Immunodeficiency and Cancer
• Cancer remains a major cause of mortality among patients with primary and acquired immunodeficiencies.
• Cancer in immunocompromised hosts is frequently associated with infectious agents, including the following:
 Epstein-Barr virus (associated with lymphoproliferative disorders and leiomyosarcoma)
Epstein-Barr virus (associated with lymphoproliferative disorders and leiomyosarcoma)
 Human herpesvirus 8 (associated with Kaposi sarcoma, Castleman disease, and pleural-based effusion lymphoma)
Human herpesvirus 8 (associated with Kaposi sarcoma, Castleman disease, and pleural-based effusion lymphoma)
 Human papillomavirus (associated with skin anal and cervical carcinomas)
Human papillomavirus (associated with skin anal and cervical carcinomas)
 Helicobacter pylori (associated with gastric carcinomas and mucosa-associated lymphoid tissue lymphomas)
Helicobacter pylori (associated with gastric carcinomas and mucosa-associated lymphoid tissue lymphomas)
• Categories of genetic immunodeficiencies with increased risk of the development of cancer include the following:
 Combined defects with T-cell dysfunction (e.g., severe combined immunodeficiency or Wiskott-Aldrich syndrome)
Combined defects with T-cell dysfunction (e.g., severe combined immunodeficiency or Wiskott-Aldrich syndrome)
 Defects that inhibit lymphoid apoptosis (e.g., autoimmune lymphoproliferative syndrome)
Defects that inhibit lymphoid apoptosis (e.g., autoimmune lymphoproliferative syndrome)
 Defects of genomic instability (e.g., ataxia telangiectasia)
Defects of genomic instability (e.g., ataxia telangiectasia)
 Acquired deficiencies in T-cell immunity with increased risk of developing cancer, such as:
Acquired deficiencies in T-cell immunity with increased risk of developing cancer, such as:
 Human immunodeficiency virus infection
Human immunodeficiency virus infection
 Delayed T-cell recovery after hematopoietic stem cell transplantation or immunosuppression to prevent and/or treat graft-versus-host disease
Delayed T-cell recovery after hematopoietic stem cell transplantation or immunosuppression to prevent and/or treat graft-versus-host disease
 Immunosuppression to treat autoimmune disorders
Immunosuppression to treat autoimmune disorders
 Immunosuppression after solid-organ transplantation
Immunosuppression after solid-organ transplantation
• Cancer can occur de novo, preexisting cancer can recur, or cancer can be transmitted by a donor.
• In general, the outcome for immunocompromised patients with cancer is inferior to the outcome for the general cancer population.
 The outcome is inferior because of the increased risk of infection and/or organ toxicity.
The outcome is inferior because of the increased risk of infection and/or organ toxicity.
 Mortality rates are decreasing with better prevention and screening, improved treatments, and supportive care.
Mortality rates are decreasing with better prevention and screening, improved treatments, and supportive care.
Introduction
In 1959, Lewis Thomas proposed the concept that immune surveillance was an active process controlling the emergence of malignant clones from somatic cells that undergo precancerous mutations during the lifetime of a normal, immune-competent individual.1 This hypothesis predicted that immune-deficient subjects should experience much higher rates of all types of cancers compared with the general population. Indeed, data from many databases have demonstrated an increased incidence of cancer but have not substantiated an increased risk of all cancer types.2–11 In many cases, de novo, reactivated, or chronic infections play a substantial role in tumor development. Epstein-Barr virus (EBV) has been associated with B-cell lymphoproliferative disease, Hodgkin lymphoma, even leiomyosarcoma.12,13 Human immunodeficiency virus (HIV)-infected patients and solid-organ allograft recipients run an increased risk of Kaposi sarcoma, which is associated with human herpesvirus 8 (HHV8), also known as Kaposi sarcoma–associated herpesvirus.14 Additionally, HIV-infected patients and solid-organ allograft recipients are at greater risk of the development of squamous cell carcinoma of the skin, cervix, and anus associated with human papillomavirus (HPV).15–17 Immunocompromised patients, especially those with abnormalities in humoral immunity, experience higher rates of gastric carcinomas and gastric mucosa–associated lymphoid tissue (MALT) lymphomas associated with Helicobacter pylori.18,19
Even among classic primary immunodeficiencies, the array of cancers varies among the specific immunodeficiency diagnoses.2,3 The delineation of precise genetic causes for many of the primary immune deficiencies has made it possible to begin to identify specific molecular pathways to explain the differences in tumors seen among patients with some of these disorders. Patients with inherited defects of genomic instability and DNA repair, which can lead to both immune deficiency and propensity to tumor development, are at increased risk of tumors of hematopoietic and epithelial origin.4,20
Cancer in Primary Immunodeficiencies
Pathogenesis of Cancer in Primary Immunodeficiencies
Advances in prevention and treatment of opportunistic infections now allow patients with primary immunodeficiencies to enjoy longer lives. However, neoplastic disorders—particularly lymphoproliferative complications—remain a major cause of premature mortality, exceeded only by infections.2 The incidence of cancer in patients with primary immunodeficiencies increases with advancing age.2,21 Table 14-1 summarizes the types of cancers observed in patients with primary immunodeficiencies. These data came from the international Immunodeficiency Cancer Registry, which evolved in the 1970s as an outgrowth of the early clinical observations of Gatti and Good3 in immunodeficient children. A retrospective review of clinical and pathological materials from the early cases reveals some imperfections in the original cataloging. For example, it is likely that a significant proportion of the males diagnosed as having hypogammaglobulinemia and in whom lymphomas developed were actually affected with X-linked severe combined immunodeficiency (SCID). Similarly, a review of slides from cases of “leukemia” in patients with SCID, hypogammaglobulinemia, and Wiskott-Aldrich syndrome (WAS) suggest that these were more likely manifestations of non-Hodgkin lymphoma (NHL). Nonetheless, the general outline of tumor types and their proportional distribution among patients with various immunodeficiencies remain relevant even today.
Table 14-1
Immunodeficiency Cancer Registry Cases: Distribution of Tumors and Immunodeficiencies
| Immunodeficiency | Adenocarcinoma (%) | Non-Hodgkin Lymphoma (%) | Hodgkin Lymphoma (%) | Leukemia (%) |
Other Tumors (%) | Total (%) |
| Severe combined immunodeficiency | 1 (2.4) | 3 (73.8) | 4 (9.5) | 5 (11.9) | 1 (2.4) | 42 (8.4) |
| X-linked agammaglobulinemia | 3 (14.3) | 7 (33.3) | 3 (14.3) | 7 (33.3) | 1 (4.8) | 21 (4.2) |
| Common variable immunodeficiency | 20 (16.7) | 55 (45.8) | 8 (6.7) | 8 (6.7) | 29 (24.2) | 120 (24.0) |
| IgA deficiency | 8 (21.1) | 6 (15.8) | 3 (7.9) | 0 (0) | 21 (55.3) | 38 (7.6) |
| Hyper-IgM syndrome | 0 (0) | 9 (56.3) | 4 (25.0) | 0 (0) | 3 (18.8) | 16 (3.2) |
| Wiskott-Aldrich syndrome | 0 | 59 (75.6) | 3 (3.8) | 7 (9.0) | 9 (11.5) | 78 (15.6) |
| Ataxia telangiectasia | 13 | 69 (46.0) | 16 (10.7) | 32 (21.3) | 20 (13.3) | 150 (30.0) |
| Other immunodeficiencies | 1 (4.0) | 12 (48.0) | 1 (4.0) | 4 (16.0) | 7 (28.0) | 25 (5.0) |
| Total immunodeficiency categories | 46 (9.2) | 252 (50.4) | 43 (8.6) | 63 (12.6) | 96 (19.2) | 500 (100) |
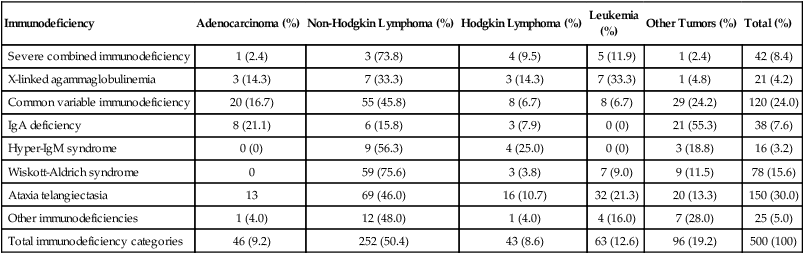
Modified from Filipovich AH, Heinitz KJ, Robison LL, Frizzera G. The immunodeficiency cancer registry: a research resource. Am J Pediatr Hematol Oncol 1987;9:183–4.
The vast majority of cancer in persons with primary immunodeficiencies are lymphoid in origin and are associated with EBV. EBV has been associated not only with B-cell malignancies but also with T-cell malignancies, Hodgkin lymphoma, and gastric carcinoma.12 However, other infectious agents are associated with cancers observed in patients with primary immunodeficiencies, such as H. pylori in MALT lymphomas in patients with humoral deficiencies, particularly IgA deficiency.18 The exception to this rule is cancers that arise in patients with an underlying defect in chromosomal repair, such as ataxia telangiectasia, Nijmegen breakage syndrome (NBS), or Bloom syndrome. In persons with these disorders, the cancers are usually not associated with infectious agents. The lack of immunosurveillance is less likely to be the primary defect leading to cancer development. Instead, the inability to correct genetic alterations is more likely the etiology for the increased risk of cancer in these patients. The following section provides a brief description of the known molecular defects in specific primary immunodeficiencies and the most common cancers observed.
Severe Combined Immunodeficiencies
SCID is a collection of more than a dozen genetically distinct disorders with severe impairment of both cellular and humoral immune function, leading to early mortality from opportunistic infections, usually during infancy.22 EBV-associated lymphomas are almost exclusively seen in patients with SCID. However, the only SCID phenotypes at risk are those in which both B cells (targets for EBV transformation) and severe quantitative or qualitative defects in T cells are present. Examples of such types of SCID include mutations in the following: X-linked common γ-chain gene of multiple interleukin (IL) receptors (IL-2RG), JAK3, IL-7 alpha-chain, CD3 delta and/or epsilon chains, and CD45.22 EBV-associated lymphomas are seen to a lesser extent in patients with purine nucleoside phosphorylase and adenosine deaminase deficiency. In these two diseases, T-cell expansion and function are impaired by accumulation of toxic intracellular metabolites; although B cells are affected to a lesser extent, absence of B cells is common. EBV-associated lymphomas are seen to a lesser extent in persons with Omenn syndrome, caused by mutations in RAG1 genes predominantly, resulting in severe restrictions on both B- and T-cell repertoire development.22
Wiskott-Aldrich Syndrome
WAS, an X-linked disorder of variable immunodeficiency and microthrombocytopenia, results from mutations in the WAS gene.23 The WAS gene encodes a large intracellular protein with several functional domains involved with cytoskeletal integrity and signal transduction. Several molecules reported to be associated with WAS are involved in normal progression through the cell cycle. WAS is expressed in cells of hematopoietic origin and in the thymus. Experimental evidence suggests that B cells in patients with WAS are resistant to apoptosis, and reports of EBV-negative B-cell lymphomas do exist, especially among men with clinically milder forms of WAS, which are sometimes termed “X-linked thrombocytopenia.”
X-Linked Lymphoproliferative Syndrome
X-linked lymphoproliferative (XLP) syndrome, a condition associated with severe or fatal complications of EBV infection and a high risk of lymphoma, results from mutations in the SH2D1A or SAP gene on the X chromosome.24,25 Clinical features of XLP syndrome include an intense immune reaction to EBV associated with hemophagocytosis and liver failure, lymphomas, aplastic anemia, and/or acquired hypogammaglobulinemia.26 Slam-associated protein, an adaptor protein linked to at least four known regulatory molecules, can alter T and natural-killer cell functions in both activating and downregulating directions and is thought to be involved in T-cell/B-cell interactions through cytokine regulation.24 Contrary to early reports, it is now known that many of the lymphomas occurring in patients with XLP syndrome are EBV negative.26
A syndrome caused by a deficiency in the XIAP gene has recently been described.27 Patients have defects in their antiapoptic functions, and the disorder has been named “deficiency of X-linked inhibitor of apoptosis” (XIAP). Because patients with deficiency of XIAP frequently have problems with EBV infections, XIAP has also been called XLP2. However, patients with deficiency of XIAP do not have an increased incidence of lymphoma.28
X-Linked CD40 Ligand Deficiency
X-linked CD40 ligand deficiency, also known as hyper-immunoglobulin M syndrome, results in failure of immunoglobulin switching by B cells (which requires signaling through CD40) and decreased development and maintenance of type 1 cell–mediated responses (including natural killer cell function) because of impaired responsiveness of CD40-expressing monocyte-derived antigen-presenting cells.29 Interestingly, patients with CD40 ligand deficiency seem to have an increased risk of EBV-associated Hodgkin lymphoma, but not NHL. Patients with CD40 ligand deficiency are also at increased risk for biliary carcinomas, because a high rate of sclerosing cholangitis occurs in patients with a history of chronic cryptosporidiosis.30
Autoimmune Lymphoproliferative Syndrome
Autoimmune lymphoproliferative syndrome (ALPS) represents a constellation of genetic apoptosis defects associated with mutations in FAS, Fas ligand, and caspase 8 genes.31 Patients with ALPS usually have an increase of circulating CD3+, αβ T-cell receptor−, CD4−CD8− T cells (so-called double-negative T cells). Characteristic clinical features of ALPS include chronic multifocal lymphadenopathy, splenomegaly, and/or autoimmune cytopenias.31 Most patients experience symptomatic improvement with immunosuppressive therapy, and autoimmune complications generally lessen in severity with advancing age. The incidence of lymphoma, B-cell, T-cell, or Hodgkin lymphoma in patients with ALPS is as high as 30%, and in some patients, more than one lymphoid tumor have developed over time.32
Cancer in Immunodeficiency-Associated Genetic Disorders of DNA Repair
Ataxia Telangiectasia
Ataxia telangiectasia (AT) is an autosomal-recessive disorder characterized by a mutation in the ataxia telangiectasia (ATM) gene, which acts as a sensor of double-stranded DNA breakage and activates numerous damage repair pathways, including cell-cycle checkpoint control, p53 activation, and DNA repair.33,34 Mutations in ATM lead to accelerated telomere loss and premature aging.35 In the context of normal lymphopoiesis, patients with AT demonstrate a 25-fold increase in nonrandom rearrangements of immunoglobulin and T-cell receptor genes compared with lymphocytes from persons without AT.36 Thymic output in persons with AT is very reduced, and a restricted T-cell repertoire emerges from oligoclonal postthymic expansion.37 Lymphoid cancers (both lymphomas and leukemias, which are usually EBV negative and can be either T cell or B cell in origin) are seen in patients with AT. However, epithelial cancers involving the skin, gastrointestinal tract, genitourinary tract, and central nervous system also can develop in persons with AT.4 Multiple tumors can be present simultaneously but more commonly develop sequentially.3
Nijmegen Breakage Syndrome
NBS is another rare autosomal-recessive syndrome that, like AT, is associated with both humoral and T-cell defects, clinical radiosensitivity, chromosomal instability, and predisposition to lymphoid and epithelial cancers.38 Like ATM, the protein that is defective in persons with NBS (NBS1, nibrin, or p95) functions to “sense” DNA double-strand breaks and activates a diversity of corrective actions.
Bloom Syndrome
The autosomal-recessive disorder called Bloom syndrome results from inherited mutations in the BLM gene.39 The BLM protein is a member of the RecQ helicase family and functions during DNA replication or in the postreplication process to resolve aberrancies incurred during replication. Immunodeficiency also develops in patients with Bloom syndrome—especially humoral defects. A propensity for colonic adenomas, epidermal carcinomas, and acute myeloid leukemia has been reported in patients with Bloom syndrome.
Treatment of Cancer in Primary Immunodeficiencies
Regardless of the etiology of the immune defect, immunodeficient children with cancer have a worse prognosis than does the general population.40–42 One potential exception is the more indolent low-grade lymphomas (e.g., MALT lymphomas).43,44 Patients with primary immunodeficiencies usually tolerate cytotoxic therapy poorly, with increased morbidity and mortality due to increased infectious complications and often increased end-organ toxicities.40–42 In the current era, improved antiviral and antifungal therapies allow most patients with primary immunodeficiencies who have cancer to be treated more aggressively. Treatment strategies differ somewhat depending on the immune defect and cancer type. For patients with primary defects in T-cell development or function (e.g., SCID, WAS, and XLP) in whom lymphoma develops, the goal is to achieve remission with the least aggressive therapy required followed by immune reconstitution with allogeneic hematopoietic stem cell transplant (HSCT), if a suitable donor is available.45 Patients with DNA repair defects (e.g., AT and NBS) are particularly difficult to treat.41,42 Although sensitivities to genotoxic agents (e.g., radiation and alkylating agents) may differ depending on the underlying mutation, the use of these agents can produce excessive toxicity in these patients and may increase the risk of secondary malignancies. Furthermore, the treatment of cancer in these patients often exacerbates other manifestations of the disease, such as neurological degeneration and/or chronic pulmonary disease. Therefore survival for more than 5 years after the diagnosis of cancer is rare.42
Cancers in Acquired Immunodeficiencies
Cancer Associated with HIV Infection
The use of combination antiretroviral therapy has been correlated with a significant decline in cancer in HIV-infected patients.46 Although a low CD4 cell count has consistently been identified as a risk factor for the development of cancer in HIV-infected patients, the specific immunologic mechanisms associated with HIV infection that increase the risk of cancer remain ill defined. It is clear that HIV leads to a lack of immunosurveillance to control viral infections, resulting in cancers associated with EBV (B-cell lymphoma, Hodgkin lymphoma, and leiomyosarcoma), HHV8 (Kaposi sarcoma, pleural effusion lymphoma, and Castleman disease), and HPV (cervical and anal neoplasm and skin cancer). See Chapter 65 for more details about HIV-associated malignancies.
Cancer in Patients with Autoimmune disease
The risk of lymphoma is increased in patients with autoimmune disease, although the overall incidence remains quite low.6,47 In patients who receive chronic immunosuppressive therapy, the pathogenesis of lymphoma is likely the same as other immune-deficient states. However, the abnormal immune response and lymphocyte proliferation to chronic antigenic stimulation may also play a role.
Persons with Sjögren syndrome have a sixfold to thirteenfold increased risk of NHL, which is the highest risk among autoimmune disorders.6,48,49 This risk has been associated with both primary and secondary disease. Of note, neither age nor use of immunosuppressive therapy appears associated with this risk.6,48 Persons with systemic lupus erythematosus appear to have a threefold to sevenfold increased risk of NHL. The risk appears to be highest in older patients.6, 49 The risk of NHL in patients with rheumatoid arthritis is increased with the use of immunosuppressive therapy, and use of anti-TNF monoclonal antibody therapy may additionally increase the risk.6,48,49 Inflammatory bowel disease is not associated with increased risk of NHL.6 However, persons with celiac disease have an increased incidence of T-cell NHL and enteropathy-type T-cell lymphoma, and persons with Crohn disease have an increased incidence of hepatosplenic T-cell lymphoma.6,50 There appears to be no increased incidence of NHL in persons with type 1 diabetes, pernicious anemia, scleroderma, myasthenia gravis, sarcoidosis, multiple sclerosis, or polymyositis/dermatomysositis.6
As opposed to NHL observed in other immune-deficient hosts, it appears that upon adjustment for co-morbidities, patients with autoimmune disease do as well as immunocompetent patients when treated with standard chemotherapy regimens,51 although the prognosis for enteropathy-type and hepatosplenic T-cell lymphomas is dismal.50,51
Cancer After Hematopoietic Stem Cell Transplantation
De novo cancers that appear after HSCT can be divided by the time of occurrence after HSCT. Early after HSCT, usually within the first 3 to 6 months, EBV-associated lymphoproliferative disease is almost exclusively seen. This disease occurs because of the delayed T-cell immune reconstitution or T-cell dysfunction associated with aggressive immunosuppression for the treatment of graft-versus-host disease.7 HSCT recipients are also at risk for malignancies that occur late, that is, years after HSCT, but these malignancies are usually a consequence of genotoxic therapies used in HSCT preparative regimens and/or pre-HSCT therapy.7 One large retrospective study identified four high-risk factors: ≥50 years of age of the recipient, ex vivo T-cell depletion, use of antithymocyte globulin (in vivo T-cell depletion), and human leukocyte antigen mismatched donor. The incidence of posttransplant lymphoproliferative disease (PTLD) was <1% with no risk factor and up to 8% for three or more risk factors.52 PTLD has been reported after autologous HSCT, usually in the setting of CD34 selection performed for tumor cell purging.53,54
A common strategy is to monitor the EBV level in peripheral blood by polymerase chain reaction in high-risk patients and initiate anti-CD20 (rituximab) as preemptive therapy to “buy time” for EBV cytotoxic T-lymphocyte reconstitution.55 Adoptive T-cell therapy with EBV-specific cytotoxic T lymphocytes is effective at preventing and treating PTLD after HSCT, but this therapy is limited to only a few centers.56 The outcome of patients who fail to respond to rituximab and/or adoptive cellular therapy is very poor.57
Posttransplant Lymphoma after Solid-Organ Transplantation
De novo Cancer in SOT Recipients
Data from several national registries have shown that SOT recipients have an increased risk of cancer compared with the general population.8–11 Table 14-2 summarizes the results from four national registries. The cumulative incidence of cancer is >30% at 20 years after SOT.9 Compared with the general population, SOT recipients tend to have more advanced stage disease and a worse outcome.58 Cancer accounts for more than 10% of the mortality 10 years or longer after SOT.9
Table 14-2
Risk of Cancer in Solid-Organ Recipients Compared with the General Population
| Cancer Type | Increased Risk vs. General Population |
| All cancers | 2.0-3.0 |
| Skin cancer | 2.5 |
| Melanoma | 1.4-3.2 |
| Nonmelanomatous | 3.0-16.0 |
| Head and neck | 3.0-5.0 |
| Gastrointestinal tract | 0.8-10 |
| Lung | 1.0-2.0 |
| Breast | 0.8-1.2 |
| Liver | 1.0-10.0 |
| Pancreatic | 0.7-2.0 |
| Kidney | 1.4-8.5 |
| Bladder | 1.1-5.1 |
| Ovarian, uterine, cervical | 0.4-6.6 |
| Prostate | 0.8-1.0 |
| Nervous system | 1.0-1.7 |
| Thyroid | 4.5-7.0 |
| Kaposi sarcoma | 9.0-26.0 |
| Leukemia | 1.0-2.6 |
| Non-Hodgkin lymphoma | 3.3-12.5 |
| Hodgkin lymphoma | 2.6-7.4 |
| Myeloma | 1.0-4.0 |
Data from Kasiske B, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant 2004;4:905-13; Villeneuva PJ, Schaubel DE, Fenton SS, et al. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant 2007;7:941-8; Collett D, Mumford L, Banner NR, et al. Comparison of incidence of malignancy in recipients of different types of organ: a UK registry audit. Am J Transplant 2010;10:1889-96; and Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005;80:S25464.
As in HSCT recipients, PTLD accounts for the majority of de novo cancers early after SOT. Although the majority of PTLD cases occur in the first 2 years after SOT, as opposed to HSCT, PTLD after SOT continues to occur over time.59 Additionally, as opposed to HSCT, PTLD after SOT is associated with EBV in only 70% to 80% of cases, with late-occurring PTLD (more than 1 year after transplant) more likely to be EBV negative.60 The use of more intensive and prolonged immunosuppression—especially use of anti–T-cell monoclonal antibody for treatment of graft rejection—has been associated with an increased PTLD risk in patients after SOT.61 The strongest risk factor for the development of PTLD is being EBV-naïve before SOT.62 Therefore the incidence of PTLD is higher in young children compared with adolescents or adults. The incidence of PTLD varies among types of allografts; with abdominal allografts (e.g., liver, kidney, and pancreas), PTLD occurs in 1% to 10% of patients, whereas with heart allografts, PTLD occurs in 5% to 10% of patients, and with lung, intestine, or multivisceral allografts, PTLD occurs in 10% to 15% of patients.63
Although the incidence of PTLD after organ transplantation has declined, the mortality rate remains problematic.63,64 Reduction of immunosuppression is the first line of therapy, and outcome is good for disease that is responsive to this intervention.65 Limited disease that is amenable to complete surgical resection is associated with a high rate of durable remissions.65,66 Rituximab and other anti–B-cell therapies have been used successfully, but relapses occur in about 25% of patients.67,68 Chemotherapy is usually reserved for the most resistant disease and in the past has been associated with a high treatment-related mortality.69,70 According to recent studies, use of chemotherapy and rituximab for patients with aggressive disease has yielded good results.71 A low-dose chemotherapy regimen in combination with or without rituximab has been shown to be effective in children with PTLD.72,73 It remains to be determined in which patients rituximab alone will be sufficient and in which patients chemotherapy is required.
Recurrent Cancer in SOT Recipients
Although SOT has been used as part of the treatment of some cancers—primarily unresectable liver tumors—concern exists about increased risk of recurrence in patients with a history of cancer after SOT. Because of a shortage of organs, patients with a history of cancer usually are required to be in remission for at least 2 years before being considered to be an SOT candidate. Table 14-3 summarizes more than 40 years of data from the Israel Penn International Transplant Tumor Registry of risk of recurrence in patients with a history of cancer who have received SOT.74
Table 14-3
Risk of Cancer Recurrence after Solid-Organ Transplant
| Risk of Recurrence after SOT | Type of Cancer |
| Low risk |
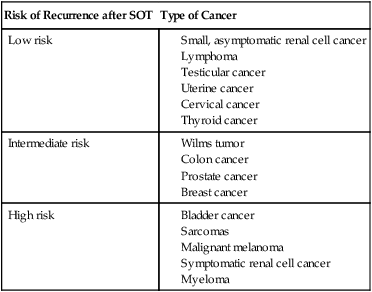
Modified from Trofe J, Beebe TM, Buell JF, et al. Posttransplant malignancy. Prog Transplant 2004;14:193–200.
Donor-Transmitted Cancer after SOT
In numerous cases it has been reported that cancer has been transmitted through organ transplantation. This transmission occurs in the setting of a donor with a history of cancer, when cancer is discovered at the time of the donation, or when genetic studies demonstrate that the cancer is of donor origin even though no cancer was known at the time of the donation. One must differentiate a donor-derived cancer, for example, a renal cell carcinoma in a transplanted kidney many years after donation, from a cancer that was transmitted at the time of donation. It is believed that occult transmission occurs via hematologic spread. If the recipient does not have the ability to “reject” the transmitted tumor cells, then cancer may develop. Data from the United Network of Organ Sharing estimate the risk of donor transmission of cancer in all recipients to be very small, that is, 0.01%.75 Because cancer may not be known or detected at the time of the donation, this figure likely underestimates the true incidence of donor-transmitted cancer. Table 14-4 summarizes data from an expert consensus panel for risk of cancer transmission based on tumor type and stage.76 If a cancer is discovered in the donor at the time of transplantation, or suspected/proven transmission is reported in another recipient from the same organ donor, it is common practice to remove the allografted organ, if possible. However, the risk of transmission versus the risk of being able to undergo a second transplant must be balanced. The outcome for patients in whom a donor-transmitted cancer develops is similar to that of transplant recipients in whom similar cancers develop de novo, that is, inferior to the general population.58
Table 14-4
Estimated Risk of Donor Transmission to Recipient in Solid-Organ Transplant
| Risk of Transmission | Tumor Type |
| No significant risk | Benign tumors |
| Minimal risk (<0.1%) |
CNS tumor with ventricular shunt, surgery other than minimal biopsy, treated with irradiation, or extra-CNS metastases
CNS tumor (WHO grade III or IV)
History of malignancy with: (1) insufficient follow-up to predict probability of cure, (2) considered incurable, or (3) probability of <90% of being cured
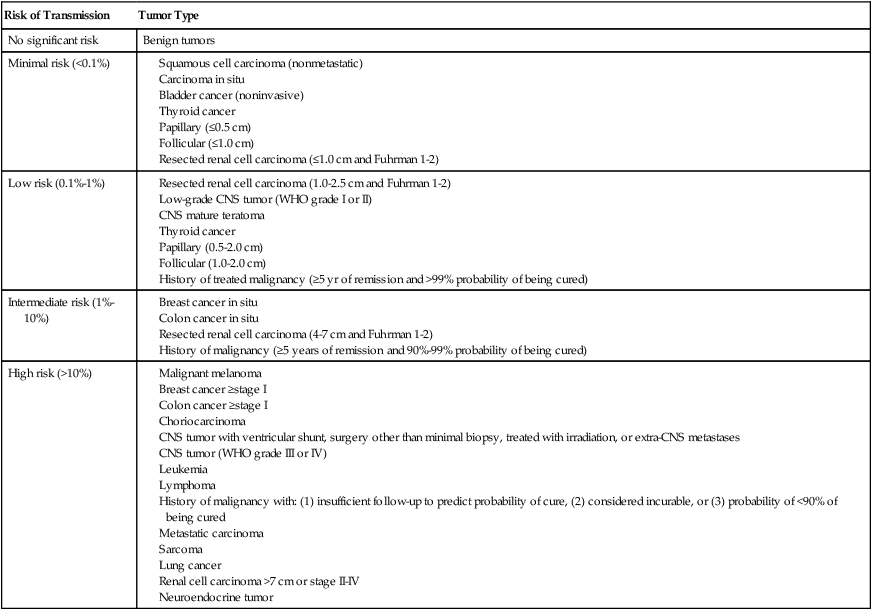
CNS, Central nervous system; WHO, World Health Organization
Modified from Nalesnik MA, Woodle ES, diMaio JM, et al. Donor-transmitted malignancies in organ transplantation: assessment of clinical risk. Am J Transplant 2011;11:1140–7.
Summary
In summary, clinical experience has provided evidence to support Thomas’ hypothesis that immune surveillance is an active process controlling the emergence of malignant clones from somatic cells during the lifetime of a normal, immune-competent individual.1 It is clear that cancers associated with infectious agents, primarily viruses such as EBV, HHV8, and HPV, are much more prevalent in immunocompromised populations. Also, it is now clear that suppression of T-cell immunity is primarily responsible for increasing the risk of developing cancer. Advances have been made to better understand molecular abnormalities in the immune system and the types of cancer to which these abnormalities predispose. Data from large databases have allowed identification of risk factors for cancer development in transplant patients. Additionally, advances have been made to improve the outcome of immunocompromised patients with cancer. However, work continues to reduce the risk of the development of cancer in these patients and to improve outcome.