee Opinion No. 258. September 2001. Obstet Gynecol. 2001;98:523–524.
110. Kuhnert M, Seelbach-Goebel B, Butterwegge M. Predictive agreement between the fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol. 1998;178:330–335.
111. Nijland R, Jongsma HW, Nijhuis JG, et al. Arterial oxygen saturation in relation to metabolic acidosis in fetal lambs. Am J Obstet Gynecol. 1995;172:810–819.
112. Salamalekis E, Bakas P, Saloum I, et al. Severe variable decelerations and fetal pulse oximetry during the second stage of labor. Fetal Diagn Ther. 2005;20:31–34.
113. Luttkus AK, Lubke M, Buscher U, et al. Accuracy of fetal pulse oximetry. Acta Obstet Gynecol Scand. 2002;81:417–423.
114. East CE, Brennecke SP, King JF, et al. The effect of intrapartum fetal pulse oximetry, in the presence of a nonreassuring fetal heart rate pattern, on operative delivery rates: a multicenter, randomized, controlled trial (the FOREMOST trial). Am J Obstet Gynecol. 2006;194:606.e1–606.e16.
115. Kuhnert M, Schmidt S. Intrapartum management of nonreassuring fetal heart rate patterns: a randomized controlled trial of fetal pulse oximetry. Am J Obstet Gynecol. 2004;191:1989–1995.
116. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049–1058.
117. Valverde M, Puertas AM, Lopez-Gallego MF, et al. Effectiveness of pulse oximetry versus fetal electrocardiography for the intrapartum evaluation of nonreassuring fetal heart rate. Eur J Obstet Gynecol Reprod Biol. 2011;159:333–337.
118. Roelants-van Rijn AM, Groenendaal F, Stoutenbeek P, van der Grond J. Lactate in the foetal brain: detection and implications. Acta Paediatr. 2004;93:937–940.
119. Borowska-Matwiejczuk K, Lemancewicz A, Tarasow E, et al. Assessment of fetal distress based on magnetic resonance examinations: preliminary report. Acad Radiol. 2003;10:1274–1282.
120. Cetin I, Barberis B, Brusati V, et al. Lactate detection in the brain of growth-restricted fetuses with magnetic resonance spectroscopy. Am J Obstet Gynecol. 2011;205:350.e1–350.e7.
121. Charles-Edwards GD, Jan W, To M, et al. Non-invasive detection and quantification of human foetal brain lactate in utero by magnetic resonance spectroscopy. Prenat Diagn. 2010;30:260–266.
122. Wolfberg AJ, Robinson JN, Mulkern R, et al. Identification of fetal cerebral lactate using magnetic resonance spectroscopy. Am J Obstet Gynecol. 2007;196:e9–11.
123. Wolfberg AJ, du Plessis AJ. Near-infrared spectroscopy in the fetus and neonate. Clin Perinatol. 2006;33:707–728.
124. Kakogawa J, Sumimoto K, Kawamura T, et al. Transabdominal measurement of placental oxygenation by near-infrared spectroscopy. Am J Perinatol. 2010;27:25–29.
125. Haydon ML, Gorenberg DM, Nageotte MP, et al. The effect of maternal oxygen administration on fetal pulse oximetry during labor in fetuses with nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2006;195:735–738.
126. Dildy GA, Clark SL, Loucks CA. Intrapartum fetal pulse oximetry: the effects of maternal hyperoxia on fetal arterial oxygen saturation. Am J Obstet Gynecol. 1994;171:1120–1124.
127. Thorp JA, Trobough T, Evans R, et al. The effect of maternal oxygen administration during the second stage of labor on umbilical cord blood gas values: a randomized controlled prospective trial. Am J Obstet Gynecol. 1995;172:465–474.
128. Gunaydin B, Nas T, Biri A, et al. Effects of maternal supplementary oxygen on the newborn for elective cesarean deliveries under spinal anesthesia. J Anesth. 2011;25:363–368.
129. Thurlow JA, Kinsella SM. Intrauterine resuscitation: active management of fetal distress. Int J Obstet Anesth. 2002;11:105–116.
130. Lin MG. Umbilical cord prolapse. Obstet Gynecol Surv. 2006;61:269–277.
131. Enakpene CA, Omigbodun AO, Arowojolu AO. Perinatal mortality following umbilical cord prolapse. Int J Gynaecol Obstet. 2006;95:44–45.
132. Katz Z, Shoham Z, Lancet M, et al. Management of labor with umbilical cord prolapse: a 5-year study. Obstet Gynecol. 1988;72:278–281.
133. Bord I, Gemer O, Anteby EY, Shenhav S. The value of bladder filling in addition to manual elevation of presenting fetal part in cases of cord prolapse. Arch Gynecol Obstet. 2011;283:989–991.
134. Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database Syst Rev. 2012;(1).
135. Puertas A, Tirado P, Perez I, et al. Transcervical intrapartum amnioinfusion for preterm premature rupture of the membranes. Eur J Obstet Gynecol Reprod Biol. 2007;131:40–44.
136. Hofmeyr GJ. Prophylactic versus therapeutic amnioinfusion for oligohydramnios in labour. Cochrane Database Syst Rev. 1996;(2).
137. Pitt C, Sanchez-Ramos L, Kaunitz AM, Gaudier F. Prophylactic amnioinfusion for intrapartum oligohydramnios: a meta-analysis of randomized controlled trials. Obstet Gynecol. 2000;96:861–866.
138. Pierce J, Gaudier FL, Sanchez-Ramos L. Intrapartum amnioinfusion for meconium-stained fluid: meta-analysis of prospective clinical trials. Obstet Gynecol. 2000;95:1051–1056.
139. Eriksen NL, Hostetter M, Parisi VM. Prophylactic amnioinfusion in pregnancies complicated by thick meconium. Am J Obstet Gynecol. 1994;171:1026–1030.
140. Cialone PR, Sherer DM, Ryan RM, et al. Amnioinfusion during labor complicated by particulate meconium-stained amniotic fluid decreases neonatal morbidity. Am J Obstet Gynecol. 1994;170:842–849.
141. Hofmeyr GJ, Xu H. Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev. 2010;(1).
142. Fraser WD, Hofmeyr J, Lede R, et al. Amnioinfusion for the prevention of the meconium aspiration syndrome. N Engl J Med. 2005;353:909–917.
143. Spong CY, Ogundipe OA, Ross MG. Prophylactic amnioinfusion for meconium-stained amniotic fluid. Am J Obstet Gynecol. 1994;171:931–935.
144. Usta IM, Mercer BM, Aswad NK, Sibai BM. The impact of a policy of amnioinfusion for meconium-stained amniotic fluid. Obstet Gynecol. 1995;85:237–241.
146. American College of Obstetricians and Gynecologists. Fetal heart rate patterns: monitoring, interpretation, and management. ACOG Technical Bulletin No. 207, July 1995. Int J Gynaecol Obstet. 1995;51:65–74.
147. Maher JE, Wenstrom KD, Hauth JC, Meis PJ. Amniotic fluid embolism after saline amnioinfusion: two cases and review of the literature. Obstet Gynecol. 1994;83:851–854.
148. Dorairajan G, Soundararaghavan S. Maternal death after intrapartum saline amnioinfusion—report of two cases. BJOG. 2005;112:1331–1333.
149. Dragich DA, Ross AF, Chestnut DH, Wenstrom K. Respiratory failure associated with amnioinfusion during labor. Anesth Analg. 1991;72:549–551.