Chapter 21 Intraoperative diagnostic techniques
Overview
Intraoperative ultrasonography (IOUS) continues to be an important component for assessing hepatic and biliary anatomy, for identifying and classifying malignant and cystic neoplasms, and for determining the resectability of malignant disease. Similarly, intraoperative cholangiography (IOC) assists the surgeon in assessing the biliary tree, its anatomy, and the occurrence of injury in both open and laparoscopic procedures. Diagnostic and staging laparoscopy (Table 21.1) have also emerged as important techniques for uncovering small-volume disease not revealed by even the most sophisticated imaging studies; however, controversy still exists regarding the true added value of the procedure (Table 21.2). This chapter will focus on these three intraoperative diagnostic modalities and their use in evaluating and managing hepatobiliary and pancreatic disease.
Table 21.2 Potential Disadvantages of Staging Laparoscopy
Intraoperative Ultrasonography
IOUS is commonly used during both open and laparoscopic procedures. Particular attention must be given to port placement when IOUS will be performed laparoscopically. Ultrasonography (US) is most useful for evaluating the liver parenchyma or for assessing the extent of mesenteric vasculature involvement of pancreatic tumors (Jakimowicz, 1993). Other common applications include localization of pancreatic endocrine tumors, assessment of biliary calculi, and ablation of hepatic tumors.
Hepatic Disease
Evaluation of the Liver
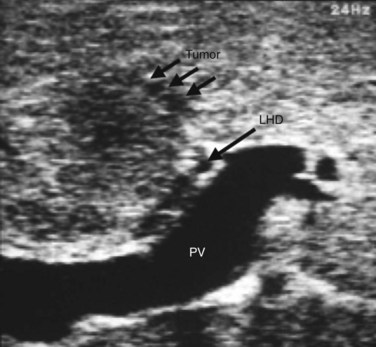
IOUS of the liver was first introduced into clinical practice in the early 1980s and rapidly became routine practice for management of malignant liver disease (Makuuchi et al, 1981; Nagasue et al, 1984; Gunven et al, 1985; Bismuth & Castaing, 1985; Belghiti et al, 1986; Gozzetti et al, 1986; Olsen, 1990; Gouillat et al, 1992; Solomon et al, 1992; Bloed et al, 2000). These early reports demonstrated that IOUS frequently altered the course of operative management of malignant hepatic tumors by more precisely delineating the relationship between tumors and major vascular and biliary structures and by detecting hepatic tumors not revealed on preoperative imaging studies (Fig. 21.1). In an analysis of 210 patients with primary and secondary hepatic malignancies, Bismuth and colleagues (1987) reported that IOUS provided additional information in 35% of cases and changed the operative plan in 20%. Parker and associates (1989) reported similar findings, with US influencing 49% of cases. A more recent analysis by Kruskal and Kane (2006) demonstrated that IOUS routinely identifies 25% to 35% more hepatic lesions than preoperative imaging studies.
It must be recognized that the yield of IOUS, particularly for identifying lesions not previously seen, depends critically on the quality of the preoperative imaging studies (see Chapters 13, 16, and 17). The results of these and other studies demonstrate the continued importance of IOUS in liver surgery for identifying and staging malignant disease.
IOUS is particularly useful for determining tumor resectability. Several reports from the 1990s have documented increased resectability rates in patients with hepatobiliary malignancy (Soyer et al, 1993; Lo et al, 1998; Jarnagin et al, 1999). More recent studies estimate that the use of IOUS will lead to changes in 15% to 25% of procedures. Regardless of these estimates, IOUS is a crucial component of the intraoperative assessment and is a useful tool in the armamentarium of liver surgeons (Zacherl et al, 2002).
Recognition of malignant disease can be challenging without the assistance of trained radiologists. Hemangiomas are typically soft and usually lack any visible flow, or they demonstrate only minimal flow compared with adjacent liver parenchyma. Metastases from colon cancer are usually hyperechoic or isoechoic to adjacent liver parenchyma and are frequently surrounded by an ill-defined hypoechoic rim that may have a bull’s-eye or target appearance. Mucinous colorectal cancer metastases may contain calcification that produces acoustic shadowing. Hepatocellular carcinoma (HCC) frequently invades major vascular structures and may be associated with porta hepatis lymphadenopathy (Jeffers et al, 1988). Documentation of lesions and their proximity to adjacent vessels will determine resectability. Resection margins of approximately 1 cm are generally sought and appear to be required for an oncologically adequate operation, although the optimal margin size is poorly defined. Keep in mind that an outline of the planned surgical margin with electrocautery of the liver capsule will produce acoustic shadowing and should be reserved until sonography is complete.
Biliary Disease
Evaluation of the Biliary Tree
As laparoscopic cholecystectomy has become the standard of care for gallbladder disease, the use of laparoscopic US during this procedure will emerge as an increasing possibility and a viable alternative to cholangiogram. Laparascopic US is now recommended as the primary screening modality for evaluating bile duct calculi because of its safety, efficiency, and overall cost effectiveness (Machi et al, 1999). IOUS is at least as good as cholangiogram, perhaps better, at detecting stones during laparoscopic cholecystectomy (Catheline et al, 1999; Siperstein et al, 1999; Tranter & Thompson, 2003). Perry and associates recently published their results from a prospective study performed between 1995 and 2005. Laparoscopic US was performed in 236 (60%) of 396 laparoscopic cholecystectomies performed for cholelithiasis. US had a positive predictive value of 100%, a negative predictive value of 99.6%, a sensitivity of 92.3%, and a specificity of 100% for detecting CBD stones (Perry et al, 2008). Proficiency with IOUS requires some time to develop, which may explain why it is not employed more liberally for biliary disease.
Laparoscopic US is a useful adjunct for oncologic staging of gallbladder carcinoma and cholangiocarcinoma. Sonographic findings not only reveal subtle liver metastases but may help to further define the local extent of invasion of these tumors into the adjacent liver bed and also outline the involvement of the ductal system, and Doppler and color flow images may help distinguish biliary sludge from polyps and other intraluminal tumors. Other applications of laparoscopic US include evaluating biliary strictures and malignant biliary obstructions and planning of surgical reconstructions such as biliary bypass procedures. These approaches and others are summarized in a review by Berber and Siperstein (2004).
Pancreatic Disease
Evaluation of the Pancreas
Other applications for US in pancreatic surgery include localizing islet cell tumors. These particularly small lesions may be difficult to visualize or palpate in the body or tail, and sonographic images may help delineate these and other malignant or premalignant tumors in the distal portions of the pancreas (Hayashibe et al, 2005). Finally, US can help identify pancreatic ductal abnormalities among patients with pancreatitis and may be useful during drainage procedures, such as cystogastrostomy.
Intraoperative Cholangiography
Technical Considerations
Intraoperative cholangiography (IOC) is most commonly used during elective cholecystectomy to assess for retained stones and to provide clarification of the biliary anatomy; rarely is it necessary or helpful in assessing extent of biliary tumors or during hepatic resection (Sotiropoulos et al, 2004). Interestingly, Mirizzi first described the procedure in 1937, as a method to help delineate the anatomy of the biliary tree in advanced cases of biliary disease (Mirizzi, 1937).
IOC is typically performed by first identifying the cystic duct at its junction with the gallbladder neck (Yamakawa, 1976). The gallbladder is retracted laterally, and the cystic duct and artery are dissected free and cleared of the fat and overlying peritoneum in the area of the triangle of Calot. A small ductotomy (less than 50% of the duct circumference) is made in the cystic duct adjacent to the gallbladder neck. The cystic duct is best approached from a right subcostal port or from the periumbilical port; we prefer the former. A 60-cm, tapered 5-Fr cholangio catheter is then advanced directly into the cystic duct through the ductotomy. A specialized cholangiogram clamp, often termed an Olsen cholangiogram clamp, secures the catheter in place (Decker et al, 2003).
Choledocholithiasis
Two factors must be considered when performing intraoperative cholangiography for the evaluation of retained bile duct stones (Hyser et al, 1999; Sigel et al, 1983; Tokumura et al, 2002). First, most stones in the biliary tree are suspected on clinical grounds or after serologic testing for liver enzyme elevation and are often visualized during preoperative US (see Chapter 13), ERCP (see Chapter 18), or MRCP (see Chapter 17). Common presentations include obstructive jaundice, cholangitis, biliary colic with abnormal liver function test results, and acute pancreatitis. The incidence of clinically silent choledocholithiasis is low, occurring in roughly one in 25 cases of biliary colic (Metcalfe et al, 2004; Nugent et al, 2005). Even when missed during cholecystectomy, these residual stones rarely if ever cause symptoms or become clinically relevant (Sarli et al, 2003a; see Chapters 30 and 35). Charfare and Cheslyn-Curtis (2003) investigated the incidence of retained biliary stones after 600 laparoscopic cholecystectomies. With a median follow-up of 3 years among 438 patients (73%), findings from this study indicate that residual bile duct stones occurred in only seven cases (1.2%).
The second factor to consider when performing IOC is that false-positive results are not uncommon, occurring up to 4% of the time (Metcalfe et al, 2004). As a result, routine application of IOC for the evaluation of unsuspected choledocholithiasis may subject patients to unnecessary bile duct explorations or ERCP studies, with considerable costs to both patients and providers (Cuschieri & Berci, 1984; Sarli et al, 2003b).
Biliary Injuries
In the late 1980s, IOC emerged as a method that could potentially help avoid major injury to the biliary system. Today laparoscopic cholecystectomy is the most commonly performed elective abdominal surgical procedure in the United States (see Chapter 34), with more than 750,000 cases performed annually. Injury to the bile duct is rare but is a major contributor to patient morbidity (see Chapter 38). These injuries are typically due to either technical error or misidentification of the duct: the technical error is that the CBD is inadvertently transected, and a surgical clip is sometimes applied to a misidentified CBD.
Four mechanisms of injury have been described (Way et al, 2003). Class I injuries involve an incision (i.e., an incomplete transection) of the CBD and typically occur when the bile duct is mistaken for a cystic duct. Class II injuries represent lateral damage to the common hepatic duct. Class III injuries are the most common and represent a CBD mistaken for a cystic duct; these are not immediately recognized intraoperatively. Finally, class IV injuries occur when a right hepatic duct is mistaken for a cystic duct.
Flum and colleagues (2003) analyzed nationwide Medicare data from 1992 to 1999. Among 1.6 million cholecystectomies performed, roughly 8000 bile duct injuries occurred overall (0.5%); these occurred in 0.39% of patients undergoing cholecystectomy with intraoperative cholangiogram and in 0.58% of patients undergoing cholecystectomy without cholangiogram (P < .001). The adjusted relative risk of bile duct injury when cholangiogram was not employed was 1.71 (95%, confidence interval [CI], 1.38 to 2.28). Inexperienced surgeons were twice as likely to cause bile duct injuries when cholangiogram was not used, as compared with their more experienced colleagues. These estimates had been observed in an earlier study (Fletcher et al, 1999).
Another benefit of intraoperative cholangiogram, with respect to biliary injuries, is the earlier recognition and correction of biliary injury (see Chapters 42A and 42B). Ideally, IOC should be performed prior to dividing the presumed cystic duct. If cannulation of the CBD, rather than the cystic duct, occurs, the cholangiogram catheter can be removed, and the ductotomy can be addressed by placing a T-tube, without the need for formal biliary reconstruction. The T-tube enables the CBD to heal without stricture formation, and the tube can be removed nonoperatively several weeks after the cholecystectomy. The alternative scenario is complete transection of the CBD, a class III injury. In the absence of cholangiogram, this transection is seldom recognized intraoperatively, typically presents postoperatively, and results in significant patient morbidity. Management undoubtedly requires an open bilioenteric anastomosis for repair, thus intraoperative cholangiogram has the potential to not only prevent bile duct injury but also to mitigate its impact if injury does occur (Giurgiu et al, 1999).
Controversies
The routine application of IOC during elective cholecystectomy has been called into question in recent years and is still a matter of considerable debate (Mills et al, 1985; Soper & Brunt, 1994; Nickkholgh et al, 2006; Metcalfe et al, 2004; Massarweh & Flum, 2007). Critics of routine cholangiogram suggest that such an approach increases the risk of ductal complications (i.e., stricture) and pancreatitis, wastes time and money, and is seldom indicated if the critical view of safety is achieved. Proponents argue that routine cholangiogram is a safe, accurate, quick, and cost-effective method for evaluating the bile duct (Amott et al, 2005; Wenner et al, 2005). Although we favor a selective approach, we also recognize that the success of either approach is highly variable and likely to depend on each surgeon’s familiarity with cholecystectomy and cholangiography. Whether a selective or routine cholangiogram policy is adopted, it is critical that surgeons become comfortable with interpreting cholangiogram results. Way and others (2003) reported that although 43 cholangiograms demonstrated a bile duct injury, only nine were correctly interpreted at the time of surgery.
Staging Laparoscopy
The growth in popularity of laparoscopy in the early 1980s added a new modality to the field of hepatobiliary and pancreatic surgery. Specialty surgeons in these disciplines frequently confront occult, unresectable disease at the time of laparotomy (de Rooij et al, 1991; Espat et al, 1999; Lillemoe et al, 1999). Reports vary, but this scenario occurs roughly 20% to 25% of the time. Jarnagin and associates (2000) demonstrated that among a cohort of 186 patients with primary and secondary hepatic malignancies, 25% were noted to harbor unresectable disease at the time of laparoscopic staging, and 65% of this group were spared an unnecessary laparotomy. Callery and colleagues (1997) similarly revealed unresectable pancreatic and periampullary disease among 11 of 50 patients, and comparable outcomes have been described by others (D’Angelica et al, 2003; Vollmer et al, 2002). Overall, these findings demonstrate that staging laparoscopy can further clarify whether curative resection is possible, confirm unresectable disease, and help specialty surgeons tailor curative and palliative operations for patients with hepatobiliary and pancreatic malignancies (see Table 21.1). Controversy still exists, however, regarding the true added value of the procedure, particularly in an era of more effective preoperative imaging (see Table 21.2).
Surgical Technique
Technical Considerations
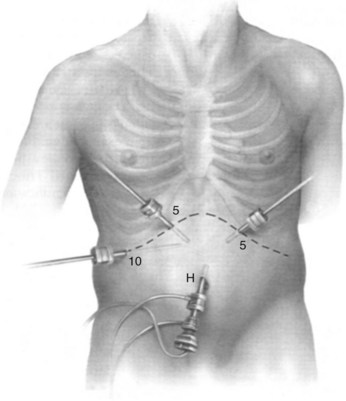
We use a 5- or 10-mm, 30-degree angled telescope to inspect the abdomen. Although we prefer a multiport technique to facilitate obtaining biopsy specimens and laparoscopic US, others may use a single-port approach. Two more ports are placed under direct vision, in the left and right upper quadrants. For pancreatic cases, another 5-mm port is inserted along the incision line to the right of the midline (Fig. 21.2). Entry of these ports is followed by a systematic examination of the peritoneal cavity; adhesions may need to be divided to adequately inspect the abdominal viscera and peritoneum. All four quadrants are examined for evidence of peritoneal deposits; biopsies are taken of any suspicious lesions using standard biopsy forceps, and samples are sent for histologic examination (Fig. 21.3). If clinically indicated, peritoneal cytology can be sampled at this time. After instillation of 200 mL of warm normal saline and gentle agitation, the irrigant is aspirated from the right and left subhepatic spaces and from the pelvis, and a sample is sent for cytologic examination.
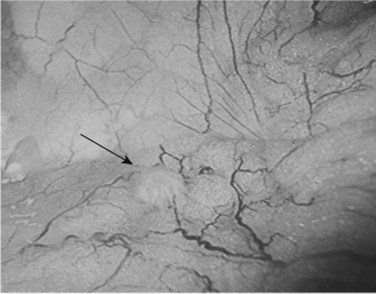
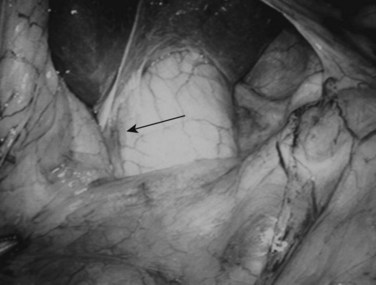
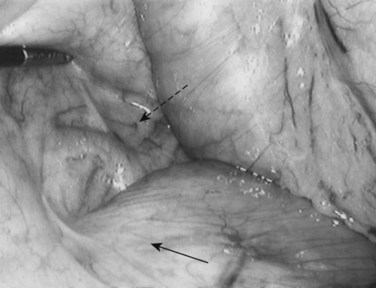
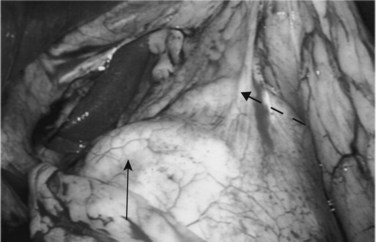
Following inspection of the peritoneum, attention is turned to the anterior and posterior surfaces of the left lateral segment of the liver and the anterior and inferior surfaces of the right lobe (Fig. 21.4). Next, the hepatoduodenal ligament, the foramen of Winslow, and hilum of the liver are examined for lymphadenopathy (Fig. 21.5). Any suspicious nodes are excised and sent for pathologic analysis. The patient is then placed in the 10-degree Trendelenburg position, and the omentum is retracted into the left upper quadrant. The ligament of Treitz and the inferior surface of the transverse mesocolon are inspected for metastatic deposits and for lymphadenopathy (Fig. 21.6).
FIGURE 21.4 Examination of the liver. A blunt 10-mm instrument is used in conjunction with a 5-mm grasper.
The patient is repositioned in the supine position, and the left lateral segment of the liver is elevated superiorly with a retractor via the left upper quadrant port. The lesser omentum is opened to visualize the caudate lobe of the liver and the vena cava. From this vantage point, the anterior aspect of the head of the pancreas, posterior wall of the stomach, and “gastric pillar,” which contains the left gastric artery and vein, can be seen (Fig. 21.7). The left gastric artery can be followed to its origin so as to allow inspection of the celiac axis (Table 21.3).
From Conlon KC, Brennan MF, 2000: Laparoscopy for Staging Abdominal Malignancies. St Louis, Mosby.
Laparoscopic Ultrasound
US can help the surgeon further assess the relationships of the primary tumor to adjacent structures (see Chapter 13). A systematic examination of the liver, biliary tree, and pancreas takes place as previously described. A 6- to 10-MHz T-shaped linear- or curvilinear-array transducer is placed over the left lateral segment to assess segments I, II, and III. The probe is moved to the right liver and placed on the dome of the liver. The vena cava is visualized posteriorly, and the hepatic and portal veins are seen as the probe is moved anteriorly.
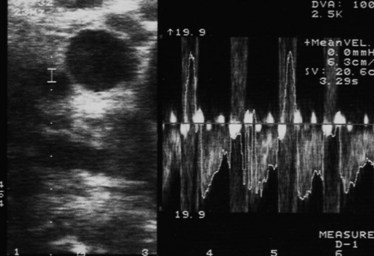
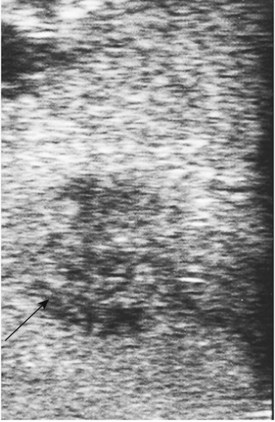
Placement of the probe in the transverse direction over the hepatoduodenal ligament allows the common hepatic duct, CBD, hepatic arteries, and portal vein to be visualized (Fig. 21.8). Similarly, at the confluence of the portal vein, the splenic and superior mesenteric veins can be identified. Finally, the superior mesenteric artery is delineated, and its relationship to any pancreatic tumor is assessed. The probe is placed on the gastrocolic omentum and advanced first caudally and then through the window in the gastrohepatic omentum. Laparoscopic US can help facilitate pathologic biopsies and needle aspirations of any suspicious lesions (Fig. 21.9, Table 21.4).
From Minnard EA, et al, 1998: Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg 228:182-187.
Complications
Morbidity associated with staging laparoscopy is uncommon, occurring in only 1% to 2% of cases. Major complications are similar to those for other laparoscopic procedures and consist of hemorrhage, visceral perforation, and intraabdominal infection. A common misconception is that suspected malignancy precludes staging laparoscopic examination. An early report raised concern about “port site” tumor implants 2 weeks after laparoscopy in a patient with malignant ascites (Dobronte et al, 1978); this concern was also expressed by later studies in the 1990s, which report rates of port-site recurrence ranging from 0.8% to 2% (Curet, 2004; Nieveen van Dijkum et al, 1999; Pearlstone et al, 1999). Shoup and colleagues (2002) examined this question in a series of 1650 diagnostic laparoscopic procedures, in which 4,299 trocars were inserted. The overall rate of port site recurrence was 0.8% (13 cases); five of those occurred in patients with local disease; the remaining eight occurred in patients with documented metastatic disease. The findings of this study suggest that recurrence is a marker of more advanced disease rather than being an isolated event directly attributable to laparoscopy. Overall, Shoup and associates concluded that staging laparoscopy can be performed safely in the setting of presumed malignancy. This concept has been confirmed by others, most recently by Velanovich (2004), who demonstrated that the incidence of port site recurrence (3%) is equivalent to that of wound recurrence in patients who had an exploratory laparotomy alone (3.9%).
Staging Laparoscopy for Potentially Resectable Disease
Hepatobiliary Malignancy
Determination of resectability of hepatobiliary malignancy begins with CT or MRI; however, laparoscopy plays an essential role in detecting occult disease (Jarnagin et al, 2001a; Jeffers et al, 1988; Lightdale, 1982; Torzilli et al, 2002). Roughly 20% to 25% of patients initially believed to have resectable disease will harbor occult disease detected by laparoscopy that precludes curative resection (Conlon et al, 2003; Weber et al, 2002). As a result, one in five patients is spared a laparotomy. When combined with laparoscopic US, these outcomes are more specific.
D’Angelica and others (2003) have provided a detailed analysis of staging laparoscopy for suspected hepatobiliary malignancy. Among 401 patients, a complete laparoscopic staging assessment was performed in 291 cases (73%), an incomplete assessment was done in 88 cases (22%), and 22 (6%) were failed examinations. Unresectable disease was discovered at the time of laparoscopy in 84 cases, for an overall incidence of 21%. Of the remaining 317 patients felt to have resectable disease during laparoscopic examinations, 69 had unresectable disease identified during open exploration, for an overall false-negative rate of 22%. This high false-negative rate was attributed primarily to failure to identify lymph node metastases or to detect vascular invasion. However, despite these outcomes, staging laparoscopy increased the resectability rate from 62% to 78%. Concomitant laparoscopic US has the potential to further increase resectability rates and to avoid unnecessary laparotomies, added benefits that have been described by several other studies (John et al, 1994; Callery et al, 1997).
Laparoscopy is most accurate for identifying peritoneal deposits (80% accuracy) and additional hepatic disease (63% accuracy), and it is least accurate for identifying nodal metastases (7% accuracy) and vascular invasion (18% accuracy). Factors that could improve the yield from laparoscopy include the surgeon’s preoperative judgment regarding the likelihood of resectability, the completeness of laparoscopic staging examinations, and the primary diagnosis; the yield was highest with biliary malignancies and lowest with colorectal metastases (D’Angelica et al, 2003).
Laparoscopic staging can be used for management of colorectal metastases (Rahusen et al, 1999; see Chapter 81A). Approximately one half of all patients with new diagnoses of colorectal cancer will subsequently develop liver metastases, yet only 20% are suitable candidates for curative hepatic resection. Determination of resectability is less straightforward for these patients, and the controversy relates to the philosophy and expertise of the surgical team. In general, however, most authors agree that hepatic cirrhosis, extrahepatic tumor spread, and significant bilobar disease are relative contraindications for hepatic resection. A recent study explored the role of staging laparoscopy in 51 patients with colorectal liver metastases (de Castro et al, 2004). Occult disease at the time of laparoscopy was discovered in 12% of patients; however, 18% of cases were understaged, and unresectable disease was found at subsequent laparoscopy. The authors suggested that adhesions from prior colon resections contribute to this false-negative rate.
In an effort to improve the yield of staging laparoscopy for hepatic colorectal metastases, a study used a clinical risk score to try to determine which patients are more likely to have nonradiologically detectable disease. The clinical risk score uses five clinical parameters: each is shown as an independent predictor of outcome after resection, and each criterion is assigned one point. Forty-two percent of patients with a clinical risk score greater than 2 had unresectable disease detected at laparoscopy versus 0% of patients with clinical risk score scores of 0 to 1 (Table 21.5; Grobmyer et al, 2004; Jarnagin et al, 2001b). Targeting laparoscopy to high-risk patients should avoid unnecessary laparoscopic staging in low-risk patients, whereas performing it in the high-risk group should prevent needless staging laparotomies and improve the yield overall from laparoscopy.
Table 21.5 Criteria for Clinical Risk Score to Determine Risks for Extrahepatic Disease
From Jarnagin WR, et al, 2001b: A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer 91:1121-1128.
Pancreatic and Periampullary Malignancy
Our approach for the staging of pancreatic adenocarcinoma is illustrated in Figure 21.10. A minority of patients (20% to 35%) with pancreatic and periampullary malignancies (see Chapter 58A, Chapter 58B, Chapter 59 ) are suitable for curative resection (Conlon & Minnard, 1997; Conlon & Brennan, 2000; Jimenez et al, 2000). Similar to management of hepatobiliary malignancies, initial diagnostic evaluation begins with high-resolution CT. Today’s studies can predict resectability in 57% to 88% of cases (Freeny, 2001; Pisters et al, 2001; Contreras et al, 2009; Mayo et al, 2009). Other useful investigative modalities that may augment CT evaluations include MRI, MRCP, ERCP, EUS, and positron emission tomography (PET).
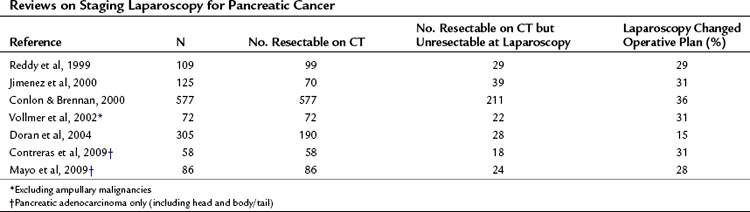
Cuschieri and colleagues first described staging laparoscopy for pancreatic malignancy in 1978; they revisited it later, in 1988. In the earlier report, laparoscopic examinations demonstrated that 42 (57%) of 73 patients had unresectable disease, mainly peritoneal or omental metastases. Since these landmark papers, technologic advances have allowed laparoscopic staging to develop so as to allow it to be comparable to open exploration in defining resectability (Table 21.6). In 1993, Conlon and associates from Memorial Sloan-Kettering Cancer Center introduced laparoscopic staging for patients with suspected adenocarcinoma of the pancreas (Conlon et al, 1996). Prior to 1993, only about one third of patients considered to harbor resectable disease after preoperative assessment ultimately underwent curative resection procedures. Between 1993 and 1998, this rate increased considerably to roughly 63%.
Table 21.6 Criteria for Unresectability in Pancreatic Cancer
Data from Conlon KC, Brennan MF, 2000: Laparoscopy for Staging Abdominal Malignancies. St Louis, Mosby; and Conlon KC, Minnard EA, 1997: The value of laparoscopic staging in upper gastrointestinal malignancy. Oncologist 2:10-17.
Other groups have reported similar findings. Warshaw and colleagues (1990) compared CT, MRI, angiography, and laparoscopy in 88 patients and found that CT failed to show peritoneal disease in 22 cases discovered at laparoscopy. MRI conferred no further benefit, and laparoscopy had a sensitivity of 96%, having understaged 1 patient. This same group reported that 39 patients (44%) were spared a laparotomy because of metastases discovered at laparoscopy not seen on spiral CT. This group performed staging laparoscopy as a day-case procedure, further reducing inpatient hospital time. Reddy and associates (1999) confirmed these findings in a later report; 29% were spared further intervention. A more recent prospective study (Doran et al, 2004) reported outcomes of 239 patients with pancreatic cancer who were submitted to staging laparoscopy; of these, 190 were considered to have resectable disease based on CT findings. After staging laparoscopy, roughly 15% of patients were spared a laparotomy. This estimate likely reflects advancements in high-resolution imaging, but it also demonstrates that despite refinements in imaging techniques, peritoneal disease is not easily revealed on CT.
Beyond these estimates, staging laparoscopy enables the surgeon to more easily detect occult metastases (Shoup et al, 2004). Contreras et al (2009) discovered further that within a 4-year period, occult metastases among 52 patients with potentially resectable pancreatic tumors were more likely to be detected during staging laparoscopy when compared with open explorations (32% vs. 18%). Many other centers have reported similar results (Table 21.7).
Laparoscopic ultrasound is a useful adjunct during staging laparoscopy. John and colleagues (1995) demonstrated that the addition of US improved the sensitivity from 65% to 80%. Callery and associates (1997) similarly reported 96% specificity and 92% sensitivity when laparoscopic US was combined with staging laparoscopy. Vollmer and others (2002) studied 72 patients with pancreatic head cancers and found that 22 had metastatic disease that precluded resection; laparoscopy alone identified 14 of these 22 patients; the remaining 8 patients had major vessel encasement or liver metastases revealed by laparoscopic US. Minnard and colleagues (1998) similarly reported that laparoscopic US altered the planned surgical treatment in 14% of patients, whose staging laparoscopic procedures were equivocal. Sonography was particularly useful in identifying venous (42%) and arterial (38%) involvement, which precludes curative resection.
Controversies
Some critics of routine staging laparoscopy believe that unresectable disease due to vascular involvement or local extension can only be confirmed during open explorations (Friess et al, 1998). Others suggest that the role for laparoscopy is limited only to those patients who do not require some form of palliation, either biliary or gastric bypass (Holzman et al, 1997; Andren-Sandberg et al, 1998). Finally, some argue that staging laparoscopy is costly, time consuming, and of decreasing diagnostic yield because of improvements in radiologic imaging techniques (Spitz et al, 1997).
Two recent studies suggested that the utility of staging laparoscopy depends on the type of malignancy (Vollmer et al, 2002; Brooks et al, 2002). Staging laparoscopy has not been shown to be useful among patients with ampullary or duodenal tumors, because these cancers tend to present earlier and are less likely to have metastatic disease at the time of presentation. In contrast, staging laparoscopy may have a role in patients with nonfunctioning islet cell tumors (Hochwald et al, 2001), which manifest with a large tumor mass and often in the presence of metastatic disease.
Amott D, et al. Prospective comparison of routine and selective operative cholangiography. Aust N Z J Surg. 2005;75:378-382.
Andren-Sandberg A, et al. Computed tomography and laparoscopy in the assessment of the patient with pancreatic cancer. J Am Coll Surg. 1998;186:35-40.
Belghiti J, et al. Surgical treatment of hepatocellular carcinoma in cirrhosis: value of preoperative ultrasonography. Gastroenterol Clin Biol. 1986;10:244-247.
Berber E, Siperstein AE. Laparoscopic ultrasound. Surg Clin North Am. 2004;84:1061-1084.
Bismuth H, Castaing D. Échographie Per-Opératoire du Foie et des Voies Biliaires. Paris: Blammarion; 1985.
Bismuth H, et al. The use of operative ultrasound in surgery of primary liver tumors. World J Surg. 1987;11:610-614.
Bloed W, et al. Role of intraoperative ultrasound of the liver with improved preoperative hepatic imaging. Eur J Surg. 2000;166:691-695.
Brooks AD, et al. The value of laparoscopy in the management of ampullary, duodenal, and distal bile duct tumors. J Gastrointest Surg. 2002;6:139-146.
Callery MP, et al. Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Surg. 1997;185:33-39.
Catheline J, et al. A comparison of laparoscopic ultrasound versus cholangiography in the evaluation of the biliary tree during laparoscopic cholecystectomy. Eur J Ultrasound. 1999;10:1-9.
Charfare H, Cheslyn-Curtis S. Selective cholangiography in 600 patients undergoing cholecystectomy with 5-year follow-up for residual bile duct stones. Ann R Coll Surg Engl. 2003;85:167-173.
Conlon KC, Brennan MF. Laparoscopy for Staging Abdominal Malignancies. St Louis: Mosby; 2000.
Conlon KC, Minnard EA. The value of laparoscopic staging in upper gastrointestinal malignancy. Oncologist. 1997;2:10-17.
Conlon KC, et al. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg. 1996;223:134-140.
Conlon R, et al. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003;16:211-216.
Contreras CM, et al. Staging laparoscopy enhances detection of occult metastases in patients with pancreatic adenocarcinoma. J Surg Oncol. 2009;100:663-669.
Curet MJ. Port site metastases. Am J Surg. 2004;187:705-712.
Cuschieri A. Laparoscopy for pancreatic cancer: does it benefit the patient? Eur J Surg Oncol. 1988;14:41-44.
Cuschieri A, Berci G. Operative biliary endoscopy. In: Cuschieri, A, Berci, G. Common Bile Duct Exploration. Boston: Martinus Nijhoff; 1984:55-59.
Cuschieri A, et al. Value of laparoscopy in the diagnosis and management of pancreatic carcinoma. Gut. 1978;19:672-677.
D’Angelica M, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183-189.
de Castro SM, et al. Diagnostic laparoscopy for primary and secondary liver malignancies: impact of improved imaging and changed criteria for resection. Ann Surg Oncol. 2004;11:522-529.
Decker G, et al. One hundred laparoscopic choledochotomies with primary closure of the common bile duct. Surg Endosc. 2003;17:12-18.
de Rooij PD, et al. Evaluation of palliative surgical procedures in unresectable pancreatic cancer. Br J Surg. 1991;78:1053-1058.
Dobronte Z, et al. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978;10:127-130.
Doran HE, et al. Laparoscopy and laparoscopic ultrasound in the evaluation of pancreatic and periampullary tumours. Dig Surg. 2004;21:305-313.
Espat NJ, et al. Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg. 1999;188:649-657.
Fletcher DR, et al. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 1999;229:449-457.
Flum DR, et al. Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA. 2003;290:2168-2173.
Freeny PC. Pancreatic carcinoma: imaging update 2001. Dig Dis. 2001;19:37-46.
Friess H, et al. The role of diagnostic laparoscopy in pancreatic and periampullary malignancies. J Am Coll Surg. 1998;186:675-682.
Giurgiu DI, et al. Laparoscopic common bile duct exploration: long-term outcome. Arch Surg. 1999;134:839-844.
Gouillat C, et al. Value of intraoperative ultrasonography in the surgical treatment of malignant tumors. Ann Chir (Paris). 1992;45:534-539.
Gozzetti G, et al. Intraoperative ultrasonography in surgery for liver tumors. Surgery. 1986;99:523-529.
Grobmyer SR, et al. Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139:1326-1330.
Gunven P, et al. Preoperative imaging of liver metastases: comparison of angiography, CT scan and ultrasonography. Ann Surg. 1985;202:573-579.
Hayashibe A, et al. A resected case of multiple intraductal papillary mucinous tumor of the pancreas with US-guided ductal branch–oriented partial pancreatectomy. Pancreatology. 2005;5:462-465.
Hochwald SN, et al. Laparoscopy predicts metastatic disease and spares laparotomy in selected patients with pancreatic nonfunctioning islet cell tumors. Ann Surg Oncol. 2001;8:249-253.
Holzman MD, et al. The role of laparoscopy in the management of suspected pancreatic and periampullary malignancies. J Gastrointest Surg. 1997;1:236-244.
Hyser MJ, et al. Laparoscopic transcystic management of choledocholithiasis. Am Surg. 1999;65:606-609.
Jakimowicz J. Review: intraoperative ultrasonography during minimal access surgery. J R Coll Surg Edinb. 1993;38:231-238.
Jarnagin WR, et al. Liver resection for metastatic colorectal cancer: assessing the risk of occult irresectable disease. J Am Coll Surg. 1999;188:33-42.
Jarnagin WR, et al. A prospective analysis of staging laparoscopy in patients with primary and secondary hepatobiliary malignancies. J Gastrointest Surg. 2000;4:34-43.
Jarnagin WR, et al. What is the yield of intraoperative ultrasound during partial hepatectomy for malignant disease? J Am Coll Surg. 2001;192:577-583.
Jarnagin WR, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91:1121-1128.
Jeffers L, et al. Laparoscopically directed fine needle aspiration for the diagnosis of hepatocellular carcinoma: a safe and accurate technique. Gastrointest Endosc. 1988;34:235-237.
Jimenez RE, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg. 2000;135:409-415.
John TG, et al. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg. 1994;220:711-719.
John TG, et al. Carcinoma of the pancreatic head and periampullary region: tumor staging with laparoscopy and laparoscopic ultrasonography. Ann Surg. 1995;221:156-164.
Kruskal JB, Kane RA. Intraoperative US of the liver: techniques and clinical applications. Radiographics. 2006;26:1067-1084.
Lightdale CJ. Laparoscopy and biopsy in malignant liver disease. Cancer. 1982;50(11 Suppl):2672-2675.
Lillemoe KD, et al. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Ann Surg. 1999;230:322-330.
Lo CM, et al. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998;227:527-532.
Machi J, et al. Laparoscopic ultrasonography versus operative cholangiography during laparoscopic cholecystectomy: review of the literature and a comparison with open intraoperative ultrasonography. J Am Coll Surg. 1999;1999:360-367.
Makuuchi M, et al. Intraoperative ultrasonic examination for hepatectomy. Jpn J Clin Oncol. 1981;11:367.
Massarweh NN, Flum DR. Role of intraoperative cholangiography in avoiding bile duct injury. J Am Coll Surg. 2007;204:656-664.
Mayo SC, et al. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era? J Am Coll Surg. 2009;208:87-95.
Metcalfe MS, et al. Is laparoscopic intraoperative cholangiogram a matter of routine? Am J Surg. 2004;187:475-481.
Mills JL, et al. Routine operative cholangiography. Surg Gynecol Obstet. 1985;161:343-345.
Minnard EA, et al. Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg. 1998;228:182-187.
Mirizzi PL. Operative cholangioraphy. Surg Gynecol Obstet. 1937;65:702-710.
Nagasue N, et al. Intraoperative ultrasonography for the surgical treatment of hepatic tumors. Acta Chir Scand. 1984;150:311-316.
Nickkholgh A, et al. Routine versus selective intraoperative cholangiography during laparoscopic cholecystectomy: a survey of 2,130 patients during laparoscopic. Surg Endosc. 2006;20:868-874.
Nieveen van Dijkum EJ, et al. Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg. 1999;189:459-465.
Nugent N, et al. Low incidence of retained common bile duct stones using a selective policy of biliary imaging. Surgeon. 2005;3:352-356.
Olsen AK. Intraoperative ultrasonography and the detection of liver metastases in patients with colorectal cancer. Br J Surg. 1990;77:998-999.
Parker GA, et al. Intraoperative ultrasound of the liver affects operative decision making. Ann Surg. 1989;209:569-577.
Pearlstone DB, et al. Laparoscopy in 533 patients with abdominal malignancy. Surgery. 1999;125:67-72.
Perry KA, et al. Laparoscopic ultrasound as the primary method for bile duct imaging during cholecystectomy. Surg Endosc. 2008;22:208-213.
Pisters PW, et al. Laparoscopy in the staging of pancreatic cancer. Br J Surg. 2001;88:325-337.
Rahusen FD, et al. Selection of patients for resection of colorectal metastases to the liver using diagnostic laparoscopy and laparoscopic ultrasonography. Ann Surg. 1999;230:31-37.
Reddy KR, et al. Experience with staging laparoscopy in pancreatic malignancy. Gastrointest Endosc. 1999;49(4 Pt 1):498-503.
Sarli L, et al. Scoring system to predict asymptomatic choledocholithiasis before laparoscopic cholecystectomy: a matched case-control study. Surg Endosc. 2003;17:1396-1403.
Sarli L, et al. Preoperative endoscopic sphincterotomy and laparoscopic cholecystectomy for the management of cholecystocholedocholithiasis: 10-year experience. World J Surg. 2003;27:180-186.
Shoup M, et al. Port-site metastasis after diagnostic laparoscopy for upper gastrointestinal tract malignancies: an uncommon entity. Ann Surg Oncol. 2002;9:632-636.
Shoup M, et al. Is there a role for staging laparoscopy in patients with locally advanced, unresectable pancreatic adenocarcinoma? J Gastrointest Surg. 2004;8:1068-1071.
Sigel B, et al. Comparative accuracy of operative ultrasonography and cholangiography in detecting common duct calculi. Surgery. 1983;94:715-720.
Siperstein A, et al. Comparison of laparoscopic ultrasonography and fluorocholangiography in 300 patients undergoing laparoscopic cholecystectomy. Surg Endosc. 1999;13:113-117.
Solomon MJ, et al. A new classification of hepatic territories using intraoperative ultrasound. Am J Surg. 1992;163:336-338.
Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am. 1994;74:953-959.
Sotiropoulos GC, et al. Intraoperative cholangioscopy in the management of biliary adenomas. J Surg Oncol. 2004;85:91-92.
Soyer P, et al. Surgical treatment of hepatic metastases: impact of intraoperative sonography. AJR Am J Roentgenol. 1993;160:511-514.
Spitz FR, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928-937.
Tokumura H, et al. Laparoscopic management of common bile duct stones: transcystic approach and choledochotomy. J Hepatobiliary Pancreat Surg. 2002;9:206-212.
Torzilli G, et al. Ultrasound-guided liver resections for hepatocellular carcinoma. Hepatogastroenterology. 2002;49:21-27.
Tranter SE, Thompson MH. A prospective single-blinded controlled study comparing laparoscopic ultrasound of the common bile duct with operative cholangiography. Surg Endosc. 2003;17:216-219.
Velanovich V. The effects of staging laparoscopy on trocar site and peritoneal recurrence of pancreatic cancer. Surg Endosc. 2004;18:310-313.
Vollmer CM, et al. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg. 2002;235:1-7.
Warshaw AL, et al. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230-233.
Way LW, et al. Cause and prevention of laparoscopic bile duct injuries: analysis of 242 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460-469.
Weber SM, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma: analysis of 100 patients. Ann Surg. 2002;235:392-399.
Wenner DE, et al. Actual time required for dynamic fluoroscopic intraoperative cholangiography. J Soc Laparoendosc Surg. 2005;9:174-177.
Yamakawa T. An improved choledocho-fiberscope and non-surgical removal of retained biliary calculi under direct visual control. Gastrointest Endosc. 1976;22:160-165.
Zacherl J, et al. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. 2002;26:550-554.