Infection and Immunity
Developmental Immunology
Except in the case of congenital infection, all pathogen encounters in the neonatal period are first-time encounters. For those first-time exposures to pathogens, neonates are dependent on the innate immune system. The innate immune system comprises cells and mechanisms that defend in a nonspecific manner. T- and B-cell responses are part of the specific (adaptive) immune system. The adaptive immune response allows the immune system to remember specific pathogens. 123
There are two types of effector CD4+ helper cells: Th1 and Th2. Each of these is responsible for eliminating a specific kind of pathogen. The Th1 response leads to cell-mediated immunity (important defense against viruses and intracellular pathogens.) Th1 responses are considered proinflammatory. Th2 responses activate B cells to make antibodies, leading to humoral immunity (important defense against extracellular bacteria, parasites, and toxins). Th2 responses are antiinflammatory. Th2 inflammatory responses are favored in the fetus, dampening the fetal immune response and preventing alloimmune reactions between mother and fetus (e.g., miscarriage). Decreased production of Th1 cytokines increases the susceptibility to infection and contributes to the poor response to vaccines. 4
 Physical barriers; intact skin and mucosal membranes
Physical barriers; intact skin and mucosal membranes
 Mononuclear inflammatory cells; particularly mast cells and tissue macrophages
Mononuclear inflammatory cells; particularly mast cells and tissue macrophages
 Soluble plasma proteins; such as cytokines and complement components 5
Soluble plasma proteins; such as cytokines and complement components 5
The vernix caseosa is a waxy coating in newborns that is secreted by fetal sebaceous glands. It contains antimicrobial peptides that act as a microbicidal shield. The lipids and acid pH of the neonatal skin inhibit microbial growth and reach maturity by week 2 to 4 in term neonates (later in premature infants). Small breaks in the integrity of the skin can serve as entry points for infection. 678
Overall activity and components of the alternative pathway are more consistently decreased than those of the classical pathway. This finding is especially problematic for neonates who are exposed to organisms with polysaccharide capsules, such as Escherichia coli K1 and group B Streptococcus (GBS) and who cannot rely on classical pathway activation owing to the lack of specific antibodies. 9
The pluripotent hematopoietic stem cells are generated from embryonic para-aortic tissue, fetal liver (by 4 weeks’ gestation) and bone marrow (by 11 weeks’ gestation). The yolk site is a major site of production of erythrocytes and phagocytes (by 3 weeks’ gestation). Liver hematopoiesis ceases by week 20 and continues only in bone marrow. All major lineages of the hematopoietic cells that are part of the immune system are present in the fetus by the beginning of the second trimester ( Fig. 13-1). 1011
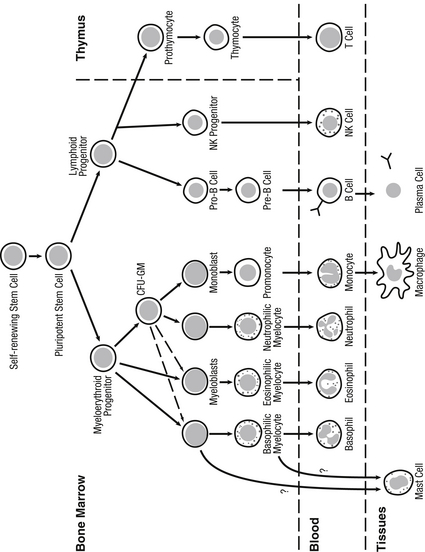
Figure 13-1 Myeloid and lymphoid differentiation and tissue compartments in which they occur. (Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn infant. 7th ed. Philadelphia: Saunders; 2011. p. 88.)
The microbicidal activity of macrophages can be regulated by cytokines (interferon gamma [IFN-gamma], granulocyte-macrophage colony-stimulating factor [GM-CSF], and tumor necrosis factor alpha [TNF-alpha]). The ability of mononuclear phagocytes to generate reactive oxygen intermediates is normal in neonates. However, IFN-gamma production and response to exogenous IFN-gamma are diminished in newborns. 12
The double-negative and double-positive cells are located in the cortex of the thymus, whereas the single-positive cells are located in the medulla, from which they migrate out of the thymus into the blood. The thymic education of T cells includes negative selection; self-reactive cells are removed to prevent autoimmune reactions and positive selection; CD4-positive and CD8-positive cells bearing antigen receptors that do not react with self MHC proteins are eliminated.
In healthy neonates the number of eosinophils increases postnatally, reaching a maximum at 3 to 4 weeks, when it represents a larger percentage (10% to 20%) of total granulocytes than in adults. This physiologic eosinophilia does not suggest the presence of allergic diseases or helminthic infections as strongly as they do in adults and is not associated with increased amounts of circulating immunoglobulin (Ig) E. 13
By week 12 of gestation, lymphocytes obtained from the human thymus respond to mitogens and foreign histocompatibility antigens. Furthermore, fetal cells stimulated with alloantigens exhibit normal antigen-specific cytotoxicity. In contrast, the phenotypic appearance and proportion of circulating cells are diminished, and the production of some cytokines such as interleukin (IL)-12 is reduced in neonates. The most significant defect appears to be a deficiency of memory T cells, which may be responsible for the deficient production of IFN-gamma in the neonate. The mother does not transfer T-cell–specific immunity to the fetus. 14
Immature polymorphonuclear neuthrophils (PMNs) (metamyelocytes and band forms) and mature bone marrow PMNs constitute a reserve pool of cells that may be rapidly mobilized into the circulation in response to inflammation. The rate of proliferation of neuthrophil precursors in the human neonate seems to be near maximal, and therefore their capacity to increase numbers in response to infection might be limited. As a result, rather than develop neutrophilia, as might occur in the septic adult, the neonate will rapidly develop neutropenia as a result of exhaustion or depletion of the storage pools. 15
Circulating PMNs increase dramatically after birth, peak at 12 to 24 hours, and then decline slowly by 72 hours in a neonate without complications. The fraction of immature forms remains constant at about 15%. A limited ability to accelerate neutrophil production in response to infection and impaired migration of PMNs to areas of microbial invasion in tissues contribute to the higher risk of developing overwhelming sepsis in neonates (especially premature infants). However, phagocytosis and microbial killing by PMNs seem not to be greatly impaired unless the neonates are critically ill. 16
The IgG content of the fetus and the newborn infant is mainly maternal in origin and is predominantly transferred during the latter third of pregnancy. Levels of all classes of IgG fall rapidly after birth, and the respective concentrations derived from maternal placental transfer and active production by the young infant are approximately equal by 2 months’ postnatal age. By 10 to 12 months of age, catabolism of passively acquired IgG is complete, and the infant produces all the circulating IgG ( Fig. 13-2).
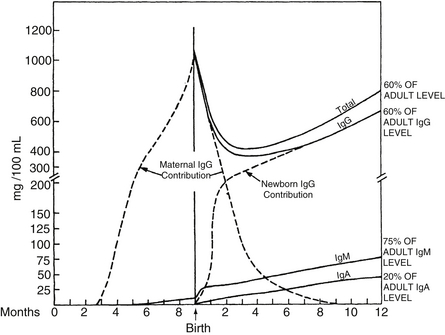
Figure 13-2 Maternal contribution of IgG to fetus and neonate. (From Wilson CB, Lewis DB, Penix LA. The physiologic immunodeficiency of immaturity. In Stiehm R, editor. Immunologic disorders in infants and children. 4th ed. Philadelphia: Saunders; 1996. p. 253–295.)
15. Why do newborn infants respond poorly to polysaccharide vaccines or encapsulated bacteria such as GBS?
In humans the antigens can be divided into three groups depending on the nature of the immune response: (1) thymus-dependent (TD) antigens, which include most protein antigens; (2) thymus-independent type 1 (TI-1) antigens, which bind directly to B lymphocytes and do not require T cells for antibody production; and (3) thymus-independent type 2 (TI-2) antigens, which are mostly polysaccharides composed of multiple identical subunits and require small numbers of T lymphocytes for antibody production to occur. The response to TI-2 antigens appears last chronologically at approximately 6 months of age, accounting for the poor neonatal response to polysaccharide vaccines and to the higher risk of infection with encapsulated organisms such as GBS. 17
NK cells appear early in gestation and reach normal numbers by mid to late gestation; however, they are largely immature in phenotype, consisting of 50% CD56-negative cells. These cells are deficient in their ability to kill virus-infected cells and to produce critical cytokines such as IFN-gamma. Furthermore, virus-specific T-cell–mediated immunity is also diminished or delayed in the neonates with decreased T-cell killing and production of IFN-gamma. Consequently, infection with HSV in the neonate can result in a rapidly progressive, fulminant, and often fatal infection. 18
An unusually severe course of infection or an unusual pathogen, recurrent pyogenic infections, and recurrent upper and lower respiratory tract infections might be caused by immunodeficiency resulting from a congenital neutropenia or dysfunctional neutrophils. A summary of the most important diseases is shown in Table 13-1. 19
TABLE 13-1
SUMMARY OF THE PRINCIPAL GRANULOCYTE DISORDERS IN NEONATES
| CATEGORY | SUBGROUP | DIAGNOSIS/PATHOGENESIS |
| Neutropenia | Iatrogenic/toxic (steroids) Immune mediated Related infections Genetic (Kostmann syndrome) |
Effect of cessation of drug. Steroids primarily affect PMN migration. Detection of auto or alloantibodies (benign neutropenia of infancy) CMV, EBV, parvovirus B19… AR mutations in the G-CSF receptor |
| Decreased motility: adhesion, rolling, migration | Leukocyte adhesion deficiencies (LAD1, LAD2, LAD 3) | LAD 1: Mutations in the beta 2 integrin gene. Delayed separation of umbilical cord, impaired lymphocytic function, and virtual absence of neutrophils in inflammatory exudates despite marked elevations of peripheral blood leukocyte counts. LAD 2: Absence of SLeX oligosaccharide. Mental retardation and periodontitis. LAD 3: Mutations in FERMT3 gene (LAD 3). Mild LAD phenotype and platelet dysfunction. |
| Decreased “sensing danger” | Toll-like receptor deficiencies (IRAK 4 deficiency, MyD88 deficiency) | Recurrent pyogenic infections. Failure to activate nuclear factor kappa-light-chain-enhancer of activated B cells impeding secretion of interleukin-1 beta and tumor necrosis factor alpha. |
| Impaired killing mechanisms | NADPH oxidase dysfunction (chronic granulomatous disease) Impaired granule formation (Chédiak–Higashi syndrome, specific granule deficiency) |
Fulminant bacterial (catalase positive) and fungi infections and chronic granulation formation. Mutations of CYBB gene (X-linked CGD) or mutation in other NADPH oxidase components (AR). Altered microtubule formation. |
Neonatal Sepsis Epidemiology
Early-onset sepsis begins at 3 days of age (or even sooner) when organisms ascend from the birth canal after overt or occult rupture of membranes. Early-onset sepsis usually manifests as fulminant systemic illness and is associated with mortality rates of 3% to 50% (highest in premature infants). Infection with gram-negative pathogens and low-birth-weight infants are at higher risk of mortality. 2021
19. How has the use of maternal intrapartum antibiotics altered the incidence of early-onset neonatal sepsis?
Since consensus guidelines were developed in 1996 and subsequently revised in 2002 and 2010, the incidence of early-onset Streptococcus agalactiae (GBS) infections has declined from 1.7 in 1000 live births to 0.28 in 1000 live births, a 70% reduction in the incidence of early-onset GBS sepsis. During the same period, however, intrapartum antibiotic administration has been associated with an increased incidence of drug-resistant neonatal sepsis, particularly ampicillin-resistant gram-negative disease in very-low-birth-weight (VLBW) infants (<1500 g) ( Fig. 13-3). Whether the increase in gram-negative sepsis is due to intrapartum antibiotic prophylaxis remains controversial. 2223
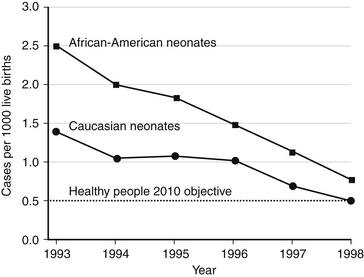
Figure 13-3 Incidence of early-onset invasive group B streptococcal diseases in African-American neonates and Caucasian neonates in four active surveillance areas (California, Georgia, Tennessee, and Maryland), 1993 through 1998. (From Schrag SJ, Phil D, Zywicki S, et al. Group B streptococcal diseases in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000;342:17.)
Among VLBW infants the incidence of early-onset sepsis increases with decreasing gestational age (15 cases in 1000 live births for preterm versus 2.5 cases in 1000 live births for term infants). Compared with data derived before the inception of guidelines for the prevention of GBS disease, there has been a significant reduction in early-onset GBS disease, from 5.9 to 1.7 cases per 1000 live births, with a concomitant increase in E. coli sepsis from 3.2 to 6.8 per 1000 live births. Approximately 85% of the E. coli isolates have been resistant to ampicillin. When the years 1991 through 1993 were compared with 1998 through 2000, there was also an increase in the incidence of early-onset fungal disease, from 0.1 to 0.4 per 1000 live births. 2425
Low parity, spontaneous labor, longer length of labor and membrane rupture, multiple digital examinations, meconium-stained fluid, internal fetal or uterine monitoring, and presence of genital tract infections. The incidence of chorioamnionitis is inversely related with gestational age. 26
The major risk factors for early-onset neonatal sepsis are preterm birth (the factor most closely associated with early-onset neonatal sepsis), maternal colonization with GBS, prolonged rupture of membranes (>18 hours before labor), and maternal signs or symptoms of chorioamnionitis. Other variables include ethnicity (e.g., African-American women have higher rates of colonization with GBS), low socioeconomic status, male sex, and low Apgar scores. Infant birth weight is inversely related to risk of early-onset sepsis ( Table 13-2).
TABLE 13-2
RISK FACTORS FOR PERINATALLY ACQUIRED NEONATAL BACTERIAL INFECTION
| MATERNAL | NEONATAL |
| Prolonged rupture of membranes >18-24 hours | Prematurity |
| Premature rupture of membranes (<37 weeks) | Low birth weight (<2500 gm) |
| Maternal fever ≥100.4°F | Male gender |
| Maternal chorioamnionitis | 5-minute Apgar score <6 |
| Maternal colonization with GBS | |
| GBS bacteriuria | |
| Previous infant with invasive GBS disease | |
| Maternal urinary tract infection at delivery | |
| Multiple gestation |
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 266.
The presence of any of these factors alone is not an indication for a complete sepsis work-up and antibiotic therapy; however, combinations of risk factors are clearly cumulative and should give rise to the suspicion of sepsis. 27
The pathogenesis of early-onset sepsis has changed over the last decades as intrapartum antibiotic prophylaxis protocols have become widely used. GBS and gram-negative enteric bacilli, predominantly E. coli, are the most common pathogens for early-onset disease. In preterm infants who weigh less than 1500 grams, E. coli is more common than GBS. Coagulase-negative staphylococci (CoNS); Staphylococcus aureus; Enterococcus, Klebsiella, and Enterobacter species; Pseudomonas aeruginosa; and fungi (especially Candida albicans) are the major pathogens for late-onset diseases (onset after 72 hours). 282930
CoNS have increased as pathogens in the NICU as the survival of extremely-low-birth-weight infants (<1000 grams) has improved. CoNS bacteremia is associated with indwelling vascular lines. CoNS produce a biofilm that facilitates adherence to the catheter and protects them from antibiotic and host immune responses. 31
26. What are the clinical differences between GBS early-onset sepsis and late or very late-onset sepsis?
Clinical manifestations of GBS late-onset neonatal sepsis are more insidious, and meningitis is frequently part of the clinical picture. Late-onset disease is associated with GBS serotype III and has a lower mortality rate than early sepsis. With increase survival of extremely-low-birth-weight neonates, very-late-onset disease (>98 days) has also been described ( Table 13-3).
TABLE 13-3
CHARACTERISTICS OF EARLY AND LATER-ONSET INFECTIONS
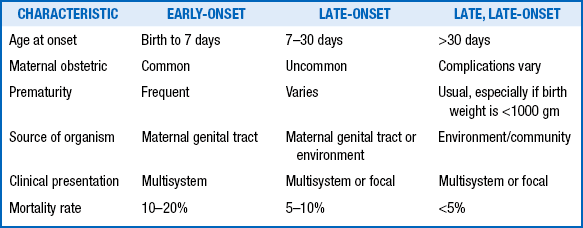
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 267.
TABLE 13-4
RISK FACTORS FOR LATE-ONSET NEONATAL SEPSIS
| RISK FACTOR | COMMENTS |
| Prematurity, low birth weight | Risk of infection is inversely related to gestational age and birth weight. |
| Intravascular catheters | Intravascular catheters provide a portal of entry for infectious organisms, and risk of infection is directly related to the number of catheter days. |
| Total parenteral nutrition (TPN) | TPN requires vascular access, which increases risk; intralipids enhance the growth of lipophilic organisms, particularly coagulase-negative staphylococci and Malassezia furfur. |
| Enteral nutrition | Human milk decreases and formula feeding increases risk. |
| Intubation, ventilation | Endotracheal intubation provides a portal of entry for colonization infection with potential pathogens. |
| Invasive procedures | These provide a portal of entry for organisms by breaking the skin and mucous membrane barriers. |
| Medications | Dexamethasone and H2 blocker use increase risk of infection; widespread and prolonged use of broad-spectrum antibiotics may predispose to infections caused by resistant organisms and fungi. |
| Hospitalization | Prolonged length of stay increases risk of exposure to hospital pathogens. |
| Overcrowding, understaffing | These increase the likelihood of poor infection-control practices (especially poor hand-washing), which increase the risk of infection. |
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 268.
Diagnosis of Neonatal Sepsis
The most sensitive criteria for the clinical diagnosis of chorioamnionitis is maternal fever higher than 38° C (100.4° F). The presence of two or more of the following criteria also supports the diagnosis; maternal leukocytosis (>15000 cells/mm3), maternal tachycardia (> 100 bpm), fetal tachycardia (>160 bpm), uterine tenderness, and foul odor of the amniotic fluid. If maternal fever and two or more of the criteria are present, there is a significant sepsis risk for the neonate, with reported attack rates ranging from 6% to 20%. This issue is further confounded by the use of epidural anesthesia, which is associated with a fourfold increased incidence of maternal fever without increasing the neonatal sepsis rate. 33
The clinical diagnosis of sepsis in the neonate is difficult because many of the signs are nonspecific. They include fever, respiratory distress, jaundice, lethargy, irritability, anorexia or vomiting, hypotonia, “not looking well,” abdominal distention, hypothermia, hypoglycemia, apnea, seizures, shock, petechiae, and purpura. There is considerable overlap with noninfectious conditions, and some infants may exhibit transiently abnormal clinical signs during the transition to postnatal life.
Diagnostic testing can assist with the decision to discontinue treatment. Isolation of the microorganism from a sterile site, such as blood or cerebrospinal fluid (CSF) remains the most valid method of diagnosis of bacterial sepsis. 3435
In studies of neonates who died, the postmortem diagnosis of sepsis was confirmed by antemortem blood cultures in only 80% of cases. The current extensive use of maternal antibiotics further confounds the reliability of the newborn blood culture. 36
In early-onset sepsis, positive urine cultures are attributable to seeding of the kidneys during an episode of bacteremia unlike the urinary tract infections (UTIs) in older infants, which are usually ascending infections. Therefore urine cultures yield very limited information about the source of infection in early sepsis and should not be part of the sepsis work-up. However, suprapubic aspiration or bladder catheterization should be performed in all infants in whom late-onset sepsis is suspected. 373839
38. Can we interpret the cell content and chemistry of neonatal CSF with the same parameters used in older children?
The cell content and chemistry of neonatal CSF differs from those of older infants. The cell content of CSF particularly in the first week is higher, and polymorphonuclear leukocytes are often present in CSF of normal newborns. In a recent study the upper reference limit of the CSF white blood cell (WBC) count was 12 cells/mm3 in preterm infants and 14 cells/mm3 in term infants. Most well infants will have cell counts lower than 10 cells/mm3. Adjusting the cell count for the number of red cells does not improve its diagnostic utility. Preterm infants have protein concentrations that are inversely correlated with their gestational age. Uninfected term newborns have protein concentrations in the CSF lower than 100 mg/dL, with a physiologic decline with postnatal age reaching values of healthy older infants before the third month of life. CSF glucose concentrations in normoglycemic uninfected neonates are similar to those of older infants (70% to 80% of a simultaneous peripheral blood glucose). Meningitis can occur in neonates with completely normal CSF values. 40
Elevated WBC counts or abnormal neutrophil indices (low absolute neutrophil counts [neutropenia], elevated band counts, and high immature-to-total neutrophil [I/T] ratios) have a poor positive predictive value for the diagnosis of early-onset sepsis. They are useful for excluding infants without infections rather than identifying infected ones. Neutropenia is the index with the best specificity. The definition of neutropenia changes with age, type of delivery, site of sampling, and altitude; peak values are reached 6 to 8 hours after delivery. A recent study suggested that the lower limits of normal WBCs at that time should be 7500/mm3 for infants born at more than 36 weeks’ gestation, 3500/mm3 for those between 28 and 36 weeks’ gestation, and 1500/mm3 for less than 28 weeks’ gestation.
A number of inflammatory mediators have been investigated as possible diagnostic tests for neonatal sepsis. IL-6, IL-8, and IL-10 have been found to have a critical role in the inflammatory response during neonatal sepsis; however, none of these mediators has sufficient sensitivity or specificity for the diagnosis of infection in this population. These mediators are currently not available for routine clinical purposes. 41
Antibiotic Treatment
Once sepsis is suspected in a neonate, antimicrobial treatment should be promptly begun after cultures have been obtained, even when there are no obvious risk factors for sepsis. Because GBS and E. coli are the most common pathogens of early-onset sepsis in the United States, a synergistic combination of ampicillin and an aminoglycoside (usually gentamicin) is suitable for the initial treatment of early-onset sepsis. Ampicillin is the antimicrobial of choice for treatment of GBS, Listeria monocytogenes, and most enterococci. Once the pathogen is identified, antimicrobial therapy should be narrowed (unless synergism is needed). 42
Third-generation cephalosporins such as cefotaxime are associated with rapid development of drug-resistant bacteria in nurseries, and extensive use has been reported to be a risk factor for invasive candidiasis. Furthermore, the third-generation cephalosporins are not active against Listeria and Enterococcus species. Because of its excellent CSF penetration, the use of cefotaxime should be restricted for infants with meningitis attributable to gram-negative organisms. 43
Stopping treatment for bacteremia without an identifiable focus of infection remains controversial, and the final decision requires consideration of antibiotic use during labor and the infant’s clinical course. Three recent observational studies have demonstrated an association between antibiotic use for longer than 5 days in infants with suspected early-onset sepsis (and negative blood cultures) with death and necrotizing enterocolitis. Therefore in a well-appearing infant antibiotics should not be continued for more than 48 hours (72 hours in certain cases).
A recent double-blind control trial of adjunctive therapy with intravenous Ig showed no effect on the outcomes (death and major disability at 2 years) of suspected or proven neonatal sepsis. 44
TABLE 13-5
MAJOR ADVERSE REACTIONS TO ANTIMICROBIALS COMMONLY USED IN NEONATES
| ANTIBIOTIC | ADVERSE EFFECTS |
| Ampicillin | Rare hypersensitivity reactions ∗ |
| Amphotericin B | Hypokalemia Reversible nephrotoxicity caused by reduced glomerular filtration rate |
| Acyclovir | Reversible renal dysfunction caused by the formation of acyclovir crystals in renal tubules † |
| Cefotaxime | Rare, occasional leukopenia |
| Ceftriaxone | Displaces bilirubin from albumin, resulting in higher bilirubin concentrations Gallbladder sludging |
| Gentamicin | Irreversible ototoxicity and reversible nephrotoxicity |
| Vancomycin | Rare nephrotoxicity, enhanced by combination with an aminoglycoside Red man syndrome (rash and hypotension) ‡ |
∗Hypersensitivity reactions are not commonly seen in the neonatal period.
†Adequate hydration helps prevent this complication.
‡Appears rapidly and resolves within minutes to hours. Lengthening infusion time usually eliminates risk for subsequent doses.
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 272–73.
Neonatal Meningitis
50. What are the mechanisms of brain injury in meningitis?
51. What factors influence antibiotic concentrations in CSF?
TABLE 13-6
FACTORS THAT INFLUENCE ANTIBIOTIC CONCENTRATIONS IN CEREBROSPINAL FLUID
| VARIABLE | EFFECT ON CNS PENETRATION | EXAMPLE |
| High degree of protein binding | Reduced | Ceftriaxone |
| Lipid solubility | Enhanced | Rifampin |
| High degree of ionization | Reduced | Beta-lactams |
| Active transport system | Enhanced | Penicillin |
| Meningeal inflammation | Enhanced ∗ | Beta-lactams, vancomycin |
∗Meningeal inflammation only influences penetration of hydrophilic antibiotics.
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 281.
53. How should gram-positive and gram-negative meningitis be treated during the neonatal period?
 Treatment of meningitis caused by enteric organisms: Cefotaxime is preferred and is often paired with an aminoglycoside. Gram-negative meningitis usually is treated for at least 3 weeks.
Treatment of meningitis caused by enteric organisms: Cefotaxime is preferred and is often paired with an aminoglycoside. Gram-negative meningitis usually is treated for at least 3 weeks.
 Treatment of meningitis caused by gram-positive organisms: Because there is synergism between ampicillin and aminoglycosides for most GBS, L. monocytogenes, and enterococci, combination therapy is recommended until the CSF is sterilized. If the GBS is determined to be a tolerant organism, combination therapy should be used for the duration of treatment (approximately 14 days). 45
Treatment of meningitis caused by gram-positive organisms: Because there is synergism between ampicillin and aminoglycosides for most GBS, L. monocytogenes, and enterococci, combination therapy is recommended until the CSF is sterilized. If the GBS is determined to be a tolerant organism, combination therapy should be used for the duration of treatment (approximately 14 days). 45
Group B Streptococcal (GBS) Infections
Between 10% and 30% of women are colonized with GBS in their birth canal. Chronic, intermittent, or transient patterns of GBS colonization have been described. Pregnant woman with GBS colonization are 25 times more likely to deliver an infant with early-onset GBS sepsis than women whose cultures are negative (though infants with early-onset GBS have been born to women with negative antenatal cultures). Affected infants became infected during labor and delivery. In the absence of intrapartum prophylaxis, 2% of infants will develop early-onset GBS sepsis. 46
Nucleic acid amplification tests (NAATs), including PCR, for GBS can be used to screen women at term with no other risk factors but should not replace traditional antenatal cultures because they have lower sensitivity. Chromogenic media can facilitate the detection of beta-hemolytic GBS, but may not detect nonhemolytic strains. 474849
57. How many serotypes of GBS have been identified? What is the clinical and immunologic significance of the serotypes?
Ten serotypes have been identified on the basis of capsular polysaccharide antigens. Early studies of GBS disease in North America demonstrated a predominance of the type III serotype, thought also to be the most virulent serotype. Currently, type III accounts for approximately 70% of isolates from infants with meningitis and is isolated in almost two thirds of infants with late-onset diseases. Since the 1970s there has been a progressive change in the predominant serotypes, with type Ia now the leading cause of early-onset infection. Types VI, VII, VIII, and IX rarely cause human diseases in the United Kingdom or the United States, but worldwide its distribution varies (e.g., types VI and VIII are the most common isolates from healthy Japanese women). From an immunologic and public health perspective, the recognition of multiple new serotypes has confounded the efforts of investigators to develop an effective multivalent vaccine to prevent this disease in newborns. 50
Penicillin (3 g [5 million units] intravenously followed by 1.5 to 1.8 g [2.5 to 3 million units] every 4 hours administered at least 4 hours before delivery) is the first-line agent for prevention of early onset GBS disease. Ampicillin is an effective alternative. Cefazolin (first-generation cephalosporin) is preferred for penicillin-allergic women at low risk for anaphylaxis. 51
59. Can clindamycin be used for intrapartum antibiotic prophylaxis (IAP) in women with penicillin allergy?
The mother’s strain should be tested for sensitivity to clindamycin and inducible resistance (D-zone test) because 25% of GBS strains are resistant to clindamycin. If the test is not available, clindamycin should not be used. Erythromycin should also not be used for IAP. Vancomycin is the recommended drug for women with severe allergic reactions if the strain has not been tested for susceptibility to clindamycin. IAP with vancomycin is probably effective but is considered inadequate (in terms of neonatal management) because of the lack of efficacy data.
 Antenatal colonization with GBS (except for women who have cesarean delivery without labor or membrane rupture)
Antenatal colonization with GBS (except for women who have cesarean delivery without labor or membrane rupture)
 Unknown GBS colonization status and any of the following: preterm labor, maternal fever (38° C or higher), prolonged rupture of membranes (18 hours or longer), or an intrapartum NAAT positive for GBS
Unknown GBS colonization status and any of the following: preterm labor, maternal fever (38° C or higher), prolonged rupture of membranes (18 hours or longer), or an intrapartum NAAT positive for GBS
 GBS bacteriuria during pregnancy (104 or more colony-forming units/mL )
GBS bacteriuria during pregnancy (104 or more colony-forming units/mL )
Pros: IAP has resulted in a dramatic reduction in incidence of early-onset disease. Figure 13-4 illustrates the decline in incidence of early-onset GBS disease over the past decade as IAP programs were implemented. The graph is based on composite data from surveillance centers of the Centers for Disease Control and Prevention (CDC), a National Institute of Child Health and Development multicenter study reviewing disease rates from 1992 to 1997, and ongoing surveillance at the author’s center. The incidences of disease from 1990 to 1993 represent the pre-IAP era, whereas data from 1993 to 1996 followed the ACOG and American Academy of Pediatrics (AAP) recommendations published in 1993. The third data set reflects the impact of the CDC recommendations published in 1996.
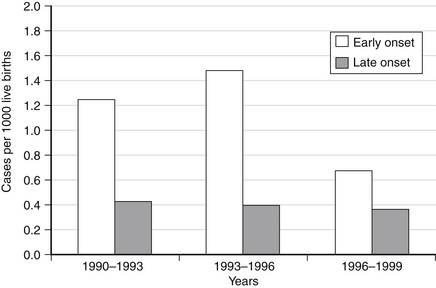
Figure 13-4 Group B streptococcal diseases in the era of intrapartum antibiotic prophylaxis (cases per 1000 live births). (From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 275.)
 IAP is not 100% effective; 20% of cases occurred despite intrapartum antibiotics
IAP is not 100% effective; 20% of cases occurred despite intrapartum antibiotics
 Screening-based strategy identifies only maximum of 85% to 90% of affected infants’ mothers
Screening-based strategy identifies only maximum of 85% to 90% of affected infants’ mothers
 Emergence of resistant organisms in mothers and infants (i.e. E. coli, Enterococcus species)
Emergence of resistant organisms in mothers and infants (i.e. E. coli, Enterococcus species)
 Increasing resistance of GBS to clindamycin and erythromycin
Increasing resistance of GBS to clindamycin and erythromycin
 Does not address other adverse outcomes of GBS infection in pregnancy (i.e. early fetal loss, preterm labor, premature rupture of fetal membranes) 5354
Does not address other adverse outcomes of GBS infection in pregnancy (i.e. early fetal loss, preterm labor, premature rupture of fetal membranes) 5354
In 2010 the CDC published new guidelines for prevention of early-onset GBS sepsis ( http://www.cdc.gov/groupbstrep/guidelines/index.html). These are summarized in Figure 13-5.
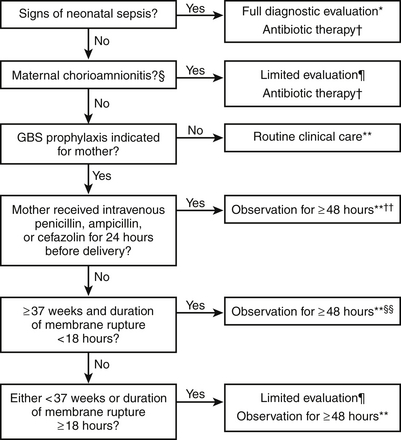
Figure 13-5 Algorithm for secondary prevention of early-onset of group B streptococcal diseases among newborns. (Randis TM, Polin RA. Early-Onset group B Streptococcal sepsis: new recommendations from the Centres for Diseases Control and Prevention. Arch Dis Child Fetal Neonatal Ed 2012;97(4):F291–4.)
∗Full diagnostic evaluation includes a blood culture, a complete blood count (CBC) including white blood cell differential and platelet counts, chest radiograph (if respiratory abnormalities are present), and lumbar puncture (if patient is stable enough to tolerate procedure and sepsis is suspected).
†Antibiotic therapy should be directed toward the most common causes of neonatal sepsis, including intravenous ampicillin for GBS and coverage for other organisms (including Escherichia coli and other gram-negative pathogens) and should take into account local antibiotic resistance patterns.
§Consultation with obstetric providers is important to determine the level of clinical suspicion for chorioamnionitis. Chorioamnionitis is diagnosed clinically and some of the signs are nonspecific.
¶Limited evaluation includes blood culture (at birth) and CBC with differential and platelets (at birth and/or at 6–12 hours of life).
∗∗If signs of sepsis develop, a full diagnostic evaluation should be conducted and antibiotic therapy initiated.
††If ≥37 weeks’ gestation, observation may occur at home after 24 hours if other discharge criteria have been met, access to medical care is readily available, and a person who is able to comply fully with instructions for home observation will be present. If any of these conditions is not met, the infant should be observed in the hospital for at least 48 hours and until discharge criteria are achieved.
§§Some experts recommend a CBC with differential and platelets at age 6–12 hours.
63. Is late maternal colonization responsible for the high number of negative GBS screens among women who subsequently deliver infants with early-onset GBS infection?
Most infants who contract late-onset disease acquire the organism outside the hospital. Mothers of these infants may have no history of genital colonization with GBS during pregnancy. 55
Staphylococcus Epidermidis
CoNS are commensal skin and mucosal flora. Nearly 99% of healthy neonates will have positive nose or umbilicus swabs for CoNS by day 4 of life. However, these organisms also account for up to one half of reported bloodstream infections in VLBW (<1500 g) infants. 56
Improved survival rates of VLBW infants have resulted in an increased risk for sepsis because of the many invasive therapies required for management, such as central venous catheters. Central lines are associated with an increased risk of CoNS bacteremia. Colonization precedes infection with this species. Therefore CoNS infections present a particular dilemma because their isolation from a single blood culture in a neonate can either reflect contamination or true bacteremia. A suggested algorithm for interpreting blood cultures caused by CoNS is shown in Figure 13-6. 57
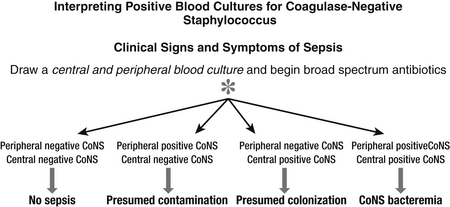
Figure 13-6 Algorithm for interpreting blood cultures caused by coagulase-negative staphylococci. (From Polin RA. The Committees on Fetus and Newborn and Infectious Diseases. Pediatrics 2012;129:e1104-9.)
Umbilical vessels and intravascular catheters are essential in the NICU, and results of blood cultures can yield ambiguous interpretations (e.g., contamination versus catheter colonization versus systemic infection). Some microbiological features can be useful assisting this decision, such as time to growth (the longer the time elapsed between obtaining the blood culture and its growth, the more likely it is it represents a contaminant), number of positive cultures (especially if obtained from different sources; peripheral and central), the organisms’ isolates (contamination is more likely when multiple specimens grow), and clinical signs. 5859
68. The most common manifestation of CoNS infection is bacteremia and sepsis, but what are the focal complications of persistent bacteremia with CoNS?
Because of concerns regarding the emergence of vancomycin-resistant organisms, routine use of prophylactic vancomycin for all neonates at risk of CoNS bacteremia is not currently recommended. 6162
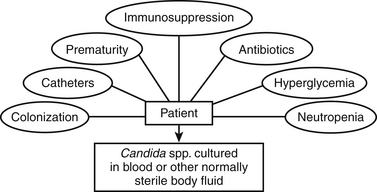
Candida
71. What are the most important risk factors for neonatal systemic candidiasis?
 Prematurity: The incidence of systemic candidiasis, particularly in the VLBW infant, has increased significantly over the past decades, with a mortality rate approaching 30%. Significant neurodevelopmental sequelae are common among survivors.
Prematurity: The incidence of systemic candidiasis, particularly in the VLBW infant, has increased significantly over the past decades, with a mortality rate approaching 30%. Significant neurodevelopmental sequelae are common among survivors.
 Long-term use of broad-spectrum antibiotics (cephalosporin or carbapenems), use of gastric-acid inhibitors (H2 blockers): Suppression of normal gastrointestinal flora enhances Candida species overgrowth.
Long-term use of broad-spectrum antibiotics (cephalosporin or carbapenems), use of gastric-acid inhibitors (H2 blockers): Suppression of normal gastrointestinal flora enhances Candida species overgrowth.
 Central intravenous catheterization and parenteral nutrition: These allow a portal of entry for the organism into the bloodstream.
Central intravenous catheterization and parenteral nutrition: These allow a portal of entry for the organism into the bloodstream.
 Prolonged steroid use: This may impair neutrophil function ( Figure 13-7). 636465
Prolonged steroid use: This may impair neutrophil function ( Figure 13-7). 636465
Approximately 1.4% of early-onset neonatal infections result from Candida species (mainly Candida albicans but increasingly from other species, such as Candida parapsilosis and Candida glabrata). For late-onset sepsis the incidence varies from 2.6% to 16.7% among VLBW infants and up to 20% for extremely-low-birth-weight infants. There is a marked inverse correlation between mortality caused by Candida species and neonatal weight; a recent analysis reported an all-cause mortality rate of 26% in infants weighing less than 1000 g with candidiasis compared with 13% in infants without candidiasis. 6667
Candida infections acquired after the first week of life might be limited to the bloodstream, urine, or CSF or may disseminate to involve one or many organ systems. Fungal abscesses may be found in the heart, bones, kidneys, bladder, eyes, or brain. The medical literature concerning end-organ evaluation after neonatal candidemia is heterogeneous; however, a retrospective study suggested potential damage from the following sources: endophthalmitis (median, 3%), meningitis (15%), brain abscess or ventriculitis (4%), endocarditis (5%), positive renal ultrasound (5%), and positive urine culture (61%). 6869
The highest risk period for ICIs in preterm neonates occurs during the first 4 to 8 weeks of life. Several studies have shown that fluconazole prophylaxis can reduce those infections among preterm infants, with highest efficacy among VLBW infants and those weighing 750 g or less. The pros and cons of antifungal prophylaxis are summarized in Table 13-7. 70
TABLE 13-7
PROS AND CONS OF ANTIFUNGAL PROPHYLAXIS FOR ELBW INFANTS
| PROS | CONS | |
| Efficacy | >80% efficacy for fluconazole prophylaxis in reducing ICI. >50% efficacy for nystatin prophylaxis. Infection, death, and neurodevelopmental impairment could be prevented even if rates are low (2% or less). A unified approach, as with GBS prophylaxis, has the most benefit. |
Rates vary by country and NICU. |
| ICI mortality | Multicenter data report >20% mortality in ELBWs (A-II) | Some single-center studies report no mortality (B-II). Empiric therapy could eliminate mortality (B-11). Appropriate treatment of documented infections could eliminate mortality. |
| NDI in survivors | 57% NDI in infants weighing <1000 g (A-II) Neither CVC removal nor empiric therapy improved NDI (A-11) |
Optimal treatment with CVC removal or empiric therapy in all patients may improve outcomes (further study needed). |
| ICI rate | 5%-10% in infants weighing <1000 g when all ICI (BSI, UTI, meningitis, peritonitis) included (A-II) 20% for infants 23 to 24 weeks’ GA (A-II) |
Some NICUs report lower rates of 2% to 3% in infants <1000 g using only BSI and meningitis (B-II). |
| Cost | Fluconazole is inexpensive. ICI increases hospital costs (A-II); >$500,000 decreased costs over 18 months in one NICU. |
Some infection-control measures are inexpensive (B-II). |
| Safety | All RCTs showed safety with no increase in liver function tests and no adverse effects; >4100 infants from all FP studies. | One retrospective study reported increased cholestasis with FP, though no significant difference at discharge. Possible concern with osmolarity of nystatin and NEC in extremely preterm infants. |
| Azole resistance | RCTs have not demonstrated increased azole resistance. Amphotericin (or a nonazole) is used for treating suspected or documented ICI. This appropriately treats ICI if resistance would occur and places less azole pressure on fungi to become resistant if exposed to high-dose fluconazole for treatment. |
There is concern that resistance may still occur over time. |
| Alternative approaches | Empiric therapy and infection-control measures are not subjected to RCTs, and impact is unknown. | Other approaches (empiric therapy, infection control measures) might be efficacious. |
From Kaufman D, Manzoni P. Strategies to prevent invasive Candida infection in extremely preterm infants. Clin Perinatol 2012;37:611–628.
75. What antifungal should be used for prophylaxis among neonates whose birth weight is below 1000 g?
Studies in more than 4000 neonates have demonstrated efficacy and safety with fluconazole prophylaxis in extremely preterm neonates, with an overall reduction of 83%. No significant increases in azole-resistant strains have been documented. Enteral fluconazole is 90% absorbed; therefore once neonates achieve enteral feeding, the dosing can be switched from intravenous to oral administration to complete 4 to 6 weeks of prophylaxis. 71
A randomized control trial of infants with birth weight below 1500 g compared oral fluconazole to oral nystatin prophylaxis started in the first 7 days of life and continued until enteral feeding was achieved. ICIs occurred in 5.3% of fluconazole-treated patients compared with 14.3% of nystatin-treated infants. The study also raised the question of safety of enteral nystatin in extremely premature infants when they were not receiving full enteral feedings. The oral nystatin suspension contains a high concentration of sucrose and is highly osmolar. This raises a theoretical concern of bacterial translocation and increased risk of necrotizing enterocolitis. Other advantages of fluconazole over nystatin are lower cost, administration twice-weekly compared with thrice daily, and ability to administer the drug intravenously when the infant is not receiving anything by mouth. 72
Although empiric or prompt standardized treatment (including prompt removal of central venous catheters) may reduce Candida-related deaths, neurodevelopmental impairment may still occur in the survivors, particularly in those weighing less than 1000 g. Strategies to reduce morbidity and mortality in NICUs are summarized in Table 13-8. 737475
TABLE 13-8
STRATEGIES TO REDUCE ICI MORTALITY AND MORBIDITY AMONG NICUs
1. Use antifungal prophylaxis (IV fluconazole) while IV access is in use (central or peripheral) for infants with birth weight less than 1000 g and/or 27 weeks’ gestational age or less (A-I) ∗
 There is B-I and B-II evidence for antifungal prophylaxis with nystatin but limited data in infants <750 g and <26 weeks gestation. Because fluconazole prophylaxis has greater efficacy compared with nystatin, efficacy in the most immature patients is less expensive and can be given to infants not feeding; the evidence currently would favor fluconazole prophylaxis in preterm infants.
There is B-I and B-II evidence for antifungal prophylaxis with nystatin but limited data in infants <750 g and <26 weeks gestation. Because fluconazole prophylaxis has greater efficacy compared with nystatin, efficacy in the most immature patients is less expensive and can be given to infants not feeding; the evidence currently would favor fluconazole prophylaxis in preterm infants.
2. Start treatment of documented infections with appropriate antifungal dosing and prompt catheter removal for candidal BSI (A-II).
3. Decrease broad-spectrum antibiotic use (B-II).
Restrict third- and fourth-generation cephalosporins and carbapenems to treatment of proven gram-negative infections.
4. Decrease H2 blocker and proton-pump inhibitor use (B-II).
Use only for proven gastritis, and restrict use to 3 days or until symptoms resolved.
Use only for severe lung disease.
∗U.S. Public Health Service Grading System for ranking recommendations in clinical guidelines: Strength of recommendation and levels of evidence. A, Good evidence; B, moderate evidence; C, poor evidence; I, at least one randomized clinical trial; II, at least one well-designed but nonrandomized trial; III, expert opinions based on experience or limited clinical reports.
From Kaufman D, Manzoni P. Strategies to prevent invasive Candida infection in extremely preterm infants. Clin Perinatol 2012(37);611–628.
According to the Infectious Diseases Society of America Guidelines published in 2009, amphotericin B deoxycholate remains the mainstay of therapy (dose is 1 mg/kg/day). Although side effects include nephrotoxicity, hypokalemia, hepatotoxicity, and bone marrow suppression, the drug appears to be well tolerated in neonates. If urinary tract involvement is excluded, the lipid formulation of amphotericin B (3 to 5 mg/kg/day) can be used. Fluconazole at 12 mg/kg/day is a reasonable alternative. The recommended length of treatment is 3 weeks. Echinocandins (e.g., caspofungin) should be used with caution among neonates and are usually reserved for situations in which resistance or toxicity precludes the use of fluconazole or amphotericin. 7677
79. What complementary investigations should be performed in a neonate with an invasive candidal infection?
The recurrence of Candida disease has been described in four immunocompetent infants after a prolonged period of latency (up to 1 year). All the infants presenting with Candida arthritis and osteomyelitis were born prematurely, had received parenteral nutrition through indwelling catheters, and had a history of systemic candidiasis during the newborn period. The pathogenesis of these latent infections remains unknown. 78
Infection Control
Neonates, especially if premature, require intensive medical care and are among the patients at highest risk for HAIs. Some series have reported that more than 20% of critically ill neonates who survive longer than 48 hours acquire a HAI, with a significant worsening of their prognosis and excessive direct health costs. 79
 Contact: Direct or indirect; from an infected person or a contaminated source. Transmission by the hands of health care workers is the most important route.
Contact: Direct or indirect; from an infected person or a contaminated source. Transmission by the hands of health care workers is the most important route.
 Droplet: Large respiratory droplets that travel 3 feet or less (e.g., pertussis).
Droplet: Large respiratory droplets that travel 3 feet or less (e.g., pertussis).
 Airborne: Smaller particles that can travel longer distances (e.g., varicella).
Airborne: Smaller particles that can travel longer distances (e.g., varicella).
Specific microorganisms can be spread by more than one route, but in most cases one mechanism predominates. Most of the HAIs are caused by the infant’s own flora. 80
Patients suspected of having tuberculosis, varicella, or measles must be placed on airborne precautions in negative-pressure rooms to prevent aerosol spread of their infection. It is important to assess the family members of such patients because they might be potential sources of the infection as well. 81
86. A nurse tells you that she has just been exposed to varicella, and she never had it as a child. What do you tell her about the period of isolation?
The following diseases require contact isolation:
 Resistant organisms, including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci
Resistant organisms, including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci
Components of standard precautions include performing proper hand hygiene and wearing gloves, gowns, masks, and other forms of eye protection.
91. What are the most frequently cited reasons that nursery personnel do not wash their hands (all invalid)?
 Hand-washing takes too much time.
Hand-washing takes too much time.
 There is a lack of soap (54%) and lack of towels (65%).
There is a lack of soap (54%) and lack of towels (65%).
 One thorough wash per day is sufficient (26%).
One thorough wash per day is sufficient (26%).
 Gloves can substitute for hand-washing (25%, including 50% of physicians).
Gloves can substitute for hand-washing (25%, including 50% of physicians).
 Hand-washing is not important if an infant is receiving antibiotics (10%).
Hand-washing is not important if an infant is receiving antibiotics (10%).
92. What are the current recommendations for hand hygiene in the NICU?
 Most experts recommend removal of hand and wrist jewelry. CDC guidelines state that staff who have direct contact with infants in NICU should not wear artificial fingernails or nail extenders. Nails should be kept less than ¼ inch long. Clear nail polish is acceptable but not nail polish with colors.
Most experts recommend removal of hand and wrist jewelry. CDC guidelines state that staff who have direct contact with infants in NICU should not wear artificial fingernails or nail extenders. Nails should be kept less than ¼ inch long. Clear nail polish is acceptable but not nail polish with colors.
 The minimum initial wash should be long enough to ensure thorough washing and rinsing of all parts of the hands and forearms (3-minute scrub without a brush to the elbow).
The minimum initial wash should be long enough to ensure thorough washing and rinsing of all parts of the hands and forearms (3-minute scrub without a brush to the elbow).
 At least a 15-second scrub should be performed before and after handling of each infant.
At least a 15-second scrub should be performed before and after handling of each infant.
 An alcohol-based hand gel should be used before and after handling infants.
An alcohol-based hand gel should be used before and after handling infants.
93. Do careful hand hygiene practices reduce the incidence of nosocomial infection?
Hand hygiene plays a key role for caregivers in the reduction of HAIs for patients.
Hand disinfection with an alcohol-based hand rub is the preferred method because of its rapid action and effectiveness. In addition, alcohol-based rubs contain emollients that serve as dermal protectors and decrease bacterial dispersal. In contrast, antiseptic skin washes can damage the skin barrier and offer no advantages. 82
The principles of family-centered care encourage liberal visitation policies in neonatal units (well-infant nurseries and NICUs). Parents and siblings should be allowed liberal visitation. Written policies should be in place to guide siblings’ visits, and parents should be encouraged to share the responsibility of protecting the newborn from contagious illness. 83
Conjuntivitis
The age at onset may suggest a specific etiology; however, there is substantial overlap among the various causes depending on obstetric factors such as prolonged rupture of membranes ( Table 13-9). 8485
TABLE 13-9
CAUSES OF NEONATAL CONJUNCTIVITIS AND TIME OF ONSET
| ETIOLOGY | USUAL TIME OF ONSET AFTER BIRTH |
| Chemical (with silver nitrate prophylaxis) | 6 to 24 hours |
| Chlamydia trachomatis | 5 to 14 days |
| Neisseria gonorrhoeae | 2 to 5 days |
| Other bacterial etiology: Staphylococcus aureus Haemophilus species Streptococcus pneumoniae Enterococcus species |
>5 days |
| Herpes simplex | 5 to 14 days |
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 283–84.
101. A 5-day-old term baby presents in the emergency room with purulent material coming from one eye. What work-up should you do?
The first step should be a Gram stain of the conjunctiva exudate. If it shows gram-negative intracellular bean-shaped diplococci, Neisseria gonorrhoeae (or other Neisseria species) should be assumed to be the cause of the eye discharge, and the infant should be admitted for urgent systemic treatment. If treatment is delayed, the infection could spread to the cornea leading to ulcerations and ultimately loss of vision. Note that the eye discharge seen in gonococcal ophthalmia is often thick, copious, and golden-yellow in color. Cultures of blood, eye discharge, and other potential sites of infection, such as CSF, should be performed to confirm the diagnosis and determine antimicrobial susceptibility. Testing for concomitant infection with C. trachomatis, Treponema pallidum, and human immunodeficiency virus (HIV) should also be done, as well as a review of hepatitis B status in the mother.
NAATs are highly sensitive and specific, but only a few are approved by the U.S. Food and Drug Administration (FDA) for conjunctival specimens; therefore the diagnosis still relies on culture. A combined DNA probe for the detection of both N. gonorrhoeae and C. trachomatis is also commercially available. Remember that C. trachomatis is an obligate intracellular organism, so the collection swab must be scraped across the conjunctiva or nasopharynx to ensure that there are adequate cells for detection. In the eye the pus should be wiped away before the conjunctiva scrapings are obtained. If herpes conjunctivitis is suspected, a PCR test for herpes simplex or culture should also be done. The identification of C. trachomatis or N. gonorrhoeae in a newborn infant indicates untreated infection in the parents. 86
Infants with chlamydial conjunctivitis are treated with oral erythromycin (50 mg/kg/day divided into four equal doses) for 14 days. Additional topical therapy is not needed. Because the efficacy of erythromycin is only 80%, a second course may be required, and follow-up of infants is recommended. Limited data suggest that azithromycin at an oral dose of 20 mg/kg given once a day for 3 days may be effective. Herpes conjunctivitis is rare and is almost always accompanied by other systemic manifestations of neonatal herpes. The treatment for neonatal herpes conjunctivitis is parenteral acyclovir plus topical therapy with 1% trifluridine solution, 0.1% iododeoxyuridine, or 3% vidarabine applied to the eye every 2 hours for 7 days or until the cornea has re-epithelialized. 87
103. Why does conjunctivitis caused by C. trachomatis not cause blindness in neonates when it causes so many cases of blindness in third-world countries?
Chlamydial Infections
105. Does C. trachomatis infection in pregnant women cause complications other than neonatal infection?
C. trachomatis is the most common sexually transmitted pathogen in Western industrialized countries. Most of the infections in adults are asymptomatic but can cause severe reproductive complications in women; chronic salpingitis caused by C. trachomatis can lead to infertility and an increased risk for ectopic pregnancy. This is in contrast with gonococcal infections, in which most infected individuals are symptomatic and therefore present acutely for care. Although studies are conflicting, C. trachomatis infection in pregnancy is weakly linked to premature rupture of membranes and premature delivery. Between 10% and 30% of women with chlamydial infections who undergo induced abortions develop late endometritis. 8889
106. What is the risk of chlamydial infection in infants born to mothers whose endocervical culture result is positive for C. trachomatis?
The remaining infants develop an apparently asymptomatic colonization of the nasopharynx, rectum, or vagina. These infants can remain colonized for up to 3 years, although most clear the infection even without treatment by 1 year of age. There is no evidence to suggest that infants with chlamydial infections should be isolated. Note that successful treatment of the mother during pregnancy with oral erythromycin or azithromycin prevents most cases of vertical transmission. 90
Mothers with positive endocervical cultures should be treated during pregnancy to prevent vertical transmission. The recommended treatment for pregnant women is azithromycin (1 g orally as single dose) or amoxicillin (1.5 g/day in 3 divided daily doses for 7 days). Repeated testing (preferably NAATs) is recommended in pregnant women 3 weeks after treatment to determine whether treatment has been successful; if not, a second course of treatment may be indicated. Sexual partners of positive women must be treated as well. Chlamydia infection in both male and female genital tracts can be asymptomatic, which is why routine screening in pregnancy is warranted. 91
Neonates with chlamydial conjunctivitis or pneumonia should receive oral erythromycin base or ethylsuccinate, 50 mg/kg/day in four divided doses, for 14 days. The efficacy of erythromycin is approximately 80%; therefore a second course may be required, and follow-up of infants is recommended. Limited data on azithromycin for treatment of chlamydial infection in infants suggest that dosing of 20 mg/kg as a single dose for 3 days may be effective. Its shorter treatment course and less severe gastrointestinal side effects could improve treatment compliance. 92
Without treatment, symptoms last an average of 6 weeks. Treatment of any previous conjunctivitis with oral erythromycin seems to prevent pneumonia, although there are case reports of treatment failures. Approximately 50% of the infants with chlamydial pneumonia do not have a history of previous conjunctivitis. 9394
Osteomyelitis and Septic Arthritis
Because most cases of neonatal osteomyelitis arise as a consequence of bacteremia, the organisms responsible for causing osteomyelitis reflect the changing trends in the ethology of neonatal sepsis as well as the different likelihood of osteoarticular shedding within pathogens.
 S. aureus; predominant organism, with increasing MRSA isolates
S. aureus; predominant organism, with increasing MRSA isolates
 GBS: second most important cause
GBS: second most important cause
 Gram-negative enteric bacilli (i.e., E. coli, Klebsiella species, Pseudomonas species, Proteus species, Enterobacter, Serratia marcescens, and Salmonella species): uncommon despite the frequency of neonatal bacteremia caused by these agents
Gram-negative enteric bacilli (i.e., E. coli, Klebsiella species, Pseudomonas species, Proteus species, Enterobacter, Serratia marcescens, and Salmonella species): uncommon despite the frequency of neonatal bacteremia caused by these agents
 Candida species: particularly in premature infants
Candida species: particularly in premature infants
 Mycoplasma hominis and Ureaplasma urealyticum: rare
Mycoplasma hominis and Ureaplasma urealyticum: rare
 T. pallidum: largely eliminated thanks to antenatal maternal screening and treatment. 9596
T. pallidum: largely eliminated thanks to antenatal maternal screening and treatment. 9596
 Extension from infection in surrounding tissues (e.g., an infected cephalohematoma spreading to the parietal bone)
Extension from infection in surrounding tissues (e.g., an infected cephalohematoma spreading to the parietal bone)
 Direct inoculation after heel-stick capillary blood sampling or fetal scalp monitoring
Direct inoculation after heel-stick capillary blood sampling or fetal scalp monitoring
 Maternal bacteremia with transplacental infection and fetal sepsis (i.e., syphilis) 97
Maternal bacteremia with transplacental infection and fetal sepsis (i.e., syphilis) 97
115. What distinct anatomic and physiologic features place the newborn infant at risk for osteomyelitis and septic arthritis?
Decompression of the primary metaphyseal abscess through the adjacent cortex also permits entrance of pus into the articular space of the bones whose metaphyses lie within the articular capsule of the joint. Suppurative arthritis of hips, shoulders, elbows, and knees is frequently seen in osteomyelitis of the humerus or the femur ( Fig. 13-8).
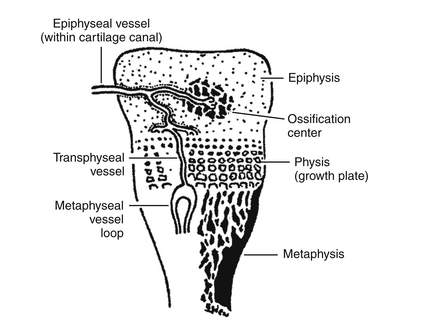
Figure 13-8 Schematic depiction of blood supply in the neonatal epiphysis. (From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 287.)
116. Why do neonates not exhibit many of the features of chronic osteomyelitis seen in older children and adults?
117. What are the manifestations of osteomyelitis in neonates?
 Systemic signs are usually absent in neonatal osteomyelitis but occasionally are present.
Systemic signs are usually absent in neonatal osteomyelitis but occasionally are present.
 In most infants the earliest presenting signs are pain, limitation of motion (pseudoparalysis), and swelling. Discoloration and increased warmth may accompany the swelling.
In most infants the earliest presenting signs are pain, limitation of motion (pseudoparalysis), and swelling. Discoloration and increased warmth may accompany the swelling.
 Feeding and weight gain are usually undisturbed if only local symptoms are present, leading to a delay in the diagnosis while bone destruction progresses.
Feeding and weight gain are usually undisturbed if only local symptoms are present, leading to a delay in the diagnosis while bone destruction progresses.
118. How often are bacterial culture results positive in neonatalosteomyelitis and septic arthritis?
 Up to 60% of blood cultures can be positive.
Up to 60% of blood cultures can be positive.
 Approximately 60% to 70% of joint and bone aspirates are positive, but previous antibiotic therapy may decrease this percentage.
Approximately 60% to 70% of joint and bone aspirates are positive, but previous antibiotic therapy may decrease this percentage.
119. Is the erythrocyte sedimentation rate (ESR) or CRP helpful in the management of osteomyelitis?
In most studies the ESR is significantly elevated on days 2 through 5. ESR values slowly return to normal within 3 weeks of therapy. In contrast, CRP rises within 6 to 12 hours of a triggering stimulus and returns to normal within 1 week of therapy. A secondary rise in either ESR or CRP could be a sign of recrudescence. Neither CRP nor ESR can be used to rule out osteomyelitis when normal. 98
121. What are the unique features of Candida bone infections?
 Unlike bacterial infections, inflammatory signs other than edema of the extremity are generally lacking.
Unlike bacterial infections, inflammatory signs other than edema of the extremity are generally lacking.
 Radiographs demonstrate “punched-out” metaphyseal lucencies that appear less aggressive than staphylococcal osteomyelitis.
Radiographs demonstrate “punched-out” metaphyseal lucencies that appear less aggressive than staphylococcal osteomyelitis.
 Affected infants often have a history of central line–related fungemia.
Affected infants often have a history of central line–related fungemia.
 Fungal septic arthritis can appear as late as 1 year after a treated fungal infection.
Fungal septic arthritis can appear as late as 1 year after a treated fungal infection.
 Fluconazole may be a good alternative to amphotericin B because of its good joint penetration.
Fluconazole may be a good alternative to amphotericin B because of its good joint penetration.
122. What is the first line of management for a suspected septic arthritis in a newborn infant?
TABLE 13-10
RADIOLOGIC STUDIES FOR THE DIAGNOSIS OF OSTEOMYELITIS
| TEST | PROS | CONS |
| Skeletal x-rays | Eventually, bony changes will be seen (i.e., punched-out lytic lesions, osseous lucencies, and periosteal elevation). Multiple sites of involvement can eventually be seen. Trauma (i.e., fracture) as a cause of swelling or pseudoparalysis can be ruled out. |
X-ray changes do not occur for 7 to 12 days. Conventional radiographs are insensitive to the destruction of <30% of the bone matrix. |
| 99mTc | Osteomyelitis can be detected earlier than on traditional skeletal surveys. With the higher-resolution gamma cameras used today, multiple sites of infection are often noted. |
Patient is exposed to radiation. False-negative studies have been reported. False-positive results result from increased metabolic bone activity. |
| Gallium bone | In equivocal 99mTc bone scans, gallium might be useful. | The radiation scan dose is significantly higher than in 99mTc bone scan. |
| Sonography | Most useful as a tool for guiding needle aspiration of fluid collections in joints or adjacent to bone. It is inexpensive. There is no radiation exposure. |
An experienced sonographer is required. Accuracy is variable in neonates. |
| MRI | Can detect inflammatory intramedullary diseases and gives excellent anatomic details in the early stages. | Requires general anesthesia |
| CT | Provides good definition of cortical bone and is sensitive for foe early detection of bone destruction, periosteal reaction and sequestra. | Requires anesthesia, radiation and lack of detection of intramedullary diseases (not however involvement of marrow compartment is uncommon in neonates) |
99mTc, 99mTechnetium; CT, computed tomography; MRI, magnetic resonance imaging.
From Polin R, Spitzer A. Fetal and neonatal secrets. 1st ed. Philadelphia: Hanley & Belfus; 2001. p. 288.
This entity can be confused with orbital cellulitis or dacryocystitis.
If the organism is identified and antibiotic sensitivities have been determined, treatment should be changed to the safest and most effective drug. Therapy should be continued for a minimum of 4 to 6 weeks. In the neonatal age group, orally administered antibiotics are not used because there are insufficient data regarding their absorption and efficacy. 99
Pyelonephritis and Urinary Tract Infection
126. A 10-day-old male infant presents with a 2-day history of fever, vomiting, lethargy, and jaundice. Examination reveals a temperature of 39° C, a blood pressure measurement of 65/40, and a pulse of 170 bpm; there are no focal abnormal physical findings. Laboratory data include the following levels: bilirubin, 7 mg/dL (direct, 2 mg/dL); creatinine, 0.2 mg/dL; WBC count, 20,000 cells/mm3; and urinalysis 60 WBCs per high-power field. What is the most likely diagnosis?
The signs and symptoms suggest an acute infectious process. The urinalysis is consistent with a diagnosis of acute pyelonephritis (assuming that the specimen has been properly obtained). The incidence of UTI in infants during the first month of life varies between 0.1% to 1% depending on the population studied. Unlike the distinction of cystitis and pyelonephritis in older infants and children, infection of the urinary tract in the neonate often involves the kidney.
The most common organism causing UTI in neonates is E. coli, which accounts for 91% of community-acquired infection in children younger than 8 weeks of age. Other organisms include Proteus, Pseudomonas, Klebsiella, and Enterococcus species or S. aureus, which may be associated with localized suppurative lesions in the urinary tract. With prolonged hospitalization, CoNS and Candida species can also cause UTIs in patients with or without urinary catheters. Candidiasis can be associated with fungal balls in the kidney and renal pelvis, which can lead to obstruction. 100
In the past, prophylactic antibiotics were often used for structural anomalies of the urinary tract or vesicoureteral reflux. However, a systematic review of randomized controlled trials revealed limited evidence for its efficacy. Moreover, antibiotic prophylaxis may increase the risk of a subsequent UTI by a resistant microrganism. 101102
133. In addition to urinalysis and urine culture, what other tests are indicated in the treatment of an infant with possible UTI?
In addition to diagnosing UTI, it is also important to evaluate the urinary tract for underlying structural or functional abnormalities that may predispose the infant to recurrent UTIs. Abdominal ultrasound is a safe and noninvasive method of evaluating structural abnormalities of the urinary tract and is the initial imaging test of choice. Intravenous pyelography can be useful in assessing the function of the kidneys. Radionuclide scans such as dimercaptosuccinic acid scans can also be used to evaluate function and structural abnormalities, specifically renal scars following UTI. Vesicoureterography to evaluate the presence or absence of vesicoureteric reflux should be performed after completion of treatment of the UTI, because transient vesicoureteral reflux commonly occurs with the acute infection. 103
Omphalitis
134. What are the presenting signs of omphalitis in neonates?
Serosanguinous drainage may be seen with a patent urachus or omphalomesenteric duct.
141. What syndrome can be associated with chronic omphalitis or delayed separation of the umbilical cord?
142. You are informed that a newborn is suspected to have funisitis. Where should you look for that infection?
Funisitis is a mild inflammation of the umbilical stump with minimal drainage and erythema in the surrounding tissue. It is a local noninvasive infection that may become invasive and lead to a severe abdominal wall inflammation associated with necrotizing fasciitis. 106
Listeriosis
143. Is L. monocytogenes still a significant pathogen to consider when evaluating sepsis in neonates?
Listeria species organisms are ubiquitous in nature. Although direct transmission to humans from infected animals has been reported, most human infections are acquired through ingestion of contaminated food. The relative resistance of Listeria organisms to high temperatures and their ability to multiply at low temperatures provide opportunities for heavy colonization of dairy products if pasteurization has been improperly carried out. Outbreaks are commonly associated with prepared meat products and seafood products. Although L. monocytogenes is probably ingested frequently, the incidence of clinical diseases in humans is relatively low, suggesting that the organism has a relatively low virulence. This is supported by the large inoculum required to cause infection in normal hosts. Nevertheless, listeriosis represents the leading cause of death from foodborne diseases in the United States. 107108
After ingestion of the microorganism, the incubation period for L. monocytogenes is less than 24 hours, but it can range from 6 hours up to 3 weeks. Invasion of the intestinal mucosal barrier leads to bacteremia, resulting in a flulike illness with fever, chills, myalgia, arthralgia, headache, and backache. Premature labor in pregnant women with listeriosis is common in approximately 70% of cases. The neonatal mortality rate, including stillbirth and abortion, is 40% to 50%. Often the placenta becomes a reservoir for bacterial proliferation, resulting in amnionitis with persistence of maternal symptoms until abortion or delivery occurs. Symptoms in the mother usually subside with or without antibiotic treatment soon after delivery. If the infection is recognized promptly, the mother may be treated effectively, preserving the pregnancy. 109110
Late-onset neonatal listeriosis commonly presents as meningitis. Affected infants may not appear particularly ill and might elude diagnosis for several days. A striking predominance of boys has been noticed in most series. Other clinical forms of diseases at this age include colitis with associated diarrhea and sepsis without meningitis. Gram stain of the CSF does not always yield the correct diagnosis because variable decoloration results in organisms that appear as either gram-negative rods or gram-positive cocci. The mortality risk of late-onset disease is generally low if treatment is started promptly. 111112113
147. What is the pathogenesis of L. monocytogenes infection, and why does insufficiency of cellular immunity in particular contribute to the development of disease?
L. monocytogenes is an intracellular, facultative anaerobic, non–spore-forming, motile gram-positive bacillus that multiplies intracellularly. Once phagocytized, invasive L. monocytogenes replicates rapidly within the cytosol, thanks to its major virulence factor, listeriolysin O. Using the cell’s own cytoskeletal actin polymerization mechanism, L. monocytogenes pushes outward on the host cell’s membrane, forming filopods, which are then injected into neighboring cells. Cell-to-cell transmission spreads rapidly without exposure to extracellular host defenses such as antibodies or neutrophils. T-lymphocytes therefore provide the only natural recognition and immunity toward L. monocytogenes, although macrophage killing (probably using nitric oxide) may also occur. Because cellular immunity is suppressed during pregnancy and is naturally deficient during early neonatal life, L. monocytogenes enjoys an advantage during these host-vulnerable periods. In hosts with adequate cellular responses, symptomatic infection is rare and self-limited. 114
Trimethoprim-sulfamethoxazole (TMP-SMX) can be considered for mothers who are sensitive to penicillin. Cephalosporins are not active against listeriosis. Iron therapy for anemia should be withheld during treatment of listeriosis because iron enhances the organism’s growth in vitro and is therefore a virulence factor, contributing to the host’s susceptibility to infection. Listeriosis is a notifiable disease in the United States. 115
Syphilis
The changing incidence of congenital syphilis over the years follows the trend of acquired syphilis in women. After a major public success in early 1990, with the lowest rates since reporting began in 1941 (10.5 cases/100,000 live births), the incidence of primary and secondary syphilis has increased since 2005, particularly in large urban areas and in the southern United States. Insufficient public health resources, use of illegal drugs (particularly crack cocaine), and coinfection with HIV are factors implicated in this increase. The World Health Organization (WHO) estimates that 1 million pregnancies are affected by syphilis worldwide. 116
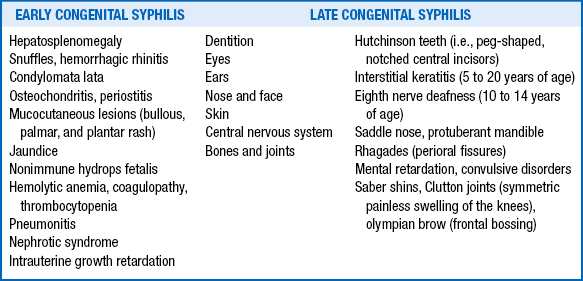
Syphilis has commonly been described as the “great imitator” because of the variety of clinical manifestations; approximately two thirds of infected newborns are asymptomatic at birth, but later (even decades later) manifestations are not uncommon. Clinical manifestations appearing within the first 2 years of life are considered early congenital syphilis, and manifestations occurring after this time are considered late congenital syphilis ( Table 13-11). 117
The findings of Hutchinson teeth, interstitial keratitis, and eighth nerve deafness constitute Hutchinson triad and are virtually pathognomonic for late congenital syphilis. 118
Nontreponemal tests for syphilis include the Venereal Diseases Research Laboratory (VDRL) test and the rapid plasma regain (RPR) test. These tests are inexpensive and rapid, so they are used mainly for screening. Quantitative results can assist in monitoring disease activity or response to treatment (preferably if performed in the same laboratory). They can be falsely negative in early primary syphilis and late congenital syphilis. Any reactive nontreponemal test needs to be confirmed with a specific treponemal test to exclude a false-positive reading, but treatment should not be delayed if the patient is symptomatic or at high risk for infection. A positive VDRL in CSF is highly specific for neurosyphilis but is insensitive. Treponemal tests include fluorescent treponemal antibody absorption (FTA-ABS) and T. pallidum particle agglutination (TP-PA). These tests will remain reactive for life even after successful treatment, and they correlate poorly with disease activity. 119
Infants should be treated if they have the following:
 Any evidence of active disease (abnormal complete blood count [CBC], physical examination findings, or x-ray)
Any evidence of active disease (abnormal complete blood count [CBC], physical examination findings, or x-ray)
 An abnormal CSF finding, regardless of serology
An abnormal CSF finding, regardless of serology
 Quantitative nontreponemal antibody titers that are at least four times greater than maternal titers
Quantitative nontreponemal antibody titers that are at least four times greater than maternal titers
Aqueous crystalline penicillin G intravenous for 10 days is the regimen of choice for congenital syphilis. 120
Human Immunodeficiency Virus
This scenario is drastically different in resource-limited countries where the prevention of mother-to-child transmission is a major public health challenge. Despite knowing the routes of transmission of the virus, approximately 500,000 children worldwide acquire the infection each year, primarily through mother-to-child transmission. Children still represent 14% of new HIV infections worldwide and almost one fifth of annual HIV-related deaths. 121
The risk of infection for an infant born to an HIV-infected mother without an intervention to prevent transmission is estimated to range between 12% and 40%. Dramatic declines in the number of HIV-infected children, to less than 2%, have been observed after the introduction of universal antenatal HIV testing, combination antiretroviral treatment during pregnancy, elective cesarean section, and avoidance of breastfeeding.
161. What factors are associated with an increased risk of perinatal transmission of HIV?
HIV infection may be transmitted in utero, intrapartum, or after birth through breastfeeding. In the absence of breastfeeding, it is believed that approximately 20% to 30% of perinatal infections occur in utero, with the remaining 70% to 80% occurring during the intrapartum period. If an infant escapes infection in utero and during delivery but is then breastfed, the risk for HIV transmission is approximately 15% when breastfeeding is continued for at least 6 months. 122
164. Why is the enzyme-linked immunosorbent assay (ELISA) HIV antibody screening test not useful for the diagnosis of HIV-exposed neonates?
165. What is the best diagnostic test for defining HIV infections in infants, and when should it be ordered?
All HIV-exposed infants should have an HIV PCR-DNA assay performed at 14 to 21 days of age; if results are negative, it should be repeated at 1 to 2 months of age and again at 4 to 6 months of age. An infant is considered infected if two separate samples test positive for DNA or RNA PCR. 123
Starting antiretroviral treatment in an infant depends on virologic, clinical, and immunologic parameters. Recommendations change with time, and consultation with a pediatric HIV expert is advised. Current guidelines are available online at http://www.aidsinfo.nih.gov.
Cytomegalovirus (CMV)
Congenital CMV infection occurs in 1% to 2% of all live births. It is transmitted transplacentally and is 40% more common after primary infections in the mother during the first half of the pregnancy than in those who have serologic evidence of previous CMV infection. Approximately 90% of infants with congenital CMV infection are asymptomatic at birth. The major concern in these babies is the 5% to 10% risk of developing hearing loss during the preschool years. 124
Approximately 10% of infected babies have symptoms at birth. Manifestations include the following:
 Intrauterine growth restriction
Intrauterine growth restriction
 Microcephaly, intracranial calcifications
Microcephaly, intracranial calcifications
173. What are the most reliable methods for diagnosing congenital CMV infection?
The diagnosis of congenital CMV is challenging because of the ubiquity of the virus. Isolation of CMV from urine during the first 2 to 4 weeks of life is the most reliable method for diagnosing congenital infection. Detection of strongly positive IgM antibodies to CMV in serum obtained within the first few days after birth is highly suggestive of congenital infection, but different assays vary in accuracy for identification of primary infection. 125
174. What methods are available to prevent the transmission of CMV to neonates through blood products?
176. What is the relationship between neonatal clinical signs and neurologic outcome in congenital CMV infection?
Long-term neurologic disability (excluding SNHL) has been reported in approximately 50% of those with manifestations and less than 5% of those without manifestations at birth. 126
Data in infants with symptomatic congenital CMV involving the CNS suggest that the prognosis at 1 to 2 years of age may be improved if infected infants are treated with parenteral ganciclovir for 6 weeks. Valganciclovir given orally provides the same systemic levels of intravenous ganciclovir. 127128
Patients treated with ganciclovir need to have their absolute neutrophil count monitored closely because as many as 60% of patients will develop significant neutropenia. If neutropenia is evident, the dose should be reduced by 50%; if neutropenia persists, the therapy should be discontinued. 129
Herpes Simplex Virus
Newborns with skin vesicles should be rapidly evaluated for the possibility of neonatal HSV infection. HSV PCR is the diagnostic method of choice, especially with CNS involvement (a reported sensitivity of 75% to 100% with CSF). HSV PCR can also be used to test serum; however, the test is not as reliable when serum is used. HSV grows readily in cultures. Babies in whom the diagnosis is established or who are strongly suspected of having neonatal HSV should be further evaluated for the possibility of disseminated infection and involvement of the CNS. Usually this means obtaining liver function tests, an ophthalmologic examination, lumbar puncture, and magnetic resonance imaging (MRI) or computed tomography (CT) scan. 130131
Until fairly recently, it was thought that women with frequent reactivation episodes of genital HSV were at greatest risk to deliver an infected infant. Prospective studies, however, indicate that the infant at greatest risk of developing neonatal HSV is born to a woman with a primary asymptomatic genital HSV infection. The risk of infection is only about 2% in infants born to mothers with recurrent HSV but 25% to 60% in infants born to women with a primary HSV at term. All women need to be examined for the presence of active lesions; however, even this will miss asymptomatic infections. The best screening test for prevention of severe neonatal HSV is a high index of suspicion when an infant develops vesicular skin lesions because more than three quarters of vertically infected infants are born from mothers with no history or clinical findings suggestive of HSV. ACOG recommends that women with active recurrent genital herpes be offered suppressive viral therapy at or beyond 36 weeks’ gestation to delivery. Universal screening is not recommended. 132
181. How is neonatal HSV classified? What is the importance of the classification with regard to prognosis?
Most often, the virus multiplies on the mucosa of the maternal genital tract, and the baby is infected during vaginal delivery. In only about 5% of infections the virus crosses the placenta to cause congenital, rather than neonatal, infection. If the diagnosis of maternal primary HSV is known at delivery and the membranes are not ruptured, infection of the newborn infant can be prevented by performing
a cesarean section. The risk that the infant will be infected is increased if the skin is broken for any reason (e.g., from a scalp monitor). Infants also can be infected with HSV type 1 if a mother has primary active HSV-1 infection, usually in the throat and mouth, at delivery. Furthermore, infants can become infected by nursing on an infected breast. Transmission from care providers, fathers, and grandparents has also been demonstrated. 133
The treatment is administration of intravenous acyclovir to all infants in whom the diagnosis of neonatal HSV is either established or suspected pending diagnostic studies. Acyclovir is an antiviral drug that interferes with the replication of HSV DNA by acting as a chain terminator and interfering with the action of DNA polymerase. Because its action occurs mainly in infected cells, it is very well tolerated. Early therapy with acyclovir has decreased mortality and morbidity resulting from serious HSV infections by 30% to 50%. Acyclovir is usually administered at a dosage of 60 mg/kg/day intravenously for 14 to 21 days, depending on the condition of the infant. 134
Approximately 50% of infants who survive neonatal HSV experience cutaneous recurrences. Use of oral acyclovir suppressive therapy for 6 months after treatment of acute neonatal HSV diseases has been shown to improve neurodevelopmental outcomes in infants with HSV CNS involvement and to prevent skin recurrences in all infants infected with HSV regardless of their neonatal manifestations. Neutrophil counts should be monitored at 2 and 4 weeks after initiation of therapy and then monthly during the acyclovir treatment. The rate of severe neutropenia (<500 cells/mL) ranges from 20% to 25% while on acyclovir suppressive therapy; in every instance, the neutropenia was reversible and no infants had associated complications. 135
Cesarean section is recommended for mothers who have active genital lesions and a suspected primary infection. Infants born to mothers with active genital HSV disease should be isolated from other infants and evaluated with “surface cultures” at 24 to 48 hours of life. Those infants born to mothers with primary disease might benefit from empiric parenteral acyclovir treatment after obtaining “surface cultures.” However, virtually no experts recommend treatment for infants born from mothers with active recurrent disease. If “surfaces cultures” are positive, the infant should be evaluated for HSV disease and should be treated to prevent progression to HSV disease. The duration of treatment is controversial. 136
Antibody titers are of little use in the diagnosis of HSV because it takes at least several days after infection for antibodies to rise. Therefore it is preferable to demonstrate the presence of virus, viral antigens, or DNA in tissues for diagnosis. Antibody titers may be useful to differentiate between maternal first infections versus recurrence because many primary infections are asymptomatic. 137
Varicella Zoster Virus
Reactivation of VZV acquired in utero is common. Thus zoster develops in approximately 15% of infants with the congenital syndrome, usually in the first few years of life. 138
189. What is the appropriate management of an infant born to a woman with varicella at term delivery?
It is possible to prevent this severe form of infant varicella by administering VZIG to the baby as soon as possible after birth. Rarely, VZIG may be ineffective in infants, so babies given this prophylaxis warrant close follow-up. Many will develop a mild form of clinical varicella with fewer than 100 skin vesicles despite VZIG. Indications for adding antiviral therapy (acyclovir) are extensive skin lesions and development of pneumonia, which suggests severe varicella. Hospitalized preterm infants exposed to VZV should receive VZIG if they are older than 28 weeks’ gestation and have mothers who lack immunity against VZV or if they are younger than 28 weeks’ gestation regardless of maternal immunity status. 139
Infants whose mothers develop varicella more than 48 hours postpartum are at significantly less risk from varicella and do not require VZIG, although some physicians may elect to administer it in infants exposed to VZV during the first 2 weeks of life whose mothers lack immunity. In this case, infection of the infant would not result from exposure to VZV in utero but rather from postpartum exposure.
With regard to isolation of infants perinatally exposed to VZV, there are two points to consider:
1. The incubation period can be as short as 10 days and is counted from the time of onset of maternal rash.
2. Infants are contagious only around the time when rash is expected to occur.
The Red Book recommends airborne and contact precautions for neonates born to women with varicella, and if the neonate is still hospitalized, the isolation should be continued until 21 or 28 days of age after exposure for those who received VZIG or intravenous Ig. 140141
Varicella in adults tends to be more severe than in children. Although the data are not conclusive, most experts believe that varicella in pregnant women is likely to be more severe than in nonpregnant women, especially in the third trimester of pregnancy. Therefore pregnant women with varicella should be closely observed, particularly for development of primary pneumonia. Pneumonia usually presents with fever, cough, dyspnea, and bilateral fluffy interstitial infiltrates on chest x-ray. Pregnant women with varicella pneumonia or even suspected varicella pneumonia should be treated with intravenous acyclovir for 7 days. Maternal acyclovir therapy has not been associated with fetal malformations, so most physicians will treat women who developed varicella or zoster within 1 day of the onset of varicella and 3 days of the onset of zoster. 142
Varicella vaccine should not be administered to pregnant women because it contains a live virus. However, no cases of congenital varicella syndrome have been reported in women who inadvertently received the vaccine. Pregnancy is not a contraindication to immunizing children in the household. If a woman lacks VZV immunity after delivery, the varicella vaccine should be administered; there is no evidence of transmission of vaccine strain through breast milk. 143
Toxoplasmosis
Toxoplasma gondii is a protozoan and obligate intracellular parasite that exists in three developmental stages: tachyzoite (in acute infections), tissue cyst containing bradyzoites in chronic latent infection, and oocyst containing sporozoites in the intestines of cats. Once infected with the tachyzoite, the organism may encyst, commonly in the skeletal muscles. Infection occurs in multiple animal species, but the cat appears to be the definitive host, and the parasite replicates sexually in the feline small intestine. Cats excrete millions of oocysts in their stool after primary infection. Oocysts must mature or sporulate in the soil (which takes at least 24 hours) before they are infectious. Sporulated oocysts can survive for a long time.
Therefore Toxoplasma infection is acquired through the ingestion of undercooked or raw meat (containing tissue cysts) or water or other foods contaminated by oocysts that have been excreted in the feces of infected domestic animals. It is possible to become infected through exposure to soil contaminated with cat feces. 144145
The incidence of congenital toxoplasmosis in the United States is estimated to be between 1 in 1000 and 1 in 10,000 live births. Congenital infection is associated with maternal primary infection in pregnancy. Transmission rates to the fetus depend on the stage of the pregnancy and treatment during pregnancy, but approximately one third of infected women will have an infected fetus. Transmission during the first trimester tends to produce a more severe disease. Maternal treatment with spiramycin may decrease the severity of sequelae in the fetus once congenital toxoplasmosis has occurred. 146
The dosage should be adjusted weekly. In addition, a CBC with differential should be performed by finger stick twice weekly to measure the absolute neutrophil count. Remember that pyrimethamine is a folic acid antagonist and therefore may cause thrombocytopenia, granulocytopenia, and anemia resulting from bone marrow suppression. The use of folinic acid can counteract this side effect. 147148
199. If maternal infection is suspected but full evaluation for toxoplasmosis in the newborn is negative except for T. gondii–specific IgG antibodies, what should be done?
In this case, transfer of maternal antibodies may be suspected as the reason for the presence of IgG antibodies. A continuous decrease in transplacental IgG antibody should be observed, with the antibody becoming undetectable by 6 to 12 months of age. The diagnosis of congenital toxoplasmosis is complex, and consultation with an infectious disease specialist is recommended.
Ureaplasma Urealitycum
Ureaplasma can be isolated from the genital tract of 40% to 80% of sexually active asymptomatic women. Colonization has been linked to younger age, lower socioeconomic status, multiple sexual partners, oral contraceptive use, recent antibiotic treatment, and African-American ethnicity. 149
201. What is the rate of vertical transmission for U. urealyticum, and what factors influence that rate?
The vertical transmission rate ranges from 25% to 60%. Transmission occurs in utero by ascending infection or during delivery through an infected birth canal. Preterm and VLBW infants are more likely to acquire U. urealyticum in the lower respiratory tract. The mode of delivery does not influence the rate of transmission, but it increases in the presence of chorioamnionitis or prolonged rupture of membranes. Neonatal colonization can last for several months, so a positive culture does not confirm causality in infections. 150
202. What are the clinical manifestations of U. urealyticum infection?
 Isolation of the organism in the amniotic fluid has been associated with histologic evidence of chorioamnionitis, even among asymptomatic women.
Isolation of the organism in the amniotic fluid has been associated with histologic evidence of chorioamnionitis, even among asymptomatic women.
 Postpartum fever, septic abortion, fetal loss, and preterm birth have been associated with Ureaplasma infection.
Postpartum fever, septic abortion, fetal loss, and preterm birth have been associated with Ureaplasma infection.
 Several case reports suggest that U. urealyticum causes pneumonia in the newborn, though its role in chronic lung disease in prematurity remains controversial.
Several case reports suggest that U. urealyticum causes pneumonia in the newborn, though its role in chronic lung disease in prematurity remains controversial.
 U. urealyticum has been isolated from CSF of newborns with meningitis, intraventricular hemorrhage, and hydrocephalus. Although several case reports describe a clinical response to treatment, its role remains unclear. 151152
U. urealyticum has been isolated from CSF of newborns with meningitis, intraventricular hemorrhage, and hydrocephalus. Although several case reports describe a clinical response to treatment, its role remains unclear. 151152
Causation has not been fully established for chronic lung disease and Ureaplasma. Furthermore, organisms are often present in healthy infants and can spontaneously disappear without treatment. Small randomized, controlled trials have not shown any benefit of treatment of U. urealyticum to prevent chronic lung disease.
Infectious Hepatitis
Several infectious agents can cause hepatitis in neonates and infants, including the TORCH pathogens: T. gondii, Other infectious etiologies (i.e., syphilis; hepatitis B, C, and D; and, rarely, hepatitis A virus), Rubella, CMV, and Herpes simplex. Additional etiologies in this age group include adenovirus, coxsackievirus, enterovirus, Epstein–Barr virus, HIV, and VZV. 153
207. What are the best ways to prevent transmission of HBV from an infected mother to a newborn?
Age at infection is the main determinant of risk for progression to chronic disease. As many as 90% of infected newborns or infants younger than 1 year of age will develop chronic infection; 25% to 50% will develop chronic infection if the infection occurs between 1 and 5 years of age; and 5% to 10% will develop chronic infection when infection happens in older children or adults. In the absence of treatment, up to 25% of infants and children who have chronic hepatitis B will die as a result of hepatocellular carcinoma or cirrhosis. 155
Maternal HCV IgG antibody can persist in a neonate for up to 18 months. Therefore diagnosis in a neonate can be made only by using reverse transcriptase PCR assays for HCV RNA, which can be detected within 1 to 2 weeks of exposure. However, because false-negative and false-positive results can occur with PCR tests, a single result is not conclusive. It is therefore recommended that infants born to infected mothers be tested at or after the first well-baby visit at 1 or 2 months of age and then undergo repeat testing. Children with persistence of HCV RNA beyond 6 months’ duration are assumed to have chronic infection. 156
Parvovirus
213. If parvovirus B19 infection occurs during pregnancy, what is the risk for an adverse fetal outcome (i.e., nonimmune hydrops fetalis or fetal demise)?
The risk of fetal death is between 2% and 6% when infection occurs during pregnancy. The greatest risk appears to occur during the first half of pregnancy. 157
214. You receive a call from a worried mother of a 2-year-old patient. The family puppy was just diagnosed with parvovirus hemorrhagic colitis. The mother is concerned about possible transmission to her toddler or herself (she is 3 months pregnant). What advice do you give?
She need not worry. Canine parvovirus is not a human pathogen.
215. Parvovirus B19 is the cause of erythema infectiosum (EI). How often do children or adults infected with parvovirus B19 develop classic EI?
Parvoviruses require actively dividing cells to replicate. Therefore it preferentially infects red blood cell (RBC) precursors. In patients with hemolytic anemia and a high RBC turnover, the viral cytopathic effect destroys bone marrow RBC precursors, resulting in reticulocytopenia. The transient arrest of erythrocyte production results in profound anemia in persons with a shortened RBC survival, such as patients with hemolytic anemia. In fetuses shortened RBC survival (60 to 80 days versus 110 to 120 days in adults) also contributes to the severity of anemia. 158
1Maródi L. Innate cellular immune responses in newborns. Clin Immunol 2006 Feb;118(2-3):137–44.
2Burgio GR, Hanson LA, Ugazio AG, editors. Immunology of the neonate. 1st ed. Berlin: Springer; 2012.
3Dzwonek AB, Neth OW, Thiébaut R, et al. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res 2008;63(6):680–5.
4Strunk T, Burgner D. Genetic susceptibility to neonatal infection. Curr Opin Infect Dis 2006;19(3):259–63.
5Nussbaum C, Sperandio M. Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol 2011;90(1):74–81.
6Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7(5):379–90.
7Härtel C, Osthues I, Rupp J, et al. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F140–5.
8Strunk T, Richmond P, Simmer K, et al. Neonatal immune responses to coagulase-negative staphylococci. Curr Opin Infect Dis. 2007;20(4):370–5.
9Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
10Nussbaum C, Sperandio M. Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol 2011;90(1):74–81.
11Yang KD, Hill HR. Granulocyte function disorders: aspects of development, genetics and management. Pediatr Infect Dis J 2001;20(9):889–900.
12Strunk T, Currie A, Richmond P, et al. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med 2011;24(1):25–31.
13Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
14Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol 2005;141(1):10–8.
15van den Berg JM, Kuijpers TW. Educational paper: defects in number and function of neutrophilic granulocytes causing primary immunodeficiency. Eur J Pediatr 2011;170(11):1369–76.
16Clapp DW. Developmental regulation of the immune system. Semin Perinatol 2006;30(2):69–72.
17Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr Res 2009;65(5 Pt 2):98R–105R.
18Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev 2004;17(1):1–13.
19Townshend J, Clark J, Cant A, et al. Congenital neutropenia. Arch Dis Child Educ Pract Ed 2008;93(1):14–8.
20Randis TM, Polin RA. Early-onset group B Streptococcal sepsis: new recommendations from the Centres for Disease Control and Prevention. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F291–4.
21Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006–15.
22Eberly MD, Rajnik M. The effect of universal maternal screening on the incidence of neonatal early-onset group B streptococcal disease. Clin Pediatr (Phila) 2009;48(4):369–75.
23Sakata H. Evaluation of intrapartum antibiotic prophylaxis for the prevention of early-onset group B streptococcal infection. J Infect Chemother 2012;18(6):853–857.
24Randis TM, Polin RA. Early-onset group B Streptococcal sepsis: new recommendations from the Centres for Disease Control and Prevention. Arch Dis Child Fetal Neonatal Ed 2012;97(4):F291–4.
25Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 2002;347(4):240–7.
26Schuchat A, Zywicki SS, Dinsmoor MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics 2000;105(1 Pt 1):21–6.
27Hyde TB, Hilger TM, Reingold A, et al. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics 2002;110(4):690–5.
28Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
29Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 2002;347(4):240–7.
30Lin F-YC, Weisman LE, Troendle J, et al. Prematurity is the major risk factor for late-onset group B streptococcus disease. J Infect Dis 2003;188(2):267–71.
31Healy CM, Baker CJ, Palazzi DL, et al. Distinguishing true coagulase-negative Staphylococcus infections from contaminants in the neonatal intensive care unit. J Perinatol 2013;33(1):52–58.
32Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol 2010;37(2):439–79.
33Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006–15.
34Jordan JA. Molecular diagnosis of neonatal sepsis. Clin Perinatol 2010;37(2):411–9.
35Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants >/=2000 grams at birth: a population-based study. Pediatrics 2000;106(2 Pt 1):256–63.
36Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed.Philadelphia: Saunders; 2010.
37Polin RA, Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 2012;129(5):1006–15.
38Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 2002;347(4):240–7.
39Lieberman E, Lang J, Richardson DK, et al. Intrapartum maternal fever and neonatal outcome. Pediatrics 2000;105(1 Pt 1):8–13.
40Srinivasan L, Shah SS, Padula MA, et al. Cerebrospinal fluid reference ranges in term and preterm infants in the neonatal intensive care unit. J Pediatr 2012;161(4):729–34.
41Venkatesh M, Flores A, Luna RA, et al. Molecular microbiological methods in the diagnosis of neonatal sepsis. Expert Rev Anti Infect Ther 2010 Sep;8(9):1037–48.
42Muller-Pebody B, Johnson AP, Heath PT, et al. Empirical treatment of neonatal sepsis: are the current guidelines adequate? Arch Dis Child Fetal Neonatal Ed 2011;96(1):F4–8.
43Hyde TB, Hilger TM, Reingold A, et al. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics 2002;110(4):690–5.
44INIS Collaborative Group, Brocklehurst P, Farrell B, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 2011;365(13):1201–11.
45Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
46Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
47Faro J, Katz A, Bishop K, et al. Rapid diagnostic test for identifying group B streptococcus. Am J Perinatol 2011;28(10):811–4.
48de Zoysa A, Edwards K, Gharbia S, et al. Non-culture detection of Streptococcus agalactiae (Lancefield group B Streptococcus) in clinical samples by real-time PCR. J Med Microbiol 2012;61(Pt 8):1086–90.
49Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347(4):233–9.
50Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult – online and print. 7th ed. Philadelphia: Saunders; 2010.
51Stafford IA, Stewart RD, Sheffield JS, et al. Efficacy of maternal and neonatal chemoprophylaxis for early-onset group B streptococcal disease. Obstet Gynecol 2012;120(1):123–9.
52Committee on Infectious Diseases, Committee on Fetus and Newborn, Baker CJ, et al. Policy statement—recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics 2011;128(3):611–6.
53Cagno CK, Pettit JM, Weiss BD. Prevention of perinatal group B streptococcal disease: updated CDC guideline. Am Fam Physician 2012;86(1):59–65.
54Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000;342(1):15–20.
55Sass L. Group B streptococcal infections. Pediatr Rev 2012;33(5):219–24–quiz224–5.
56Kilbride HW, Wirtschafter DD, Powers RJ, et al. Implementation of evidence-based potentially better practices to decrease nosocomial infections. Pediatrics 2003;111(4 Pt 2):e519–33.
57Craft A, Finer N. Nosocomial coagulase negative staphylococcal (CoNS) catheter-related sepsis in preterm infants: definition, diagnosis, prophylaxis, and prevention. J Perinatol 2001;21(3):186–92.
58Healy CM, Baker CJ, Palazzi DL, et al. Distinguishing true coagulase-negative Staphylococcus infections from contaminants in the neonatal intensive care unit. J Perinatol 2013;33(1):52–8.
59Struthers S, Underhill H, Albersheim S, et al. A comparison of two versus one blood culture in the diagnosis and treatment of coagulase-negative staphylococcus in the neonatal intensive care unit. J Perinatol 2002;22(7):547–9.
60Isaacs D, Australasian Study Group for Neonatal Infections. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Arch Dis Child Fetal Neonatal Ed 2003;88(2):F89–93.
61Kilbride HW, Powers R, Wirtschafter DD, et al. Evaluation and development of potentially better practices to prevent neonatal nosocomial bacteremia. Pediatrics 2003;111(4 Pt 2):e504–18.
62Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988-1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics 2000;106(6):1387–90.
63Robinson JA, Pham HD, Bloom BT, et al. Risk factors for persistent candidemia infection in a neonatal intensive care unit and its effect on mortality and length of hospitalization. J Perinatol 2012;32(8):621–5.
64Benjamin DK, Ross K, McKinney RE, et al. When to suspect fungal infection in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics 2000;106(4):712–8.
65Chitnis AS, Magill SS, Edwards JR, et al. Trends in Candida central line-associated bloodstream infections among NICUs, 1999-2009. Pediatrics 2012;130(1):e46–52.
66Miranda LDN, Rodrigues ECA, Costa SF, et al. Candida parapsilosis candidemia in a neonatal unit over 7 years: a case series study. BMJ Open 2012;2(4).
67Ali GY, Algohary EHSS, Rashed KA, et al. Prevalence of Candida colonization in preterm newborns and VLBW in neonatal intensive care unit: role of maternal colonization as a risk factor in transmission of disease. J Matern Fetal Neonatal Med 2012;25(6):789–95.
68Benjamin DK, Poole C, Steinbach WJ, et al. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112(3 Pt 1):634–40.
69Karlowicz MG, Giannone PJ, Pestian J, et al. Does candidemia predict threshold retinopathy of prematurity in extremely low birth weight (</=1000 g) neonates? Pediatrics 2000 May;105(5):1036–40.
70Kaufman DA, Manzoni P. Strategies to prevent invasive candidal infection in extremely preterm infants. Clin Peritanol 2010;37(3):611–28.
71Leibovitz E. Strategies for the prevention of neonatal candidiasis. Pediatr Neonatal 2012;53(2):83–9.
72Kaufman D, Boyle R, Hazen KC, et al. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 2001;345(23):1660–6.
73Kaufman DA, Manzoni P. Strategies to prevent invasive candidal infection in extremely preterm infants. Clinics in Perinatology 2010;37(3):611–28.
74Hsieh E, Smith PB, Jacqz-Aigrain E, et al. Neonatal fungal infections: when to treat? Early Hum Dev 2012;88 Suppl 2:S6–S10.
75Greenberg RG, Benjamin DK, Gantz MG, et al. Empiric antifungal therapy and outcomes in extremely low birth weight infants with invasive candidiasis. J Pediatr 2012;161(2):264–9.
76Manzoni P, Benjamin DK, Franco C, et al. Echinocandins for the nursery: an update. Curr Drug Metab 2013;14(2):203–207.
77Ascher SB, Smith PB, Watt K, et al. Antifungal therapy and outcomes in infants with invasive Candida infections. Pediatr Infect Dis J 2012;31(5):439–43.
78Harris MC, Pereira GR, Myers MD, et al. Candidal arthritis in infants previously treated for systemic candidiasis during the newborn period: report of three cases. Pediatr Emerg Care 2000;16(4):249–51.
79Clark R, Powers R, White R, et al. Nosocomial infection in the NICU: a medical complication or unavoidable problem? J Perinatol 2004;24(6):382–8.
80Polin RA, Denson S, Brady MT, et al. Epidemiology and diagnosis of health care-associated infections in the NICU. Pediatrics 2012;129(4):e1104–9.
81Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult – online and print. 7th ed. Philadelphia: Saunders; 2010.
82Polin RA, Denson S, Brady MT, et al. Strategies for prevention of health care-associated infections in the NICU. Pediatrics 2012;129(4):e1085–93.
83Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
84Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis 2005;16(4):235–44.
85Shah S. Pediatric practice infectious diseases. 1st ed. Pennsylvania: McGraw-Hill Professional; 2009.
86Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
87Thordsen JE, Harris L, Hubbard GB. Pediatric endophthalmitis. A 10-year consecutive series. Retina (Philadelphia, Pa.) 2008;28(3 Suppl):S3–7.
88Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
89Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis 2011;53 (Suppl 3):S99–102.
90Silva MJPM de A, Florêncio GLD, Gabiatti JRE, et al. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Braz J Infect Dis 2011;15(6):533–9.
91Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
92Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
93Bellulo S, Bosdure E, David M, et al. Chlamydia trachomatis pneumonia: two atypical case reports. Arch Pediatr 2012;19(2):142–5.
94Horvat JC, Starkey MR, Kim RY, et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol 2010;125(3):617–25.
95Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases: expert consult—online and print. 4th ed. Philadelphia: Saunders; 2012.
96Zhang J, Lee BH, Chen C. Gram-negative neonatal osteomyelitis: two case reports. Neonatal Netw 2011;30(2):81–7.
97Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
98Dessì A, Crisafulli M, Accossu S, et al. Osteo-articular infections in newborns: diagnosis and treatment. J Chemother 2008;20(5):542–50.
99Ecury-Goosssen GM, Huysman MA, Verhallen-Dantuma JCTM, et al. Sequential intravenous-oral antibiotic therapy for neonatal osteomyelitis. Pediat Infect Dis J 2009;28(1):72–3.
100Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult – online and print. 7th ed. Philadelphia: Saunders; 2010.
101Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases: expert consult—online and print. 4th ed. Philadelphia: Saunders; 2012.
102Montini G, Rigon L, Zucchetta P, et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics 2008;122(5):1064–71.
103Beetz R. Evaluation and management of urinary tract infections in the neonate. Curr Opin Pediatr 2012;24(2):205–11.
104Mullany LC, Saha SK, Shah R, et al. Impact of 4.0% chlorhexidine cord cleansing on the bacteriological profile of the newborn umbilical stump in rural Sylhet District, Bangladesh: a community-based, cluster-randomized trial. Pediatr Infect Dis J 2012;31(5):444–450.
105Shah S. Pediatric practice infectious diseases. 1st ed. Pennsylvania: McGraw-Hill Professional; 2009.
106Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
107Taillefer C, Boucher M, Laferrière C, et al. Perinatal listeriosis: Canada’s 2008 outbreaks. J Obstet Gynaecol Can 2010;32(1):45–8.
108Jackson KA, Iwamoto M, Swerdlow D. Pregnancy-associated listeriosis. Epidemiol Infect 2010;138(10):1503–9.
109Jackson KA, Iwamoto M, Swerdlow D. Pregnancy-associated listeriosis. Epidemiol Infect 2010;138(10):1503–9.
110Lamont RF, Sobel J, Mazaki-Tovi S, et al. Listeriosis in human pregnancy: a systematic review. J Perinat Med 2011;39(3):227–36.
111Remington JS, Klein JO, Wilson CB. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010
112Le Monnier A, Abachin E, Beretti J-L, et al. Diagnosis of Listeria monocytogenes meningoencephalitis by real-time PCR for the hly gene. J Clin Microbiol 2011;49(11):3917–23.
113Jiao Y, Zhang W, Ma J, et al. Early onset of neonatal listeriosis. Pediatr Int 2011;53(6):1034–7.
114Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases: expert consult—online and print. 4th ed. Philadelphia: Saunders; 2012.
115Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
116Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult – online and print. 7th ed. Philadelphia: Saunders; 2010.
117Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult – online and print. 7th ed. Philadelphia: Saunders; 2010.
118Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases: expert consult—online and print. 4th ed. Philadelphia: Saunders; 2012.
119Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
120Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
121Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA 2012;308(4):343–4.
122Nielsen-Saines K, Watts DH, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med 2012;366(25):2368–79.
123Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
124Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother 2009;63(5):862–7.
125Bradford RD, Cloud G, Lakeman AD, et al. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J Infect Dis 2005;191(2):227–33.
126Oliver SE, Cloud GA, Sanchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J Clin Virol 2009;46(Suppl 4):S22–6.
127Kimberlin DW, Lin C-Y, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003;143(1):16–25.
128Kimberlin DW, Acosta EP, Sanchez PJ, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis 2008;197(6):836–45.
129Kimberlin DW, Lin C-Y, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003;143(1):16–25.
130Kimberlin DW. Herpes simplex virus infections in neonates and early childhood. Semin Pediatr Infect Dis 2005;16(4):271–81.
131Kimberlin DW. Herpes simplex virus infections of the newborn. Semin Perinatol 2007;31(1):19–25.
132Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
133Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
134Kimberlin DW, Whitley RJ, Wan W, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med 2011;365(14):1284–92.
135Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
136Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr 2012;161(1):134–8.
137Kimberlin DW. Diagnosis of herpes simplex virus in the era of polymerase chain reaction. Pediatr Infect Dis J 2006;25(9):841–2.
138Mandelbrot L. Fetal varicella—diagnosis, management, and outcome. Prenat Diagn 2012;32(6):511–8.
139Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
140Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
141Kellie SM, Makvandi M, Muller ML. Management and outcome of a varicella exposure in a neonatal intensive care unit: lessons for the vaccine era. Am J Infect Control 2011;39(10):844–8.
142MacMahon E. Investigating the pregnant woman exposed to a child with a rash. BMJ 2012;344:e1790.
143Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
144Kaye A. Toxoplasmosis: diagnosis, treatment, and prevention in congenitally exposed infants. J Pediatr Health Care 2011;25(6):355–64.
145Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363(9425):1965–76.
146Olariu TR, Remington JS, McLeod R, et al. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 2011;30(12):1056–61.
147SYROCOT (Systematic Review on Congenital Toxoplasmosis) study group, Thiébaut R, Leproust S, et al. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet 2007;369(9556):115–22.
148Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
149Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2010.
150Fonseca LT, Silveira RC, Procianoy RS. Ureaplasma bacteremia in very low birth weight infants in Brazil. Pediatr Infect Dis J 2011;30(12):1052–5.
151Kafetzis DA, Skevaki CL, Skouteri V, et al. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clinical Infectious Diseases 2004;39(8):1113–22.
152Kasper DC, Mechtler TP, Böhm J, et al. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J Perinatol Med 2011;39(3):331–6.
153Long SS, Pickering LK, Prober CG. Principles and practice of pediatric infectious diseases: expert consult—online and print. 4th ed. Philadelphia: Saunders; 2012.
154Tran TT. Hepatitis B: treatment to prevent perinatal transmission. Clin Obstet Gynecol 2012;55(2):541–9.
155Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
156Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red Book Report of the Committee on Infectious Diseases. 29th ed. American Academy of Pediatrics; 2012.
157Dijkmans AC, de Jong EP, Dijkmans BAC, et al. Parvovirus B19 in pregnancy: prenatal diagnosis and management of fetal complications. Curr Opin Obstet Gynecol 2012;24(2):95–101.
158Remington JS, Klein JO, Wilson CB, et al. Infectious diseases of the fetus and newborn: expert consult—online and print. 7th ed. Philadelphia: Saunders; 2