Chapter 27 Interventional endoscopy
Technical aspects
Overview
The field of therapeutic biliary and pancreatic endoscopy has evolved rapidly since the first reports of endoscopic sphincterotomy in 1974. A spectrum of interventional procedures has gained widespread acceptance as therapeutic alternatives to operative management (Table 27.1; Brugge & Van Dam, 1999). The minimally invasive nature of endoscopic retrograde cholangiopancreatography (ERCP) coupled with its ability to be performed under conscious sedation have resulted in its rapid dissemination around the world. Refinements in procedural technique and improvements in accessory equipment have improved results and safety. This chapter focuses on these technical advances. The indications, contraindications, and complications of interventional biliary and pancreatic endoscopy are presented elsewhere (see Chapter 14, Chapter 18, Chapter 36, Chapter 37, Chapter 50D, Chapter 54, Chapter 63B ) and are not directly addressed here.
Table 27.1 Interventional Endoscopy in Biliary and Pancreatic Disease
Cannulation
Selective cannulation of the bile and pancreatic ducts is a prerequisite for interventional procedures. Cannulation usually can be achieved with a standard cannulating catheter, using contrast injection to confirm successful cannulation. Use of a sphincterotome for cannulation has the advantage of enabling immediate sphincterotomy after cannulation. The sphincterotome also enables upward bowing in a caudocranial axis to assist in selective cannulation of the bile duct (Fig. 27.1). Wire-guided sphincterotome cannulation is associated with a lower rate of pancreatitis (Lee et al, 2009).
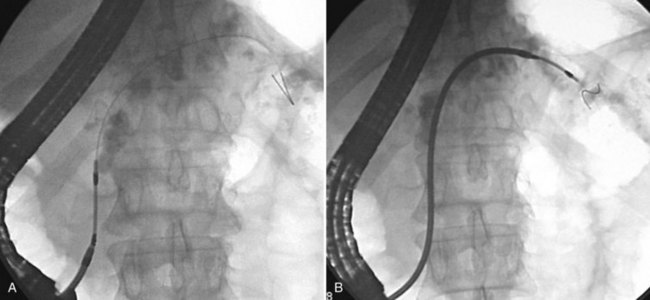
If free cannulation with a cannulating catheter fails, guidewire cannulation can be attempted. Hydrophilic guidewires with a special slippery coating are ideal for cannulation, because they minimize trauma to the papilla and more effectively seek out the path to the desired duct. The guidewire is extended several millimeters from the tip of the cannula or sphincterotome, and the papillary orifice is cautiously probed with the wire, until it slips into the duct (Fig. 27.2). When deep cannulation is achieved, the cannulating catheter can be advanced over the wire into the duct.
Precutting
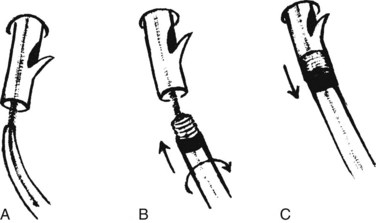
Precutting is performed with either a needle-knife catheter (Fig. 27.3, top) or Erlangen-type precut papillotome (Fig. 27.3, bottom). Most of the published experience has been with the needle-knife catheter, which consists of a retractable bare diathermy wire that extends 5 mm from the tip of the catheter; the needle is inserted at or just above the papillary orifice, and the cut is directed upward in the 11 to 12 o’clock position (Fig. 27.4). Biliary cannulation success rates exceeding 90% have been reported (Misra, 2009). The needle knife can also be used to create a supraampullary fistula in patients with biliary obstruction as a result of an ampullary malignancy. Another application is to free an impacted stone at the papilla; an incision is made directly into the most bulging portion of the papilla against the surface of the impacted stone. Higher complication rates with precut sphincterotomy for stone extraction have been reported (Pereira-Lima et al, 2001).
Precutting is not without risk, but some series suggest that in expert hands, it does not increase the risks of pancreatitis (Lawrence et al, 2009). Two large multicenter studies identified precutting as a significant risk factor for postsphincterotomy pancreatitis, and there have been isolated reports of perforation. Practice guidelines have established that precutting requires appropriate training and expertise and should be reserved for patients with a strong indication for sphincterotomy (Carr-Locke, 2004).
Rendezvous Procedure
Biliary cannulation can be aided by a combined percutaneous and endoscopic approach, known as a rendezvous procedure. A guidewire is inserted via the percutaneous transhepatic route into the duodenum and is subsequently grasped at the endoscopic end. The guidewire is pulled through the working channel of the endoscope and serves as a guide rail to enable retrograde biliary cannulation, sphincterotomy, and further endoscopic intervention (Calvo et al, 2001).
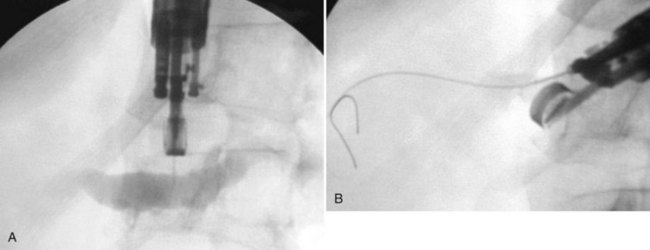
A rendezvous approach also can be used to accomplish biliary or pancreatic duct cannulation under endoscopic ultrasound guidance (Mallery et al, 2004). The bile or pancreatic duct is punctured transduodenally or transgastrically with a standard fine aspiration needle loaded with a guidewire. The wire is advanced anterogradely across the stricture into the duodenum (Fig. 27.5), and the stent is placed over the wire in the standard retrograde fashion.
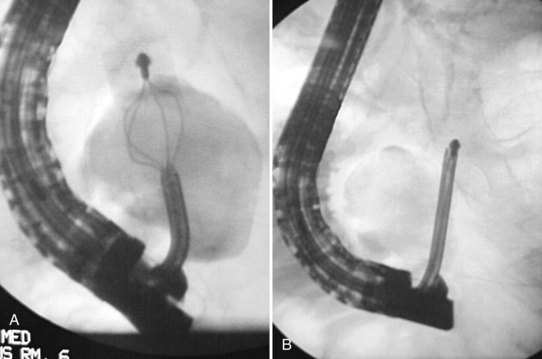
A further variation of the rendezvous approach can be used to achieve cannulation of the pancreatic duct (Bataille & Deprez, 2002). The minor papilla is used to pass a wire from the duct of Santorini into the duct of Wirsung and then across the major papilla. When cannulation of the major papilla is achieved, the wire is rerouted from the minor papilla to the main pancreatic duct (Fig. 27.6).
Juxtapapillary Diverticula
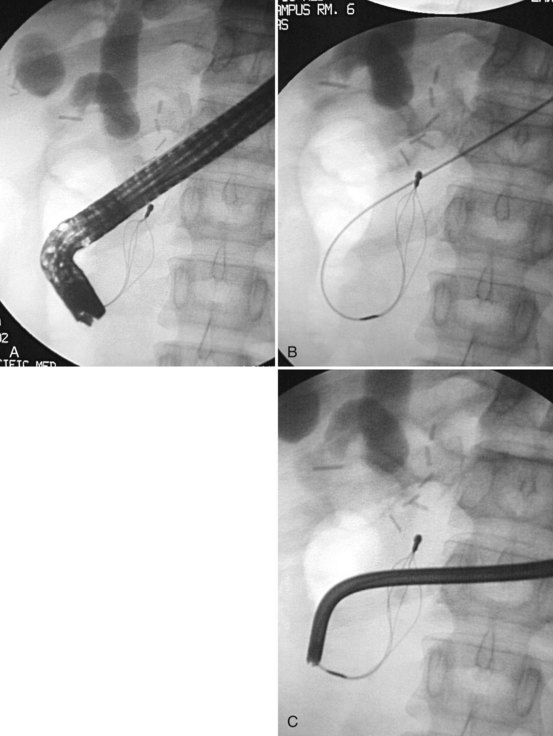
Frequently found in elderly patients, juxtapapillary duodenal diverticula alter the position and anatomic boundaries of the papilla and displace the course of the bile and pancreatic ducts, making cannulation more difficult (Panteris et al, 2008). Accessing the papilla is a problem when it is located within the diverticulum, but various ancillary maneuvers may improve access. Aspirating air from the diverticulum may help “evert” the diverticulum, bringing the papilla into a more favorable position for cannulation. Altering the patient’s position and applying abdominal pressure may also be helpful. Failing these, a small depot of normal saline can be injected into the submucosal plane of the mucosa adjacent to the papilla. The injection should be made on the diverticular side of the papilla in an attempt to “tilt” it toward the endoscopist (Fig. 27.7). The use of two accessories, forceps and cannula, inserted together through the working channel to enable the endoscopist to pull the papilla out of the diverticulum and cannulate the papilla simultaneously also has been described (Fujita et al, 1998).
Deep cannulation of the bile duct adjacent to a large juxtapapillary diverticulum may be difficult, because the diverticulum distorts the course of the distal common bile duct. It is helpful to alter the direction of cannulation after entering the biliary orifice according to the anatomy of the diverticulum (Fig. 27.8). The direction of cannulation may be caudal rather than cranial; a guidewire tends to straighten the course of bile and pancreatic ducts and may facilitate deep cannulation (Draganov et al, 2005).
Billroth II Gastrectomy
A patient who has undergone a Billroth II gastrectomy poses a special challenge to the endoscopist. Accessing the papilla is the first obstacle. When the afferent loop is long, it can be difficult to intubate, and the papilla can be difficult to reach. Cannulation of the Billroth II papilla is the second obstacle. Because the endoscope approaches the papilla from below rather than above, the papillary anatomy in a Billroth II patient appears reversed. The bile duct is cannulated by orienting the catheter downward, toward the 5 to 6 o’clock position, rather than toward the standard 11 to 12 o’clock position. It is helpful to use a new, straight catheter or mold a downward bow into the catheter to achieve this (Fig. 27.9). A rotatable sphincterotome can also aid in the cannulation of the bile duct (Kim et al, 2008). Cannulation of the pancreatic duct is achieved by inserting the catheter at the 11 o’clock position and aiming it upward in a right-to-left orientation. The bile duct is usually easier to cannulate than the pancreatic duct, because the catheter is automatically directed in the axis of the bile duct. The placement of a guidewire into the bile duct aids in the placement and performance of the sphincterotomy (Dolay & Soylu, 2008).
Sphincterotomy
The basic technique of sphincterotomy has changed little since its initial description. The standard sphincterotome, the Erlangen “pull-type” model, consists of a catheter containing a cautery wire exposed 20 to 25 mm near the tip of the instrument. The leading tip distal to the wire, the “nose,” is 5 to 10 mm in diameter. After deep bile duct cannulation, the sphincterotome is retracted slowly, until one fourth to one half of the wire length is exposed outside the papilla. The sphincterotome is slightly bowed so that the wire is in contact with the roof, but not excessively, to avoid a “zipper” incision. The incision is made by gently lifting the sphincterotome against the papillary roof using the elevator and up–down controls while applying short bursts of current. The power settings vary according to the type of diathermy unit used. It is debated whether blended or pure-cut current should be used. Theoretically, pure-cut current would be associated with less edema of the ampulla and may decrease the risk of pancreatitis, but the risk of postsphincterotomy hemorrhage might be greater. In a prospective randomized study, the use of pure-cut current was associated with a lower incidence of pancreatitis than with blended-current sphincterotomy (Elta et al, 1998). In addition, the use of blended current after pure cutting reduced the risk of bleeding without increasing the risk of pancreatitis (Gorelick et al, 2001). Another randomized study found that pure-cut current did not reduce the frequency of pancreatitis and was associated with a significantly increased risk of minor bleeding (Macintosh et al, 2004). If blended current is used, the cut should be monitored for excessive tissue blanching and edema, because this is likely to increase the risk of pancreatitis.
The length of a bile duct sphincterotomy should be tailored to the indication. A small incision (≤1 cm) is adequate for stent insertion, whereas stone extraction usually requires a larger incision, depending on the size of the stone to be extracted (Fig. 27.10A). The extent of the sphincterotomy is limited by the length of the intraduodenal portion of the common bile duct. The length of a pancreatic duct sphincterotomy should not exceed 1 cm because of the perpendicular insertion at the ampulla (Fig. 27.10B). In rare cases, a biliary sphincterotomy can be performed with a sphincterotome placed through a percutaneously placed choledochoscope (Itoi et al, 2004). The adequacy of a sphincterotomy can be gauged by the ability to move the bowed sphincterotome across the opening. In the presence of a juxtapapillary diverticulum, the incision should be performed in millimeter increments, paying close attention to the boundaries of the incision and the course of the intraduodenal segment of the bile duct. Late stenosis has been reported to be more common after pancreatic sphincterotomy than after biliary sphincterotomy and is easily treated with repeat endoscopic sphincterotomy (Veldkamp et al, 2007).
In a Billroth II patient, the standard pull-type sphincterotome is unsuited for sphincterotomy because of the difficult approach to the papilla. Special sphincterotomes and techniques have been developed to accommodate this anatomic variation (Dolay & Soylu, 2008). As an alternative to the sphincterotome, a needle knife can be used to perform the sphincterotomy over a previously inserted stent that serves as a guide rail for the incision (Nakahara et al, 2009; Fig. 27.11).
Sphincterotomy of the minor papilla in a patient with pancreas divisum requires greater caution because of the smaller size of the papilla. Wire-guided cannulation will aid in the performance of a minor ampulla sphincterotomy (Maple et al, 2009). The incision length is generally 3 to 5 mm, but it may be larger if the minor papilla is very prominent. The incision is made at the 12 o’clock position. A needle knife can also be used to perform the sphincterotomy over a 5- or 7-Fr stent (Fig. 27.12; Kwan et al, 2008), and balloon dilation sphincteroplasty can be used to aid in the extraction of pancreatic duct stones (Maydeo et al, 2009). A reduction in symptoms and episodes of pancreatitis is often reported after sphincterotomy in older patients with a history of recurrent pancreatitis and a dilated pancreatic duct (Fukumori et al, 2007; Borak et al, 2009).
Sphincteroplasty
Balloon dilation of the sphincter muscle using high-pressure hydrostatic balloons 6 or 8 mm in diameter has been employed as an alternative to sphincterotomy (Fig. 27.13; MacMathuna et al, 1994). The theoretical advantage of this approach is the preservation of the sphincter function. The drawback of balloon dilation is a more limited size of the papillary opening, which may limit the size of the stone that can be extracted using the balloon or a basket catheter. Stones measuring greater than 8 mm often require mechanical lithotripsy to enable transpapillary extraction. The risk of pancreatitis seems to be higher after a sphincteroplasty compared with a sphincterotomy, but balloon dilation after a limited sphinctertomy does not increase the risk of pancreatitis (Kowalski et al, 2009).
Stone Extraction
Several series have shown that 85% to 90% of bile duct stones can be removed effectively after endoscopic sphincterotomy with a basket and/or balloon catheter (see Chapters 36 and 37). The choice of basket or balloon is largely dictated by stone size: baskets are sturdier and provide better traction for removal of a larger stone (Fig. 27.14); a balloon catheter occludes the lumen and is ideal for removing small stones or sludge (Fig. 27.15).
Several technical points may increase the success rate of stone extraction. The sphincterotomy should be generous enough to enable extraction of the stone. The adequacy of the incision can be assessed by passing an inflated balloon catheter through the sphincterotomy opening. If multiple medium or large stones are present, it is important to remove stones individually, beginning with the lowermost stone, to avoid stone impaction. If a stone is mistakenly captured, it can be dislodged from the basket by pushing it upward into the hepatic duct confluence, where the basket tends to fold over or advance into the hepatic duct, thereby releasing the stone. When extracting a stone, the direction of traction should be in the axis of the bile duct, which can be verified on fluoroscopy. The success rate of stone extraction after balloon sphincteroplasty is very high (Garcia-Cano et al, 2009). Lithotripsy is indicated for the extraction of stones too large for extraction through the sphincteroplasty (Attasaranya et al, 2008).
Intrahepatic stones pose a special challenge, because they are difficult to access via the retrograde transpapillary route (see Chapters 39 and 44). These stones usually form above strictures and are tightly impacted, and a wire-guided basket may be helpful to gain access to such stones. Intrahepatic stones also can be removed by the percutaneous transhepatic route (see Chapters 28 and 44).
Pancreatic duct stones are a common finding in obstructive chronic pancreatitis. Pancreatic duct stones tend to be firmly impacted or form proximal to a stricture, requiring preliminary fragmentation using lithotripsy techniques. Stone fragments and debris are extracted with a basket or balloon (Fig. 27.16). A recent study comparing surgical and endoscopic treatment of benign pancreatic disease suggests surgery provides a superior long-term outcome (Cahen et al, 2007).
Lithotripsy
Mechanical Lithotripsy
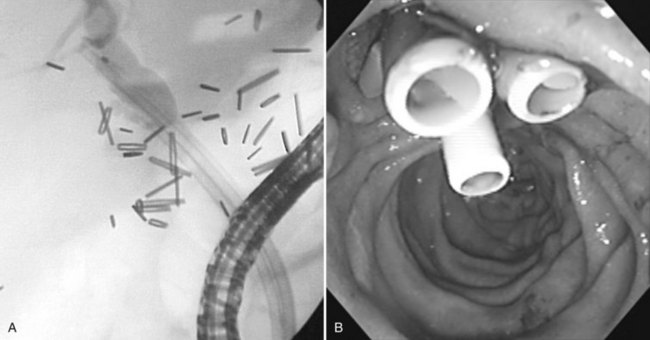
Using mechanical lithotripsy, the stone is forcefully crushed in the arms of a Dormia basket after entrapment. This method is the simplest and most cost-effective of the lithotripsy techniques, with success rates of 80% to 90%. There are two variations to the technique of mechanical lithotripsy: a nonendoscopic method and a through-the-endoscope method. Using the nonendoscopic method, the stone is captured within a standard Dormia basket, the basket handle is cut off, and the endoscope is removed. A coiled metal sheath is inserted over the wire, until its tip is in contact with the stone, and mechanical lithotripsy is performed (Fig. 27.17). Using the through-the-endoscope method, a special lithotripsy basket contained within a metal sheath is inserted through the endoscope working channel (Fig. 27.18). The stone is captured in the basket, and lithotripsy is performed. Because the basket is part of a single-unit lithotriptor device, this method presumes that the endoscopist anticipates the need for mechanical lithotripsy before an attempt is made to extract the stone. If stone extraction is attempted with a standard Dormia basket, and the stone and basket become impacted in the bile duct, the endoscopist must use the nonendoscopic method to effect fragmentation.
Intraductal Shock-Wave Lithotripsy
Failure of mechanical lithotripsy is usually due to an inability to capture the stone in the basket, which may occur if the stone is firmly impacted or very large. An alternative modality to achieve stone fragmentation is to use shock waves, which can be delivered intraductally or extracorporeally. Intraductal lithotripsy is performed using flexible probes that can be introduced into the bile or pancreatic duct. The shock waves are generated using electrohydraulic or laser technology and are applied directly to the stone surface. Because shock waves can cause injury to the duct wall, intraductal lithotripsy generally is performed under endoscopic guidance using a “baby scope” inserted into the duct via the duodenoscope, or “mother scope” (Fig. 27.19; Swahn et al, 2009). The lithotripsy probe is inserted through the operating channel of the baby scope, and shock waves are fired under visual guidance, until adequate stone fragmentation is achieved (Fig. 27.20). In patients with an altered anatomy, a nonoperative approach can be taken, using a holmium laser delivered through a choledochotomy (Bark et al, 2009).
Extracorporeal Shock-Wave Lithotripsy
Extracorporeal shock-wave lithotripsy (ESWL) has been shown to be an effective and safe method to fragment bile and pancreatic duct stones that defy endoscopic extraction. The technique can be used for fragmentation of bile duct stones using fluoroscopic or ultrasound guidance (Amplatz et al, 2007). However, if the stone is not visible by fluoroscopy or ultrasound, ESWL is not possible (Merrett & Desmond, 1990). After fragmentation of any stones, the residual fragments must be removed, usually aided by endoscopic sphincterotomy (Tandan et al, 2009). The need for ESWL has decreased as the effectiveness of mechanical lithotripsy has improved. Pancreatic stone fragmentation with ESWL requires multiple sessions and endoscopic management that includes pancreatic sphincterotomy and stent placement (Brand et al, 2000).
Chemical Dissolution
Monooctanoin and methyl tert-butyl ether (MTBE) have been used to dissolve common bile duct stones via nasobiliary drainage catheters and T-tubes (Tandon et al, 1990). Despite encouraging initial reports, this approach has been abandoned because of the cumbersome and time-consuming technique, poor overall results, and adverse effects related to leakage of the solvent into the duodenum. The administration of ursodeoxycholic acid in combination with endoscopic stent placement was shown in one study to soften and decrease the size of common bile stones, facilitating endoscopic extraction (Han et al, 2009). This approach is taken when initial stone extraction and fragmentation is unsuccessful.
Stricture Dilation
Stricture dilation can be performed using push-type dilation catheters (bougies) or high-pressure hydrostatic balloons (see Chapter 28). Hydrostatic balloons are commonly used to dilate benign strictures caused by chronic pancreatitis, sclerosing cholangitis, or operative injury (Freeman et al, 2001). When inflated, the balloon applies radial dilating force, and balloons are available in varying lengths (2, 3, and 4 cm) and diameters (4, 6, and 8 mm). The balloon is inflated with diluted contrast material to the maximum atmospheric pressure allowable, and it is kept inflated until the stricture “waist” disappears, generally after 30 to 60 seconds. Dilation is painful, and this may limit the duration of inflation; sequential brief dilations may be better tolerated. Balloon dilation is usually used in conjunction with stent placement except in liver transplantation patients, in whom chronic stenting may be associated with complications (Kulaksiz et al, 2008). The duration of stenting required to achieve a permanent dilation effect varies. Postoperative biliary strictures and pancreatic strictures secondary to chronic pancreatitis may require more than a year of stenting (Weber et al, 2009b), and bile duct strictures associated with chronic calcified pancreatitis are less likely to respond to plastic stenting (Kahl et al, 2003). The placement of multiple stents by adding a stent at 3-month intervals may optimize the dilation effect (Fig. 27.21; Sakai et al, 2009). Similar approaches have been successful for strictures in the ducts of Santorini and Wirsung (Costamagna et al, 2006).
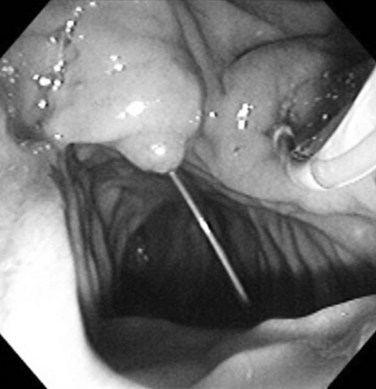
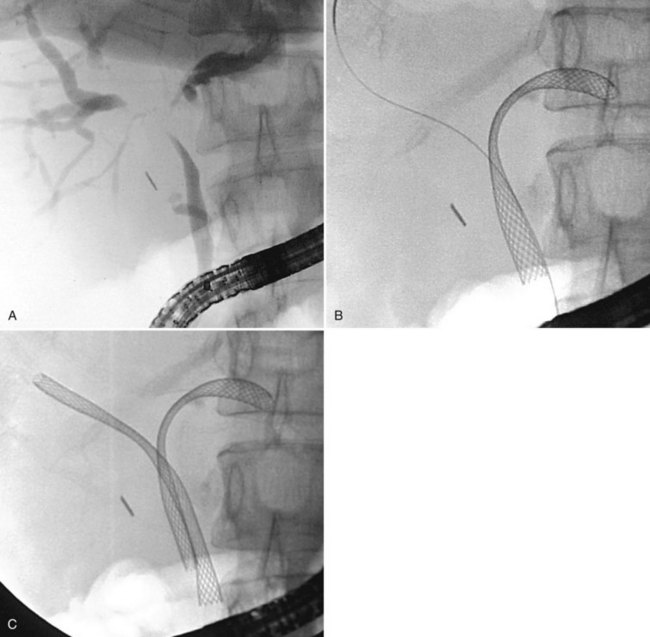
Tight strictures that do not permit passage of a bougie or balloon catheter can be opened with the Soehendra Stent Retriever (Cook Medical, Winston-Salem, NC) (Brand et al, 2000). The device has a threaded tip at its end with a central lumen for coaxial guidewire insertion, and it is rotated to core a path through a high-grade stricture. After placement of a guidewire across the stricture, the Retriever is coaxially inserted until it engages the stricture. The rotational device is rotated clockwise at the handle by the assistant, while the endoscopist advances the device across the stricture (Fig. 27.22). Strictures should be sampled with brushings for cytology or forceps for histologic material (Higashizawa et al, 2002).
Stenting
Endoscopic transpapillary stent placement is a well-standardized technique that can be carried out with an overall success rate of greater than 90%. Stenting has become the treatment of choice for the palliative decompression of malignant distal biliary obstruction (see Chapter 50B, Chapter 58A, Chapter 58B, Chapter 59, Chapter 63B ), and it may be an alternative to surgery for the treatment of benign distal biliary and pancreatic strictures, although not all authors agree regarding the long-term efficacy (see Chapter 42A, Chapter 42B ). Stenting also has been found to be an effective treatment for bile duct leaks after surgery or trauma (Coelho-Prabhu & Baron, 2009); a biliary sphincterotomy should be performed at the time of the stent placement and may be used as the sole treatment (Aksoz et al, 2009). Pancreatic duct leaks are also effectively treated, particularly with stenting across the duct defect.
Types of Stents
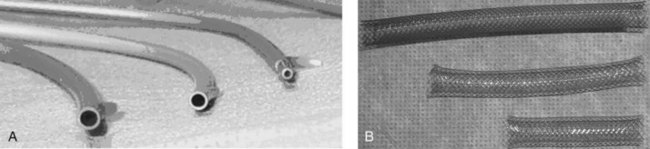
Two types of stents are used, made of either plastic and expandable metal (Fig. 27.23). Plastic stents may be straight, with flaps for anchorage, or they may be a single or double pigtail (Jain et al, 2000). Expandable metal stents may be uncovered or covered. The covering of the expandable stent may block drainage of a neighboring duct, so covered stents should be restricted to distal bile duct strictures and must be used cautiously in patients with a gallbladder. Covered stents may extend patency rates by preventing tumor or hyperplastic tissue ingrowth, but this requires further study. Plastic stent migration out of the stricture is more commonly seen in benign strictures requiring the use of longer stents (Arhan et al, 2009), and the frequency of stent occlusion is related to the diameter of the metal stent (Loew et al, 2009). Recently, the use and removal of covered metal stents in benign disease has been demonstrated but is associated with a complication rate of 14%. Special “pancreatic” stents made of soft plastic with multiple side holes for drainage of side branches are used in the pancreatic duct. Pancreatic duct stents with large side holes remain patent for longer periods than stents with small side holes (Buscaglia et al, 2009); however, most pancreatic stents are placed for short-term prophylaxis to maintain sphincter patency (Chahal et al, 2009). Long-term use of fully covered metal stents in pancreatic duct strictures has been reported, but the migration rates are high (Park et al, 2008).
Technique of Stent Placement
After diagnosing the type and site of the obstruction with a diagnostic ERCP, a small sphincterotomy is often performed to facilitate insertion of instruments. The stricture is negotiated with a guidewire; use of short guidewires may decrease the duration of the procedure and time spent on wire exchanges (Reddy & Draganov, 2009). The technical success of stent placement has been enhanced by the use of hydrophilic wires, which allow easier and less traumatic passage through strictures. In addition, various maneuvers may facilitate the negotiation of a tight stricture: The angle of access to the stricture can be changed by adjusting the position of the guide catheter tip, and an angulated guidewire tip may be helpful for negotiating eccentric strictures. For concentric strictures, an inflated balloon catheter inserted over the guidewire centers the guidewire in the lumen. When the stricture is passed with a wire, a dilating catheter is coaxially inserted to dilate the stricture and obtain a complete cholangiogram. Cytology brushings can be obtained at this point to confirm malignant obstruction. A stent (plastic or expandable metal) is inserted using the Seldinger technique. Decompression of the obstructed biliary tree is typically indicated by a gush of dark stagnant bile into the duodenum.
Hilar Strictures
Hilar strictures are often difficult to stent, because they are further away from the endoscope and tend to be tortuous and sclerotic (see Chapters 28 and 50D). For obstructing hilar cholangiocarcinoma (Klatskin tumors), the success rate of transpapillary stenting varies according to the level of obstruction. For type I strictures (involvement of the common hepatic duct), the success rate is around 86%, but it decreases to 45% for type II strictures (involvement of both main hepatic ducts) and 15% for type III strictures (involvement of secondary intrahepatic branches). Controversy exists as to whether both lobes of the liver need to be drained when a bifurcation lesion obstructs both lobes. Although some studies suggest that unilateral stenting is associated with a high frequency of jaundice resolution, two subsequent studies showed bilateral drainage to be associated with a significantly lower complication rate (Naitoh et al, 2009). Both lobes should be drained in type II and III obstruction if contrast medium fills both hepatic ductal systems and fails to drain. Endoscopic transpapillary stenting may need to be complemented by percutaneous transhepatic stenting when drainage is not complete and jaundice fails to resolve adequately (see Chapters 28 and 50D; Paik et al, 2009).
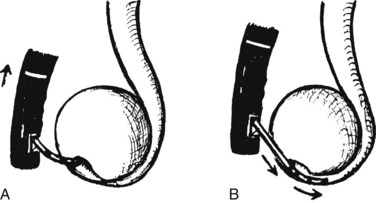
Dual-stent or multiple-stent insertion for hilar strictures requires a clear outline of the ductal anatomy on cholangiography. The anatomy is best appreciated with the patient in the supine position, because the right and left ductal branches are separated. A wire is advanced into each duct for which stent drainage is planned. A super-stiff 0.038-inch Amplatz guidewire (Boston Scientific, Natick, MA) assists stenting of a tight, angulated stricture. After wire placement, the strictures are dilated with a balloon to facilitate subsequent stent insertion; a 6 mm balloon is generally used. If a balloon catheter cannot be negotiated across a tight stricture, a 7-Fr Soehendra Stent Retriever can be used to core a path through the stricture (van Someren et al, 1996). The left hepatic duct should be stented first, because the left duct has a more acute angle of takeoff from the common hepatic duct (Fig. 27.24).
For malignant hilar strictures, self-expandable metal stents have numerous advantages over plastic stents. Drainage is superior because of the larger diameter and open mesh design that provides drainage of side branches. A sphincterotomy is not required when the distal (bottom) ends of metal stents are positioned within the duct, which may reduce the risk of poststenting cholangititis. Many authors disagree with endoscopic approaches to hilar strictures and advocate intubation for obstruction at this level by percutaneous methods (see Chapter 52; Paik et al, 2009).
Chronic Pancreatitis (See Chapter 55A, Chapter 55B )
Focal strictures of the main pancreatic duct may be stented to improve drainage and provide pain relief (Ponchon et al, 1995). As a rule, large diameter stents should be left in place, until the stricture either resolves or is significantly improved, but only in the face of well-established chronic pancreatitis. Persistent stenting has been used to achieve an adequate dilation effect with normalization of ductal caliber (Fig. 27.25). If the stricture is persistent or recurs, multiple stents can be placed to achieve greater dilation. Despite symptomatic improvement, the use of intraductal stents for prolonged periods is associated with a significant risk of duct damage (Somogyi & Forsmark, 1998). Migrated pancreatic duct stents can be managed with endoscopic balloon retrieval (Price et al, 2009).
Stent Clogging
Clogging of plastic stents is a common problem that necessitates a stent exchange. The mean duration of patency of plastic biliary stents is approximately 5 months. Exploring ways to prolong stent patency has been the subject of considerable research over the years and remains unresolved. Expandable metal stents can be inserted through the duodenoscope in a compressed state and can expand to a diameter threefold that of plastic stents and are less prone to clogging (Weber et al, 2009b). Small diameter metal stents occlude more rapidly than large diameter stents (Loew et al, 2009). Conformable stents do not offer any better patency rate than nonconformable metal stents (Yang et al, 2009); and despite a need for fewer procedures with the use of metal stents, cost savings may not be significant (Yoon et al, 2009). Uncovered metal stents are not removable, which may present a problem if they become occluded, or if surgery is considered at a later time, and expandable stents should be reserved for unresectable malignant strictures. Covered metal stents may be removed, although with a significant complication rate (Mahajan et al, 2009).
Pancreatic stents occlude over time, but only a few patients become symptomatic (Buscaglia et al, 2009). The low incidence of pain or pancreatitis despite stent occlusion suggests that the stent functions as a wick around which pancreatic fluid continues to drain. In contrast to the bile duct, infection of the pancreatic duct (ductitis) is rarely seen as a complication of stent clogging.
Replacement of a clogged plastic stent can be performed by either removing the clogged stent with a snare or forceps and inserting a new stent or by exchanging the stent over a guidewire using a stent retriever (Fig. 27.26; Chaurasia et al, 1999). Foreign body forceps are used for biliary stents impacted in the wall of the duodenum. The metal spiral stent retriever is used when guidewire access to the bile duct is necessary.
Endoprosthesis for Stone Impaction
Failure to clear the duct of stones after sphincterotomy and ductal instrumentation places the patient at substantial risk for ductal infection (cholangitis, pancreatitis) and sepsis. Stent placement provides persistent drainage despite the presence of bile duct stones (Sharma et al, 2009). The placement of a 7- or 10-Fr endoprosthesis may be used as a temporizing measure to maintain drainage and prevent stone impaction pending more definitive management (Sharma et al, 2009). Stent placement also may be preferable to attempting stone extraction in a septic patient; clearance of the duct can be performed when the sepsis has resolved, and the patient’s condition has stabilized (Chopra et al, 1996). Stents should not be left in place as definitive treatment or over an extended time, because late complications, mainly cholangitis, have been reported. Surgical treatment of the problem must be considered when endoscopic techniques fail (see Chapters 29, 34, and 35).
Photodynamic Therapy
Photodynamic therapy has been used in conjunction with biliary stenting to improve biliary drainage in patients with nonresectable cholangiocarcinoma. A photosensitizing cytotoxic agent is administered intravenously that is selectively taken up by tumor cells. Subsequent exposure to laser light of the proper wavelength activates the photosensitizing agent and results in tumor destruction. Application of laser light is performed with thin laser fibers. A recent randomized trial found that stenting plus photodynamic therapy was superior to stenting alone in prolonging survival in patients with advanced cholangiocarcinoma (Kahaleh et al, 2008). Laser light therapy can be delivered through a metal stent (Wang et al, 2008).
Sphincter of Oddi Manometry (See Chapter 12)
Sphincter of Oddi manometry is used in some centers to diagnose sphincter of Oddi dysfunction (SOD), a poorly understood entity associated with recurrent abdominal pain and at times with abnormal liver function tests and serum amylase values. The technique of sphincter of Oddi manometry has become fairly standardized, using special endoscopic manometry catheters introduced into the bile or pancreatic duct (Fig. 27.27). Solid-state catheters provide results nearly identical to water-perfused catheters (Draganov et al, 2009); the bile and pancreatic sphincter pressures are measured separately. Sphincterotomy has been shown to result in symptomatic improvement of biliary pain in patients documented to have SOD and abnormal liver function tests (Elmi & Silverman, 2009). High-risk patients should have a prophylactic pancreatic stent placement and needle-knife fistulotomy (Madacsy et al, 2009). Small diameter pancreatic duct stents, as small as 3 Fr, are safer and have a higher rate of spontaneous passage (Rashad et al, 2004). Unsuccessful pancreatic stent placements are associated with a high rate of pancreatitis (Freeman et al, 2004). Pancreatic sphincterotomy, often in conjunction with biliary sphincterotomy, is performed for patients with recurrent pancreatitis using a wire-guided papillotome (Joo et al, 2009).
Ampullectomy
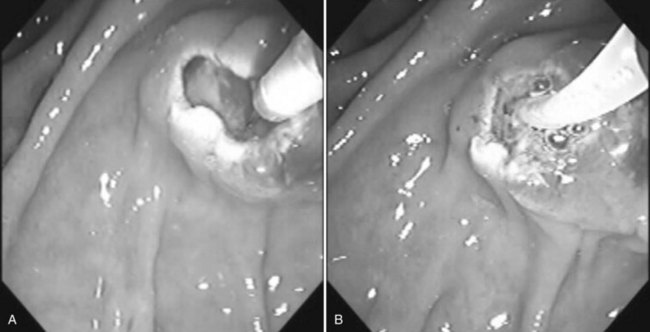
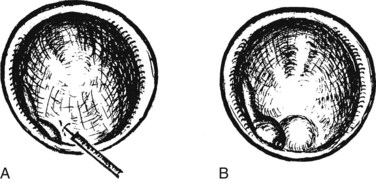
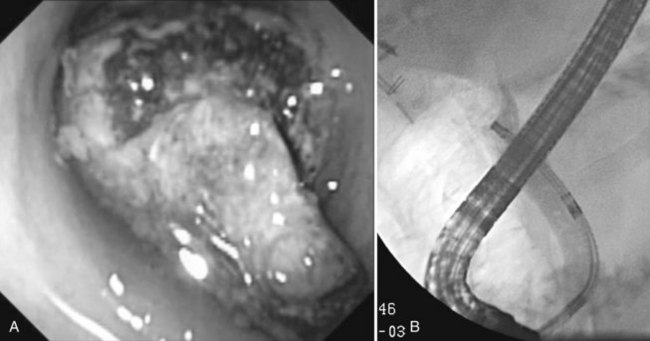
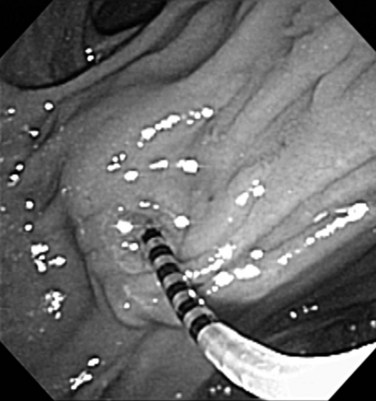
Benign adenomas of the papilla of Vater can be removed together with the papilla of Vater using a diathermic snare in an analogous manner to colon polypectomy (snare ampullectomy). Endoscopic ultrasonography may aid in assessing these tumors and for selecting patients suited to this approach (see Chapter 14) The technique entails snaring of the papilla flush with the duodenal wall (Fig. 27.28; Menees et al, 2009). Saline does not need to be injected prior to the ampullectomy. This is an alternative to local surgical resection, if complete excision of the adenoma and ampulla is achieved; however, it is crucial that the excised tissue specimen be retrieved and submitted for complete histologic evaluation. Surgical resection is indicated if the specimen shows malignancy or complete excision cannot be achieved endoscopically (see Chapter 59). Prophylactic pancreatic stent placement is used to prevent pancreatitis (Yamao et al, 2009; Harewood et al, 2005). Periodic endoscopic surveillance is mandatory to evaluate for adenoma recurrence.
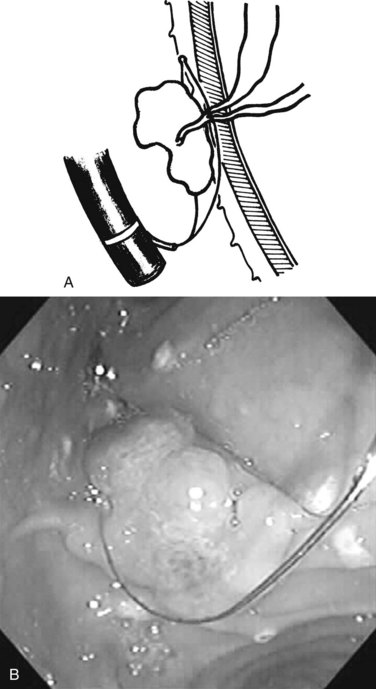
FIGURE 27.28 Schematic (A) and endoscopic (B) views showing the technique of snare ampullectomy of a benign ampullary adenoma.
Pseudocysts and Leaks
To determine whether a cyst communicates with the pancreatic duct, ERCP may be indicated in patients with pseudocysts that fail to resolve or those that recur (Lakhtakia & Reddy, 2009). Outflow obstruction secondary to a stricture, stone, or ampullary stenosis should be treated with sphincterotomy and/or stenting (Habashi & Draganov, 2009). Stenting also has been found to be effective in the management of partial and complete ductal leaks, fistulas, and ascites (Fig. 27.29; Kozarek et al, 1997). Endoscopic approaches to internal drainage of nonneoplastic pancreatic cysts are described in Chapter 14.
Aksoz K, et al. Endoscopic sphincterotomy alone in the management of low-grade biliary leaks due to cholecystectomy. Dig Endosc. 2009;21:158-161.
Amplatz S, et al. Extracorporeal shock wave lithotripsy for clearance of refractory bile duct stones. Dig Liver Dis. 2007;39:267-272.
Arhan M, et al. Migration of biliary plastic stents: experience of a tertiary center. Surg Endosc. 2009;23:769-775.
Attasaranya S, et al. Large-diameter biliary orifice balloon dilation to aid in endoscopic bile duct stone removal: a multicenter series. Gastrointest Endosc. 2008;67:1046-1052.
Bark K, et al. Operative choledochoscopic laser lithotripsy for impacted intrahepatic gallstones: a novel surgical approach. Surg Endosc. 2009;23:221-224.
Bataille L, Deprez P. A new application for therapeutic EUS: main pancreatic duct drainage with a “pancreatic rendezvous technique.”. Gastrointest Endosc. 2002;55:740-743.
Borak GD, et al. Long-term clinical outcomes after endoscopic minor papilla therapy in symptomatic patients with pancreas divisum. Pancreas. 2009;38:903-906.
Brand B, et al. Prospective evaluation of morphology, function, and quality of life after extracorporeal shockwave lithotripsy and endoscopic treatment of chronic calcific pancreatitis. Am J Gastroenterol. 2000;95:3428-3438.
Brugge WR, Van Dam J. Pancreatic and biliary endoscopy. N Engl J Med. 1999;341:1808-1816.
Buscaglia JM, et al. Are large side holes associated with reduced rates of pancreatic stent occlusion? Results of a prospective study. JOP. 2009;10:496-500.
Cahen DL, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676-684.
Calvo MM, et al. The rendezvous technique for the treatment of choledocholithiasis. Gastrointest Endosc. 2001;54:511-5113.
Carr-Locke DL. Biliary access during endoscopic retrograde cholangiopancreatography. Can J Gastroenterol. 2004;18:251-254.
Chahal P, et al. Short 5 Fr vs long 3 Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2009;7:834-839.
Chaurasia OP, et al. Endoscopic techniques for retrieval of proximally migrated biliary stents: the Amsterdam experience. Gastrointest Endosc. 1999;50:780-785.
Chopra KB, et al. Randomised study of endoscopic biliary endoprosthesis versus duct clearance for bile duct stones in high-risk patients. Lancet. 1996;348:791-793.
Coelho-Prabhu N, Baron TH. Assessment of need for repeat ERCP during biliary stent removal after clinical resolution of postcholecystectomy bile leak. Am J Gastroenterol. 2010;105:100-105.
Costamagna G, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-259.
Dolay K, Soylu A. Easy sphincterotomy in patients with Billroth II gastrectomy: a new technique. Turk J Gastroenterol. 2008;19:109-113.
Draganov P, Devonshire DA, Cunningham JT. A new technique to assist in difficult bile duct cannulation at the time of endoscopic retrograde cholangiopancreatography. JSLS. 2005;9:218-221.
Draganov PV, Kowalczyk L, Forsmark CE. Prospective trial comparing solid-state catheter and water-perfusion triple-lumen catheter for sphincter of Oddi manometry done at the time of ERCP. Gastrointest Endosc. 2009;70:92-95.
Elmi F, Silverman WB. Biliary sphincter of Oddi dysfunction type I versus occult biliary microlithiasis in post-cholecystectomy patients: are they both part of the same clinical entity? Dig Dis Sci. 2010;55:842-846.
Elta GH, et al. Pure cut electrocautery current for sphincterotomy causes less post-procedure pancreatitis than blended current. Gastrointest Endosc. 1998;47:149-153.
Freeman ML, Cass OW, Dailey J. Dilation of high-grade pancreatic and biliary ductal strictures with small-caliber angioplasty balloons. Gastrointest Endosc. 2001;54:89-92.
Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointest Endosc. 2004;59:8-14.
Fujita N, et al. ERCP for intradiverticular papilla: two-devices-in-one-channel method. Gastrointest Endosc. 1998;48:517-520.
Fukumori D, et al. An endoscopic sphincterotomy of the minor papilla in the management of symptomatic pancreas divisum. Hepatogastroenterology. 2007;54:561-563.
Garcia-Cano J, et al. Biliary sphincterotomy dilation for the extraction of difficult common bile duct stones. Rev Esp Enferm Dig. 2009;101:541-545.
Gorelick A, et al. First cut, then blend: an electrocautery technique affecting bleeding at sphincterotomy. Endoscopy. 2001;33:976-980.
Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol. 2009;15:38-47.
Han J, et al. Effect of biliary stenting combined with ursodeoxycholic acid and terpene treatment on retained common bile duct stones in elderly patients: a multicenter study. Am J Gastroenterol. 2009;104:2418-2421.
Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367-370.
Higashizawa T, et al. Biliary guidewire facilitates bile duct biopsy and endoscopic drainage. J Gastroenterol Hepatol. 2002;17:332-336.
Itoi T, et al. A novel technique for endoscopic sphincterotomy when using a percutaneous transhepatic cholangioscope in patients with an endoscopically inaccessible papilla. Gastrointest Endosc. 2004;59:708-711.
Jain SK, et al. Pigtail stents: an alternative in the treatment of difficult bile duct stones. Gastrointest Endosc. 2000;52:490-493.
Joo YW, et al. Endoscopic pancreatic sphincterotomy: indications and complications. Korean J Intern Med. 2009;24:190-195.
Kahaleh M, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290-297.
Kahl S, et al. Risk factors for failure of endoscopic stenting of biliary strictures in chronic pancreatitis: a prospective follow-up study. Am J Gastroenterol. 2003;98:2448-2453.
Kim GH, et al. Endoscopic removal of bile duct stones by using a rotatable papillotome and a large-balloon dilator in patients with a Billroth II gastrectomy (with video). Gastrointest Endosc. 2008;67:1134-1138.
Kowalski T, et al. Post-sphincterotomy transampullary balloon dilation is a safe and effective technique. Dig Dis Sci. 2009;54:670-674.
Kozarek RA, et al. Transpapillary stenting for pancreaticocutaneous fistulas. J Gastrointest Surg. 1997;1:357-361.
Kulaksiz H, et al. Is stenting necessary after balloon dilation of post-transplantation biliary strictures? Results of a prospective comparative study. Endoscopy. 2008;40:746-751.
Kwan V, et al. Minor papilla sphincterotomy for pancreatitis due to pancreas divisum. A N Z J Surg. 2008;78:257-261.
Lakhtakia S, Reddy DN. Pancreatic leaks: endo-therapy first? J Gastroenterol Hepatol. 2009;24:1158-1160.
Lawrence C, et al. Post-ERCP pancreatitis rates do not differ between needle-knife and pull-type pancreatic sphincterotomy techniques: a multiendoscopist 13-year experience. Gastrointest Endosc. 2009;69:1271-1275.
Lee TH, et al. Can wire-guided cannulation prevent post-ERCP pancreatitis? A prospective randomized trial. Gastrointest Endosc. 2009;69:444-449.
Loew BJ, et al. Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc. 2009;70:445-453.
Macintosh DG, Love J, Abraham NS. Endoscopic sphincterotomy by using pure-cut electrosurgical current and the risk of post-ERCP pancreatitis: a prospective randomized trial. Gastrointest Endosc. 2004;60:551-556.
MacMathuna P, Lennon J, Crowe J. Balloon sphincteroplasty vs endoscopic papillotomy for bile duct stones. Lancet. 1994;343:486.
Madacsy L, et al. Prophylactic pancreas stenting followed by needle-knife fistulotomy in patients with sphincter of Oddi dysfunction and difficult cannulation: new method to prevent post-ERCP pancreatitis. Dig Endosc. 2009;21:8-13.
Mahajan A, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc. 2009;70:303-309.
Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: report of 6 cases. Gastrointest Endosc. 2004;59:100-107.
Maple JT, et al. Wire-assisted access sphincterotomy of the minor papilla. Gastrointest Endosc. 2009;69:47-54.
Maydeo A, Bhandari S, Bapat M. Endoscopic balloon sphincteroplasty for extraction of large radiolucent pancreatic duct stones (with videos). Gastrointest Endosc. 2009;70:798-802.
Menees SB, et al. A survey of ampullectomy practices. World J Gastroenterol. 2009;15:3486-3492.
Merrett MN, Desmond PV. Extracorporeal shock wave lithotripsy for bile duct stones: an Australian experience. J Gastroenterol Hepatol. 1990;5:537-541.
Misra SP. Pre-cut sphincterotomy: does the timing matter? Gastrointest Endosc. 2009;69:480-483.
Naitoh I, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552-557.
Nakahara K, et al. Therapeutic endoscopic retrograde cholangiopancreatography using an anterior oblique-viewing endoscope for bile duct stones in patients with prior Billroth II gastrectomy. J Gastroenterol. 2009;44:212-217.
Paik WH, et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62.
Panteris V, et al. Influence of juxtapapillary diverticula on the success or difficulty of cannulation and complication rate. Gastrointest Endosc. 2008;68:903-910.
Park H, et al. Feasibility and safety of placement of a newly designed, fully covered self-expandable metal stent for refractory benign pancreatic ductal strictures: a pilot study (with video). Gastrointest Endosc. 2008;68:1182-1189.
Pereira-Lima JC, Rynkowski CB, Rhoden EL. Endoscopic treatment of choledocholithiasis in the era of laparoscopic cholecystectomy: prospective analysis of 386 patients. Hepatogastroenterology. 2001;48:1271-1274.
Ponchon T, et al. Endoscopic stenting for pain relief in chronic pancreatitis: results of a standardized protocol. Gastrointest Endosc. 1995;42:452-456.
Price LH, et al. Good stents gone bad: endoscopic treatment of proximally migrated pancreatic duct stents. Gastrointest Endosc. 2009;70:174-179.
Rashdan A, et al. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin Gastroenterol Hepatol. 2004;2:322-329.
Reddy SC, Draganov PV. ERCP wire systems: the long and the short of it. World J Gastroenterol. 2009;15:55-60.
Sakai Y, et al. Long-term prognosis of patients with endoscopically treated postoperative bile duct stricture and bile duct stricture due to chronic pancreatitis. J Gastroenterol Hepatol. 2009;24:1191-1197.
Sharma BC, et al. Endoscopic biliary drainage by 7 Fr or 10 Fr stent placement in patients with acute cholangitis. Dig Dis Sci. 2009;54:1355-1359.
Somogyi L, Forsmark CE. Pancreatic duct stenting in benign pancreatic disease. Semin Gastrointest Dis. 1998;9:73-79.
Swahn F, et al. Ten years of Swedish experience with intraductal electrohydraulic lithotripsy and laser lithotripsy for the treatment of difficult bile duct stones: an effective and safe option for octogenarians. Surg Endosc. 2010;24:1011-1016.
Tandan M, et al. Extracorporeal shock wave lithotripsy of large difficult common bile duct stones: efficacy and analysis of factors that favor stone fragmentation. J Gastroenterol Hepatol. 2009;24:1370-1374.
Tandon RK, Nijhawan S, Arora A. Management of retained common bile duct stones in patients with T-tube in situ: role of endoscopic sphincterotomy. Am J Gastroenterol. 1990;85:1126-1131.
van Someren RN, et al. A novel technique for dilating difficult malignant biliary strictures during therapeutic ERCP. Gastrointest Endosc. 1996;43:495-498.
Veldkamp MC, et al. Iatrogenic ampullary stenosis: history, endoscopic management, and outcome in a series of 49 patients. Gastrointest Endosc. 2007;66:708-716.
Wang LW, et al. Self-expandable metal stents and trans-stent light delivery: are metal stents and photodynamic therapy compatible? Lasers Surg Med. 2008;40:651-659.
Weber A, et al. Long-term outcome of endoscopic therapy in patients with bile duct injury after cholecystectomy. J Gastroenterol Hepatol. 2009;24:762-769.
Weber A, et al. Self-expanding metal stents versus polyethylene stents for palliative treatment in patients with advanced pancreatic cancer. Pancreas. 2009;38:e7-e12.
Yamao T, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119-124.
Yang KY, et al. A comparison of the Niti-D biliary uncovered stent and the uncovered Wallstent in malignant biliary obstruction. Gastrointest Endosc. 2009;70:45-51.
Yoon WJ, et al. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effectiveness in a country with a low ERCP cost. Gastrointest Endosc. 2009;70:284-289.