Hematology and Transfusion Medicine
Normal Erythrocyte Values of Neonates
1. A term newborn infant on the day of birth has a hemoglobin (Hgb) of 11.8 g/dL. Is that value low or is it within the expected (normal) reference range?
Expected values, also called “reference ranges,” for Hgb and hematocrit on the day of birth are a function of gestational age, increasing gradually through the second and third trimesters. Studies with very large sample sizes of neonates on the day of birth reveal no differences in Hgb or hematocrit associated with the infants’ sex. Reference ranges for blood Hgb concentrations are shown as Figure 12-1. The fifth percentile value (the lowest expected limit) at term is 14 g/dL. Thus the value of 11.8 g/dL in this patient is low. 1
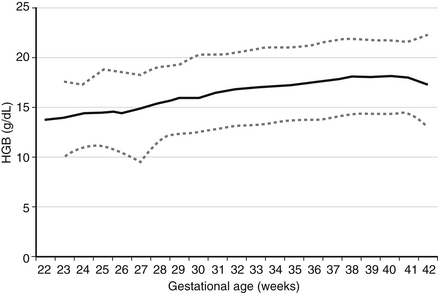
Figure 12-1 Reference range for blood hemoglobin concentration on the first day after birth. The lower and upper dashed lines represent the 5th and the 95th percentile values, respectively, and the solid line represents the mean value. (From Jopling et al. Pediatrics 2009;123(2):e333–337.)
2. A term newborn infant on the day of birth has an Hgb of 24 g/dL. Is that value high, or is it within the expected (normal) reference range?
The 95th percentile reference range at term is 22.5 g/dL (see Figure 12-1). The value of 24 g/dL in this patient is therefore high.
3. Are Hgb and hematocrit values of newborn infants higher when obtained from capillary blood than when obtained from venous or arterial blood?
4. Which one of the following RBC measurements in a fetus or newborn infant does not normally diminish gradually with maturation during the second and third trimesters of pregnancy?
The MCV and the MCH both diminish gradually through the second and third trimesters, as shown in Figure 12-2. The MCHC, however, does not change during this period but remains in the range of 31 to 34 g/dL. MCHC values greater than 36 g/dL should alert you to the possibility of hereditary spherocytosis or pyropoikilocytosis, two conditions that generally present with a low MCV and a high MCHC. They commonly also demonstrate hyperbilirubinemia and sometimes a diminishing Hgb concentration. If the MCV is consistently greater than 36 g/dL you should assess the morphology of the RBCs, and you will need to ask whether other family members have abnormally shaped RBCs and have had anemia, neonatal jaundice, or early cholelethiasis (bilirubin stones).
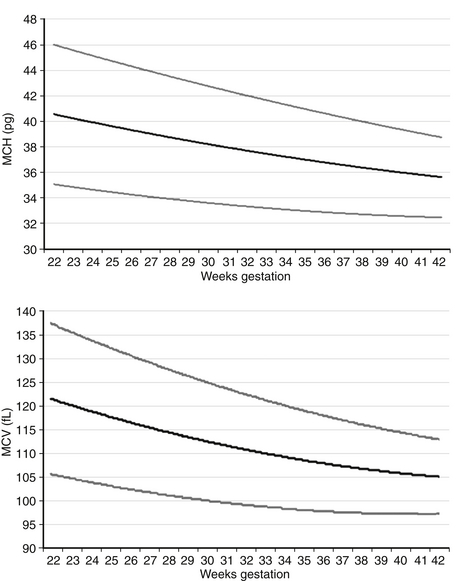
Figure 12-2 Reference ranges for mean corpuscular volume (MCV) in fL and mean corpuscular hemoglobin (MCH) in pg on the first day after birth. The lower and upper lines represent the 5th and the 95th percentile values, respectively, and the center line represents the mean value. (From Christensen, et al. J Perinatol 2008;28:24–28.)
5. The complete blood count (CBC) of a term neonate is reported to show greater than 100 nucleated red blood cells (NRBCs) per 100 white blood cells (WBCs). Is this value high, or is it within the expected (normal) reference range?
NRBCs can be reported as the number of NRBCs per 100 WBCs or as NRBCs per μL. The latter provides a more accurate accounting because of changing WBC counts over the first several days. The former is more commonly used in clinical practice. Reference ranges for NRBCs per 100 WBCs are shown in Figure 12-3. For term infants on the day of birth, the 95th percentile (highest expected limit) is 15 per 100 WBCs. Therefore the value of 100 in this patient is abnormally high.
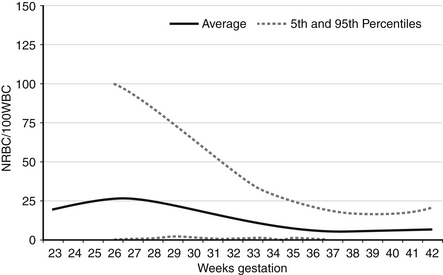
Figure 12-3 Reference ranges for nucleated red blood cells (NRBCs) per 100 white blood cells on the first day after birth. The lower and upper dashed lines represent the 5th and the 95th percentile values, respectively, and the center line represents the mean value. (From Christensen, et al. Neonatol 2011;99:289–294.)
6. A newborn infant at 28 weeks’ gestation has no NRBCs per 100 WBCs. Is that value low, or is it within the expected (normal) reference range?
7. You are asked to evaluate the result of a Hgb electrophoresis from a state metabolic screen, drawn on a nontransfused, extremely-low-birth-weight (less than 1 kg) neonate. The report reads as follows: 85% Hgb F, 5% Hgb A, and 10% Hgb Barts. What is your interpretation?
Hgb is a tetramer of globin chains, usually of two distinct types, bound to a heme moiety. Adult hemoglobin (Hgb A) consists of two alpha chains and two beta chains, whereas fetal hemoglobin (Hgb F) consists of two alpha chains and two gamma chains ( Figure 12-4).
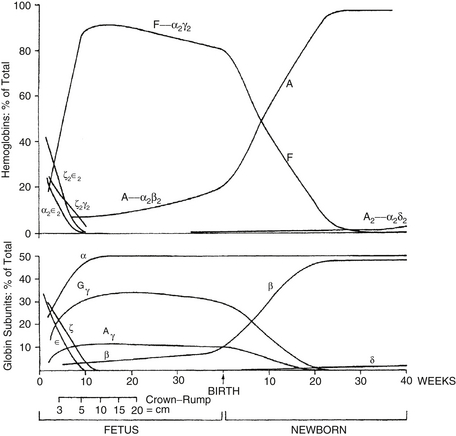
Figure 12-4 Changes in hemoglobin tetramers (top) and in globin subunits (bottom) during human development from embryo to early infancy. (From Bunn HF, Forget BG. Hemoglobin: molecular, genetic, and clinical aspects. Philadelphia: Saunders; 1986.)
Embryonic Hgbs are present in the first 8 weeks after conception and consist of Hgb Gower 1 (zeta 2, epsilon 2), Hgb Gower 2 (alpha 2, epsilon 2), and Hgb Portland (zeta 2, gamma 2). Hgb Barts consists of four gamma chains and occurs in the absence or deficiency of alpha chains. Thus 10% of the Hgb observed as Barts is abnormal and suggests a deficiency of at least one, and probably two, of the four alpha chain genes. Deletion of one alpha gene results in a phenotypically normal individual, and deletion of two can result in mild microcytic anemia with the presence of Barts Hgb during the fetal and early newborn period. Deletion of three genes gives rise to Hgb H disease; deletion of all four gives rise to a lethal syndrome of hydrops fetalis with all or most of the Hgb being Barts, and no Hgb A or F or alpha 2 (because there are no alpha chains). 2
Hgb F binds oxygen more avidly. The oxyhemoglobin dissociation curve for Hgb F is shifted to the left of the adult curve. The higher affinity for oxygen facilitates transfer of oxygen from maternal Hgb A but also results in decreased oxygen release to the fetal tissues. The latter situation is not a disadvantage, however, because fetal tissues use oxygen primarily for growth; metabolic functions are mostly handled by the mother.
Anemia in the Fetus and Newborn Infant
9. A term neonate has a total serum bilirubin (TSB) level of 18.8 mg/dL measured at 48 hours after birth as part of a pre–hospital-discharge bilirubin screening program. A CBC indicates that the Hgb is 11.2 g/dL, the MCV is 88 fL, and the MCHC is 38.2 g/dL. Which of the four values (bilirubin, Hgb, MCV, MCHC) is normal for age?
None of the four is normal. The bilirubin and the MCHC are high, and the Hgb and the MCV are low.
10. Which of the following would be appropriate diagnostic and management steps at this point?
 Initiate intensive phototherapy.
Initiate intensive phototherapy.
 Take a careful family history of neonatal jaundice and anemia, chronic jaundice and anemia, and gallstones (bilirubin cholelethiasis) at an early age.
Take a careful family history of neonatal jaundice and anemia, chronic jaundice and anemia, and gallstones (bilirubin cholelethiasis) at an early age.
 Determine the maternal and neonatal blood type and the direct antiglobulin test (Coombs test).
Determine the maternal and neonatal blood type and the direct antiglobulin test (Coombs test).
 Review the blood film, specifically looking for erythrocyte morphologic abnormalities.
Review the blood film, specifically looking for erythrocyte morphologic abnormalities.
 Obtain a reticulocyte count, urine analysis (looking for free Hgb), and serum haptoglobin.
Obtain a reticulocyte count, urine analysis (looking for free Hgb), and serum haptoglobin.
 Intensive phototherapy and careful follow-up of the TSB are needed because the TSB plots well into the “high risk” zone ( Figure 12-5).
Intensive phototherapy and careful follow-up of the TSB are needed because the TSB plots well into the “high risk” zone ( Figure 12-5).
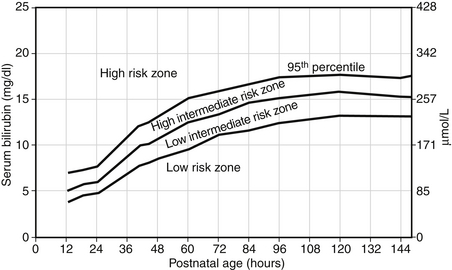
Figure 12-5. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103:6-14.
 The family history might reveal that the father or mother has hereditary spherocytosis (HS). Approximately two thirds of cases of HS are inherited in an autosomal dominant fashion. HS in this neonate would be compatible with the laboratory findings given. Between 40% and 50% of neonates with HS have a mutation in ANK1 (at 8p11.2) encoding the RBC cytoskeletal protein component ankyrin 1. Between 20% and 35% have a mutation in SLC4A1 (at 17q21) encoding band 3, and 15% to 30% have a mutation in SPTB (at 14q23-24.1) encoding beta-spectrin.
The family history might reveal that the father or mother has hereditary spherocytosis (HS). Approximately two thirds of cases of HS are inherited in an autosomal dominant fashion. HS in this neonate would be compatible with the laboratory findings given. Between 40% and 50% of neonates with HS have a mutation in ANK1 (at 8p11.2) encoding the RBC cytoskeletal protein component ankyrin 1. Between 20% and 35% have a mutation in SLC4A1 (at 17q21) encoding band 3, and 15% to 30% have a mutation in SPTB (at 14q23-24.1) encoding beta-spectrin.
 Alternatively, the family history might reveal that either father or mother has hereditary elliptocytosis (see the discussion later in this chapter explaining how this could explain this neonate’s findings). On the other hand, the family history might be completely unrevealing because about one third of HS cases in neonates are either de novo mutations or autosomal recessive varieties. The latter includes mutations in SPR1 (1q22-23) encoding alpha-spectrin and EPB42 (at 15q15-21) encoding protein 4.2. The latter mutation is more likely among neonates of Japanese descent.
Alternatively, the family history might reveal that either father or mother has hereditary elliptocytosis (see the discussion later in this chapter explaining how this could explain this neonate’s findings). On the other hand, the family history might be completely unrevealing because about one third of HS cases in neonates are either de novo mutations or autosomal recessive varieties. The latter includes mutations in SPR1 (1q22-23) encoding alpha-spectrin and EPB42 (at 15q15-21) encoding protein 4.2. The latter mutation is more likely among neonates of Japanese descent.
 Type and Coombs testing should be done when the TSB falls in the “high risk” zone. The MCHC can be high in ABO hemolytic disease associated with spherocytes, but it generally does not exceed 36.5 fL. The value greater than 38 in this case suggests that HS is more likely.
Type and Coombs testing should be done when the TSB falls in the “high risk” zone. The MCHC can be high in ABO hemolytic disease associated with spherocytes, but it generally does not exceed 36.5 fL. The value greater than 38 in this case suggests that HS is more likely.
 It is appropriate to examine the blood film for the presence of spherocytes or other morphologic abnormalities. Although microspherocytes can be seen in ABO hemolytic disease (sometimes presenting a dilemma between HS and ABO hemolytic disease in neonates with Coombs-positive jaundice), the high MCHC in this case suggests that HS is more likely. Figure 12-6 shows an example of a blood smear of a neonate with HS.
It is appropriate to examine the blood film for the presence of spherocytes or other morphologic abnormalities. Although microspherocytes can be seen in ABO hemolytic disease (sometimes presenting a dilemma between HS and ABO hemolytic disease in neonates with Coombs-positive jaundice), the high MCHC in this case suggests that HS is more likely. Figure 12-6 shows an example of a blood smear of a neonate with HS.
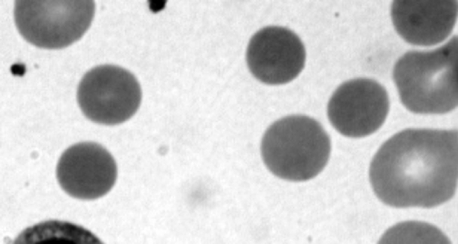
Figure 12-6 Photomicrograph of a Wright-stained blood film of a newborn infant with autosomal dominant hereditary spherocytosis. Note that many erythrocytes lack a zone of central pallor.
 Hereditary elliptocytosis (HE) in a neonate does not generally result in significant hemolytic jaundice and anemia, and the RBC indices are not generally abnormal. However, a related condition called pyropoikilocytosis does indeed present similarly to this case, including early jaundice, anemia, low MCV, and high MCHC. Pyropoikilocytosis generally occurs when a father or mother has HE, consisting of an alpha-spectrin deficiency, and the other spouse has an asymptomatic alpha-spectrin defect. As a result, the neonate has a genetic condition similar to autosomal recessive inheritance as a compound heterozygote. Figure 12-7 shows an example of a blood film of HE, and Figure 12-8 shows an example of neonatal pyropoikiolocytosis. 3
Hereditary elliptocytosis (HE) in a neonate does not generally result in significant hemolytic jaundice and anemia, and the RBC indices are not generally abnormal. However, a related condition called pyropoikilocytosis does indeed present similarly to this case, including early jaundice, anemia, low MCV, and high MCHC. Pyropoikilocytosis generally occurs when a father or mother has HE, consisting of an alpha-spectrin deficiency, and the other spouse has an asymptomatic alpha-spectrin defect. As a result, the neonate has a genetic condition similar to autosomal recessive inheritance as a compound heterozygote. Figure 12-7 shows an example of a blood film of HE, and Figure 12-8 shows an example of neonatal pyropoikiolocytosis. 3
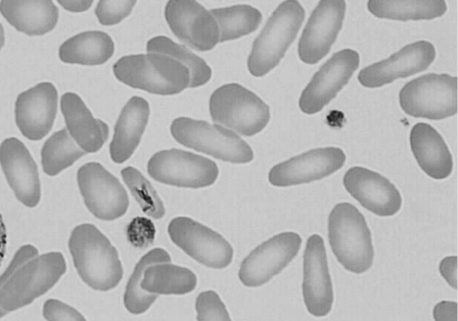
Figure 12-7 Photomicrograph of a Wright-stained blood film of a newborn infant with hereditary elliptocytosis. Note that most of the erythrocytes do have a zone of central pallor, but they vary in shape from round to oval to elliptical.
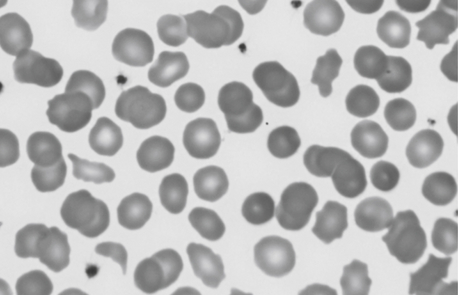
Figure 12-8 Photomicrograph of a Wright-stained blood film of a newborn infant with the diagnosis of pyropoikilocytosis. The mother had autosomal dominant hereditary elliptocytosis, and the father had a “silent” mutation in alpha-spectrin. Neither parent had problematic jaundice during the neonatal period or subsequently, but the baby required phototherapy for more than 1 week. Note that some of the erythrocytes appear normal, but many have abormal shapes, varying from spherocytes to schistocytes to acanthocytes. This high degree of poikilocytosis was termed pyropoikilocytosis because the cells resemble those after thermal burns (“pyro”).
11. Following a long labor of a term primipara, vacuum extraction is successfully accomplished. A capillary blood gas reading obtained within a few minutes of delivery was normal, including an Hgb count of 16 g/dL. Over the next hour the site of the vacuum attachment to the crown of the head becomes progressively larger and more fluctuant. A repeat Hgb count is still 16 g/dL. One clinician suggests that this finding might represent a subgaleal hemorrhage, but another states that the stable Hgb level is more likely to represent caput succedaneum. Which of the following would be appropriate diagnostic and management decisions?
 Order continuous heart rate and respiratory rate monitoring with frequent blood pressure measurements, looking for any signs of compensated hypovolemia.
Order continuous heart rate and respiratory rate monitoring with frequent blood pressure measurements, looking for any signs of compensated hypovolemia.
 Obtain a CBC (at least a Hgb and hematocrit) now and again in about 2 hours.
Obtain a CBC (at least a Hgb and hematocrit) now and again in about 2 hours.
 Obtain a blood type and cross match in case an early transfusion is needed.
Obtain a blood type and cross match in case an early transfusion is needed.
 Wrap the head very tightly with an elastic bandage to prevent further head swelling.
Wrap the head very tightly with an elastic bandage to prevent further head swelling.
 Consider imaging the head to determine whether the swelling is consistent with blood in the subgaleal space.
Consider imaging the head to determine whether the swelling is consistent with blood in the subgaleal space.
All neonates who had a “spontaneous” subgaleal hemorrhage (not delivered by vacuum or forceps extraction) lacked signs of shock, had no transfusions, and generally had a good outcome. Thus vacuum delivery is the most significant risk factor for developing a neonatal subgaleal hemorrhage. 4
13. A subgaleal hemorrhage following vacuum extraction delivery is rare, occurring in fewer than 1 percent of all vacuum deliveries (true or false).
14. If a subgaleal hemorrhage is diagnosed, the expected mortality rate is about 25% (true or false).
False. Some publications describing cases from the 1980s and earlier did indeed report a mortality rate this high, but more recent series suggest the mortality rate is 5% to 10%. Regardless, this injury is devastating. Vigilance and aggressive management are likely responsible for the observed improvement in outcome.
15. Hemolytic disease of the fetus and newborn (HDFN) can occur when a woman has immunoglobulin G antibodies directed against paternal RBC antigens inherited by the fetus. What is a typical clinical presentation for a case of HDFN?
Neonatal hemolytic jaundice is typical, particularly manifesting in the following ways:
 Hyperbilirubinemia typically results in an elevated cord blood TSB; also, a TSB in the “high risk” zone within 24 or 48 hours of birth is possible.
Hyperbilirubinemia typically results in an elevated cord blood TSB; also, a TSB in the “high risk” zone within 24 or 48 hours of birth is possible.
 Reticulocytosis: After day of life one or two, the reticulocyte count generally falls to near zero percent. However, in most cases of HDFN the reticulocyte count remains elevated, sometimes higher than 10%.
Reticulocytosis: After day of life one or two, the reticulocyte count generally falls to near zero percent. However, in most cases of HDFN the reticulocyte count remains elevated, sometimes higher than 10%.
 Hemolysis: Evidence includes a falling Hgb, free Hgb in the urine, and an absent serum haptoglobin concentration.
Hemolysis: Evidence includes a falling Hgb, free Hgb in the urine, and an absent serum haptoglobin concentration.
 Erythroblastosis: Most cases other than anti-D have a normal or only a slightly elevated NRBC count. Severe cases of HDFN can have marked anemia, erythroblastosis, and hydrops fetalis. 5
Erythroblastosis: Most cases other than anti-D have a normal or only a slightly elevated NRBC count. Severe cases of HDFN can have marked anemia, erythroblastosis, and hydrops fetalis. 5
16. HDFN arising from maternal anti-Kell (Kell1) antibody can present in a manner very different from the typical presentation described previously. What is that presentation, and why is that variety of HDFN different from the rest?
True. Women lacking the A and the B erythrocyte antigens often have anti-A and anti-B antibodies even before pregnancy. In the case of women with blood type O, their anti-A and anti-B antibodies are sometimes of the immunoglobulin G type and therefore can cross the placenta and bind to fetal antigens. Unlike the situation observed with maternal anti-D, in neonates with ABO hemolytic disease the principal problem is usually jaundice. Anemia, erythroblastosis, and hydrops are all very rare. The ABO locus is on chromosome 9 and has three main allelic forms: A, B, and O. The A and B alleles encode glycosyltransferases. The O allele differs from the A allele by deletion of only one nucleotide—guanine at position 261. This deletion causes a frame shift and results in premature termination of translation of the mRNA. 6
The H antigen is the precursor to the ABO blood group antigens. The H locus is on chromosome 19 and encodes the H antigen, which is expressed on the RBC surface. The H antigen is then modified by the A or the B antigen to produce the final A, B, or O antigen. Very rarely an individual lacks the H antigen because of a mutation in the H gene. This results in type O blood, but because the precursor molecule (the H antigen) is also missing, even type O blood cannot be transfused because the individual recognizes the H antigen in the type O blood as foreign. This unusual O blood type is called Bombay blood group and occurs in approximately four per million people, except in parts of India where it may be as common as 1 in 10,000. Neonates who are type O on the basis of Bombay can hemolyze if transfused with type O blood.
19. A mother about to deliver at 29 weeks’ gestation requests that the obstetrician and the neonatal team do everything possible to avoid an RBC transfusion in the neonate. What options are available to help the family with this request?
 Delay clamping of the umbilical cord, or cord “stripping” or “milking.” These maneuvers, roughly equivalent in terms of the volume of fetal blood transferred from the placenta to the fetus, can be expected to result in an Hgb concentration of about 2 g/dL. Ask the obstetrician to consider these approaches.
Delay clamping of the umbilical cord, or cord “stripping” or “milking.” These maneuvers, roughly equivalent in terms of the volume of fetal blood transferred from the placenta to the fetus, can be expected to result in an Hgb concentration of about 2 g/dL. Ask the obstetrician to consider these approaches.
 Draw all laboratory tests on NICU admission (e.g., blood culture, CBC, state metabolic screen) using fetal blood in the placenta after placental delivery, thereby removing no blood from the neonate initially.
Draw all laboratory tests on NICU admission (e.g., blood culture, CBC, state metabolic screen) using fetal blood in the placenta after placental delivery, thereby removing no blood from the neonate initially.
 Carefully consider the need for all blood tests you order during the first several days to weeks, with the understanding that many early RBC transfusions in very-low-birth-weight infants are generally needed on the basis of anemia that results from phlebotomy for laboratory testing.
Carefully consider the need for all blood tests you order during the first several days to weeks, with the understanding that many early RBC transfusions in very-low-birth-weight infants are generally needed on the basis of anemia that results from phlebotomy for laboratory testing.
 Consider slightly lowering the Hgb value you consider as a “transfusion trigger.” For instance, if your guidelines call for RBC transfusion at 10 g/dL or lower, consider lowering it to 9 g/dL or lower for this patient.
Consider slightly lowering the Hgb value you consider as a “transfusion trigger.” For instance, if your guidelines call for RBC transfusion at 10 g/dL or lower, consider lowering it to 9 g/dL or lower for this patient.
 Consider administering the long-acting erythropoietin analog darbepoetin (Aranesp) once in the first few days and again 1 week later. Use 10 μg/kg as a unit dose.
Consider administering the long-acting erythropoietin analog darbepoetin (Aranesp) once in the first few days and again 1 week later. Use 10 μg/kg as a unit dose.
 Let the parents know that despite your best efforts to help them with their request, the baby’s best interests might force you to administer an RBC transfusion if the baby would be critically compromised otherwise. Explain all the steps that you are taking to avoid transfusing the infant. 7
Let the parents know that despite your best efforts to help them with their request, the baby’s best interests might force you to administer an RBC transfusion if the baby would be critically compromised otherwise. Explain all the steps that you are taking to avoid transfusing the infant. 7
20. A twin-twin transfusion is expected on the basis of discordant-sized monochorionic twins. Fetal ultrasonography indicates the likelihood of anemia in the smaller twin because of the middle cerebral artery blood flow. It appears that the larger twin has pleural fluid and ascites, although these are subtle findings. You are anticipating that the smaller twin will be anemic and the larger twin may be polycythemic, but what other hematologic differences do you anticipate?
The donor (anemic, smaller) twin is more likely to have a hyporegenerative neutropenia, similar to that seen in neonates born after pregnancy-induced hypertension. This situation is likely to present with no left shift (a normal immature-to-total neutrophil ratio) and a duration of only about 2 or 3 days. Similarly, the donor twin is more likely to have a moderately low platelet count with a normal mean platelet volume (MPV). The pathogenesis of these findings is not known with certainty but likely relates to the accelerated erythropoietic effort in the anemic twin, with a concomitant temporary reduction in platelet and neutrophil production. Also, the anemic twin will usually have a higher NRBC count, generally above the reference range for age (see Figure 12-3). 8
21. RBC transfusion can be life-saving for neonates with acute hemorrhage or severe anemia. However, before ordering any RBC transfusion, the clinician must assess the potential risks and potential benefits. What are some of the risks associated with RBC transfusion in the NICU?
Typical transfusion reactions (i.e., those commonly reported for adult recipients) are only rarely observed in transfused neonates. These include febrile nonhemolytic reactions, urticarial (allergic) reactions, hypothermia, circulatory overload, hypotensive reactions, citrate toxicity (e.g., peripheral paresthesia, tingling, buzzing, cramps, nausea, vomiting), and acute hemolysis resulting from undetected incompatibility. Transfusion-transmitted diseases include bacterial contamination, which is considerably more common than the hepatitis and other viruses transmitted in past decades, before development and implementation of modern hemovigilance techniques and procedures.
Adverse associations with transfusions that are unique to neonates include transfusion-associated necrotizing enterocolitis (generally very-low-birth-weight neonates 3 to 4 weeks old receiving a “late” transfusion) and severe intraventricular hemorrhage in extremely-low-birth-weight neonates after an “early” transfusion. Transfusion-related acute lung injury (TRALI) reactions involve acute onset of (or acute worsening of) respiratory distress after transfusion. All plasma-containing blood products have been implicated in TRALI reactions. TRALI is now among the three leading causes of transfusion-related fatalities, along with ABO incompatibility and bacterial contamination, but it is rarely reported (perhaps because it is rarely recognized) in neonatal transfusion recipients. 910
22. After a double-volume exchange transfusion for extreme hyperbilirubinemia in a term neonate, you obtain a CBC. The exchange was performed with packed RBC reconstituted with plasma to a hematocrit concentration of approximately 60%. Before the exchange transfusion a CBC revealed a hematocrit concentration of 30%, an Hgb of 10 g/dL, a platelet count of 230,000/μL, and an absolute neutrophil count of 2000/μL. What significant differences do you anticipate finding in the CBC after the exchange?
 The hematocrit and Hgb will be higher than before the exchange. If they are not higher, you might want to check the hematocrit and Hgb in the remaining unused reconstituted unit to ensure that the product you received approximated what you ordered.
The hematocrit and Hgb will be higher than before the exchange. If they are not higher, you might want to check the hematocrit and Hgb in the remaining unused reconstituted unit to ensure that the product you received approximated what you ordered.
 The erythrocyte indices, particularly the MCV and MCH, will fall because adult donor erythrocytes have partly replaced erythrocytes of the neonate. The MCHC will likely be about the same because this measurement is generally in the same range in neonates and adults.
The erythrocyte indices, particularly the MCV and MCH, will fall because adult donor erythrocytes have partly replaced erythrocytes of the neonate. The MCHC will likely be about the same because this measurement is generally in the same range in neonates and adults.
 The platelet count will be considerably lower after the exchange because of the lack of platelets in the reconstituted donor unit. It is not uncommon to find a platelet count below 100,000/μL after an exchange transfusion. The count will not likely fall to a level requiring a platelet transfusion, however, unless you must repeat the double-volume exchange transfusion within a few hours. Indeed, if a repeat exchange transfusion is needed soon after the first, be aware that the platelet count after the second exchange might fall to exceedingly low levels. Because there is no large marrow ready reserve of platelets, the platelet count will not rebound rapidly (within hours) of the exchange.
The platelet count will be considerably lower after the exchange because of the lack of platelets in the reconstituted donor unit. It is not uncommon to find a platelet count below 100,000/μL after an exchange transfusion. The count will not likely fall to a level requiring a platelet transfusion, however, unless you must repeat the double-volume exchange transfusion within a few hours. Indeed, if a repeat exchange transfusion is needed soon after the first, be aware that the platelet count after the second exchange might fall to exceedingly low levels. Because there is no large marrow ready reserve of platelets, the platelet count will not rebound rapidly (within hours) of the exchange.
 The neutrophil count, already on the low side (2000/μL) before the exchange will be lower still afterward. Similar to the anticipated fall in platelet count, the reconstituted donor unit will lack neutrophils, and the count will fall. It would be expected that the post–exchange transfusion neutrophil count would be below 1000/μL in this patient.
The neutrophil count, already on the low side (2000/μL) before the exchange will be lower still afterward. Similar to the anticipated fall in platelet count, the reconstituted donor unit will lack neutrophils, and the count will fall. It would be expected that the post–exchange transfusion neutrophil count would be below 1000/μL in this patient.
Polycythemia: Diagnosis and Management
Technically, these two words describe different aspects of illness, but in neonates the two tend to occur together. In older children and adults marked increases in blood concentrations of leukocytes and serum proteins can result in hyperviscous blood, even with a normal hematocrit level, and they can therefore have hyperviscosity without polycythemia. Neonates with leukocyte concentrations up to and over 100,000/μL, however, have been reported to have normal blood viscosity measurements. In neonates hyperviscosity is nearly always secondary to polycythemia, and polycythemia (particularly a “central” hematocrit exceeding 70%) essentially always indicates hyperviscosity. 11
A hematocrit (or blood Hgb concentration) exceeding the 95th percentile limit (see Fig. 12-1) on the first day of life is, by definition, abnormal. However, not all neonates with a hematocrit above the 95th percentile need a reduction transfusion. If they did, 5% of all neonates would be subjected to a reduction transfusion. A general recommendation is that if the central (noncapillary) value exceeds 70% and the neonate has physiologic disturbances consistent with hyperviscosity, a reduction transfusion is warranted. Those disturbances include tachypnea, tachycardia, plethora, hypoglycemia, and tremulousness. An additional general recommendation is that if the central hematocrit exceeds 75%, a reduction transfusion may be warranted even if the neonate is asymptomatic. Ideally, avoiding the signs associated with hyperviscosity by reducing the hematocrit before intravascular problems result is preferable.
Normal Platelet Values of Mothers and Neonates
26. You are informed that an apparently healthy term neonate was delivered to a woman with “mild” thrombocytopenia, and you wonder whether it would be of value to obtain a platelet count on the neonate. The mother’s platelet count on her first prenatal visit (at 10 weeks) was 205,000/μL, but it was 132,000/μL at delivery; her MPV was 9.2 fL at 10 weeks and again at delivery. Her pregnancy, labor, and delivery were completely normal. Is her platelet count low, or is it within the expected (normal) reference range? Is it good practice to check a platelet count on her normal-appearing neonate on the basis of her count of 132,000/μL?
Reference ranges for platelet counts during pregnancy are shown in Figure 12-9. The 5th percentile value (the lowest expected limit) at term is 100,000/μL; thus a value of 135,000/μL is within the normal range. As the figure shows, the reference range diminishes steadily throughout gestation, generally falling by an increment of about 50,000/μL from early pregnancy to term. This fall is at least partly caused by the normal hemodilution of pregnancy. In contrast, no change in MPV normally occurs during pregnancy. Neonates born to women who have platelet counts in the range of 100,000 to 150,000/μL do not have an increased risk of neonatal thrombocytopenia. Therefore if the neonate appears healthy, it is not necessary to obtain a platelet count on the basis of the mother’s count of 135,000/μL. 12
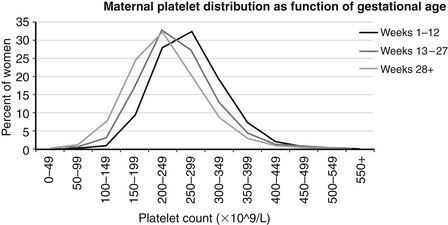
Figure 12-9 Reference ranges are shown for platelet counts of women during pregnancy. The three lines represent the mean values at 1 to 12, 13 to 27, and more than 28 weeks’ gestation, as documented on 92,518 pregnant women. (From Jensen, et al. Am J Perinatol 2010;28:597–604.)
27. A neonate delivered at 30 weeks’ gestation is admitted to the NICU. Aside from mild respiratory distress, the patient seems stable. A CBC on NICU admission reveals a platelet count of 129,000/μL with an MPV of 8.8 fL. Is this platelet count low, or is it within the expected (normal) reference range?
Reference ranges for platelet counts on the day of birth, according to gestational age, are shown in Figure 12-10. The 5th percentile (lower) expected range at 30 weeks’ gestation is about 110,000/μL, and thus the observed count of 129,000/μL is normal.
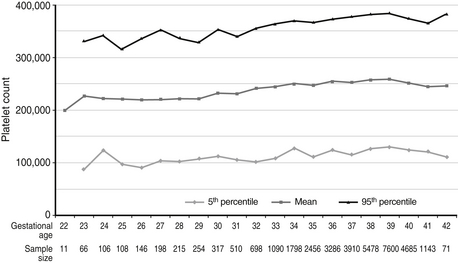
Figure 12-10 Reference ranges are shown for platelet counts of neonates on the day of birth, according to gestational age. The lower and upper lines represent the 5th and the 95th percentile values, respectively, and the center line represents the mean value. (From Wiedmeier, et al. J Perinatol 2009;29:130–136.)
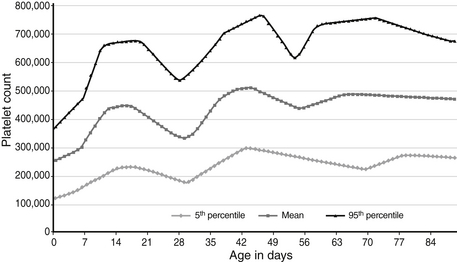
28. Approximately 3 weeks later, the same neonate discussed in Question 27 is doing well and growing appropriately, but a CBC shows that the platelet count is now 595,000/μL. Is that value high, or is it within the expected (normal) reference range?
Reference ranges for platelet counts during the first 90 days after birth are shown in Figure 12-11. The 95th percentile (upper limit) value at 3 weeks is about 650,000/μL, and the value of 595,000/μL is therefore within the expected reference range. The reference range for platelets increases after birth, reaching a first peak at 14 to 20 days. This change is most likely due to the physiologic thrombopoietin surge that occurs at birth. This surge—and the subsequent increase in platelet count—occurs after either vaginal or cesarean delivery and in preterm as well as term neonates. A comparable increase in MPV also occurs during the first 2 to 3 weeks, consistent with increased platelet production. The cause of the second peak in platelet count at 40 to 50 days (see Figure 12-11) is not known.
Thrombocytopenia and Platelet Transfusion
29. A 39-week-gestation, appropriately grown male neonate is noted to have petechiae shortly after birth. The platelet count is 9000/μL, and the MPV is 8.2 fL. The remainder of the CBC is normal. Appropriate actions include which of the following?
 Repeat the platelet count, and examine the blood film to obtain an estimimate of whether the platelets are abnormally small, normal in size, or abnormally large.
Repeat the platelet count, and examine the blood film to obtain an estimimate of whether the platelets are abnormally small, normal in size, or abnormally large.
 Order a platelet transfusion, and obtain a repeat platelet count after the transfusion.
Order a platelet transfusion, and obtain a repeat platelet count after the transfusion.
 Have a platelet count performed on the mother (if not already done).
Have a platelet count performed on the mother (if not already done).
 Look for malformations, syndromes, and evidence of congenital infection.
Look for malformations, syndromes, and evidence of congenital infection.
All the preceding steps can be appropriate depending on the circumstances.
In this case, an x-ray ( Figure 12-12) of the forearms showed radioulnar synostosis, making ATRUS the likely diagnosis (a mutation in HOXA11 mapping to 7p15). ATRUS is an autosomal dominant condition, but neonates can be much more severely affected than their affected parent. Generally, the affected parent has radioulnar synostosis with limited forearm pronation and supination, but his or her platelet counts tend to be only moderately low (50,000/μL) with minimal clinical bleeding problems. Neonates with ATRUS are likely to be dependent on platelet transfusion, and marrow or cord blood transplantation is the only known curative procedure. 13
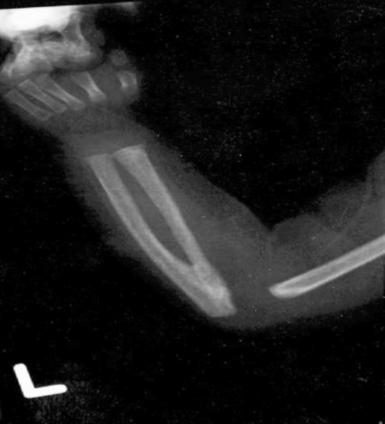
Figure 12-12 An x-ray of the forearm of a 37-weeks’-gestation female infant who, shortly after birth, was noted to have scattered petechiae and a platelet count of 9000/μL. She was otherwise well-appearing and in no distress. Fusion of the proximal radius and ulna is seen. This is a case of ATRUS (Amegakaryocytic Thrombocytopenia with RadioUlnar Synostosis) (From Sola, et al. J Perinatol 2004;24:528–530.)
30. What is the expected rise in platelet count after a platelet transfusion? What is the average survival time of transfused platelets in a neonate?
In cases of ATRUS, the rise in platelet count following transfusion and the disappearance of the transfused platelets should conform to these principles, because the kinetic mechanism responsible for the thrombocytopenia is reduced platelet production. However, in cases of neonatal thrombocytopenia caused by a platelet consumptive process, such as DIC, a propagating thrombus, or immune-mediated thrombocytopenia, the platelet count will not increase by the expected amount after transfusion and will fall much more rapidly than previously described. 14
Reference Ranges for Neutrophil Counts and Neutropenia
32. Why are neutrophil counts of neonates much higher at high altitude? Is the difference of any clinical significance?
Newborn nurseries in Colorado, New Mexico, and Utah, 4000 to 5500 feet above sea level, have reported much higher reference ranges for blood neutrophil concentrations during the first 3 days after birth than nurseries at or near sea level. It is curious that hematocrit/Hgb levels and NRBC/μL are not higher at the high-altitude hospitals; the mechanism explaining the difference in neutrophil counts is not known. Figure 12-13 shows the reference ranges for high-altitude and sea-level centers superimposed. One value in recognizing that these differences exist is that NICUs at high altitude can falsely label neonates as neutrophilic if the sea-level range is used. Perhaps the opposite is also true; neonates at sea level could have the diagnosis of neutrophilia missed if the reference range for high-altitude centers is used.
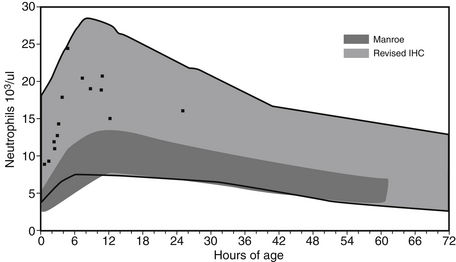
Figure 12-13 Reference ranges are shown for blood neutrophil counts during the first 3 days after birth. The light-gray scale indicates the reference range for neutrophil counts obtained from neonates at high altitude (identified as Revised IHC). The dark-gray scale indicates the reference range for counts obtained from neonates near sea-level (identified as Manroe). The black dots represent 14 healthy term neonates in Utah who would have been termed “neutrophilic” according to the Manroe chart but were seen to be well within the high-altitude reference ranges. (From Lambert, et al. J Perinatol 2009;29:822–825.)
33. A neonate delivered at 29 weeks’ gestation is unstable on a mechanical ventilator, and the first blood gas reading indicates severe metabolic acidosis. Hypotension and poor perfusion are present. The infant is small for gestational age with asymmetric growth retardation; birth weight is below 5th percentile, length is at the 20th percentile, and the occipital-frontal circumference falls at the 40th percentile. His mother has been diagnosed with pregnancy-induced hypertension (PIH). The first CBC, obtained on the neonate just after birth, shows neutropenia, with 750 neutrophil/μL and a marked left shift, with 1% polymorphonuclear neutrophil, 21% band neutrophils and 4% metamyelocytes. A senior neonatologist notes that the neutropenia in this patient is the very common variety seen in small-for-gestational-age neonates born after PIH; that this variety is transient, resulting from reduced neutrophil production that will improve in the next day or two; and that antibiotic treatment is not needed. What do you think about this advice?
34. A well-appearing term neonate has a screening CBC performed as part of a protocol for asymptomatic neonates delivered to women testing positive for Group B Streptococcus who did not receive intrapartum antibiotics. The CBC is normal, with the exception of a neutrophil count of 550/μL. No band forms were seen, and the platelet count is normal. The baby has no dysmorphic features and appears totally healthy. Which of the following steps would be appropriate for evaluating the neutropenia in this neonate?
 Repeat the CBC, and examine the blood film to estimate whether the neutrophil count indeed appears low on the smear and whether the neutrophils appear morphologically normal.
Repeat the CBC, and examine the blood film to estimate whether the neutrophil count indeed appears low on the smear and whether the neutrophils appear morphologically normal.
 Examine the neonate carefully to ensure that no subtle or early signs of infection are present, such as respiratory distress, tachycardia, and poor perfusion.
Examine the neonate carefully to ensure that no subtle or early signs of infection are present, such as respiratory distress, tachycardia, and poor perfusion.
 Have a CBC performed on the mother (if not already done), and inquire whether she has a past history of neutropenia or an autoimmune disorder (e.g., systemic lupus erythematosus) and whether neonatal neutropenia was recognized in any of her other children.
Have a CBC performed on the mother (if not already done), and inquire whether she has a past history of neutropenia or an autoimmune disorder (e.g., systemic lupus erythematosus) and whether neonatal neutropenia was recognized in any of her other children.
All the preceding actions are appropriate.
35. The repeat CBC on the healthy term baby described in Question 34 is similar to the first. The pathologist reports that the neutrophil concentration on the blood film is indeed low, that the rare neutrophils present appear mature and morphologically normal, and that the other leukocytes and the erythrocytes and platelets also appear normal. You find that the mother had a normal CBC before delivery. She never had a diagnosis of neutropenia or an autoimmune disorder, and her two previous children were healthy with no known medical problems. The next morning the CBC on this neonate is essentially unchanged, with an absolute neutrophil count of 490/μL. He still appears to be healthy and is breastfeeding well. Can you construct a reasonable differential diagnosis for this variety of neonatal neutropenia?
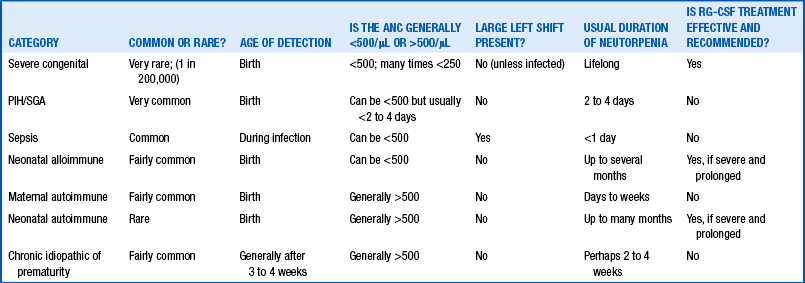
Table 12-1 categorizes neonatal neutropenia into subtypes. Some of these varieties are very common, and others are exceedingly rare. Some are the result of reduced neutrophil production, and others the result of accelerated neutrophil utilization (sepsis) or destruction (immune mediated). Although this could be one of the subtypes of severe congenital neutropenia, those are extremely rare. Given that the patient is not ill and has no left shift, this is not the neutropenia of overwhelming sepsis. Because this infant is not small for gestational age and the mother did not have PIH, it is not that variety. Most likely this is a case of alloimmune neonatal neutropenia (ANN), wherein the mother has immunoglobulin G antibody against a neutrophil antigen she lacks but that is expressed by father and fetus. 15
36. Because ANN is high on the list of possibilities, what can you cite about the pathogenesis, diagnostic tests, natural history, and treatment options for that condition?
Other ELANE mutations result in cyclic neutropenia, which can also be categorized as a type of severe congenital neutropenia, wherein cyclic drops in the absolute neutrophil count occur on an every-3-to-4-week cycle. The ELANE mutations causing cyclic neutropenia are generally single nucleotide substitutions that produce a defective neutrophil elastase protein retaining some level of function. 1617
Eosinophilia
39. The CBC of a newborn infant admitted to the NICU after birth at 33 weeks’ gestation has an eosinophil count (WBCs multiplied by the percentage of eosinophils) of 1000/μL. Is that value high, or is it within the expected (normal) reference range?
Expected values, also called reference ranges, for eosinophil counts on the day of birth are a function of gestational age, increasing gradually through the second and third trimesters. Reference ranges for eosniophil counts at birth are shown in Figure 12-14. The 95th percentile value (the highest expected limit) at 34 weeks is about 1100/μL. Thus a value of 1000/μL is normal. 18
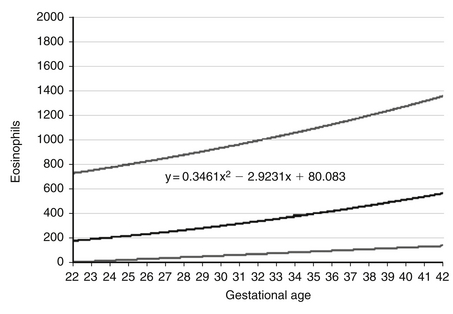
Figure 12-14 Reference ranges are shown for eosinophil counts of neonates on the day of birth, according to gestational age. The lower and upper lines represent the 5th and the 95th percentile values, respectively, and the center line represents the mean value. (From Christensen, et al. J Perinatol 2010;30:540–545.)
40. The CBC of the neonate in discussed in Question 39 is now 27 days old. The infant is about to be discharged home. The eosinophil count is now 1500/μL. Is that value high, or is it within the expected (normal) reference range?
Although the eosinophil count has increased significantly from that measured on the day of birth, the value is within the expected range. The reference range for blood eosinophil concentration during the first 28 days after birth is shown in Figure 12-15.
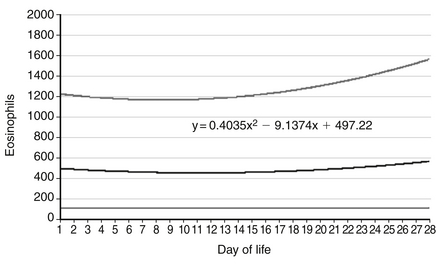
Figure 12-15 Reference ranges are shown for eosinophil counts of neonates for the first 28 days after birth. The lower and upper lines represent the 5th and the 95th percentile values, respectively, and the center line represents the mean value. (From Christensen, et al. J Perinatol 2010;30:540–545.)
41. If the eosinophil count of the neonate discussed in Questions 39 and 40 were 3500/μL, the term eosinophilia would properly apply. What are the more common conditions that might be associated with such a high eosinophil count in this neonate?
Eosinophils are effector cells involved in allergic and nonallergic inflammatory conditions. Circulating eosinophils are derived from myelocytic progenitors within the marrow and within extramedullary sites as well. After exiting the site of production and entering the blood, eosinophils circulate for approximately 1 day (T½ 18 hours), after which they transmigrate to tissues, primarily in the gastrointestinal tract, where they produce cytokines and chemokines. Several pathologic conditions in neonates are associated with eosinophilic tissue infiltration, and these conditions are often accompanied by blood eosinophilia. The conditions include erythema toxicum, neonatal eosinophilic pustulosis, and bronchopulmonary dysplasia. Other inflammatory conditions associated with eosinophilia are neonatal eosinophilic esophagitis, eosinophilic colitis, subcutaneous fat necrosis with eosinophilic granules, a variety of infectious diseases, and necrotizing enterocolitis after erythrocyte transfusion. A slight increase can occasionally be expected in preterm infants corresponding to the time weight gain is established.
Coagulation
42. You are asked to see a healthy term female newborn shortly after birth because the mother has von Willebrand disease. The parents want to discuss with you the possibility that the child might also have this condition. What facts would be helpful for you to give them?
First, you might inquire if the mother knows the type of von Willebrand disease that she has. You should also find out if she had any bleeding problems as a baby and about her own bleeding history. Perhaps the parents already know that von Willebrand disease is the most common inherited disorder of coagulation, with a prevalence as high as 1% of the general population. They might also already know that von Willebrand disease is not a single disorder and that the most common variety, type 1 (the most likely type the baby may have inherited from mother), is hardly ever problematic during the newborn period. Only type 3, a very rare autosomal recessive variety completely lacking von Willebrand factor antigen and activity, is likely to result in excessive bleeding in infants. Table 12-2 reviews the features of von Willebrand subtypes.19
TABLE 12-2
TYPES OF VON WILLEBRAND DISEASE, GENERALLY THE RESULT OF MUTATIONS AFFECTING THE QUALITY OR QUANTITY OF VWF PROTEIN.
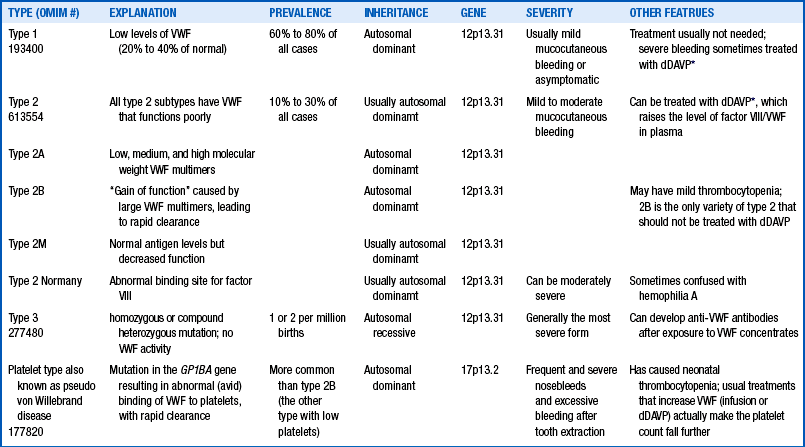
∗dDAVP (1-deamino-8-D-arginine vasopressin) is not commonly used in the neonatal period because of the risks of hyponatremia and edema.
43. You are asked to see a healthy term female newborn shortly after birth because the mother has the factor V Leiden mutation. The parents want to discuss with you the possibility that the child might also have this condition. What facts would be helpful for you to give them?
44. You are asked to see a healthy term male newborn on the day of birth because two of the mother’s brothers have hemophilia A. The mother does not know whether she is a hemophilia carrier, and the parents elected not to have fetal diagnostic tests performed. However, now they would like to discuss with you the possibility that their newborn son might have hemophilia, and they want to know whether he does before they proceed with a circumcision. How can you help them?
The gene F8 encoding factor VIII is located at Xq28. Bleeding severity in patients with hemophilia A correlates with the plasma levels of factor VIII. Patients are expected to have “mild” bleeding problems with levels in the range of 6% to 30% of normal, “moderate” with levels 2% to 5% of normal, and “severe” with levels less than 1% of normal.
45. An ill 32-week-gestation male newborn, approximately 24 hours old, is being managed with mechanical ventilation for severe respiratory distress and is being treated with dopamine because of hypotension. He is also receiving antibiotics because of the possibility of bacterial sepsis. You notice a rather sudden appearance of bright red blood in the endotracheal tube, and you see oozing around the umbilical catheters and at venipuncture sites. You suspect the patient has developed DIC. How do you proceed?
In case reports recombinant factor VIIa has been used successfully to treat life-threatening bleeding in neonates with DIC. Also, activated protein C is under investigation as an adjunctive treatment for neonates with DIC, but the risks of either of these potential treatments are great, and risk-to-benefit evaluations are needed.
1Christensen RD, Henry E, Jopling J, et al. The CBC: reference ranges for neonates. Semin Perinatol 2009;33:3–11.
2Kemper AR, Knapp AA, Metterville DR, et al.Weighing the evidence for newborn screening for Hemoglobin H disease. J Pediatr 2011;158:780–3.
3Cohen RS, Wong RJ, Stevenson DK. Understanding neonatal jaundice: a perspective on causation. Pediatr Neonatol 2010;51:143–8.
4Reid J. Neonatal subgaleal hemorrhage. Neonatal Netw 2007;26(4):219–27.
5Wennberg RP, Ahlfors CE, Aravkin AY. Guidelines for neonatal hyperbilirubinemia: an evidence based quagmire. Curr Pharm Des 2009;15:2939–45.
6Geaghan SM. Diagnostic laboratory technologies for the fetus and neonate with isoimmunization. Semin Perinatol 2011;35(3):148–54.
7McPherson RJ, Juul SE. Erythropoietin for infants with hypoxic-ischemic encephalopathy. Curr Opin Pediatr 2010;22:139–45.
8Mosquera C, Miller RS, Simpson LL. Twin-twin transfusion syndrome. Semin Perinatol 2012;36:182–9.
9Strauss RG. How I transfuse red blood cells and platelets to infants with the anemia and thrombocytopenia of prematurity. Transfusion 2008;48:209–17.
10Strauss RG. Anaemia of prematurity: pathophysiology and treatment. Blood Rev 2010;24:221–5.
11deAlarcón PA, Werner EJ, Christensen RD, editors. Neonatal hematology. Cambridge: Cambridge University Press; 2012.
12Wiedmeier SE, Henry E, Sola-Visner MC, et al. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J Perinatol 2009;29:130–6.
13Strauss RG. Platelet transfusions in neonates: questions and answers. Expert Rev Hematol 2010;3:7–9.
14Christensen RD. Platelet transfusion in the neonatal intensive care unit: benefits, risks, alternatives. Neonatology 2011;100:311–8.
15Maheshwari A, Christensen RD, Calhoun DA. Immune neutropenia in the neonate. Adv Pediatr 2002;49:317–39.
16Maheshwari A, Christensen RD, Calhoun DA. Immune neutropenia in the neonate. Adv Pediatr 2002;49:317–39.
17Boztug K, Klein C. Genetic etiologies of severe congenital neutropenia. Curr Opin Pediatr 2011;23:21–6.
18Christensen RD, Jensen J, Maheshwari A, et al. Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63,000 records in a multihospital health-care system. J Perinatol 2010;30:540–5.
19Saxonhouse MA, Manco-Johnson MJ. The evaluation and management of neonatal coagulation disorders. Semin Perinatol 2009;33:52–65.