Chapter 11 Infections in liver, biliary, and pancreatic surgery
Infection
Infection is defined as a pathologic state resulting from invasion of the body by pathogenic microorganisms—bacteria, yeast, parasites, or viruses. These agents can challenge host defense mechanisms because of either an infectious organism’s virulence or a weakness in host defenses. In most instances, infection results when normal host defense mechanisms fail to prevent tissue penetration and the subsequent local propagation and/or systemic dissemination of the injurious agent. All infections can be complicated by sepsis, which is the invasion of a micoorganism or its toxins into the bloodstream, coupled with the host response to that invasion. More specifically, sepsis is a systemic inflammatory response syndrome caused by infection (American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee, 1992). When sepsis remains untreated, altered host inflammatory and coagulation cascades—intracellular homeostasis, cellular hypoxia, endothelial dysfunction, apoptosis, and eventually cardiovascular collapse—lead to multiorgan failure and death. Despite recent advances in treatment (Rivers et al, 2001), the incidence of sepsis and septic shock has been increasing (Angus et al, 2001). Furthermore, the associated mortality rate remains at 30% among all septic patients, with rates increasing to 85% in the setting of multiple organ failure (Angus et al, 2001).
Patients undergoing hepatopancreatobiliary surgery are at particular risk for infection and sepsis because of the severity of their disease process, often complicated by comorbid medical conditions and exacerbated by the stress of the surgical treatment itself. Hepatopancreatobiliary diseases that place patients at particular risk include infected necrotizing pancreatitis and sepsis associated with uncontrolled leakage from pancreatic–enteric anastamosis following pancreatoduodenectomy (Howard et al, 2007; Adams, 2009). This chapter reviews the applicable host defense mechanisms as well as their alteration by hepatopancreatobiliary diseases and operations.
Host Defenses
The host defense mechanisms preventing the entry of infectious organisms into the liver, biliary tract, and pancreas can be organized into three broad categories (Krige & Bornman, 2000): physical, chemical, and immunologic (Table 11.1). Although these mechanisms are distinct, significant overlap occurs. Furthermore, optimal defense often requires coordination of all three systems.
Table 11.1 Hepatobiliary and Pancreatic Host Defense Mechanisms
| Hepatobiliary | Pancreatic | |
|---|---|---|
| Physical | Biliary sphincter | Pancreatic sphincter |
| Hepatic tight junctions | Pancreatic tight junctions | |
| Bile flow | Pancreatic juice flow | |
| Mucus | Mucus | |
| Cilia | Cilia | |
| Chemical | Bile salts | Pancreatic fluid |
| Immunologic | Kupffer cells | — |
| Immunoglobulin A | Immunoglobulin A | |
| Fibronectin | — | |
| Complement | Complement | |
| CD40 receptor | CD40 receptor | |
| Blood supply | Blood supply |
Infectious microorganisms can enter the liver either hematogenously or retrograde via the biliary ductal system. The most common hematogenous route of infectious entry is the portal venous system. Under normal circumstances, the portal system drains all abdominal gastrointestinal venous blood directly into the liver before it enters the systemic circulation. As a result, portal venous blood typically contains enteric pathogens and toxins, which are cleared and neutralized by the liver (see Chapter 9). This activity is primarily regulated by Kupffer cells, which act as the resident hepatic macrophages. Consequently, Kupffer cells are not only important determinants of normal hepatic physiology and hemostasis but also of any inflammatory response. The liver therefore acts as the ultimate barrier to infection for all gastrointestinal tract and peritoneal cavity microorganisms attempting to access the systemic circulation. Perturbation of this flow, in particular portal venous obstruction, may occur from causes that are presinusoidal (portal vein thrombosis, surgery, trauma, cancer), sinusoidal (hepatitis, cirrhosis), or postsinusoidal (Budd-Chiari syndrome, right heart failure). Such obstructions often lead to portal hypertension with subsequent portosystemic shunting (see Chapter 70A). This new access to the systemic circulation allows a bypass of the liver’s defenses against enteric pathogens and toxins, thereby increasing the risk of bacteremia and sepsis. Although less common, direct hematogenous spread of microorganisms to the liver may also occur via the hepatic arterial system (e.g., bacterial endocarditis, intravenous drug use).
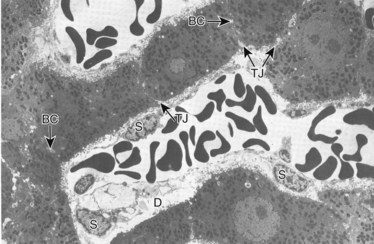
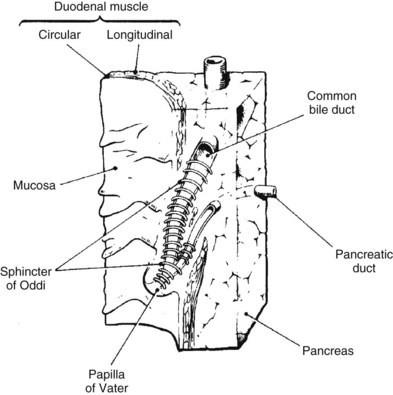
Under normal circumstances, an intact sphincter of Oddi provides an effective mechanical barrier that prevents bacteria and other enteric organisms from entering the liver (retrograde) via the biliary system (see Chapter 1B). Ascending infection from the intestine can access the biliary tree when the sphincter mechanism is destroyed, endoscopically or surgically; bypassed, as in a biliary-enteric anastomosis; or violated, such as via a transsphincteric stent. The biliary system can also be directly contaminated by percutaneous access via either the transhepatic route or with operatively placed drains (e.g., T-tubes). Fortunately, the presence of bacteria within bile does not uniformly result in an ascending biliary infection, because the main physical barrier to biliary sepsis remains: intrahepatic tight junctions (Fig. 11.1), which form a barrier between bile canaliculi and hepatic sinusoids and act to keep bile separated from the bloodstream. Under pathologic conditions, mixing of these two compartments can result in seeding of biliary organisms into the hepatic blood supply. Another physical hepatic defense mechanism is the flow of bile itself. Bile is produced by hepatocytes and excreted into bile canaliculi, which then coalesce to form larger ductules, major sectoral ducts, and ultimately extrahepatic biliary ducts. Bile flow out of the liver helps prevent ascending infection by its mechanical flushing action; however, interruption of flow via obstruction or stasis reduces this protective mechanism (see Chapter 43).
As previously discussed, the main physical host defense against the reflux of enteric contents, and adherent bacteria, into the biliary system is the biliary sphincter within the sphincter of Oddi (Fig. 11.2; see Chapter 1A, Chapter 1B ). Similar to the liver, bile flow also assists the biliary system by flushing and helping prevent free reflux of duodenal contents into the extrahepatic ducts. The bile duct epithelium within the ductules has a single cilium, which facilitates propulsion of bile toward the extrahepatic biliary ducts (Gilroy et al, 1995; Itoshima et al, 1977). Bile flow is further propelled toward the duodenum by gallbladder contraction. A final physical barrier within the biliary tract is mucus produced by the epithelial cells throughout the extrahepatic bile ducts. Mucus promotes distal flow by preventing adherence of bacteria and debris to the epithelial cell membrane (see Chapter 7).
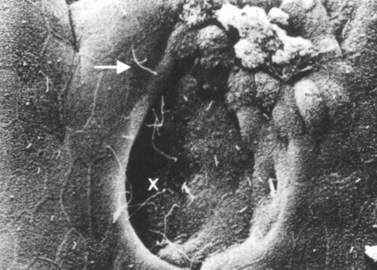
Similar to the biliary system, the main physical host defense mechanism for the pancreas is the pancreatic sphincter within the sphincter of Oddi. Although a true anatomic pancreatic sphincter is present in only one third of patients, all normal glands have a pressure zone at the end of the pancreatic duct that relaxes in response to secretin (Carr-Locke et al, 1985). This pressure zone prevents reflux of enteric contents into the pancreatic ductal system. Similar to the liver, the pancreas also contains paracellular seals or tight junctions between acinar and intralobular ductal epithelial cells. The integrity of the pancreatic tight junctions can be compromised, however, in experimental states of inflammation or pancreatitis, and possibly when ductal pressures are elevated (Fallon et al, 1995; Schmitt et al, 2004). The pancreatic ductal system is also aided by the flow of pancreatic duct fluid (flushing) to prevent reflux of duodenal contents. To facilitate this flow, the epithelium in the secondary ducts has a single cilium (Fig. 11.3) that propels secretion into the main pancreatic ductal system. Similar to the biliary system, the pancreatic ductal epithelium also secretes mucus, which acts as a barrier to the adherence of bacteria and debris (Moniaux et al, 2004; Trede & Carter, 1997).
Chemical host defense mechanisms also exist in the hepatic and pancreatobiliary systems. Bile salts within the biliary ducts have been shown to have bacteriostatic and bactericidal properties (Stewart et al, 1986; Sung et al, 1993). The maintenance of intestinal flora by bile salts is also crucial to host defenses, because bile salts have the ability to suppress pathogenic bacteria that threaten the integrity of the mucosal barrier, and they promote translocation of bacteria into the portal circulation (Bradfield, 1974; Clements et al, 1993; Deitch et al, 1990). Conditions of either bile salt loss or administration of prolonged courses of antibiotics may interfere with the balance of the intestinal flora and weaken this important host defense. Bile salts have also been shown to have antifungal effects, particularly against Candida albicans (Marshall et al, 1987). Furthermore, their trophic impact on epithelial cells increases both their integrity and ability to withstand insult from infectious pathogens (Stewart et al, 1986). Finally, bile salts posses an antiendotoxin effect (Stewart et al, 1986).
Pancreatic ductal fluid also contains inherent antibacterial properties (Rubinstein et al, 1985) independent of its enzymatic activity. Data suggest this fluid is bactericidal against multiple bacteria, including Escherichia coli, Shigella and Salmonella species, and Klebsiella pneumoniae (Kruszewska et al, 2004). Pancreatic fluid is also bacteriostatic against Staphylococcus aureus, S. epidermidis, and Pseudomonas aeruginosa; however, Bacteroides fragilis and Streptococcus faecalis are resistant to effects of pancreatic ductal fluid (Rubinstein et al, 1985). According to studies in swine, the antibacterial activity of pancreatic fluid appears to be independent of digestive enzymes. It is highest before eating and lowest during digestion of food, suggesting regulation through alternative signaling pathways (Holowachuk et al, 2004). By virtue of its antibacterial properties, pancreatic ductal fluid also regulates the colonization of enteric bacteria throughout the intestine by allowing the flora to maintain a normal homeostatic balance (Kruszewska et al, 2004).
In addition to its antibacterial effects, pancreatic ductal fluid appears to enhance the inherent action of certain bactericidal antibiotics (e.g., quinolones, trimethoprim, chloramphenicol, tetracycline) in studies comparing microbiologic media with those containing pancreatic ductal fluid (Mett et al, 1984). This enhanced activity has been shown toward many species of bacteria, including E. coli, Salmonella, Serratia, Proteus, and Staphylococcus. An unidentified, low-molecular-weight factor (independent of complement or lysozyme) in the pancreatic ductal fluid has been implicated (Mett et al, 1984). Although this factor is unknown, reproducible data show enhanced antibacterial activity in combination with commonly employed antibiotics, such as mezlocillin, ceftriaxone, gentamicin, ciprofloxacin, ofloxacin, and imipenem (Minelli et al, 1996). The relative degree of enhanced activity from pancreatic juice also depends on the specific bacteria (Minelli et al, 1996). Pancreatic ductal fluid contains fungistatic properties, in particular toward C. albicans (Kruszewska et al, 2004; Rubinstein et al, 1985), as well as growth factors (e.g., epidermal growth factor) that have a trophic effect on pancreatic epithelial cells. This helps preserve their integrity to pathogens (Alvarez et al, 1997). It is unknown whether pancreatic ductal fluid offers any antiendotoxin effects.
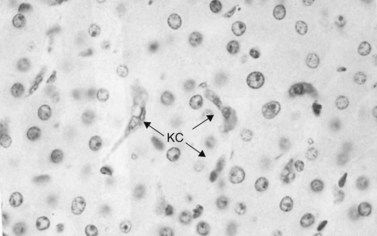
Immunologic host defense mechanisms also exist in the hepatic and pancreatobiliary systems. Hepatic cellular defense is provided by a large phagocyte population termed Kupffer cells (Fig. 11.4) as well as a plethora of other immunologically active cells (see Chapters 9 and 10). Kupffer cells are strategically located in the sinusoids of the liver, where they filter endotoxins from the gut, phagocytose bacteria, regulate sinusoidal vascular responsiveness, process and present antigens to T cells, and secrete cytokines, such as interleukins (ILs), interferons (INFs), and tumor necrosis factor (TNF) (Laskin, 1990). Kupffer cells represent one third of all nonparenchymal liver cells and 85% of all mononuclear phagocytes in the body (Kimmings et al, 1995; Wang et al, 1993). With the exception of circulating mononuclear cells, there is no evidence of a functionally equivalent resident phagocyte population in the pancreas.
Humoral defenses also exist within the hepatic and pancreatobiliary systems. Immunoglobulin (IG) A is secreted into the bile from the gallbladder and intrahepatic/extrahepatic biliary epithelium and plays an important role in barrier protection from infectious pathogens (Emmrich et al, 1998; Scott-Conner & Grogan, 1994) and is also present in pancreatic ductal fluid. The concentration of IgA is inversely related to pancreatic ductal fluid volume (Ohshio et al, 2001). In disease states characterized by exocrine insufficiency (e.g., chronic pancreatitis), a higher concentration of IgA is also observed in the remaining human pancreatic ductal fluid (Emmrich et al, 1998).
Other immunologic molecules in the bile include fibronectin, which opsonizes pathogens and facilitates biliary epithelial defense against bacteria (Wilton et al, 1987). Complement—in particular, C3, C4, and factor B—is also present and functionally active in the bile at levels similar to serum (Sumiyoshi et al, 1997). As expected, clinical conditions of C3 deficiency are associated with increased susceptibility to infection in the biliary system and elsewhere (Homann et al, 1997). Complement is also present in pancreatic ductal fluid (Andoh et al, 1996; Sumiyoshi et al, 1997). Specifically, C3, C4, and factor B are synthesized in the pancreatic ductal epithelium and secreted in response to proinflammatory cytokines IL-1β, TNF-α, and INF-γ (Andoh et al, 1996; Sumiyoshi et al, 1997). These cytokines act as the primary drivers for sepsis (Cohen, 2002).
The biliary and pancreatic epithelium also may have some inherent immunologic ability. Normally, the CD40 receptor is expressed on regenerating or inflamed bile duct epithelium. CD40-CD40 ligand interactions may be responsible for immunity to certain pathogens. CD40 is also expressed on pancreatic ductal epithelial cells and may play a similar role (Vosters et al, 2004). Patients with deficient CD40 ligand (X-linked immunodeficiency with hyper-IgM) show a higher incidence of hepatopancreatobiliary infections, inflammation, and carcinoma (Hayward et al, 1997).
Host Defenses Challenged by Underlying Hepatopancreatobiliary Conditions in Surgical Patients
Liver
Underlying hepatic conditions that may challenge host defenses in a surgical patient include hepatitis, cirrhosis, liver failure, portal hypertension, and hepatocellular toxin–induced dysfunction (see Chapters 64 and 70A). Hepatitis, cirrhosis, and liver failure all represent relative degrees of hepatic dysfunction, insufficiency, and compromise. As hepatic function deteriorates and ultimately leads to liver failure, physical, chemical, and immunologic hepatic host defenses also become compromised. With decreased bile production capacity, the physical defense of flushing bile flow is less efficient. The effect of primary liver cell dysfunction on hepatic tight junction integrity is unknown, but these junctions are altered in biliary obstructive causes of liver dysfunction. Bile salt deficiency associated with liver dysfunction leads to inadequate chemical defenses of bile salt–mediated antibacterial and antiendotoxin effects, as well as epithelial tropism, which results in reduced integrity of the epithelial barrier. The bacteriologic flora of the intestine is also altered, which may increase the quantity and change the type of bacteria that translocate into the portal system (Clements et al, 1993; Deitch et al, 1990). Liver dysfunction also leads to primary and secondary decreases in Kupffer cell function and to depressed synthesis of immunologically active proteins, which impairs cellular and humoral immunologic defense. Finally, cellular immunity may also be affected by the altered number and type of T cells present within the liver in hepatitis and cirrhosis (Deignan et al, 2002).
Portal hypertension is responsible for secondary decreases in Kupffer cell function. Resultant portosystemic shunting in patients with cirrhosis may compromise reticuloendothelial cell function by allowing enteric pathogens to be moved away from Kupffer cells. Portal hypertension therefore contributes to an increased risk of infection in animals with liver dysfunction (Basista et al, 1994). Local blood flow is also substantially altered in portal hypertension and cirrhosis, as intrahepatic sinusoidal resistance is increased. Sinusoidal responsiveness to endogenous vasodilators is depressed, whereas responsiveness to endogenous vasoconstrictors is increased (Birney et al, 2003). Endothelin and nitric oxide are two significant endogenous mediators of stellate cell contractility that modulate the local intrahepatic resistance in portal hypertension (Rockey, 2001).
Biliary System
The most well-studied underlying biliary condition that can interfere with host defenses in a surgical patient is biliary obstruction (see Chapter 7). This condition may occur from causes that are intrinsic (tumors, stones, strictures), extrinsic (pancreatitis, tumors), or functional (sphincter of Oddi dysfunction). The increased pressure within the biliary system as a consequence of biliary obstruction leads to weakened hepatic tight junctions. This effect is even more dramatic when pressure increases rapidly, such as with biliary tract manipulation (Raper et al, 1989). Leaky tight junctions result in bile reflux into the hepatic sinusoids (i.e., cholangiovenous reflux). Other studies suggest that cholangiovenous reflux may occur directly from bile ductules, through the spaces of Mall and Disse (see Fig. 11.1), into the hepatic sinusoids. This pathway measures 1.7 to 10 µm and offers less resistance to bacteria and toxins than higher resistance pathways such as hepatic tight junctions, biliary canaliculi, or hepatocytes (Stewart et al, 1988). During states of biliary obstruction, the reduced flow of bile also diminishes the physical barrier of the flushing action of bile. Obstruction causes decreased bile salt delivery to the intestine, resulting in reduced bile salt–mediated bacteriostatic and bactericidal activity and therefore colonic bacterial overgrowth. This overgrowth disturbs the protective bacterial flora and promotes local inflammation, injury, and subsequent increased rates of translocation into the portal circulation (Clements et al, 1993; Deitch et al, 1990). The increased bacterial load is not adequately cleared in the liver, allowing entry into the systemic circulation (Bradfield, 1974).
In addition to the direct local effect, jaundice associated with biliary obstruction can substantially alter host defenses. It causes depressed Kupffer cell function by reducing clearance capacity. This results in an increased bacterial load, which overwhelms the hepatic sinusoids and increases bacterial entry into the bloodstream (Clements et al, 1993; Ding et al, 1992; Katz et al, 1991; Megison et al, 1991; Pain et al, 1987).
The cause of Kupffer cell dysfunction in biliary obstruction and jaundice is unclear. One plausible theory relates increased bacterial translocation from the ileum and colon into the portal venous system, thereby directly overwhelming Kupffer cell clearance. Other theories include direct effects of bile salts on Kupffer cell membranes (Takiguchi & Koga, 1988), ineffective opsonins (e.g., fibronectin), decreased sinusoidal size and blood flow, and reduced major histocompatibility complex expression (Clements et al, 1994a, 1994b; Ding et al, 1992; Takiguchi & Koga, 1988).
The role of endotoxins on Kupffer cell function is also poorly understood, and animal studies suggest that biliary obstruction and portal vein endotoxinemia may generate oxygen free radicals in the liver that reduce the ability of Kupffer cells to clear toxins (Ogura et al, 1996; Takahashi et al, 1996). The use of oxygen free-radical scavengers, such as coenzyme Q10 or styrene-co-maleic acid superoxide dismutase in animal models, has been shown to interrupt the detrimental effects of endotoxin on Kupffer cell clearance and hepatic immunologic reserve (Ogura et al, 1996; Takahashi et al, 1996). Biliary obstruction results in less IgA production by the liver and lowers bile IgA levels. Endoscopic drainage of patients with biliary obstruction resolves this IgA deficiency in the bile (Sung et al, 1995); however, prolonged biliary obstruction eventually leads to hepatocyte dysfunction and necrosis, impairing liver synthetic function. Many proteins with immunologic importance are produced less efficiently, resulting in an increased susceptibility to infection.
Clinical relevance of the host challenge associated with biliary obstruction is evident in the higher probability of morbidity and mortality in patients with obstructive jaundice who undergo invasive procedures (Blamey et al, 1983; Pitt et al, 1981; Povoski et al, 1999; Schmidt et al, 2004).
Numerous controlled studies have suggested that preoperative external biliary drainage is associated with increased infectious morbidity. This is likely related to bile loss and the risk of infection and inflammation with catheterization of the biliary tree (Hatfield et al, 1982; Hochwald et al, 1999; Kimmings et al, 2000; McPherson et al, 1984; Pitt et al, 1985; Povoski et al, 1999; Smith et al, 1984; Sohn et al, 2000; van der Gaag et al, 2009). Internal stenting offers a theoretic benefit, with avoidance of external catheters and a return of bile to the gastrointestinal tract (eneterohepatic cycle). Unfortunately, multiple retrospective studies have reported mixed results (Trede & Schwall, 1988; Sohn et al, 2000; Schmidt et al, 2004), and four prospective randomized controlled trials provide conflicting data. These trials typically include patients undergoing pancreaticoduodenectomy (periampullary or pancreatic head adenocarcinoma), randomized to either preoperative endoscopic biliary drainage or surgery without drainage (see Chapters 50B, 50C, 62A, 90B, and 90C). Because of varied findings, however, the authors’ conclusions have ranged from recommending routine preoperative endoscopic biliary drainage for patients with biliary obstruction (Lygidakis et al, 1987) to suggesting that although liver function improves with internal stenting, there is no difference in infectious outcomes (Lai et al, 1994). The most recent and methodologically sound trial reports that routine preoperative biliary drainage actually increases the rate of complications related to surgery (van der Gaag et al, 2010). Finally, a Cochrane review, as well as a meta-analysis, concluded no positive effect was seen from preoperative endoscopic biliary stenting in obstructed patients (Saleh et al, 2002; Wang et al, 2008).
Differences in the perioperative risk of pancreatectomy and major partial hepatectomy in jaundiced patients must be recognized. Contrary to the results in pancreas, some evidence shows improved outcome with decompressing the future liver remnant in patients with proximal biliary tumors (see Chapters 50C and 90C).
Theoretically, internal biliary stenting is advantageous over externalized stents, because it avoids the loss of bile and may reduce bacterial contamination. In animal models of obstructive jaundice, biliary decompression via internal stenting promotes Kupffer cell recovery (Clements et al, 1996), as evidenced by increased cell clearance capacity and normalization of endotoxin and anti–core glycolipid antibody concentrations (Greve et al, 1992). It has been suggested that the inconsistent results of clinical trials may be due to inadequate time allowed for liver recovery. The duration of biliary decompression needed to affect these parameters is unknown (van der Gaag et al, 2009). Some animal studies suggest several weeks (Katz et al, 1991; Megison et al, 1991; Parks et al, 1996; Roughneen et al, 1986; Scott-Conner & Grogan, 1994; Thompson et al, 1990), which may not be feasible in the clinical setting for cancer patients. In contrast, studies of limited duration in bile duct ligation models suggest Kupffer cell clearance recovers within days (Ding et al, 1992).
In addition to the effects of biliary obstruction on hepatic tight junctions, bile salt loss, bacterial overgrowth, and Kupffer cell dysfunction as described earlier, it may also be associated with the development of cholangitis. Although bacteria may colonize the biliary tract without producing clinically significant cholangitis, the risk of subsequent infection is directly related to the pressure within the biliary system. More specifically, greater degrees of obstruction lead to higher pressures (Pitt et al, 1990). This increase has a more dramatic effect when pressure increases quickly, as with biliary tract manipulation or common bile duct stone obstruction (Raper et al, 1989). In addition, specific virulence factors may explain how some bacteria species colonize and others cause sepsis. Slime production, for example, is necessary for some bacteria to colonize, but too much prevents cholangiovenous reflux and sepsis (Stewart et al, 2003). Bacteria that create complement are thought to induce an enhanced cholangiovenous reflux from resulting TNF-α production (Stewart et al, 2002). P1 fimbriae, α-galactosyl residues, and the absence of mannose-specific fimbriae also have been cited as distinguishing pathogenic bacteria from colonizing bacteria (Wetter et al, 1994). As a result, the degree and rapidity of obstruction and bacterial virulence play important roles in determining whether biliary pathogens will promote cholangitis.
Pancreas
Patients who present with acute necrotizing pancreatitis are at significant risk of developing a pancreatic infection. Translocation of bacteria and endotoxin is thought to play a major role in the cause of these infectious complications, both before and after operative debridements. This theory is based on experimental (Foitzik et al, 1995; Medich et al, 1993; Widdison et al, 1994) and clinical data (Buchler et al, 1992). Interestingly, the bacteriology of infected pancreatic tissue has changed substantially in recent years from predominantly gut flora to a greater emphasis on gram-positive microorganisms as well as on fungus (Kochhar et al, 2009; Besselink et al, 2009). This is presumed to be a consequence of improved therapies with a resultant increase in the microorganisms (gram-positive and fungal) associated with advanced/prolonged critical care treatments (central line infections). Finally, bacteria may exist within pancreatic pseudocysts, which if drained ineffectively can result in pancreatic abscess that may progress to systemic infection.
Numerous prospective randomized controlled trials have attempted to address the role of prophylactic antibiotics in preventing pancreatic infection during acute pancreatitis. The methodologic heterogeneity in these studies is striking, given their variations in patient numbers (underpowered), entry criteria, antibiotic selection, outcome measures, and lack of appropriate controls. They have also often ignored patients with significant necrosis, who are at greatest risk for infection. Although older trials employing prophylactic antibiotics (Foitzik et al, 1995; Luiten et al, 1995; Pederzoli et al, 1993; Sainio et al, 1995) reported reductions in the incidence of infectious complications, subsequent operations, and mortality, two larger contemporary trials found no significant benefit to the early use of prophylactic antibiotics in patients with severe acute pancreatitis (Dillinger et al, 2007; Isenmann et al, 2004). These data highlight the impact of proinflammatory mediators (cytokines) in inducing a systemic inflammatory response syndrome during the early phase (onset to 2 weeks) of pancreatitis and therefore a shift in philosophy from previous reviews and meta-analyses (Bassi et al, 2003; Nathens et al, 2008). As a result, currently no compelling evidence supports the use of early prophylactic antibiotics in these patients (Howard, 2007).
Host Defenses Challenged by Hepatopancreatobiliary Surgery
Liver
Surgery.
The main surgical procedures of the liver involve resections of various extents in patients with variable degrees of hepatic reserve. Liver resection may be accompanied by biliary–enteric reconstructive procedures, such as with proximal cholangiocarcinomas (see Chapter 50B). Various hepatic ablative techniques are also being increasingly utilized, such as radiofrequency ablation, cryoablation, and microwave ablation (see Chapter 85A, Chapter 85B, Chapter 85C, Chapter 85D ).
Host Defense.
The main effect of liver resection on host defense is the removal of functional liver mass, which results in hepatocyte loss, disruption of the biliary drainage system, and reduced portal venous drainage (increased portal pressure, reduced portal outflow). Loss of functional liver mass has a direct and proportional effect on the capacity of the reticuloendothelial system to respond to infection. Bacterial and endotoxin clearance is also suppressed after hepatectomy. Normally, the existing reserve of a healthy liver can accommodate this loss, however, a patient with impaired liver function may not be able to compensate. In recent years, the role of preoperative portal vein embolization (PVE) of the hepatic lobe to be resected has been increasing (see Chapter 93A, Chapter 93B ). This allows preoperative hypertrophy of the remaining lobe, which can be beneficial in patients with limited hepatic reserve.
Despite the overall loss of Kupffer cell quantity, function of the remaining liver is not depressed (Andersson & Foss, 1991; Gross et al, 1985; Vo & Chi, 1988). Loss of functional liver mass also results in less overall production of bile and bile salts with their trophic (Borghi et al, 1991), antibacterial, antiendotoxin, and immunologic effects (Cahill et al, 1987; Dahlgren et al, 1986). Loss of reticuloendothelial system capacity, portal venous drainage, and protective effects of bile salts presents significant challenges to host defenses. Preexisting liver dysfunction and postoperative liver failure further exacerbate these challenges.
Although ablative procedures may preserve more hepatic mass than formal resections, the loss of functional volume remains important. More specifically, ablative techniques can result in two distinct challenges to host defenses: First, they create necrotic tissue, which is not surgically removed. Cryoablation causes hepatocellular necrosis by inducing formation of ice crystals, whereas radiofrequency and microwave ablation result in necrosis via heat-induced coagulation. Necrotic tissue from either source can act as a nidus for infection. Second, ablative techniques may have systemic effects, as cryotherapy has been shown to induce “cryoshock” in 1% of patients (Seifert & Morris, 1999). This can result in a systemic inflammatory response, coagulopathy, and multiorgan dysfunction or failure, particularly of the renal and pulmonary systems. More recent studies have suggested that patients undergoing radiofrequency ablation do not display the systemic inflammatory responses with Kupffer cell–mediated cytokine release of proinflammatory cytokines (TNF-α, IL-1) characteristic of cryoablation (Schell et al, 2002; Jansen et al, 2010). Other authors have confirmed a reduced cytokine response after radiofrequency ablation but suggest that it still has a significant response compared with resection alone. This group also reported that the renal and lung dysfunction characteristic of cryoablation does not occur in swine treated with radiofrequency ablation (Ng et al, 2004). In contrast, other authors have suggested the systemic inflammatory response characteristic of cryoablation is not detrimental but rather leads to inhibition of tumor growth (Joosten et al, 2001, 2003).
Although the acceptance of laparoscopic techniques for hepatic resection is rapidly increasing, data outlining the immune host response to this approach are minimal. Porcine models suggest that open procedures may be associated with more immune suppression than laparoscopic approaches (Burpee et al, 2002), but to date no studies have evaluated differences in relevant clinical end points, such as postoperative infection.
Infections.
Septic complications represent the most frequent cause of morbidity after liver resection (D’Amico & Cillo, 1999; Melendez et al, 1998) with the most common issue being intraabdominal abscesses (D’Amico & Cillo, 1999; Melendez et al, 1998). Intraabdominal infection can lead to sepsis and organ failure. Mortality from infectious complications after liver resection is significant and depends on age, comorbidities, hepatic reserve, and extent of liver resection (Garwood et al, 2004).
Pathophysiology of Infection.
Translocation of bacteria and endotoxin is thought to play a major role in the cause of sepsis after liver resection. Bacteria are present in the mesenteric lymph nodes, portal venous system, and systemic vasculature after resection, regardless of whether a biliary–enteric drainage is constructed (Wang et al, 1992, 1994, 1995; Yeh et al, 2003). Decreased bile flow through the intestine, bacterial overgrowth with preference for E. coli (Wang et al, 1993), and postoperative ileus associated stasis, overgrowth, and bacterial adherence to the intestine appear to be important factors. Finally, experimental evidence indicates that decreased blood flow to the intestine results in enterocyte protein loss, mucosal atrophy, and loss of villous height (Wang et al, 1993). This combination is thought to promote bacterial and endotoxin translocation in the setting of decreased functional Kupffer cell mass. As noted earlier, ablative therapies may also promote infection through the formation of necrotic liver tissue and its effects on systemic immunity and end-organ function. All of these factors combine to promote infection in patients following liver surgery.
Risk Factors.
One of the risk factors for infection in patients undergoing hepatic resection may relate to the amount of ischemic/necrotic liver at the resection margin (Yanaga et al, 1986) or ablation site. Intraperitoneal blood and bile, which accumulate in the surgical field, also inhibit peritoneal macrophage function (Andersson et al, 1989, 1990a, 1990b). Other risk factors relate to the extent of hepatic resection—specifically, the loss of functional liver mass—patient functional reserve, preoperative liver sepsis, iatrogenic introduction of bacteria (stenting, biliary-enteric anastomosis), blood transfusion, and shock (Andersson et al, 1990a, 1990b; Hochwald et al, 1999; Pace et al, 1989; Yanaga et al, 1986).
Causative Organisms.
Most organisms isolated from intraabdominal sepsis after liver resection are enteric gram-negative aerobes, most commonly E. coli, and Enterococcus. Multiple bacterial isolates are present in 50% to 75% of cases (Pace et al, 1989; Wang et al, 1992). Common isolates associated with higher mortality in current studies also include fungus and gram-positive organisms, particularly methicillin-resistant S. aureus (Garwood et al, 2004).
Prevention and Treatment.
Prophylactic antibiotics that cover enteric organisms—gram-negative aerobes, anaerobes, and enterococci—should be administered prior to any hepatobiliary manipulation. In addition, prophylactic antibiotics to cover skin flora should be included. Minimizing the amount of necrotic tissue at the margin of resection is optimal, and methods of sparing functional liver mass (parenchymal-sparing segmental resections) can be preventive (Jarnagin et al, 2002). Techniques using low central venous pressure and intermittent Pringle maneuvers reduce blood loss and thereby decrease the subsequent need for transfusion. Meticulous hemostasis, intraoperative identification of bile leaks, and judicious use of saline irrigation to remove bile, blood, and succus entericus from the peritoneal cavity and wound at the time of surgery are also essential.
After liver resection, early enteral feeding has been shown to reduce the rate of all infectious complications (Shirabe et al, 1997). It is thought to preserve and promote the gut mucosal integrity by maintaining nutrition and normal microbiologic flora. Animal studies have also shown that ethylhydroxyethyl cellulose (Wang et al, 1993, 1995), dextran 70 (Wang et al, 1997), and probiotics (Seehofer et al, 2004) can reduce bacterial translocation after liver resection. Ethylhydroxyethyl cellulose and dextran 70 act as cellular surfactants that may enhance endothelial cell resistance (Wang et al, 1995, 1997). Probiotics are bacterial cultures (e.g., lactic acid bacteria) that promote the normal microbiologic flora (Seehofer et al, 2004). Proper validation of these interventions still await human trials (Rayes et al, 2009).
Abdominal drains after liver resection do not prevent infection and may be associated with greater infectious complications based on randomized prospective studies (Belghiti et al, 1993; Burt et al, 2002; Fong et al, 1996; Liu et al, 2004). Techniques of preoperative PVE and hepatic arterial embolization may be employed to promote hypertrophy of the existing functional liver mass before resection. A prospective trial in 55 patients undergoing right hepatectomy showed that PVE significantly decreased postoperative complications in patients with chronic liver disease. Notably, a trend was seen (P < .08) toward decreased septic complications (Farges et al, 2003).
By creating a greater functional liver volume, the remaining mass after resection may better support any infectious challenge to host defenses. A study comprising 181 patients in whom mesenteric lymph nodes were cultured before and after liver resection showed that the only factor associated with bacterial translocation and subsequent postoperative infection was intraoperative blood transfusion (Yeh et al, 2003). Avoidance of blood-product transfusion during liver resection deserves strong consideration based on data indicating higher complication rates, longer hospital stays, and early mortality (Kooby et al, 2003; Dixon et al, 2009).
Biliary System
Host Defense.
In comparison to laparoscopic techniques, data suggest that open procedures may be associated with significant suppression of cell-mediated immune response, such as delayed-type hypersensitivity (Altamura et al, 2002; Whelan et al, 2003; Boo YJ et al, 2007). Additionally, the degree of intraabdominal pressure during laparoscopic cholecystectomy may regulate interleukin release (Basgul et al, 2004). To date, few trials have shown differences in immunologic parameters and relevant clinical end points, such as postoperative infection (Jacobi et al, 2002; Kooby, 2006).
Infections.
The most common significant postoperative infection is the intraabdominal abscess; however, cholangitis may also occur when indwelling stents are introduced into the biliary ductal system. Wound infection may comprise skin flora, but more commonly, it involves enteric organisms when bile is infected, or when a biliary-enteric anastomosis has been performed (Lewis et al, 1987; Wells et al, 1989).
Risk Factors.
Bactibilia is an important risk factor for infectious complications in biliary surgery. The presence of bacteria in the bile does not predict a patient’s risk of infection; however, evidence of bactibilia was found in 10% to 15% of patients at low risk for positive gallbladder bile cultures, for example, patients undergoing elective open cholecystectomy (Lewis et al, 1987; Reiss et al, 1982; Wells et al, 1989). Bactibilia is present in more than 80% of patients at high risk for positive cultures, such as those with acute cholecystitis (Claesson, 1986).
In patients undergoing laparoscopic cholecystectomy with positive gallbladder bile cultures, no significant difference in postoperative infectious complications were seen when a several-day course of preoperative antibiotics was employed for those at high risk (Al-Abassi et al, 2001). In contrast, the presence of bactibilia in the setting of common bile duct disease, and specifically increased biliary pressure and biliary obstruction, does predispose patients to postoperative infectious complications (Cox et al, 1978; Pitt & Couse, 1990). The greater the concentration of bacteria and the higher the degree and rapidity of obstruction, the more severe the septic complications (Pitt & Couse, 1990; Raper et al, 1989). As expected, in patients with acute suppurative cholangitis, bactibilia is present in nearly 100% of cases. This translates into a high risk of postoperative infection (Lee & Chung, 1997). Other risk factors for infection include the patient’s functional reserve, preoperative sepsis, introduction of foreign bodies (stents, drains), disruption of efficient blood supply, blood transfusion, and shock.
Causative Organisms.
Bile is normally sterile, so the type and concentration of biliary bacteria depend on the degree of obstruction or stasis, the presence of foreign bodies, and whether the obstruction is related to malignant or benign causes. The most common biliary pathogens are enteric gram-negative aerobes (Lee & Chung, 1997). Polymicrobial cultures are present in 67% of infected bile cultures (Leung et al, 1994). In patients with previous biliary instrumentation, and particularly after long-term antibiotic treatment, Pseudomonas, Serratia, Streptococcus, Enterococcus, and Enterobacter species may be present (Lipsett & Pitt, 1990; Nomura et al, 2000; Thompson et al, 1994). Gram-positive organisms, especially Enterococcus faecalis, are more common in patients who have undergone percutaneous instrumentation of the biliary tree. Anaerobes are present only occasionally but typically are found in patients with poor functional reserve, complex biliary problems, or long-term biliary stenting (Doty & Pitt, 1986; Krige et al, 1992; van den Hazel et al, 1994a, 1994b). Bile cultures in patients with malignant causes of biliary obstruction more commonly display Klebsiella, Enterobacter, Streptococcus, Enterococcus, and Candida species compared with benign biliary obstruction (Nomura et al, 2000; Thompson et al, 1994). Whether E. faecalis is truly a biliary pathogen is unclear. Antibiotic use is known to destabilize E. faecalis in the enteric flora by killing susceptible strains. Multiple antibiotic-resistant enterococcal strains, such as E. faecalis strain V583, are able to colonize the gut (Gilmore & Ferretti, 2003). In a study of patients with biliary malignancy undergoing preoperative biliary decompression, only the incidence of enterococcal bactibilia increased after antibiotic use (16% before and 63% after antibiotic use). When analyzing the correlation between preoperative bile bacteria and postoperative complications, only Enterococcus species were associated with the occurrence of complications (Nomura et al, 2000). Whether E. faecalis works independently or synergistically with other biliary bacteria as a primary pathogenic mechanism is unknown.
Pancreas
Host Defense.
The main effect of resection on host defense is the removal of functional pancreatic tissue mass. Pancreatectomy results in less production of pancreatic ductal fluid with its trophic, antibacterial, and immunologic effects. Pancreatic exocrine insufficiency following resection can also cause malnutrition and result in long-term depressed immunity without sufficient supplementation of exogenous pancreatic enzymes. In patients with preexisting pancreatic exocrine insufficiency, these host defenses are suppressed even further. Pancreatectomy may also result in pancreatic endocrine dysfunction secondary to loss of islet cell mass or in patients with inadequate islet cell function. Although the precise target range is somewhat controversial, uncontrolled perioperative hyperglycemia is a significant predisposing factor for postoperative infection and mortality (Capes et al, 2000; Grey & Perdrizet, 2004; Lipshutz & Gropper, 2009). Also, when pancreatic surgery involves enteric drainage, the effect on host defense results from exposure to enteric pathogens via biliary–enteric or pancreaticoenteric anastomoses. Finally, the impact of laparoscopic techniques for pancreatic resection on host immune status is unclear. In dog models, however, laparoscopic distal pancreatectomy results in significantly less stress as measured by both IL-1 and serum cortisol levels, when compared with conventional open procedures (Naitoh et al, 2002).
Infections.
The most common cause of intraabdominal sepsis in pancreatic surgery is intraabdominal abscess with or without pancreatic fistula or pancreaticoenteric anastomotic leak. Wound infection may occur with skin flora or enteric flora when bile is infected, or when an enteric anastomosis is performed (Lewis et al, 1987; Wells et al, 1989). Surgical procedures to address the complications of acute necrotizing pancreatitis, infected pancreatic necrosis, or pancreatic abscess are frequently accompanied by sepsis and the systemic inflammatory response syndrome.
Causative Organisms.
Enteric gram-negative aerobes arising in the pancreatic ductal fluid, either from instrumentation or via biliary-enteric or pancreaticoenteric drainage, are the main source of bacterial contamination. In acute necrotizing pancreatitis, gram-negative organisms are more commonly isolated in patients with biliary pancreatitis, whereas gram-positive organisms are more frequent in those with alcoholic pancreatitis (Raty et al, 1998). The incidence of gram-positive organisms may also be higher in necrotizing cases, when sampling of fluid collections or necrosis is performed. Candida and Pseudomonas, as in biliary bacteriology, occur during long hospital and intensive care unit stays and are typically associated with prolonged courses of antibiotics and subsequent increased mortality (Bittner et al, 1987; Kochhar et al, 2009; Agvald-Ohman C, 2008).
Prevention and Treatment.
Prevention of the infectious risks associated with pancreatic surgery entails counteracting the loss of functional pancreatic tissue. For pancreatic exocrine insufficiency, adequate exogenous pancreatic enzyme supplementation should begin postoperatively with initiation of diet. Early feeding prevents the breakdown of gut integrity (Kimmings et al, 1995), and for pancreatic endocrine insufficiency (diabetes), reasonable perioperative control of hyperglycemia with insulin should be routine. Appropriate prophylactic antibiotics must be employed that address enteric gram-negative aerobes, anaerobes, and skin flora. Prophylactic antibiotics that address translocation flora and penetrate pancreatic tissue (e.g., imipenem, cilastatin, piperacillin/tazobactam) remain important preventive measures, particularly in patients with underlying pancreatic necrosis.
ACCP/SCCM Consensus Conference Committee. Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-874.
Adams DB. The pancreatic anastomosis: the danger of a leak, which anastomotic technique is better? J Gastrointest Surg. 2009;13:1182-1183.
Agvald-Ohman C, et al. Invasive candidiasis in long-term patients at a multidisciplinary intensive care unit: Candida colonization index, risk factors, treatment and outcome. Scand J Infect Dis. 2008;2008:145-153.
Al-Abassi AA, et al. Infection after laparoscopic cholecystectomy: effect of infected bile and infected gallbladder wall. Eur J Surg. 2001;167:268-273.
Altamura M, et al. A comparative study between conventional and laparoscopic cholecystectomy: evaluation of phagocytic and T cell–mediated antibacterial activities. J Clin Gastroenterol. 2002;34:135-140.
Alvarez C, et al. The pancreatic duct epithelium in vitro: bile acid injury and the effect of epidermal growth factor. Surgery. 1997;122:476-484.
Andersson R, Foss A. Abdominal sepsis following liver resection in the rat. Hepatogastroenterology. 1991;38:547-549.
Andersson R, et al. Factors influencing the outcome of E. coli peritonitis in rats. Acta Chir Scand. 1989;155:155-157.
Andersson R, et al. Effect of bile on peritoneal morphology in Escherichia coli peritonitis. Scand J Gastroenterol. 1990;25:405-411.
Andersson R, et al. Intraabdominal abscess formation after major liver resection. Acta Chir Scand. 1990;156:707-710.
Andoh A, et al. Local secretion of complement C3 in the exocrine pancreas: ductal epithelial cells as a possible biosynthetic site. Gastroenterology. 1996;110:1919-1925.
Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
Basgul E, et al. Effects of low and high intra-abdominal pressure on immune response in laparoscopic cholecystectomy. Saudi Med J. 2004;25:1888-1891.
Basista MH, et al. Effect of isolated portal hypertension on Kupffer cell function. Dig Dis Sci. 1994;39:46-50.
Bassi C, et al, 2003: Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev (4)CD002941.
Belghiti J, et al. Drainage after elective hepatic resection: a randomized trial. Ann Surg. 1993;218:748-753.
Besselink MG, et al. Timing and impact of infections in acute pancreatitis. Br J Surg. 2009;96:267-273.
Birney Y, et al. Eicosanoids in cirrhosis and portal hypertension. Prostaglandins Other Lipid Mediat. 2003;72:3-18.
Bittner R, et al. Pancreatic abscess and infected pancreatic necrosis: different local septic complications in acute pancreatitis. Dig Dis Sci. 1987;32:1082-1087.
Blamey SL, et al. Prediction of risk in biliary surgery. Br J Surg. 1983;70:535-538.
Boo YJ, et al. Systemic immune response after open versus laparoscopic cholecystectomy in acute cholecystitis: a prospective randomized study. Scand J Clin Lab Invest. 2007;67:207-214.
Borghi E, et al. Alcoholic hepatopathy, deficiency pathology and serum transaminases: presentation of 2 clinical cases. Rec Prog Med. 1991;82:331-333.
Bradfield JW. Control of spillover: the importance of Kupffer-cell function in clinical medicine. Lancet. 1974;2:883-886.
Buchler M, et al. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology. 1992;103:1902-1908.
Burpee SE, et al. The metabolic and immune response to laparoscopic vs open liver resection. Surg Endosc. 2002;16:899-904.
Burt BM, et al. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441-445.
Cahill CJ, et al. Bile salts, endotoxin and renal function in obstructive jaundice. Surg Gynecol Obstet. 1987;165:519-522.
Capes SE, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773-778.
Carr-Locke DL, et al. Effects of exogenous secretin on pancreatic and biliary ductal and sphincteric pressures in man demonstrated by endoscopic manometry and correlation with plasma secretin levels. Dig Dis Sci. 1985;30:909-917.
Claesson BE. Microflora of the biliary tree and liver-clinical correlates. Dig Dis. 1986;4:93-118.
Clements WD, et al. Morphometric evidence of Kupffer cell activation in biliary obstruction. Br J Surg. 1994;81:1814-1815.
Clements WD, et al. Biliary decompression promotes Kupffer cell recovery in obstructive jaundice. Gut. 1996;38:925-931.
Clements WDB, et al. Extrahepatic biliary obstruction promotes bacterial translocation. Am J Surg. 1993;165:749.
Clements WDB, et al. Major histocompatibility class II antigen expression in experimental biliary obstruction. Br J Surg. 1994;81:757-758.
Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885-891.
Cox JL, et al. The relationship between biliary tract infections and postoperative complications. Surg Gynecol Obstet. 1978;146:233-236.
Dahlgren UI, et al. Antibodies to Escherichia coli and anti-adhesive activity in paired serum, hepatic and gall bladder bile samples. Scand J Immunol. 1986;24:251-260.
D’Amico D, Cillo U. Impact of severe infections on the outcome of major liver surgery: a pathophysiologic and clinical analysis. J Chemother. 1999;11:513-517.
Deignan T, et al. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J Hepatol. 2002;37:101-108.
Deitch EA, et al. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79-84.
Dillinger EP, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674-683.
Ding JW, et al. Effect of biliary decompression on reticuloendothelial function in jaundiced rats. Br J Surg. 1992;79:648-652.
Dixon E, et al. Blood loss in surgical oncology: neglected quality indicator? J Surg Oncol. 2009;99:508-512.
Doty J, Pitt HA. Management of empyema of the gallbladder. Infect Surg. 1986;5:271-297.
Emmrich J, et al. Secretory immunoglobulin A in pancreatic juice and pancreatic tissue of patients with chronic pancreatitis. Gut. 1998;42:436-441.
Fallon MB, et al. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863-1872.
Farges O, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217.
Foitzik T, et al. Pathogenesis and prevention of early pancreatic infection in experimental acute necrotizing pancreatitis. Ann Surg. 1995;222:179-185.
Fong Y, et al. Drainage is unnecessary after elective liver resection. Am J Surg. 1996;171:158-162.
Garwood RA, et al. Infectious complications after hepatic resection. Am Surg. 2004;70:787-792.
Gilmore MS, Ferretti JJ. Microbiology: the thin line between gut commensal and pathogen. Science. 2003;299:1999-2002.
Gilroy C, et al. Cilia in the porcine bile ductule: motile or sensory? Histol Histopathol. 1995;10:301-304.
Greve JW, et al. Complications in obstructive jaundice: role of endotoxins. Scand J Gastroenterol. 1992;194(Suppl):8-12.
Grey NJ, Perdrizet GA. Reduction of nosocomial infections in the surgical intensive-care unit by strict glycemic control. Endocr Pract. 2004;10(Suppl 2):46-52.
Gross K, et al. Bacterial clearance in the intact and regenerating liver. J Pediatr Surg. 1985;20:320-323.
Hatfield AR, et al. The microbiology of direct bile sampling at the time of endoscopic retrograde cholangiopancreatography. J Infect. 1982;4:119-125.
Hayward AR, et al. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J Immunol. 1997;158:977-983.
Hochwald SN, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261-266.
Holowachuk SA, et al. Nonparallel secretion of antibacterial activity and protein in porcine pancreatic juice. Pancreas. 2004;28:E32-E38.
Homann N, et al. Effects of acetaldehyde on cell regeneration and differentiation of the upper gastrointestinal tract mucosa. J Natl Cancer Inst. 1997;89:1692-1697.
Howard TJ. As good as it gets: the study of prophylactic antibioics in severe acute pancreatitis. Ann Surg. 2007;245:684-685.
Howard TJ, et al. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. Gastrointest Surg. 2007;11:43-49.
Isenmann R, et al. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997-1004.
Itoshima T, et al. Scanning electron microscopy of the bile ductule. Gastroenterol Jpn. 1977;12:476-482.
Jacobi CA, et al. Immunologic changes during minimally invasive surgery. Dig Surg. 2002;19:459-463.
Jansen MC, et al. Cryoablation iduces greater inflammatory and coagulative responses than radiofrequency ablation or laser-induced thermotherapy in a rat liver model. Surgery. 2010;147(5):686-695.
Jarnagin WR, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-407.
Joosten JJ, et al. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49-58.
Joosten JJ, et al. Cryosurgery of tumor tissue causes endotoxin tolerance through an inflammatory response. Anticancer Res. 2003;23:427-432.
Katz S, et al. Impaired hepatic bacterial clearance is reversed by surgical relief of obstructive jaundice. J Pediatr Surg. 1991;26:401-406.
Kimmings AN, et al. Inflammatory and immunologic effects of obstructive jaundice: pathogenesis and treatment. J Am Coll Surg. 1995;181:567-581.
Kimmings AN, et al. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725-731.
Kochhar R, et al. Prevalence and outcome of fungal infection in patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2009;24:743-747.
Kooby DA. Laparoscopic surgery for cancer: historical, theortetical, and technical considerations. Oncology. 2006;20:917-927.
Kooby DA, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860-869.
Krige JE, et al. Prospective, randomized study comparing amoxycillin-clavulanic acid and cefamandole for the prevention of wound infection in high-risk patients undergoing elective biliary surgery. J Hosp Infect. 1992;22(Suppl A):33-41.
Krige JEJ, Bornman PC. Infections in hepatic, biliary and pancreatic surgery. In: Blumgart LH, Fong Y, editors. Surgery of the Liver and Biliary Tract, vol 1. Philadelphia: Saunders; 2000:151-166.
Kruszewska D, et al. Effect of the antibacterial activity of pig pancreatic juice on human multiresistant bacteria. Pancreas. 2004;28:191-199.
Lai EC, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195-1198.
Laskin DL. Nonparenchymal cells and hepatotoxicity. Semin Liver Dis. 1990;10:293-304.
Lee DW, Chung SC. Biliary infection. Baillieres Clin Gastroenterol. 1997;11:707-724.
Leung JW, et al. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest Endosc. 1994;40:716-721.
Lewis RT, et al. Biliary bacteria, antibiotic use, and wound infection in surgery of the gallbladder and common bile duct. Arch Surg. 1987;122:44-47.
Lipsett PA, Pitt HA. Acute cholangitis. Surg Clin North Am. 1990;70:1297-1312.
Lipshutz AK, Gropper MA. Perioperative glycemic control: an evidence-based review. Anesthesiology. 2009;110:408-421.
Liu CL, et al. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194-201.
Luiten EJ, et al. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995;222:57-65.
Lygidakis NJ, et al. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1987;153:665-668.
Marshall SE, et al. Aspects of the effect of bile salts on Candida albicans. J Med Vet Mycol. 1987;25:307-318.
McPherson GA, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71:371-375.
Medich DS, et al. Pathogenesis of pancreatic sepsis. Am J Surg. 1993;165:46-52.
Megison SM, et al. Effects of relief of biliary obstruction on mononuclear phagocyte system function and cell-mediated immunity. Br J Surg. 1991;78:568-571.
Melendez JA, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620-625.
Mett H, et al. Duodeno-pancreatic secretions enhance bactericidal activity of antimicrobial drugs. Antimicrob Agents Chemother. 1984;26:35-38.
Minelli EB, et al. Antimicrobial activity of human pancreatic juice and its interaction with antibiotics. Antimicrob Agents Chemother. 1996;40:2099-2105.
Moniaux N, et al. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633-1638.
Naitoh T, et al. Gastro-intestinal transit and stress response after laparoscopic and open surgery. Surg Endosc. 2002;16:1615-1619.
Nathens AB, et al. Severe acute pancreatitis: a review. Surg Infect. 2008;9:573-578.
Ng KK, et al. Comparison of systemic responses of radiofrequency ablation, cryotherapy, and surgical resection in a porcine liver model. Ann Surg Oncol. 2004;11:650-657.
Nomura T, et al. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci. 2000;45:2183-2186.
Ogura Y, et al. Pathophysiological effect of hepatic ischemia and reperfusion after hepatectomy in dogs with obstructive jaundice, focusing on the effect of coenzyme Q10 and styrene-co-maleic acid superoxide dismutase. J Gastroenterol. 1996;31:379-386.
Ohshio G, et al. Immunoglobulin A secretion into pancreatic juice as a novel marker of local immune defense and exocrine pancreatic function. Dig Dis Sci. 2001;46:2140-2146.
Pace RF, et al. Intra-abdominal sepsis after hepatic resection. Ann Surg. 1989;209:302-306.
Pain JA, et al. Reticuloendothelial system phagocytic function in obstructive jaundice and its modification by a muramyl dipeptide analogue. Eur Surg Res. 1987;19:16-22.
Parks RW, et al. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345-1349.
Pederzoli P, et al. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480-483.
Pitt HA, et al. Factors affecting mortality in biliary tract surgery. Am J Surg. 1981;141:66-72.
Pitt HA, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545-553.
Pitt HA, et al. Biliary sepsis and toxic cholangitis. In: Surgical Treatment of Digestive Disease. Chicago: Year Book Medical Publishers; 1990:332-350.
Povoski SP, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg. 1999;3:496-505.
Raper SE, et al. Anatomic correlates of bacterial cholangiovenous reflux. Surgery. 1989;105:352-359.
Raty S, et al. Difference in microbes contaminating pancreatic necrosis in biliary and alcoholic pancreatitis. Int J Pancreatol. 1998;24:187-191.
Rayes N, et al. Prebiotics, probiotics, synbiotics in surgery: are they only trendy, truly effective or even dangerous? Langenbecks Arch Surg. 2009;394:547-555.
Reiss R, et al. Septic complications and bile cultures in 800 consecutive cholecystectomies. World J Surg. 1982;6:195-199.
Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
Rockey DC. Cellular pathophysiology of portal hypertension and prospects for management with gene therapy. Clin Liver Dis. 2001;5:851-865.
Roughneen PT, et al. Impaired specific cell-mediated immunity in experimental biliary obstruction and its reversibility by internal biliary drainage. J Surg Res. 1986;41:113-125.
Rubinstein E, et al. Antibacterial activity of the pancreatic fluid. Gastroenterology. 1985;88:927-932.
Sainio V, et al. Early antibiotic treatment in acute necrotizing pancreatitis. Lancet. 1995;346:663-667.
Saleh MM, et al. Preoperative endoscopic stent placement before pancreaticoduodenectomy: a meta-analysis of the effect on morbidity and mortality. Gastrointest Endosc. 2002;56:529-534.
Schell SR, et al. Pro- and antiinflammatory cytokine production after radiofrequency ablation of unresectable hepatic tumors. J Am Coll Surg. 2002;195:774-781.
Schmidt CM, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718-727.
Schmitt M, et al. Disruption of paracellular sealing is an early event in acute caerulein-pancreatitis. Pancreas. 2004;28:181-190.
Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res. 1994;57:316-336.
Seehofer D, et al. Probiotics partly reverse increased bacterial translocation after simultaneous liver resection and colonic anastomosis in rats. J Surg Res. 2004;117:262-271.
Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109-114.
Shirabe K, et al. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection: the results of a randomized prospective study. Hepatogastroenterology. 1997;44:205-209.
Smith R, et al. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized controlled trial examining renal function. Surgery. 1984;96:641-647.
Sohn TA, et al. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg. 2000;4:258-268.
Stewart L, et al. Antibacterial activity of bile acids against common biliary tract organisms. Surg Forum. 1986;37:157-159.
Stewart L, et al. Cholangiovenous reflux pathways as defined by corrosion casting and scanning electron microscopy. Am J Surg. 1988;155:23-28.
Stewart L, et al. Gram-negative bacteria killed by complement are associated with more severe biliary infections and produce more tumor necrosis factor-alpha in sera. Surgery. 2002;132:408-414.
Stewart L, et al. Cholangitis: bacterial virulence factors that facilitate cholangiovenous reflux and tumor necrosis factor-alpha production. J Gastrointest Surg. 2003;7:191-199.
Sumiyoshi K, et al. Biosynthesis and secretion of MHC class III gene products (complement C4 and factor B) in the exocrine pancreas. J Gastroenterol. 1997;32:367-373.
Sung JJ, et al. Biliary IgA secretion in obstructive jaundice: the effects of endoscopic drainage. Gastrointest Endosc. 1995;42:439-444.
Sung JY, et al. Antibacterial activity of bile salts against common biliary pathogens: effects of hydrophobicity of the molecule and in the presence of phospholipids. Dig Dis Sci. 1993;38:2104-2112.
Takahashi K, et al. Pathophysiological changes caused by occlusion of blood flow into the liver during hepatectomy in dogs with obstructive jaundice: effects of intestinal congestion. J Gastroenterol Hepatol. 1996;11:963-970.
Takiguchi S, Koga A. Effects of bile acids and endotoxin on the function and morphology of cultured hamster Kupffer cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54:303-311.
Thompson J, et al. An analysis of infectious failures in acute cholangitis. HPB Surg. 1994;8:139-145.
Thompson RL, et al. Development and reversibility of T lymphocyte dysfunction in experimental obstructive jaundice. Br J Surg. 1990;77:1229-1232.
Trede M, Carter D, 1997: The complications of pancreatoduodenectomy and their management. In Trede M, Carter D (eds): Surgery of the Pancreas. Philadelphia, Churchill Livingstone, pp 675-692.
Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39-47.
van der Gaag NA, et al. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. J Gastrointest Surg. 2009;13:814-820.
van der Gaag NA, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Eng J Med. 2010;362:129-137.
van den Hazel SJ, et al. Role of antibiotics in the treatment and prevention of acute and recurrent cholangitis. Clin Infect Dis. 1994;19:279-286.
van den Hazel SJ, et al. The pathogenesis of bacterial cholangitis. Eur J Gastroenterol Hepatol. 1994;6:1053-1057.
Vo NM, Chi DS. Effect of hepatectomy on the reticuloendothelial system of septic rats. J Trauma. 1988;28:852-854.
Vosters O, et al. CD40 expression on human pancreatic duct cells: role in nuclear factor-kappa B activation and production of pro-inflammatory cytokines. Diabetologia. 2004;47:660-668.
Wang Q, et al, 2008: Preoperative biliary drainage for obstructive jaundice. Cochrane Database Sys Rev 3:CD005444.
Wang X, et al. Bacterial translocation after major hepatectomy in patients and rats. Arch Surg. 1992;127:1101-1106.
Wang X, et al. Water-soluble ethylhydroxyethyl cellulose prevents bacterial translocation induced by major liver resection in the rat. Ann Surg. 1993;217:155-167.
Wang X, et al. Bacterial translocation and intestinal capillary permeability following major liver resection in the rat. J Surg Res. 1995;58:351-358.
Wang X, et al. The role of intravenous administration of dextran 70 in enteric bacterial translocation after partial hepatectomy in rats. Eur J Clin Invest. 1997;27:936-942.
Wang XD, et al. Bacterial translocation, intestinal ultrastructure and cell membrane permeability early after major liver resection in the rat. Br J Surg. 1994;81:579-584.
Wells GR, et al. Relationship between bile colonization, high-risk factors and postoperative sepsis in patients undergoing biliary tract operations while receiving a prophylactic antibiotic. West of Scotland Surgical Infection Study Group. Br J Surg. 1989;76:374-377.
Wetter LA, et al. Differences in outer membrane characteristics between gallstone-associated bacteria and normal bacterial flora. Lancet. 1994;343:444-448.
Whelan RL, et al. Postoperative cell-mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc. 2003;17:972-978.
Widdison AL, et al. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut. 1994;35:1306-1310.
Wilton PB, et al. Complement in local biliary tract defense: dissociation between bile complement and acute phase reactants in cholecystitis. J Surg Res. 1987;42:434-439.
Yanaga K, et al. Intraperitoneal septic complications after hepatectomy. Ann Surg. 1986;203:148-152.
Yeh DC, et al. Bacterial translocation after cirrhotic liver resection: a clinical investigation of 181 patients. J Surg Res. 2003;111:209-214.