Infection in the Patient with Cancer
Alison G. Freifeld and Daniel R. Kaul
• Risk assessment is an important tool for evaluation and treatment in patients with cancer who have fever and neutropenia. Patients who are expected to have neutropenia lasting more than 7 days, including those undergoing allogeneic stem cell transplantation or therapy for acute leukemia, are considered to be at high risk for infectious complications. Most patients with solid tumors will have neutropenia lasting fewer than 7 days and are considered to be at low risk.
• Other risk factors for infection in patients with cancer, beside chemotherapy- and disease-related neutropenia, are the presence of indwelling catheters, comorbid medical conditions such as diabetes or chronic obstructive pulmonary disease, recent surgery, malnutrition, and cellular and humoral immune defects from an underlying tumor and its treatment.
• Fever during neutropenia necessitates immediate evaluation, assessment of risk as high or low, appropriate cultures, and prompt institution of empirical broad-spectrum antibacterial therapy with a defined regimen that covers Pseudomonas aeruginosa and enteric gram-negative organisms, as well as common institutional pathogens. Gram-positive active agents are not a standard component of the empirical treatment regimen for fever and neutropenia.
• Antibiotic prophylaxis with levofloxacin has been shown to decrease fever and infection in high-risk patients with acute leukemia or those undergoing stem cell transplantation, with neutropenia (<1000 neutrophils/mm3) lasting more than 7 days, although no morality benefit has been consistently shown.
• Posaconazole prophylaxis reduces the incidence of invasive fungal infections and mortality in patients undergoing induction for acute leukemia and reduces fungal infections in patients treated for higher grade graft-versus-host disease. Voriconazole has been shown to be equivalent to fluconazole for prevention of fungal infections in allogeneic stem cell transplant recipients.
• Newer agents used to treat a variety of lymphoproliferative disorders (e.g., alemtuzumab and purine analogs) result in prolonged suppression of cellular immunity and predispose persons to certain infections; patients receiving these agents may benefit from prophylaxis against opportunistic infections.
• An epidemic strain of Clostridium difficile has emerged as a major cause of morbidity and mortality in many centers, and fluoroquinolone use is a risk factor. Metronidazole is recommended for mild disease, but oral vancomycin should be given for more severe symptoms of C. difficile infection.
• Numerous antifungal agents may be used for empirical antifungal therapy after 4 to 7 days of broad-spectrum antibiotic use in patients who are still febrile; however, some debate continues regarding whether all patients definitely require empirical antifungal therapy. A high-resolution computed tomography scan of the chest and serial galactomannan assay results may identify possible mold infections and guide antifungal management.
• Patients scheduled for allogeneic hematopoietic stem cell transplantation should be screened for evidence of latent herpesvirus and hepatitis virus infections, and prophylaxis should be instituted accordingly.
Introduction
Numerous disease-related and chemotherapy-induced factors render patients with cancer at increased risk for infection.1,2 These factors include the type of cancer (e.g., a solid tumor versus a lymphoma or acute leukemia), the severity and duration of neutropenia, and impairments in cellular function caused by cytotoxic or immunosuppressive drugs; breaches in the integument from surgical procedures, the presence of indwelling plastic venous catheters, or mucositis of the gastrointestinal tract as a result of chemotherapy; and comorbid conditions such as malnutrition, deconditioning, or medical problems such as chronic obstructive lung disease or diabetes. Approaches to prevention, diagnosis, and management of infectious complications in patients with cancer are greatly influenced by the cumulative burden of these risk factors. Neutropenia remains one of the most important predisposing factors for infection, overriding many of the others. This chapter addresses current standards for the management of fever during neutropenia and also highlights contemporary guidelines for the prevention and treatment of common infectious complications in patients with cancer.
Infection Risk Factors
Neutropenia as a Risk Factor for Infection
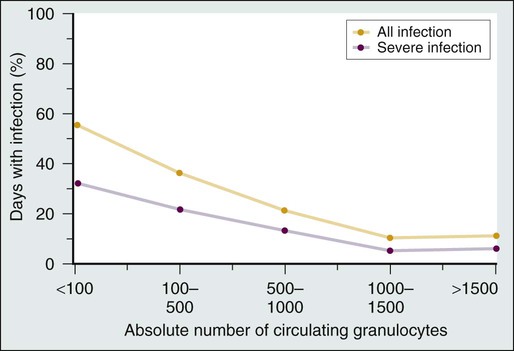
The association between neutropenia and increased infection risk was first demonstrated by Bodey and colleagues3 in 1966 in a study of leukemic patients undergoing cytotoxic therapy. The data show that the frequency of infectious complications is inversely related to the degree and duration of neutropenia (Fig. 36-1). Infection risk starts to increase when the absolute neutrophil count (ANC) decreases to less than 1000 cells/mm3 and increases dramatically when the ANC count is less than 500 cells/mm.3 Fewer than half of the neutropenic patients who become febrile will have an identified infection. In roughly 10% to 20% or more of patients with neutrophil counts less than 100 cells/mm3, a bloodstream infection will be identified. More than 50% of all episodes of fever and neutropenia represent “fever of undetermined origin,” with no clear infection source on physical and radiographic examination and cultures.3,4 In addition to the severity of the ANC nadir, the duration of neutropenia is also an important determinant of both infection risk and infection type. Brief durations of neutropenia, particularly for less than 7 days, are usually associated with a rapid and favorable response to empirical antibiotic therapy. Fever often may be of unknown origin during this early phase of neutropenia, but if a causative pathogen is identified, bacteria and viruses predominate. A neutrophil count persistently less than 500 cells/mm3 for more than 7 days is associated with greater risk for infection-related morbidity and mortality and is the setting in which Aspergillus and other invasive fungal infections most often occur. Other pathogens that may cause infection during the course of prolonged neutropenia (i.e., after 7 days) include antibiotic-resistant bacteria, Candida species, and other molds. Deaths during the state of neutropenia usually are due to these subsequent infections. Overall mortality rates during the state of fever and neutropenia are less than 1% for low-risk patients and approximately 5% for those with more prolonged duration of neutropenia.5–9
Other Risk Factors for Infection
Disruption of integumentary, mucosal, and mucociliary barriers by cytotoxic therapies provides opportunities for invasion by colonizing bacteria on the skin, in the gastrointestinal tract, or in the mucous membranes.10 Shifts in normal colonizing microbial flora at these sites occur as a result of chemotherapy, antibiotic use, and nosocomial exposures, leading to increased colonization with gram-negative and antibiotic-resistant pathogens. Indwelling catheters create a significant breach in host defense, permitting direct access of skin flora and other pathogens to blood or subcutaneous tissue. Tumor growth can disrupt normal anatomic structures, and cancer surgery may result in anatomic alterations and wounds, providing local sites for pathogen entry.
Steroid therapy induces a broad immunosuppressive effect, including impaired chemotaxis and killing by neutrophils, impaired T-cell function, and alterations in skin and mucosal barriers. Long-term or high-dose steroid therapy is a significant risk factor for invasive fungal infections in particular (i.e., Aspergillus and Cryptococcus), as well as P. jiroveci (usually seen with tapering of the steroid regimen). Steroid therapy also may predispose affected patients to development of bacterial infections and Mycobacterium tuberculosis reactivation. High-dose cytosine arabinoside therapy causes mucositis that may predispose to life-threatening streptococcal bacteremias.10 Fludarabine and alemtuzumab cause prolonged suppression of CD4+ lymphocytes and attendant susceptibility to infections with Listeria, P. jiroveci, and herpesviruses, as well as bacterial infections.11,12 The administration of temozolomide along with radiation for glioblastoma also is associated with increased susceptibility to P. jiroveci, as well as profound myelotoxicity.13 Patients with myeloma who are treated with the proteasome inhibitor bortezomib are at increased risk for herpes zoster, and prophylaxis should be administered.14
Sources of Infection
Colonization by pathogenic bacteria, fungi, or viruses generally is a prerequisite for infection. Accordingly, endogenous bacterial and fungal flora and latent herpesvirus infections account for a majority of initial infections in neutropenic patients with cancer.17–17 These infections include skin colonizers such as Staphylococcus aureus and coagulase-negative staphylococci, viridans streptococci, and herpes simplex from the oropharynx and gram-positive bacteria, as well as enteric gram-negative bacteria, from the gut. Candida albicans infections often are derived from the skin or the gastrointestinal or female genital tract. Latent infections that may reactivate during immunosuppression include those due to herpes simplex or varicella-zoster virus, Epstein-Barr virus (EBV), and cytomegalovirus (CMV), as well as hepatitis B and C viruses, M. tuberculosis, and Toxoplasma gondii.
Exogenous sources of infection often are found in the hospital and home environments. Contaminated blood products, hospital equipment, water sources, and nosocomial spread of organisms from health care workers represent less common, albeit significant, sources of infection. Common nosocomially spread infections include those due to Clostridium difficile, respiratory viruses, vancomycin-resistant enterococci, and other multiresistant bacteria. Water sources such as faucets and shower heads have been implicated in the spread of Legionella and Aspergillus.18 Outbreaks of Klebsiella and Enterobacter infection related to intravenous solutions have been well documented.21–21
Foods can be a potential source of infection, particularly unwashed fruits and vegetables. Neutropenic patients generally are advised to follow safe food-handling guidelines, which include washing fruits and vegetables thoroughly, cooking eggs until the whites and yolks are completely hard, and cooking meat until it is well done.22 Potted plants, mulch, and excavation and building or renovation sites have been implicated as sources of Aspergillus and other molds that may cause disease.23
Approach to Fever in the Neutropenic Patient
Fever often is the only reliable sign of significant underlying infection in the neutropenic patient. No specific clinical features, such as hypotension, chills or the magnitude of the increase in body temperature can accurately distinguish between fever due to an infection and that due to a noninfectious cause. Nor are laboratory tests such as C-reactive protein or procalcitonin levels considered specific or sensitive or enough to be relied on for making decisions about the use of antibiotics at the time of presentation.26–26 Therefore all febrile neutropenic patients should receive empirical broad-spectrum antibiotics, ideally within 1 hour of presentation. Although a clinically or microbiologically documented source of fever is not found in most febrile neutropenic patients, the rapid initiation of empirical antibiotic therapy remains an important standard of care for all patients in this setting. The choice of specific antibiotic regimens depends on an assessment of the patient’s infection risk during neutropenia, which depends on the duration of neutropenia, the underlying cancer, and comorbid medical conditions. Conditions and findings that may alter the empirical antibiotic regimen are shown in Table 36-1.
Table 36-1
Approach to Management of Fever and Neutropenia
| History and Physical Findings | Indicated Modifications in Antimicrobial Coverage or Diagnostic Test |
| Fever during neutropenia | Empiric antibiotic regimen (see text); if the patient is receiving antibiotic prophylaxis, then empiric therapy must be based on an agent of a different class from that used for prophylaxis (e.g., if the patient is receiving fluoroquinolone-based prophylaxis, then switch to a beta-lactam–based regimen) |
| Hypotension, signs of sepsis | Broad-spectrum antimicrobial coverage: a triple-antibiotic regimen (e.g., carbapenem plus vancomycin plus an aminoglycoside) is recommended; if cultures are negative for gram-positive organisms, consider discontinuing vancomycin after 3 days |
| Fever that is persistent or recrudescent on or after day 5 of antibiotic therapy | Empirical antifungal therapy: add an amphotericin B product, an echinocandin, or voriconazole if the patient is not already receiving prophylaxis with a mold-active agent; a CT scan of the chest may help identify fungal infection; consider a CT scan of the chest and frequent galactomannan testing during neutropenia to guide preemptive treatment as an alternative strategy to empiric antifungal treatment |
| Severe oral or esophageal mucositis | Send a swab for viral (HSV) culture; add antiviral coverage (if not already being given); consider antiviral resistance to prophylaxis if esophagitis occurs late in the course of neutropenia; add an antifungal agent for possible Candida esophagitis; switch streptococcal coverage to vancomycin; esophagoscopy may be indicated |
| Catheter exit site or tunnel erythema, tenderness, or discharge or cellulitis at any site | Culture any discharge; add vancomycin; for tunnel infection (erythema and tenderness 2 cm above exit site), catheter removal and surgical débridement generally are required |
| Possible anaerobic infection | Add metronidazole if broad-spectrum antibiotics without activity against anaerobes are being used |
| Abdominal pain, especially right lower quadrant, suggestive of neutropenic enterocolitis; oropharyngeal or neck or soft-tissue swelling | Perform a CT scan of the affected area; supportive care: avoid surgical intervention if possible in a neutropenic patient |
| New pulmonary infiltrate | Bronchoscopy (with or without biopsy) is the preferred method for evaluating new infiltrates in high-risk patients; Nodular: add mold coverage with voriconazole, posaconazole, or amphotericin B preparation; Alveolar: broaden gram-negative coverage and add Legionella coverage (quinolone or macrolide); Interstitial: send specimen for diagnostic studies for both respiratory viruses, PCP, and herpesviruses, particularly CMV; Review patient history for risk factors for tuberculosis or infection with endemic fungi |
| Upper respiratory symptoms of coryza, congestion during fall/winter | Send nasal wash or swab for respiratory viral studies (e.g., culture, PCR, rapid antigen testing) |
| Hemorrhagic cystitis | Indicated studies include urine viral culture and BK virus PCR assay (for hematopoietic stem cell transplant recipients) |
Definitions
Guidelines for evaluating antimicrobial therapy for fever and neutropenia have been developed by the Infectious Diseases Society of America and the National Comprehensive Cancer Network.2,26 Fever is defined as a single temperature measurement of 38.3°C (101°F) or greater in the absence of other obvious causes; a temperature of 38°C or greater for an hour or longer is considered to represent a “febrile state” that also requires prompt evaluation and intervention in the setting of neutropenia.2,26 Neutropenia is defined as an ANC less than 1000 cells/mm3 in some centers but more often is designated by a neutrophil count of less than 500 cells/mm3, which is a level associated with a much higher risk for infection. For practical purposes, an ANC less than 500 cells/mm3, or a count that is anticipated to fall below that level within 48 hours, constitutes a state of neutropenia.
Initial Evaluation
Initial laboratory evaluation should include a complete blood cell count and differential white cell count to determine the degree of neutropenia, liver and renal function tests, oxygen saturation determination, and urinalysis. Chest radiography should be reserved only for patients who have symptoms of respiratory tract infection. Note that radiographic findings may be minimal in neutropenic patients with pneumonia. At least two blood culture samples, each consisting of 20 to 40 mL of blood, should be obtained from both a peripheral vein and from each catheter lumen.2,4 An adequate volume of blood taken for culture will enhance the chances of recovering a pathogen.27
Drawing blood samples from both a vascular catheter and a peripheral vein can help determine whether the catheter is a source of infection. If the differential time to culture positivity between catheter- and venipuncture-derived cultures is greater than 2 hours, the catheter is implicated as a source.28 In patients with cancer, however, one study showed that catheter-drawn blood cultures without concomitant peripheral cultures do have very good negative predictive value and fairly good sensitivity (89%) and specificity (95%).29 A positive culture result for coagulase-negative staphylococci—a common contaminant—requires two positive blood culture sets to be considered a “true positive.”28
Risk Assessment
Patients who present with fever and neutropenia may have a variety of clinical outcomes. Most receive broad-spectrum empirical antibiotics and survive the episode without major incident. In a minority of patients, significant infections will develop or they will experience other life-threatening medical events. Numerous studies have sought to stratify patients at presentation into those with high versus low risk for complications.2,8,26,30–37
Risk assessment, using a scheme based on clinical criteria, should be performed as part of the initial evaluation to classify patients as high or low risk for serious complications during a febrile episode.2,26 This risk assessment will help determine which empirical antibiotics are appropriate and in what venue—that is, the hospital or outpatient clinic—the patient may be treated for fever and neutropenia. A patient’s risk is driven largely by the duration of neutropenia expected as a consequence of chemotherapy and by certain historical and physical characteristics of the neutropenic patient who presents with fever (Table 36-2). Clinical features of low-risk patients include neutropenia anticipated to last less than 7 days, absence of serious medical comorbidity, and outpatient status at onset of fever. Typically, these patients are receiving chemotherapy for solid tumors. Oral antibiotics given outside of the traditional hospital setting may be appropriate for a subset of low-risk patients. High-risk patients, in contrast, generally have an expected duration of neutropenia of 7 days or longer and/or have serious medical comorbid conditions. They should be always admitted for antibiotic therapy. Recipients of allogeneic hematopoietic stem cell transplants, patients with acute leukemia who are receiving induction or consolidation therapy, and patients with myelodysplasia or aplastic anemia are considered at high risk for complications during neutropenia. Persons who have received autologous hematopoietic stem cell transplants seem to be at intermediate risk for infections, even though they may have prolonged durations of neutropenia (i.e., longer than 7 days). Similarly, patients with lymphoma or CLL and recipients of purine analog therapy (e.g., fludarabine) or those undergoing mini-allogeneic transplantation may be considered to be at intermediate risk for severe infection because they tend to have only brief periods of neutropenia, or none at all, but they may acquire significant impairments of cellular immunity that make them susceptible to certain types of infections.
Table 36-2
Risk for Infectious Complications after Cytotoxic Chemotherapy: Clinical Features Defining Risk Status and Risk Prediction in Patients with Fever and Neutropenia
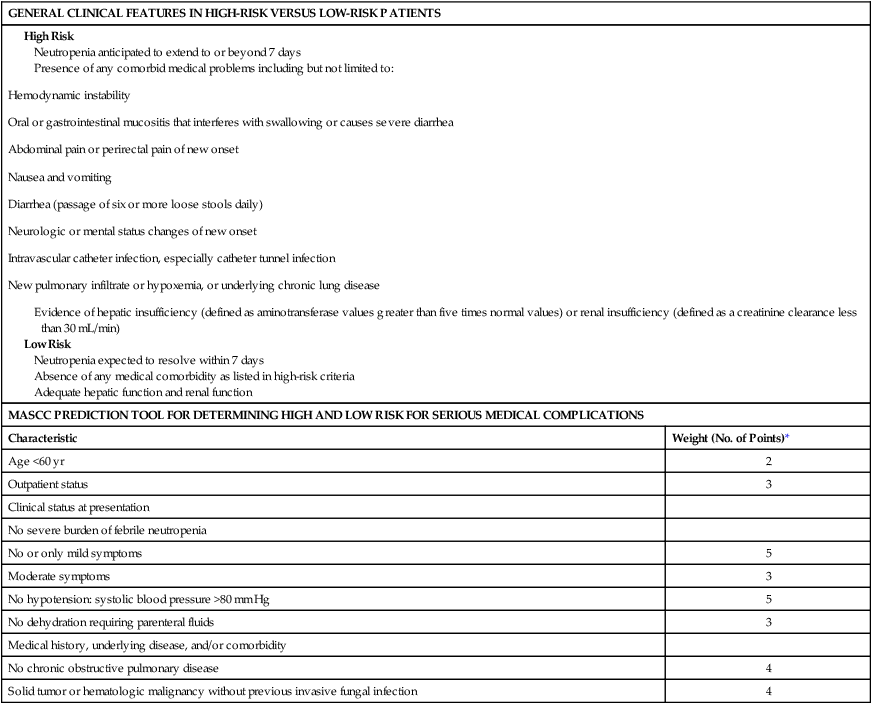
MASCC, Multinational Association for Supportive Care in Cancer.
*If relevant, points are added to yield a sum score. If the score is >20, the patient is predicted to be at low risk (<10%) for the development of serious medical complications during the course of febrile neutropenia.
Adapted from Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011;52(4):e56-93.
Although many clinicians rely on the criteria shown in Table 36-2, these low- and high-risk features have not been clinically validated.2 The Multinational Association of Supportive Care in Cancer (MASCC) has carefully developed a validated clinical prediction rule that can distinguish high-risk from low-risk patients with substantial accuracy; this rule is shown in Table 36-2.8,35–37 Use of these MASCC prediction criteria should be considered a standard method of distinguishing between low-risk and high-risk patients.2,26 The criteria provide a summation of weighted risk factors, including patient age, history, outpatient or inpatient status, acute clinical signs, the presence of medical comorbid conditions, and severity of fever and neutropenia as assessed by “burden of illness.” Low-risk patients are identified by a cumulative score >21 points (Table 36-2). A fundamental difficulty with the MASCC system is the nebulous nature of one of its major criteria: the “burden of febrile neutropenia” and symptoms associated with that burden. This burden is interpreted to be a measure of how “sick” the patient appears to be on presentation.34 Nonetheless, in a validation study of the MASCC assessment tool, the rate of serious medical complications during the course of neutropenia was only 5% among 441 febrile neutropenic adult patients initially classified as low risk.8 Of the patients with episodes that were predicted to be low risk, 189 (43%) were eligible for oral treatment, but only 79 patients (18%) met additional stringent criteria for discharge from the hospital and receipt of outpatient therapy (clinically stable or improving and with an adequate home environment and psychosocial status) after at least 24 hours of observation in the hospital. Only three patients required readmission to the hospital for fever or other reasons, and no adverse events occurred among the carefully selected outpatient subgroup.
Empiric Antibiotic Therapy
Monotherapy with a broad-spectrum antipseudomonal agent such as an extended-spectrum antipseudomonal cephalosporin (e.g., cefepime or ceftazidime), piperacillin-tazobactam, or a carbapenem (e.g., imipenem-cilastatin or meropenem), constitutes a standard approach to the management of fever and neutropenia in both high-risk and low-risk patients with cancer.2,8,26,38–44 The choice of agent should be based on a review of local institutional bacterial susceptibility patterns. Reports of high rates of multidrug-resistant gram-negative bacteremias in patients with cancer underscores the need to select agents based on a review of local institutional bacterial susceptibility patterns.47–47 Some institutions routinely use combination therapy with either (1) an aminoglycoside plus either an extended-spectrum antipseudomonal cephalosporin or piperacillin-tazobactam or (2) ciprofloxacin plus an antipseudomonal penicillin. No clear benefit of combination therapy over monotherapy has been demonstrated, and increased renal toxicity has been observed with aminoglycoside use.2,8,26,38–44 In an era of increasing bacterial resistance, however, circumstances may arise in which initial empirical antibiotic combinations are advisable.48 With the possibility of infection as a result of methicillin-resistant S. aureus, for example, the addition of vancomycin is prudent. If a pneumonia is identified at the outset, then adding an antipseudomonal fluoroquinolone (i.e., ciprofloxacin or levofloxacin) ensures a broadened spectrum against potentially resistant gram-negative or atypical pathogens, as well as “atypical” organisms such as Legionella.49 The initial antibiotic regimen may be modified according to clinical, radiographic, or culture data available at the time of presentation, as outlined in Table 36-1.
High-risk patients should receive intravenous antibiotics in the inpatient setting. Again, the choice of specific regimen is highly dependent on institutional profiles of pathogen susceptibility and on potential infection sites in a specific patient. Notably, many of these patients are receiving fluoroquinolone prophylaxis and are therefore at increased risk for resistant gram-negative and gram-positive infections.47 It is not clear, however, whether different antibiotic regimens are required for these patients at the onset of fever.
Low-risk patient who can tolerate oral agents may be treated with a combination of oral ciprofloxacin plus amoxicillin-clavulanate. In two randomized double-blind trials, this combination proved as effective as intravenous broad-spectrum monotherapy in selected low-risk patients.33,34 Ciprofloxacin should not be used as a solo agent because of its poor coverage of gram-positive organisms.2,7 Levofloxacin has better activity against gram-positive organisms but less potent antipseudomonal activity than does ciprofloxacin, and it appears to be a reasonable alternative to combination oral empirical therapy in low-risk patients.50
An outpatient treatment course with oral or intravenous (IV) antibiotics may be considered after a brief inpatient stay, during which IV therapy is initiated, fulminant infection is excluded, the patient is deemed to be clinically stable and at low risk for complications, assessment of family support is completed, and the status of initial culture specimens may be ascertained.2,26,31,35 In one large series, oral outpatient treatment for low-risk fever and neutropenia was deemed to be successful in 80% of patients, with 20% of patients requiring readmission to the hospital, primarily for persistent fever. Factors predicting readmission included age greater than 70 years, grade of mucositis greater than 2, poor performance status, and ANC less than 100 cells/mm3 at the outset of fever.51
Use of Vancomycin or Other Gram-Positive Agents
Vancomycin or other agents with potent activity against drug-resistant gram-positive pathogens are not recommended for routine inclusion in the initial empirical regimen. Although a predominance of gram-positive pathogens is isolated in blood cultures from febrile neutropenic patients, these organisms rarely cause life-threatening infections.52 A large, prospective, randomized trial from the European Organization for Research and Treatment of Cancer failed to show a clinical advantage for using a regimen containing vancomycin compared with one that did not contain vancomycin in adults with fever and neutropenia.53 Widespread use and prolonged courses of vancomycin are linked to the emergence of vancomycin-resistant organisms, especially enterococci. To limit the spread of vancomycin resistance, the Centers for Disease Control and Prevention has issued guidelines to limit vancomycin use.54 These guidelines suggest specific indications for vancomycin therapy in febrile, neutropenic patients with cancer (Table 36-3). Other agents active against gram-positive pathogens include daptomycin, quinupristin-dalfopristin, ceftaroline, and linezolid. Use of these drugs should be similarly limited to distinct indications, rather than broadly applied. Caution is advised when prescribing these agents because each has significant adverse effects; of note, linezolid may cause myelosuppression when used for a prolonged course. Myelotoxicity has been associated with prolonged linezolid use, primarily in the form of thrombocytopenia and occasionally neutropenia.55 However, an analysis of hematologic parameters after stem cell transplantation revealed no delay in the times to neutrophil or platelet engraftment between control patients and those receiving linezolid for a median of 16 days.56
Table 36-3
Appropriate Use of Gram-Positive Active Antibiotics in the Neutropenic Patient with Cancer
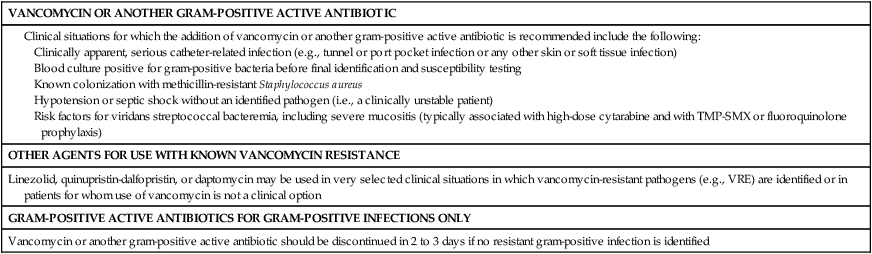
TMP-SMX, Trimethoprim-sulfamethoxazole; VRE, vancomycin-resistant enterococci.
Documented serious infections that are likely to be caused by gram-positive pathogens, such as cellulitis or obvious catheter-related infections, should be treated with vancomycin or another gram-positive active agent. Many catheter-related bloodstream infections are caused by coagulase-negative staphylococcal isolates with high-level beta-lactam antibiotic resistance, and thus vancomycin is indicated. Methicillin-resistant S. aureus (MRSA) has emerged as a major pathogen in hospitalized patients.57 If a neutropenic patient is known to be colonized with MRSA, it is reasonable to add vancomycin to the initial regimen until blood and other cultures are proved negative for this organism. Studies are lacking to confirm whether this strategy is effective. If vancomycin is started empirically for concerns about drug-resistant gram-positive infections but cultures and examination do not identify such an organisms within 2 or 3 days, then empirical vancomycin therapy should be discontinued.2,26,54,58
Substantial mucosal damage increases risk for infection with viridans streptococci, of which 18% to 29% of are beta-lactam resistant.59–62 High-dose cytarabine or intensive therapy that damages oropharyngeal mucosal barriers has been associated with an increased risk of such overwhelming sepsis due to viridans streptococcal infections. Prophylaxis with ciprofloxacin or trimethoprim-sulfamethoxazole (TMP-SMX) also has been associated with an increased risk of gram-positive infections, particularly those due to viridans streptococci.47,59–62 Viridans streptococcal breakthrough infections also have been observed with levofloxacin prophylaxis.61,62
Initial Empirical Therapy for Patients Who Are Clinically Unstable
Hypotension or septic shock in a neutropenic patient necessitates very broad empirical antibiotic coverage. Broad coverage is warranted in view of the high mortality rate in neutropenic patients with the systemic inflammatory response syndrome.2,26 Patients should receive multidrug therapy with an antipseudomonal beta-lactam (e.g., cephalosporin, carbapenem, or penicillin, depending on local susceptibilities) plus an aminoglycoside or ciprofloxacin (if the patient is not already receiving fluoroquinolone prophylaxis) and vancomycin or another gram-positive active agent with MRSA activity until culture results are available.
Subsequent Modifications of Empiric Antibiotic Regimens
No empirical antibiotic regimen initially administered for fever and neutropenia can be expected to cover all possible infections that may occur during the course of neutropenia. Therefore modifications of the initial regimen—that is, additions or changes of antimicrobial agents—sometimes are required. An important consideration in the management of cancer-related infection is that the mean time to defervescence for febrile patients with neutropenia who receive appropriate initial antibiotic therapy ranges from 2 to 7 days.2,5–7 Therefore at least 3 to 4 days of the initial antibiotic regimen should be given to otherwise stable patients, regardless of continued fever, to determine effectiveness. For patients with a documented infection, it is important to note that tissue-based infections such as pneumonia may take longer to respond to antimicrobial therapy.7
Patients who have a fever that persists beyond 4 days of initial antimicrobial therapy and who do not have an identifiable site or source of infection should undergo reassessment of their initial antimicrobial therapy. Any change in antimicrobial agents should be based on the patient’s clinical status, results of examination and cultures, and also the likelihood of early marrow recovery. Although resolution of fever may be slow, persistent fever may suggest a nonbacterial infection, a bacterial infection that is resistant to empirical antibiotics, the emergence of a secondary infection, a closed-space infection, inadequate antimicrobial serum levels, or drug fever. A careful search for these etiologic conditions should be conducted. Frequent and arbitrary antibiotic changes for persistent fever in an otherwise stable patient are to be discouraged. The clinically stable patient with persistent fever may be safely watched without altering the initial antibacterial therapy. If vancomycin was started earlier, it should be discontinued if the patient does not meet the criteria for its use.2,26,54 The addition of vancomycin without specific indications in a blind effort to suppress persistent fever has not been shown to be effective.58 If the fever persists beyond 4 to 7 days, initiation of empirical antifungal therapy should be considered (see the next section on Empiric Antifungal Treatment).
Clinically unstable patients who are persistently febrile should be evaluated for changes in antibacterial antibiotics. Double gram-negative bacillary coverage, as reviewed earlier, is recommended. The addition of vancomycin should also be considered, particularly if the clinical indications outlined in Table 36-3 are present.
Empiric Antifungal Therapy
Empiric antifungal therapy traditionally is started when neutropenic patients demonstrate continued or recrudescent fever after 4 days or more of an empiric antibacterial regimen because the risk of invasive fungal disease, particularly invasive aspergillosis, increases with prolonged duration of neutropenia.63,64 The empiric use of amphotericin B in early studies during the 1970s and 1980s showed a reduction in breakthrough fungal infections.38,65,66 Since then, numerous comparative studies have demonstrated that less toxic alternatives, including liposomal amphotericin B, itraconazole, and caspofungin, are not inferior to amphotericin B desoxycholate.67–72 Voriconazole is also effective in reducing breakthrough fungal infections.73 The other available echinocandins—anidulafungin and micafungin—are less well studied but presumably are effective in this setting. Itraconazole has been demonstrated to have efficacy in this setting as well, but concerns about liver and cardiac toxicities have discouraged use of this drug.67,72
Whether all patients with persistent fever require empiric antifungal therapy, and when it should be started, are subjects of long-standing debate. Invasive fungal infections are now relatively infrequent during neutropenia with the advent of more effective antifungal prophylaxis and the use of colony-stimulating factors to abbreviate duration of neutropenia. Furthermore, fever is obviously a nonspecific surrogate marker for fungal infection. Currently, guidelines suggest that an antifungal agent be started at day 4 to 7 in high-risk patients who are not receiving antifungal prophylaxis.2,26 Candidemia is the greatest concern in these patients, and fluconazole, amphotericin B preparation, caspofungin, itraconazole, or voriconazole are considered acceptable choices. If the patient is already receiving fluconazole prophylaxis (which is commonly the case), certain non-albicans species of Candida, including C. glabrata and C. krusei, along with mold infections such as invasive aspergillosis, must be considered. Fluconazole has reduced activity against these Candida species and lacks activity against molds. At present, no data are available to guide the use of empirical antifungal therapy in patients already receiving antimold prophylaxis, such as with voriconazole, posaconazole, or an echinocandin. In all cases, symptoms and signs of invasive fungal pneumonia or sinusitis should be sought; the workup may include computed tomography (CT) scans of chest or sinuses (or both), with follow-up nasal endoscopy or bronchoalveolar lavage as indicated. Biopsy and culture of any suspected lesions should be pursued aggressively to make a definitive mycologic diagnosis that can guide therapy.
A CT scan of the chest is increasingly being used to evaluate the possibility of invasive aspergillosis in patients with prolonged fever and neutropenia; in one study, 90% of persons with proven active disease showed radiographic abnormalities by CT.74 Macronodules with or without a halo sign are characteristic of invasive aspergillosis, with the halo sign being an especially important early clue.75,76 Other manifestations include nodular, wedge-shaped, peripheral, multiple, and cavitary lesions. An air-crescent sign generally is a late finding. Initiation of antifungal agents on the basis of finding a halo sign has been associated with significantly better response to therapy and improved survival.76,77 Maertens and colleagues78 used intensive monitoring for clinical symptoms and signs, serial galactomannan testing (discussed later in this section), and early CT scanning to identify a group of patients with persistent fever while receiving fluconazole prophylaxis in whom antimold therapy did not appear to be required. These data, as well as increasing clinical experience, have led some experts to suggest withholding empiric antifungal therapy if the patient is clinically stable and has no clinical or chest CT scan signs of fungal infection, if no Candida or Aspergillus organisms are recovered from any site, and if neutrophil recovery is imminent. A high-resolution chest CT scan may be a useful screening tool in patients with persistent fever and neutropenia; any abnormalities should be investigated with nasal endoscopy or bronchoalveolar lavage; biopsy and culture of any suspected lesions should be pursued aggressively to make a definitive diagnosis; and treatment with an antimold agent should be initiated.
The serum galactomannan assay specifically detects Aspergillus organisms but not other common fungal pathogens.79 Sensitivity varies widely in different patient populations. A recent metaanalysis reported sensitivities of 64% to 78% and specificities of 81% to 95% in patients with neutropenia or neutrophil dysfunction.80 Many conditions affect the performance of the galactomannan assay, especially the use of concomitant piperacillin-tazobactam (false-positive) or antimold antifungal agents (false-negative). Although prospective serial galactomannan monitoring may be useful for patients at high risk for Aspergillus infections, routine use in low-risk patients is not advised.79,80 Furthermore, a single negative test result is of little value in ruling out invasive aspergillosis, although a positive result in concert with abnormalities on the chest CT scan makes the diagnosis highly probable. Galactomannan testing of bronchoalveolar lavage fluid appears to be specific and more sensitive than culture for identifying invasive aspergillosis.79,81,82
Duration of Antibiotic Therapy
It generally is recommended that empiric antibiotic therapy continue until recovery of the ANC to more than 500 neutrophilic cells/mm3 on one occasion, as long as the patient is clinically stable. In a stable patient who remains febrile despite the return of higher counts, it usually is safe to discontinue antimicrobial drugs and look for a source of fever. In patients who are afebrile and clinically stable but have continued neutropenia, some clinicians recommend a 2-week course of antibiotics at a minimum and then stopping the antibiotics, with close observation.2,26
Documented infections should be treated for a minimum duration of 7 to 14 days with an antibiotic regimen that is narrowed and directed toward any organisms that have been documented microbiologically. Activity against Pseudomonas aeruginosa should be maintained in most patients who remain neutropenic. Most experts prefer to continue antibiotics at least until the ANC returns to more than 500 cells/mm3. A longer course is given if clinically necessary (for example, if a documented infection is ongoing) regardless of neutrophil recovery. Persistent fever of unknown source is not a reason to continue empiric antibiotics beyond ANC recovery.2,26
For low-risk patients who typically have brief periods of neutropenia, some groups of investigators have examined the practice of stopping antibiotics before the ANC reaches 500 cells/mm3. With a clear and steady increase in the ANC or, alternatively, in the absolute phagocyte count (antigen presenting cells = polymorphonuclear neutrophils + bands + monocytes), it appears that the discontinuation of antibiotics before a count of 500 neutrophilic cells/mm3 is achieved can be safe as long as no complicating comorbid conditions are present.51,83–87
Adjunctive Therapies
Hematopoietic Growth Factors
Prophylactic use of hemopoietic growth factors is common in the setting of intensive chemotherapy regimens such as stem cell transplantation. Treatment of fever or infection with growth factors, however, is not standard practice. No consistent benefit has been demonstrated in terms of morbidity (i.e., duration of fever and use of antimicrobial agents) or mortality among several randomized controlled trials of the addition of growth factors to an antibiotic regimen at the time of fever during neutropenia.90–90 The routine use of growth factors in treating febrile neutropenic patients is discouraged. Therapy with colony-stimulating factors, however, could be considered for severely neutropenic patients who have serious uncontrolled infections such as bacterial sepsis, pneumonia, severe cellulitis or sinusitis, systemic fungal infections, hypotensive episodes, or multiorgan dysfunction as a result of sepsis. Prophylactic use of the myeloid growth factors is recommended in patients at greater than a 20% risk of febrile neutropenia and in patients with important factors that increase the individual risk of neutropenic complications.90
Granulocyte Transfusions
Granulocyte transfusion may potentially increase the response to antimicrobial therapy in selected cases of uncontrolled fungal or gram-negative infection during prolonged neutropenia. Granulocyte colony-stimulating factor use in donors has increased the granulocyte yield approximately fourfold. Multiple recent studies have shown that granulocyte transfusions can be helpful in controlling the progression of severe infections despite the use of appropriate antibiotics, with a response rate of 40% to 80%.91 Variability in results is a consequence of patient characteristics. This benefit is limited to a select patient population, because the incidence of prolonged yet reversible neutropenia is relatively low. Rare adverse events associated with granulocyte transfusions include transmission of human CMV, alloimmunization associated with fever, graft-versus-host reactions if granulocytes are not irradiated, and progressive platelet refractoriness.
Infections in the Patient with Cancer
Bacteremia
Bloodstream infections occur in about 10% to 20% of febrile patients during neutropenia, most often when neutrophil counts are less than 100 cells/mm3.2,26,45–47,92 The risk of bacteremia is related to the depth of neutropenia, the presence of gastrointestinal mucositis or of an indwelling catheter, or the concomitant presence of a specific site of infection such as pneumonia or soft tissue process. Most bacteremias are due to gram-positive organisms, with coagulase-negative staphylococci and streptococci predominating. Gram-negative pathogens, including Escherichia coli, Enterobacter, Klebsiella, and P. aeruginosa, are important albeit less frequently occurring pathogens.16
A classification system for bacteremias in febrile neutropenic patients has been developed by Elting and colleagues,7 based on the size and presence of associated tissue involvement. Complex bacteremias were those associated with deep-tissue infections of the lung, the liver or spleen, the kidney, the colon, bone and joints, veins and the heart, meninges, and soft tissues with necrosis, or affected skin or soft tissue, wounds, or cellulitis greater than 5 cm in diameter. Simple bacteremias were associated with less tissue involvement (e.g., bacteruria, otitis, pharyngitis, and an affected area of soft tissue less than 5 cm in diameter). The prognostic significance of a complex infection associated with bacteremia on survival was dramatic. At 21 days, 20% of patients with complex infections were dead, compared with only 5% of patients with simple bacteremias (P < .0001). Profoundly neutropenic patients with simple bacteremias had a much higher response rate to antibiotics than was noted in patients with complex bacteremias (94% vs. 70%; P < .0001). The median time to defervescence for patients with simple bacteremias was half that observed for patients with complex bacteremias (2.5 days vs. 5.3 days; P < .0001).
With increasing incidence of MRSA in hospitals and the community, it is recommended that vancomycin be added to standard empirical antibiotic coverage if gram-positive cocci in clusters are isolated from a blood culture, pending identification.16,48,52–54,57 Vancomycin-resistant enterococcus (VRE) also is common in high-risk cancer patients at some institutions. If gram-positive cocci in pairs and chains are identified in blood cultures in settings where VRE is prevalent, then empiric addition of an antimicrobial active against VRE (e.g., linezolid or daptomycin) is prudent, pending definite identification and susceptibility profile. Gram-negative bacteremias in the neutropenic patient are increasingly prevalent in some institutions and are associated with high mortality unless treated promptly and with appropriate antibiotics.95–95 A variety of resistance mechanisms are evolving among gram-negative organisms. Therefore if gram-negative rods are isolated in blood cultures during fever and neutropenia, an aminoglycoside (which is preferred if the patient was heavily pretreated with fluoroquinolones) or a fluoroquinolone should be added to empiric therapy regimens to ensure coverage of resistant strains until a full susceptibility report is available.
Pulmonary Infections
Pulmonary infections are associated with the greatest morbidity and mortality, even when a specific pathogen is not identified. Approximately 25% of all febrile episodes in the neutropenic patient, and 50% of documented infections, are pneumonias.98–98 Numerous infectious and noninfectious causes of pulmonary infiltrates are found in patients with cancer who have fever and neutropenia. Noninfectious causes include the underlying disease itself, radiation therapy, drug toxicity, pulmonary hemorrhage, and leukostasis. Infectious involvement of the lungs may be caused by bacteria (pneumococci, members of Enterobacteriaceae, Pseudomonas spp., and Legionella spp.), fungi (Candida, Aspergillus, and Fusarium spp.), viruses (influenza virus, parainfluenza virus, respiratory syncytial virus [RSV], CMV, and HSV), and protozoa (Toxoplasma, Cryptococcus, and Strongyloides).
Auscultatory abnormalities in the chest may be minimal or absent, and the chest radiograph appears normal at the onset of clinical manifestations in 30% of neutropenic patients with subsequently identified pneumonia.97 A CT scan of the chest is essential to better define a pulmonary process. Neutropenia will decrease the diagnostic advantage normally provided by the sputum examination, because purulent sputum production is rare in this setting. Bronchoalveolar lavage may increase the diagnostic yield, especially for P. jiroveci and viruses, but less so for bacteria once antibiotics are started.99 The yield for molds such as Aspergillus with this procedure has been mostly in the range of 50% for suspected cases, and thus a negative result on bronchoalveolar lavage sampling does not rule out an invasive fungal process. PCR assay or galactomannan testing of lavage fluid may increase these yields.7,79,81,82,92 If the clinical picture, radiographic appearance, and findings on lavage fluid analysis are nondiagnostic, a thoracoscopic or CT-guided lung biopsy should be considered if the patient’s platelet count is adequate.
The radiographic appearance of a pulmonary infiltrate may give clues to the etiology (Table 36-4).74–77,98 Focal lesions are suggestive of bacterial etiology and should be treated accordingly with broad-spectrum antibiotics. Nodular lesions, particularly those with surrounding areas with a ground-glass appearance (i.e., halo lesions), are suggestive of mold infection, such as with Aspergillus. This presentation is of particular concern in the two patient groups that are at particularly high risk for mold infection: patients with graft-versus-host disease (GVHD) taking high-dose steroids and patients with prolonged neutropenia, such as those undergoing induction treatment for acute myelogenous leukemia. In these types of patients, even minimal symptoms of low-grade fever, pleuritic chest pain, or nasal stuffiness should prompt an immediate CT scan of the chest and sinuses to look for signs of invasive aspergillosis.
Table 36-4
Pulmonary Infiltrates and Their Association with Specific Infectious and Noninfectious Disorders
| Radiologic Sign | Potential Etiologic Disorder(s) |
| Interstitial infiltrates | Pulmonary edema |
| Diffuse alveolar damage |
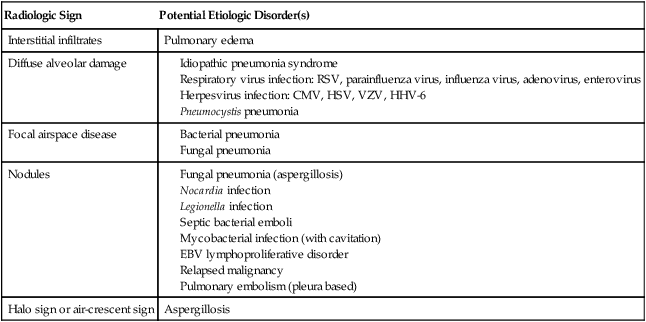
A diffuse interstitial picture is suggestive of a noninfectious etiology such as pulmonary edema or diffuse alveolar damage, or it may represent an atypical pneumonia, such as that due to Legionella, community respiratory viruses (e.g., parainfluenza virus, RSV, or influenza virus), Mycoplasma, or opportunistic infections such as P. jiroveci and CMV infection in the susceptible host (discussed later in this chapter). The addition of a fluoroquinolone or a macrolide will cover most atypical bacterial pathogens. For pneumocystis pneumonia, TMP-SMX at high doses (15 to 20 mg/kg per day) is standard treatment. CMV pneumonia requires initial treatment with intravenous ganciclovir plus administration of intravenous immunoglobulin as an adjuvant treatment.100
Pneumonia in a patient undergoing treatment for cancer is considered a health care–associated process. Accordingly, the empirical antimicrobial regimen is directed toward a range of pathogens. Current guidelines from the American Thoracic Society specify a combination of an antipseudomonal beta-lactam agent (cefepime, ceftazidime, a carbapenem, or piperacillin-tazobactam) plus vancomycin or linezolid plus an antipseudomonal fluoroquinolone or aminoglycoside.101
Fungal Infections
Fungal infections are due to yeast or mold pathogens. Candida organisms typically may colonize the gastrointestinal tract and are the most common yeast pathogens in patients with cancer. The spectrum of disease caused by Candida spp. ranges from mild mucocutaneous lesions (e.g., thrush) to disseminated deep tissue involvement (e.g., hepatosplenic candidiasis). An episode of candidemia probably precedes all tissue-invasive infections. Not all candidemias are clinically or microbiologically detected, however, and end organ disease may be the first manifestation of invasive candidiasis. Blood cultures lack sensitivity for Candida and are positive in only perhaps 50% to 70% of the patients with invasive disease.79,102 PCR of fungal genetic material and metabolite or fungal cell wall component detection methods are rapidly being developed for clinical laboratory use and will be much more sensitive.79
Neutropenia, the presence of an indwelling venous catheter, and chemotherapy-associated gastrointestinal mucosal injury are important risk factors for invasive candidiasis, rendering patients with cancer at especially high risk. Despite the introduction of newer antifungal agents, recent data showed that the Candida-related overall 30-day crude mortality rate was 38% and the attributable mortality rate was 19% in patients with a hematologic malignancy—similar to older rates.103 Because of the difficulty in diagnosing this infection and the associated high mortality, any blood culture found to grow even a single colony of Candida must be regarded as a sign of true infection. Every patient with Candida isolated from the bloodstream should receive at least a 2-week course of antifungal therapy, and in most cases, any indwelling catheters should be removed. Clinical signs of candidemia may range from fever alone to fulminant sepsis. Pustular skin lesions may be a first sign of candidemia. C. albicans accounts for about half of the cases, but non-albicans species, especially C. glabrata and C. krusei, are increasing in incidence. C. glabrata is associated with the highest mortality in patients with cancer. Of note, C. glabrata and C. krusei are somewhat resistant and fully resistant, respectively, to fluconazole and appear to be linked to increased mortality in patients with cancer.103,104
Catheter-related candidemia should be treated with antifungal therapy, and the catheter should be removed in most cases.28,104,105 Although some studies advocate leaving the catheter in place if the source of candidemia is thought to be the gastrointestinal tract, a recent metaanalysis (n > 1900 patients) indicates that catheter removal is associated with decreased mortality.106
The availability of echinocandins (e.g., caspofungin, micafungin, and anidulafungin) and newer generation triazoles (e.g., posaconazole and voriconazole), combined with increasing prophylactic use of antifungal agents in certain populations (e.g., those with acute leukemia or GVHD) and the emergence of resistant Candida species in some institutions, has changed the treatment paradigm for candidemia. Certain non-albicans species (e.g., C. glabrata and C. krusei) predictably have reduced susceptibility to fluconazole. Early empiric treatment with an echinocandin (e.g., caspofungin, micafungin, and anidulafungin) is associated with lower mortality.106 Candidemia that develops during prophylaxis with an azole should be treated with an echinocandin or a amphotericin B preparation. Complications of fungemia may include endocarditis, endophthalmitis, vertebral osteomyelitis, and hepatosplenic candidiasis.106
Hepatosplenic candidiasis is a form of chronic disseminated candidiasis that almost exclusively affects patients undergoing leukemia induction or stem cell transplantation.15,106–109 It generally manifests only on recovery from neutropenia, as persistent fevers unresponsive to antibacterial agents. Patients may have right upper quadrant tenderness and demonstrate a variety of other gastrointestinal signs and symptoms. Consistent laboratory findings include marked elevation of alkaline phosphatase, with normal or mildly elevated bilirubin and transaminases and a rebound leukocytosis after neutrophil recovery. Blood cultures are almost always negative for fungal growth, as are liver biopsy specimens. Suspected hepatosplenic candidiasis is confirmed by the presence of multiple characteristic well-defined lesions in the liver or spleen, and occasionally in the kidneys on CT scan or ultrasound examination. A prolonged course of therapy, initially with an amphotericin B formulation or an echinocandin, then with oral fluconazole for a number of months, has been advocated. For patients with acute leukemia and hepatosplenic candidiasis, repeated cycles of chemotherapy may be given once the infection is stabilized, and the antifungal agents are continued through the courses of cytotoxic therapy.
Aspergillus spp. are the most common mold pathogens in patients with cancer, but they are seen almost exclusively in two specific settings: during periods of prolonged neutropenia or in patients with GVHD after allogeneic transplantation.110 As discussed earlier (in the Pulmonary Infections section), the vast majority of Aspergillus and other mold infections occur in the lung, whereas fewer organisms disseminate to sinuses, skin, or abdominal organs. Dissemination to the brain occurs in approximately 5% of patients. Unfortunately, the sensitivity of diagnostic tests used to diagnose invasive aspergillosis remains very poor, and delay in diagnosis is all too common, contributing to poor outcome.110 The CT scan has become an essential diagnostic tool for earlier diagnosis of invasive aspergillosis, with characteristic lesions such as nodules, the halo sign, and the crescent sign having high predictive value for the disease.74,76,77 Despite some improvements in antifungal therapy in recent years, invasive aspergillosis is still associated with at least a 50% mortality rate in patients with cancer.110 Voriconazole has been shown to be superior to amphotericin B for the treatment of proven or probable invasive aspergillosis, and it is now the agent of choice.2,26,111
On the basis of a few small retrospective studies, combination antifungal treatment appears to be safe and has promising efficacy.111,112 Preliminary results from a randomized multicenter study comparing voriconazole to voriconazole combined with anidulafungin in stem cell transplant and hematology patients with proven or probable invasive aspergillosis demonstrated no statistically significant difference in 6-week survival, but a trend favoring the combination arm was found.113 More definitive clarification of the efficacy of this combination awaits final publication of the results of this study. The best course in patients who do not respond to first-line therapy is unknown; one open-label trial suggested that oral posaconazole was associated with a 42% response rate in patients who are refractory to or intolerant of conventional therapy for Aspergillus infection, although this response rate is not better than results seen with voriconazole.114
Fusarium is notable for causing tender, red skin nodules and positive blood cultures for mold in approximately half of the cases of this infection.115 Infection with any of the Zygomycetes (Mucor, Rhizopus, and Rhizomucor) is characterized by later onset (after day 90 of the transplant) and a longer median duration of survival. Sinus disease with erosion through tissue planes is a feature of Zygomycetes infections but also may occur with Aspergillus infection. The mortality rate is approximately 80% for transplant recipients with proven infection. In up to 60% of persons who have undergone colonization, progression to invasive infection will occur.116 Other less common molds, including Fusarium, Scedosporium/Pseudallescheria, and Trichosporon, appear to be more susceptible to voriconazole than to amphotericin B. Of note, however, one report recently indicated successful treatment of Fusarium infection with a combination of both agents, and many centers use dual therapy for serious Fusarium infection in high-risk patients.117
An increased incidence of infection with voriconazole-resistant molds such as Rhizopus or Mucor species has been described. This increase may to be related to “breakthrough” infections from voriconazole prophylaxis in high-risk patients with cancer.118,119 Amphotericin B products have been standard therapy for infections due to these pathogens, but the triazole posaconazole has potent in vitro activity against the Zygomycetes and has quickly become an important drug in battling infections with these molds.120 It is available only in an oral formulation and has significantly less toxicity than that observed with amphotericin products. Posaconazole has not been evaluated as a first-line agent for treatment of zygomycoses, but reports of posaconazole for salvage therapy are encouraging, and it is considered the second-line agent of choice.120 Patients in whom pulmonary lesions with radiologic characteristics suggestive of mold infection (e.g., the halo sign, as noted earlier) develop despite voriconazole prophylaxis should receive treatment for zygomycoses with amphotericin B or posaconazole pending microbiological or histologic diagnosis. Aggressive and repeated surgical débridement is a critically important adjunct to the treatment of zygomycoses of the sinuses.116
Gastrointestinal Infections
Upper Gastrointestinal Tract
Lower Gastrointestinal Tract
Enteritis, clinically manifested by diarrhea, is common in patients with cancer. Diarrhea may be due to chemotherapy-induced or infection-induced mucositis. C. difficile is the most common pathogen to cause diarrhea in patients with cancer. During the past decade, the emergence of an epidemic strain of C. difficile has resulted in a dramatic increase in the incidence and severity of C. difficile infection at many centers.121,122 Hematopoietic stem cell transplant recipients are at particularly high risk for C. difficile–associated disease in the early period after transplantation. The epidemic strain is resistant to fluoroquinolones, and use of this class of antibiotics has been implicated as a risk factor in institutional outbreaks.123 Consequently, fluoroquinolones for antibacterial prophylaxis in high-risk patients are likely to contribute to the development of C. difficile–associated disease.124 Because of an increased rate of failure of oral metronidazole noted in recent studies, guidelines recommend oral vancomycin for patients with evidence of severe disease (e.g., a white blood cell count greater than 15,000 cell/µL, shock, renal failure, and the need for transfer to the intensive care unit), combined with intravenous metronidazole.125 For patients unable to tolerate oral therapy (because of ileus or toxic megacolon, for example), intravenous metronidazole and vancomycin administered by nasogastric tube or by enema should be considered.125 Colectomy may be necessary in seriously ill, nonresponding patients. Although no proven approach is recognized, a slowly tapering course of oral vancomycin or metronidazole over 6 weeks often is used for patients with multiple relapses. Intestinal microbiota therapy (“stool transplant”) appears to be the most effective option for treating relapse, although experience in patients with cancer is limited.126,127 Outpatients must be alerted to the possibility of recurrence so that retreatment can be instituted quickly. Recurrences may be decreased by primary treatment with fidaxomicin, compared with vancomycin. Prolonged tapering courses of oral vancomycin are often used to treat frequently recurrent C. difficile–associated disease.125,127
Other bacterial causes of diarrhea are uncommon in patients with cancer unless exposure to such pathogens has occurred. CMV infection can cause protracted or hemorrhagic diarrhea, especially in children or in patients undergoing allogeneic hematopoietic stem cell transplant. Adenovirus may cause severe diarrhea in allogeneic transplant recipients, and stool culture techniques and plasma PCR assay aid in diagnosis.128
Typhlitis, also known as neutropenic enterocolitis, is a unique and potentially life-threatening syndrome occurring in febrile neutropenic patients, particularly those with leukemia or who have had intensive cytotoxic therapy.131–131 Abdominal pain, especially in the right lower quadrant, often with attendant rebound tenderness, and decreased bowel sounds, fever, and diarrhea are typical presenting features. Typhlitis may be limited to the cecum but can involve the entire intestine. A CT scan is the diagnostic study of choice and usually demonstrates thickening of the bowel wall, sometimes with pneumatosis coli.131 C. septicum, P. aeruginosa, enteric gram-negative organisms, and anaerobes are the most common pathogens associated with this syndrome. C. difficile occasionally is associated with typhlitis, and thus treatment for C. difficile infection (i.e., with oral vancomycin or metronidazole) should be included in the initial antibiotic regimen for typhlitis, along with a broad-spectrum intravenous antimicrobial cocktail. Severe sepsis, bowel perforation, and hemorrhage may accompany or follow typhlitis. Therapy consists of nasogastric suction, bowel rest, intravenous fluids, and broad-spectrum antibiotics to cover gram-negative organisms and anaerobes (e.g., piperacillin/tazobactam, a carbapenem). Antifungal agents also should be part of the initial treatment regimen for typhlitis. In approximately 5% of patients with typhlitis, complications develop that require surgical intervention, including uncontrolled sepsis, lower gastrointestinal tract bleeding, and perforation.130
Central Nervous System Infections
In the patient with cancer who demonstrates new mental status changes, focal neurologic signs, or neck stiffness, evaluation for central nervous system (CNS) infection is necessary, because infection is a common cause of neurologic complications.132,133 The initial evaluation begins with cerebral magnetic resonance imaging or contrast-enhanced CT, followed immediately by lumbar puncture if no contraindication exists. Empiric treatment with antibacterial agents with adequate CNS penetration, such as cefepime or meropenem at high doses, is indicated. Of note, neutropenic patients may have few white cells in the cerebrospinal fluid (CSF), even in the setting of meningitis. Impaired T-cell function (e.g., corticosteroids, purine analogs, GVHD, and alemtuzumab) but not neutropenia is a risk factor for Listeria meningitis.120 Ampicillin should be added if Listeria infection is suspected in a patient with T-cell impairment. Patients with varicella-zoster virus (VZV) or HSV meningoencephalitis may have suggestive skin lesions; if such infection is suspected, then acyclovir, 10 mg/kg every 8 hours, should be added and a CSF sample sent for PCR testing for both viruses. Cryptococcal meningitis often manifests with fever and subtle mental status changes over several days, and very low CSF cell counts often are seen; serum and CSF cryptococcal antigens are sensitive diagnostic tests. False-positive test results at low titers may occur in patients with plasma cell dyscrasias. Treatment is with amphotericin B preparations combined with 5-fluorocytosine (with monitoring for thrombocytopenia, leukopenia, and diarrhea) initially. A repeat spinal fluid examination must be performed after 2 weeks of therapy to determine if the CSF is culture-negative; if it is, then the patient may be switched to oral fluconazole. Heavily immunosuppressed patients (e.g., recent recipients of an allogeneic hematopoietic stem cell transplant and patients with chronic extensive GVHD) are at risk for meningoencephalitis from reactivation of pathogens such as human herpesvirus–6 (HHV-6), EBV, or T. gondii, although such cases are very rare. CSF PCR assay for these specific herpesviruses or Toxoplasma is used to make the diagnosis; CSF culture typically is negative. Ganciclovir or foscarnet generally is used to treat HHV-6 infection. T. gondii reactivations often are rapidly fatal despite combination therapy with pyrimethamine and sulfadiazine or clindamycin treatment.134
Treatment of cerebral aspergillosis generally requires voriconazole, which attains high brain tissue levels.135 Limited information suggests that posaconazole may be an effective agent for treatment of CNS fungal infections caused by Aspergillus spp, Cryptococcus neoformans, and Pseudallescheria boydii.136 Zygomycosis of the CNS is associated with a very high mortality rate; liposomal amphotericin B is considered the best available therapy.137
Vascular Access Devices
Indwelling venous access devices are commonly required in patients with cancer for the administration of chemotherapy, blood products, and parenteral nutrition, as well as for withdrawal of blood for therapy monitoring and microbiological evaluation. Infection is a common complication of venous access devices. The risk of infection varies with the device used, the duration of placement, and the extent of the patient’s immunosuppression. Local signs and symptoms, such as erythema and tenderness, are unreliable indicators of catheter infection even in the immunocompetent patient. The evolution of these signs over time, however, is suggestive of infection. Venous access device infections are categorized as entry site infections, tunnel or pocket infections, and catheter-associated bloodstream infections.28,138 (See Chapter 26 on Establishing and Maintaining Vascular Access.)
Determining whether a bloodstream infection is related to the venous access device often is difficult, because frequently, no evidence of local catheter inflammation is seen. The concept of “differential time to positivity” has been used to distinguish venous access device–related infections from other types of infection, as follows: If the times at which blood cultures become positive (by machine detection in the clinical microbiology laboratory) are more than 2 hours apart for simultaneously obtained catheter and peripheral vein blood cultures, the catheter is then strongly implicated as the source of infection.138 Although this differentiation may help determine whether a catheter can be retained or must be removed, most indwelling catheter–related infections will respond to antimicrobial therapy alone, without catheter removal. Certain exceptions are notable: Catheter removal is advisable for patients with catheters as the likely source of bloodstream infections caused by fungi (yeasts and molds) and nontuberculous mycobacteria (e.g., M. chelonae, M. fortuitum, and M. abscessus). Because S. aureus bacteremia is more likely to result in metastatic seeding and endocarditis, guidelines also suggest catheter removal for patients with S. aureus bacteremia.28 The value of transesophageal echocardiography in the setting of any S. aureus bloodstream infections has been well demonstrated to determine duration of therapy.139 Patients with S. aureus bacteremia of brief duration with no evidence of endocarditis by transesophageal echocardiography, no metastatic seeding, and prompt catheter removal may receive a short course (e.g., 14 days) of treatment. Patients who do not meet those criteria should receive 4 to 6 weeks of treatment.28 For other bacteria, the decision concerning the need for catheter removal will depend on the severity of the clinical picture, the degree of immunosuppression, and the availability of an alternative vascular access site in a given patient. In general, if blood cultures remain positive despite appropriate antimicrobial therapy for more than 48 hours, or if the patient is clinically unstable, the catheter should be removed independent of the etiology.
Viral Infections
The incidence of HSV reactivation infections has been reduced with the widespread use of acyclovir prophylaxis. Reactivations not involving the CNS are treated with lower doses of intravenous acyclovir (e.g., 5 mg/kg every 8 hours) or similar drugs given orally, whereas VZV infections are treated with higher doses (e.g., intravenous acyclovir, 10 mg/kg every 8 hours, or oral valacyclovir, 1000 mg every 8 hours). Acyclovir resistance is very uncommon among patients with cancer.140 HSV infections that break through acyclovir prophylaxis, however, should be considered to be acyclovir-resistant until viral sensitivity testing can be performed, and consideration should be given to initiating treatment with foscarnet or cidofovir. Reports of acyclovir-resistant varicella are extremely rare.141
Patients at highest risk of CMV reactivation include recipients of allogeneic bone marrow transplants in the early period after engraftment (i.e., days 30 to 100 after transplantation), during which time reactivation will develop in 60% of seropositive patients.142 In addition, patients receiving alemtuzumab and those receiving high-dose corticosteroids for GVHD are at high risk. Universal prophylaxis and preemptive strategies using ganciclovir or valganciclovir have markedly reduced the incidence of CMV disease in these patient groups (see the following discussion on CMV prevention).143
End-organ CMV disease most commonly involves the lungs, gastrointestinal tract, or liver.144,145 Definitive diagnosis requires histopathological evidence, but compatible clinical findings (e.g., colonic ulcers and an interstitial pattern on chest radiography) in the setting of CMV viremia diagnosed by PCR or antigen testing is suggestive of invasive disease. CMV treatment is initiated with induction doses of the antiviral agent for at least 2 weeks, followed by a variable course of maintenance dosing, which is half the induction dose. The total duration of treatment depends on clinical response, the results of viremia or antigenemia assays that reflect viral replication activity, and the patient’s overall state of immunosuppression. Intravenous ganciclovir (i.e., an induction dose of 5 mg/kg every 12 hours and a maintenance dose of 5 mg/kg once daily) or oral valganciclovir if absorption is not an issue (900 mg twice daily for at least a 2-week induction and then 900 mg once daily) are first-line treatments when end-organ disease is documented. Foscarnet is a second-line option typically used when the risk of hematologic toxicity precludes the use of ganciclovir or valganciclovir. Electrolyte abnormalities, seizures, and renal dysfunction complicate the use of foscarnet. Cidofovir is a third-line option that is less effective and more toxic than foscarnet.146
When the end-organ manifestation of CMV infection is pneumonitis, many centers use intravenous immune globulin (IVIG) administered at a dose of 500 mg/kg and typically added on an every-other-day basis for the duration of induction.100,144 When CMV infection manifests in an organ other than the lungs, there is less evidence for the efficacy of IVIG, and thus it is rarely used. However, IVIG can be added if the patient’s total immunoglobulin G (IgG) level is below 400 mg/dL. CMV hyperimmune globulin (prepared from CMV seropositive recipients) has not been shown to be superior to standard IVIG in the treatment of persons with CMV disease, and studies show conflicting results regarding the anti-CMV activity of IVIG versus CMV hyperimmune globulin.16,17,100,144,147,148 A typical dose for centers choosing to use CMV hyperimmune globulin is 150 mg/kg on days 1, 4, and 11, with additional doses on day 18 and 25 in critically ill patients.
HHV-6, a virus acquired by 95% of humans during childhood, reactivates asymptomatically in up to 50% of patients after allogeneic hematopoietic stem cell transplant.149,150 Clinical disease is uncommon but may include interstitial pneumonitis, encephalitis, rash, cytopenias, and delayed engraftment.149,150 PCR assay performed on plasma, tissue biopsy material, or CSF can be useful in suggesting HHV-6–associated disease. HHV-6 has 60% DNA homology with CMV, and treatment of documented infection usually is initiated with induction doses of foscarnet or ganciclovir, although responses are variable and it is unclear which drug is more effective.151
RSV and influenza virus infections generally are seasonal, whereas parainfluenza and adenovirus infections may occur year round. Upper respiratory infections may progress to pneumonia, with mortality rates between 6.6% and 80% in heavily immunosuppressed patients.152,153 Preengraftment hematopoietic stem cell transplant recipients are likely at the highest risk of mortality from RSV infection. Inhaled ribavirin reduces RSV shedding, but clinical efficacy has not been demonstrated154; furthermore, difficulty of administration and occupational exposure issues limit use of this agent. Oral ribavirin at a dose of 600 mg three times a day is more convenient and has been safely used, but efficacy is unknown155 A neuraminidase inhibitor commonly is used to treat influenza and may prevent progression from upper respiratory infection to pneumonia, but prolonged shedding and the development of resistance may occur.156 Adenovirus may cause pneumonia, colitis, hemorrhagic cystitis, hepatitis, or disseminated disease with a sepsis-like presentation in patients undergoing allogeneic hematopoietic stem cell transplant.128,157 Asymptomatic reactivation is common in adults, but rising levels of viremia or isolation from multiple sites has been associated with an increased risk of severe disease. No standard treatment has been validated, but cidofovir (1 mg/kg three times a week or 5 mg/kg weekly) has been associated with a reduction in levels of viremia.158
Reactivation of the BK virus in the uroepithelium may lead to hemorrhagic cystitis in allogeneic transplant recipients, and a quantitative PCR assay for BK virus in the urine and plasma may be helpful both diagnostically and in assessing response to treatment.159,160 Low-dose cidofovir (1 mg/kg weekly) has been used for treatment, but its efficacy is unknown.161
Prevention of Infections in Selected Risk Groups
General guidelines for prevention of infections are shown in Table 36-5.
Table 36-5
Prophylaxis for Selected Risk Categories
| Risk Category | Bacterial | Duration | Viral* | Duration | Fungal | Duration | PCP† |
| Most solid tumors | None | — | None‡ | Neutropenic period | None | — | None |
| Acute leukemia/MDS | Fluoroquinolone | Neutropenic period | HSV, VZV | Neutropenic period | Posaconazole | Neutropenic period | Only patients with ALL |
| Autologous HSCT (neutropenic period) | None | — | HSV, VZV | Neutropenic period and 30 days after | Fluconazole | Neutropenic period | For 90-180 days after transplantation |
| Allogeneic HSCT | Penicillin | Minimum 1 yr after transplantation | HSV, VZV, CMV | HSV and VZV during neutropenia, some centers continue for up to 1 yr, CMV monitoring to day 100 minimum | Fluconazole (some centers use a mold-active agent) | 75 days after transplantation | For at least 6 mo after transplantation |
| GVHD | Penicillin | Until resolution of GVHD and off immunosuppression | HSV, VZV, CMV | Until resolution of GVHD and off immunosuppression | Posaconazole | Until off significant immunosuppression | Until resolution of GVHD |
| Alemtuzumab | None | None | HSV, VZV, CMV | At least 2 months and CD4+ ≥100 cells/µL | Consider | None | At least 2 mo and CD4+ ≥200 cells/µL |
| Purine analogs§ | None | None | HSV, VZV | At least 2 mo | None | None | At least 2 mo and CD4+ ≥200 cells/µL |
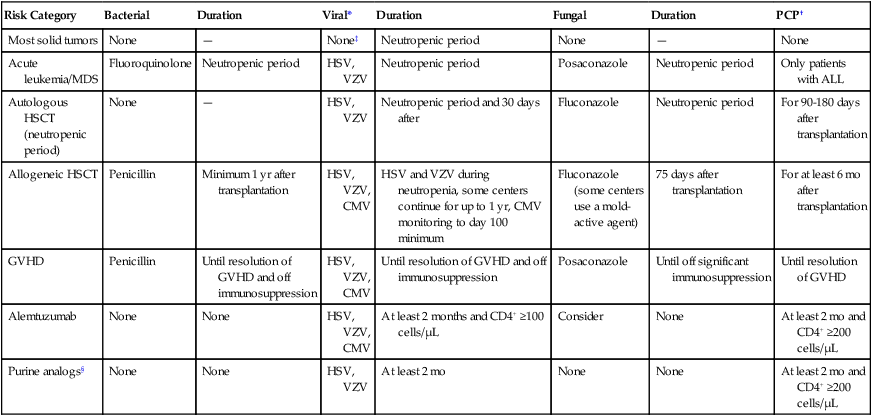
*HSV or VZV prophylaxis with acyclovir, valacyclovir, or famciclovir; see text (under Prevention of Infections in Selected Risk Groups) for CMV prophylaxis strategy.
†Agents used for PCP prophylaxis include trimethoprim-sulfamethoxazole, dapsone, atovaquone, and inhaled pentamidine.
‡HSV prophylaxis for patients with a history of HSV reactivation.
Low-Risk Patients
Patients categorized as low risk for complications while having fever and neutropenia include most patients with solid tumors. These patients are at lower risk for serious infections, and thus they do not generally require any prophylaxis against bacteria, fungi, or viruses. Although one large randomized study demonstrated that levofloxacin prophylaxis led to a statistically significant decrease in febrile episodes, hospitalizations, and possible infections in a group of largely low-risk patients (e.g., those with breast cancer, testicular cancer, or small cell lung cancer), the reductions seen were of marginal clinical significance. Consequently, this practice is discouraged by most experts.3,26,162,163 The absolute risk reduction for a first febrile episode was only 4.4%, documented infections were rare, and mortality was unaffected by prophylaxis.162 Concerns about antimicrobial resistance and an increasing incidence of severe C. difficile infections with widespread fluoroquinolone use are also reasons to limit the use of these agents. Furthermore, because fluoroquinolone-based outpatient treatment of fever during neutropenia is an option for many low-risk patients, routine fluoroquinolone prophylaxis in these patients would eliminate that attractive possibility. Routine fungal or viral prophylaxis also is not required for low-risk patients, with the exception of HSV prophylaxis in patients with a history of HSV reactivation.
Patients with Acute Leukemia
Patients undergoing induction or consolidation therapy for acute leukemia have a prolonged period of neutropenia, and the use of routine antibacterial prophylaxis during the period of neutropenia has been controversial. In a recent large trial, patients with expected duration of neutropenia longer than 7 days were randomly assigned to receive either treatment with 500 mg of levofloxacin daily or placebo. Among patients with acute leukemia, a reduction in febrile episodes, microbiologically documented infection, and bacteremia was observed with levofloxacin treatment; however, no mortality benefit was seen.164 A metaanalysis of clinical trials of fluoroquinolone prophylaxis in high-risk patients did show a survival advantage.165 The National Comprehensive Cancer Network currently recommends antibacterial prophylaxis with a fluoroquinolone (preferably levofloxacin) for patients with acute leukemia with expected duration of neutropenia of greater than 7 days.2 Potential problems associated with routine antibacterial prophylaxis include C. difficile colitis, fungal superinfections, and the development of antimicrobial resistance. In particular, when the rate of fluoroquinolone resistance in common gram-negative organisms (e.g., E. coli and Klebsiella spp.) exceeds 20%, continued application of fluoroquinolone prophylaxis may not be effective and may promote further cross-resistance, limiting the effectiveness of broad-spectrum empiric antibiotics if fever occurs.46,166
Similarly, in patients with acute leukemia, a recent trial suggested a morbidity and mortality benefit with the use of antimold prophylaxis with posaconazole. This trial, conducted in more than 600 patients with acute leukemia or myelodysplastic syndrome, randomly assigned patients to prophylaxis with itraconazole, fluconazole, or posaconazole until recovery from neutropenia and complete remission of disease were obtained.167 Patients who received treatment with posaconazole experienced significantly fewer invasive mold infections and had a survival benefit. Based on these data, posaconazole should be considered the agent of choice for antifungal prophylaxis in neutropenic patients with acute leukemia. Many centers use voriconazole for this purpose because its activity against Aspergillus spp. also is potent, but no comparative study has been performed to show equivalence of voriconazole and posaconazole for this purpose.
Patients Undergoing Autologous Hematopoietic Stem Cell Transplantation
Because of prolonged neutropenia and mucosal toxicity of the conditioning regimen, patients undergoing autologous hematopoietic stem cell transplantation are at intermediate risk of infection. Because GVHD does not occur in this population, recipients of autologous transplants experience fewer late infections than do allogeneic hematopoietic stem cell transplant recipients. Most centers use preventative protocols similar to those used for patients with acute leukemia (e.g., variable use of fungal and bacterial prophylaxis until the resolution of neutropenia). Further, because reconstitution of cellular immune function may take many months, 1 year of acyclovir (to prevent VZV reactivation or primary varicella) and 3 to 6 months of prophylaxis against PCP is recommended.143 Autologous transplant recipients also require revaccination (Table 36-6).
Table 36-6
Vaccine Schedule after Hematopoietic Stem Cell Transplantation
| Vaccine | 12 mo | 14 mo | 24 mo |
| INACTIVATED-ORGANISM VACCINES | |||
| Begin reimmunization at 1-yr anniversary visit | |||
| Diphtheria, tetanus, pertussis* | X | X | X |
| Haemophilus influenzae type b conjugate | X | X | X |
| Hepatitis B | X | X | X |
| Pneumococcal 13 valent conjugate vaccine† | |||
| Inactivated polio | X | X | X |
| Influenza | Seasonal if >6 mo after transplantation | ||
| LIVE-VIRUS VACCINES | |||
| Reimmunize at 2-yr anniversary visit if no active graft-versus-host disease or immunosuppressive therapy | |||
| Mumps-measles-rubella | X | ||
| Varicella | X | ||
*One of these three boosters should be the Tdap tetanus–diphtheria–acellular pertussis vaccine.
†First dose 3 to 6 months after transplantation followed by two doses at 2-month interval; an optional fourth dose with the polysaccharide 23 valent vaccine can be given at 12 to 18 months.
Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation
A comprehensive guideline for preventing infectious complications among hematopoietic cell transplantation recipients was recently published and is an excellent resource for practioners.143 Bacterial infections may cause more than 25% of fevers in the preengraftment phase of allogeneic hematopoietic stem cell transplant, and thus antibacterial prophylaxis (usually with a newer fluoroquinolone) is often used during this period168 (Fig. 36-2). This practice has been shown to reduce the incidence of infections, particularly those due to gram-negative organisms, in other high-risk groups (see Risk Assessment), but it has not been consistently associated with a decrease in mortality.164,165 Because of the risk of resistant organisms and C. difficile infection, some centers choose not to use routine prophylaxis for allogeneic transplant recipients.169,170 After engraftment, prophylaxis against encapsulated organisms is indicated. Penicillin prophylaxis has decreased the incidence of infection-related morbidity and mortality from Streptococcus pneumoniae, H. influenzae type b, and N. meningitidis significantly. Prophylaxis generally is discontinued 1 or 2 years after transplantation or later if immunosuppression is ongoing at the 2-year point. Prophylaxis with TMP-SMX (or atovaquone, dapsone, or aerosolized pentamidine, if the patient is intolerant to TMP-SMX) for 6 months after transplantation is indicated to prevent pneumocystis pneumonia. Prophylaxis is generally extended if GVHD develops.
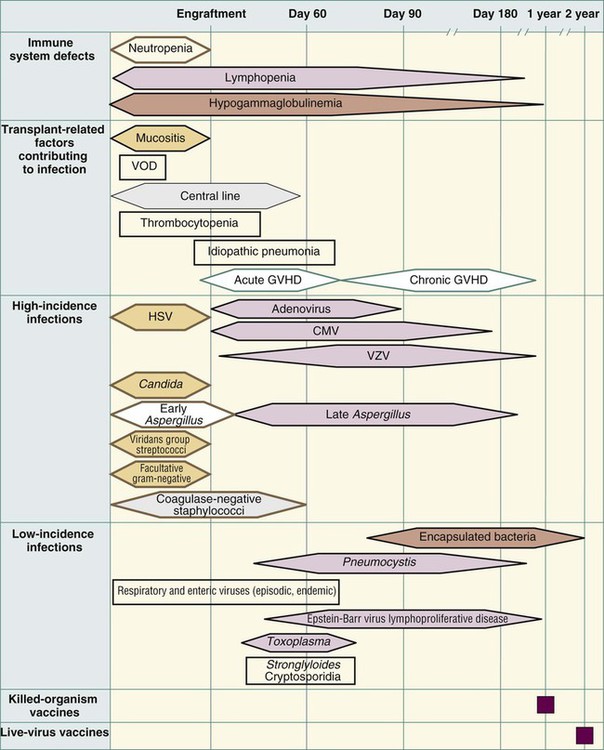
Fluconazole prophylaxis has been clearly demonstrated to prevent candidemia and hepatosplenic candidiasis among allogeneic transplant recipients, with treatment generally continuing to day 75 after transplantation.171–174 Fluconazole, however, does not have activity against yeasts such as C. glabrata (some strains) and C. krusei or against molds such as Fusarium, Aspergillus, and the Zygomycetes. Some centers use prophylaxis with an agent active against mold, such as voriconazole, in high-risk patients (i.e., mismatched or matched unrelated donor transplants). A recent trial demonstrated no statistically significant benefit of voriconazole compared with fluconazole for the prevention of fungal infections when it was administered for the first 100 days (180 days for those receiving prednisone at a dose of ≥1 mg/kg/day or for recipients of T-cell–depleted grafts with CD4+ counts <200 at day 100) after transplantation.175
Prevention of CMV disease after allogeneic hematopoietic stem cell transplantation can be effectively accomplished with use of either universal prophylaxis or preemptive therapy with ganciclovir or valganciclovir.2 The preemptive strategy uses weekly or twice monthly blood antigenemia or PCR viral load monitoring tests to guide initiation of antiviral agents, whereas universal prophylaxis involves providing the drug to all patients at risk (all but seronegative donors and recipients). In the absence of prophylaxis, CMV will reactivate in about 60% of CMV seropositive recipients142 and a much smaller percentage of CMV seronegative recipients. However, a majority of transplant recipients will initially present with CMV reactivation silently; this reactivation typically is detected by the weekly monitoring test before the development symptoms (e.g., fever) or end-organ disease. Severe end-organ disease can occur if subclinical reactivations are not treated. The preemptive strategy is preferred to universal CMV prophylaxis for two reasons: First, it decreases exposure to this marrow-toxic drug, and second, in allowing for some low-level replication of CMV, recovery of CMV-specific immunity may be enhanced. Both strategies, however, appear to shift the timing of CMV reactivations to later (after day 100, when monitoring often stops) in the postengraftment course, after it is discontinued in transplant recipients at higher risk. Strategies for preemptive therapy vary; typically, intravenous ganciclovir or oral valganciclovir is administered at induction dosing for 7 to 14 days and then reduced to maintenance dosing if a reduction in viremia or antigenemia is observed.176 Treatment may be continued for 2 to 3 weeks after a negative result on a viremia or antigenemia test, and then weekly monitoring is resumed. In most centers, weekly monitoring is continued for at least 100 days, and longer if immunosuppression for GVHD is needed. In some patients receiving intense immunosuppression to treat GVHD, CMV viremia or antigenemia may respond poorly to initial treatment. Management of these patients is difficult, but as long as induction doses are maintained, the development of end-organ disease appears to be uncommon.177
Vaccination after autologous or allogeneic hematopoietic stem cell transplant should be initiated once cellular immunity is thought to be reconstituted, which typically occurs at approximately 12 to 18 months after transplantation if GVHD does not occur.143 A full immunization series, similar to that given to young children, is recommended because posttransplant patients are considered immunologically naïve.
Because of the high risk of invasive disease with pneumococcus and evidence of serologic response when a conjugate vaccine is administered earlier, many centers begin pneumococcal vaccination 3 to 6 months after transplantation. In order to provide protection against serotypes not included in the conjugate vaccine, some centers administer a fourth dose with the polysaccharide vaccine 12 to 18 months after the initial three-shot conjugate vaccination series is completed.143,178 Some centers will check titers for response, but this approach is not standardized. Because of poor response, most centers defer influenza vaccination until at least 6 months after transplantation. Hepatitis B surface antibody should be checked after completion of the vaccine series, with revaccination using a double dose a reasonable approach for persons who do not seroconvert. Autologous transplant recipients also require revaccination, and most centers use a similar schedule as is used for allogeneic transplant recipients. Table 36-6 shows a standard immunization schedule to be used after transplantation.
Patients with Graft-versus-Host Disease
GVHD and its treatment result in significant suppression of the cell-mediated immune system and high risk of certain infections. The use of penicillin to prevent infection with encapsulated organisms (e.g., S. pneumoniae) is widely accepted.2,26,143 Invasive mold infections, particularly invasive aspergillosis, constitute a serious threat during GVHD. A large randomized trial comparing the new triazole posaconazole with fluconazole in patients with grade II or IV acute GVHD or chronic extensive GVHD demonstrated a reduction in overall breakthrough invasive fungal infections, including invasive Aspergillus infections with posaconazole.179 No effect on mortality was observed, however. On the basis of this trial, guidelines recommend posaconazole prophylaxis in patients who are undergoing immunosuppressive treatment for severe GVHD.2,26 Posaconazole currently is available only as an oral suspension that must be taken three times daily with a high-fat meal or at least a carbonated beverage, which is critical for adequate absorption of the drug. Many centers monitor levels to ensure adequate absorption, and patients undergoing acid suppression therapy may have particular difficulty achieving adequate levels. Patients with GVHD of the gut may not be able to tolerate this regimen. Voriconazole and echinocandins are potential alternatives but are not well studied for prophylaxis in this setting.
Antiviral prophylaxis with acyclovir or an equivalent drug (e.g., valacyclovir and famciclovir) is widely used to prevent HSV- or VZV-related disease. Most centers have adopted a preemptive monitoring approach, as discussed previously (see the earlier section entitled Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation), to decrease the incidence of CMV-related disease. TMP-SMX or an alternative agent for intolerant patients (e.g., dapsone, inhaled pentamidine, and atovaquone) is routinely used as prophylaxis against PCP while patients continue to undergo immunosuppression.
Prophylaxis with Other Immunosuppressive Therapies
Alemtuzumab (Campath) has been used with increasing frequency in patients with a variety of lymphoproliferative disorders, with the primary effect being prolonged suppression of cellular immunity. In one study of patients with B-cell CLL, the median time to reach a CD4+ count greater than 100 cells/µL was 4 months, and the median CD4+ count was less than 25% of baseline at 9 months.180 A high frequency of CMV reactivation and bacterial infections have been reported during alemtuzumab therapy, and opportunistic infections similar to those seen in patients with late-stage AIDS (e.g., P. jiroveci, progressive multifocal leukoencephalopathy, cryptococcosis, disseminated histoplasmosis, and cerebral toxoplasmosis) also have been described.181,182 Prophylaxis against P. jiroveci with TMP-SMX or an alternative agent should be given for at least 2 months after completion of alemtuzumab therapy and probably should be continued until the CD4+ count is above 200 cells/µL. Weekly monitoring for CMV infection (i.e., antigenemia or PCR) should be instituted until the CD4+ cell count is greater than 100 cells/µL, and positive results should be treated (see the Viral Infections section). Acyclovir or an equivalent agent (famciclovir or valacyclovir) also should be given throughout the course of cellular immunosuppression to reduce the risk of HSV or VZV reactivation. Although serious bacterial and fungal infections also appear to be increased with alemtuzumab, it is thus far unclear what type of prophylaxis should be undertaken in these patients.183
Purine analogs (e.g., fludarabine, cladribine, and pentostatin) also cause a depletion of T-cell populations and an increased risk of opportunistic infections compared with traditional therapy with alkylating agents. The rate of opportunistic infection varies considerably, but patients who have received previous therapy, patients who respond partially or nonresponding patients, and patients receiving other agents (e.g., alkylating agents and corticosteroids) are at higher risk for such infections.12 Prophylaxis against PCP for 2 months and until the CD4+ count is greater than 200 cells/µL is recommended. Antiviral prophylaxis with acyclovir or an equivalent drug should be considered in these higher risk patients. The role of antifungal prophylaxis with fluconazole or an agent active against mold in this population is unclear. Patients with myeloma who are treated with the proteasome inhibitor bortezomib are at increased risk for herpes zoster, and prophylaxis should be administered.14
Pretransplantation Measures to Prevent Infection
Pretransplantation Serostatus Blood Work
Stem cell or marrow donor infectious diseases screening protocols (similar to those for solid organ donors) aim to identify donors who may transmit latent infections to the recipient.143,184 Several serostatus test results, if positive, would not constitute an indication to cancel the transplant but would lead to a different action plan.184 If the indirect screening test for syphilis, such as the rapid plasma reagin or Venereal Disease Research Laboratory test, yields a positive result and is confirmed by a direct test, that is, the fluorescent treponemal antibody test, then the patient should receive high-dose penicillin treatment for 10 to 14 days.
Hepatitis B (core antibody, surface antibody, and surface antigen) and C serologic studies are performed in donor and recipient before transplantation; if results are positive, viral load testing should be performed. Because of the decreased sensitivity of hepatitis C serologies in immunosuppressed patients, some centers screen with nucleic acid tests. Hepatitis B or (much less commonly, hepatitis C) may flare after transplantation, leading to life-threatening hepatic dysfunction.187–187 Recipients with detectable hepatitis B DNA should be treated with antiviral medications, possibly in combination (e.g., tenofovir, lamivudine, adefovir, entecavir) if possible before transplantation and for 6 months after transplantation (longer if GVHD occurs).143 Patients with a history of hepatitis B but no detectable hepatitis B DNA may receive prophylactic antiviral agents or be monitored for hepatitis flare. Treatment for hepatitis C in a patient requiring stem cell transplantation is usually not practical prior to transplantation. In this circumstance, transplantation is not contraindicated, but an assessment of hepatic status before transplantation and an attempt to reduce the hepatoxicity of the conditioning regimen is reasonable in patients with cirrhosis or significant fibrosis.
A person infected with a hepatitis virus may serve as a donor if no alternative donor is available or if the intended recipient is already seropositive. The risk of transmission is lowest when a hepatitis B-positive or hepatitis C-positive donor has an undetectable viral load. Accordingly, donors found to have high viral loads on testing for hepatitis B or C should receive treatment with appropriate antiviral agents to reduce viral loads before transplantation and before donation, in conjunction with hepatology consultation.185–189 Recipients who receive cells from a seropositive donor should have viral load levels monitored before and after transplantation and also may require treatment.
CMV, HSV, and VZV serologic studies are routinely performed before transplantation. If either the donor or the recipient is seropositive, a prevention strategy (as presented earlier) is used.143 If both the recipient and the donor are CMV seronegative, seroconversion with blood products is possible, and thus CMV seronegative or filtered, leukoreduced blood products are commonly used.190,191 Patients seropositive for HSV or VZV (or with a reliable history of chickenpox) receive prophylaxis as discussed. VZV-seronegative patients should receive varicella-zoster immune globulin prophylaxis within 96 hours of significant exposure.143 If varicella-zoster immune globulin is not available, most experts would treat with IVIG, acyclovir, or valacyclovir for 3 weeks.
Knowing the EBV serostatus before transplantation is not mandatory because 95% of adult patients can be assumed to be seropositive for this virus. EBV-driven PTLD occurring as a consequence of EBV reactivation is in the differential diagnosis for any space-occupying mass after transplantation, but this disease usually appears several months after the neutropenic phase.192 Major risk factors for the development of PTLD include primary EBV infection after transplantation in a seronegative recipient, the use of antithymocyte globulin or anti-CD3 monoclonal antibodies for immunosuppression, and CMV primary infection or reactivation.195–195 Mismatched or unrelated donor stem cell grafts or T-cell–depleted grafts also are associated with an increased risk for PTLD. Although the incidence of PTLD after allogeneic hematopoietic stem cell transplant is as low as 0.5% in patients without major risk factors, it increases to 22% in patients with three or more risk factors. Routine monitoring of EBV viral load appears to be of limited value after transplantation.195 However, in patients with high-risk profiles for PTLD and a rising viral load, preemptive rituximab treatment appears to effectively reduce EBV viral load and, accordingly, the incidence of PTLD after transplantation.196,197
Historically, 15% of patients receiving transplants in the United States are seropositive for T. gondii, but this percentage may be higher in European centers.198 The risk of reactivation leading to disease among seropositive patients is 2% to 6%, and parasitemia can be detected by PCR in 16% of seropositive recipients and may precede disease, leading to an opportunity for a preemptive prevention strategy.200–200 Because serologies for T. gondii may be unreliable after transplantation, serologies should be obtained before transplantation because this information can be useful in evaluating patients in whom syndromes compatible with toxoplasmosis (e.g., a CNS mass lesion) later develop. It is likely that low-dose sulfa-based regimens such as those used to prevent PCP also are effective in preventing Toxoplasma infection.199,201
Environmental Measures to Prevent Infection during and after Transplantation
Handwashing, or preferably the use of alcohol-based hand-rub disinfectant, is the mainstay of infection prevention in the hospital or clinic.202 Persons entering the patient room to perform an examination or who touch the patient (including visitors and health care workers) should wash or disinfect their hands outside the room.17 Some hematology units with very high-risk patients (e.g., patients with acute leukemia and patients who have undergone a hematopoietic stem cell transplant) do not allow children as visitors during the respiratory virus season (although visits may be arranged off the unit), and certainly staff and visitors with evidence of respiratory viral infections should not have contact with high-risk patients. It should be stressed, however, that routine use of gown, gloves, and masks is not required in the presence of a neutropenic patient.
Some infectious situations necessitate use of special isolation procedures.203 Contact isolation (i.e., gloves and gowns) is indicated for encounters with patients with adenovirus or methicillin-resistant S. aureus or C. difficile infection. Droplet precautions are added to contact precautions for respiratory virus or varicella infection. Carriers of vancomycin-resistant enterococci are placed in contact isolation until they meet federally determined criteria for discontinuation of isolation, including a negative result on culture from the original site of a positive culture, if the site is still available for culture (as in a wound or urine), plus three consecutive negative results on cultures of rectal swabs taken at least 1 week apart.54
High-efficiency particulate air (HEPA) filtration has replaced laminar air flow as the means of prevention of infection through ventilation in most transplant centers.203 With at least 12 air exchanges per hour, HEPA filters are capable of removing particles larger than 0.2 mm in diameter, such as mold spores. In addition, it is recommended that room air pressure be maintained continuously above that of the corridor (i.e., a positive-pressure environment should be maintained). These environmental measures are recommended for allogeneic transplant recipients; it does not appear that autologous transplant recipients require this level of protection. For individual patients, such as those who will receive T-cell–depleted transplants, an “upgrade” to a laminar air flow environment may be considered. Not all centers, however, continue to maintain laminar air flow patient rooms, and the use of these facilities remains controversial.203
Review of Commonsense Measures That Will Assist in the Prevention of Infection
Safe food-handling practices are encouraged for all immunosuppressed patients. A “neutropenic diet” is no longer recommended.22 Food-borne infections constitute an increasingly difficult problem in the United States. Ground meat products must be cooked thoroughly so that bacteria distributed onto meat in the grinding process, such as E. coli O 157:H7, are killed. Any fruits or vegetables that cannot be peeled must be washed thoroughly before being eaten, and patients should be aware that salad bars are associated with occasional transmission of Salmonella, Shigella, and E. coli infections.204,205 Food products that inherently contain infectious organisms should be avoided, including undercooked eggs (Salmonella), miso soup (Aspergillus), and soft cheeses or unpasteurized dairy products (Listeria) or blue cheeses (molds). Yogurt containing Lactobacillus has been found to cause lung infection on rare occasions, possibly after aspiration events.206
No particular restrictions apply to travel, but strategies to minimize transmission of infectious diseases have been summarized.143 Some social situations, such as sitting in a crowded movie theater or classroom, increase the risk of acquiring a viral illness. Turning away from people who are coughing or sneezing, or even quickly donning a mask, may be helpful in preventing transmission of infection in the first months after transplantation, until immunosuppression is stopped. Patients should be instructed to practice appropriate infection prevention by washing their hands as soon as possible after being close to someone with a cold. In view of outbreaks of infection with noroviruses (Norwalk-like viruses) on cruise ships and other types of outbreaks (e.g., staphylococcal infection) commonly associated with the close living quarters typical with this type of vacation, cruise ships should not be high on the list for vacation choices.207,208