Chapter 22 Intraoperative and immediate postoperative management
Overview
Improvements in patient selection criteria and advances in hepatic surgical techniques and perioperative care have expanded the pool of patients who are candidates for hepatobiliary surgical procedures. Patients with known underlying hepatic parenchymal disease are at greater operative risk, particularly for liver-related and biliary tract–related procedures (see Chapters 2, 70B, and 90A). More recent studies have documented the progressive safety of liver resection in noncirrhotic patients (Belghiti et al, 2000; Jarnagin et al, 2002; Mullen et al, 2007). The anesthetic problems in connection with liver transplantation (Chapter 97B) and cirrhosis (Chapter 90A) are discussed elsewhere in this book. This chapter addresses the unique anesthetic considerations of noncirrhotic patients undergoing hepatobiliary operations, with an emphasis on problems related to joint anesthetic and surgical management.
Preoperative Evaluation
With the advent of modern techniques, the anesthetic risk attributed to any surgical procedure depends heavily on the preoperative status of the patient. In hepatobiliary patients, the presence of preoperative pulmonary, cardiac, or hepatic disease significantly augments the incidence of postoperative morbidity and mortality. Although in the past, the presence of comorbidities favored elective intensive care unit (ICU) admission, currently they more appropriately designate a select group of patients who may potentially fail fast tracking. These patients need to be recognized at the preoperative visit and clinically optimized before surgery. Postoperative morbidity adversely affects long-term outcome after hepatic resection, therefore efforts aimed at reducing perioperative morbidity will likely further enhance the therapeutic benefit of resection (Ito et al, 2008).
Cardiac Evaluation
For most stable patients, cardiac risk can be derived from the Revised Cardiac Risk Index (Lee et al, 1999b). This simple index identifies six independent risk factors: 1) high-risk type of surgery, 2) history of ischemic heart disease, 3) history of congestive heart failure, 4) history of cerebrovascular disease, 5) diabetes mellitus, and 6) renal insufficiency. The number of clinical risk factors, the risk level of the surgery, and the functional capacity of the patient will determine the need for further testing prior to surgery or pharmacologic intervention perioperatively (Poldermans et al, 2008). For those patients with clinical risk factors, the 2007 American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery are an excellent framework for evaluating cardiac risk in the perioperative period for patients undergoing liver surgery (Fleischer et al, 2007).
Over the years, a shift in perioperative management has been seen, away from treating coronary obstruction with interventions such as surgery and stents and toward medical therapy aimed at stabilizing coronary plaque and preventing myocardial oxygen supply and demand mismatch. Today, preoperative cardiac testing, cardiac stenting, and coronary revascularization are performed for the same indications as those used in the nonoperative setting (Poldermans et al, 2008), but β-blockers, statins, and aspirin are now widely used in the perioperative setting. Compelling evidence supports perioperative β-blocker therapy, unless contraindicated, in patients with coronary artery disease. β-Blocker therapy has been shown to reduce the incidence of perioperative ischemic events and myocardial infarction (Goldman, 2001; Lee et al, 1999a). Recently the risk benefit of this therapy in patients without coronary artery disease has been challenged. The perioperative ischemic evaluation (POISE) study showed an increased incidence of ischemic stroke in combination with intraoperative bradycardia and hypotension (Devereaux et al, 2006). In a retrospective review of patients who underwent noncardiac surgery, perioperative β-blockers showed no benefit—and possible harm—in low-risk patients, but in high-risk patients, a benefit was seen (Lindenauer et al, 2005).
Communication among the patient’s cardiologist, surgeon, and anesthesiologist is essential for the management of hepatobiliary surgical patients who have coronary artery stents. Clinical judgment is of the utmost importance to balance the risk–benefit ratio of antiplatelet therapy interruption versus continuation. Aspirin should never be interrupted, unless the risk of bleeding far outweighs the risk of stent thrombosis (Newsome et al, 2008).
In hepatobiliary patients with a history of alcohol abuse, cardiac assessment should include the evaluation of myocontractile function. Cirrhotic cardiomyopathy classically occurs in men 30 to 55 years old who have been alcohol abusers for more than 10 years (Braunwald, 1997). Putative mechanisms of damage are direct toxic effects of 1) ethanol and its metabolite acetaldehyde; 2) nutritional deficiencies, particularly of thiamine (beriberi); and 3) additives in commercial products, such as cobalt in beer. Alcohol and acetaldehyde have been shown to decrease calcium binding and transport, myocardial lipid metabolism, myocardial synthesis, and myocardial adenosine triphosphatase (ATPase) activity. The two basic patterns of cardiac dysfunction are 1) left ventricular dilation with impaired systolic function and 2) left ventricular hypertrophy with diminished compliance and normal or increased contractile performance. Presenting manifestations range from insidious onset to catastrophic left-sided cardiac failure. Anginal chest pain does not occur, unless the patient has concomitant coronary artery disease or aortic stenosis.
Pulmonary Evaluation
Despite steady advances in care, patients with respiratory disease remain at increased risk for postoperative pulmonary complications (PPCs). Indeed postoperative pulmonary complications may rival cardiovascular complications in frequency and severity (Lawrence et al, 1995). Studies that examine risk factors for PPCs have limitations, but some consistent patterns are seen. The three most important risk factors for PPCs are 1) pulmonary disease, 2) cigarette smoking, and 3) the site of surgery, with abdominal surgery being one of the highest risk sites (Warner, 2000). Of note, pulmonary function testing per se is not useful in predicting risk (McAlister et al, 2003); thus routine pulmonary function testing is not justified unless it is part of an effort to optimize preoperative pulmonary status. The definition of “optimization” depends on the type of respiratory disease and the individual patient. Consultation with the surgeon and pulmonologist will help determine whether a course of systemic corticosteroids or antibiotics is warranted perioperatively. Despite the increased risk of PPCs in patients with preexisting pulmonary disease, no prohibitive level of pulmonary function has been established for which surgery is contraindicated (Nunn et al, 1988; Kroenke et al, 1993). A prospective study did not find an elevated Paco2 level to be a risk factor among surgical candidates, hence clinicians should not use arterial blood gas analyses to identify patients for whom the risk of surgery is prohibitive (Kearney et al, 1994).
Obese patients are at risk of suffering from a number of respiratory derangements, including obstructive sleep apnea (OSA), obesity hypoventilation syndrome, and restrictive impairment. The increase in body mass also results in increased oxygen consumption and carbon dioxide production. With these isssues in mind, it is not surprising that acute PPCs are twice as likely in obese and OSA patients (Rose et al, 1994; Dindo et al, 2003). Many patients with OSA are undiagnosed, but a strong relationship exists between obesity and OSA (Strohl et al, 1996). The American Society of Anesthesiologists addressed this issue with practice guidelines to assess patients for possible OSA before surgery and to provide careful postoperative monitoring for those suspected to be at risk (Gross et al, 2006).
An association was also found between obesity and OSA and several medical conditions, such as increased risk of venous stasis, pulmonary embolism, hypertension, cerebral vascular acccidents, cardiomyopathy, arrythmias, and ischemic heart disease (Dominguez et al, 1993; Strohl et al, 1996; Gupta et al, 2001). With these issues in mind, the anesthesiologist should have an informed discussion with the patient about the increased risk of morbidity and mortality and should also work with other members of the patient care team to determine whether any interventions need to be initiated before surgery in an effort to minimize complication risk.
Polysomnography is the gold standard for diagnosis of OSA, but it is an expensive and limited resource. The most reasonable approach is to check room air pulse oximetry. If the patient has an oxygen saturation level less than 96%, further evaluation is warranted; a 2-week period of continuous positive airway pressure therapy has been shown to be effective in correcting abnormal ventilatory drive and improving cardiac function (Lin, 1994; Loadsman et al, 2001). In addition, deep venous thrombosis prophylaxis should be discussed with the surgeon.
Hepatic Evaluation
Risk factors and symptoms of liver disease are not as well defined as those in other organ systems. The diagnosis of liver disease requires a high degree of suspicion and careful probing of the clinical history to identify specific risk factors for liver disease, such as previous blood transfusions, jaundice, travel, tatoos, high-risk sexual behavior, illicit drug use, excessive alcohol intake, and chemotherapy (Suman & Carey, 2006).
In North America and Europe, most hepatic resections are performed for metastatic disease. The operative outcome for hepatobiliary surgery is linked to severity of hepatic parenchymal disease and the extent of postoperative functional reduction (see Chapter 2). In patients with mild or well-compensated chronic hepatic disease, operative outcome is likely to be indistinguishable from the outcome in the general population. Patients with hepatocellular carcinoma (HCC) have poorer outcome than those with metastatic disease given the propensity of HCC to develop in the cirrhotic liver (Fattowich, 2003).
The goal of preoperative screening is to determine the presence of preexisting liver disease without the need for extensive or invasive monitoring. Liver function tests can measure different aspects of hepatic function, but as a group of tests, they lack specificity and are often affected by nonhepatic function. These biochemical markers cannot quantify the hepatic disease. Another issue is finding abnormal hepatic function test results in asymptomatic patients. In general, for asymptomatic patients with mildly elevated alanine and aspartate aminotransferase levels and a normal bilirubin concentration, cancellation of surgery is rarely indicated. In patients with significant abnormalities, detailed investigation is warranted to evaluate for underlying cirrhosis, given the high perioperative risk observed in patients with cirrhosis. The need for further investigation in these patients is highlighted by studies that report an incidence of undiagnosed cirrhosis of 34% in asymptomatic patients with abnormal liver function results (Hay et al, 1989; Hultcrantz et al, 1986). It is important to identify patients with underlying hepatic impairment, because they may be at increased risk for further injury from alterations in splanchnic blood flow that occur during anesthesia and intraabdominal surgery.
Measurement of metabolic liver function would provide the necessary predictive information to assess this risk. Metabolic liver function tests (see Chapter 2), such as the aminopyrine breath test and indocyanine green clearance, may have predictive value in this regard but are not routinely performed. To date, risk assessment has been primarily performed in patients with known liver disease (Child-Turcotte-Pugh score), but the quantification of risk in asymptomatic patients should not be extrapolated from these studies. The risk of postoperative hepatic insufficiency is closely related to the volume and function of the remnant liver, and several methods for liver volume determination are available. Most use computerized tomography (CT) combined with three-dimensional CT volumetry (Denys et al, 2002). When the functional liver remnant volume is below a certain threshold, generally 25% to 30% for normal liver and 40% for diseased liver, the risk of postoperative hepatic failure is high, and portal vein embolization may be indicated prior to hepatic resection (Vauthey et al, 2000).
Bloodless Surgery
In the early years of hepatic resection, major intraoperative blood loss was common, transfusion of blood products was routine, and morbidity and mortality were unacceptably high (Foster & Berman, 1977). General improvements in hepatic resection techniques have reduced intraoperative blood loss and transfusion rates; nevertheless, perioperative transfusion remains a potent predictor of increased perioperative morbidity and mortality (Jarnagin et al, 2002). In recent years, liver resection without the need for blood transfusion has become increasingly possible (Belghiti et al, 2000; Cunningham et al, 1994; Fan et al, 1999; Jarnagin et al, 2002). Patient blood management is based on three pillars: 1) detecting and treating preoperative anemia, 2) reducing the loss of red blood cells perioperatively, and 3) optimizing the treatment of anemia (Spahn et al, 2008).
Thorough preoperative planning is essential to avoid perioperative allogeneic transfusion. Any history of bleeding disorders and management of anticoagulation must be evaluated, including discontinuation of drugs that adversely affect clotting (e.g., acetylsalicylic acid, nonsteroidal antiinflammatory drugs, and anticoagulants). In patients with anemia, recombinant human erythropoietin or iron therapy may optimize the starting operative hemoglobin (Monk, 1996). Preoperative autologous donation also has been used to reduce the need for allogeneic red blood products (Brecker & Goodnough, 2001). Preoperative autologous donation may not avoid allogeneic blood, however, because almost half of the patients who donate blood before surgery are anemic on the day of operation, and preoperative strategies to augment the red blood cell mass require more time than is generally reasonable for optimal efficacy. Patients with low hemoglobin levels at the start of surgery are at an increased risk of receiving allogeneic blood (Armas-Loughran et al, 2003). In addition, preoperative autologous donation is costly; it can be associated with clerical errors; and for every 2 units donated, usually only 1 unit gets transfused (Goldman et al, 2002). If the patient is optimized and surgery is bloodless, the autologous units are discarded. Other blood conservation strategies, such as intraoperative blood salvage (cell saver) and acute normovolemic hemodilution (ANH) have been used successfully for hepatobiliary surgery in Jehovah’s Witness patients (Jabbour et al, 2005). ANH has been shown in two prospective studies to reduce the amount of red blood cells transfused per patient in major liver resections (Matot et al, 2002a; Jarnagin et al, 2008).
Intraoperative Management
Hepatic blood flow in surgical patients can be altered by a variety of factors, including arterial blood pressure, posture changes, carbon dioxide levels, intravascular volume shifts, positive pressure ventilation, positive end-expiratory pressure, regional anesthesia, and volatile anesthetics (Gelman, 1992). Surgical stimulation and manipulation of the liver markedly increases hepatic oxygen extraction ratio and splanchnic vasoconstriction (see Chapter 4) (Kainuma et al, 1991; Whittle & Moncada, 1998). A 16% decrease in hepatic blood flow is associated with anesthesia and mechanical ventilation (Gelman, 1976), and a further 40% decrease is seen with splanchnic surgery. This decrease may be the direct effect of sympathetic control of the hepatic venous bed mediated through the hepatic innervation (Greenway et al, 1986).
Positive end-expiratory pressure decreases hepatic blood flow in a stepwise manner. The response cannot be explained by a decrease in cardiac output alone; investigators postulate a vasoconstrictive response in the preportal vasculature. Intraperitoneal insufflation and head-up tilt result in impairment of hepatic blood flow secondary to decreases in cardiac output (Berendes et al, 1996b; Eleftheriadis et al, 1996). The hepatic blood flow decrease caused by hypotensive spinal anesthesia is attenuated by ephedrine administration (Nakayama et al, 1993). Two human studies using the indocyanine green plasma disappearance rate to evaluate the effects of high lumbar epidural anesthesia (EDA) on hepatic blood flow have found a decrease of 25% to 35% despite a constant cardiac output (Kennedy et al, 1971; Tanaka et al, 1997). Patients treated with norepinephrine to compensate for the EDA-induced decrease in arterial blood pressure had an additional marked decrease in hepatic blood flow (Meierhenrich et al, 2009).
It is controversial whether catecholamines increase hepatic blood flow and oxygen supply, but dobutamine does not cause a significant increase in hepatic arterial blood flow (Kainuma et al, 1992). Total hepatic blood flow and portal venous blood flow are increased, however, resulting in an augmentation of hepatic oxygen delivery. The increase in hepatic oxygen delivery is countered by an increase in hepatic oxygen uptake with no overall improvement in hepatic oxygen supply–uptake ratio. Dopamine and norepinephrine increase hepatic venous oxygen saturation, suggesting that the vasoactive treatment of patients in septic shock may not compromise splanchnic oxygenation (Ruokonen et al, 1991, 1993). Hepatic portal flow does not decrease despite a 20% to 60% reduction in blood pressure as long as cardiac index is maintained during sodium nitroprusside hypotension (Chauvin et al, 1985). A well-planned anesthetic maximizes the relationship between oxygen transport and oxygen use with the premise that reductions in systemic pressure would reduce hepatic blood flow. Anything resulting in significant reductions of systemic pressure and cardiac output–induced hypotension, hypovolemia, and anesthetic overdoses should be avoided.
Volatile Anesthetics
Anesthetic Hepatotoxicity
Fulminant hepatic necrosis and jaundice after halothane, so-called halothane hepatitis, is rare—one in 6000 to 35,000 anesthetics—but often fatal. Halothane hepatitis is an immunologic phenomenon initiated by halothane metabolism and the binding of its metabolite to liver proteins to form trifluoroacetylated proteins, which stimulate antibodies in susceptible individuals. Upon subsequent halothane reexposure, these antibodies mediate massive hepatic necrosis. Because the extent of metabolism of enflurane, isoflurane, and desflurane is so much less than that of halothane, fulminant hepatitis from these anesthetics is far less common than with halothane (Elliot & Strunin et al, 1993). In 1987, the U.S. Food and Drug Administration determined that there was no conclusive association between isoflurane exposure and postoperative hepatitis (Shingu et al, 1983; Stoelting et al, 1987). Sevoflurane metabolism is different from that of the other volatile anesthetics, however, because it does not result in trifluoroacetyled liver proteins, and immune-based hepatitis after sevoflurane has not been reported. With the disappearance of halothane and enflurane from clinical practice in developed countries, and lack of hepatotoxicity from either isoflurane, desflurane or sevoflurane, anesthetic volatile hepatotoxicity is not a significant concern (Elliot & Strunin, 1993).
Hemodynamics
Volatile anesthetics reduce hepatic blood flow in a dose-dependent fashion by affecting cardiac output and systemic pressure. Surgical manipulation causes an additional decrease in estimated hepatic blood flow. Isoflurane has been considered the agent of choice in cases in which preservation of splanchnic blood flow is required, and liver blood flow and the hepatic artery buffer response are maintained better in the presence of isoflurane than with any other volatile anesthetic agent (Berendes et al, 1996a). In addition, isoflurane is shown to attenuate the increases in hepatic oxygen consumption associated with surgery and liver manipulation.
Desflurane is shown to have no deleterious effects on liver function and hepatocyte integrity, and it is associated with significantly greater gut blood flow than equipotent isoflurane. This difference cannot be explained by systemic hemodynamics alone. There is no difference in total hepatic flow between isoflurane and desflurane groups, however, which suggests that an intact hepatic arterial supply buffers response with desflurane (O’Riordan et al, 1997). Sevoflurane is similar to isoflurane and desflurane with a few exceptions (Ebert et al, 1995). Indocyanine green clearance is better preserved during sevoflurane anesthesia. In Beagles subjected to sevoflurane anesthesia and ligation of the hepatic artery, hepatic oxygen supply–uptake ratio, hemoglobin oxygen saturation, and oxygen partial pressure in hepatic venous blood were significantly lower than with halothane or isoflurane.
Nitrous oxide is used extensively in patients with hepatic disease (Khalil et al, 1994; Lampe et al, 1990). Although it has not been shown to contribute to hepatic disease exacerbation, the sympathomimetic effects of nitrous oxide may increase hepatic metabolic requirements.
Intravenous Anesthetics and Muscle Relaxants
Inhaled anesthetics supply all the aspects needed for anesthesia in one package, but today most anesthesiologists choose multiple drugs to reach their goals: immobility, amnesia, suppression of autonomic reflexes, muscle relaxation, and analgesia. Over the past several decades, dramatic advances have been made in intravenous anesthesia with the result that total intravenous anesthesia is now a workable alternative to the traditional inhalation anesthetic. Anesthesiologists using multiple drugs take advantage of interactions among drugs that have different mechanisms of action but similar therapeutic effects. The therapeutic goal of the anesthetic can often be achieved with less toxicity and faster recovery than when individual drugs are used alone in higher doses. The safety of intravenous anesthetics and muscle relaxants in patients having hepatobiliary surgery is well documented (Gelman, 1992).
The main mechanisms that affect hepatic elimination of a drug are changes in hepatic blood flow and changes in the ability of the liver cells to biotransform and/or excrete a drug. These two mechanisms, hepatic blood flow and hepatocyte function, play an important role in the choice of anesthetics for patients undergoing hepatobiliary surgery. High-extraction drugs (ketamine, flumazenil, morphine, fentanyl, sufentanil, lidocaine) are directly related to liver blood flow and are essentially cleared as they pass through the liver. Protein binding, enzymatic induction, intrahepatic shunting, and the effect of anesthetics on liver blood flow may influence the elimination of drugs with a high extraction rate. Reduced metabolic clearance results in increased peak drug level with minimal change in the elimination half-life. Drugs with a low extraction rate (benzodiazepines) differ from high-extraction drugs in that concentration changes little after passage through the liver, and intrinsic clearance depends on hepatic metabolic function as determined by liver size and total enzyme capacity (Colli et al, 1988). In patients with impaired liver function, such drugs have a prolonged length of activity with no increase in peak levels.
Special Anesthetic Considerations
Anesthesia for Hepatectomy
Fluid loading before resection is commonly practiced in preparation for hepatic resection. This fluid loading often results in a distended and tense vena cava and dilated hepatic veins, both of which contribute to increased blood loss during liver mobilization and parenchymal transection. A relationship exists between extent of intraoperative blood loss and morbidity; blood loss greater than 5 L in an adult is associated with prohibitive mortality (Yanaga et al, 1988). Inadvertently, fluid loading to provide hemodynamic stability during episodes of uncontrolled bleeding may cause technical difficulties and handicap repair of venous injuries.
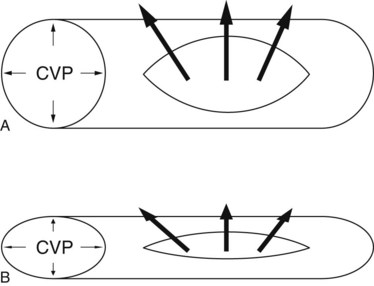
Another approach, low central venous pressure (CVP) anesthesia, is designed to preclude vena caval distension to facilitate mobilization of the liver and dissection of retrohepatic vena cava and major hepatic veins (Cunningham et al, 1994; Melendez et al, 1998). This surgical anesthetic technique compares favorably with other techniques for major hepatic resection (Delva et al, 1989; Segawa et al, 1993; Yanaga et al, 1988). Low CVP anesthesia is performed in combination with surgical inflow and outflow vascular control before parenchymal transection. Such an approach requires close cooperation between the anesthesiologist and the surgeon. Low CVP anesthesia minimizes hepatic venous bleeding during parenchymal transection and facilitates control of inadvertent venous injury. The blood loss resulting from a vascular injury is directly proportional to the pressure gradient across the vessel wall and the fourth power of the radius of the injury. If the CVP is lowered from 15 mm Hg to 3 mm Hg, the blood loss through a vena caval injury consequently falls by a factor greater than 5. Lowering CVP lessens the pressure component of the equation and also minimizes the radial component of flow by reducing vessel distension (Fig. 22.1).
Fluid restriction is an important aspect of the low CVP anesthesia technique. Preoperative overnight fluid replacement, a maneuver commonly performed before or immediately after the induction of general anesthesia, is withheld. Strict fluid restriction is practiced until the liver resection is completed. Intermittently, small fluid boluses may be given to maintain hemodynamic stability. To guarantee the safety of patients, appropriate cannulation is carried out, and a high level of vigilance is maintained. Transfusion equipment, such as the Woods pump coupled to a level 1 system, is an important aid to emergency fluid resuscitation, although this is infrequently needed. Intravascular hypovolemia is counteracted with 15-degree head-down tilt. Steep head-down tilt preserves hemodynamic stability and renal function by improving venous return. In animals, head-down tilt increases glomerular filtration rate, sodium excretion, and urine output lasting 8 hours (McCombs et al, 1996). Prolonged head-down tilt improves venous return and yields a 70% increase in plasma atrial natriuretic protein (Hughson et al, 1995; Terai et al, 1995). Although some investigators report intraoperative oliguria during low CVP anesthesia, no increase in the incidence of postoperative renal failure has been observed (Melendez et al, 1998).
Anesthesia is maintained with a combination of isoflurane in oxygen and narcotics, because isoflurane provides vasodilation with minimal myocardial depression (Schwinn et al, 1990). Elimination of nitrogen from the anesthetic gas mixture is necessary to permit expiratory nitrogen monitoring for air emboli. Restriction of nitrous oxide in the gas mixture prevents the diffusion-mediated increase in the volume of circulating air. Consistent with its minimal effect on cardiac output and systemic pressure, fentanyl has no effect on liver blood flow and oxygen delivery. In combination with isoflurane, fentanyl has the least effect on hepatic hemodynamics of any opiate, making it the narcotic of choice for anesthetics. In most patients, fentanyl is the ideal narcotic to aid in the maintenance of anesthesia, providing analgesia with minimal hypotension (Rosow et al, 1982). In a small group of patients, the anesthetic is supplemented with morphine to achieve the desired CVP. Morphine reduces CVP by inducing venous vasodilation caused by histamine release and µ3 receptor activation (Grossman et al, 1996; Rosow et al, 1982; Stefano et al, 1995). The combination of these anesthetics readily provides the favorable low CVP environment for hepatic resection. With this technique, intravenous nitroglycerin to lower CVP to less than 5 mm Hg is rarely necessary.
The risk of intraoperative air emboli is likely to increase under low CVP anesthesia. Transesophageal echocardiography can be used to monitor air emboli, but this technology is sensitive and overdiagnoses clinically insignificant events. With surgical vigilance and rapid occlusion of open venous channels (see Chapters 90A and 92), low CVP anesthesia results in a low incidence (0.4%) of clinically significant air emboli. More complex low CVP anesthesia management techniques have been described (Rees et al, 1996). They employ epidural blockade and intravenous nitroglycerin. These patients may require intraoperative dopamine for systemic pressure support, a technique this is cumbersome and adds an unnecessary level of complexity to an already challenging situation.
Bloodless Surgery
Red blood cell transfusions are indisputably associated with an increase in mortality, major adverse cardiac and noncardiac outcome, acute lung injury, nosocomial infection, tumor growth, duration of hospitalization, and cost (Murphy et al, 2007; Beattie et al, 2009; Kulier et al, 2007; Marik et al, 2008; Atzil et al, 2008; Shander et al, 2007). Therefore it is important not to confuse the momentary helpful effect of red blood cell transfusion on hypotension and hypovolemia with an outcome benefit. Intraoperative blood cell salvage, hemodilution, and tolerance of normovolemic anemia (Gozzetti et al, 1995) all may contribute to reduced allogeneic red blood cell transfusion in major liver surgery. Autologous blood cell salvage (intraoperative autotransfusion) involves recovery of the patient’s shed blood from a surgical wound. The blood is then washed or filtered and reinfused into the patient. Hepatectomies often are performed for cancer but previously, cell salvage had been excluded in oncologic surgery because of the concern for potential dissemination of cancer cells. This concern has been challenged because of the availability of leukocyte-depleting filters and irradiation of blood removed during cancer surgery (Hansen et al, 1999).
Another transfusion-sparing technique is acute normovolemic hemodilution (ANH), a recycling technique that can be performed intraoperatively by the anesthesiologist. Blood is removed from the patient after induction and is replaced with crystalloid or colloid fluid. By removing the patient’s blood and replacing it with acellular fluid, the blood loss is diluted. The previously removed blood is stored at room temperature in the operating room and is returned to the patient at the conclusion of the operation. Two randomized studies of ANH in major hepatic resection showed a significant reduction in the percentage of patients requiring allogeneic red blood cell transfusion (Matot et al, 2002a; Jarnagin et al, 2008).
Epidural Anesthesia and Analgesia
Epidural block is widely used to manage major abdominal surgery and postoperative analgesia, but its risks and benefits are uncertain. Studies do not support the routine use of epidural anesthesia (EDA), rather than parenteral analgesia, to prevent postoperative mortality (Fanzca et al, 2002; Park et al, 2001; Wijeysundera et al, 2008). There are, however, other reasons to prefer EDA, including better postoperative pain relief and reduced pulmonary complications (Fanzca et al, 2002). In contrast to EDA for other abdominal surgery, the use of an indwelling epidural catheter and the timing of its removal in patients undergoing liver resection remain controversial (Matot, et al, 2002b).
The hemostatic abnormalities associated with liver resection are not always predictable and/or reversible. Significant disturbances of prothrombin time (PT) can be seen in patients undergoing uncomplicated liver resection. In patients who undergo minor liver resections, the PT usually returns to control levels within 1 day. In patients having major liver resections, the disturbances in PT can be prolonged and, as a result, the epidural catheter cannot be removed without administering fresh frozen plasma to normalize the coagulation profile because of a theoretically increased risk of spinal hematoma (Siniscalchi et al, 2004). In addition, thoracic EDA is associated with a decrease in hepatic blood flow and, if combined with continuous infusion of norepinephrine to counteract the EDA-related decrease in systemic arterial blood pressure, hepatic blood flow is decreased further (Meierhenrich et al, 2009; Tanaka et al, 1997). Hepatic hypoperfusion is regarded as an important factor in the alteration of hepatic oxygen balance and metabolic activity and the pathophysiology of perioperative liver injury. Furthermore, it has been hypothesized that hypoperfusion of the liver may initiate or contribute to the development of a systemic inflammatory response syndrome that may lead to multiple organ failure (Maynard et al, 1997). The decision to insert an epidural catheter for either intraoperative anesthesia or postoperative analgesia in patients undergoing hepatic resection should be made with care.
Obstructive Jaundice
Bile duct obstruction affects hepatic hemodynamics. Acute biliary obstruction is associated with an increase in liver blood flow (see Chapter 4), but flow is decreased with chronic obstruction. Relief of long-term obstruction is not associated with a return to normal pressures and may be associated with hemodynamic embarrassment and shock. The exact cause of hemodynamic derangement in the face of biliary obstruction is unknown, but increased portal resistance is believed to play a role. Biliary sepsis may contribute to the exacerbation of the hemodynamic embarrassment. In these cases, aggressive hemodynamic monitoring may be lifesaving. Either by percutaneous or endoscopic approaches, preoperative biliary drainage does not necessarily improve the perioperative morbidity of patients who show no evidence of infection, and it is therefore not recommended as a routine (Lai et al, 1994; see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ). When there is clinical concern for the development of acute cholangitis, rapid biliary decompression and intravenous antibiotics should be administered preoperatively, and surgery should be delayed until the infection resolves (Lai et al, 1992).
Anesthesia for Vascular Isolation (See Chapter 91A, Chapter 91B )
Prolonged inflow occlusion with or without hepatic vascular isolation is an alternative surgical approach for liver resection. Hepatic vascular isolation induces significant hemodynamic changes. The anesthetic management is more complex, and many patients require pulmonary artery catheters during the procedure. Hepatic vascular isolation typically causes a decrease in venous return with a resultant decrease in cardiac index and an increase in systemic vascular resistance. Mean arterial pressure is maintained by infusing large volumes of fluid to keep the CVP high. Some patients require vasopressor support to maintain adequate perfusion during the cross-clamping period. Most patients tolerate these hemodynamic changes reasonably well, although in some cases, persistent hypotension or low cardiac index or both demand that the procedure be abandoned. Several studies of hepatic vascular isolation are summarized in Table 22.1 (Belghiti et al, 1996; Delva et al, 1989; Emond et al, 1995; Emre et al, 1993; Habib et al, 1994; Hannoun et al, 1993; Melendez et al, 1998). In these studies, blood loss and transfusion requirements were greater than those reported by the authors using portal triad clamping with extrahepatic control of the hepatic veins. Hepatic vascular isolation is more complex and has not yielded better results than portal triad clamping and extrahepatic control of the hepatic veins (Belghiti et al, 1996; Cunningham et al, 1994).
Postoperative Care
Mortality and morbidity after hepatobiliary surgery have decreased as a result of surgical and anesthetic advances and a better understanding of hepatic physiology (Belghiti et al, 2000; Jarnagin et al, 2002; Melendez et al, 1998; Ryan et al, 1982). Hepatobiliary surgery is increasingly common at major medical centers, and postoperative recovery is becoming indistinguishable from that after other major abdominal surgery. At Memorial Sloan-Kettering Cancer Center, most patients recover after major surgery in the postanesthesia care unit (day 1) and then are transferred to the general ward without any need for intensive care.
Immediate Postoperative Concerns
Some of the problems that cause metabolic and functional changes after hepatobiliary surgery are common to all major intraabdominal procedures (Aronsen et al, 1969). Others are unique and require an in-depth understanding of liver physiology. Immediate postoperative concerns are bleeding, intravascular volume, renal function, and oxygenation. Important laboratory tests are hematocrit, electrolytes, serum creatinine, blood urea nitrogen, PT/partial thromboplastin time, liver enzymes, and chest radiograph.
Unique postoperative concerns in patients after hepatobiliary surgery include phosphorus replacement, anesthetic elimination, drug metabolism, coagulation, and postoperative pain control. Data suggest that hepatocellular regeneration may be critically dependent on cellular ATPase stores (Campbell et al, 1990; Greene, 1981). Liver regeneration after partial hepatectomy involves rapid cell division 24 to 72 hours after liver resection. Standard therapy for posthepatic resection should include the supplementation of intravenous fluids with sodium or potassium phosphate. Particular attention must be paid to anesthetic elimination and residual drug effect—especially sedative, analgesic, and neuromuscular blockers—in patients who have altered drug pharmacodynamics and pharmacokinetics. Postoperative pain management must be adapted to this population. Patient-controlled analgesia, commonly employed for postoperative pain management, should be employed initially without a basal narcotic infusion rate. After hepatic resection, patients experience an unpredictable reduction in the metabolism of narcotics.
The half-life of coagulation factors is 6 hours, so coagulation factor deficiency is likely to evolve rapidly. Routine parenteral administration of vitamin K commonly corrects the coagulation abnormalities within 48 hours. More rapid correction may be accomplished by fresh frozen plasma but is rarely needed, unless the PT or international normalized ratio (INR) exceeds 2, or if there are concerns of ongoing postoperative blood loss (Martin et al, 2003).
Delayed Perioperative Complications
Identifying patients at high risk for complications is an important but difficult component of the overall management strategy. A study from Memorial Sloan-Kettering Cancer Center showed that when postoperative hepatobiliary patients required admission to the intensive care unit, mortality ranged from 37% to 79% regardless of admission diagnosis (Melendez et al, 1998). A systematic proactive approach that identifies early deviations from the norm would be preferable to having to treat organ failure (Sesto et al, 1987).
Jaundice
The differential diagnosis of postoperative jaundice is listed in Table 22.2. Mild jaundice in the early postoperative period after partial hepatectomy is relatively common, particularly in patients who undergo a major resection and have a transient, self-limited hyperbilirubinemia in the first few days after operation, which may be exacerbated by hematoma reabsorption and hemolysis of transfused blood. Progressive jaundice that does not resolve portends a more significant problem.
| Increased Bilirubin Load | Biliary Obstruction | Hepatocellular Injury |
|---|---|---|
| Blood transfusion Hematoma Hemolytic anemia Extracorporeal circulation hemolysis |
Intrahepatic cholestasis Drug induced Infection induced Extrahepatic cholestasis Bile duct injury Pancreatitis Retained gallstones |
Hepatic hypoxemia Exacerbated chronic hepatitis Acute hepatitis Viral Alcoholic Gilbert disease Sepsis Trauma Toxins, drugs |
Modified from Brown BR, 1988: Postoperative jaundice. In Brown BR (ed): Anesthesia in Hepatic and Biliary Disease. Philadelphia, FA Davis, pp 265-273; and Gelman S, 1992: Anesthesia and the liver. In Barash PG, et al, (eds): Clinical Anesthesia, 2nd ed. Philadelphia, Lippincott, pp 1185-1214.
One suggested definition of postoperative hepatic insufficiency is a peak bilirubin greater than 7 mg/dL. In a cohort of 1059 noncirrhotic patients undergoing hepatic resection, a postoperative bilirubin greater than 7 mg/dL was associated with 30% chance of dying of hepatic insufficiency (Mullen et al, 2007). On the other hand, in the same cohort, 70% survived; this highlights the importance of identifying any correctable problem that may be contributing to the hyperbilirubinemia.
Ascites
Ascites (see Chapter 74) occurs as a result of several factors: 1) impaired albumin and protein synthesis; 2) increased hydrostatic pressure in hepatic sinusoids and splanchnic capillaries; 3) overproduction of hepatic and splanchnic lymph, leading to a transudation of lymph into the peritoneal space; 4) limited or reduced reabsorption of water and protein by peritoneal lymphatics; 5) sodium retention by the kidney secondary to hyperaldosteronism, increased sympathetic stimulation, and alterations in metabolism of prostaglandins and kinins; and 6) impaired renal water excretion, partially owing to increased concentrations of antidiuretic hormone. The decreased serum albumin 1 to 2 weeks after hepatobiliary surgery, by itself, is generally unimportant clinically (Grundmann & Heistermann, 1985), and even in the presence of severe hypoalbuminemia, albumin infusion has no beneficial effect.
Renal Failure
After hepatobiliary surgery, rapidly progressive renal failure may occur (see Chapter 23), although this is uncommon after routine resections (Belghiti et al, 2000; Jarnagin et al, 2002; Melendez et al, 1998). Hepatic resection using low CVP anesthesia is associated with a very low incidence of postresection renal dysfunction, as shown by Melendez and colleagues (1998). The principal differential diagnoses include acute tubular necrosis, prerenal azotemia, and hepatorenal syndrome, which is the development of otherwise unexplained renal failure in patients with advanced liver disease.
In hepatorenal syndrome, urine sodium is typically less than 10 mEq/L with hyperosmolar urine, oliguria (<400 mL/24 h), fractional excretion of sodium less than 1, and urine creatinine/plasma creatinine ratio greater than 30 : 1. The pathophysiology of hepatorenal syndrome is based on the pooling of blood in the splanchnic bed and decreasing plasma volume (Hartleb et al, 1997). The kidney perceives a decreased glomerular filtration rate, and the resulting vasoconstriction shunts blood away from the renal cortex. In some cases, the precipitating event can be attributed to hemodynamic instability, nephrotoxic drugs, infection, or overzealous diuresis and paracentesis. Although no specific treatment for hepatorenal syndrome is available, it should always be assumed that the primary pathophysiologic event is circulatory, which requires transfer of the patient to the ICU.
Pulmonary Care
Minor pulmonary complications, such as atelectasis or pleural effusion, are common after liver resection. With aggressive postoperative pulmonary toilet and early mobilization, such problems resolve without the need for further intervention. Perioperative pulmonary complications include pneumonia, bronchospasm, atelectasis, hypoxemia, exacerbation of underlying chronic lung disease, and respiratory failure, usually defined as the need for mechanical ventilatory support. Patients with significant hepatic parenchymal disease are at potential risk for hepatopulmonary syndrome, which is defined as a triad of signs that comprise 1) liver disease, 2) increased alveolar-arterial gradients, and 3) evidence of intrapulmonary vascular resistance (Krowka & Cortese, 1994). Autopsy specimens of human and animal lung have shown precapillary pulmonary dilations and direct arteriovenous communications. Hepatopulmonary syndrome is rare after hepatic resection, and these patients generally require management in the ICU.
Sepsis
Mortality after hepatic resection is often caused by abdominal sepsis (Bozzetti et al, 1992). In one series, 6 of 14 deaths were the result of intraabdominal sepsis (Melendez et al, 1998). Biliary manipulation and placement of biliary stents, culture-positive bile, retained gallstones, ascites, and blood loss all may predispose to abdominal sepsis. Patients undergoing hepatic resection for hilar cholangiocarcinoma are at particular risk for postoperative infective complications. Other sources of sepsis include the respiratory and urinary systems. Upper abdominal incision, pleural effusion, chronic obstructive pulmonary disease, and poor pulmonary toilet may promote the development of pneumonia, so wound inspections and central line catheter changes should be performed routinely.
Gastrointestinal Bleeding
Although the incidence of stress-related gastric mucosal lesions is high, the actual incidence of bleeding ulcers is 5% with a mortality of 50% reported. In practice, clinically significant gastrointestinal bleeding after hepatic resection is rare (Jarnagin et al, 2002). Endoscopy allows identification of the bleeding site, and treatment is largely supportive with transfusions, antacids, and H2 blockers. Prophylactic therapy for prevention of stress ulceration should be considered in all patients after major hepatobiliary procedures.
Encephalopathy
A sudden change in a patient’s mental status and the onset of asterixis, a flapping tremor related to loss of extensor tone, are signs of hepatic insufficiency that should precipitate a search for the cause and the start of therapy for hepatic encephalopathy (Gitlin, 1998). Precipitating factors include gastrointestinal bleeding, infection, drugs, diet, and dehydration. The exact pathogenesis of hepatic encephalopathy is unknown, but accumulation of endogenous ammonia is associated with the onset of encephalopathy and has been suggested as causative. Other possible contributory factors include 1) changes in the blood–brain barrier permeability, 2) abnormal neurotransmitter balance, 3) altered cerebral metabolism, 4) impairment of neuronal Na+-K+-ATPase activity, and 5) increased endogenous benzodiazepines. Standard therapy is designed to reduce levels of ammonia and other potentially toxic metabolites, and short-term benefit from flumazenil administration has been reported (Ferenci et al, 1989). Dietary protein restriction and lactulose, however, remain the gold standard of care.
Armas-Loughran B, et al. Evaluation and management of anemia and bleeding disorders in surgical patients. Med Clin North Am. 2003;87:229-242.
Aronsen KF, et al. Metabolic changes following major hepatic resection. Ann Surg. 1969;169:102-110.
Atzil S, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008;109:989-997.
Beattie WS, et al. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574-581.
Belghiti J, et al. Portal triad clamping or hepatic vascular exclusion for major liver resection: a controlled study. Ann Surg. 1996;224:155-161.
Belghiti J, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38-46.
Berendes E, et al. Effects of enflurane and isoflurane on splanchnic oxygenation in humans. J Clin Anesth. 1996;8:456-468.
Berendes E, et al. Effects of positive end-expiratory pressure ventilation on splanchnic oxygenation in humans. J Cardiothorac Vasc Anesth. 1996;10:598-602.
Bozzetti F, et al. Morbidity and mortality after surgical resection of liver tumors: analysis of 229 cases. Hepatogastroenterology. 1992;39:237-241.
Braunwald, E, editor. Heart Disease: A Textbook of Cardiovascular Medicine, 5th ed. Philadelphia: Saunders. 1997:1793-1799.
Brecker ME, Goodnough LT. The rise and fall of preoperative autologous blood transfusion. Transfusion. 2001;41:145.
Campbell KA, et al. In vivo 31P NMR spectroscopic changes during liver regeneration. J Surg Res. 1990;49:244-247.
Chauvin M, et al. Hepatic plasma flow during sodium nitroprusside–induced hypotension in humans. Anesthesiology. 1985;63:287-293.
Colli A, et al. Disposition of a flow-limited drug (lidocaine) and a metabolic capacity-limited drug (theophylline) in liver cirrhosis. Clin Pharmacol Ther. 1988;44:642-649.
Cunningham JD, et al. One hundred consecutive hepatic resections: blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050-1056.
Delva E, et al. Vascular occlusion for liver resections: operative management and tolerance to hepatic ischemia in 142 cases. Ann Surg. 1989;209:211-218.
Denys A, et al. Indications for and limitations of portal vein embolization before major hepatic resection for hepatobiliary malignancy. Surg Oncol Clin N Am. 2002;11:955-968.
Devereaux PJ, et al. Rationale, design, and organization of the perioperative ischemic evaluation (POISE) trial; a randomized controlled trial of metoprolol versus placebo in patients undergoing noncardiac surgery. Am Heart J. 2006;152:223-230.
Dindo MK, et al. Obesity in general elective surgery. Lancet. 2003;361:2032-2035.
Dominguez OD, et al. Sleep apnea and the risk for perioperative myocardial infarction. Ann Intern Med. 1993;119:953.
Ebert TJ, et al. Cardiovascular responses to sevoflurane: a review. Anesth Analg. 1995;81(Suppl 6):S11-S22.
Eleftheriadis E, et al. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
Elliot RH, Strunin L. Hepatotoxicity of volatile anaesthetics: implications for halothane, enflurane, isoflurane, sevoflurane, and desflurane. Br J Anaesth. 1993;70:339-348.
Emond J, et al. Total vascular occlusion for major hepatectomy in patients with abnormal liver parenchyma. Arch Surg. 1995;130:824-831.
Emre S, et al. Liver resection under total vascular isolation: variations on the theme. Ann Surg. 1993;217:15-19.
Fan ST, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322-330.
Fanzca JR, et al. Epidural anaesthesia and anlagesia and outcome of major surgery: a randomized trial. Lancet. 2002;359:1276-1282.
Fattowich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47-58.
Ferenci P, et al. Successful long-term treatment of portal-systemic encephalopathy by the benzodiazepine antagonist flumazenil. Gastroenterology. 1989;96:240-243.
Fleischer LE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation for noncardiac surgery. Circulation. 2007;116:1971-1996.
Foster JH, Berman MM. Solid liver tumors. Major Prob Clin Surg. 1977;22:1-342.
Gelman S. Disturbances in hepatic blood flow during anesthesia and surgery. Arch Surg. 1976;111:881-883.
Gelman S. Anesthesia and the liver. In: Barash, PG, et al. Clinical Anesthesia. 2nd ed. Philadelphia: Lippincott; 1992:1185-1214.
Gitlin N. Hepatic encephalopathy. In: Zakim, D, Boyer, TD. Hepatology: A Textbook of Liver Disease. 3rd ed. Philadelphia: Saunders; 1998:605-617.
Goldman L. Assessing and reducing cardiac risks of noncardiac surgery. Am J Med. 2001;110:320-323.
Goldman M, et al. Declining value of preoperative autologous donation. Transfusion. 2002;42:819-823.
Gozzetti G, et al. Liver resection without blood transfusion. Br J Surg. 1995;82:1105-1110.
Greene NM. Anesthesia risk factors in patients with liver disease. Contemp Anesth Pract. 1981;4:87-109.
Greenway CV, et al. Effects of circulating catecholamines on hepatic blood volume in anesthetized cats. Am J Physiol. 1986;250(6 Pt 2):H992-H997.
Gross JB, et al. Practice guidelines for the perioperative management of patients with sleep apnea. Anesthesiology. 2006;104:1081-1093.
Grossman M, et al. Morphine-induced venodilation in humans. Clin Pharmacol Ther. 1996;60:554-560.
Grundmann R, Heistermann S. Postoperative albumin infusion therapy based on colloid osmotic pressure: a prospectively randomized trial. Arch Surg. 1985;120:911-915.
Gupta RM, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement. Mayo Clin Proc. 2001;76:897-905.
Habib N, et al. Liver resection with total vascular exclusion for malignant tumors. Br J Surg. 1994;81:1181-1184.
Hannoun L, et al. Liver resection with normothermic ischaemia exceeding 1 h. Br J Surg. 1993;80:1161-1165.
Hansen E, et al. Blood irradiation for intraoperative autotransfusion in cancer surgery: demonstration of efficient elimination of contaminating tumor cells. Transfusion. 1999;39:608-615.
Hartleb M, et al. The role of nitric oxide in portal hypertensive systemic and portal vascular pathology. Acta Gastroenterol Belg. 1997;60:222-232.
Hay JE, et al. The nature of unexplained chronic transaminotransferase elevations of a mild to moderate degree in asymptomatic patients. Hepatology. 1989;9:193-197.
Hughson RL, et al. Investigation of hormonal effects during 10-h head-down tilt on heart rate and blood pressure variability. J Appl Physiol. 1995;78:583-596.
Hultcrantz R, et al. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scan J Gastroenterol. 1986;21:109-113.
Ito H, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994-1002.
Jabbour N, et al. Transfusion-free surgery: Single institution experience of 27 consecutive liver transplants in Jehovah’s witnesses. J Am Coll Surg. 2005;201:412-417.
Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397-407.
Jarnagin WR, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248:360-369.
Kainuma M, et al. Monitoring hepatic venous hemoglobin oxygen saturation in patients undergoing liver surgery. Anesthesiology. 1991;74:49-52.
Kainuma M, et al. The effect of dobutamine on hepatic blood flow and oxygen supply–uptake ratio during enflurane nitrous oxide anesthesia in humans undergoing liver resection. Anesthesiology. 1992;77:432-438.
Kearney DJ, et al. Assessment of operative risk in patients undergoing lung resection: importance of predicted pulmonary function. Chest. 1994;105:753-759.
Kennedy WF, et al. Simultaneous systemic and hepatic hemodynamic measurements during high peridural and anesthesia in normal man. Anesth Analg. 1971;50:1069-1078.
Khalil M, et al. Pharmacokinetics and pharmacodynamics of rocuronium in patients with cirrhosis. Anesthesiology. 1994;80:1241-1247.
Kroenke K, et al. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest. 1993;104:1445-1451.
Krowka MJ, Cortese DA. Hepatopulmonary syndrome: current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537.
Kulier A, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116:471-479.
Lai EC, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1585.
Lai EC, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195-1198.
Lampe GH, et al. Nitrous oxide does not impair hepatic function in young or old surgical patients. Anesth Analg. 1990;71:606-609.
Lawrence VA, et al. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995;10:671-678.
Lee TH, et al. Reducing cardiac risk in noncardiac surgery. N Engl J Med. 1999;341:1838-1840.
Lee TH, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
Lin CC. Effect of CPAP on ventilatory drive in normocapnic and hypercapnic patients with obstructive sleep apnea syndrome. Eur Respir J. 1994;7:2005-2010.
Lindenauer PK, et al. Perioperative beta-blocker therapy and mortality after noncardiac surgery. N Eng J Med. 2005;353:349-361.
Loadsman JA, et al. Anesthesia and sleep apnea. Br J Anaesth. 2001;86:254-266.
Marik PE, et al. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667-2674.
Martin RC, et al. The use of fresh frozen plasma after major hepatic resection for colorectal metastasis: is there a standard for transfusion? J Am Coll Surg. 2003;196:402-409.
Matot I, et al. Effectiveness of acute normovolemic hemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology. 2002;97:794-800.
Matot I, et al. Epidural anesthesia and analgesia in liver resection. Anesth Analg. 2002;95:1179-1181.
Maynard ND, et al. Liver function and splanchnic ischemia in critically ill patients. Chest. 1997;111:180-187.
McAlister FA, et al. Accuracy of preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am J Respir Crit Care Med. 2003;167:741-744.
McCombs GB, et al. Effects of thoracic volume expansion on cardiorenal function in the conscious rat. Aviat Space Environ Med. 1996;67:1086-1091.
Meierhenrich R, et al. The effect of thoracic epidural anesthesia on hepatic blood flow in patients under general anesthesia. Anesth Analg. 2009;108:1331-1337.
Melendez JA, et al. Peri-operative outcome of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion and the risk of post-operative renal dysfunction. J Am Coll Surg. 1998;178:620-625.
Monk T. Improving the outcome of acute normovolemic hemodilution with epoetin alfa. Semin Hematol. 1996;33(Suppl 2):49-50.
Mullen JT, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-862.
Murphy GJ, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544-2552.
Nakayama M, et al. Effects of ephedrine on indocyanine green clearance during spinal anesthesia: evaluation by the finger piece method. Anesth Analg. 1993;77:947-949.
Newsome LT, et al. Coronary artery stents: II. Perioperative considerations and management. Anesth Analg. 2008;107:570-590.
Nunn JF, et al. Respiratory criteria of fitness for surgery and anaesthesia. Anaesthesia. 1988;43:543-551.
O’Riordan J, et al. Effects of desflurane and isoflurane on splanchnic microcirculation during major surgery. Br J Anaesth. 1997;78:95-96.
Park WY, et al. Effect of epidural anesthesia and analgesia on perioperative outcome: a randomised controlled Veterans Administration study. Ann Surg. 2001;234:560-571.
Poldermans D, et al. Pre-operative risk assessment and risk reduction before surgery. J Am Coll Cardiol. 2008;51:1913-1924.
Rees M, et al. One hundred and fifty hepatic resections: evolution of technique towards bloodless surgery. Br J Surg. 1996;83:1526-1529.
Rose DK, et al. Critical respiratory events in the postanesthesia care unit: patient, surgical and anesthetic factors. Anesthesiology. 1994;81:410-418.
Rosow CE, et al. Histamine release during morphine and fentanyl anesthesia. Anesthesiology. 1982;56:93-96.
Ruokonen E, et al. Effect of vasoactive treatment on the relationship between mixed venous and regional oxygen saturation. Crit Care Med. 1991;19:1365-1369.
Ruokonen E, et al. Regional blood flow and oxygen transport in septic shock. Crit Care Med. 1993;21:1296-1303.
Ryan WH, et al. Reduction in the morbidity and mortality of major hepatic resection: experience with 52 patients. Am J Surg. 1982;144:740-743.
Schwinn DA, et al. Isoflurane-induced vasodilation: role of the adrenergic nervous system. Anesth Analg. 1990;71:451-459.
Segawa T, et al. Operative results in 143 patients with hepatocellular carcinoma. World J Surg. 1993;17:663-667.
Sesto ME, et al. Hepatic resection in 128 patients: a 24-year experience. Surgery. 1987;102:846-851.
Shander A, et al. Estimating the cost of blood: past, present and future directions. Best Pract Res Clin Anaesthesiol. 2007;21:271-289.
Shingu K, et al. Hepatic injury induced by anesthetic agents in rats. Anesth Analg. 1983;62(2):140-145.
Siniscalchi A, et al. Increased prothrombin time and platelet counts in living donor right hepatectomy: implications for epidural anesthesia. Liver Transpl. 2004;10:1144-1149.
Spahn DR, et al. Patient blood management: the pragmatic solution for the problems with blood transfusion. Anesthesiology. 2008;109:951-953.
Stefano GB, et al. Presence of the µ3 opiate receptor in endothelial cells. J Biol Chem. 1995;270:30290-30293.
Stoelting RK, et al. Hepatic dysfunction after isoflurane anesthesia. Anesth Analg. 1987;66(2):147-153.
Strohl KP, et al. Recognition of obstuctive sleep apnea. Am J Resp Crit Care Med. 1996;154:279-289.
Suman A, Carey WD. Assessing the risk of surgery in patients with liver disease. Cleve Clin J Med. 2006;73:398-404.
Tanaka N, et al. The effect of dopamine on hepatic blood flow in patients undergoing epidural anesthesia. Anesth Analg. 1997;85:286-290.
Terai C, et al. Effect of mild Trendelenburg on central hemodynamics and internal jugular vein velocity, cross-sectional area, and flow. Am J Emerg Med. 1995;13:255-258.
Vauthey JN, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519.
Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000;92:1467-1472.
Whittle BJR, Moncada S. Nitric oxide: the elusive mediator of the hyperdynamic circulation of cirrhosis. Hepatology. 1998;16:1089-1092.
Wijeysundera D, et al. Epidural anaesthesia and survival after intermediate-to-high non-cardiac surgery: a population-based cohort study. Lancet. 2008;10:61121-61126.
Yanaga K, et al. Hepatic resection for hepatocellular carcinoma in elderly patients. Am J Surg. 1988;155:238-244.